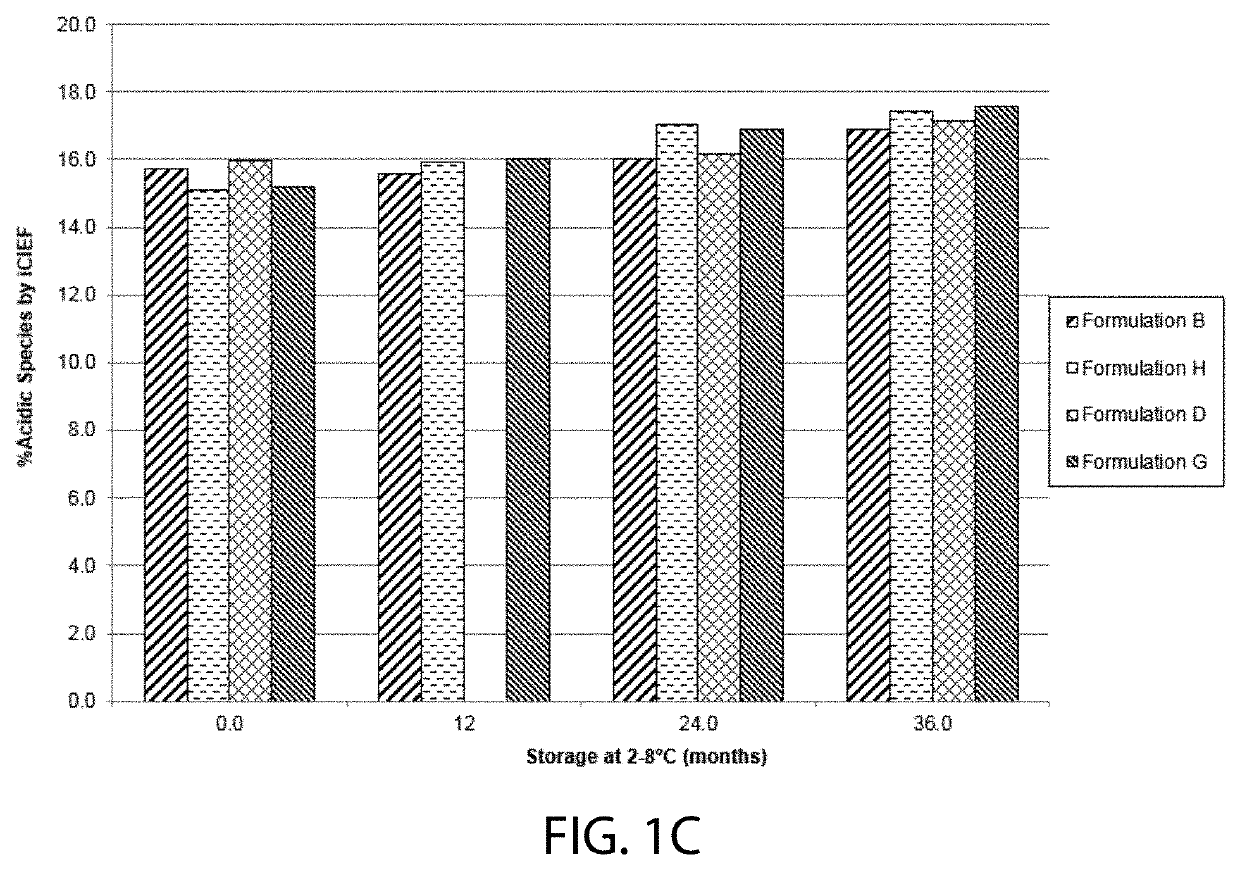
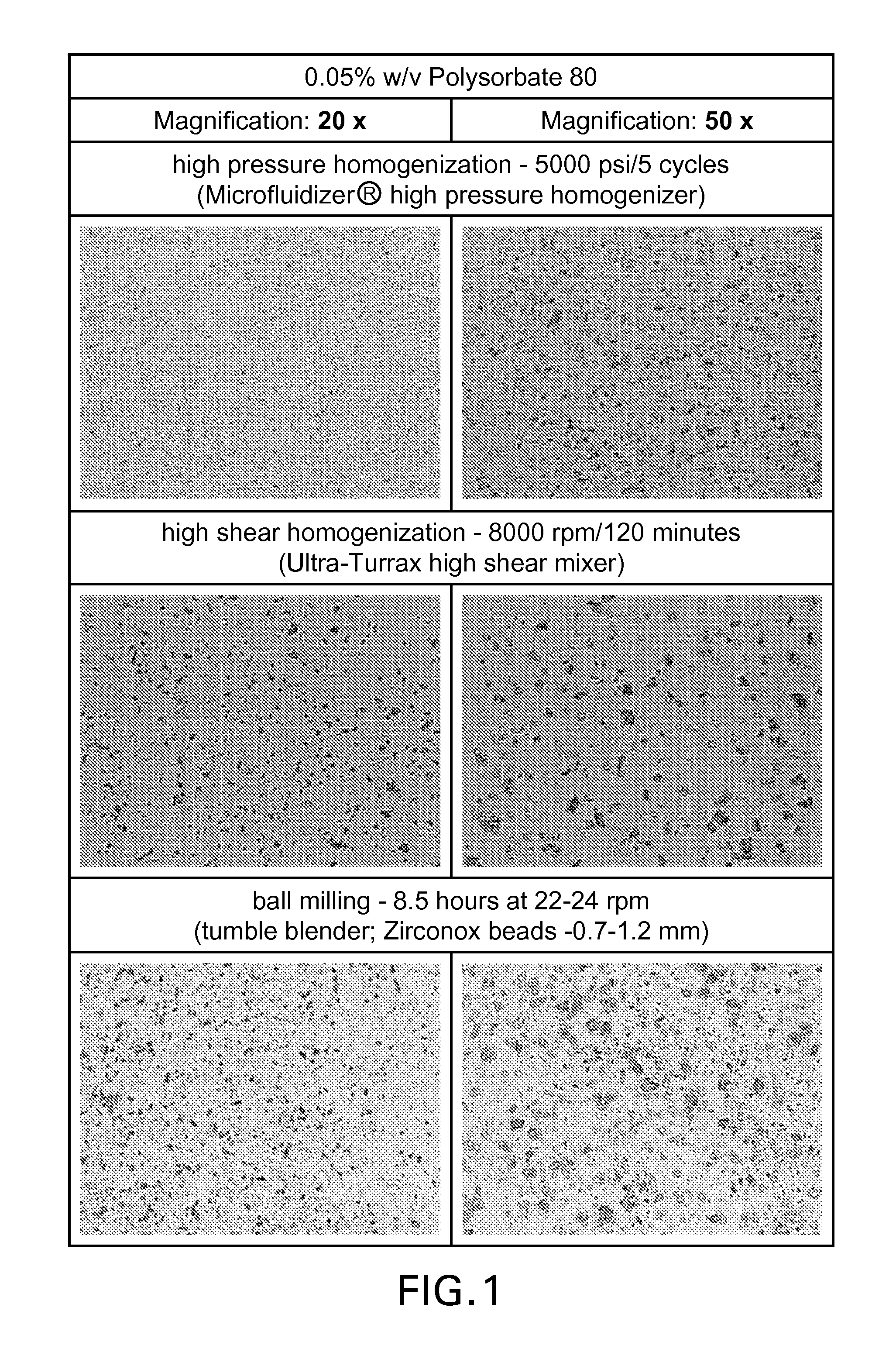
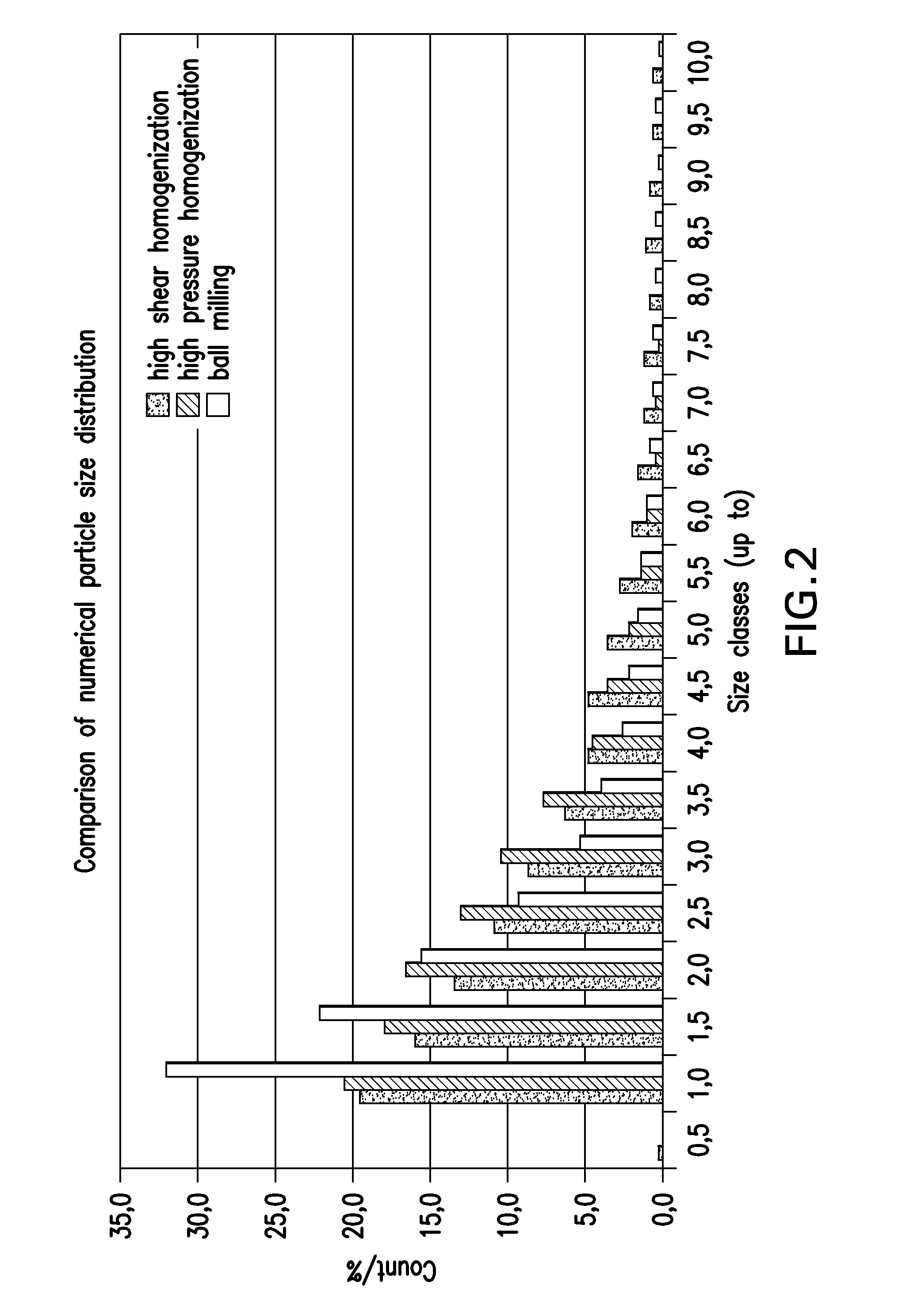
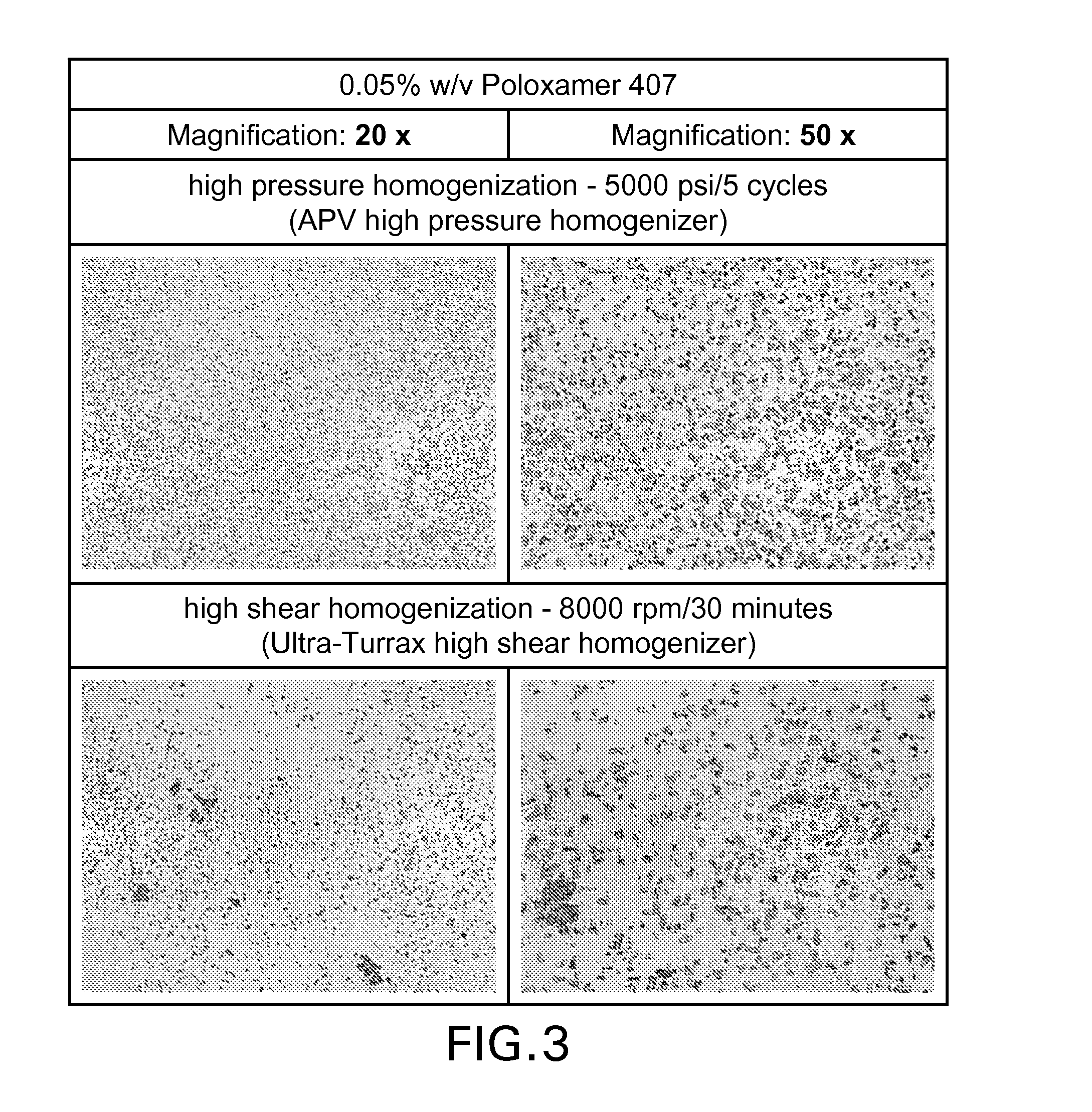
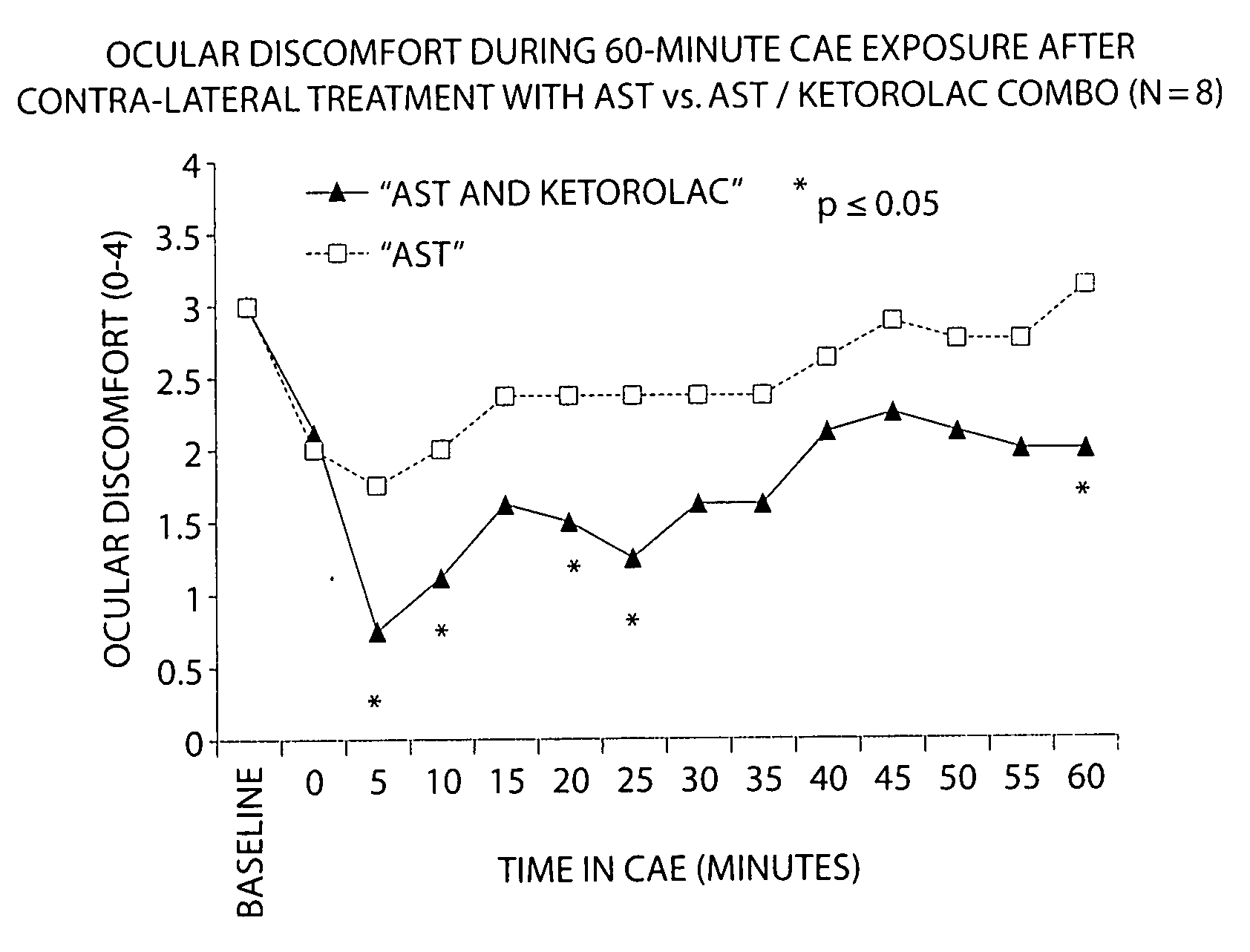
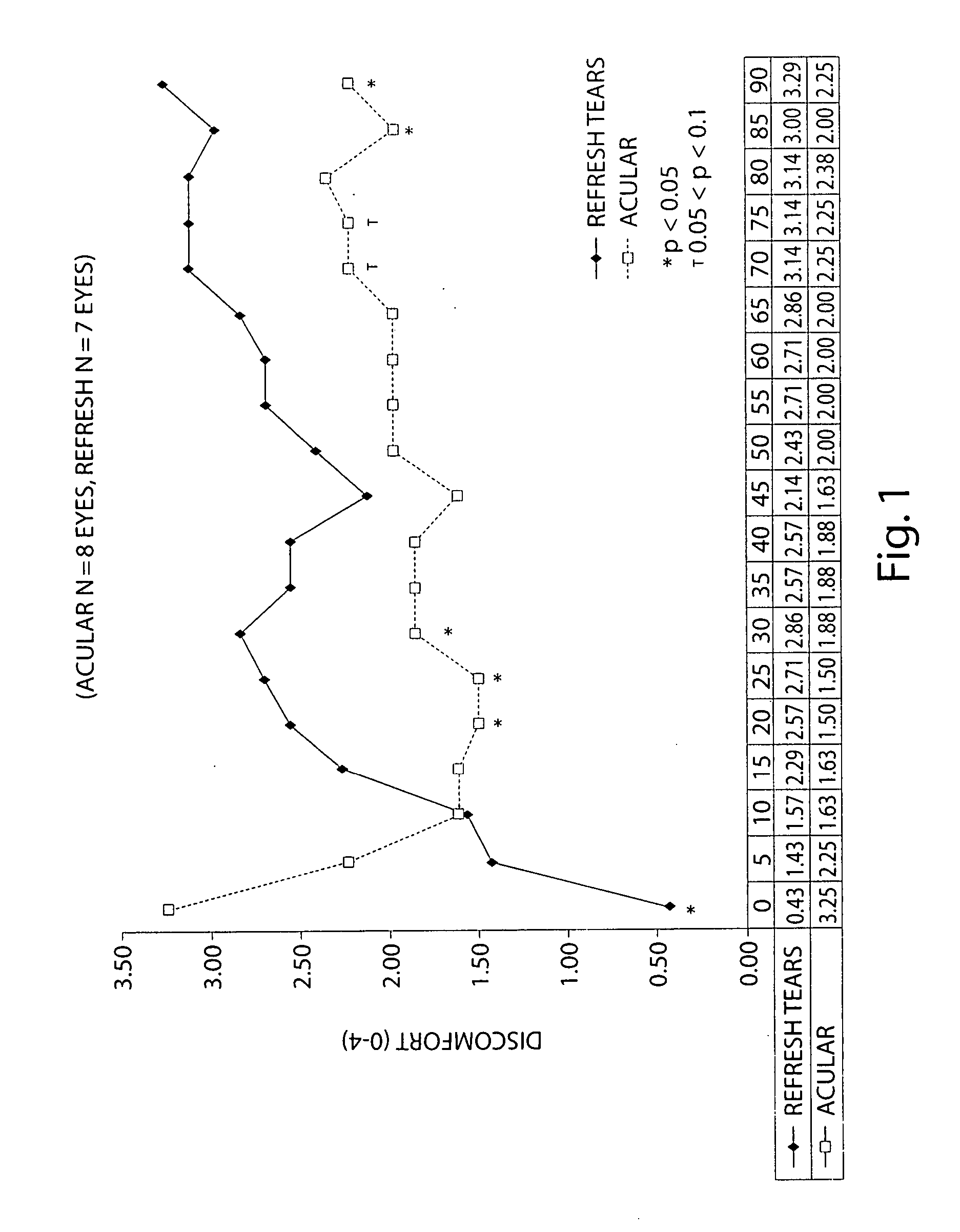
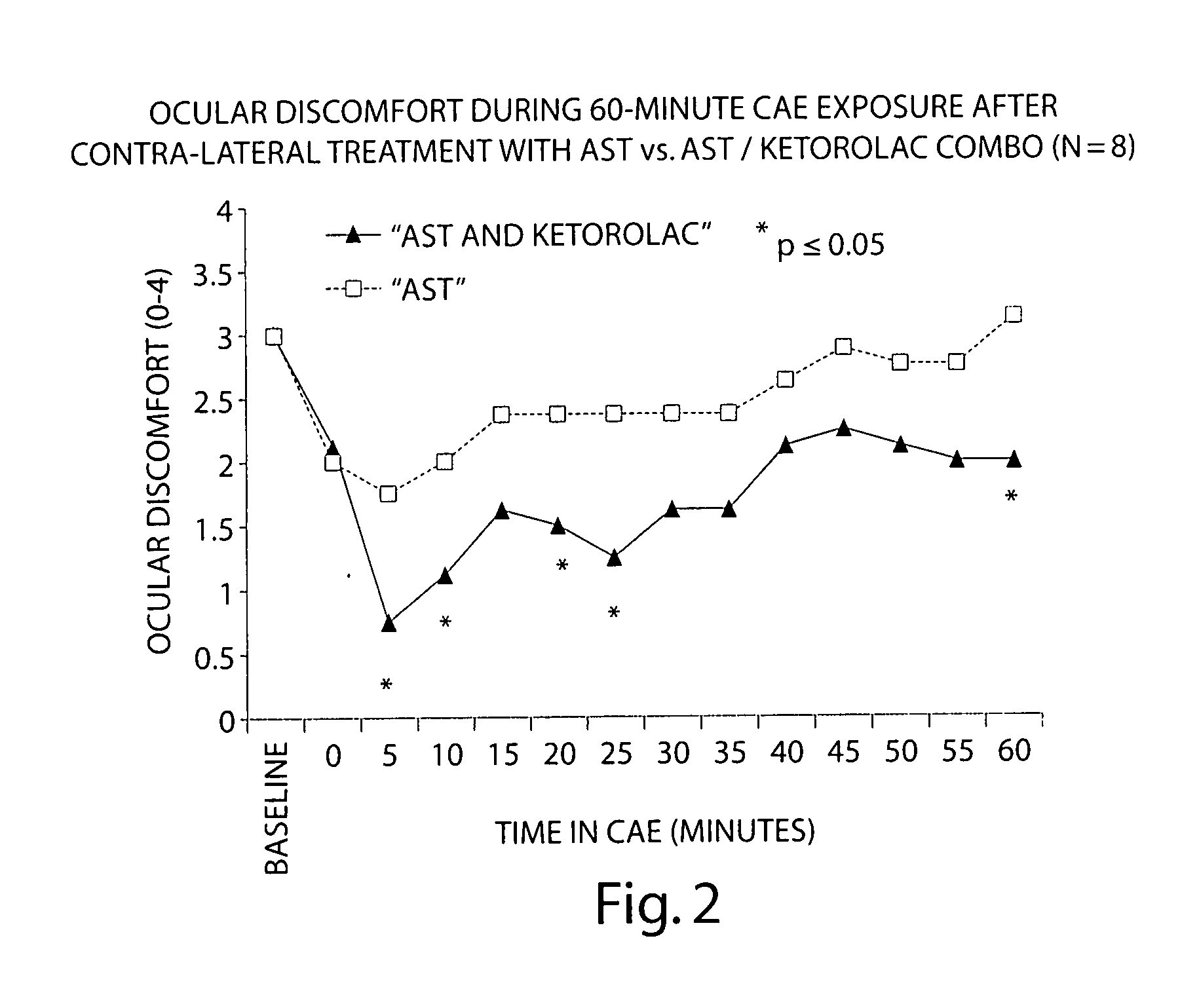
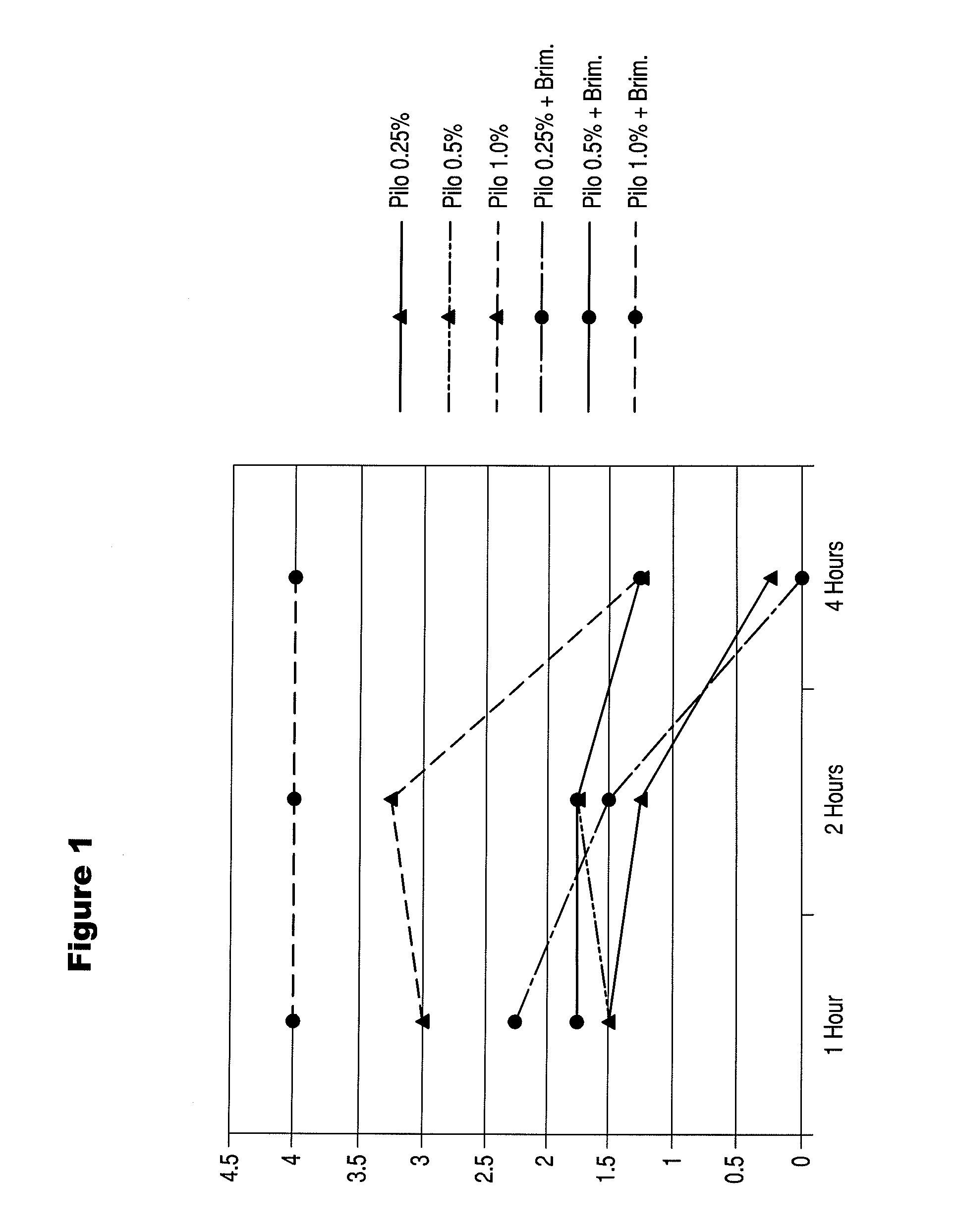
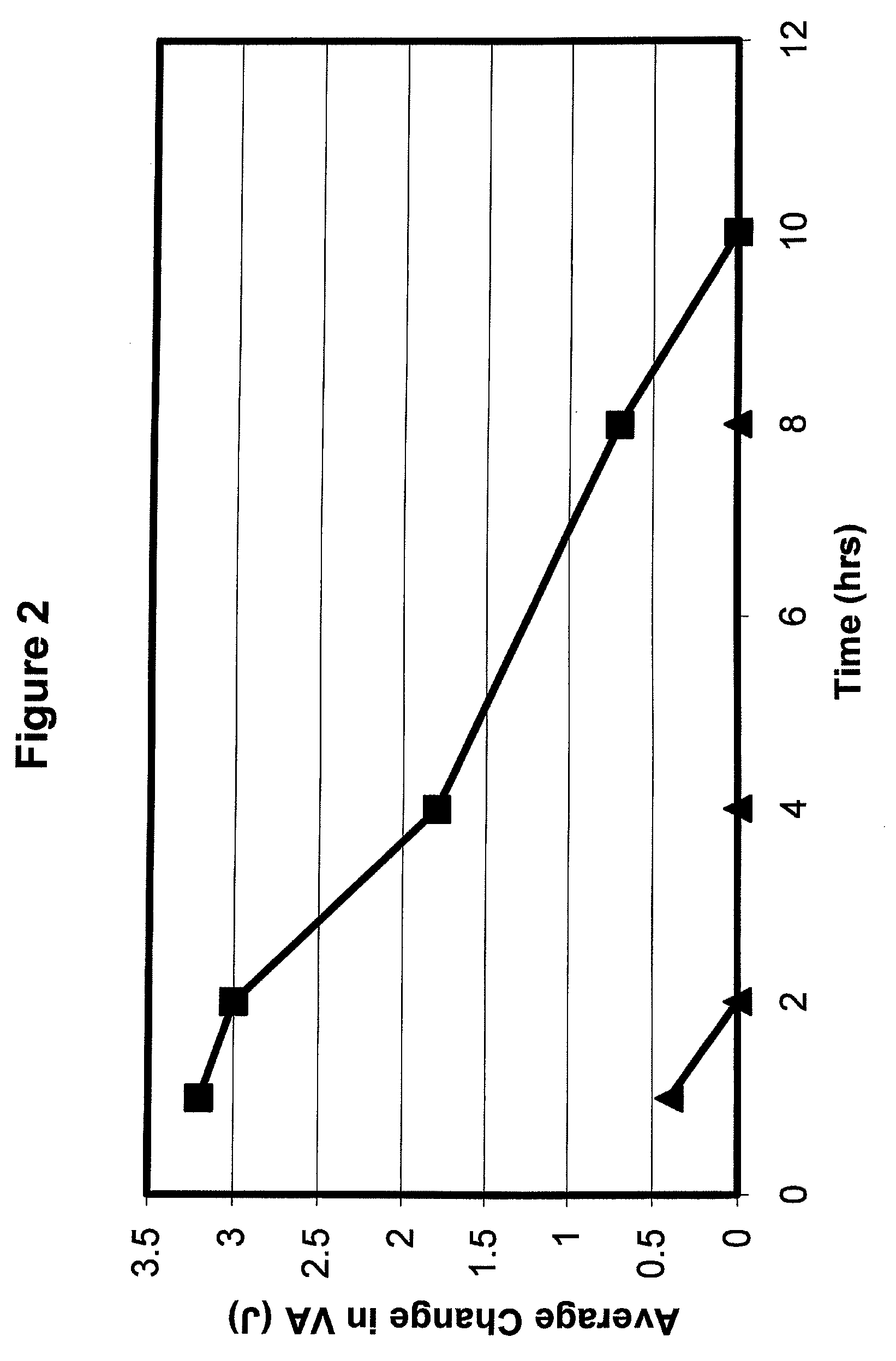
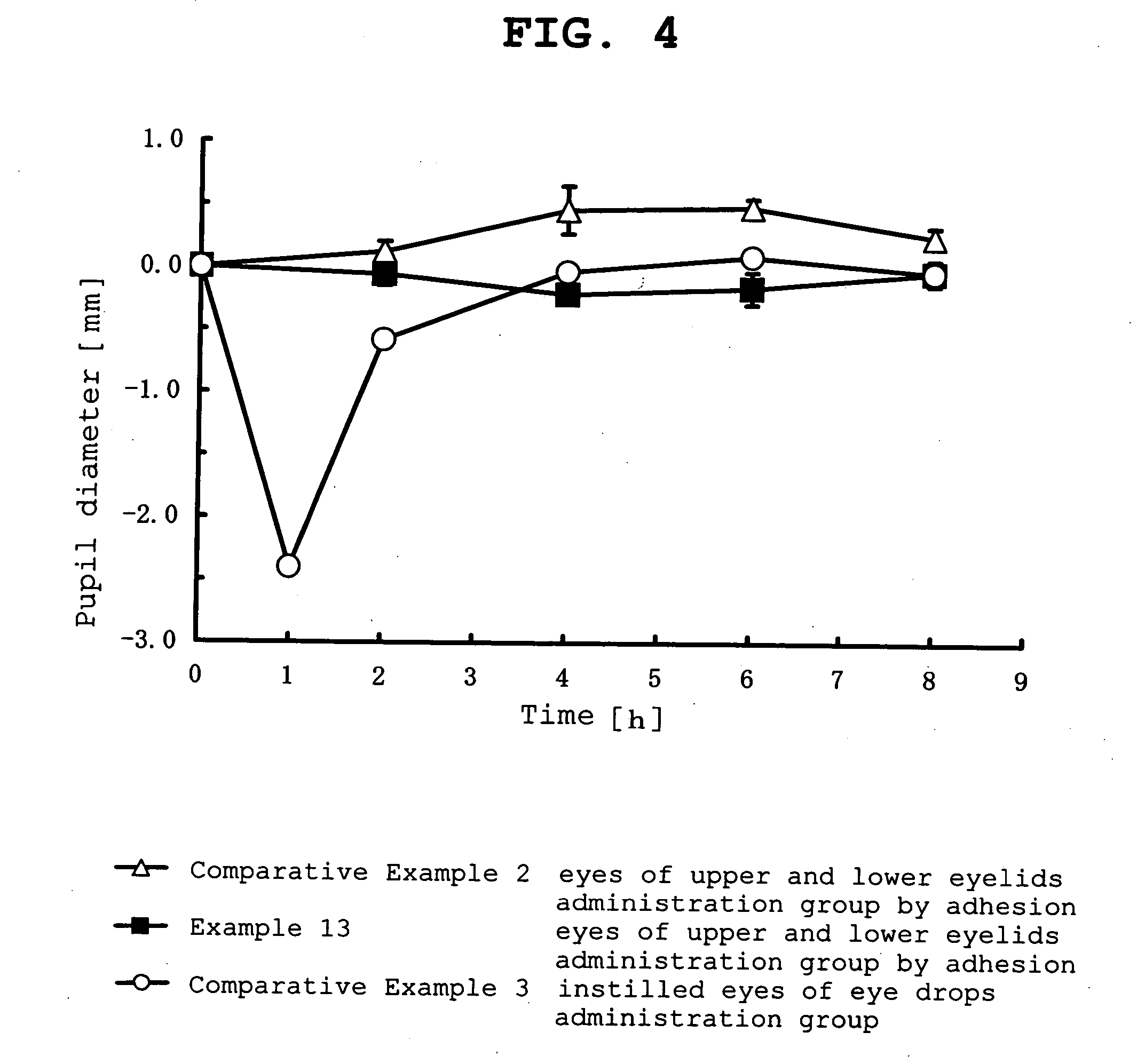
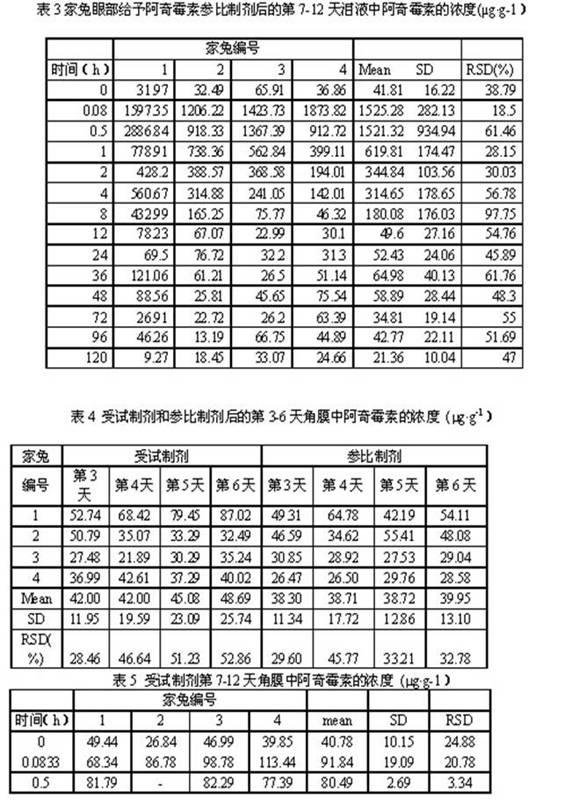
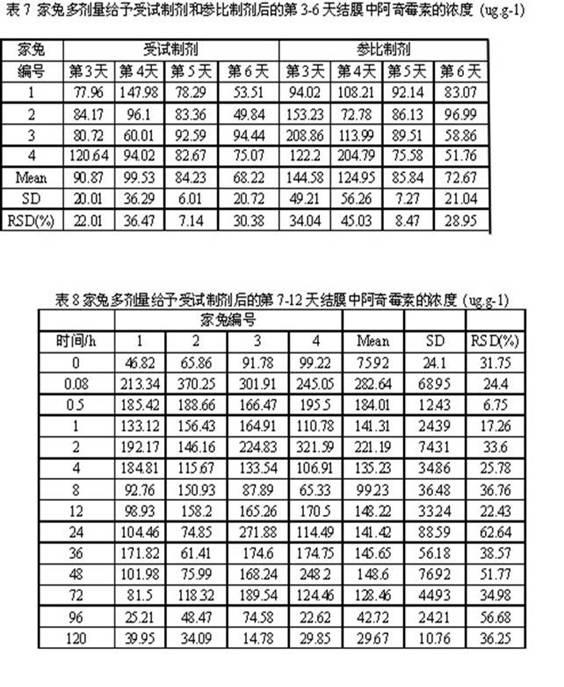
