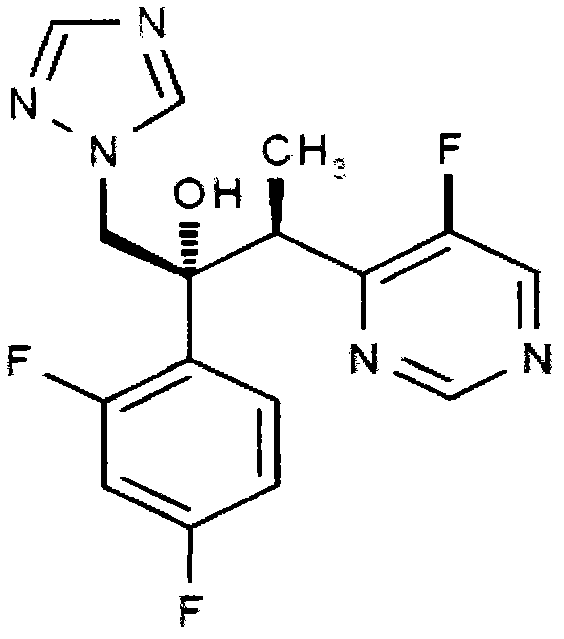
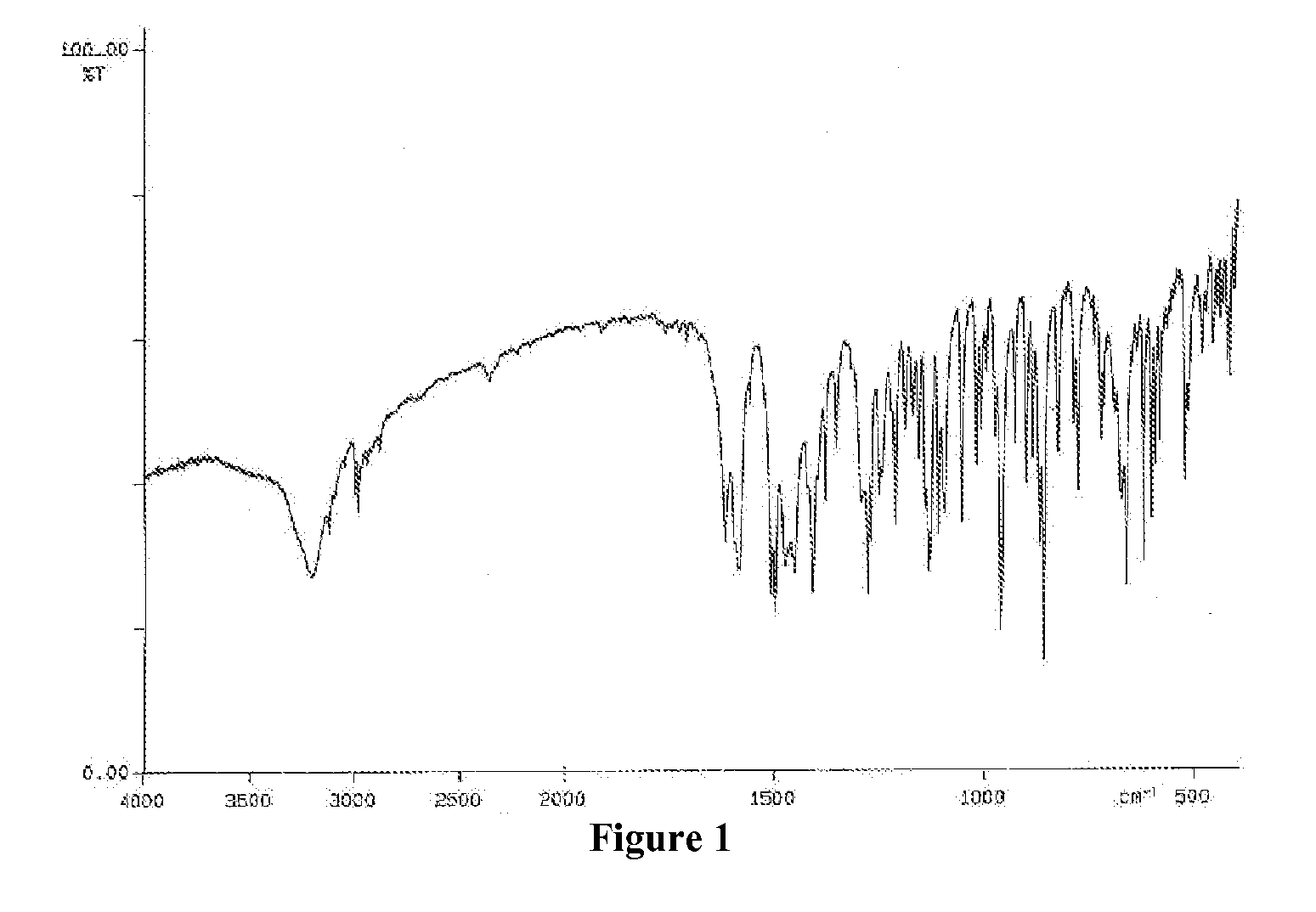
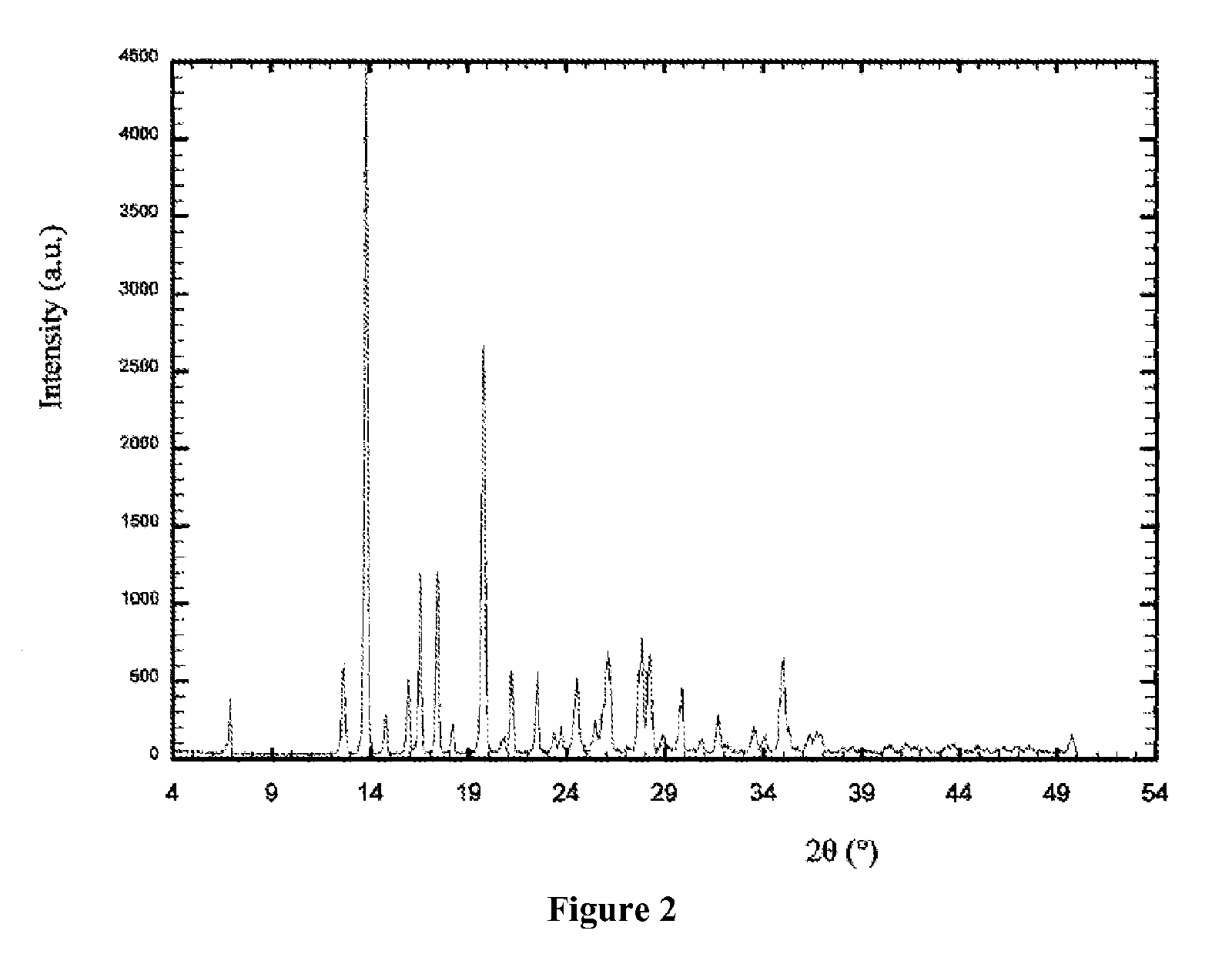
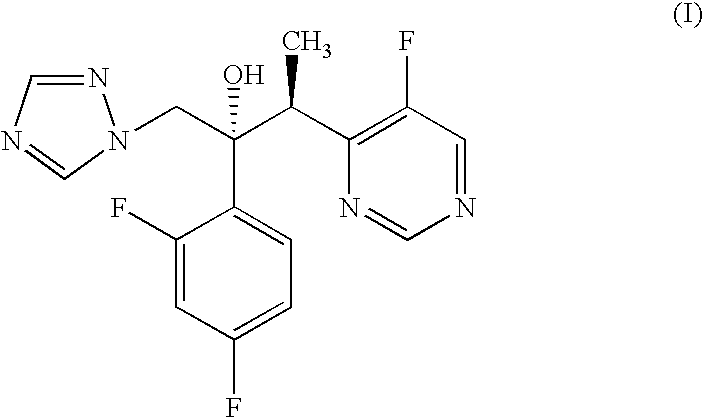
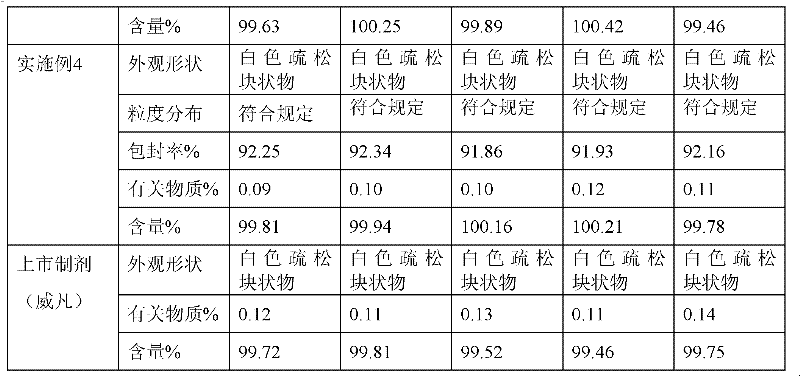
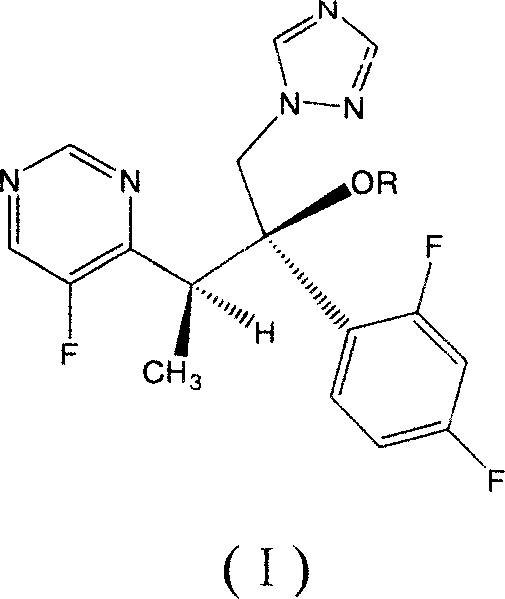
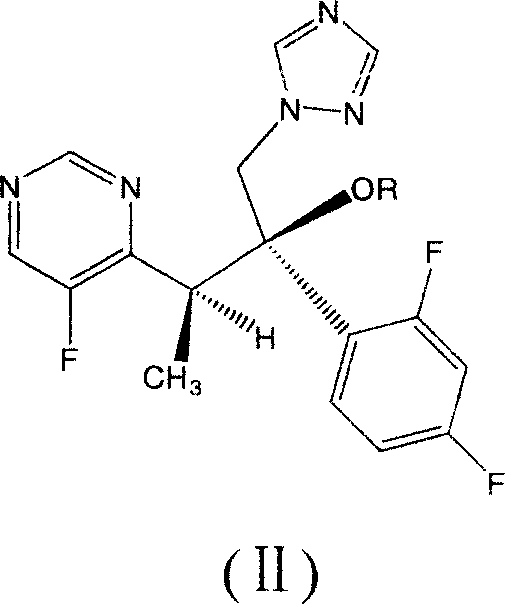
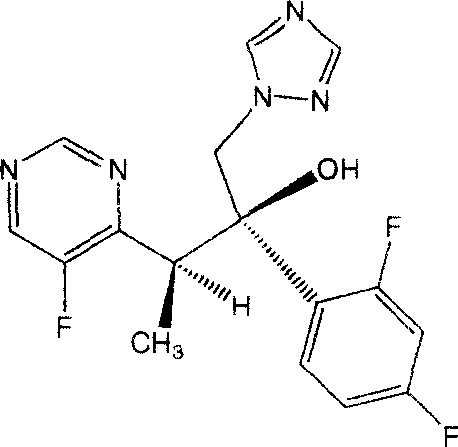
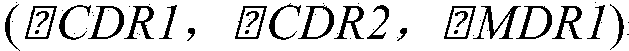Patents
Literature
164 results about "Voriconazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
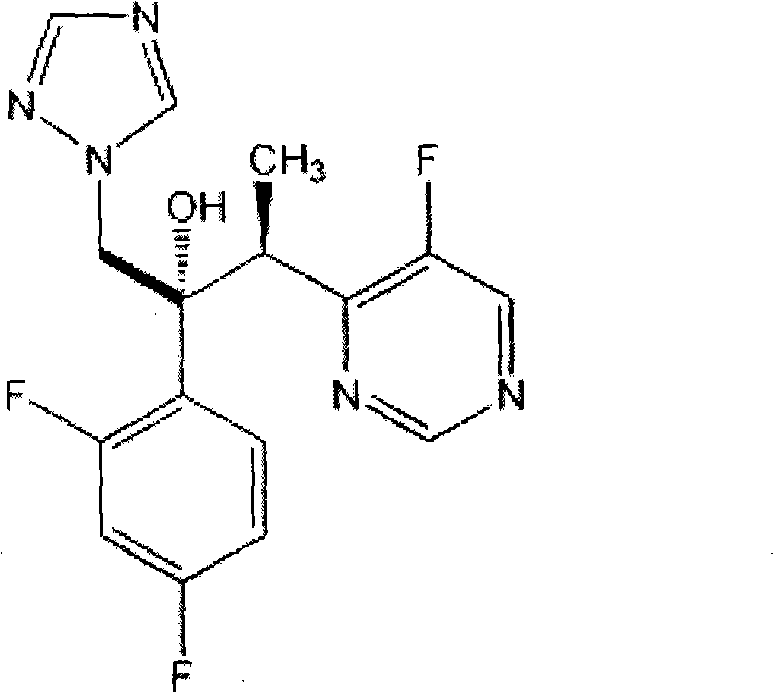
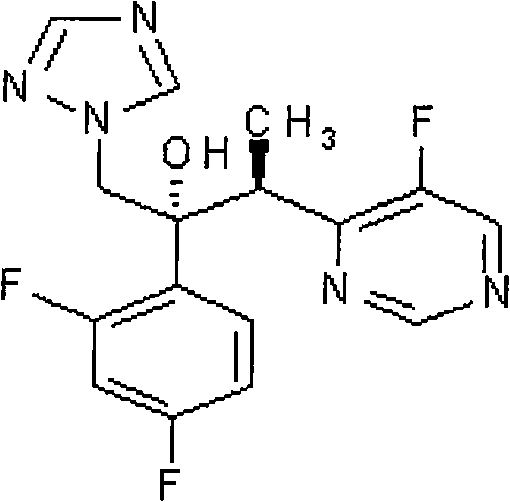
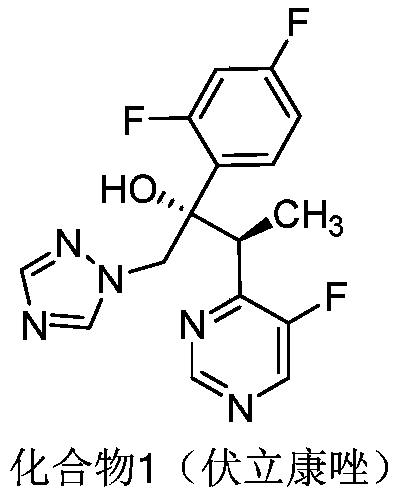
Voriconazole is used to treat a variety of fungal infections.
Voriconazole freeze-drying powder injection and its preparation process
The present invention relates to one kind of freeze dried voriconazole composition including voriconazole in effectively treating amount and hydroxypropyl-beta-cyclodextrin. Each of the preparation unit contains voriconazole in 50-400 mg, pregreably 200 mg and hydroxypropyl-beta-cyclodextrin of 5-40 time, preferably 15-25 times, weight of voriconazole.
Owner:大道隆达(北京)医药科技发展有限公司
Voriconazole eye drops and preparation method thereof
InactiveCN101849905AImprove comfortGood for antifungal effectOrganic active ingredientsSenses disorderMedicineBromine
The invention discloses voriconazole eye drops. Every 1000 milliliters of eye drops contain 3-50g of voriconazole powder, 20-500g of hydroxypropyl-beta-cyclodextrin, 0.1-0.3g of bacteriostat, 0.5-2.5g of sodium hyaluronate and the balance of water for injection, wherein the bacteriostat is benzalkonium chloride, benzalkonium bromine or ethylparaben. The invention also discloses a preparation method of the voriconazole eye drops. The invention greatly increases the solubility of voriconazole in water to 0.3-5%, so that the medicine can exist in