Patents
Literature
47 results about "Ophthalmic drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ophthalmic Ointment. This medication is used to treat eye infections. This product contains neomycin, bacitracin, and polymyxin, antibiotics that work by stopping the growth of bacteria. This medication treats only bacterial eye infections. It will not work for other types of eye infections (e.g., infections caused by viruses, fungi, mycobacteria).
Drug delivery device and syringe for filling the same
InactiveUS20040230183A1Little changeSenses disorderPharmaceutical delivery mechanismOphthalmic drugBiomedical engineering
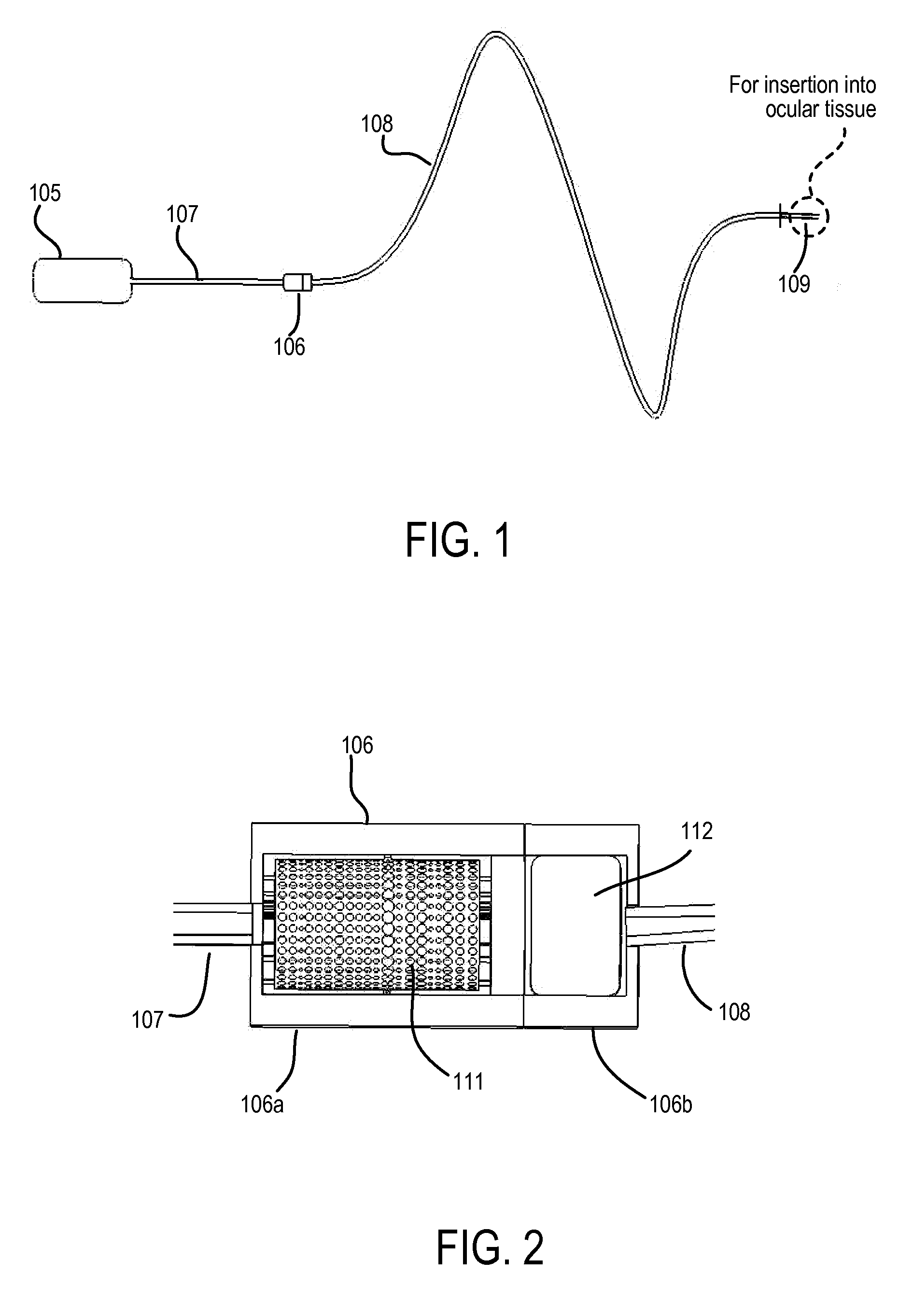
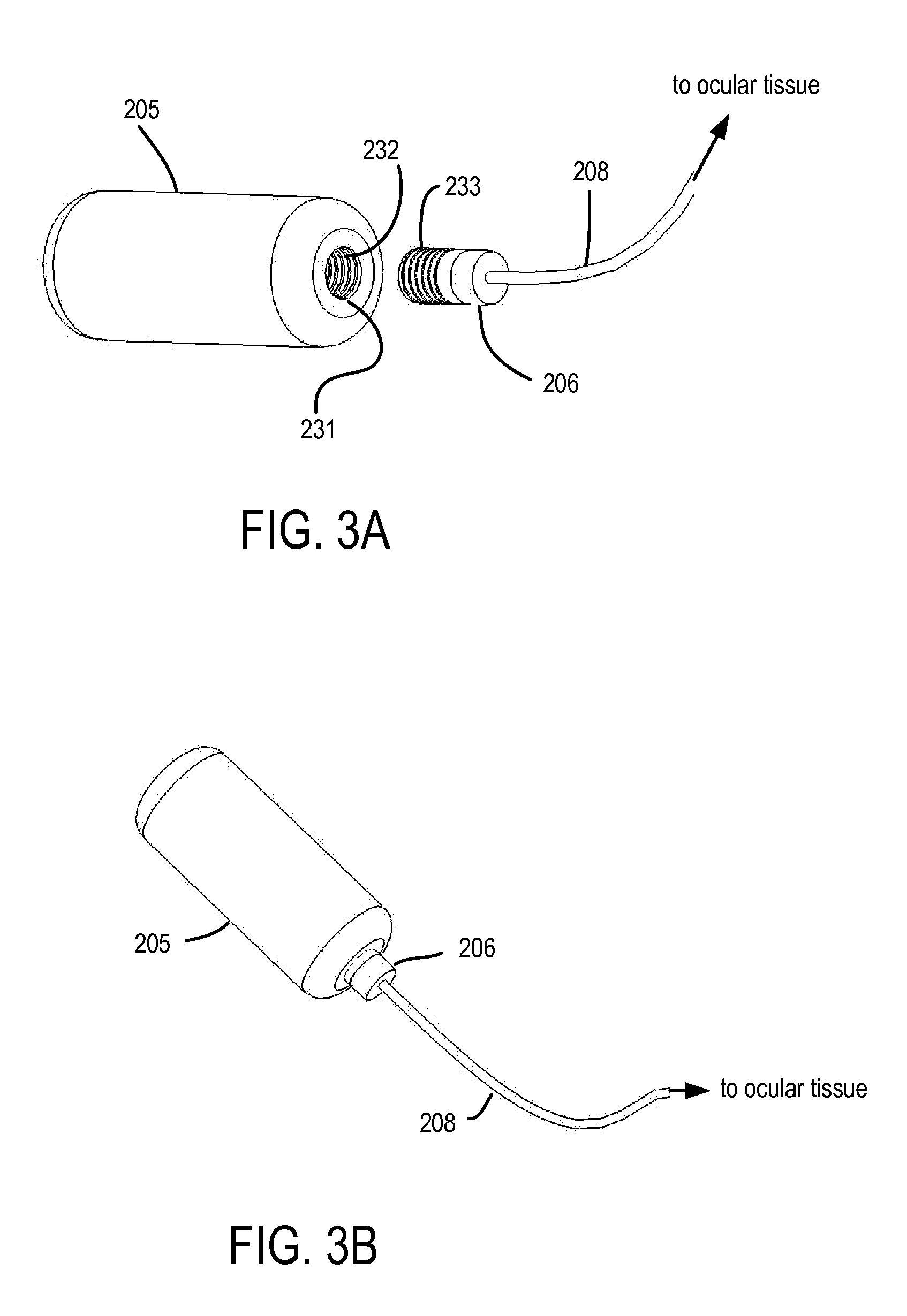
The invention relates to the field of drug delivery devices. More particularly, the invention relates to implantable, refillable drug delivery devices which provide for drug delivery over sustained time periods. The present invention has particular application for ophthalmic drug delivery applications.
Owner:(OSI) EYETECH INC
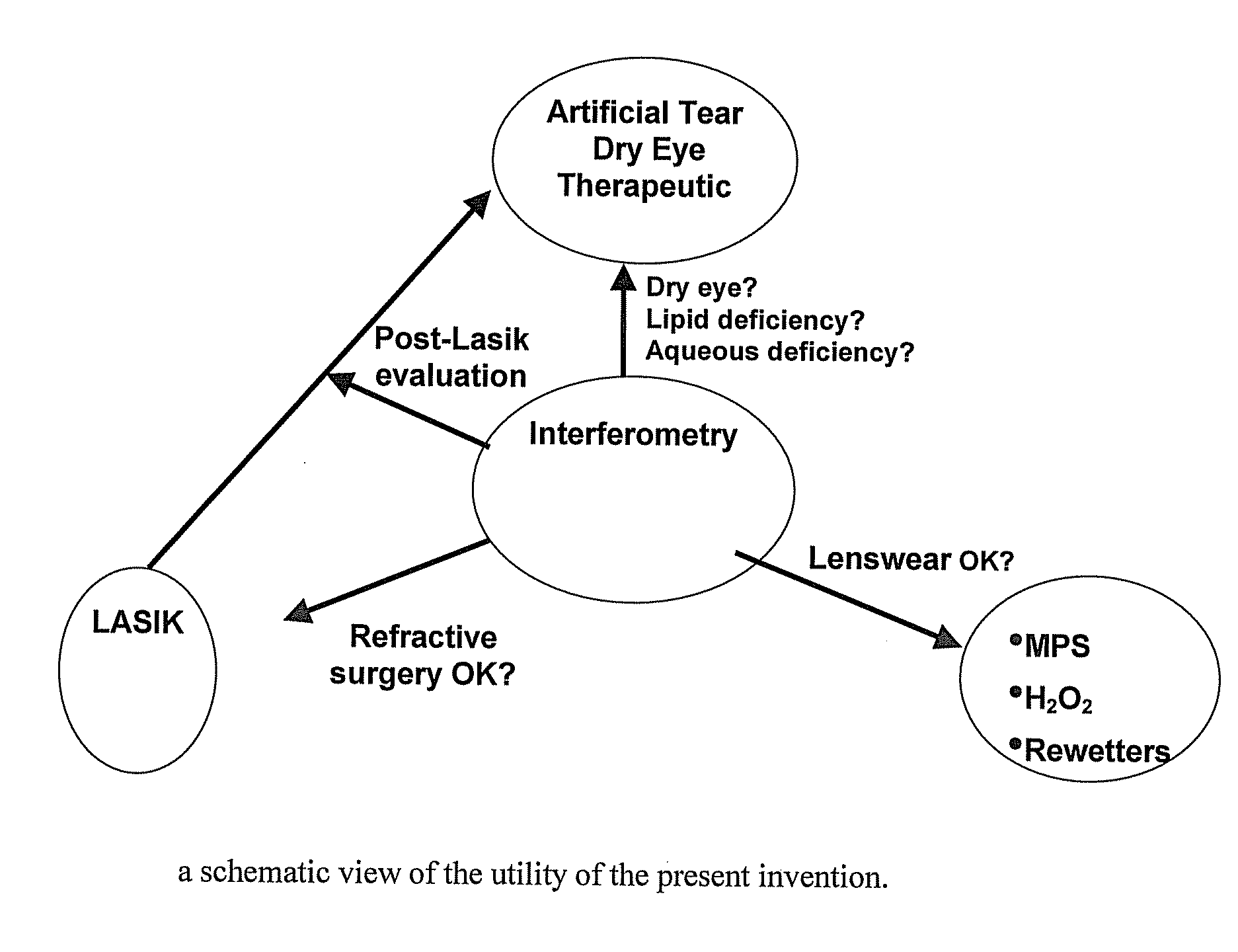
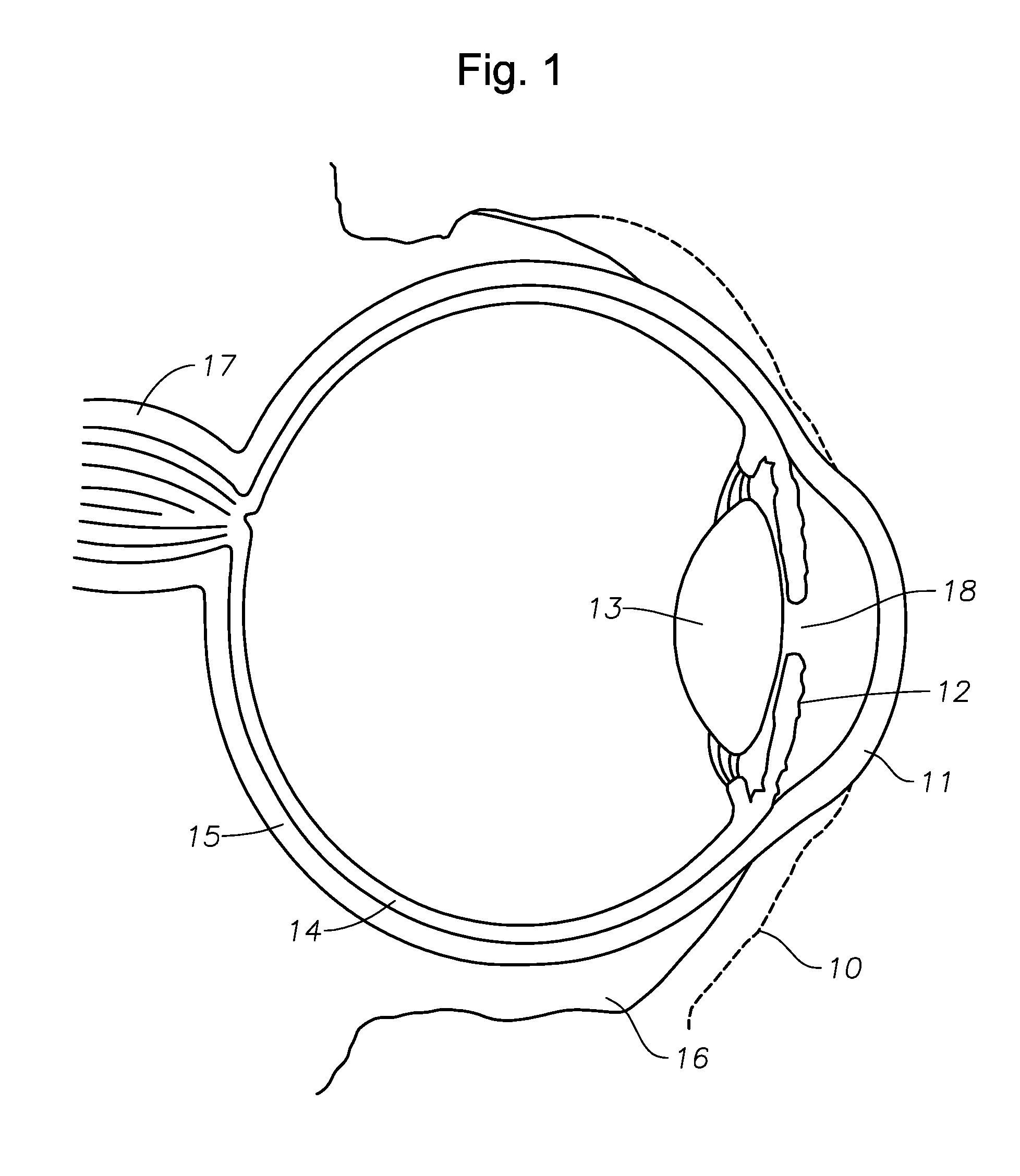
Methods and devices for measuring tear film and diagnosing tear disorders
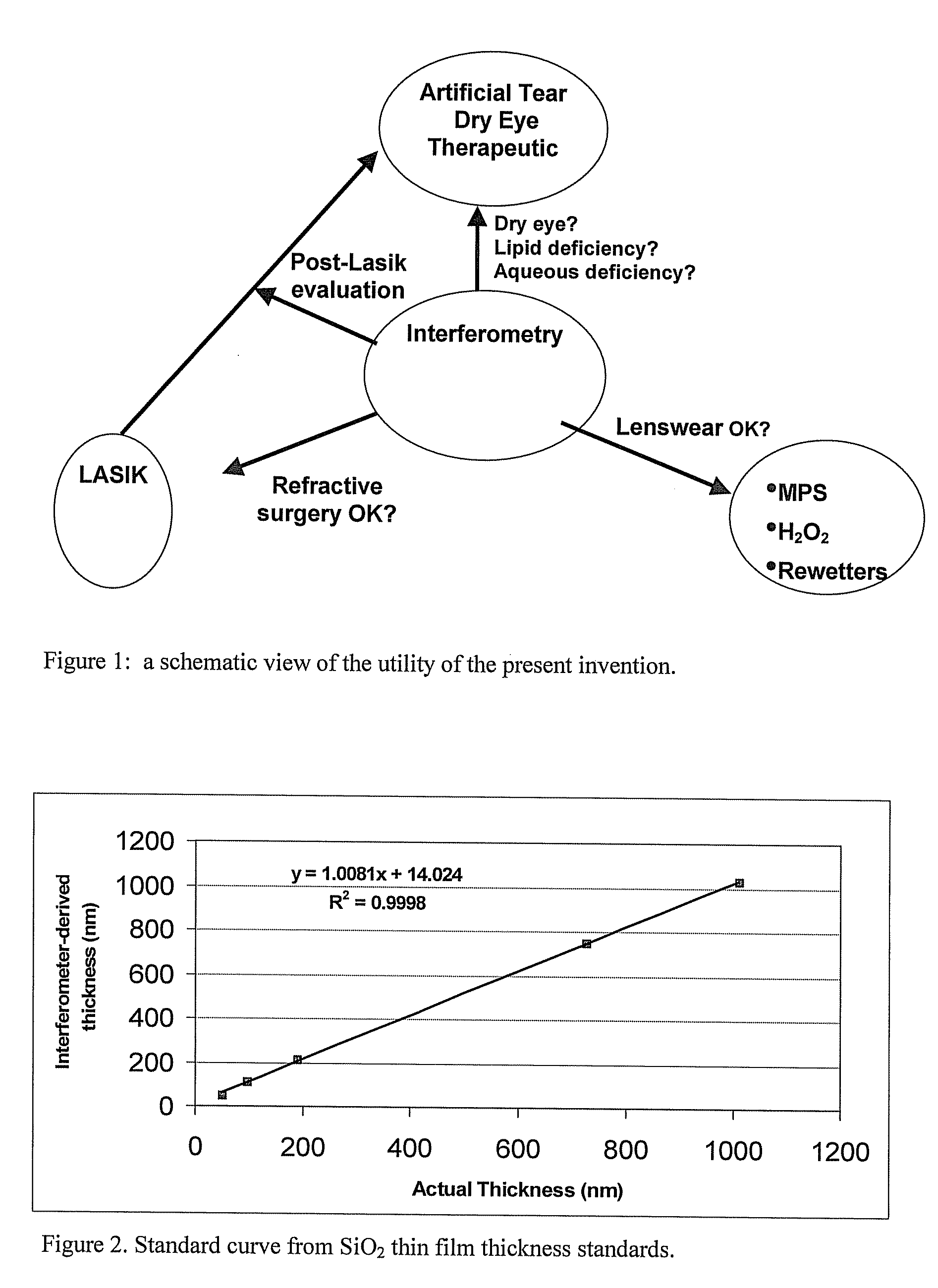
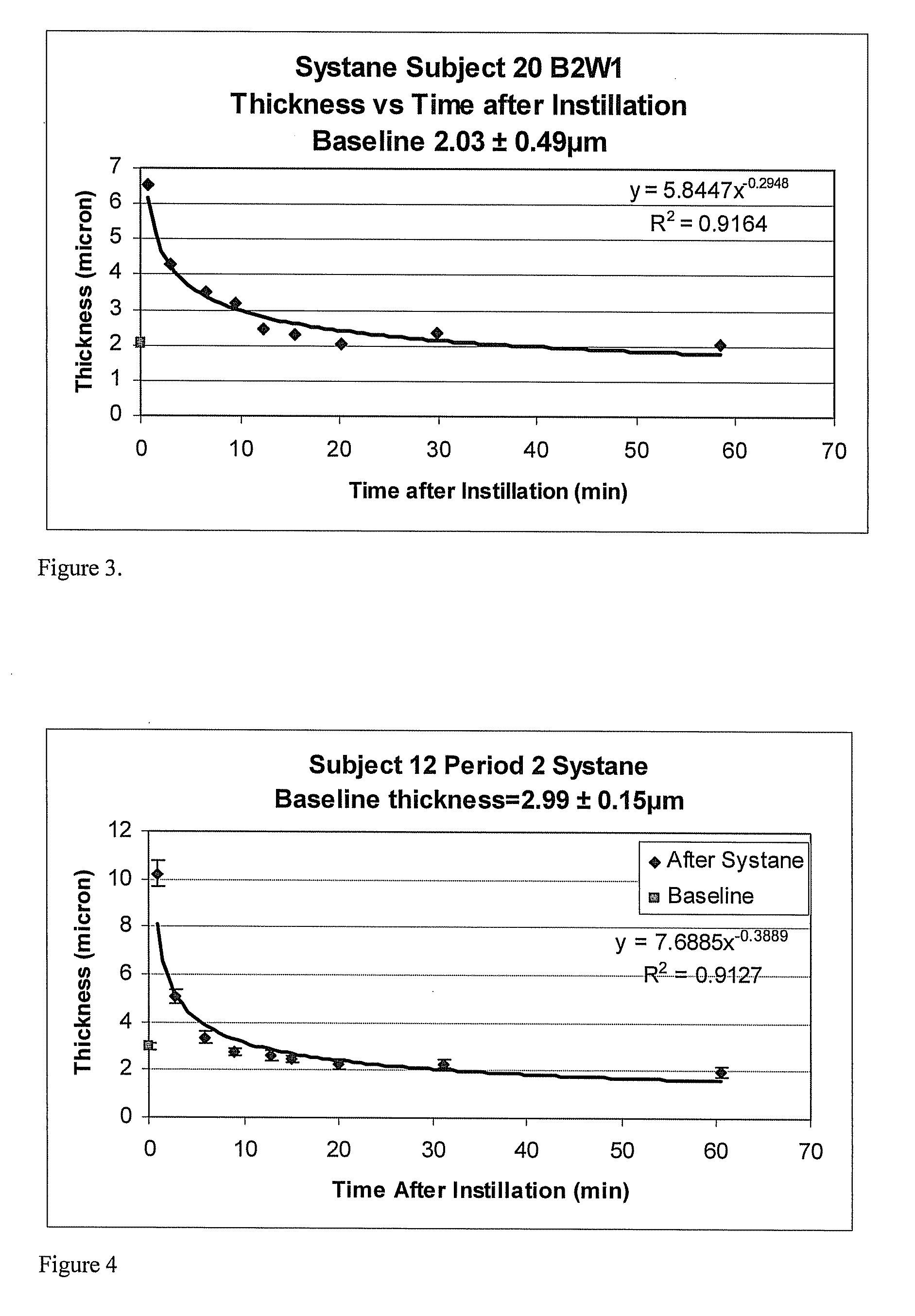
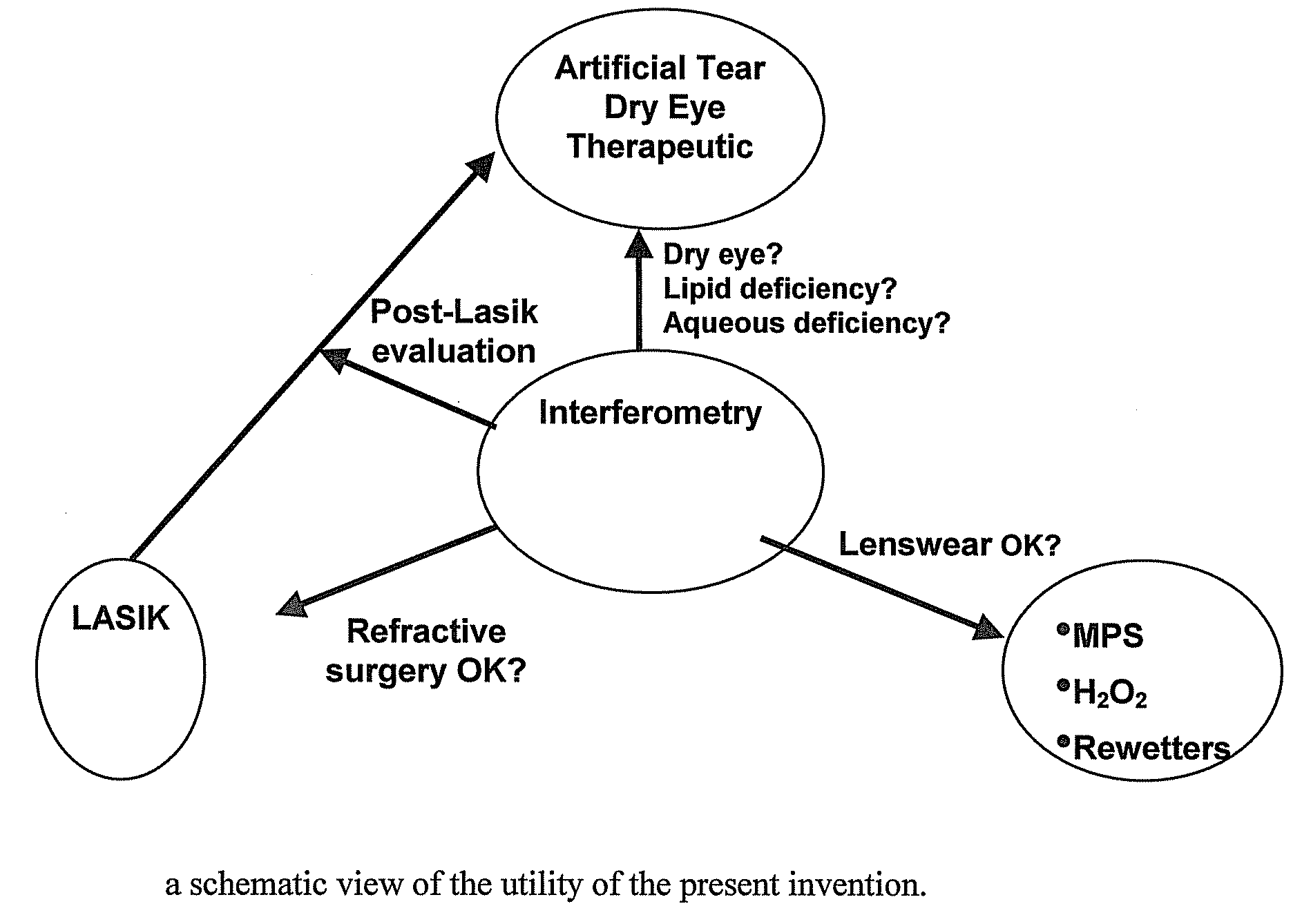
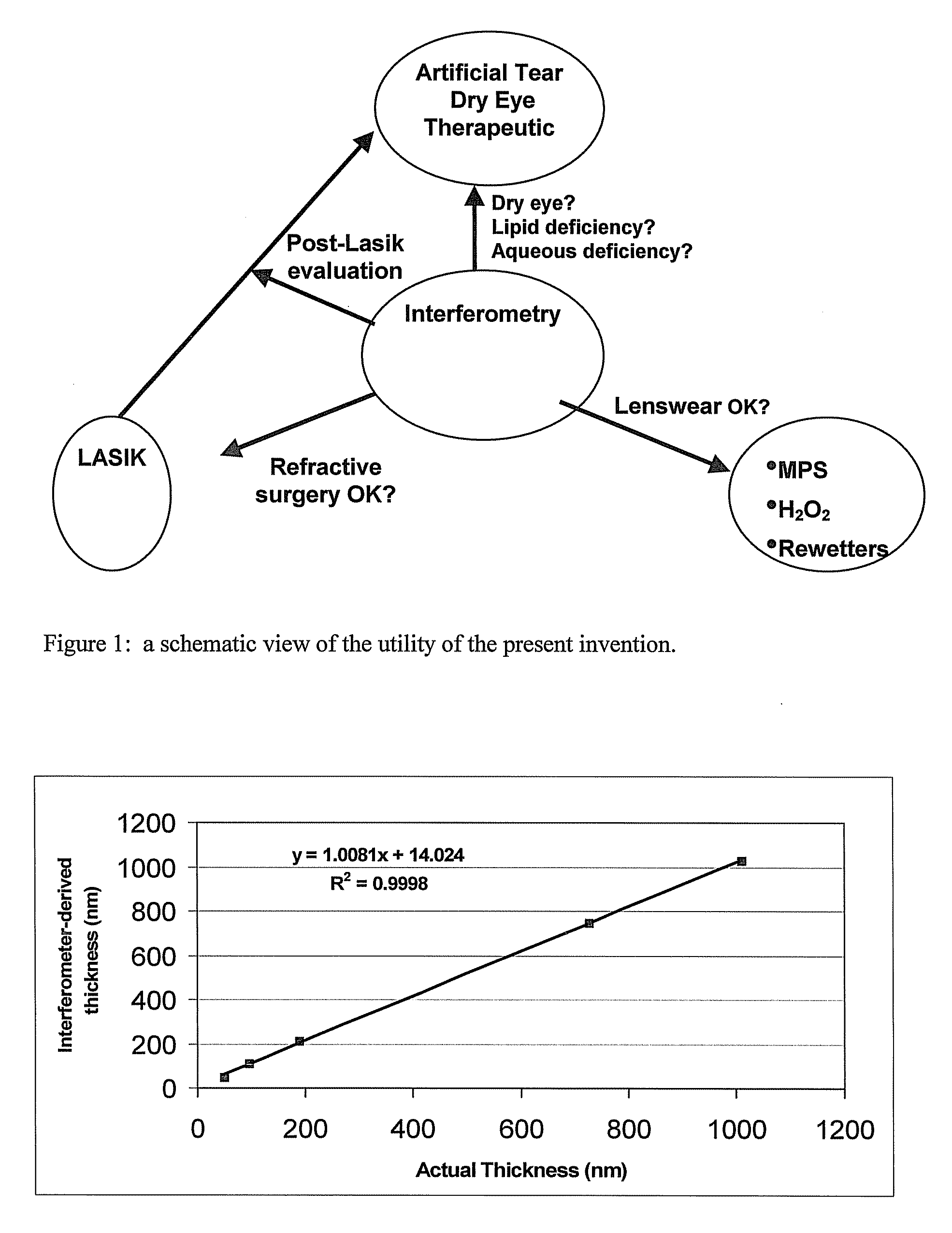
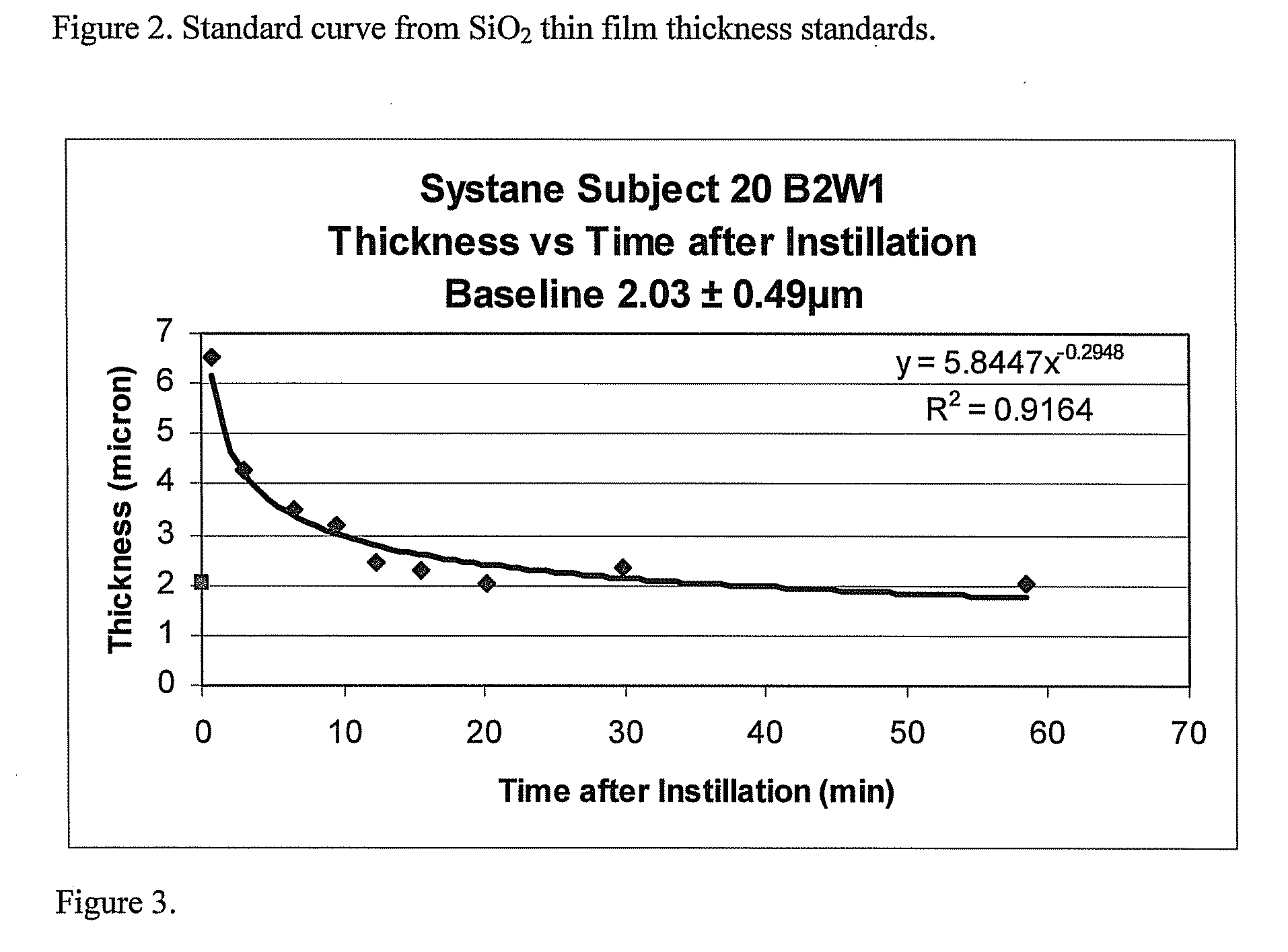
Methods and devices measure eye blinks and tear film lipid and aqueous layer thickness before and following ophthalmic formula application onto the ocular surface, especially wherein the ophthalmic formula is an artificial tear. The methods and devices are suitable for dry eye diagnosis. The methods and devices are suitable for use to evaluate ophthalmic formula effects on the tear film and to use such information to diagnose ophthalmic formula treatment of ocular disease conditions such as dry eye in the absence of contact lens wear or post-surgical eye drop treatment and diagnosis. The methods and devices are also suitable for use in the optimization of ophthalmic drug dosage forms and sustained drug release.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Devices, Systems and Methods for Ophthalmic Drug Delivery
