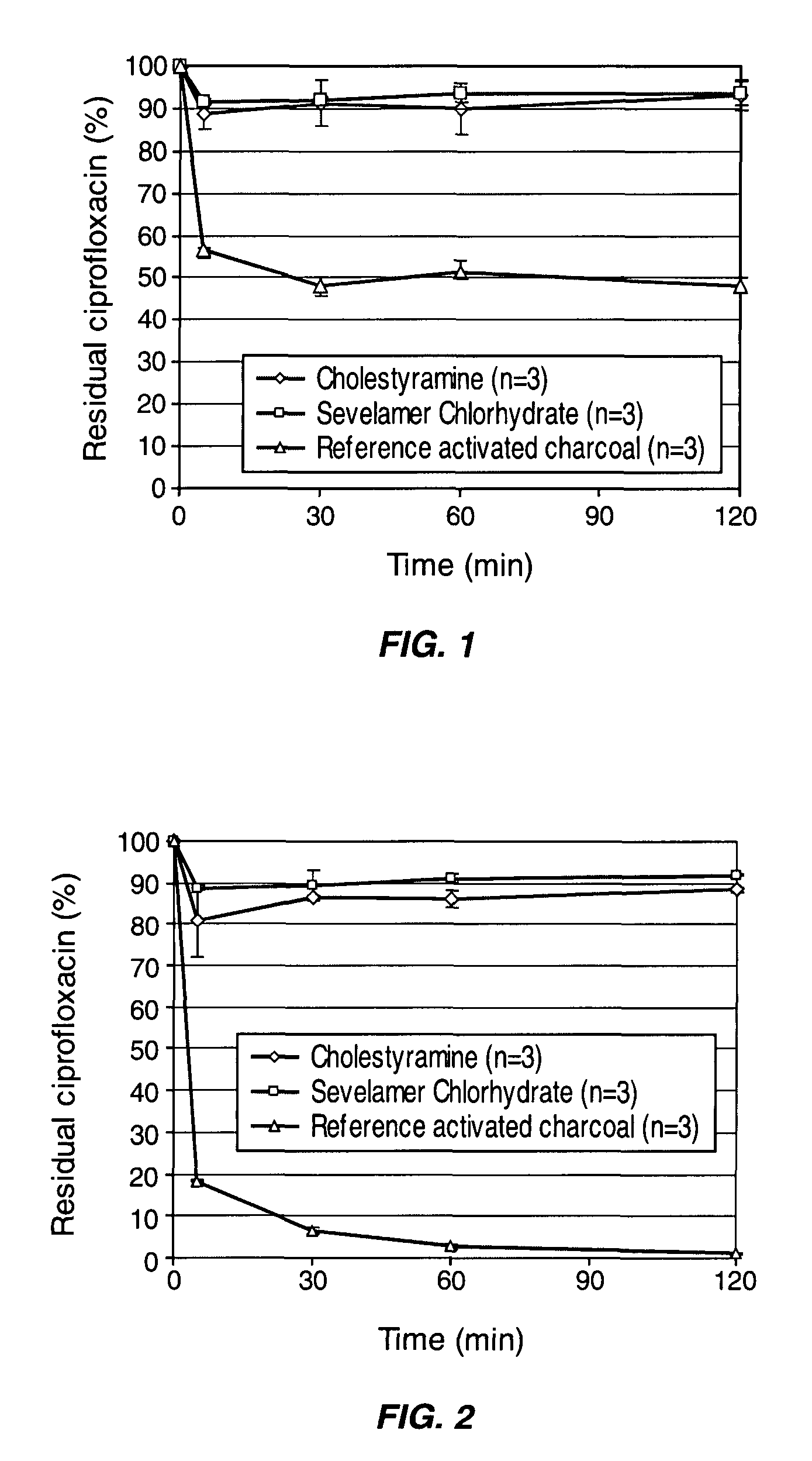
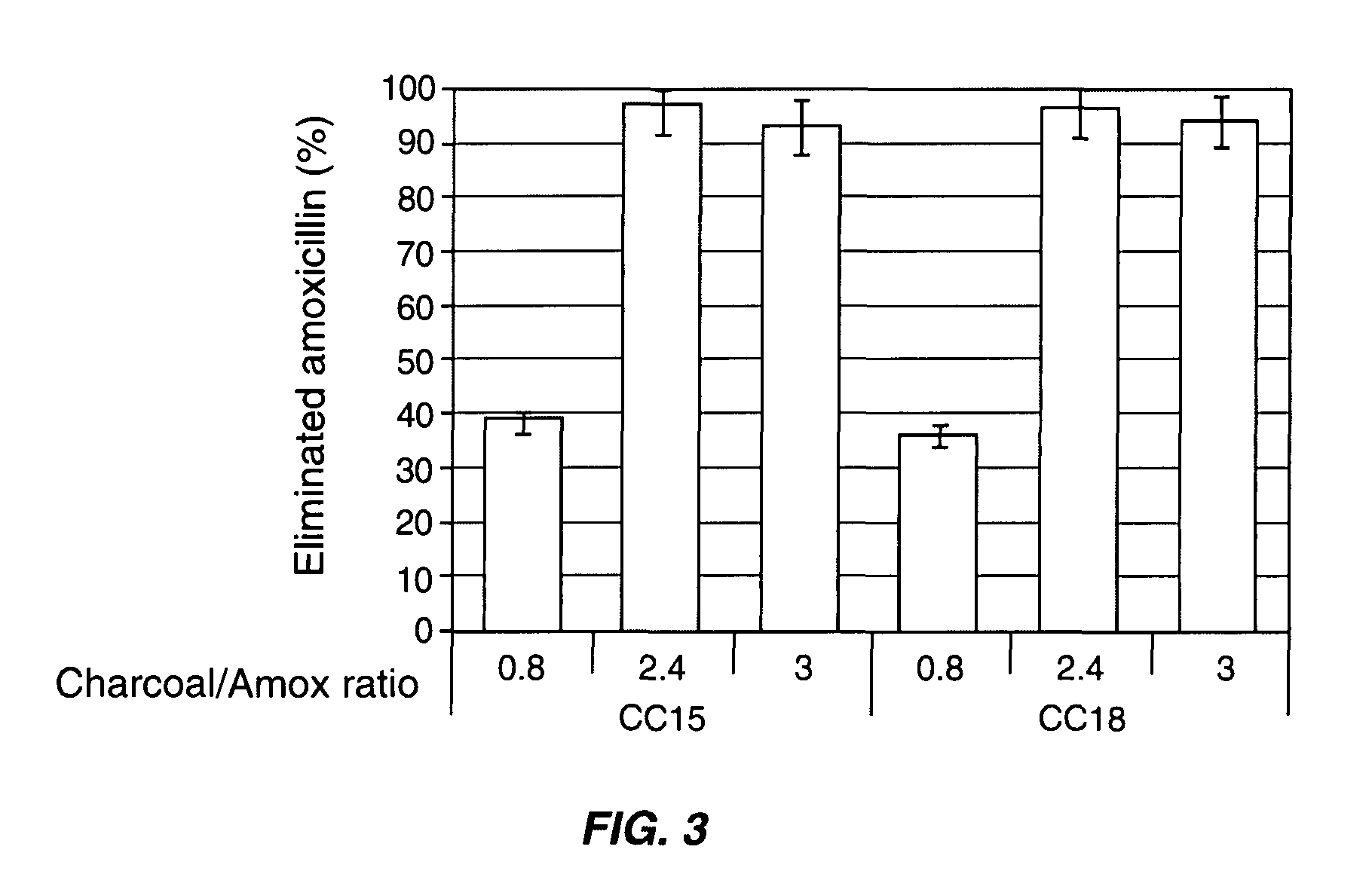
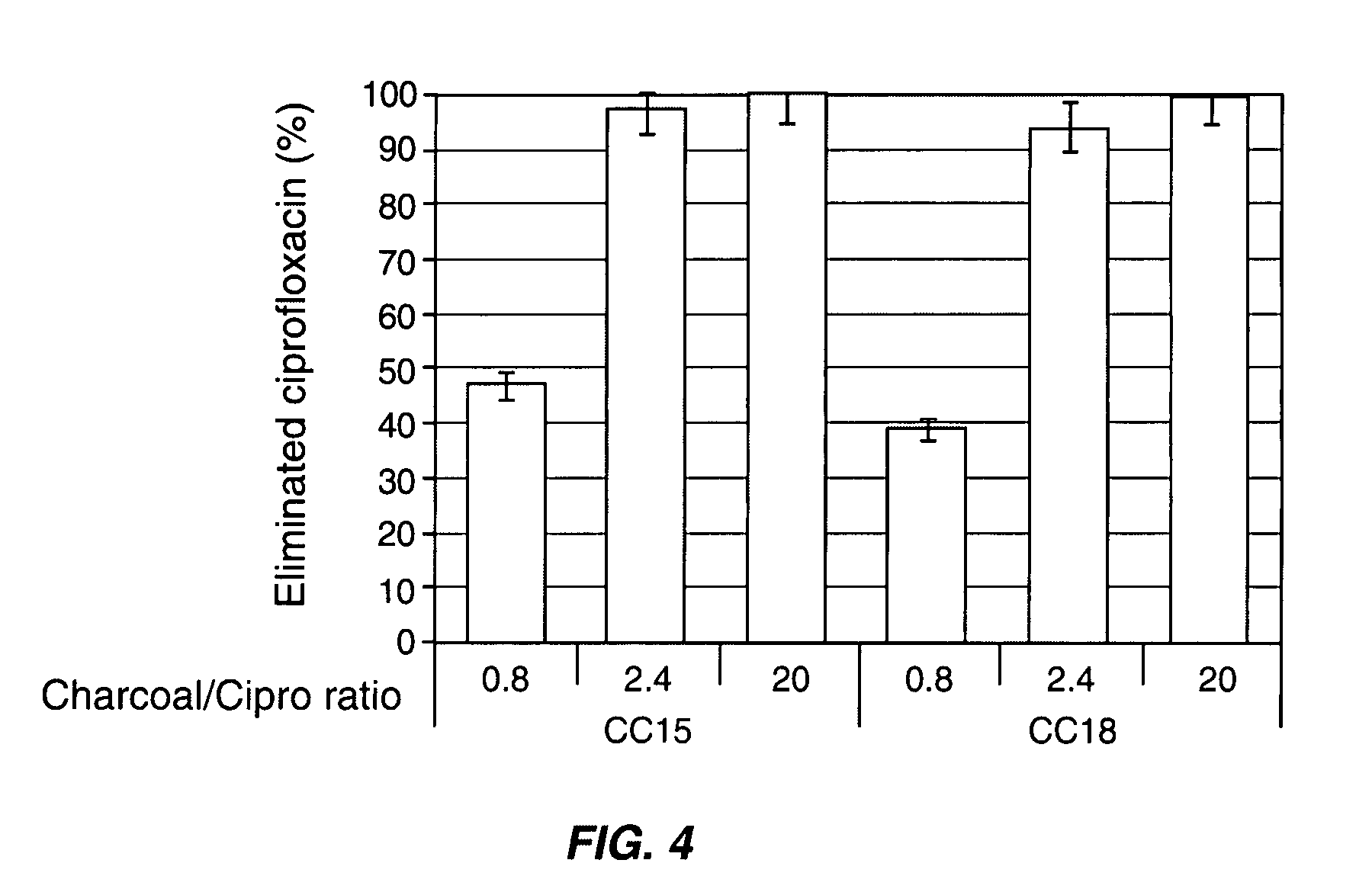
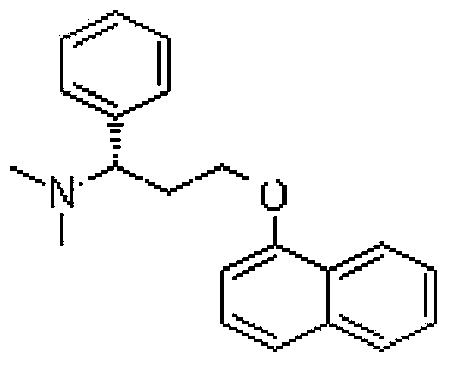
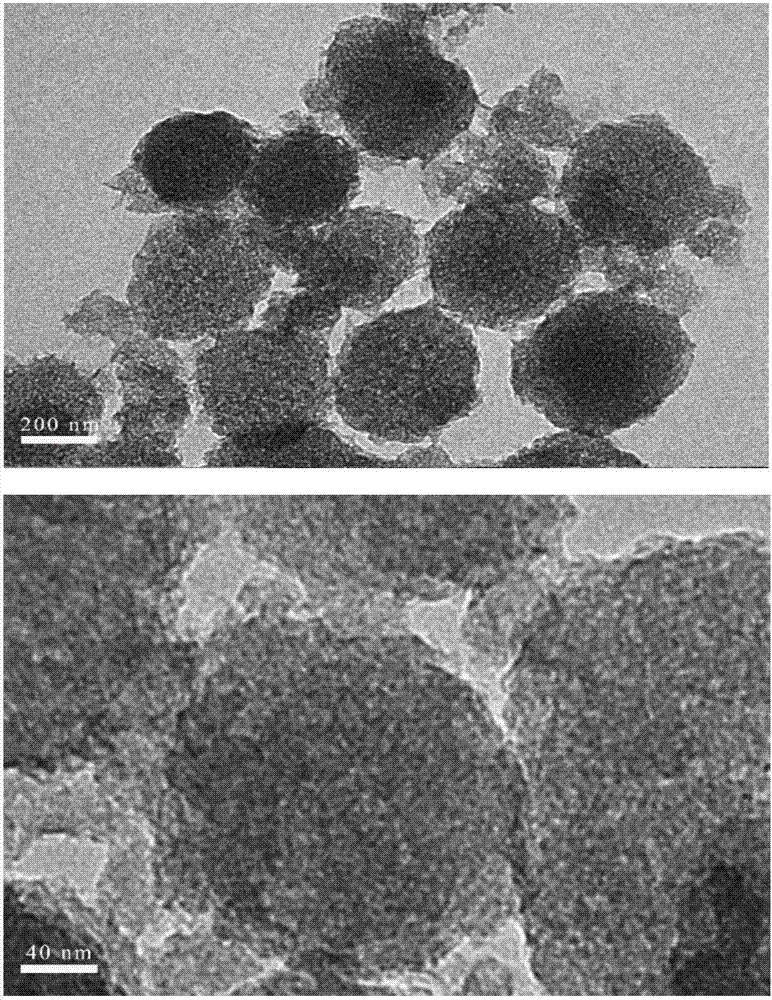
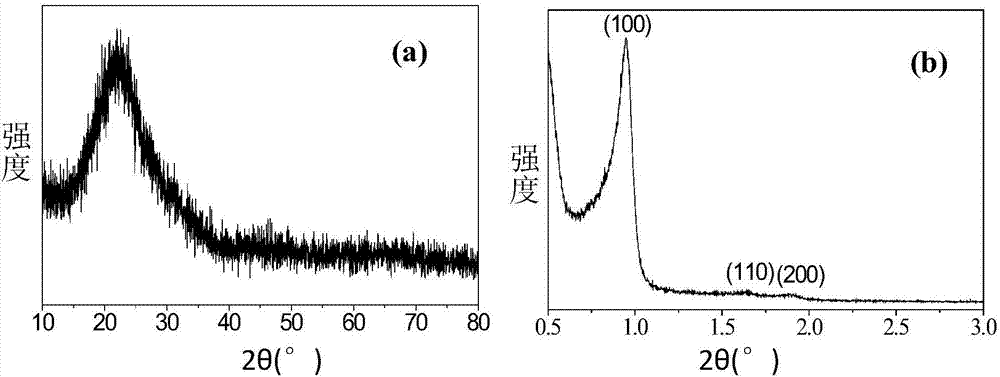
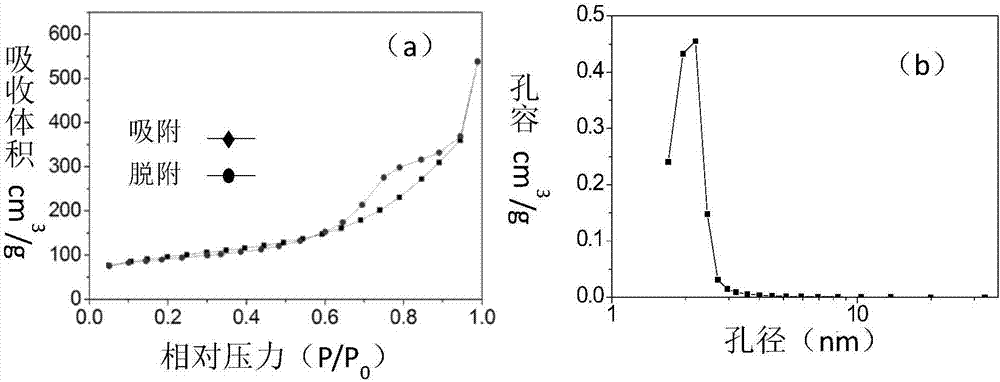
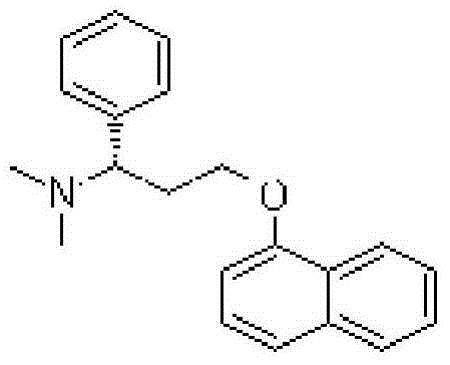
Patents
Literature
45 results about "Drug adsorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug Adsorption (Hapten) Hypothesis. In the drug adsorption hypothesis, the drug binds to the RBC membrane and the antibody is largely directed against the drug itself or against a metabolite of the drug. The DAT is positive with IgG and possibly complement and occasionally results in extravascular hemolysis.
Site-specific intestinal delivery of adsorbents, alone or in combination with degrading molecules
InactiveUS8048413B2Reduce concentrationImprove side effectsAntibacterial agentsBiocideDrug adsorptionMetabolite
Compositions which deliver adsorbents, alone or in combination with active drug “degrading molecules,” in a site-specific manner to the intestine, and which eliminate or at least lower the concentration of residual unwanted material within the intestine, are disclosed. Methods of treatment using the compositions are also disclosed. The material to be eliminated can include residual active antibiotics, metabolites, bacterial or other toxins, and drugs which cause side effects in the gastrointestinal tract. The adsorbents can be formulated in capsules, tablets or any acceptable pharmaceutical composition, and are ideally designed to specifically release the adsorbents in a programmed manner at a specific site of the intestinal tract. The programmed delivery prevents adsorbents from interfering with the normal absorption process of a given molecule after oral absorption, until it reaches the lower part of the small intestine. The compositions can be used to adsorb, and therefore remove, any residual drug, metabolite thereof, or bacterial toxin after oral or parenteral administration which would otherwise cause adverse effects in the lower intestine and / or colon.
Owner:CENT NAT DE LA RECHERCHE SCI +3