water solution in the form of molecules, thereby providing convenience for exerting the antifungal action of the medicine. Meanwhile, when being applied to eyes, the invention provides convenience for exerting the antifungal action of the medicine through cornea. The preparation method can be carried out at room temperature without affecting the medicine stability, and has the advantages of simple and easy realization and low cost.
Owner:河南省眼科研究所
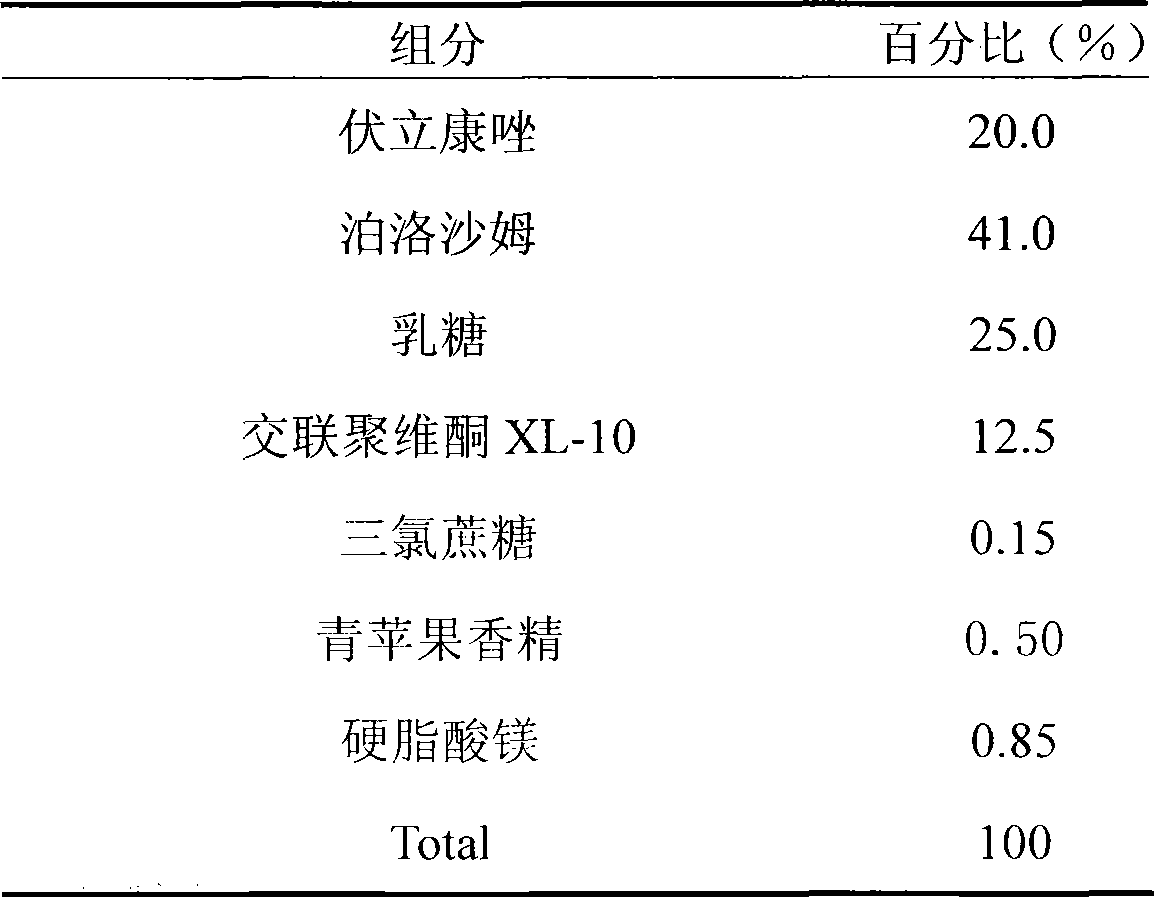
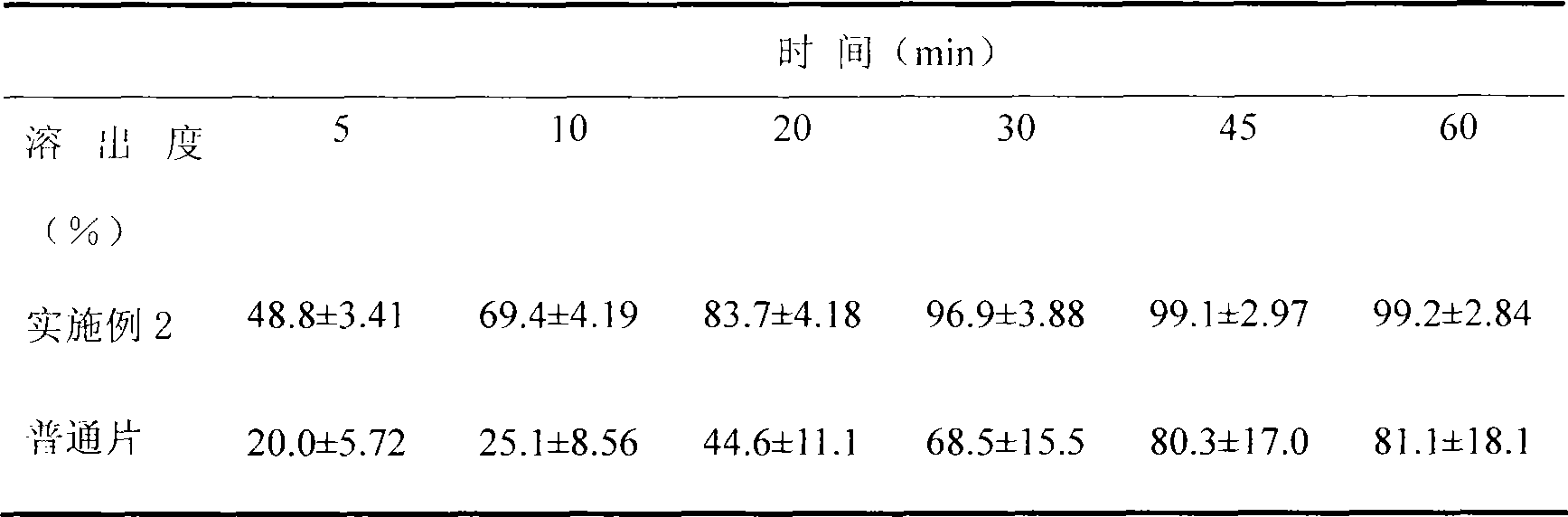
Voriconazole containing tablets
ActiveCN102133202AImprove bioavailabilityGood curative effectOrganic active ingredientsAntimycoticsMedicineDissolution
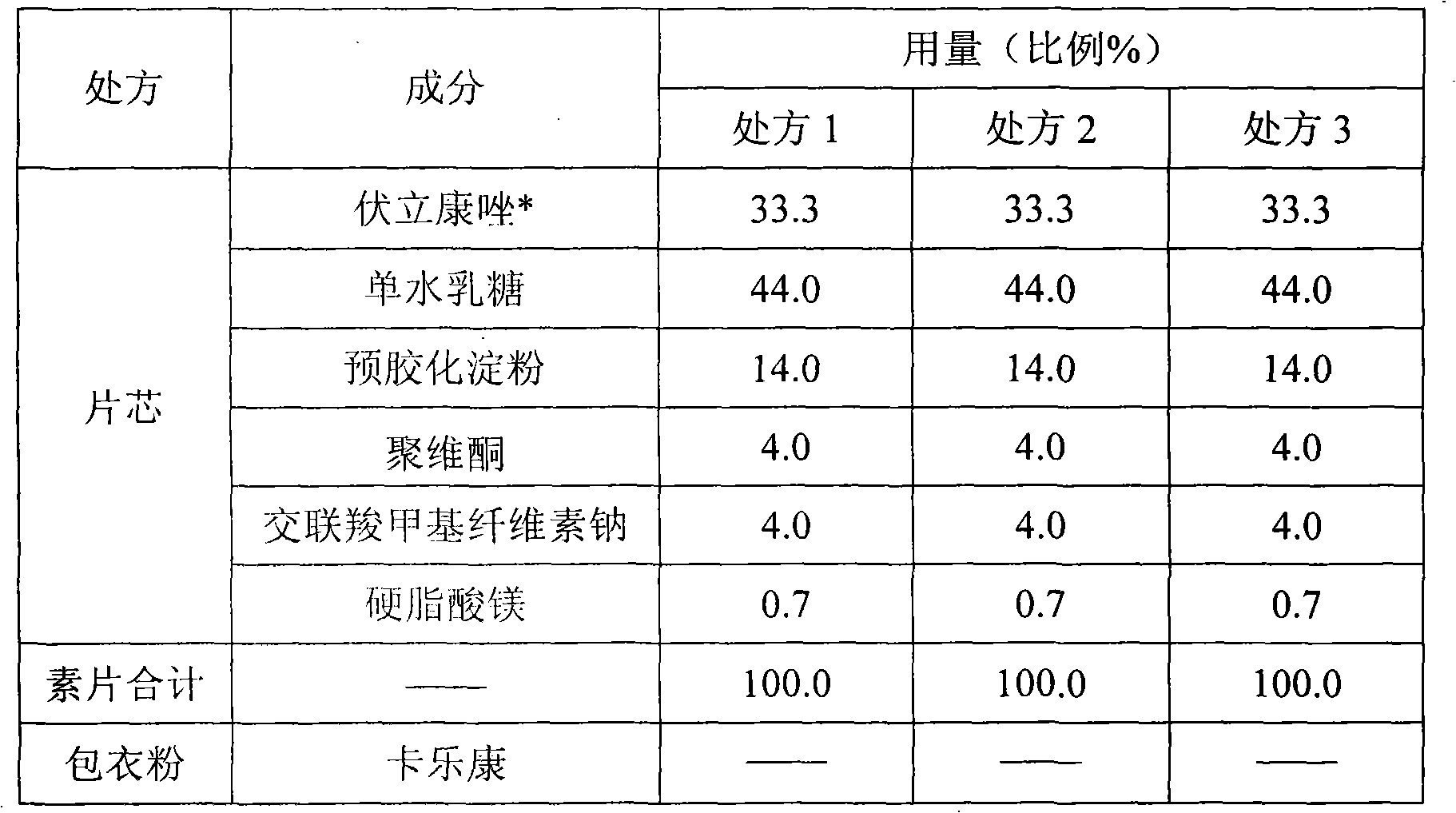
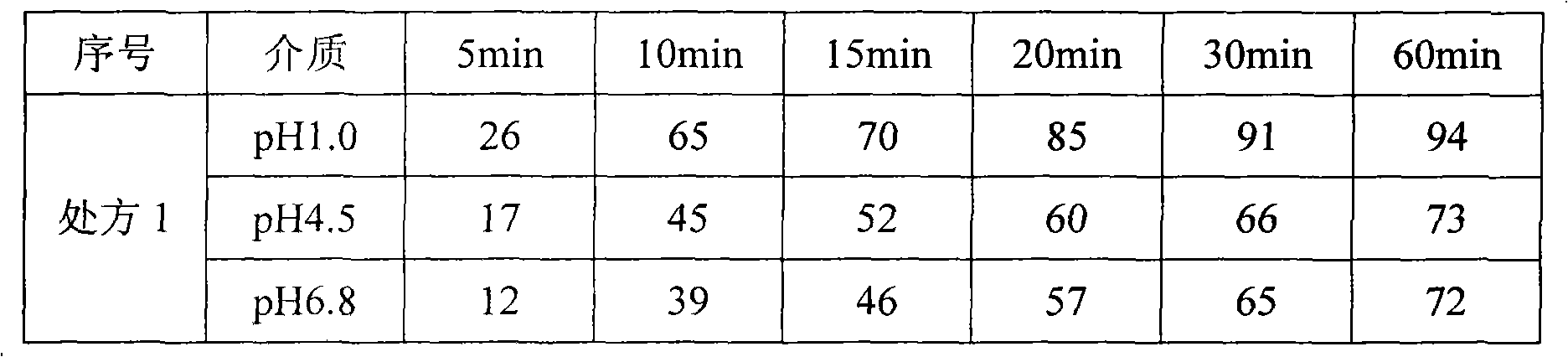
The invention relates to voriconazole tablets. The particle size of voriconazole in the tablets is controlled in a range of D(V, 0.9) <= 100 mu m, and the content of a disintegrant accounts for 2-6% of the total weight of the tablets. According to the method provided by the invention, voriconazole tablets with a high dissolution rate, small dissolution difference and small dissolution fluctuation among different batches under different pH conditions can be obtained, thus improving the quality and bioavailability of the voriconazole tablets. The invention adopts a conventional wet granulation process which is simple and suitable for commercial production to prepare the voriconazole tablets.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Solid medicine composition containing voriconazole
InactiveCN101390861AGood disintegration timeGood dissolutionOrganic active ingredientsAntimycoticsDiseaseOrally disintegrating tablet
