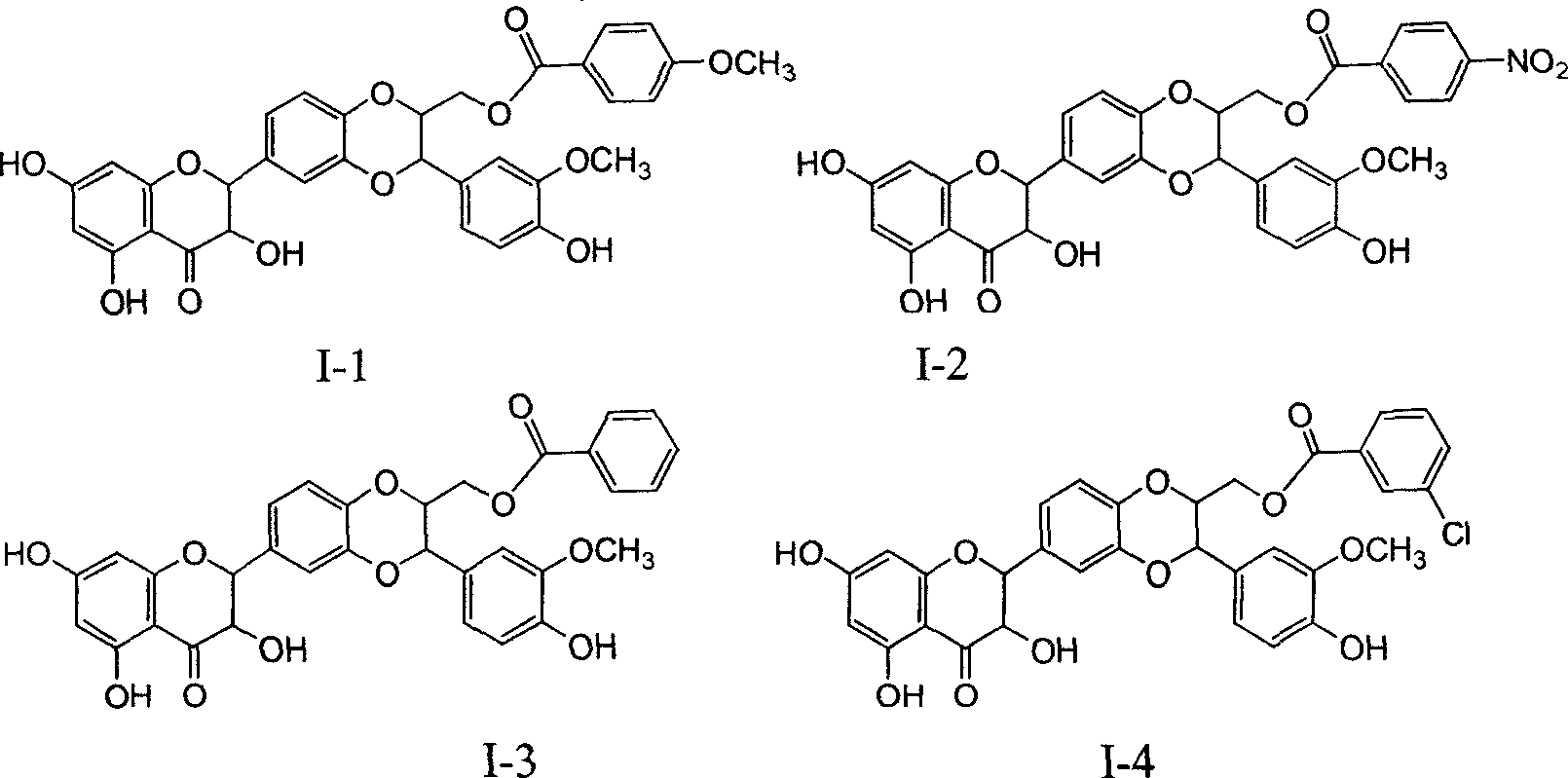
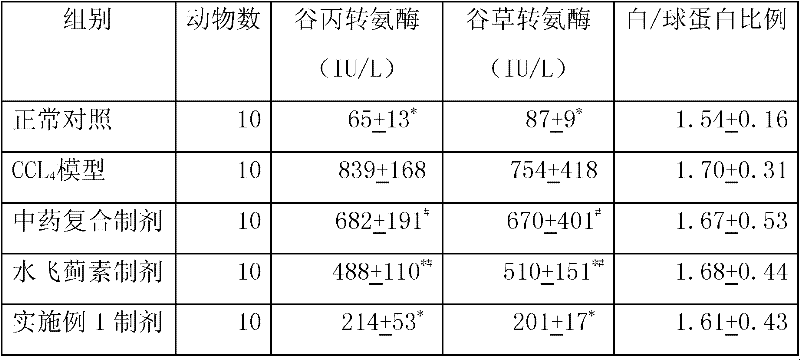
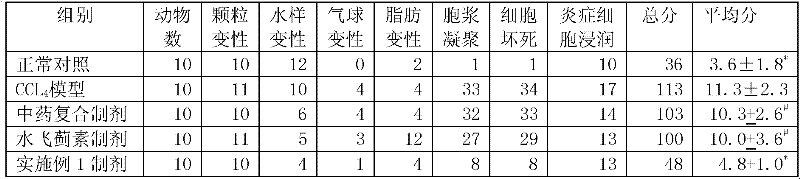
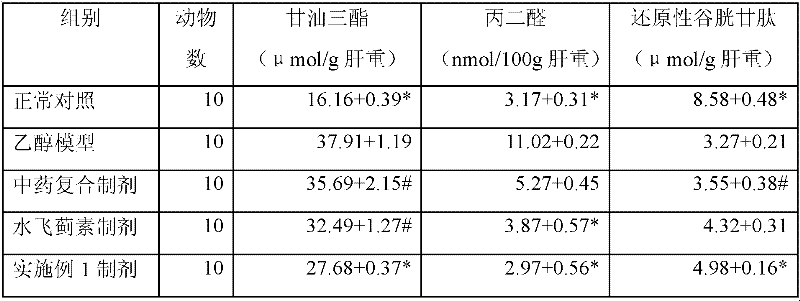
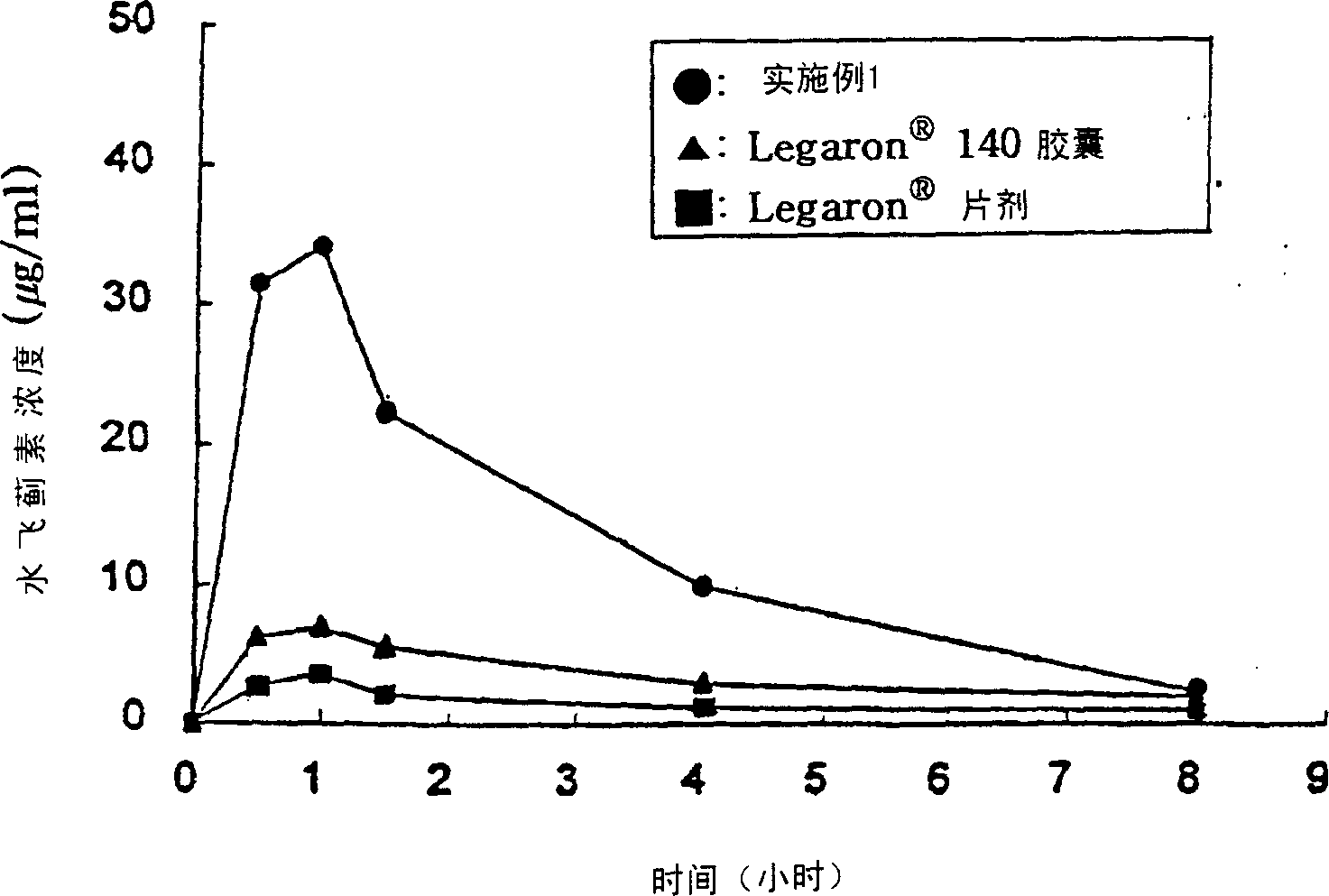
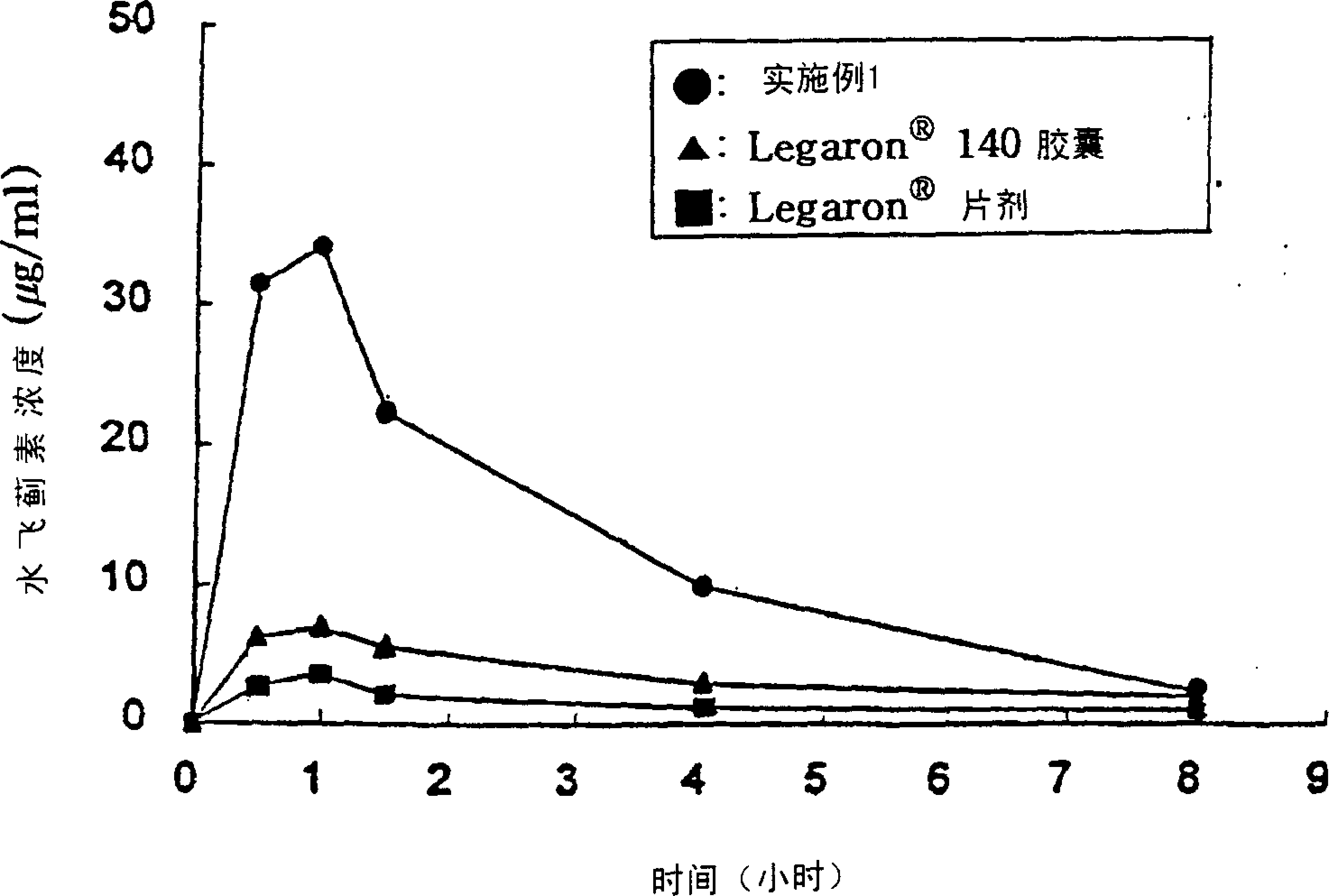
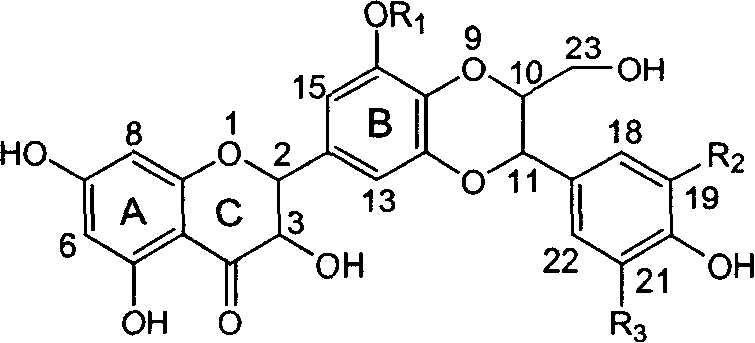
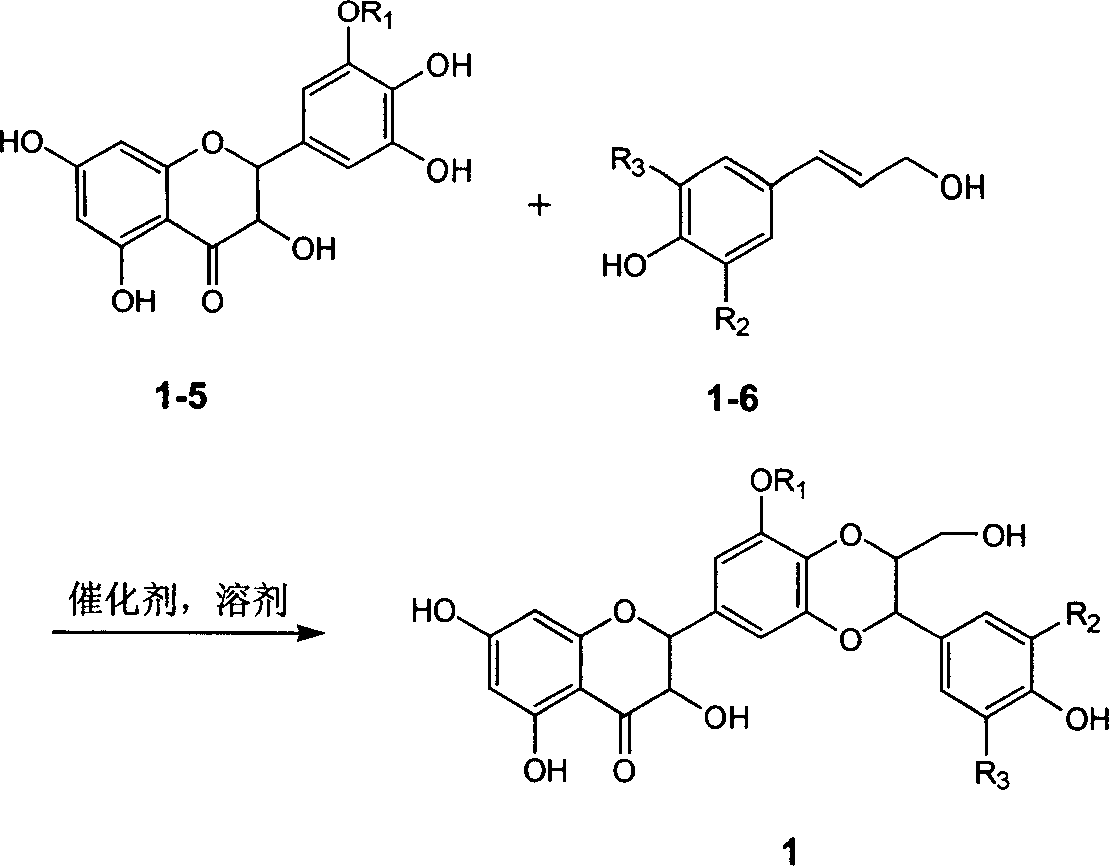
Patents
Literature
335 results about "Silibinin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
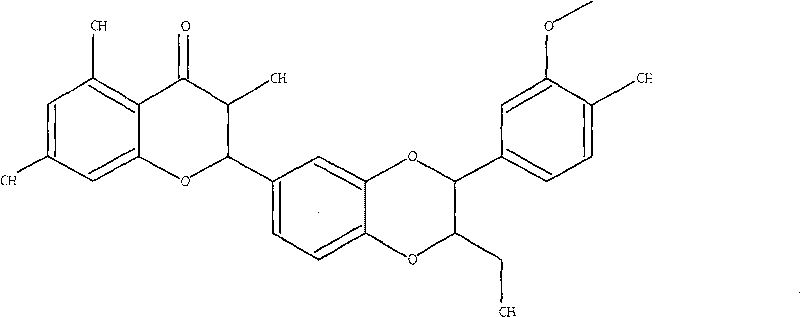
Silibinin (INN), also known as silybin (both from Silybum, the generic name of the plant from which it is extracted), is the major active constituent of silymarin, a standardized extract of the milk thistle seeds, containing a mixture of flavonolignans consisting of silibinin, isosilibinin, silicristin, silidianin, and others. Silibinin itself is a mixture of two diastereomers, silybin A and silybin B, in approximately equimolar ratio. The mixture exhibits a number of pharmacological effects, particularly in the liver, and there is some clinical evidence for the use of silibinin as a supportive element in alcoholic and child grade 'A' liver cirrhosis.
Compositions and methods useful for treating and preventing chronic liver disease, chronic HCV infection and non-alcoholic steatohepatitis
InactiveUS20050123628A1Reduce oxidative stressReduce lipid peroxidationBiocideDipeptide ingredientsChronic viral hepatitis CLipid peroxidation
The invention relates generally to compositions comprising antioxidants useful for reducing oxidative stress and lipid peroxidation, and treating chronic liver disease, chronic hepatitis C virus infection and non-alcoholic steatohepatitis. In particular, the invention relates to the preparation and oral administration of compositions comprising glycyrrhizin, schisandra, ascorbic acid, L-glutathione, silymarin, lipoic acid, and d-alpha-tocopherol. The invention also relates to the preparation and parenteral administration of compositions comprising glycyrrhizin, ascorbic acid, L-glutathione, and vitamin B-complex, preferably by infusion or intravenous injection. The invention further relates to methods of using the antioxidants, oral compositions and parenteral compositions.
Owner:ZABRECKY GEORGE
Compositions for Regulation of Hair Growth
InactiveUS20080254055A1Reduce frequencyImprove shaving efficiencyBiocideCosmetic preparationsPersonal carePhytic acid
Personal care composition comprising at least one hair growth regulating compound selected from the group consisting of glyceryl dilaurate, apigenin, tetrahydrocurcumin, oleanolic acid, azelaic acid, sulforaphane, canavanine, pyridoxal 5-phosphate, phytic acid, tannic acid, grape seed extract, NG-nitro-L-arginine-methyl ester, benzamidine, sodium butyrate, betulinic acid, polyornithine, polyarginine, fisetin, jasmonates, methyl-jasmonate, cis-jasmone, caffeic acid phenethyl ester, delphinidin, ethyl abietate, esculetin, sorbic acid methyl ester, canaline, N-formyl-methionine, N-formyl-alanine, taurine, palmitoyl carnitine, undecanol, undecylenic acid, rutin, fusidic acid, phenyl pyruvic acid, L-isoleucine, phenyl glycine, silibinin, silymarin, L-ascorbic acid-6-palmitate, N-undecylenoyl-L-phenylalanine, and salts, derivatives and mixtures of any of the foregoing; and a dermatologically-acceptable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
