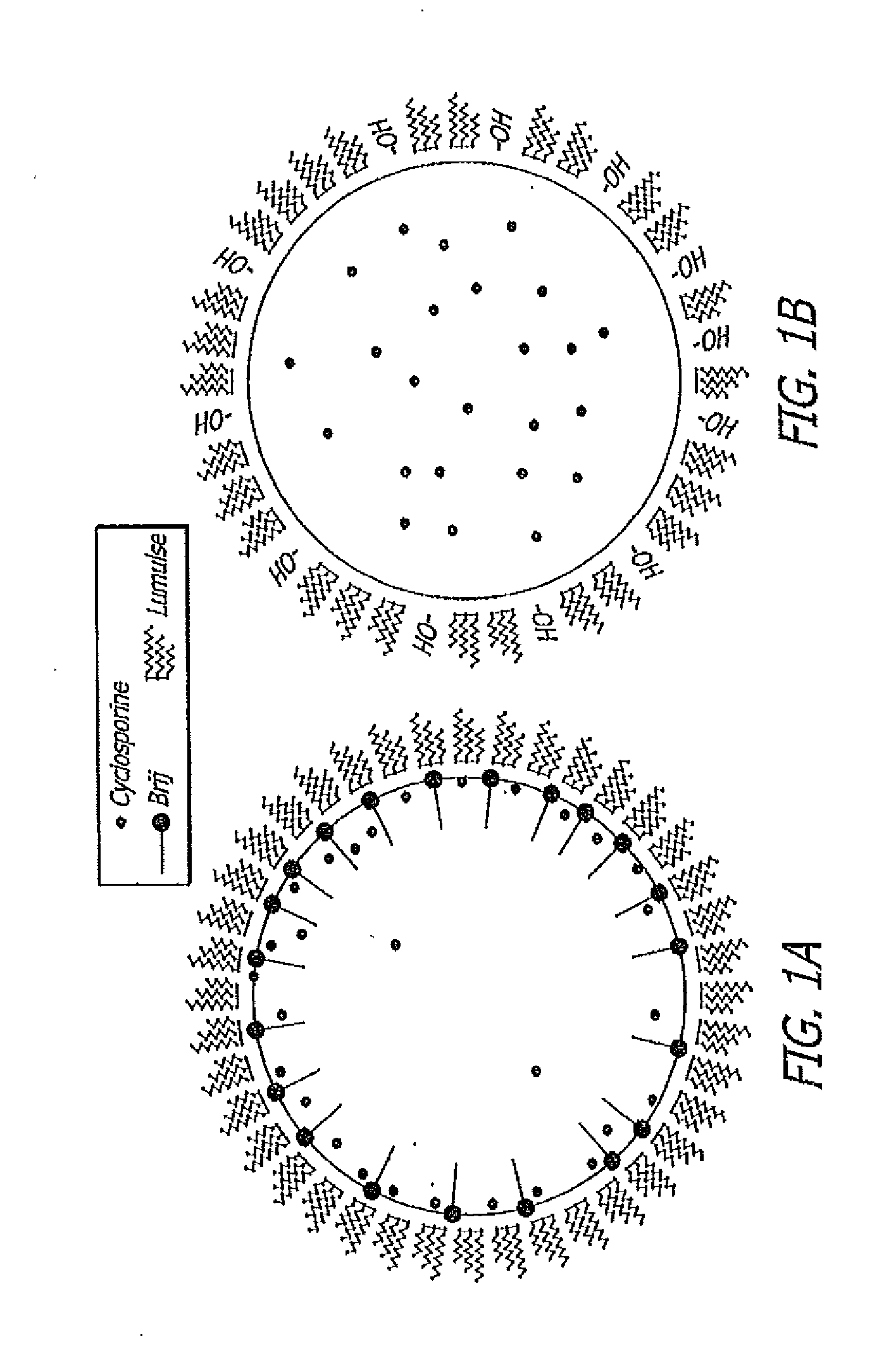
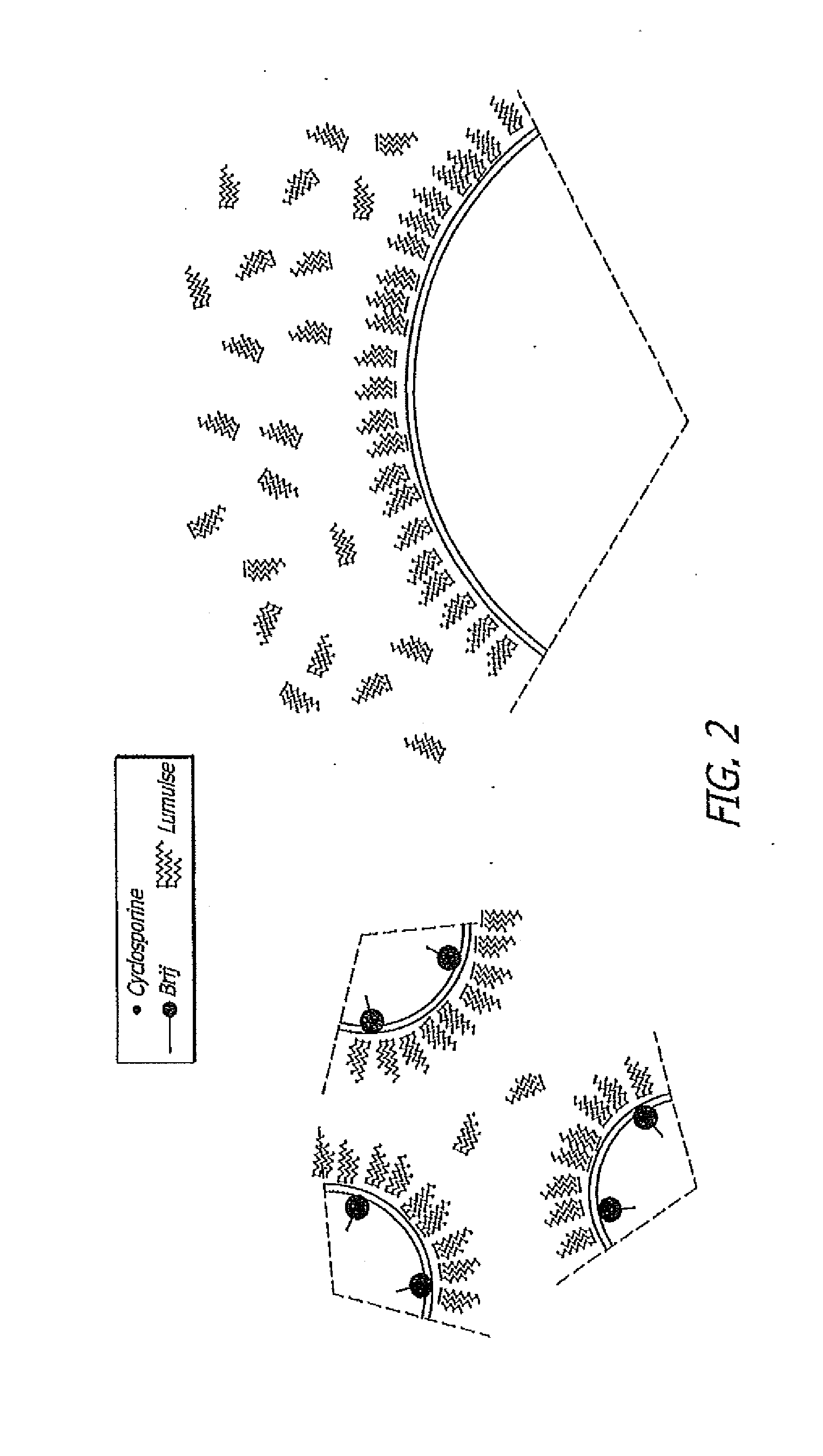
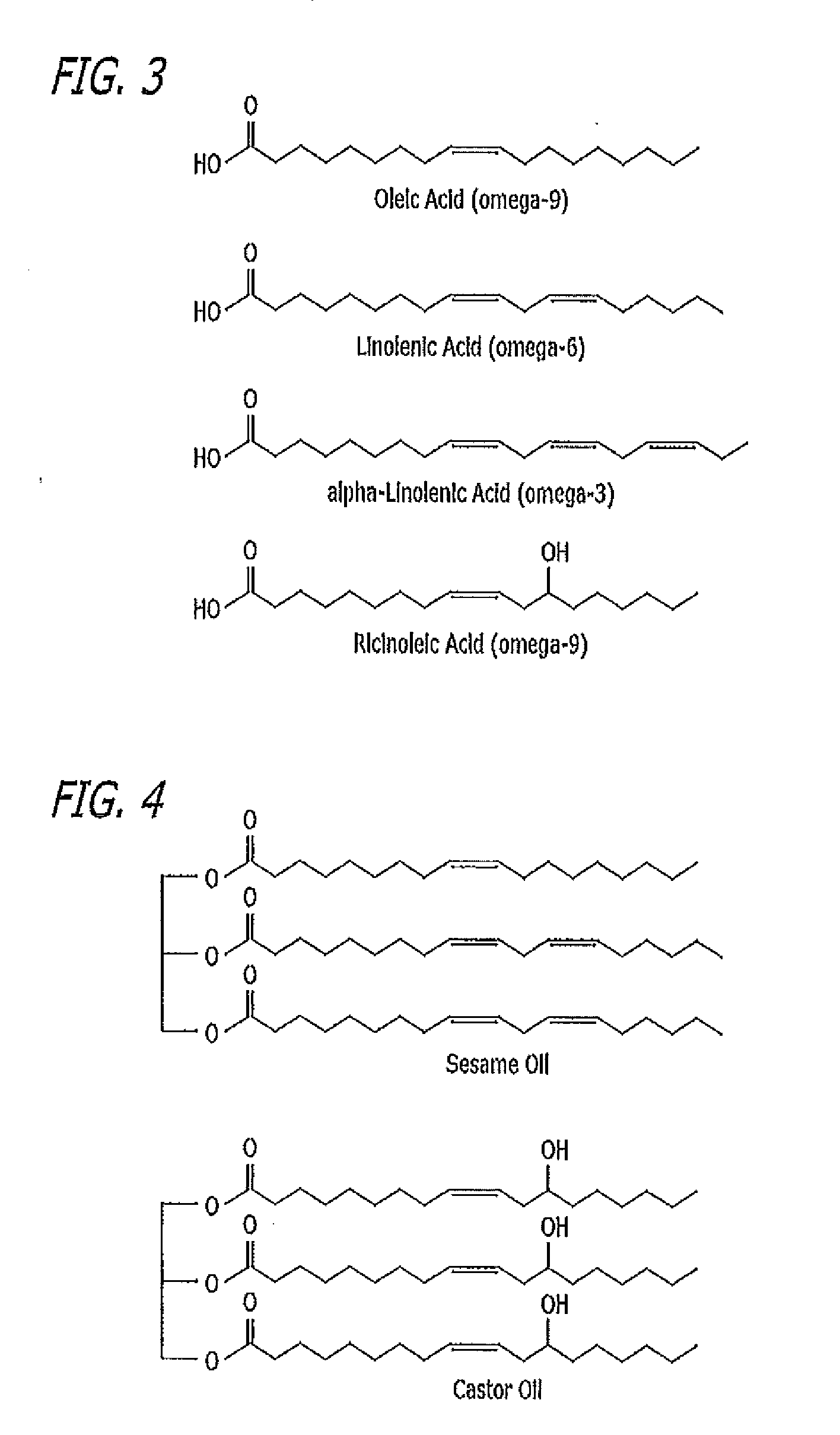
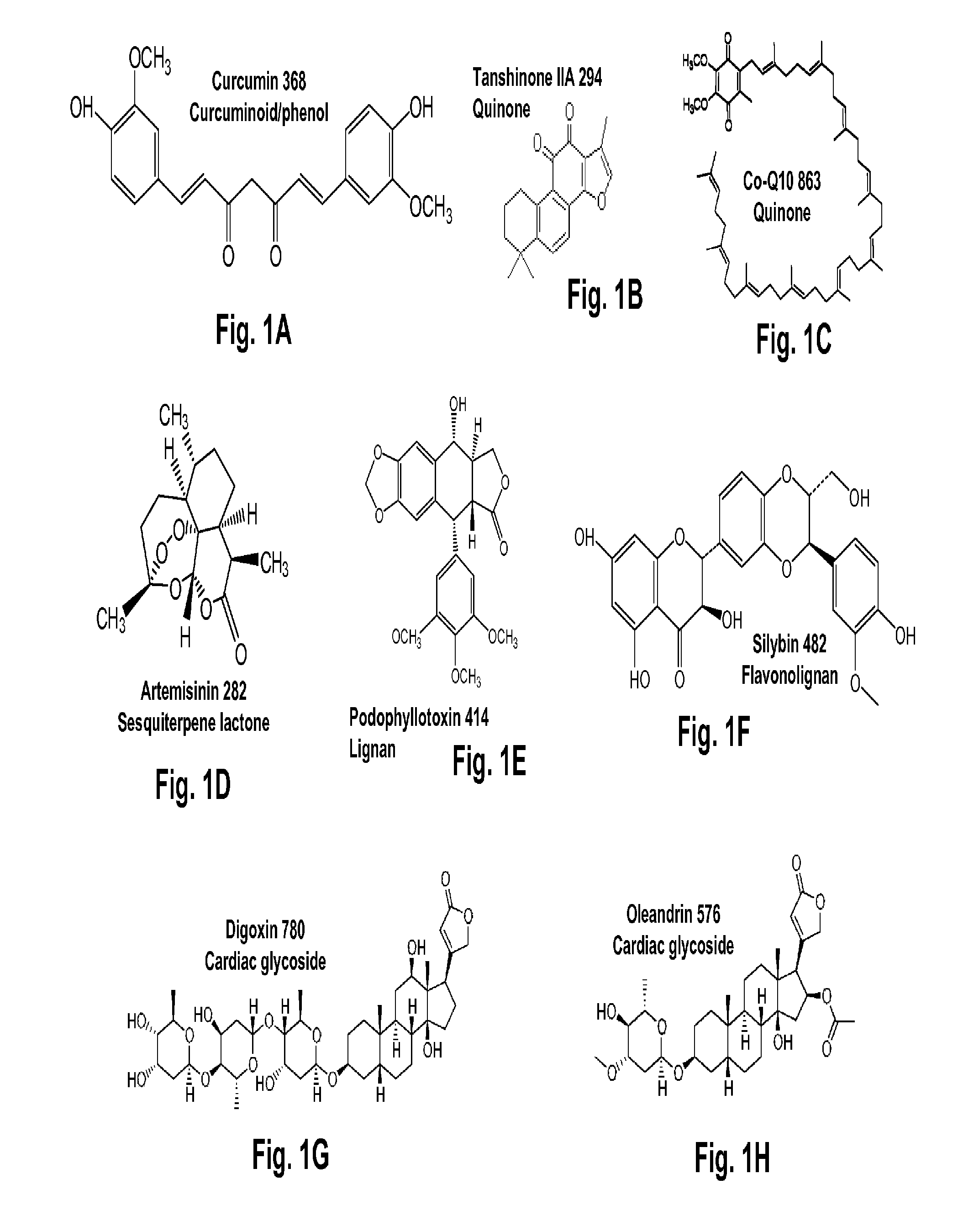
Patents
Literature
115 results about "Ciclosporin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
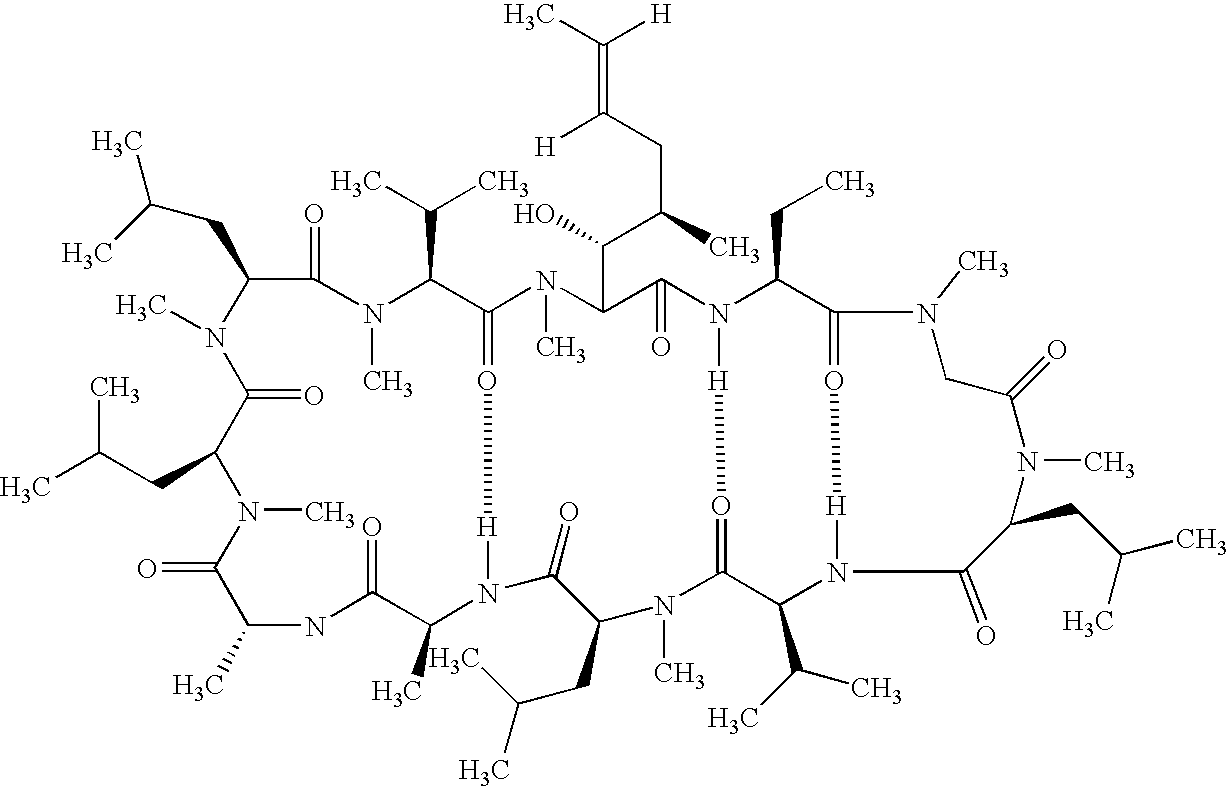
Cyclosporine is used to prevent organ rejection in people who have received a liver, kidney, or heart transplant.
