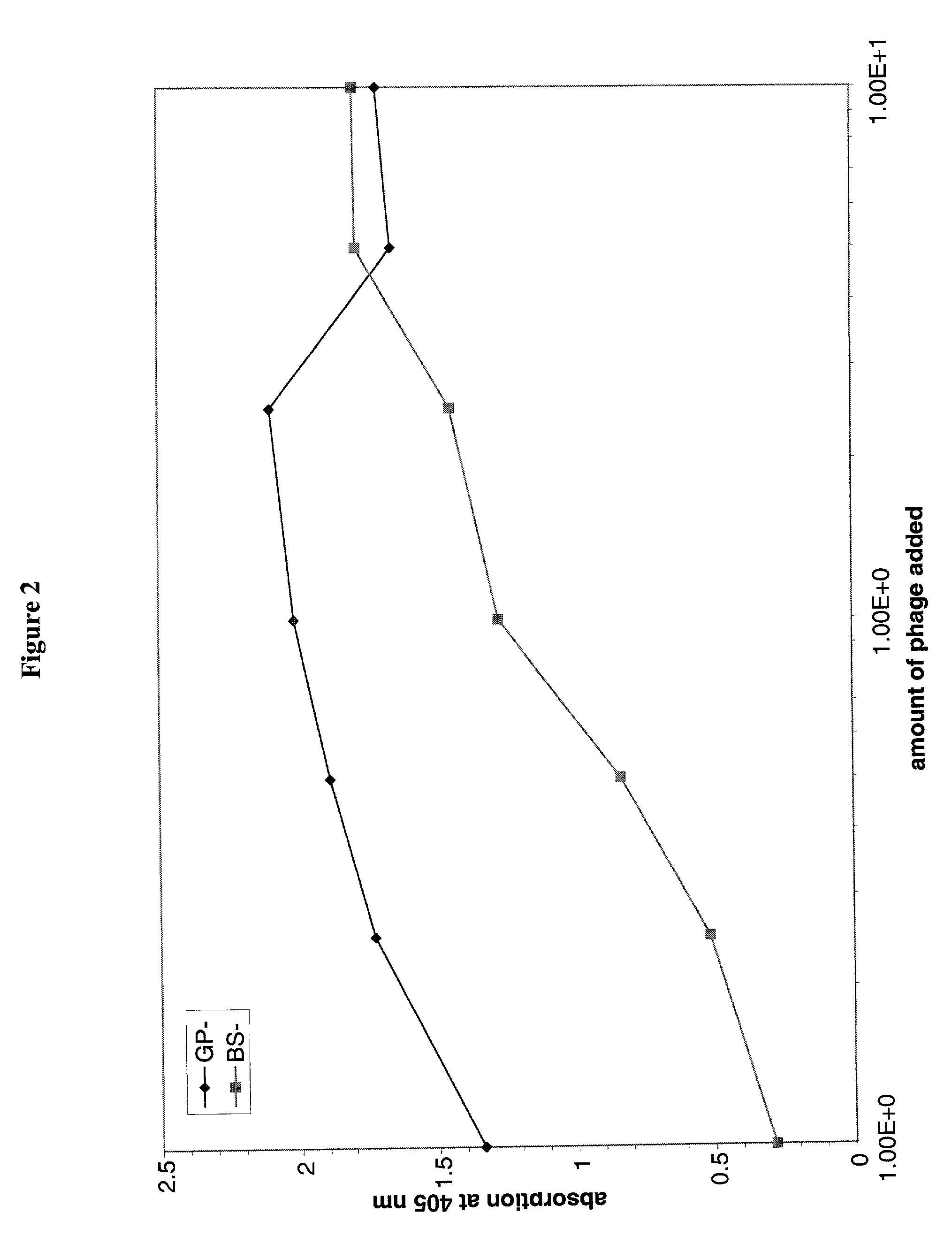
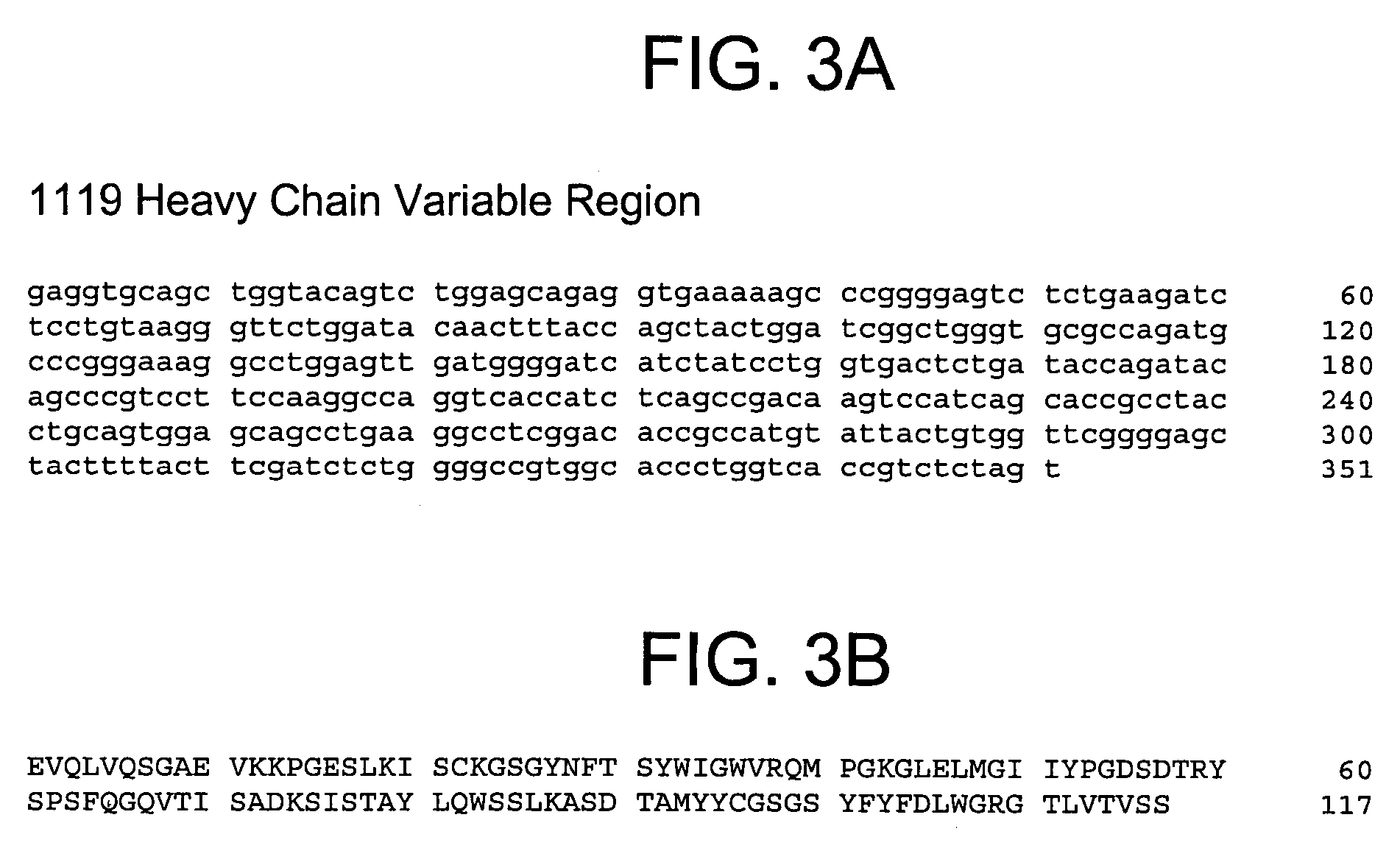
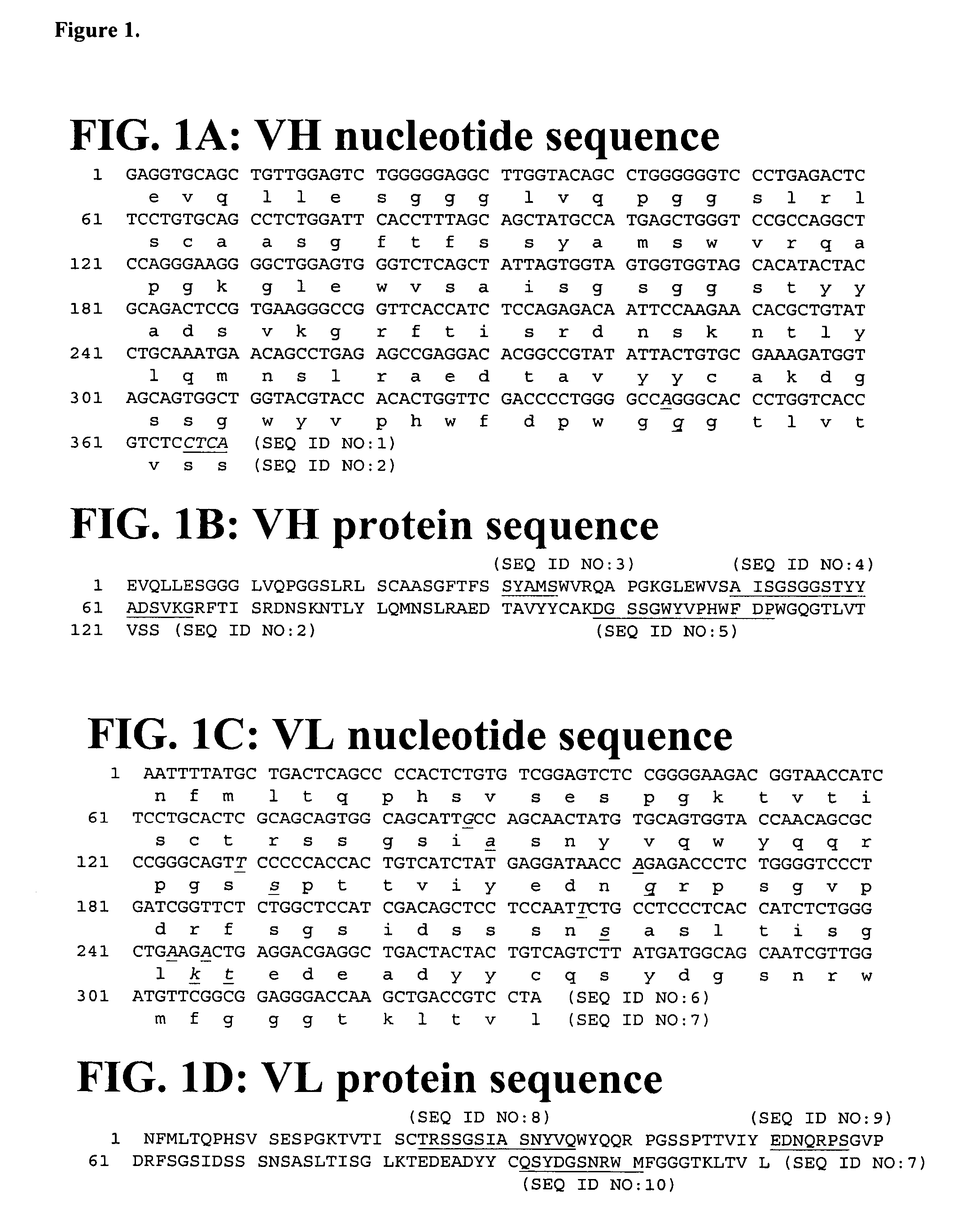
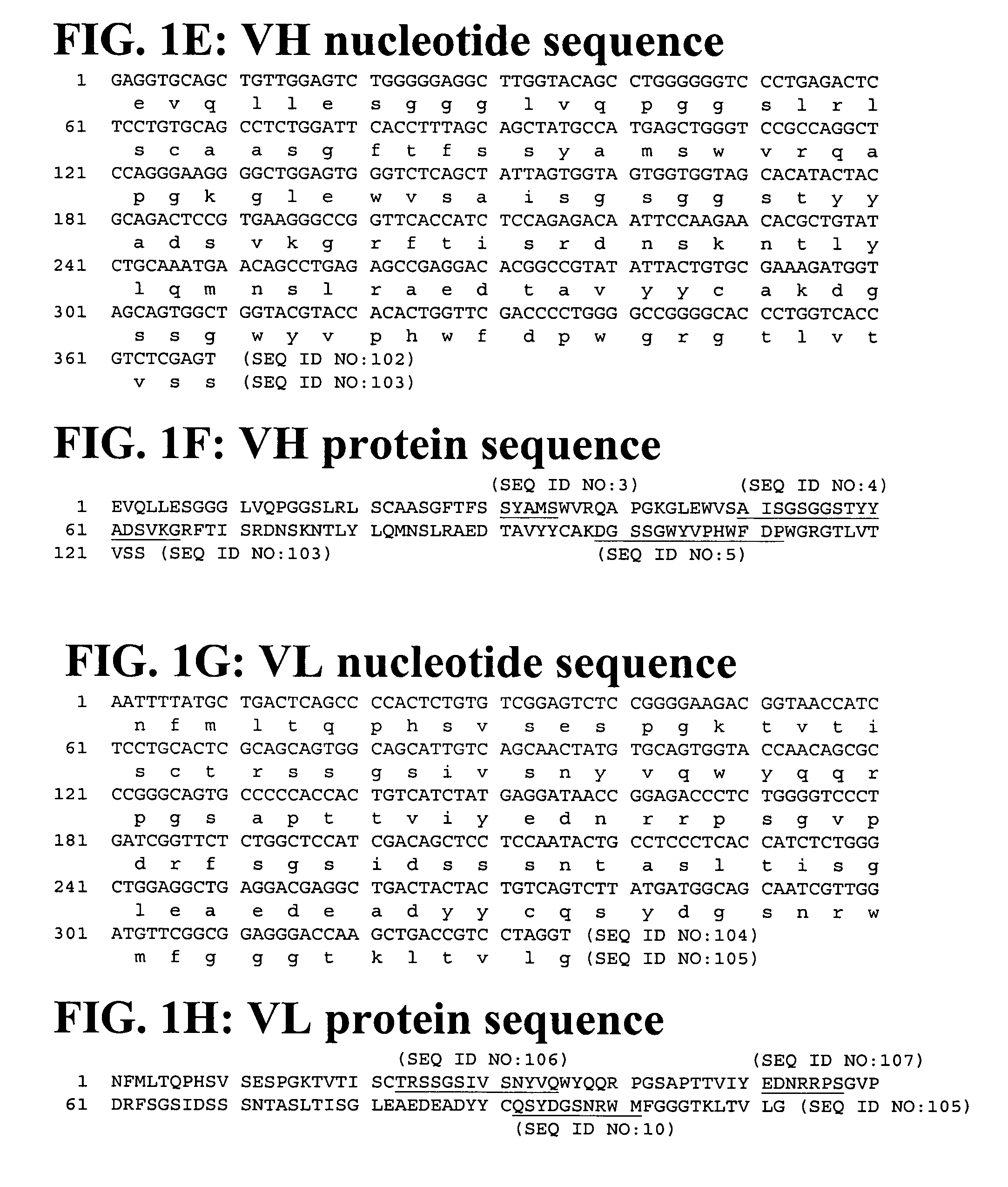
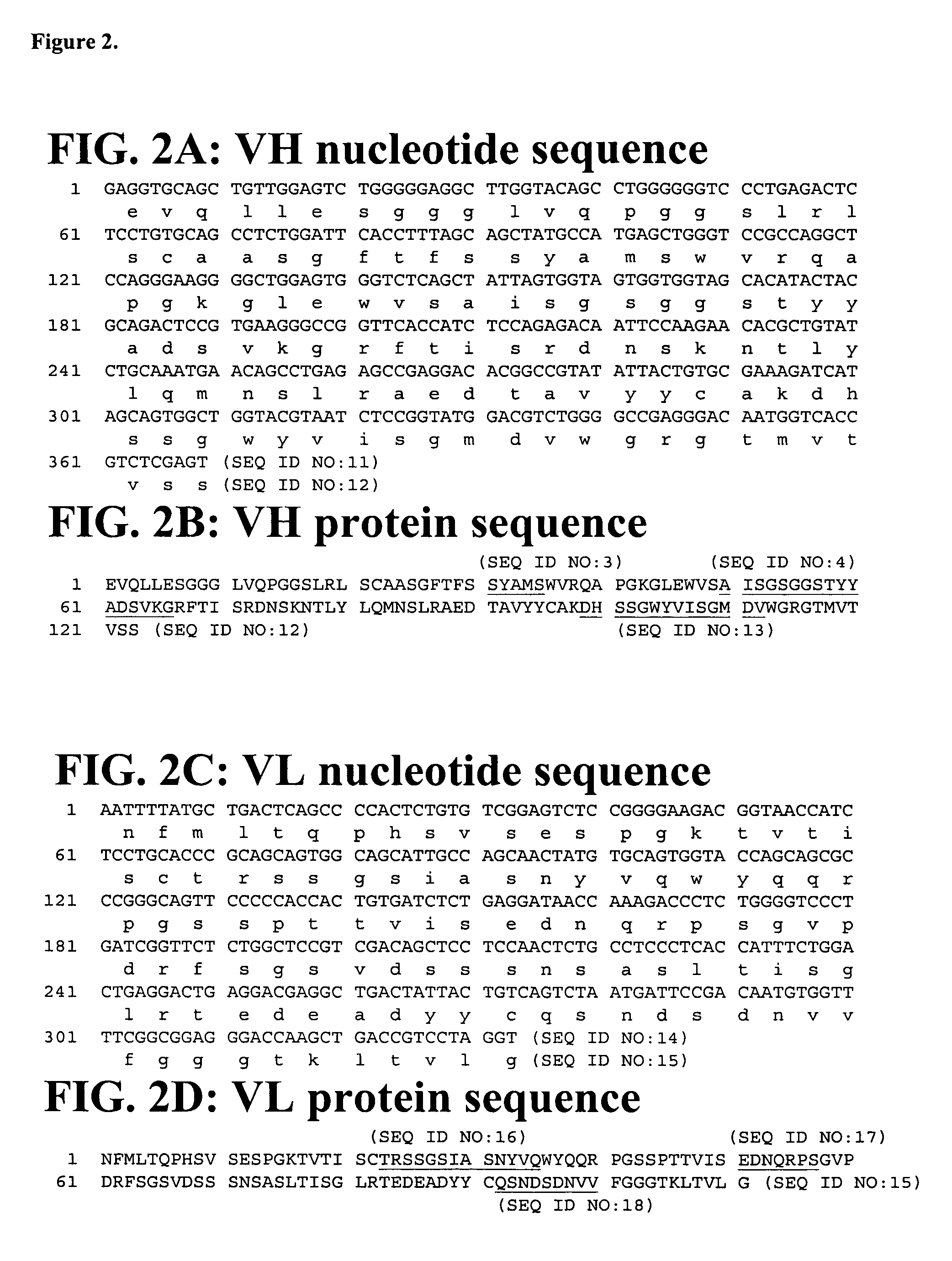
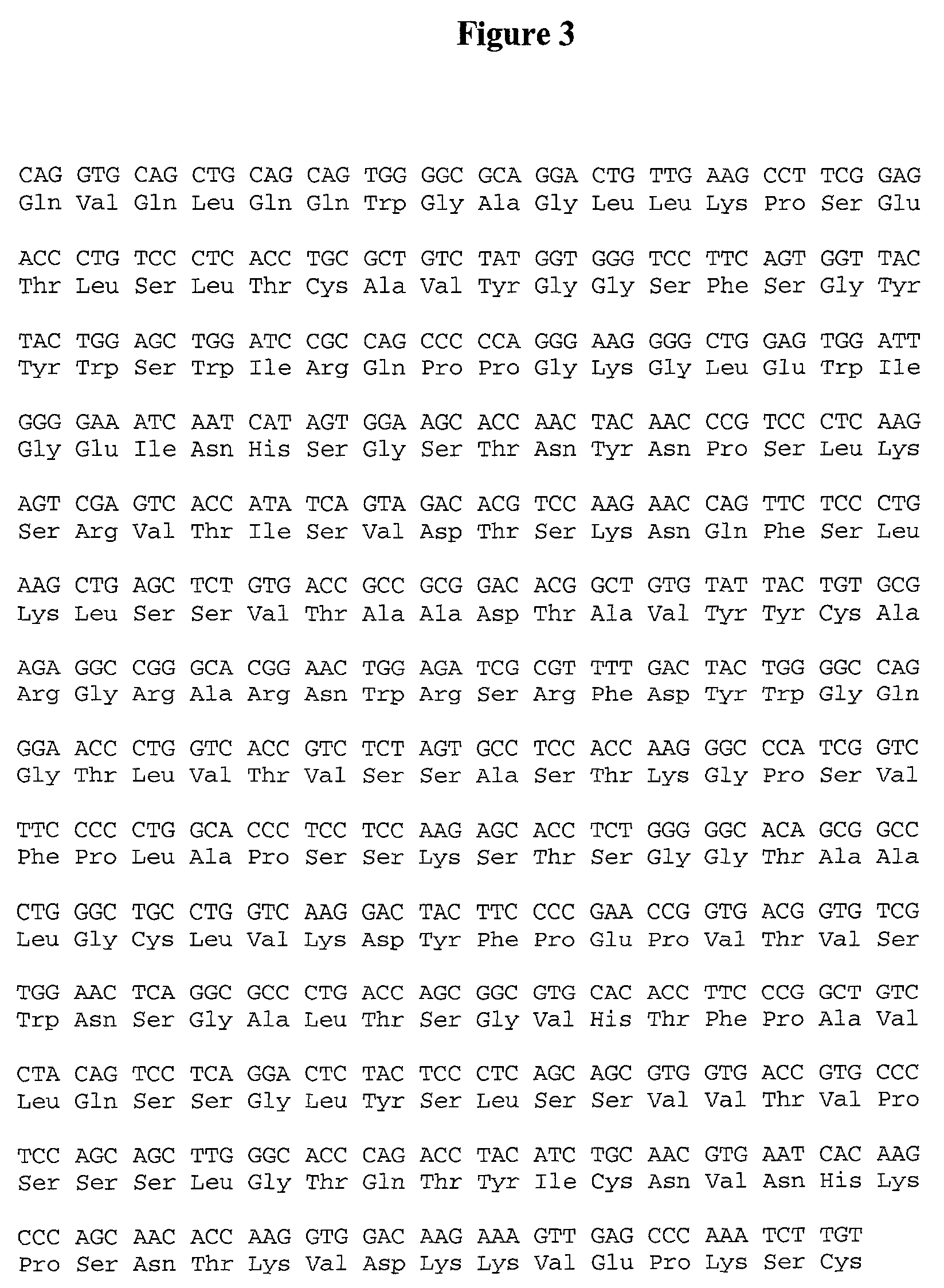Patents
Literature
333 results about "Interferon gamma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to reduce the frequency and severity of serious infections due to chronic granulomatous disease, a disorder that runs in families. This drug is often used along with antibiotics to help prevent these serious infections. This medication is also used to slow the worsening of malignant osteopetrosis, another disorder that runs in families, that affects bones, nerves, and blood.
