Patents
Literature
62 results about "Purine Antimetabolite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An antimetabolite is a chemical that inhibits the use of a metabolite, which is another chemical that is part of normal metabolism. Such substances are often similar in structure to the metabolite that they interfere with, such as the antifolates that interfere with the use of folic acid.
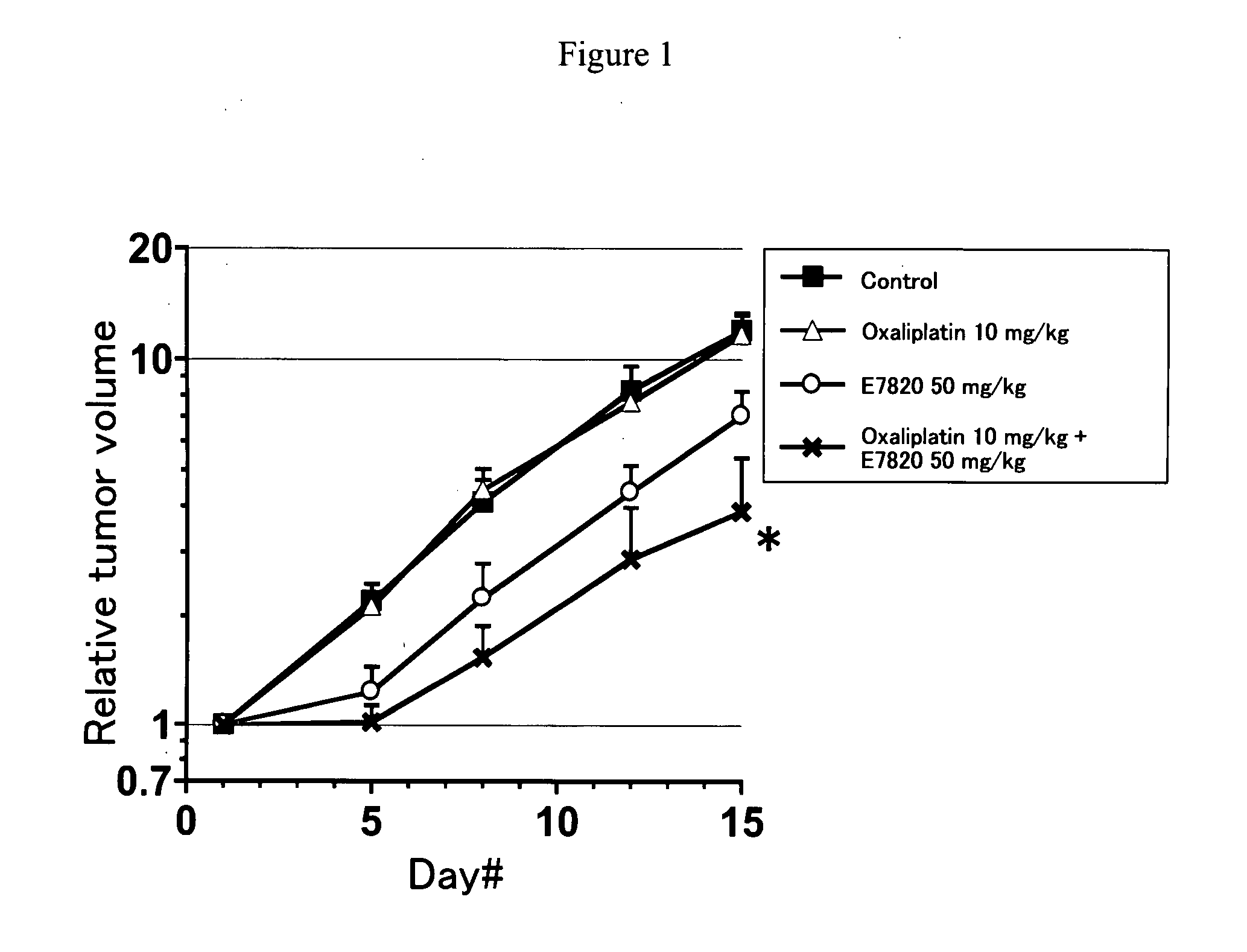
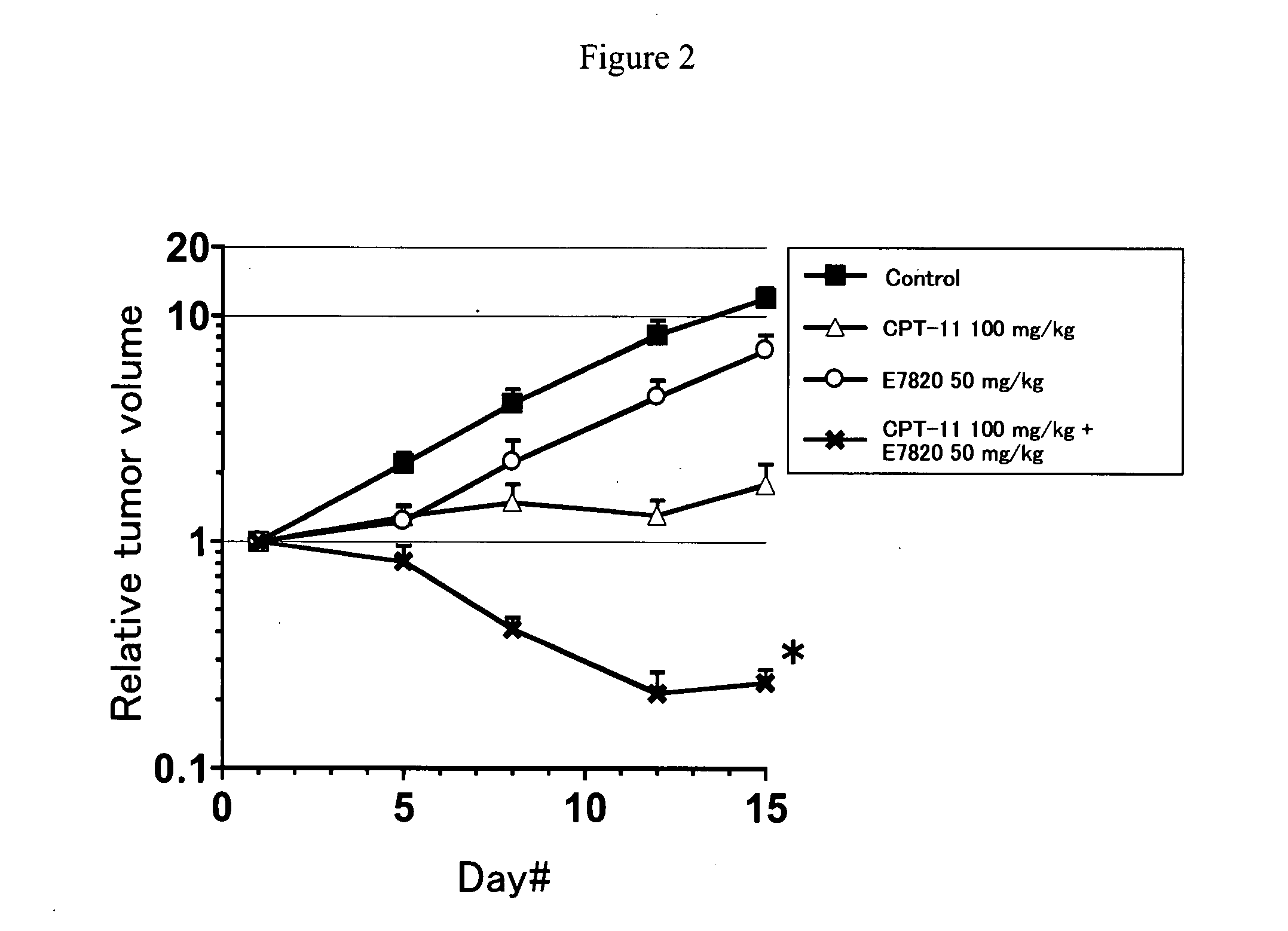
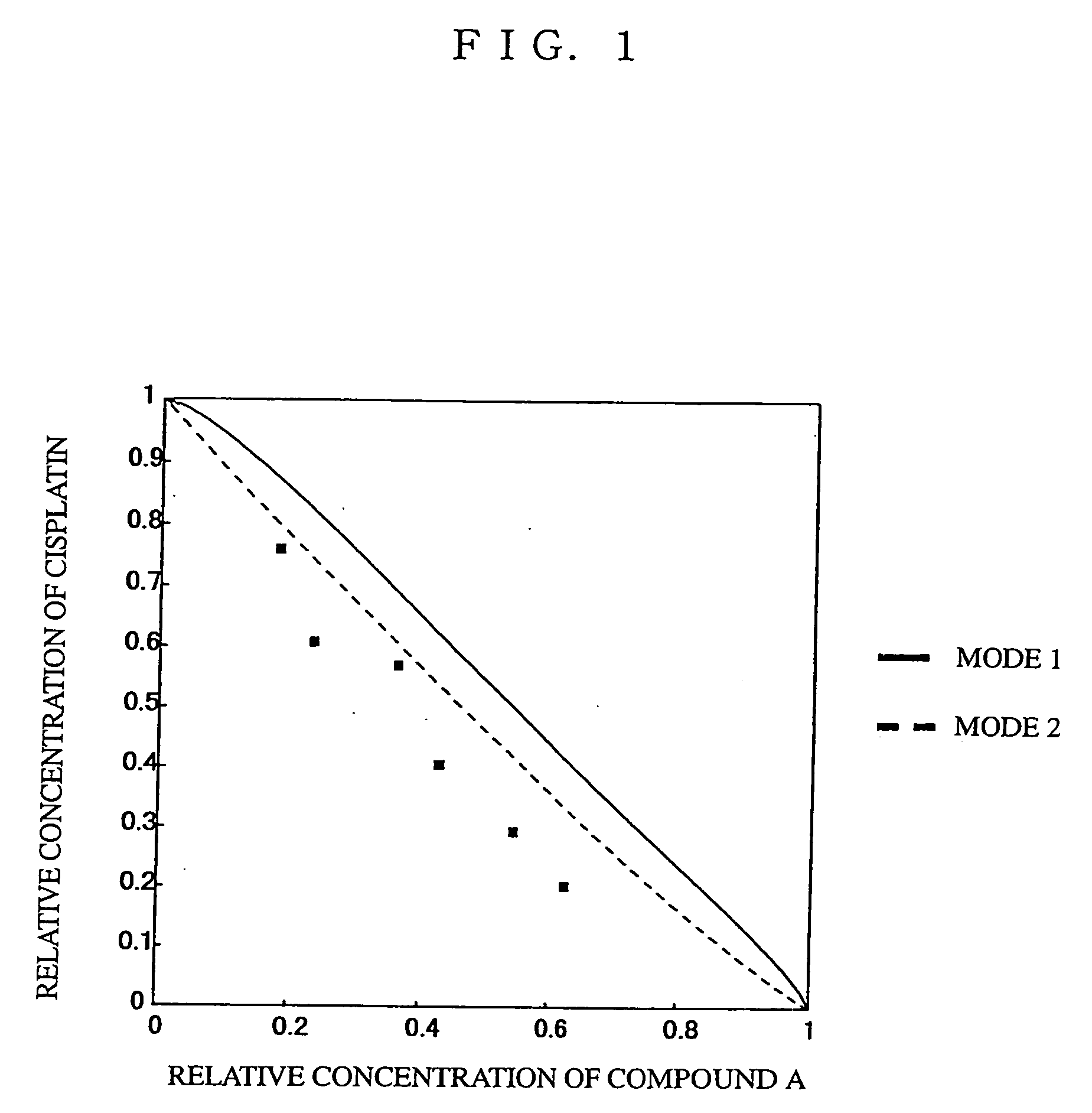
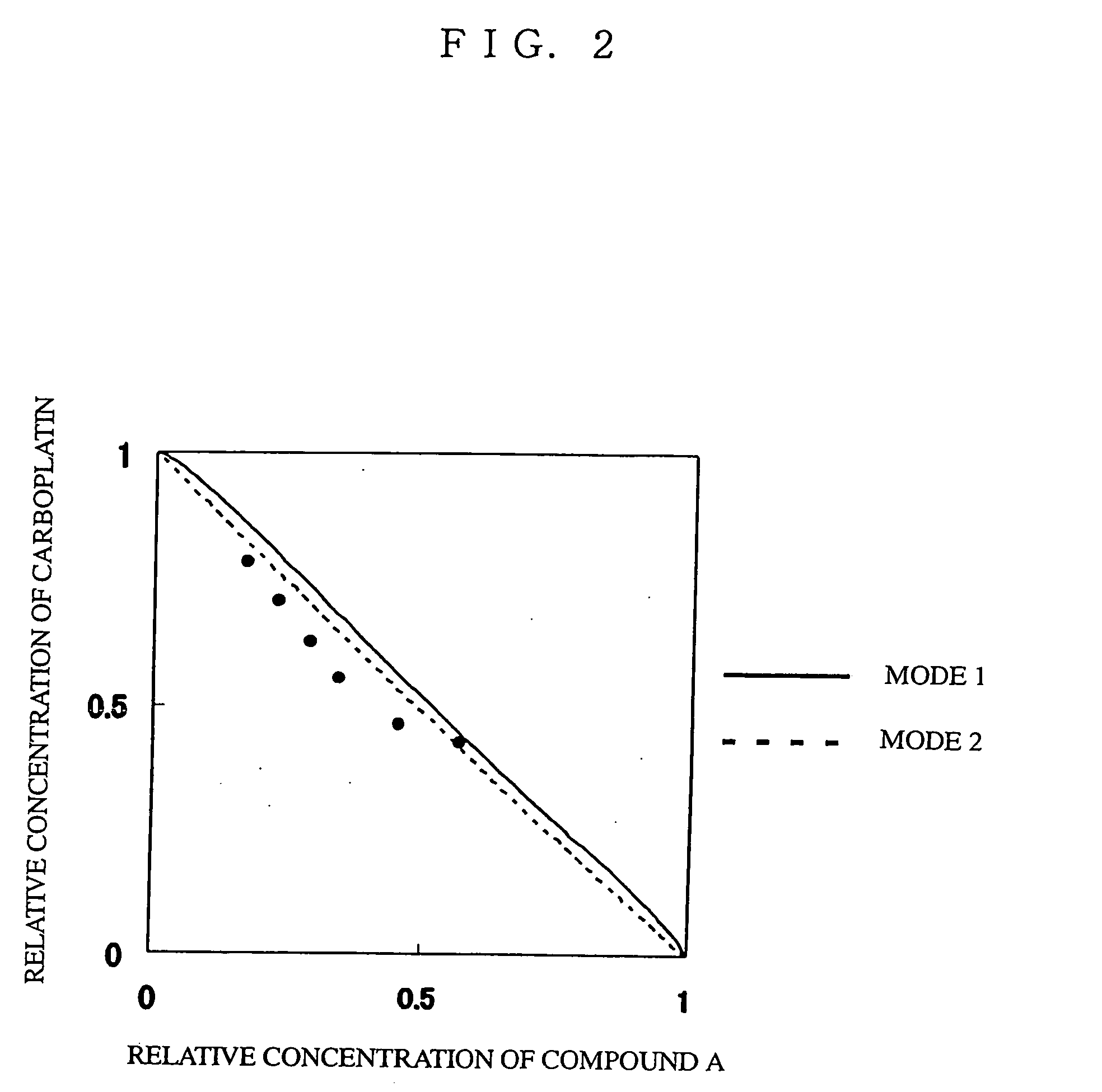
Novel Concomitant Use of Sulfonamide Compound with Anti-Cancer Agent
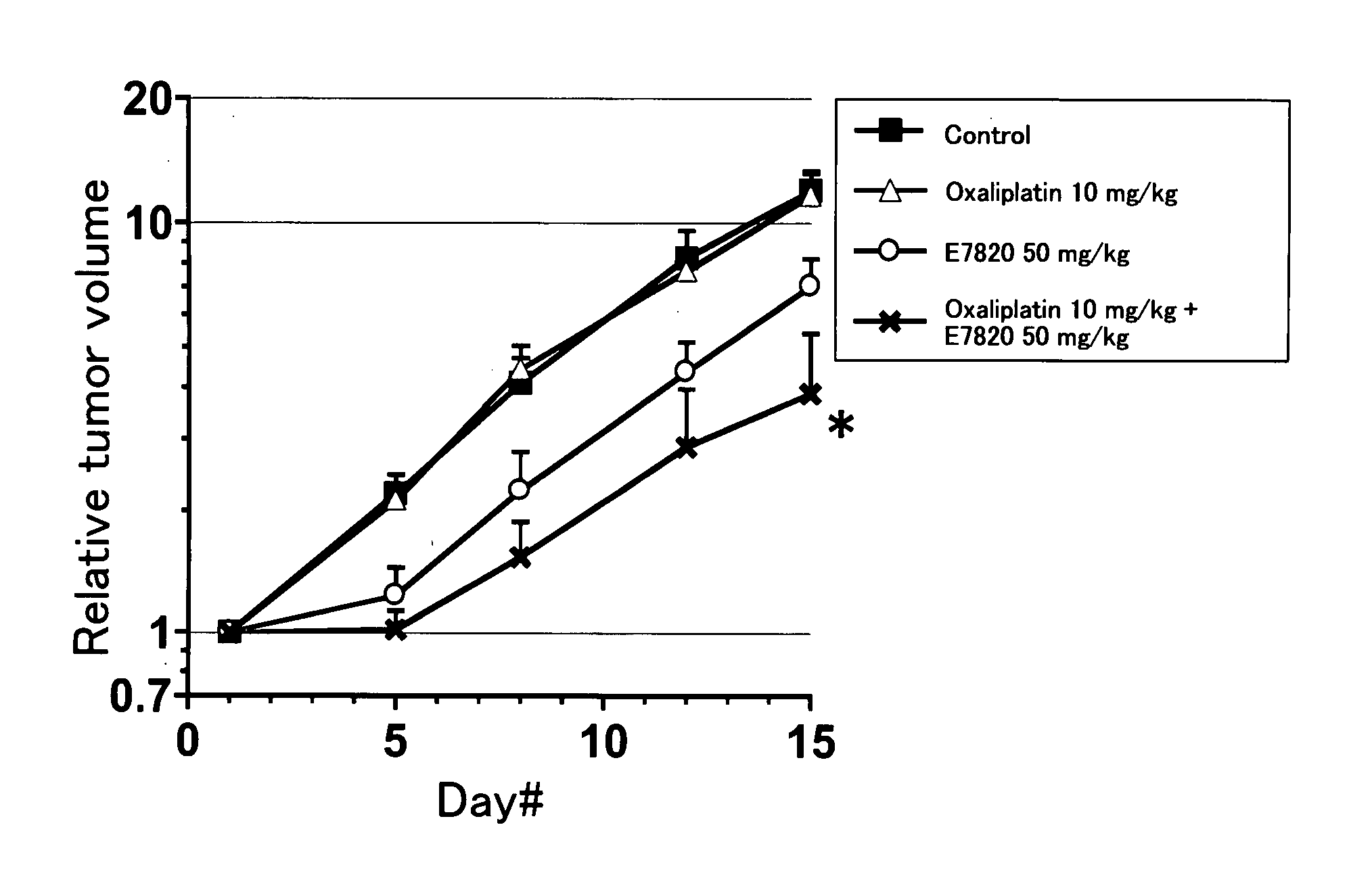
InactiveUS20090047365A1Improve anti-tumor activityGood activity angiogenesis inhibiting activityHeavy metal active ingredientsBiocidePlatinum complexTopoisomerase-I Inhibitor
The present invention relates to a pharmaceutical composition, a kit, a method of treating cancer and / or a method of inhibiting angiogenesis comprising a sulfonamide compound in combination with a platinum complex, a DNA-topoisomerase I inhibitor, an antimetabolite, a microtubule inhibitor or an antibiotic.