Compositions and methods useful for treating and preventing chronic liver disease, chronic HCV infection and non-alcoholic steatohepatitis
InactiveUS7078064B2Reduce peroxidationReduce stressBiocideDipeptide ingredientsChronic viral hepatitis CLipid peroxidation
The invention relates generally to compositions comprising antioxidants useful for reducing oxidative stress and lipid peroxidation, and treating chronic liver disease, chronic hepatitis C virus infection and non-alcoholic steatohepatitis. In particular, the invention relates to the preparation and oral administration of compositions comprising glycyrrhizin, schisandra, ascorbic acid, L-glutathione, silymarin, lipoic acid, and d-alpha-tocopherol. The invention also relates to the preparation and parenteral administration of compositions comprising glycyrrhizin, ascorbic acid, L-glutathione, and vitamin B-complex, preferably by infusion or intravenous injection. The invention further relates to methods of using the antioxidants, oral compositions and parenteral compositions.
Owner:ZABRECKY GEORGE
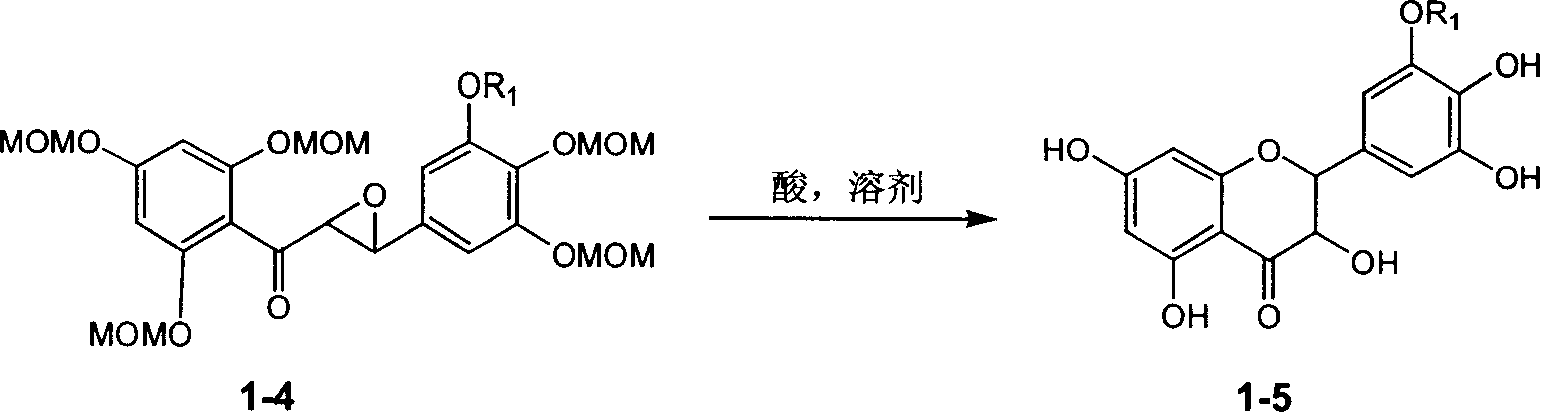
Silybin esters derivatives and preparation and use thereof
The invention relates to a silibinin monoester derivative and its medicine salt or solvates. The invention also relates to its preparation method and its drug combination and medical application. The compound can inhibit activity of hepatitis b virus, so it is expected to be used as drug to treat hepatitis b virus and relevant virus diseases. The compound can protect liver and is expected to be used as drug preventing liver damage. The compound possesses effect anti free radical and is expected to be used as drug treating diseases caused by free radical.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Composition with auxiliary protection effect on chemical hepatic injury and alcoholic hepatic injury and health care food thereof
The invention provides a composition with auxiliary protection effect on chemical hepatic injury and alcoholic hepatic injury and health care food thereof. The composition or the health care food comprises the following components in percentage by weight: 8-15% of silymarin, 10-20% of wolfberry fruit extract, 15-30% of licorice root extract, 15-20% of kudzuvine root extract, 15-20% of wolfberry fruit extract, and 12-25% of turmeric extract. The health care food provided by the invention has the comprehensive functions of silymarin, wolfberry fruit extract, licorice root extract, kudzuvine root extract, wolfberry fruit extract and turmeric extract, and the components take synergistic action, so as to greatly improve the administration effect of a single component. Functional tests prove that the health care food has auxiliary protection effect on chemical hepatic injury and alcoholic hepatic injury. The preparation method of the health care food is simple, which meets the need of large-scale industrial production.
