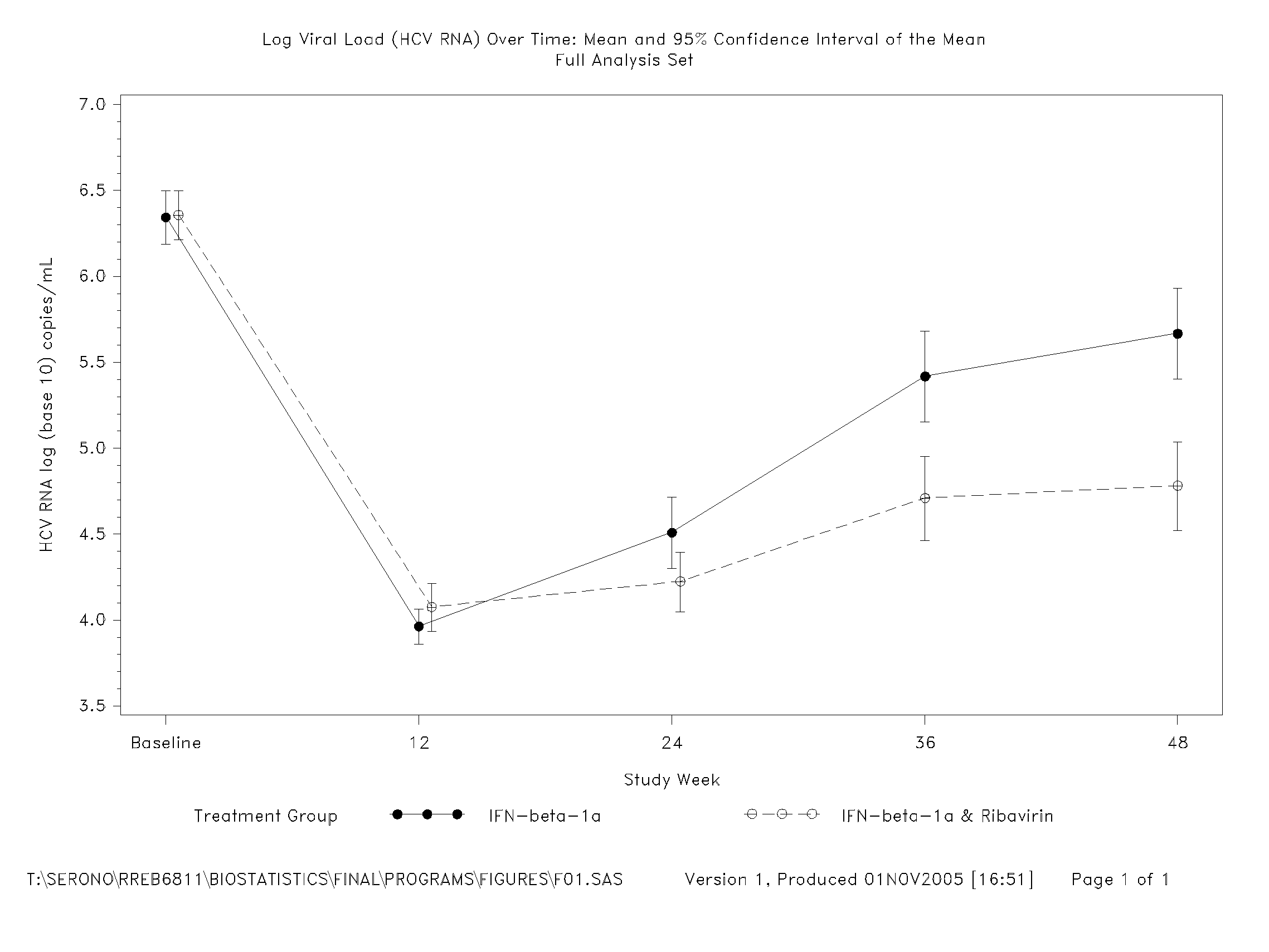
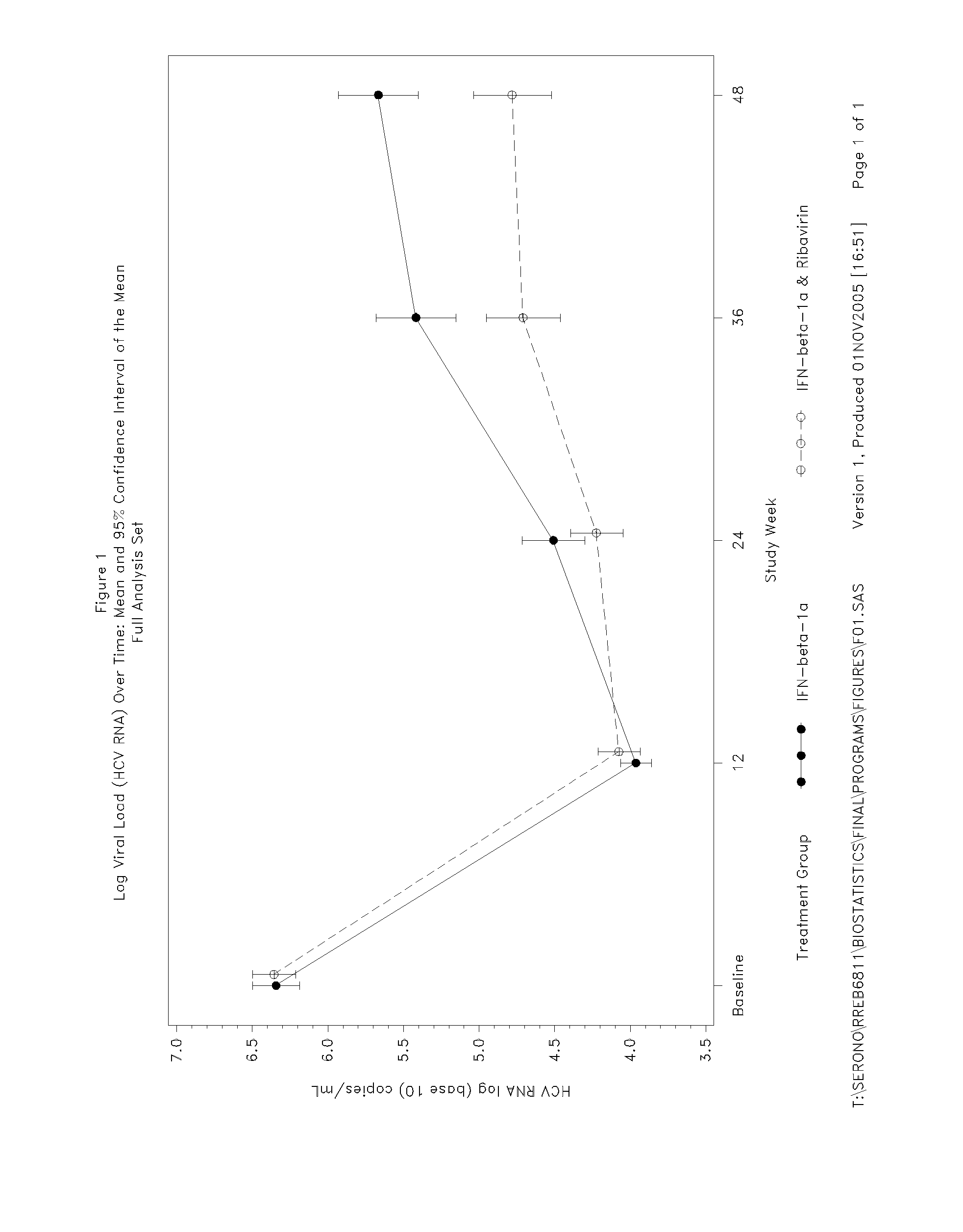
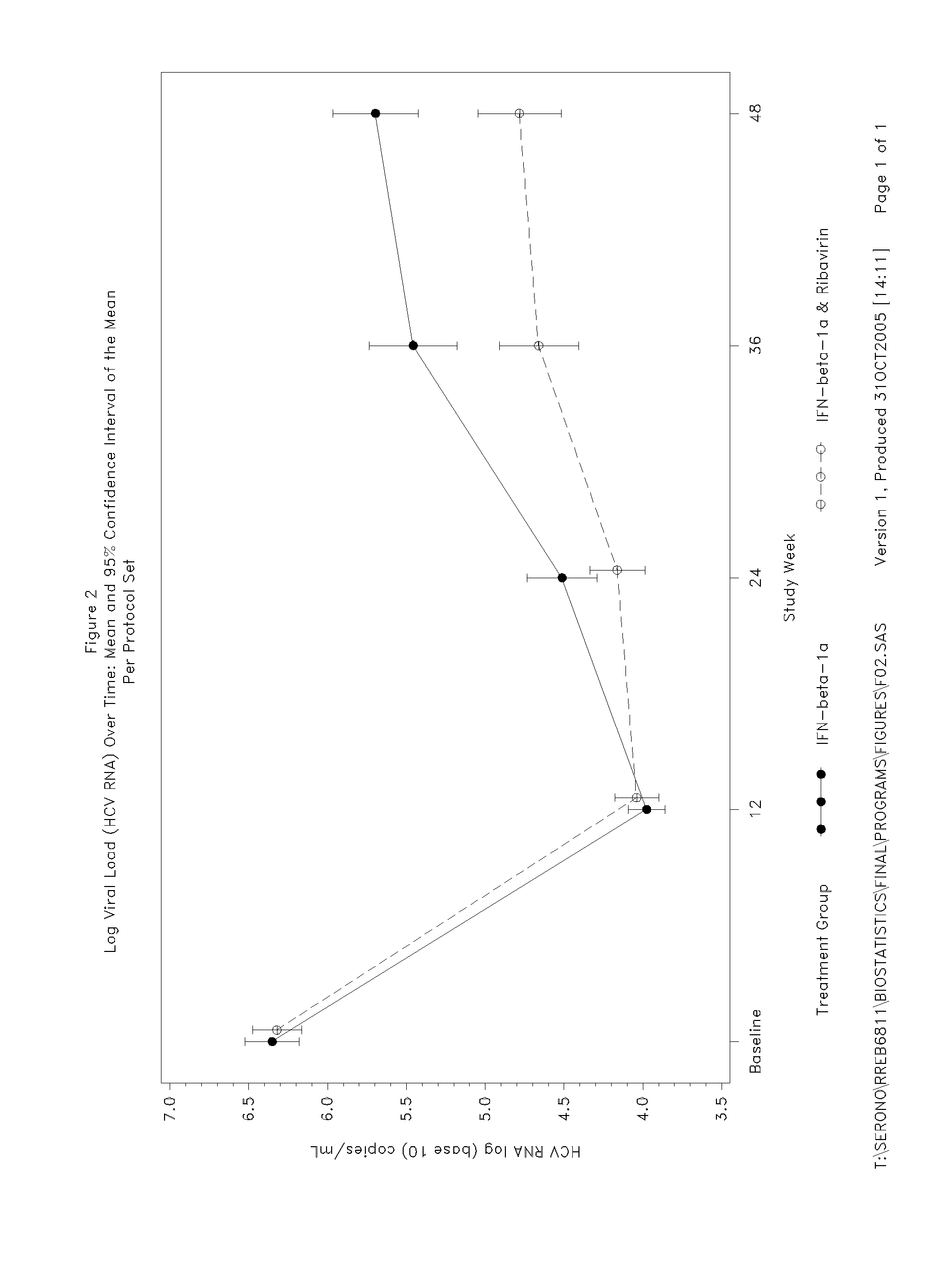
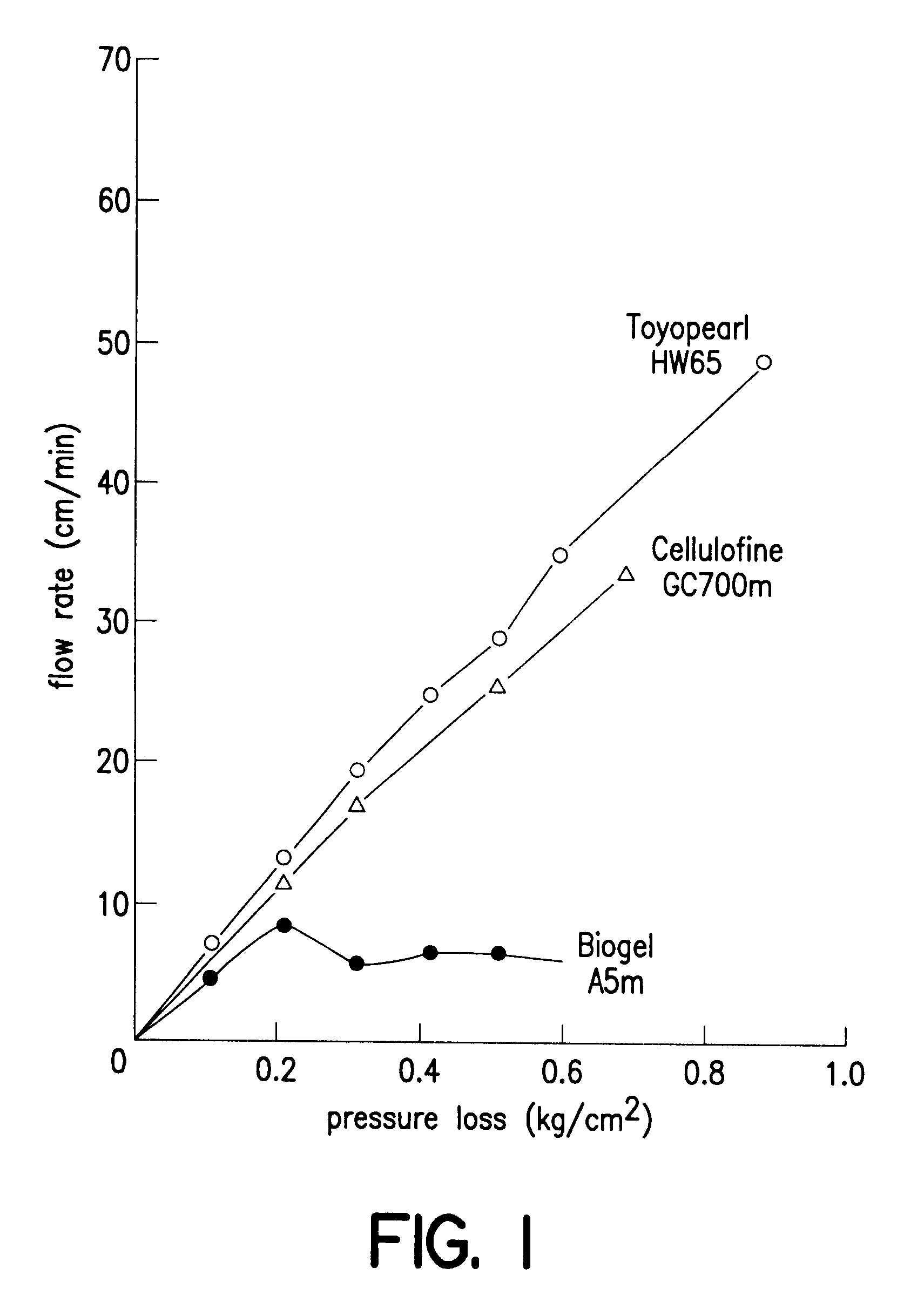
Patents
Literature
133 results about "Interferon therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods for treating viral infection using IL-28 and IL-29
ActiveUS7135170B2Reduction in viral infection levelReduce viral infectionBiocidePeptide/protein ingredientsInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Method of treating tuberculosis with interferons
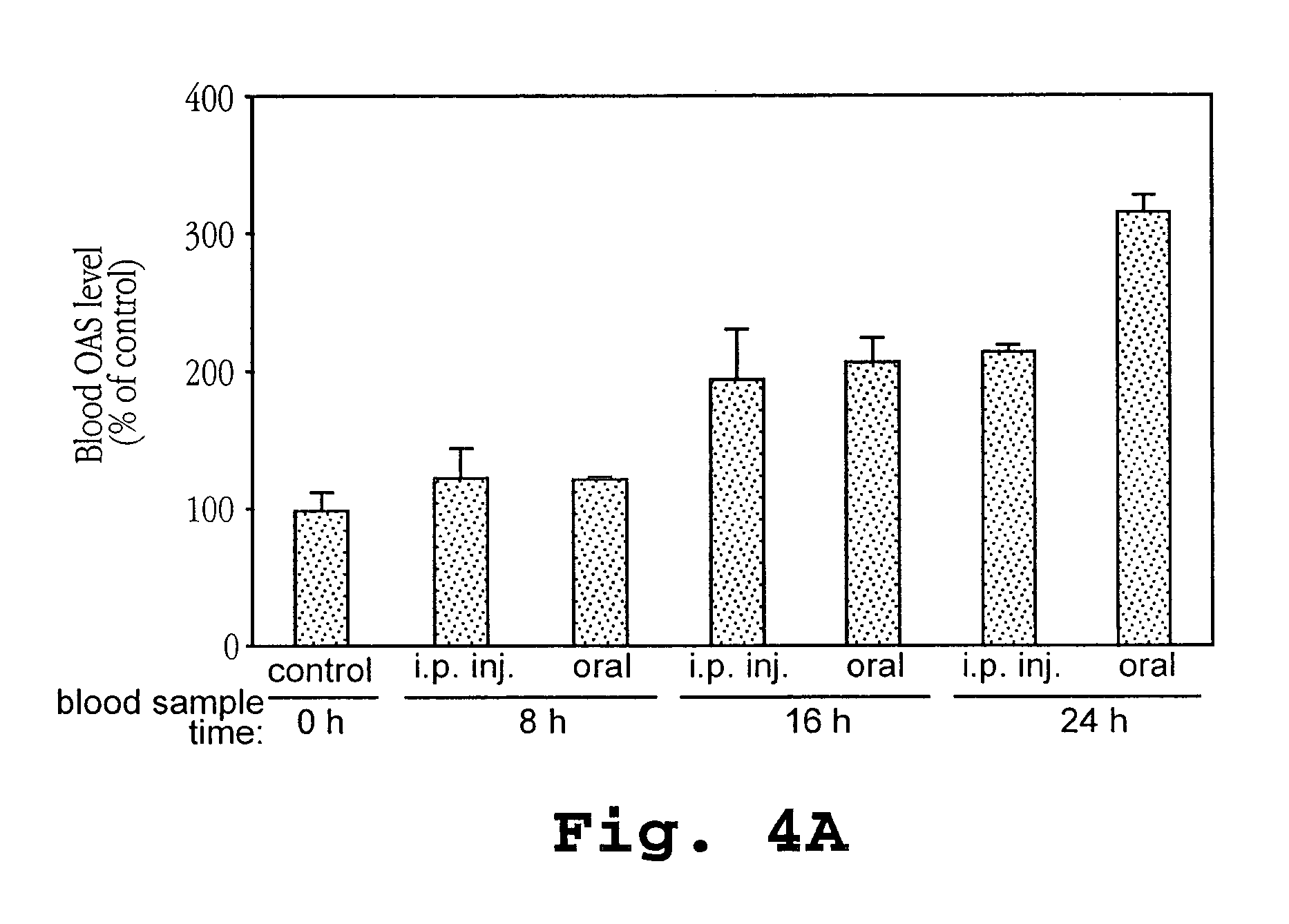
InactiveUS20100098660A1Increase appetiteReduction in wheezingAntibacterial agentsBiocideInterferon therapyInterferon alpha
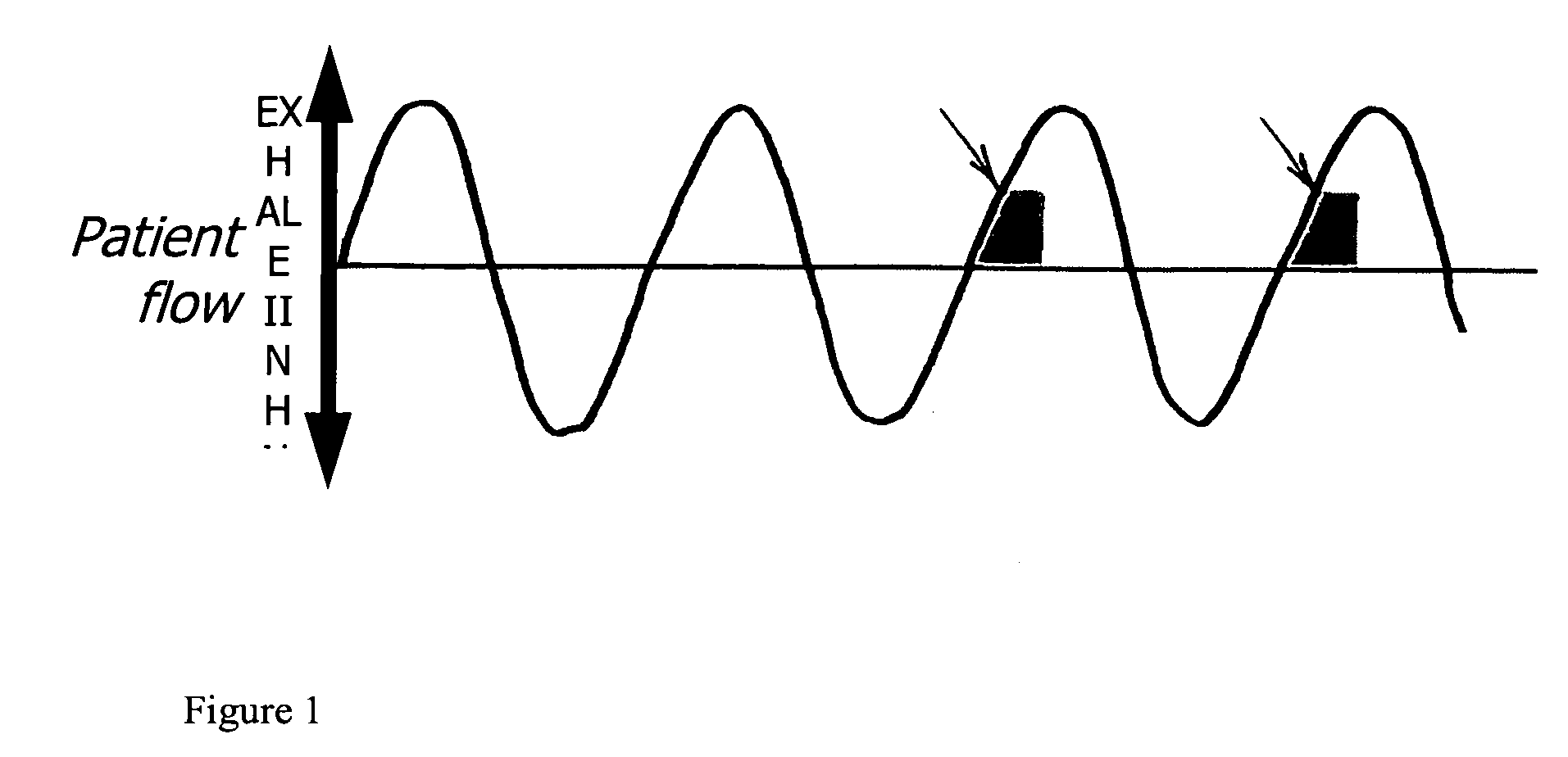
A method of treating tuberculosis comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Further, a method of reducing the infectivity of tuberculosis or reducing the number of infectious organisms present in the lungs of a patient suffering from tuberculosis comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Also, pharmaceutical compositions of one or more aerosolized interferon(s) are provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
IL28 and IL29 TRUNCATED CYSTEINE MUTANTS AND ANTIVIRAL METHODS OF USING SAME
InactiveUS20070053933A1Organic active ingredientsPeptide/protein ingredientsInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Methods for treating viral infection using il-28 and il-29 cysteine mutants
InactiveUS20080075693A1Prolonged Circulatory Half-LifeLow immunogenicityPeptide/protein ingredientsAntipyreticInterferon therapyHematopoietic cell
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Method of treating interferon non-responders using HCV protease inhibitor