The invention discloses voriconazole solid combination. The solid dispersion technology is adopted in the preparation process of the combination. The preparation forms include dispersible tablets, orally disintegrating tablets and chewable tablets. The voriconazole solid combination is used for the treatment of the diseases caused by fungal infection.
Owner:AVENTIS PHARMA HAINAN
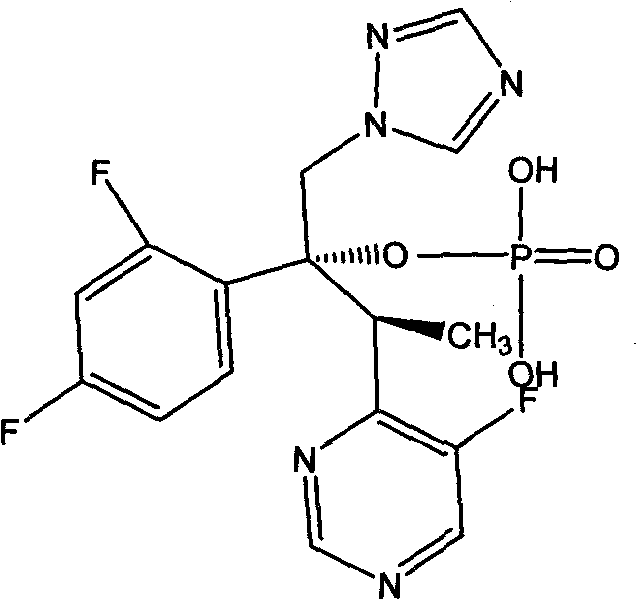
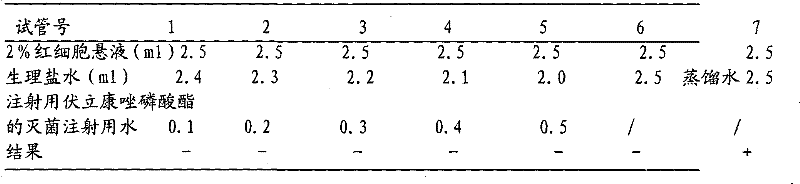
Voriconazole phosphate ester for injection and preparation method thereof
ActiveCN101744778AImprove performanceNo pollution in the processOrganic active ingredientsPowder deliveryPhosphateMedical prescription
The invention provides voriconazole phosphate ester for injection and a medicinal salt thereof and a preparation method for the voriconazole phosphate ester for injection and the medicinal salt thereof. The preparation method comprises the following steps: adding 5 to 98 percent water for injection in a liquid preparation container; adding 90 to 110 percent of the accurate formula dosage of voriconazole phosphate ester and the medicinal salt thereof in the container; stirring the mixture; slowly dropwise adding a pH value regulator; regulating pH to between 6.0 and 11; supplementing water to the full dosage and then adding 0.01 to 1.0 percent (weight in volume) medicinal carbon into the product; stirring the mixture for 15 to 60 minutes; using a sand filter stick to carry out rough filtration and decarburization on the obtained product, and using a 0.22mum millipore filter to carry out fine filtration on the product until the clarity is qualified; after determining that the content of the midbody is qualified, determining the filling quantity and subpackaging the finished product in the vial; adding the semi-plug; carrying out freezing and drying on the sample; controlling the moisture content between 1 and 8 percent; pressing the plug; and carrying out capping.