Owner:HUANGJIN DADANG BIOLOGICAL SCI & TECH SHANGHAI +1
Herbal composition for treating hangovers
InactiveUS20080075710A1Alleviate and reduce effect of hangoverBiocidePeptide/protein ingredientsAdditive ingredientPotassium
An herbal composition is adapted to treat hangovers. In one embodiment, the herbal composition comprises potassium, thiamin (vitamin B-1), magnesium, silymarin and N-acetyl-L-cysteine (acetylcysteine), or any pharmaceutically acceptable salts thereof. In a method of treating hangovers, an herbal composition is provided that comprises potassium, thiamin, magnesium, silymarin and N-acetyl-L-cysteine. The herbal composition is placed in the body so as to alleviate or reduce the effects of a hangover.
Owner:LIQUID POTIONS
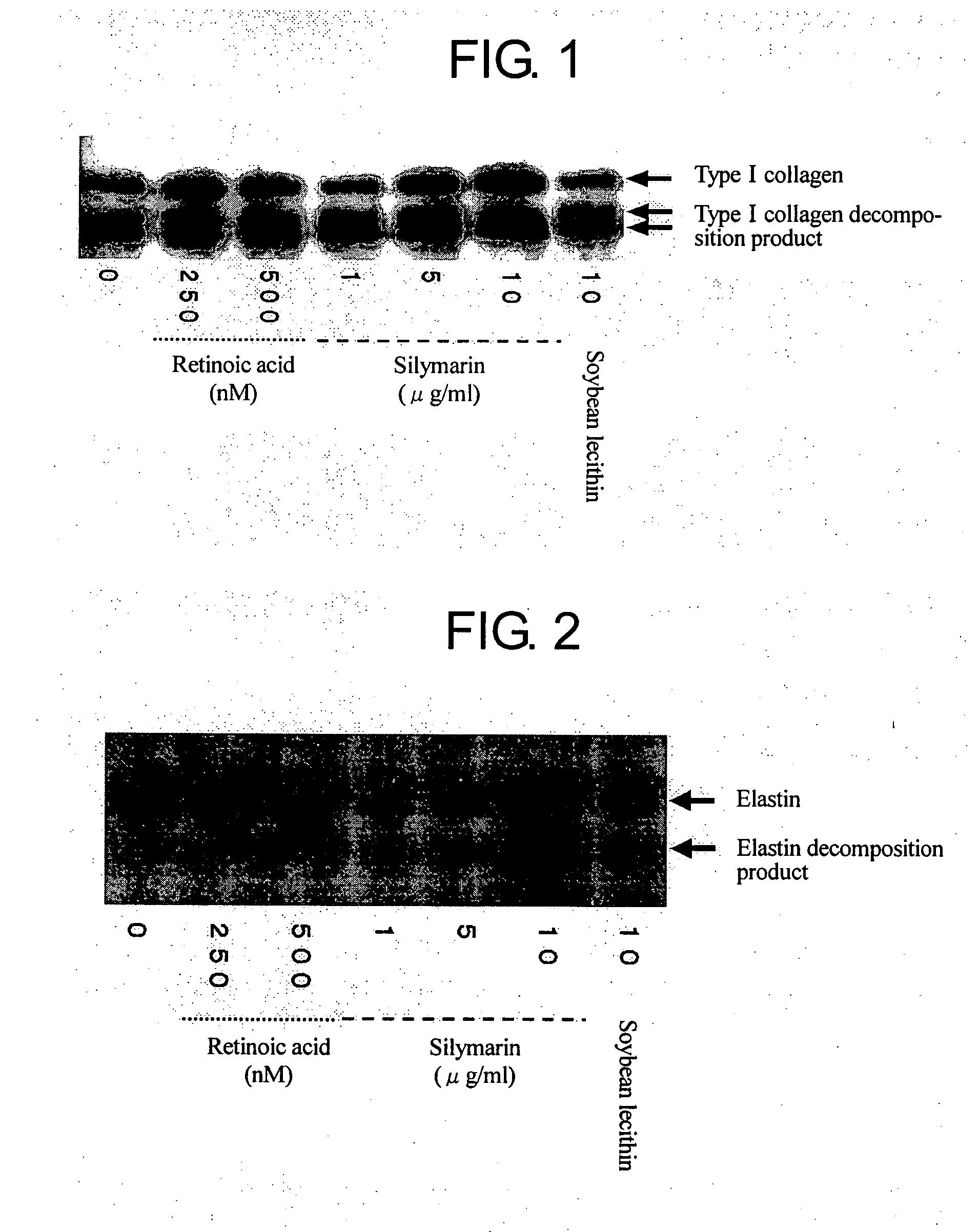
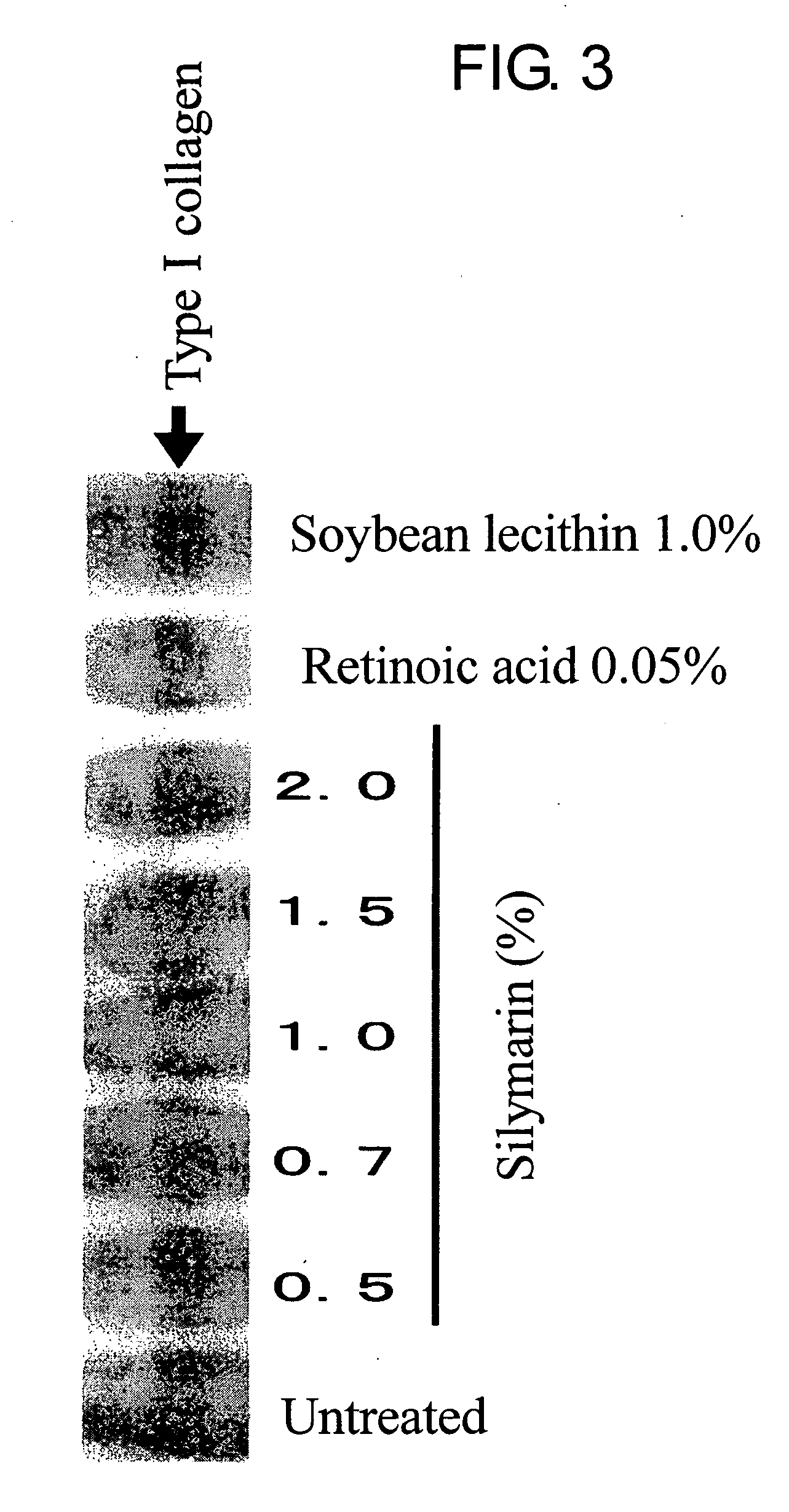
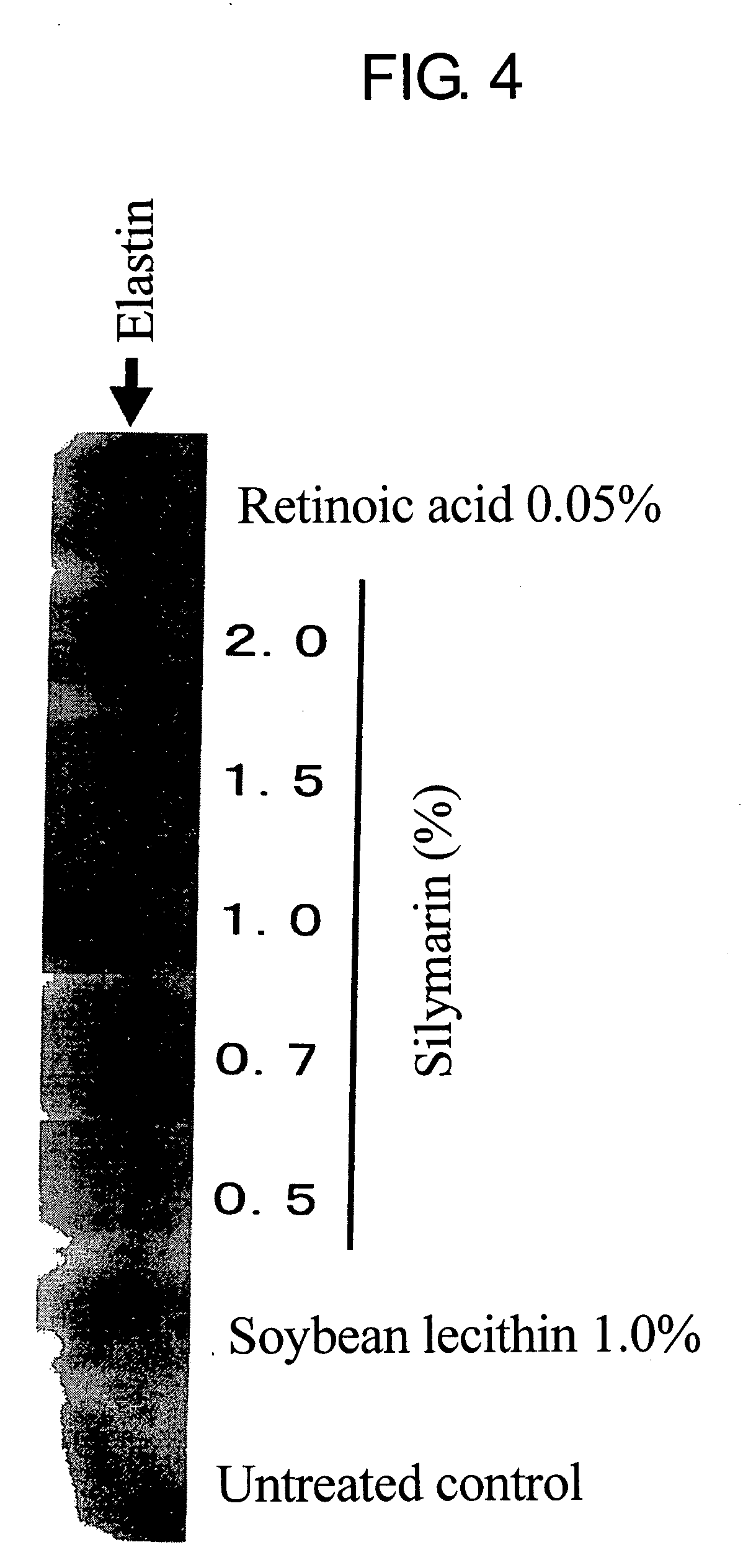
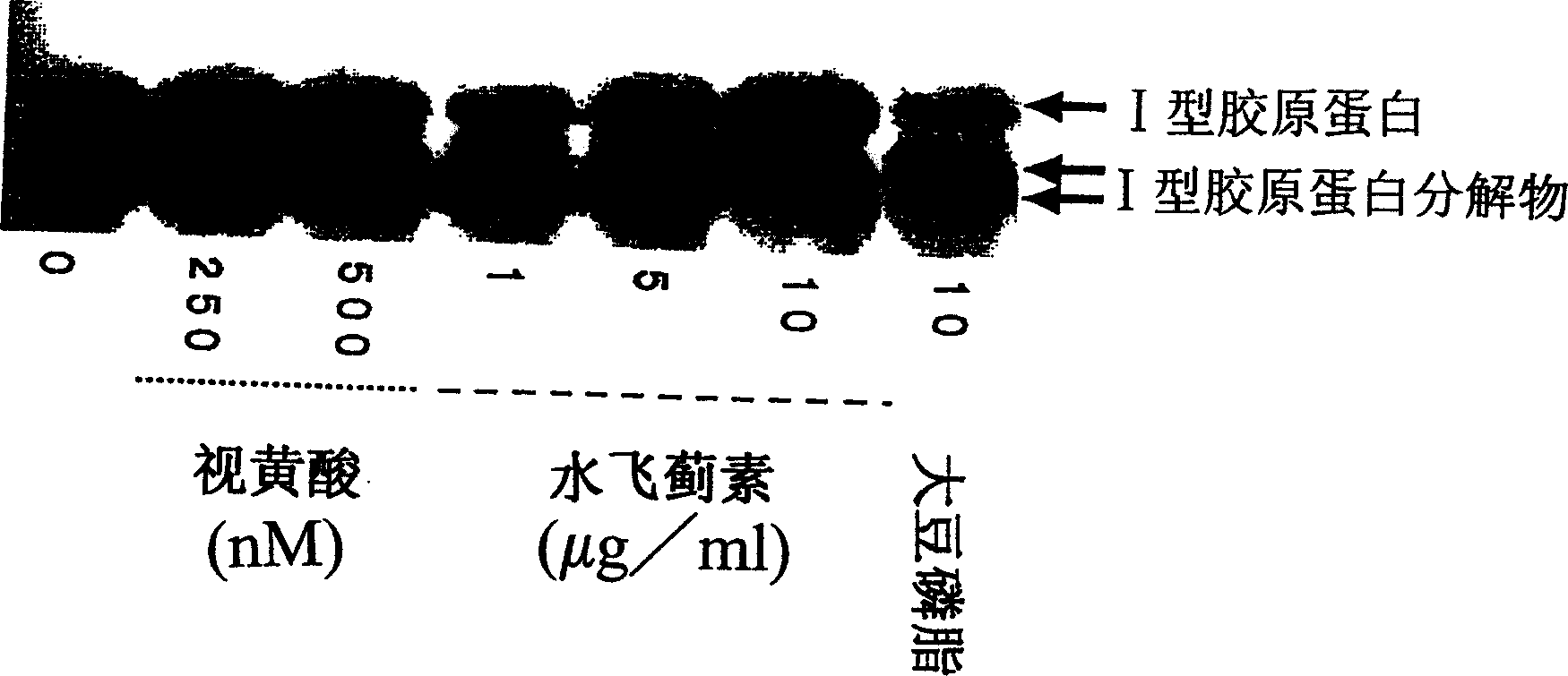
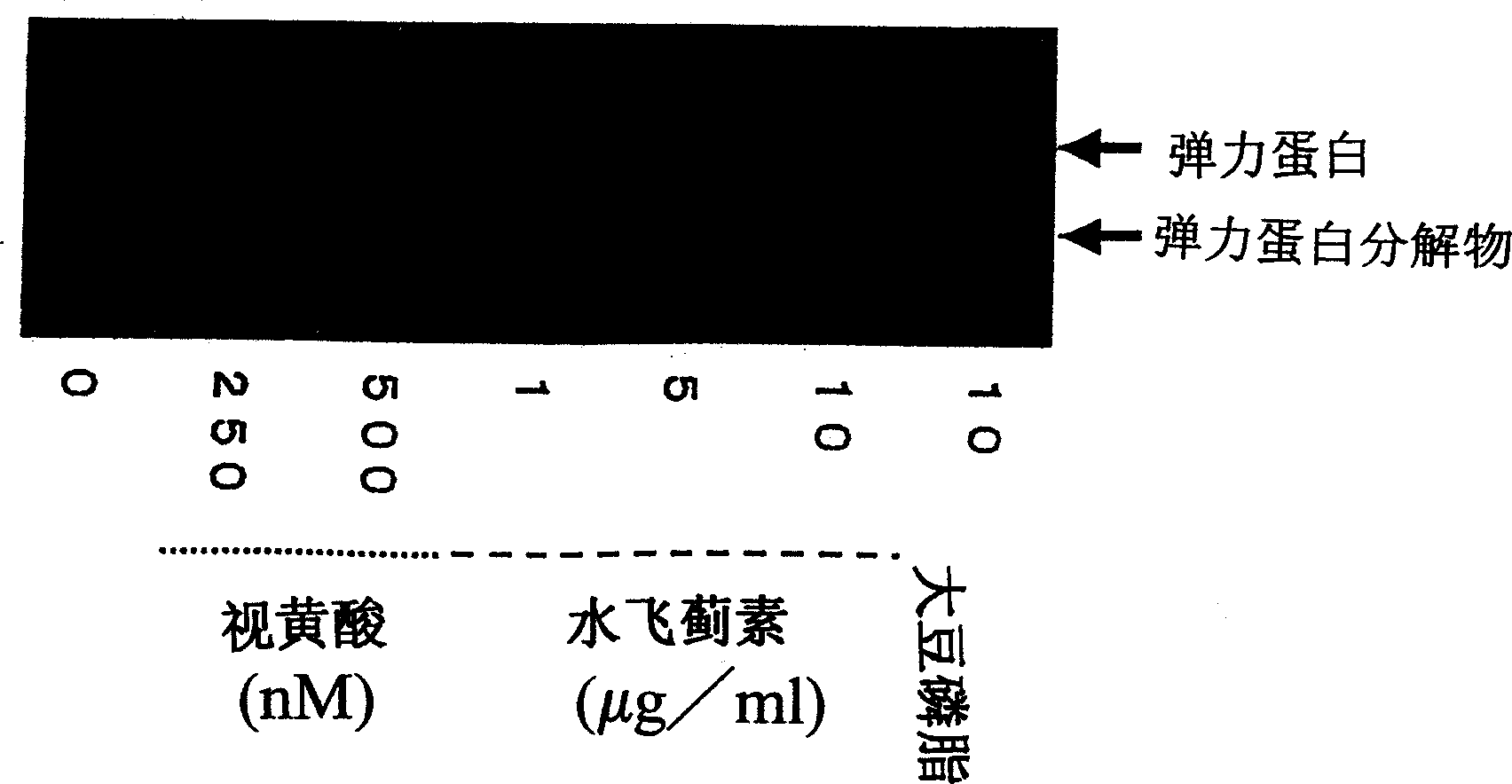
Composition for promoting production of type 1 collagen and/or elastin
InactiveUS20060233738A1Easy to produceImproves suppleness and elasticity of skinBiocideCosmetic preparationsWrinkle skinMedicine
This invention aims to provide a composition that promotes the production of type I collagen and / or elastin in the human skin fibroblast cells, wherein the composition improves the suppleness and elasticity of the skin, is amply effective in preventing and improving wrinkles and sagging, and is also very safe to the skin. The present invention relates to a composition that contains silymarin, which is a general term for flavonolignans such as silybin, silydianin, silychristin and isosilybin, wherein the aforementioned composition has