Owner:EISIA R&D MANAGEMENT CO LTD
Use of antitumor indolopyrrolocarbazole derivative and other anticancer agent in combination
InactiveUS20050171036A1Improve anti-tumor effectBiocideHeavy metal active ingredientsAnticarcinogenHalogen
This invention relates to a combined preparation for simultaneous, separate, or sequential administration in the treatment of cancer, comprising two separate preparations: a preparation comprising, in combination with a pharmaceutically acceptable carrier or diluent, at least one compound of general formula I: wherein R1 and R2 each independently represent a hydrogen atom, lower alkyl, or the like, and G represents pentosyl or the like, X1 and X2 each independently represent a hydrogen atom, a halogen atom, or the like or a pharmaceutically acceptable salt thereof; and a preparation, in combination with a pharmaceutically acceptable carrier or diluent, such as antitumor alkylating agents, antitumor antimetabolites, antitumor antibiotics, or plant-derived antitumor agents (a preparation comprising at least one compound of general formula I or a pharmaceutically acceptable salt thereof may be combined with two or more other antitumor agents), and a method for cancer treatment comprising the administration of these preparations in combination.
Owner:BANYU PHARMA CO LTD
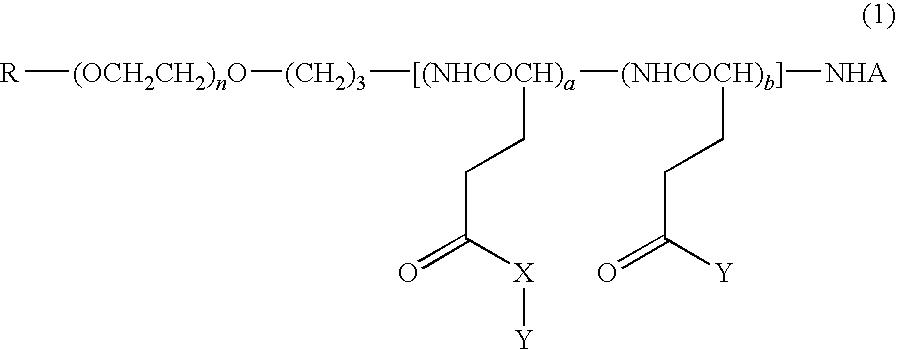
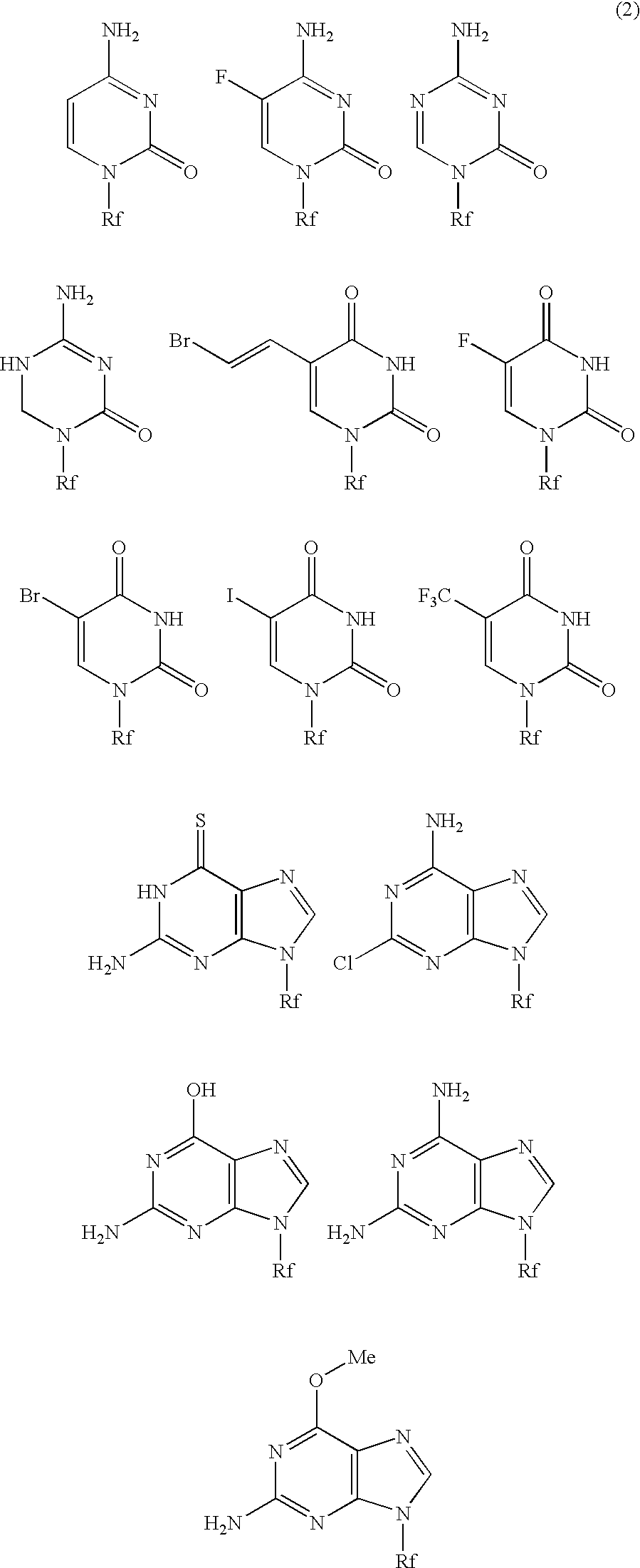
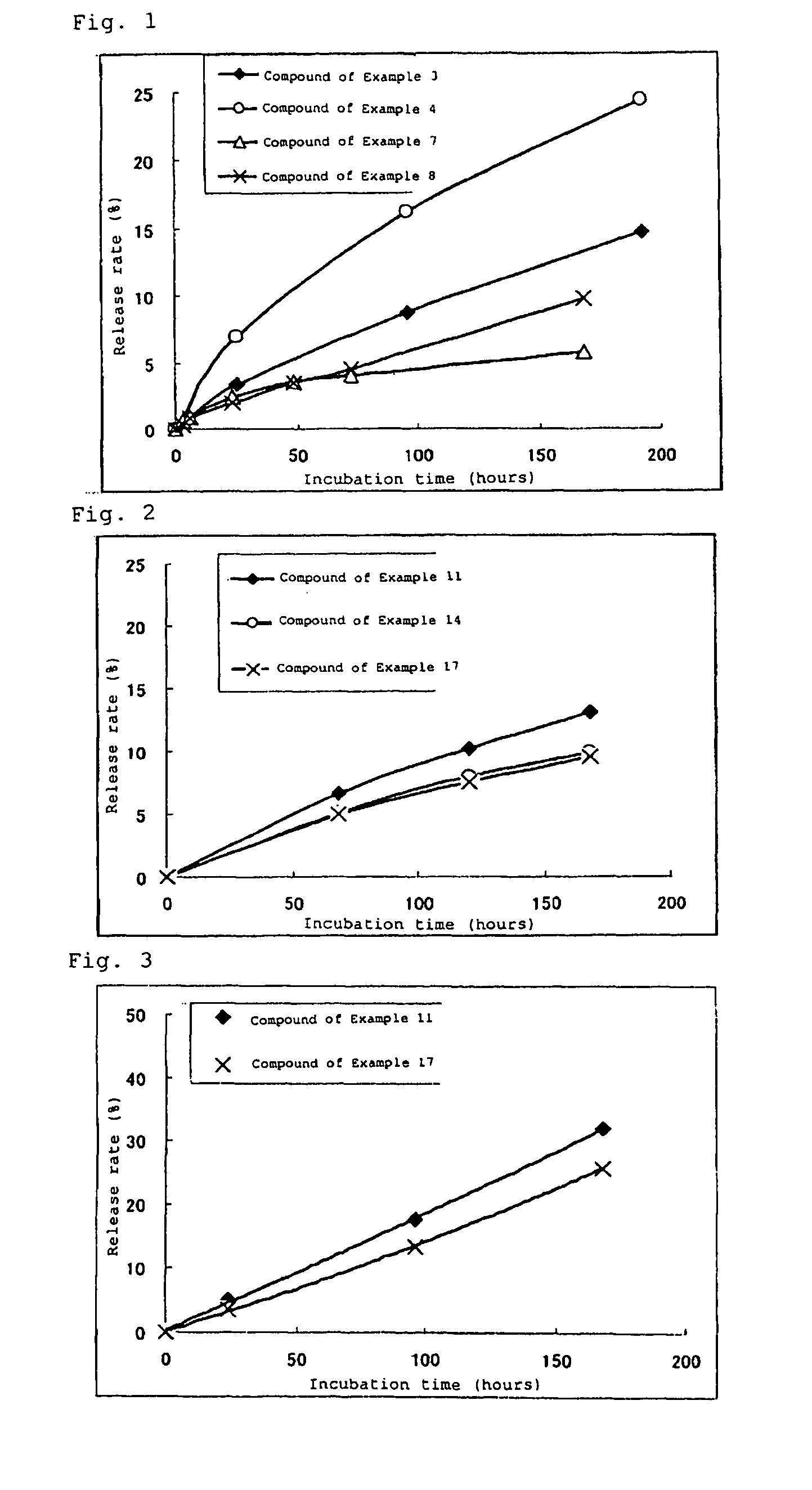
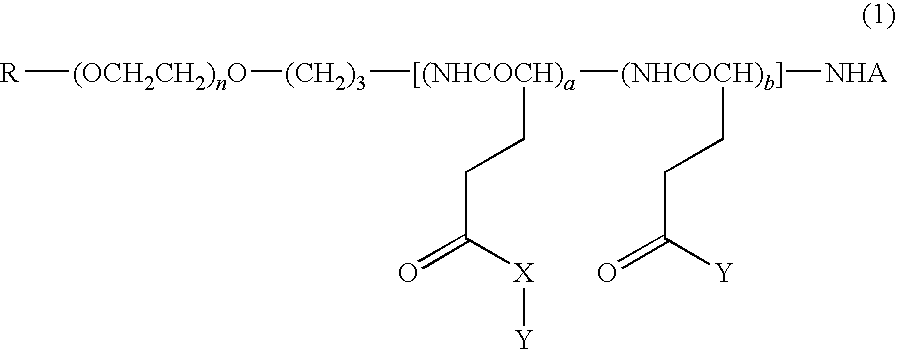
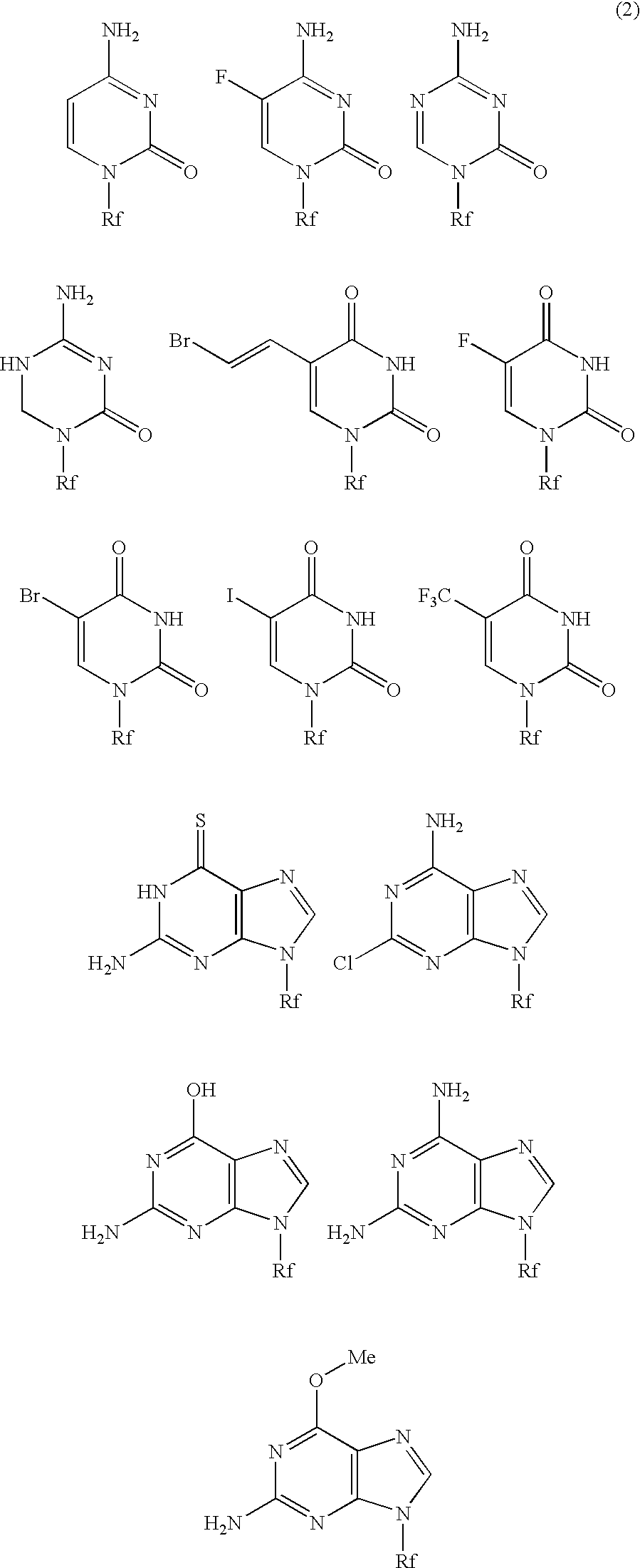
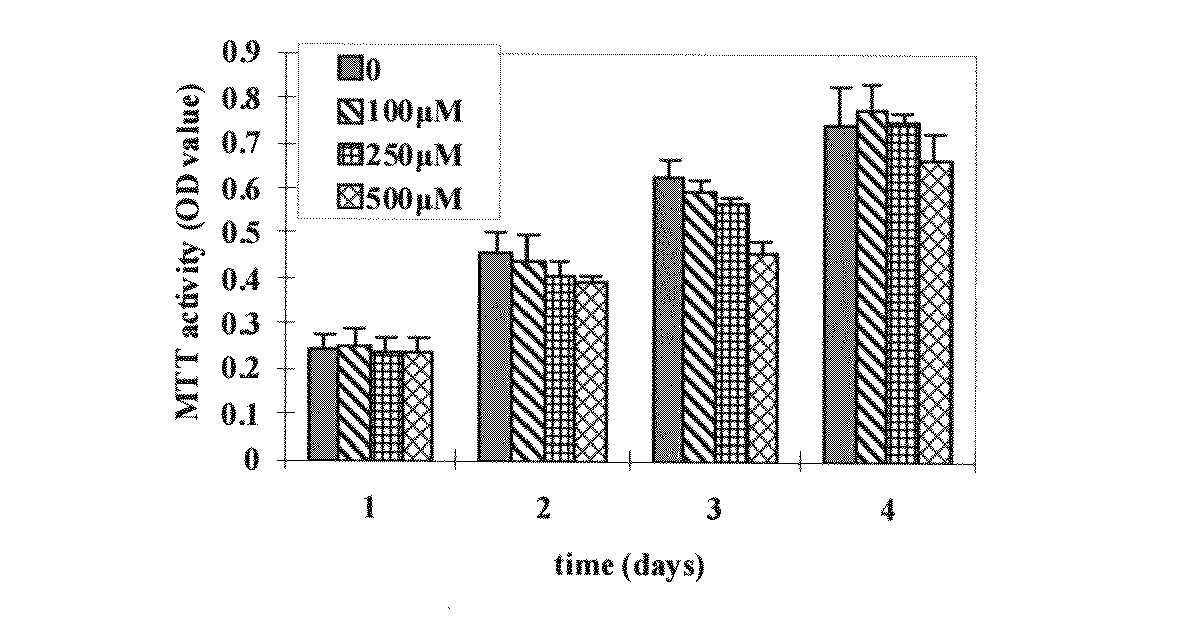
High molecular weight derivative of nucleic acid antimetabolite
ActiveUS20100029849A1Good effectFew adverse effectOrganic active ingredientsSugar derivativesSide chainPolyethylene glycol
[Problems] A derivative of a nucleic acid antimetabolite is demanded which can show a higher therapeutic effect at a lower dose.[Means for Solving Problems] Disclosed is a high molecular weight derivative a nucleic acid antimetabolite, which is characterized by comprising a high molecular weight compound comprising a polyethylene glycol moiety and a polymer moiety having a carboxyl group in a side chain and a nucleoside derivative which can act as a nucleic acid antimetabolite, wherein the nucleoside derivative is bound to the carboxyl group in the side chain of the high molecular weight compound via a highly hydrophobic linker.
Owner:NIPPON KAYAKU CO LTD
High molecular weight derivative of nucleic acid antimetabolite
ActiveUS8188222B2Good effectGood treatment effectOrganic active ingredientsSugar derivativesSide chainPolyethylene glycol
Owner:NIPPON KAYAKU CO LTD
Application of 2-bromide-isovanillin for the manufacture of a medicament for anti-cancer or/and radiation/chemotherapy sensitization
ActiveUS20080221221A1Induce apoptosisHigh sensitivityBiocideAldehyde active ingredientsSide effectTherapeutic effect
Use of 2-bromo-isovanillin in the preparation of an anticancer medicament and / or radio- and chemotherapy sensitizing medicament is disclosed. The medicament for the treatment of cancers and / or for radio- and chemotherapy sensitization comprising 2-bromo-isovanillin as active ingredient provided herein has the following features: (1) low toxicity, without evident adverse effects; (2) significant therapeutic effect, with remarkable proliferation inhibiting and pro-apoptotic effects in tumor cells; (3) a broad-spectrum anticancer activity; (4) suitable to be used in combination with antimetabolites, to enhance the effects and meanwhile lower the toxicity, and also to reduce multi-drug resistance; (5) convenient and safe administration, the main route being oral.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Therapeutic agents for pancreatic cancer
InactiveUS20130149302A1Good treatment effectReduce lesionsOrganic active ingredientsDigestive systemTherapeutic effectOncology
We achieved the present invention on the basis of the finding that an excellent therapeutic effect against pancreatic cancer can be obtained by administering an XL-6 inhibitor and an antimetabolite to pancreatic cancer patients. We also found that metastatic lesions from human pancreatic cancer can be reduced and ascites can be eliminated.
Owner:NAT CANCER CENT +1
Therapeutic agents for pancreatic cancer
InactiveUS20160022812A1Good treatment effectReduce lesionsOrganic active ingredientsDigestive systemTherapeutic effectOncology
We achieved the present invention on the basis of the finding that an excellent therapeutic effect against pancreatic cancer can be obtained by administering an IL-6 inhibitor and an antimetabolite to pancreatic cancer patients. We also found that metastatic lesions from human pancreatic cancer can be reduced and ascites can be eliminated.
