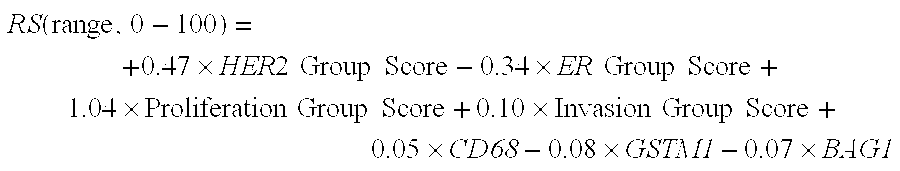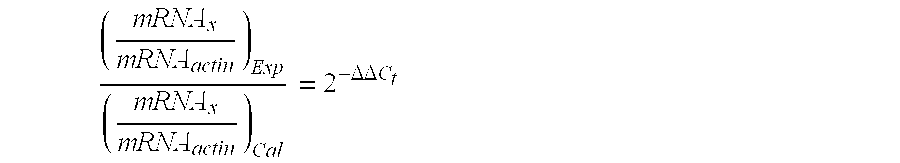Patents
Literature
395 results about "Treatment response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
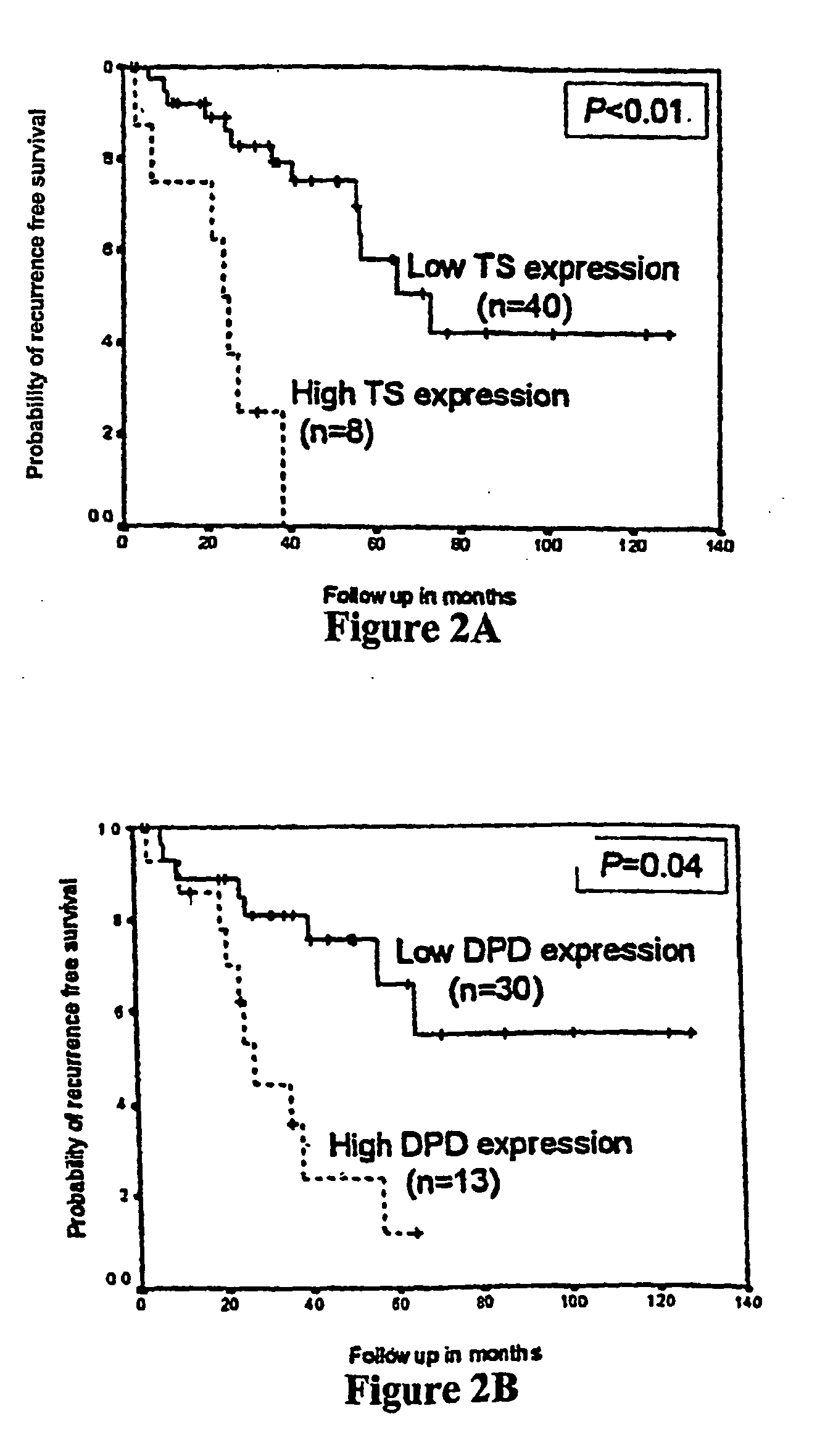
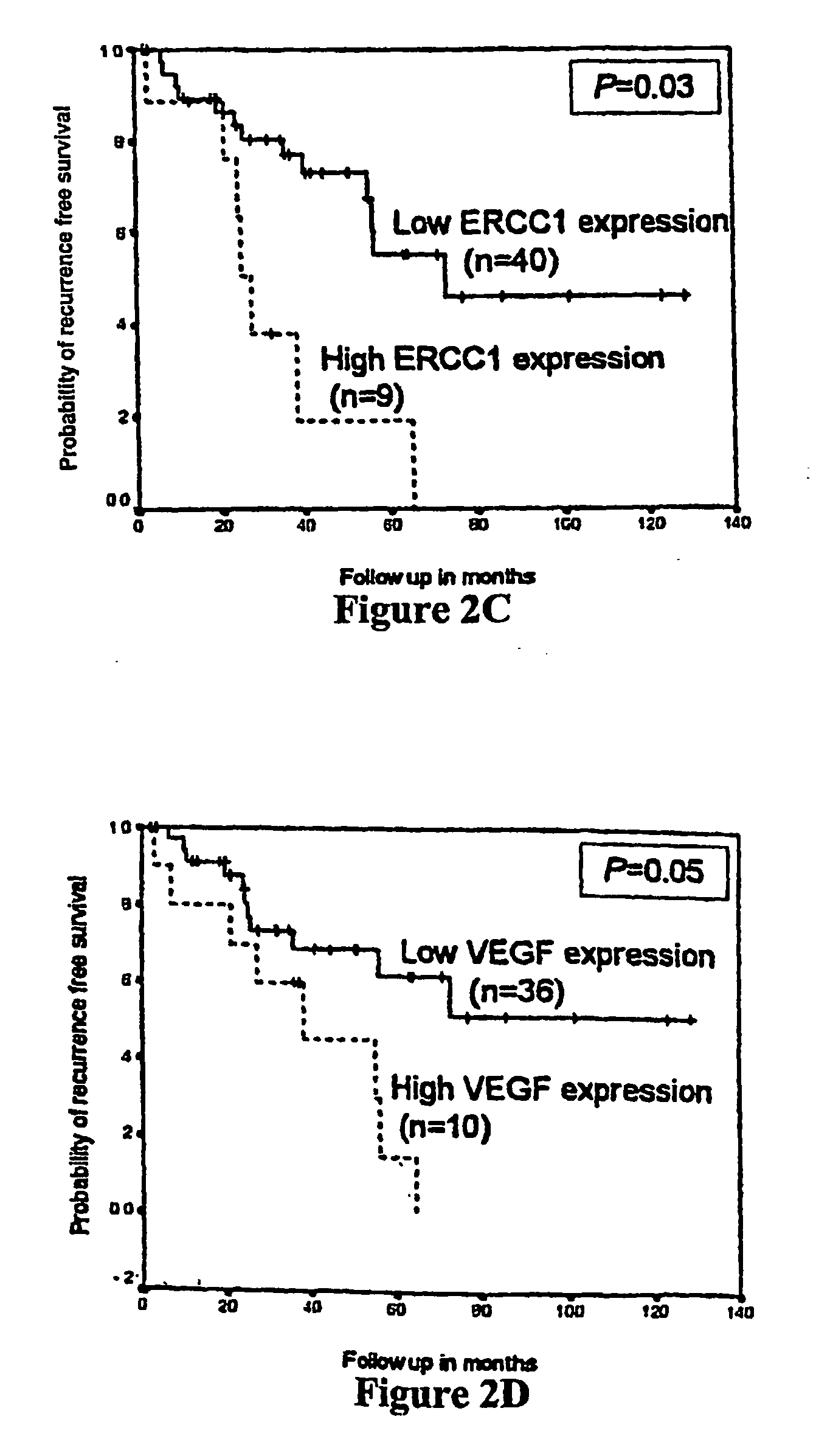
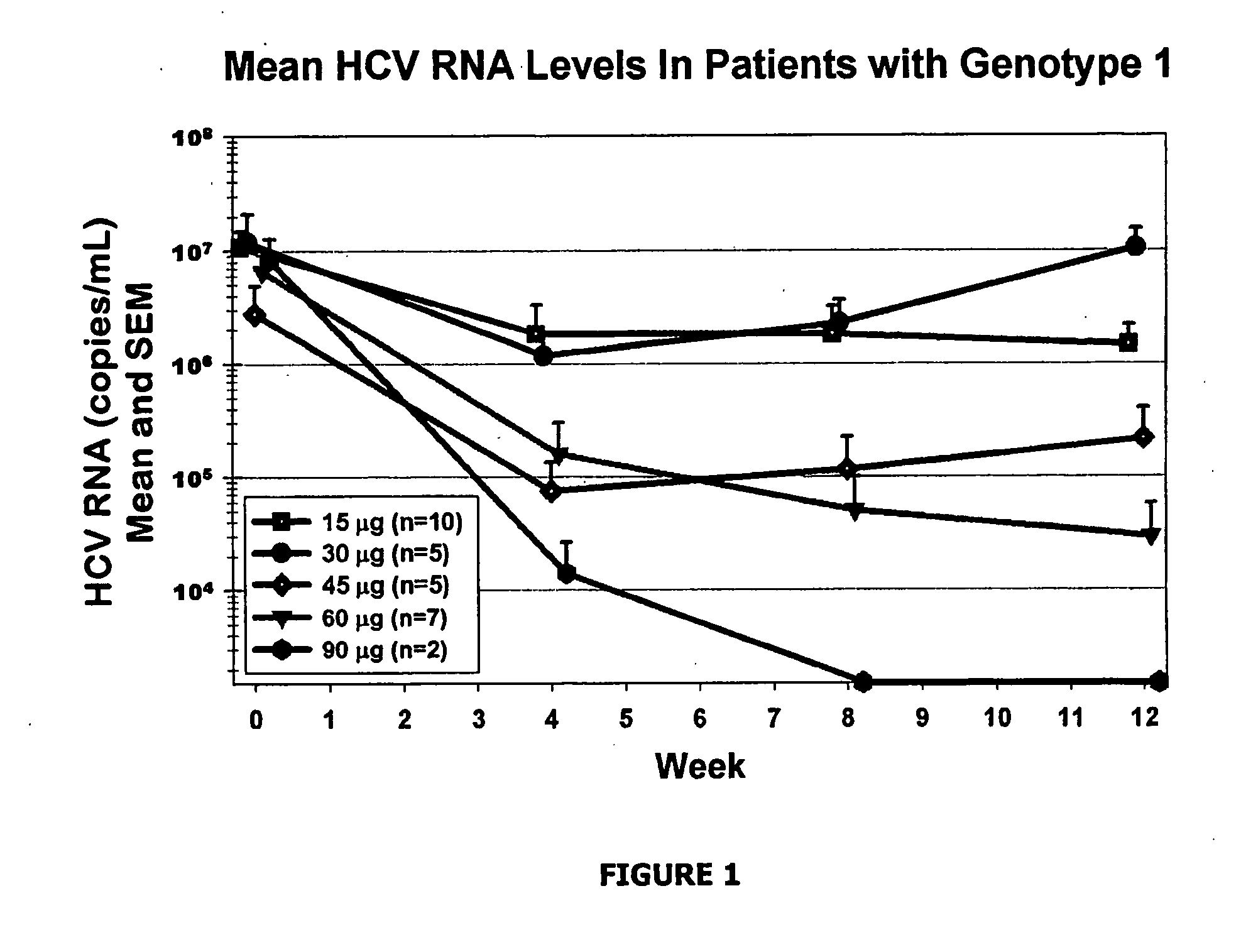
Treatment Response. The period after treatment when a WM patient has experienced either stabilization of disease, an improvement in disease status, or even, unfortunately, disease progression is called a “response”.
Methods of delivering energy to body portions to produce a therapeutic response
Owner:BOSTON SCI SCIMED INC
Method for diagnosing heart disease, predicting sudden death, and analyzing treatment response using multifractal analysis
InactiveUS6993377B2Reducing minimum sizeIncreased analytic detailMedical simulationMedical report generationTest batterySudden death
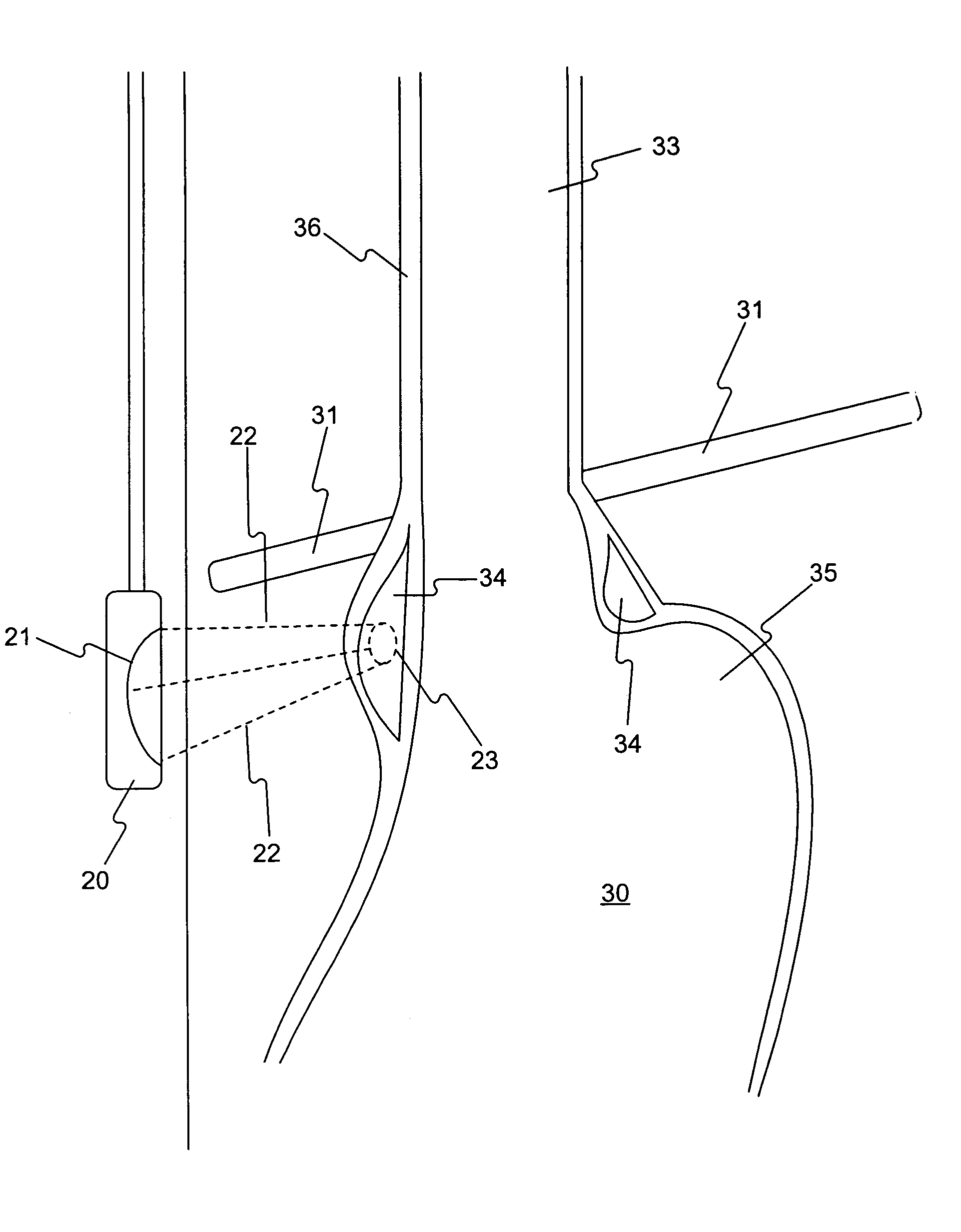
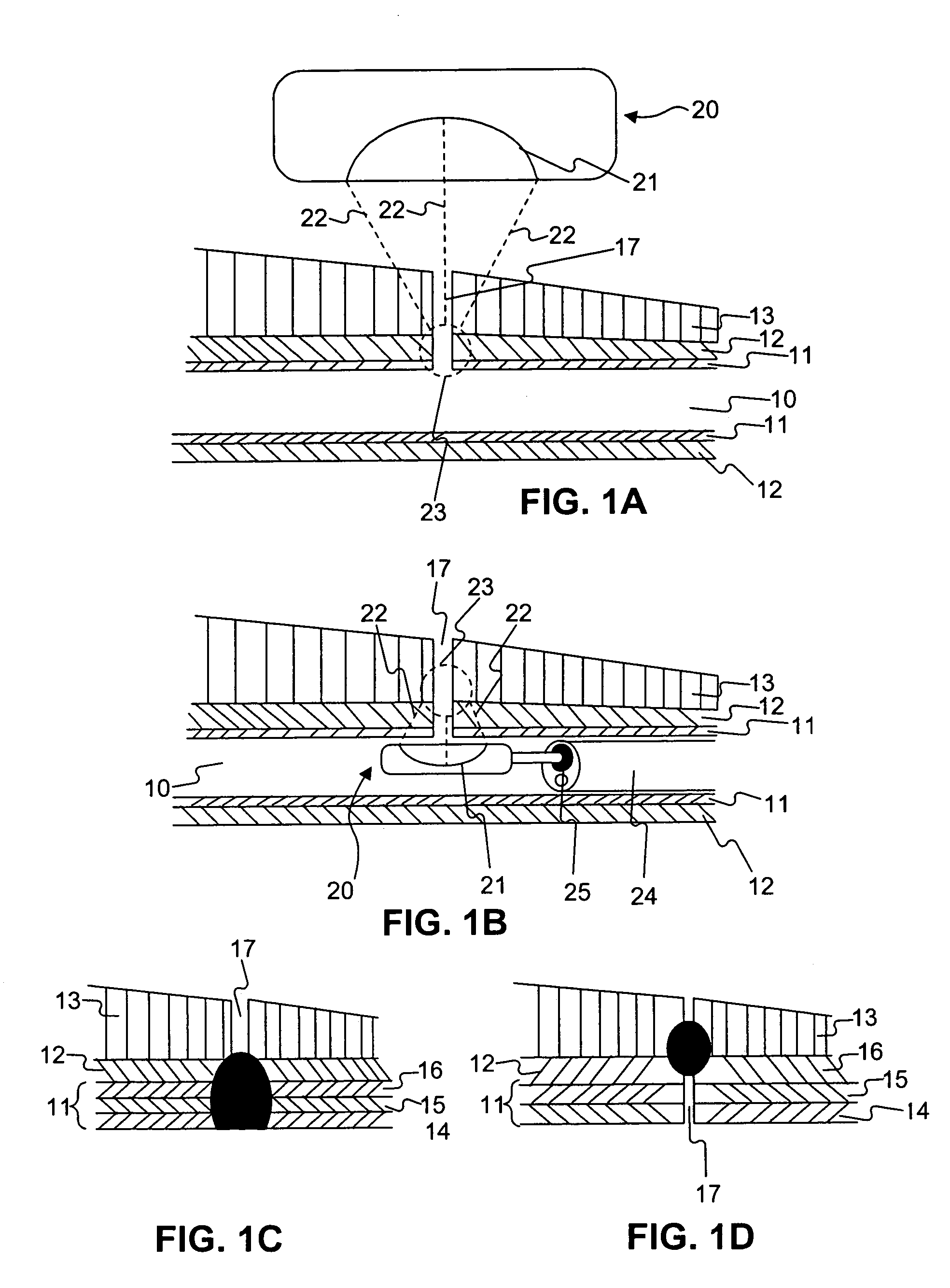
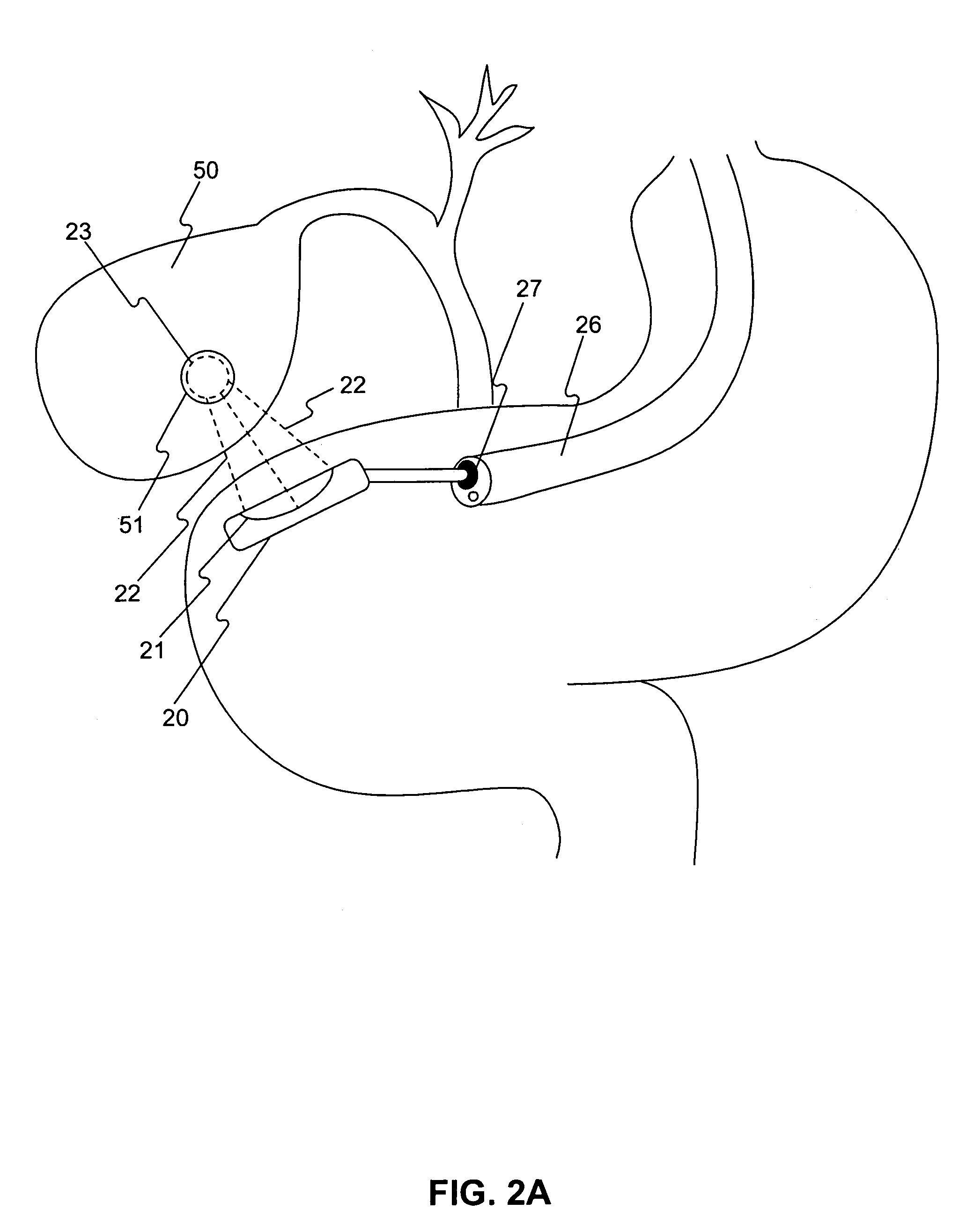
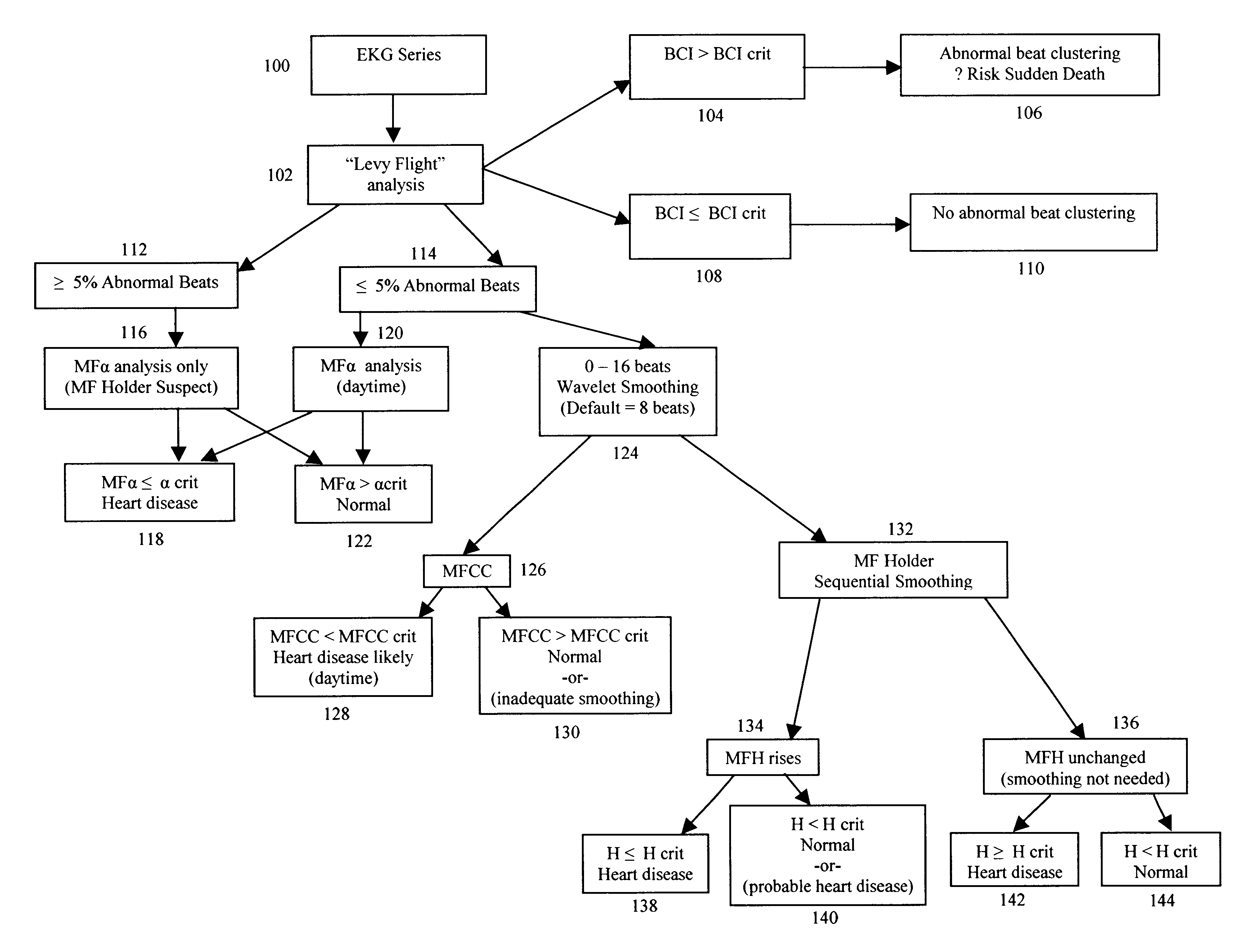
A method of analyzing electrocardiogram (EKG) data for use in the diagnosis of heart disease, prognosis of cardiac conditions, and the monitoring of heart disease therapies is disclosed. The method utilizes a wavelet-based multifractal analysis with one or more of (1) a discrete wavelet smoothing step to remove the effects of abnormal beats; (2) “Levy flight” analysis to detect the frequency of abnormal beats known to adversely affect the multifractal (MF) analysis; and (3) MF alpha analysis, a multifractal extension of monofractal short term (ST) alpha analysis. The invention further comprises an EKG test battery comprising Levy flight anomalous beat / beat cluster screening, followed by (ST) MF alpha analysis and MF Holder analysis (when validated by the Levy flight analysis). The wavelet smoothing step can also be used to classify human EKGs by observing the effect of sequential smoothing on the MF Holder coefficient. Alternative choices to the wavelet smoothing approach to removal of abnormal beat effects include probability distribution function analysis to determine the MF Holder coefficient directly, abnormal beat ridge skeleton removal to remove the offending beats based on a direct multifractal spectrum calculation, and the calculation of various types of entropy coefficients for the EKG time series.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
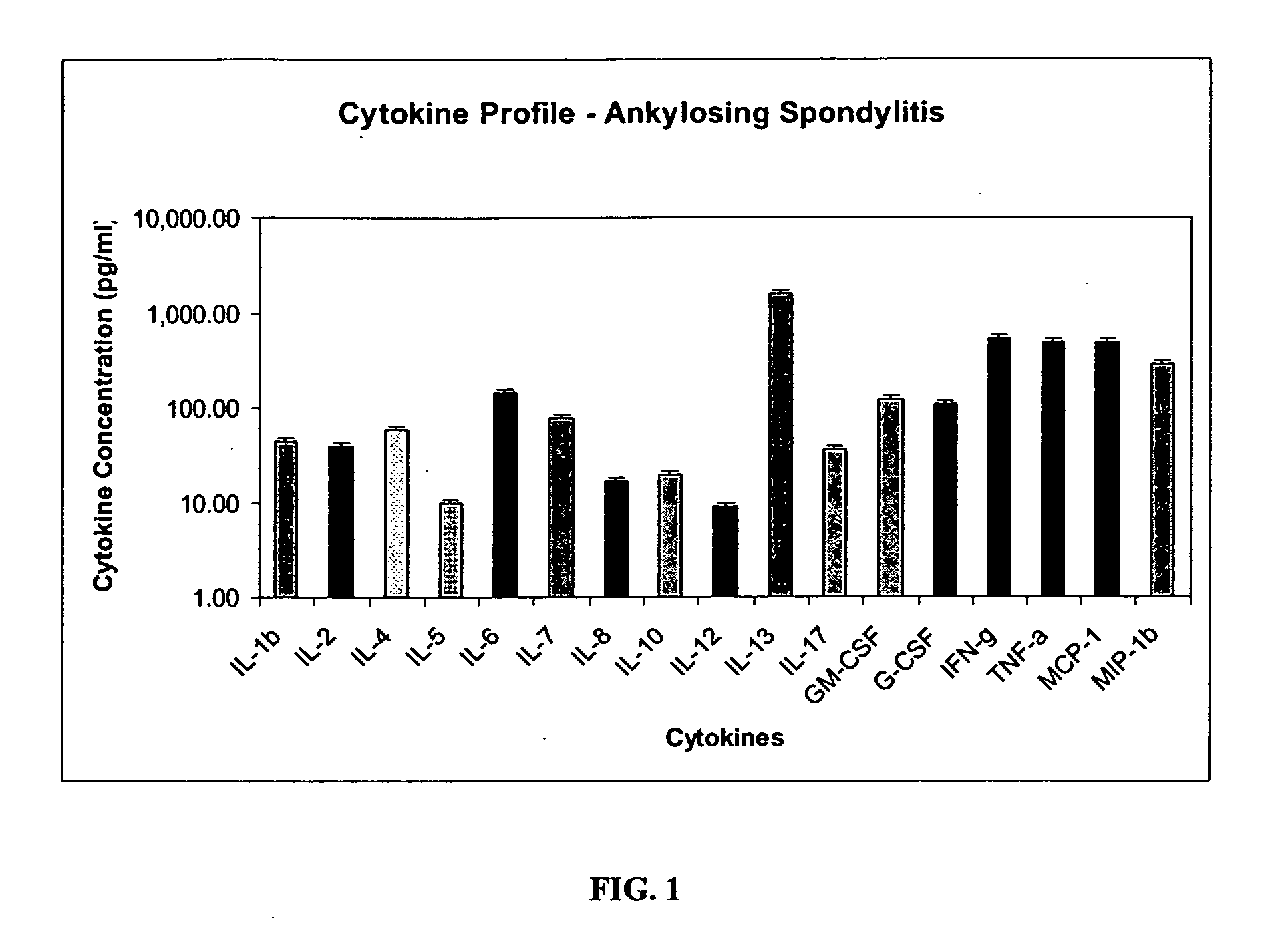
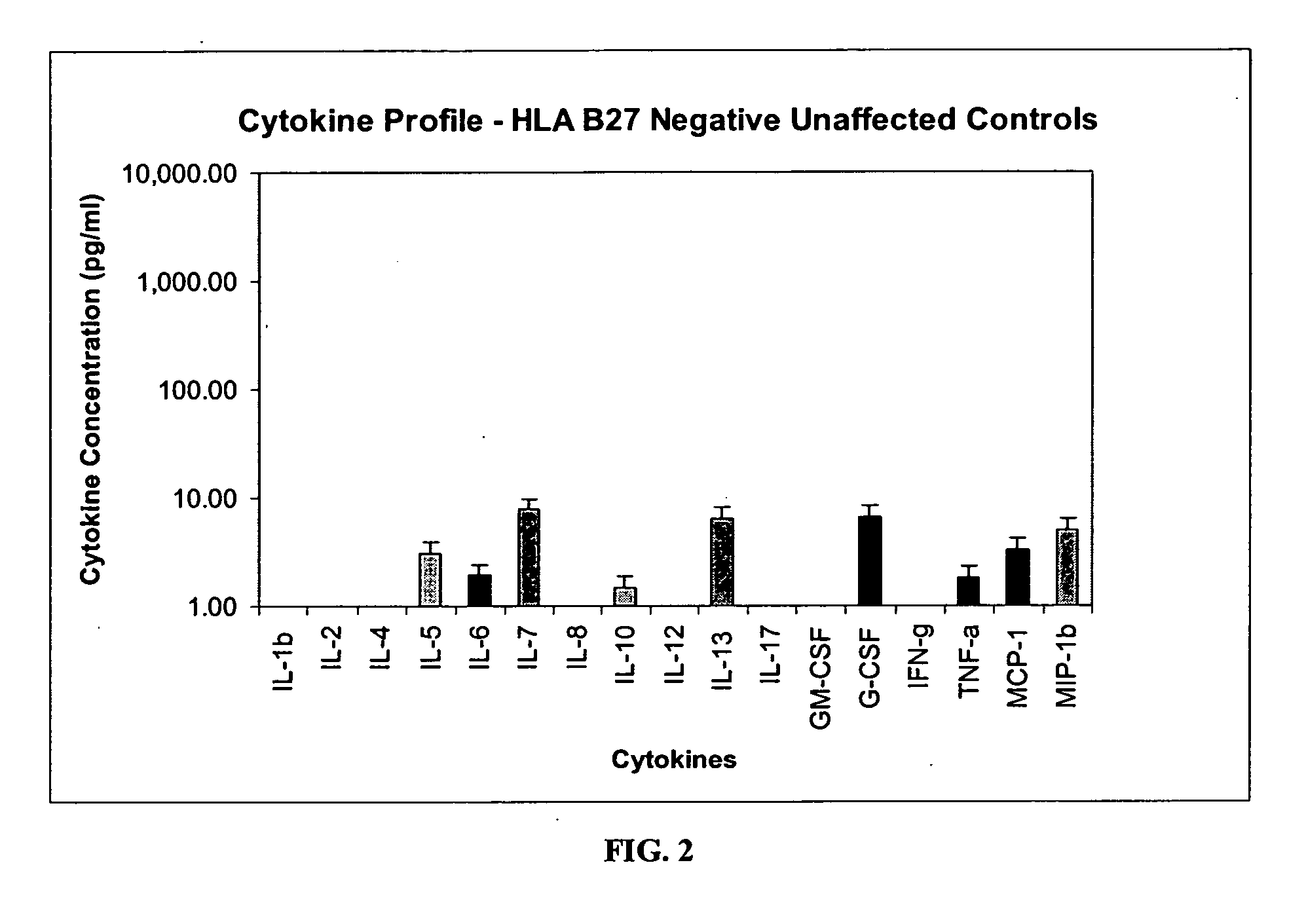
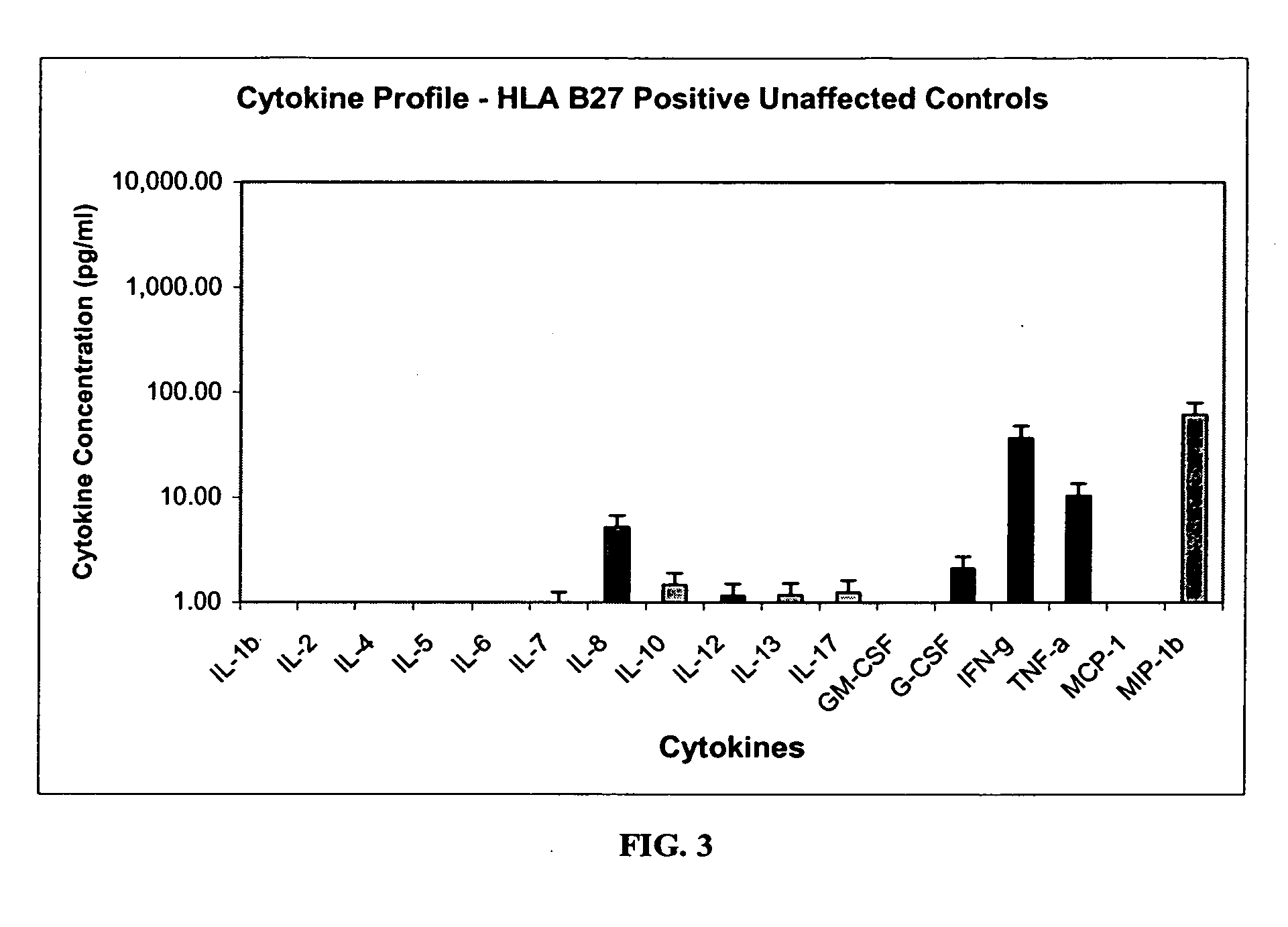
Method of using cytokine assays to diagnose treat, and evaluate inflammatory and autoimmune diseases
InactiveUS20060094056A1Snake antigen ingredientsDisease diagnosisAutoimmune conditionAutoimmune disease
The invention provides methods for diagnosing, treating, or evaluating inflammatory and autoimmune diseases by sampling peripheral blood, serum, plasma, tissue, cerebrospinal fluid, or other bodily fluids from a human subject having a suspected diagnosis. The sample is analyzed for the presence and amount of certain cytokines, which provides the diagnosis, prognosis or evaluation of therapeutic response.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA +1
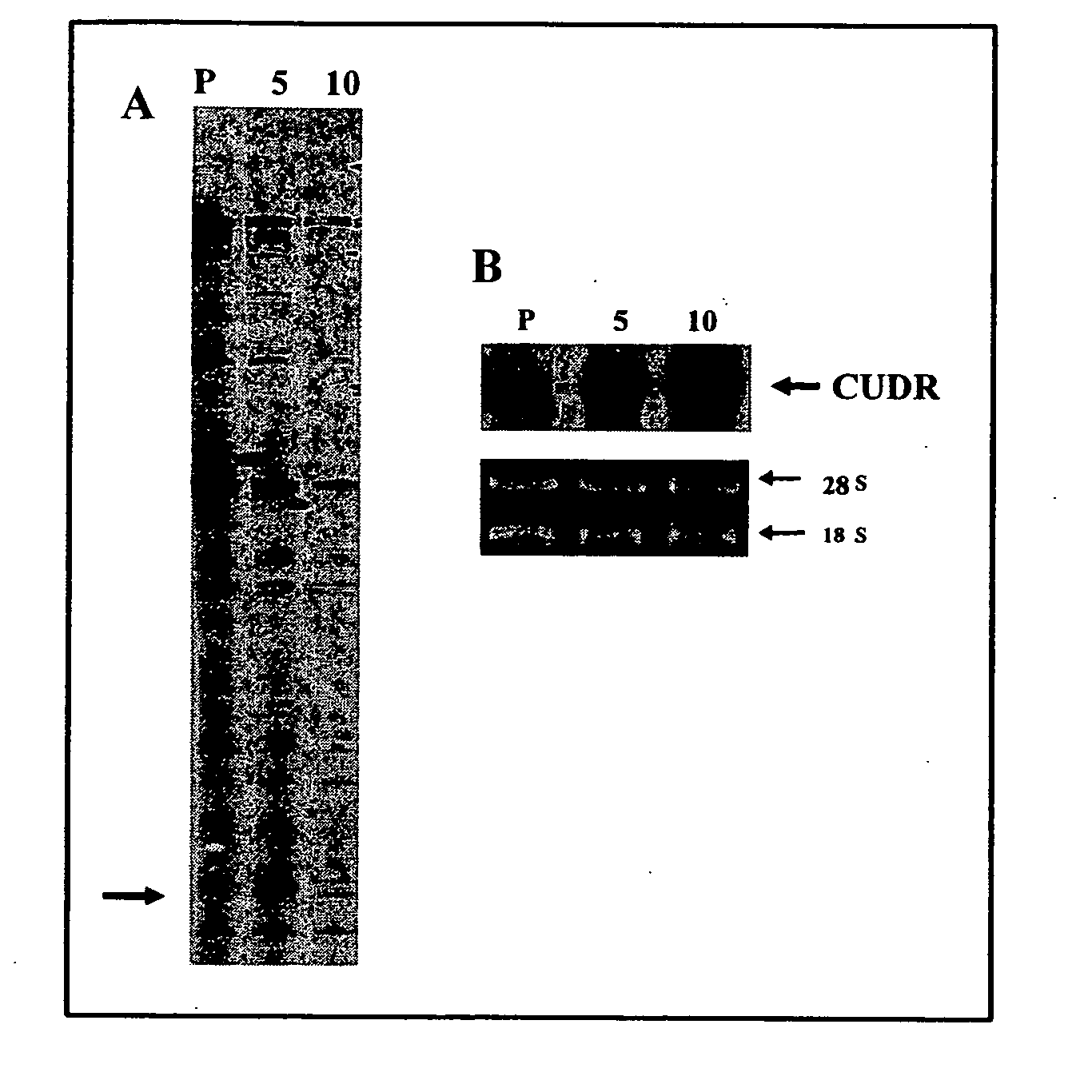
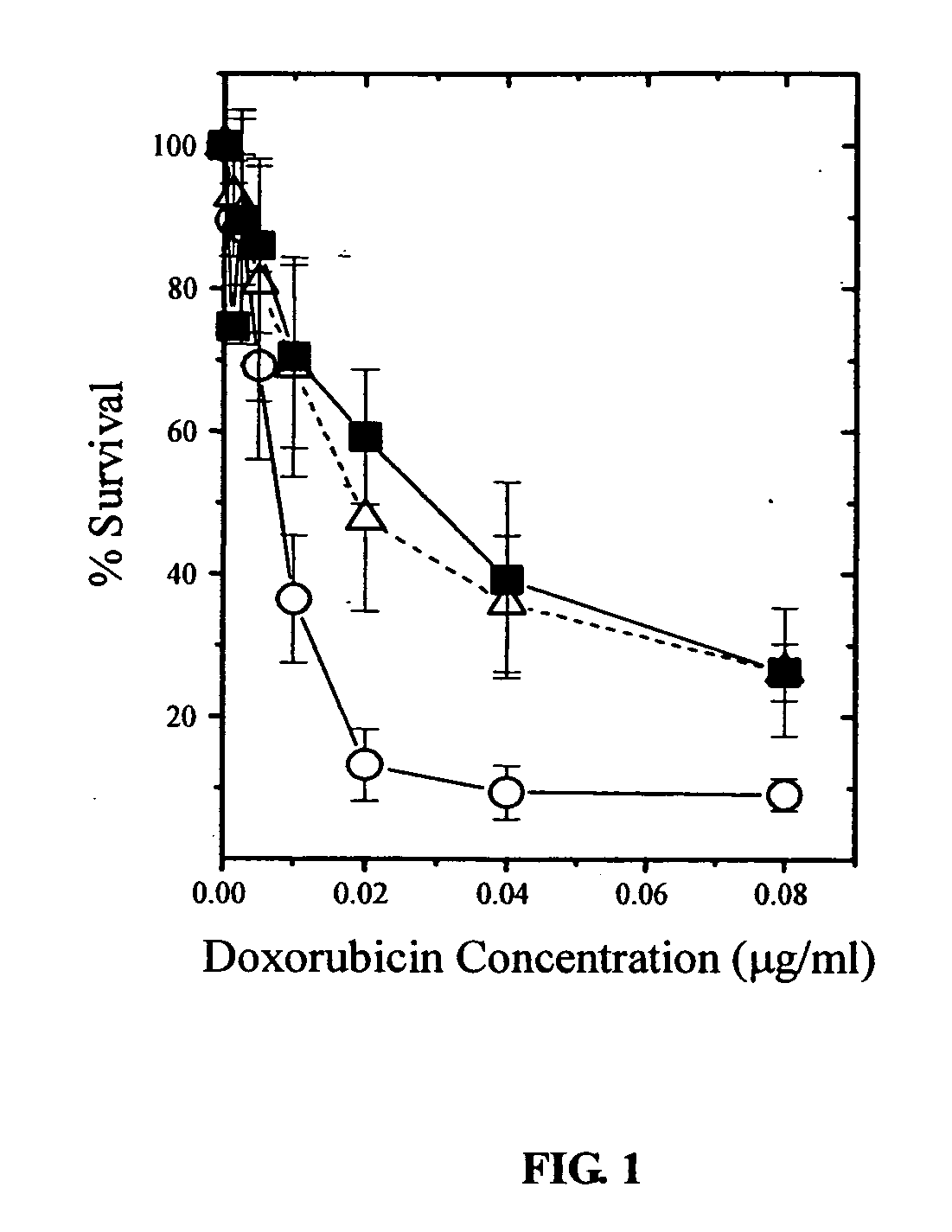
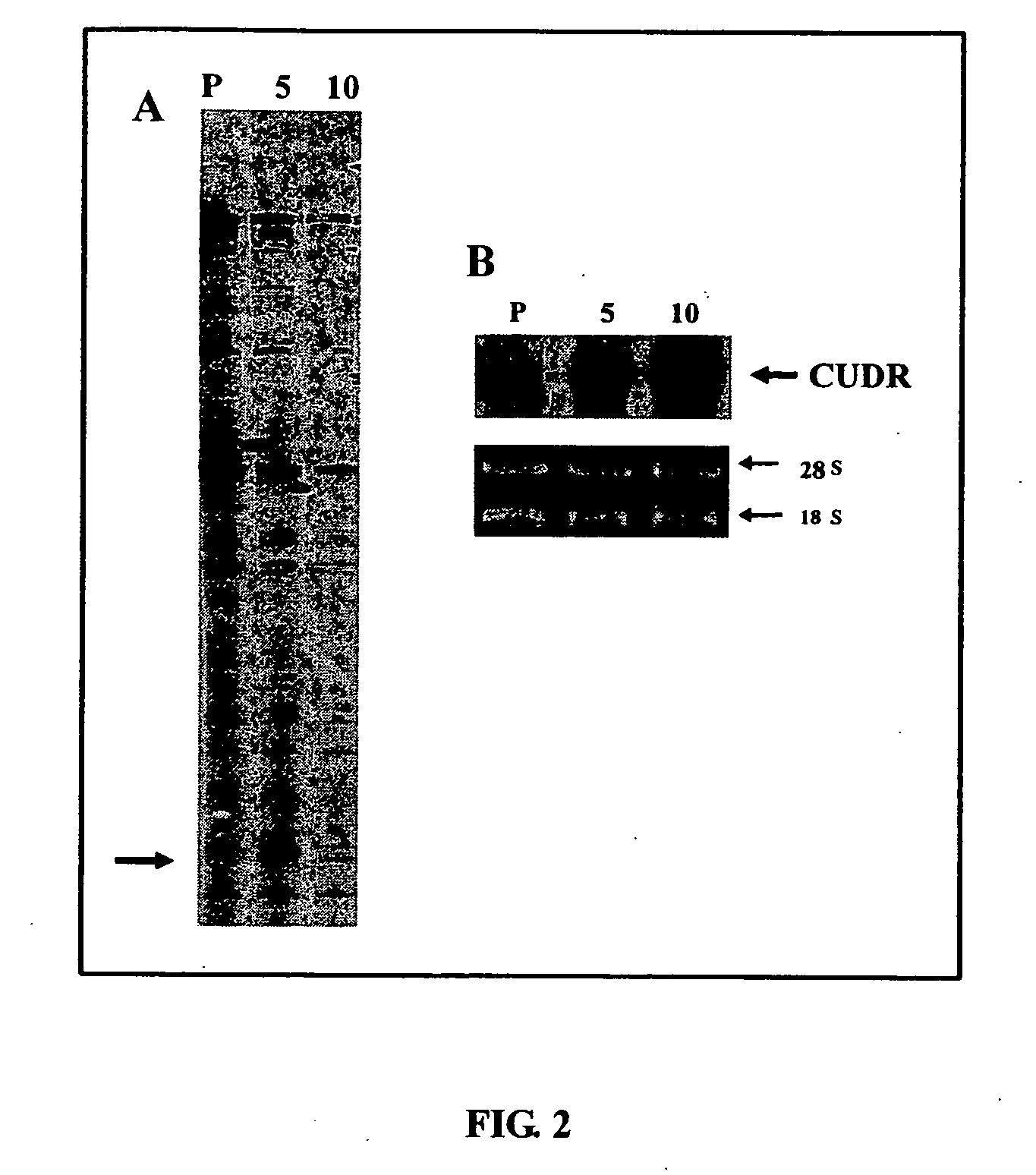
CUDR as biomarker for cancer progression and therapeutic response
InactiveUS20080044828A1Sugar derivativesMicrobiological testing/measurementHuman cancerCancer therapy
