Preparation and application of biodegradable bupivacaine microspheres with high drug loading
A bupivacaine and biodegradation technology, which is applied in the directions of drug combinations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. The problem of large drug volume, etc., to achieve the effect of reducing the number of doses and toxic and side effects, the process is mature, and the action time is prolonged
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: Bupivacaine polylactic acid microspheres (BUP-PLLA-MS)
[0039] PLLA (IV=2.0dl / g) was used as the carrier, the weight ratio of bupivacaine to carrier material (BUP:PLLA) was 70:30, and the volume ratio of the oil-water phase was 1:136. The formulation composition is shown in Table 1.
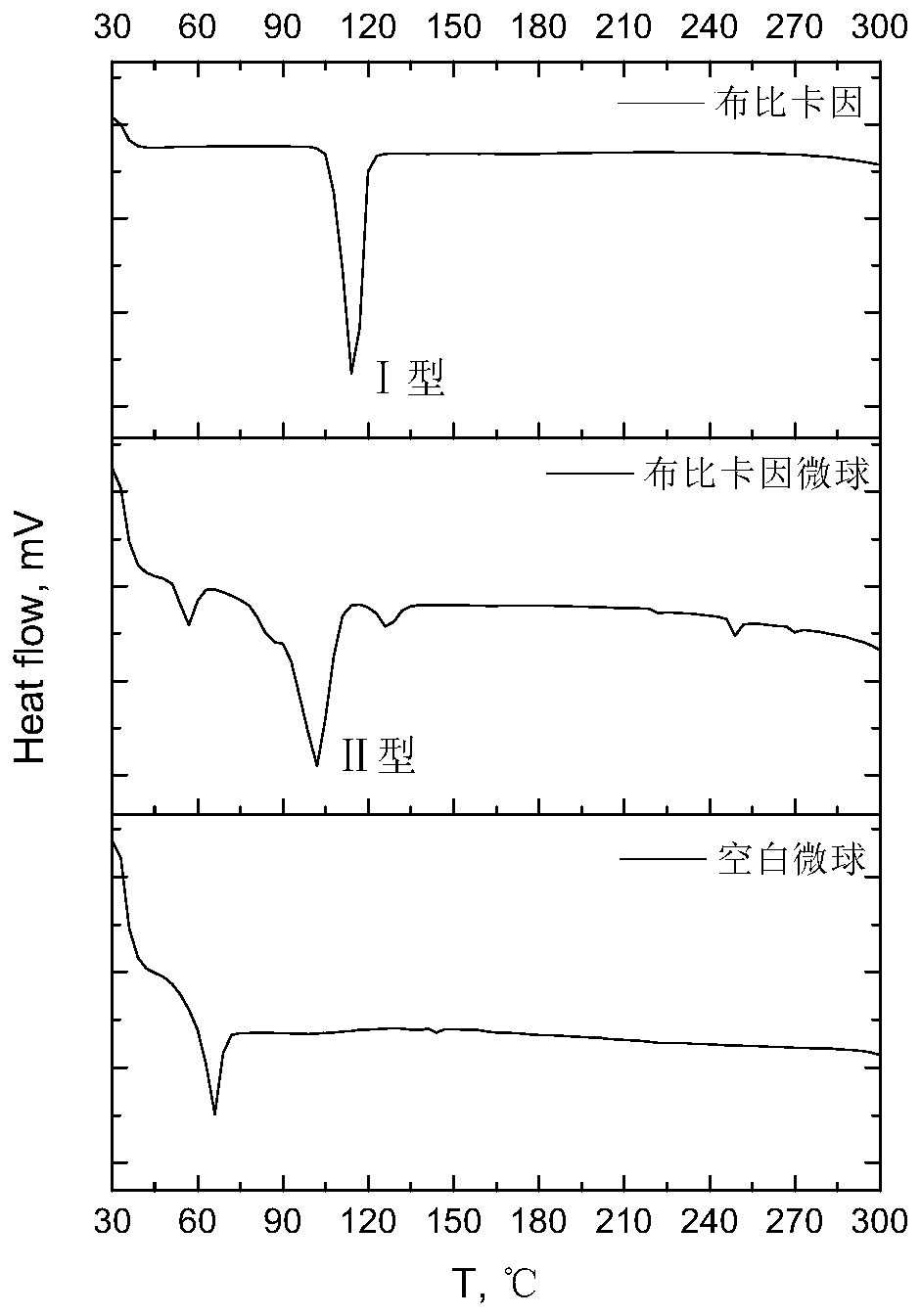
[0040]Preparation method: Dissolve PLLA and BUP in DCM as organic phase or dispersed phase, 50ml of 1% PVA and 0.6% Tween 80 aqueous solution as aqueous phase or continuous phase, quickly add organic phase to aqueous phase, high speed shear at 8000rpm Cut and emulsify for 5 minutes to form O / W type colostrum, then disperse into 700ml 1% PVA aqueous solution to form a uniform and stable emulsion, and evaporate at 40°C for 20 minutes to remove DCM to obtain solidified microspheres, which are washed, filtered, and dried to obtain cloth Pivacaine poly(L-lactic acid) microspheres. The particle size is 34.54±9.73 μm, and the drug loading is 68.86%. Scanning electron microscopy (...
