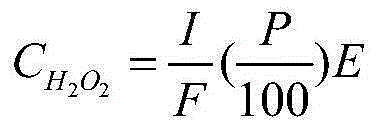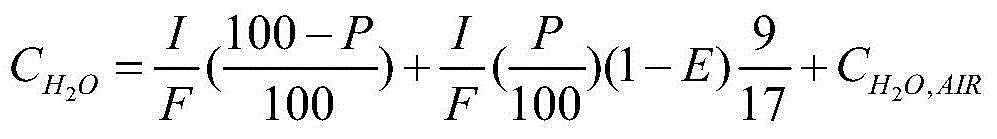Patents
Literature
103 results about "Vaporized hydrogen peroxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaporized hydrogen peroxide (trademarked VHP, also known as hydrogen peroxide vapor, HPV) is a vapor form of hydrogen peroxide (H₂O₂) with applications as a low-temperature antimicrobial vapor used to decontaminate enclosed and sealed areas such as laboratory workstations, isolation and pass-through rooms, and even aircraft interiors.
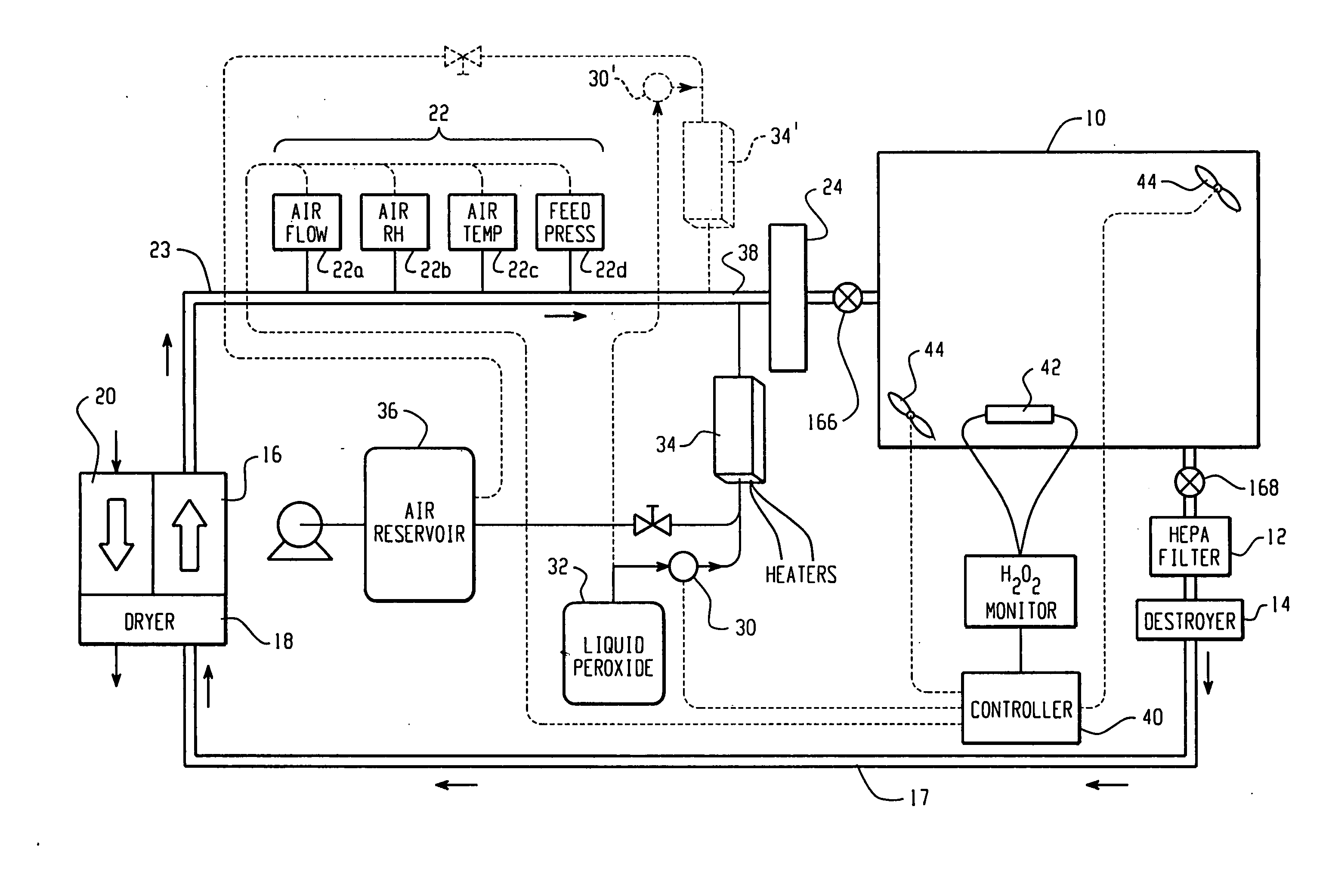
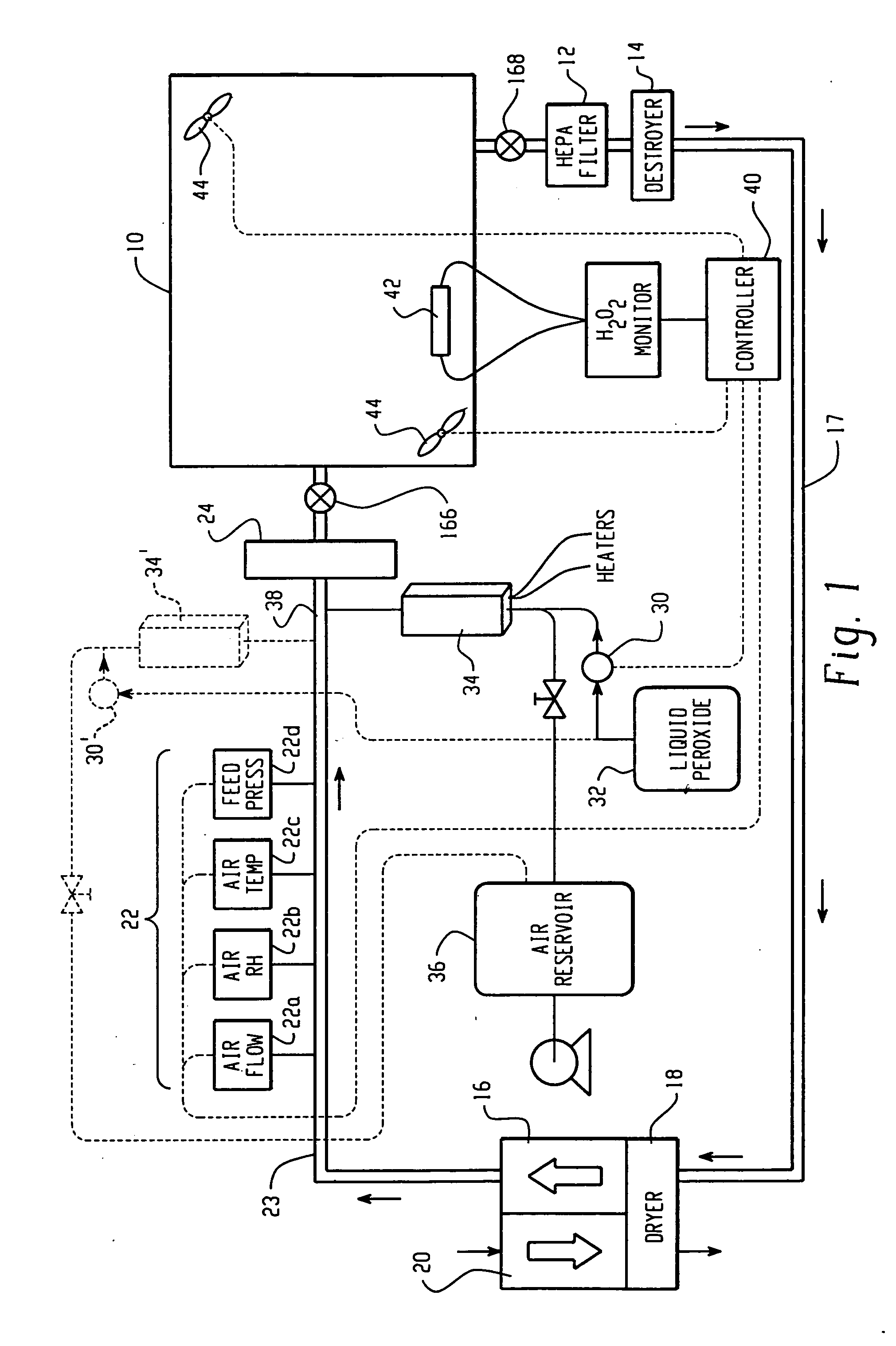
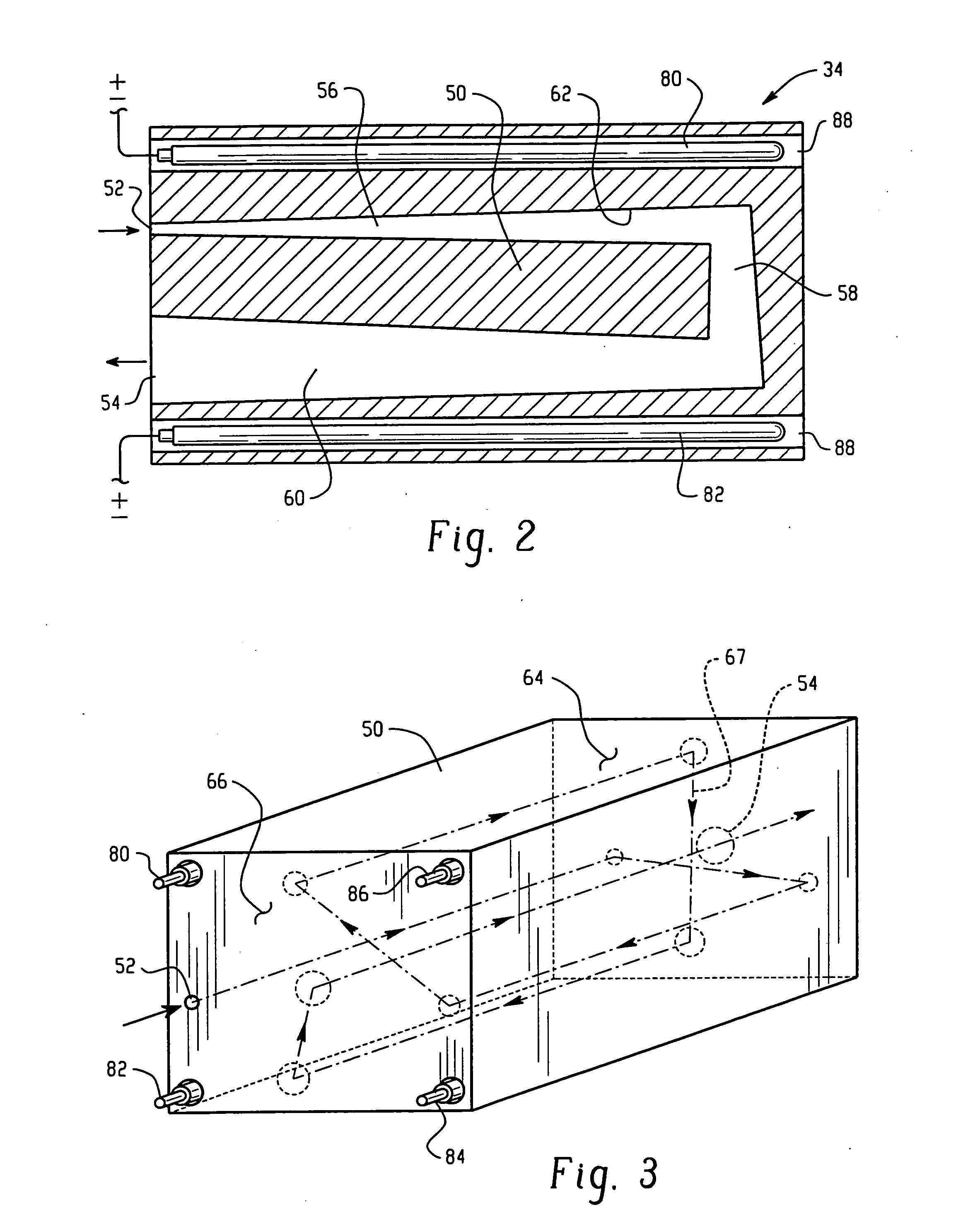
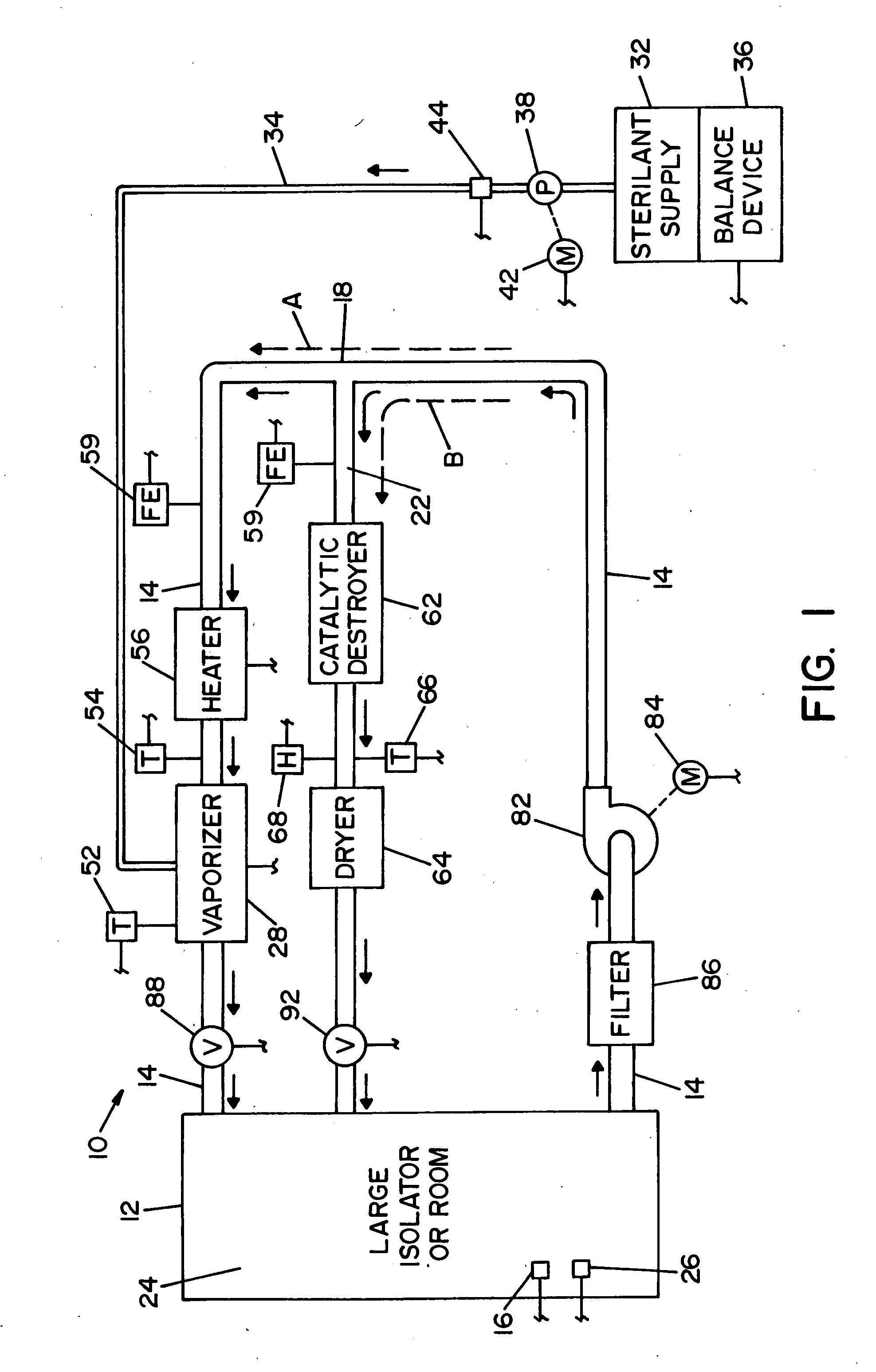
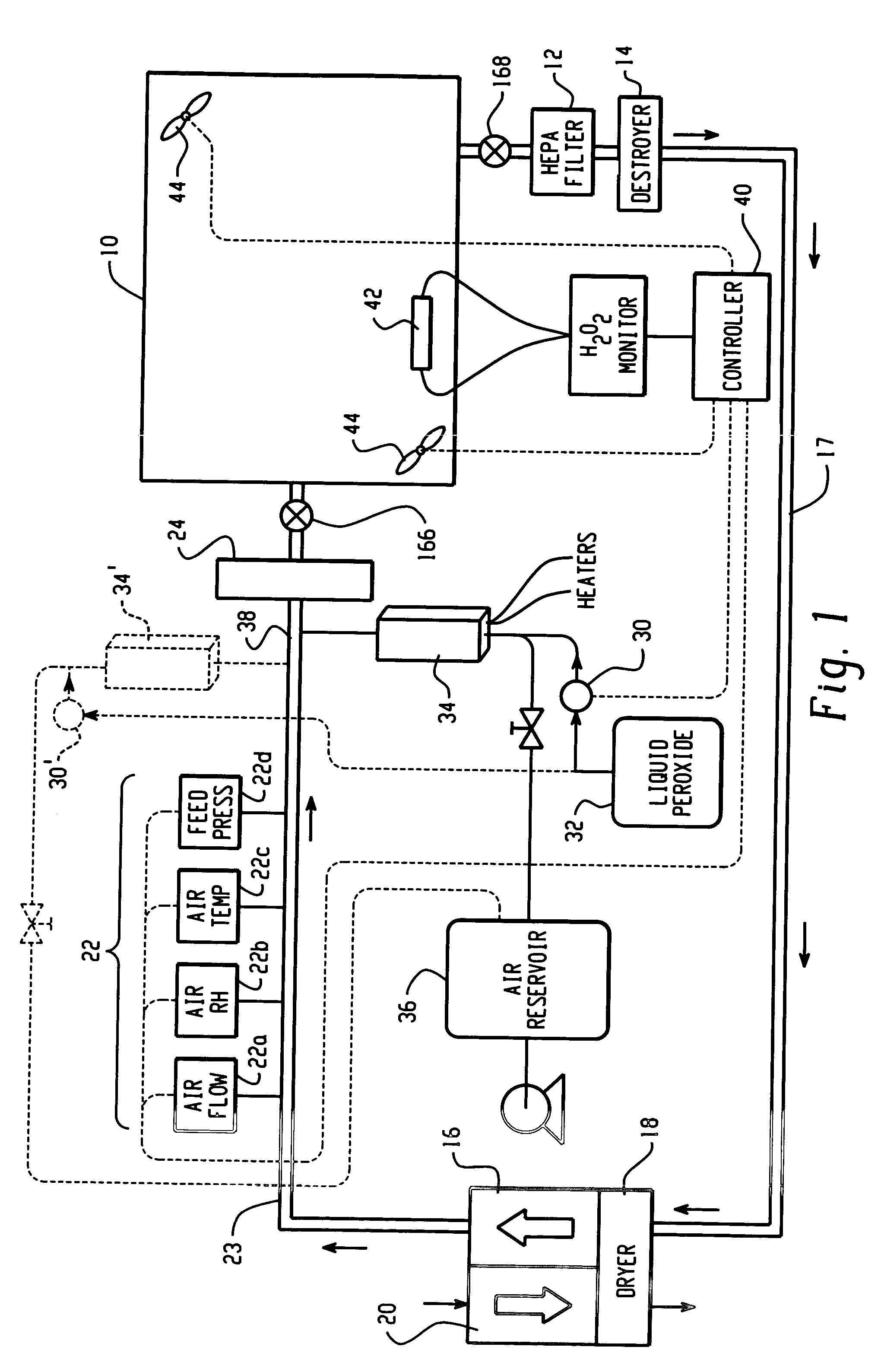
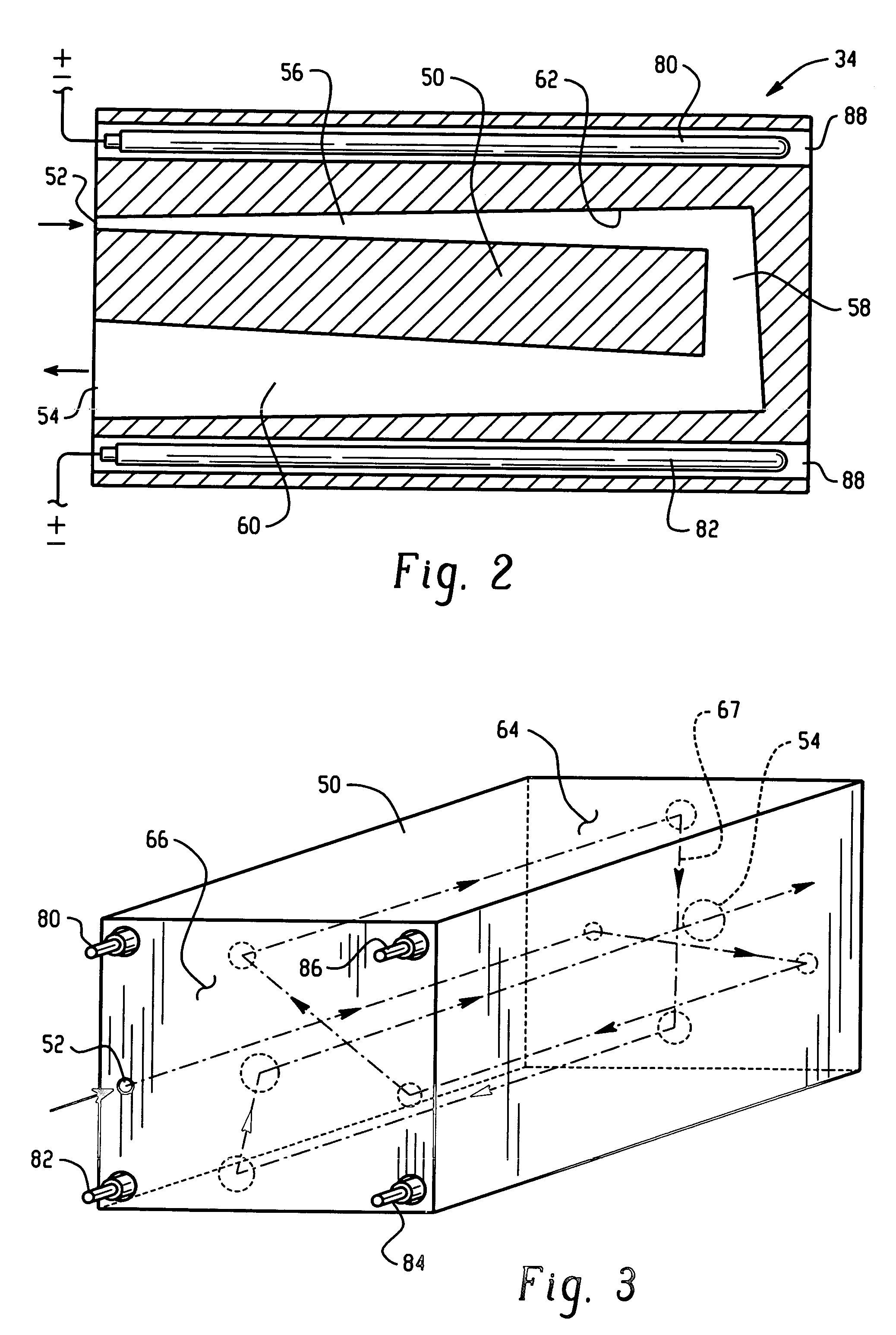
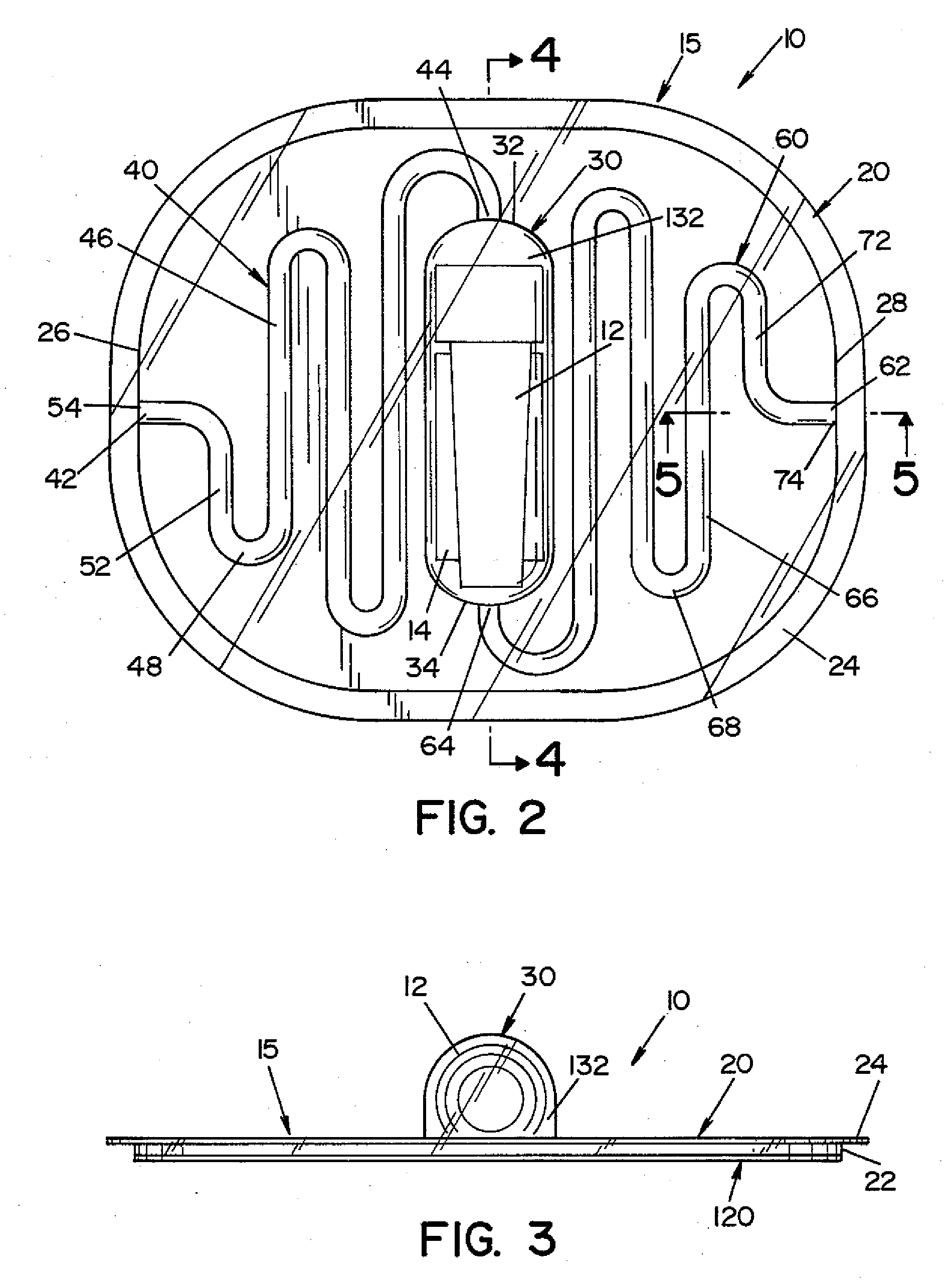
