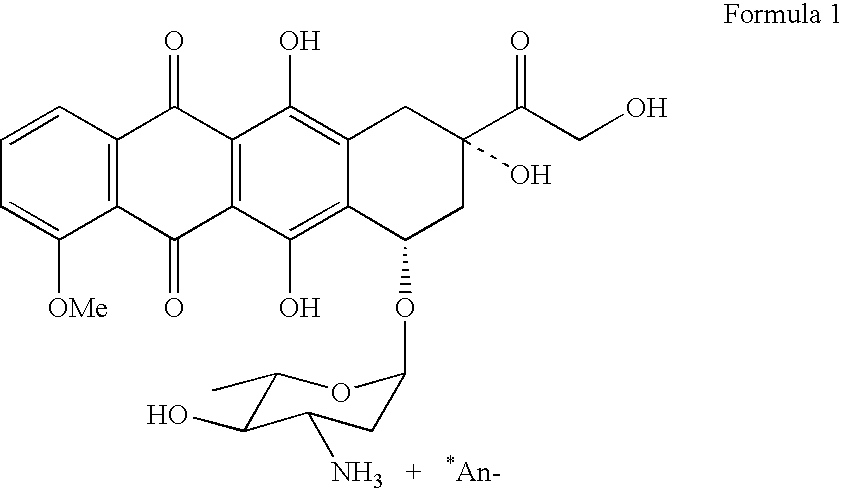
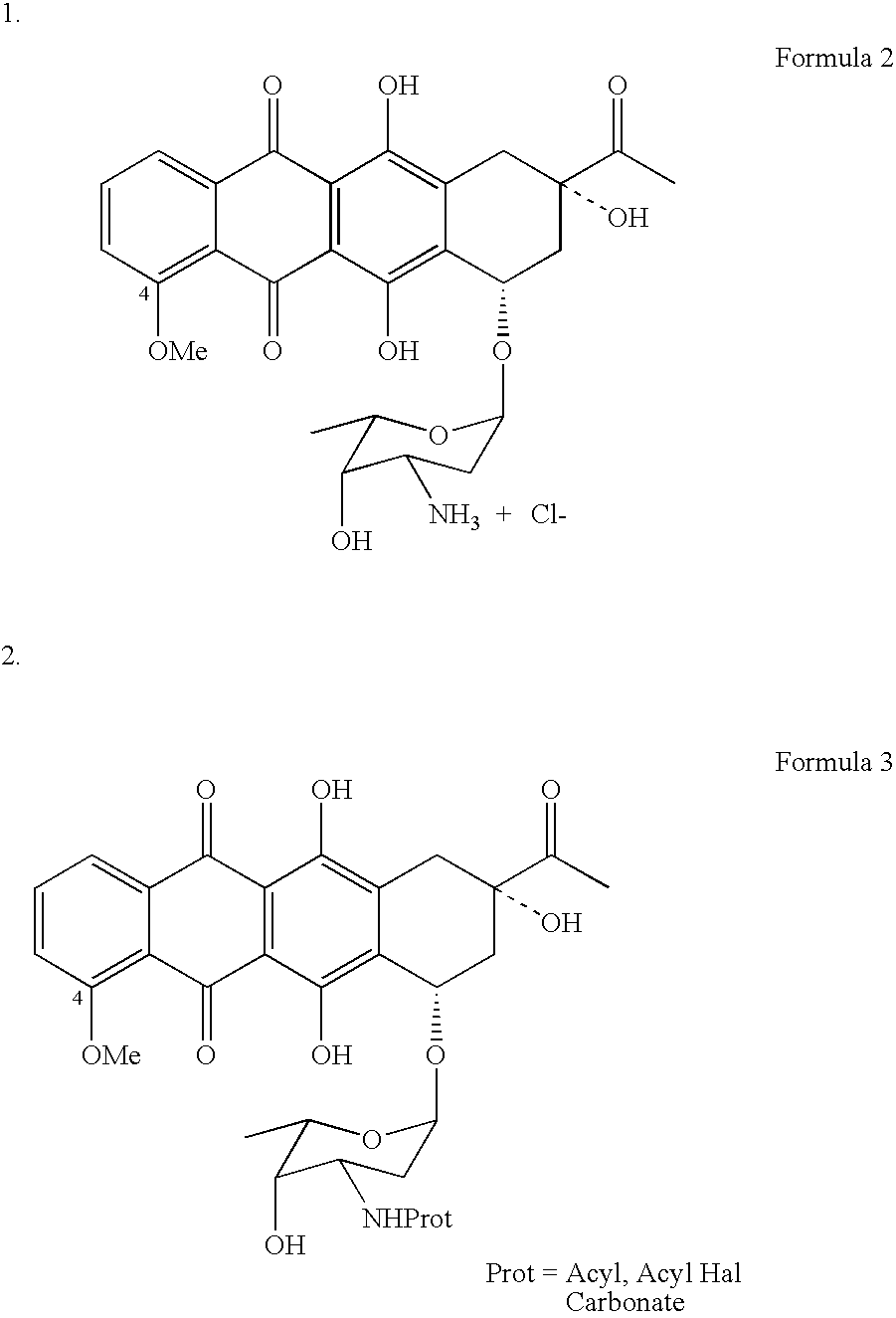
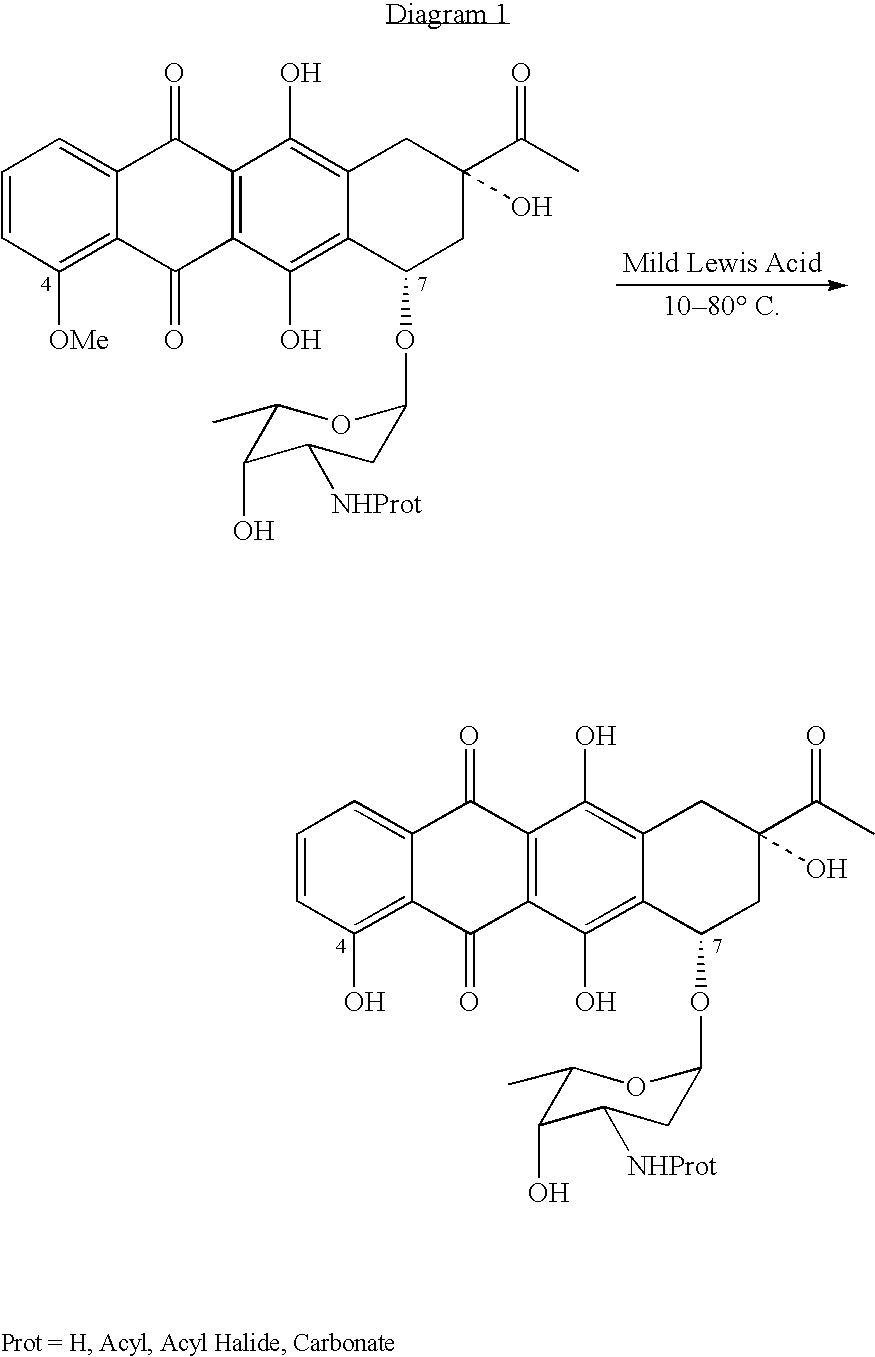
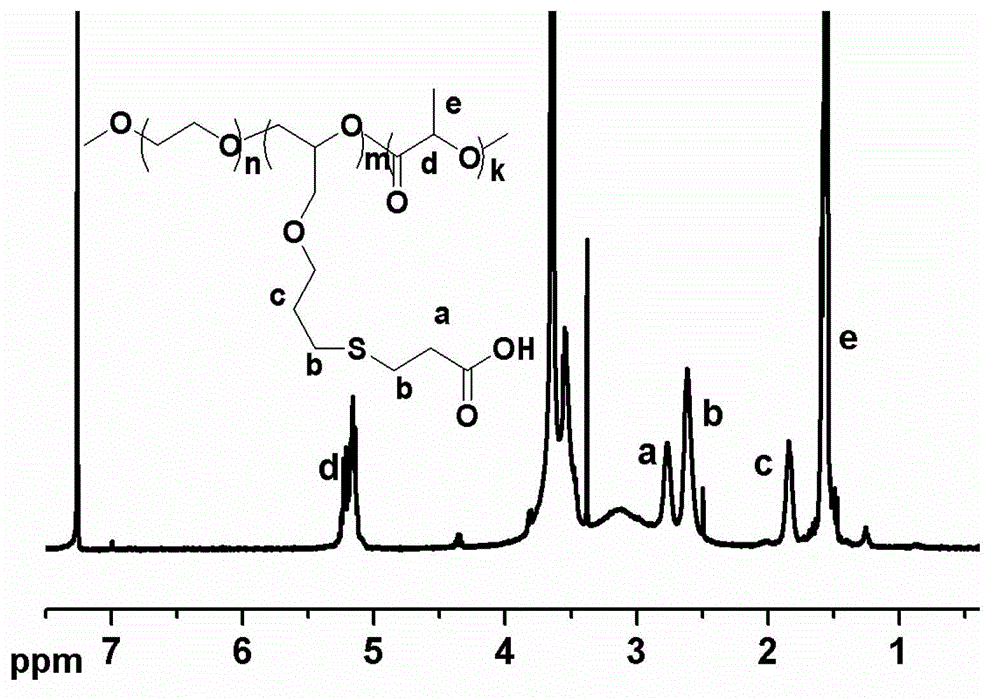
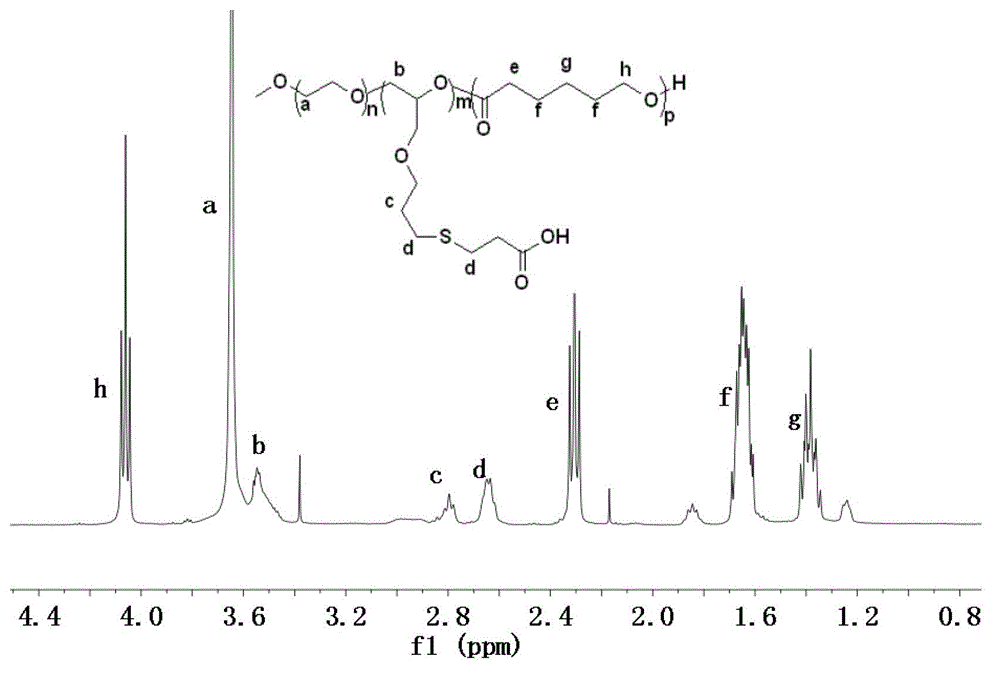
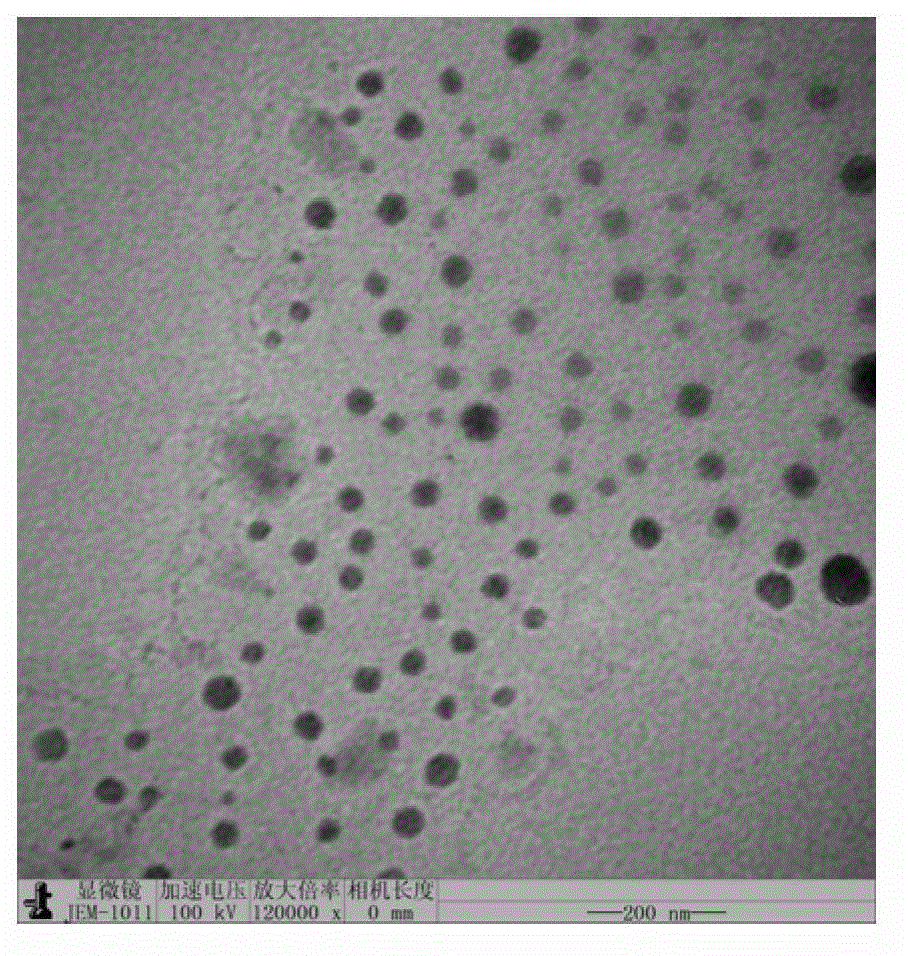
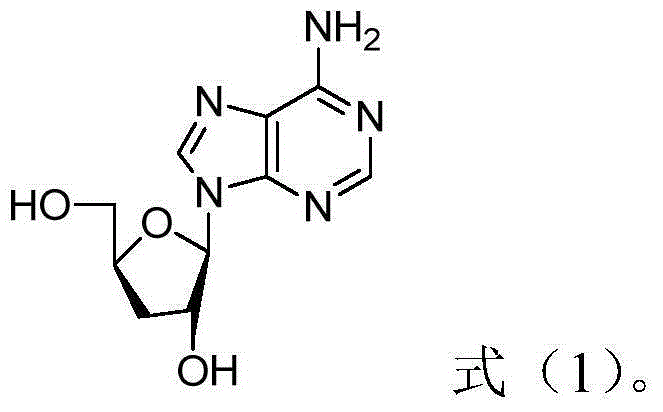
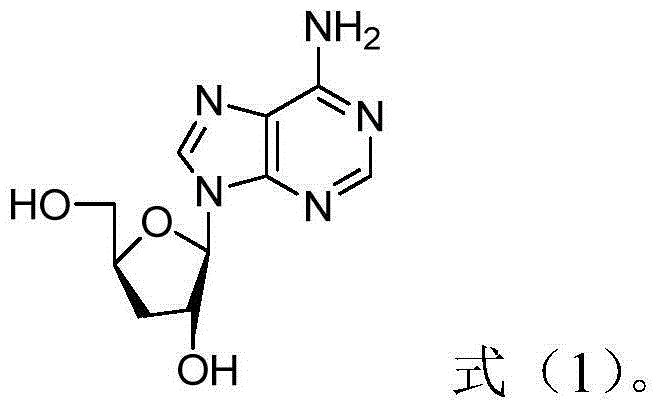
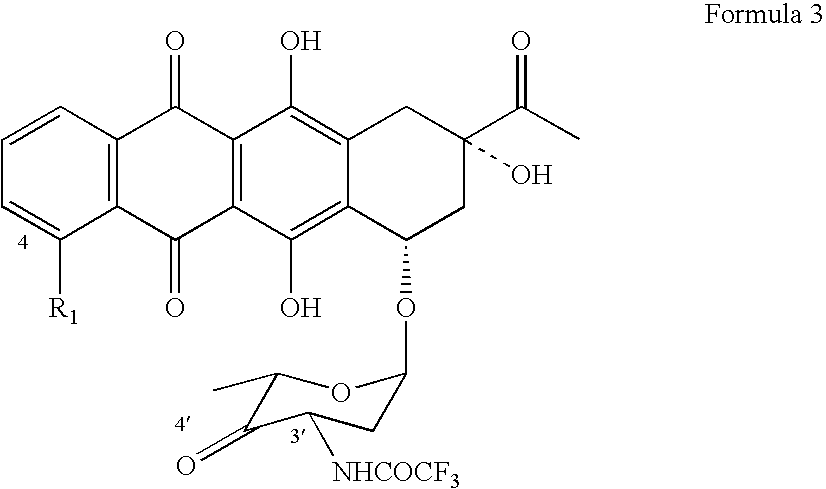
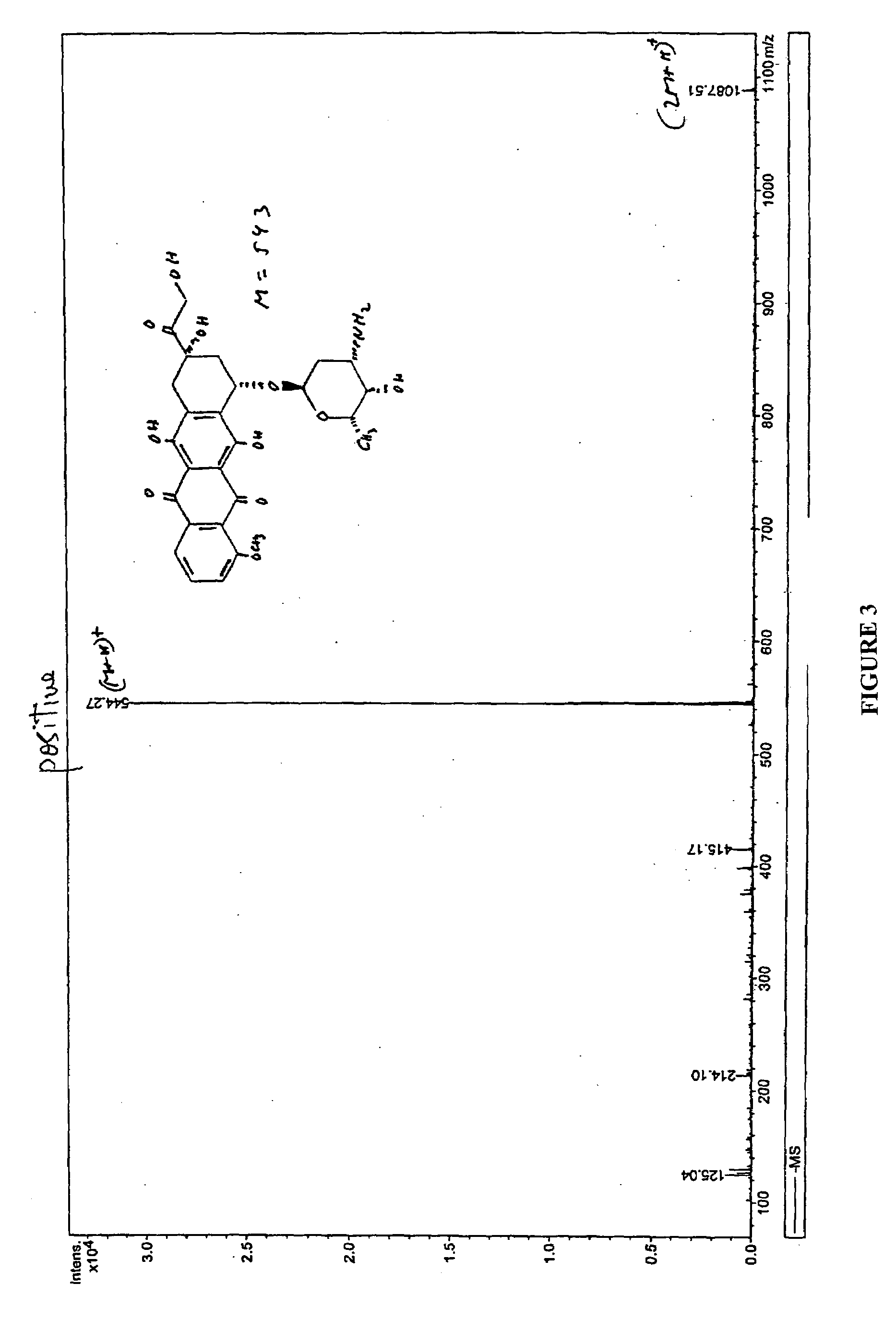
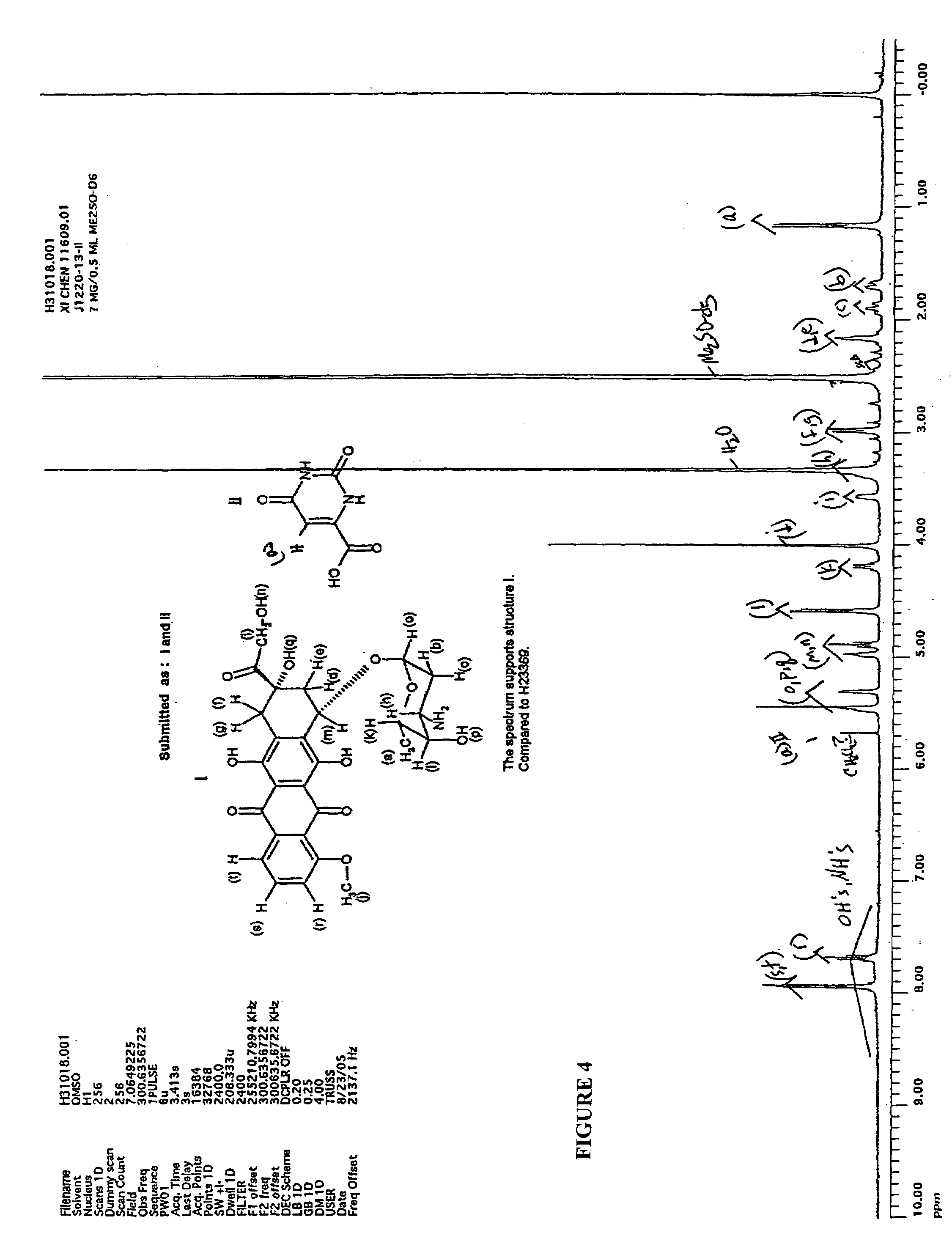
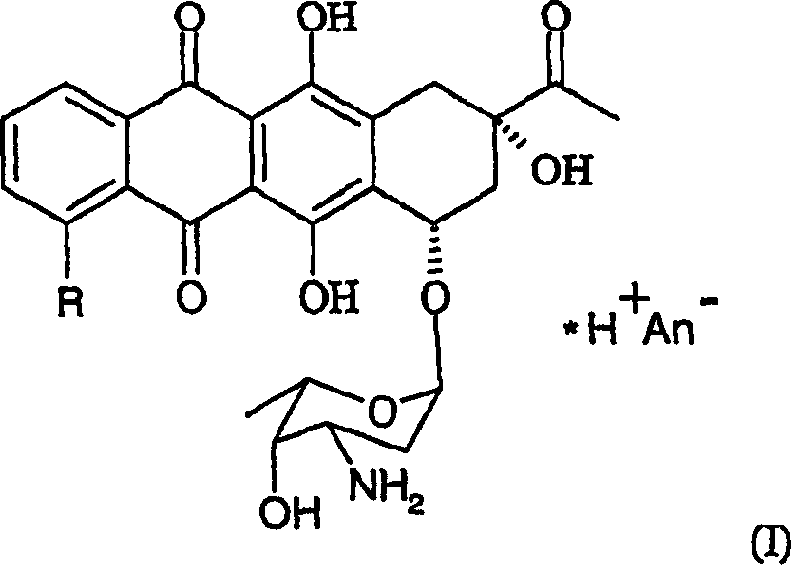
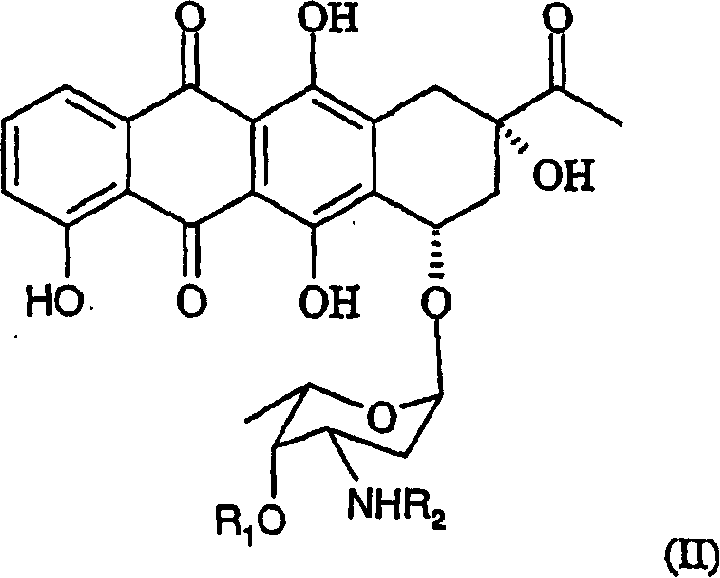
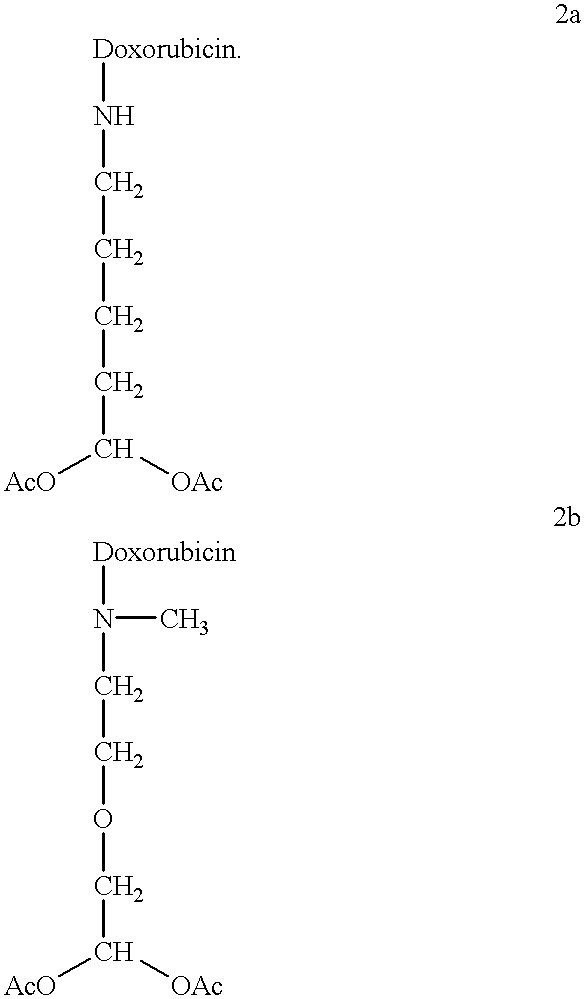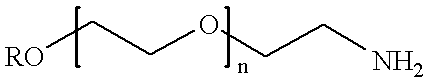Patents
Literature
99 results about "Daunorubicin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
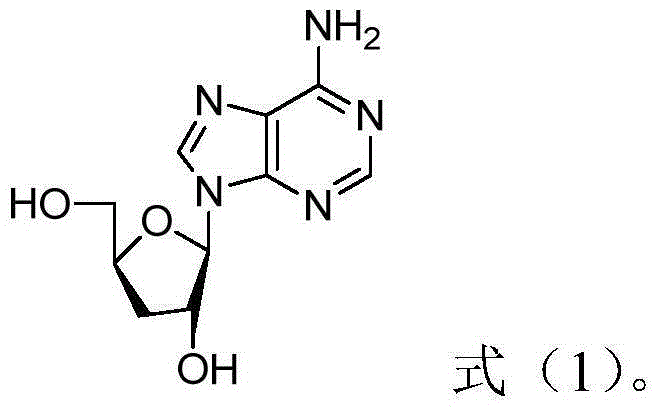
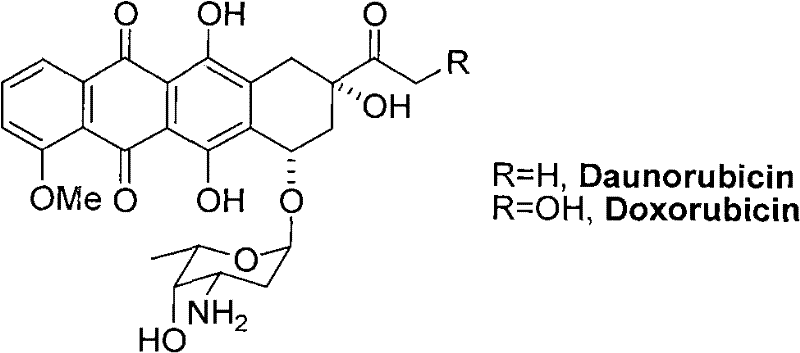
Daunorubicin is used to treat leukemia and other cancers.
Antineoplastic conjugates of transferrin, albumin and polyethylene glycol
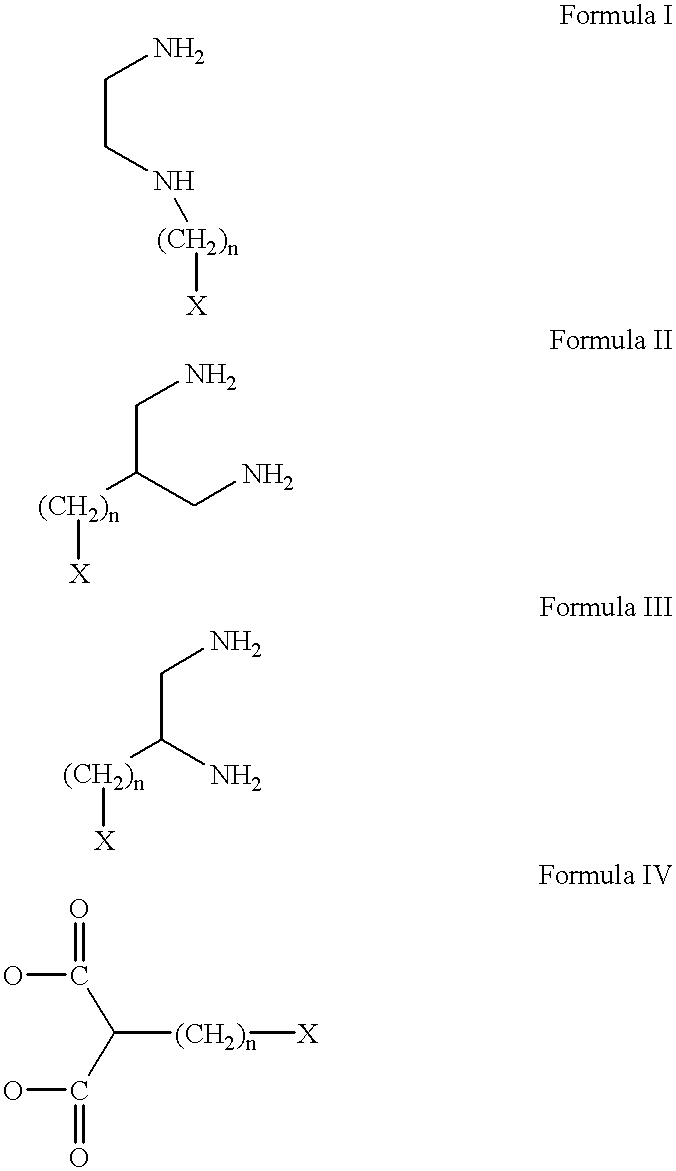
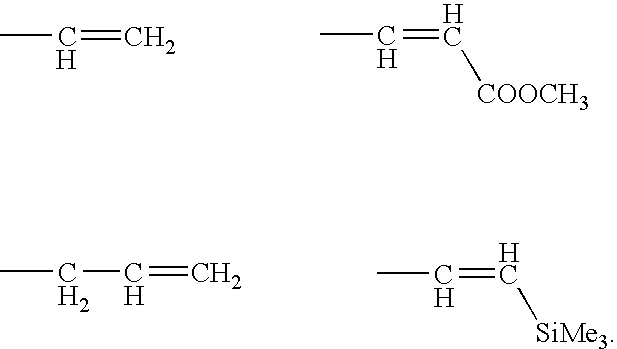
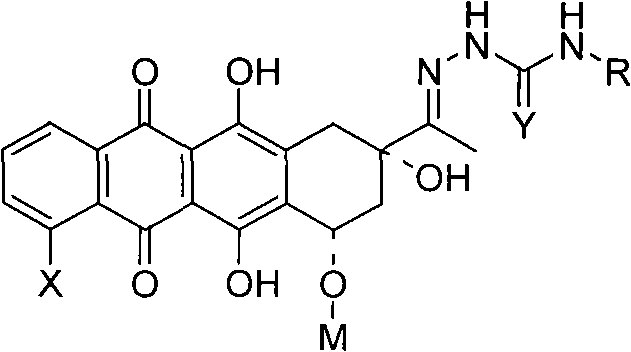
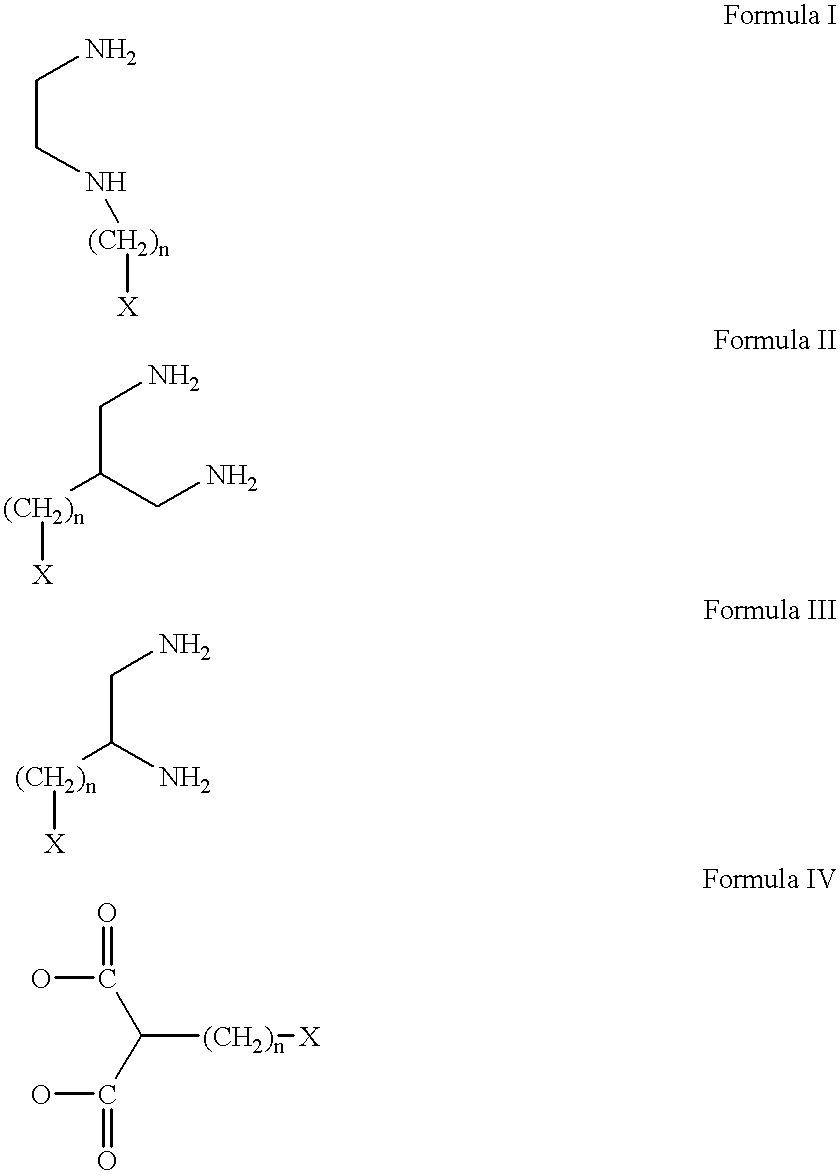
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R* H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Liposomal formulations of anthracycline agents and cytidine analogs
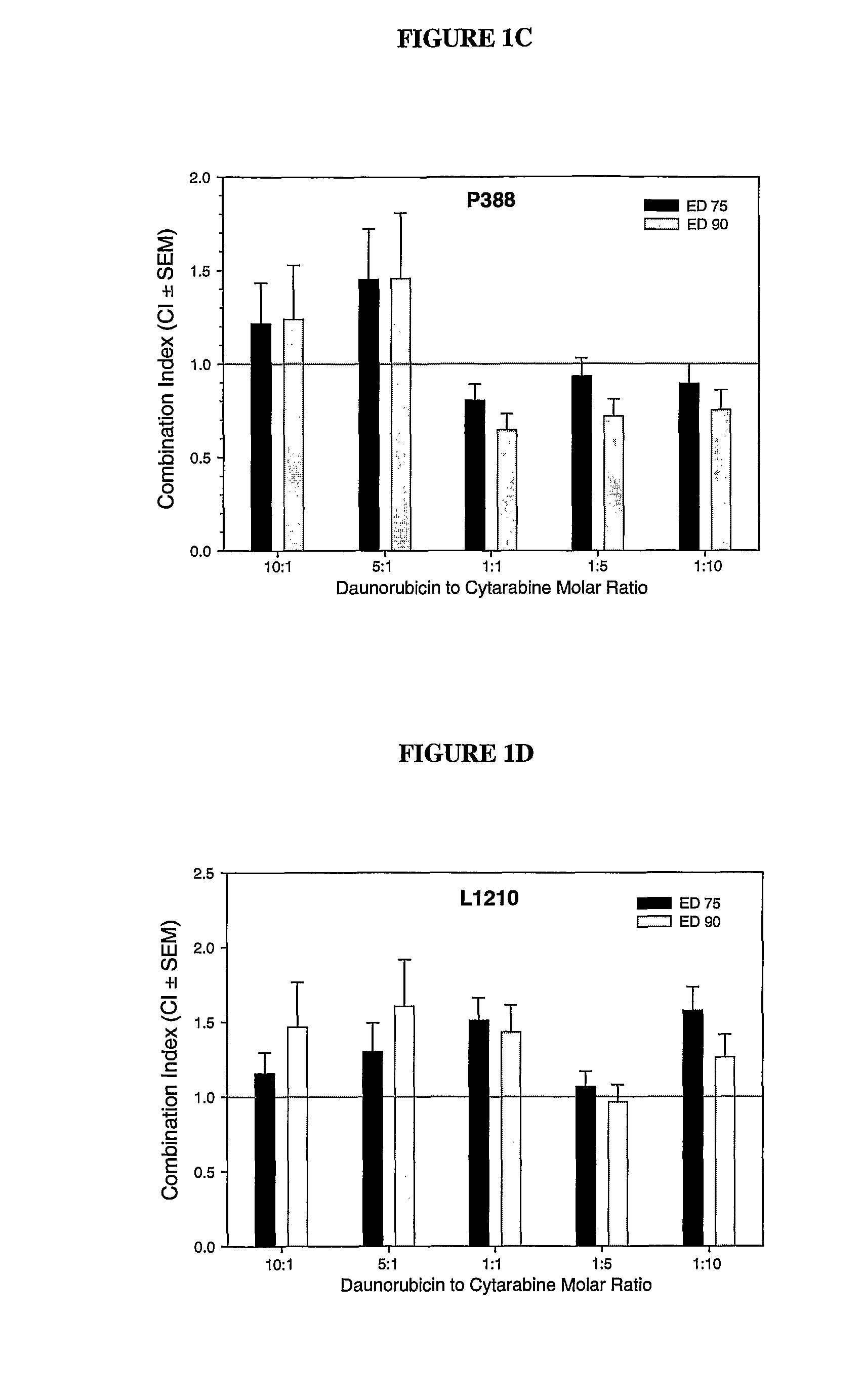
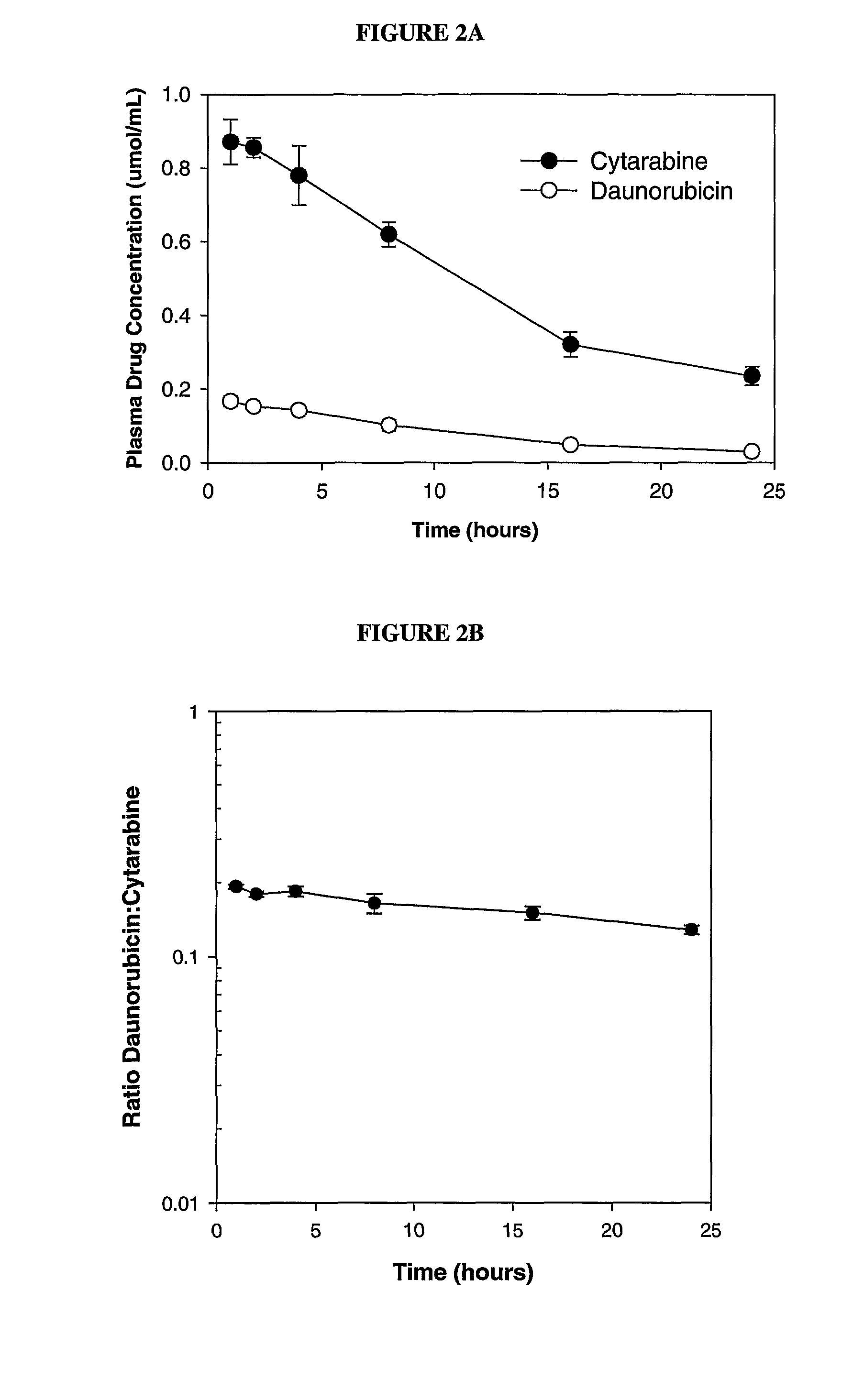
Compositions which comprise an anthracycline agent, and a cytidine analog are encapsulated in liposomal carriers. The preferred anthracycline agent is selected from the group of daunorubicin, doxorubicin, and idarubicin, while the preferred cytidine analog is selected from the group of cytarabine, gemcitabine, or 5-azacytidine. The combination of the anthracycline agent and cytidine analog encapsulated in said liposomal carriers are useful in achieving a drug retention and a sustained drug release for each therapeutic agent.
Owner:CELATOR PHARMA INC
Dual target liposome and preparation method and application thereof
InactiveCN101816629AIncrease drug concentrationResearch to aid in non-invasive treatmentsOrganic active ingredientsMacromolecular non-active ingredientsDaunorubicinNon invasive
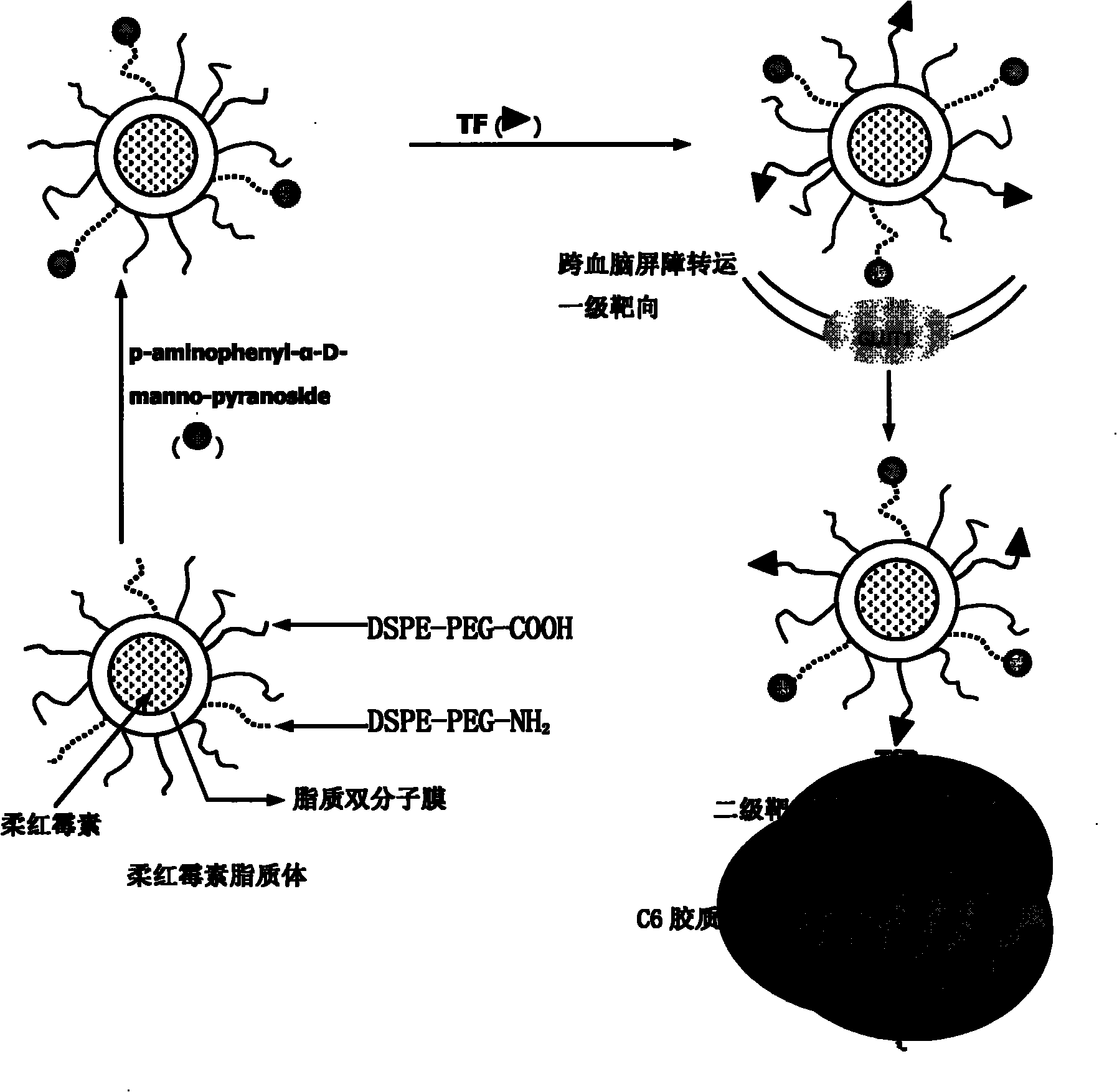
