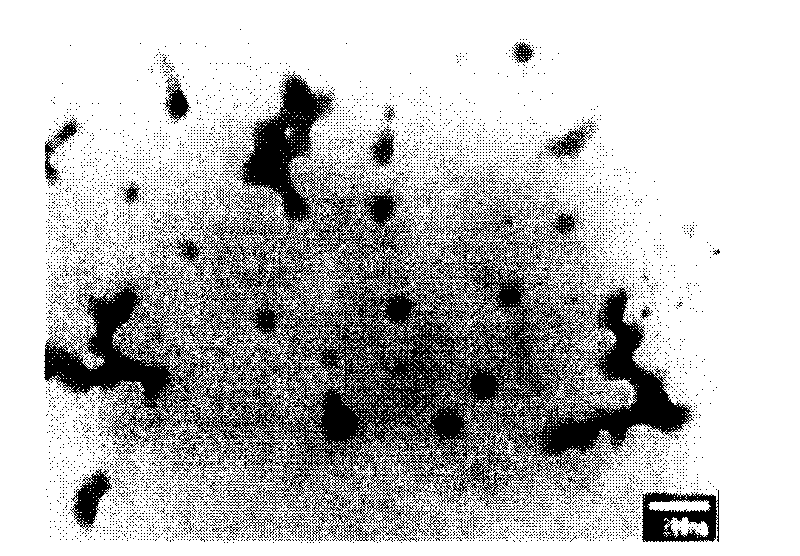
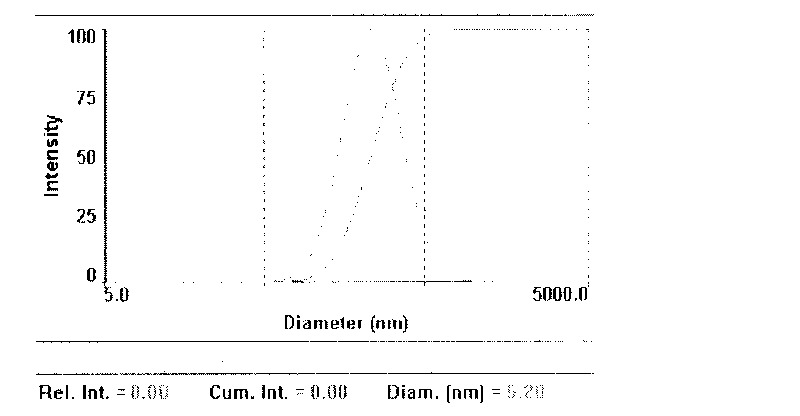
Intraocular nano particle freeze-dried powders and preparation method thereof
A freeze-dried powder and nanoparticle technology, applied in the field of medicine, can solve the problems of short duration of drug effect, increase the incidence of complications such as intraocular hemorrhage, cataract and retinal detachment, etc., and achieve the effects of prolonging the action time, small volume, and prolonging the release rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A freeze-dried powder of nanoparticles for intraocular use, which comprises the following components:
[0021] The mass percent of polylactic acid polyglycolic acid copolymer PLGA is 80.8%;
[0022] The mass percent of dexamethasone acetate DA is 19.2%.
[0023] A preparation method for intraocular nanoparticle freeze-dried powder, comprising the steps of:
[0024] First, dissolve 50 mg of the glucocorticoid dexamethasone acetate DA in 1 ml of acetone as the inner water phase; dissolve 200 mg of poly(lactic-co-polyglycolic acid) PLGA in 9 ml of dichloromethane, and the concentration of PLGA is 2.0%, as the oil phase; the two are mixed under ice-bath conditions, while stirring at a high speed, the stirring speed is 30000 rpm for emulsification for 60 seconds to form water-in-oil type colostrum, internal water phase: oil phase=1: 9;
[0025] Next, under ice-bath conditions, the above-mentioned water-in-oil colostrum was added dropwise to 2.5% (w / v) polyvinyl alcohol PV...
Embodiment 2
[0037] A freeze-dried powder of nanoparticles for intraocular use, which comprises the following components:
[0038] The mass percent of polylactic acid polyglycolic acid copolymer PLGA is 54.7%;
[0039]The mass percent of dexamethasone acetate DA is 45.3%.
[0040] A freeze-dried powder of nanoparticles for intraocular use and a preparation method thereof, comprising the steps of:
[0041] First, dissolve 200 mg of the glucocorticoid dexamethasone acetate DA in 3 ml of acetone as the inner aqueous phase; dissolve 200 mg of polylactic acid polyglycolic acid copolymer PLGA in 9 ml of dichloromethane, and the concentration of polylactic acid polyglycolic acid copolymer PLGA is 2.2%, as the oil phase; the two are mixed under ice bath conditions, while stirring at a high speed, the stirring speed is 30000 rpm, forming water-in-oil colostrum, internal water phase: oil phase=1:3;
[0042] Next, under ice-bath conditions, the above-mentioned water-in-oil colostrum was added dropw...
Embodiment 3
[0054] A freeze-dried powder of nanoparticles for intraocular use, which comprises the following components:
[0055] The mass percent of polylactic acid PLA is 70%;
[0056] The mass percent of dexamethasone acetate DA is 30%.
[0057] A freeze-dried powder of nanoparticles for intraocular use and a preparation method thereof, comprising the steps of:
[0058] First, the glucocorticoid dexamethasone acetate DA 50mg was dissolved in 1ml acetone as the inner water phase; 100mg polylactic acid PLA was dissolved in 4.5ml methylene chloride, and the weight ratio concentration of polylactic acid PLA was 2.0%, as the oil phase; Mix the two under ice bath conditions, and at the same time, stir at a high speed of 30,000 rpm for emulsification for 60 seconds to form water-in-oil colostrum, the inner water phase: oil phase = 1:5;
[0059] Next, under ice-bath conditions, the above-mentioned water-in-oil type colostrum was added dropwise to 2.5% (v / w) polyvinyl alcohol PVA 40ml, and 40...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com