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Gene expression markers for predicting response to chemotherapy
ActiveUS20050260646A1Useful predictionSugar derivativesMicrobiological testing/measurementHepsinDHPS
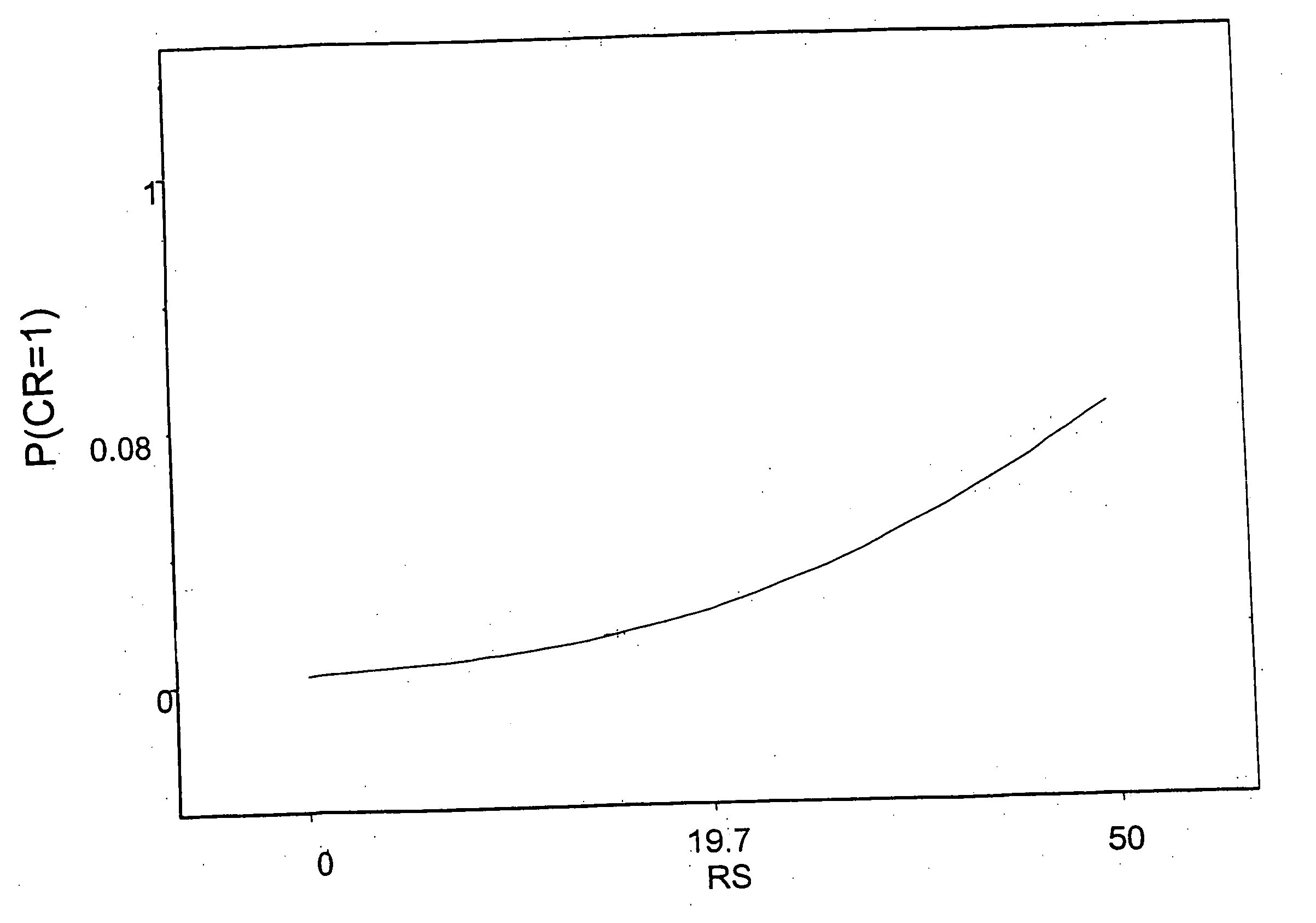
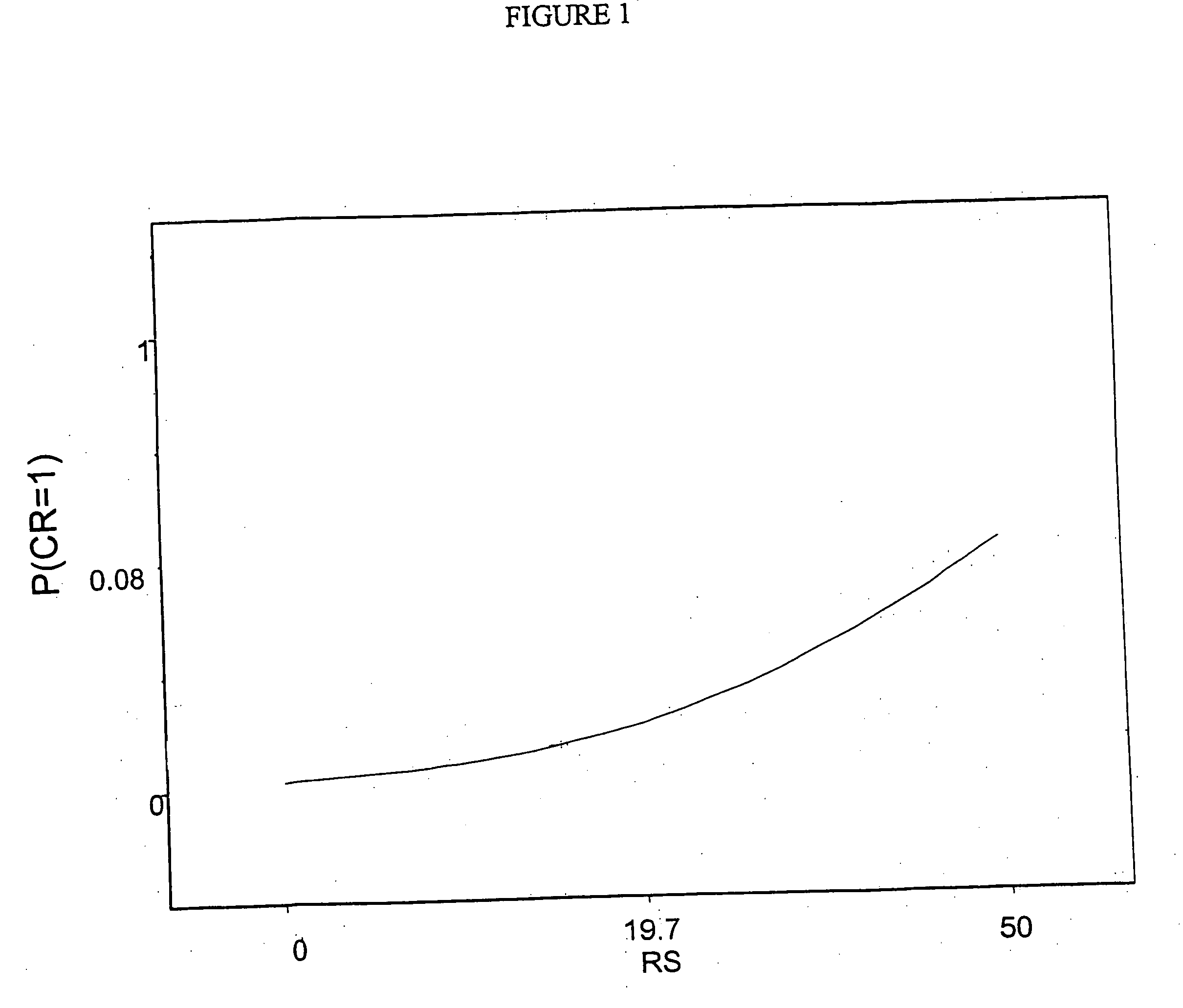
The present invention provides sets of genes the expression of which is important in the prognosis of cancer. In particular, the invention provides gene expression information useful for predicting whether cancer patients are likely to have a beneficial treatment response to chemotherapy.FHIT; MTA1; ErbB4; FUS; BBC3; IGF1R; CD9; TP53BP1; MUC1; IGFBP5; rhoC; RALBP1; STAT3; ERK1; SGCB; DHPS; MGMT; CRIP2; ErbB3; RAP1GDS1; CCND1; PRKCD; Hepsin; AK055699; ZNF38; SEMA3F; COL1A1; BAG1; AKT1; COL1A2; Wnt.5a; PTPD1; RAB6C; GSTM1, BCL2, ESR1; or the corresponding expression product, is determined, said report includes a prediction that said subject has a decreased likelihood of response to chemotherapy.
Owner:GENOMIC HEALTH INC +1
Gene expression markers for predicting response to chemotherapy
InactiveUS20050064455A1Reduce the possibilityDecreased likelihood of responseMicrobiological testing/measurementAntineoplastic agentsCancer researchTreatment response
The invention provides sets of genes the expression of which predicts whether cancer patients are likely to have a beneficial treatment response to chemotherapy.
Owner:GENOMIC HEALTH INC
Method to optimize drug selection, dosing and evaluation and to help predict therapeutic response and toxicity from immunosuppressant therapy
InactiveUS20060253263A1Good curative effectMinimizing toxic side effectMicrobiological testing/measurementProteomicsMaintenance therapyAutoimmune condition
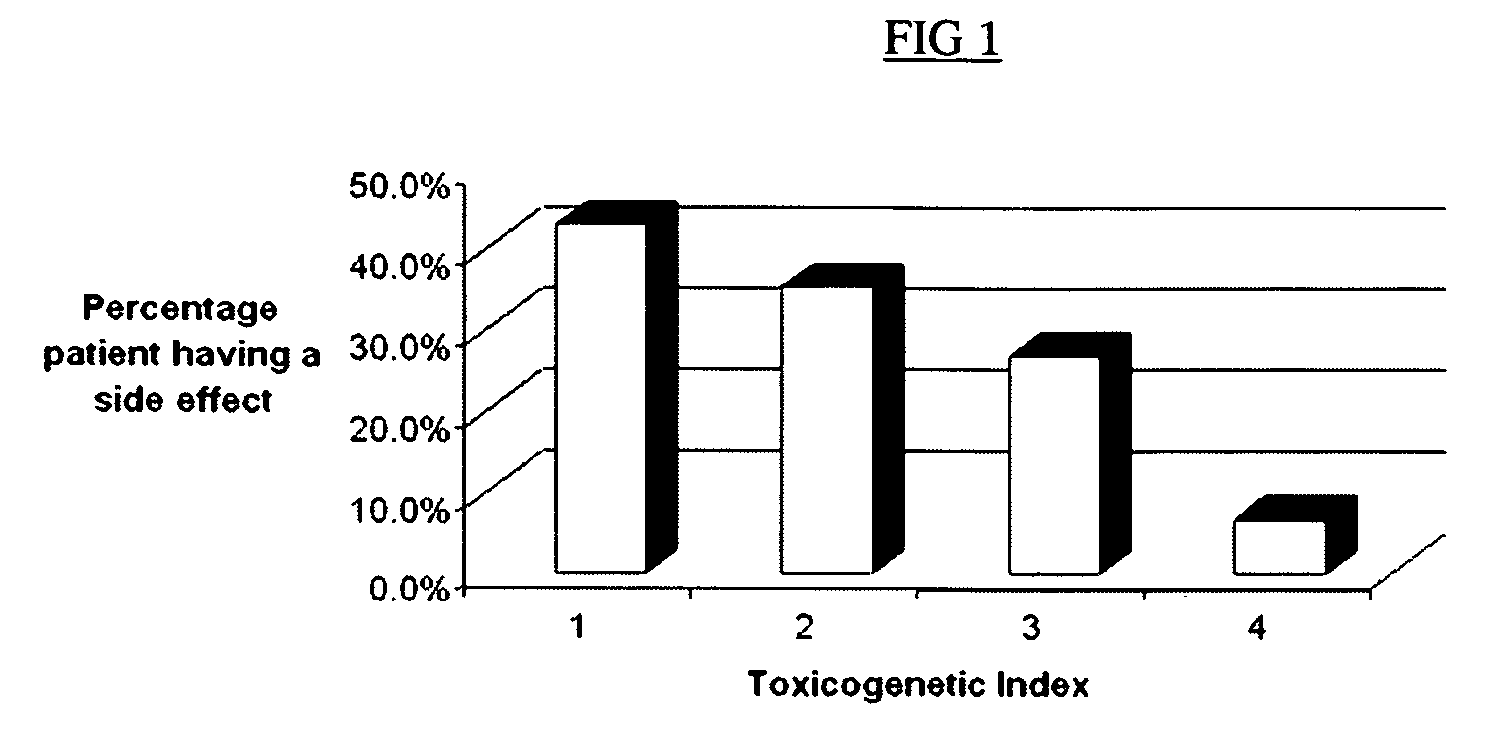
The present invention provides a method of effectively measuring risk for therapeutic toxicity of a subject having an autoimmune disorder or cancer and predicting and evaluating therapeutic efficacy of immunosuppressive therapies for autoimmune diseases and cancers before or after starting therapy. The present invention also provides for determining a drug metabolite level of a subject during therapy and measuring periodically the drug metabolite level of a subject on maintenance therapy to ensure treatment compliance and continued therapeutic response by measuring minimal clinical important differences (MCID) in the drug metabolite levels. The present invention also provides for a method to effectively optimize the selection and dose of immunosuppressive therapies of a subject having an autoimmune disease or cancer to improve therapeutic efficacy and reduce therapeutic toxicity prior to starting concomitant biologic therapy and before or after the subject has failed to respond to the at least one immunosuppressive agent.
Owner:MESHKIN BRIAN JAVAADE
Tape stripping methods for analysis of skin disease and pathological skin state
ActiveUS7183057B2Less traumaticEfficient methodBioreactor/fermenter combinationsBiological substance pretreatmentsCuticleAdhesive
The present invention provides non-invasive methods for detecting, monitoring, and diagnosing skin disease and pathological skin states such as irritated skin and psoriasis. The methods include using tape stripping to analyze expression in epidermal samples, of one or more skin markers. In illustrative examples, the tape stripping is performed using pliable tape that has a rubber adhesive. Furthermore, the present invention provides methods for predicting and monitoring response to therapy for a skin disease, such as psoriasis or dermatitis. Finally, the methods can include the use of a microarray.