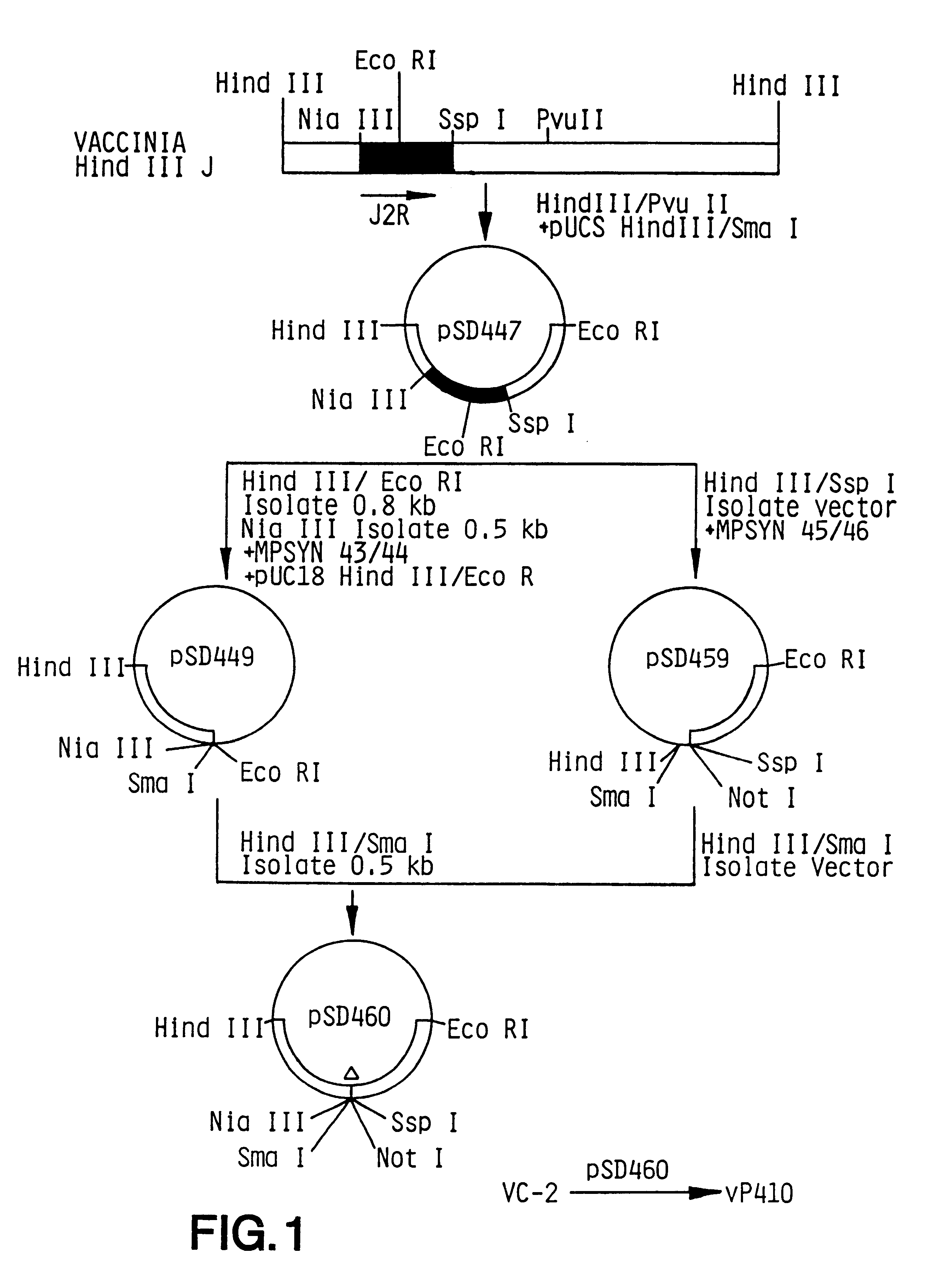
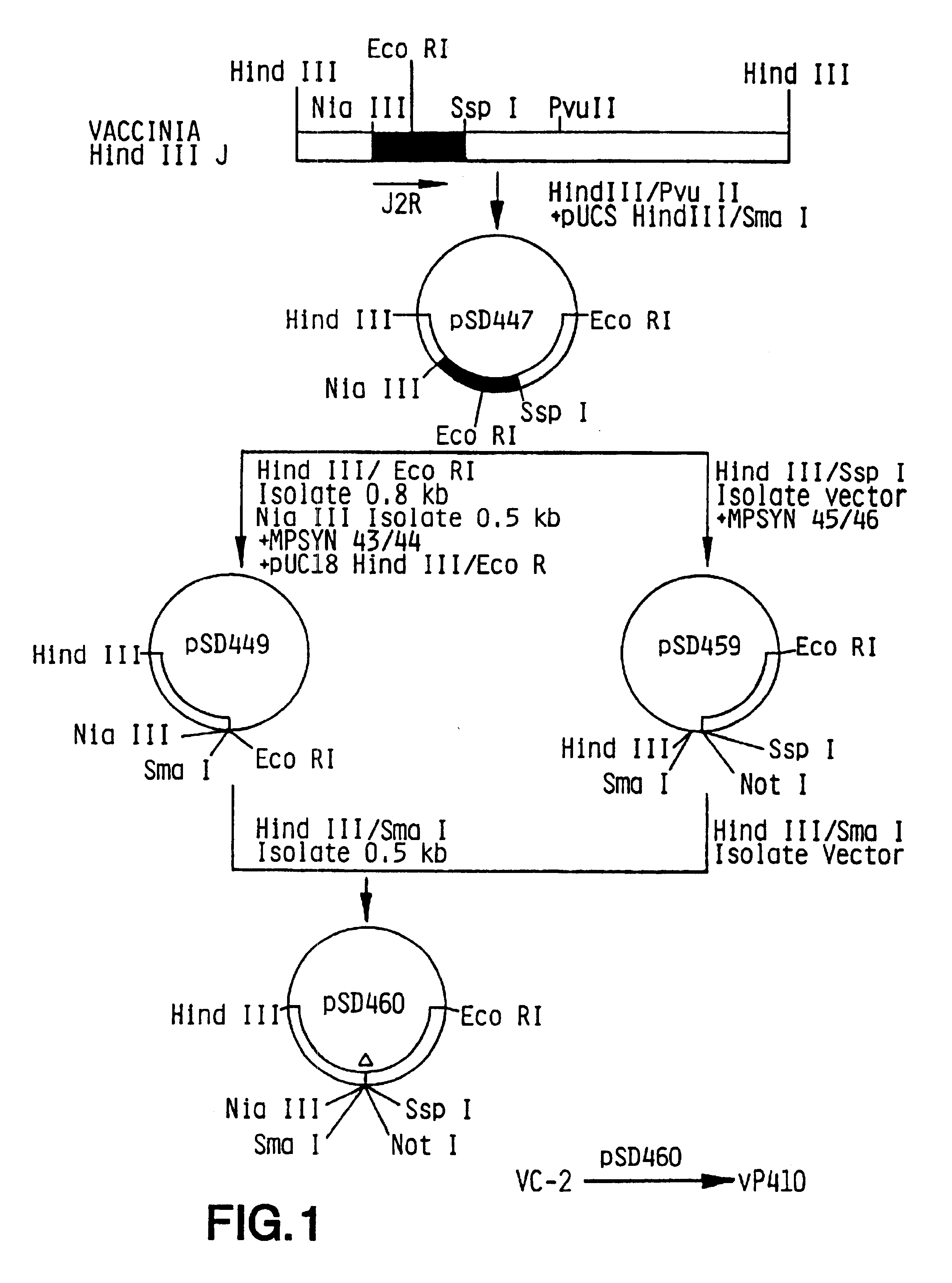
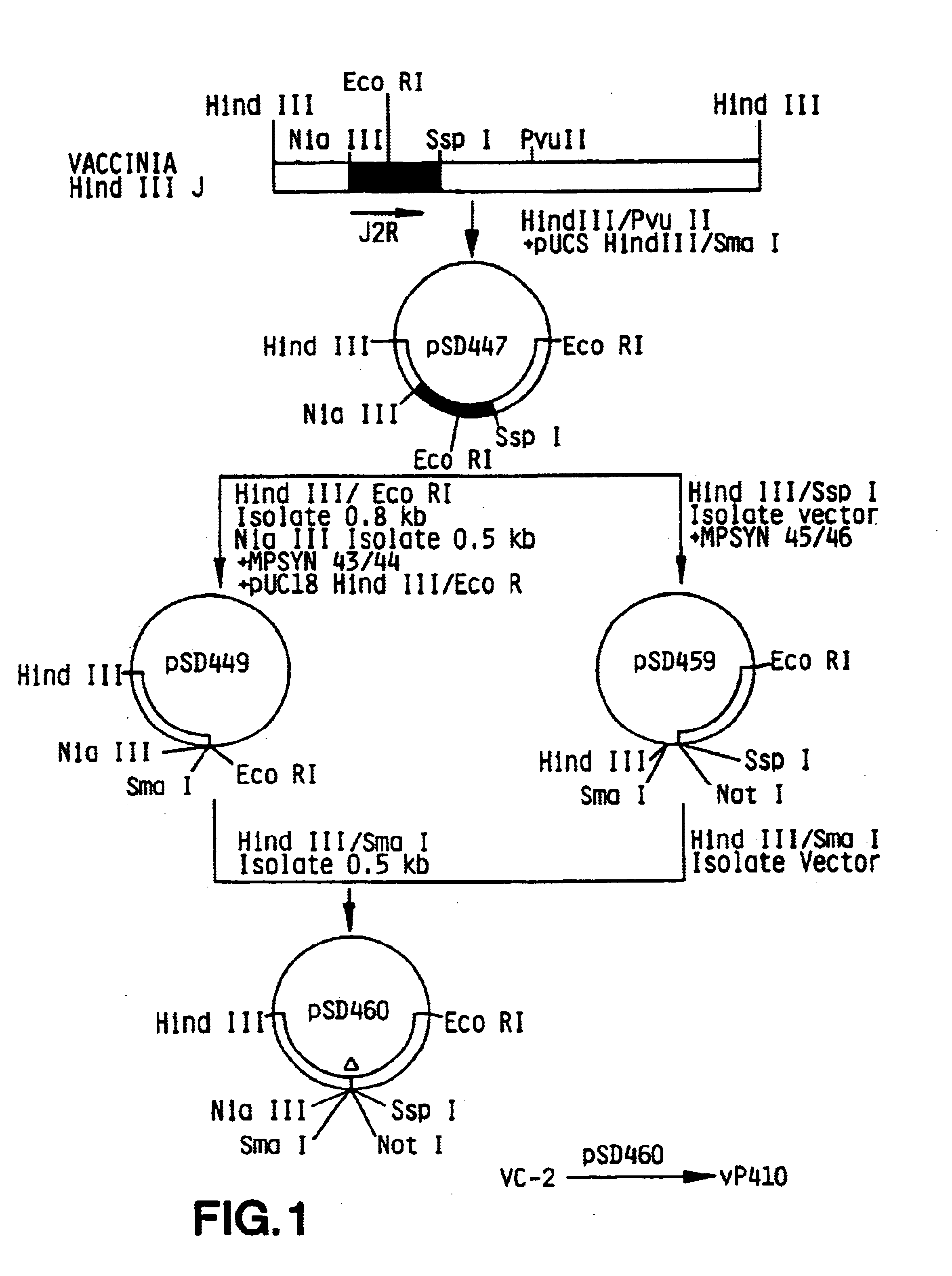
Pox virus containing DNA encoding a cytokine and/or a tumor associated antigen
InactiveUS6265189B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Attenuated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least on of: human tumor necrosis factor; nuclear phosphoprotein p53, wildtype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GNCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:VIROGENETICS
Fully human antibody Fab fragments with human interferon-gamma neutralizing activity
InactiveUS7084257B2Peptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsDNA-binding domainAntigen binding
Selective binding agents of interferon-gamma (IFNγ) are provided by the invention. More particularly, the invention provides for antibodies and antigen binding domains which selectively bind to IFNγ and may be used to prevent or treat conditions relating to autoimmune and inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus and multiple sclerosis. Nucleic acid molecules encoding said antibodies and antigen binding domains, and expression vectors and host cells for the production of same are also provided.