Devices, systems and techniques for delivering drugs to an ocular tissue are described. In at least some embodiments, a terminal component (e.g., a needle or open end of a catheter) is implanted in an ocular tissue and used to deliver one or more drugs. The delivered drugs may come from a source which is also implanted, or may be introduced from an external source (e.g., via a port). Both solid and liquid drug formulations can be used. Ocular implants can alternatively include a thin film coating that releases a drug into an ocular tissue.
Owner:NEUROSYSTEC CORP
Ophthalmic drug delivery system
ActiveUS7638137B2Inhibited DiffusionInhibit migrationSpectales/gogglesPowder deliveryOphthalmic drugOphthalmology
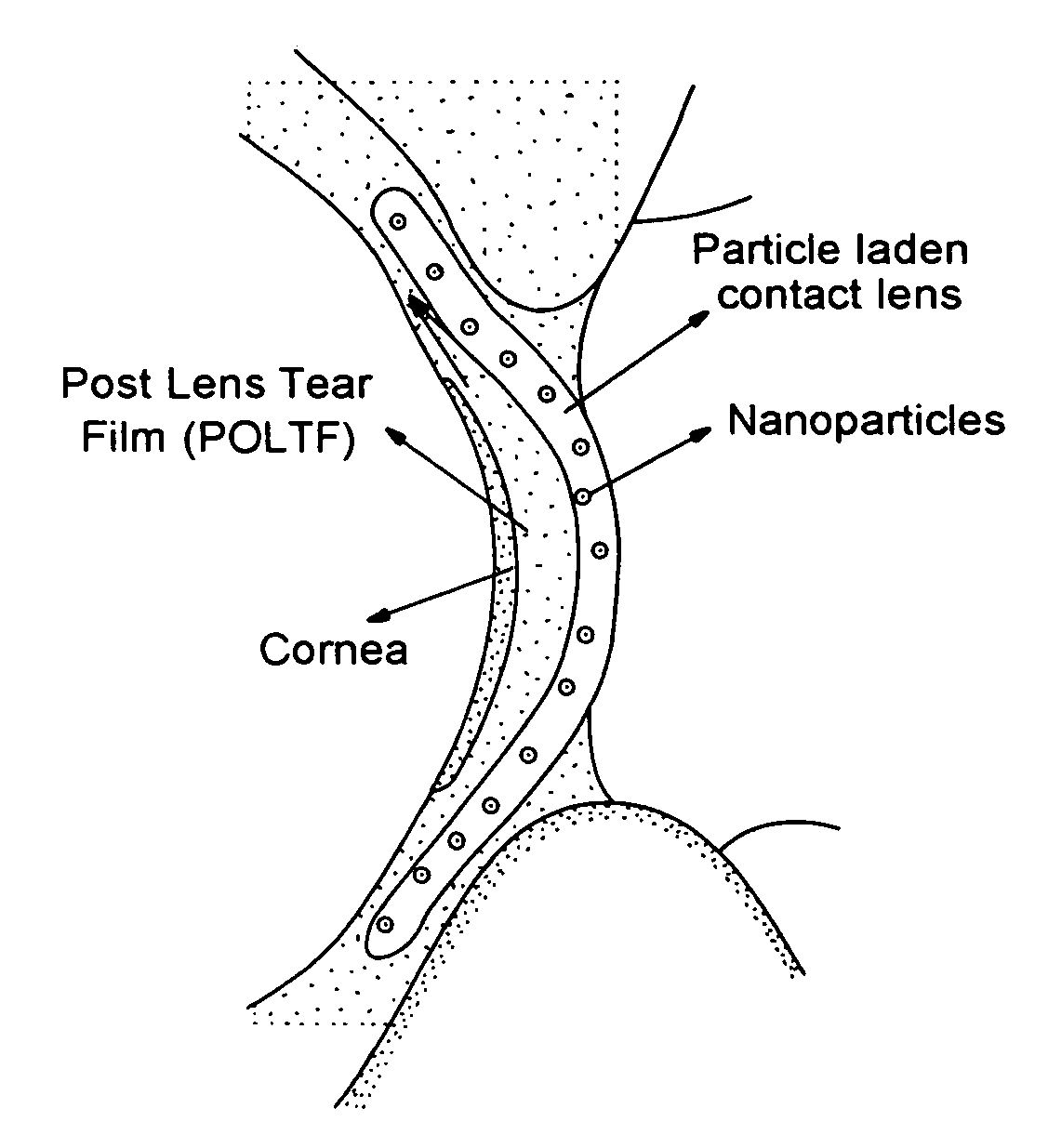
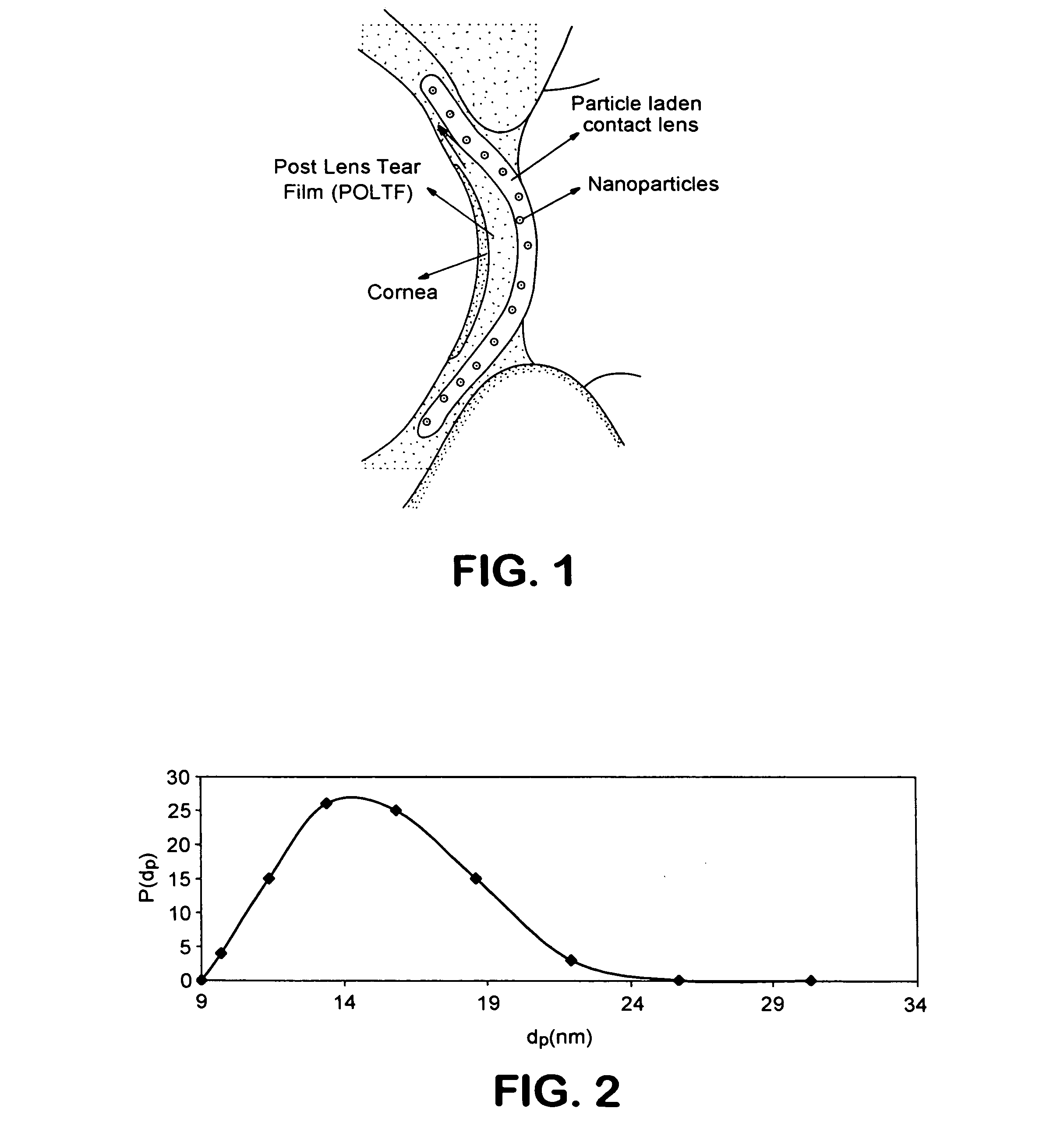
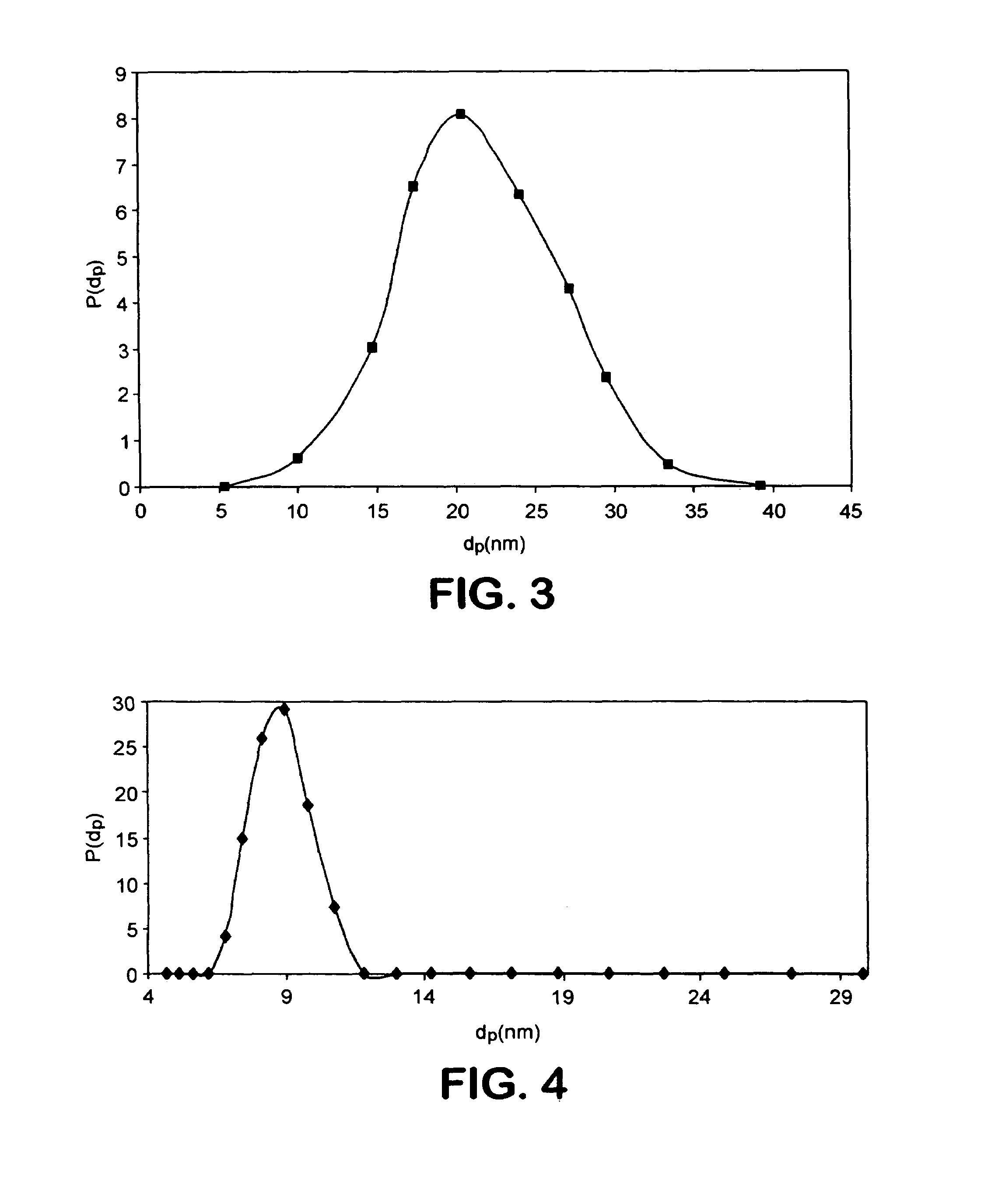
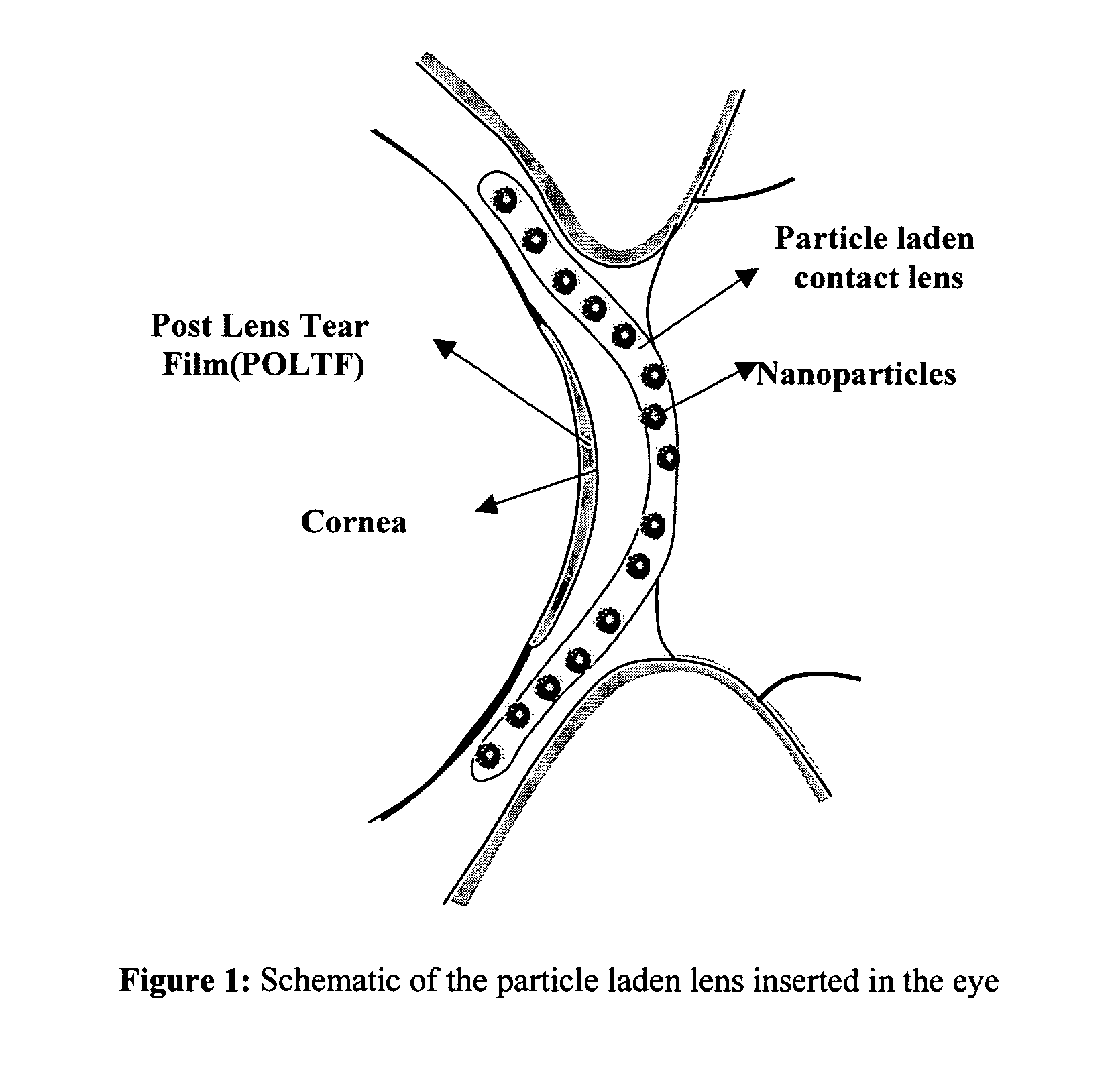
A drug delivery system comprising a contact lens having dispersed therein as nanoparticles having a particle size less than about 50 nm, an ophthalmic drug nanoencapsulated in a material from which said ophthalmic drug is capable of diffusion into and migration through said contact lens and into the post-lens tear film when said contact lens is placed on the eye.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Methods and devices for measuring tear film and diagnosing tear disorders
Methods and devices measure eye blinks and tear film lipid and aqueous layer thickness before and following ophthalmic formula application onto the ocular surface, especially wherein the ophthalmic formula is an artificial tear. The methods and devices are suitable for dry eye diagnosis. The methods and devices are suitable for use to evaluate ophthalmic formula effects on the tear film and to use such information to diagnose ophthalmic formula treatment of ocular disease conditions such as dry eye in the absence of contact lens wear or post-surgical eye drop treatment and diagnosis. The methods and devices are also suitable for use in the optimization of ophthalmic drug dosage forms and sustained drug release.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Ophthalmic drug delivery system
A fluid atomizer is disclosed. The fluid atomizer comprises a body having a proximal end and a distal end. A removable reservoir containing a fluid disposed therein is connected to the body. A discharge plate is disposed at the distal end, wherein the discharge plate includes a plurality of openings extending therethrough. An ultrasonic oscillator is disposed in the body for transmitting the fluid from the reservoir to the discharge plate and through the plurality of openings, wherein transmission of the fluid through the plurality of openings atomizes the fluid.
