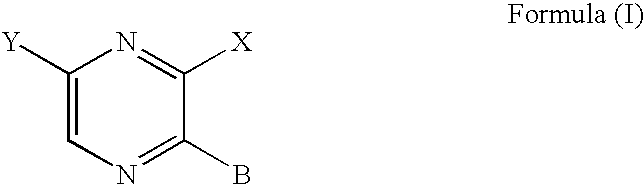Patents
Literature
88 results about "Ophthalmic drugs" patented technology
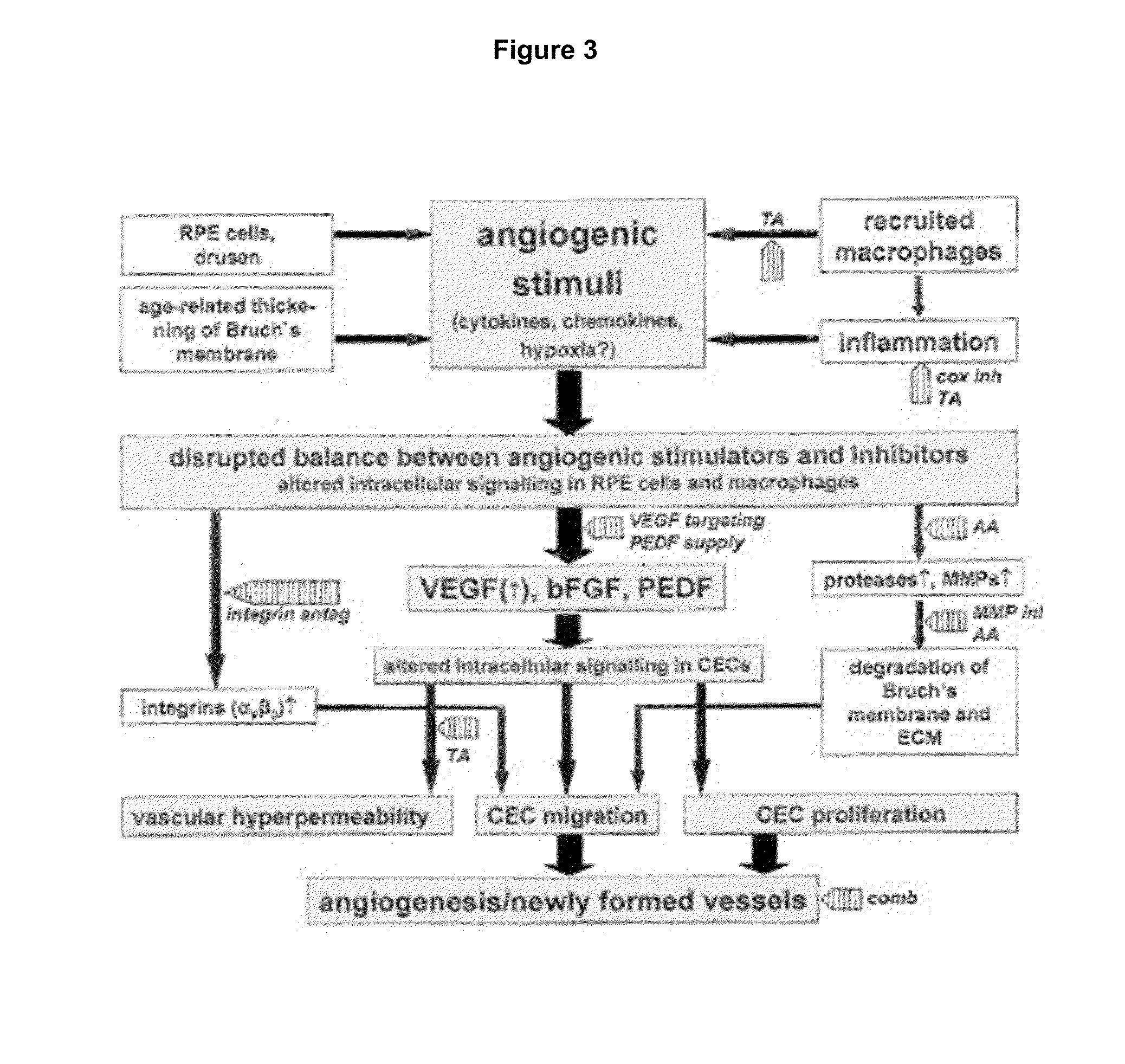
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
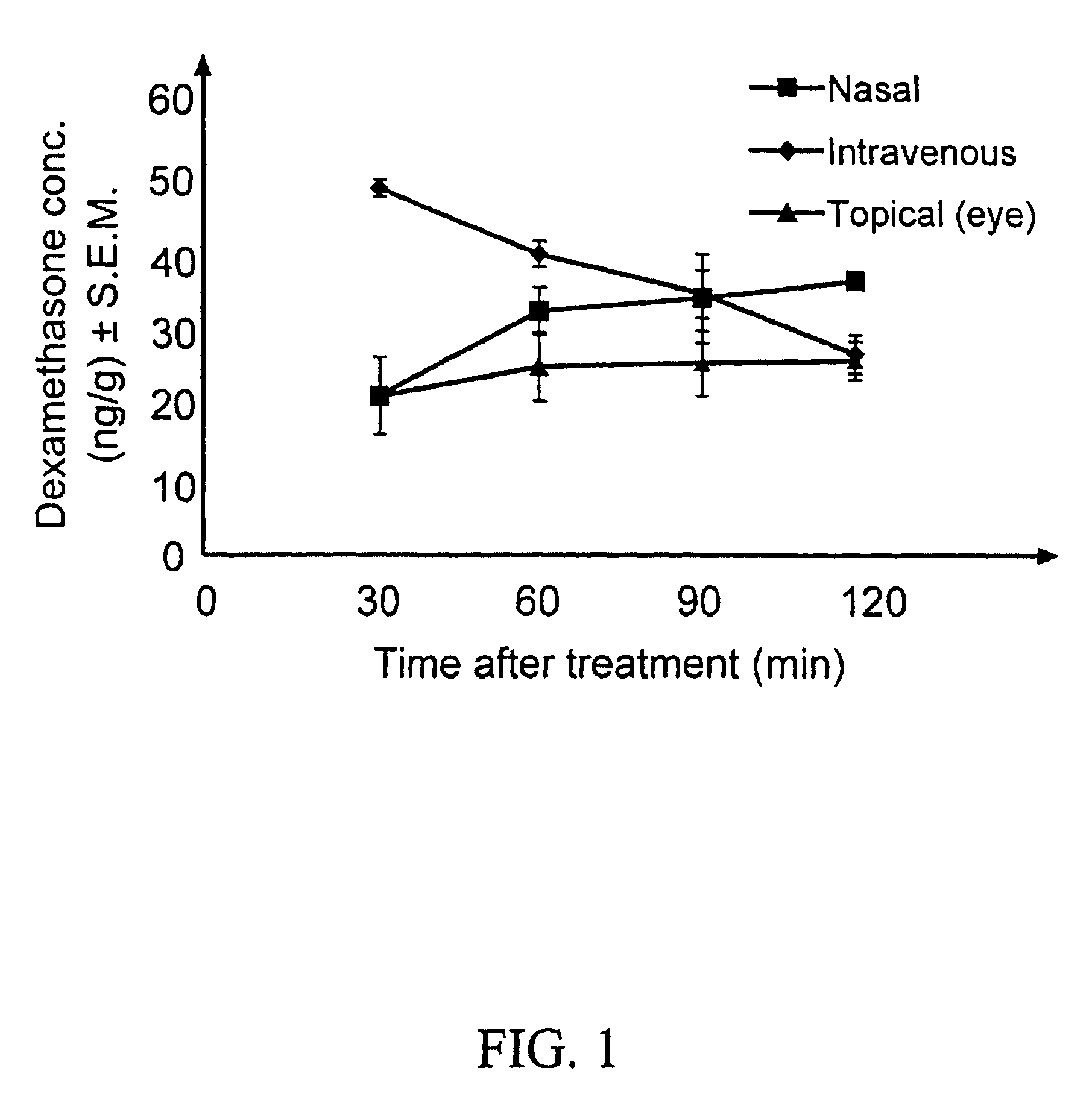
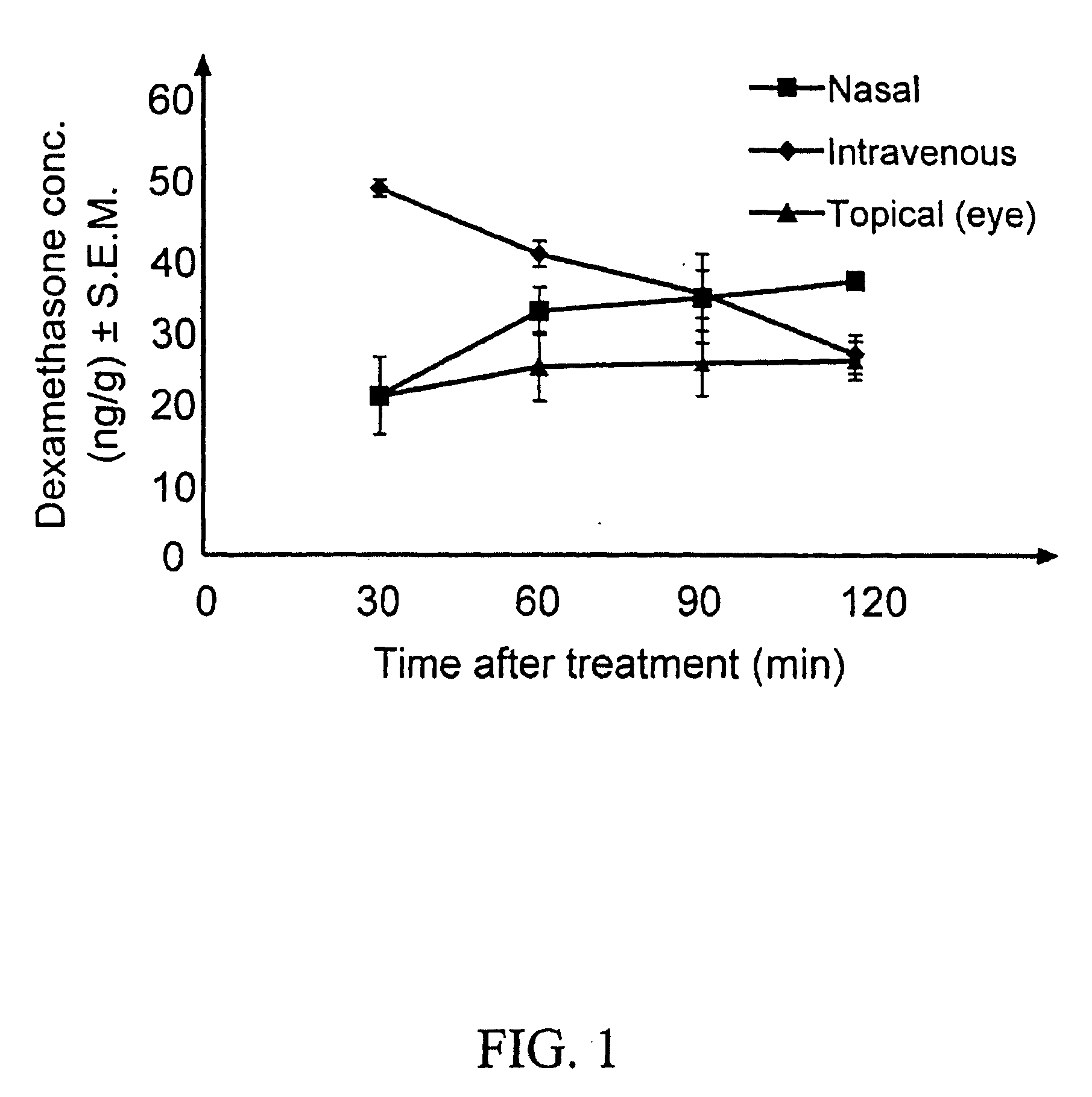
Dexamethasone ophthalmic is in a class of drugs called corticosteroids. It inhibits processes in the body that cause inflammation. Therefore, the swelling and pain of inflammatory conditions is decreased.
Cyclodextrin nanotechnology for ophthalmic drug delivery
The invention provides an ophthalmic composition which is an aqueous suspension comprising drug, cyclodextrin and water, the composition having an aqueous phase of from about 0.1% (w / v) to about 90% (w / v) of the drug in solution, as dissolved free drug and as dissolved drug / cyclodextrin complex(es), and a solid phase of from about 10% (w / v) to about 99.9% (w / v) of the drug as solid