A method of treating, preventing or ameliorating one or more symptoms associated with hepatitis C virus (HCV) in a patient in whom either the HCV is of Genotype 1 and / or the patient was previously treated with interferon and the previous interferon therapy was ineffective to treat the one or more symptoms associated with HCV, comprising administering to such a patient an effective amount of at least one compound of formulae I-XXVI of which the following structural formula is exemplary or a pharmaceutically acceptable salt, solvate or ester thereof. Optional combined administration of said at least one compound with an interferon or pegylated interferon and / or ribaviron is also contemplated.
Owner:SCHERING CORP
Immunotherapy for chronic hepatitis c virus infection
InactiveUS20110256098A1Increase the number ofReduce in quantitySsRNA viruses positive-sensePeptide/protein ingredientsChronic viral hepatitis CInterferon therapy
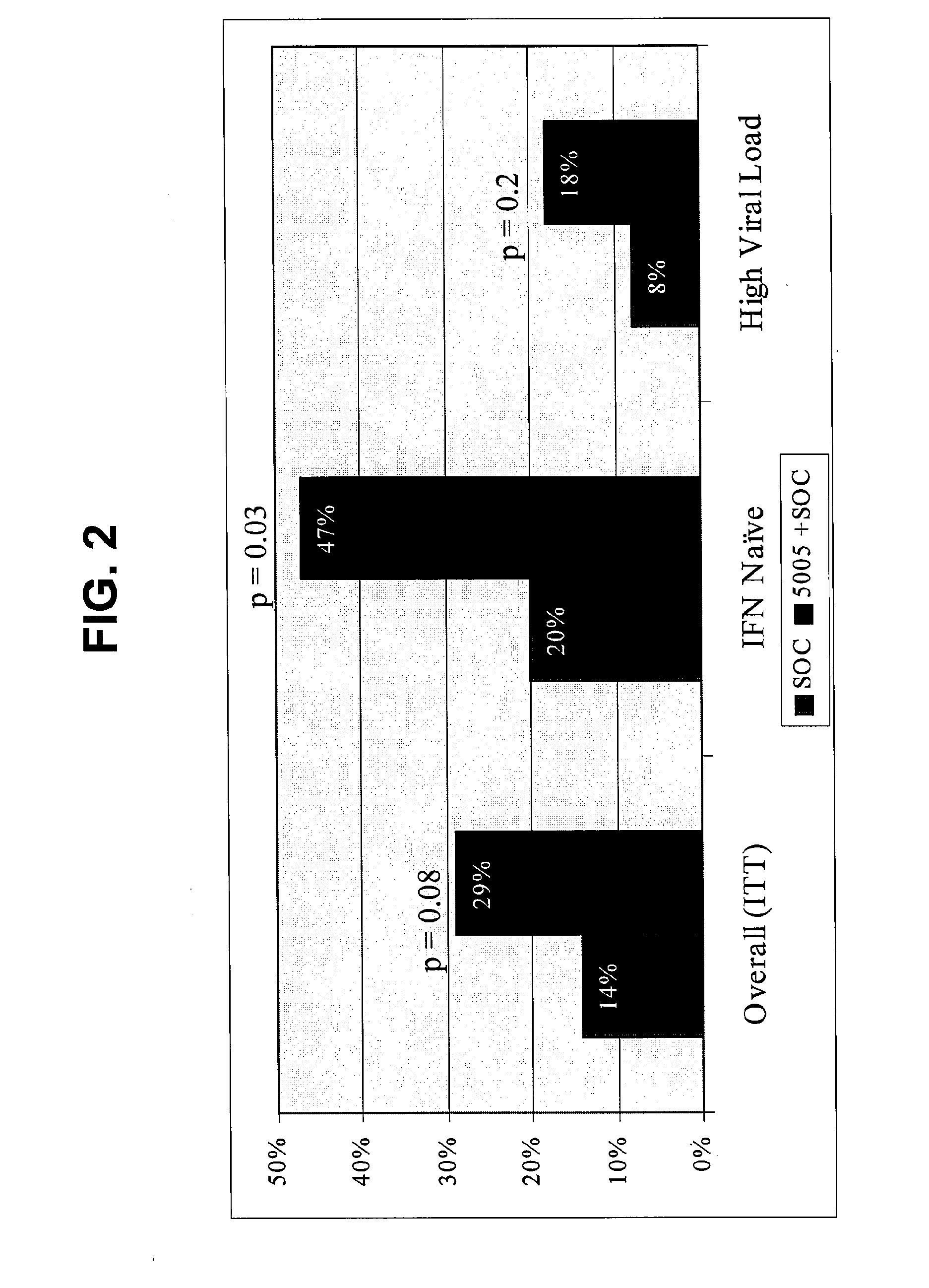
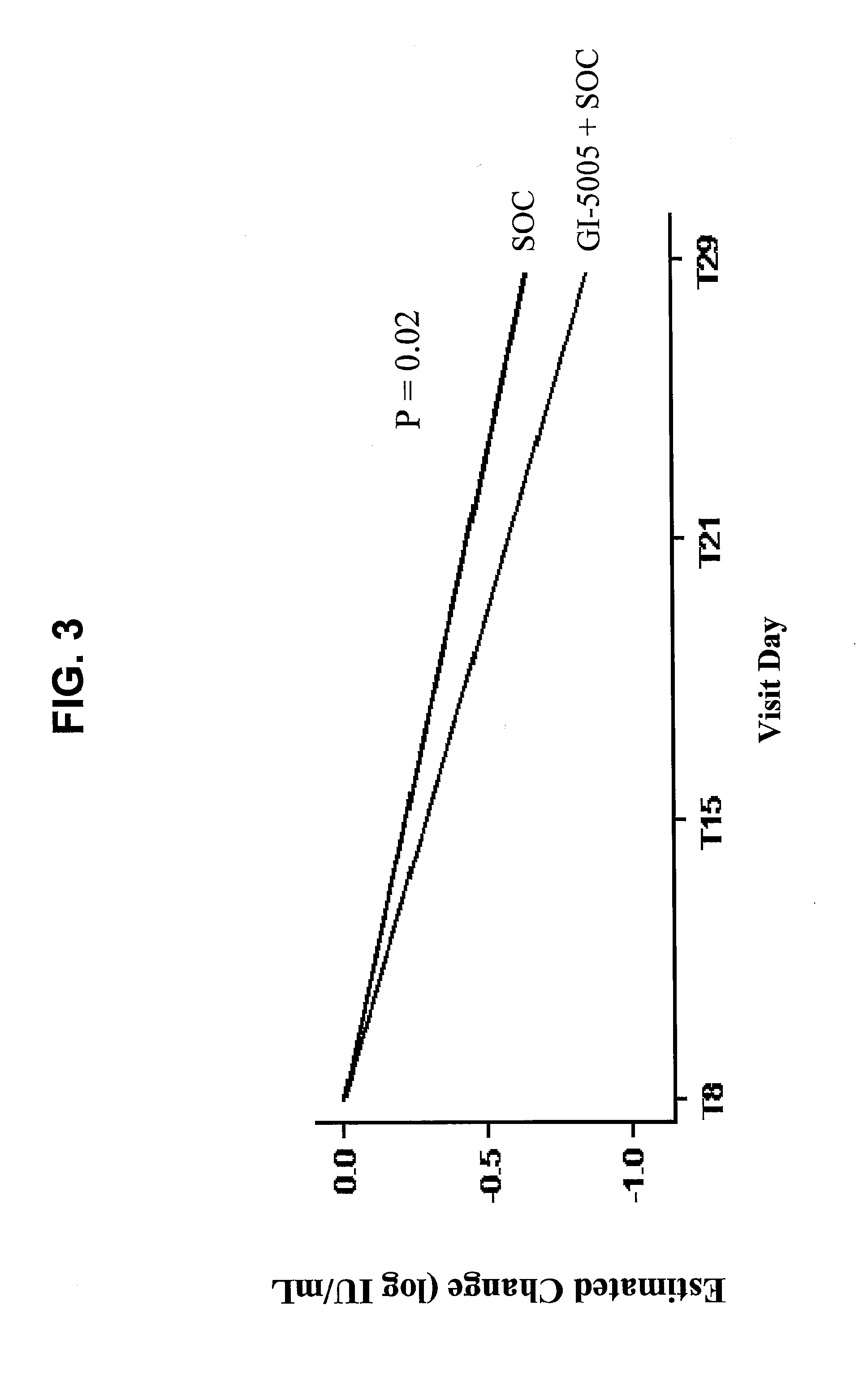
Disclosed are uses of immunotherapeutic compositions in combination with Standard of Care (SOC), or interferon therapy combined with anti-viral therapy, for the improved treatment of chronic hepatitis C virus (HCV) infection and related conditions, including liver function. The compositions, kits and uses of the invention, as compared to the use of SOC therapy alone: improves the rate of early response to therapy as measured by early virologic markers (e.g., RVR and EVR), enlarges the pool of patients who will have sustained responses to therapy over the long term, offers shortened courses of therapy for certain patients, enables “rescue” of patients who are non-responders or intolerant to SOC therapy, improves liver function and / or reduces liver damage in patients, and enables the personalization of HCV therapy for a patient, which can result in dose sparing, improved patient compliance, reduced side effects, and improved long term therapeutic outcomes.
