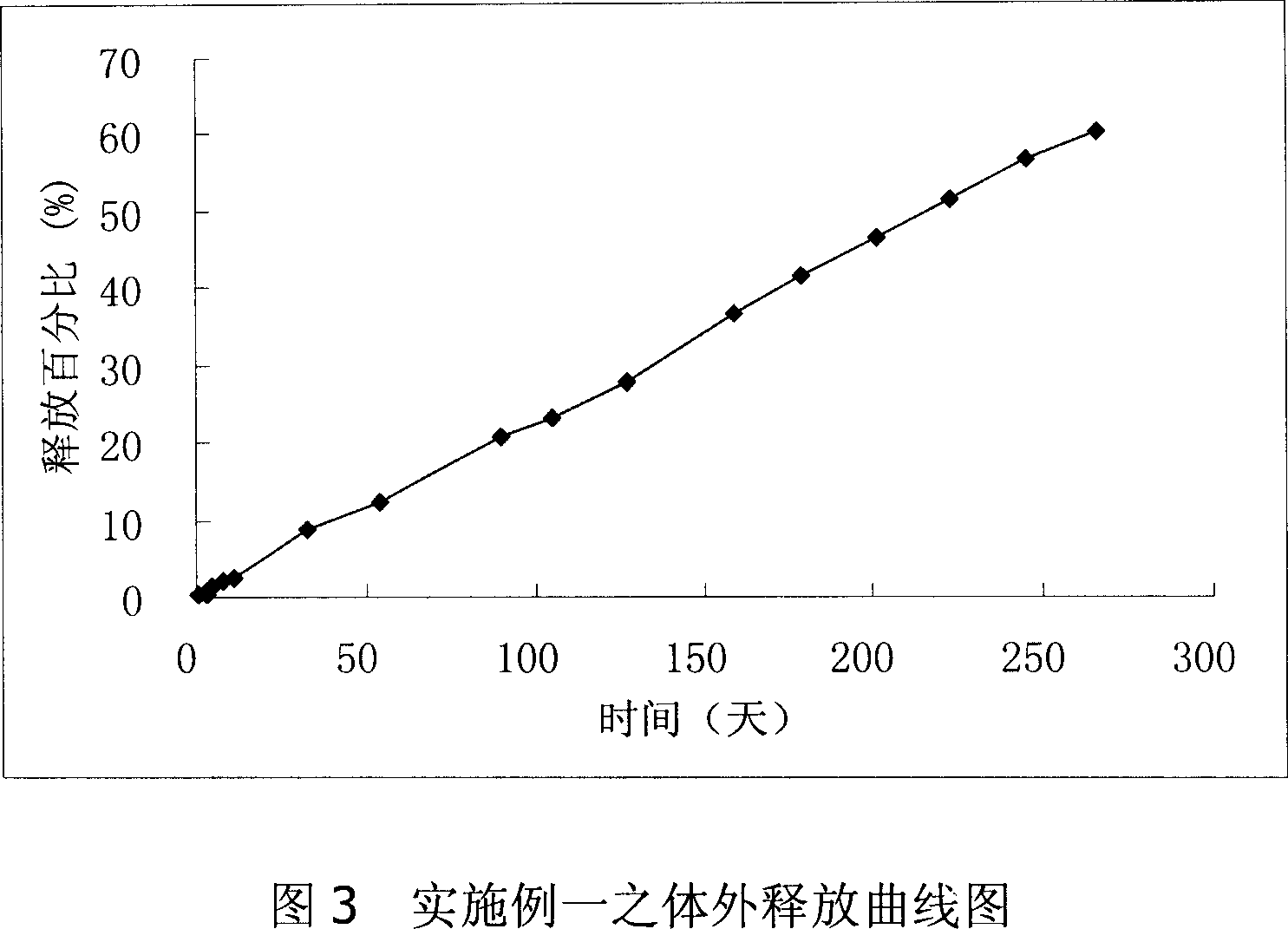
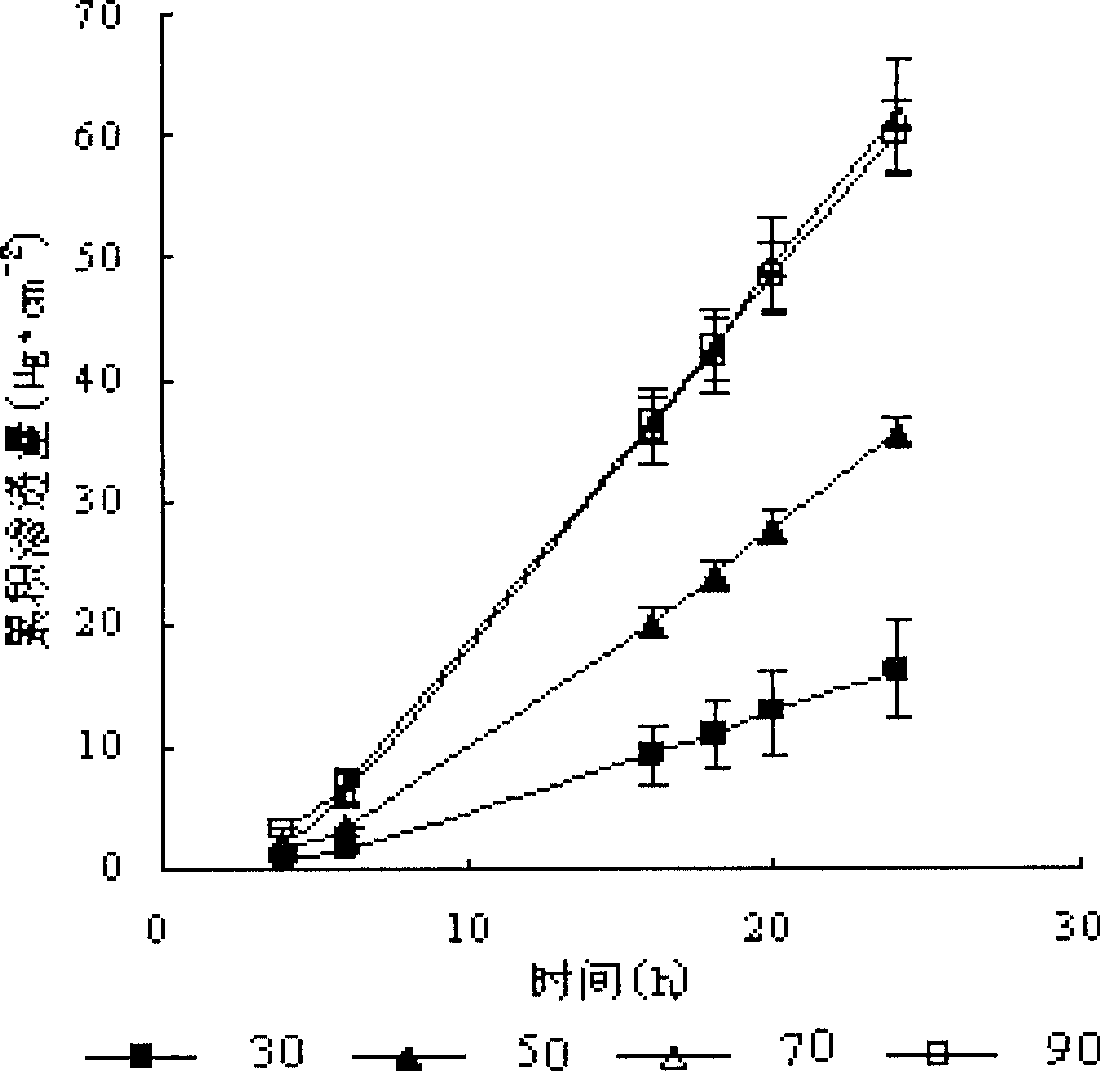
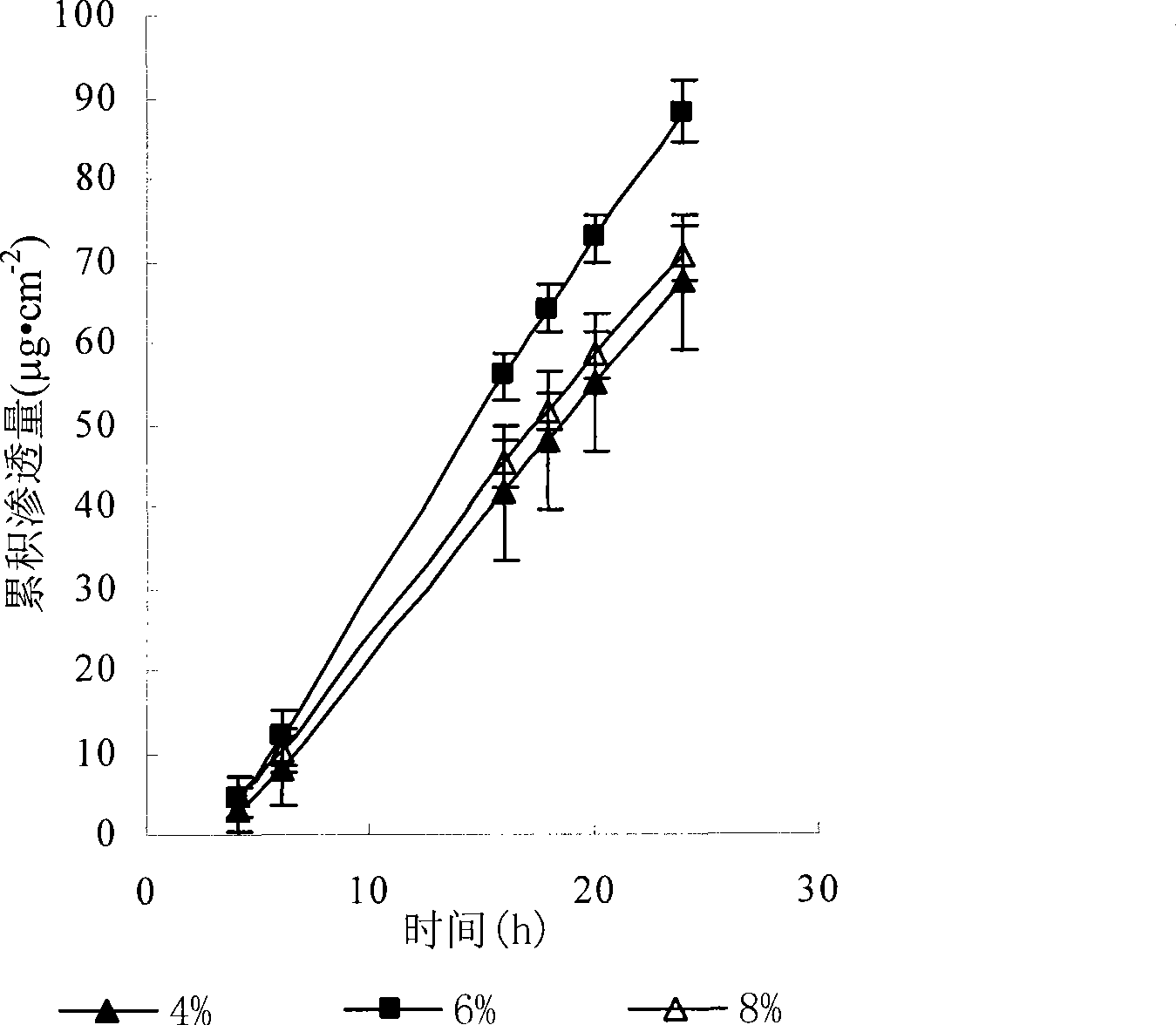
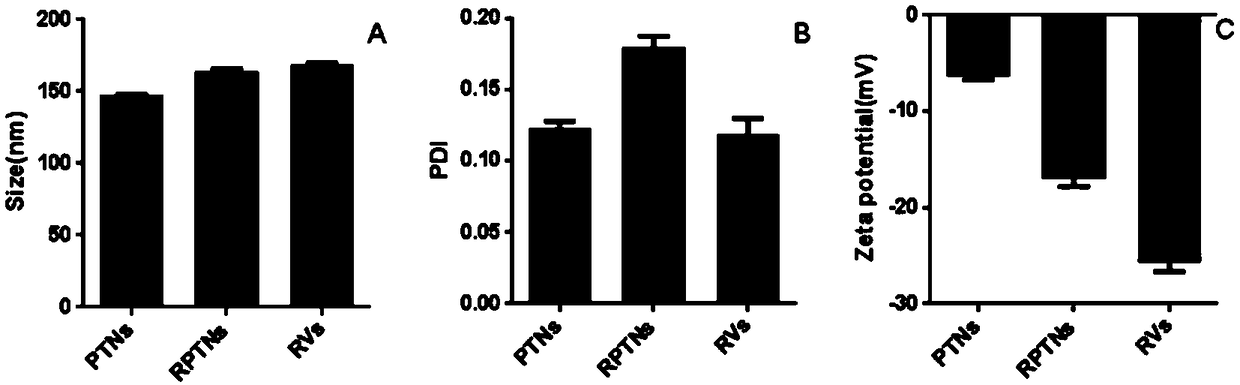
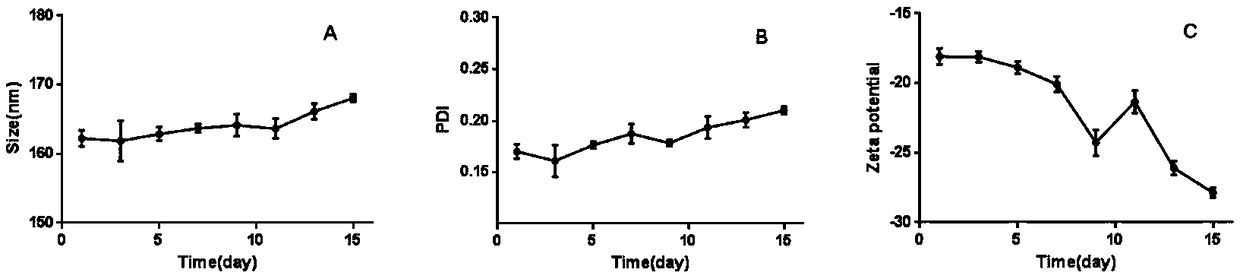
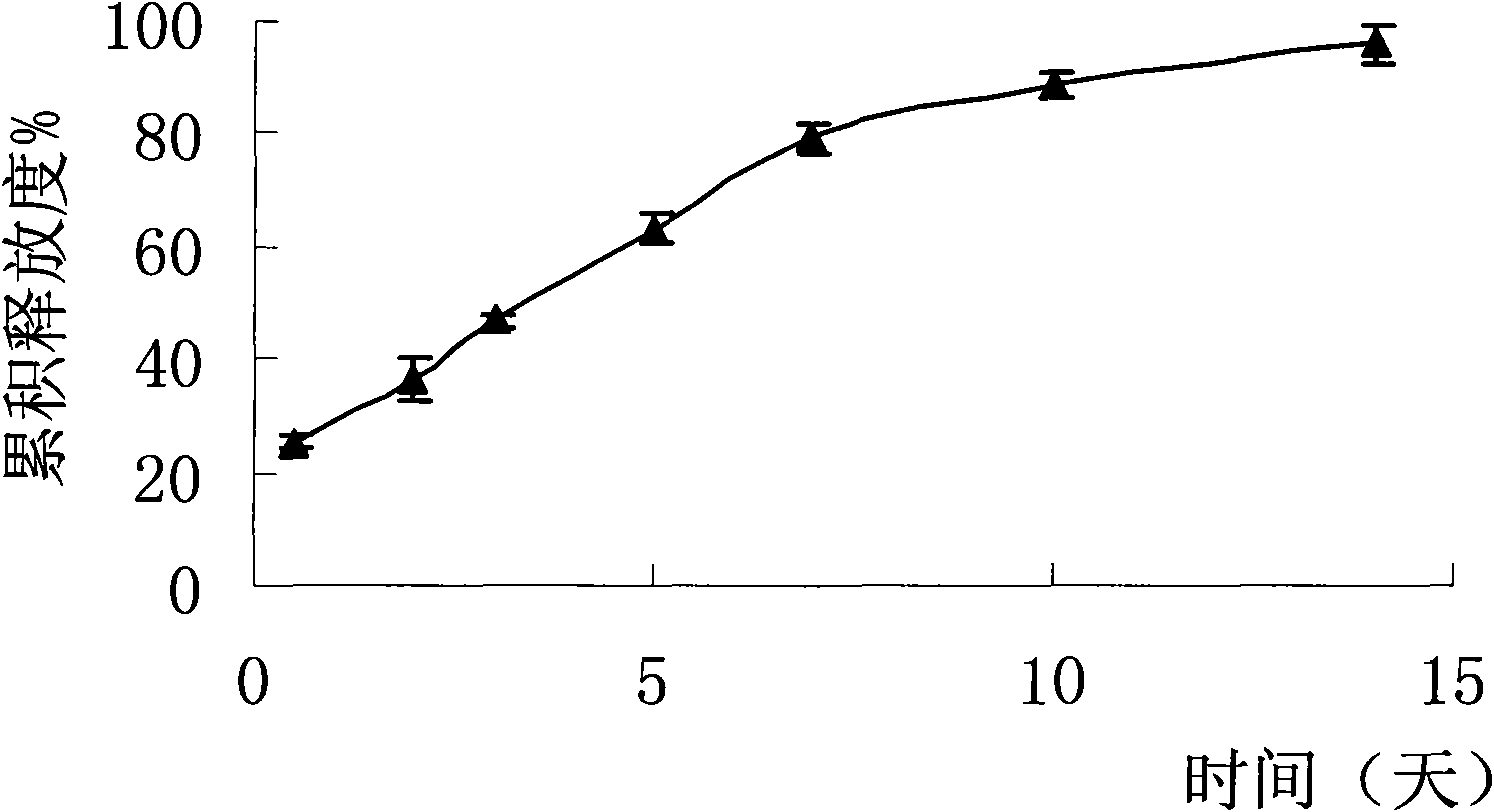
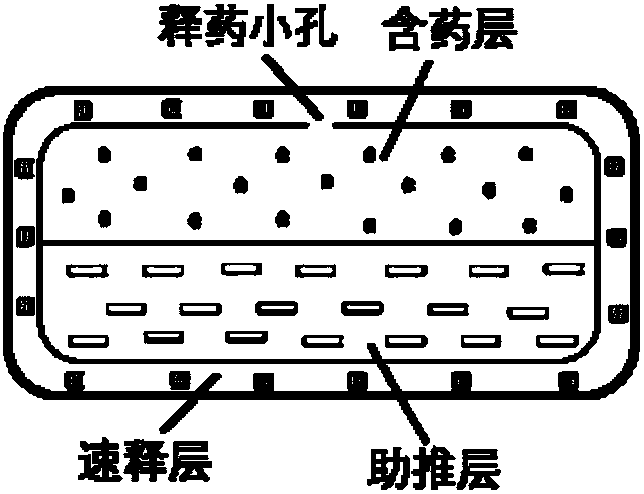
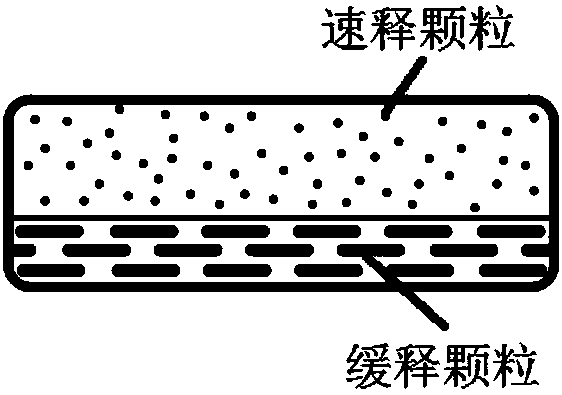
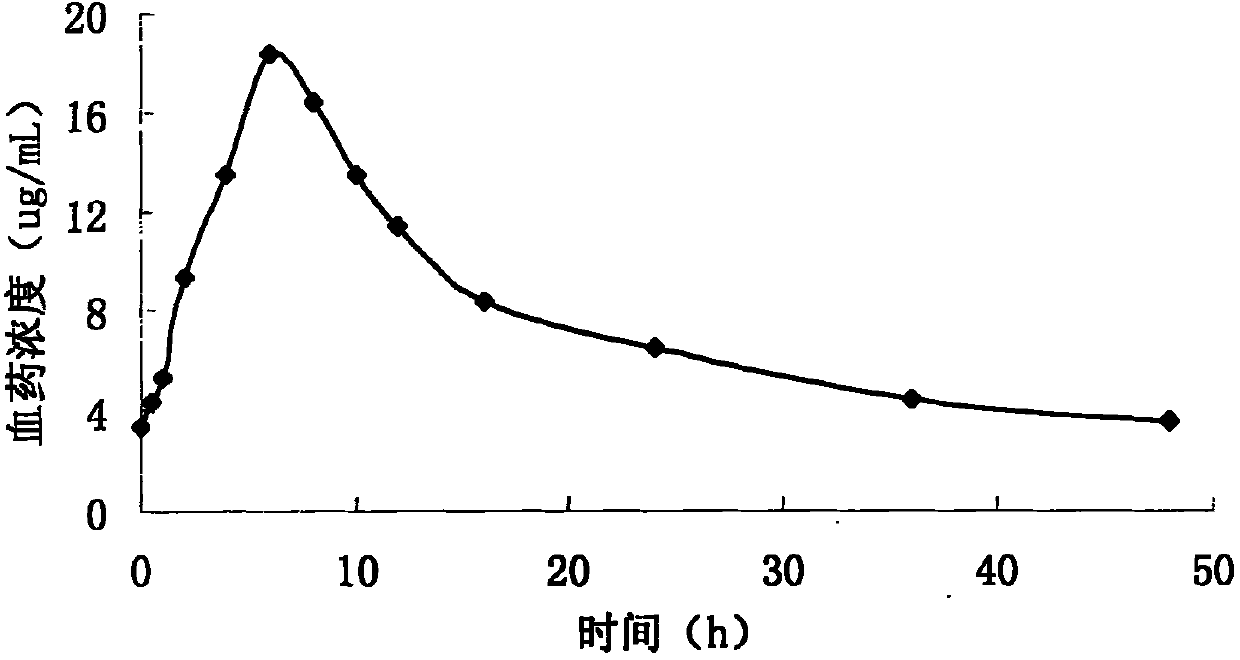
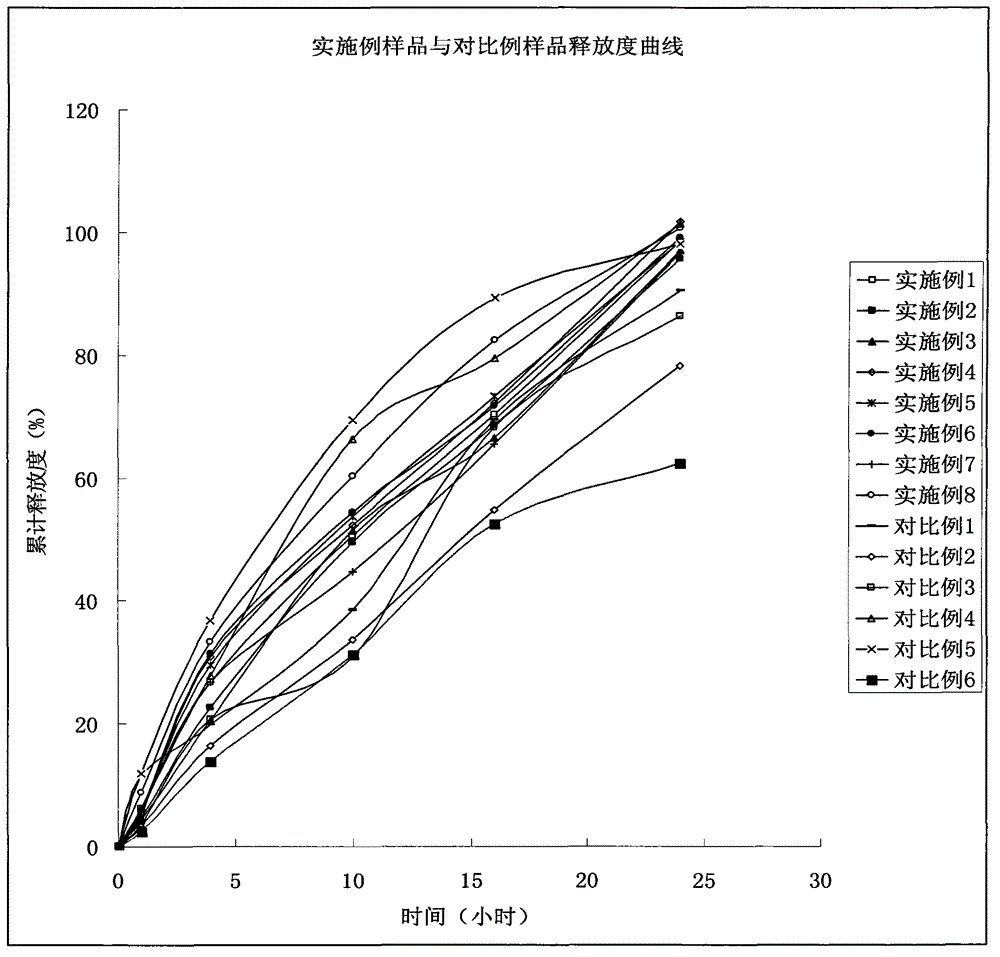
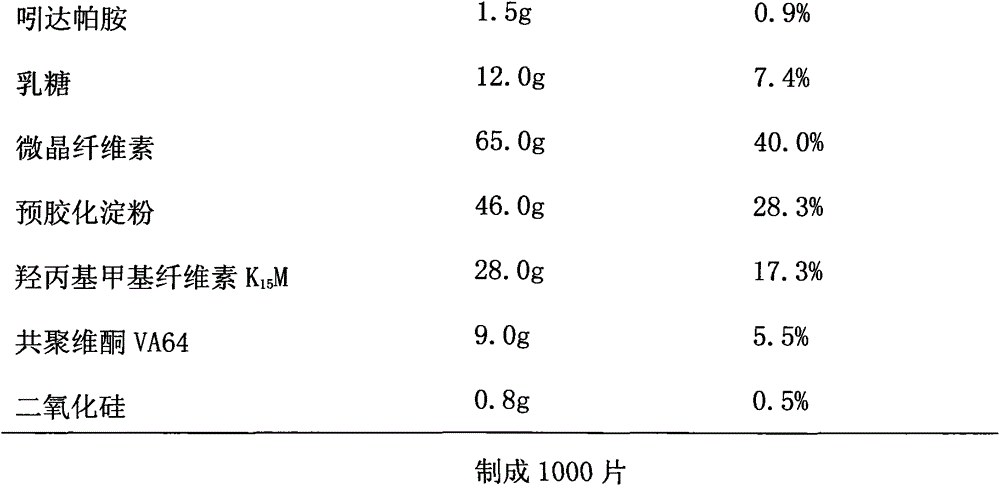
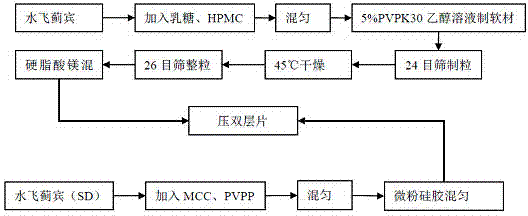
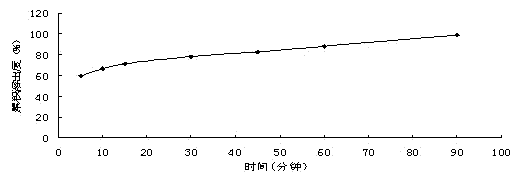
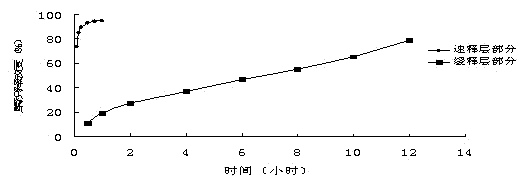
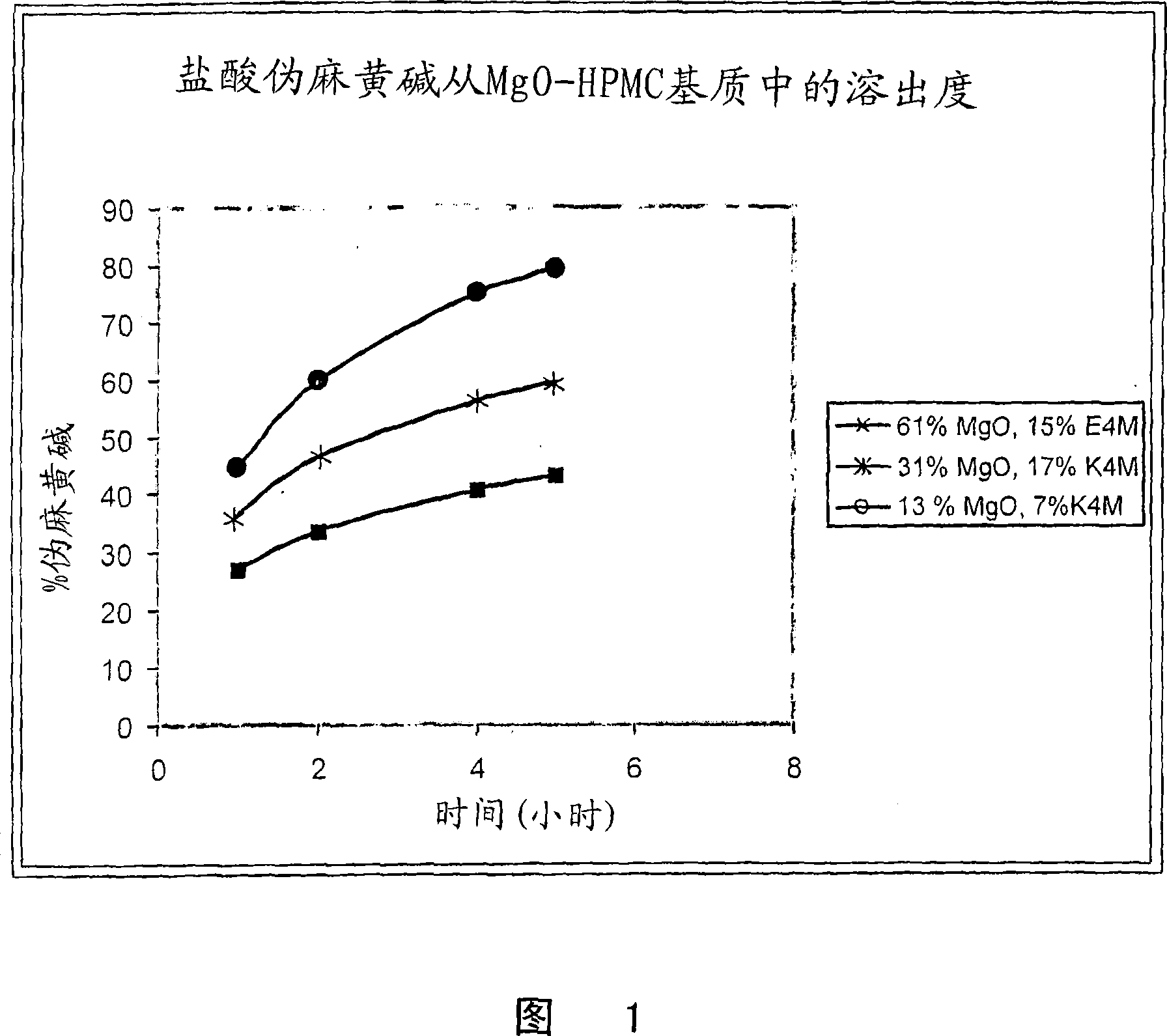
Patents
Literature
147 results about "Blood drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biologically active substance intended for use in diagnosis, treatment, or prevention of blood disorders.
Sustained release preparation of licardipine hydrochloride and its preparing process
InactiveCN101011395AGood slow releaseQuick effectOrganic active ingredientsPharmaceutical product form changeSide effectSustained Release Capsule
The invention relates to a method for preparing Licardipine Hydrochloride slow-release agent, which can be used to treat hypertension, coronary disease or the like. The inventive slow-release agent is formed by quick-release stomach-soluble micro drop and slow-release enteric-soluble micro drop at 1:0.5-5 ratios in the