Owner:OPTIMYST SYST
Devices And Methods For Ophthalmic Drug Delivery
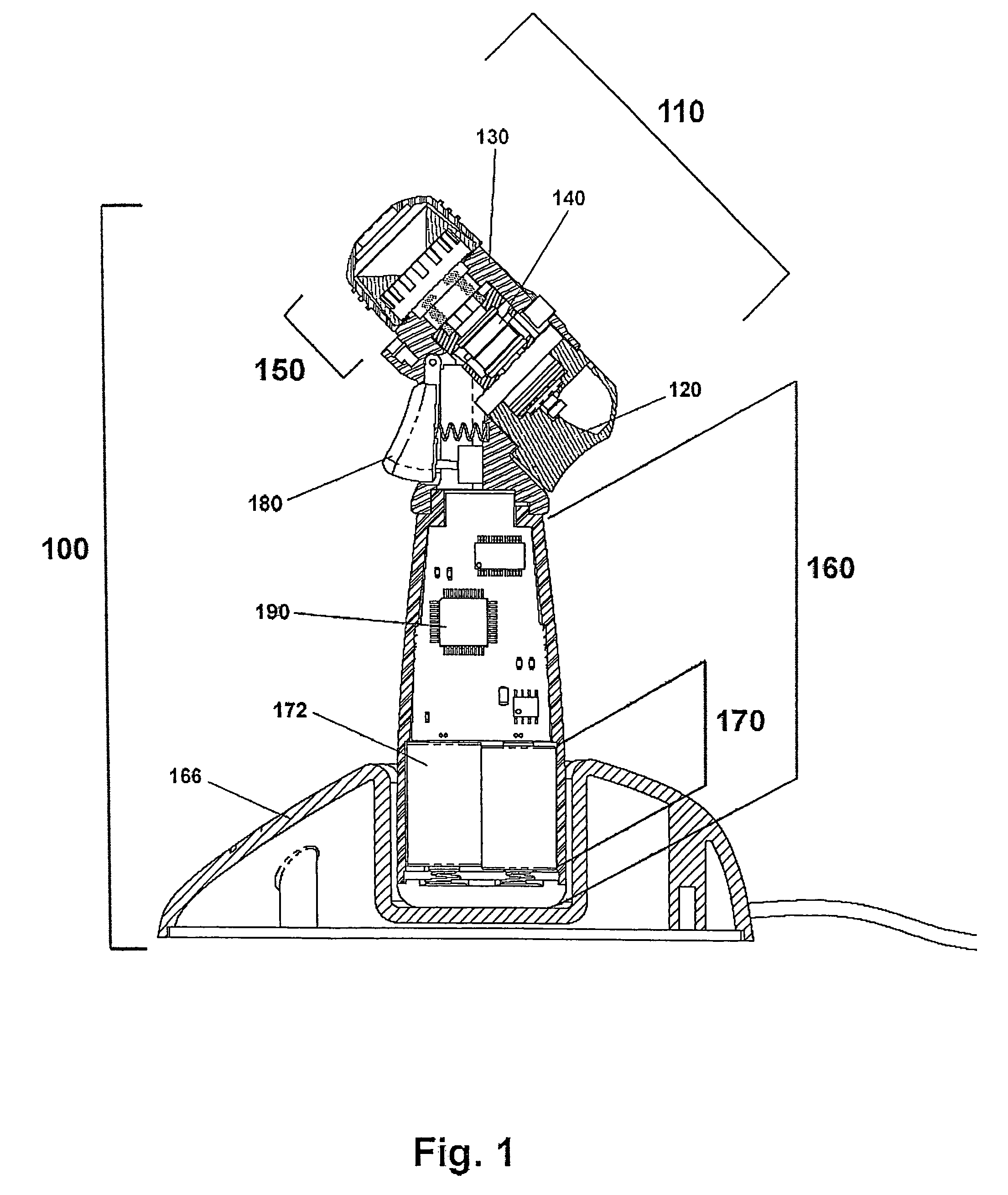
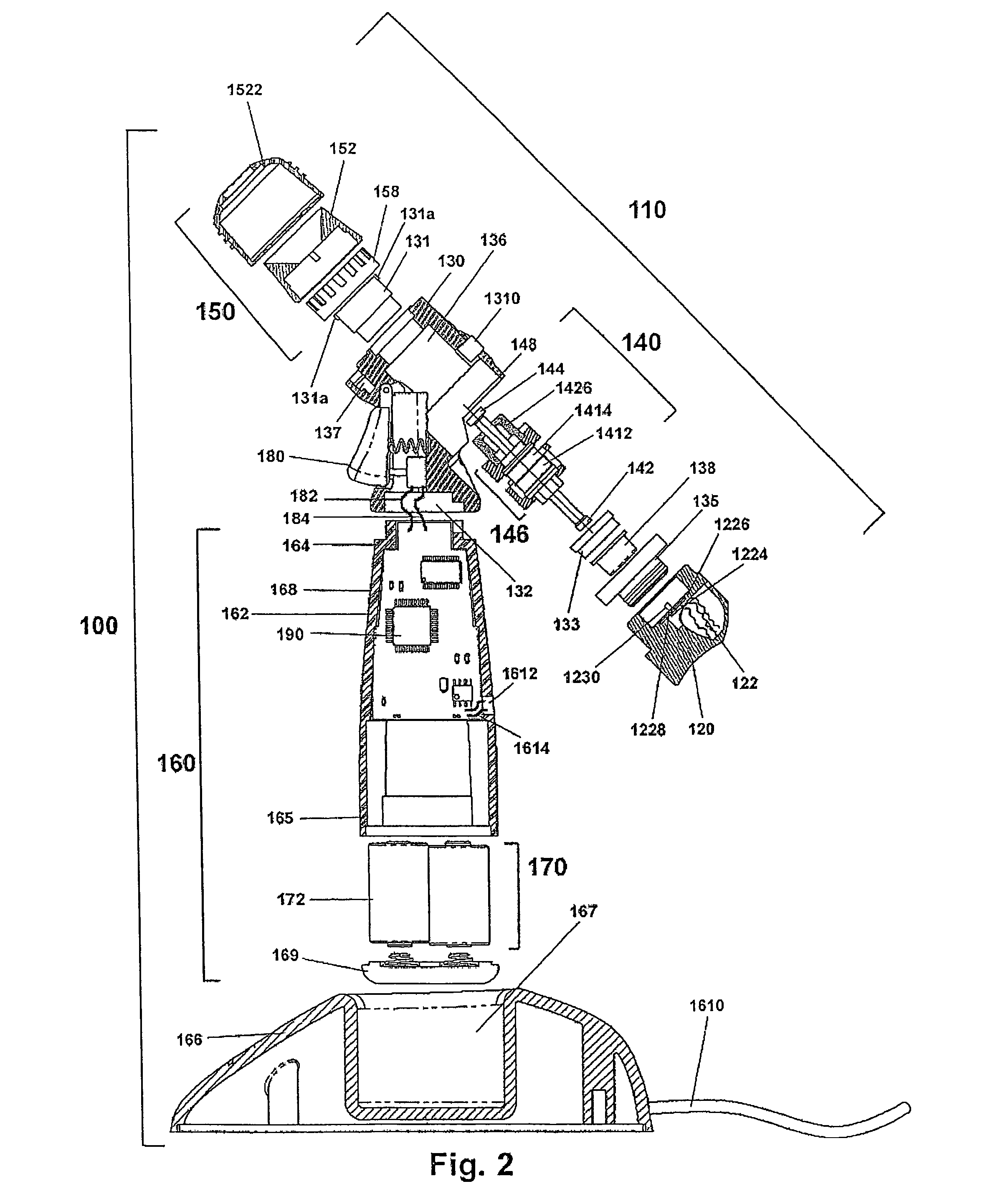
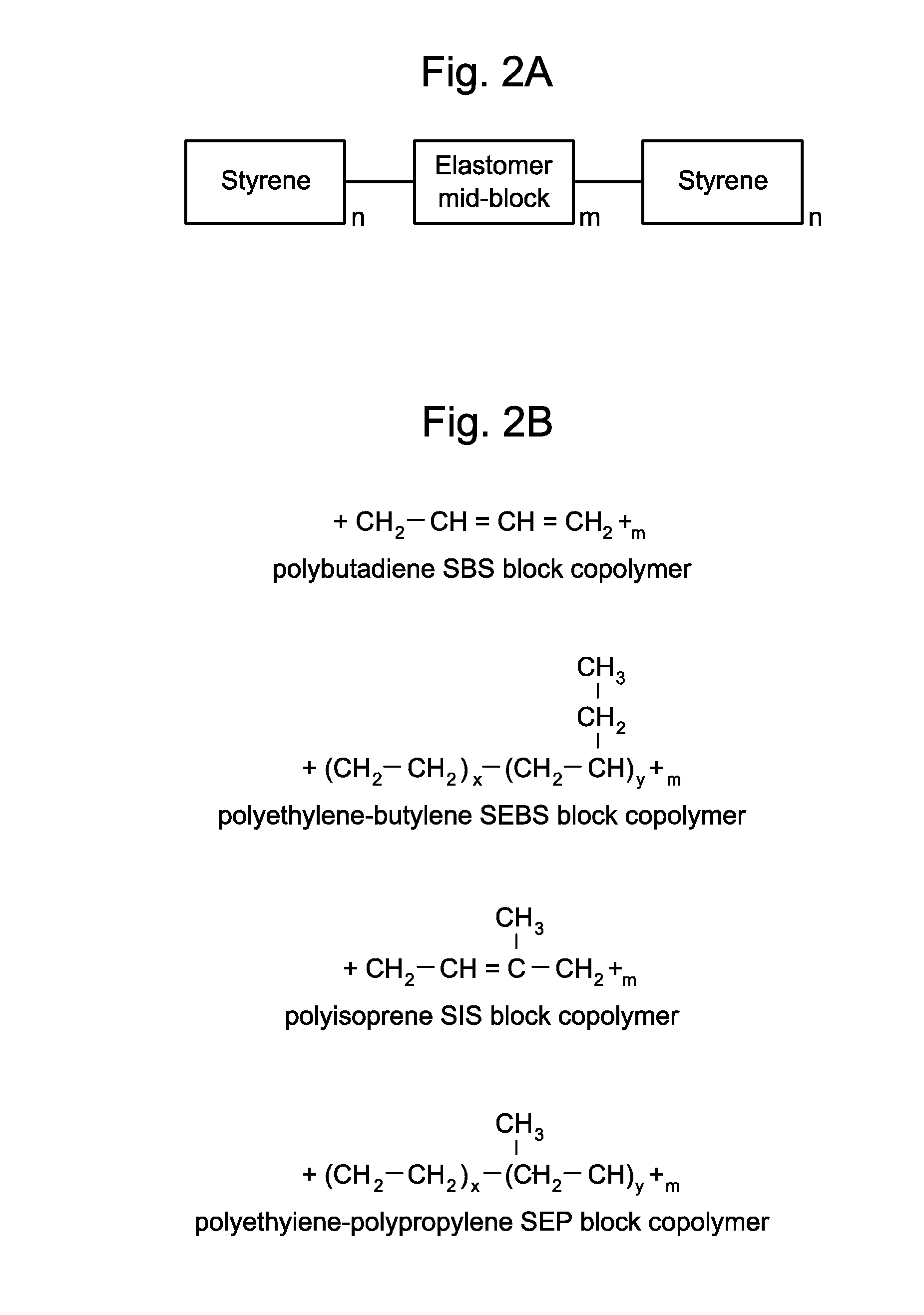
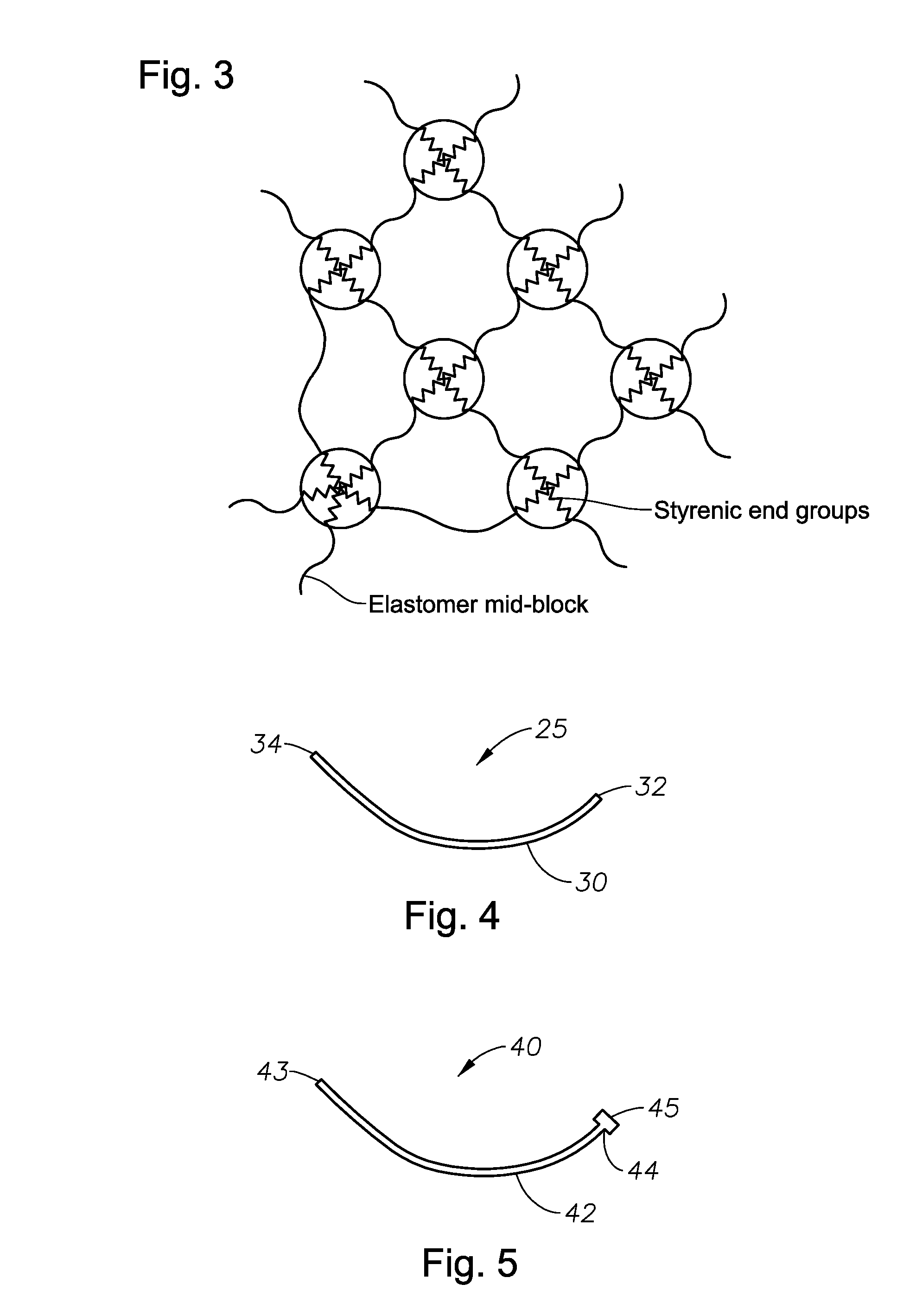
InactiveUS20100266664A1Easy to manufactureWithout effectOrganic active ingredientsSenses disorderElastomerOphthalmic drug
Disclosed are ophthalmic drug-delivery devices, comprising a body having a proximal end and a distal end, wherein the body includes a styrene elastomer matrix and a drug in contact with the matrix. Also disclosed are methods of treating or preventing an eye disease in a subject, that involve contacting an eye of the subject with an ophthalmic drug delivery device comprising a body having a proximal end and a distal end, wherein the body comprises a styrene elastomer matrix and a drug in contact with the matrix, wherein release of the drug from the device occurs over time following contacting of the device with the eye of the subject.
