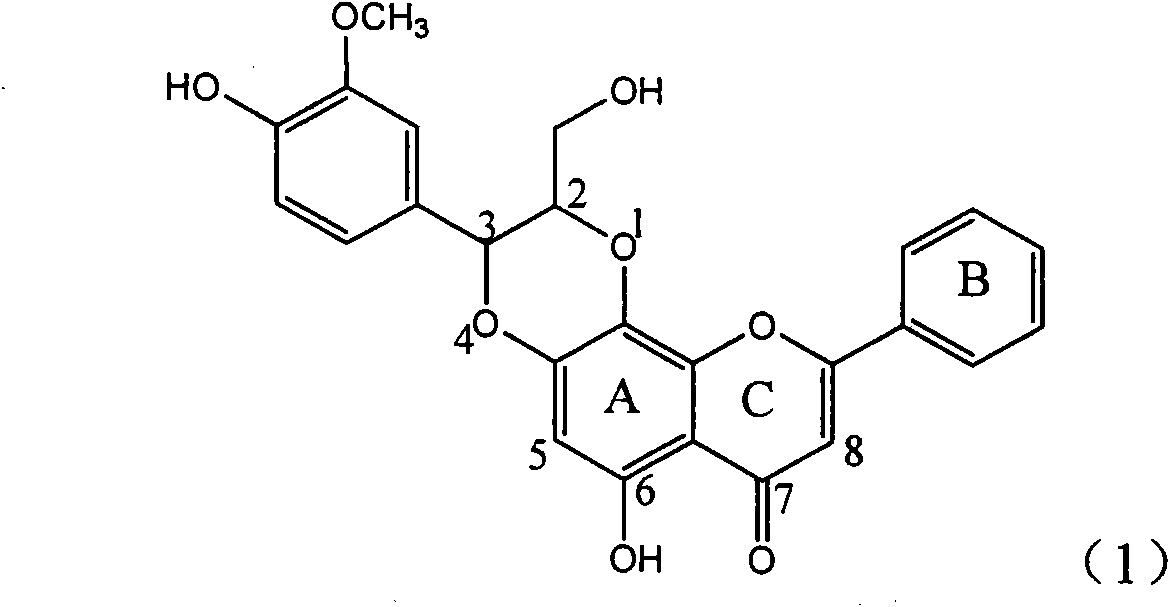
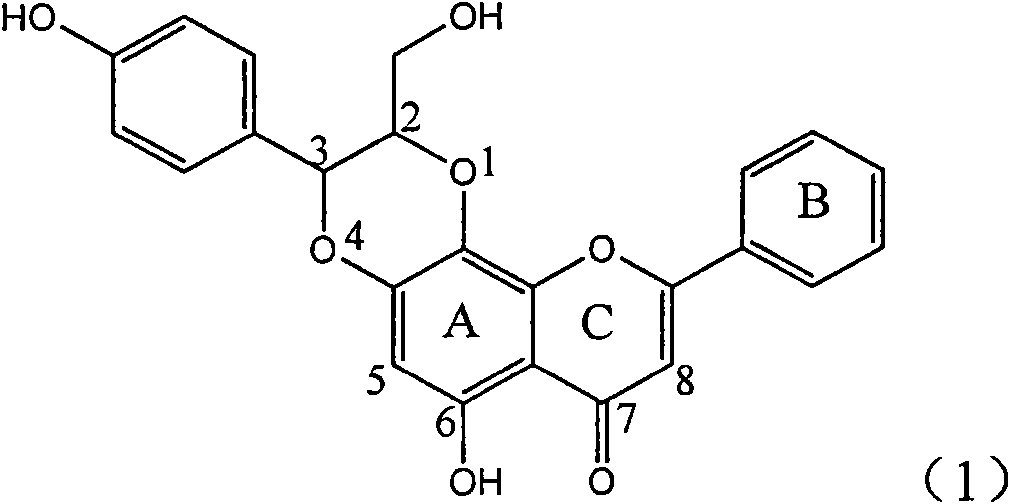
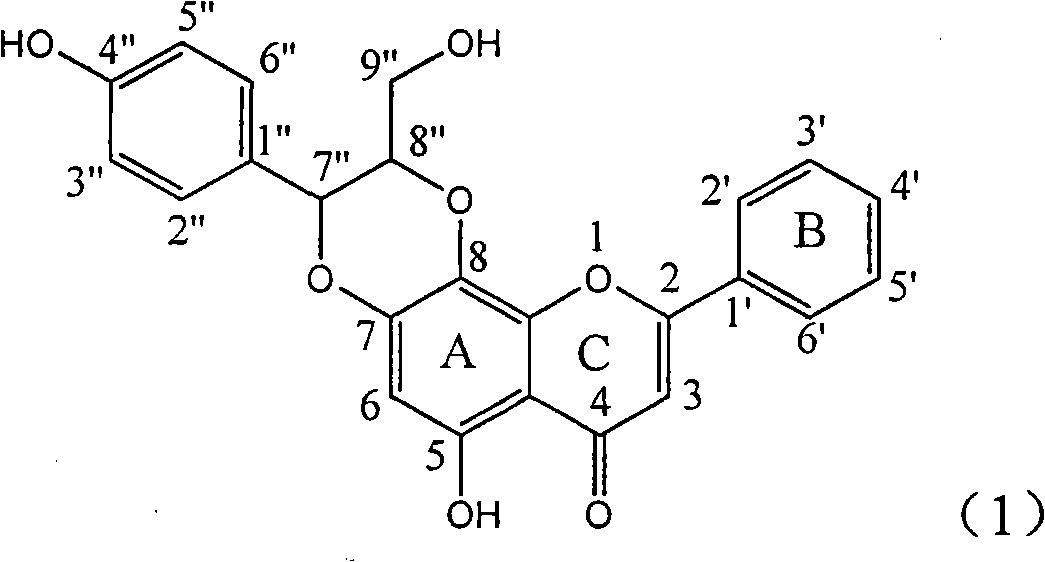
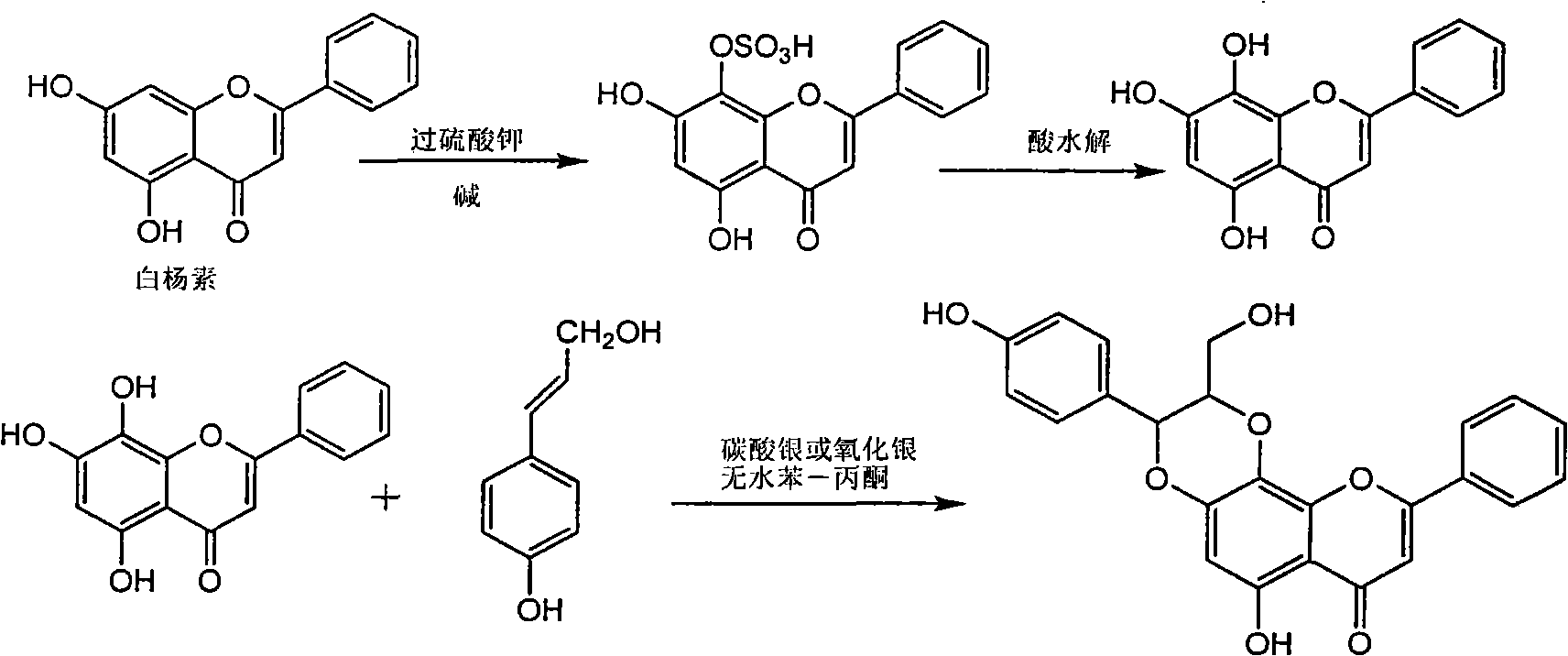
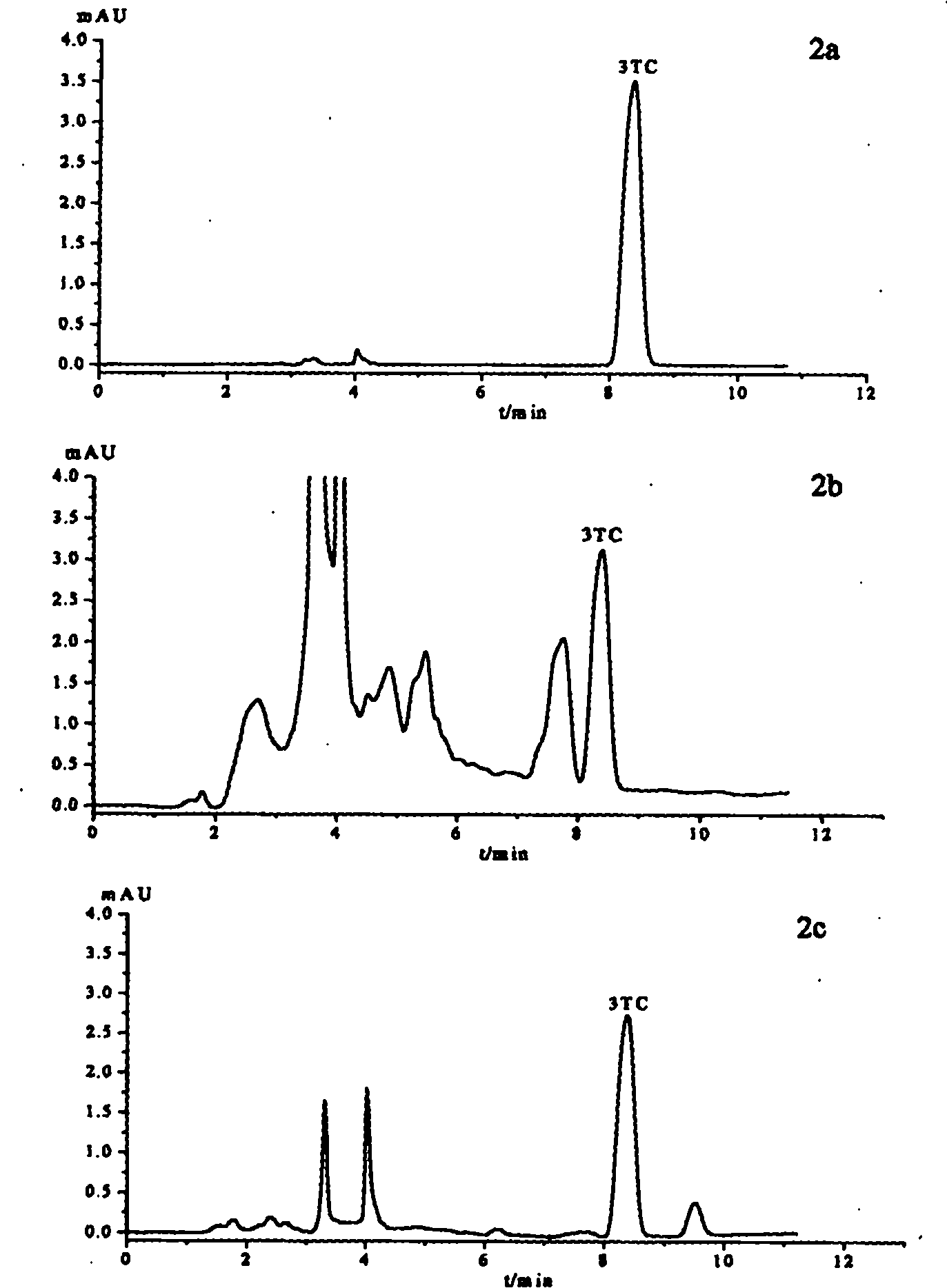
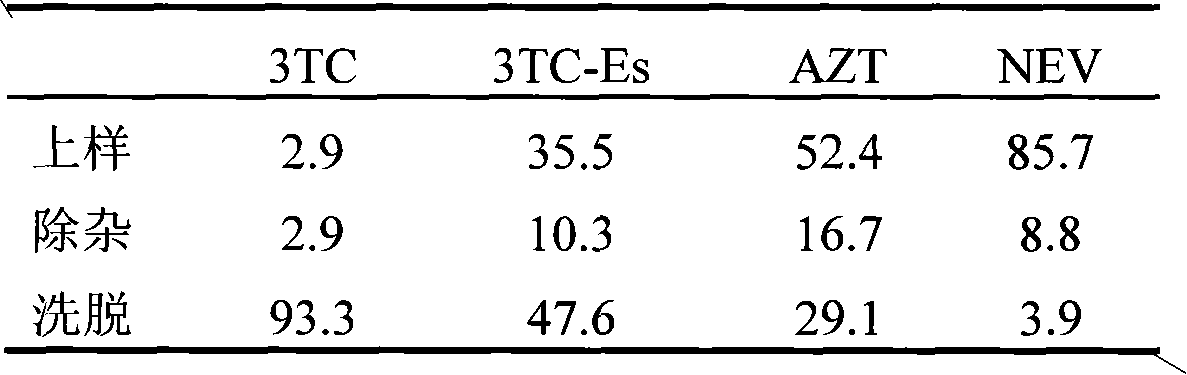
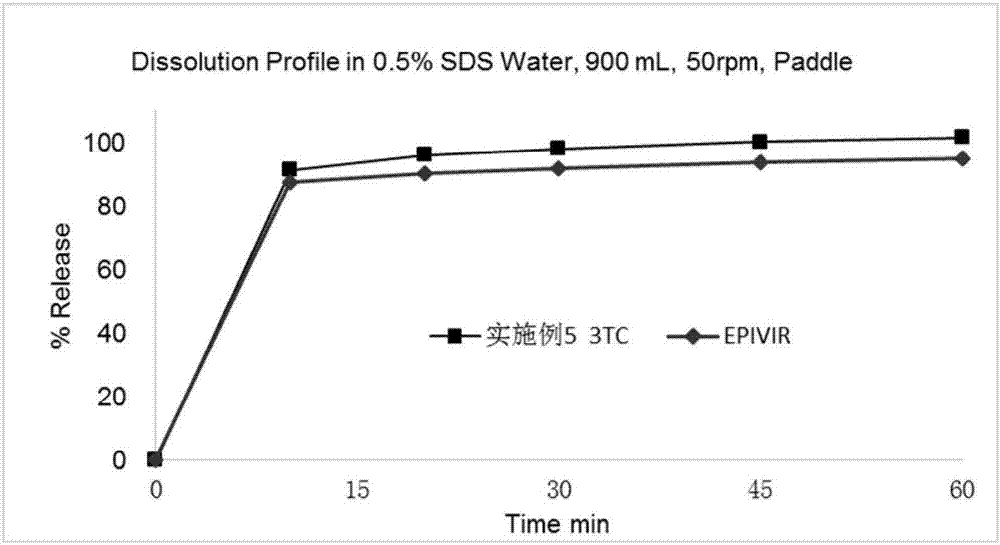
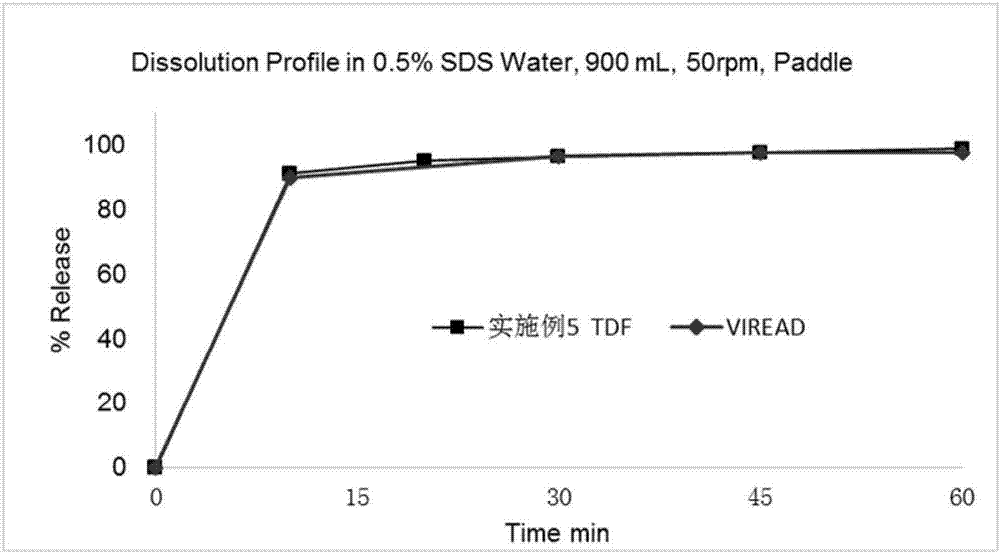
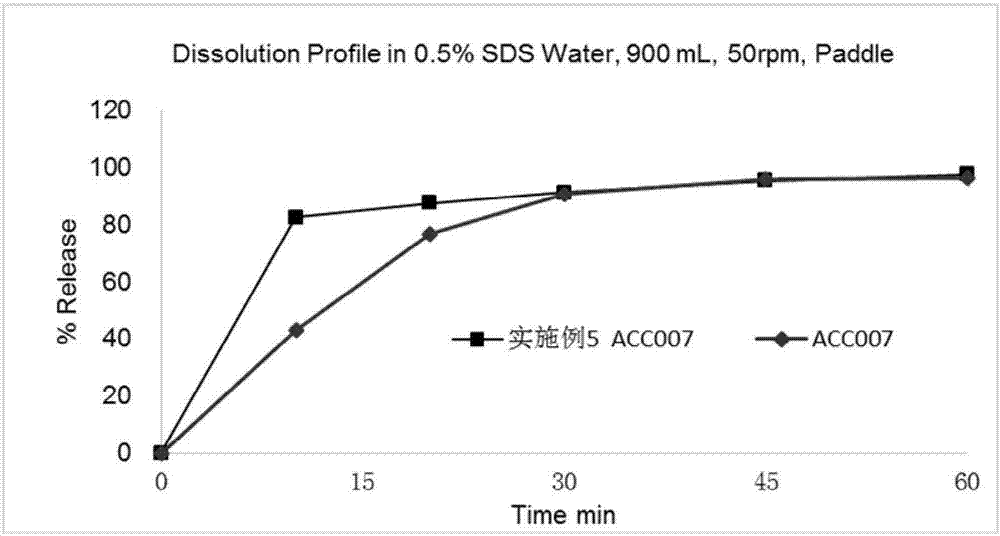
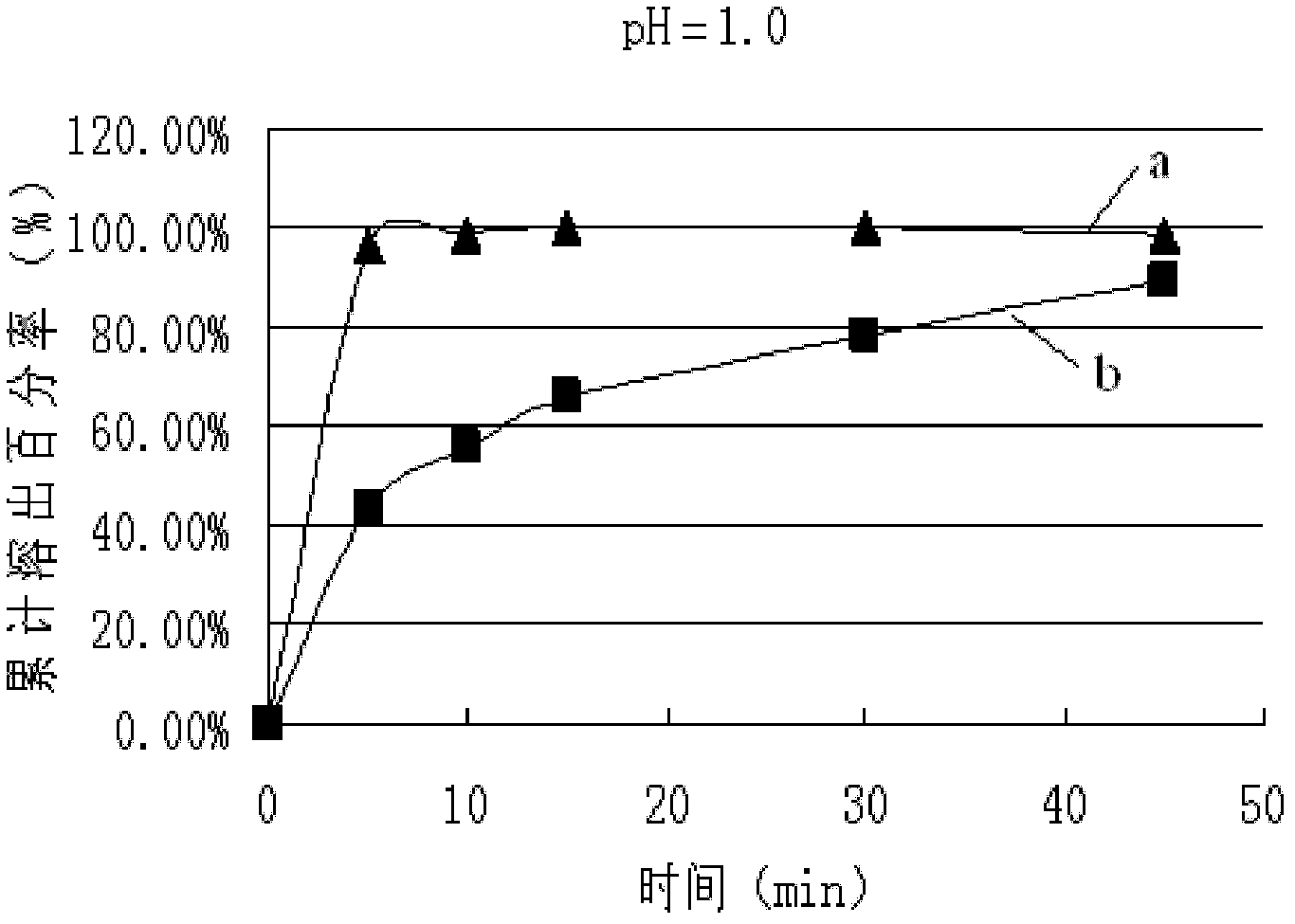