a property to promote the production of type I collagen and / or property to promote the production of elastin. It also relates to a composition containing silymarin derived from a silymarin-containing plant and / or extract of such plant, wherein the aforementioned composition also has a property to promote the production of type I collagen and / or property to promote the production of elastin.
Owner:FUAN KERU
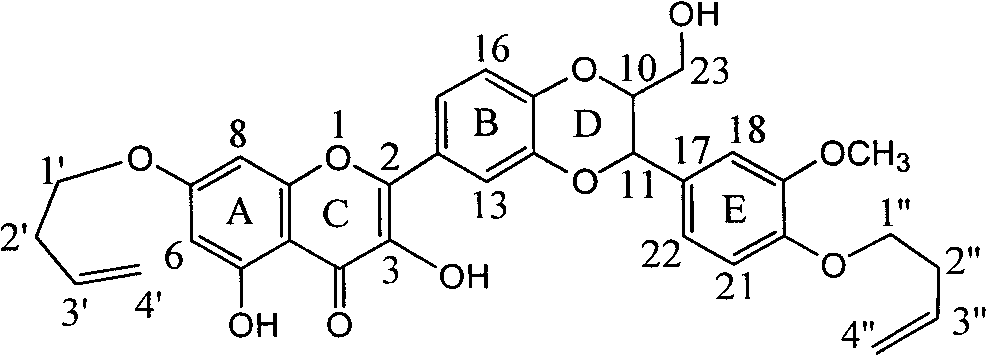
Use of acetamide dehydrogenation silibinin as medicament for treating viral hepatitis B
InactiveCN101829091APowerful removalInhibitory activityOrganic active ingredientsDigestive systemAntigenDisease
The invention relates to the use of acetamide dehydrogenation silibinin as a medicament for treating viral hepatitis B, in particular to the use of dehydrogenation silibinin esters flavonoid lignanoid replaced by A ring methoxy formyl amine or pharmaceutically acceptable salt as the medicament for eliminating HBsAg (hepatitis B surface antigen) and HBeAg (hepatitis Be antigen) and restraining copy of HBV DNA. The cetamide dehydrogenation silibinin can obviously restrain the HBsAg and HBeAg activity, and the strengths for eliminating the HBsAg and HBeAg are 90.5% and 63.6% at the concentration of 20 microgramme / milliter and are 5.6 times and 3.8 times more than positive contrast medicament alpha-interferon. Meanwhile, the restraining rate to the HBV DNA is 90.4% at the concentration, is 12% higher than lamivudine, and is 2.4 times more than a- interferon. Therefore, the flavonoid lignanoid or the pharmaceutically acceptable salt can be expected for treating hepatitis B virus infection as the non-nucleoside medicament.
