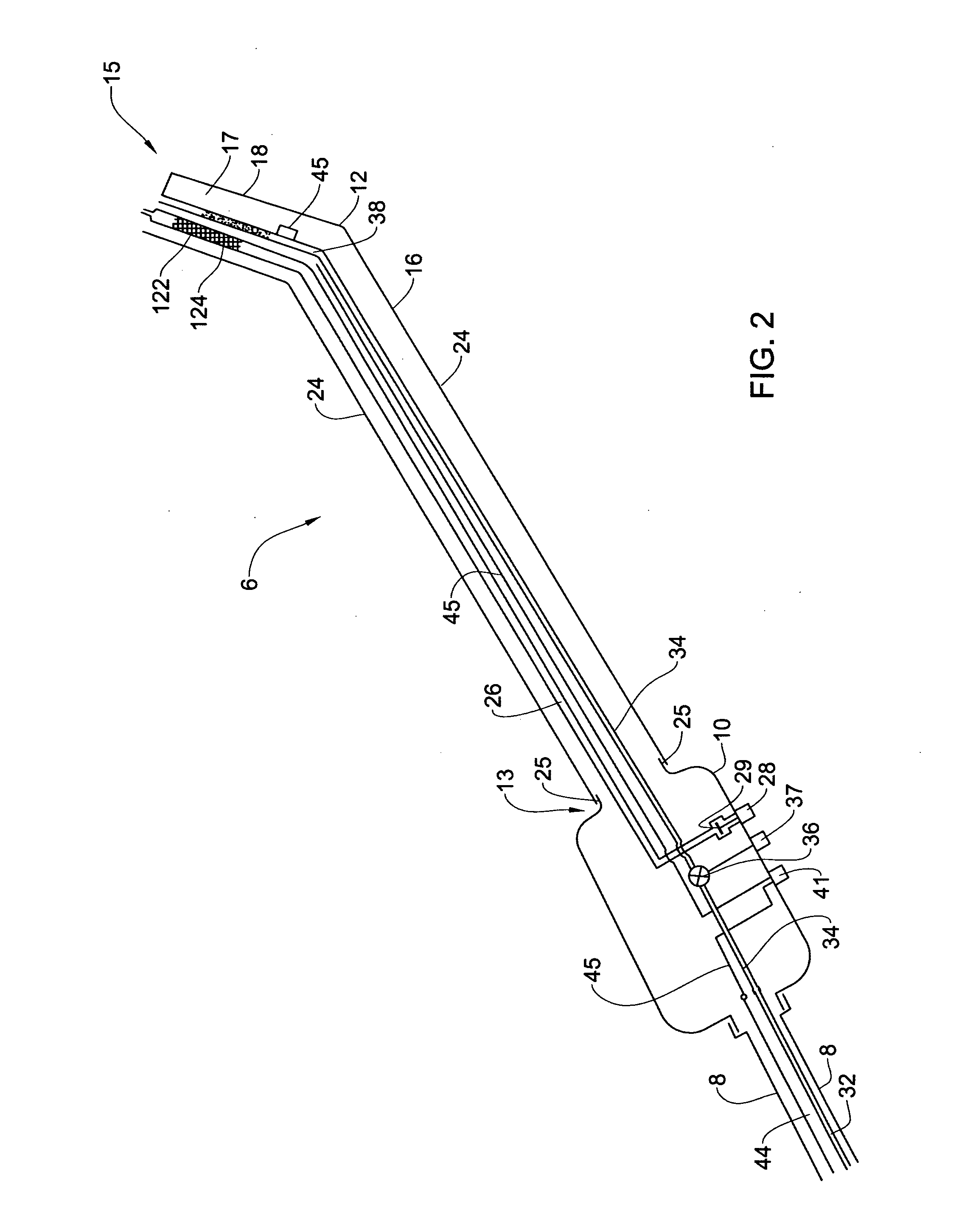
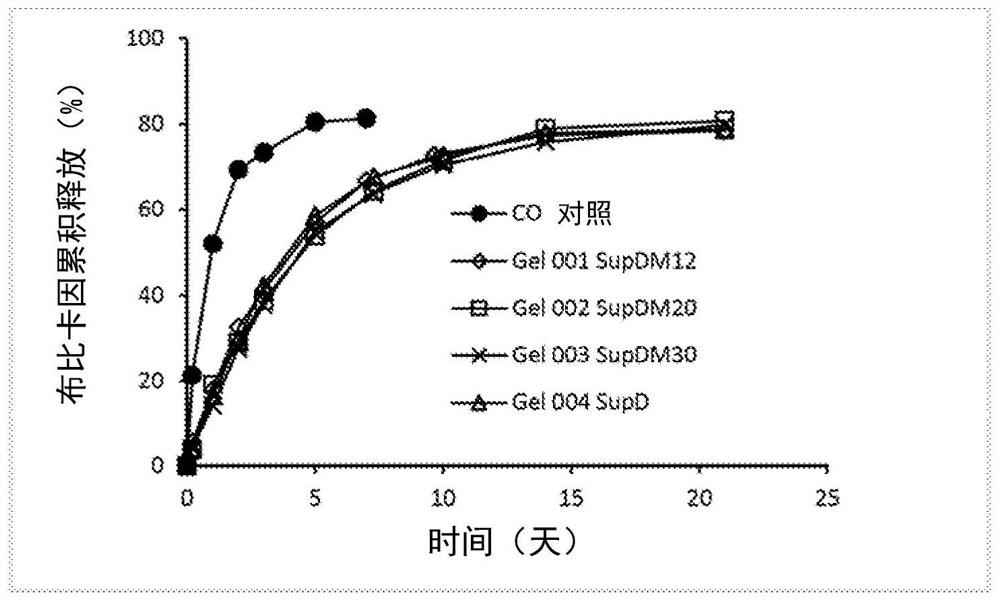
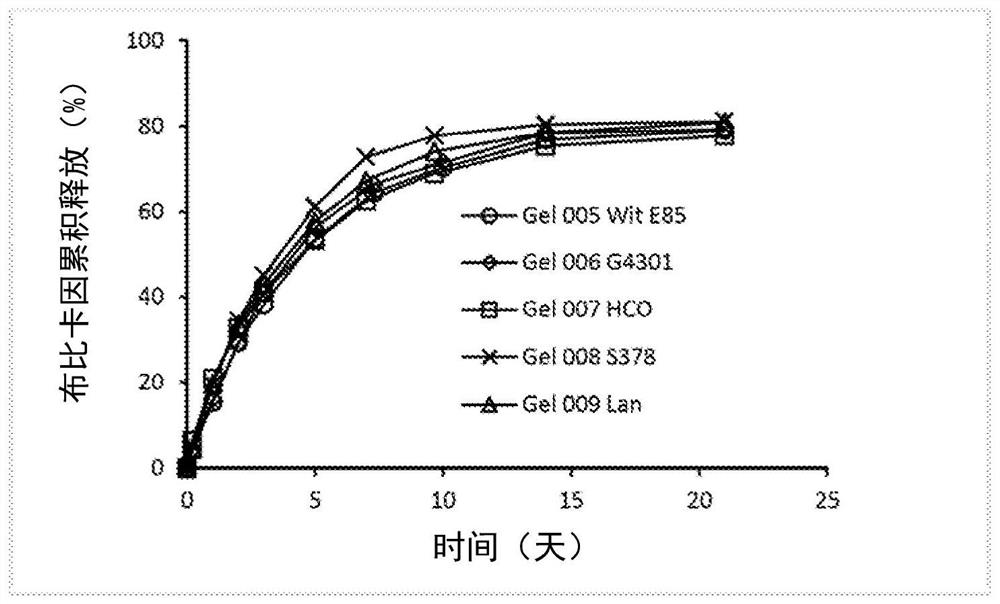
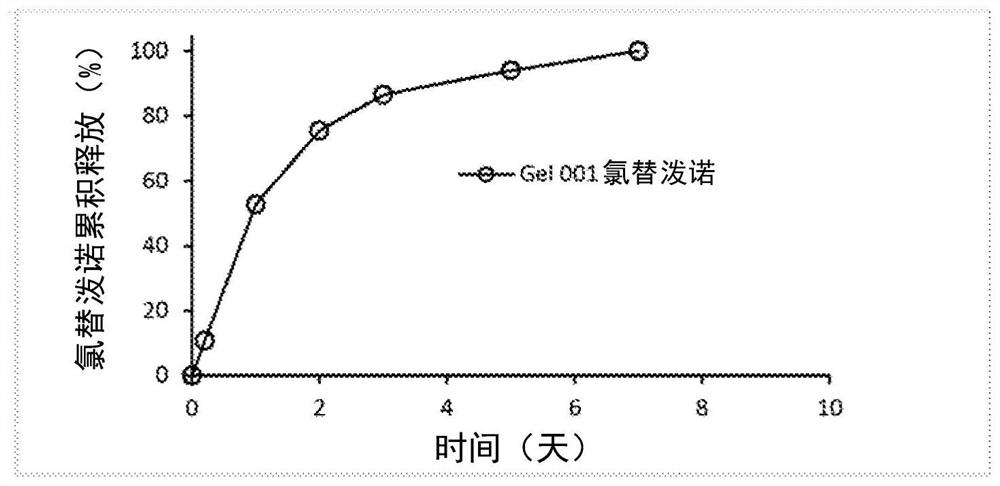
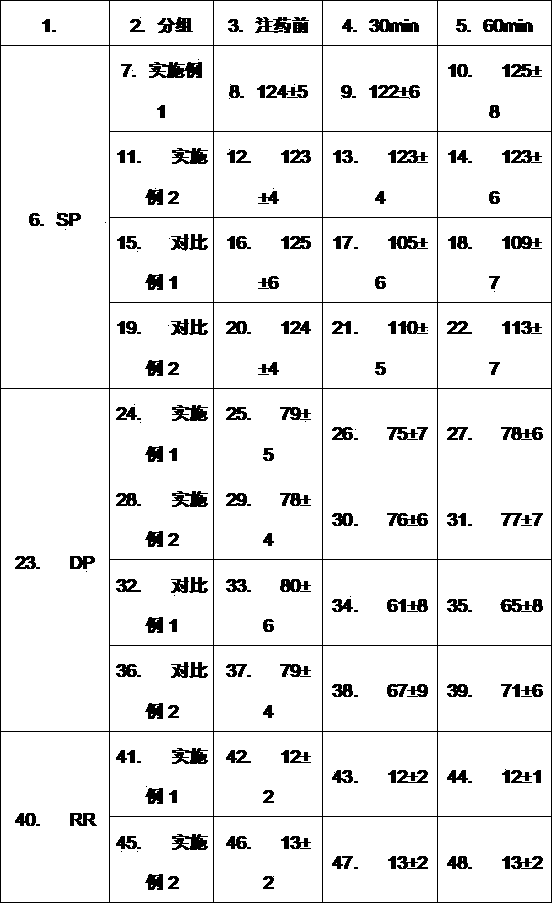
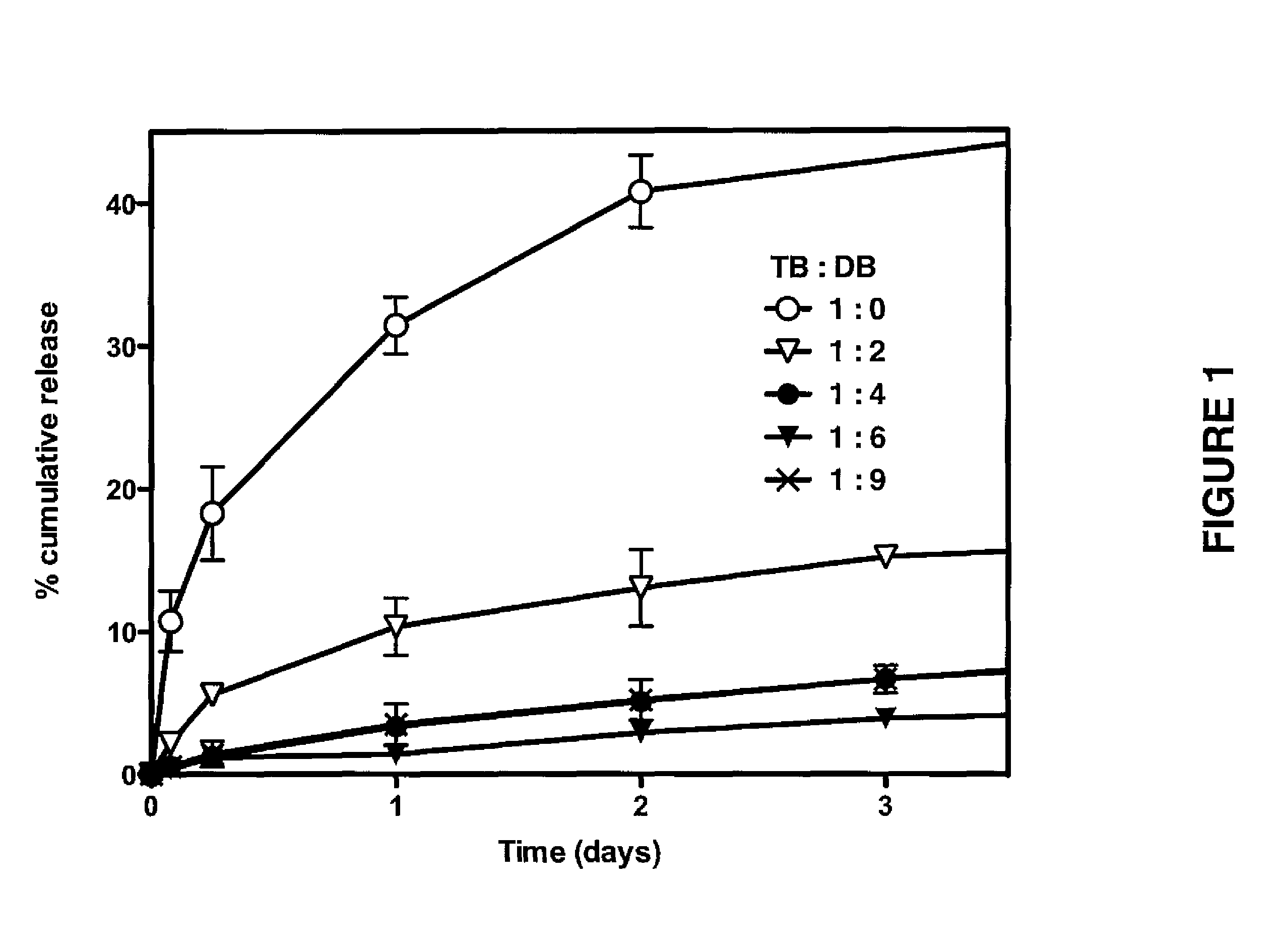
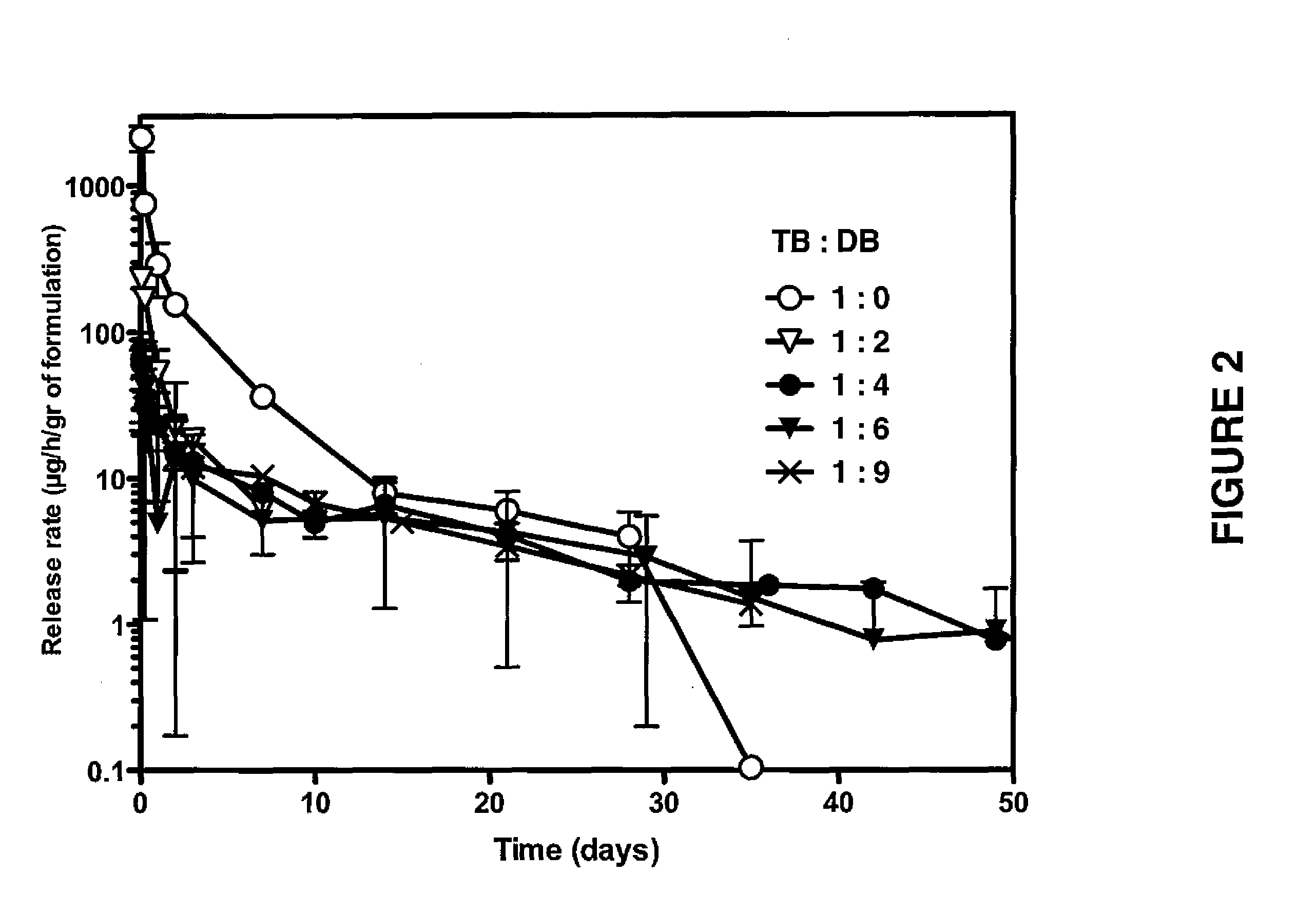
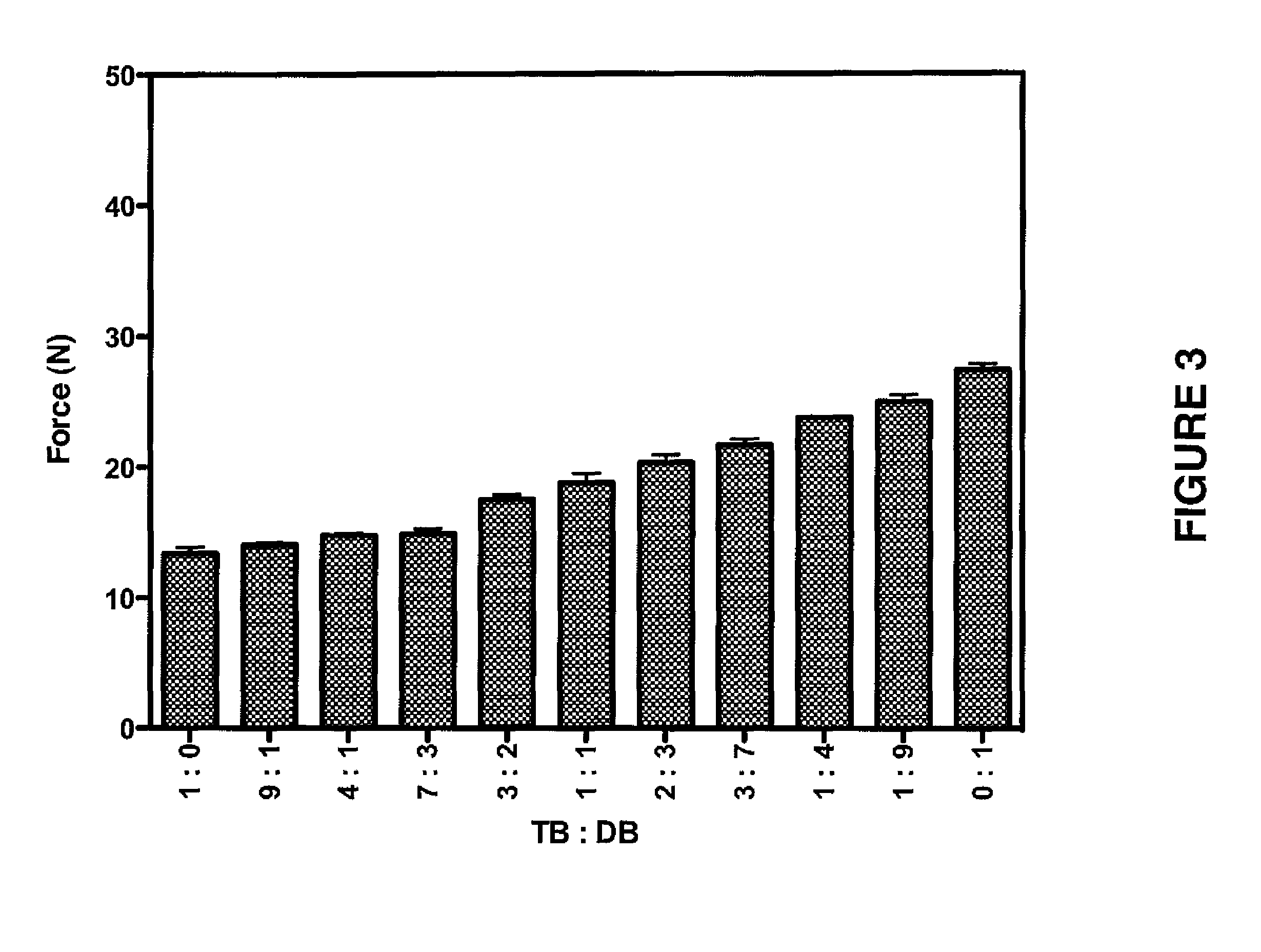
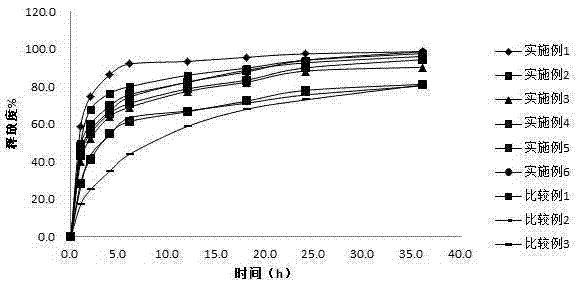
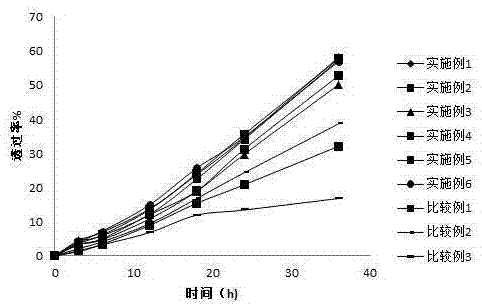
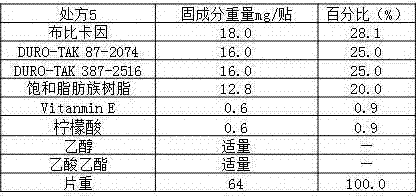
Patents
Literature
72 results about "Bupivacaine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bupivacaine, marketed under the brand name Marcaine among others, is a medication used to decrease feeling in a specific area. In nerve blocks, it is injected around a nerve that supplies the area, or into the spinal canal's epidural space. It is available mixed with a small amount of epinephrine to increase the duration of its action. It typically begins working within 15 minutes and lasts for 2 to 8 hours.
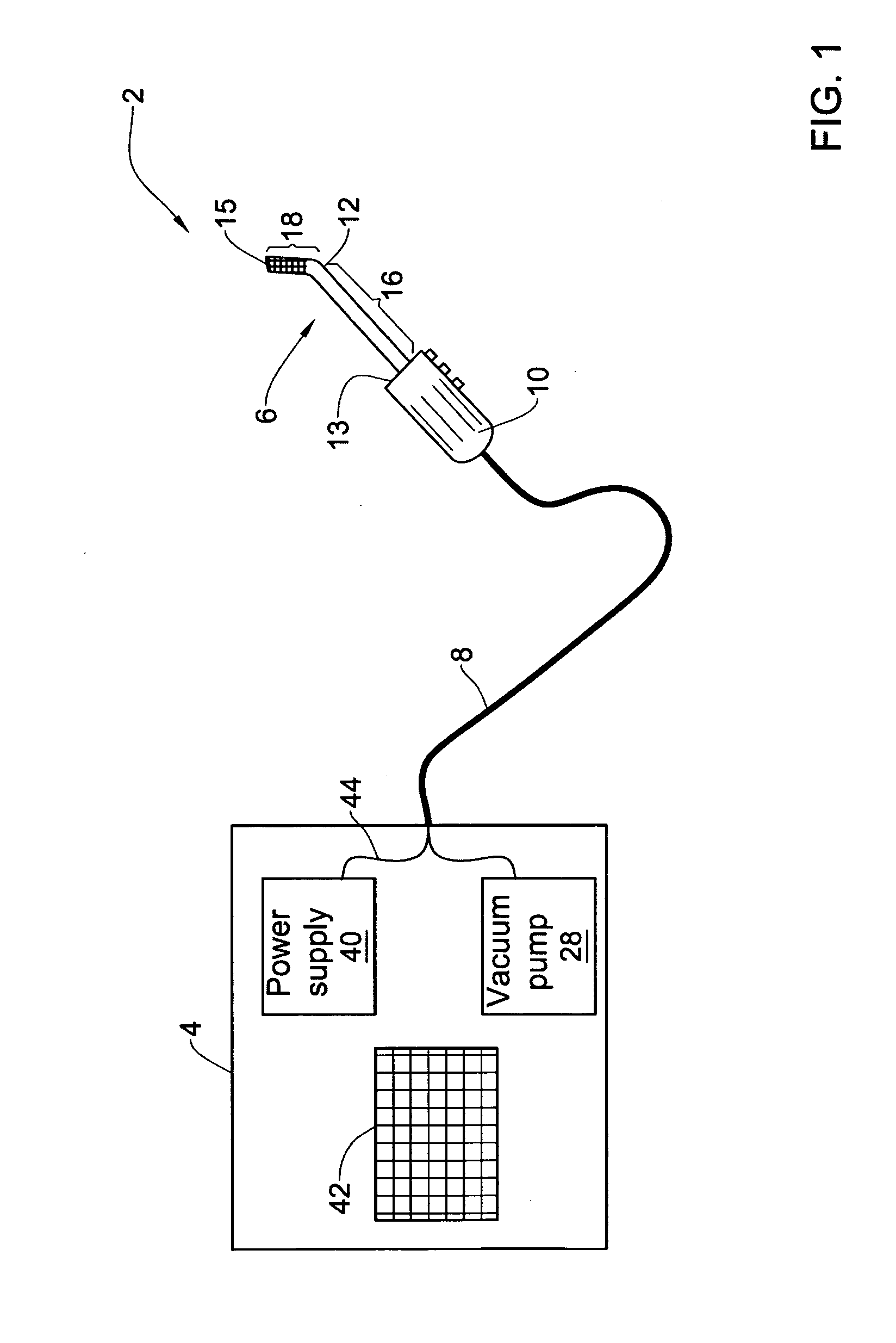
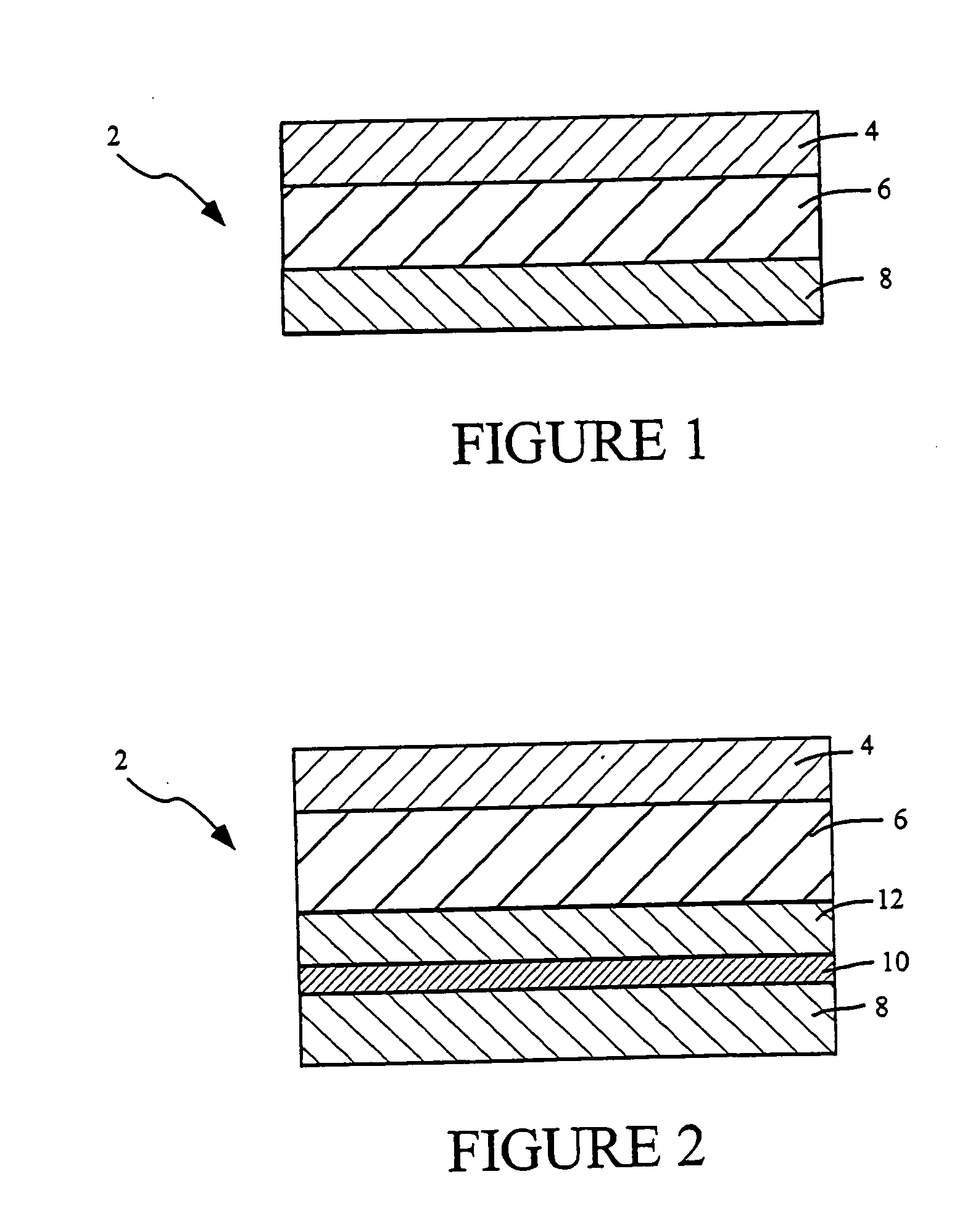
Pharmaceutical composition and system for permeabilizing fetal membranes
Provided is a pharmaceutical composition for permeabilizing fetal membranes including an active ingredient having a log K in the range of 2 to 4, where K is the octanol / water partition coefficient. The active ingredient may be, for example, bupivacaine, sodium lauryl sulfate or oleic acid. Further provided is a system for transfetal membrane transport. The system includes a probe unit adapted for insertion into a female reproductive tract and releasing a substance onto fetal membranes that permeabilizes the membranes. The system is also configured to apply ultrasound radiation to the fetal membranes to further increase the membrane permeability.
