Medical AMTPS material, medical instrument and preparation method
A technology of medical equipment and raw materials, which is applied in the field of medical equipment and can solve problems such as hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
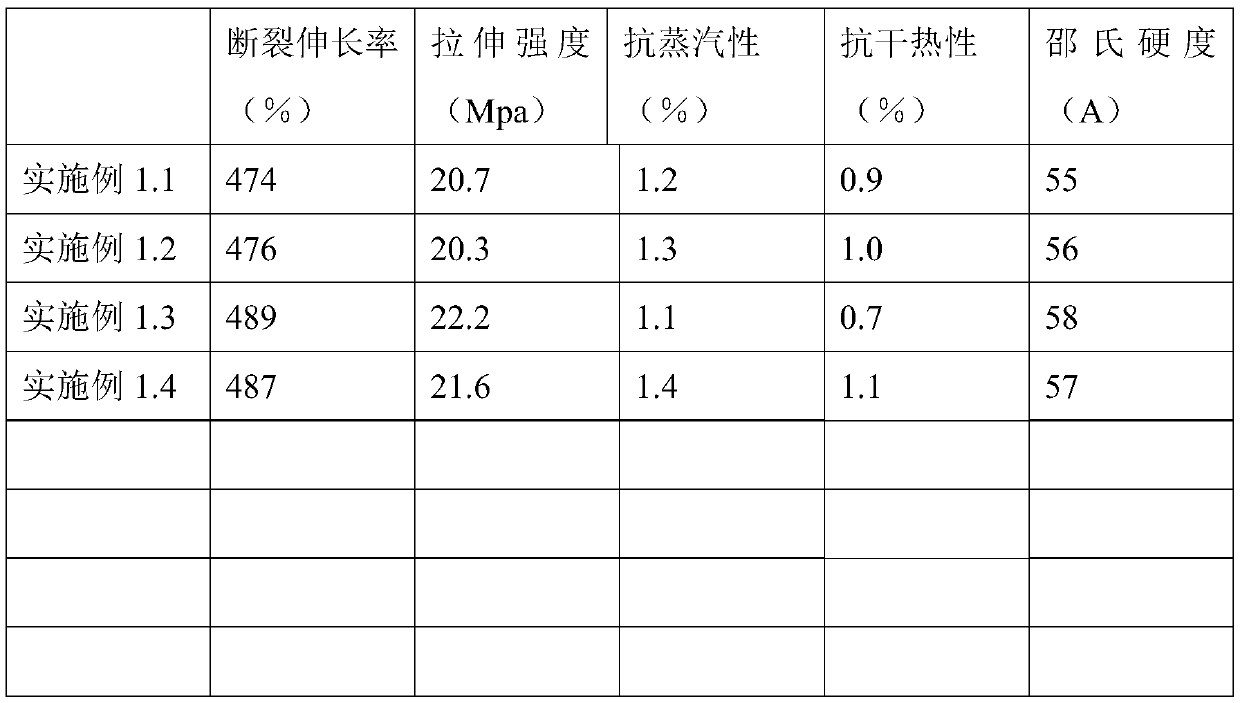
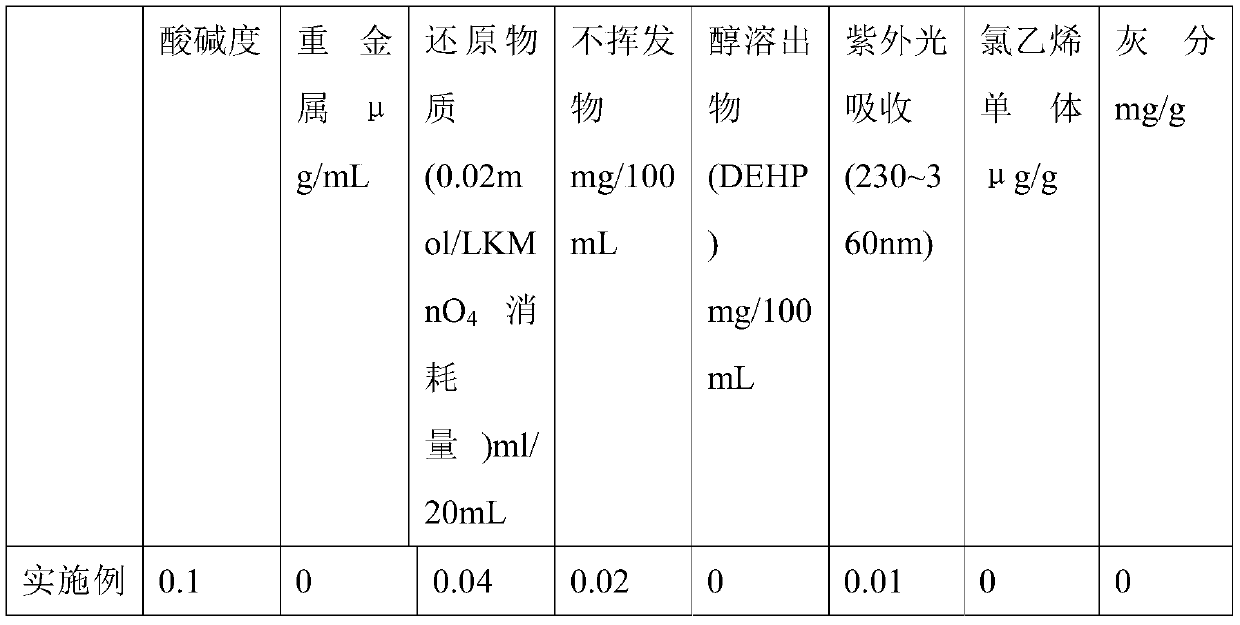
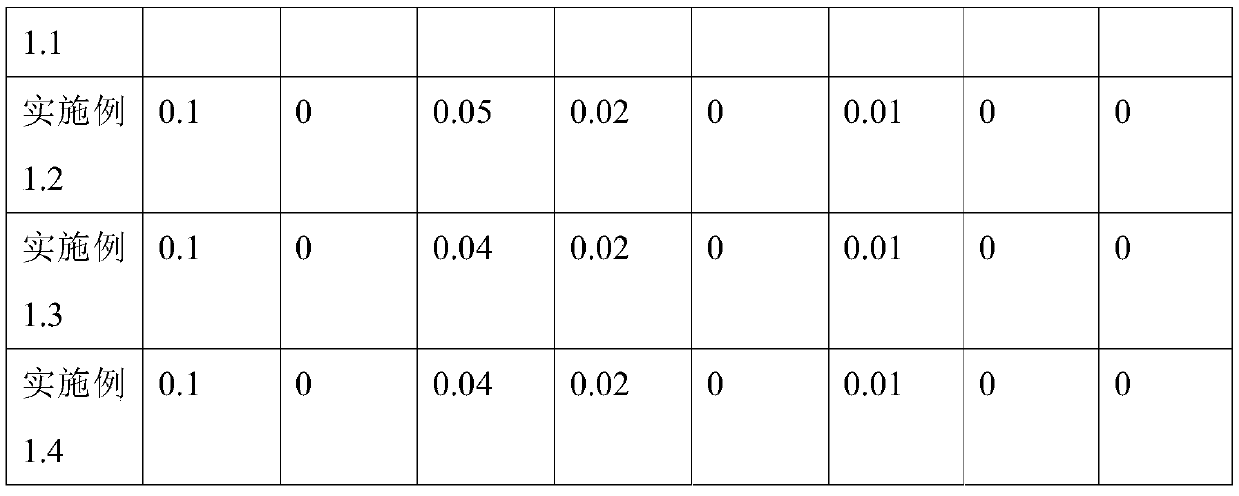
[0051] A medical AMTPS material, comprising the following raw materials in weight ratio: 70 parts by weight of styrene-butadiene-styrene block copolymer SEBS, 30 parts by weight of reinforced resin polypropylene PP, 0.055 parts by weight of green composite antioxidant ECLL , 0.1 parts by weight of slip agent, 0.01 parts by weight of anti-tack agent, and 0.005 parts by weight of antibacterial agent.
[0052] In this embodiment, the styrene-butadiene-styrene block copolymer is a hydrogenated styrene-butadiene-styrene block copolymer. The reinforced resin polypropylene PP includes isotactic polypropylene, syndiotactic polypropylene and random polypropylene.
[0053] The preparation method of the green compound antioxidant ECLL is as follows: uniformly mixing vitamin E, vitamin C, lecithin, chlorogenic acid, and isopropyl myristate in a pulverized oatmeal. Vitamin E: Vitamin C: Lecithin: Chlorogenic Acid: Isopropyl Myristate = 2:0.2:2:1:1.
[0054] The slip agent includes erucam...
Embodiment 12
[0065] A kind of medical AMTPS antibacterial material, same as embodiment 1.1, difference is, comprise the raw material of following weight ratio: styrene-butadiene-styrene block copolymer SEBS 66 weight parts, reinforced resin polypropylene PP 34 weight 0.105 parts by weight of green composite antioxidant ECLL, 0.1 parts by weight of slip agent, 0.01 parts by weight of antisticking agent, and 0.008 parts by weight of antibacterial agent. The antibacterial agent is chlorogenic acid.
Embodiment 13
[0067] A kind of medical AMTPS antibacterial material, identical with embodiment 1.1, difference is, styrene-butadiene-styrene block copolymer SEBS 62 weight parts, reinforced resin polypropylene PP 38 weight parts, green composite antioxidant ECLL 0.2 parts by weight, 0.1 parts by weight of slip agent, 0.01 parts by weight of anti-sticking agent, and 0.01 parts by weight of antibacterial agent. The antibacterial agent is an equal proportion mixture of octenidine and chlorogenic acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| brittleness temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com