The invention discloses a dual target liposome and a preparation method and application thereof. The target liposome provided by the invention consists of liposome and modifiers on the surface of the liposome, wherein the modifiers on the surface of the liposome comprise p-aminophenyl-alpha-D-manno-pyranoside and transferrin. The invention also discloses a medicament-loaded liposome, which is obtained by wrapping daunorubicin by using the target liposome. The obtained target liposome has good capability of crossing the blood brain barrier, targets brain glioma, and can be used as a medicament carrier. The medicament-loaded liposome can target the medicament to a brain glioma site after crossing the blood brain barrier so as to greatly increase the concentration of the medicament at a tumor site and improve the effect of chemotherapy. The target liposome provides a new measure for brain glioma chemotherapy, contributes to the research of non-invasive therapy of the brain glioma, and has important theoretical meaning and clinical meaning.
Owner:PEKING UNIV
Method for preparing graphene oxide double-targeting medicine carrier material, and loaded medicine
InactiveCN102552932AAchieve targeted deliveryStrong targetingMacromolecular non-active ingredientsAntineoplastic agentsMedicineDrug carrier
The invention relates to a method for preparing a graphene oxide double-targeting medicine carrier material, and a medicine loaded by the carrier material. The method is used for preparing the carrier material and the loaded medicine. The invention solves the problems that the conventional nano-medicine carrier has a complicated structure, is high in synthetic cost and low in efficiency, and cannot be massively prepared easily. The method for preparing the graphene oxide double-targeting medicine carrier material comprises the following steps of: preparing carboxymethylated graphene oxide; preparing a graphene oxide-biologically targeting molecule composite; and preparing the graphene oxide double-targeting medicine carrier material, wherein the loaded medicine is one or more of doxorubicin hydrochloride, paclitaxel, hydroxycamptothecine and daunomycin.
Owner:HARBIN INST OF TECH
Method of preparing 4-R-substituted 4-demethoxydaunorubicin
ActiveUS7053191B2Reduce in quantityImprove processing yieldSugar derivativesSugar derivatives preparationPtru catalystAcyl group