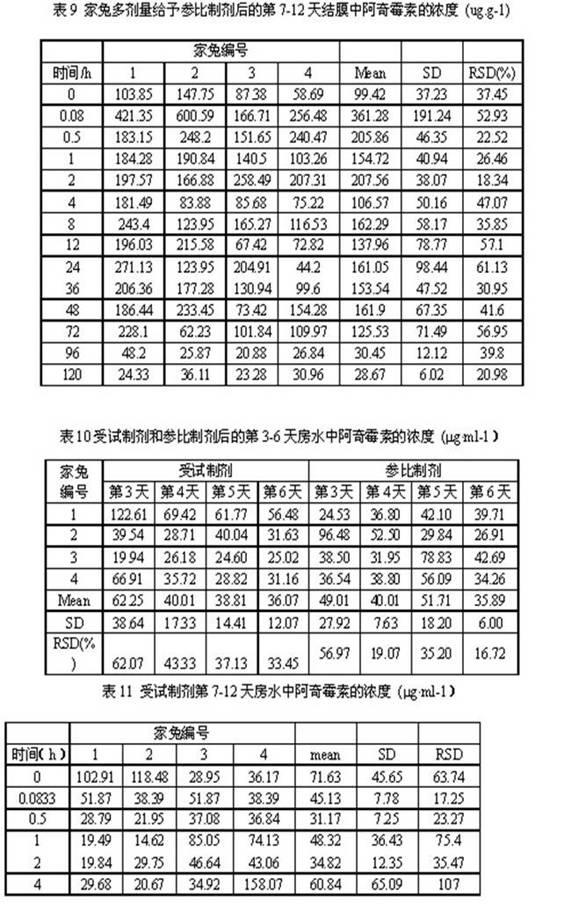
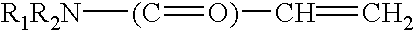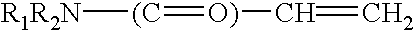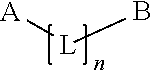Patents
Literature
267 results about "Ophthalmic preparations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ophthalmic preparations are agents especially designed to be applied to the eyes. The eye is extremely sensitive and is easily irritated if the composition of the ophthalmic preparation is not appropriate.