hollow capsule. The inventive capsule has slow-release effect in 12 hours. The slow-release enteric-soluble micro drop comprises Licardipine Hydrochloride, medical macromolecule materials, drug release adjusting agent and some medical finding. The micro drops are prepared by extruding-rolling technique. The invention can quickly approach the blood drug density to treatment object and hold the density stably, with low side effect.
Owner:SOUTHEAST UNIV
Long acting slow releasing drug addiction eliminating prepn and its prepn process and use
ActiveCN1973840ASolve the sudden release problemRelease stabilityOrganic active ingredientsNervous disorderMicrosphereSolvent
The present invention provides the preparation process and usage of slow released naltrexone (NTX) preparation, which is used in the rehabilitation after eliminating opium addiction. The NTX preparation includes NTX as the opium receptor antagonist and matrix of biodegradable polymer material polylactic acid. The preparation process includes emulsification and solvent volatilization to prepare microsphere, pressing into tablet, coating with polylactic acid and other steps. The NTX preparation has great medicine carrying amount and high encapsulating rate, and may reach blood medicine concentration for over 360 days. When it is used, the NTX preparation is injected with special injector into subcutaneous fat for NTX to release slowly and persistently to maintain the effective blood medicine concentration.
Owner:SHENZHEN SCIENCARE MEDICAL INDUSTRIES CO. LTD.
Colchicines gastric floating sustained-release tablet and method for preparing same
InactiveCN101536990ASchematic diagram of the preparation processOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletSide effect
The invention discloses a colchicines gastric floating sustained-release tablet, which comprises active components of colchicines and pharmaceutic adjuvant according to the weight ratio of 1:24-1,999, wherein the pharmaceutic adjuvant comprises a hydrophilic gel framework material, a effervescing agent, a floating assistant material, a filler, a pH value regulator and a lubricant. The colchicines gastric floating sustained-release tablet can swell quickly in gastric juice or a similar gastric juice medium and can float on the gastric juice for at least 4 hours. The invention also relates to a method for preparing the colchicines gastric floating sustained-release tablet. The colchicines gastric floating sustained-release tablet can reach the effective blood-drug concentration quickly after being taken and then release drugs slowly, and can maintain the balanced blood-drug concentration so as to reduce the dose times, relieve the toxic side effect and improve the bioavailability.