Functionalization mesoporous molecular sieve used in adsorption and sustained-release alkaline drug method
InactiveCN101259104AAdjust loadRegulated release rateAntibacterial agentsPowder deliveryMolecular sieveControlled release
The invention relates to a functionalized mesoporous molecular sieve and a method to use the functionalized mesoporous molecular sieve for adsorption and controlled release of alkaline drugs, namely, an alkaline drug is added to water or organic solution to obtain solution A, ensuring that the concentration of the alkaline drug is 1.0-100.0 mg / mL; the functionalized mesoporous molecular sieve is added to the solution A, ensuring that the functionalized mesoporous molecular sieve / solution A is equal to 0.1-0.5g / 10.0-500.0 mL, stirred for 0.5 to 3 days and then filtered, the obtained solid is dried, thus obtaining functionalized mesoporous molecular sieve B capable of absorbing drugs; -CH3 group is grafted on the surface of the unctionalized mesoporous molecular sieve B capable of absorbing drugs by adopting a post-processing method, so as to obtain double-functionalized solid pulvis with absorbed alkaline drug. The functionalized mesoporous molecular sieve of the invention has the advantages of well-distributed drug, large drug loading capacity, availability for convenient adjusting and long time of drug release.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Non-PVC material for medical infusion products and preparation method thereof
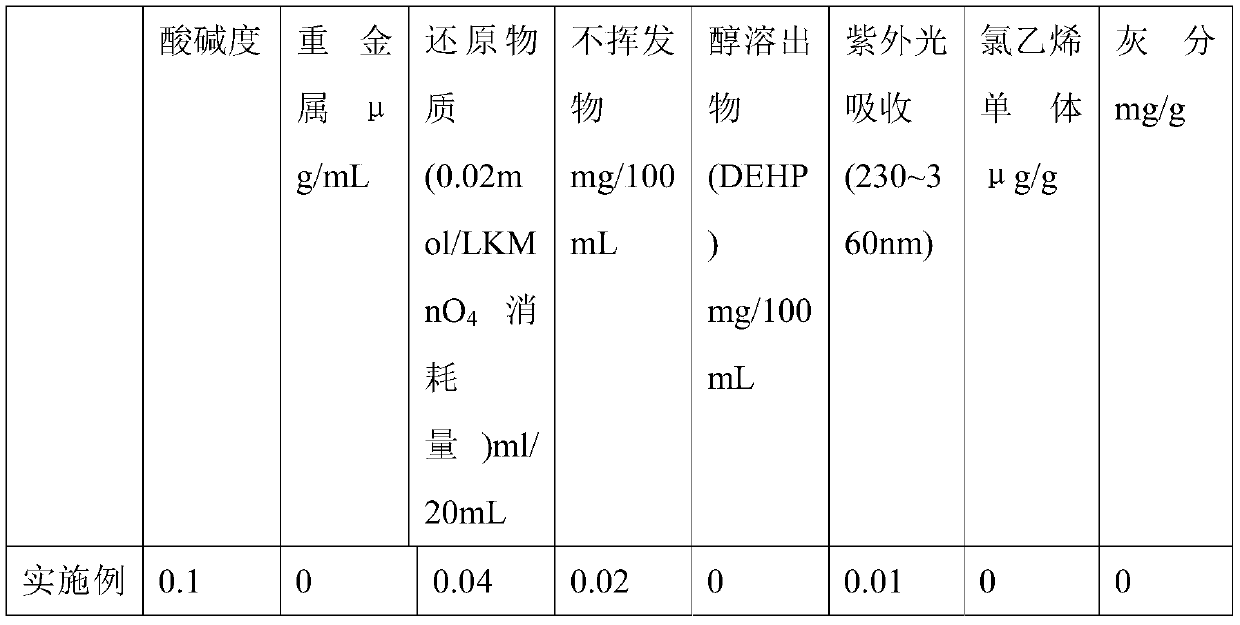
The invention relates to a non-PVC material for medical infusion products and a preparation method thereof. The non-PVC material is characterized by comprising the following components by weight percent: 25-30% of SEBS, 45-49% of filling oil, 17-20% of PP, 6-7% of filler and 0.4-0.6% of antioxidant 1076. A disposable bag infusion set or a medical infusion product produced by the invention has the advantages of less precipitate, low drug adsorption, high transparency, light weight, more convenient treatment after use, environmental protection and the like. The non-PVC material does not react with paclitaxel and other fat emulsion anti-cancer drugs.