Spontaneous emulsions containing cyclosporine
A pharmaceutical composition contains cyclosporine as the active ingredient. More specifically, the composition is an orally administered pharmaceutical formulation in the form of a spontaneous emulsion comprising cyclosporine, ethanol ethyl oleate and polyoxyethylene glycerol trioleate. A method for preparing an orally administered pharmaceutical composition involves first dissolving cyclosporine in ethanol. Polyoxyethylene glycerol trioleate and an oil component are then added, mixed and diluted in an aqueous media to form a spontaneous emulsion.
Owner:WOCKHARDT EU OPERATIONS SWISS
Diterpene Glycosides as Natural Solubilizers
InactiveUS20110033525A1Improve solubilityRetain activityBiocideHydroxy compound active ingredientsItraconazoleCapsaicin
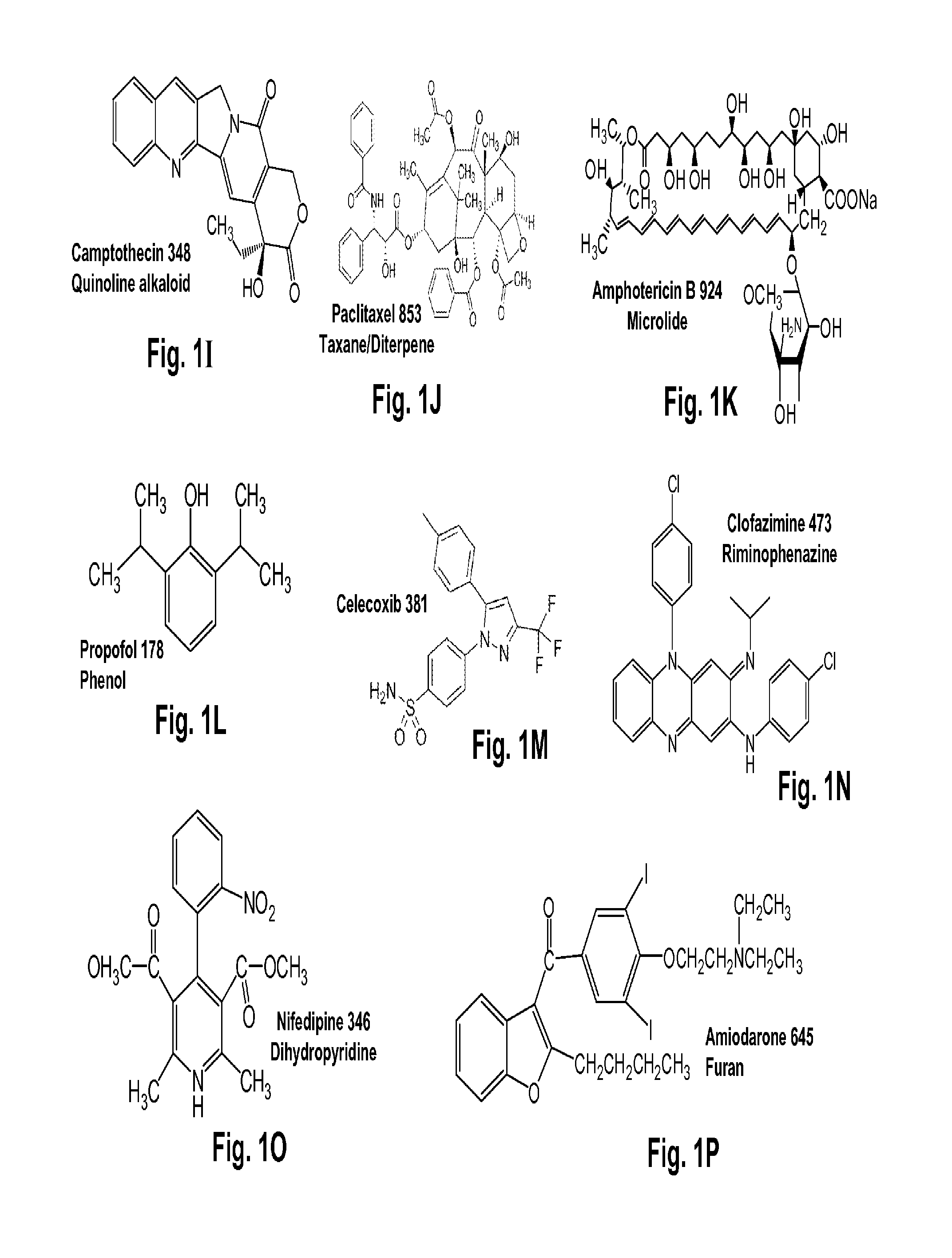
Several diterpene glycosides (e.g., rubusoside, rebaudioside, steviol monoside and stevioside) were discovered to enhance the solubility of a number of pharmaceutically and medicinally important compounds, including but not limited to, paclitaxel, camptothecin, curcumin, tanshinone HA, capsaicin, cyclosporine, erythromycin, nystatin, itraconazole, and celecoxib. The use of the diterpene glycoside rubusoside increased solubility in all tested compounds. The diterpene glycosides are a naturally occurring class of water solubility-enhancing compounds that are non-toxic and that will be useful as new complexing agents or excipients in the pharmaceutical, agricultural (e.g., solubilizing pesticides), cosmetic and food industries. Aqueous solutions by using rubusoside to increase the solubility of otherwise insoluble drugs will have several new routes of administration. In addition, aqueous solutions of therapeutic compounds with rubusoside were shown to retain the known pharmacological activity of the compounds.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Composition
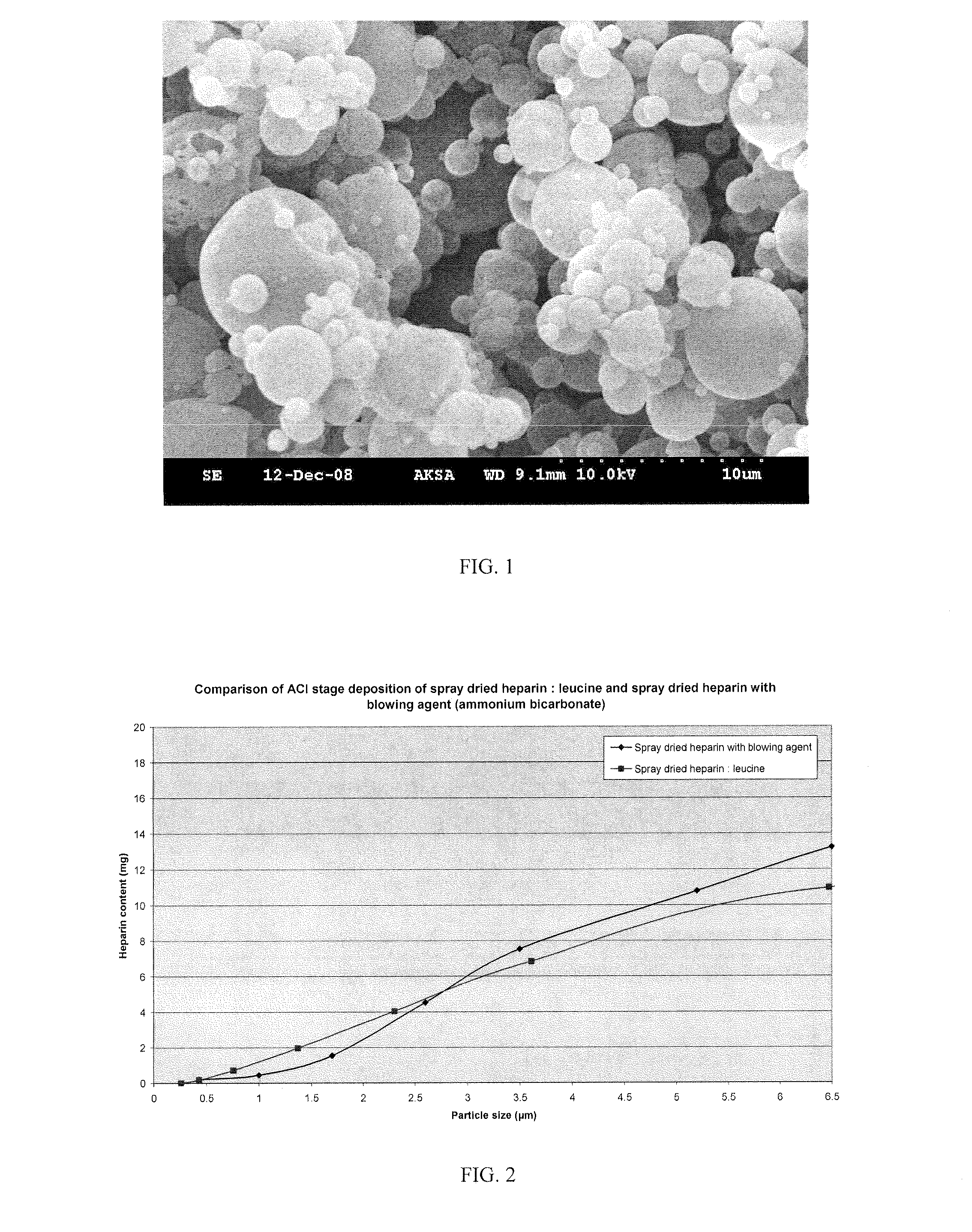
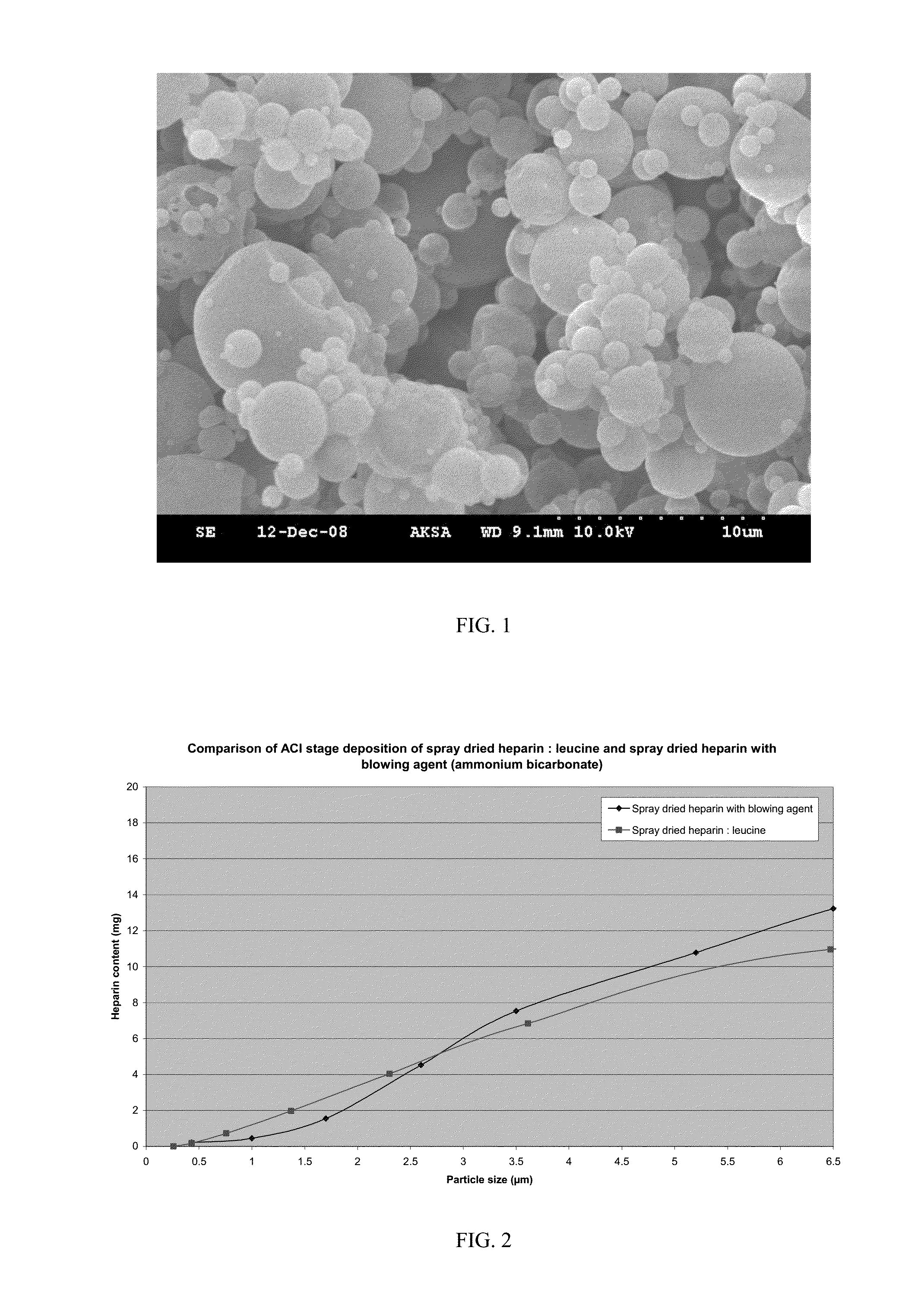
The invention provides microparticles comprising an immunosuppressant, such as tacrolimus, sirolimus, pimecrolimus, ciclosporin, everolimus or a derivative thereof, and optionally a pharmaceutically acceptable excipient or carrier, such as a saccharide, amino acid, a sugar alcohol or a mixture thereof, and having a median geometric diameter of less than, or equal to, about 10 μm and which have a tap density of less than or equal to about 0.3 g / cm3.
