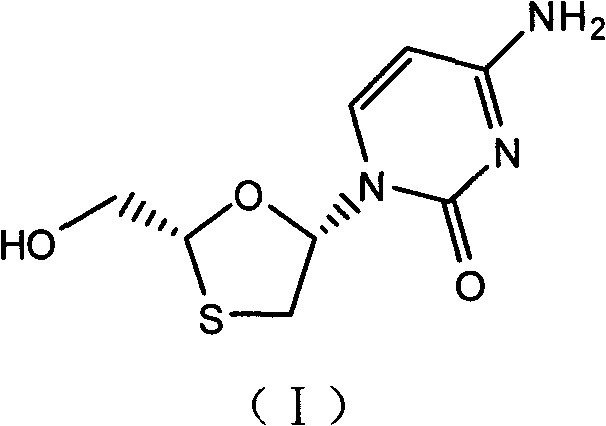
Industrial preparation method for lamivudine
A technology of lamivudine and its compound, which is applied in the field of industrial preparation of anti-hepatitis B virus drug lamivudine, can solve the problems of waste gas, cumbersome operation process, and high corrosion of equipment, and achieve easy implementation, simple preparation, and corrosion resistance big effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
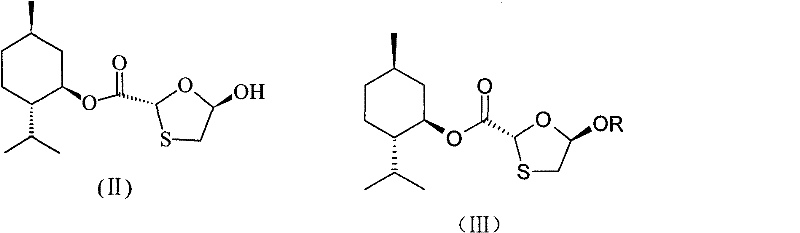
[0066] (5R)-Acetoxy-1,3-oxathiolane-(2R)-carboxylic acid-[(1′R, 2′S, 5′R)-5′-methyl-2′- Preparation of (1-methylethyl)cyclohexyl] ester (IIIA)
[0067] Compound (II) 5-hydroxyl-1,3-oxathiolane-2-carboxylic acid [(1'R, 2'S, 5'R)-5'-methyl-2'-(1 -Methylethyl)cyclohexyl]ester (200g) was put into a 2L three-necked flask, DMAP (10.16g) and tetrahydrofuran 600ml were added, stirred and dissolved, cooled to -25~-20°C, and diethyl ether was added dropwise within 2~3 hours. Acid anhydride 99ml, continue to react for 45-60min after the dropwise addition, then slowly add 10% sodium carbonate aqueous solution dropwise to adjust the pH to 7, transfer it to a separatory funnel, let stand to separate layers, extract the water layer twice with 100ml tetrahydrofuran, collect organic layer, dried over anhydrous magnesium sulfate overnight, filtered, the filter cake was washed with a small amount of tetrahydrofuran, the filtrate was concentrated under reduced pressure at 60°C, recrystallized wi...
Embodiment 2
[0069] (5R)-propionyloxy-1,3-oxathiolane-(2R)-carboxylic acid-[(1′R, 2′S, 5′R)-5′-methyl-2′ Preparation of -(1-methylethyl)cyclohexyl]ester (IIIB)
[0070] Compound (II) 5-hydroxyl-1,3-oxathiolane-2-carboxylic acid [(1'R, 2'S, 5'R)-5'-methyl-2'-(1 -Methylethyl)cyclohexyl]ester (200g) was put into a 2L three-necked flask, DMAP (10.16g) and tetrahydrofuran 600ml were added, stirred and dissolved, cooled to -25~-20°C, and propane was added dropwise within 2~3 hours. Acid anhydride 134ml, continue to react for 45-60min after the dropwise addition, then slowly add 10% sodium carbonate aqueous solution dropwise to adjust the pH to 7, transfer it to a separatory funnel, let stand to separate layers, extract the water layer twice with 110ml tetrahydrofuran, collect organic layer, dried over anhydrous magnesium sulfate overnight, filtered, the filter cake was washed with a small amount of tetrahydrofuran, the filtrate was concentrated under reduced pressure at 60°C, recrystallized wit...
Embodiment 3
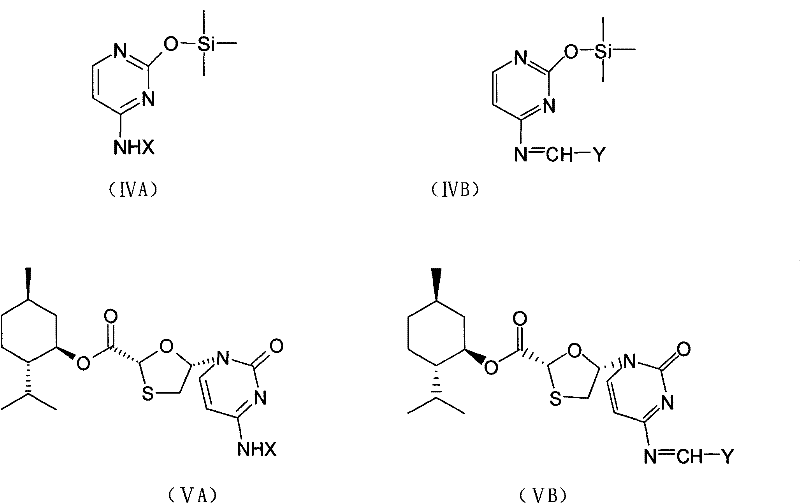
[0075] 5S-[5-(4-acetylamino-2-oxo-1(2H)-pyrimidine base)]-1,3-oxathiolane-2R-carboxylic acid-[(1′R,2 Preparation of 'S, 5'R)-5'-methyl-2'-(1-methylethyl)cyclohexyl]ester (VA)
[0076] Put 25.5g of N-acetylcytosine, 0.77g of ammonium sulfate, 22.0ml of hexamethyldisilazane and 500ml of chloroform into a 1L three-necked flask, stir and raise the temperature and reflux for 2 hours until the reaction solution is dissolved and clear, and cool to the internal temperature 0°C to obtain a chloroform solution of compound (IVA); under nitrogen protection, add 44.0ml of iodotrimethylsilane dropwise to the chloroform solution of compound (IVA) while stirring while maintaining the internal temperature at 0°C, dropwise within 1.5 to 2 hours After the addition, 100ml of compound (IIIA) (48.0g) in chloroform was added dropwise within 2 hours, and the temperature was raised to 30-35°C to react for 15h. The reaction solution was concentrated to dryness under reduced pressure, and 100ml of methy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com