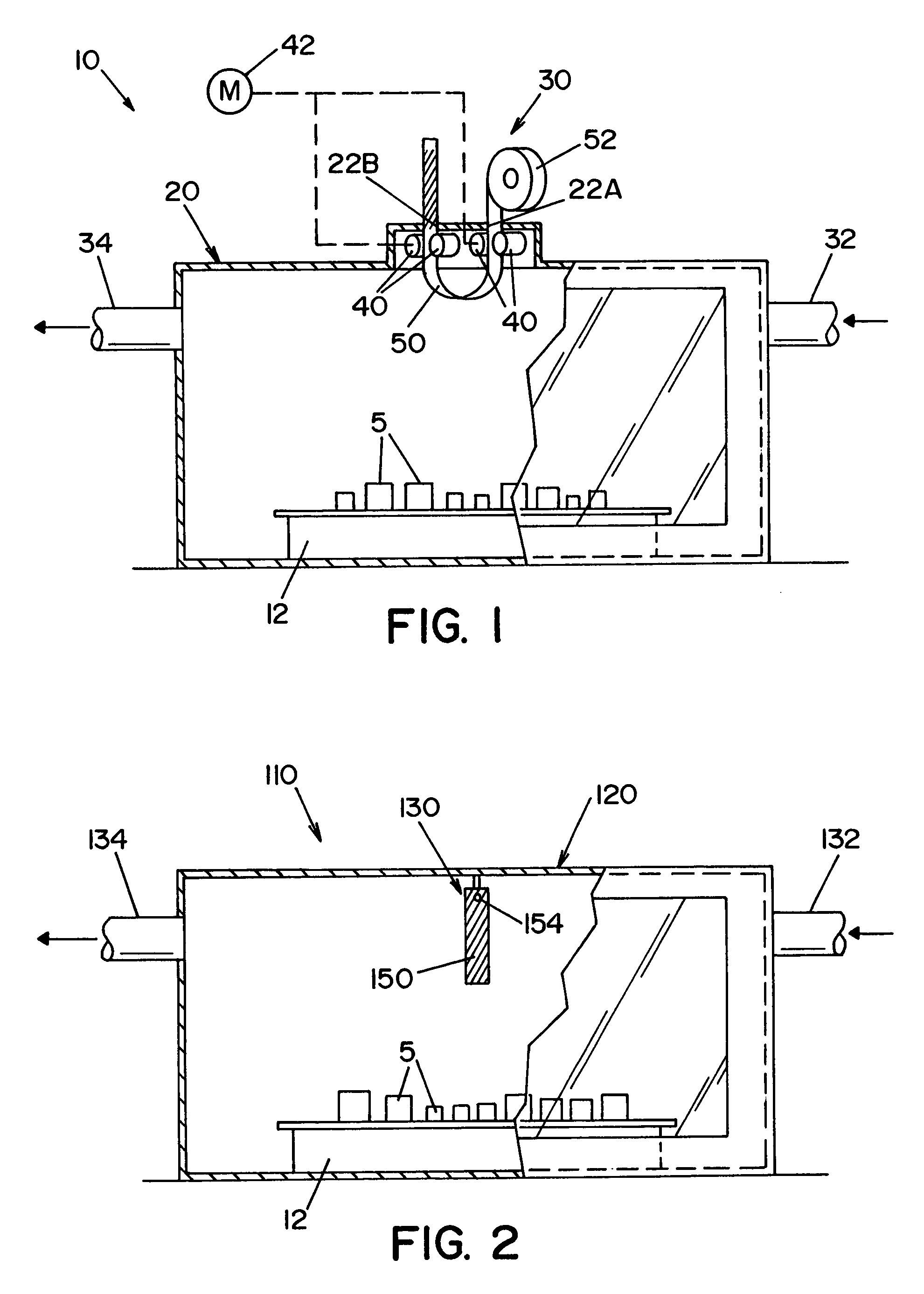
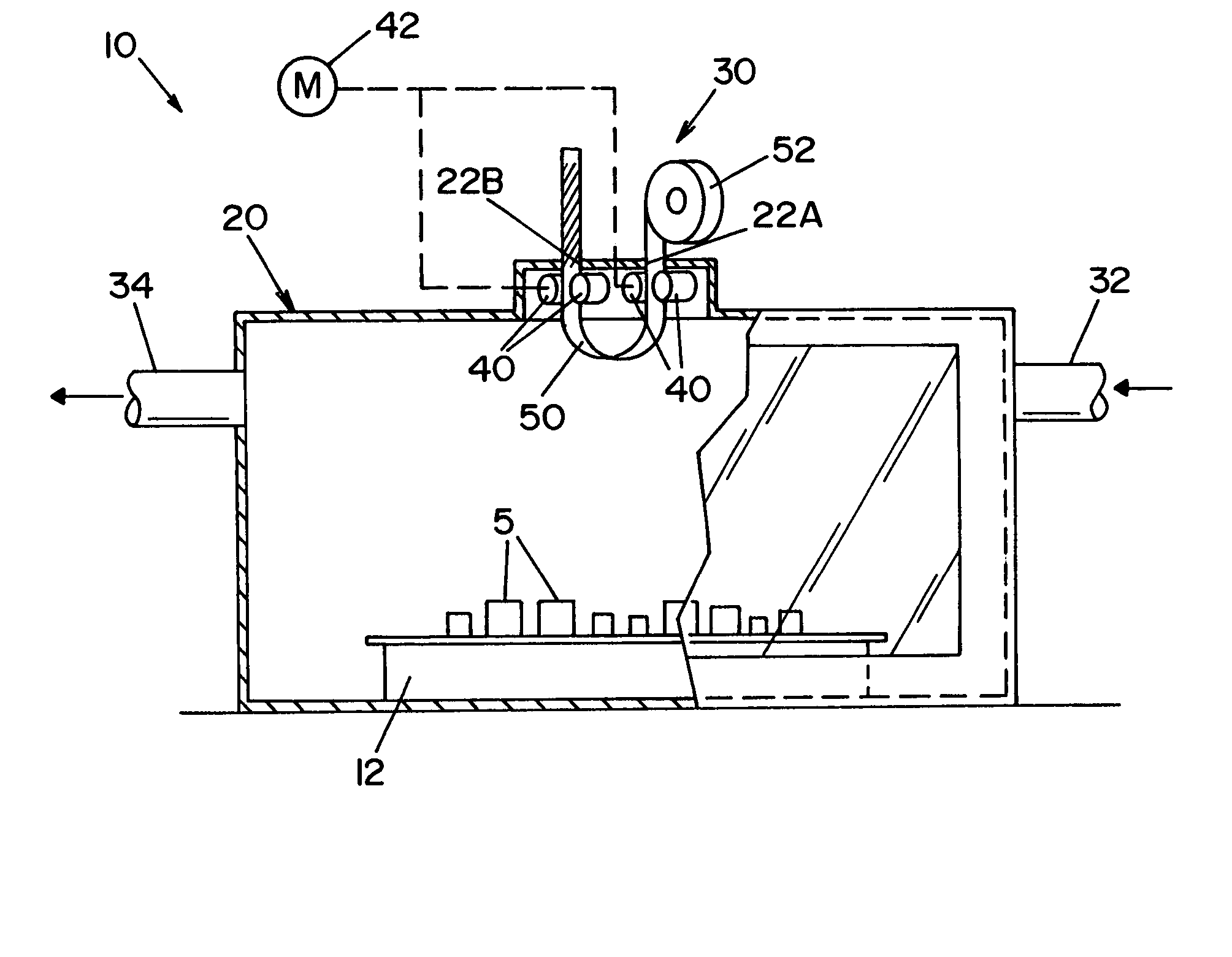
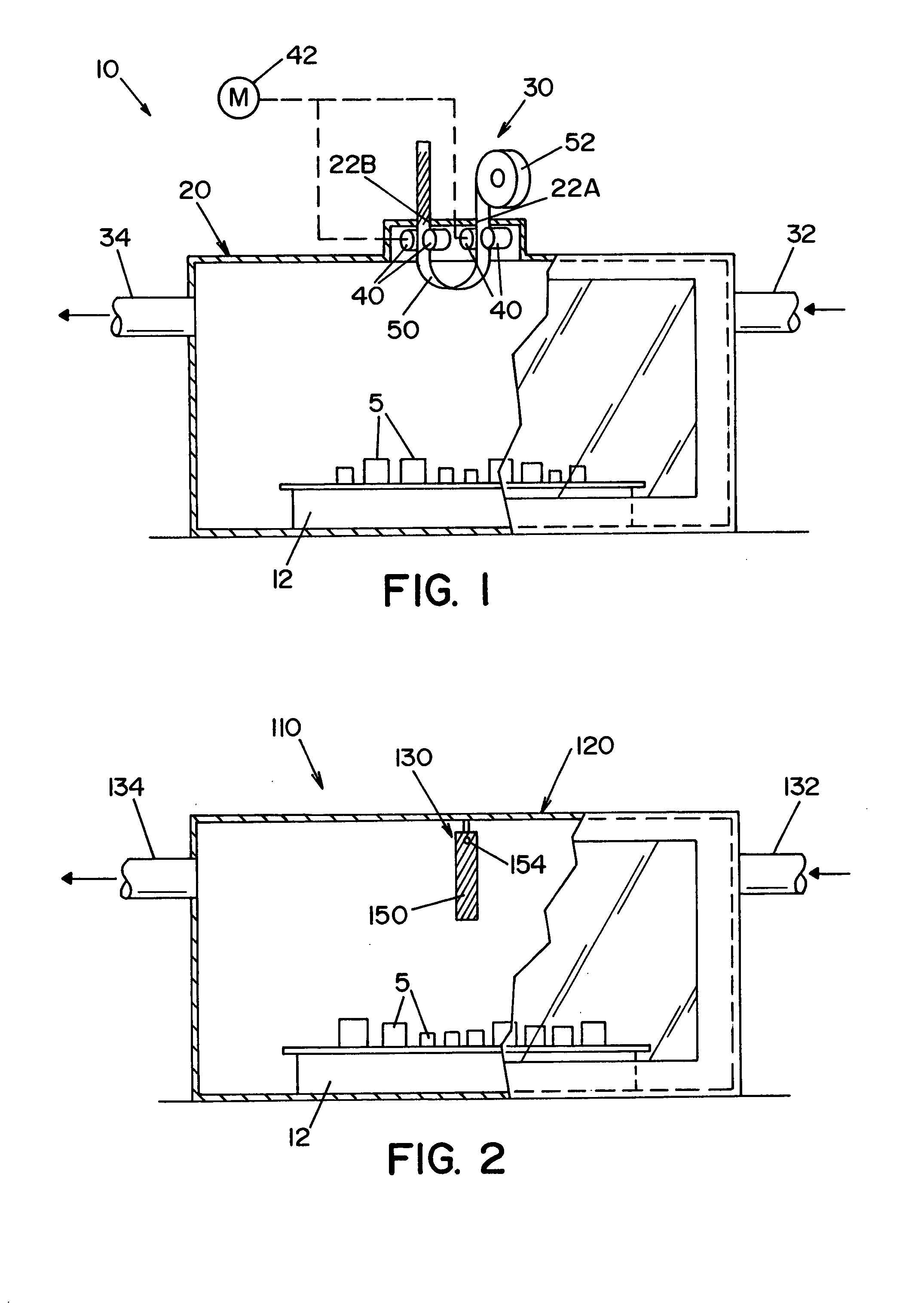
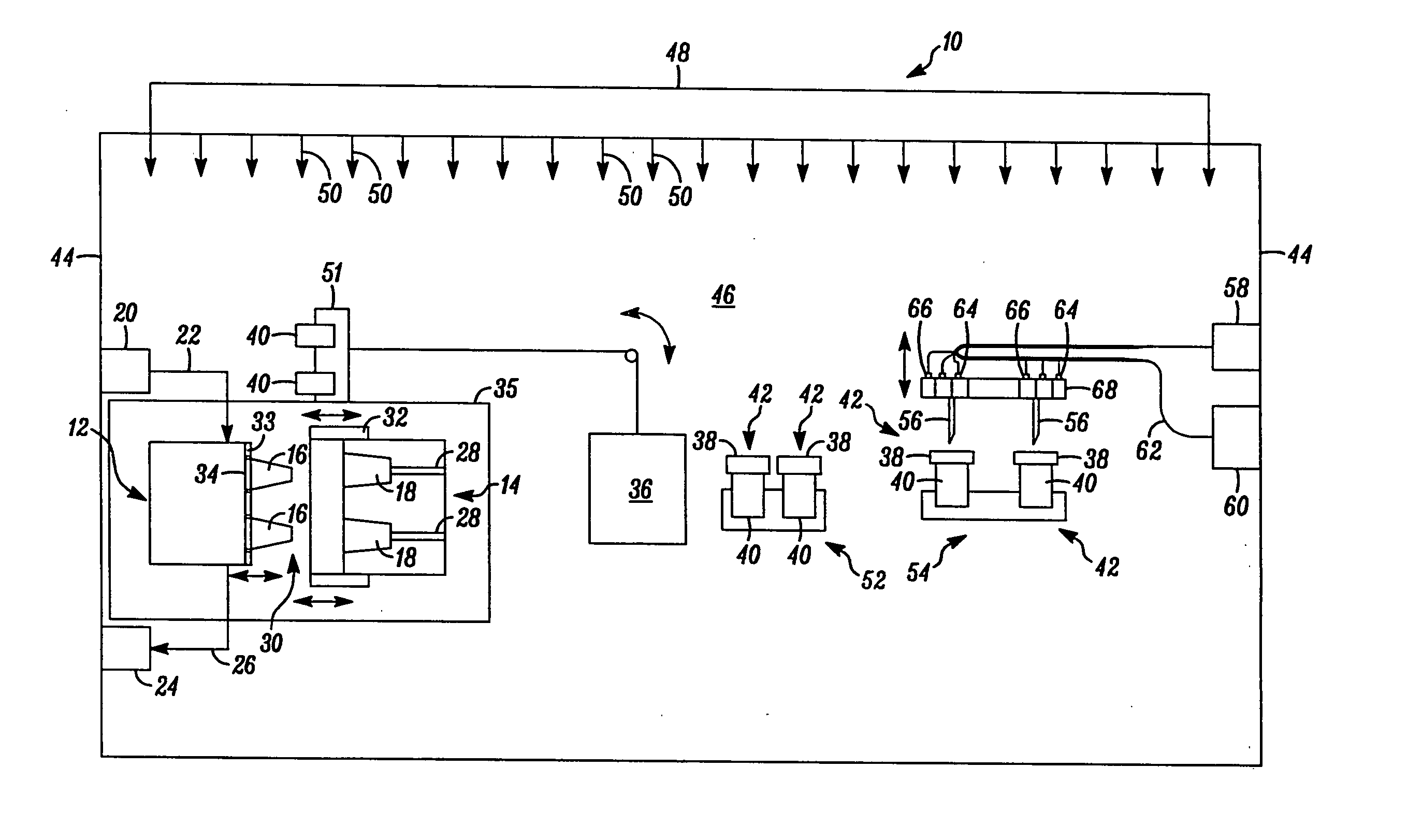
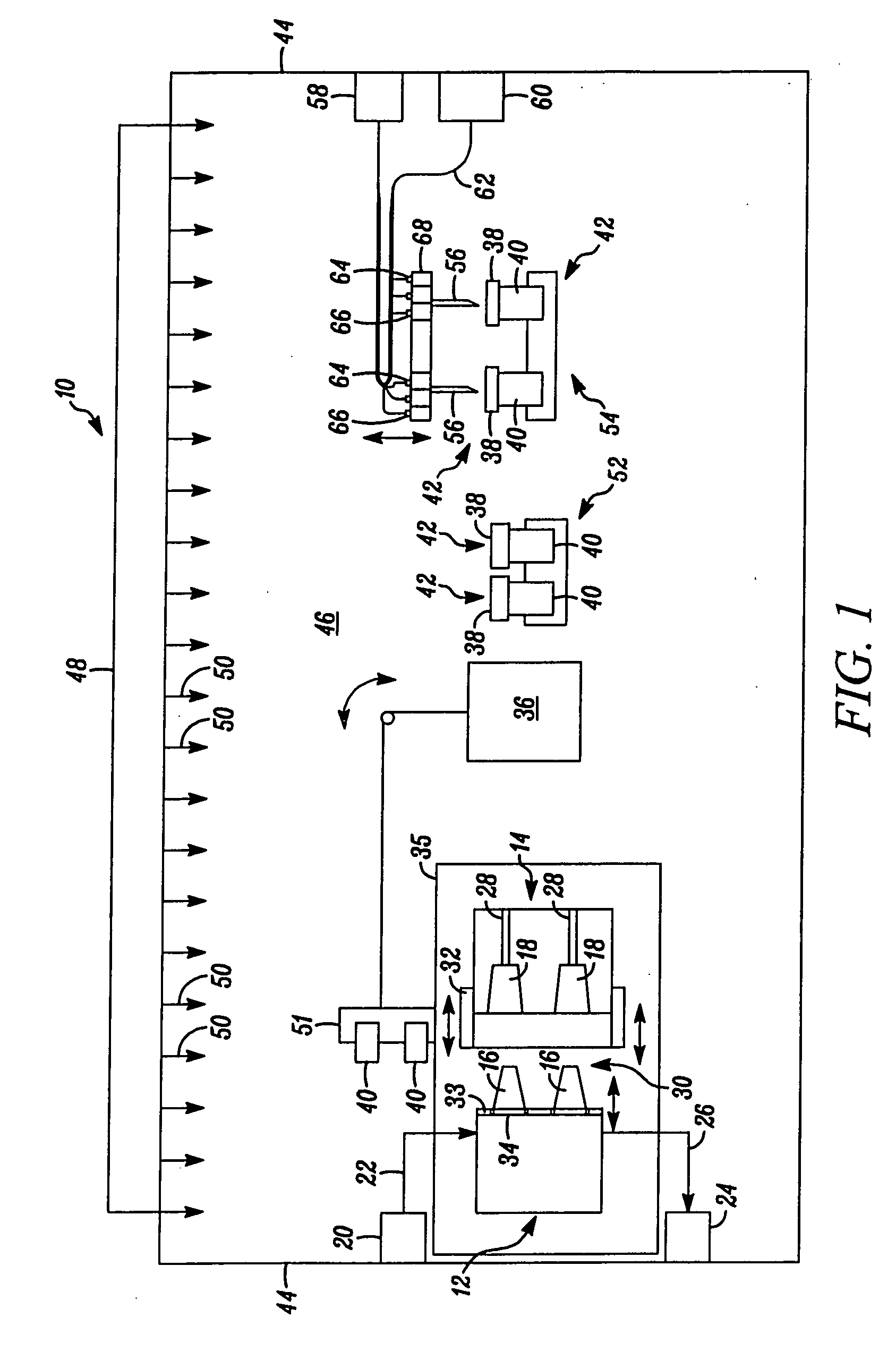
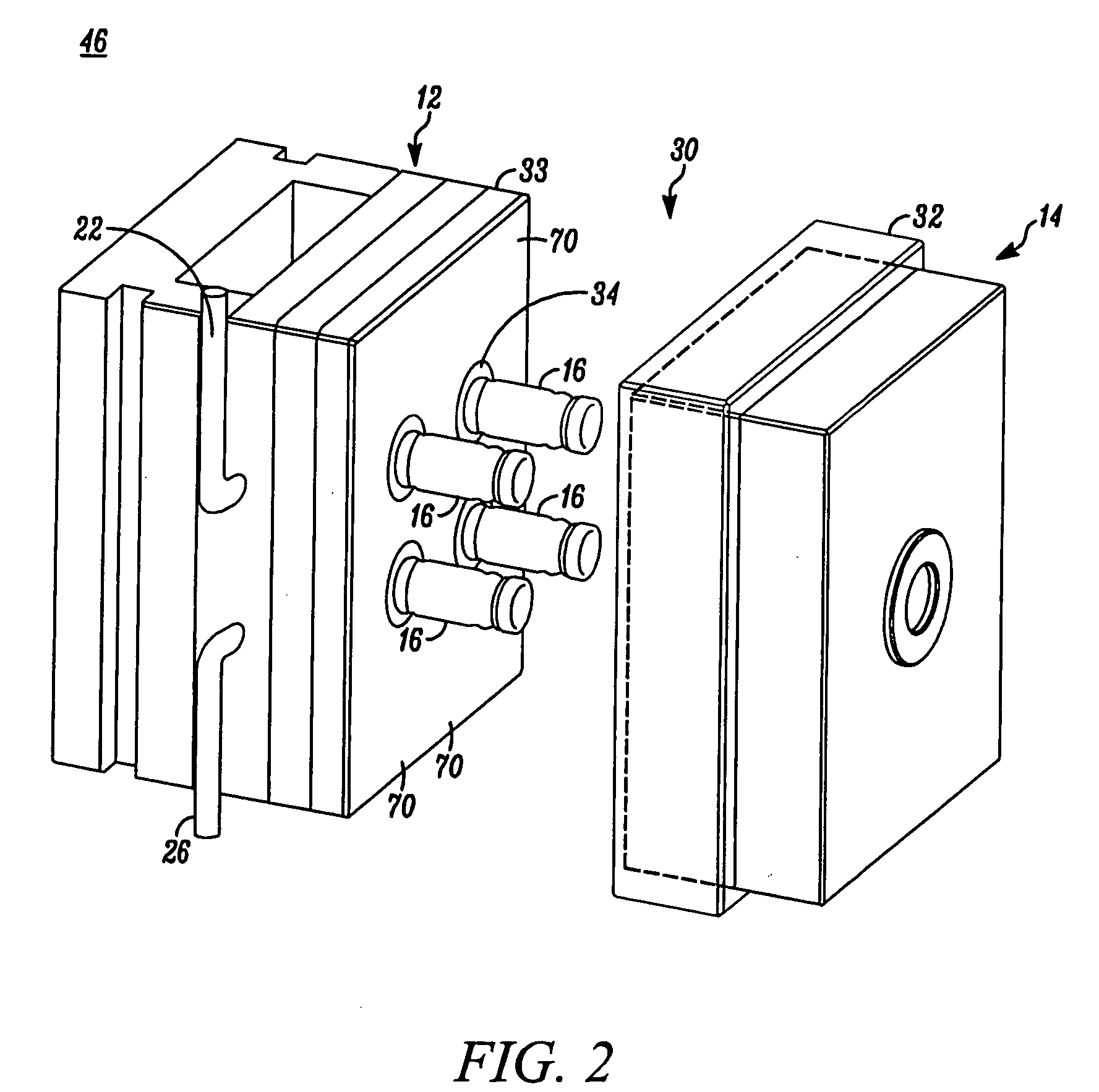
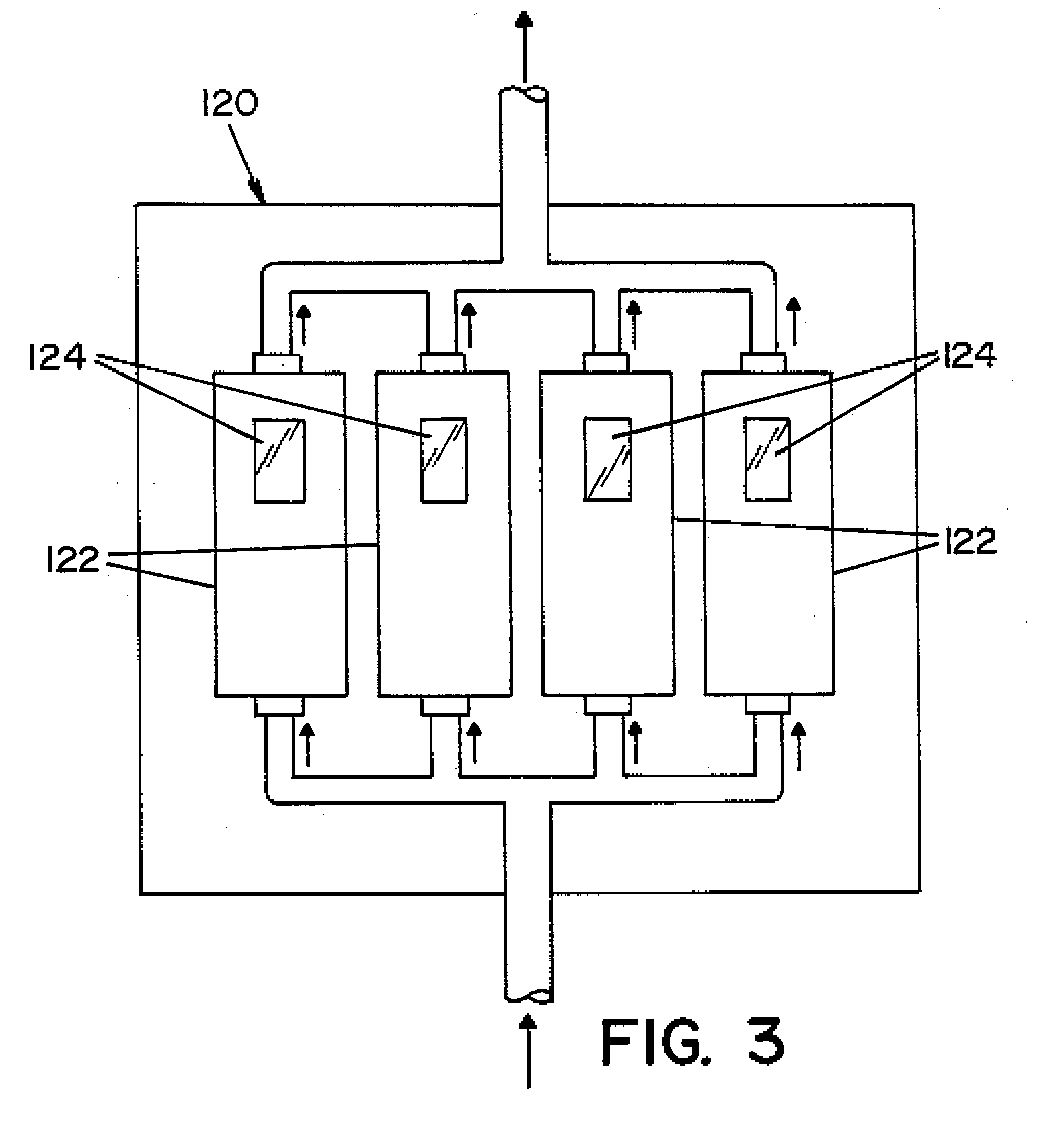
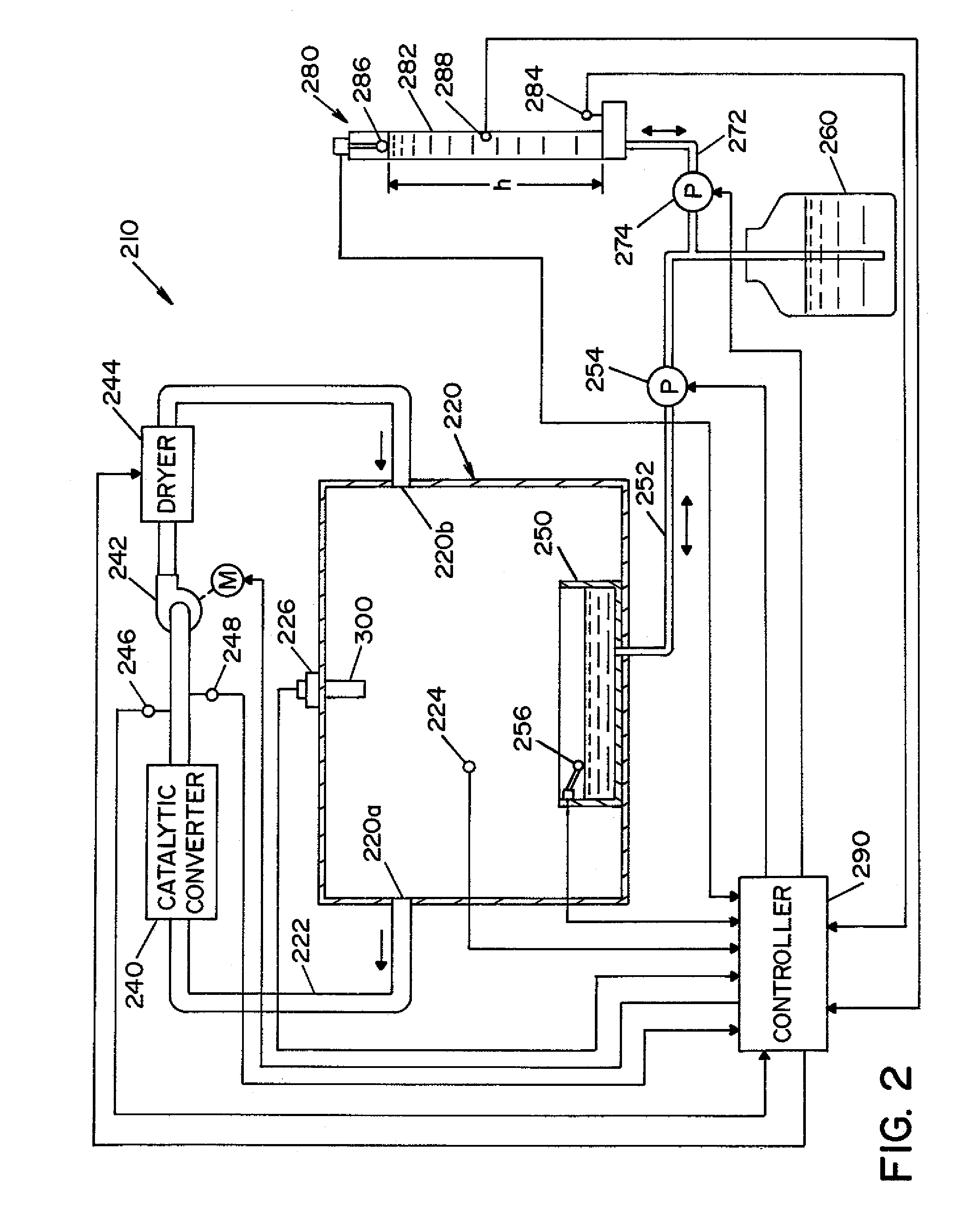
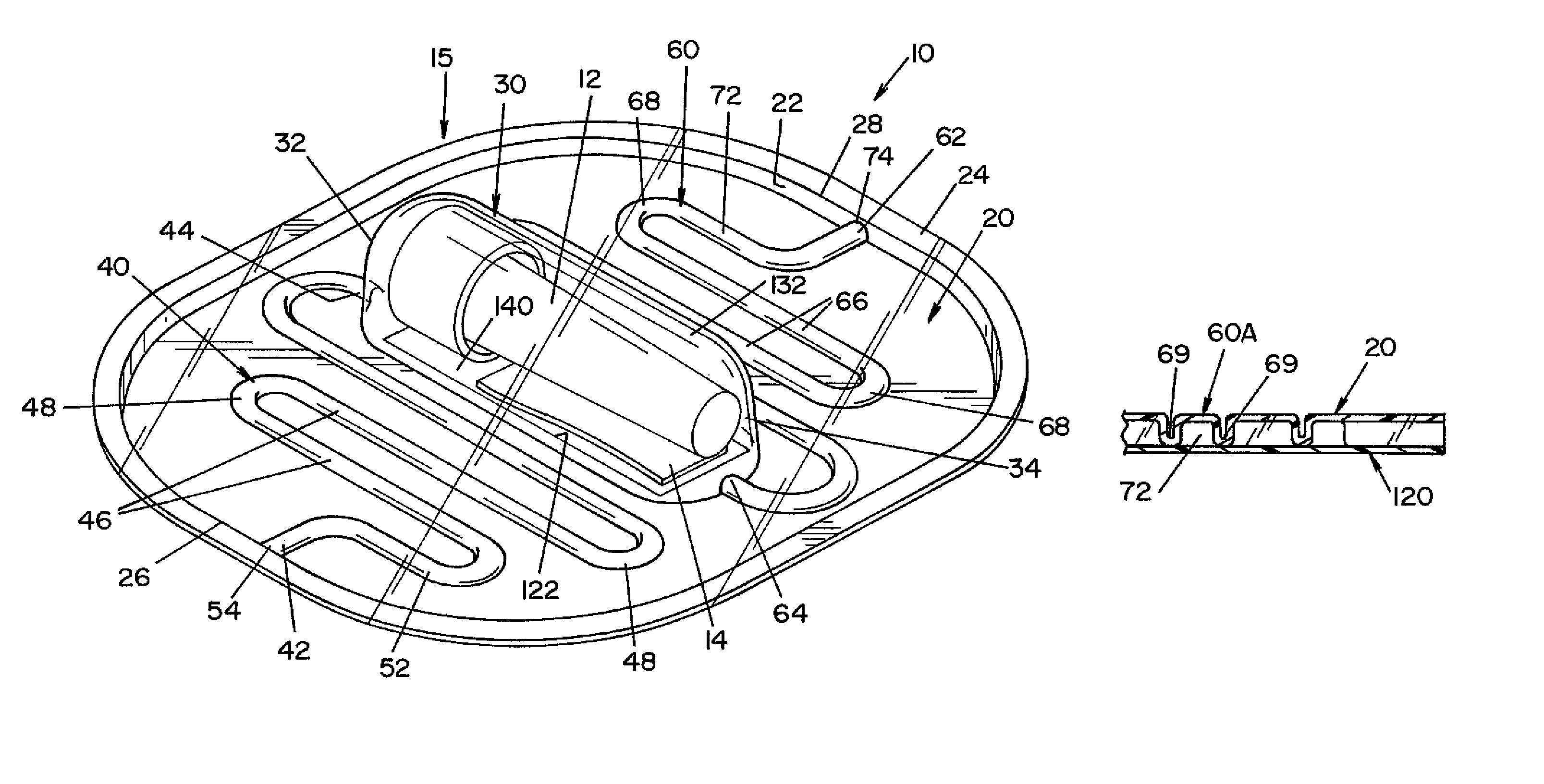
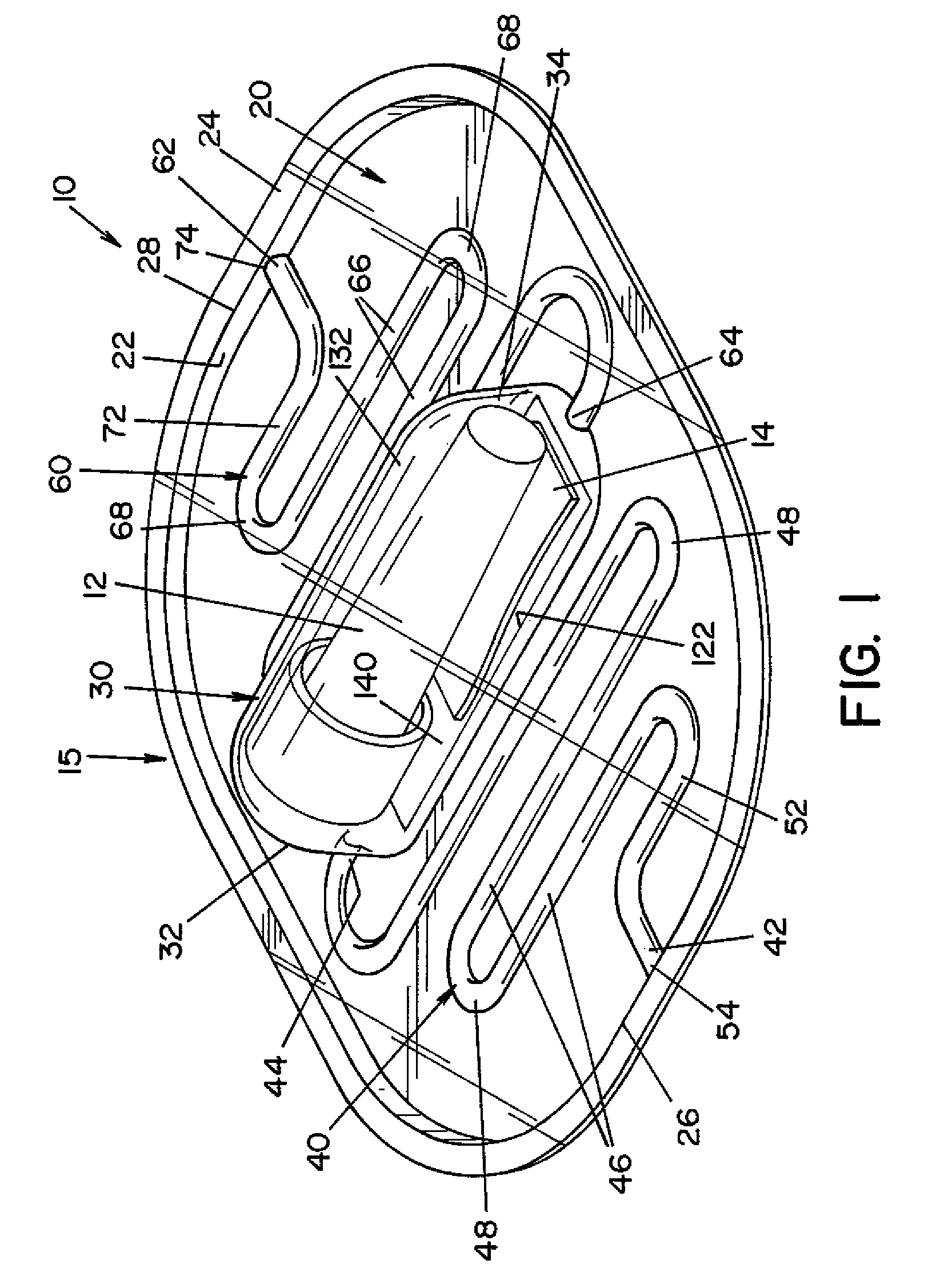
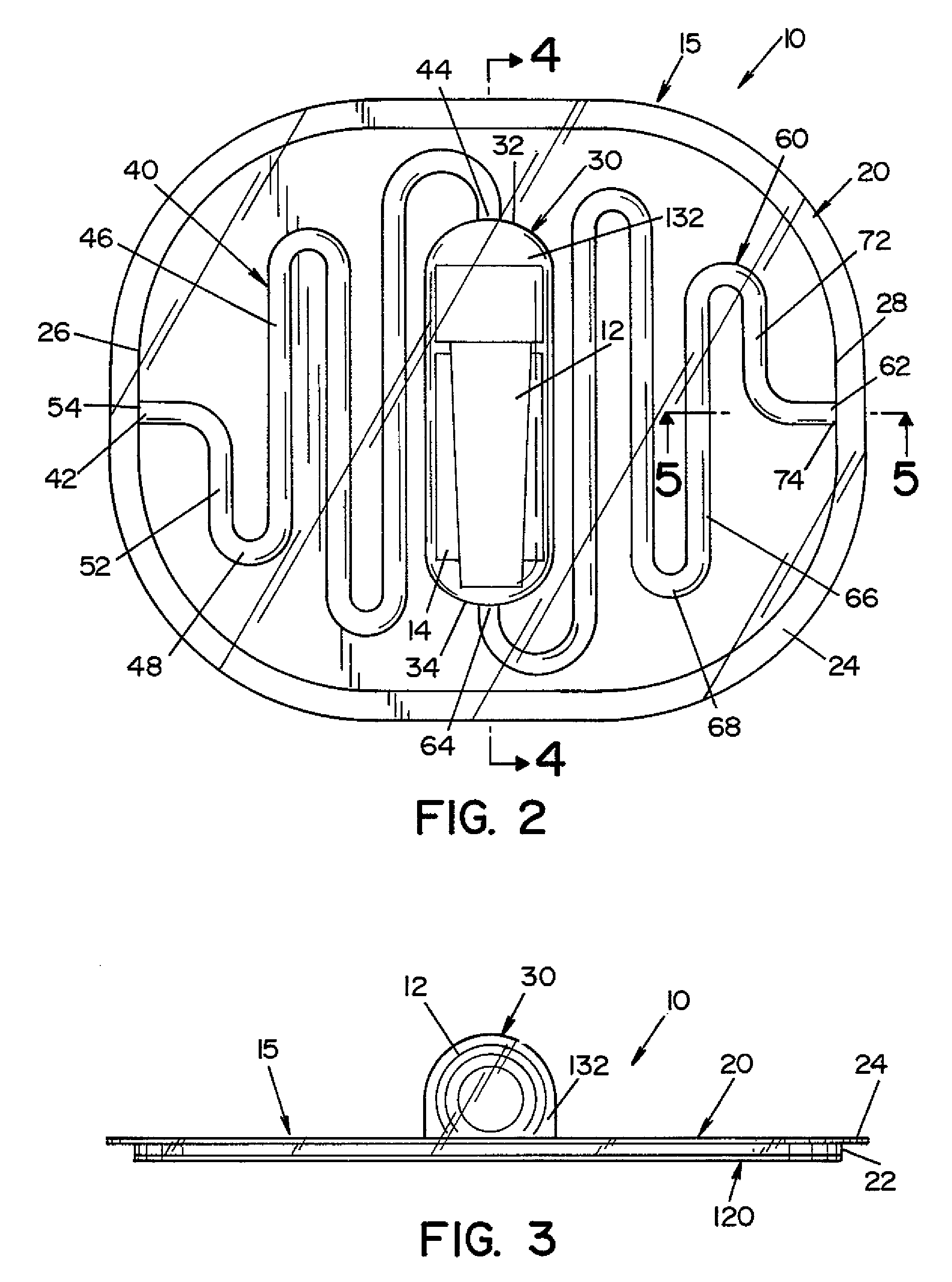
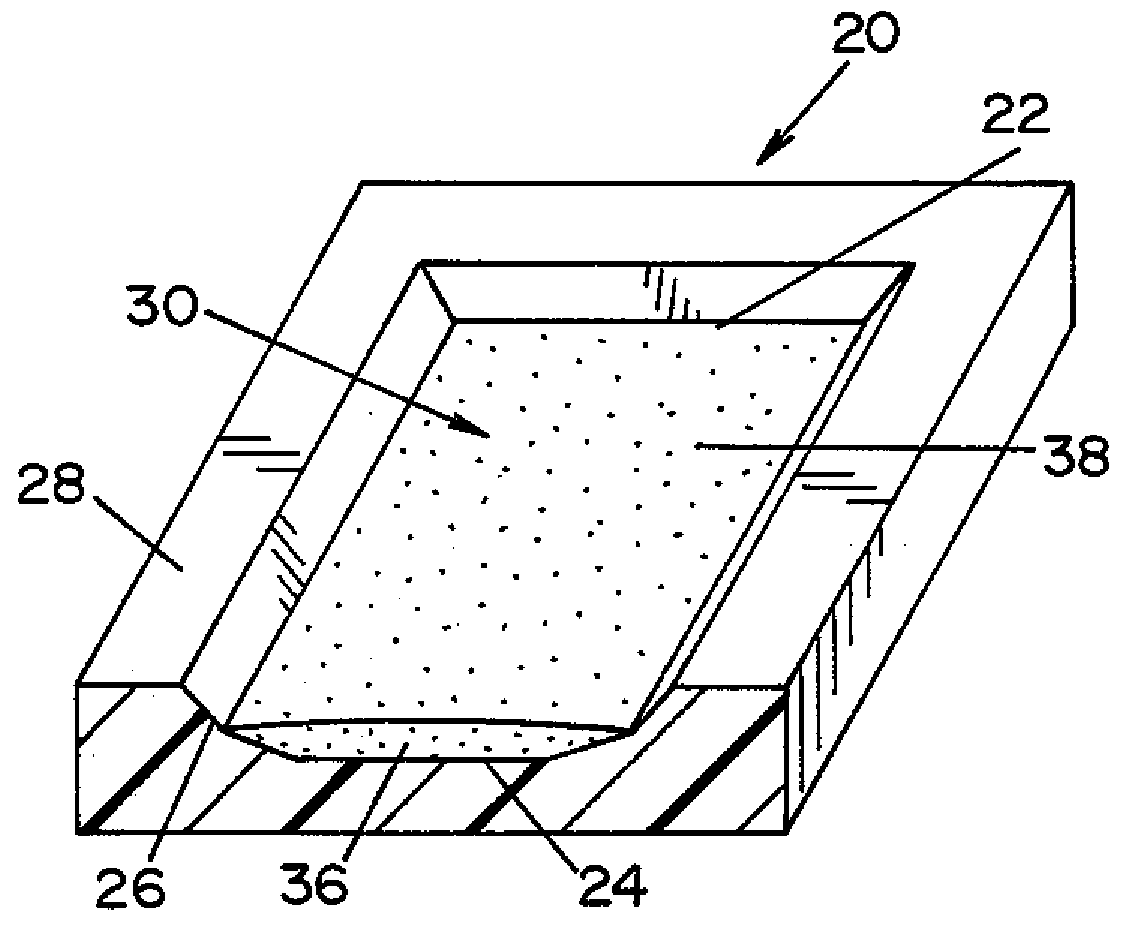
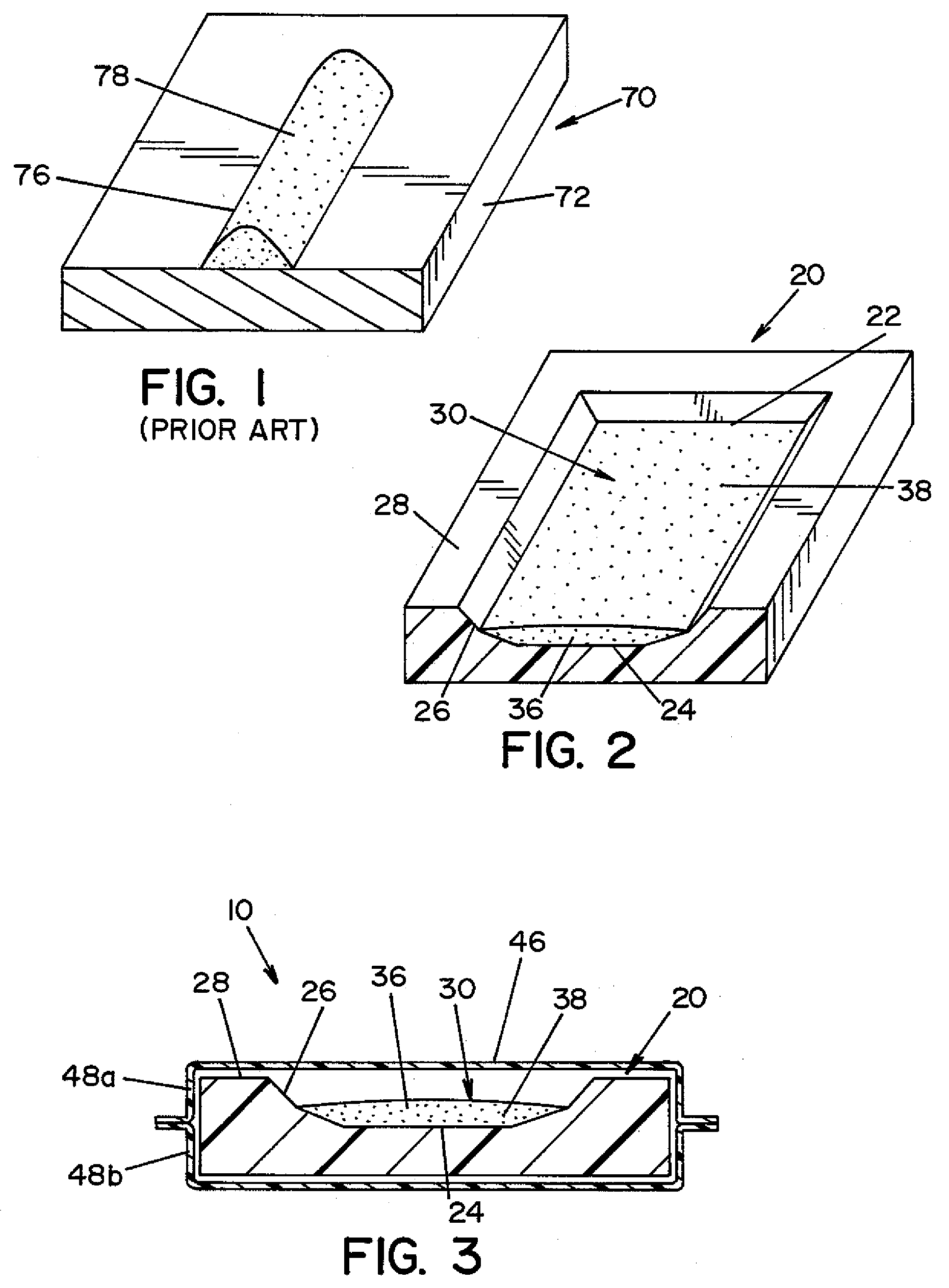
High capacity flash vapor generation systems
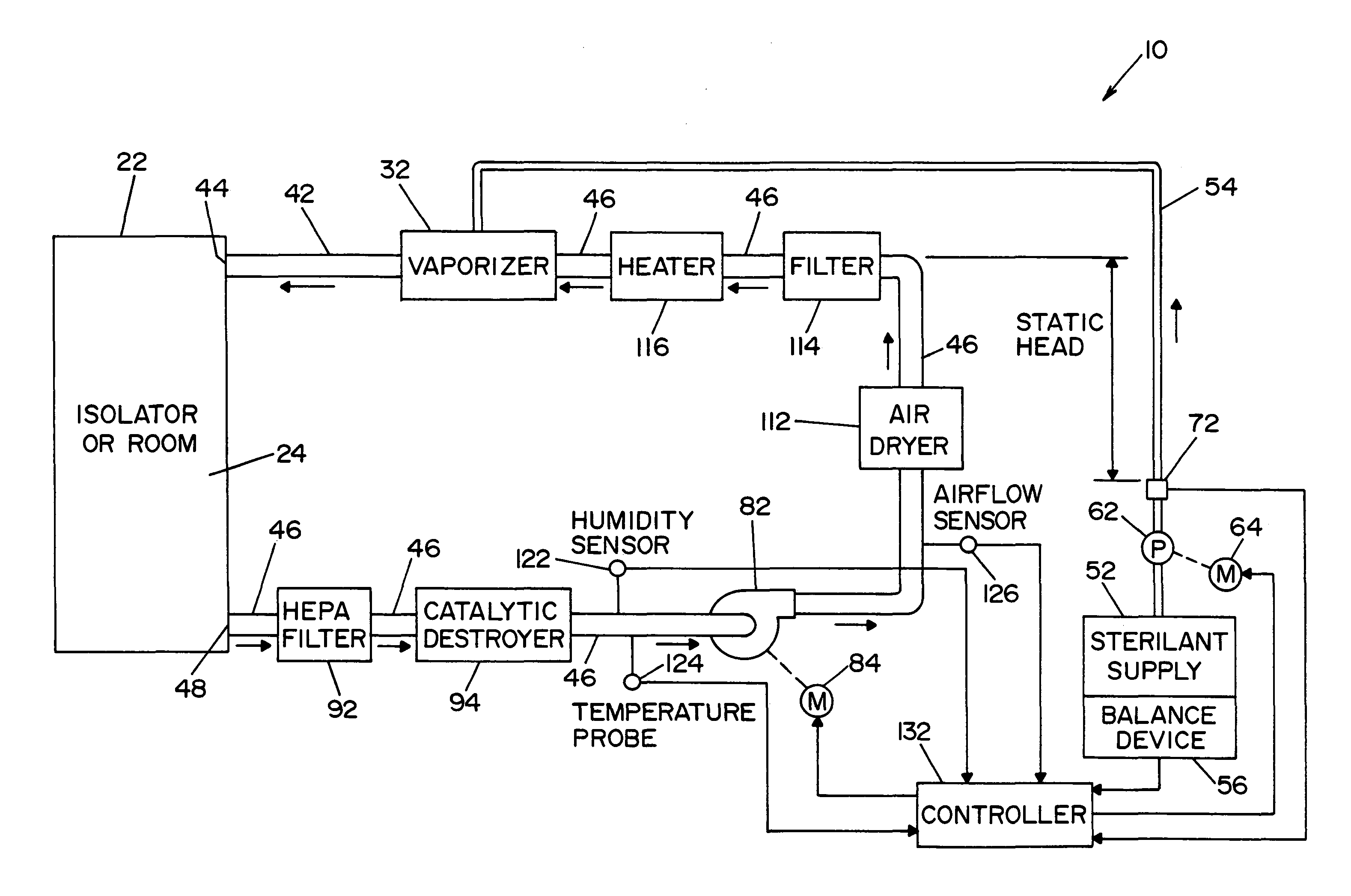
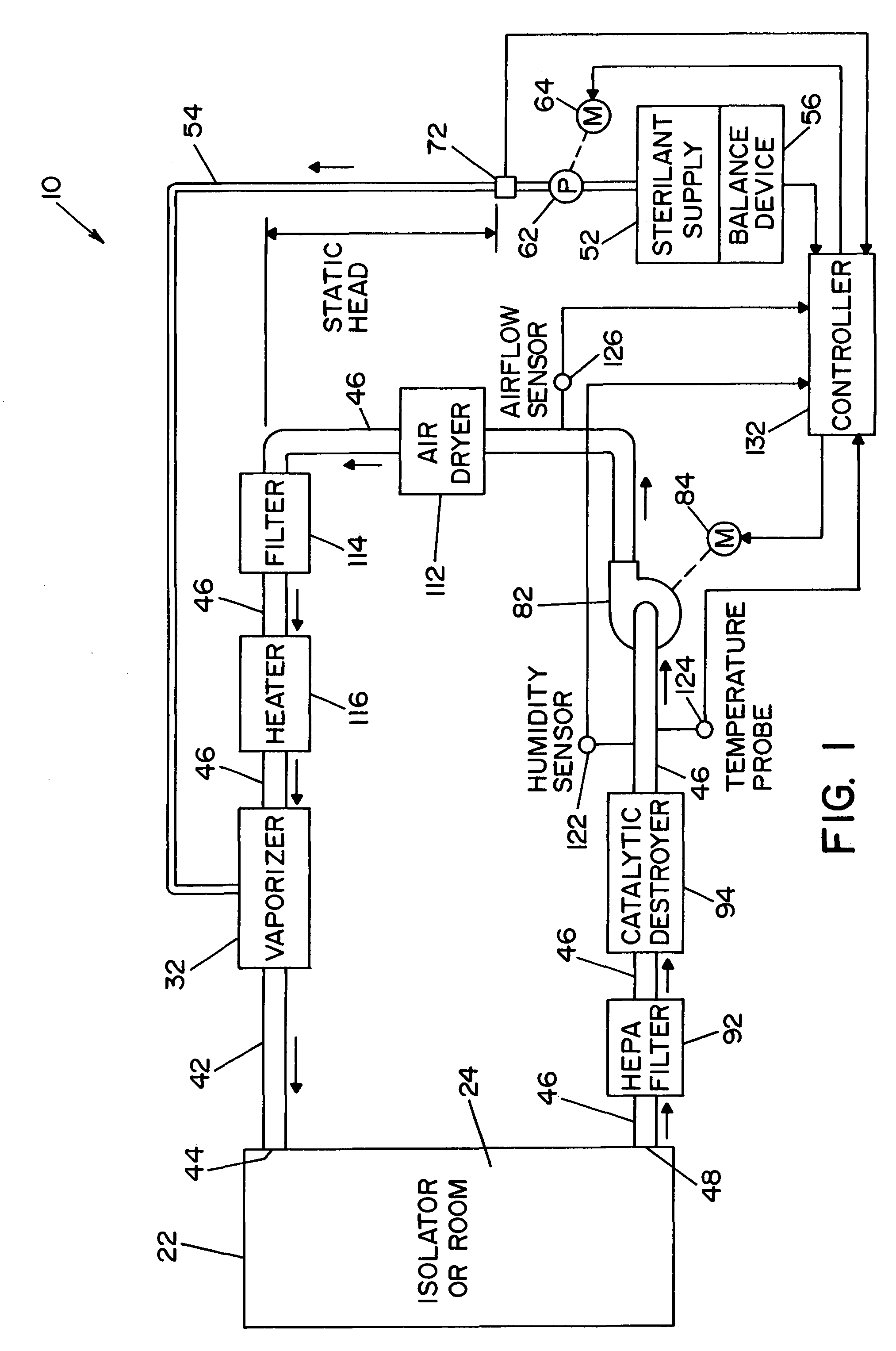
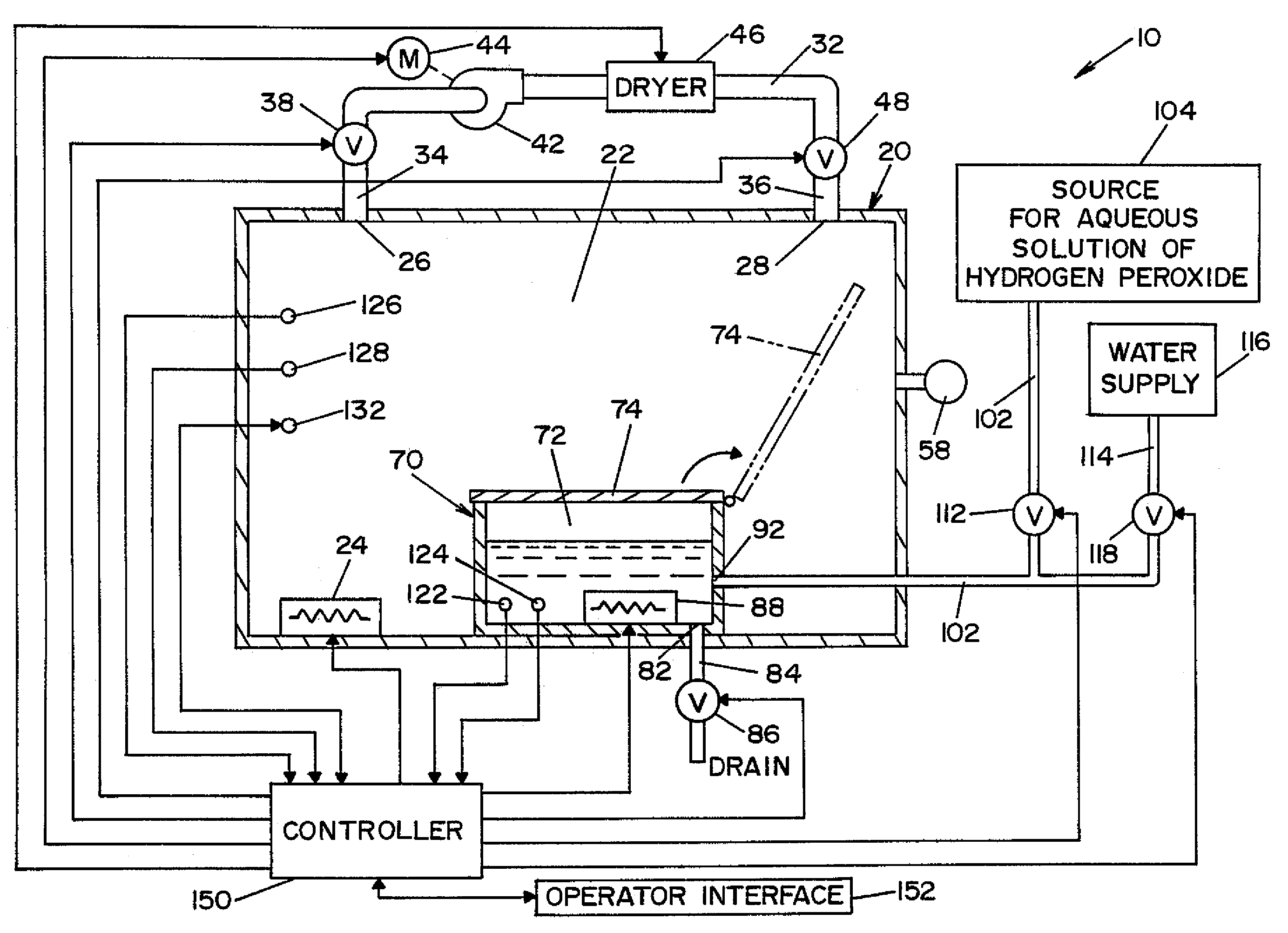
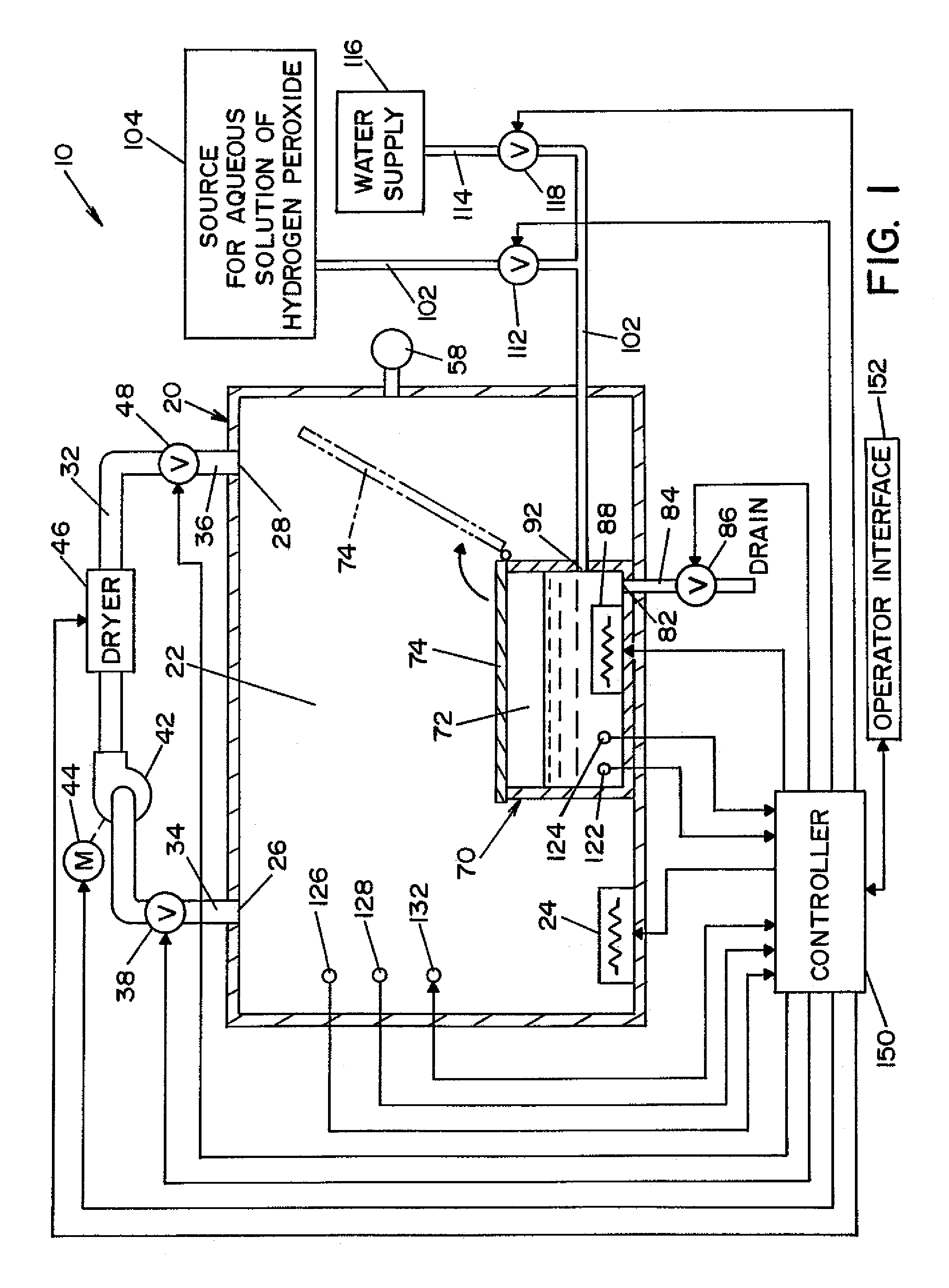