Owner:INNOVATA LTD
Fatty acid compositions and methods of use
InactiveUS20090011012A1Easy to carryConvenient travelBiocideCapsule deliveryCyclosporine toxicityAntioxidant
The invention relates to highly concentrated DHA and EPA formulations in a soft gel capsule. A capsule may contain at least 80% omega-3 fatty acids, salts or derivatives thereof, where EPA and DHA are present in relative amounts of greater than or equal to 3:1 or less than or equal to 1:3, and constitute at least 75% to greater than 95% of the total fatty acids present in the capsule. Capsules of the invention may be provided in a blister package so as to provide clean and protected oils that are easy to travel with. Compliance is improved with one-pill-a-day dosing and the days of the week imprinted on the foil packing. Anitoxidant protection may be provided by rosemary and vitamin C. The invention also provides a methods of treatment, modulation or prophalaxis of coronary disease, altering serum LDL-cholesterol and / or HDL-cholesterol, lowering serum triglycerides, lowering blood pressure, pulse rate, altering the activity of the blood coagulation factor VII complex, mild hypertension, protection from cyclosporine toxicity in kidney transplant, rheumatoid arthritis, development and progression of retinopathy, hypertriglyceridemia, and neurological disorders in a subject.
Owner:BAUM SETH J
Ocular solutions
InactiveUS7083803B2Reduce inflammationReduce bacterial growthBiocideSenses disorderDiseaseEverolimus
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease.
Owner:PEYMAN GHOLAM A DR
Ocular solutions
InactiveUS7087237B2Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusMacrolide resistance
Containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
Nanoparticulate and Controlled Release Compositions Comprising Cyclosporine
InactiveUS20080124389A1Reduces and eliminates developmentImprove complianceBiocidePowder deliveryDiseaseAutoimmune disease
The present invention is directed to a composition comprising a nanoparticulate cyclosporine having improved bioavailability. The nanoparticulate cyclosporine particles of the composition have an effective average particle size of less than about 2000 nm in diameter and are useful in the prevention and treatment of organ transplant rejection and autoimmune diseases such as psoriasis, rheumatoid arthritis, and other related diseases. The invention also relates to a controlled release composition comprising a cyclosporine or a nanoparticulate cyclosporine that in operation delivers the drug in a pulsed or bimodal manner for the prevention and treatment of organ transplant rejection and autoimmune diseases such as psoriasis, rheumatoid arthritis, and other related diseases.
Owner:ELAN PHRMA INT LTD
Methods to measure immunosuppressive tacrolimus, sirolimus, and cyclosporin a complexes in a blood sample
The present invention provides methods, diagnostic assays, and diagnostic kits based on said methods, to determine levels of immunosuppressive complexes containing immunosuppressive drugs tacrolimus, sirolimus and cyclosporine A separately and in combination, formed in the blood of a drug-treated patient or in a patient candidate to immunosuppressive drug therapy. These methods, assays and kits are especially useful when using automated systems.
Owner:ABBOTT LAB INC
Pharmaceutical preparation with cyclosporin a
InactiveUS20010014665A1Reduce nephrotoxicityReduce impactOrganic active ingredientsBiocideAlcoholVegetable oil
The invention relates to a pharmaceutical preparation which consists of or contains cyclosporin A, an emulsifying .alpha.-tocopherol derivative, an ethoxylation product of vegetable oils, fatty acids or fats as a further emulsifier and a pharmaceutically customary alcohol.
Owner:HEXAL AG
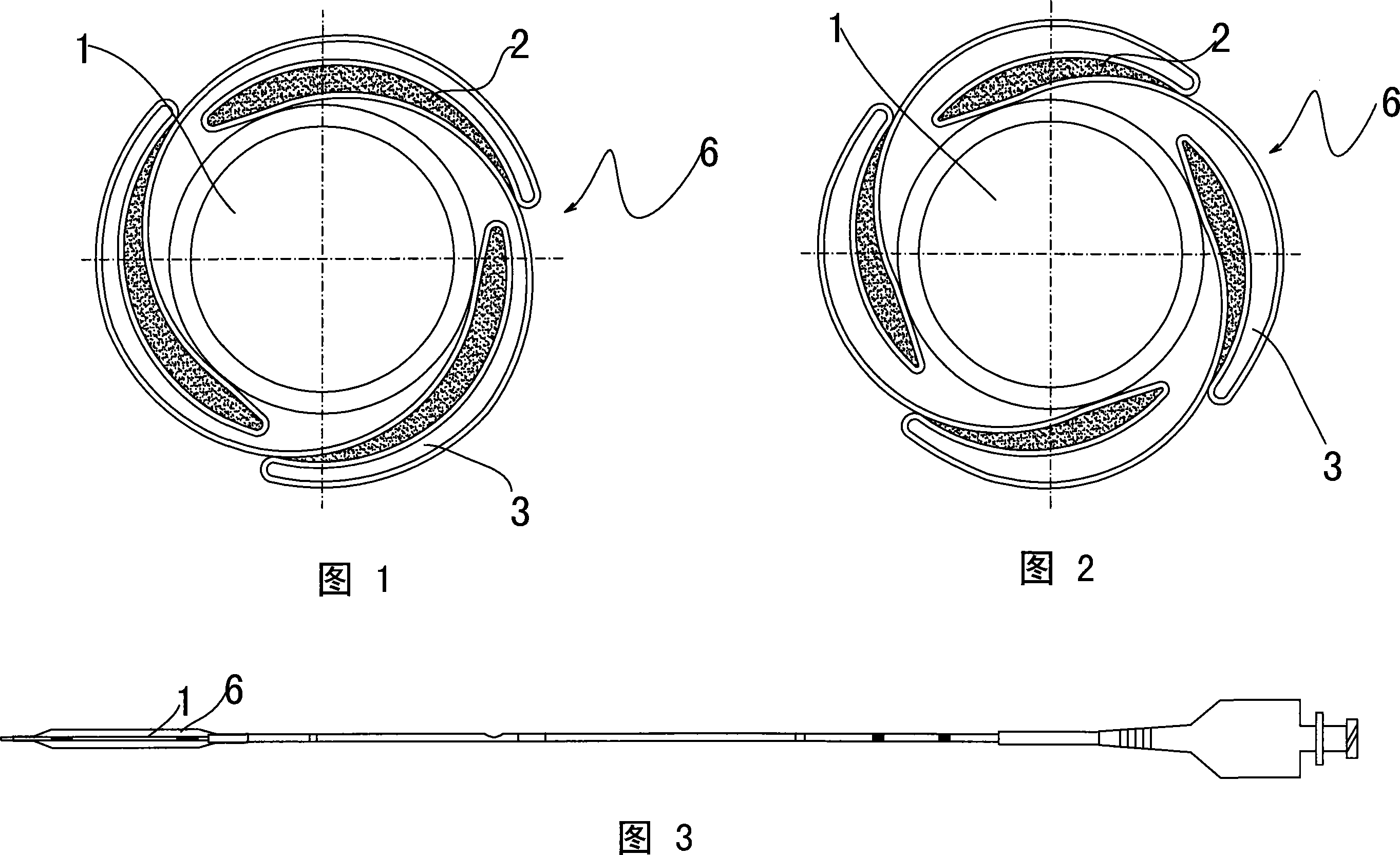
Novel sacculus dilating catheter
The present invention provides a new type balloon dilation catheter which includes ballon and medication material coated on stent. Said medication material comes from one or two and more than two mixtures of heparin sodium, fiber degrading enzyme, serine proteinase, batroxobin, aspirin, genistein, hirudin and its recombined product, colchicine, sirolimus, biolimus, zotarolimus, tracrolimus, pimecrolimus, simvastatin, atorvastatin, pravastatin, ciclosporin, Anti-CD34, dexamethasone, bleomycin, plicamycin, daunomycin, mitomycin C, actinomycin D, taxol, celastrol, methopterin, 5-fluorouracil, cytarabine and 6-purinethol. The balloon is made of macromolecule nylon material, and the stimulation to blood vessel is far lower than the stent with metal structure.