Owner:普尔药物科技开发(深圳)有限公司
Transdermal plaster of rivastigmine and preparation process thereof
InactiveCN1994290AEasy to useClear curative effectOrganic non-active ingredientsEster active ingredientsTransdermal patchRivastigmine
The invention relates to a method for preparing kabalatin adhere agent for treating senile dementia, wherein said adhere agent can stably hold blood drug density and reduce feeding time, with high safety.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
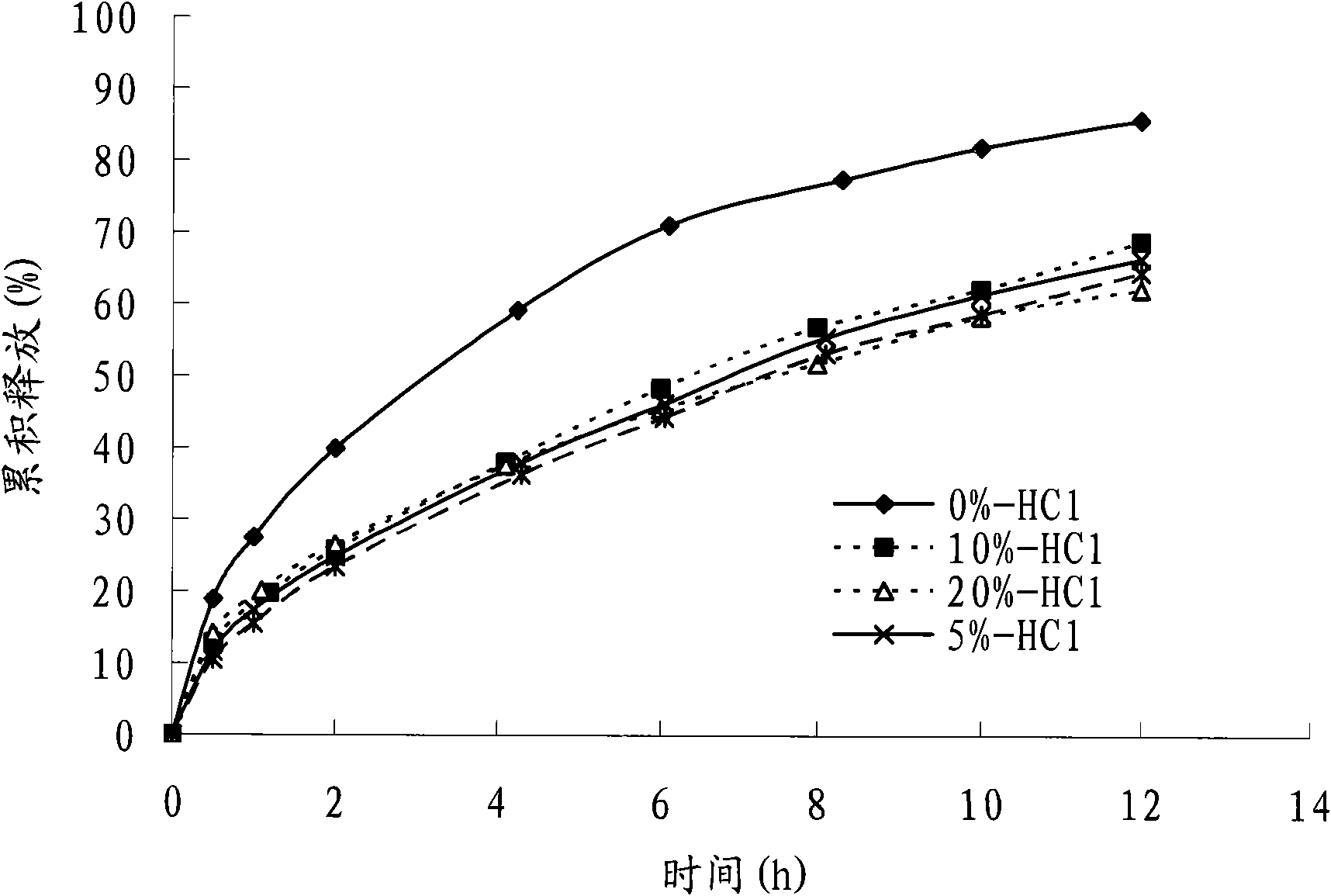
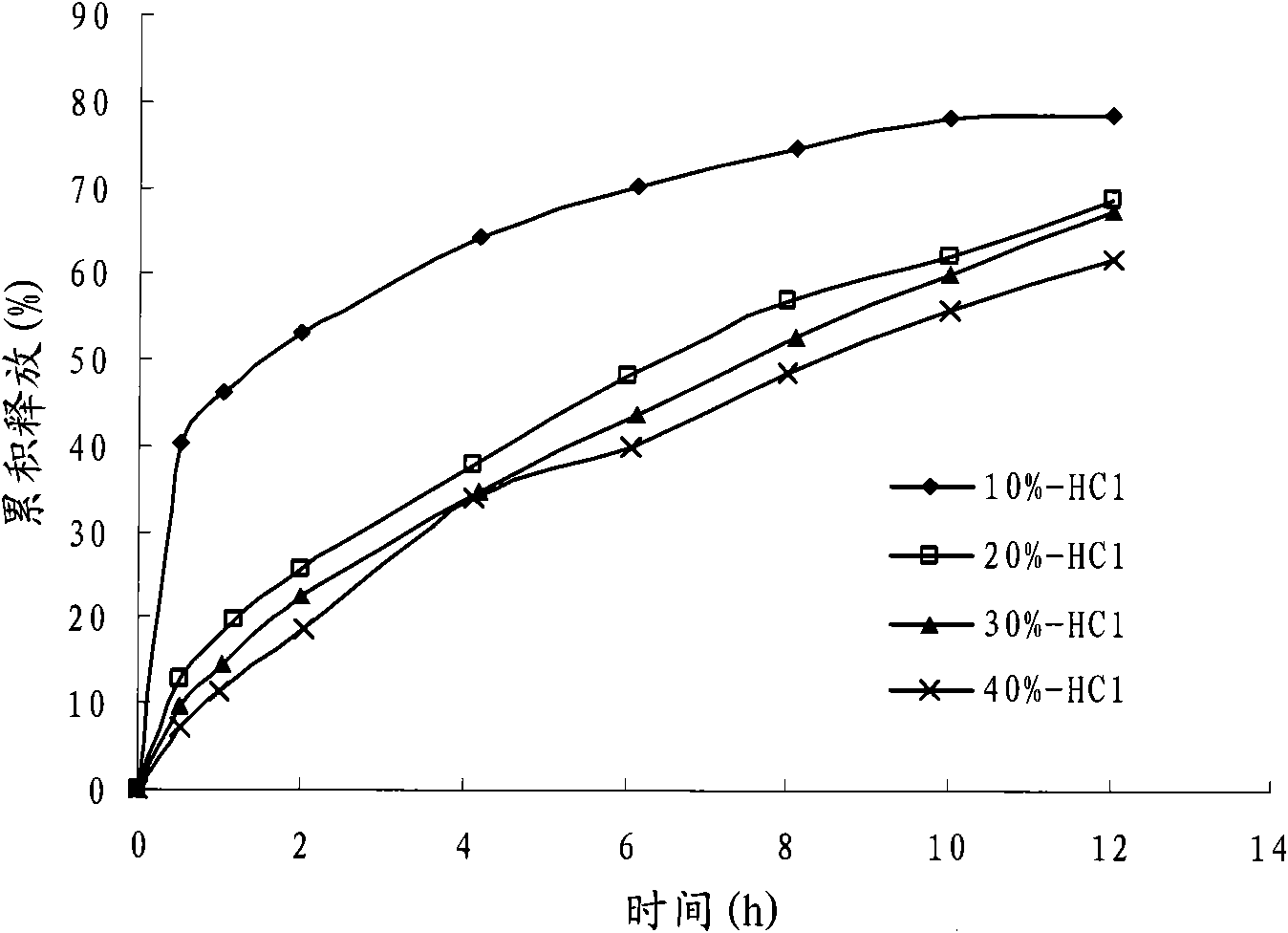
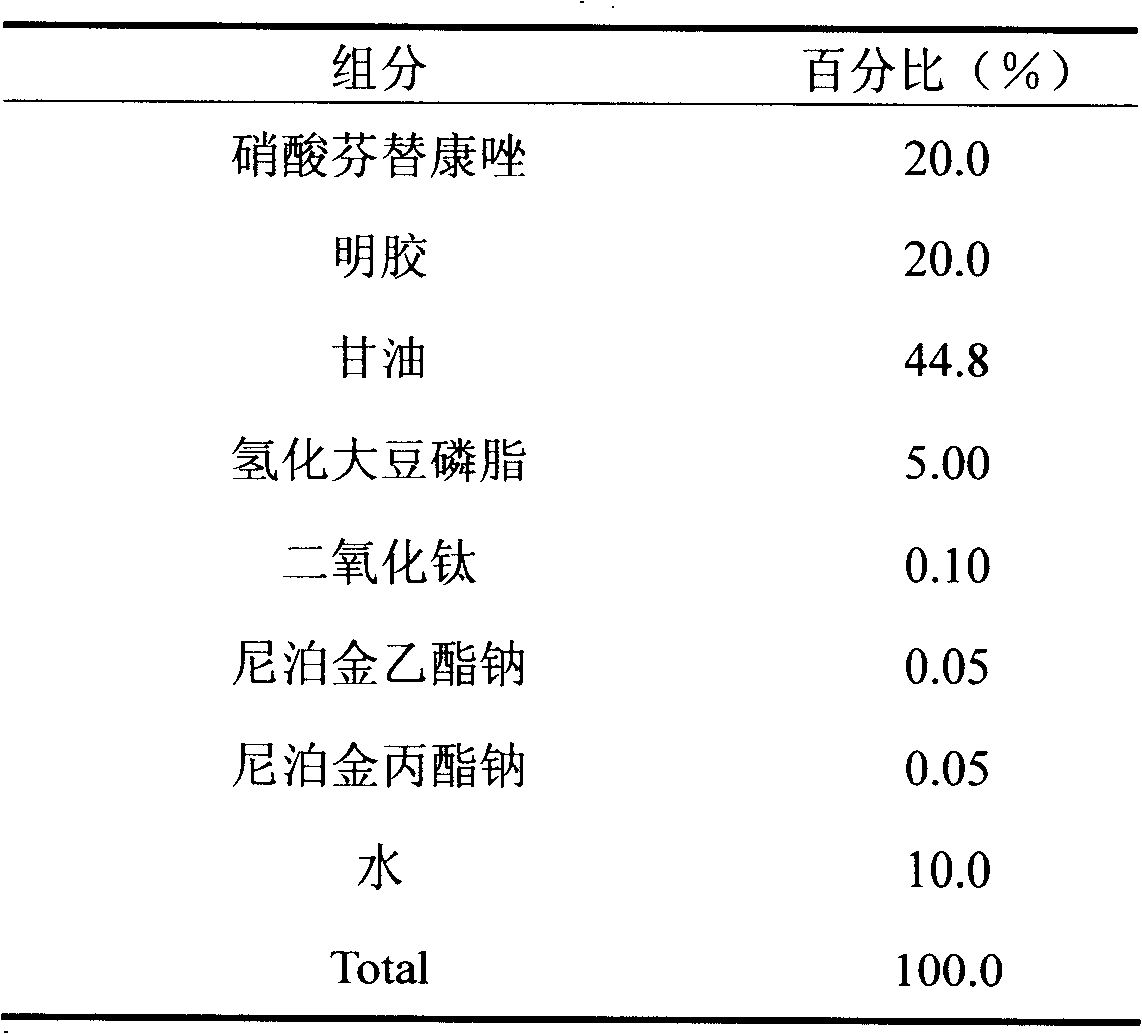
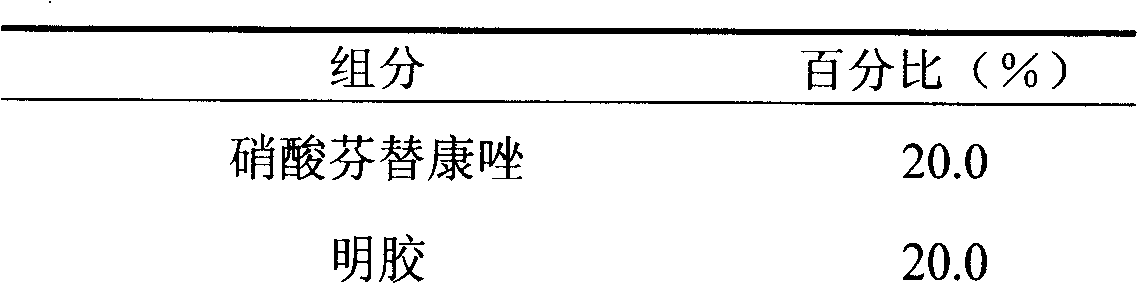
Fenticonazole suppository for treating exterior colpitis
The invention discloses a suppository for treating vulvovaginitis, comprising fenticonazole nitrate, glycerogelatin matrix and additives capable of playing a part in slow release. The suppository can have the effects of inhibiting the rapid rise of the blood drug level and prolonging the acting time.
Owner:BEIJING D VENTUREPHARM TECH DEV
Novel dosage form of sinomenine medicament or hydrochlorate thereof and preparation technique thereof
The invention discloses a sinomenine or an enteric-coated controlled-release tablet of hydrochloride thereof. The prepared enteric-coated controlled-release tablet hardly releases the drug in artificial simulated gastric juice, but can slowly and smoothly release the drug in artificial simulated intestinal juice; the sustained release time of the drug can achieve more than 12 hours or even 24 hours; the enteric-coated controlled-release tablet is taken once or twice daily, the plasma drug concentration in vivo is smooth, and the peak-valley phenomenon of the plasma drug concentration is reduced; as the prepared enteric-coated controlled-release tablet hardly releases the drug in stomach, the contacted concentration of the drug with the gastric mucosa is small, the stimulation of the stomach caused by the drug is alleviated. As the prepared enteric-coated controlled-release tablet sustainedly slowly releases the drug in intestinal tract, the times of the drug administration are reduced, and the patient compliance is improved, thereby being applicable to the needs of the clinical development.
Owner:HUNAN ZHENGQING PHARM GRP CO LTD
Risperidone percutaneous absorption paster
InactiveCN101366705AProlong the action timeLittle side effectsOrganic active ingredientsNervous disorderTectorial membraneSide effect
The invention discloses a risperidone transdermal absorbing patch, which consists of a back lining layer, a protective film and a substrate layer which is arranged between the back lining layer and the protective film and contains medicines. The compositions of the substrate layer containing the medicines by weight percent are 65 to 95 percent of polyacrylate pressure sensitive adhesive, 4 to 15 percent of risperidone and 1 to 20 percent of transdermal enhancer. The patch delivers the risperidone by means of transdermal impregnation, can prolong the acting time of the medicine, maintains stable blood drug level, reduces the side effect of the medicine, is convenient to use, and can be taken as a medicine for treating various mental sickness such as schizophrenia, mania or dementia and so on.
