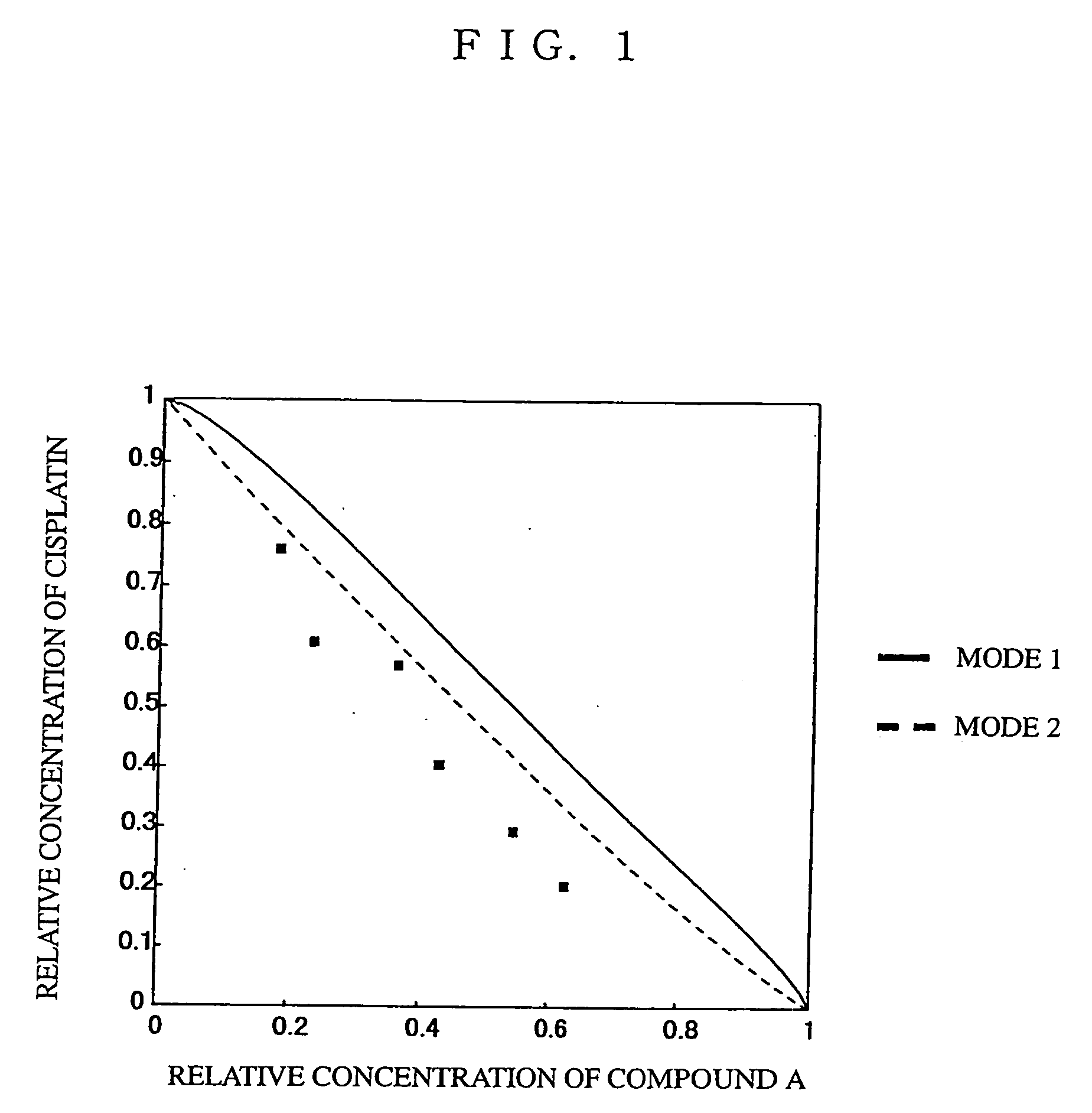
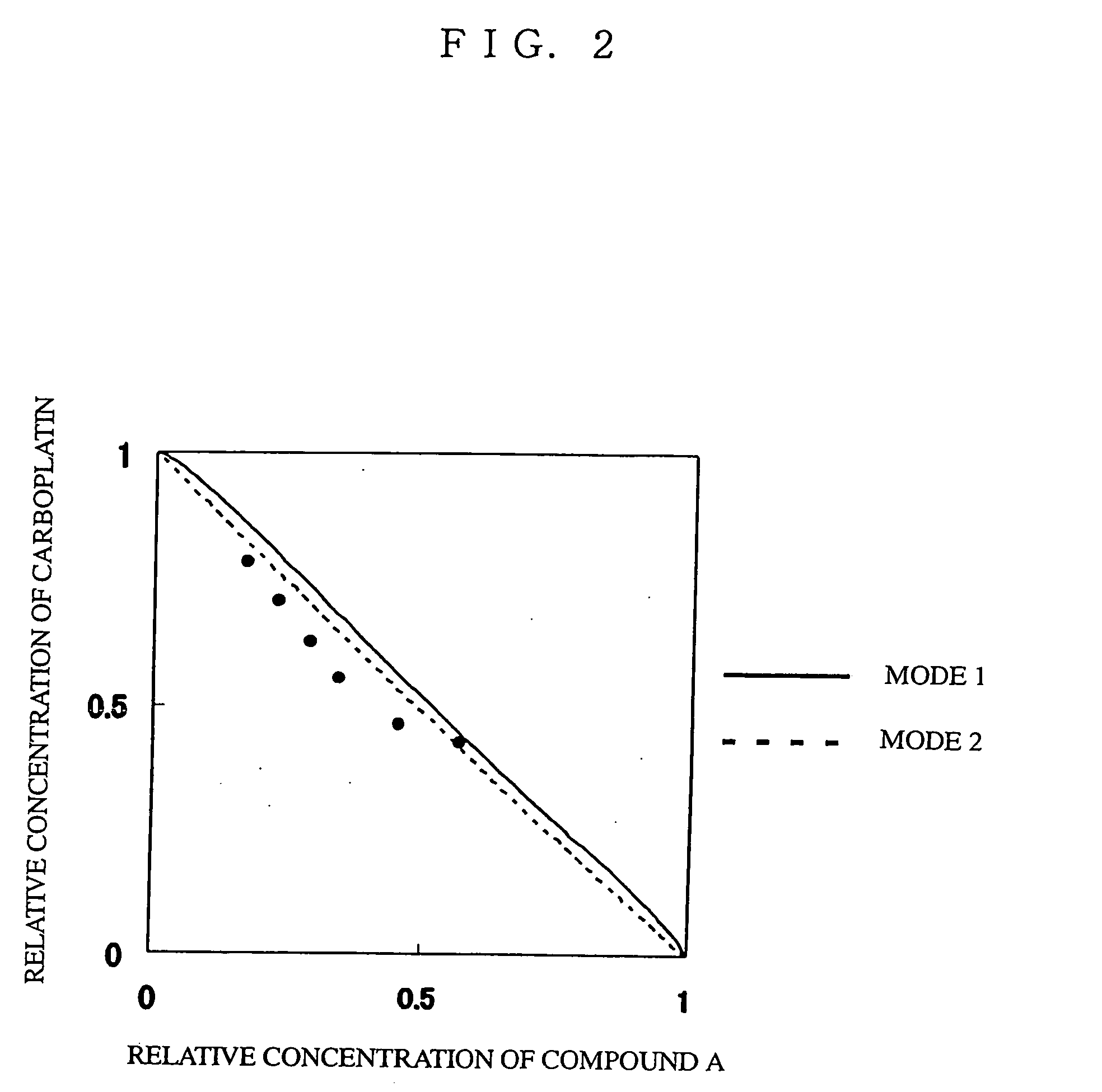
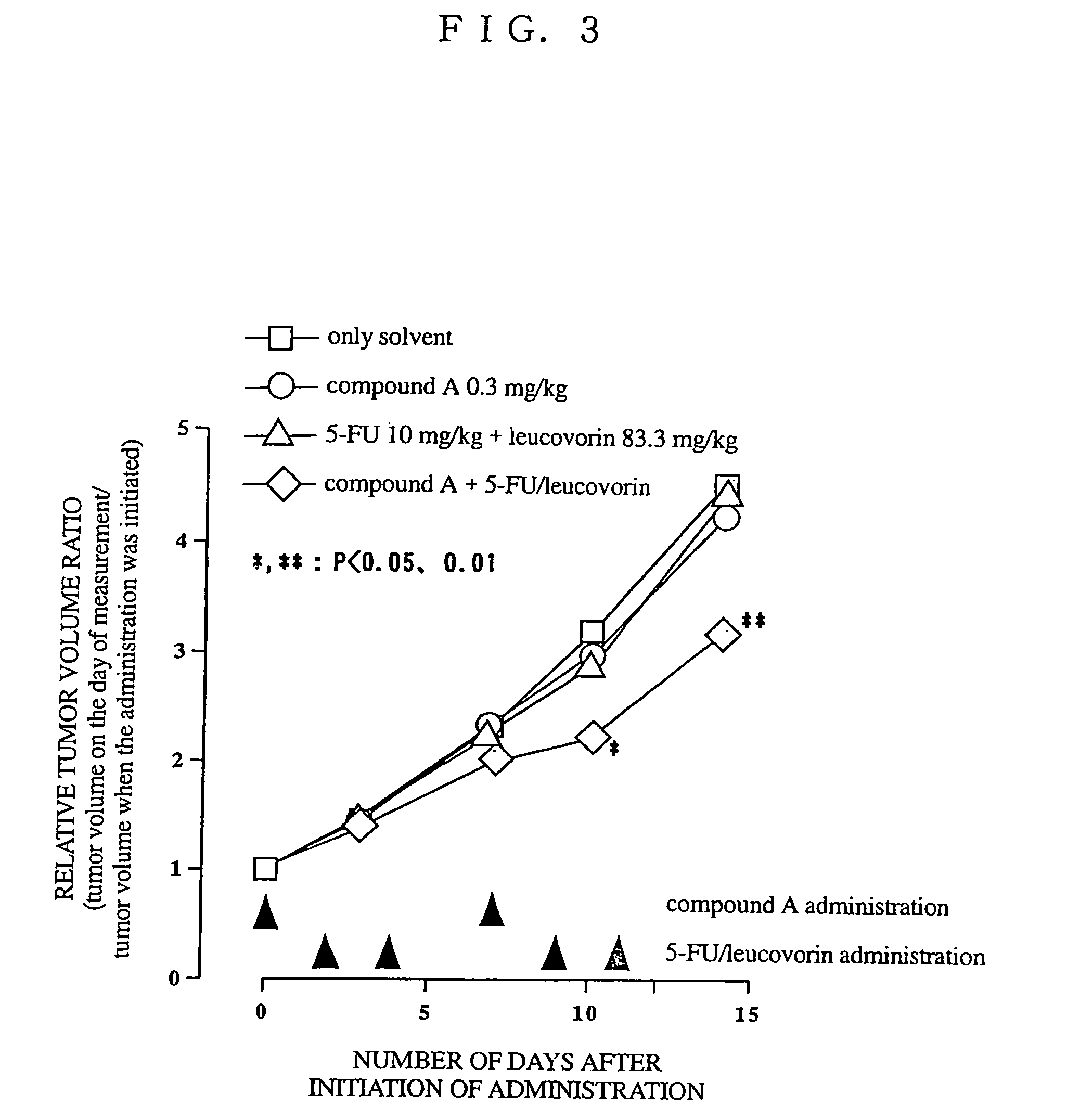
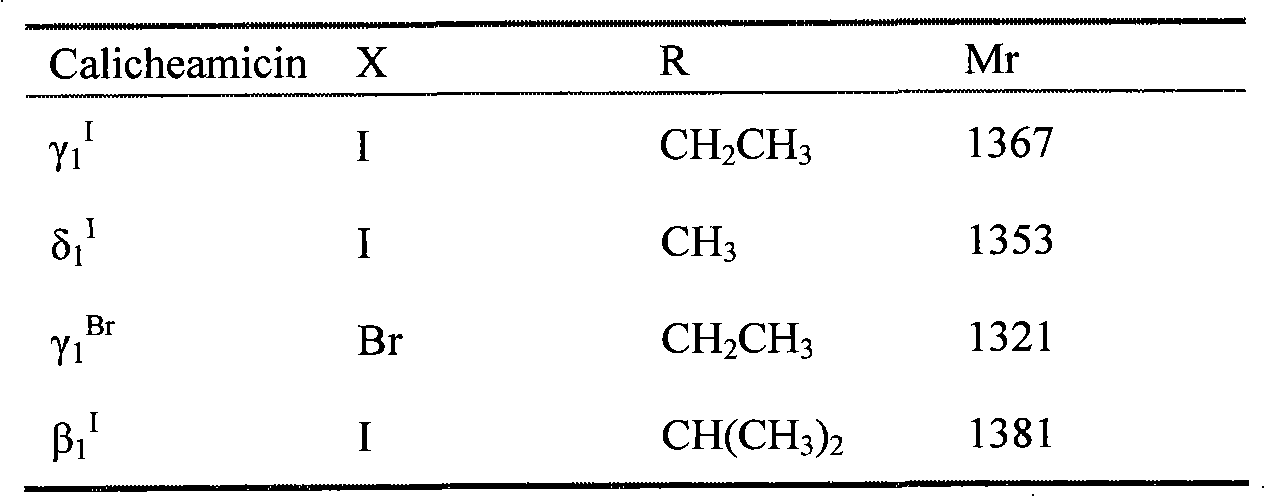
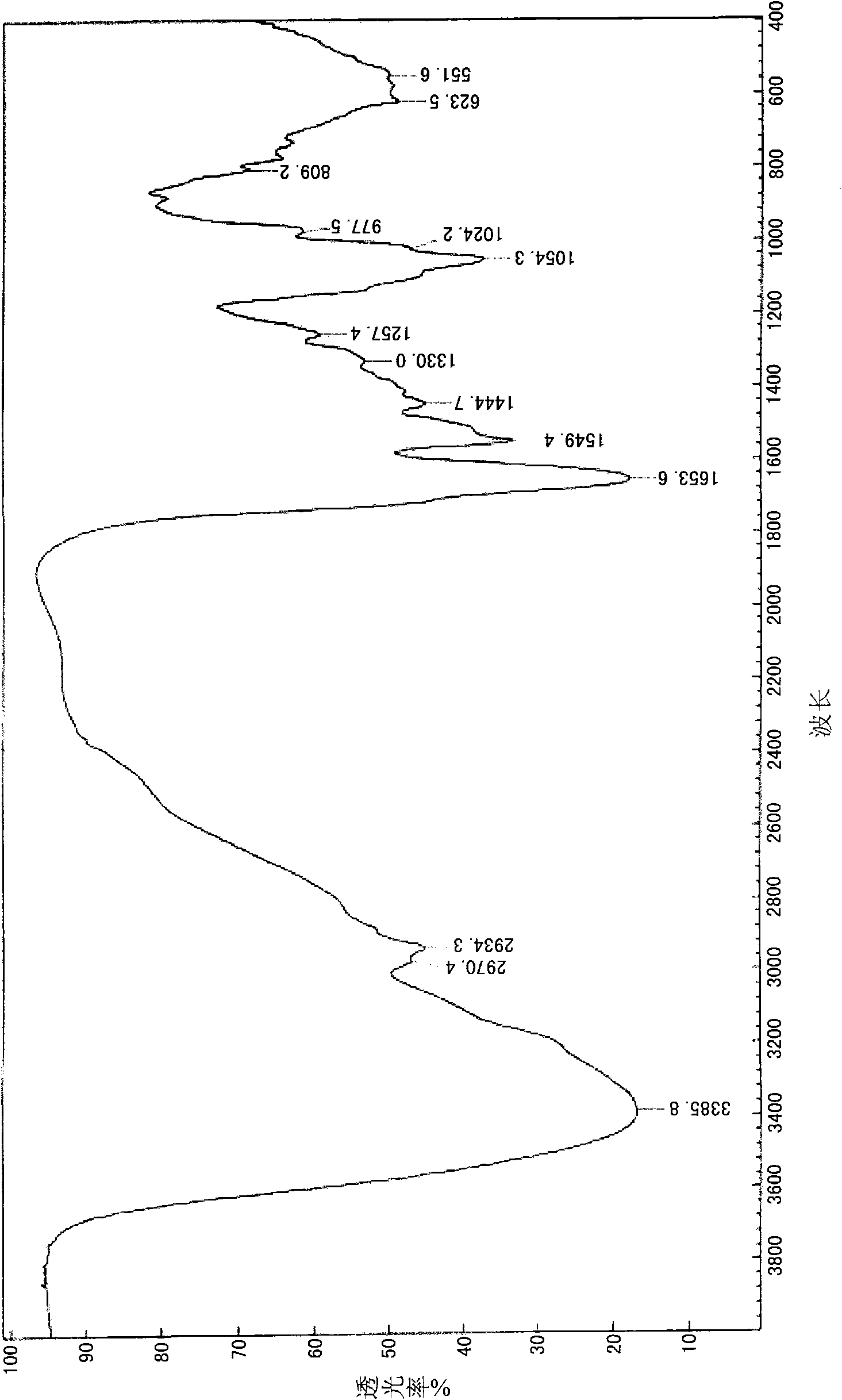
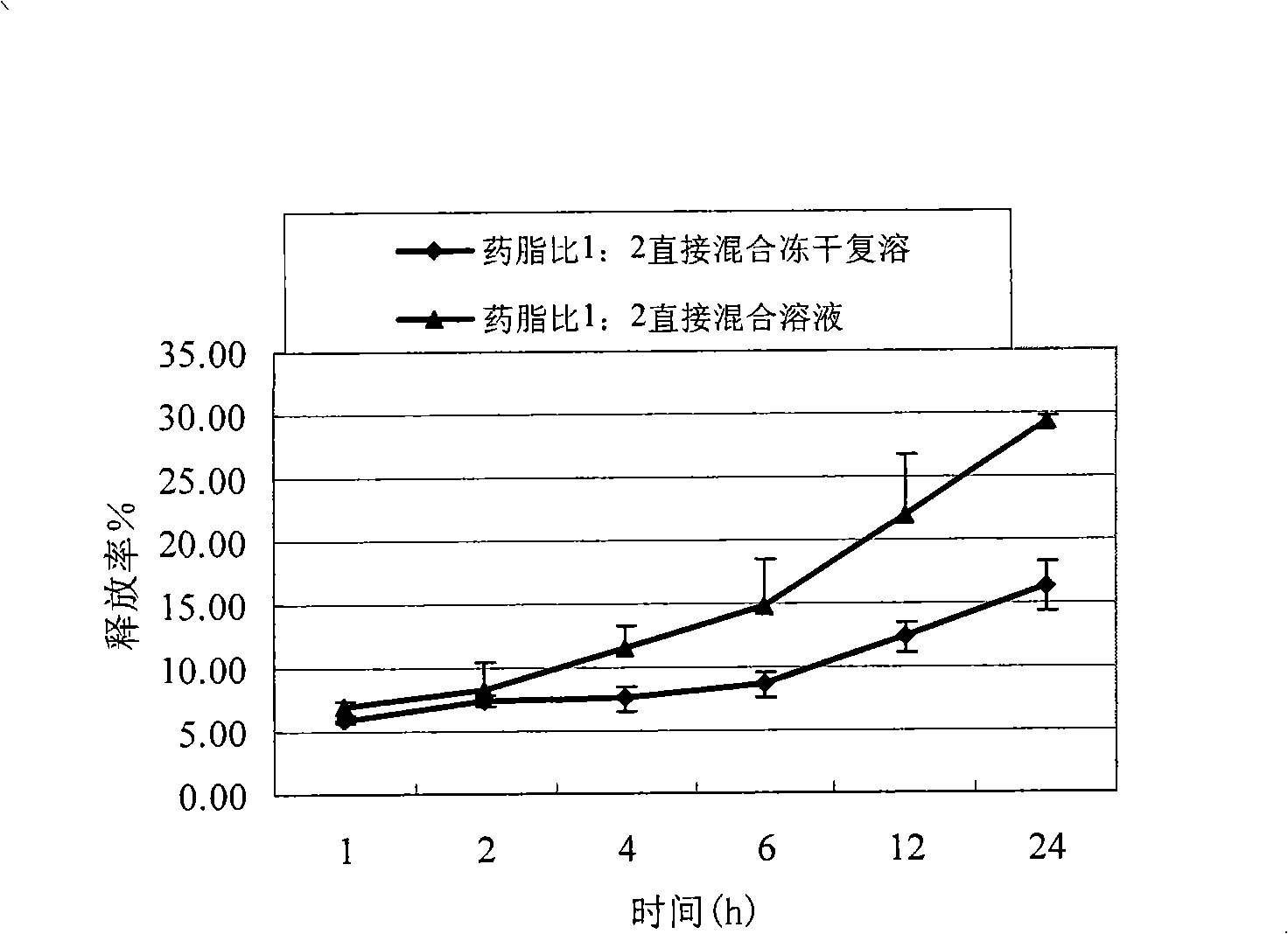
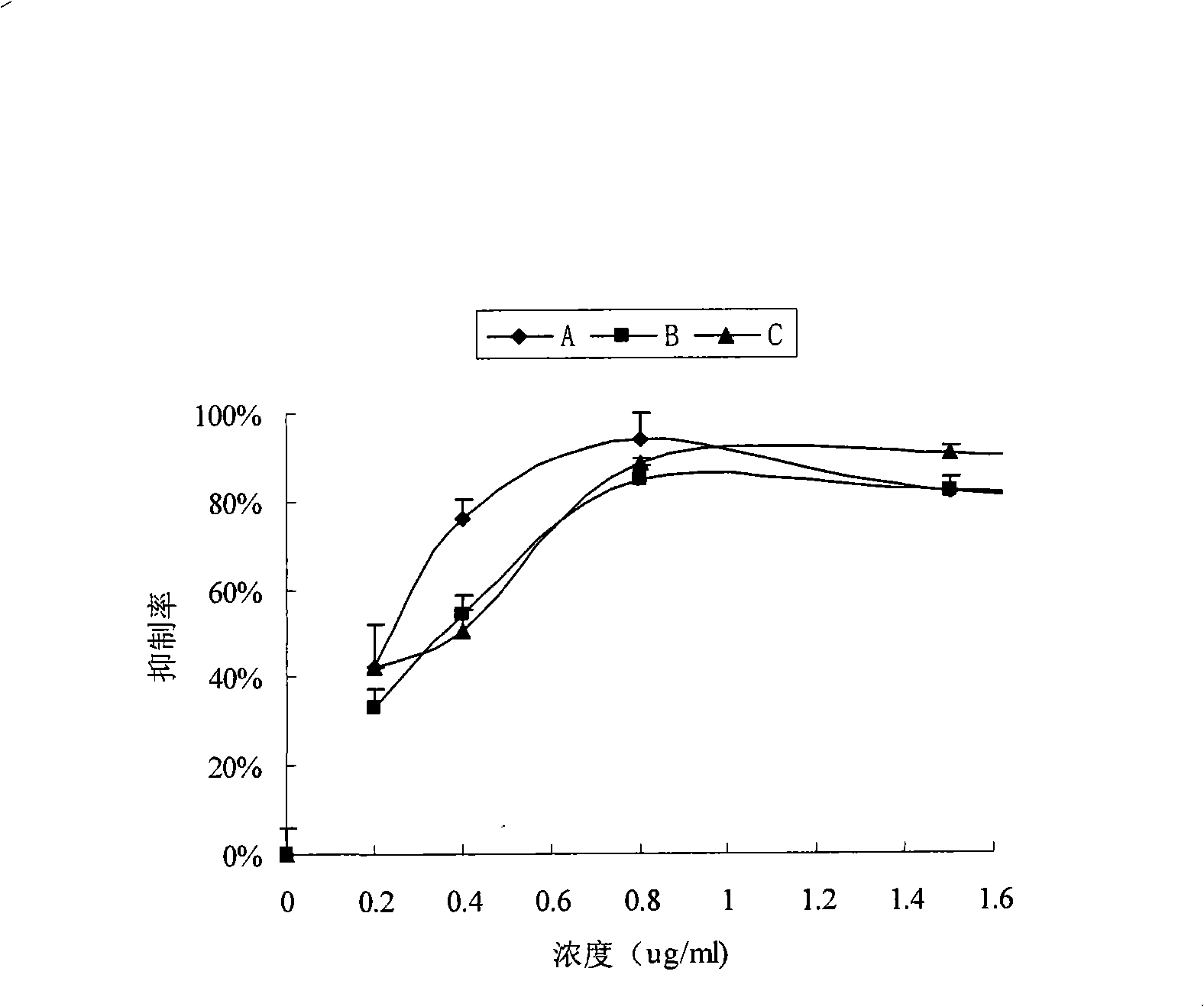