Owner:B G NEGEV TECH & APPL LTD
Method and pharmaceutical to treat spinal discs
Methods for reducing chronic pain caused by a disrupted spinal disc are described. In one method, a physiologically acceptable amount of an injectable is injected into the disc. The injectable is obtained from a stock solution comprising chondroitin sulphate, glucosamine HCl, aqueous solution of dextrose; sodium carboxymethylcellulose, and a buffer substance in quantity to bring the pH of the stock solution to a value above about 6.0. Water is also added to dilute the stock solution. The stock solution may further comprise an anesthetic such as bupivicaine.
Owner:NOTOGEN INC
Transdermal delivery systems
Owner:DURECT CORP
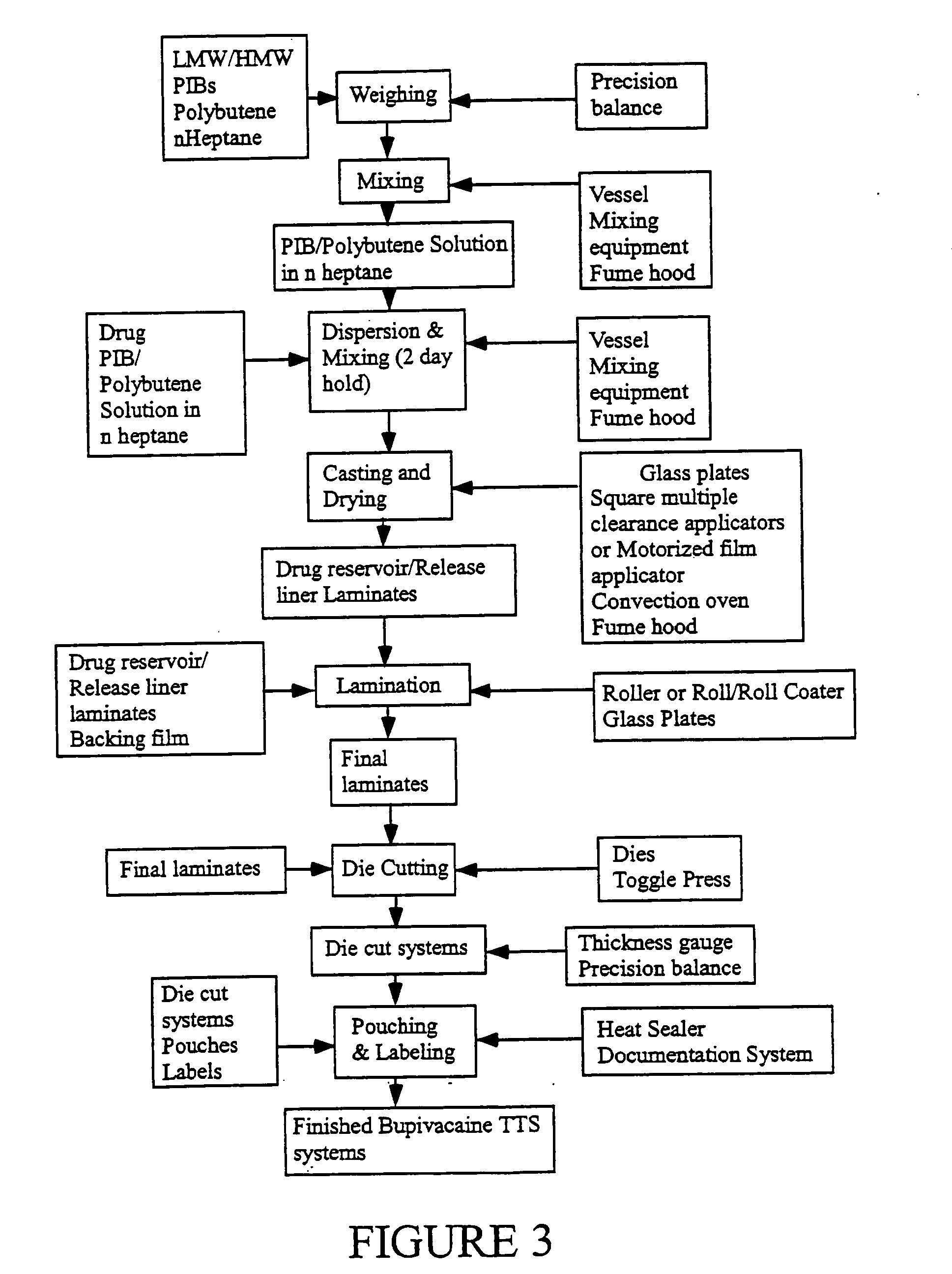
Local anesthetic phospholipid-mixed solvent-oil sustained-release drug delivery system and preparation method thereof
ActiveCN108743952AExtended release timeLess irritatingAntipyreticAnalgesicsVitamin E AcetatePhospholipid
The invention relates to a local anesthetic sustained-release preparation with phospholipid-mixed solvent-oil as a carrier, and a preparation method thereof. The sustained-release preparation is prepared from local anesthetic as an active ingredient and a mixture of phospholipid-mixed solvent-oil as a sustained-release drug delivery system, and selectively is prepared from an antioxidant, whereinthe local anesthetic is selected from one of bupivacaine or ropivacaine free base or a mixture thereof; the mixed solvent is a mixture of benzyl benzoate and one or two of benzyl alcohol, ethanol; theantioxidant is vitamin E acetate, lipoic acid and the like; in the phospholipid-mixed solvent-oil mixture, the content of phospholipid does not exceed 50%, the content of benzyl benzoate does not exceed 10%, and the rest is oil.