Owner:GLOBE IMMUNE INC
Pharmaceutical Compositions for Treatment of HCV Patients that are Poor-Responders to Interferon
InactiveUS20100330035A1Inhibitory activityOrganic active ingredientsPeptide/protein ingredientsInterferon therapyPrimate
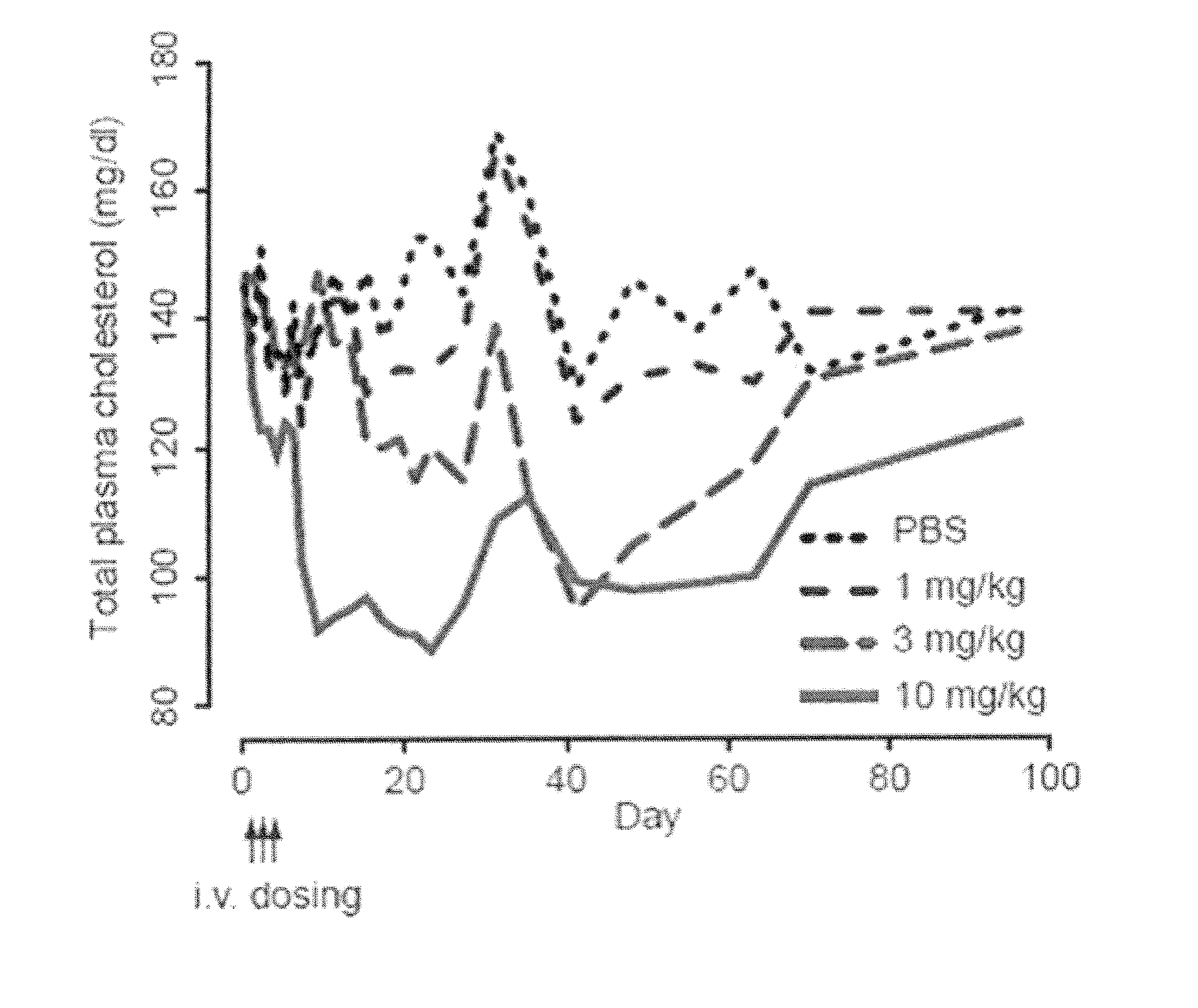
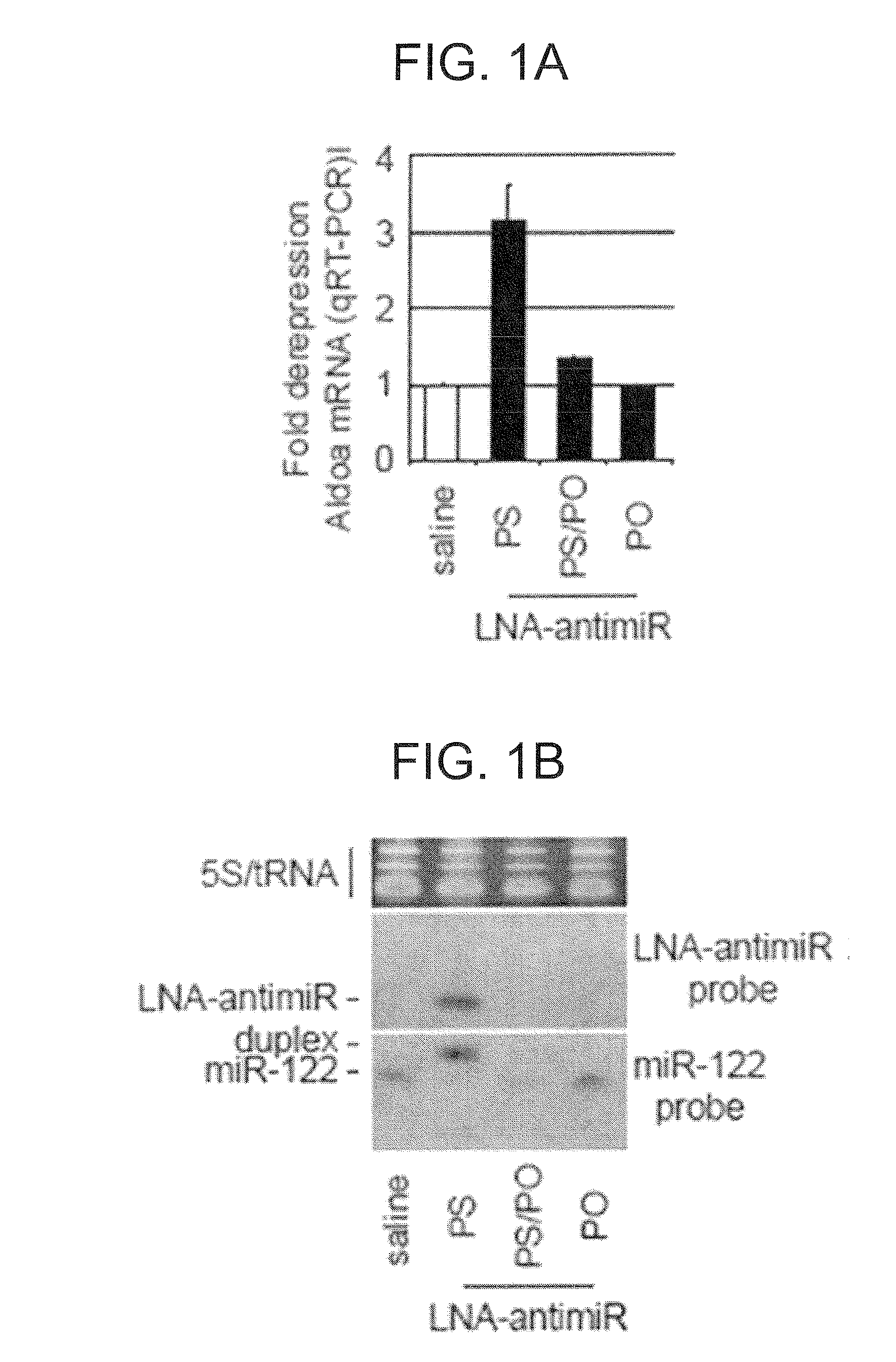
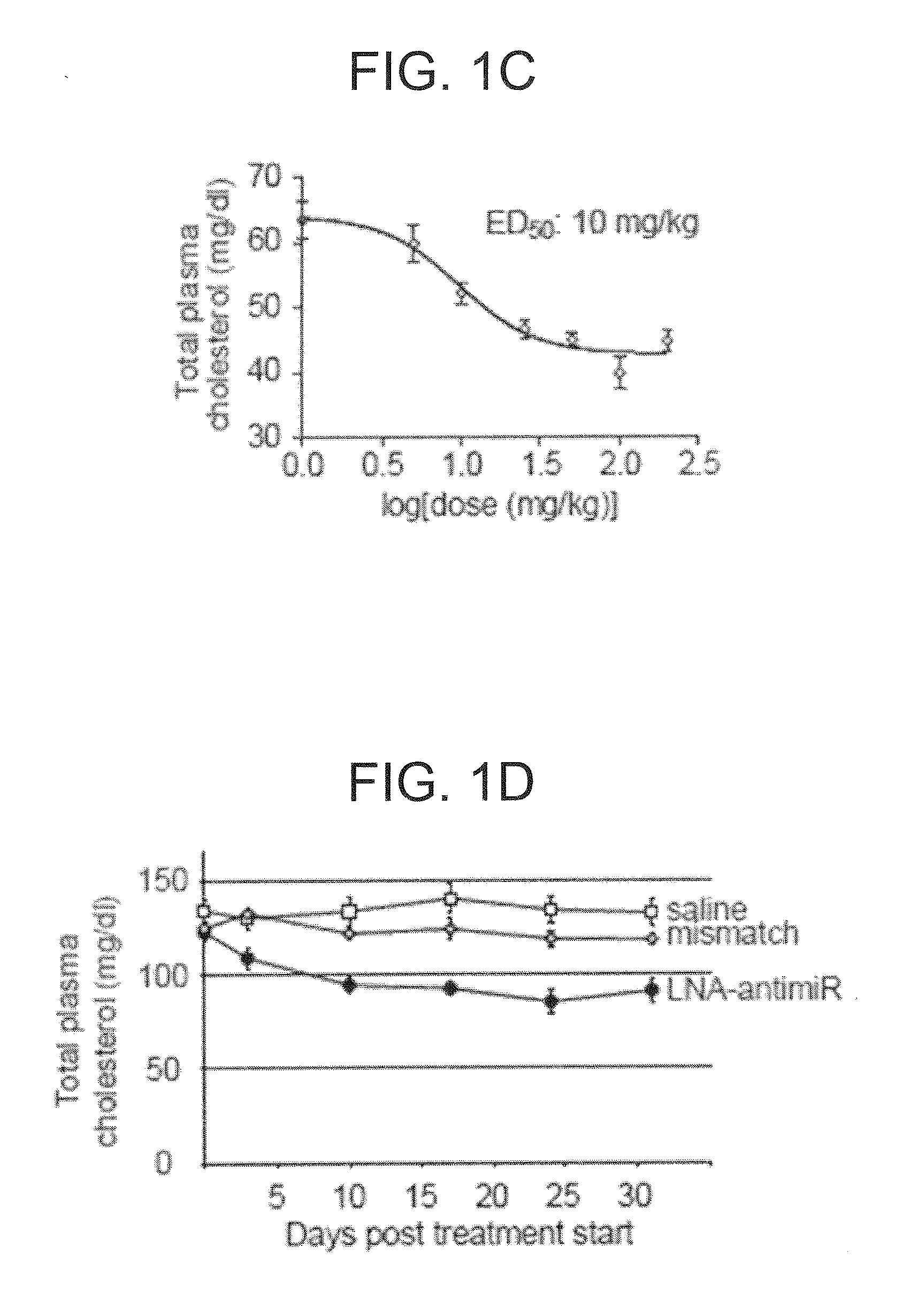
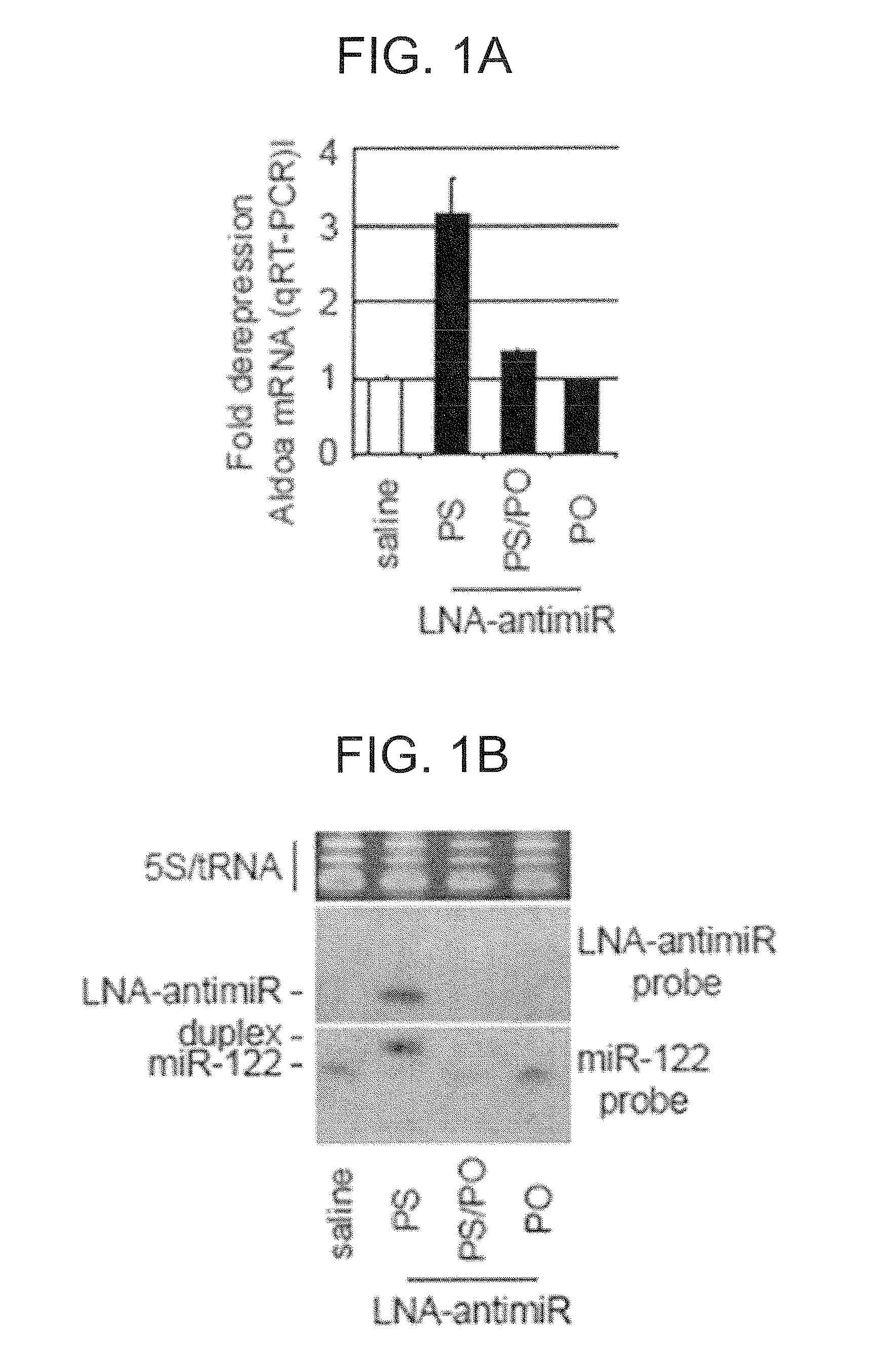
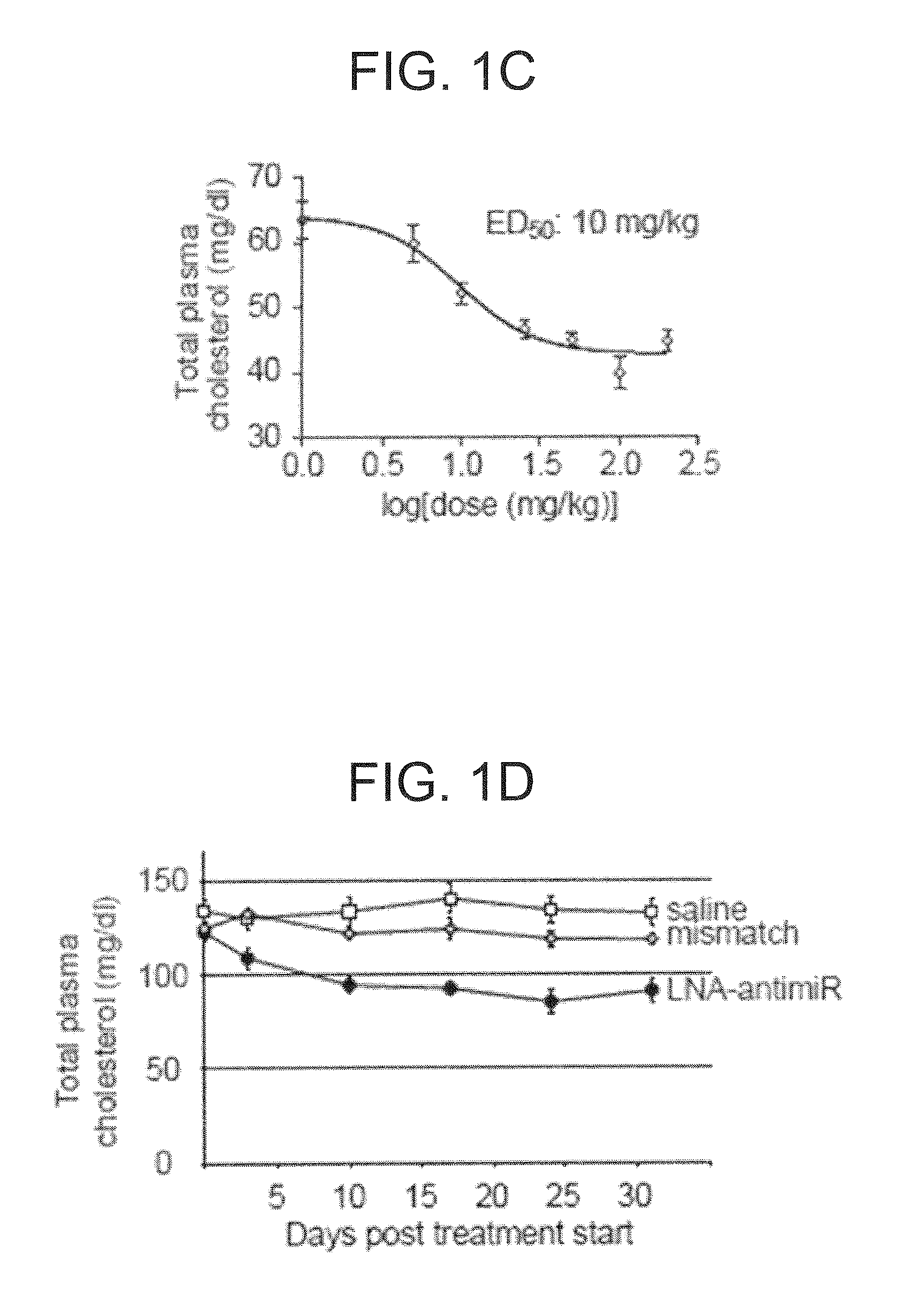
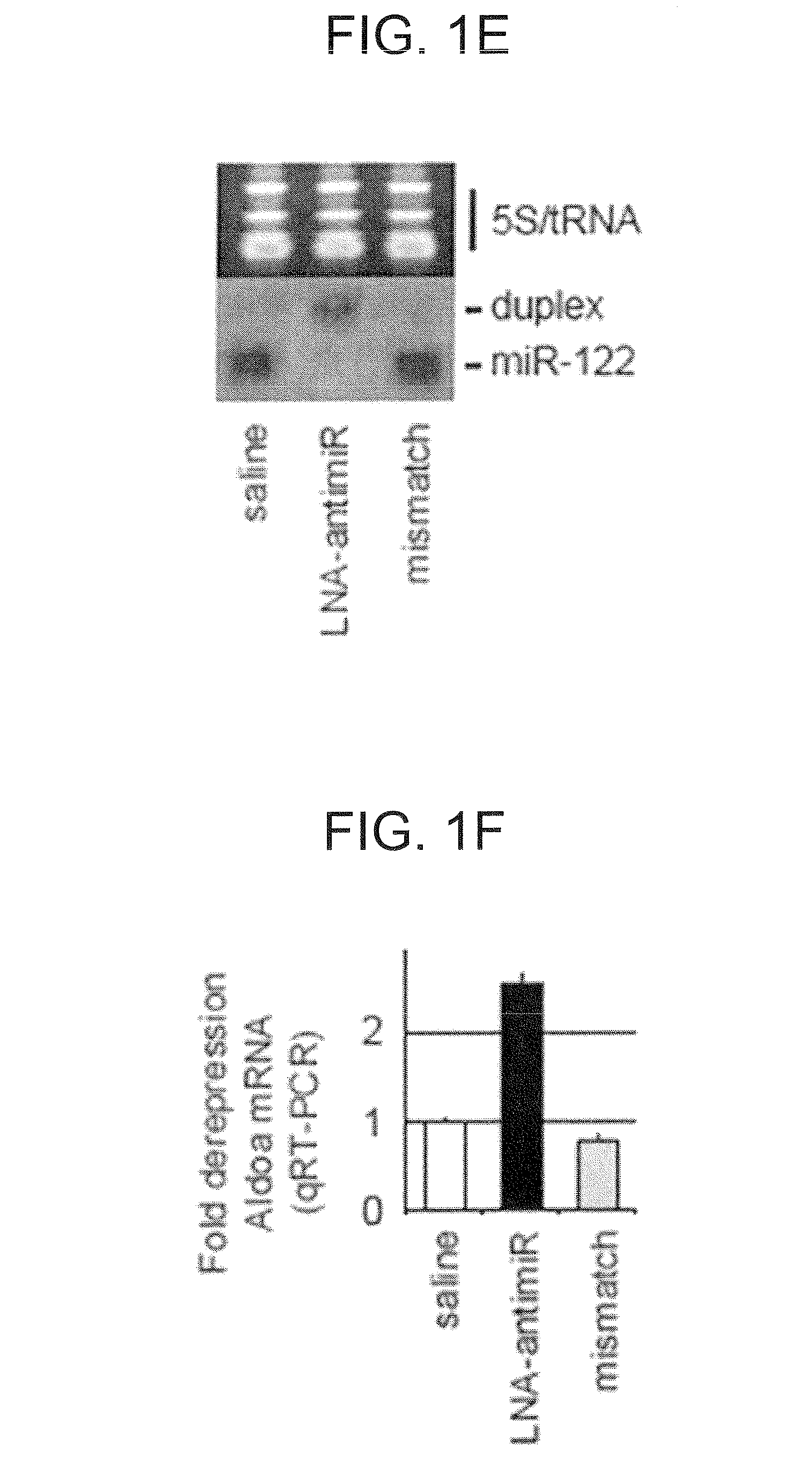
The present invention provides compositions and methods of treatment of HCV infected subjects that are not sensitive to interferon treatment. Further, compositions and methods are provided for prevention of organ transplant rejection. The compositions of the invention comprise an anti microRNA-122 oligonucleotide, and are made for administration to a primate.
Owner:ROCHE INNOVATION CENT COPENHAGEN
Methods and compositions for interferon therapy
InactiveUS20050025742A1Long contact timeKeep for a long timePeptide/protein ingredientsGenetic material ingredientsGene deliveryInterferon therapy