InactiveUS20050084415A1Increase productionIncrease in sizePackage sterilisationLavatory sanitoryAntimicrobial compoundVaporized hydrogen peroxide
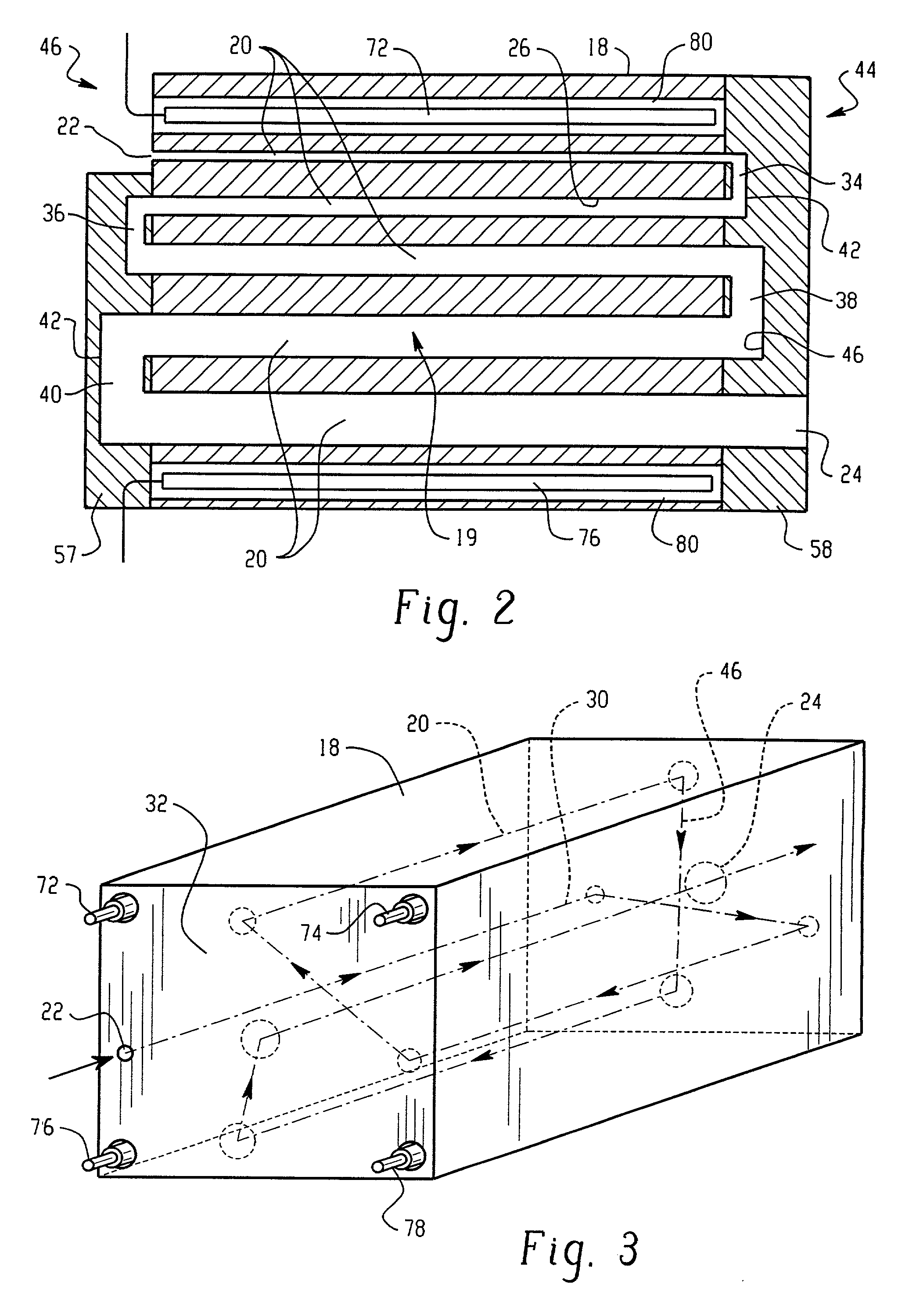
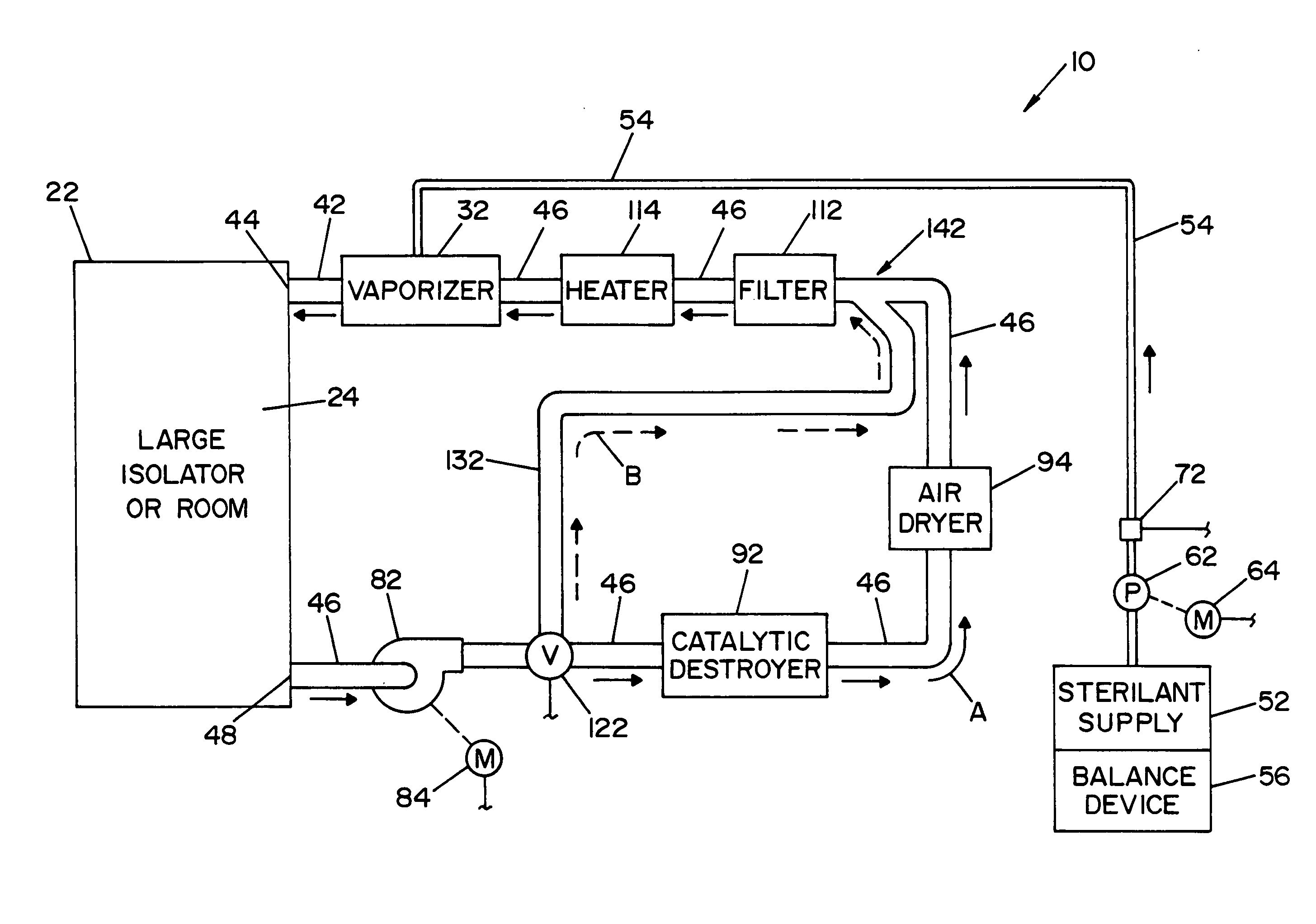
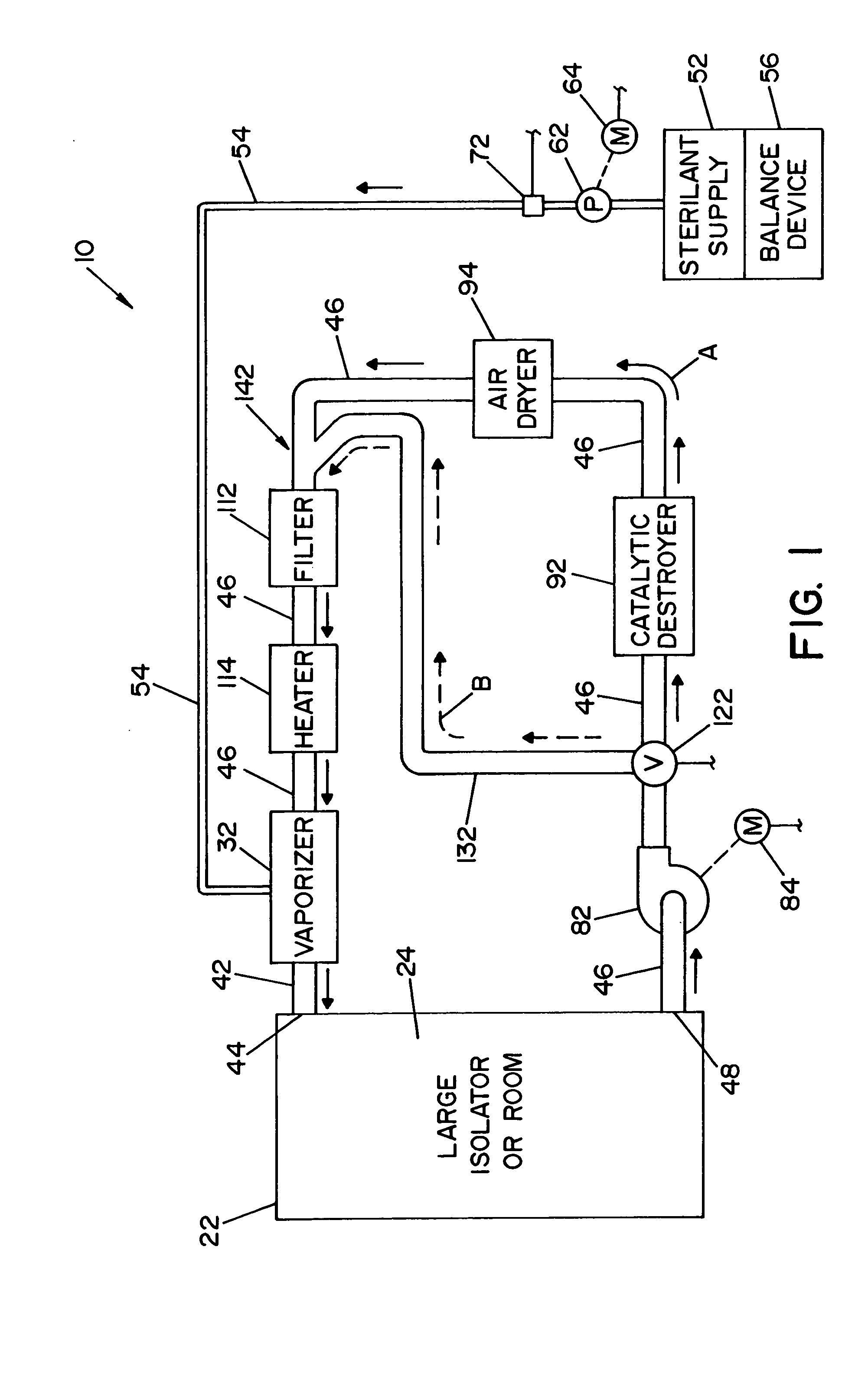
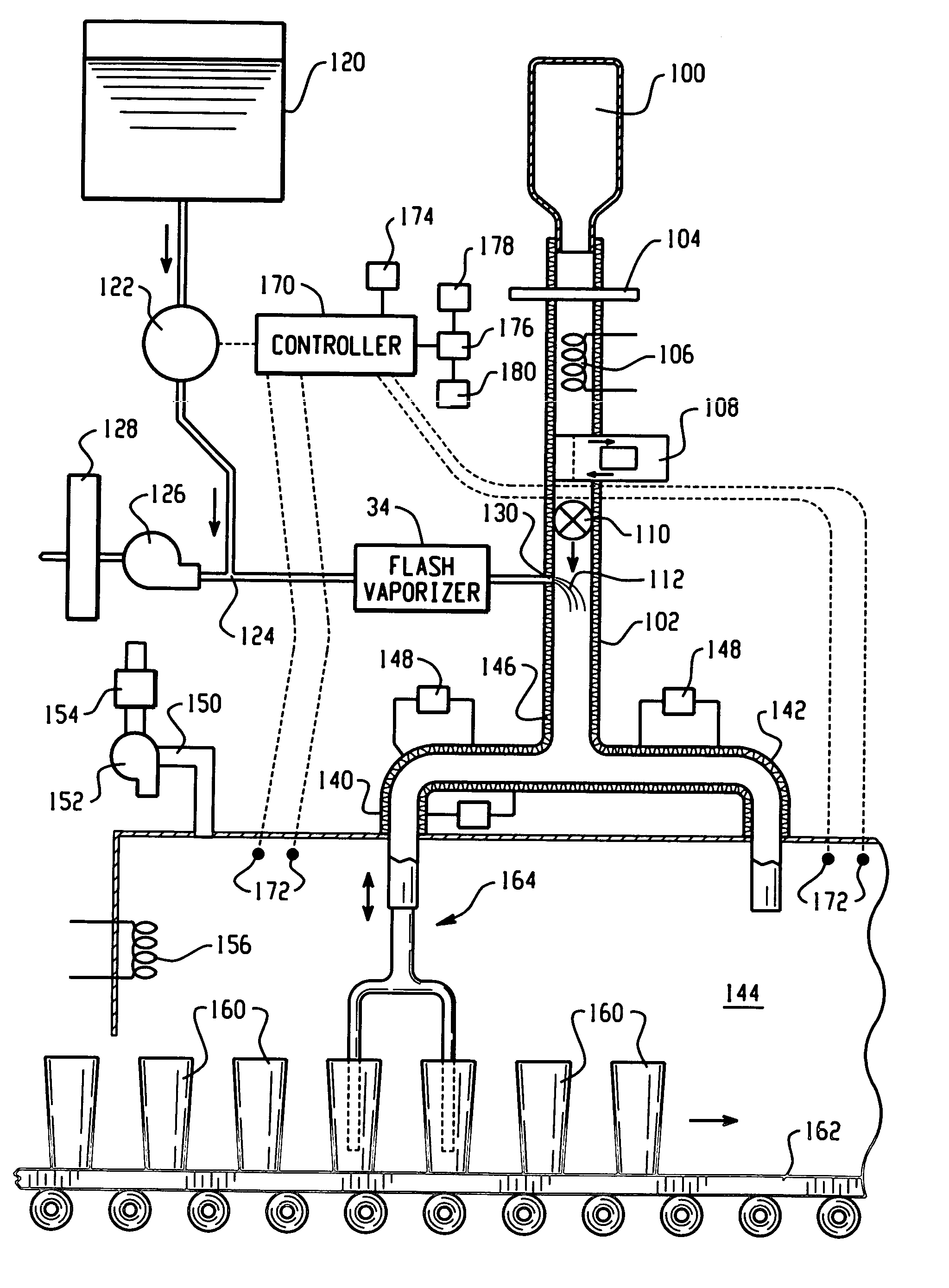
A flash vaporizer (34) provides a constant flow of vaporized hydrogen peroxide or other antimicrobial compounds for rapidly sterilizing large enclosures (10), such as rooms or buildings. The vaporizer includes a heated block (50) which defines an interior bore or bores (70, 72, 74). The flowpath created by the bore or bores increases in cross sectional area as the hydrogen peroxide passes through the block to accommodate the increase in volume during the conversion from liquid to gas. The vapor is injected into dry air in a duct that circulates it to the large enclosure.
