Phosphoryl N-fatty acyl nucleoside analogue for treating viral hepatitis and liver cancer
A technology for fatty acyl nucleosides and nucleoside analogs, applied in the field of phosphoryl N-fatty acyl nucleoside analogs, which can solve the problems of drug leakage into the external water phase and low encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
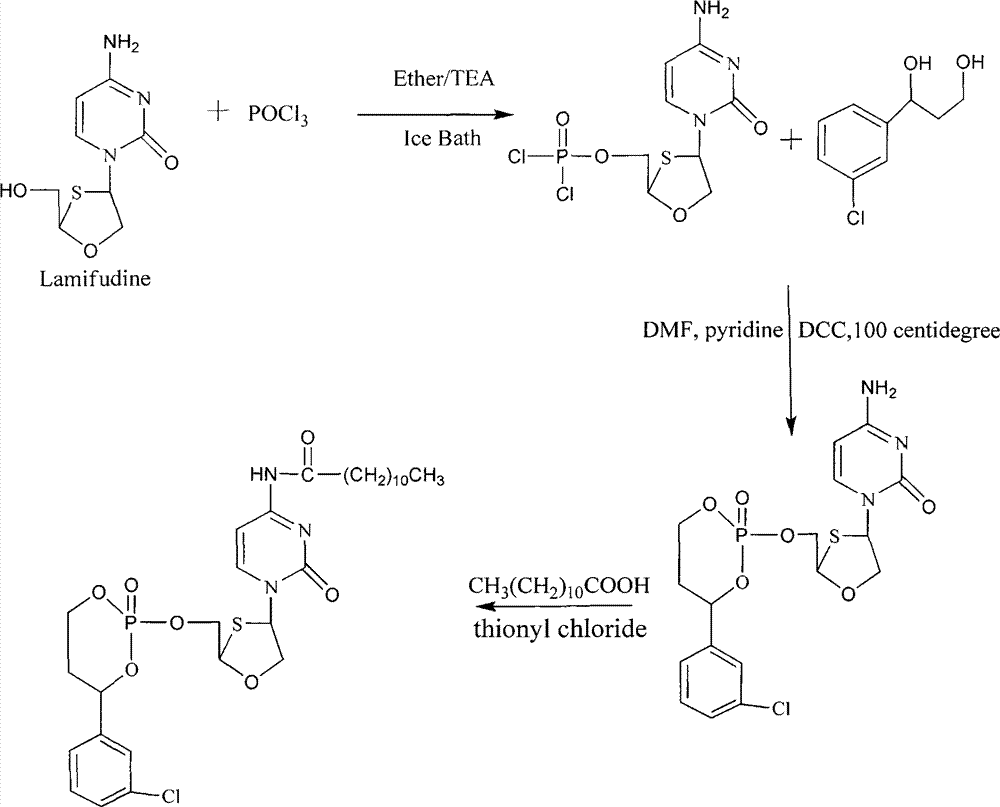
[0033] Embodiment 1. the synthesis of phosphoryl N-fatty acyl gemcitabine
[0034] The structural formula of phosphoryl N-fatty acyl gemcitabine is:
[0035]
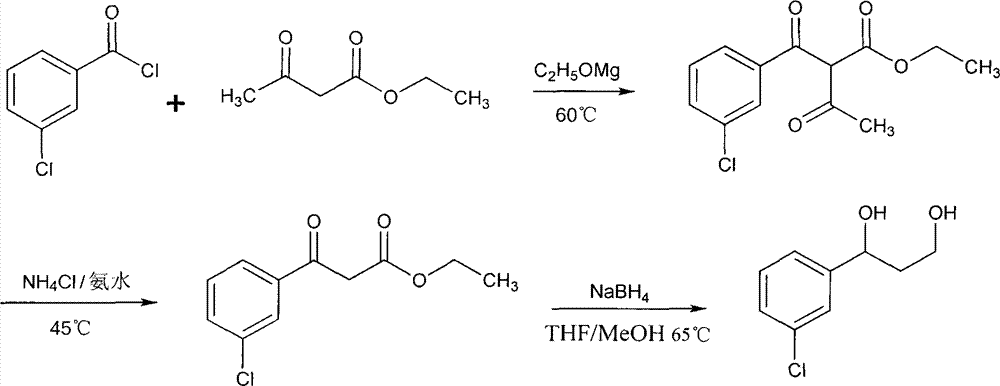
[0036] Wherein R is a single-chain aliphatic chain, and R=C 7 h 15 -, C 9 h 19 -, C 11 h 23 -, C 13 h 27 -, C 15 h 31 - or C 17 h 35 - 1-(3-Chlorophenyl)-1,3-propanediol is first synthesized. Weigh magnesium powder (6.3g, 0.26mol), add ethanol (27ml) and carbon tetrachloride (3ml), and stir under ice-bath conditions to obtain a white solid. A mixture of 33 ml of ethyl acetoacetate (0.26 mol), 40 ml of ethanol and 30 ml of diethyl ether was added dropwise. After 0.5h, m-chlorobenzoyl chloride (33ml, 0.26mol) was added dropwise, in an oil bath at 60°C for 2h, after the reaction liquid was cooled, it was poured into 150ml of cold water, and the pH value was adjusted to 4 with 3M hydrochloric acid solution. Take the organic phase and wash with 5% (w / v) NaHCO 3 and water three times respectively, dried and ...