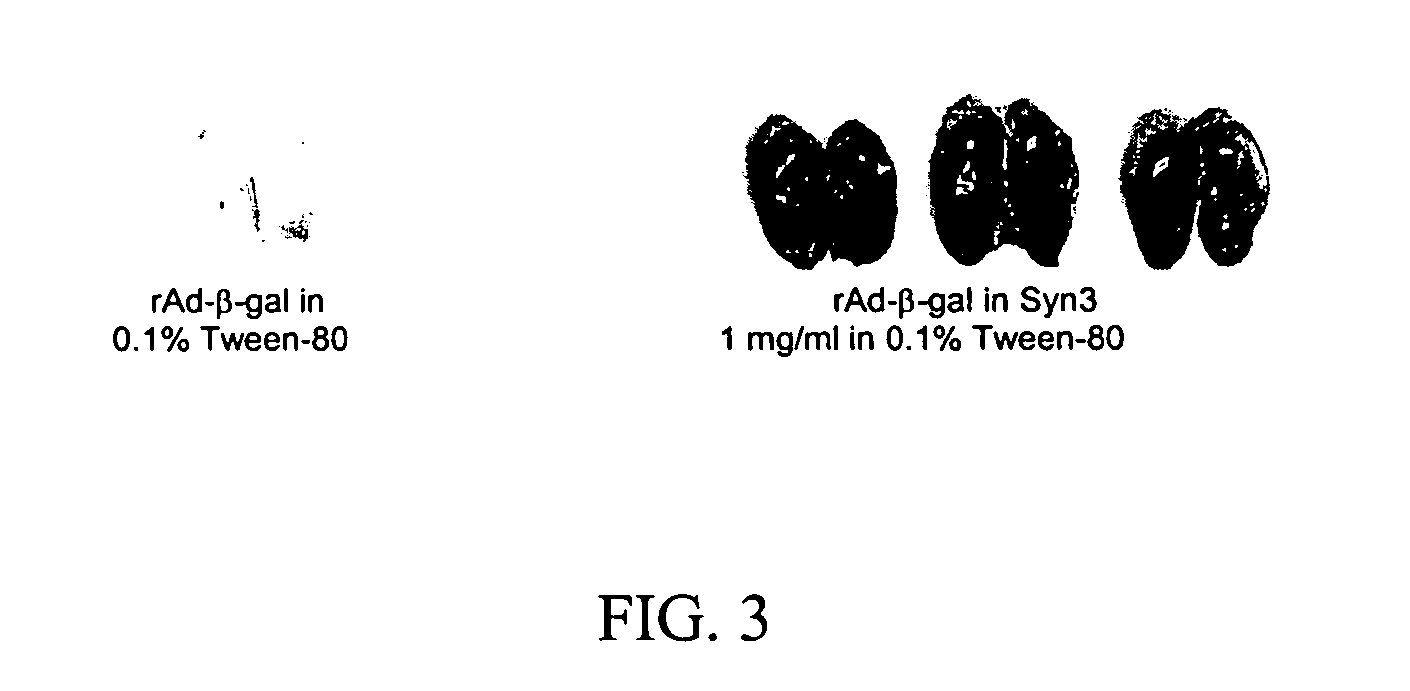
Methods and pharmaceutical compositions for administering protein or gene therapy to tissues or organs having an epithelial cell layer are provided. A protein or nucleic acid encoding the protein is administered to the target tissue or organ in combination with treatment with a delivery enhancing agent which increases the delivery of the interferon or nucleic acid to the cells of the target tissues or organs. The methods and combinations are particularly useful in the treatment of cancers and other conditions responsive to interferon therapy. An exemplary method comprises the transurethral intravesical administration to the bladder of a therapeutically effective amount of a pharmaceutical composition comprising an alpha-interferon or a gene delivery system encoding the interferon and SYN3 or a SYN3 homolog or analog. In the urinary bladder, as much as a 10 to 1000 fold increased in interferon levels and activity may be observed with the use of a SYN3 formulation as opposed to a PBS formulation of the same interferon protein or interferon gene delivery system.
