Method for obtaining alimentary absorption characteristic of medicament
A technology of absorption characteristics and digestive tract, which is applied in the field of obtaining the absorption characteristics of drugs in the digestive tract, can solve the problems of unreliable research data, poor repeatability, and unreliable location, and achieve high reliability, easy operation, and reliability of data provided Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The purpose of this example is to obtain the absorption characteristics of the drug to be studied in the human colon. The drug to be studied is aminophylline, and the chemical name of aminophylline is 1,3-dimethyl-3,7-dihydro-1H-purine-2,6-diketone-1,2-ethylenediamine salt dihydrate substance, the molecular formula is C 2 h 8 N 2 (C 7 h 8 N 4 o 2 ) 2 2H 2 O, with a molecular weight of 456.46, is mainly used clinically for bronchial asthma, wheezing bronchitis, obstructive emphysema, etc. to relieve wheezing symptoms, and can also be used for asthma caused by cardiogenic pulmonary edema. The test subjects were 8 male healthy volunteers, the dosage form was powder dosage form, and the planned drug release location was the transverse colon.
[0022] The specific method steps are:
[0023] 1. The dosage form preparation of test medicine, takes by weighing 8 parts of aminophylline powders, every part of 150mg,
[0024] 2. The encapsulation of the test drug, select...
Embodiment 2
[0033] The purpose of this example is to obtain the drug absorption characteristics of the drug to be studied in the digestive tract of volunteers. The drug to be studied is carbamazepine, chemical name: 5H-dibenzo[b,f]azepine-5-carboxamide, molecular formula: C15H12N20 molecular weight: 236.27. The test subjects were selected as 8 male healthy volunteers, and the dosage form of the test drug was an aqueous solution.
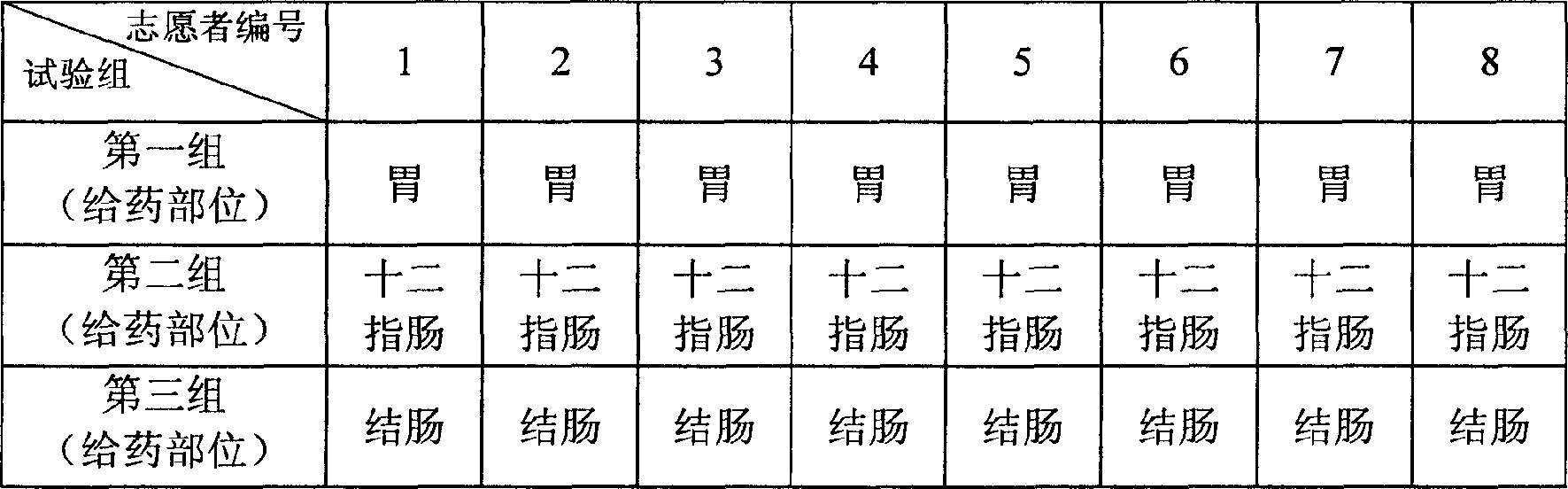
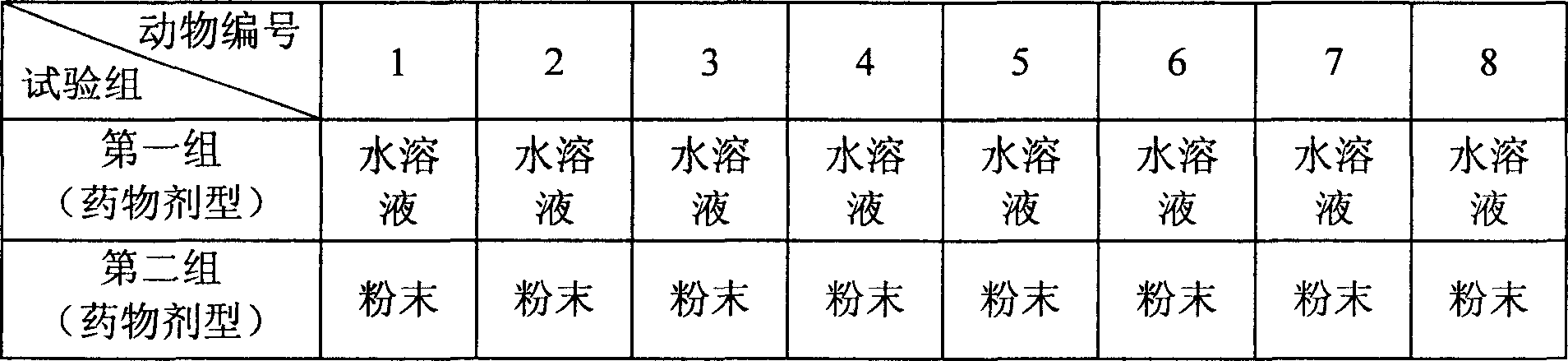
[0034] The specific implementation steps of this embodiment include three sets of steps as described in Example 1, each set of steps is for the fixed-point release of drugs in a specific part of the digestive tract, namely the stomach, duodenum, and colon. As shown in Table 1. After a set of test procedures is completed, after a certain drug elimination period (generally 7 days), the next set of tests is carried out.
[0035] The specific method steps of each group of experiments are as follows:
[0036] 1. The preparation of the dosage form of test medicine,...
specific Embodiment 3
[0047] The purpose of this example is to obtain the absorption characteristics of the drug to be studied in the colon of Beagle dogs. The drug to be studied is matrine. Matrine is an alkaloid extracted from the roots of Sophora flavescens and Sophora flavescens. Its molecular formula is C15H24N2O and its molecular weight is 248.36. It is mainly used clinically to treat cancer, Viral hepatitis, leukopenia, bronchial asthma and asthmatic bronchitis, etc.
[0048] In the specific implementation steps of this embodiment, two groups of steps as described in Example 1 are included, each step adopts a dosage form of the medicine, and the dosage form is an aqueous solution dosage form or a powder dosage form, and each group of steps requires 8 ratios. Greyhound. Each group of steps performs fixed-point drug release of a drug dosage form, and the drug release site is the colon, as shown in Table 2. After a set of test procedures is completed, after a certain drug elimination period (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com