Ophthalmic formulations and uses thereof
Provided by the present invention are compositions or formulations suitable for application to a patient's eyes which utilizes a topical ophthalmically-acceptable formulation comprising a therapeutically-effective amount of an ophthalmically-active antimicrobial agent, and an ophthalmically-active anti-inflammatory or steroidal agent in combination with physiologic levels of serum electrolytes in an ophthalmic formulation for the treatment of changes in the normal eye condition. The invention also includes methods of treating patient's having an ophthalmic disease, injury or disorder, utilizing the compositions or formulations. Also provided are kits comprising the compositions or formulations and a means of applying the compositions or formulation to the patient's eyes.
Owner:BAUSCH & LOMB INC
Sustained release and long residing ophthalmic formulation and the process of preparing the same
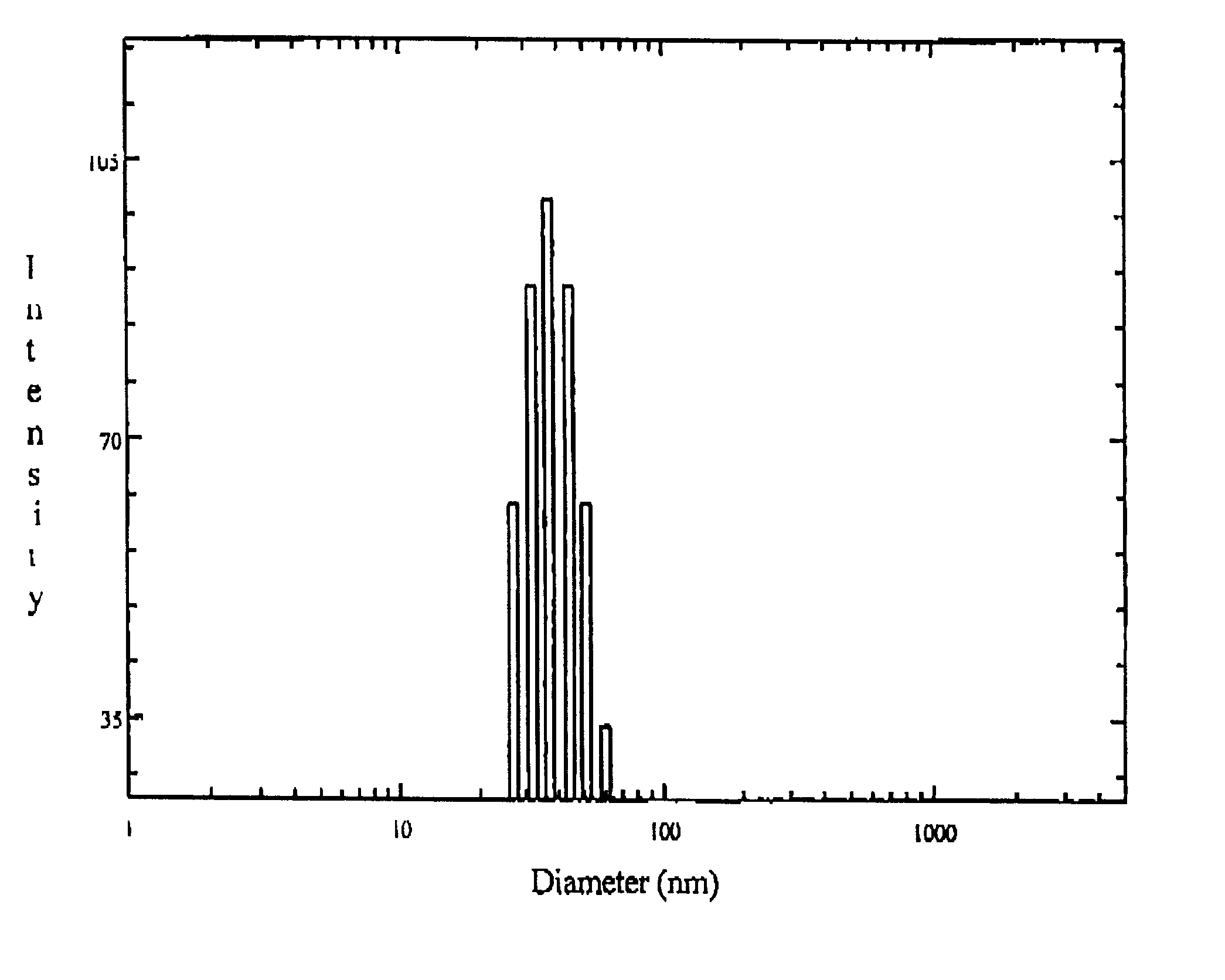
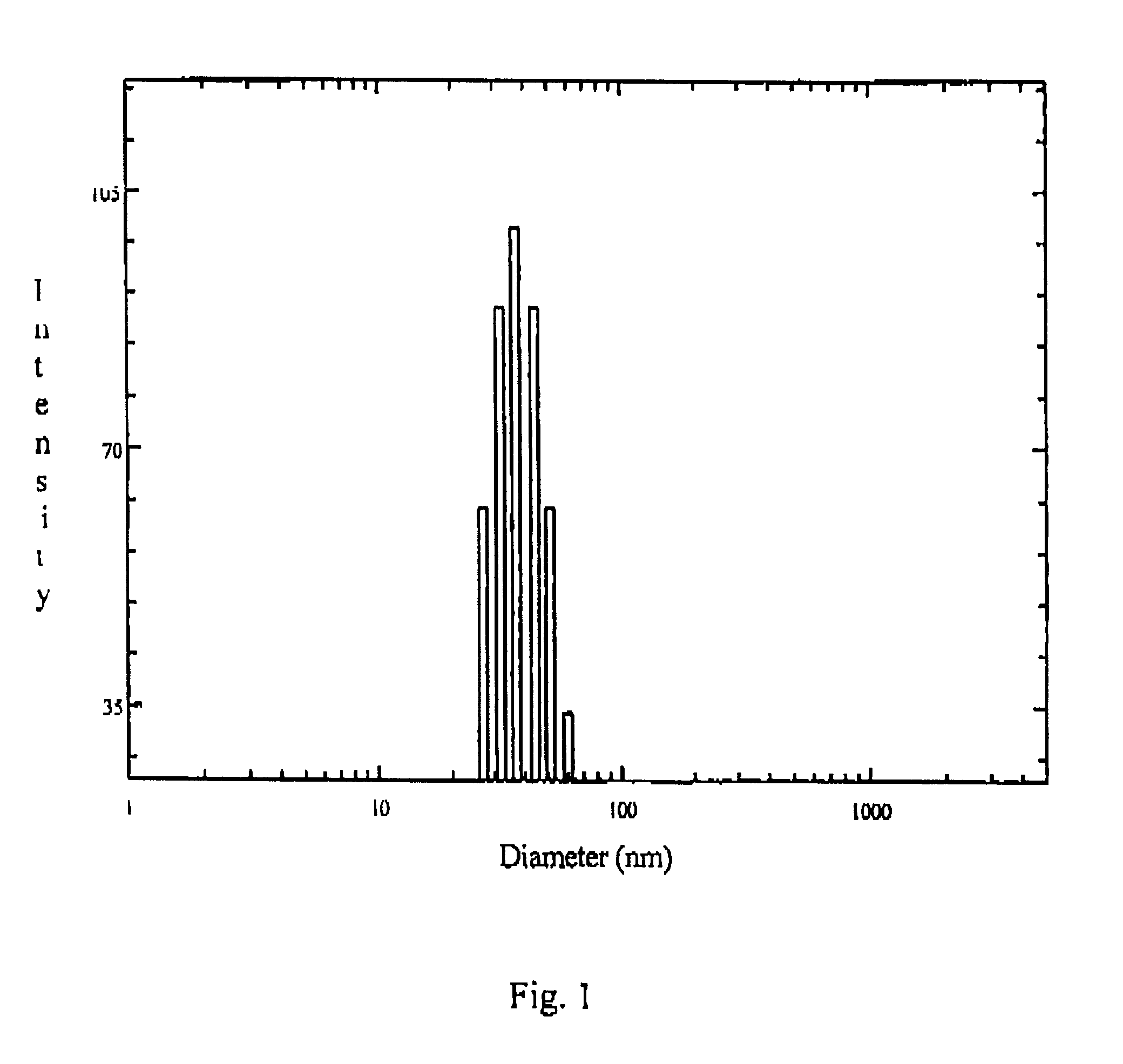
The present invention relates to sustained release and long residing opthalmic formulation having thermosensitivity, mucoadhesiveness, hydro gel properties and small particle size. The said formulation comprises micelle solution of random block co-polymer having a hydrophobic component and a hydrophillic component of general formula -(X+Y+Z-)m, and at least one hydrophobic drug with the block co-polymer solution. The invention also provides a process of preparing said formulation.
Owner:UNIVERSITY OF DELHI
Sustained release and long residing ophthalmic formulation and the process of preparing the same
The present invent relates to sustained release and long residing ophthalmic formulation having thermosensitivity, mucoadhesiveness, hydro gel properties and small particle size. The said formulation comprises of micelle solution of random block co-polymer having a hydrophobic component and a hydrophilic component of general formula -(X+Y+Z-)m, wherein m is an integer greater than 2 X is a monomer which will provide hydrogel formation properties of the co-polymer to reduce the irritability of the eye and is selected from vinyl group of compounds Y is a monomer which will provide thermosensitivity properties of the co-polymer having a general formula R1-R2N-(C=O)-CH=CH2, R1=a proton or CnH2n+1 in which n may have the value from 3 to 6 and R2=alkyl group having chain length of C3 to C6 Z is a monomer which will provide mucoadhesiveness and pH-sensitivity properties to the co-polymer and is selected from acrylate based monomers at least one hydrophobic drug with the said block co-polymer solution; The invention also provides a process of preparing said formulation.
Owner:UNIVERSITY OF DELHI
Preparations and methods for ameliorating or reducing presbyopia
This application relates to the use of one or more parasympathomimetic drugs in combination with one or more alpha agonists to create optically beneficial miosis to, for example, temporarily treat presbyopia. The invention provides a pharmaceutical preparation comprising a therapeutically effective amount of one or more parasympathomimetic drugs or cholinesterase inhibitors, or a pharmaceutically acceptable salt thereof, in combination with one or more alpha agonists or antagonists, or a pharmaceutically acceptable salt thereof. The invention further provides for a method for treating, ameliorating or reducing presbyopia of a patient having an eye, comprising administering to said eye a pharmaceutically effective amount of the ophthalmic preparation.
Owner:VISUS THERAPEUTICS INC
Ophthalmic formulations, methods of manufacture, and methods of using same
InactiveUS20100311688A1Increased tear film stabilityIncreasing tear film break up timeOrganic active ingredientsBiocideOphthalmologyOphthalmic preparations
The present invention provides compositions for treating and / or preventing signs and symptoms associated with dry eye and / or ocular irritation, and methods of use thereof. Such compositions are provided in novel ophthalmic formulations that are comfortable upon instillation in the eye. Methods of manufacture are also provided.