Owner:ZHEJIANG UNIV
Erythrocyte membrane-encapsulated tetrandrine PLGA nanoparticle and preparation method and application thereof
InactiveCN109394733AGood water solubilityReduce releaseOrganic active ingredientsAntipyreticErythrocyte membraneSide effect
The invention belongs to the technical field of pharmacy and particularly relates to an erythrocyte membrane-encapsulated tetrandrine PLGA nanoparticle and a preparation method and application thereof. The nanoparticle structurally includes an erythrocyte membrane, PLGA and tetrandrine, the tetrandrine is combined with PLGA to form a tetrandrine-PLGA nanocore, and the erythrocyte membrane is encapsulated outside the tetrandrine-PLGA nanocore. The preparation method is simple and efficient. The nano preparation improves the biocompatibility of a whole drug delivery system, long circulation andsustained release of the tetrandrine in the human body are achieved, toxic and side effects caused by too high blood medicine peak concentration of common injections are avoided, and the nanoparticlehas a good application prospect.
Owner:SHANGHAI JIAO TONG UNIV
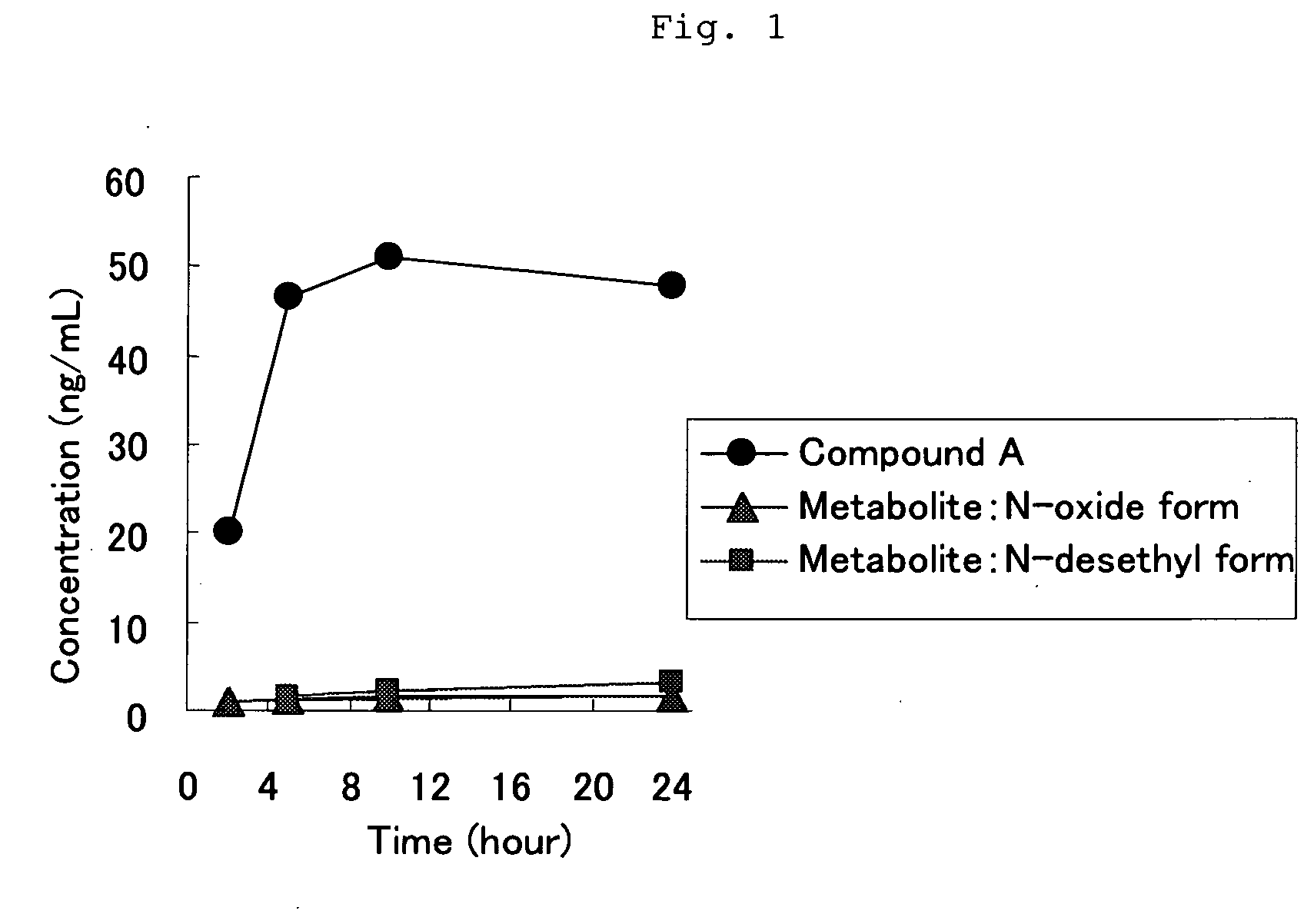
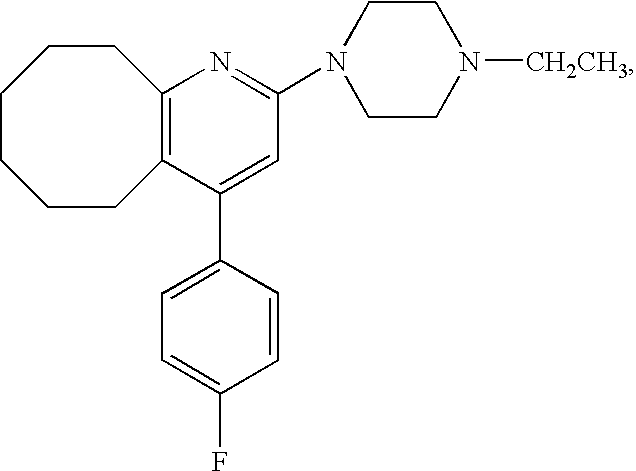
Novel Tape Preparation
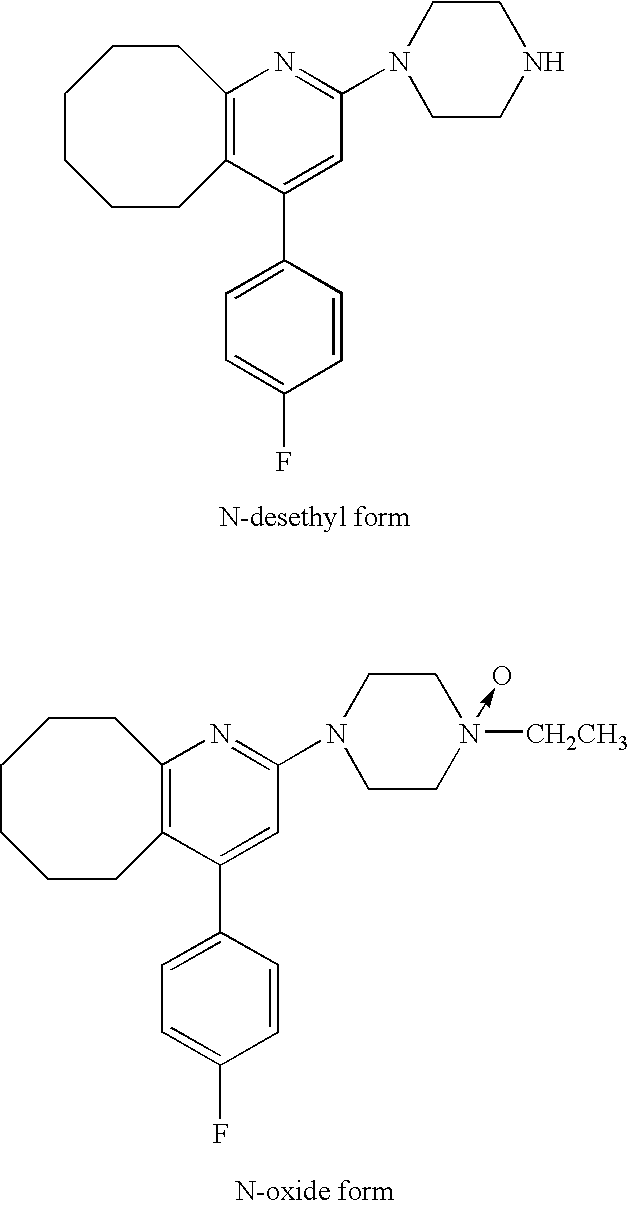
InactiveUS20090169605A1Avoid it happening againGood skin permeabilityOrganic active ingredientsNervous disorderCompound aMetabolite
It is intended to provide a preparation for percutaneous administration of 2-(4-ethyl-1-piperazinyl)-4-(4-fluoro-phenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridine (Compound A), which inhibits the generation of a metabolite and is capable of continuously maintaining a blood drug level. Specifically, a tape preparation comprising an adhesive layer formed on one surface of a support, characterized in that the adhesive layer contains (1) Compound A or a physiologically acceptable acid addition salt thereof, and (2) an acrylic adhesive, or (1) Compound A or a physiologically acceptable acid addition salt thereof, (2) an acrylic adhesive, and (3) a permeation enhancer is provided.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Dexibuprofen slow-release capsule and production method thereof
The invention discloses a dexibuprofen slow-release capsule. Pellets are filled in the capsule, each pellet consists of a pellet core and four layers of materials wrapped outside the pellet core, the four layers of materials are sequentially an inner isolation layer, a first coating layer, a second coating layer and a third coating layer from inside to outside, and the pellet core, the inner isolation layer, the first coating layer, the second coating layer and the third coating layer respectively have the following compositions: the pellet core is prepared by medicine auxiliary materials, the inner isolation layer is stearic acid, the first coating layer is a coating mixture, the second coating layer comprises the coating mixture and the stearic acid, the third coating layer is the coating mixture, and the coating mixture consists of dexibuprofen and polyvidone K30. The invention also provides a production method of the dexibuprofen slow-release capsule. Compared with an ordinary capsule, the dexibuprofen slow-release capsule disclosed by the invention has the same absorption degree, but the dexibuprofen blood maximum concentration (Cmax) of the dexibuprofen slow-release capsule disclosed by the invention in human bodies is lower, the maximum time (Tmax) from the administration to the blood Cmax reaching is longer, and a good slow release effect is realized.
Owner:武汉长联来福制药股份有限公司
Medicinal gelatin microspheres and preparation method thereof
ActiveCN102475684AMeet the needs of delayed release and prolonged efficacyImprove efficacyOrganic active ingredientsPharmaceutical product form changeCross-linkDissolution
The invention relates to medicinal gelatin microspheres and a preparation method thereof, and belongs to the field of medicine preparation. The active ingredient of the medicinal gelatin microspheres is trimetazidine or a medicinal salt of the trimetazidine. The preparation of the microspheres adopts a chemical cross-linking method, and comprises steps of emulsification, deoiling and dewatering, chemical cross-linking, washing and drying, and the like. According to the preparation method, the particle size, the appearance, the encapsulation efficiency, the drug loading rate are adopted as indexes to comprehensively optimize the carrier, the oil phase, the dewatering and deoiling agent, the cross-linking curing agent, and other reagents added in the process, wherein the carrier, the oil phase, the dewatering and deoiling agent, the cross-linking curing agent, and other reagents are suitable for preparation of the microspheres of the present invention. The prepared microspheres have characteristics of smooth surface, particle size of 50-200 mum, drug loading rate of 30-35%, and has a slow release effect under the common dissolution conditions. In addition, the blood drug level of the microspheres is stable in vivo, and is better than the blood drug level of ordinary tablets.