Owner:NAT CANCER CENT +1
Combination cancer therapy with an hsp90 inhibitor and an antimetabolite
InactiveUS20140296176A1Increasing side effect profileSurprising biological activityBiocideCarbohydrate active ingredientsCytarabineHsp Inhibitor
The invention provides a method of treating a subject with cancer, particularly leukemia, lymphoma, solid cancer such as colorectal cancer, gastric cancer, bladder cancer, non-small cell lung cancer, and breast cancer, comprising administering to the subject a compound of formulae (I) 40 or (Ia) in combination with an antimetabolite such as methotrexate, pemetrexed, cytarabine or nelarabine, or 5-fluorouracil, or capecitabine or their derivatives.
Owner:SYNTA PHARMA CORP
Agents for intravitreal administration to treat or prevent disorders of the eye
Methods and preparatons for treating disorders of the eye and / or causing posterior vitreous disconnection or disinsertion. Preparations containing a) urea, b) urea derivatives (e.g., hydroxyurea, thiourea), c) a non-steroidal anti-inflamatory agents, d) antmetabolites, e) urea, urea derivatives, non-enzymatic proteins, nucleosides, nucleotides and their derivatives (e.g., adenine, adenosine, cytosine, cytadine, guanine, guanitadine, guanidinium, thymidine, thimitadine, uradine, uracil, cystine), uric acid, calcium acetal salicylate, ammonium sulfate or other compound capable of causing non-enzymatic dissolution of the hyaloid membrane or e) any of the possible combinations thereof, are administered to the eye in therapeutically effective amounts.
Owner:KATO PHARMA
9h-pyrimido[4,5-b]indoles, 9h-pyrido[4',3':4,5]pyrrolo[2,3-d]pyridines, and 9h 1,3,6,9 tetraaza-fluorenes as chk1 kinase function inhibitors
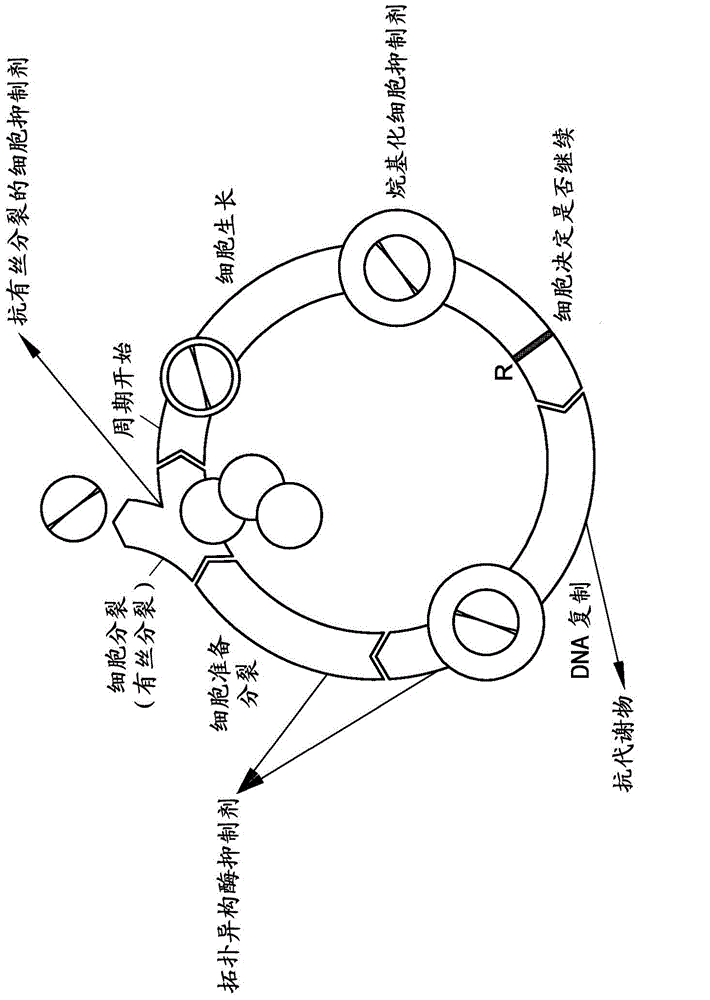
The present invention pertains generally to the field of therapeutic compounds, and more specifically to certain tricyclic compounds (referred to herein as TC compounds), and especially certain 9H-pyrimido[4,5-b]indole, 9H-pyrido[4′,3′:4,5]pyrrolo[2,3-d]pyridine, and 9H-1,3,6,9-tetraaza-fluorene compounds, which, inter alia, inhibit Checkpoint Kinase 1 (CHK1) kinase function. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit CHK1 kinase function, and in the treatment of diseases and conditions that are mediated by CHK1, that are ameliorated by the inhibition of CHK1 kinase function, etc., including proliferative conditions such as cancer, etc., optionally in combination with another agent, for example, (a) a DNA topoisomerase I or II inhibitor; (b) a DNA damaging agent; (c) an antimetabolite or TS inhibitor; (d) a microtubule targeted agent; and (e) ionising radiation.
Owner:CANCER RES TECH LTD
Therapeutic Oxy-Phenyl-Aryl Compounds and Their Use
The present invention pertains generally to the field of therapeutic compounds, and more specifically to certain oxy phenyl aryl compounds (referred to herein as OPA compounds), as described herein, which, inter alia, inhibit Checkpoint Kinase 2 (CHK2) kinase function. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit CHK2 kinase function, and in the treatment of diseases and conditions that are mediated by CHK2, that are ameliorated by the inhibition of CHK2 kinase function, etc., including proliferative conditions such as cancer, etc., optionally in combination with another agent, for example, (a) a DNA topoisomerase I or II inhibitor; (b) a DNA damaging agent; (c) an antimetabolite or TS inhibitor; (d) a microtubule targeted agent; and (e) ionising radiation.
Owner:CANCER RES TECH LTD
Compositions and methods for treating pterygium
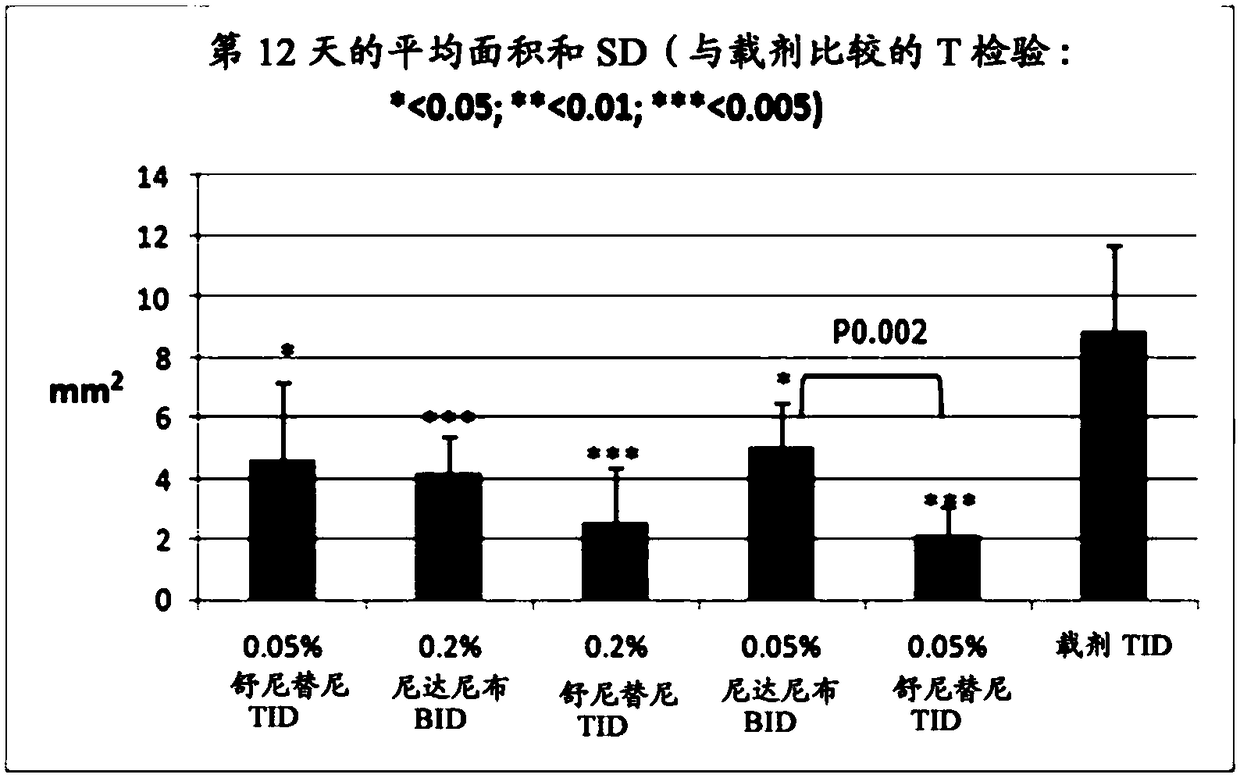
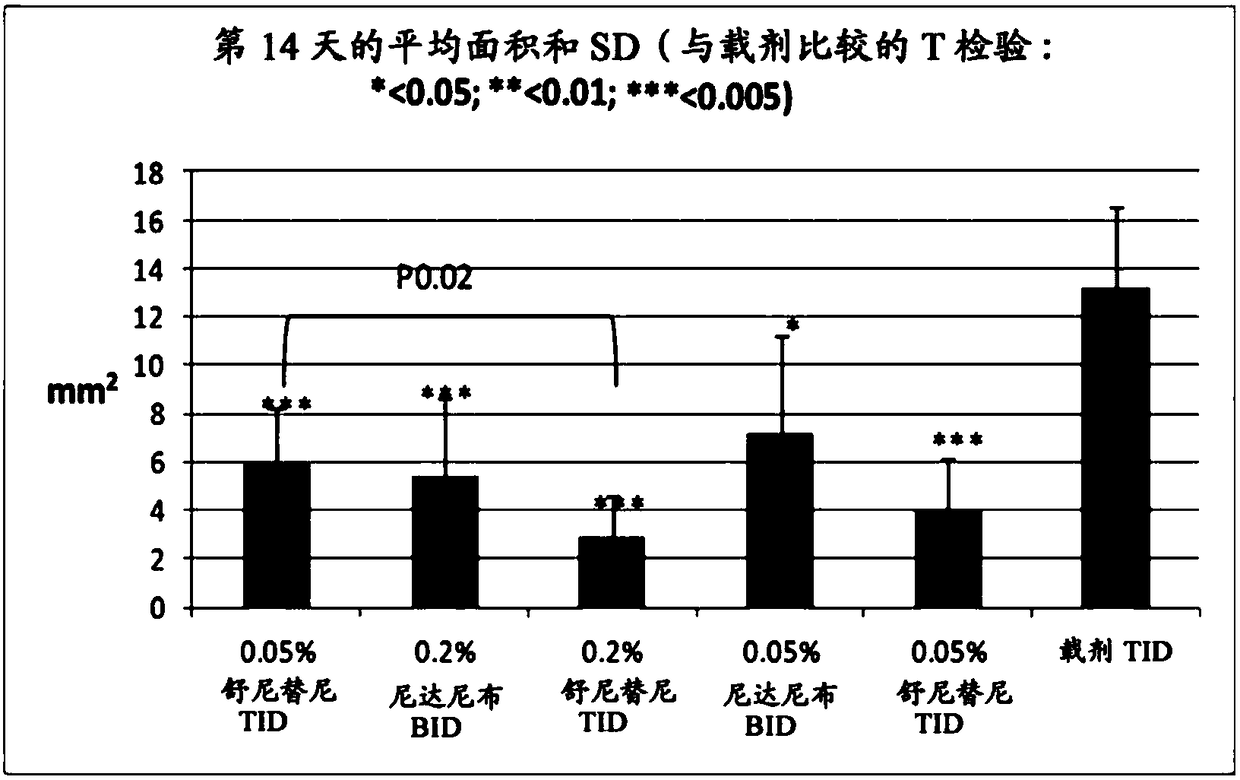
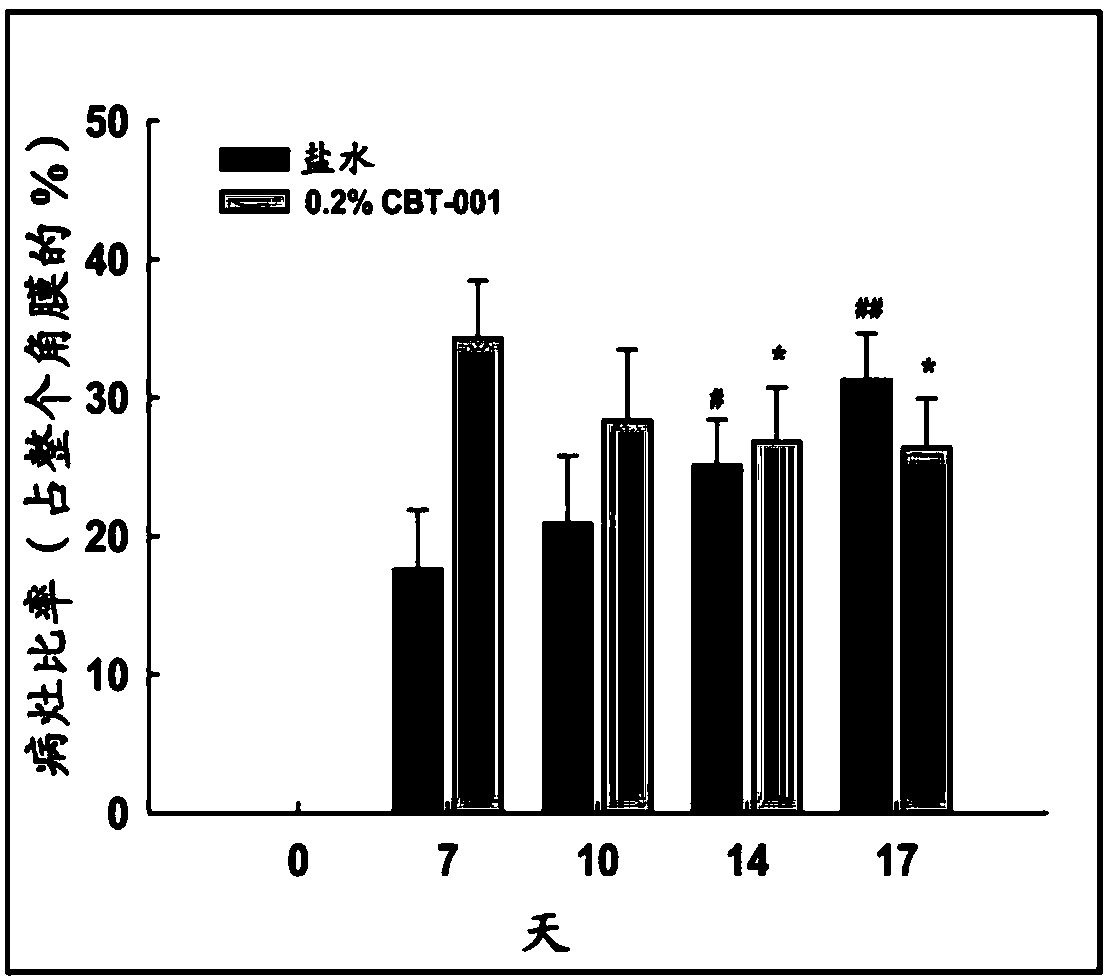
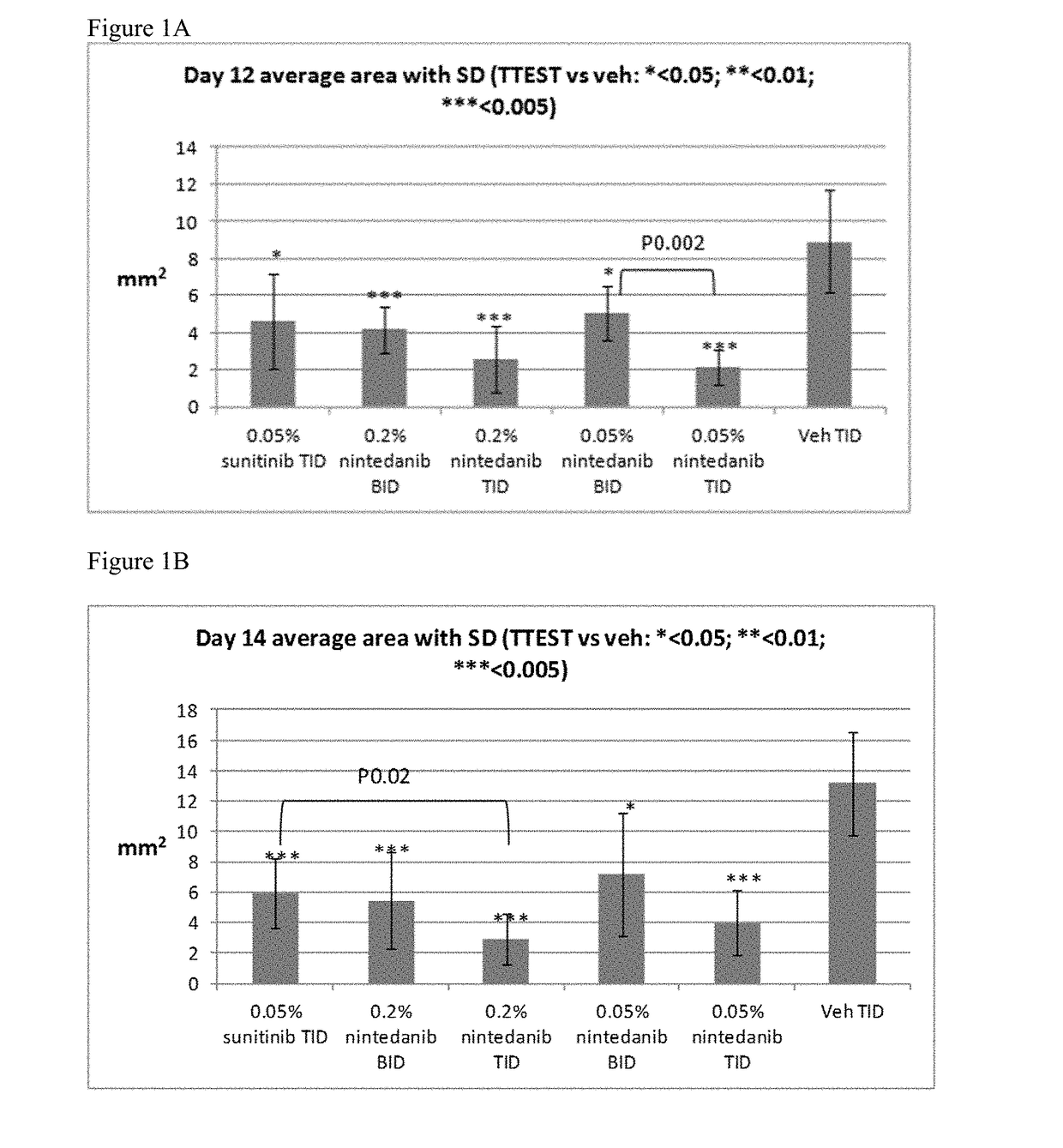
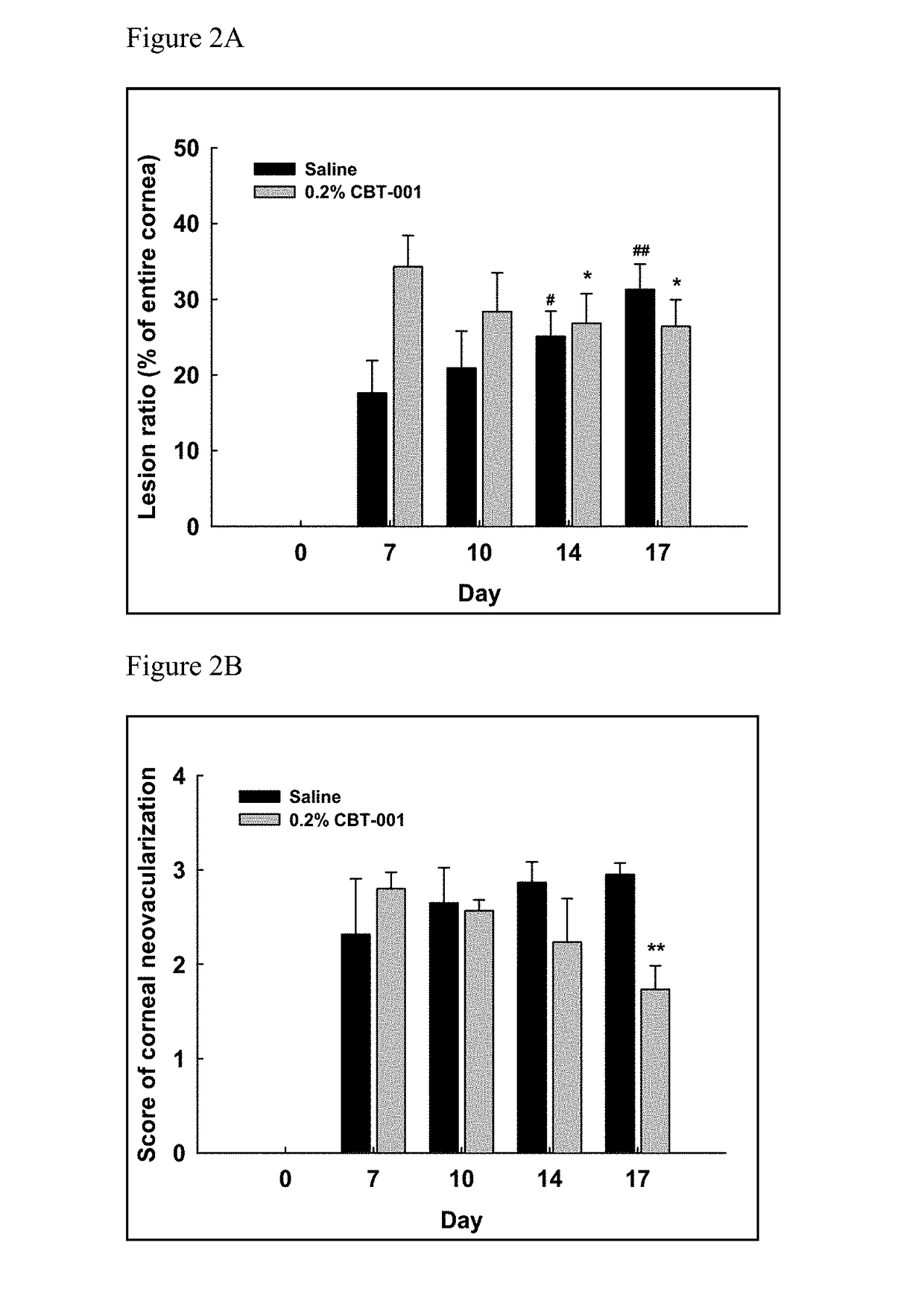
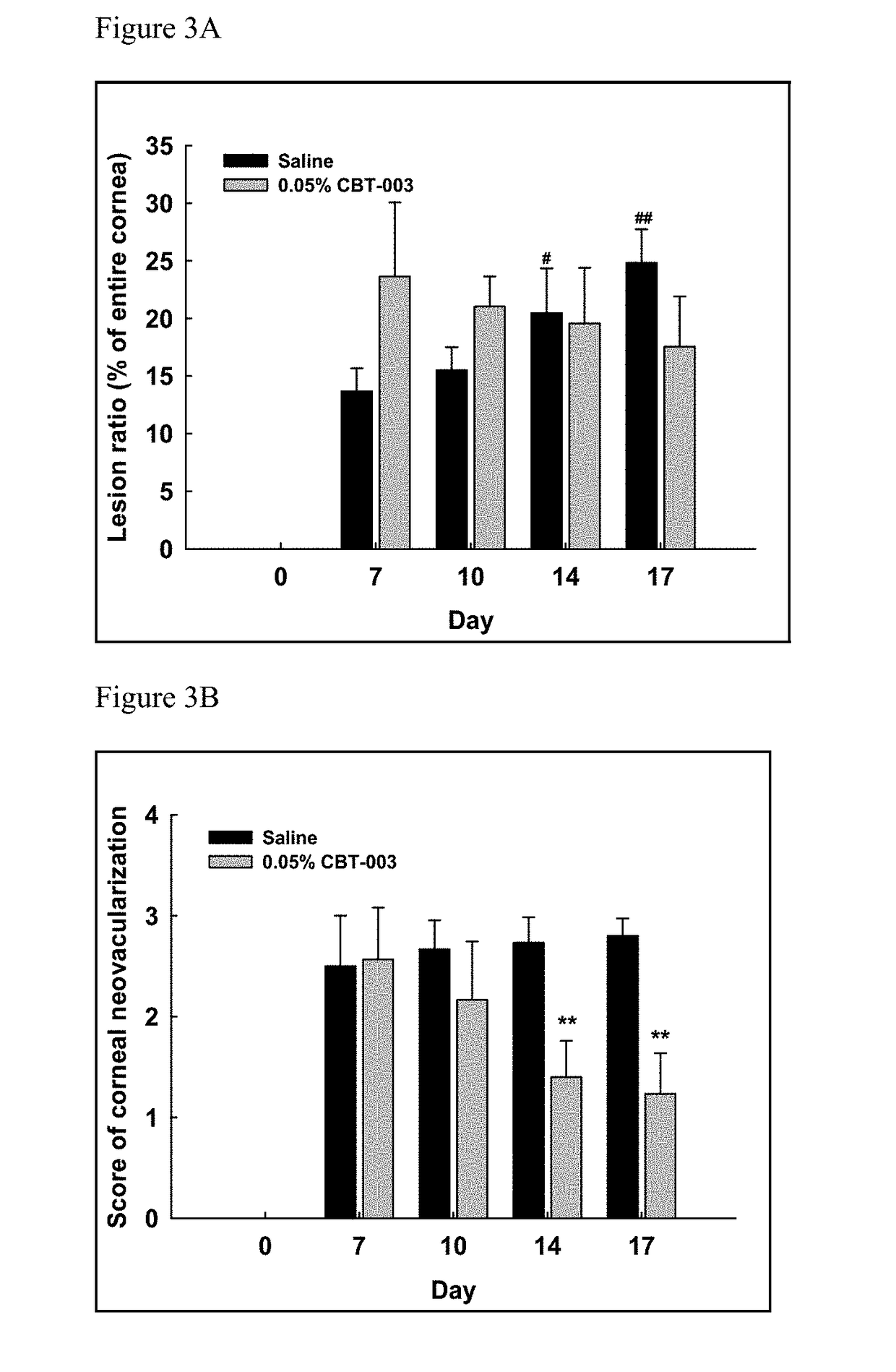
ActiveUS9980901B2Stabilization of pterygium sizeStabilizing pterygiumOrganic active ingredientsSenses disorderPurine AntimetaboliteSurgery
Compositions and methods for inducing pterygium regression from visual axis / central cornea, stabilizing pterygium, treating hyperemia and symptoms in pterygium patients, and treating pterygium recurrence following pterygiectomy are disclosed. The methods include administration of a multikinase inhibitor, an antimetabolite or a combination thereof to patients in need thereof.
Owner:CLOUDBREAK THERAPEUTICS LLC
Bicyclylaryl-aryl-amine compounds and their use
The present invention pertains generally to the field of therapeutic compounds, and more specifically to certain bicyclylaryl-aryl-amines compounds of the following formula (referred to herein as BCAA compounds), which, inter alia, inhibit Checkpoint Kinase 1 (CHK1) kinase function. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit CHK1 kinase function, and in the treatment of diseases and conditions that are mediated by CHK1, that are ameliorated by the inhibition of CHK1 kinase function, etc., including proliferative conditions such as cancer, etc., optionally in combination with another agent, for example, (a) a DNA topoisomerase I or II inhibitor; (b) a DNA damaging agent; (c) an antimetabolite or TS inhibitor; (d) a microtubule targeted agent; and (e) ionising radiation:
Owner:CANCER RES TECH LTD
Slow released anticancer injection with both antimetabolite and its synergist