Owner:AMGEN INC
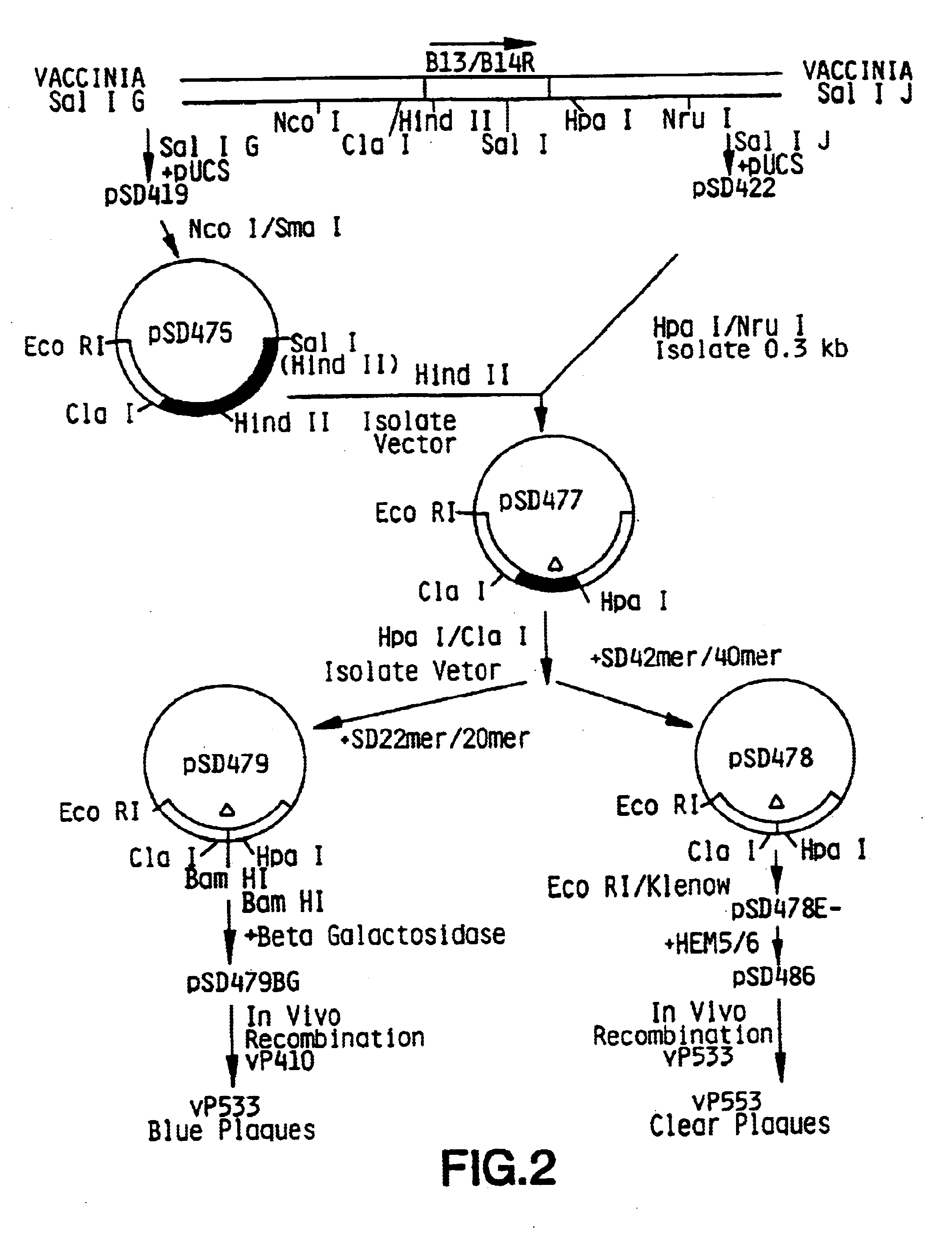
Vaccina virus comprising cytokine and/or tumor associated antigen genes
InactiveUS6537594B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Owner:VIROGENETICS
Methods and compositions for inducing antigen-specific immune responses
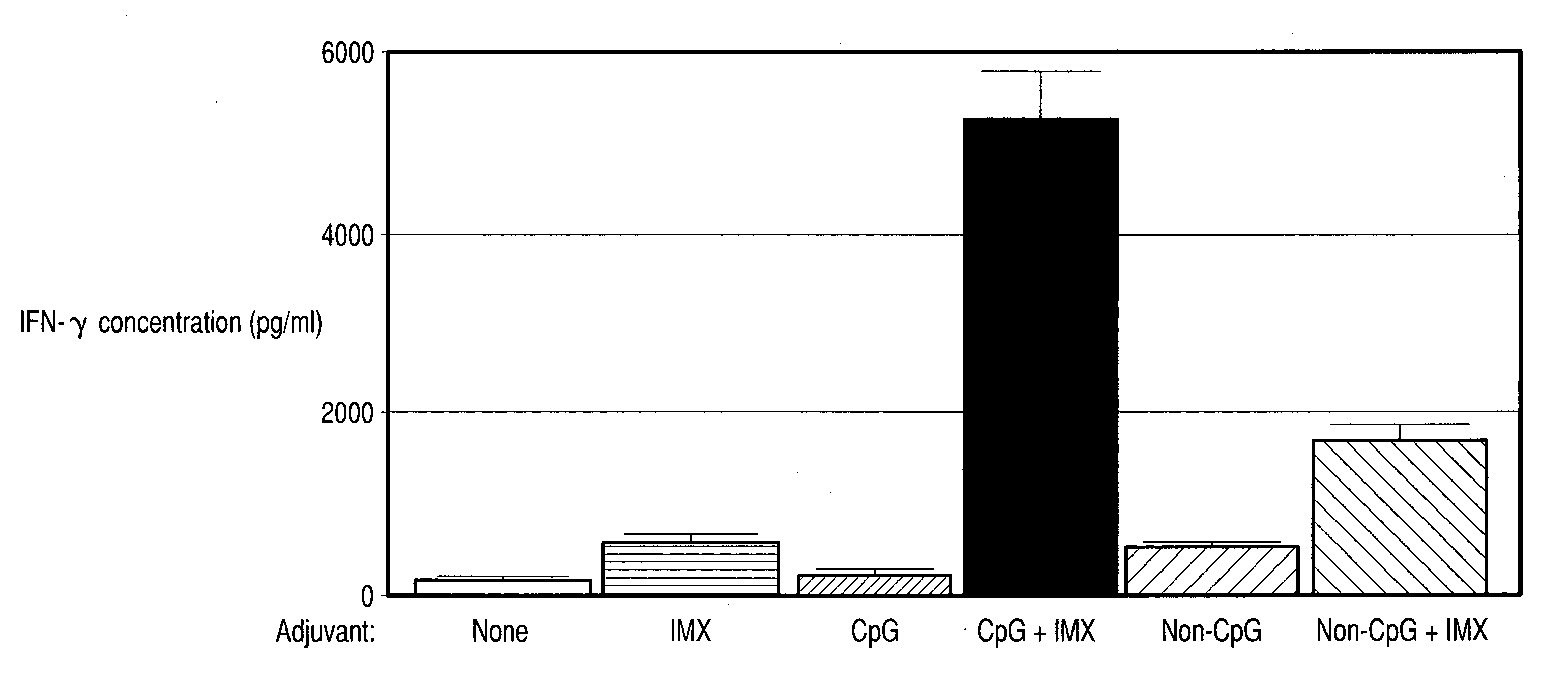
InactiveUS20060287263A1Enhanced IFN-gamma productionIncrease productionAntibacterial agentsBiocideImmunostimulating ComplexesSpecific immunity
Vaccine compositions comprising (a) an oligonucleotide, (b) and immune stimulating complex and (c) an antigen induce a strong interferon-gamma immune response. Both oligonucleotides containing immune stimulatory motifs and oligonucleotides lacking immune stimulatory motifs contribute to an interferon-gamma response when administered with an immune stimulating complex.
Owner:COLEY PHARM GRP INC +1
Method and composition for treatment of renal failure with antibodies and their equivalents as partial or complete replacement for dialysis
InactiveUS7504106B2Lower Level RequirementsSlow onsetBiocidePeptide/protein ingredientsInterleukin 6Creatinine rise
A method for treating patients with renal failure includes administering to them an effective amount of antibody or of a functional equivalent thereof to at least two of urea, creatinine, tumor necrosis factor alpha, interferon gamma, interleukin 6 and interleukin 1 beta. Soluble cytokine receptors also can be employed. The method can be used as a supplement to or as partial or complete replacement for dialysis. A pharmaceutical composition includes antibody or functional equivalent thereof to urea, creatinine, or both; antibody, functional equivalent or soluble cytokine receptor to tumor necrosis factor alpha, interferon gamma, interleukin 6, interleukin 1 beta or any combination thereof The composition can be included in a kit.
Owner:SKURKOVICH BORIS +2
Method of treating tuberculosis with interferons
InactiveUS20100098660A1Increase appetiteReduction in wheezingAntibacterial agentsBiocideInterferon therapyInterferon alpha
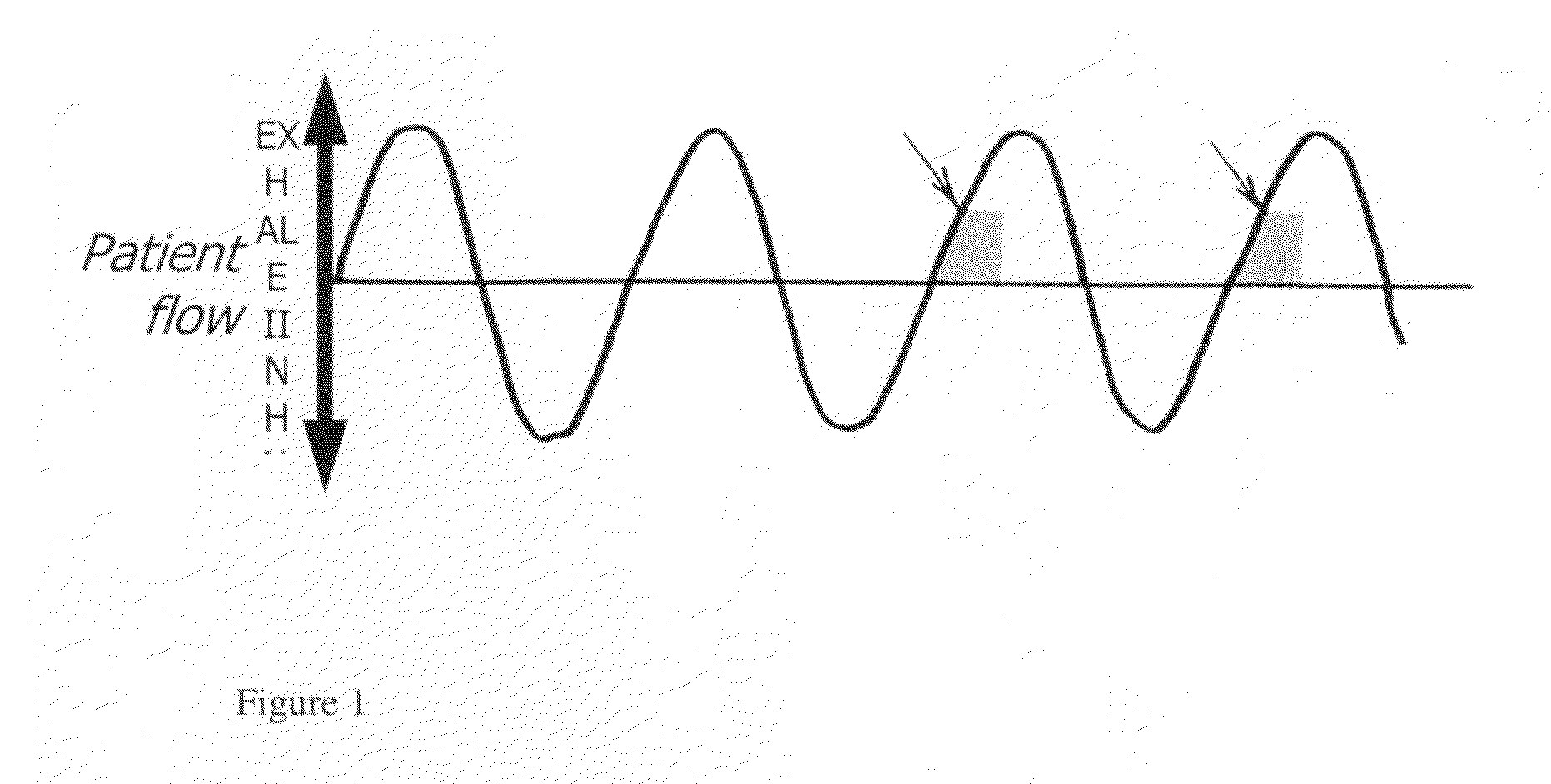
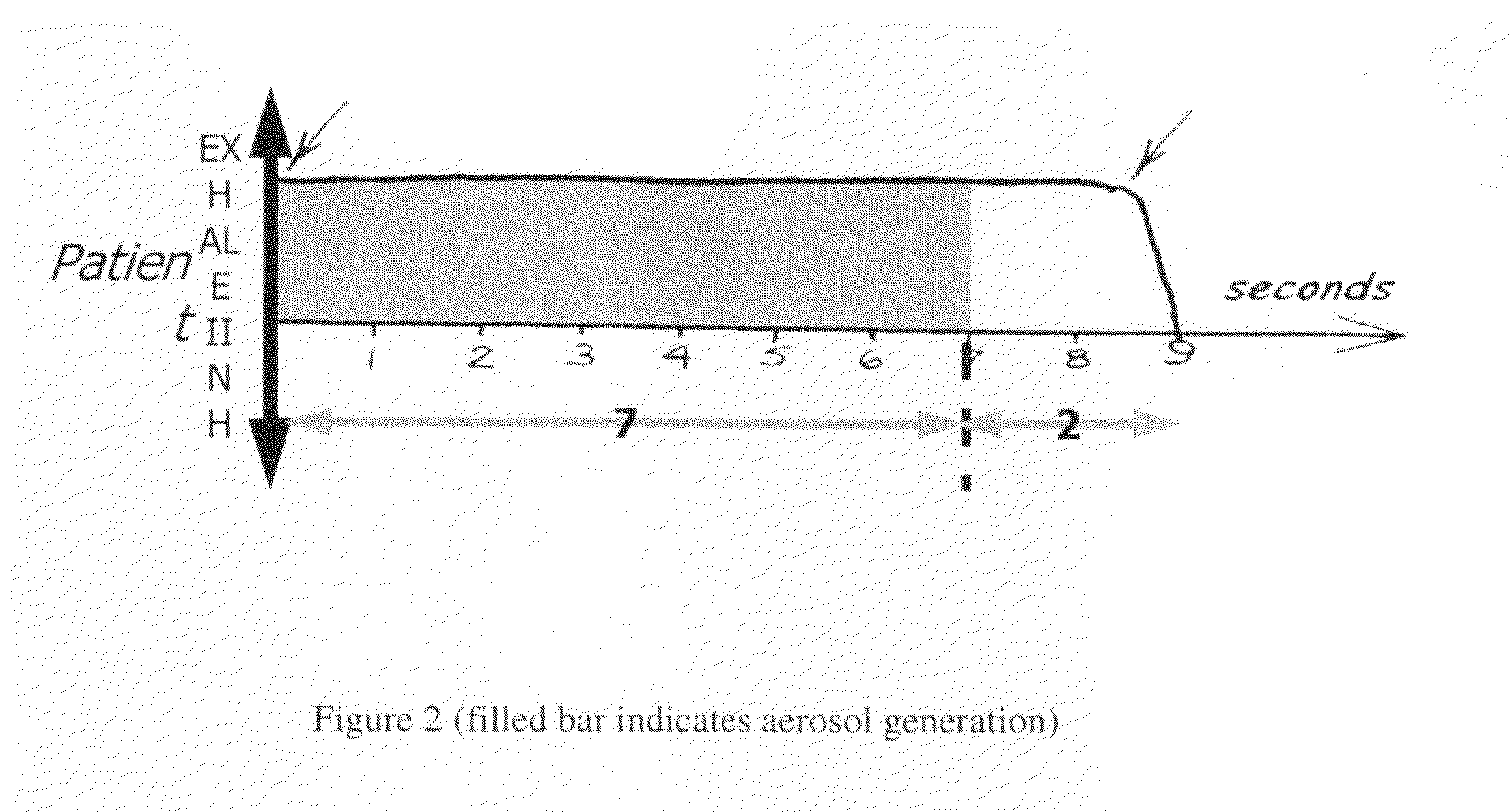
A method of treating tuberculosis comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Further, a method of reducing the infectivity of tuberculosis or reducing the number of infectious organisms present in the lungs of a patient suffering from tuberculosis comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Also, pharmaceutical compositions of one or more aerosolized interferon(s) are provided.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Pox virus comprising DNA sequences encoding CEA and B7 antigen
Attenutated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: human tumor necrosis factor; nuclear phosphoprotein p53, wiltype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GMCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:AVENTIS PASTEUR LTD
Human anti-IFN-γ neutralizing antibodies as selective IFN-γ pathway inhibitors
ActiveUS7335743B2Prevents and antagonizes inhibitionNot inhibit or modulate the biological activityNervous disorderAntipyreticNeutralizing antibodyDisease cause
This invention provides antibodies that interact with or bind to human interferon-gamma (IFN-γ) and methods for treating IFN-γ mediated diseases by administering a pharmaceutically effective amount of antibodies to IFN-γ. Methods of detecting the amount of IFN-γ in a sample using antibodies to IFN-γ are also provided.