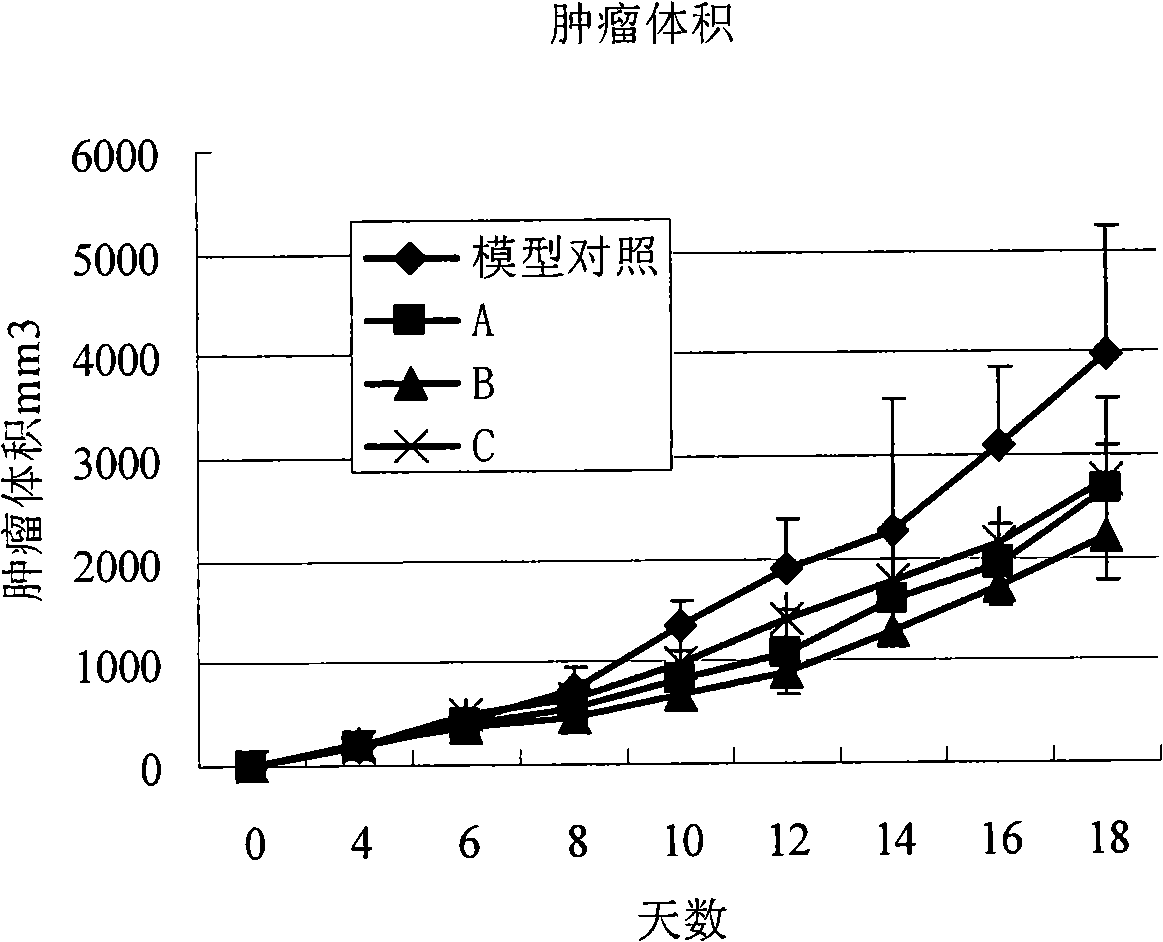
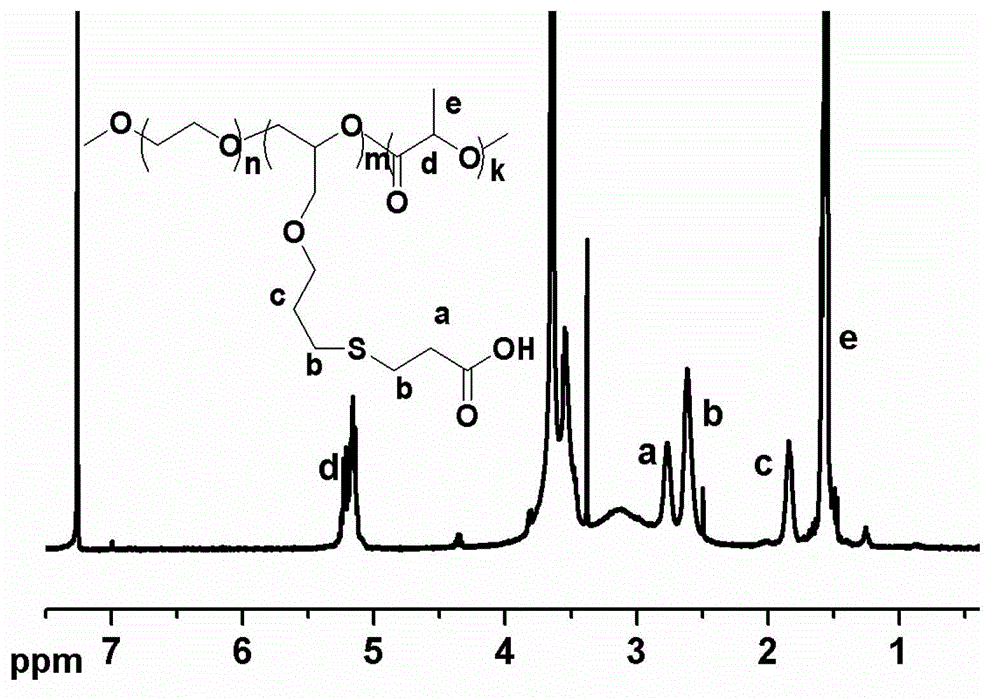
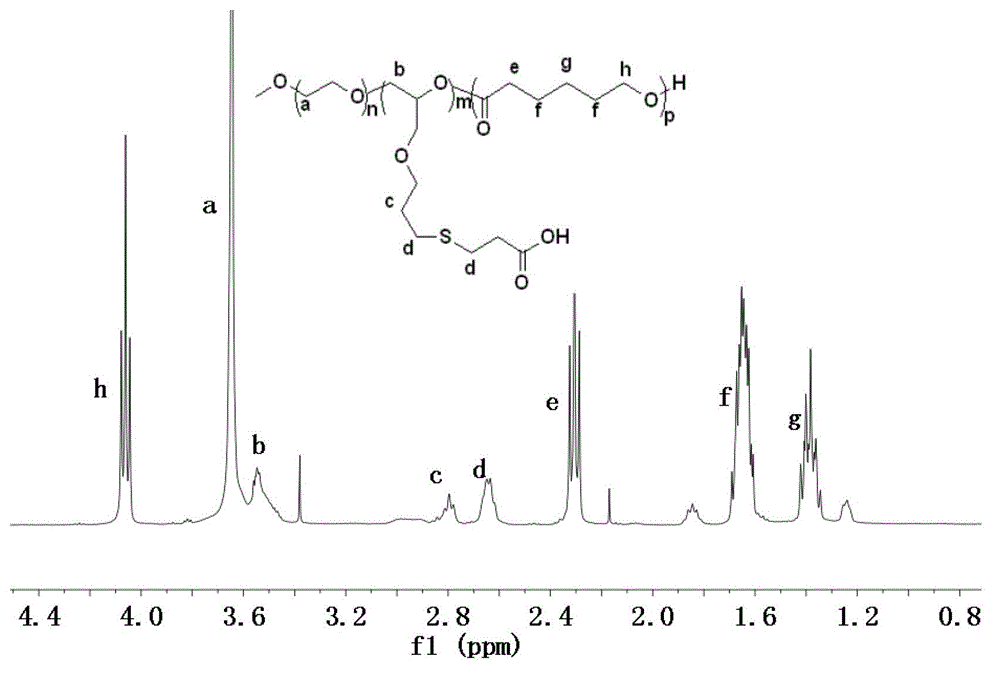
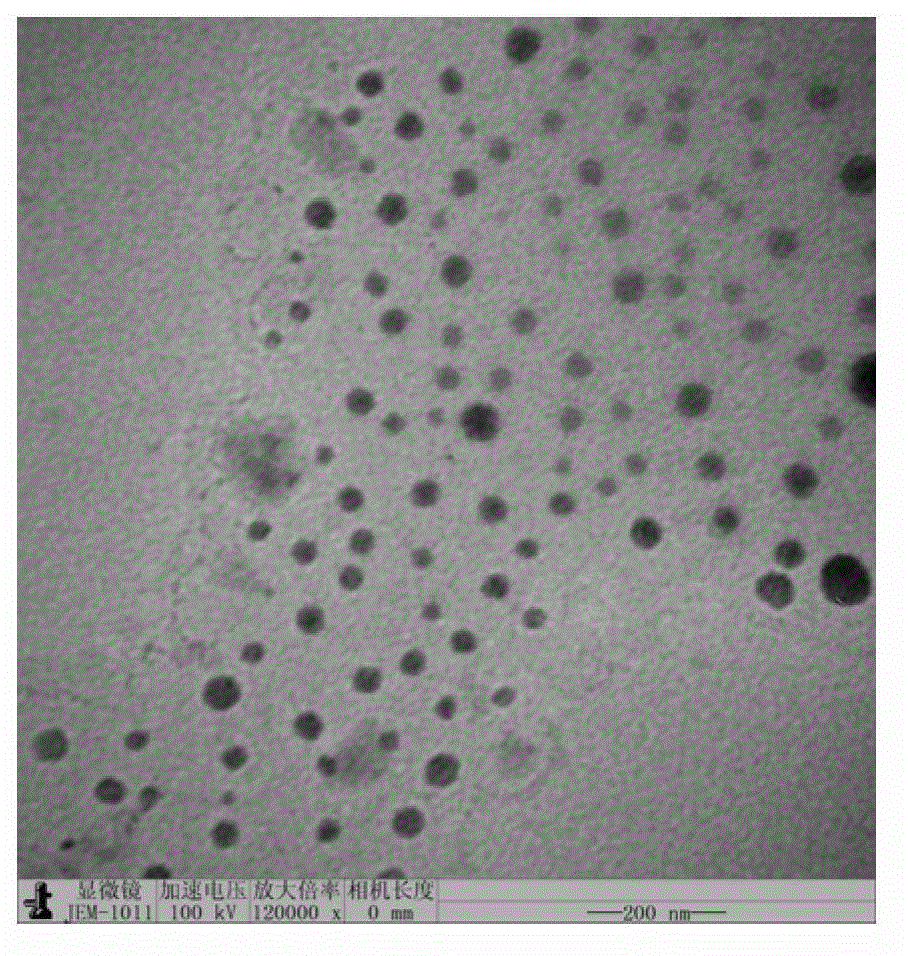
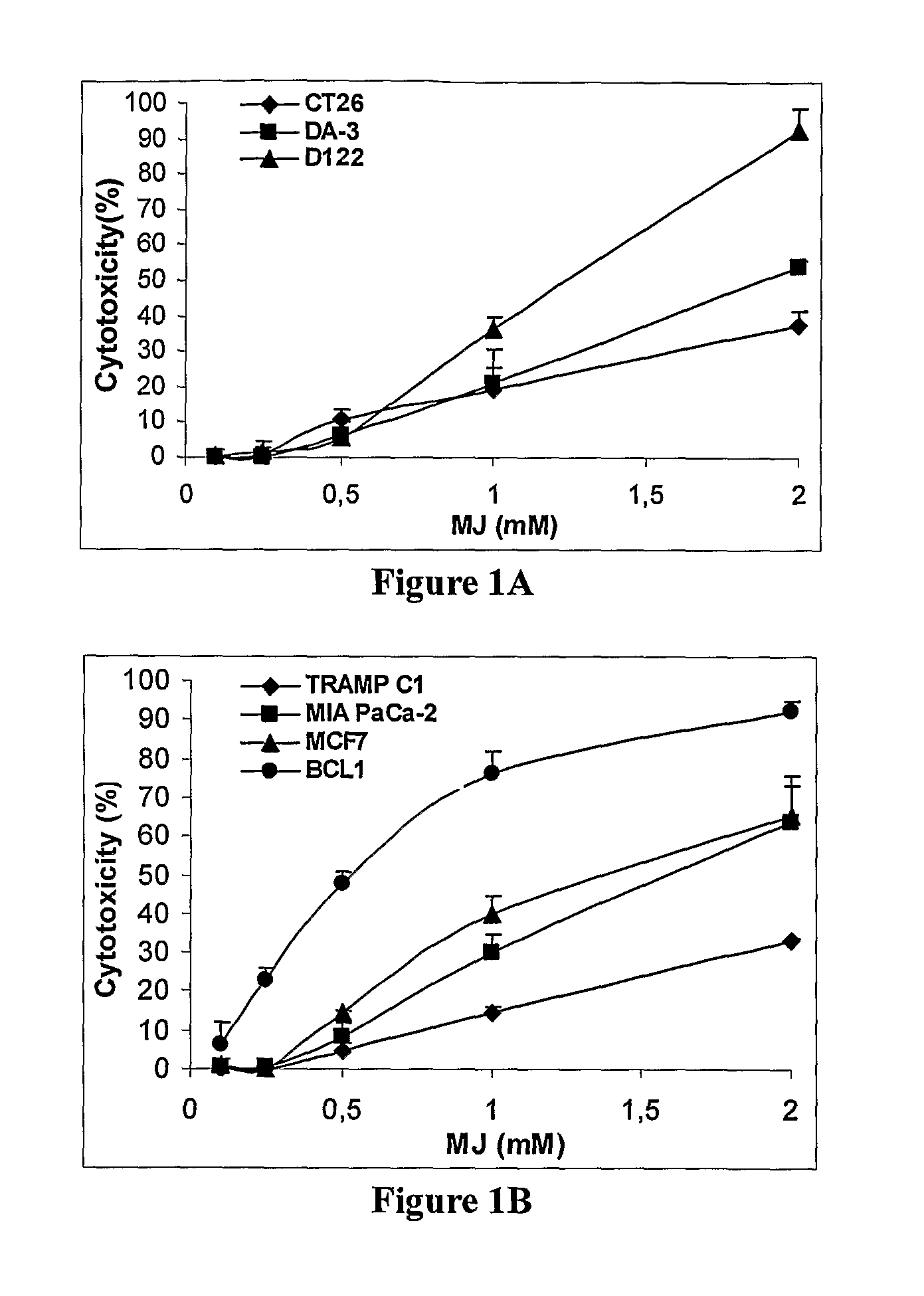
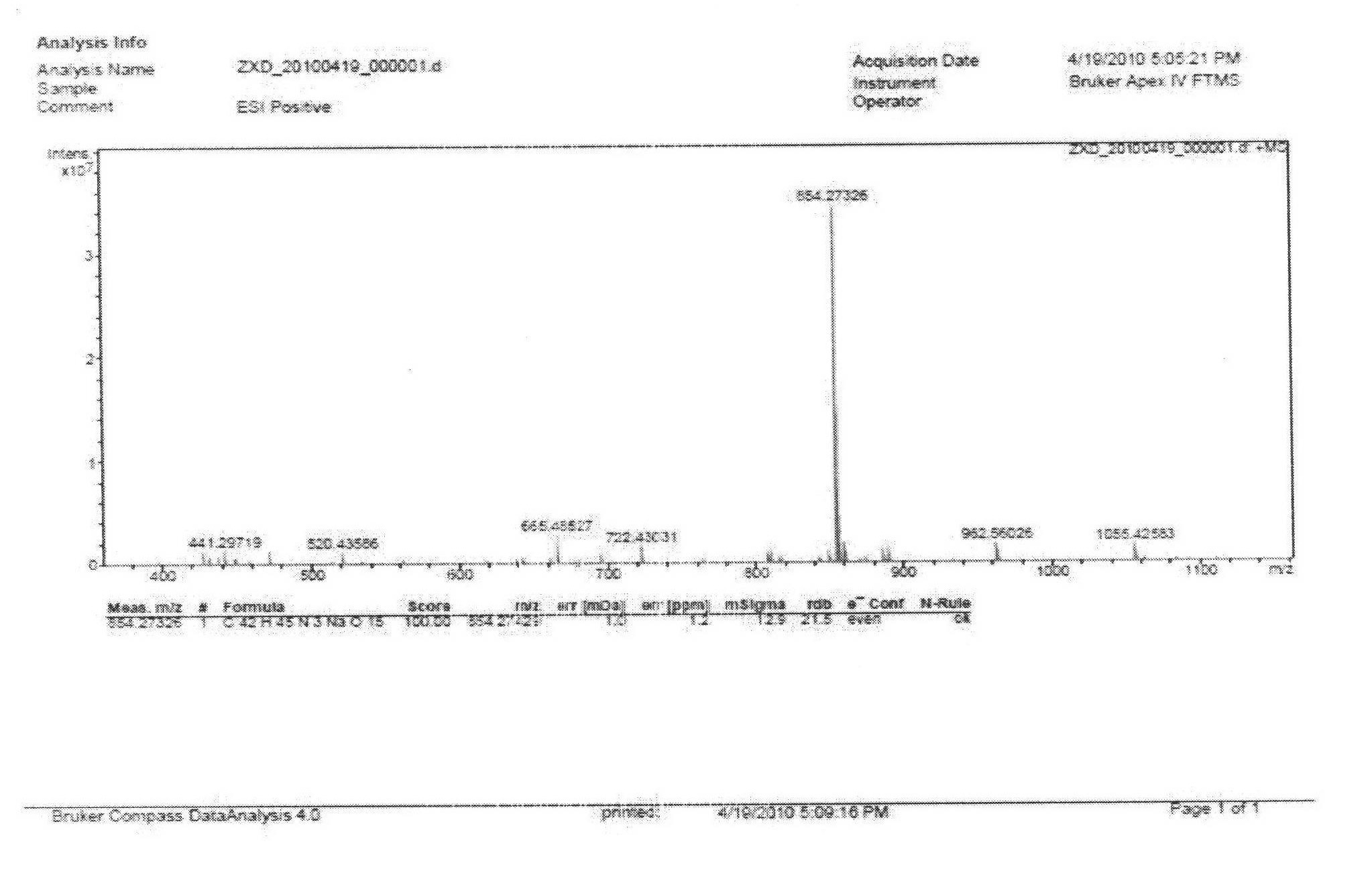
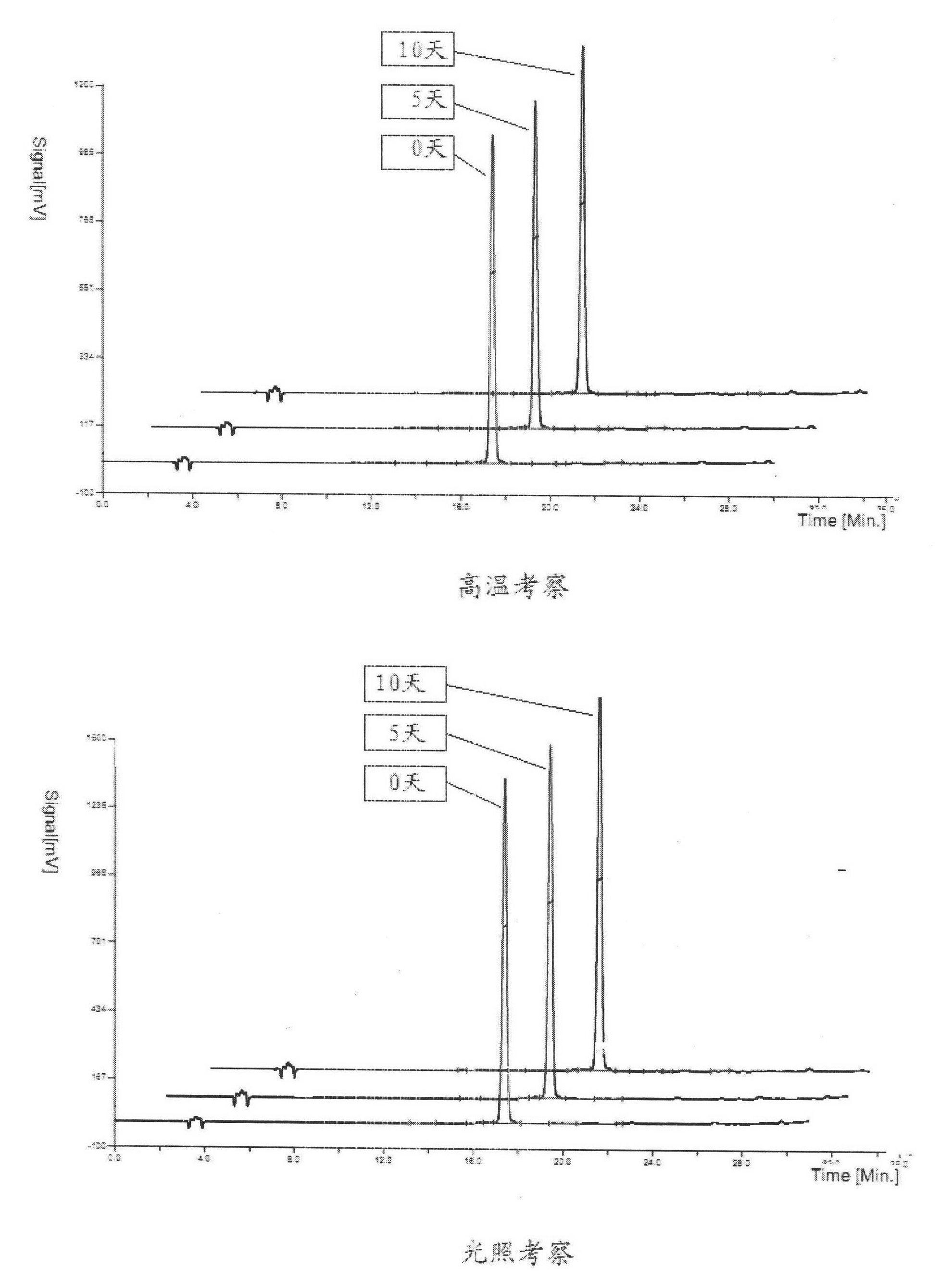
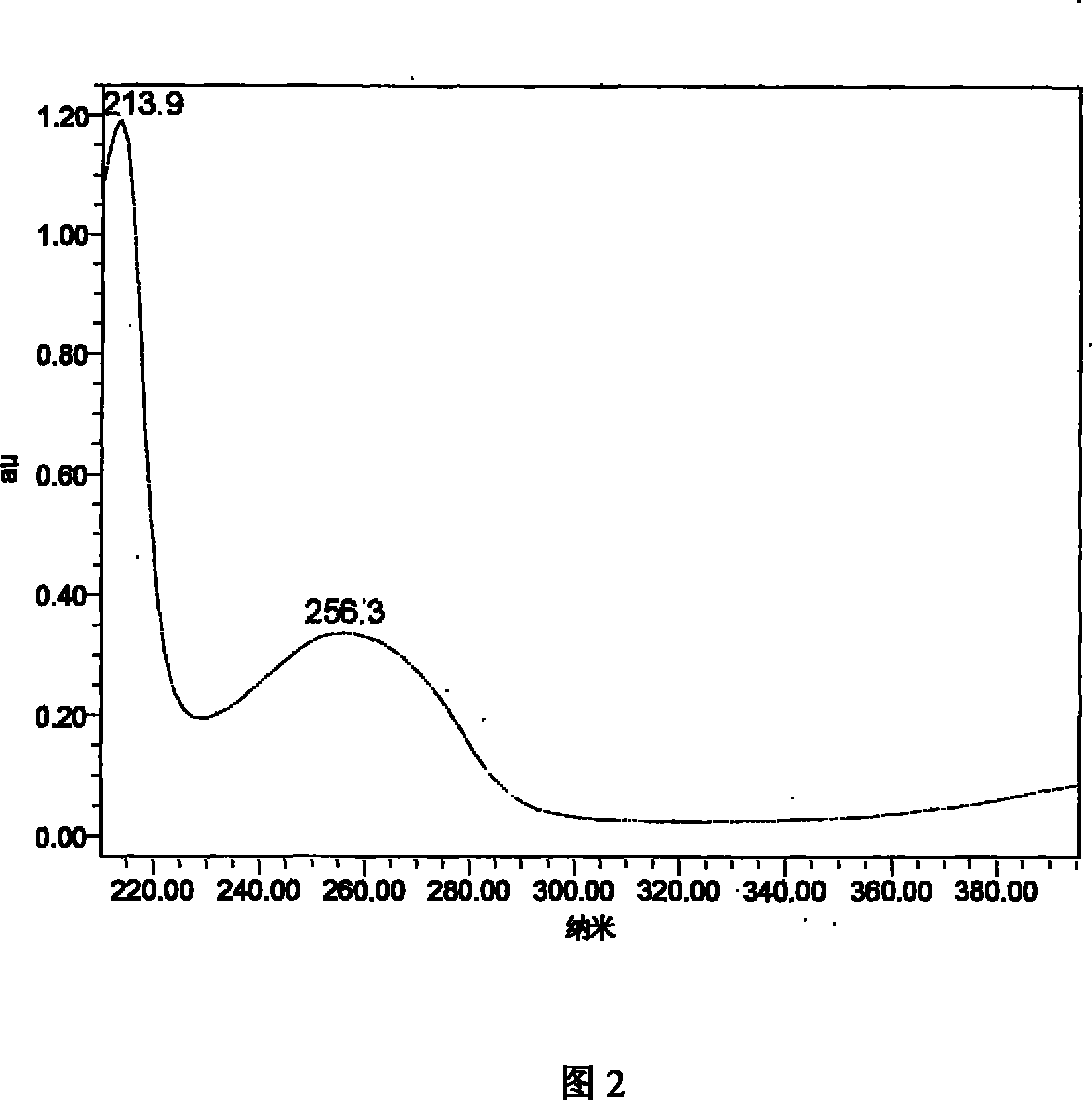
