Patents
Literature
81 results about "Vitreous cavity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
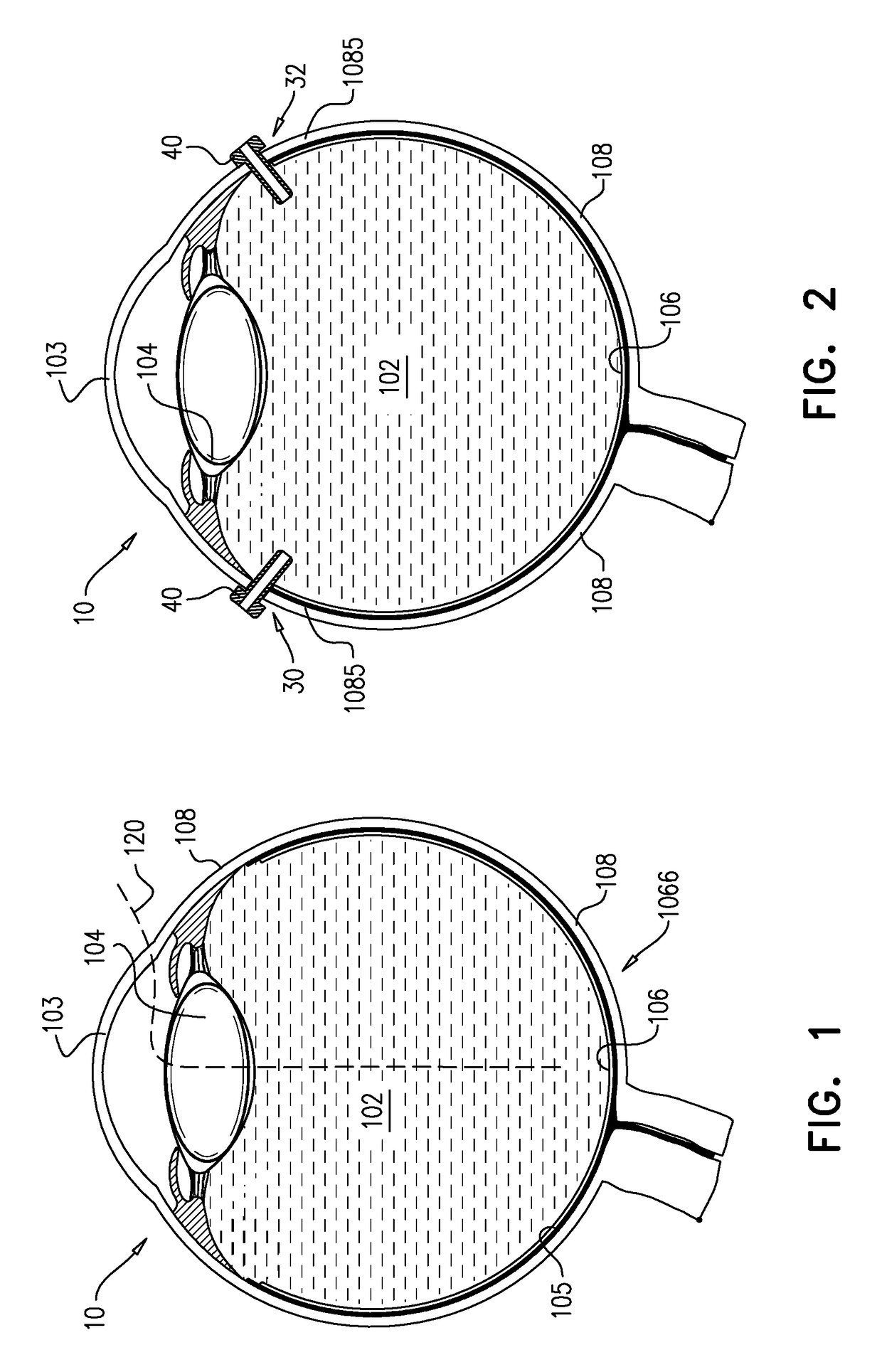
Vitreous cavity. Etymology: L, vitreus, glassy, cavum, cavity. the cavity in the eye posterior to the lens that contains the vitreous body and vitreous membrane and is transected by the vestigial remnants of the hyaloid canal.
Treatment of retinal detachment
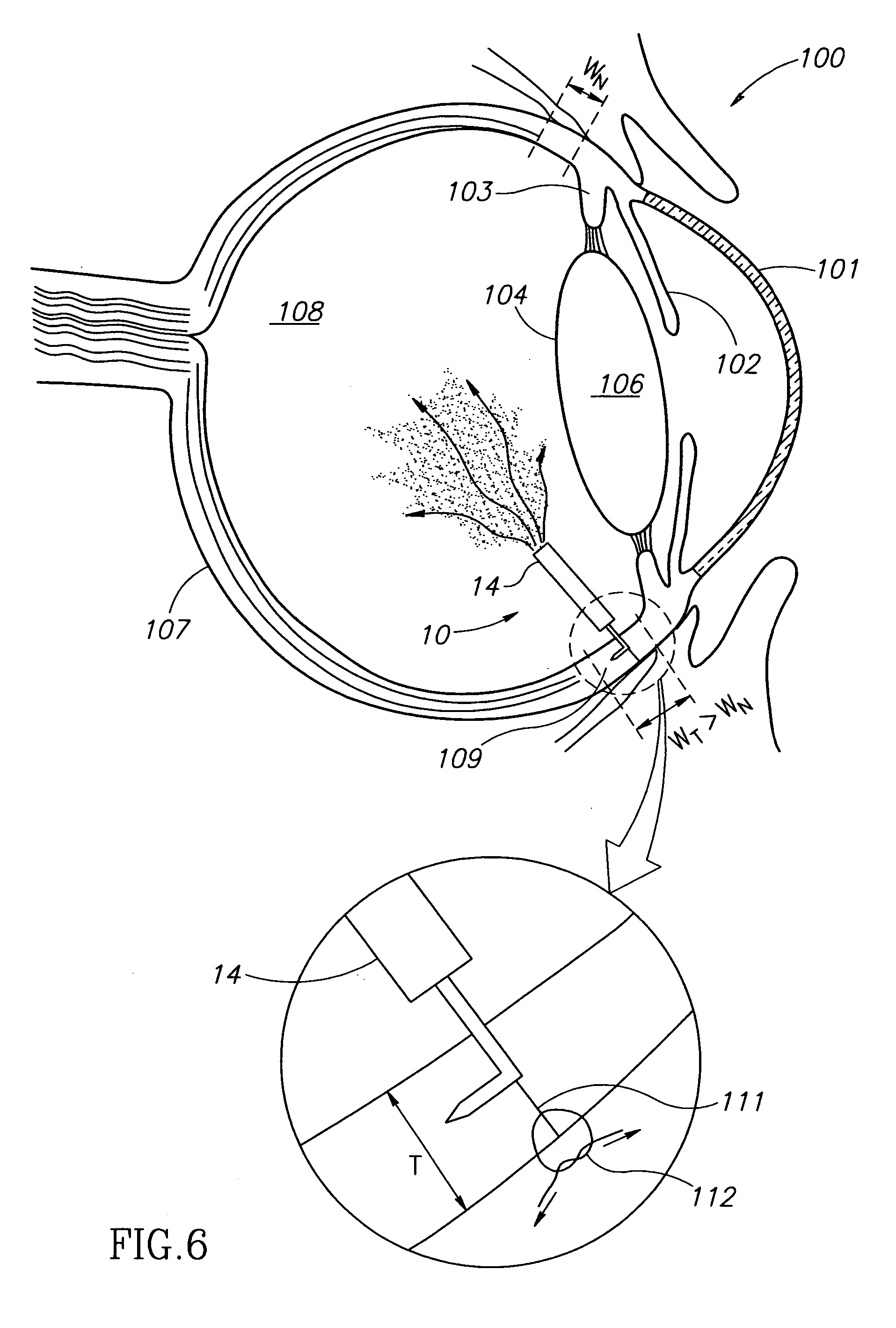
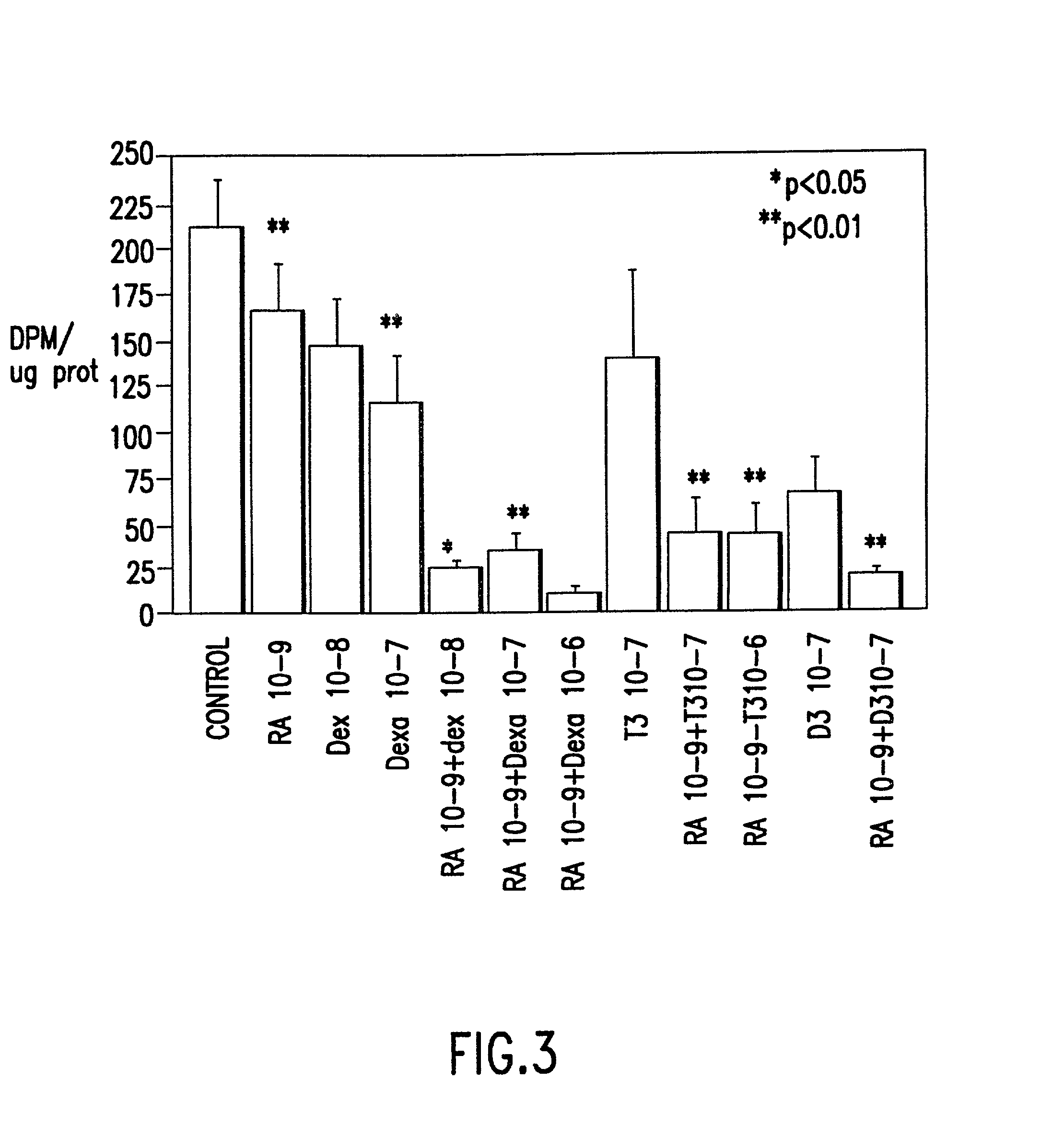
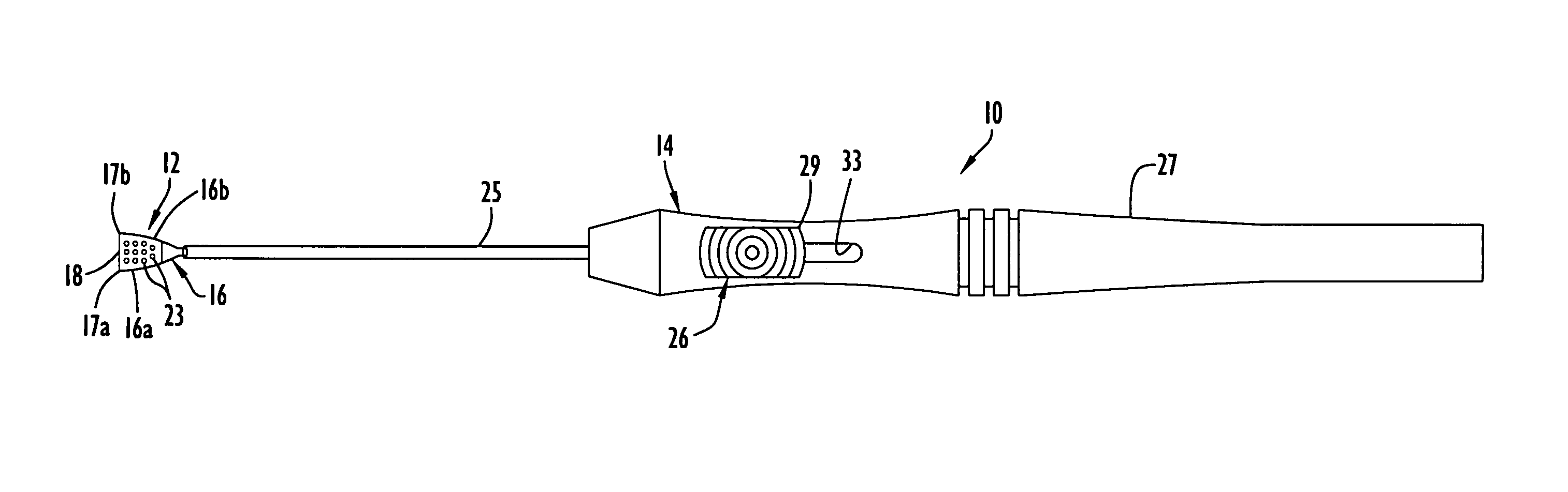
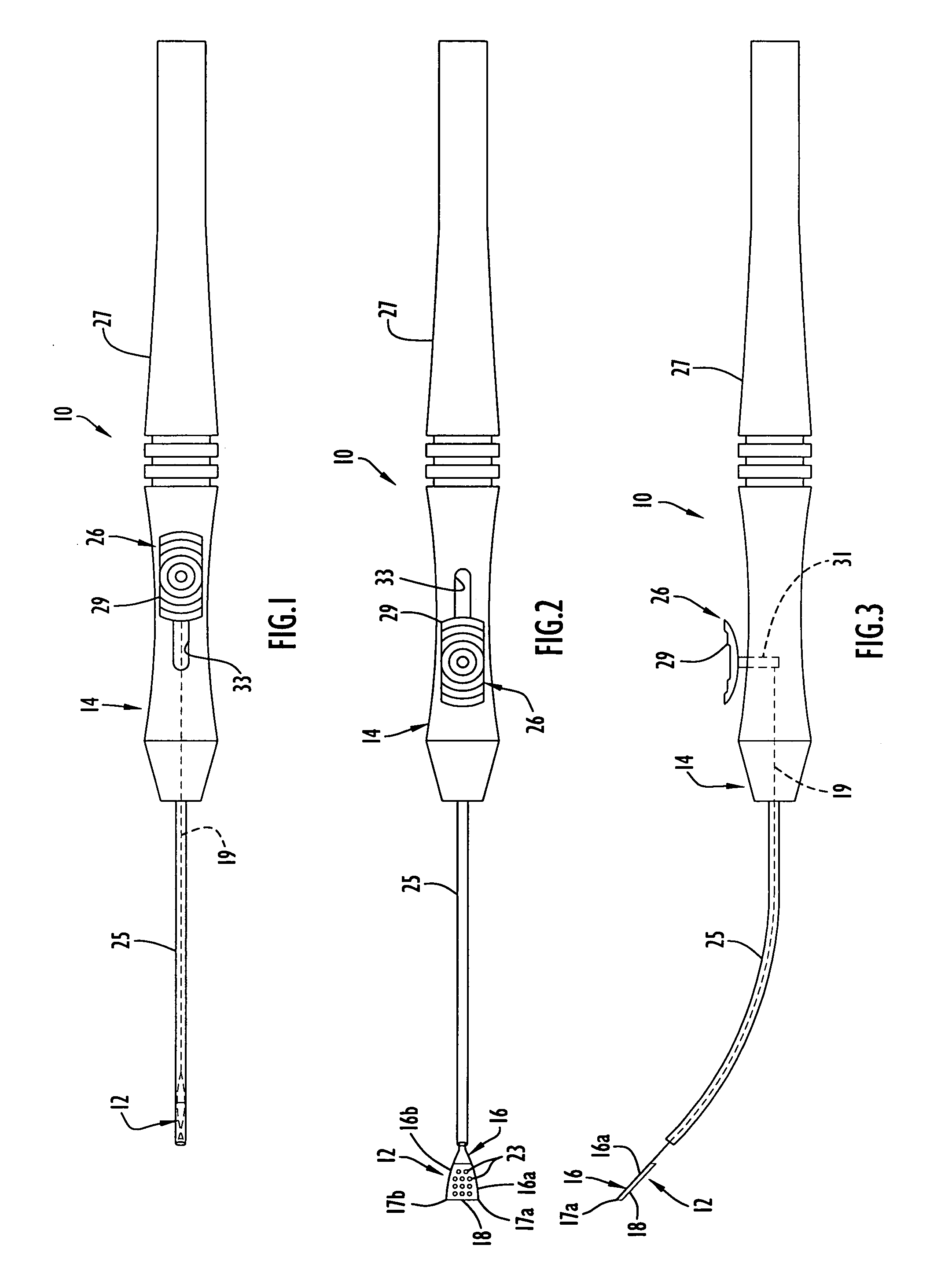
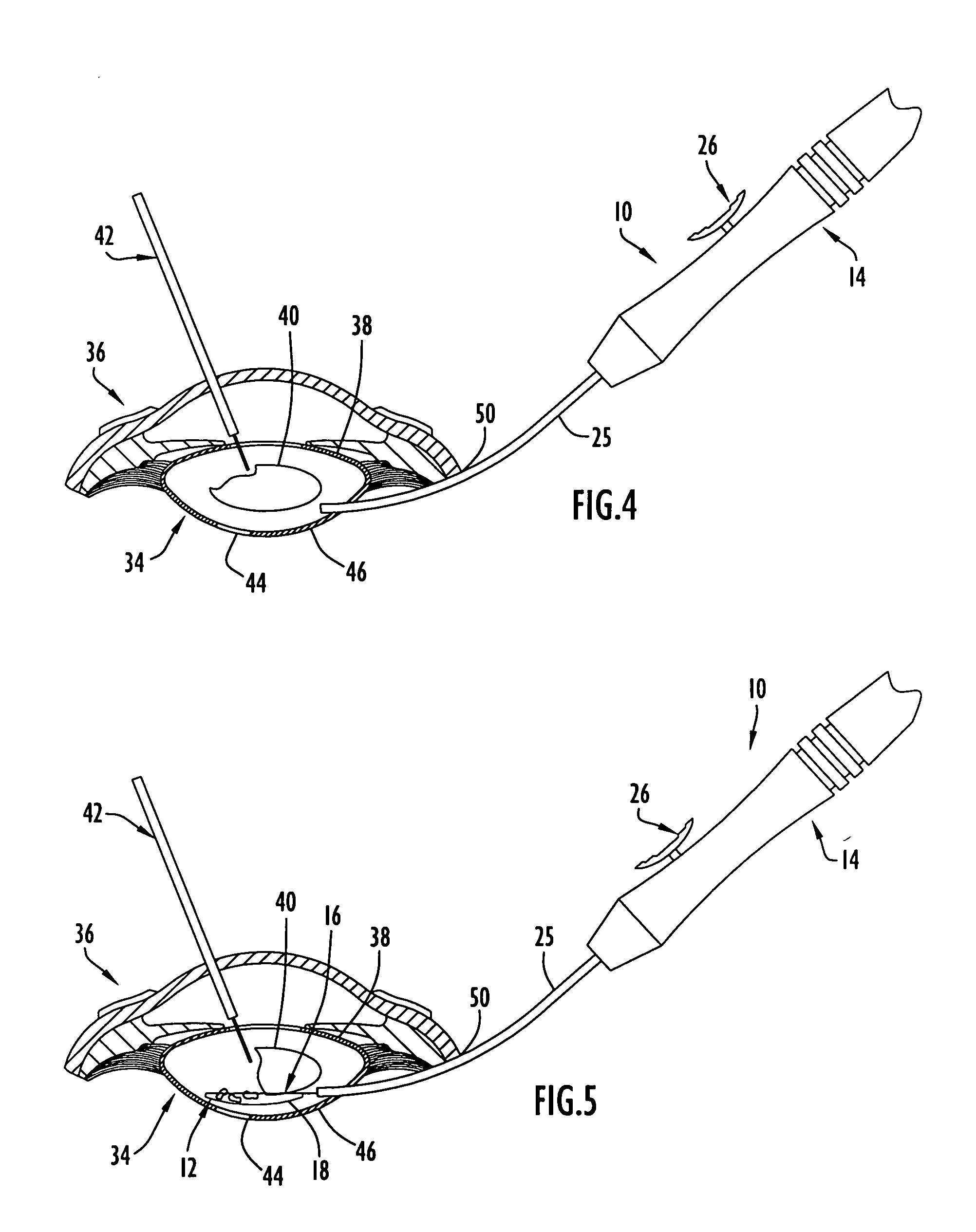
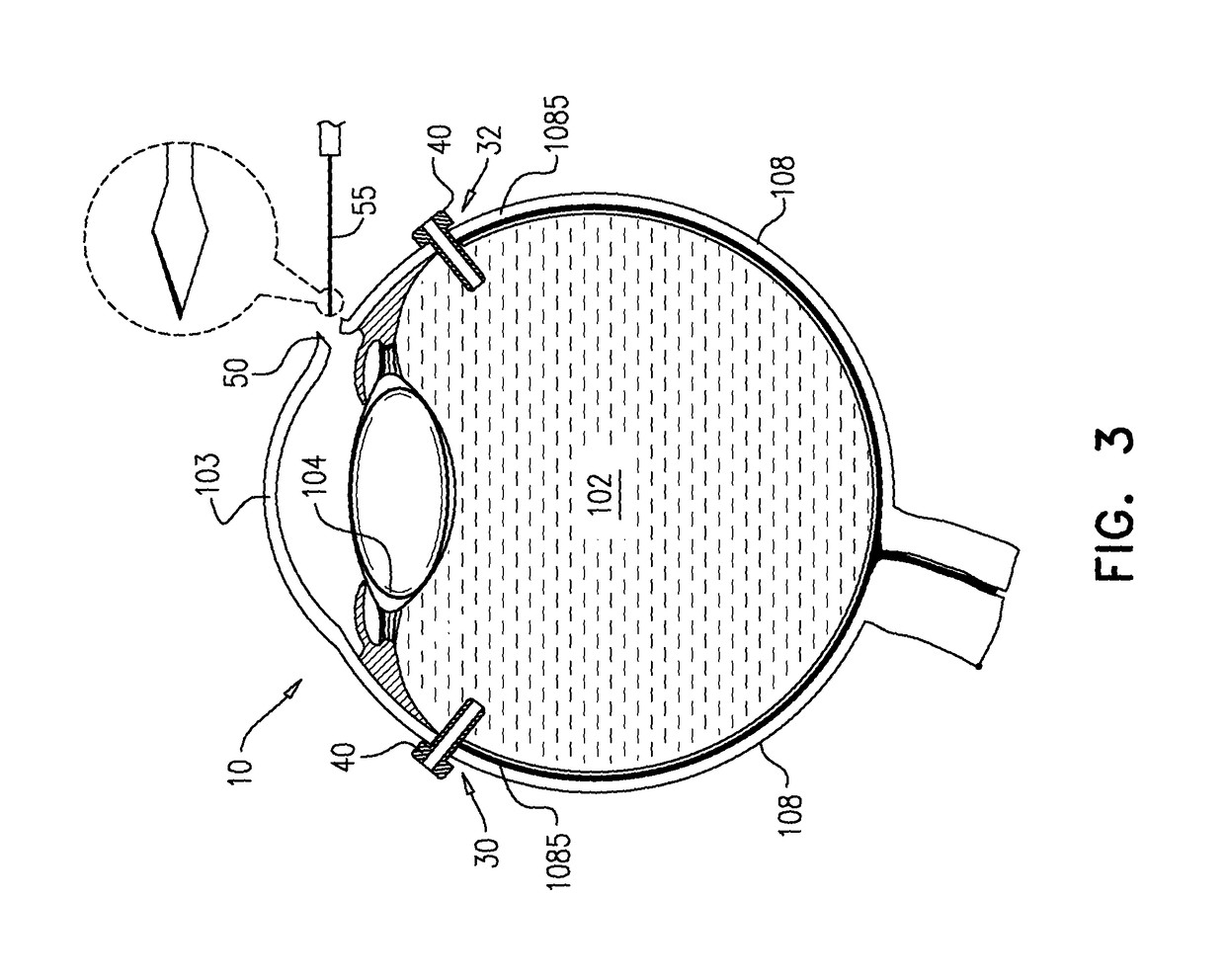
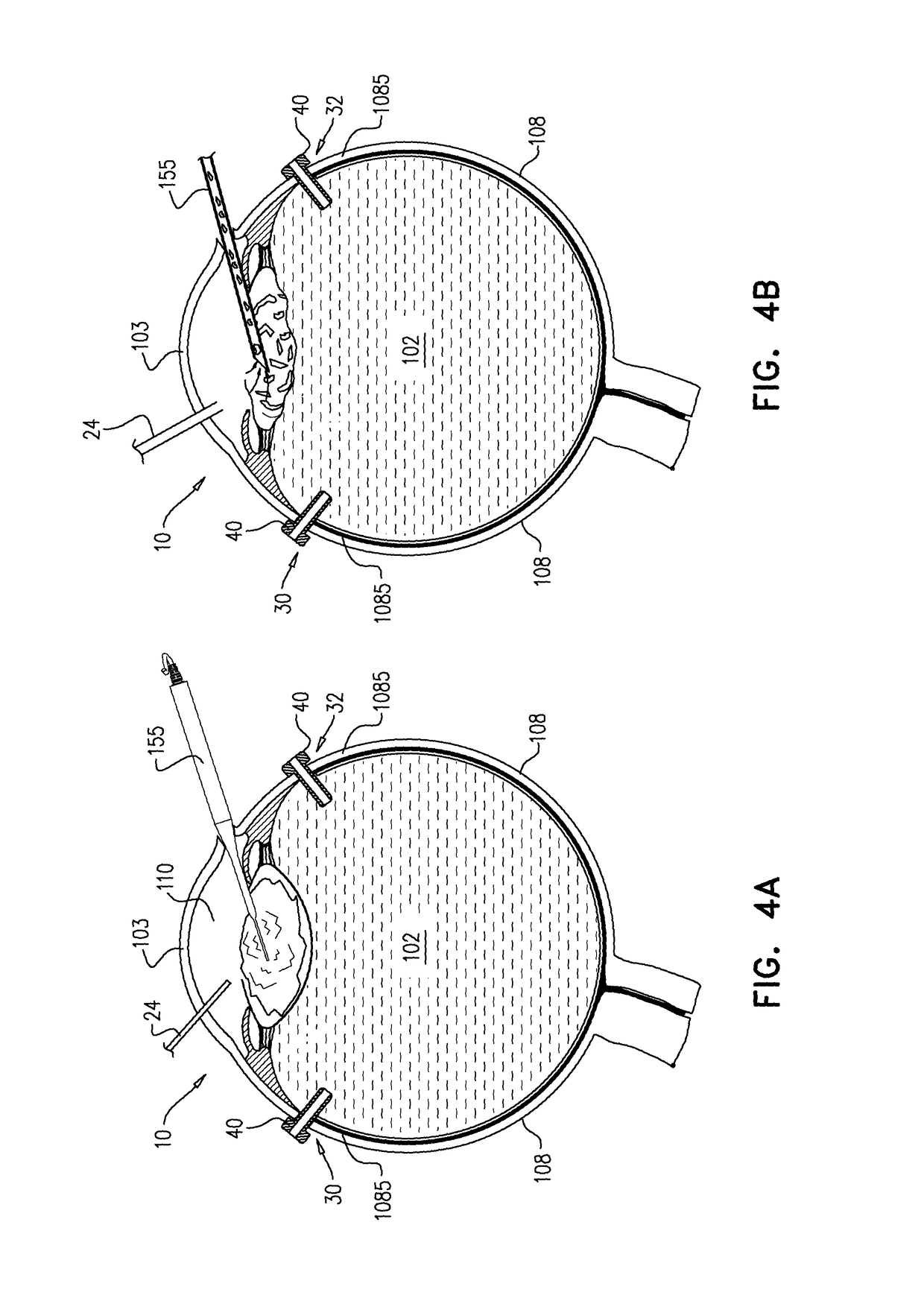
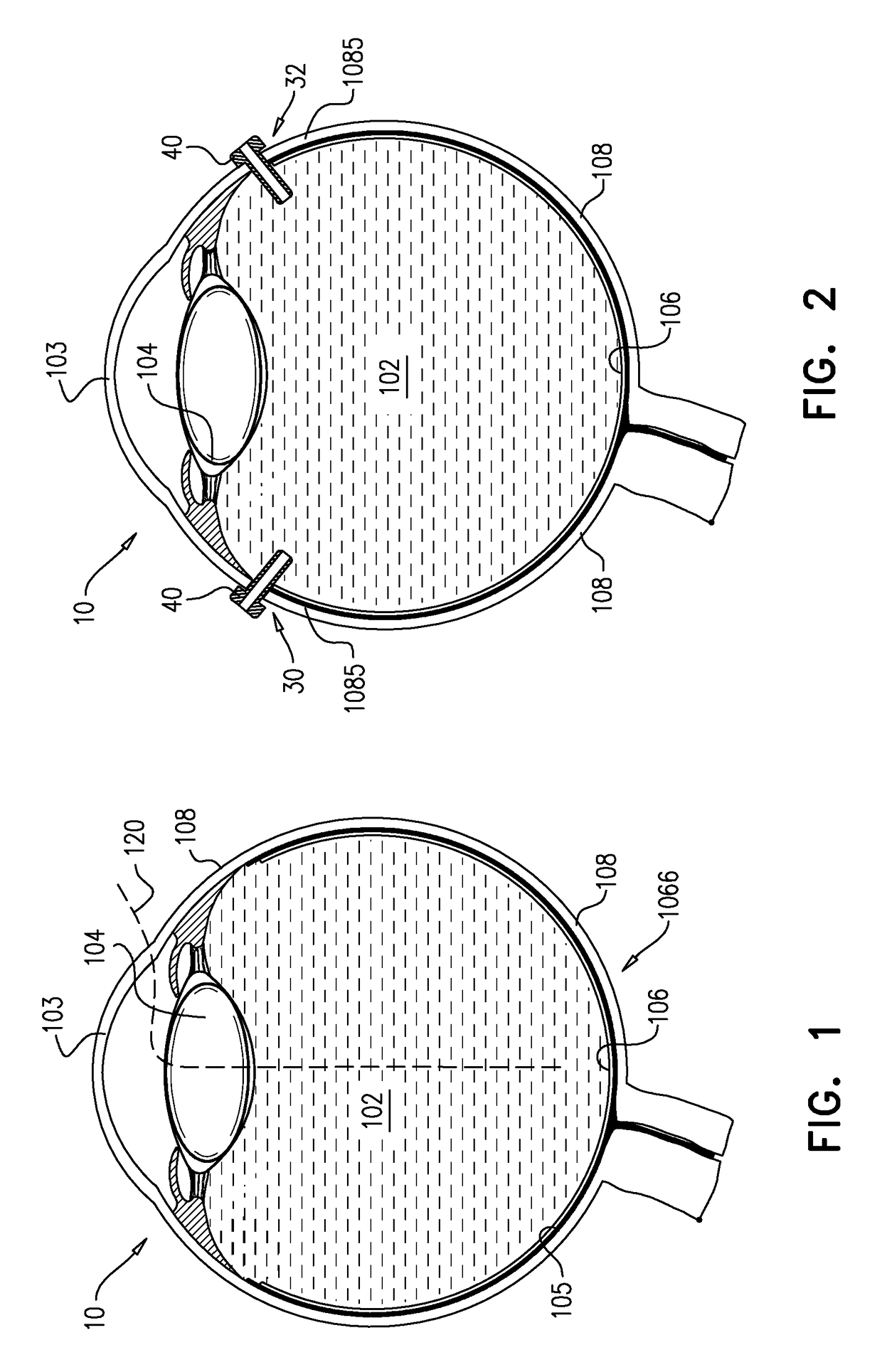
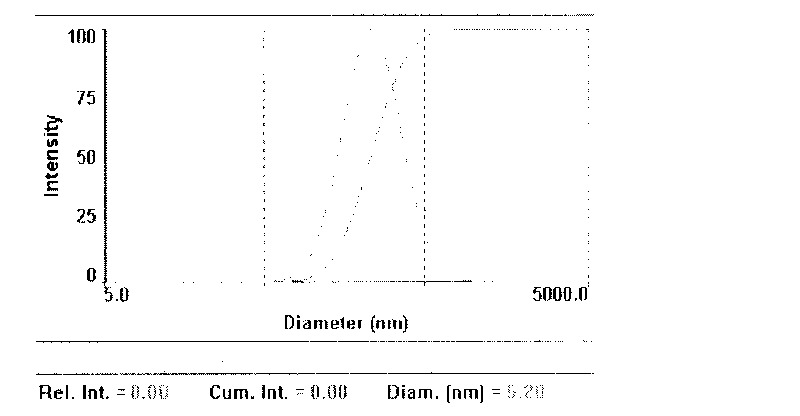
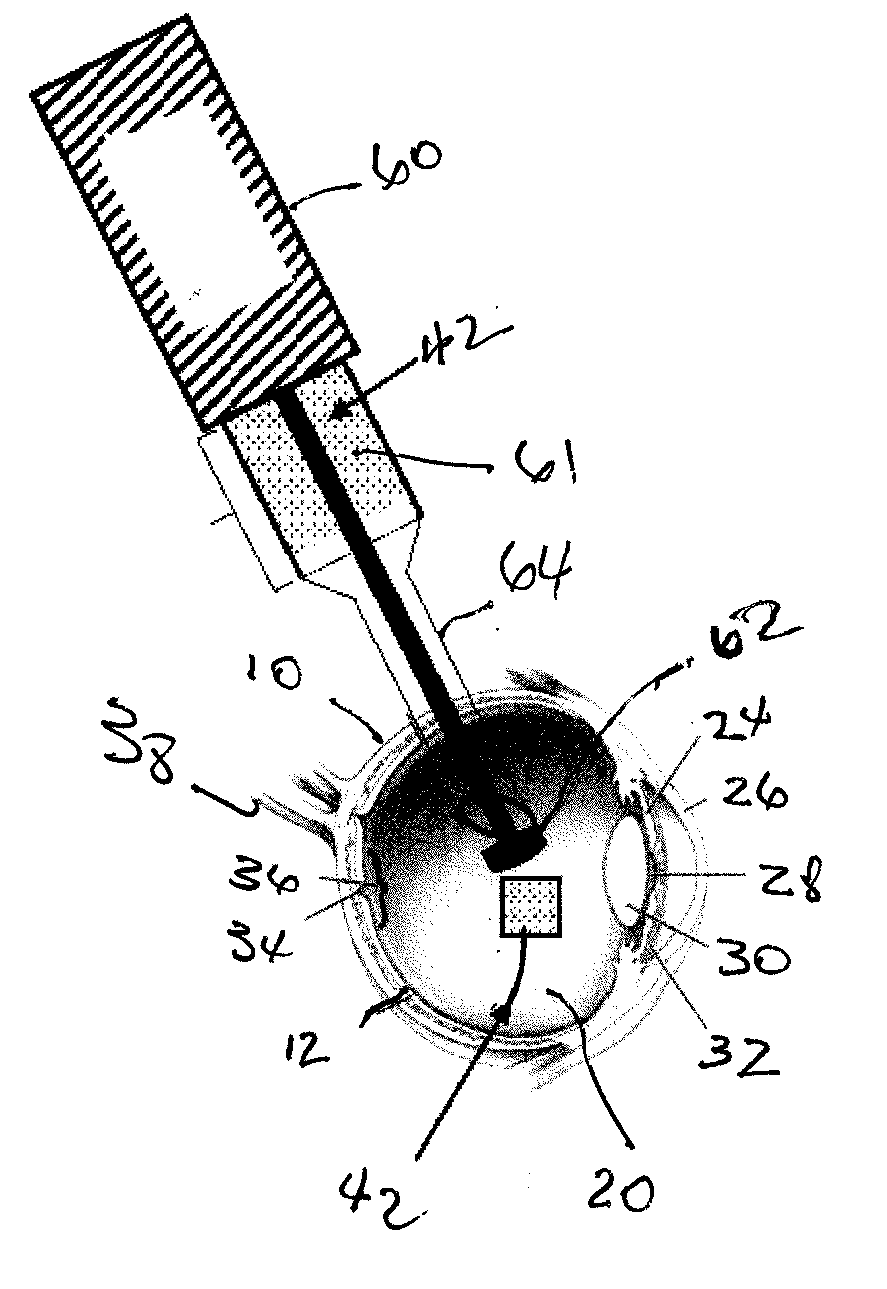
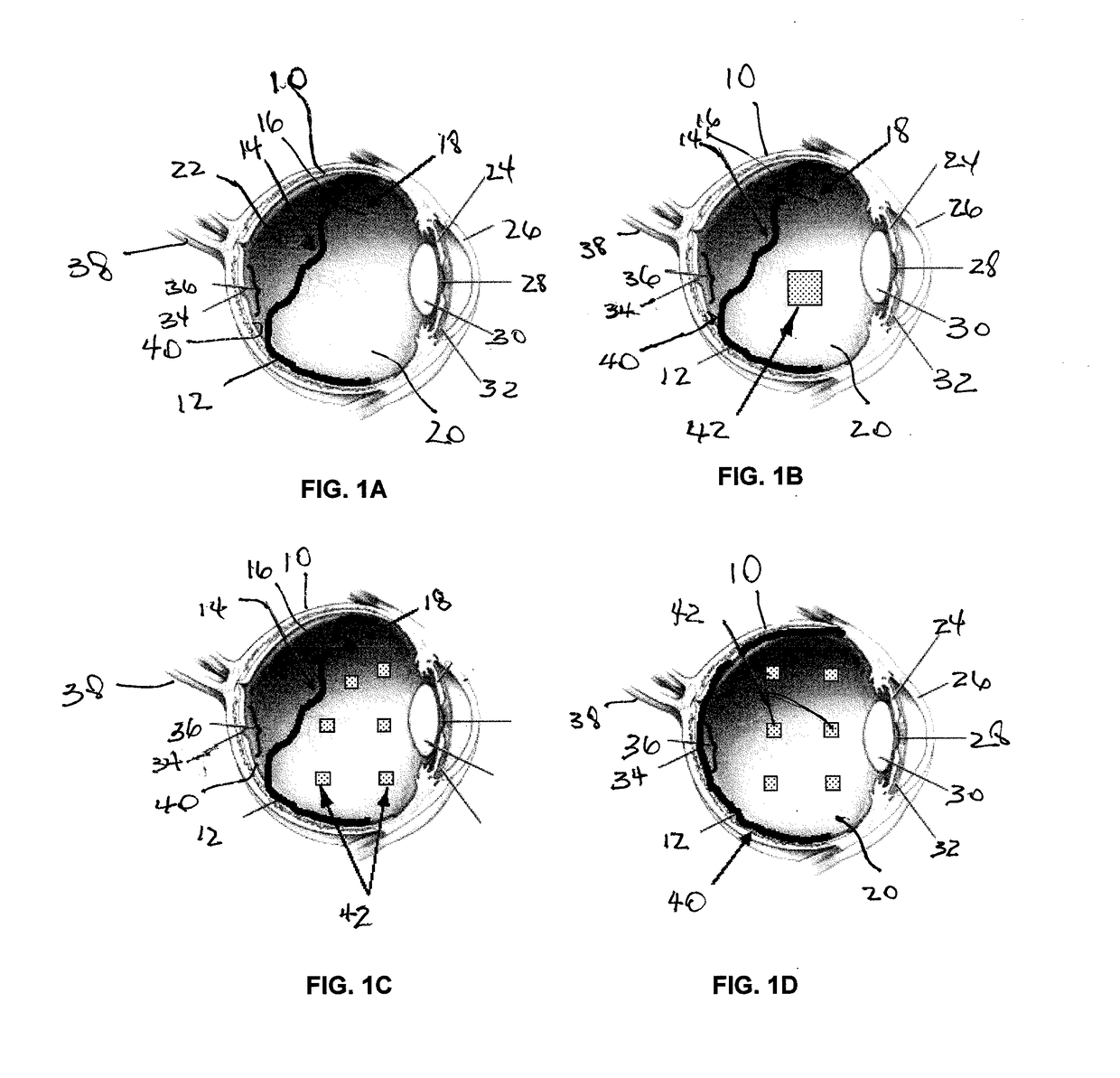