Owner:ER SQUIBB & SONS INC
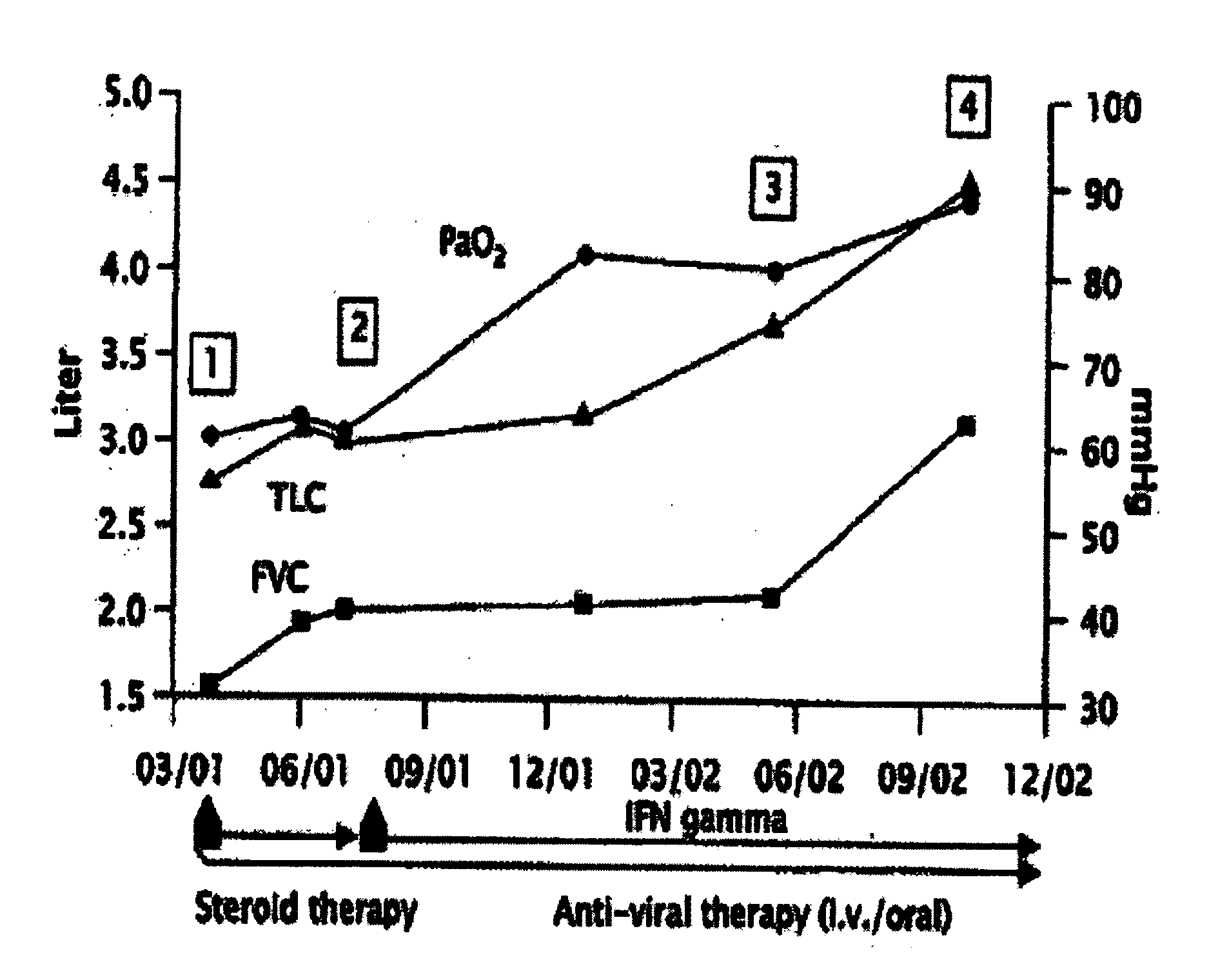
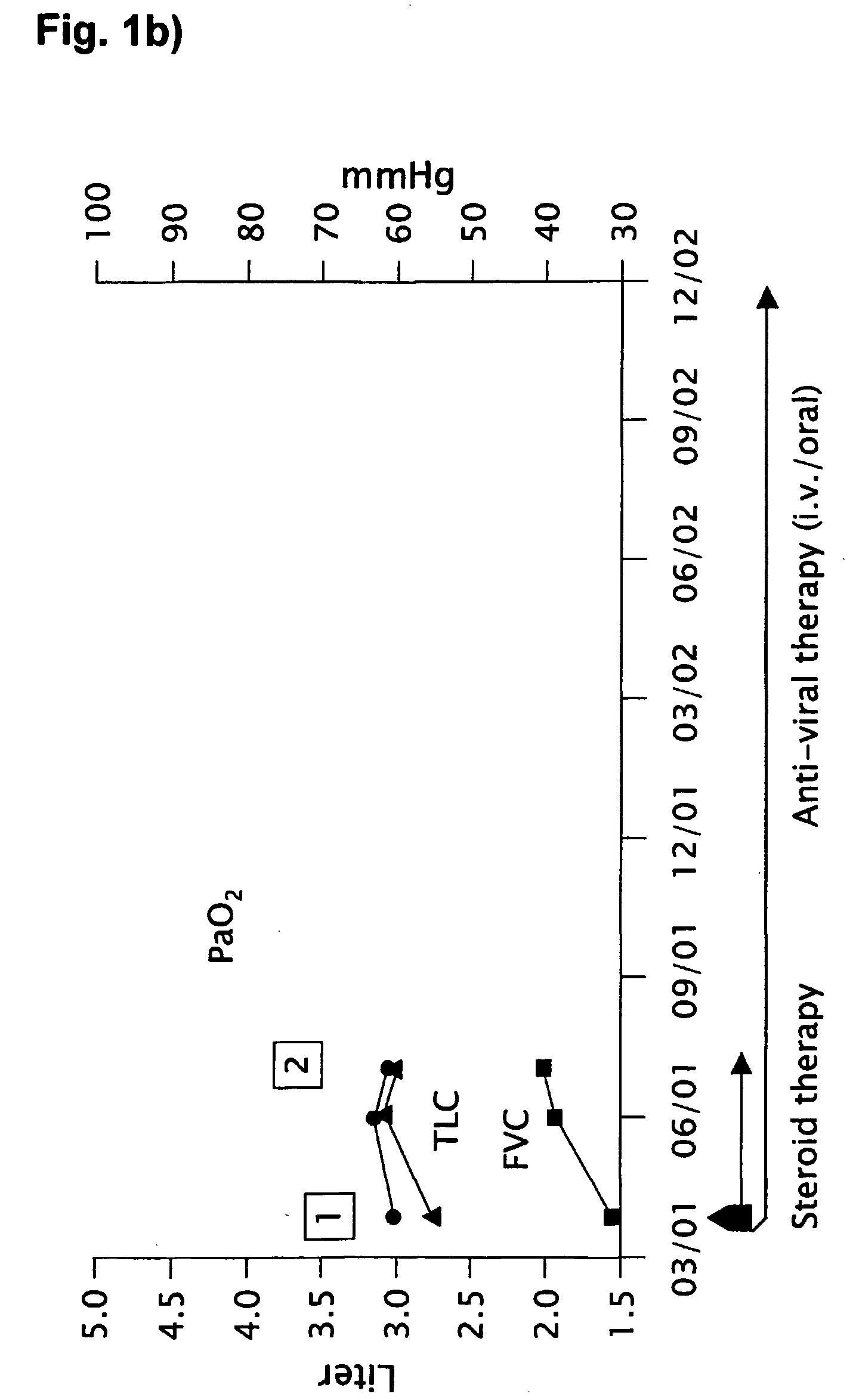
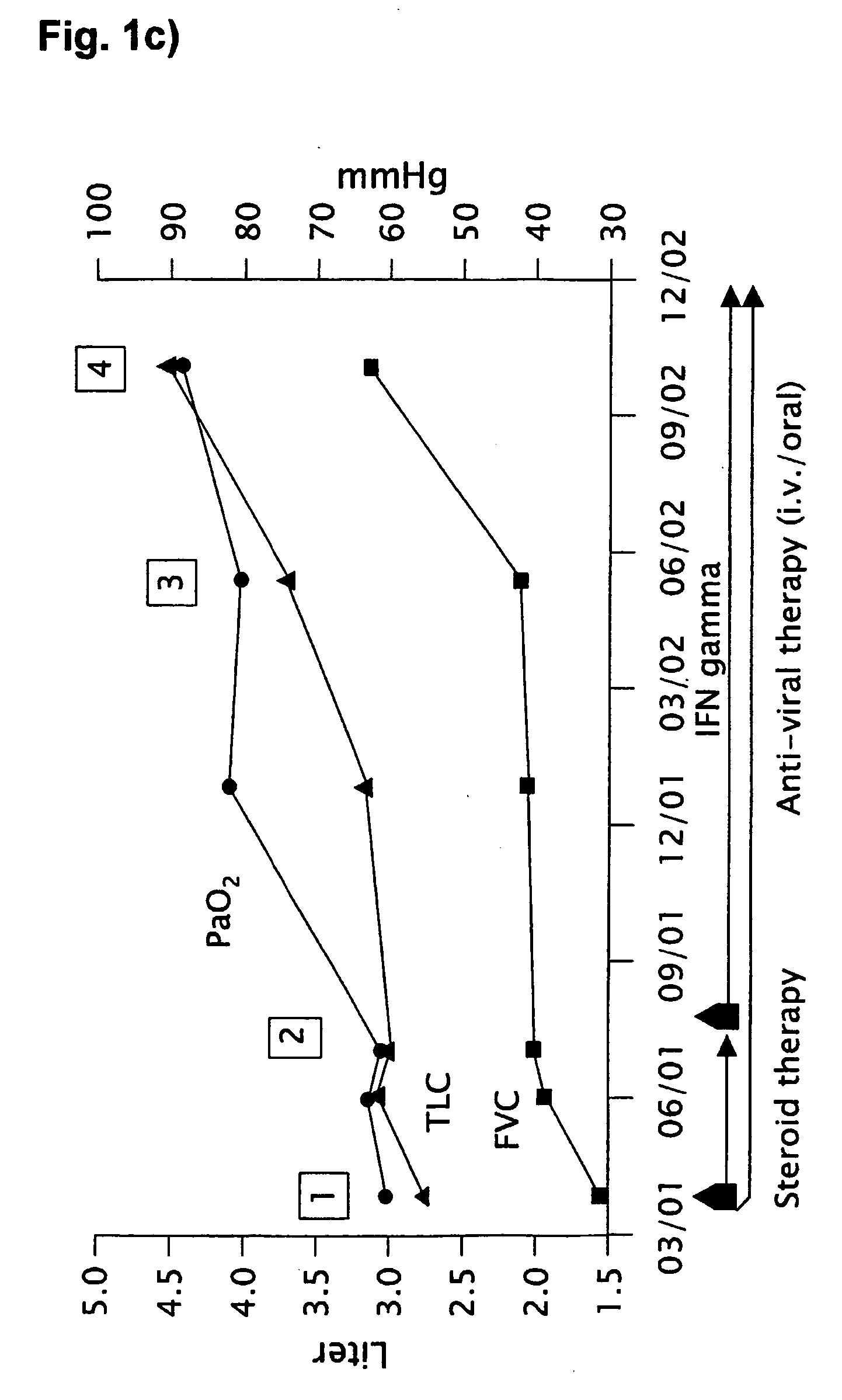
Novel pharmaceutical composition of interferon gamma or pirfenidone with molecular diagnostics for the improved treatment of interstitial lung diseases
InactiveUS20090142301A1Bioreactor/fermenter combinationsBiocideInterstitial lung diseaseInterferon alpha
The present invention relates to a novel pharmaceutical composition of compounds having the biological activity of interferon gamma (IFN-γ) or pirfenidone in combination with a diagnostic array of candidate polynucleotides for the improved treatment of all forms of interstitial lung disease, in particular of idiopathic pulmonary fibrosis (IPF).