The slow released anticancer injection consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent. The effective anticancer component is antimetabolite Zalcitabine, Emtricitabine, etc and / or antimetabolite synergist selected from hormone anticarcinogen and / or platinum compound. The slow releasing supplementary material is selected from difatty acid-sebacic acid copolymer, poly (erucic acid dipolymer-sebacic acid), poly(fumaric acid-sebacic acid), etc or their composition. The suspending agent is carboxymethyl cellulose, etc. and has viscosity of 80-3000 cp at 20-30 deg.c. The slow released microsphere may be also prepared into slow released implanting agent for use alone or together with chemotherapeutic medicine, radiotherapeutic medicine, etc.
Owner:DASEN BIOLOGICAL PHARMA CO LTD
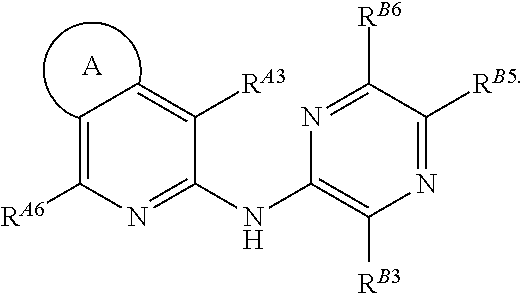
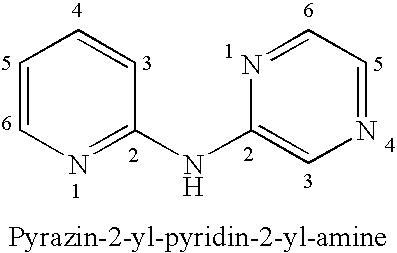
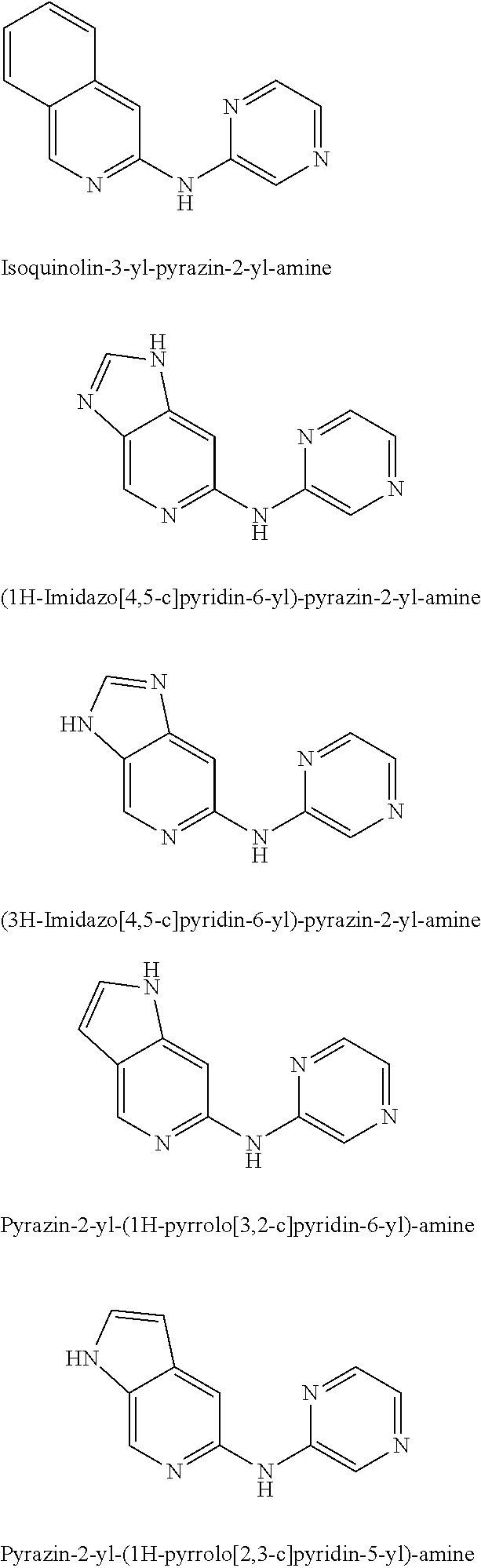
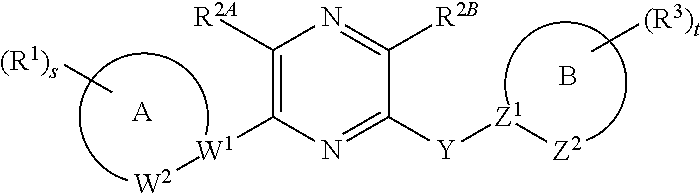
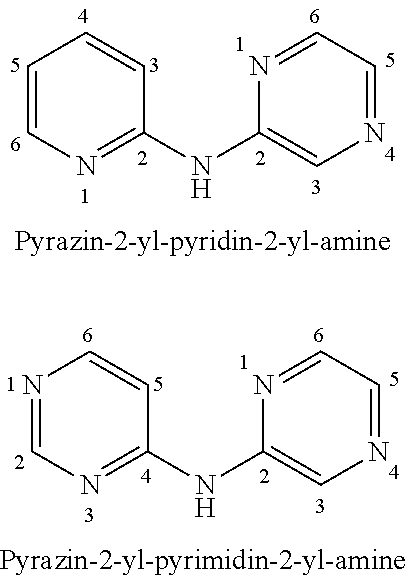
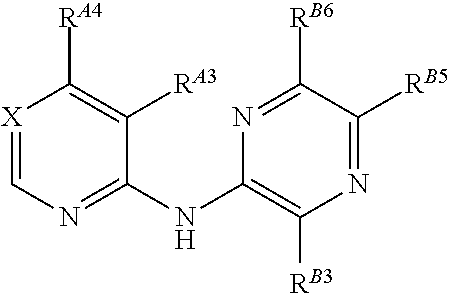
Pyrazin-2-yl-pyridin-2-yl-amine and pyrazin-2-yl-pyrimidin-4-yl-amine Compounds and Their Use
The present invention pertains generally to the field of therapeutic compounds, and more specifically to certain biarylamine compounds (referred to herein as BAA compounds), and especially certain pyrazin-2-yl-pyridin-2-yl-amine and pyrazine-2-yl-pyrimidin-4-yl-amine compounds, which, inter alia, inhibit Checkpoint Kinase 1 (CHK1) kinase function. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit CHK1 kinase function, and in the treatment of diseases and conditions that are mediated by CHK1, that are ameliorated by the inhibition of CHK1 kinase function, etc., including proliferative conditions such as cancer, etc., optionally in combination with another agent, for example, (a) a DNA topoisomerase I or II inhibitor; (b) a DNA damaging agent; (c) an antimetabolite or TS inhibitor; (d) a microtubule targeted agent; and (e) ionising radiation.
Owner:CANCER RES TECH LTD
5-[[4-[[morpholin-2-yl]methylamino]-5-(trifluoromethyl)-2-pyridyl]amino]pyrazine-2-carbonitrile and therapeutic uses thereof
ActiveUS20150126471A1Increased endogenous replicative stressIncreased endogenous activationBiocideOrganic chemistryDiseasePyrazine
The present invention pertains generally to the field of therapeutic compounds. More specifically the present invention pertains to 5-[[4-[[morpholin-2-yl]methylamino]-5-(trifluoromethyl)-2-pyridyl]amino]pyrazine-2-carbonitrile compounds (referred to herein as “TFM compounds”) which, inter alia, inhibit Checkpoint Kinase 1 (CHK1) kinase function. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit CHK1 kinase function, and in the treatment of diseases and conditions that are mediated by CHK1, that are ameliorated by the inhibition of CHK1 kinase function, etc., including proliferative conditions such as cancer, etc., optionally in combination with another agent, for example, (a) a DNA topoisomerase I or II inhibitor; (b) a DNA damaging agent; (c) an antimetabolite or a thymidylate synthase (TS) inhibitor; (d) a microtubule targeted agent; (e) ionising radiation; (f) an inhibitor of a mitosis regulator or a mitotic checkpoint regulator; (g) an inhibitor of a DNA damage signal transducer; or (h) an inhibitor of a DNA damage repair enzyme.
Owner:CANCER RES TECH LTD
9H-pyrimido[4,5-B]indoles, 9H-pyrido[4',3':4,5]pyrrolo[2,3-D]pyridines, and 9H 1,3,6,9 tetraaza-fluorenes as CHK1 kinase function inhibitors
The present invention pertains generally to the field of therapeutic compounds, and more specifically to certain tricyclic compounds (referred to herein as TC compounds), and especially certain 9H-pyrimido[4,5-b]indole, 9H-pyrido[4′,3′:4,5]pyrrolo[2,3-d]pyridine, and 9H-1,3,6,9-tetraaza-fluorene compounds, which, inter alia, inhibit Checkpoint Kinase 1 (CHK1) kinase function. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit CHK1 kinase function, and in the treatment of diseases and conditions that are mediated by CHK1, that are ameliorated by the inhibition of CHK1 kinase function, etc., including proliferative conditions such as cancer, etc., optionally in combination with another agent, for example, (a) a DNA topoisomerase I or II inhibitor; (b) a DNA damaging agent; (c) an antimetabolite or TS inhibitor; (d) a microtubule targeted agent; and (e) ionising radiation.
Owner:CANCER RES TECH LTD
Therapeutic agents for pancreatic cancer
ActiveUS20180236068A1Good treatment effectReduce lesionsOrganic active ingredientsDigestive systemTherapeutic effectOncology
We achieved the present invention on the basis of the finding that an excellent therapeutic effect against pancreatic cancer can be obtained by administering an IL-6 inhibitor and an antimetabolite to pancreatic cancer patients. We also found that metastatic lesions from human pancreatic cancer can be reduced and ascites can be eliminated.
Owner:NAT CANCER CENT +1
Anticancer medicine composition containing antimetabolite
The anticancer medicine composition connecting antimetabolite includes effective anticancer component and medicinal supplementary material, and features the effective anticancer component, which includes antimetabolite and antimetabolite synergist selected from bichlorethamm medicine, taxol anticarcinogen, and tetrazine. The medicinal supplementary material is mainly biocompatible and degradable absorptive polymer. The anticancer medicine composition is mainly slow released implantation preparation or slow released injection. The slow released injection consists of slow released microballoon and solvent. Implanting or injecting the slow released preparation to local tumor part can lower the systematic toxic reaction of the medicine and raise the medicine concentration of local tumor part selectively to raise the treating effect.