Owner:XIAN LIBANG PHARMA TECH
Method for reducing pain
ActiveUS7268109B2Nervous disorderPeptide/protein ingredientsPharmaceutical formulationAnalgesic agents
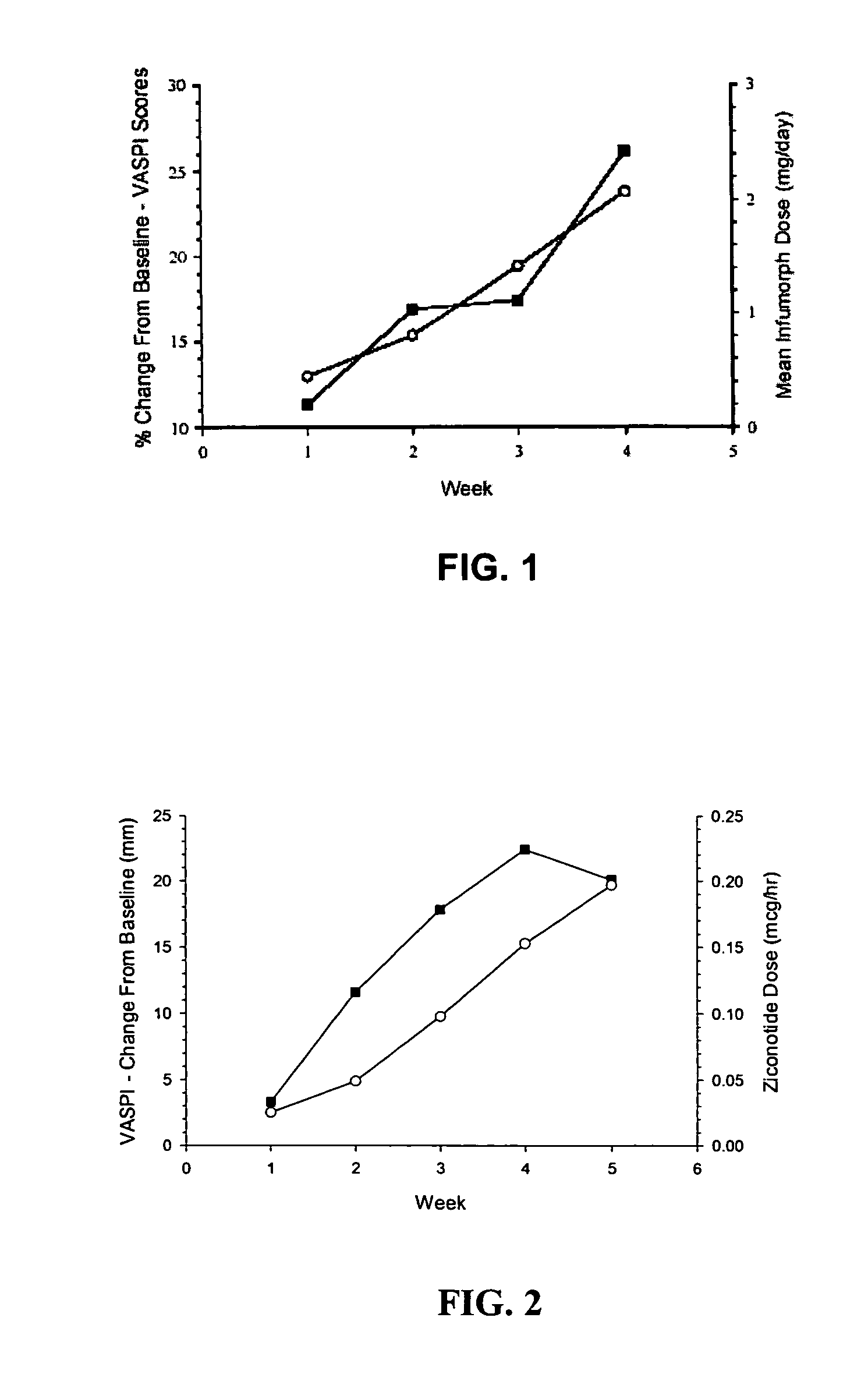
The present invention is direct to a method of producing analgesia in a mammalian subject. The method includes administering to the subject an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyil, buprenorphine, and sufentanil, or its pharmaceutically acceptable salts thereof, wherein the ω-conopeptide retains its potency and is physically and chemically compatible with the analgesic compound. A preferred route of administration is intrathecal administration, particularly continuous intrathecal infusion. The present invention is also directed to a pharmaceutical formulation comprising an omega conopeptide, preferably ziconotide, in combination with an analgesic selected from the group consisting of morphine, bupivicaine, clonidine, hydromorphone, baclofen, fentanyl, buprenorphine, and sufentanil.
Owner:TERSERA THERAPEUTICS LLC
Dermal compositions of substituted amides and the use thereof as medication for pain and pruritus
Dermal compositions comprising topical formulations of bupivacaine or ropivacaine, characterized by effective dermal absorption and long duration of dermal anesthetic activity, and intended for use in patients suffering from pruritus and dermal pain, including neuropathic pain, are provided. Compositions containing both bupivacaine and capsaicin are provided. Methods of alleviating pain by the topically administration of these compounds are also provided.
Owner:BRIDGE PHARMA INC
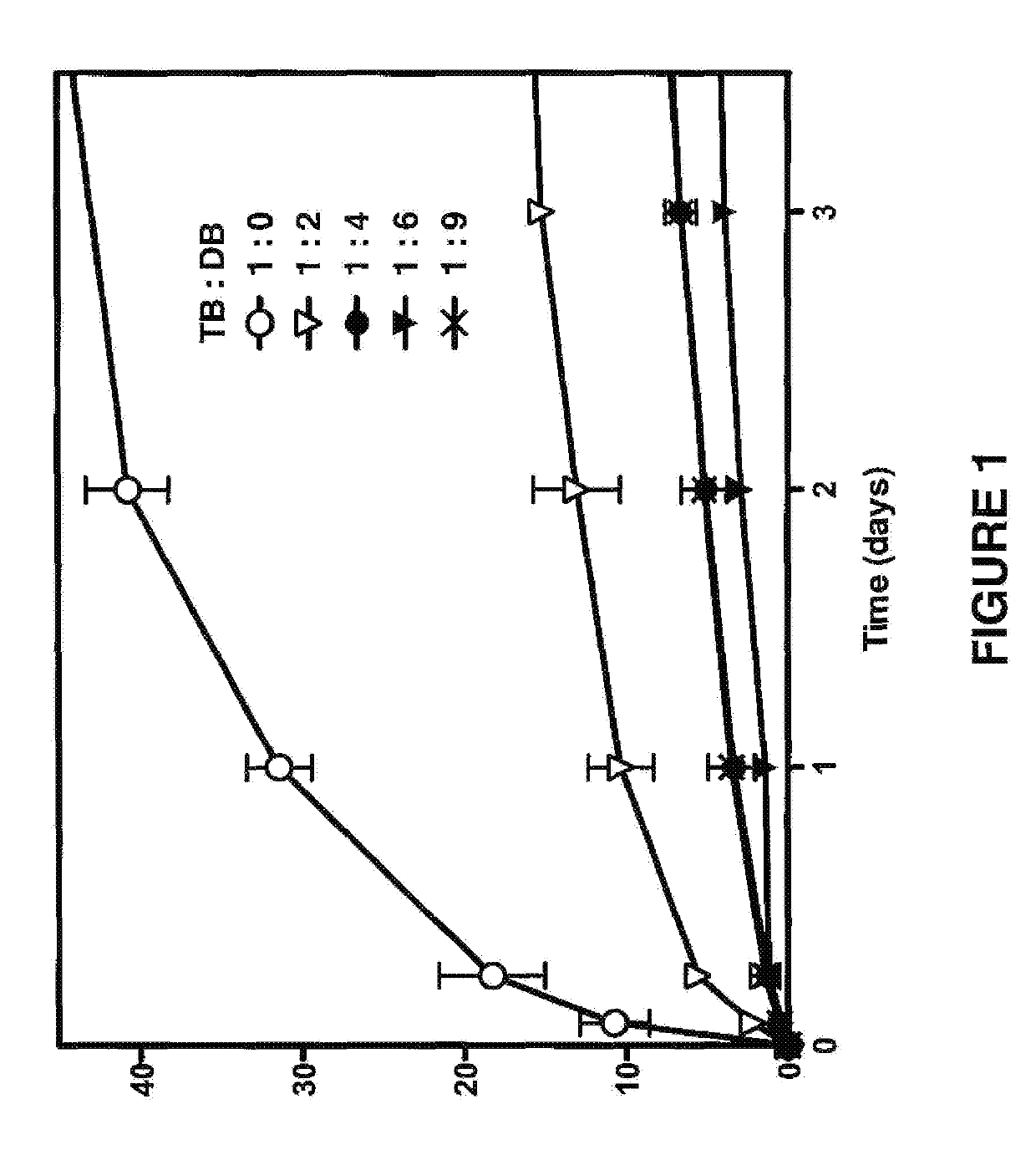
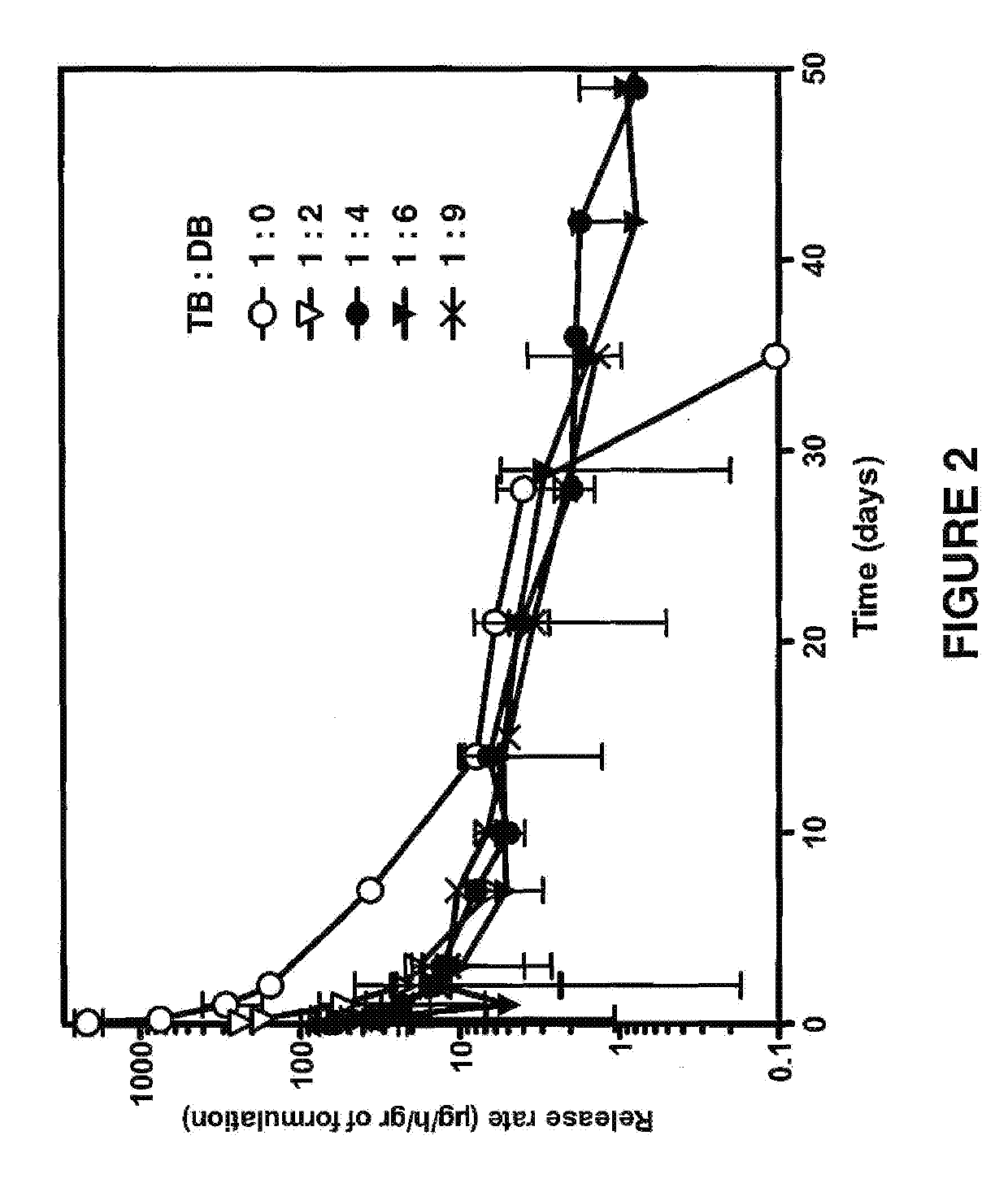
Neosaxitoxin combination formulations for prolonged local anesthesia
ActiveUS8975281B2Low toxicityProlongs the duration of complete blockade to a mechanical stimulusBiocideNervous disorderChannel blockerEpinephrine binding
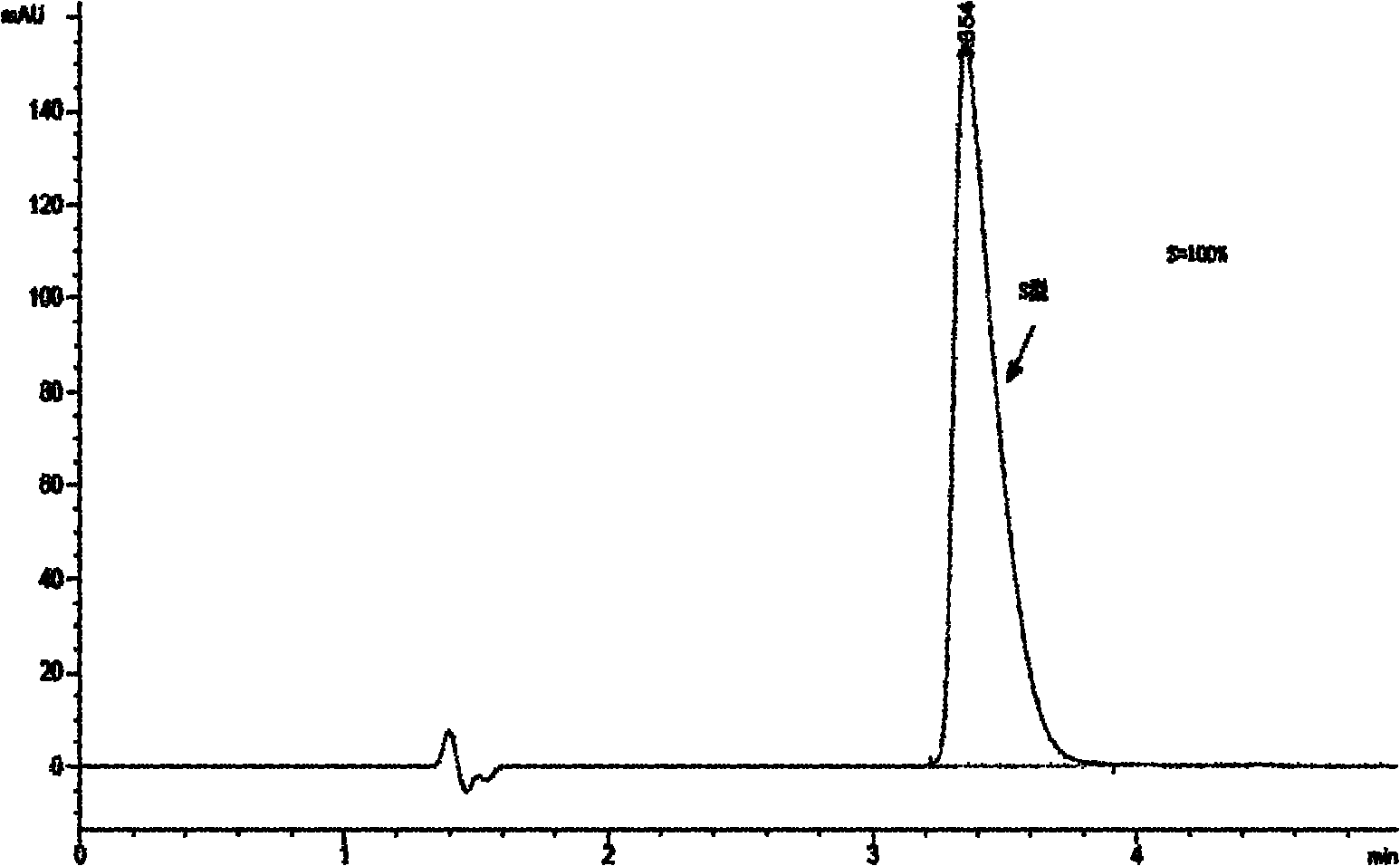
Since each of the site I sodium channel blockers have a unique activity and cannot be used to extrapolate the same effective dosage for another site I sodium channel blocker, studies were conducted to identify dosages of neosaxitoxin (“NeoSTX”) and bupivacaine, alone or in combination with epinephrine, to provide two to three days of pain relief in humans. Bupivacaine-NeoSTX combinations produce more reliable blockade and longer duration blockade compared to NeoSTX alone. The three-way combination of NeoSTX-bupivacaine-epinephrine produces more prolonged local anesthesia than the two-way combination of NeoSTX-bupivacaine. Addition of epinephrine to this NeoSTX-bupivacaine combination dramatically prolongs the duration of complete blockade to a mechanical stimulus. These results led to development of specific combination dosage formulations.