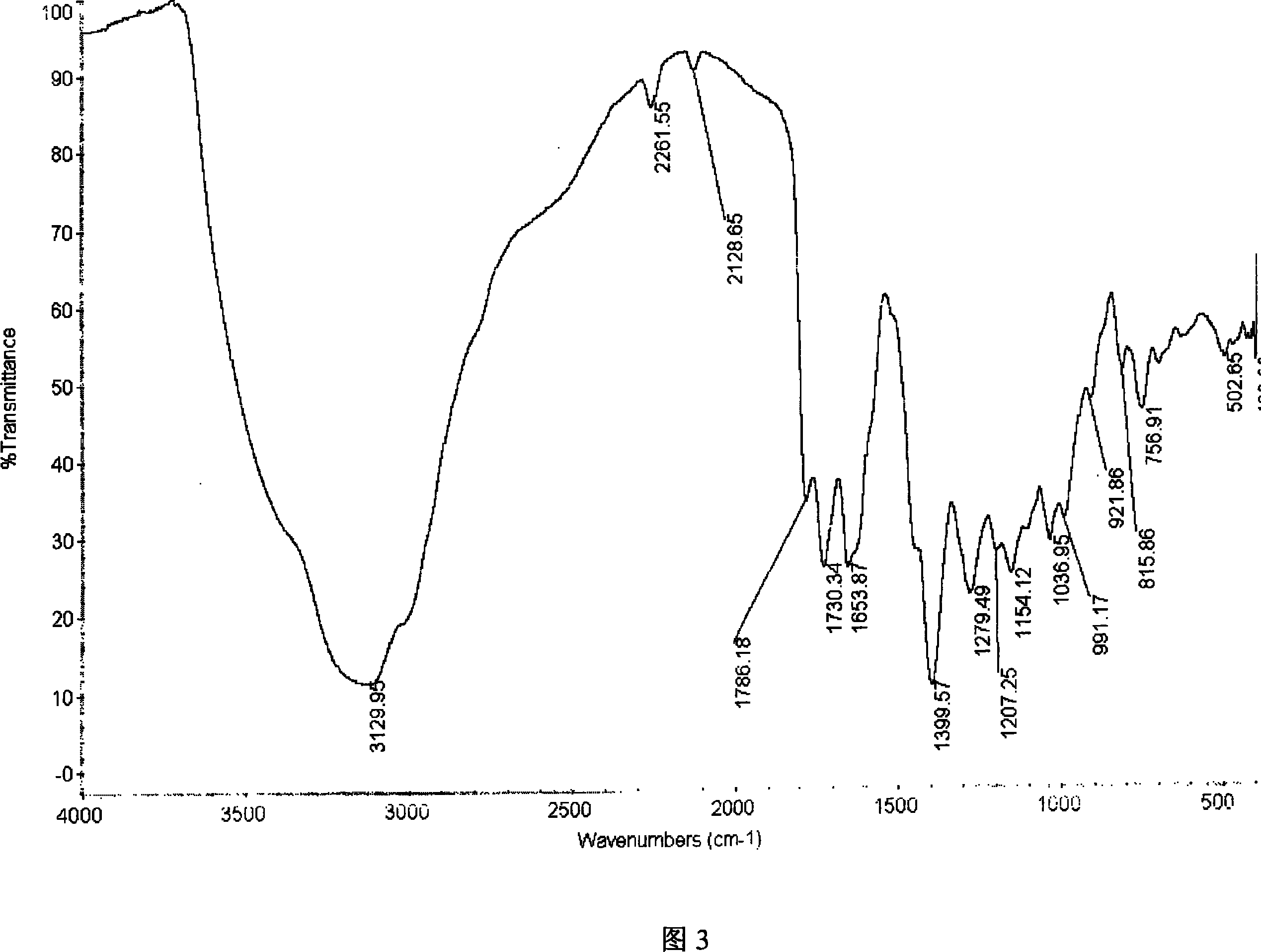
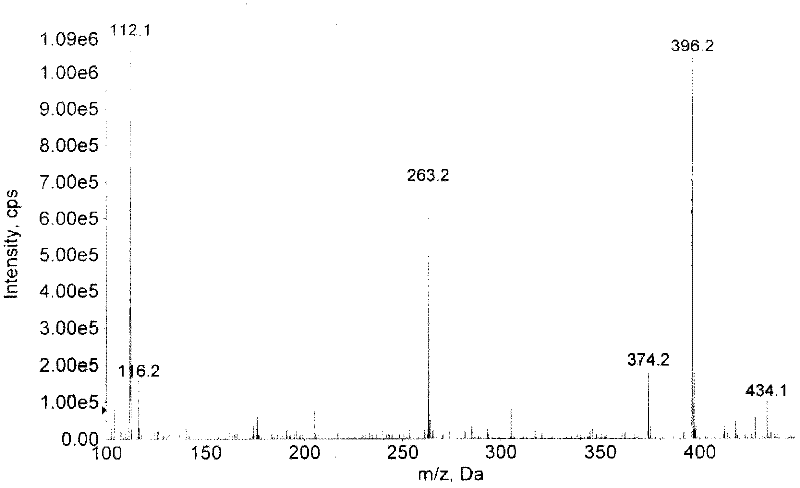
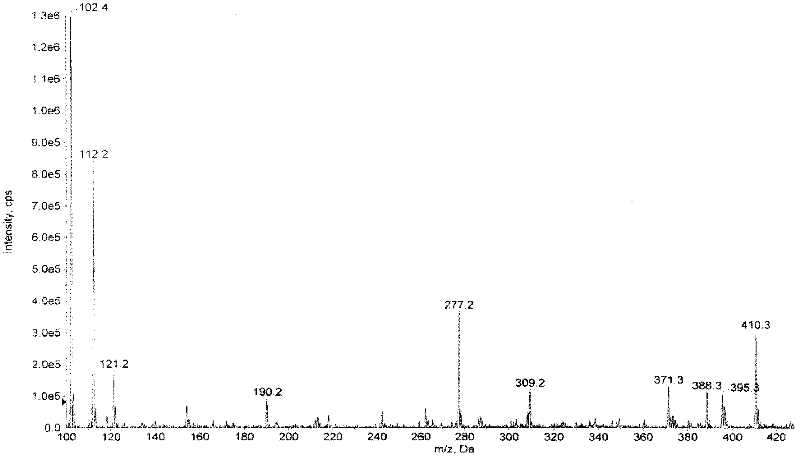
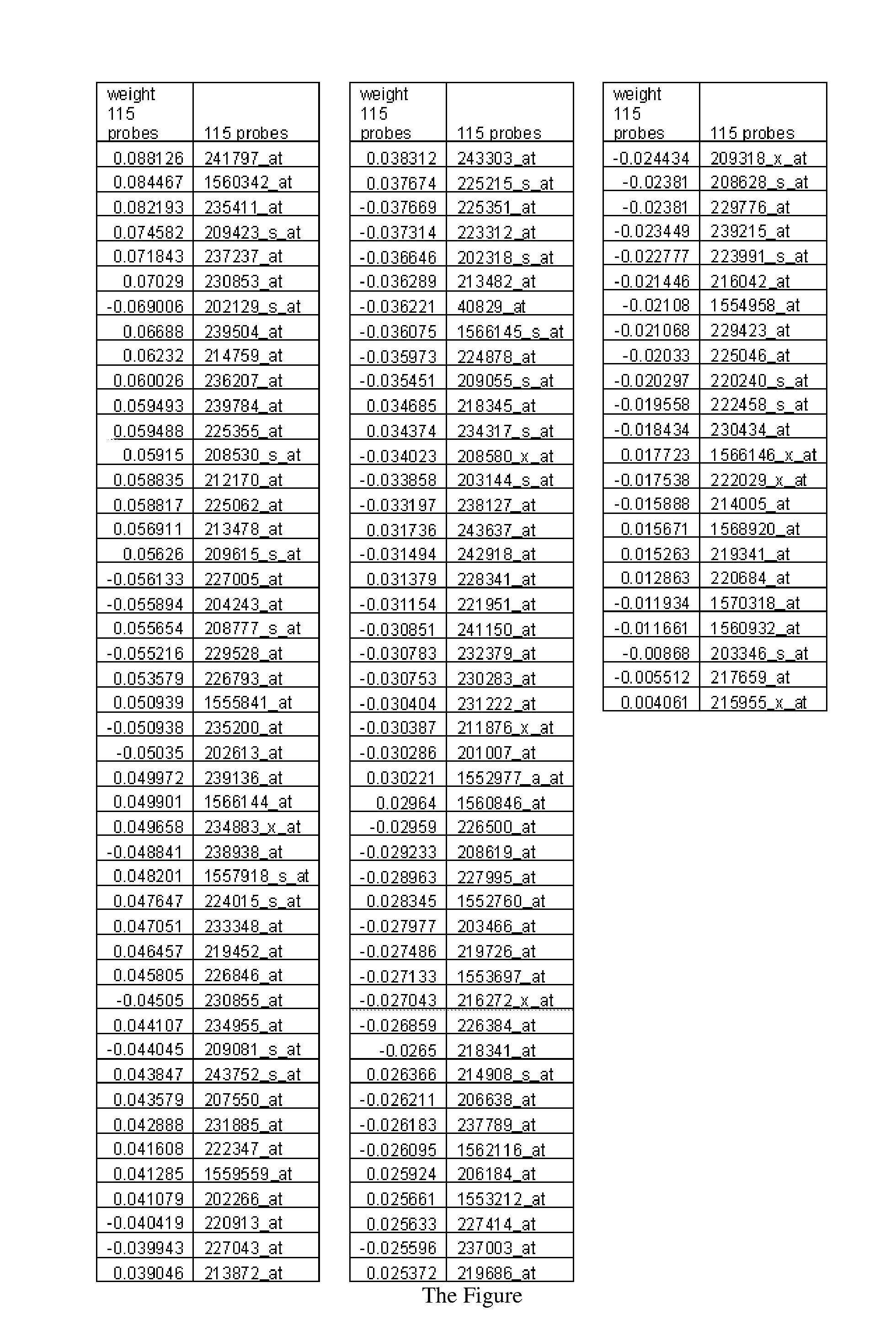
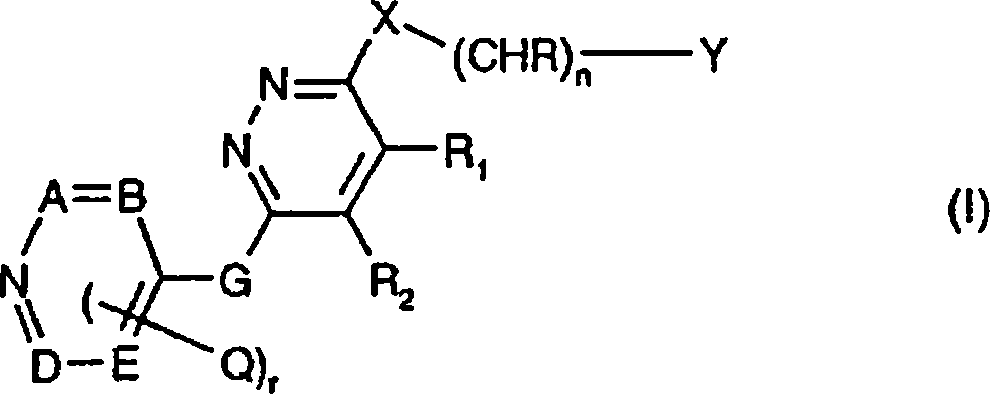
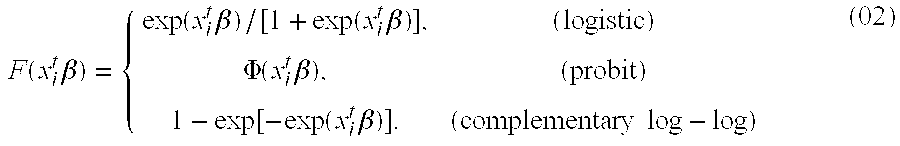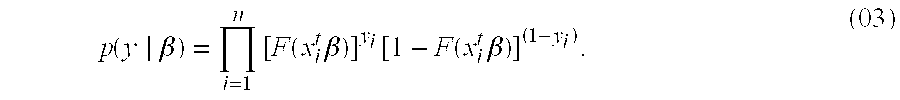Patents
Literature
61 results about "Antitumor Antibiotics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antitumor antibiotic. [‚an·tē′tüm·ər ‚an·tē‚bī′äd·ik] (microbiology) A substance, such as actinomycin, luteomycin, or mitomycin C, which is produced by microorganisms and is effective against some forms of cancer.