drug / cyclodextrin particles, suspended in the aqueous phase; the size of the solid particles being from about 10 nm to about 1 mm, the drug / cyclodextrin particles being capable of dissolving in aqueous tear fluid within 24 hours of application to the eye surface. The aqueous eye suspension can be in the form of eye drops, eye gel or eye mist. Further, the invention provides a method for treating a condition of the posterior segment and / or anterior segment of the eye comprising applying to the eye surface, in an amount which delivers to said segment or segments a therapeutically effective amount of a drug suitable for treating said condition, an ophthalmic composition which is as defined above. Nasal compositions and methods and ophthalmic and nasal compositions in powder form are also provided.
Owner:OCULIS EHF
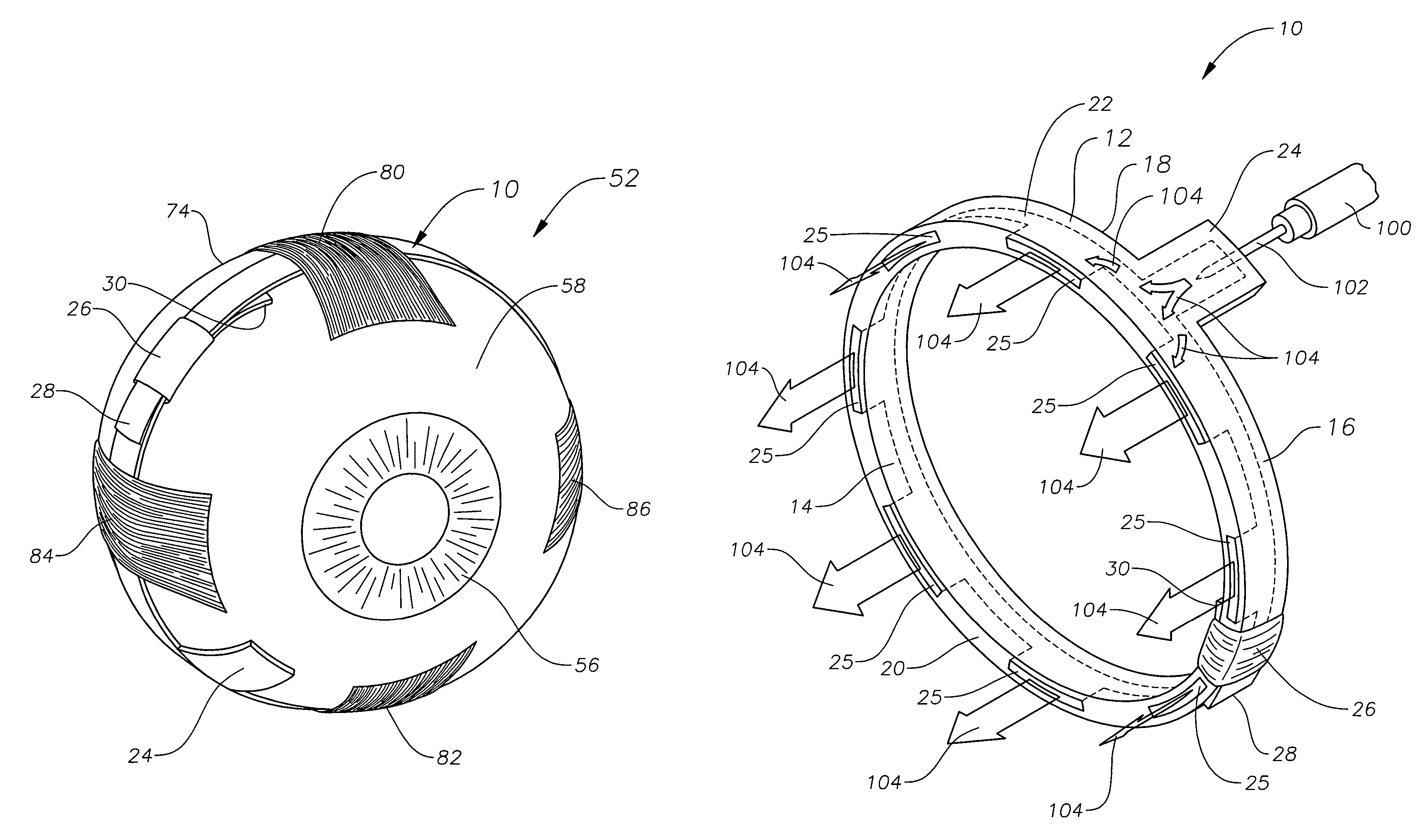
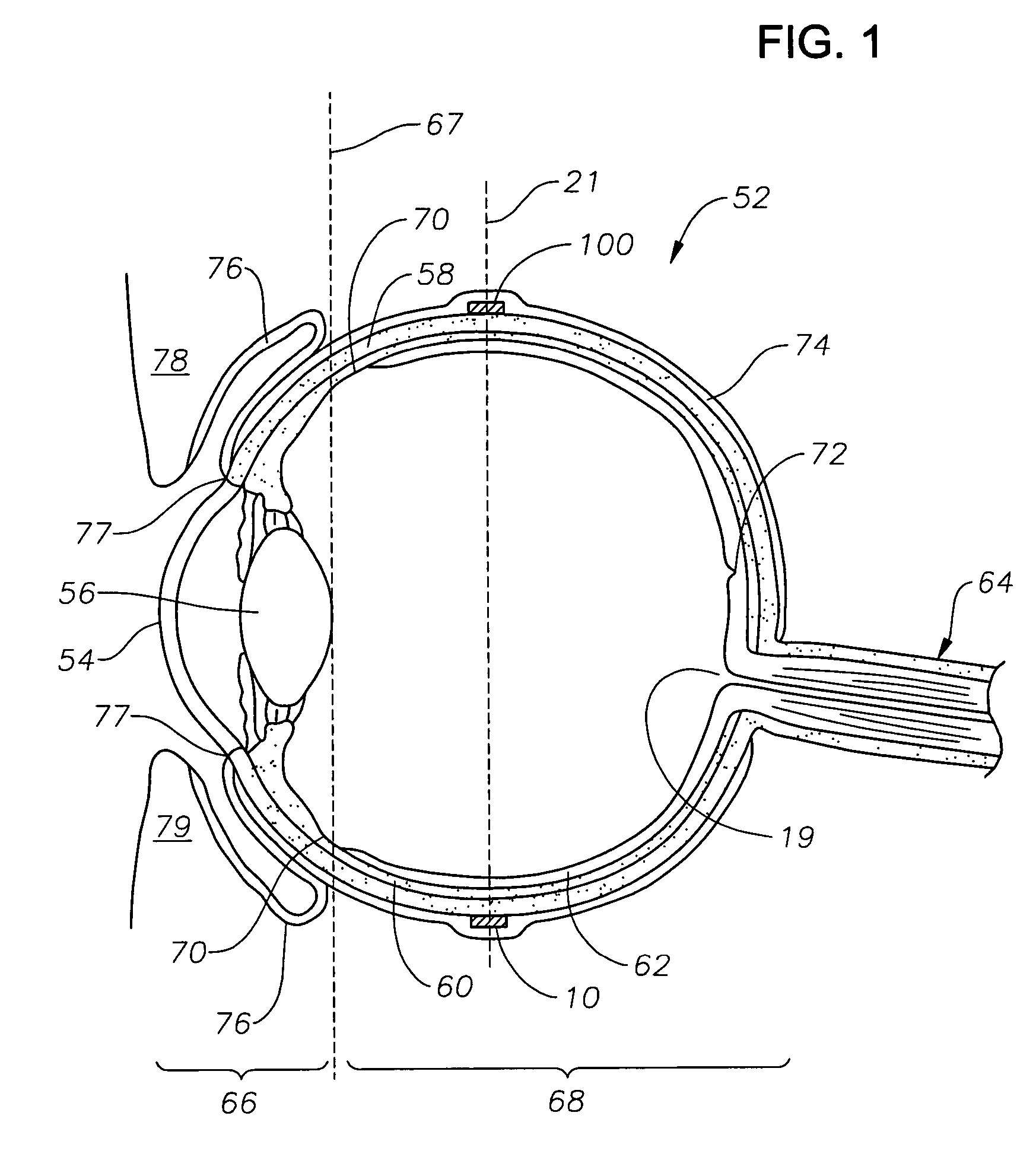
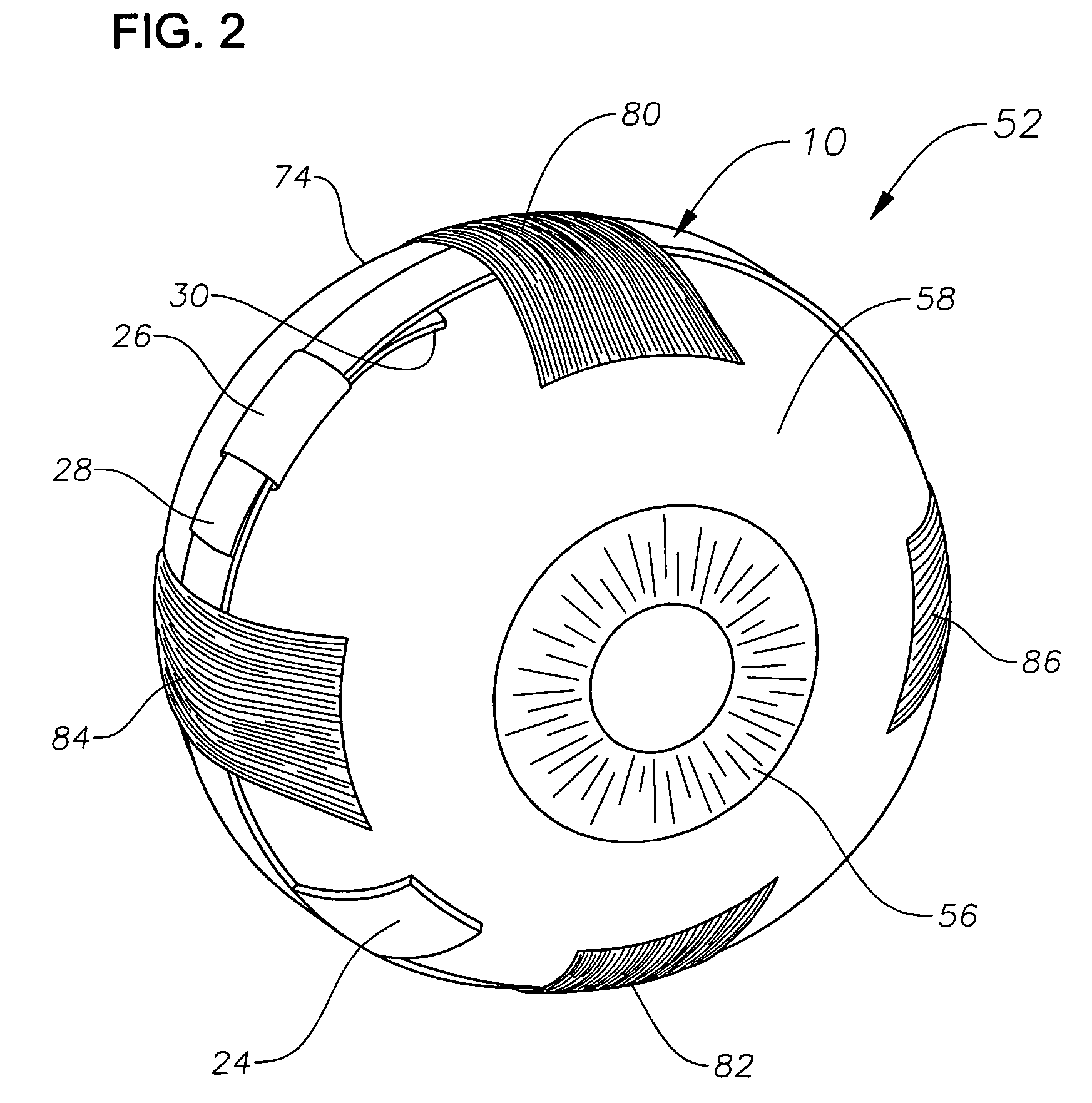