Owner:DALI UNIV
Application of diamine formyl dehydrogenated silybin serving as medicament for curing viral hepatitis B
InactiveCN101829090AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of diamine formyl dehydrogenated silybin serving as a medicament for curing viral hepatitis B, in particular to application of a flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or pharmaceutically acceptable salts thereof in preparation of a medicament for clearing HBsAg and HBeAg and a medicament for inhibiting HBV DNA replication. The flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents has extremely high HBsAg and HBeAg inhibiting activities; when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at a concentration of 20 mu g / ml, the inhibition rates of the HBsAg and the HBeAg are respectively 94.4 percent and 95.7 percent which exceed 5.9 times and 5.7 times those of a positive control alpha-interferon; and simultaneously the inhibition rate of the HBV DNA is 99.7 percent when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at the same concentration, and the inhibition activity of the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is higher than that of lamivudine and the alpha-interferon. In summary, the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or the pharmaceutically acceptable salts thereof can be expected for preparing non-nucleoside medicaments for clearing the HBsAg and the HBeAg, inhibiting the HBV DNA replication, and curing the hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of aromatic carbamoyl dehydro-silibinin as medicament for treating viral hepatitis B
InactiveCN101829086AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of aromatic carbamoyl dehydro-silibinin as a medicament for treating viral hepatitis B, in particular to application of todehydro-silibinin flavonolignans with a ring A and a ring E which are substituted by double base aromatic carbamoyl methoxyl and pharmaceutically acceptable salt thereof for preparing medicaments for removing HBsAg and HBeAg and medicaments for inhibiting HBV DNA. The todehydro-silibinin flavonolignans has extremely obvious activity on inhibiting the HBsAG and the HBeAg, has the intensity of 46.2 percent and 68.9 percent for respectively removing the HBsAG and the HBeAg in the presence of the concentration of 100 microgram / milliliter, which is 2.9 times and 4.1 times higher than that of positive control medicament alpha-interferon, and has the inhibition ratio of 96 percent on HBV DNA in the presence of the concentration of 100 microgram / milliliter, which is higher than that of lamivudine and the alpha-interferon. Accordingly, the flavonolignans and the pharmaceutically acceptable salt thereof can be expected to be used for preparing non-nucleoside medicaments applied for removing HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
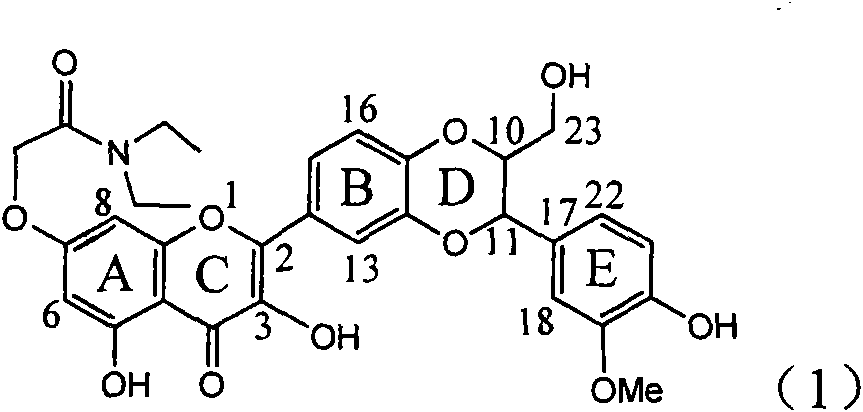
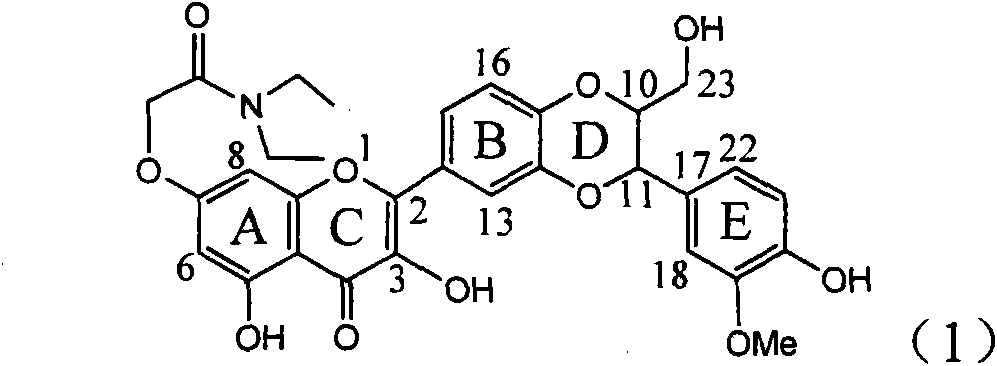
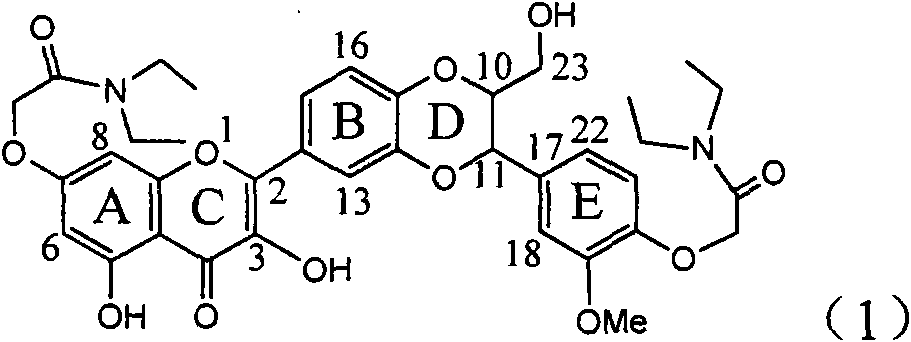
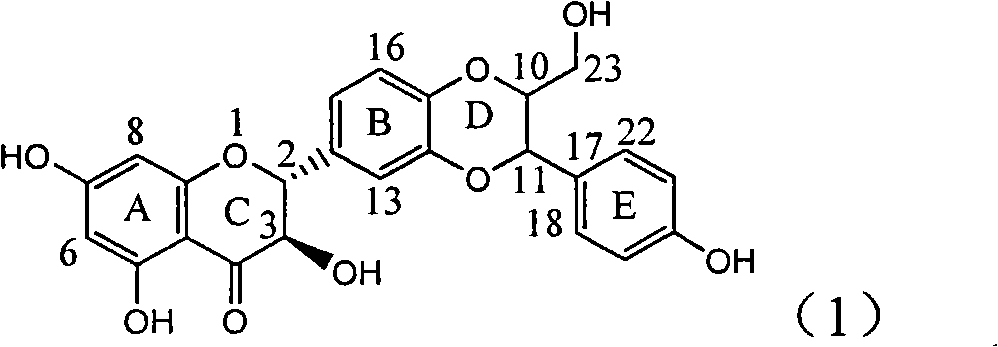
Application of E-ring demethoxy-silibinin for preparing medicament for treating viral hepatitis B
InactiveCN101912383AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of E-ring demethoxy-silibinin for preparing medicaments for treating viral hepatitis B, and particularly to application of compound in formula (1) and pharmaceutically acceptable salt thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV DNA replication. The invention has extremely superactive activity for suppressing the HBsAg and HBeAg; in the presence of the concentration of 20 microgram per millilitre, the intensities for clearing the HBsAg and HBeAg are 95.0% and 34.4% respectively, which are 5.9 and 2.0 times corresponding activity of a positive control medicament alpha-interferon; and it should be noticed that the suppression ratio of the medicament for HBV DNA at the concentration is about 91.5%, which is 13% higher than lamivudine and 2.4 times alpha-interferon suppression activity. In summary, the flavonolignans or pharmaceutically acceptable salt thereof can be prospectively used for preparing non-nucleoside medicaments for clearing the HBsAg and HBeAg, suppressing HBV DNA replication and treating hepatitis B virus infection disease.
Owner:DALI UNIV
Application of dehydrogenated silibinin diether in preparation of medicaments for preventing and treating leukemia
InactiveCN101785767ASimple methodShort stepsOrganic active ingredientsAntineoplastic agentsMyeloid leukemiaLeukemia
The invention provides application of dehydrogenated silibinin diether in the preparation of medicaments for preventing and treating leukemia and relates to medical application of lignin flavone silibinin to the prevention and treatment of chronic myeloid leukemia. Particularly, the invention relates to application of dehydrogenated silibinin diether, of which the seventh bit and the twentieth bit are substituted by 1-butylene, or pharmaceutically acceptable salts thereof in the preparation of medicaments for preventing and treating chronic myeloid leukemia. Natural products of the silibinin are treated by simple steps to synthesize the compound. The pharmacological tests prove that the compound can potently inhibit the in-vitro proliferation of human chronic myeloid leukemia cell strains(K562) and adriamycin drug-resistant strains (K562 / ADR), and the IC50 values are 11.9+ / -1.6 micromoles and 15.9+ / -1.2 micromoles respectively.
Owner:DALI UNIV
Production method of silymarin
ActiveCN102079745AReduce the amount of fatReduce the use effectOrganic chemistrySilybum Marianum SeedSolvent
The invention provides a production method of silymarin, which comprises the following steps: shelling silybum marianum seeds; filling the silybum marianum shells into a softening tank, and softening the silybum marianum shells with vapor; filling the softened silybum marianum shells into a flat-turn extractor, and adding ethanol to carry out extraction, thereby obtaining an extracting solution; concentrating the extracting solution to a certain degree to obtain a concentrated solution; adding aqueous alkali into the concentrated solution, stirring and standing to precipitate; and filtering the precipitate, drying, pulverizing and screening. In the extraction process, no toxic or harmful reagent is adopted, and no solvent residue which is harmful to the product can be produced; the ultraviolet detection indicates that the total flavone content in the product can be higher than 70%, the contents of silybin and isosilybin can be higher than 30%, and the product yield is 3-4% of the silybum marianum seeds; and therefore, the invention has the advantages of high extraction efficiency, simple technique and low solvent consumption.
Owner:内蒙古昶辉生物科技股份有限公司
Process for isolation of hepatoprotective agent silymarin from the seeds of the plant Silybum marianum
The invention relates to a novel process for the isolation of a hepatoprotective agent Silymarin from the seeds of the plant Silybum marianum comprising (i) Precooling the seeds to (-) 20° C. for 24 hours in a deep freezer / cold room. (ii) Powdering the cooled seeds in a hammer mill, fitted with about 40 mesh discharge screen. (iii) Defatting the seeds by extracting with hexane in a soxhlet type extractor to remove the total quantity of fatty oil without using a scrain. (iv) Extracting the defatted seeds with acetonitrile at 20-30° C. to extract silymarin fraction. (v) Concentration of the sensitive silymarin fraction under vacuum in a agitated wiped thin film evaporator (vi) stirring the silymarin such dry powder with cold dichloromethane at 5° C. followed by filtration and drying with a slow purge of nitrogen gas. (vii) Further purification of silymarin by suspending in 5 times its weight of acetonitrile and precipitating by 8-12 times its weight of water at 20° C. (viii) Filtering the precipitated silymarin in a closed vacuum filter and having 1-2 mum screen washing the cake three times with distilled water (ix) Drying of Silymarin cake in vacuum oven to obtain substantially pure silymarin.
Owner:COUNCIL OF SCI & IND RES
Frozen powder injection of silybin and its preparing method
Owner:SHANDONG GREENERY NATURAL MEDICINE RES & DEV
Silybin injection containing cyclo dextrin or its derivatives
InactiveCN1391894ASolve the problem of insolubleOrganic active ingredientsDigestive systemWater insolubleCyclodextrin
The present invention features that silybin is included by cyclodextrin or its derivative so as to dissolve in water to prepare injection. The present invention solves the problem of water insoluble silybin for being suitable for intravenous injection.
Owner:广州瑞济生物技术有限公司
Process for extracting water grind thisvine using alcohole as single organic dissolvent
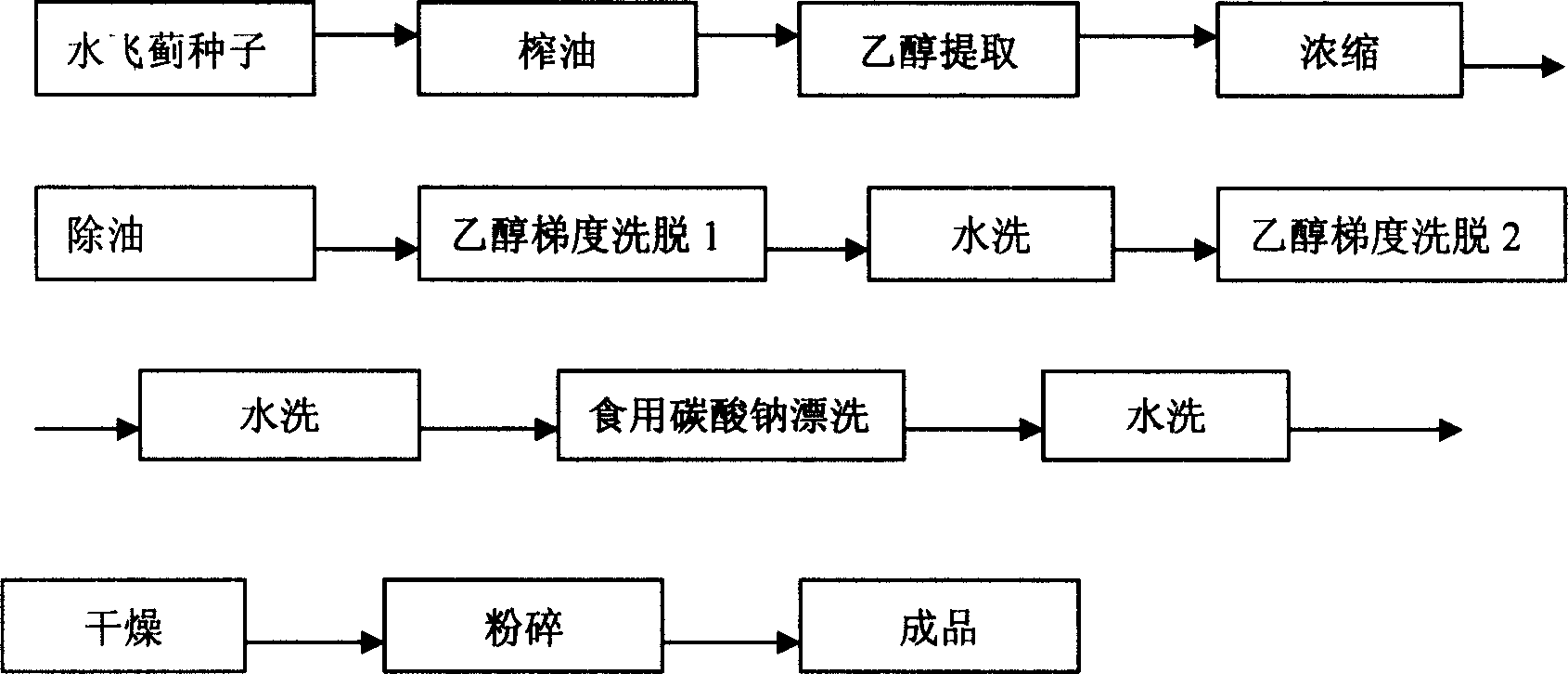
The invention discloses a process of extracting silymarine by using alcohol as a single organic solvent, adopting edible alcohol to extract silymarine, then adopting alcohol and water to wash twice so as to eliminate oil and impurities, using edible sodium carbonate for elution, drying the silymarine solids obtained by fulling and then crushing and obtaining the final product, where by the UV determination, the total flavone content in the silymarine has mass percent concentration of 70%-90%, the product yield is 2-3% of the quantity of silymarine seeds from which the oil has been extracted. It solves the problem of harmful solvent residues in current silymarine products, and it can obtain the silymarine products without harmful solvent residues.