Owner:CHENGDU XINJIN SHIFENG MEDICAL APP ANDINSTR CO LTD
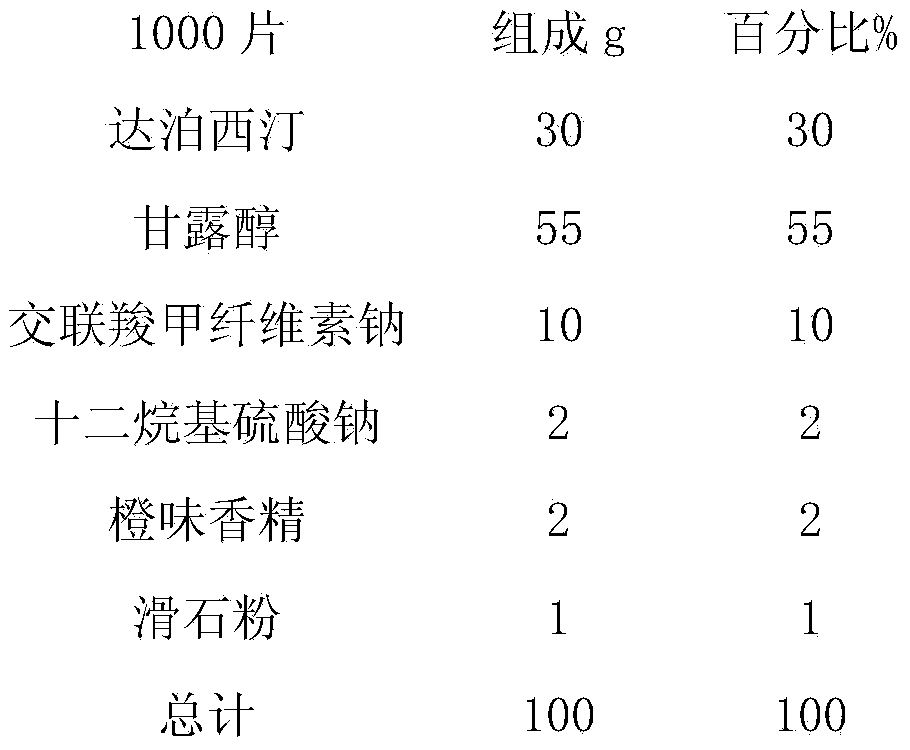
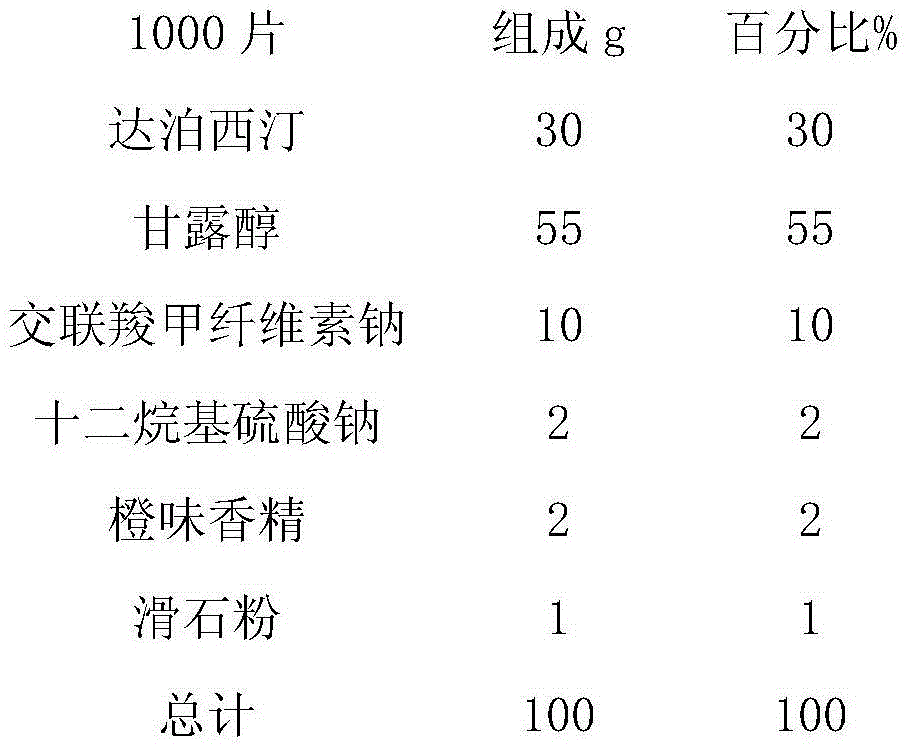
Dapoxetine tablets and preparation method thereof
ActiveCN103735525AFast absorptionImprove bioavailabilityOrganic active ingredientsPill deliveryDrug adsorptionSide effect
The invention relates to dapoxetine tablets and a preparation method thereof. The dapoxetine tablets comprise the following components in percentage by weight: 10 to 50 percent of dapoxetine, 30 to 80 percent of a filling agent, 3 to 20 percent of a disintegrant, 0.5 to 1 percent of a lubricant and 0.2 to 2.5 percent of a flavoring agent. The preparation method comprises the following steps: micronizing the dapoxetine and the filling agent and controlling the particle size to be 0.5-20 [mu]m; preparing the fine powder obtained in the previous step and a part of the disintegrant into a soft material by ethanol water and pelletizing by a 24-mesh sieve; drying the obtained fine particles at the temperature of 50 to 70 DEG C and straightening by a 20-mesh sieve; sequentially adding the rest disintegrant, the flavoring agent and the lubricant into the dried particles, carrying out blending for two times, and until intermediates are qualified by detection, and carrying out tabletting, so that the dapoxetine tablets are obtained, wherein blending is carried out for one time before the lubricant is added and then is carried out again after the lubricant is added. The dapoxetine tablets are coated or uncoated conventional tablets, chewable tablets and orally disintegrating tablets, are used for treating male prospermia, high in drug adsorption speed and bioavailability, and convenient to take and have small side effects.
Owner:JIANGSU TIANZHAO PHARMA
Method for preparing natural silk functional dressings
InactiveCN101181645AImprove drug adsorptionAchieve reinclusionAbsorbent padsBandagesDrug adsorptionCyclodextrin
The present invention relates to a preparation method of silk functional dressing, which is to graft cyclodextrin to silk fiber through chemical bonds, and then use β-phenylacrylic acid as a guest molecule to prepare silk functional dressing products; it can be used for development Anti-infection and anti-inflammation functional dressing products; it uses the three-dimensional chiral cavity microenvironment of cyclodextrin as a storage space for drugs, which can greatly increase the drug adsorption capacity of the material, and its adsorption of β-phenylacrylic acid The amount can reach 2.178mg / g.
Owner:ZHEJIANG SCI-TECH UNIV
Azoxystrobin suspension concentrate with particle sizes of less than 100nm and preparation method of azoxystrobin suspension concentrate
ActiveCN107410295ALarge particle sizeNarrow dispersionBiocideFungicidesDrug adsorptionOrganic solvent
The invention discloses azoxystrobin suspension concentrate with particle sizes of less than 100nm and a preparation method of the azoxystrobin suspension concentrate and belongs to the technical field of production of nano-pesticides. The method comprises the steps of dissolving azoxystrobin into a water-soluble organic solvent as an organic phase, adopting water as a water phase and adding a surfactant to at least one phase of water phase or organic phase; and mixing the water phase with turbulent flow of the organic phase through a fast jet hedging blending method, carrying out collection by using water or surfactant-containing water to prepare the azoxystrobin suspension concentrate. The suspension concentrate prepared by adopting the method is stable in particle size and narrow in dispersion, is smaller than 100nm, is conducive to drug adsorption on the blade surface and is capable of withstanding blowing and rain washing, osmosis and conduction to a blade can be strengthened, and the azoxystrobin suspension concentrate has the characteristics of being friendly to environment, prone to environmental degradation and high in medicinal effect.
Owner:YANGZHOU UNIV
Preparation method for botanical pesticide for specially killing cabbage caterpillars during organic vegetable planting
The invention discloses a preparation method for a botanical pesticide for specially killing cabbage caterpillars during organic vegetable planting. The preparation method comprises the following steps: 1), preparing raw materials, wherein the raw materials comprise the following components in parts by weight: 10-15 parts of radix sophorae flavescentis, 7-10 parts of melia toosendan, 12-19 parts of melia toosendan bark, 10-15 parts of tripterygium glycosides, 18-24 parts of raw kusnezoff monkshood roots, 4-6 parts of radix euphorbiae lantu, 6-8 parts of pericarpium citri reticulatae and 13 parts of tobacco; 2), adding 3 to 4 times of water by weight in the raw materials, decocting in a sealing manner at the temperature of 90-100 DEG C for 100 to 120 minutes, filtering, and concentrating the filtrate to be 1 / 3-1 / 4 of the original used water amount to obtain the botanical pesticide for specially killing pierisrapae linne. The botanical pesticide can targetedly and specially kill cabbage caterpillars by scientific compatibility of the traditional Chinese medicine components, and is high in permeability, conducive to plant uptake, low in cost, free of three-evil, residue, pollution and drug resistance, high in drug adsorption, and long in drug effect duration.