Owner:CHANGZHOU SIMM DRUG RES & DEV CENT
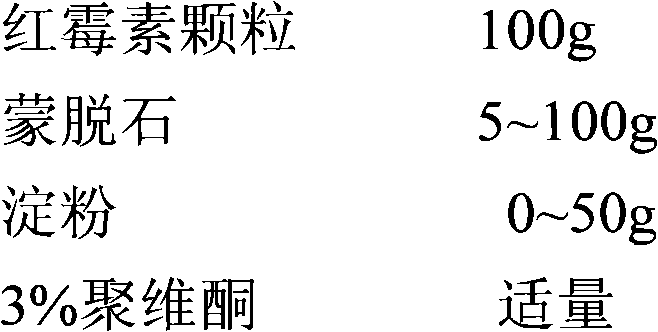
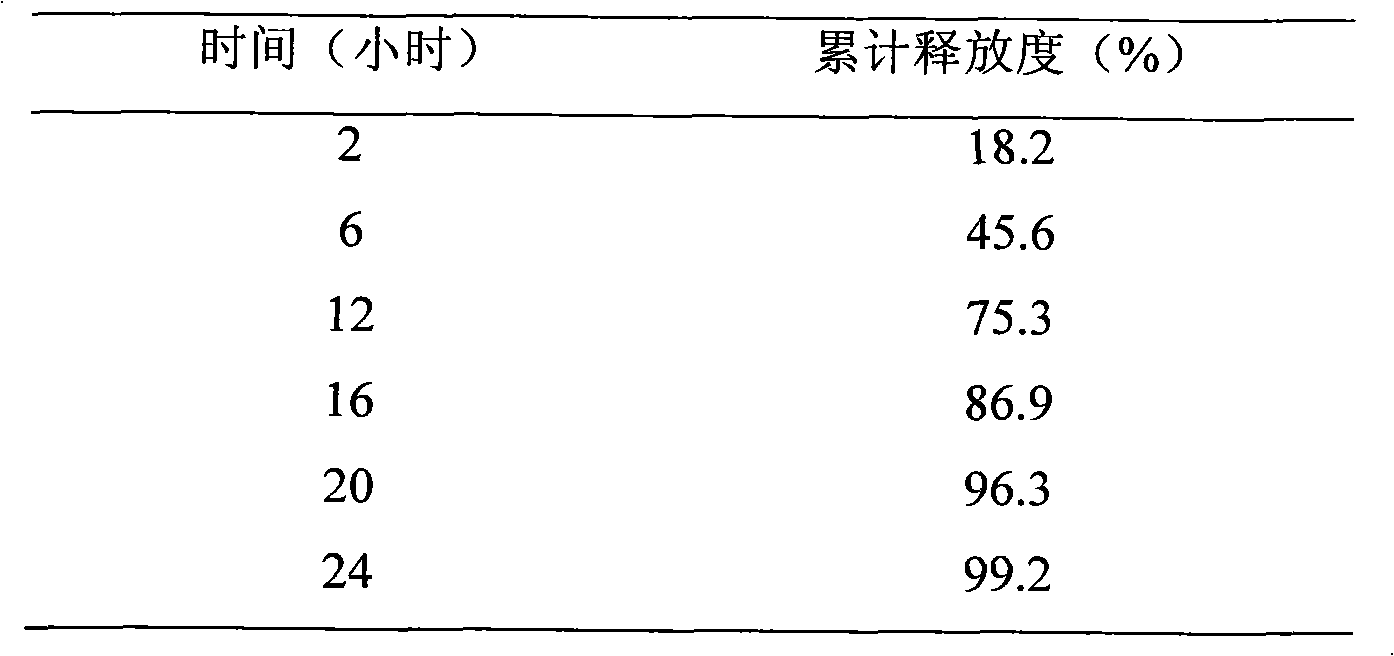
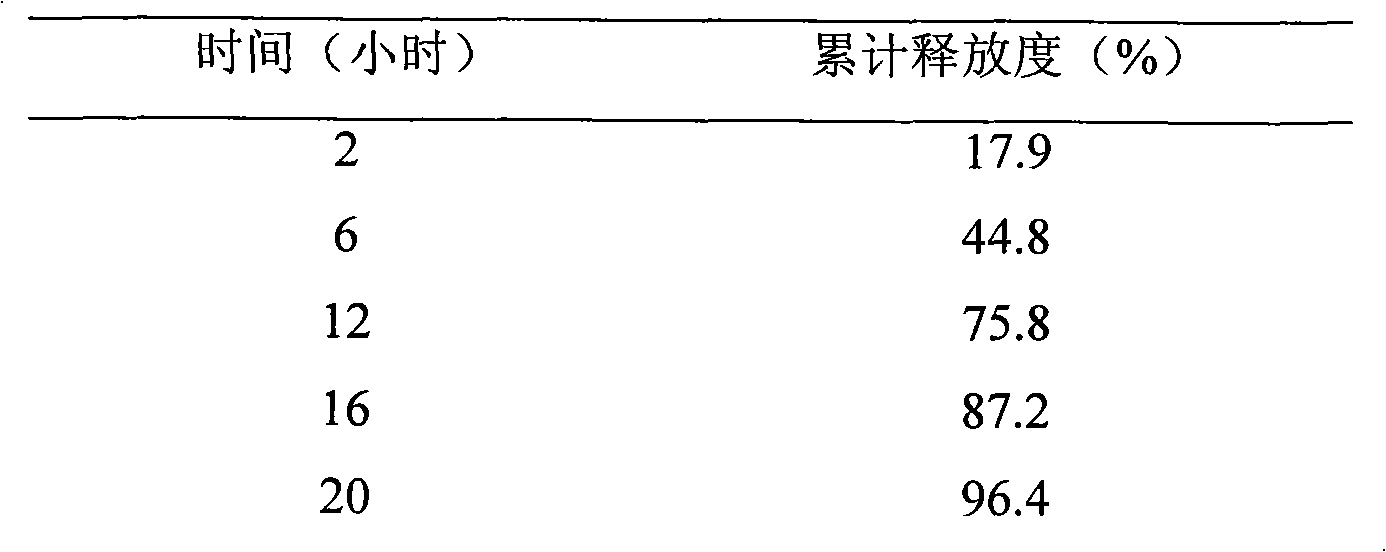
New preparation of erythrocin and relevant drug thereof and preparation method of new preparation
The invention relates to a preparation method of new preparation of erythrocin, which is characterized in that an endothelin core of erythrocin is prepared, and then an isolating layer, a protective layer, a second isolating layer and an improved enteric-coating material layer are applied one by one. In this way, new preparation of the erythrocin which has certain feature of releasing (dissolving) in acid solution (hydrochloric acid solution 9 to 1000) can be formed. The technology of the new preparation can also be widely applied to drugs which, like erythrocin, when being taken orally by a patient, cause the patient to suffer the side effects of stimulation, sickness and the like after degradation in the stomach of the patient or contact with the stomach of the patient, and drugs which the patient needs to take orally to let the blood concentration to reach the peak value in a short time. Such drugs include macrolides of azithromycin, metronidazole of nitroimidazoles, tinidazole, acyclovir as an antiviral drug, ammonium chloride as a phlegm eliminating drug, bromhexine, chloroquine as an antimalarial, nitroquine, artemisinin, dihydroartemisinin, artesunate, primaquine, pyrimethamine, carbarsone and emetine amebicides and so on.
Owner:胡昌勤 +1
Cordyceps sinensis polysaccharide liposome medicament and preparation thereof
InactiveCN101401789APromote absorptionOrganic active ingredientsMetabolism disorderCholesterolAdditive ingredient
The invention relates to liposome of extract of cordyceps and a method for preparing the same. The main composition of the extract is polysaccharide. The liposome of the cordyceps is prepared by using phospholipid and cholesterol as membrane materials, and the prepared liposome of the cordyceps can be further prepared into dosage forms of tablet, injection, oral agent, capsule, granule, freeze-dried powder and the like. The method for preparing the liposome of the cordyceps can adopt a reverse evaporation method, a film ultrasound method and an ethanol injection method, the encapsulation rate of the methods can reach more than 40 percent, and the in vitro burst rate is low. The preparing process improves the stability of the drug. After the polysaccharide of the cordyceps is coated with the liposome, the polysaccharide of the cordyceps can be absorbed and used better in human body; and through the slow release effect of the liposome, the effective blood drug level of the drug can be maintained for a longer time.
Owner:JILIN UNIV
Rosuvastatin calcium sustained-release preparation and preparation method thereof
InactiveCN101889975AOvercome the defect of "peak valley" fluctuation in blood drug concentrationImprove securityOrganic active ingredientsMetabolism disorderRosuvastatin CalciumBlood drug
The invention relates to a rosuvastatin calcium sustained-release preparation and a preparation method thereof. The rosuvastatin calcium sustained-release preparation basically contains 5 to 10mg of rosuvastatin calcium, and the balance of sustained-release framework material and other pharmaceutical excipients. The preparation method is simple; and all the materials are proportioned and the preparation is prepared by the preparation method for common tablets, granules or capsules. The rosuvastatin calcium sustained-release preparation prepared by the method avoids adverse reactions such as rhabdomyolysis, proteinuria, nephrosis, kidney failure, hepatotoxicity and the like caused by overdosage of medicaments; meanwhile, due blood concentration and time for treating diseases after the medicaments are taken can be maintained, and the peak valley phenomenon of the blood concentration is effectively avoided.
Owner:BEIJING HONGWAN PHARMA TECH
Production method of enteric-coated kitasamycin for feed
ActiveCN101611766AProtect weak alkaline antibioticsProlong the action timeAnimal feeding stuffAccessory food factorsAcrylic resinMicroparticle
The invention discloses a production method of enteric-coated kitasamycin for feed, which comprises the following process steps: step one, the preparation of drug-loaded pellets; step two, inner layer sustained-release coating of the drug-loaded pellets, which prolongs the release and acting time of kitasamycin; and steps three, outer layer enteric coating of the drug-loaded pellets, which ensures the release in succus entericus and small or no release in gastric juice. The method has the advantages that the kitasamycin is subjected to pellets pelletizing and 99 percent of the prepared granulums can pass through 24 meshes, so the dust is greatly reduced and the fluidity is increased; the coating of the inner layer sustained-release agent (HPMC) prolongs the release and acting time of the kitasamycin, reduces medication times and reduces the medication cost; and a layer of enteric substance, namely acrylic resin-III is sprayed and coated on the outer layer of particles. The substance protects the kitasamycin which is a weakly alkaline antibiotic from being damaged by gastroc acid in stomach, quickly disintegrates after entering enteric canal and releases the kitasamycin; and then the kitasamycin is absorbed by gastrointestinal mucosa into blood drug to play a role of restraining the reproduction of pathogenic microorganism and preventing diarrhea. Insoluble in the stomach, kitasamycin coating formulations have no pessimal stimulation on the stomach, and cannot result in regurgitation and vomiting. In addition, the sustained-release formulation, namely the kitasamycin prolongs the acting time so the medication times is reduced, the medication cost of farmers is reduced, and the economic benefit is improved.
Owner:WUXI ZHENGDA POULTRY
Monosialotetrahexosyl ganglioside sodium for injection and preparation method thereof
ActiveCN102641281AImprove complianceImprove the quality of lifeOrganic active ingredientsNervous disorderLife qualityBlood concentration
The invention belongs to the technical field of medicines and specifically relates to monosialotetrahexosyl ganglioside sodium for injection and a preparation method thereof. The monosialotetrahexosyl ganglioside sodium for injection provided in the invention contains GM1, gelatin and PLGA (Polylacticcoglycollic Acid). A microsphere injection prepared from the monosialotetrahexosyl ganglioside sodium for injection provided by the invention is good in reproducibility and encapsulation ratio; the drug-loading rate is improved; the drug release is continuous and stable; the dosage at a time is obviously reduced and the foreign body sensation is reduced; effective blood concentration can be maintained for a long time; the shortcoming of administration every day is avoided; the life quality of a patient is improved; and the problem of the fluctuation of the blood concentration caused by the conventional preparations is solved.
Owner:SHANDONG NEWTIME PHARMA
Method for synchronously measuring blood drug concentrations of multiple antidepressants
InactiveCN102175778AAccurate measurementSensitive and reliableComponent separationMedicineMass spectrometry detector
The invention discloses a method for synchronously measuring blood drug concentrations of multiple antidepressants, wherein the method comprises the steps of: pre-processing a sample to be measured, analyzing the chromatographic column separation, and performing the detection by a mass detector. The method has the advantages that the samples are a few, the pre-processing procedure is simple, the analysis period is short, the detection sensitivity is high, the blood drug concentrations of a plurality of antidepressants can be detected synchronously, the application is wide, the cost is low, and the method is suitable for monitoring conventional blood drug concentrations.