Owner:MONDOBIOTECH AG
Method of stabilizing useful protein and useful protein compositions
InactiveUS6391296B1Improve biological activityActivity of protein can be maintainedPeptide/protein ingredientsWort preparationCrystallographyProtein composition
A method of stabilizing a useful protein by mixing a useful protein and an aqueous solution of a compound having the basic structure of arabic acid, and a stabilizied useful protein compositon containing a useful protein and a compound having the basic structure of arabic acid; gum arabic is preferred as a compound having the basic structure of arabic acid, and examples of a useful protein include cytokine and interferon; and a production method for canine interferon-gamma and stabilization thereof.
Owner:TORAY IND INC
Method and pharmaceutical composition for the treatment of multiple sclerosis
InactiveUS20030166589A1Peptide/protein ingredientsGenetic material ingredientsDiseaseAutoimmune responses
Methods and pharmaceutical compositions are disclosed, effective in breaking-down immunological tolerance the CXC chemokine interferon gamma-inducible protein 10 (IP-10), resulting in the generation of self specific immunity to IP-10, for the treatment of diseases, such as autoimmune diseases, in which IP-10 plays a pivotal role in disease onset and / or progression, e.g., in multiple sclerosis (MS).
Owner:KARIN NATHAN
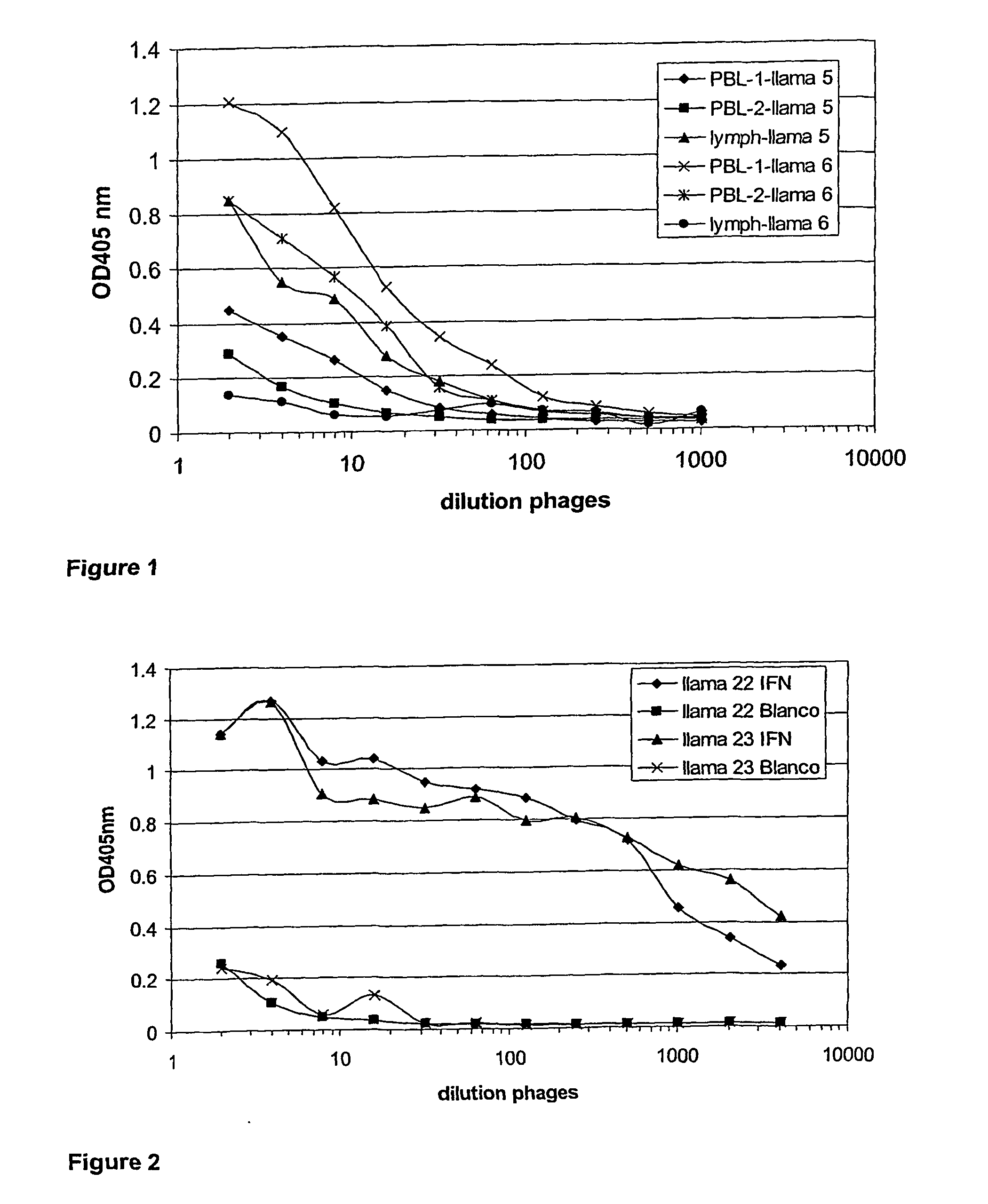
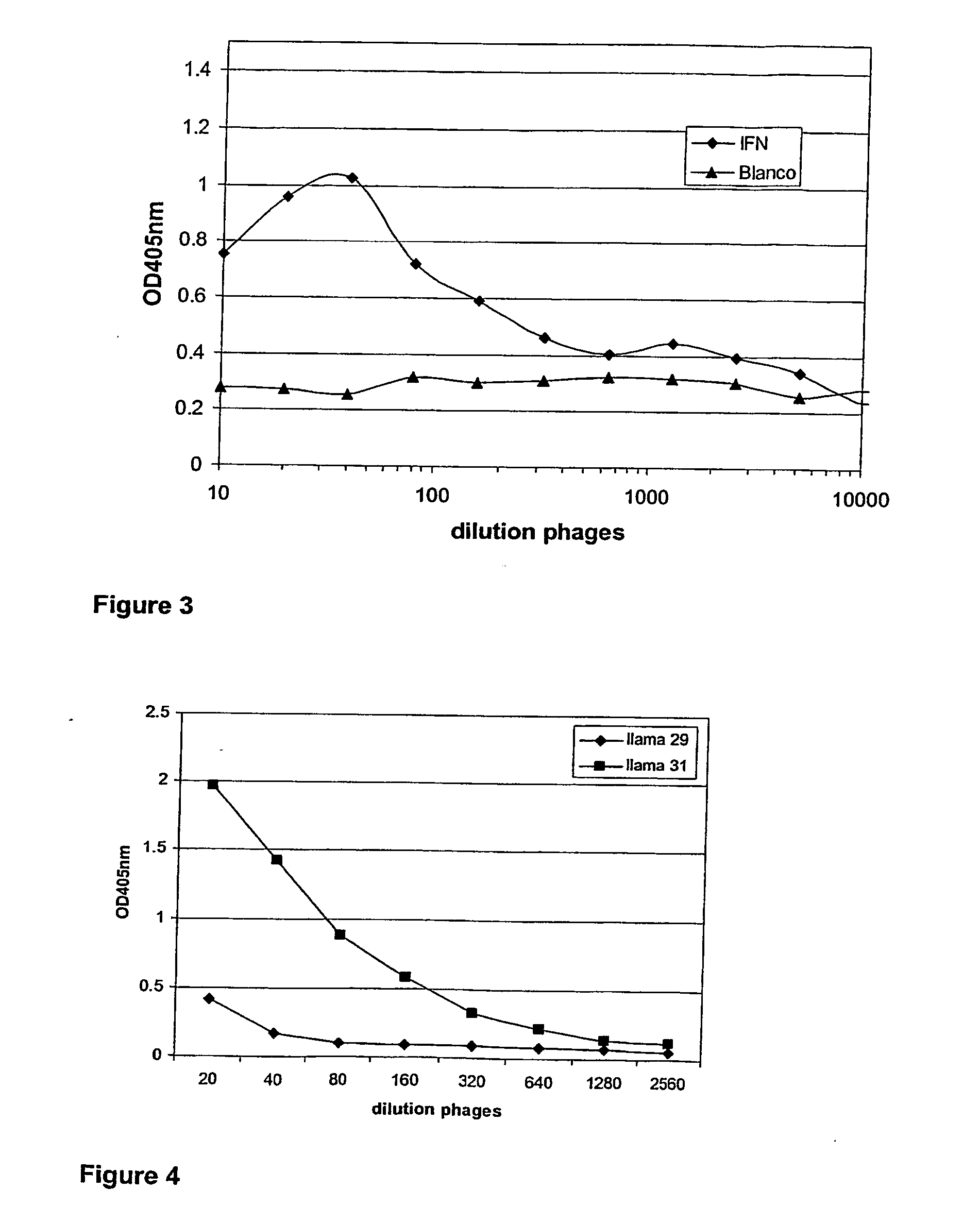
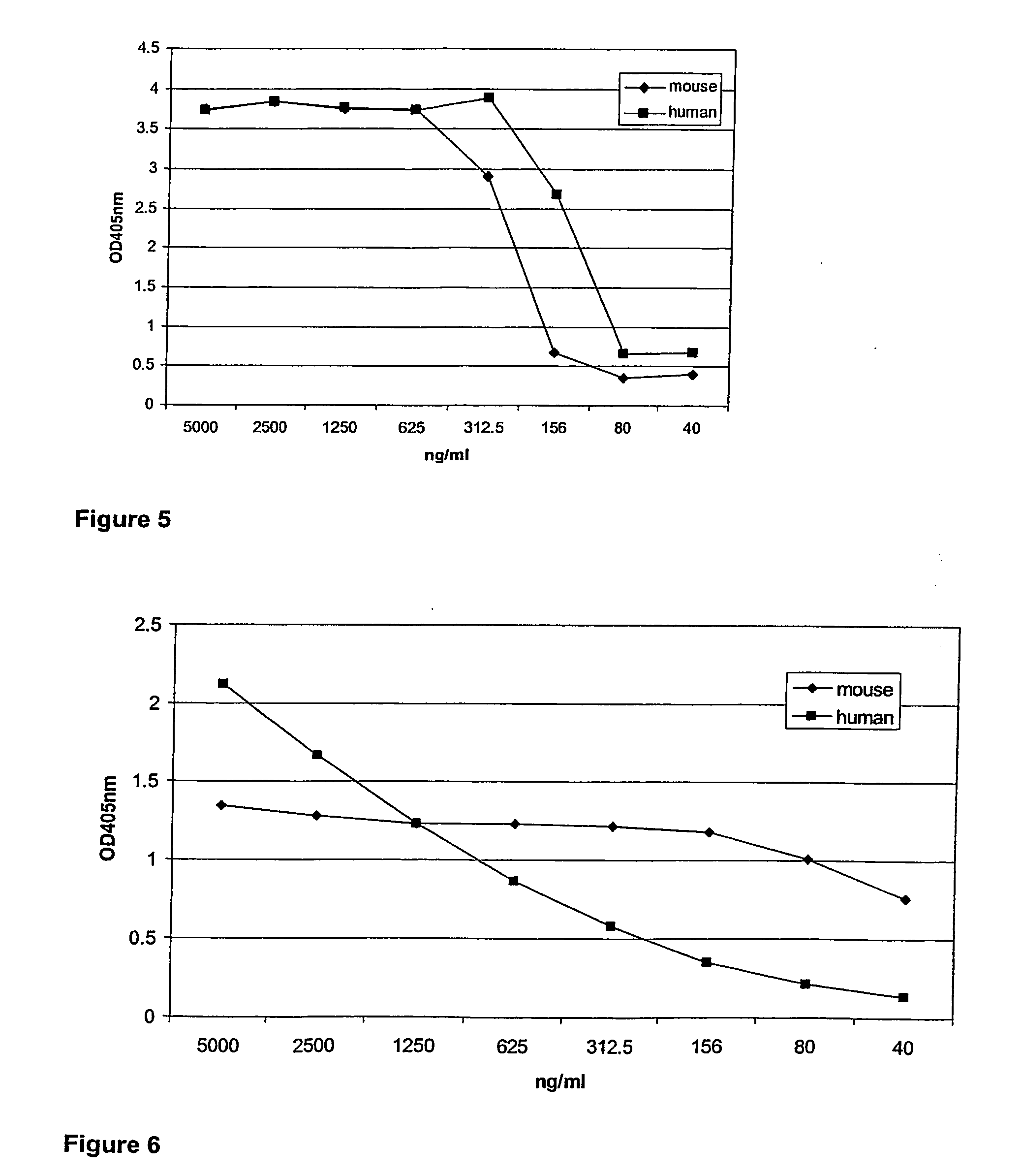
Single domain antibodies directed against interferron-gamma and uses therefor
InactiveUS20060034833A1Improve breathabilityImmunoglobulins against blood coagulation factorsImmunoglobulins against cytokines/lymphokines/interferonsSingle-domain antibodyInterferon gamma
The present invention relates to polypeptides derived from single domain heavy chain antibodies directed to interferon gamma. It further relates to single domain antibodies that are Camelidae VHHs. It further relates to methods of administering said polypeptides. It further relates to protocols for screening for agents that modulate the IFN-gamma receptor, and the agents resulting from said screening.