Owner:ZHEJIANG HENGZE ECOLOGICAL AGRI SCI & TECH LIMITED
Nanostructured liposome vector with highly effective antineoplastic activity
InactiveCN101011358AIncrease intakeGood curative effectOrganic active ingredientsPharmaceutical non-active ingredientsDrug adsorptionLipid formation
The invention relates to a nanometer liposome carrier with high-effect anti-tumor activity, which carries the anti-tumor Paclitaxel, while the liposome is formed by single lipid and liquid lipid, wherein, the liquid lipid is 5-20% of liposome, the Paclitaxel is 3.1-3.6% of solid liposome nanometer particles, and the diameter is 481-532nm. The invention has high-effect cell extraction and cell-pulp hold function via the nanometer liposome carrier, while the package on the anti-tumor drug whose molecule target is at the cell pulp can improve the adsorption of anti-tumor drug and improve the drug density at target part. The invention can improve the drug adsorption of tumor cell, reduce the drug distribution at normal cell, reduce the side effect and toxicity, and improve the effect.
Owner:ZHEJIANG UNIV
Powder preparation with diatomite as carrier and application method
The invention provides a powder preparation with diatomite as a carrier and an application method. The powder preparation is prepared by employing one or some pesticides or bactericides as effective constituents, employing diatomite as a main carrier, being added with a certain amount of auxiliaries, and being subjected to fluid energy milling to certain fineness. After being applied, the powder preparation has good suspension effects in air. Because of long suspension time, the powder preparation can be attached to positions where cannot be reached by spray application and the attached evenness on leaves is high. The powder preparation can be used for control of a plurality of diseases and insect pests of greenhouse vegetables and flowers. In field experiments, when the method is compared to traditional spray application methods, the powder preparation has better control effects on diseases and insect pests, and has beneficial effects of less dosage, high drug adsorption rate, simple technology, low cost and the like.
Owner:INST OF VEGETABLE & FLOWERS CHINESE ACAD OF AGRI SCI
Monocrystalline silicon precision infusion filter membrane, preparation method thereof, filter and infusion device
InactiveCN109092076AGuaranteed mechanical strengthShort filter pathSemi-permeable membranesFiltering accessoriesDrugs infusionDrug adsorption
The invention discloses a monocrystalline silicon precision infusion filter membrane, a preparation method thereof, a filter and an infusion device. The monocrystalline silicon precision infusion filter membrane comprises a body. The body is provided with a monocrystalline silicon filter membrane and a monocrystalline silicon grid support body arranged on the side of the monocrystalline silicon filter membrane and integrally connected to the monocrystalline silicon filter membrane. The monocrystalline silicon filter membrane is provided with a plurality of penetrating filtering holes with thediameter of 0.2-5 microns and the depth of 2-50 microns. The filter membrane has good bonding strength and sealing performance, ensures the sound condition under the stress state, solves the problemsof low thickness and brittleness, has the advantages of short filtering path, high structural strength, good sealing performance, low filtration pressure difference, low filter membrane swelling and less drug adsorption, and can better meet the ultrafiltration requirements of different types of drug infusion.
Owner:常州费曼生物科技有限公司
Drug balloon
PendingCN110575607ALarge specific surface areaImprove adsorption capacityBalloon catheterCoatingsPorosityFiber
The invention discloses a drug balloon. The drug balloon comprises a balloon body and a drug coating, wherein the surface of the balloon body is coated with the drug coating; the drug coating is prepared from a nanofiber net membrane and drug particles, and the interior and the surface of the nanofiber net membrane are evenly coated with the drug particles; and the thickness of the drug coating is5-500 [mu]m. The drug balloon has the beneficial effects that firstly, the specific surface area of the nanofiber net membrane is large, drugs are adsorbed to the surface of the fiber membrane through electrostatic acting force, the drug adsorption capacity is fundamentally improved, the drug adsorption amount is increased greatly, and meanwhile in the balloon conveying and expanding processes, the drugs adsorbed to the outer surface of the balloon body can be kept as far as possible; and secondly, the porosity of the nanofiber net membrane is high and can reach 70% or above, through a uniqueporous interconnection structure, the drugs can be located in the nanofiber net membrane, and in the balloon conveying and expanding processes, the loss of the drugs can be reduced as far as possible.
Owner:JIANGSU NOWYON MEDICAL CO LTD
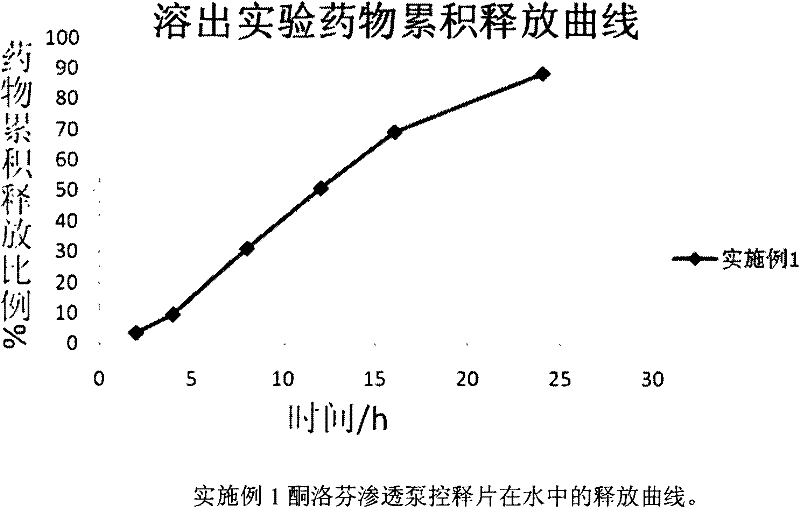
Ketoprofen osmotic pump type controlled release preparation and preparation method thereof
InactiveCN102670575ALasting effectGood curative effectOrganic active ingredientsAntipyreticDrug adsorptionSide effect
The invention relates to a ketoprofen osmotic pump type controlled release preparation and a preparation method thereof. The ketoprofen osmotic pump type controlled release preparation comprises an osmotic pump tablet core and a semipermeable controlled release coat film coated outside the tablet core, wherein the osmotic pump tablet core comprises a remedium cardinale, an osmotic active substance, a latent solvent, a retarding agent, a filling agent and a lubricating agent; and the semipermeable controlled release coat film comprises a coating material, a pore-foaming agent and a plasticizing agent. The invention also relates to the preparation method of the osmotic pump type controlled release preparation. The ketoprofen osmotic pump type controlled release preparation disclosed by the invention is prepared by a simple conventional preparation method of general tablets and is mainly characterized in that a single-layer osmotic pump tablet technology is applied to preparation of insoluble drug osmotic pump tablet; the tablet core is tabletted by adopting a conventional method without determining a punching surface; the drug release is safer, so that the toxic or side effects and the administration frequencies of the drug can be reduced; and the influence on the drug adsorption due to food intake and in vivo environment can be avoided, and thus the compliance of patients is improved.
Owner:CHINA PHARM UNIV
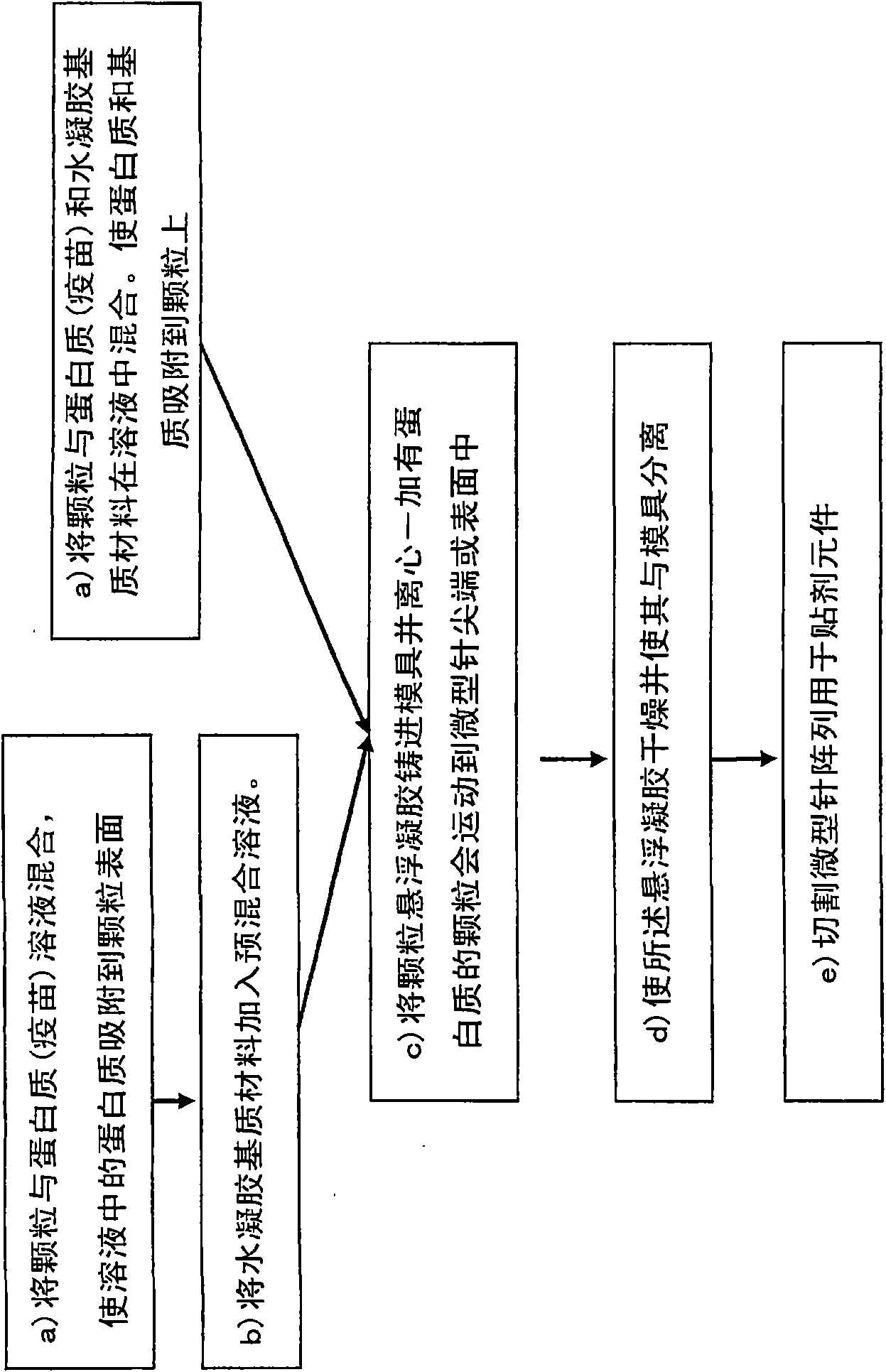
Solid solution perforator containing drug particle and/or drug-adsorbed particles
Owner:THERAJECT INC.
Silicon nitride infusion filter membrane, preparation method thereof, filter and infusion device
InactiveCN109092077APrecise thickness controlPrecise Control of ConsistencySemi-permeable membranesFiltering accessoriesDrug adsorptionFiltration
The invention discloses a silicon nitride infusion filter membrane, a preparation method thereof, a filter and an infusion device. The silicon nitride infusion filter membrane comprises a body. The body is provided with a monocrystalline silicon grid support body and a silicon nitride filter membrane arranged on the top surface of the monocrystalline silicon grid support body. The silicon nitridefilter membrane is provided with a plurality of penetrating filtering holes with the diameter of 0.2-1 micron and the depth of 0.1-0.5 micron. The silicon nitride filter membrane is integrally connected with the monocrystalline silicon grid support body, has good bonding strength and sealing performance, ensures the sound condition under the stress state, and has high strength and good elasticity.The filter membrane thickness is controlled below 1 micron. The filter membrane further has the advantages of good biocompatibility, short filtering path, low filtration pressure difference, low filter membrane swelling and less drug adsorption, and can better meet the nano ultrafiltration infusion requirements of different types of drugs due to the fact that the filter membrane surface modification is more convenient.