Use of antitumor indolopyrrolocarbazole derivative and other anticancer agent in combination
InactiveUS20050171036A1Improve anti-tumor effectBiocideHeavy metal active ingredientsAnticarcinogenHalogen
This invention relates to a combined preparation for simultaneous, separate, or sequential administration in the treatment of cancer, comprising two separate preparations: a preparation comprising, in combination with a pharmaceutically acceptable carrier or diluent, at least one compound of general formula I: wherein R1 and R2 each independently represent a hydrogen atom, lower alkyl, or the like, and G represents pentosyl or the like, X1 and X2 each independently represent a hydrogen atom, a halogen atom, or the like or a pharmaceutically acceptable salt thereof; and a preparation, in combination with a pharmaceutically acceptable carrier or diluent, such as antitumor alkylating agents, antitumor antimetabolites, antitumor antibiotics, or plant-derived antitumor agents (a preparation comprising at least one compound of general formula I or a pharmaceutically acceptable salt thereof may be combined with two or more other antitumor agents), and a method for cancer treatment comprising the administration of these preparations in combination.
Owner:BANYU PHARMA CO LTD
Preparation method of efficient antitumor antibiotic calicheamicin
InactiveCN102732580ADetoxificationAids in biosynthesisMicroorganism based processesFermentationMicromonospora echinosporaAntitumor Antibiotics
The present invention provides a fermentation method of high-yield antitumor antibiotic calicheamicin. The method of the invention employs Micromonospora echinospora for fermentation production of calicheamicin in a fermentation medium added with different kinds of macroporous adsorption resins. The method of the present invention has stable process and substantially increased fermentation yield.
Owner:ZHEJIANG HISUN PHARMA CO LTD
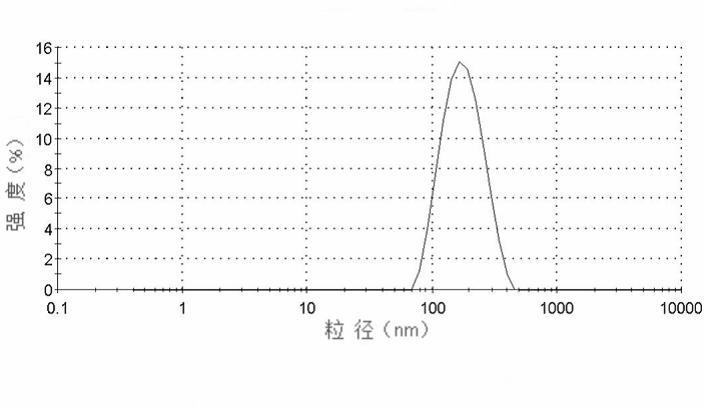
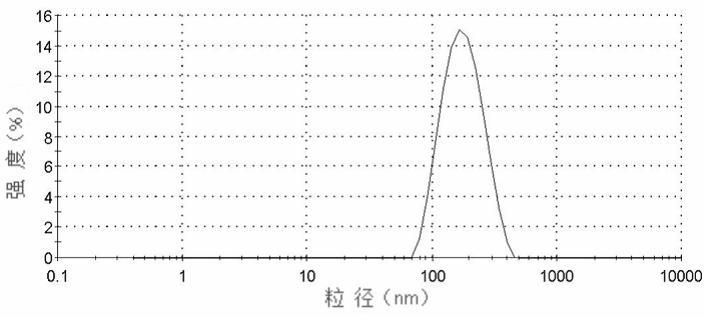
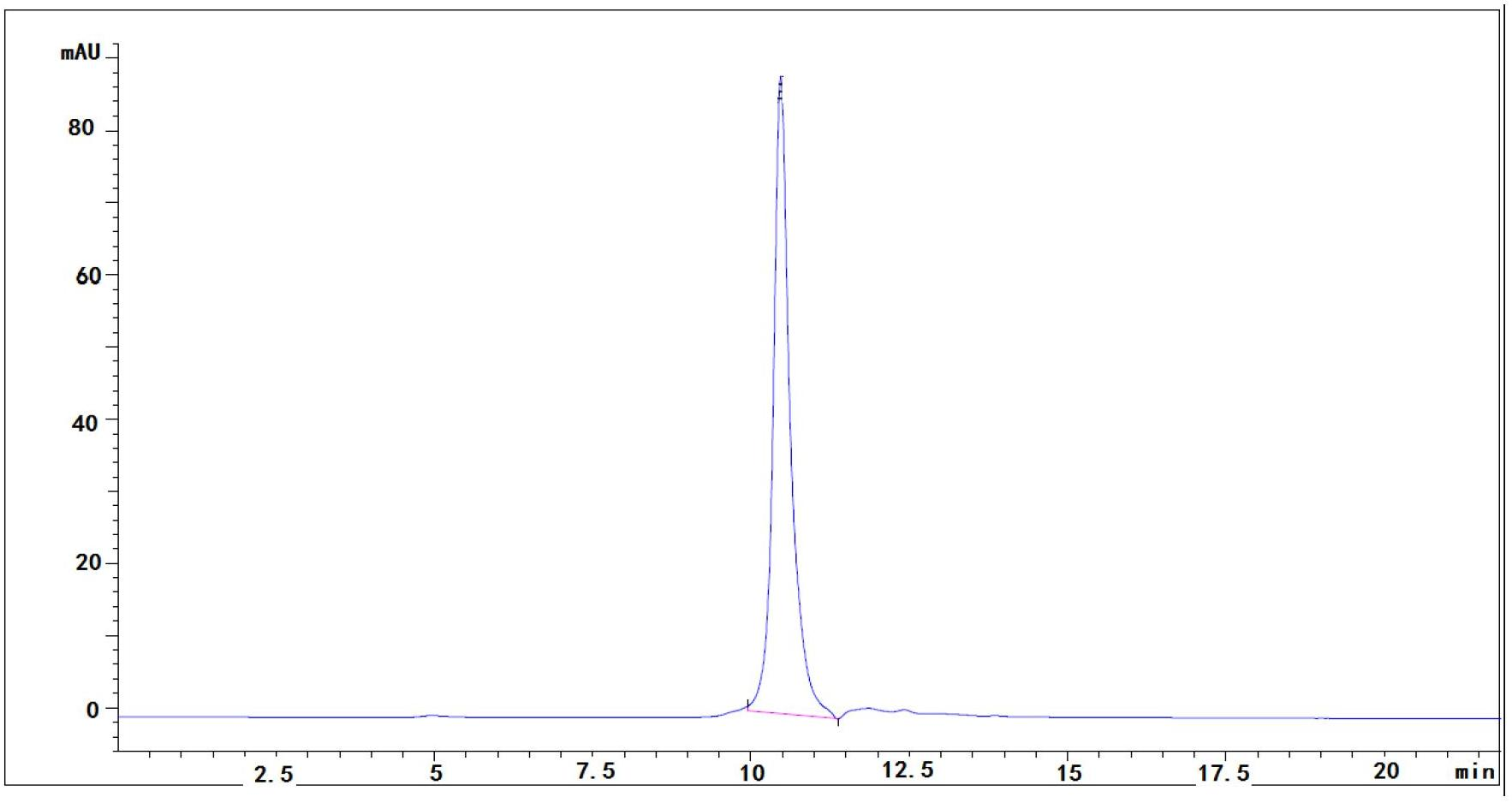
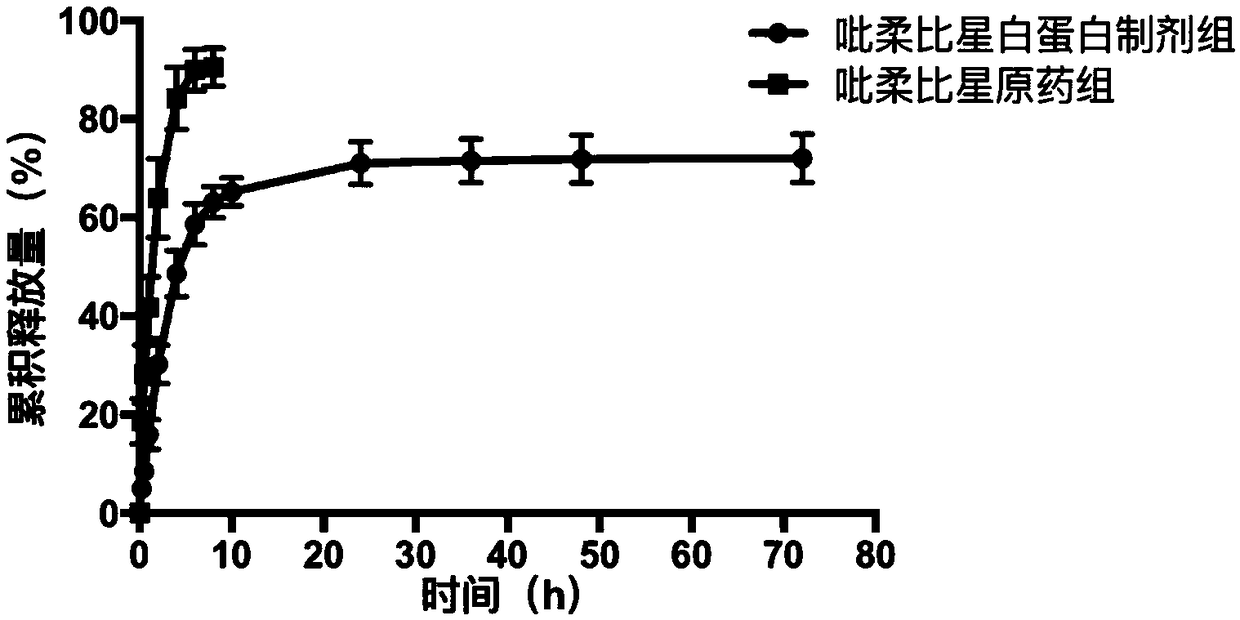
Albumin nanoparticle preparation for anthracycline antitumor antibiotic oleic acid compound
ActiveCN102552154AProlong the action timeTo achieve the purpose of targeted deliveryPowder deliveryAntinoxious agentsTumor targetingBiology
The invention provides a method for preparing an anthracycline antitumor antibiotic oleic acid compound and an oil-water double phase for preparing the compound and albumin, and the anthracycline antitumor antibiotic oleic acid compound is prepared into an albumin nanoparticle preparation. The albumin nanoparticle preparation prepared by the method is little in using amount of auxiliary materials, high in medicine-carrying dose and high in stability, and a preparation process is simple and is suitable for large-scale industrial production; and the albumin nanoparticle preparation has the tumor targeting and good clinical application value.
