Patents
Literature
86 results about "After cataract" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An after cataract, also know as a posterior capsular opacity, is a gradual clouding of the container (or capsule) that holds the implant in place after cataract surgery. The capsule was the structure that once held the natural lens in place behind the pupil.
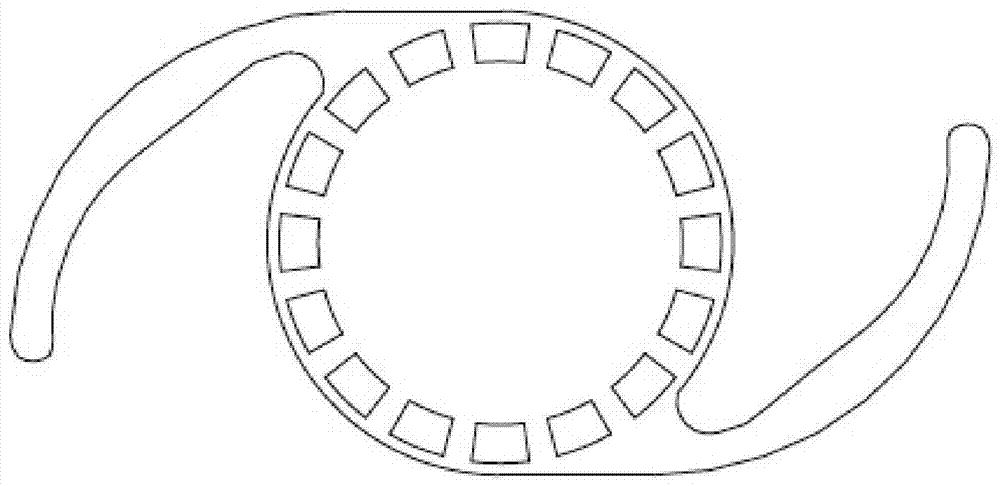
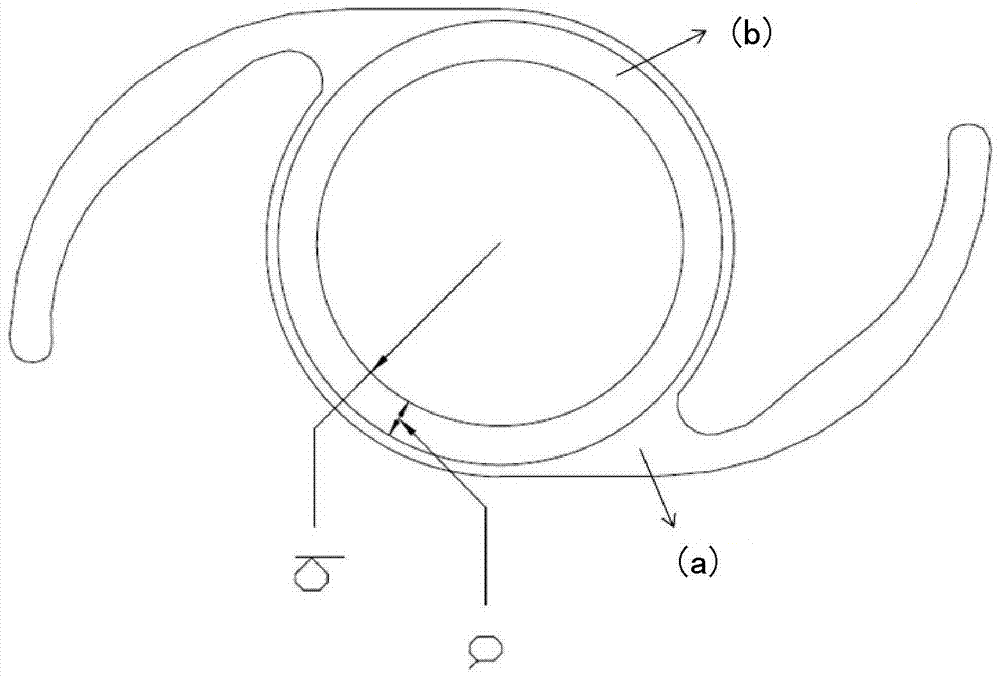
Intraocular Device to Restore Natural Capsular Tension after Cataract Surgery
InactiveUS20130304206A1Restoring natural tensionRestore tensionEye surgeryIntraocular lensIntraocular lensCapsular bag
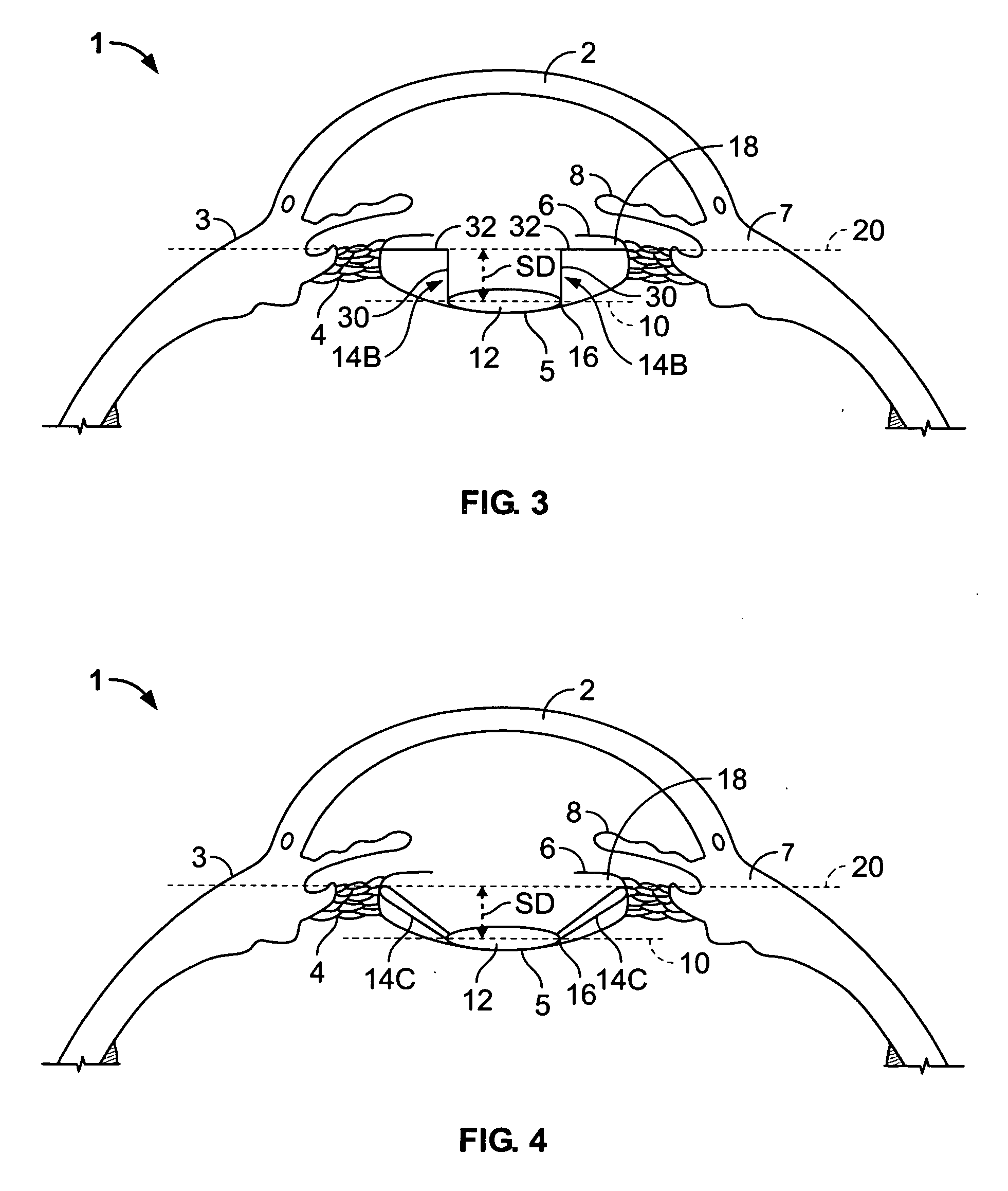
Provided herein are a devices, ophthalmic lens systems and methods for restoring natural tension and anatomy of a lens capsule post-surgically in an eye of a subject. The device generally comprises an inward tensioning ring-like structure having a shape configured to circumferentially fit within and be anchored to a post-surgical lens capsule of the eye. The device may have one or both of external and internal grooves formed to receive the lens capsule and one or both of an intraoptical lens or a tensioning element. The ophthalmic lens system generally comprises the device and an intraocular lens inserted therein. The anchored device provides tension to an equatorial area of the capsule resulting in a decrease in equatorial diameter.
Owner:PALLIKARIS IOANNIS
Method and device for performing online aberrometrie in refractive eye correction indices
The invention relates to a method and a device for the complete correction of sight defects in the human eye. Combinations of measuring, and processing methods are described which when applied as disclosed in the invention, make it possible to fully correct sight defects in the human eye. Measuring methods are used which can precisely scan the surface