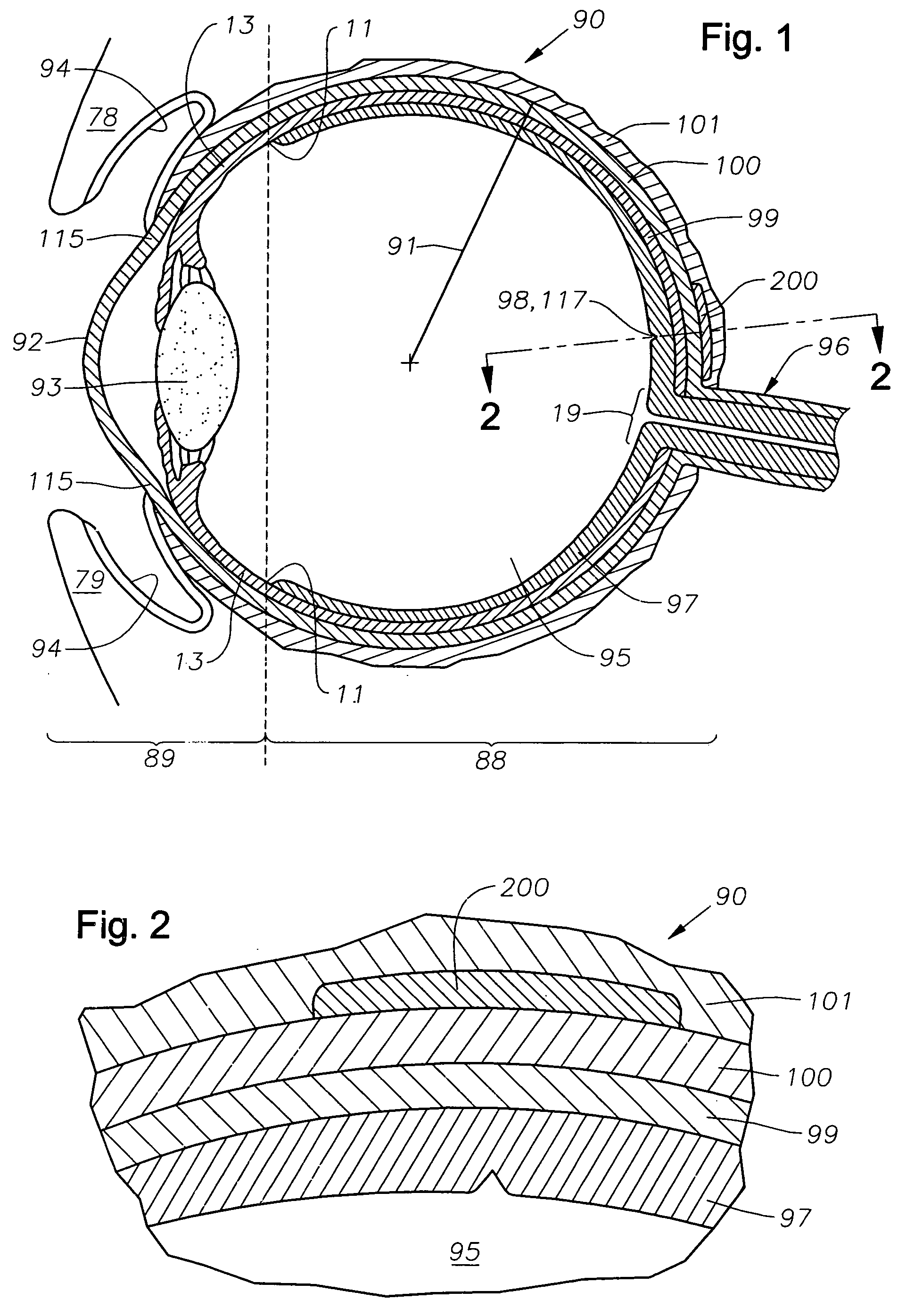
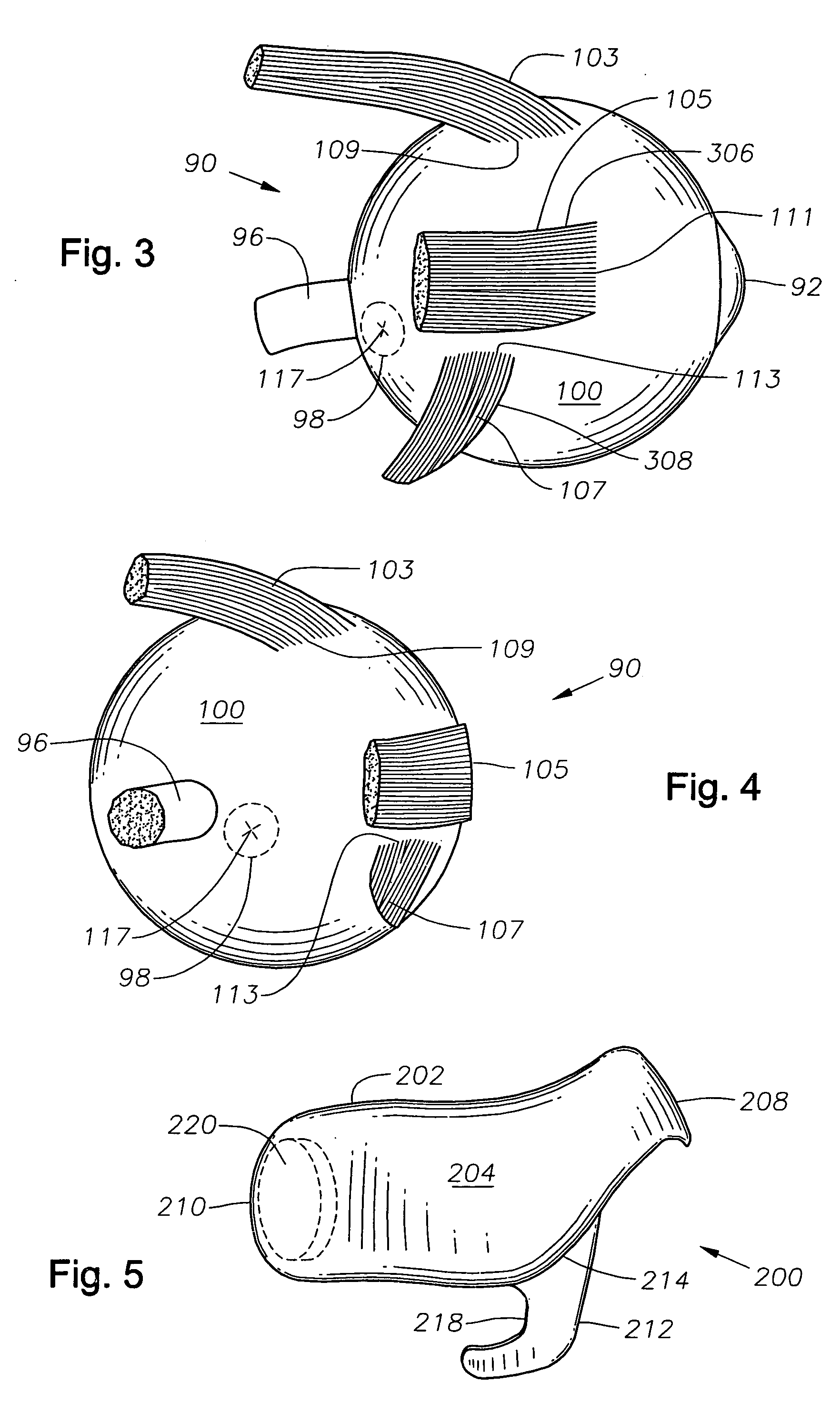
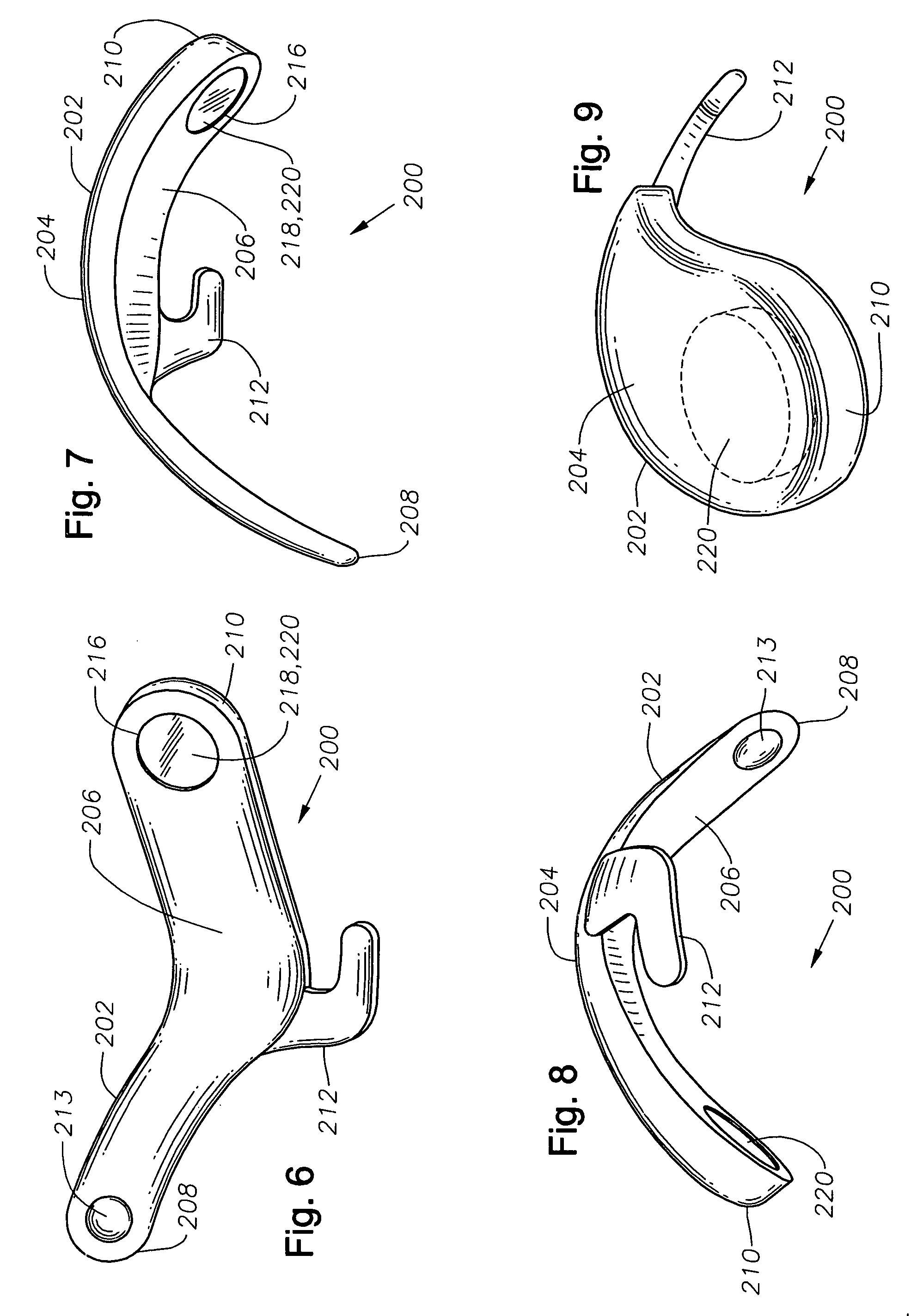
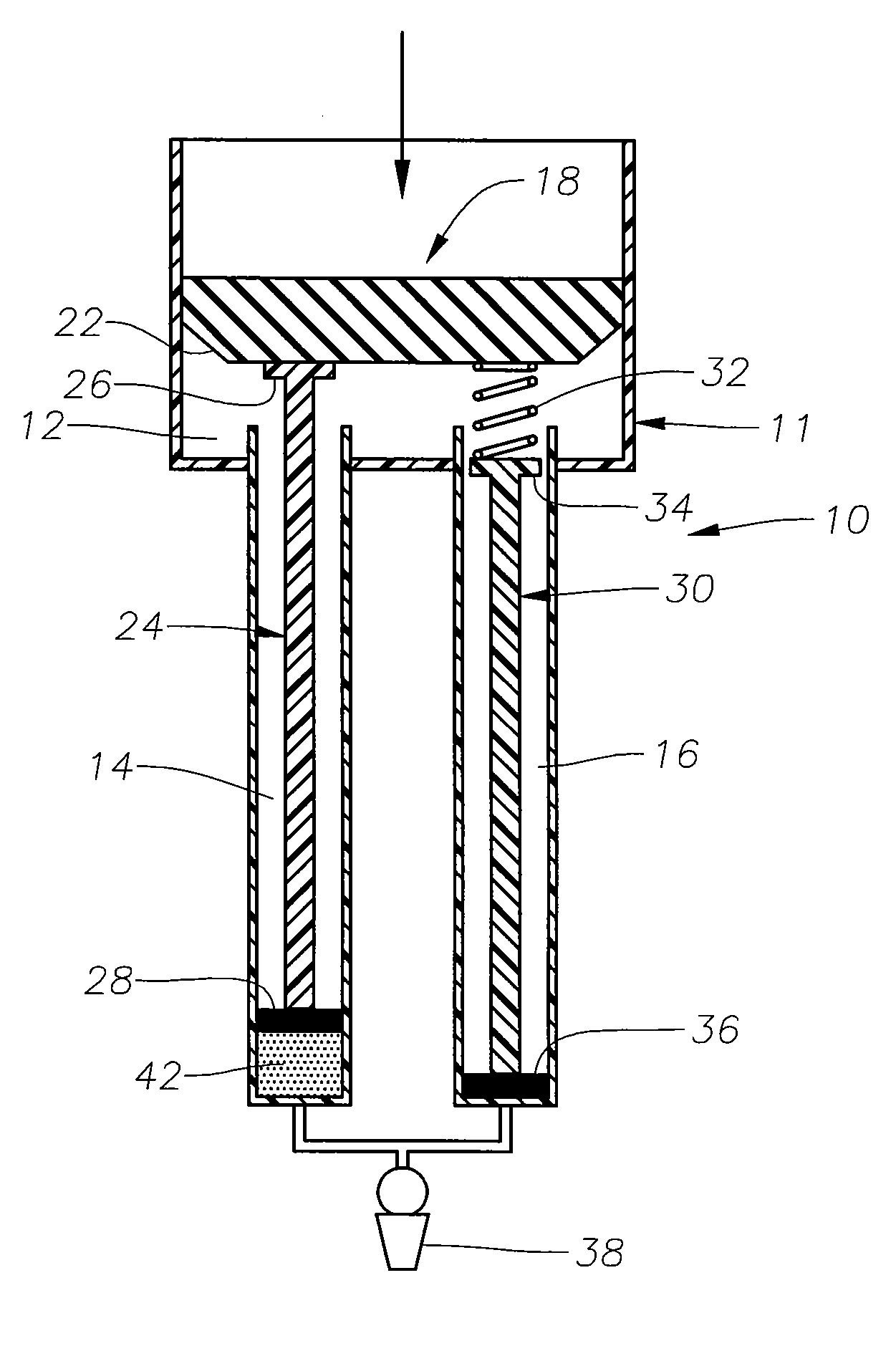
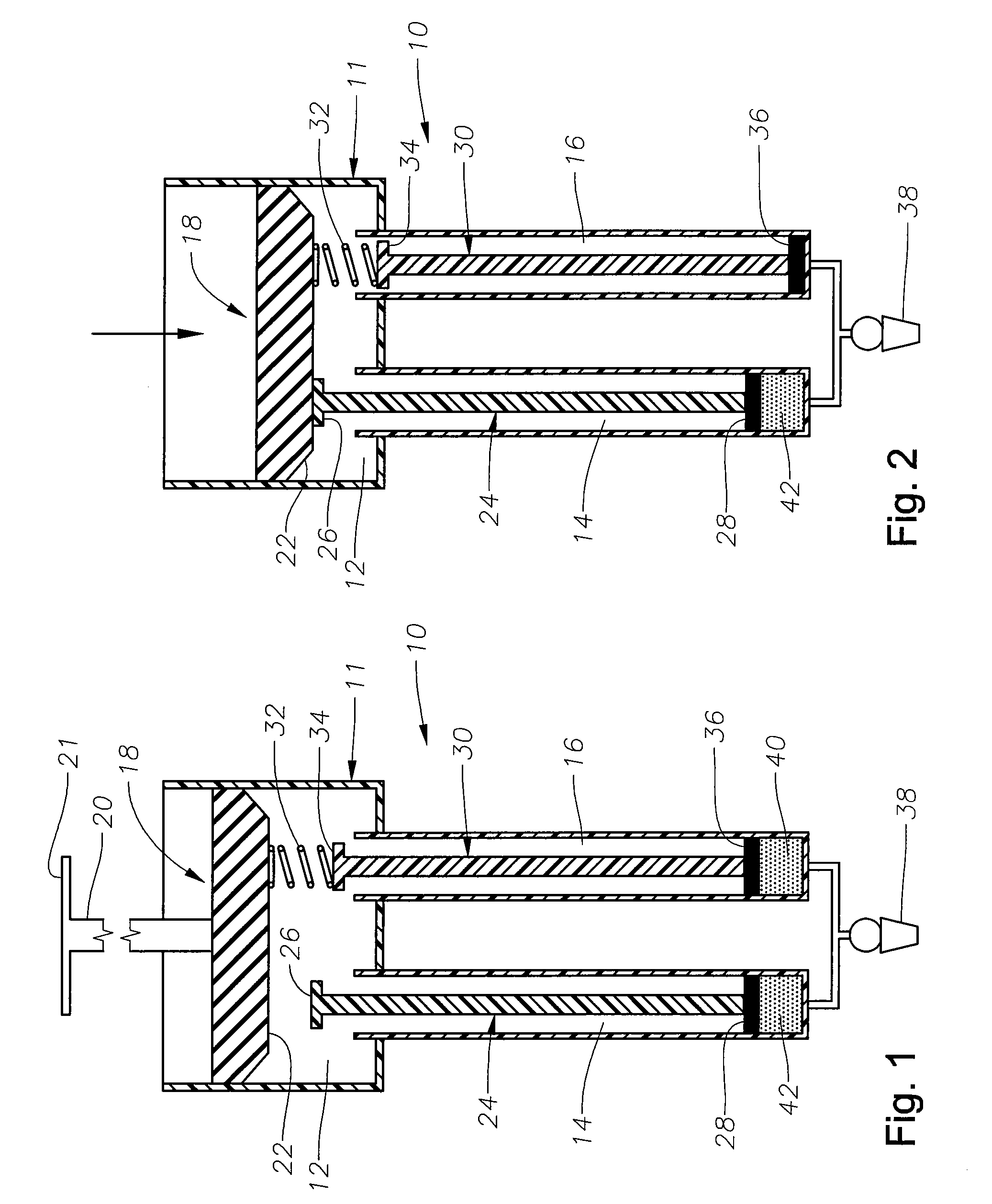
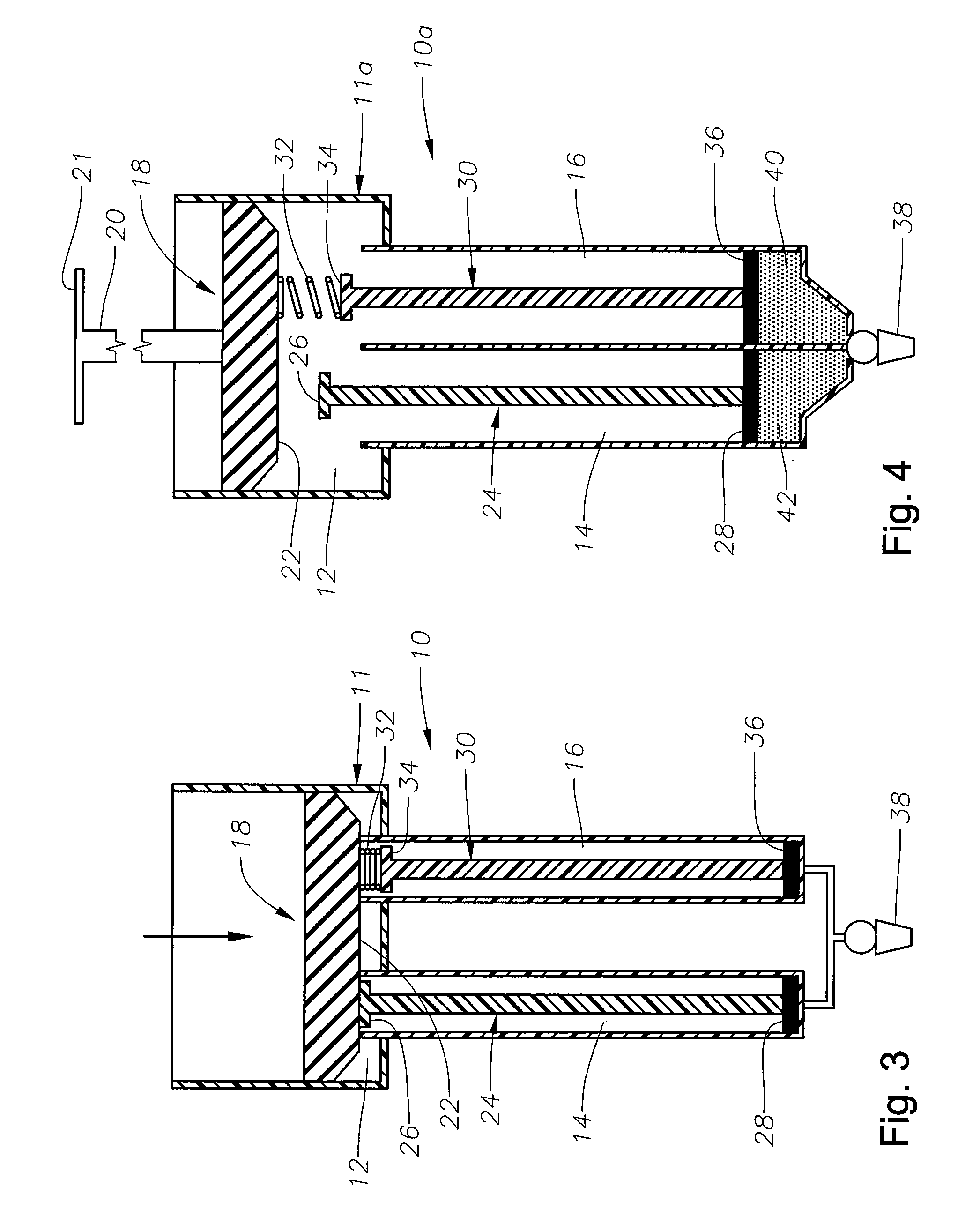
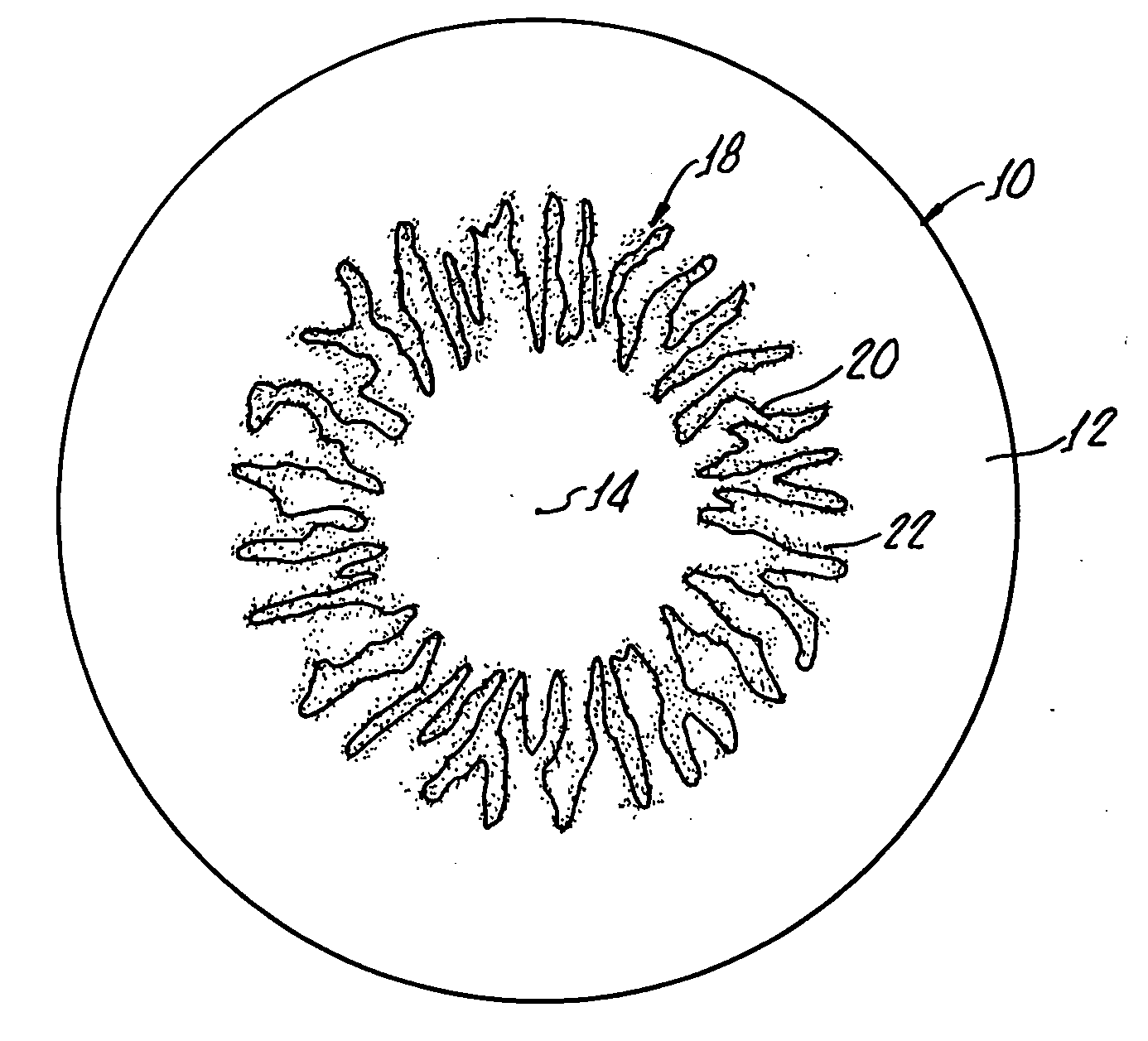
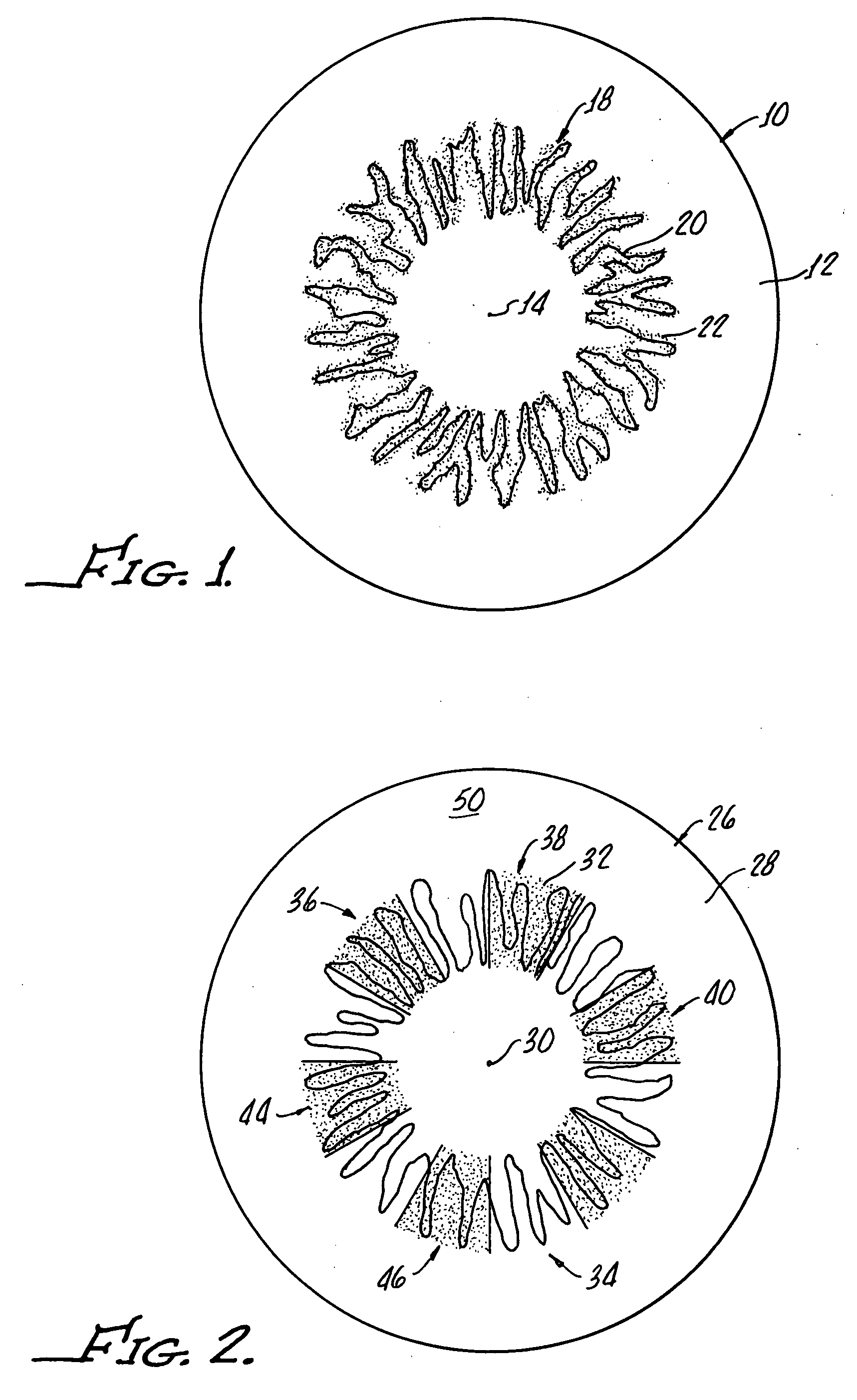
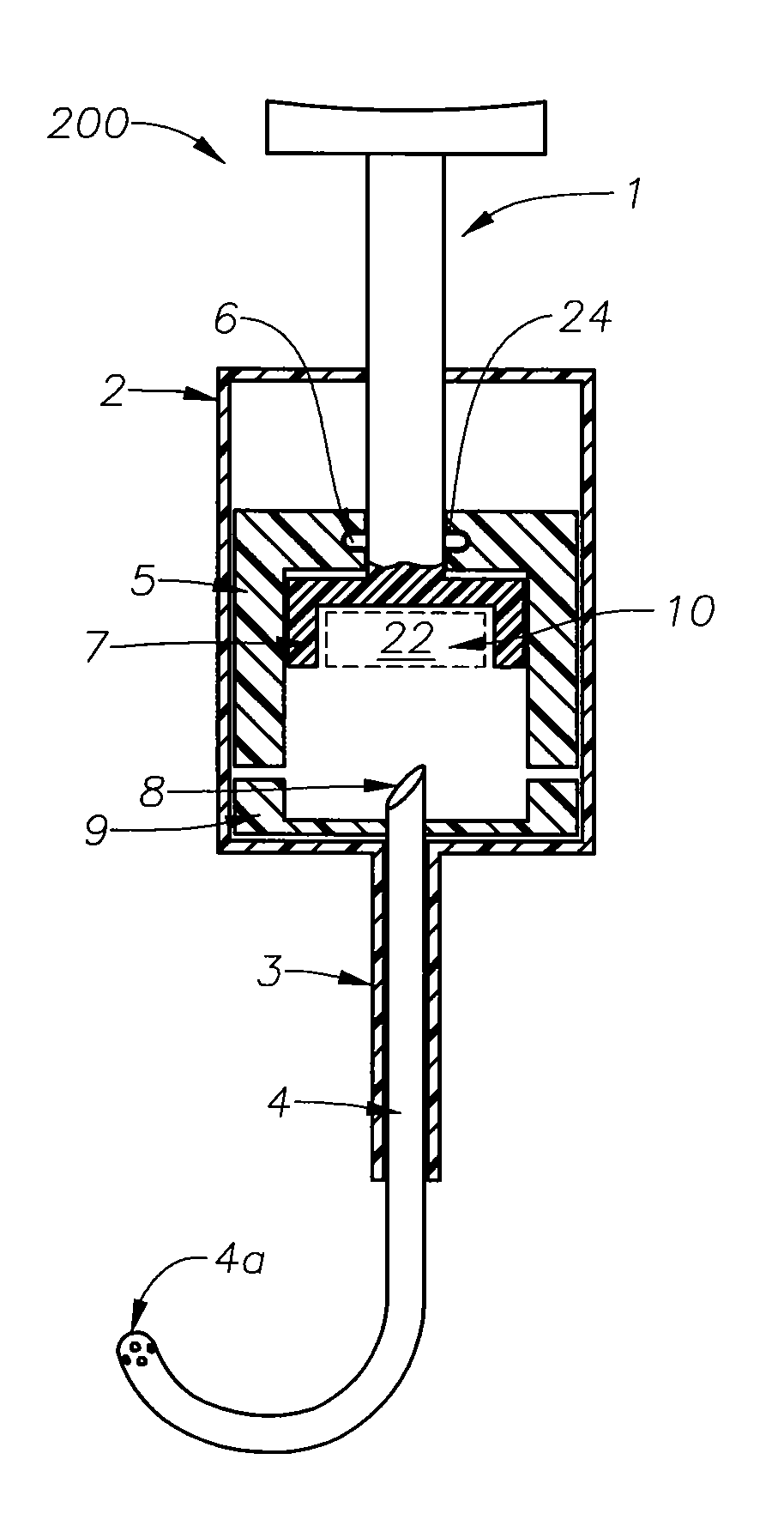
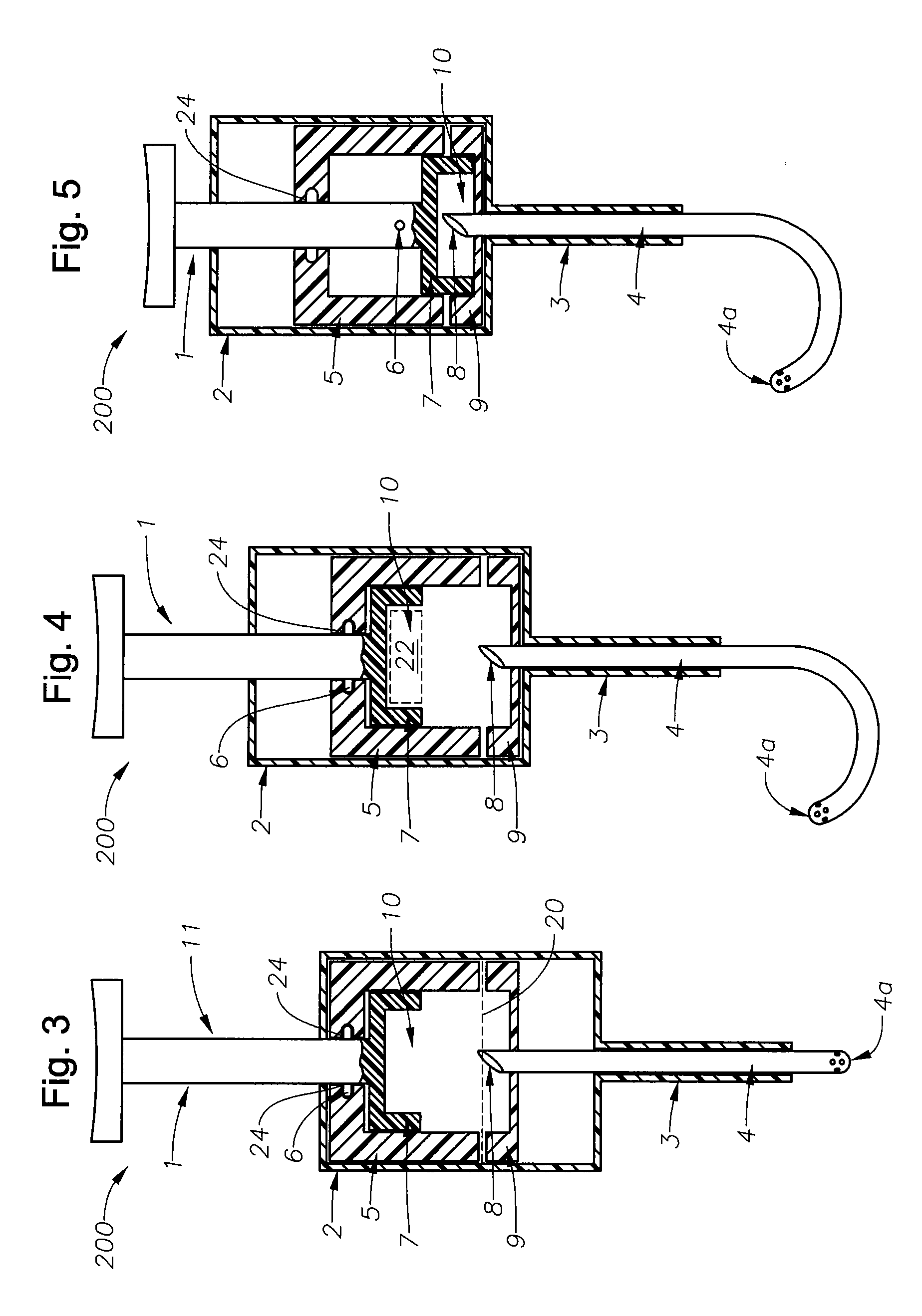
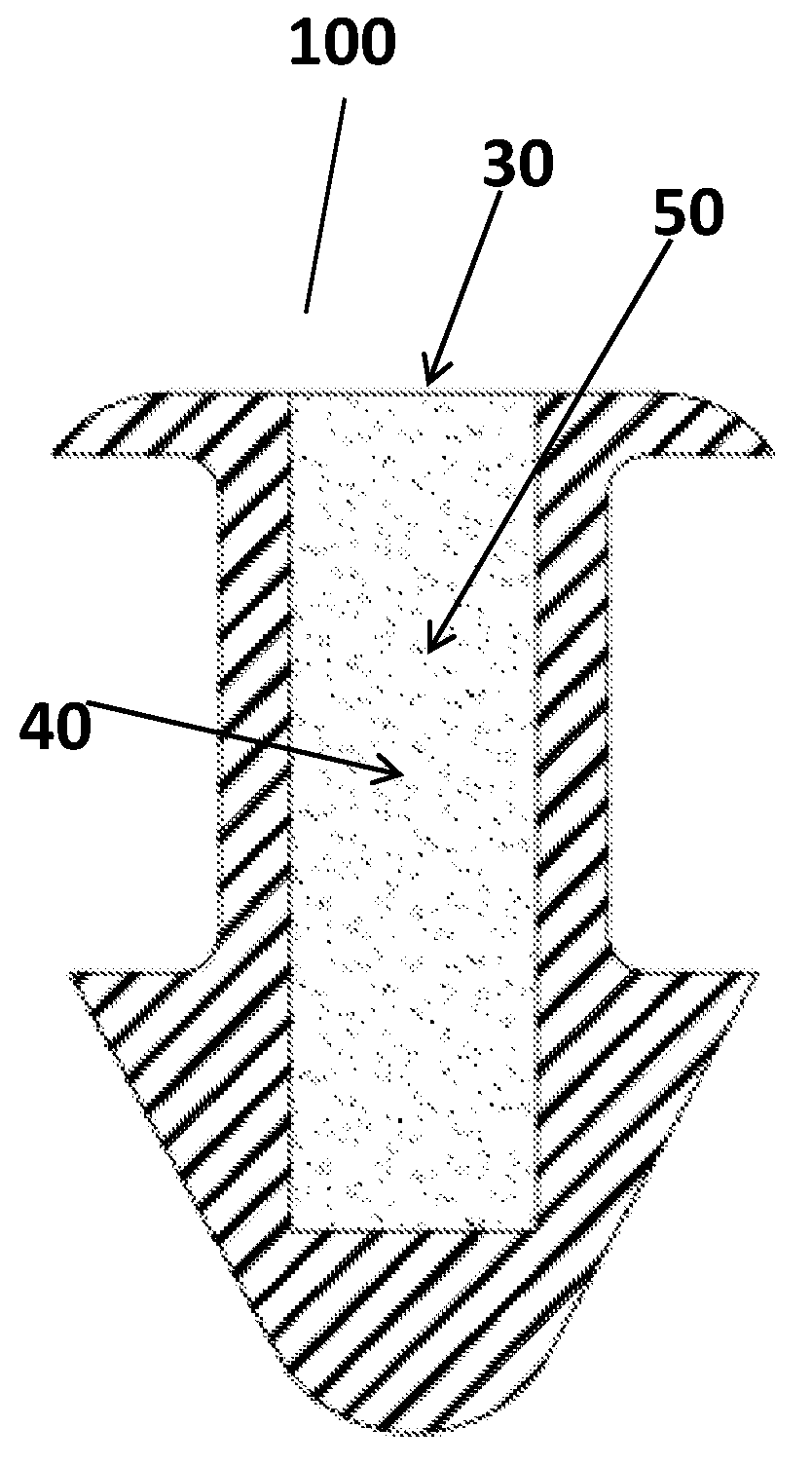
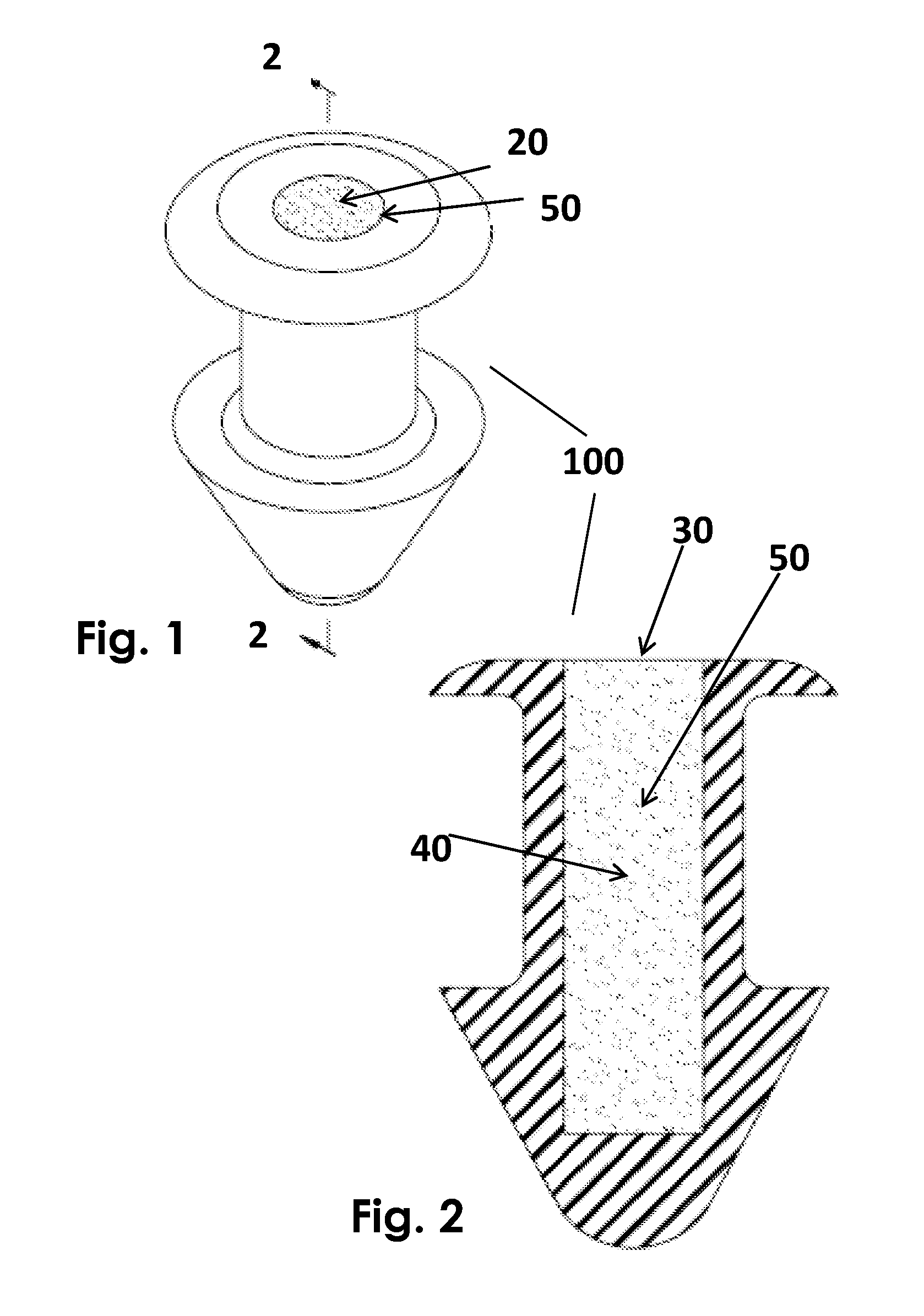
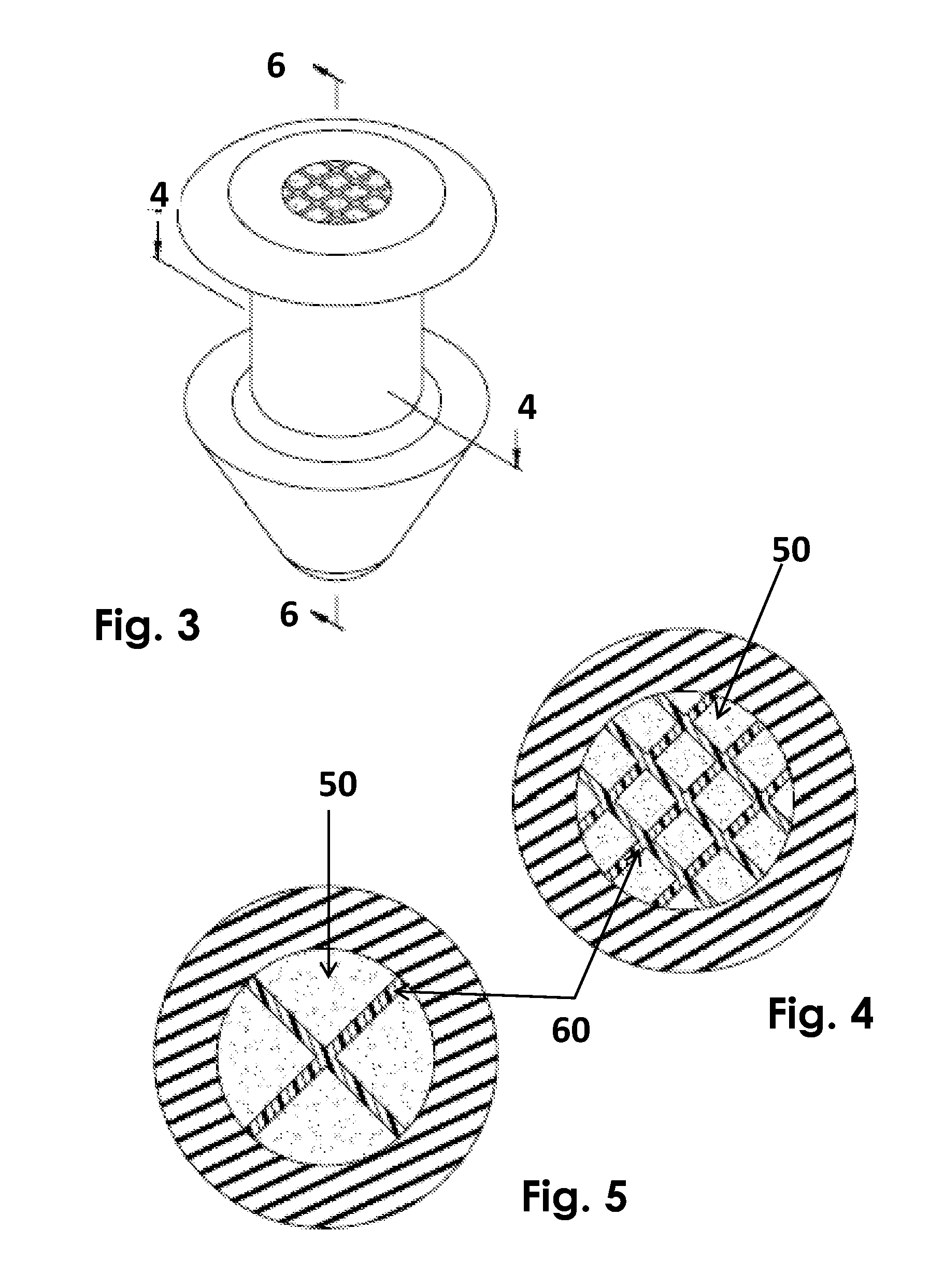
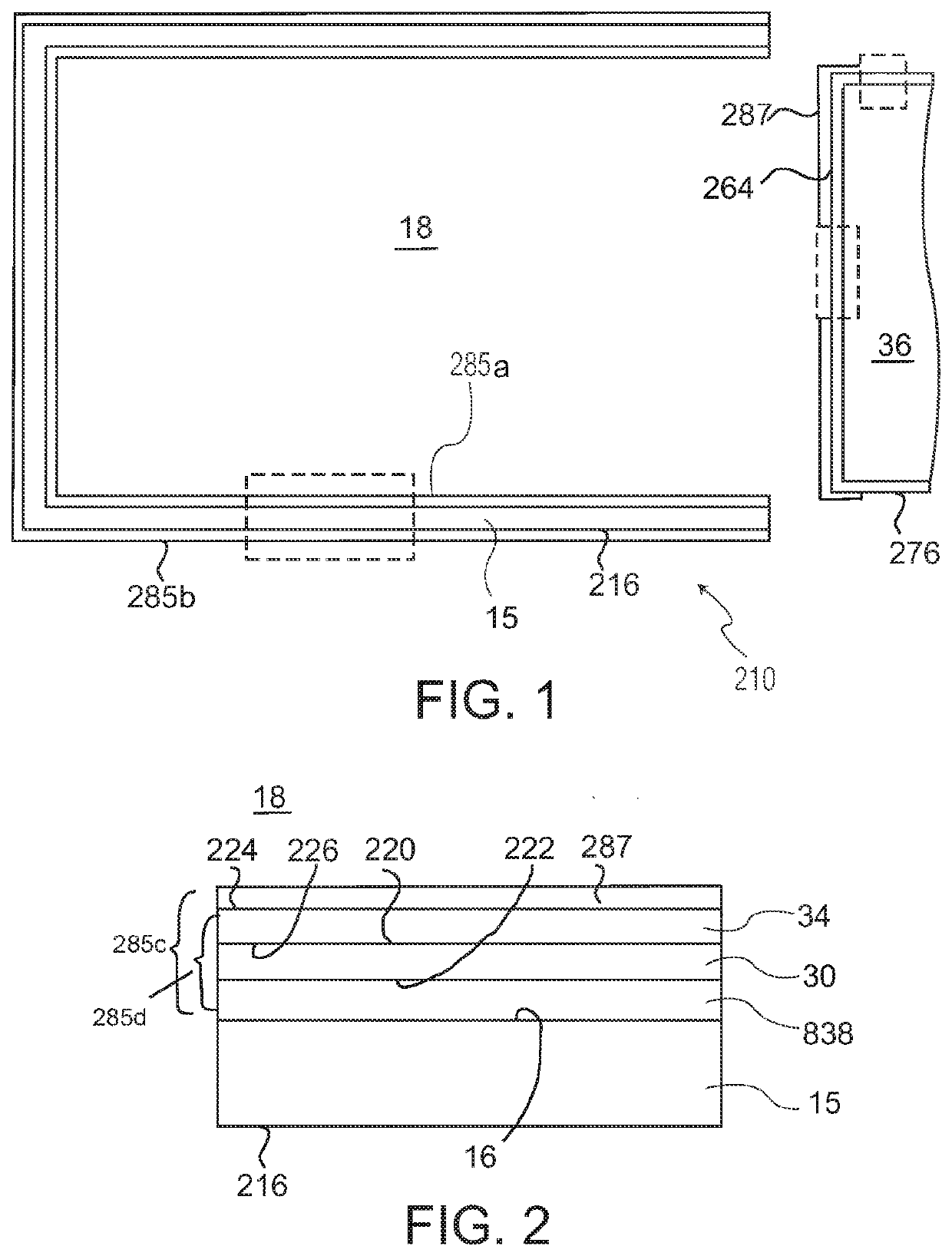
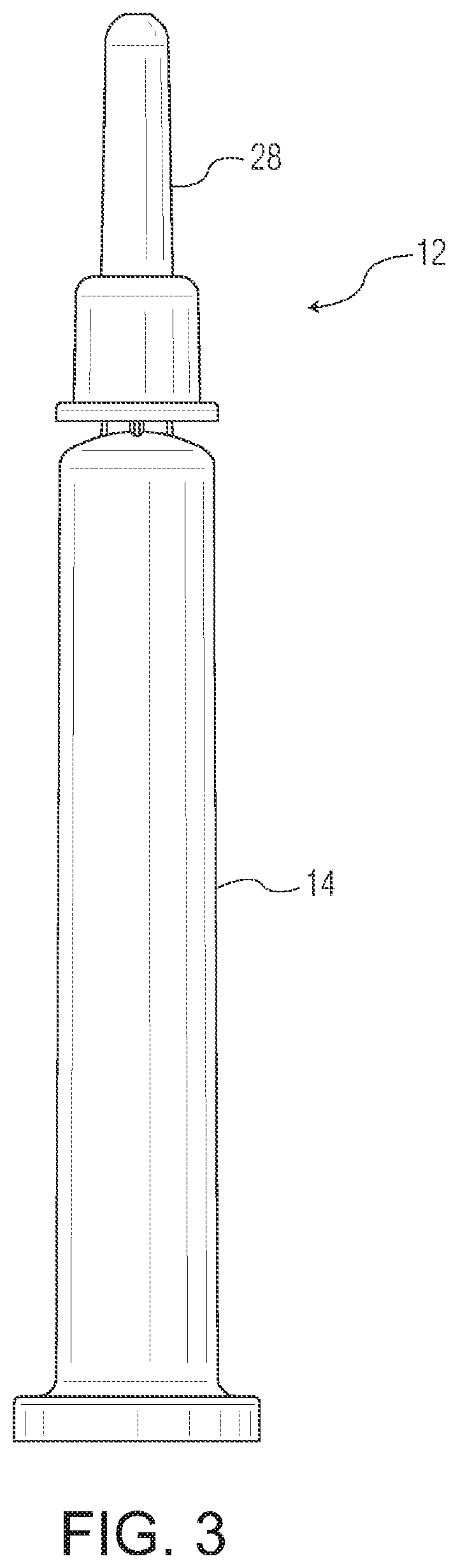
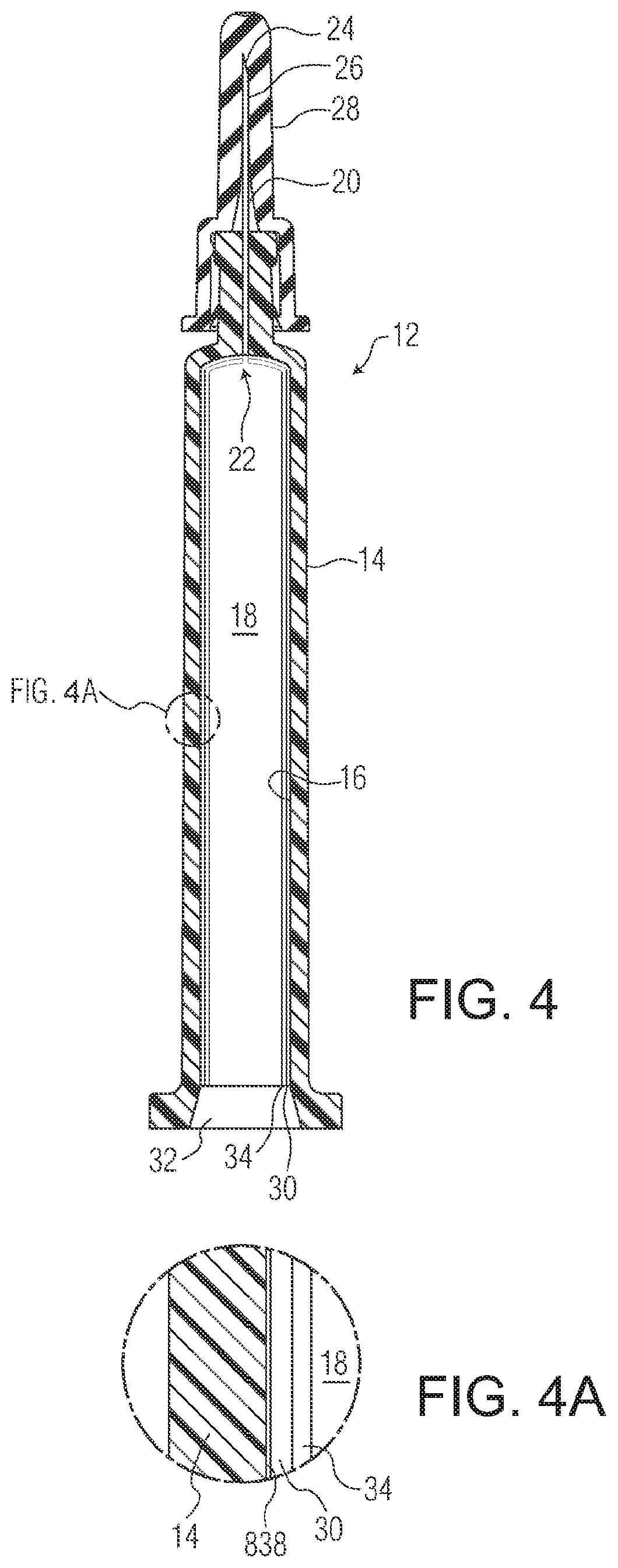
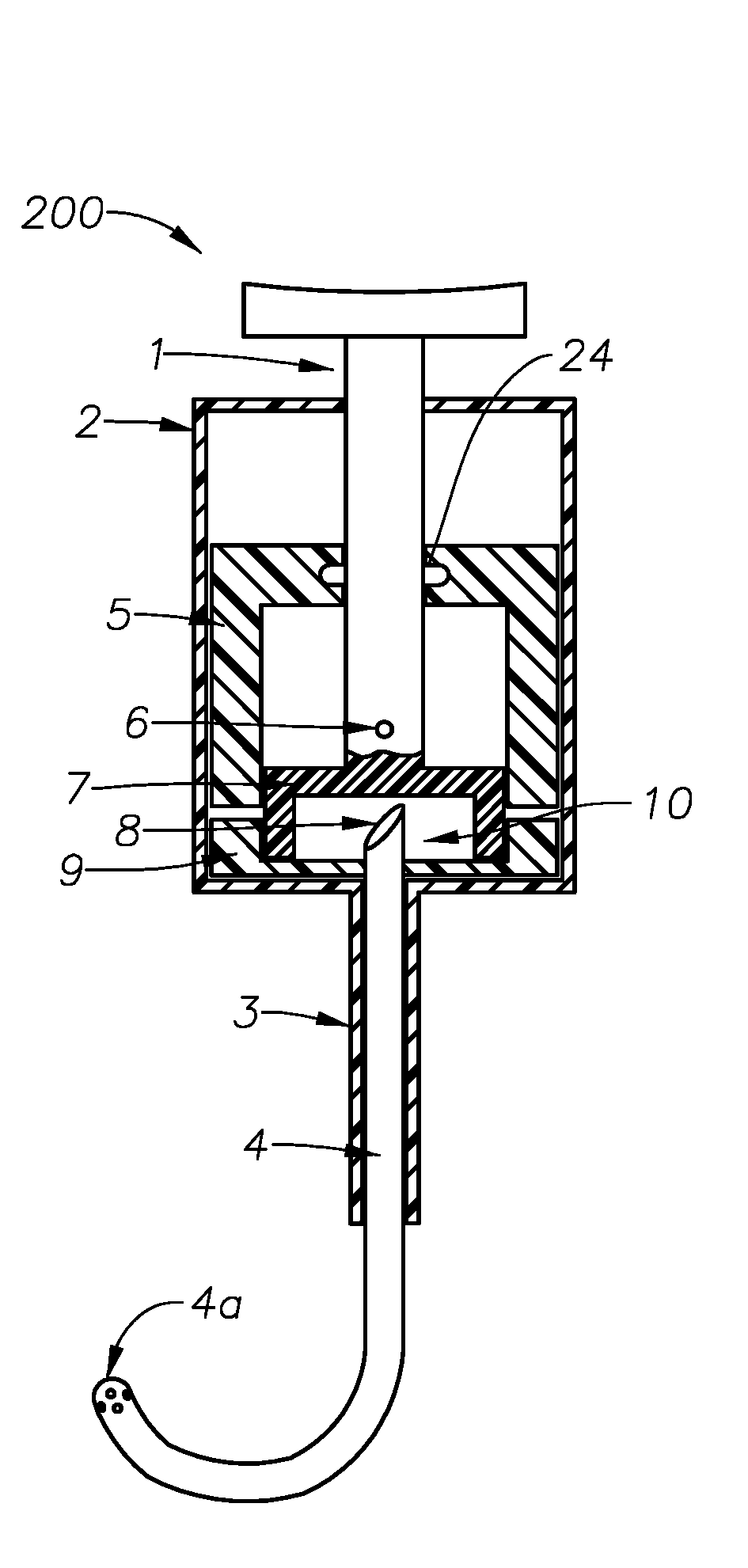
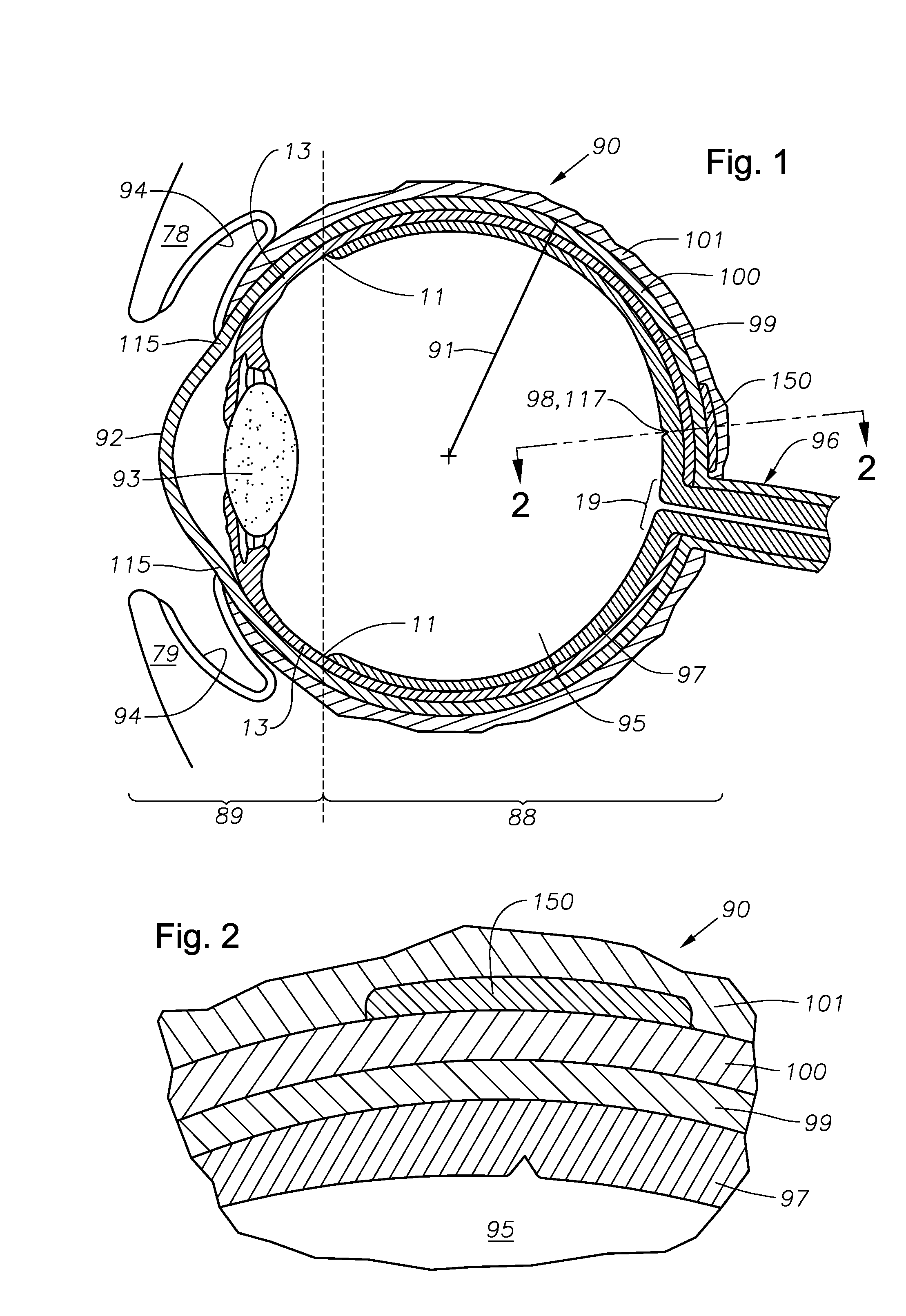
Ophthalmic drug delivery device
An ophthalmic drug delivery device having a scleral surface, an orbital surface, an injection port on the orbital surface, and a fluid conducting passageway disposed within