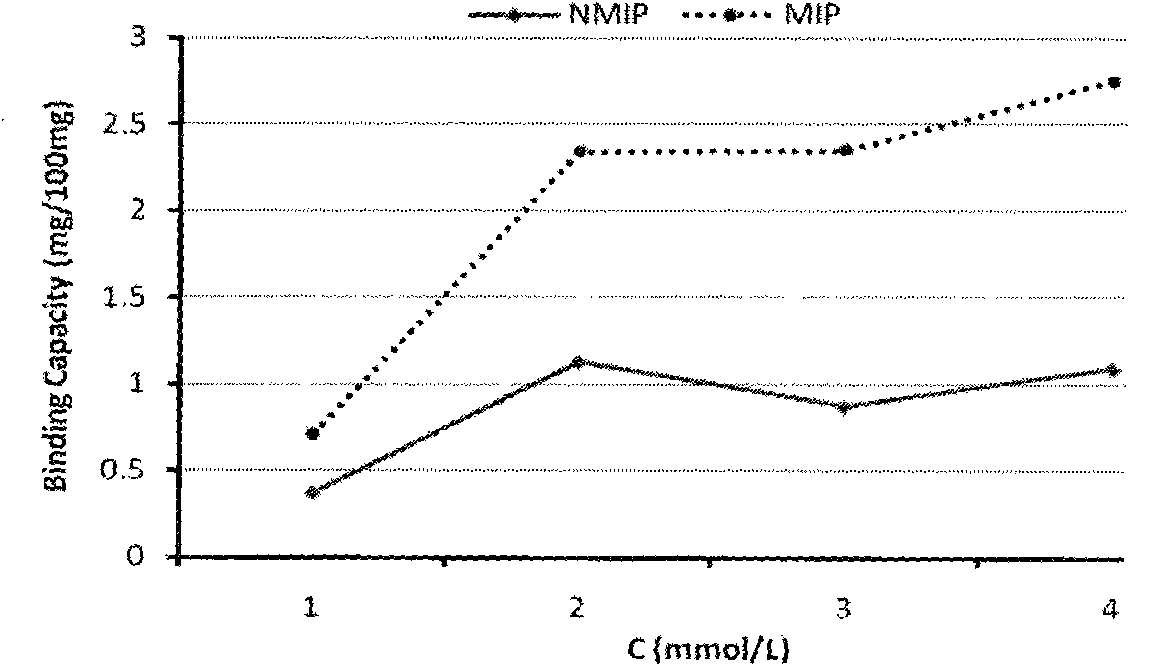
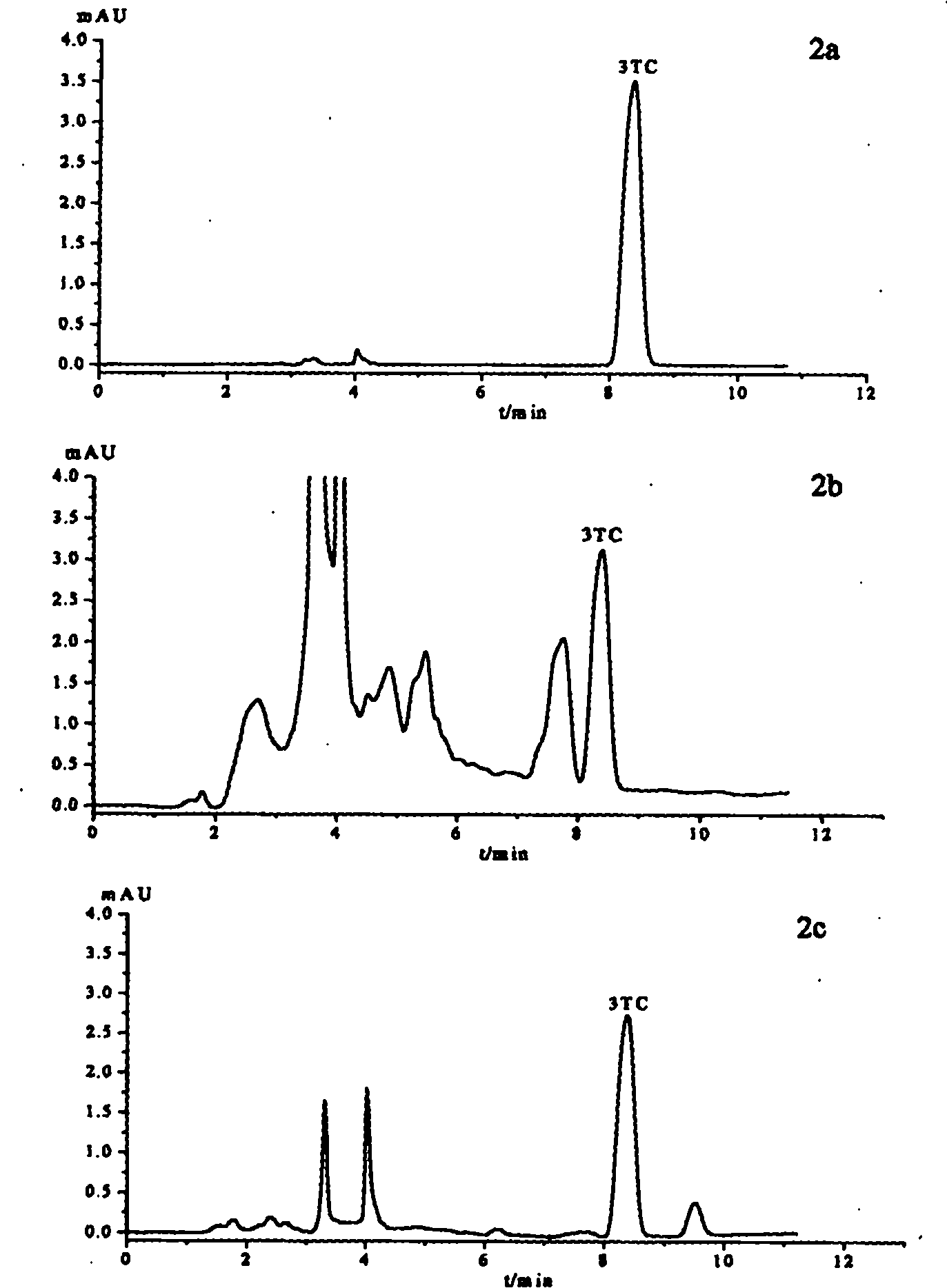
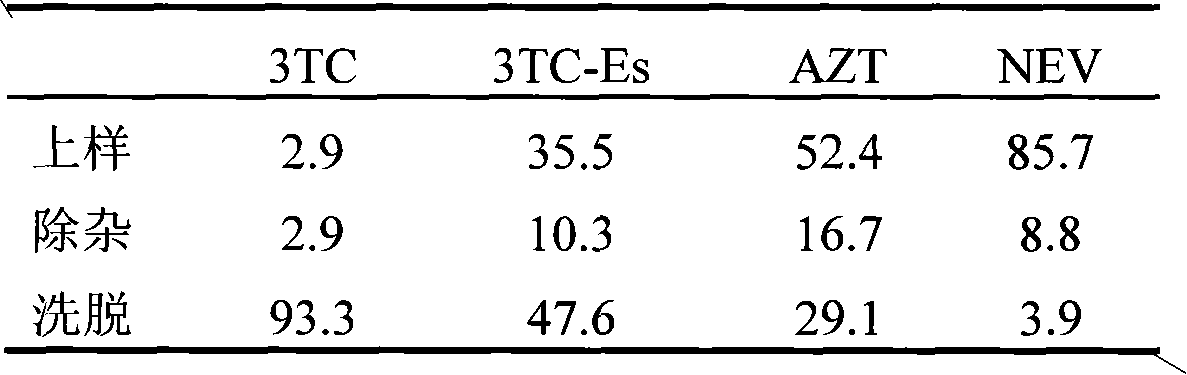
Lamivudine molecularly imprinted solid phase extraction column prepared by using template substituting method and applications thereof
A solid-phase extraction column, lamivudine technology, applied in material separation, analysis of materials, ion exchange and other directions, can solve the problem of preparation that has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0010] A) Synthesis of lamivudine ester compound (3TC-Es): 2.4 mL of pyridine and 1.0 g of lamivudine are placed in a reaction flask, fully dissolved, 2 mL of acetic anhydride is added, stirred at room temperature for 2 hours, and evaporated Solvent and unreacted acetic anhydride, dissolve the white crude product in chloroform, extract with water, collect the chloroform layer, continue to extract the water layer with chloroform for 3 times, combine the chloroform solution, and evaporate the chloroform to obtain a white solid. Ethanol recrystallization obtains white flocculent lamivudine esterification product;
[0011] B) Put the template, functional monomer, cross-linking agent, 40 mg of initiator and 16 mL of porogen chloroform at a ratio of 1 mmol: 4 mmol: 20 mmol into a 50 mL round-bottomed flask, fully dissolve and mix well, and ultrasonically degas for 5 minutes , nitrogen for 15 minutes, and heated in a 45°C constant temperature oil bath for 24 hours.
[0012] C) Grind...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com