Owner:DERMTECH INT
Biomarkers of cyclin-dependent kinase modulation
InactiveUS20070105114A1Modulate cdk activityMicrobiological testing/measurementBiological material analysisMammalCancer research
Biomarkers having expression patterns that correlate with a response of cells to treatment with one or more cdk modulating agents, and uses thereof. Also provided are methods for testing or predicting whether a mammal will respond therapeutically to a method of treating cancer that comprises administering an agent that modulates cdk activity.
Owner:BRISTOL MYERS SQUIBB CO
Tape stripping methods for analysis of skin disease and pathological skin state
ActiveUS20050221334A1Less traumaticEfficient methodBioreactor/fermenter combinationsBiological substance pretreatmentsAdhesiveNon invasive
The present invention provides non-invasive methods for detecting, monitoring, and diagnosing skin disease and pathological skin states such as irritated skin and psoriasis. The methods include using tape stripping to analyze expression in epidermal samples, of one or more skin markers. In illustrative examples, the tape stripping is performed using pliable tape that has a rubber adhesive. Furthermore, the present invention provides methods for predicting and monitoring response to therapy for a skin disease, such as psoriasis or dermatitis. Finally, the methods can include the use of a micro array.
Owner:DERMTECH INT
Genetic markers for predicting disease and treatment outcome
InactiveUS20060115827A1Reduce and delay recurrenceProlong survival timeBiocideMicrobiological testing/measurementNucleic Acid ProbesIncreased risk
The invention provides compositions and methods for determining the increased risk for recurrence of certain cancers and the likelihood of successful treatment with one or both of chemotherapy and radiation therapy. The methods comprise determining the type of genomic polymorphism present in a predetermined region of the gene of interest isolated from the subject or patient. Also provided are nucleic acid probes and kits for determining a patient's cancer risk and treatment response.
Owner:UNIV OF SOUTHERN CALIFORNIA
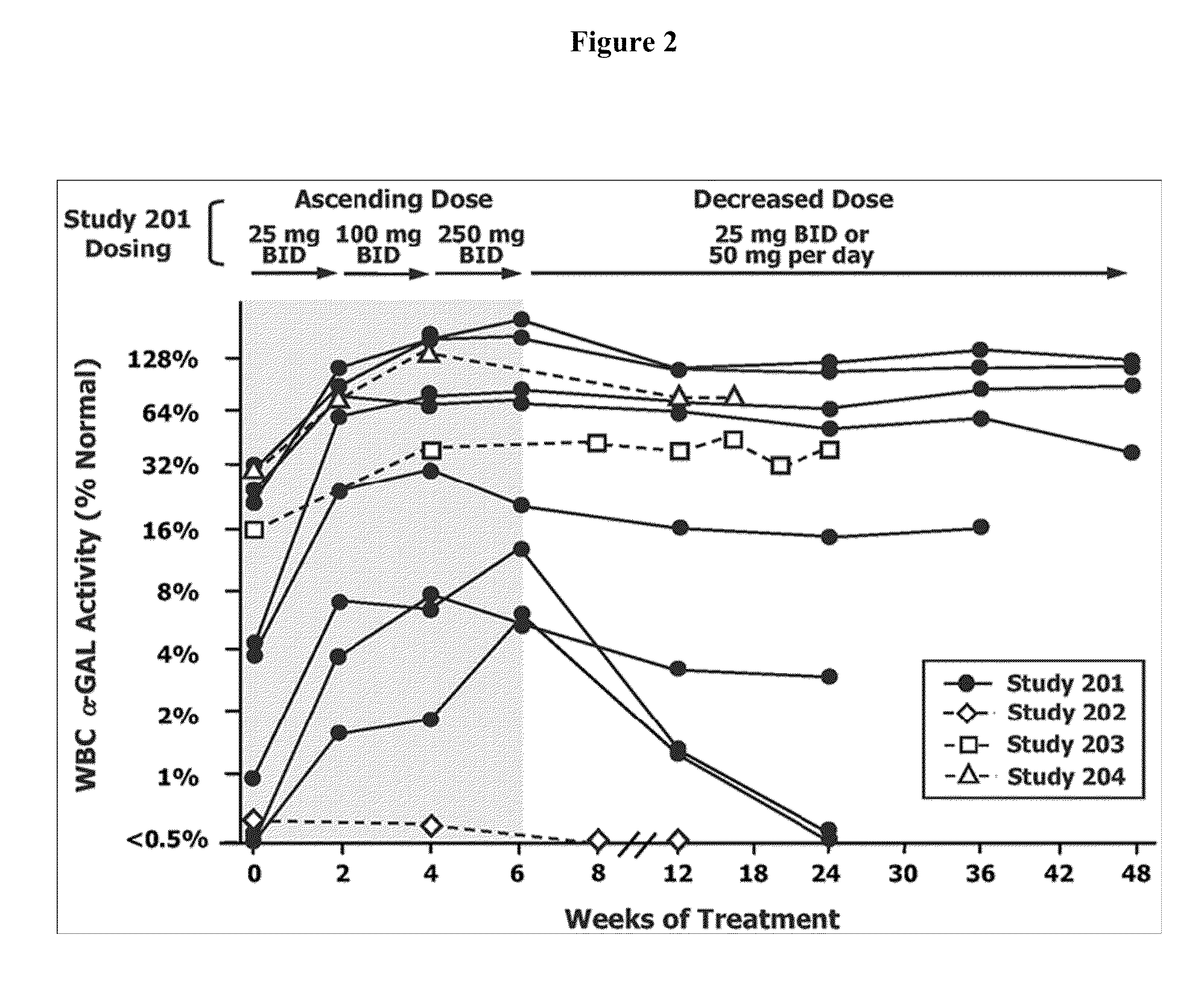
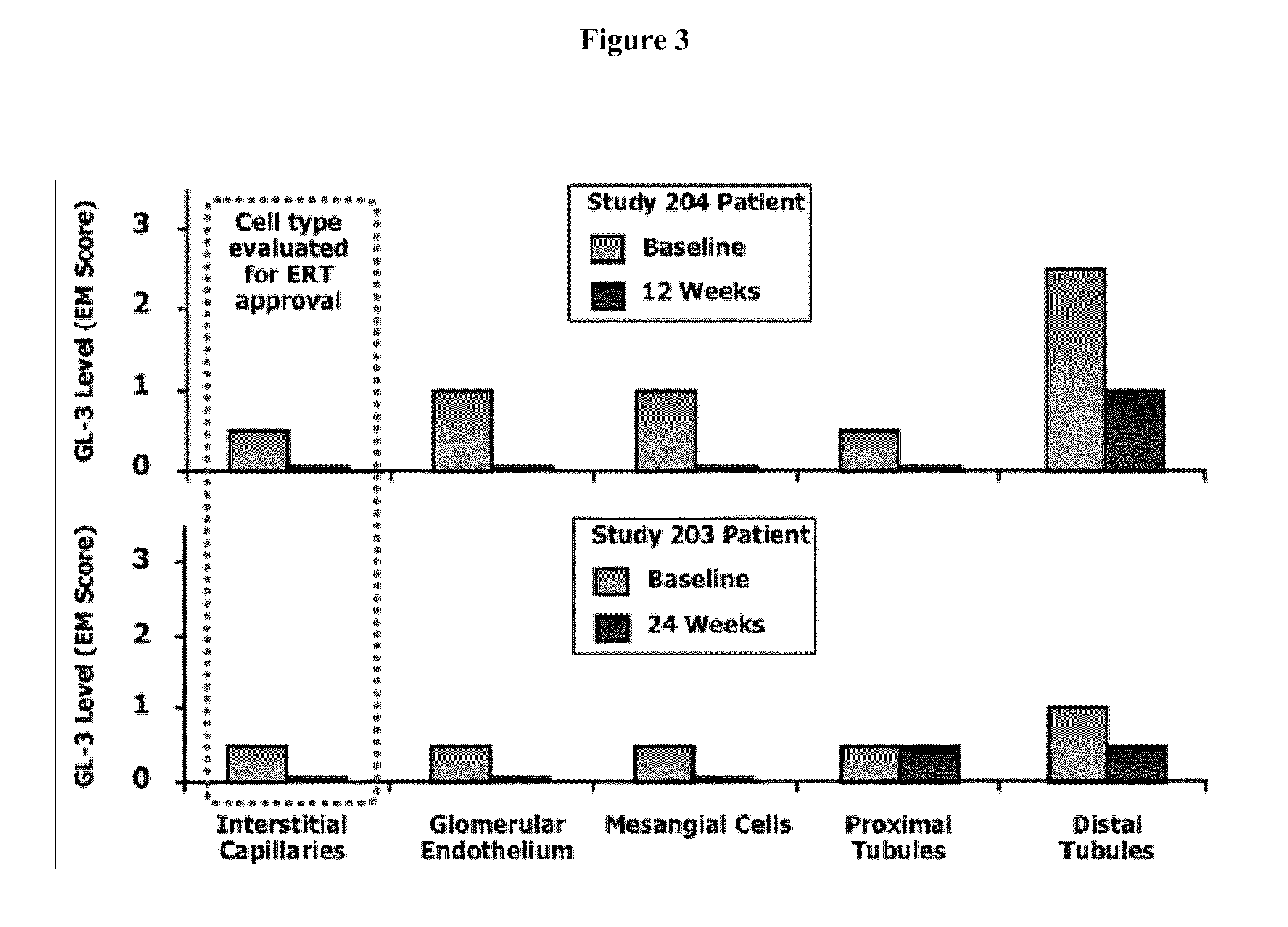
Method for the treatment of fabry disease using pharmacological chaperones
InactiveUS20100113517A1High activityHigh protein activityBiocidePeptide/protein ingredientsPharmacometricsCytoplasmic staining
The present invention provides a method treating a patient with Fabry disease by determining whether there is an improvement of a surrogate marker that is associated with Fabry disease following administration of a specific pharmacological chaperone of α-galactosidase A. The method includes administering an effective amount of 1-deoxygalactonojirimycn to the individual, wherein the 1-deoxygalactonojirimycin binds to alpha-galactosidase A in an amount effective to increase activity of the alpha-galactosidase A. The present invention also provides a method for monitoring and increasing a therapeutic response of a patient with Fabry disease following administration of a specific pharmacological chaperone of α-galactosidase A by evaluating the effect on the cytoplasmic staining pattern of a cell from the patient, wherein detection of a staining pattern in the cell that is similar to the staining pattern in a cell from a healthy individual indicates that the individual with Fabry disease is a responder.
