Solid medicine composition containing voriconazole
A voriconazole and composition technology, applied in the field of new antifungal pharmaceutical compositions, can solve the problems of poor patient compliance, large difference in dissolution rate between batches, poor water solubility of voriconazole, etc., and achieve high bioavailability and fast absorption in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
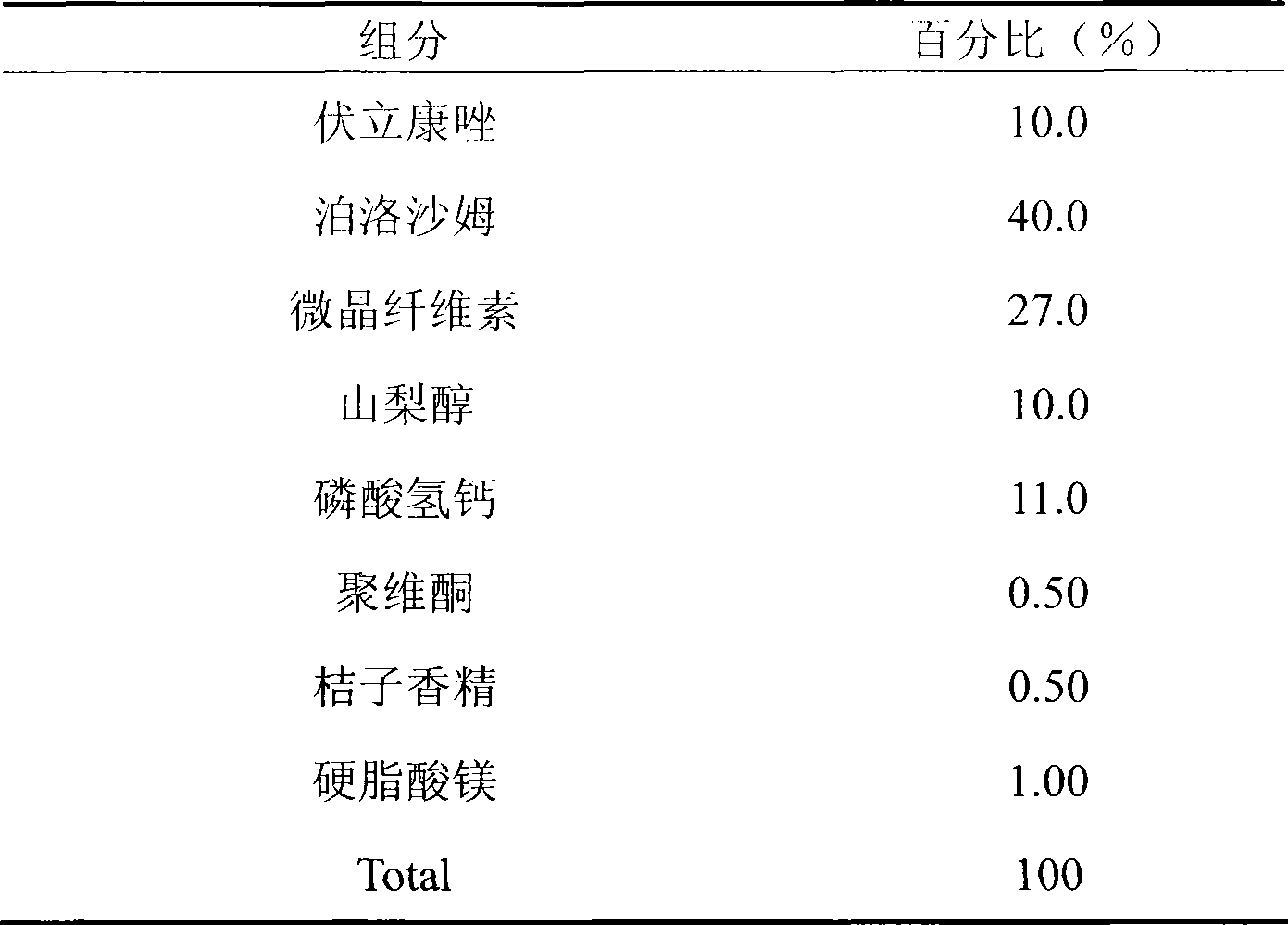
[0017] Example 1: It is in the form of a chewable tablet
[0018]
[0019] Preparation process: Weigh voriconazole and poloxamer according to the prescription amount to prepare solid dispersion, weigh the rest of the excipients, pass through 100 mesh, mix well, add 10% povidone aqueous solution as adhesive, make soft material, pass through 20 mesh Sieve and granulate, dry, granulate with a 24-mesh sieve; add magnesium stearate and orange flavor according to the prescription ratio, mix well, and compress into tablets.