Owner:SICHUAN BAILI PHARM CO LTD
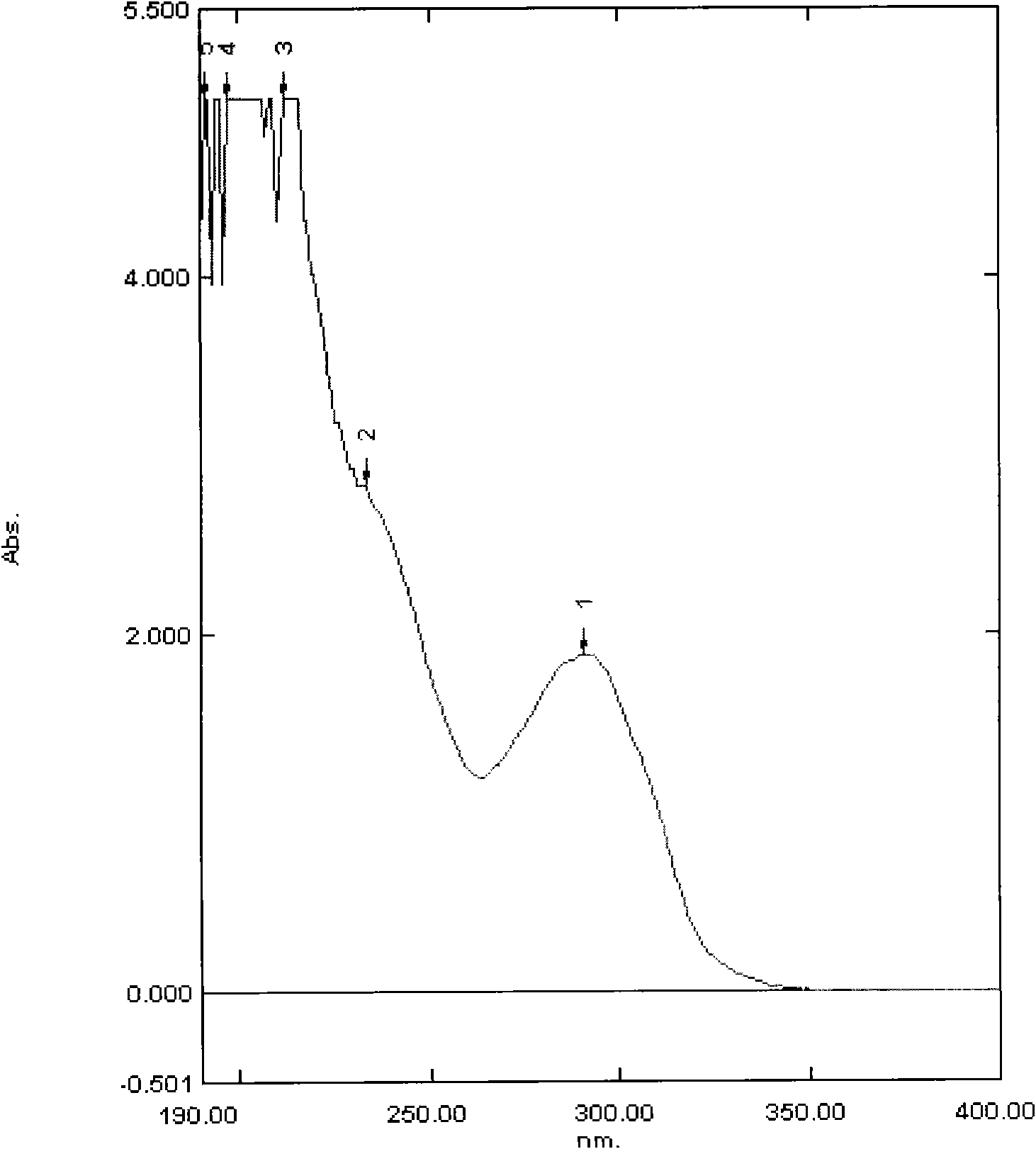
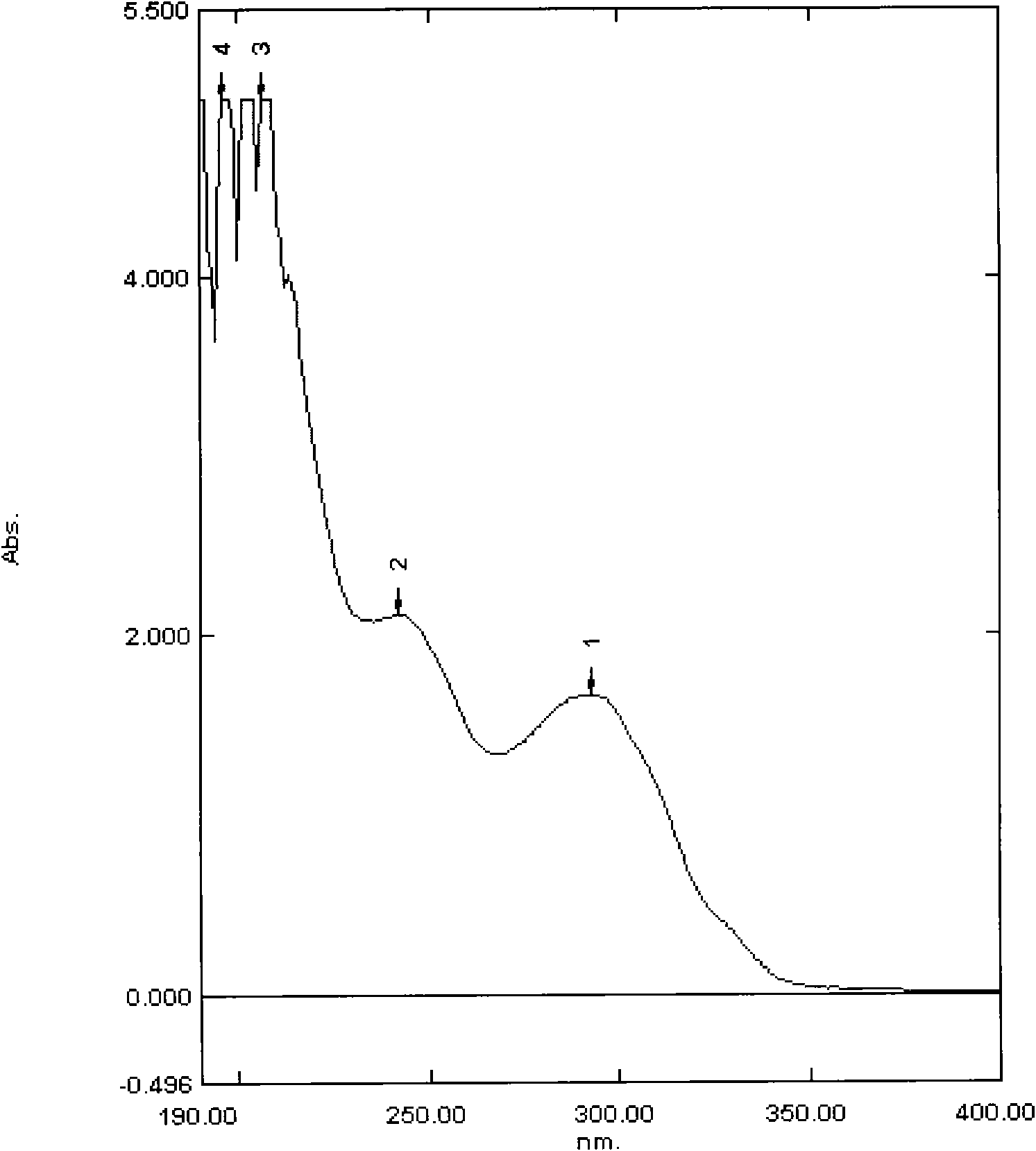
Antitumor antibiotics, pharmaceutically acceptable salts thereof, preparation method thereof and use thereof
InactiveCN101628931AMicroorganism based processesSaccharide peptide ingredientsAntitumor AntibioticsStructural formula
The invention provides a novel compound having a structural formula (I), and a pharmaceutically acceptable salts formed by the compound. The compound is obtained by fermentation culture of producing bacteria thereof. The compound is the novel compound of a bleomycin group, has better antitumor activity than that of the clinical bleomycin, and can be used for preparing an antitumor medicament.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Method for preparing nano micelle formulation of anthracene nucleus antineoplastic antibiotic
ActiveCN101322681ASimple processSuitable for industrial productionOrganic active ingredientsPharmaceutical delivery mechanismAnthraceneOrganic solvent
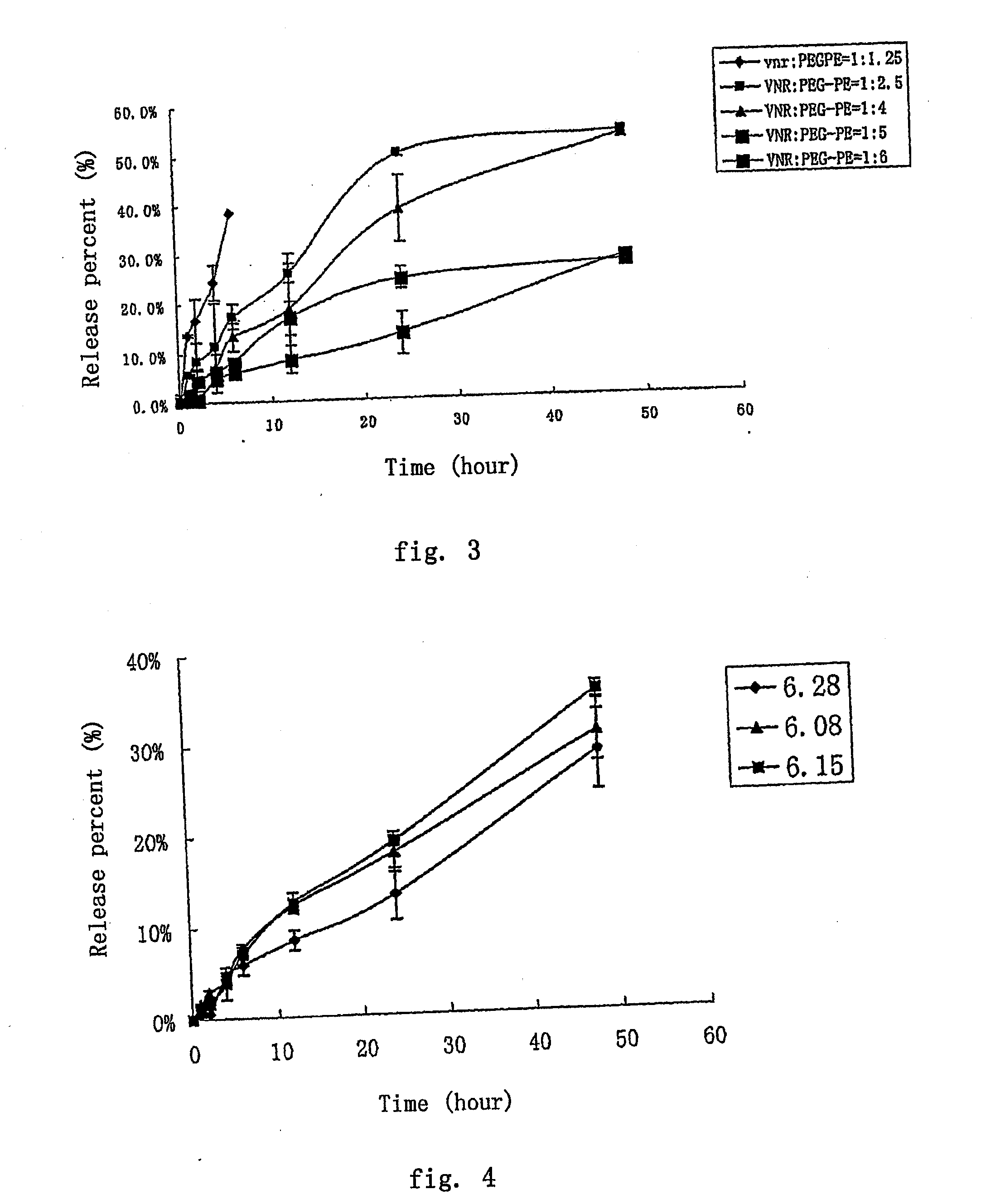
The invention provides a method for preparing a nano micelle preparation of anthracycline antitumor antibiotics, including the following steps: (1) dissolving anthracycline antitumor antibiotics in water or medicinal buffer salt solution; (2) dissolving polyethylene glycol-phospholipid in water or medicinal buffer salt solution; (3) mixing the solution in step (1) and the solution in step (2) and hydrating the mixture at 25 DEG C to 60 DEG C to obtain the nano micelle of anthracycline antitumor antibiotics. Compared with the prior art, the method of the invention is simple and easy to operate, does not need complex large-scale instruments, and is suitable for industrial production, and the obtained nano micelle preparation does not contain nano organic solvent.