of the cornea and also register other imaging defects in the light path up to the retina. Computer-aided of said measuring results determined when combined with calculation of ideally corrected ocular lenses (for example after cataract operations) or ideally corrected surfaces of the cornea opens up the possibility of manufacturing a patient-specific lens and / or achieving ideal correction of the cornea using preferably a topography-supported spot-scanning-excimer laser system.
Owner:CARL ZEISS MEDITEC AG
Ocular solutions
InactiveUS7083803B2Reduce inflammationReduce bacterial growthBiocideSenses disorderDiseaseEverolimus
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease.
Owner:PEYMAN GHOLAM A DR
Ocular solutions
InactiveUS20050063996A1Reduce turbidityReduce inflammationAntibacterial agentsBiocideEverolimusCell migration
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
Maintaining preoperative position of the posterior lens capsule after cataract surgery
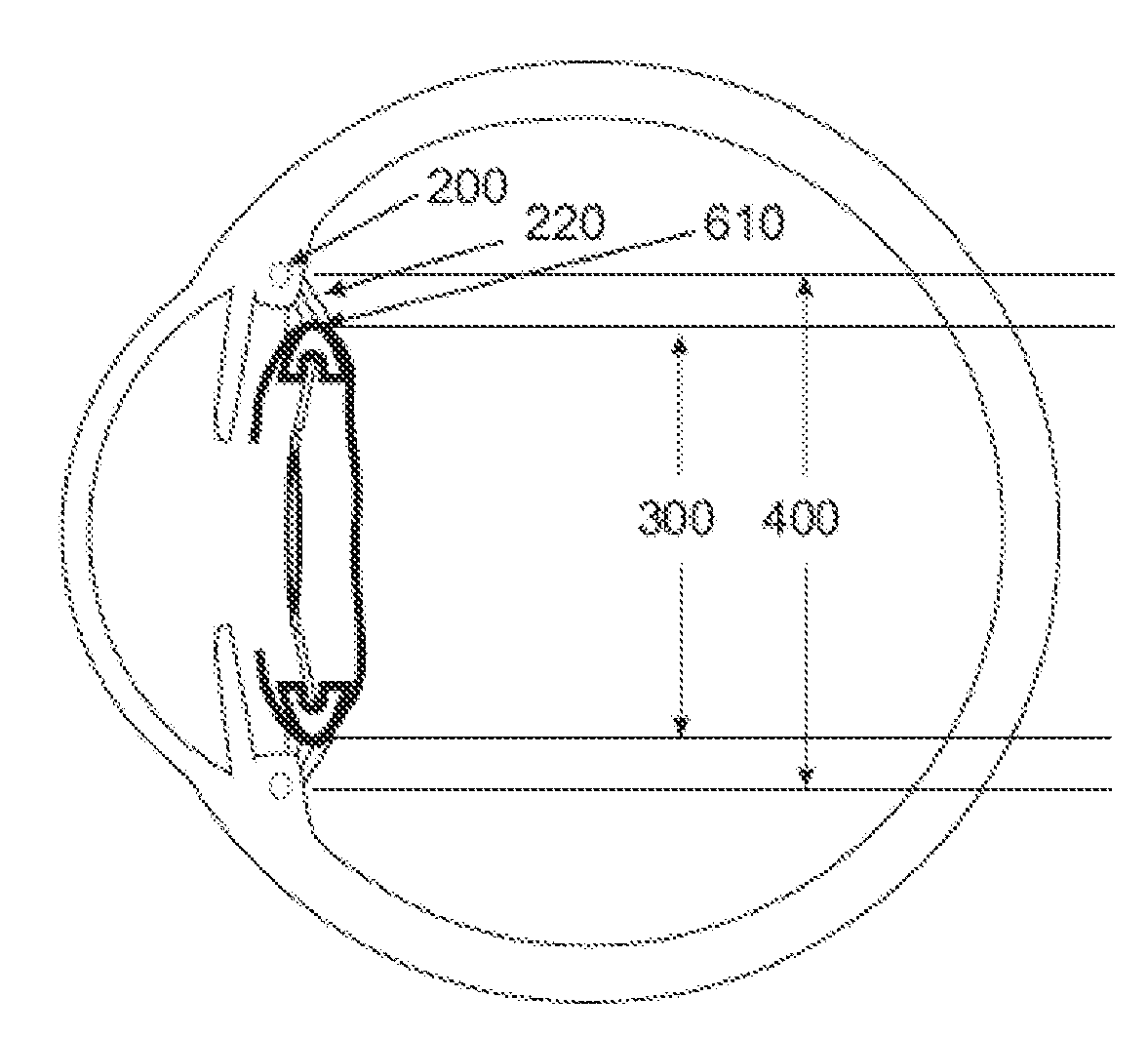
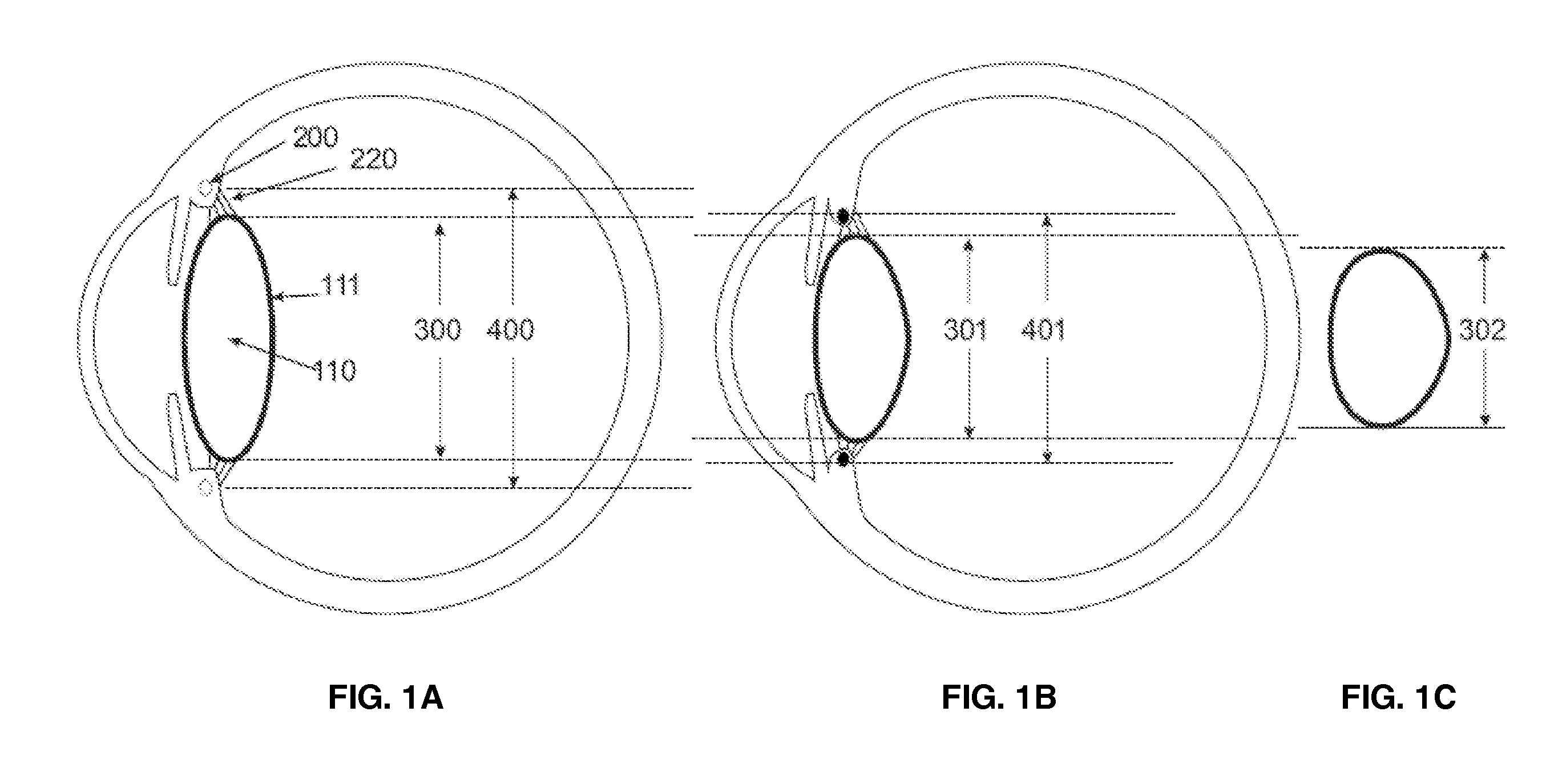
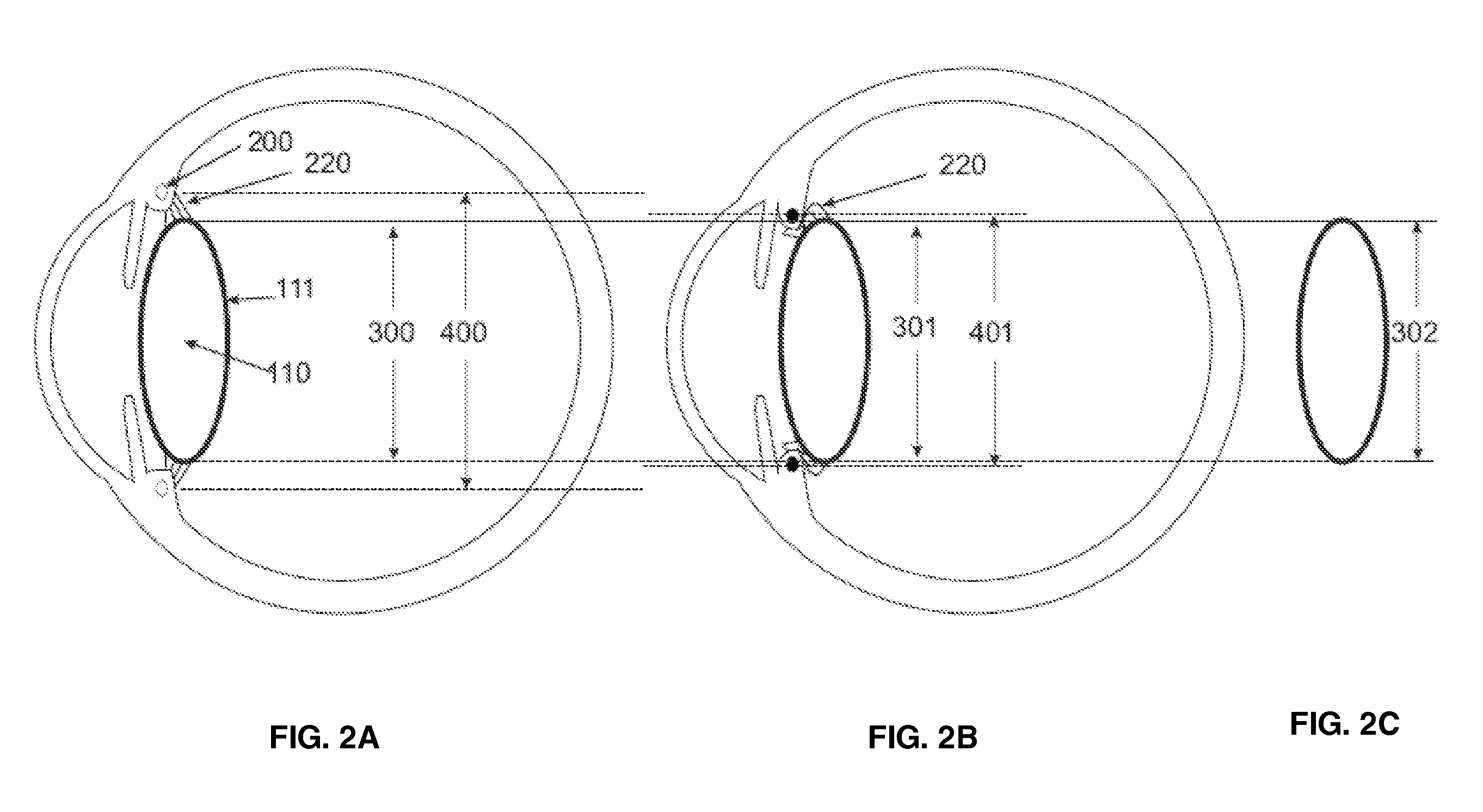
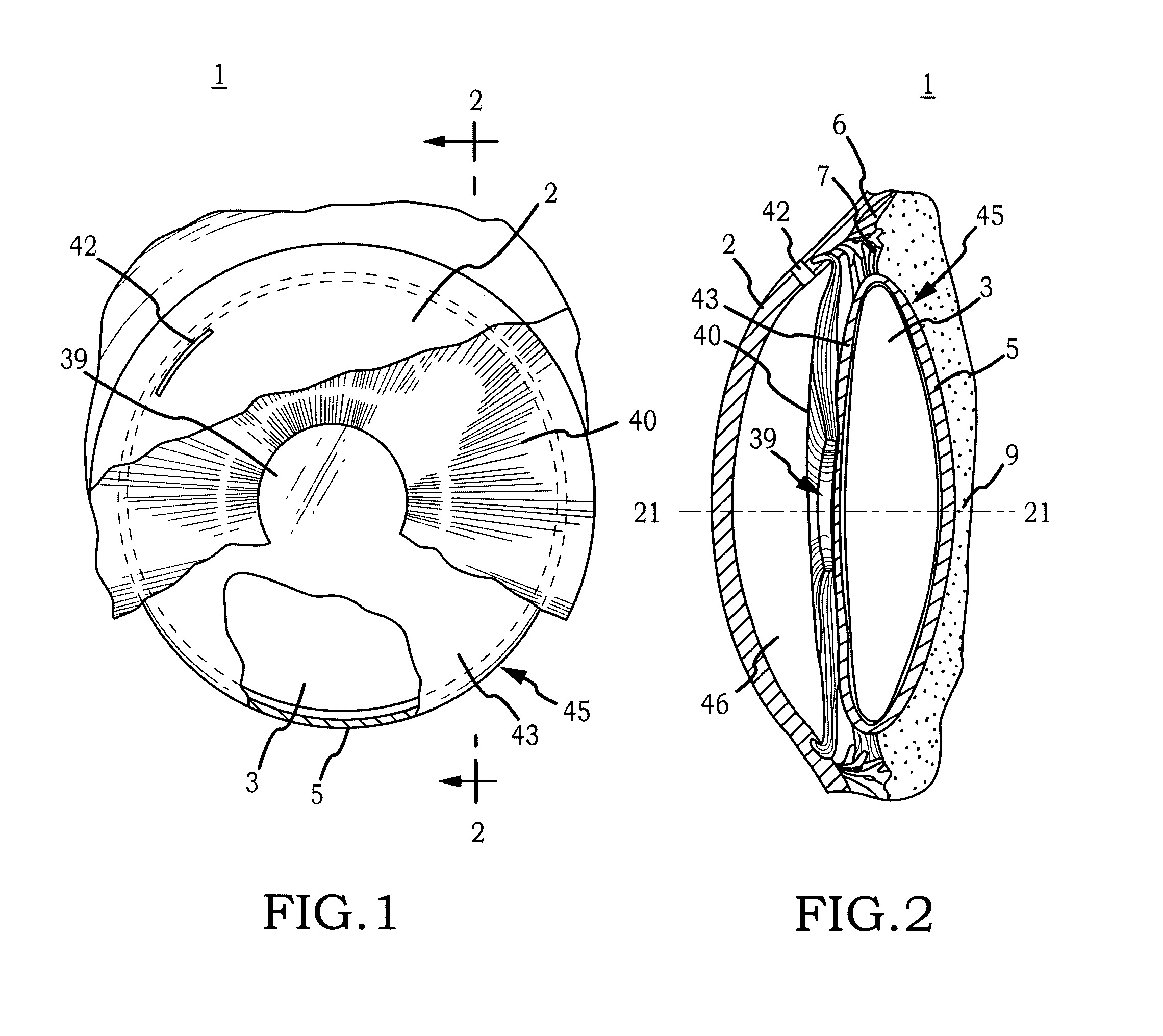
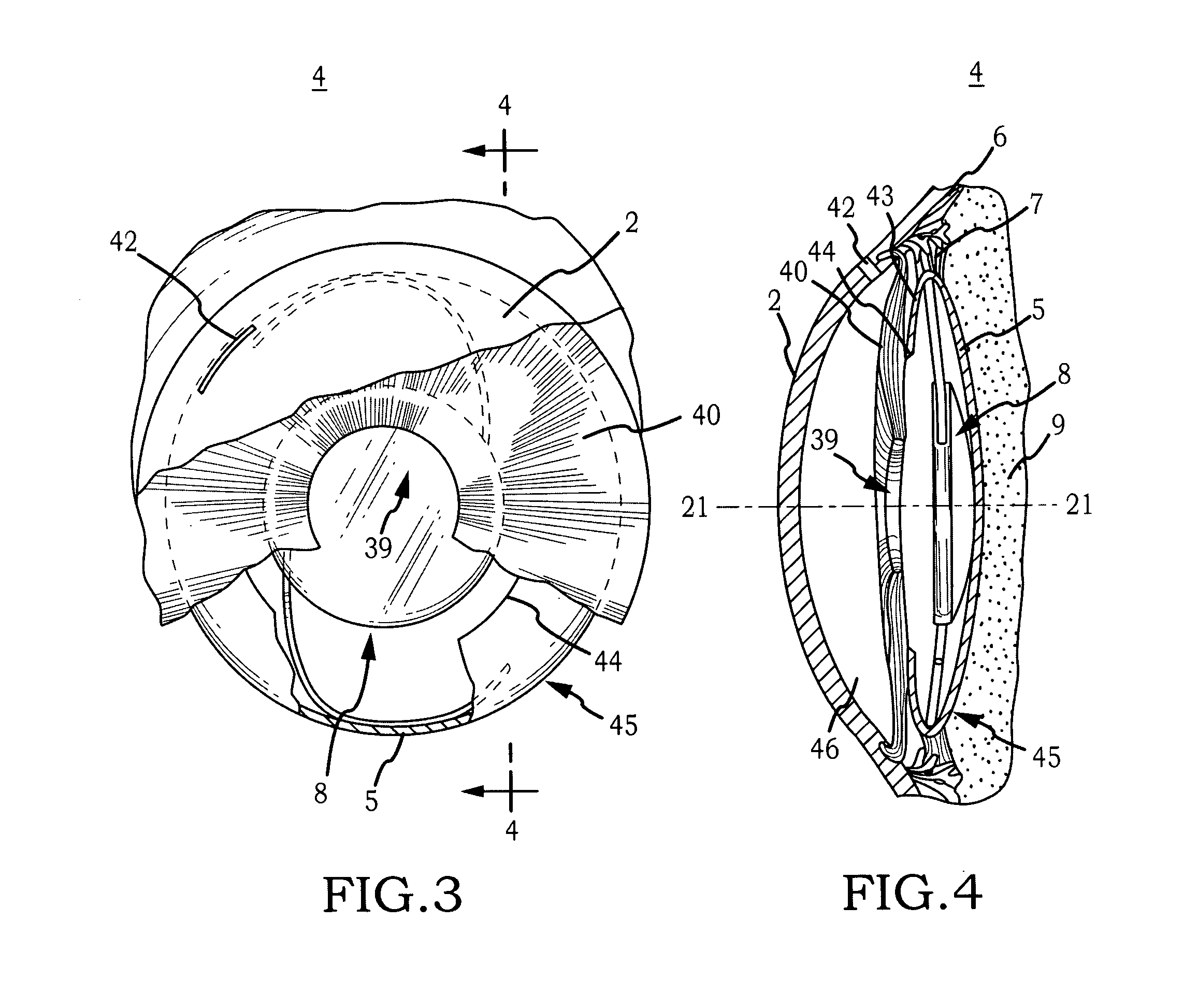
Intraocular lens implant that includes a lens optic and lens haptics configured to maintain a preoperative position of the posterior lens capsule after cataract removal and insertion of a lens implant. The lens haptics have proximal and distal portions, with the distal portions lying in a common plane and the lens optic extending in a lens optic plane. The distance between the planes may be at least substantially the same dimension as or larger than a shift distance that the posterior lens capsule would otherwise traverse between its normal anatomical location and its shifted anatomical location where it not constrained. The shifted anatomical location arises naturally after both removal of cataract lens material and removal of a portion of an anterior capsule.