Owner:HC SYNTHETIC PHARMA CO LTD
Voriconazole slow-release suppository and preparation method thereof
ActiveCN102058519AReasonable workmanshipSmall toxicityOrganic active ingredientsAntimycoticsMicrosphereWhole body
The invention discloses a voriconazole slow-release suppository and a preparation method thereof. The voriconazole slow-release suppository disclosed by the invention is prepared from voriconazole, a solubilizer, a slow-release material, an emulsifier, a dispersing medium and an excipient; and the preparation method disclosed by the invention comprises the following steps of: preparing slow-release microspheres by solubilizing the extremely indissolvable voriconazole, carrying out cast pouring and cooling to obtain the voriconazole slow-release suppository. The preparation method disclosed bythe invention is simple, the prepared suppository has a slow-release characteristic, the first pass effect generated by oral administration and the toxic action of intravenous administration for the organs of a whole body are avoided, and particularly for the patients of intensive care units, the patients with serious fungous infection and child and senile patients, the pain brought to the patients by frequent administration is reduced, the administration compliance is improved, and the curative effect of medicaments is improved.
Owner:苏州特瑞药业股份有限公司
Novel oriented synthesis method of voriconazole, medicinal salt and intermediate thereof
InactiveCN1919846AReduce pollutionEasy to operateOrganic active ingredientsAntimycoticsAntibiotic DrugsKetone
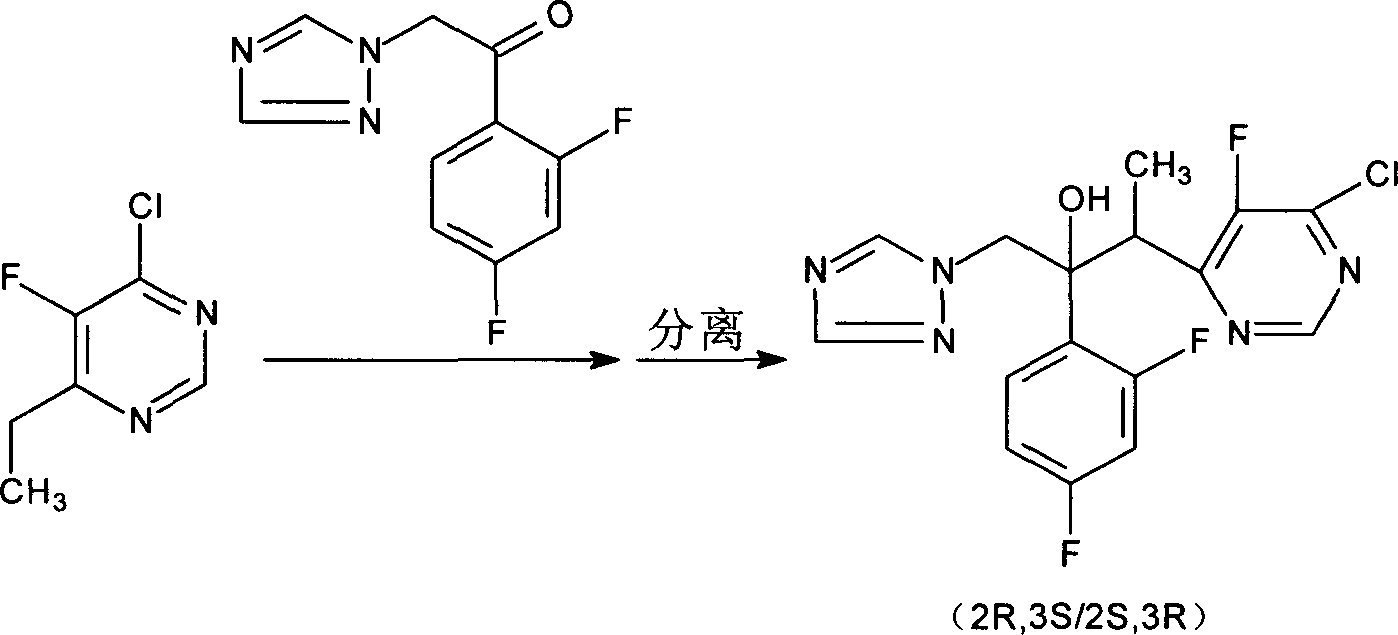
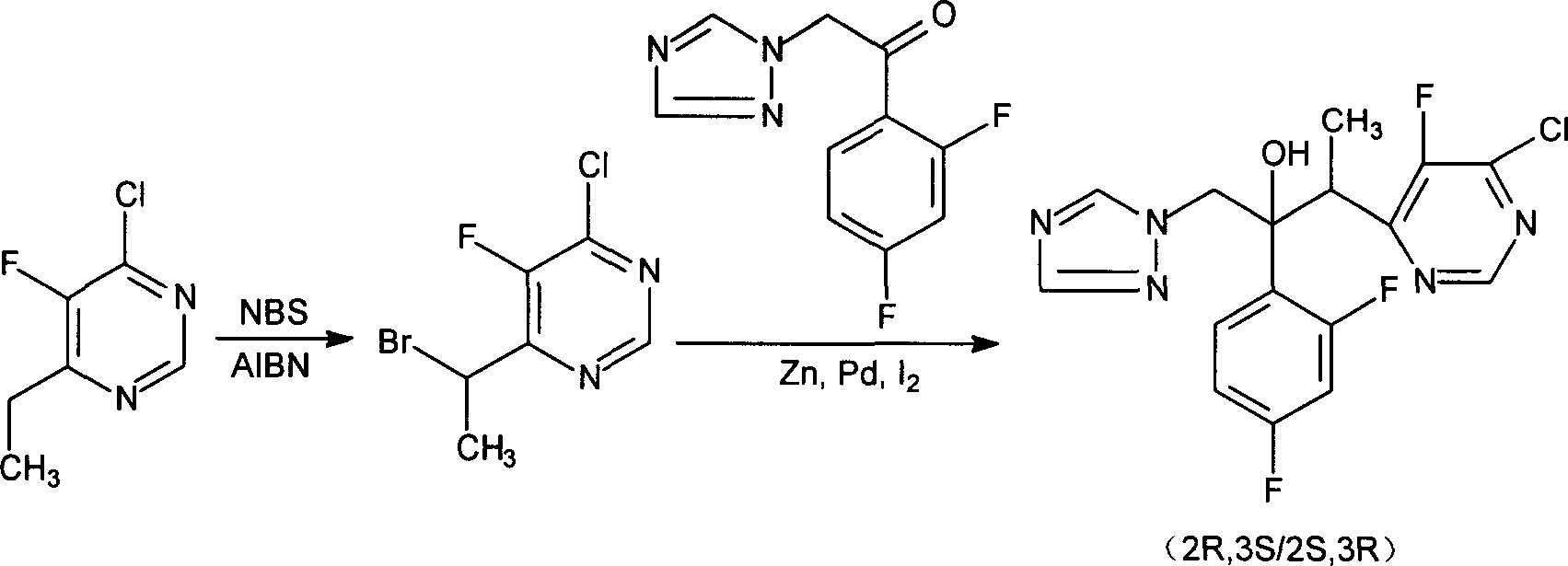
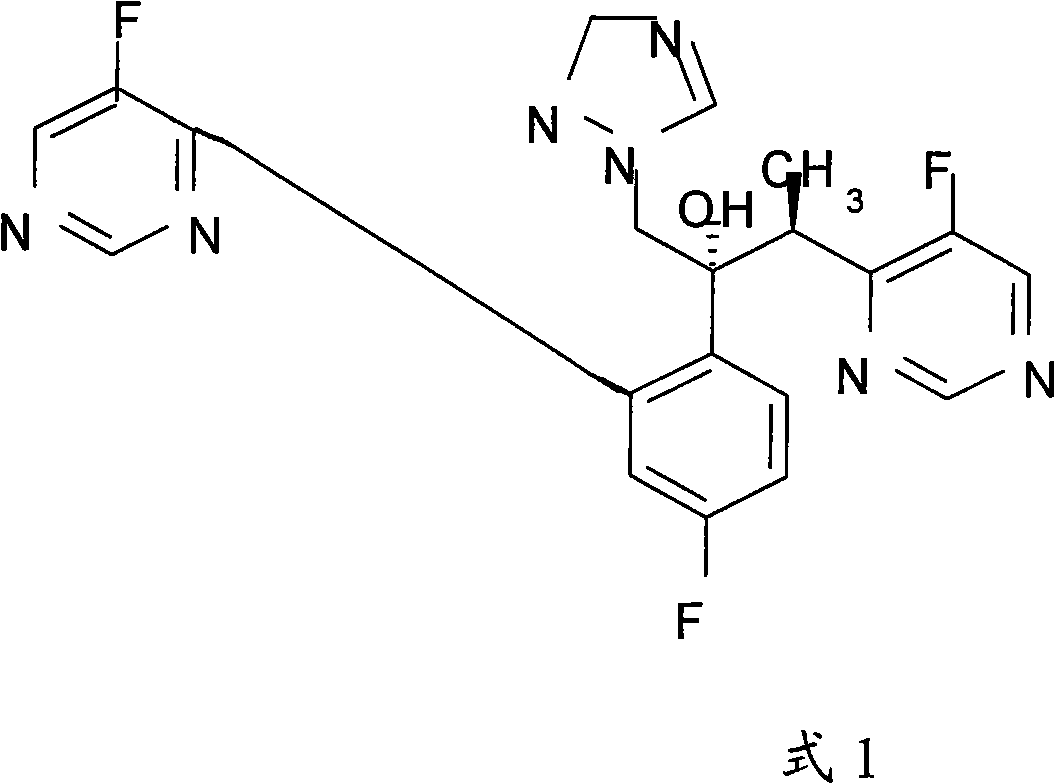
The invention discloses a new-typed antibiotic drug and medical salt and preparing method of one new directional intermediate, which is characterized by the following: adopting directional ketone synthetic technology corresponding to metal organic compound; preparing key intermediate (2R,3S / 2S,3R)-2-(2,4 difluorobenzene-3-(4-chlorin-5-fluoxydin-6-radical)-1-(1H-1, 2, 4-ribavirin-1-radical)-2-butanol or salt; reducing; dechlorinating; detaching to obtain voltyokonoside.