Owner:SHANGHAI WHITTLONG PHARMA INST
Antharcycline antitumor antibiotics loaded nano-micelle preparation and preparation method thereof
InactiveCN102973525AAvoid dissociationHigh drug loadingOrganic active ingredientsPowder deliveryPolyesterProtein molecules
The invention discloses an antharcycline antitumor antibiotics loaded nano-micelle preparation, which comprises antharcycline antitumor antibiotics, A-B-C type triblock polymer and assisting agents, wherein the antharcycline antitumor antibiotics is one or more of adriamycin, daunorubicin, pharmorubicin, perarubicin or lacinomycin; and the A-B-C type triblock polymer is polyethylene glycol-polyethylene glycol containing carboxyl on side chain-polyester. According to the preparation, drugs are coated in the nano-micelle by virtue of the coaction of self-assembly of segmented copolymer and electrostatic adsorption, the nano-micelle has uniform and stable grain diameter, the encapsulation efficiency reaches 99 percent, and the prepared nano-micelle preparation is of a spherical structure with grain diameter between 20 and 300nm; and the shell of the micelle is made from polyethylene glycol molecules, so that drugs are prevented from being contacted with enzyme and other protein molecules in blood or identified and swallowed by a reticuloendothelial system in the body, so that the circulating period of micelle in the body can be prolonged.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI +1
Combination methods of treating cancer
InactiveUS20100003346A1Prevent proliferationSynergistic effectHeavy metal active ingredientsBiocideNitrosoTreatment effect
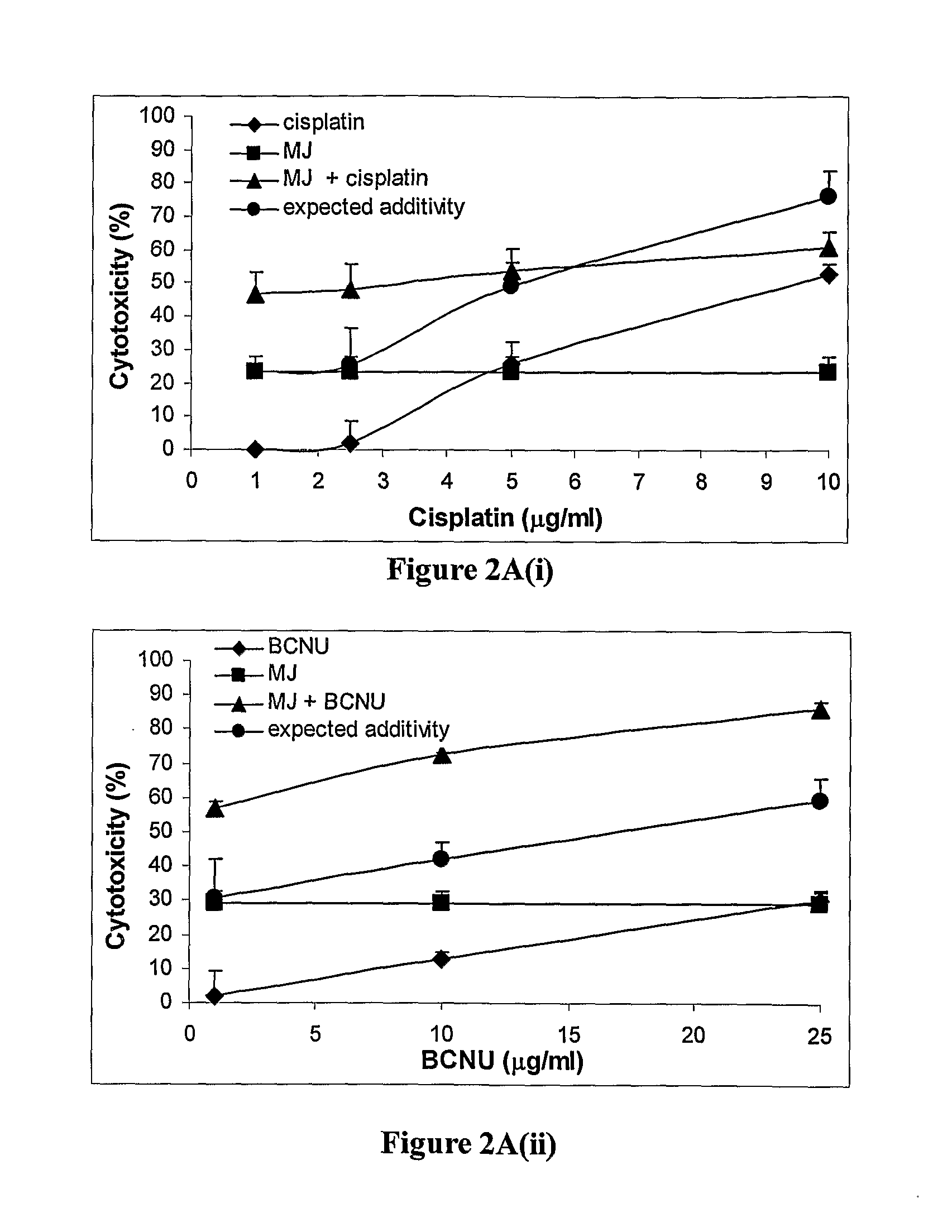
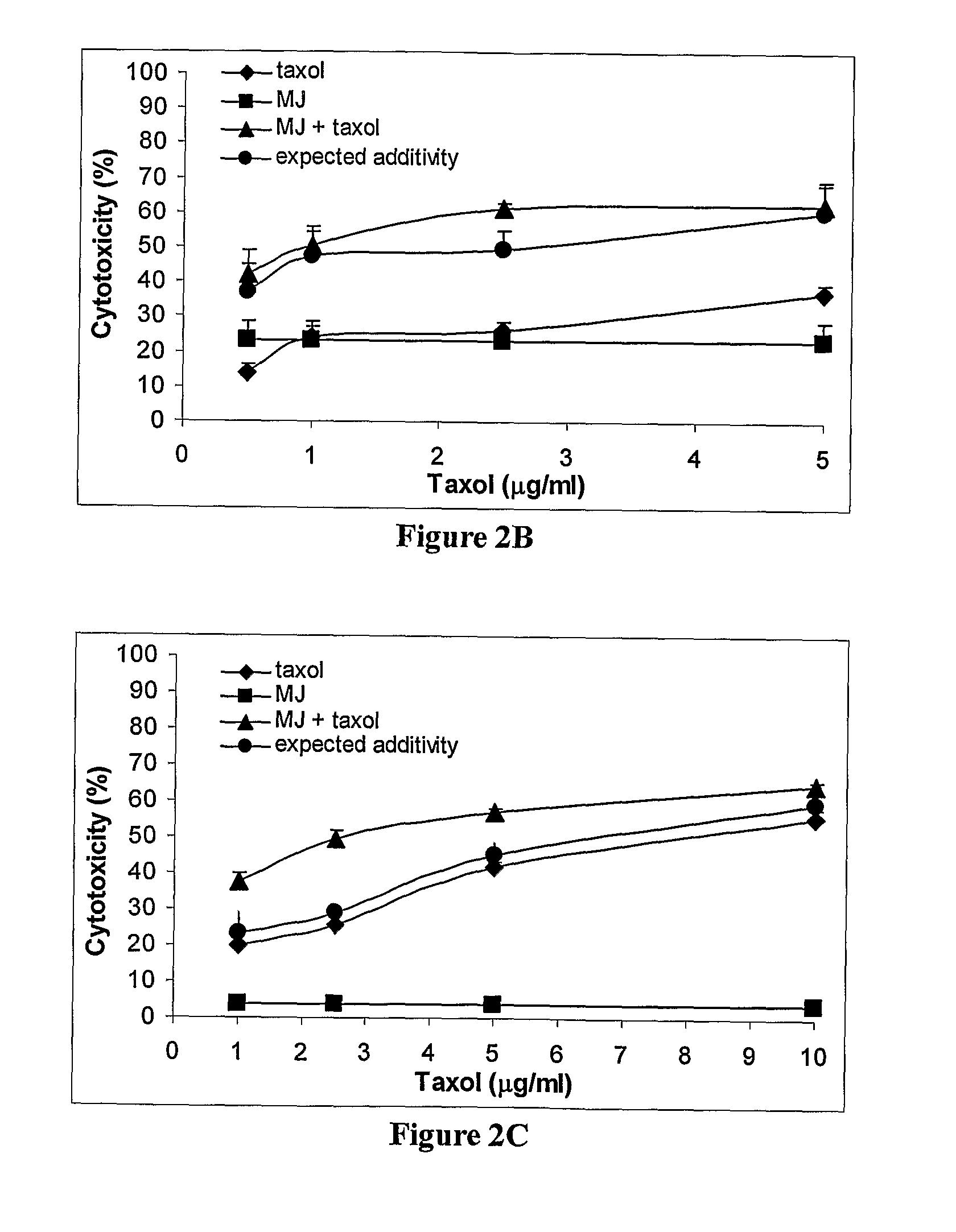
The present invention relates to compositions and methods for treating cancer, by administering a combination comprising a jasmonate derivative (e.g., methyl jasmonate or a compound of any of formulae I through VII or any of the jasmonate derivatives exemplified by such formulae) and at least one other agent selected from a chemotherapeutic agent (e.g., a nitroso-urea, a platinum compound, a taxane derivative, an antitumor antibiotic) and an inhibitor of glycolysis (e.g., 2-deoxy-D-glucose). The jasmonate derivative and the at least one other agent together provide a therapeutic effect, which is preferably synergistic (cooperative).
Owner:SEPAL PHARMA SA +1
Lipid nano particle preparation containing composite formed fatty acid and anthracyclines antitumor antibiotics and preparation method thereof
ActiveCN101810570AIncrease fat solubilityLow toxicityOrganic active ingredientsPowder deliveryLipid formationFatty acid formation
The invention provides a lipid nano particle preparation containing a composite formed fatty acid and anthracyclines antitumor antibiotics and a preparation method thereof. The lipid nano particle preparation comprises a composite formed by the anthracyclines antitumor antibiotics and fatty acid, and phospholipid and lipoid of lipid nano particle carrier ingredients, wherein the weight ratio of the composite, the phospholipid and the lipoid is 1:1-10:1-10:1-1000.
Owner:SICHUAN BAILI PHARM CO LTD
A pharmaceutical composition for targeted tumor therapy and its preparation method
The patent of the present invention relates to a pharmaceutical composition containing antitumor antibiotic-dipeptide derivatives that can be used for targeted tumor therapy and a preparation method thereof, belonging to the technical field of pharmaceutical preparations. The pharmaceutical composition is an injection composed of antitumor antibiotic-dipeptide derivatives, phospholipids, stabilizers, organic solvents, antioxidants, surfactants, and pH regulators. Dry powder needle. The pharmaceutical composition greatly increases the solubility and stability of the antitumor antibiotic-dipeptide derivatives with targeted antitumor effects in aqueous solution, and can be effectively used for malignant epithelial tumors (such as breast cancer, ovarian cancer, Targeted therapy for lung cancer, colon cancer, pancreatic cancer, skin melanoma) and other solid tumors.
Owner:重庆寰瑞生物技术有限公司
Slow released compound anticancer injection containing bendamustine
InactiveCN1887261AEasy injectionIncrease drug concentrationOrganic active ingredientsSolution deliveryMicrospherePolyethylene glycol
The slow released compound anticancer injection containing bendamustine consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent. The effective anticancer component is the composition of bendamustine and bendamustine synergist selected from antitumor antibiotic and antimetabolite. The slow releasing supplementary material is polylactic acid and its copolymer, polifeprosan, EVAc, etc or their composition. The .suspending agent has viscosity of 80-3000 cp. The slow released microsphere may be also prepared into slow released implanting agent. The present invention can inhibit the growth of tumor and enhance the curative effect of chemotherapy, radiotherapy and other non-operative treatment.
Owner:JINAN SHUAIHUA PHARMA TECH
Method for determination of doxorubicin hydrochloride in body fluid based on magnetic nano-adsorbent
InactiveCN103464109AEasy to makeMake fastComponent separationOther chemical processesFluorescenceSorbent
The invention provides a preparation method of a trimethylstearylammonium bromide (TSAB)-coated Fe3O4 magnetic nano-adsorbent, and a method for determination of doxorubicin hydrochloride in blood plasma and urine by the TSAB-coated Fe3O4 magnetic nano-adsorbent. The method for determination of doxorubicin hydrochloride in blood plasma and urine comprises the following steps of preparing the TSAB-coated Fe3O4 / SiO2 in a layer-by-layer assembly way by a chemical coprecipitation method, adding the TSAB-coated Fe3O4 / SiO2 into doxorubicin hydrochloride-containing blood plasma and urine samples, adjusting a pH value to 4-5 by a phosphate buffer, carrying out vortex oscillation for 3min, carrying out magnetic separation, removing a supernatant, adding an eluent into the magnetic separation product, carrying out elution, carrying out magnetic separation, collecting a supernatant and carrying out analysis by a high performance liquid chromatography-fluorescence detection method. The magnetic nano-adsorbent obtained by the preparation method has good stability and large adsorption quantity, can be recycled, has a strong capability of extracting anthracene ring antitumor antibiotics such as adriamycin from blood plasma and urine samples, and can realize fast and effective separation and determination of doxorubicin hydrochloride in the samples.
Owner:SHANXI UNIV
Nano-micellar preparation of anthracylcline antitumor antibiotics encapsulated by the phosphatide derivatized with polyethylene glycol
InactiveUS20090232900A1Improve stabilityEnhanced drug distributionBiocideCarbohydrate active ingredientsAdjuvantProtein molecules
The present invention provides a nano-micellar preparation of anthracycline antitumor antibiotics for intravenous injection, which comprises a therapeutically effective amount of anthracycline antitumor antibiotics, a phosphatide derivatized with polyethylene glycol, together with pharmaceutically acceptable adjuvants. The preparation is prepared by encapsulating the medicament with a nano-micelle to obtain the nano-micellar preparation of anthracycline antitumor antibiotics for injection. The anthracycline antitumor antibiotics and the phosphatide derivatized with polyethylene glycol form a nano-micelle with a highly homogeneous particle size. In the micelle, the hydrophobic core of encapsulated medicament is surrounded by polyethylene glycol molecules to form a hydrophilic protective layer, so that the medicament is prevented from contacting with the enzymes and other protein molecules in blood and being recognized and phagocytozed by reticuloendothelial system in the body, and the circulation time in vivo of the micelle is prolonged.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Combination of EPA, DPA and/or DHA with a chemotherapeutic agent
The present invention refers to a combination of an eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and / or docosahexanoic acid (DHA), or a combination of a nutritional composition comprising EPA, DPA, and / or DHA and a protein, a carbohydrate, a fat, an amino acid, a fatty acid, a dietary fibre, a vitamin, a mineral, a trace element, ß-carotenoid, a flavonoid, a nucleotide, L- carnitin, choline, inositol, taurine, creatine, and / or a co-enzyme, wherein EPA is in an amount of 1 to 1000 mg / 100ml, preferably 100 to 700 mg / 100ml, DPA is in an amount of >50 mg / 100ml, preferably 50 to 1000 mg / 100ml, preferably 6 to 800 mg / 100ml, more preferably 80 to 500 mg / 100ml and / or DHA is in an amount of 1 to 500 mg / 100ml, preferably 1 to 300 mg / 100ml and a chemotherapeutic agent selected from the group consisting of an alkylating drug, an antimetabolite, an antimytotic cytostatic, a topoisomerase inhibitor, antitumor antibiotic, and any other cytostatic, and / or a radiotherapy. These combinations are successful for use in treating a neoplastic disease like the method for treating a neoplastic disease comprising EPA, DPA, and / or DHA or a nutritional composition comprising EPA, DPA, and / or DHA and and a protein, a carbohydrate, a fat, an amino acid, a fatty acid, a dietary fibre, a vitamin, a mineral, a trace element, ß-carotenoid, a flavonoid, a nucleotide, L- carnitin, choline, inositol, taurine, creatine, and / or a co-enzyme in combination with a chemotherapeutic agent, and / or radiotherapy.
Owner:NV NUTRICIA
Cancer Chemotherapeutic Agent/Formulation, Manufacture and Use Thereof
ActiveUS20160023996A1Reduce tumor volumeInhibit cell proliferationBiocideCarboxylic acid nitrile preparationCytotoxicityPharmaceutical formulation
A cancer chemotherapeutic agent that is particularly kinase suppressing and / or any other signaling pathway interfering agents and pharmaceutical formulations / compositions involving the same and its process of manufacture is provided. A potential the cancer chemotherapeutic agent is provided which apart from stated anticancer activity as a proven kinase suppressing and / or any other signaling pathway interfering agent could also involve specific potential binding affinity towards the intramolecular G-Quadruplex DNA structure and / or other potential quadruplex forming sequences over duplex DNA structures favours further diverse end use and application including but not limited to antiaging, antiangiogenic, antiproliferative, antitumor, antibiotic, antiviral, antifungal and multiple anticancer therapeutics, and also possesses favourable cytotoxicity values towards uncontrollably proliferative cells by inducing apoptosis irrespective of cells' p53 status, without being cytotoxic to normal cells.