Owner:AMERICAN STERILIZER CO
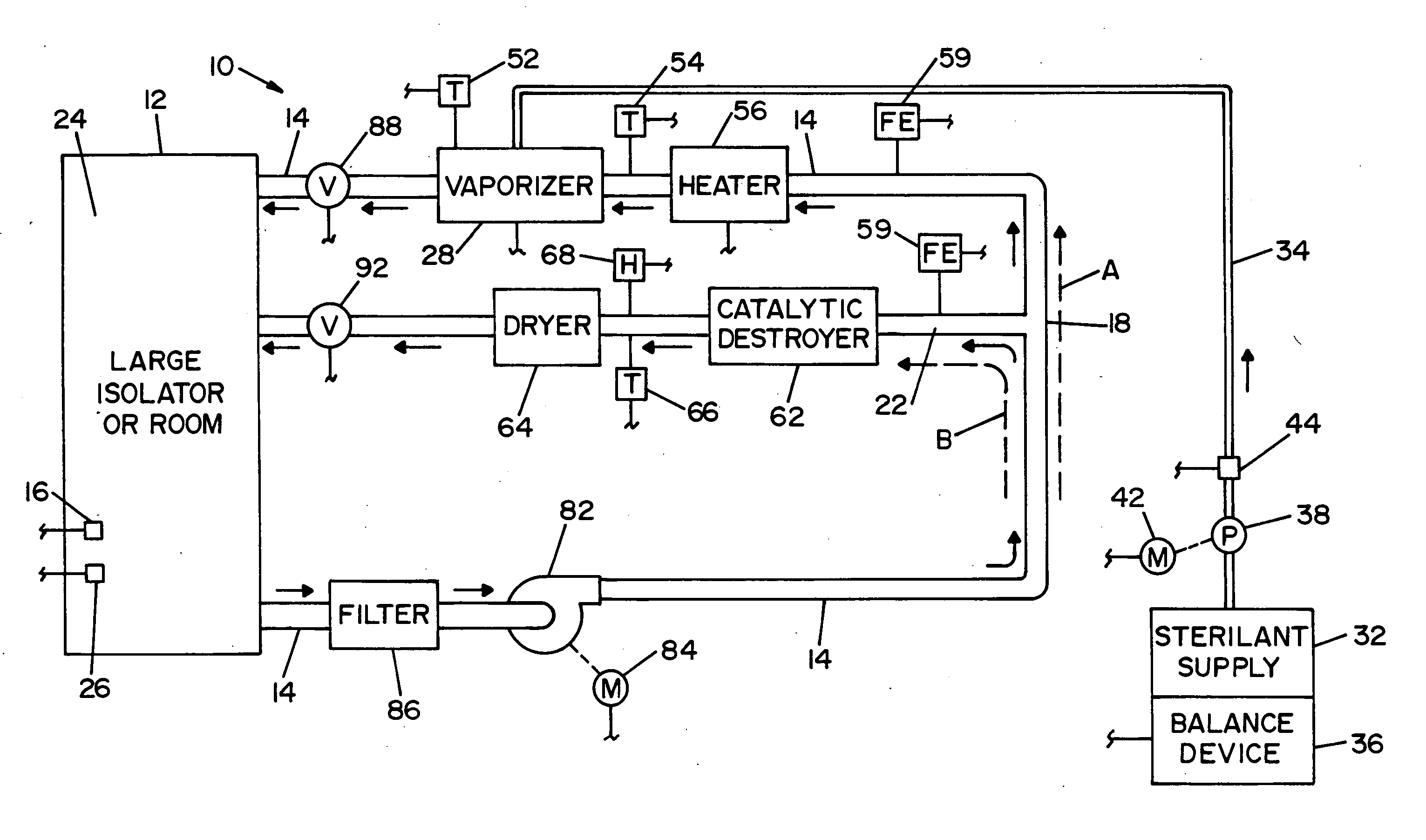
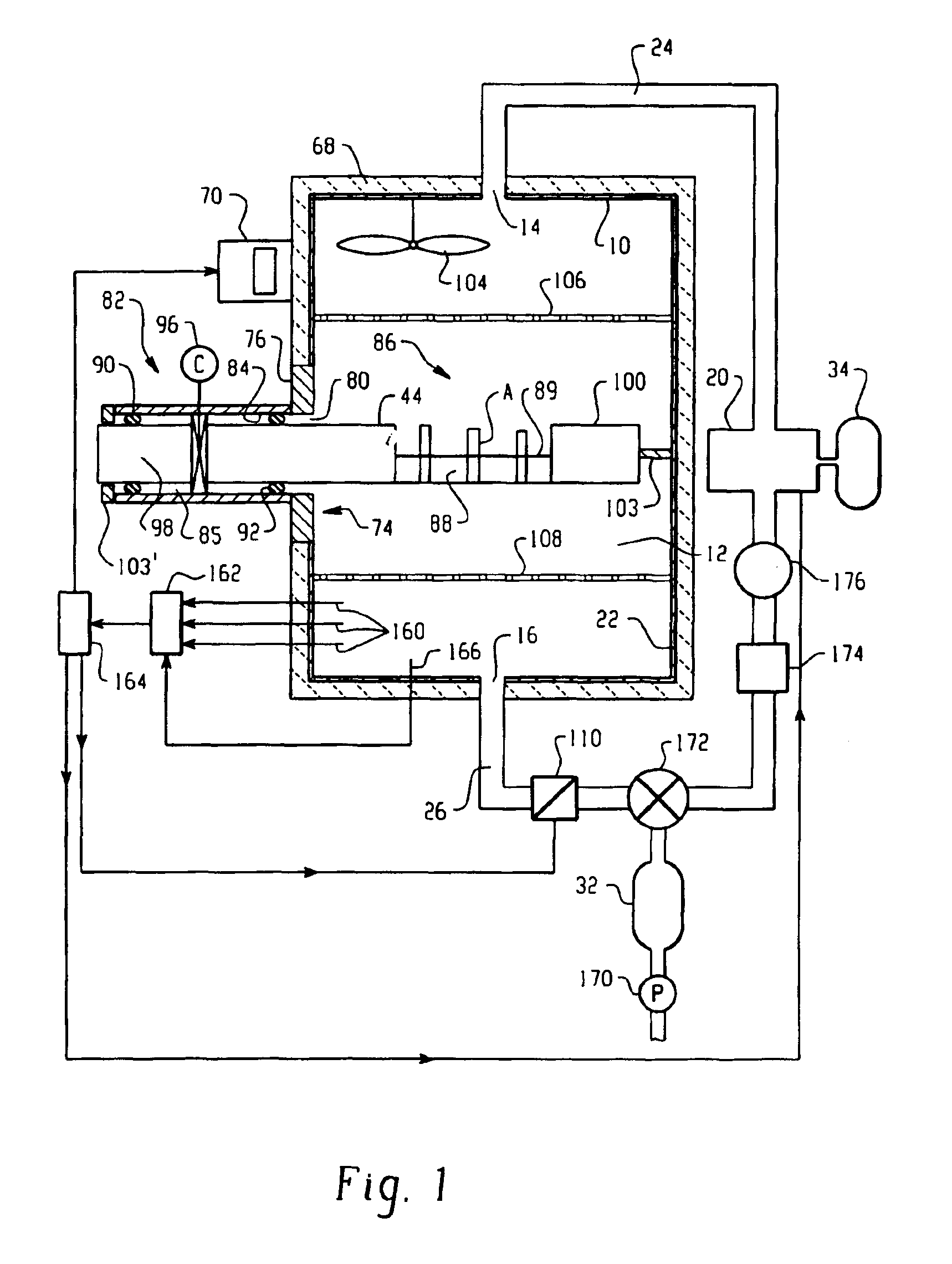
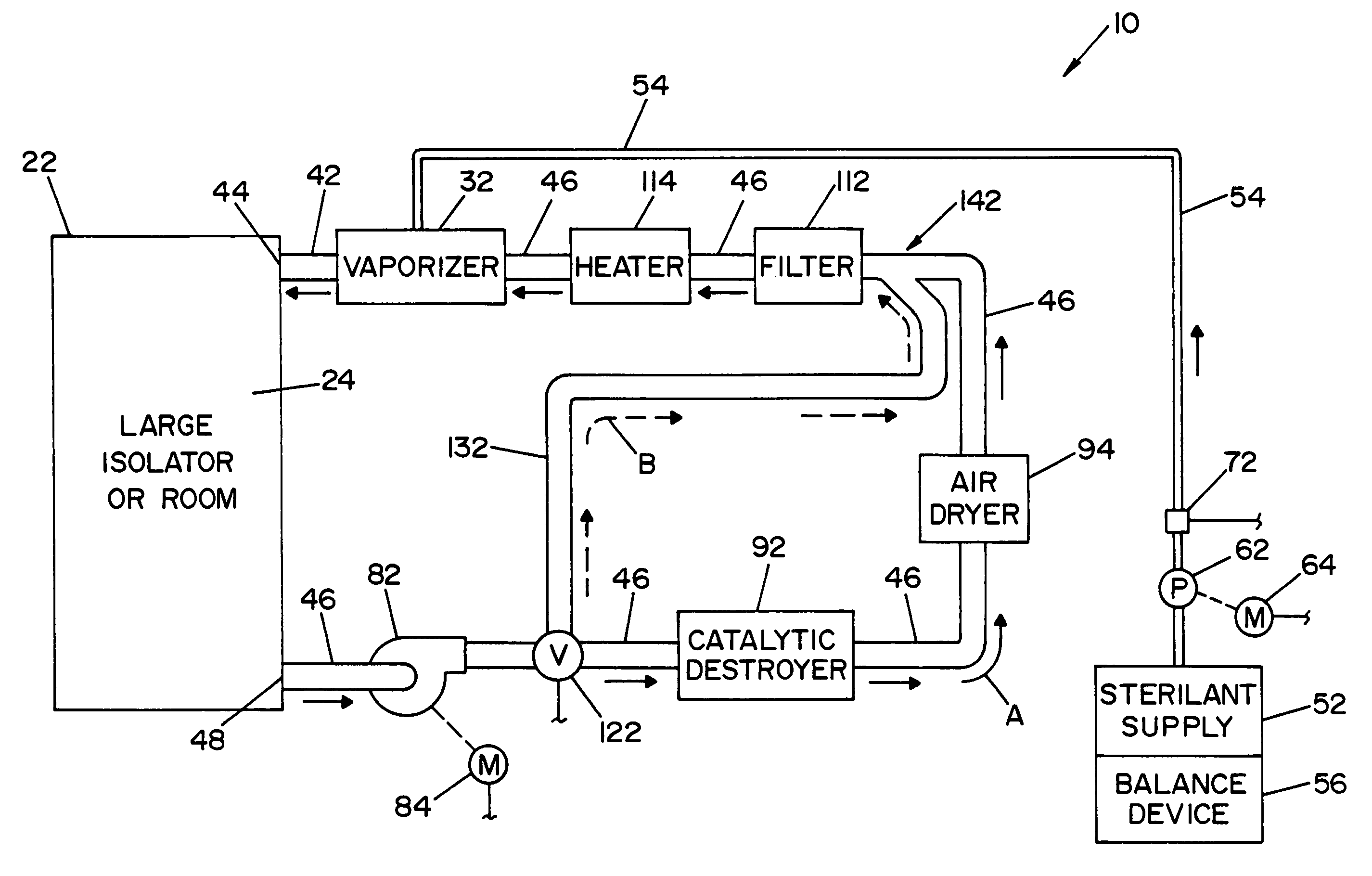
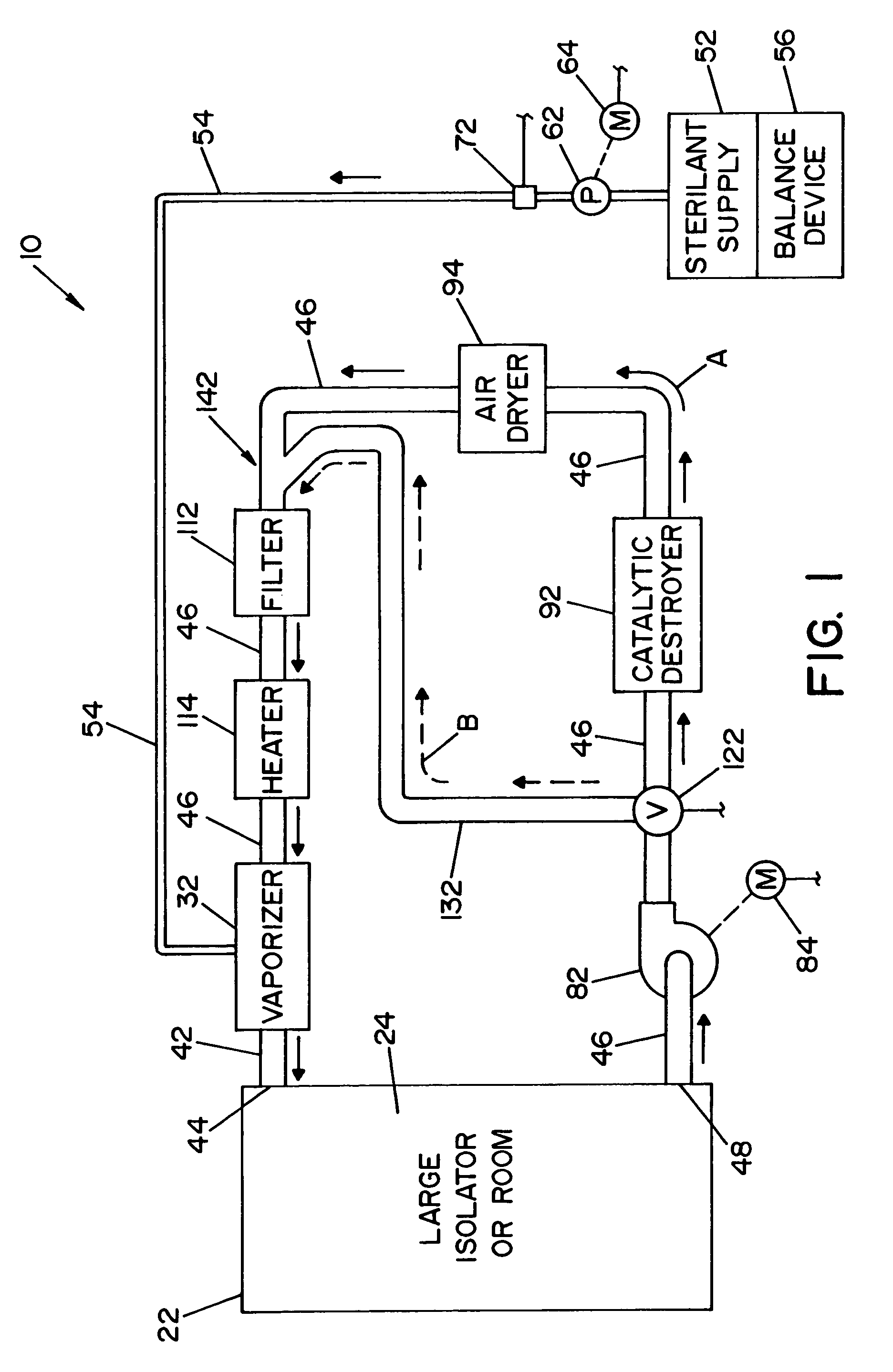
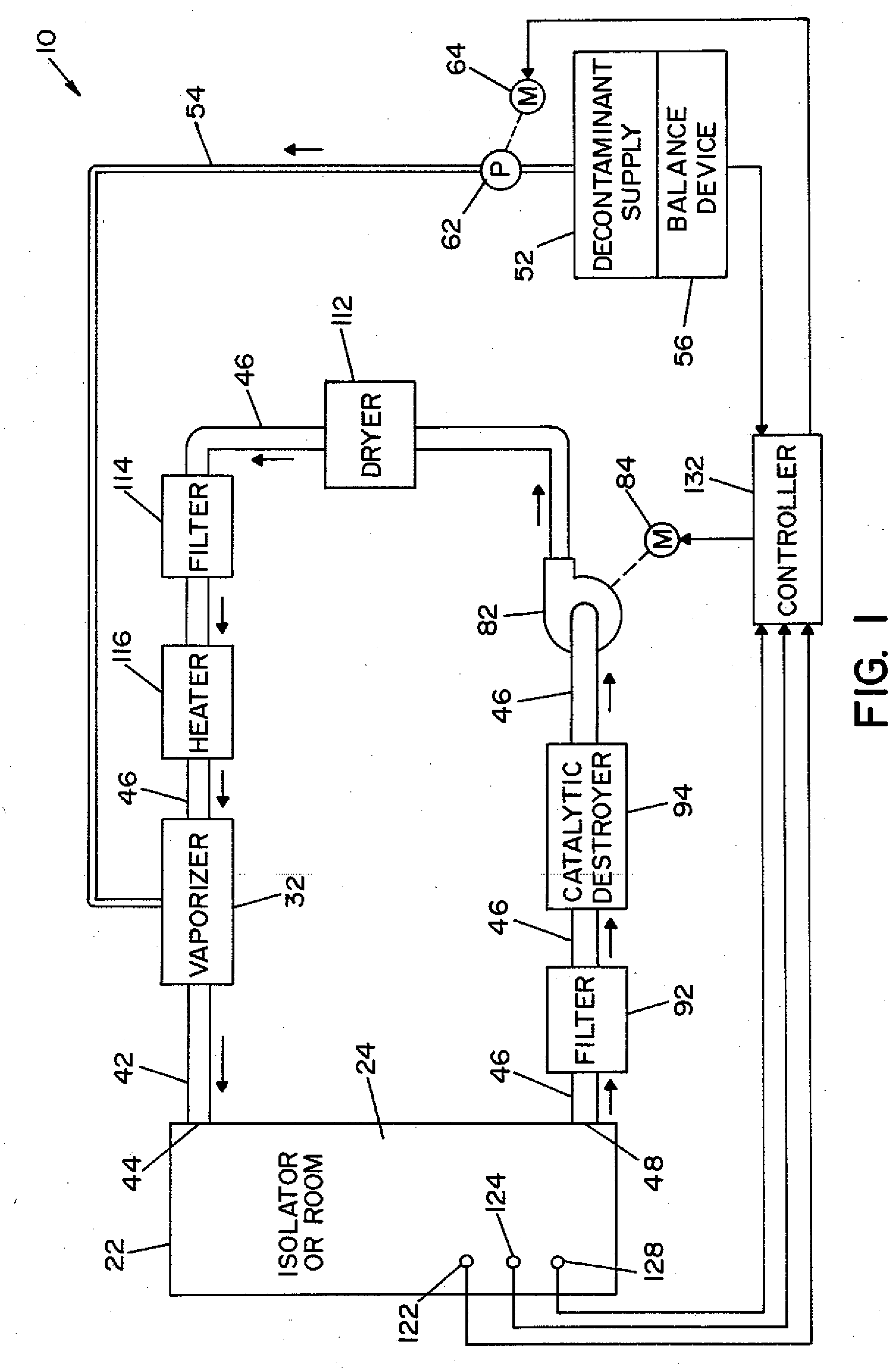
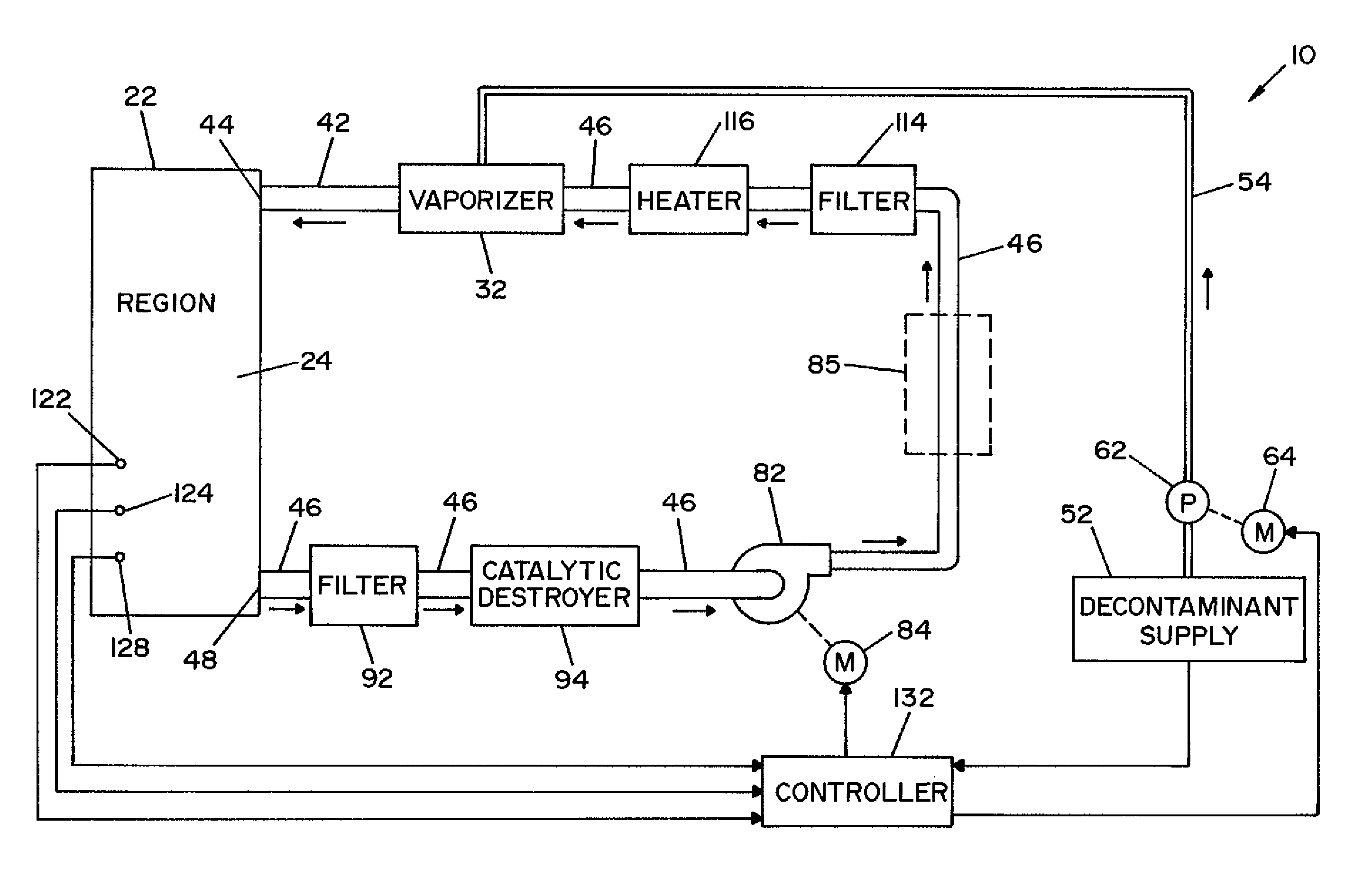
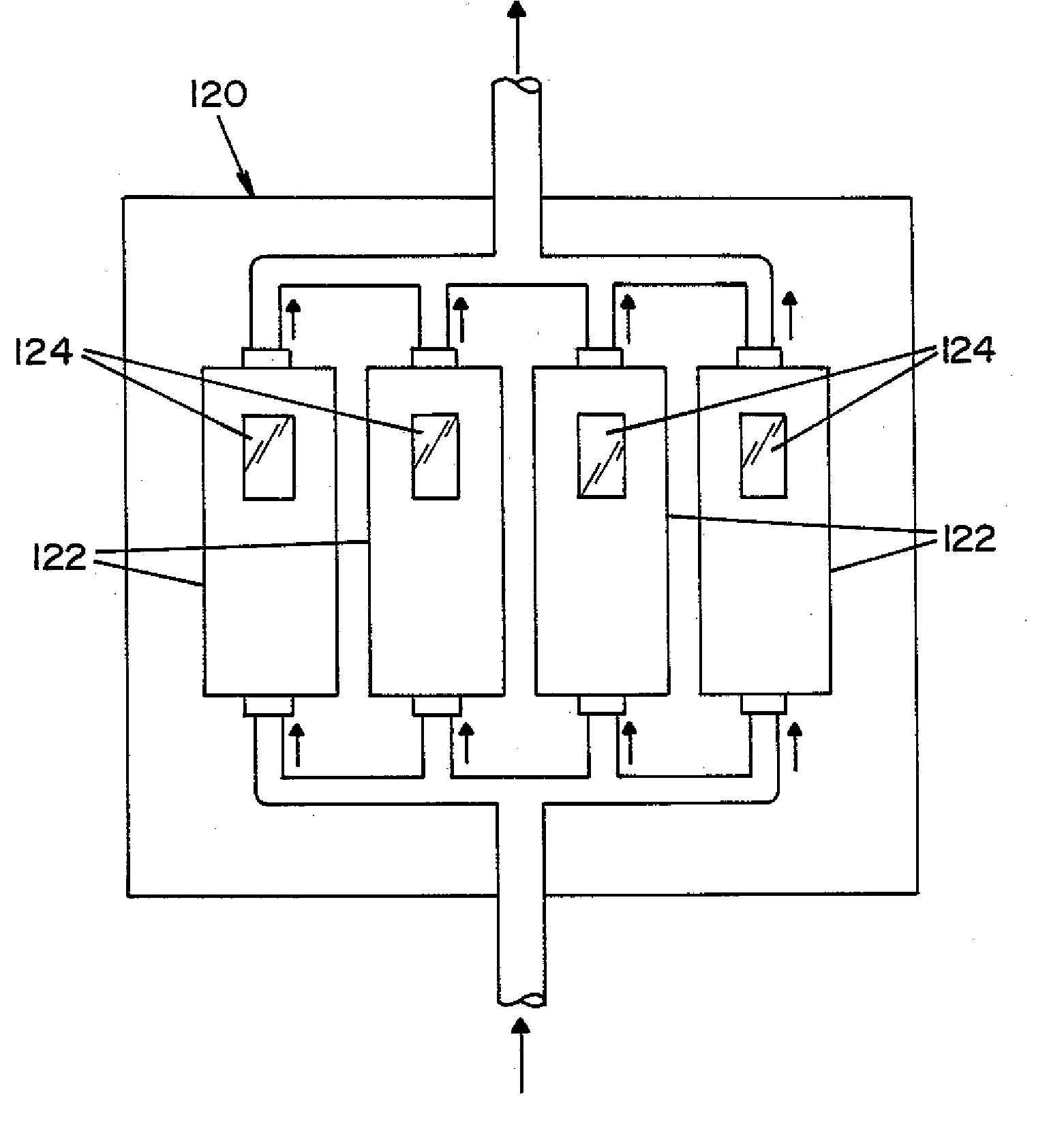
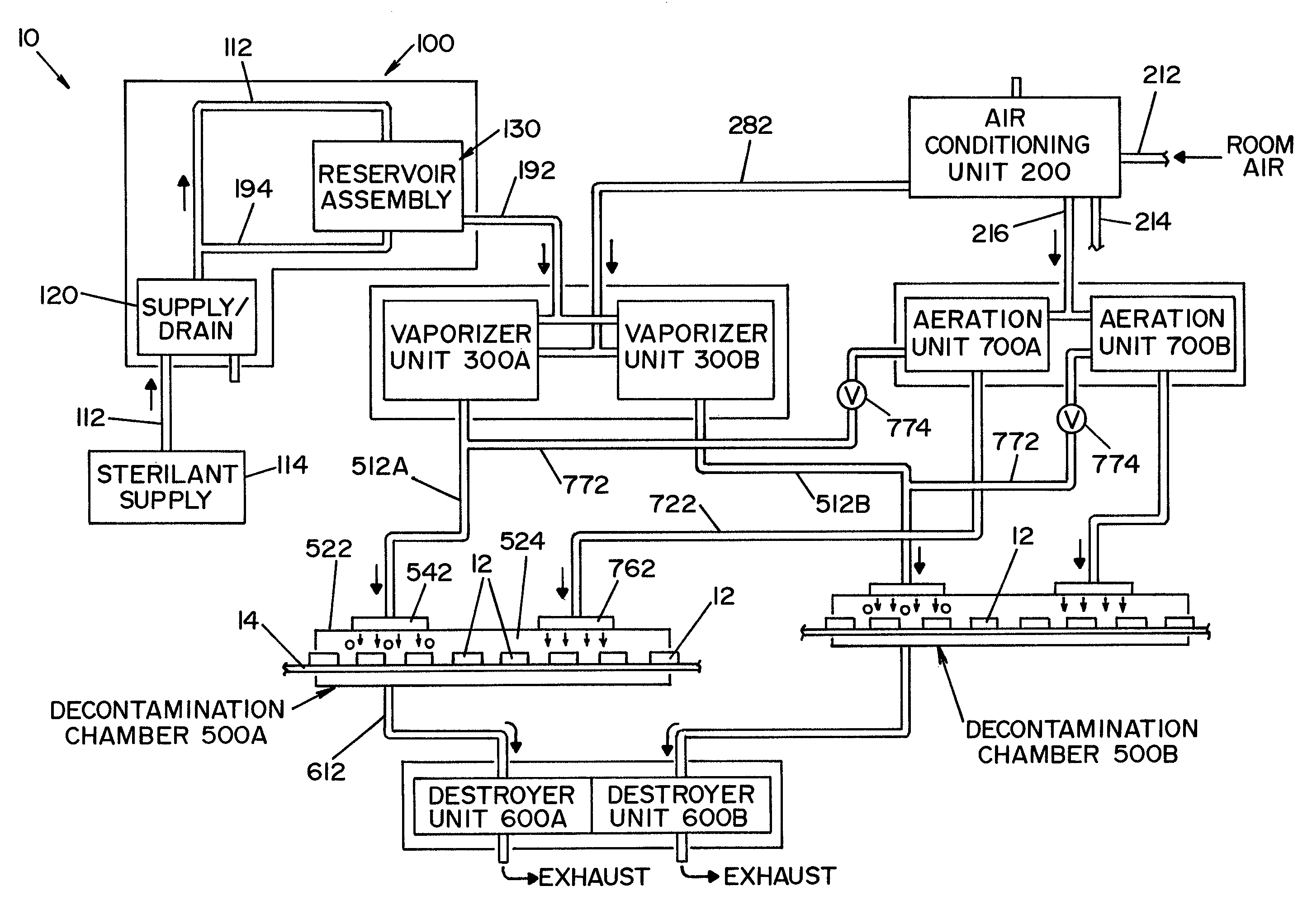
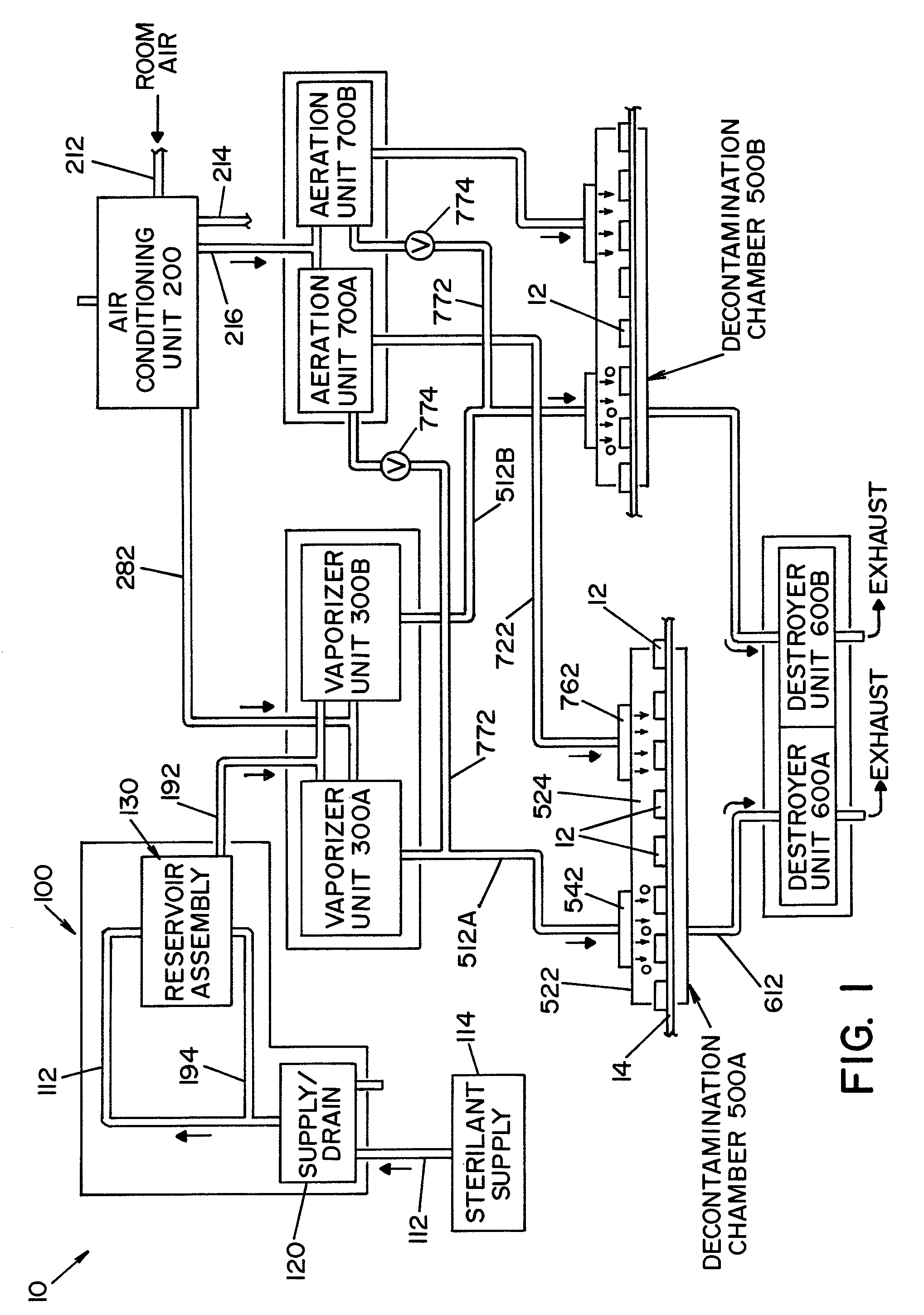
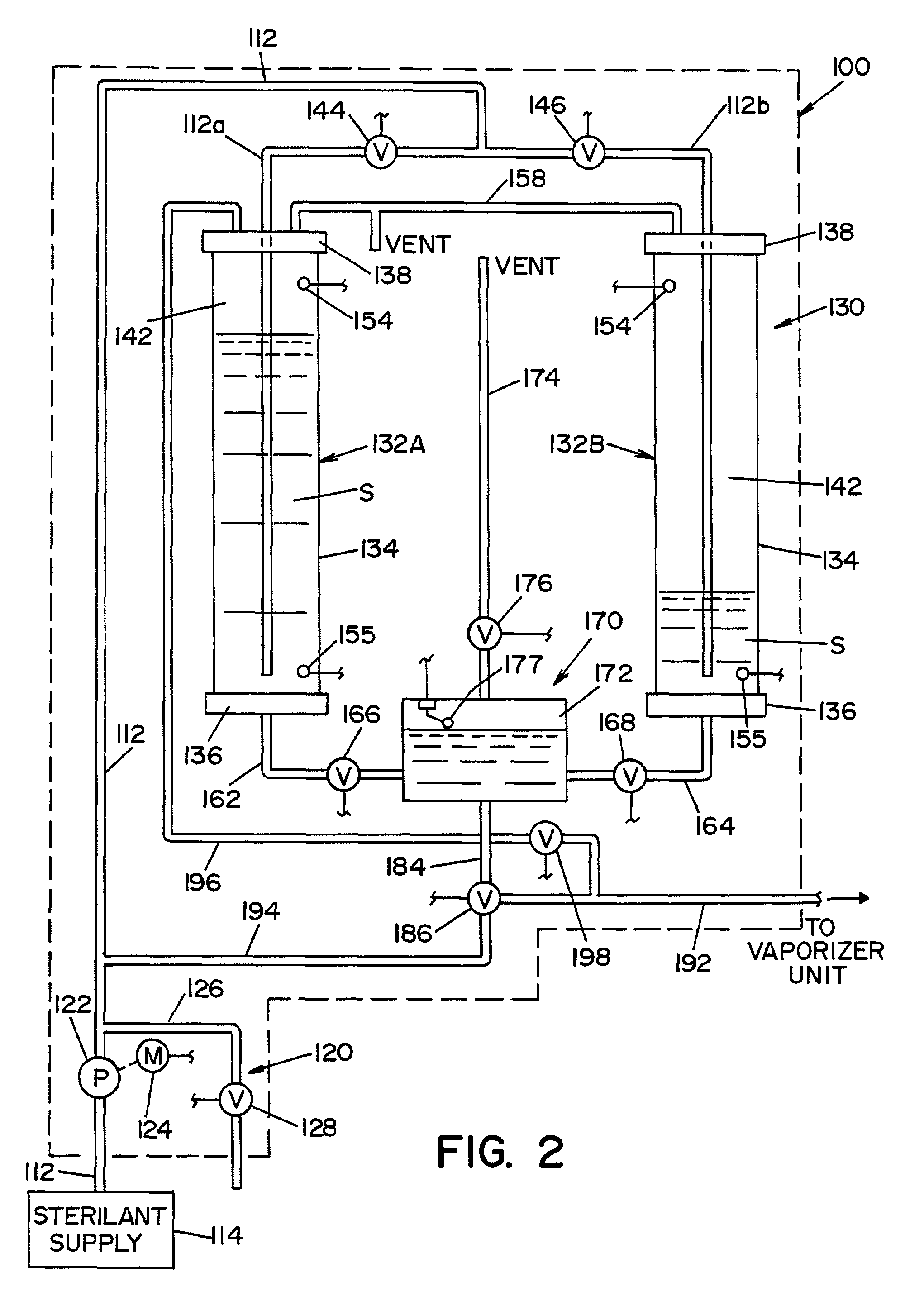
Parallel flow VHP decontamination system
InactiveUS20070098592A1Lower overall pressure dropEnsure efficient flowLavatory sanitoryDeodrantsClosed loopEngineering
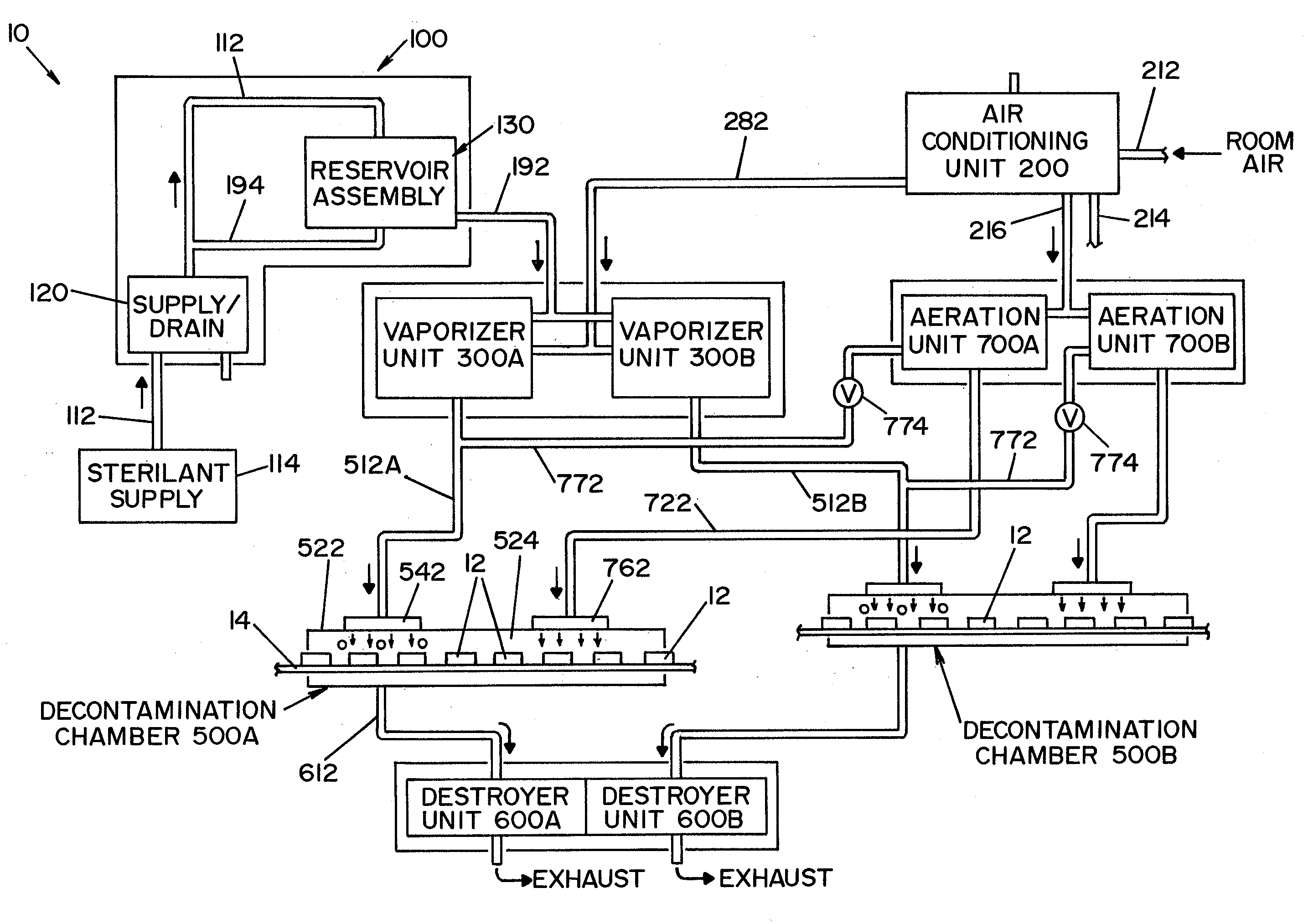
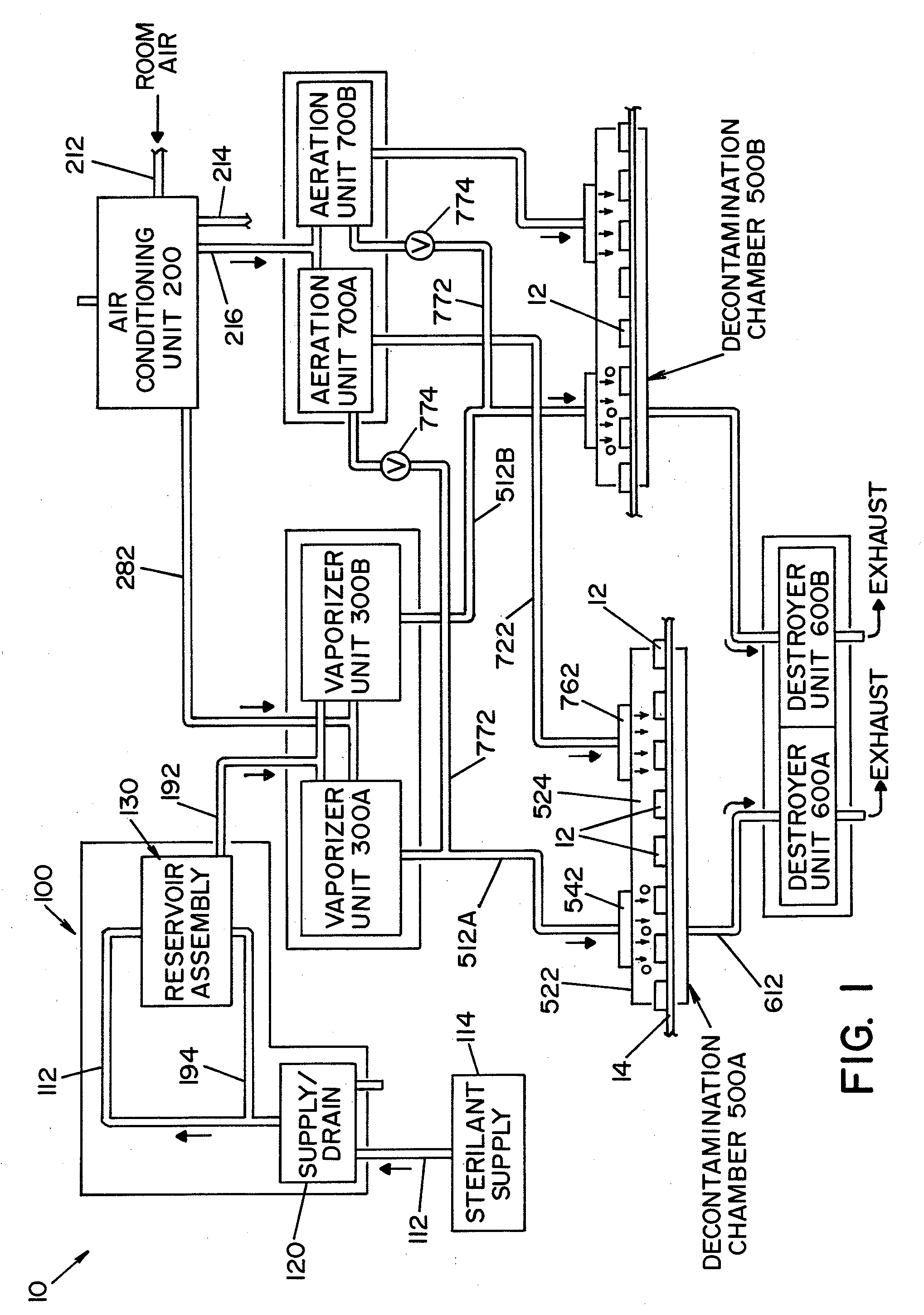
A closed loop vapor decontamination system for decontaminating a defined region. A chamber defines the region. A first fluid flow path connects at both ends to the chamber to define a closed loop path through the chamber. A second fluid flow path connects at both ends to the chamber to define a closed loop path through the chamber. The system has a means for conveying a carrier gas simultaneously along the first and second fluid flow paths. A generator generates vaporized hydrogen peroxide and is disposed along the first fluid flow path for introducing vaporized hydrogen peroxide into the carrier gas as it circulates through the first fluid flow path. A destroyer converts the vaporized hydrogen peroxide into water and oxygen and is disposed along the second fluid flow path for breaking down the vaporized hydrogen peroxide in the carrier gas as it circulates through the second fluid flow path. A controller operates to control the amount of the carrier gas flowing along the first and second fluid flow paths.
Owner:AMERICAN STERILIZER CO
Vapor phase decontamination of containers
ActiveUS7186374B2Increase productionLarge throughputPackage sterilisationLavatory sanitoryVapor phaseVapor generator
A flash vapor generator (10) provides a constant flow of vaporized hydrogen peroxide for rapidly sterilizing a large decontamination tunnel (11) with a high container throughput. The vaporizer includes a heated block which defines an interior bore or bores. The conditions within the decontamination tunnel are carefully monitored to avoid condensation of the vapor while maintaining the vapor as close as possible to the saturation limit.