A method of synthesizing 4-R-substituted anthracyclines and their corresponding salts from 4-demethyldaunorubicin includes the steps of treating 4-demethyldaunorubicin with a sulfonylating agent to form 4-demethyl-4-sulfonyl-R3-daunorubicin. 4-Demethyl-4-R3-sulfonyl-daunorubicin is then subject to a reducing agent in the presence of a transition metal catalyst in a temperature range of about 30° C. to about 100° C. in a polar aprotic solvent in an inert atmosphere. Protected 4-demethoxy-4-R-daunomycin then undergoes hydrolysis in a basic solution to form the 4-R-substituted anthracyclines. The novel method lacks the step of forming a stereospecific glycoside bond between aglycone and aminoglycoside. The method also increases the yield of the final product up to 30 to 40%.
Owner:SYNBIAS PHARMA
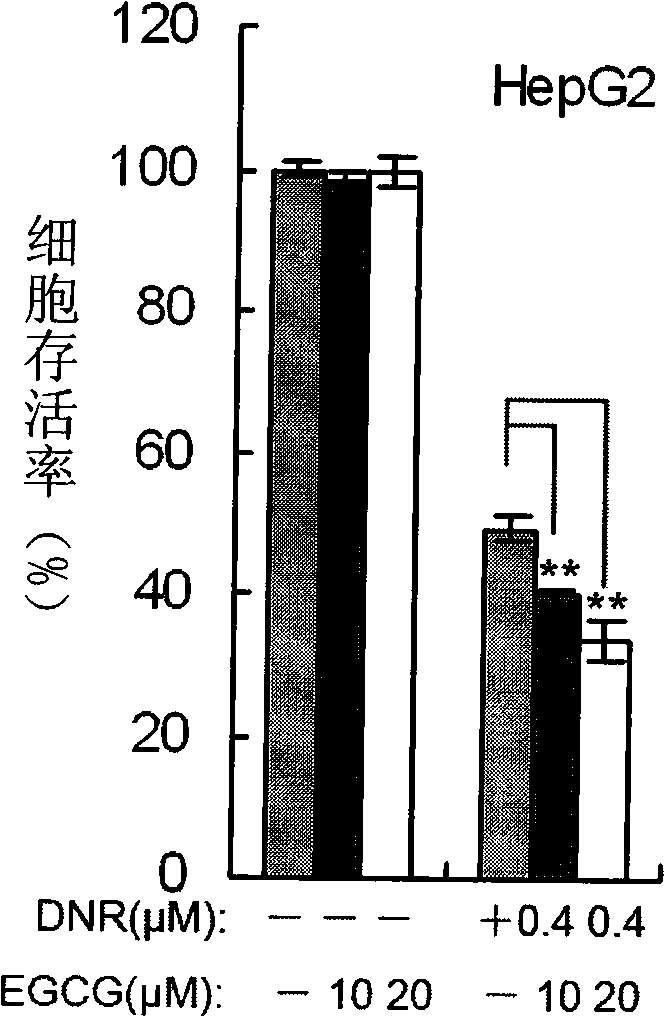
Combination of epigallocatechin-3-gallate and cerubidin and use thereof
InactiveCN101507730AReduce usagePrevent proliferationOrganic active ingredientsAntineoplastic agentsSide effectMass ratio
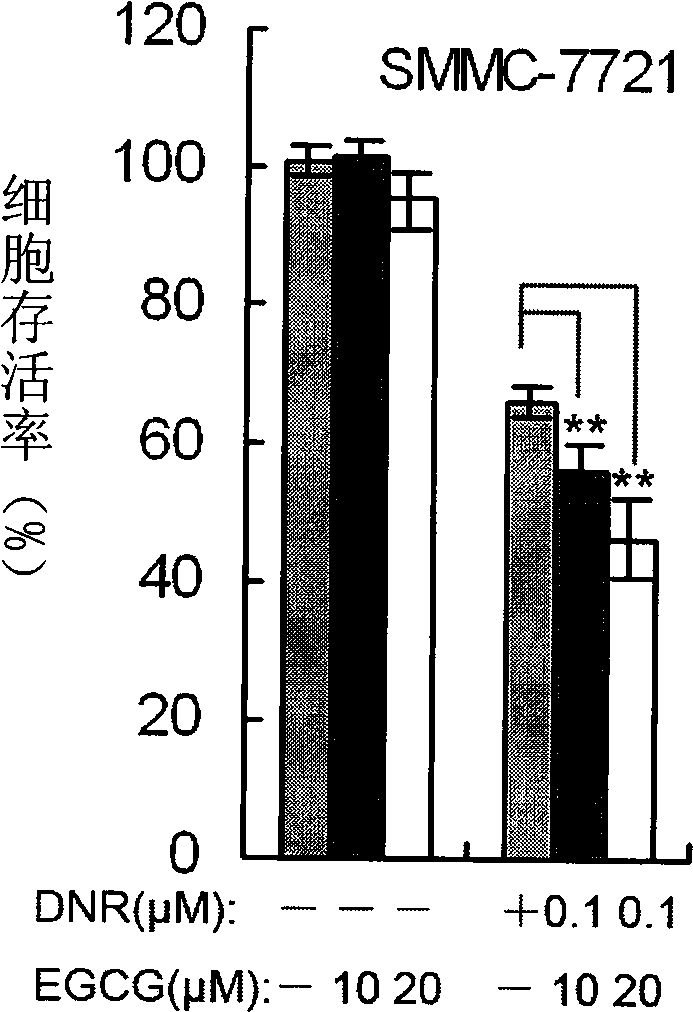
The invention belongs to the field of medicine and gene engineering, and provides a composition for inhibiting the proliferation of tumor cells. The composition contains epigallocatechin gallate (EGCG) and daunorubicin (DNR), and the mass ratio of the epigallocatechin gallate to the daunorubicin is 600: 1-1: 2. The combination of the EGCG and the DNR, on one hand, ensures that the effect of inhibiting the proliferation of the tumor cells is more obvious than the superposition of single use effects, on the other hand, the combination of the EGCG and the DNR obviously reduces the using amount of the DNR, greatly reduces the cardiac toxicity of the DNR, and strengthens the anti-tumor effect of the DNR at the same time. The EGCG is a natural tea extract, has rich resource and low price, and almost has no toxic side effect after more than one thousand years of drinking, so the combination of the EGCG and the DNR relatively reduces the using amount of the DNR and drug cost. The invention provides a high-efficiency cheap anti-tumor pharmaceutical composition, which improves the inhibition rate of tumor and reduces the treatment cost of tumor patients at the same time.