Owner:大道隆达(北京)医药科技发展有限公司
Prepn process and prepn of new soluble voriconazole salt
InactiveCN1847243AImprove solubilitySimple preparation processOrganic active ingredientsPowder deliverySolubilityAlcohol
The preparation process of soluble Voriconazole salt includes the following steps: 1. dissolving Voriconazole of 1-10 weight portions in alcohol of 60-90 weight portions at 15-40 through stirring; 2. dropping acid of 2-5 weight portions through stirring for 20-40 min; and 3. recrystallizing at normal temperature, filtering, washing with small amount of alcohol, and drying. The prepared salt is added with pharmaceutically acceptable supplementary material to prepare medicine powder for injection, emulsion for intravenous injection and injection. The present invention has high solubility and no toxicity.
Owner:大道隆达(北京)医药科技发展有限公司
Novel voriconazole broad-spectrum antifungal medicine compound, broad-spectrum antifungal medicine composition and application thereof
ActiveCN101575330ABroad and potent antifungal activityImprove mechanical propertiesOrganic active ingredientsAntimycoticsMonilinia laxaItraconazole
The invention relates to a novel broad-spectrum antifungal medicine compound, a broad-spectrum antifungal medicine composition, an application of the compound or the composition in the preparation of a broad-spectrum antifungal medicine, and an application of the compound or the composition in the preparation of a medicine used for treating severe invasive infection (comprising Candida krusei) caused by invasive aspergillosis and / or Fluconazole-resistant Monilia and / or severe infection caused by Scedosporium and fusarium. The novel broad-spectrum antifungal medicine compound and the broad-spectrum antifungal medicine composition have extensive and strong antifungal activity and better dynamic property and safety. For deep mycotic infection, the novel broad-spectrum antifungal medicine compound and the broad-spectrum antifungal medicine composition are better than the original voriconazole in the aspects of the antibiotic activity and the medicine resistance and is better than amphotericin B in the aspects of safety and effectiveness; and compared with the formerly applied voriconazole, Fluconazole, itraconazole and amphotericin B, the novel broad-spectrum antifungal medicine compound and the broad-spectrum antifungal medicine composition have reinforced medicine effect, less adverse reaction and good safety.
Owner:LIVZON PHARM GRP INC
Freezing-dried preparation containing voriconazole and its preparation method
The present invention discloses a voriconazole freezing dry preparation and the preparation method, which comprises the voriconazole, the oiliness component, the emulsifier and the freezing dry preparation. In the present invention, the voriconazole is packageed into the lipid micro sphere by the voriconazole freezing dry preparation. The melting speed is fast and the safety property is reliable.
Owner:张文芳
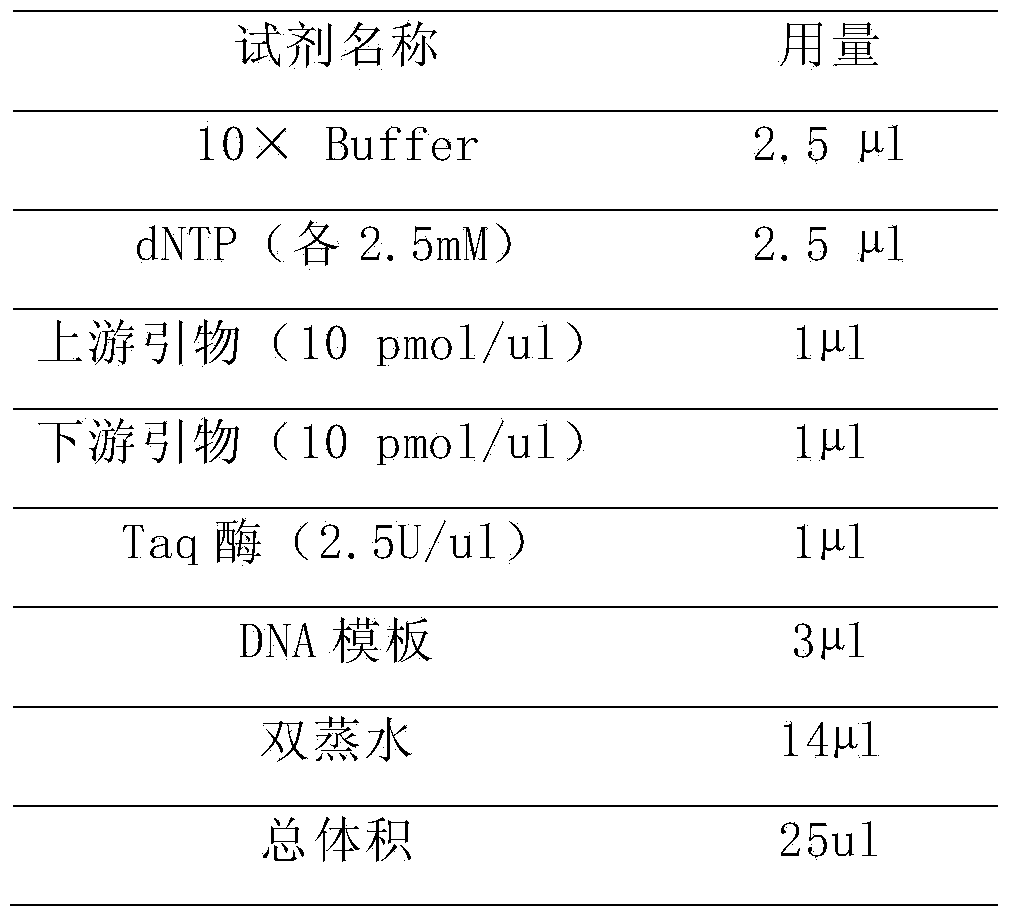
Pair of specific primers and probe for detection of CYP2C19 gene chip
InactiveCN103525925AStrong specificityGood signal to noise ratioMicrobiological testing/measurementDNA/RNA fragmentationSide effectCurative effect
The invention relates to the molecular biology field, and discloses a specific oligonucleotide probe for detection of SNP loci of rs4244285681G / A and rs4986893636G / A of a CYP2C19 gene and a pair of specific primers for amplification of the CYP2C19 gene during detection of the above SNP loci. The probe can hybridize with different genotypes of the CYP2C19 gene specifically, and the genotypes comprise a CYP2C19*1 type, a CYP2C19*2 type and CYP2C19*3 type. The products can be used for detection of metabolic abilities for relevant medicines (such as plavix, omeprazole, voriconazole and the like) and of Chinese population, clinical medication schemes can be guided and adjusted, clinical personalized medication can be provided with a basis, the curative effects can be raised and the risks of toxic and side effects can be reduced.
Owner:SHANGHAI BAIO TECH
Process for the preparation of voriconazole
InactiveUS20100056784A1High yieldHigh enantiomeric purityAntimycoticsOrganic chemistryMedicinal chemistryVoriconazole
Owner:MEDICHEM
Compound voriconazole eardrops and preparation method thereof
InactiveCN103156862AGood effectHas antibacterial activityAntibacterial agentsOrganic active ingredientsSide effectIrritation
The invention relates to eardrops, and in particular relates to compound voriconazole eardrops and a preparation method thereof. The compound voriconazole eardrops is characterized in that 0.1-2.0g of voriconazole, 0.1-3.0g of hydrocortisone, 5-40g of hydroxypropyl-beta-cyclodextrin, 10-80ml of polyethylene glycol, 0.1-3.0g of menthol, 0.1-2.0g of sodium chloride, 0.01-0.1g of ethylparaben, 5-60g of glycerin and 10-100ml of ethanol are contained in per 100-1000ml of the eardrops. The compound voriconazole eardrops disclosed by the invention has the beneficial effects that the composing prescription is reasonable and scientific and the voriconazole with the broadest antifungal spectrum is selected as the main medicine, so that the eardrops has antibacterial activity on all candida; the toxic and side effects of systemic administration can be reduced through local application and in the meantime, the eardrops is convenient for application, capable of increasing the curative effect and low in price when being directly used as the eardrops; and the eardrops is stable in quality and little in irritation and adverse reaction.
Owner:孙国栋 +1
Voriconazole nano-micro composite powder and preparation method thereof
ActiveCN103126991AImprove securityNo side effectsPowder deliveryOrganic active ingredientsHigh concentrationOrganic solvent
The invention discloses voriconazole nano-micro composite powder, wherein a safe and reliable pharmaceutical auxiliary material is adopted to replace cyclodextrin, tween and other auxiliary materials generally-used in the current voriconazole preparations or physiologically toxic organic solvents as a solvent so as to improve product safety, the composite powder can be dispersed in water so as to obtain a transparent dispersion, and the drug has characteristics of high concentration, small particle size and easy absorption by human body. The present invention provides a voriconazole nano-micro composite powder preparation method, which has characteristics of simple operation, easy enlargement and industrial production, and good application prospect.
Owner:BEIJING WINSUNNY PHARMA CO LTD +1
Pharmaceutical Formulations Comprising Voriconazole and Processes for Preparation Thereof
ActiveUS20110257197A1Improve solubilityNot affect stability of drugOrganic active ingredientsBiocideMedicinePharmaceutical formulation
The present invention provides a pharmaceutical formulation comprising voriconazole or a pharmaceutically acceptable derivative thereof, and an excipient of formula (I), i.e., monomethoxy poly(ethylene glycol)-poly (D,L-lactic acid) block copolymers (mPEG-PDLLA). The pharmaceutical formulation of the present invention has been shown to be stable and safe by experiments.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Preparation method for voriconazole and voriconazole intermediate
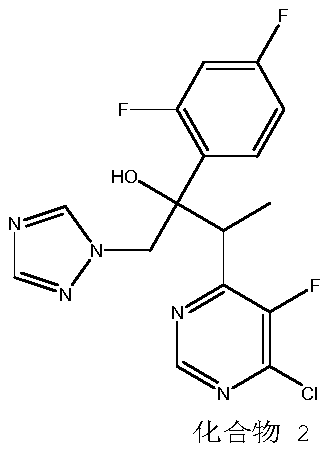
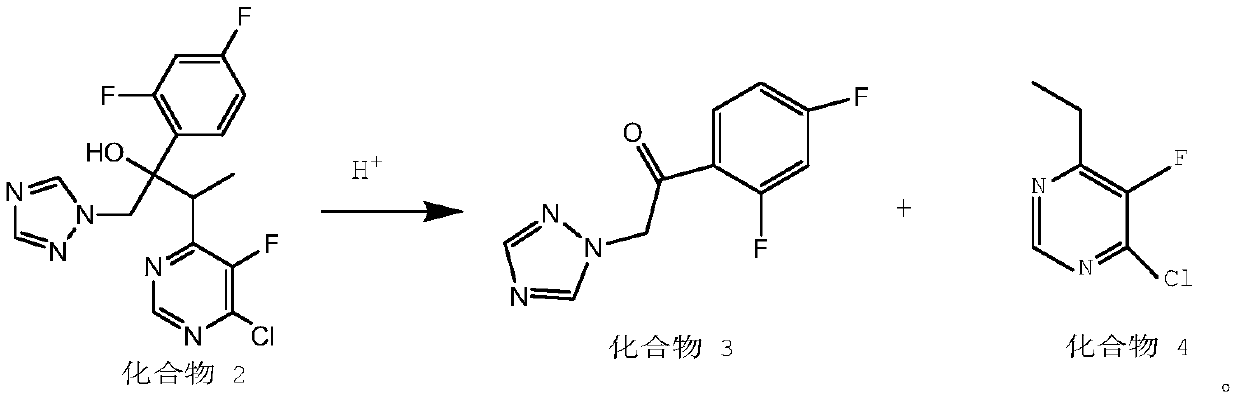
The invention discloses a preparation method for voriconazole and a voriconazole intermediate. According to the invention, with a voriconazole condensate isomer as a raw material, 4-chloro-6-ethyl-5-fluoropyrimidine and 2'4'-difluoro-2-[1-(1H-1,2,4-triazolyl)]acetophenone are obtained through recovery under an acidic condition, and can be further used for preparation of the voriconazole. By adoption of the method provided by the invention, the utilization rates of raw and auxiliary materials for preparation of the voriconazole in the prior art can be greatly improved; and cost is reduced.