the device that is fluidily coupled to the injection port and terminates in an opening for communicating the fluid to an outer surface of the sclera is disclosed. The fluid contains a pharmaceutically active agent useful for the treatment of a disease of the posterior segment of the eye.
Owner:NOVARTIS AG
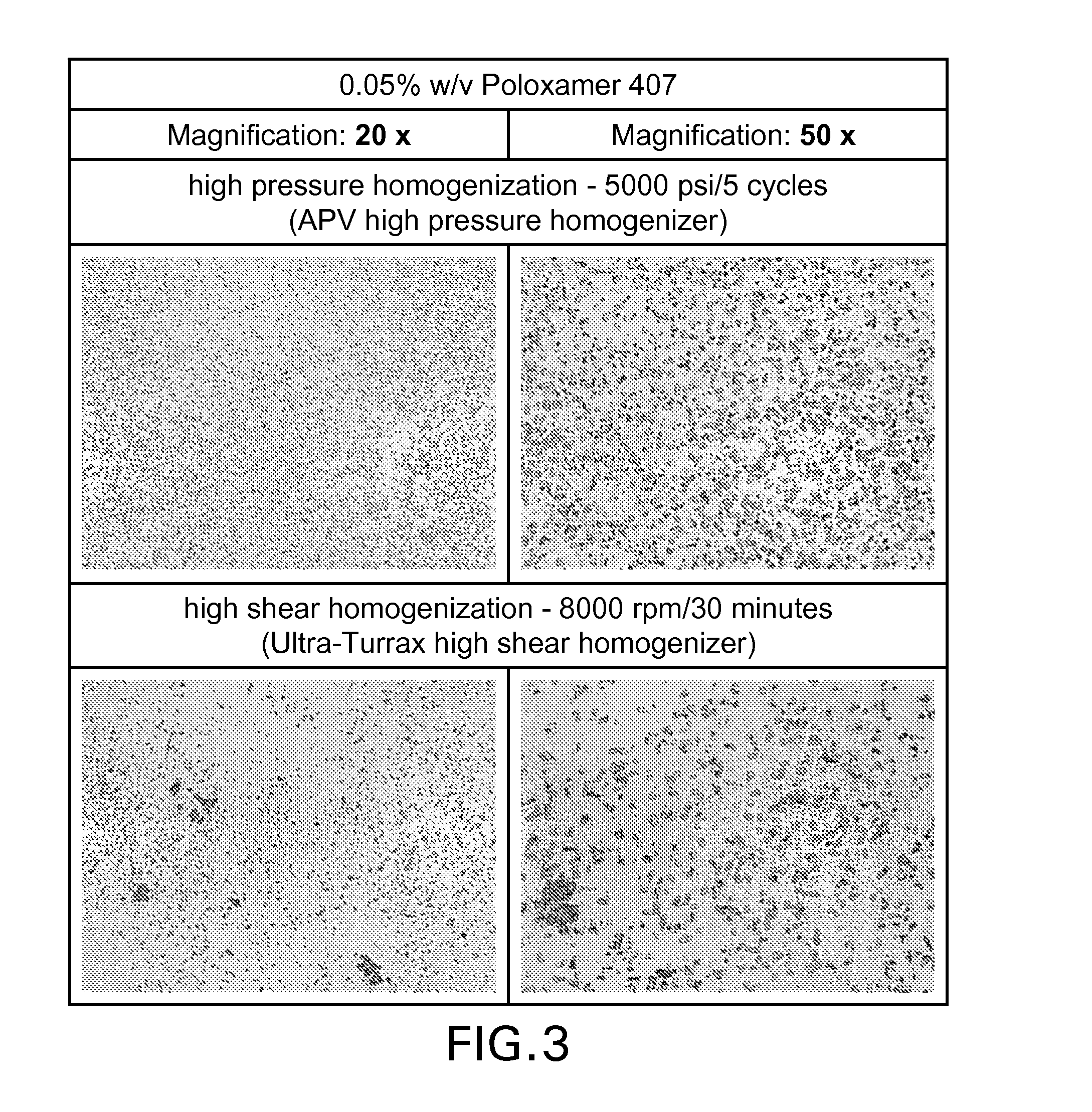
Suspension formulations of nepafenac and other ophthalmic drugs for topical treatment of ophthalmic disorders
Topical aqueous suspension compositions of sparingly soluble ophthalmic drugs are disclosed. The compositions comprise a combination of a poloxamer or meroxapol surfactant and a glycol tonicity-adjusting agent such as propylene glycol.
Owner:NOVARTIS AG
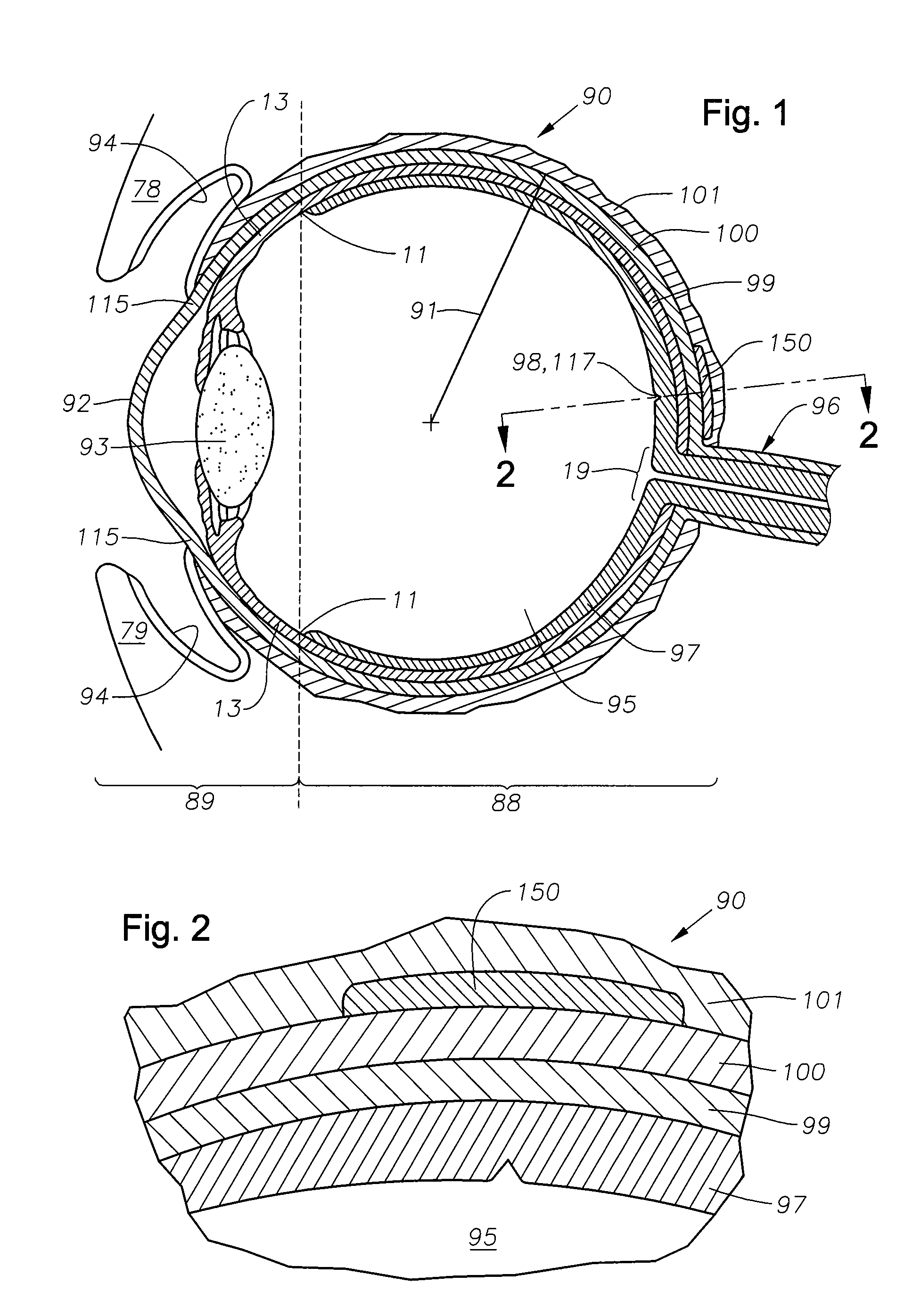
Juxtascleral Drug Delivery and Ocular Implant System
Ophthalmic drug delivery devices useful for delivery of pharmaceutically active agents to the posterior segment of the eye are disclosed.