Owner:JINAN SHUAIHUA PHARMA TECH
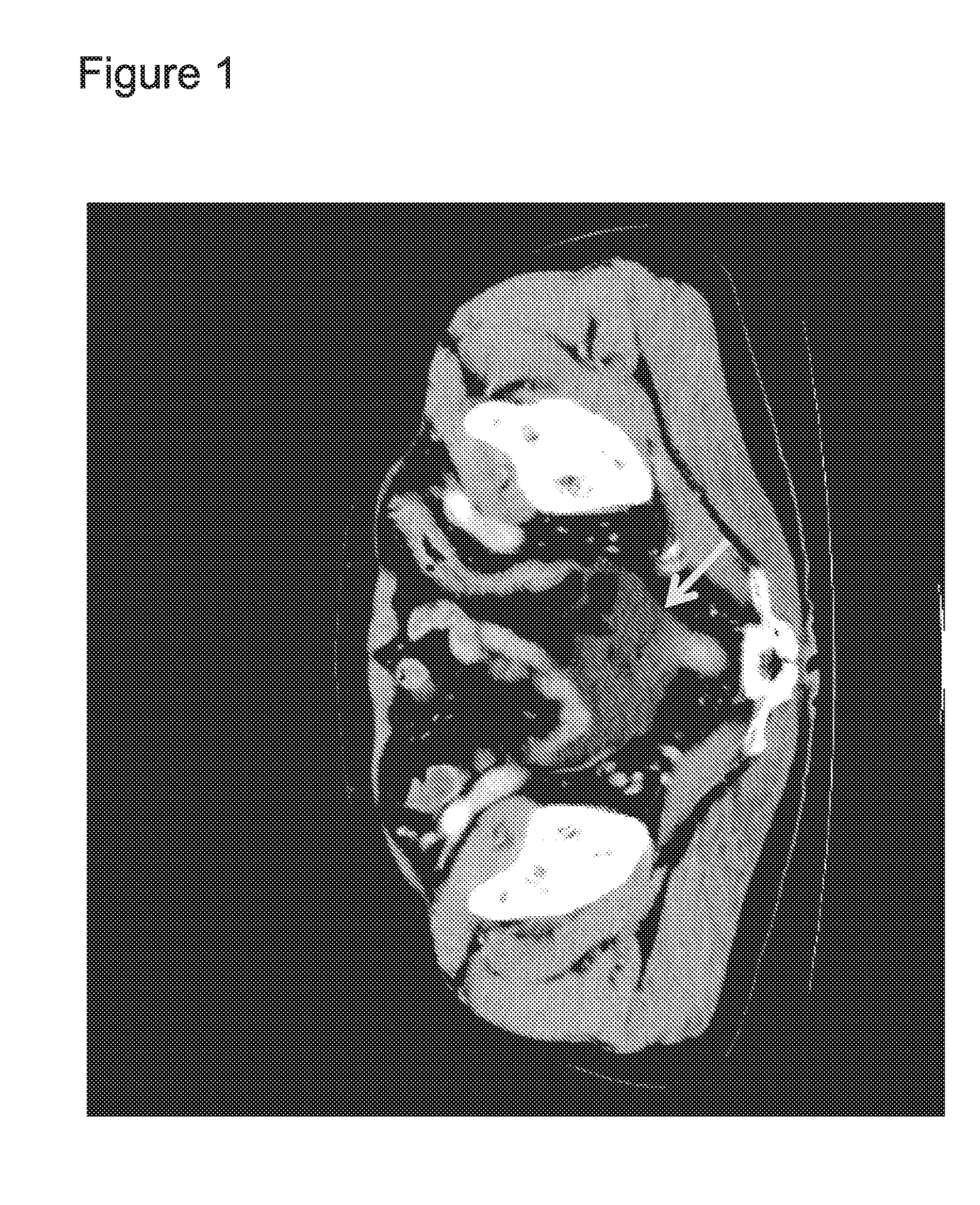
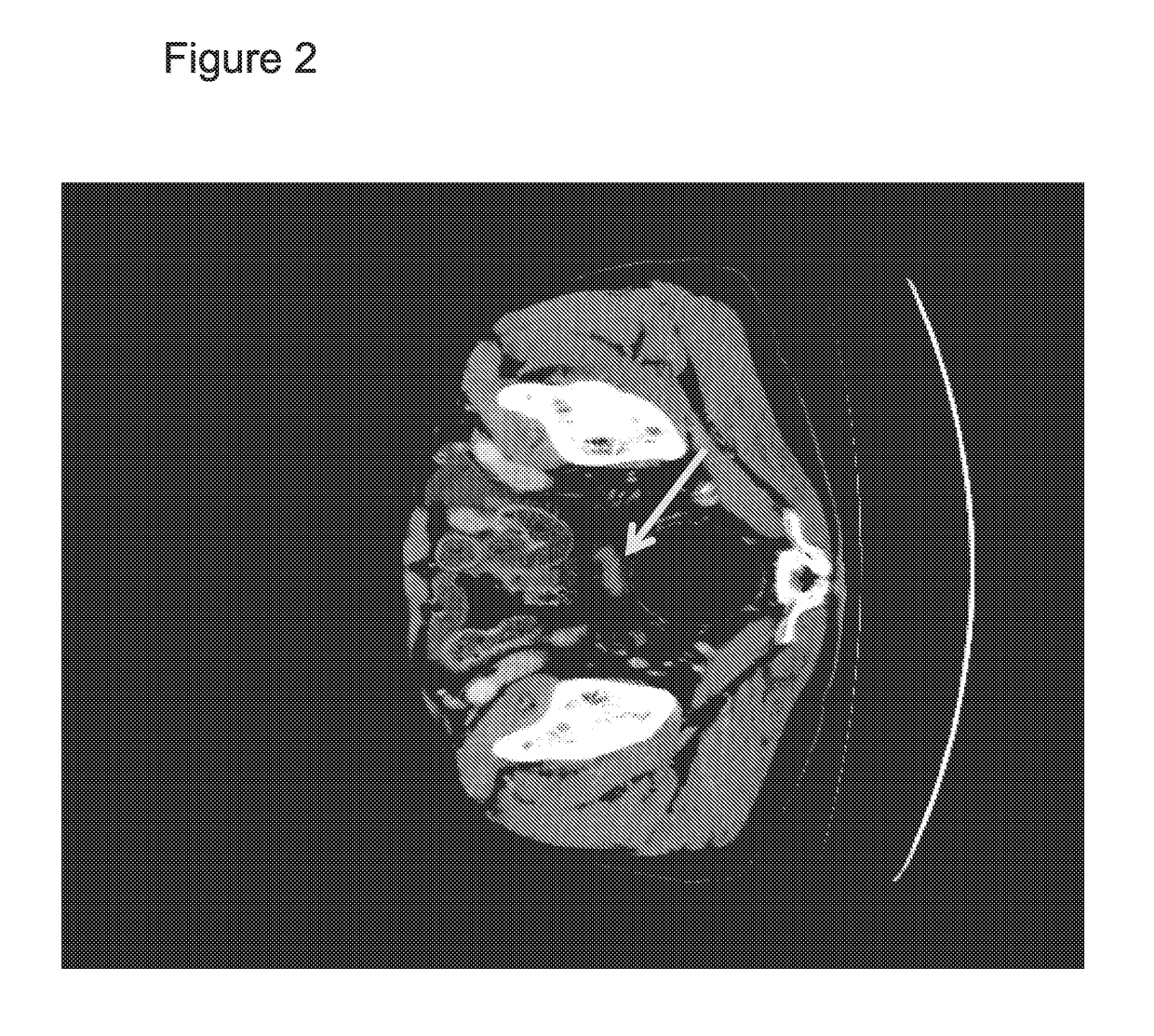
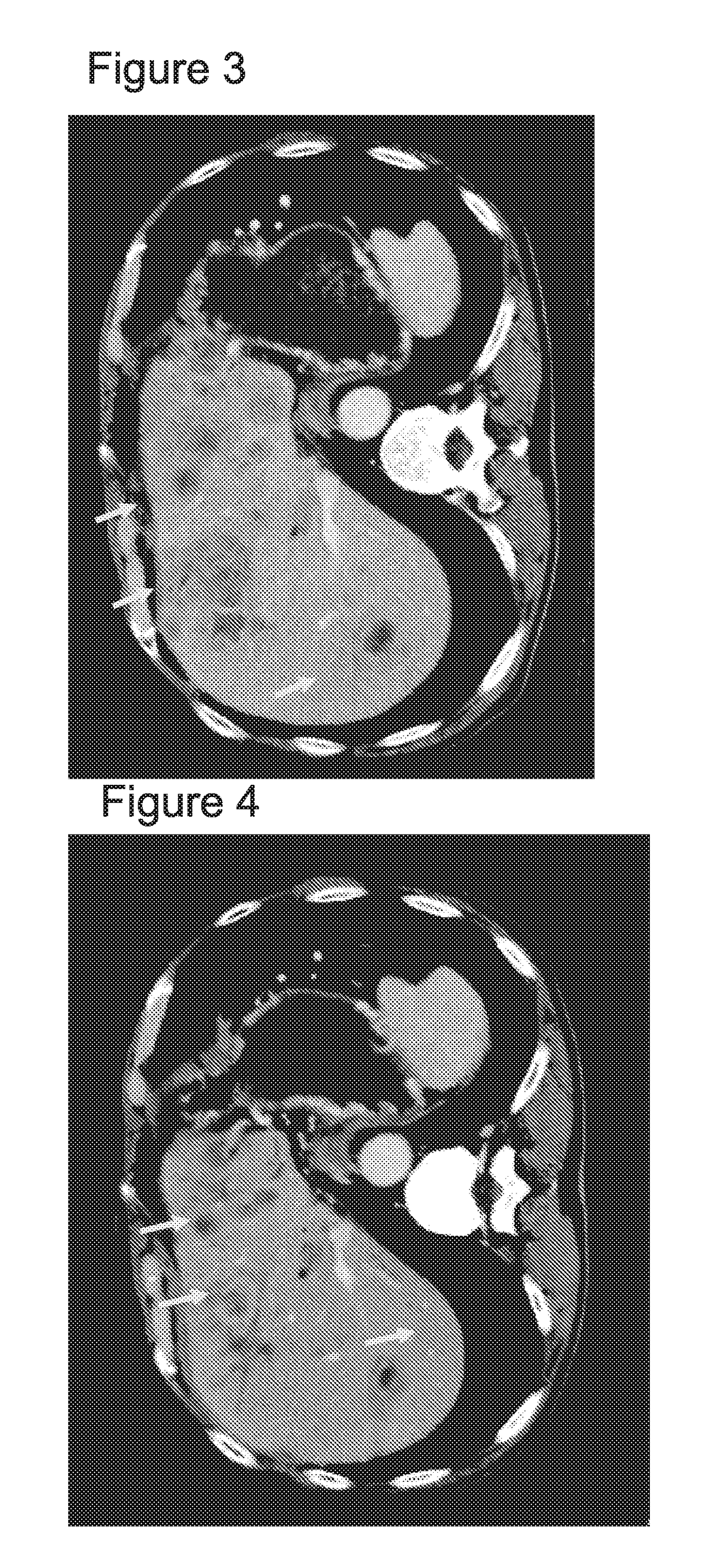
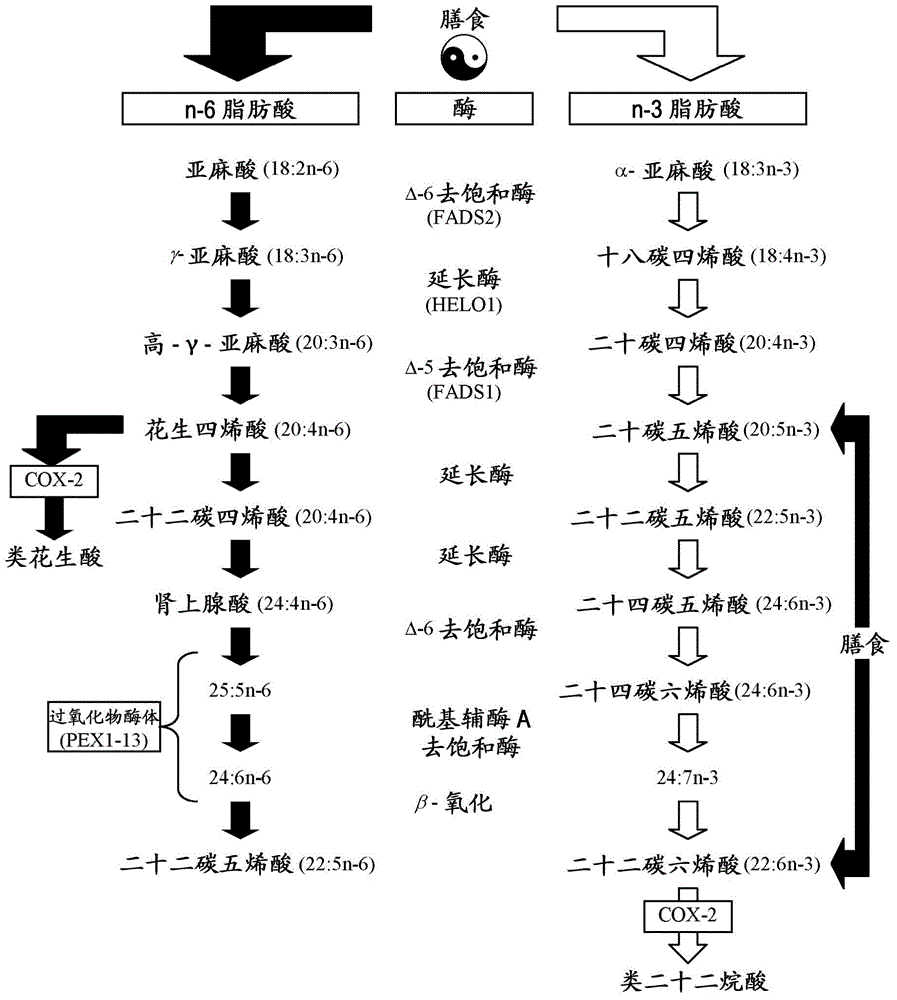
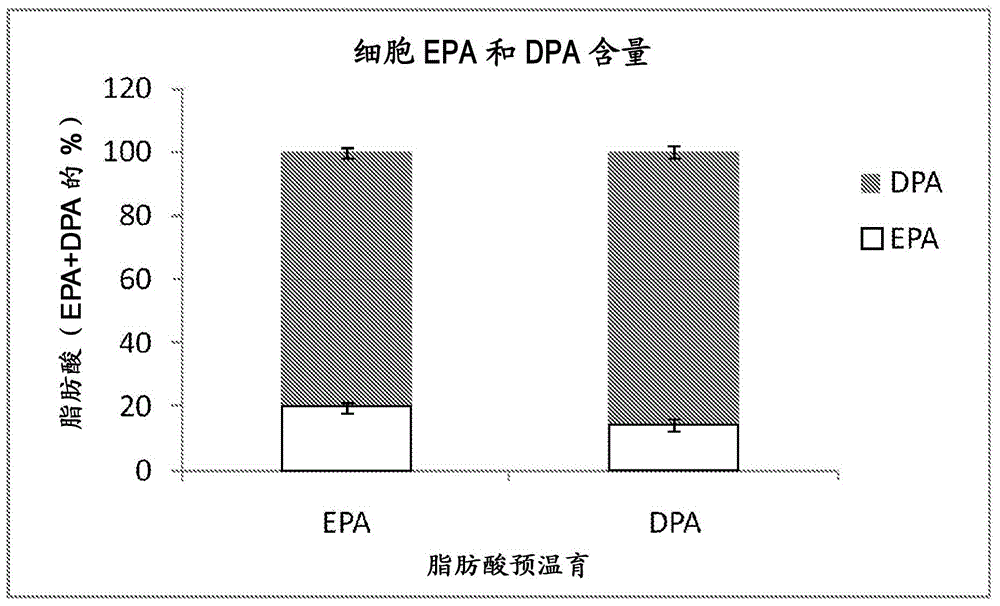
Combination of EPA, DPA and/or DHA with a chemotherapeutic agent
The present invention refers to a combination of an eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and / or docosahexanoic acid (DHA), or a combination of a nutritional composition comprising EPA, DPA, and / or DHA and a protein, a carbohydrate, a fat, an amino acid, a fatty acid, a dietary fibre, a vitamin, a mineral, a trace element, ß-carotenoid, a flavonoid, a nucleotide, L- carnitin, choline, inositol, taurine, creatine, and / or a co-enzyme, wherein EPA is in an amount of 1 to 1000 mg / 100ml, preferably 100 to 700 mg / 100ml, DPA is in an amount of >50 mg / 100ml, preferably 50 to 1000 mg / 100ml, preferably 6 to 800 mg / 100ml, more preferably 80 to 500 mg / 100ml and / or DHA is in an amount of 1 to 500 mg / 100ml, preferably 1 to 300 mg / 100ml and a chemotherapeutic agent selected from the group consisting of an alkylating drug, an antimetabolite, an antimytotic cytostatic, a topoisomerase inhibitor, antitumor antibiotic, and any other cytostatic, and / or a radiotherapy. These combinations are successful for use in treating a neoplastic disease like the method for treating a neoplastic disease comprising EPA, DPA, and / or DHA or a nutritional composition comprising EPA, DPA, and / or DHA and and a protein, a carbohydrate, a fat, an amino acid, a fatty acid, a dietary fibre, a vitamin, a mineral, a trace element, ß-carotenoid, a flavonoid, a nucleotide, L- carnitin, choline, inositol, taurine, creatine, and / or a co-enzyme in combination with a chemotherapeutic agent, and / or radiotherapy.