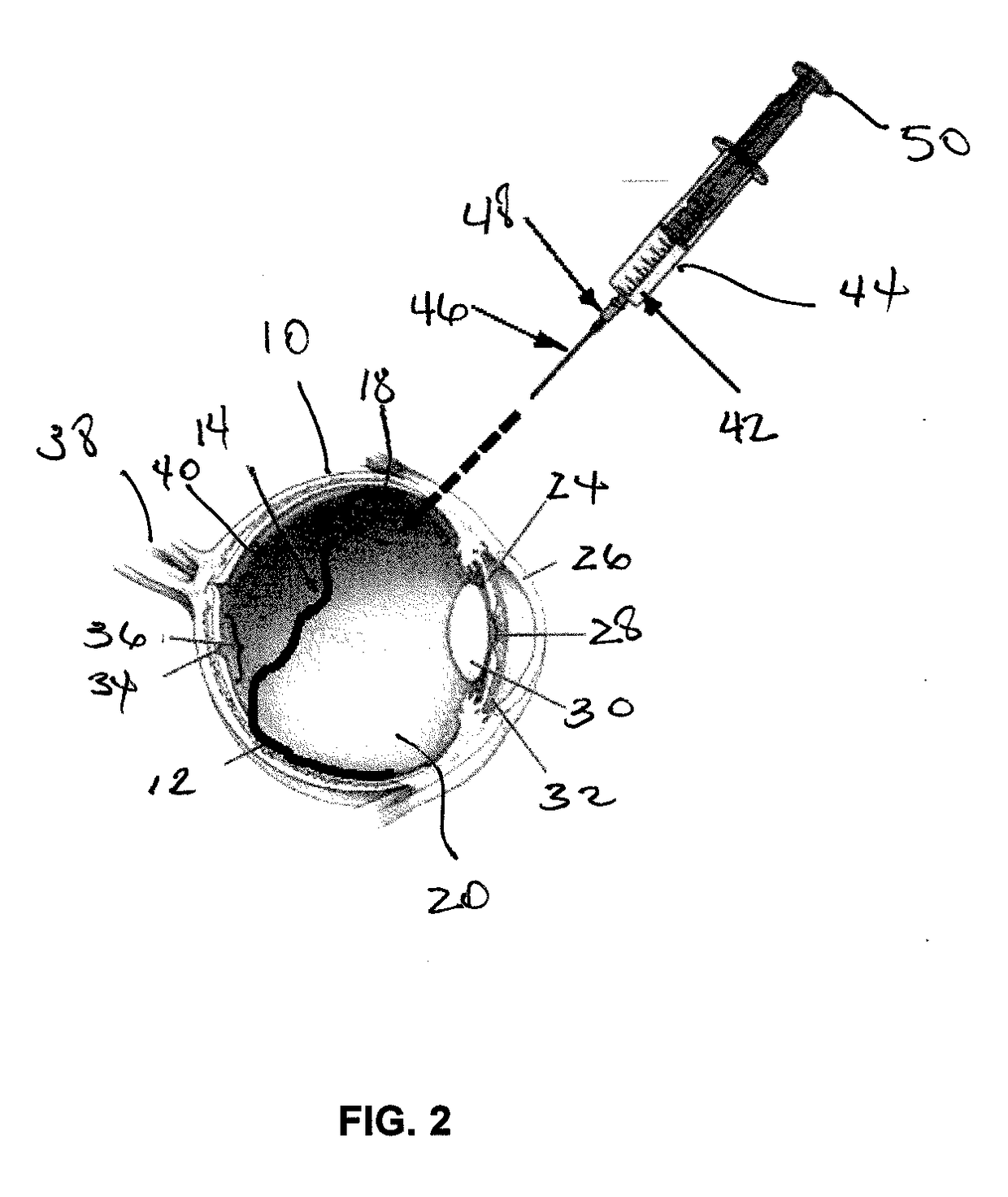
An apparatus and minimally invasive method for removing fluid from a subretinal space to allow a detached retina to flatten. An apparatus, comprising a fluid withdrawal device and a guide for advancing and placing the device, is positioned on the exterior eye surface at the detachment site. Various embodiments of the apparatus are disclosed. Using the guide, the surgeon advances the device into the fluid-filled space and drains fluid, allowing the retina to flatten. Additional injection of saline or gas into the vitreous cavity normalizes intraocular pressure, and the patient is ambulatory immediately afterward. Unlike other retinal attachment techniques, in the inventive procedure the patient receives only a local anesthetic, and has no restraint on head movement.