Owner:AMICUS THERAPEUTICS INC
Fabrication of Microfilters and Nanofilters and Their Applications
ActiveUS20120183946A1Bioreactor/fermenter combinationsSolvent extractionReactive-ion etchingDisease progression
Micro- and nanofilters with precision pore sizes and pore layout have applications in many fields including capturing circulating tumor cells and fetal cells in blood, water treatment, pathogen detection in water, etc. Methods to fabricate micro- and nanofilters not using track etching or reactive ion etching are provided, allowing easy fabrication of single layer or stack of films simultaneously, and / or stack of films on rolls. Microfilter can be made using one or more layers of material. Invention enables mass production of microfilters with lithographic quality at low cost. Isolation, enumeration and characterization of circulating tumor cells using microfilters provides (i) guides to cancer treatment selection and personalize dosage, (ii) low cost monitoring for treatment response, disease progression and recurrence, (iii) assessment of pharmacodynamic effects, (iv) information on mechanisms of resistance to therapy, and (v) cancer staging. Microfabrication methods are also applicable to fabrication of any free standing patterned polymeric films.
Owner:CREATV MICROTECH
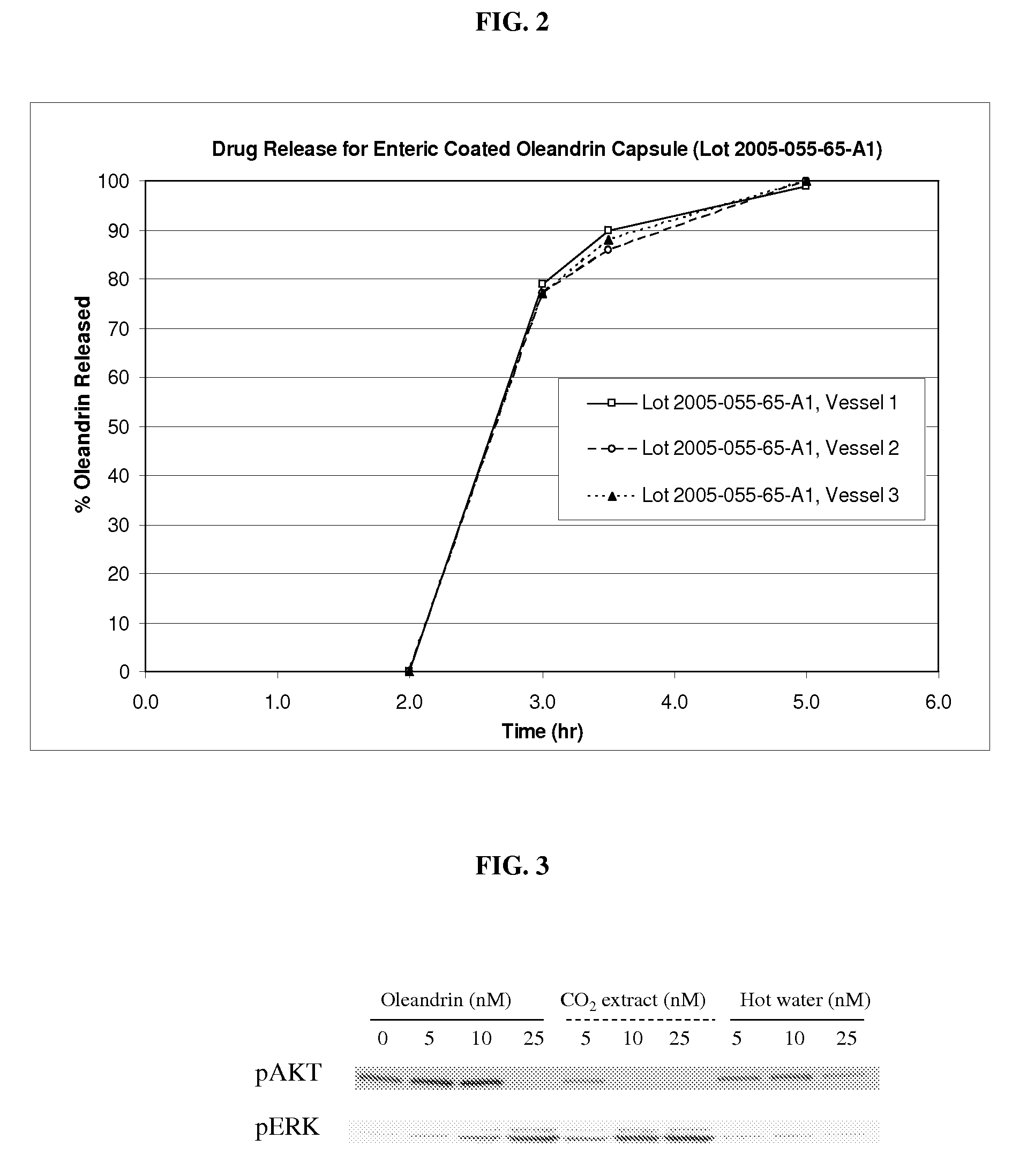
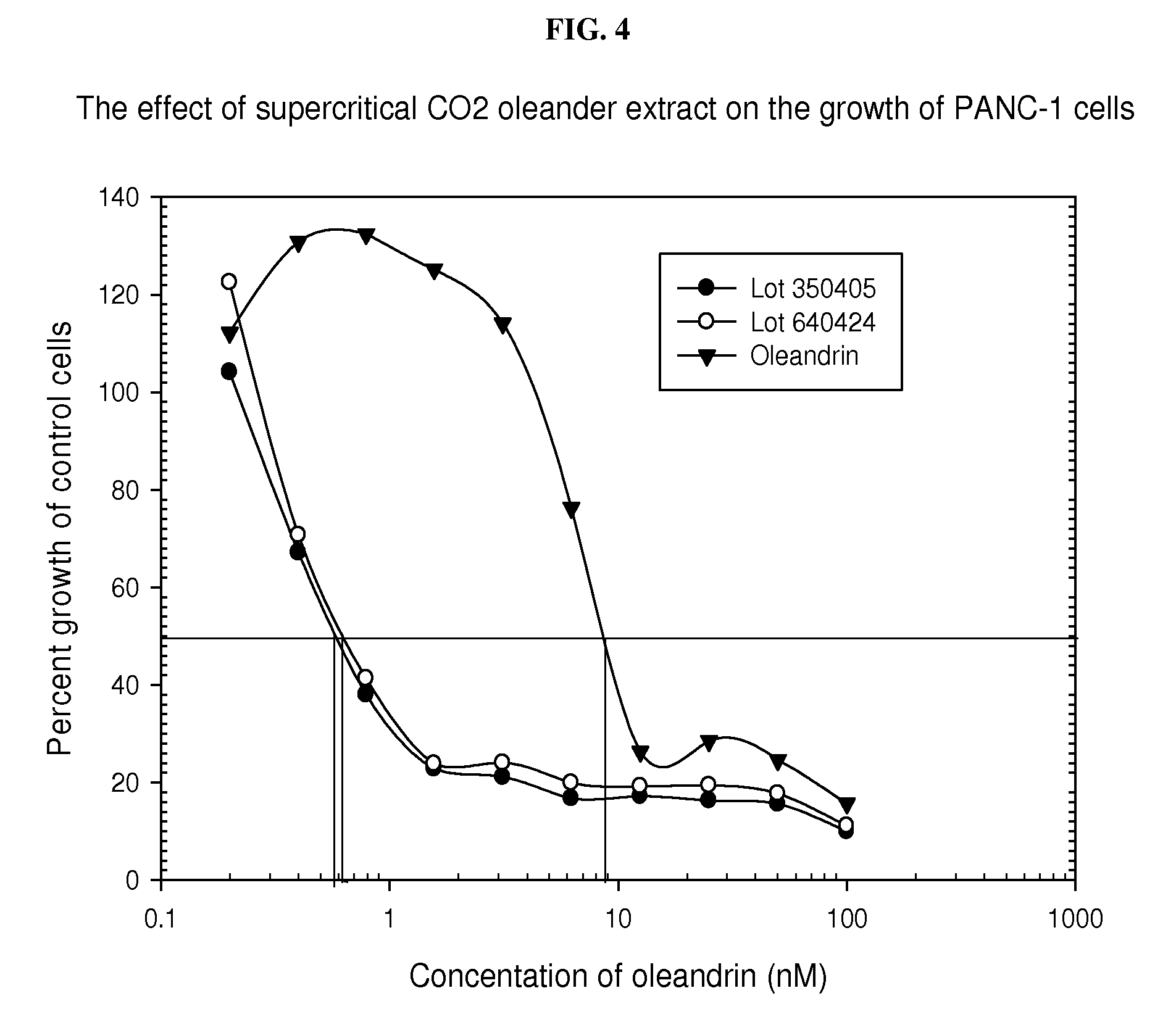
SCF extract containing cardiac glycoside
A supercritical fluid (SCF) extract of a cardiac glycoside-containing plant mass is provided. The extract can be included in a pharmaceutical composition containing an extract-solubilizing amount of solubilizer. Oleandrin is included within the extract when a cardiac glycoside-containing plant, such as Nerium oleander, is extracted by SCF extraction. The extract can also contain one or more other SCF extractable pharmacologically active agents. The composition can be used to treat a wide range of disorders that are therapeutically responsive to a cardiac glycoside.
Owner:PHOENIX BIOTECH INC +1
Means and methods for the prediction of treatment response of a cancer patient
ActiveUS20130330325A1Enhance their cancer-fighting capacityIncrease capacityMicrobiological testing/measurementDisease diagnosisSurgeryTreatment response