Owner:NOVARTIS AG
Intraocular Lens Cell Migration Inhibition System
InactiveUS20120232649A1Inhibit migrationReduce posterior capsule opacificationEye treatmentTissue regenerationPosterior capsular opacificationLens epithelial cell
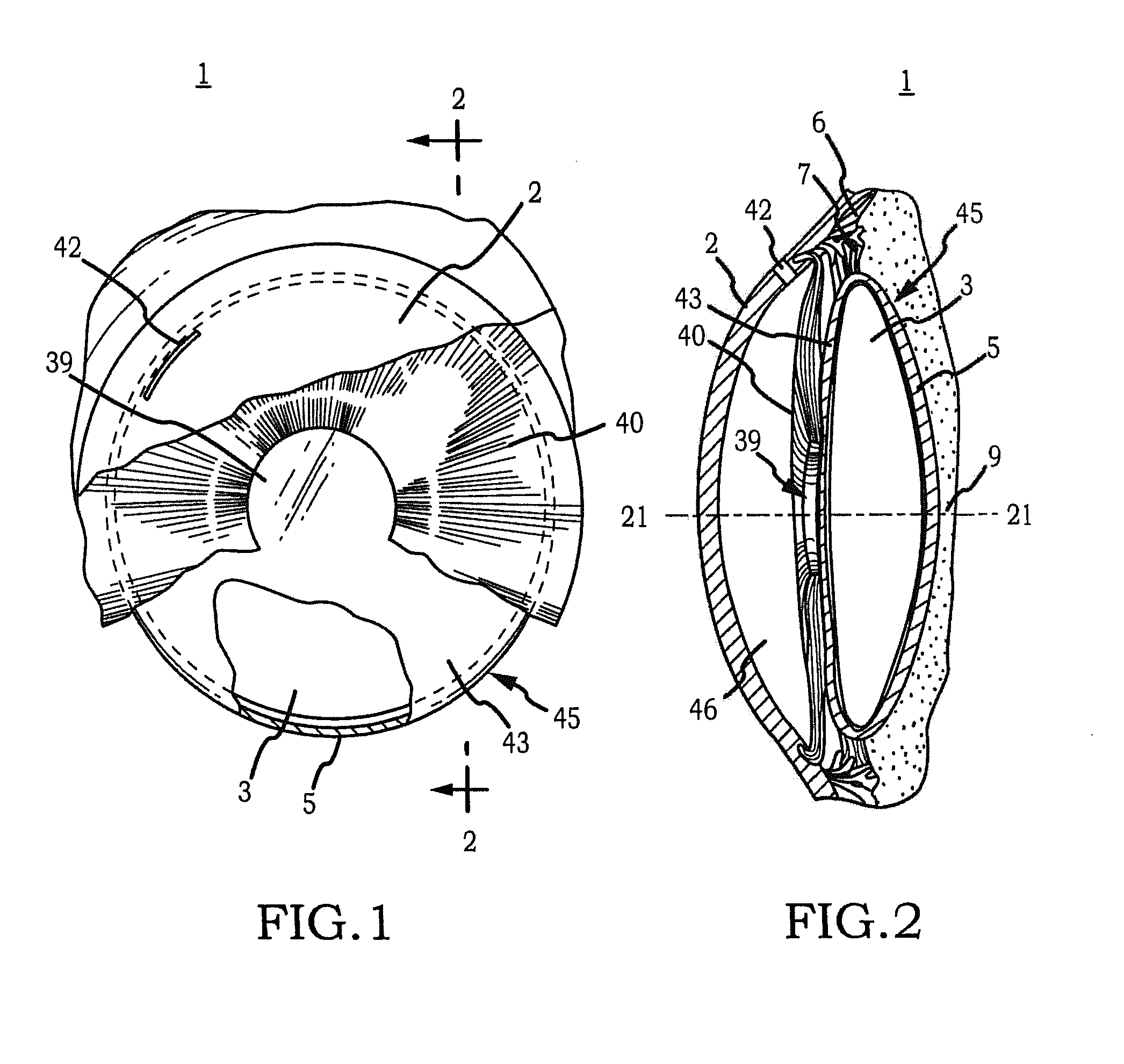
Generally, an intraocular implant and methods for treating an ocular condition. In particular, an intraocular implant which implanted between an intraocular lens and the surface of the posterior capsule of the eye inhibits migration of residual lens epithelial cells after cataract surgery by providing structural barriers to reduce posterior capsule opacification of the eye.
Owner:INSIGHT INNOVATIONS
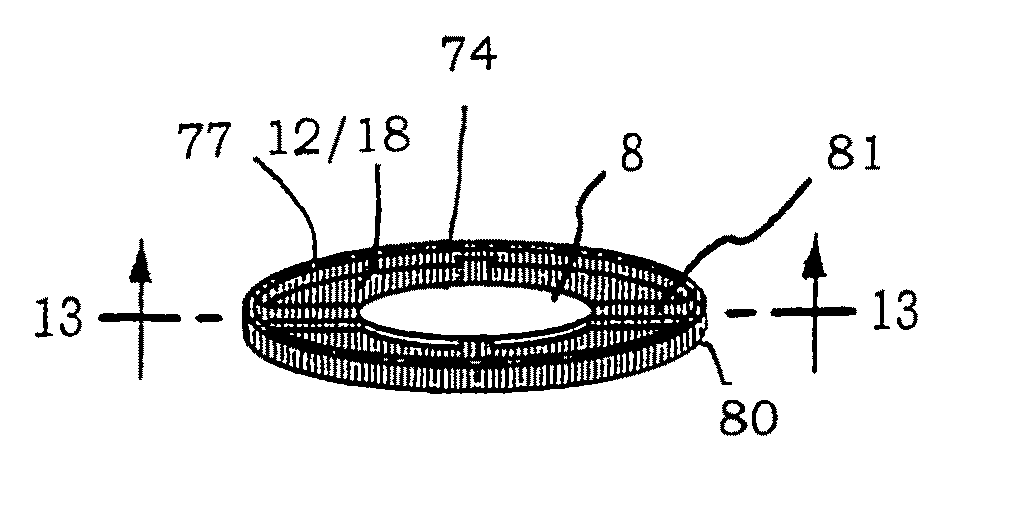
Biocompatible biodegradable intraocular implant system
InactiveUS20110230963A1Prevent proliferationPharmaceutical delivery mechanismEye treatmentIntraocular lensActive agent
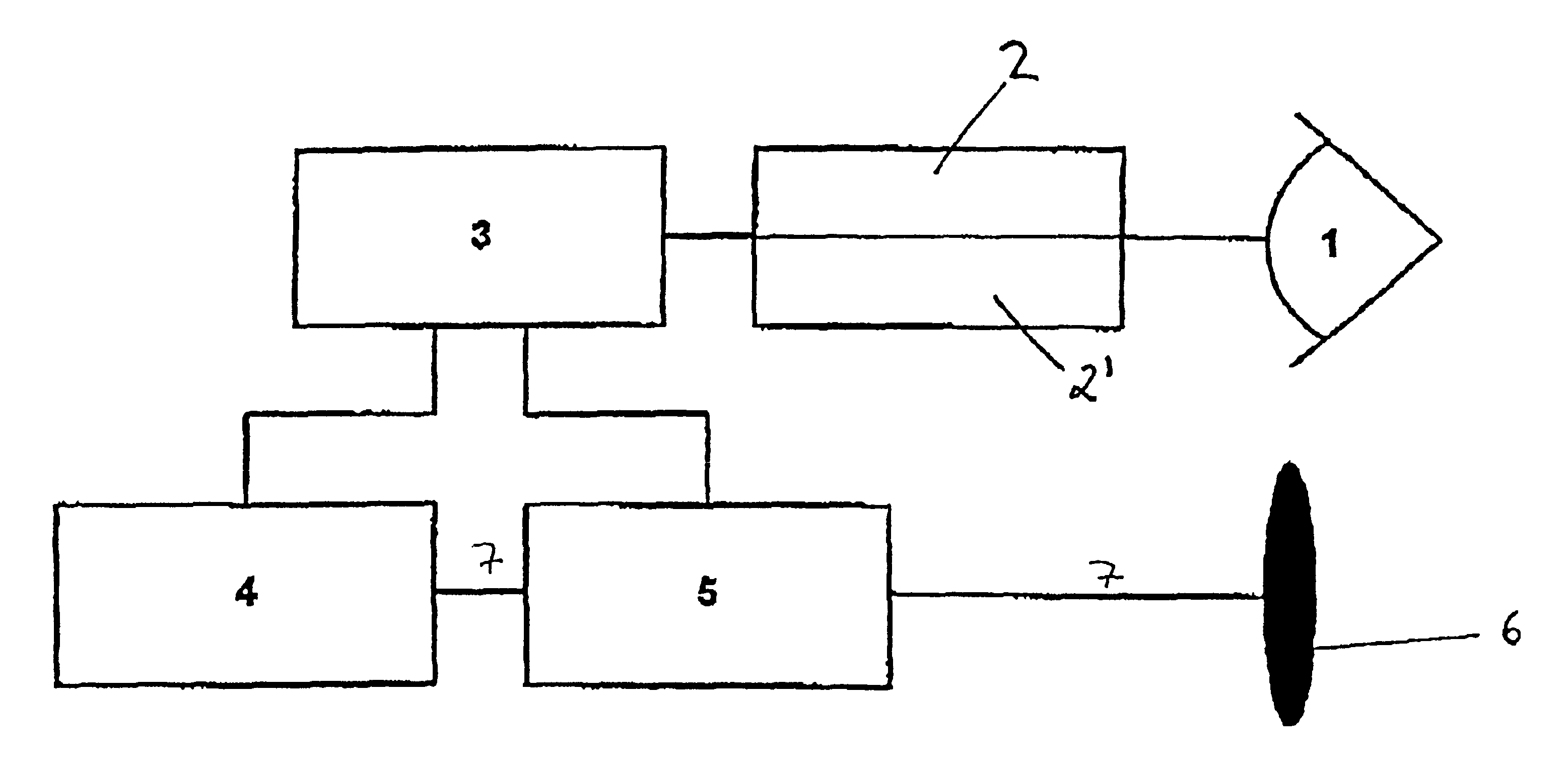
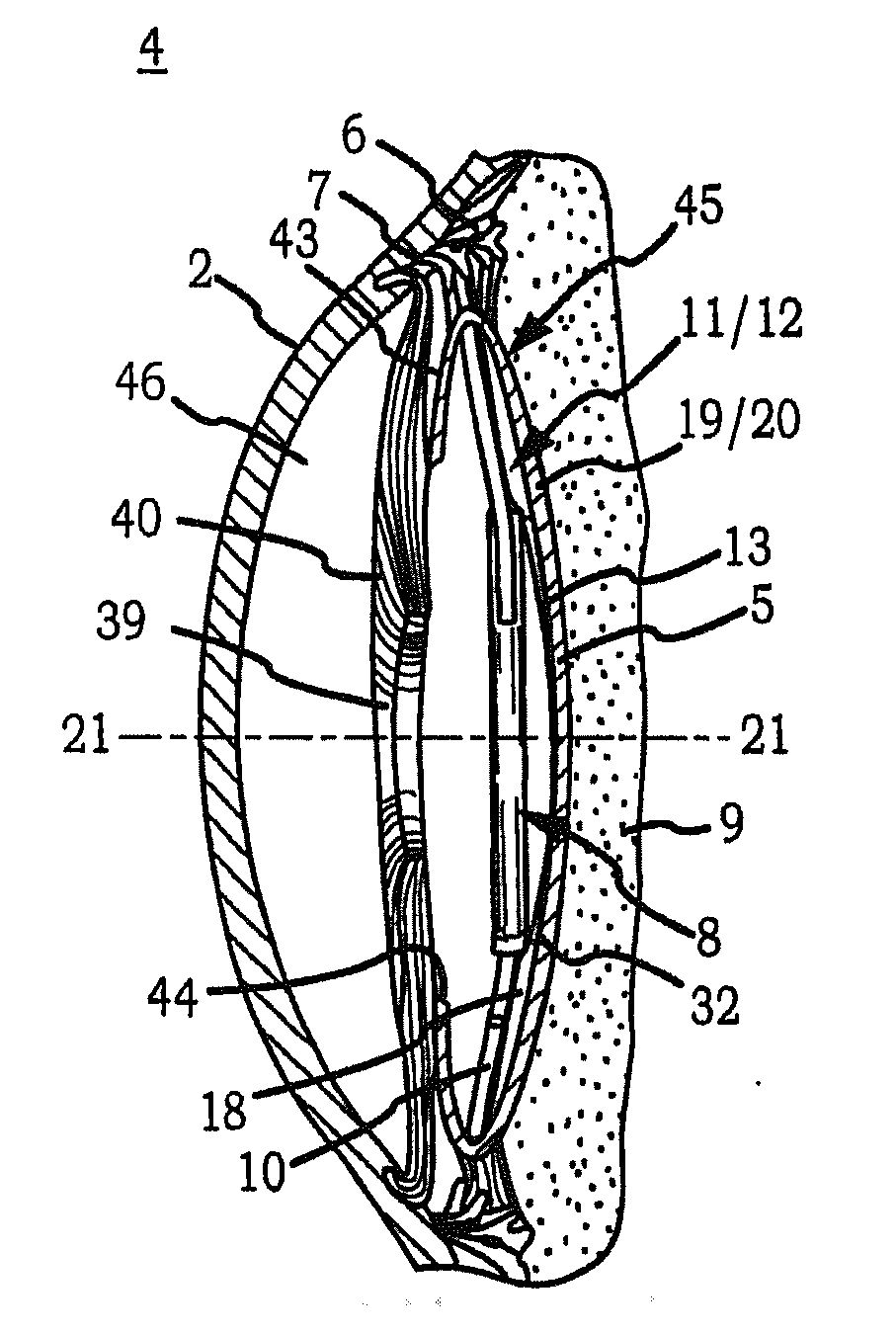
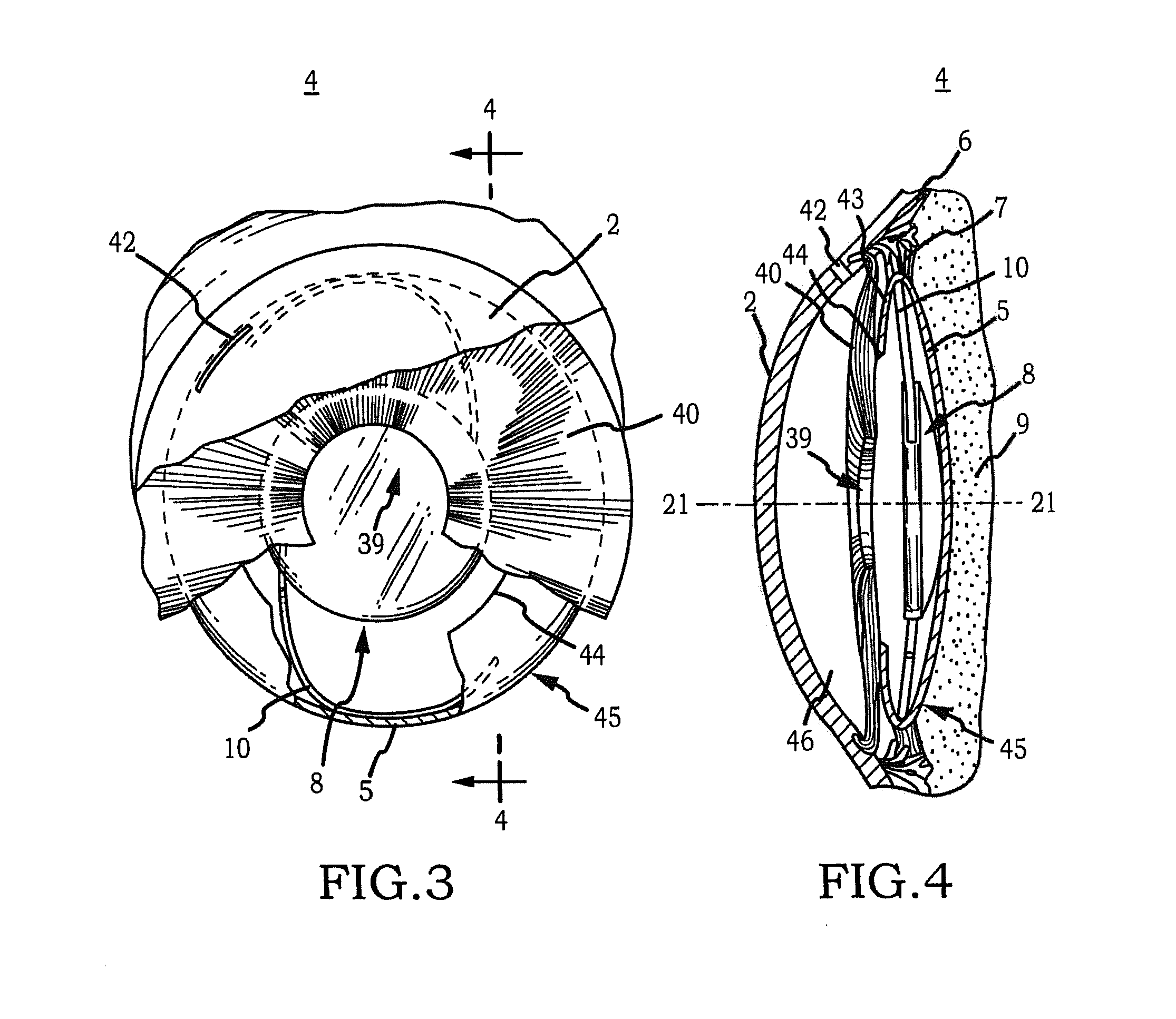
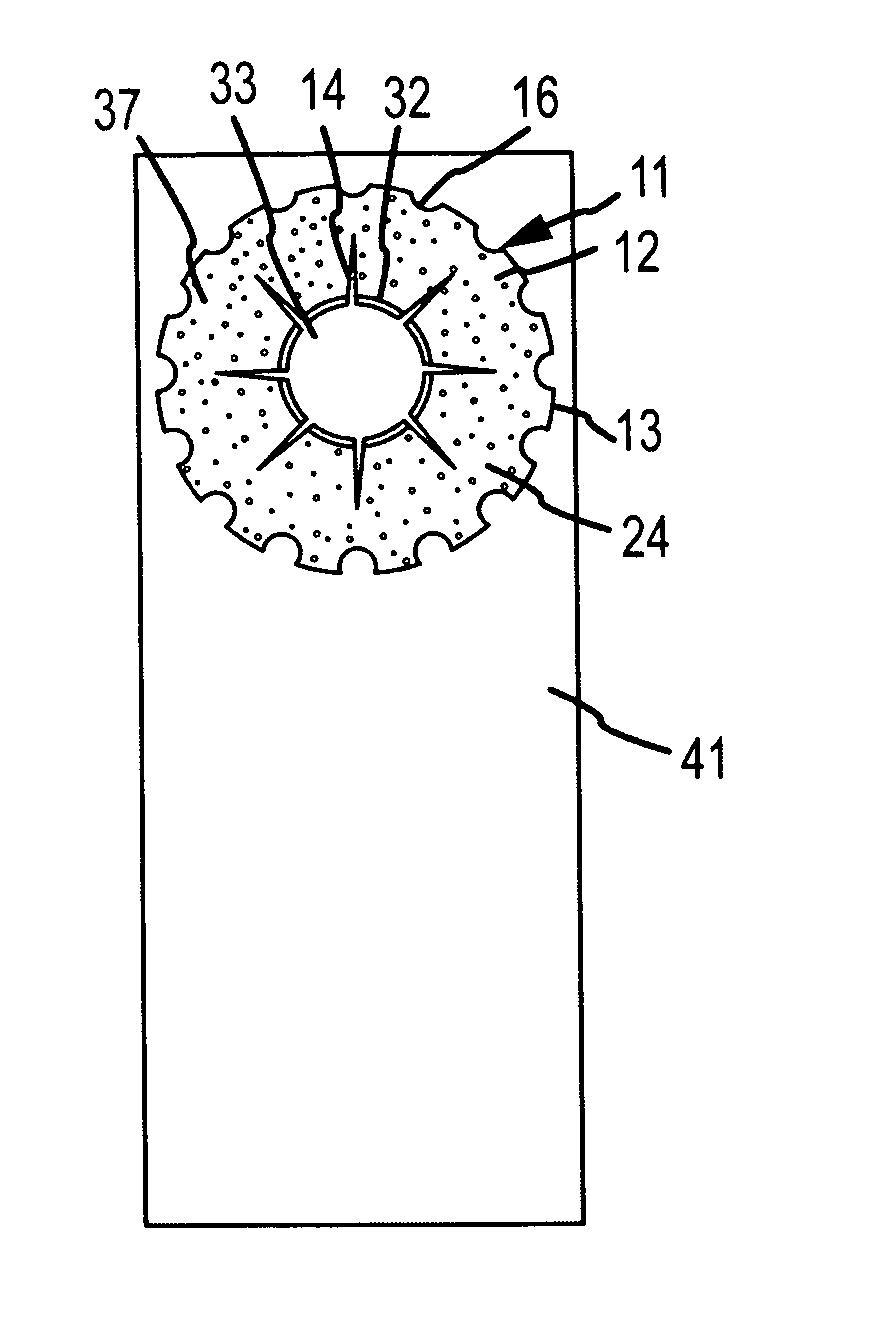
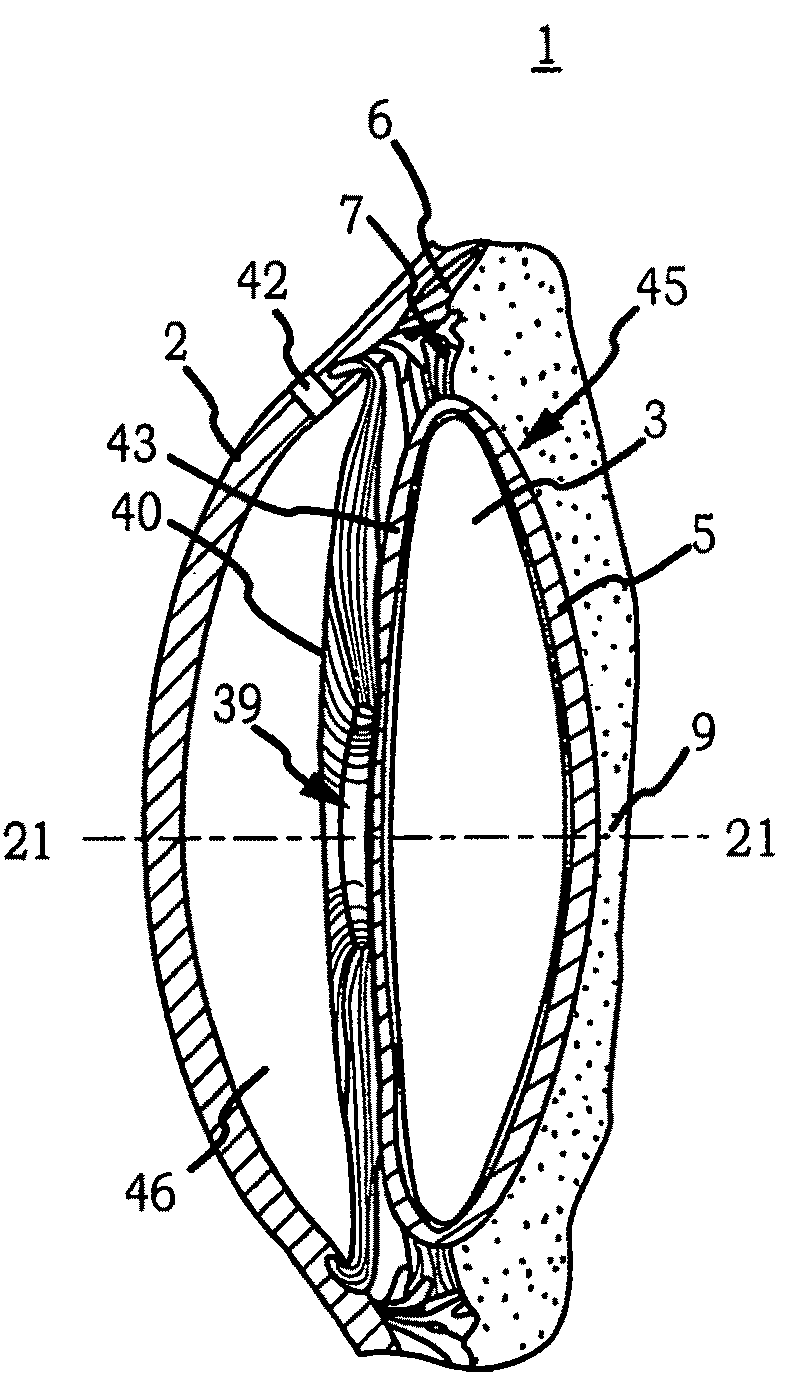
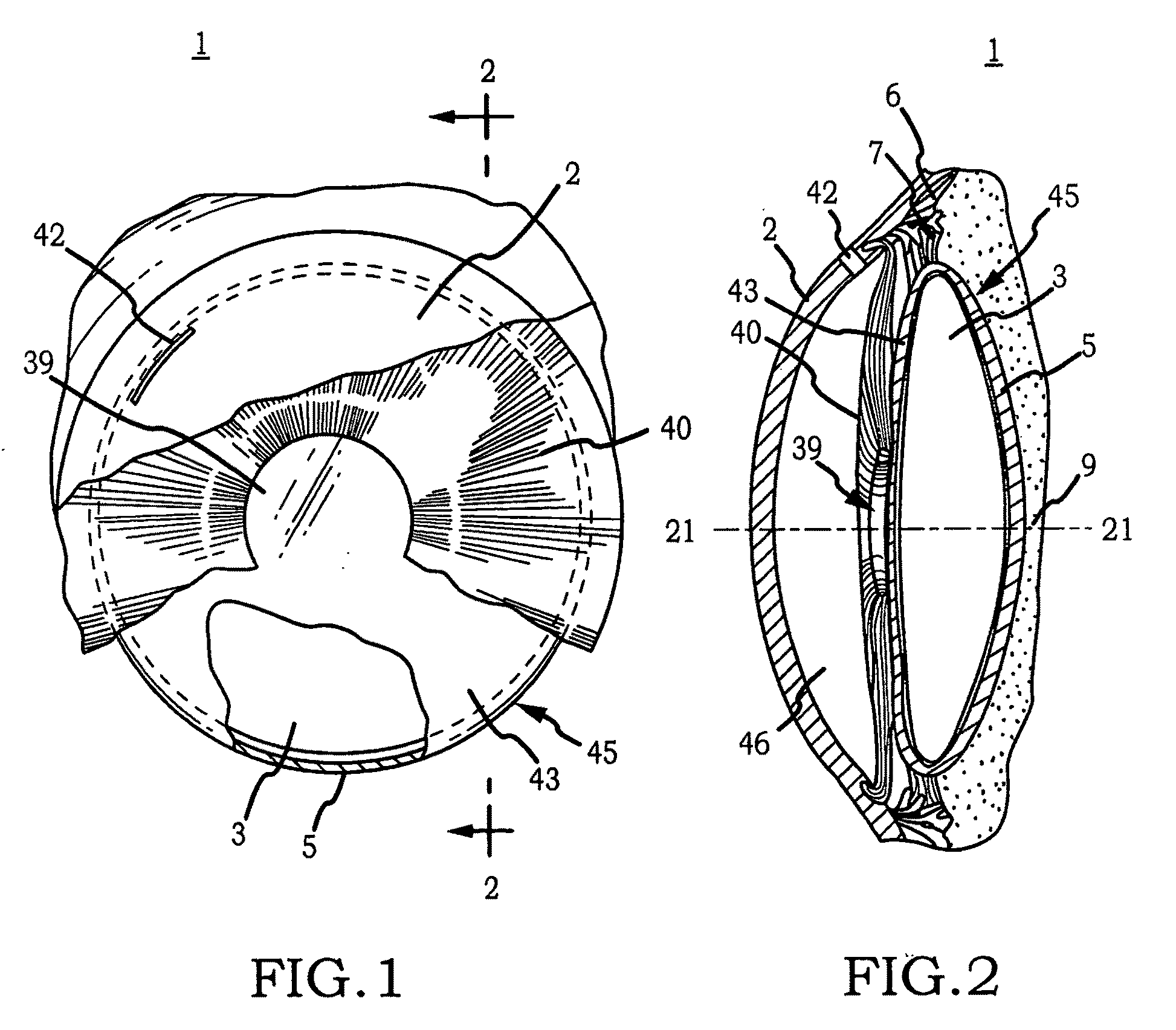
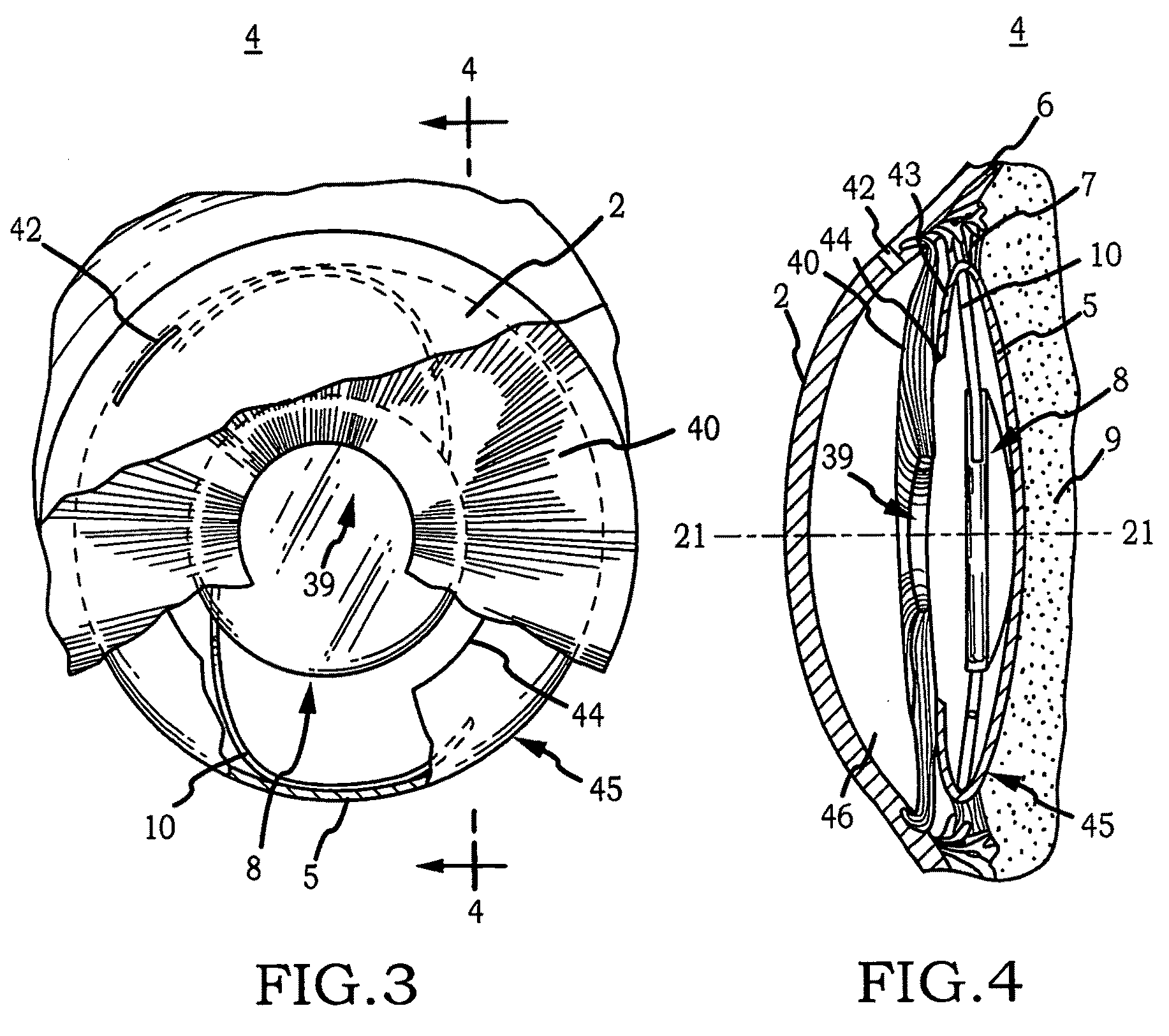
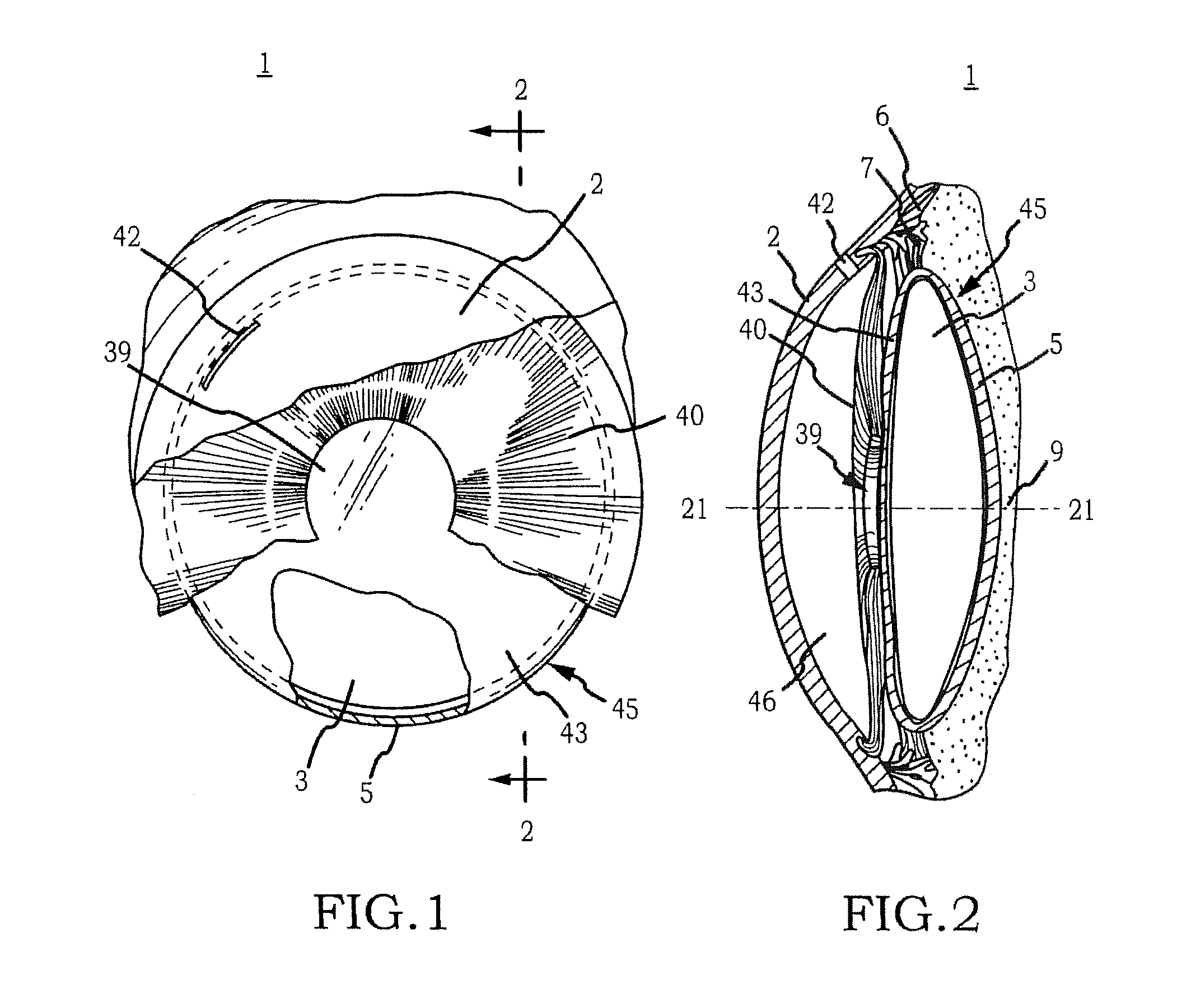
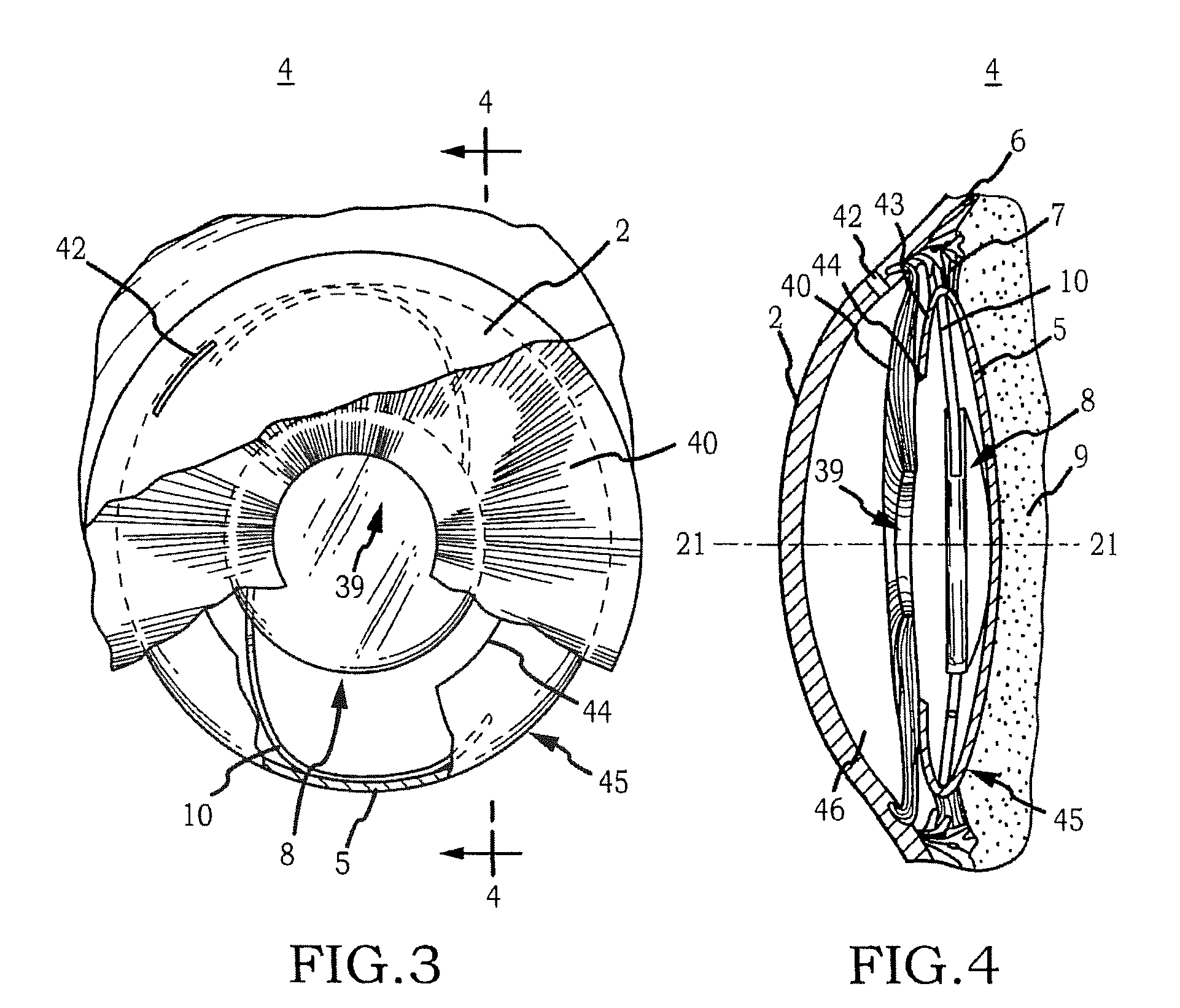
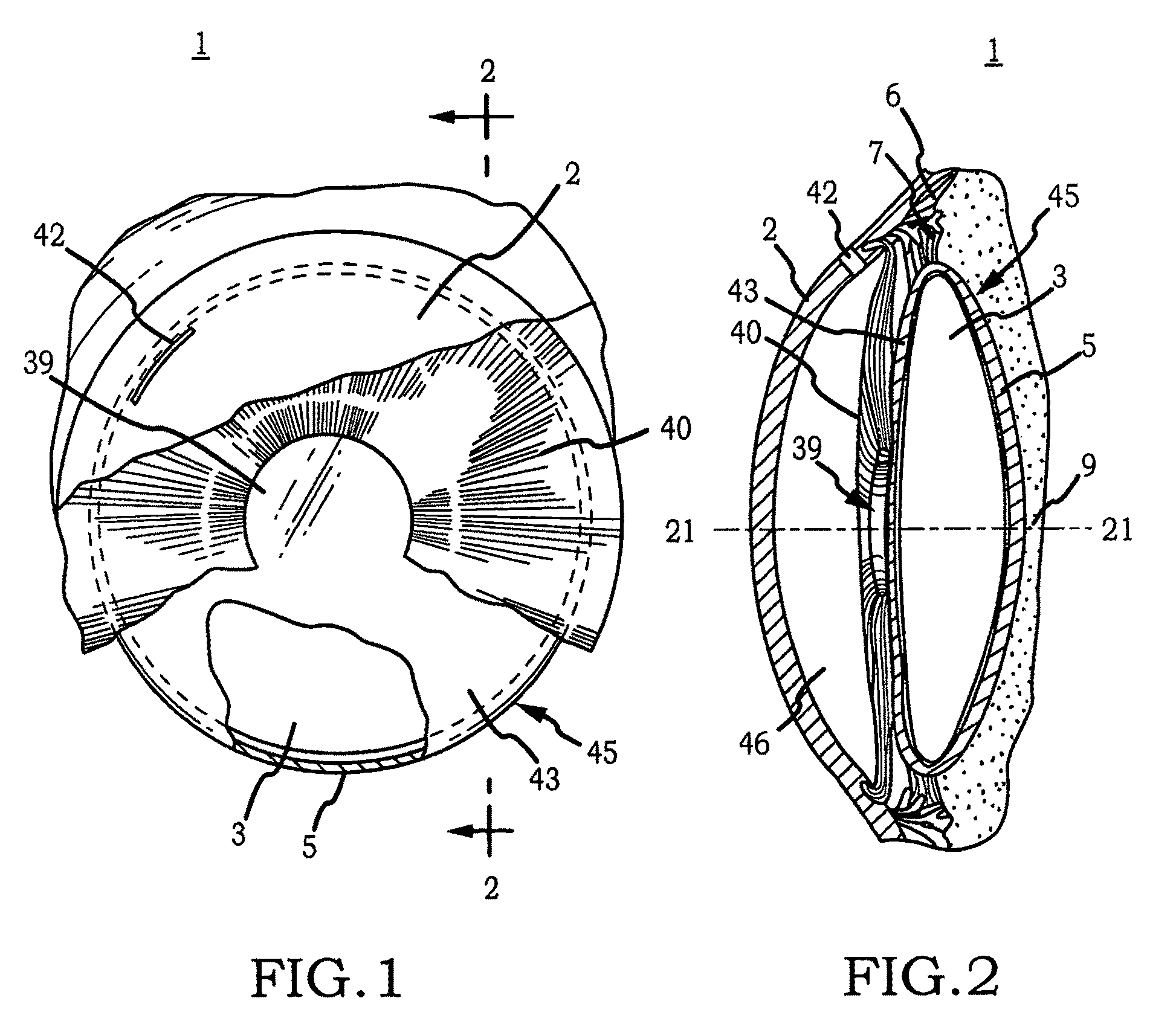
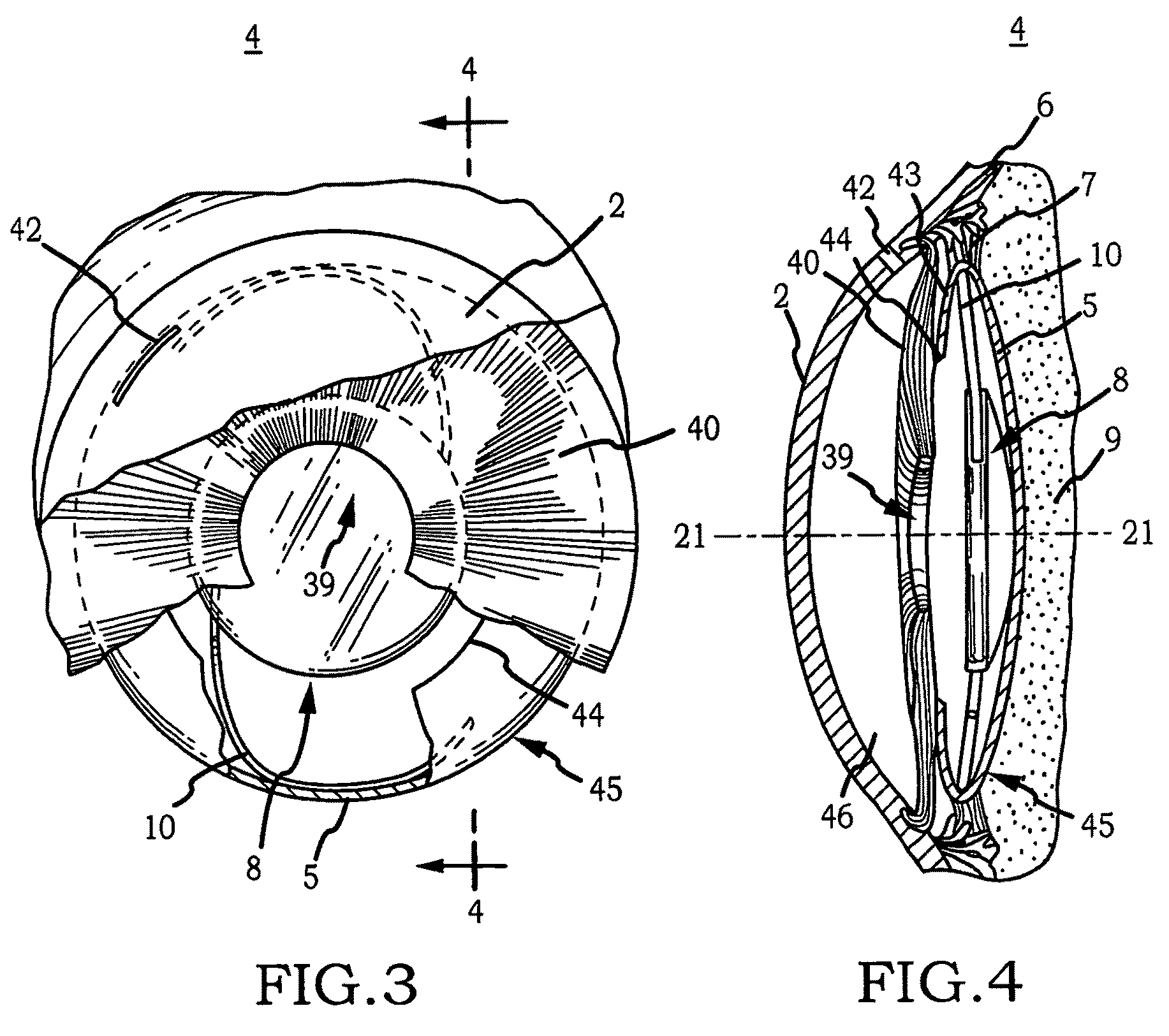
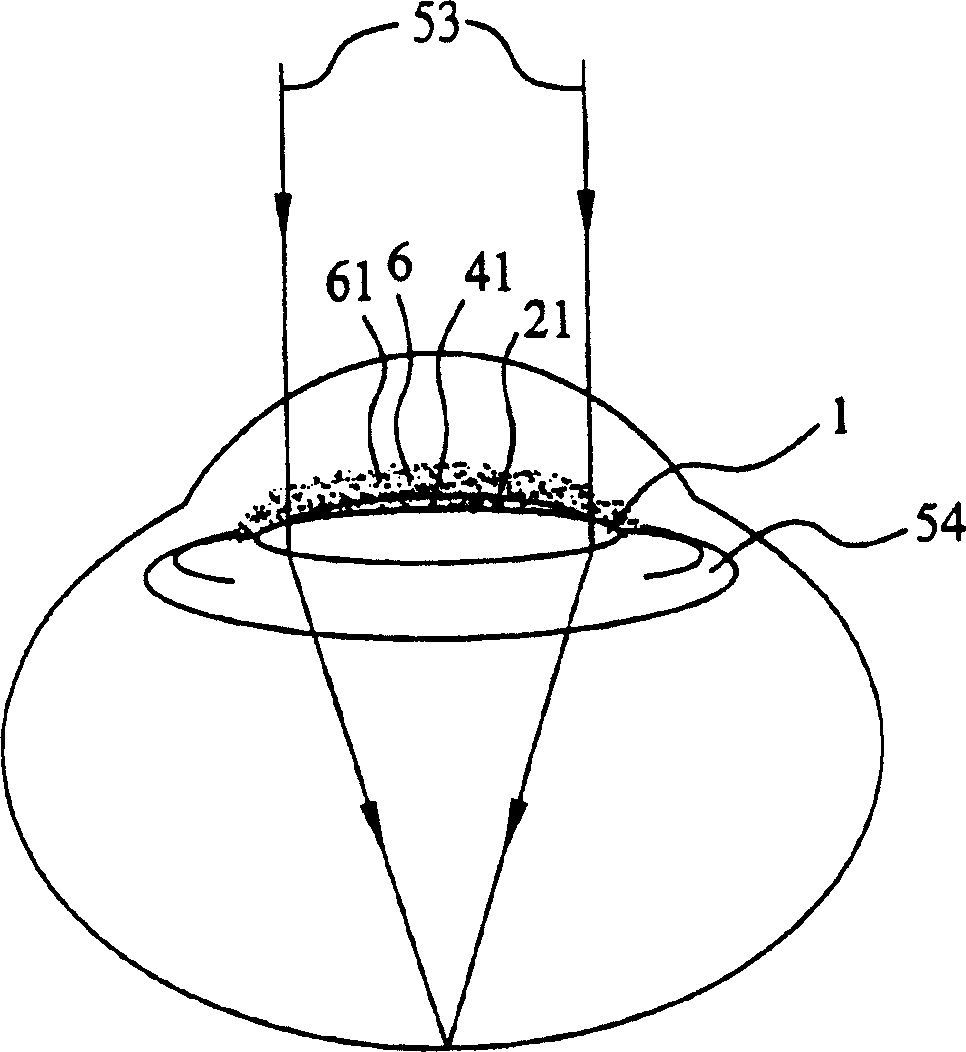
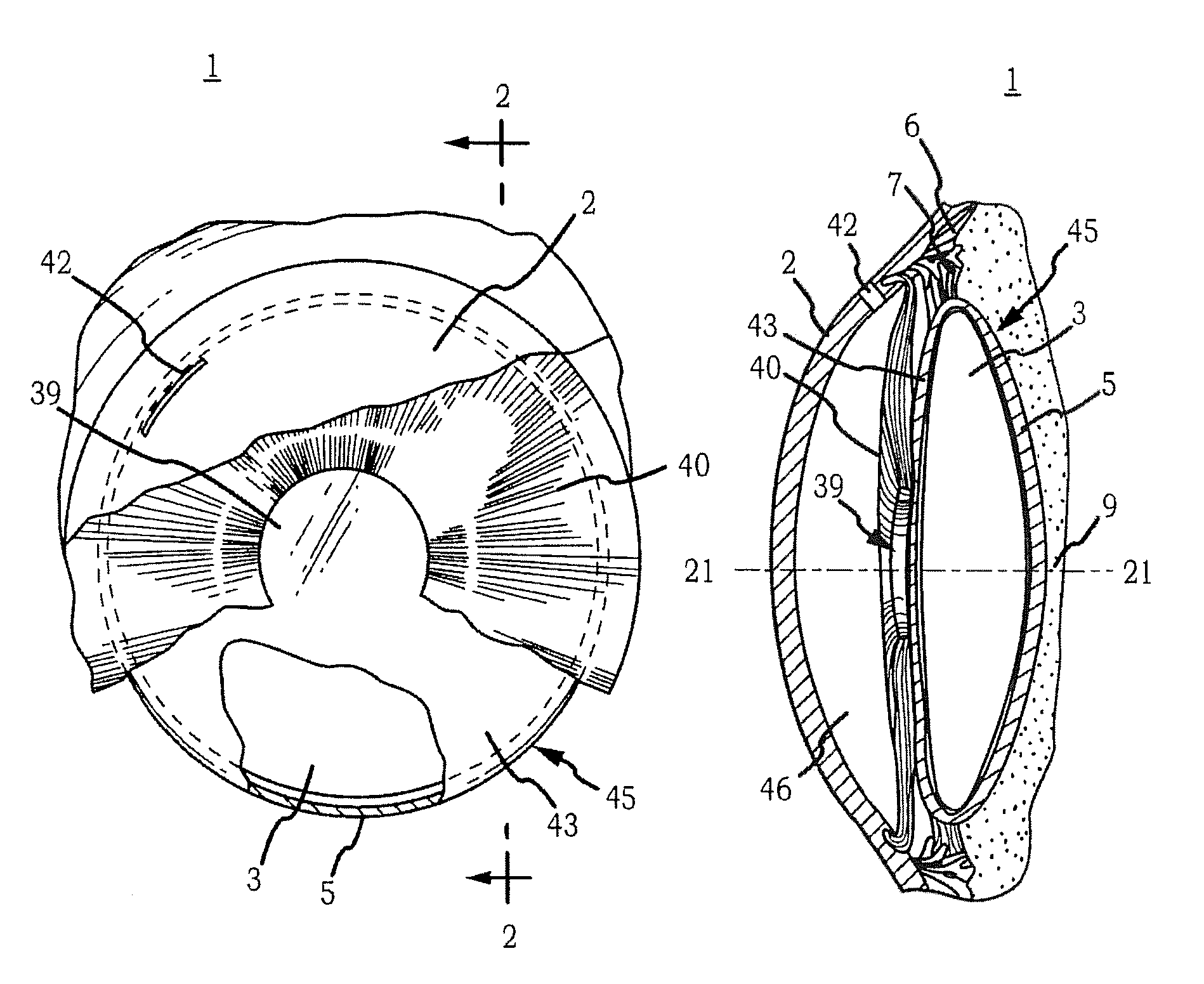
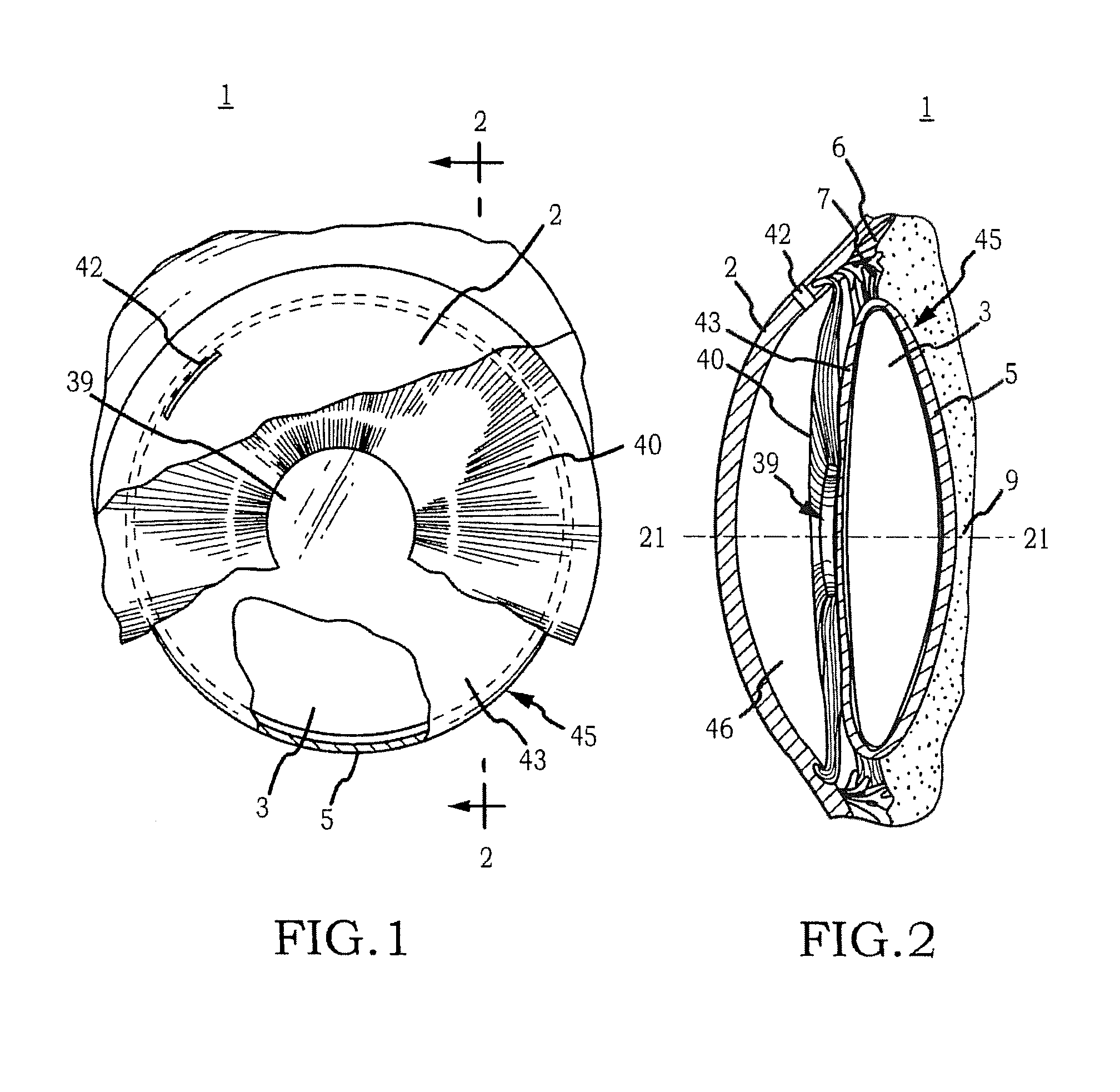
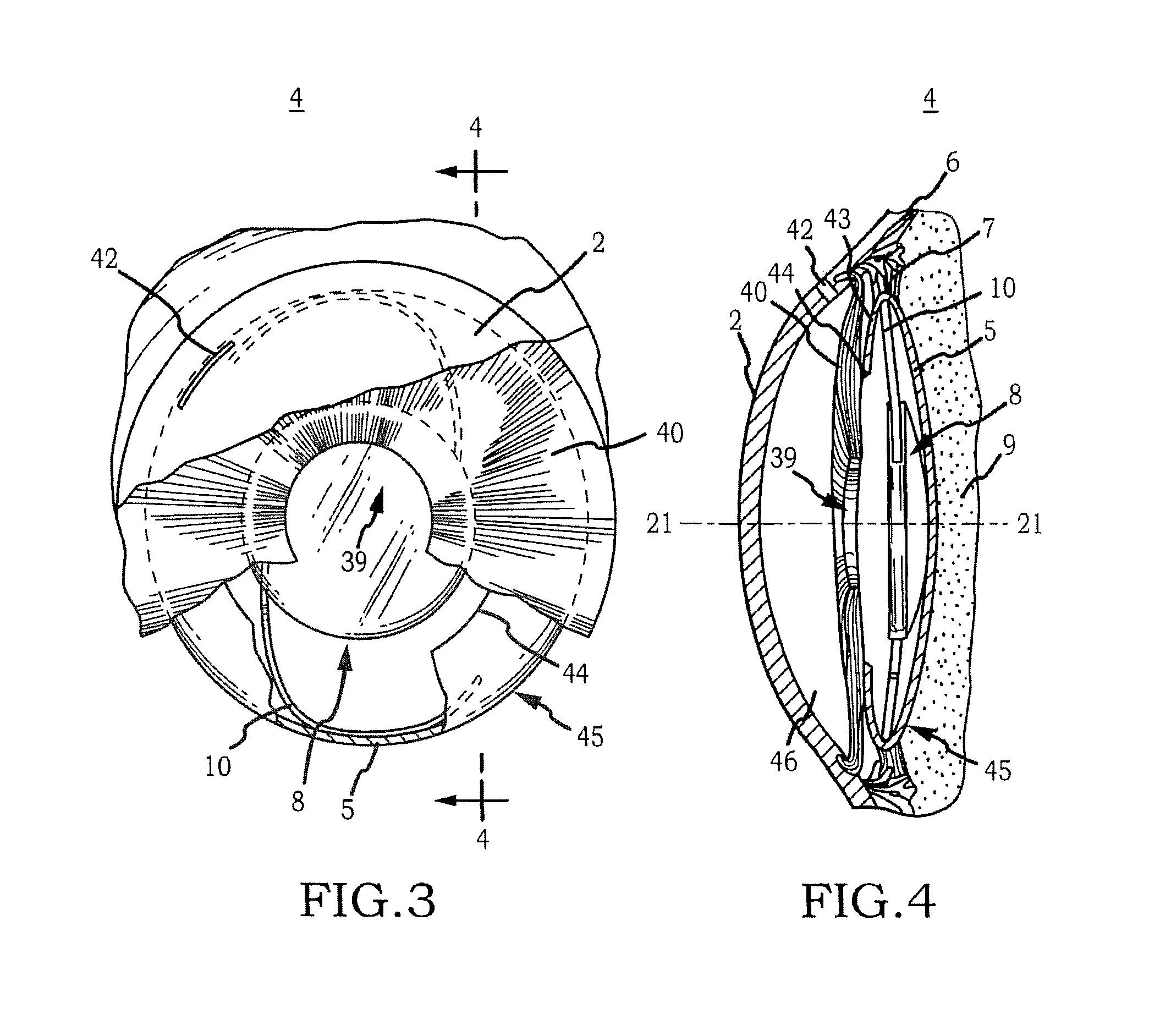
Generally, an intraocular implant and methods for treating an ocular condition. As to certain embodiments, an intraocular biocompatible biodegradable implant (11) which can provide a biocompatible biodegradable material in the form of a flexible membrane (12) containing an active agent (24) which implanted between an intraocular lens (8) and the surface of the posterior capsule (5) of the eye (1)(4) inhibits migration of residual lens epithelial cells after cataract surgery by providing structural or pharmaceutical barriers to reduce posterior capsule (5) opacification of the eye (1)(4).