Owner:PEYMAN GHOLAM A
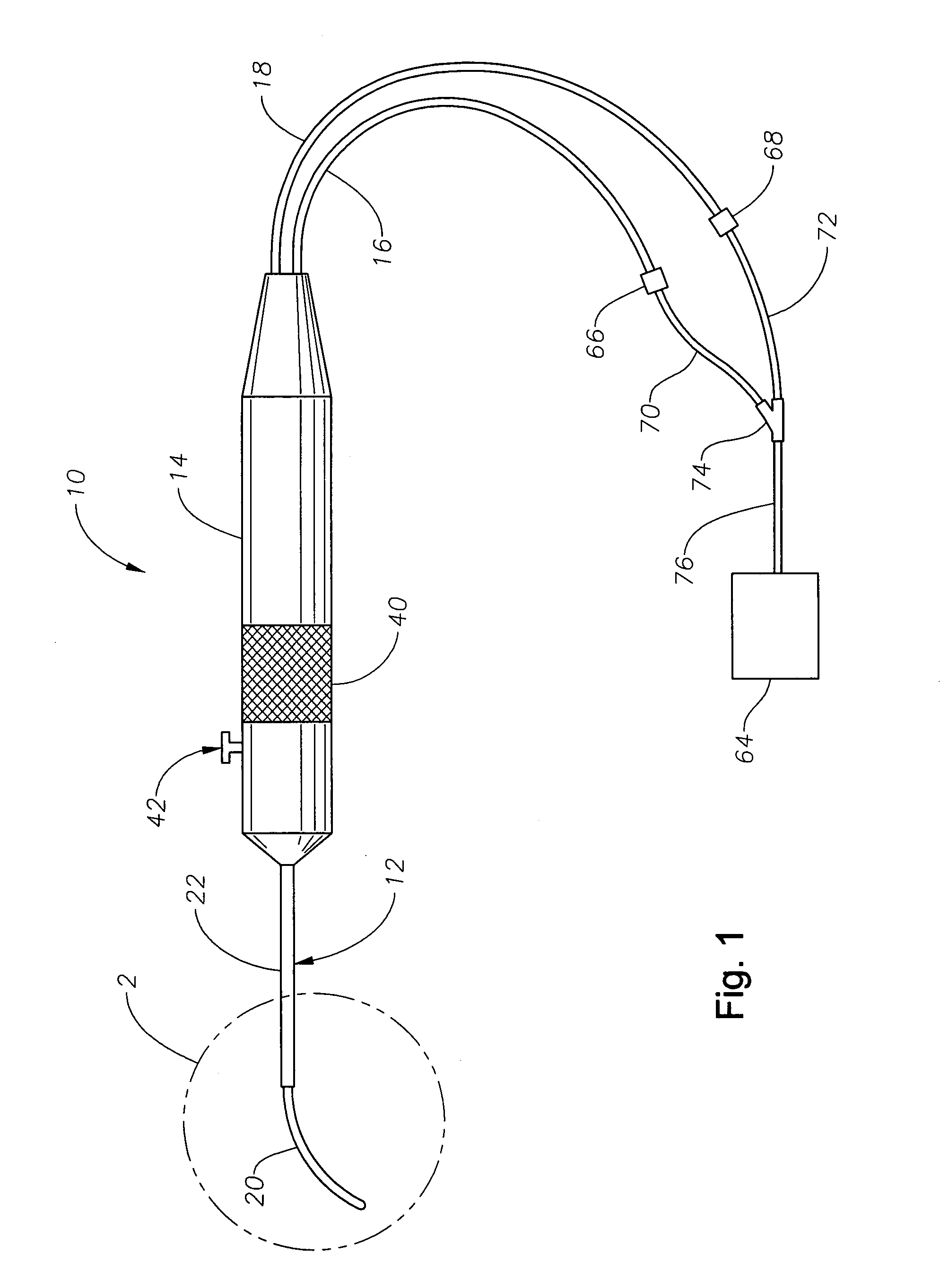
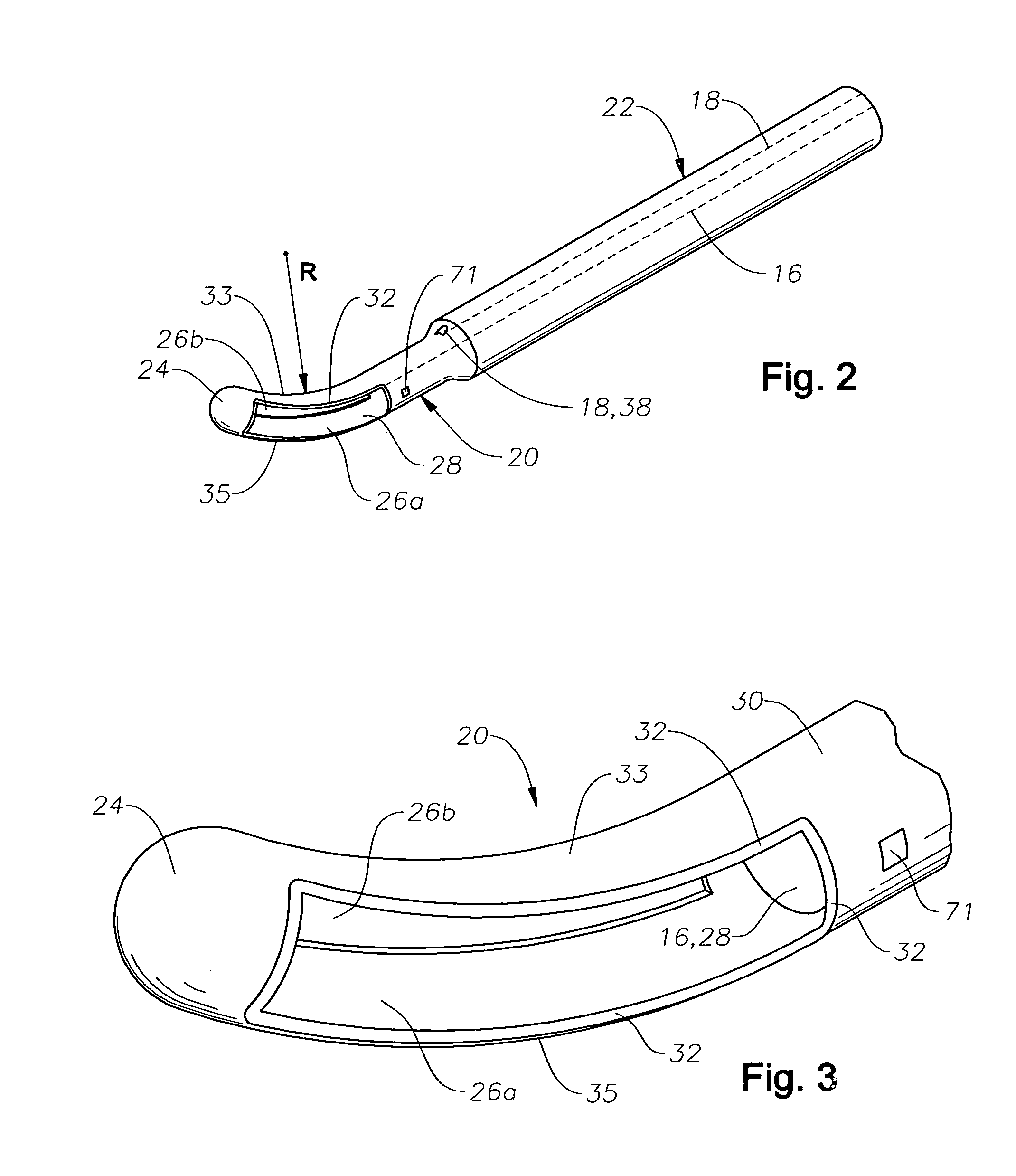
Vitreoretinal instrument
An improved instrument for removing subretinal fluid and performing fluid exchanges in vitreoretinal surgery is disclosed. The instrument includes a cannula with a curved distal portion and a side port on the curved distal portion for aspirating subretinal fluid. The instrument also includes a second port for aspirating a second fluid from the vitreous cavity during a fluid exchange.
Owner:ALCON INC
Compound subretinal prostheses with extra-ocular parts and surgical technique therefore
InactiveUS20070250135A1Minimize damageHighly controlled introductionElectrotherapyEye surgeryRetinal implantRetinaculotomy
In a method for introducing a retinal implant to a position within a subretinal region of an eye, the following steps are performed: a) preparing a fornix-based scleral flap at a distance from the limbus; b) detaching the retina by subretinal injection of balanced salt solution, from the vitreous cavity and creating a localized bubble in the area of the scleral flap; c) performing in the upper hemisphere of the eye a peripheral retinotomy and detaching a part of the retina; d) advancing the implant into the subretinal space and placing an inner portion of said implant on the retinal pigment epithelium onto the desired position; and e) closing the sclera flap.
Owner:RETINA IMPLANT
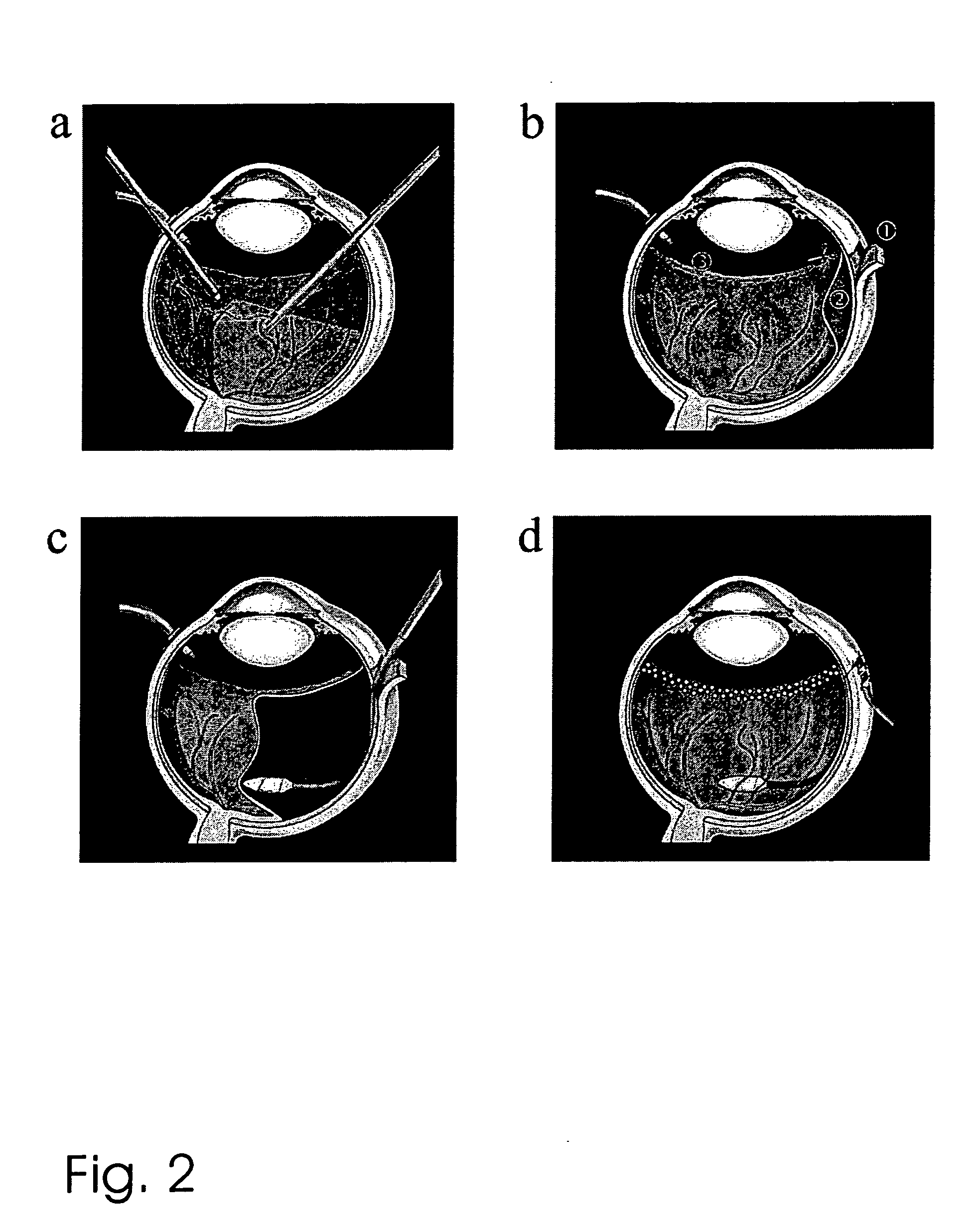
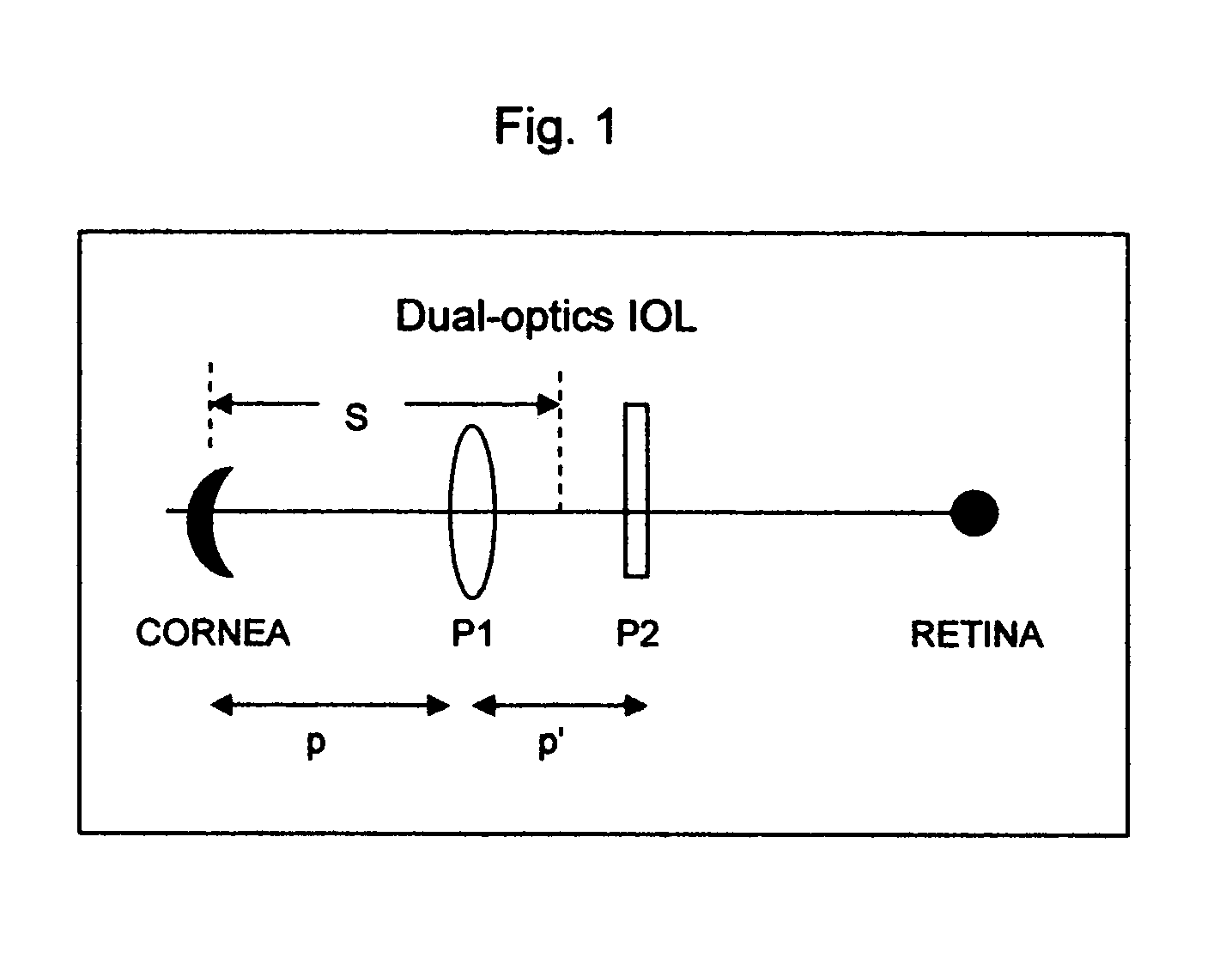
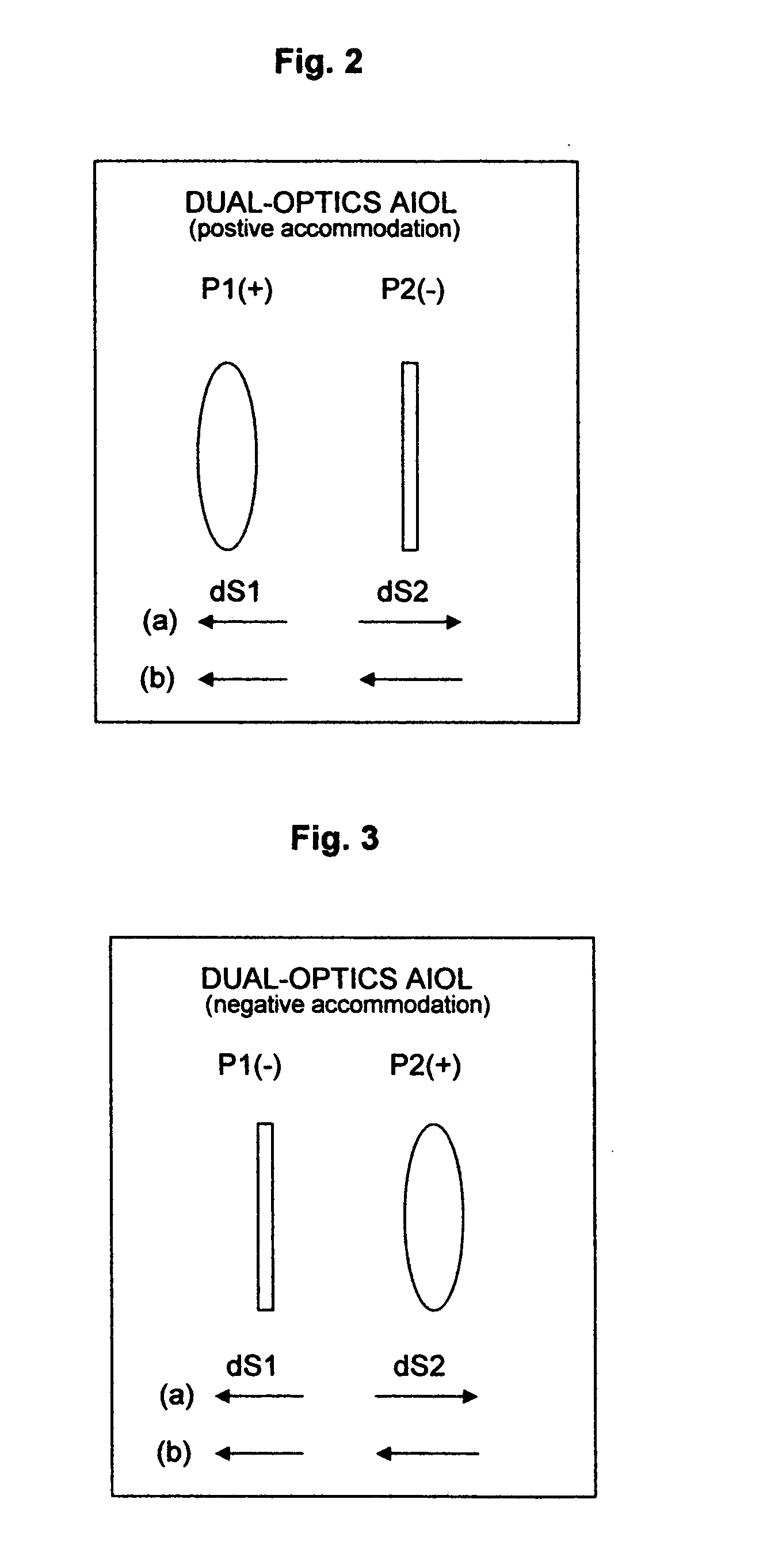
Method and device for vision correction via dual-optics accommodating intraocular lens
Method and design of a dual-optics accommodating intraocular lens (IOL) for vision correction of adult and pediatric eyes after cataract surgeries are disclosed. For adult eyes, a positive accommodation amplitude greater than 3.5 diopter and preferably 4.0 to 10.0 diopter may be achieved by optimal configurations having the positive-power front-optics moves toward the cornea, whereas the negative power back-optics moves in the opposite direction. In contrast, a negative accommodation is required for pediatric eyes and may be achieved by a reversed configurations. The enhanced efficiency, up to 500%, is proposed by preferred embodiments based on new lens design formulas and calculation steps for the IOL power pre-determined by the measured ocular parameters including the corneal power, IOL position and the vitreous cavity length of the eye.
Owner:LIN J T
Accommodating Intraocular Lens
ActiveUS20110313519A1Reduce widthProcess stabilityIntraocular lensIntraocular lensMuscle contraction
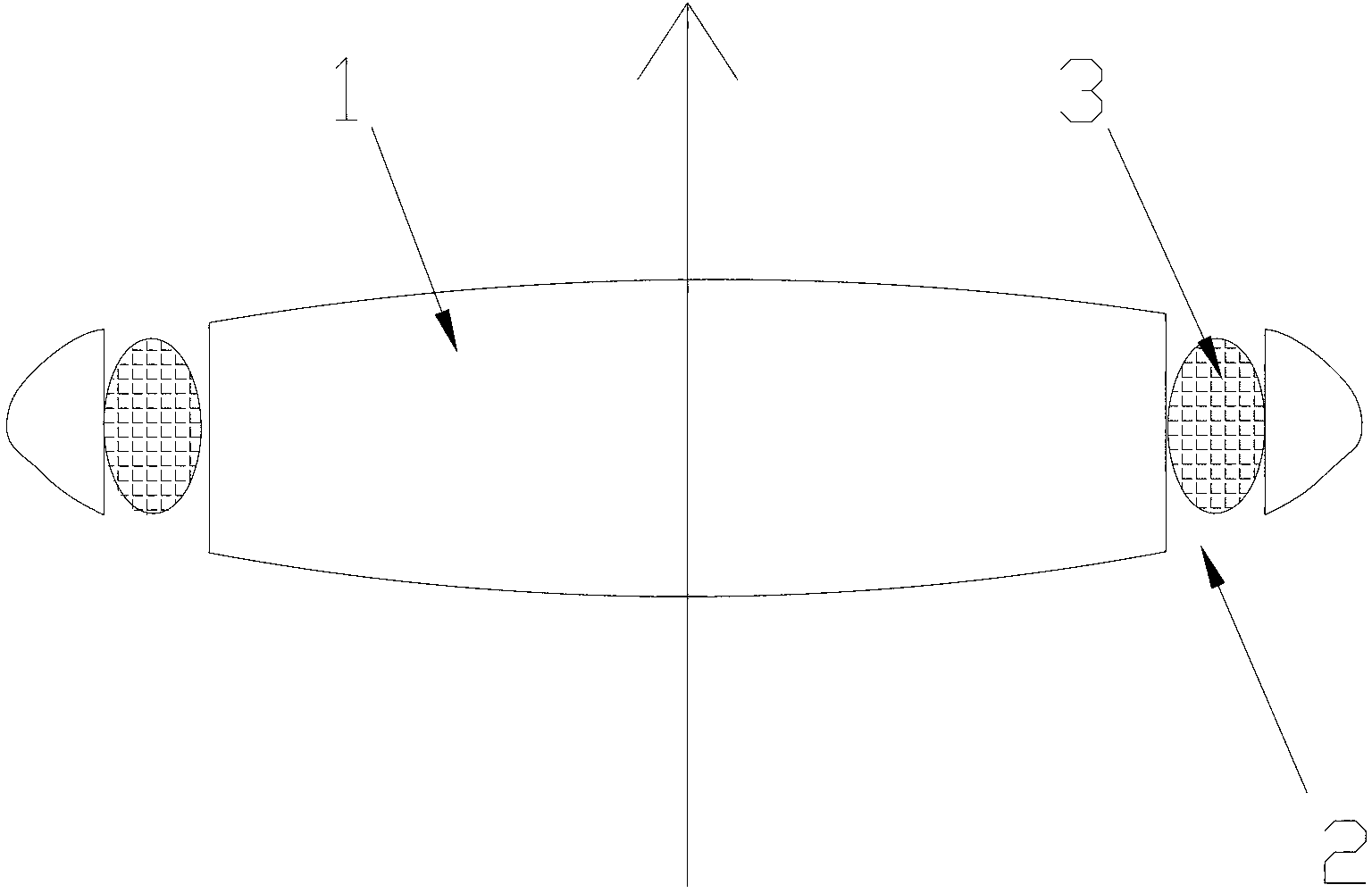
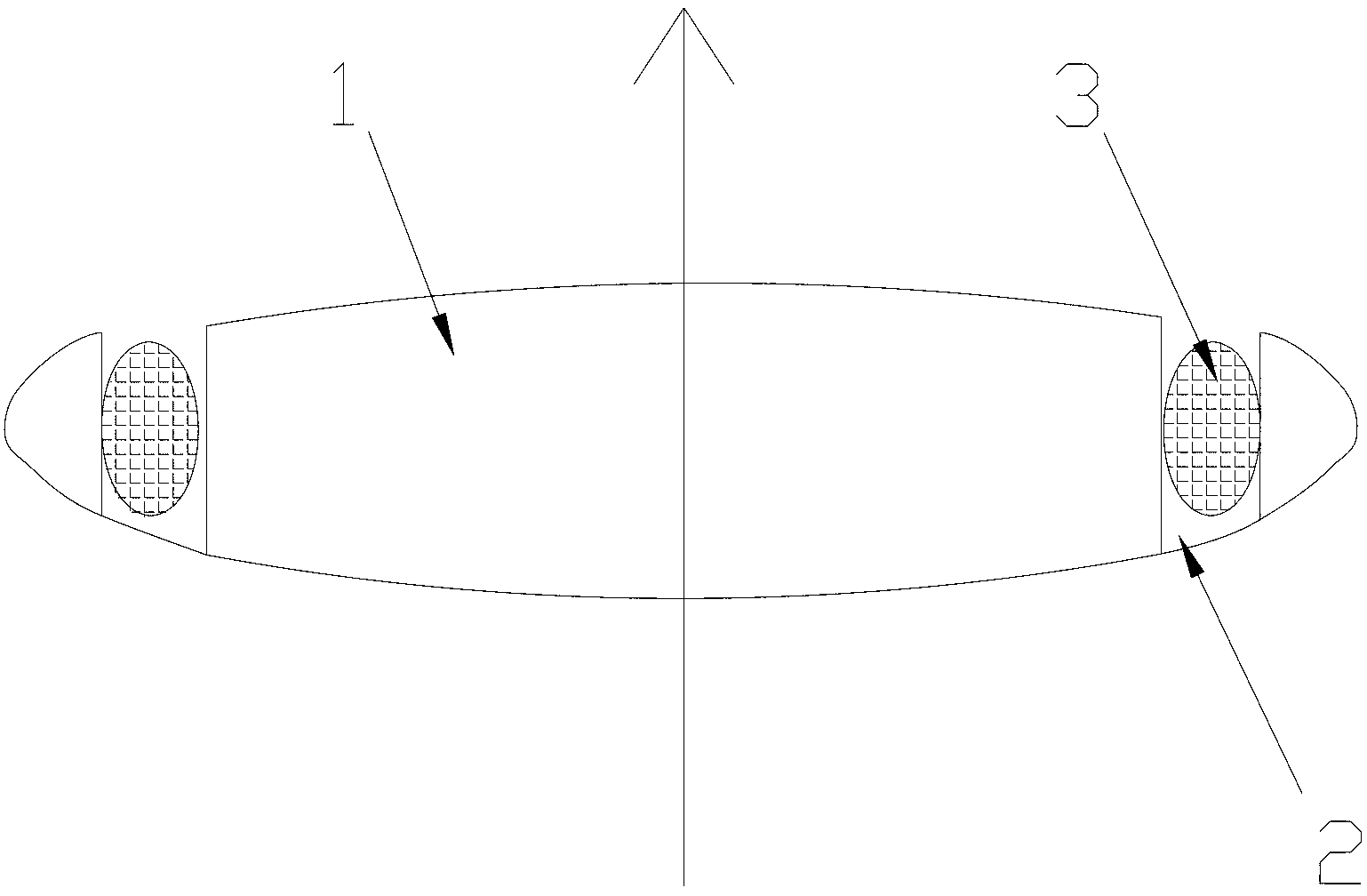
An intraocular lens for insertion into a capsular bag of an eye comprises: an optic; and at least one plate haptic coupled to the optic by one or more flexible connecting members. The plate haptic with flexible finger extensions is designed to engage the periphery of the capsular bag. An increase in radial pressure upon constriction of the ciliary muscle causes the rigid posteriorly vaulted plate haptics to move centrally to further increase the vitreous cavity pressure with constriction of the ciliary muscle: the optic with its thin stretchable hinge across the connecting member is then displaced anteriorly along the axis of the eye. The haptic includes a longitudinally rigid frame to restrict deformation of the haptic in a longitudinal direction while permitting deformation in a transverse direction. Furthermore, the flexible connecting members include one or more hinged straps that extend radially and / or longitudinally from the optic. The optic can move forwards and backwards, relative to both the distal and proximal ends of the plate haptics, in response to ciliary muscle contraction and relaxation with an increase and decrease of vitreous cavity pressure. Finger-like projection extends from the plate haptic to engage the capsular bag to center and fixate the accommodating lens within the capsular bag.