The present invention relates to the field of treatment efficacy prediction in patients with malignant diseases. More precisely, this invention relates to the prediction of the efficacy of a treatment in cancer patients, based on the precise quantification of several biological markers that are related to the innate and adaptive immune response of said patient against said cancer.
Owner:GRABE NIELS +3
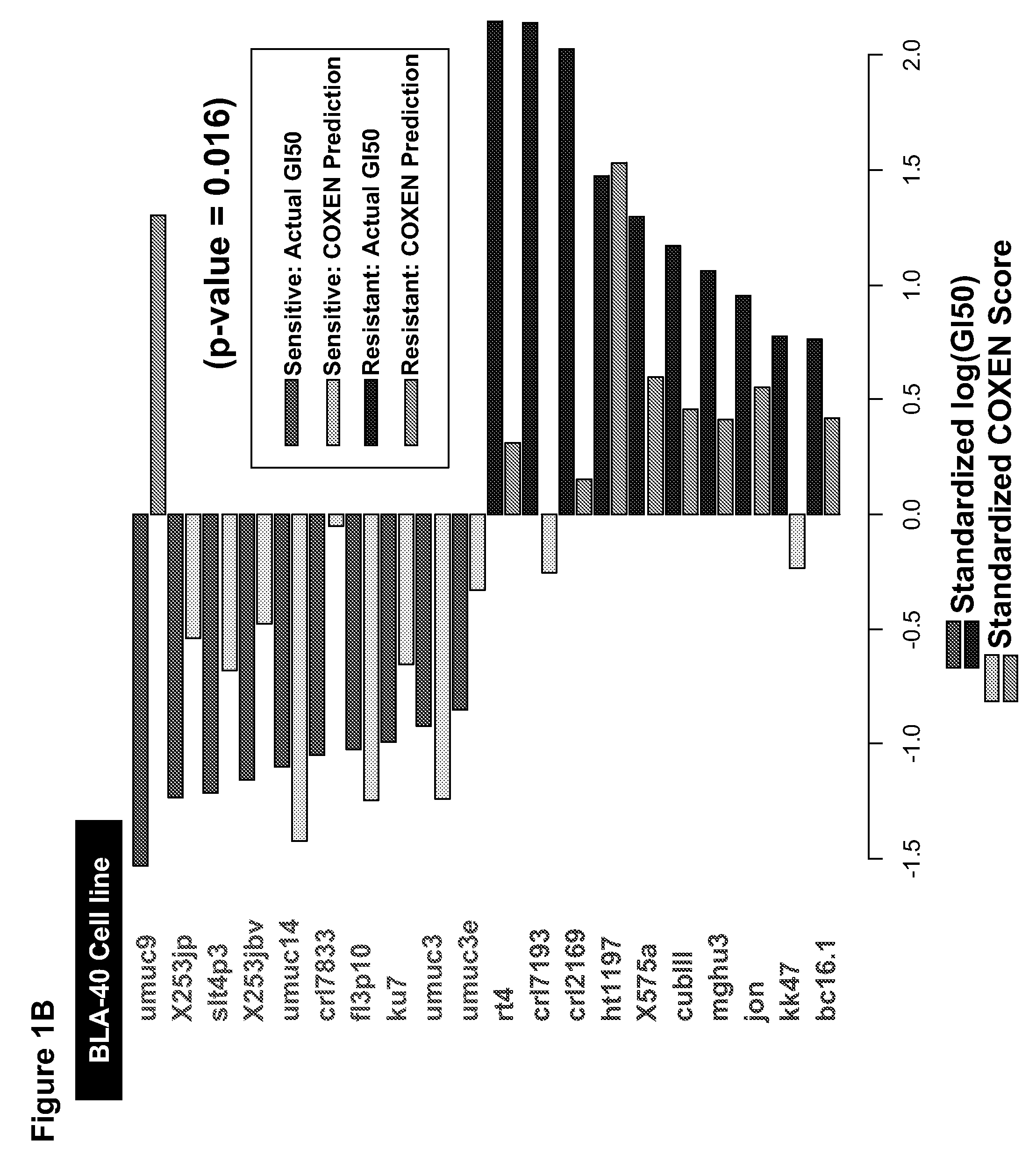
Prediction of an agent's or agents' activity across different cells and tissue types
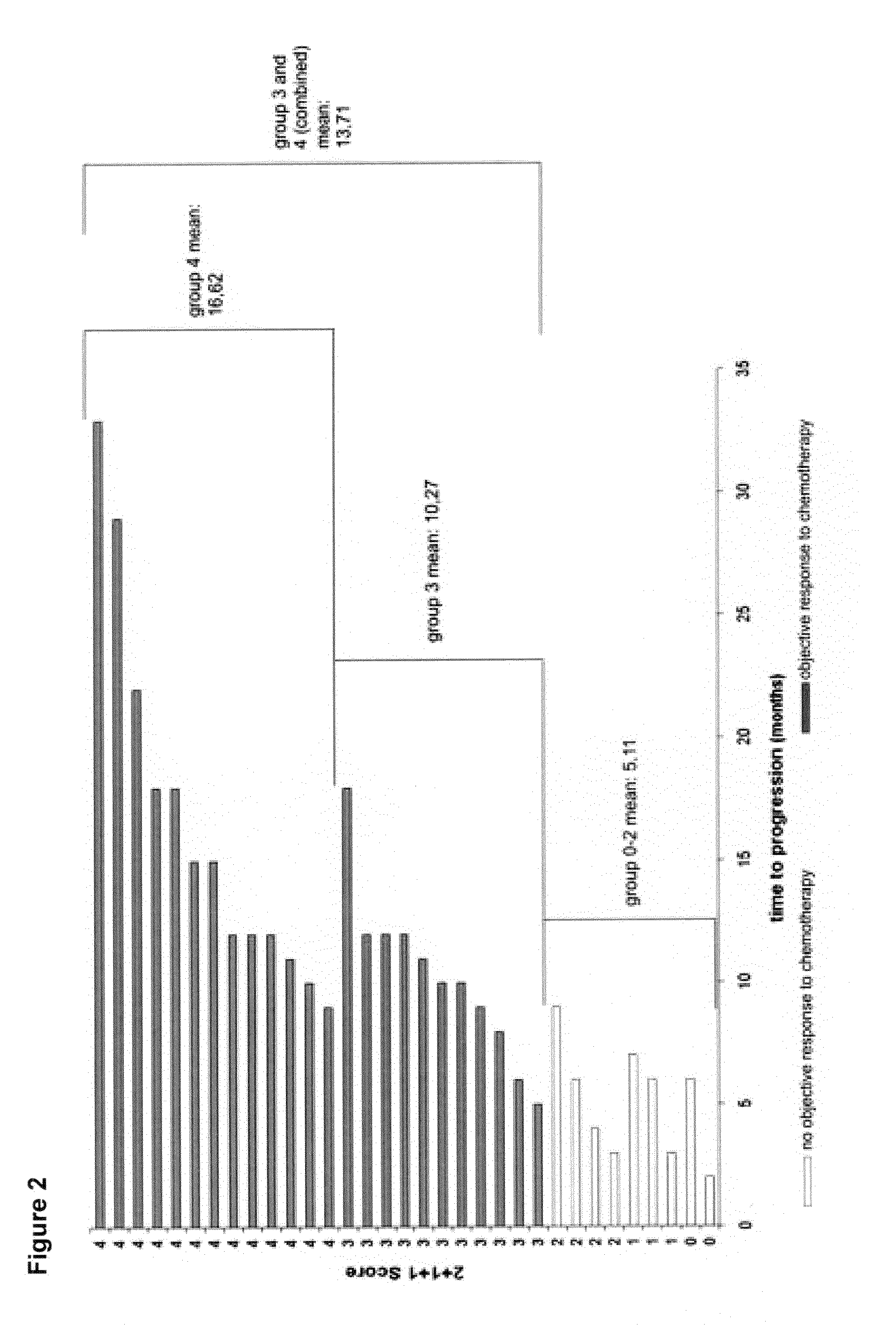
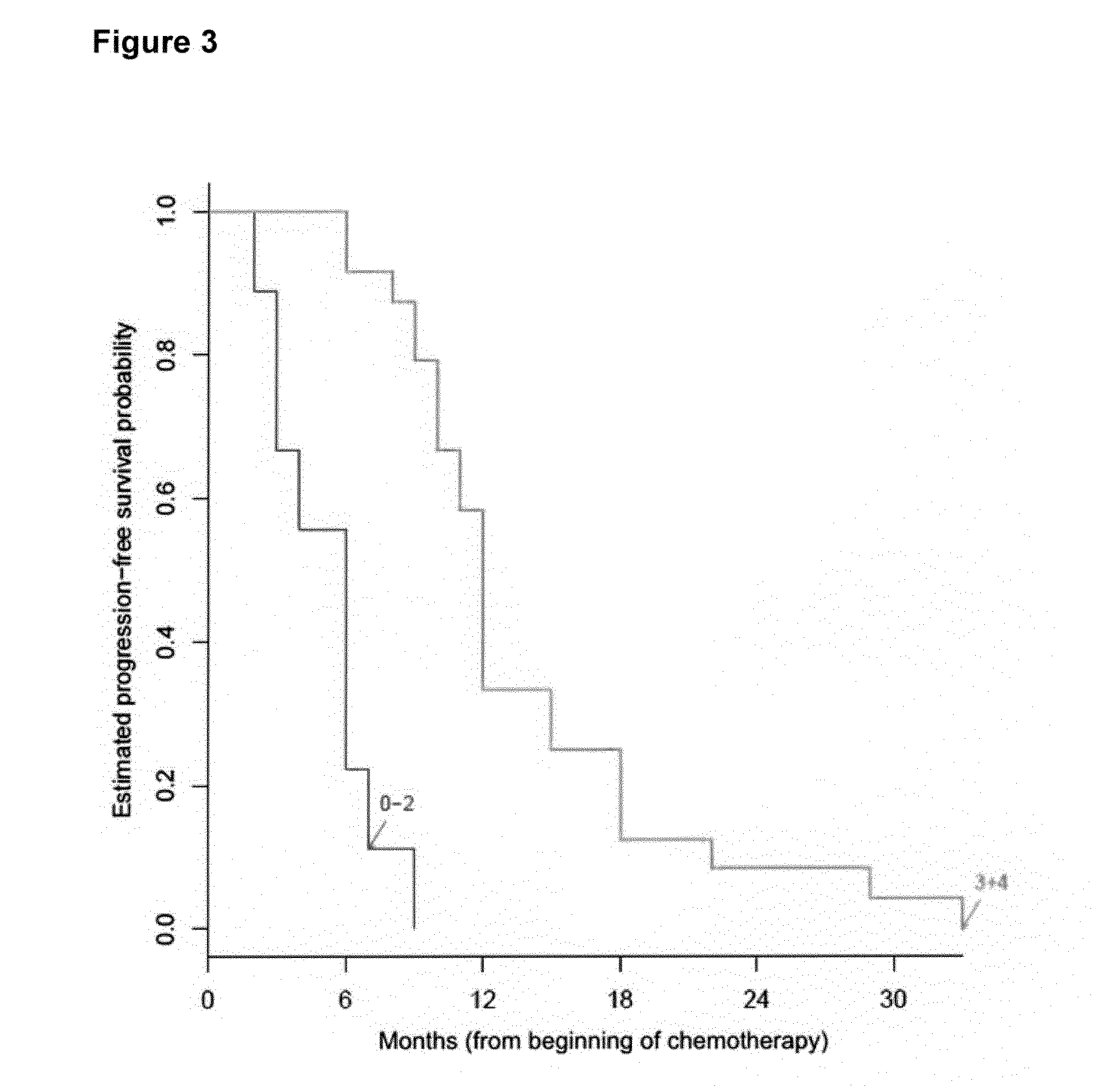
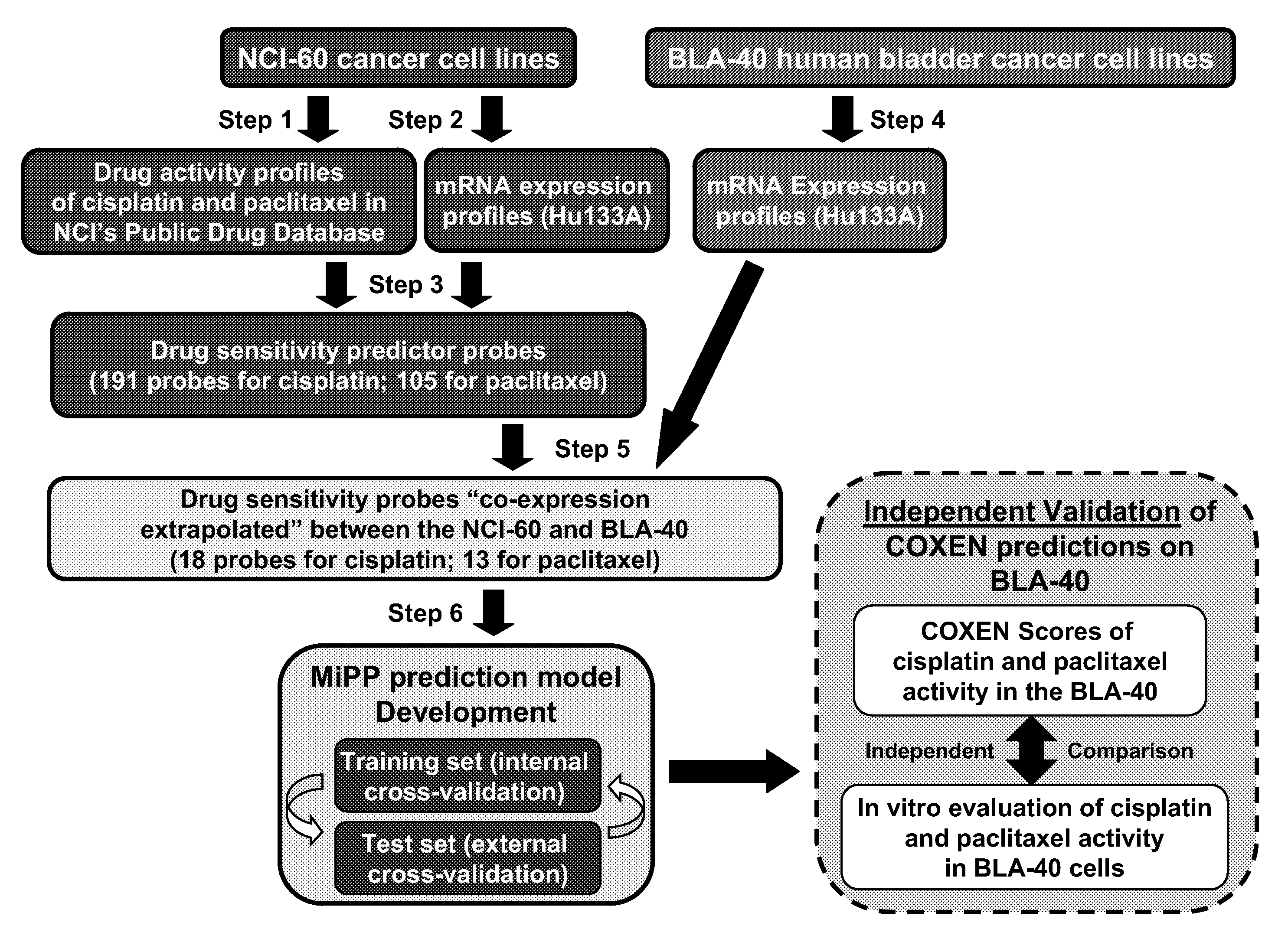
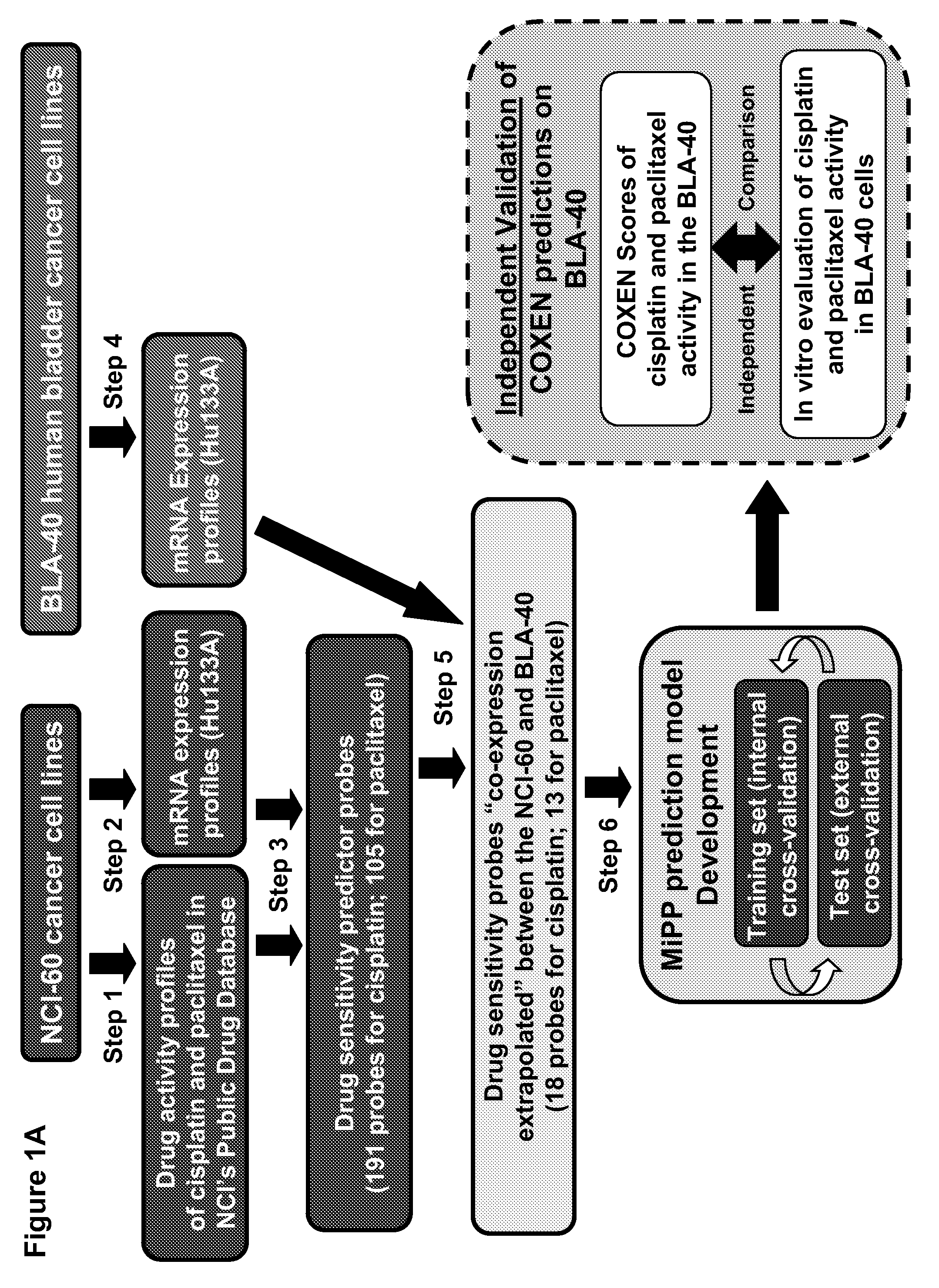
The present invention relates to a novel algorithm that uses molecular profile signatures to extrapolate the physiological processes of one type of cell set (e.g., cell line, tissue, normal or diseased) to predict the activity of an agent or agents against another type of cell set that has never been exposed to the agent in question (drug efficacy prediction). The novel algorithm also allows one to predict the therapeutic response of a patient to a therapeutic regimen even though the patient (or patients) may have never been exposed to that agent before, thereby allowing for selecting a therapeutic agent or combination of agents that would best suit the patient (i.e., personalized medicine). The present invention also relates to methods of using the agents identified by the novel algorithm to treat a variety of diseases, including cancer.
Owner:THEODORESCU DAN +1
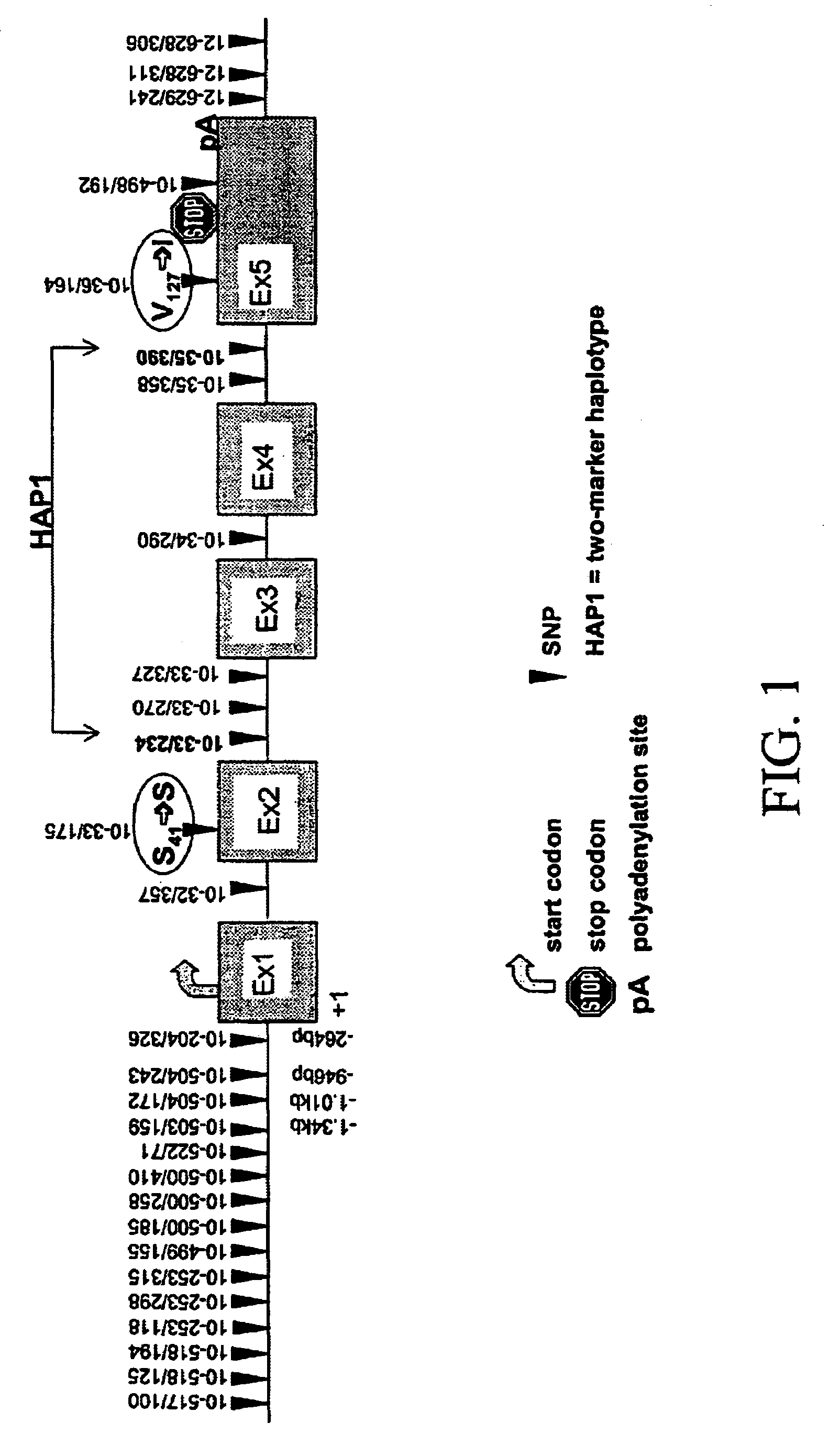
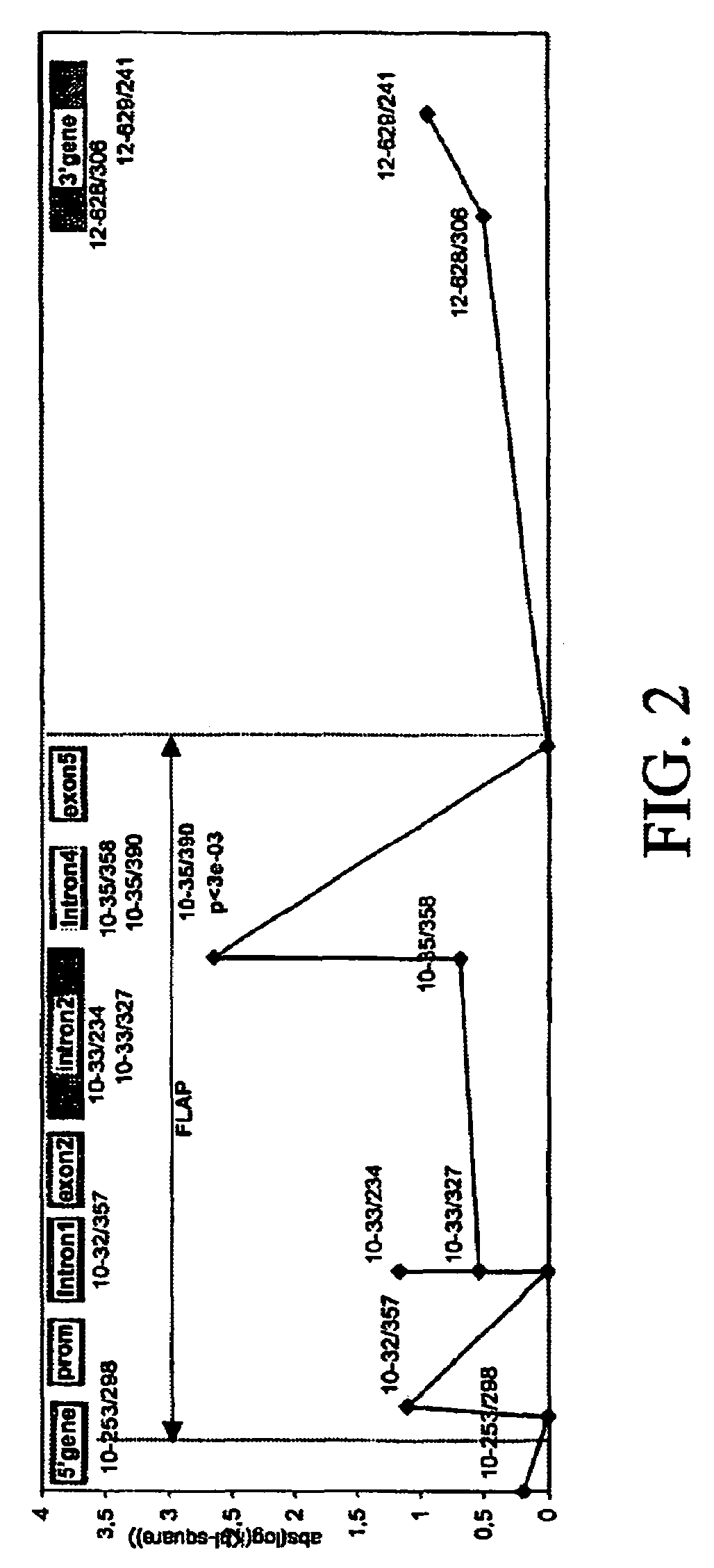
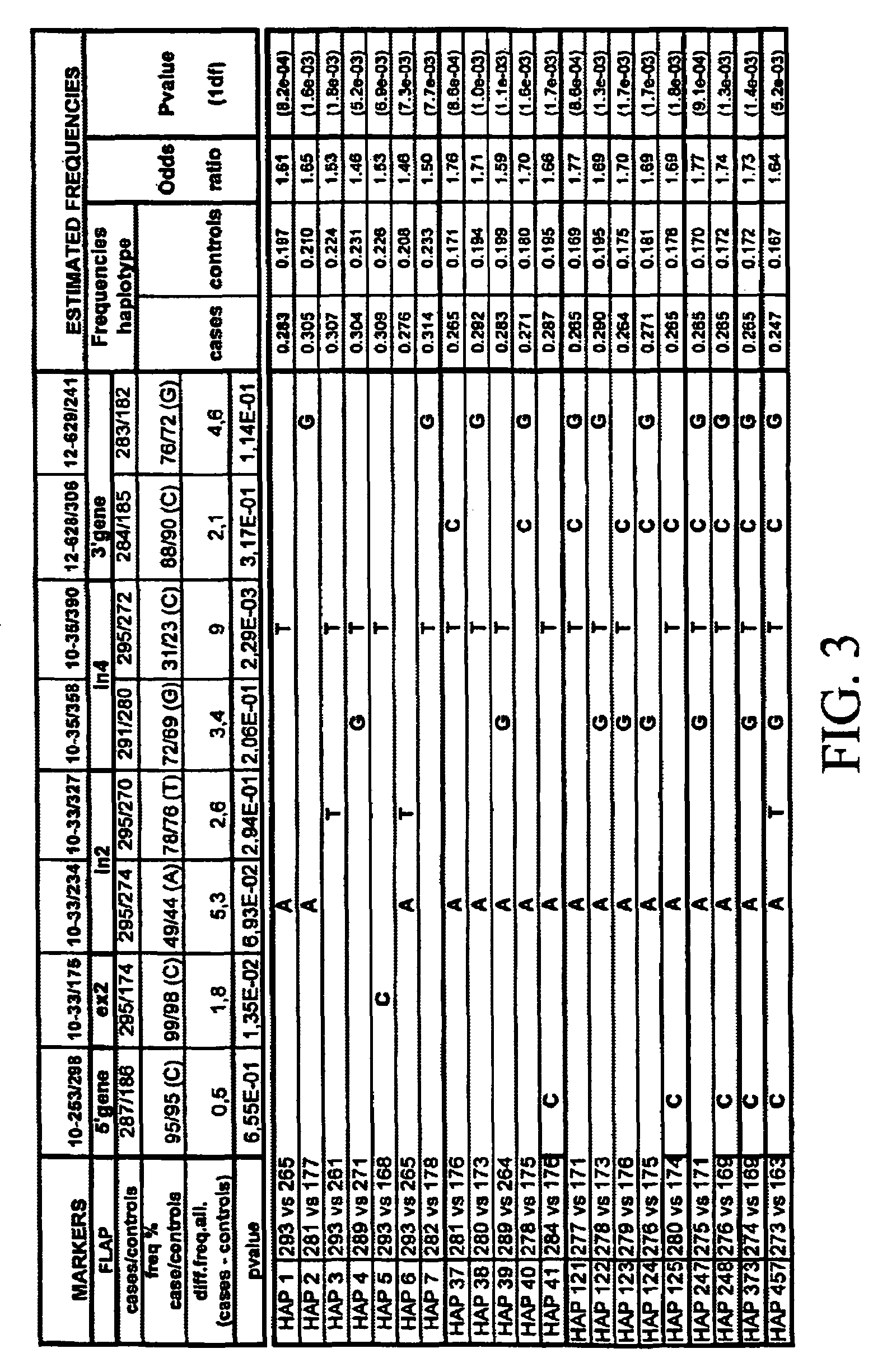
Genomic sequence of the 5-Lipoxygenase-activating protein (FLAP), polymorphic markers thereof and methods for detection of asthma
The invention concerns the genomic sequence of the FLAP gene. The invention also concerns biallelic markers of a FLAP gene and the association established between these markers and diseases involving the leukotriene pathway such as asthma. The invention provides means to determine the predisposition of individuals to diseases involving the leukotriene pathway as well as means for the diagnosis of such diseases and for the prognosis / detection of an eventual treatment response to agents acting on the leukotriene pathway.