Owner:ZHEJIANG HUAHAI LICHENG PHARMA CO LTD +1
Freeze-dried voriconazole micelle preparation and preparation method thereof
InactiveCN102335118AGood biocompatibilityImprove securityOrganic active ingredientsAntimycoticsSolubilityMixed micelle
The invention discloses a freeze-dried voriconazole micelle preparation and a preparation method thereof. The freeze-dried voriconazole micelle preparation consists of the following components in part by weight: 1 part of voriconazole, 2 to 10 parts of cholate, 1.5 to 5 parts of phospholipid, and 1 to 10 parts of freeze-drying supporting agent. According to the preparation, the dissolubility of the voriconazole is increased, and the dissolution degree of the voriconazole in water is improved; and the preparation can be diluted into intravenous injection by using injection water for clinical use. The particle diameter of the prepared voriconazole micelles is between 10 and 100 nanometers, the particle diameter of most micelles is about 20 nanometers, the particle size is uniform, the frozen and dried voriconazole micelles are high in re-dissolution speed, a transparent and colorless solution is formed after the voriconazole micelles are re-dissolved, and the particle diameter has no obvious change, so the safety of clinical application is enhanced. According to the prepared freeze-dried voriconazole micelle preparation, because the voriconazole is wrapped inside by the mixed micelles, influence of light, heat and moisture is avoided, and the stability of the voriconazole in a long-term storage process is improved.
Owner:JINAN KANGHE MEDICAL TECH
Preparation method for injectable hydrogel for controlled release of voriconazole intraocular drug
ActiveCN108434093ARelieve painReduce economyOrganic active ingredientsSenses disorderHalf-lifeBiocompatibility Testing
The invention discloses a preparation method for injectable hydrogel for controlled release of a voriconazole intraocular drug, and relates to the preparation method for injectable hydrogel. The preparation method aims to solve the problems that an existing voriconazole preparation is fast in metabolism and needs to be repeatedly supplied, the body pain and economic burden of a patient are increased, and an existing voriconazole sustained release system is poor in biocompatibility and poor in long-acting controlled-release effect. The method comprises the following steps of 1, preparing whiteflocculent polyaldehyde dextran; 2, preparing water-soluble linear poly-beta-cyclodextrin; and 3, firstly preparing a PBS solution of the water-soluble linear poly-beta-cyclodextrin with voriconazoleloaded in a cyclodextrin molecular cage, and then dissolving carboxymethyl chitosan into the PBS solution so as to obtain the injectable hydrogel for controlled release of the voriconazole intraoculardrug. According to the preparation method, the half-life of the prepared injectable hydrogel for controlled release of the voriconazole intraocular drug in a vitreous cavity is 30-60 days; and the injectable hydrogel for controlled release of the voriconazole intraocular drug can be obtained.
Owner:QINGDAO UNIV
Composition and method for killing aspergillus fumigatus
The invention provides a composition and a method for killing aspergillus fumigatus, and belongs to the technical field of biology. The method for killing aspergillus fumigatus comprises the following steps: preparing a medicine; collecting a strain; preparing a vector and a spore suspension; performing a medicine sensitivity test; preparing an aspergillus fumigatus bio-membrane vector; acting the medicine to the aspergillus fumigatus bio-membrane; detecting the metabolic activity by virtue of an XTT (2,3bis(2methoxy4nitro5sulfophenyl)5[(phenylamino)carbonyl]2htetrazolium hydroxide) reduction method; preparing a fluorescent dye liquor, and performing fluorescent dyeing and observation; combining chlorogenic acid and voriconazole to kill aspergillus fumigatus. According to the composition and the method for killing aspergillus fumigatus, aspergillus fumigatus in bio-membranes can be killed, and the problem that an antifungal drug cannot kill aspergillus fumigatus in a bio-membrane can be solved.
Owner:GUANGXI MEDICAL UNIVERSITY
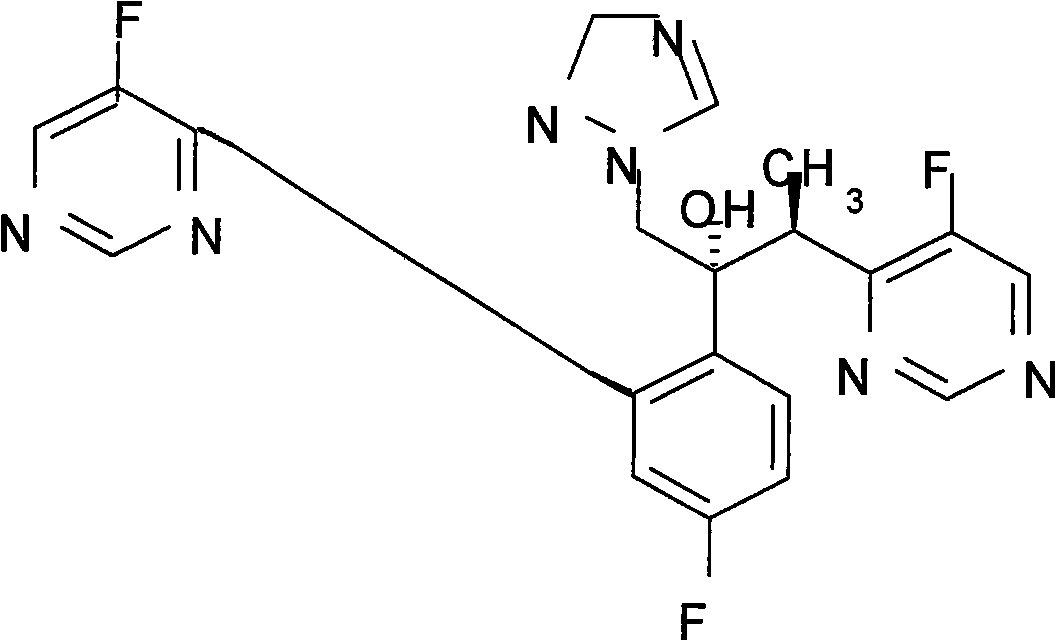
Voriconazole derivate and preparation process thereof
InactiveCN100999518ATo achieve asymmetric synthesisHigh yieldOrganic active ingredientsAntimycoticsPropanoic acidEnantiomer
This invention involves Voriconazole derivatives. The invention also involves Voriconazole derivatives preparation methods, including the following steps : 2 - (5-fluoro-4-yl) acetate and ethanol for esterification, generate 2 -(5-fluoro-4-yl) ethyl acetate, in alkaline conditions takes reaction with methylation agent, generate 2 - (5-fluoro-4-yl) ethyl propionate; hydrolysis of 2 - (5-fluoro-4-yl) propionic acid, through split gain S-2 - (5-fluoro-4-yl) propionic acid; for chlorination to gain S-2 - (5-fluoro-4-yl) propionyl chloride; occurred Friedel-Crafts reaction, generating S-1 - 2, 4-difluoro-2 - (5-fluoro-4-yl) acetone; with 1-methyl - 1-H-1 ,2,4 - triazol under alkali conditions to take reaction to gain voriconazole and the series of voriconazole derivatives. The methods described in this invention has short routes, only use of a pair of enantiomers separation, the overall yield has been greatly improved, is a simple and easy method for synthesis of voriconazole and its derivatives.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
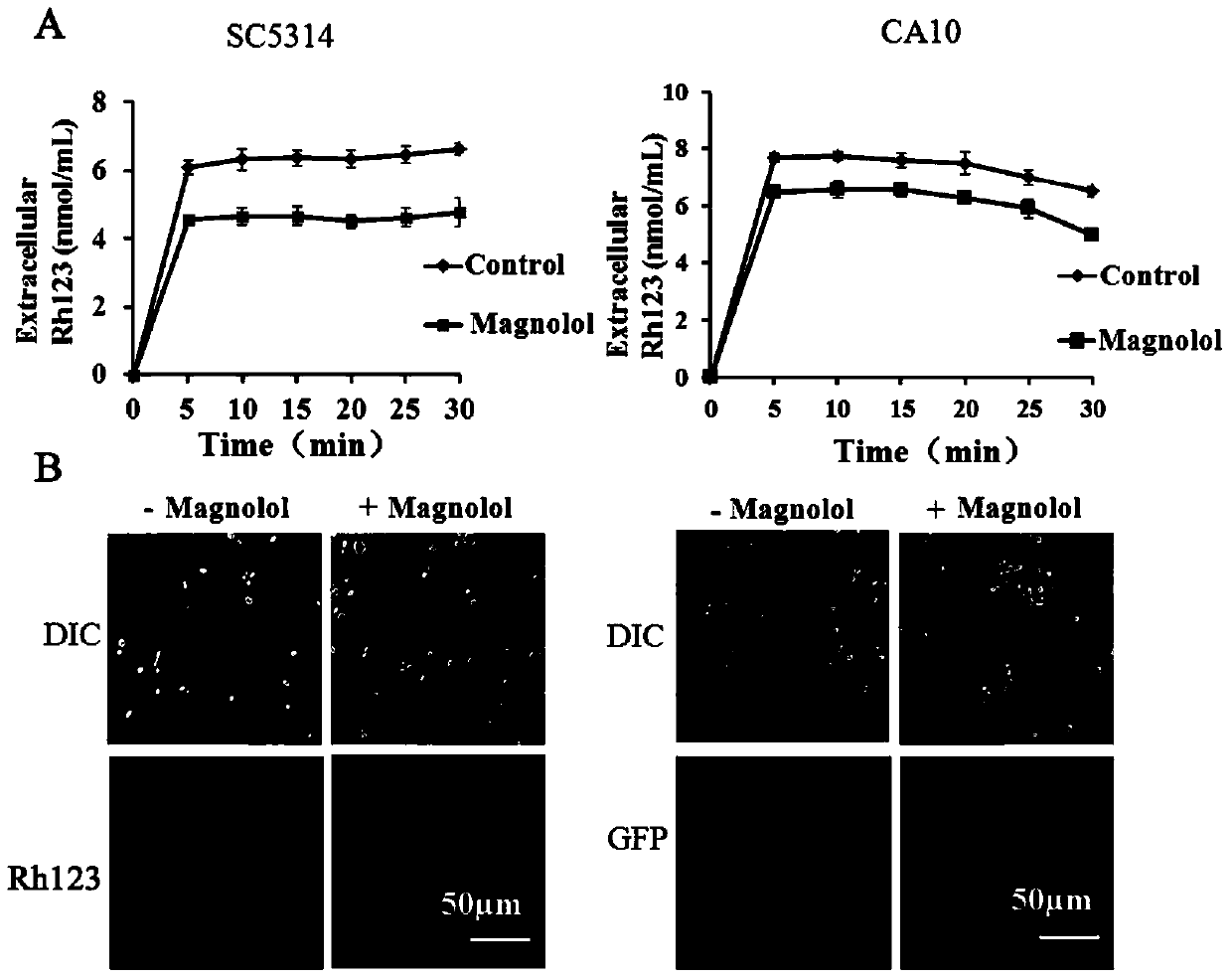
Application of magnolol and azole medicines to preparation of antifungal combined medicines
InactiveCN104188962AGood curative effectInhibit synthesisAntimycoticsHydroxy compound active ingredientsHonokiolMedicine
The invention provides application of magnolol and azole medicines to preparation of antifungal combined medicines. The application indicates that azole medicines such as fluconazole, ketoconazole, itraconazole, miconazole and voriconazole are used together with magnolol to generate a synergetic antifungal effect; the magnolol serving as an efflux pump Cdrlp substrate is utilized to compete with the azole medicines for an efflux system, so that the concentration of efflux medicines can be reduced, and the curative effect of medicines can be improved. Furthermore, magnolol acts on the synthetic pathway of ergosterol and is combined with azole medicines to form a double-edged sword to inhibit synthesis of ergosterol together.