Owner:NV NUTRICIA
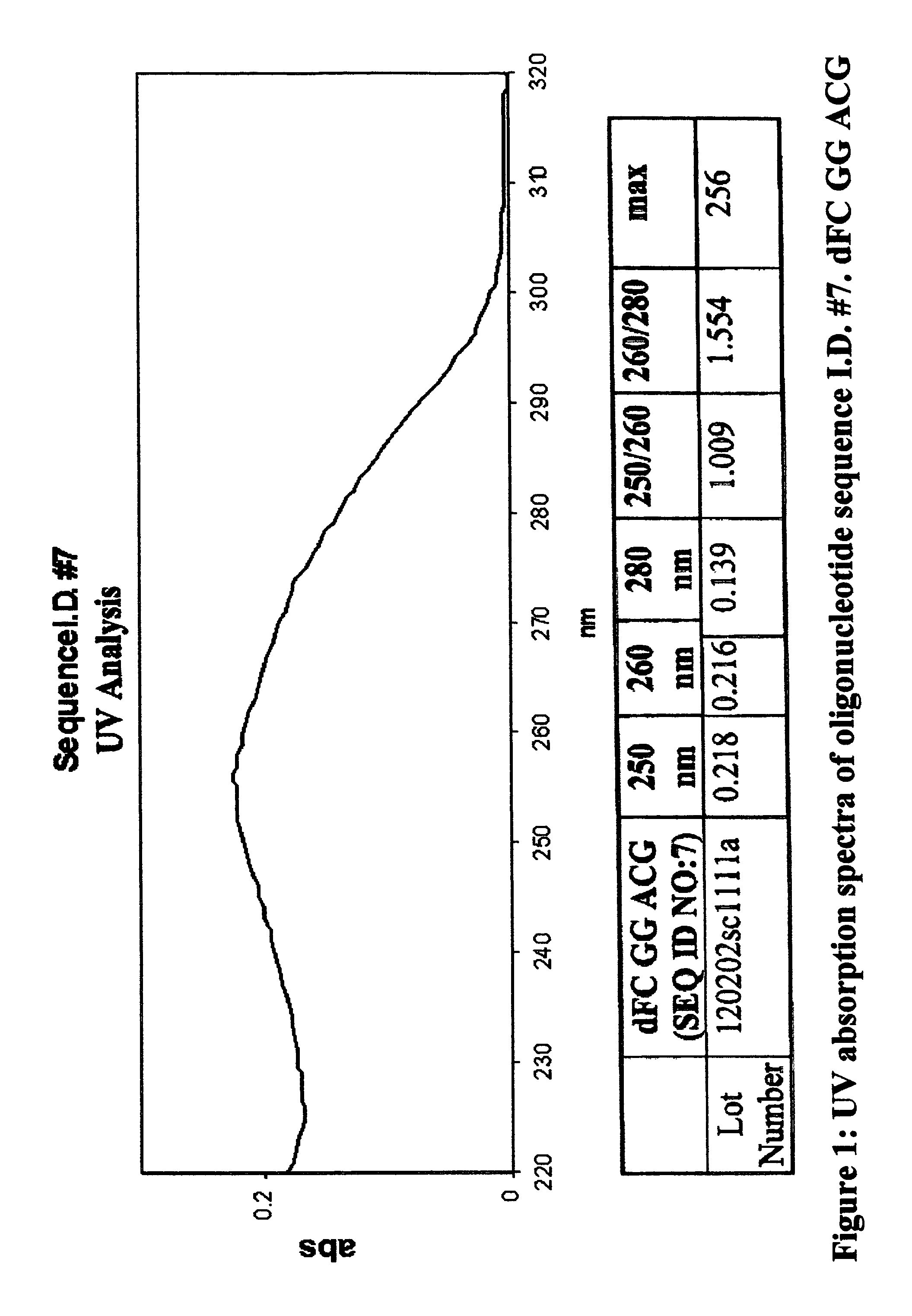
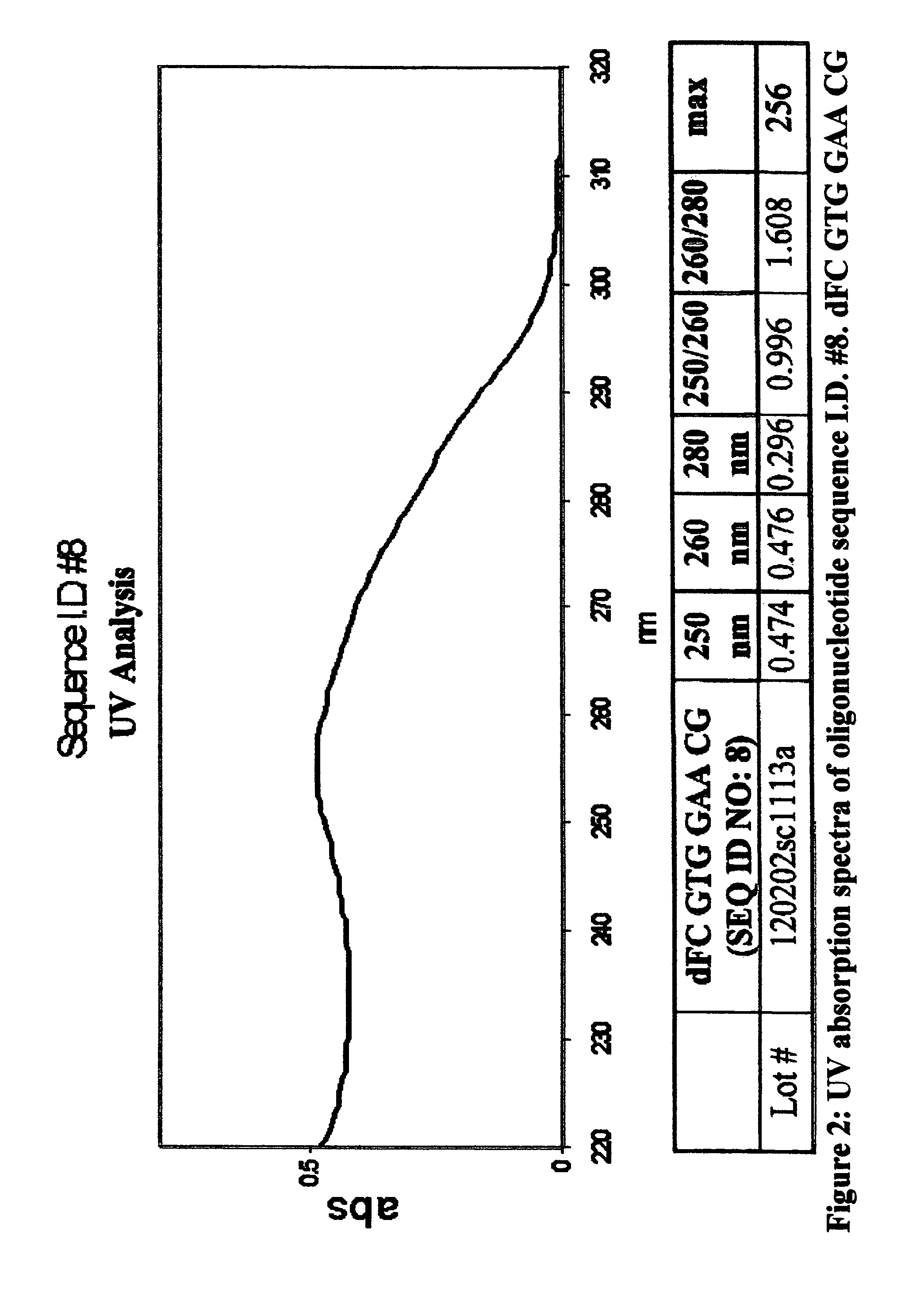
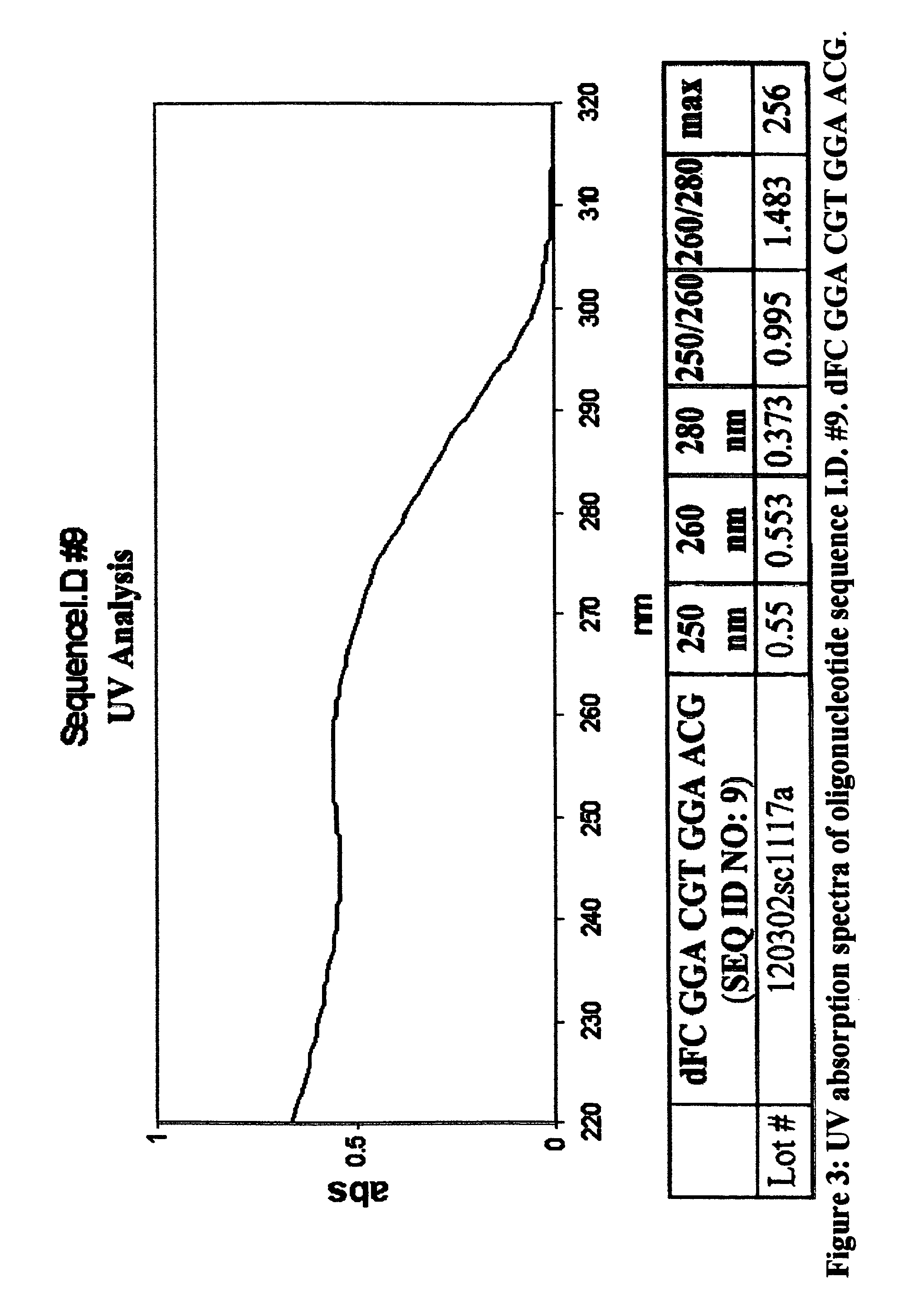
Oligonucleotides and related compounds
ActiveUS7846436B2Prevent necrosis and apoptosisHigh activitySugar derivativesGenetic material ingredientsPyrimidine NucleotidesPurine Antimetabolite
The present invention relates generally to oligonucleotides and more specifically to oligonucleotides which have a sequence including at least two CpG dinucleotides and a prodrug of an antimetabolite. The prodrug can be part of a CpG dinucleotide or may be attached elsewhere on the oligonucleotide.
Owner:CHEMGENES CORP
Compositions and methods for treating pterygium
Compositions and methods for inducing pterygium regression from visual axis / central cornea, stabilizing pterygium, treating hyperemia and symptoms in pterygium patients, and treating pterygium recurrence following pterygiectomy are disclosed. The methods include administration of a multikinase inhibitor, an antimetabolite or a combination thereof to patients in need thereof.
Owner:BOYUN BIOMEDICAL TECH GUANGZHOU CO LTD
Method for decreasing inflammation and stress in a mammal using glucose antimetaboltes, avocado or avocado extracts
InactiveCN101711158AReduce inflammationReduced responseNervous disorderAntipyreticGlucose polymersD-Glucose
The present invention is directed to a method for decreasing inflammation and stress in a mammal comprising; administration to a mammal a composition comprising a glucose antimetabolite; and wherein said composition comprises amounts of the glucose anti-metabolite sufficient to lower a level of a C-reactive protein in the blood of the mammal subsequent to administration of the glucose anti-metabolite.
Owner:IAMS
Agents for intravitreal administration to treat or prevent disorders of the eye
Methods and preparations for treating disorders of the eye and / or causing posterior vitreous disconnection or disinsertion. Preparations containing (a) urea, (b) urea derivatives (e.g., hydroxyurea, thiourea), (c) a non-steroidal anti-inflamatory agent, (d) antimetabolites, (e) urea, urea derivatives, non-enzymatic proteins, nucleosides, nucleotides and their derivatives (e.g., adenine, adenosine, cytosine, cytadine, guanine, guanitadine, guanidinium, thymidine, thimitadine, uradine, uracil, cystine), uric acid, calcium acetal salicylate, ammonium sulfate or other compound capable of causing non-enzymatic dissolution of the hyaloid membrane or (e) any of the possible combinations thereof, are administered to the eye in therapeutically effective amounts.
Owner:透明视网膜技术公司
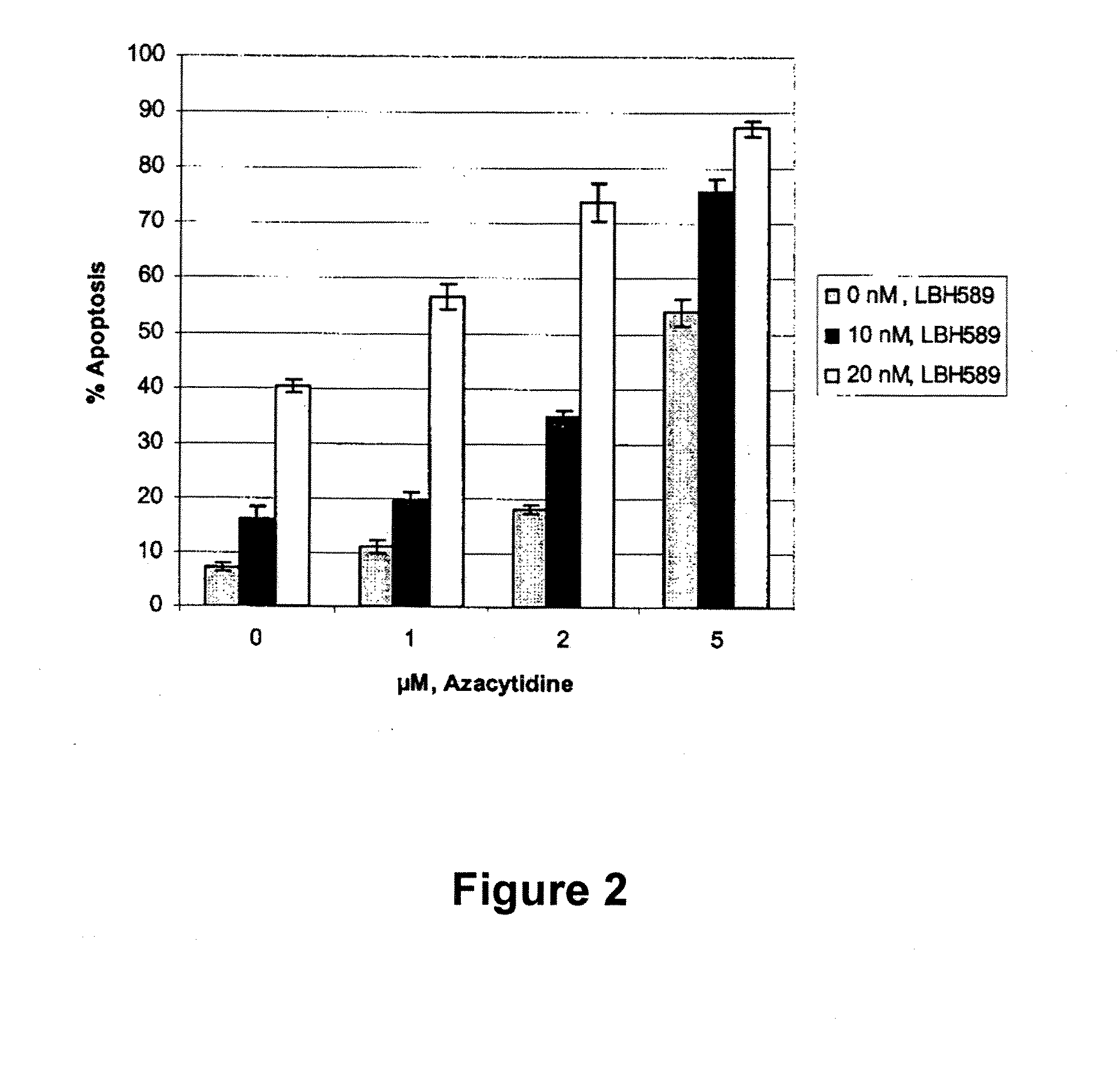
Combination of an HDAC inhibitor and an antimetabolite
The invention relates to a combination which comprises:(a) a HDAI; and(b) an anti-metabolite,for simultaneous, concurrent, separate or sequential use, especially for use in the treatment of proliferative diseases, more specifically MDS or AML. The invention also relates to pharmaceutical compositions comprising such a combination and to a method of treating MDS or AML, in a mammal, particularly a human, with such a combination. The present invention further also relates to a commercial package or product comprising such a combination.