Owner:CHILDRENS MEDICAL CENT CORP
Bis-[6-oxa-(2-carboxylbenzenesulfonyl-butanedioic acid 1,4 monoester-4)-beta-cyclodextrin, preparation method and application thereof
ActiveCN101928356AAchieve separationComponent separationMaterial analysis by electric/magnetic meansProcaineButanedioic acid
The invention relates to bis-[6-oxa-(2-carboxylbenzenesulfonyl-butanedioic acid 1,4 monoester-4)-beta-cyclodextrin, a preparation method and application thereof as chiral selective agent in high performance capillary electrophoresis (HPCE), namely being prepared into a chiral electrophoresis column and a mobile phase chiral additive for detachment of chiral substances. The molecular formula of the bis-[6-oxa-(2-carboxylbenzenesulfonyl-butanedioic acid 1,4 monoester-4)-beta-cyclodextrin is determined to be C64H84O49S2; beta-CD-B2 is used as the HPCE mobile phase chiral additive to separate chiral substances of phenylglycinol, anisodamine, isoprenaline and propafenone to realize baseline separation; beta-CD-B2 is used for preparing a chiral HPCE column to separate chlortrimeton, bupivacaine, procaine, atenolol, anisodamine, propafenone, lobeline and other chiral substances to realize the baseline separation. Therefore, a novel HPCE quantitative measurement method for a single enantiomerof various chiral substances can be established.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Method for simultaneously determining of concentration multi anesthesia medicament in blood plasma
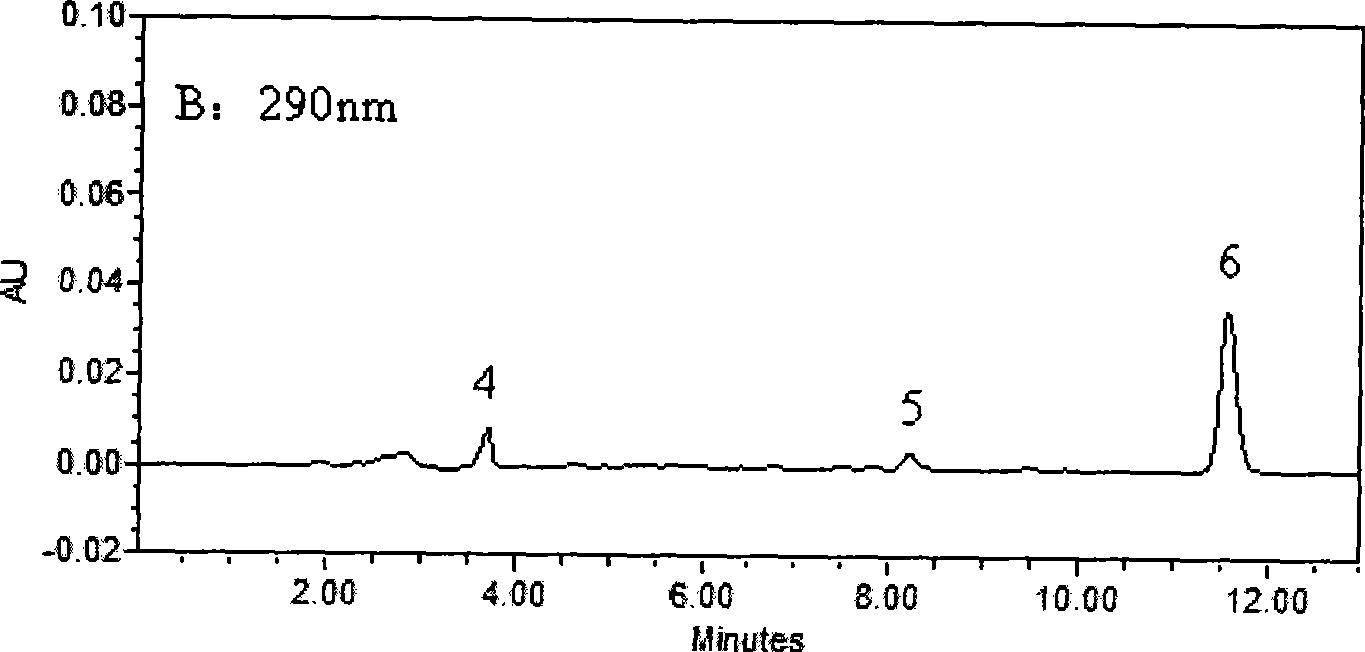
InactiveCN101393196ASimple and fast operationEasy to operateComponent separationMaterial analysis by optical meansProcaineUltraviolet absorption
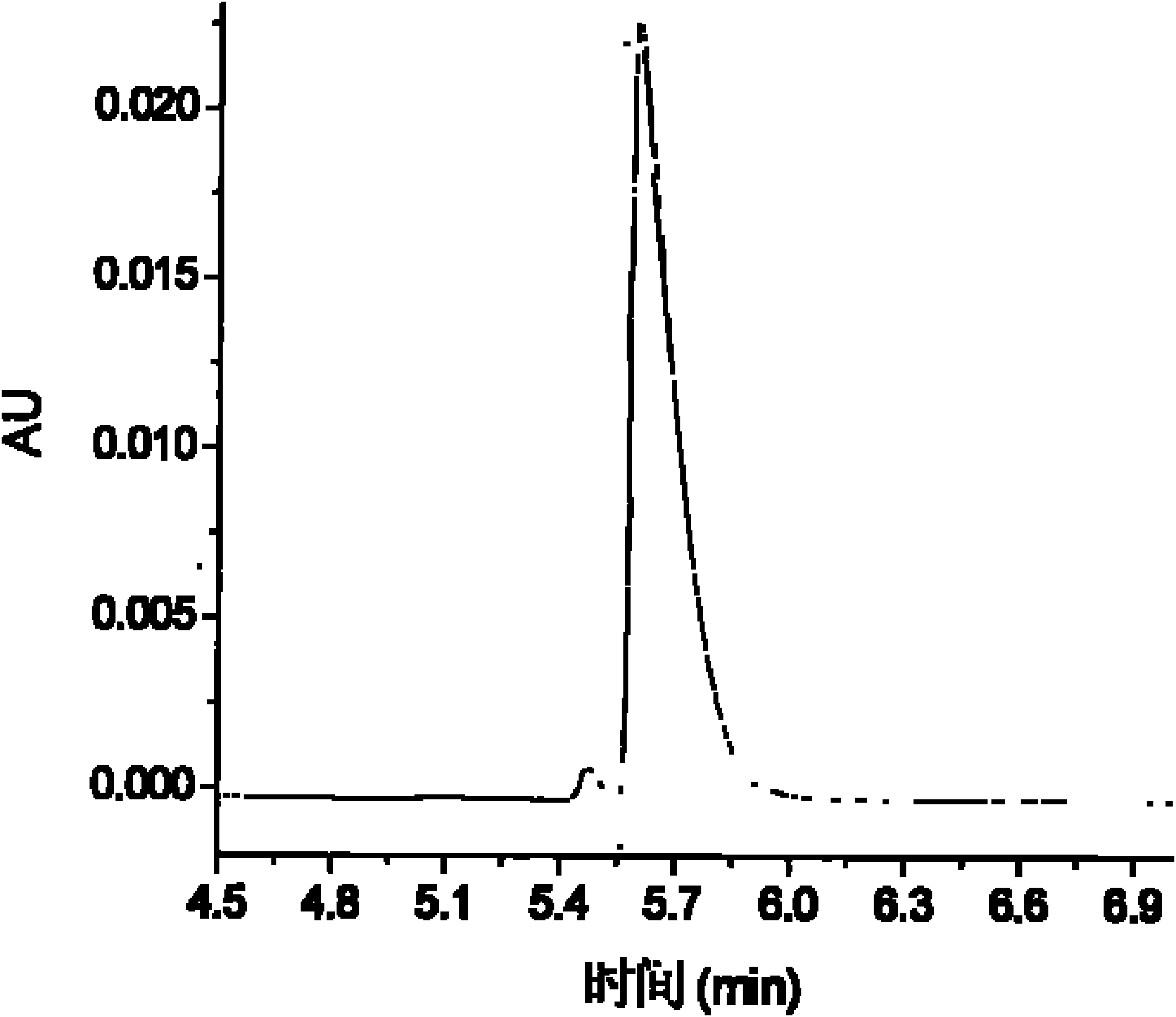
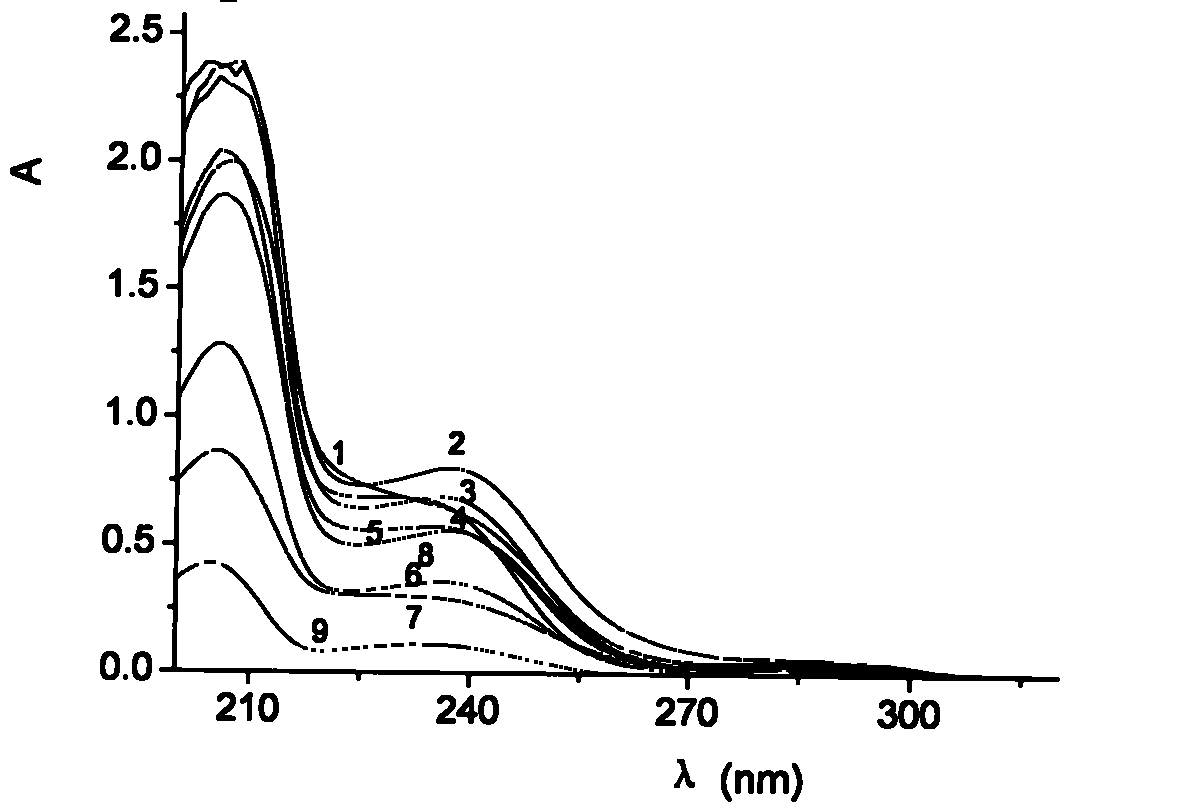
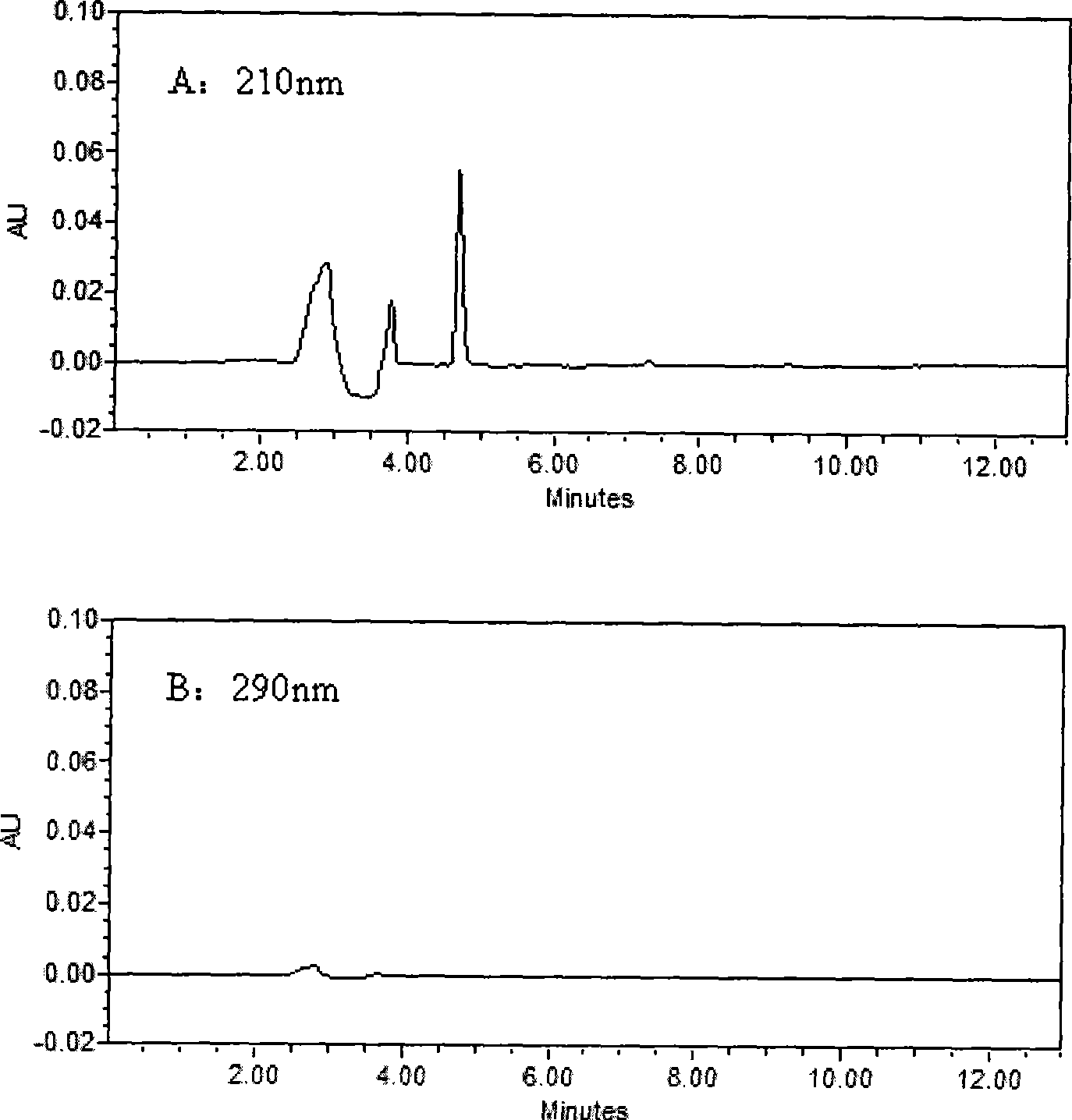
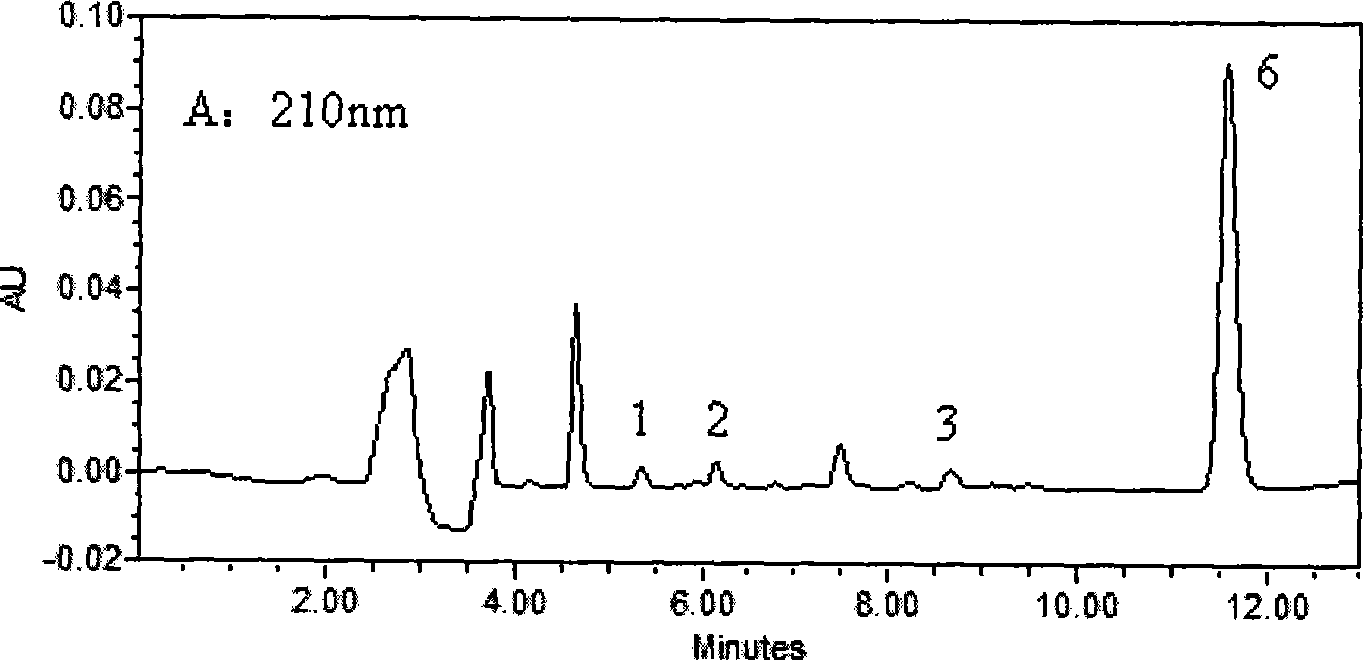
The invention belongs to the field of medical examination and relates to a method capable of synchronously determining the concentrations of a plurality of local anesthetic drugs in human plasma. The method adopts a plasma cholineesterase inhibitor to inhibit the activity of plasma cholinesterase under ice bath condition below 3 DEG C, which controls the hydrolysis of totokaine and assures the accuracy of the method; by utilizing characteristics that lidocaine, ropivacaine and bupivacaine have stronger characteristic of ultraviolet absorption at wavelength of 210nm, and procaine and the totokaine have stronger characteristic of ultraviolet absorption at wavelength of 290nm, an ultraviolet dual-wavelength method is used to detect after the separation of an acid mobile phase at a chromatographic column; and the method can ensure that the sensitivity of synchronous determination of the local anesthetic drugs is greatly improved. The method has less sampling from samples and simple, quickand sensitive pretreatment, does not need expensive equipment or reagents, has short analysis period and low cost, and is suitable for the monitoring of clinical conventional blood concentration of aplurality of drugs.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Microneedle devices and methods
A medical device, comprising: an array of microneedles, and a coating disposed on the microneedles, wherein the coating comprises: a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of tetracaine, ropivacaine, bupivacaine, procaine and a combination thereof; wherein the local anesthetic is present in an amount of at least 1 wt-% based upon total weight of solids in the coating, and wherein the local anesthetic and dose-extending component are in a non-eutectic weight ratio; a medical device, comprising an array of dissolvable microneedles, the microneedles comprising: a dissolvable matrix material; at least 1 wt-% of a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of tetracaine, ropivacaine, bupivacaine, procaine and a combination thereof; wherein the local anesthetic and dose-extending component are in a non-eutectic weight ratio, and wherein wt-% is based upon total weight of solids in all portions of the dissolvable microneedles which contain the local anesthetic; a method of extending a topically delivered local anesthetic dose in mammalian tissue using the devices; and methods of making the devices are provided.
