Compositions and methods for treating pterygium
A technology of pterygium and implant, applied in the field of compositions and methods for treating pterygium, capable of solving problems such as visual impairment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
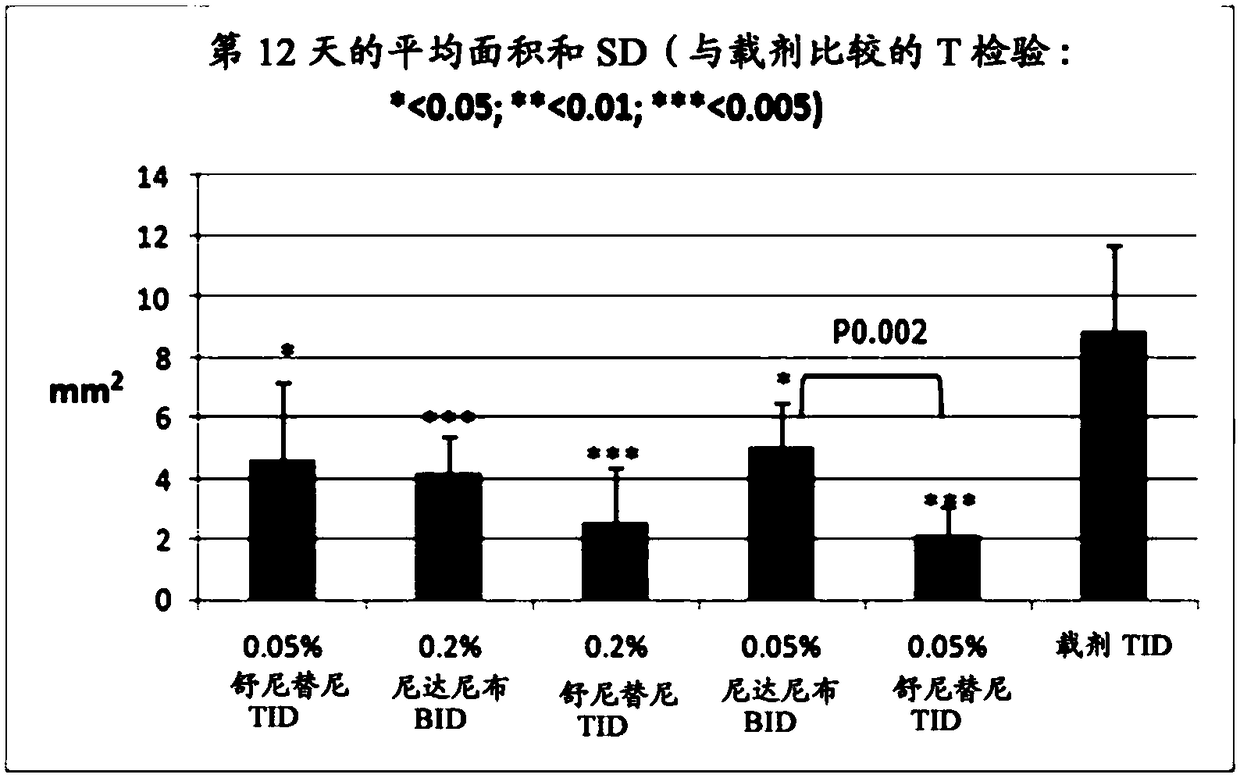
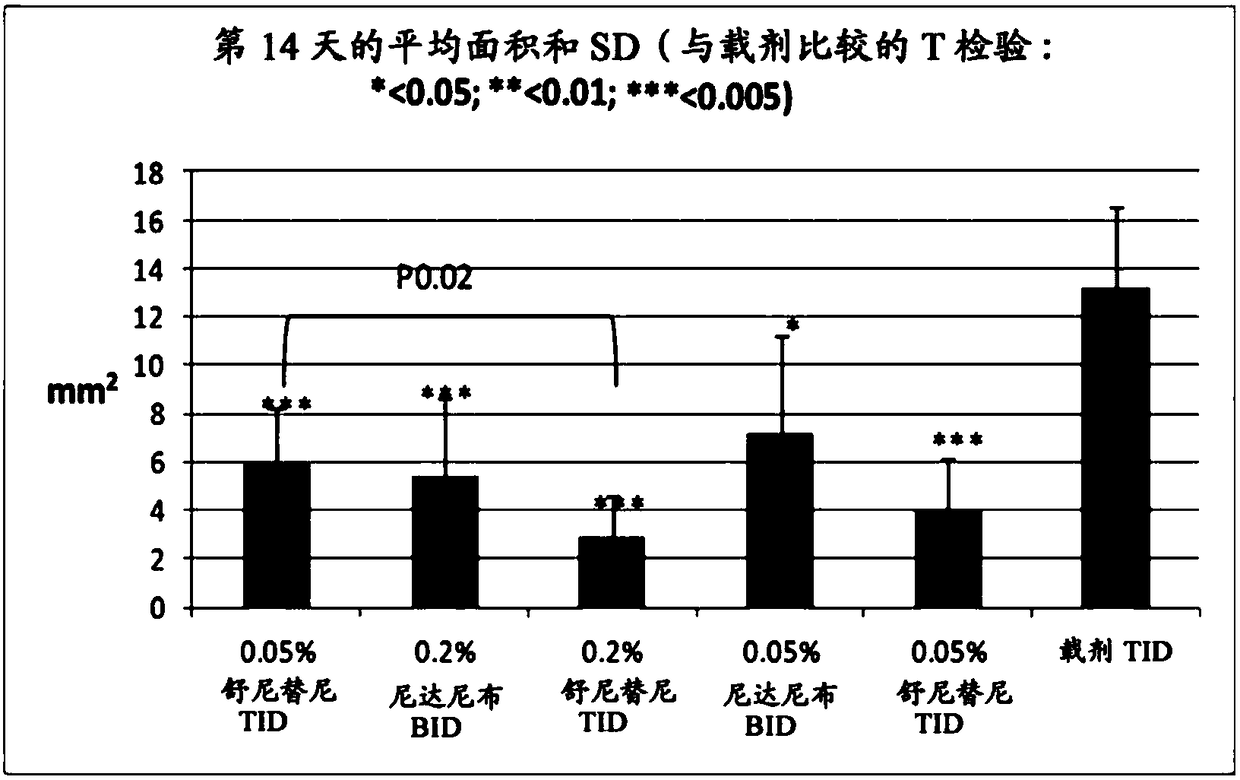
[0085] Example 1: Rabbit corneal suture model of neovascularization and hyperemia
[0086] Establish a rabbit corneal suture model to evaluate the effect of drugs on corneal neovascularization (Ko et al., Cornea.2013; 32(5):689-695; Pérez-Santonja et al., Am J Ophthalmol.2010;150(4):519 -528). The anti-angiogenic activity of nintedanib was investigated in this model.
[0087] Topical Ophthalmic Preparations
[0088] Topical compositions were prepared comprising 0.2% or 0.05% nintedanib in phosphate buffered saline (pH 7.4) containing 10% 2-hydroxypropyl beta cyclodextrin. In addition, a composition containing 0.05% sunitinib was prepared in the same vehicle as a positive control.
[0089] Animals and Treatment Procedures
[0090]Thirty-six female New Zealand White rabbits were used for the study. Briefly, five sutures were placed in the superior cornea of the right eye of each animal on day 1 to induce neovascularization. Both eyes of the animals were treated as de...