Owner:ACIEX THERAPEUTICS INC
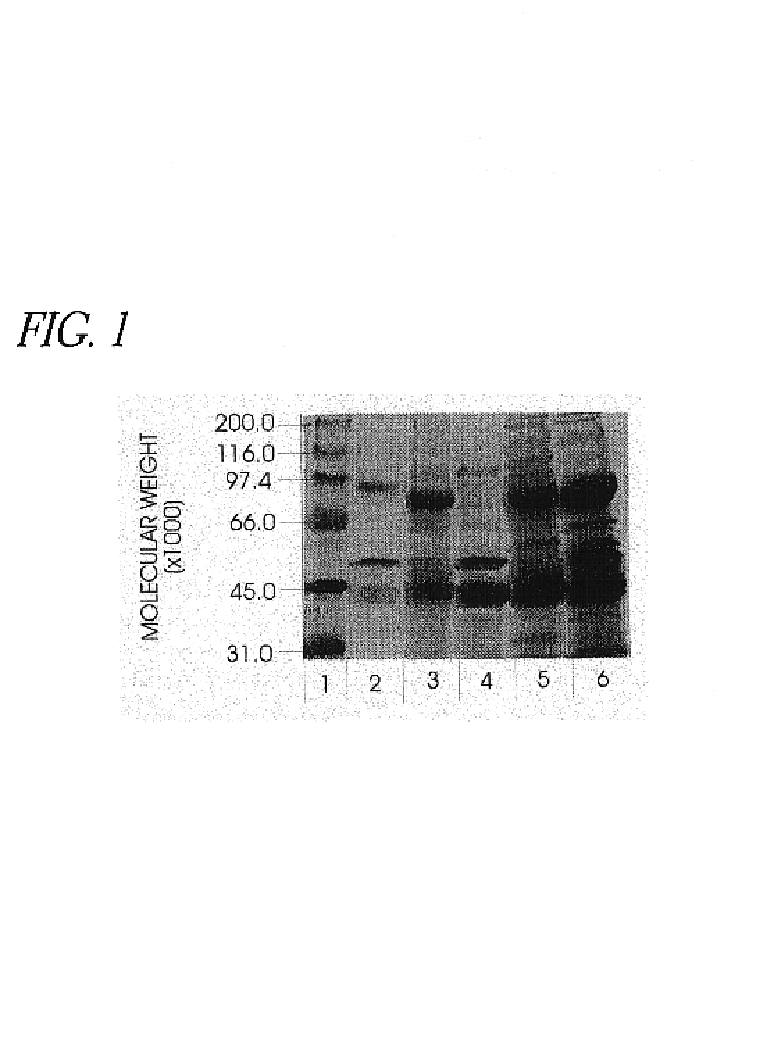
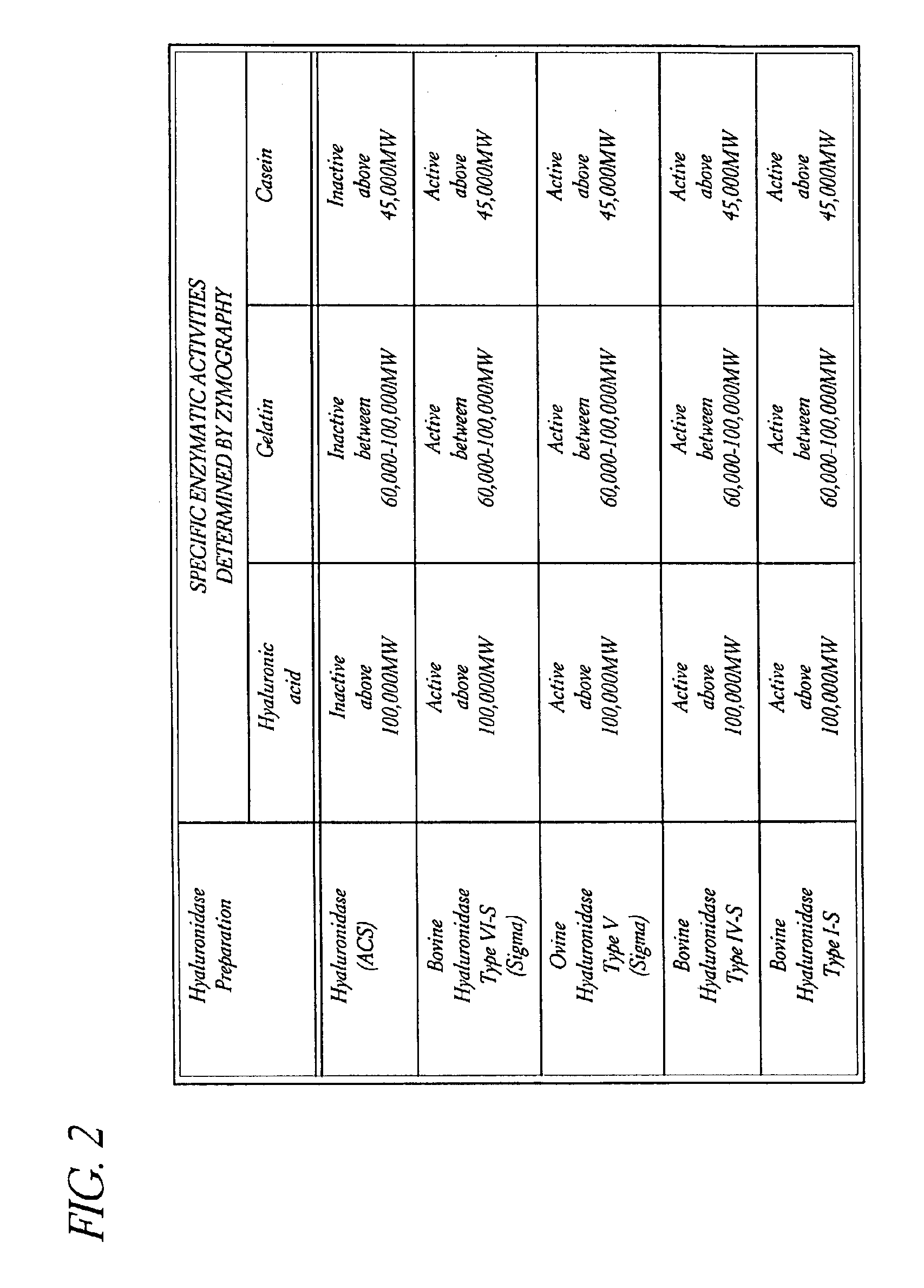
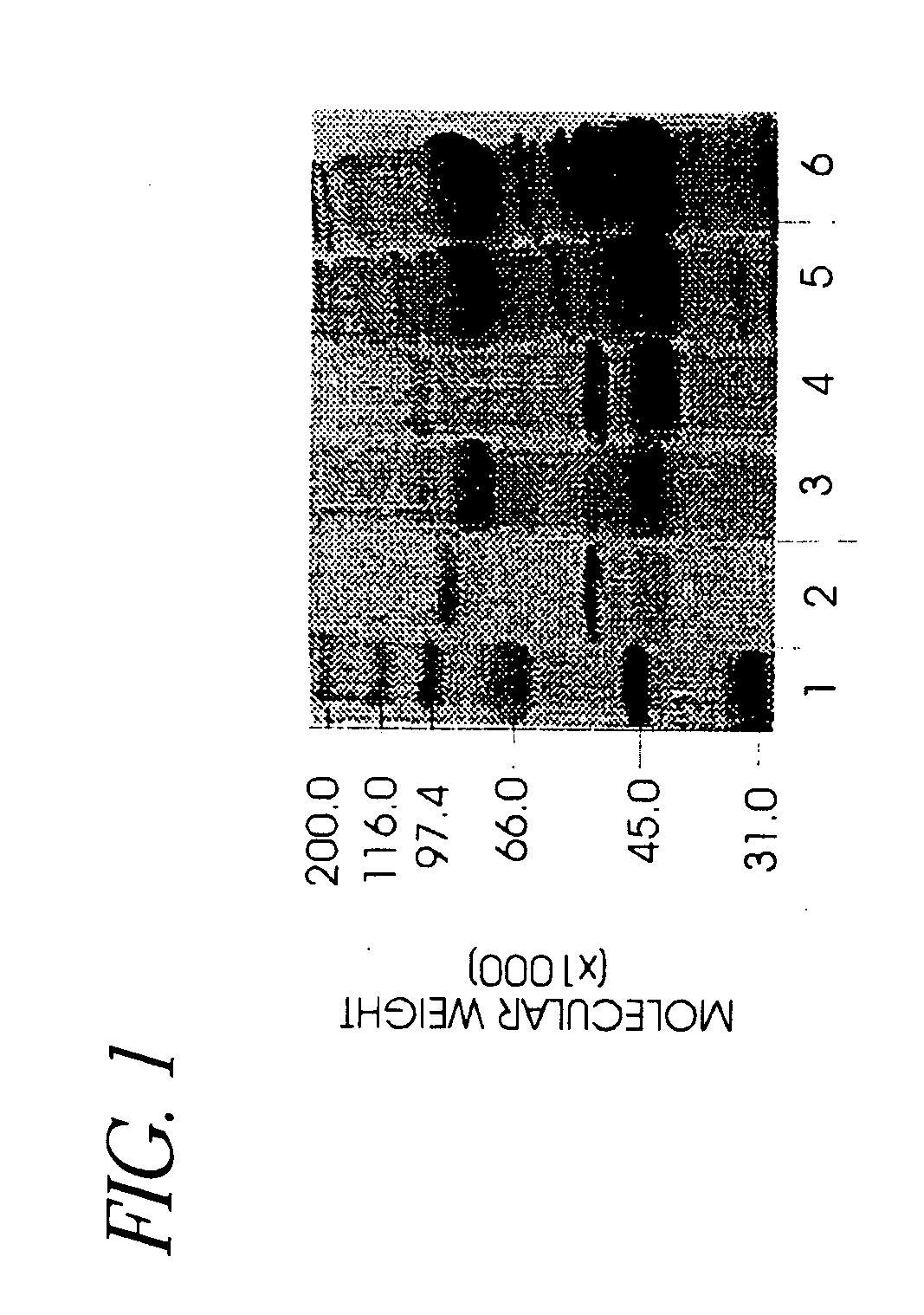
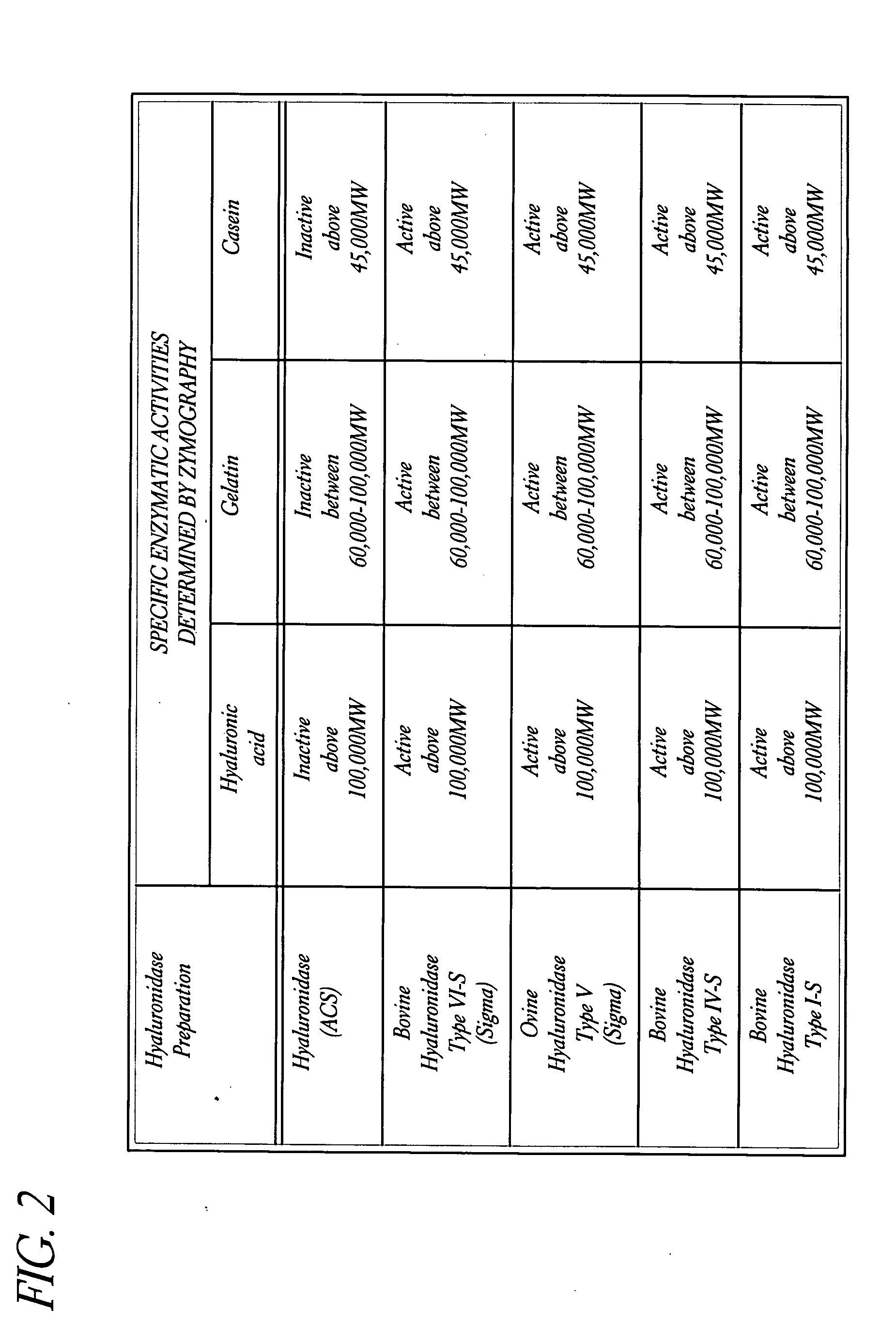
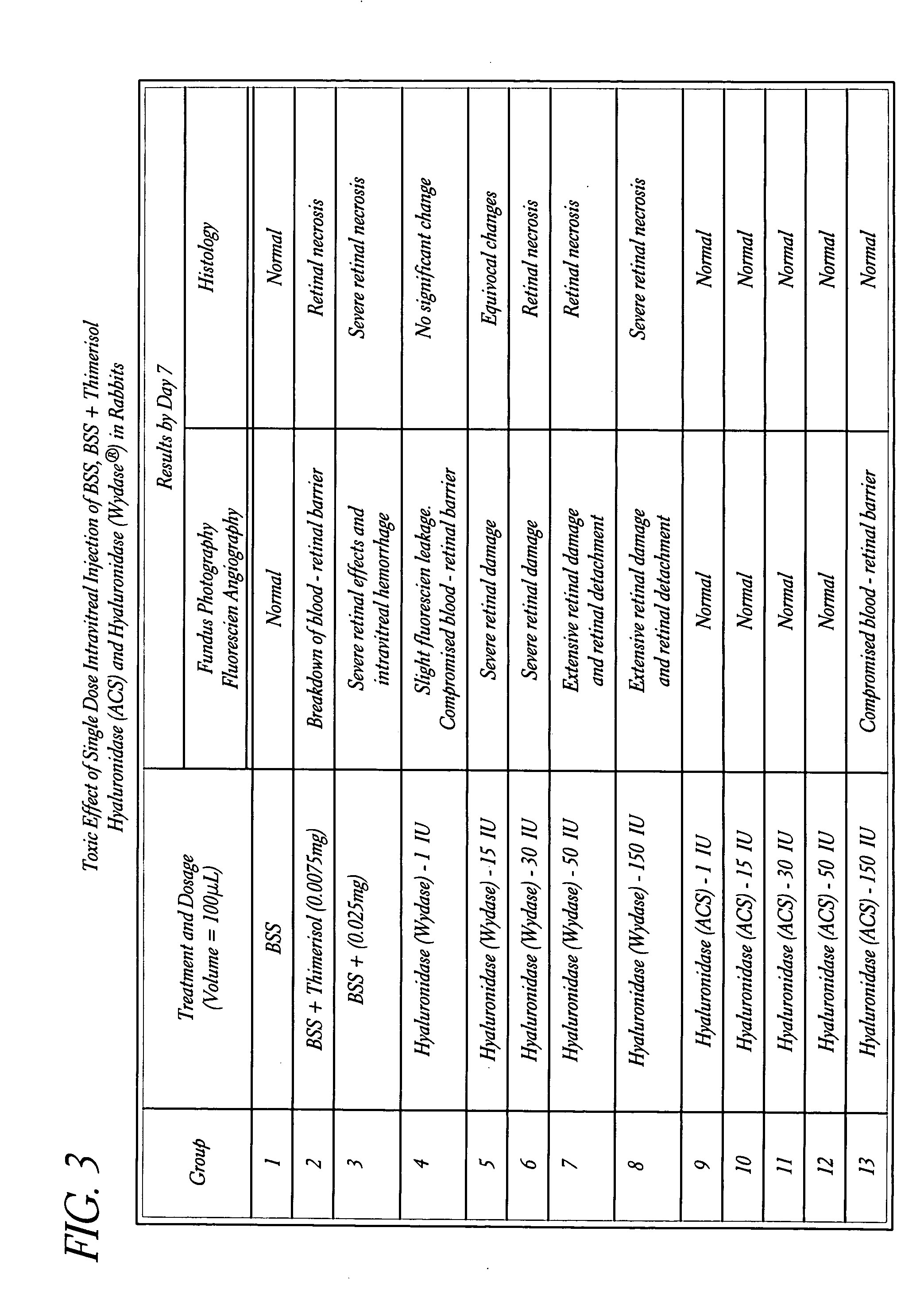
Use of hyaluronidase in the manufacture of an ophthalmic preparation for liquefying vitreous humor in the treatment of eye disorders
InactiveUS6863886B2Improve clearance rateIncreases rate of liquid exchangeBiocidePeptide/protein ingredientsDiseaseHyaluronidase
An enzymatic method is provided for treating ophthalmic disorders of the mammalian eye. Prevention of neovascularization and the increased rate of clearance from the vitreous of materials toxic to retina is accomplished by administering an amount of hyaluronidase effective to liquefy the vitreous humor of the treated eye without causing toxic damage to the eye. Liquefaction of the vitreous humor increases the rate of liquid exchange from the vitreal chamber. This increase in exchange removes those materials and conditions whose presence causes ophthalmological and retinal damage.
Owner:BAUSCH & LOMB PHARMA HLDG
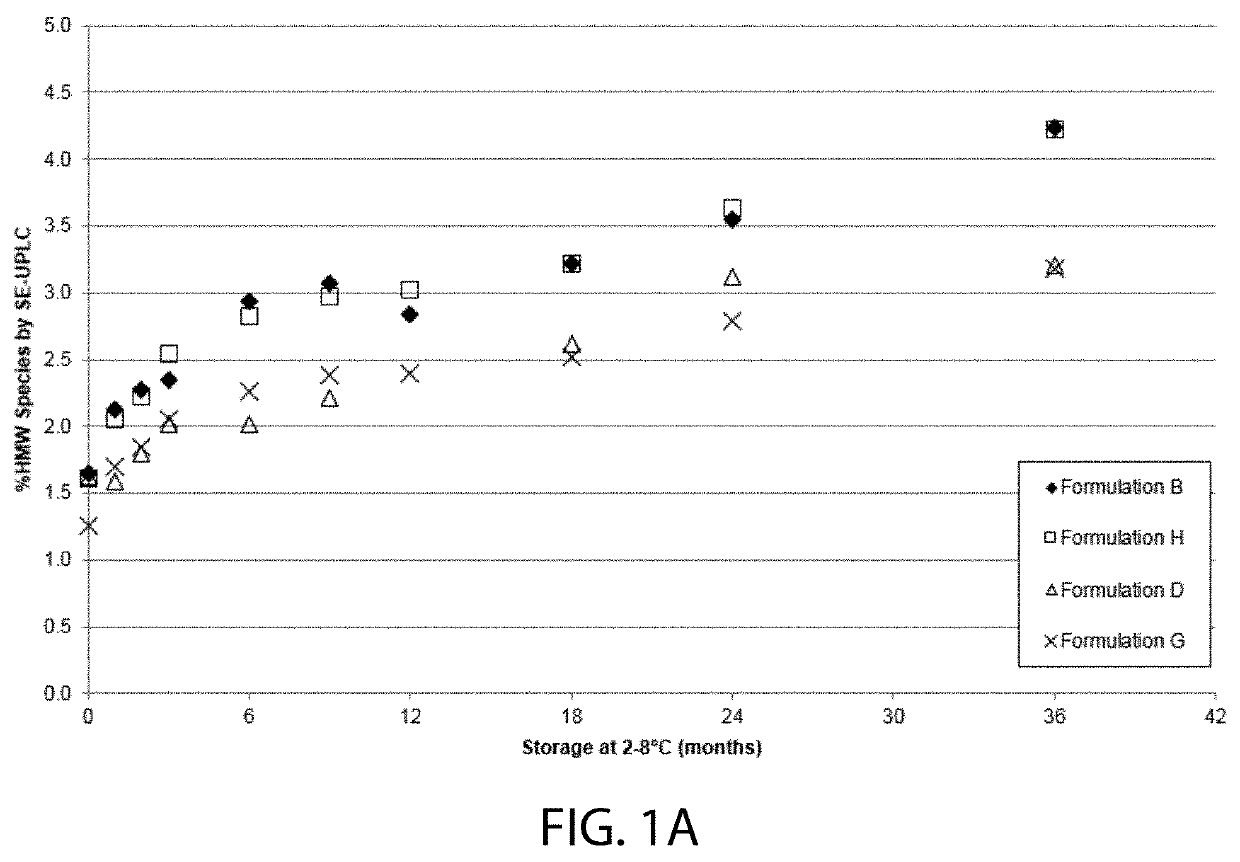
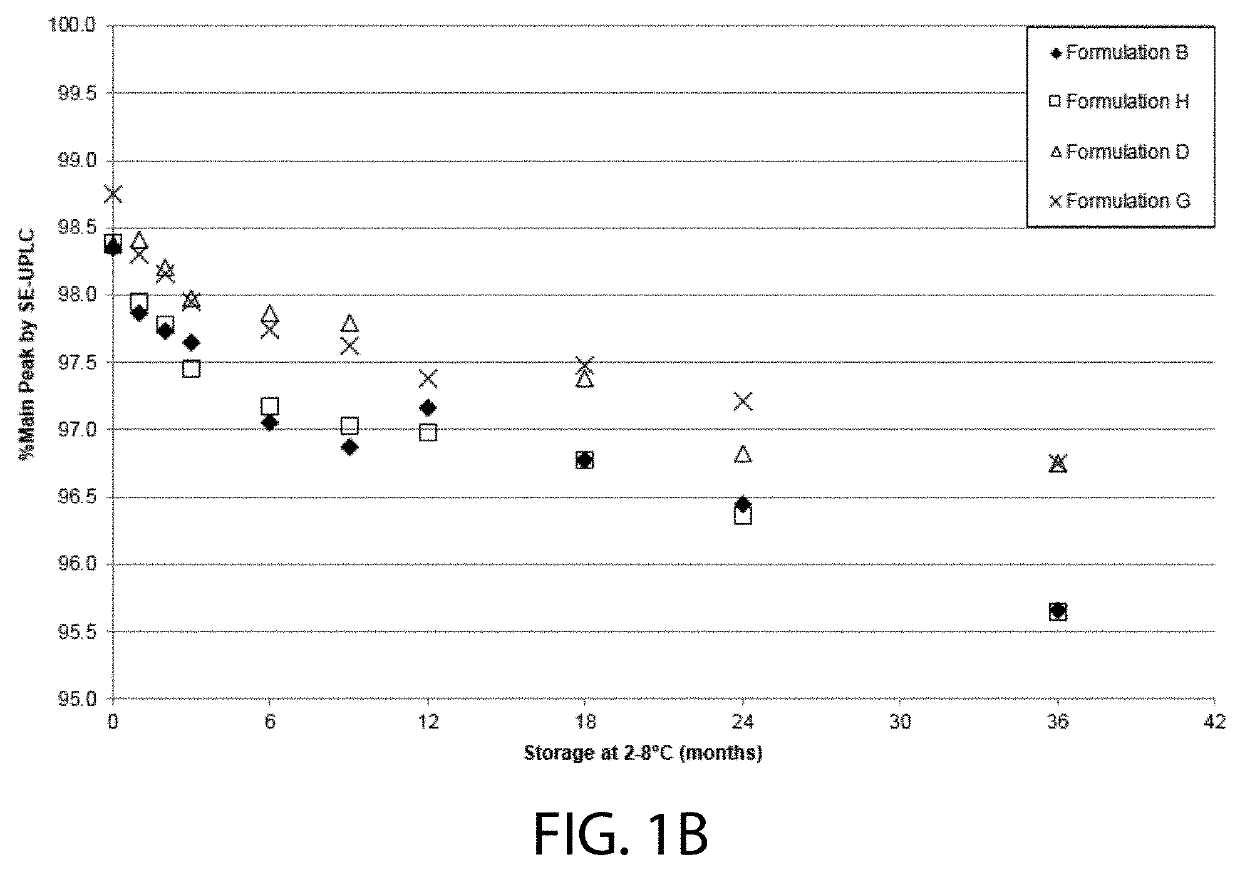
High concentration VEGF receptor fusion protein containing formulations
ActiveUS20190343918A1Long-term manufacturing and storage stabilityReduce dosageSenses disorderPeptide/protein ingredientsHigh concentrationVEGF receptors
The present invention provides ophthalmic formulations having high concentrations of vascular endothelial growth factor (VEGF) receptor fusion protein and high stability during storage. Methods for treating angiogenic eye disorders using the high concentration formulations are also provided.