Owner:BEIRNAERT ELS
Method of treating pulmonary disease with interferons
InactiveUS20070065367A1Improve toleranceDecrease in TNF-aPowder deliveryDispersion deliveryInterferon therapyDisease
A method of treating a pulmonary disease such as, for instance idiophathic pulmonary fibrosis (IPF) and asthma, comprising administering an aerosolized interferon such as interferon α, interferon β or interferon γ in a therapeutically effective amount is provided herein. Also, pharmaceutical compositions of one or more aerosolized interferon(s) are provided.
Owner:NEW YORK UNIV +1
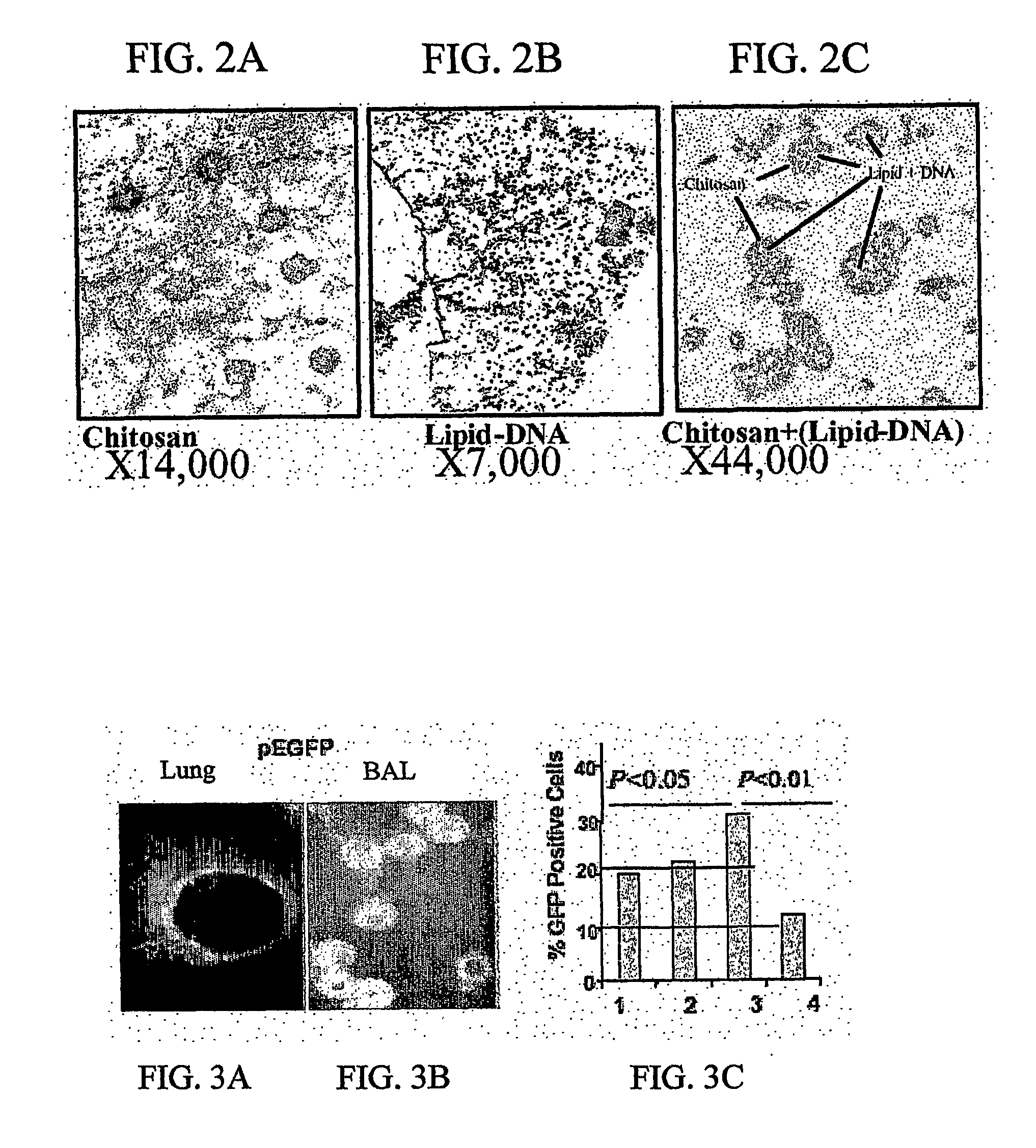
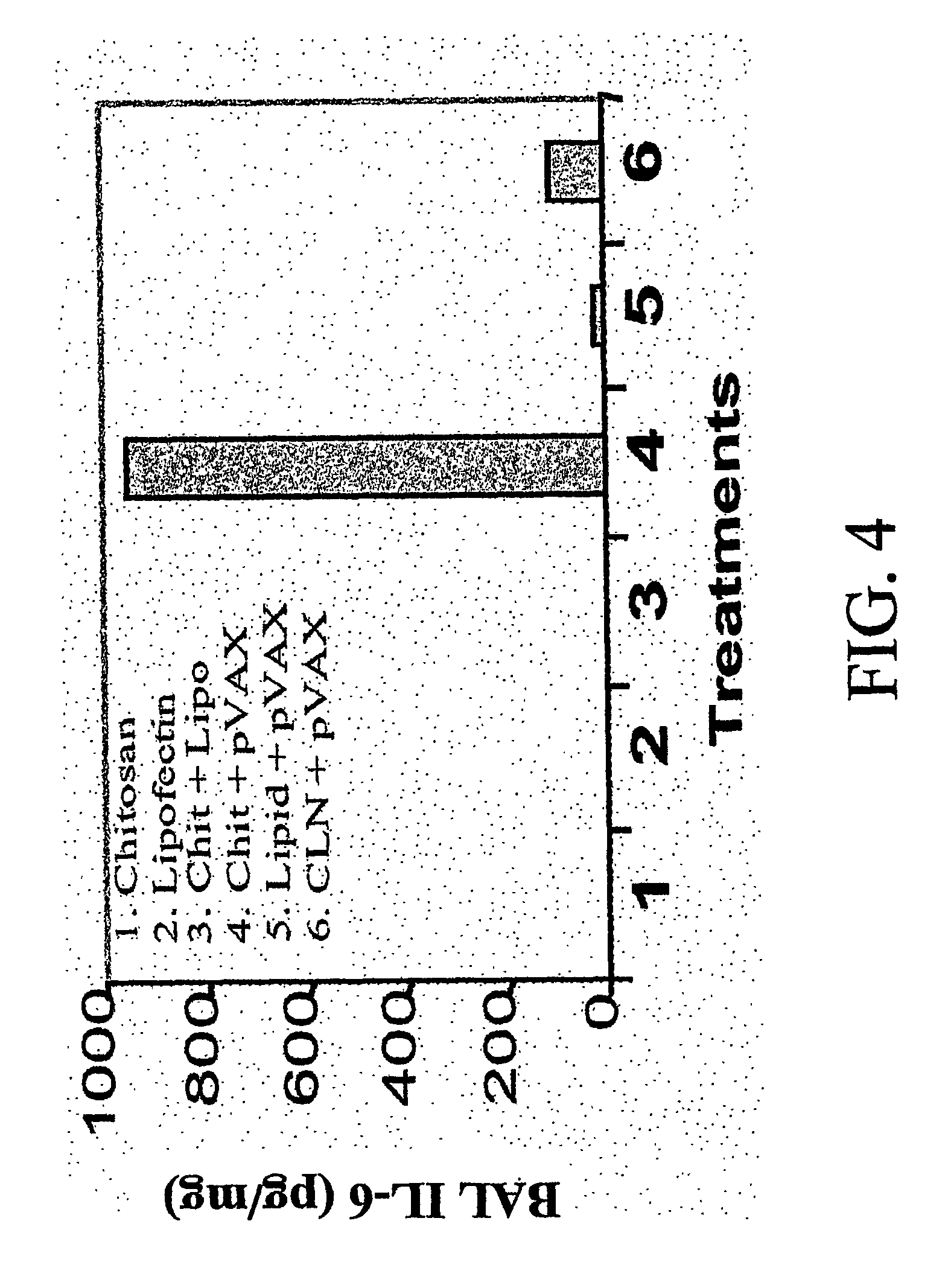
Chitosan-microparticles for ifn gene delivery
InactiveUS20070116767A1Enhancing interferon-gamma expressionProduction process specificationPowder deliveryBiocideGene deliveryDelivery vehicle
The present invention provides particles comprising chitosan, or a derivative thereof, useful as delivery vehicles for polynucleotides encoding polypeptides, compositions comprising such particles and a pharmaceutically acceptable carrier, and methods for delivering polynucleotides using such particles. Optionally, the particles of the invention further comprise a lipid component. The present invention further provides a method for enhancing interferon-gamma expression to regulate the production of cytokines secreted by T-helper type 2 (Th2) cells within a subject by administering an effective amount of a particle of the subject invention to the subject, wherein the particle comprises a polynucleotide encoding interferon-gamma.