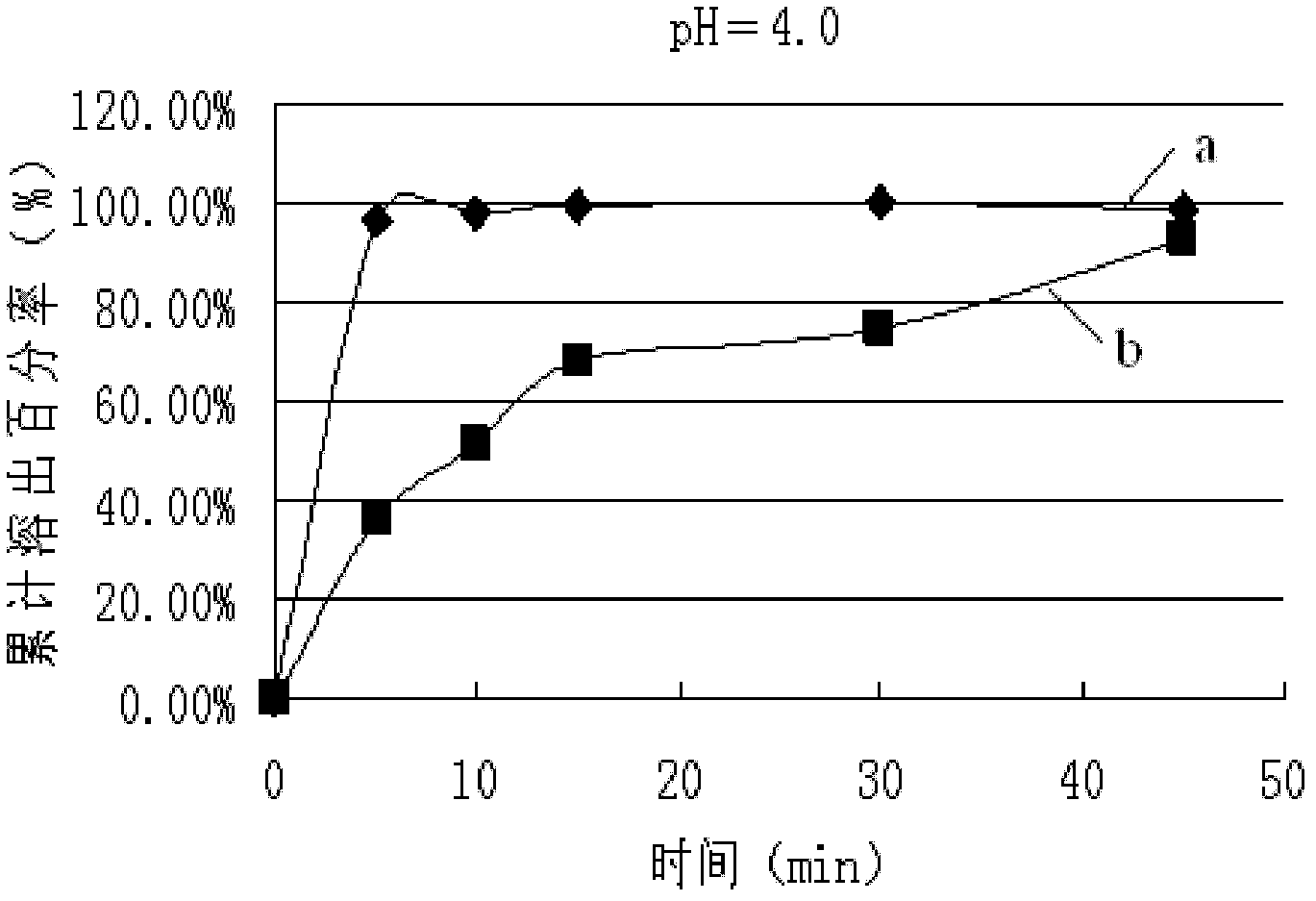
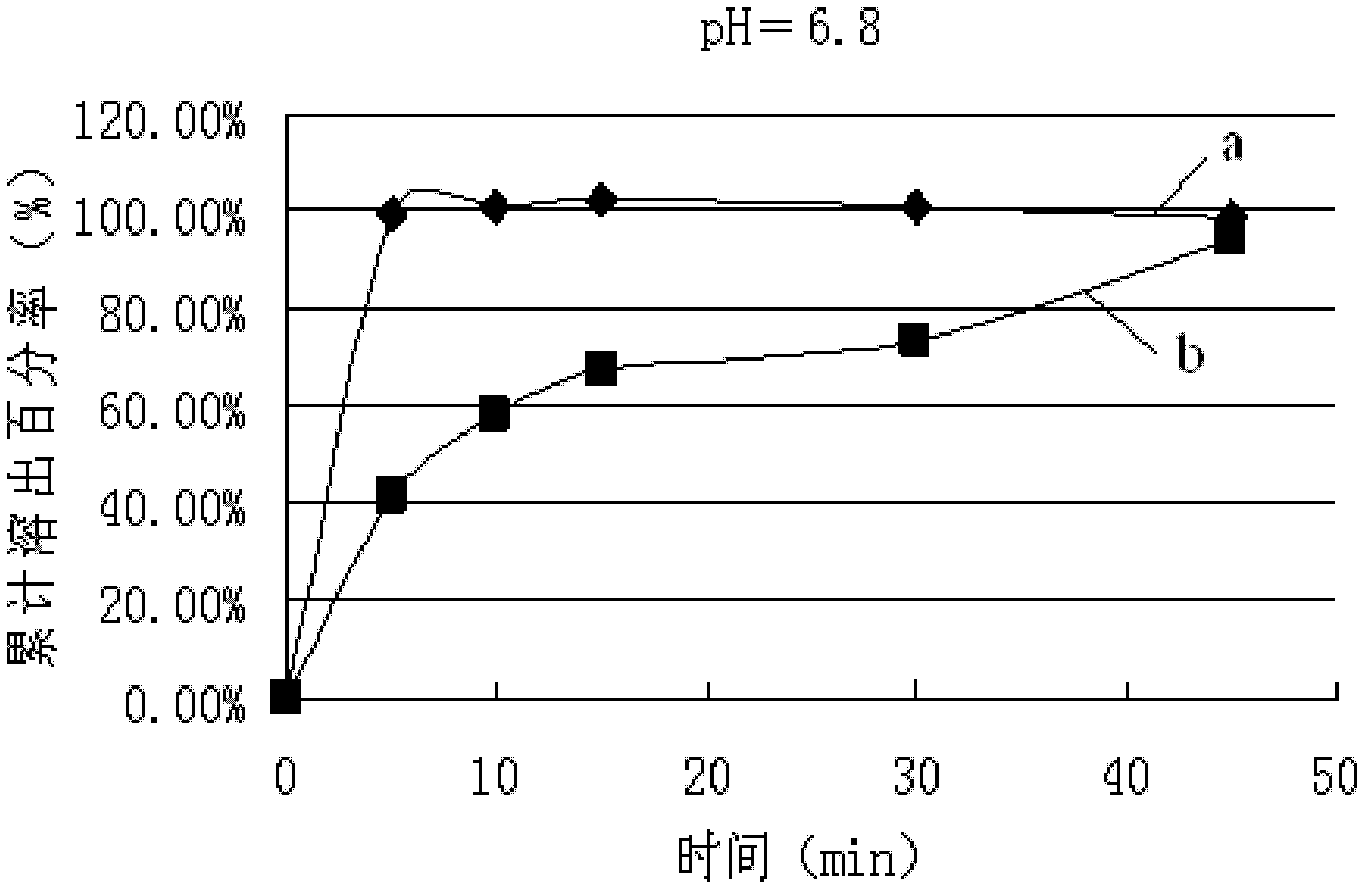
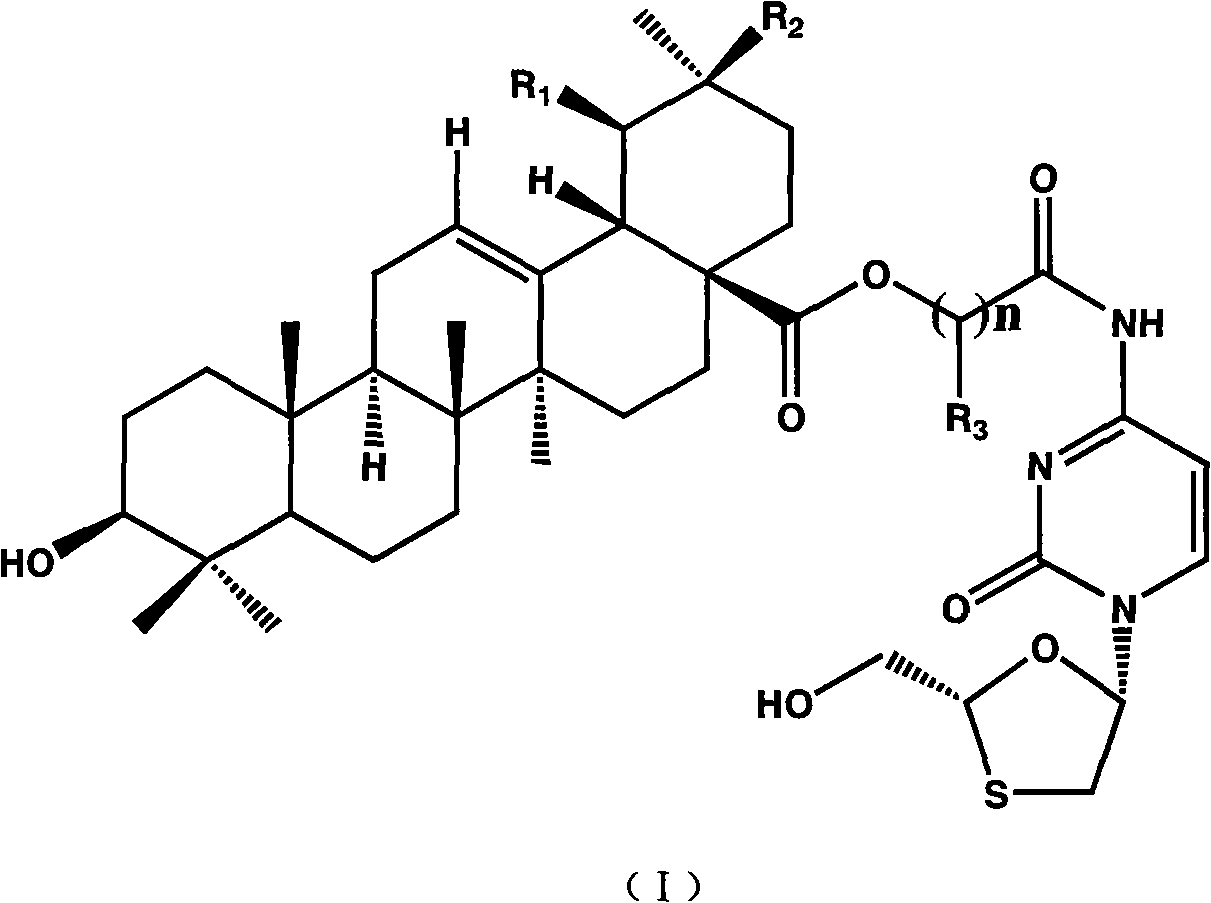
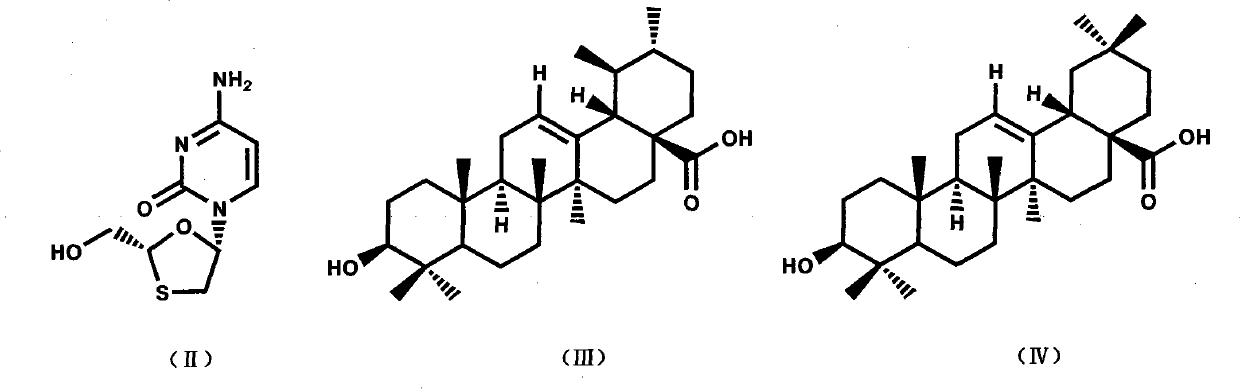
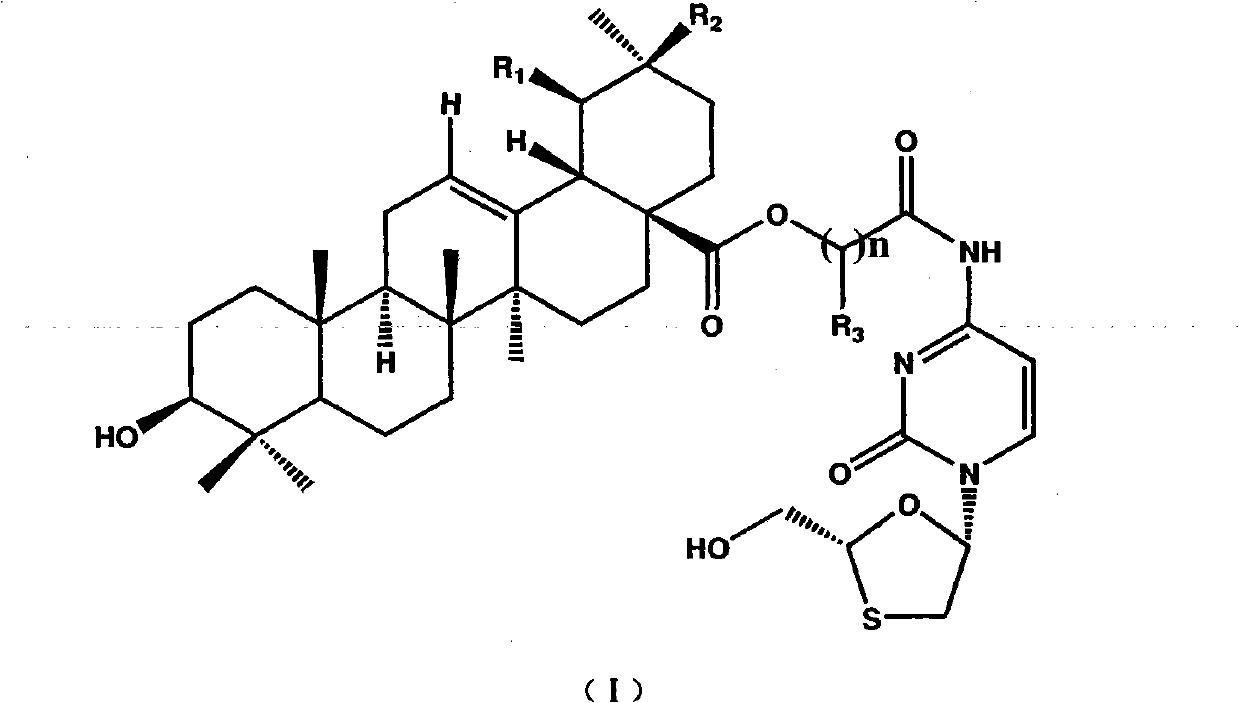
Patents
Literature
261 results about "Lamivudine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
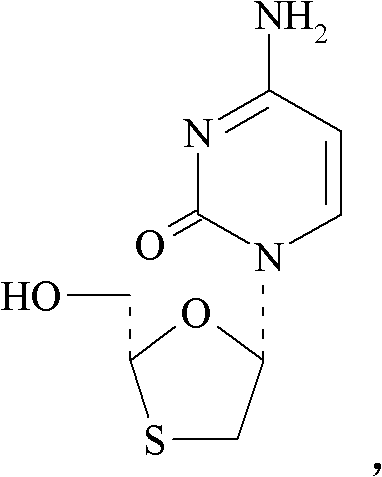
Lamivudine-HBV is used to treat hepatitis B infection.