Owner:XIAN UNIV OF TECH
Pharmaceutical composition with liver-protecting effect and preparation method thereof
ActiveCN103655929AStrong fat oxidationGood anti-inflammatory effectDigestive systemPlant ingredientsBlack teaSilybum marianum extract
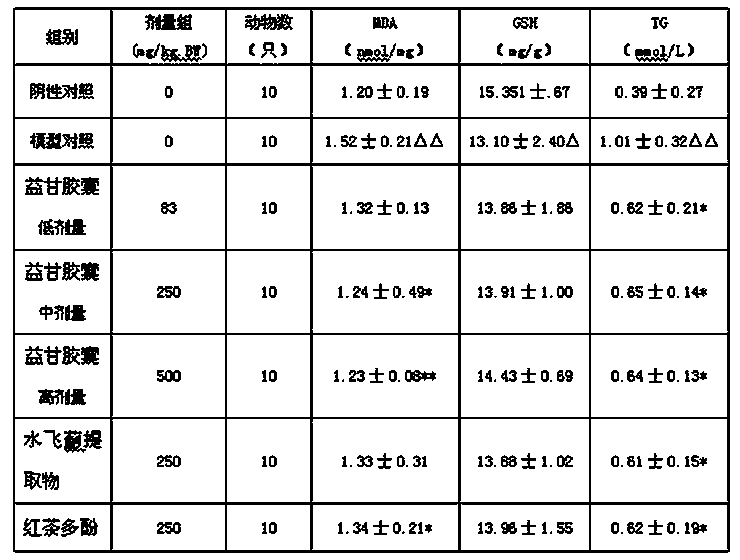
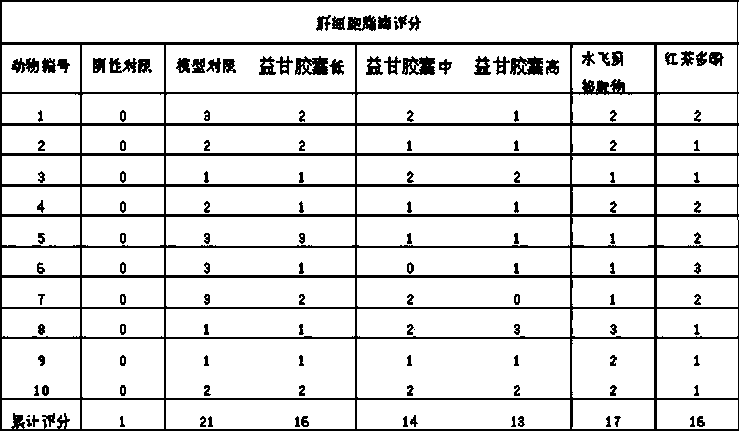
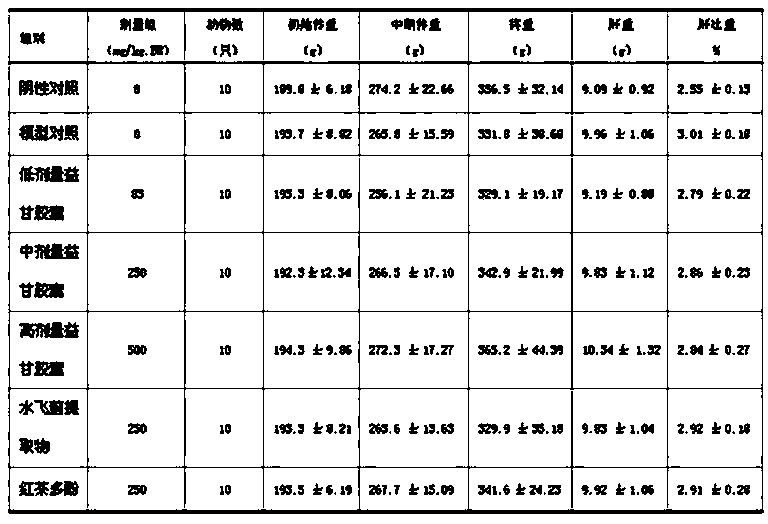
The invention relates to a pharmaceutical composition with liver-protecting effects. The composition comprises the following substances by weight: 6-15 parts of black tea polyphenol and 2-6 parts of a Silybum marianum extract. The silymarin and black tea polyphenol with different drug effects have very clear roles in prevention and treatment of chemical liver injury from different targets and the ways of, and are complementary with each other to effectively prevent and treat chemical liver injury. The two medicines are combined to perform the efficacies of strengthening and nourishing the liver, coursing the liver, detoxifying and immunoloregulation. Tests have proved that the pharmaceutical composition provided by the invention can prevent liver injury.
Owner:JIANGSU DEHE BIOTECH
Process for preparing silymarin
InactiveCN1463970AAvoid active adverse effectsThe extraction and separation process is simpleOrganic chemistryBulk chemical productionSILYBUM MARIANUM SEED OILOrganic solvent
The preparation process of silybin with silybum mariamum as material includes supercritical CO2 extraction of silybum mariamum seed oil, organic solvent extraction of silybin and the separation of extractive liquid in supercritical CO2 environment to obtain high-activity silybin. The said process is superior to traditional process with local high temperature and incomplete deoiling to affect product quality and yield, and has simplified extraction and separation process, short extraction period, and high silybin content in product. The silybum mariamum seed oil product of the present invention is clear and transparent health food oil with excellent commercial foreground.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
Oral micro-emulsion composition of silybin
An oral micro-emulsion composition comprising a Carduus marianus extract, silybin or a silybin derivative; an organic solvent; a surfactant; and an oil provides a high in vivo bioavailability of silybin.
Owner:HANMI PHARMA
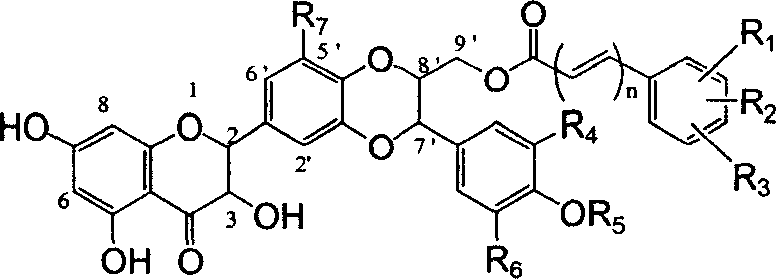
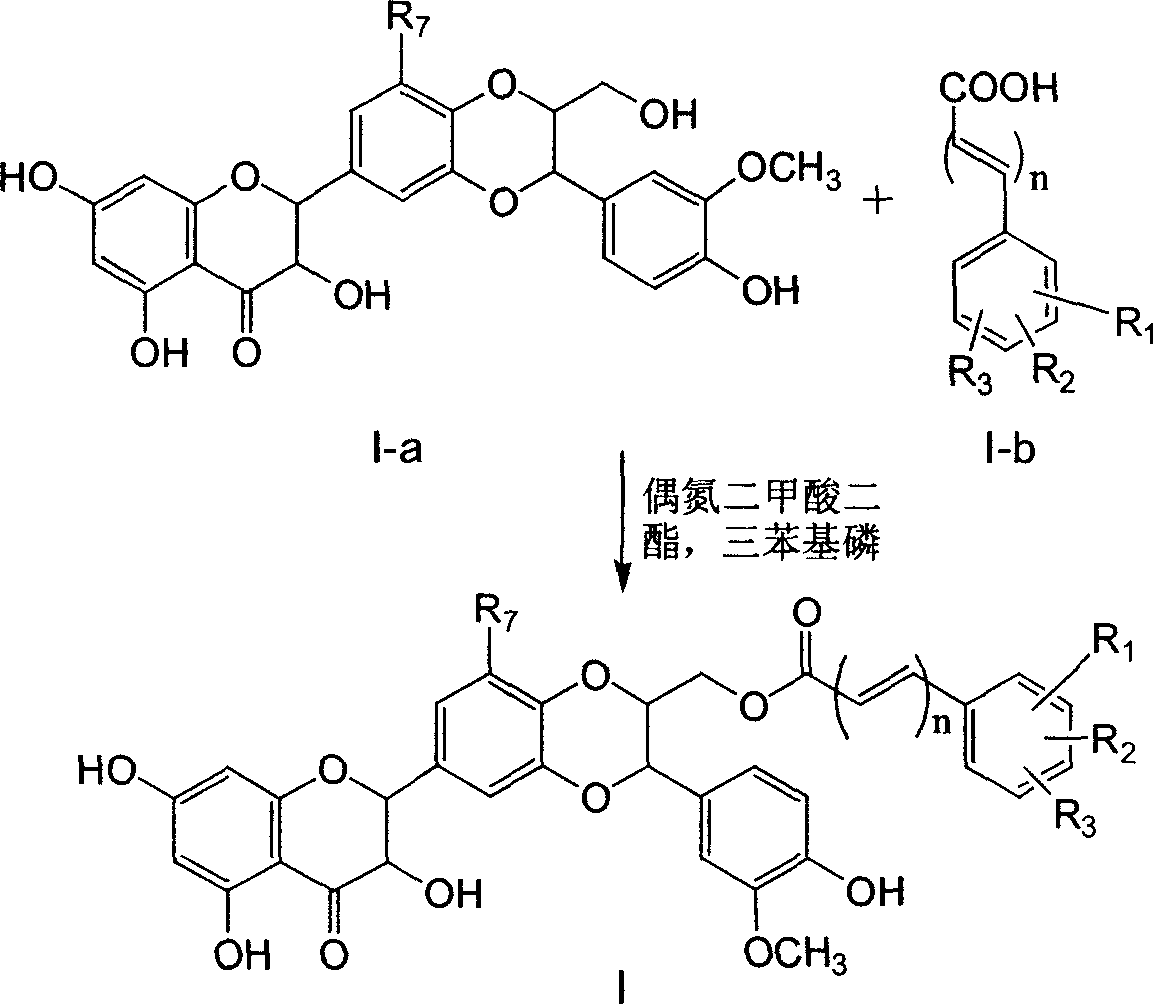
Silybin flavonolignan and their production method and use
The invention relates to a silibinin flavonolignan and its medicine salt or solvates. The invention also relates to the method preparing its important mediate and its drug combination and medical application. The compound can protect primary hepatocyte from oxidative damage for SD newly born rat hepatitis virus, so it is expected to be used as drug preventing liver damage. The compound can remove superoxide anion free radical and diphenyl picryl phenylhydrazine free radical, inhibit free radical from inducing generation of fat oxidatant, protect PC 12 cell from being damaged by free radical, so it is expected to be used as drug treating diseases caused by free radical.