Owner:FUDAN UNIV
Methods of treatment with anthracycline derivatives
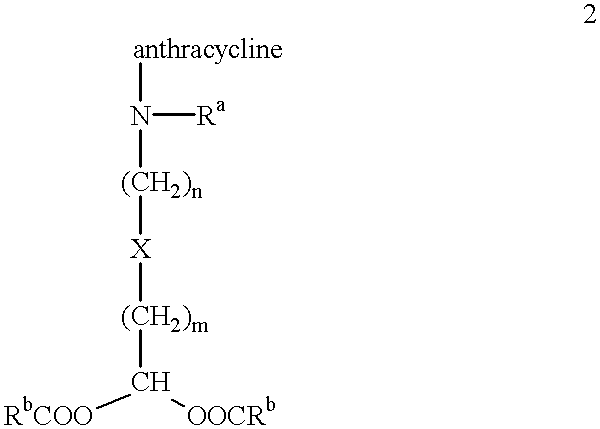
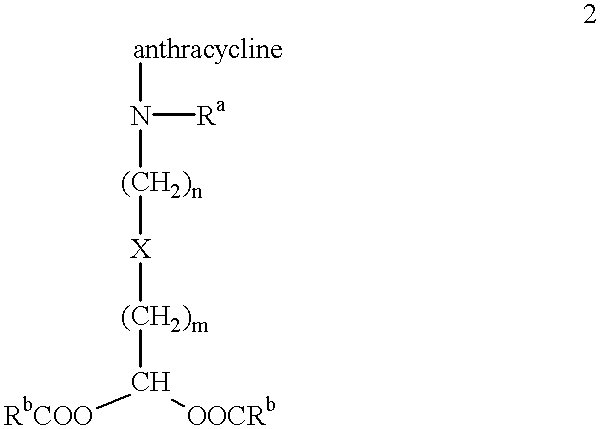
The present invention is a compound having the structurewhere:anthracycline is doxorubicin, daunorubicin or a derivative thereof;N is the 3' nitrogen of daunosamine;Ra is H or alkyl;X is, O, S, CRc2 or NRc where Rc is H or alkyl;Rb is alkyl or aryl;n is 1 to 6; andm is 0 to 6.Ra and Rc are preferably H, methyl, ethyl, propyl or butyl, although other alkyl substituents are usable. Rb is alkyl or aryl. The compound of the present invention as described above is activatable in vivo by esterases and spontaneous dehydration to form an aldehyde. The aldehyde may couple to nucleophiles of intracellular macromolecules. The compounds of the present invention are cytotoxically effective in the inhibition of human myeloma cells.
Owner:PRO NEURON INC
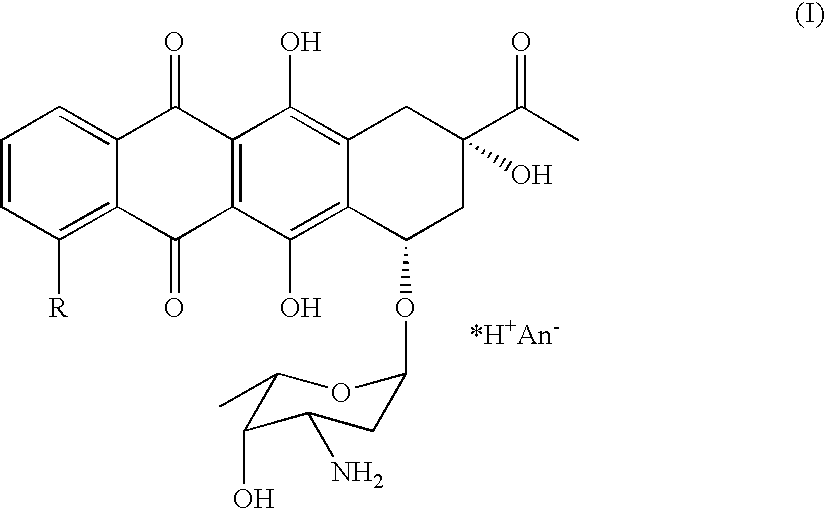
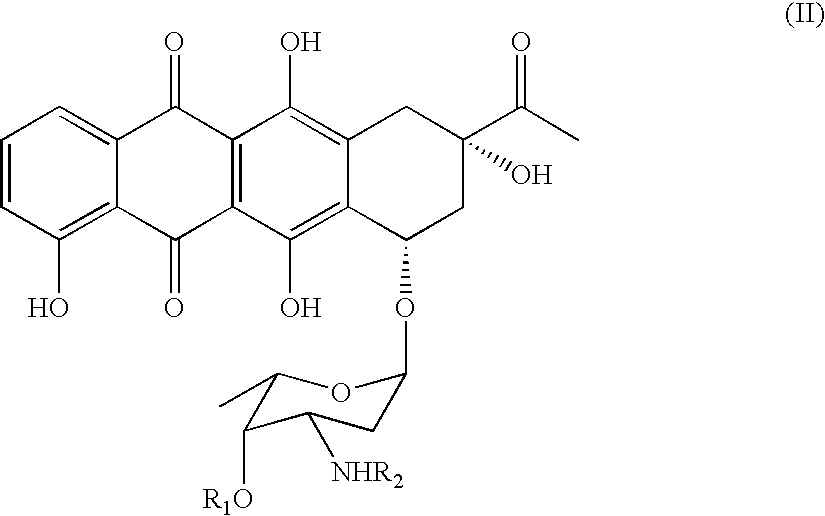
Derivative of highly active tetra cyclic anthroquinones antibiotics and preparation and application thereof
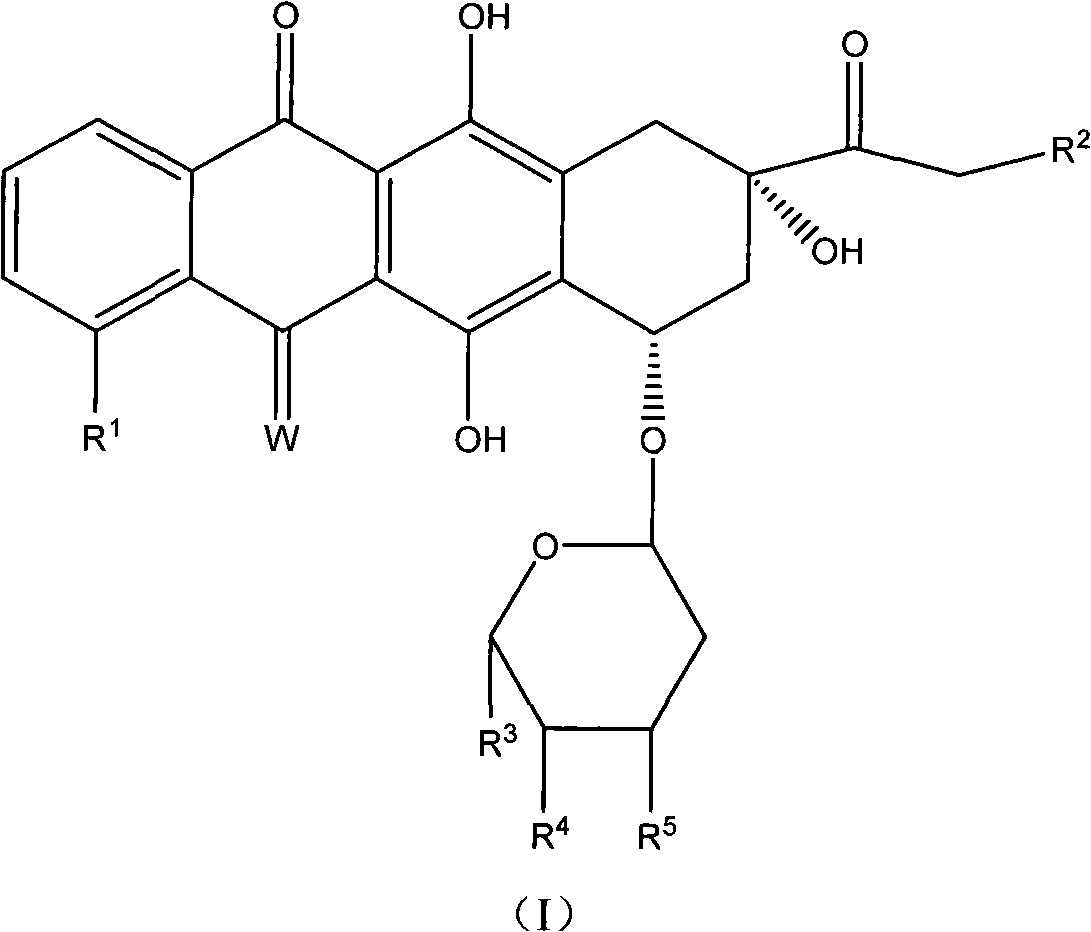
The invention relates to a derivative of tetra cyclic anthroquinones antibiotics with anti-cancer activity, namely, the compound shown by formula (I). The derivative of the tetra cyclic anthroquinones antibiotics has the activity equal to or even higher than that of the known medicines such as doxorubicin, daunorubicin and the like at cell level and simultaneously has better tolerance than the doxorubicin on animal bodies.
Owner:TIANJIN HEMAY ONCOLOGY PHARMA CO LTD
Method for preparing 4-demethyldaunorubicin
InactiveUS20070135624A1Reduce in quantitySugar derivativesAntineoplastic agentsOrganic acidOrganic solvent
A method of preparing the anthracyclin carminomycin using a starting material comprising daunorubicin. The method comprises reacting daunorubicin or N-protected daunorubicin with soft Lewis acids for the demethylation of the 4-methoxy group, resulting in a reaction mass. The reaction mass is treated with an aqueous solution of a strong organic acid or a mineral acid. After decomposition of the resulting carminomycin and Lewis acids reactive complex, the reaction mass is extracted using a water insoluble organic solvent. As a result, carminomycin is extracted as a base.
Owner:SYNBIAS PHARMA
Antharcycline antitumor antibiotics loaded nano-micelle preparation and preparation method thereof
InactiveCN102973525AAvoid dissociationHigh drug loadingOrganic active ingredientsPowder deliveryPolyesterProtein molecules
The invention discloses an antharcycline antitumor antibiotics loaded nano-micelle preparation, which comprises antharcycline antitumor antibiotics, A-B-C type triblock polymer and assisting agents, wherein the antharcycline antitumor antibiotics is one or more of adriamycin, daunorubicin, pharmorubicin, perarubicin or lacinomycin; and the A-B-C type triblock polymer is polyethylene glycol-polyethylene glycol containing carboxyl on side chain-polyester. According to the preparation, drugs are coated in the nano-micelle by virtue of the coaction of self-assembly of segmented copolymer and electrostatic adsorption, the nano-micelle has uniform and stable grain diameter, the encapsulation efficiency reaches 99 percent, and the prepared nano-micelle preparation is of a spherical structure with grain diameter between 20 and 300nm; and the shell of the micelle is made from polyethylene glycol molecules, so that drugs are prevented from being contacted with enzyme and other protein molecules in blood or identified and swallowed by a reticuloendothelial system in the body, so that the circulating period of micelle in the body can be prolonged.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI +1
Application of cordycepin in preparation of pharmaceuticals cooperating with radiotherapy and/or chemotherapy to treat tumors, and pharmaceutical composition
ActiveCN105106959AImprove efficiencyLittle side effectsOrganic active ingredientsAntineoplastic agentsSide effectDaunorubicin
The invention relates to the field of medicine and discloses application of cordycepin in preparation of pharmaceuticals cooperating with radiotherapy and / or chemotherapy to treat tumors. The cordycepin has a structure shown in the following formula (1). The invention further discloses a pharmaceutical composition cooperating with radiotherapy and / or chemotherapy to treat tumors, wherein the pharmaceutical composition comprises active ingredients including the cordycepin and at least one of daunorubicin, dactinomycin and doxorubicin. The doxorubicin has high acting efficiency and low side effect in the application to the preparation of pharmaceuticals cooperating with radiotherapy and / or chemotherapy to treat tumors.
Owner:SHENZHEN BEIANG BIOTECH CO LTD
Liposomal Formulations of Anthracycline Agents and Cytidine Analogs
Compositions which comprise an anthracycline agent, and a cytidine analog are encapsulated in liposomal carriers. The preferred anthracycline agent is selected from the group of daunorubicin, doxorubicin, and idarubicin, while the preferred cytidine analog is selected from the group of cytarabine, gemcitabine, or 5-azacytidine. The combination of the anthracycline agent and cytidine analog encapsulated in said liposomal carriers are useful in achieving a drug retention and a sustained drug release for each therapeutic agent.
Owner:CELATOR PHARMA INC
Method for production of non-natural antibiotic
Disclosed is a transformant prepared by introducing a ketoreductase gene involved in the biosynthesis of L-epivancosamine into an actinobacterium originally capable of producing daunorubicin. Also disclosed is a process of efficiently producing a non-natural daunorubicin derivative using the transformant. The transformant is capable of efficiently producing a non-natural daunorubicin derivative such as epidaunorubicin.