Owner:AMERICAN STERILIZER CO
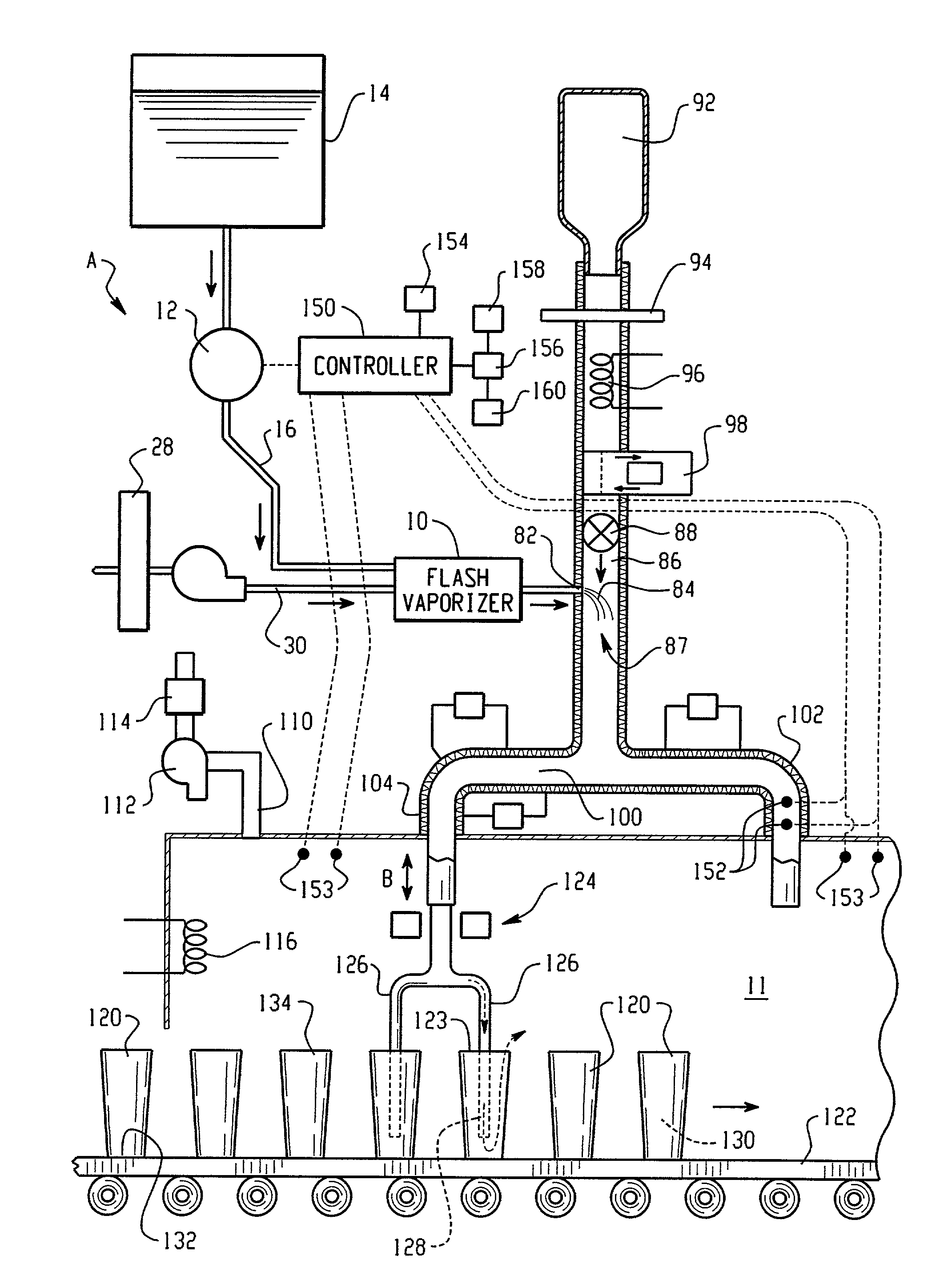
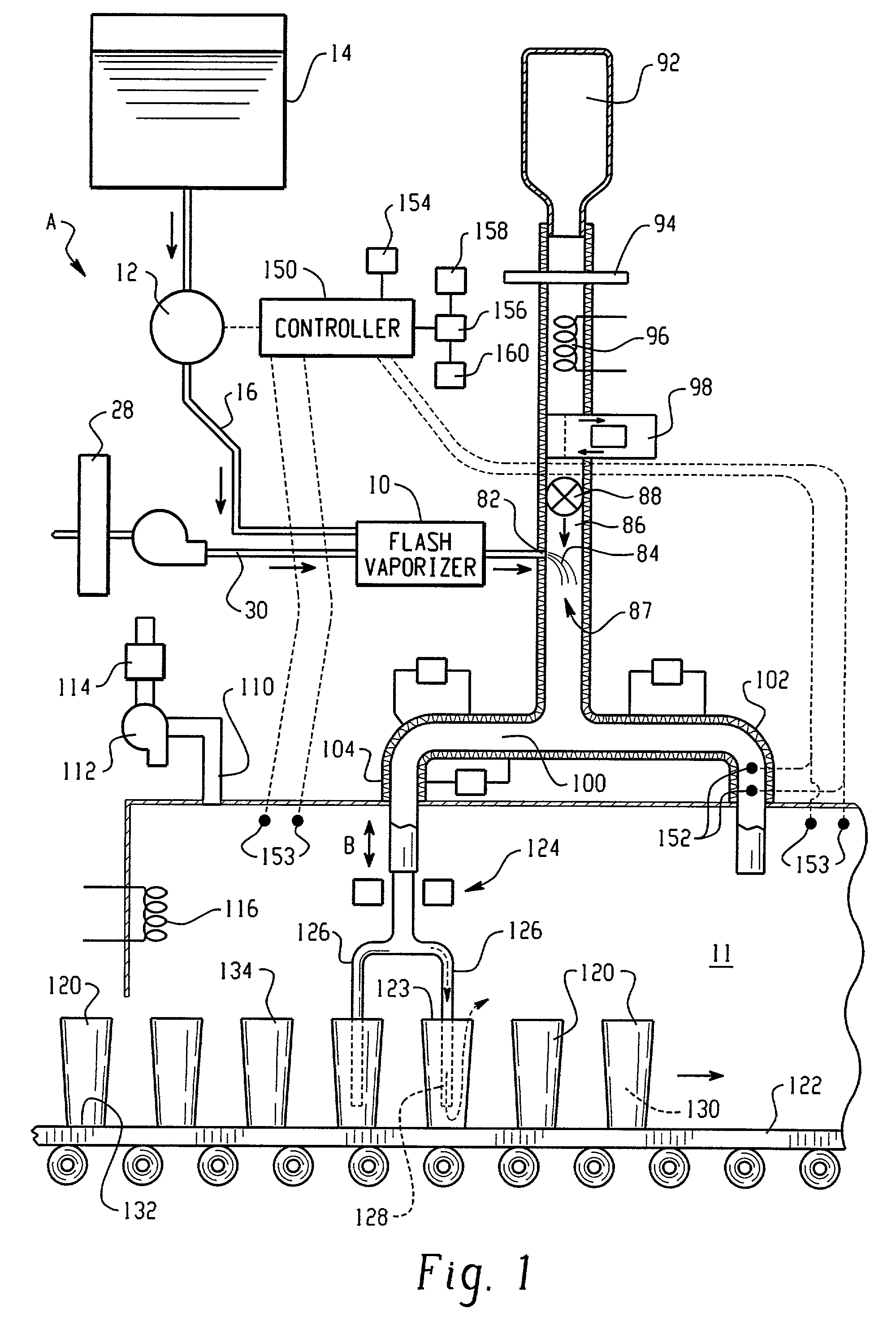
Hydrogen peroxide vaporizer
ActiveUS20070253859A1Modifies flowModifies temperatureLavatory sanitoryDeodrantsLiquid hydrogenEngineering
A method of decontaminating articles, comprising the steps of:(a) moving a plurality of articles having a known temperature along a first path;(b) conveying a carrier gas along a second path that includes an elongated plenum, the second path intersecting the first path downstream from the plenum;(c) heating the carrier gas to a temperature of at least about 105° C. at a location upstream of the plenum;(d) introducing into the carrier gas in the plenum an atomized mist of a liquid hydrogen peroxide of known concentration; and(e) controlling the following:(1) the volumetric flow of carrier gas along the second path;(2) the volume of hydrogen peroxide introduced into the carrier gas; and(3) the temperature of the carrier gas introduced into the plenum, such that the concentration of the vaporized hydrogen peroxide in the carrier gas where the first path intersects the second path has a dew point temperature below the known temperature of the articles.
Owner:AMERICAN STERILIZER CO
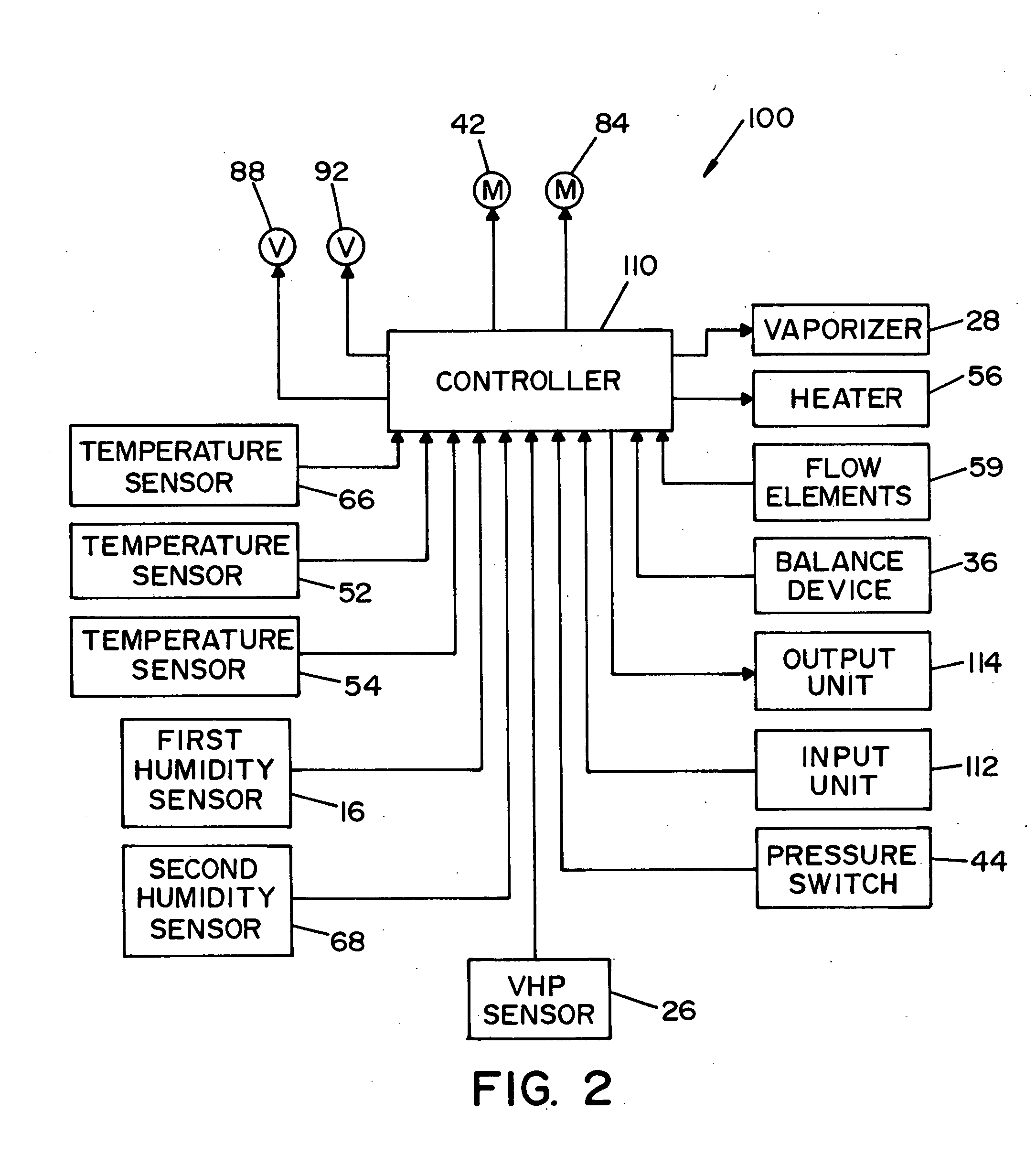
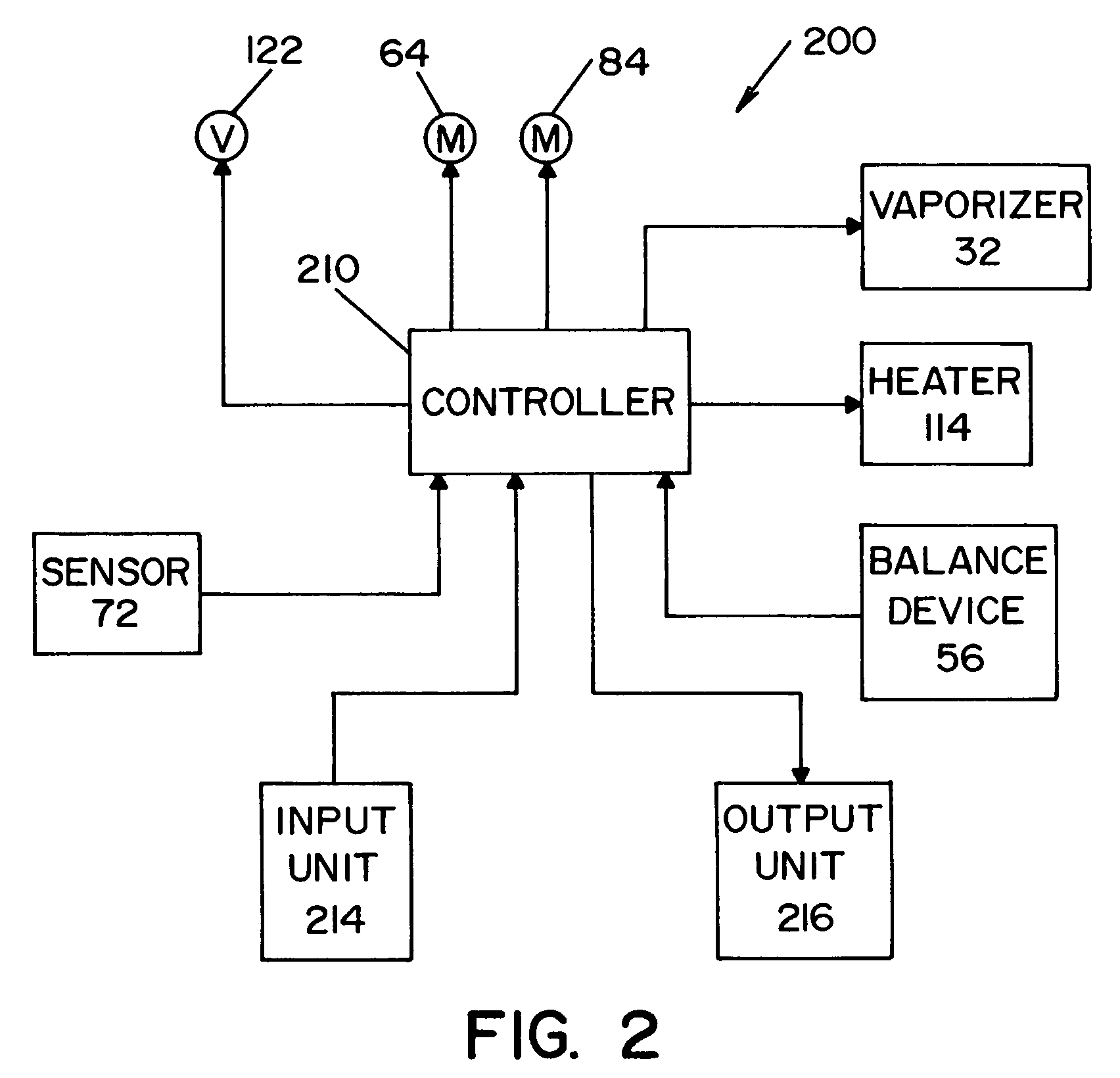
System and method for determining concentration of sterilant
InactiveUS6953549B2Material analysis by optical meansLavatory sanitoryClosed loopProcess engineering
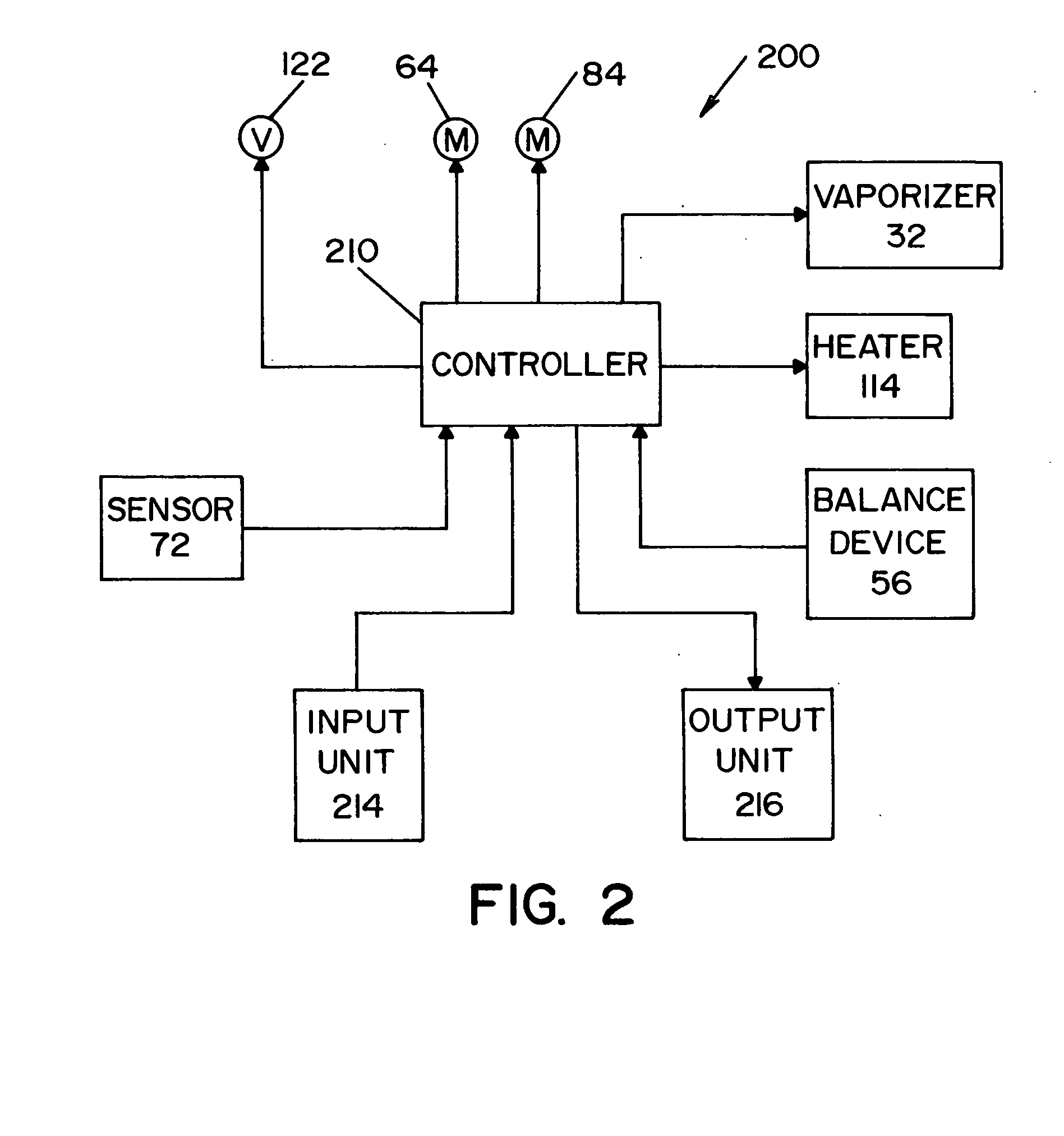
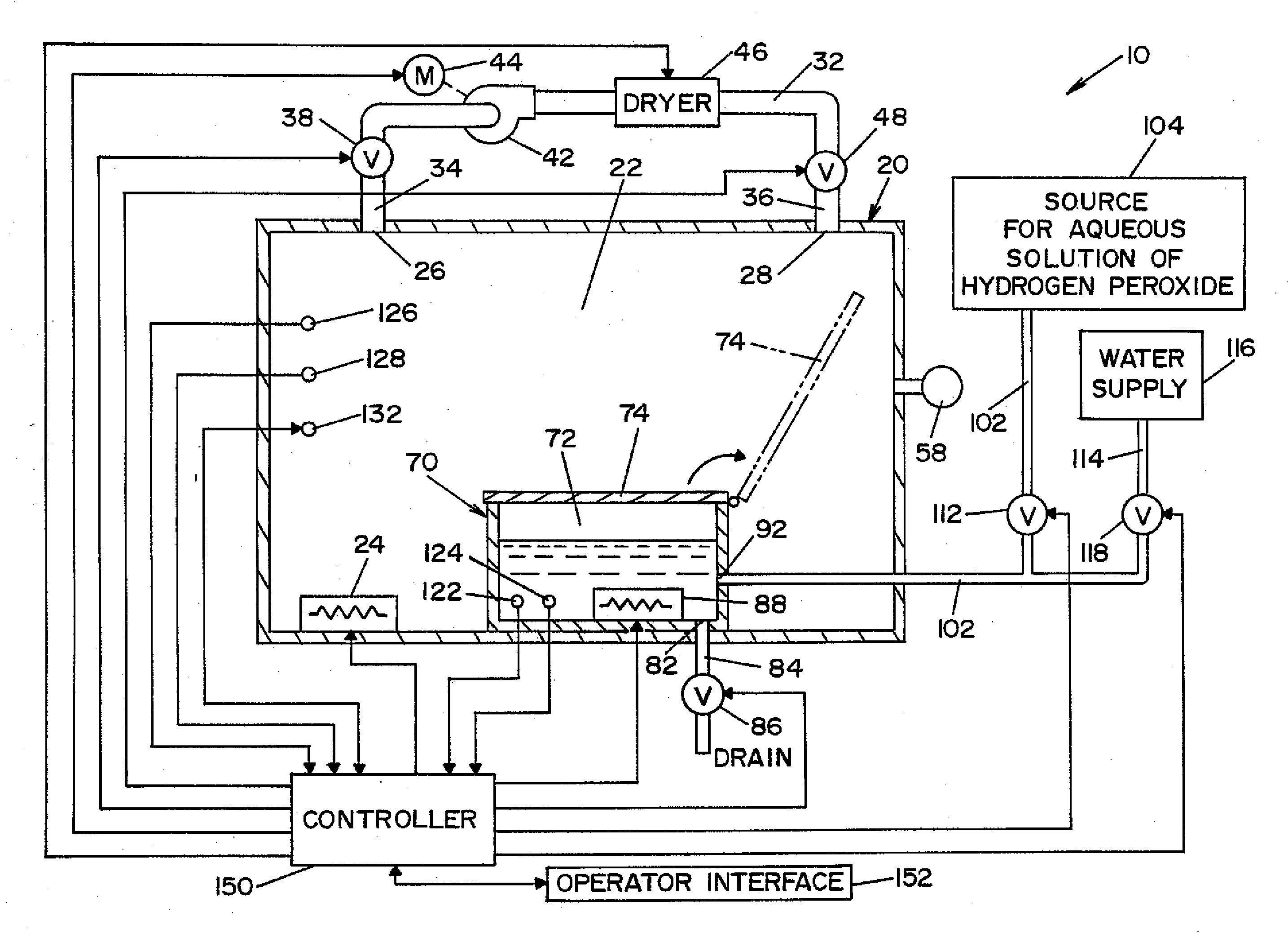
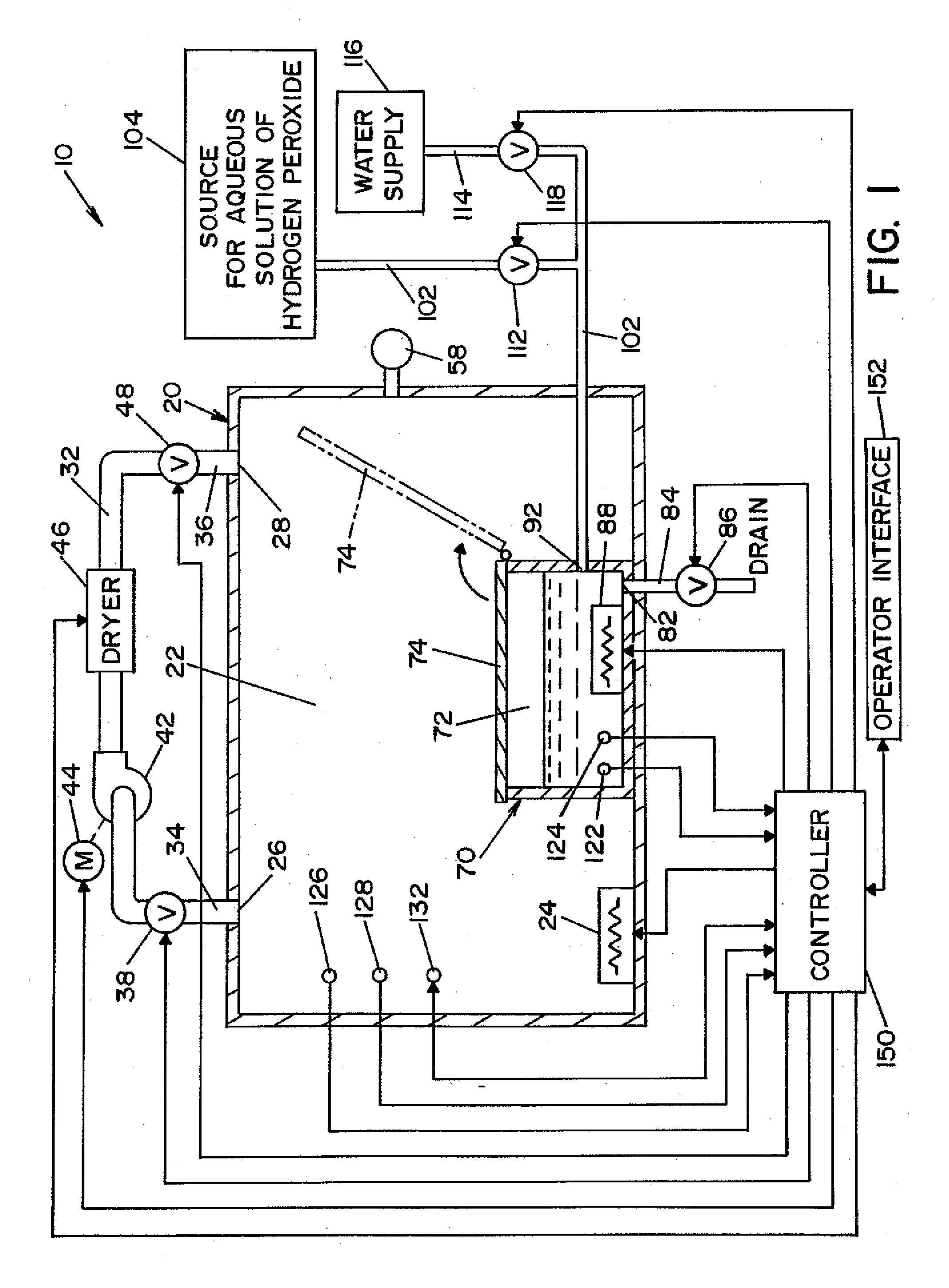
A vapor decontamination system for decontaminating a defined region. The system is comprised of a chamber defining a region, and a generator for generating vaporized hydrogen peroxide from a solution of hydrogen peroxide and water. A closed loop circulating system is provided for supplying the vaporized hydrogen peroxide to the region. A destroyer breaks down the vaporized hydrogen peroxide, and a sensor downstream from the destroyer is operable to sense moisture in the system and provide electrical signals indicative thereof. A controller determines the presence of vaporized hydrogen peroxide in the region based upon the electrical signals from the sensor.
Owner:AMERICAN STERILIZER CO
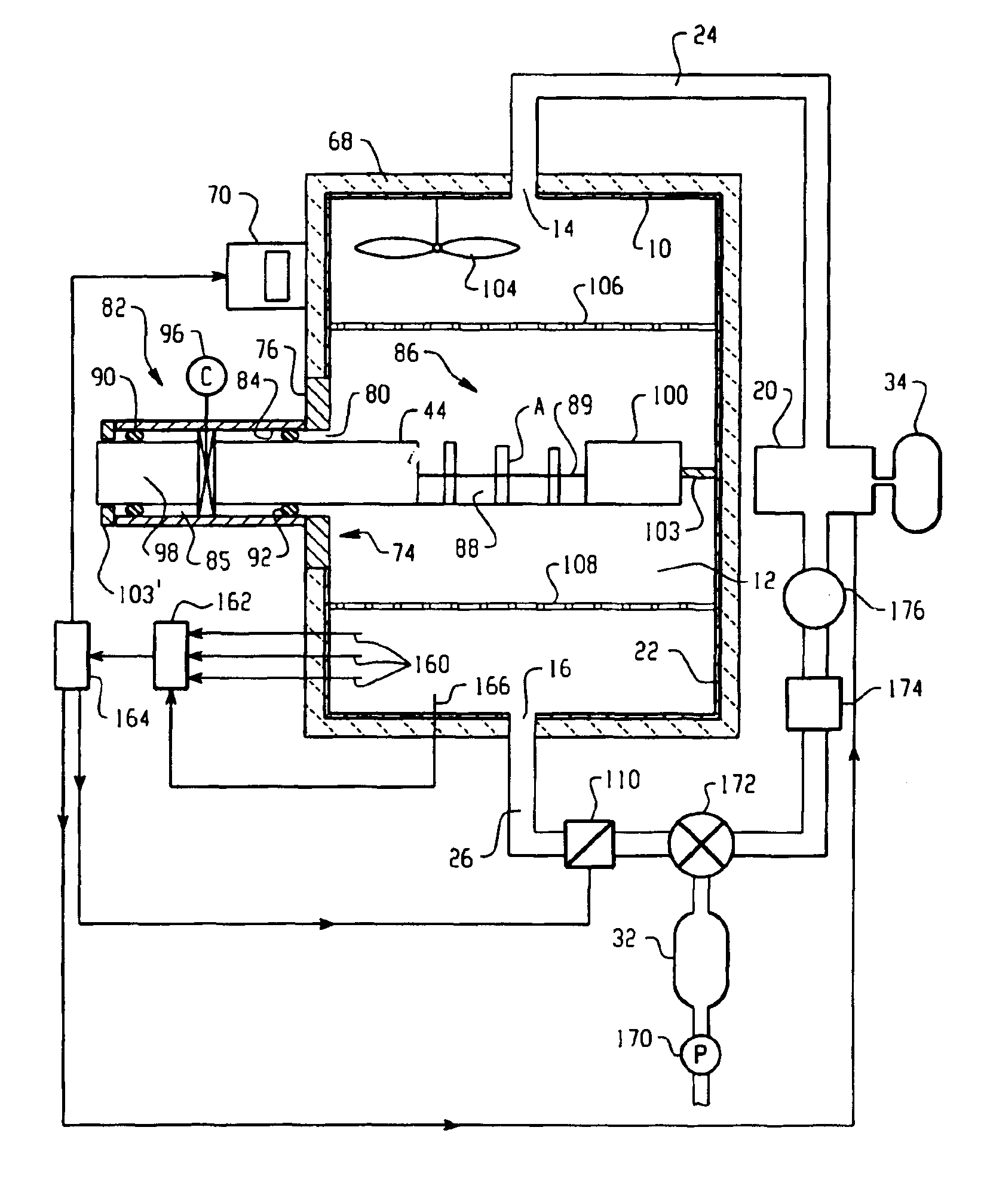
Vapor phase decontamination process biological indicator evaluator resistomer (BIER) vessel
InactiveUS6936434B2Evenly distributedApparent advantageBioreactor/fermenter combinationsBiological substance pretreatmentsEngineeringVapor phase
A BIER vessel evaluates biological indicators for sterilization processes. By flowing gaseous sterilant, such as vaporized hydrogen peroxide, through a chamber (12) before, during, and after introducing the indicators, the indicators are instantaneously exposed to preselected steady state conditions, allowing accurate and reproducible evaluation of the indicator response. A door (32) to an opening (30) in the chamber opens for introducing the indicators to the chamber without appreciably disturbing the steady state conditions therein. After a preselected time, the biological indicators are removed and evaluated for remaining biological activity.
Owner:AMERICAN STERILIZER CO
System and method for determining concentration of sterilant
InactiveUS20050013726A1Material analysis by optical meansLavatory sanitoryClosed loopProcess engineering
A vapor decontamination system for decontaminating a defined region. The system is comprised of a chamber defining a region, and a generator for generating vaporized hydrogen peroxide from a solution of hydrogen peroxide and water. A closed loop circulating system is provided for supplying the vaporized hydrogen peroxide to the region. A destroyer breaks down the vaporized hydrogen peroxide, and a sensor downstream from the destroyer is operable to sense moisture in the system and provide electrical signals indicative thereof. A controller determines the presence of vaporized hydrogen peroxide in the region based upon the electrical signals from the sensor.
Owner:AMERICAN STERILIZER CO
Hydrogen peroxide sterilization device and method
InactiveCN104984378AReduce the temperatureSuitable for sterilizationGaseous substancesChemicalsUltrasonic nozzleAir blower
A hydrogen peroxide sterilization device comprises a hydrogen peroxide liquid storage barrel, an ultrasonic atomizer, an electrical control unit, a vaporizing chamber, a metering pump, an solenoid-controlled valve, an air blower, a heater, a filter device, an air inlet and a steam outlet pipe; the ultrasonic atomizer is further provided with an ultrasonic nozzle provided with an air flue to evenly diffuse atomized hydrogen peroxide microdroplets in the vaporizing chamber, the vaporization efficiency is improved, the atomized hydrogen peroxide microdirplets can be vaporized rapidly at a lower temperature, and the energy is saved. Indoor air serves as carrier gas, outside air is not introduced, and secondary pollution is avoided. Hydrogen peroxide big droplets which are not vaporized in the vaporizing chamber can drop back on the heater under the action of gravity to be vaporized again. By controlling various related parameters, a dew point temperature of output vaporized hydrogen peroxide is made to be lower than the indoor temperature all the time, a steady flow is guaranteed for the vaporized hydrogen peroxide, and the water mist condensation phenomenon which occurs in the transport process are avoided.
Owner:ZHANGJIAGANG INST OF IND TECH SOOCHOW UNIV
System and method for increasing concentration of sterilant in region
ActiveUS20050084431A1Quickly increasing concentrationImprove concentrationLavatory sanitoryDeodrantsClosed loopRadiochemistry
A vapor decontamination system for decontaminating a defined region. The system is comprised of a chamber defining a region, and a generator for generating vaporized hydrogen peroxide from a solution of hydrogen peroxide and water. A closed loop circulating system is provided for supplying the vaporized hydrogen peroxide to the region. A destroyer within the closed loop circulating system breaks down the vaporized hydrogen peroxide. A bypass conduit is provided to bypass the destroyer. A controller causes vaporized hydrogen peroxide from the generator to bypass the destroyer during a predetermined phase of operation.
Owner:AMERICAN STERILIZER CO
High capacity flash vapor generation systems
ActiveUS7157046B2Increase productionIncrease in sizePackage sterilisationFire rescueAntimicrobial compoundLarge capacity
A flash vaporizer (34) provides a constant flow of vaporized hydrogen peroxide or other antimicrobial compounds for rapidly sterilizing large enclosures (10), such as rooms or buildings. The vaporizer includes a heated block (50) which defines an interior bore or bores (70, 72, 74). The flowpath created by the bore or bores increases in cross sectional area as the hydrogen peroxide passes through the block to accommodate the increase in volume during the conversion from liquid to gas. The vapor is injected into dry air in a duct that circulates it to the large enclosure.
Owner:AMERICAN STERILIZER CO
Process challenge device for assessing the effective performance of a biocontamination deactivation process
ActiveUS20080261296A1Reduce condensationIncrease loopBioreactor/fermenter combinationsAnalysis using chemical indicatorsBiomedical engineeringVaporized hydrogen peroxide
A process challenge device (PCD) for determining the effectiveness of a microbial deactivation process that uses a vaporous deactivating agent (e.g., vaporized hydrogen peroxide) as a deactivating agent. The PCD includes first and second layers that are joined together to form (1) a chamber dimensioned to receive a biological and / or chemical indicator, and (2) first and second conduits fluidly connecting the chamber with a region outside the PCD. Each conduit has one end in communication with the region outside the PCD and another end in communication with the chamber. A removable seal member seals the biological and / or chemical indicator inside the chamber.
Owner:AMERICAN STERILIZER CO
System and method for increasing concentration of sterilant in region
ActiveUS7238330B2Quickly increasing concentrationImprove concentrationLavatory sanitoryDeodrantsClosed loopVaporized hydrogen peroxide
A vapor decontamination system for decontaminating a defined region. The system is comprised of a chamber defining a region, and a generator for generating vaporized hydrogen peroxide from a solution of hydrogen peroxide and water. A closed loop circulating system is provided for supplying the vaporized hydrogen peroxide to the region. A destroyer within the closed loop circulating system breaks down the vaporized hydrogen peroxide. A bypass conduit is provided to bypass the destroyer. A controller causes vaporized hydrogen peroxide from the generator to bypass the destroyer during a predetermined phase of operation.