Owner:ZHEJIANG HISUN PHARMA CO LTD
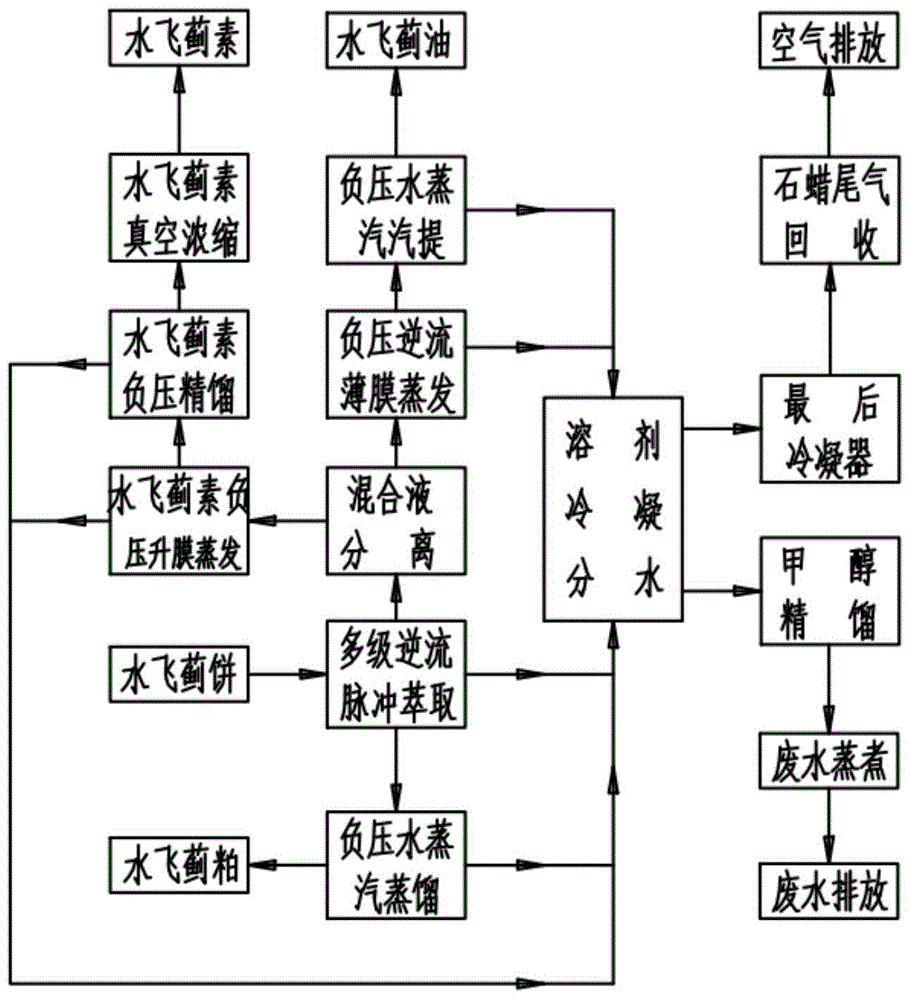
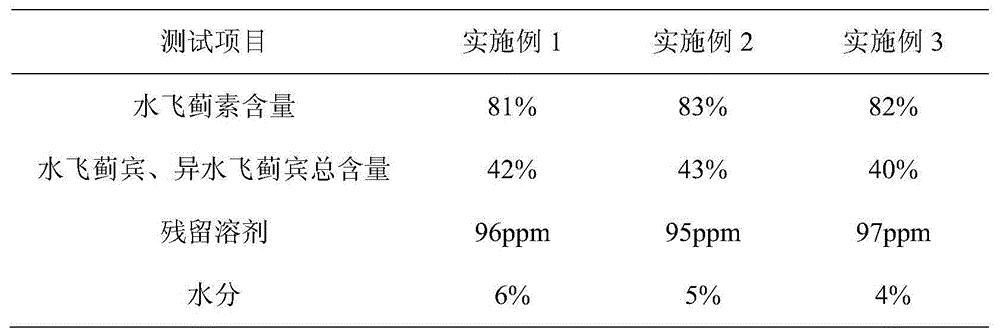
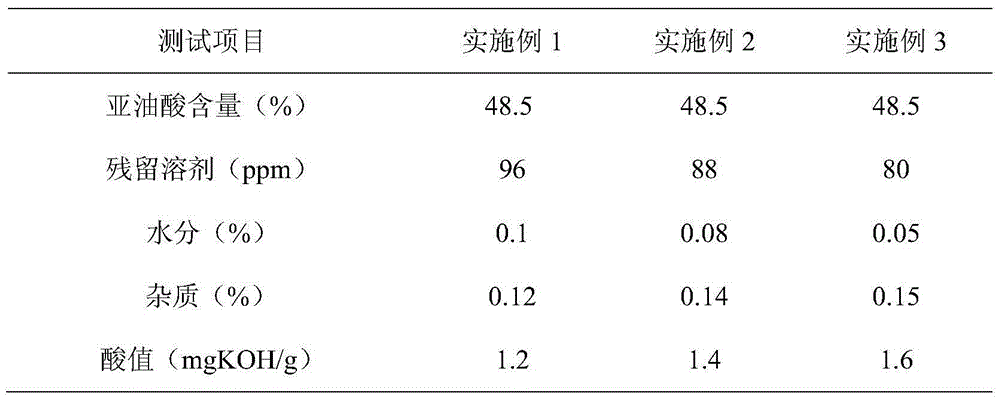
Method for simultaneously extracting silybum marianum oil and silymarin from silybum marianum cake
ActiveCN104673486AHigh extraction rateAchieve scaleOrganic chemistryFatty-oils/fats productionWater vaporEvaporation
The invention discloses a production method for simultaneously extracting silybum marianum oil and silymarin from a silybum marianum cake. The method comprises the following steps: (a) performing multistage pulse counter-current extraction on the silybum marianum cake to obtain a mixed solution containing the silybum marianum oil and the silymarin, wherein the solvent used in the extracting process is a mixed solvent of the methanol and the normal hexane at a mass ratio of (0.6-1):1; (b) filtering and separating the mixed solution containing the silybum marianum oil and the silymarin to obtain the silybum marianum oil normal hexane mixed solution and the silymarin methanol mixed solution; (c) performing negative-pressure counter-current film evaporation and negative-pressure water vapor steam distillation on the silybum marianum oil normal hexane mixed solution to obtain the silybum marianum oil; and (d) performing negative-pressure climbing film evaporation, negative-pressure rectification and spray dehydration on the silymarin methanol mixed solution to obtain the silymarin. By adopting the method provided by the invention, the extracting rate of the silymarin is greatly increased and the problems of large input of the solvent and low extracting rate in the silymarin production process can be solved; the production process is free of pollution and low in cost, so that the large-scale, industrial and continuous production of the silymarin can be realized.
Owner:安徽嘉旗粮油工程技术有限公司
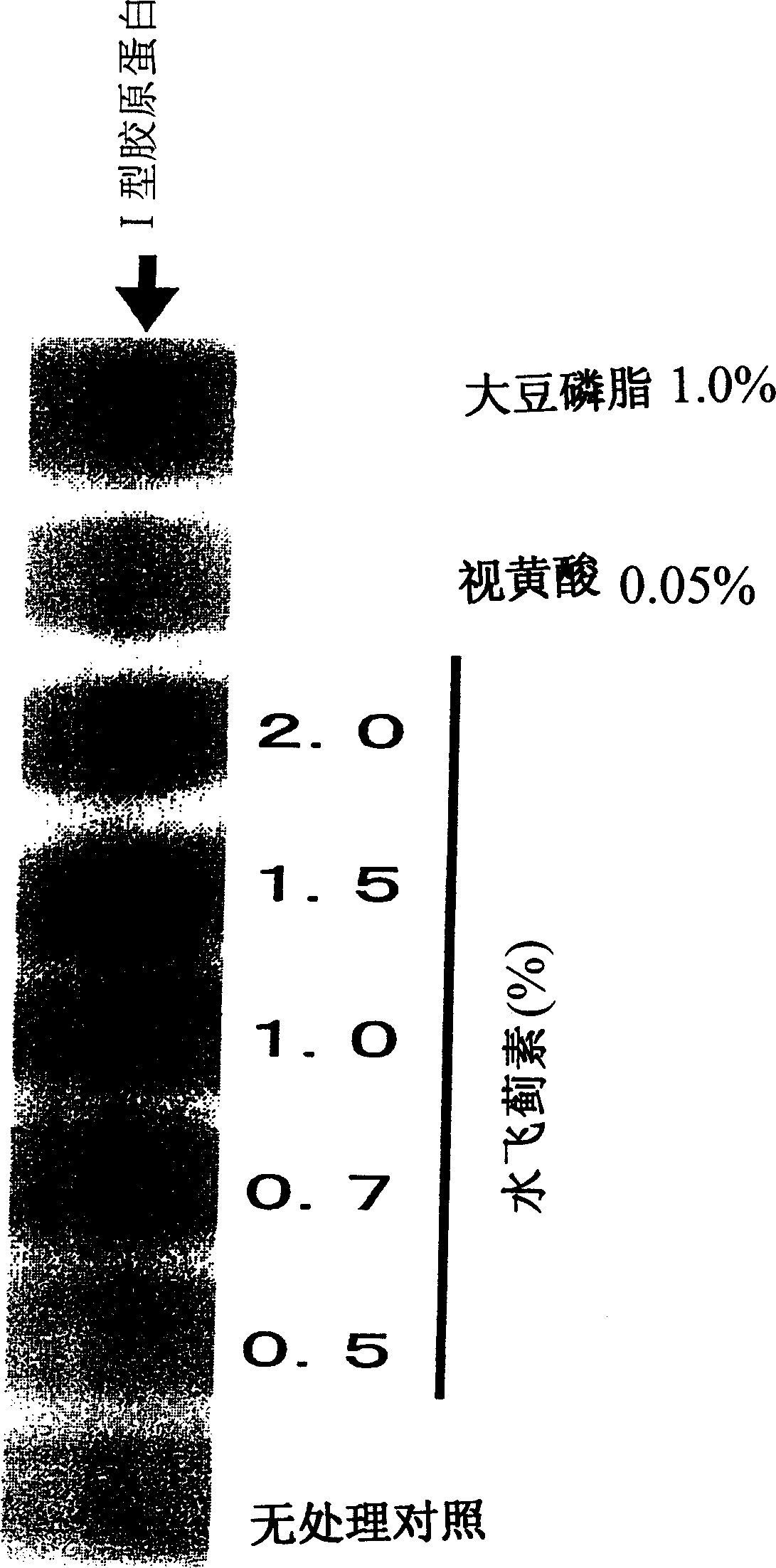
Composition for promoting production of type I collagen and/or elastin
It is intended to provide a composition promoting the production of type I collagen and / or elastin in human skin fibroblasts to thereby provide a composition for preventing the skin from aging which can improve skin tension and elasticity, is sufficiently efficacious in preventing, inhibiting or relieving wrinkles and sagging in the skin and yet has a high safety to the skin. A composition characterized by containing silymarins, (i.e., a general term for flavonolignans such as silybin, silydianin, silychristin and siosilybin) and having an effect of promoting the production of type I collagen and / or elastin; and a composition characterized in that silymarins contained therein originate from a silymarin-containing plant and / or an extract of the plant and having an effect of promoting the production of type I collagen and / or elastin.
Owner:FUAN KERU
Method for preparing Silymarin
InactiveCN101759687AHigh purityConducive to mass production operationsOrganic chemistryBulk chemical productionFiltrationPetroleum ether
The invention relates to a method for preparing Silymarin. The method has the advantages that the operation is simple and convenient, the pollution is low and the equipment investment is small. The technological steps are that: Silybum marianum fine powder is taken and is added in a CO2 supercritical extractor, methanol is used as entrainer, the entrainer accounts for 2-6 percent of the total volume of extraction solvent, the extraction pressure is 10-40MPa, the extraction temperature is 30-60 DEG C, CO2 flow is 1-5ml per gram crude drug per minutes, the extraction time is 150-250min, the extract is obtained, acetone is added, agitation is conducted to enable the acetone to be dissolved, filtration is conducted, the filtered liquid is obtained and is concentrated, the obtain liquid is filled in a high-efficiency countercurrent extractor, water-acetone-chloroform (1:5:2) solvent system is used, the bottom phase is a fixed phase, the top phase is a flowing phase, Silymarin segments are collected and are concentrated, crystals are precipitated and are separated, and acetone-petroleum ether (0.5-2:1) are added for recrystallization. The invention has the advantages that the purity of the prepared Silymarin is high and the industrialization amplification can be realized easily.
Owner:SUZHOU PAITENG BIOLOGICAL MEDICAL TECH
New method for purifying silymarin
The invention belongs to the field of natural organic chemistry, and relates to a method for preparing high purity silymarin by the following steps: adopting biological wall breaking and microwave pretreatment to obtain oil, carrying out microwave countercurrent extraction and macroporous resin purification to obtain the high purity silymarin. The method has the following advantages that: 1, a step-by-step method is adopted to carry out degreasing, seeds are pretreated, microwave countercurrent extraction is performed, and isoelectric point precipitation is performed to remove proteins, such that complete dissolution of effective components of the silymarin is prompted; 2, with the microwave extraction, the extraction time is reduced to 60-80 minutes so as to improve extraction speed and increase effective component content, and reduce herb consumption and energy consumption; 3, the macroporous resin adsorption technology is adopted so as to reduce the amount of the solvent, and the macroporous resin can be conveniently and rapidly regenerated, such that the cost is substantially reduced; and 4, two products of silybum marianum oil and silymarin can be obtained. With the method, the biological wall breaking treatment is performed on the silybum marianum seeds at the early stage so as to shorten the microwave pretreatment time, such that the seed oil can be extracted in just a few minutes; with the two-step microwave extraction, the extraction time is shortened; and the disadvantages of long conventional extraction time, low extract purity and single extract are overcome.