Embodiment 2
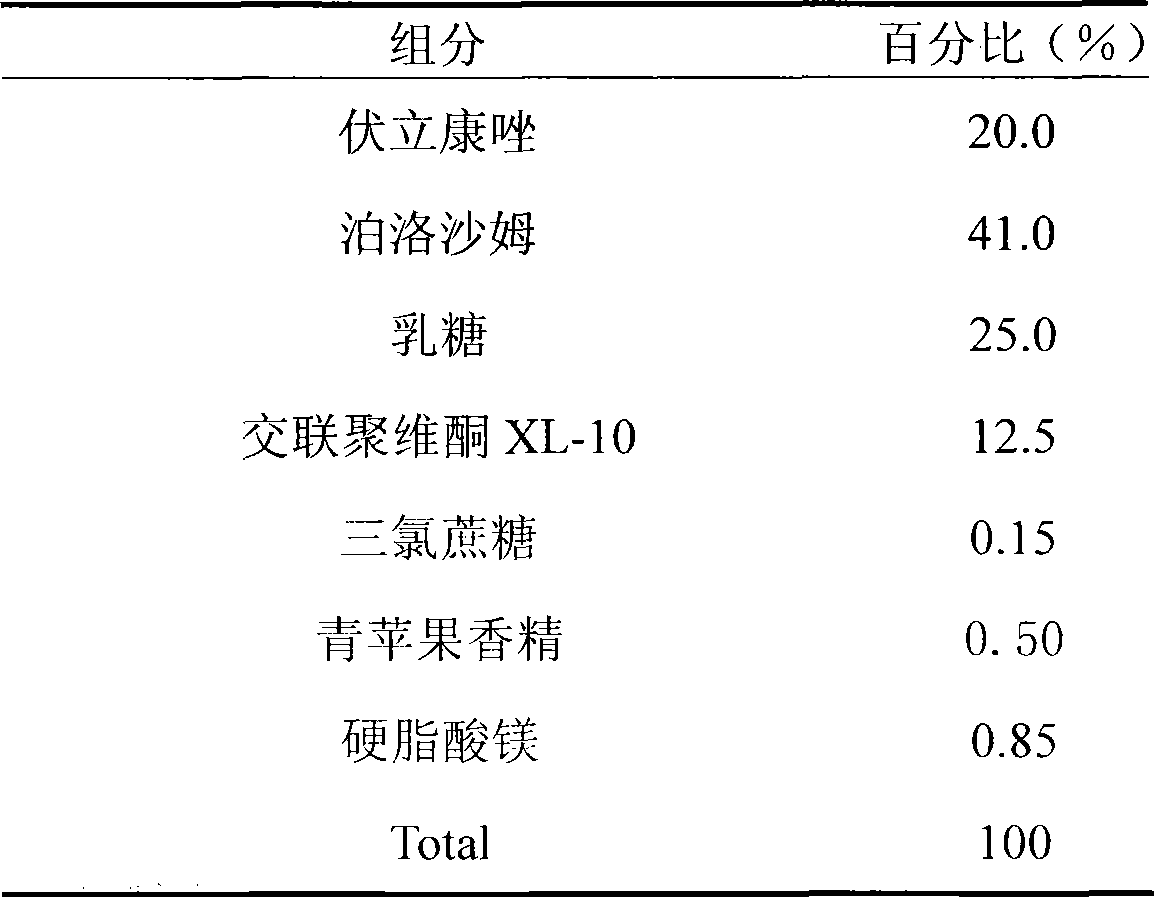
[0020] Embodiment 2: It exists in the form of dispersible tablets
[0021]
[0022] Preparation process: Weigh voriconazole and poloxamer according to the prescription amount to prepare solid dispersion, weigh the rest of the excipients, pass through 100 mesh, mix well, add sucralose aqueous solution as adhesive, make soft material, pass through 20 mesh sieve granules, dried, sieved with 24 meshes; add magnesium stearate and orange flavor according to the prescription ratio, mix well, and compress into tablets.