Owner:NOVARTIS AG
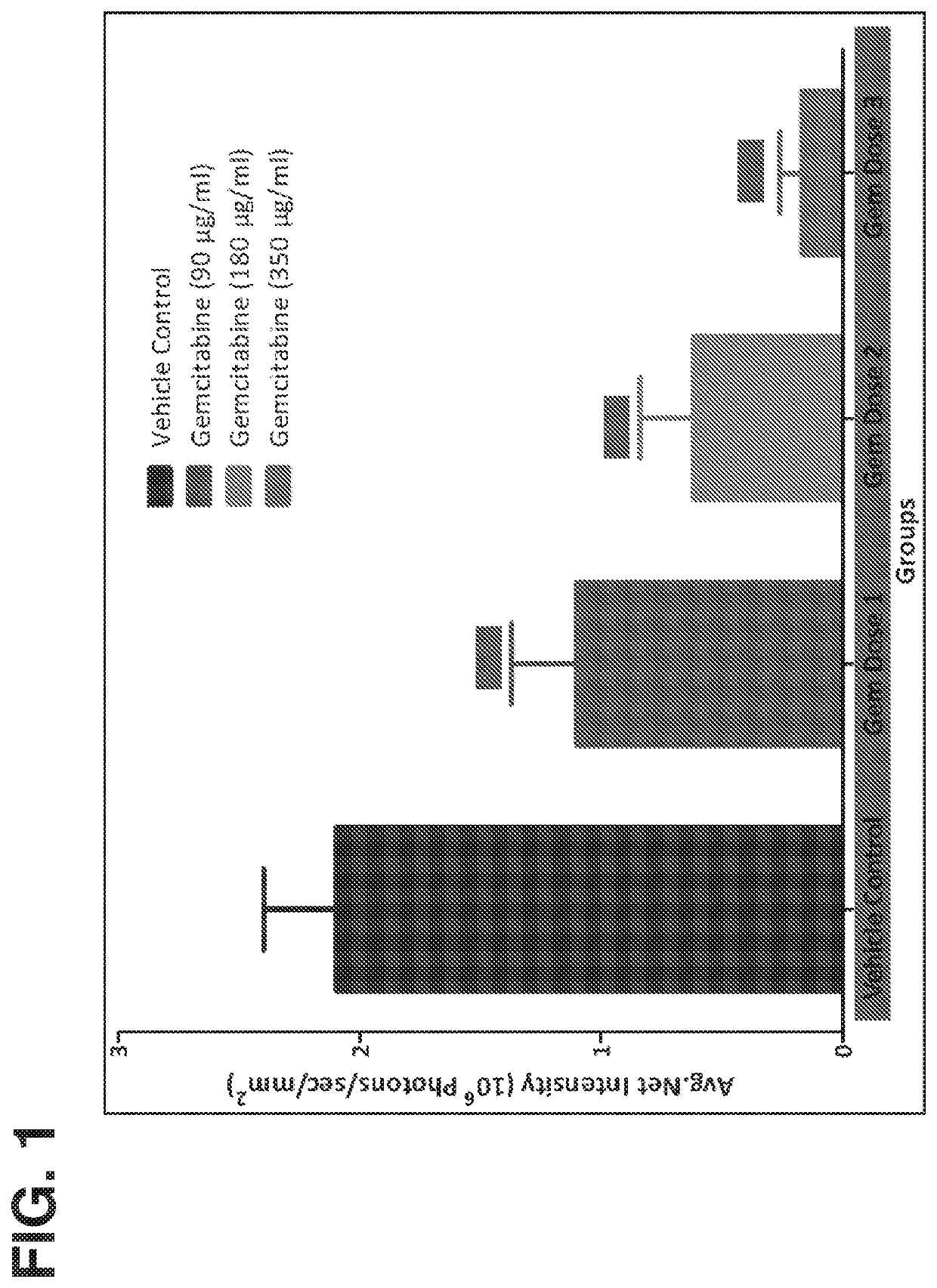
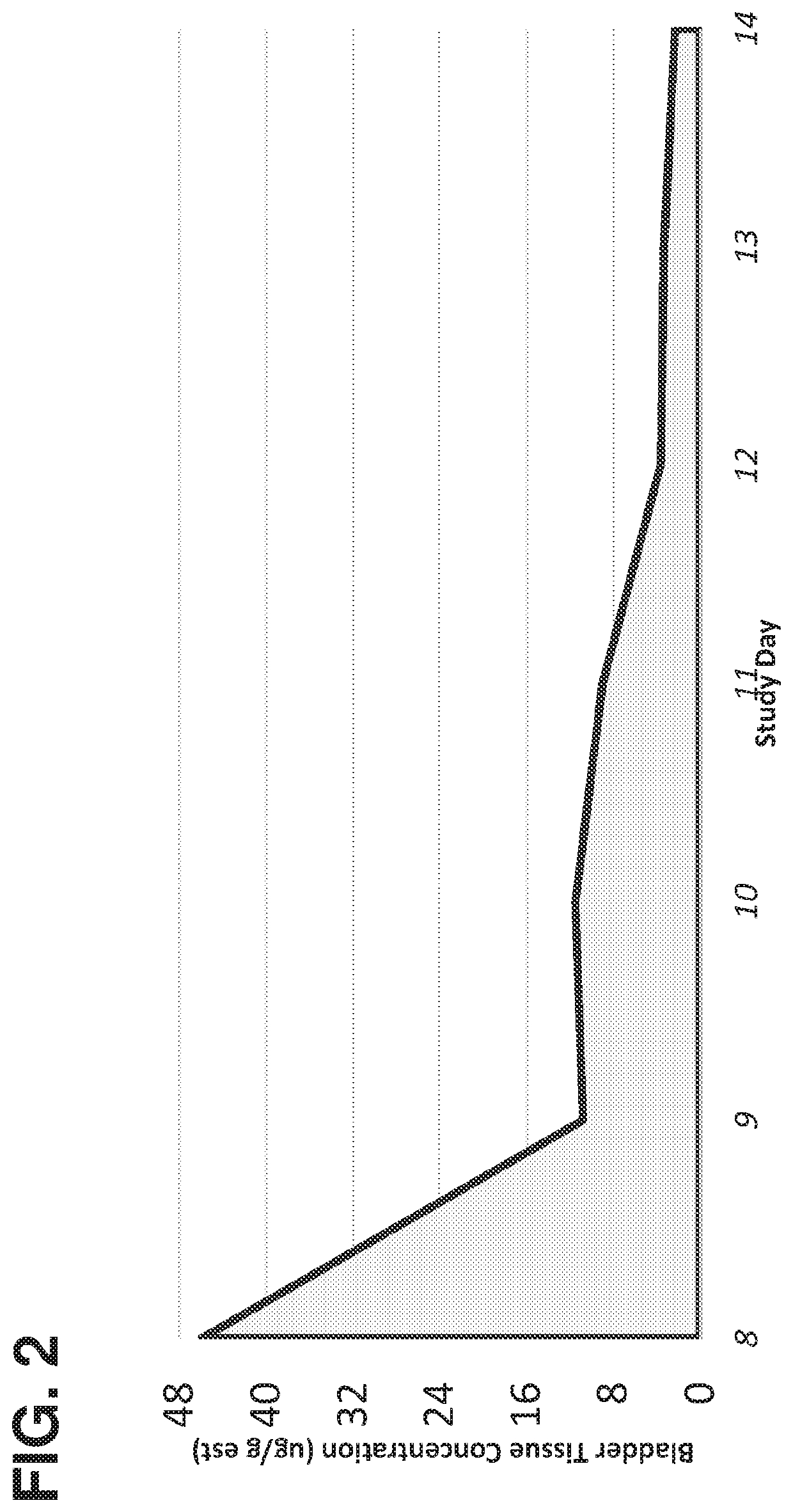
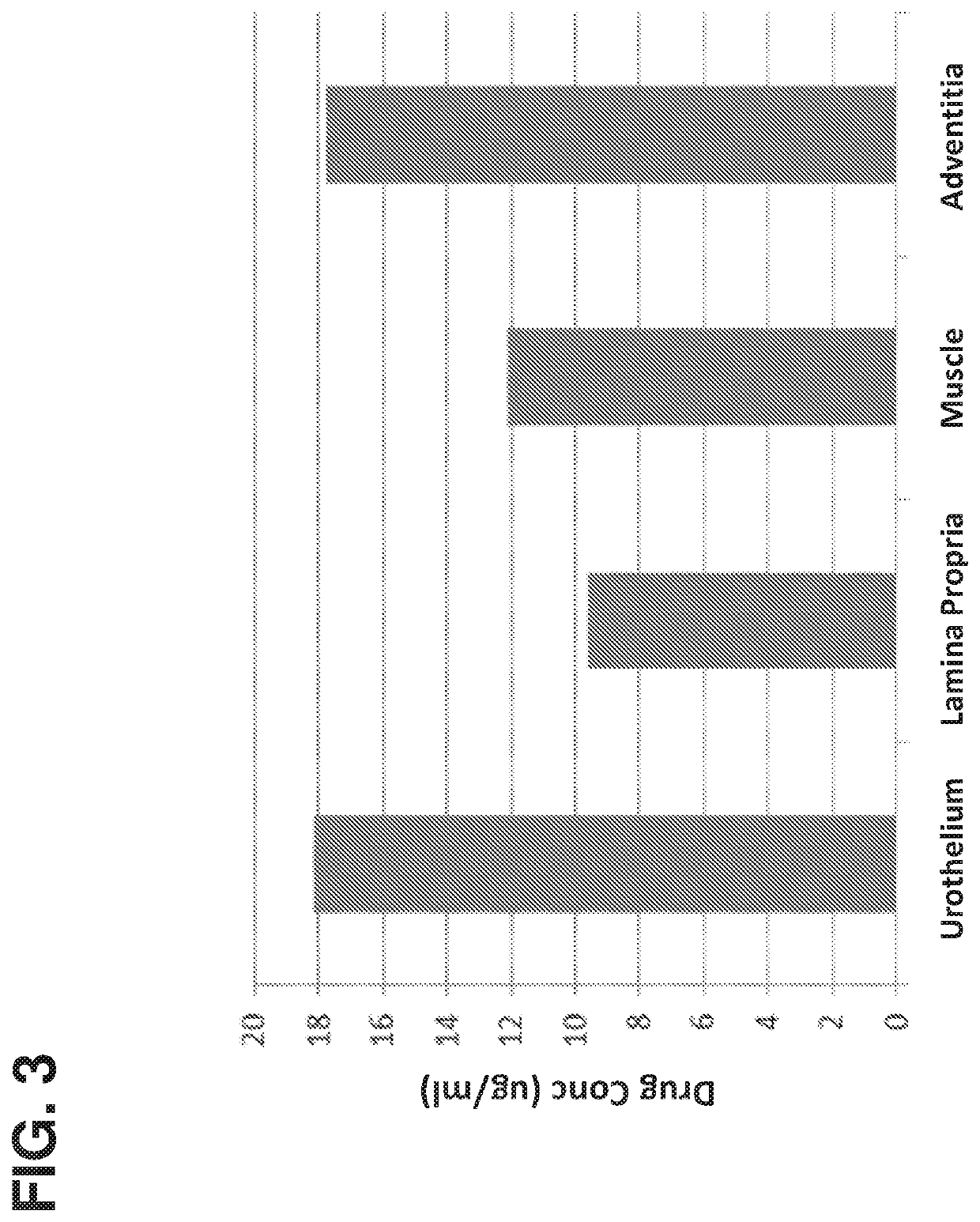
Method of treating lower tract urothelial cancer
PendingUS20190388338A1Reduce recurrenceShorten the progressOrganic active ingredientsPharmaceutical delivery mechanismImmunomodulating AgentAntineoplastic Antimetabolites
The present invention provides methods, devices, and kits related to treatment of urothelial carcinomas of the lower tract with antimetabolite (such as gemcitabine). In some aspects the methods, devices and kits relate to treatment of urothelial carcinomas of the lower tract with an antimetabolite (such as gemcitabine) and an immunomodulating agent.
Owner:TARIS BIOMEDICAL
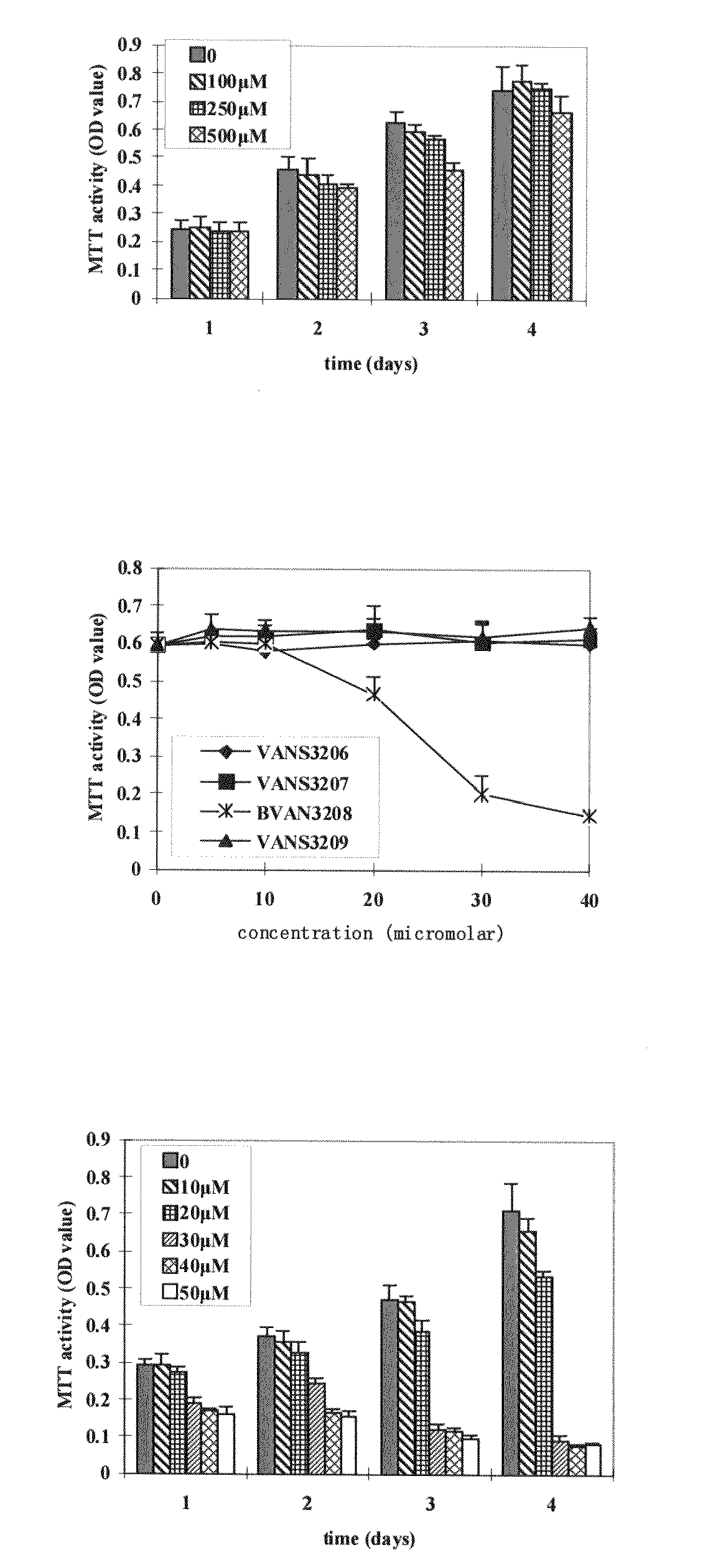
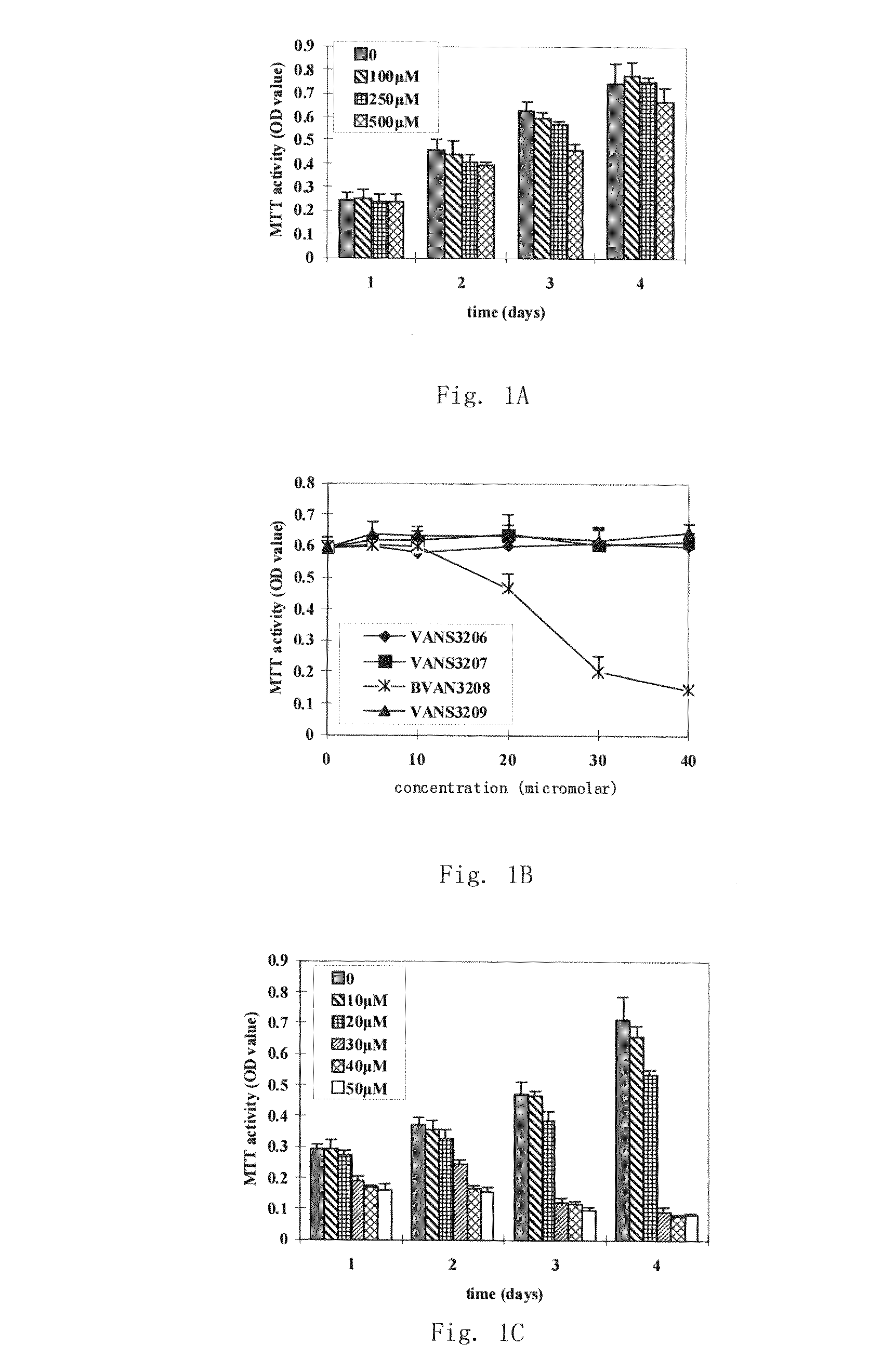
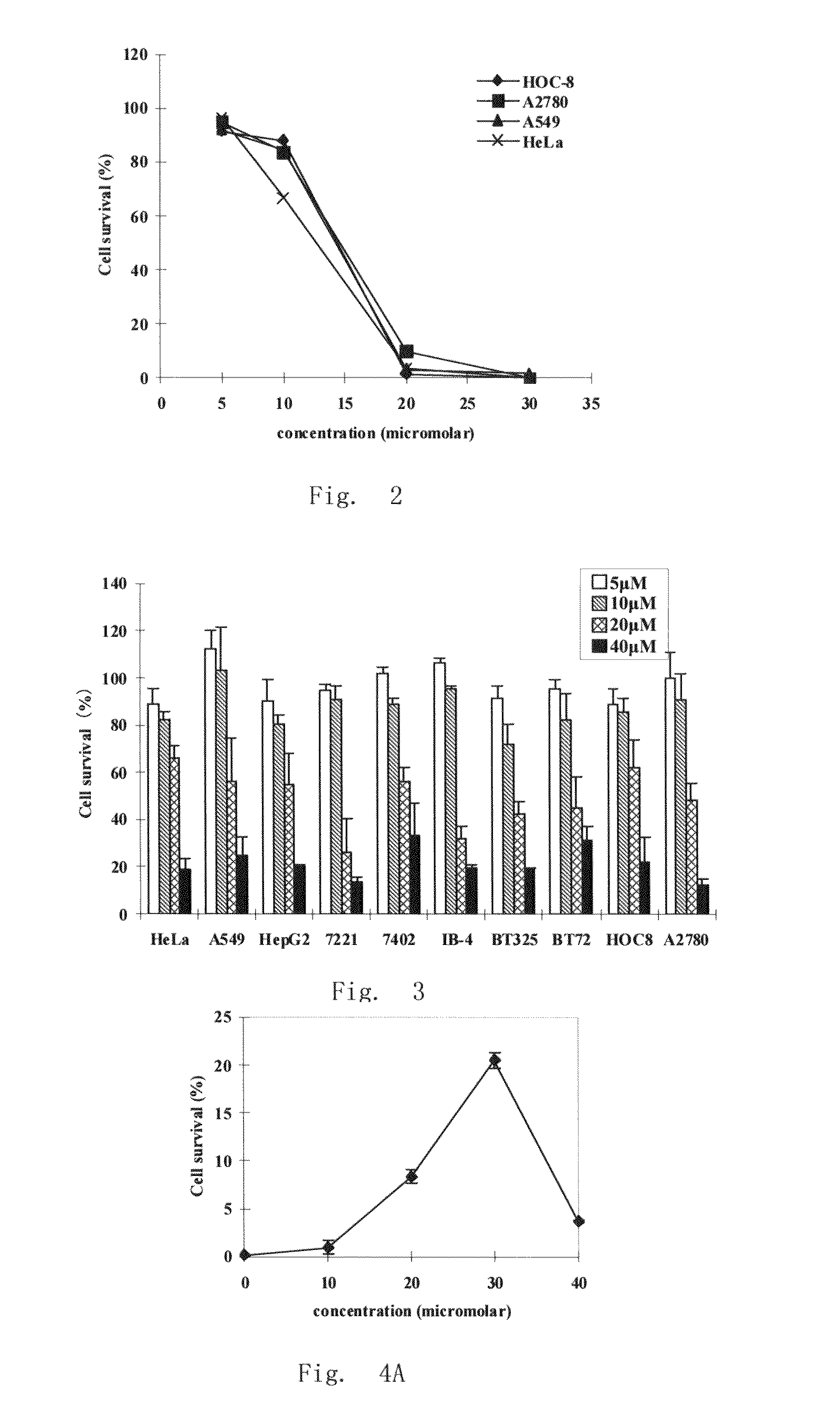
Gemcitabine-containing anti-cancer medicine sustained-release injection
The invention relates to cancer therapy drug sustained-release injection containing gemcitabine. The injection comprises sustained-release microspheres and dissolvent, wherein, the sustained-release microspheres comprise active ingredients for cancer therapy and sustained-release auxiliary materials, and the dissolvent is special dissolvent containing suspending agent. The active ingredients for cancer therapy comprise antimetabolite of gemcitabine or antimetabolite selected from zalcitabine, emtritabine, galocitabine, ibacitabine, ancitabine, decitabine, flurocitabine, enocitabine, imidazoletabine, capecitabine, gemcitabine, fludarabine or cladribine, and / or antimetabolite synergist selected from phosphoinositide 3-kinase inhibitor, pyrimidine analogs and / or DNA repair enzyme inhibitor; the sustained-release auxiliary materials comprise polifeprosan, bi-fatty acid, decanedioic copolymer, polylactic copolymer and EVAc; the viscocity of the suspending agent is 100cp to 3000cp (at 20 to 30 DEG C) and the suspending agent is selected from sodium carboxymethyl cellulose. The sustained-release microspheres can also be produced into sustained-release implant, and by injecting or positioning the sustained release agent in tumor or tumor margin, the efficacy of non-operative treatment such as radiation treatment and chemical treatment can be enhanced.