Owner:常州费曼生物科技有限公司
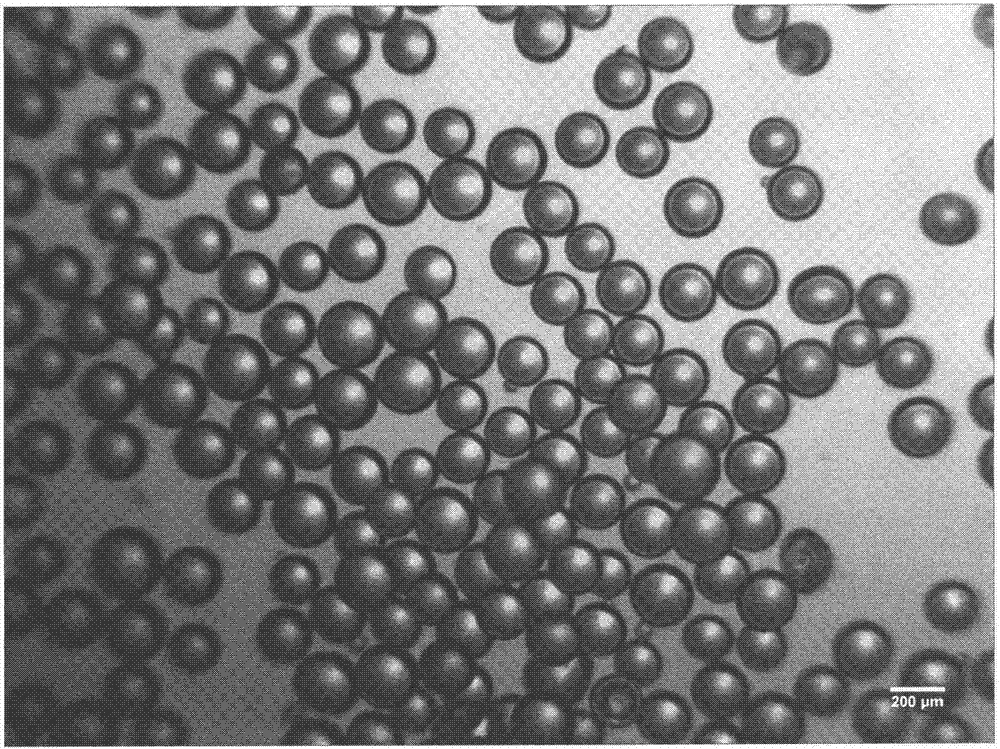
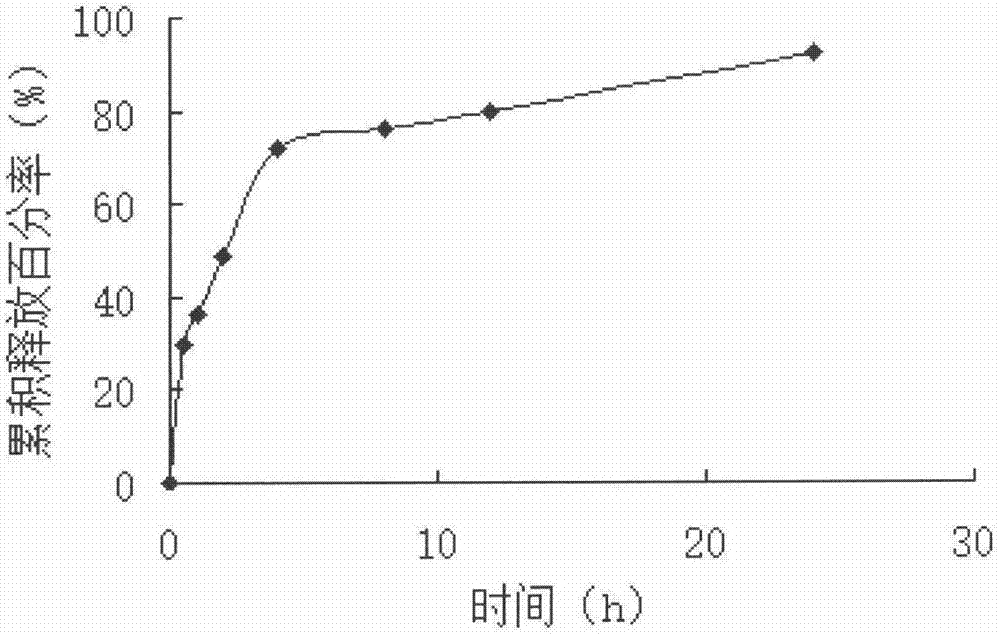
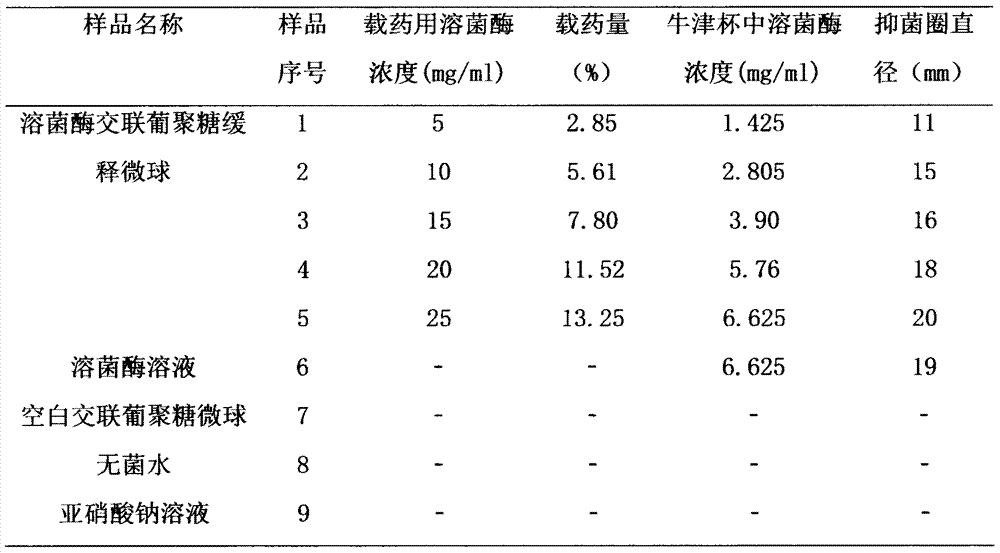
Lysozyme cross-linked dextran sustained-release microspheres and preparation method thereof
InactiveCN107049960AAnti-inflammatoryGive full play to the antiviral effectAntibacterial agentsPeptide/protein ingredientsCross-linkDrug adsorption
The invention belongs to the field of drug delivery systems of macromolecular drugs and in particular relates to lysozyme cross-linked dextran sustained-release microspheres and a preparation method thereof. The lysozyme cross-linked dextran sustained release microspheres are characterized by being prepared from the following components: cross-linked dextran microspheres and lysozyme. The preparation method comprises the following steps: weighing quantitative lysozyme and adding the lysozyme into purified water; after the lysozyme is completely dissolved, adding quantitative dextran microspheres into a solution; sufficiently stirring on a magnetic stirrer to completely disperse the microspheres; standing at room temperature and carrying out adsorption and drug loading; filtering and collecting the microspheres; freezing and drying in vacuum to obtain the lysozyme cross-linked dextran sustained-release microspheres. The sustained-release microspheres provided by the invention have good sustained-release effect, water swelling effect and biological adhesion effect and can be used for mucosal drug delivery and skin drug delivery so as to better express anti-inflammatory, antibacterial and anti-virus effects of the lysozyme.
Owner:CHINA PHARM UNIV
Noninvasive gynaecological nursing drug use device
InactiveCN105597223AGuaranteed pressure balanceImprove elimination effectMedical devicesLight therapyDrug adsorptionTreatment effect
The invention discloses a noninvasive gynaecological nursing drug use device which comprises a drug administration shield. The drug administration shield is a pipe-shaped shield and one end of the drug administration shield is closed; an open end of the drug administration shield is matched with a mounting groove at the right side of a junction board for installation; the left side of the junction board is connected with a hand-held end; a battery, a light source, a light condensing device and an optical fiber are sequentially and fixedly mounted inside the hand-held end from left to right; the optical fiber runs through a through hole in the middle of the junction board to be connected with a beam splitter mirror; the beam splitter mirror is fixedly mounted inside the drug administration shield; the power supply is connected with the battery by a wire; a ring-shaped drug distribution groove is formed inside the junction board; the ring-shaped drug distribution groove is connected with a cover body by a flexible catheter; the cover body is connected with a squeezing ball by a screw thread; a drug administration passage is formed inside a pipe wall of the drug administration shield; the drug administration passage is communicated with the ring-shaped drug distribution groove. The device disclosed by the invention adopts a noninvasive drug administration mode, meanwhile, an effect of a power light-wave therapeutic apparatus is combined, a drug adsorption speed is improved, and a treatment effect is promoted.
Owner:侯荣
Bionic cell membrane-inner core nanoparticle as well as preparation method and application thereof
PendingCN114099466ASimple manufacturing methodReduce concentrationNanomedicinePharmaceutical non-active ingredientsAntithrombotic AgentDrug adsorption
The invention relates to a biomimetic cell membrane-inner core nanoparticle as well as a preparation method and application thereof. The biomimetic cell membrane-inner core nanoparticle comprises an inert inner core and a biomimetic cell membrane coated outside the inert inner core. The invention develops the antithrombotic drug reversal agent which is effective for various antithrombotic drugs, high in safety, convenient to operate and low in preparation cost, the inert core is used as a support skeleton, and the bionic cell membrane is used as an antithrombotic drug adsorption layer; the receptor expressed by the bionic cell membrane is used as a bait to be combined with an antithrombotic drug to reduce the blood concentration in a free form, so that the antithrombotic effect can be effectively reduced; on the other hand, the biocompatibility of the nano particles can be enhanced through wrapping of the bionic cell membrane, and the probability that the nano particles are cleared by an organism is reduced.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Nanoporous magnesium silicate microsphere/PBS (poly(butylene succinate)) composite scaffold, composite scaffold coated with protein, preparation methods and application
InactiveCN107412856AHigh porosityImprove water absorptionPharmaceutical delivery mechanismTissue regenerationDrug adsorptionMicrosphere
The invention discloses a nanoporous magnesium silicate microsphere / PBS (poly(butylene succinate)) composite scaffold, a composite scaffold coated with protein, preparation methods and an application. A preparation method of a genipin crosslinking protein coating supported drug-nanoporous magnesium silicate microsphere / PBS composite scaffold comprises the following steps: PBS is mixed with an organic solvent, the mixture is then mixed with nanoporous magnesium silicate microspheres and a pore-foaming agent, the mixture is subjected to pressing forming, the organic solvent and the pore-foaming agent are removed, and the composite scaffold is obtained after drying; the composite scaffold is soaked in a resveratrol buffer solution, and the resveratrol supported composite scaffold is obtained after drying; a gliadin solution is added to the resveratrol supported composite scaffold, the gliadin protein coating supported composite scaffold is obtained after drying and then mixed with a genipin solution for a crosslinking reaction, and a product is obtained. The composite scaffolds have higher in-vitro degradation performance and bioactivity, good cell compatibility, high drug adsorption capacity and good drug slow release effect, and the effective acting time of drugs can be prolonged.