Owner:上海赢生医疗科技有限公司
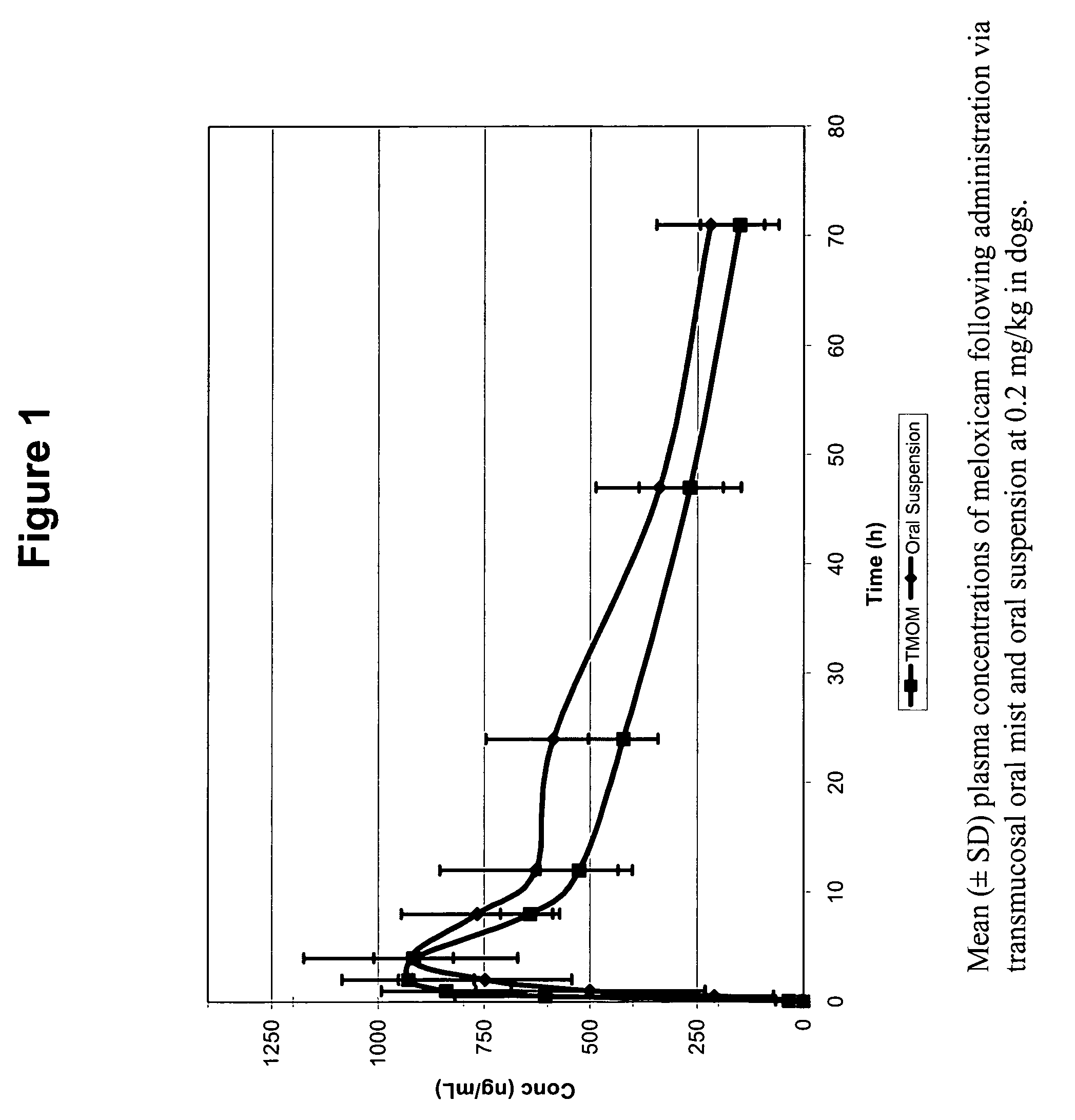
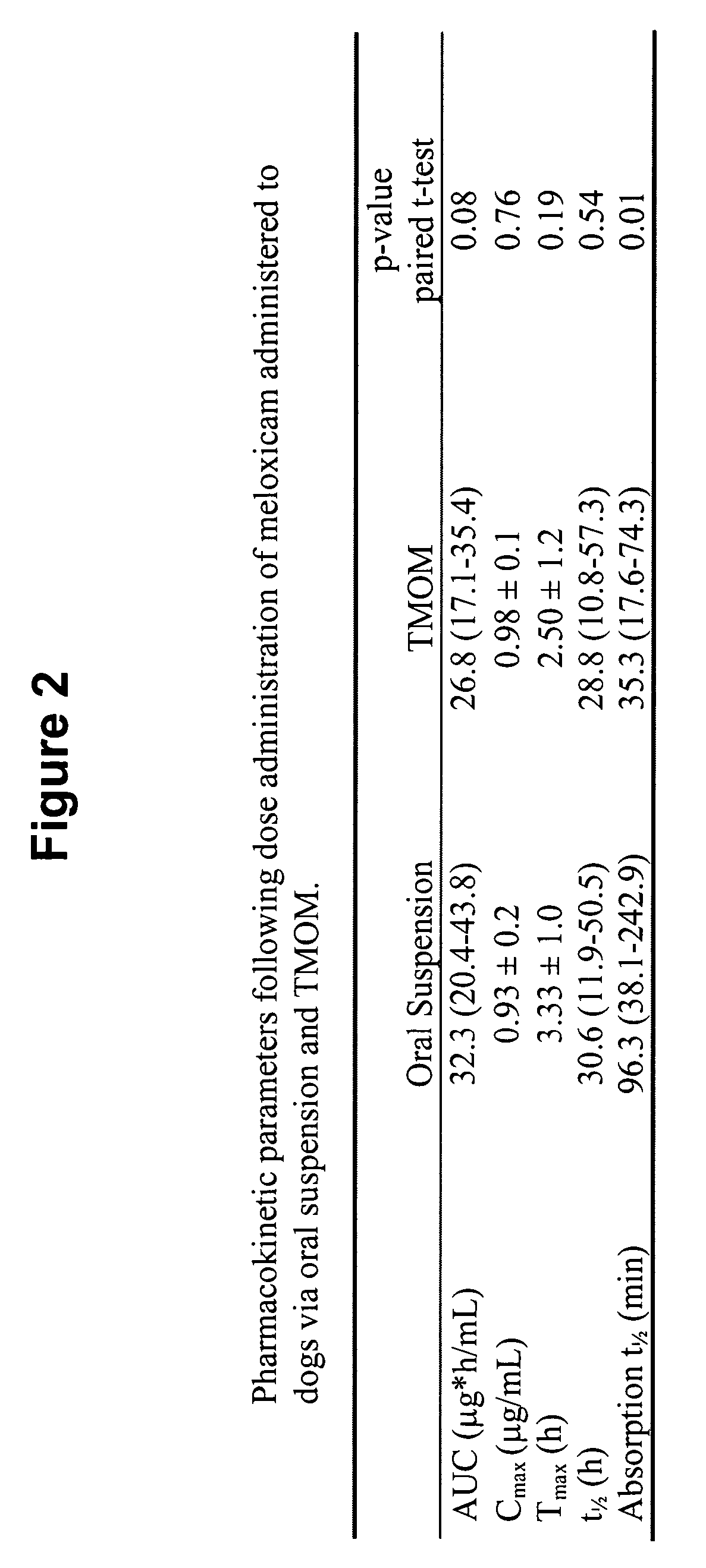
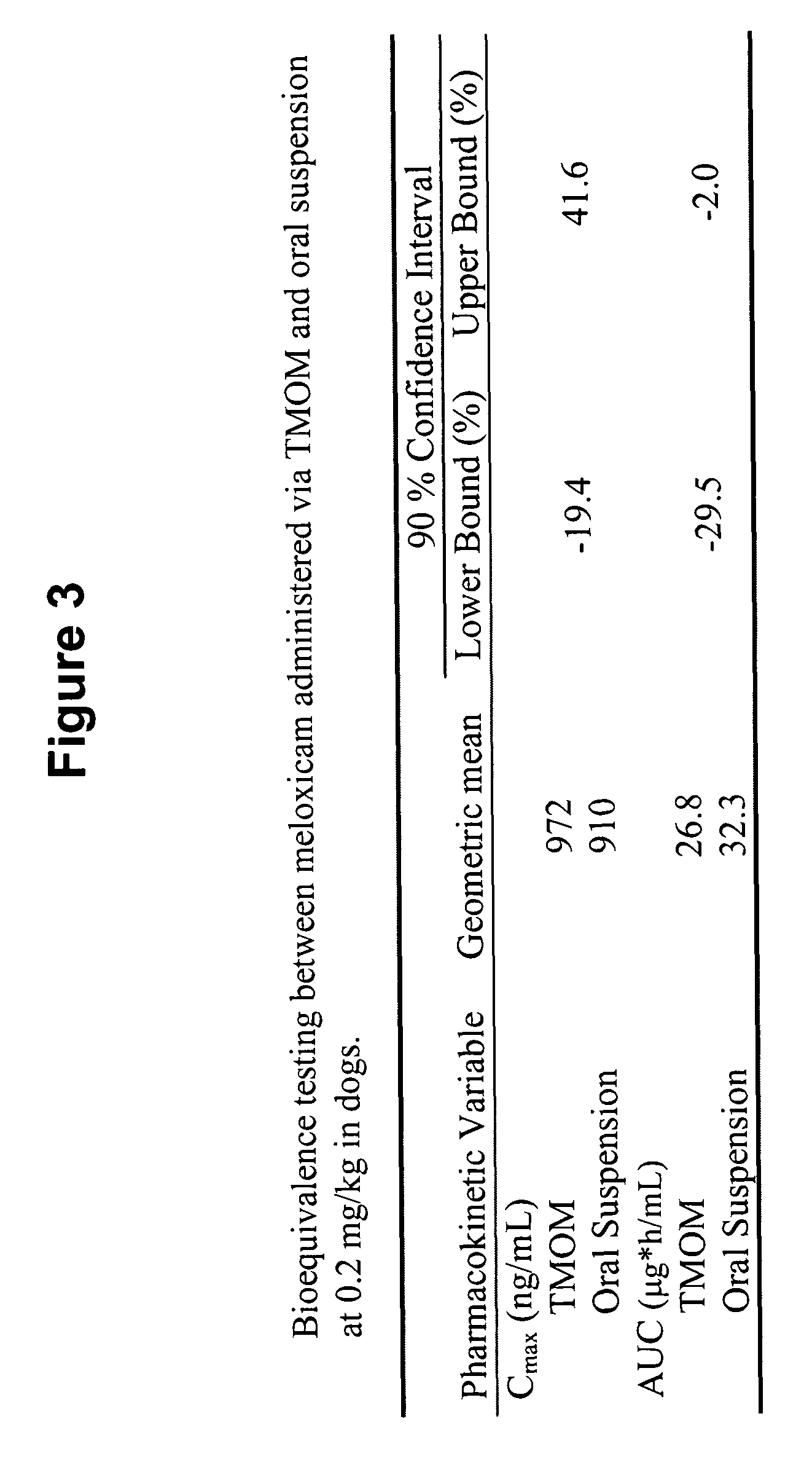
Transmucosal administration of meloxicam compositions for treating and preventing disorders in non-human domesticated animals
The invention includes compositions for transmucosal administration to an animal comprising at least one active agent and a pharmaceutically acceptable carrier. A preferred active agent is selected from the group consisting of meloxicam, carprofen, enrofloxacin, clemastine, diphenhydramine, digoxin, levothyroxine, cyclosporine, ondansetron, lysine, zolpidem, propofol, nitenpyram, ivermectin, milbemycin, and pharmaceutically acceptable salts, solvates and esters thereof. In another embodiment, the invention includes methods of treating or preventing a condition in an animal comprising transmucosally administering a composition comprising a therapeutically or prophylactically effective amount of an active agent and a pharmaceutically acceptable carrier.
Owner:ZOTTIS BELGIUM
Ciclosporin eye gel and preparation method thereof
ActiveCN103735495AImprove solubilityImprove bioavailabilitySenses disorderAerosol deliveryWater basedSolubility
The invention belongs to the field of medicines, and discloses ciclosporin eye gel and a preparation method thereof. The ciclosporin eye gel is water-based eye gel which is prepared from the raw materials: ciclosporin A, a nonionic surfactant, a water-based gel matrix, a wetting agent, a pH modifier and water. The ciclosporin A of the ciclosporin eye gel has high solubility in water, and the corneal permeability of the ciclosporin A is good. Meanwhile, the ciclosporin eye gel is the water-based eye gel, the adhesiveness of a mucous membrane is good, the corneal retention time is long, and the bioavailability of ciclosporin A is high. In addition, the ciclosporin eye gel does not contain an oil-based solvent and a preservative and has slight irritation to eyes and low damage to the ocular surface. The preparation method of the ciclosporin eye gel is simple for operation and is suitable for large scale production.
Owner:ZHAOKE GUANGZHOU OPTHALMIC DRUG
Preparation method of ciclosporin ophthalmic solution
InactiveCN103656617ASolve solubilitySmall local irritationSenses disorderPharmaceutical delivery mechanismSolubilityEmulsion
The invention relates to an ophthalmic solution which comprises ciclosporin. Due to adoption of a micro emulsion technique, the problem of solubility of ciclosporin in water is solved. The ophthalmic solution is applicable to ophthalmic preparations, and has the characteristics of small irritation and good stability. The ophthalmic solution provided by the invention is reasonable in formula and stable in process, and is applicable to industrial production and clinical application.
Owner:SHENYANG XINGQI PHARM CO LTD
Ophthalmic ciclosporin emulsion
InactiveCN105726479AHigh partition coefficientHigh content of active ingredientsSenses disorderCyclic peptide ingredientsEmulsionCiclosporin
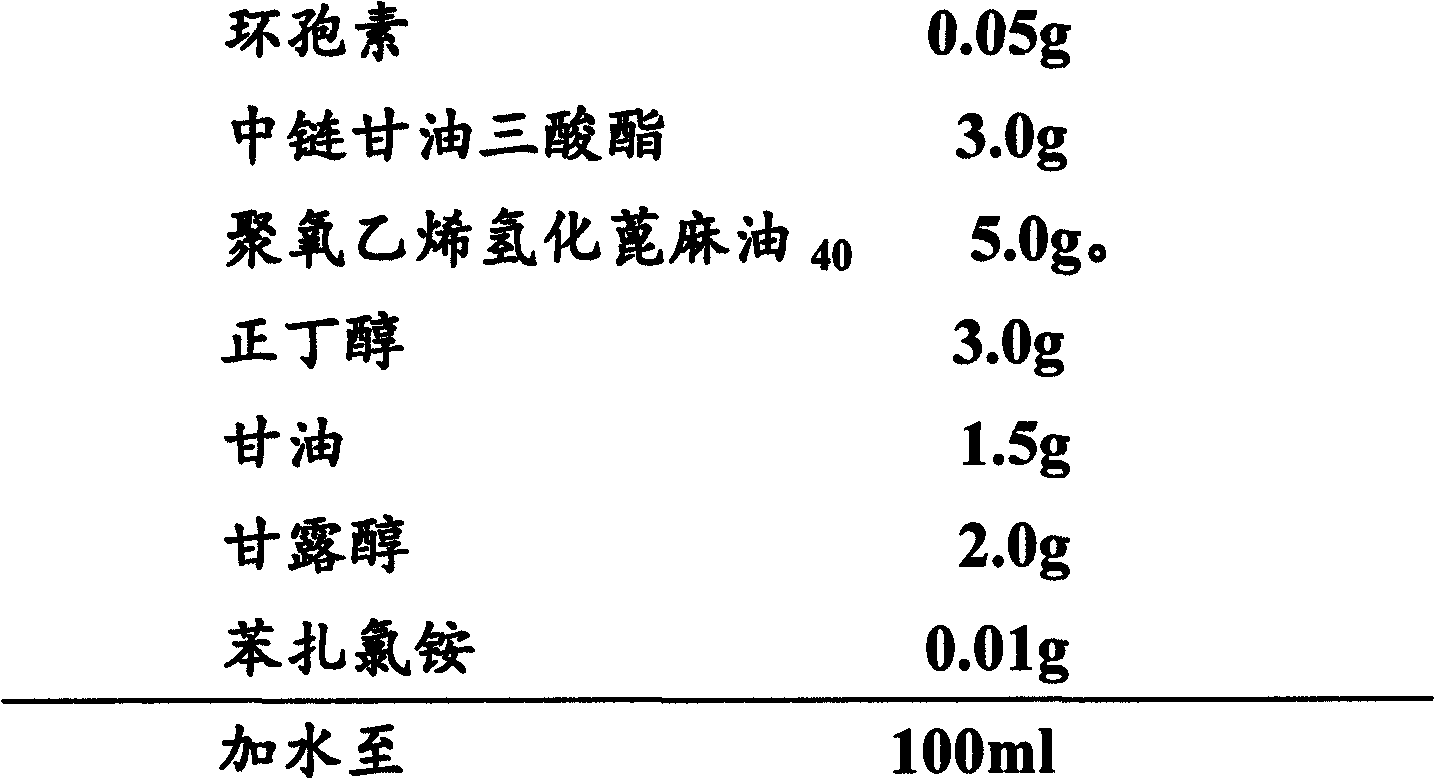
The invention provides ophthalmic ciclosporin emulsion. The ophthalmic emulsion is characterized by comprising the following components by weight percent: 0.02-0.1% of ciclosporin, 2.5-4% of polyoxyethylated castor oil, 1-3% of middle-chain fatty glyceride, 2-5% of glycerin, 0.2-0.5% of sodium alginate and the balance of water.