Owner:REGENERON PHARM INC
Ophthalmic formulations and processes for their preparation
InactiveUS20130065888A1Suspension stabilityUniform particle size distributionSenses disorderPharmaceutical delivery mechanismPharmacologyOphthalmic drugs
The invention relates to a process for preparing ophthalmic formulations and to formulations containing a suspension of an ophthalmic drug in an aqueous vehicle. The invention further relates to the production of stable ophthalmic formulations that have a minimal propensity to form drug aggregates.
Owner:TEVA PHARM USA INC
Contact lens and eye drop rewetter compositions and methods
Stable ophthalmic formulations comprising hyaluronic acid (sodium hyaluronate) as the primary active demulcent ingredient, stabilized oxy-chloro complex (available commercially as OcuPure(tm) from Advanced Medical Optics, Purite® from Allergan, and Purogene from Biocide) for preservative efficacy, balanced salts mimicking the tear film, and sodium borate as a buffer are disclosed. In one embodiment, preferred stable formulations may be used in the human eye with or without contact lenses. In another embodiment preferred formulations may also be used as a storage and conditioning solution for contact lenses following disinfection.
Owner:ADVANCED MEDICAL OPTICS
Pharmaceutical composition for use in medical and veterinary ophthalmology
ActiveUS20120094962A1Prevents irreversible absorptionPrevents reversible and irreversible absorptionAntibacterial agentsBiocideDiseaseAntioxidant
The invention relates to the manufacturing and use of pharmaceutical compositions of medicines (ophthalmic preparations) comprising a mitochondria-addressed antioxidant and a set of auxiliary substances providing effective treatment for ophtalmological diseases in humans and animals.
Owner:ESSEX BIO INVESTMENT LTD
Self-preserved antibacterial nasal, inhalable, and topical ophthalmic preparations and medications
InactiveUS20050080043A1Avoid tissue damageImprove drug deliveryBiocideOrganic active ingredientsDermatologyOphthalmic preparations
Self-preserved nasal, inhalable and topical ophthalmic preparations and medications which destroy, inhibit or therapeutically significantly limit microbial growth within said preparations or medications. The nasal, inhalable, and topical ophthalmic preparations and medications have been found to be stable without buffer and maintain a stable pH at pH 3.5 or lower. They are well tolerated by patients.
Owner:SHAHINIAN LEE JR
Formulations and methods for treating dry eye
UndeterminedUS20070299124A1Relieve ocular discomfortImprove integrityBiocideSenses disorderOphthalmic preparationsSigns and symptoms
The present invention provides compositions for treating and / or preventing signs and symptoms associated with dry eye and / or ocular irritation, and methods of use thereof. Such compositions are provided in novel ophthalmic formulations that are comfortable upon instillation in the eye.
Owner:OPHTHALMIC RES ASSOCS +1
Preparations and Methods for Ameliorating or Reducing Presbyopia
ActiveUS20110152274A1Ameliorating and reducing presbyopiaEfficient productionBiocideSenses disorderCholinesteraseAgonist
This application relates to the use of one or more parasympathomimetic drugs in combination with one or more alpha agonists to create optically beneficial miosis to, for example, temporarily treat presbyopia. The invention provides a pharmaceutical preparation comprising a therapeutically effective amount of one or more parasympathomimetic drugs or cholinesterase inhibitors, or a pharmaceutically acceptable salt thereof, in combination with one or more alpha agonists or antagonists, or a pharmaceutically acceptable salt thereof. The invention further provides for a method for treating, ameliorating or reducing presbyopia of a patient having an eye, comprising administering to said eye a pharmaceutically effective amount of the ophthalmic preparation.
Owner:VISUS THERAPEUTICS INC
Contact lens and eye drop rewetter compositions and methods
Stable ophthalmic formulations comprising hyaluronic acid (sodium hyaluronate) as the primary active demulcent ingredient, stabilized oxy-chloro complex (available commercially as OcuPure™ from Advanced Medical Optics, Purite® from Allergan, and Purogene from Biocide) for preservative efficacy, balanced salts mimicking the tear film, and sodium borate as a buffer are disclosed. In one embodiment, preferred stable formulations may be used in the human eye with or without contact lenses. In another embodiment preferred formulations may also be used as a storage and conditioning solution for contact lenses following disinfection.
Owner:ADVANCED MEDICAL OPTICS
Pirenzepine ophthalmic gel
Owner:VF PHARMA
Preparations and Methods for Ameliorating or Reducing Presbyopia
InactiveUS20100298335A1Ameliorating and reducing presbyopiaEfficient productionBiocideSenses disorderCholinesteraseAgonist
Owner:KAUFMAN HERBERT E
Ophthalmic composition
InactiveUS20130053374A1Good water solubilitySafely employedBiocideSenses disorderCarteololBeta blocker
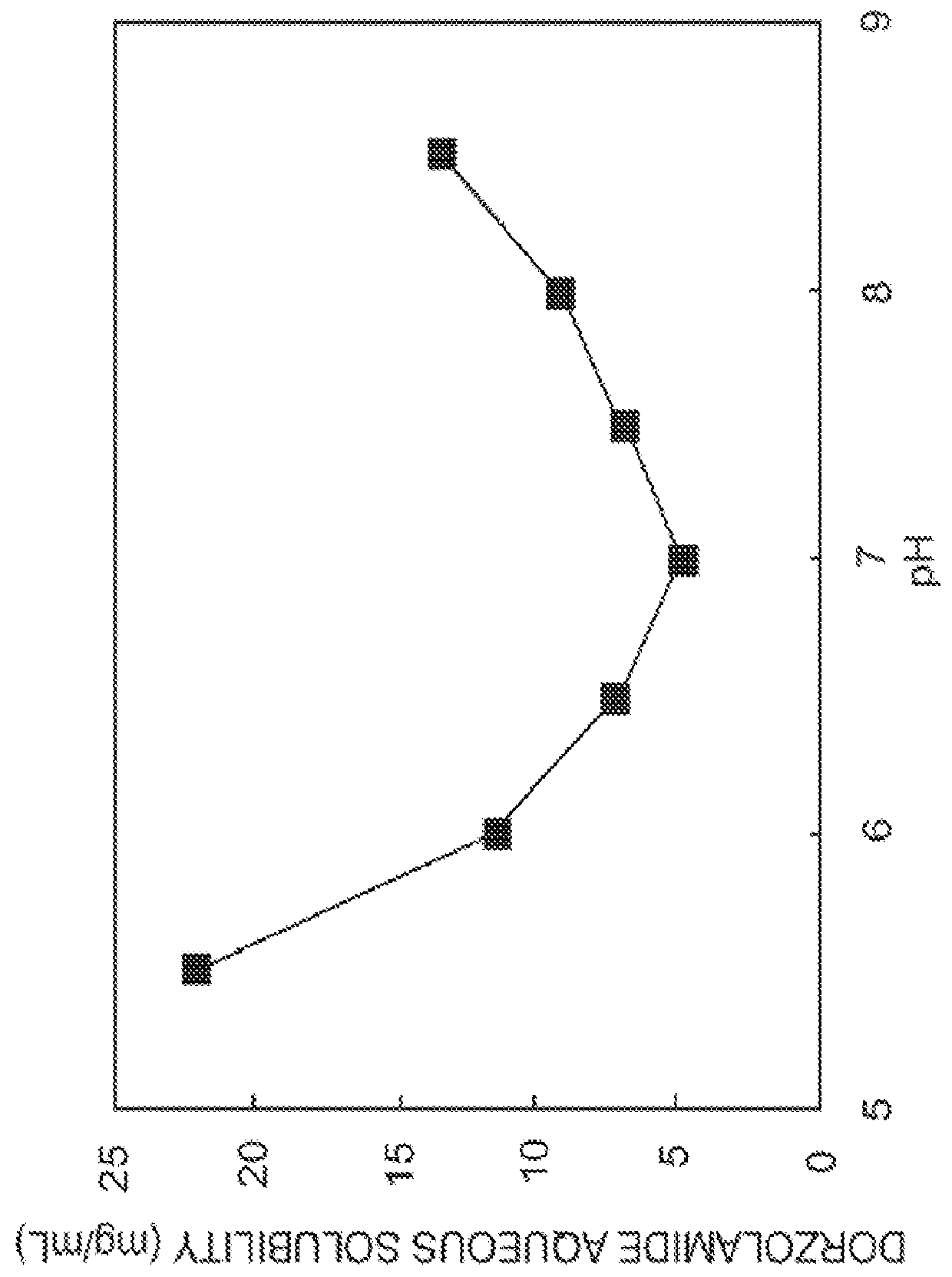
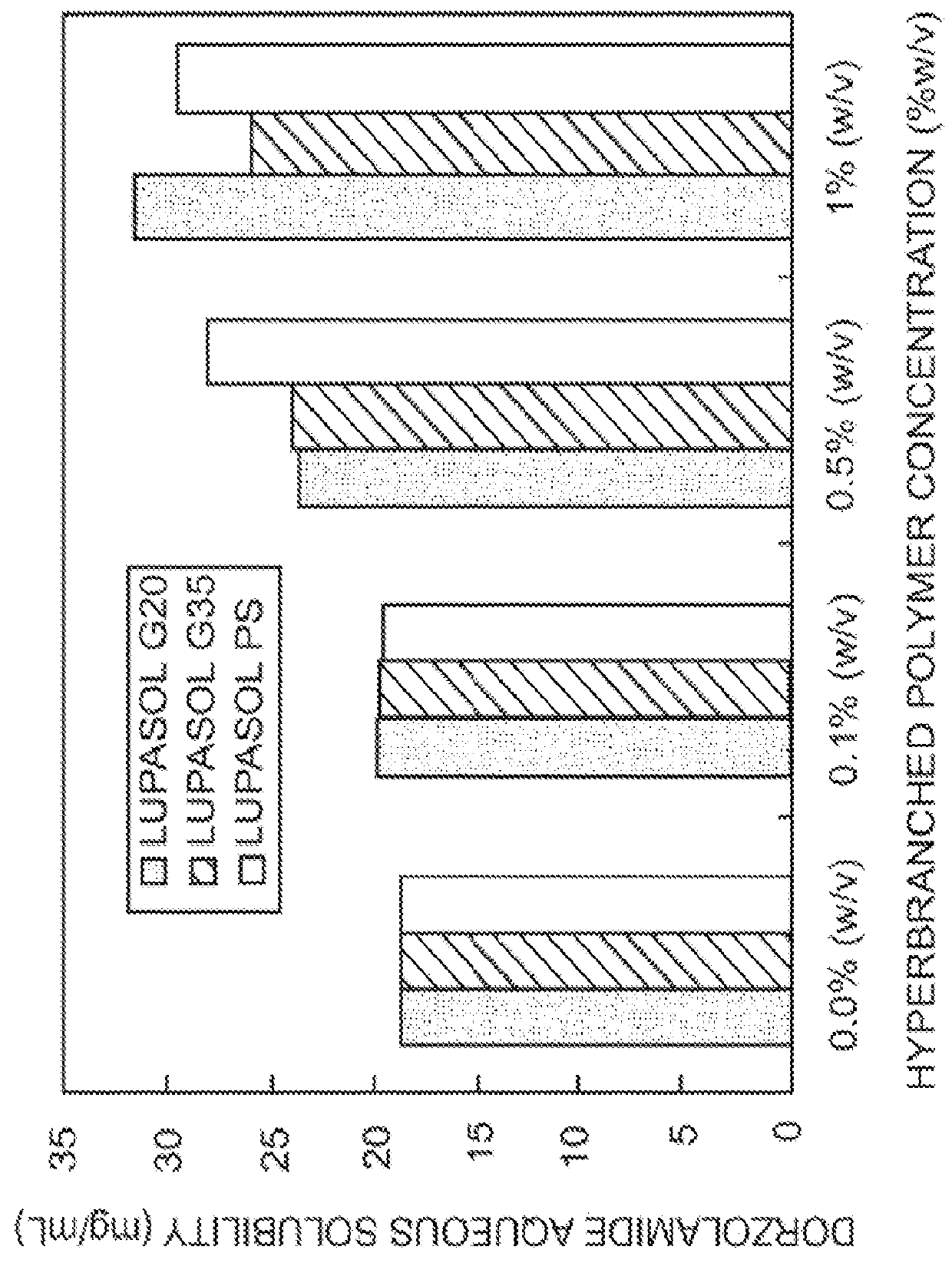
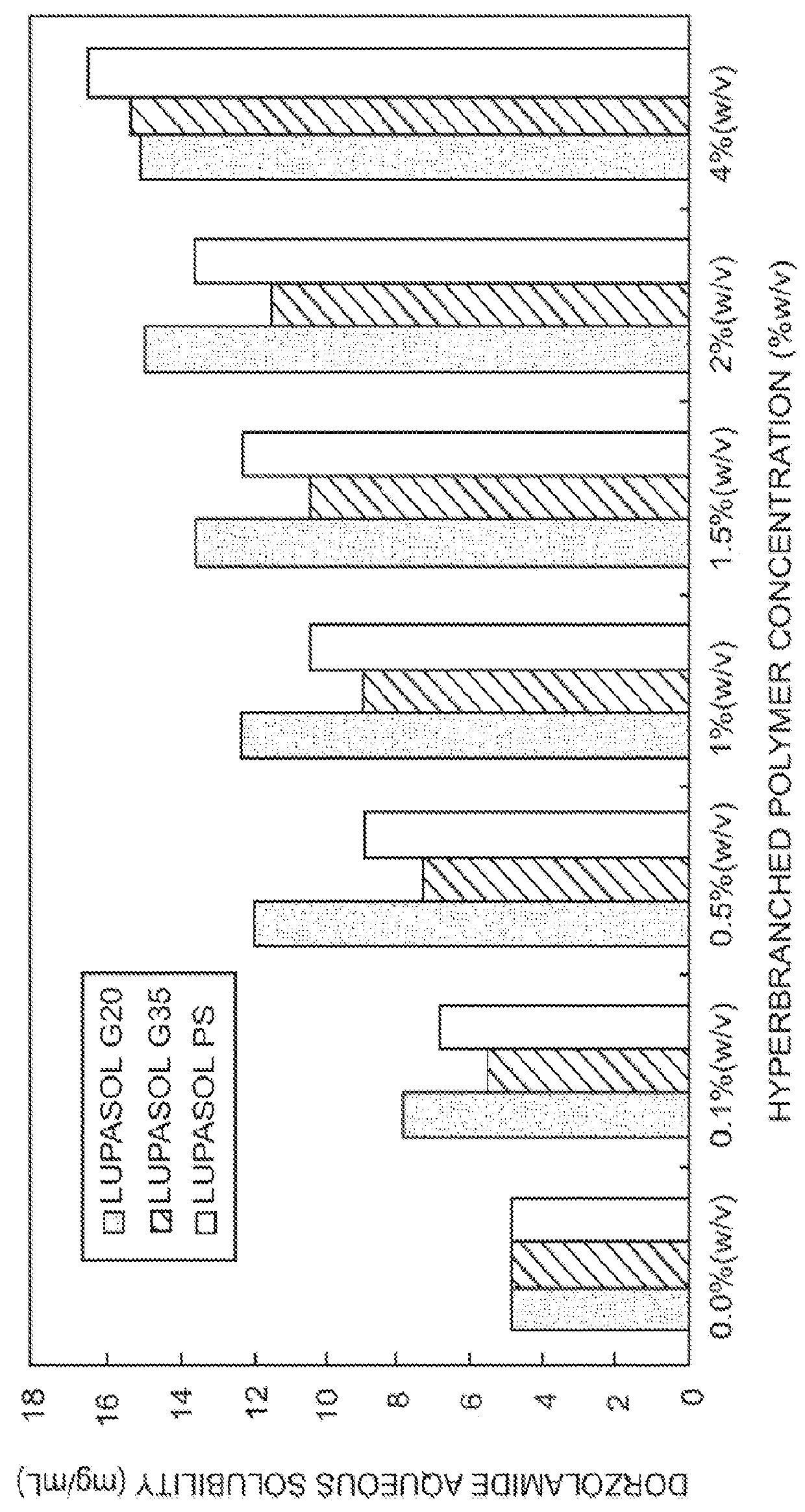
The present invention provides an ophthalmic composition comprising a hyperbranched polyester. The ophthalmic compositions may also comprise carbonic anhydrase inhibitors, wherein the hyperbranched polyester increases the aqueous solubility of the carbonic anhydrase inhibitor, and increases corneal permeation of the active agent. The ophthalmic compositions may also comprise non-ionic surfactants, such as PEG, Polysorbate, HPMC or HEC, and beta-blockers, such as Carteolol, Levobunolol, Betaxolol, Metipranolol, Timolol or Propranolol. The concentration of the hyperbranched polyester in the ophthalmic formulation should be less than or equal to 4% (w / v) in order to avoid any cytotoxic effects on human corneal cells and thus the eye irritation.