Owner:CANJI
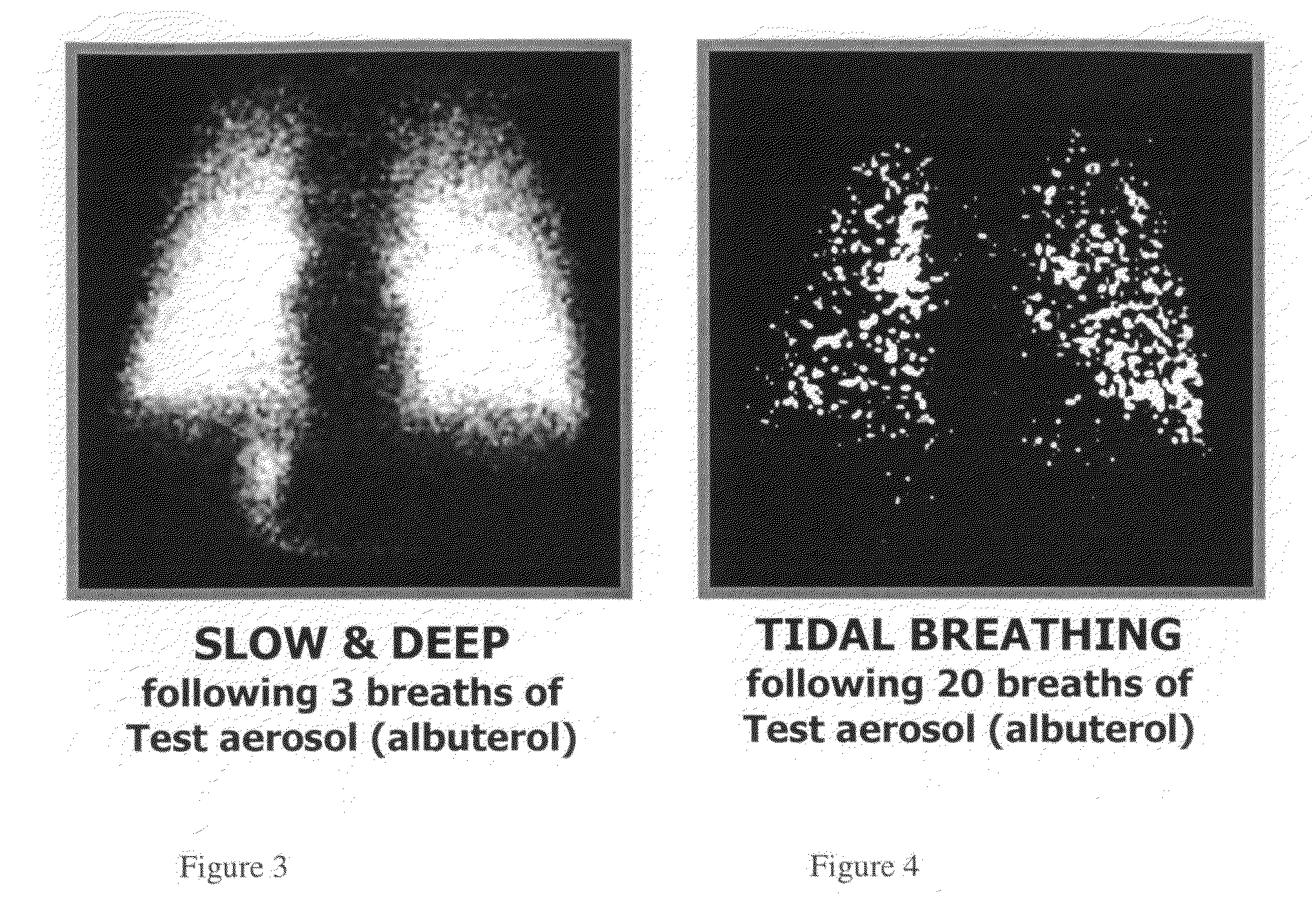
Method of treating pulmonary disease with interferons
InactiveUS20070065367A1Improve toleranceDecrease in TNF-aPowder deliveryDispersion deliveryInterferon therapyDisease
A method of treating a pulmonary disease such as, for instance idiophathic pulmonary fibrosis (IPF) and asthma, comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Also, pharmaceutical compositions of one or more aerosolized interferon(s) are provided.
Owner:NEW YORK UNIV +1
Adsorbent for eliminating hepatitis c virus, adsorber, and adsorption method
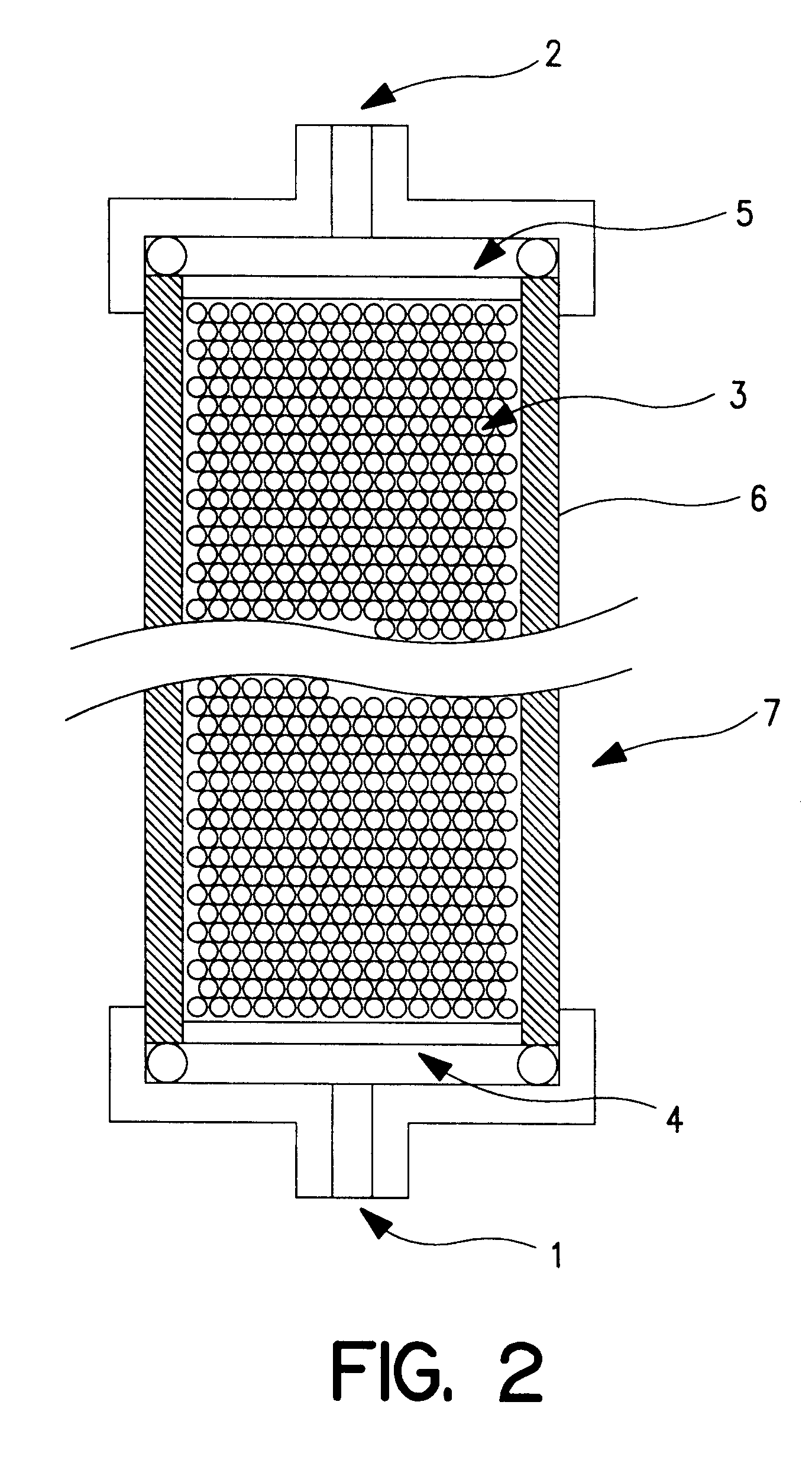
InactiveUS20030044769A1High adsorbing affinityImprove performanceOrganic active ingredientsBiocideInterferon therapySorbent
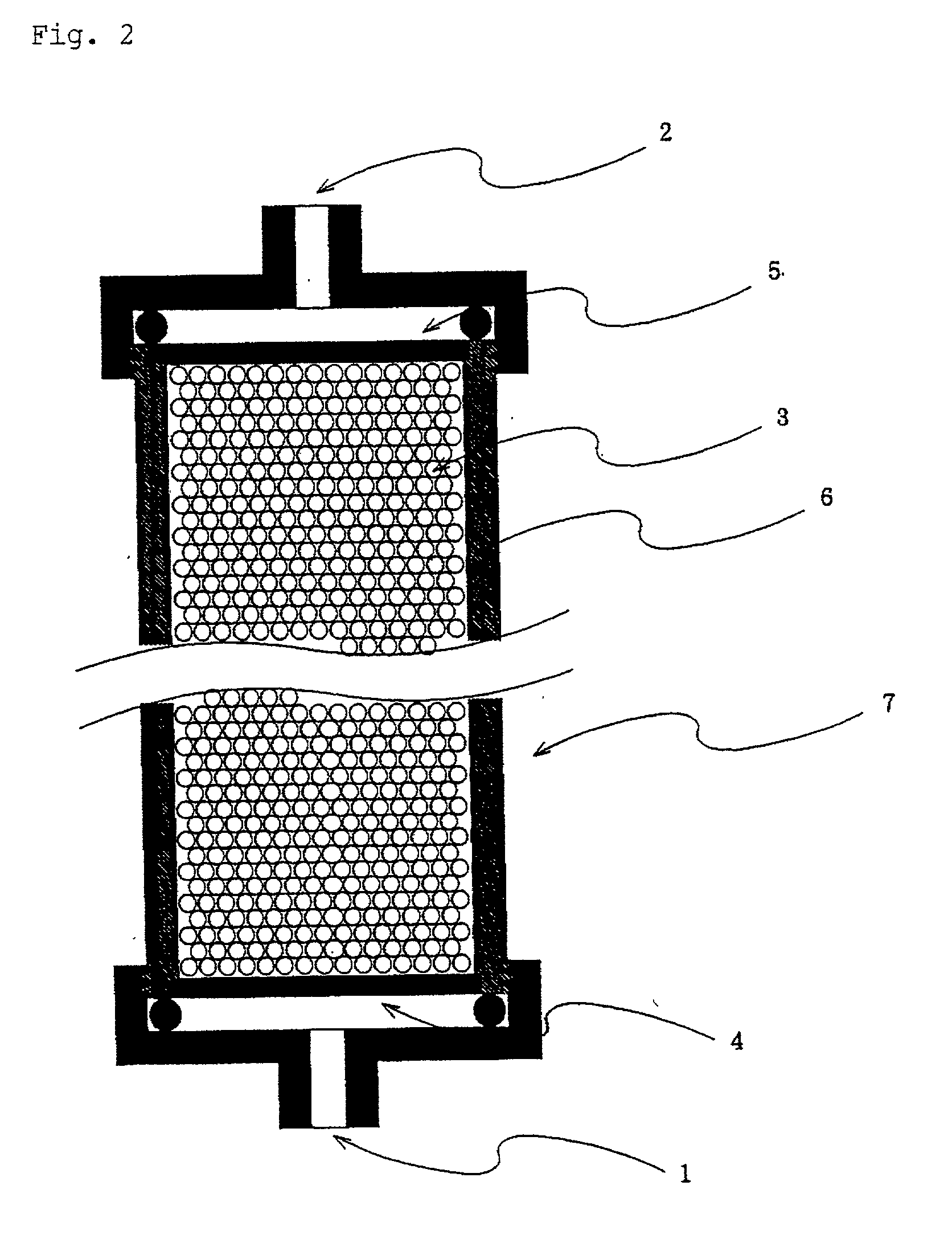
An adsorbent for removing hepatitis C virus which has the ability to adsorb HCV particles, particularly immune-complex HCV particles, from a patient's body blood safely and with high efficiency and high selectivity for enhancing the efficacy of interferon therapy, an HCV adsorption apparatus including said adsorbent, and a adsorbing method for removing HCV are provided. An adsorbent for removing hepatitis C virus which comprises a compound capable of adsorbing hepatitis C virus as immobilized on a water-insoluble carrier, an adsorption apparatus including said adsorbent, and an adsorbing method for removing HCV.
Owner:KANEKA CORP
Methods for treating psychosis associated with interferson-alpha therapy
ActiveUS20060063748A1Promote mental healthSufficient effectBiocideNervous disorderInterferon therapyPharmacology
The present invention relates to the treatment of psychosis associated with interferon-therapy. The present invention further relates to kits for the treatment of Hepatitis C in a patient.
Owner:CORCEPT THERAPEUTICS INC
Method for treating diseases with omega interferon
InactiveUS20070248572A1Change levelAntibacterial agentsOrganic active ingredientsInterferon therapyTolerability
A method of treating an immunologic, proliferative, or infectious disease in a warm-blooded animal is disclosed. The method comprises administering to the animal omega interferon (IFN) at a dosage and activity for the disease state treated sufficient to induce a therapeutic response in the animal, which dosage and activity for the disease state treated is higher than would be well-tolerated based on data for non-omega IFN's. The omega IFN is administered alone or In combination with a therapeutically effective amount of at least one adjunctive therapeutic agent. Also disclosed is an article of manufacture useful for treating an immunologic, proliferative, or infectious disease, which article comprises (1) omega IFN in a form suitable for administering a therapeutically effective amount of the omega IFN to the subject in order to induce the desired therapeutic response (2) instructions for administering the omega IFN as desired, that is higher than would be well-tolerated based on data for non-omega IFNs.
Owner:INTARICA THERAPEUTICS
Treatment Of Hepatitis C In The Asian Population With Interferon-Beta
InactiveUS20080075696A1Good effectBiocidePeptide/protein ingredientsGreek letter betaInterferon therapy
The invention relates to the use of IFN-beta in the manufacture of a medicament for the treatment of an HCV infection in a treatment-naïve patient belonging to the Asian population.
Owner:MERCK SERONO SA
Adsorbent for eliminating hepatitis C virus, adsorber, and adsorption method
InactiveUS6600014B2High adsorbing affinityImprove performanceBioreactor/fermenter combinationsBiological substance pretreatmentsInterferon therapyImmune complex deposition
An adsorbent for removing hepatitis C virus which has the ability to adsorb HCV particles, particularly immune-complex HCV particles, from a patient's body blood safely and with high efficiency and high selectivity for enhancing the efficacy of interferon therapy, an HCV adsorption apparatus including said adsorbent, and a adsorbing method for removing HCV are provided.An adsorbent for removing hepatitis C virus which comprises a compound capable of adsorbing hepatitis C virus as immobilized on a water-insoluble carrier, an adsorption apparatus including said adsorbent, and an adsorbing method for removing HCV.