Owner:INSIGHT INNOVATIONS
Ocular solutions
InactiveUS20060228394A1Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusOcular structure
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease. The agent(s) may be formulated with polymers or other components for extended or slow release to provide a substantially constant concentration over the course of treatment.
Owner:MINU
Ocular solutions
InactiveUS7087237B2Reduce inflammationReduce bacterial growthAntibacterial agentsBiocideEverolimusMacrolide resistance
Containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions.
Owner:PEYMAN GHOLAM A DR
Intraocular lens with iris diaphragm
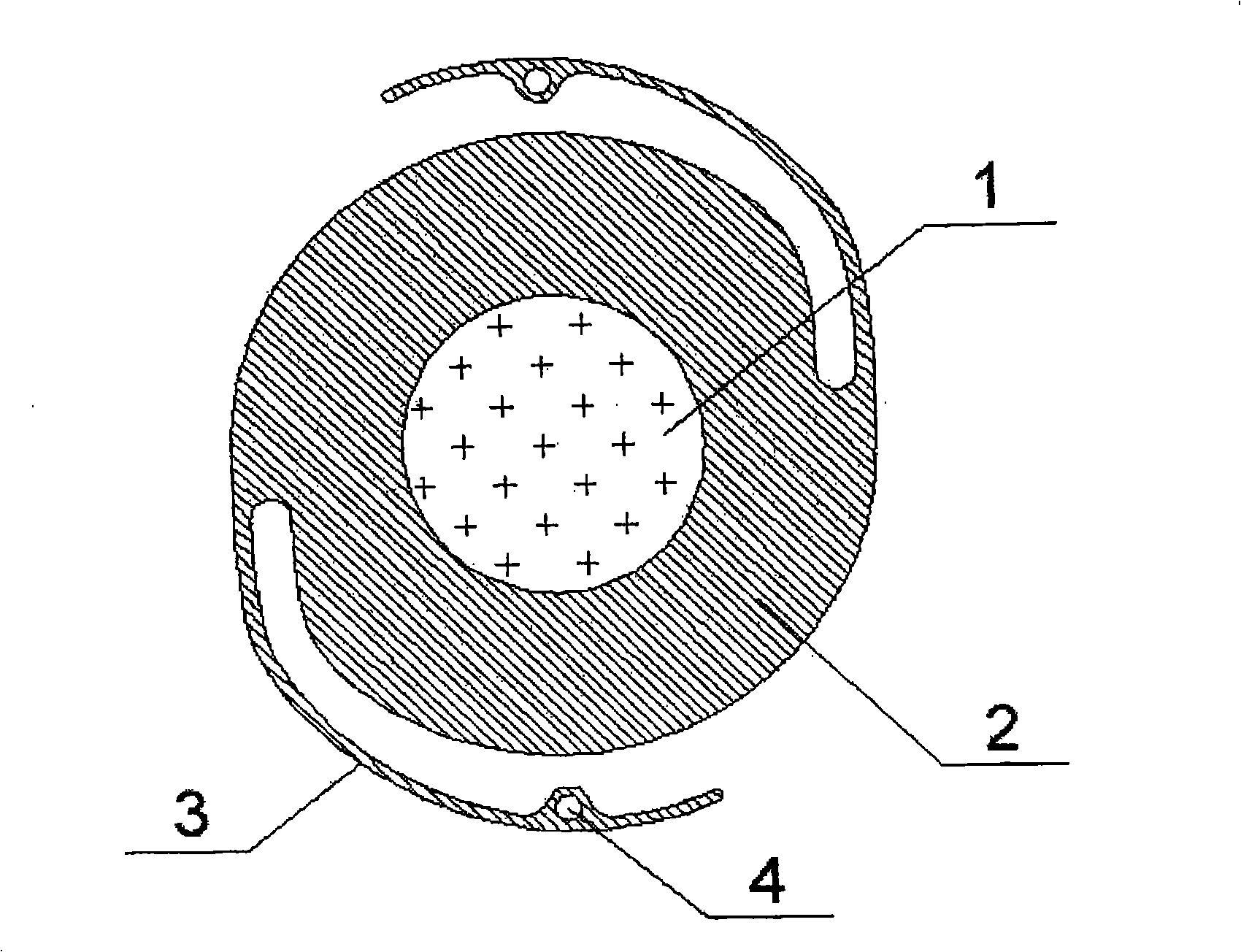
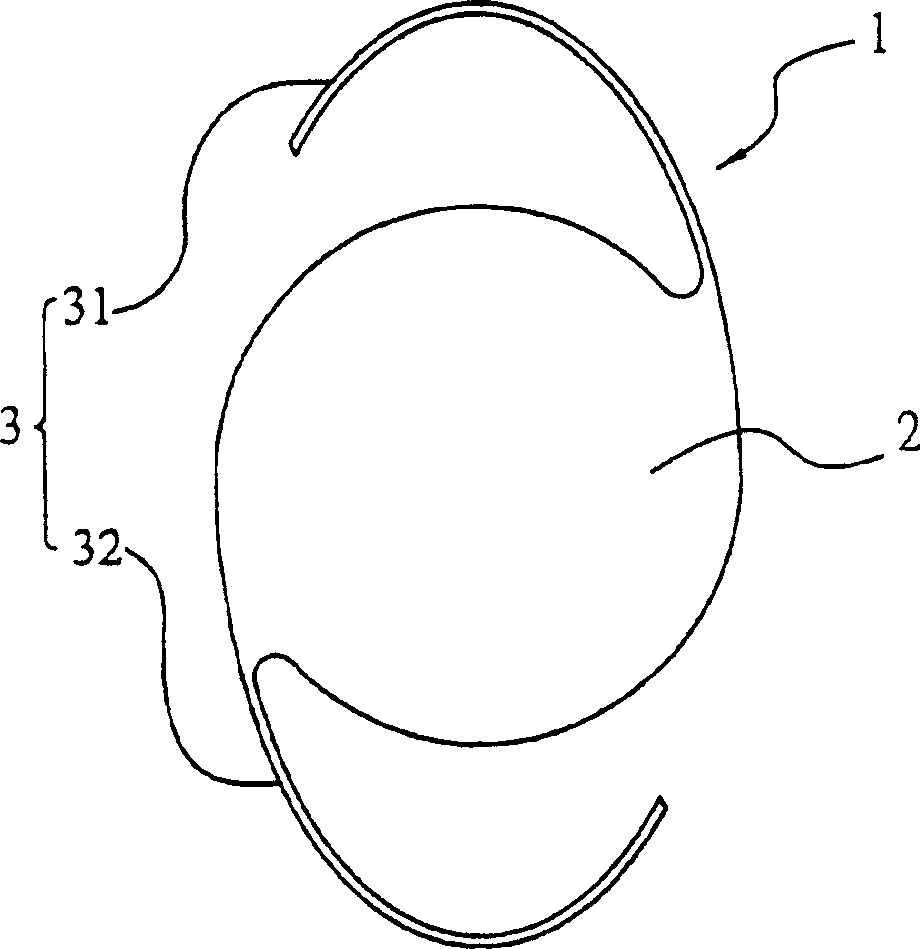
The invention discloses an artificial lens with an iris diaphragm, comprising an optical zone (1) which is transparent at the center, the iris diaphragm (2) surrounding the optical zone (1) and a loop (3); the artificial lens is in an integrated style and is composed of an entire block of material, the material is selected from foldable materials such as hydrophobic acrylic ester, hydrophilic acrylic ester, silica gel, etc. The artificial lens with the iris diaphragm of the invention has soft texture, can be filled or implanted in the eyes by a small incision after being folded; in addition, the artificial lens has high refractive index, thin lens, small incision and slow formation of after cataract and can slowly restore in 2-30 seconds after being implanted, thus cyst membrane is not easy to be damaged; moreover, the artificial lens has the advantages of little astigmia developed in post-operation, quick healing, fast recovery of visual performance and little postoperative treatment; a brown iris, which is the natural color iris of Chinese, can be adopted, and the eye implanted with such an artificial lens looks more natural.