Owner:SENJU USA
Compounding use of sodium hyaluronate for eye preparation
InactiveCN1488404AImprove stabilityImprove water holding capacitySenses disorderPharmaceutical non-active ingredientsGlycerolWater soluble
The invention refers to the compound applied technique of two components of adjunct medical material-sodium hyaluronate and glycerin, in ophthalmic preparation. Its main technique: the compound base material is added to the ophthalmic preparation to make into stable water-soluble transparent eye drops; sodium hyaluronate is 0.05%-0.5% (g / ml) and glycerin 0.1%-2.5% (g / ml), and the mixed proportion: the former : the latter=1:1-1:4, and the optimal project: the former 0.05%-0.25%, the latter 0.1%-0.5%, and the mixed proportion: 1:2-1:4, and they can be added in multiple eye drops.
Owner:刘继东
2-pyrrolidone derivatives for preservation of ophthalmic, otic and nasal compositions
2-Pyrrolidone derivatives are provided as preservatives for topical formulations, particularly for ophthalmic, otic, and nasal formulations. N-octyl-2-pyrrolidone, without any other preservative or preservative aid in an isotonic pH 7.5 formulation, provided sufficient preservation to comply with the European Pharmacopoeia 5.0 standards in addition to the United States Pharmacopoeia 24 standards for parenteral and ophthalmic preparations. On the other hand, N-dodecyl-2-pyrrolidone requires a second anti-microbial agent to achieve such preservation standards.
Owner:ALCON RES LTD
Cranberry extract eye ophthalmic preparation and preparation method and uses thereof
InactiveCN103860625AGood effectMeet the strong demand for eye protectionSenses disorderAnthropod material medical ingredientsLingonberry extractDisease
The invention relates to a cranberry extract eye ophthalmic preparation and a preparation method and uses thereof, and belongs to the technical field of eye ophthalmic preparations. The technical problem to be solved is to provide a cranberry extract eye ophthalmic preparation. The cranberry extract eye ophthalmic preparation comprises an active ingredient of cranberry extract. The cranberry extract eye ophthalmic preparation can effectively treat, prevent or alleviate cataract, glaucoma, vitreous opacity, macular degeneration, retinopathy, optic atrophy, keratitis, epiphora induced by wind, presbyopia, myopia, visual fatigue, blurred vision, conjunctival burn, conjunctivitis, corneal ulcer, palpebral ecgema, floaters or trachoma and other eye diseases. A novel preparation is provided for treating cataract, glaucoma, vitreous opacity, macular degeneration, retinopathy, optic atrophy, keratitis, epiphora induced by wind, presbyopia, myopia, visual fatigue, blurred vision, conjunctival burn, conjunctivitis, corneal ulcer, palpebral ecgema, floaters or trachoma and other eye diseases, and meets the strong demand of people for loving and protecting eyes.
Owner:CHENGDU JIANKABIN SCI & TECH
Use of hyaluronidase in the manufacture of an ophthalmic preparation for liquefying vitreous humor in the treatment of eye disorders
InactiveUS20050069531A1Improve clearance rateIncreases rate of liquid exchangePeptide/protein ingredientsDrug compositionsDiseaseHyaluronidase
An enzymatic method is provided for treating ophthalmic disorders of the mammalian eye. Prevention of neovascularization and the increased rate of clearance from the vitreous of materials toxic to retina is accomplished by administering an amount of hyaluronidase effective to liquefy the vitreous humor of the treated eye without causing toxic damage to the eye. Liquefaction of the vitreous humor increases the rate of liquid exchange from the vitreal chamber. This increase in exchange removes those materials and conditions whose presence causes ophthalmological and retinal damage.
Owner:KARAGEOZIAN HAMPAR +5
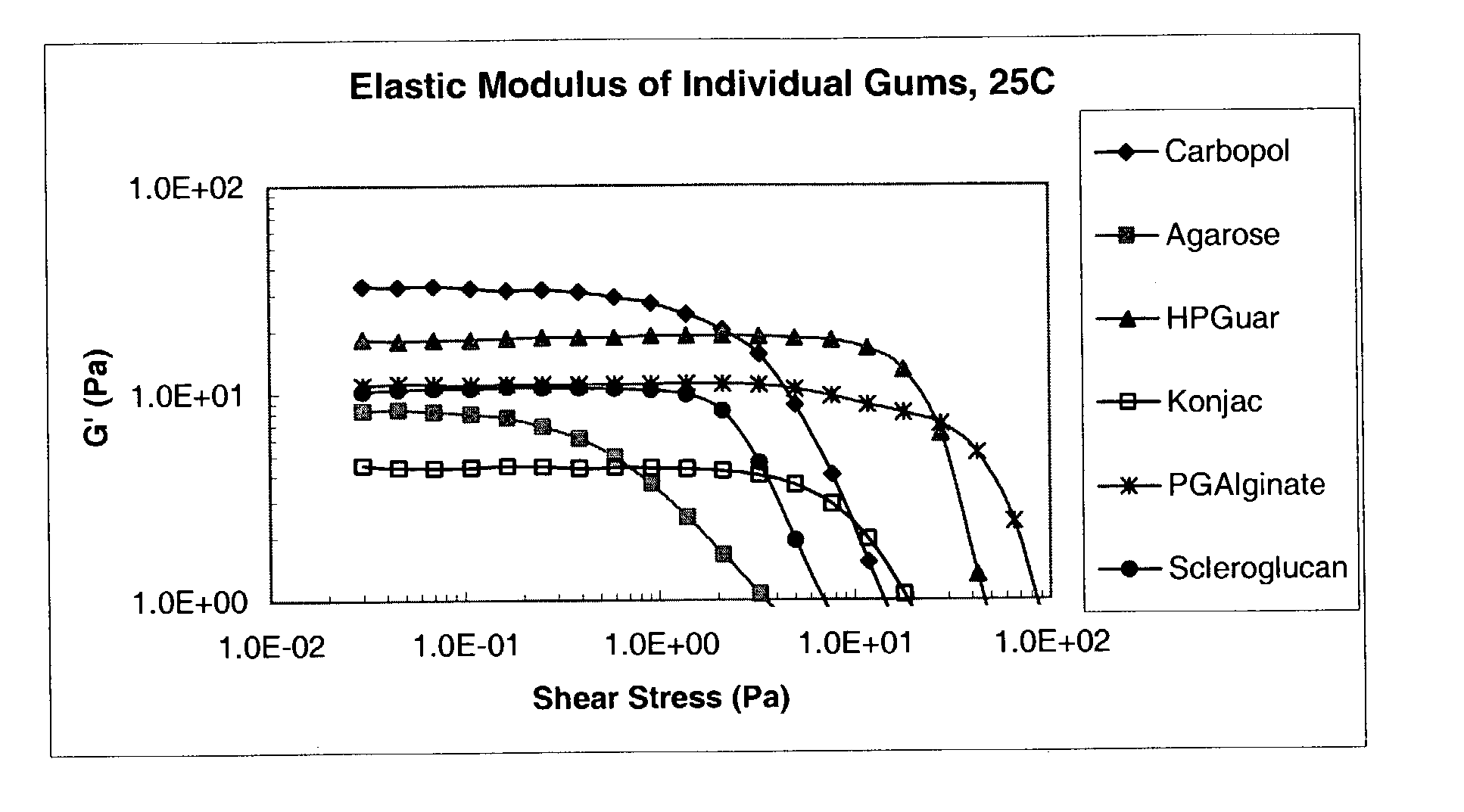
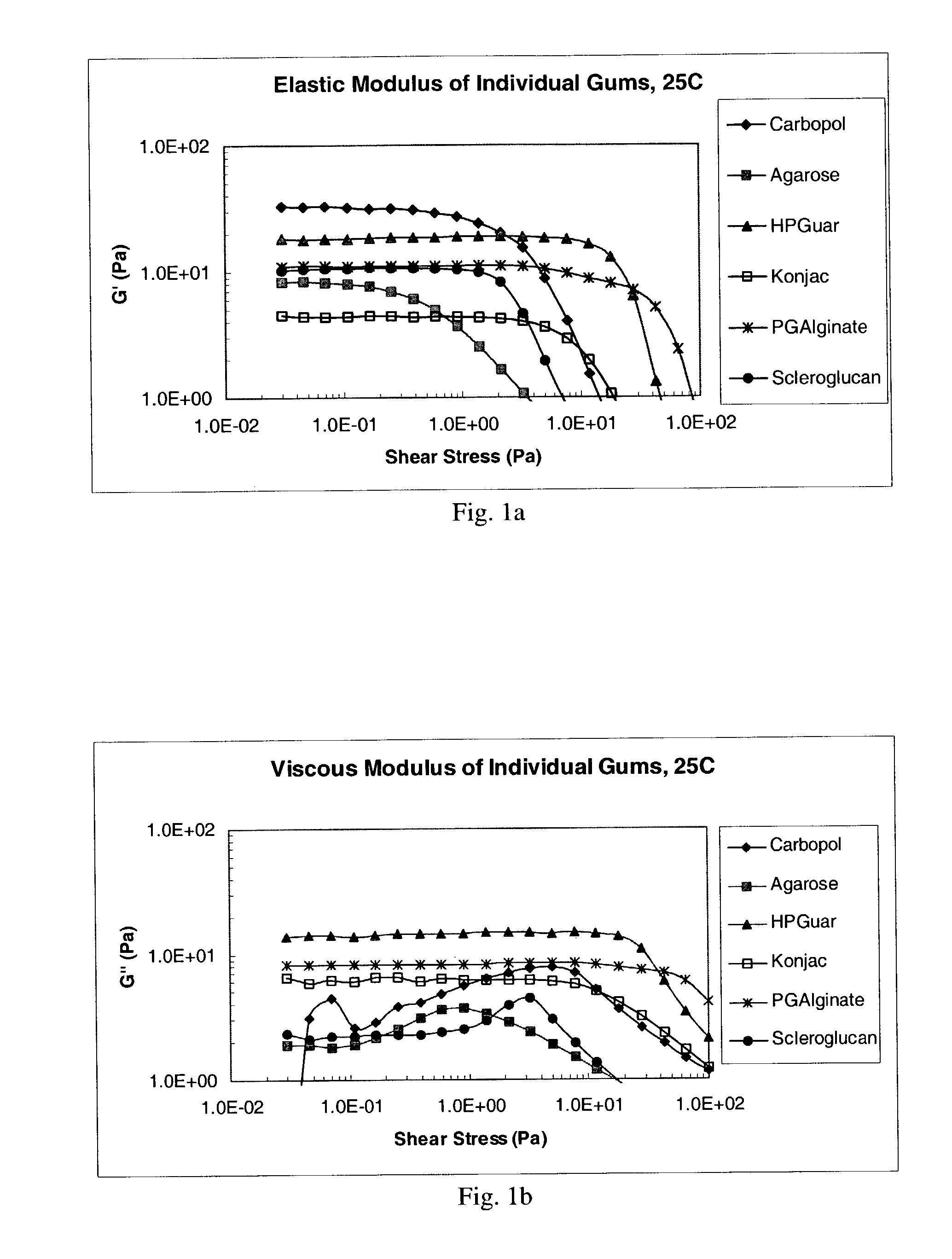
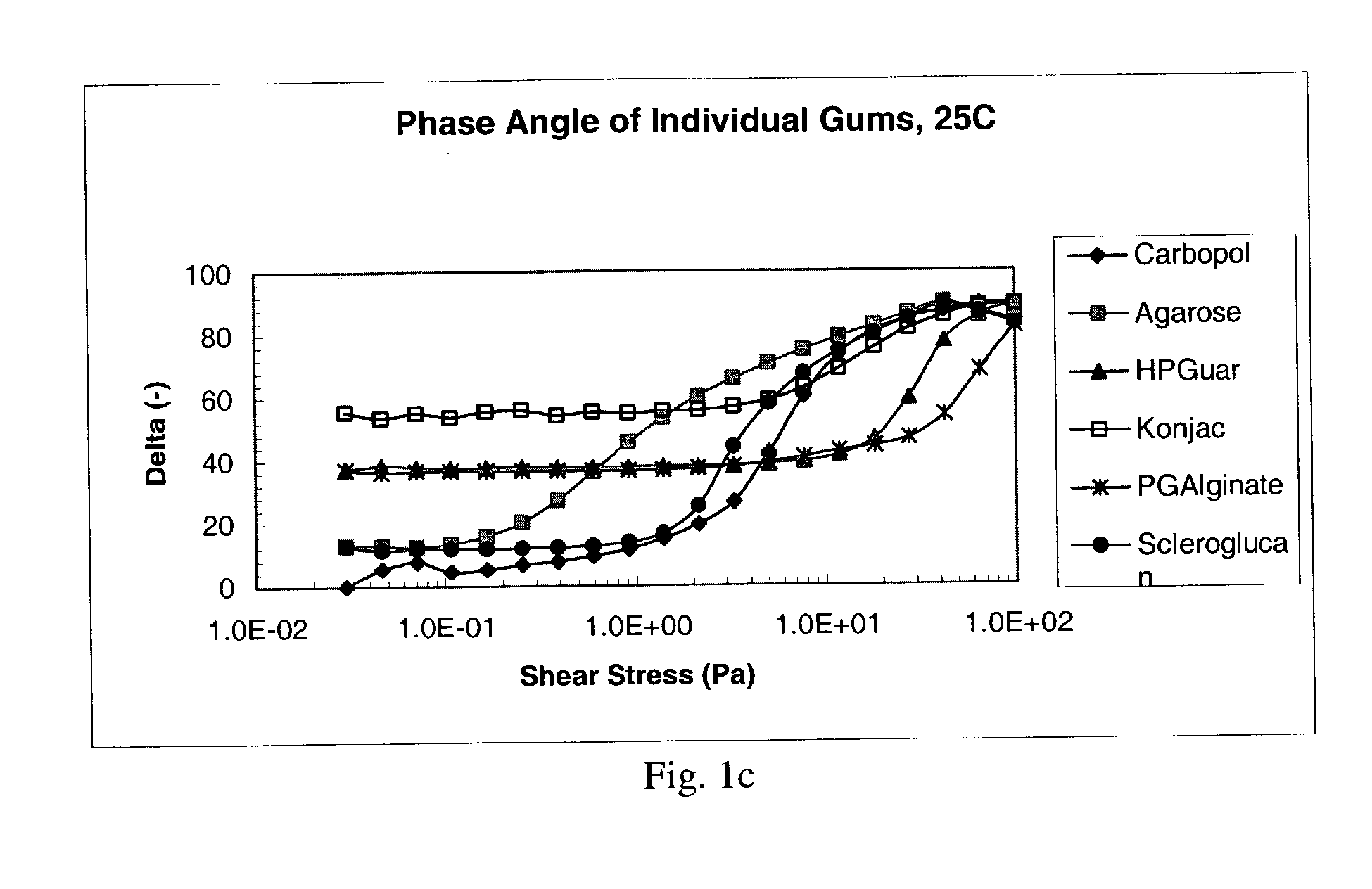
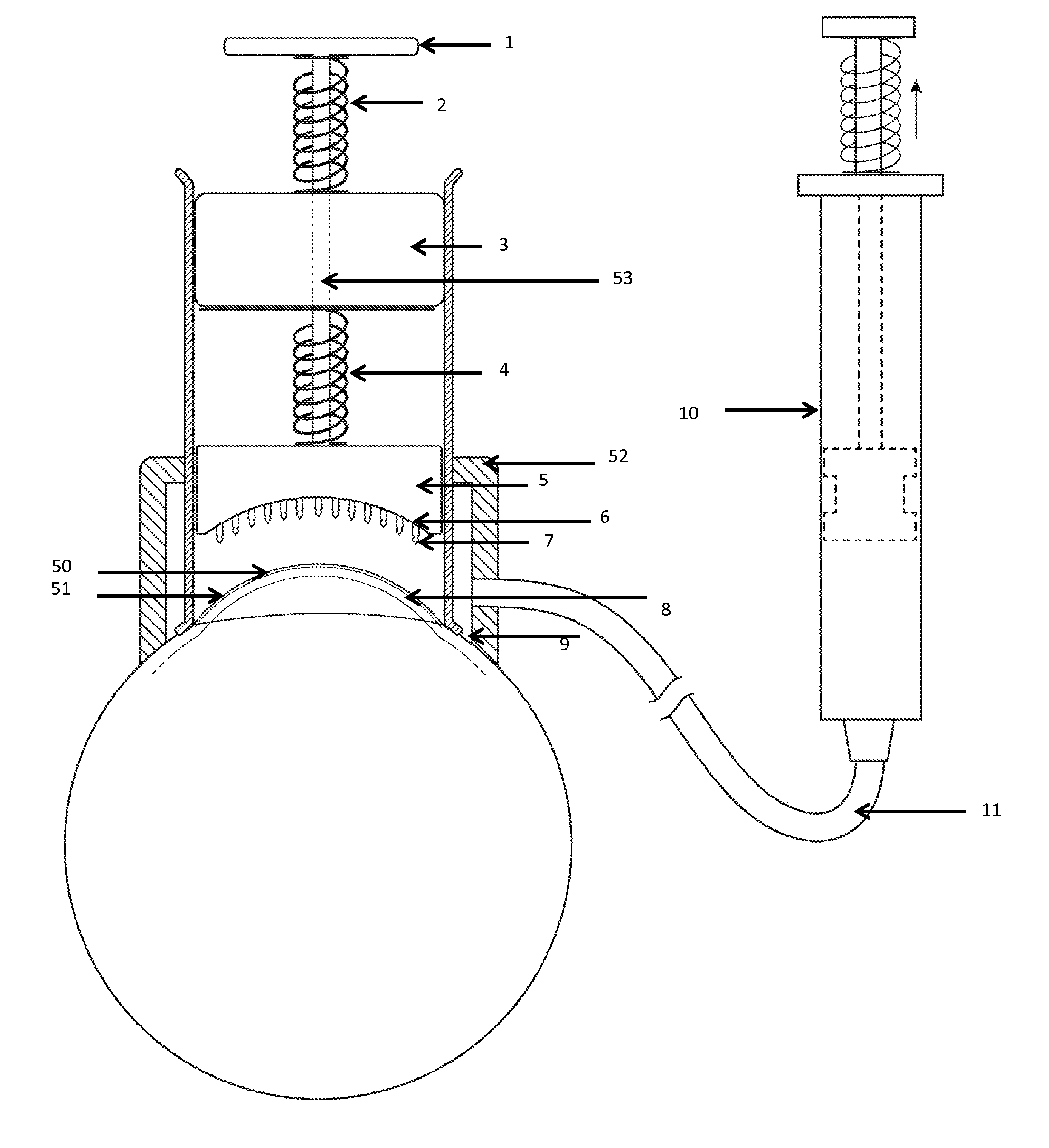
Ophthalmic formulation with novel gum composition
InactiveUS7128928B2High viscosityEnhanced gel forming capabilityBiocidePowder deliveryActive agentRetention time
There is provided a pharmaceutical composition suitable for topical administration to an eye, the composition comprising (a) a pharmacologically effective concentration of an active agent and (b) a combination of at least two ophthalmically compatible polymers comprising a novel gum system. In preferred embodiments of the present invention, the compositions increase the retention time of the active agent in the eye, when compared to compositions with other gums or gum systems.