Owner:SERONO GENETICS INST SA
Systems and methods for predicting effectiveness in the treatment of psychiatric disorders, including depression
InactiveUS20090306534A1ElectroencephalographyMicrobiological testing/measurementPsychoactive drugPsychopathology
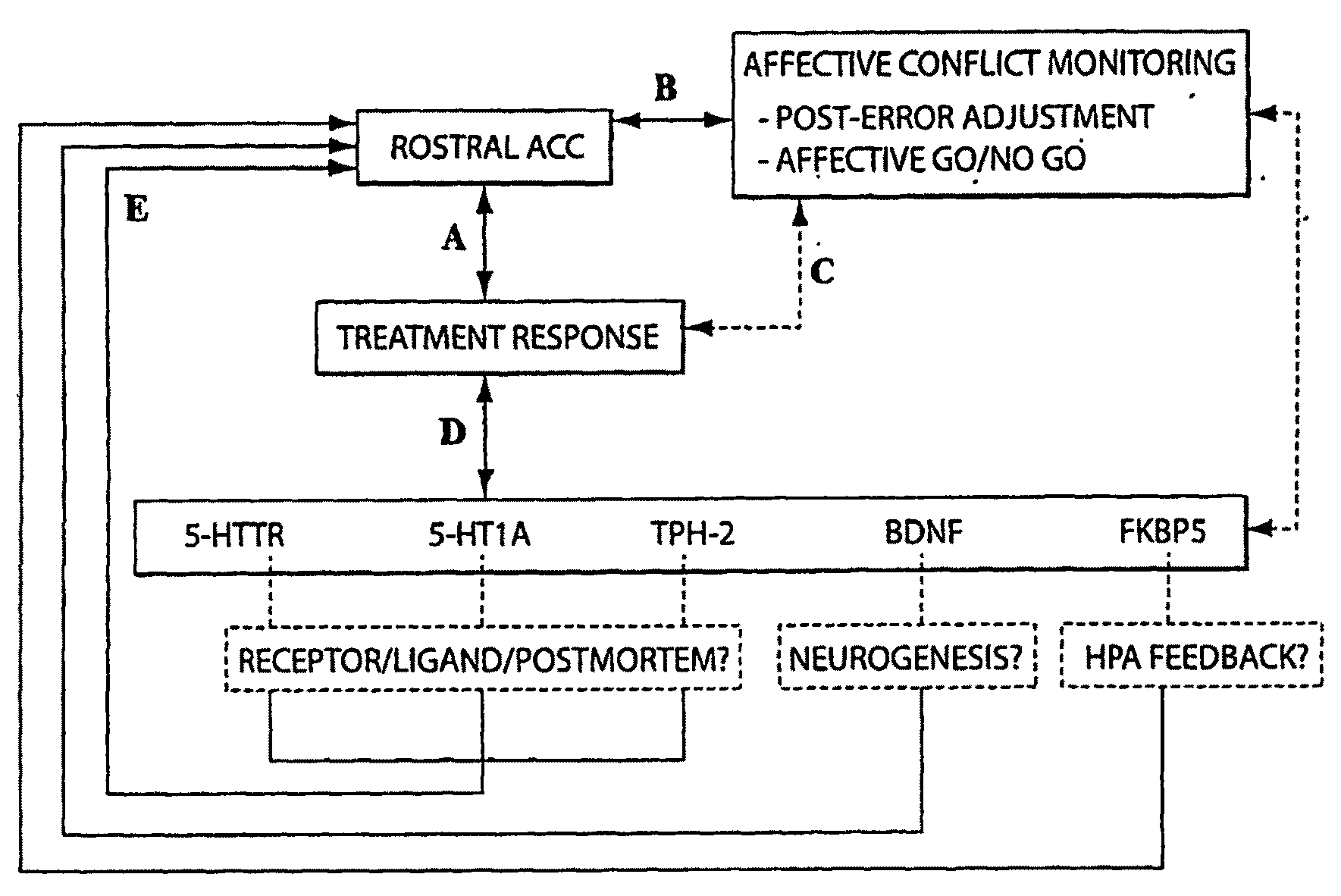
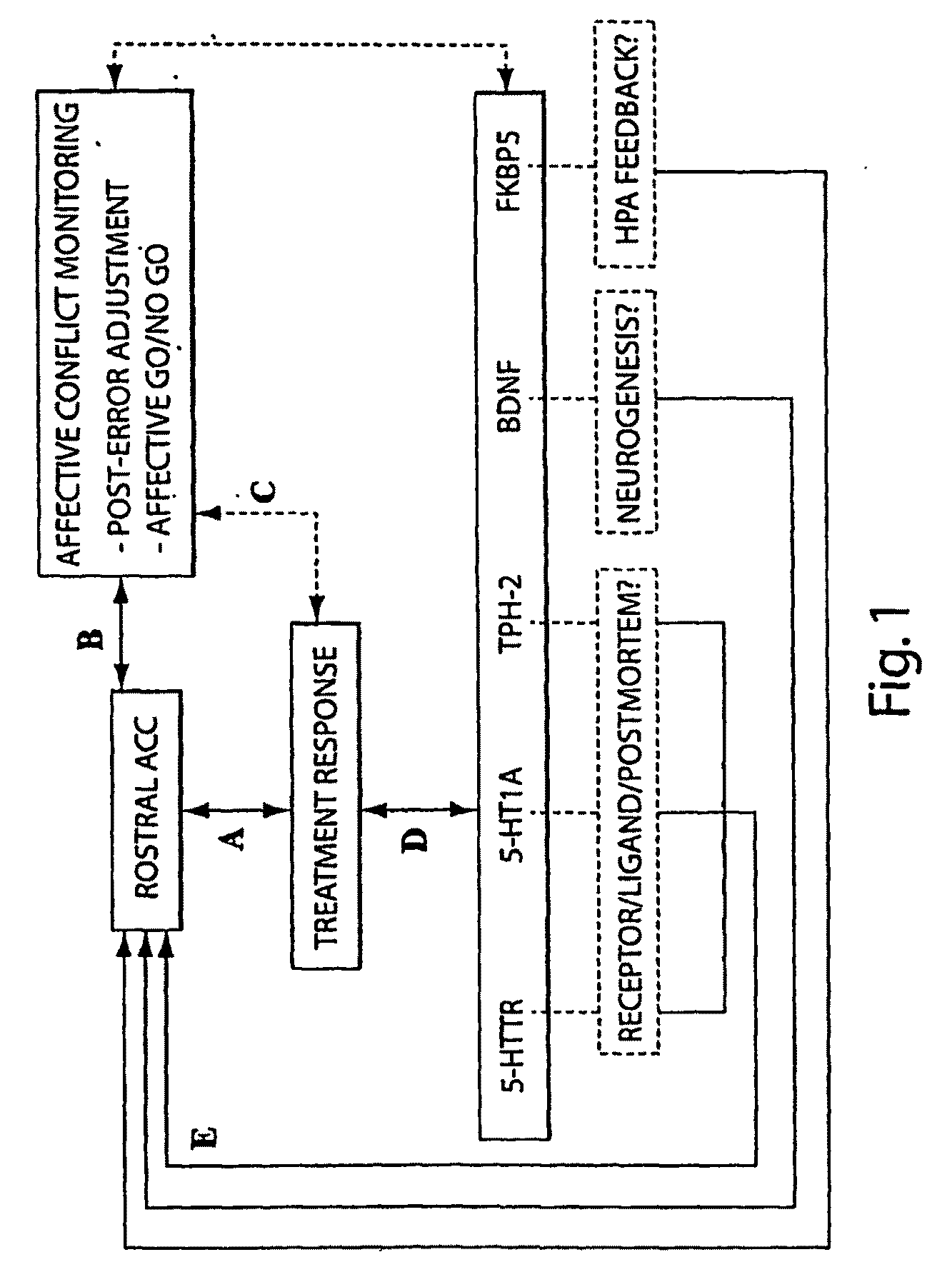
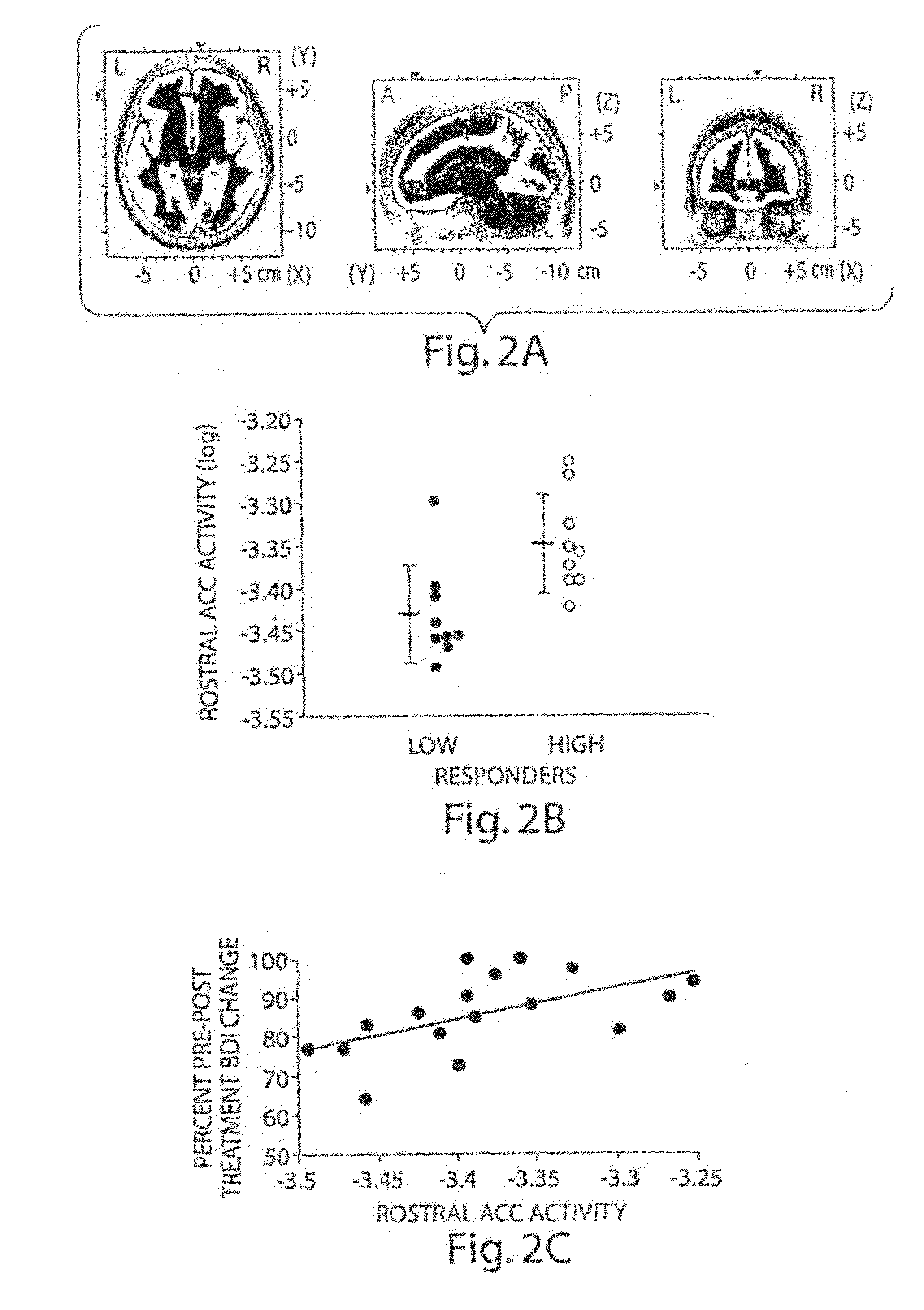
The present invention pertains to the evaluation and / or treatment of psychopathological diseases. In one aspect, information regarding brain activity, coupled with behavior tests (e.g., determining performance in a computer-based task), may be used to predict the response of a subject to psychological treatment, e.g., with a psychoactive drug. For example, the subject may be one suffering from depression, or other disturbances of the rostral anterior cingulate cortex. Another aspect of the present invention is directed to methods for analyzing neurobiological predictors through integration of information gathered from one or more levels of analyses: (1) behavior, (2) brain function, and / or (3) genes. In one aspect, the methods of the present invention can comprise any one of these components (i.e., behavior, brain function, and genes), or a combination of two or more of these components, and / or other components. Through these methods, development of novel algorithms for improving the ability to identify biological surrogate markers of treatment response are disclosed, according to certain embodiments of the invention. Still other aspects of the present invention are directed to systems and methods for implementing such evaluation techniques, analyzing such evaluation techniques, promotion of such evaluation techniques, and the like.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
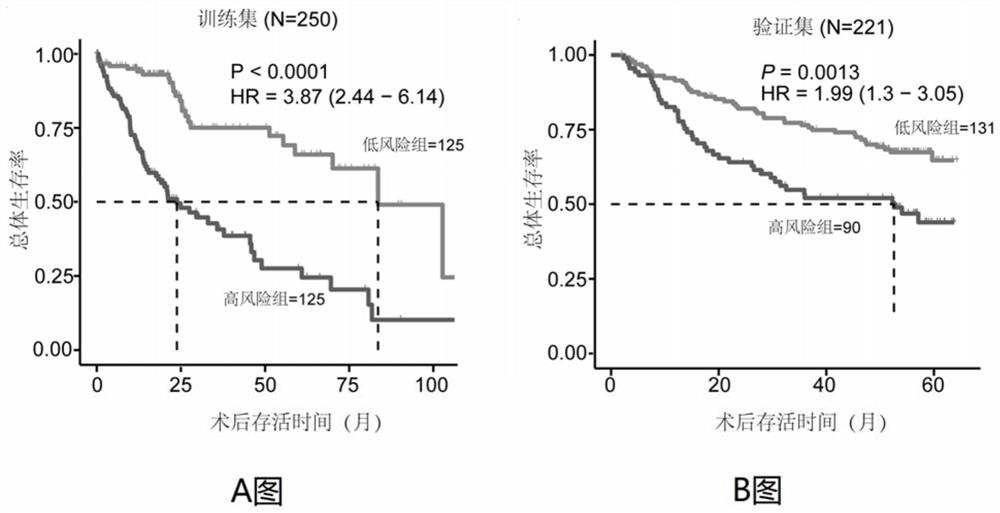
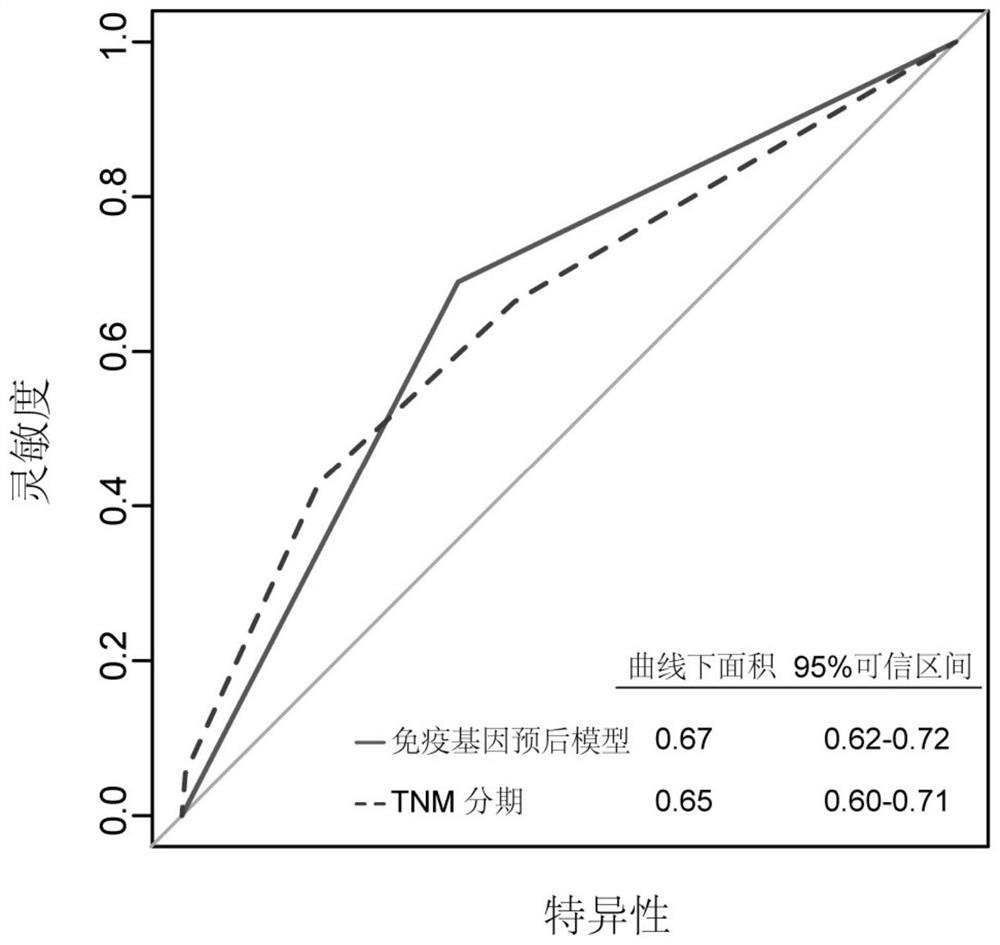
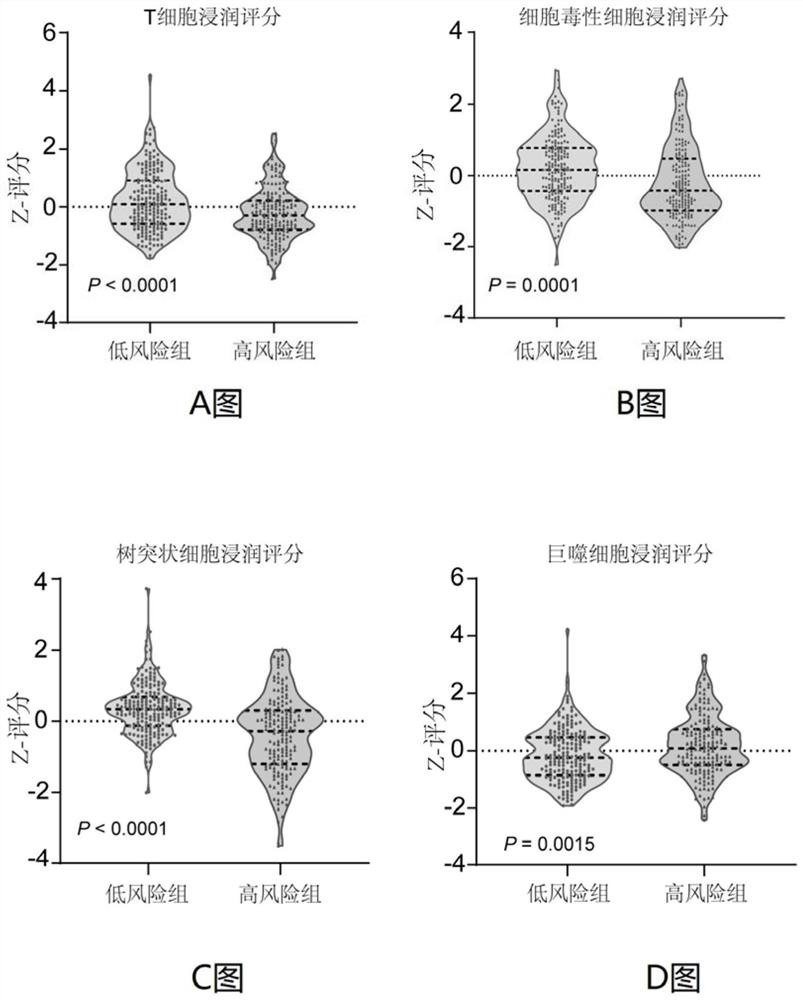
Immune gene prognosis model for predicting hepatocellular carcinoma tumor immune infiltration and postoperative survival time
ActiveCN112011616APromote the implementation of precision medicineObjective assessment of infiltrationMicrobiological testing/measurementBiostatisticsTNM staging systemMicroarray cgh
The invention relates to an immune gene prognosis model for predicting hepatocellular carcinoma tumor immune infiltration and postoperative survival time, and belongs to the technical field of biological medicines. The model can be used for evaluating the infiltration degree of immune cells in a tumor in clinical practice by detecting the expression levels of 22 specific immune related genes of ahepatocellular carcinoma patient, so that the model can be used for predicting hepatocellular carcinoma tumor immune infiltration in clinical practice and improve the prediction capability of the liver cancer immunotherapy response. The model can be used for judging the postoperative overall survival risk of a patient and guiding the formulation of a postoperative treatment strategy, and the corresponding microarray chip kit can realize the standardization and convenience of detection. Meanwhile, the immune gene prognosis model provided by the invention can increase the prediction accuracy andthe clinical net income of a hepatocellular carcinoma TNM staging system on the total survival time of three years and five years after operation. As a molecular marker for objectively and accuratelyevaluating the tumor immune state and poor prognosis risk of hepatocellular carcinoma, the model can realize accurate implementation of hepatocellular carcinoma immunotherapy and accurate prognosis prediction.
Owner:上海顿慧医疗科技发展有限公司
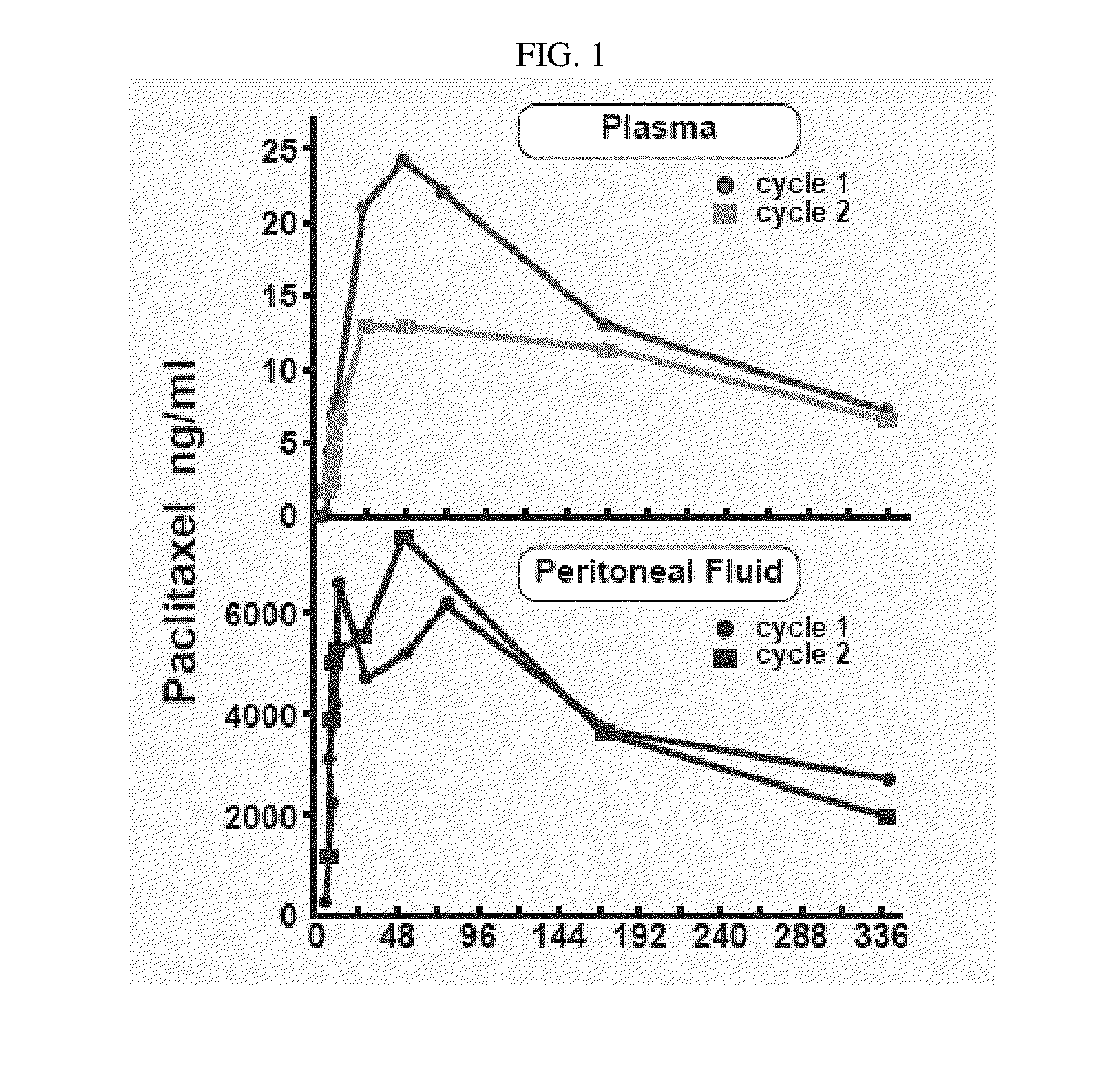
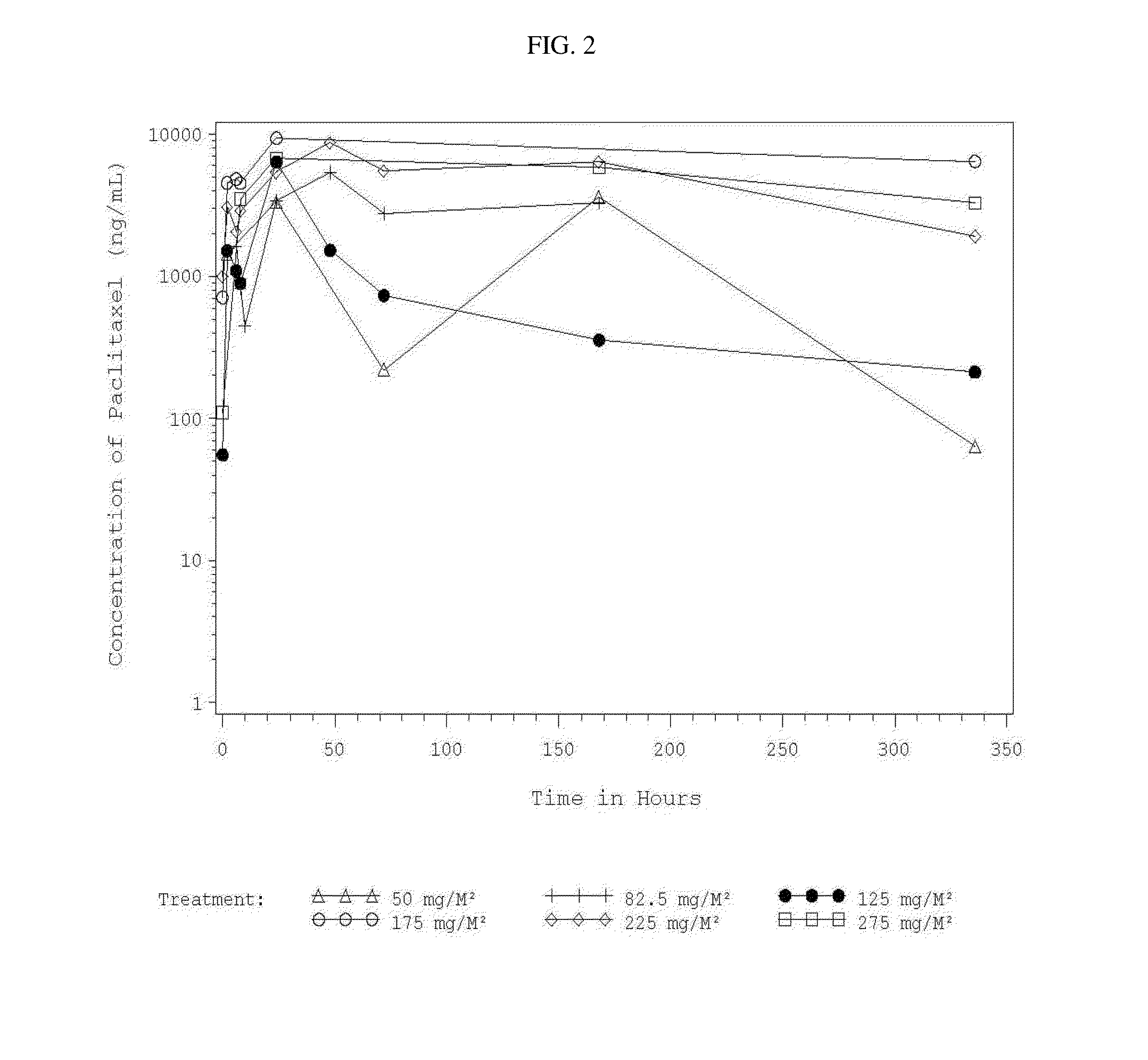
Use of Paclitaxel Particles
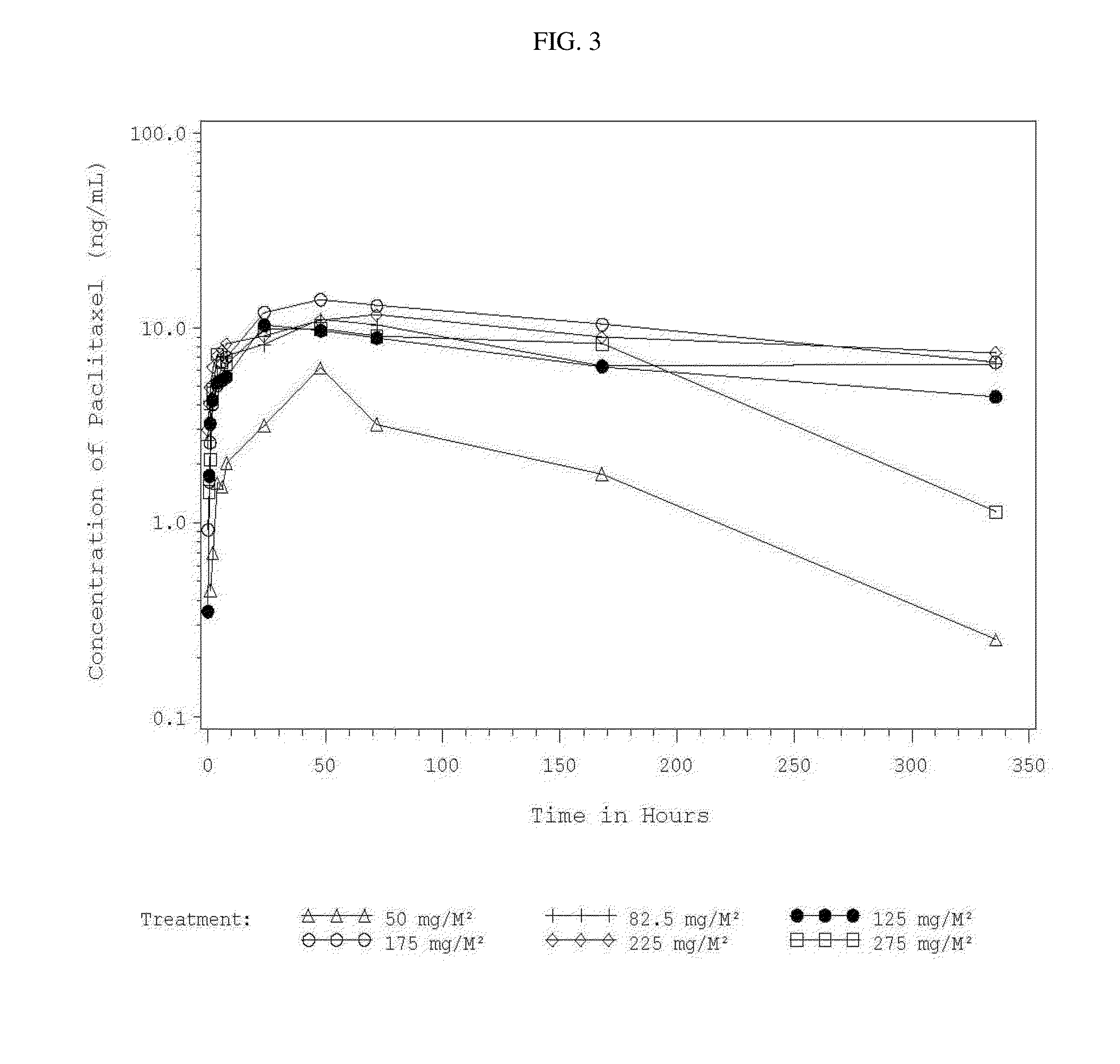
InactiveUS20150342872A1Improve progressImproved pharmacokinetic parametersBiocideOrganic active ingredientsDiseaseMedicine
A method of administering a suspension of paclitaxel particles into the peritoneum is provided. A corresponding method of treating a disease, disorder or condition that is therapeutically responsive to paclitaxel is also provided. The methods provide improvements over comparator formulations containing an equivalent dose of paclitaxel.