Owner:SOUTHEAST UNIV
HPLC-MSMS method for determining concentrations of two antitumor drugs in human plasma
ActiveCN110045048AGuaranteed curative effectHigh selectivityComponent separationMetaboliteMetabolic enzymes
The invention discloses a method for determining the concentrations of two anti-tumor drugs, metabolites and endogenous substances related to the activity of a metabolic enzyme in human plasma, whichcomprises the following steps: preprocessing, respectively taking voriconazole and bromouracil as internal standards, separating drug components in supernate by using high performance liquid chromatography in a preprocessed sample, performing drug targeting detection by using a high-resolution mass spectrum multi-reaction monitoring mode, and quantifying to realize the analysis and determination of the concentrations of the two anti-tumor drugs, the metabolites and the endogenous substances related to the activity of the metabolic enzyme in the plasma. The method has the advantages of rapidness, extremely high targeting property, high flux, high sensitivity, strong specificity, good precision and accuracy, good stability, high extraction recovery rate, no obvious matrix effect and dilutioneffect and the like. The method for determining the concentrations of two anti-tumor drugs, metabolites and endogenous substances related to the activity of the metabolic enzyme in the human plasma can be used for monitoring the blood concentration of irinotecan and metabolites thereof and the blood concentration of fluorouracil and endogenous substances related to the activity of the metabolic enzyme thereof clinically.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Antifungal oral dosage forms and the methods for preparation
The present invention relates to pharmaceutical dosage forms which include an antifungal having poor solubility. The pharmaceutical dosage forms of the present invention further comprise non-spherical granules, which do not contain a coated core region and may be formed into pharmaceutically acceptable dosage forms. The antifungal active pharmaceutical ingredients include itraconazole, saperconazole, ketoconazole, voriconazole and fluconazole.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Voriconazole dried suspension and preparation method thereof
InactiveCN102232929ALarge distribution areaFast absorptionOrganic active ingredientsAntimycoticsDifficult swallowingSuspending Agents
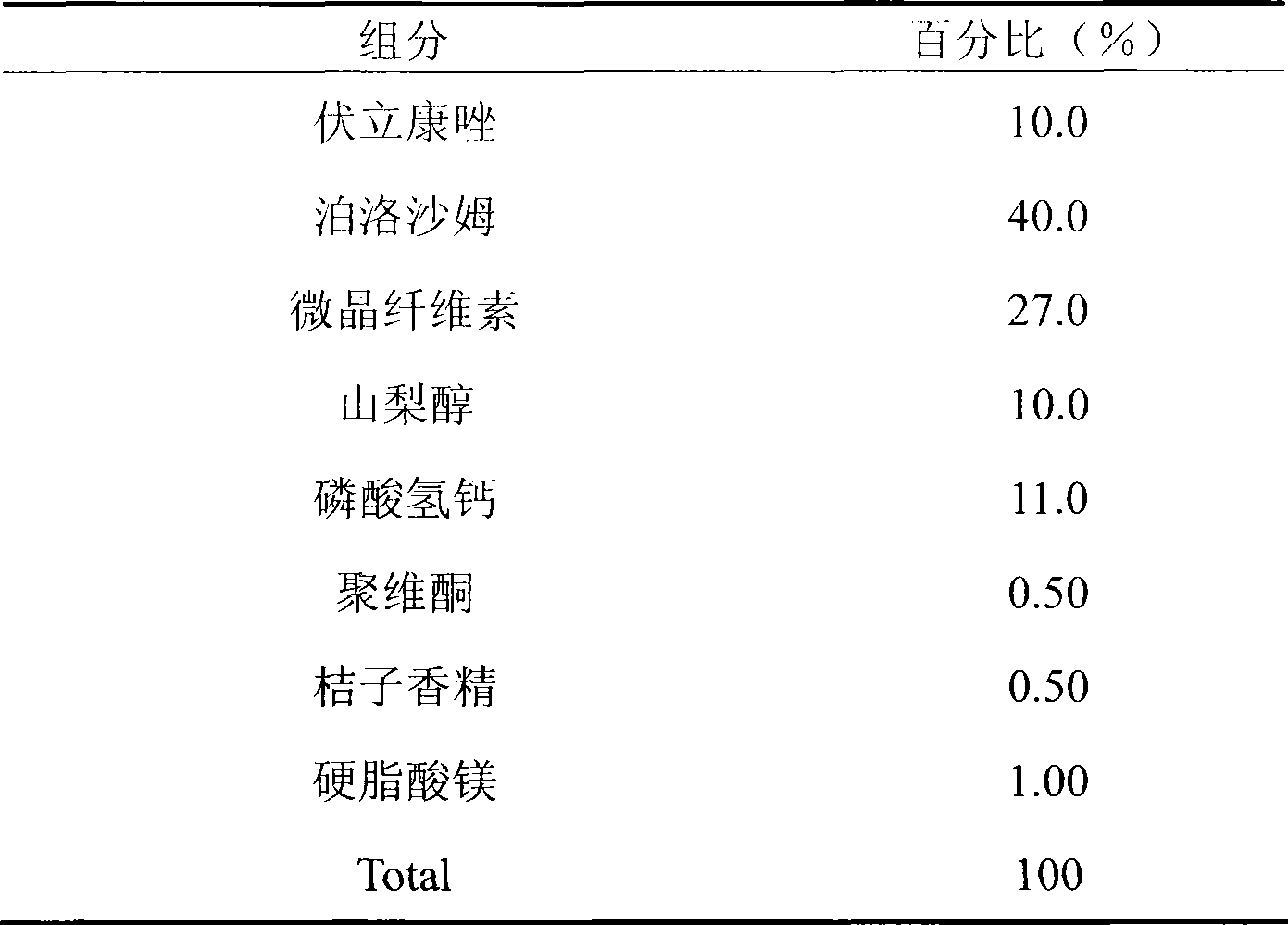
The invention relates to a Voriconazole dried suspension, comprising 5-15wt% of Voriconazole, 3-20wt% of suspending agent, 30-60wt% of filling agent, 20-50wt% of sweetening agent, 0.1-10wt% of PH regulator, 0.2-2wt% of lubricant, 0.1-2wt% of flavoring agent and 1-5wt% of opacifier. The Voriconazole dried suspension is a fine particle dosage form with more advantages than tablet and other solid dosage forms, has the advantages of large distribution area in gastrointestinal tract, fast absorption and high bioavailability, and can avoid the disadvantage of difficult swallowing of the tablet. Dried suspension is stored as solid, and is given through the mouth in suspension state, so as to be suitable for drugs with low water stability, and for children, the old and the patients who has difficult in swallowing.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +2
New method for prepraring Voriconazole intermediate of antifungal drug
The invention describes a process for preparing the triazole derivatives or acid-addition salts of formula (I). The said compounds can be used to prepare antifungal drugs such as Voriconazole.
Owner:北京德众万全医药科技有限公司
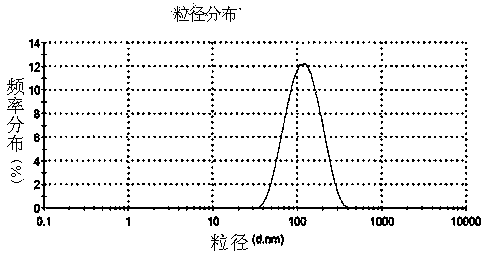
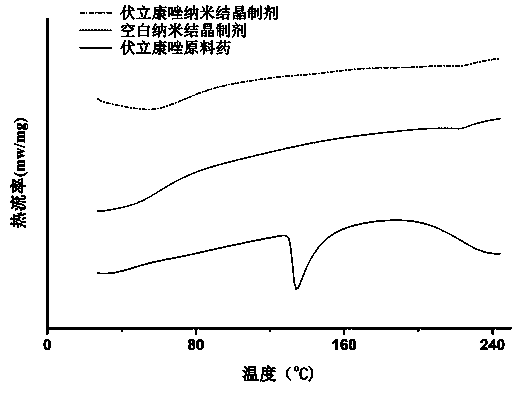
Eye use voriconazole nanocrystal preparation and preparation method thereof
ActiveCN104188904AImprove solubilityImprove bioavailabilityOrganic active ingredientsPowder deliveryBioavailabilityPharmaceutical Substances
The invention provides an eye use voriconazole nanocrystal preparation and a preparation method thereof. The eye use voriconazole nanocrystal preparation is prepared from voriconazole, biodegradable polymer auxiliary material, a penetration enhancer, an osmotic pressure conditioning agent, a pH conditioning agent and distilled water, and is characterized in that the solubility of indissolvable voriconazole is improved by adopting the nanocrystal technique and using the eye use penetration enhancer, so as to enhance the adhesion and permeability between the preparation and the corneas. The voriconazole nanocrystal preparation takes EudragitRS100, PVA, and the like as main carrier materials and the precipitation method is adopted to prepare the voriconazole nanocrystal preparation; the novel eye use penetration enhancer with good safety and small stimulation is used to enhance the permeability of the cornea of the system; the voriconazole nanocrystal preparation can be used for eye administration of fat-soluble voriconazole; the preparation is good in stability, high in permeability and high in bioavailability and has no stimulation.