Owner:AMERICAN STERILIZER CO
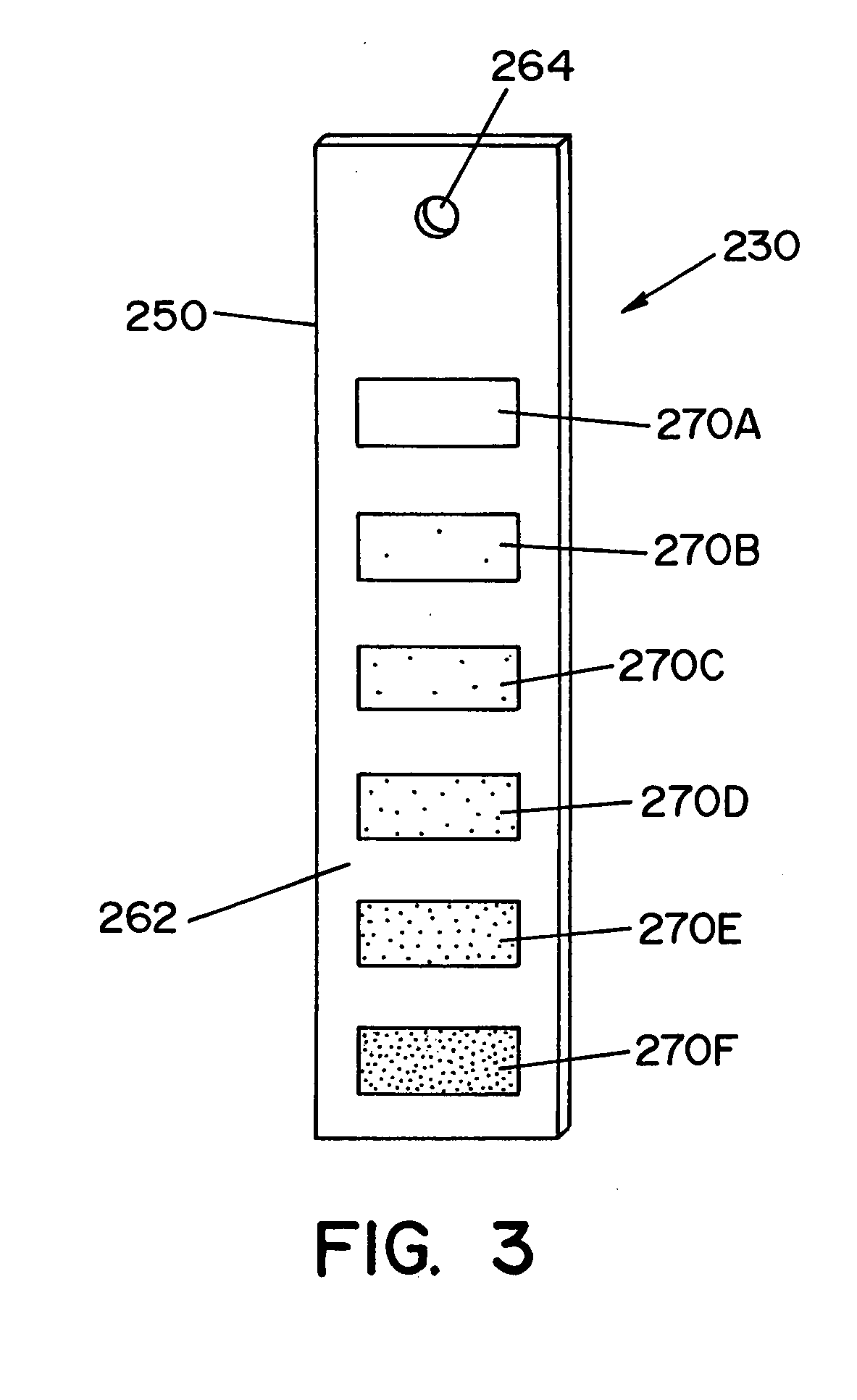
Visual detector for vaporized hydrogen peroxide
A method and apparatus for sensing the concentration of a gaseous sterilant in a sealable enclosure. An indicator is provided that has a chemistry such that the indicator changes color when exposed to vaporized hydrogen peroxide (VHP). The chemistry is adapted to react when exposed to a specific minimum concentration of vaporized hydrogen peroxide for a specific minimum period of time. In this manner, it can be visually determined whether articles (e.g., medical instruments and like devices) located within the sealable enclosure have been exposed to a minimum threshold of vaporized hydrogen peroxide.
Owner:STERIS CORP
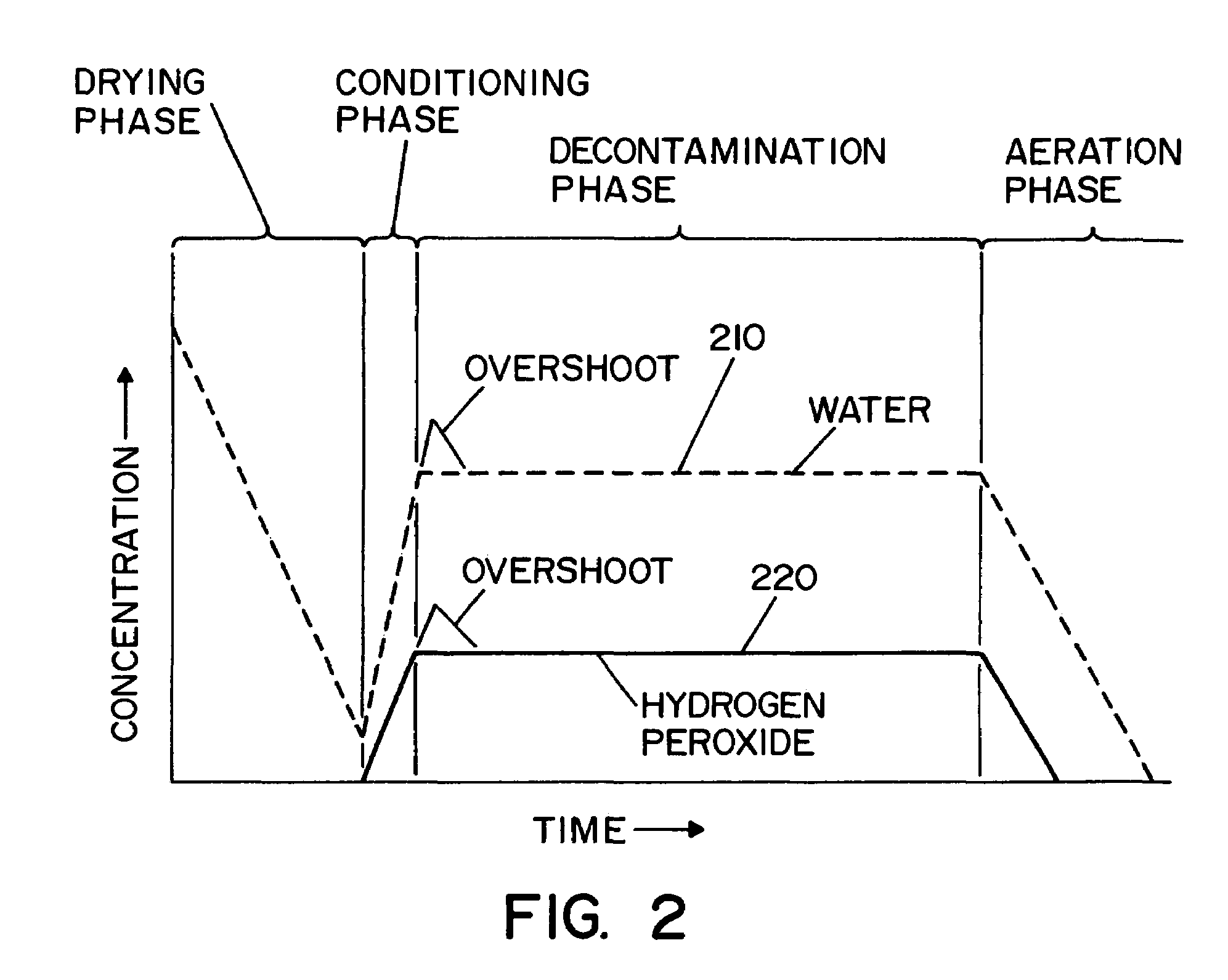
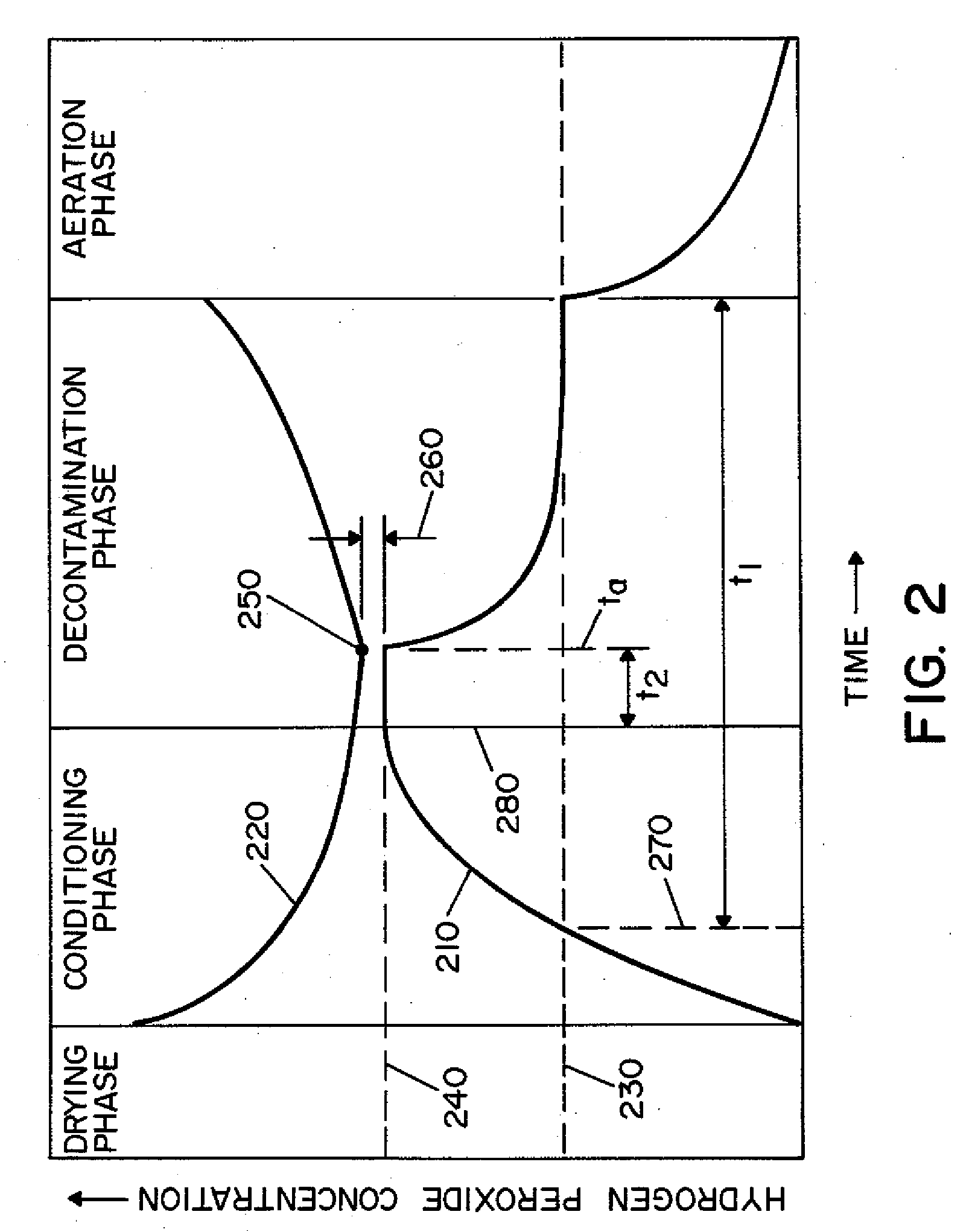
Vaporized hydrogen peroxide decontamination system with concentration adjustment mode
ActiveUS20080267818A1Avoid condensationPrevent operating conditionLavatory sanitoryDeodrantsEnvironmental engineeringH2O2 - Hydrogen peroxide
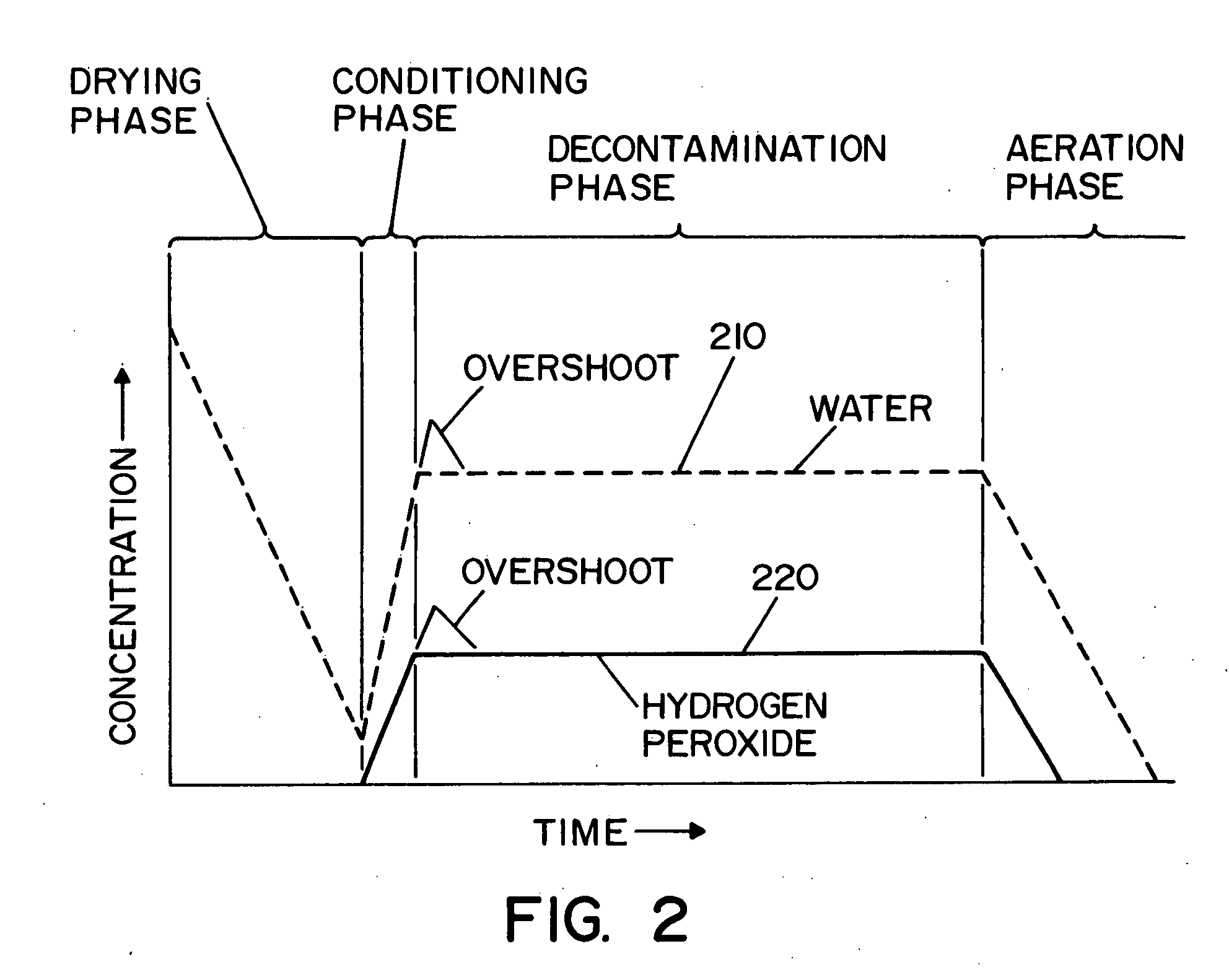
A decontamination system for decontaminating a region with a vaporized decontaminant, such as vaporized hydrogen peroxide. The concentration of the vaporized decontaminant within the region is modified in response to operating conditions. The decontamination system adjusts the concentration of the vaporized decontaminant in response to the monitored saturation concentration of the decontaminant, thereby preventing condensation of the vaporized decontaminant during a decontamination cycle. The decontamination system also adjusts the concentration of the vaporized decontaminant in order to minimize the time required to complete a successful decontamination operation.
Owner:AMERICAN STERILIZER CO
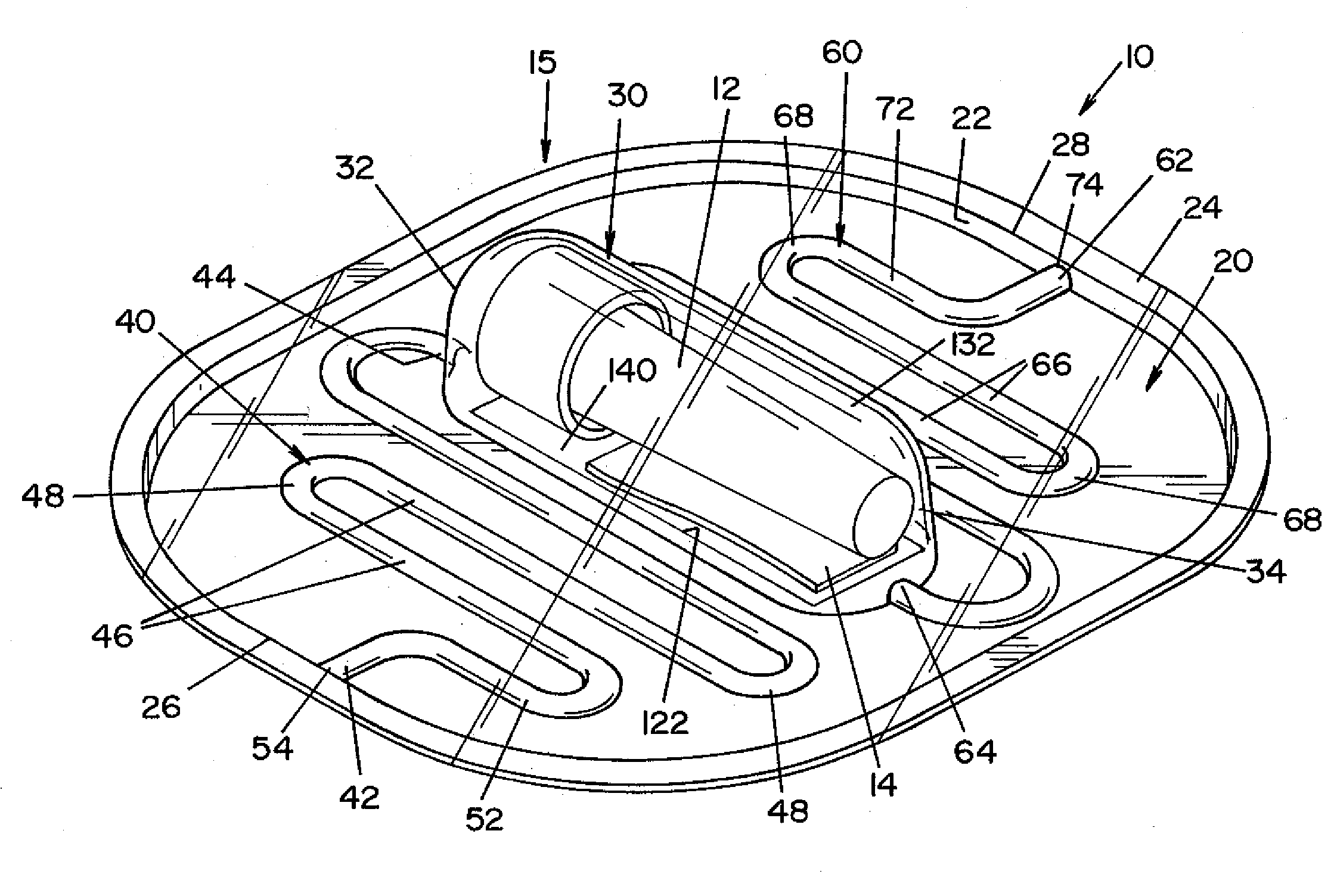
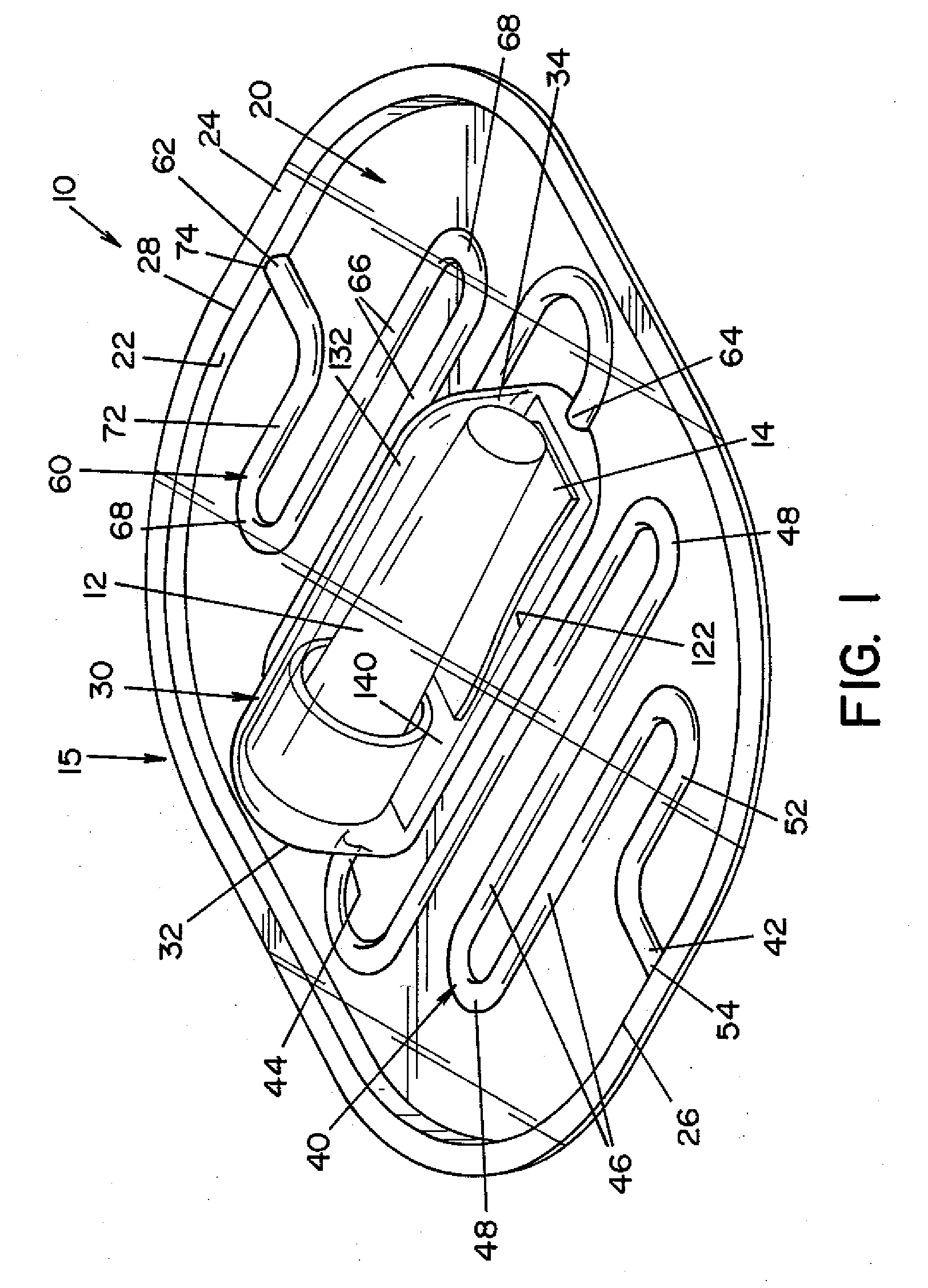
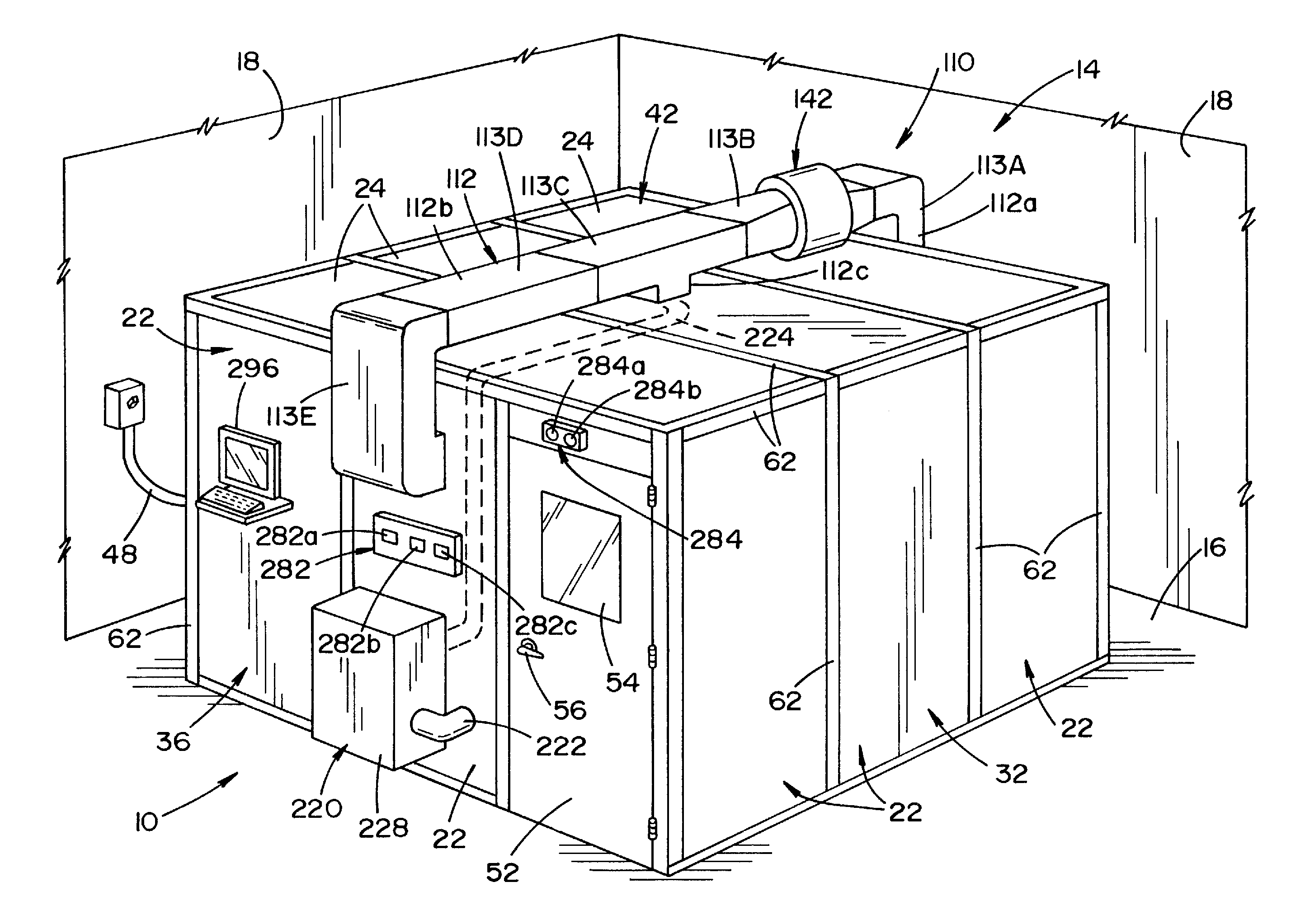
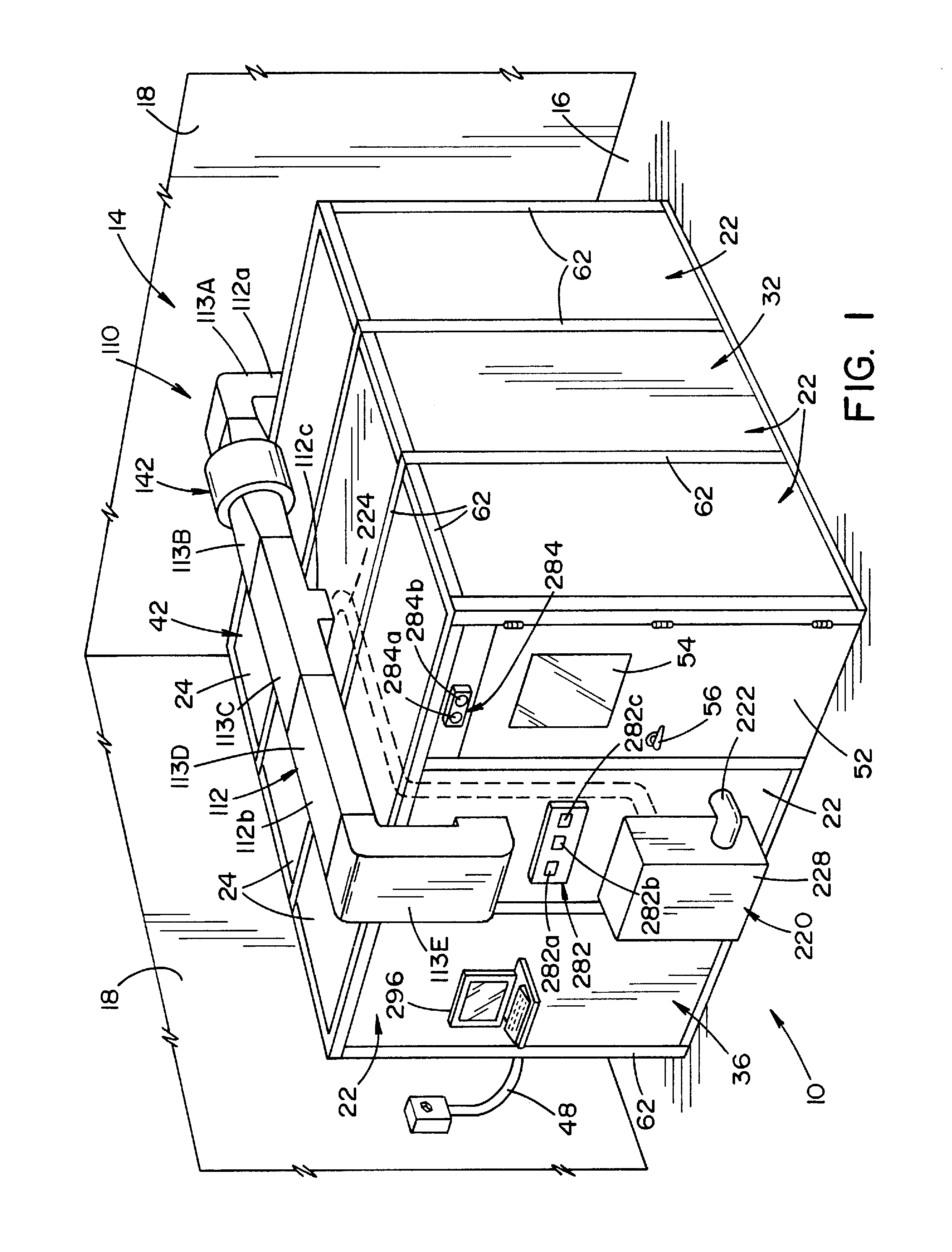
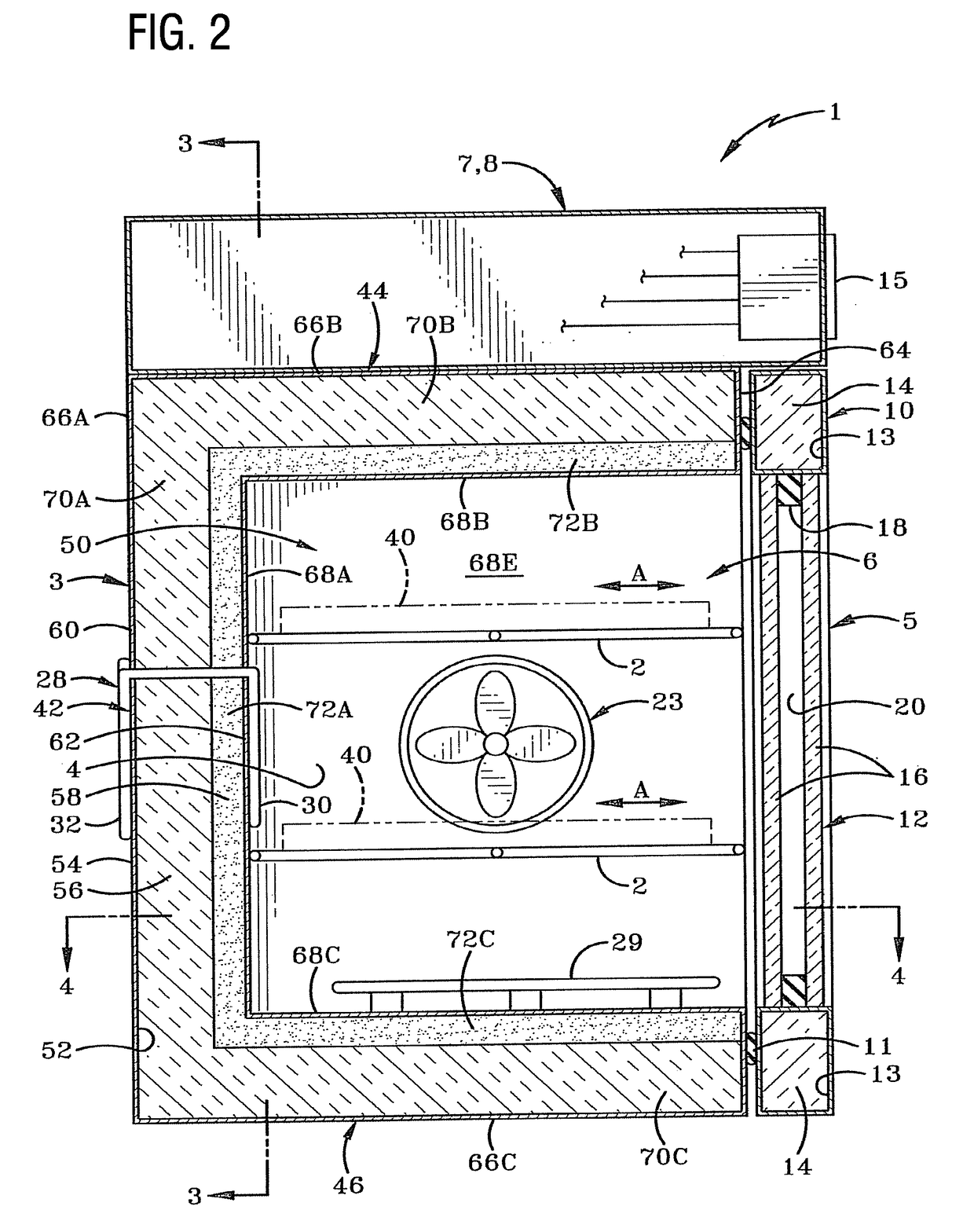
Vaporized hydrogen peroxide decontamination structure
ActiveUS20130216438A1Avoid accessExcellent decontaminationGaseous chemical processesSamplingEngineeringVaporized hydrogen peroxide
A decontamination enclosure, comprised of a plurality of preformed panels joined together to form a structure defining a totally enclosed chamber. A door is formed in at least one of the panels, the door being movable between an opened position and a closed position to allow access to the chamber. A circulation system is attached to the structure for circulating vaporized hydrogen peroxide through the chamber. A controller is provided for controlling the amount of vaporized hydrogen peroxide introduced into the chamber.
Owner:AMERICAN STERILIZER CO
Visual detector for vaporized hydrogen peroxide
ActiveUS20050019206A1Analysis using chemical indicatorsSamplingChange colorVaporized hydrogen peroxide
A method and apparatus for sensing the concentration of a gaseous sterilant in a sealable enclosure. An indicator is provided that has a chemistry such that the indicator changes color when exposed to vaporized hydrogen peroxide (VHP). The chemistry is adapted to react when exposed to a specific minimum concentration of vaporized hydrogen peroxide for a specific minimum period of time. In this manner, it can be visually determined whether articles (e.g., medical instruments and like devices) located within the sealable enclosure have been exposed to a minimum threshold of vaporized hydrogen peroxide.