Owner:ALCON RES LTD
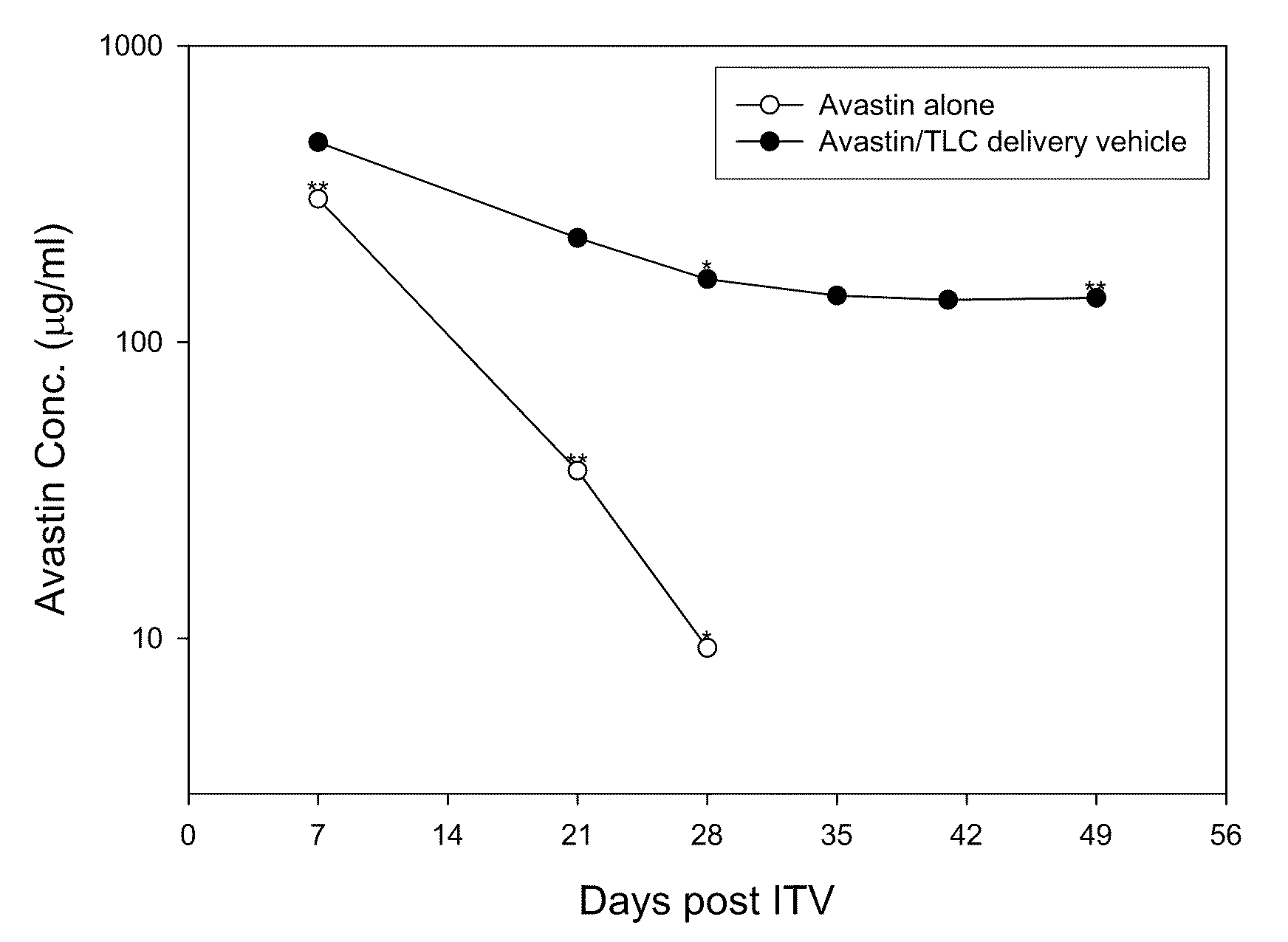
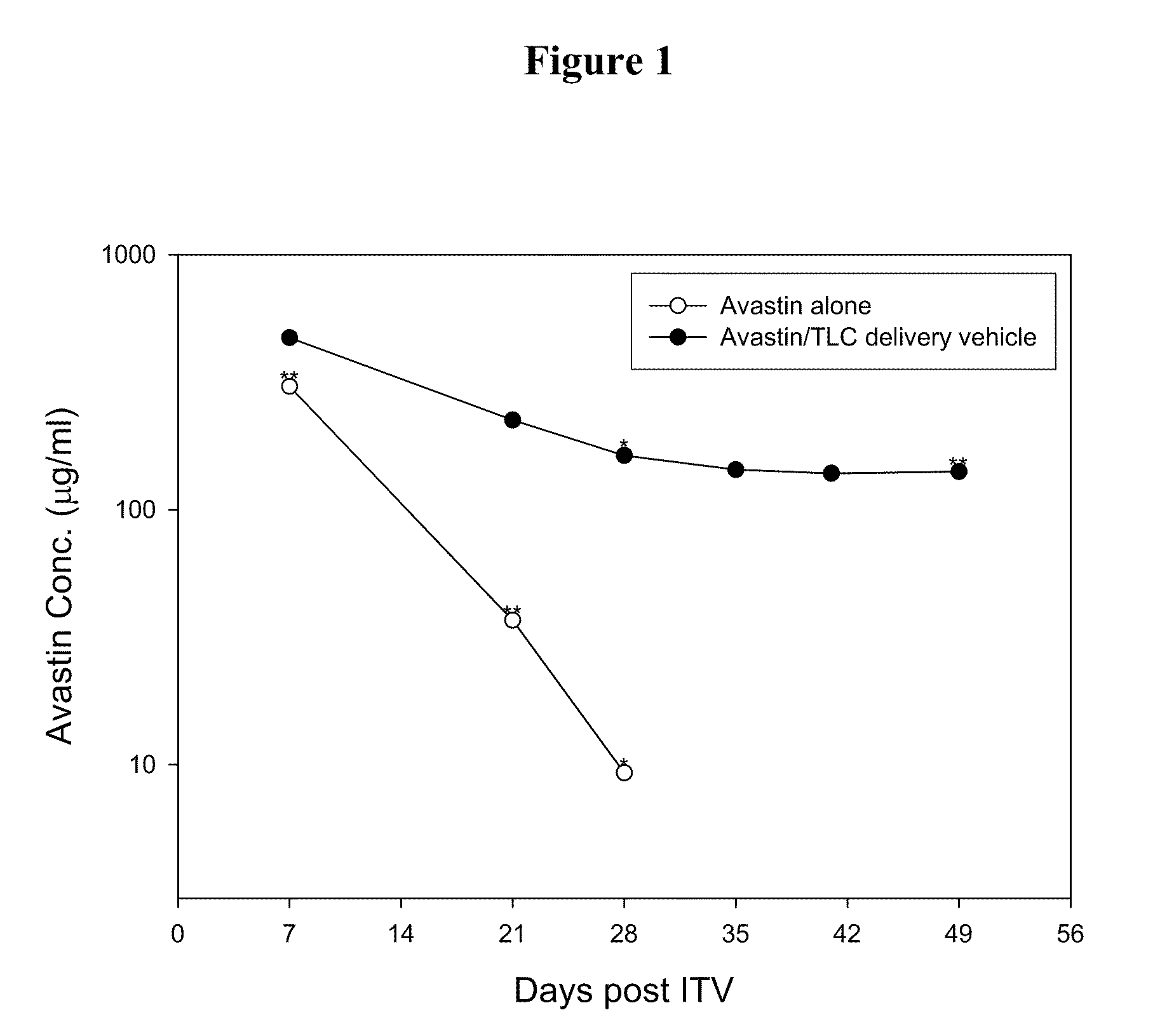
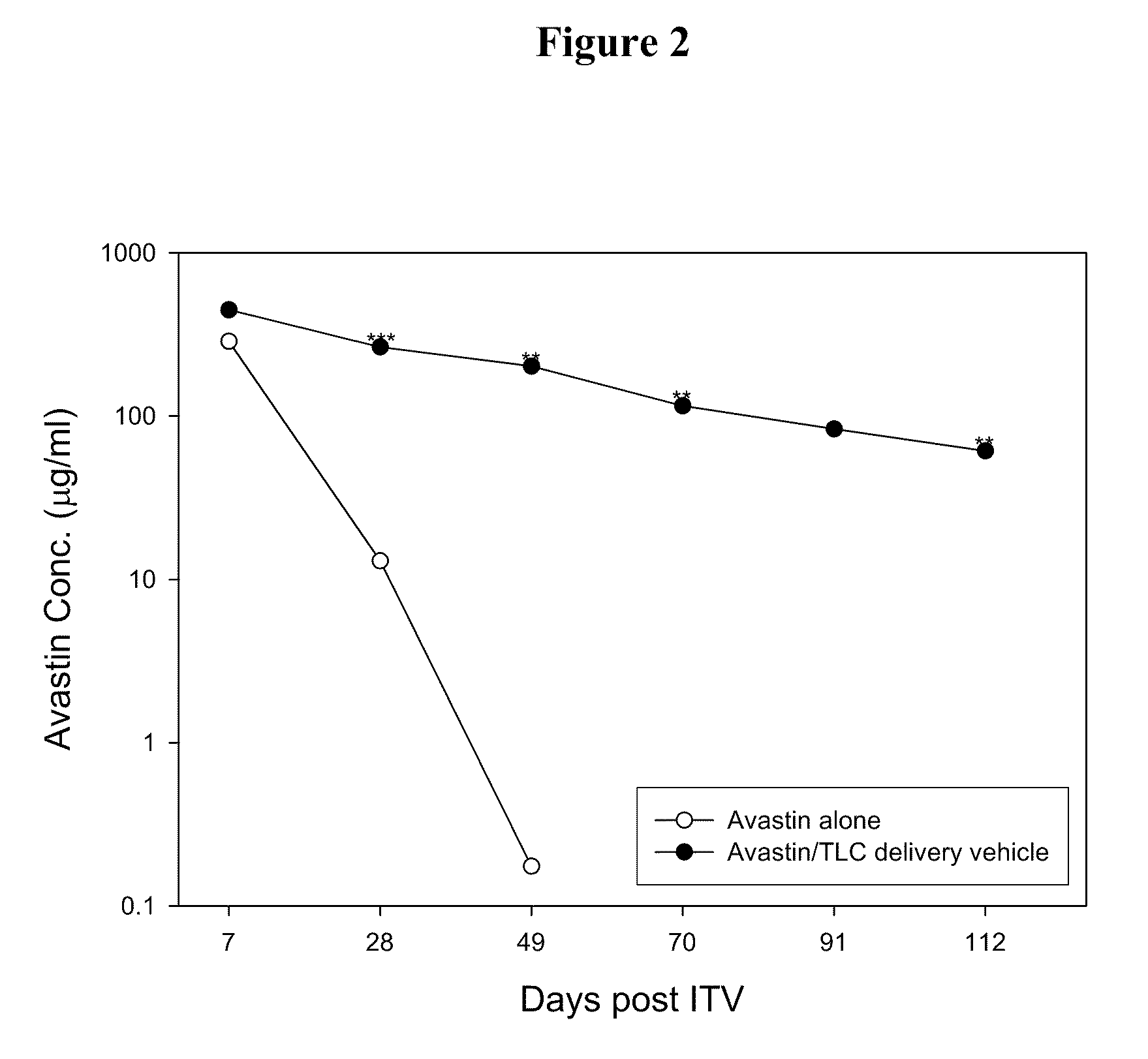
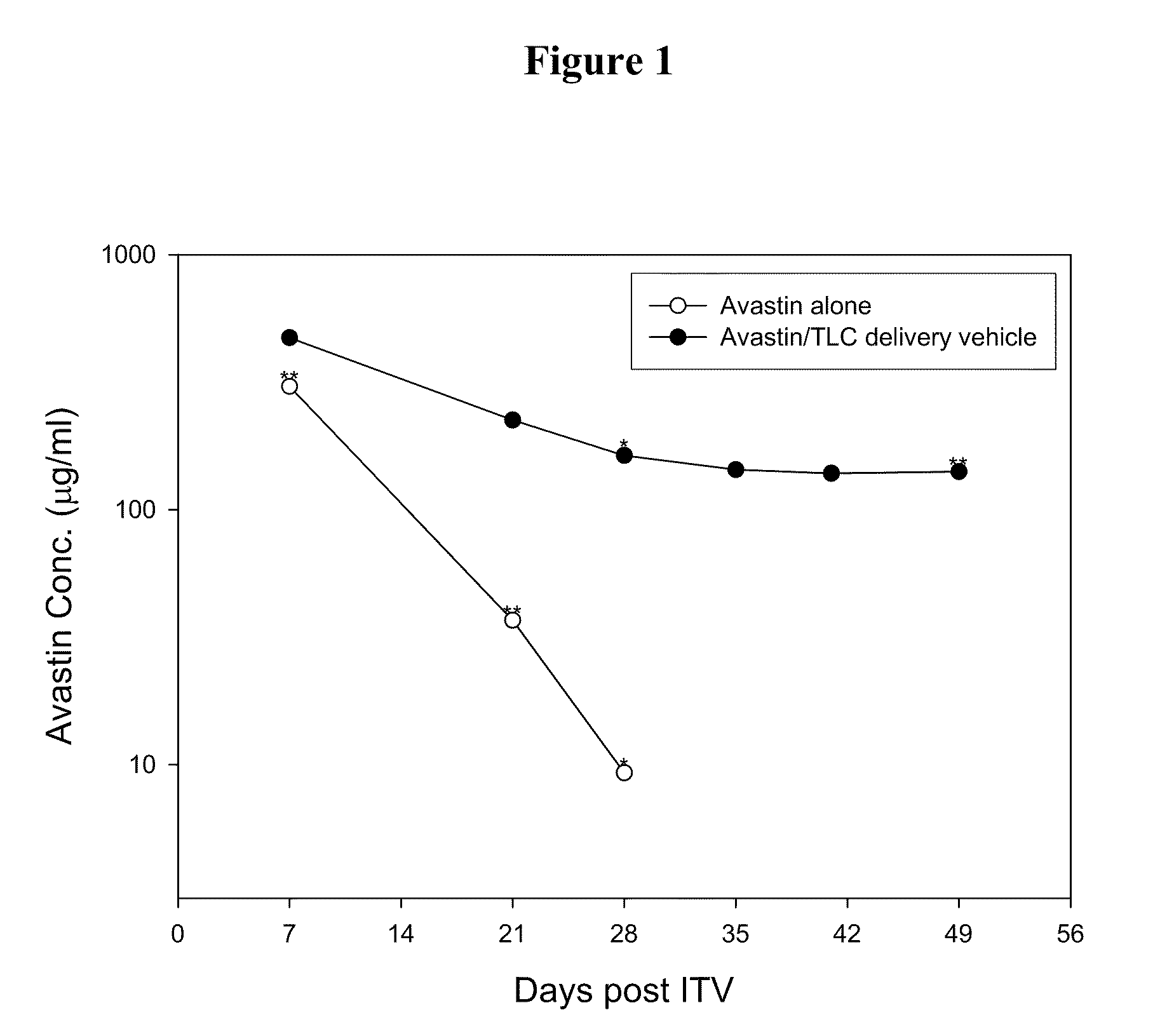
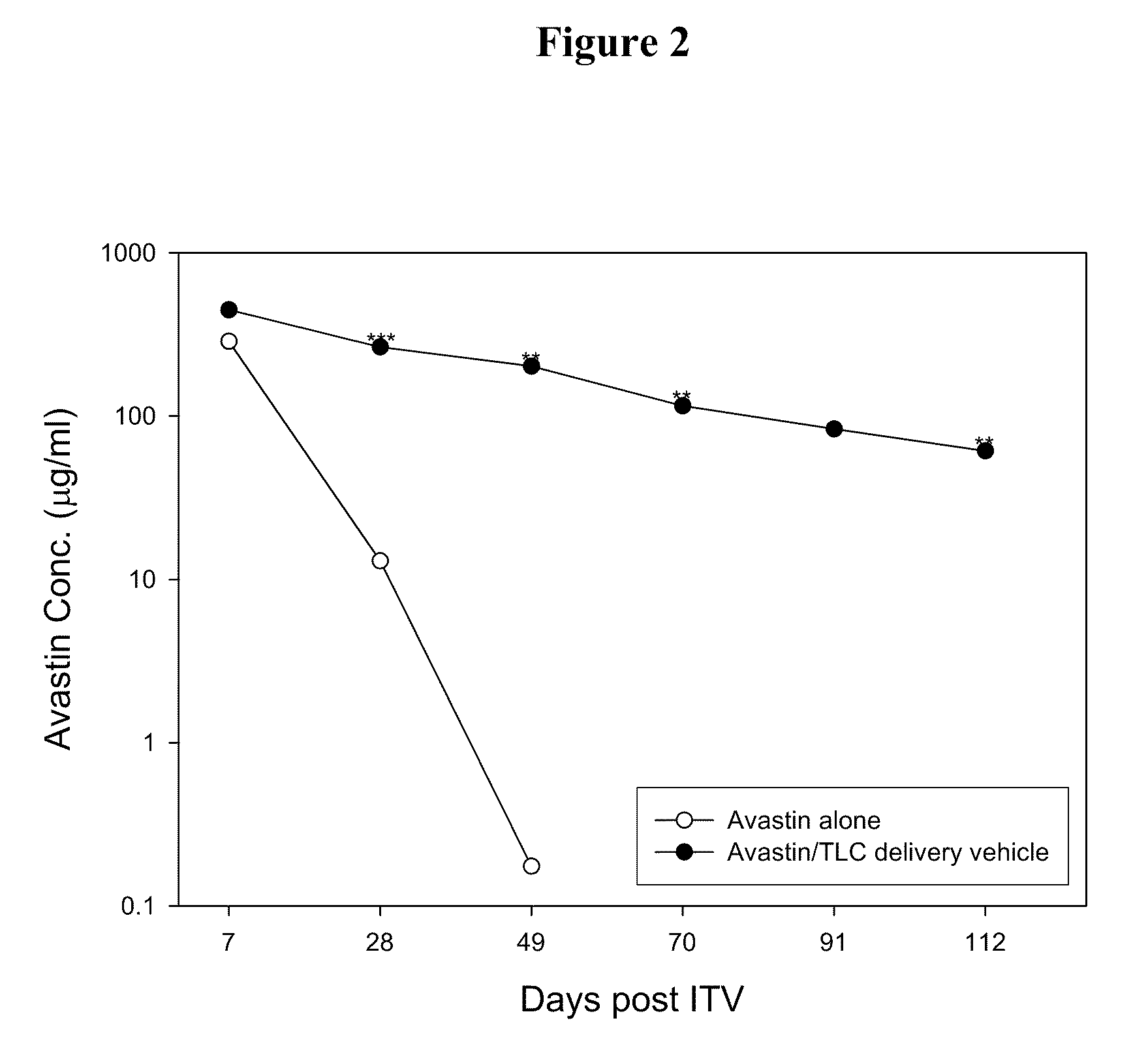
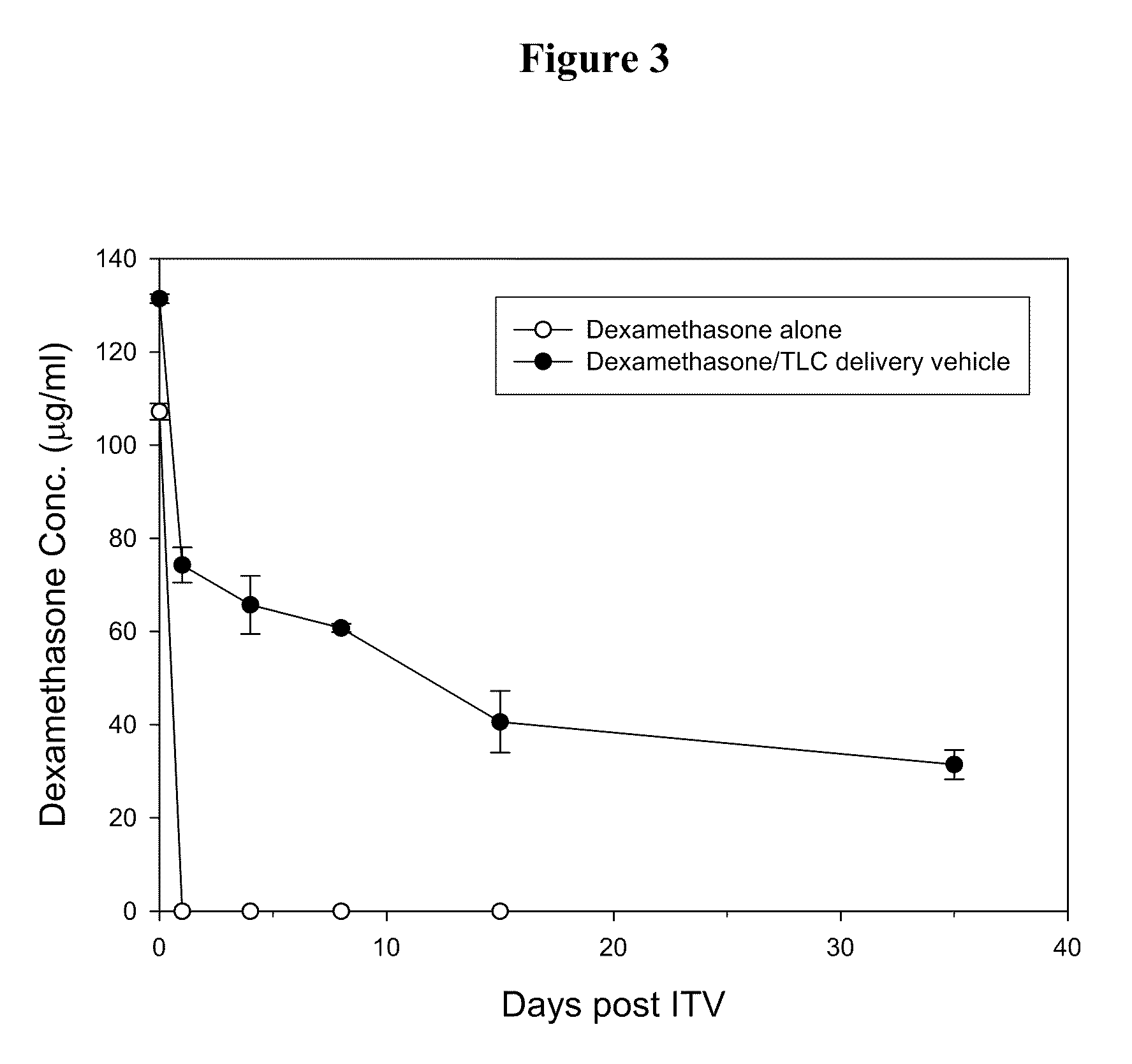
Ophthalmic Drug Delivery System Containing Phospholipid and Cholesterol
An ophthalmic drug delivery system that contains phospholipid and cholesterol for prolonging drug lifetime in the eyes.
Owner:TLC PHARMA INC +1
Inhibition of irritating side effects associated with use of a topical ophthalmic medication
InactiveUS6933289B2Reduced bioavailabilityEliminate the effects ofBiocidePowder deliverySide effectOphthalmic drug
This invention relates to a method of reducing an irritating or adverse side effect associated with the topical use of an active ophthalmic drug comprising incorporating an effective amount of a cyclodextrin or cyclodextrin derivative into a formulation to complex the active drug such that the concentration of the free active drug is reduced below a tolerable threshold, and incorporating an effective amount of a viscosity increasing agent in said formulation such that the bioavailability of said drug is high enough to be therapeutically effective, wherein the cyclodextrin or cyclodextrin derivative is not required to solubilize the active drug.Another aspect of this invention relates to topical ophthalmic formulations comprising an active drug, a cyclodextrin or cyclodextrin derivative, and a viscosity-enhancing agent, in effective amounts as stated above.
Owner:ALLERGAN INC
Ophthalmic drug delivery system
ActiveUS8273366B2Inhibited DiffusionInhibit migrationEmulsion deliveryMicrocapsulesOphthalmic drugNanoparticle
A drug delivery system comprising a contact lens having dispersed therein as nanoparticles having a particle size less than about 200 nm, an ophthalmic drug nanoencapsulated in a material from which said ophthalmic drug is capable of diffusion into and migration through said contact lens and into the post-lens tear film when said contact lens is placed on the eye.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Ophthalmic drug delivery
The present invention includes and provides a method of delivering a medicament to an eye of a subject in need thereof a solution, the method comprising: (a) providing droplets containing the medicament with a specified average size and average initial ejecting velocity; and (b) delivering the medicament to the eye, where the droplets deliver a percentage of the ejected mass of the droplets to the eye.
Owner:EYENOVIA
Ophthalmic Drug Delivery
The present invention includes and provides a method of delivering a medicament to an eye of a subject in need thereof a solution, the method comprising: (a) providing droplets containing the medicament with a specified average size and average initial ejecting velocity; and (b) delivering the medicament to the eye, where the droplets deliver a percentage of the ejected mass of the droplets to the eye.
Owner:EYENOVIA
Ophthalmic drug delivery system and applications
InactiveUS20100233241A1The relative position is appropriateAppropriate stabilityOrganic active ingredientsSenses disorderDiseaseOphthalmic drug
An ocular device for insertion into an eye is provided and includes a body having an anterior surface and a posterior surface for placement on one of superior sclera and inferior sclera of the eye. The posterior surface is defined by a base curve that is substantially identical to a radius of curvature of the one of the superior sclera and inferior sclera of the eye. In one embodiment, the ocular device serves as an ocular drug delivery system and contains an active pharmaceutical agent, a lubricant, etc. In a second embodiment the ocular device can be constructed in such a manner to treat a wide variety of ocular conditions and diseases.