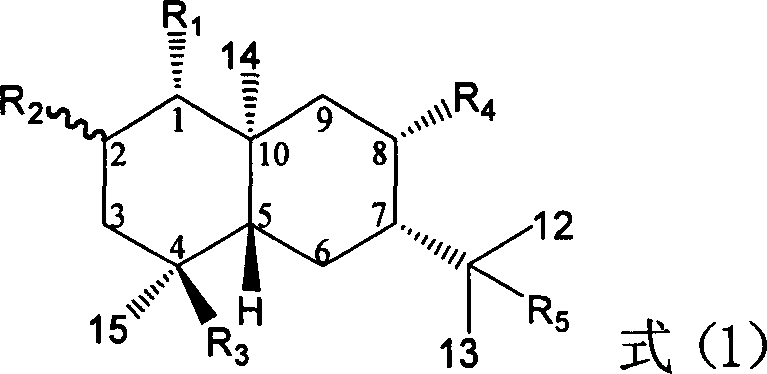
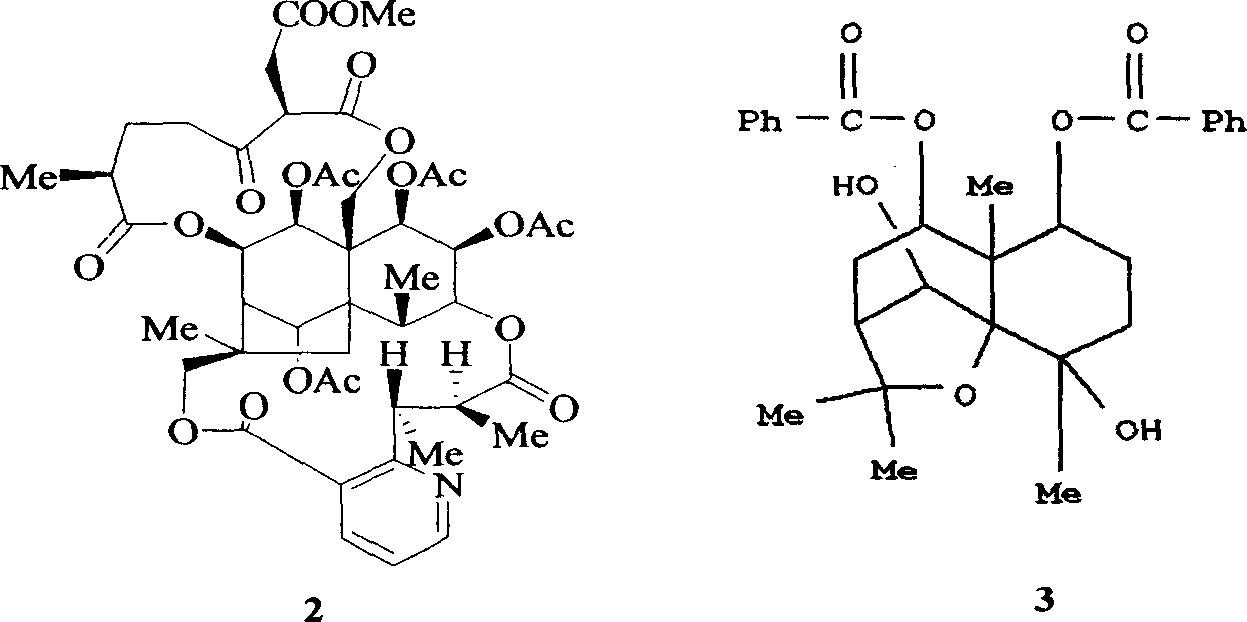
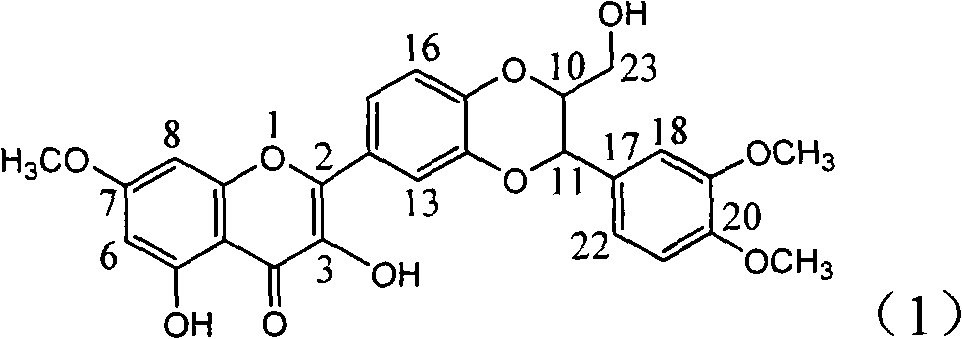
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
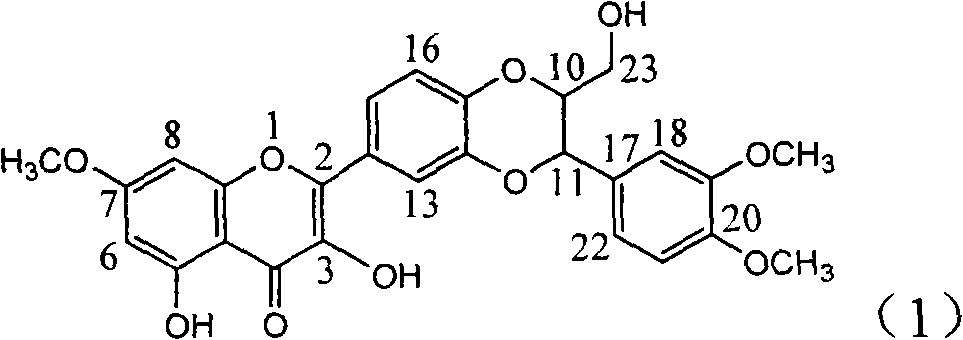
Medical use of 1beta-keto-5, 11(13)-diene eudesmane-12-acid for inhibiting hepatitis B virus
InactiveCN1927197APrevention and treatment of viral hepatitis BHBsAg reductionOrganic active ingredientsSugar derivativesDiseasePositive control
The invention involves A 1- beta-keto-5, 11(13)-diene eudesmane-12-acidum who has the structure as formula (1) shows and its medical salt, or solvate and its drug combinations and its medical usage in reducing hepatitis B surface antgien and inhibiting replication activity of aethyl- hepatovirus HBV-DNA medicinal. The invention compounds has strong inhibitory action in hepatitis B surface antgien (HBsAg) externalized by HepG2.2.15 cells and replication of aethyl- hepatovirus deoxyribonucleotide (HBV-DNA) in vitro, its inhibiting ability against HBsAg surpasses that of positive control Lamivudine in the same dose; it has obvious inhibiting ability against replication of aethyl- hepatovirus HBV-DNA under the concentration of 100 mu g / mL,20 mug / mL and 4 mug / mL DNA, it belongs to anti- aethyl- hepatovirus natural products of superactive non-nucleoside, can be expected for producing drugs for treating aethyl- hepatovirus infected disease.
Owner:WENZHOU MEDICAL UNIV
Use of acetamide dehydrogenation silibinin as medicament for treating viral hepatitis B
InactiveCN101829091APowerful removalInhibitory activityOrganic active ingredientsDigestive systemAntigenDisease
The invention relates to the use of acetamide dehydrogenation silibinin as a medicament for treating viral hepatitis B, in particular to the use of dehydrogenation silibinin esters flavonoid lignanoid replaced by A ring methoxy formyl amine or pharmaceutically acceptable salt as the medicament for eliminating HBsAg (hepatitis B surface antigen) and HBeAg (hepatitis Be antigen) and restraining copy of HBV DNA. The cetamide dehydrogenation silibinin can obviously restrain the HBsAg and HBeAg activity, and the strengths for eliminating the HBsAg and HBeAg are 90.5% and 63.6% at the concentration of 20 microgramme / milliter and are 5.6 times and 3.8 times more than positive contrast medicament alpha-interferon. Meanwhile, the restraining rate to the HBV DNA is 90.4% at the concentration, is 12% higher than lamivudine, and is 2.4 times more than a- interferon. Therefore, the flavonoid lignanoid or the pharmaceutically acceptable salt can be expected for treating hepatitis B virus infection as the non-nucleoside medicament.
Owner:DALI UNIV
Application of diamine formyl dehydrogenated silybin serving as medicament for curing viral hepatitis B
InactiveCN101829090AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
The invention relates to application of diamine formyl dehydrogenated silybin serving as a medicament for curing viral hepatitis B, in particular to application of a flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or pharmaceutically acceptable salts thereof in preparation of a medicament for clearing HBsAg and HBeAg and a medicament for inhibiting HBV DNA replication. The flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents has extremely high HBsAg and HBeAg inhibiting activities; when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at a concentration of 20 mu g / ml, the inhibition rates of the HBsAg and the HBeAg are respectively 94.4 percent and 95.7 percent which exceed 5.9 times and 5.7 times those of a positive control alpha-interferon; and simultaneously the inhibition rate of the HBV DNA is 99.7 percent when the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is at the same concentration, and the inhibition activity of the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents is higher than that of lamivudine and the alpha-interferon. In summary, the flavonolignan of dehydrogenated silibinin esters of which the ring A and the ring E have diamine formyl-methoxyl substituents or the pharmaceutically acceptable salts thereof can be expected for preparing non-nucleoside medicaments for clearing the HBsAg and the HBeAg, inhibiting the HBV DNA replication, and curing the hepatitis B virus infection diseases.