Owner:STERIS CORP
Simultaneous flue gas desulfurization and denitrification system and method based on hydrogen peroxide
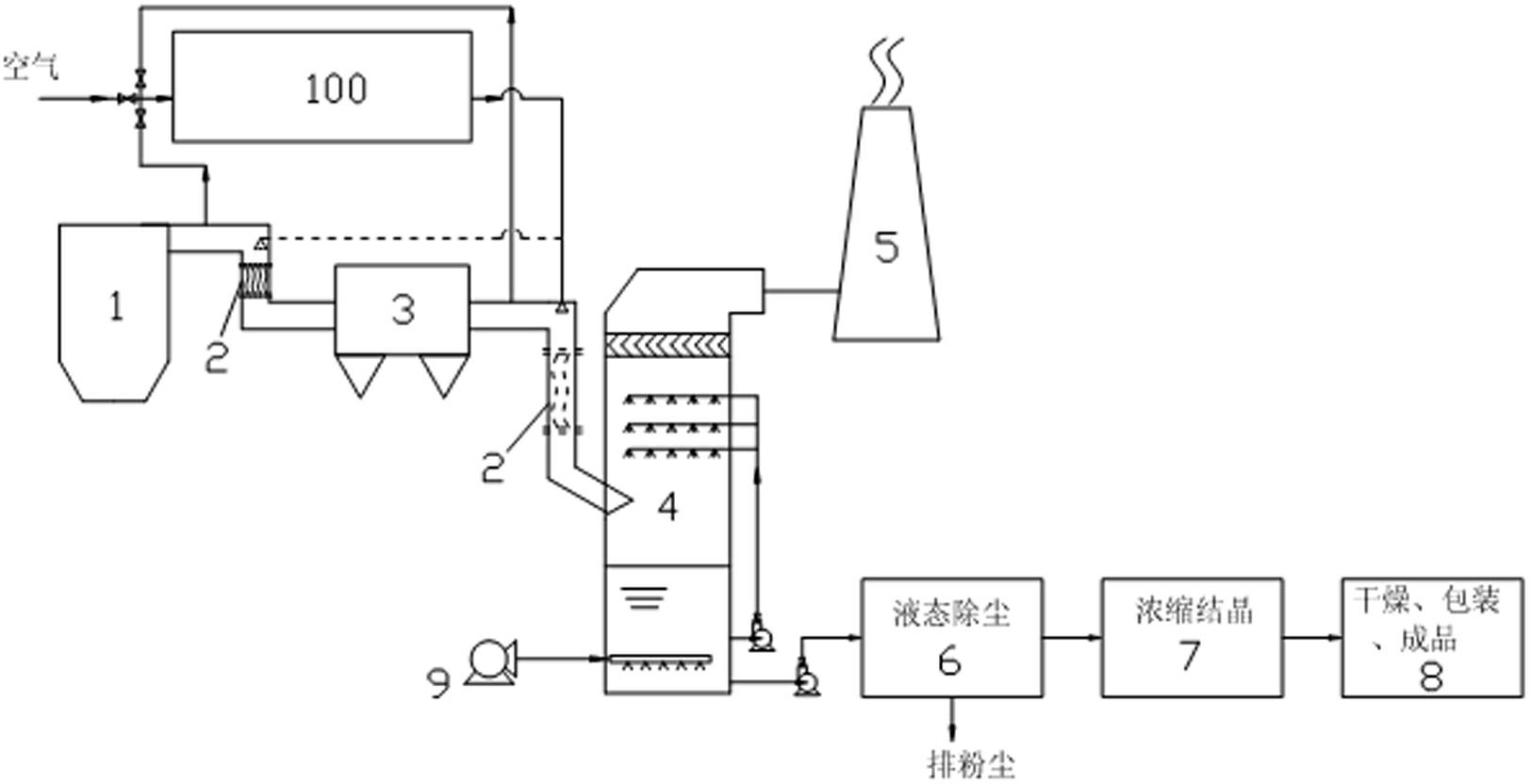
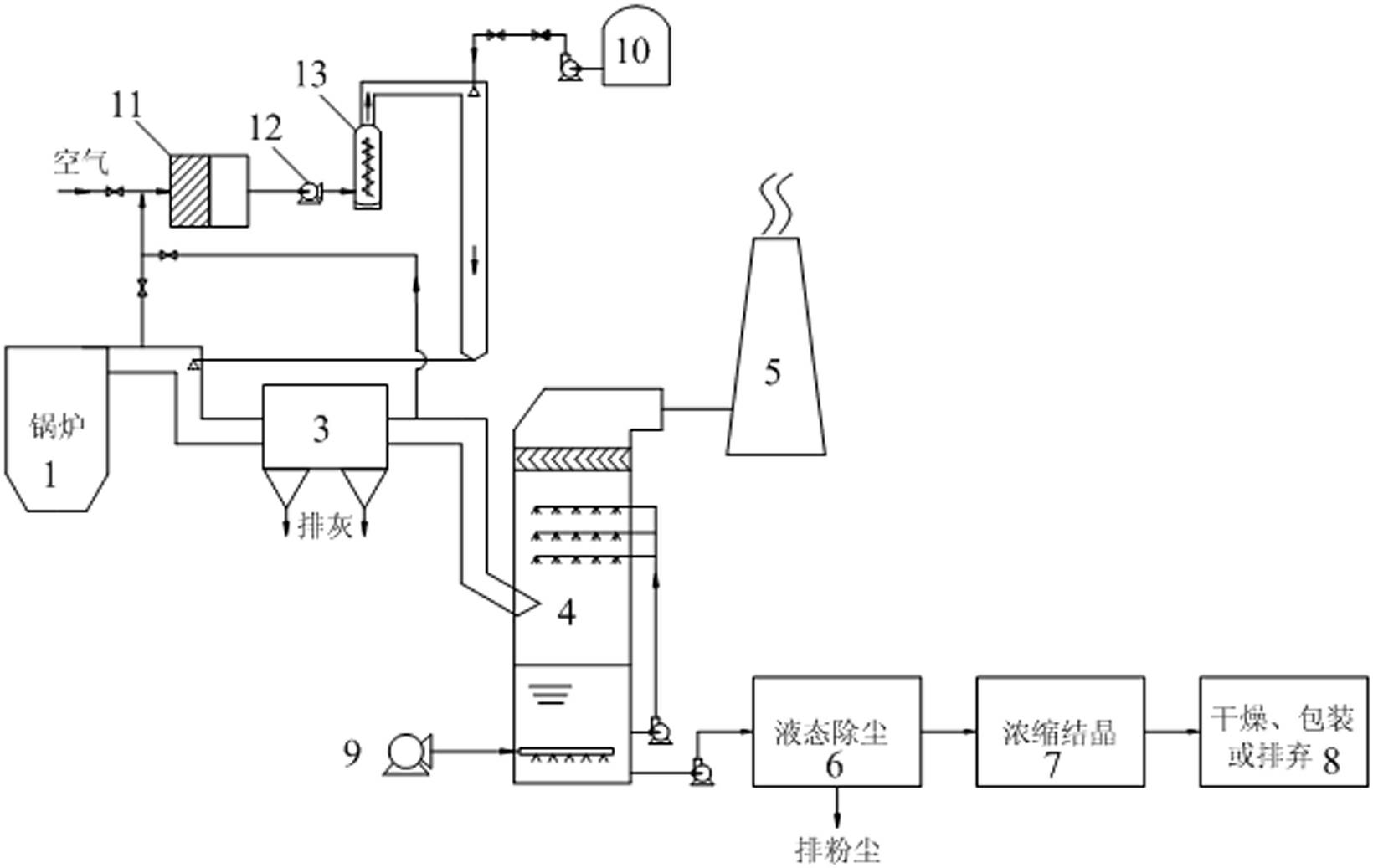
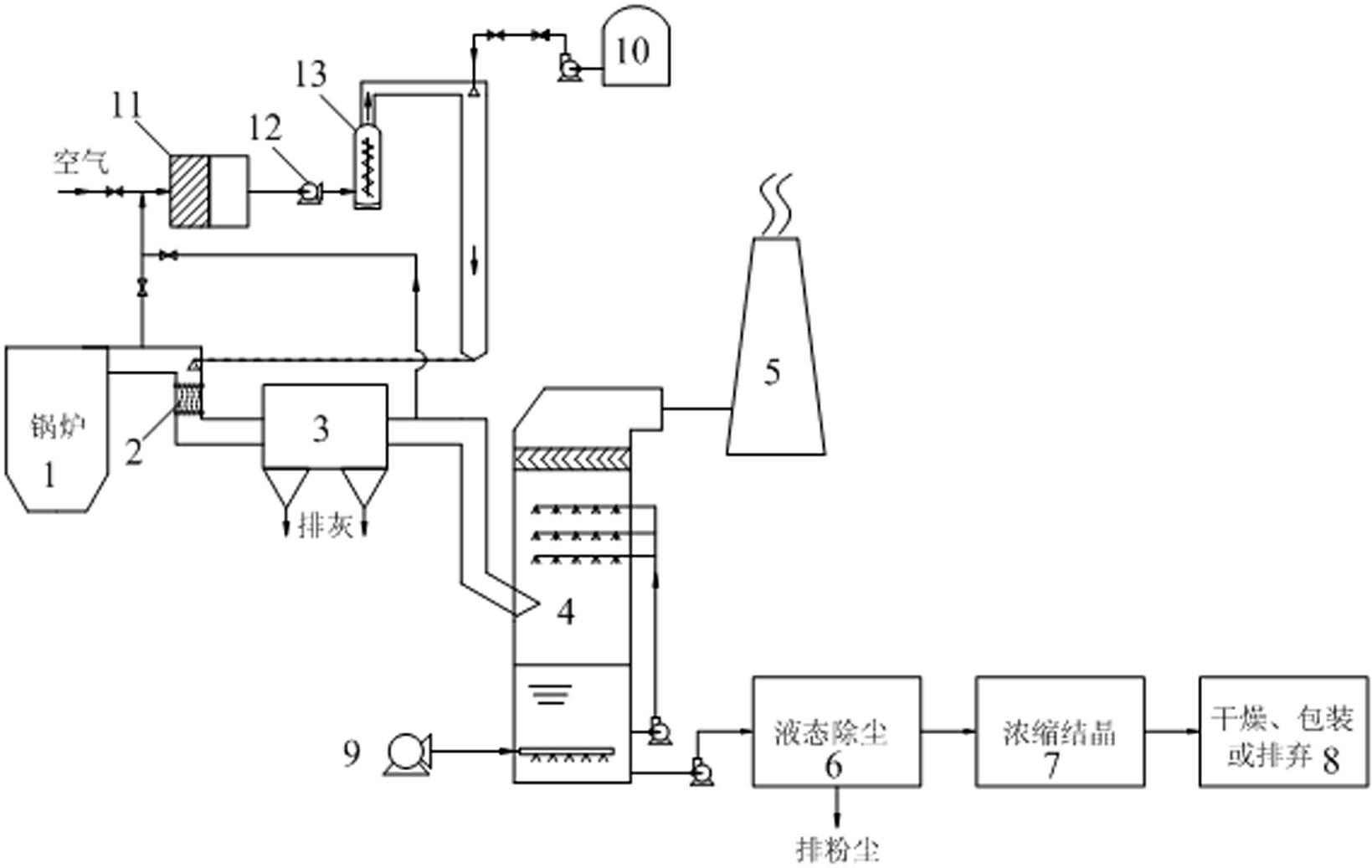
InactiveCN102274681AReduce energy consumptionLow investment costDispersed particle separationAbsorption columnEngineering
The invention relates to a system and method for simultaneous desulfurization and denitrification of flue gas based on the action of hydrogen peroxide. The system includes a flue gas generating device, a dust collector connected to the flue gas generating device through a flue, an absorption tower connected to the dust collector through the flue, the top of the absorption tower is connected to a chimney, and the bottom of the absorption tower is connected to an oxidation fan and Liquid dedusting device, the liquid dedusting device is connected with a salt solution concentration and crystallization device, the salt solution concentration and crystallization device is connected with a finished product packaging device, and the flue is connected with a hydrogen peroxide vaporization system, which is used to provide peroxide hydrogen gas. The simultaneous flue gas desulfurization and denitrification system based on hydrogen peroxide in the present invention can make the flue gas achieve a denitrification rate of more than 85% and a desulfurization rate of more than 95%. The system has low investment cost, low energy consumption and convenient use. It is an SCR It is incomparable with the denitrification method of the ozone oxidation method.
Owner:ZHONG POLERIS GREENTECH HLDG
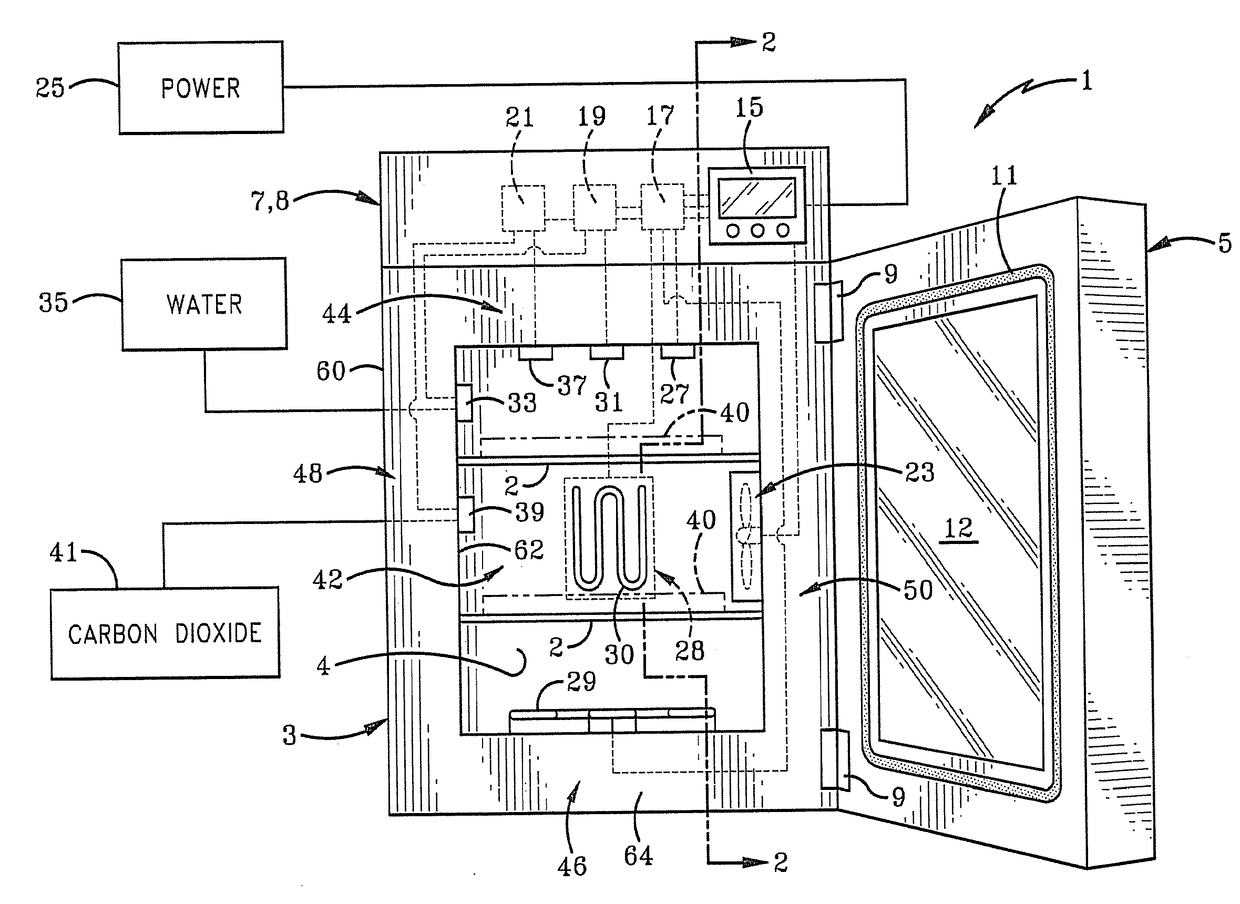
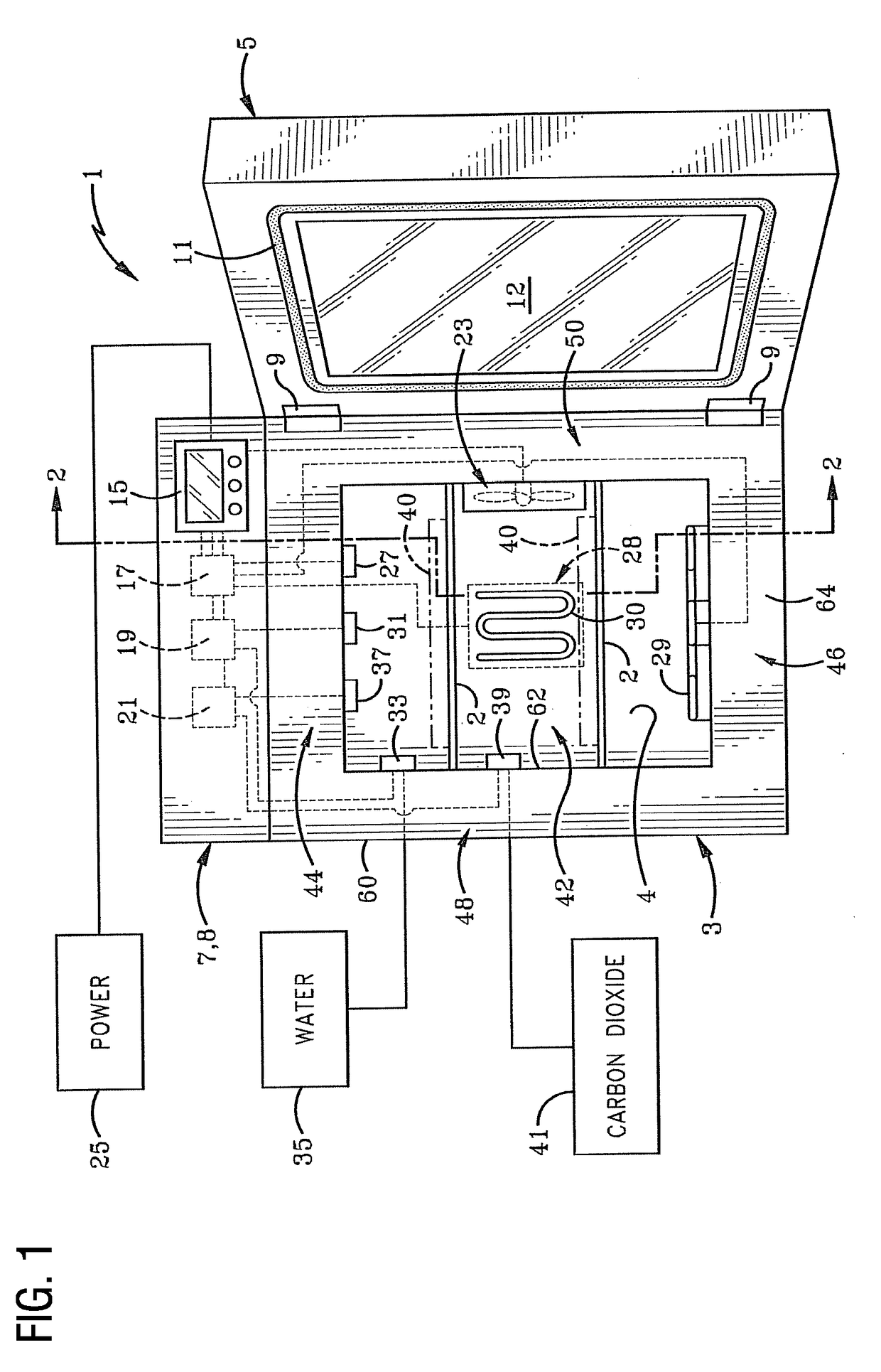
System and Method for Vaporized Hydrogen Peroxide Cleaning of An Incubation Chamber
ActiveUS20170175069A1Bioreactor/fermenter combinationsBiological substance pretreatmentsNuclear engineeringRelative humidity
A method is provided for vaporized hydrogen peroxide cleaning of a chamber. The method includes altering a temperature of air in the chamber from an initial temperature to a sterilization temperature over a first time period. The method also includes injecting vaporized hydrogen peroxide into air in the chamber to alter a relative humidity of hydrogen peroxide vapor in the chamber to a sterilization level over the first time period. The method also includes maintaining the temperature at the sterilization temperature and the relative humidity at the sterilization level over a second time period. The method also includes reducing the relative humidity from the sterilization level to a safe level over a third time period. In one embodiment, the method is provided for vaporized hydrogen peroxide cleaning of an interior chamber of an incubation container. In other embodiments, the incubation chamber featured in the method is provided.
Owner:CARON PROD & SERVICES
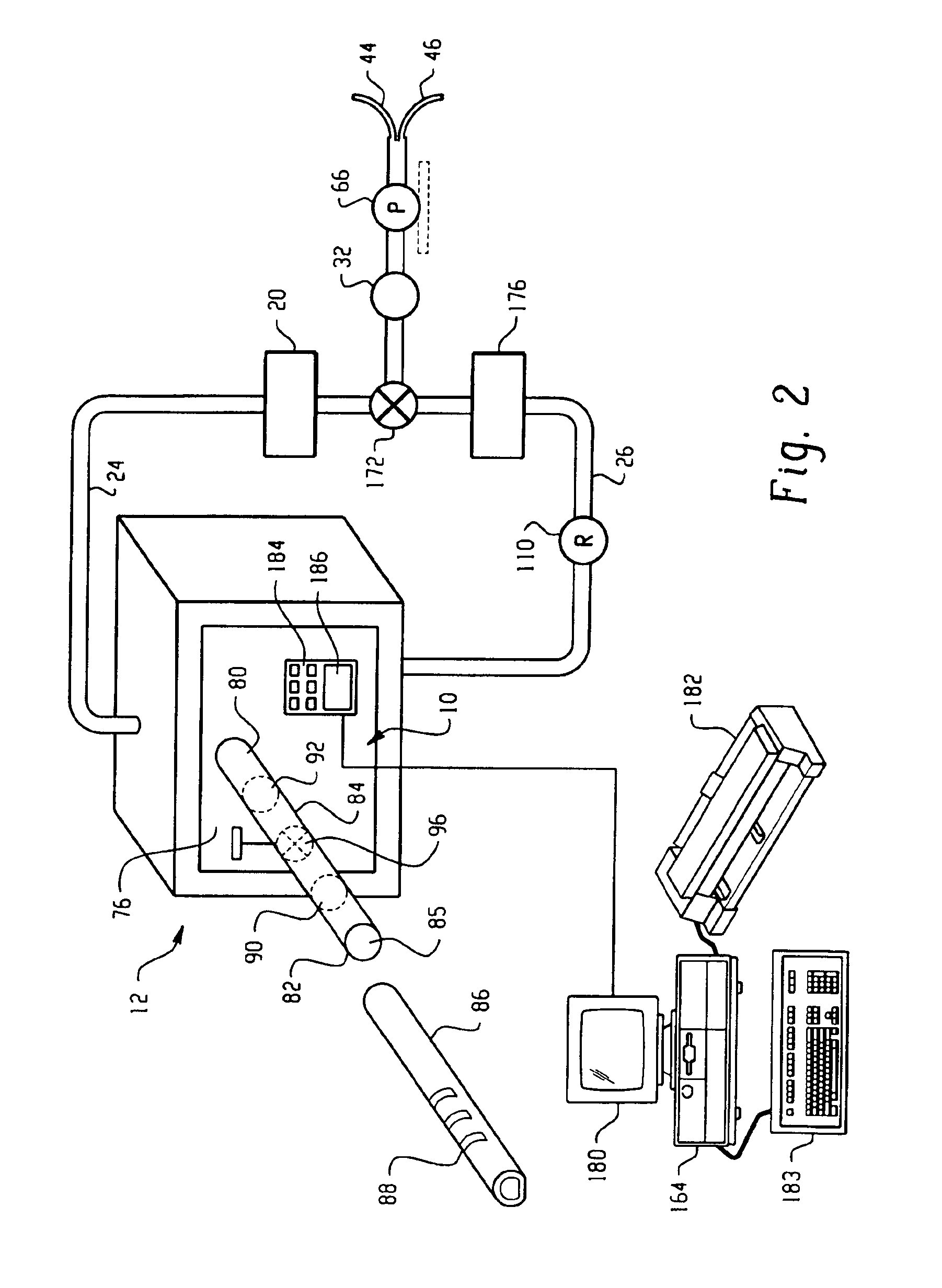
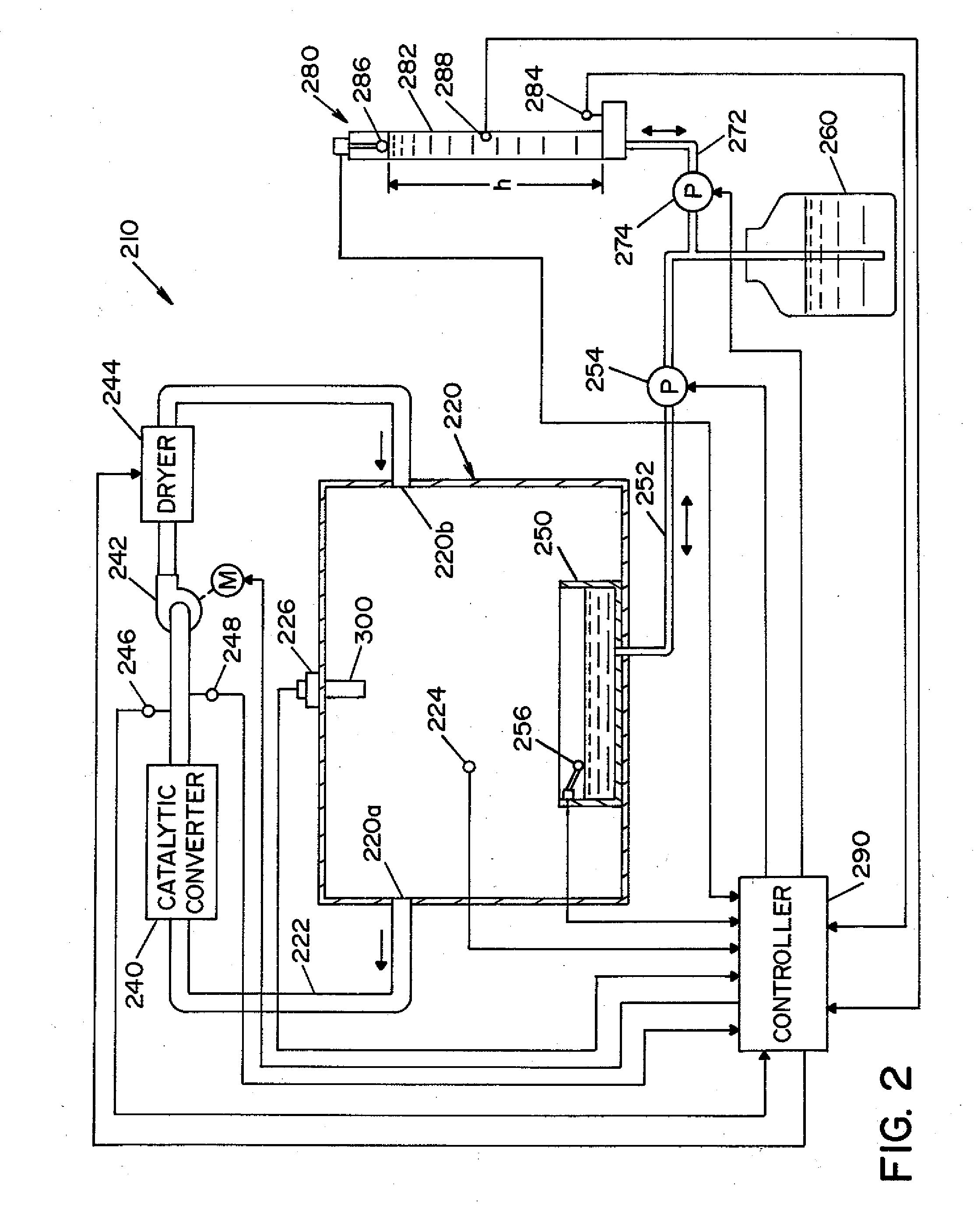
Vaporized hydrogen peroxide probe calibration rig
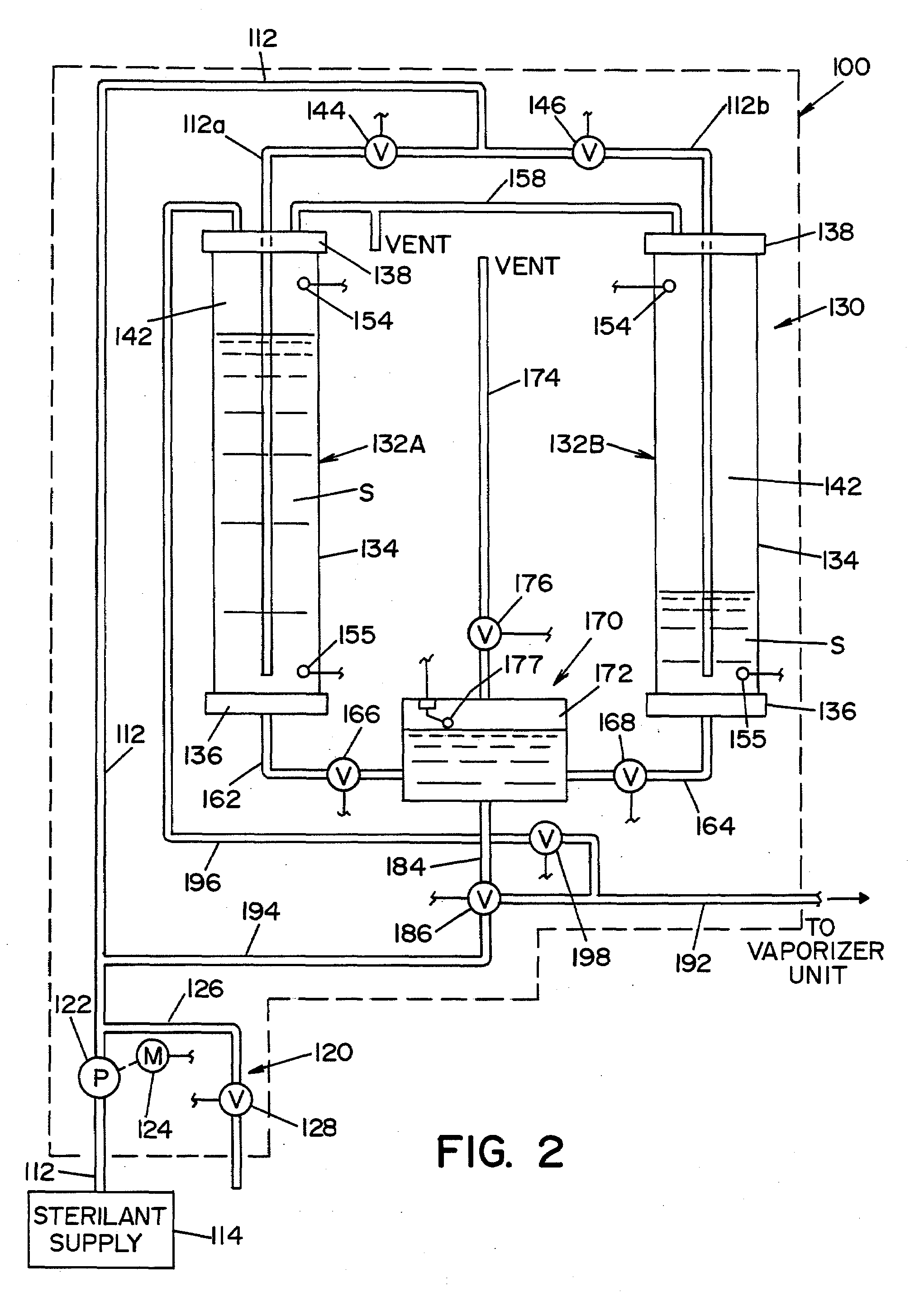
ActiveUS20080264140A1Accurate CalibrationMaterial analysis by electric/magnetic meansMaterial analysis by optical meansLiquid hydrogenAqueous solution
A method and apparatus for calibrating a sensor used to sense the concentration of vaporized hydrogen peroxide (VHP). A concentration of liquid hydrogen peroxide in an aqueous solution is determined and correlated with a corresponding concentration of vaporized hydrogen peroxide indicative of an “actual” vaporized hydrogen peroxide concentration. An error value is determined by comparing the “actual” vaporized hydrogen peroxide concentration to a “measured” vaporized hydrogen peroxide concentration, indicated by the sensor being calibrated. The error value is used to properly calibrate the sensor.
Owner:AMERICAN STERILIZER CO
Sterile de-molding apparatus and method
InactiveUS20070114690A1Avoid pollutionAvoid partialDischarging arrangementMouldsContact formationMaterials science
An apparatus and method are provided for molding sterile parts. The apparatus has a first mold portion and a second mold portion. At least one of the first and second mold portions defines a mold cavity configured to receive a molten plastic and form therefrom at least one molded part At least one of the first and second mold portions is movable relative to the other between (i) a closed position for sealing the mold cavity or cavities and molding at least one part therein, and (ii) an open position defining a fluid passageway between the first and second mold portions and permitting the passage of a fluid sterilant therein. A fluid source that contains or otherwise generates a fluid sterilant, such as vaporized hydrogen peroxide, is connectable in fluid communication with the fluid passageway for introducing the sterilant into the fluid passageway with at least one of the first and second mold portions in the open position, and in turn contacting with the sterilant the surfaces of the first and second mold portions forming the fluid passageway and located adjacent to the at least one mold cavity, but not contacting an interior surface of a molded part within the mold cavity, to sterilize the exposed mold surfaces and thereby prevent contamination of the molded part.
Owner:MEDINSTILL DEV
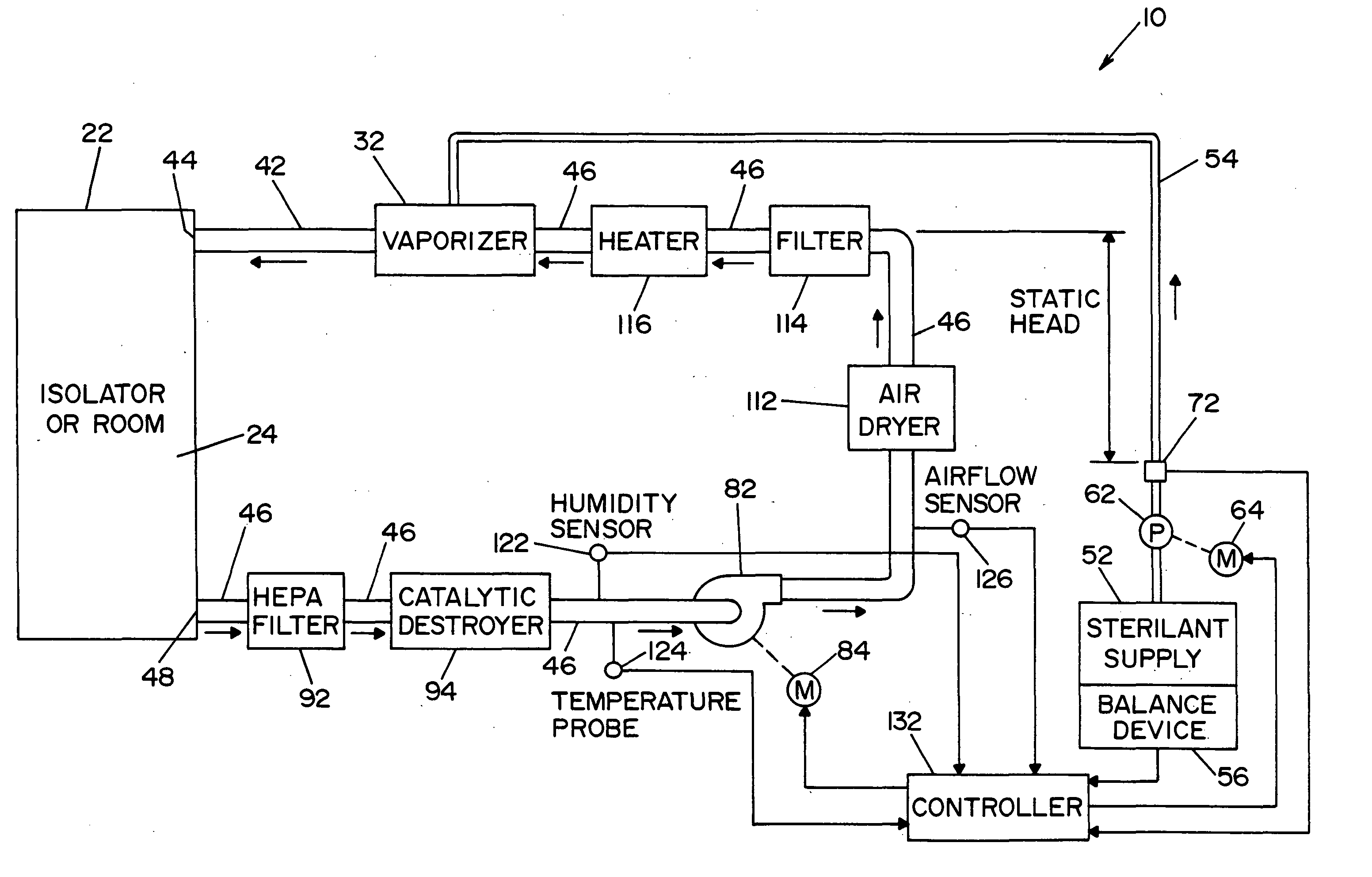
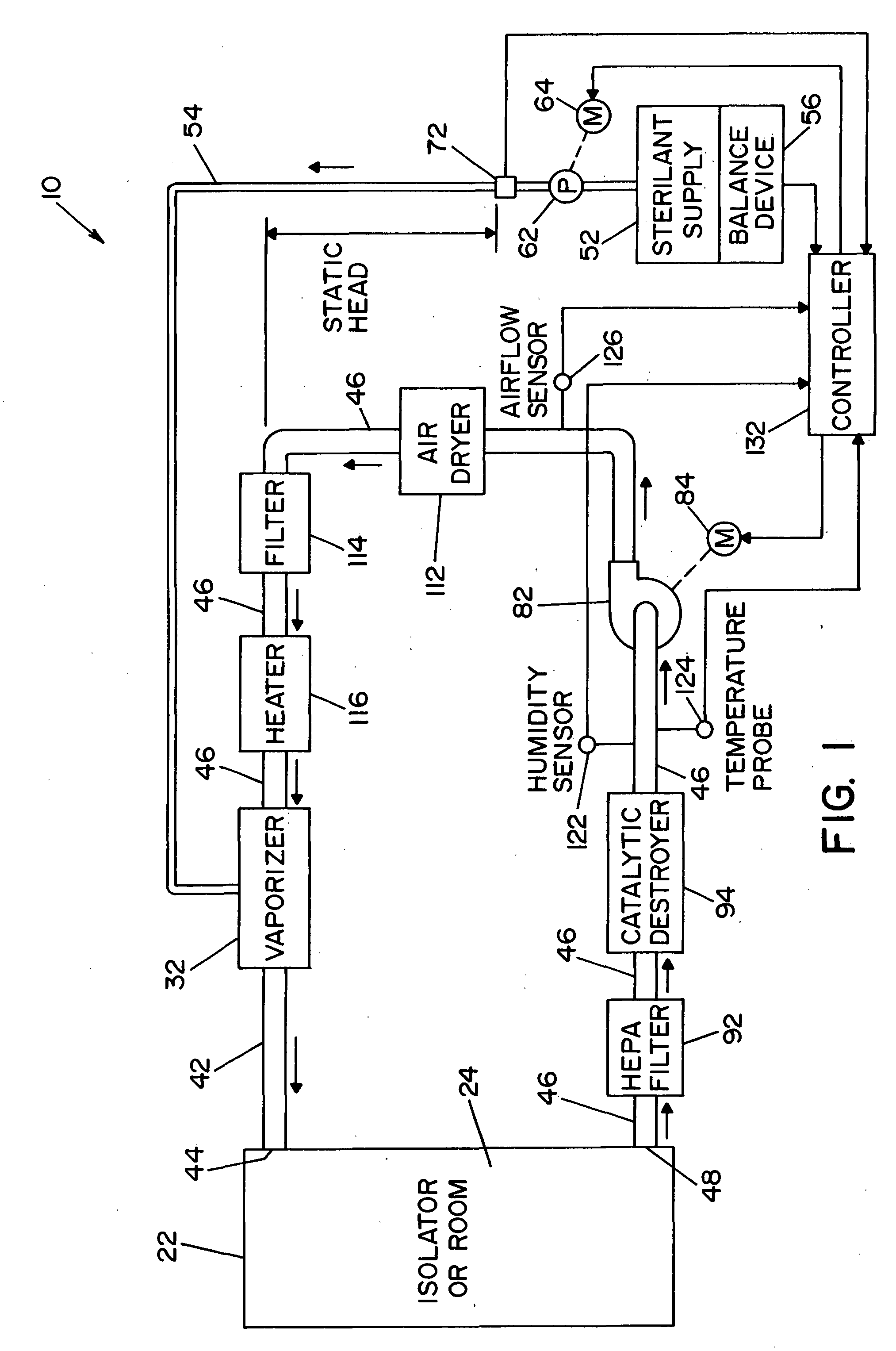
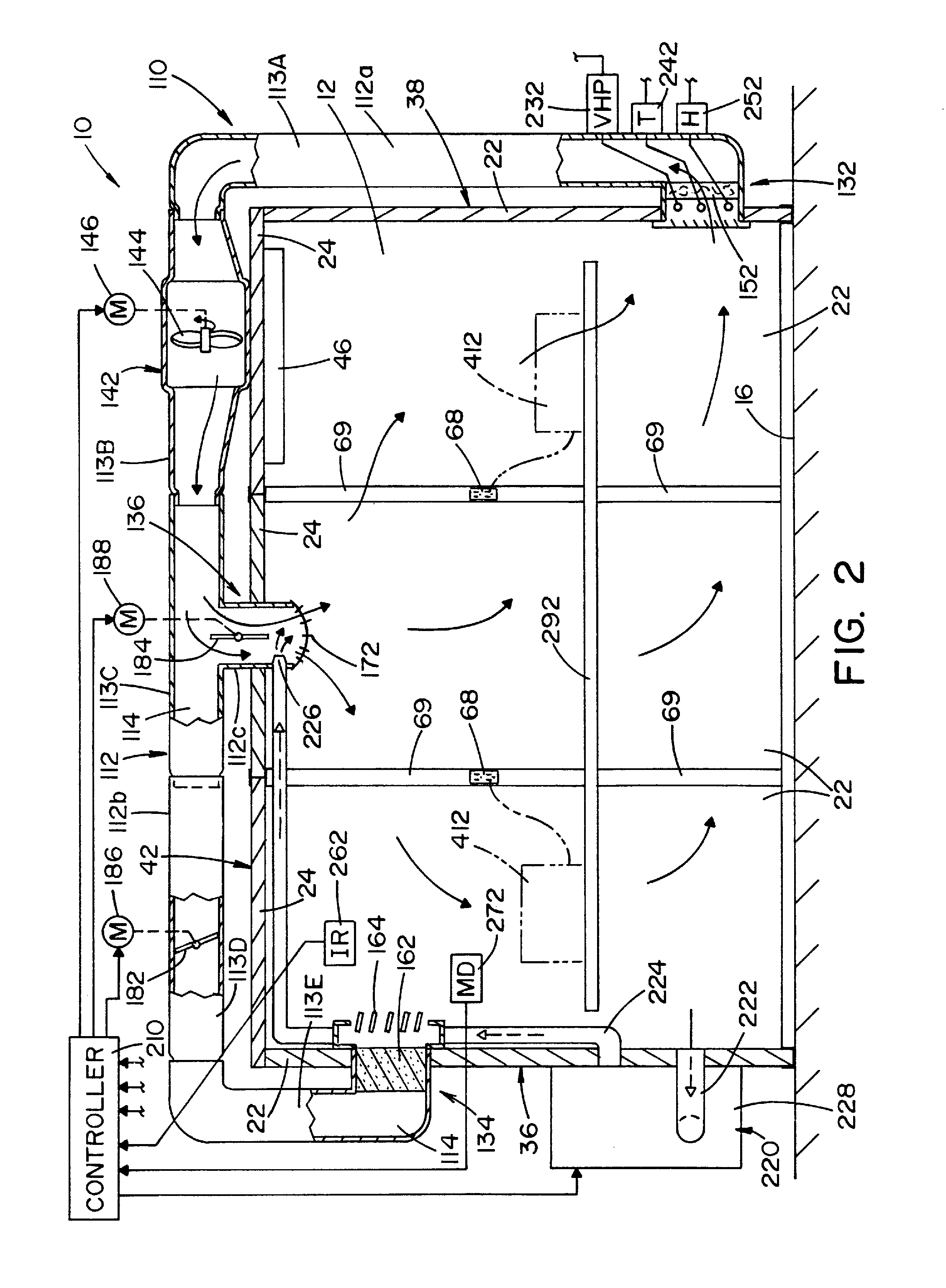
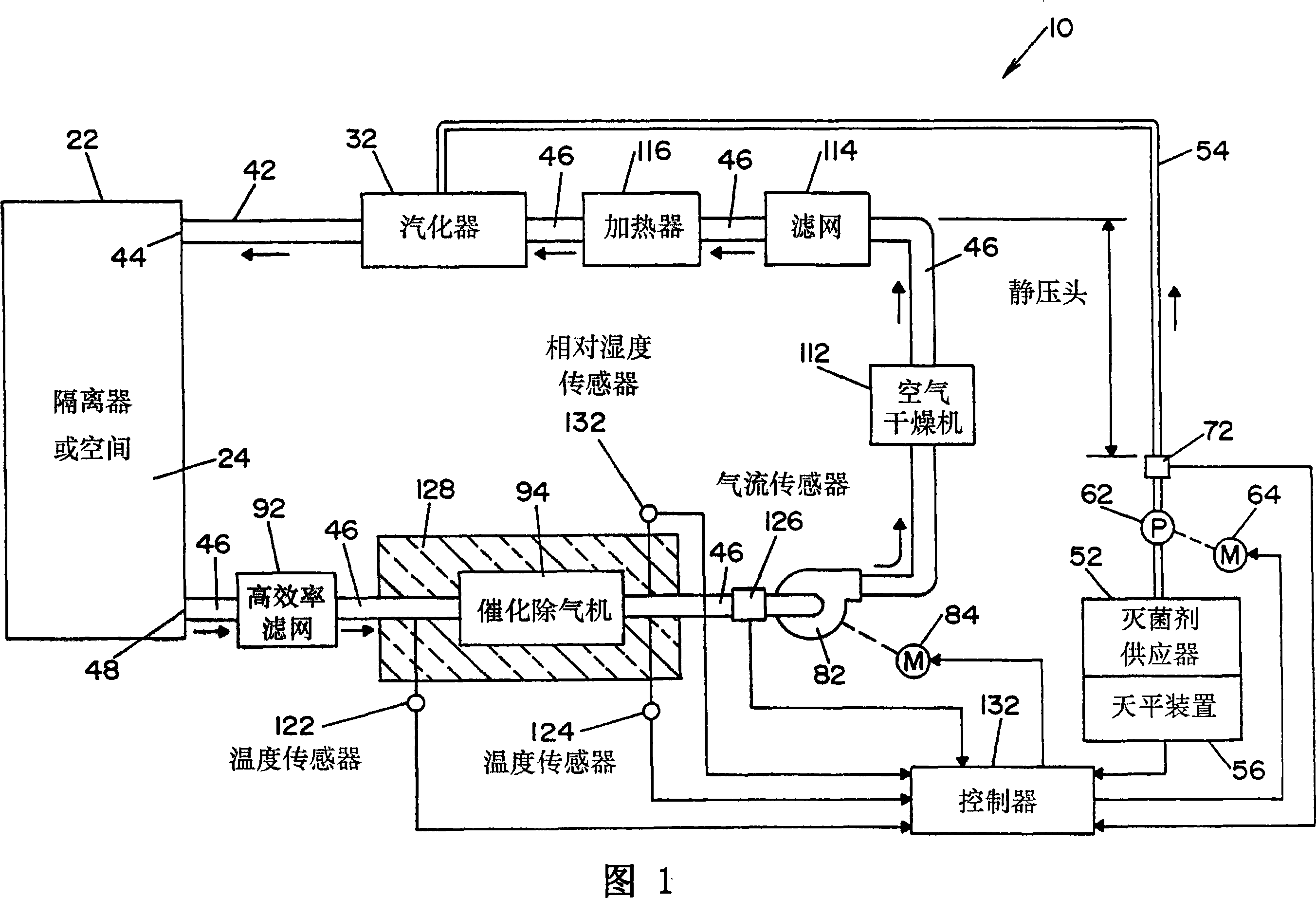
Method and apparatus for decontaminating a region without dehumidification
ActiveUS8007717B2Continuous monitoringExhaust apparatusElement comparisonSuccessful completionBioburden
A method and apparatus for decontaminating a region with a gaseous or vaporous decontaminant (e.g., vaporized hydrogen peroxide) without dehumidification (e.g., without the use of a dryer). The saturation concentration of the decontaminant inside the region is monitored, and the concentration of the decontaminant inside the region is regulated to prevent condensation of the decontaminant. The bioburden reduction is continuously monitored during a decontamination phase to ascertain successful completion of a decontamination process in accordance with a target bioburden reduction.