Owner:ALCON RES LTD
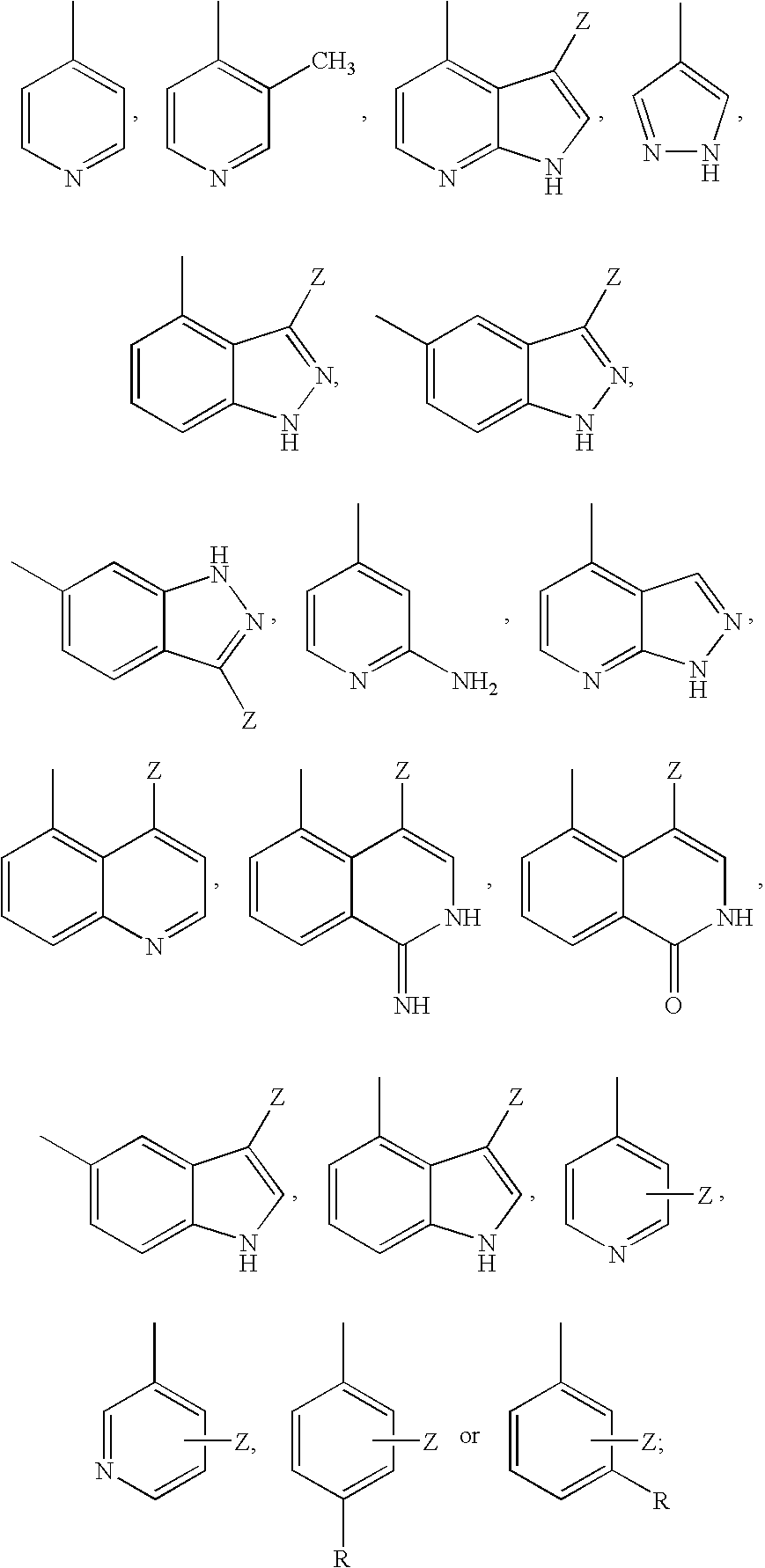
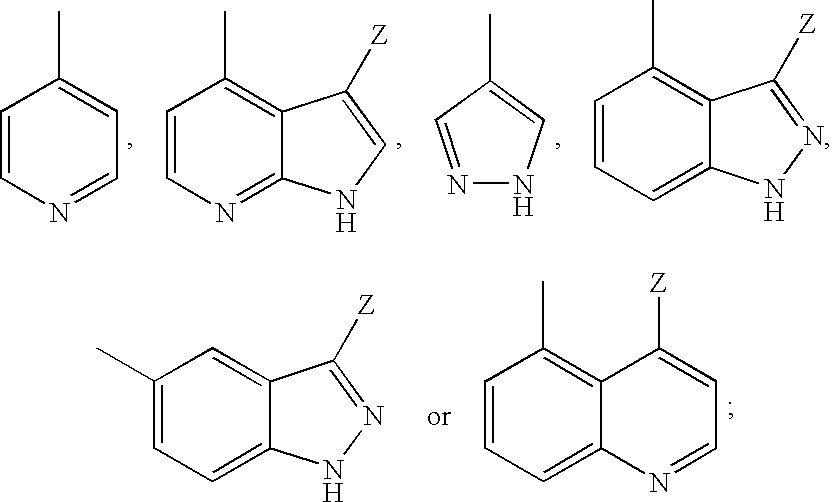
6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS
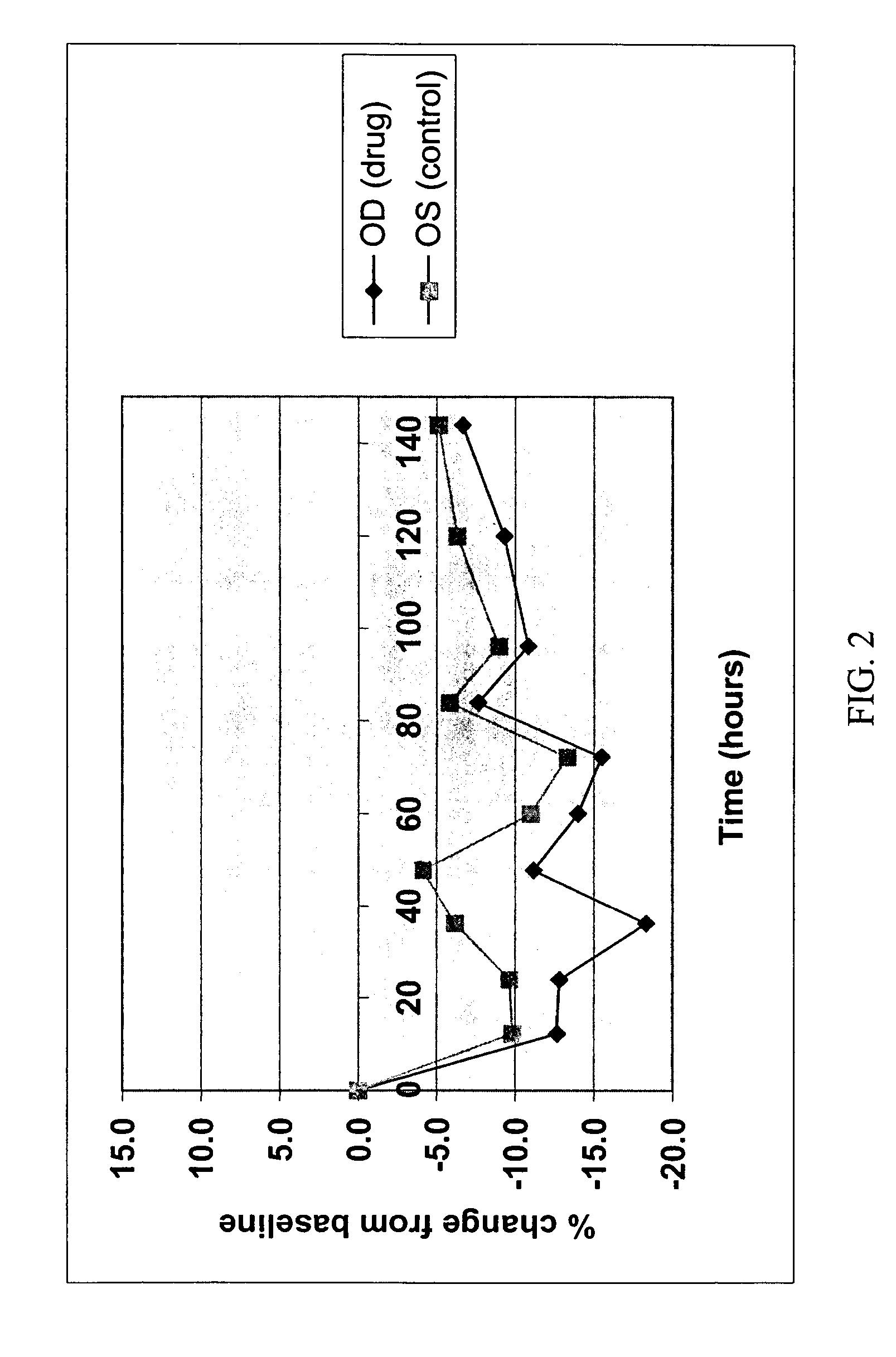
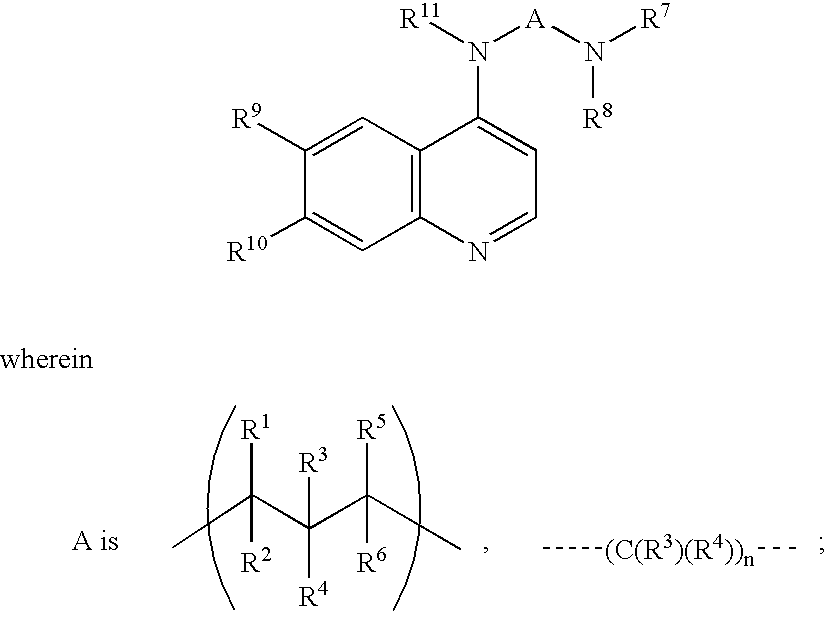
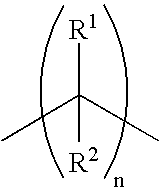
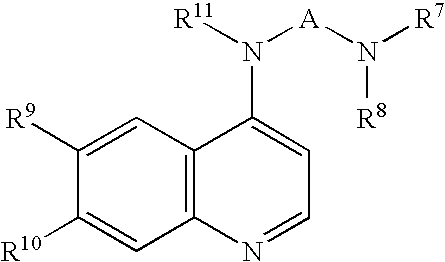
Methods for using 6-aminoimidazo[1,2-b]pyridazine analogs are disclosed herein to treat rho kinase-mediated diseases or rho kinase-mediated conditions, including controlling intraocular pressure and treating glaucoma, are disclosed. Ophthalmic pharmaceutical compositions useful in the treatment of eye diseases such as glaucoma, and additionally useful for controlling intraocular pressure, the compositions comprising an effective amount of 6-aminoimidazo[1,2-b]pyridazine analogs, are disclosed herein.
Owner:ALCON RES LTD
Ophthalmic drug delivery device
An ophthalmic drug delivery device having a first end and a second end, an injection port, a reservoir, and a sleeve is disclosed. The injection port is for sealingly engaging a needle of a syringe, which is for providing a fluid comprising a pharmaceutically active agent. The reservoir is disposed within the device, is fluidly coupled to the injection port, and has an opening for communicating the fluid to an outer surface of a sclera of an eye. The sleeve is for engaging the device proximate overlapping portions of the first end and the second end for forming a generally ring-shaped three-dimensional geometry upon implantation of the device on the outer surface of the sclera. The device is useful for the treatment of a disease of the posterior segment of the eye.
Owner:NOVARTIS AG
Cyclodextrin nanotechnology for ophthalmic drug delivery
The invention provides an ophthalmic composition which is an aqueous suspension comprising drug, cyclodextrin and water, the composition having an aqueous phase of from about 0.1% (w / v) to about 90% (w / v) of the drug in solution, as dissolved free drug and as dissolved drug / cyclodextrin complex(es), and a solid phase of from about 10% (w / v) to about 99.9% (w / v) of the drug as solid drug / cyclodextrin particles, suspended in the aqueous phase; the size of the solid particles being from about 10 nm to about 1 mm, the drug / cyclodextrin particles being capable of dissolving in aqueous tear fluid within 24 hours of application to the eye surface. The aqueous eye suspension can be in the form of eye drops, eye gel or eye mist. Further, the invention provides a method for treating a condition of the posterior segment and / or anterior segment of the eye comprising applying to the eye surface, in an amount which delivers to said segment or segments a therapeutically effective amount of a drug suitable for treating said condition, an ophthalmic composition which is as defined above. Nasal compositions and methods and ophthalmic and nasal compositions in powder form are also provided.
Owner:OCULIS EHF
Ophthalmic drug delivery device
InactiveUS20060039952A1Inhibit migrationEasy to implantEye treatmentProsthesisOphthalmologyActive agent
Ophthalmic drug delivery devices useful for delivery of pharmaceutically active agents to the posterior segment of the eye are disclosed. The devices may include extensions, immobilizing structures, and / or geometries to help properly locate, and prevent migration of, the devices.
Owner:YAACOBI YOSEPH
Ophthalmic Drug Delivery
The present invention includes and provides a method of delivering a medicament to an eye of a subject in need thereof a solution, the method comprising: (a) providing droplets containing the medicament with a specified average size and average initial ejecting velocity; and (b) delivering the medicament to the eye, where the droplets deliver a percentage of the ejected mass of the droplets to the eye.
Owner:EYENOVIA
Percutaneous absorption preparation for treating ophthalmic disease, use thereof and method for migration of ophthalmic remedy into topical tissue in eye
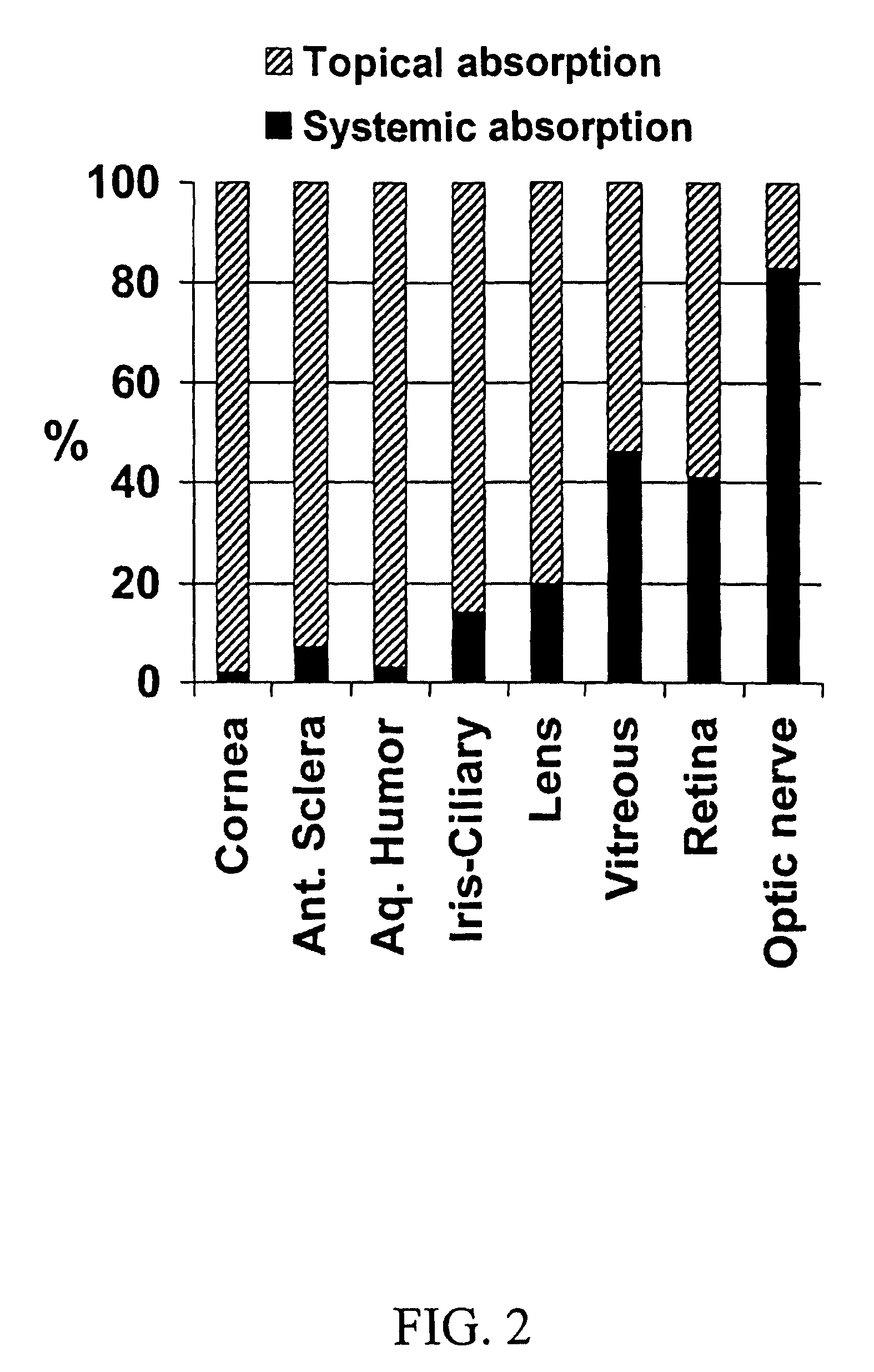
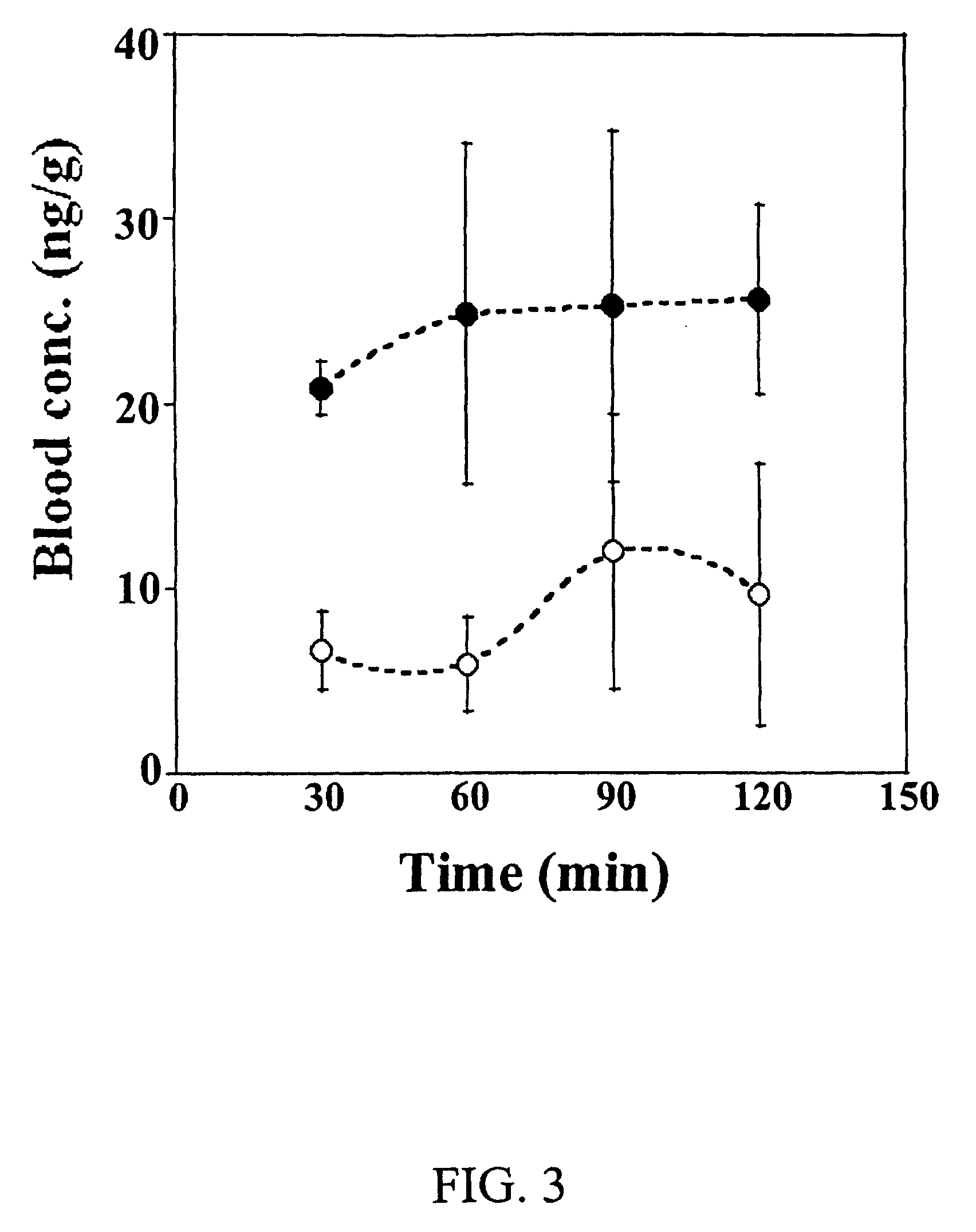
InactiveUS20060036220A1Efficacy can be sustainedly developedCan be sustainedly developedSenses disorderAntipyreticDiseaseEyelid
A transdermal drug delivery system for treatment of ophthalmic diseases comprising a structure that a plaster layer containing a remedy for ophthalmic diseases is provided on a support, wherein the system is applied to a skin surface including a front surface of an eyelid to administer the remedy for ophthalmic diseases in the plaster layer to an ophthalmic topical tissue by percutaneous permeation substantially without being administered through a systemic blood flow. Use of the transdermal drug delivery system for treatment of ophthalmic diseases, comprising applying the transdermal drug delivery system to a skin surface including a front surface of an eyelid to transfer the remedy for ophthalmic diseases in the plaster layer to an ophthalmic topical tissue by percutaneous permeation substantially without being administered through a systemic blood flow, and a method for transferring the remedy for ophthalmic diseases to the ophthalmic topical tissue.
Owner:SENJU PHARMA CO LTD
Device for Ophthalmic Drug Delivery
An ophthalmic drug delivery device having two actuation assemblies for dispensing incompatible dosage forms and facilitating the prevention of dosage form reflux.
Owner:ALCON INC
Ophthalmic Lipophilic and Hydrophilic Drug Delivery Vehicle Formulations
InactiveUS20140378401A1Increase shear forcePhase-low viscosityBiocideSenses disorderSolubilitySide effect
The ophthalmic drug delivery vehicles provide comfort and compliance; drug solubility, residence time and permeability; and reduce side effects. In addition, the delivery vehicle can be slightly modified to provide an artificial tear formulation.
Owner:PS THERAPIES LTD
Iris design as a drug depot for zonal drug delivery by contact lens
A contact lens providing zonal drug delivery includes a lens body having an optical axis and an opaque simulated iris pattern applied to the lens body, about the optical axis, with the pattern including an ophthalmic drug.
Owner:ALLERGAN INC
Ophthamological drugs
InactiveUS20060135609A1Improve permeabilityEsterified saccharide compoundsBiocideMedicineElevated intraocular pressure
The present invention relates generally to ophthamological drugs. More specifically, the inventon relates to a method of modifying (derivatizing) ophthamological drugs so as to increase their penetration through the cornea. The invention also relates to drugs modified (derivatized) in accordance with the instant method and to the use of same in treating conditions associated with elevated intraocular pressure, particularly, glaucoma.
Owner:DUKE UNIV
Opthalmic pharmaceutical compositions and methods for treating ocular inflammation
InactiveUS20060014786A1Improve efficiencySustained levelBiocidePharmaceutical delivery mechanismOphthalmology4-Aminoquinoline
The present invention relates to novel ophthalmic pharmaceutical compositions comprising an inflammation-treating amount of a 4-aminoquinoline compound, derivative, isomers, or chemical salts, and methods for using these compositions for the treatment of ocular inflammatory conditions by topical administration directly to the eye.
Owner:RAUT RAJEEV
Juxtascleral drug delivery and ocular implant system
Ophthalmic drug delivery devices useful for delivery of pharmaceutically active agents to the posterior segment of the eye are disclosed.
Owner:ALCON RES LTD
Aminopyrazine analogs for treating glaucoma and other rho kinase-mediated diseases and conditions
InactiveUS20060142307A1Control IOPOrganic active ingredientsSenses disorderIntraocular pressureKinase
Methods for using aminopyrazine analogs to treat rho kinase-mediated diseases or rho kinase-mediated conditions, including controlling intraocular pressure and treating glaucoma, are disclosed. Ophthalmic pharmaceutical compositions useful in the treatment of eye diseases such as glaucoma, and additionally useful for controlling intraocular pressure, the compositions comprising an effective amount of aminopyrazine analogs, are also disclosed.