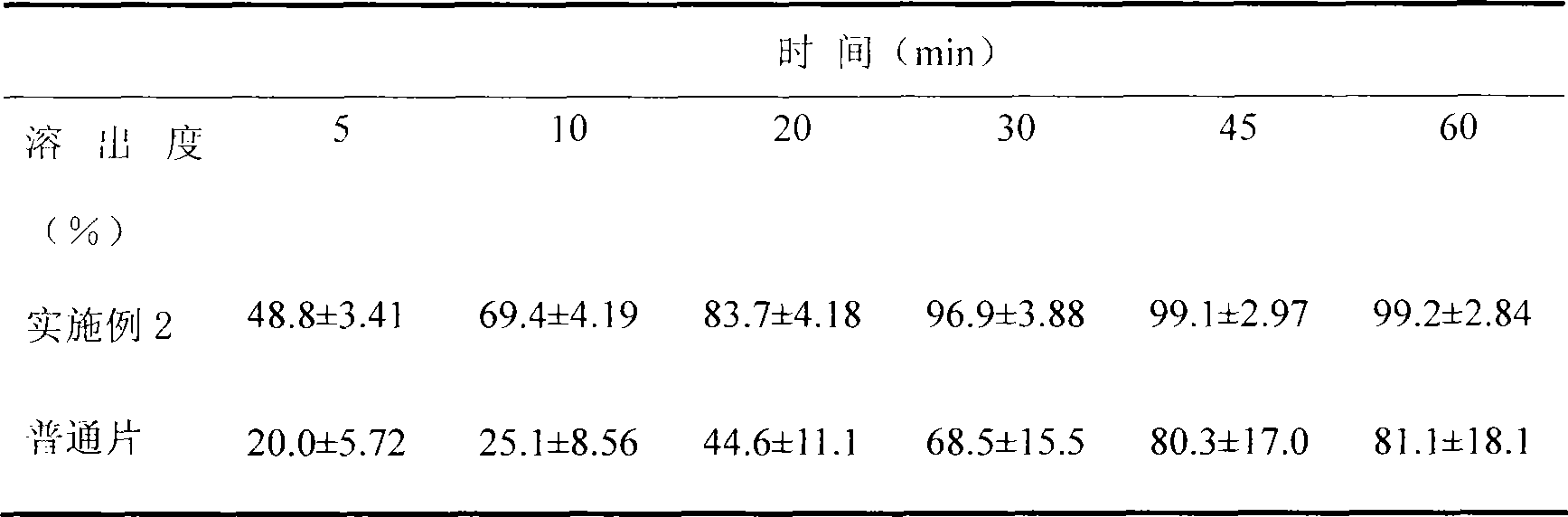
[0023] Embodiment 2 is compared with the dissolution rate of commercially available common tablet (n=6, X ± SD)
[0024]
[0025] The results show that: the dissolution of the sample prepared in Example 2 at each time point is faster than that of the common tablet.
Embodiment 3
[0026] Embodiment 3: It exists in the form of an orally disintegrating tablet
[0027]
[0028] Preparation process: Weigh voriconazole and poloxamer according to the prescription amount to prepare solid dispersion, weigh the rest of the excipients, pass through 100 mesh, mix well, use water as binder, make soft material, pass through 20 mesh sieve to granulate, dry, Sieve through a 24-mesh sieve; add magnesium stearate and orange flavor according to the prescription ratio, mix well, and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com