Owner:北京茗泽中和药物研究有限公司
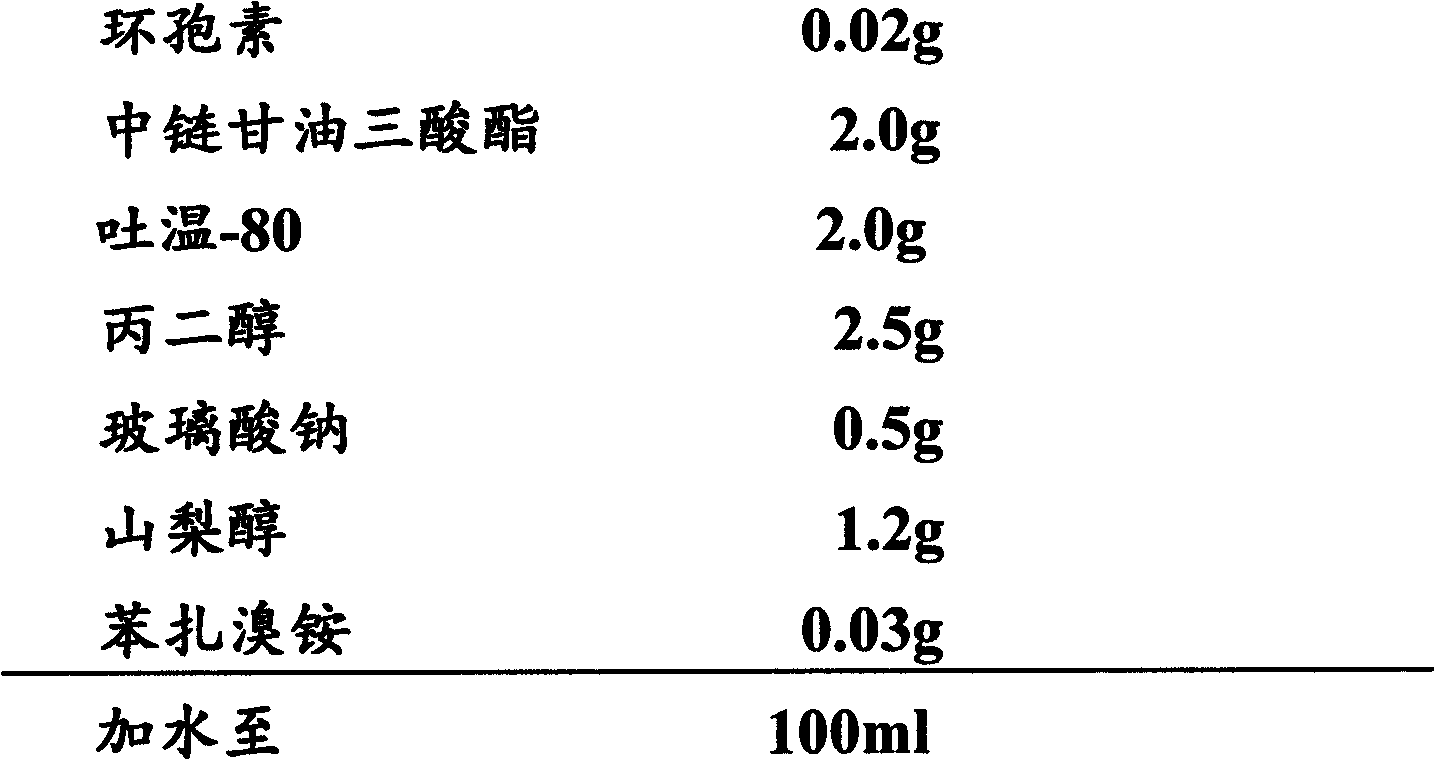
Stable cyclosporine containing ophthalmic emulsion for treating dry eyes
ActiveUS20120093894A1Relieve dry eye symptomsEfficiently relievedBiocideSenses disorderVegetable oilSide chain
Disclosed herein are stable oil-in-water emulsion ophthalmic topical liquid compositions having an average particle size less than 1 μm including at least one plant-derived oil other than castor oil wherein the oil comprises only aliphatic side chains free of polar pendent groups. The oil-in-water emulsion ophthalmic topical liquid compositions also include a hydrophilic surfactant having an HLB value between approximately 10 and 14, a vegetable oil-derived hydrophobic non-co-block surfactant having unsaturated side chains that contain less than four oxygen atoms having an HLB value between approximately 4 and 6 and is a liquid at room temperature. The oil-in-water emulsion ophthalmic topical liquid compositions disclosed herein can further include an amount of cyclosporine A or polyphenol in an amount effective to relieve dry eye symptoms.
Owner:JOHNSON & JOHNSON SURGICAL VISION INC
Terpene Glycosides and Their Combinations as Solubilizing Agents
ActiveUS20120121696A1Improve solubilityBiocideHydroxy compound active ingredientsChemistryCiclosporin
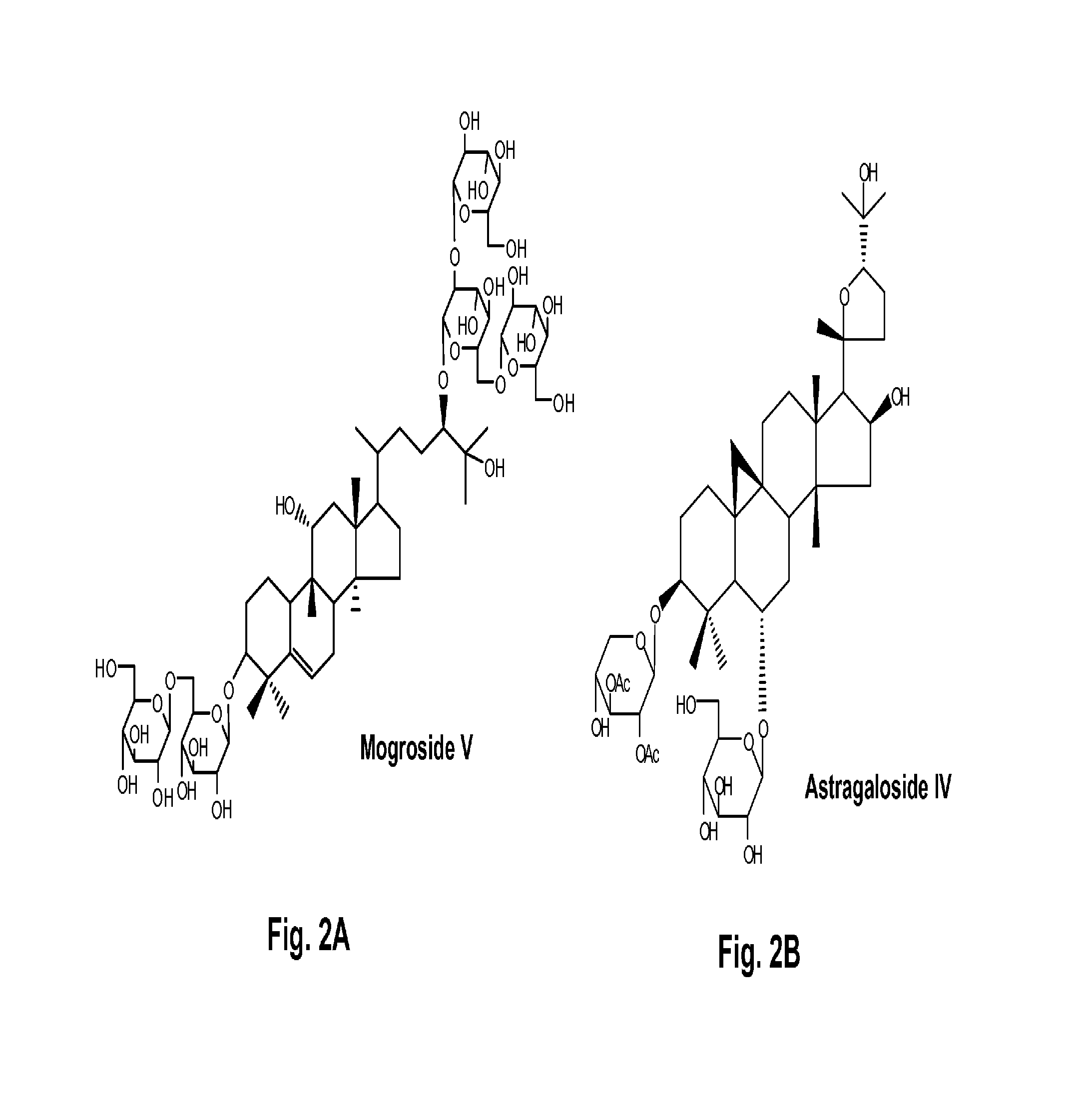
Several terpene glycosides (e.g., mogroside V, paenoiflorin, geniposide, rubusoside, rebaudioside A, steviol mono-side and stevioside) were discovered to enhance the solubility of a number of pharmaceutically and medicinally important compounds, including but not limited to, paclitaxel, camptothecin, curcumin, tanshinone HA, capsaicin, cyclosporine, erythromycin, nystatin, itraconazole, celecoxib, clofazimine, digoxin, oleandrin, nifedipine, and amiodarone. The use of the diterpene glycoside rubusoside and monoterpene glycoside paenoiflorin increased solubility in all tested compounds. The terpene glycosides are a naturally occurring class of water solubility-enhancing compounds that are non-toxic and that will be useful as new complexing agents or excipients in the pharmaceutical, agricultural (e.g., solubilizing pesticides), cosmetic and food industries.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Application of HMGCoA reductase inhibitor in preparation of medicine used for treating xerophthalmia
InactiveCN103007284AImprove permeabilityPromote secretionSenses disorderEster active ingredientsSide effectOphthalmic drug
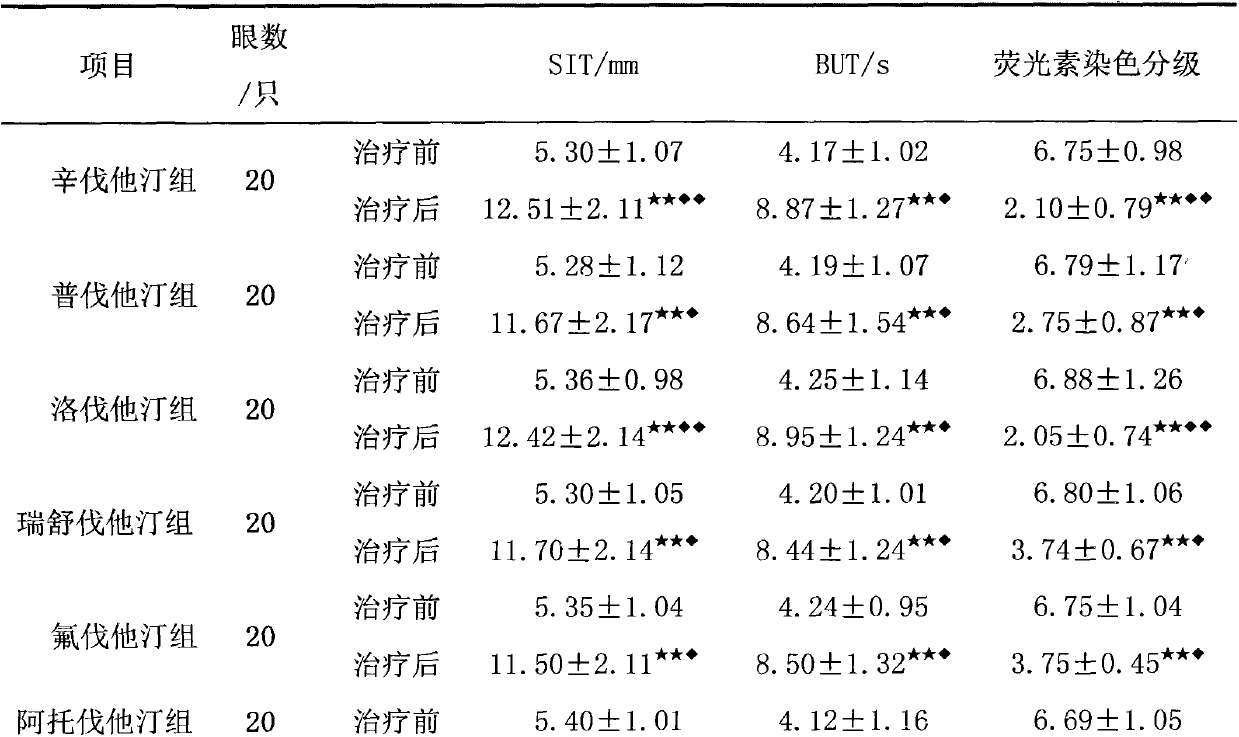
The invention relates to an application of an HMGCoA reductase inhibitor in preparation of a medicine used for treating xerophthalmia and belongs to the field of medicines. The inventor finds that a frequently-used lipid regulation medicine, the HMGCoA reductase inhibitor, can obviously prolong SIT time and BUT time and obviously reduce an FLS score when being used for treating the xerophthalmia, and the HMGCoA reductase inhibitor is better than ciclosporin or artificial tears in the aspect of improving the indexes, wherein simvastatin and lovastatin have the best treatment effect. The HMGCoA reductase inhibitor also can be prepared into oral preparation used for treatment or adjuvant therapy of the xerophthalmia. The HMGCoA reductase inhibitor has a definite curative effect and less toxic and side effects, compliance of a patient is high, and clinical ophthalmic drugs are further enriched.
Owner:闫莹
Cyclosporine emulsion and the preparing method
The present invention provides one kind of self-emulsified ciclosporin preparation and its preparation process. The self-emulsified ciclosporin preparation contains ciclosporin 5-25 wt%, vitamin E-TPGS 25-70 wt% and Pharmasolve 5-50 wt%. It has high stability, high medicine concentration, great capacity of self emulsifying, high bioactivity and easy taking, and may be used as orally taken liposoluble medicine.
Owner:SHANGHAI KAIZHAO PHARMA TECH
Cyclosporin a compositions
InactiveUS20070219127A1Stable and long-term shelf-livesLong shelf-livesPowder deliveryBiocideDiseaseCiclosporin
A composition comprising cyclosporin A and a nonaqueous, physiologically acceptable liquid carrier, said composition being suitable for topical administration to an eye of a mammal is disclosed herein. Methods of treating disease related thereto are also disclosed.
Owner:ALLERGAN INC
Composition of ciclosporin A and amphipathic chitosan derivatives and preparation thereof
InactiveCN102120027AHigh drug loadingHigh encapsulation efficiencyPharmaceutical delivery mechanismCyclic peptide ingredientsCholic acidSide effect
The invention relates to the field of pharmaceutic preparations, and discloses a composition of ciclosporin A and amphipathic chitosan derivatives (N,O-carboxymethyl N-cholic acid chitosan) and a preparation method thereof. The composition has the characteristics of high medicament loading rate, high stability and high possibility of being absorbed, and can be used for overcoming the defects such as serious toxic or side effect of the existing ciclosporin A. The preparation method of the composition is simple, and the process is mature, thus the preparation method id applicable to large-scale industrial production.