Owner:CUMMING JAMES STUART
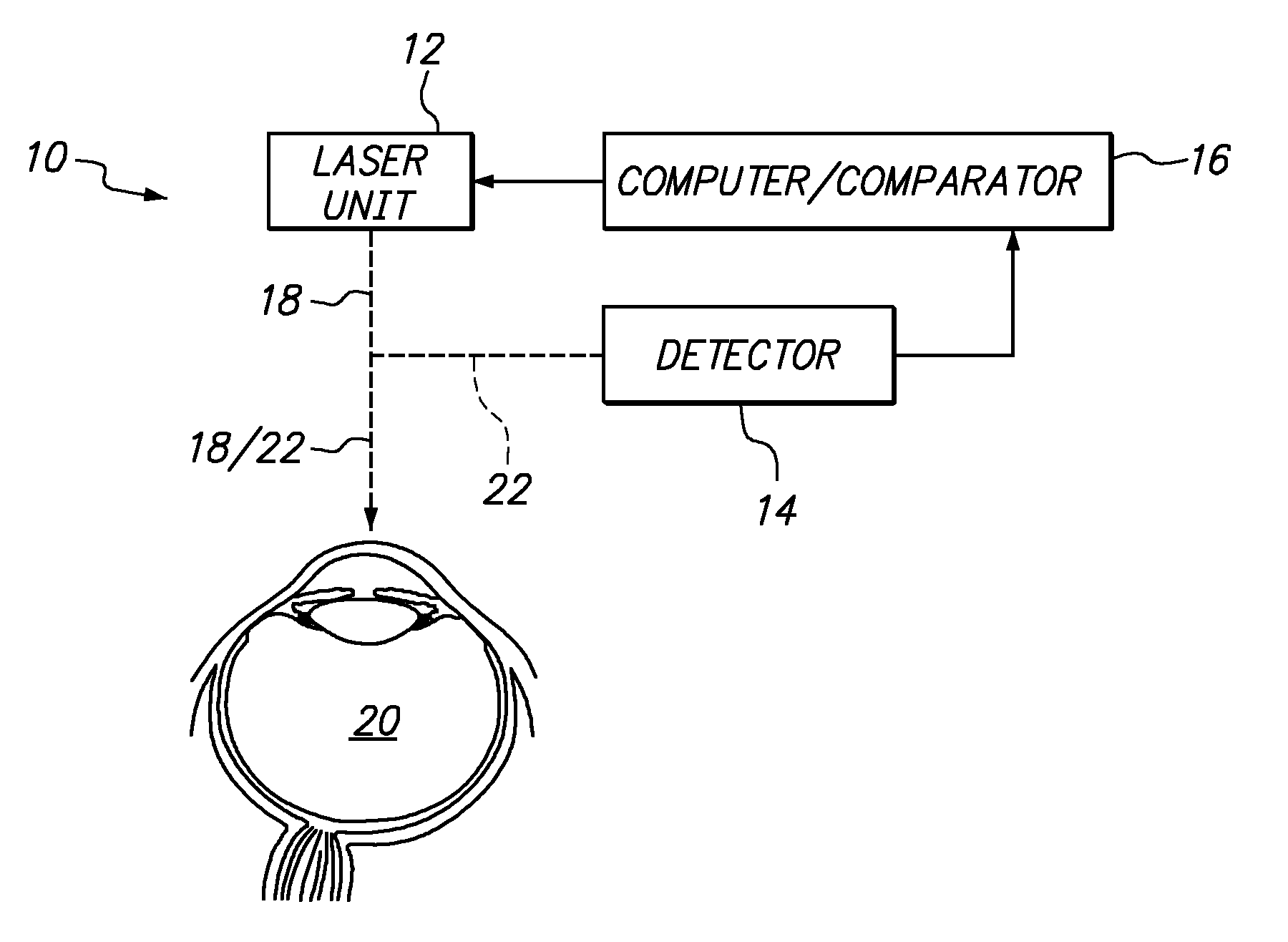
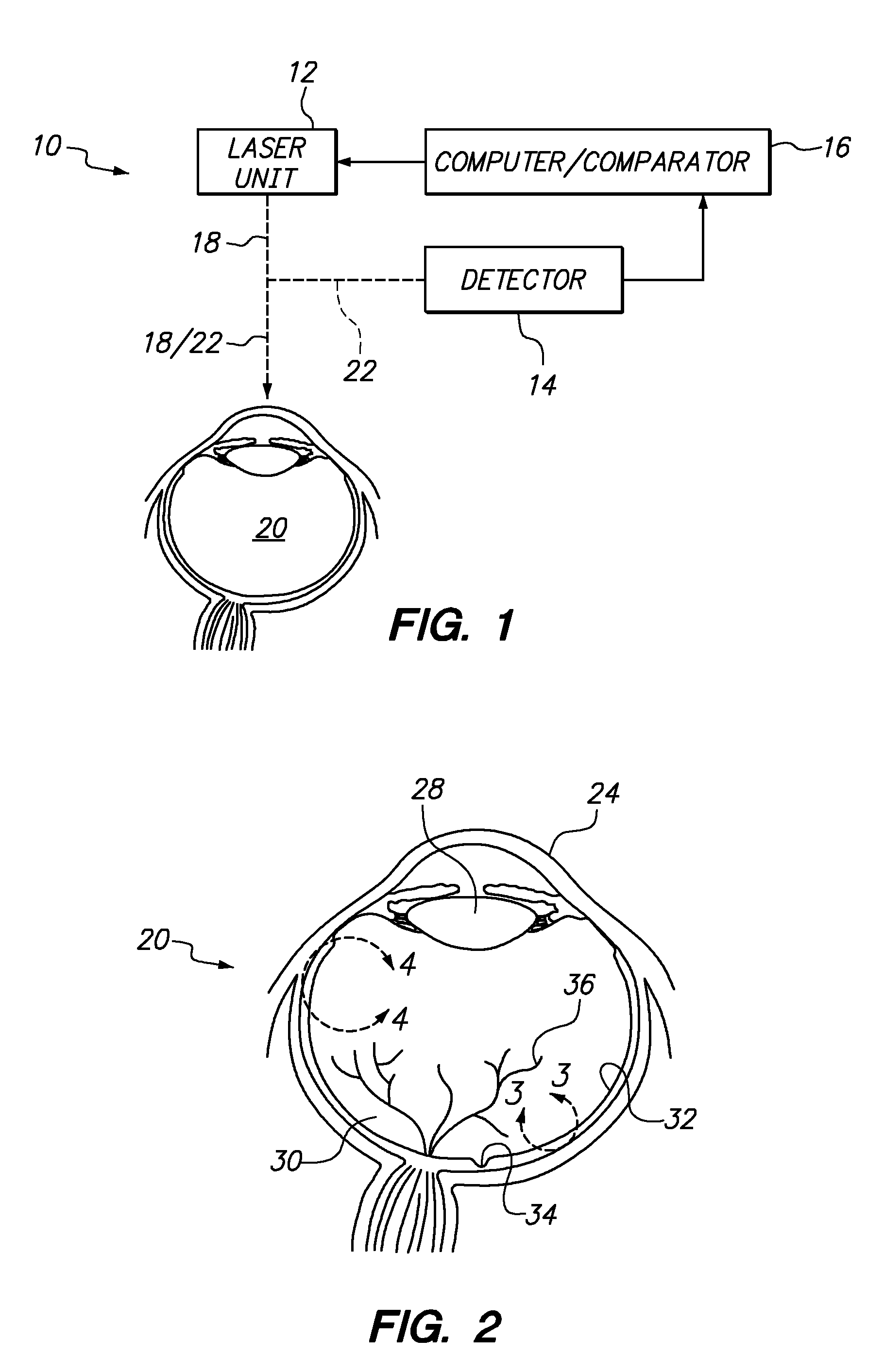
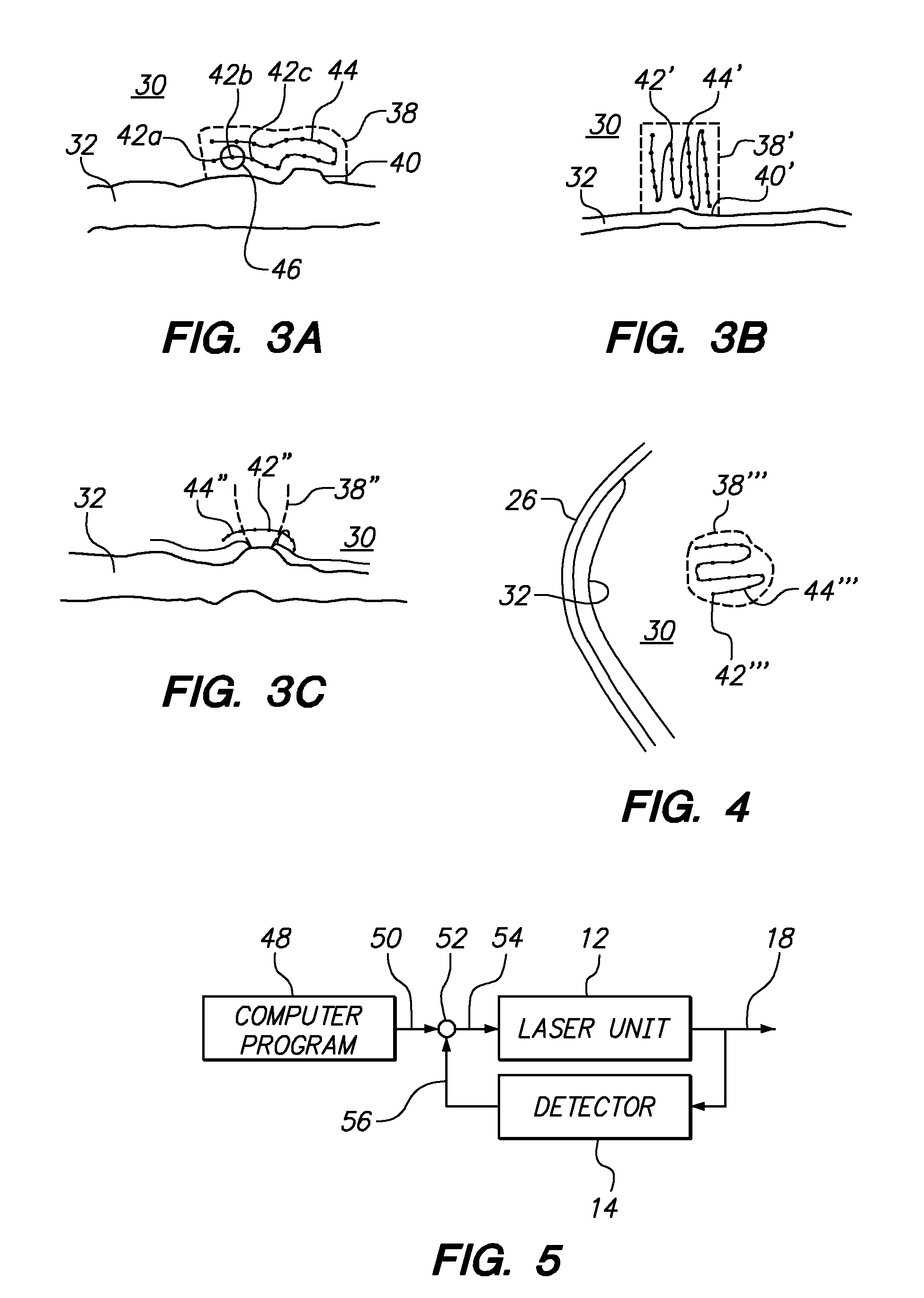
Systems and methods for treating target tissue in the vitreous cavity
A system and its method for treating targeted tissue in the vitreous cavity of an eye include a laser unit for generating a laser beam and a detector for creating an image of the targeted tissue. The system also includes a computer which defines a focal spot path for emulsifying the targeted tissue. A comparator that is connected with the computer then controls the laser unit to move the focal spot of the laser beam. This focal spot movement is accomplished to treat the targeted tissue, while minimizing deviations of the focal spot from the defined focal spot path.
Owner:TECHNOLAS PERFECT VISION
Transretinal implant and method of implantation
A retinal implant device to stimulate a retina of an eye thereby producing a specific effect in an eye, such as vision or drug treatment of a chronic condition is described. The retinal device is made of a retinal implant that is positioned subretinally and that contains a multitude of stimulation sites that are in contact with the retina. A connection carries the stimulating electrical signal or drug. The connection passes transretinally through the retina and into the vitreous cavity of the eye, thereby minimizing damage to the nutrient-rich choroid. The lead is attached to a source of drugs or electrical energy, which is located outside the eye. The lead passes through the sclera at a point near the front of the eye to avoid damage to the retina.