Owner:3M INNOVATIVE PROPERTIES CO
Synthesis method of bupivacaine
InactiveCN105418489AHigh yieldReduce pollutionOrganic chemistryDimethylaniline N-oxideSynthesis methods
The invention belongs to a synthesis method of bupivacaine. The method comprises: adding 2 piperidinecarboxylicacid into aqueous alkali, dropwise adding Cbz and alkaline water, after finishing dropwise adding at normal temperature, reacting for 12 hours, after reaction, extracting with diethyl ether, washing a water layer to be weak-acid with 18% of diluted hydrochloric acid, extracting with the diethyl ether again, combining an diethyl ether layer, drying and filtering, and concentrating to obtain a dried product; adding the dried product into a DMF solvent, then adding a catalyst for reaction for 1 hour at normal temperature, then adding 2,6-dimethylaniline, reacting for 18hours at normal temperature, adding water and ethyl acetate for washing, taking an organic layer, drying and filtering, and concentrating to obtain a dried concentrated product; adding the dried concentrated product into a solvent, then adding a catalyst, pressurizing and introducing hydrogen, filtering after reaction and concentrating to obtain a dried product; adding the product in the above step into a solvent, dropwise adding bromo-n-butane at normal temperature, after dropwise adding, rising temperature to 80 DEG C for reacting for 12 hours, adding diluted hydrochloric acid, slowing cooling to normal temperature, and crystallizing, filtering and drying to obtain the product. The synthesis method has the advantages of higher yield, smaller pollution and low equipment requirement.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
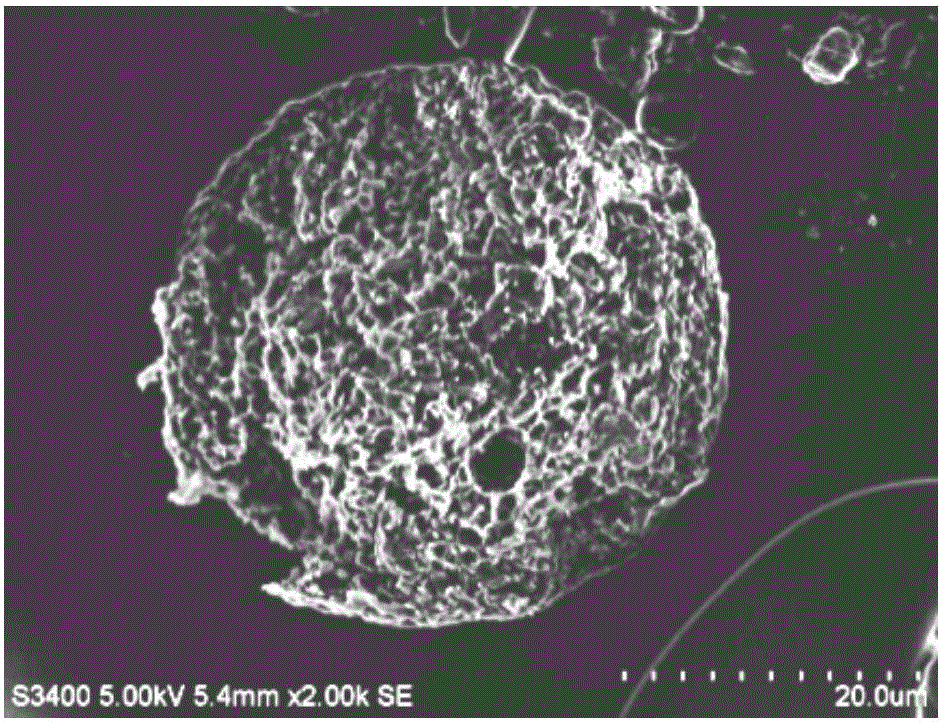
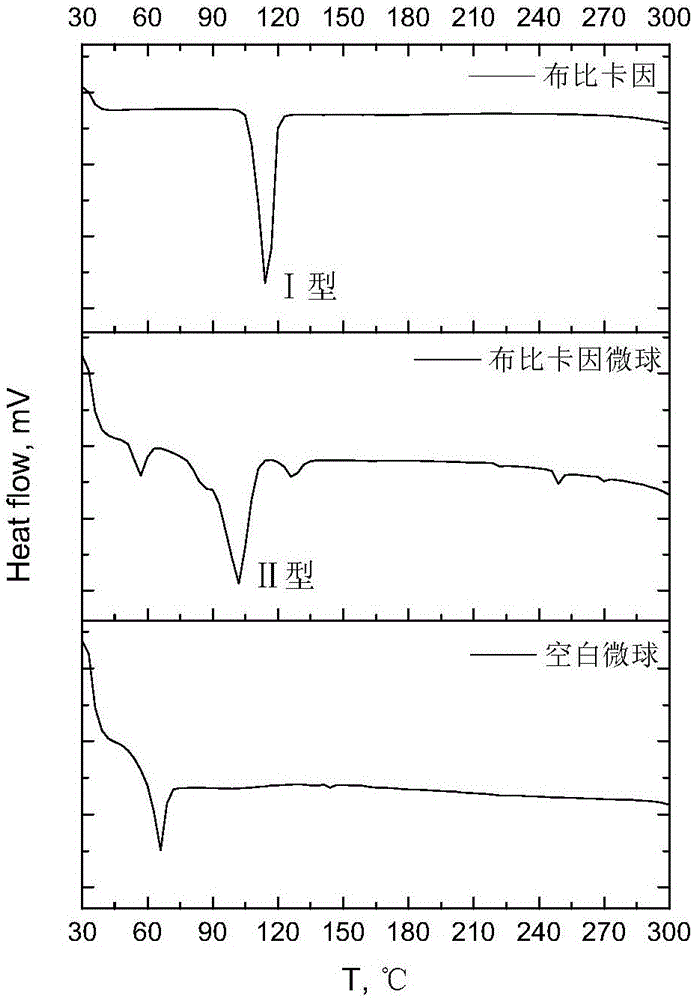
Preparation and application of biodegradable bupivacaine microspheres with high drug loading capacity
ActiveCN106344521AThe emulsification-solvent evaporation method is simple and easyMature technologyAnaestheticsPharmaceutical non-active ingredientsMedicineMicrosphere
The invention relates to the technical field of pharmaceutical preparations, and provides a preparation method of biodegradable bupivacaine microspheres with high drug loading capacity. Bupivacaine microspheres entrapping free alkali are prepared by utilizing biodegradable high-molecular-weight polylactic acid as a carrier material according to an emulsified solvent evaporation method. The frozen-dried bupivacaine microspheres are dispersed in a medium for injection so as to be subjected to local injection administration, and is used for postoperative analgesia or is used for relieving local pains; the weight ratio of bupivacaine to the carrier material is (75:25) to (85:15), the drug loading capacity of the bupivacaine in the microspheres is not less than 60%, and medicaments can be completely released within 5-7 days.
Owner:SHENYANG PHARMA UNIVERSITY
Methods of administering drugs in an implantable multi-chamber pump
InactiveUS20140296830A1Relieve painReducing severe and chronic painMedical devicesPressure infusionNeuropathic painZiconotide
One embodiment of the present invention is a method for reducing pain using a multi chamber pump to separately administer multiple drugs. For example, one chamber may contain an omega conopeptide, such as ziconotide, and the other chamber or chambers may contain one or more other drugs, which may include of morphine, hydromorphone, fentanyl, sufentanil, bupivacaine, baclofen, clonidine, and buprenorphine, or their pharmaceutically acceptable salts thereof. Other applications of the present invention include methods for treating severe chronic pain due to cancer, failed back syndrome, CRPS, neuropathic pain, mixed neuropathic and nociceptive pain.
Owner:JAZZ PHARMA
Medicine composition with functions of narcotizing in operation and alleviating pain after operation
InactiveCN101732524AHas an anesthetic effectRelieve painNervous disorderHydroxy compound active ingredientsSurgical operationMenthol
The invention discloses a medicine composition with the functions of narcotizing in an operation and alleviating pain after the operation. The medicine composition comprises tetracaine, bupivacaine, mastic, rhizoma cyperi, girald daphne bark, white peony root and menthol. The medicine composition is added with conventional auxiliary materials in pharmacy according to a conventional process to prepare a clinically acceptable medicament form. The medicine composition is a medicine for injecting and narcotizing in the operation and alleviating pain in a long acting way after the operation and is already applied to clinic successfully throughout the years. The medicine composition can enable the surgical field to be clear, can also avoid pain, sagging distention, frequent defecation and uroschesis caused by the operation, can loosen the anal canal fully and is convenient for the surgical operation.
Owner:刘振起
Biodegradable drug delivery for hydrophobic compositions
InactiveUS20190160171A1Cyclic peptide ingredientsPharmaceutical non-active ingredientsPolyesterPolymer science
A biodegradable drug delivery compositions comprising a triblock copolymer containing a polyester and a polyethylene glycol and a diblock copolymer containing a polyester and an end-capped polyethylene glycol, as well as at least one pharmaceutically active principle or hydrophobic active principle such as medroxyprogesterone acetate, levonorgestrel, cyclosporine, progesterone or bupivacaine is disclosed.