Owner:CHINA PHARM UNIV
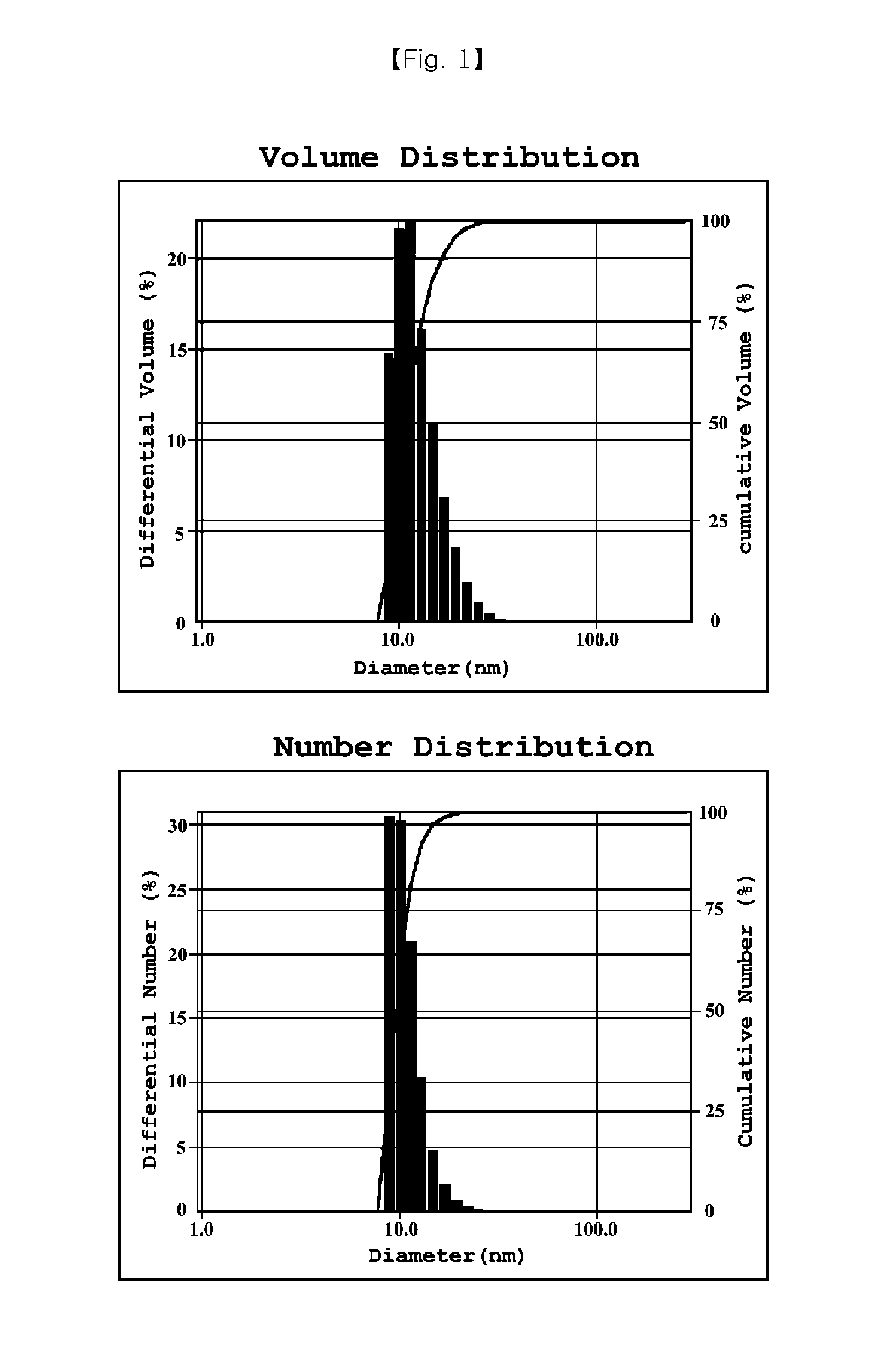
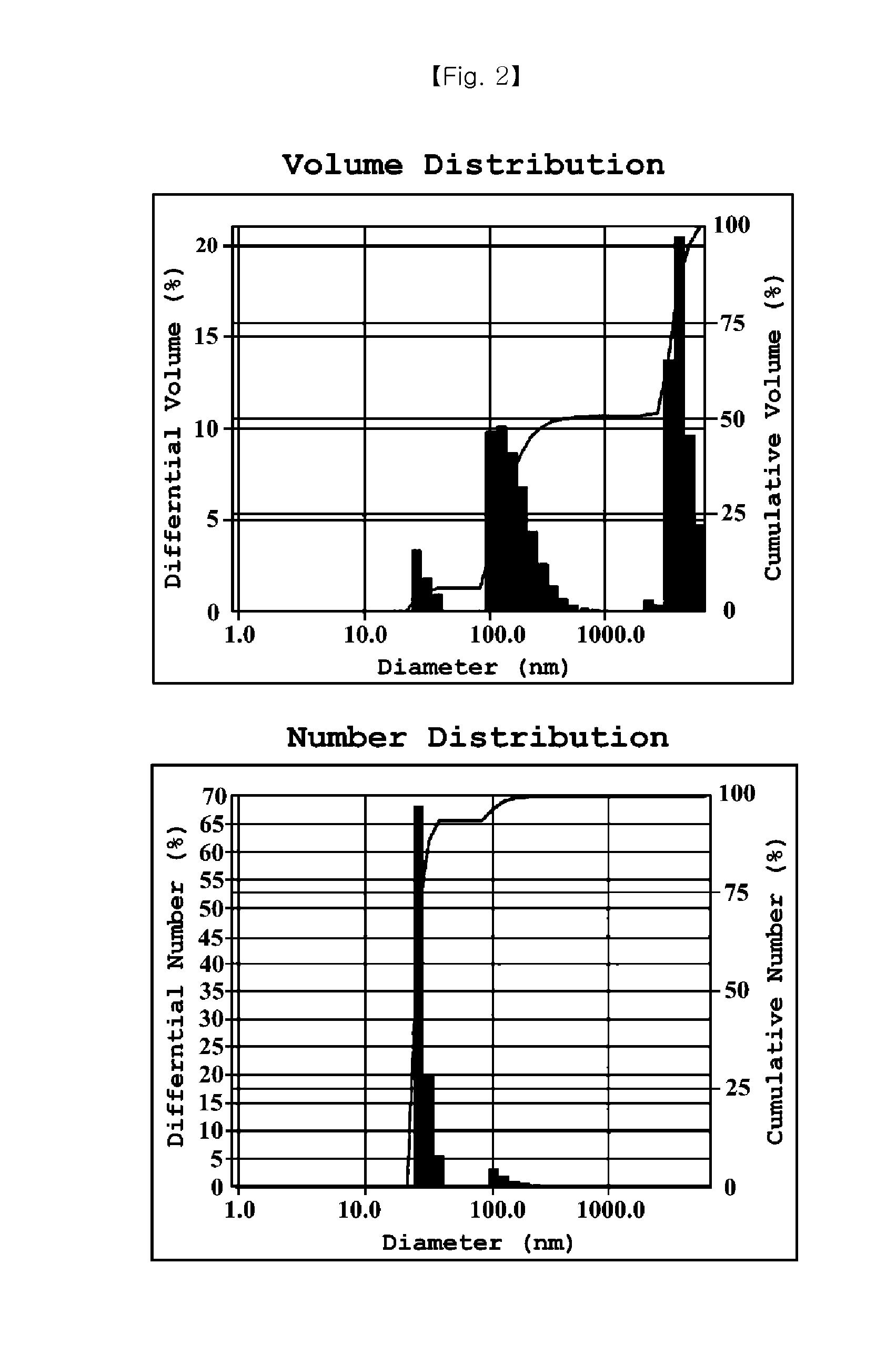
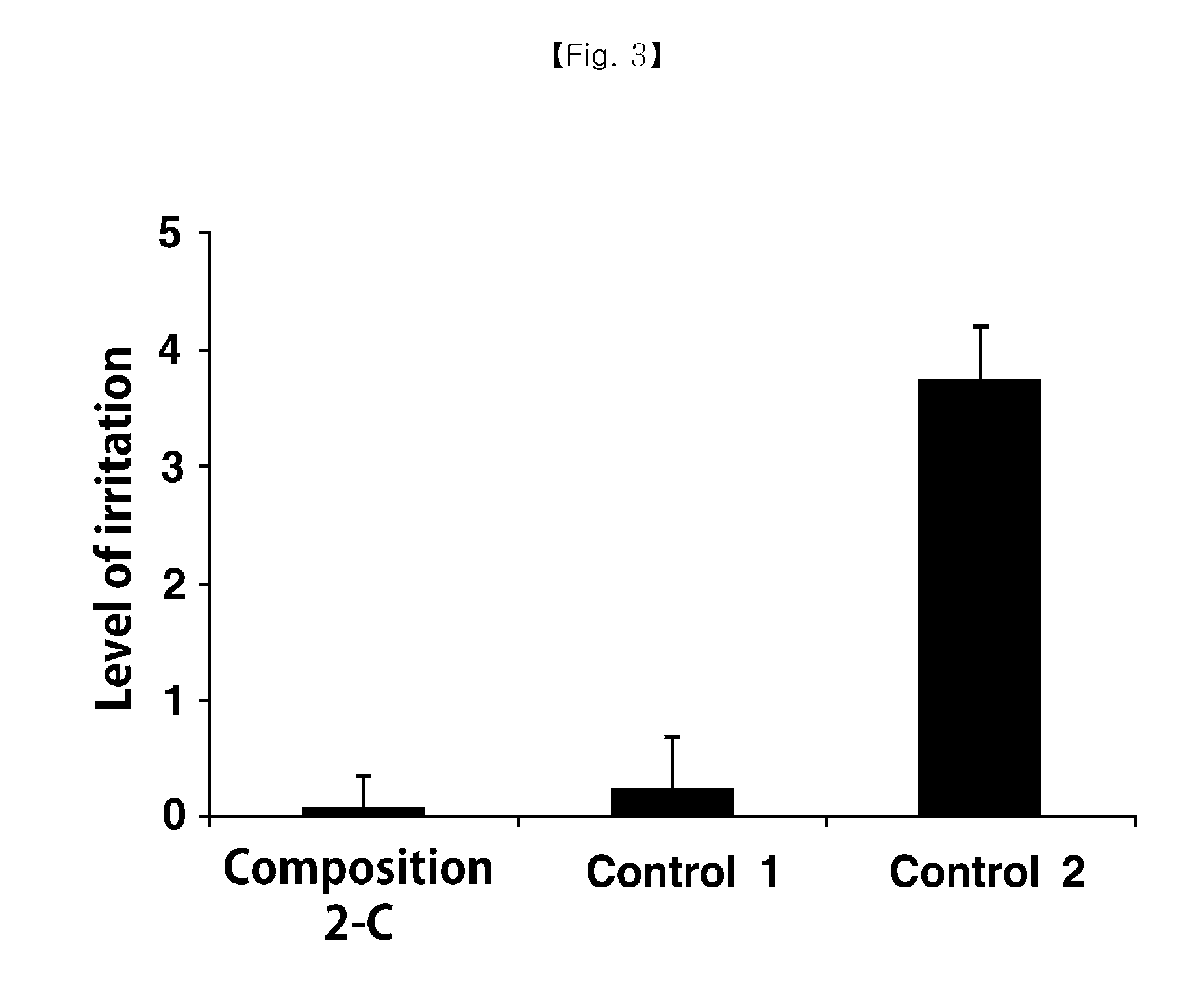
Cyclosporine-Containing Non-Irritative Nanoemulsion Ophthalmic Composition
ActiveUS20150125494A1No irritationEqually distributedSenses disorderSolution deliveryMedicineIrritation
Provided is an ophthalmic composition containing cyclosporine as an active ingredient and including polyethoxylated castor oil or polyethoxylated hydrogenated castor oil, and a method of preparing the same. Particularly, the ophthalmic composition is prepared as a nanoemulsion having a particle diameter of 100 nm or less simply by mixing and stirring an oil phase and an aqueous phase without using a high speed stirring or shearing machine, so that it is very physiochemically stable and storable for a long time. In addition, the ophthalmic composition causes no irritation to eyes.