Owner:MEIJI SEIKA PHARMA CO LTD
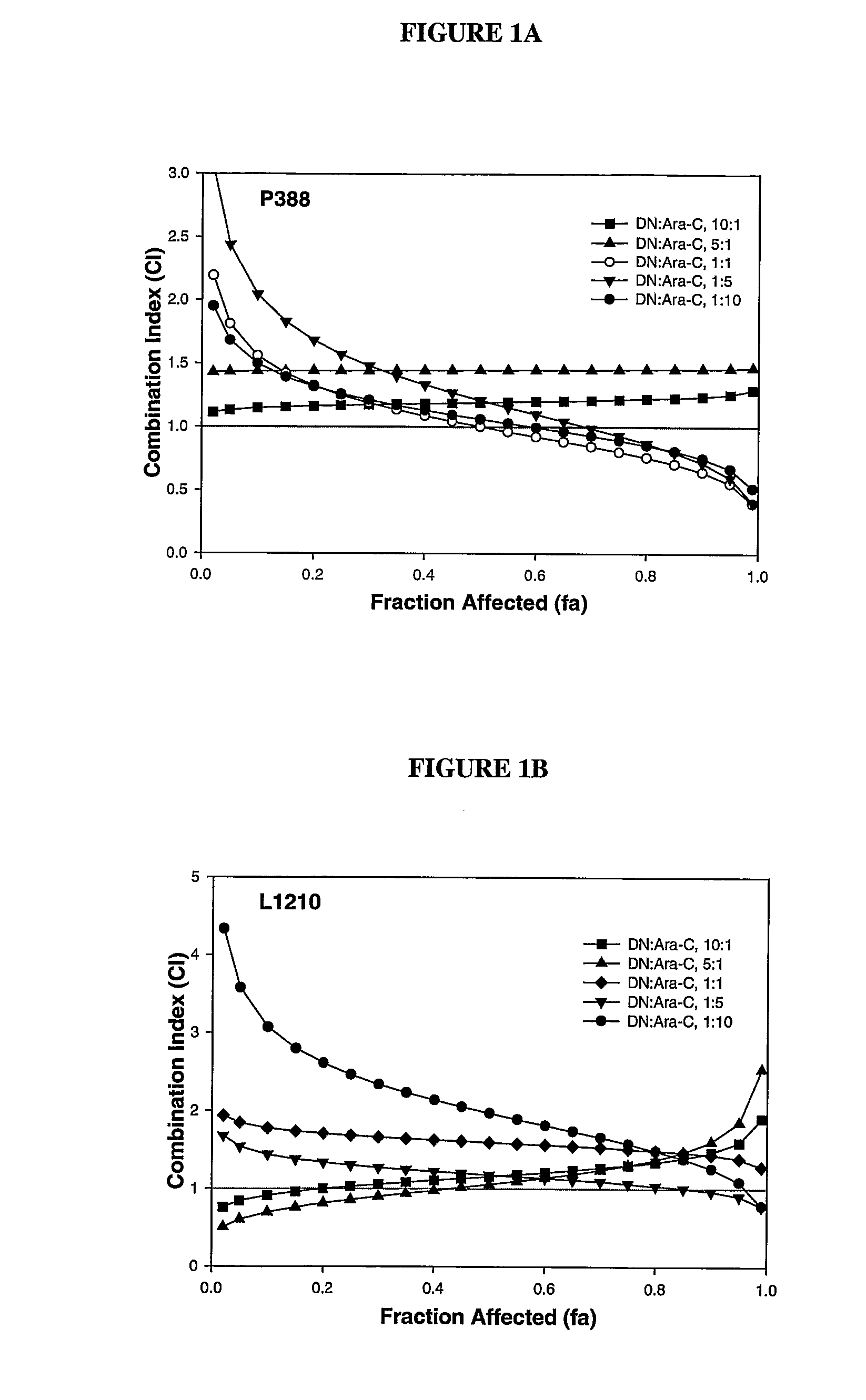
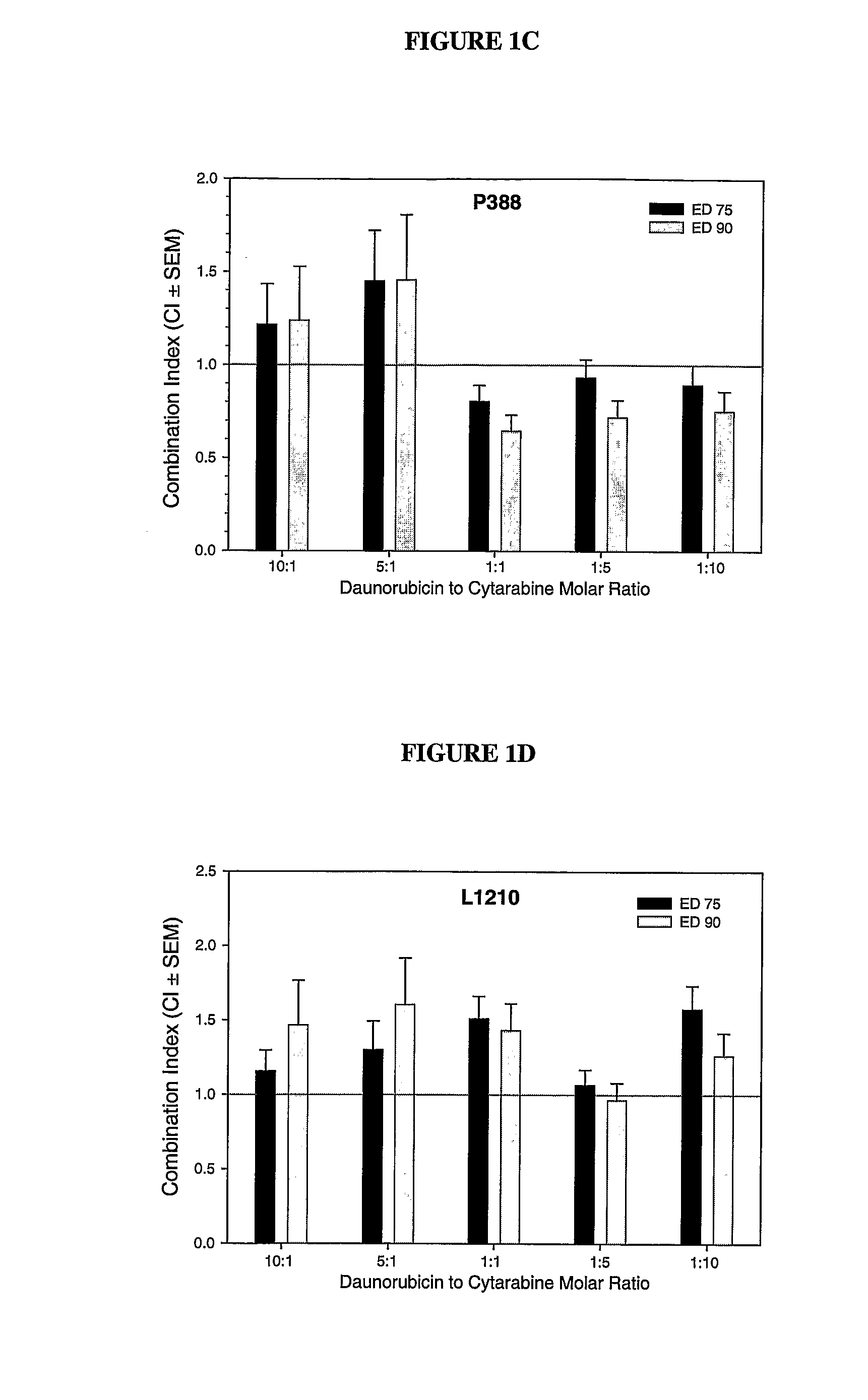
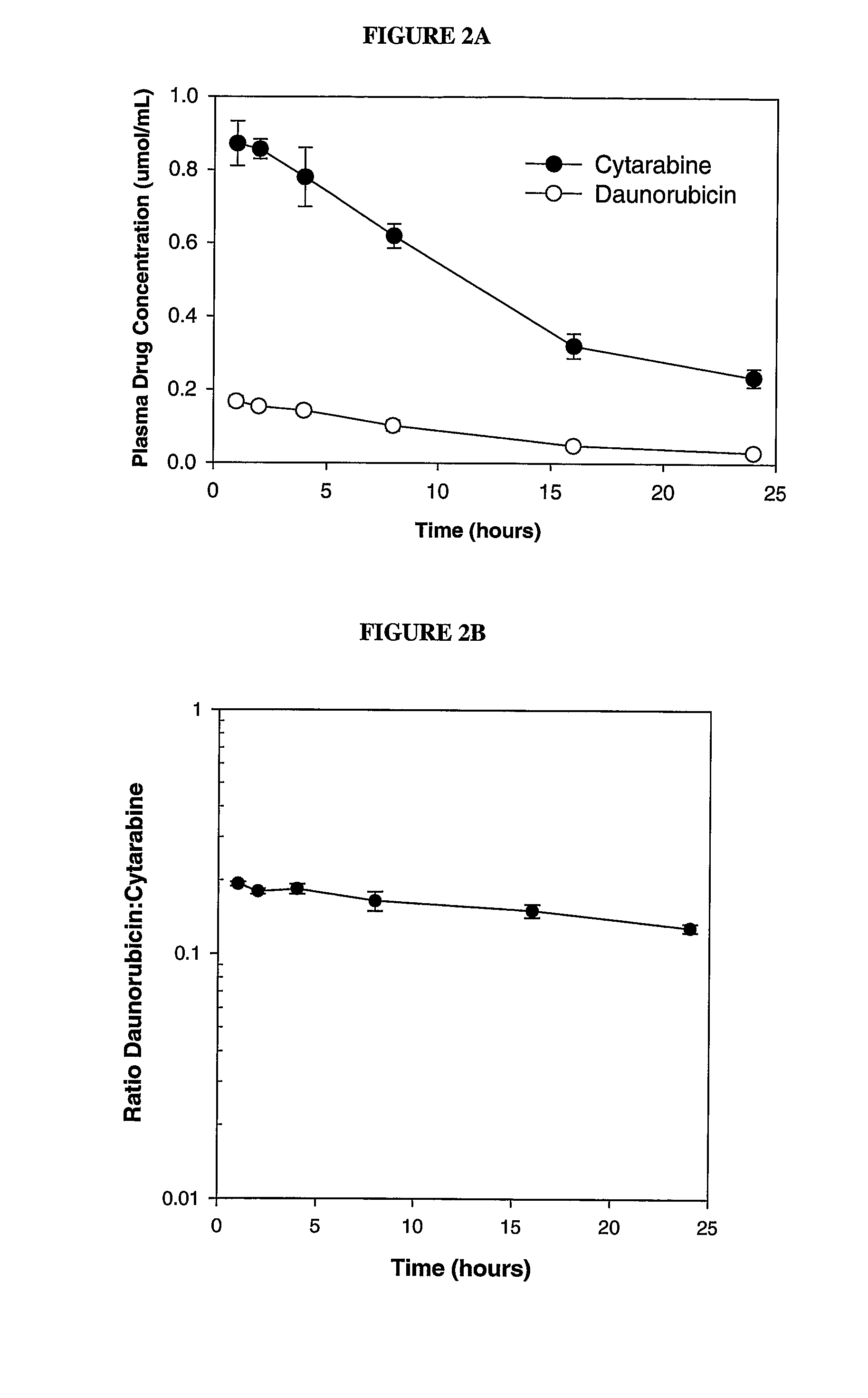
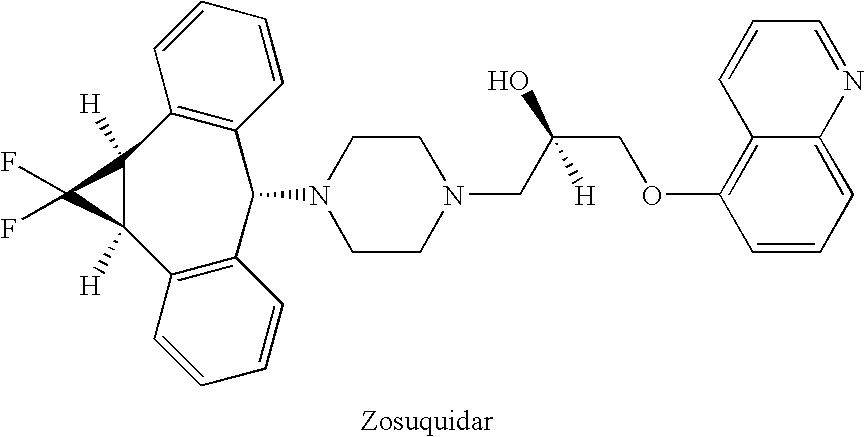
Zosuquidar, daunorubicin, and cytarabine for the treatment of cancer
InactiveUS20070010465A1Strong therapeutic activityImprove completion rateBiocideCarbohydrate active ingredientsCytarabineRecurrent acute
The present invention relates to a method of treating patients with solid tumors, leukemias, and other malignancies using a combination of zosuquidar, daunorubicin, and cytarabine. The invention is also directed to pharmaceutical formulations comprising zosuquidar, daunorubicin, and cytarabine. The formulations are particularly effective in treating relapsed Acute Myelogenous Leukemia (AML).
Owner:KANISA PHARMA INC
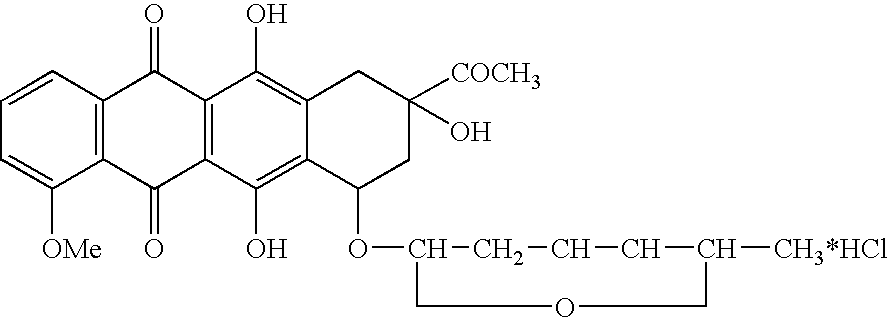
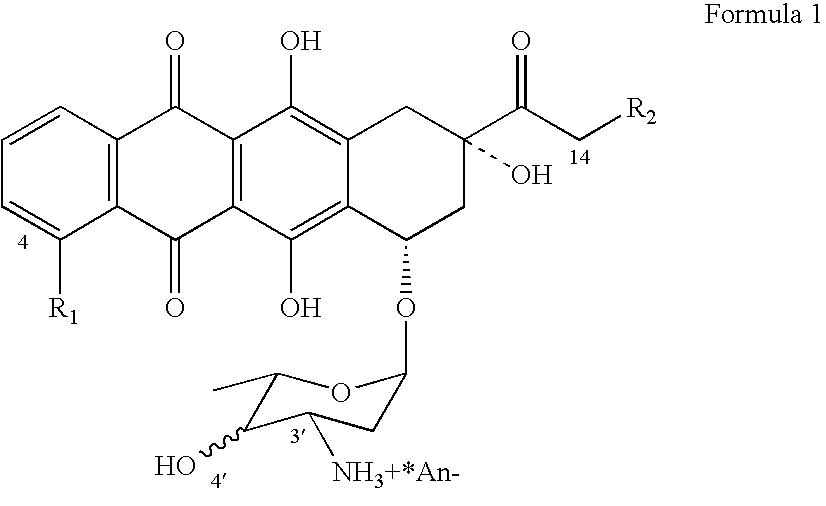
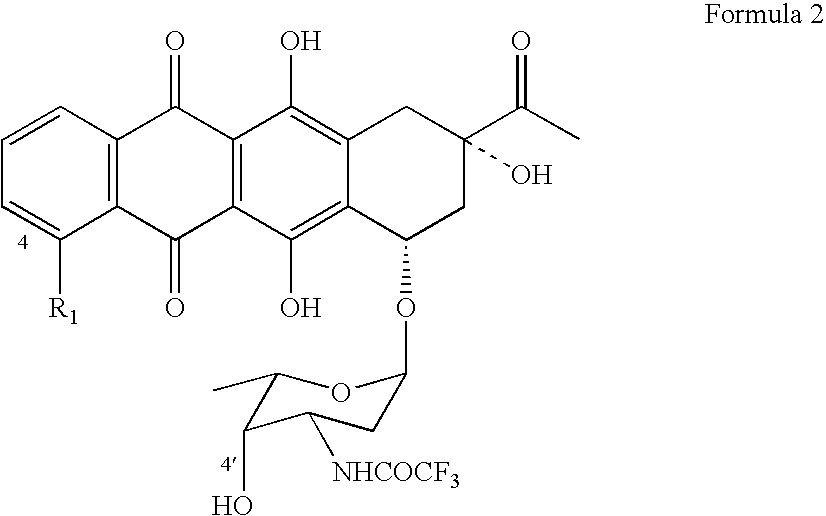
Epimerization of 4'-C bond and modification of 14-CH3-(CO)-fragment in anthracyclin antibiotics
ActiveUS20060223766A1Reduce in quantitySimplification of extractionBiocideSugar derivativesFormateAntibiotic Y
A method of synthesizing R1, R2-substituted-4′ (ax. or eq.)-OH anthracyclines and their corresponding salts of Formula (1) from daunorubicin or N-Trifluoroacetyl-4-R1-derivatives of daunorubicin, wherein R1 is defined as H, OH, and 4′-HO is defined as ax[ial]. The method includes producing N-Trifluoroacetyl daunorubicin and treating the N-Trifluoroacetyldaunorubicin or N-Trifluoroacetyl-4-R1-derivatives of daunorubicin, wherein R1 is defined as H, OH, with dimethylsulfoxide activated by different acylating agents. The attained intermediate product is then treated with a strong base (ex. tertiary amines) resulting in the 4′-keto-N-Trifluoroacetyl-4-R1 daunorubicin wherein R1 is defined as H, OH, OMe. The 4′-keto-N-Trifluoroacetyl-4-R1-daunorubicin is reacted with a reducing agent, a derivative of a borohydride of an alkaline metal MHBL3 , to produce N-Trifluoroacetyl-4′-epi-4-R1-daunorubicin. The N-Trifluoroacetyl-4′-epi-4-R1-daunorubicin undergoes hydrolysis in a basic solution to produce a derivate of an anthoacyclin which is halogenized [by complex halogenides] to form a 14-Hal-derivative. This result is then hydrolyzed by well-known methods in the presence of a formate of an alkaline metal to form the desired final compound.
Owner:SYNBIAS PHARMA
Compounds and compositions for the treatment of cancer
New uses for phenylketone carboxylate compounds and substituted aromatic compounds of Formula I, Formula I.1, Formula I.2, Formula IA, Formula IB, Formula IC and Formula II and their pharmaceutical acceptable salts for the treatment of cancer. The use of a combination of two of these compounds is described and the use of the combination of one of these compounds with an anticancer agent such as decarbazine, doxorubicin, daunorubicin, cyclophosphamide, busulfex, busulfan, vinblastine, vincristine, bleomycin, etoposide, topotecan, irinotecan, taxotere, taxol, 5-fluorouracil, methotrexate, gemcitabine, cisplatin, carboplatin and chlorambucil.
Owner:PROMETIC PHARMA SMT LTD
Zosuquidar, daunorubicin, and cytarabine for the treatment of cancer
InactiveUS20070010478A1Improve completion rateStrong therapeutic activityBiocideCarbohydrate active ingredientsCytarabineRecurrent acute
The present invention relates to a method of treating patients with solid tumors, leukemias, and other malignancies using a combination of zosuquidar, daunorubicin, and cytarabine. The invention is also directed to pharmaceutical formulations comprising zosuquidar, daunorubicin, and cytarabine. The formulations are particularly effective in treating relapsed Acute Myelogenous Leukemia (AML).
Owner:KANISA PHARMA INC
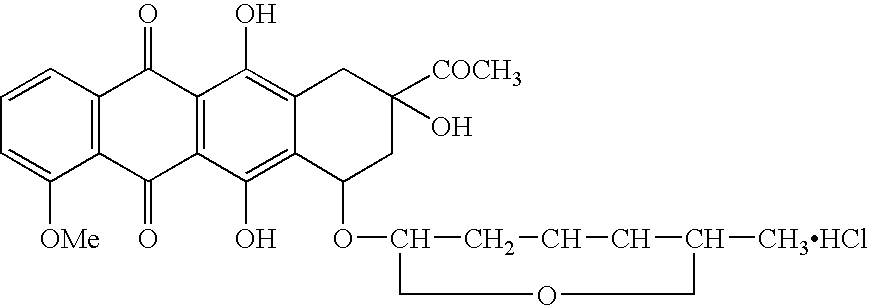
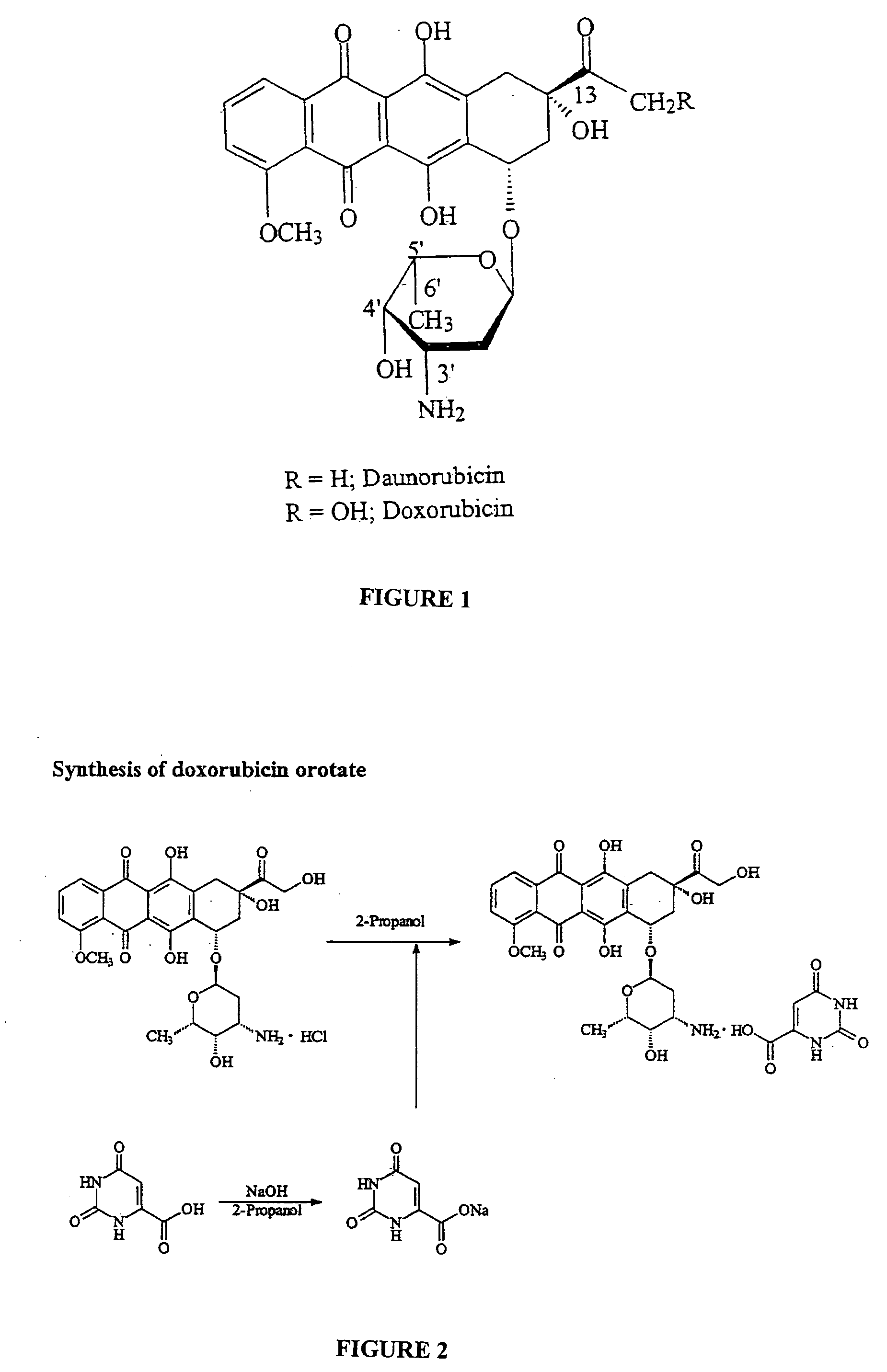
Compositions and methods of reducing tissue levels of drugs when given as orotate derivatives
ActiveUS20080025949A1Lower drug concentrationLow toxicityBiocideSalicyclic acid active ingredientsSide effectTissue toxicity
This invention is in the field of chemical restructuring of pharmaceutical agents known to cause tissue toxicity as a side effect, by producing their orotate derivatives. More particularly, it concerns orotate derivatives of the anthracyclines, doxorubicin and daunorubicin, that are found to reduce levels of the pharmaceutical agent in noncancerous tissues. There orotate derivatives are equally efficacious in inhibiting the SCCAKI-1 kidney tumor in animals and the reduction in the heart tissue of doxorubicin compared with doxorubicin HCl suggests a reduction in toxicity induced by free radical generation by the anthrracyclines.