Owner:KANEKA CORP
Methods of predicting responsiveness to interferon treatment and treating hepatitis c infection
InactiveUS20120009148A1Prediction of responsivenessPeptide/protein ingredientsMicrobiological testing/measurementInterferon therapyHLA-G
Provided are methods of predicting responsiveness of a subject to interferon treatment, comprising comparing a level of expression in a cell of the subject of at least one gene selected from the group consisting KIR3DL3, KIR3DL2, KIR3DL1, KIR2DL1, KIR2DL2, KIR2DL3, KLRG1, KIR3DS1, CD160, HLA-A, HLA-B, HLA-C, HLA-F, HLA-G and IFI27 to a reference expression data of the at least one gene obtained from at least one interferon responder subject and / or at least one interferon non-responder subject. Also provided are methods and pharmaceutical compositions for treating a subject in need of interferon treatment.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Pharmaceutical compositions for treatment of HCV patients that are poor-responders to interferon
Owner:ROCHE INNOVATION CENT COPENHAGEN
Recombinant human interferon-like proteins
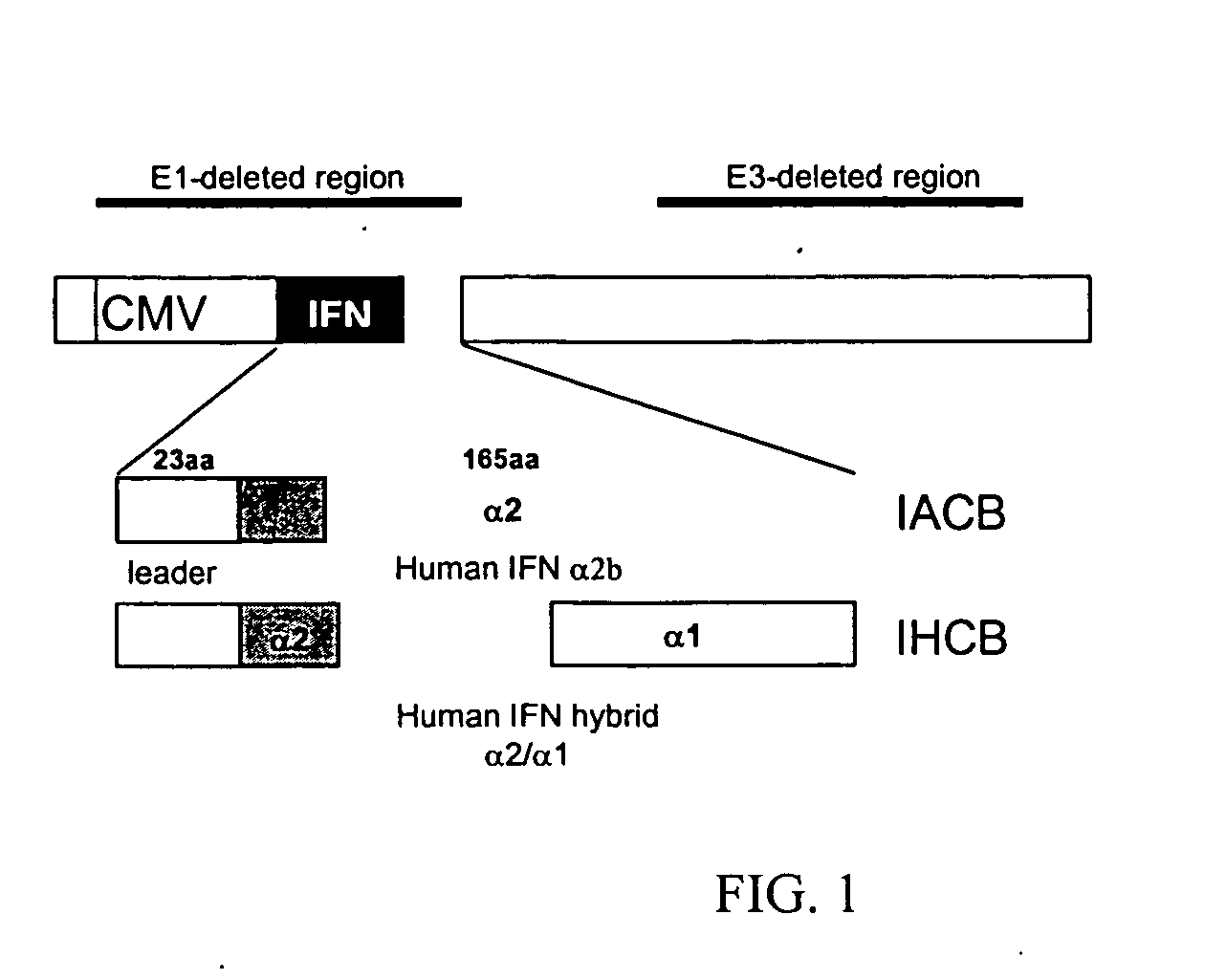
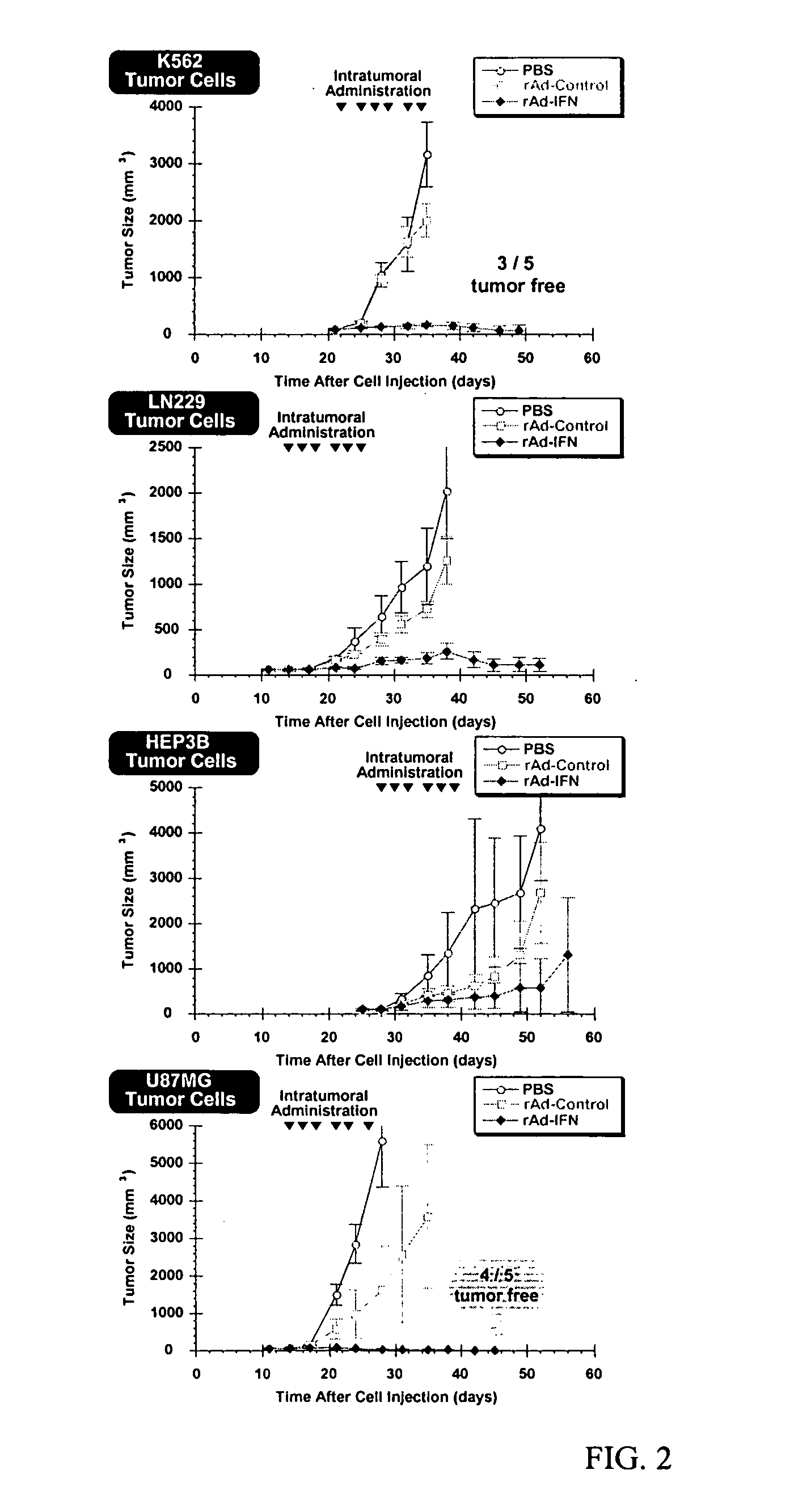
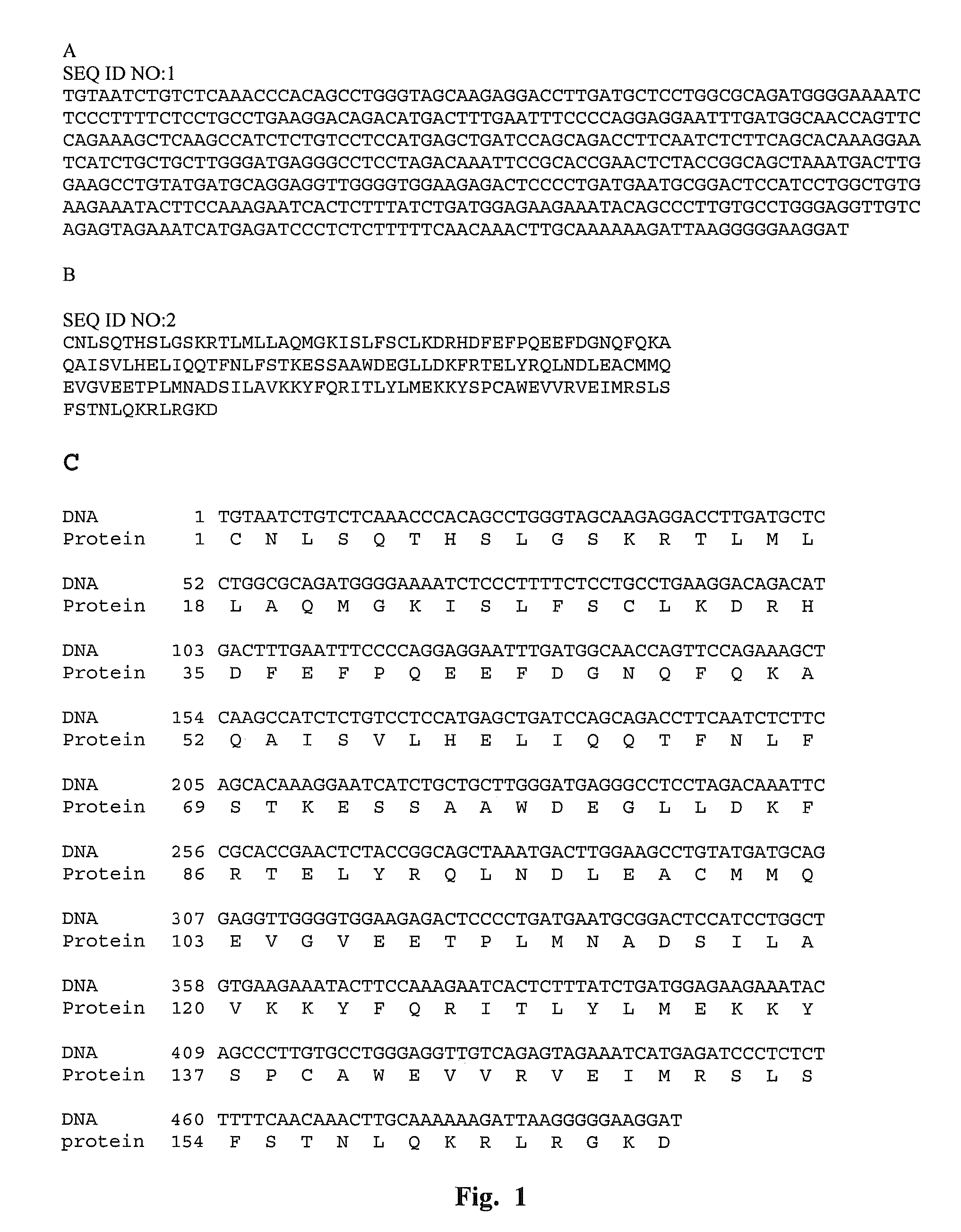
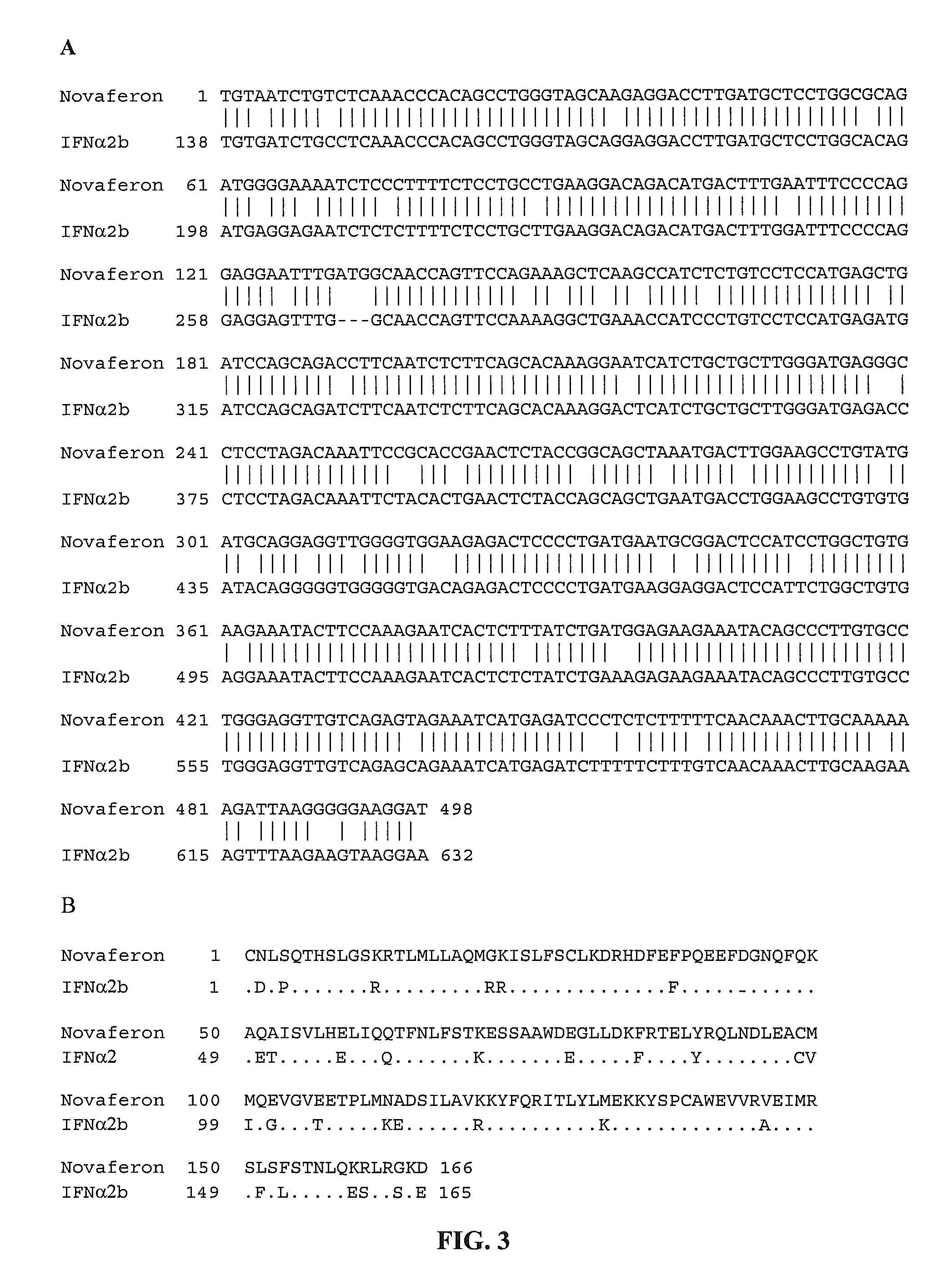
This application relates to recombinant human interferon-like proteins. In one embodiment a recombinant protein created by gene shuffling technology is described having enhanced anti-viral and anti-proliferative activities in comparison to naturally occurring human interferon alpha 2b (HuIFN-α2b). The invention encompasses a polynucleotide encoding the protein and recombinant vectors and host cells comprising the polynucleotide. Preferably the polynucleotide is selected from the group of polynucleotides each having a sequence at least 93% identical to SEQ ID: No. 1 and the protein is selected from the group of proteins each having an amino acid sequence at least 85% identical to SEQ ID No: 2. The proteins and compositions comprising the proteins can be used for treatment of conditions responsive to interferon therapy, such as viral diseases and cancer.