Owner:MEDINCELL SA
Amide compound, preparation method and application thereof
ActiveCN112574098AReduce dosageImprove securityOrganic chemistryAntipyreticPharmaceutical drugNarcotic
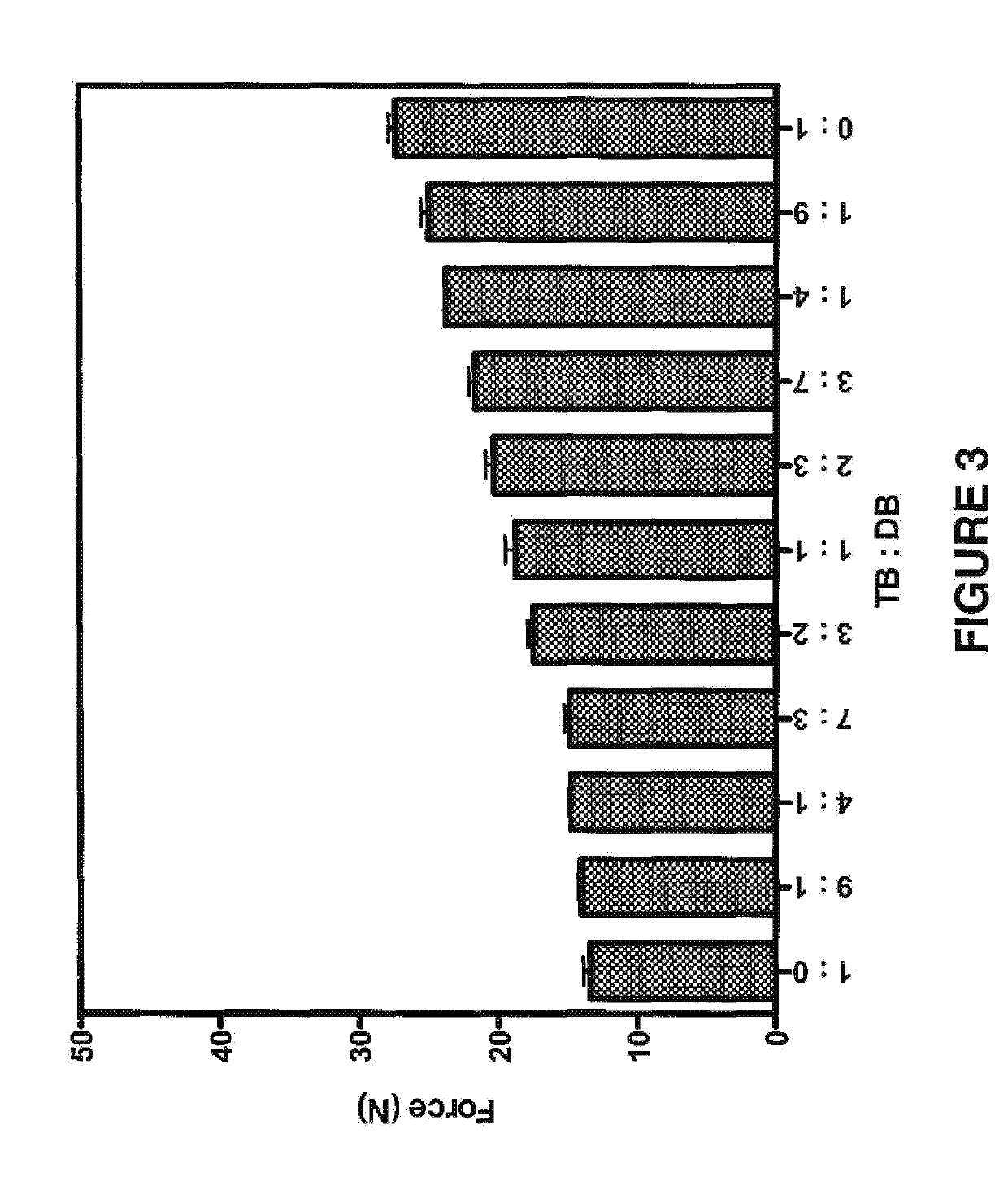
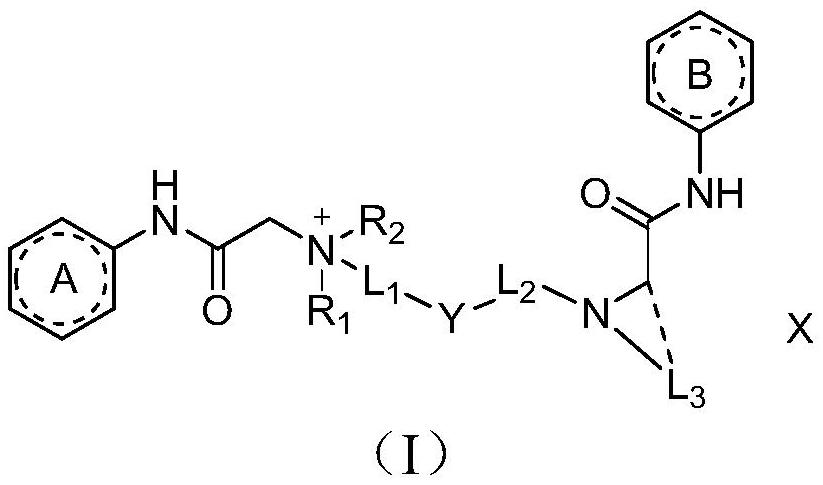
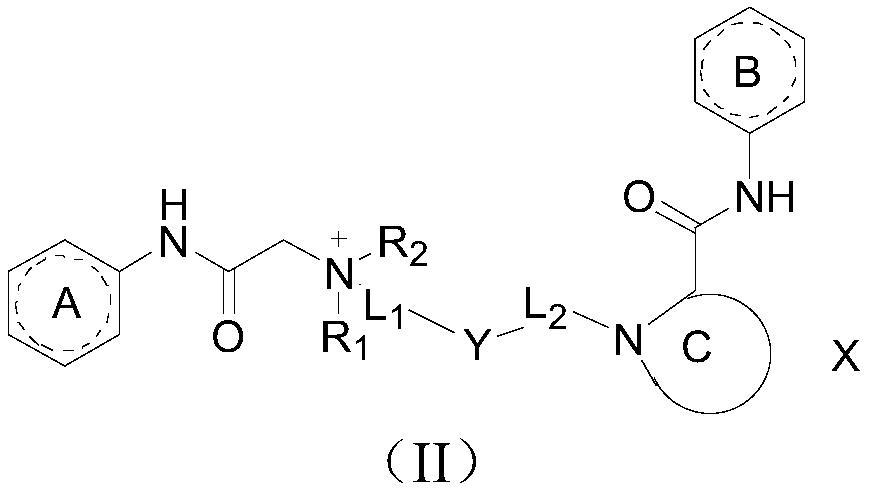
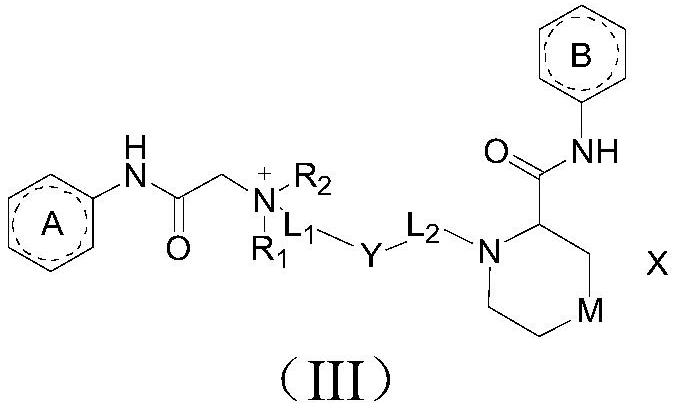
The invention discloses an amide compound, a preparation method and application thereof, and particularly provides a compound as shown in a formula (I), or a salt thereof, or an isotope replacement form thereof, or an optical isomer thereof, or a solvate thereof, or a crystal form thereof, or a prodrug thereof. According to the invention, the local anesthetic effect duration of the compound provided by the invention is obviously longer than that of a contrast drug bupivacaine; and compared with bupivacaine, the compound provided by the invention is higher in titer and better in safety, can bedecomposed and removed from plasma more quickly, and has a very good application prospect in preparation of local anesthetic drugs or local analgesic drugs.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Sustained-release analgesic drug
The invention relates to a sustained-release analgesic drug, which is microsphere of a topical anesthesia agent. The topical anesthesia agent comprises lidocaine, tetracaine, bupivacaine, oxybuprocaine and procaine. The sustained-release microsphere provided by the invention can be prepared into a sterile microsphere injection, a powder, a spray, a tablet, a capsule, a lozenge, a soft capsule, a pill, a syrup, an external ointment, an emulsion, a bandage, a binder and a gauze. The sustained-release microsphere can be prepared by a multiple double emulsion method, a non-aqueous method, a low temperature spray extraction method or a phase coacervation method. The sustained-release analgesic drug provided by the invention can be used for pain killing of skin wounds with small area, such as cuts, abrasions and burns, and operation wounds.
Owner:伍丽娟
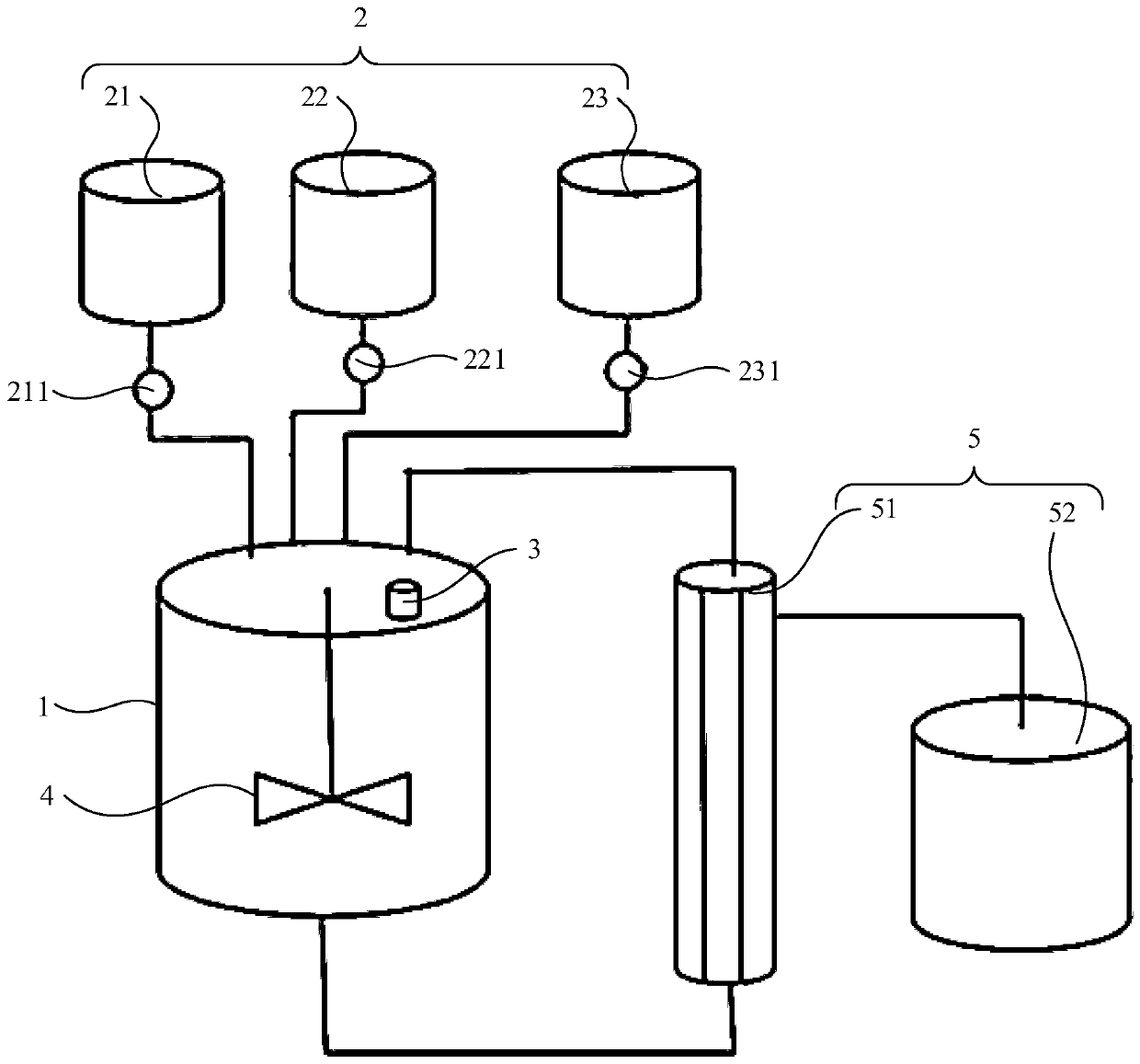
High-concentration bupivacaine multivesicular liposome, and preparation method and liquid distribution system thereof
PendingCN110179752AReduce in quantityReduce control difficultyTransportation and packagingMixer accessoriesHigh concentrationNitrogen generator
The invention belongs to the technical field of preparations, and specifically relates to a high-concentration bupivacaine multivesicular liposome as well as a preparation method and a liquid distribution system thereof. The liquid distribution system comprises a main liquid distribution tank, a plurality of auxiliary liquid distribution tanks which are arranged in parallel on the main liquid distribution tank, and a heating apparatus and an air exhaust apparatus which are respectively located on the main liquid distribution tank, wherein the heating apparatus is suitable for heating test liquid in the main liquid distribution tank and the air exhaust apparatus is suitable for maintaining vacuum degree in the main liquid distribution tank, thereby allowing extraction of volatile organic solvents from the test liquid; and thus, nitrogen generator and gas sterile filtration are not required while sterility control of gas is not needed, so that, difficulty of control of aseptic process isreduced with aseptic environment in liquid distribution process improved. Therefore, the liquid distribution system is suitable for industrial production.
Owner:常州吾合生物医药有限责任公司
Method for enriching piperidine-2-formanilide optically active compound
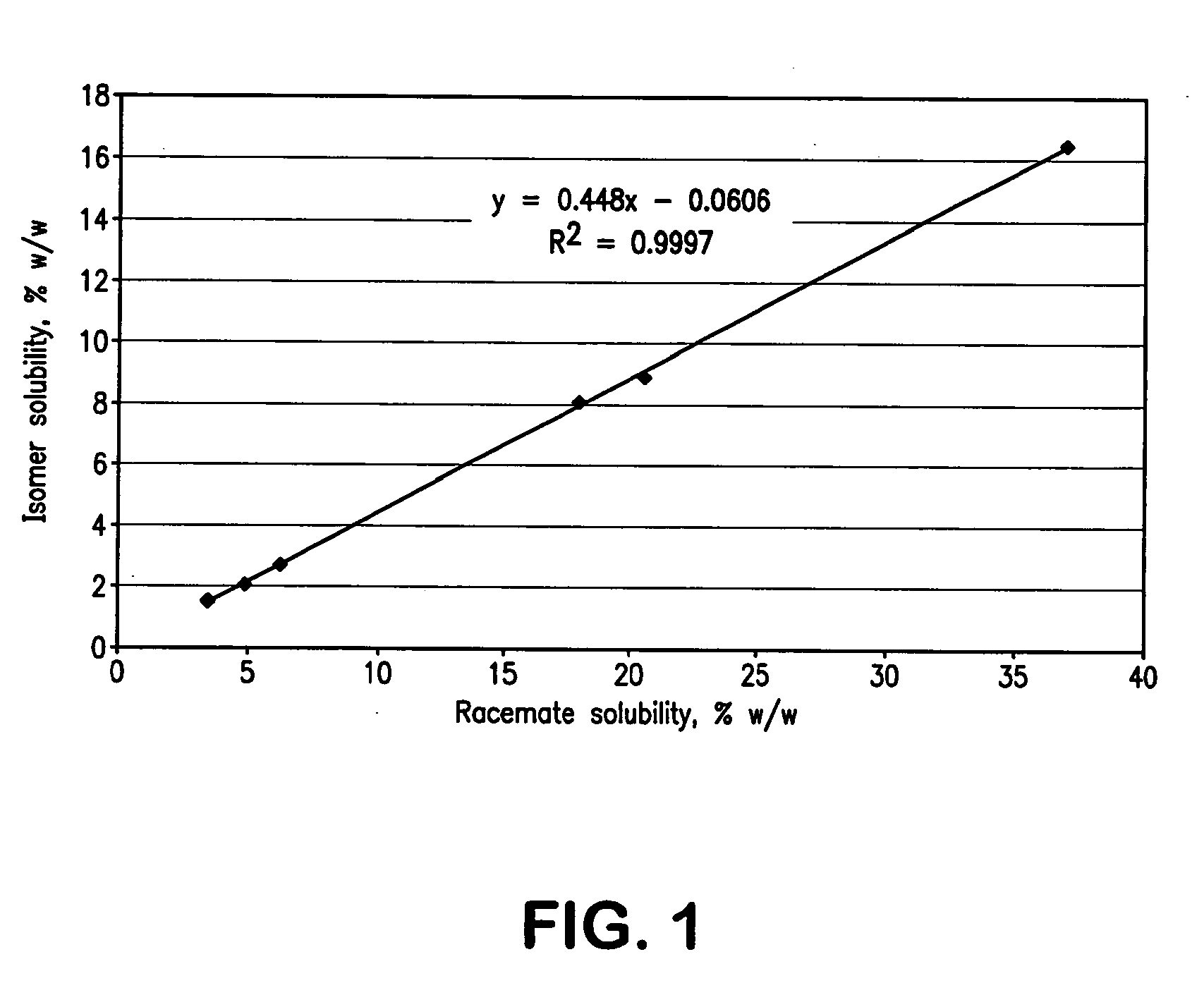
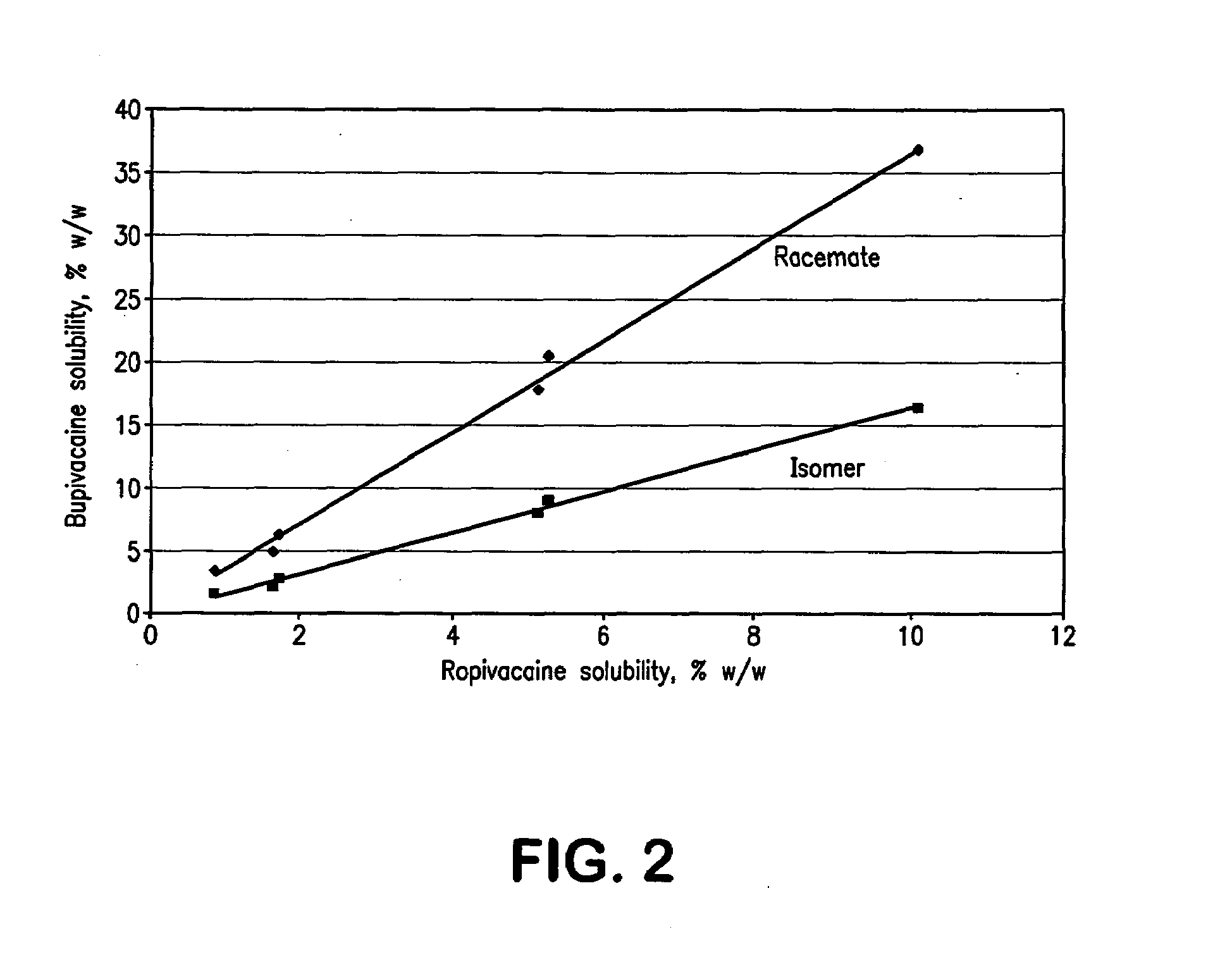
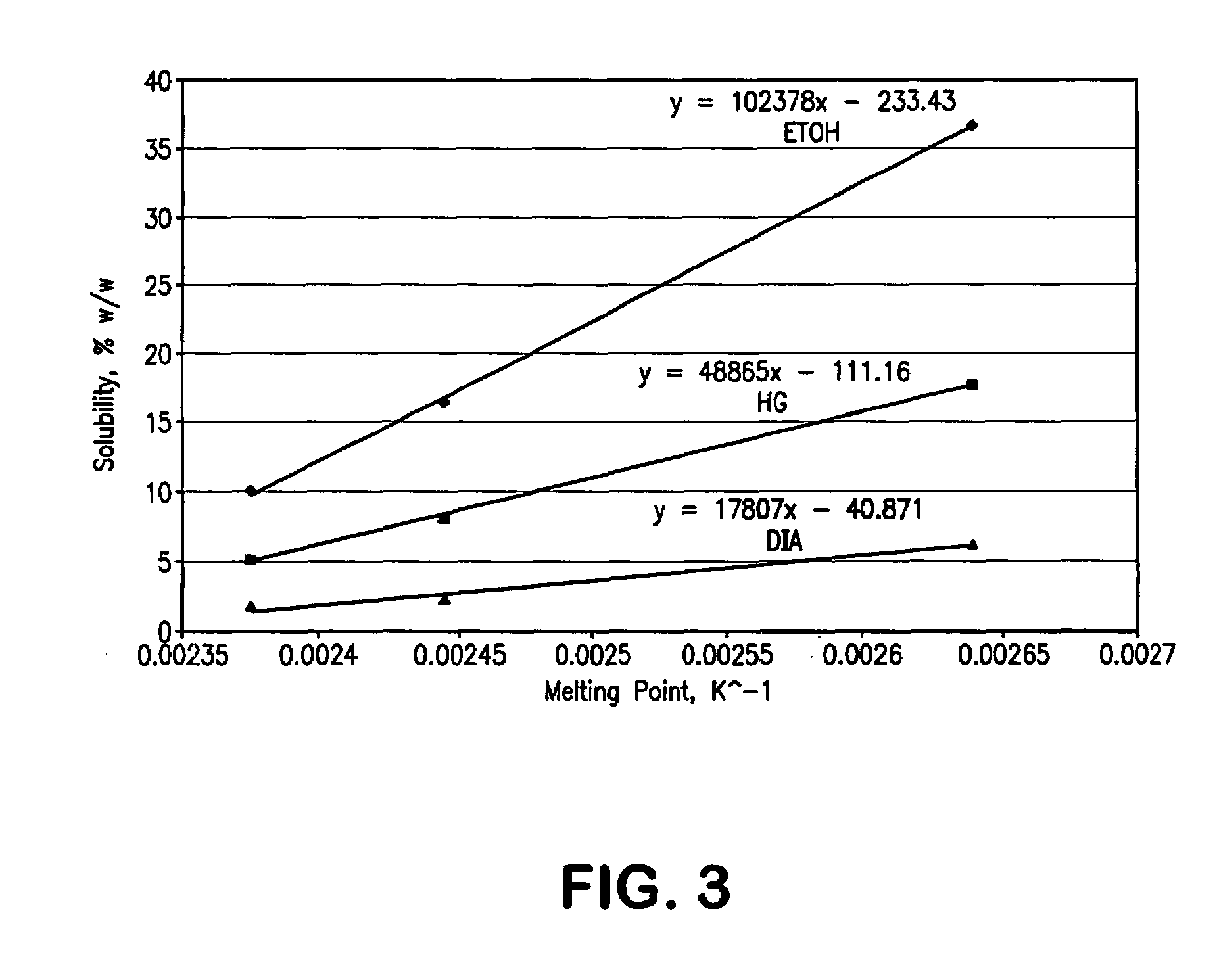
The invention relates to a method for enriching a piperidine-2-formanilide optically active compound. The method comprises the following steps of: mixing R type ropivacaine and bupivacaine or an intermediate thereof with an inert solvent, an initiating agent and mercaptan and carrying out a racemization reaction to obtain a reaction solution containing RS configuration ropivacaine, bupivacaine orthe intermediate thereof; adding acid to the reaction solution to carrying out salifying reaction, bleaching and extracting through an organic solvent, drying and dewatering an organic phase, and carrying out spinning evaporating to obtain racemized ropivacaine, bupivacaine or the intermediate solid thereof; adding the obtained solid salt to acetone to separate out DBTA (Dibenzoyl Tartaric Acid) salt which is an S type enantiomer, wherein at the moment, a large quantity of white precipitates are generated in the solution, and bleaching to obtain white solid; and dissolving the solid into alkali, and then bleaching to obtain an enriched piperidine-2-formanilide optically active compound. The method provided by the invention shortens the time and reduces the temperature of the racemization reaction, reduces pollution to environment, caused by wastes and improves the production efficiency.