Owner:SAVVIPHARM INC
Method of preparing 4-R-substituted 4-demethoxydaunorubicin
The method for synthesizing 4-R-substituted anthracycline antibiotics and corresponding salts thereof from 4-demethyldaunorubicin comprises the following steps: treating 4-demethyldaunorubicin with a sulfonating agent to form 4-demethyldaunorubicin Methyl-4-sulfonyl-R3-daunorubicin; then in an inert atmosphere, in an aprotic polar solvent, in the temperature range of about 30-100 ° C, in the presence of a transition metal catalyst to make 4- Demethyl-4-R3-sulfonyl-daunorubicin reacts with reducing agent. Then, the protected 4-desmethoxy-4-R-daunorubicin is hydrolyzed in alkaline solution to form 4-R-substituted anthracycline antibiotics. The new method eliminates the step of forming a stereospecific glycoside between the aglycone and the aminoglycoside. The method also increases the yield of the final product by up to 30 to 40%.
Owner:苏洛克股份有限公司
Method for preparing high-purity 4'-epi-daunorubicin
InactiveCN102190691AHigh puritySimple methodSugar derivativesSugar derivatives preparationAqueous acetoneDaunorubicin
The invention provides a method for preparing high-purity 4'-epi-daunorubicin, which comprises the following steps of: a) loading the treated 4'-epi-daunorubicin fermentation liquor on macroporous weak acid resin, and eluting by using aqueous solution of acetone or methanol added with hydrochloric acid; b) evaporating the 4'-epi-daunorubicin eluent to dryness, dissolving in water, and extracting by using chloroform; and c) loading the 4'-epi-daunorubicin extracting solution on nonpolar adsorption resin, and eluting by using aqueous solution of acetone to obtain the 4'-epi-daunorubicin. The method has simple steps, is suitable for large-scale industrial production, and has high commercial value; and the final purity of the prepared 4'-epi-daunorubicin is improved to over 97 percent.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Degradable polymer supported nanometer Daunorubicin microsphere and its prepn process
InactiveCN1973843AGood dispersionGood inhibition rateOrganic active ingredientsPowder deliveryPhosphateMicrosphere
The present invention is degradable polymer supported nanometer Daunorubicin microsphere and its preparation process. The microsphere has size of 200-800 nm, Daunorubicin carrying rate of 3.05-18.4 %, encapsulating rate of 53.2-88.3 %, and degradable polymer polylactic acid or polylactic acid-polyglycolic acid copolymer. Experiment shows that the nanometer Daunorubicin microsphere may be released continuously in buffering phosphate solution for 2-8 weeks and the releasing rate and period may be controlled through changing the sort and molecular weight of the polymer. The nanometer Daunorubicin microsphere is superior to conventional Daunorubicin preparation, and has higher acute promyelocytic leukemia cell ingesting efficiency, higher tumor cell inhibiting rate and long effective period.
Owner:HUAZHONG UNIV OF SCI & TECH
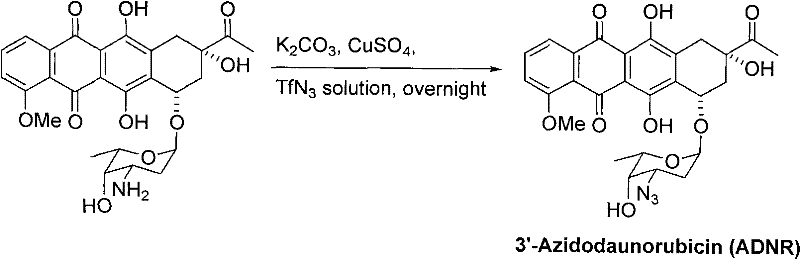
New 3'-azido daunorubicin-13-thiosemicarbazone compound with cancer resistance and preparation method thereof
InactiveCN102603824AEnhanced inhibitory effectOrganic active ingredientsSugar derivativesCancer cellSulfur
The invention discloses a new 3'--azido daunorubicin-13-thiosemicarbazone compound with cancer resistance and a preparation method thereof. The preparation method comprises the following specific steps: reacting 3'-azido daunorubicin with various thiosemicarbazide to synthesize the new 3'-azido-daunorubicin-13-thiosemicarbazone compound. The compound shows high activity in the activity test of cancer cells.
Owner:HENAN NORMAL UNIV
Temperature controlled sustained-release injection containing anti-cancer medicine
InactiveCN101273965APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectVinorelbine
The invention relates to a temperature-controlled sustained-release injection containing an anti-cancer drug, which consists of the anti-cancer drug and an amphiphilic block copolymer hydrogel and has the temperature-sensitive gelatinization feature, the temperature-controlled sustained-release injection is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, thus allowing the drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the temperature-controlled sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be vincristine, vinorelbine, navelbine, vindesine, vinleurosine, vinrosidine, cephalotaxine, bleomycin, daunomycin, aclarubicin, epirubicin, idarubicin, pirarubicin, valrubicin, mitomycin C, actinomycin D, losoxantrone, mitoxantrone, mitozolomide, temozolomide and so on.
Owner:SHANDONG LANJIN PHARMA +1
Antineoplastic conjugates of transferin, albumin and polyethylene glycol
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R*H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Synthesis method for valrubicin
InactiveCN103694291AAvoid it happening againAvoid generatingSugar derivativesSugar derivatives preparationState of artSynthesis methods
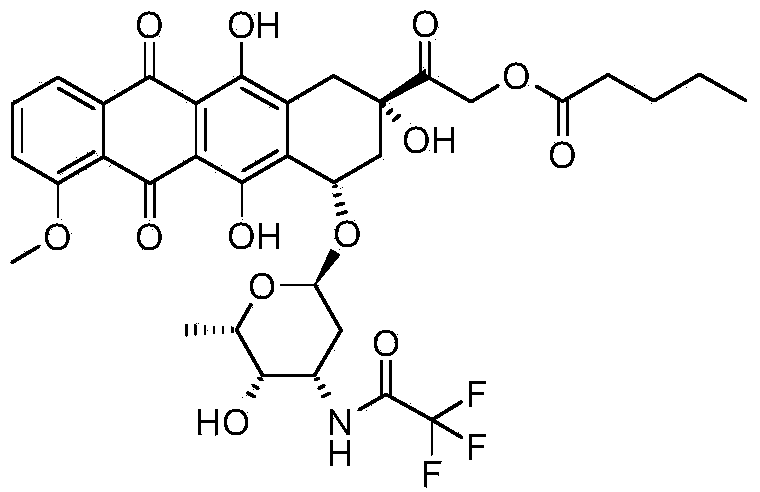
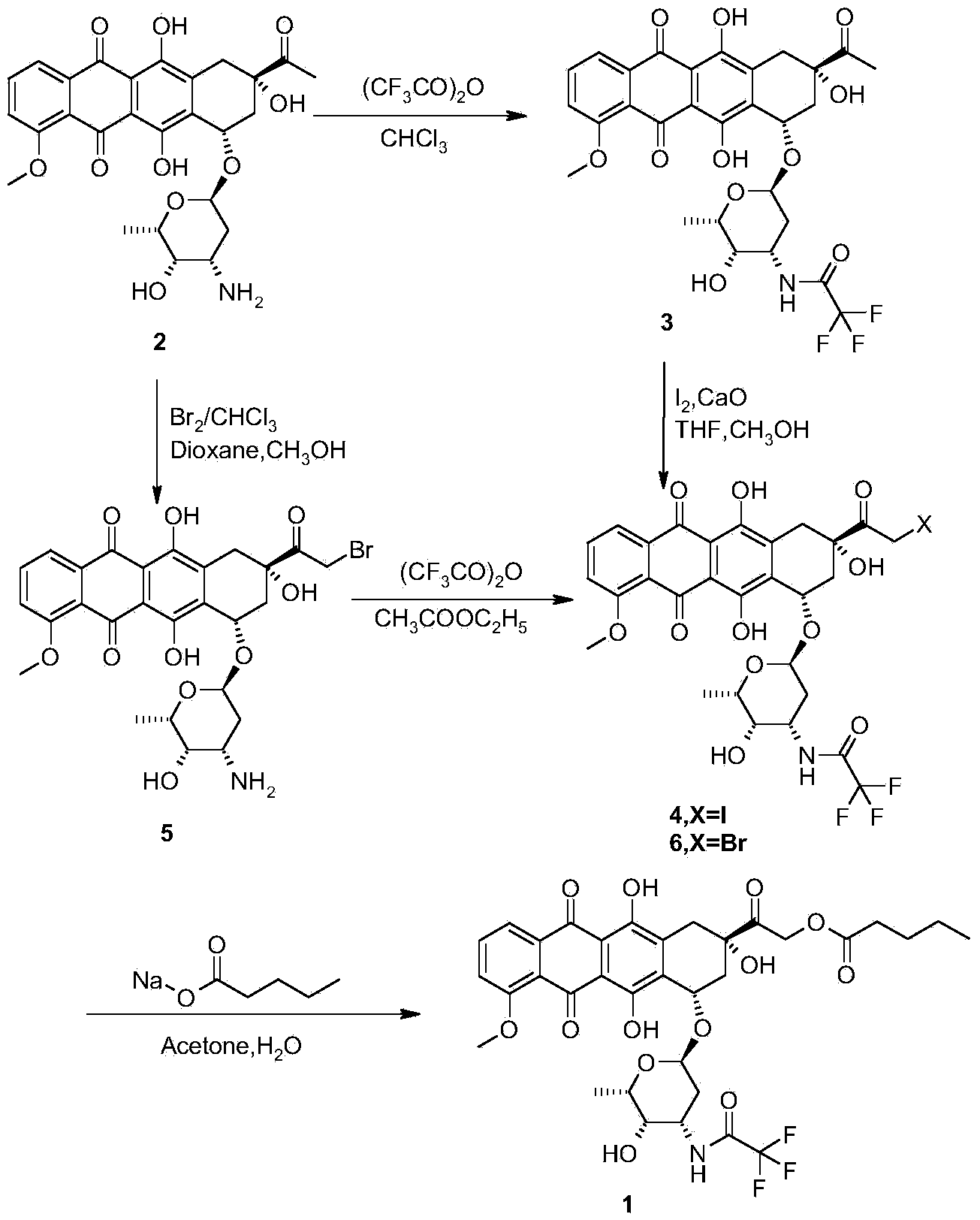
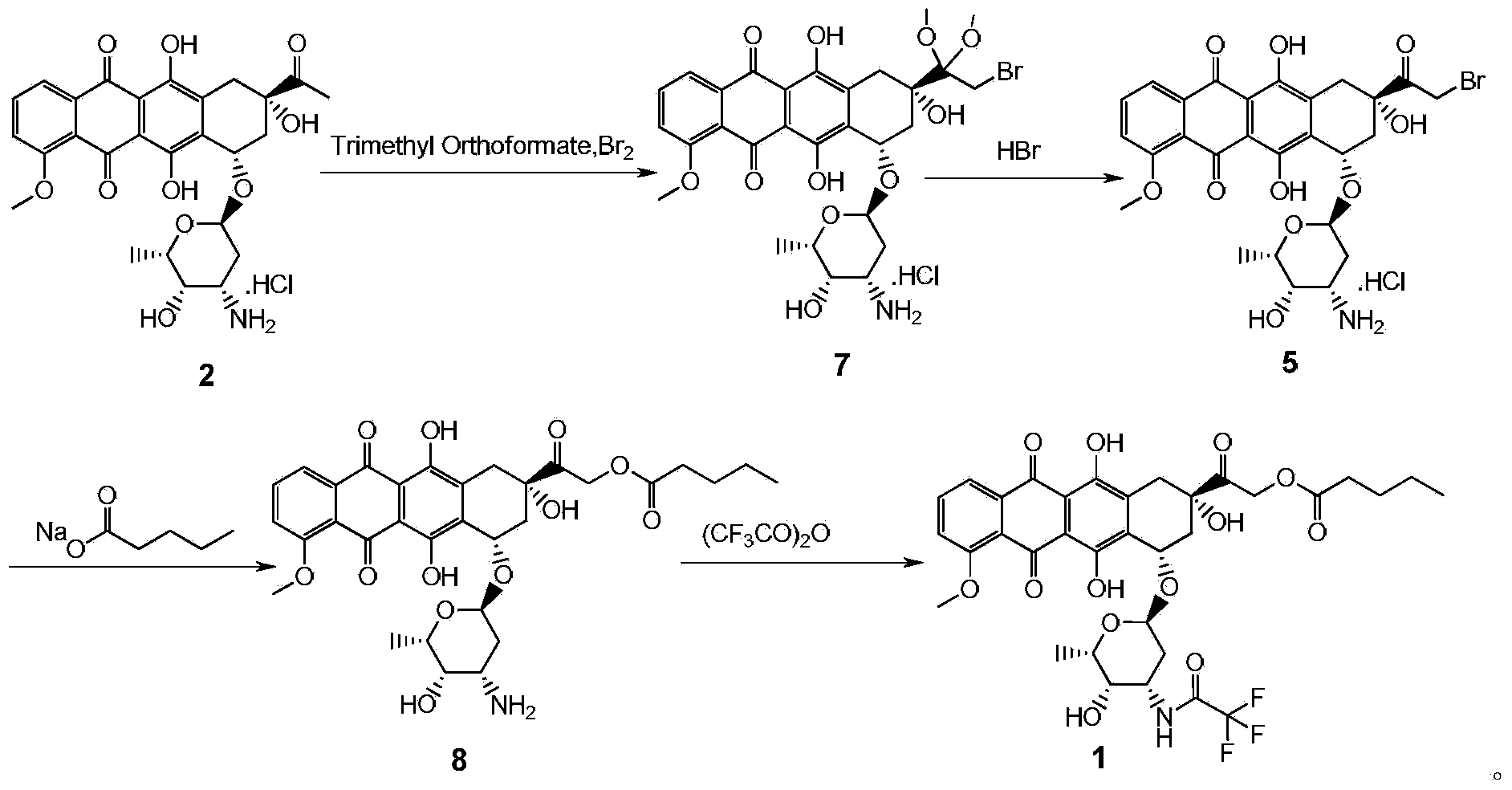
The invention provides a synthesis method for valrubicin. Daunorubicin is used as a starting material. The method comprises the following steps of selectively protecting carbonyls, performing bromination reaction, removing carbonyl protection, performing reaction with sodium n-valerate, and performing reaction with trifluoroaceticanhydride to obtain amide to prepare the valrubicin. Compared with the prior art, the synthesis method has the advantages that intermediate compounds 5 and 8 obtained by such a synthesis route are high in purity and low in impurity content, so that a target product valrubicin with purity of over 99 percent is prepared, and is high in yield and suitable for industrial production.