Owner:VISTA SCI
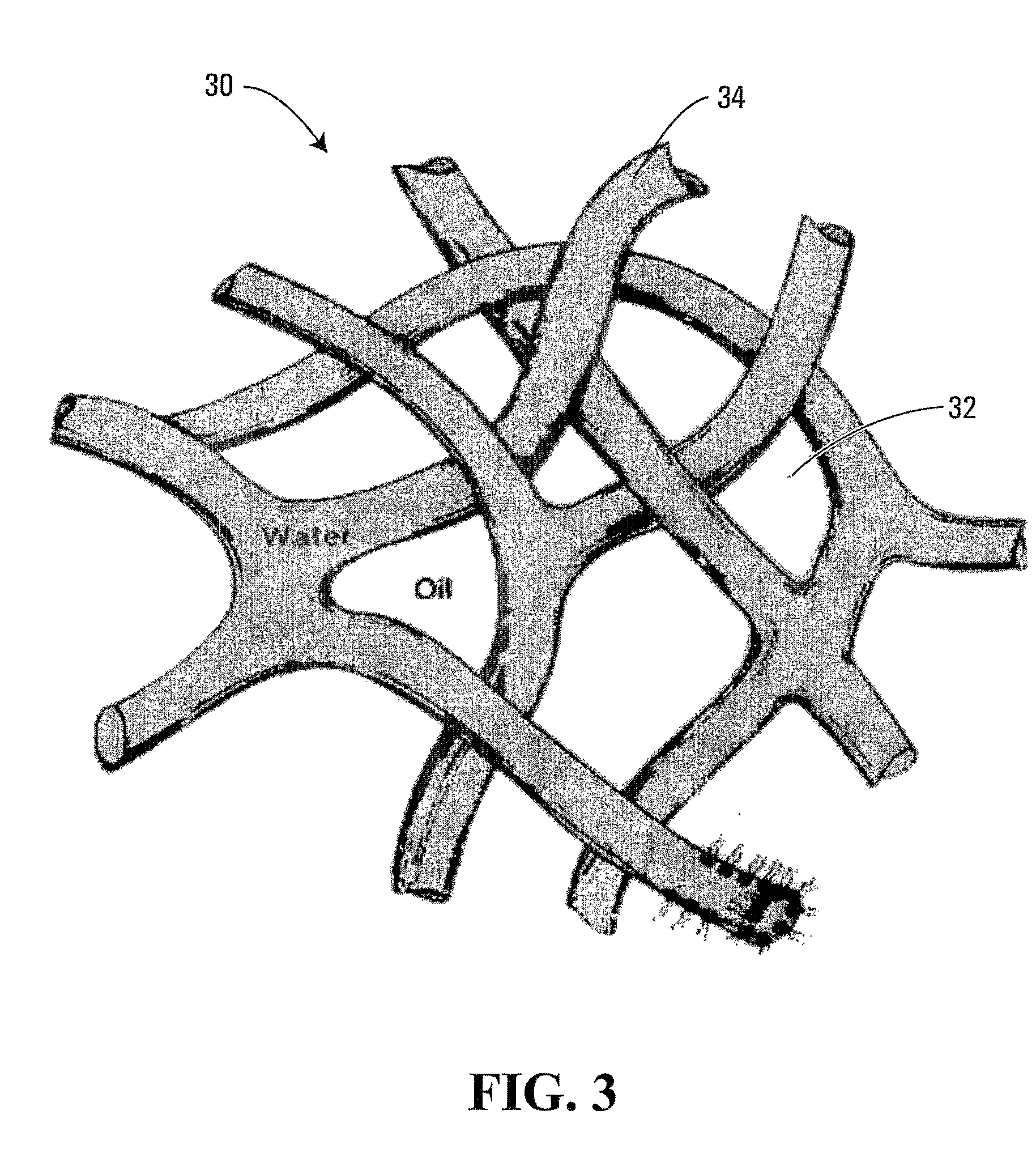
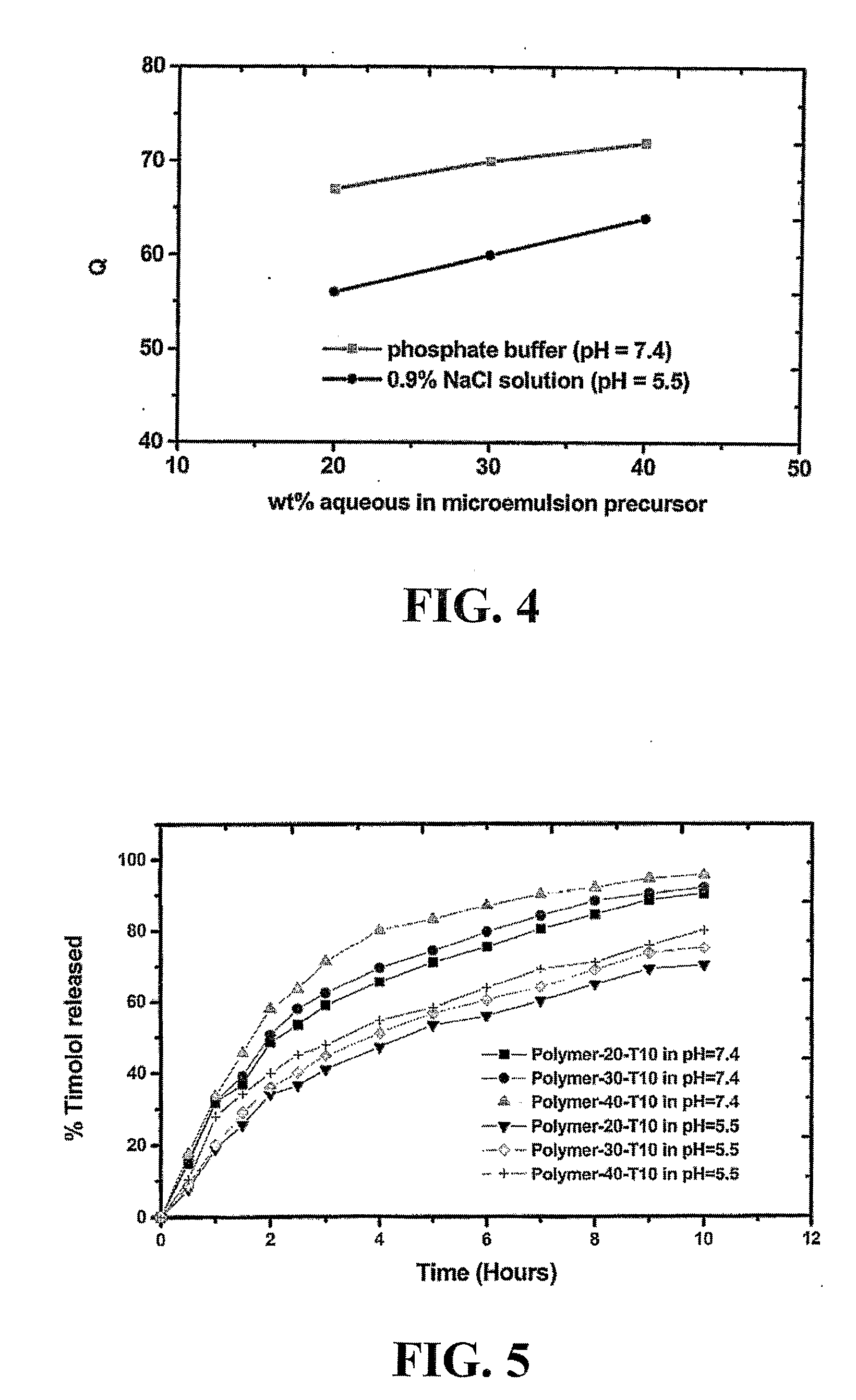
Polymer having interconnected pores for drug delivery and method
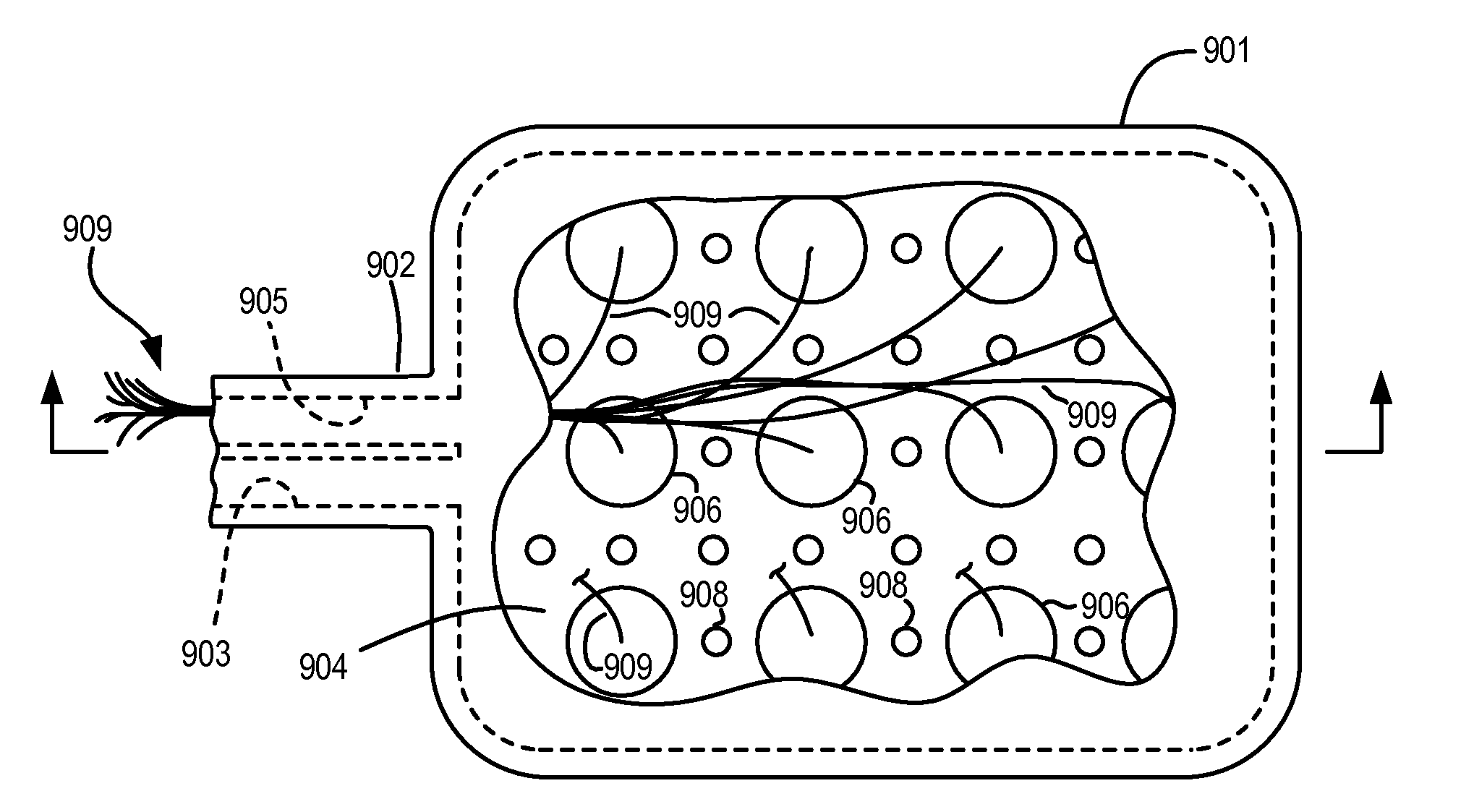
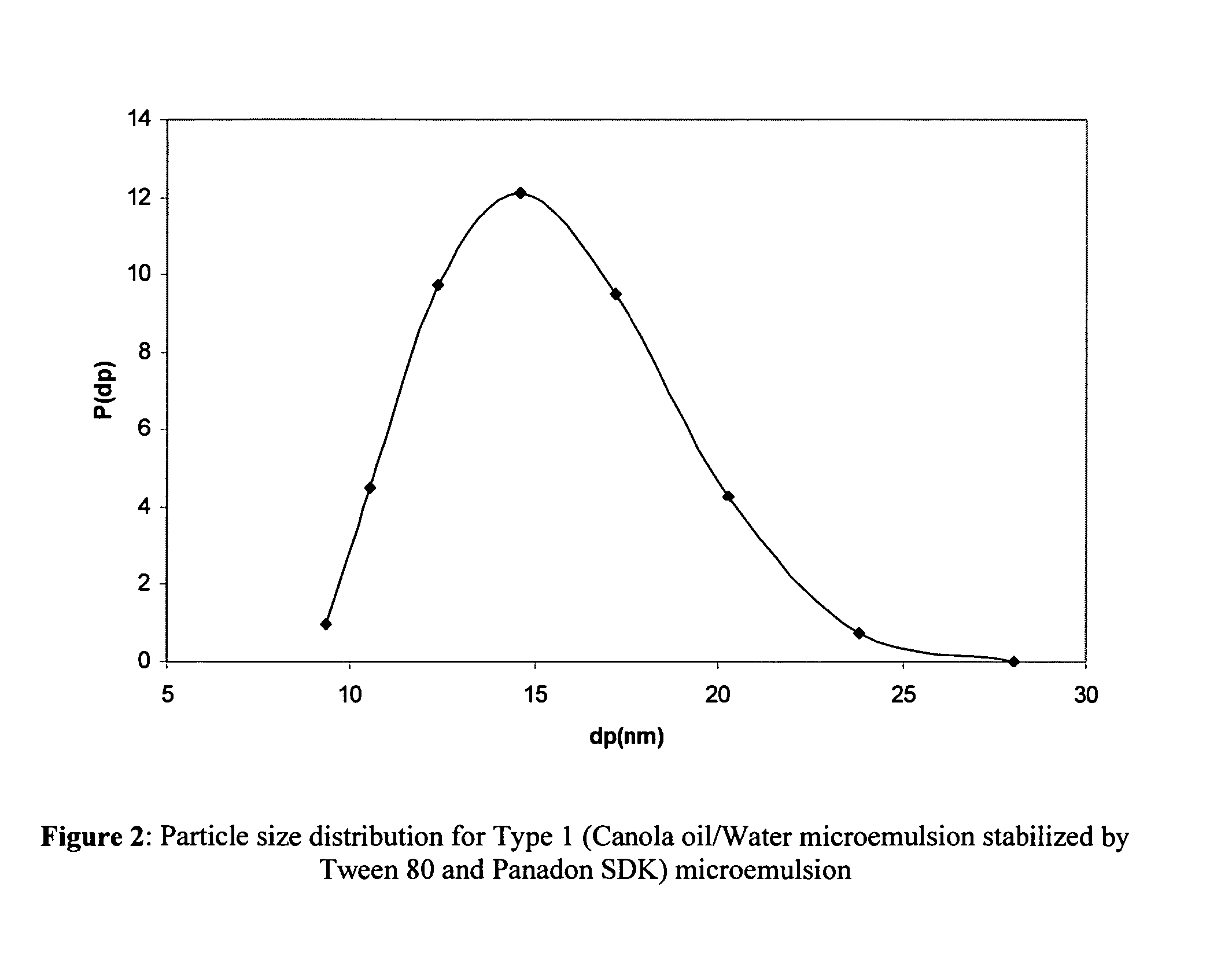
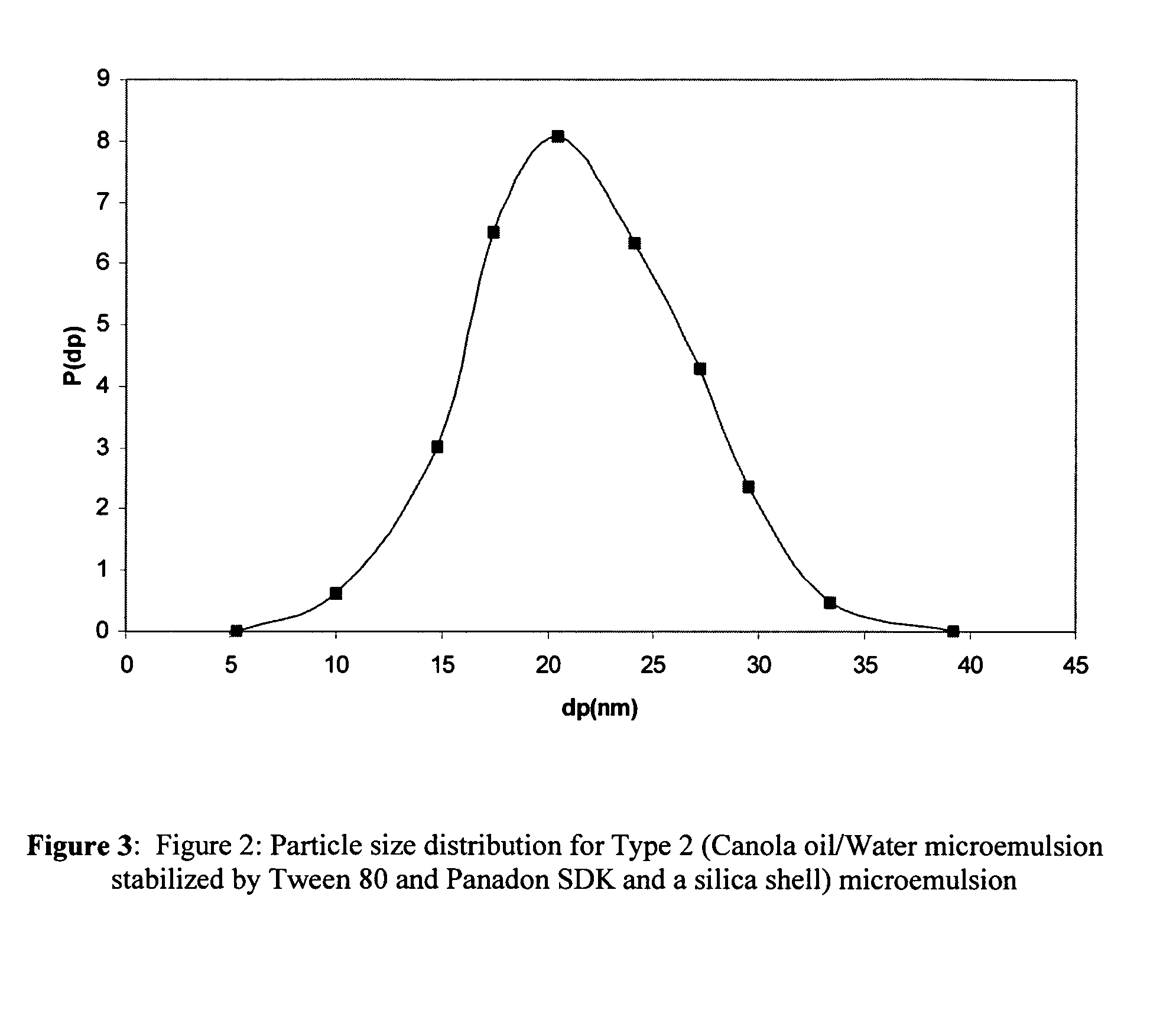
A bicontinuous microemulsion of water, a monomer, and a surfactant copolymerizable with the monomer is polymerized to form a transparent and porous polymer defining interconnected pores. The pores may have a pore diameter in the range of 10 to 100 mm. The microemulsion may further include a drug such that, when the polymer is formed, the drug is dispersed in one or both of the polymer and the pores and is releasable therefrom when the polymer is in contact with a liquid. The drug may be an ophthalmic drug and the polyer can be used to form drug delivery devices, such as contact lenses and artificial corneas.