Embodiment 2
[0041] Embodiment 2: Bupivacaine polylactic acid microspheres (BUP-PLLA-MS)
[0042] PLLA (IV=2.0dl / g) was used as the carrier, the weight ratio of bupivacaine to carrier material (BUP:PLLA) was 75:25, and the oil-water phase volume ratio was 1:150. The composition of the prescription is shown in Table 1.
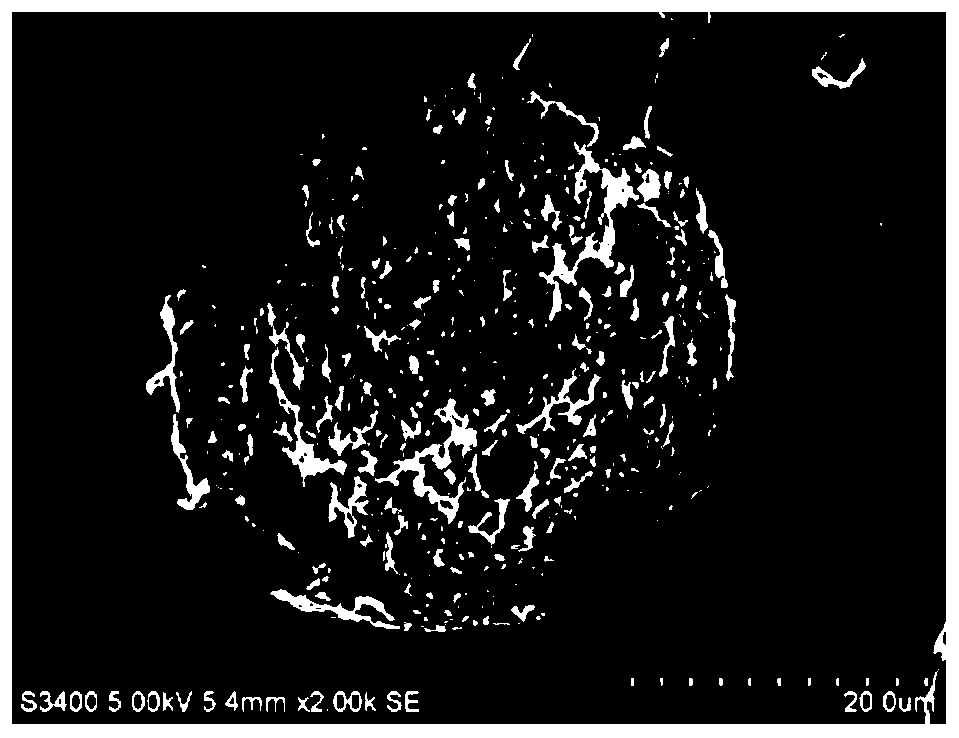
[0043] The microsphere preparation method in Example 1 was adopted to obtain bupivacaine poly(L-lactic acid) microspheres. The particle size is 33.29±9.58 μm, and the drug loading is 70.97%. SEM observation shows that the shape of the microspheres is round, the surface is smooth, and there are tiny holes (see image 3 ). In the pH 7.4 phosphate buffer solution in vitro, the microspheres can continuously and slowly release the drug, and the cumulative release of bupivacaine in 7 days is 75% (see Figure 4 ).
Embodiment 3
[0044] Embodiment 3: Bupivacaine polylactic acid microspheres (BUP-PLLA-MS)
[0045] PLLA (IV=2.0dl / g) was used as the carrier, the weight ratio of bupivacaine to carrier material (BUP:PLLA) was 80:20, the volume ratio of the oil-water phase was 1:187.5, and the composition of the prescription was shown in Table 1.
[0046] The microsphere preparation method in Example 1 was adopted to obtain bupivacaine poly(L-lactic acid) microspheres. The particle size is 31.83±5.36 μm, and the drug loading capacity is 74.94%. SEM observation shows that the microspheres are round in shape, smooth in surface and free of drug adsorption (see image 3 ). In the pH7.4 phosphate buffer solution in vitro, the microspheres can release the drug continuously and slowly, and the cumulative release of bupivacaine in 5 days is greater than 90%. The prescription microspheres have a high drug loading capacity and are suitable for sustained release (see Figure 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com