Owner:DAXINGANLING LINGOBERRY BOREAL BIOTECH CO LTD
Water-soluble silymarin and preparation method thereof
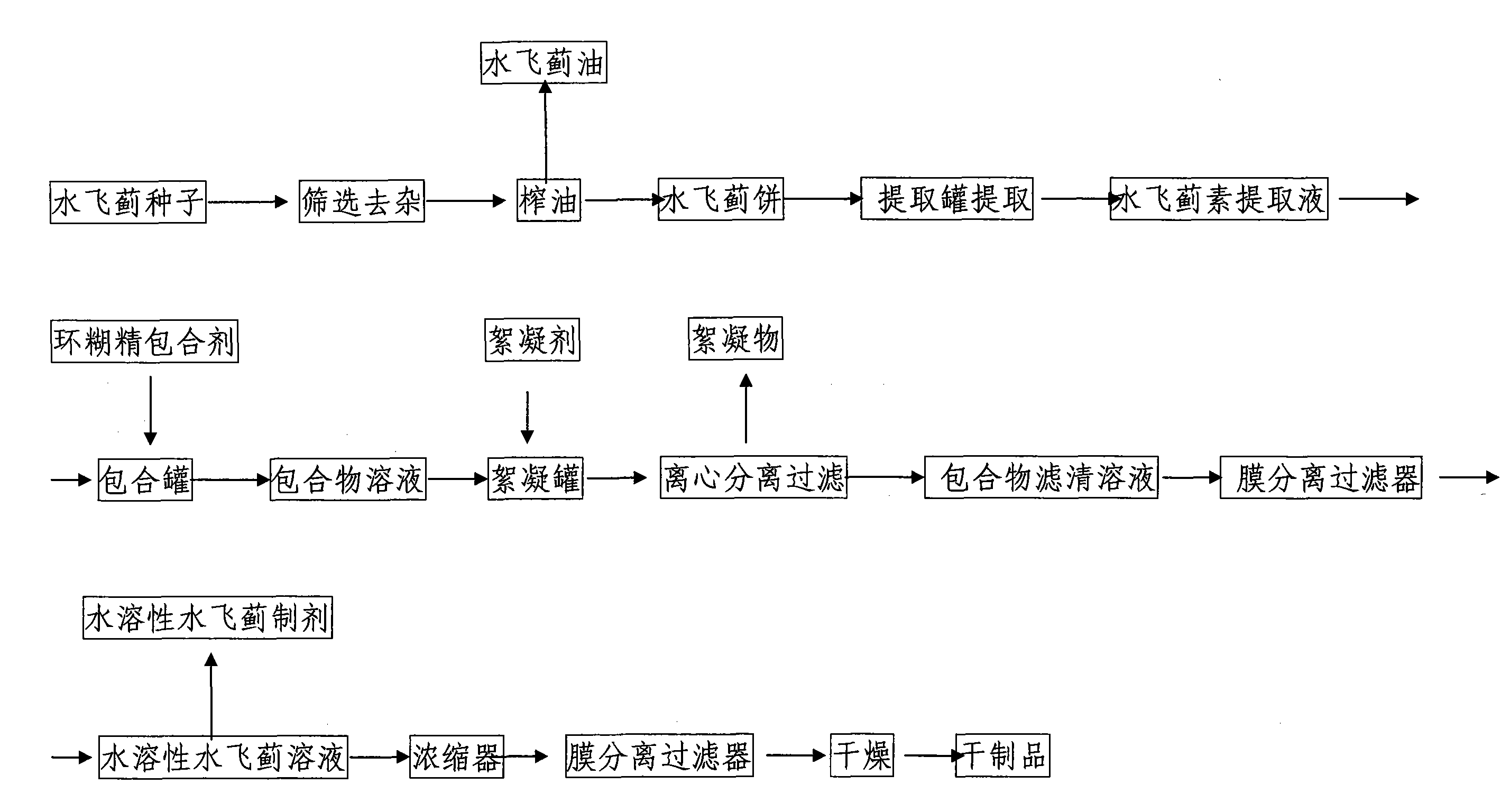
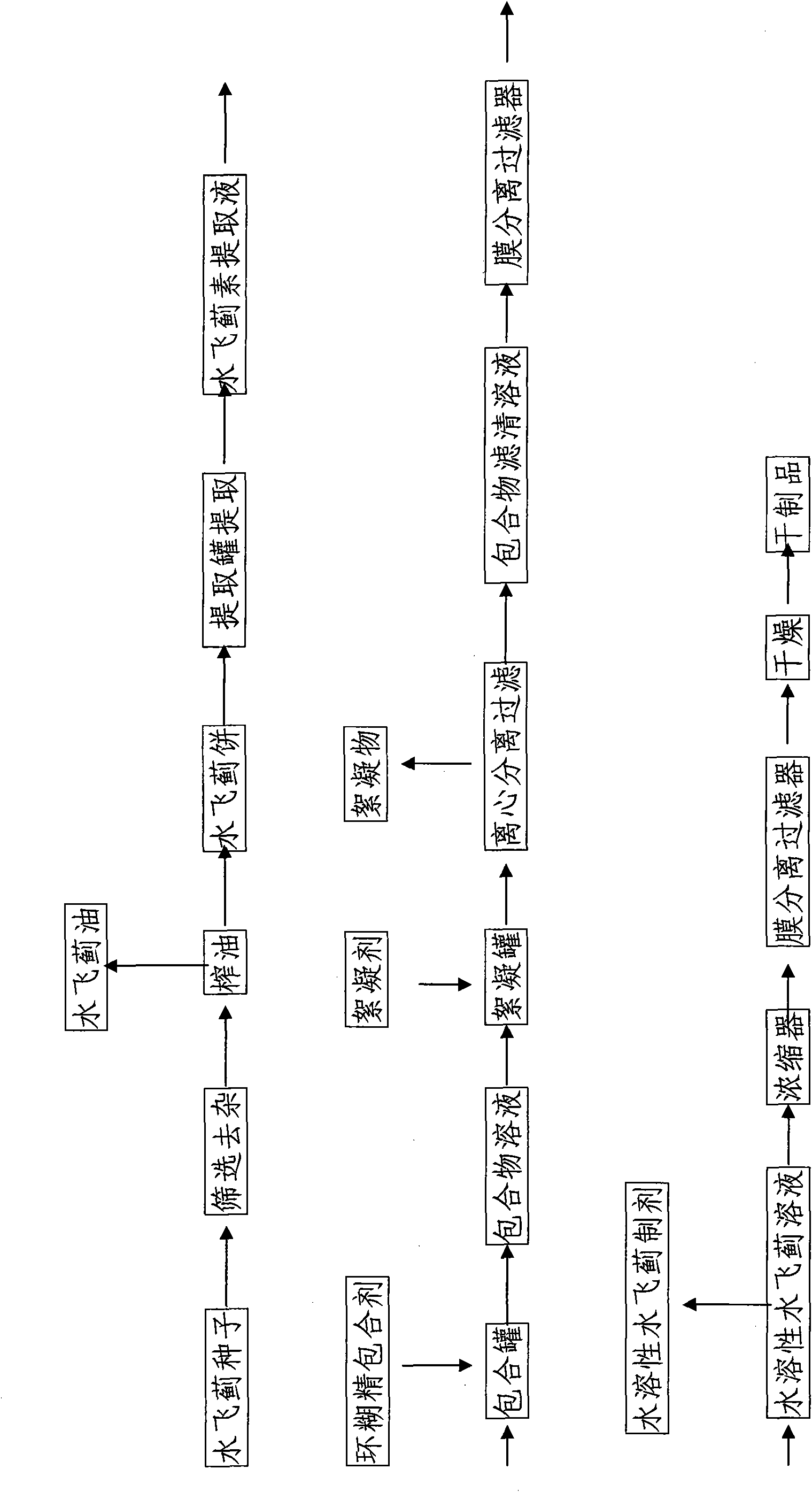
InactiveCN101810660ANo residueImprove qualityOrganic active ingredientsMetabolism disorderSolubilitySilybum Marianum Seed
The invention discloses water-soluble silymarin, which is an inclusion compound that takes cyclodextrin as main molecules and silymarin as guest molecules; and the inclusion compound contains 12 to 20wt % of silybinin. The preparation method thereof comprises the following steps: silybum marianum seeds are squeezed into silybum marianum cakes; the silybum marianum cakes are added into alkali water ethanol solution and are heated to 75DEG C to 85DEG C, the constant temperature is kept for 1.5-2.5 hours, and silybum marianum extracting solution is prepared after 2-4 times of continuous extraction; the silybum marianum extracting solution is added with the cyclodextrin for inclusion, thus obtaining inclusion compound solution; flocculant is added, standing after being well stirred; after flocculate is filtered out, membrane separation process is carried out, thus obtaining water-soluble silymarin solution; and after the membrane separation process, the water-soluble silymarin solution is concentrated and dried, thus obtaining the dried product of the water-soluble silymarin. The water-soluble silymarin prepared by the method has the advantages of high solubility, good quality, good drug stability and high bioavailability.
Owner:江苏健佳药业有限公司
Compositions for prevention and treatment of symptoms associated with ethyl alcohol consumption
InactiveUS6913769B2Reduce negative impactReduce the impactSalicyclic acid active ingredientsHeavy metal active ingredientsMedicineManganese
Described is a composition for the prevention and treatment of symptoms associated with ethyl alcohol consumption. The composition comprises silymarin, salicin, at least one B vitamin, magnesium, molybdenum and manganese. Treatment of symptoms associated with the consumption of ethyl alcohol involves ingesting the described composition prior to, during, or after the consumption of the alcohol.
Owner:BETTER LIVING CHEM
Epidermal growth factor compound lipidosome as well as preparation method and application thereof
ActiveCN103505403AStrong absorption capacityReduce fatigueCosmetic preparationsMake-upBiotechnologyCholesterol
The invention discloses epidermal growth factor compound lipidosome as well as a preparation method and application thereof, and belongs to the field of traditional Chinese medicines. The epidermal growth factor compound lipidosome is prepared by the following raw materials in parts by mass: 0.1-5 parts of epidermal growth factors, 5-30 parts of silibinin, 5-30 parts of coix seed oil, 5-30 parts of yeast extracts, 5-30 parts of microbial polysaccharide, 30-200 parts of hydrogenated lecithin, 10-80 parts of cholesterol and 5-30 parts of glycerinum. The epidermal growth factor compound lipidosome has the advantages of simple technique, safety, no poison, little skin irritation, mild property and stable effect.
Owner:GUANGZHOU GIALEN COSMETICS
Silybinin injection and its preparation method
InactiveCN1762345AOral bioavailability is lowImprove bioavailabilityOrganic active ingredientsDigestive systemPharmaceutical medicineMethylamines
The invention relates to a silybin injection and its preparation method, which comprises silybin meglumine, glucosamine, alkaline amino acid solubiliser and pharmaceutically acceptable solvent, or compound of silybin and alkaline solubiliser and pharmaceutically acceptable solvent, the pH is 4-11. The injection also comprises one or more of pharmaceutically acceptable solubilizer, pH regulator, isotonic conditioning agent, painkiller, anti-oxidant agent, preservative and complexing agent.
Owner:丛晓东 +1
Method for extracting silymarin from milk thistle seed coat
InactiveCN102219781AQuality exceedsEliminate potential safety hazardsOrganic chemistryOrganic solventMILK THISTLE SEED
The invention provides a method for extracting silymarin from milk thistle seed coat. The objective of the invention is to solve the following problem in the prior art: the extraction of silymarin from milk thistle exotesta uses the integral body of milk thistle as the raw material, and therefore the extracted coarse silymarin contains considerable impurities and has to be refined; the process of refinement is cumbersome and consumes considerable organic solvents. The method provided in the invention comprises the following steps: cleaning milk thistle seeds; decorticating the milk thistle seeds with a decorticator so as to obtain milk thistle seed coats and seed kernels respectively; carrying out extraction on the milk thistle seed coats with a solvent; recovering the solvent by condensation so as to obtain silymarin. According to the invention, silymarin extracted from milk thistle seed coats has a quality far surpassing the international standard.
Owner:邸凤阁
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com