Owner:SHANGHAI CHANGHAI HOSPITAL
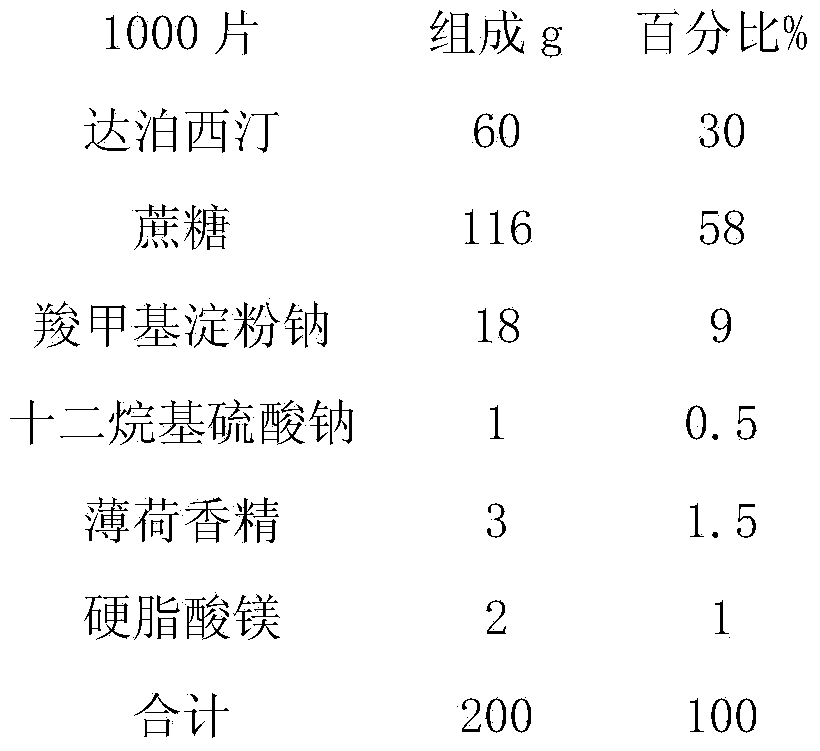
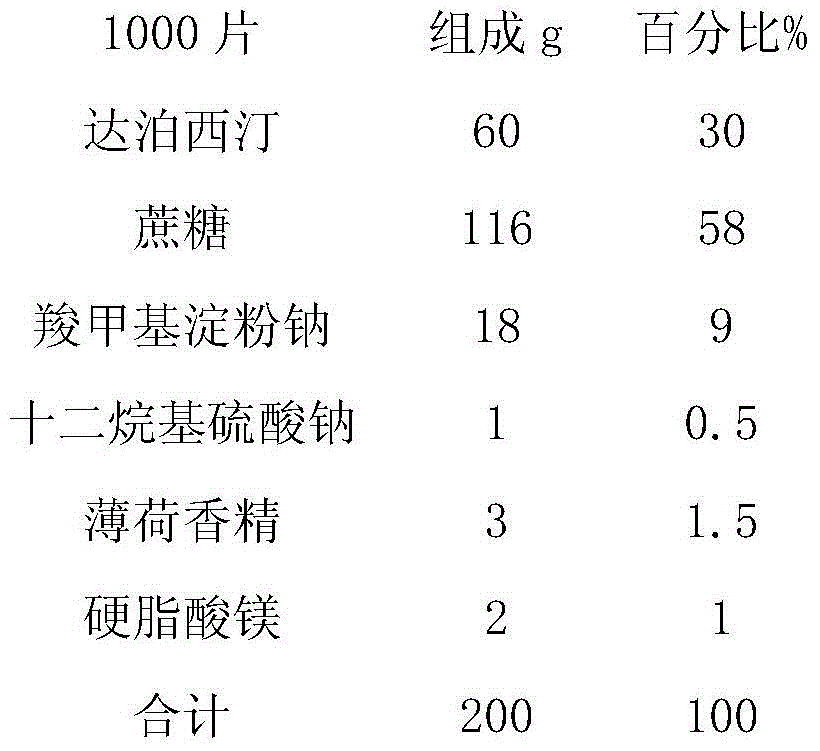
Dapoxetine tablets and preparation method thereof
ActiveCN103735525BFast absorptionImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsDrug adsorptionSide effect
The invention relates to dapoxetine tablets and a preparation method thereof. The dapoxetine tablets comprise the following components in percentage by weight: 10 to 50 percent of dapoxetine, 30 to 80 percent of a filling agent, 3 to 20 percent of a disintegrant, 0.5 to 1 percent of a lubricant and 0.2 to 2.5 percent of a flavoring agent. The preparation method comprises the following steps: micronizing the dapoxetine and the filling agent and controlling the particle size to be 0.5-20 [mu]m; preparing the fine powder obtained in the previous step and a part of the disintegrant into a soft material by ethanol water and pelletizing by a 24-mesh sieve; drying the obtained fine particles at the temperature of 50 to 70 DEG C and straightening by a 20-mesh sieve; sequentially adding the rest disintegrant, the flavoring agent and the lubricant into the dried particles, carrying out blending for two times, and until intermediates are qualified by detection, and carrying out tabletting, so that the dapoxetine tablets are obtained, wherein blending is carried out for one time before the lubricant is added and then is carried out again after the lubricant is added. The dapoxetine tablets are coated or uncoated conventional tablets, chewable tablets and orally disintegrating tablets, are used for treating male prospermia, high in drug adsorption speed and bioavailability, and convenient to take and have small side effects.
Owner:JIANGSU TIANZHAO PHARMA
Drug Delivery Carrier
InactiveUS20110136722A1Improve bioavailabilityLow water solubilitySugar derivativesPeptide/protein ingredientsDrug adsorptionPharmaceutical Substances
The present disclosure relates to a method for the sustained release of a drug, comprising the steps of: (a) preparing a biocompatible polymer having a hydrophobic group conjugated to the biocompatible polymer; and (b) contacting the biocompatible polymer to the drug for adsorbing the drug to the hydrophobic group of the biocompatible polymer, thereby obtaining a drug delivery carrier for the sustained release of the drug; wherein the drug is a protein, a peptide or a non-hydrophilic chemical drug; wherein when the drug adsorbed to the hydrophobic group of the biocompatible polymer is administered to a mammal, it shows a sustained release profile in the mammal. The drug delivery carrier according to the present disclosure having the hydrophobic group conjugated to the biocompatible polymer may be useful for adsorption of synthetic drugs having very low solubility in water. Further, it may regulate discharge rate of adsorbed drugs by regulating a portion of hydrophobic groups conjugated to the polymeric material. Thus, the present disclosure provides a broad-spectrum platform technology applicable to new hydrophobic synthetic drugs to be developed in the future as well as those that have been developed already but face difficulties due to low bioavailability.
Owner:PRONEXX
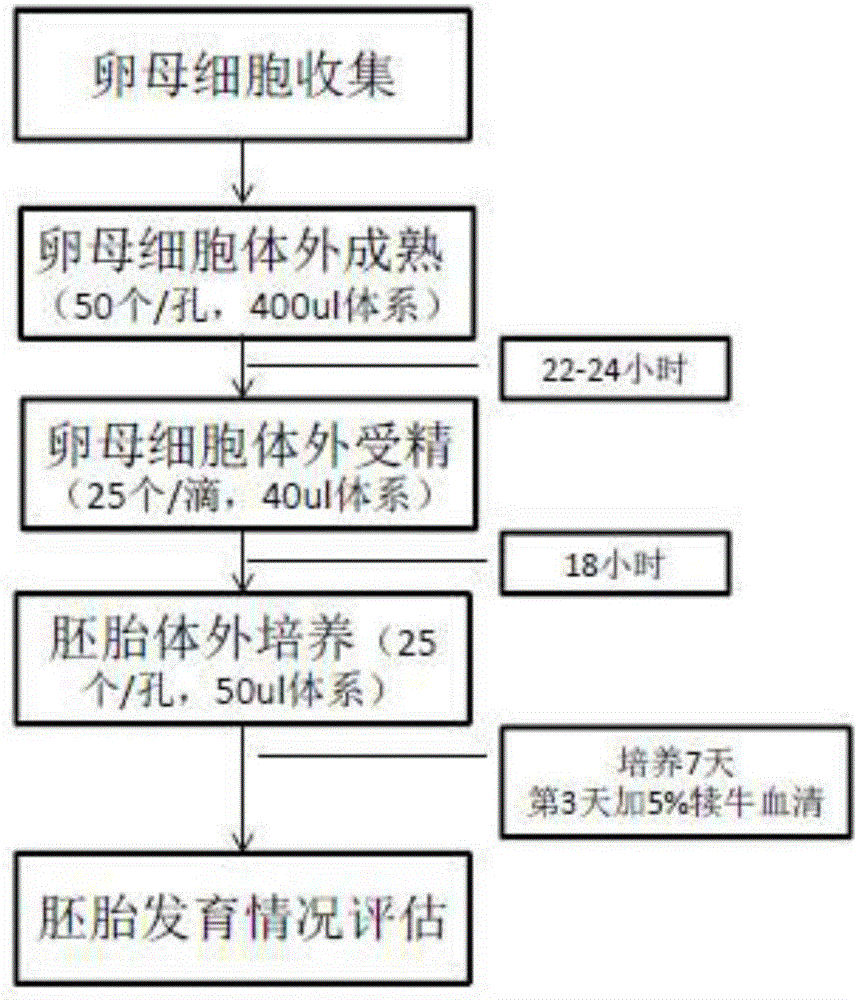
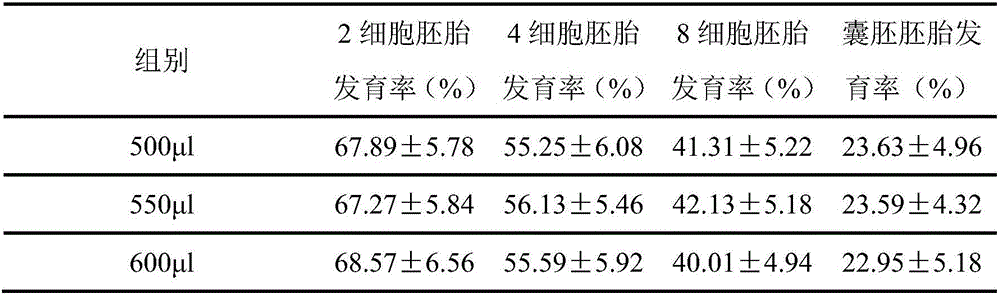
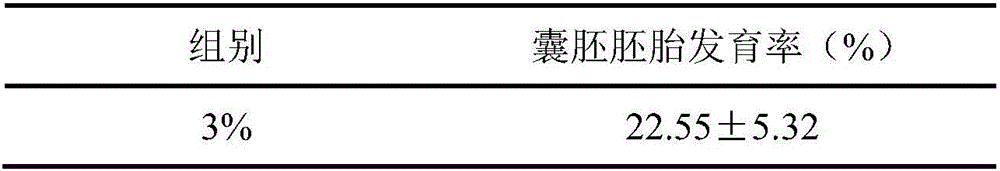
In-vitro embryo culture method
ActiveCN107523538AAddressing the effects of adsorptionReflecting the real effectCulture processCell culture active agentsBlood serumBlastocystis
The invention discloses an in-vitro embryo culture method which specifically comprises the following steps: 1) placing an embryo culture medium in an atmosphere of CO2, O2 and balanced N2 in a culture dish before embryo culture; 2) placing a zygote into a hyaluronidase, allowing standing still, absorbing part of supernatant, abandoning the part of the supernatant and oscillating remaining liquid with an oscillator, so as to obtain mixed solution; 3) adding SOF-HEPES into the mixed solution, transferring the mixed solution to a plate, washing an embryo for several times, placing the washed embryo into the culture dish in step 1), adding 5% of serum into the culture dish on the third day of culture and waiting for blastocyst formation. In the in-vitro embryo culture method, mineral oil is mainly eliminated, and the influence of the covered mineral oil on adsorption of added medicines, so that the actual functions of the added medicines are reflected; meanwhile, a culture system without addition of the mineral oil can also achieve a desired effect of in-vitro embryo culture.