Owner:DALI UNIV
Application of aromatic carbamoyl dehydro-silibinin as medicament for treating viral hepatitis B
InactiveCN101829086AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
The invention relates to application of aromatic carbamoyl dehydro-silibinin as a medicament for treating viral hepatitis B, in particular to application of todehydro-silibinin flavonolignans with a ring A and a ring E which are substituted by double base aromatic carbamoyl methoxyl and pharmaceutically acceptable salt thereof for preparing medicaments for removing HBsAg and HBeAg and medicaments for inhibiting HBV DNA. The todehydro-silibinin flavonolignans has extremely obvious activity on inhibiting the HBsAG and the HBeAg, has the intensity of 46.2 percent and 68.9 percent for respectively removing the HBsAG and the HBeAg in the presence of the concentration of 100 microgram / milliliter, which is 2.9 times and 4.1 times higher than that of positive control medicament alpha-interferon, and has the inhibition ratio of 96 percent on HBV DNA in the presence of the concentration of 100 microgram / milliliter, which is higher than that of lamivudine and the alpha-interferon. Accordingly, the flavonolignans and the pharmaceutically acceptable salt thereof can be expected to be used for preparing non-nucleoside medicaments applied for removing HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
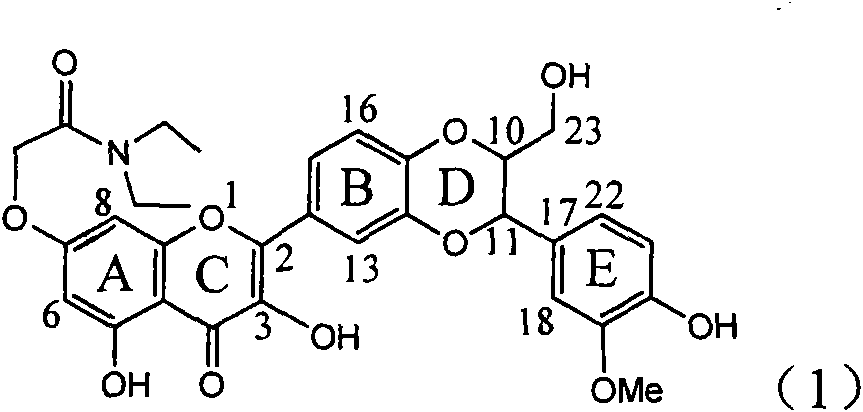
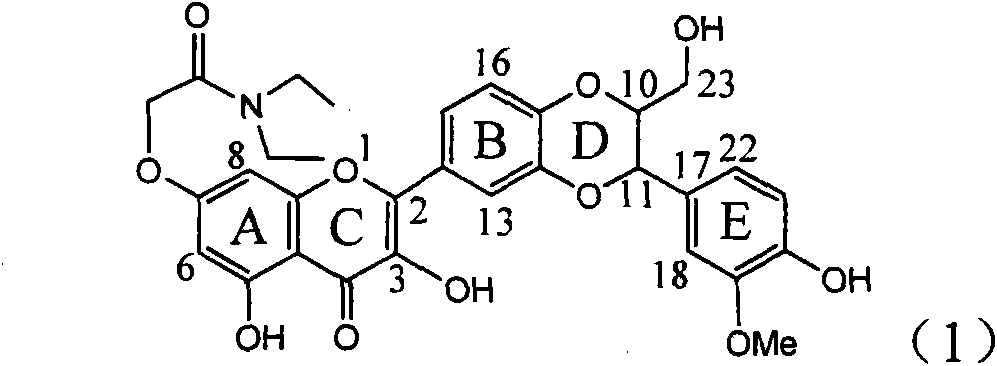
Application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B
InactiveCN101829104AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
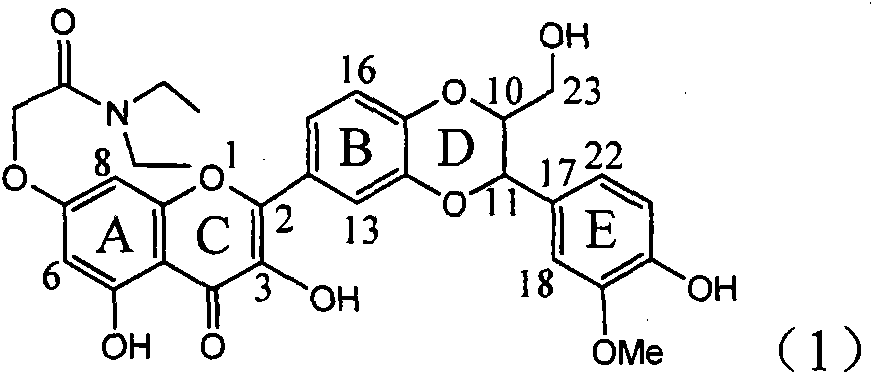
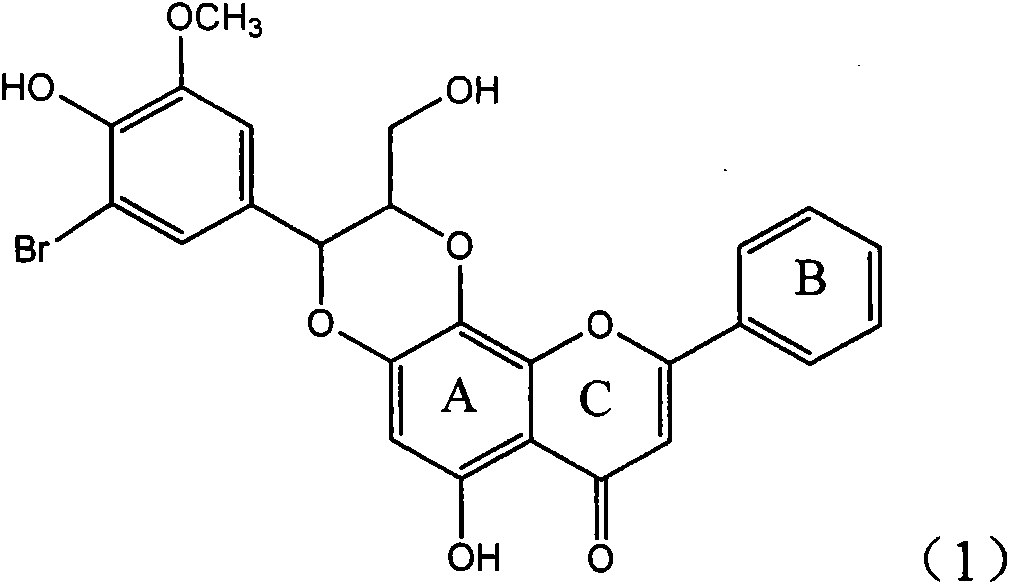
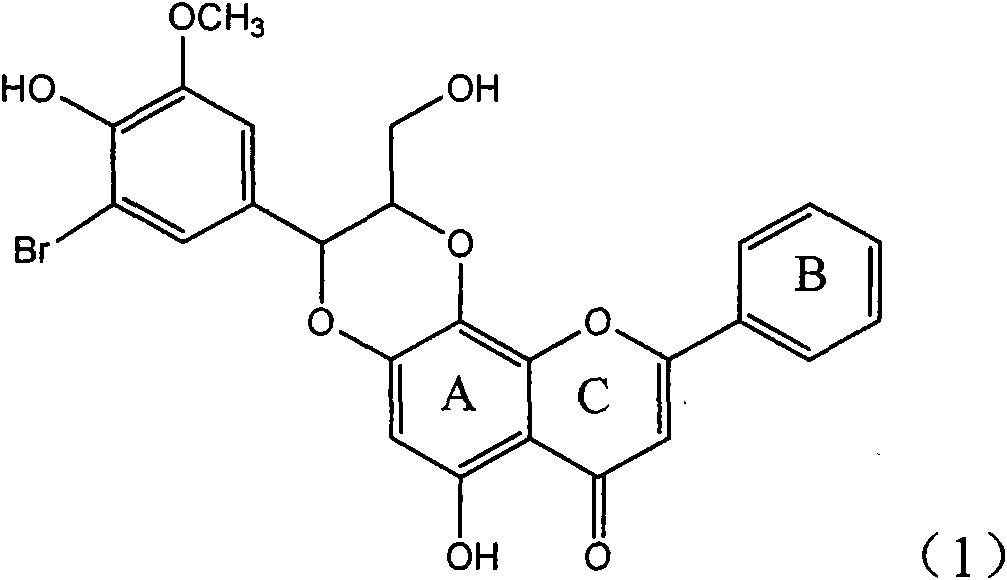
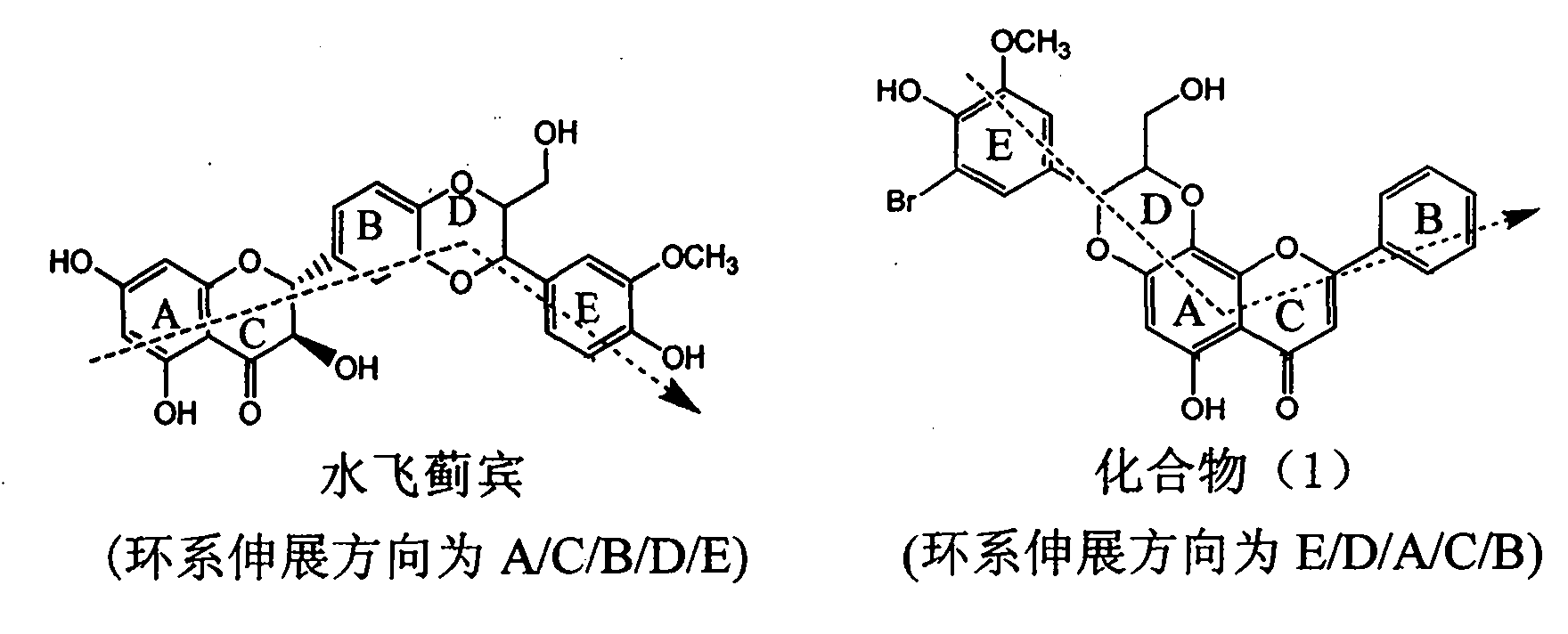
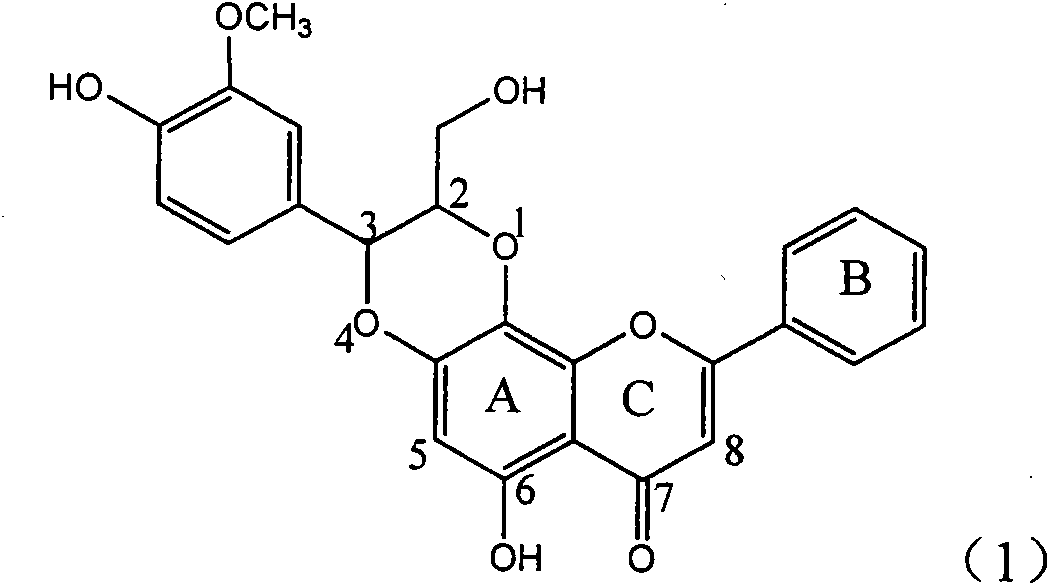
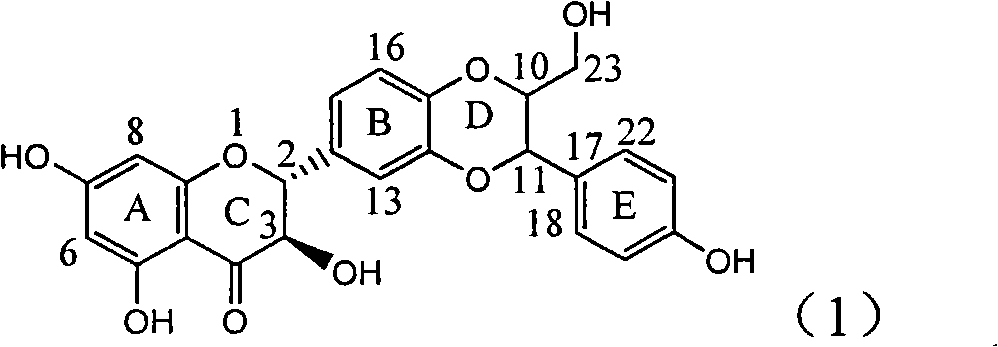
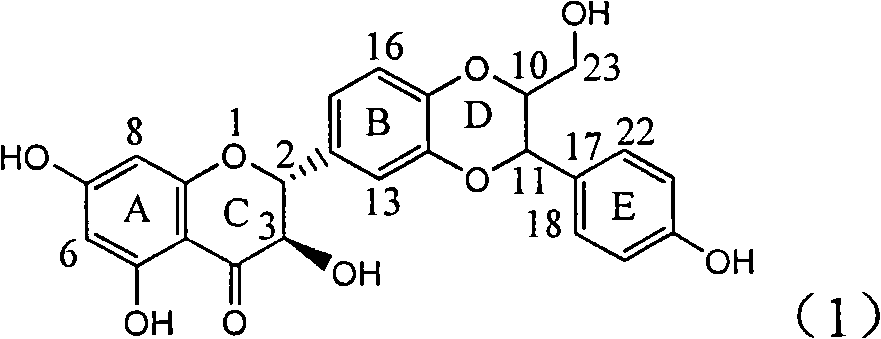
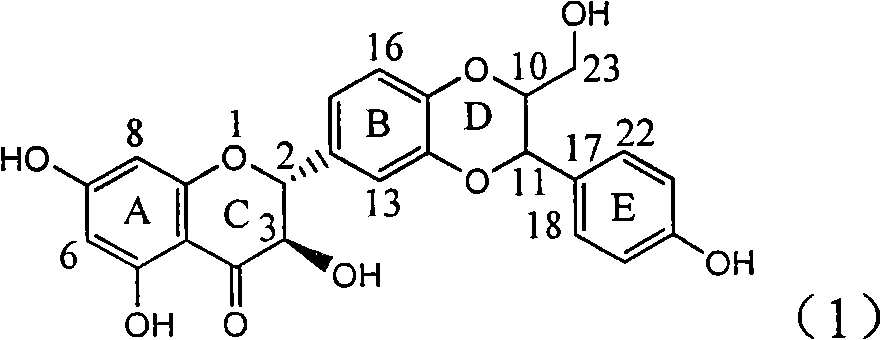
The invention relates to application of ring A coupling flavonolignan in preparing medicaments for treating viral hepatitis B, in particular to application of a compound of the formula (1) or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away HBsAg (Hepatitis B Surface Antigen) and HBeAg (Hepatitis B e Antigen) and suppressing the HBV (Hepatitis B Virus) DNA replication. The intensities of the flavonolignan for clearing away the HBsAg and the HBeAg are respectively 29.4 percent and 29.1 percent in the presence of a concentration of 20 micrograms / milliliter, which is respectively 1.8 times and 1.7 times of the corresponding activity of a positive control medicament (10,000 units / milliliter of alpha-interferon). What is even more exciting is that in the presence of the concentration, the suppression rate of the flavonolignan to the HBV DNA is higher than 83 percent, which is higher than that of Lamivudine which is a positive control and is 2.2 times of that of the alpha-interferon to the HBV DNA. Accordingly, the flavonolignan and the pharmaceutically acceptable salt thereof are indicated to be capable of being expected to be used for preparing non-nucleoside medicaments for clearing away the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infection diseases.
Owner:DALI UNIV
Application of flavone lignan (+/-) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B
InactiveCN101953827AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseLignan
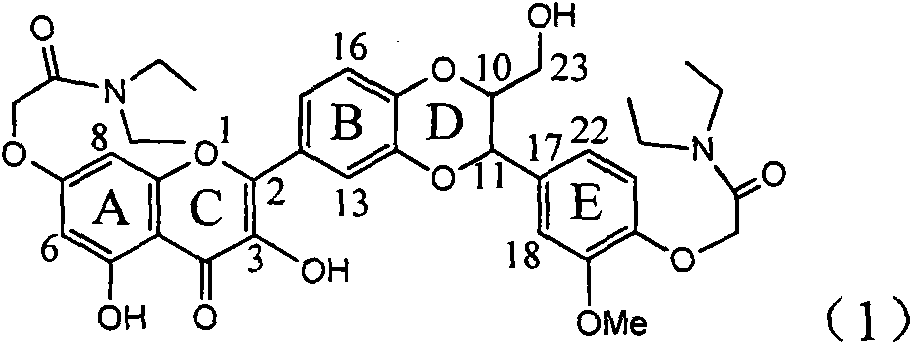
The invention relates to application of flavone lignan (+ / -) Scutellaprostin A in preparing medicaments for treating viral hepatitis type B, in particular to a compound with the formula (1) or pharmaceutically-acceptable salts thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV (Hepatitis B Virus) DNA replication. In the invention, the intensities of the compound for clearing the HBsAg and the HBeAg under the concentration of 20 micrograms / milliliter respectively reach 81.8 percent and 81.9 percent, which are respectively 5.1 times and 4.8 times as high as the corresponding activity of alpha-interferon used as a positive contrast medicament; and what is more exciting, when the compound has the concentration, the compound performs a suppression ratio higher than 81 percent, and the value is also higher than that of both lamivudine and alpha-interferon. Accordingly, the flavone lignan or the pharmaceutically-acceptable salts can be expectably used for preparing nucleoside medicaments for clearing the HBsAg and the HBeAg, suppressing the HBV DNA replication and treating HBV infected diseases.
Owner:DALI UNIV
Application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV)
InactiveCN101829085AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseasePositive control
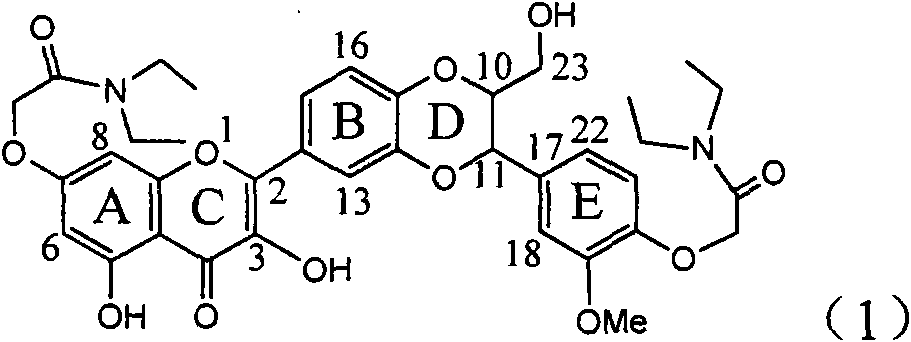
The invention relates to application of ring A dioxane flavonolignan in preparing medicaments for resisting hepatitis B viruses (HBV), in particular to application of ring A dioxane coupling type flavone lignan or a pharmaceutically acceptable salt thereof in preparing medicaments for clearing away hepatitis B e antigen (HBeAg), suppressing the HBV DNA replication and treating HBV infection diseases. The flavonolignan has certain activity on resisting the HBeAg, and the intensity of the flavonolignan for clearing away the HBeAg is higher than that of Lamivudine which is a positive control and close to that of 10,000 units / milliliter of alpha-interferon. Meanwhile, the suppression ratio of the compound to the HBV DNA replication is higher than 80 percent in the presence of a concentration of 100 micrograms / milliliter. The pharmacodynamical results indicate that the flavonolignan or the pharmaceutically acceptable salt thereof can be expected to be used for preparing the medicaments for clearing away the HBeAg, suppressing the HBV DNA replication and treating the HBV infection diseases.