Owner:CRITITECH INC
ECG acquisition and treatment-response system for treating abnormal cardiac function
ActiveUS20130281816A1Prevent unintended medicatingAvoid overdoseElectrocardiographySensorsMicrocontrollerCardiac feature
A method and system for detecting and treating abnormal cardiac function. The patient's cardiac activity is substantially continuously monitored via a portable wearable device having electrodes and a microcontroller. Features in the monitored cardiac activity indicative of abnormal cardiac function are automatically detected. In response to the detection of an abnormal detected cardiac function, at least one person is automatically alerted of the detected abnormal cardiac function of the patient, and medication may be automatically caused to be administered to the patient. The portable wearable device preferably includes a plurality of electrodes and a microcontroller in communication with the electrodes, adapted to receive sensed cardiac activity signals and create digital signals enabling identification of at least one cardiac parameter. A remote computer server is in communication with the portable wearable device and compares values of the identified cardiac parameter with a range of normal values for the cardiac parameter.
Owner:CARDIMETRIX
Immunization of animals by topical applications of a salmonella-based vector
InactiveUS20030125278A1Efficient methodSimple and effective and economical and immunizationSsRNA viruses negative-senseBacterial antigen ingredientsGene deliveryWhole body
The present invention relates to techniques of skin-targeted non-invasive gene delivery to elicit immune responses and uses thereof. The invention further relates to methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal following topical application of vectors, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal as well as such a method further including disposing the vector in and / or on the delivery device. The vector can be gram negative bacteria, preferably Salmonella and most preferably Salmonella typhimurium.
Owner:UAB RES FOUND
Polymorphisms for predicting disease and treatment outcome
The invention provides compositions and methods for determining the increased risks for recurrence of certain cancers and the likelihood of successful treatment with one or both of chemotherapy and radiation therapy. The methods comprising determining the type of genomic polymorphism present in a predetermined region of the gene of interest isolated from the subject or patient. Also provided are nucleic acid probes and kits for determining a patient's cancer risk and treatment response.
Owner:UNIV OF SOUTHERN CALIFORNIA
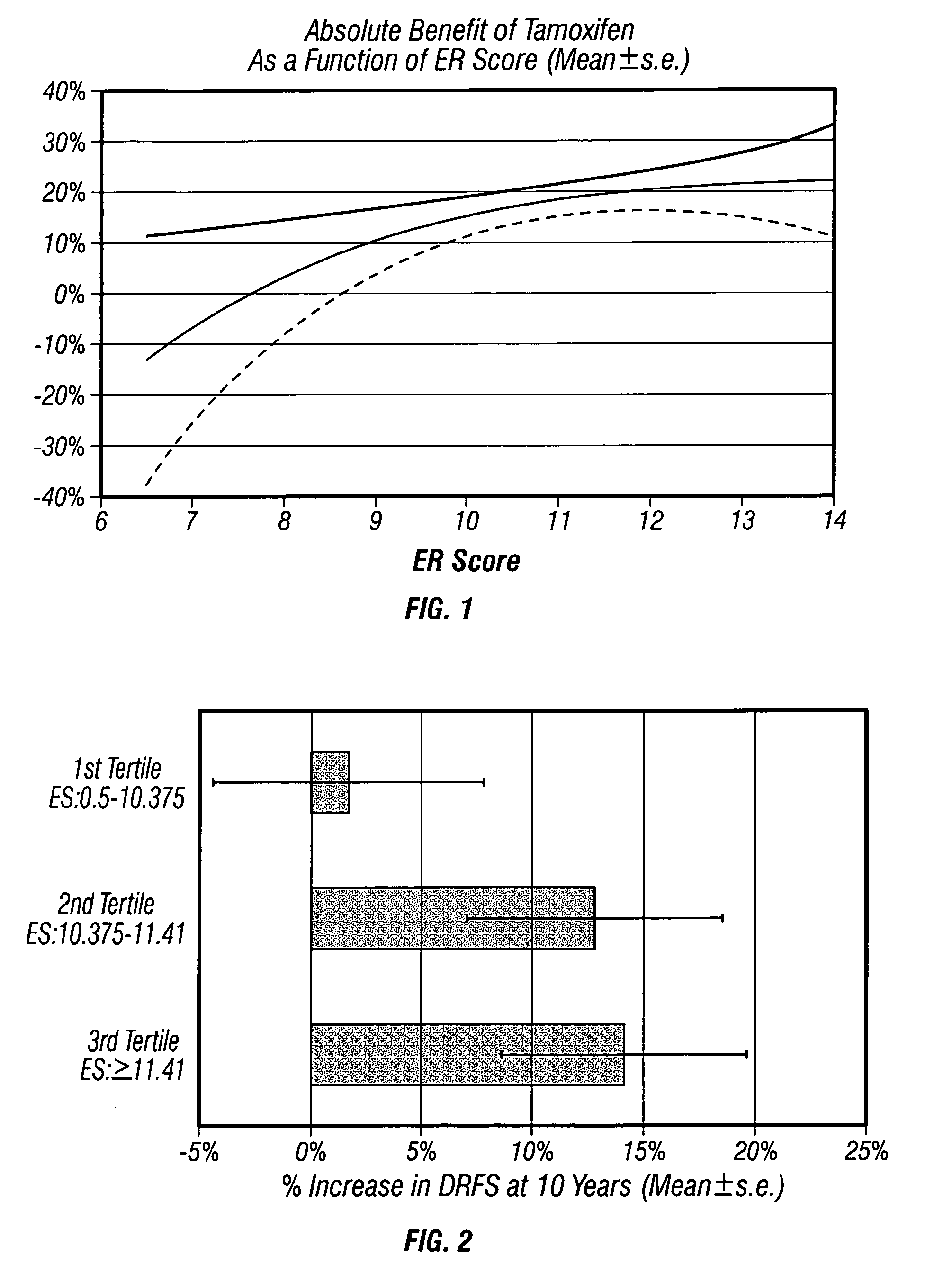
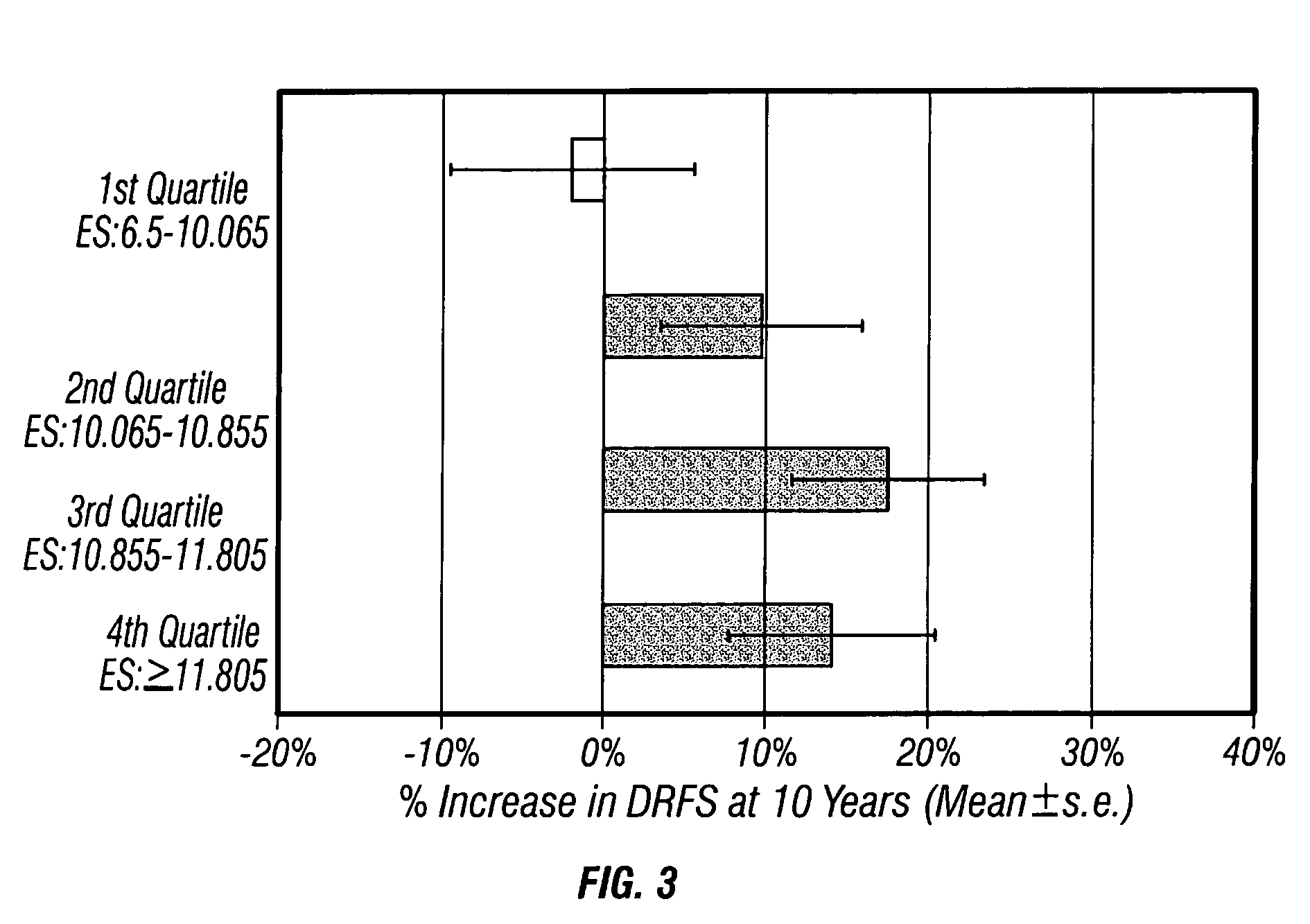
Molecular indicators of breast cancer prognosis and prediction of treatment response
ActiveUS7622251B2Reduce riskMicrobiological testing/measurementProteomicsAdjuvantLymph node negative
The present invention relates to quantitative molecular indicators that can guide clinical decisions in breast cancer, such as estrogen receptor (ESR1)-positive, lymph node-negative breast cancer. In particular, the invention concerns certain genes, the varied expression of which indicates the likelihood of recurrence of surgically resected breast cancer in patients who are not treated with a therapeutic agent in the adjuvant setting. In addition, the invention concerns the use of quantitative measurement of the expression of certain genes, including the ESR1 gene, that measure as a continuous variable, to determine (a) the likelihood of a beneficial response to the anti-estrogen therapeutic agent, such as tamoxifen; and (b) the potential magnitude of beneficial response to chemotherapy.
Owner:MICROSOFT CORP +2
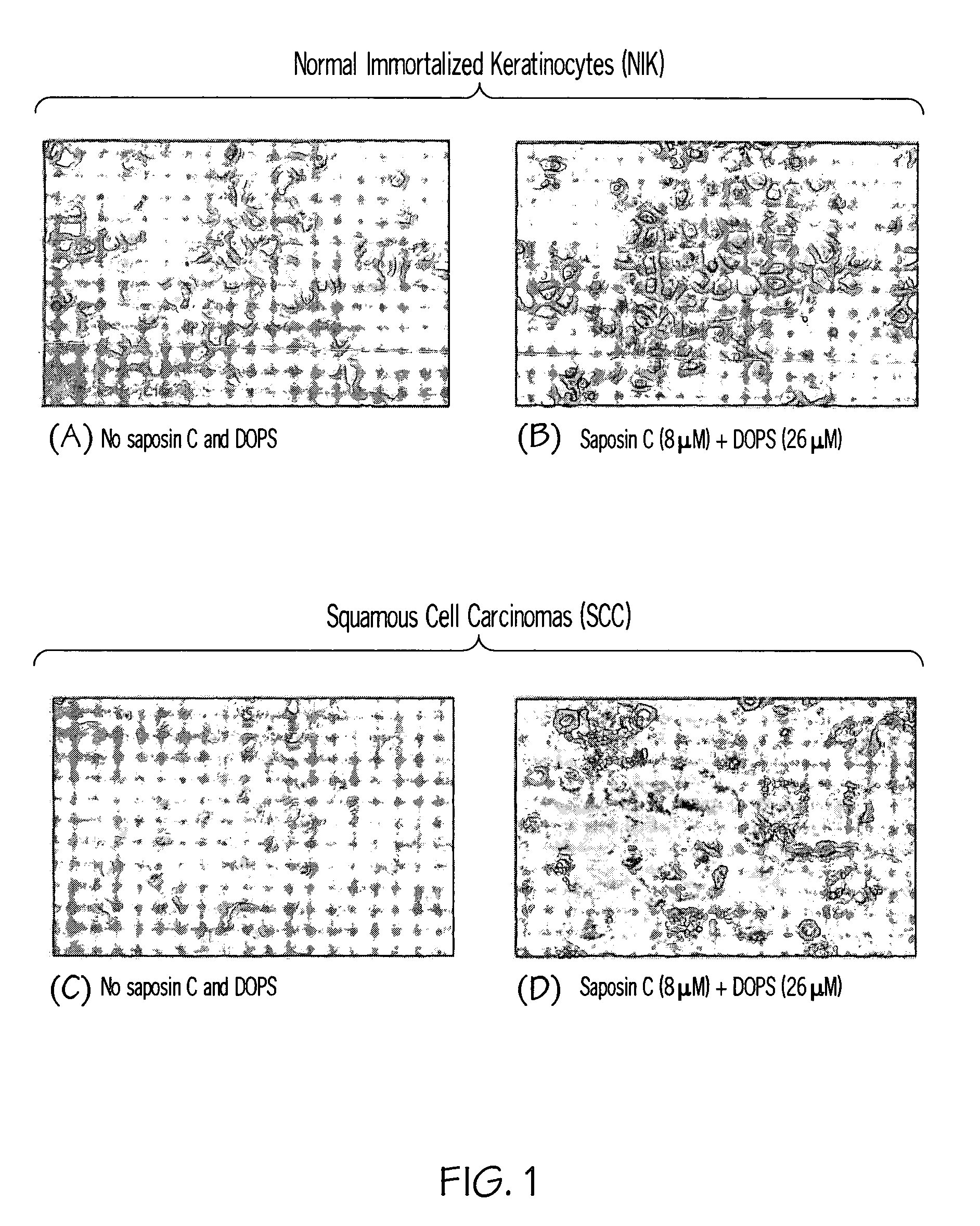
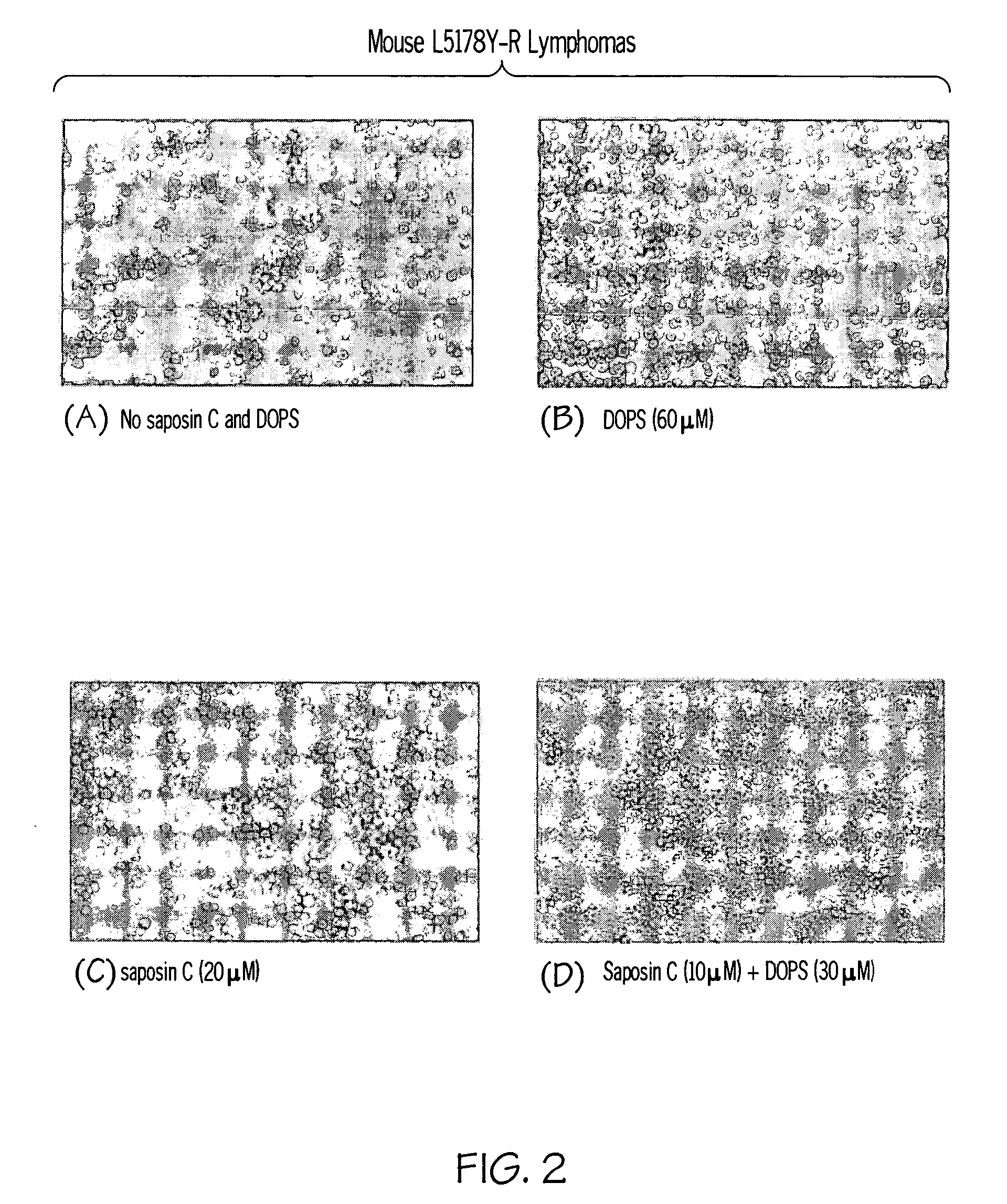
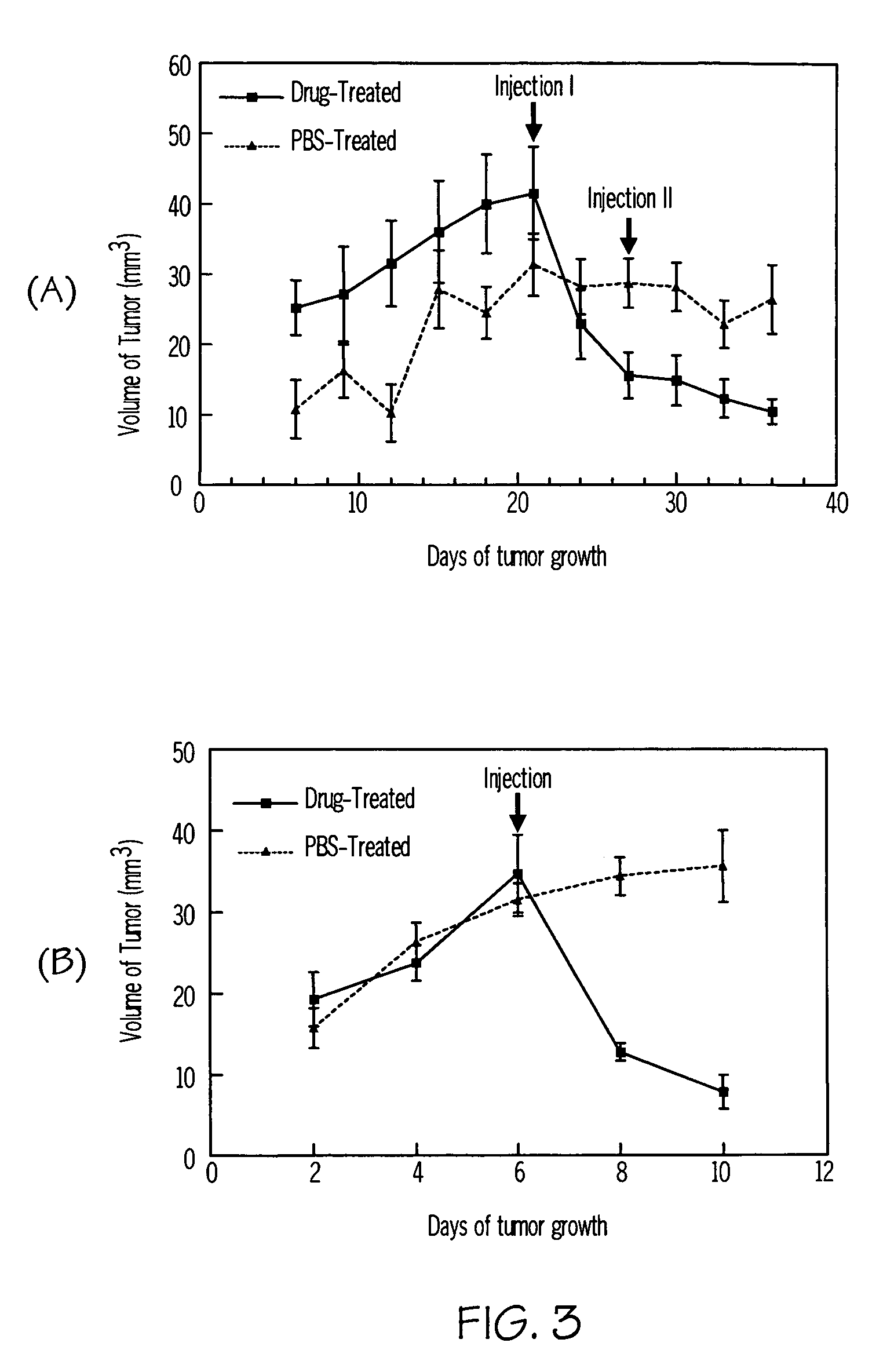
Saposin C-DOPS: a novel anti-tumor agent
Compositions and methods for treating subjects with disorders characterized by hyper-proliferating cells such as tumors and cancers are provided. The compositions comprise agents that are combinations of saposin C (or prosaposin-related polypeptides) and dioleoylphosphatidylserine (or inner leaflet components). This anti-tumor agent is administered in the methods of the invention according to a dosing regimen. Administering an agent of the invention results in a positive therapeutic response in a subject with a tumor.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Method of handling subscriber services in a wireless intelligent network
InactiveUS6615042B1Interconnection arrangementsSpecial service for subscribersHome subscriber serverTreatment response
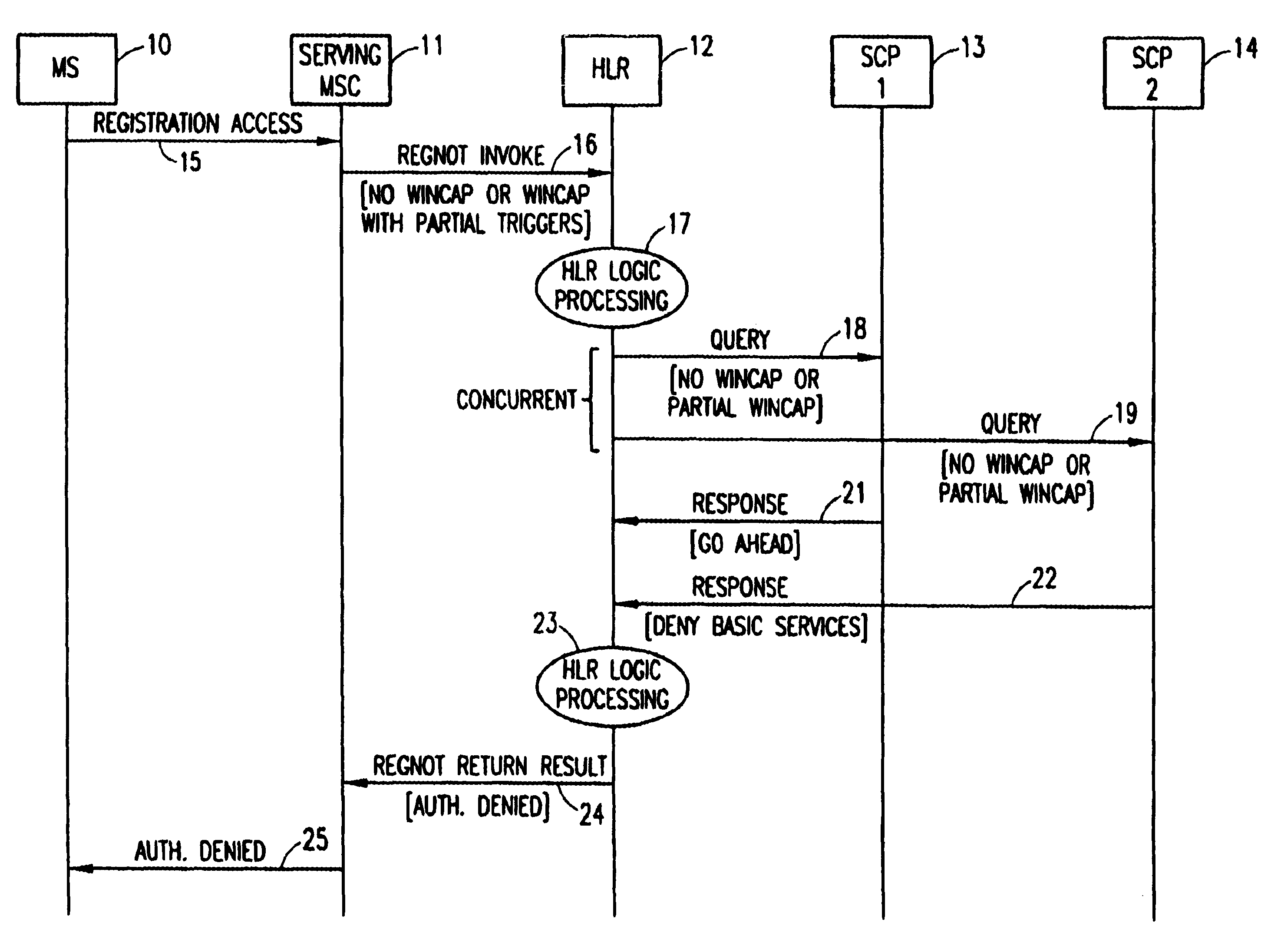
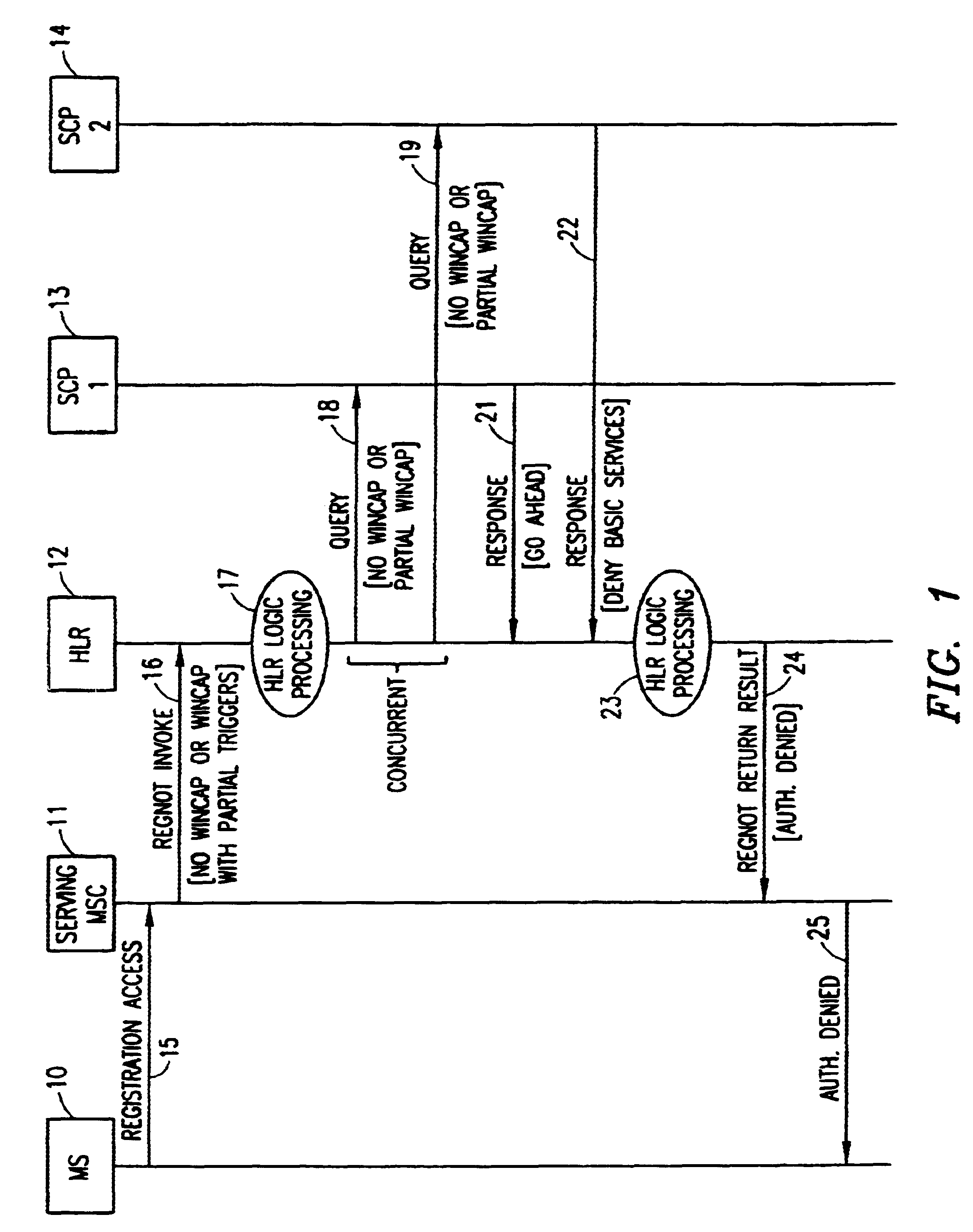
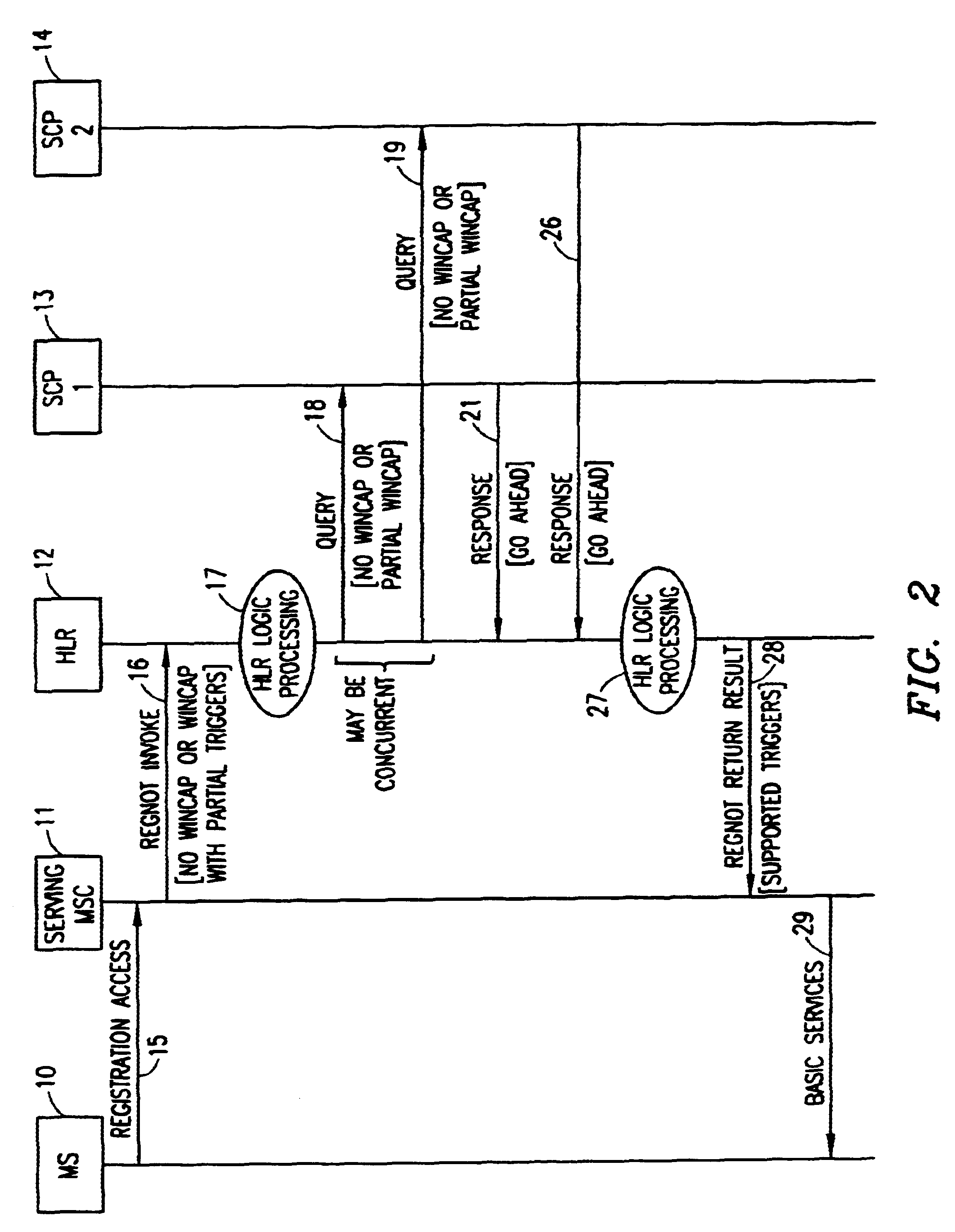