Embodiment 2
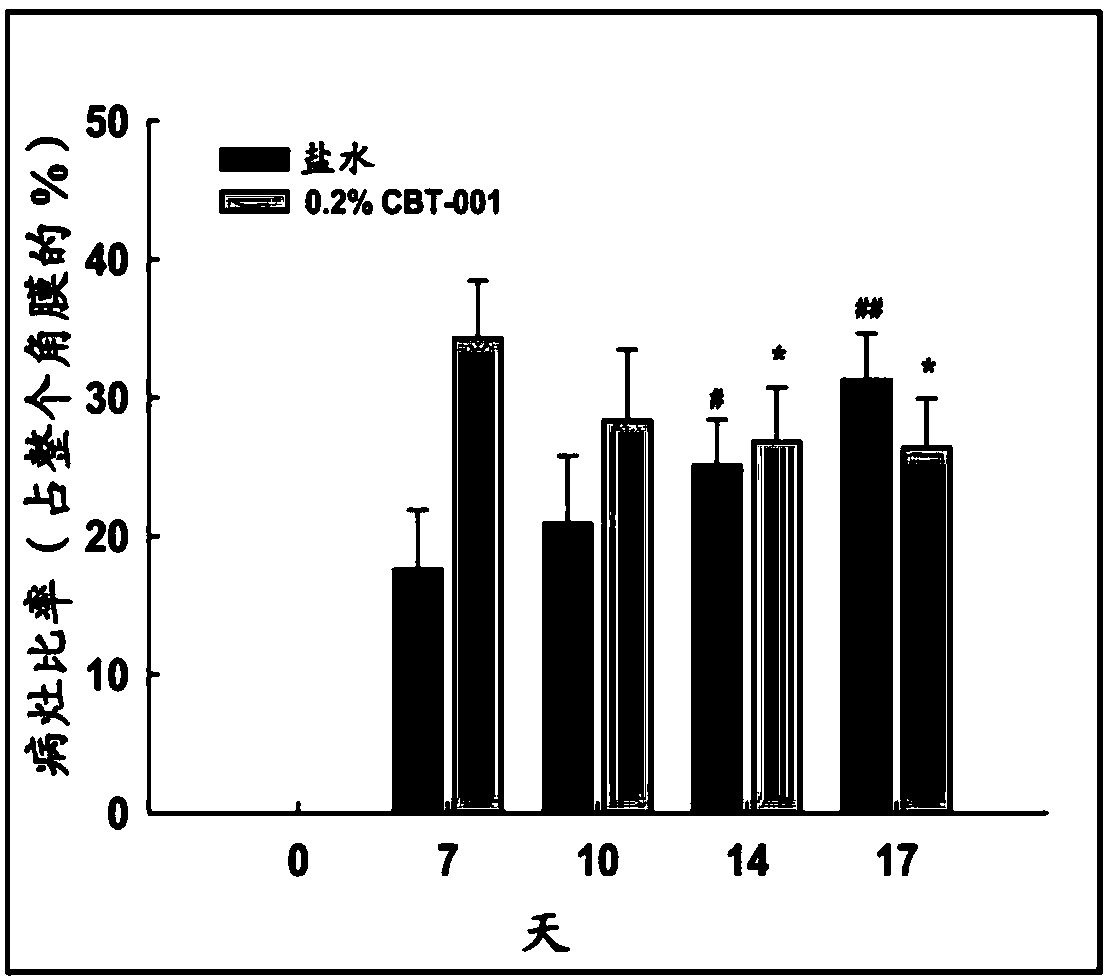
[0107] Embodiment 2: human pterygium mouse model
[0108] A mouse model of human pterygium was described to assess the growth of human pterygium on the cornea of immunodeficient mice (Lee et al., Graefes Arch Clin Exp Ophthalmol. 2014;252(4):609-18). This study examined the effect of several drugs on the growth of pterygium. The drugs were nintedanib, sunitinib, and mitomycin C.
[0109] Topical Ophthalmic Preparations
[0110] The analytes were prepared in phosphate buffered saline (pH 7.4) containing 10% 2-hydroxypropyl beta-cyclodextrin. Details of the formulations are disclosed in the following sections.
[0111] animal
[0112] Seven-week-old male athymic nude mice were acclimatized in filter-top closed cages under pathogen-free conditions.
[0113] Human pterygium primary cell culture
[0114] Human pterygium epithelial cells (hPEC) were isolated from specimens collected after surgical resection and cultured. All participants provided written informed cons...
Embodiment 3
[0135] Example 3: Formulation
[0136] Nintedanib Ophthalmic Solution
[0137] The drug product is an isotonic ophthalmic solution prepared in 2-hydroxypropyl beta-cyclodextrin or other similar cyclodextrin and a buffered solution (pH range from 5.5 to 8.0). Other viscosity builders, lubricants, preservatives may be added to enhance the functionality of the formulation. See Table 6 for the composition of the ophthalmic solution.
[0138] Table 6: Nintedanib Ophthalmic Solution
[0139]
[0140]
[0141] Nintedanib Ophthalmic Suspension
[0142] The drug product is an isotonic ophthalmic suspension prepared in sodium carboxymethylcellulose and buffer solution (pH range from 5.5 to 8.0). The drug particle size is reduced to less than 40 microns. Other viscosifiers, lubricants, solubilizers and preservatives may be added to enhance the functionality of the formulation suspension. The composition is shown in Table 7.
[0143] Table 7: Nintedanib Ophthalmic Suspension ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com