Owner:DALI UNIV
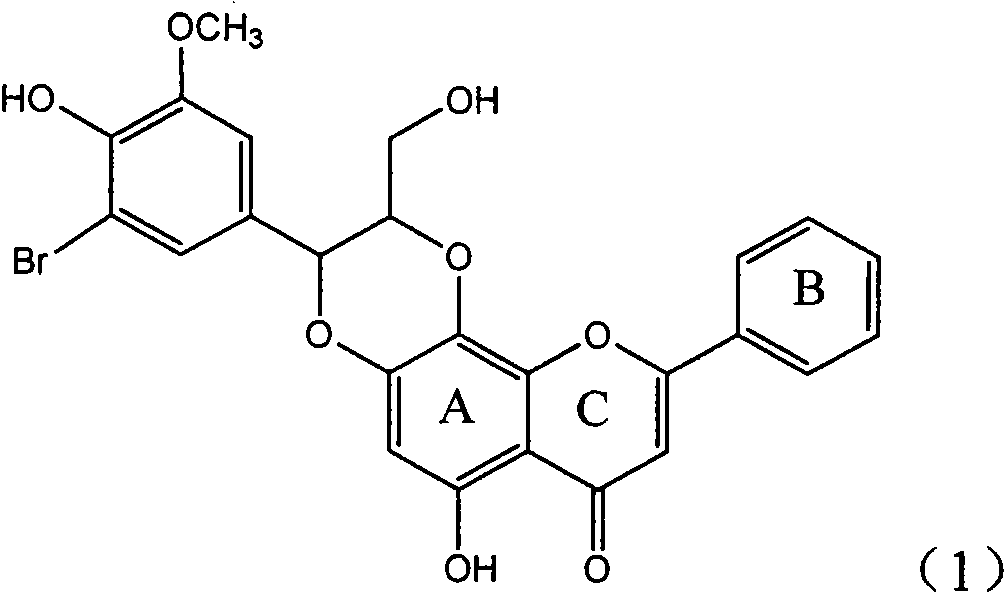
Application of E-ring demethoxy-silibinin for preparing medicament for treating viral hepatitis B
InactiveCN101912383AConvenient sourceThe source is easy to getOrganic active ingredientsDigestive systemDiseasePositive control
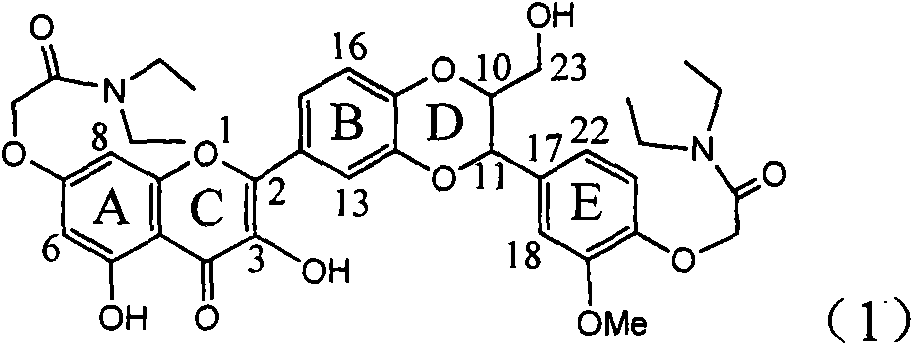
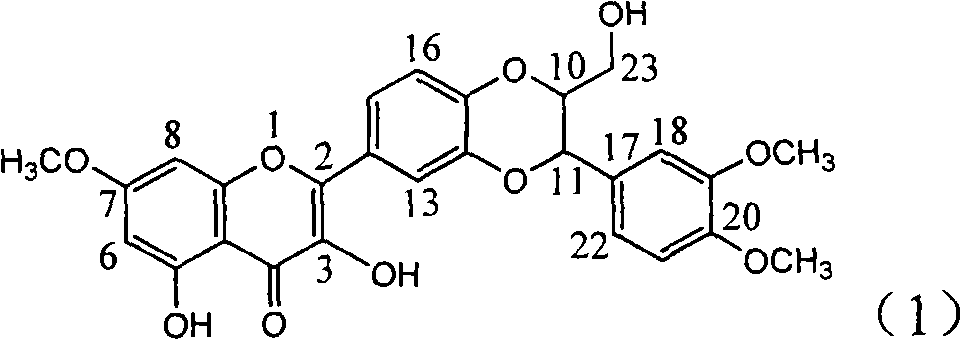
The invention relates to application of E-ring demethoxy-silibinin for preparing medicaments for treating viral hepatitis B, and particularly to application of compound in formula (1) and pharmaceutically acceptable salt thereof for preparing medicaments for clearing HBsAg and HBeAg and suppressing HBV DNA replication. The invention has extremely superactive activity for suppressing the HBsAg and HBeAg; in the presence of the concentration of 20 microgram per millilitre, the intensities for clearing the HBsAg and HBeAg are 95.0% and 34.4% respectively, which are 5.9 and 2.0 times corresponding activity of a positive control medicament alpha-interferon; and it should be noticed that the suppression ratio of the medicament for HBV DNA at the concentration is about 91.5%, which is 13% higher than lamivudine and 2.4 times alpha-interferon suppression activity. In summary, the flavonolignans or pharmaceutically acceptable salt thereof can be prospectively used for preparing non-nucleoside medicaments for clearing the HBsAg and HBeAg, suppressing HBV DNA replication and treating hepatitis B virus infection disease.
Owner:DALI UNIV
Application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B
InactiveCN101912385AConvenient sourceThe source is easy to getOrganic active ingredientsAntiviralsDiseaseNucleoside Reverse Transcriptase Inhibitor
The invention relates to application of dimethyl dehydrated silybin in preparing medicaments for treating virus hepatitis B, in particular to the application of 7 and 20-position methyl substituted dehydrated silybin or pharmaceutically acceptable salts thereof in preparing medicaments for removing HBsAG and HBsAg and medicaments for inhibiting HBV DNA replication. The dehydrated silybin has remarkably HBsAg and HBeAg inhibiting