Owner:JIANGSU POLYTECHNIC COLLEGE OF AGRI & FORESTRY
Solid liposome nano granule with antineoplastic activity
InactiveCN101011359AIncrease intakeGood curative effectOrganic active ingredientsPharmaceutical non-active ingredientsDrug adsorptionSide effect
The invention relates to a nanometer liposome carrier with high-effect anti-tumor activity, which carries the anti-tumor Paclitaxel, while the Paclitaxel is 3.1-3.6% of solid liposome nanometer particles, and the diameter is 481-532nm. The invention has high-effect cell extraction and cell-pulp hold function via the nanometer liposome carrier, while the package on the anti-tumor drug whose molecule target is at the cell pulp can improve the adsorption of anti-tumor drug and improve the drug density at target part. The invention can improve the drug adsorption of tumor cell, reduce the drug distribution at normal cell, reduce the side effect and toxicity, and improve the effect.
Owner:ZHEJIANG UNIV
Transfusion liquid heater
The invention relates to a transfusion heater, wherein the invention comprises tubular thermal-insulated sheath which contains transfusion duct; the sheath extends into chamber through the liquid medium; the chamber has heating medium tube through to the temperature-adjustable liquid medium heater; the heater has micro pump; the sheath is made from temperature-resistant soft or rigid plastic, and its side has switch slim. The invention can adjust liquid heating temperature or heating voltage via the drug speed, drug temperature and condition temperature, to hold liquid at 20-35Deg. C, to improve drug adsorption.
Owner:王富华
Method for obtaining alimentary absorption characteristic of medicament
InactiveCN1966086AGain absorbing propertiesImprove reliabilityIn-vivo testing preparationsMaterial analysisDrug adsorptionDrug metabolism
The invention relates to a method for obtaining the drug enteron adsorption property, wherein it comprises that: (1), preparing the agent of drug; (2), packing said agent into storage chamber of remote-control electric capsule; (3), test animal or people takes said capsule; (4), tracking the position of capsule in the body; (5), remote-control releasing drug; (6), collecting sample of body fluid or blood and preparing; (7), analyzing blood drug density; (8), extracting the drug metabolism kinetics parameters. The inventive method can obtain the drug adsorption property in enteron accurately, with high reliability.
Owner:CHONGQING UNIV
Surface-modified microsphere composition, application of the same, and method of preparing the same
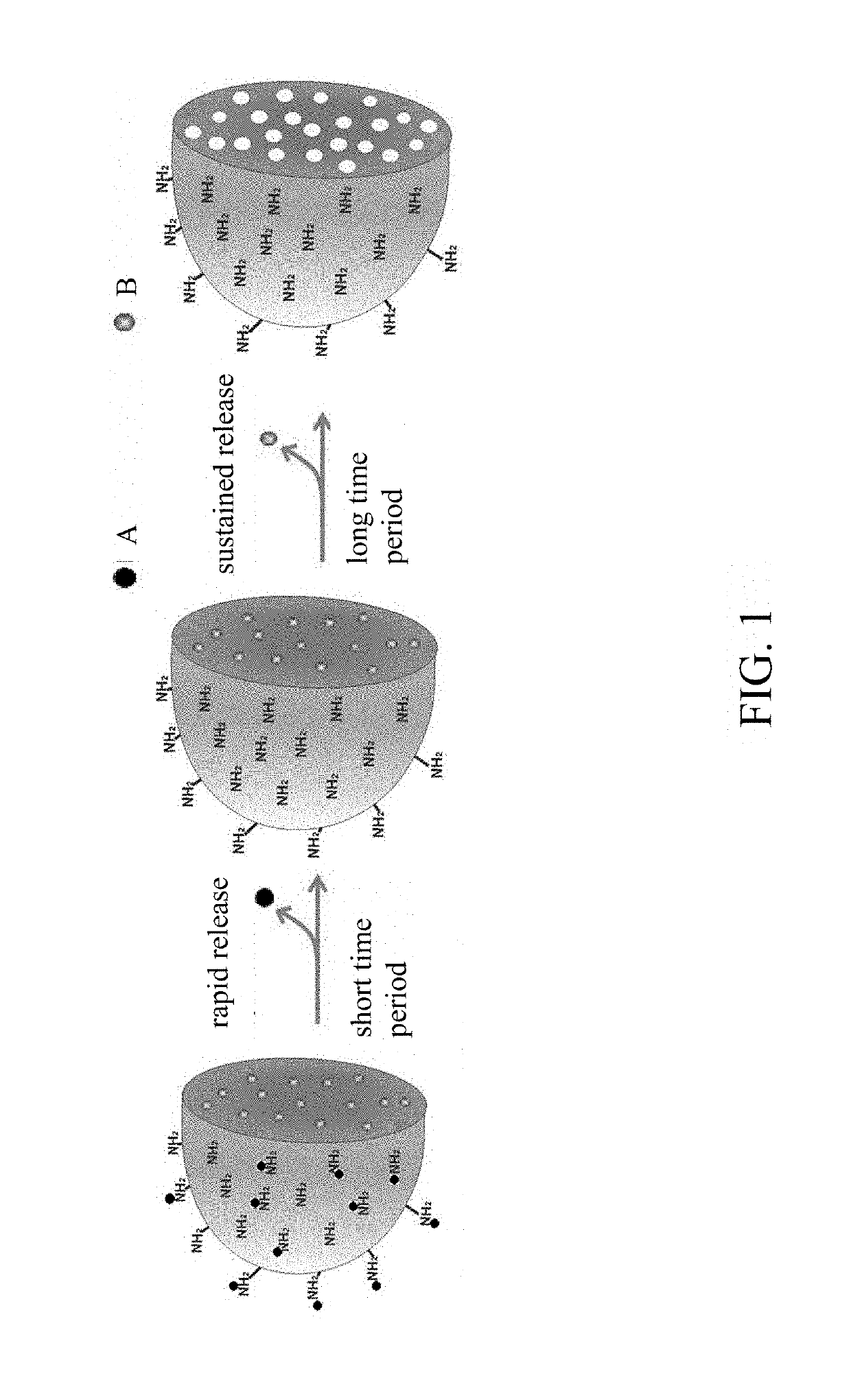
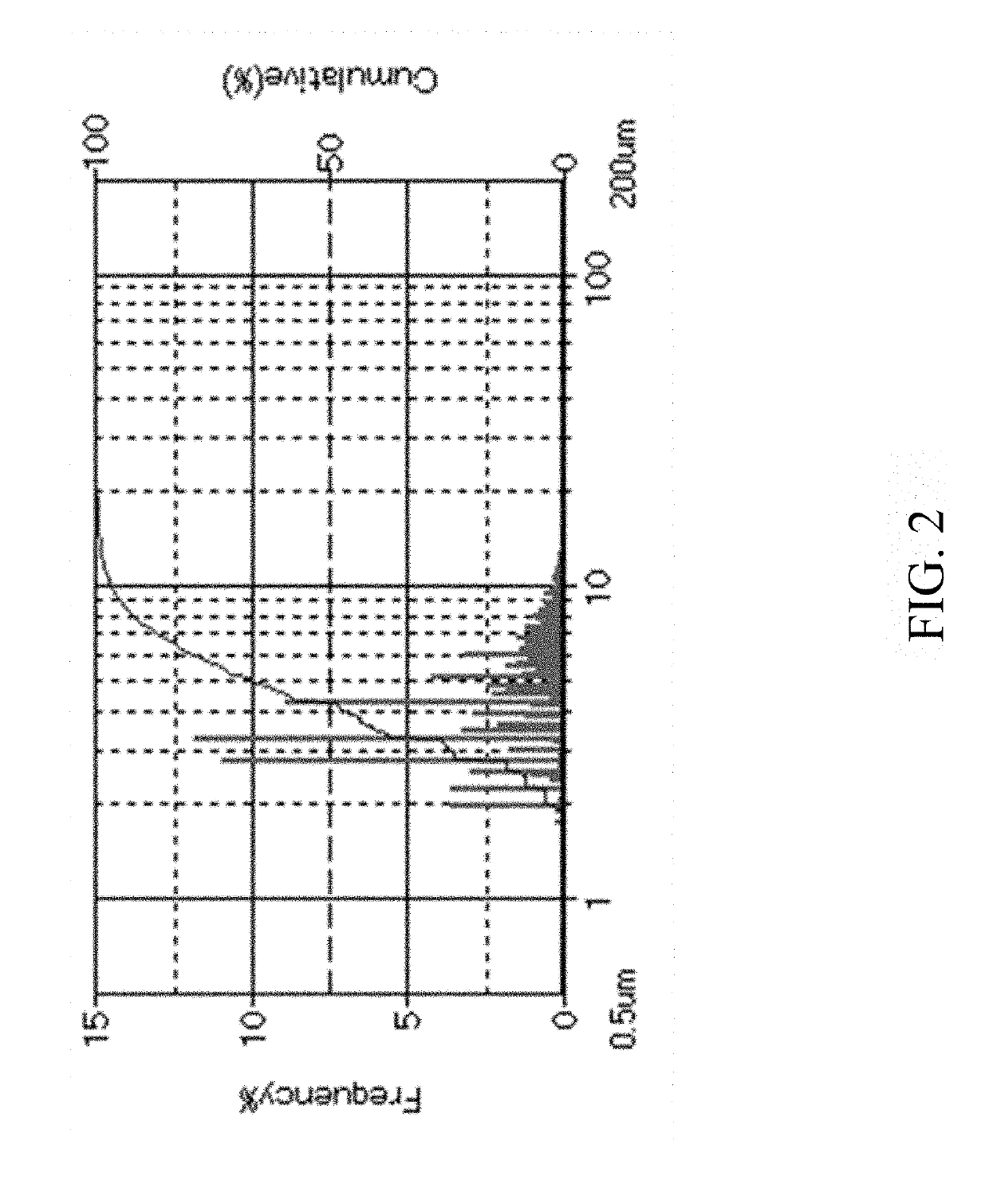
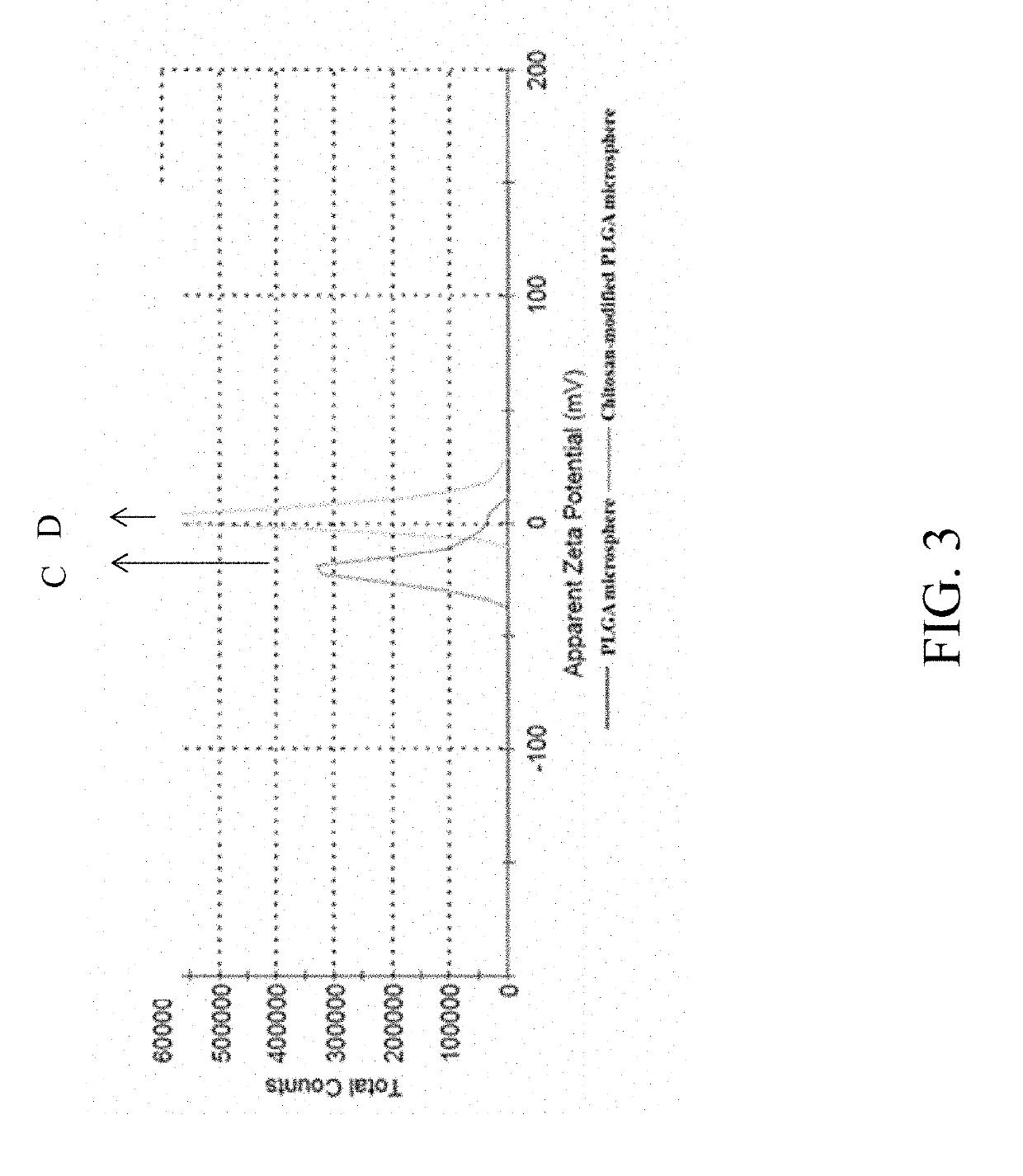
A surface-modified microsphere composition comprises poly(lactic-co-glycolic acid) (PLGA), chitosan, hydrophobic drug, and hydrophilic drug. The PLGA forms a microsphere. The chitosan is formed on a surface of the microsphere. The hydrophobic drug is encapsulated by the microsphere. The hydrophilic drug is adsorbed on the surface of the microsphere. The surface-modified microsphere composition is for treating arthritis. A method of preparing a surface-modified microsphere composition involves producing by an oil-in-water emulsion method microspheres formed from PLGA, covered with chitosan, and adapted to encapsulate hydrophobic drug, and having hydrophilic drug adsorbed on their surfaces. The surface-modified microsphere composition enables drug to be released to achieve efficacy both instantly and persistently.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Infusion set filtration membrane and preparation method, infusion set filtration membrane structure and preparation process, filter and infusion set
PendingCN108854590AReduce swellingReduce adsorptionMembranesUltrafiltrationDrug adsorptionFiltration membrane
The invention discloses an infusion set filtration membrane and a preparation method, an infusion set filtration membrane structure and a preparation process, a filter and an infusion set. The infusion set filtration membrane comprises a membrane body. The membrane body is made of aluminum oxide and provided with a plurality of through filtration holes. A filtration hole pattern formed by the multiple filtration holes is defined by an imprinting mold through an imprinting transfer method. After anodization, the diameter of each obtained filtration hole is 0.1-1 micrometer, the depth of each filtration hole is 2-100 micrometers, and the distance between the filtration holes is 0.5-10 times the diameter of each filter hole. The infusion set filtration membrane has more accurate particle diameter filtration capacity. Meanwhile, the distance between the holes can be freely regulated, so that the infusion set filtration membrane has good mechanical strength, and it is ensured that the infusion set filtration membrane remains undamaged under the normal filtration pressure. Meanwhile, the infusion set filtration membrane also has the characteristics of short filtration path, high structural strength, good sealing performance, small filtration differential pressure, less swelling, less drug adsorption, etc., and can better meet the nano ultrafiltration requirements of infusion of different types of drugs.