Owner:HUBEI UNIV OF TECH
Preparation method of voriconazole
InactiveCN104744441AEasy to prepareReduce manufacturing costOrganic chemistrySodium acetateFormamidine acetate
The invention relates to a preparation method of voriconazole, wherein the preparation method comprises the following specific steps: adding alpha-fluoropropionyl ethyl acetate and a methanol solution of formamidine acetate into a methanol solution of sodium methylate, carrying out cyclization to obtain 6-ethyl-5-fluoropyrimidine-4(3H)-one, chloridizing to obtain 4-chloro-6-ethyl-5-fluoropyrimidine, dissolving 4-chloro-6-ethyl-5-fluoropyrimidine in tetrahydrofuran, dropwise adding into a tetrahydrofuran solution of lithium diisopropylamide, adding a tetrahydrofuran solution of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazole-1-yl)ethyl ketone, carrying out a reaction to obtain 3-(4-chloro-5-fluoropyrimidine-6-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazole-1-yl)butyl-2-ol, dissolving in ethanol, adding 10% palladium-carbon and sodium acetate, and then splitting to obtain voriconazole. According to the preparation method of voriconazole, the preparation method is simple, and the production cost is low.
Owner:李磊
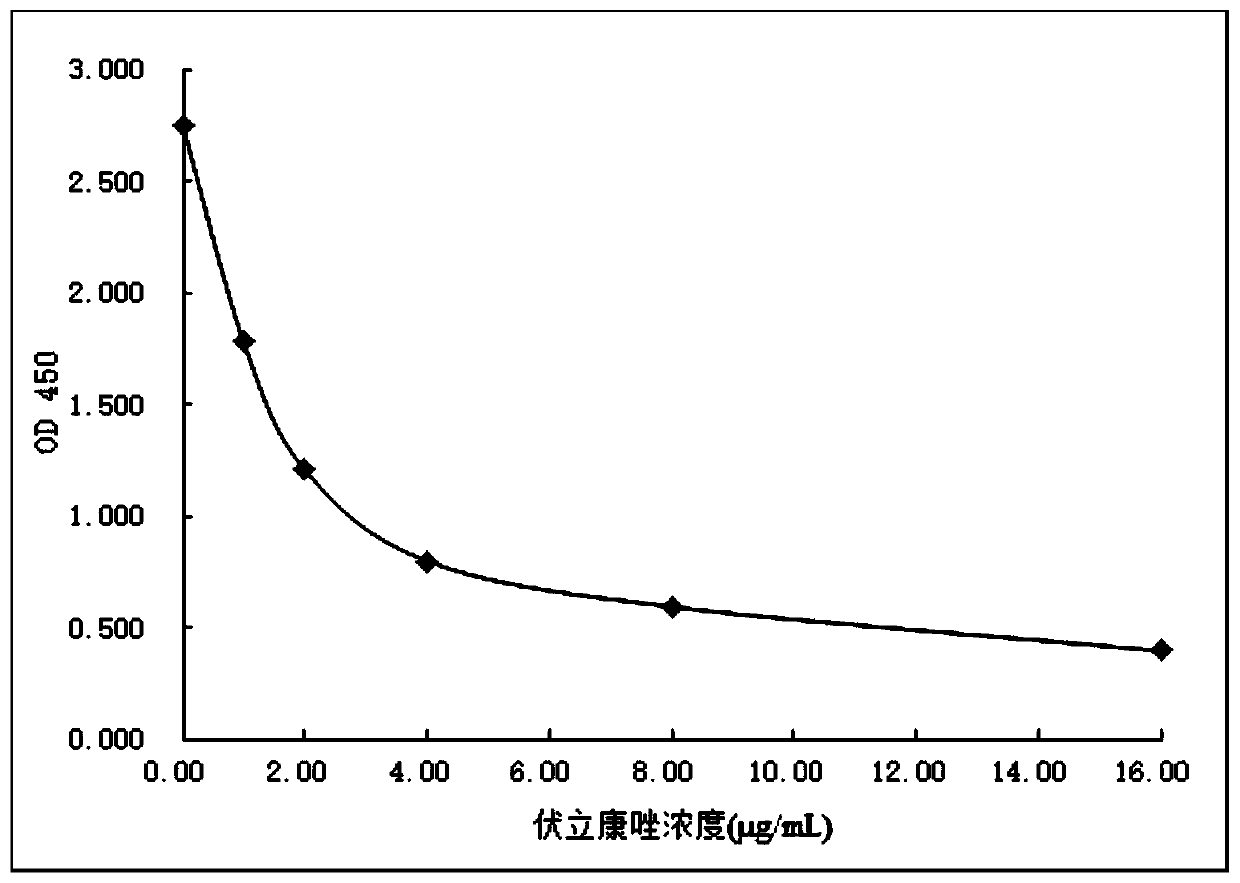
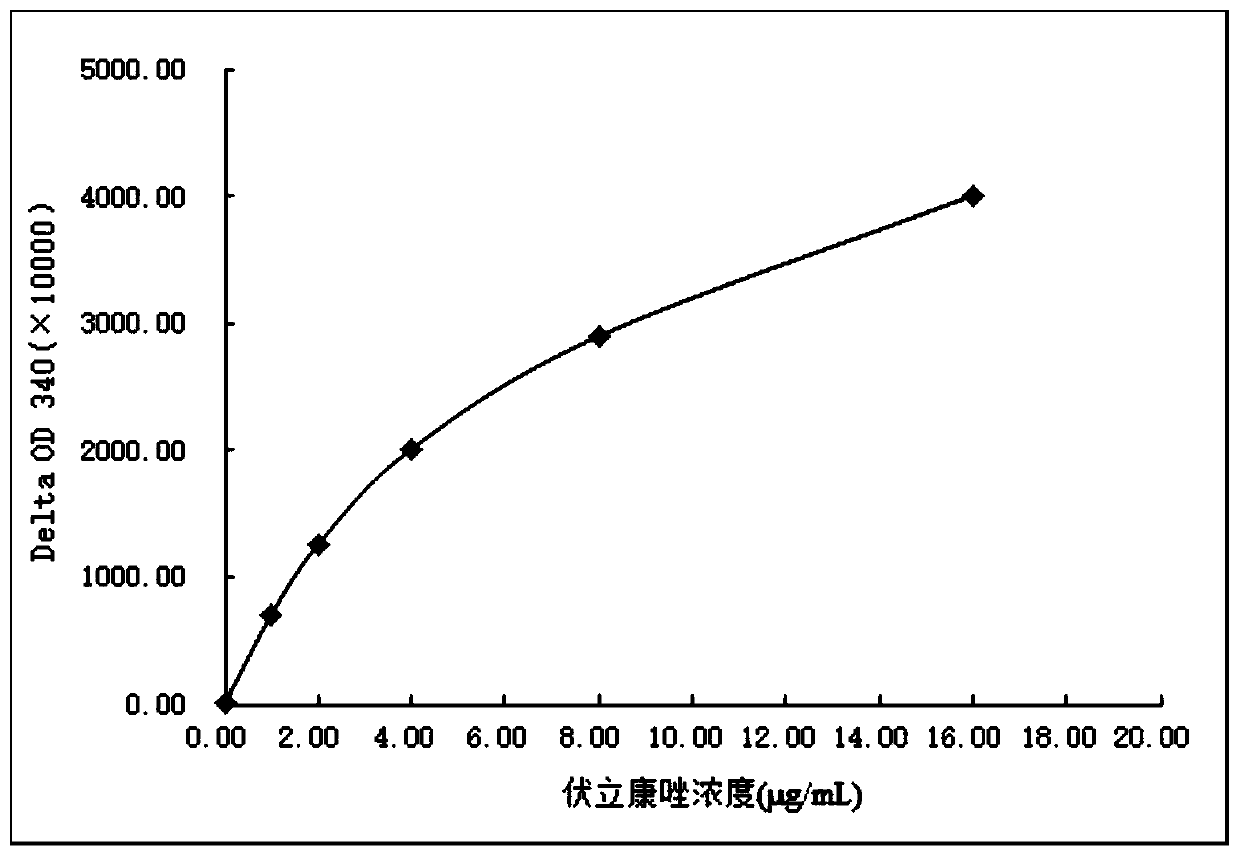
Voriconazole derivative, synthesis method thereof and voriconazole immunogen, preparation method and application thereof
InactiveCN109928958AHigh sensitivityStrong specificitySerum albuminDepsipeptidesSynthesis methodsTrue positive rate
The invention discloses a voriconazole derivative, a synthesis method thereof and a voriconazole immunogen, a preparation method and application thereof. An anti-voriconazole specific antibody prepared from the voriconazole derivative has strong specificity and high titer, also has no cross reaction with 92 common interferents, thus reflecting high accuracy, precision, sensitivity and specificity.The voriconazole immunogen can be applied to preparation of an anti-voriconazole specific antibody and the anti-voriconazole specific antibody can be applied to preparation of a voriconazole detection reagent. The detection reagent can realize high throughput and rapid detection of voriconazole on a fully automatic biochemical analyser.
Owner:苏州博源医疗科技有限公司
Method for simultaneously determining blood concentrations of six first-line antituberculosis drugs and antifungal drugs voriconazole in blood plasma
PendingCN112326824AEnables high-throughput assaysStrong specificityComponent separationAntituberculosis drugAntituberculous drugs
The invention discloses a method for simultaneously determining blood concentrations of six first-line antituberculosis drugs and antifungal drugs voriconazole in blood plasma, and relates to the technical field of clinical blood concentration monitoring and control. According to the method, an HPLC MS / MS method is adopted, acetaminophen is used as an internal standard, the blood plasma is subjected to protein precipitation through using methyl alcohol, is subjected to elution and separation through HPLC under the specific gradient elution condition according to a specific mobile phase, then enters a mass spectrum system and undergoes multi-reaction monitoring and analysis, therefore, accurate quantification of the blood concentration of seven antibiotics is achieved at the same time. Themethod has the advantages of strong specificity, high sensitivity, good precision and accuracy, good stability, high extraction recovery rate, no obvious matrix effect, simple operation, convenience and rapidness. The method provided by the invention can realize high-throughput determination of clinical samples of tuberculosis patients and guide clinical individualized medication.
Owner:中国人民解放军总医院第八医学中心
Voriconazole ear drop and preparation method and application thereof
ActiveCN102526056AImprove the feeling of stuffy earsImprove complianceOrganic active ingredientsSenses disorderRegimenPatient compliance
The invention provides an ear drop which comprises voriconazole and one or more than one of its pharmaceutical salts, ambroxol and one or more than one of its pharmaceutical salts, and pharmaceutical adjuvants. The ear drop of the invention can effectively improve patient feeling of earache and ear fullness; the ear drop is prepared by the combination of ambroxol hydrochloride and voriconazole, and thus the application of the ear drop has significantly better effect than single application of a voriconazole ear drop or ambroxol hydrochloride, and has higher patient compliance than single application of a voriconazole ear drop, has a shorter treatment course; and it is concluded that the combination of a voriconazole ear drop and ambroxol hydrochloride for the ear drop has synergistic effect on disease cause elimination and function recovery.
Owner:LIVZON GROUP LIVZON PHARMA FACTORY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com