Owner:ALCON INC
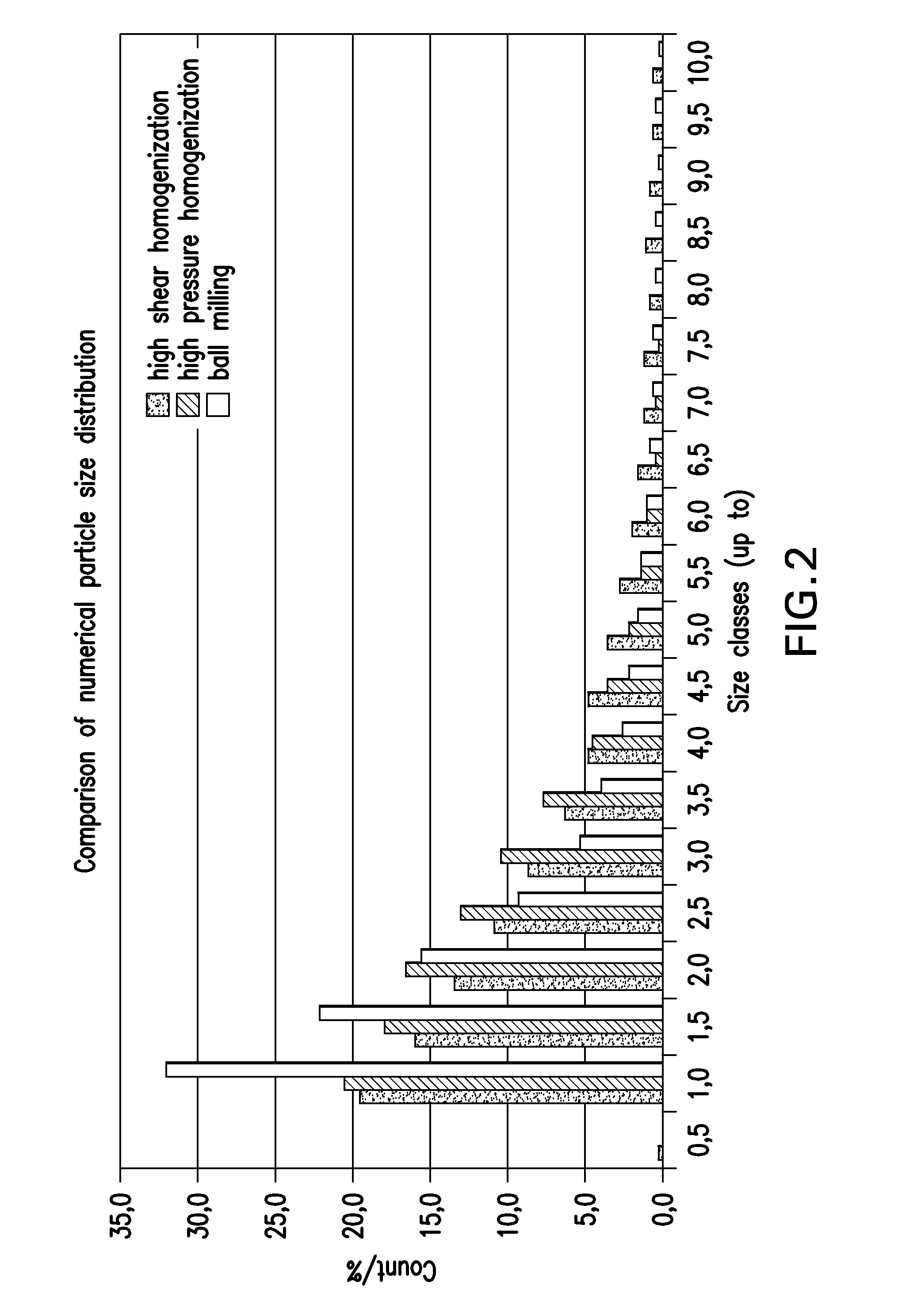
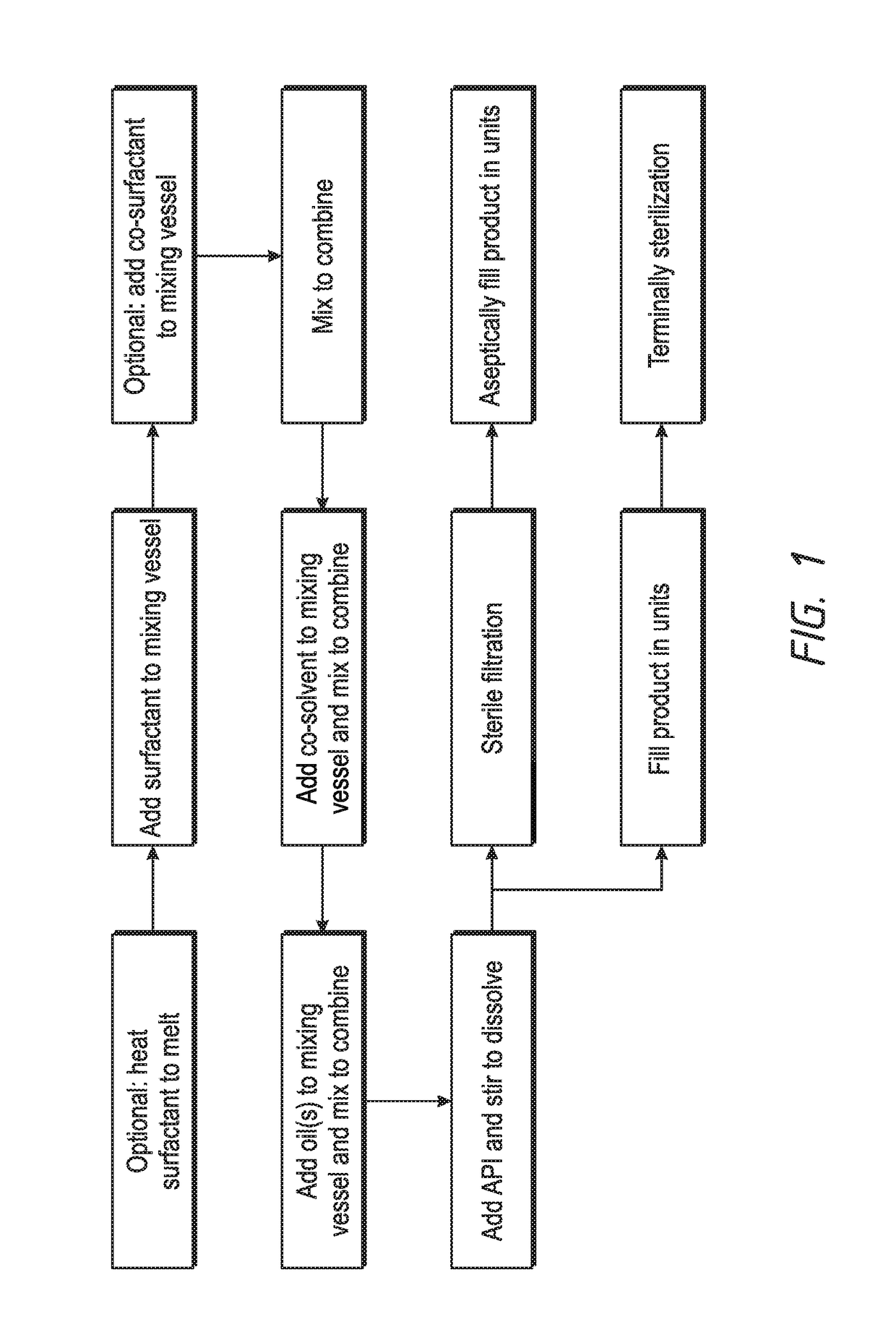
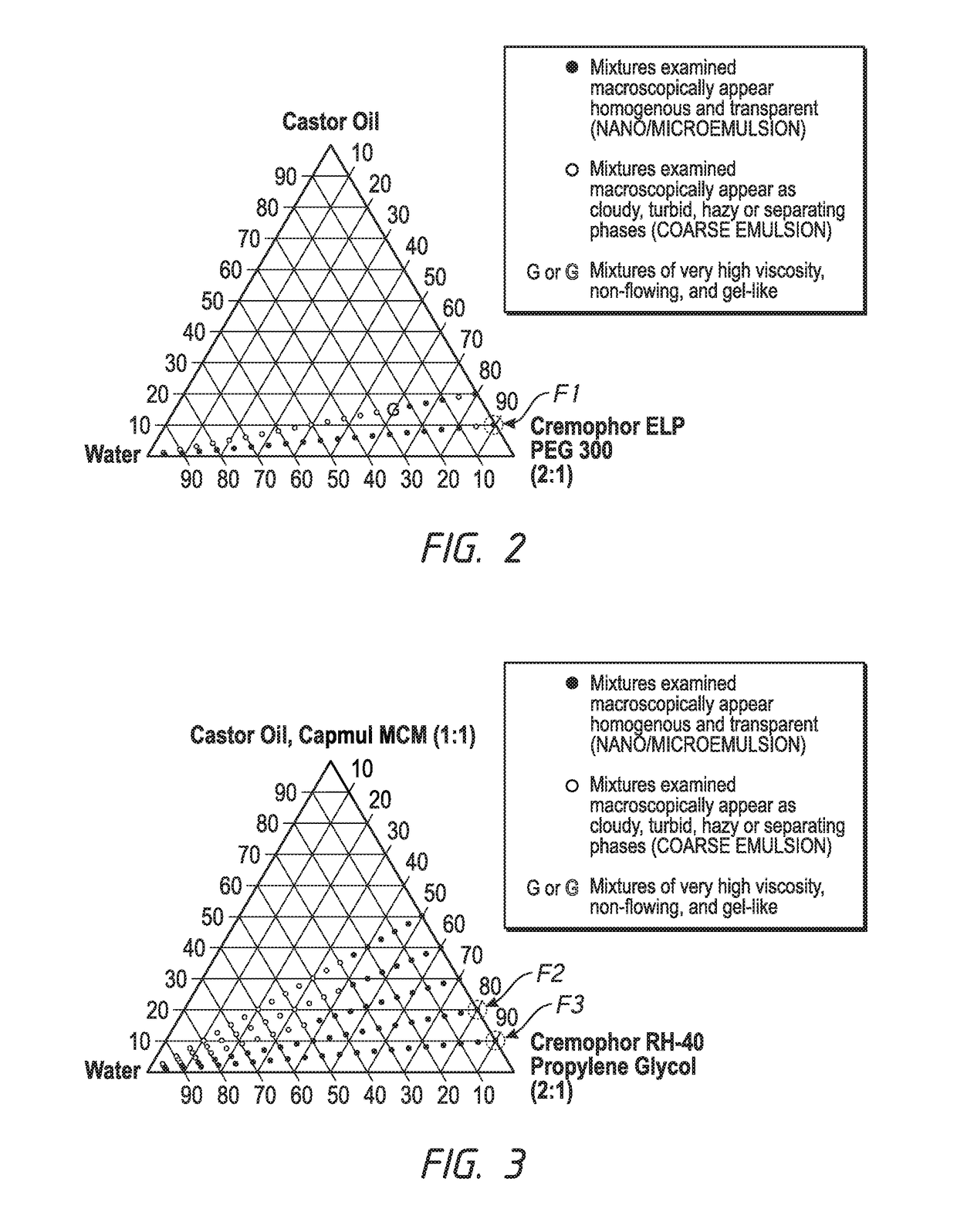
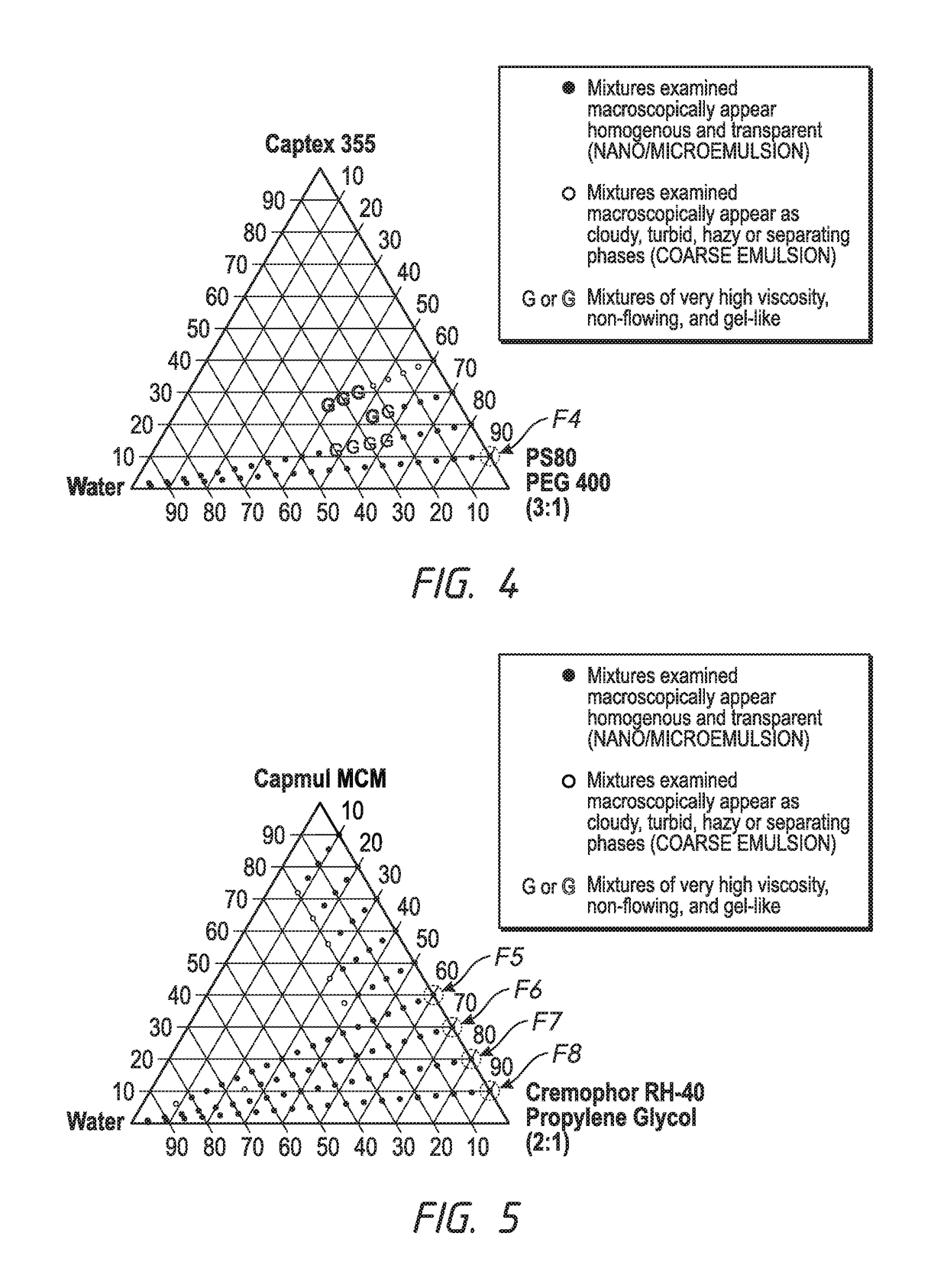
Ophthalmic formulations and processes for their preparation
InactiveUS20130065888A1Suspension stabilityUniform particle size distributionSenses disorderPharmaceutical delivery mechanismPharmacologyOphthalmic drugs
The invention relates to a process for preparing ophthalmic formulations and to formulations containing a suspension of an ophthalmic drug in an aqueous vehicle. The invention further relates to the production of stable ophthalmic formulations that have a minimal propensity to form drug aggregates.
Owner:TEVA PHARM USA INC
Ophthalmic drug delivery
The present invention includes and provides a method of delivering a medicament to an eye of a subject in need thereof a solution, the method comprising: (a) providing droplets containing the medicament with a specified average size and average initial ejecting velocity; and (b) delivering the medicament to the eye, where the droplets deliver a percentage of the ejected mass of the droplets to the eye.
Owner:EYENOVIA INC
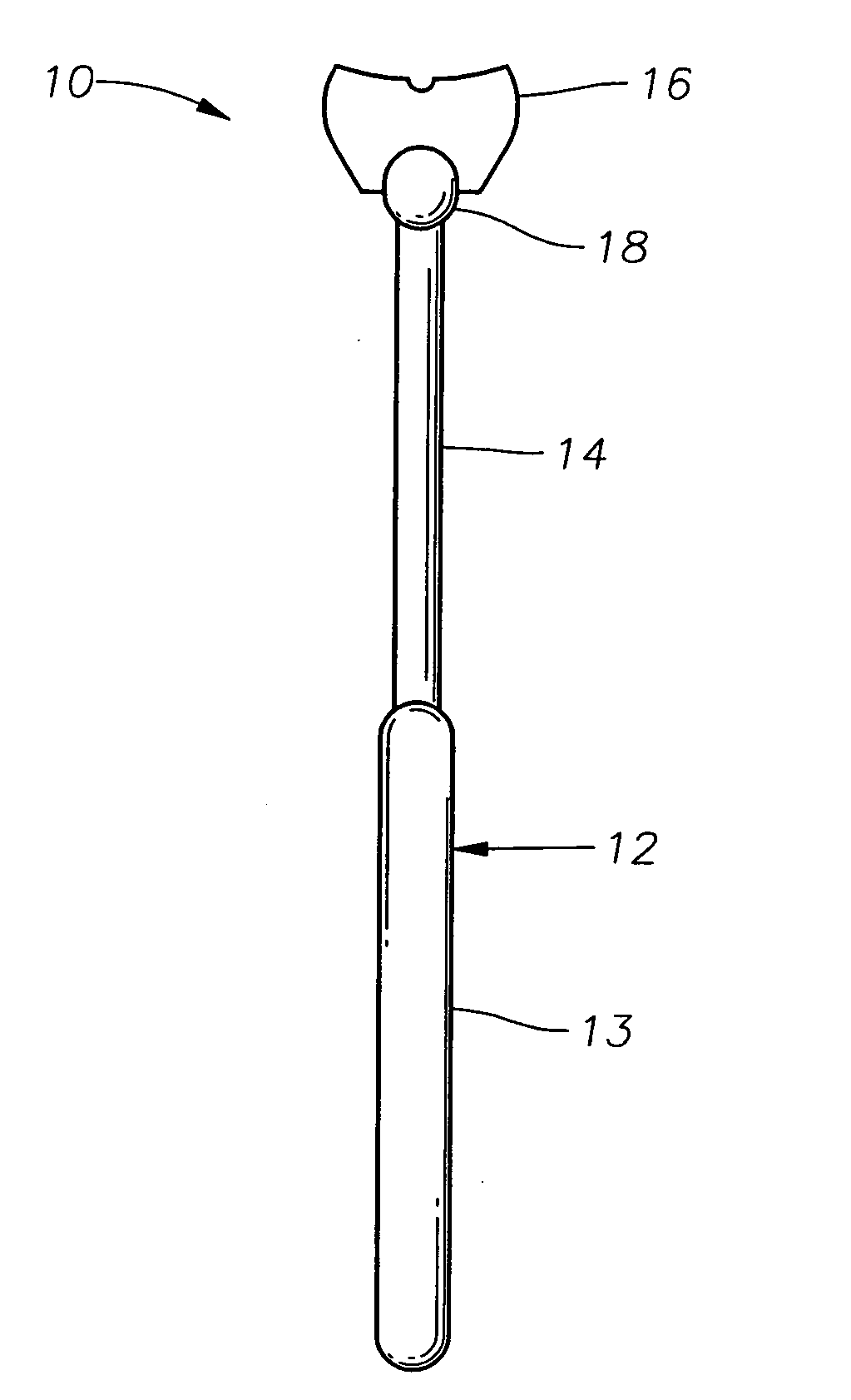
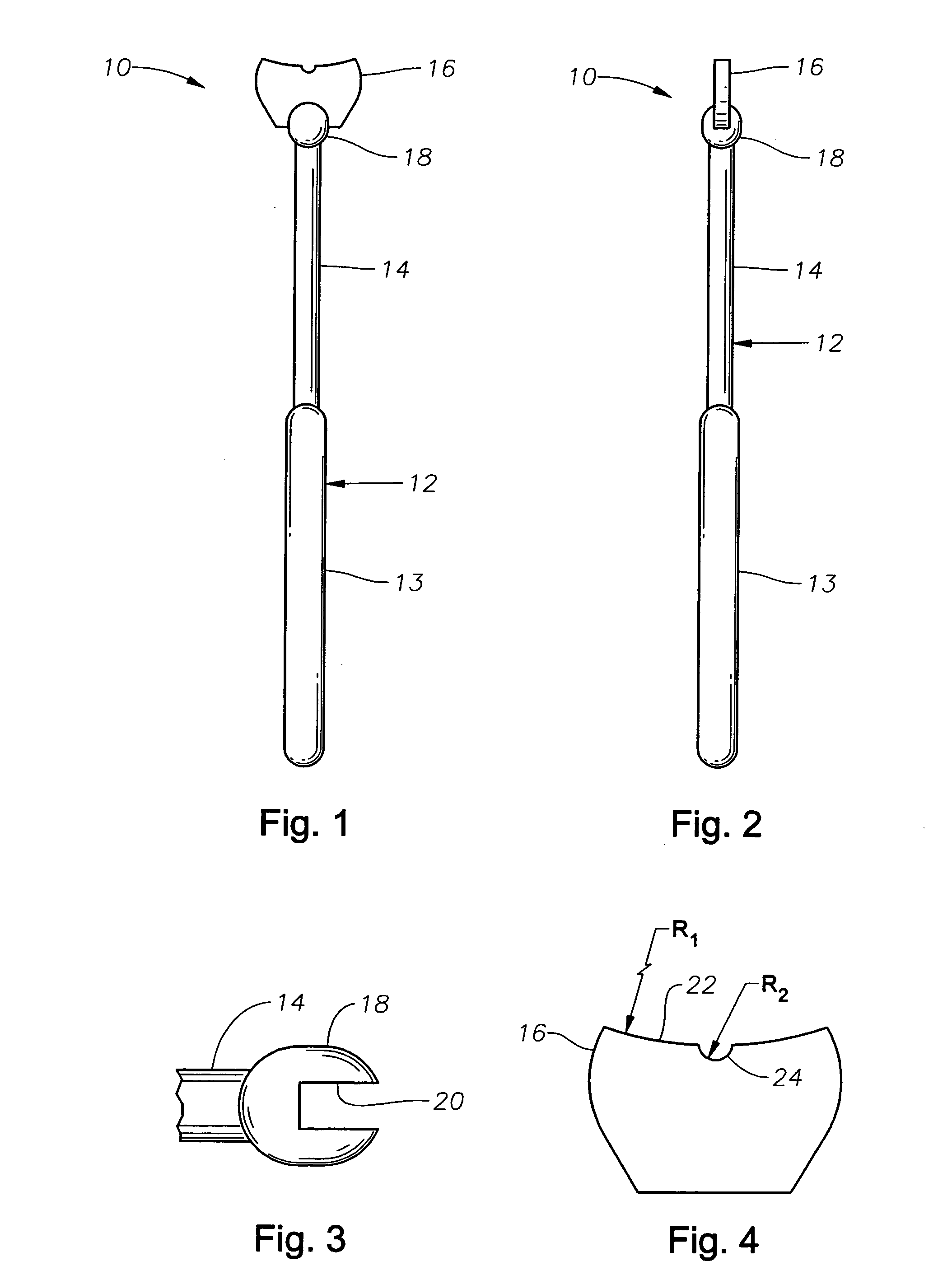
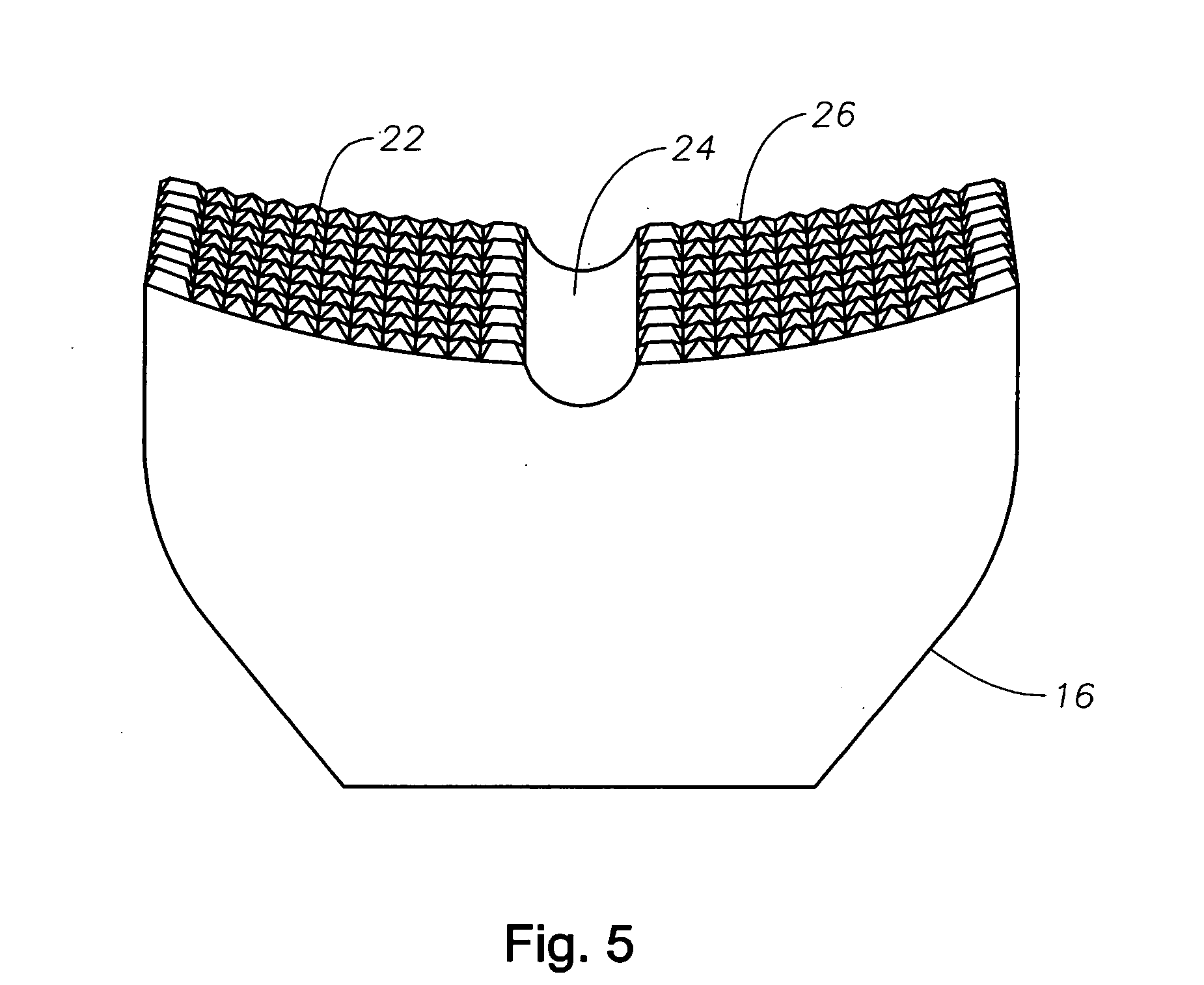
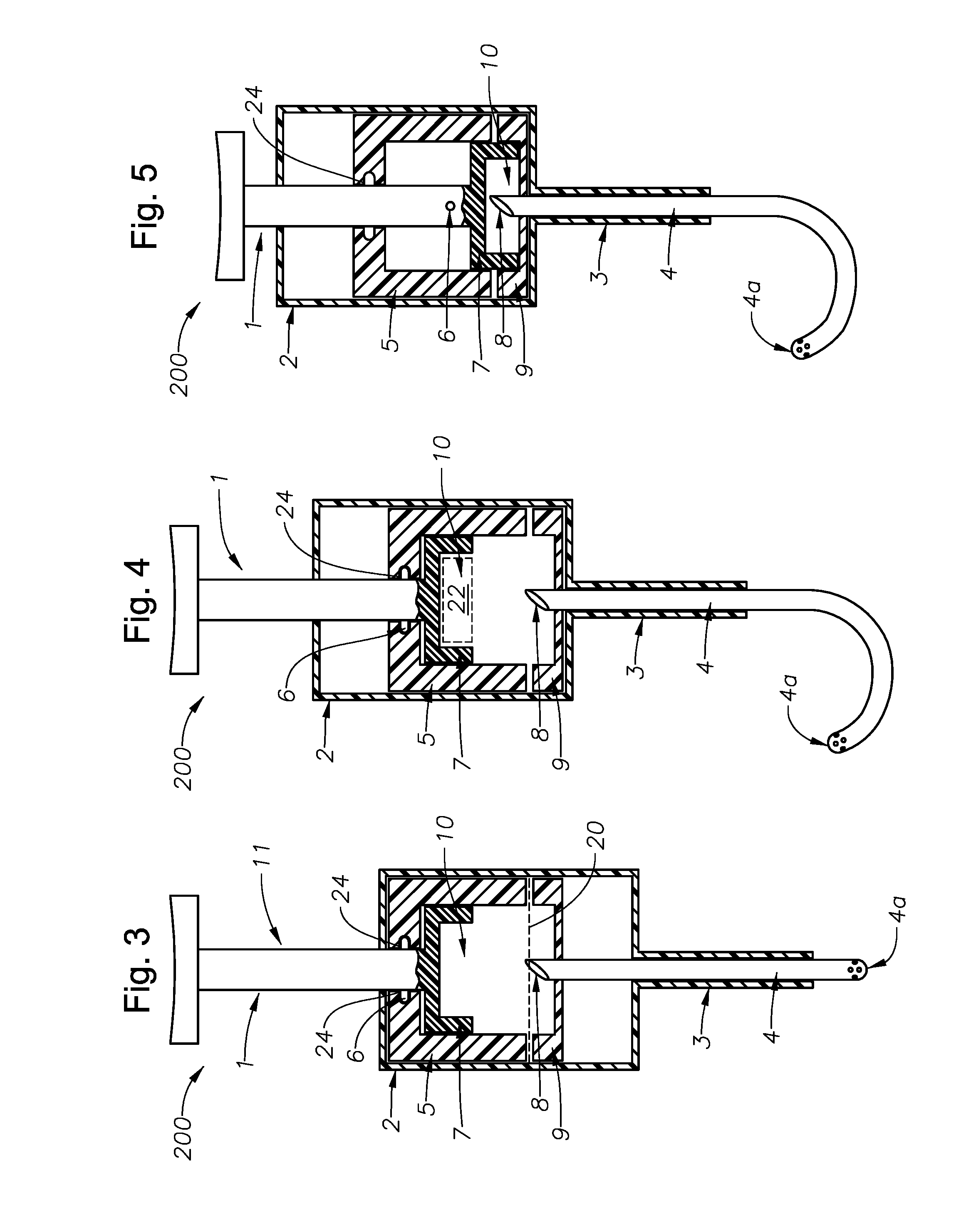
Counter pressure device for ophthalmic drug delivery
InactiveUS20060047255A1Facilitates drug placementPreventing and minimizingSurgical instrument detailsMedical applicatorsRefluxConjunctiva
A counter pressure device for ophthalmic drug delivery. The device includes a handle and a head coupled to a distal end of the handle. The head comprises a curved, concave surface for contacting the conjunctiva and having a notch for removably receiving a cannula. The device minimizes or prevents drug reflux and facilitates drug placement during ophthalmic drug delivery.