Owner:AGENCY FOR SCI TECH & RES
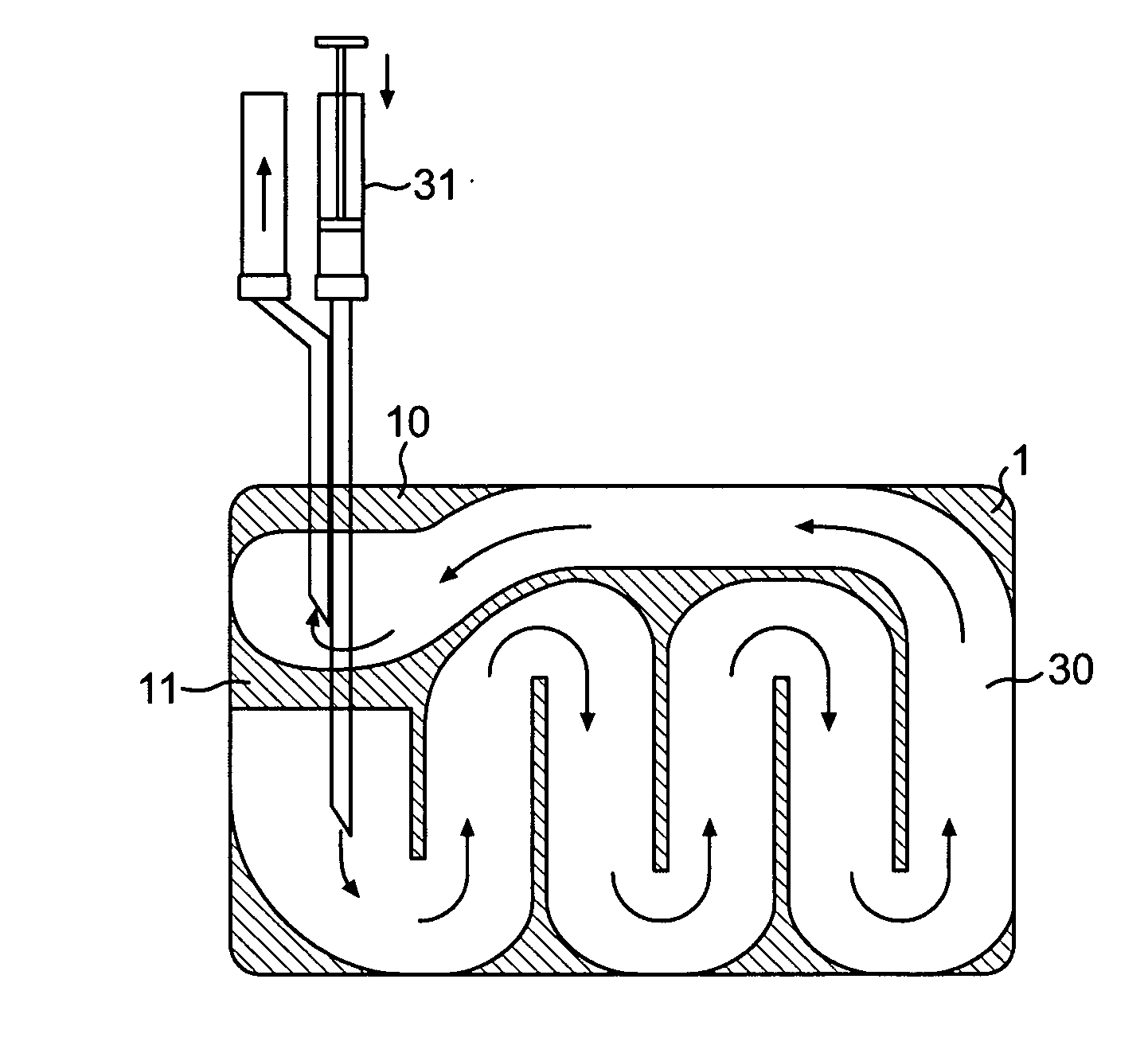
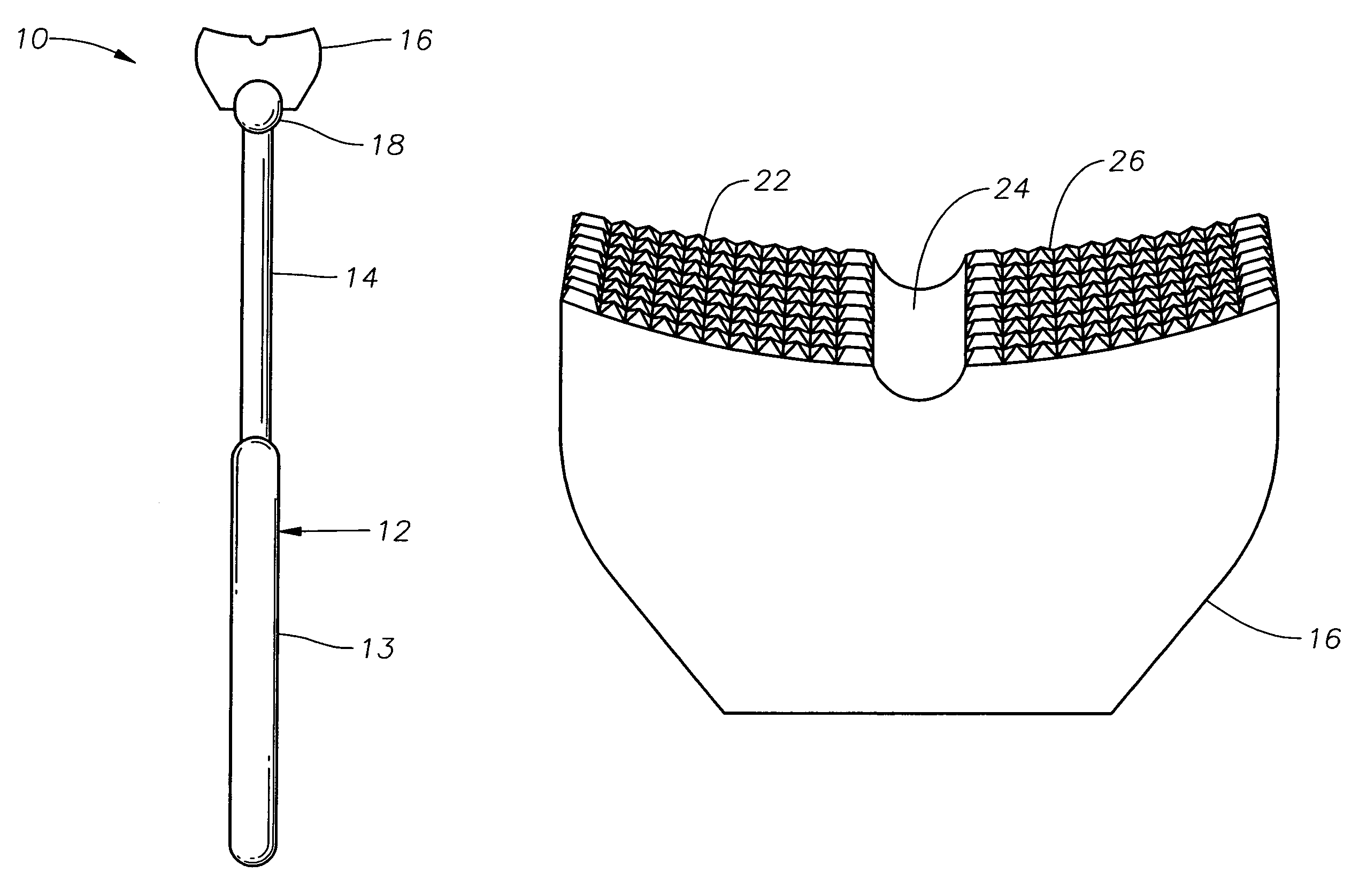
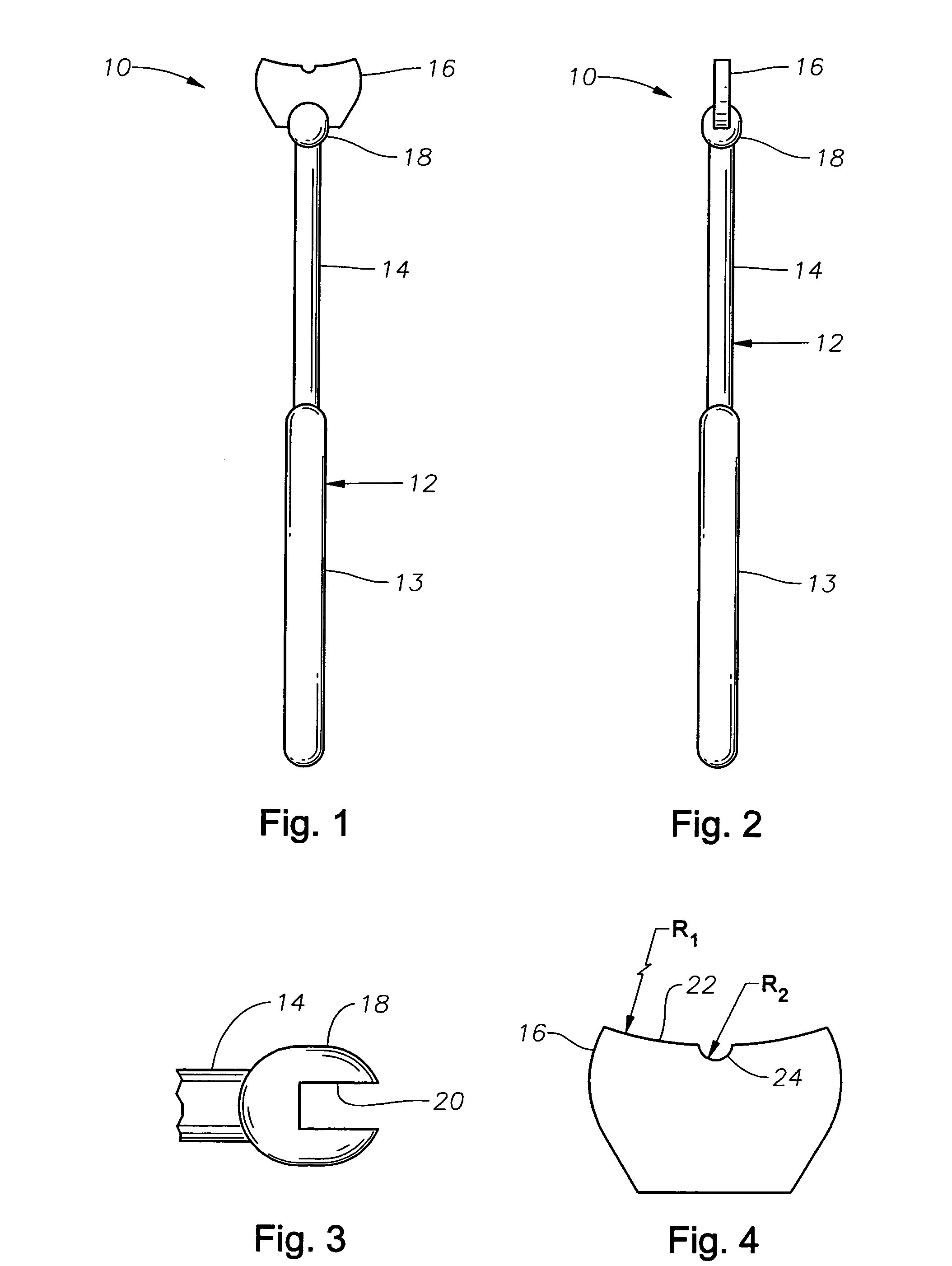
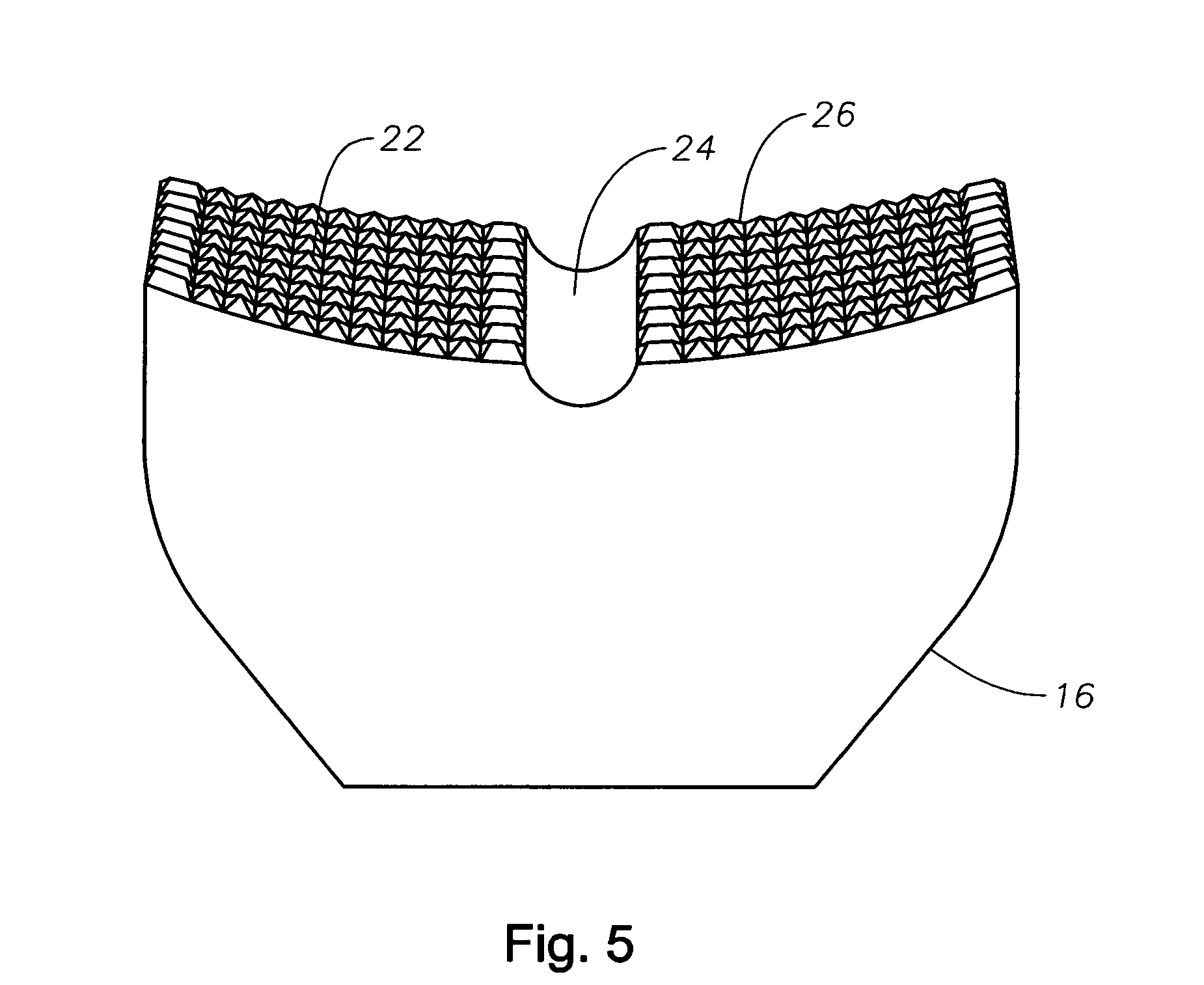
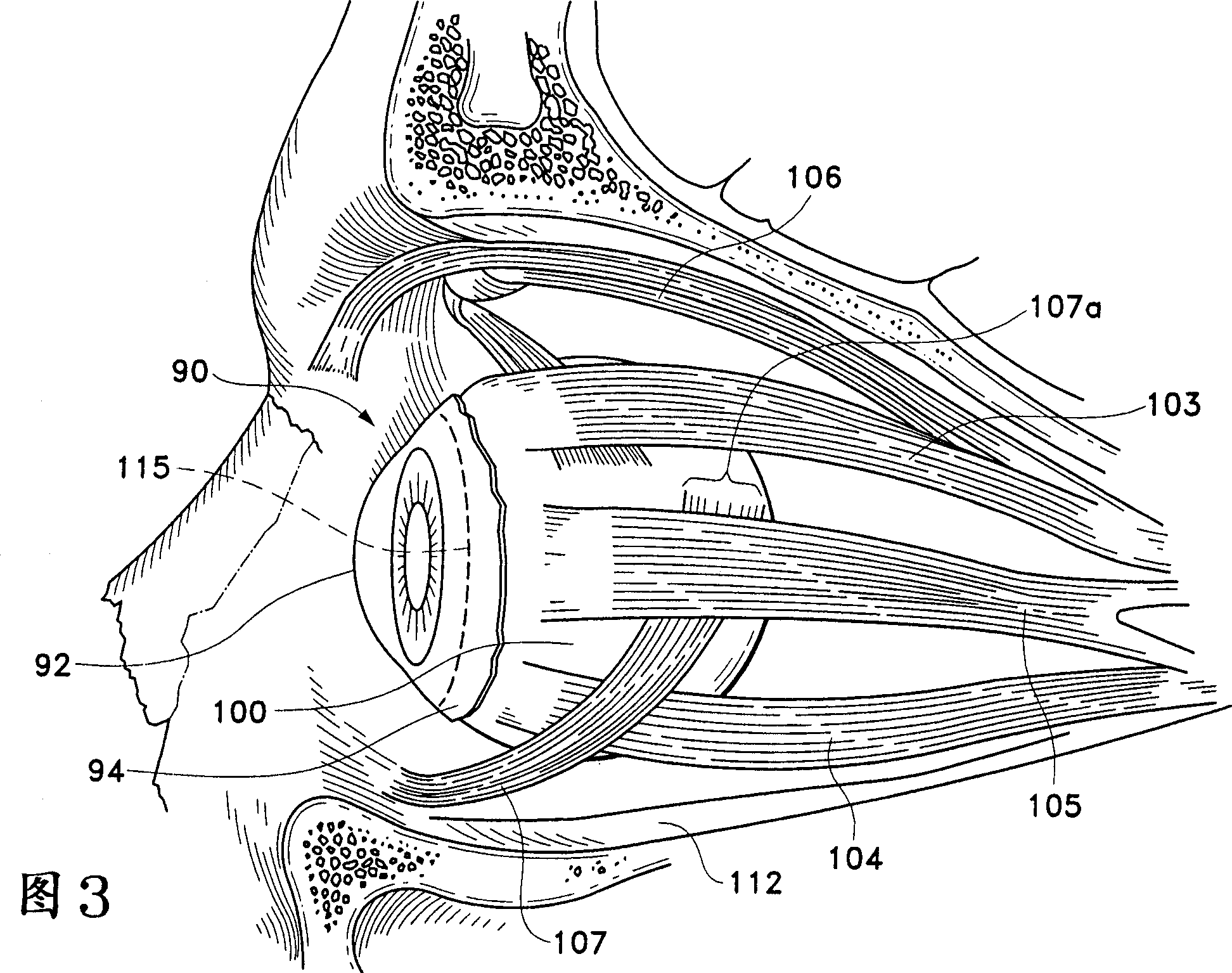
Counter pressure device for ophthalmic drug delivery
InactiveUS7402156B2Facilitates drug placementPreventing and minimizingSurgical instrument detailsMedical applicatorsConjunctivaReflux
A counter pressure device for ophthalmic drug delivery. The device includes a handle and a head coupled to a distal end of the handle. The head comprises a curved, concave surface for contacting the conjunctiva and having a notch for removably receiving a cannula. The device minimizes or prevents drug reflux and facilitates drug placement during ophthalmic drug delivery.
Owner:NOVARTIS AG
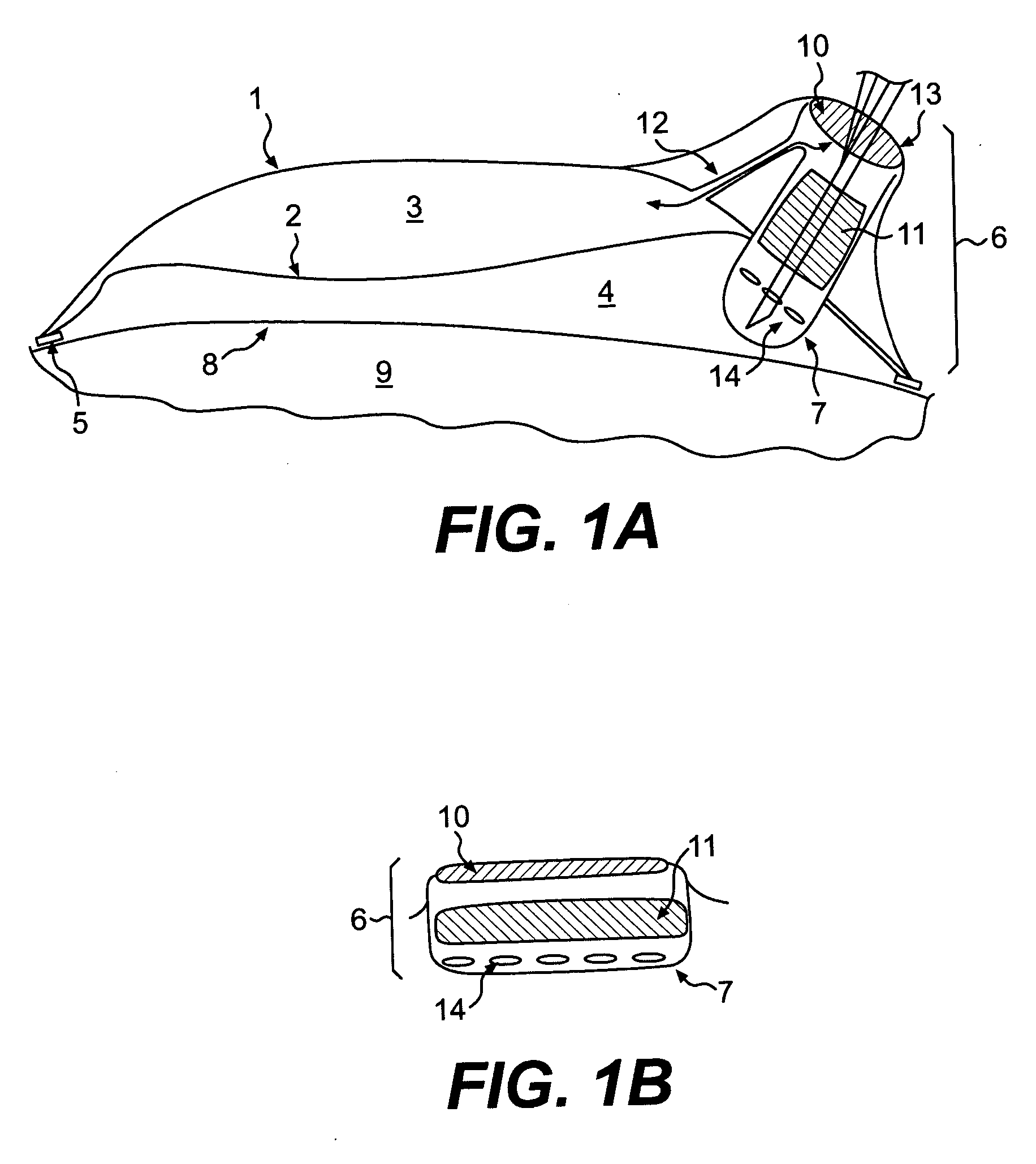
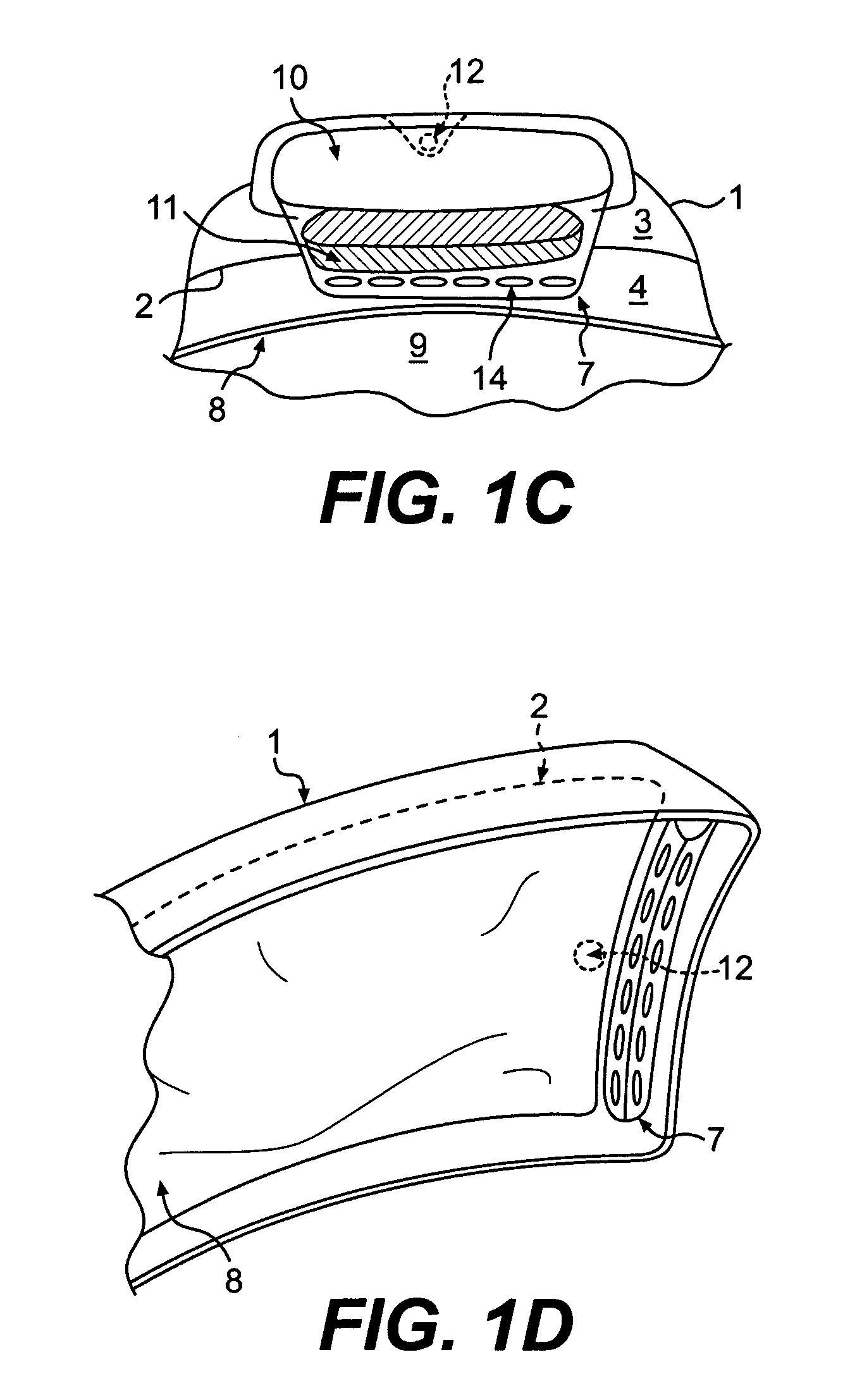
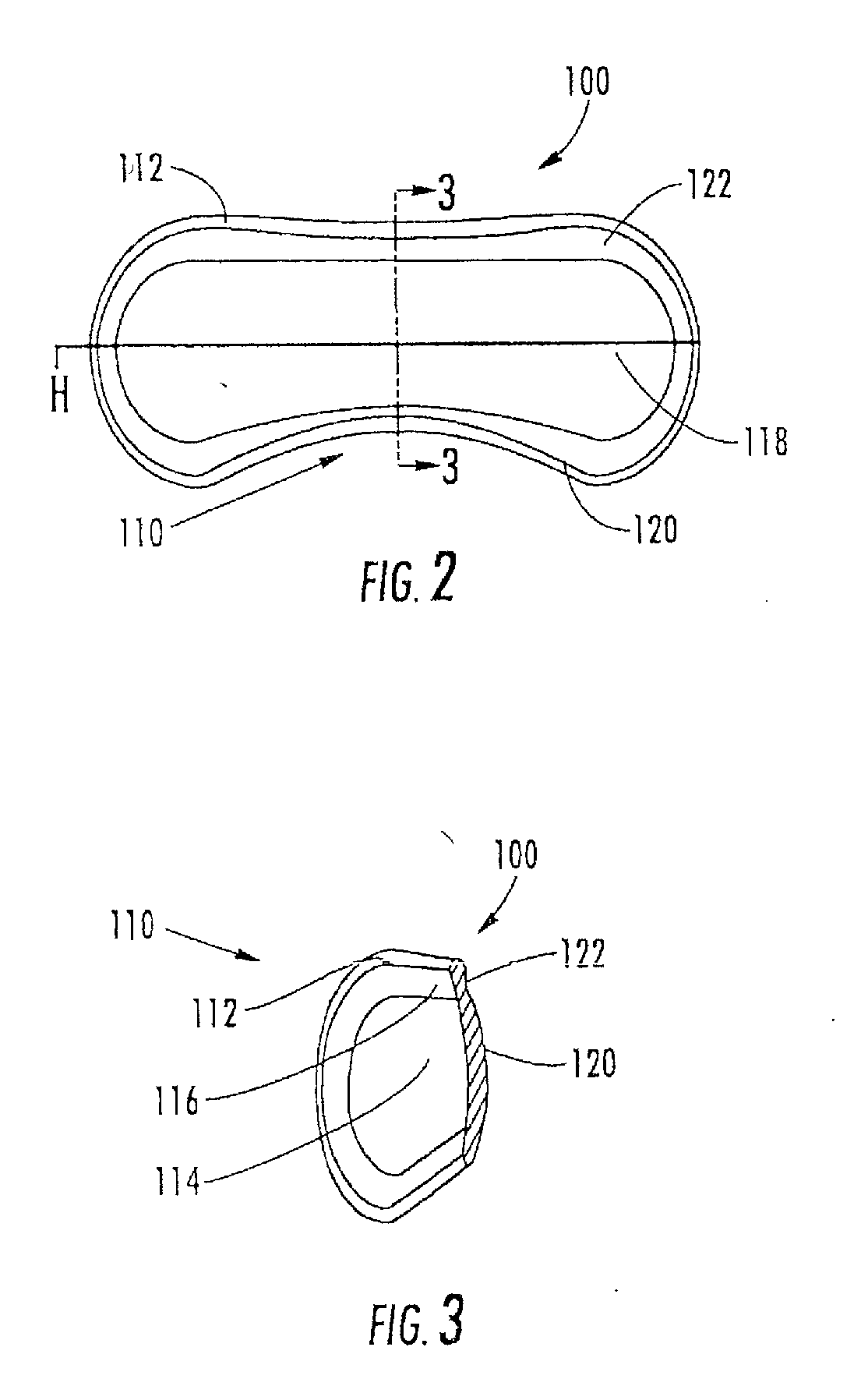
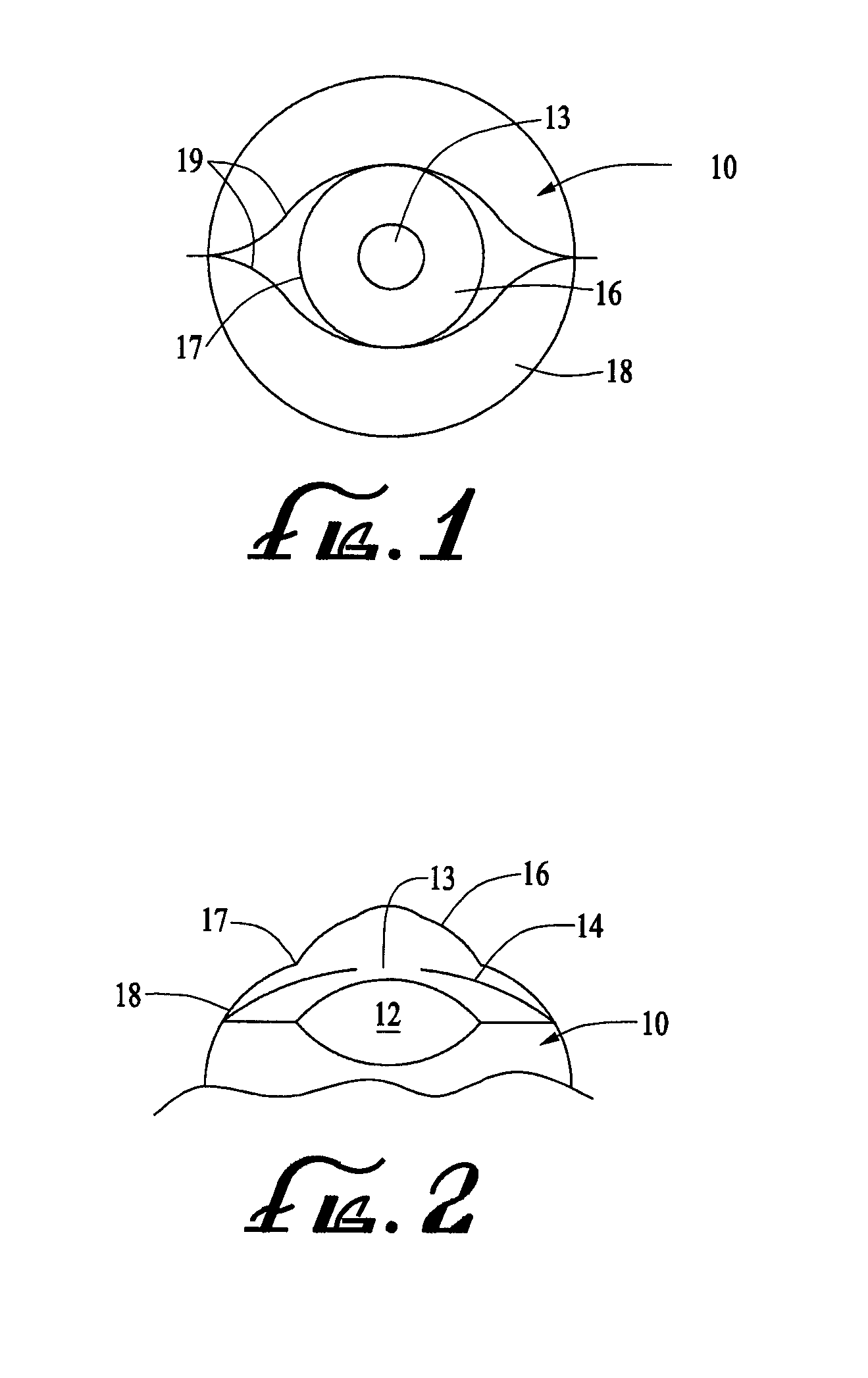
Ring shaped contoured collagen shield for ophthalmic drug delivery
A collagen corneal shield for placement on the outer surface of the eye for protection of the eye, for delivery of drugs to the eye, or for both protection and drug delivery to the eye, functions without obstructing vision. The shield has a first, central portion comprising non-crosslinked collagen capable of rapid decomposition in the presence of enzymes in the tears, and a second portion comprising crosslinked collagen which decomposes slowly in the presence of enzymes in the tears so that it remains on the eye for a period of time longer than the first portion. The first portion of the shield covers the central portion of the cornea and the second portion of the shield extends outward from the first portion. As a result of decomposition of the first portion the corneal shield becomes a ring or washer shape around the central portion and at least a portion of the cornea is no longer covered by the shield so that vision is not obstructed. The corneal shield can also be contoured so that it has one or more base curve radii selected to provide retention of the shield on the eye.