Owner:DOHENY EYE INST +1
Eye wall anchored fixtures
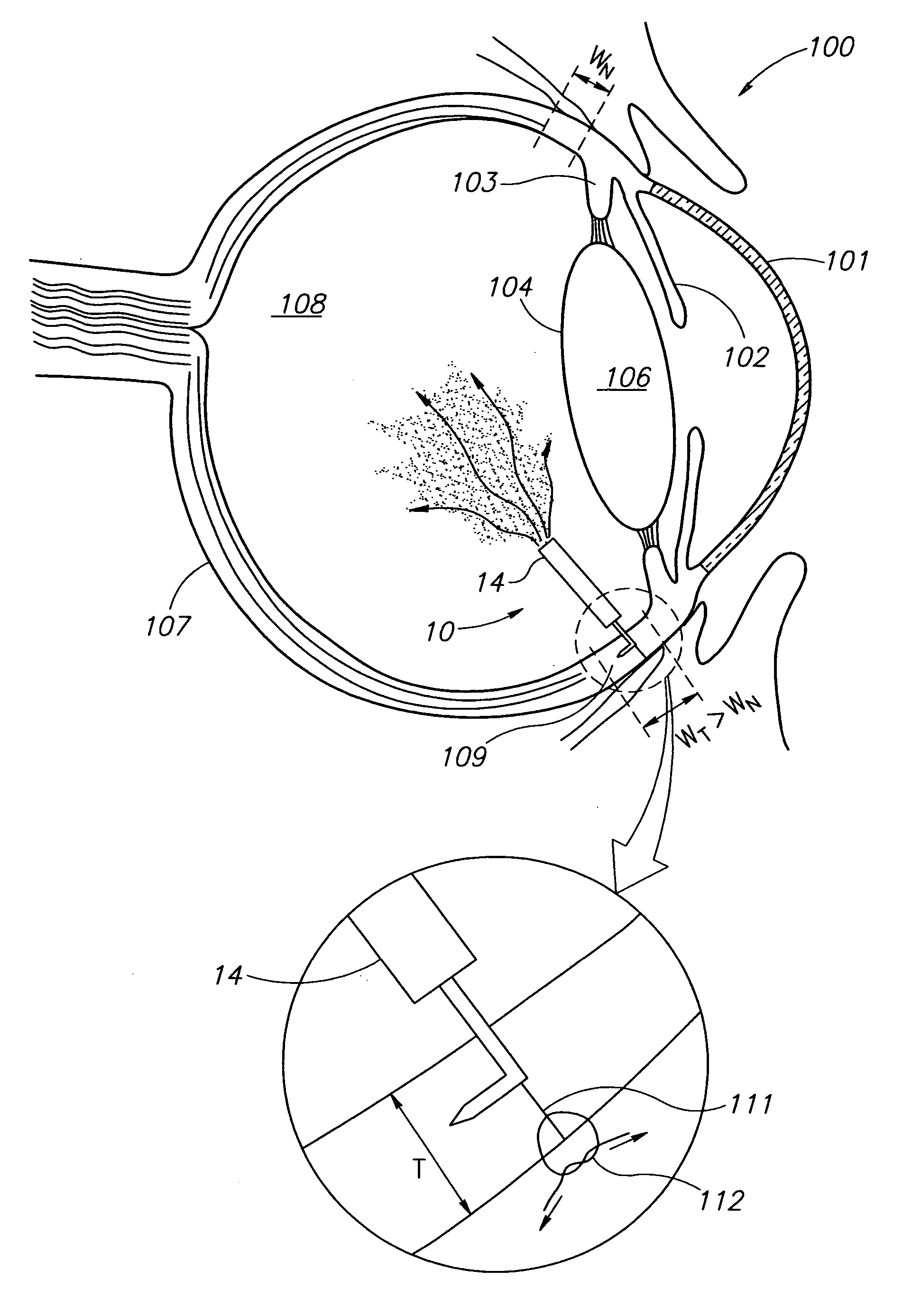
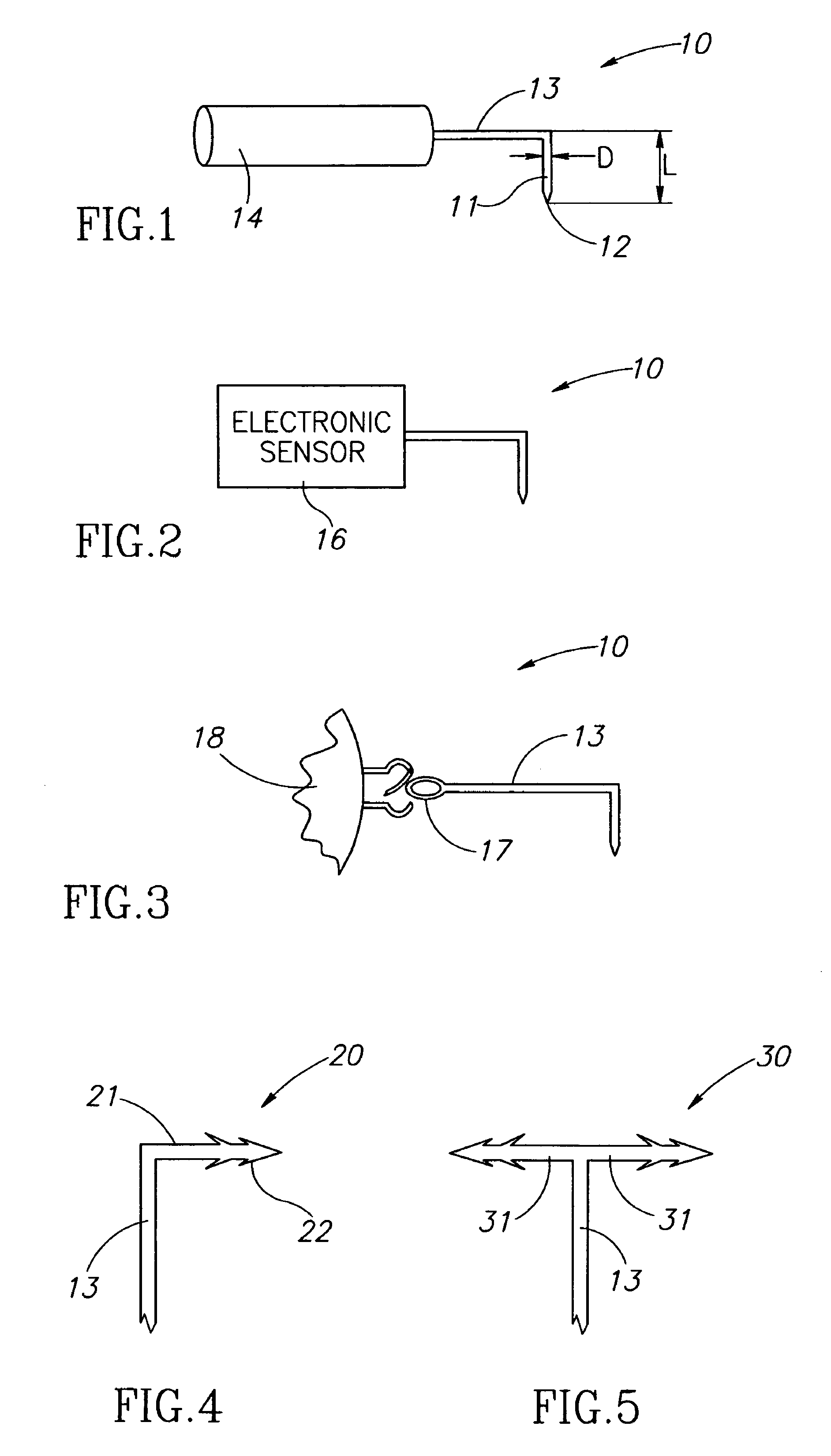
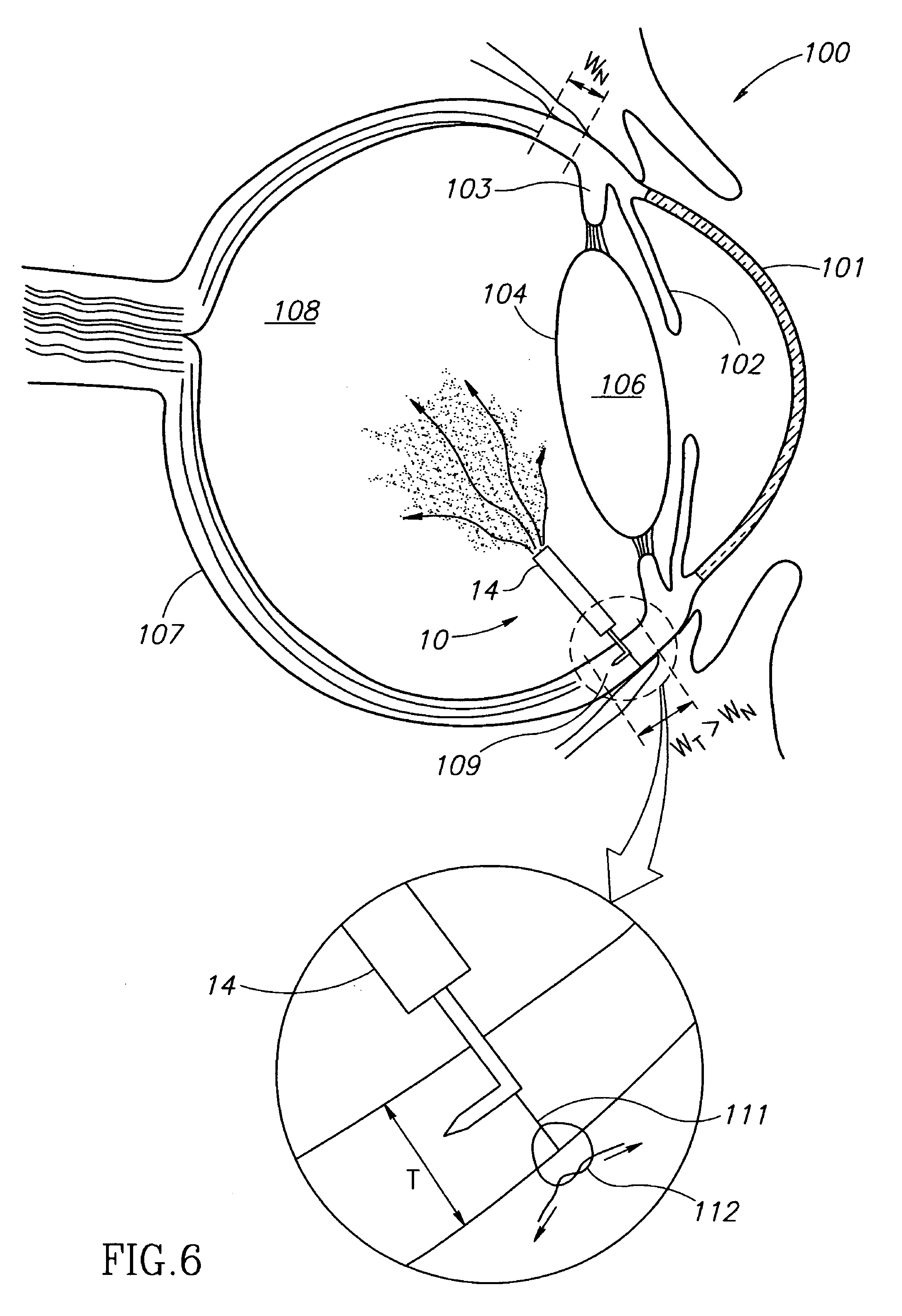
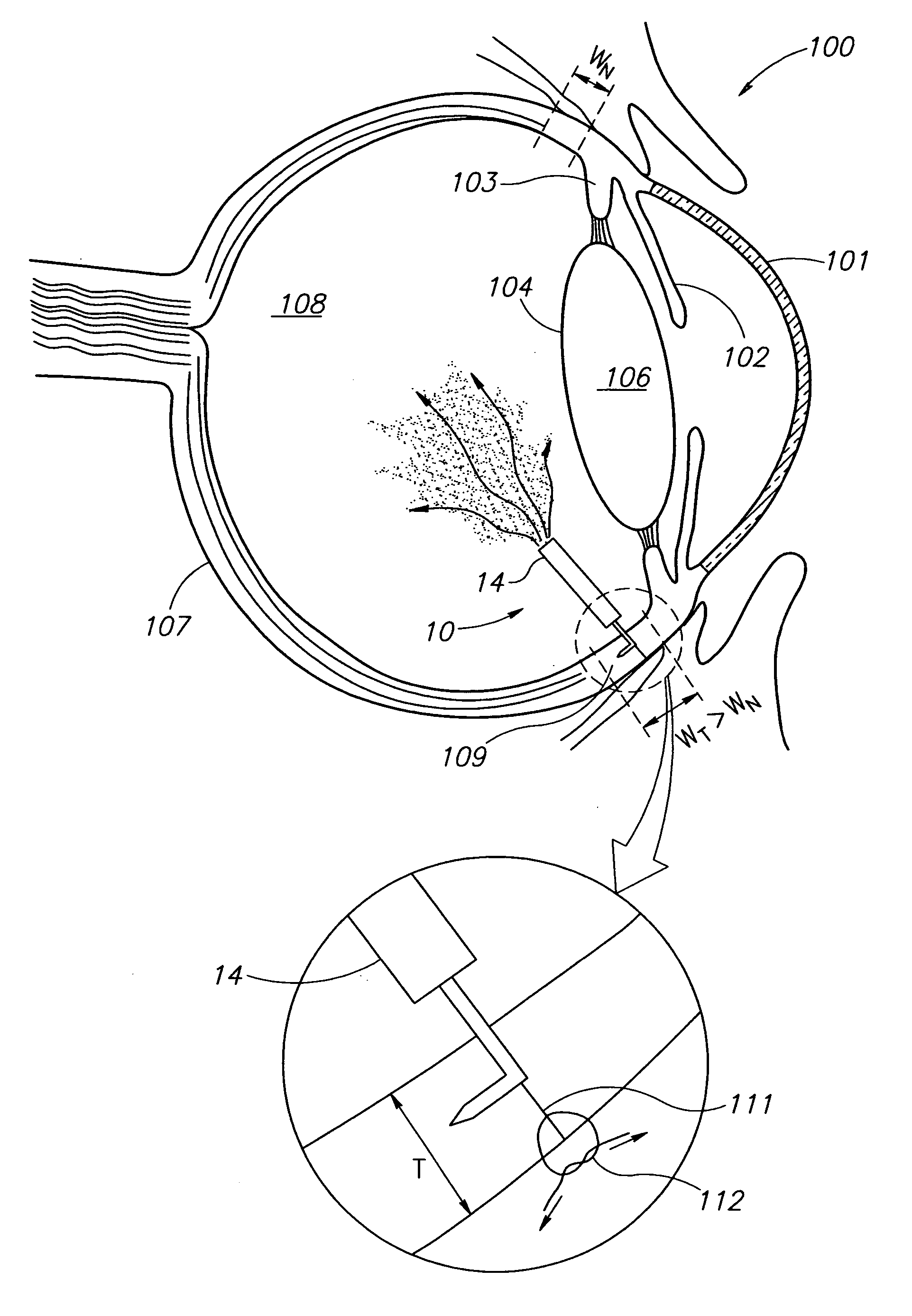
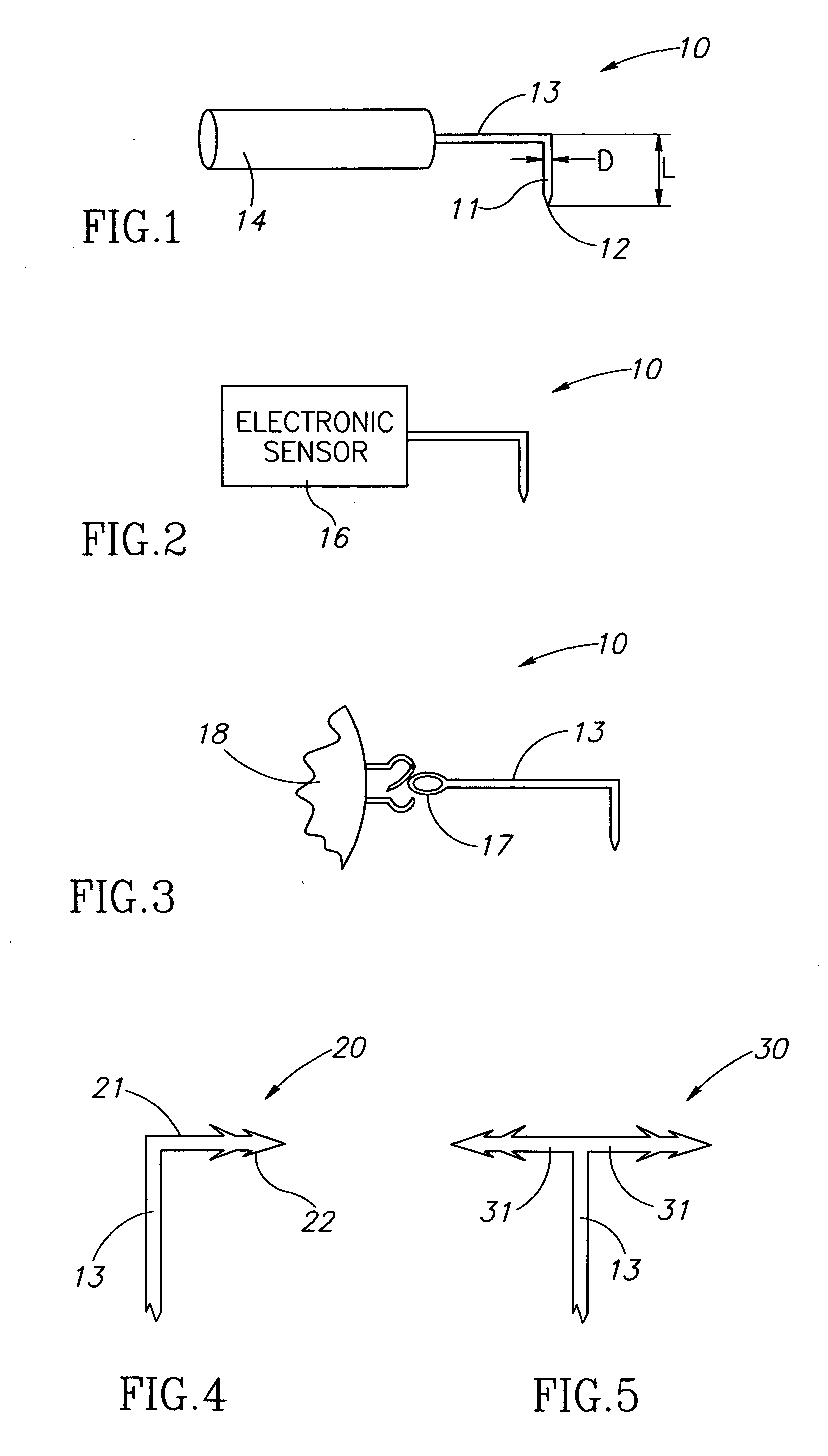
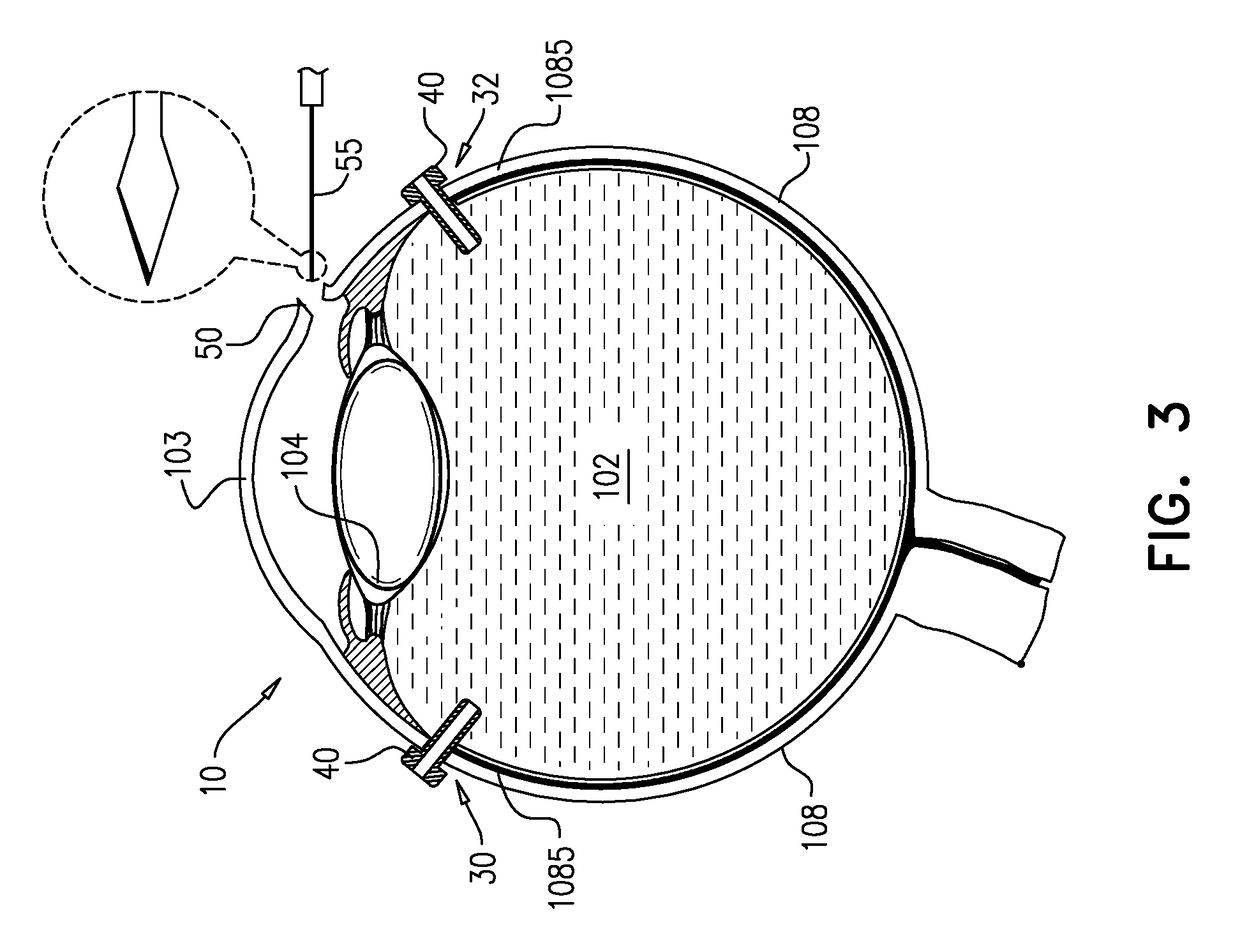
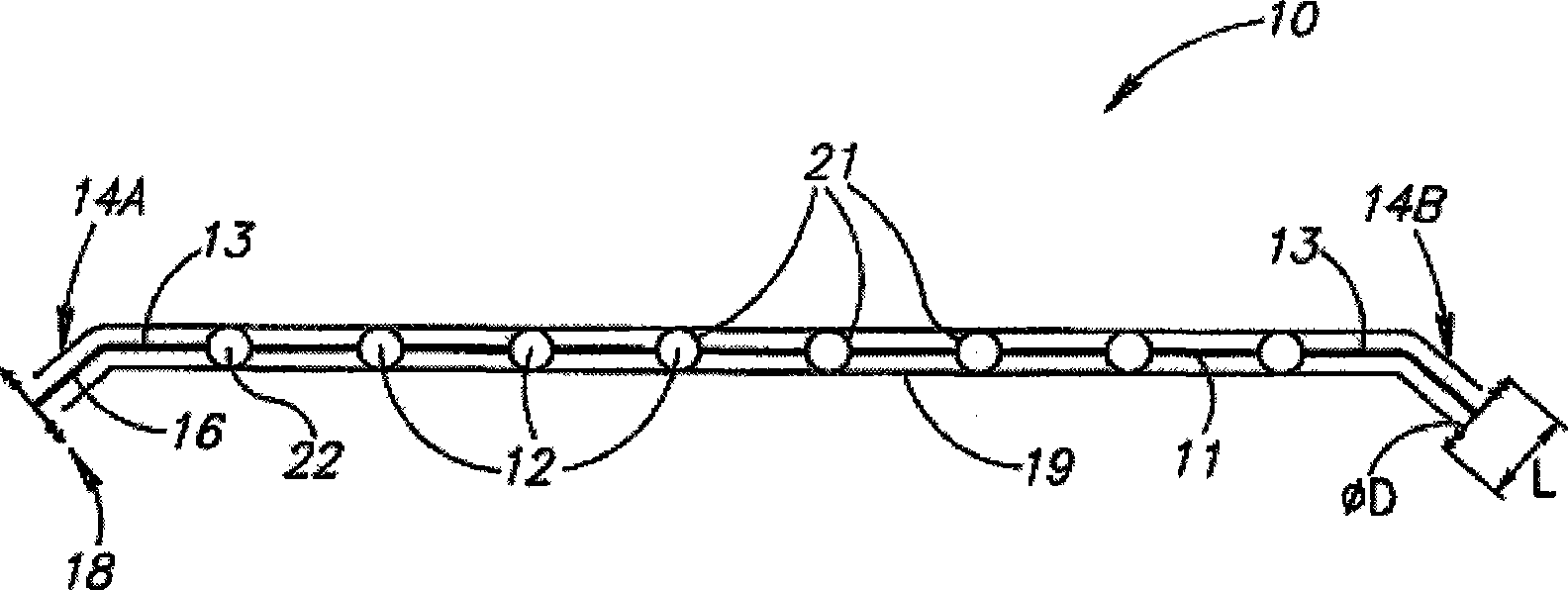
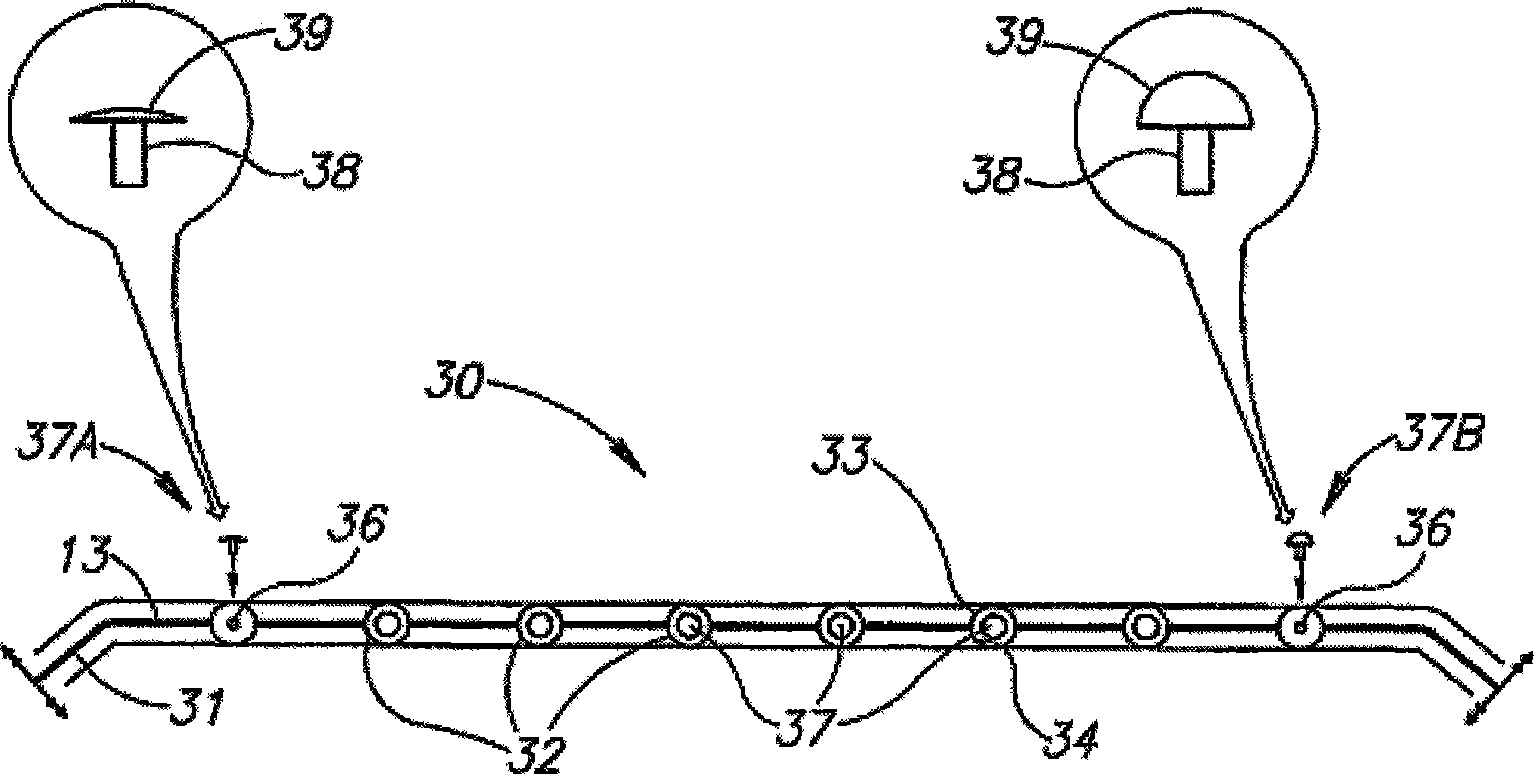
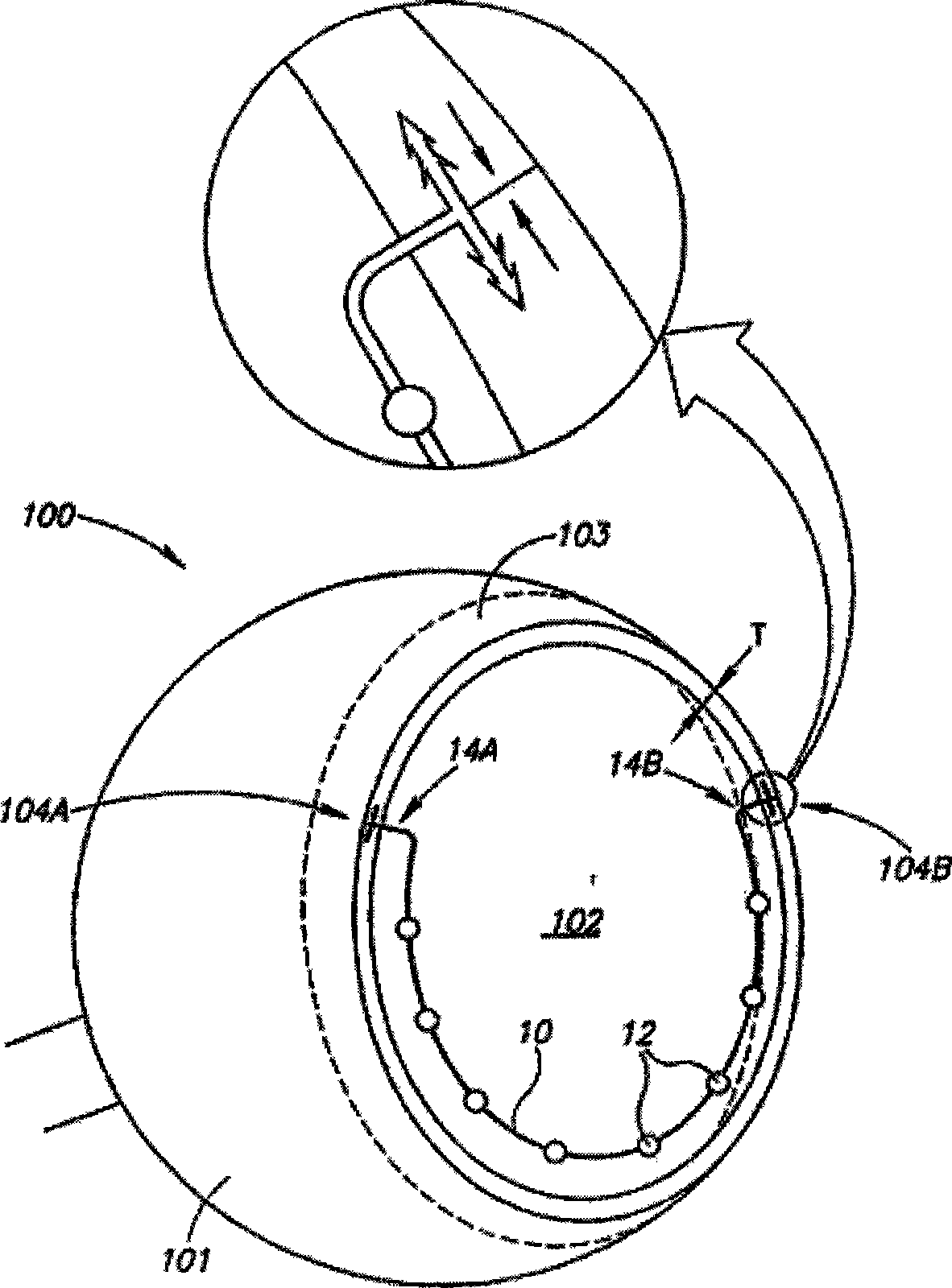
Eye wall anchored fixtures each including at least one elongated anchor member for driven lengthwise insertion into an eye's eye wall in a transverse direction to its thickness for supporting an intraocular device in the eye's vitreous cavity. Fixtures are preferably anchored in circumferential incisions in a human adult eye's pars plana preferably 3.5 mm posterior to its corneal limbus, and perpendicular to a circumferential incision. The fixtures are either generally L-shaped with a single elongated anchor member designed for withdrawal from an eye wall or self-anchoring thereinto, or generally T-shaped with a pair of oppositely directed self-anchoring elongated anchor members for sealing a throughgoing incision. Intraocular devices can be designed for intraocular drug delivery, for acquiring intraocular physiological measurements, and the like.