activity, wherein the strength for removing the HBsAg and the HBeAg at the concentration of 20 milligram / milliliter is 88.9 percent and 84.1 percent respectively, which are 5.5 times and 5.0 times that of a positive contrast medicament. More importantly, the dehydrated silybin shows the HBV DNA inhibition ratio of about 99.6 percent at the concentration of 20 milligram / milliliter, the activity exceeds lamivudine by 23 percent, which is 2.6 times that of interferon. Therefore, favonolignan or pharmaceutically acceptable salts thereof can be predictably used for preparing the non-nucleoside reverse transcriptase inhibitor medicaments for removing the HBsAg and HBeAg, inhibiting HBV DNA replication and treating hepatitis B virus infection diseases.
Owner:DALI UNIV
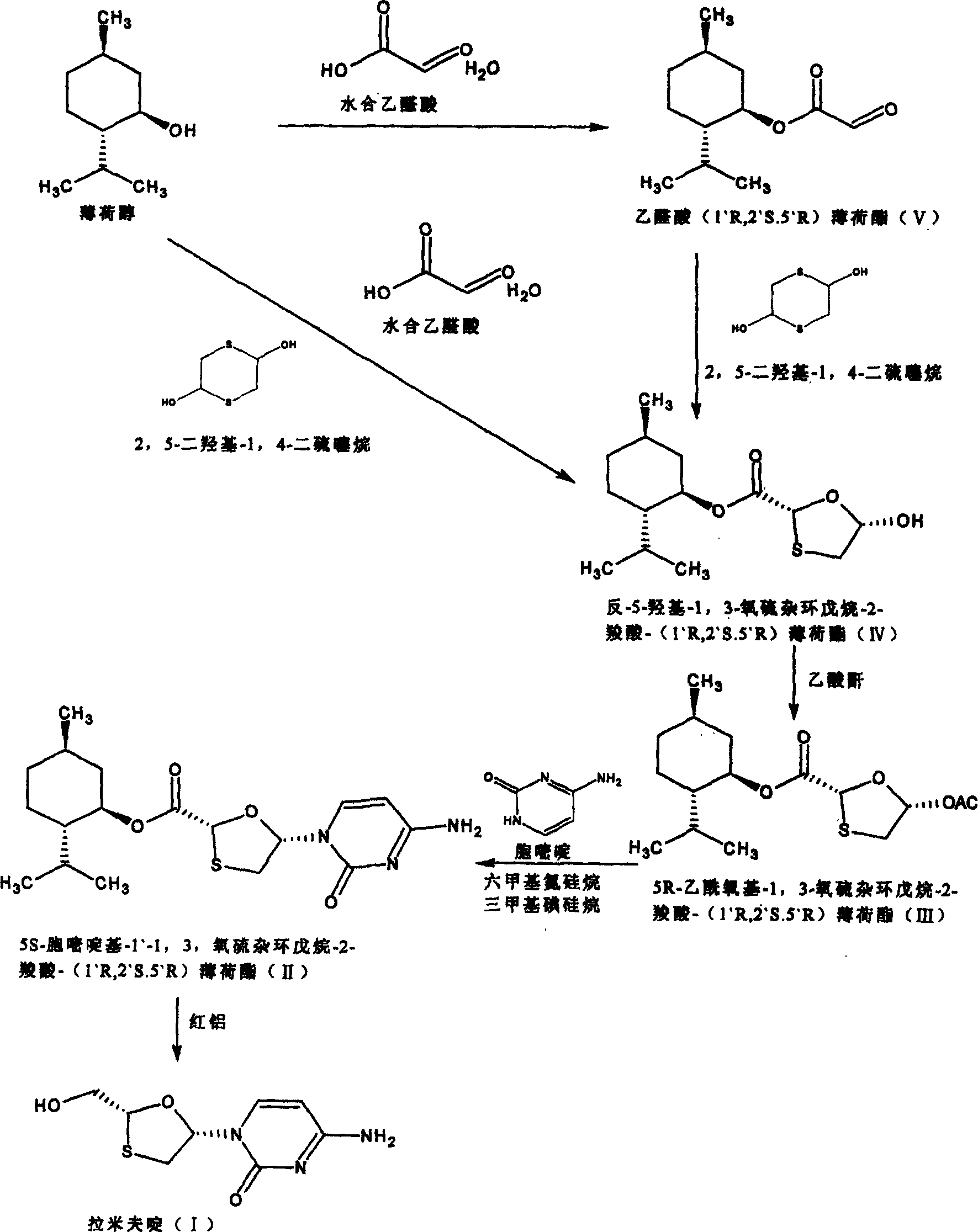
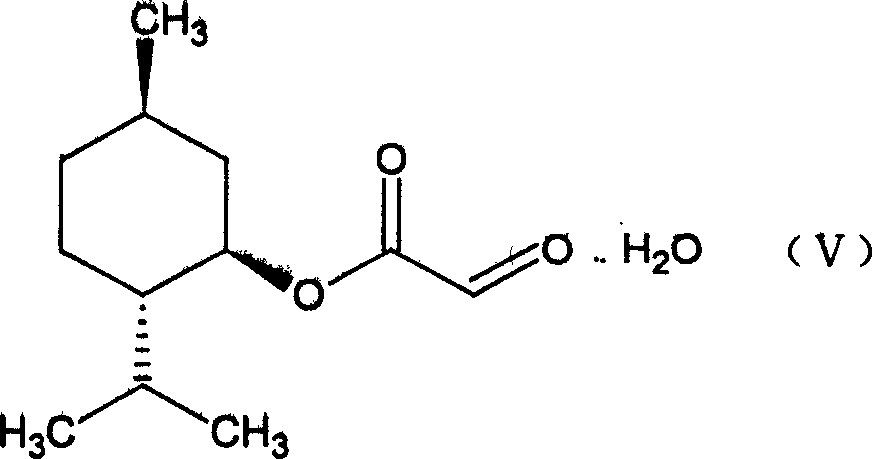
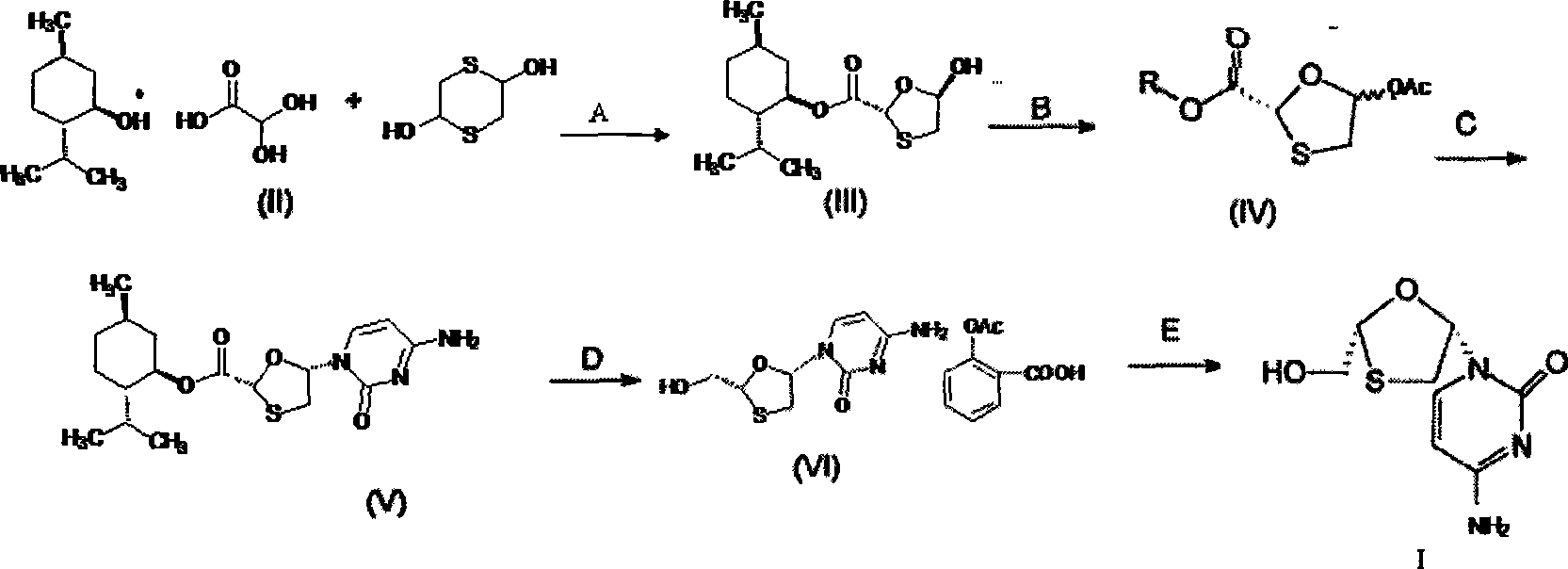
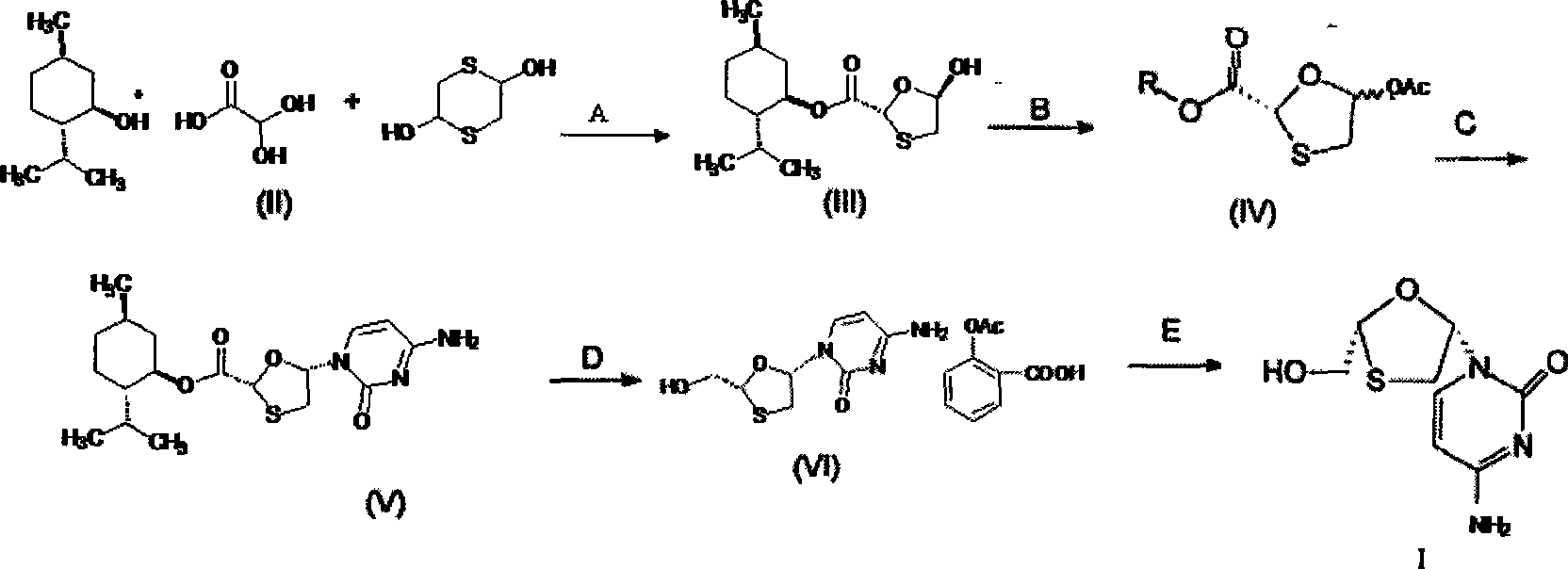
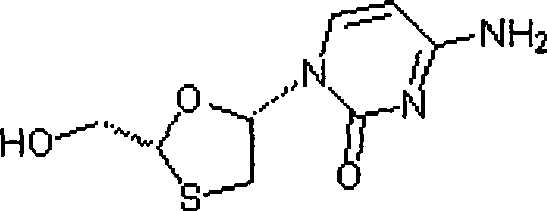
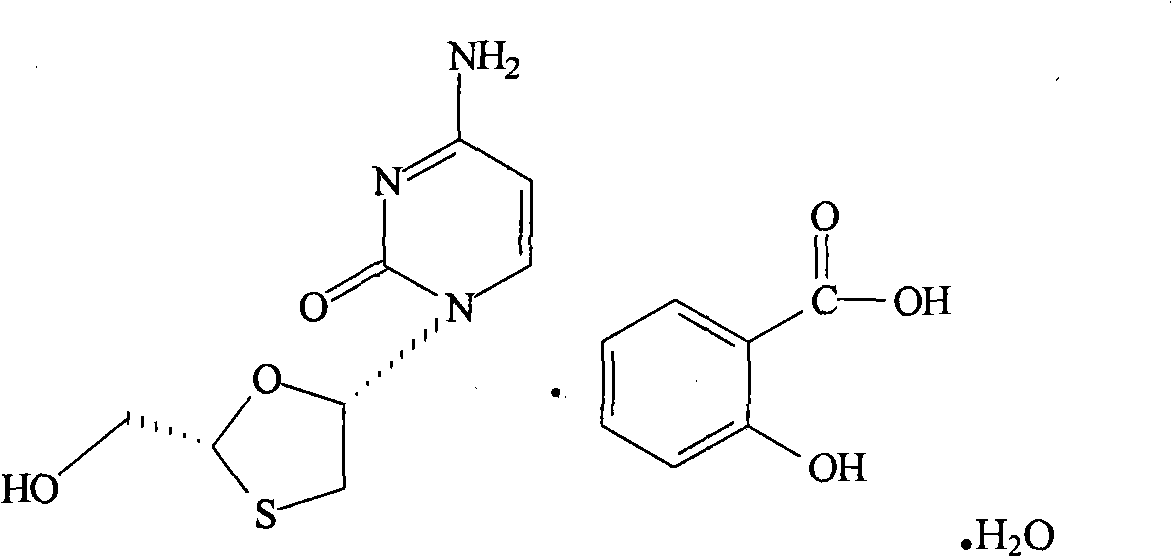
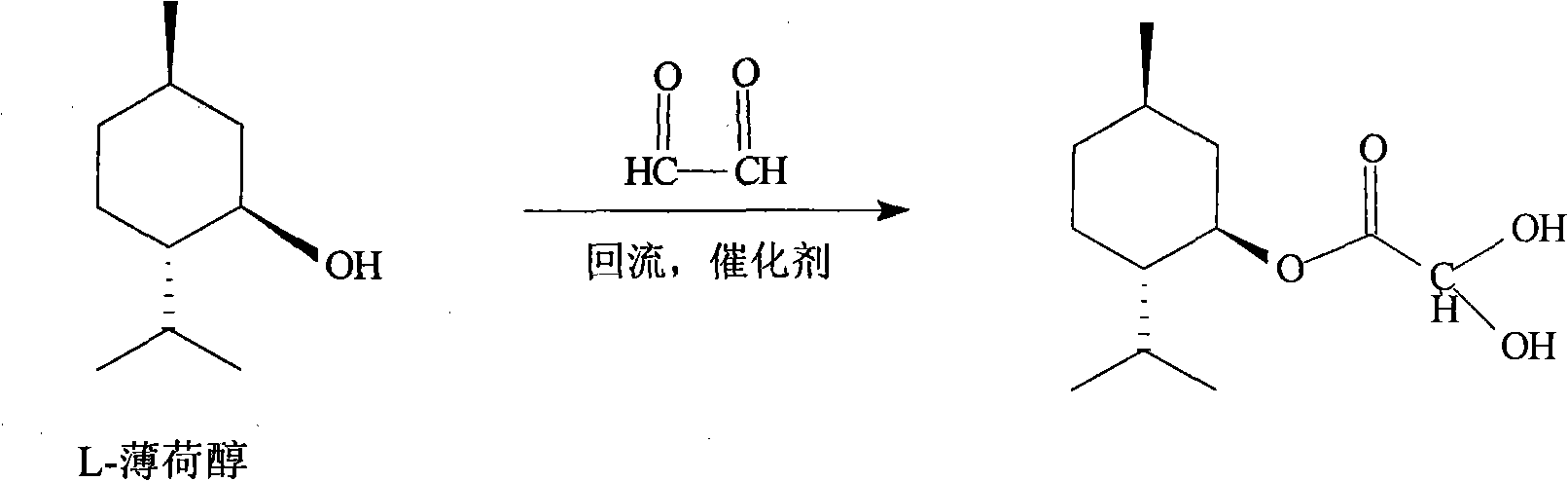
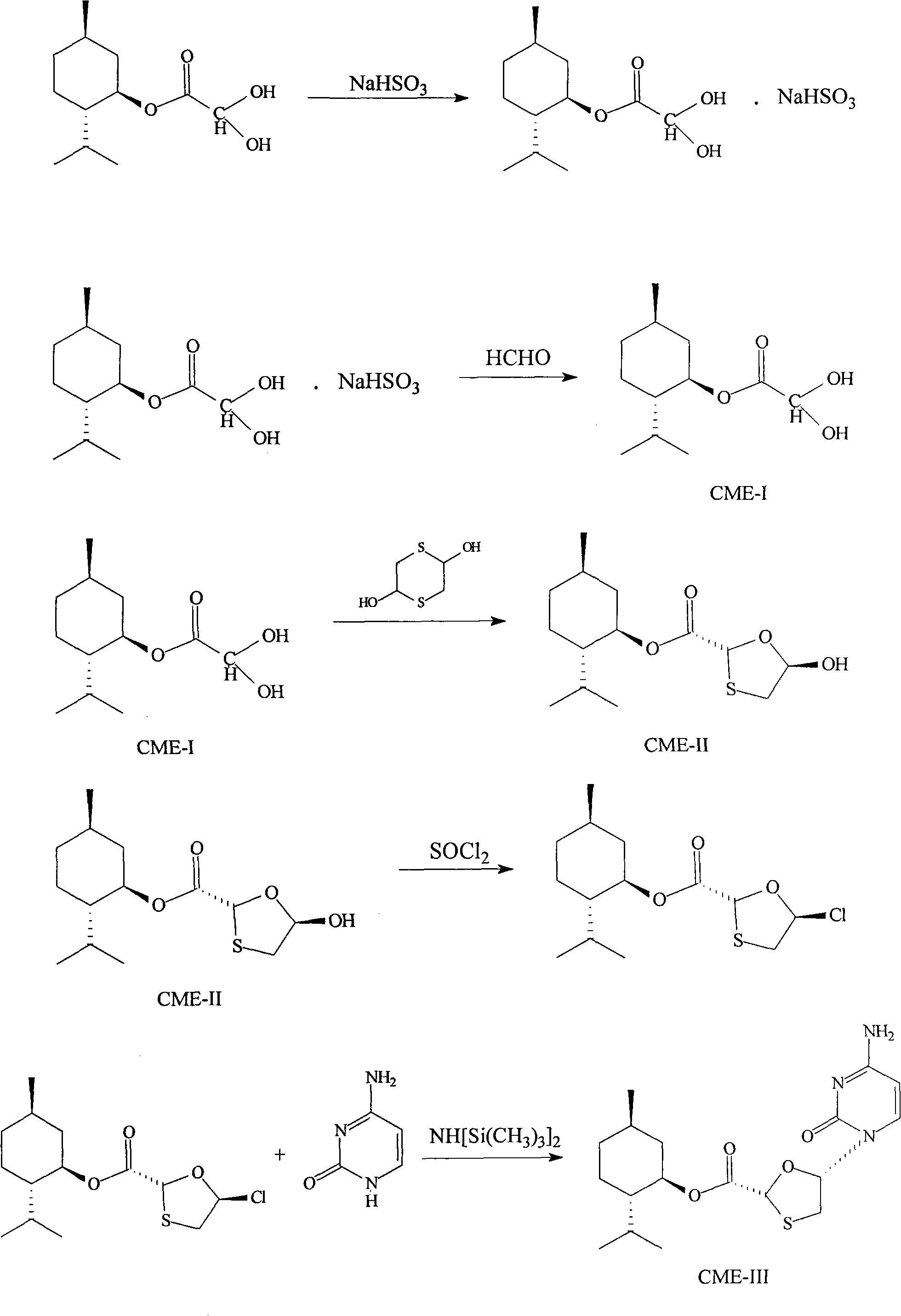
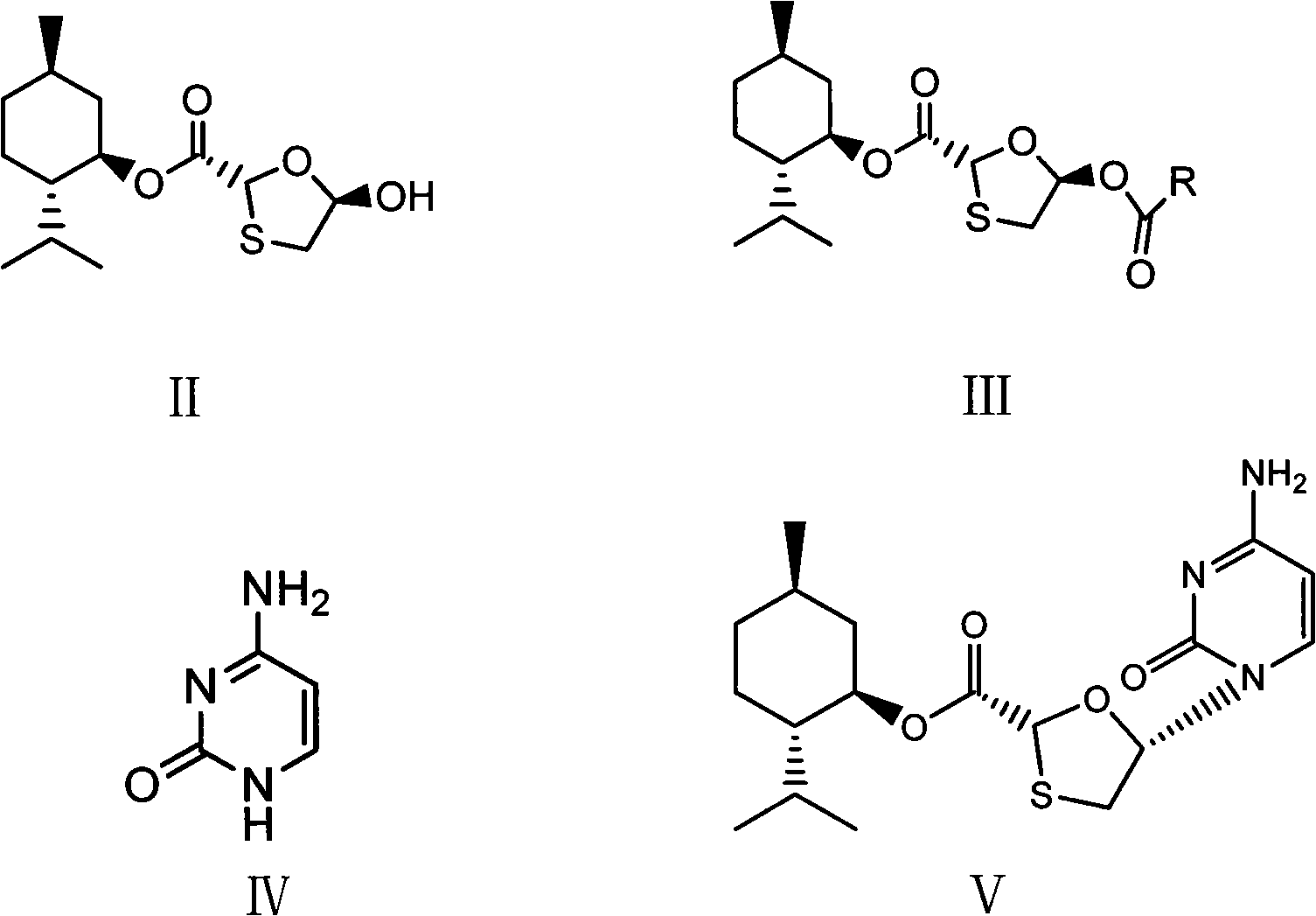
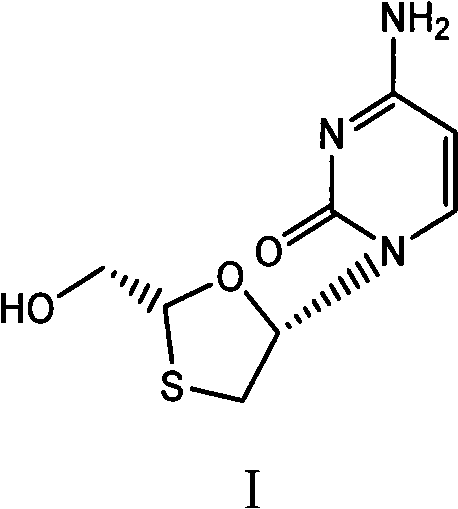
Suitable industrialized method of preparing Lamivudine
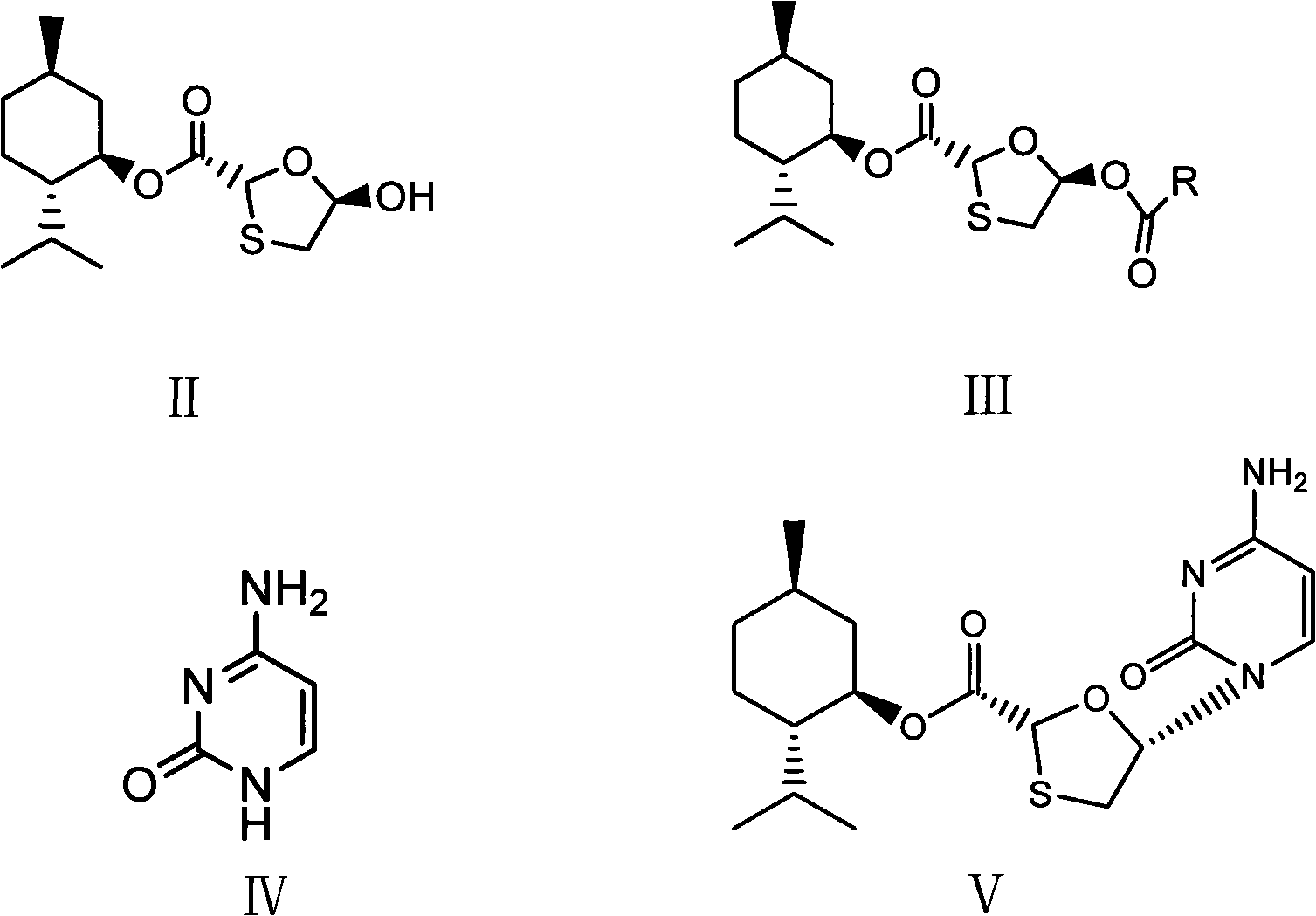
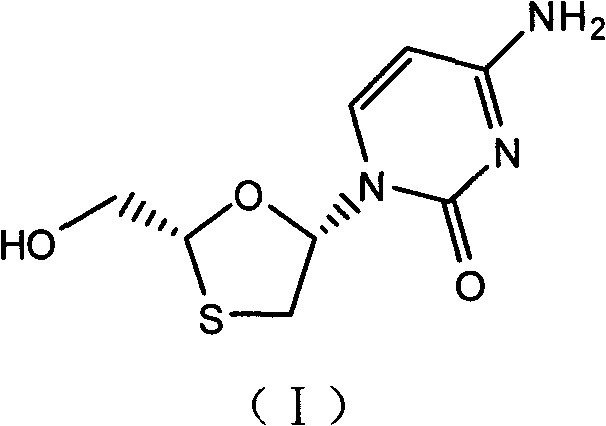
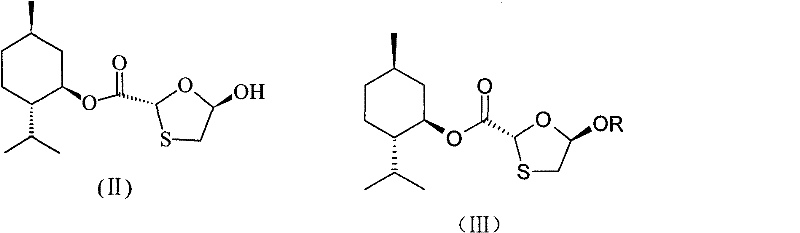
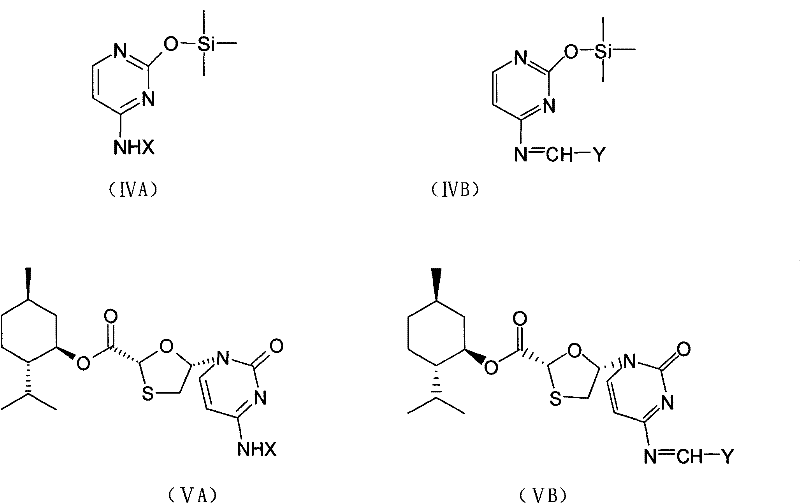
This invention prepn. method consists procedures (as shown in route chart) of: (1). hydrated glyoxalic acid is reacted with menthol in solvent and catalyst to product stable intermediate glyoxalic (1, R, 2.5, 5, R) menthol (V); then being reacted with 2,5-dihydroxy-1,4-dithiothiane to produce product and then proceeding crystallation to obtain menthol ester (IV); (2). proceeding acidylation by using hydroxy to obtain menthol ester (III); (3) being condensed by cytosine under protection of silanized reagent, to obtain menthol ester (II); (4) obove-said product is reduced by reducing agent to obain final invented product Lamifudin (I). This invention has advantages of: available raw material, high yield, high safety, and commercialization prodn.