Owner:OASIS RES
Ophthalmic Drug Dispensing Tip
InactiveUS20080039807A1Increase in sizeSave both the patient and the healthcare system moneyMedical applicatorsEye treatmentOphthalmic drugBottle
A dispensing tip apparatus for an eye drop dispenser to administer topical ophthalmic solutions is described. The apparatus integrates an ophthalmic solution-dispensing tip with an optical gauging assembly. The tip provides continuous visual feedback about it orientation and relationship to the eye. The dispensing tip when attached to any standard topical ophthalmic solution dispensing bottle or reservoir enables the user to view a target, visually align the dispenser tip, and administer an eye drop with precision. There is also a visual feedback by which the dispenser tip is prevented from gaining too close proximity and contacting the eye, thus preventing contamination of the medication and its dispenser. The visual feedback can also contain textual or graphic information that serves as a promotional advertisement.
Owner:PINE JERROLD SCOTT
Self-emulsifying drug delivery (SEDDS) for ophthalmic drug delivery
InactiveUS20180036233A1Easy to prepareFew stepsAntibacterial agentsOrganic active ingredientsNano sizeEmulsion
Provided herein are topical ophthalmic preparations which comprise a non-aqueous, self-emulsifying system which can spontaneously give rise to either nanosized emulsions upon contact with an aqueous phase. Also provided herein are methods for the preparation of the same and their use in formulating and delivering poorly water soluble drugs.
Owner:ALLERGAN INC
Arificial tears containing trehalose as well as preparation and application method thereof
InactiveCN101181279AEffective treatmentStable physical and chemical propertiesOrganic active ingredientsSenses disorderDiseaseOphthalmic drug
The invention relates to artificial tear containing trehalose and the preparation and application method, which pertains to the technical field of pharmaceutical manufacture. The main component of the invention is the trehalose, the basic composition of the artificial tear is the trehalose, simulating mucin protein and conventional ophthalmic pharmaceutical excipients; the weight ratio of the three is that 0.3 to 97 percent trehalose, 0.3 to 97 percent simulating mucin protein and 2 to 98 percent conventional ophthalmic pharmaceutical excipients. The pH and the osmotic pressure of the artificial tear of the invention are equivalent to physiologic tear, the invention contains rich electrolyte ions, the type and the content of the ions are equivalent to the physiologic tear, and the invention can be used in the treatment of dry eye syndrome and the adjuvant treatment of diseases which are caused by other dry eye syndromes. At the same time, the invention can be used as the drug delivery system of various ophthalmic drugs.
Owner:黑龙江海昌生物技术有限公司 +1
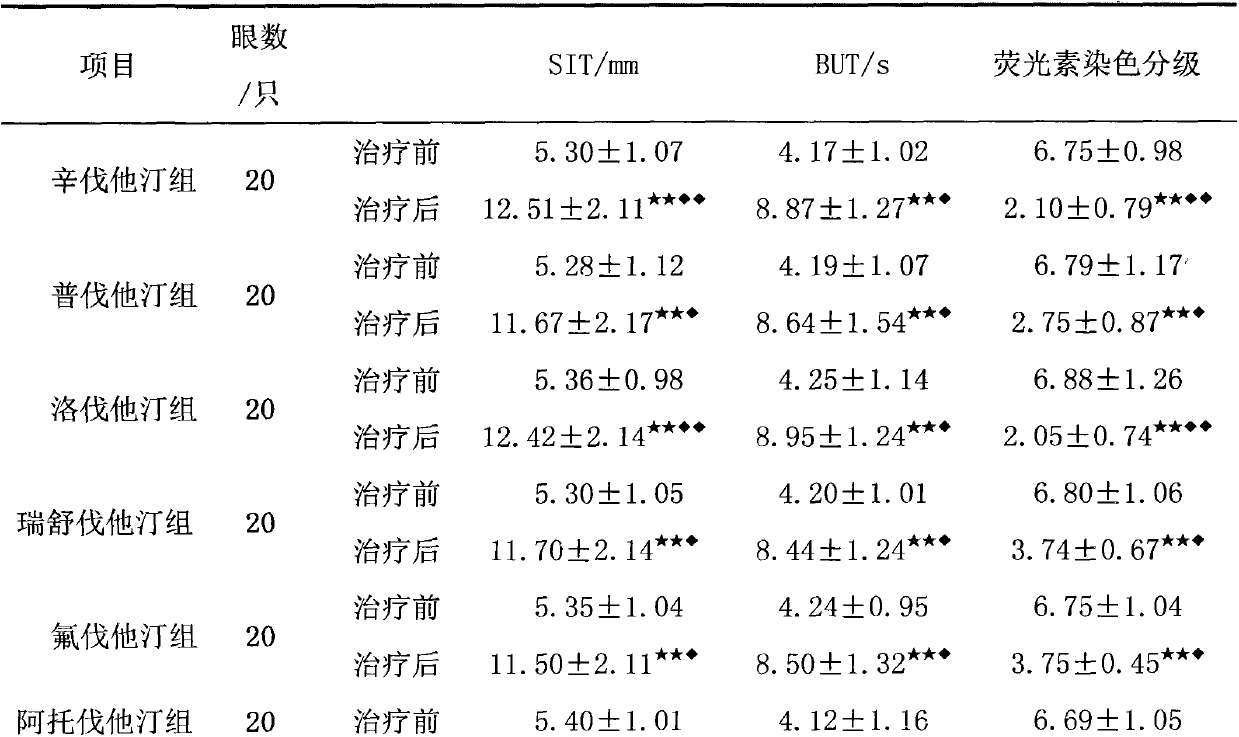
Application of HMGCoA reductase inhibitor in preparation of medicine used for treating xerophthalmia
InactiveCN103007284AImprove permeabilityPromote secretionSenses disorderEster active ingredientsSide effectOphthalmic drug
The invention relates to an application of an HMGCoA reductase inhibitor in preparation of a medicine used for treating xerophthalmia and belongs to the field of medicines. The inventor finds that a frequently-used lipid regulation medicine, the HMGCoA reductase inhibitor, can obviously prolong SIT time and BUT time and obviously reduce an FLS score when being used for treating the xerophthalmia, and the HMGCoA reductase inhibitor is better than ciclosporin or artificial tears in the aspect of improving the indexes, wherein simvastatin and lovastatin have the best treatment effect. The HMGCoA reductase inhibitor also can be prepared into oral preparation used for treatment or adjuvant therapy of the xerophthalmia. The HMGCoA reductase inhibitor has a definite curative effect and less toxic and side effects, compliance of a patient is high, and clinical ophthalmic drugs are further enriched.
Owner:闫莹
Ophthalmic drug delivery system containing phospholipid and cholesterol
An ophthalmic drug delivery system that contains phospholipid and cholesterol for prolonging drug lifetime in the eyes.
Owner:TLC PHARMA INC +1
Gel useful for the delivery of ophthalmic drugs
InactiveCN101534794AKeep stickingExtended stayPowder deliveryOrganic active ingredientsDiseaseMedicine
It is described a gel composed of a mixture of a polymer which forms a gel, a buffer; a saccharide and one or more active ingredients useful for treating diseases of the eyes.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Ophthalmic drug combination and preparation and application methods thereof
The invention relates to an ophthalmic drug combination and preparation and application methods thereof. The ophthalmic drug combination comprises N-(2)-L-alanyl-L-glutamine which is suspended or dissolved inside eye-acceptable isotonic solution. The ophthalmic drug combination is low in irritation, good in stability and high in safety.
Owner:SHENGYUAN PHARMA GUANGZHOU LTD
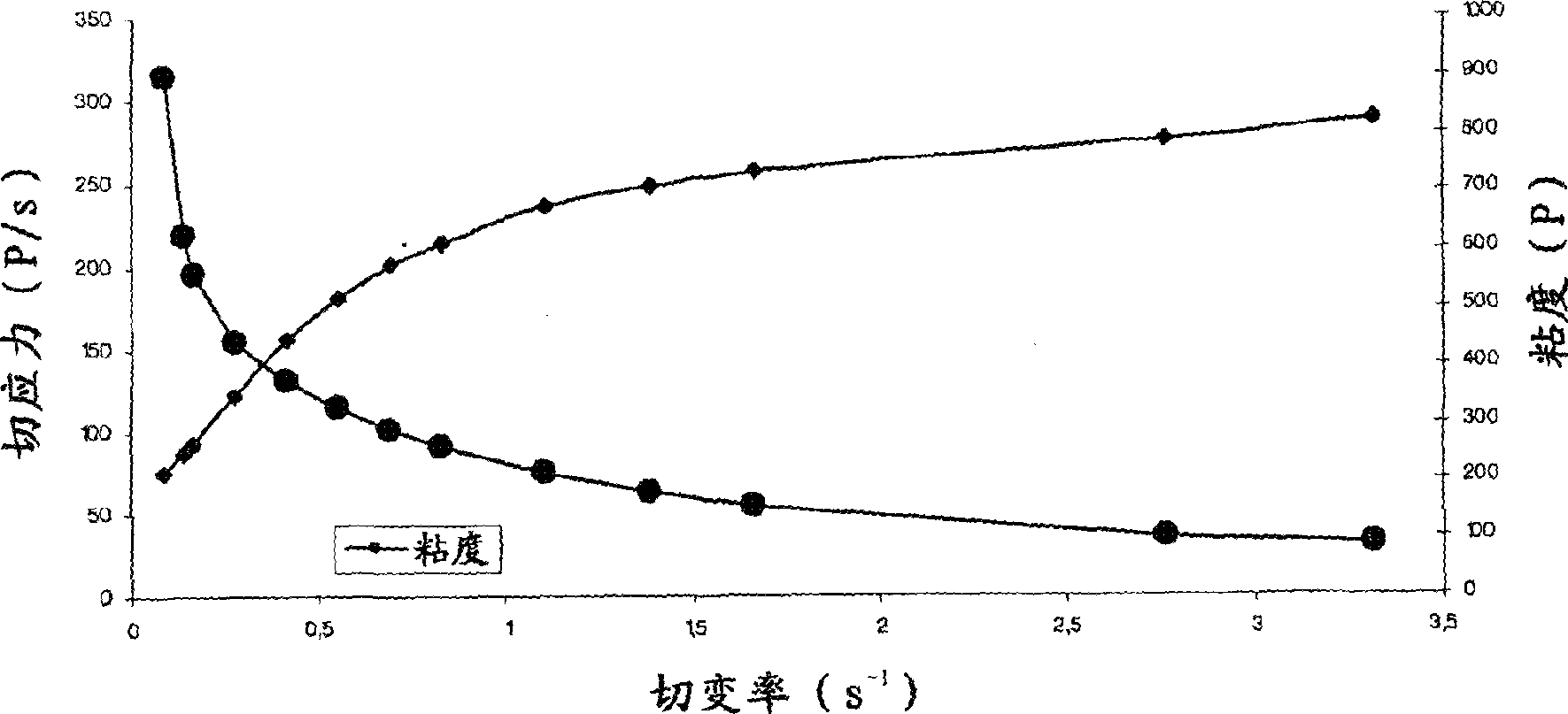
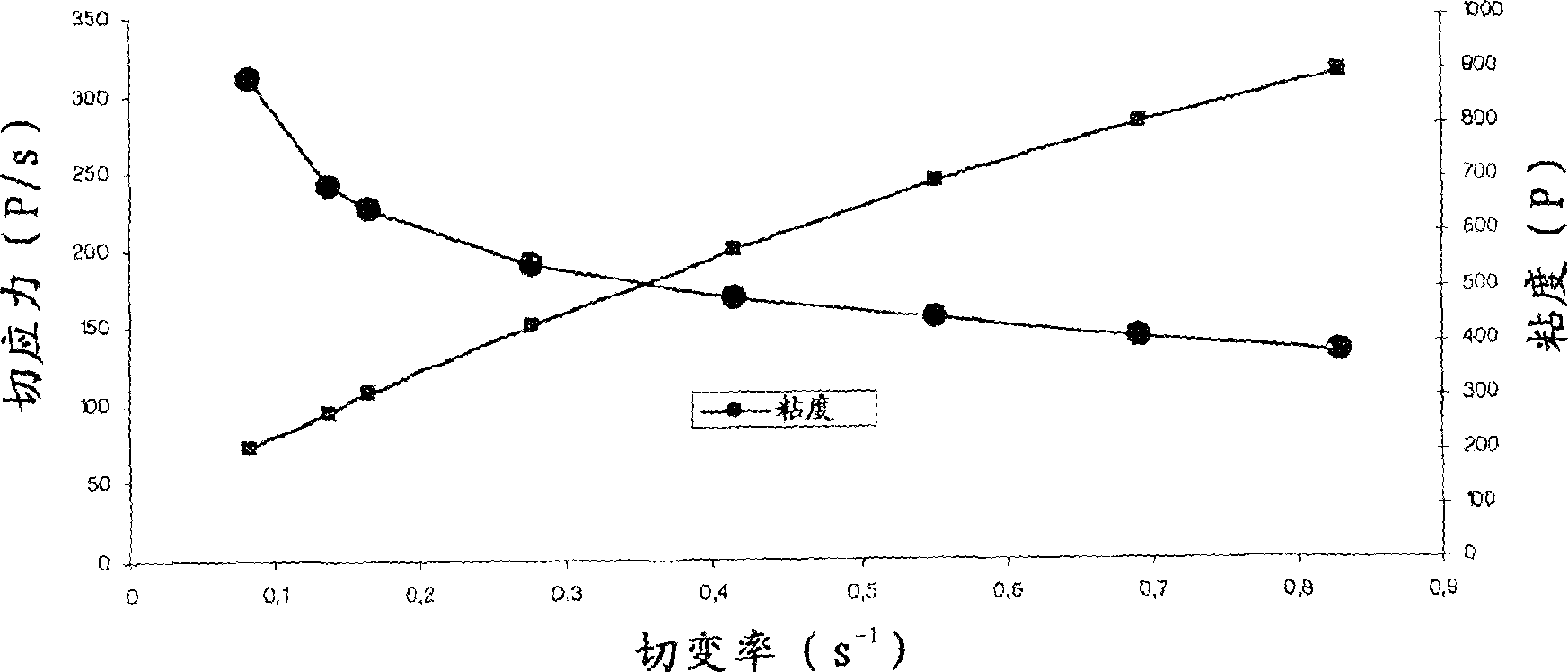
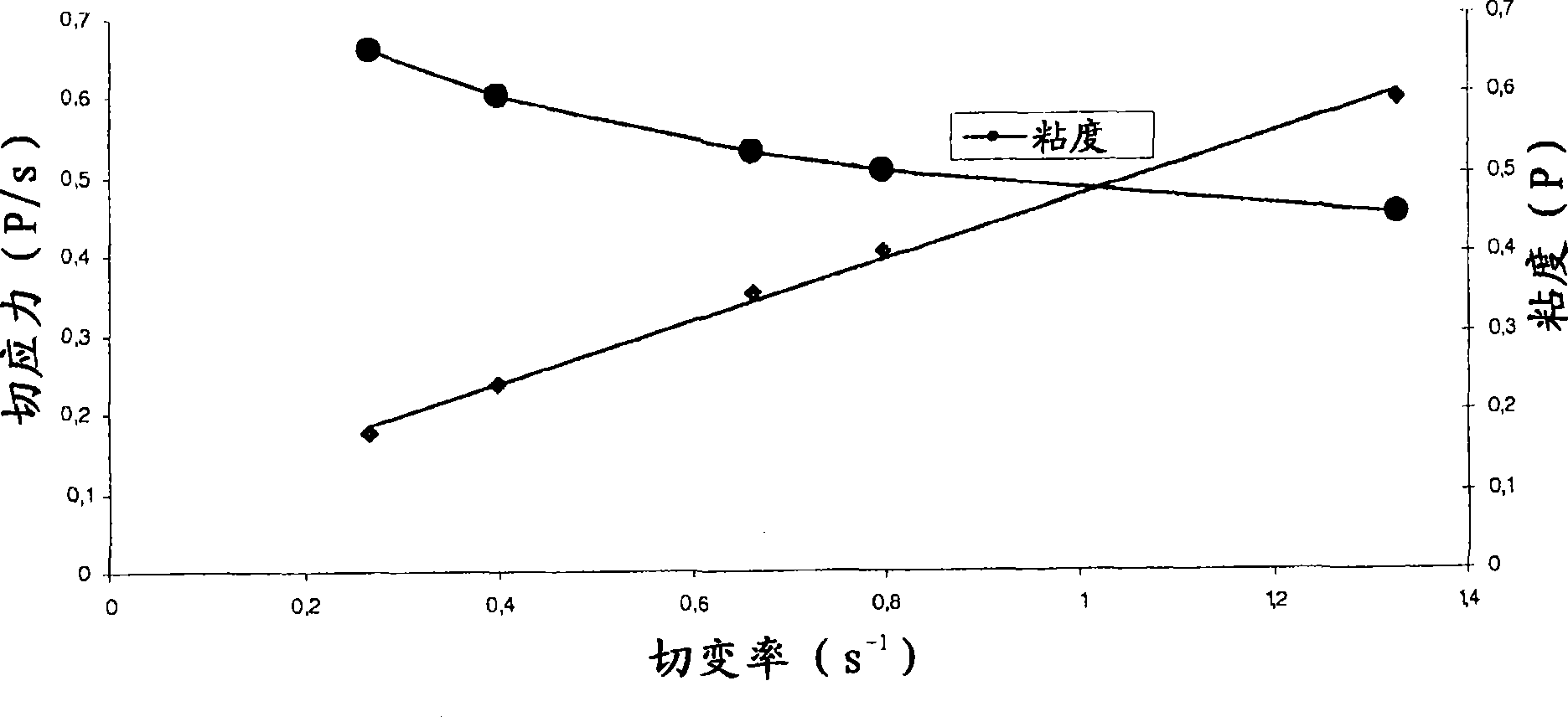
Ophthalmic Lipophilic Drug Delivery Vehicle Formulations
ActiveUS20140378391A1Increase shear forcePhase-low viscosityBiocideInorganic non-active ingredientsSolubilitySide effect
The ophthalmic drug delivery vehicles provide comfort and compliance; drug solubility, residence time and permeability; and reduce side effects. In addition, the delivery vehicle can be slightly modified to provide an artificial tear formulation.