Owner:NOVAGEN HLDG CORP
Treatments for viral infections using IFN cytokines and ribavirin, alone or in combination
InactiveUS20060024271A1Reduce dosageReduced pro-inflammatory responseBiocidePeptide/protein ingredientsCytokineViral infection
A treatment or prophylaxis for viral infection using interferon (IFN) cytokines alone or in combination with ribavirin is provided. The treatments and prophylaxis allow for lowered dosages of IFNs, reduced pro-inflammatory responses, and delays the initiation time and reduced frequency of the IFN treatment required.
Owner:AFG BIOSOLUTIONS
NS5A nucleotide sequence variation as a marker for interferon response
InactiveUS20050260567A1Raise the possibilitySustained responseSsRNA viruses positive-senseMicrobiological testing/measurementInterferon therapyNucleotide variation
Methods and reagents for determining a nucleotide variation at position 937 of the HCV-1a NS5A gene useful in predicting an individual's response to interferon treatment are presented.
Owner:ROCHE PALO ALTO LLC
Recombinant human interferon-like proteins
This application relates to recombinant human interferon-like proteins. In one embodiment a recombinant protein created by gene shuffling technology is described having enhanced anti-viral and anti-proliferative activities in comparison to naturally occurring human interferon alpha 2b (HuIFN-α2b). The invention encompasses a polynucleotide encoding the protein and recombinant vectors and host cells comprising the polynucleotide. Preferably the polynucleotide is selected from the group of polynucleotides each having a sequence at least 93% identical to SEQ ID: No. 1 and the protein is selected from the group of proteins each having an amino acid sequence at least 85% identical to SEQ ID No: 2. The proteins and compositions comprising the proteins can be used for treatment of conditions responsive to interferon therapy, such as viral diseases and cancer.
Owner:NOVAGEN HLDG CORP
Method of treatment using interferon-tau
InactiveUS7105154B2Improve bindingStabilization and protectionSenses disorderNervous disorderInterferon therapyInterferon tau
Owner:PEPGEN CORP
Methods for treating viral infection using IL-28 and IL-29 cysteine mutants
InactiveUS20050244423A1Peptide/protein ingredientsViral antigen ingredientsInterferon therapyCysteine thiolate
IL-28A, IL-28B, IL-29, and certain mutants thereof have been shown to have antiviral activity on a spectrum of viral species. Of particular interest is the antiviral activity demonstrated on viruses that infect liver, such as hepatitis B virus and hepatitis C virus. In addition, IL-28A, IL-28B, IL-29, and mutants thereof do not exhibit some of the antiproliferative activity on hematopoietic cells that is observed with interferon treatment. Without the immunosuppressive effects accompanying interferon treatment, IL-28A, IL-28B, and IL-29 will be useful in treating immunocompromised patients for viral infections.
Owner:ZYMOGENETICS INC
Forecasting method of treating effect of interferon on treating chronic hepatitis B
InactiveCN101908096ARaise the ratioSuitable for predicting efficacy before treatmentSpecial data processing applicationsInterferon therapyPredictive methods
The invention relates to a forecasting method of the treating effect of interferon on chronic hepatitis B. The forecasting method comprises the following steps of: collecting information related to chronic hepatitis B patients; inputting treatment outcome influencing factors of the patients; dividing the treating effect evaluation into three classes; determining specific scores of all levels of the influencing factors by taking the influencing factors as independent variable and the treating effect as dependent variable and building a rating scale and a model; randomly dividing collected illness cases into a training set and a test set, building a rating scale and a model by taking the training set as a sample, and detecting the accuracy of the rating scale and the model by taking the test set as a sample; taking the Youden index which is shown as a formula: sensitivity+specificity-1 as the evaluation index of the rating scale and the accurate model performance, and when the Youden index takes a maximum value, prompting that the forecasting performance is optimal; and enabling the forecasting performance to be optimal by adjusting parameters of mutation rate, mating rate, sub-algebra generation, and the like of a genetic algorithm as well as parameters of kernel training parameters, outlier penalty coefficients, and the like of a support vector machine.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Method of treating tuberculosis with interferons