Owner:深圳万乐药业有限公司
Antitumor agent and process for producing the same
InactiveUS7682630B2Improving selective solid targeting capacityEliminate side effectsBiocideHeavy metal active ingredientsCancer cellDaunorubicin
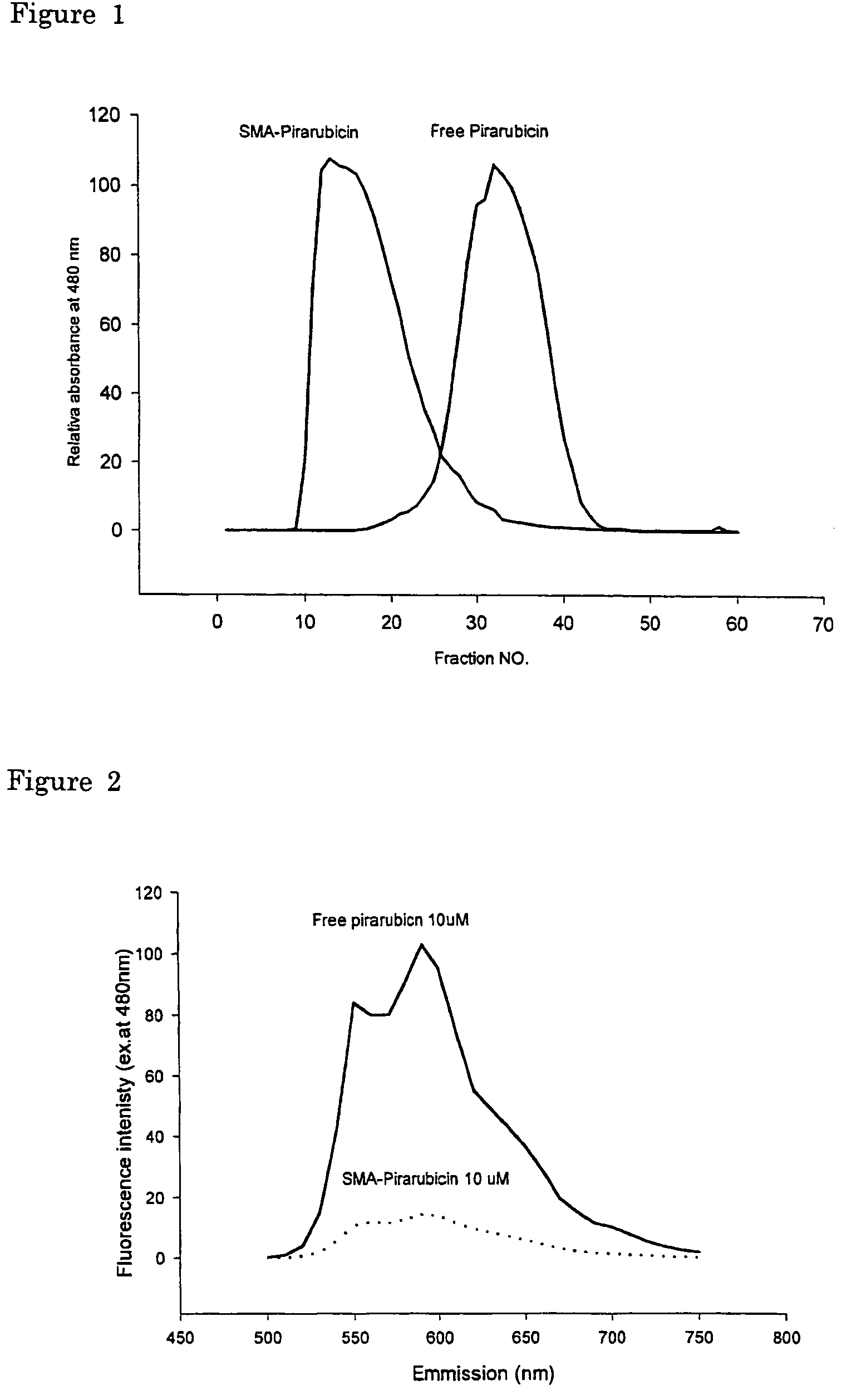
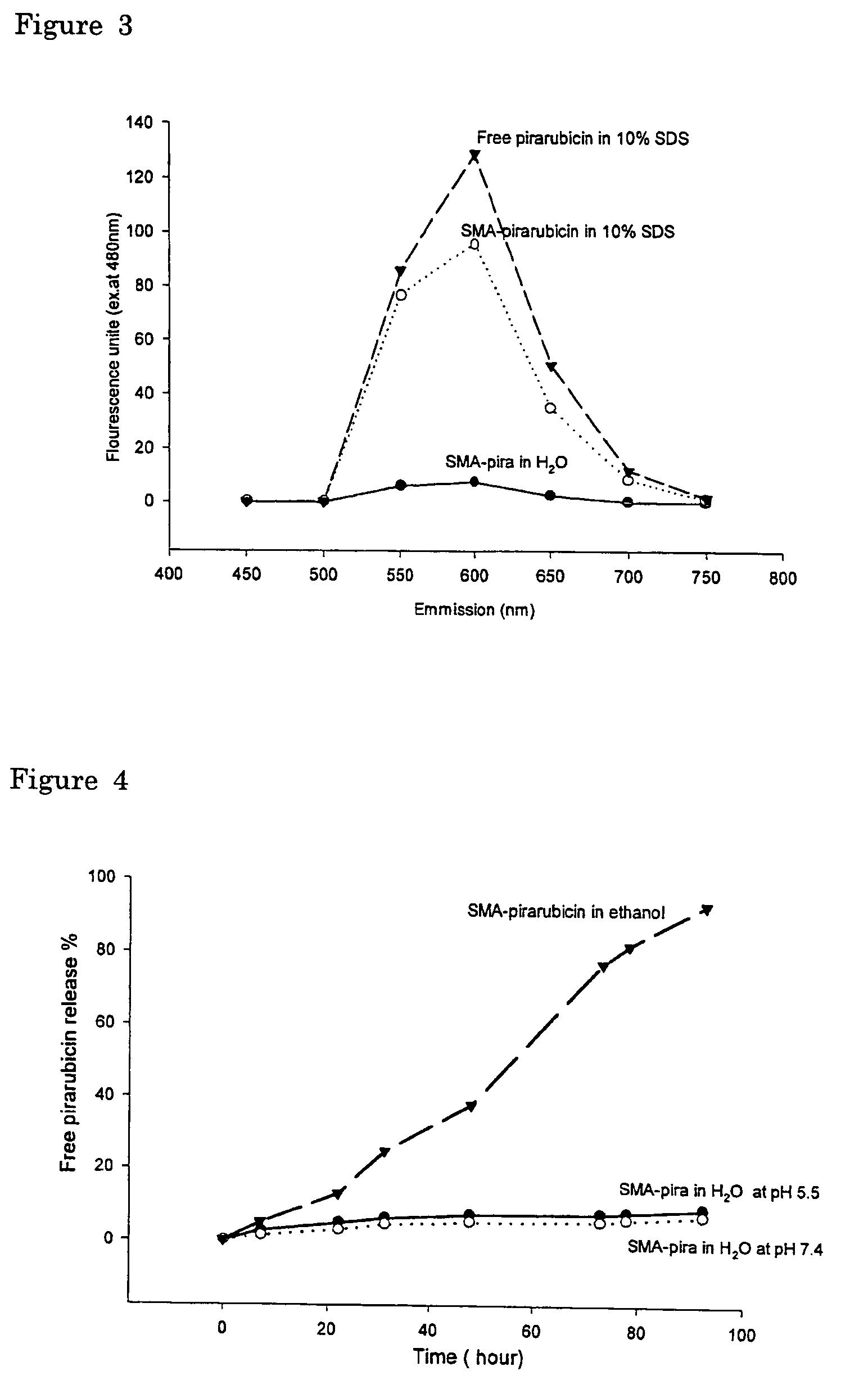
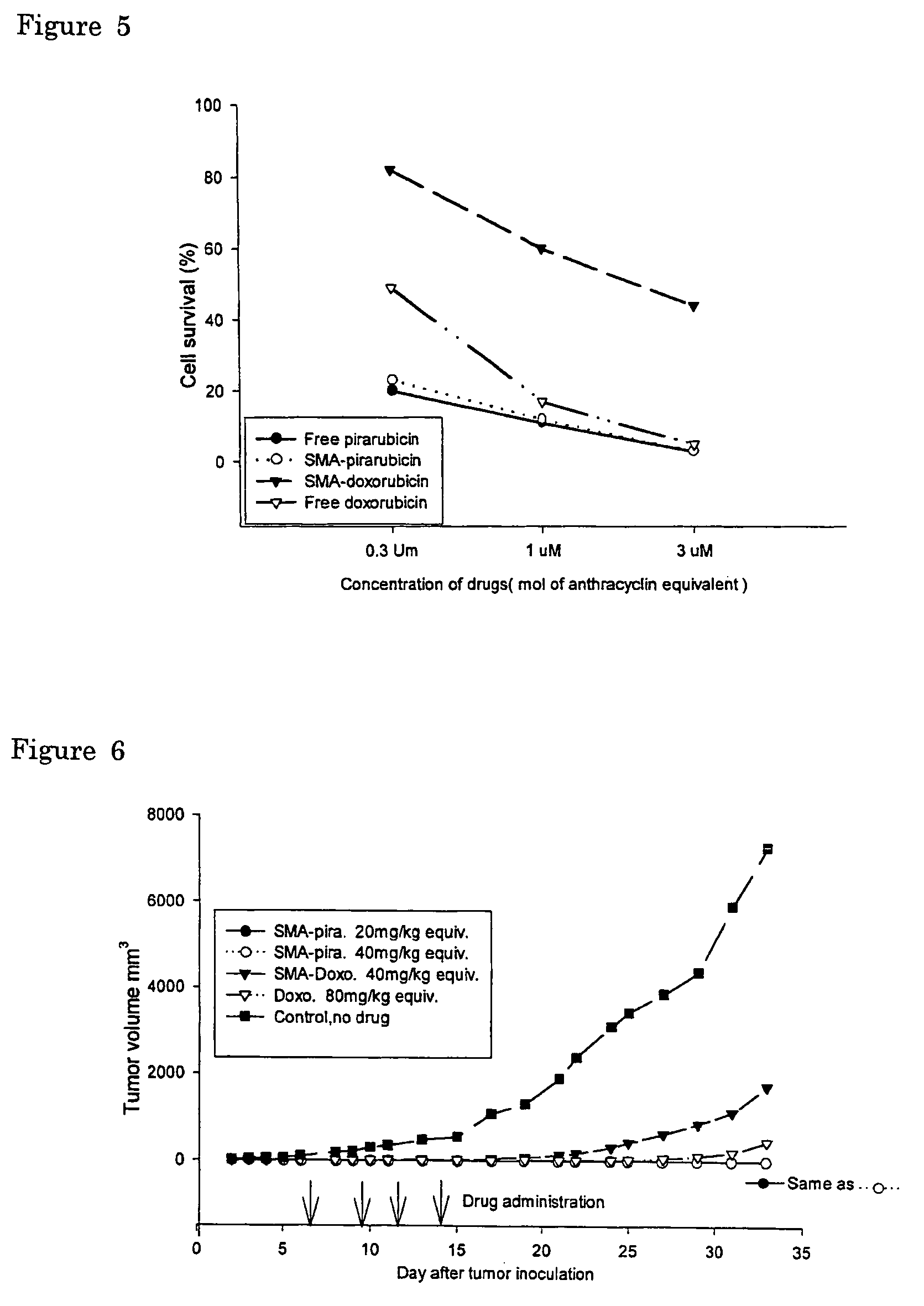
The present invention relates to polymeric antitumor agent which is formed in polymeric micelle complex by intermolecular bonding or mutual interaction between styrene maleic acid copolymer (SMA) and low molecule antitumor agent which is anthracyclins drug such as pirarubicin, doxorubicm, epirbicin, daunorbicin, acralbicin, or alkaloid antitumor agent such as cis-platinum, and taxol These polymeric antitumor agents may improve specificity to cancer cells so that it improves antitumor effect, while it may not be concentrated at normal organ or tissue, so that adverse effect may be diminished. These polymeric antitumor agents may be prepared by dissolving SMA and low molecule antitumor agent in aqueous solution, then in the presence of aqueous soluble carbodiimide, amino acids, or polyamine, adjusting pH to form micelle complex and recovering polymer fraction.
Owner:MAEDA HIROSHI
Targeted anti-tumor drug system for drug-resistant tumor cells and construction method thereof
ActiveCN109620969AGood biocompatibilityMitigation of lethalityPowder deliveryOrganic active ingredientsUltrasound contrast mediaPorphyrin
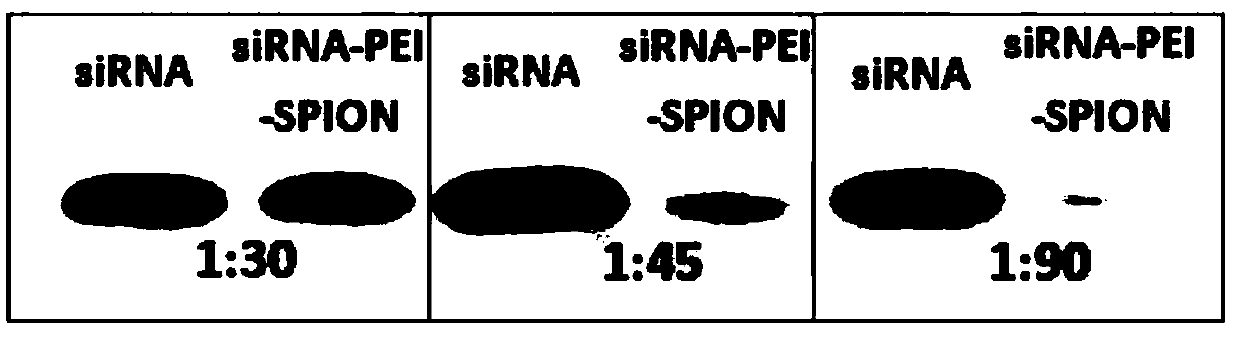
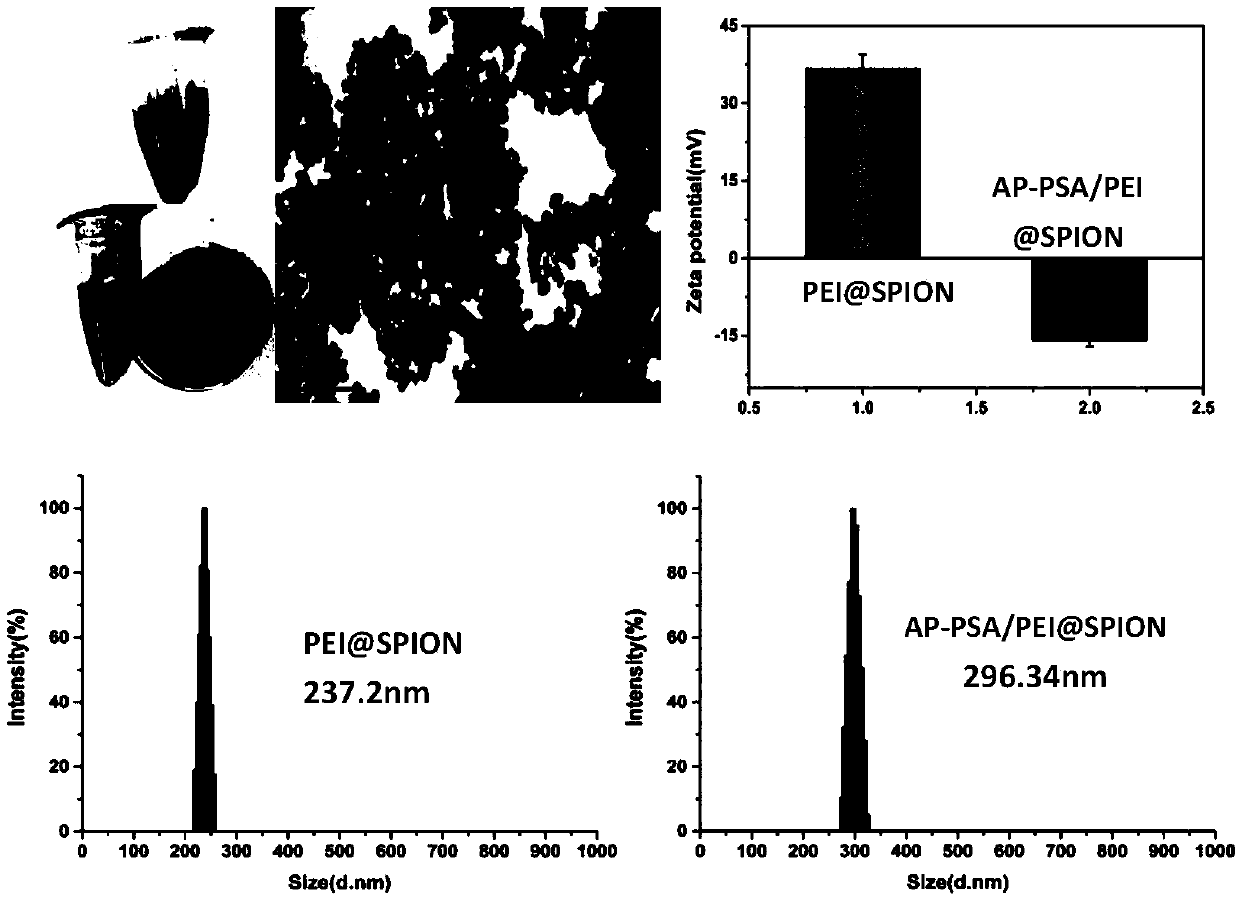
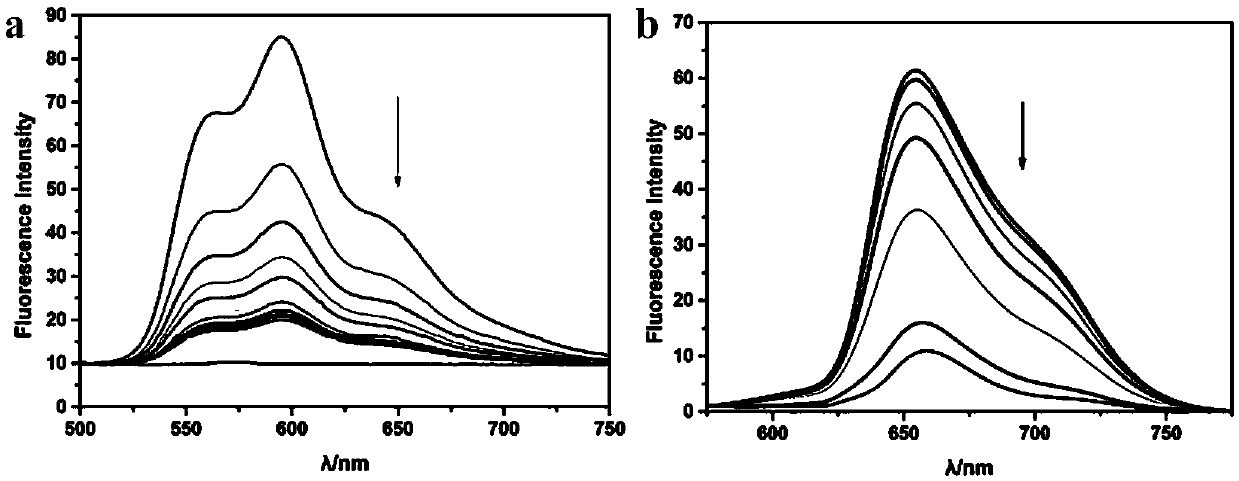
The invention discloses a magnetic targeted anti-tumor drug system for drug-resistant tumor cells and a construction method thereof. The drug system comprises a small interfering RNA-nucleic acid aptamer carrier, an anti-tumor drug immobilized by the carrier, and polyethylene imine-modified superparamagnetic iron tetraoxide nanoparticles. The expression of a corresponding protein is down-regulatedby using small interfering RNA, and the magnetic targeted anti-tumor drug system has high specificity and high efficiency; after the magnetic targeted anti-tumor drug system combines a single chemotherapeutic drug daunromycin with a nucleic acid aptamer, the biocompatibility of the drug is increased, and targeting is endowed, to provide a possibility to reduce the killing effect of chemotherapeutic drugs on normal cells; and at the same time, a photosensitizer porphyrin is loaded to achieve the dual tumor killing effect of chemotherapy and photodynamic therapy. PEI-modified superparamagneticferroferric oxide nanoparticles are used as anticancer drugs / gene carriers for synergistic enhancement of therapeutic efficiency, and as effective ultrasound contrast agents for in vitro tumor imaging.
Owner:GUANGDONG PHARMA UNIV
Dual sustained-release anticancer injection
InactiveCN101301264APharmaceutical delivery mechanismPharmaceutical non-active ingredientsMitozolomideMicrosphere
A double sustained release anticancer gel sustained release injection consists of anticancer medicine and amphiphilic block copolymer hydrogel, wherein the anticancer medicine comprises vincristine, vinorelbine, vinblastine, daunomycin, mitoxantrone, mitozolomide and temozolomide, etc., and exists in sustained release preparation injection in the forms of sustained release microsphere, microsphere or micropowder, i.e. the anticancer medicine in anticancer useful quantity is partly or completely wrapped inside the sustained release microsphere. Sustained release gel has temperature-sensitive gelling characteristics and is in the state of fluxible liquid in an environment with the temperature lower than body temperature; moreover, the sustained release gel can be automatically converted into non-flowing water-insoluble gel capable of biodegradation and absorption inside the body of a warm blood so as to slowly release medicine inside part of a tumor; the sustained release microsphere is propitious to release medicine smoothly and slowly, and double sustained release is propitious to control tumor cells entering a dormancy stage; moreover, the medicine which exists in the sustained release gel in the form of micropowder is propitious to release the medicine relatively faster and to control cells in faster proliferation. The double sustained release anticancer gel sustained release injection can used together with radiotherapeutic particle, etc.