Owner:SHANDONG WEIFANG PHARMA FACTORY
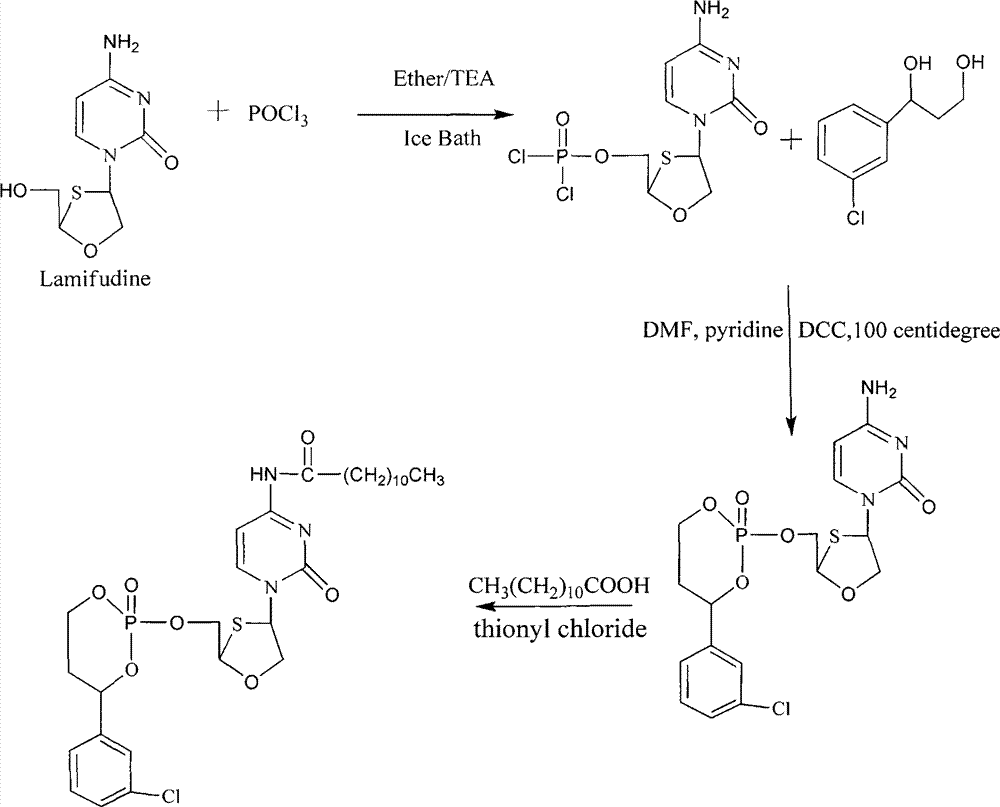
Phosphoryl N-fatty acyl nucleoside analogue for treating viral hepatitis and liver cancer
The invention discloses a phosphoryl N-fatty acyl nucleoside analogue for treating viral hepatitis and liver cancer. The phosphoryl N-fatty acyl nucleoside analogue is characterized in that a nucleoside analogue is modified by a cyclophosphoryl group and then is connected to aliphatic chains having different numbers of carbon atoms. The phosphoryl N-fatty acyl nucleoside analogue can be used for convenient preparation of a nanometer transmission system and has obvious hepatocyte and tumor targeting. The nanometer transmission system comprises liposome, nonionic surfactant niosomes, nanoparticles, nano-emulsion and a self-assembled transmission system. The nucleoside analogue is selected from lamivudine, adenine arabinoside, cidofovir, gemcitabine, cytosine arabinoside, azacitidine and fludarabine. After intravenous administration, the nanometer transmission system of the phosphoryl N-fatty acyl nucleoside analogue has effects of targeting treatment on viral hepatitis and liver cancer.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Lamy stationary slice and preparing method thereof
InactiveCN101199491AImprove yieldSimple processOrganic active ingredientsAntiviralsSlurryMagnesium stearate
The invention discloses a lamivudine tablet and the related preparation method. The lamivudine tablet is made of 150 portions(based on weight) of lamivudine, 70 to 90 portions of starch, 5 to 10 portion of dry starch, 1 to 1.5 portions of magnesium stearate and 15 to 20 portions of starch slurry with a concentration of 10%. The invention has the advantages of high finished product rate and low cost.
Owner:ZHUHAI HUAAO IMPORT & EXPORT CORP
Lamivudine diastereoselective synthesis method
A lamivudine diastereoselective synthesis method, which takes chiral auxiliary agent L-menthol as the initial material, synthesizes trans-5-hydroxyl-1, 3-oxygen thiacyclopentane-2-carboxylic acid-(1'R, 2'S, 5'R) menthol ester under the action of concentrated sulfuric acid, choose triethanolamine to obtain trans-isomer trans-5-hydroxyl-1, 3-oxygen thiacyclopentane-2-carboxylic acid-(1'R, 2'S, 5'R) menthol ester, and let the trans-isomer to react with acylating agent to obtain trans-5-acetoxy-1, 3-oxygen thiacyclopentane-2-carboxylic acid-(1'R, 2'S, 5'R) menthol ester, glycosidate with cytosine under the action of alkali to obtain 5S-cytosine-1'-radical-1, 3-oxygen thiacyclopentane-2-carboxylic acid-(1'R, 2'S, 5'R) menthyl ester, and then deoxidize with a deoxidizer, and salifying with aspirin, to ionize and liberate lamivudine. Since triethanolamine is added as in the course of reaction interconverting agent, the yield of lamivudine is increased greatly. Aspirin is added in the course of reaction, so that the lamivudine forms an aspirin salt that has poor water solubility, and therefore can effectively separate and liberate lamivudine from the medium.
Owner:湖南千金湘江药业股份有限公司
Synthesis and preparation process of lamivudine intermediate HDMS
InactiveCN101830893ALow costStep synthesis yield increaseOrganic chemistryOrganic synthesisGas phase
The invention discloses a synthesis and preparation process of a lamivudine intermediate HDMS, which belongs to the field of organic synthetic medicaments. The synthesis and preparation process is characterized in that a preparation method of glyoxylic acid menthol ester comprises the following steps of: under the catalysis of concentrated sulfuric acid, performing a reflux reaction on L-menthol and glyoxylic acid, wherein a reactive solvent is cyclohexane; judging the reaction end point through a gas phase chromatography, and then cooling and washing the mixture; reacting an organic layer with solution of sodium bisulfite at the temperature of between 20 and 30 DEG C; judging the reaction end point through the gas phase chromatography, and then washing the mixture by using the cyclohexane; reacting an aqueous layer with methanol at the temperature of between 20 and 30 DEG C; and washing the mixture after filtering and drying the mixture to obtain the glyoxylic acid menthol ester. By using the synthesis and preparation process, the condition is milder, the operation is simpler and more convenient and the reaction processes are easier to control, so the product production yield and the quality stability are improved, and the cost of the lamivudine intermediate HDMS is greatly reduced.
Owner:ANHUI PROVINCE YIFAN SPICE
Disoproxil fumarate, lamivudine and efavirenz tri-combination compound mini-pill tablet and preparation method thereof
ActiveCN103908456AGuaranteed stabilityGood compressibilityOrganic active ingredientsAntiviralsDissolutionTableting
The invention discloses a tri-combination compound mini-pill tablet co-prepared by disoproxil fumarate DF coated mini-pills, lamivudine coated mini-pills and efavirenz mini-pills and a preparation method thereof. The invention solves the problems that effective component degradation and dissolution delaying phenomena are generated due to interaction among the tri-combination compound components, tabletting is not facilitated during preparation and patient swallowing is not facilitated; and the single-layer tablet having the three component drugs without mutual contact is prepared by a mini-pill tabletting method, so as to obtain good stability and fast dissolving rate.
Owner:ANHUI BIOCHEM BIO PHARMA
Preparation method of lamivudine and intermediate thereof
ActiveCN101597281AOperational securityThe process steps are simpleOrganic chemistryAntiviralsCytimidineStereochemistry
The invention discloses a preparation method of lamivudine and an intermediate thereof; (2R,5R)-5-hydroxyl-[1,3]oxathiolane-2-carboxylicacid(2S-isopropyl-5R-methyl-1R-cyclohexyl)ester, the structure of which is shown in formula (II) is used as a raw material; acyl compound, the structure of which is shown in formula (III) is obtained by the acylation reaction of acyl; acyl compound (III) reacts with cytimidine, the structure of which is shown in formula (IV) by condensation reaction to prepare the intermediate of lamivudine (V) and the intermediate is reducted to obtain lamivudine (I). The invention is characterized by safe and reliable operation, simplified process, low production cost and the like, thus being suitable for industrialized production.
Owner:SHANDONG WEIFANG PHARMA FACTORY
Application of lignan of biphenyl cyclooctene series in preparing anti-hepatitis B virus medicament
InactiveCN101375842AOrganic active ingredientsDigestive systemTest comparisonDibenzocyclooctadiene lignan
The invention belongs to the traditional Chinese medicine pharmaceutical field and relates to a new use of a dibenzocyclooctadiene lignan in the preparation of anti-HBV drugs. The invention extracts the dibenzocyclooctadiene lignan from S.wilsoniana of Schisandra plant and confirms the anti-HBV activity thereof through a test. The result of the pharmacological test comparison of the dibenzocyclooctadiene lignan with the active controlled lamivudine proves that the dibenzocyclooctadiene lignan has significant anti-HBV role, low effective concentration and smaller cell toxicity, and the dibenzocyclooctadiene lignan can be used as an active ingredient for preparing the drugs for the treatment of hepatitis B.
Owner:FUDAN UNIV
Easy-dissolution lamivudine tablet and preparation method thereof
ActiveCN102144984AFully contactedExcellent water dissolution propertiesOrganic active ingredientsDigestive systemCarboxymethyl starchMedicine
The invention relates to an easy-dissolution lamivudine tablet and a preparation method thereof. The lamivudine tablet comprises the following raw materials in parts by weight: 100 parts of lamivudine, 95-105 parts of microcrystalline cellulose, 5-8 parts of sodium carboxymethyl starch, 1.5-3 parts of magnesium stearate and 90-120 parts of 2% hydroxypropyl methylcellulose (E15) aqueous solution.
Owner:FUJIAN COSUNTER PHARMA CO LTD
Lamivudine molecularly imprinted solid phase extraction column prepared by using template substituting method and applications thereof
InactiveCN102008946AQuick analysisEfficient separationIon-exchange process apparatusComponent separationMethacrylateSolid phase extraction
The invention relates to the preparation of an absorption material and the application to medicament separation and enrichment, in particular to the preparation methods of a Lamivudine molecularly imprinted polymer and a Lamivudine molecularly imprinted solid phase extraction column and the applications thereof. The Lamivudine esterified ester is obtained through esterification synthesis. The molecularly imprinted polymer is synthesized by using the Lamivudine esterified ester as a template, methacrylic acid as a monomer, trimethoxy propane trimethyl acrylate as a crosslinker and chloroform as a porogen by adopting a body polymerization method; and molecularly imprinted polymer particles are uniformly filled in the solid-phase extraction column to obtain the Lamivudine molecular imprinting solid-phase extraction column. The invention realizes the efficient separation, enrichment and purification of Lamivudine in a biological sample and has high selectivity as compared with traditional related technologies, such as a common solvent extraction method, a C18 solid-phase extraction method, and the like. Moreover, the Lamivudine molecularly imprinted solid phase extraction column has low cost by being repeatedly used and can become a necessary method in the Lamivudine pretreatment in biological samples.
Owner:XINJIANG UNIVERSITY
Anti-HIV compound preparation and preparation method and application thereof
ActiveCN106860414AGood curative effectImprove stabilityOrganic active ingredientsAntiviralsTreatment effectNervous system
The invention discloses anti-HIV compound preparation. The anti-HIV compound preparation is prepared from the following ingredients in weight percentage: 10 to 35% of lamivudine, 10 to 35% of fumaric acid tenofovir ester, 5 to 20% of 3-((3-ethyl-2,6-dioxo-5-(propyl-2-radical)-1,2,3,6-tetrahydropyrimidine-4-radical)carbonyl)-5-tolunitrile and 10 to 75% of excipient. The anti-HIV compound preparation disclosed by the invention is single-layer tablet or double-layer tablet; in preparation, the fumaric acid tenofovir ester and compound of the 3-((3-ethyl-2,6-dioxo-5-(propyl-2-radical)-1,2,3,6-tetrahydropyrimidine-4-radical)carbonyl)-5-tolunitrile are independently pelletized and then totally mixed and compressed into tablets. The anti-HIV compound preparation disclosed by the invention has the advantages of good stability, a simple preparation technology and controllable quality, can restrain HIV virus from different targets, improves a treating effect of single drug use to AIDS, avoids side effects on a nervous system and has small toxicity and a wide safety dosage range.
Owner:YANGZHOU AIDEA BIOTECH +1
Detection method of hepatitis B virus genome drug resistance mutation
ActiveCN1786189AAccurate and effective identificationAccurately and effectively distinguishMicrobiological testing/measurementWild typeDrug resistance
The invention relates to examination mutant hepatitis virus method, especially the method of using DNA reverse dot blot hybridization technique to quickly and exactly distinguish clinic blood sample wild type and rummy fuding tolerance mutant hepatitis b virus. And it also relates to clinic measuring kit.
Owner:上海达安医学检验所有限公司
Lamivudine tablet and preparation method thereof
ActiveCN103181910AAvoid interventionReduce manufacturing costOrganic active ingredientsPharmaceutical delivery mechanismActive componentCurative effect
The invention discloses a lamivudine tablet, which is characterized in that a tablet core contains the followed raw materials by weight: 100 parts of lamivudine, 70-150 parts of filler, 0.1-10 parts of disintegrating agent, 0-5 parts of flow aid, and 0.1-3 parts of lubricant. The invention further provides a method for preparing the lamivudine tablet. The tablet core of the lamivudine tablet of the invention is prepared by directly pressing a mixture of active component lamivudine and accessories, so the dissolving-out speed is faster. Further, the tablet core of the invention is coated with a film coating, thereby preventing the tablet core form moisture absorption, and guaranteeing the medicine curative effect. The stability of the tablet is better. The method for preparing the lamivudine tablet in the invention, has advantages of simple technology, convenient operation and low production cost.
Owner:BEIJING UNION PHARMA FACTORY
Chiral chromatographic fixed phase stuffing of vancomycin phenylisocyanate and its prepn
The chiral chromatographic fixed phase stuffing of vancomycin phenylisocyanate is prepared through bonding vancomycin as glycopeptide macrocyclic antiseptic chemical onto silicon gel carrier, and the subsequent derivation of phenylisocyanate to obtain chiral chromatographic fixed phase stuffing. The silicon gel carrier is first reacted with 3-aminopropyl triethoxy silane for silanation and then space arm activated; and the activated silicon gel carrier is bonded with vancomycin and finally derivated with phenylisocyanate. The chiral chromatographic fixed phase stuffing of vancomycin phenylisocyanate after assembled to column may be used in forward phase, reverse phase and polar organic phase chromatographic condition. The present invention may be used in simple polar organic phase mode to resolve the medicine antimer of zolmitriptan, lamivudine, etc.
Owner:ZHEJIANG UNIV
Industrial preparation method for lamivudine
The invention discloses an industrial preparation method for lamivudine (3TC). The method is characterized in that: 5-hydroxyl-1,3-oxathiolane-2-carboxylic acid [(1<,>R, 2<,>S, 5<,>R)-5<,>-methyl-2<,>-(1-methylethyl) cyclohexyl] ester reacts with a acylating agent to obtain a acylate; the acylate and monosilylated acyl cytosine or monosilylated cytosine schiff's base are subjected to a glycosylation through a catalysis of lewis acid to obtain a 3TC intermediate, followed by reducing and deacylating or hydrolyzing and reducing to obtain the 3TC. The method provided by the present invention has advantages of simple, available and cheap raw materials, simple operation, safe production, high yield, less waste pollution, and is applicable for industrial production.