Owner:NOVARTIS AG
Pharmaceutical package for ophthalmic formulations
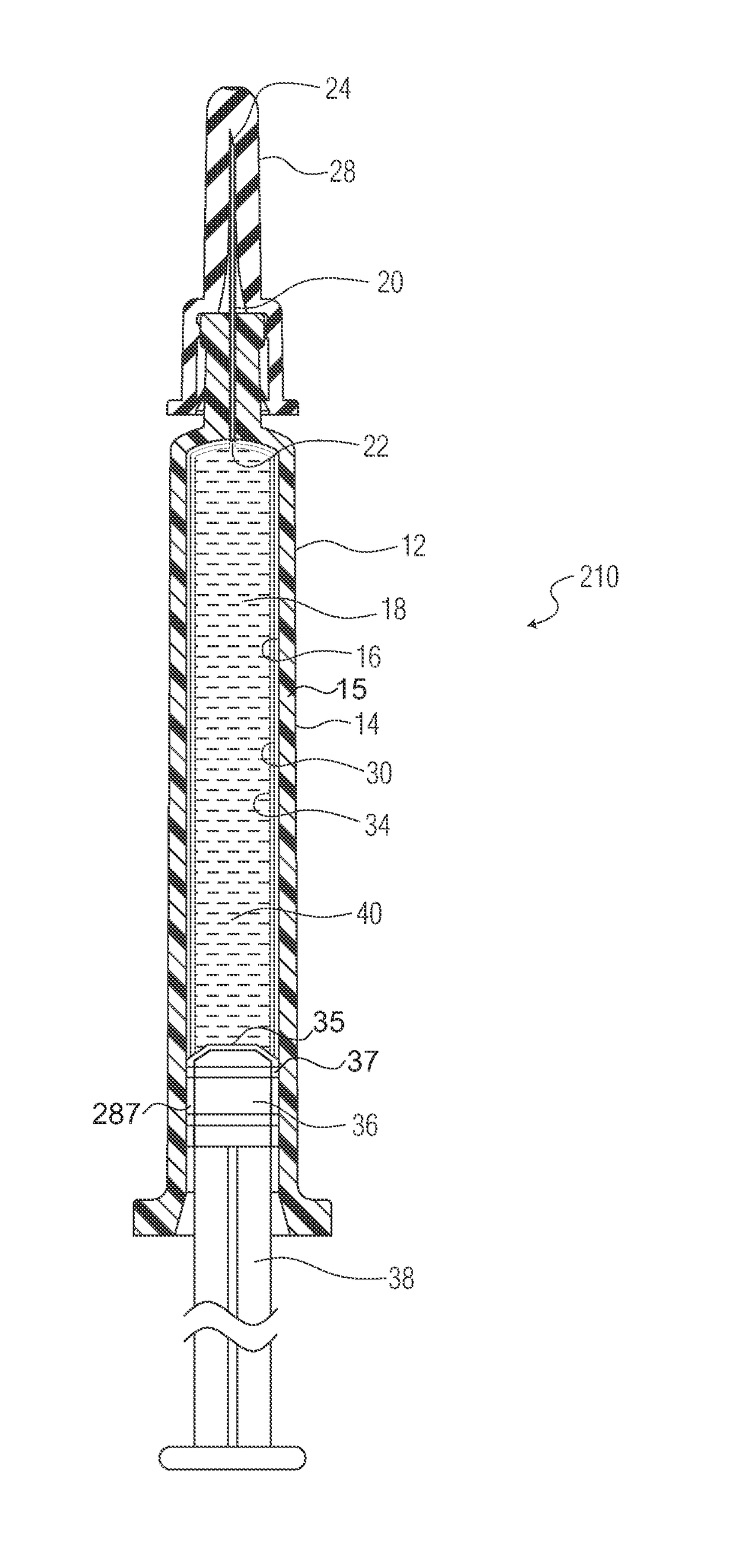
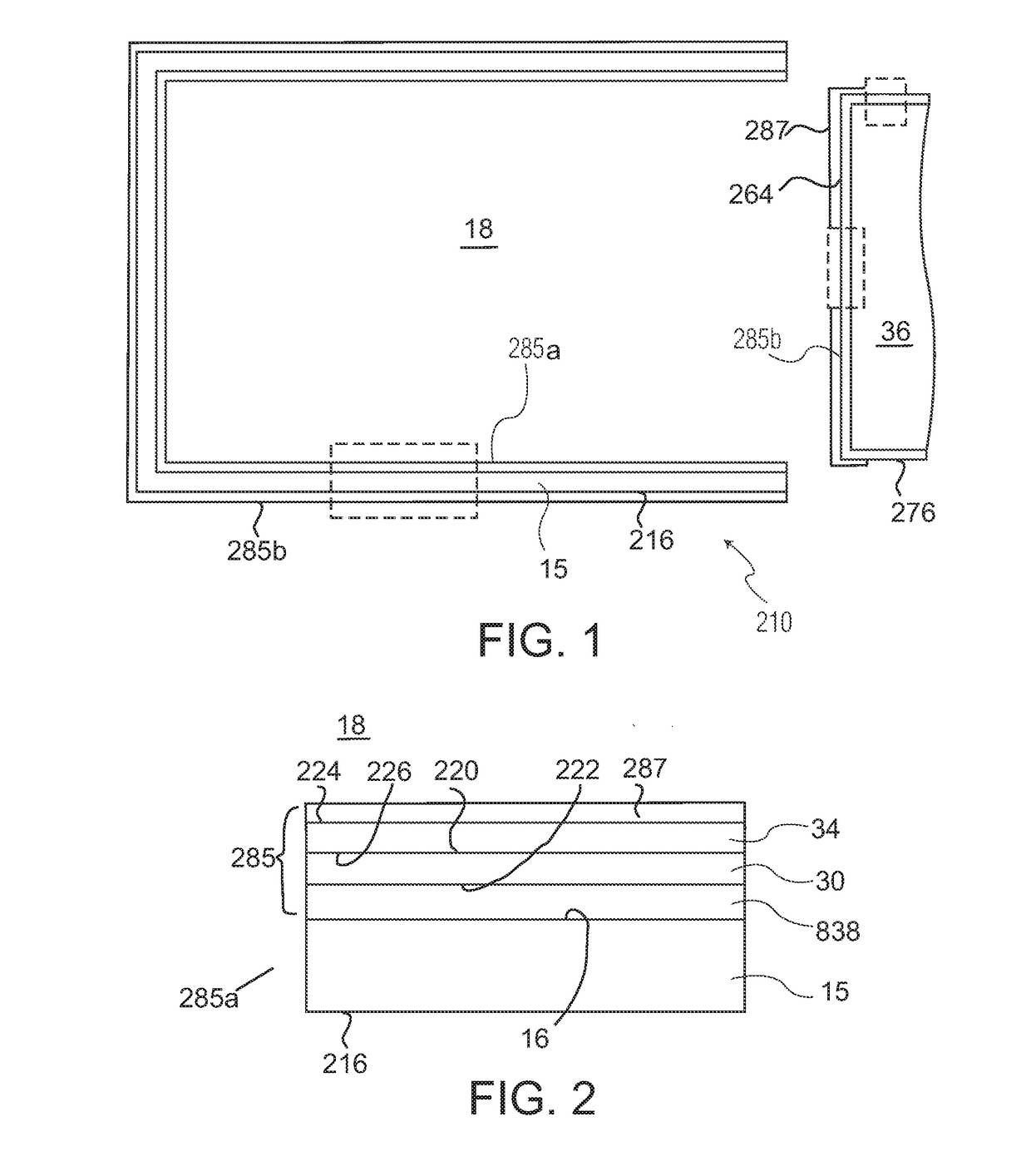
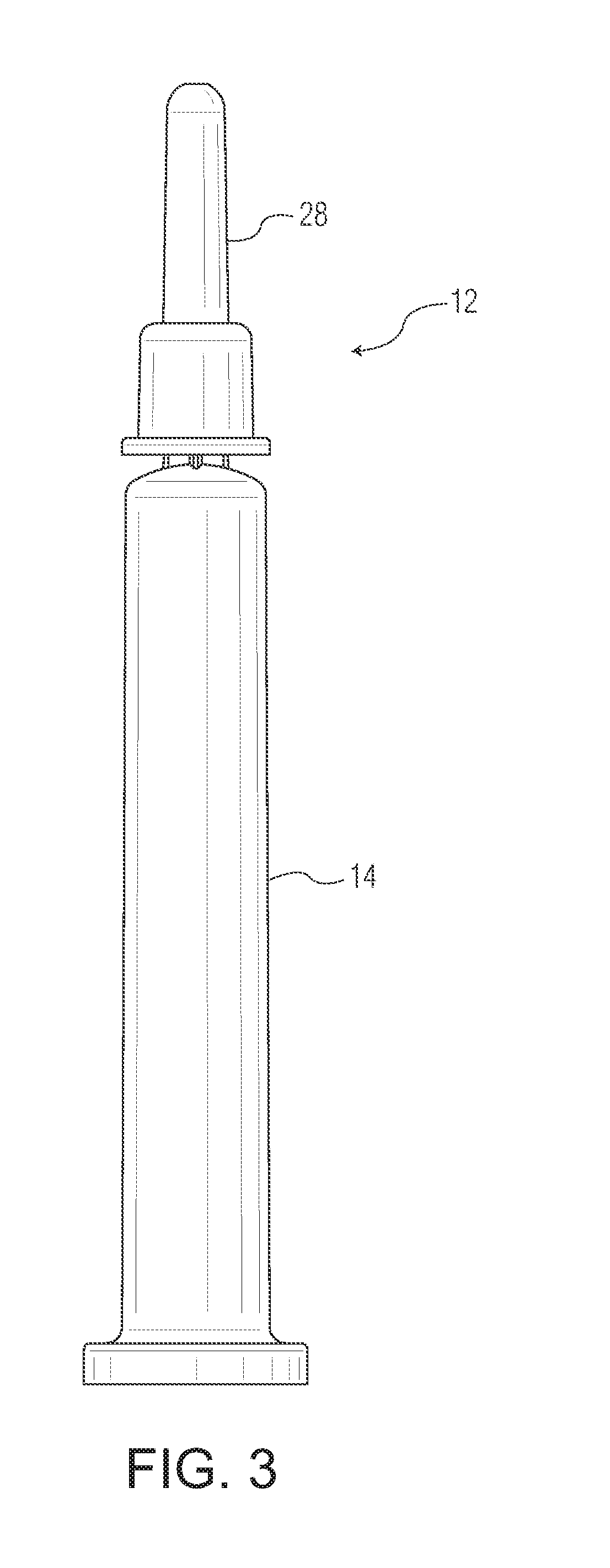
ActiveUS20180325728A1Senses disorderPeptide/protein ingredientsBiomedical engineeringOphthalmic drugs
A liquid formulation of an ophthalmic drug in a pharmaceutical package, for example a syringe, cartridge, or vial, made in part or in whole of a thermoplastic polymer, coated on the interior with a tie coating or layer, a barrier coating or layer, a pH protective coating or layer, and optionally a lubricity coating or layer.
Owner:SI02 MEDICAL PRODS
Ophthalmic pharmaceutical compositions for the treatment of neoangiogenic pathologies of the eye
The present invention relates to ophthalmic pharmaceutical compositions comprising Sorafenib, its derivatives or active metabolites, for the treatment and prevention of ocular neoangiogenic pathologies.
Owner:S I F I SPA
Ophthalmic percutaneous absorption type preparation
The present invention provides an ophthalmic percutaneous absorption type preparation containing an ophthalmic drug and a vasoconstrictor, which can increase the amount of the ophthalmic drug transferred through the eyelid to a topical area in the eye, particularly the anterior segment of the eye such as conjunctiva, lacrimal fluid, aqueous humor, cornea and the like by administration to the skin surface of an eyelid.
Owner:SENJU PHARMA CO LTD
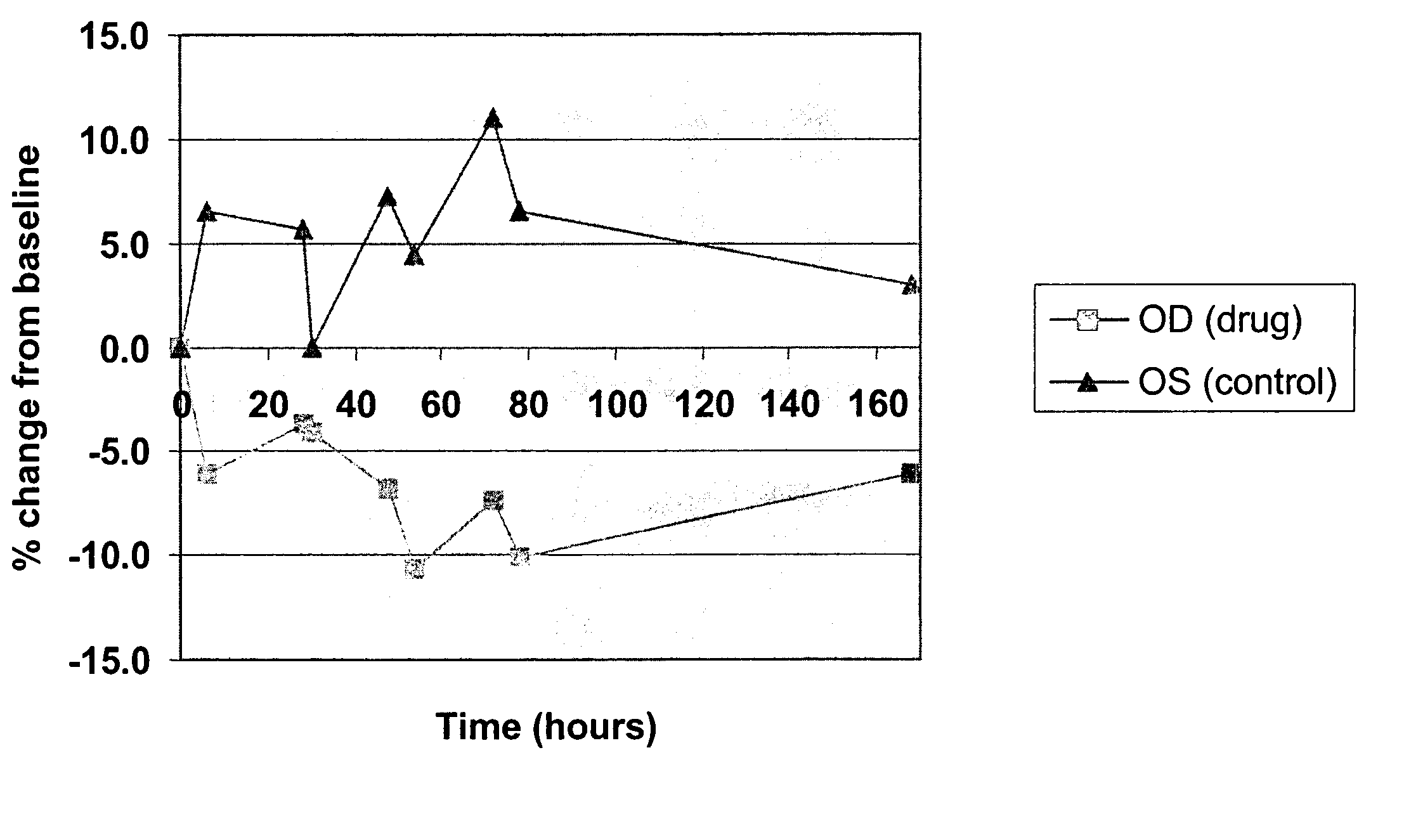
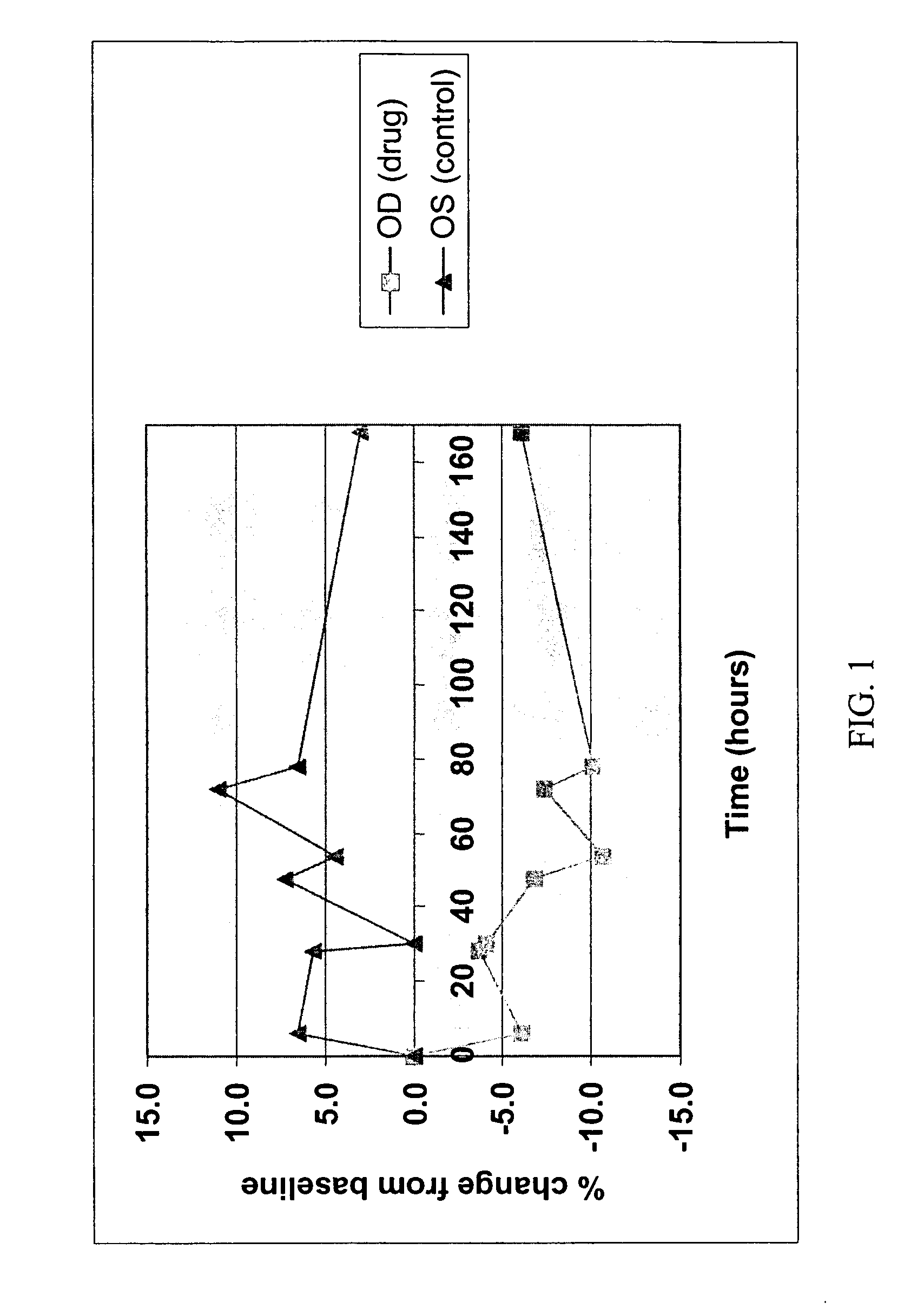
Method for delivering ophthalmic drugs
This invention relates to a method of delivering ophthalmic drugs, specifically prostaglandins and prostamides. This method may also be applied to other types of intraocular pressure (IOP)-lowering drugs (e.g. carbonic anhydrase inhibitors, beta blockers, alpha-adrenergic agonists, and parasympathomimetics) as well as other types of ophthalmic drugs for other indications (e.g. dry eye, inflammation, and infection).