Owner:PHARMACIA CORP
Method and apparatus for the delivery of photo-chemical (cross-linking) treatment to corneal tissue
Owner:SEROS MEDICAL
Ophthalmic formulation and method for ameliorating presbyopia
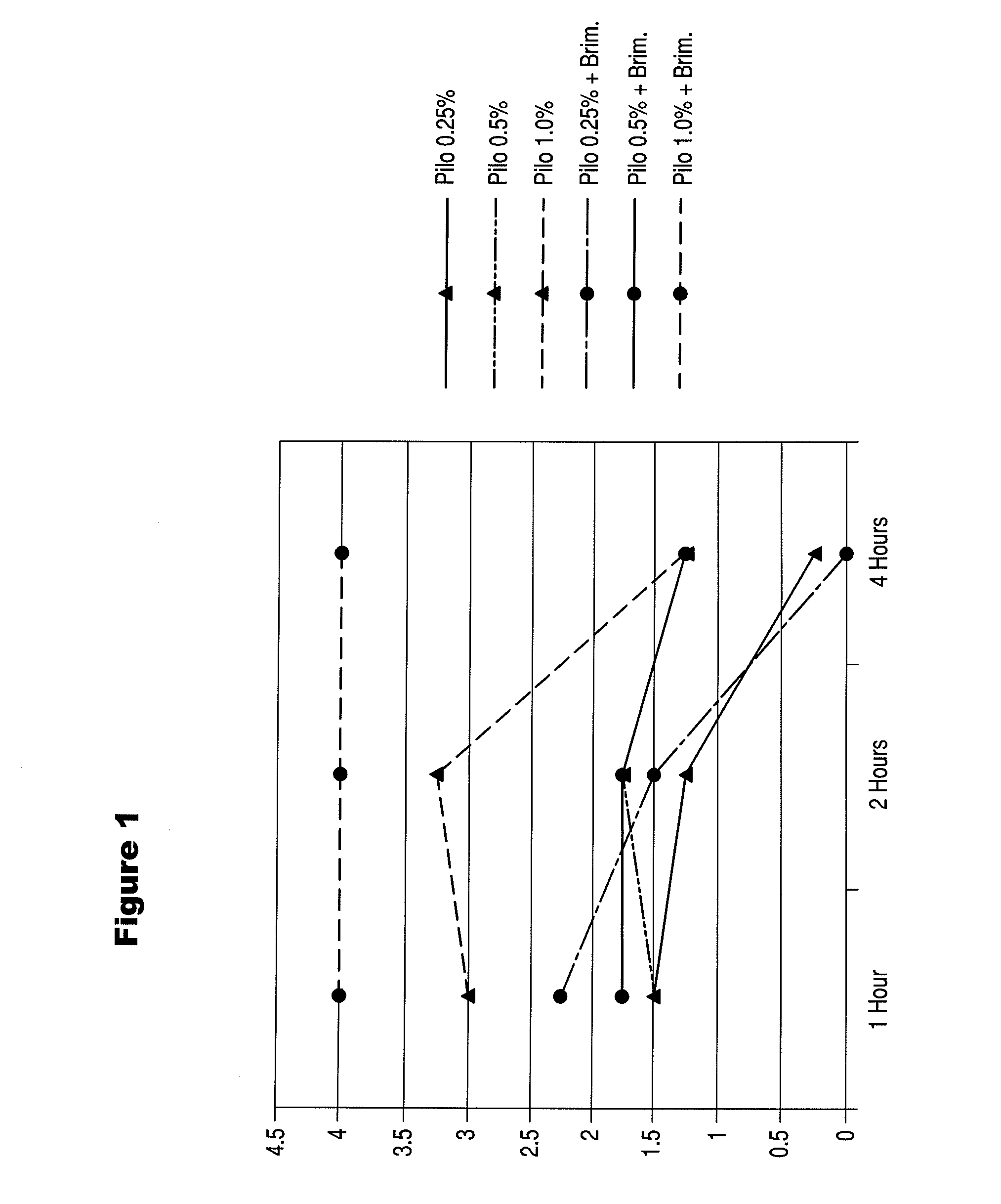
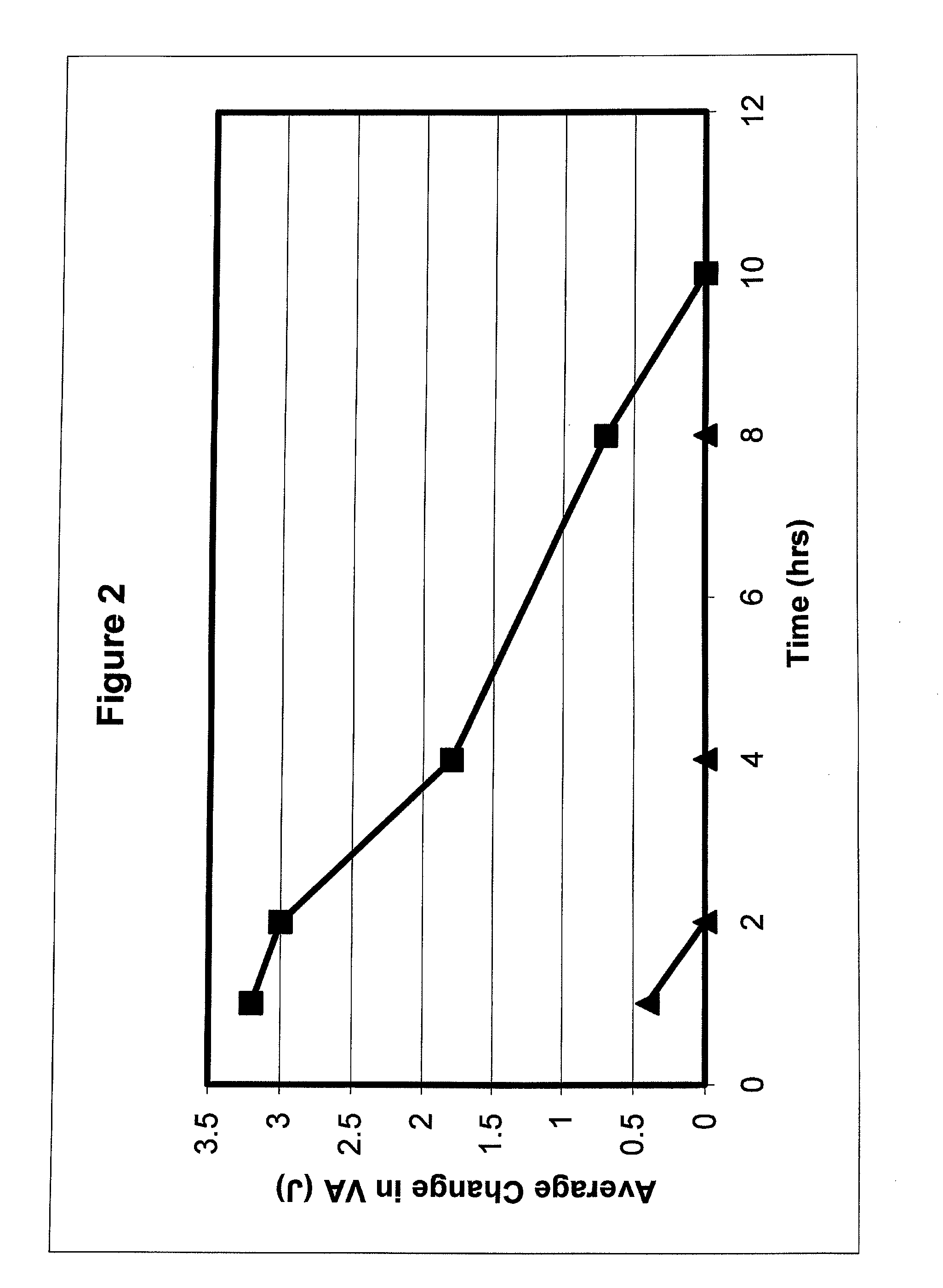
An ophthalmic formulation having an effective amount of a parasympathomimetic agent comprising pilocarpine, or a pharmaceutically acceptable salt thereof, and one or more α1 adrenergic agonists or antagonists is disclosed. The ophthalmic formulation may enable treatment of conditions adversely affecting the visual acuity of a patient, including presbyopia. A method of using the disclosed ophthalmic formulation to treat or ameliorate symptoms of presbyopia is also disclosed.
Owner:VEJARANO RESTREPO LUIS FELIPE
Ophthalmic percutaneously absorbed preparation containing muscarinic receptor agonist
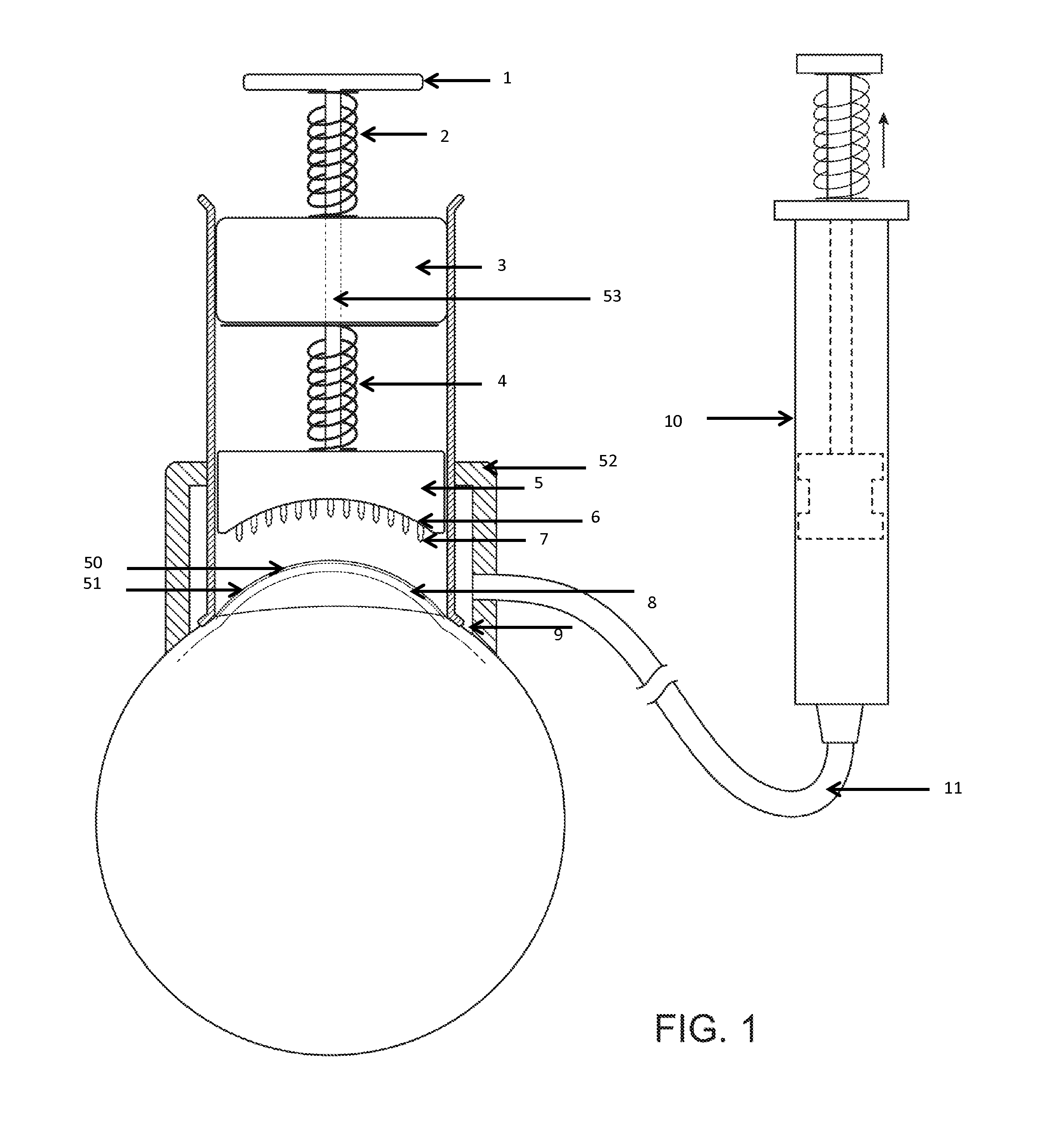
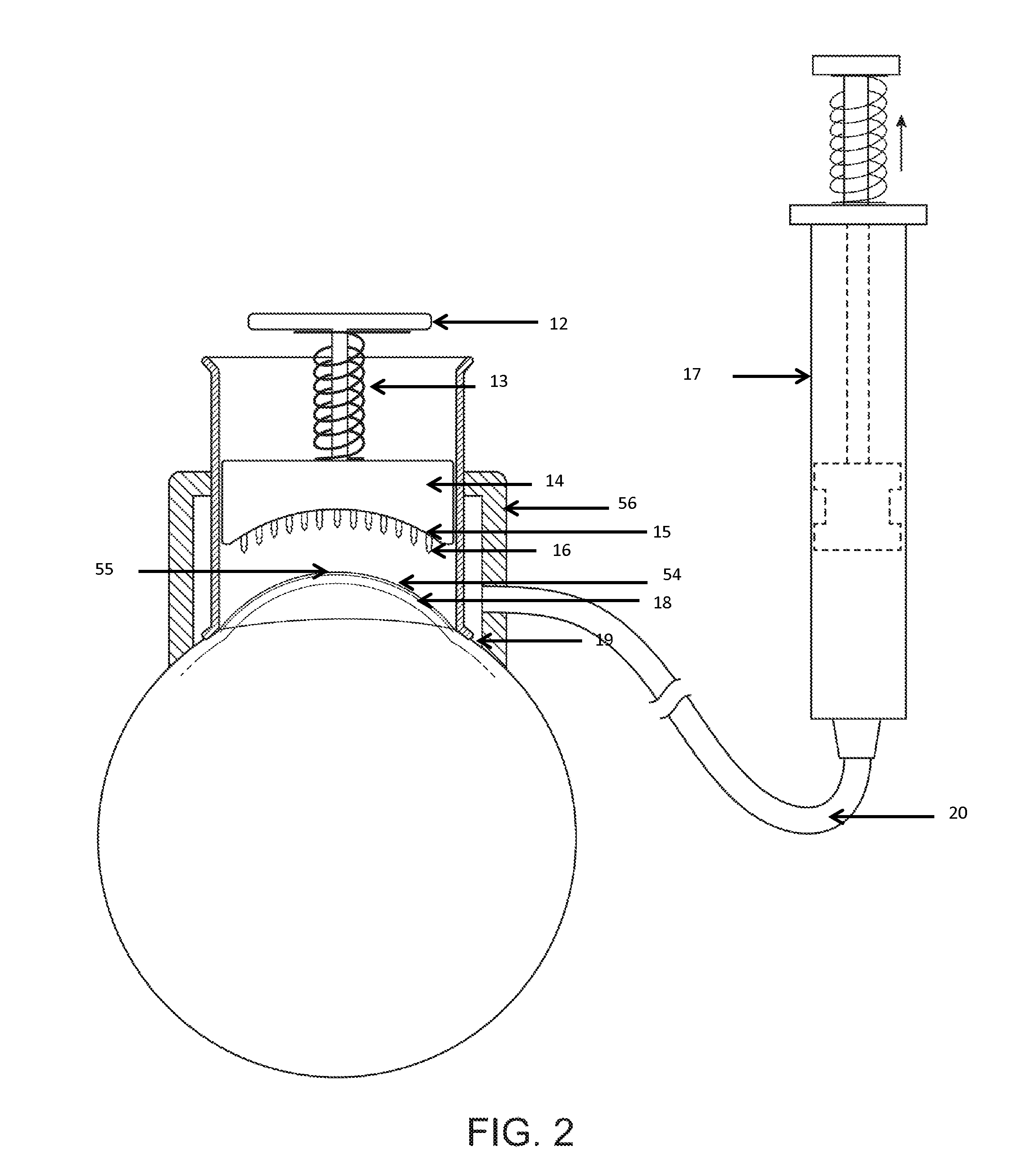
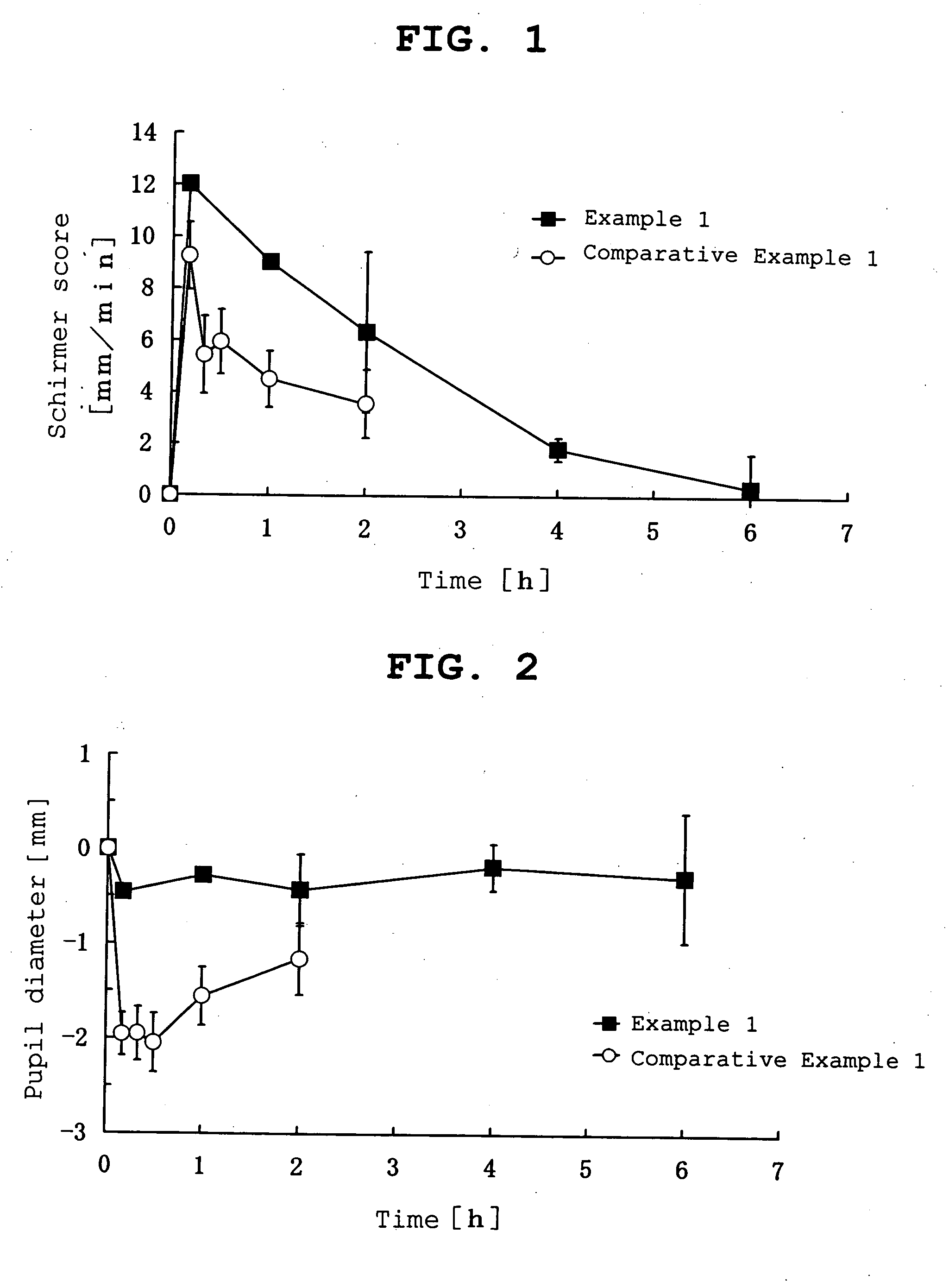
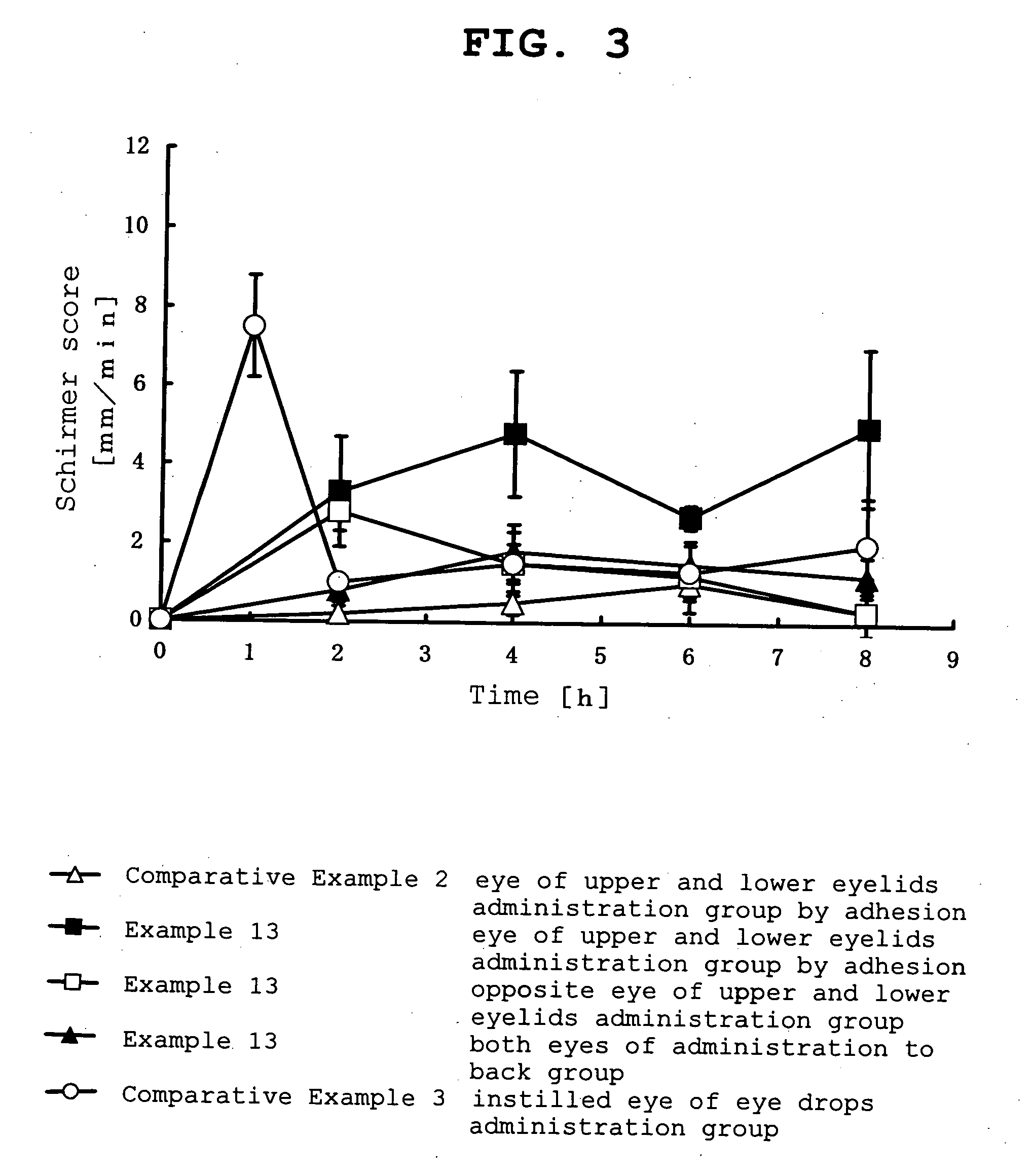
InactiveUS20070053964A1Promote secretionSustained effectOrganic active ingredientsBiocideEyelidSide effect
A percutaneous absorption type ophthalmic preparation comprising muscarinic receptor agonist is prepared, which can promote lacrimal fluid secretion by administration to the skin surface of the eyelid, and which causes fewer side effects such as miosis, thereby to provide a percutaneous absorption type ophthalmic preparation capable of maintaining a therapeutically effective concentration of a muscarinic receptor agonist for promoting lacrimal fluid secretion, which is associated with fewer side effects such as miosis, and a method of promoting lacrimal fluid secretion by administering a percutaneous absorption type ophthalmic preparation containing a muscarinic receptor agonist to the skin surface of the eyelid.