Owner:BEIJING GUGE MEDICINE DEV CO LTD
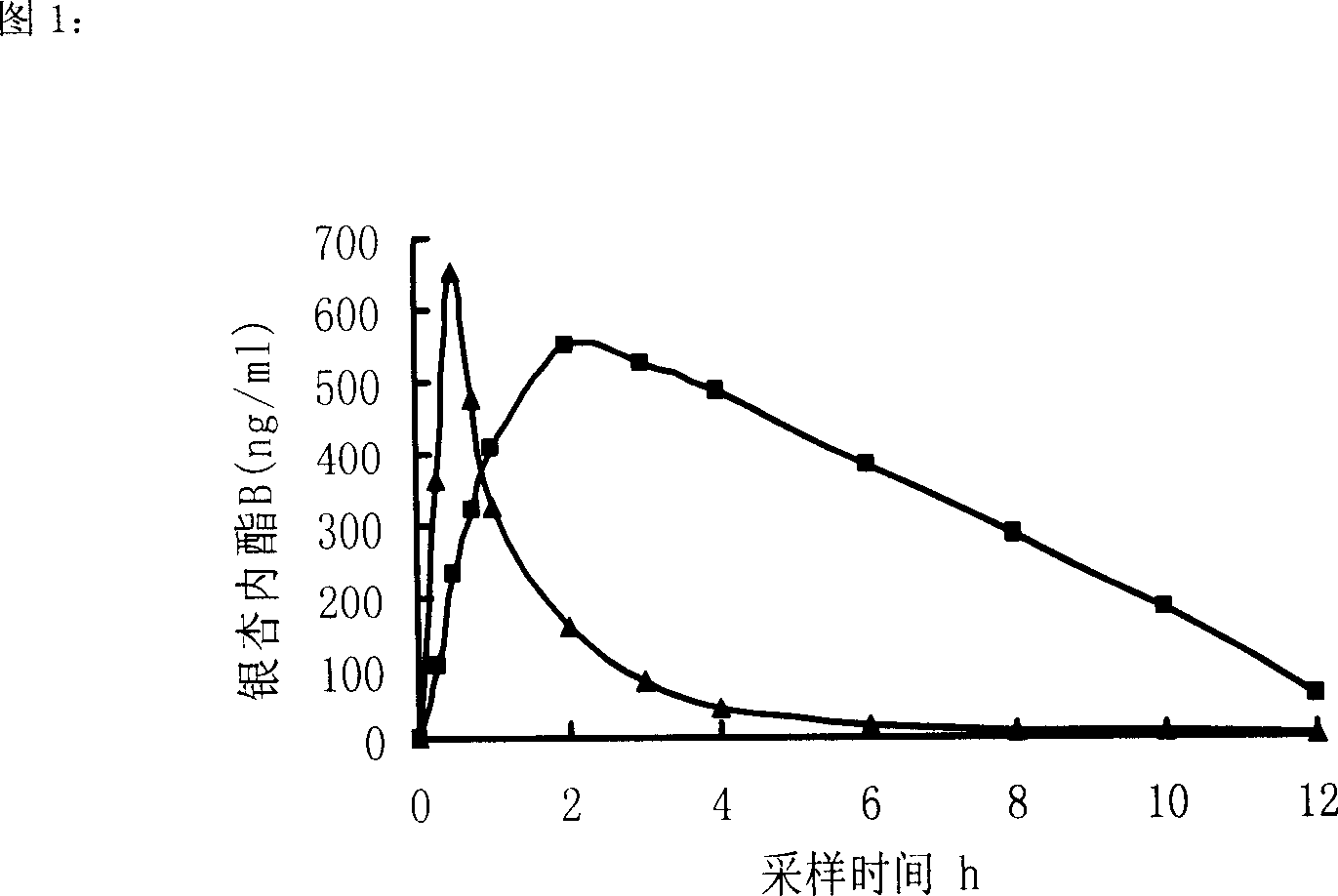
Sustained release formulation of bilobalide-B and preparation process thereof
The invention relates to a bilobalide B slow-release agent and relative production, wherein it is formed by bilobalide B, slow-release material, and other findings. The invention can stabilize blood drug density, improve treatment effect, and reduce side effect. It is portable.
Owner:王爱民
Compound control-released percutaneous medicine plaster for treating high blood pressure and its preparation method
InactiveCN1633994AImprove treatment efficiencyImprove securityPharmaceutical active ingredientsSheet deliveryBlood drugTreatment hypertension
The invention relates to a compound control-released percutaneous medicine plaster for treating high blood pressure and its preparation method, wherein the paste comprises laminated back lining layer, a pressure-sensitive adhesive framework form medicament-containing layer and a protective film, wherein the compound medicament comprises diuretics, central alpha-acceptor excitant and cirumferential alpha-receptor blocking agent, beta-receptor blocking agent, calcium antagonist and any two of the five categories of medicament for influencing angiotensin II formation and treating high blood pressure. The invention may realize medicine administration once in front of chest or behind the ears, wherein homeostasis blood concentration can be reached.
Owner:王睿 +3
Olaparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof
InactiveCN108201536AAchieve absorptionAccurate blood drug concentration in vivoOrganic active ingredientsPill deliveryControl releaseEnzyme inhibition
The present invention relates to an olaparib oral controlled-release and sustained-release pharmaceutical composition, which contains dissolution form improved olaparib and a matrix polymer for release rate adjustment. According to the present invention, the in vivo absorption behavior, the blood drug level and the PARP enzyme inhibition level of the pharmaceutical composition can be controlled, the pharmaceutical composition has the improved olaparib loading and / or oral absorption and / or bioavailability and / or blood drug concentration control and / or enzyme inhibition level control, and can beused as the sole preparation or can be combined with other treatment methods in the treatment of cancer.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Method for determining human plasma antiviral drug concentration
InactiveCN101105478ASimple and fast operationEasy to operateComponent separationFluorescence/phosphorescenceAntiviral drugMedicine
The invention belongs to medical detection field, relates to an analysis detection method of drug in the body of a person, and specifically relates to the method that the densities of antiviral drugs in blood plasma of the person such as acyclovir, ganciclovir and penciclovir can be detected at the same time. The method in the invention is characterized in that pilot sample is pretreated; as acyclovir, ganciclovir and penciclovir have the character of strong fluorescence absorption, acyclovir, ganciclovir and penciclovir can be separated from each other in an acidity flowing phase chromatographic column and be detected by a fluorescence detector. The method in the invention has the advantages of little sample, simple, swift and sensitive pretreatment, short analysis period and low cost; furthermore, the invention doesn't need expensive equipment and reagent and is suitable for the detection of clinical conventional blood drug density of acyclovir, ganciclovir and penciclovir.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Phellinus linteus polysaccharide oral liposome medicine and preparation technology thereof
ActiveCN102631319AIncrease blood concentrationExtended half-lifePowder deliveryOrganic active ingredientsMedicineLiposome
The present invention relates to a Phellinus linteus polysaccharide oral liposome medicine and a preparation technology thereof. By encapsulating the Phellinus linteus polysaccharide by a liposome, after the Phellinus linteus polysaccharide is orally taken and enters the digestive system, the encapsulated Phellinus linteus polysaccharide is prevented from being destroyed by gastric juice, bile and the like, and effectively delivered to the absorption site, therefore, the blood drug level of the Phellinus linteus polysaccharide is increased, and the half-life period of the Phellinus linteus polysaccharide is prolonged. Compared with the Phellinus linteus polysaccharide which is not encapsulated by the liposome, the Phellinus linteus polysaccharide encapsulated by the liposome has the advantages that the blood drug level is increased and the half-life period is obviously prolonged.
Owner:山东省循证医学研究院
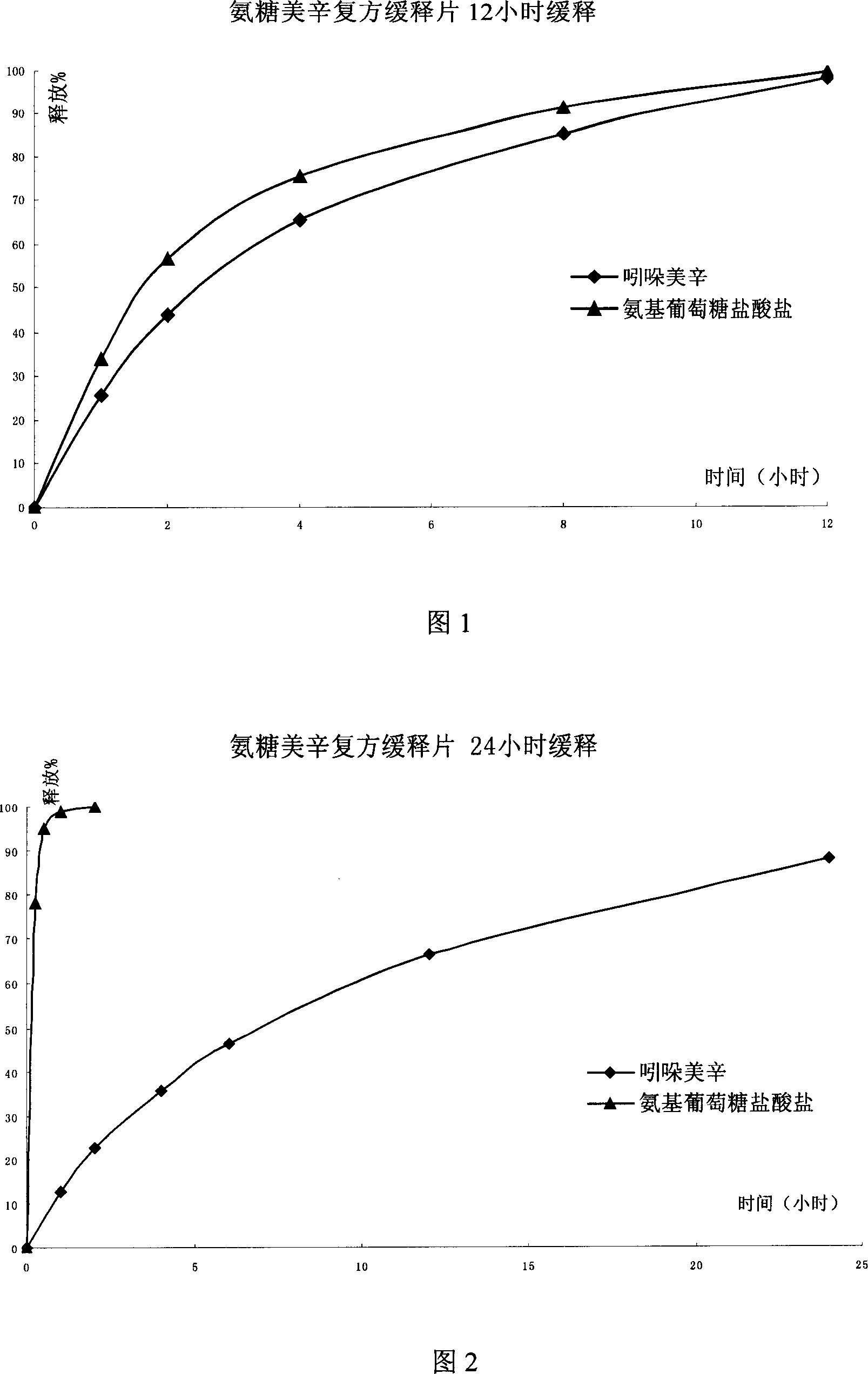
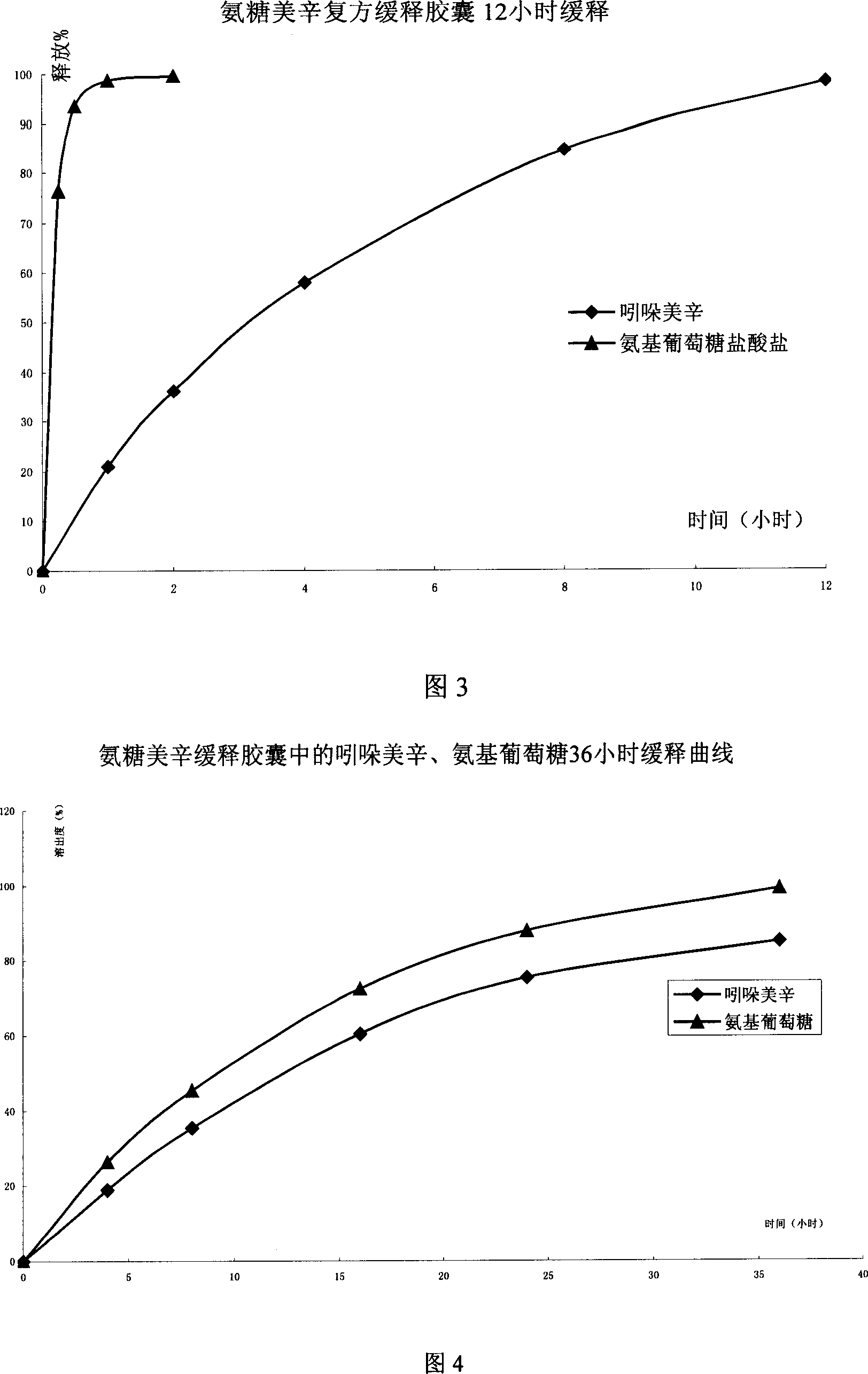
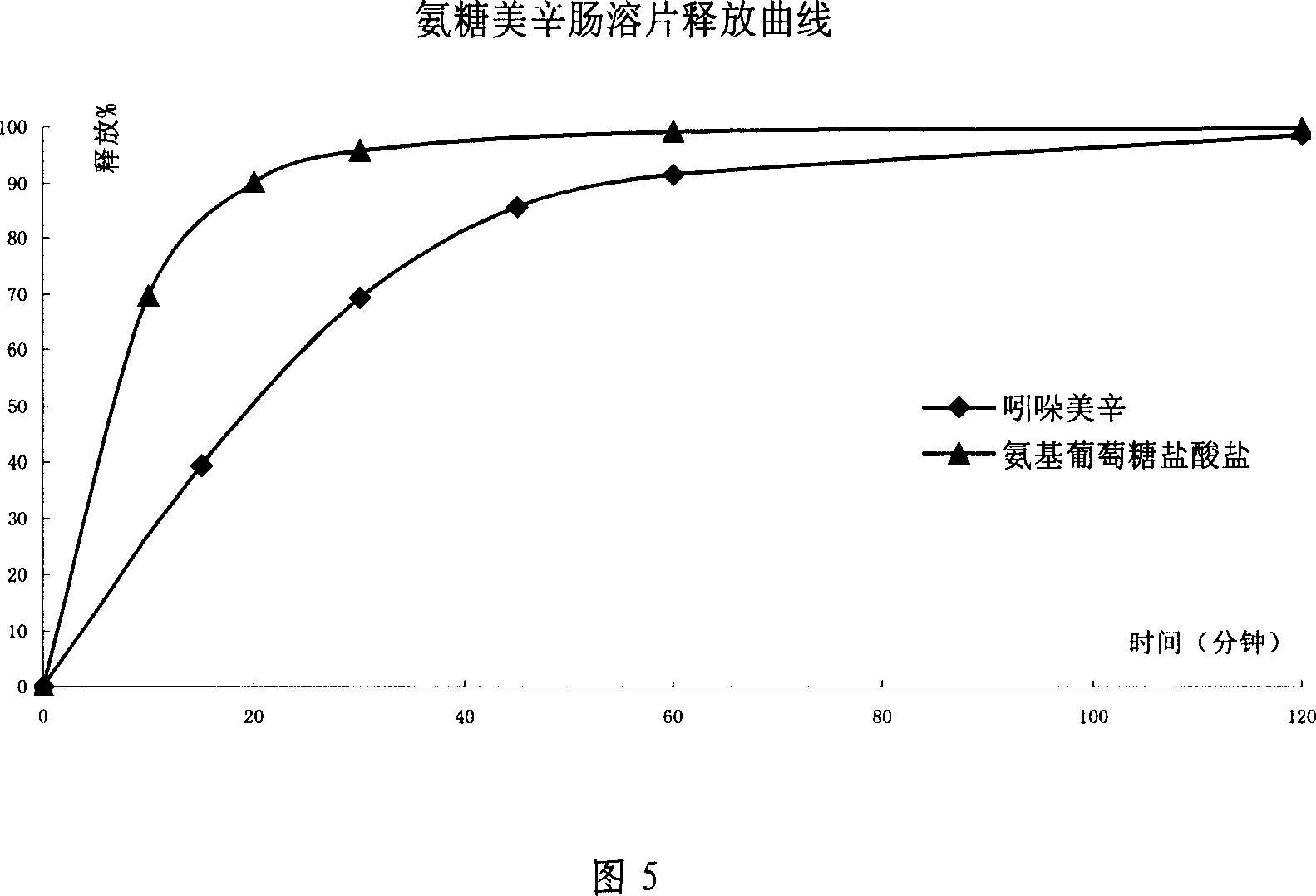
Sustained releasing formulation of compound glucosamine, preparation process and application thereof
InactiveCN1961887ASafe releaseSmooth releaseOrganic active ingredientsAntipyreticSide effectVolumetric Mass Density
The invention relates to a composite slow-release agent, wherein it contains indomethacin, aminoglucose salt, slow-release material and other findings, while their ratios are 5-45%, 20-80%, 1-75%, and the left is findings. Its preparation comprises that mixing indomethacin and / or aminoglucose salt, and slow-release material and / or packs, then adding other findings, using general slow-release technique to obtain one or several skeleton or mould-contoro slow-release agent. The invention can be made into particle, oral, tablet or capsule. The invention can keep stable blood drug density in 8-36h, reduce side effect.
Owner:ZHEJIANG HAILISHENG PHARM CO LTD
Aceclofenac in extended-released tablets and method of manufacturing the same
ActiveCN101108170AReduce stimulationSmall fluctuations in blood concentrationOrganic active ingredientsAntipyreticSustained Release TabletAdditive ingredient
The invention relates to a drug sustained-release preparation and its preparation method, in particular to an aceclofenac sustained-release tablet and its preparation method, which comprises the following ingredients according to the weight percentage: aceclofenac of 60 to 95 per cent, skelecton retarder of 3 to 30 per cent, adhesive of 1 to 10 per cent and lubricant of 0.5 to 15 per cent. The hydroxypropylmethyl cellulose and carboxyvinyl polymer are adopted as optimized skelecton retarder. With such a technical proposal in the invention, the one aceclofenac sustained-release tablet can be taken a day to effectively reduce the fluctuation of blood drug level, prolong the maintenance duration of effective blood drug level and lower down the incitement to gastrointestinal tract. Besides, the invention also provides the preparation method of the aceclofenac sustained-release tablet.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
Medication reminding method and device and compute readable storage medium
ActiveCN109718112AAvoid false positivesMedical data miningHealth-index calculationDiseaseEmergency medicine