Owner:济南基福医药科技有限公司
Kit for preparation of nano-targeted liposome drug in combined radionuclide therapy and chemotherapy
InactiveUS20080226546A1Simple and convenient and effectiveSimple and convenient and effective and easyOrganic active ingredientsRadioactive preparation carriersCholesterolDspe peg
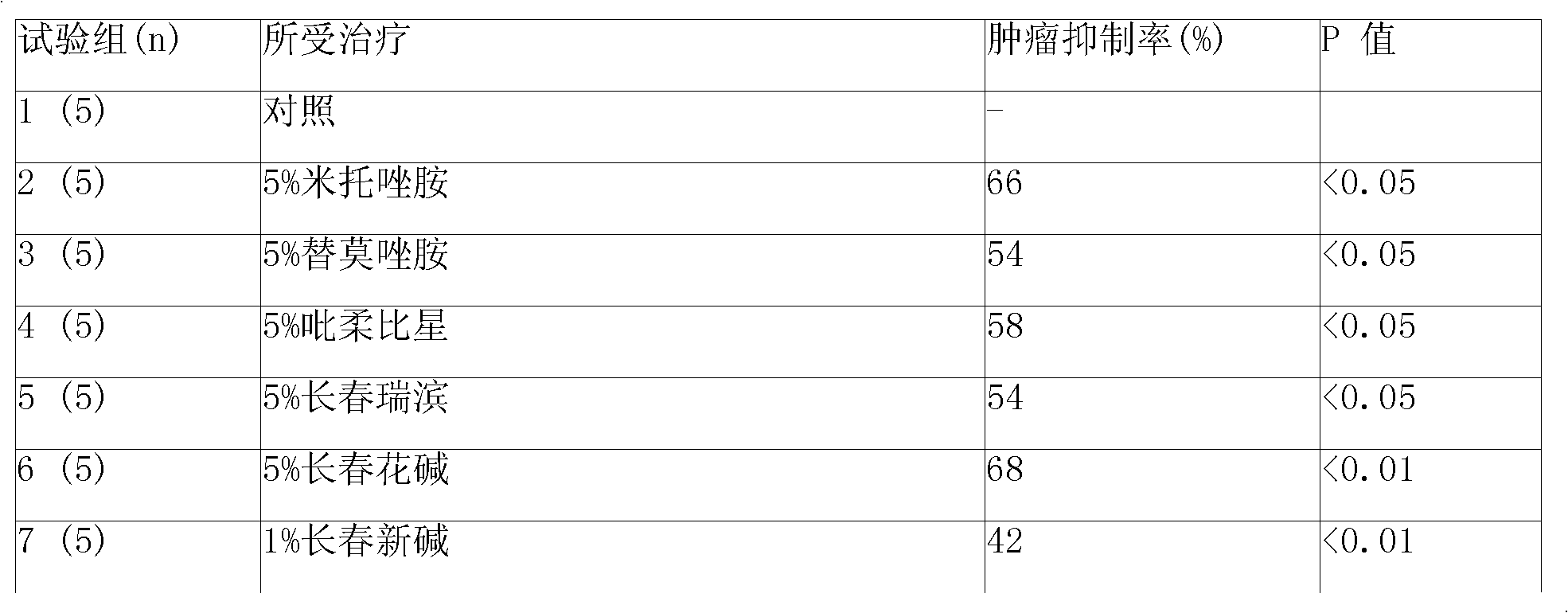
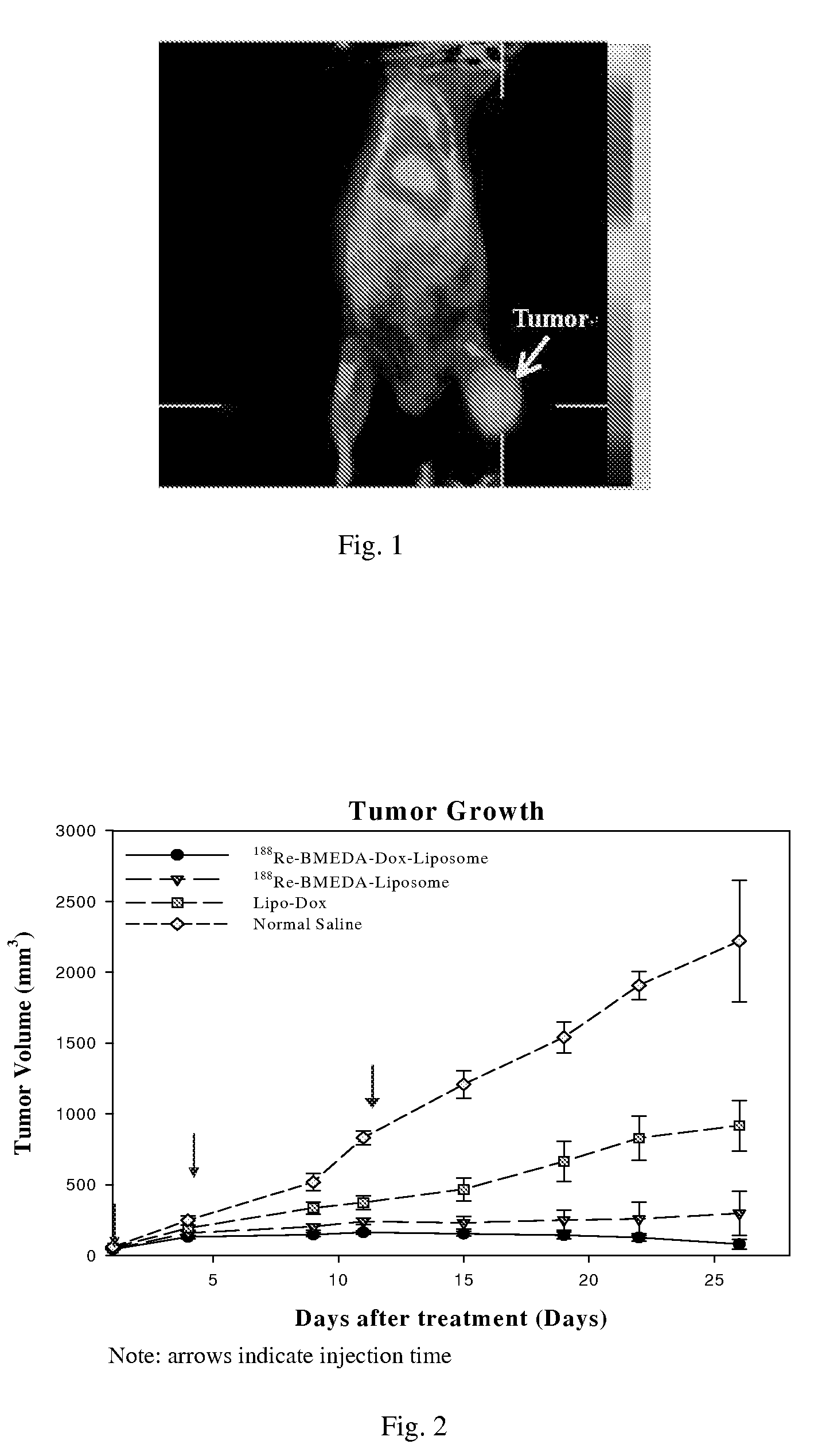
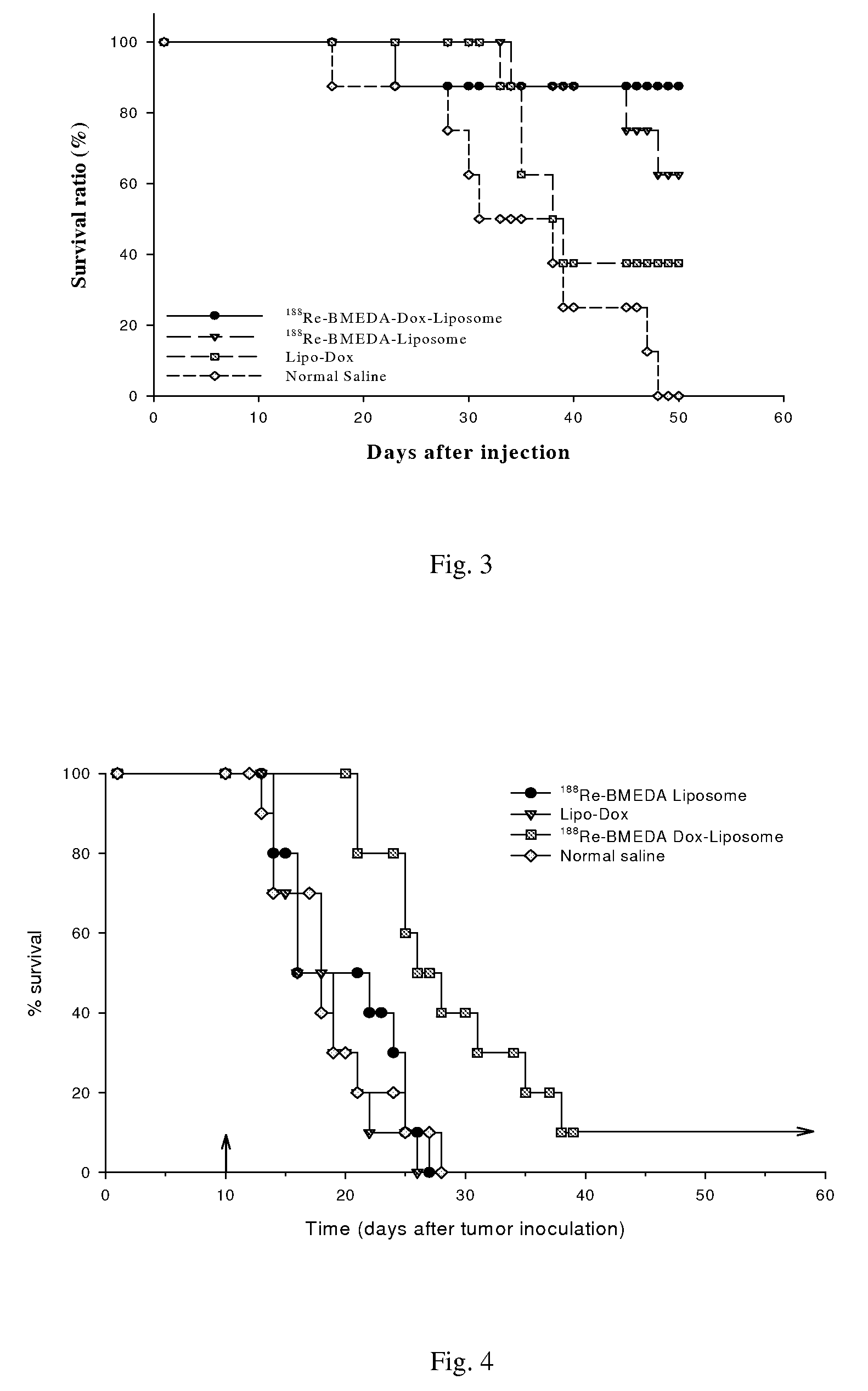
This invention is to manufacture a kit for preparation of nano-targeted liposome drugs in combined chemotherapy and radionuclide therapy. It is a kit consisting of three components: (1) A 10 ml vial A which contains BMEDA, gluconate acetate, SnCl2. (2) A 10 ml vial B which contains DSPC, cholesterol, DSPE-PEG, and Doxorubicin(DXR) (or Daunorubicin, Vinolbine). (3) A 10 ml vial C which contains 188ReO4− (or 186ReO4−) solution. The procedure of using the kit is as follows: (1) Remove the contents of the 188ReO4− (or 186ReO4−) solution from vial C. (2) Inject the 188ReO4− (or 186ReO4−) solution into the vial A, and the mixtures react in appropriate temperature. (3) Remove the contents of the 188Re-BMEDA (or 186Re-BMEDA) solution from vial A. (4) Inject the 188Re-BMEDA (or 186Re-BMEDA) solution into the vial B, and the mixtures react in appropriate temperature. The reconstituted solution in the vial B is applied to combine bimodality radiochemotherapy for treatment of tumor and ascites.
Owner:INST NUCLEAR ENERGY RES ROCAEC +1
Compound serving as anti-tumor medicine synergist and reversal agent
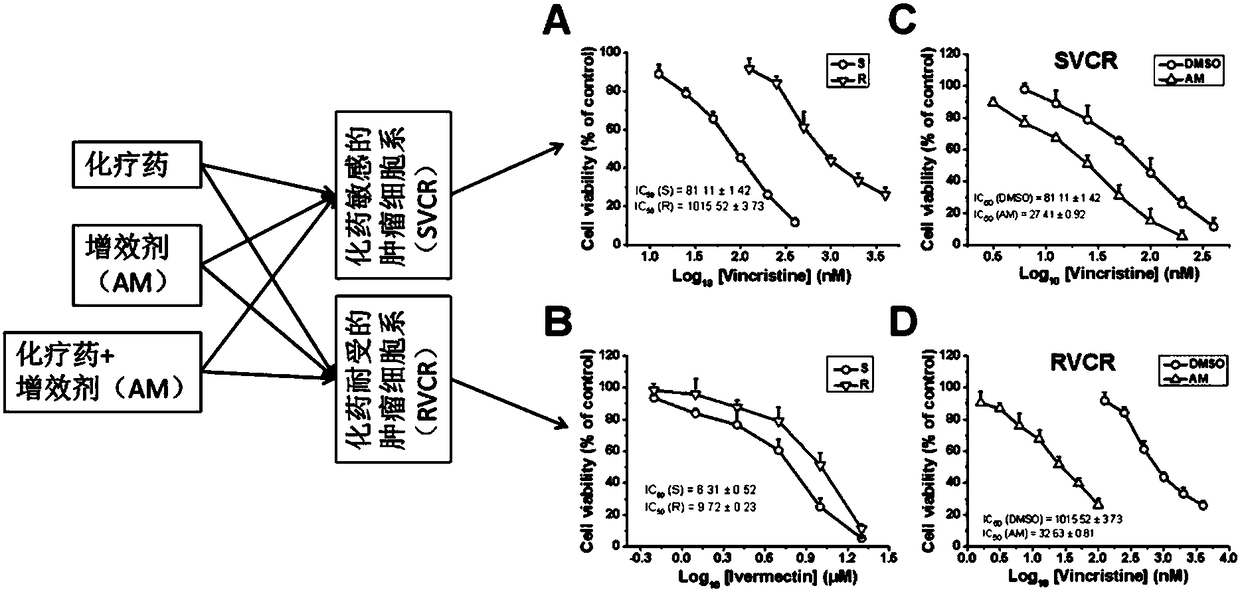
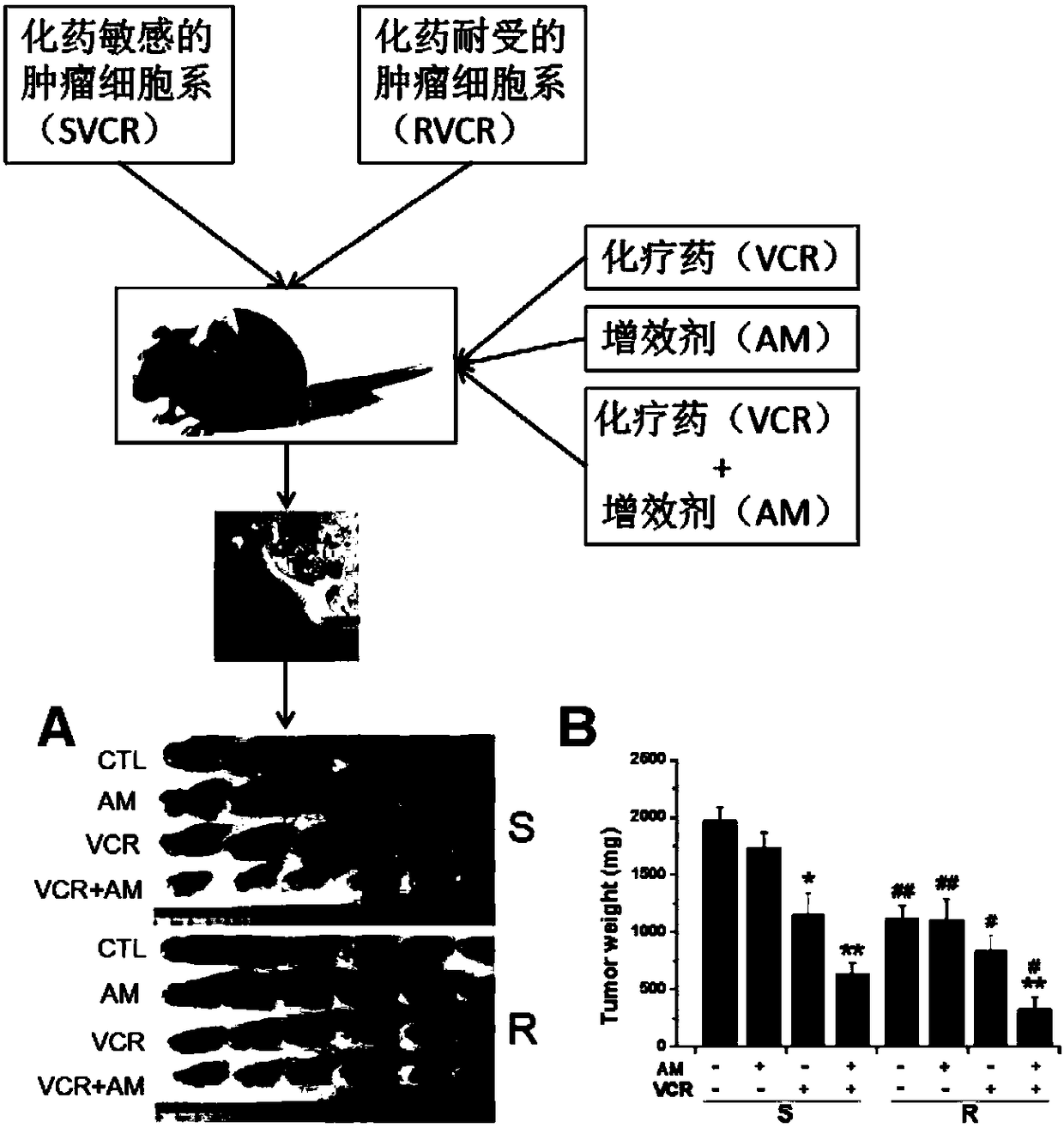
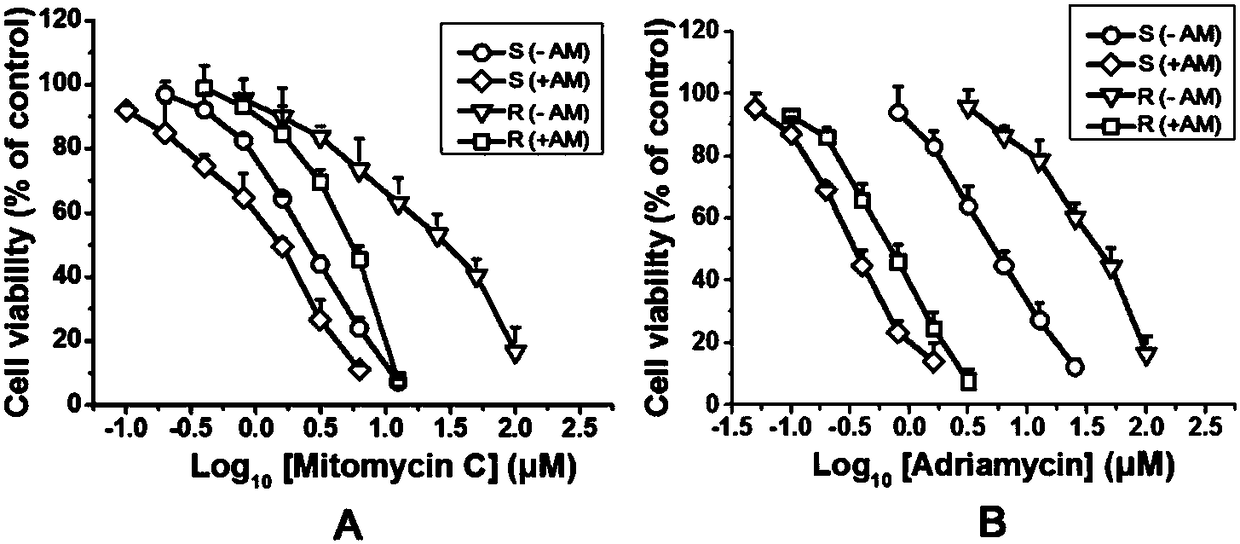
InactiveCN108524533AIncreased sensitivityLower doseOrganic active ingredientsAntineoplastic agentsAvermectinDaunorubicin
The invention discloses a compound serving as an anti-tumor medicine synergist and a reversal agent and belongs to the technical field of medicines. The invention provides application of an avermectinmedicine to preparation of an anti-tumor medicine and application of the avermectin medicine to preparation of the anti-tumor medicine synergist or the reversal agent. The invention also provides ananti-tumor medicine composition which comprises one or more anti-tumor medicines and one or more avermectin medicines as effective components. The avermectin medicine is avermectin, ivermectin, epiomycin, doramectin or eprinomectin, and the anti-tumor medicine is vincristine and salt thereof, daunorubicin, taxol and salt thereof or doxorubicin. The avermectin medicine can improve the sensibility of tumors to chemotherapeutic medicines and thus can be used as the anti-tumor medicine synergist; and the avermectin medicine has an excellent effect on reversing the medicine resistance of tumor cells to the anti-tumor medicine.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com