Owner:HUONS
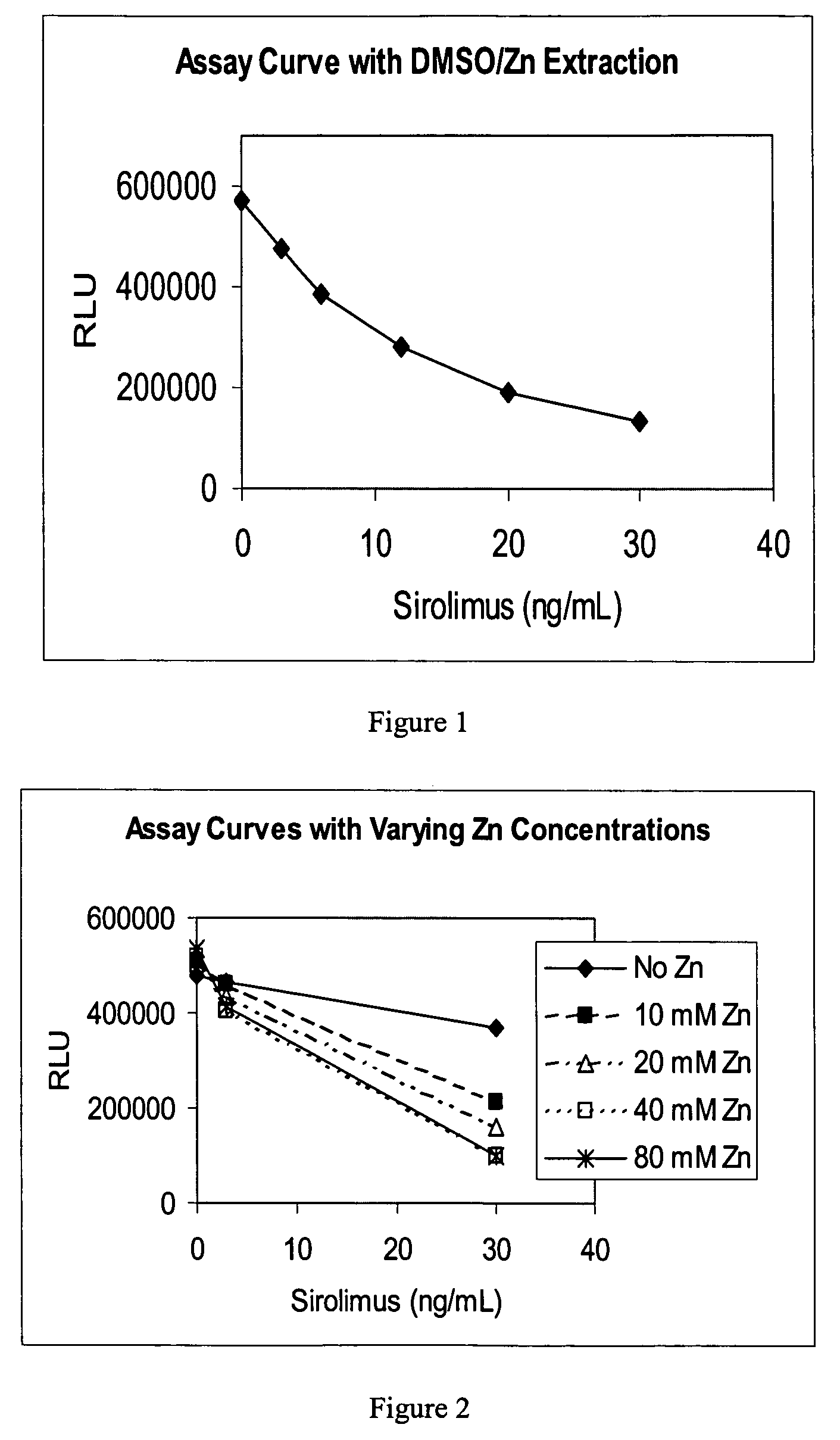
Immunosuppressant drug extraction reagent for immunoassays
ActiveUS7883855B2High sensitivity immunoassayAccurate measurementEarth material testingImmunoglobulinsImmunochemistryChemistry
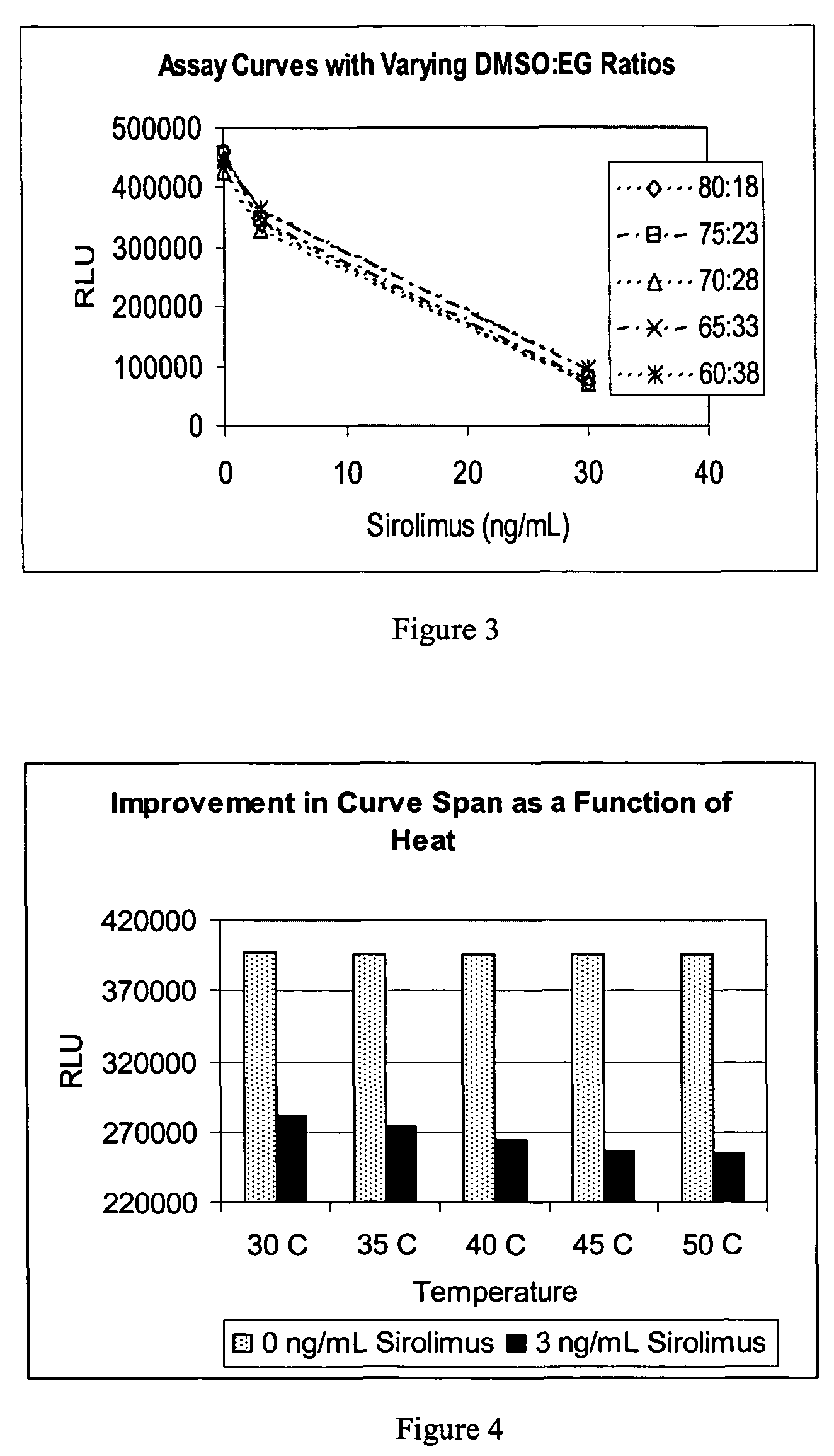
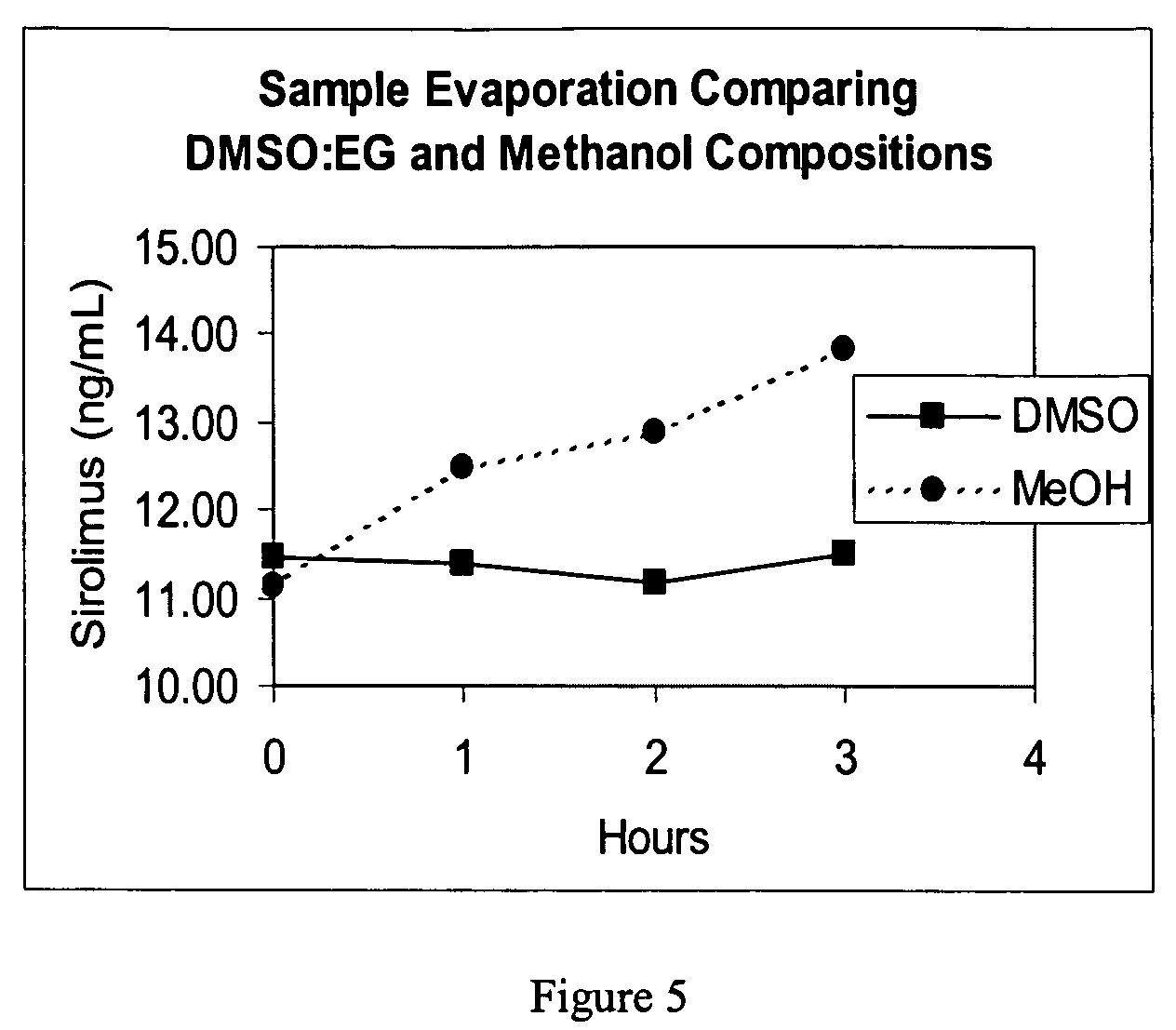
An improved extractive reagent composition and method for extracting an immunosuppressant drug, such as sirolimus, tacrolimus or cyclosporine, from blood samples while yielding a test sample extract that has low vapor pressure and is compatible with immunoassay components. The inventive reagent composition comprises dimethyl sulfoxide (DMSO), at least one divalent metal salt and water. The sample extracts resulting from use of each of these combinations have low vapor pressure and are compatible with immunochemistry assays.
Owner:ABBOTT LAB INC
Composition
The invention provides microparticles comprising an immunosuppressant, such as tacrolimus, sirolimus, pimecrolimus, ciclosporin, everolimus or a derivative thereof, and optionally a pharmaceutically acceptable excipient or carrier, such as a saccharide, amino acid, a sugar alcohol or a mixture thereof, and having a median geometric diameter of less than, or equal to, about 10 μm and which have a tap density of less than or equal to about 0.3 g / cm3.
Owner:INNOVATA LTD
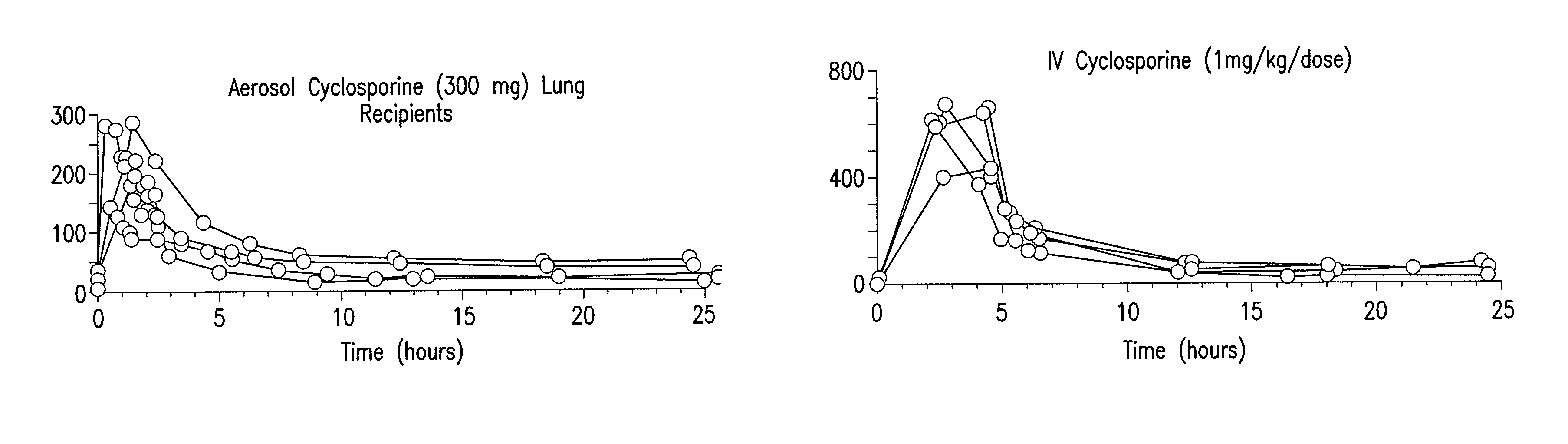
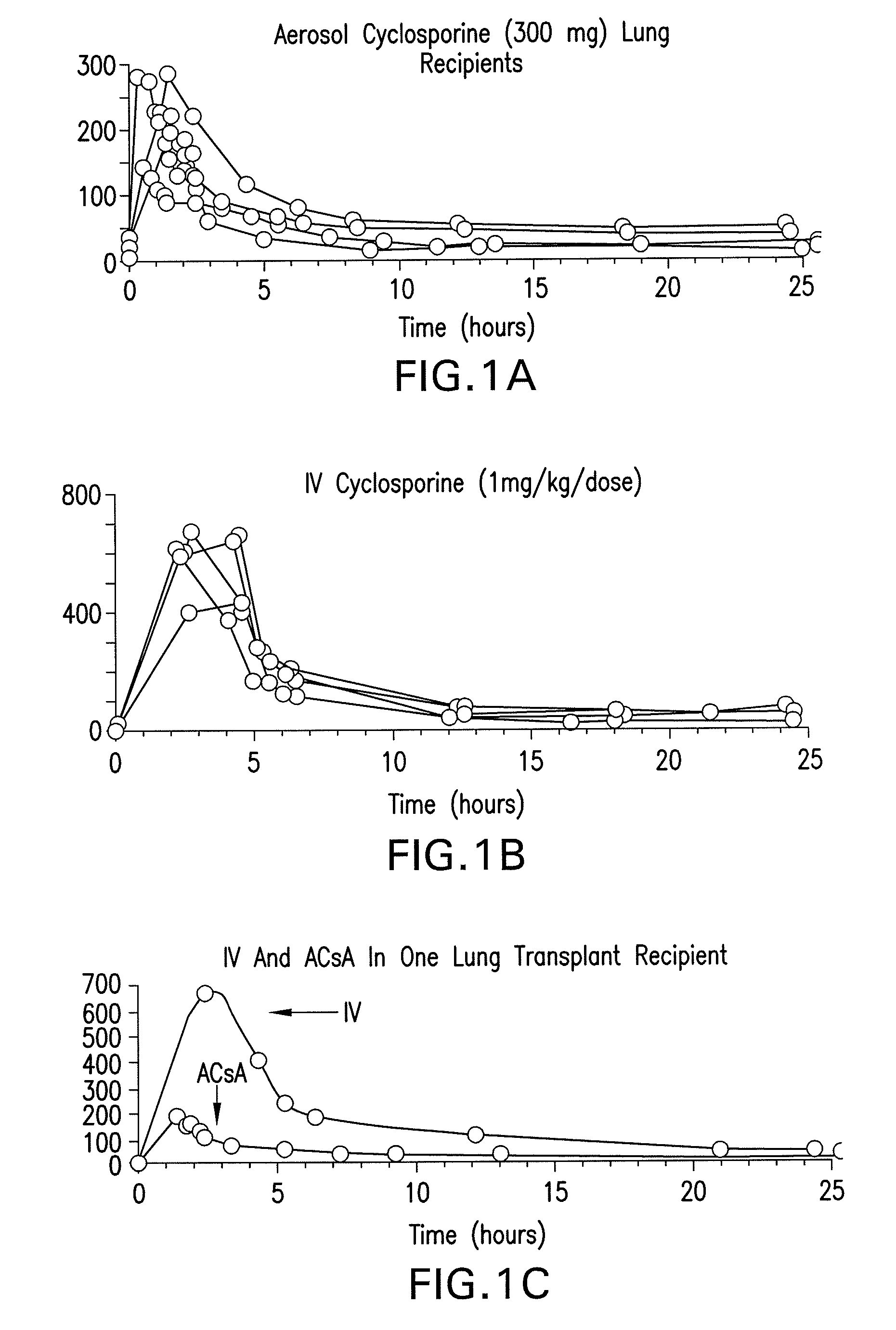
Use of aerosolized cyclosporine for prevention and treatment of pulmonary disease
InactiveUS8158110B2Easy to controlLow toxicityAntibacterial agentsBiocideDiseaseInflammation Process
The present invention relates to methods and compositions for prevention of graft rejection in lung transplant recipients and for treatment of subjects with pulmonary disorders. Specifically, the methods and compositions of the invention provide a means for inhibiting immune response mediated inflammatory processes in the lungs. The method of the invention comprises the administration of aerosolized cyclosporine for prevention of acute and / or chronic refractory rejection in lung transplant patients. The invention further provides for the use of aerosolized cyclosporine to treat subjects having immunologically mediated inflammatory pulmonary disorders including, but not limited to, asthma, cystic fibrosis, idiopathic pulmonary fibrosis, chronic bronchitis and allergic rhinitis. The present invention, by enabling a method for the use of aerosolized cyclosporine for inhibiting pulmonary inflammation leading to prevention of graft rejection and treatment of pulmonary disorders, provides a safer and less toxic treatment than those methods that utilize systemic administration of cyclosporine.
