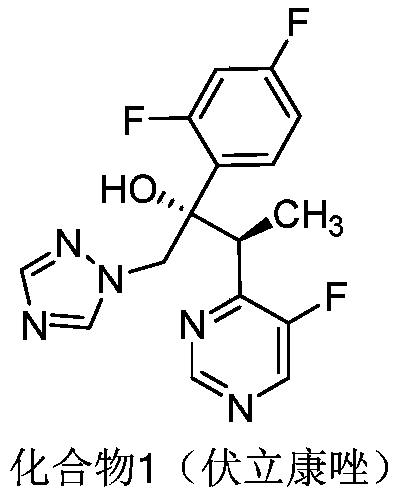
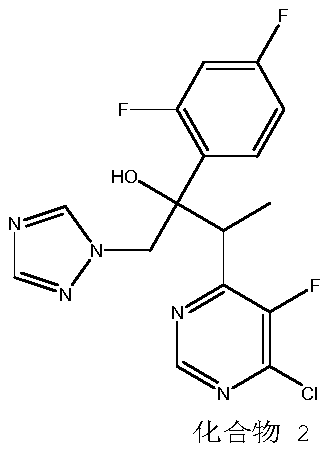
Preparation method for voriconazole and voriconazole intermediate
A technology of voriconazole and intermediates, which is applied in the field of preparation of voriconazole and its intermediates, can solve the problems of being unable to meet the requirements of industrial production cost control, unable to greatly improve the utilization rate of raw and auxiliary materials, expensive transition metal catalysts, etc., and achieve easy scale The effect of industrial production, low cost, and short synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
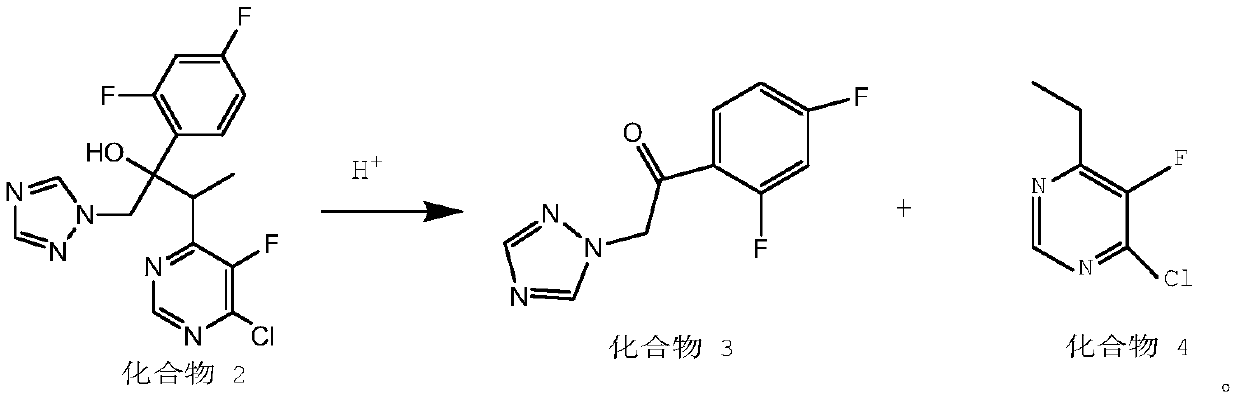
[0031] Preparation of 4-chloro-6-ethyl-5-fluoropyrimidine and 2'4'-difluoro-2-[1-(1H-1,2,4-triazolyl)]acetophenone
[0032] Add 38.3 g of 2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1,2,4-triazol-1-yl)butan- 2-alcohol (configuration 2S, 3S / 2R, 3R, containing a small amount of 2R, 3S / 2S, 3R), in 500ml of 1mol / L dilute hydrochloric acid, heat up to 60-70°C for 6-7 hours, and the reaction is over After cooling down to 0°C, adjust the pH value to 7-8 with ionic membrane liquid alkali, extract with dichloromethane to obtain a mixture of compound 3 and compound 4, and distill under reduced pressure to obtain 22g of compound 4 with a purity of 92% and a yield of 98.7% . The collected fractions were subjected to atmospheric distillation to remove the solvent, and then vacuum distillation to obtain 14.2 g of compound 3 with a purity of 99.1% and a yield of 88.4%.
Embodiment 2
[0034] Preparation of 4-chloro-6-ethyl-5-fluoropyrimidine and 2'4'-difluoro-2-[1-(1H-1,2,4-triazolyl)]acetophenone
[0035] Add 38.3 g of 2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1,2,4-triazol-1-yl)butan- 2-alcohol (configuration 2S, 3S / 2R, 3R, containing a small amount of 2R, 3S / 2S, 3R), in 380ml 3mol / L sulfuric acid, heat up to 40-50°C for 10 hours, cool down to room temperature after the reaction , ionic membrane liquid base to adjust the pH value to 7-8, extracted with ethyl acetate to obtain a mixture of compound 3 and compound 4, and concentrated to obtain 21 g of compound 4 with a purity of 90% and a yield of 94.2%. The collected fractions were subjected to atmospheric distillation to remove the solvent, and then vacuum distillation to obtain 13.6 g of compound 3 with a purity of 99.0% and a yield of 84.6%.
Embodiment 3
[0037] Preparation of 4-chloro-6-ethyl-5-fluoropyrimidine and 2'4'-difluoro-2-[1-(1H-1,2,4-triazolyl)]acetophenone
[0038] Add 38.3 g of 2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1,2,4-triazol-1-yl)butan- 2-alcohol (configuration 2S, 3S / 2R, 3R, with a small amount of 2R, 3S / 2S, 3R), add 380ml dioxane, 11.5g trifluoroacetic acid, heat up to 70-90°C for 3-5 hours After the reaction was completed, the temperature was lowered to room temperature, and the pH value was adjusted to 7-8 by ion-exchange membrane liquid alkali. The mixture of compound 3 and compound 4 was obtained by extraction with dichloromethane, and concentrated to obtain 20 g of compound 4 with a purity of 91% and a yield of 89.6%. The collected fractions were subjected to atmospheric distillation to remove the solvent, and then vacuum distillation to obtain 13.7 g of compound 3 with a purity of 99.3% and a yield of 85.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com