Owner:苏州六六视觉科技股份有限公司
Intraocular implant cell migration inhibition system
InactiveUS20110295367A1Inhibit migrationReduce posterior capsule opacificationEye surgeryTissue regenerationPosterior capsular opacificationLens epithelial cell
Generally, an intraocular implant and methods for treating an ocular condition. In particular, an intraocular implant which implanted between an intraocular lens and the surface of the posterior capsule of the eye inhibits migration of residual lens epithelial cells after cataract surgery by providing structural barriers to reduce posterior capsule opacification of the eye.
Owner:INSIGHT INNOVATIONS
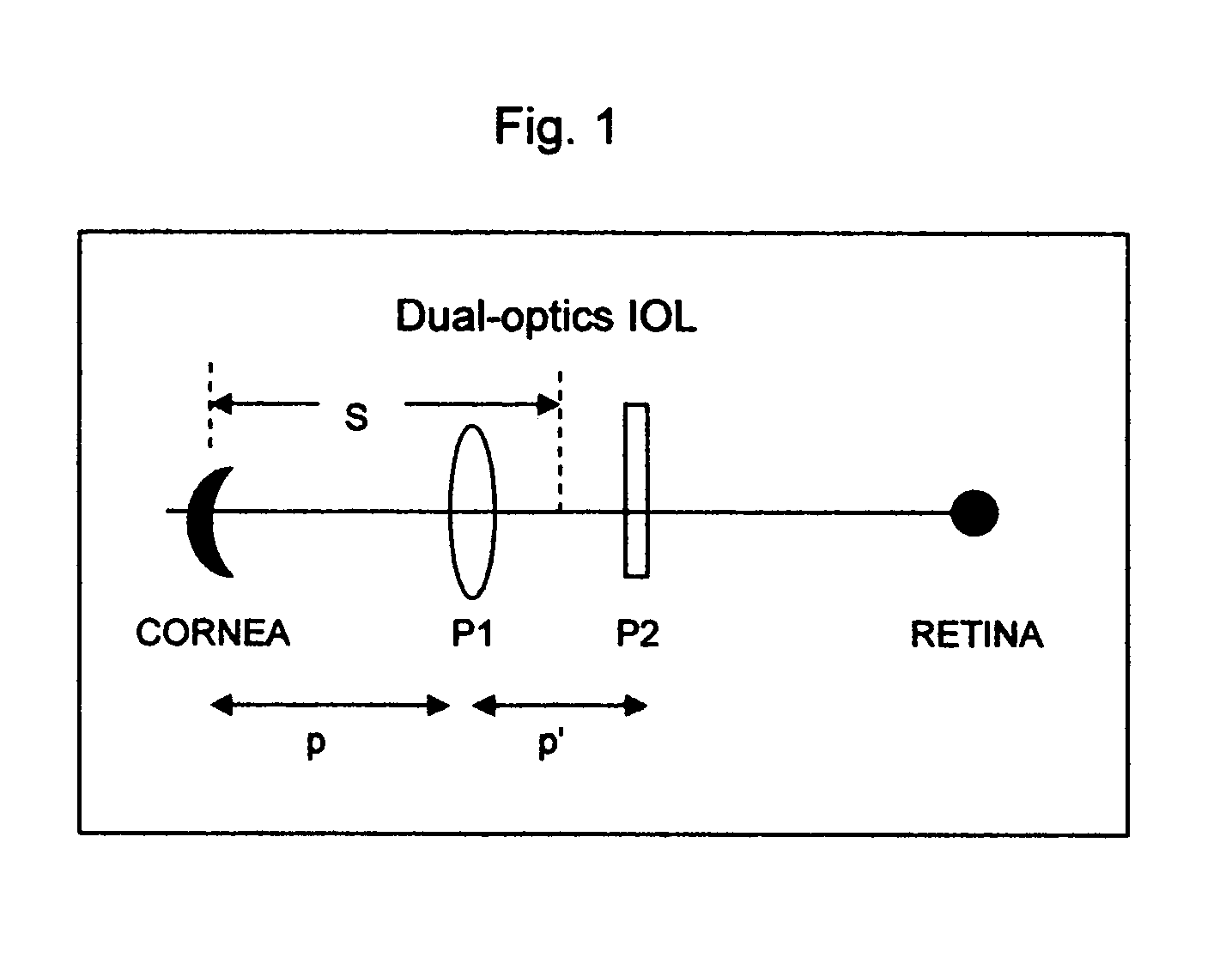
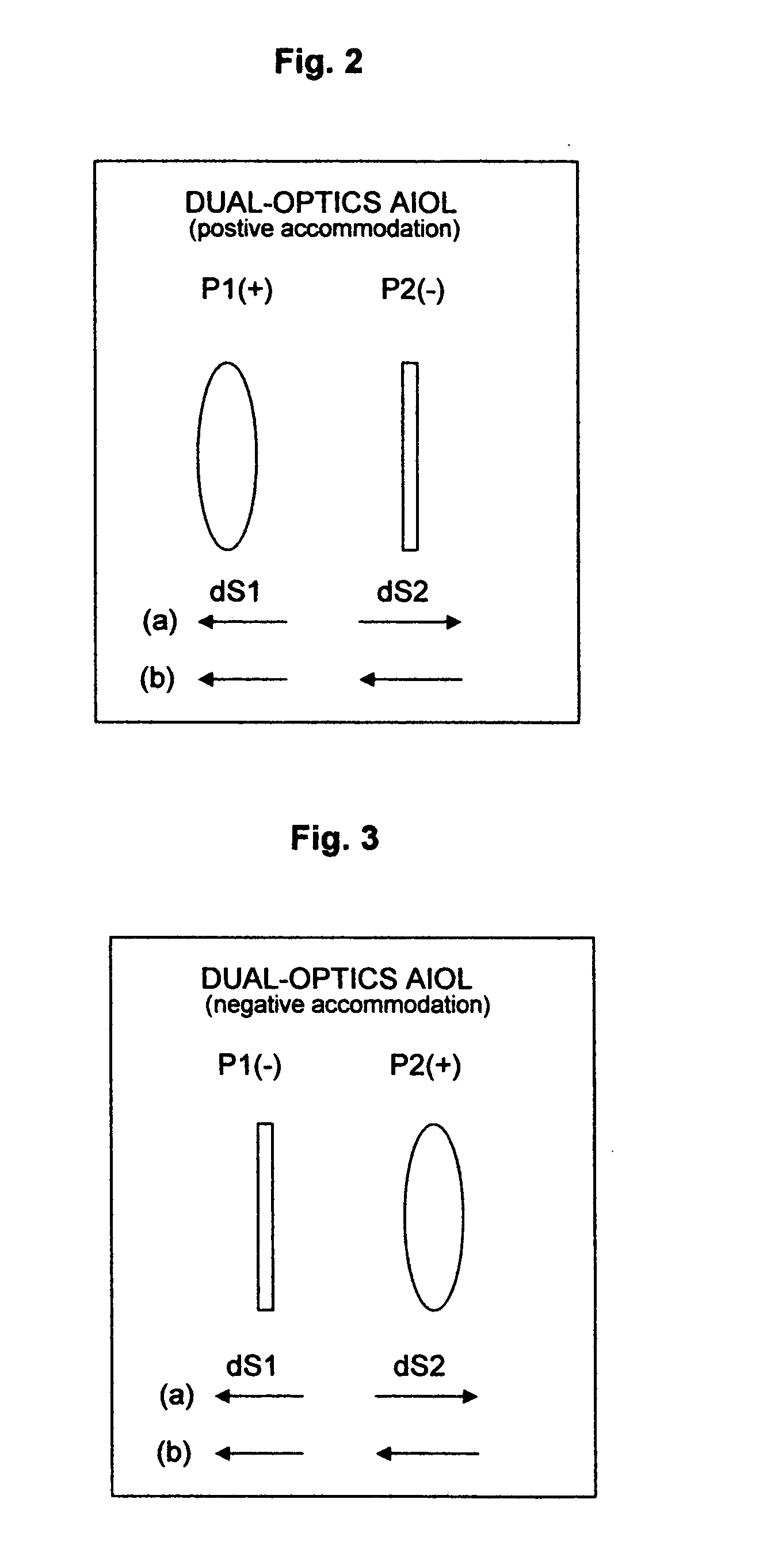
Method and device for vision correction via dual-optics accommodating intraocular lens
Method and design of a dual-optics accommodating intraocular lens (IOL) for vision correction of adult and pediatric eyes after cataract surgeries are disclosed. For adult eyes, a positive accommodation amplitude greater than 3.5 diopter and preferably 4.0 to 10.0 diopter may be achieved by optimal configurations having the positive-power front-optics moves toward the cornea, whereas the negative power back-optics moves in the opposite direction. In contrast, a negative accommodation is required for pediatric eyes and may be achieved by a reversed configurations. The enhanced efficiency, up to 500%, is proposed by preferred embodiments based on new lens design formulas and calculation steps for the IOL power pre-determined by the measured ocular parameters including the corneal power, IOL position and the vitreous cavity length of the eye.