Embodiment 2
[0041] Example 2.1-(3-chlorophenyl)-1,3-propanediol-N-dodecanoyl lamivudine phosphate
[0042] This product is the product of lamivudine and 1-(3-chlorophenyl)-1,3-propanediol to generate phosphate, and then amidation with dodecanoic acid. Molecular formula C 28 h 41 ClN 3 o 7 PS, molecular weight 642.14. First, 1-(3-chlorophenyl)-1,3-propanediol is synthesized, and the specific steps are as follows. Take magnesium powder (6.3g, 0.26mol) and add it to a round bottom flask, add ethanol (27ml) and carbon tetrachloride (3ml), and stir and mix under ice bath conditions. A solution of ethyl acetoacetate (33ml, 0.26mol) in ethanol (40ml) and diethyl ether (30ml) was added dropwise thereto. After reacting for 0.5h, the mixture was cooled to 0°C, and m-chlorobenzoyl chloride (33ml, 0.26mol) was added dropwise, reacted at 60°C for 2h, and cooled. The reaction solution was poured into cold water (150ml) and acidified to pH4 with hydrochloric acid solution. Separate the organic ph...
Embodiment 3
[0046] Embodiment 3. Preparation of phosphoryl N-lauroyl gemcitabine liposomes
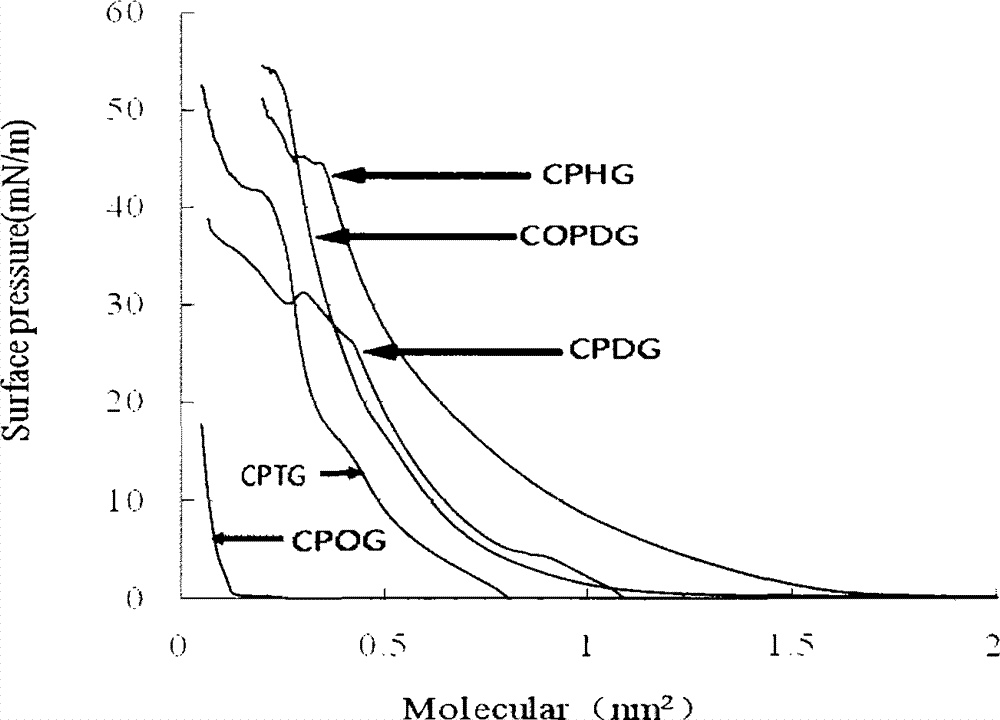
[0047] Take phosphoryl N-lauroyl gemcitabine (25 mg) and soybean lecithin (0.1 g) in a 250 ml flask, dissolve them in 20 ml of dichloromethane, and evaporate under reduced pressure to obtain a layer of organic fat-soluble film, and add phosphoric acid with a pH of 7.4 Shake 10ml of saline buffer solution, most of the membranes fall off, sonicate at 50°C until a uniform suspension is obtained, and observe under a microscope, most of the particles are less than 1 micron in diameter, which are phosphoryl N-dodecanoyl gemcitabine liposomes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com