Owner:FORSIGHT VISION5 INC
Eye wall anchored fixtures
Eye wall anchored fixtures each including at least one elongated anchor member for driven lengthwise insertion into an eye's eye wall in a transverse direction to its thickness for supporting an intraocular device in the eye's vitreous cavity. Fixtures are preferably anchored in circumferential incisions in a human adult eye's pars plana preferably 3.5 mm posterior to its corneal limbus, and perpendicular to a circumferential incision. The fixtures are either generally L-shaped with a single elongated anchor member designed for withdrawal from an eye wall or self-anchoring thereinto, or generally T-shaped with a pair of oppositely directed self-anchoring elongated anchor members for sealing a throughgoing incision. Intraocular devices can be designed for intraocular drug delivery, for acquiring intraocular physiological measurements, and the like.
Owner:FORSIGHT VISION5 INC
Method of preventing proliferation of retinal pigment epithelium by retinoic acid receptor agonists
InactiveUS20020128291A1Prevent proliferationPotent ability AP1-dependantBiocidePowder deliveryDiseaseLiposome
Proliferation of retinal pigment epithelium following surgery or trauma or resulting in ocular diseases associated with choroidal neovascularization, such as age related macular degeneration and histoplasmosis syndrome, is prevented by contacting retinal pigment epithelium cells with a therapeutic amount of a retinoic acid receptor (RAR agonist, preferably one with specific activity for retinoic acid receptors. Preferably the RAR agonist is also a potent antagonist of AP1-dependent gene expression. Alternatively, the proliferation of retinal pigment epithelium is ameliorated with a therapeutic amount of an AP-1 antagonist, alone or in combination with an RAR agonist. The drug can be administered by bolus injection into the vitreous cavity using a dosage from about 50 to 150 .mu.g. Or by slow release from liposomes or an oil tamponade injected into the vitreous cavity. Formulations for preventing proliferation of retinal pigment epithelium are also provided.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
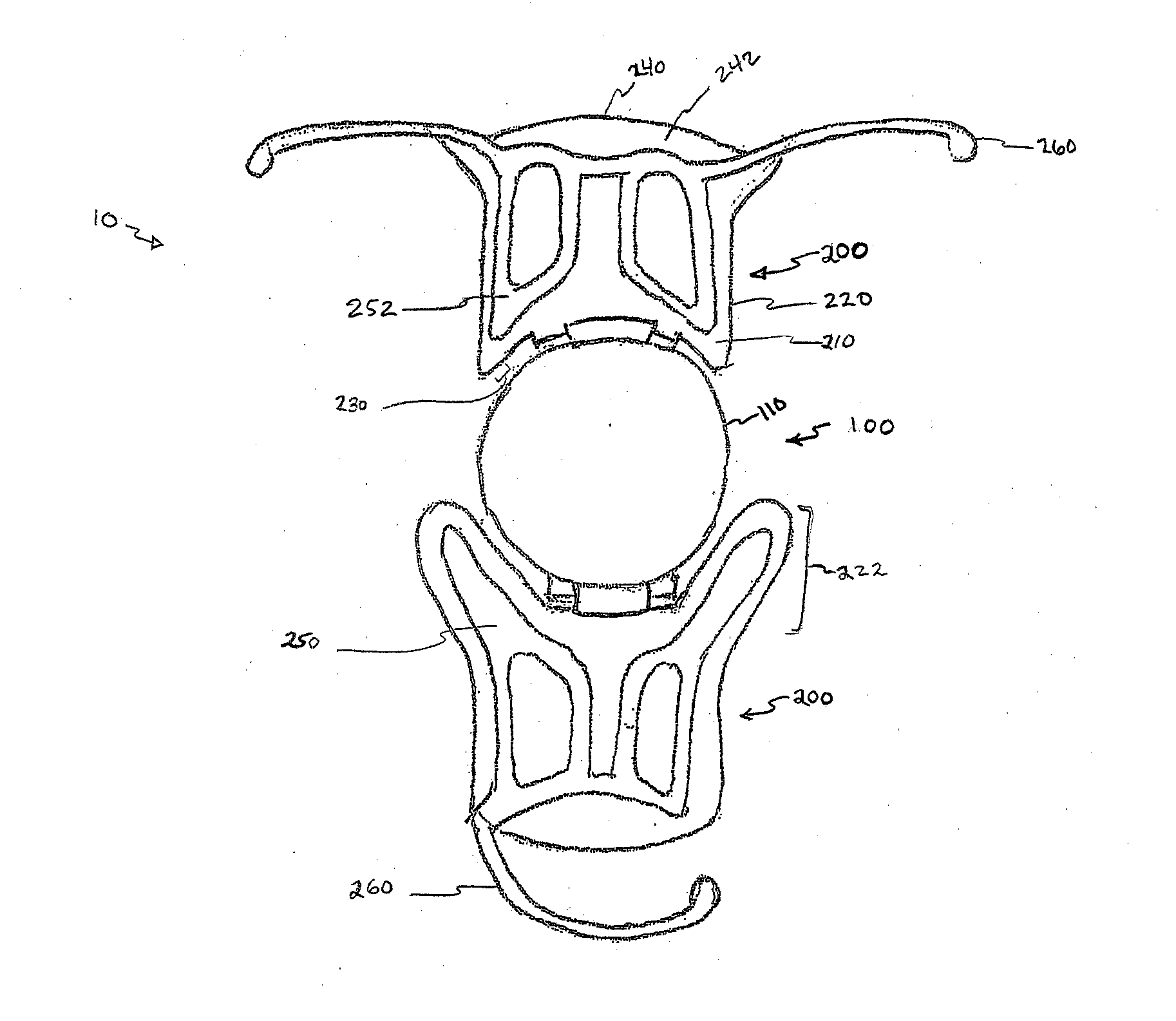
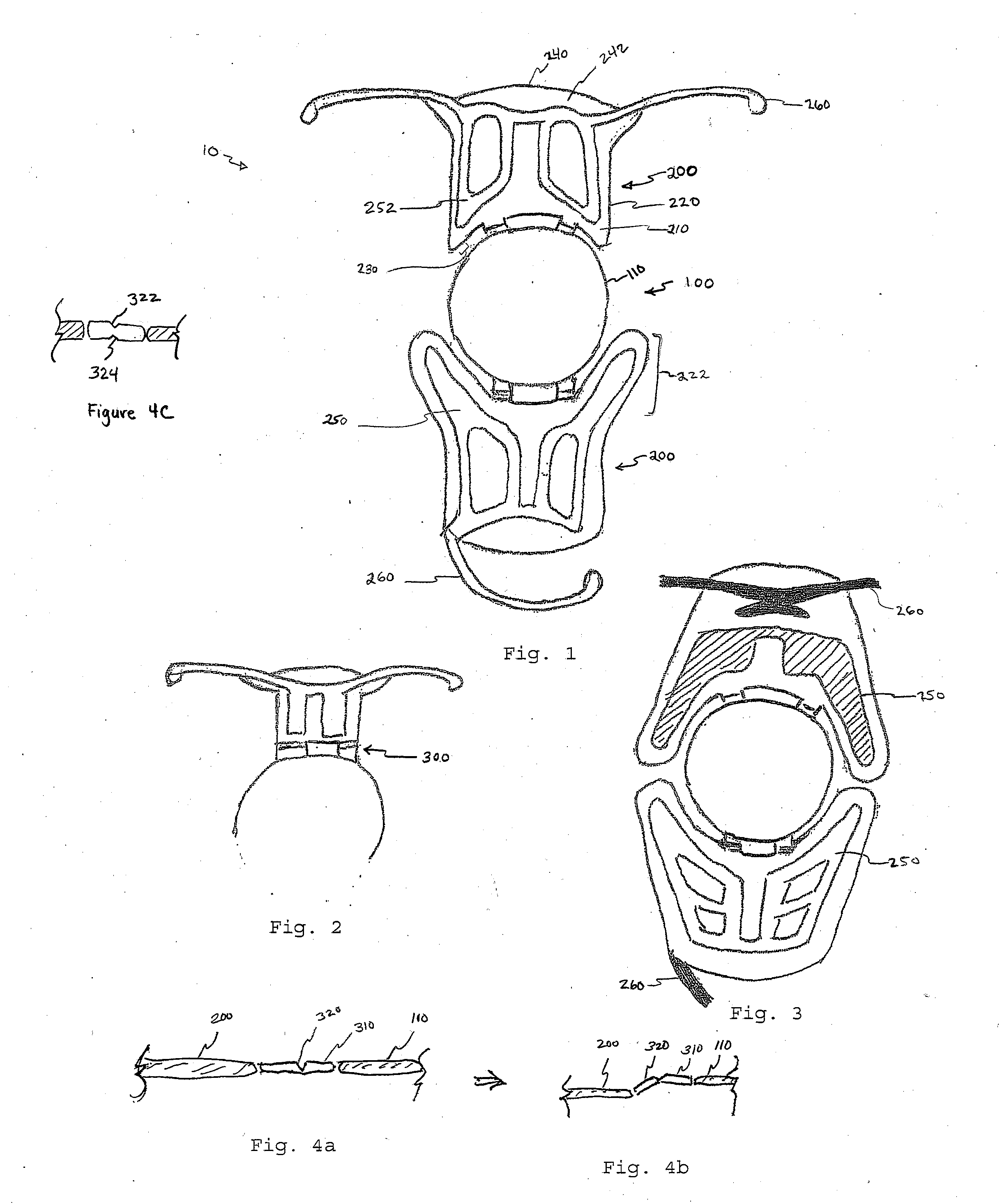
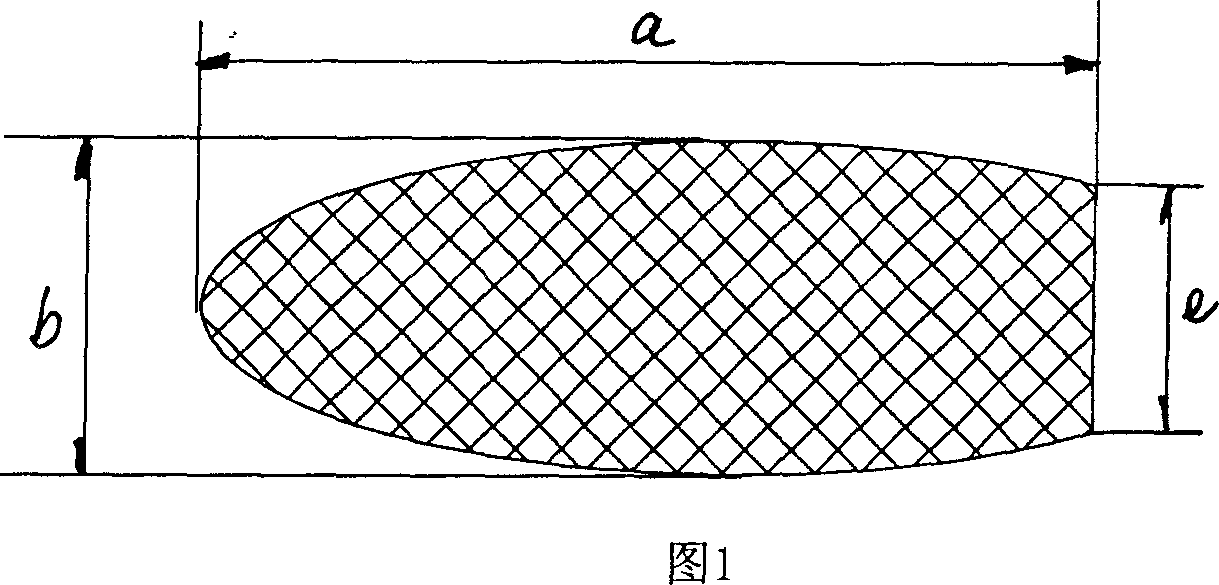
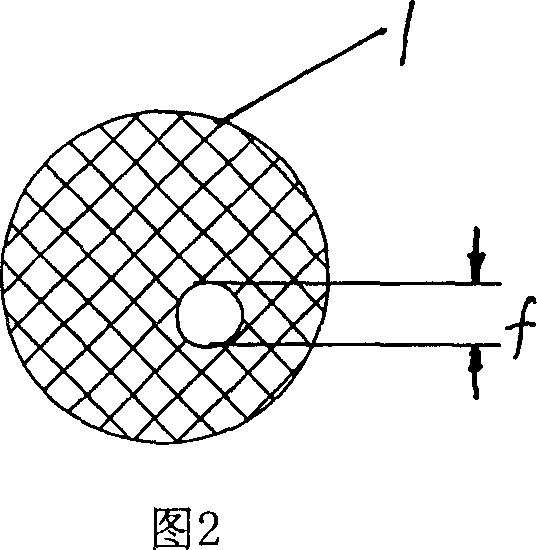
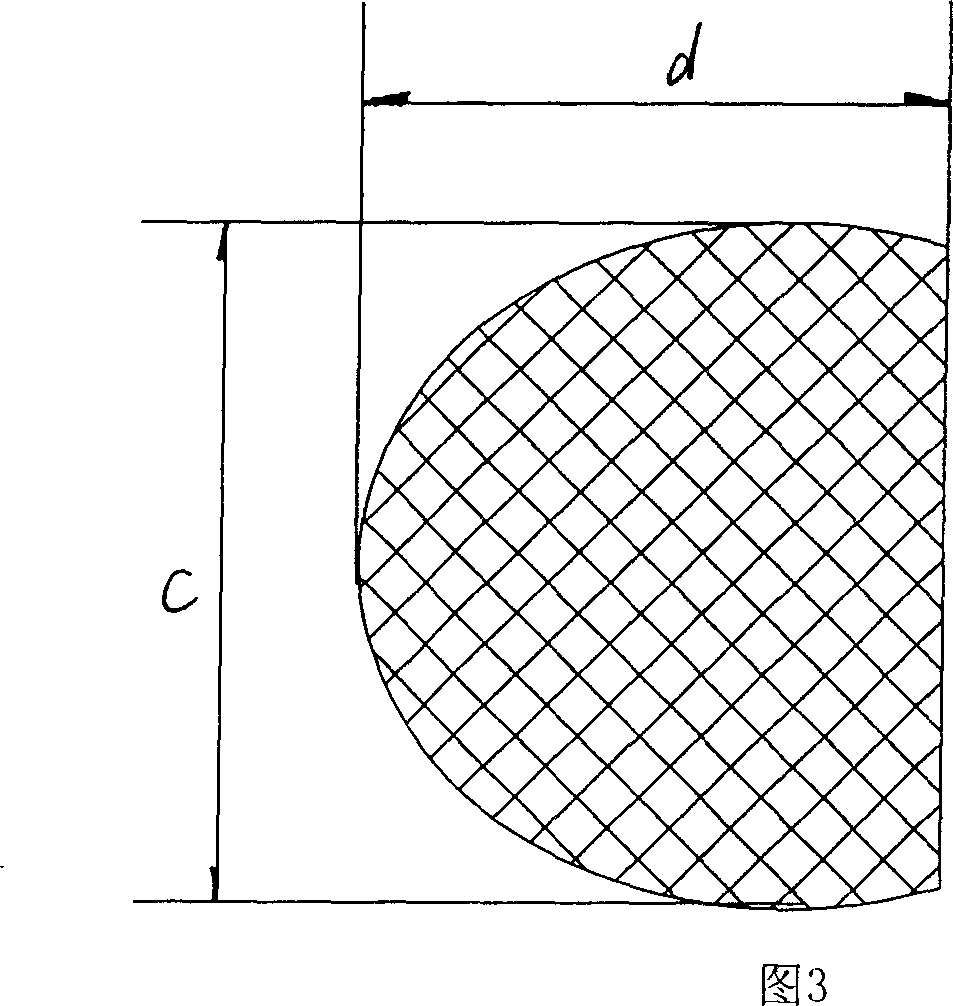
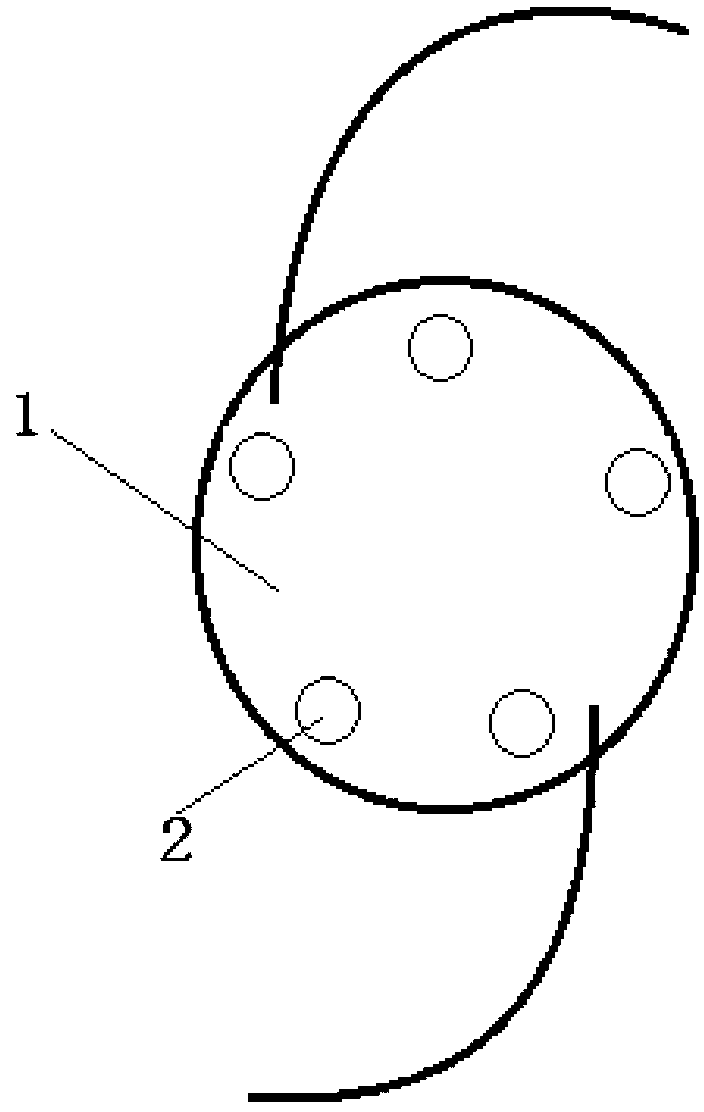
Bracket of vitreous body cavity for curing retina disease, and manufacturing method
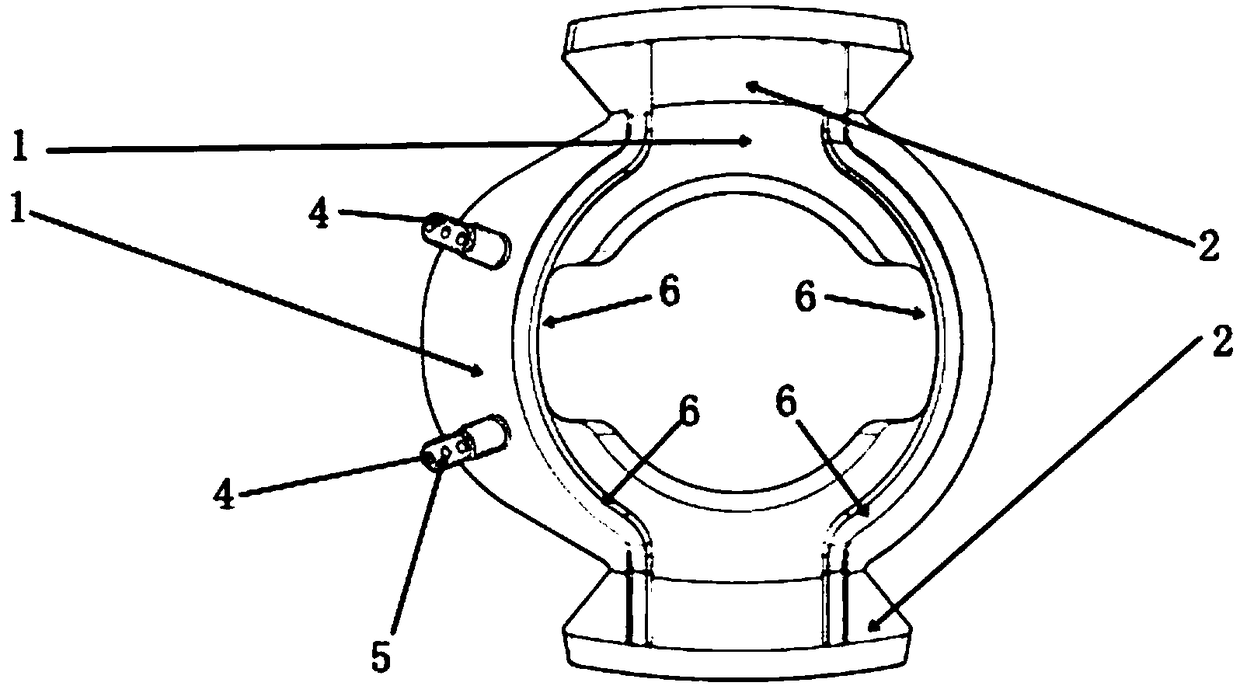
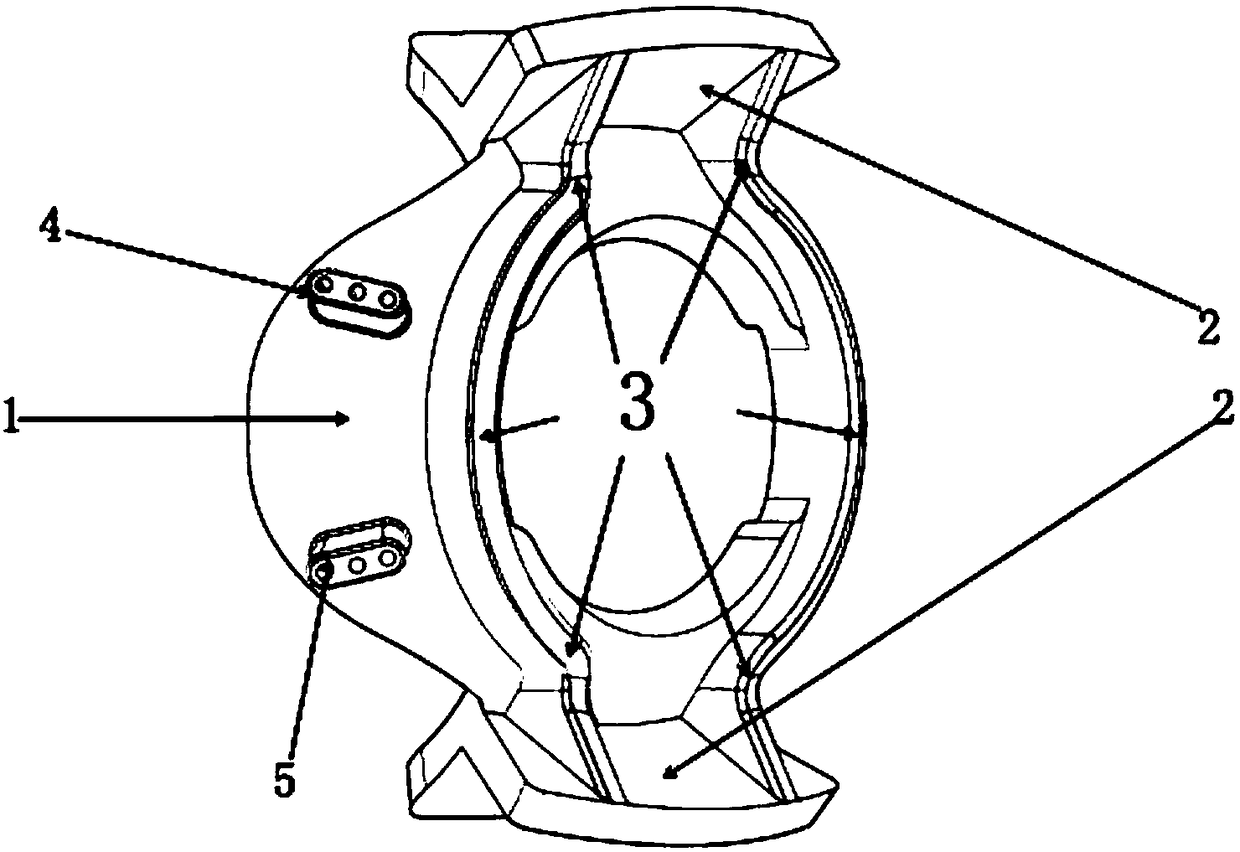
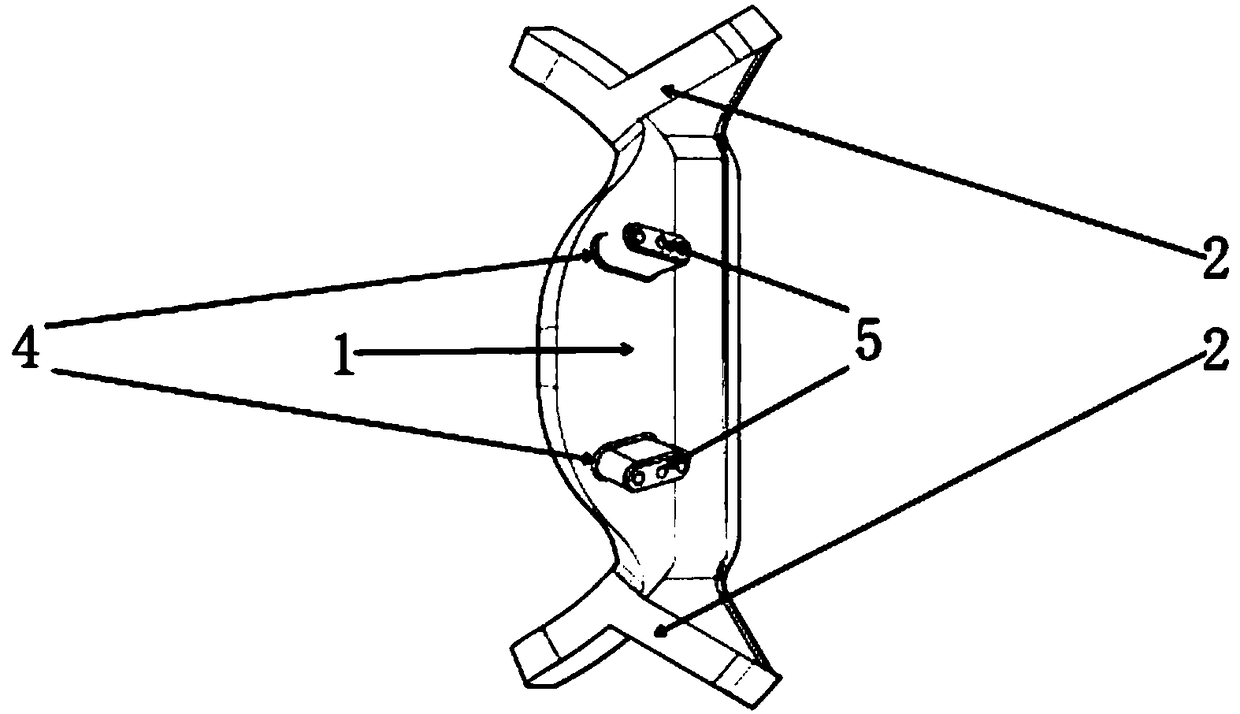
A supporting frame of vitreous body cavity for treating retinopathy is a Rugby football-shaped single-layer netted structure with a front opening (1.0-2.2 mm in diameter) and a rear yellow spot hole (0.5-1.5 mm in diameter). Said netted structure is made of Ni-Ti marmem. Its preparing method is also disclosed.