Owner:LIN J T
Artificial crystalline len with transforming growth factor resistant beta2 antibody membrane on surface and manufacturing method thereof
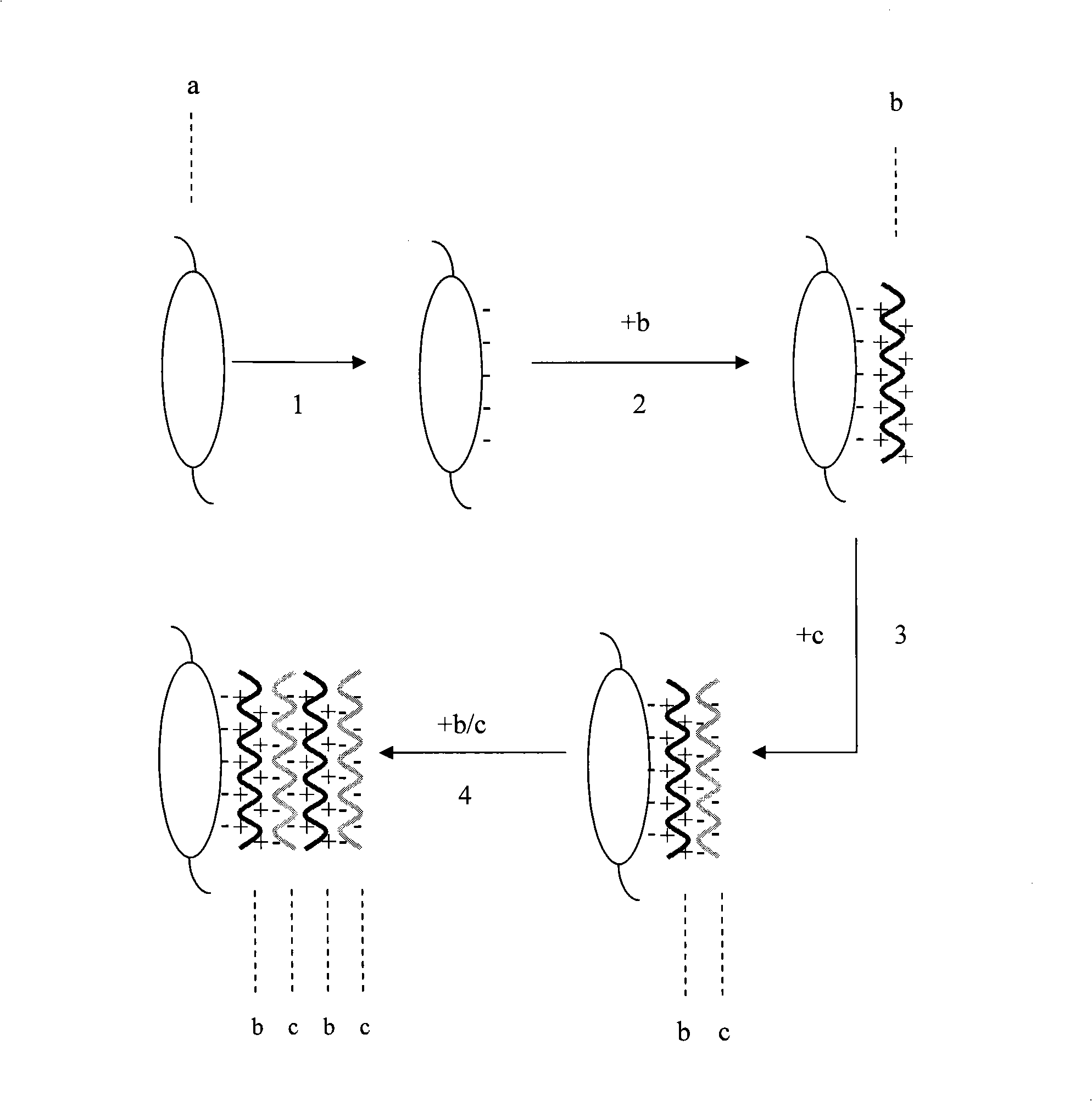
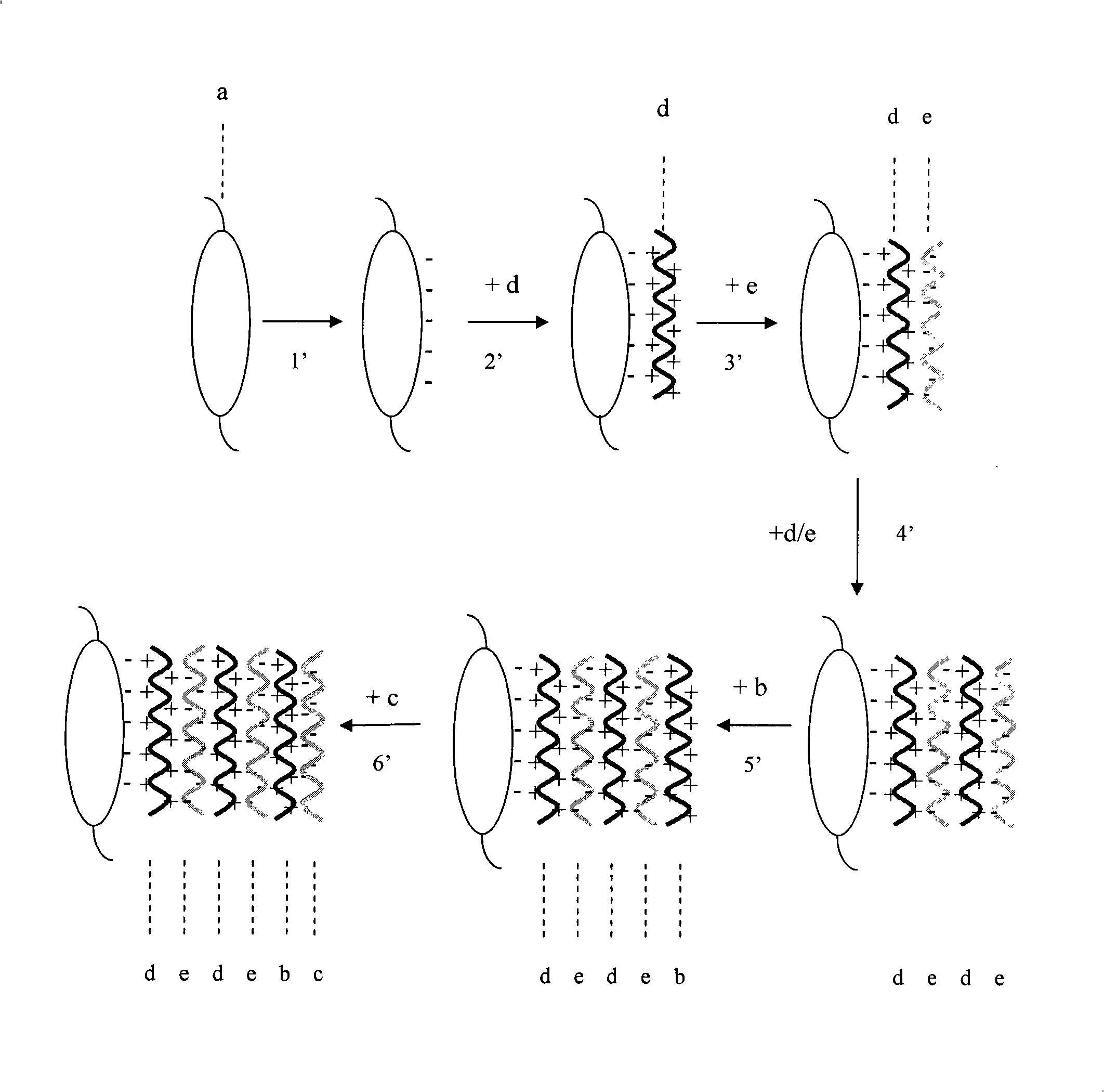
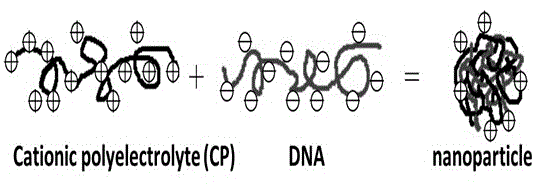
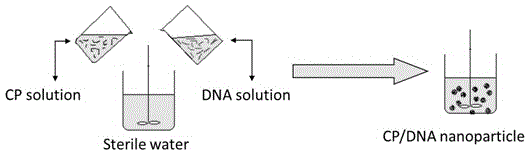
The invention provides an artificial lentis, which contains anti-transforming growth factor beta 2 antibody membrane on the surface and can inhibit intercurrent post-cataract after cataract surgery, and also provides a the production method thereof. The production method includes the steps that: the artificial lentis is charged with positive electricity or negative electricity after the artificial lentis is cleaned, dried, and pretreated on the surface; the artificial lentis is soaked in a polyelectrolyte solution the charge of which is opposite to the surface charge of the artificial lentis for adsorbing, and rinsing the artificial lentis by deionized water, and drying the artificial lentis by nitrogen gas; the artificial lentis is soaked in a phosphate buffering solution of anti-transforming growth factor beta 2 antibody, the pH value of which is 4-10, and the carried charge of which is opposite to that of the polyelectrolyte, for adsorption; finally, the artificial lentis is rinsed by phosphate buffering solution, and the artificial lentis is dried by nitrogen gas; the alternating assembly steps are repeated. The artificial lentis of the invention can inhibit the transformation and differentiation as well as cyst membrane shrinkage of the lentis epithelial cells in a target way, and then interdicts the occurrence of the post-cataract, and has excellent biocompatibility. The production method of the invention is scientific and simple, and can ensure the activity under a dry state and the safety and reliability during medical transplantation of the anti-transforming growth factor beta 2.
Owner:SECOND AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
Nanometer fluorouracil coat artificial crystalloid and the preparing method
InactiveCN101036804ASuppress turbidityLow toxicityCoatingsIntraocular lensPosterior capsule opacificationChitosan nanoparticles
The invention relates to a intraocular lens. At present the Poly-Methyl Methacrylate (PMMA) intraocular lens conventional for clinical treatment of cataract always causes inflammatory treaction after implantation. Posterior capsule opacification is also a major compalication after cataract surgery. The invention selects fluorouracil to solve the above problems. Based on weak penetrating force of chitosan nanoparticles in eyes and the relation between the phagocytosis amount of conjunctival epithelial cells to nanoparticles and the particle size of nanoparticles, the fluorouracil nanoparticles preparation is prepared using chitosan-poly(acrylic acid) as carrier, composite coated on the surface of PMMA intraocular lens. The invantion also provides a preparation method thereof. The said intraocular lens not only increases the biocompatibility of the intraocular lens, but also inhibites posterior capsule opacification pafter cataract surgery. The said intraocular lens can also prevent anterior membrane after implantation of intraocular lens and after-cataract.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Ocular solutions
InactiveUS20050063997A1Reduce turbidityReduce inflammationBiocideSenses disorderEverolimusOcular structure
Ocular solutions containing at least one macrolide antibiotic and / or mycophenolic acid provide anti-inflammatory, anti-cell proliferation, anti-cell migration, anti-angiogenesis, antimicrobial, and antifungal effects. In one embodiment, the solution is administered intraocularly after cataract surgery before insertion of a replacement intraocular lens, resulting in reduced posterior capsular opacification which may eliminate the need for a subsequent surgery. The solution may be one that is invasively administered, for example, an irrigation or volume replacement solution containing at least one macrolide antibiotic such as tacrolimus, sirolimus, everolimus, cyclosporine, and ascomycin, or mycophenolic acid. The solution may be one that is non-invasively or topically administered in the form of drops, ointments, gels, creams, etc. and may include eye lubricants and contact lens solutions. The solution may contain a supratherapeutic concentration of agent(s) so that a therapeutic concentration of a topically administered solution accumulates in a diseased ocular structure sufficient to treat the disease. The agent(s) may be formulated with polymers or other components for extended or slow release to provide a substantially constant concentration over the course of treatment.
Owner:PEYMAN GHOLAM A DR
Artificial crystal with antiproliferous medicine coating for preventing and treating after-cataract forming
InactiveCN101053680AGrowth inhibitionGood treatment effectIntraocular lensTreatment effectAntiproliferative Agents
An artificial lens having stents coated with antiproliferative agents for prevention and treatment of the posterior capsule opacification, the invention relates to an artificial lens having stents coating for solving problems of time of present artificial lens loaded with medicine acting on cornea is short. The artificial lens having stents coated with antiproliferative agents for prevention and treatment of the posterior capsule opacification is prepared by ordinary artificial lens and coating with antiproliferative agents, wherein the coating with antiproliferative agents adheres on surface of ordinary artificial lens, thickness of the coating is 3 to 20 mum. The invention adopts macomolecule coating material to coat antiproliferative agents on artificial lens, slowly releases agents in capsules and ambitus cells, represses generation of lens epithelial cell, prevents and cures formation of the posterior capsule opacification. The agents of the invention act on cornea long time, capable of releasing effectively 90 to 120 days, have good therapeutic, patients use the artificial lens of the invention, formation rate of the posterior capsule opacification reduced greatly from more than 60% to 10% and without untoward reaction.
Owner:刘红玲
Micropatterned Intraocular Implant
InactiveUS20150342725A1Inhibit migrationInhibit growth and migrationTissue regenerationIntraocular lensPosterior capsule opacificationLens epithelial cell
Generally, an intraocular implant having on the external surface a plurality of pattern surface elements disposed in spaced apart relation defining a tortuous pathway adapted to control a flow of fluid, or a flow of particles suspended in a fluid, or inhibits the growth or migration of cells. In particular, an intraocular implant which implanted between an intraocular lens and the surface of the posterior capsule of the eye inhibits growth or migration of residual lens epithelial cells after cataract surgery by providing structural barriers to reduce posterior capsule opacification of the eye.