Owner:常州费曼生物科技有限公司
Medical AMTPS material, medical instrument and preparation method
The invention discloses a medical AMTPS material, a medical instrument and a preparation method. The medical AMTPS material comprises, by weight, 50-80 parts of styrene-butadiene-styrene segmented copolymer, 25-50 parts of reinforced resin polypropylene PP, 0.05-0.3 part of green composite antioxidant ECLL, 0.05-0.2 part of slipping agent, 0.005-0.2 part of anti-adherent or enhancer and 0.002-0.02part of antibacterial agent. A plasticizer DEHP is not added, so that damage to a patient caused by metal ions is avoided, and the medical AMTPS material is safe, nontoxic, free of drug adsorption and capable of ensuring drug treatment effect. Carcinogenic dioxin gas is not generated during burning, and performance of an injector and an infusion tube can meet using standards existing medical infusion tubes; by adding the composite antioxidant and the antibacterial agent, the medical AMTPS material has an antibacterial function, risk that the injector and the infusion tube are polluted by bacteria in the process of transportation and storage can be prevented, and safety of the patient is ensured.
Owner:四川洁凯医疗器械有限公司
Polyolefin elastomer for peristaltic pumps and preparation method thereof
InactiveCN108003542AImprove deformation recovery performanceImprove wear resistanceDrug adsorptionPeristaltic pump
The invention discloses a polyolefin elastomer for peristaltic pump pipes and a preparation method thereof. The polyolefin elastomer is prepared from, by weight, 20-40 parts of styrene elastomer, 60-80 parts of olefin block copolymer, 0.2-0.5 part of complex antioxidant and 0.3-0.5 part of lubricant, wherein the styrene elastomer is styrene-butadiene-styrene block polymer or hydrogenated styrene-butadiene-styrene polymer or styrene-isoprene-styrene block polymer. Compared with polyvinyl chloride peristaltic pump pipes widely used at present, the polyolefin elastomer has the advantages of no toxicity, low drug adsorption, excellent creep resistance, wear resistance and deformation recovery capacity and the like. The polyolefin elastomer for peristaltic pump pipes has superhigh resilience, and thus the relative flow deviation of pump pipes made of the polyolefin elastomer is smaller than 6% in 4 h.
Owner:深圳恒方大高分子材料科技有限公司 +2
Methods and apparatus for passive, proportional, valveless gas sampling and delivery
ActiveUS11259717B2Avoid dilutionEasy to useHealth-index calculationMedical devicesDrug adsorptionAerosolize
A fluid dynamic valve passively allows fluid flow out of a moving stream in one flow direction and not in the reverse. This allows the collection of fluid from a single direction of an AC fluid flow. The siphoned portion of the flow has a flow rate proportional to the mainstream flow. This device can collect exhaled breath or selective entrenchment during inhale. In one orientation, it can meter aerosolized particles into an inhale breath stream for pulmonary delivery, without complicated breath timing or drug loss due to drug adsorption to the back of the throat. Alternatively, a user can breathe through the device and a proportional amount, relative to the volumetric flow rate, of each exhale can flow into an auxiliary chamber for analysis. In addition, the device has a low respiratory burden and is comfortable to use.
Owner:MASSACHUSETTS INST OF TECH
Preparation method of plant insecticide specially used for killing cabbage caterpillar in organic vegetable planting process
The invention discloses a preparation method for a botanical pesticide for specially killing cabbage caterpillars during organic vegetable planting. The preparation method comprises the following steps: 1), preparing raw materials, wherein the raw materials comprise the following components in parts by weight: 10-15 parts of radix sophorae flavescentis, 7-10 parts of melia toosendan, 12-19 parts of melia toosendan bark, 10-15 parts of tripterygium glycosides, 18-24 parts of raw kusnezoff monkshood roots, 4-6 parts of radix euphorbiae lantu, 6-8 parts of pericarpium citri reticulatae and 13 parts of tobacco; 2), adding 3 to 4 times of water by weight in the raw materials, decocting in a sealing manner at the temperature of 90-100 DEG C for 100 to 120 minutes, filtering, and concentrating the filtrate to be 1 / 3-1 / 4 of the original used water amount to obtain the botanical pesticide for specially killing pierisrapae linne. The botanical pesticide can targetedly and specially kill cabbage caterpillars by scientific compatibility of the traditional Chinese medicine components, and is high in permeability, conducive to plant uptake, low in cost, free of three-evil, residue, pollution and drug resistance, high in drug adsorption, and long in drug effect duration.
Owner:ZHEJIANG HENGZE ECOLOGICAL AGRI SCI & TECH LIMITED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com