Owner:ZHENGZHOU UNIV
Lenticular net instruments
A lenticular net instrument comprises an elongate handle coupled with a lenticular net movable to contracted and expanded configurations via an actuator of the handle. The net in the contracted configuration has a narrow profile for insertion in and removal from a lens capsule through a small incision in the eye. The net is movable to the expanded configuration within the lens capsule behind a cataractous nucleus. The net has a plurality of openings therein of a size to prevent fragments of the cataractous nucleus produced by fragmentation with a fragmenting instrument from passing therethrough such that the fragments do not pass into the vitreous cavity. A method of cataract surgery involves deploying a net behind a cataractous nucleus upon the occurrence of a rupture in the capsular wall.
Owner:SABET SINA J
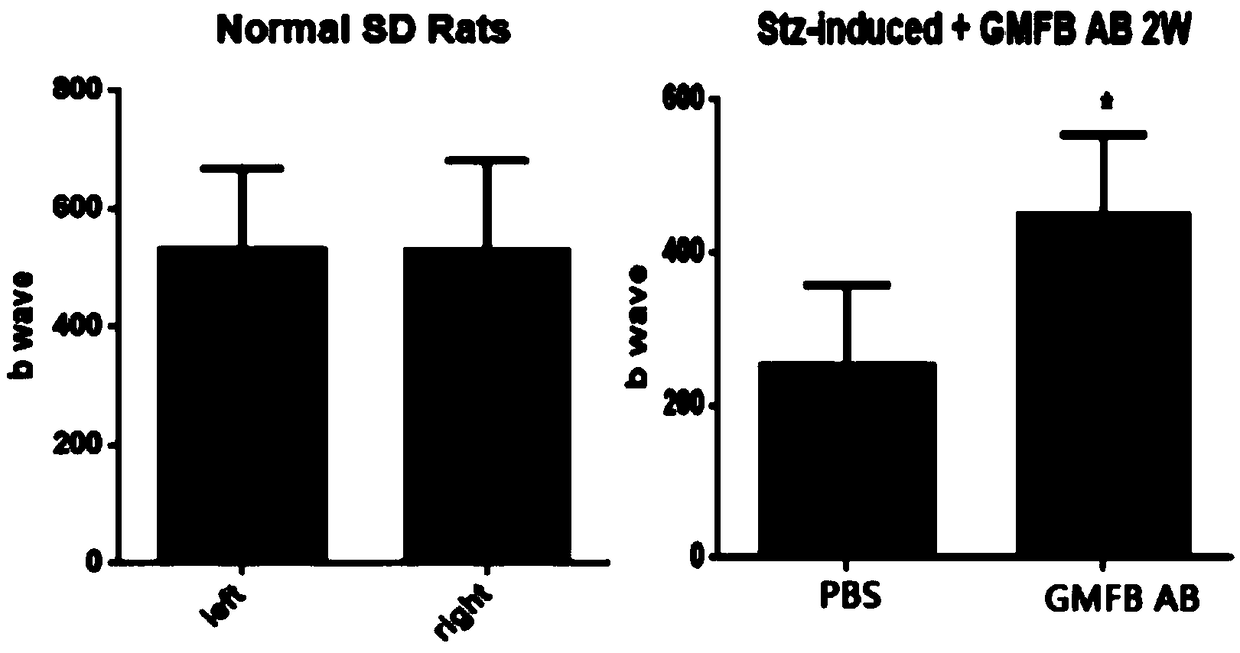
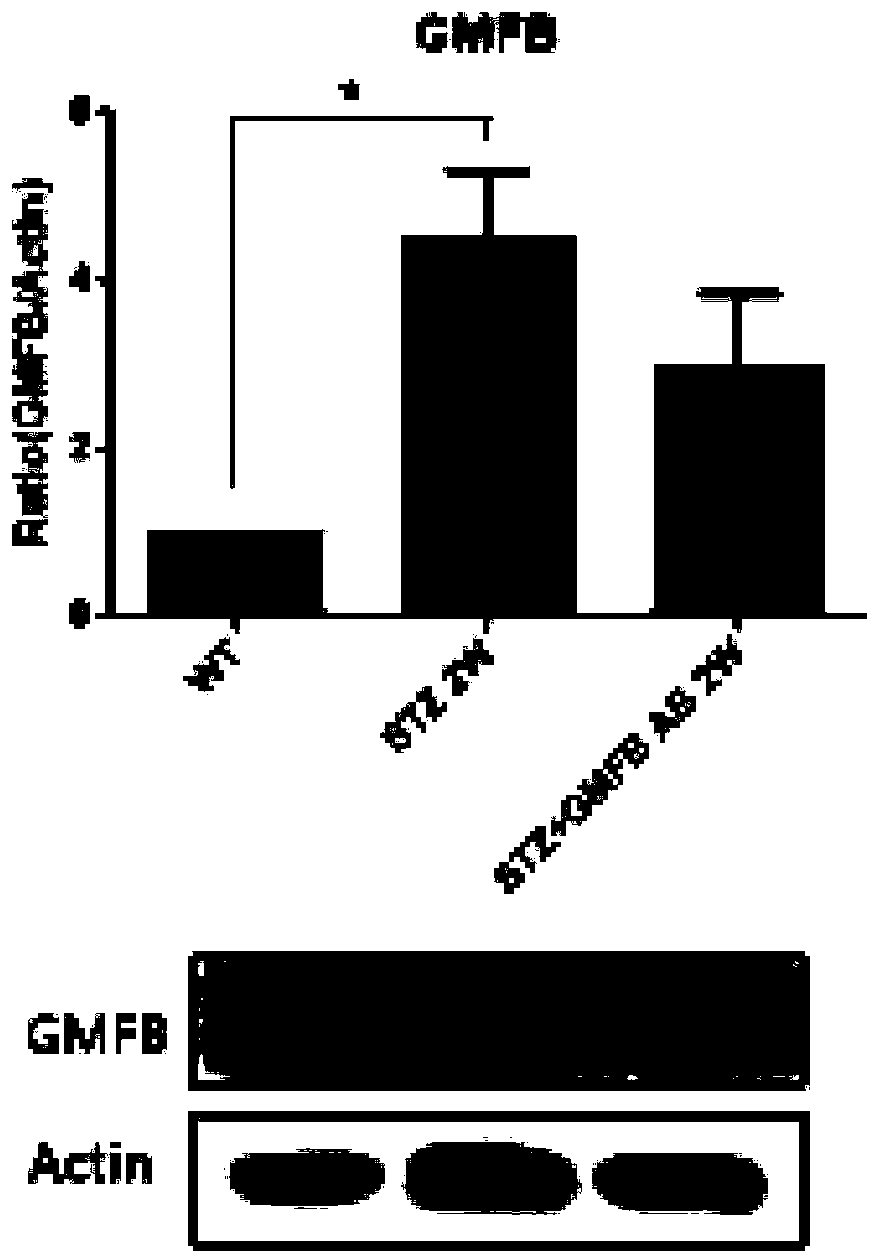
Application of GMFB antibody in preparation of drugs for treating diabetic retinopathy
PendingCN108939066AReduced glial reactionDemonstrating a neuroprotective roleSenses disorderMetabolism disorderDiabetes retinopathyApoptosis
The invention relates to an application of a GMFB antibody, in particular, an application of the GMFB antibody in the preparation of drugs for treating diabetic retinopathy. The GMFB antibody is injected into the right eye of an STZ induced DM rat by a vitreous cavity injection technology, then PBS with an equal amount is injected into the left eye, after two weeks, the difference of visual functions of two eyes of the rat is detected by an ERG method, and the result shows that the visual function of the eyeball that is injected with the GMFB antibody is obviously improved, compared with thatof the eyeball that is not injected with the GMFB antibody. Then people find that the GFAP expression of the retina of the eyeball with the GMFB antibody is prominently reduced, TUNEL dyeing for detecting cell apoptosis is also weakened, and the result shows that the GMFB antibody injected into the vitreous cavity can be used as a target of DR early intervention to protect the visual function.
Owner:TONGJI UNIV
Preparation method for injectable hydrogel for controlled release of voriconazole intraocular drug
ActiveCN108434093ARelieve painReduce economyOrganic active ingredientsSenses disorderHalf-lifeBiocompatibility Testing
The invention discloses a preparation method for injectable hydrogel for controlled release of a voriconazole intraocular drug, and relates to the preparation method for injectable hydrogel. The preparation method aims to solve the problems that an existing voriconazole preparation is fast in metabolism and needs to be repeatedly supplied, the body pain and economic burden of a patient are increased, and an existing voriconazole sustained release system is poor in biocompatibility and poor in long-acting controlled-release effect. The method comprises the following steps of 1, preparing whiteflocculent polyaldehyde dextran; 2, preparing water-soluble linear poly-beta-cyclodextrin; and 3, firstly preparing a PBS solution of the water-soluble linear poly-beta-cyclodextrin with voriconazoleloaded in a cyclodextrin molecular cage, and then dissolving carboxymethyl chitosan into the PBS solution so as to obtain the injectable hydrogel for controlled release of the voriconazole intraoculardrug. According to the preparation method, the half-life of the prepared injectable hydrogel for controlled release of the voriconazole intraocular drug in a vitreous cavity is 30-60 days; and the injectable hydrogel for controlled release of the voriconazole intraocular drug can be obtained.
Owner:QINGDAO UNIV
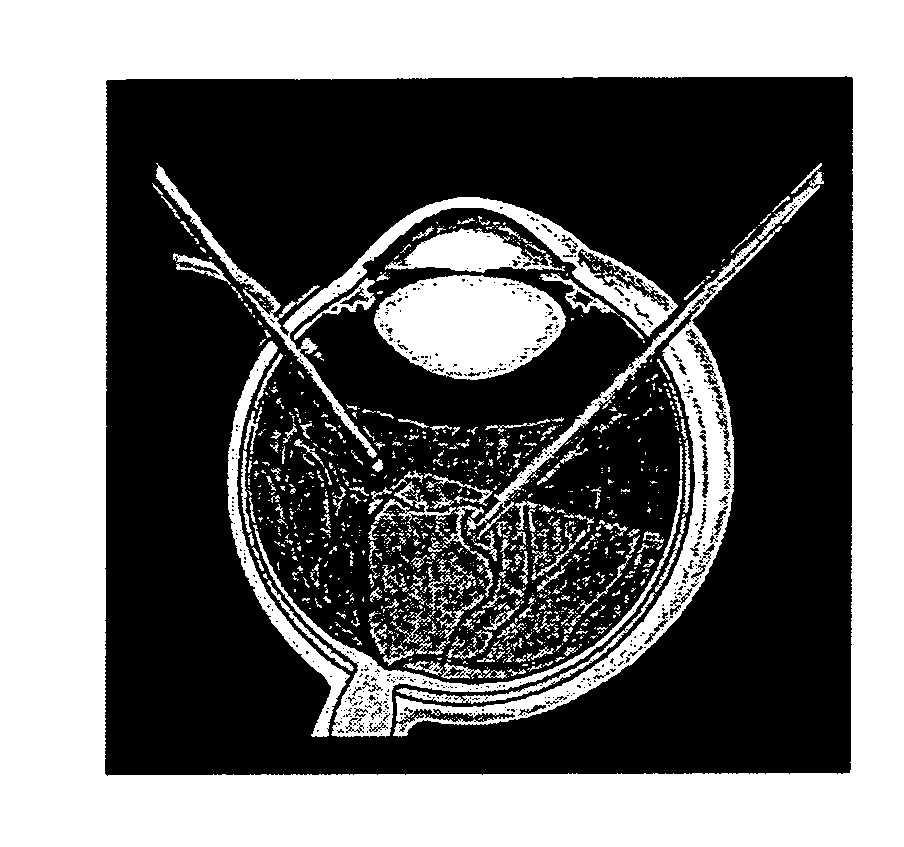
Surgical techniques for implantation of a retinal implant
A method is provided for implanting, in an eye, apparatus having an electrode array including stimulating electrodes shaped to define distal tips protruding from the array, a plurality of photosensors, and driving circuitry, configured to drive the electrodes to apply currents to the retina. The method includes removing a lens of the eye and a vitreous body of the eye, creating a corneoscleral incision and inserting the apparatus through the corneoscleral incision into a vitreous cavity of the eye. During the inserting, an orientation of the distal tips is maintained pointing towards a cornea of the eye. Subsequently to the inserting, the apparatus is rotated such that the distal tips point towards a macula of the eye. Subsequently to the rotating, the apparatus is positioned epi-retinally, such that the distal tips of the electrodes penetrate the retina. Other applications are also described.
Owner:NANO RETINA LTD
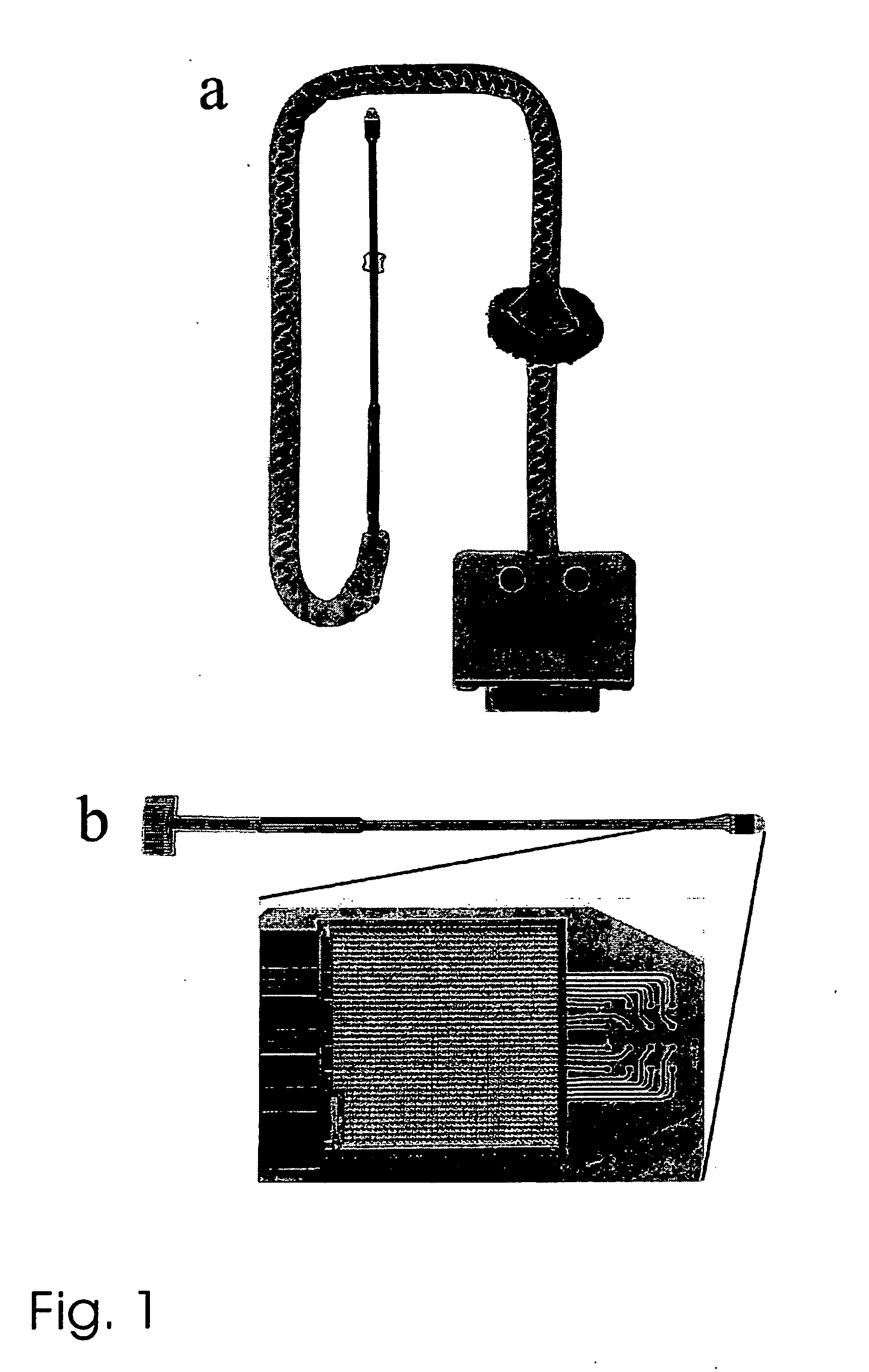
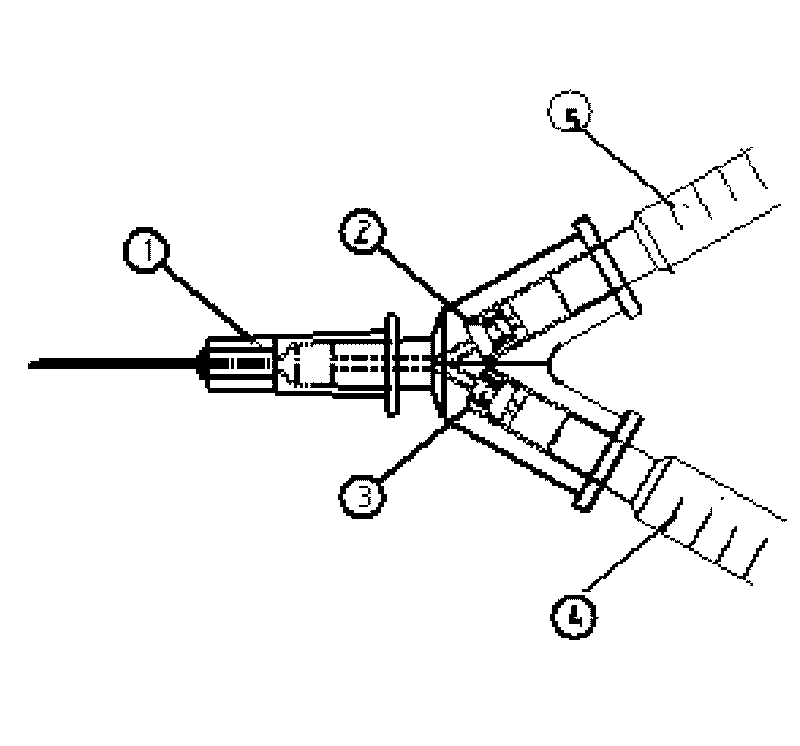
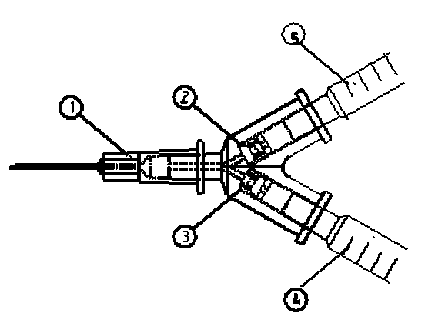
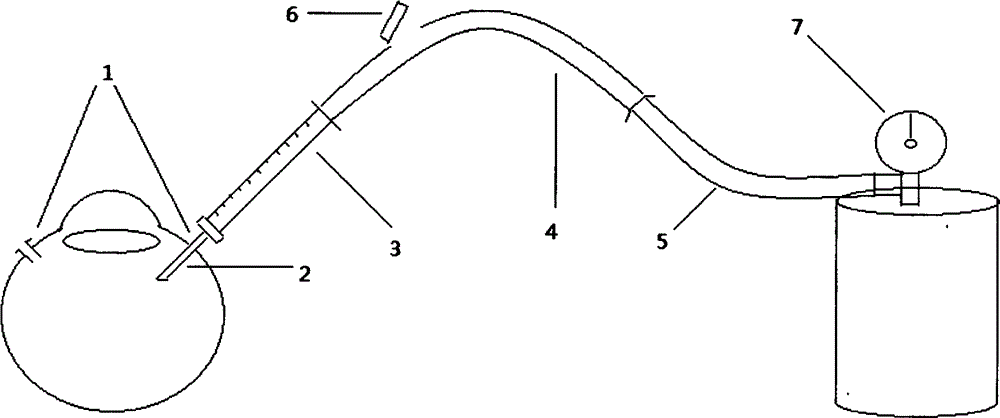
Medical syringe with vitreous cavity
The invention relates to a medical syringe with a vitreous cavity, which is provided with a three-way tube, two built-in one-way valves and two 1 ml syringes. The single tube end of the three-way tube is connected with a 30G syringe needle; the dual tube ends are respectively connected with two 1 ml syringes through the built-in one-way valves; and the control flow directions of the two one-way valves are respectively a direction opposite to the three-way tube and the direction entering the three-way tube. Therefore, a medicinal liquid can be injected directly through the other syringe without withdrawal of a liquid drawing needle tube. The medical syringe with the vitreous cavity has the characteristics of simple structure, convenient operation, low cost, good safety performance and capability of avoiding cross-infection effectively.