Owner:SHARKLET TECH +2
Acrylic acid ester shape-memory intraocular lens material and preparation method thereof
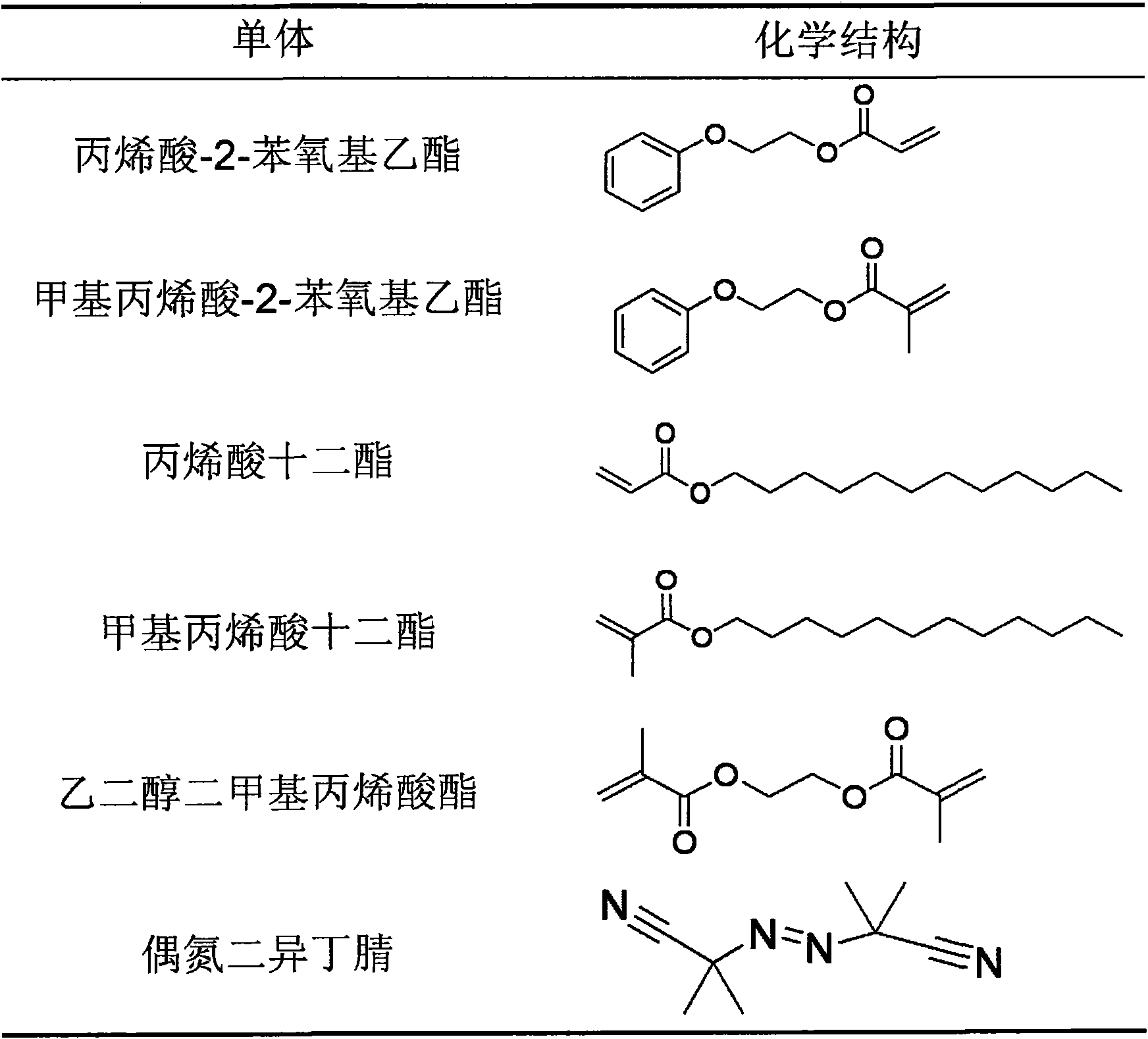
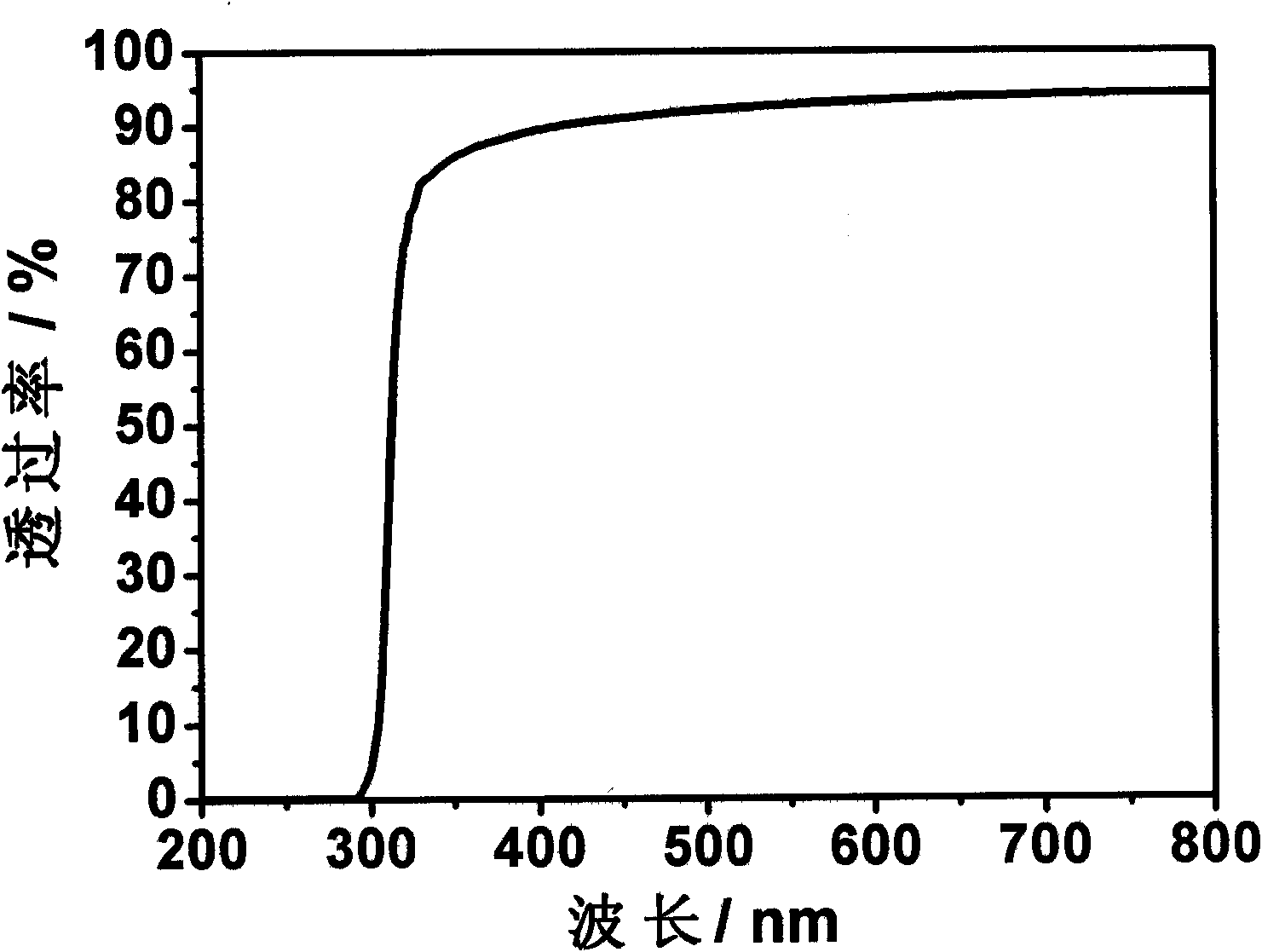
The invention provides an acrylic acid ester shape-memory intraocular lens material and a preparation method thereof, belonging to the field of biomedical material. The intraocular lens comprises the following basic materials of acrylic acid ester monomer crylic acid-2-phenoxy and methacrylic acid-2-phenoxy ethyl that are capable of being thermally polymerized and have a mass ratio of 3 / 1-9 / 1; furthermore, a cross linking agent and a thermal initiator are added in the basic materials; and the alkyl ester acrylate added in the material can control the thermodynamic performance of the polymerisate. The thermal-polymerization monomer, the thermal initiator and the cross linking agent can be uniformly mixed under the protection of the nitrogen and injected to a die for thermal polymerization reaction at the temperature of 70 DEG C-90 DEG C for 24-48 hours; and the shape-memory intraocular lens material is obtained by removing unreacted monomer and oligomer with a Soxhlet extraction method. The material has shape-memory performance; furthermore, the memory performance conversion temperature of the material can be adjusted to a body temperature, the deformation quantity of the material is large and the light transmittance at the visible light area is higher. The intraocular lens material can effectively reduce surgical incision and increase the effective optical area of intraocular lens simultaneously, and reduces the occurrence of complication such as clinical after-cataract, glare, etc.
Owner:UNIV OF SCI & TECH BEIJING
Intraocular lens for preventing and treating after cataract, and preparation method thereof
The present invention relates to medical devices, particularly to an intraocular lens and a preparation method thereof, more particularly to a medical device for light therapy, specifically to an intraocular lens for preventing and / or treating after cataract, and a preparation method thereof.
Owner:EYEBRIGHT MEDICAL TECH BEIJING +2
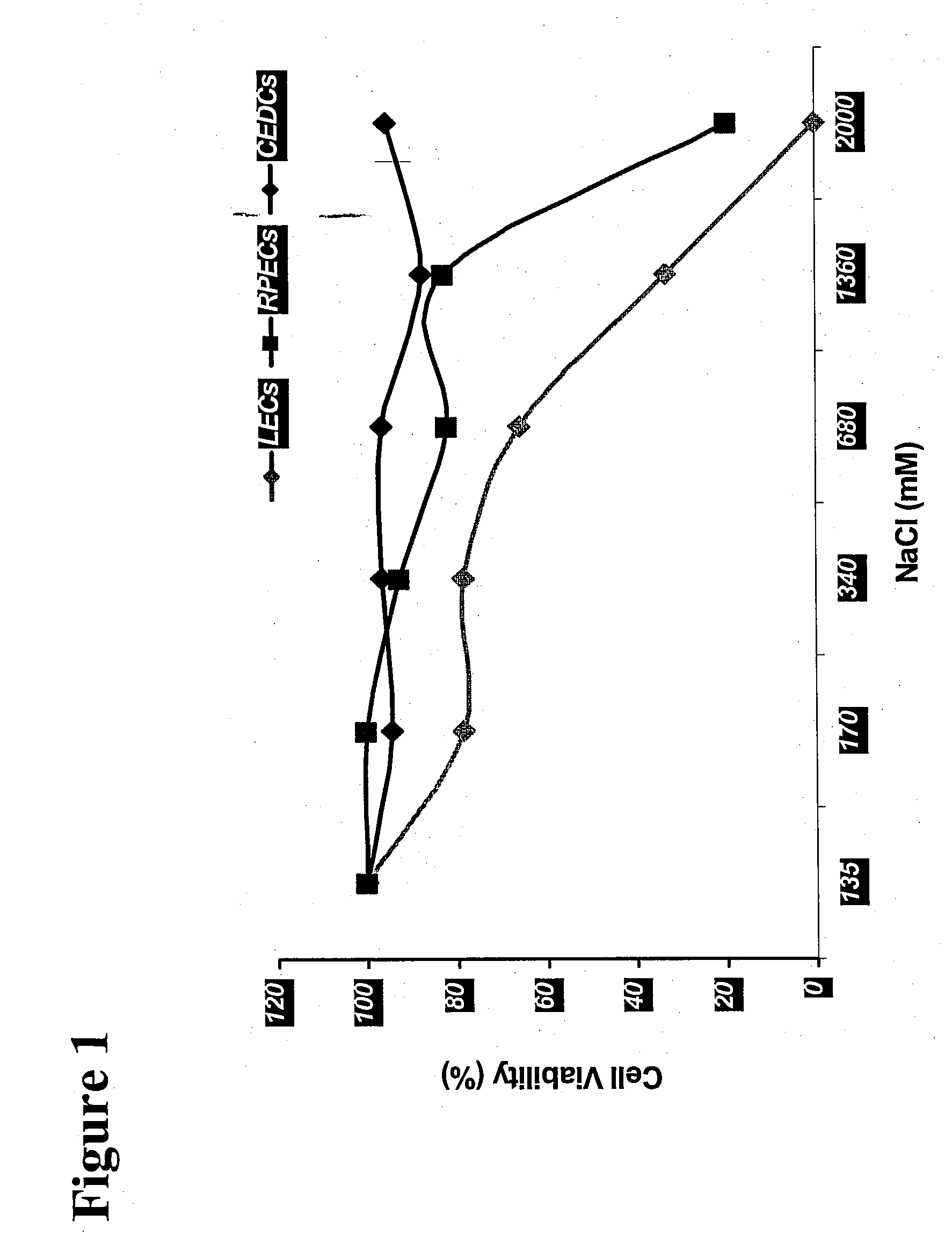
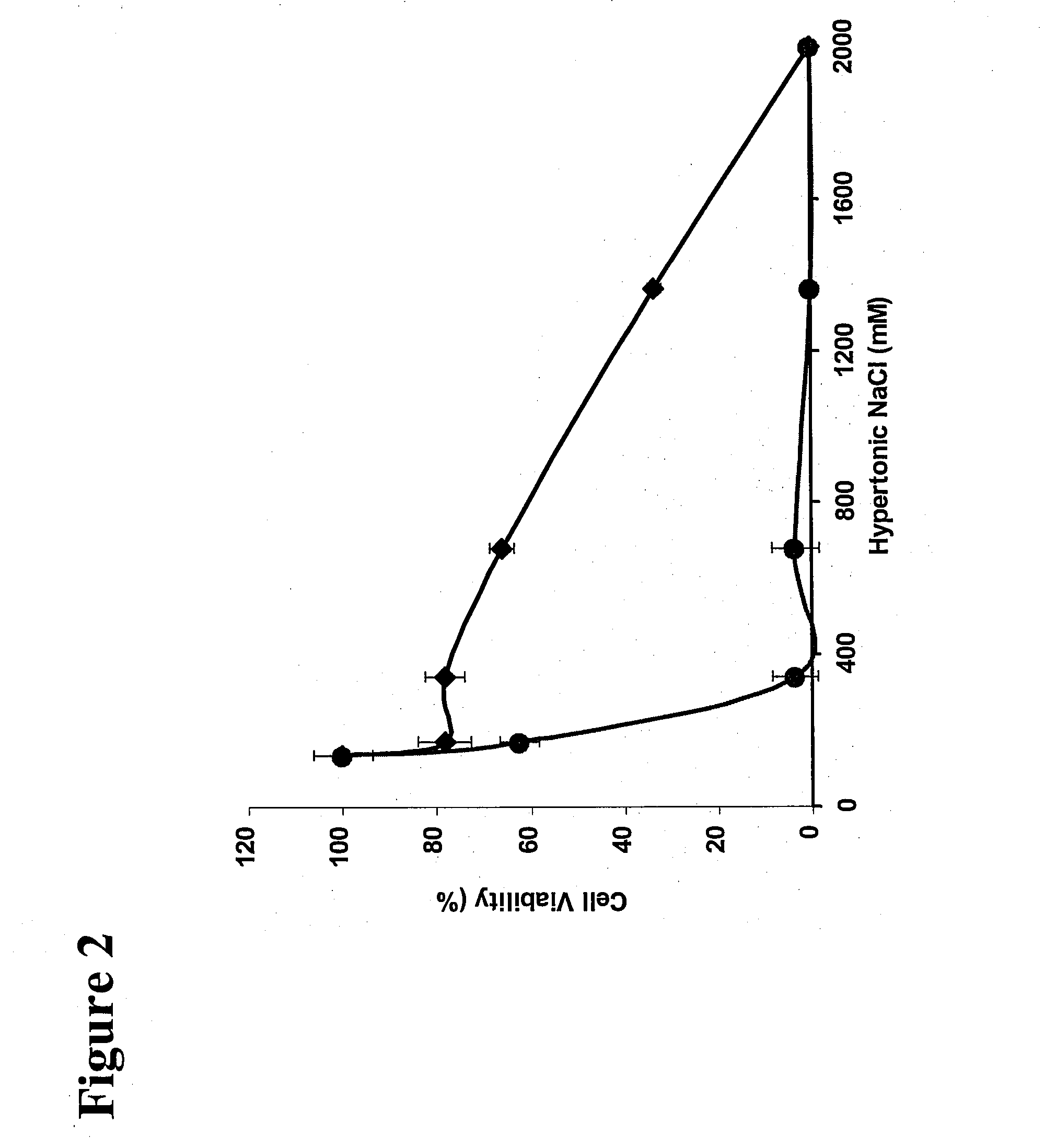
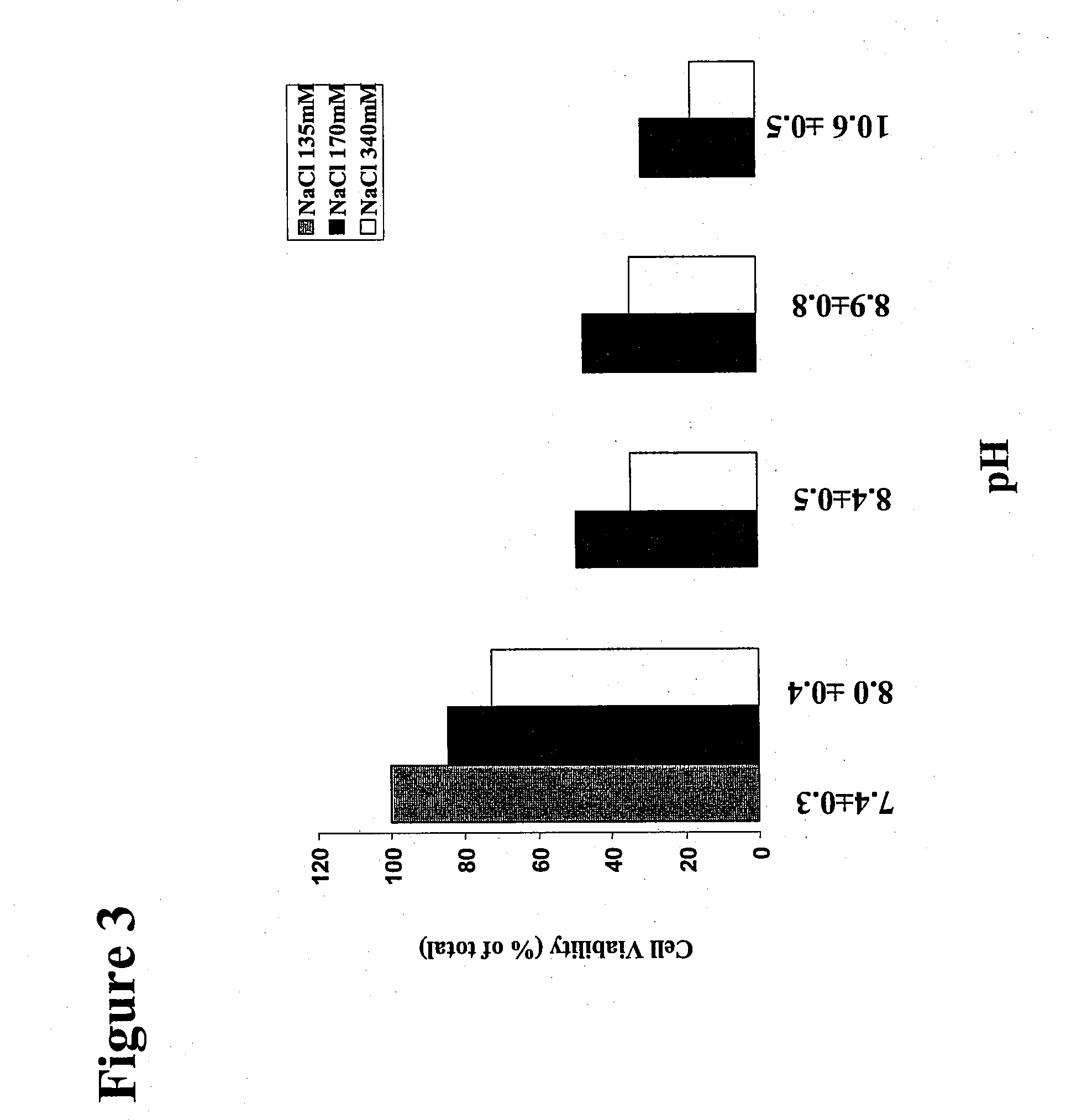
Treatment Solution and Method for Preventing Posterior Capsular Opacification by Selectively Inducing Detachment And/Or Death of Lens Epithelial Cells
InactiveUS20070129286A1Great incidencePrevent PCOBiocideSenses disorderIon distributionCellular mechanism
A treatment solution used to prevent posterior capsular opacification is applied or introduced into the lens capsular bag before, during, or after cataract surgery. The treatment solution comprises an ion transport mechanism interference agent, which either alone or in combination with other treatment agents such as an osmotic stress agent and an agent to establish a suitable pH, selectively induces detachment and / or death of lens epithelial cells such that posterior capsular opacification is prevented. While the ion transport mechanism interference agent is capable of interfering with the cellular mechanisms and cell ion distribution of a broad range of cells, a concentration of agent is selected such that the treatment solution interferes selectively with the cellular mechanisms of lens epithelial cells while leaving other ocular cells substantially unharmed. The treatment solution selectively induces cellular death and / or detachment of lens epithelial cells while other ocular cells and tissue remain substantially unharmed and without lengthy preoperative pre-treatment.
Owner:ABBOTT MEDICAL OPTICS INC
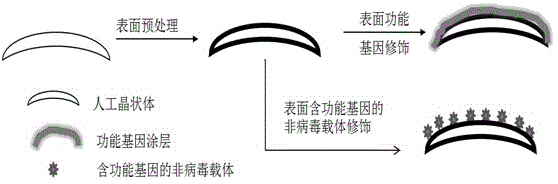
Surface-mediated gene therapy type artificial lens and preparation method for same
ActiveCN104825249APrevent proliferationReduce incidenceIntraocular lensTransdifferentiationViral Genes
The invention discloses a surface-mediated gene therapy type artificial lens and a preparation method for the same. A plasmid DNA (Deoxyribonucleic Acid) containing a functional gene or a non-viral gene vector loaded with the plasmid DNA containing the functional gene is fixedly arranged on the surface of the artificial lens. The artificial lens and the preparation method for the same have the advantages that a surface-deviated gene therapy vector is obtained by a surface gene modification method, so that the epithelial cell proliferation and transdifferentiation of the lens are suppressed by a surface-mediated gene therapy method, and the artificial lens capable of effectively suppressing after cataract is obtained.
Owner:WENZHOU MEDICAL UNIV
Hydrophilic shape memory hydrogel serving as artificial lens material
ActiveCN105536053AImprove mechanical propertiesGood optical performancePharmaceutical delivery mechanismTissue regeneration(Hydroxyethyl)methacrylateEquilibrium moisture content
The invention discloses a hydrophilic shape memory hydrogel serving as an artificial lens material, belonging to the field of biomedical materials. The invention aims at solving the problems of glare, eccentric displacement and easy induction of after cataract of the existing foldable artificial lenses. The hydrophilic shape memory hydrogel is prepared from a polymeric monomer and an initiator, wherein thepolymeric monomer refers to hydroxyethyl methacrylate, 2-phenoxyethyl acrylate, polyethylene glycolmethacrylate and triethylene glycoldiacrylate; and the initiator is azodiisobutyronitrile. The hydrophilic shape memory hydrogel disclosed by the invention serves as the artificial lens material, the equilibrium moisture content is over 30%, the refraction index is greater than 1.4, the light transmittance can go beyond 90%, the deformation recovery rate exceeds 96%, and the mechanical and optical properties are good; and the artificial lens can simulate a normal human lens in diameter and thickness, thus the whole lens capsule can be filled withthe implanted artificial lensto effectively reduce the complications such as after cataract, glare and eccentric displacement of artificial lens.
