Biological indicator for use with vaporous microbial deactivating agents and method for making same
a technology of microbial deactivating agent and biological indicator, which is applied in the field of biological indicator, can solve the problems of biological indicator that does not accurately indicate the efficacy of a microbial deactivation process, and the microorganisms disposed on the flat upper surface of the carrier come into contact with the package, so as to improve the permeability of vaporous microbial deactivating agent, reduce the number of microorganisms, and reduce the effect of bacterial population
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023]It should be understood that as used herein the term “vaporous” deactivating agents also includes “gaseous” deactivating agents. By way of example, and not limitation, the deactivating agents may include vaporized hydrogen peroxide, ozone, chlorine dioxide, and ethylene oxide.
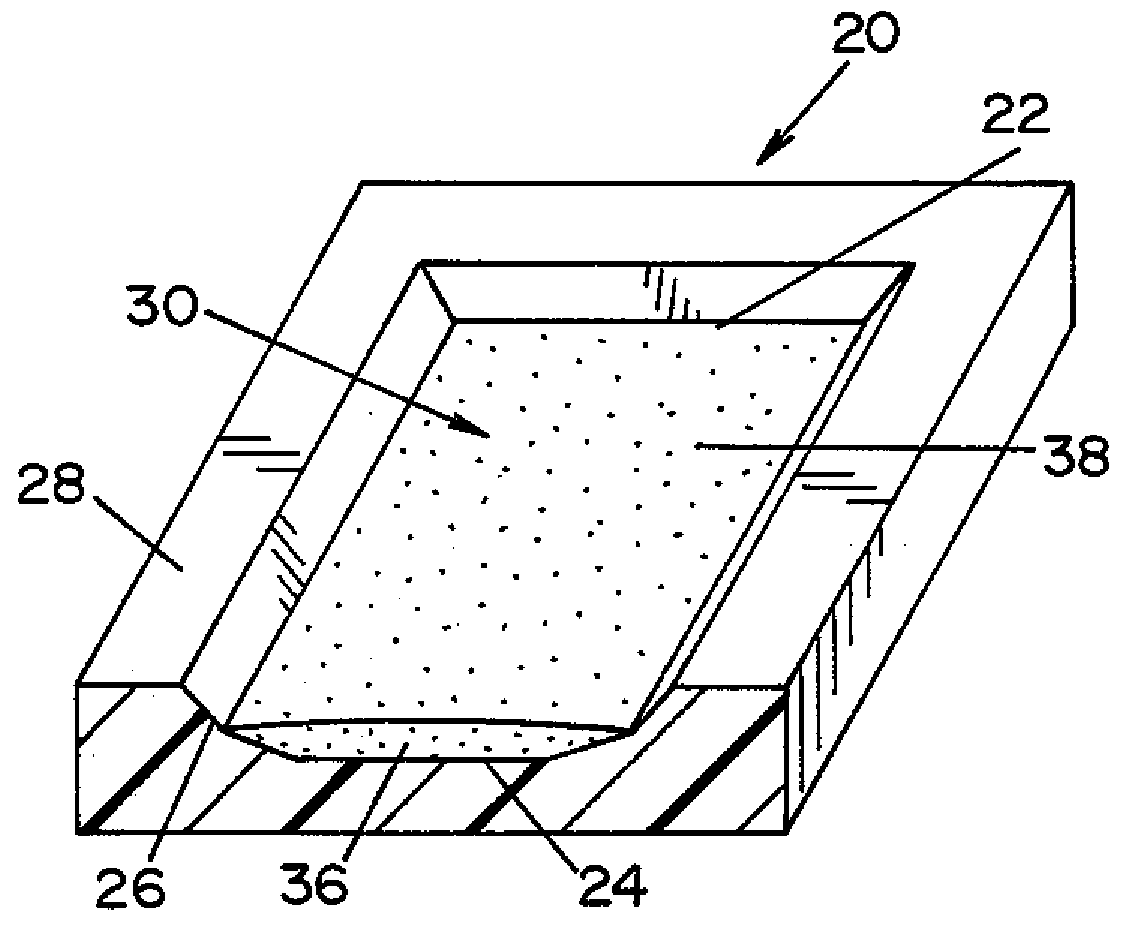
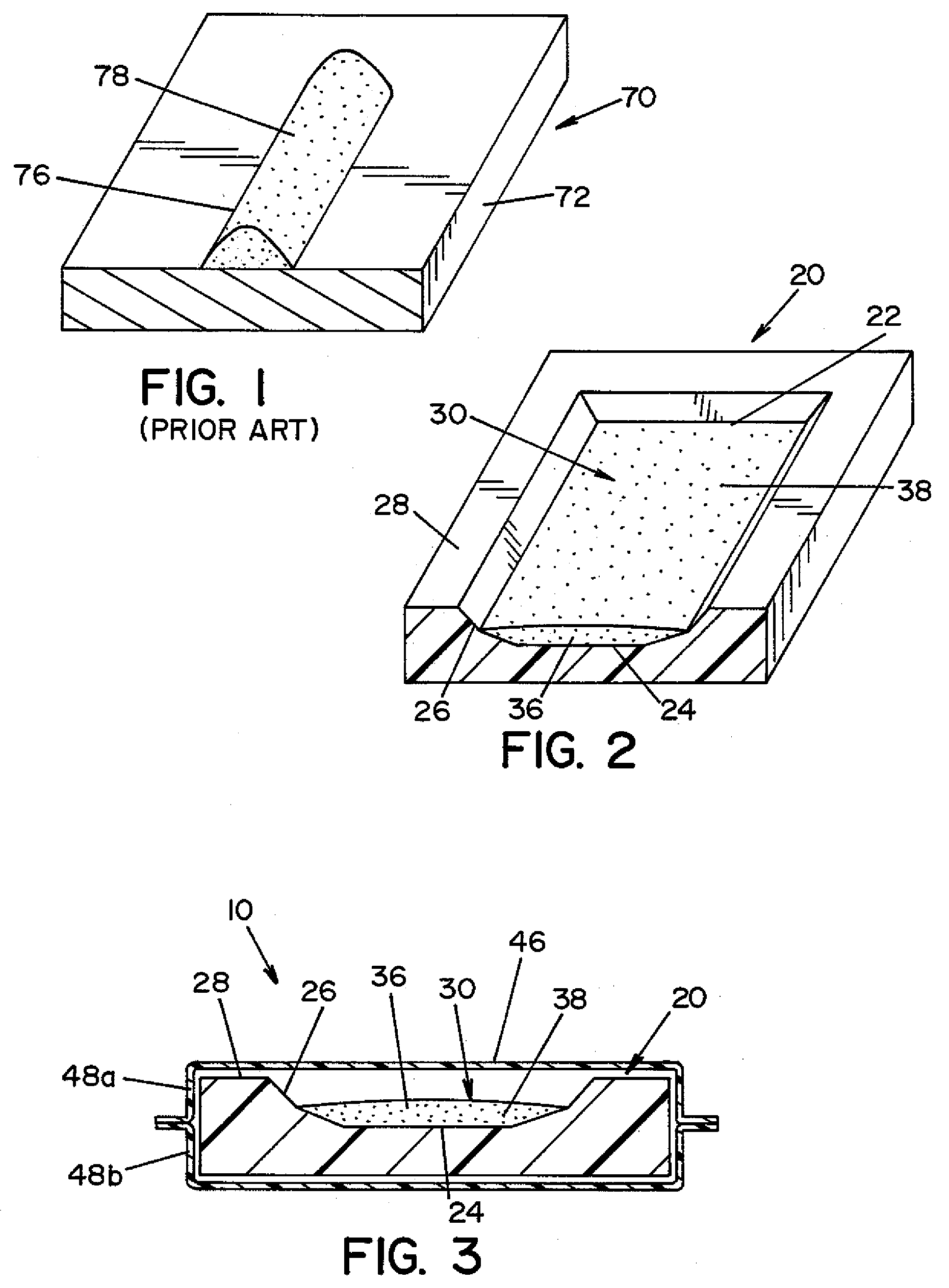
[0024]Referring now to the drawings wherein the showings are for the purpose of illustrating a preferred embodiment of the invention only, and not for the purpose of limiting same, FIG. 3 shows a biological indicator (BI) 10 according to an embodiment of the present invention. BI 10 is generally comprised of a carrier 20 (best seen in FIG. 2); an inoculum 30 comprised of a plurality of microorganisms 38 suspended in a suspension medium 36; and an envelope 46. Inoculum 30 is prepared by suspending microorganisms 38 within suspension medium 36. Inoculum 30 is deposited onto carrier 20. Thereafter, carrier 20 is sealed within envelope 46. Each component of BI 10 is described in detail below.
[0025]In the illu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| permeable | aaaaa | aaaaa |
| wetting | aaaaa | aaaaa |
| impermeable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com