Owner:耿燕
Drug-carrying type artificial lens capable of being opened by laser
InactiveCN103169548ASmall regulationEasily exposedMedical devicesIntraocular lensDiseaseMultiple injection
The invention discloses a drug-carrying type artificial lens capable of being opened by a laser. The drug-carrying type artificial lens capable of being opened by the laser comprises an artificial lens main body, a plurality of drug storing holes with preset intervals are formed in the peripheral surface of the artificial lens main body, and micro-capsules loading active drug and capable of being punched through or resolved by the laser are embedded in the drug-storing holes. A plurality of drug micro-capsules are embedded into the peripheral portion of the artificial lens, the lens is opened to release by the laser on a suitable opportunity to achieve a treatment goal, and relative to a drug sustained releasing system, side effects brought by long-term sustained drug releasing is avoided. Besides the treatment goal of post-operation inflammatory resistance and posterior capsule opacification resistance, the drug-carrying type artificial lens capable of being opened by the laser can be embedded to achieve the purpose of controlling eye diseases aiming at certain diseases of age related macular degeneration, diabetic retinopathy and the like together with the complication of a cataract, and the complications caused by multiple injections of a vitreous cavity and the side effects of systemic administration are avoided or reduced.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Surgical techniques for implantation of a retinal implant
A method is provided for implanting, in an eye, apparatus having an electrode array including stimulating electrodes shaped to define distal tips protruding from the array, a plurality of photosensors, and driving circuitry, configured to drive the electrodes to apply currents to the retina. The method includes removing a lens of the eye and a vitreous body of the eye, creating a corneoscleral incision and inserting the apparatus through the corneoscleral incision into a vitreous cavity of the eye. During the inserting, an orientation of the distal tips is maintained pointing towards a cornea of the eye. Subsequently to the inserting, the apparatus is rotated such that the distal tips point towards a macula of the eye. Subsequently to the rotating, the apparatus is positioned epi-retinally, such that the distal tips of the electrodes penetrate the retina. Other applications are also described.
Owner:NANO RETINA LTD
Intra-ocular release system of voriconazole
InactiveCN101390825BReduce dosageReduce the number of dosesOrganic active ingredientsSenses disorderLactideVitreous base
The invention relates to a long-acting voriconazole intraocular medicine release system which contains voriconazole and is characterized in that the intraocular medicine release system also contains biodegradable medicinal high-molecular auxiliary material; voriconazole and biodegradable medicinal high-molecular auxiliary material are mixed to form homogenized mixture; the weight ratio of biodegradable high-molecular auxiliary material and voriconazole is 0.1:0.9-0.9-0.1; the molecular weight of the biodegradable high-molecular auxiliary material is 5000-1000000Dalton. The biodegradable high-molecular auxiliary material is selected from poly(lactide-co-glycolide) and polylactic acid. The release period of the voriconazole intraocular medicine release system is a week to a year, has less medicine dosage, less times of administration, reduces the adverse reaction caused by the repeated injection of vitreous cavity and greatly improve the compliance of patients; if the vitreous body baseis implanted simultaneously during the excision operation of the central vitreous body, the complications of intraocular hemorrhage and retinal detachment caused by the repeated vitreous body puncture medicine-injection.
Owner:SHANDONG EYE INST
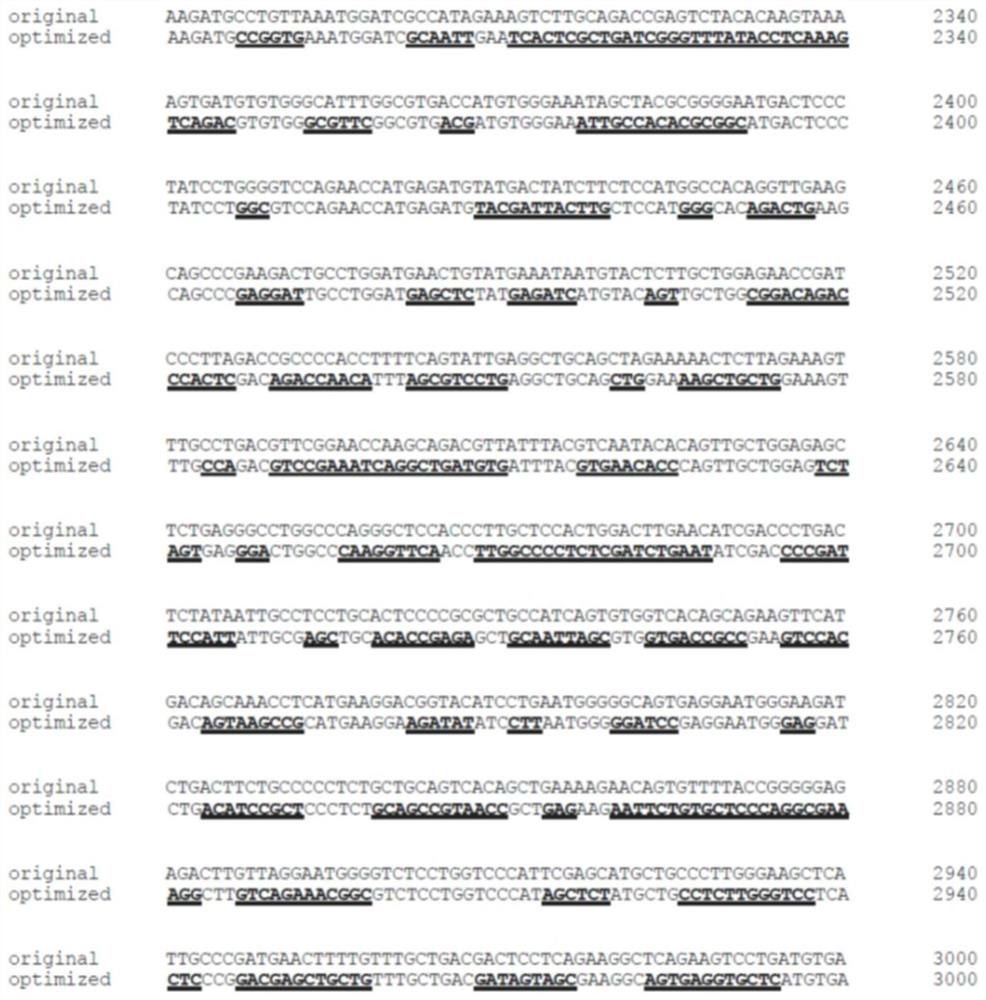
Nucleotide sequence for coding human receptor tyrosine kinase Mer and application of nucleotide sequence
ActiveCN111826378AImprove expression efficiencyPrevent or treat degenerationSenses disorderTransferasesNucleotideTyrosine
The invention relates to the technical field of biomedical gene treatment, and discloses a nucleotide sequence for coding human receptor tyrosine kinase Mer and application thereof. The nucleotide sequence disclosed by the invention and the nucleotide sequence shown in SEQ ID NO: 3 have more than or equal to 95% identity. The application proves that by using an AAV-MERTK drug for treatment, the retinal pathology symptoms of rats with retinitis pigmentosa caused by MERTK mutation can be remarkably improved, and retinal function recovery can be remarkably improved. The AAV-MERTK drug is injectedinto a vitreous cavity, MERTK can be efficiently expressed in a retinal pigment epithelium layer, and the thickness of a retinal outer nuclear layer is increased. Meanwhile, a retinal potential diagram shows that compared with a control group, the rats in an AAV-MERTK drug treatment group have strong response to stimulation. Therefore, the AAV-MERTK drug has the effect of preventing or treating the retinitis pigmentosa.
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
Intraocular nano particle freeze-dried powders and preparation method thereof
InactiveCN101711745AImprove permeabilityIncreased release ratePowder deliveryOrganic active ingredientsGlucocorticoidMethylene Dichloride
The invention relates to intraocular nano particle freeze-dried powders, which comprises polylactic acid (PLA) or polylactic-co-glycolic acid (PLGA) and glucocorticoids. The powders are prepared by the following steps: firstly, dissolving the glucocorticoids in acetone to serve as an inner water phase; dissolving the biodegradable polymer PAL or PLGA in methylene dichloride to serve as an oil phase; under the ice-bath condition, mixing the two mixtures, and stirring at high speed to form a water-in-oil type primary emulsion; secondly, under the ice-bath condition, dropwise adding polyvinyl alcohol (PVA) serving as an outer water phase in the primary emulsion to form a water-in-oil-in-water type multiple emulsion through the action of ultrasonication; and finally, stirring the water-in-oil-in-water type multiple emulsion in a stink cupboard and volatilizing an organic phase under reduced pressure, collecting and washing the sediments after centrifuging the product, removing the dissociative glucocorticoids and PVA by washing with distilled water, and then preparing and storing the freeze-dried powders. The intraocular nano particle freeze-dried powders have the characteristics of having large medicament-loading rate and uniform size distribution and capacities of slowly releasing the glucocorticoids, reducing times of repeated medicament administration for the vitreous cavity and reducing the generation of ocular complications.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Methods and devices for treating a retinal detachment
ActiveUS20180185288A1Reduce probabilityDiffusion slowPowder deliverySenses disorderOphthalmologySubretinal fluid
A method of treating a detachment of a retina of an eye, the method including the steps of injecting a substance into a vitreous cavity of the eye, the substance slowing flow of fluid through the vitreous cavity into a subretinal space to reduce a rate of accumulation of a subretinal fluid in the subretinal space to below a rate of removal of the subretinal fluid from the subretinal space by retinal pigment epithelium; and permitting the retinal pigment epithelium to reattach the retina. The invention also includes devices for practicing the method.
Owner:VITREAN INC
Auxiliary device for vitreous cavity injection
The invention discloses an auxiliary device for vitreous cavity injection, which is composed of a fixing ring, an eyelid support and a support beam connecting the eyelid and the eyelid. The drug can be injected into the vitreous cavity safely and effectively according to the size of the eyeball, the position and depth of the drug administration are relatively fixed, the use is convenient, the operation is simple, the operation risk is reduced, the operation efficiency is improved, and the requirement of ergonomics is met.
Owner:LONGHUA HOSPITAL SHANGHAI UNIV OF TRADITIONAL CHINESE MEDICINE
Method for fetching silicone oil from eyes in minimally invasive, quantitative and controllable manner
The invention discloses a minimally invasive quantitative controllable design for fetching silicone oil from eyes, provides a method for fetching silicone oil from eyes in a minimally invasive, quantitative and controllable manner, and relates to improvement of methods for medical operation. The method has the advantages that the method mainly aims to accurately control negative pressures in real time when the silicone oil is fetched on the premise that special equipment is omitted, the negative pressures can be quickly released when liquid of vitreous cavities is mistakenly sucked, and accordingly severe problems of mistaken suction of ocular tissues, collapse of eyeballs and the like can be solved; double negative pressure release links of a manual valve are regulated and controlled by a conventional negative-pressure sucker and the manual valve of a negative-pressure tube when continuous quantitative negative-pressure suction assistance is required, so that the method mainly can be implemented, and tissue injury can be reduced by the aid of 23G minimally invasive incision; the 23G puncture incision is adopted in the method, the head of a 1 ml syringe is connected with a 22G intravenous indwelling canula to enter the eyes to suck the silicone oil, a disposable sputum sucking tube is connected with the tail of the syringe, the tail of the sputum sucking tube is further connected with a disposable suction connecting tube and then is connected with the conventional wall type or vertical negative-pressure sucker, negative-pressure values are set by the negative-pressure sucker, and operators can flexibly adjust the negative pressures by means of mastering an opening degree of the manual valve; all service materials are common articles for operation rooms, and accordingly the method is convenient to popularize.
Owner:李毓敏 +2
Antifungal activity ophthalmopathy slow-release microparticle and use thereof
InactiveCN101390836ARemove malabsorptionImprove compliancePowder deliveryOrganic active ingredientsWhole bodyLactide
The invention relates to an anti-fungal ophthalmopathy sustained-release milli-microcapsule and the application thereof. The anti-fungal ophthalmopathy sustained-release milli-microcapsule contains voriconazole and is characterized in that the sustained-release milli-microcapsule also contains biodegradable pharmaceutical high-molecular auxiliary material; the voriconazolen and the biodegradable pharmaceutical high-molecular auxiliary material construct homogenized milli-microcapsule with the diameter of 100nm-100um; wherein, the voriconazolen accounts for 2%-50% of the total weight of the milli-microcapsule; the molecular weight of biodegradable pharmaceutical high-molecular auxiliary material is 5000-1000000Dolton. The pharmaceutical high-molecular auxiliary material is selected from poly(lactide-co-glycolide), polylactic acid, polylactic acid-glycolic acid. The anti-fungal ophthalmopathy sustained-release milli-microcapsule is widely applicable to the direct medicine delivery treatment in vitreous cavity or the ocular fungal infection prevention. The anti-fungal ophthalmopathy sustained-release milli-microcapsule treats fungal endophthalmitis through direct sustained-release vitreous cavity medicine delivery, needs less medicine dosage and effectively maintains the medicine concentration for 3 months after once medicine delivery so that the medicine economy is greatly improved. The medicine dosage applied on the ocular part is less when common voriconazolen injection is delivered systematically; patients cannot endure if the direct vitreous cavity injection needs to be conducted 3 times a day.
Owner:SHANDONG EYE INST
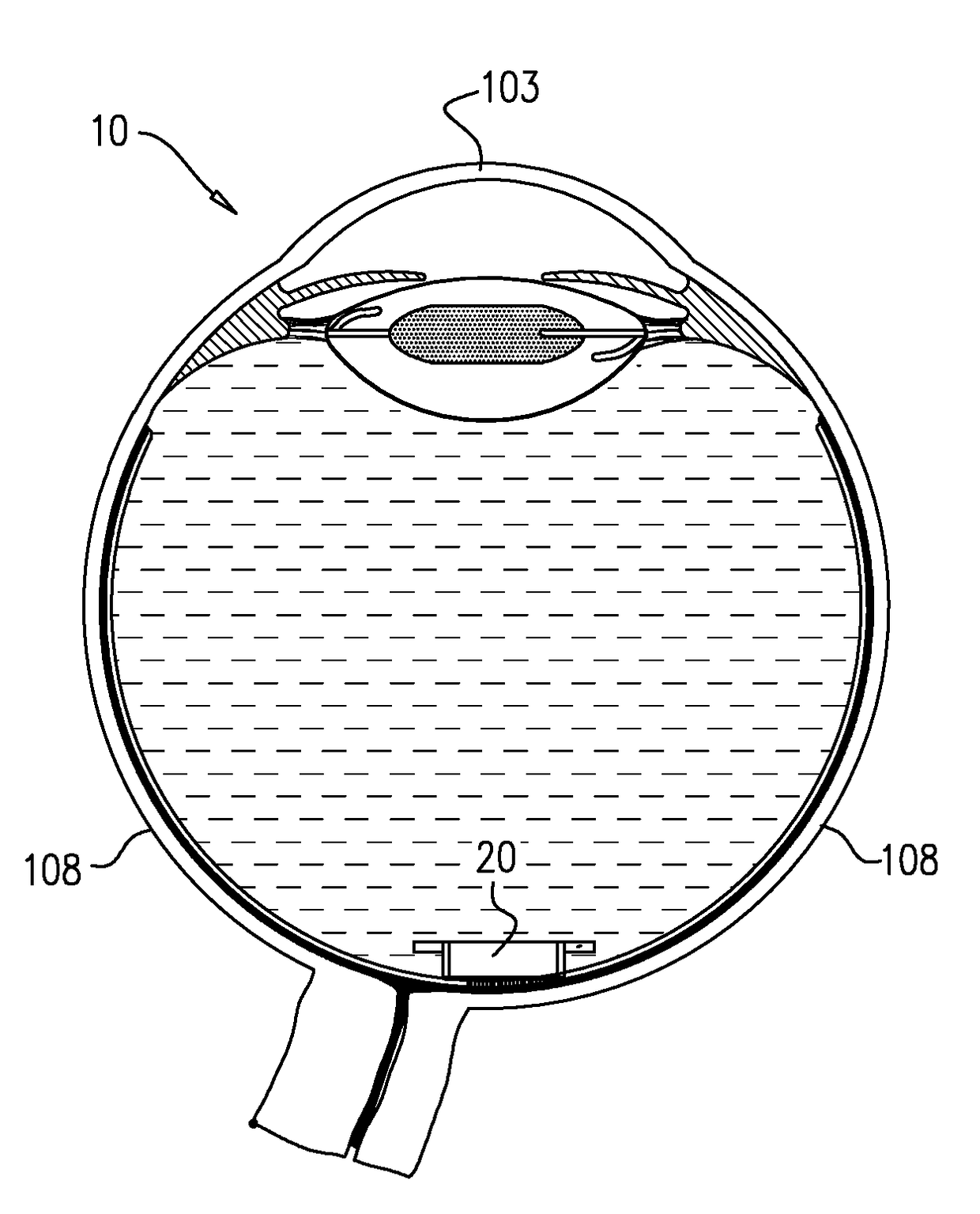
Intraocular drug dispenser
InactiveCN101389295ASenses disorderPharmaceutical delivery mechanismControlled releaseMedication Dispenser
Intraocular drug dispenser includes an elongated support member with a pair of opposite ends for anchoring to opposite surfaces of an eye's eye wall, and a string of discrete drug containing capsules for individual time controlled release of their drug contents into an eye's vitreous cavity.
Owner:NULENS
Recombinant adeno-associated virus-nadh dehydrogenase subunit 4 gene full-length and agent for the treatment of leber hereditary optic neuropathy
ActiveCN104450747BEfficient transfectionHigh expressionSenses disorderNervous disorderNucleotideMitophagy
The invention discloses a recombinant adeno-associated virus-NADH dehydrogenase subunit 4 gene full length and a medicament for treating Leber hereditary optic neuropathy. The full length of the gene is shown in the nucleotide sequence of SEQ ID NO: 1. Wherein, the nucleotide sequence has a full length of 3824bp, which consists of a CAG promoter sequence, a coding sequence of ND4 with a Cox10 mitochondrial localization sequence and a UTR with a length of 625bp. The medicament is injected into the vitreous cavity of the eye for the treatment of Leber's hereditary optic neuropathy. The medicament of the present invention is injected into the vitreous cavity, can maintain vitality in the vitreous cavity, and can be efficiently transfected into optic nerve cells. The signal peptide at the front end of the protein N, Orientation guides the protein into the mitochondria, and the mature ND4 protein enters the mitochondria to play a role. Therefore, drugs are effective in the treatment of Leber hereditary optic neuropathy.
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
Application of met-RANTES in preparing medicine used for treating inherited retinal degeneration
ActiveCN103357017APracticalTechnically difficultSenses disorderPeptide/protein ingredientsDiseaseReceptor activation
The invention relates to an application of a beta chemokine receptor inhibitor or a composition comprising the beta chemokine receptor inhibitor in preparing a medicine used for treating inherited retinal diseases or for protecting inherited retinal degeneration photoreceptor cells. The beta chemokine receptor inhibitor blocks the combination of beta chemokine and the receptor thereof, and further blocks the signaling pathways after receptor activation and the caused oxidative damage reaction, so as to treat the inherited retinal diseases or to protect the inherited retinal degeneration photoreceptor cells. Preferably, the beta chemokine receptor inhibitor is administrated through vitreous cavity injection. Preferably, the beta chemokine receptor inhibitor is selected from met-RANTES. Preferably, the disease is inherited retinal degeneration. The intervention of the beta chemokine receptor inhibitor against inherited retinal degeneration diseases is substantially made earlier. Therefore, the application is an ideal means for treating early-stage RP. According to the application, a specific gene defect type is not considered. The beta chemokine receptor inhibitor provided by the invention has the advantages such as better practicality, wider application, and the like.
Owner:北京同仁医学科技有限责任公司
Foscarnet sodium eye vitreous intracavity controlled-release medicine
ActiveCN104906587AAvoid harmActing as sustained release drugOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolFoscarnet
The invention discloses a foscarnet sodium eye vitreous intracavity controlled-release medicine. The controlled-release medicine is characterized in that the controlled-release medicine comprises an ingredient A and an ingredient B; the ingredient A is a uniform an mixed solution formed by foscarnet sodium and a biodegradable medicinal polyethylene glycol auxiliary material, and the ingredient B is a peptide cross-linking agent solution. The ingredient A and the ingredient B can form a gel-like implant rapidly after the ingredient A and the ingredient B are injected into an eye vitreous cavity, rapid release of foscarnet sodium is retarded, therefore the medicine controlled-release effect is achieved and dosage interval time is prolonged. The reaction can be achieved under physiological conditions, namely, the reaction can be carried out in a normal saline or a buffer with a pH value of 7.4, special organic solvents or a strong acidic condition needed by a traditional implant or gelata are not needed, safety is high, and damage to human tissues is avoided effectively. Through control of the concentration of 4arm-PEG-MAL in the solution and the concentration of triethylanmine in the solution, reaction time can be controlled effectively, and the controlled-release medicine can be used for injection of an extremely fine 33G needle.
Owner:QINGDAO UNIV
Retinal progenitor cell and preparation thereof having function of treating degenerative retinal diseases
ActiveCN106318909AProliferation effect is goodImprove featuresSenses disorderNervous system cellsPotential changeBiology
The invention relates to the technical field of stem cells, in particular to a retinal progenitor cell and a preparation thereof having a function of treating degenerative retinal diseases. The preservation number of the retinal progenitor cell provided by the invention is CGMCC NO.10419; and the retinal progenitor cell, which can keep a good proliferation ability and stem cell properties, can take a good curative effect on treating the degenerative retinal diseases. Experiments show that the retinal progenitor cell that the preservation number is CGMCC NO.10419, as being injected into the vitreous cavities of RCS rats, can take a protective effect and achieve multi-focal visual electric-physiology display on retinal outer nuclear layer cells; and in dark adaptation, potential change conducted by retinal nerve photoreceptor cells in a retinal progenitor cell injection group is more obvious than that in a group which is just injected with an HBSS (Hanks balanced salt solution) buffer solution. A water maze test also indicates that the visual function of the rats in the retinal progenitor cell injection group is improved. In addition, all rats injected with the retinal progenitor cell that the preservation number is CGMCC NO.10419 are free from abnormity.
Owner:HE EYE HOSPITAL SHENYANG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com