A method of treating tuberculosis comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Further, a method of reducing the infectivity of tuberculosis or reducing the number of infectious organisms present in the lungs of a patient suffering from tuberculosis comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Also, pharmaceutical compositions of one or more aerosolized interferon(s) are provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Method of predicting therapeutic effect of interferon treatment on hepatitis C
InactiveCN101750474AEfficient managementHigh cure rateBiological testingTesting medicinal preparationsInterferon therapyPatient management
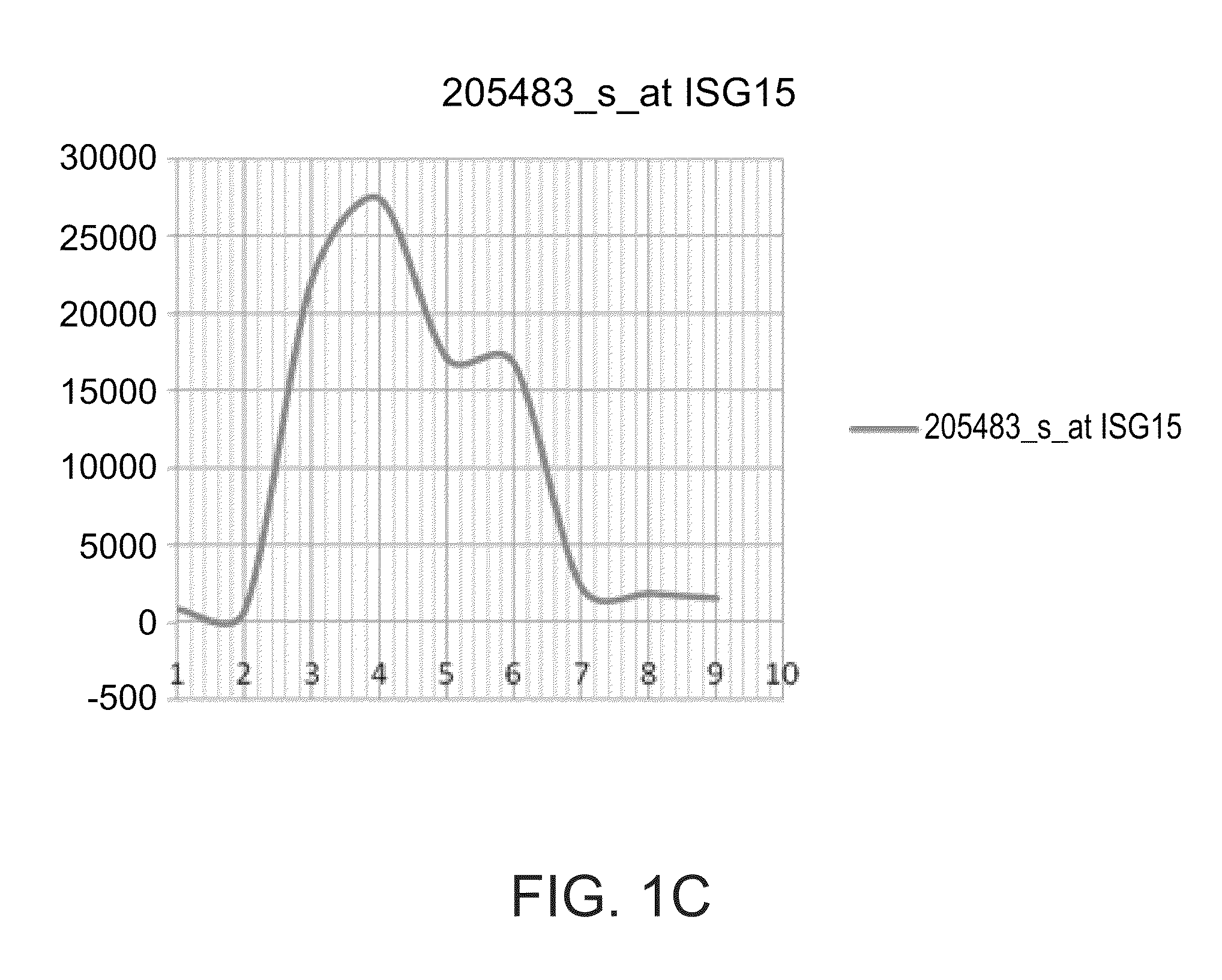
The invention relates to an in vitro method of predicting the therapeutic effect of interferon treatment on a patient with hepatitis C, which belongs to the in vitro diagnostic reagent category of the biological medicine and is a substantive improvement based on a patent being applied for at present. The interferon treatment is effective only for 50 percent of patients with hepatitis C. The clinical operation is short of a method of predicting the therapeutic effect before the treatment of hepatitis C. According to the positioning and analysis of the expression of the ISG15 or MxA protein in the liver tissue through the immunohistochemical technique before the treatment of patients with hepatitis C, the distributions of ISG15 or MxA are different in the liver tissues of the patients for whom the interferon treatment is effective and the patients for whom the interferon treatment is not effective: the expression of the genes in the liver cells indicates that the interferon treatment is not effective for the patients; and the expression of the genes in the macrophage cells indicates that the interferon treatment is effective for the patients. The method is used to predict the therapeutic effects of the interferon treatment on 31 patients with hepatitis C, and the rate of accuracy reaches 100 percent. The invention can predict whether the patient with hepatitis C is sensitive to the interferon treatment before the interferon treatment, thereby optimizing the patient management.
Owner:陈利民
Method of treating tuberculosis with interferons
ActiveUS20080292559A1Increase appetiteReduction in wheezingAntibacterial agentsOrganic active ingredientsInterferon therapyInterferon alpha
A method of treating tuberculosis comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Also, pharmaceutical compositions of one or more aerosolized interferon(s) are provided.
Owner:NEW YORK UNIV +1
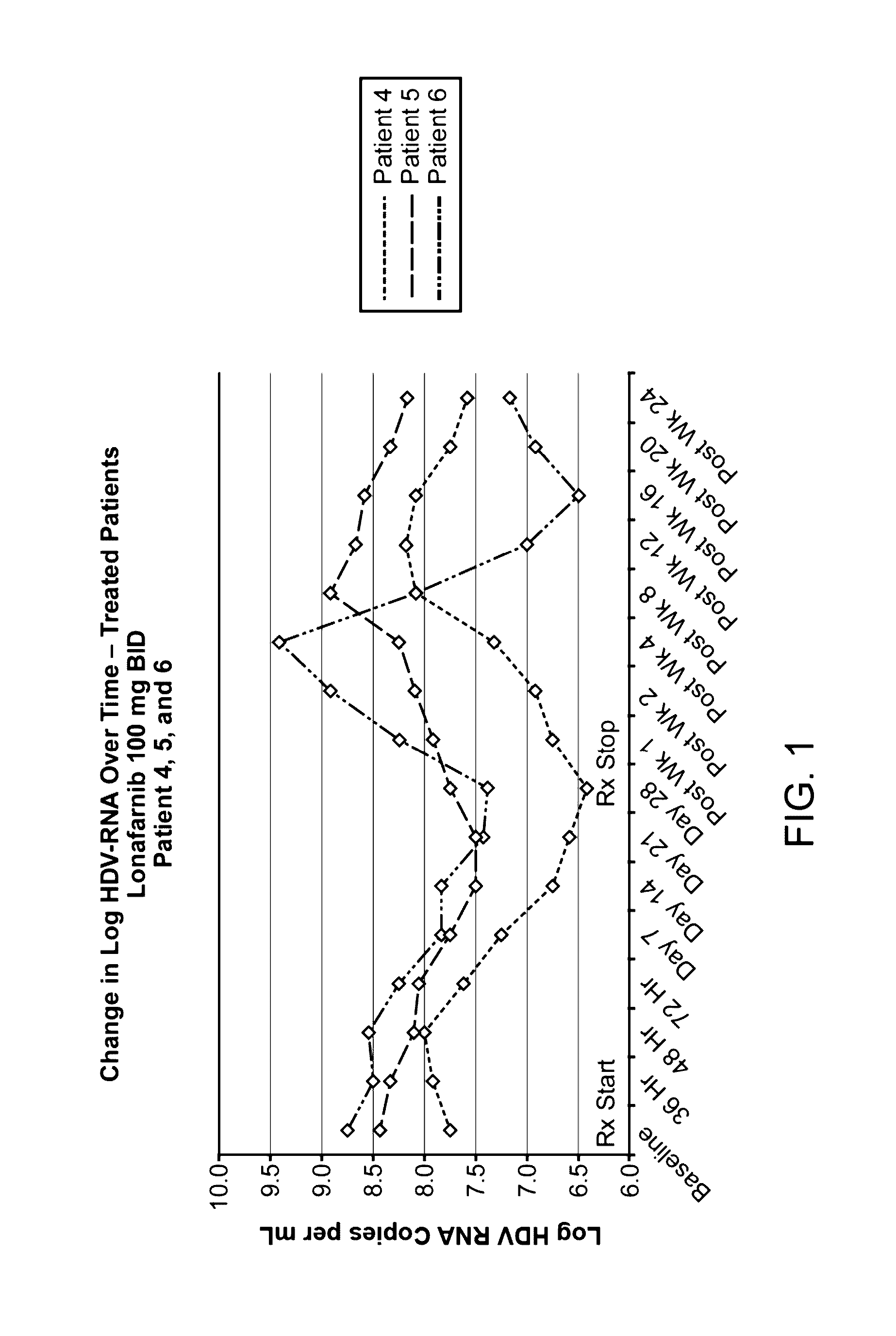
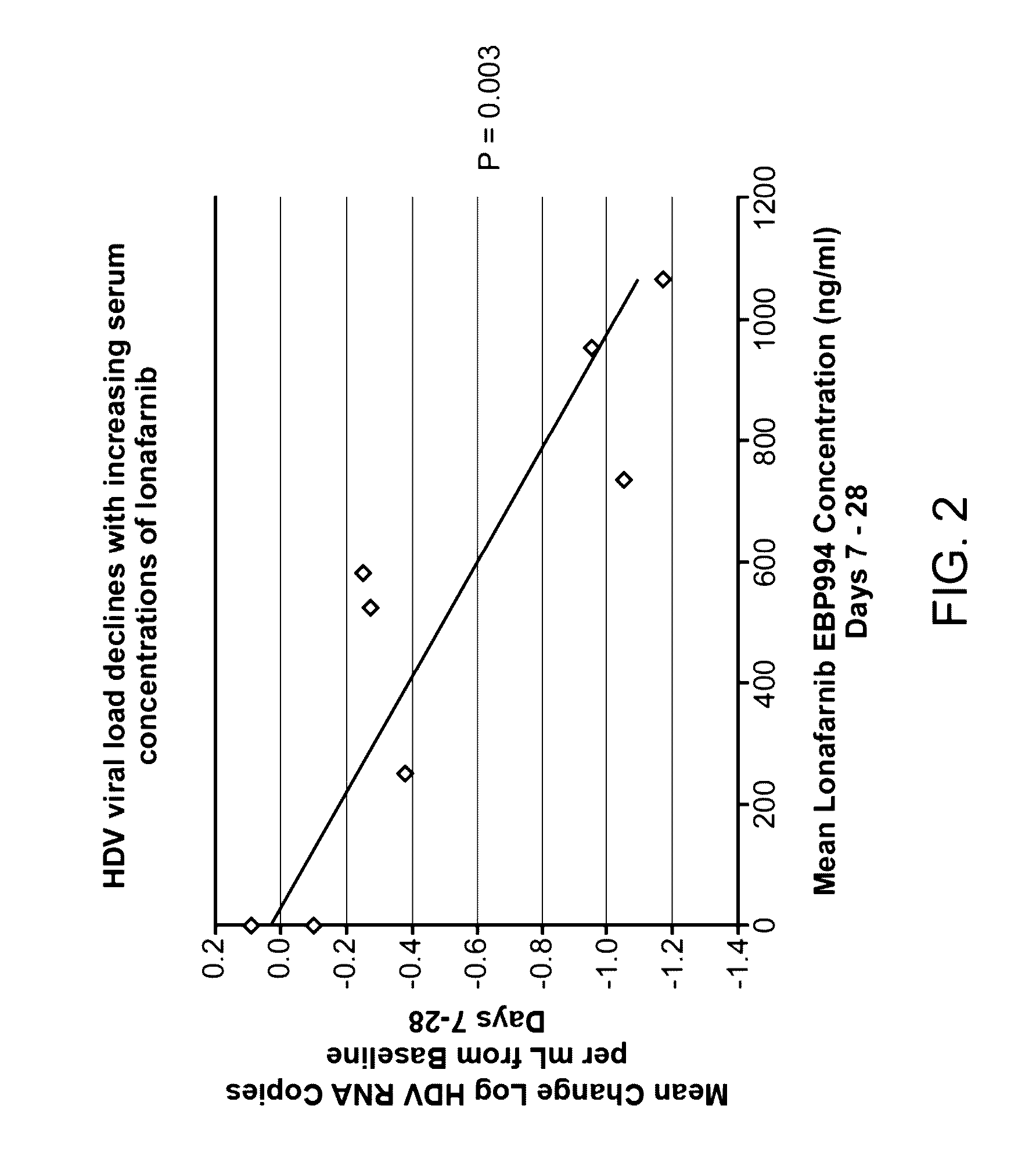
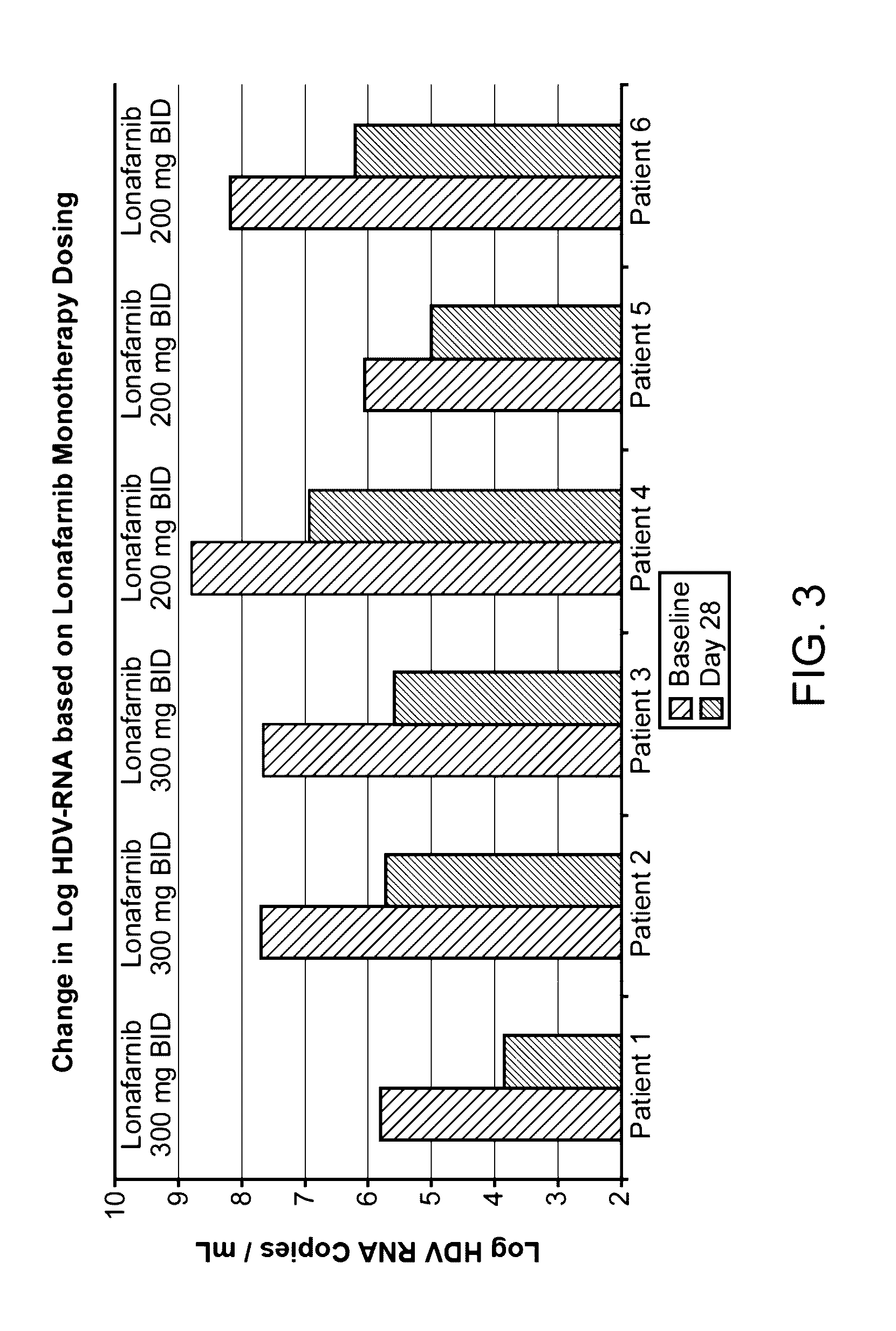
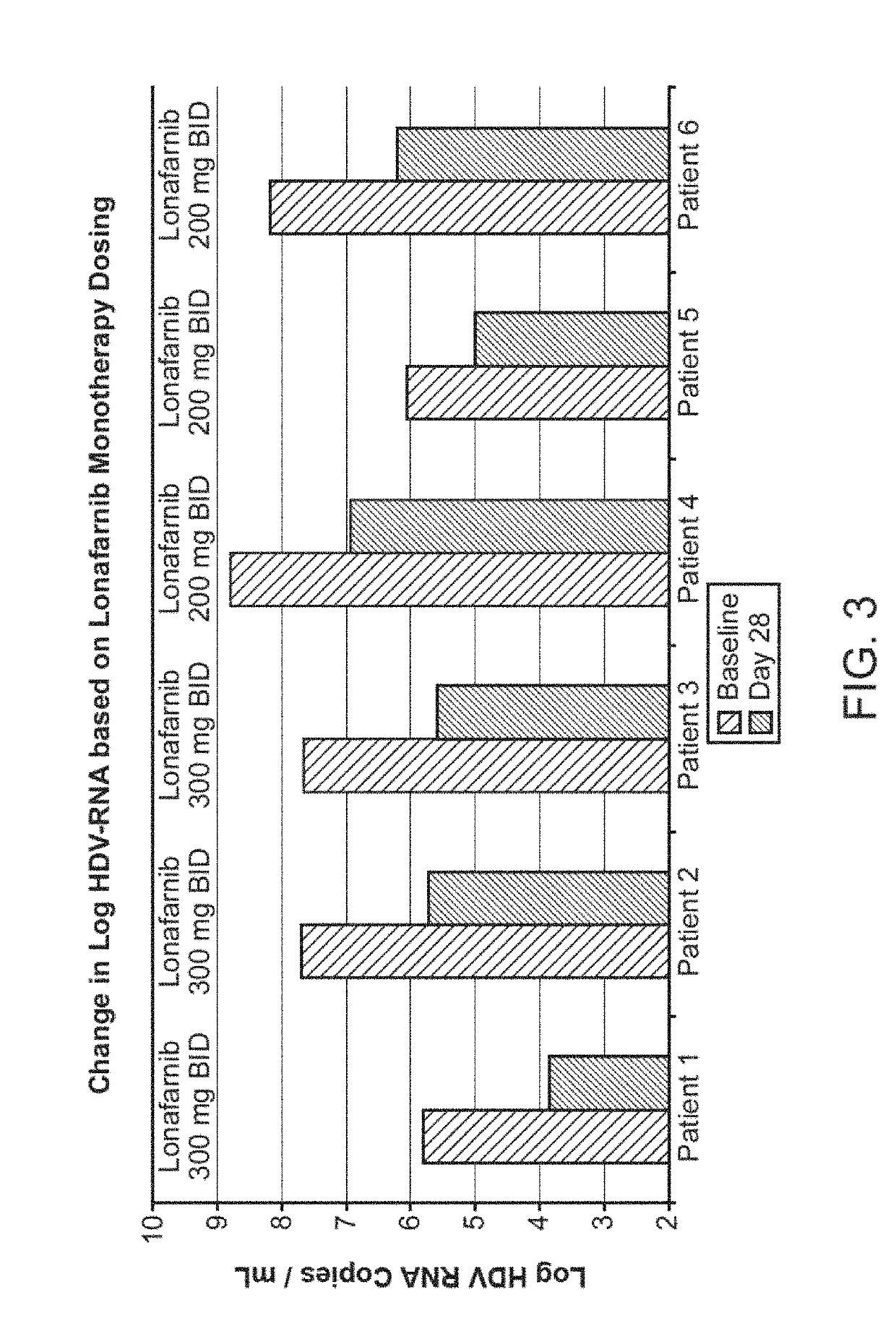
Treatment of hepatitis delta virus infection
ActiveUS20170042862A1Organic active ingredientsPowder deliveryInterferon therapyDeltaretrovirus Infections
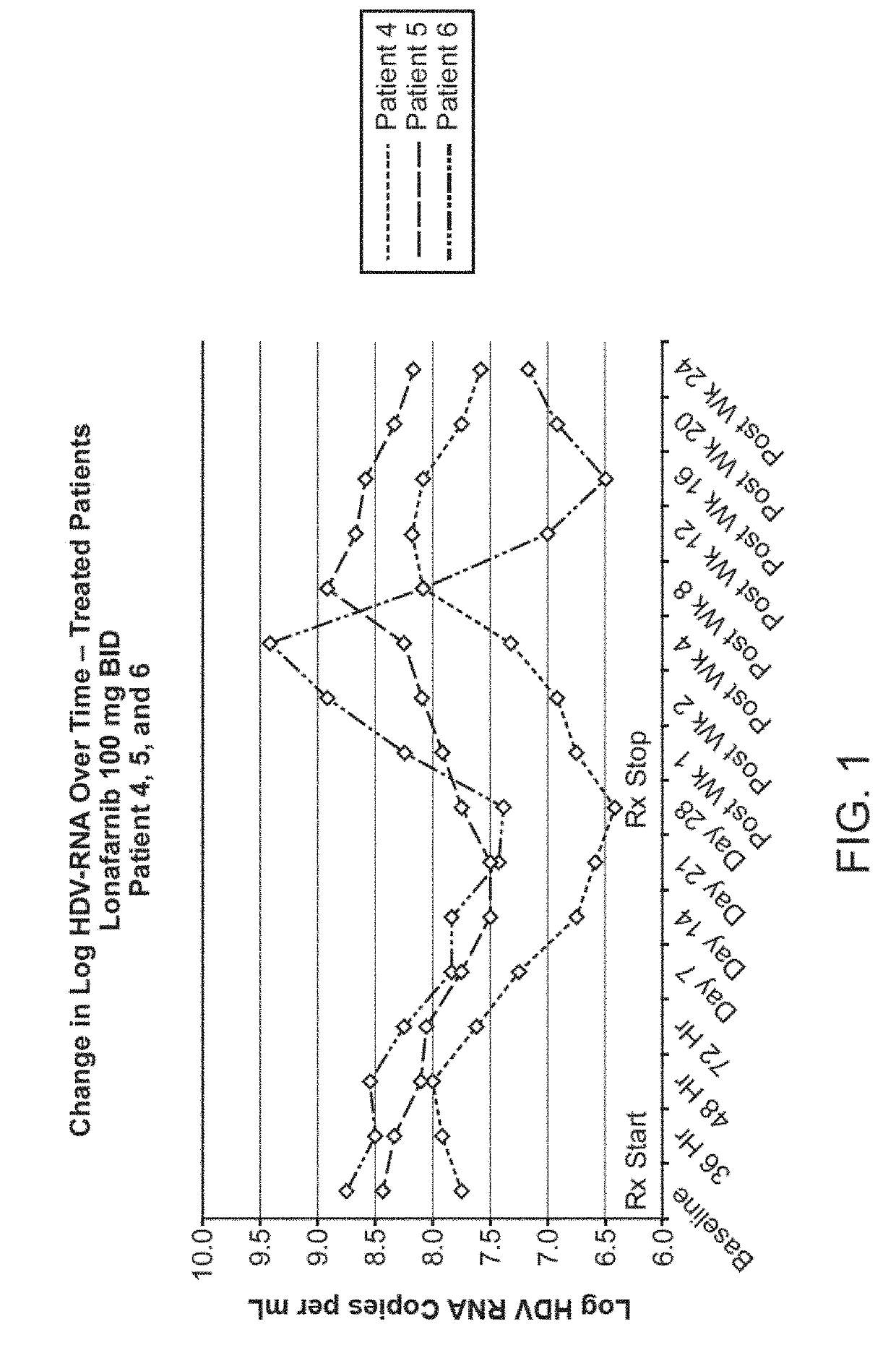
Methods of reducing hepatitis delta virus (HDV) viral loads in a patient are provided. In some embodiments, the method comprises treating the patient with lonafarnib-ritonavir co-therapy. In some embodiments, the method further comprises treating the patient with an interferon.
Owner:EIGER BIOPHARMLS
Treatment of hepatitis delta virus infection
ActiveUS20190167646A1Powder deliveryOrganic active ingredientsInterferon therapyDeltaretrovirus Infections
Methods of reducing hepatitis delta virus (HDV) viral loads in a patient are provided. In some embodiments, the method comprises treating the patient with lonafarnib-ritonavir co-therapy. In some embodiments, the method further comprises treating the patient with an interferon.
Owner:EIGER BIOPHARMLS
Treatment of hepatitis c in the asian population with interferon-beta
InactiveCN101123979APeptide/protein ingredientsDigestive systemInterferon therapyPharmaceutical drug
The invention relates to the use of IFN-beta in the manufacture of a medicament for the treatment of an HCV infection in a treatment-nave patient belonging to the Asian population.
Owner:MERCK SERONO SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com