Owner:PS THERAPIES LTD +1
Non-aqueous oil delivery system for ophthalmic drugs
InactiveUS20100323978A1Comfortable to useImprove effectivenessBiocideSenses disorderWhite petrolatumVegetable oil
The present invention relates to a delivery system for ophthalmic drugs, and more particularly, to a non aqueous oil delivery system. Low concentrations of ophthalmic drugs suspended in an oil vehicle delivery system are as therapeutically effective in man and animals as the corresponding higher concentrations of ophthalmic drugs that are commercially used in aqueous solutions. Eye drops that utilize this nonaqueous oil delivery system, when used in man, are comfortable to use and produce little ocular irritation, have a longer shelf-life, low systemic toxic potential, and only short term blurring of vision. Using this nonaqueous oil delivery system, a single drop of ophthalmic drug with a concentration that is 10 times less than the same drug used in commercially available aqueous eye drops is as effective as the commercially available aqueous ophthalmic eye drops that require many applications to be effective. In addition, utilizing the nonaqueous oil delivery system as eye drops produces no ocular sensation of burning, stinging or excessive tearing that is commonly associated with the commercially available aqueous eye drops. This very dilute ophthalmic drug preparation has a greatly reduced systemic toxicity potential as compared to the commercially used aqueous ophthalmic drops. The vehicle includes castor oil, corn oil, glycerol, mineral oil USP, vegetable oil, white petrolatum USP, and mixtures, there of. The ophthalmic class of drugs includes antimicrobials, miotics, mydriatics, mydriatic-cycloplegics, mydriatic-cycloplegic reversal agents and topical anesthetics.
Owner:HANNA CALVIN
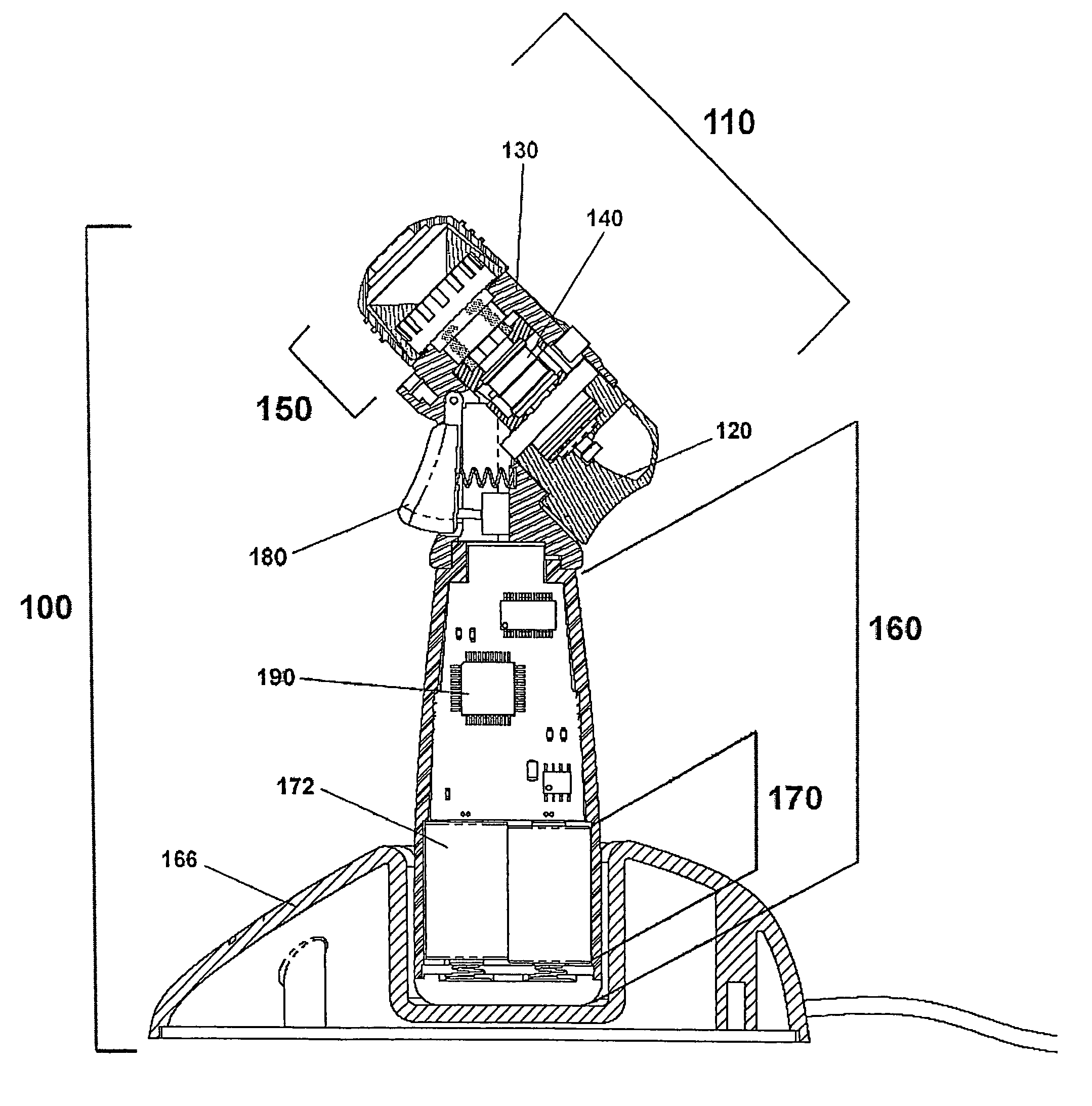
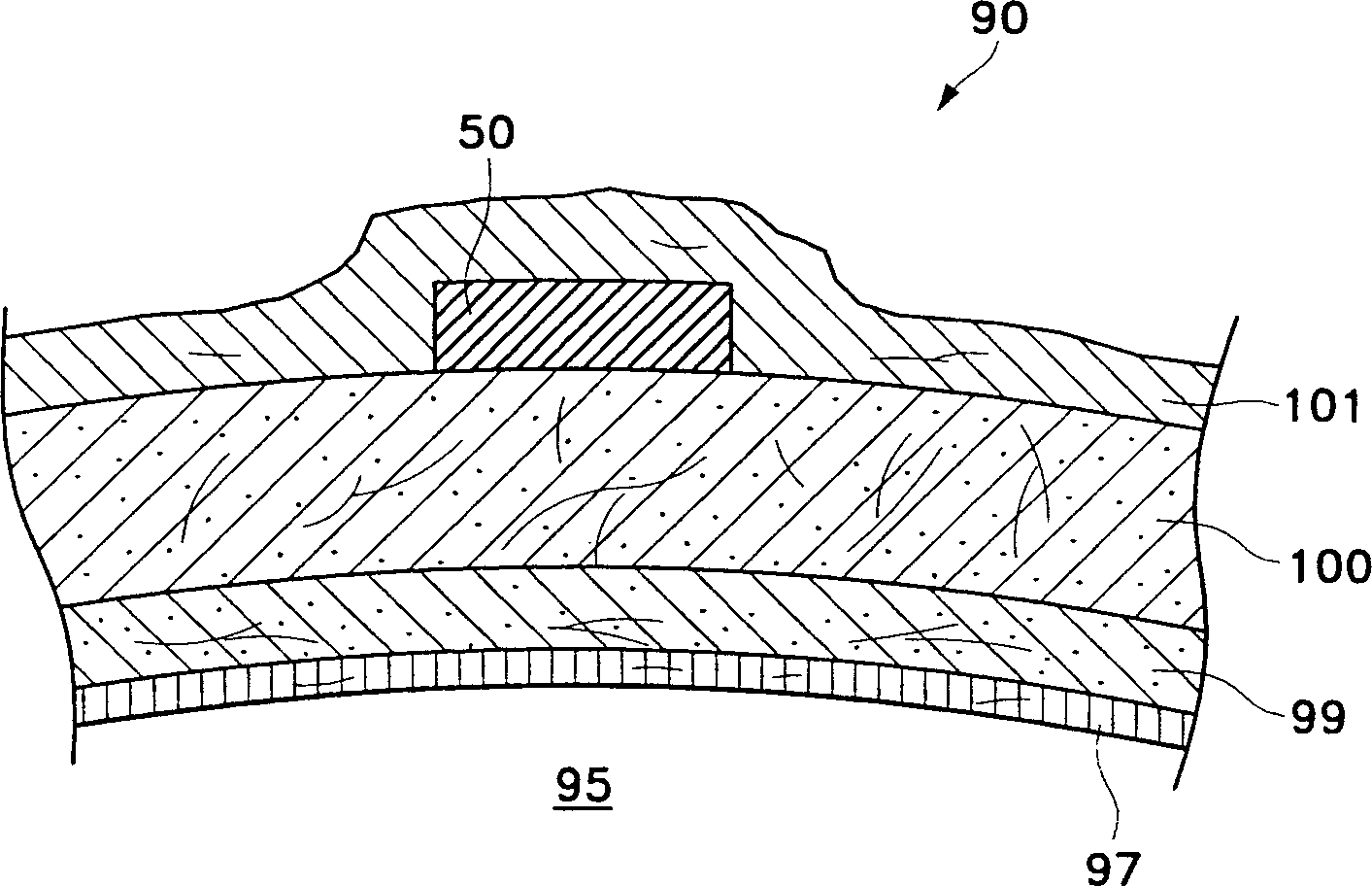
Ophthalmic drug delivery device
The present invention relates to a drug release device for the human eye. The human eye has a sclera, an inferior oblique muscle, and a macula. The device of the present invention includes a pharmaceutically active agent, and a geometry that facilitates implanting the device on an outer surface of the sclera, below the inferior oblique muscle, and deploying the pharmaceutically active agent to the macula above. Also disclosed is a method of delivering a pharmaceutically active agent to the back of the human eye.
Owner:ALCON INC
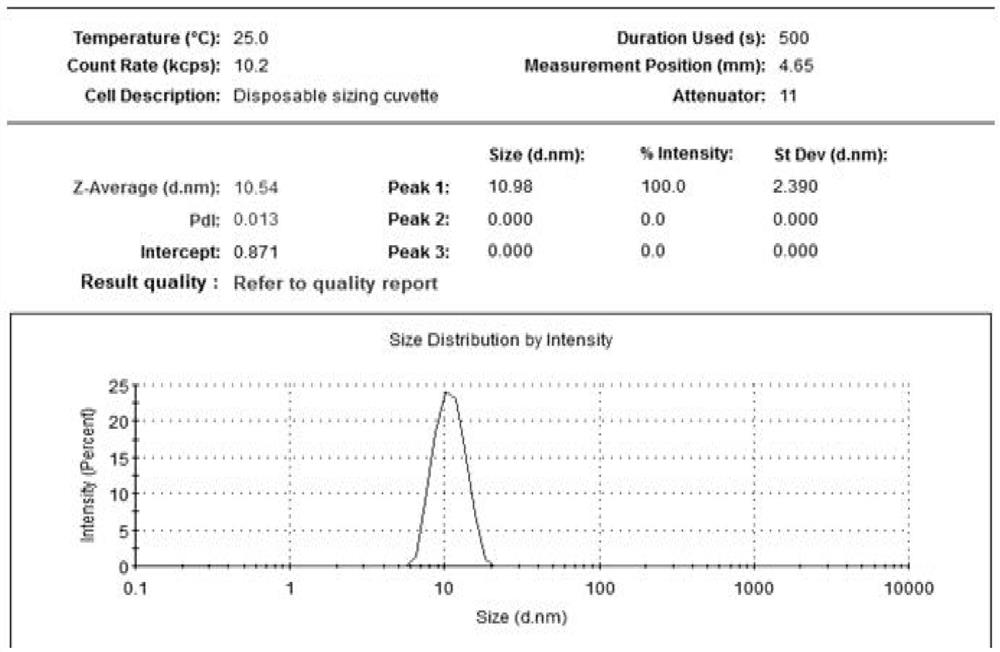
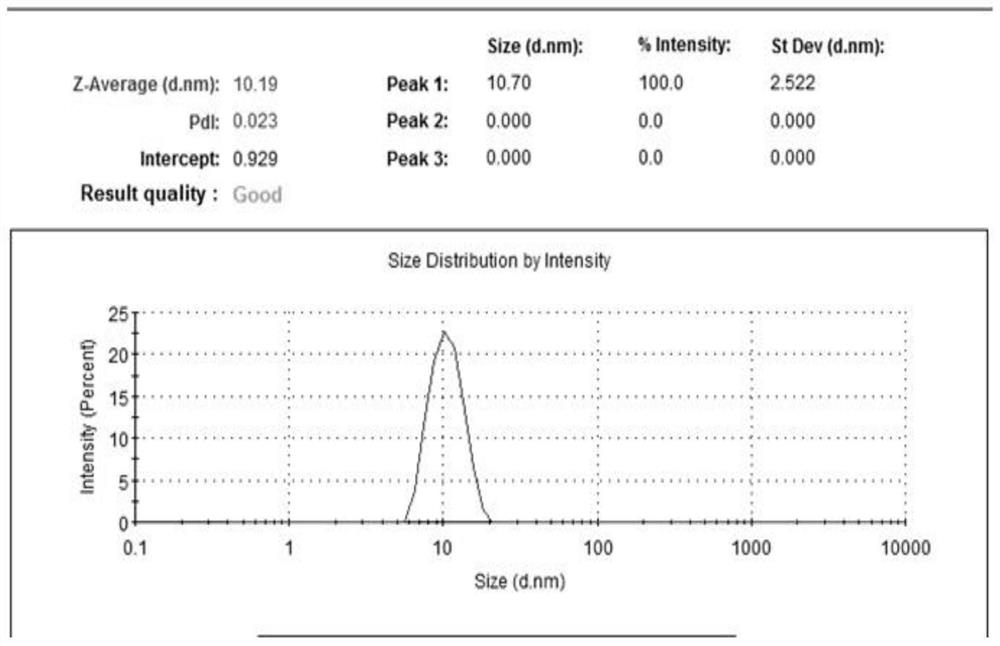
In-situ gel containing cyclosporin micelle as sustained-release ophthalmic drug delivery system
PendingCN112516084AImproved membrane transportImprove stabilitySenses disorderAntipyreticOphthalmic drugOphthalmology
The invention provides an in-situ gel containing cyclosporin micelles as a sustained release ophthalmic drug delivery system. The in-situ gel contains 0.01 wt% to 5 wt% of an aqueous ophthalmic formulation of cyclosporin present in the form of a micelle with the particle size of no greater than 20 nm.
Owner:IVEW THERAPEUTICS (ZHUHAI) CO LTD
Preparation and application of novel antithrombotic polymer nano-drug
InactiveCN106729748AEasy to makeEasy to operateNanomedicineKetone active ingredientsSolubilityOphthalmic drug
The invention relates to a novel antithrombotic polymer nano-drug. The novel antithrombotic polymer nano-drug is prepared by connecting a hydrophobic antithrombotic substance with a hydrophilic antithrombotic substance by means of a simple chemical reaction; the novel antithrombotic polymer nano-drug can be separately used as a polymer nano-drug, and can be also prepared into a polymer nano-drug composition by being physically coated with other pharmaceutically active or pharmacologically active molecules so as to improve the antithrombotic activity of the pharmaceutically active or pharmacologically active molecules. The antithrombotic polymer nano-drug not only improves the water solubility and stability of the hydrophobic antithrombotic substance, prolongs the action time of the hydrophobic antithrombotic substance and increases the concentration of the drug reaching the part with thrombus, but also reduces the bleeding risk of heparin of the hydrophilic antithrombotic substance and derivatives thereof and realizes the cooperative utilization of two different action mechanisms, thus giving play to higher antithrombotic activity. By adding corresponding medical auxiliary materials, the antithrombotic polymer nano-drug and a composition thereof can be prepared into corresponding preparations used for injection, oral administration or ophthalmic drug delivery. The preparation method of the novel antithrombotic polymer nano-drug is simple and lower in cost, thus being suitable for large-scale continuous production.
Owner:CHINA PHARM UNIV
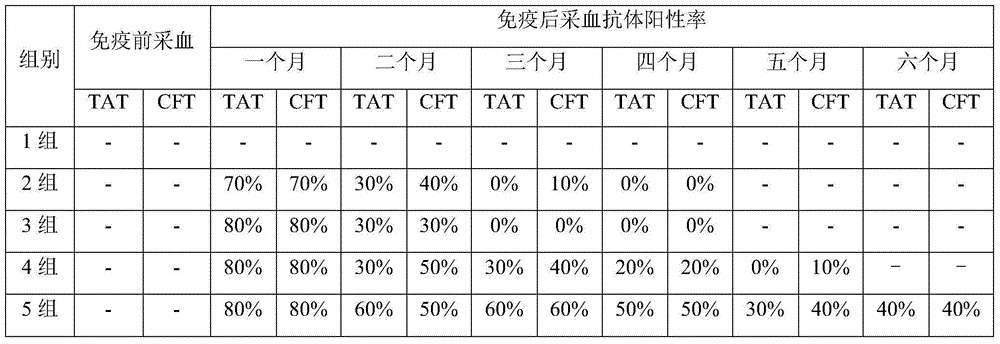
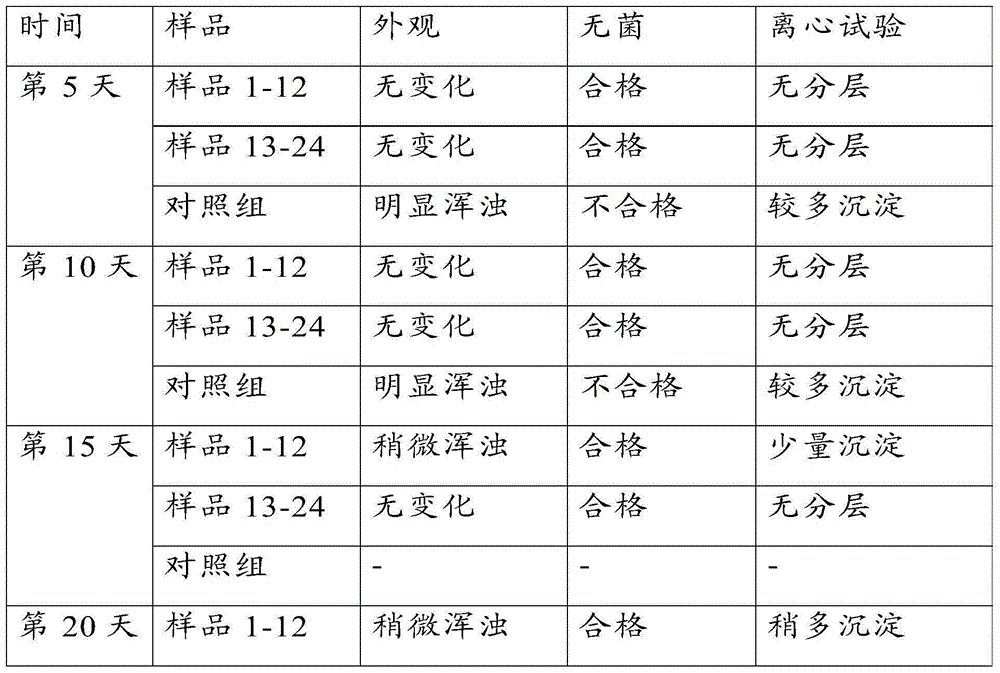
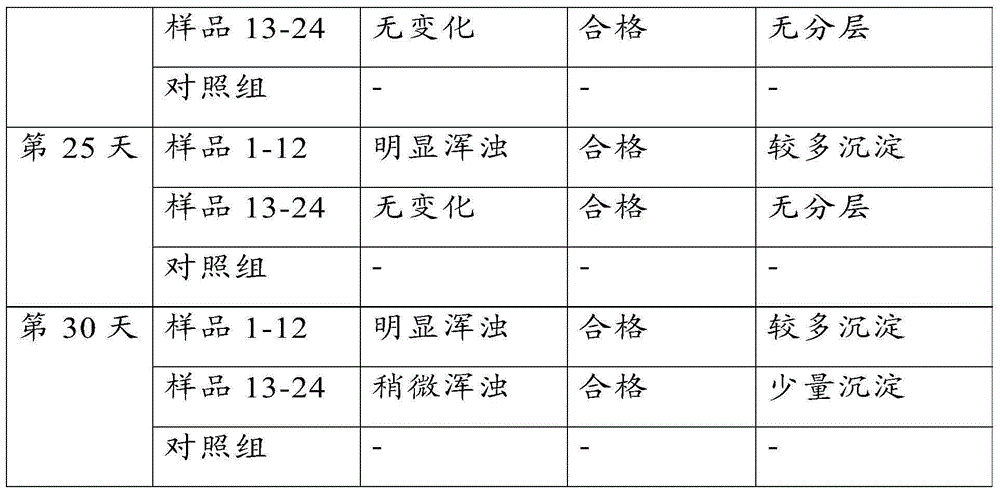
Vaccine for preventing brucellosis by means of ophthalmic drug delivery
InactiveCN105056222ASolve the problem of detoxificationAntibacterial agentsBacterial antigen ingredientsCow milkingHypodermoclysis
The invention relates to a vaccine for preventing brucellosis by means of ophthalmic drug delivery. Through adoption of the vaccine for preventing brucellosis by means of ophthalmic drug delivery, the problems of continuous production of antibodies by immune cows, abortion of pregnant cows and bacterium excretion in adult cow milk due to conventional subcutaneous injection of a bacterium burgeri vaccine are solved.
Owner:QILU ANIMAL HEALTH PROD
Polyvinyl alcohol composition
ActiveCN104983786APromote healingRestore barrierSenses disorderHydroxy compound active ingredientsOphthalmic drugCurative effect
The invention relates to the field of ophthalmic drugs, in particular to a polyvinyl alcohol composition, which is mainly prepared by following raw materials in parts by weight: 8-15 parts of polyvinyl alcohol, 0.1-0.5 part of polyvinylpyrrolidone, 0.5-5 parts of xanthan gum and 0.5-3 parts of nonionic surfactant. According to the polyvinyl alcohol composition, the polyvinyl alcohol, polyvinylpyrrolidone, xanthan gum and nonionic surfactant are specifically selected for match usage, a moisturizing action is enhanced, action time thereof in eyes is prolonged, healing of corneal epithelial cells is better promoted, an epithelial cell barrier is recovered, a treatment effect is improved, a finally obtained preparation is stable in performance and high in use comfortable level, and has obvious treatment effect for dry eyes, eyestrain and the like. In the preparation process, respective raw materials are directly mixed in water, and matched with one another, and the defect that polyvinylpyrrolidone is easily caked is overcome.
Owner:泊诺(天津)创新医药研究有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com