Preparation and application of biodegradable bupivacaine microspheres with high drug loading capacity
A bupivacaine biodegradation technology, applied in the direction of drug combination, non-active ingredient medical preparations, pharmaceutical formulas, etc., can solve problems such as prolonging the action time of bupivacaine, limited application fields, and large administration volume , to achieve the effects of reducing the number of administrations and side effects, mature technology, and prolonging the action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: Bupivacaine polylactic acid microspheres (BUP-PLLA-MS)
[0039] PLLA (IV=2.0dl / g) was used as the carrier, the weight ratio of bupivacaine to carrier material (BUP:PLLA) was 70:30, and the volume ratio of the oil-water phase was 1:136. The formulation composition is shown in Table 1.
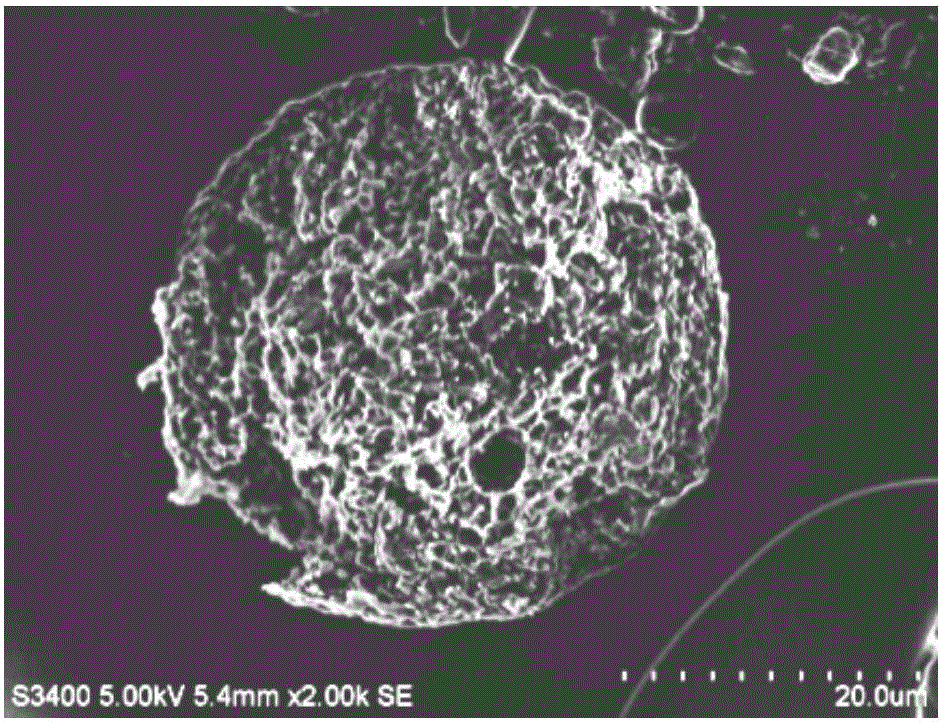
[0040]Preparation method: Dissolve PLLA and BUP in DCM as organic phase or dispersed phase, 50ml of 1% PVA and 0.6% Tween 80 aqueous solution as aqueous phase or continuous phase, quickly add organic phase to aqueous phase, high speed shear at 8000rpm Cut and emulsify for 5 minutes to form O / W type colostrum, then disperse into 700ml 1% PVA aqueous solution to form a uniform and stable emulsion, and evaporate at 40°C for 20 minutes to remove DCM to obtain solidified microspheres, which are washed, filtered, and dried to obtain cloth Pivacaine poly(L-lactic acid) microspheres. The particle size is 34.54±9.73 μm, and the drug loading is 68.86%. Scanning electron microscopy (...
Embodiment 2
[0041] Embodiment 2: Bupivacaine polylactic acid microspheres (BUP-PLLA-MS)
[0042] PLLA (IV=2.0dl / g) was used as the carrier, the weight ratio of bupivacaine to carrier material (BUP:PLLA) was 75:25, and the oil-water phase volume ratio was 1:150. The composition of the prescription is shown in Table 1.
[0043] The microsphere preparation method in Example 1 was adopted to obtain bupivacaine poly(L-lactic acid) microspheres. The particle size is 33.29±9.58 μm, and the drug loading is 70.97%. SEM observation shows that the shape of the microspheres is round, the surface is smooth, and there are tiny holes (see image 3 ). In the pH 7.4 phosphate buffer solution in vitro, the microspheres can continuously and slowly release the drug, and the cumulative release of bupivacaine in 7 days is 75% (see Figure 4 ).
Embodiment 3
[0044] Embodiment 3: Bupivacaine polylactic acid microspheres (BUP-PLLA-MS)
[0045] PLLA (IV=2.0dl / g) was used as the carrier, the weight ratio of bupivacaine to carrier material (BUP:PLLA) was 80:20, the volume ratio of the oil-water phase was 1:187.5, and the composition of the prescription was shown in Table 1.
[0046] The microsphere preparation method in Example 1 was adopted to obtain bupivacaine poly(L-lactic acid) microspheres. The particle size is 31.83±5.36 μm, and the drug loading capacity is 74.94%. SEM observation shows that the microspheres are round in shape, smooth in surface and free of drug adsorption (see image 3 ). In the pH7.4 phosphate buffer solution in vitro, the microspheres can release the drug continuously and slowly, and the cumulative release of bupivacaine in 5 days is greater than 90%. The prescription microspheres have a high drug loading capacity and are suitable for sustained release (see Figure 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com