Owner:UNIVERSITY OF PITTSBURGH
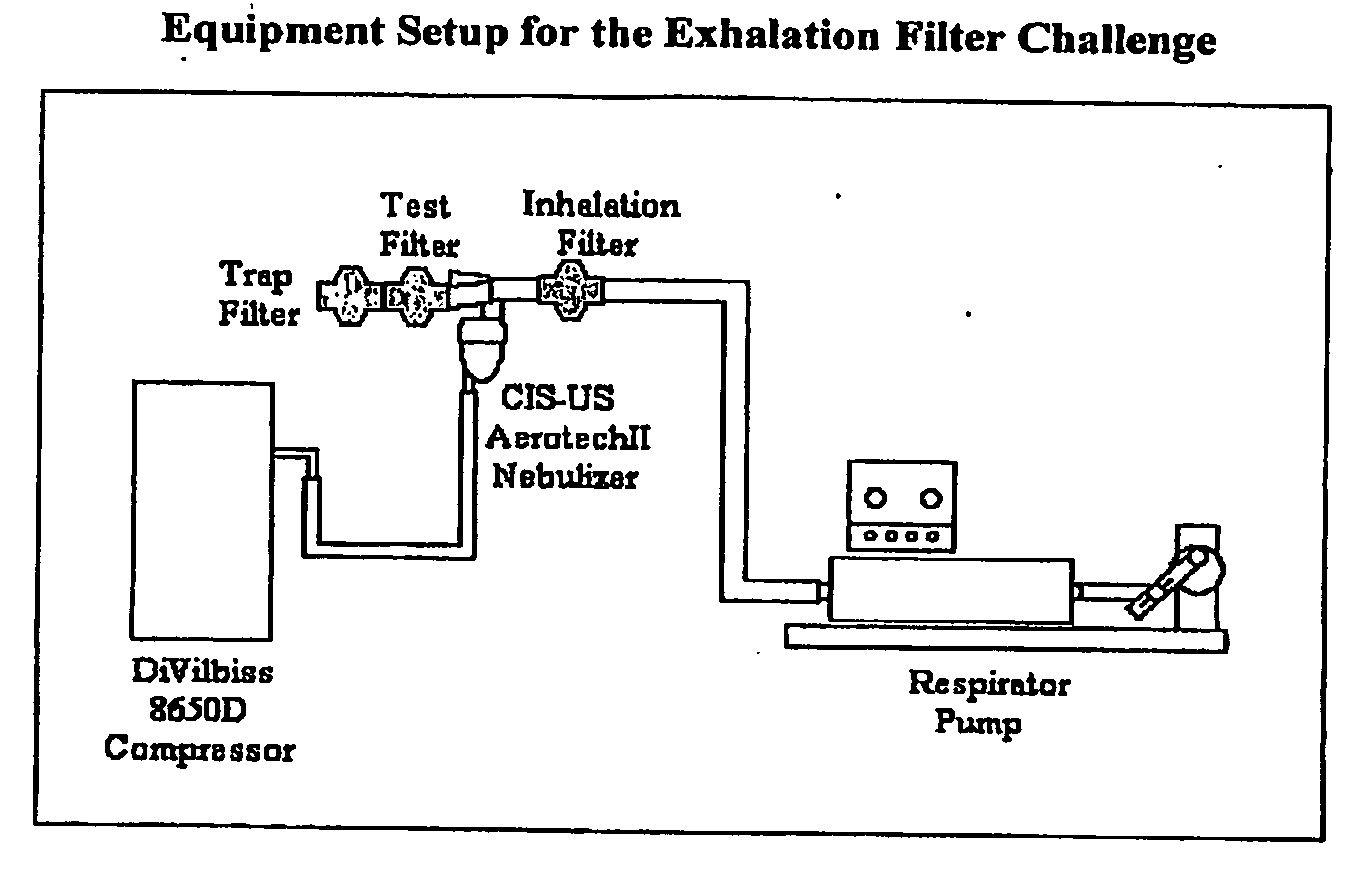
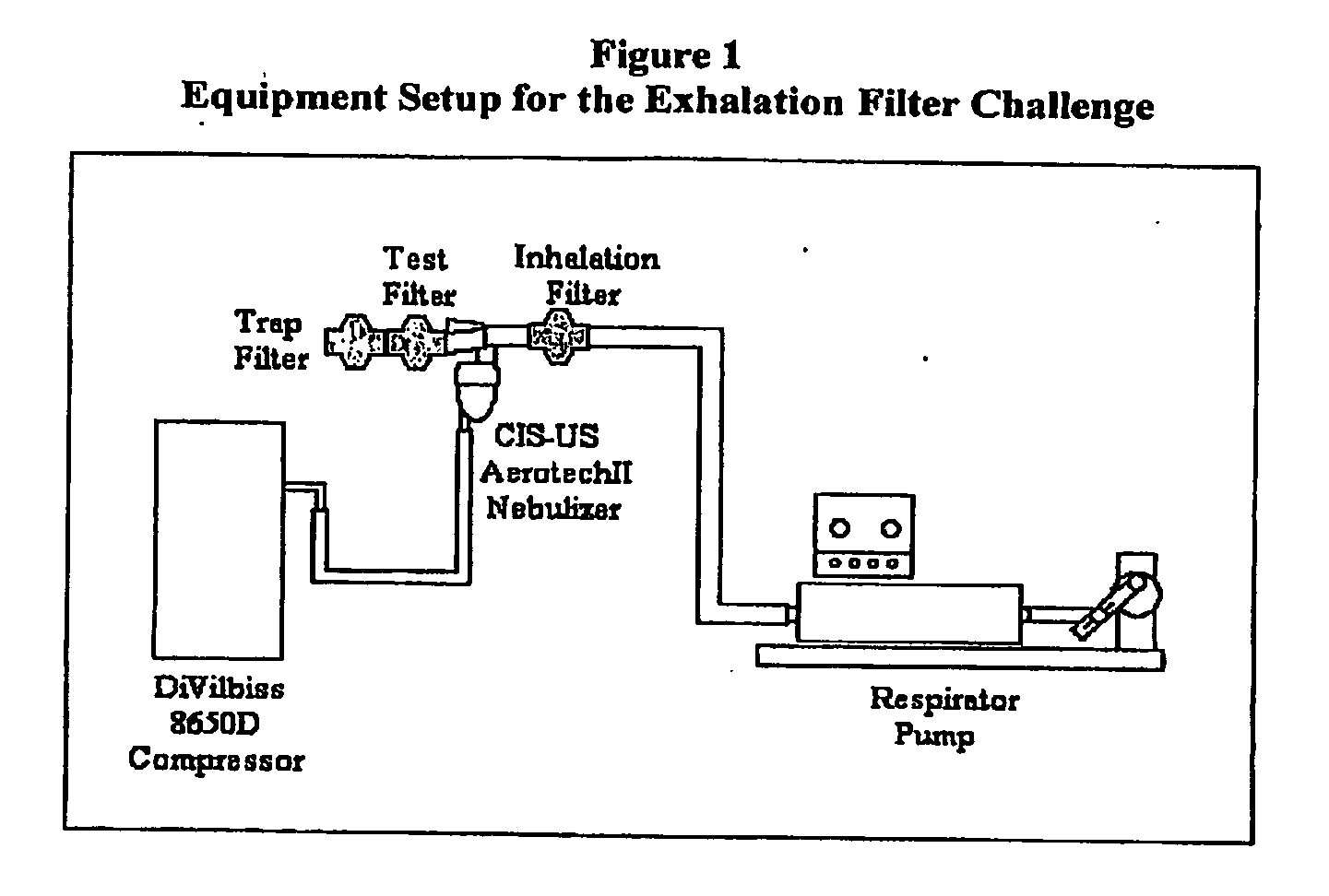
System for delivering nebulized cyclosporine and methods of treatment
InactiveUS20100163021A1Minimizes and prevents escapeEffective filteringRespiratorsRespiratory disorderEngineeringSolvent
Systems comprising a pressurized delivery device and a formulation of cyclosporine coupled to an exhalation filter or trap that is capable of preventing cyclosporine from escaping into the local environment are provided. An apparatus for use in the system comprises either an exhalation filter and a pressurized delivery device, wherein the exhalation filter is capable of providing high filter efficiency and maintaining low filter resistance after usage with the formulation, or a trap which provides a means for expired gas to be released into a solvent chamber containing a solvent with a high affinity for cyclosporine. These systems may be used to treat patients with pulmonary disorders, organ transplant patients such as lung transplant patients, and other immune-related disorders.
Owner:NOVARTIS AG
Method for extracting umbilical cord blood hematopoietic stem cells under low-oxygen environment
ActiveCN107475197AOvercoming non-physiological oxygen stressPrecise temperature controlCulture processBlood/immune system cellsHydroxyethyl starchBlood collection
The invention discloses a method for extracting umbilical cord blood hematopoietic stem cells under a low-oxygen environment. The method specifically comprises the following steps: adding 80ng / ml to 120ng / ml of ciclosporin A into an anticoagulant added into a sterile blood collection bag; adding 30ng / ml to 70ng / ml of the ciclosporin A into a hydroxyethyl starch solution sedimentation agent; carrying out a whole-process extraction process of the hematopoietic stem cells in a sealed low-oxygen working station. The low-oxygen working station is provided with independent operation platforms at two sides and a transferring gate; the oxygen concentration of the operation platforms at two sides is set to 3 percent to 8 percent; the temperature of the operation platform at one side is set to 3 DEG C to 10 DEG C and the operation platform is used for carrying out preparation and sub-packaging of a reagent, transferring umbilical cord blood and cryopreserving the umbilical cord blood hematopoietic stem cells; the temperature of the operation platform at the other side is set to 18 DEG C to 25 DEG C and the operation platform is used for carrying out sedimentation of the umbilical cord blood, carrying out blood serum separation on the umbilical cord blood and centrifuging after the blood serum separation. According to the method disclosed by the invention, the hematopoietic stem cells with more quantity, more original state and strong proliferation and differentiation capabilities can be extracted.
Owner:希瑞干细胞科技有限公司
Method for detecting plasma concentration of ciclosporin A by adopting competition method
The invention relates to a method for detecting plasma concentration of ciclosporin A by adopting a competition method, which comprises the steps of: coupling the ciclosporin A on the surface of a magnetic bead, adding a plasma sample after anticoagulation and dilution in a sample hole, adding a ciclosporin A monoclonal antibody marked by fluorescein, eluting the mixture, detecting the fluorescence intensity under excitation of exciting light, and obtaining the plasma concentration of ciclosporin A. The method for detecting plasma concentration of ciclosporin A is simple to operate, has high detection speed, uses less samples and has low cost, higher sensitivity and wider linear range compared with an ELISA (Enzyme-Linked Immuno Sorbent Assay) method, and good application prospect.
Owner:SHANGHAI GENEXT MEDICAL TECH
Medicinal composition as well as preparation and application thereof
InactiveCN103920138APromote secretionGood treatment effectOrganic active ingredientsSenses disorderXerophthalmiaTreatment effect
The invention relates to the technical field of medicines, and in particular relates to a medicinal composition as well as a preparation and application thereof. The medicinal composition comprises a component a and a component b; the component a is rebamipide, pharmaceutically acceptable salt or isomeride thereof; and the component b is ciclosporin A, pharmaceutically acceptable salt or isomeride thereof. Experiments prove that a synergistic effect can be achieved when the rebamipide and the ciclosporin A are used together. The composition significantly improves the effects of promoting lacrimal secretion and eliminating inflammation compared with the effects achieved when the rebamipide and the ciclosporin A are used separately (P<0.05). Therefore, when the rebamipide and the ciclosporin A are compounded to prepare the medicament for treating xerophthalmia, the treatment effect can be improved.
Owner:ZHAOKE PHARMA GUANGZHOU
Injection for treating hyperthyroidism
InactiveCN101474397AReduce volumeHigh remission rateOrganic active ingredientsCyclic peptide ingredientsLarynxSevere hypothyroidism
The invention relates to an injection medicament for treating hyperthyroid, which comprises the following components by weight part: 2 to 5 milligrams of dexamethasone injection, 0.5 to 1 milligram of octreotide injection, and 10 to 20 milligrams of ciclosporin A. Immune preparations such as the dexamethasone injection, the octreotide injection, the ciclosporin A and the like are adopted for treating the hyperthyroid by locally injecting the thyroid, the volume of the tumid thyroid is remarkably shrunk, and the function of the thyroid basically recovers within two months, so the injection medicament has remarkable curative effect, and can remarkably improve the remission rate of hyperthyroid and reduce the rate of relapse. Serious abnormal change, hypothyroidism and parathyroid hypofunction are not caused, and laryngeal recurrent nerve injury is also not caused. Through more than 580 cases of clinical therapy in related hospitals, the volume of the tumid thyroid is remarkably shrunk after therapy, clinical symptoms disappear, allergic response and complications are not caused, the effective percentage is nearly 100 percent, and the cure rate is as high as 98 percent.
Owner:陈小国
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com