Owner:JOHNSON & JOHNSON VISION CARE INC
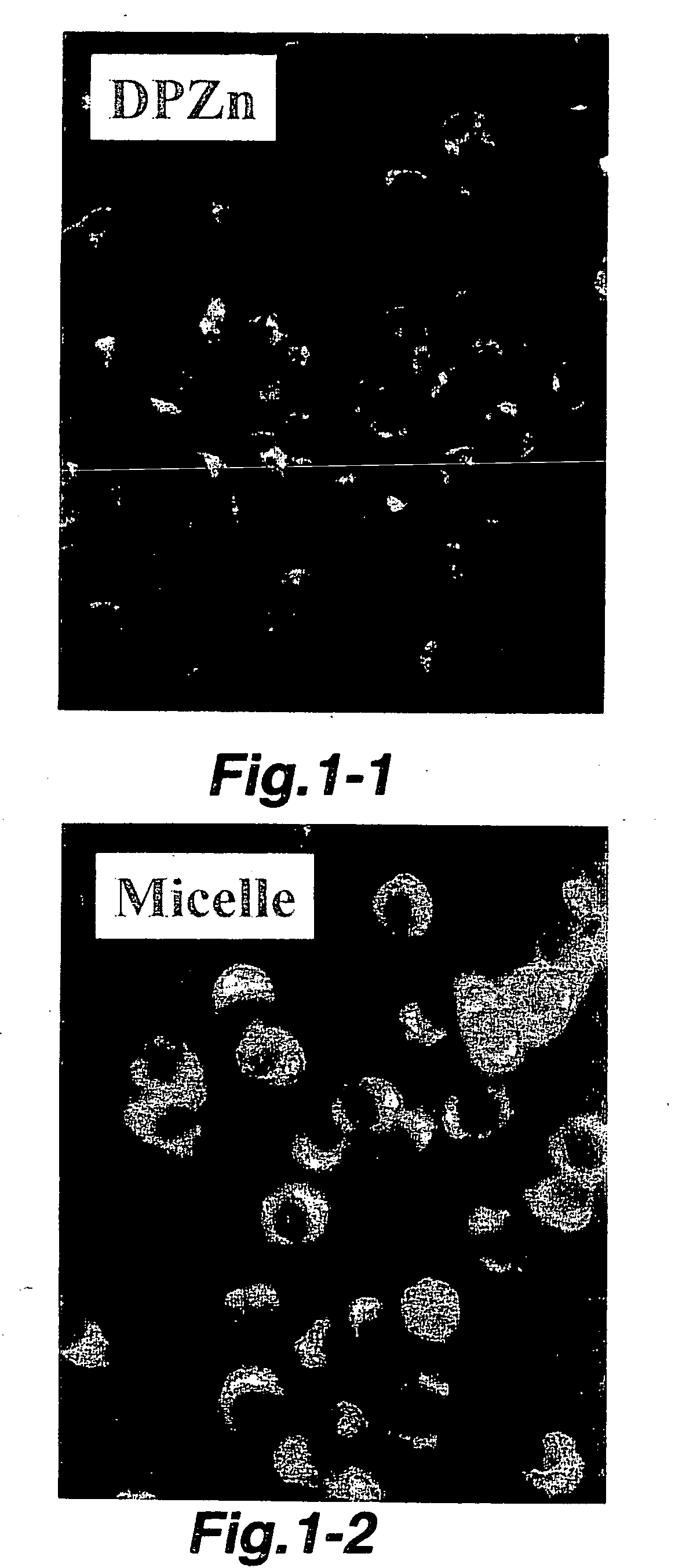
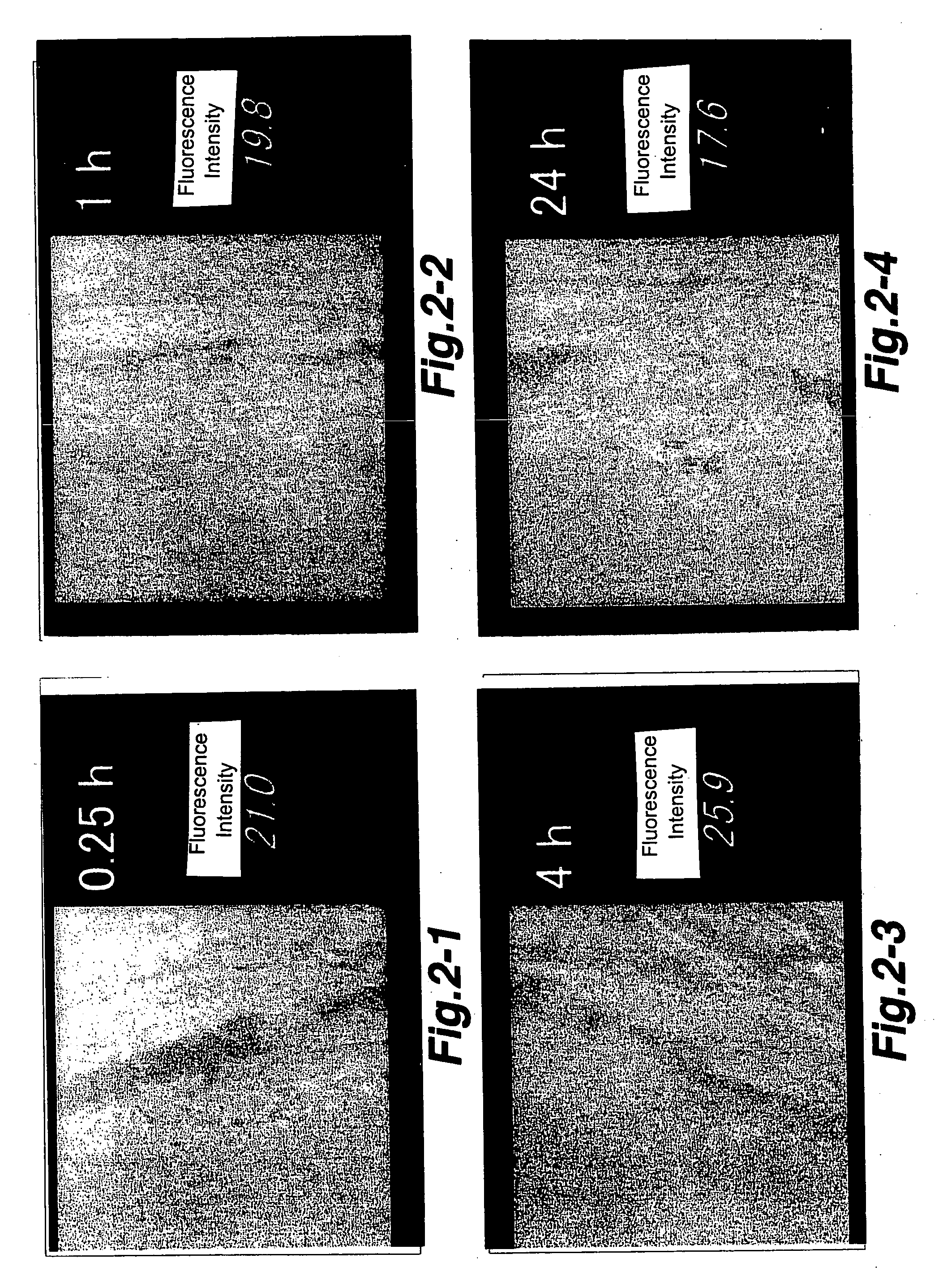
Ophthalmic drug delivery system using polymer micell
InactiveUS20060127481A1Improve accumulation abilityEasy to usePowder deliveryBiocideSenile macular degenerationWhole body
Owner:KATAOKA +3
Nano lipid cubic crystal preparation and its preparation method and application
InactiveCN102274175AImprove stabilityAchieve autoclave sterilizationSenses disorderSolution deliveryLipid formationMedicine
The invention discloses a nanometer lipid cubic crystal preparation, which comprises fat-soluble medicine, amphiphilic lipid, stabilizer and water. The invention also discloses a preparation method of the nanometer lipid cubic crystal preparation and an application of the nanometer lipid cubic crystal in preparation of an ophthalmic drug preparation for treating ophthalmic diseases. The nano lipid cubic crystal preparation of the present invention can significantly improve the corneal penetration ability of drugs, has better stability than liposomes and nanoparticles, can realize high-pressure high-temperature sterilization, has good drug release characteristics, and has broad clinical applications and market prospects.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Sterilizable pharmaceutical package for ophthalmic formulations
PendingUS20200171244A1Reduced stabilityMinimize the numberMedical devicesPharmaceutical delivery mechanismChlorine dioxideOphthalmology
A liquid formulation of an ophthalmic drug in a pre-filled pharmaceutical package, for example a syringe, cartridge, vial or any other vessel made in part or in whole of a thermoplastic polymer, coated on the interior with a tie coating or layer, a barrier coating or layer, a pH protective coating or layer, and optionally a lubricity coating or layer. A blister, a pouch, a bag, a tray or a tub may encompass as a secondary packaging the syringe, vial, cartridge, tube or any other vessel. The package is suitable for sterilization (e.g., surface and / or terminal sterilization) with sterilization gas residuals being minimal and / or lower than required by ISO 10993-7; and / or the stability of the ophthalmic drug is maintained, during a prolonged time period following the sterilization. The sterilization gas may be EO, propylene oxide, chlorine dioxide, nitrogen dioxide, or vaporized hydrogen peroxide (VHP), among others.
Owner:SI02 MEDICAL PRODS
Pharmaceutically Stable Compound Consisting of Timolol, Dorzolamide and Brimonidine
The present invention is related to ophthalmic formulations for the treatment of ocular ailments. More specifically, it is related to the pharmaceutical industry in the production of ophthalmic medication for the treatment of ocular hypertension. The advantage of the present invention over other state of the art treatments is that it achieves a composition of Dorzolamide Hydrochloride, Timolol Maleate and Brimonidine Tartrate with excellent properties of stability; it does not give rise to chemical reactions which produce modifications in the active molecules; with no antagonistic effects among the components. The present invention consists of a stable pharmaceutical composition for the treatment of ocular hypertension characterized by consisting of the following excipients: Polyoxyl 40 Stearate, Sodium Borate crystals, Sodium Chloride, Mannitol and Benzalkonium chloride.
Owner:BAYARDO ARTURO JIMENEZ
Self-emulsifying drug delivery (SEDDS) for ophthalmic drug delivery
InactiveUS20180036233A1Easy to prepareFew stepsAntibacterial agentsOrganic active ingredientsNano sizeEmulsion
Provided herein are topical ophthalmic preparations which comprise a non-aqueous, self-emulsifying system which can spontaneously give rise to either nanosized emulsions upon contact with an aqueous phase. Also provided herein are methods for the preparation of the same and their use in formulating and delivering poorly water soluble drugs.
Owner:ALLERGAN INC
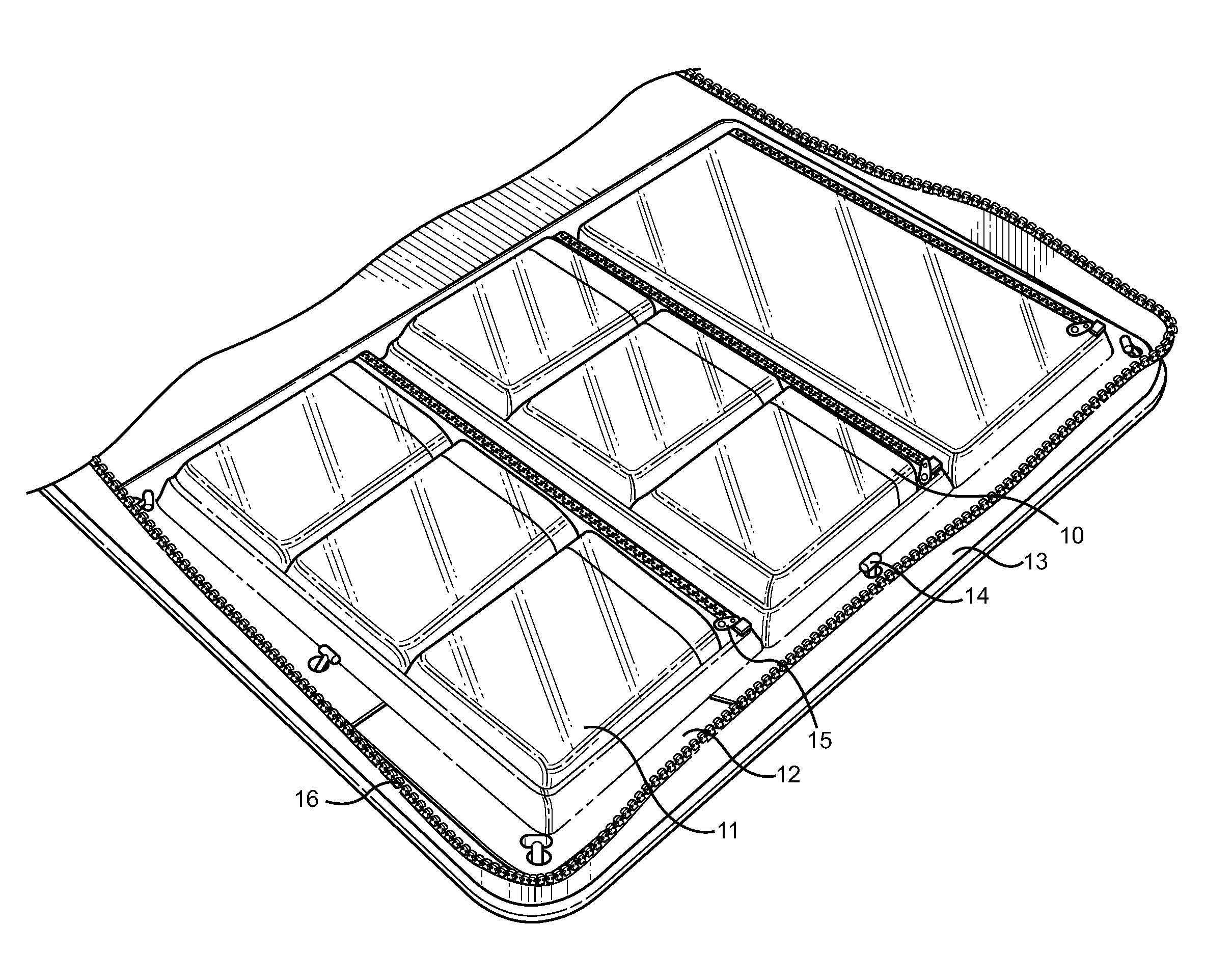
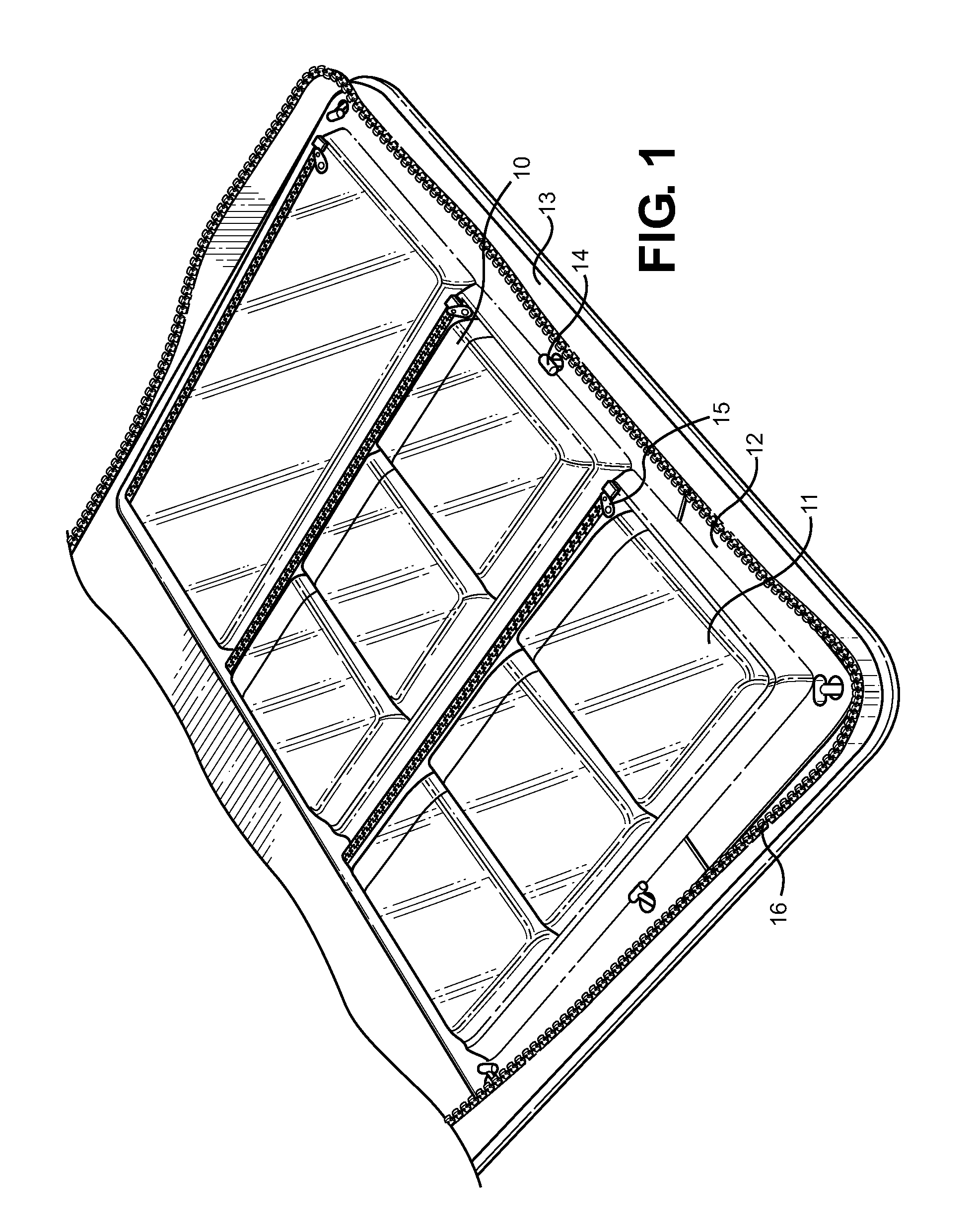
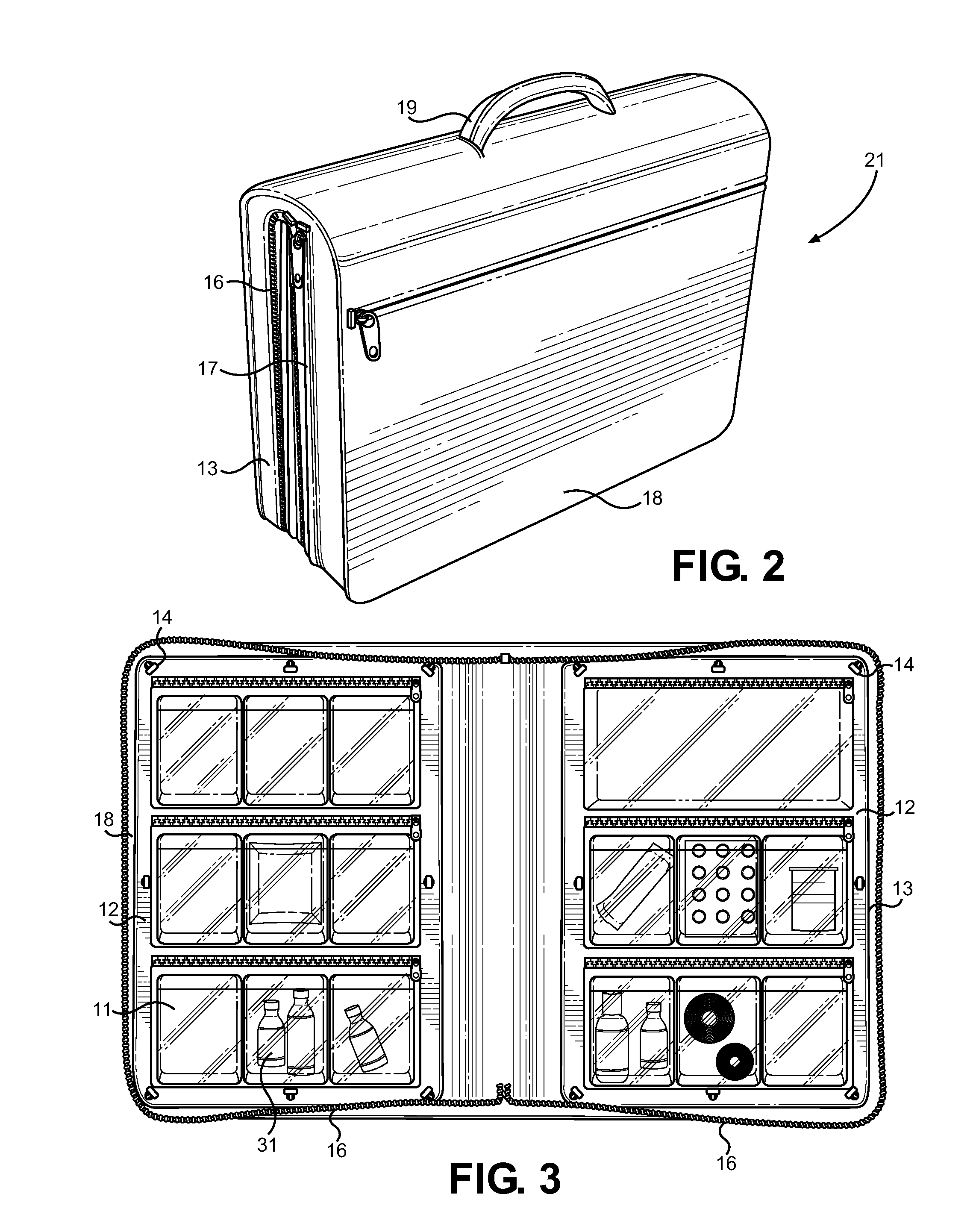
Ophthalmic Medication and Treatment Case
ActiveUS20130277247A1Efficient storageEfficient, organized and modularSurgical furniturePharmaceutical containersVisual acuityOphthalmic drugs
Owner:CARUTH DEBBIE JOANNE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
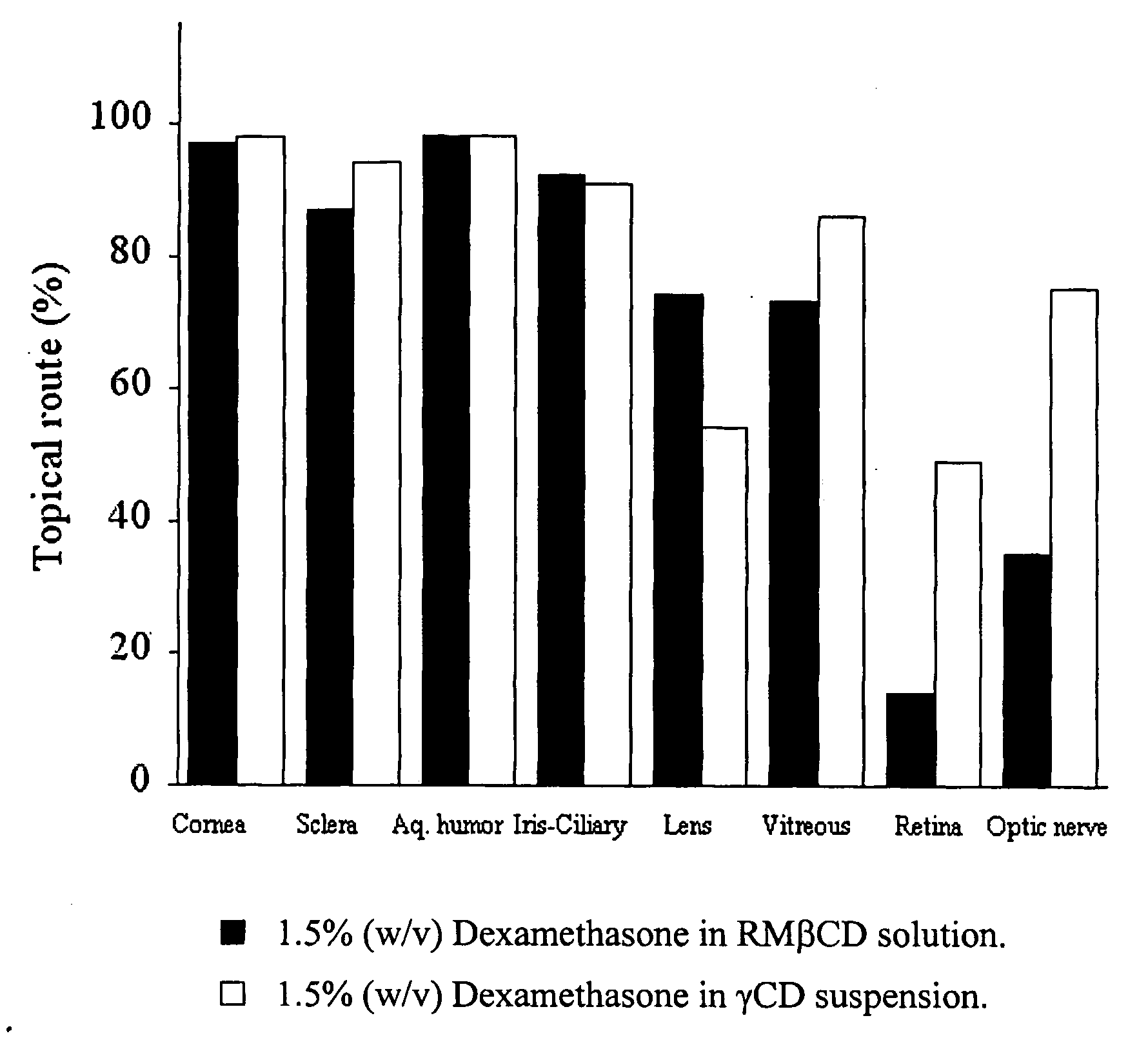
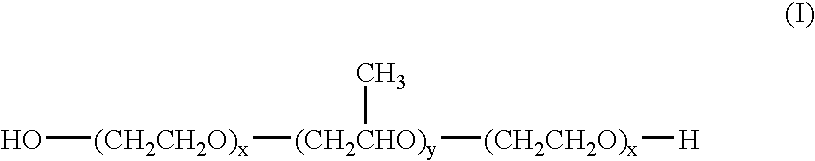
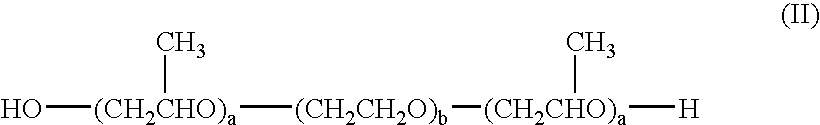
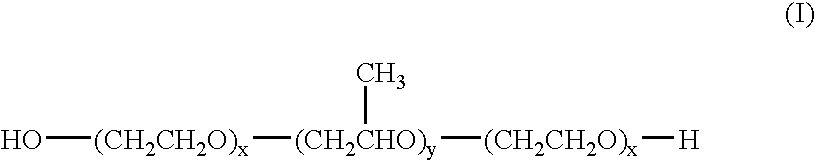
![6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS 6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS](https://images-eureka.patsnap.com/patent_img/3dc8f53b-639a-4691-adea-803f412a77c0/US20080153813A1-20080626-C00001.png)
![6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS 6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS](https://images-eureka.patsnap.com/patent_img/3dc8f53b-639a-4691-adea-803f412a77c0/US20080153813A1-20080626-C00002.png)
![6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS 6-AMINOIMIDAZO[1,2-b]PYRIDAZINE ANALOGS AS RHO KINASE INHIBITORS FOR THE TREATMENT OF RHO KINASE-MEDIATED DISEASES AND CONDITIONS](https://images-eureka.patsnap.com/patent_img/3dc8f53b-639a-4691-adea-803f412a77c0/US20080153813A1-20080626-C00003.png)