Owner:吉斯凯(苏州)制药有限公司
Daucane type sesquiterpenes and preparation method and application thereof
InactiveCN101375841AHydroxy compound active ingredientsDigestive systemTest comparisonBULK ACTIVE INGREDIENT
The invention belongs to the traditional Chinese medicine pharmaceutical field and relates to a dancane sesquiterpene, a preparation method thereof and a new use in the preparation of anti-HBV drugs. The invention extracts the dancane sesquiterpene from S.wilsoniana of Schisandra plant and confirms the anti-HBV activity thereof. The result of the pharmacological test comparison of the compound with the active controlled lamivudine proves that the compound has significant anti-HBV role, low effective concentration and smaller cell toxicity, and the dancane sesquiterpene compound can be used as an active ingredient for preparing the drugs for the treatment of hepatitis B.
Owner:FUDAN UNIV
Lamivudin stearate and synthesis method and application
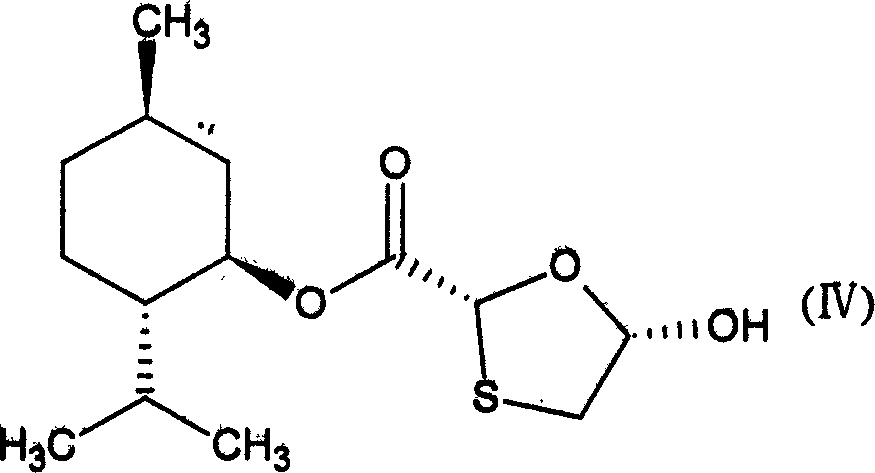
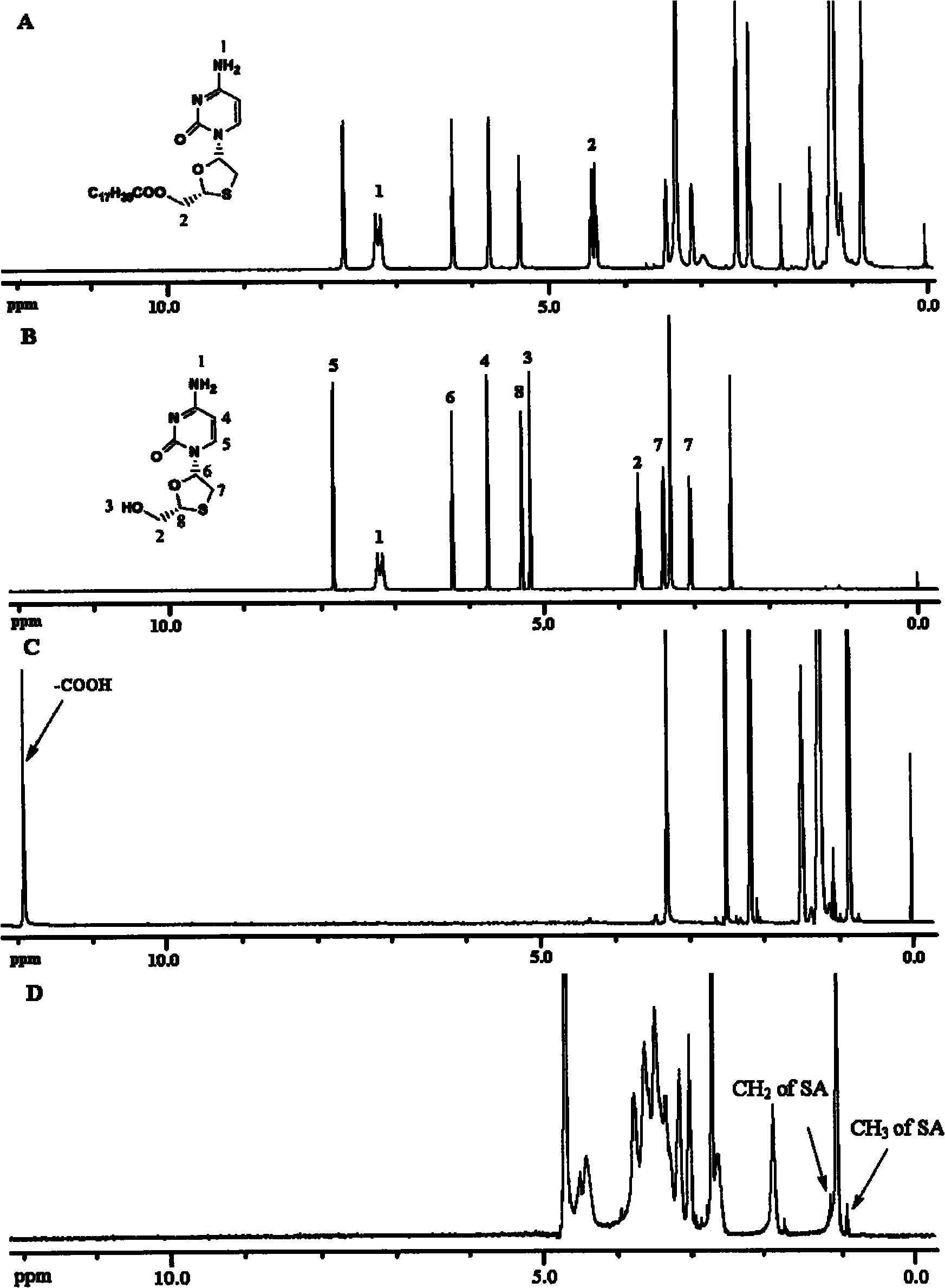
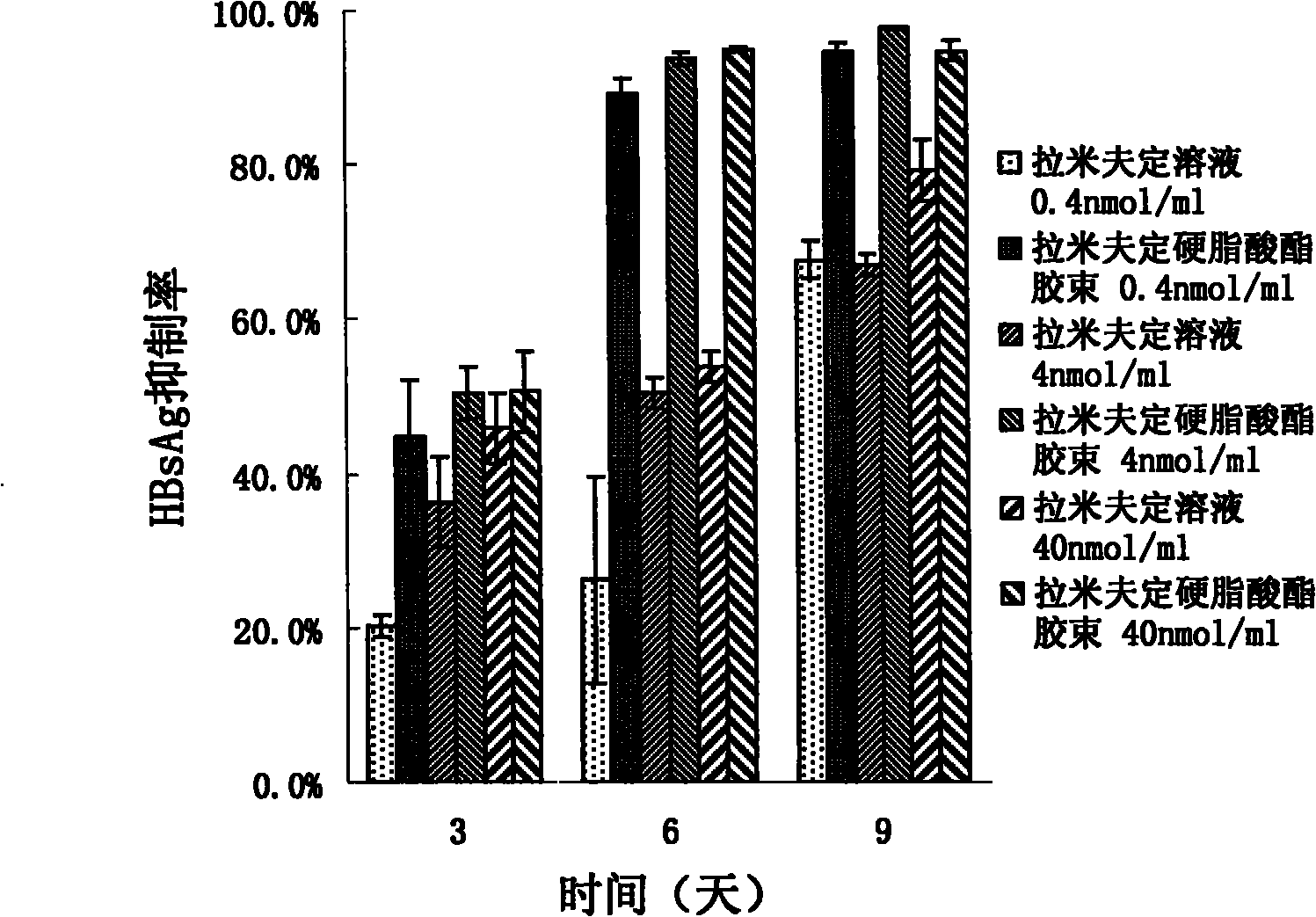
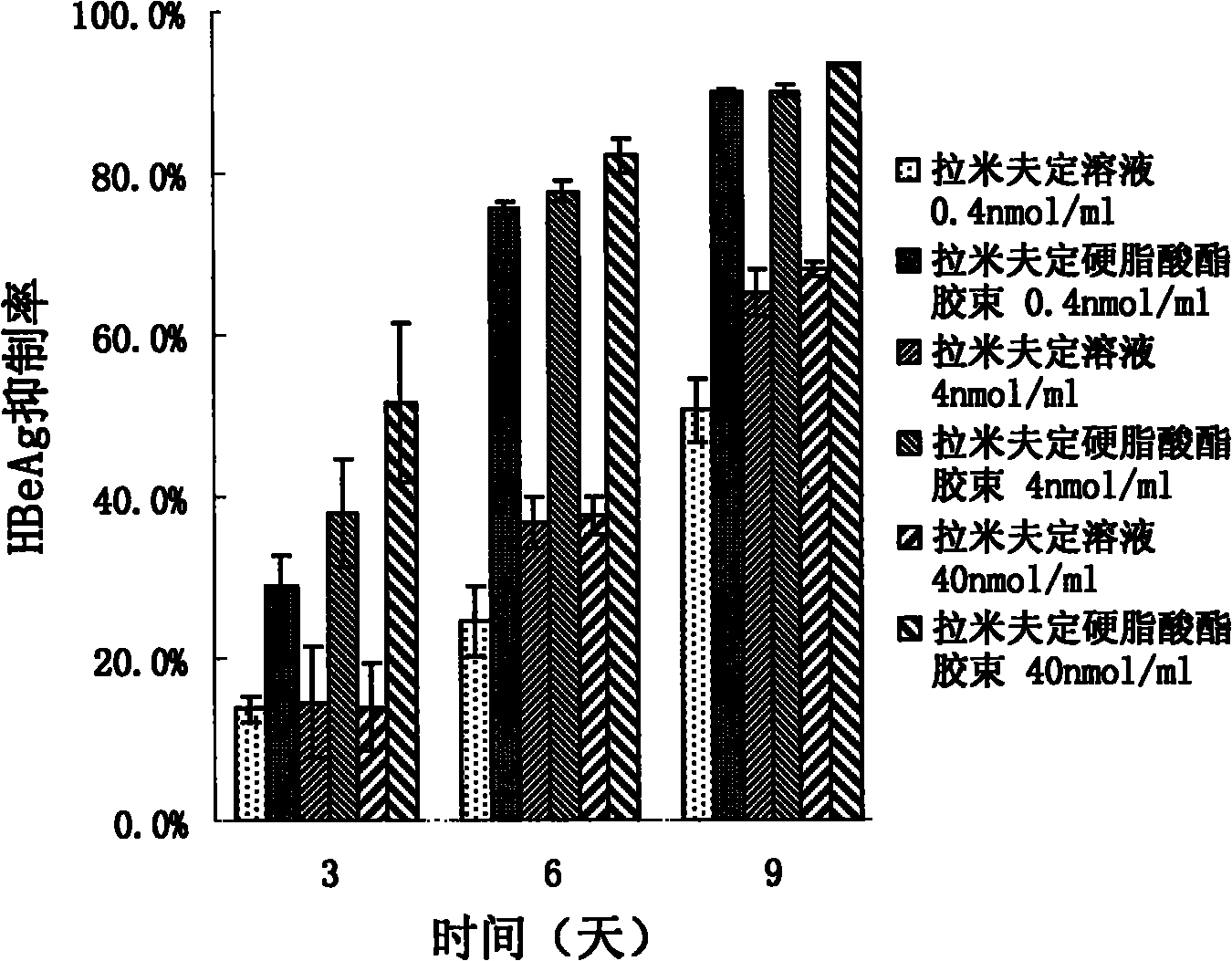
InactiveCN101805334AConducive to loadIncrease intakeOrganic active ingredientsOrganic chemistrySide effectSynthesis methods
The invention provides lamivudin stearate. The lamivudin stearate is characterized in that the lamivudin stearate is synthesized through lipophilicity modification of lamivudin, which is favorable for medicinal loads of targeted carrier materials; chitosan-stearate graft micelles having efficient cell intake and low toxicity are used for encapsulating antiviral medicaments of molecular targets incells so as to greatly increase medicament intake of viral cells and medicament concentration at the medicinal molecular targets; the increased medicament intake of the viral cells is favorable for reducing the distribution of the medicaments in normal tissues or cells and toxic and side effects of the medicaments; the increased medicament concentration at the medicinal molecular targets is favorable for improving the effects of the antiviral medicaments; and the lamivudin stearate can be applied to preparing the medicaments having efficient anti-HBV activity. The lamivudin stearate has the following chemical structural formula.
Owner:ZHEJIANG UNIV
Preparation method and applications of lamivudine twin drug
InactiveCN101766632AHas antiviral effectImprove liver functionOrganic active ingredientsDigestive systemHepatic inflammationPropanoic acid
The invention provides a preparation method and applications of a lamivudine twin drug. Lamivudine, and ursolic acid or oleanolic acid are condensed into the lamivudine-ursolic acid or lamivudine-oleanolic acid twin drug at a low temperature by using ethyl chloroacetate (ethyl bromoacetate) or ethyl chloropropionate (ethyl bromopropionate) as the linking group. The twin drug possibly has a dual-action mechanism; and the twin drug has the action of antivirus, and also has the actions of resisting inflammations, protecting the liver cell membranes, improving the liver function and resisting fibrosis. Thus, the invention organically combines the antivirus therapy and the liver protection treatment and provides a new concept for the research and development of drugs for treating hepatitis.
Owner:CHINA PHARM UNIV
Application of lindley eupatorium herb in preparing anti-hepatitis B virus medicaments
The invention discloses an application of lindley eupatorium herb in preparing anti-hepatitis B virus medicaments. Lindley eupatorium herb ethanol extract, lindley eupatorium herb flavone part, lindley eupatorium herb hemiterpene part, lindley eupatorium herb 70% ethanol water elution part and chain diterpene are prepared from lindley eupatorium herb and are subjected to hepatitis B virus (HBV) testing, and testing results indicate that the four extracts and the chain diterpene have the effect of remarkably inhibiting hepatitis B surface antigen (HBsAg) and hepatitis B core antigen (HBeAg), and have better drug activities than those of clinical medicament lamivudine (3TC) for treating hepatitis B. The lindley eupatorium herb can be used for preparing medicaments for resisting hepatitis B virus.
Owner:SUZHOU UNIV
Novel anti- hepatitis B virus medicine compounds-lamivudine and protocatechuic acid
InactiveCN101224209AGood curative effectReduce the risk of drug resistanceOrganic active ingredientsDigestive systemHepatitis B Virus AntigenSide effect
The invention provides an anti-HBV medicine combination which has more remarkable treatment effect and safety and no toxic and side effect and can evidently increase the negative conversion ratio of HBSAg. The invention takes HepG2.2.15 as a target cell and adds lamivudinevir, protocatechuic acid and the compatibility preparation of lamivudinevir and protocatechuic acid with a proper concentration to cell culturing medium. The cell is cultured by a regular method and HBsAg and HBeAg secreted by the cell is detected by ELISA method, HBV DNA content is measured by fluorescent quantitation PCR, HBV resistance effect of the drug is judged by depression rate calculation method to determine the medical treatment effect on HBV and the preparation proportion of medicine combination. The proportion of lamivudinevir to protocatechuic acid is between 1 to 50 and 1 to 100 (mass ratio) and generally the dosage of lamivudinevir is 0.05-5Mug and the protocatechuic acid is 5-500 Mug. The medicine combination can evidently improve the depression effect of the lamivudinevir on Hepatitis B antigen and reduce the toxic and side effect of the lamivudinevir when being used separately.
Owner:HUBEI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com