Silibinin double-layer slow-release tablets and preparation method thereof
A technology of silibinin and sustained-release tablets, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, pill delivery, etc., can solve the problems of low drug content, achieve good reproducibility, reduce the number of times of taking medicine, Effect of Uniform Effective Plasma Concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
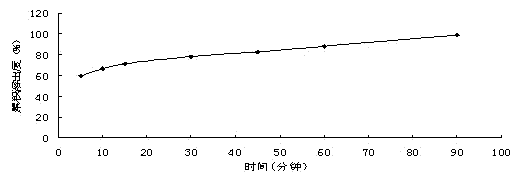
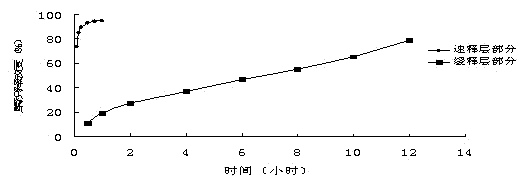
Examples
Embodiment 2
[0048]
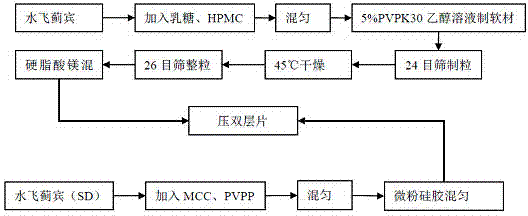
[0049] Preparation process: Mix silibinin solid dispersion, MCC, PVPP, and L-HPC through an 80-mesh sieve, add 6g of micropowder silica gel and mix evenly to obtain immediate-release layer granules; Part of the raw material of Binbin is replaced by the same amount of lactose, and 20ml of 5% PVPk30 ethanol solution is added to make a soft material, passed through a 26-mesh sieve to granulate, dried at 45°C, granulated with a 24-mesh sieve, and added with 1% magnesium stearate Mix evenly to obtain blank slow-release layer granules. The sustained-release granules and immediate-release granules are punched into a double-layer sustained-release tablet with an 8 mm shallow concave.
Embodiment 3
[0051]
[0052] Preparation process: Mix the quick-release part of the excipients evenly by sieving, replace the SLB solid dispersion with an equivalent amount of MCC, and obtain the quick-release layer granules; mix the silybin raw material drug, HPMCk4M, and lactose evenly by grinding, add 20ml of 5% PVPk30 ethanol solution is used to make soft materials, passed through a 26-mesh sieve to granulate, dried at 45°C, sized with a 24-mesh sieve, added 1% magnesium stearate and mixed evenly to obtain sustained-release layer granules. The sustained-release granules and immediate-release granules are punched into a double-layer sustained-release tablet with an 8 mm shallow concave.
Embodiment 4
[0054]
[0055] Preparation process: First pass the main and all auxiliary materials through a 80-mesh sieve. Mix the silibinin solid dispersion in the quick-release layer with other excipients in the quick-release layer evenly by sieving, then add micropowdered silica gel and mix well to obtain the quick-release granules; take the silybin in the slow-release layer Mix thisbinin with HPMCk4M and lactose evenly by grinding, make soft material with 10ml of 5% PVPk30 ethanol solution, granulate through a 26-mesh sieve, dry at 45°C, granulate with a 24-mesh sieve, add magnesium stearate and mix evenly. Sustained-release granules are obtained. The sustained-release granules and immediate-release granules are punched into a double-layer sustained-release tablet with an 8 mm shallow concave.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com