Owner:BOSE INSTITUTE +1
Antitumor antibiotics and its production
InactiveCN101074234AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryNocardiaAntitumor Antibiotics
A pyranyl nequinone anti-neoplastic antibiotic is prepared by fermenting, culturing Nocardiopsis sp. YIM 80133, separating out from fermented liquid, purifying extract to obtain final product with molecular formula C21H20010 and molecular weight 432.38. It has strong inhibiting function of human progranuleukemic tumor cell.
Owner:YUNNAN UNIV
Slow released compound anticancer prepn containing bendamustine
InactiveCN1887264AEasy to operateGood repeatabilityOrganic active ingredientsSolution deliveryMicrospherePolyethylene glycol
The slow released compound anticancer injection containing bendamustine consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent. The effective anticancer component is the composition of bendamustine and bendamustine synergist selected from antitumor antibiotic and antimetabolite. The slow releasing supplementary material is polylactic acid / glycolic acid copolymer, polyethylene glycol / polylactic acid, EVAc, etc. The .suspending agent is carboxymethyl cellulose, etc. and has viscosity of 80-3000 cp at 20-30 deg.c. The slow released microsphere may be also prepared into slow released implanting agent. The present invention can inhibit the growth of tumor effectively and enhance the curative effect of chemotherapy, radiotherapy and other non-operative treatment.
Owner:JINAN SHUAIHUA PHARMA TECH
Antitumor antibiotic ancomycin and its derivative
ActiveCN102250176ASpecial chemical structureDefinitely anti-tumor effectOrganic active ingredientsSugar derivativesChemical synthesisIn vivo
The invention relates to derivatives of a cytidine peptide compound, and specifically relates to ancomycin methyl ester, isopropyl ester and isopentyl ester. Wherein, carboxyl in position 5' of the hexose ring in the fermentation product ancomycin of streptomyces albulus C-9095 is connected with the methyl, isopropyl and isopentyl respectively for esterification through different chemosynthesis methods, thus obtaining the relevant ancomycin ester derivatives. Experimental research both in vivo and in vitro proves that the derivatives have inhibitory effects on relevant tumor cells, thus being hopeful to be developed as antitumor medicaments.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Slow-released anticancer injection
The slow released anticancer injection consists of slow released microballoon and solvent. The slow released microballoon includes effective anticancer component and slow releasing supplementary material, and the solvent is common solvent or special solvent containing suspending agent. The effective anticancer component is 5-FU synergist or the combination of 5-FU and 5-FU synergist selected from antitumor antibiotic, antimetabolite, taxane and plant alkaloid; the slow releasing supplementary material is PLA, PLGA, EVAc, etc or their composition; and the suspending agent is sodium carboxymethyl cellulose, mannitol, sorbitol, Tween-80 or their composition. The slow released microballoon may be also prepared into slow released implantation preparation. Implanting or injecting the slow released preparation to local tumor part can lower the systematic toxic reaction of the medicine and raise the medicine concentration of local tumor part selectively to raise the treating effect.
Owner:SHANDONG LANJIN PHARMA
Combination methods of treating cancer
Owner:RAMOT AT TEL AVIV UNIV LTD +1
Method of predicting chemotherapeutic responsiveness of cancer
ActiveUS20100292087A1% efficiencyPredict prognosisLibrary screeningAnalogue computers for chemical processesAlkaloidWilms' tumor
Disclosed is a method of predicting clinical tumor outcome by providing gene expression from a tumor sample. The method utilizes a novel genetic screen to identify genes that contribute to chemotherapeutic responsiveness, using formalin fixed paraffin embedded clinical samples of epithelial cancer, specifically serous ovarian cancer. The method is useful in predicting tumor responsiveness to chemotherapeutics, including alkylating agents, cisplatin, antimetabolites, plant alkaloids, and antitumor antibiotics. A microarray screen showed formalin fixed paraffin embedded samples can identify genes related to chemotherapeutic response with 86% efficiency.
Owner:UNIV OF SOUTH FLORIDA
Treatment of aml
InactiveCN101076338AOrganic active ingredientsAntineoplastic agentsConventional chemotherapyAntitumor Antibiotics
The present Invention relates to a method of treating a warm-blooded animal having acute myeloid leukemia (AML) which is resistant to conventional chemotherapy, comprising administering to said animal a therapeutically effective amount of a compound of formula (I), wherein the radicals and symbols have the meanings as defined in the specification, together or in combination with a conventional compound or compound mixture useful in AML treatment, in particular a topoisomerase II inhibitor, an antimetabolite, or an antitumor antibiotic, for simultaneous, separate or sequential use; and to a pharmaceutical composition and a commercial package comprising said combination.
Owner:齐肯胡伊斯-格罗宁根学院(UMCG)
Compound anticancer sustained-release injection containing vascular inhibitor
InactiveCN101352414AOrganic active ingredientsPharmaceutical delivery mechanismAdditive ingredientDepressant
The invention discloses a compound anticancer slow-release injection which contains blood vessel inhibitor and consists of slow-release microspheres and solvent; wherein, the slow-release microspheres comprise anticancer effective ingredients and slow-release auxiliary materials; the solvent is common solvent or special solvent containing a suspending agent; the anticancer effective ingredients are synergists of the blood vessel inhibitor such as gefitinib, erlotinib, lapatinib and futalanib, etc. and the blood vessel inhibitor which is selected from antitumor antibiotics and / or antimetabolic drugs; the slow-release auxiliary materials are selected from one or combination of copolymer of bi-fatty acid and sebacic acid and copolymer of poly-(erucic acid dimmer-sebacic acid), poly-(fumaric acid-sebacic acid), polifeprosan, polyactic acid, polyglycolic acid and glycolic acid, and copolymer of ethylene-vinylacetate; the suspending agent is selected from carboxymethyl cellulose, etc. and has 80cp-3000cp (with the temperature of 20 DEG C to 30 DEG C) of viscosity; the slow-release microspheres also can be made into a slow-release implant, is injected or placed in tumor or around the tumor and is applied separately or is applied with the non-operative treatment of chemoradiotherapy, and the like, in a combining way.
Owner:JINAN KANGQUAN PHARMA TECH
Method for preparing nano micelle formulation of anthracene nucleus antineoplastic antibiotic
ActiveCN101322681BImprove stabilityImprove distributionOrganic active ingredientsPharmaceutical delivery mechanismAnthraceneOrganic solvent
The invention provides a method for preparing a nano micelle preparation of anthracycline antitumor antibiotics, including the following steps: (1) dissolving anthracycline antitumor antibiotics in water or medicinal buffer salt solution; (2) dissolving polyethylene glycol-phospholipid in water or medicinal buffer salt solution; (3) mixing the solution in step (1) and the solution in step (2) andhydrating the mixture at 25 DEG C to 60 DEG C to obtain the nano micelle of anthracycline antitumor antibiotics. Compared with the prior art, the method of the invention is simple and easy to operate, does not need complex large-scale instruments, and is suitable for industrial production, and the obtained nano micelle preparation does not contain nano organic solvent.
Owner:SHANGHAI WHITTLONG PHARMA INST
Antitumor antibiotic gaddie mycin generated by streptosporangium C-3560
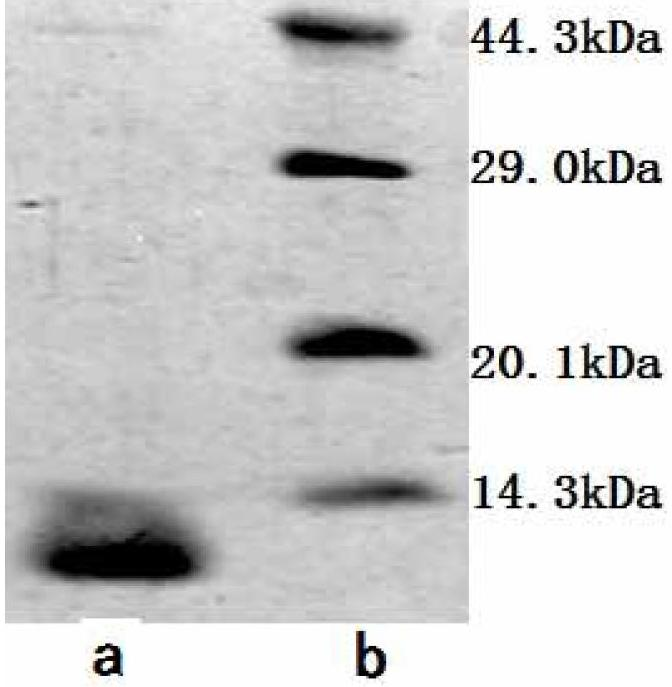
ActiveCN102675431AExpand sourcePeptide/protein ingredientsMicroorganism based processesActive proteinAntitumor Antibiotics
The invention relates to an antitumor active substance generated by streptosporangium C-3560. The antitumor active protein molecular weight is 10.8kDa, and the N-end sequence is shown as a sequence table SEQ ID No.1. Active substance biology experimental study results prove that obvious antitumor activity is realized on various kinds of tumor cells in-vitro culture, the tumor cell apoptosis can be induced, and in addition, the cell cycle arrest is generated. In-vitro animal experiments prove that the tumor growth of mouse H22 liver cancer can be obviously inhibited, and the antitumor active substance is hopeful to further develope into clinical efficient antitumor medicine.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Compound anti-cancer slow-release injected containing blood vessel inhibitor
InactiveCN1850037APharmaceutical delivery mechanismMacromolecular non-active ingredientsCelluloseAnticarcinogen
The present invention relates to a compound anticarcinogen slow-released injection containing vasoinhibitor and its synergist. It is composed of slow-released microsphere and solvent. The slow-released microsphere includes anticancer effective component and slow-released auxiliary material, the solvent is general solvent or special solvent containing suspension adjuvant. The anticancer effective component is combination of vasoinhibitor and vasoinhibitor synergist of antitumor antibiotic or antimetabolite medicine, the slow-released auxiliary material is one selected from racemic polylactic acid and its copolymer, monomethyl polyethylene glycol and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer, carboxyl terminated polylactic acid and glycollic acid copolymer and ethylene-vinylacetate, etc. or their combination. The viscosity of suspension adjuvant is 100 cp-3000 cp, said suspension adjuvant is selected from sodium cellulose glycollate. The slow-released microsphere also can be made into slow-released implant preparation. Said invention also provides its application method for curing various tumors and cancers.
Owner:SHANDONG LANJIN PHARMA +1
Pharmaceutical composition for Anti-vascular diseases and Anti-tumor and use thereof
ActiveUS20170087208A1Relieves local pain stimulationIncreased riskPowder deliveryOrganic active ingredientsAbnormal tissue growthGlucocorticoid
An anti-vascular diseases and antitumor pharmaceutical composition is provided in the present invention, and it includes effective ingredients including bleomycin antitumor antibiotic, adrenal glucocorticoid, epinephrine or pharmaceutically acceptable salts thereof in a weight ratio of (1-8):(2-5):(0.00005-0.001). The pharmaceutical composition provided can be used for treatment of vascular diseases and tumors.
Owner:GUANGZHOU YIDAI PHARMA +1
Albumin-binding anthracycline antitumor antibiotic preparation and preparation method thereof
ActiveCN108743956ANon-immunogenicBiodegradableMacromolecular non-active ingredientsAntineoplastic agentsMedicineAntitumor Antibiotics
The present invention discloses an anthracycline antitumor antibiotic albumin conjugate comprising an anthracycline antitumor antibiotic or a pharmaceutically acceptable salt thereof and albumin in aweight ratio of 1:1 to 1000. The invention also discloses lyophilized powder for injection, a preparation, application and a preparation method of the anthracycline antitumor antibiotic albumin conjugate. The anthracycline antitumor antibiotic albumin conjugate has high efficiency, low toxicity, good drug stability, simple preparation method and convenient clinical use.
Owner:SICHUAN BAILI PHARM CO LTD
Slow released injection containing satraplatin and its synergist
The slow released anticancer injection containing both satraplatin and its synergist consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material carrier, and the solvent is special solvent containing suspending agent. The effective anticancer component includes satraplatin, Cisplatin, Carboplatin, etc and satraplatin synergist selected from antitumor antibiotic, toporase inhibitor and / or tetrazine compound. The slow releasing supplementary material is selected from difatty acid-sebacic acid copolymer, polylactic acid, etc. The suspending agent is carboxymethyl cellulose sodium, etc. and has viscosity of 100-3000 cp at 20-30 deg.c. The slow released microsphere may be also prepared into slow released implanting agent for use to raising chemotherapeutic and radiotherapeutic effect.
Owner:JINAN KANGQUAN PHARMA TECH
Slow released compound anticancer injection containing blood vessel inhibitor
The slow released compound anticancer injection containing blood vessel inhibitor and its synergist consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is common solvent or special solvent containing suspending agent. The effective anticancer component is blood vessel inhibitor and / or blood vessel inhibitor synergist selected from antitumor antibiotic and antimetabolite. The slow releasing supplementary material is selected from difatty acid-sebacic acid copolymer, poly(erucic acid dipolymer-sebacic acid), poly(fumaric acid-sebacic acid), etc or their composition. The suspending agent is carboxymethyl cellulose, etc. and has viscosity of 80-3000 cp at 20-30 deg.c. The slow released microsphere may be also prepared into slow released implanting agent for use alone or together with chemotherapeutic and / or radiotherapeutic medicine..
Owner:JINAN SHUAIHUA PHARMA TECH
Anticancer medicine slow-release preparation containing platinum compound and its synergist
The present invention relates to an anticarcinogen slow-released injection containing platinum compound and its synergist. It is composed of microsphere and solvent, in which the microsphere includes anticancer effective component and slow-released auxiliary material, the solvent is general one or special solvent containing suspension adjuvant. The anticancer effective component includes platinum compound selected from heptylplatin, loplatin, neidaplatin, oxaliplatin, denaplatin or enloplatin and / or platinum compound synergist selected from antitumor antibiotic, antimetabolite medicine, taxane and / or plant alkaloid; the slow-released auxiliary material is selected from polylactic acid copolymer, ethylene-vinylacetate copolymer, difatty acid and sebacic acid polymer and poly (fumaric acid-sebacic acid) copolymer. The suspension adjuvant is selected from sodium cellulose glycollate, and its viscosity is 100 cp-3000 cp (20 deg.C-30 deg.C). The slow-released microsphere also can be made into slow-released implant preparation.
Owner:JINAN SHUAIHUA PHARMA TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com