Owner:SENJU PHARMA CO LTD
A kind of azithromycin gel eye drop and its preparation process
ActiveCN102283799AStrong practical significanceStrong application valueAntibacterial agentsOrganic active ingredientsBacterial ConjunctivitisAntioxidant
The invention discloses azithromycin gel eye drops and a preparation process thereof. The eye drops are prepared from a main medicine, namely azithromycin and excipients such as an adhesive, a gel matrix, an isotonic regulator, a preservative, an antioxidant, a buffering agent and the like. The adhesive, namely polycarbophil in the eye drops can increase the biological adhesion of the eye drops, so that the detention time of medicines in eyes is further prolonged. The invention provides a practical, convenient and reliable ophthalmic preparation for treating bacterial conjunctivitis, and solves the problems that the detention time of medicines in an eye drop formulation in the eyes is short, the medicines are not easy to absorb, the bioavailability is low and the like.
Owner:北京乐维生物技术有限公司
Eyes preparation for divergent pupil and its making method
InactiveCN101073556AImprove complianceImprove toleranceSenses disorderPharmaceutical delivery mechanismGellan gumPreservative
The invention is concerned with the method of preparation of the Mydriasis ophthalmic preparation. It is the kind of ophthalmic preparation that in liquid form before dripping into eyes but turns to gelatin postural after. It contents Mydriasis agent as the active material and other supplementary materials, such as poloxamer, carbomer, Deacetylcephalomannine Gellan Gum, transition regulator and medicinal acceptable antiseptic, isoosmotic adjustment, PH regulator and injection usage water. It can use of optometry, pre-operation naydriasis, etc. The advantages of the invention are: highly biocompatibility, low pungency, and long lasting.
Owner:RUIQIAO MEDICAL SCI & TECH SUZHOU
Ophthalmic formulation and method of manufacture thereof
Provided herein is an ophthalmic formulation that comprises a fine particle of an A1 agonist in an aqueous suspension and a manufacturing process thereof. More specifically, provided herein is a topically applied ophthalmic aqueous suspension which is obtainable by suspending a fine particle of an A1 agonist in a surfactant and preservative; a method of reduction of intraocular pressure using the formulation and a manufacturing process of the aqueous suspension thereof.
Owner:INOTECK PHARMA CORP
Method and ophthalmic formulation for eye protection or treatment
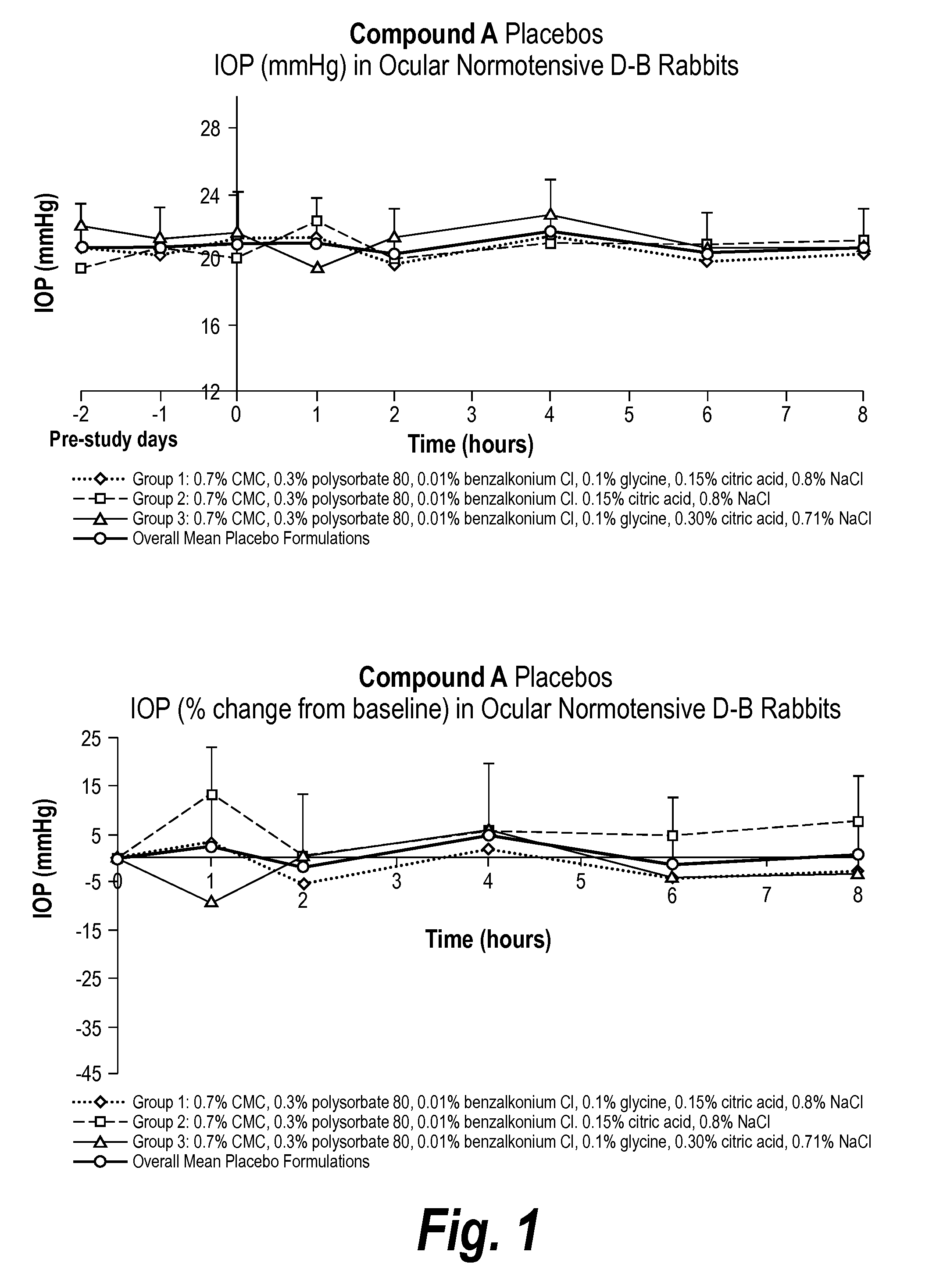
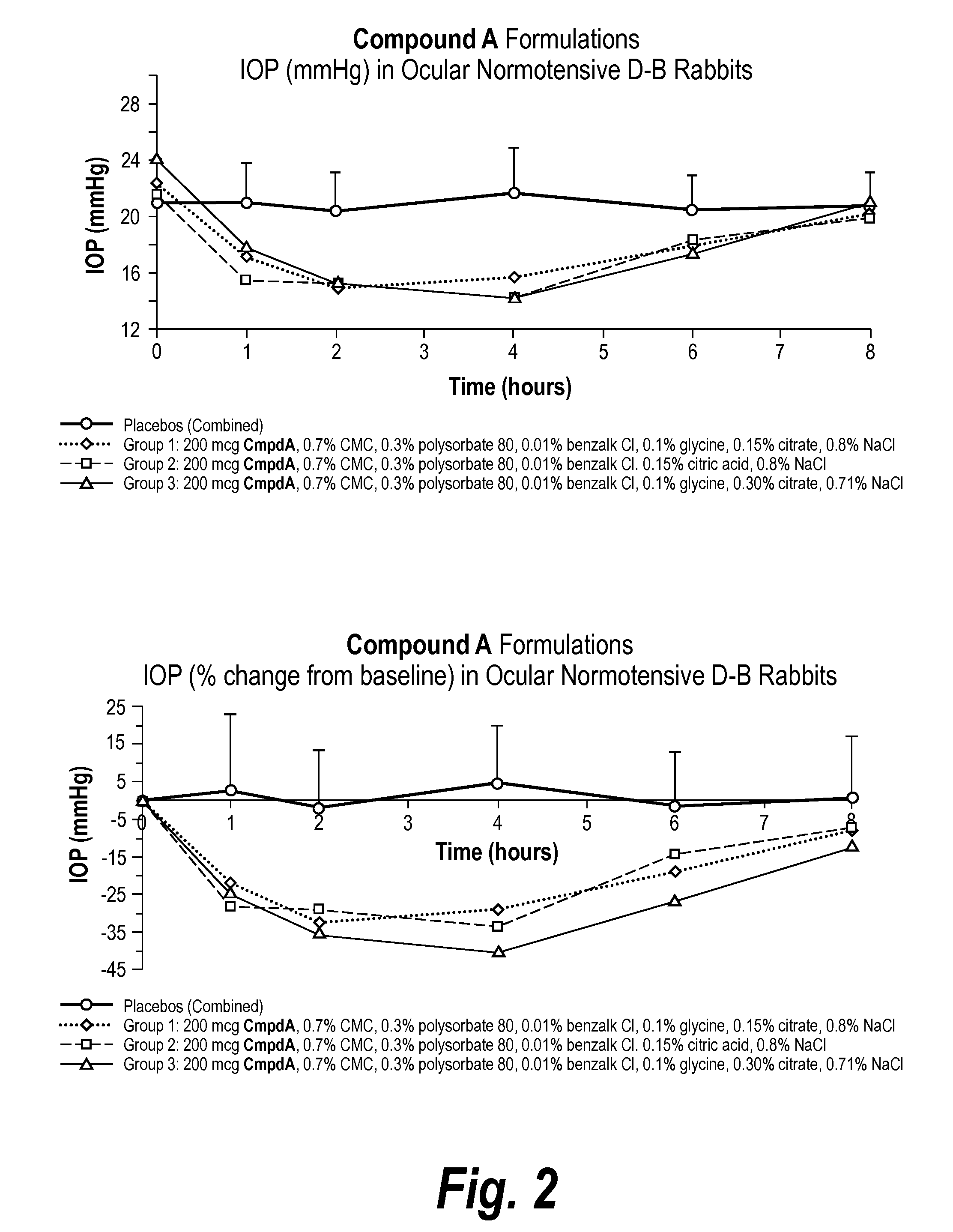
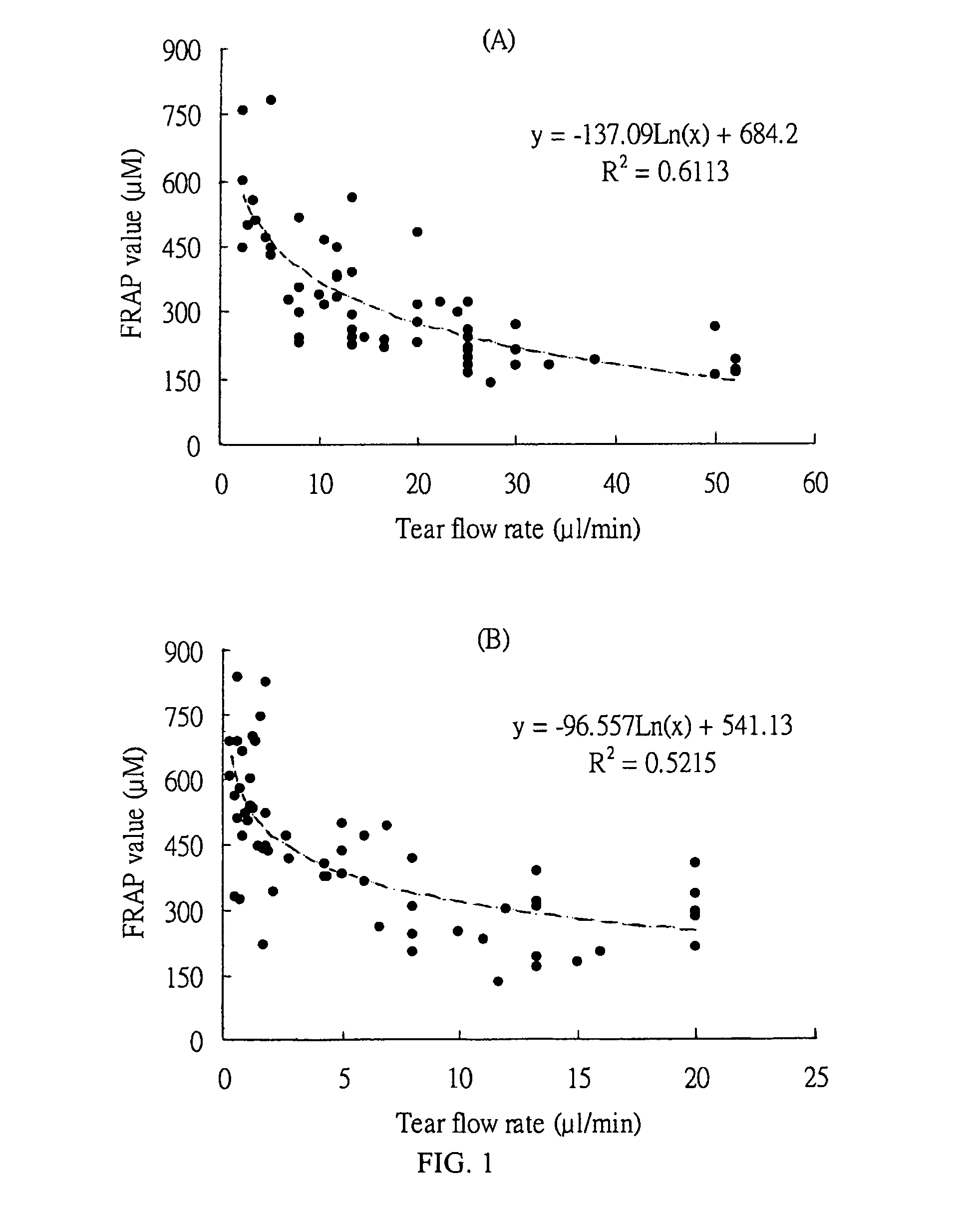
An ophthalmic composition for artificial tears useful for treating dry eye symptoms or for general eye protection. The composition includes a physiologically balanced salt solution and one or more anti-oxidative ingredients which collectively confer on said ophthalmic composition a total antioxidant capacity as a FRAP value of greater than 100 μmol / l, preferably greater than 150 μmol / l. The composition also has peak UV absorption in the range of 200-350 nm. Suitable anti-oxidative ingredient includes ascorbate (ascorbic acid), urate (uric acid), cysteine, glutathione, tyrosine, lactoferrin, transferrin, albumin, caeruloplasmin and lacrimal gland peroxidase.
Owner:THE HONG KONG POLYTECHNIC UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com