Owner:SHANDONG LANJIN PHARMA +1
Application of 2-bromide-isovanillin for the manufacture of a medicament for anti-cancer or/and radiation/chemotherapy sensitization
ActiveUS20110098362A9Induce apoptosisHigh sensitivityBiocideAldehyde active ingredientsSide effectTherapeutic effect
Use of 2-bromo-isovanillin in the preparation of an anticancer medicament and / or radio- and chemotherapy sensitizing medicament is disclosed. The medicament for the treatment of cancers and / or for radio- and chemotherapy sensitization comprising 2-bromo-isovanillin as active ingredient provided herein has the following features: (1) low toxicity, without evident adverse effects; (2) significant therapeutic effect, with remarkable proliferation inhibiting and pro-apoptotic effects in tumor cells; (3) a broad-spectrum anticancer activity; (4) suitable to be used in combination with antimetabolites, to enhance the effects and meanwhile lower the toxicity, and also to reduce multi-drug resistance; (5) convenient and safe administration, the main route being oral.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
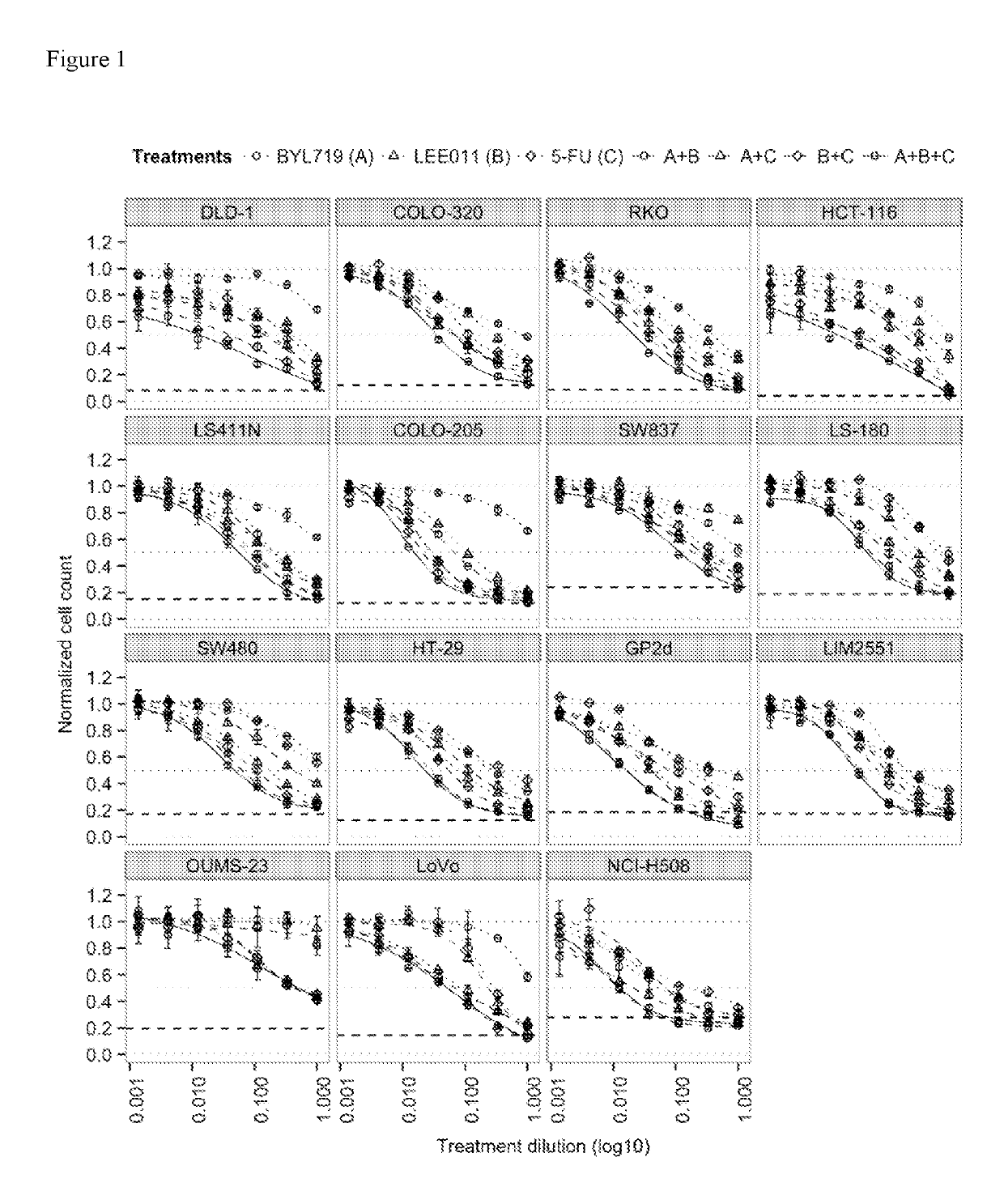
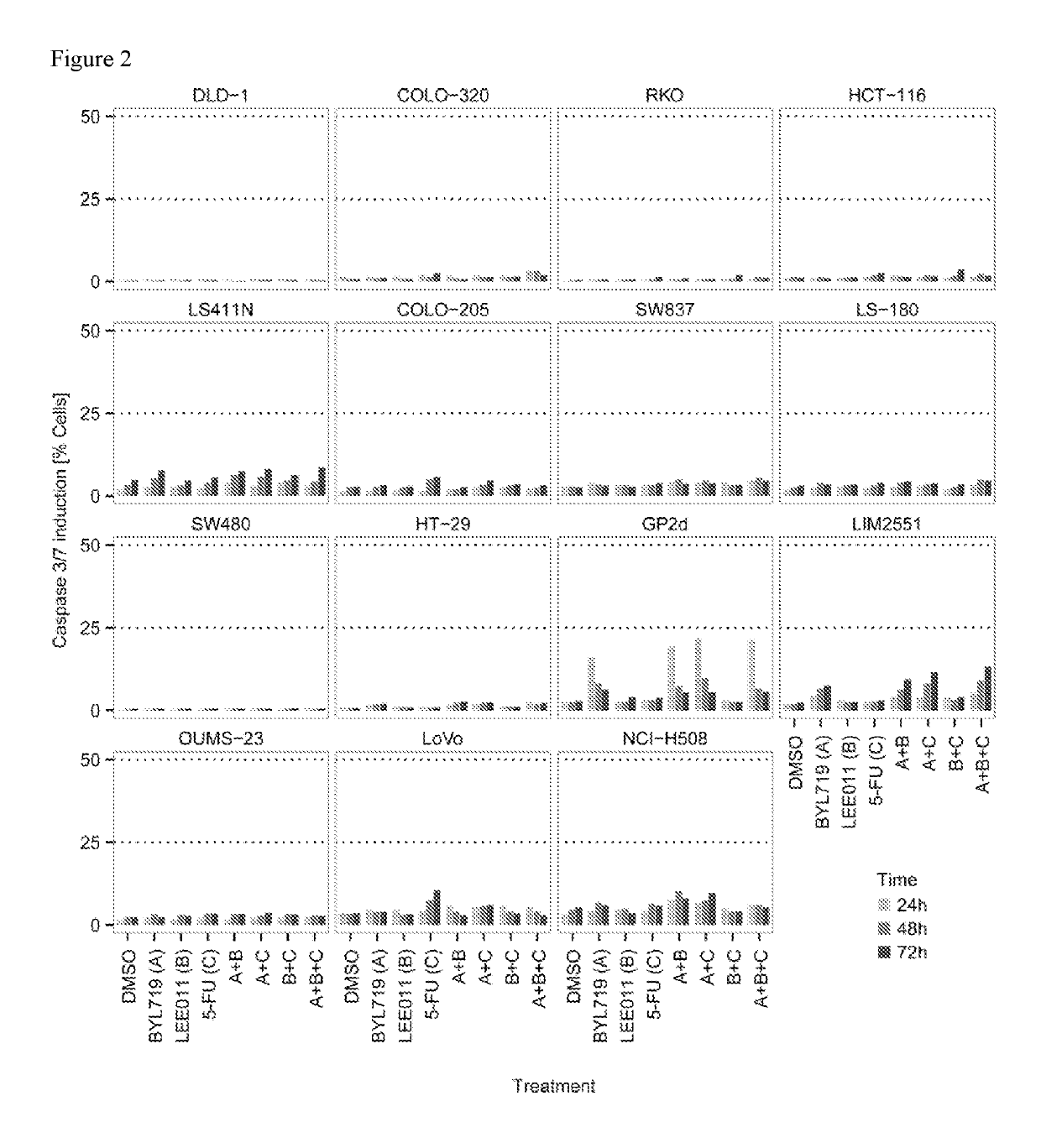
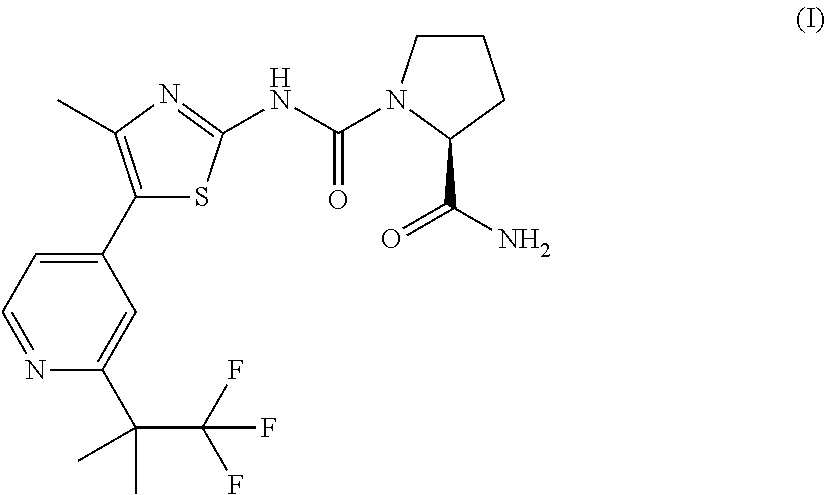
Pharmaceutical combination comprising the PI3K inhibitor alpelisib and the CDK4/6 inhibitor ribociclib, and the use thereof in the treatment/prevention of cancer
InactiveUS10328066B2Inhibit progressAvoid symptomsAntineoplastic agentsHeterocyclic compound active ingredientsCancer preventionRibociclib
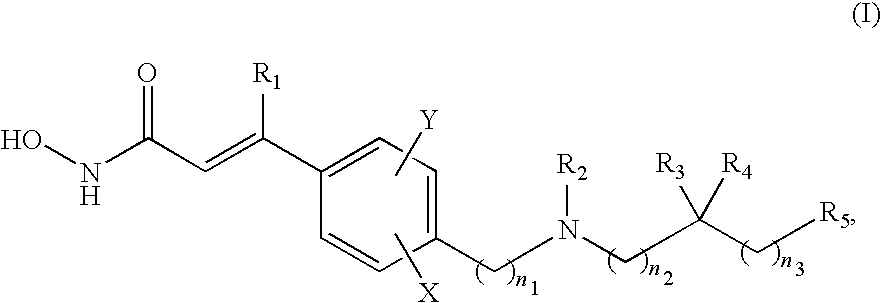
The present disclosure pertains to a pharmaceutical combination comprising (a) an alpha-isoform specific PI3K inhibitor, (b) a cyclin dependent kinase 4 / 6 (CDK4 / 6) inhibitor, and (c) an antimetabolite antineoplastic agent; combined preparations and pharmaceutical compositions thereof; the uses of such a combination in the treatment or prevention of cancer; and methods of treating or preventing cancer in a subject comprising administering a therapeutically effective amount of such combination.
Owner:NOVARTIS AG
Agents for intravitreal administration to treat or prevent disorders of the eye
Methods and preparations for treating disorders of the eye and / or causing posterior vitreous disconnection or disinsertion. Preparations containing (a) urea, (b) urea derivatives (e.g., hydroxyurea, thiourea), (c) a non-steroidal anti-inflamatory agent, (d) antimetabolites, (e) urea, urea derivatives, non-enzymatic proteins, nucleosides, nucleotides and their derivatives (e.g., adenine, adenosine, cytosine, cytadine, guanine, guanitadine, guanidinium, thymidine, thimitadine, uradine, uracil, cystine), uric acid, calcium acetal salicylate, ammonium sulfate or other compound capable of causing non-enzymatic dissolution of the hyaloid membrane or (e) any of the possible combinations thereof, are administered to the eye in therapeutically effective amounts.
Owner:透明视网膜技术公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![9h-pyrimido[4,5-b]indoles, 9h-pyrido[4',3':4,5]pyrrolo[2,3-d]pyridines, and 9h 1,3,6,9 tetraaza-fluorenes as chk1 kinase function inhibitors 9h-pyrimido[4,5-b]indoles, 9h-pyrido[4',3':4,5]pyrrolo[2,3-d]pyridines, and 9h 1,3,6,9 tetraaza-fluorenes as chk1 kinase function inhibitors](https://images-eureka.patsnap.com/patent_img/5fbea4bb-82fb-48c5-b01c-ccdb074062e2/US20100210639A1-20100819-C00001.png)
![9h-pyrimido[4,5-b]indoles, 9h-pyrido[4',3':4,5]pyrrolo[2,3-d]pyridines, and 9h 1,3,6,9 tetraaza-fluorenes as chk1 kinase function inhibitors 9h-pyrimido[4,5-b]indoles, 9h-pyrido[4',3':4,5]pyrrolo[2,3-d]pyridines, and 9h 1,3,6,9 tetraaza-fluorenes as chk1 kinase function inhibitors](https://images-eureka.patsnap.com/patent_img/5fbea4bb-82fb-48c5-b01c-ccdb074062e2/US20100210639A1-20100819-C00002.png)
![9h-pyrimido[4,5-b]indoles, 9h-pyrido[4',3':4,5]pyrrolo[2,3-d]pyridines, and 9h 1,3,6,9 tetraaza-fluorenes as chk1 kinase function inhibitors 9h-pyrimido[4,5-b]indoles, 9h-pyrido[4',3':4,5]pyrrolo[2,3-d]pyridines, and 9h 1,3,6,9 tetraaza-fluorenes as chk1 kinase function inhibitors](https://images-eureka.patsnap.com/patent_img/5fbea4bb-82fb-48c5-b01c-ccdb074062e2/US20100210639A1-20100819-C00003.png)
![5-[[4-[[morpholin-2-yl]methylamino]-5-(trifluoromethyl)-2-pyridyl]amino]pyrazine-2-carbonitrile and therapeutic uses thereof 5-[[4-[[morpholin-2-yl]methylamino]-5-(trifluoromethyl)-2-pyridyl]amino]pyrazine-2-carbonitrile and therapeutic uses thereof](https://images-eureka.patsnap.com/patent_img/17857fa6-d003-4440-8cbc-3466e04970e6/US20150126471A1-20150507-C00001.PNG)
![5-[[4-[[morpholin-2-yl]methylamino]-5-(trifluoromethyl)-2-pyridyl]amino]pyrazine-2-carbonitrile and therapeutic uses thereof 5-[[4-[[morpholin-2-yl]methylamino]-5-(trifluoromethyl)-2-pyridyl]amino]pyrazine-2-carbonitrile and therapeutic uses thereof](https://images-eureka.patsnap.com/patent_img/17857fa6-d003-4440-8cbc-3466e04970e6/US20150126471A1-20150507-C00002.PNG)
![5-[[4-[[morpholin-2-yl]methylamino]-5-(trifluoromethyl)-2-pyridyl]amino]pyrazine-2-carbonitrile and therapeutic uses thereof 5-[[4-[[morpholin-2-yl]methylamino]-5-(trifluoromethyl)-2-pyridyl]amino]pyrazine-2-carbonitrile and therapeutic uses thereof](https://images-eureka.patsnap.com/patent_img/17857fa6-d003-4440-8cbc-3466e04970e6/US20150126471A1-20150507-C00003.PNG)
![9H-pyrimido[4,5-B]indoles, 9H-pyrido[4',3':4,5]pyrrolo[2,3-D]pyridines, and 9H 1,3,6,9 tetraaza-fluorenes as CHK1 kinase function inhibitors 9H-pyrimido[4,5-B]indoles, 9H-pyrido[4',3':4,5]pyrrolo[2,3-D]pyridines, and 9H 1,3,6,9 tetraaza-fluorenes as CHK1 kinase function inhibitors](https://images-eureka.patsnap.com/patent_img/20dd10c3-0cd5-48e9-9961-d4f922e92e48/US08618121-20131231-C00001.png)
![9H-pyrimido[4,5-B]indoles, 9H-pyrido[4',3':4,5]pyrrolo[2,3-D]pyridines, and 9H 1,3,6,9 tetraaza-fluorenes as CHK1 kinase function inhibitors 9H-pyrimido[4,5-B]indoles, 9H-pyrido[4',3':4,5]pyrrolo[2,3-D]pyridines, and 9H 1,3,6,9 tetraaza-fluorenes as CHK1 kinase function inhibitors](https://images-eureka.patsnap.com/patent_img/20dd10c3-0cd5-48e9-9961-d4f922e92e48/US08618121-20131231-C00002.png)
![9H-pyrimido[4,5-B]indoles, 9H-pyrido[4',3':4,5]pyrrolo[2,3-D]pyridines, and 9H 1,3,6,9 tetraaza-fluorenes as CHK1 kinase function inhibitors 9H-pyrimido[4,5-B]indoles, 9H-pyrido[4',3':4,5]pyrrolo[2,3-D]pyridines, and 9H 1,3,6,9 tetraaza-fluorenes as CHK1 kinase function inhibitors](https://images-eureka.patsnap.com/patent_img/20dd10c3-0cd5-48e9-9961-d4f922e92e48/US08618121-20131231-C00003.png)