Owner:YICHANG HUMANWELL PHARMA
Neosaxitoxin Combination Formulations for Prolonged Local Anesthesia
ActiveUS20140329841A1Low toxicityProlongs the duration of complete blockade to a mechanical stimulusBiocideNervous disorderMechanical irritationChannel blocker
Since each of the site I sodium channel blockers have a unique activity and cannot be used to extrapolate the same effective dosage for another site I sodium channel blocker, studies were conducted to identify dosages of neosaxitoxin (“NeoSTX”) and bupivacaine, alone or in combination with epinephrine, to provide two to three days of pain relief in humans. Bupivacaine-NeoSTX combinations produce more reliable blockade and longer duration blockade compared to NeoSTX alone. The three-way combination of NeoSTX-bupivacaine-epinephrine produces more prolonged local anesthesia than the two-way combination of NeoSTX-bupivacaine. Addition of epinephrine to this NeoSTX-bupivacaine combination dramatically prolongs the duration of complete blockade to a mechanical stimulus. These results led to development of specific combination dosage formulations.
Owner:CHILDRENS MEDICAL CENT CORP
Method of changing muscle lengths with anesthetic drugs
According to one aspect, the fibers of a muscle to be shortened or lengthened are exposed to a local anesthetic drug such as bupivacaine. The concentration and volume of the local anesthetic drug are sufficient to causes the muscle fibers to be damaged by the myotoxicity of the drug. The muscle is then kept at a different length, shorter or longer, during the ensuing period of muscle fiber regeneration, resulting in a shortened or lengthened muscle. In one embodiment, the shortened length of the treated muscle improved the position and the movement of the eye to which it was attached.
Owner:SCOTT ALAN BROWN
A kind of bupivacaine multivesicular liposome preparation device
ActiveCN108158998BAvoid breakingRound shapePharmaceutical product form changePharmaceutical non-active ingredientsMedicineOrganosolv
Owner:GUANGZHOU BOSITAO CONTROLLED RELEASE PHARMA CO LTD
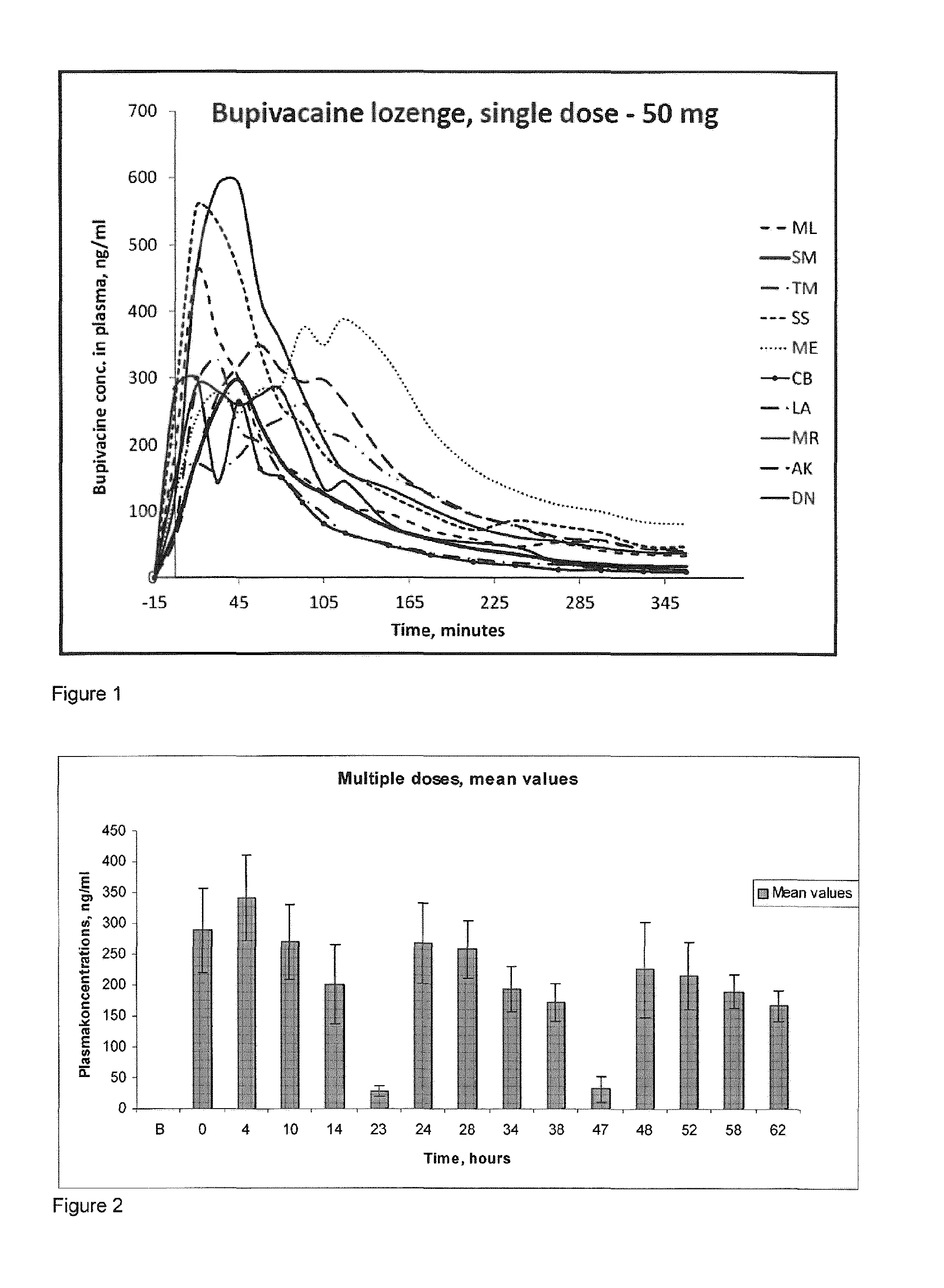
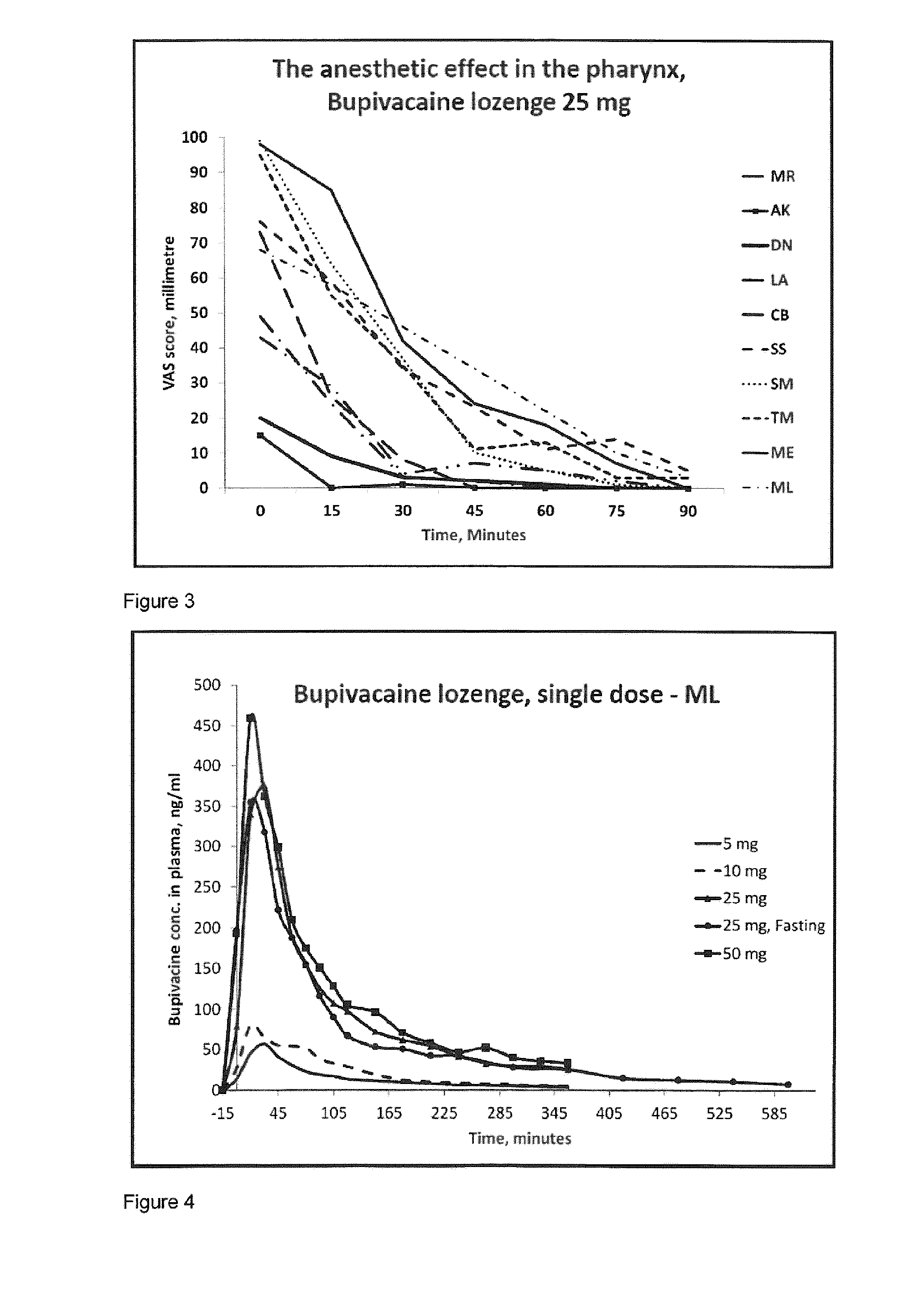
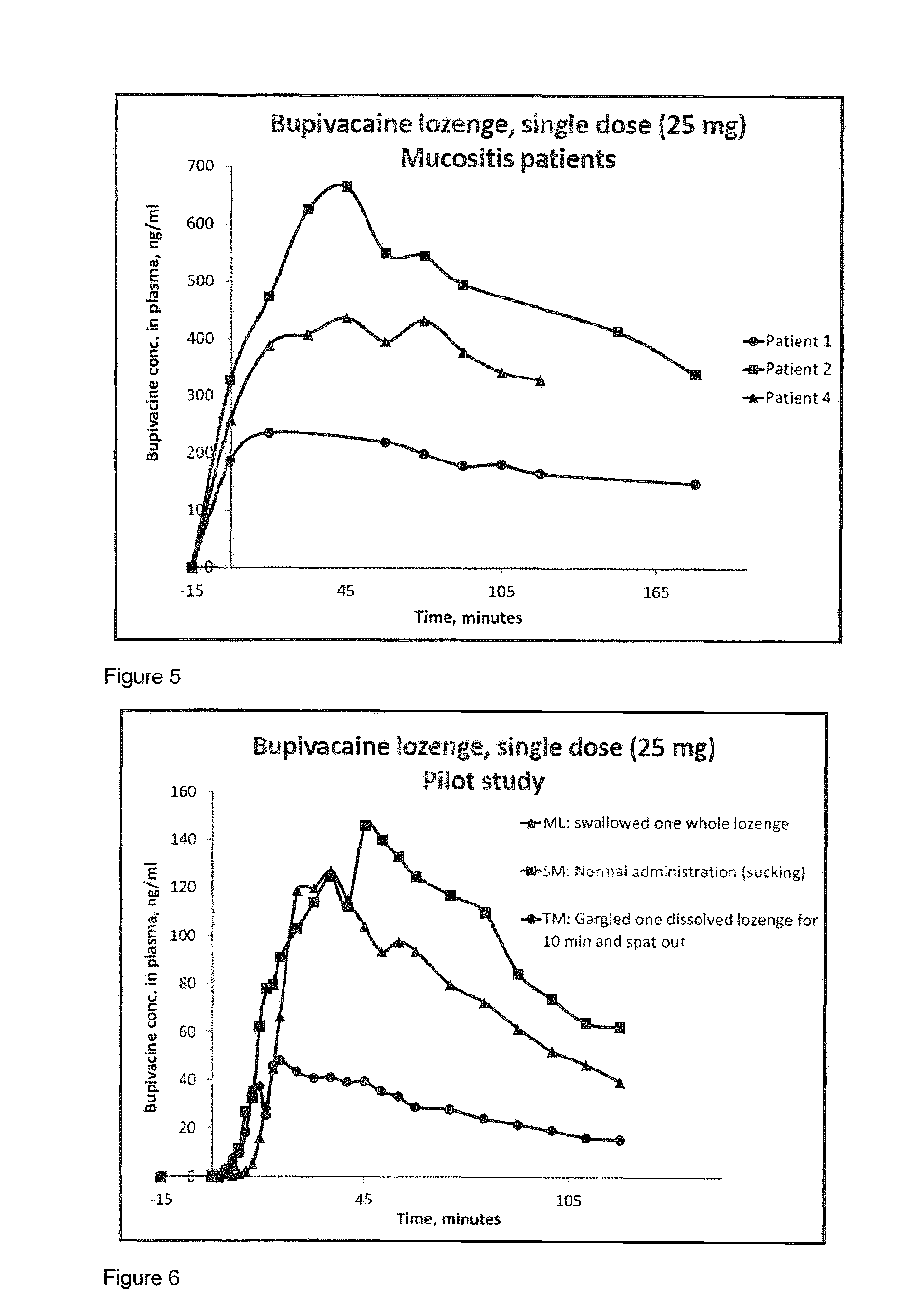
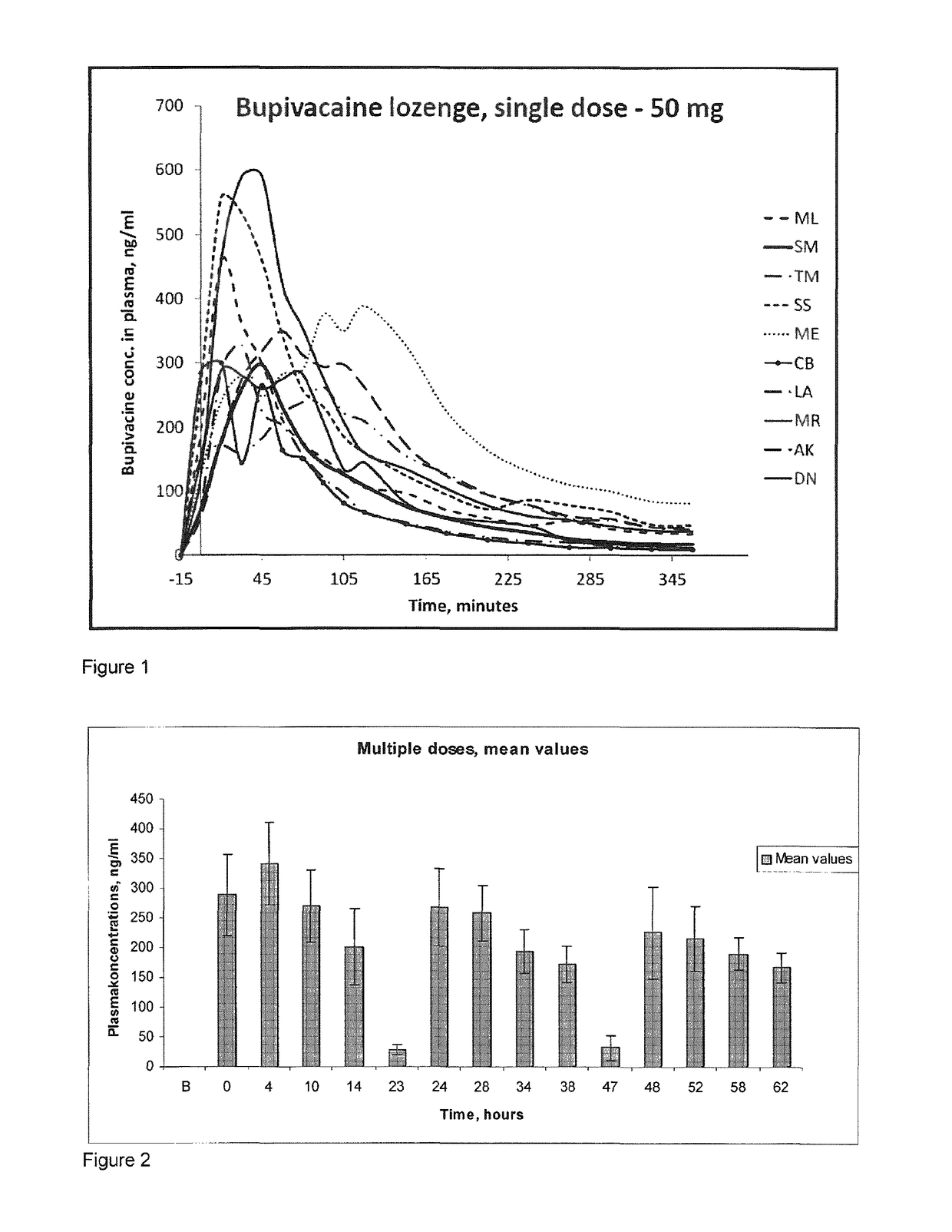
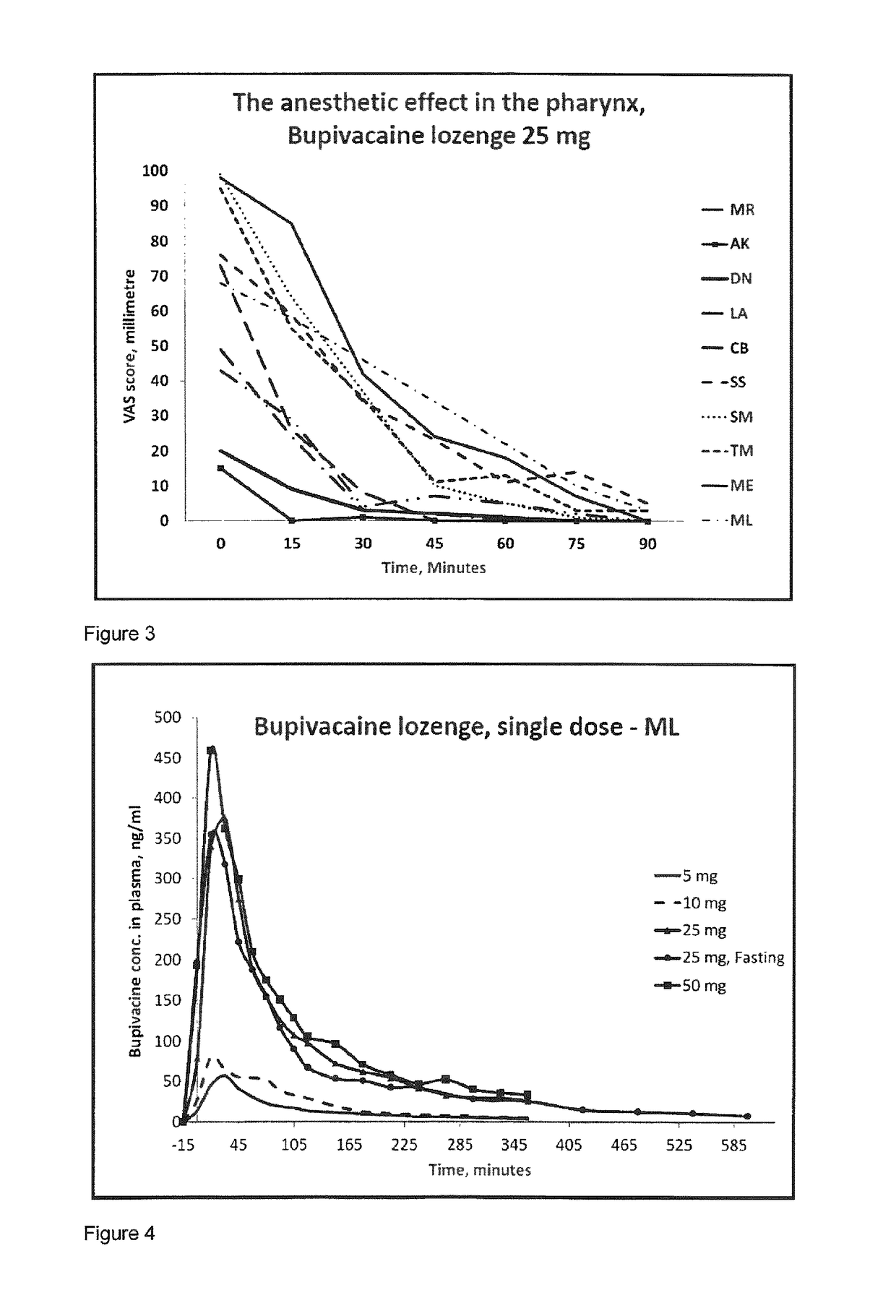
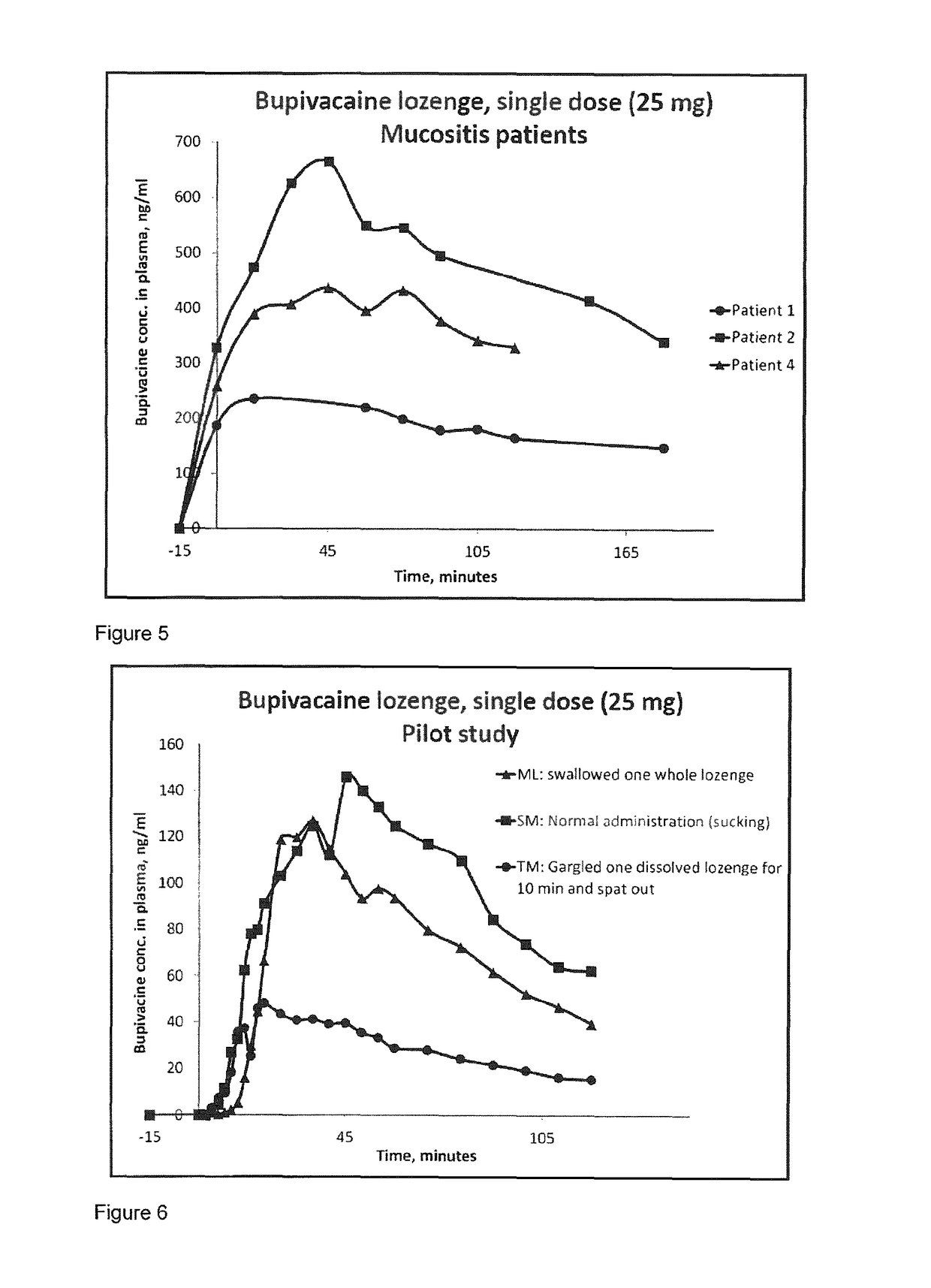
Pharmaceutical compositions comprising a local anaesthetic such as bupivacaine for local administration to the mouth or throat
ActiveUS20140296293A1Extended shelf lifeImprove stabilityBiocidePharmaceutical delivery mechanismDiseaseS syndrome
The present invention relates to compositions comprising a lipophilic local anaesthetic, preferably bupivacaine or a pharmaceutically active salt thereof, which are formulated for local administration to the mouth or throat of a subject. The compositions are useful in the treatment or alleviation of pain, burning or xerostomia of the oral cavity, pharynx, oral mucosa and pharyngeal mucosa or for use in providing local anesthesia of the oral cavity, pharynx, oral mucosa and pharyngeal mucosa. In particular in the treatment of pain, burning or xerostomia, which is caused by a disease such as oral mucositis, Burning Mouth Syndrome, Sjogren's syndrome, xerostomia, periodontitis, toothache, tonsillectomy, throat infection or mononucleosis, canker sores and aphthous stomatitis.
Owner:ONCOZENGE AB
Pharmaceutical compositions comprising a local anaesthetic such as bupivacaine for local administration to the mouth or throat
ActiveUS9956211B2Fast absorptionExtended maintenance periodPharmaceutical delivery mechanismAnaestheticsDiseaseS syndrome
The present invention relates to compositions comprising a lipophilic local anaesthetic, preferably bupivacaine or a pharmaceutically active salt thereof, which are formulated for local administration to the mouth or throat of a subject. The compositions are useful in the treatment or alleviation of pain, burning or xerostomia of the oral cavity, pharynx, oral mucosa and pharyngeal mucosa or for use in providing local anesthesia of the oral cavity, pharynx, oral mucosa and pharyngeal mucosa. In particular in the treatment of pain, burning or xerostomia, which is caused by a disease such as oral mucositis, Burning Mouth Syndrome, Sjogren's syndrome, xerostomia, periodontitis, toothache, tonsillectomy, throat infection or mononucleosis, canker sores and aphthous stomatitis.
Owner:ONCOZENGE AB
A kind of preparation method of bupivacaine multivesicular liposome and bupivacaine multivesicular liposome preparation
ActiveCN108078929BAvoid breakingRound shapePharmaceutical non-active ingredientsLiposomal deliveryOrganosolvDrugs preparations