The invention discloses a medication reminding method, device and system and a compute readable storage medium, and belongs to the field of computers. The method comprises the following steps after apatient takes a target drug, obtaining physiological index parameters of the patient, wherein the physiological index parameters are used for representation of the disease treated with the target drug; when the physiological index parameters exceed a preset parameter scope, obtaining the medication time, wherein the medication time is a time period between the time when the patient takes the target drug and the current time; according to the medication time period, determining blood drug level of patient at the current time; and when the blood drug level is lower than a preset level threshold,prompting the patient to take the target drug. The medication remaining method and device can prevent medication reminding from operating by mistake.
Owner:BOE TECH GRP CO LTD
Pyridostigmine bromide sustained-release tablet and preparation method thereof
InactiveCN102258496AWell mixedOrganic active ingredientsDigestive systemSustained Release TabletSide effect
The invention belongs to the technical field of medicinal preparations, and discloses a formula of pyridostigmine bromide sustained-release tablets taken twice every day and used for treating myasthenia gravis, postoperative functional intestinal tympanites and uroschesis and a preparation process. The preparation is in a form of sustained-release tablets consisting of skeleton tablet cores and coatings, wherein the skeleton tablet core consists of pyridostigmine bromide, sustained-release agent and the like. The pyridostigmine bromide sustained-release tablets can overcome the defects of current marketed common medicinal tablets, realize sustained release to ensure relatively stable blood concentration and longer acting time, have the advantages of low toxic or side effects and convenience for administration, and can keep effective blood concentration for relatively long time, reduce the administration times, improve the compliance of patients and reduce the side effect caused by over high peak concentration. The preparation process of the pyridostigmine bromide sustained-release tablets is simple, low in cost, easy to control and easy for industrialized production.
Owner:CHONGQING MEDICAL UNIVERSITY
Indapamide sustained-release drug composite and preparation method thereof
InactiveCN103142529AAvoid sudden releaseRelease stabilityOrganic active ingredientsPharmaceutical delivery mechanismDrug release rateAdhesive
The invention discloses an indapamide sustained-release drug composite and a preparation method thereof. The indapamide sustained-release drug composite comprises the following ingredients: indapamide serving as an active ingredient and a proper amount of filler, framework material, lubricant and copovidone (VA64). The indapamide sustained-release tablet disclosed by the invention is used for treating primary hypertension and is characterized in that the VA64 is added in the prescription so that the burst release of the drug is prevented, the stable release of the drug is guaranteed and the hypokalemia caused by overhigh blood concentration is avoided; in the process, the indapamide is micronized to below 50 micrometers so that the release of the drug is improved and the delayed release of the drug is avoided; and the direct powder compression process is adopted so that the problems that the framework material coheres to form sticky balls due to adhesive and the homogeneity of drug releasing rate is influenced are avoided. The prepared indapamide sustained-release tablet has the advantages that the drug releasing rate is stable; the homogeneity of the releasing rate is good; the drug bioavailability is increased; and the drug quality is guaranteed.
Owner:KANGYA OF NINGXIA PHARMA
Silibinin double-layer slow-release tablets and preparation method thereof
InactiveCN102846573AImprove solubilityImprove bioavailability in vivoOrganic active ingredientsDigestive systemProlonged-release tabletImmediate release
The present invention discloses silibinin double-layer slow-release tablets, which comprise an immediate release layer and a slow-release layer, wherein the immediate release layer mainly comprise a silibinin solid dispersion, a filler, a disintegrating agent and a lubricant, the slow-release layer mainly comprises silibinin, a slow-release material, a filler, a lubricant and a binder, a weight ratio of the silibinin content in the immediate release layer to the silibinin content in the slow-release layer is 2:8-3:7, and a weight ratio of the immediate release layer to the slow-release layer is (1.5-2.4):1. A purpose of the present invention is to provide the silibinin double-layer slow-release tablets with characteristics of good patient compliance, low side effect, rapid effect, and lasting maintenance of stable effective blood drug level. The present invention further discloses a preparation method for the silibinin double-layer slow-release tablets. The method of the present invention has characteristics of good reproducibility and high production efficiency, and is suitable for industrial mass production. In addition, the prepared double-layer slow-release tablets have good release uniformity.
Owner:SICHUAN ACAD OF CHINESE MEDICINE SCI
Preparation method for degradable microcapsules with disulfide bonds
InactiveCN105214070AAltered metabolic kineticsReduce the peak-to-valley concentration differencePeptide/protein ingredientsDigestive systemCross-linkStable state
The invention discloses a preparation method for degradable microcapsules with disulfide bonds. According to the preparation method, disulfide bond covalent cross-linked (FITC-PAH / DPA)5 microcapsules are prepared by taking MnCO3 micro-particles as templates, taking 3,3'-dithiobispropionate as a cross-linking agent, and adopting a layer-by-layer assembly method; the prepared microcapsules can be kept in a stable state in an external environment, and can also release a drug directionally in cells, so that the drug ingredients of the microcapsules are protected to prolong the residence time of the drug ingredients in the cells; the characteristics of pharmacokinetics are changed; the peak-valley concentration difference of blood drug in vivo is reduced; adverse reactions are reduced; the preparation method is simple; the raw materials are chip; the preparation method is applicable to batch production.
Owner:ZHEJIANG SCI-TECH UNIV
Multi-layer tablet comprising non-steroidal anti-inflammatory drugs, decongestants and non-sedating antihist amines
The present invention is directed to a pharmaceutical composition and method for treatment of rhinitis and cold like symptoms, the composition includes a non-steroidal anti-inflammatory drug (NSAID), a decongestant and an antihistamine preferably in a multi-layer tablet comprising an immediate release layer and an extended release layer to optimize the delivery profile and match the duration of biological action of the multiple active agents.
Owner:WYETH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com