Owner:AMERICAN STERILIZER CO
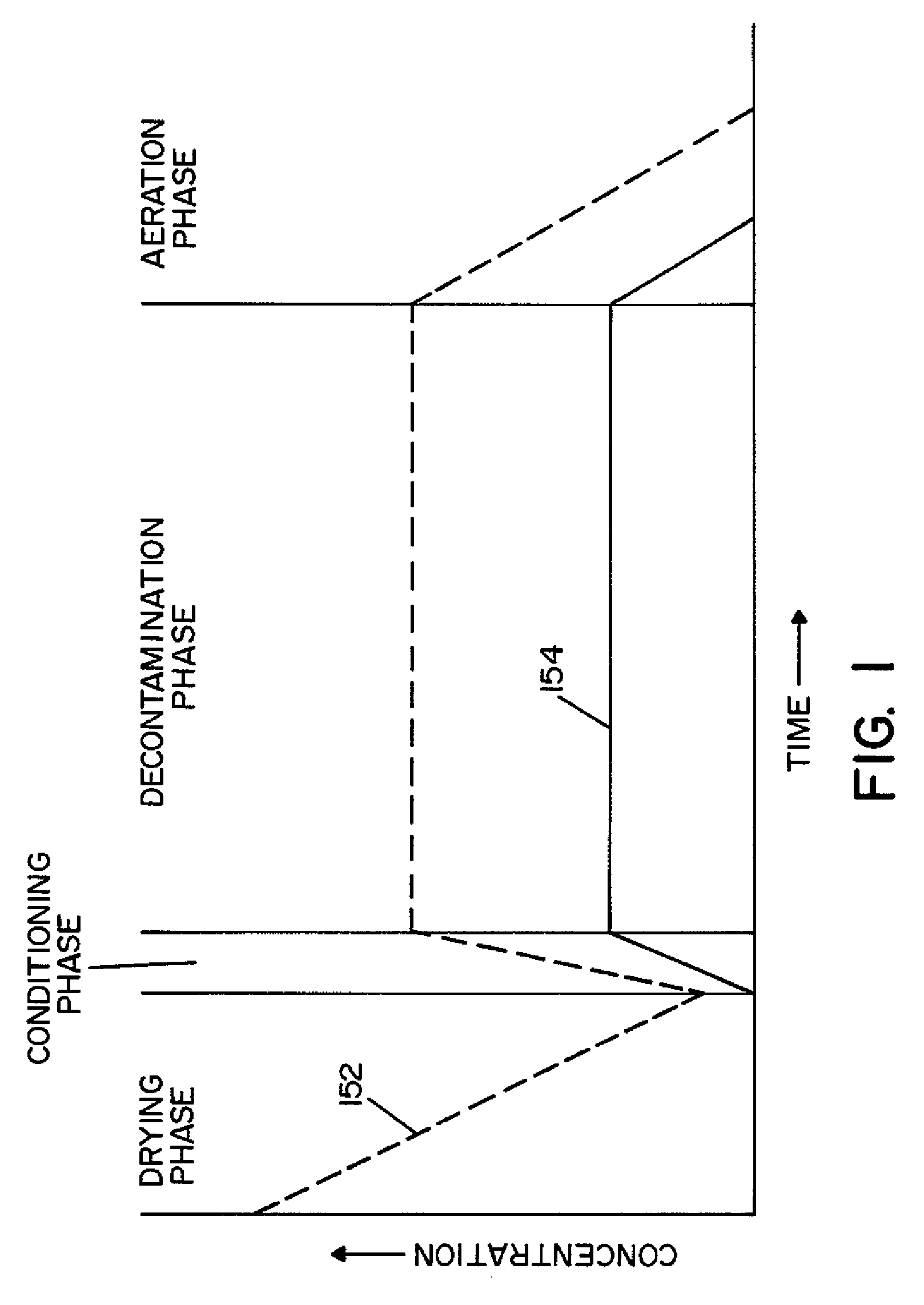
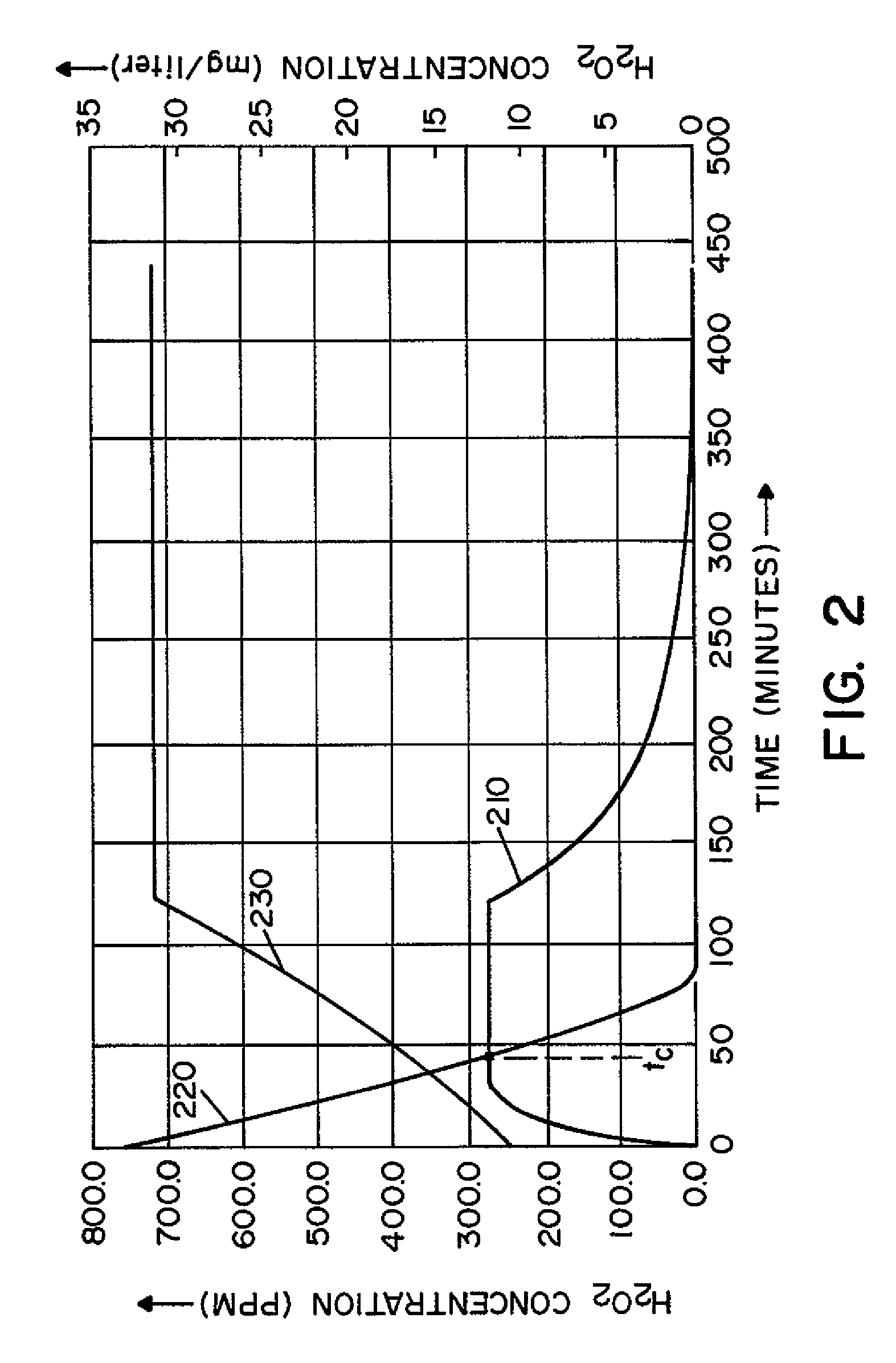
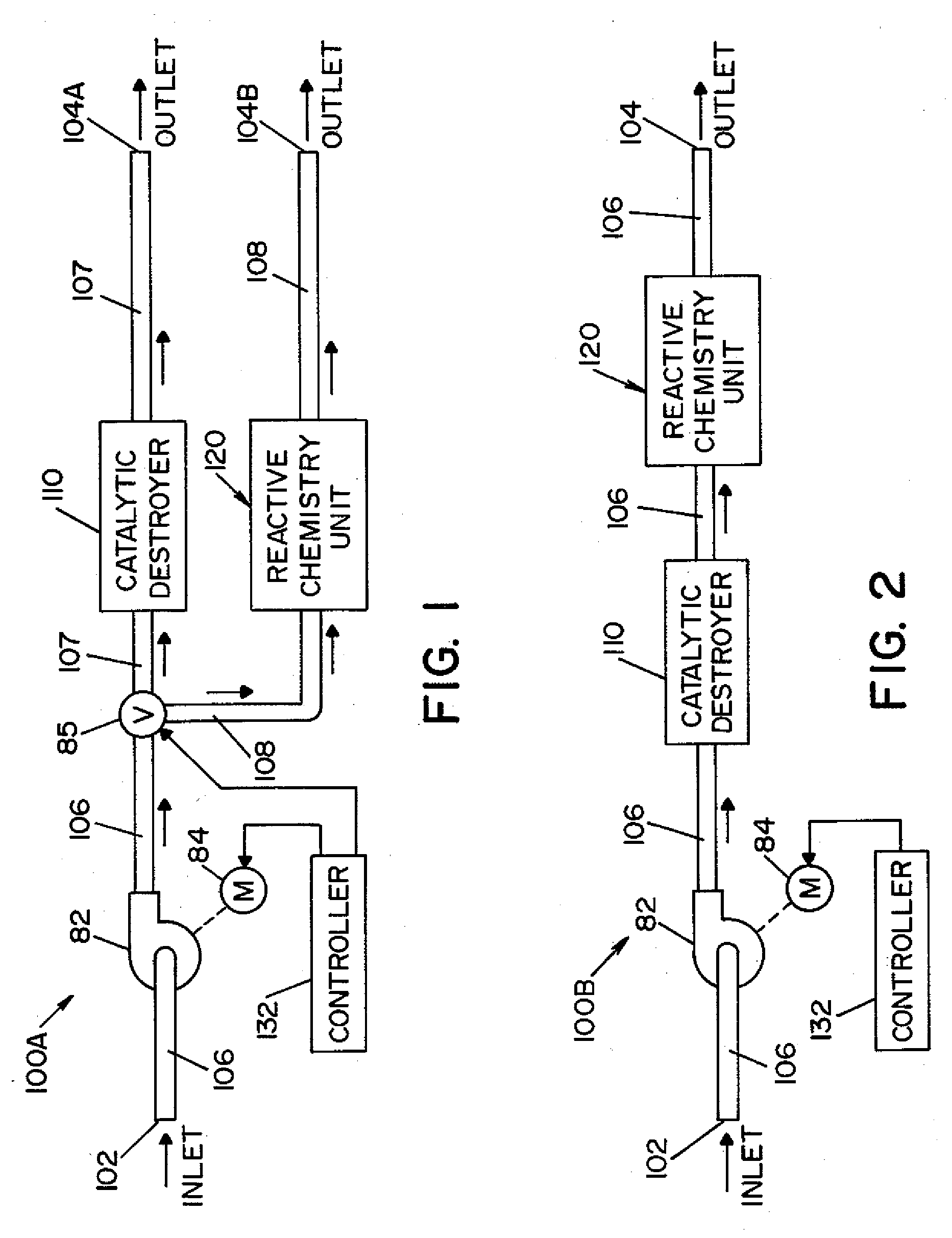
Method and apparatus for removal of vaporized hydrogen peroxide from a region
A method and apparatus for aerating a region exposed to a gaseous / vaporous sterilant. A catalytic destroyer and a reactive chemical unit are used to reduce the concentration of the gaseous / vaporous sterilant within the region. The reactive chemical unit includes a chemistry that is chemically reactive with the gaseous / vaporous sterilant. In one embodiment, the gaseous / vaporous sterilant is vaporized hydrogen peroxide and the chemistry of the reactive chemical unit includes thiosulfate and iodide.
Owner:AMERICAN STERILIZER CO
Vaporized hydrogen peroxide concentration detector
InactiveCN101080242AJudgment concentrationNo need to interveneLavatory sanitoryFire rescueEnvironmental engineeringHydrogen peroxide generation
A vapor decontamination system for decontaminating a defined region. The system is comprised of a chamber defining a region, and a generator for generating vaporized hydrogen peroxide from a solution of hydrogen peroxide and water. A closed loop circulating system is provided for supplying the vaporized hydrogen peroxide to the region. A destroyer breaks down the vaporized hydrogen peroxide, and sensors upstream and downstream from the destroyer are operable to sense moisture in the system and provide electrical signals indicative thereof. A controller determines the presence of vaporized hydrogen peroxide in the region based upon the electrical signals from the sensors.
Owner:AMERICAN STERILIZER CO
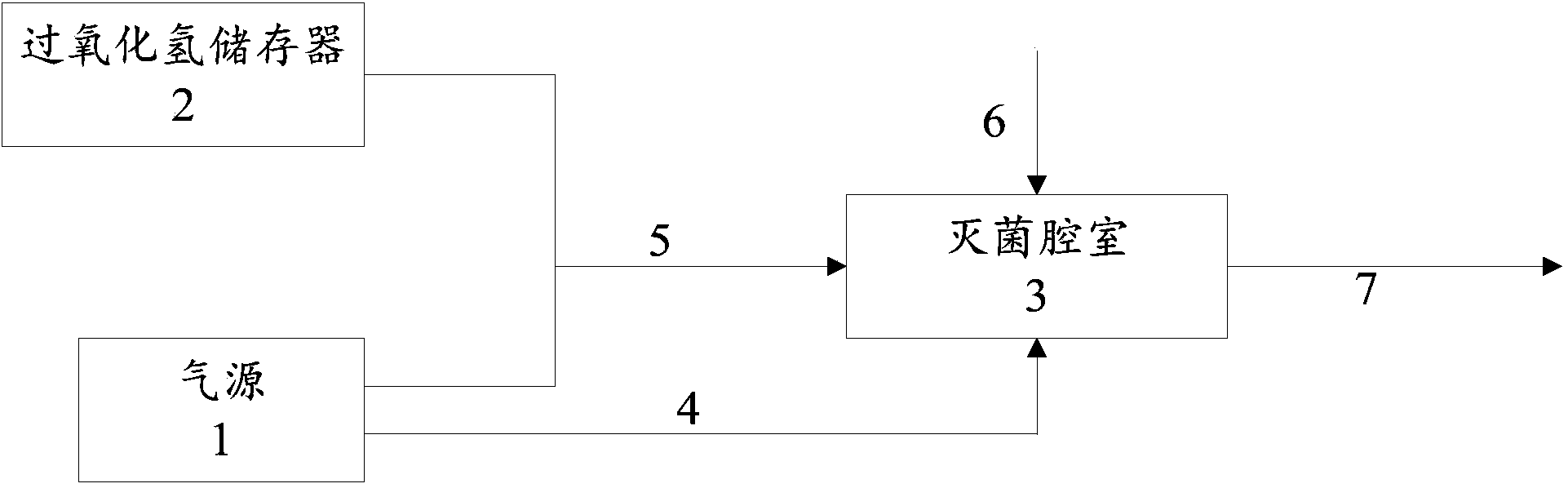
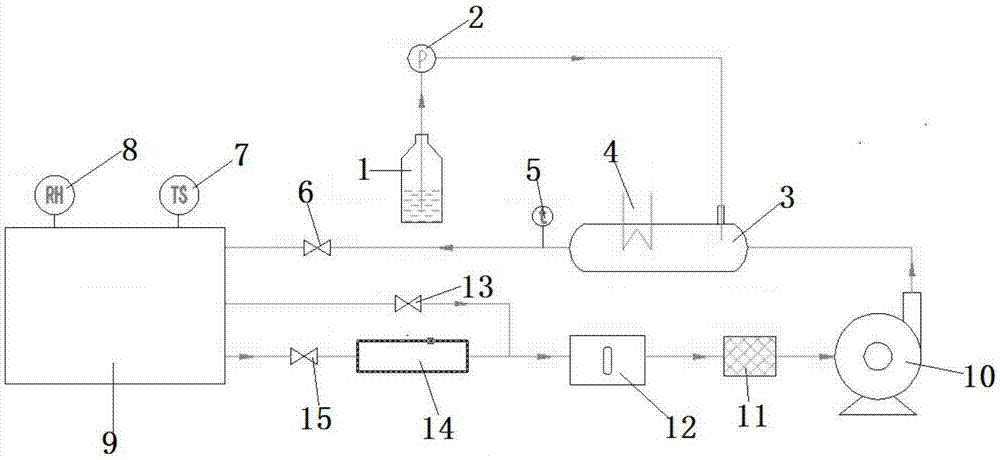
VHP sterilization device and method
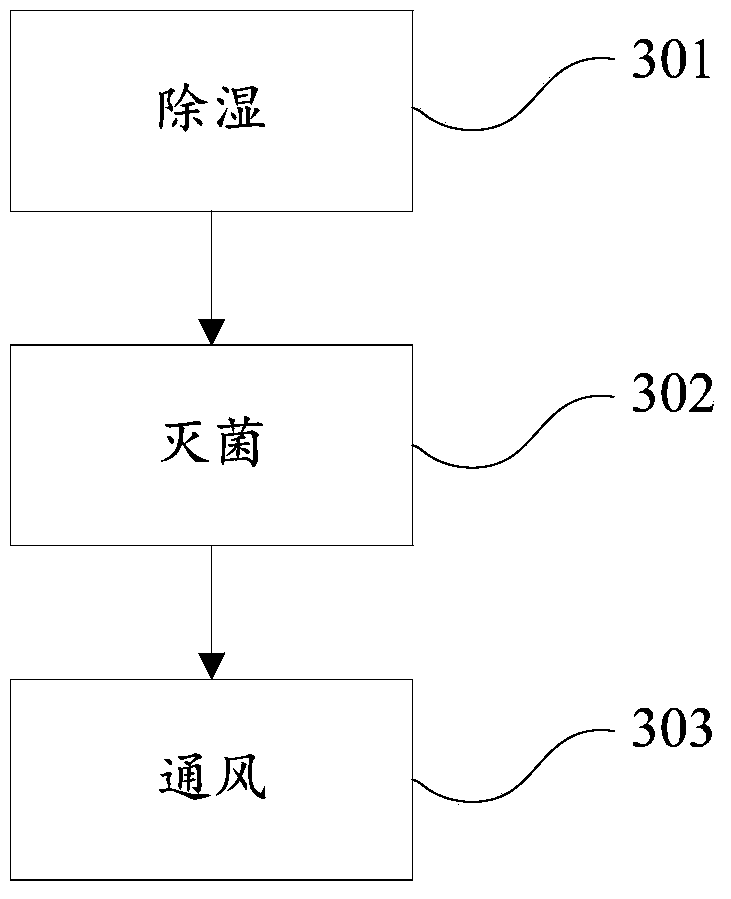
ActiveCN104174058AImprove sterilization effectImprove securitySpace heating and ventilation safety systemsLighting and heating apparatusVaporized hydrogen peroxideWaste management
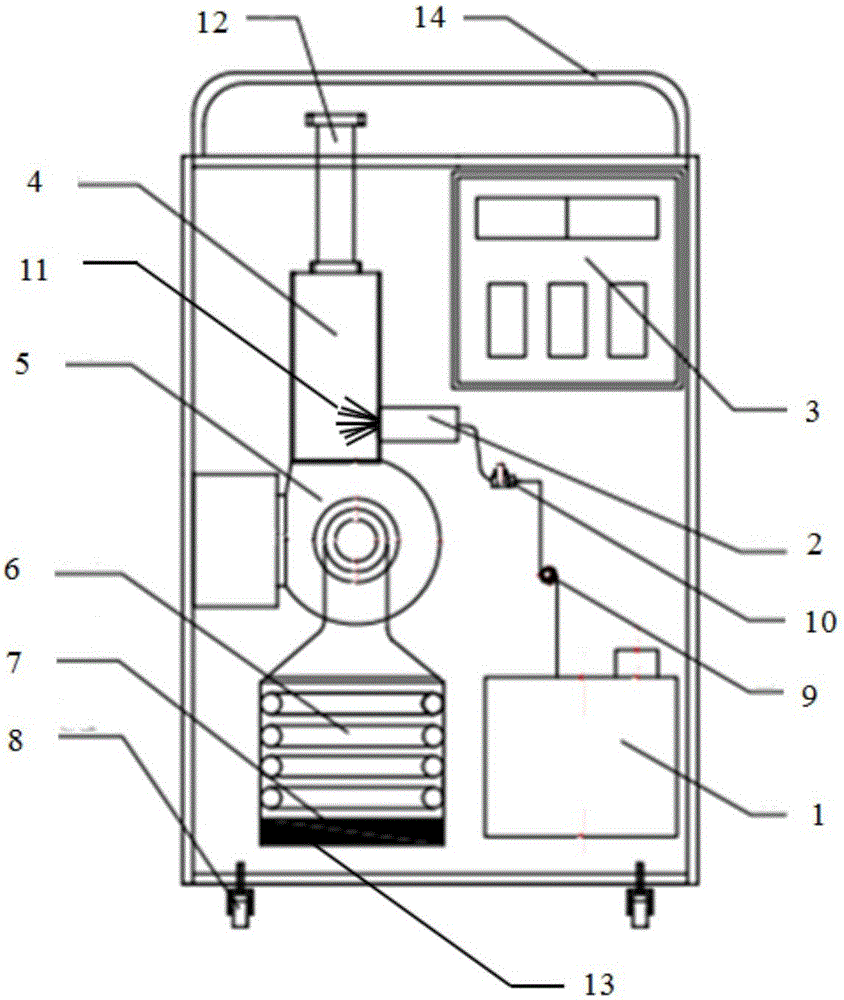
The invention discloses a VHP sterilization device and method. The sterilization device is arranged between a material area and a sterile area, and comprises an air source, a hydrogen peroxide storage device, a sterilization chamber, a dehumidification pipeline, a sterilization pipeline, a vent line and an air exhaust pipeline, wherein the dehumidification pipeline is connected with the air source and the sterilization chamber; the sterilization pipeline is connected with the air source, the hydrogen peroxide storage device and the sterilization chamber; the vent line is connected with the sterile area and the sterilization chamber; and the air exhaust pipeline is connected with the material area and the sterilization chamber. The sterilization method utilizing the sterilization device comprises three steps of dehumidifying, sterilizing and venting. Compared with the prior art, the scheme disclosed by the invention has the advantages that vapor hydrogen peroxide serves as a sterilizing medium, dehumidification, sterilization and ventilation are performed due to reasonable pipeline arrangement, and the sterilization effect and safety are effectively improved.
Owner:SHANGHAI YANFU PHARMA SYST ENG
Vaporized hydrogen peroxide probe calibration rig
ActiveUS7640782B2Accurate CalibrationMaterial analysis by electric/magnetic meansMaterial analysis by optical meansLiquid hydrogenAqueous solution
A method and apparatus for calibrating a sensor used to sense the concentration of vaporized hydrogen peroxide (VHP). A concentration of liquid hydrogen peroxide in an aqueous solution is determined and correlated with a corresponding concentration of vaporized hydrogen peroxide indicative of an “actual” vaporized hydrogen peroxide concentration. An error value is determined by comparing the “actual” vaporized hydrogen peroxide concentration to a “measured” vaporized hydrogen peroxide concentration, indicated by the sensor being calibrated. The error value is used to properly calibrate the sensor.
Owner:AMERICAN STERILIZER CO
Hydrogen peroxide vaporizer
ActiveUS8071021B2Modifies flowModifies temperatureLavatory sanitoryDeodrantsLiquid hydrogenEngineering
A method of decontaminating articles, comprising the steps of:(a) moving a plurality of articles having a known temperature along a first path;(b) conveying a carrier gas along a second path that includes an elongated plenum, the second path intersecting the first path downstream from the plenum;(c) heating the carrier gas to a temperature of at least about 105° C. at a location upstream of the plenum;(d) introducing into the carrier gas in the plenum an atomized mist of a liquid hydrogen peroxide of known concentration; and(e) controlling the following:(1) the volumetric flow of carrier gas along the second path;(2) the volume of hydrogen peroxide introduced into the carrier gas; and(3) the temperature of the carrier gas introduced into the plenum, such that the concentration of the vaporized hydrogen peroxide in the carrier gas where the first path intersects the second path has a dew point temperature below the known temperature of the articles.
Owner:AMERICAN STERILIZER CO
Process challenge device for assessing the effective performance of a biocontamination deactivation process
ActiveUS7927866B2Reduce condensationIncrease loopBioreactor/fermenter combinationsAnalysis using chemical indicatorsBiomedical engineeringVaporized hydrogen peroxide
Owner:AMERICAN STERILIZER CO
Biological indicator for use with vaporous microbial deactivating agents and method for making same
InactiveUS20080206801A1Minimal materialMinimizes stackingApparatus sterilizationMicrobiological testing/measurementMicroorganismChlorine dioxide
A biological indicator and method of making same. The biological indicator includes a carrier having a recess formed therein in order to restrict movement of an inoculum deposited onto the carrier. The inoculum includes microorganisms (e.g., bacterial spores) suspended in a suspension medium. The microorganisms are prepared by removing extraneous material and subjecting the microorganisms to sonication to break up agglomerations. The suspension medium includes a wetting agent to reduce surface tension, thereby facilitating flow of the suspension medium to prevent stacking of microorganisms on the surface of the carrier, and to allow the inoculum to more evenly “plate out” on carrier surfaces. The carrier, with inoculum deposited thereon, is enclosed in an envelope made of a material permeable to a vaporous deactivating agent (e.g., vaporized hydrogen peroxide, ozone, chlorine dioxide, ethylene oxide, etc.), thereby facilitating exposure to the vaporous deactivating agent.
Owner:AMERICAN STERILIZER CO
Hydrogen peroxide sterilization apparatus and hydrogen peroxide sterilization method
InactiveCN106963962ASafe and controllable processSterilization is safe and efficientLavatory sanitoryGaseous substancesPeristaltic pumpInlet valve
The present invention belongs to the field of sterilization apparatuses and sterilization methods, and particularly relates to a hydrogen peroxide sterilization apparatus and a hydrogen peroxide sterilization method. With the hydrogen peroxide sterilization apparatus and the hydrogen peroxide sterilization method, vaporized hydrogen peroxide can be produced so as to effectively sterilize the space and the object requiring sterilization, and the hydrogen peroxide residue can be rapidly cleared after the sterilization is completed. The hydrogen peroxide sterilization apparatus of the present invention comprises a liquid storage container, a peristaltic pump, a vaporizer, a heating device, a temperature sensor, a sterilization gas inlet valve, a sterilization space, and a related control system.
Owner:HANZHOU YINGTIAN SCI INSTR
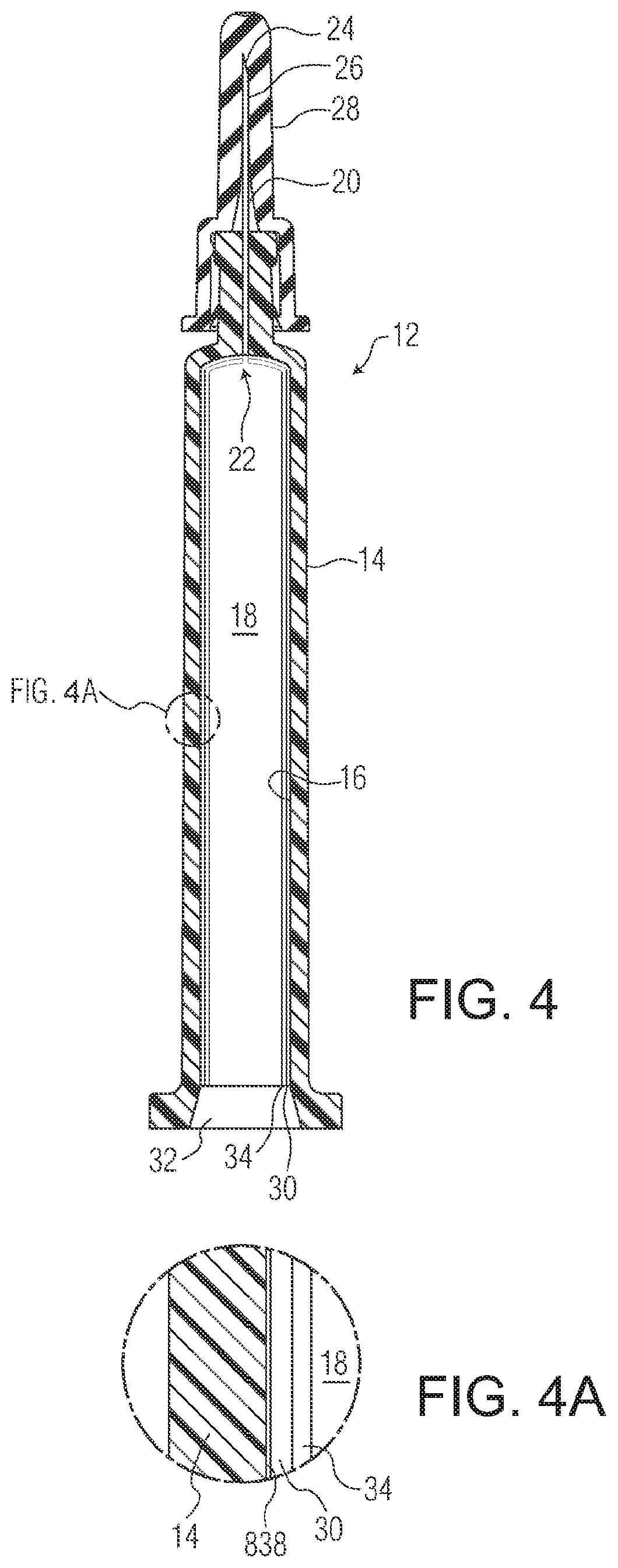
Sterilizable pharmaceutical package for ophthalmic formulations
PendingUS20200171244A1Reduced stabilityMinimize the numberMedical devicesPharmaceutical delivery mechanismChlorine dioxideOphthalmology
A liquid formulation of an ophthalmic drug in a pre-filled pharmaceutical package, for example a syringe, cartridge, vial or any other vessel made in part or in whole of a thermoplastic polymer, coated on the interior with a tie coating or layer, a barrier coating or layer, a pH protective coating or layer, and optionally a lubricity coating or layer. A blister, a pouch, a bag, a tray or a tub may encompass as a secondary packaging the syringe, vial, cartridge, tube or any other vessel. The package is suitable for sterilization (e.g., surface and / or terminal sterilization) with sterilization gas residuals being minimal and / or lower than required by ISO 10993-7; and / or the stability of the ophthalmic drug is maintained, during a prolonged time period following the sterilization. The sterilization gas may be EO, propylene oxide, chlorine dioxide, nitrogen dioxide, or vaporized hydrogen peroxide (VHP), among others.
Owner:SI02 MEDICAL PRODS
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com