A method in a Wireless Intelligent Network (WIN) of handling subscriber services when a serving Mobile Switching Center (MSC) does not support all WIN triggers. The WIN includes a Home Location Register (HLR) having a subscriber profile that indicates the WIN triggers required to support a particular subscriber's active services. When an invoke message is sent from the MSC to the HLR, a WIN Capabilities (WINCAP) parameter is included and indicates the WIN triggers supported by the MSC. The HLR identifies a Service Control Point (SCP) for each subscriber service that requires a WIN trigger and is not supported by the MSC. The HLR then queries each identified SCP for call-treatment instructions. If an SCP determines that its service is essential, a response is returned to the HLR with an instruction to deny service to the subscriber. If an SCP determines that its service is not essential, a response is returned to the HLR with an instruction to skip the unsupported service and allow basic services to the subscriber. The HLR collates the responses and determines a call-treatment response to return to the MSC.
Owner:OPTIS WIRELESS TECH LLC
Method for treating diseases with omega interferon
InactiveUS20070248572A1Change levelAntibacterial agentsOrganic active ingredientsInterferon therapyTolerability
A method of treating an immunologic, proliferative, or infectious disease in a warm-blooded animal is disclosed. The method comprises administering to the animal omega interferon (IFN) at a dosage and activity for the disease state treated sufficient to induce a therapeutic response in the animal, which dosage and activity for the disease state treated is higher than would be well-tolerated based on data for non-omega IFN's. The omega IFN is administered alone or In combination with a therapeutically effective amount of at least one adjunctive therapeutic agent. Also disclosed is an article of manufacture useful for treating an immunologic, proliferative, or infectious disease, which article comprises (1) omega IFN in a form suitable for administering a therapeutically effective amount of the omega IFN to the subject in order to induce the desired therapeutic response (2) instructions for administering the omega IFN as desired, that is higher than would be well-tolerated based on data for non-omega IFNs.
Owner:INTARICA THERAPEUTICS
Multi-scale complex systems transdisciplinary analysis of response to therapy
Described herein are methods and systems to measure dynamics of disease progression, including cancer growth and response, at multiple scales by multiple techniques on the same biologic system. Methods and systems according to the invention permit personalized virtual disease models. Moreover, the invention allows for the integration of previously unconnected data points into an in silico disease model, providing for the prediction of disease progression with and without therapeutic intervention.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
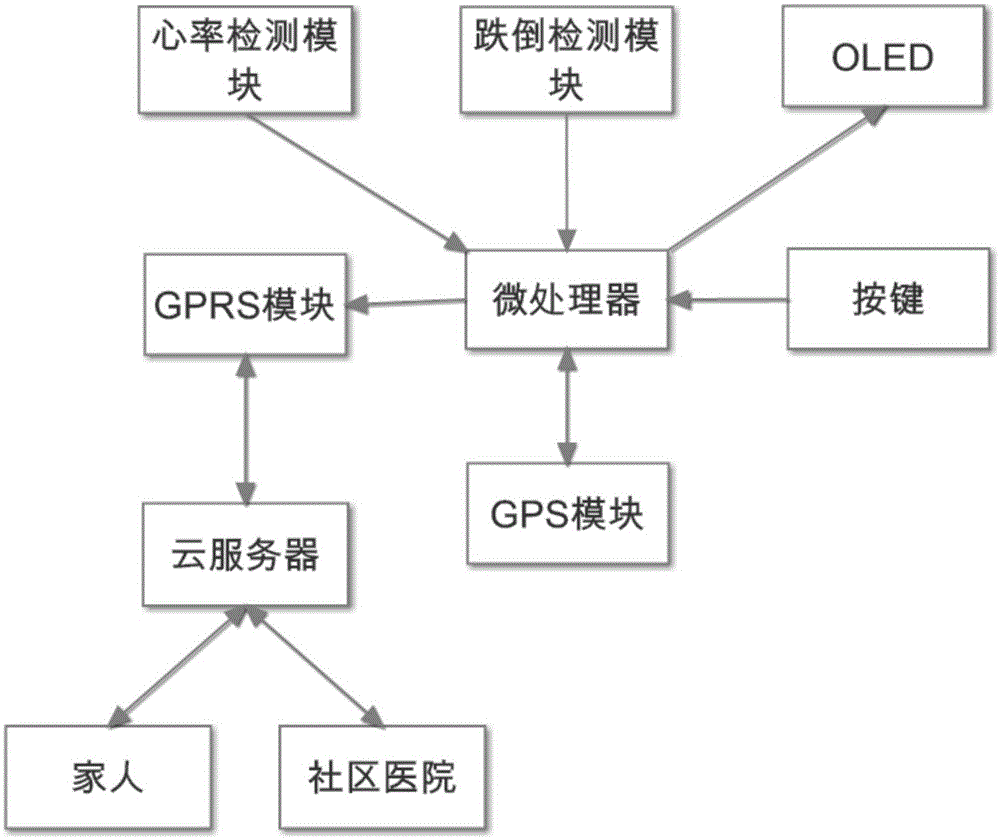
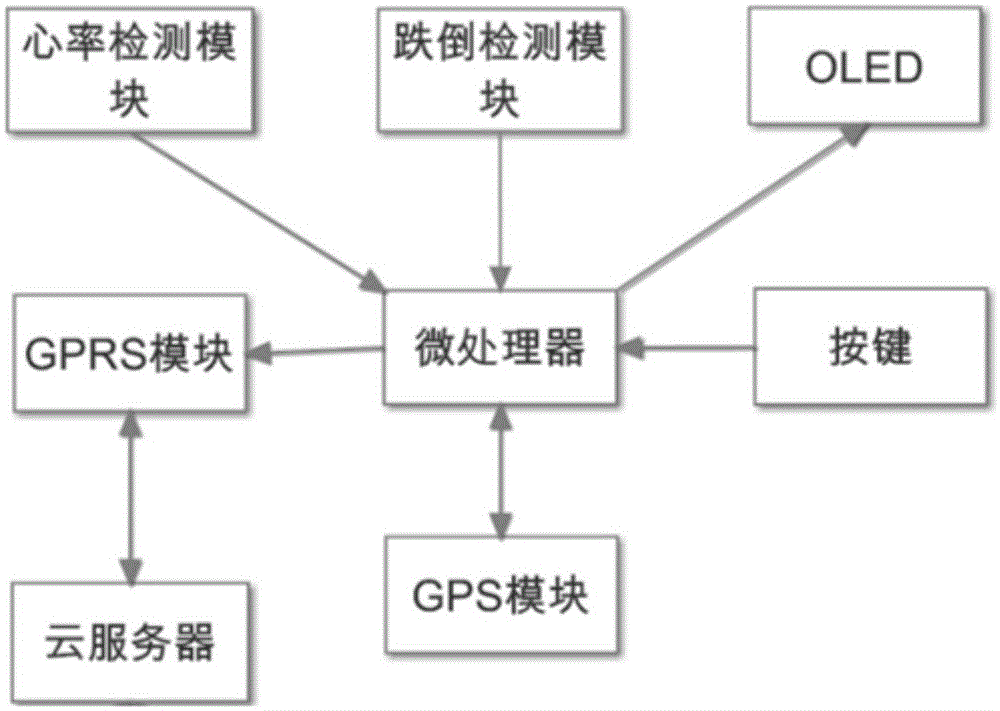
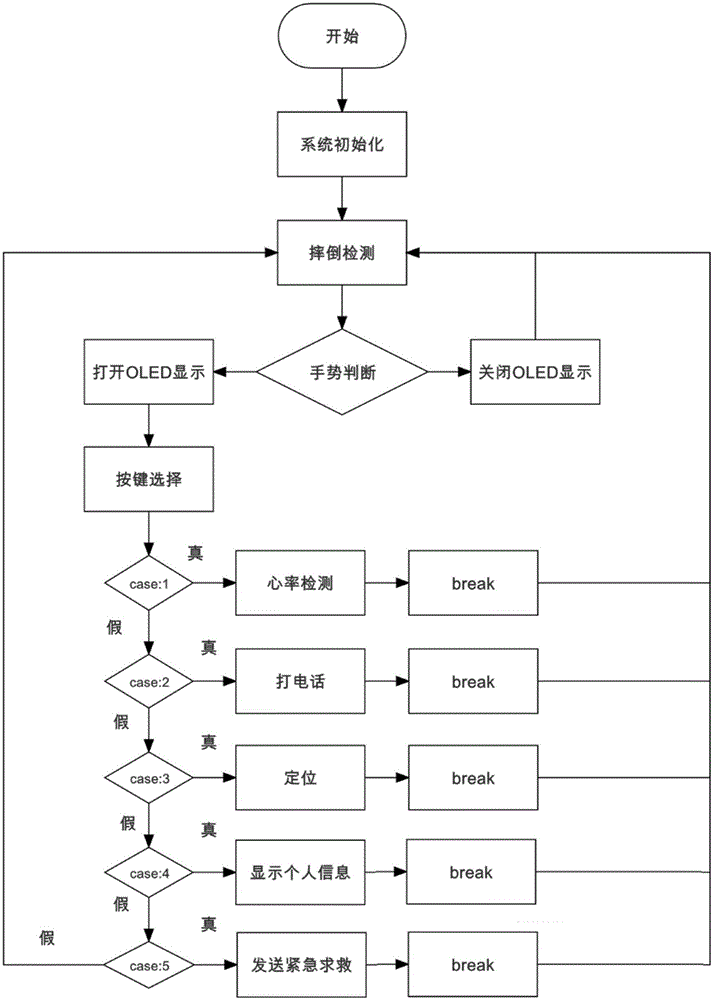
Health data detection bracelet for old people
InactiveCN105054894AStrong targetingHigh implementabilityDiagnostic recording/measuringBraceletsDiseasePersonalization
The present invention discloses a health data detection bracelet for old people. The health data detection bracelet comprises heart rate detection, fall detection and GPS positioning. By using the bracelet, a user can call for help from families and hospitals through a network cloud end server. Fall detection is added compared with a conventional health bracelet so as to prevent severe injuries easily caused by falls of the old people. Community hospitals are added as a third party, diseases of unhealthy old people can be timely intervened and treated, thereby greatly shortening treatment response time and raising treatment initiatives. Through cloud storage of health data, related medical researches and individualized health service customization based on big data become convenient. With addition of a WeChat public platform of a hand-held terminal and a mobile phone app, the families can timely learn about health conditions of the old people in the family whenever and wherever possible. And with further addition of an energy-saving display, the service life of the health data detection bracelet for old people is prolonged.
Owner:BEIJING NORMAL UNIV ZHUHAI
Gene Expression Markers for Predicting Response to Chemotherapy
The present invention provides sets of genes the expression of which is important in the prognosis of cancer. In particular, the invention provides gene expression information useful for predicting whether cancer patients are likely to have a beneficial treatment response to chemotherapy. FHIT; MTA1; ErbB4; FUS; BBC3; IGF1R; CD9; TP53BP1; MUC1; IGFBP5; rhoC; RALBP1; STAT3; ERK1; SGCB; DHPS; MGMT; CRIP2; ErbB3; RAP1GDS1; CCND1; PRKCD; Hepsin; AK055699; ZNF38; SEMA3F; COL1A1; BAG1; AKT1; COL1A2; Wnt.5a; PTPD1; RAB6C; GSTM1, BCL2, ESR1; or the corresponding expression product, is determined, said report includes a prediction that said subject has a decreased likelihood of response to chemotherapy.
Owner:GENOMIC HEALTH INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com