The invention discloses a preparation method of bupivacaine multivesicular liposome and a bupivacaine multivesicular liposome preparation, and relates to the field of bupivacaine medicinal preparations. The preparation method of the bupivacaine multivesicular liposome comprises the following steps: adding a neutral fat into a preemulsion formed from an organic solvent-containing oil phase and a bupivacaine-containing first water phase to obtain a first solution for forming a compound emulsion. Compared with the conventional preparation method of the bupivacaine multivesicular liposome, the preparation method provided by the invention has the advantages as follows: through the step of adding the neutral fat into the preemulsion formed from the organic solvent-containing oil phase and the bupivacaine-containing first water phase, the problem of breakage of the multivesicular liposome in the subsequent organic solvent removing step can be overcome, so that the prepared multivesicular liposome is round in shape and basically free of phospholipid fragments, and thus the long-acting slow-release effect of a bupivacaine medicine is improved.
Owner:GUANGZHOU BOSITAO CONTROLLED RELEASE PHARMA CO LTD
Injectable long-acting local anesthetic semi-solid gel formulations
What is disclosed is a controlled release pharmaceutical composition comprising a biocompatible and bioerodible semi-solid gel comprising a triglyceride of ricinoleic acid, a gelling agent, bupivacaine and, optionally, a corticosteroid, an analgesic, or an anti-inflammatory agent.
Owner:HUZHOU HUI ZHONG JI SHI BIOTECHNOLOGY CO LTD
Composition containing bupivacaine for local anaesthesia and application thereof
InactiveCN103933041AImprove securityHigh precisionAnaestheticsHeterocyclic compound active ingredientsIndometacinTetrahydropalmatine
The invention relates to the field of medicines, and particularly relates to a pharmaceutical composition which has anesthetic action in operation. The composition is prepared from 1.0-3.0mg of bupivacaine, 1-5mu g of sufentanil, 15-30mu g of tetrahydropalmatine, 15-30 mu g of indometacin and 0.1-0.5mg of ropivacaine. The anesthetic composition is preferably prepared from 3.0mg of bupivacaine, 3mu g of sufentanil, 20mu g of tetrahydropalmatine, 20mu g of indometacin and 0.3mg of ropivacaine.
Owner:QINGDAO CENT HOSPITAL
Biodegradable drug delivery for hydrophobic compositions
A biodegradable drug delivery compositions comprising a triblock copolymer containing a polyester and a polyethyl-ene glycol and a diblock copolymer containing a polyester and an end-capped polyethylene glycol, as well as at least one pharmaceutically active principle or hydrophobic active principle such as medroxyprogesterone acetate, levonorgestrel, cyclosporine, progesterone or bupivacaine is disclosed.
Owner:MEDINCELL SA
Skin topical preparation containing bupivacaine or pharmaceutical salt thereof
ActiveCN107028916AReduced content of active ingredientsAnaestheticsPharmaceutical non-active ingredientsChemical reactionTopical preparation
The invention discloses a skin topical preparation containing bupivacaine or pharmaceutical salt thereof, and provides an external preparation for local administration. The preparation contains active pharmaceutical ingredients, a pressure-sensitive adhesive binder, a diluent and other pharmaceutically acceptable excipients. The adopted pressure-sensitive adhesive binder is an acrylic acid pressure-sensitive adhesive containing hydroxyl, or a silicone pressure-sensitive adhesive or a mixture of the two. The pressure-sensitive adhesive enables the active pharmaceutical ingredients to exist stably and does not chemically react with the active pharmaceutical ingredients, so that drug stability is improved, and clinical application of the preparation is safer and more reliable. Meanwhile, the pressure-sensitive adhesive material can effectively avoid crystallization of a patch, and can also control the viscosity of the patch on the basis of certain content, so that the patch does not fall off easily in use, skin can not be injured when the patch is peeled off, no residue is left on skin, pollution is avoided, and great convenience is brought to patients in use.
Owner:BEIJING TIDE PHARMA
Process for improving calf beef production property by using growth hormone releasing factor gene expression plasmid
The invention discloses a calf beef producing property improving method, which comprises the following steps: 1) injecting expressive plasmid of growth hormone releasing factor gene in the calf musculus semitendinosus positing, 2) setting the fitful agent quantity at 5-7mg per calf, 3) adopting the density of injection dilution liquid of expressive plasmid at 0.25% bupivacaine PSS, 4) improving beef producing property without influencing quality and safety.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com