Owner:HARBIN INST OF TECH
Intraocular Lens Cell Migration Inhibition System
ActiveUS20130304205A1Inhibit migrationReduce posterior capsule opacificationEye treatmentTissue regenerationPosterior capsular opacificationLens epithelial cell
Generally, an intraocular implant and methods for treating an ocular condition. In particular, an intraocular implant which implanted between an intraocular lens and the surface of the posterior capsule of the eye inhibits migration of residual lens epithelial cells after cataract surgery by providing structural barriers to reduce posterior capsule opacification of the eye.
Owner:INSIGHT INNOVATIONS
A method and apparatus for image analysis of aft-onset cataract
InactiveCN109102494AEasy to analyzeQuick analysisImage enhancementImage analysisPattern recognitionImaging analysis
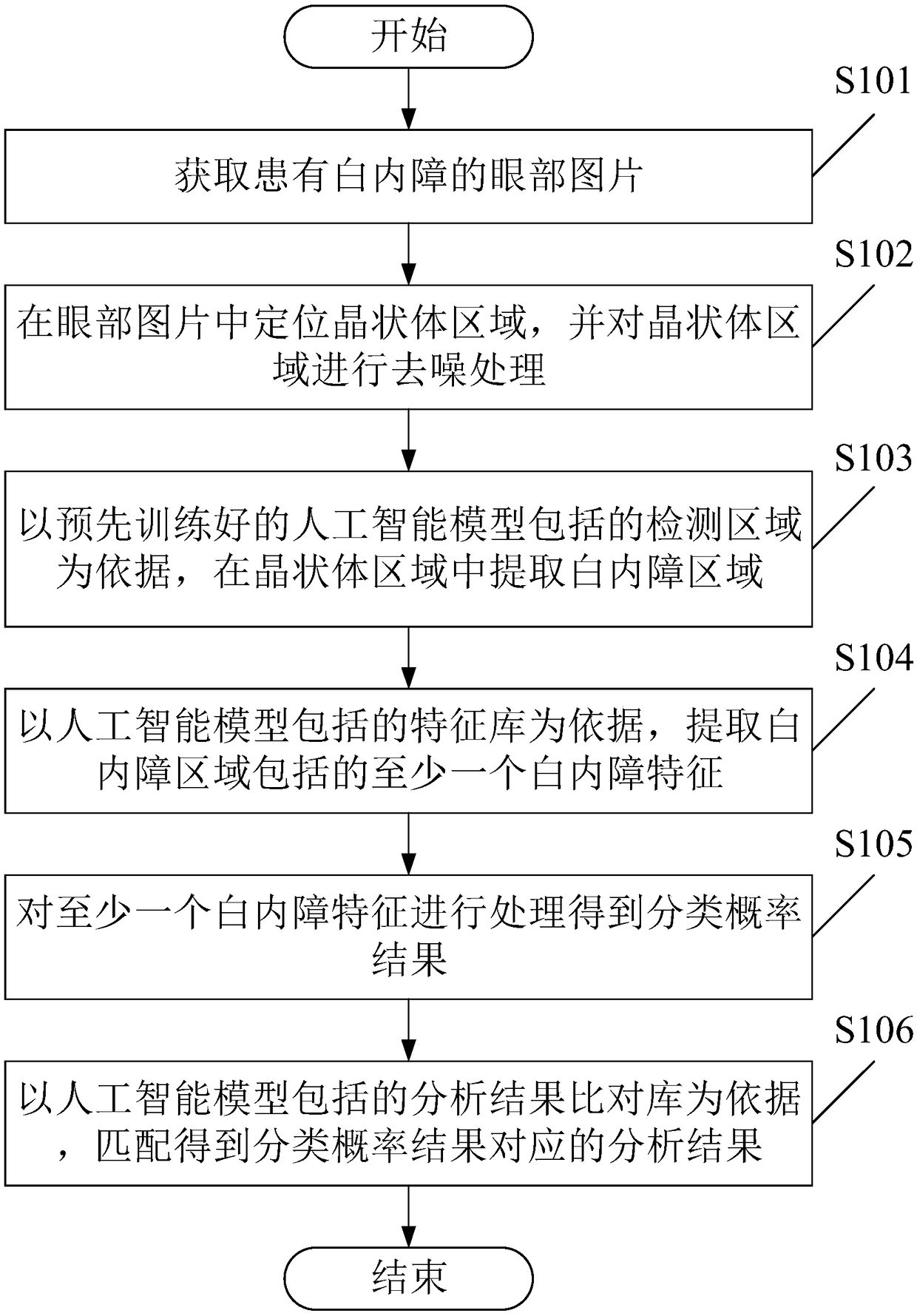
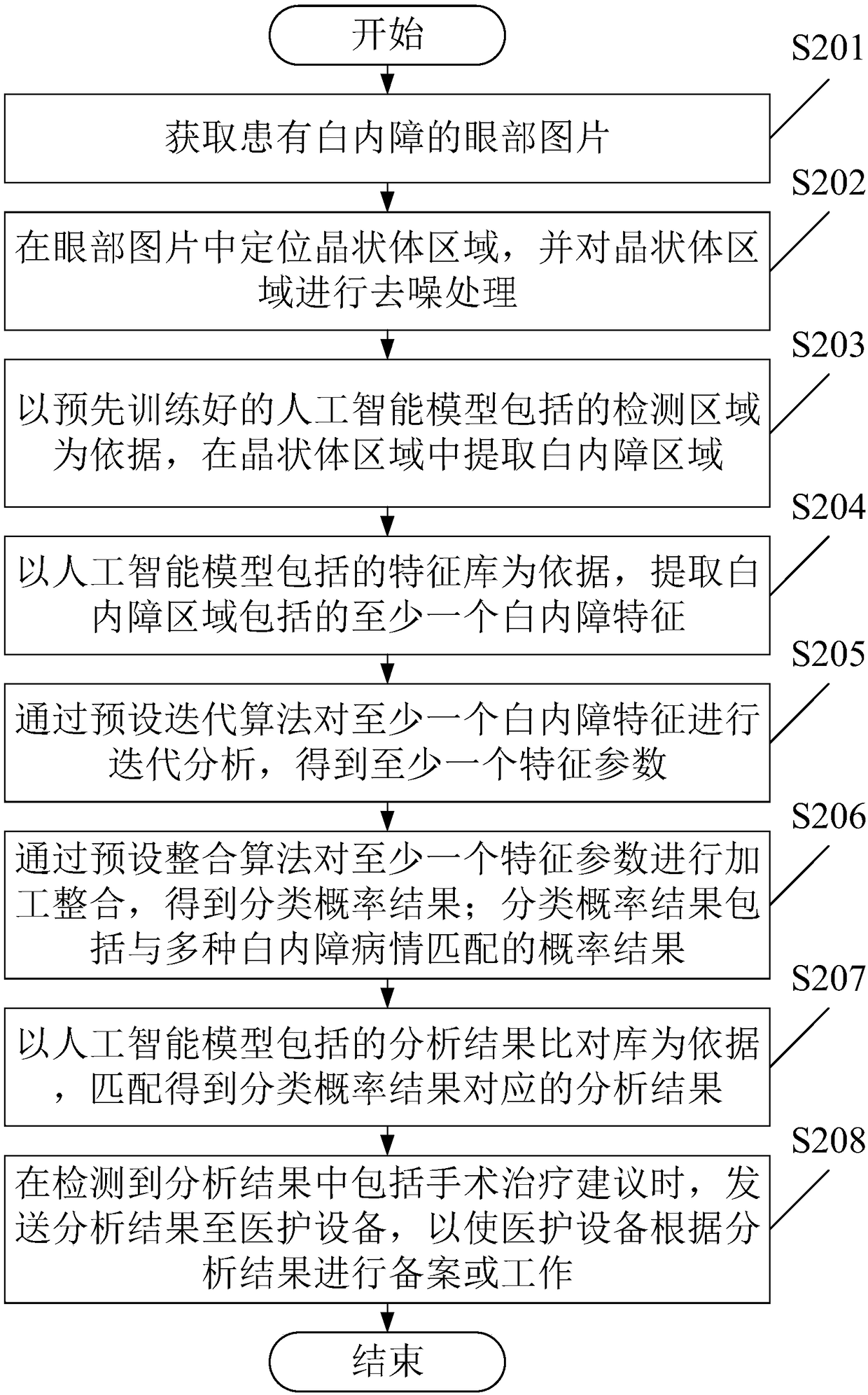
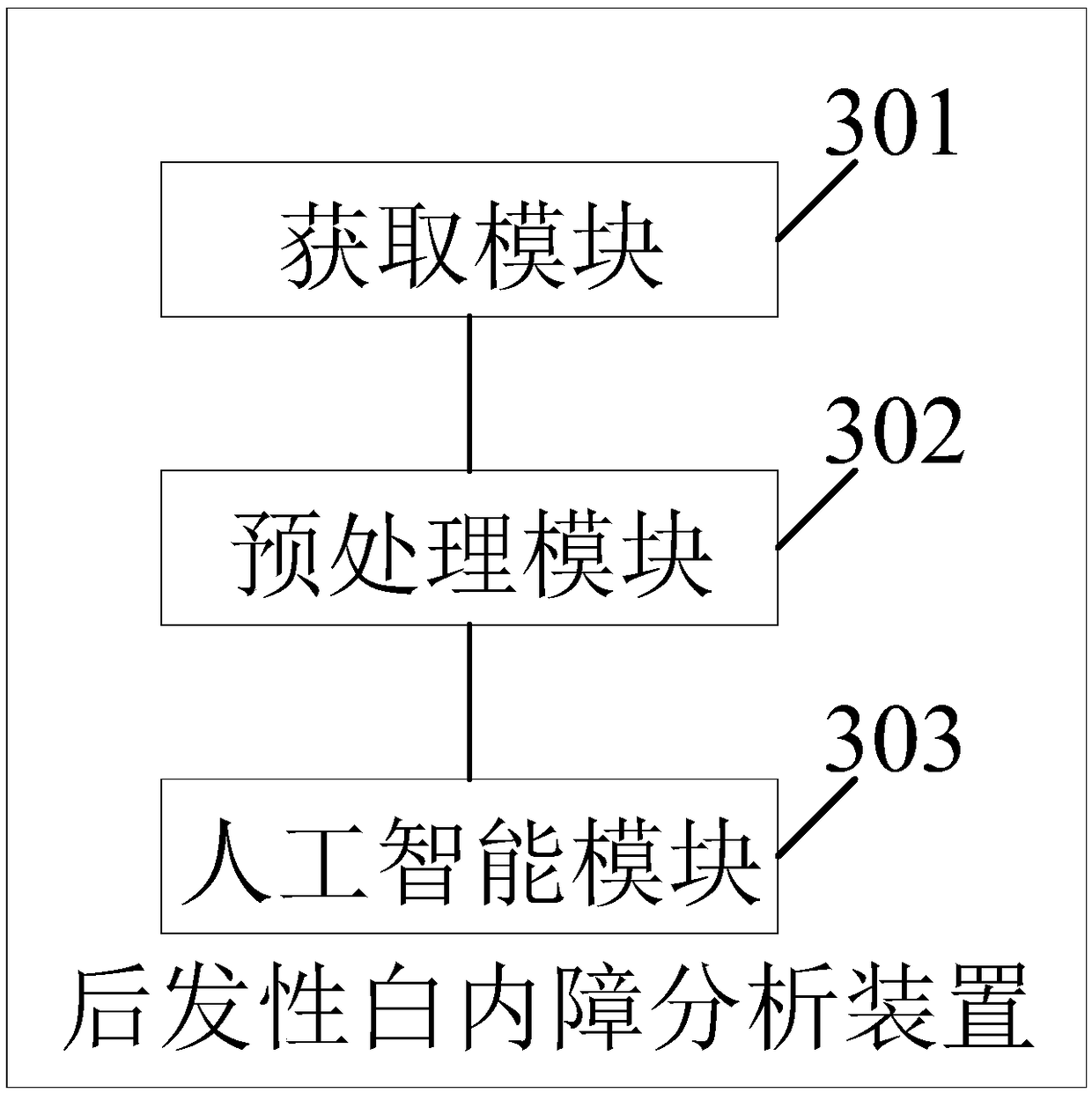
The invention provides an image analysis method and a device for after-cataract, which comprises the following steps of: receiving an eye picture of after-cataract; a posterior capsule hyperplasia region of after-cataract is located and processed by slit-lamp posterior radiography; the area of posterior capsule proliferation is extracted from the area of posterior capsule proliferation according to the detection area included in the artificial intelligence model, wherein, the artificial intelligence model is pre-trained; at least one after-cataract feature included in the after-cataract regionis extracted based on the feature library included in the artificial intelligence model; at least one after cataract feature is processed to obtain a classification probability result; based on the analysis results comparison database included in the artificial intelligence model, matching results corresponding to the classification probability results are obtained. It can be seen that the methodmentioned above can simply, quickly and accurately determine the after-cataract of the patient, and give the corresponding analysis results to the user, so as to improve the efficiency of the user toseek medical treatment.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Artificial crystalline lens with one or more additional parts
The invention relates to medical devices, in particular to a medical device for treating cataract, and particularly an artificial crystalline lens used for preventing and / or treating after cataract. More particularly, the artificial crystalline lens with one or more additional parts comprises a, a body, and b, an annular additional body, and the annular additional body is connected to the artificial crystalline lens.
Owner:EYEBRIGHT MEDICAL TECH BEIJING
Intraocular implant cell migration inhibition system
InactiveUS8551167B2Inhibit migrationReduce posterior capsule opacificationEye surgeryTissue regenerationPosterior capsular opacificationLens epithelial cell
Owner:INSIGHT INNOVATIONS
Artificial crystalline lens with optical catalytic coating
The present invention provides an artificial crystalline which includes an optical crystalline body and an optical catalytic coating that coats on at least one portion of the surface of the optical crystalline body. The artificial crystalline of the invention can prevent and restrain the intraocular inflammation and ensuing cataract after cataract operation.
Owner:蔡明霖 +3
Cell Migration Inhibition System
InactiveUS20140288645A1Inhibit migrationReduce posterior capsule opacificationEye treatmentTissue regenerationPosterior capsular opacificationLens epithelial cell
Generally, an intraocular implant and methods for treating an ocular condition. In particular, an intraocular implant which implanted between an intraocular lens and the surface of the posterior capsule of the eye inhibits migration of residual lens epithelial cells after cataract surgery by providing structural barriers to reduce posterior capsule opacification of the eye.
Owner:INSIGHT INNOVATIONS
Remedy for corneal failure
InactiveUS20060234922A1Recovers corneal sensitivityImproves condition of dry eyeBiocideSenses disorderNeuroparalytic keratopathyCorneal surgery
The present invention provides a new type of pharmaceutical agent that recovers corneal sensitivity after corneal surgery or improves the condition of dry eye. Application of a somatostatin receptor agonist is expected to provide an improvement effect on decreased corneal sensitivity after cataract surgery or LASIK surgery, decreased corneal sensitivity and dry eye associated with corneal neurodegeneration such as neuroparalytic keratopathy, corneal ulcer, diabetic keratopathy and the like.
Owner:SENJU PHARMA CO LTD
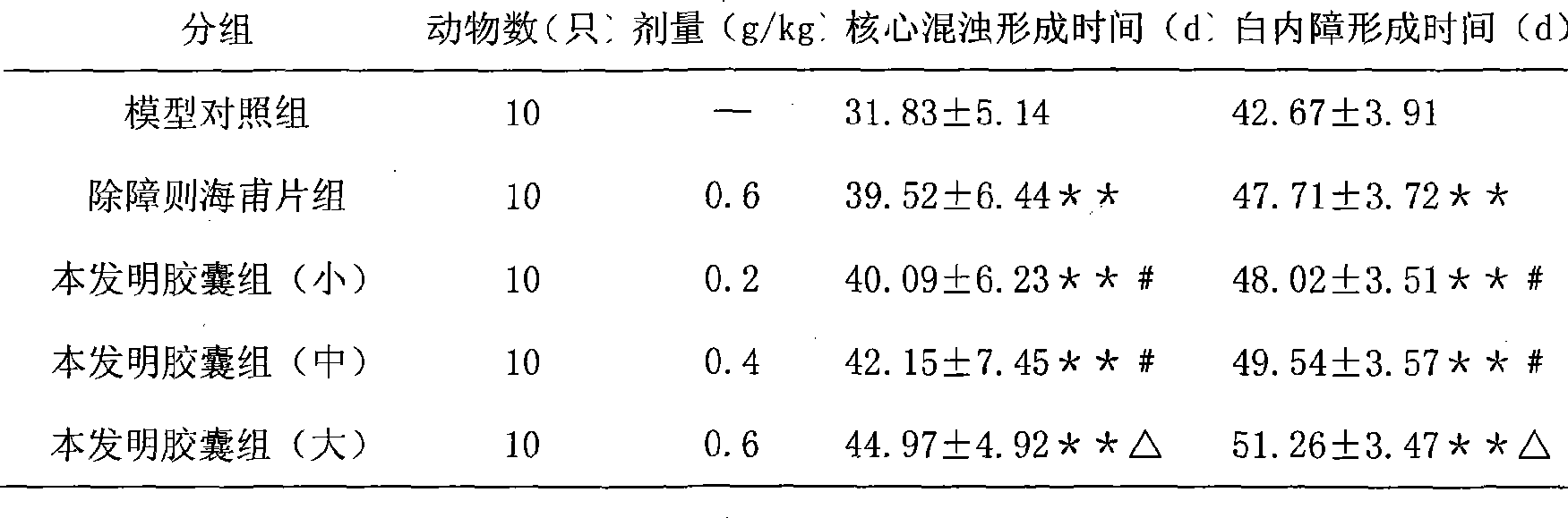
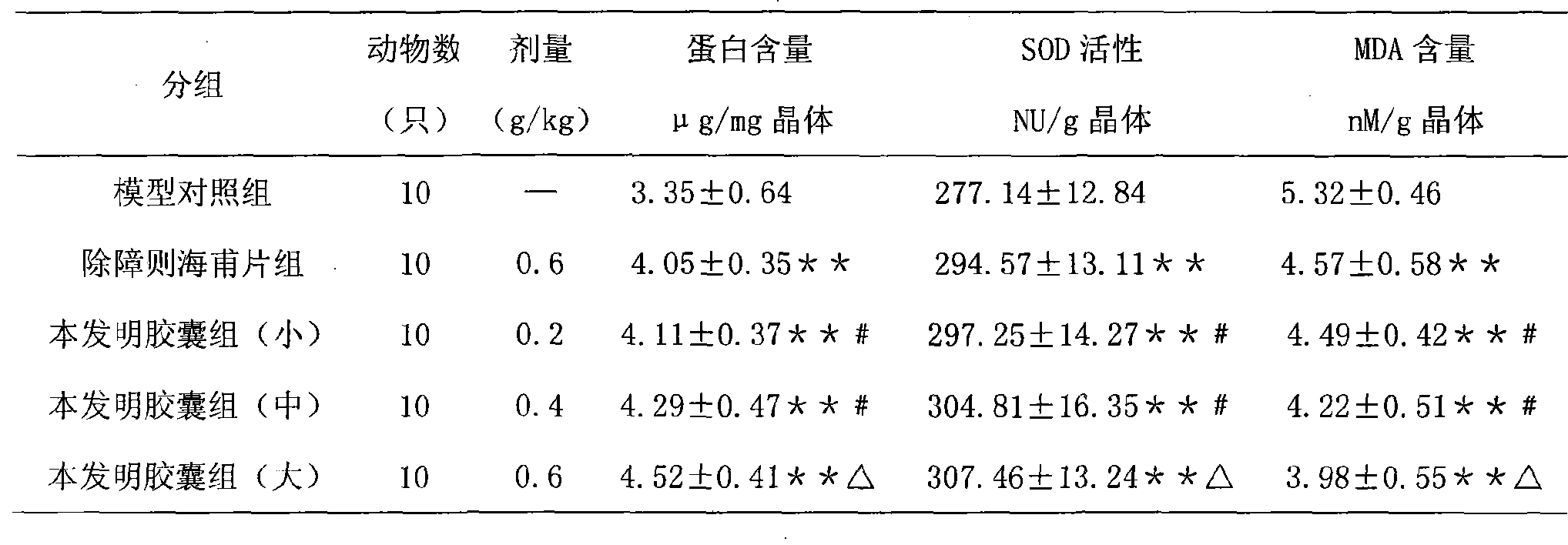
Traditional Chinese medicine formulation for treating cataract and preparation method thereof
ActiveCN101361892ALong-term medication is safe and reliableNo toxic reaction was foundSenses disorderPill deliveryLuteinAdditive ingredient
The invention discloses a Chinese medicine for curing cataract and a preparation method thereof. The medicine is prepared by processing the mixture of active pharmaceutical ingredients obtained from processing aloe, operculina turpethum, rose flowers, medicine terminalia fruit, mastic, saffron, Secmonia, and lutein, and a carrier acceptable by the drug according to a certain proportion. The drug carrier is simple syrup and starch. Compared with the prior art, the Chinese medicine provided by the invention is used for curing cataract induced by the degeneration of yellow spots and the abnormal savda and bile, and after-cataract, with a noticeable efficacy.
Owner:陕西东泰制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com