Owner:UNIV OF SOUTH FLORIDA
Interferon antagonists useful for the treatment of interferon related diseases
InactiveUS20030138404A1Extended half-lifeLow immunogenicityBiocideNervous disorderDiseaseAutoimmune responses
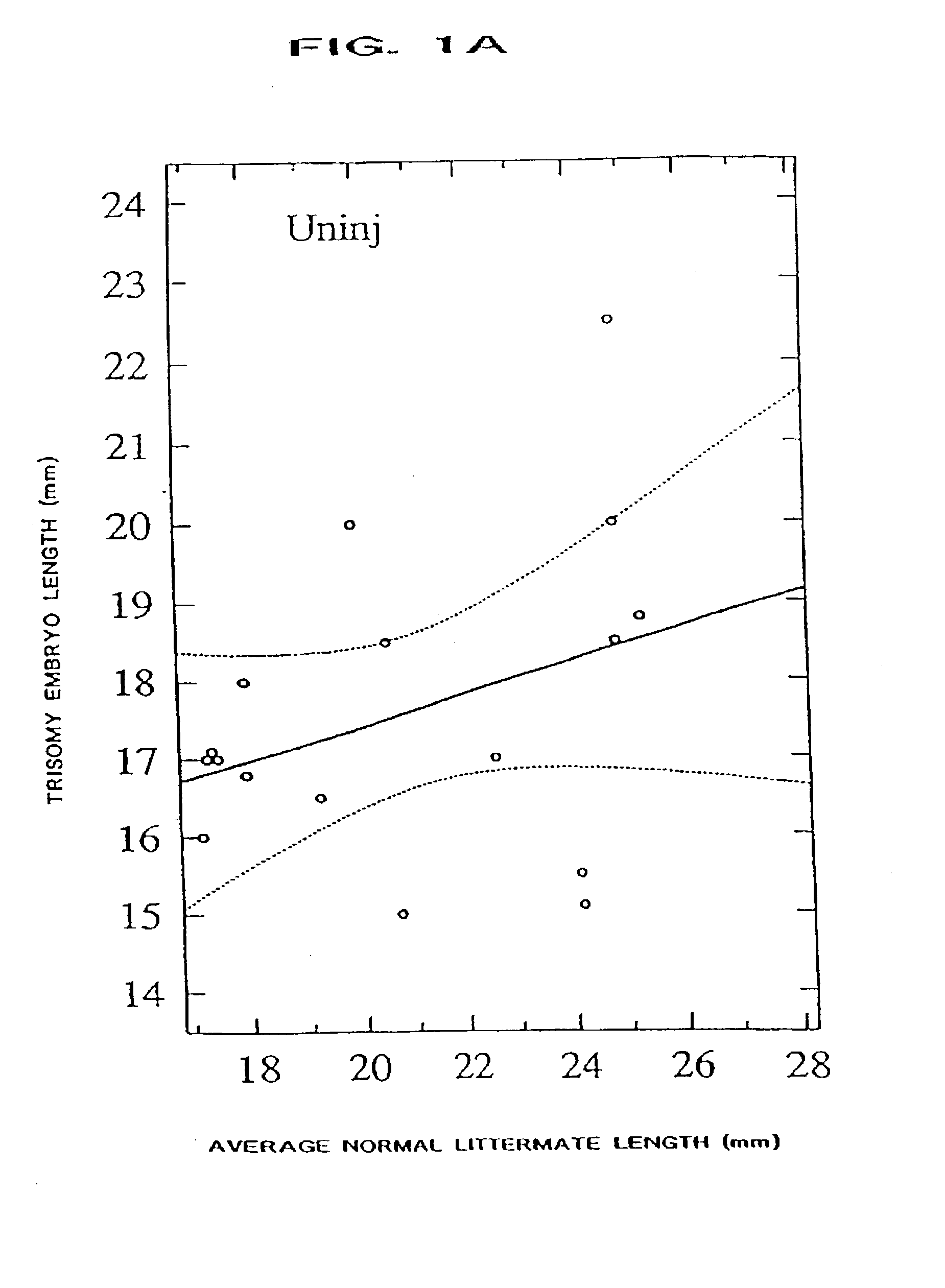
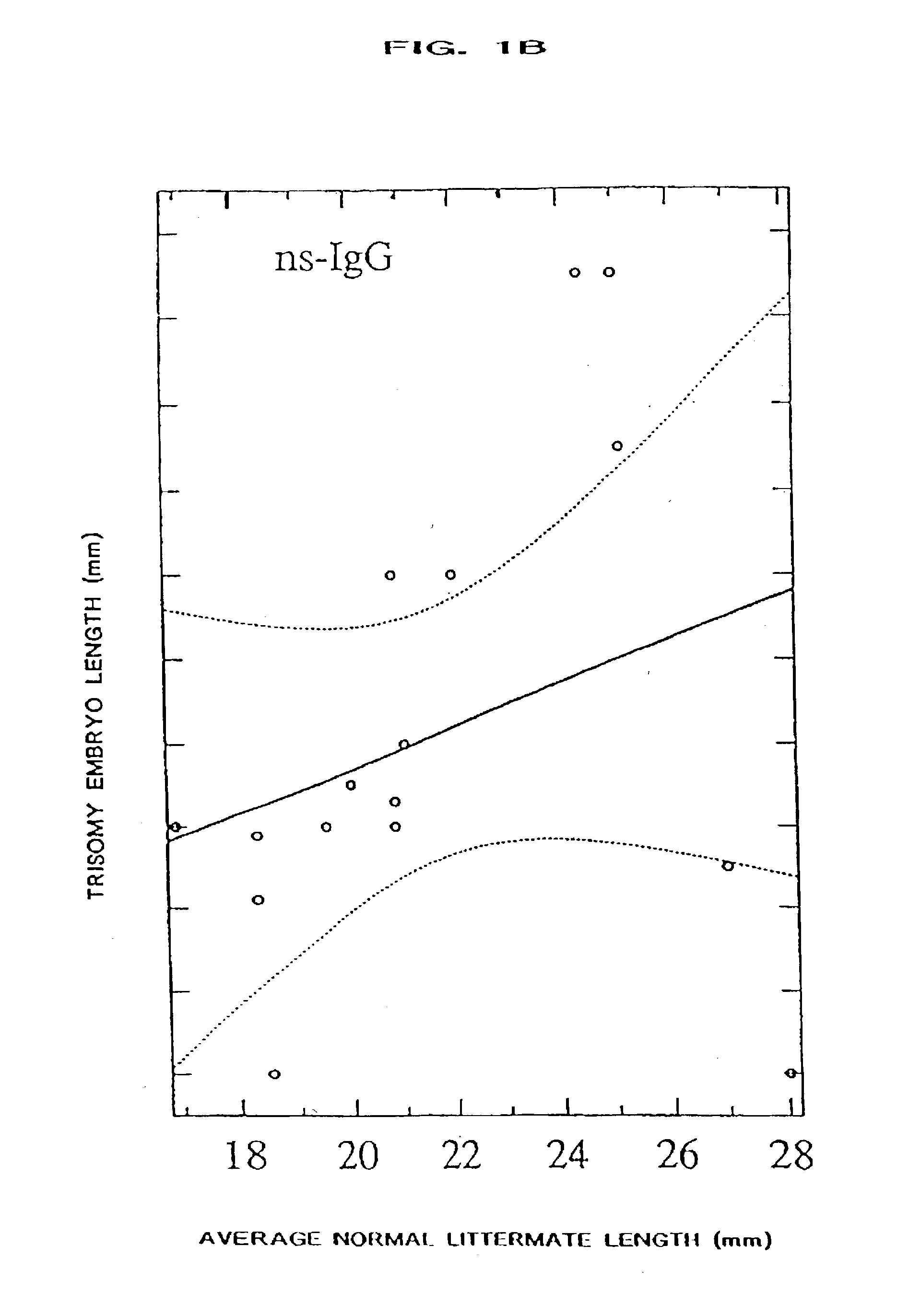
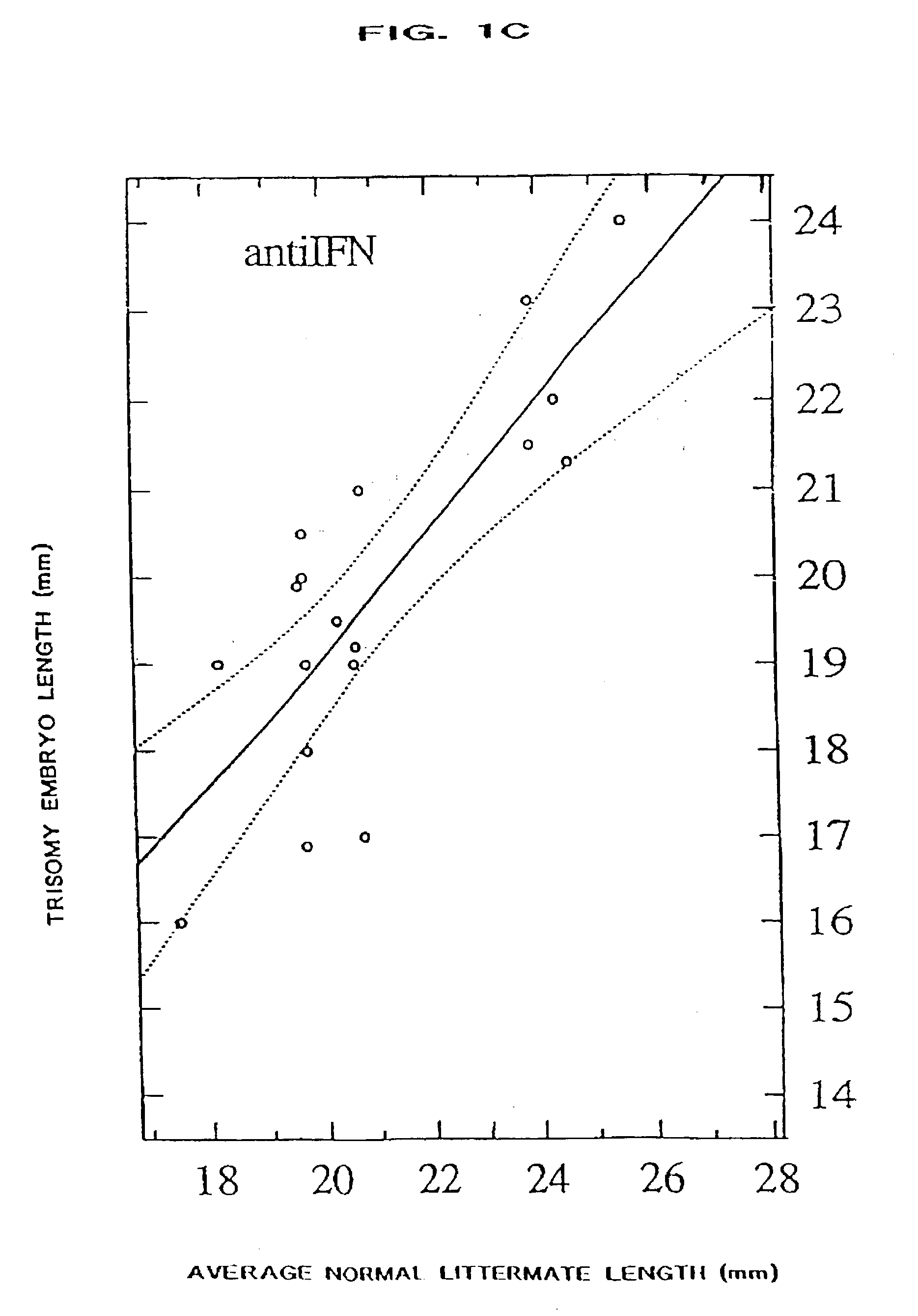
The present invention relates to a process for ameliorating or preventing diseases that are caused, in part, by an increased level of, and / or an abnormal responsivity to, interferon. Alzheimer's disease, HIV infection, Down syndrome, transplant rejection, autoimmune disease, and infant encephalitis are examples of such diseases. Specifically, the invention provides a method for treating subjects suffering from, or at risk for, such diseases by the administration of a pharmacological preparation of interferon binding proteins of mammalian and / or viral origin that antagonize interferon's action. This invention comprises compositions of interferon binding proteins that can inhibit the activity of interferon gamma plus interferon alpha such compositions along with their method of production and modification being described herein.
Owner:MEIOGEN BIOTECH CORP
Stabilizing alkylglycoside compositions and methods thereof
ActiveUS8084022B2Convenient treatmentReduce aggregationPeptide/protein ingredientsMetabolism disorderInterferon alphaImmunogenicity
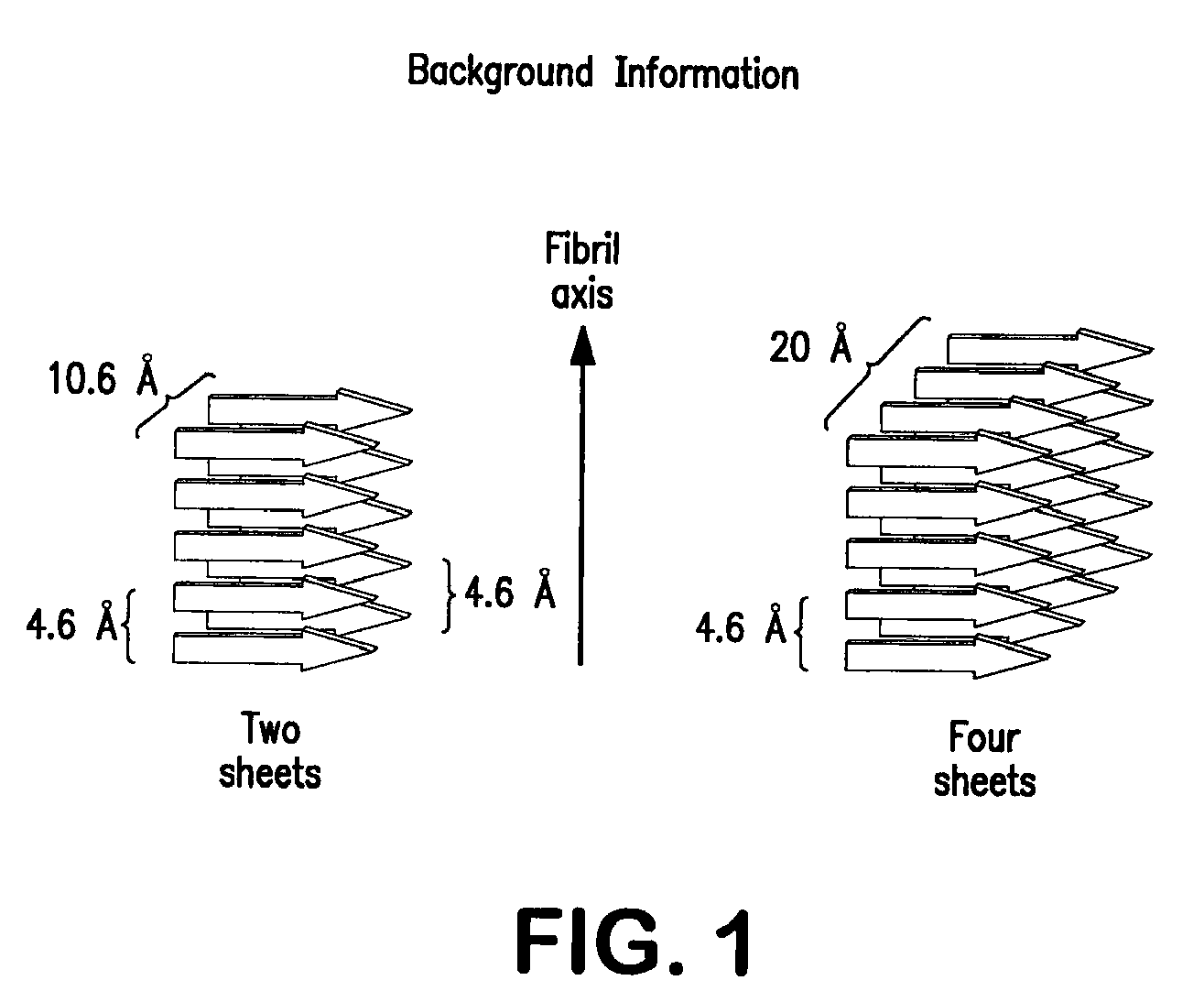
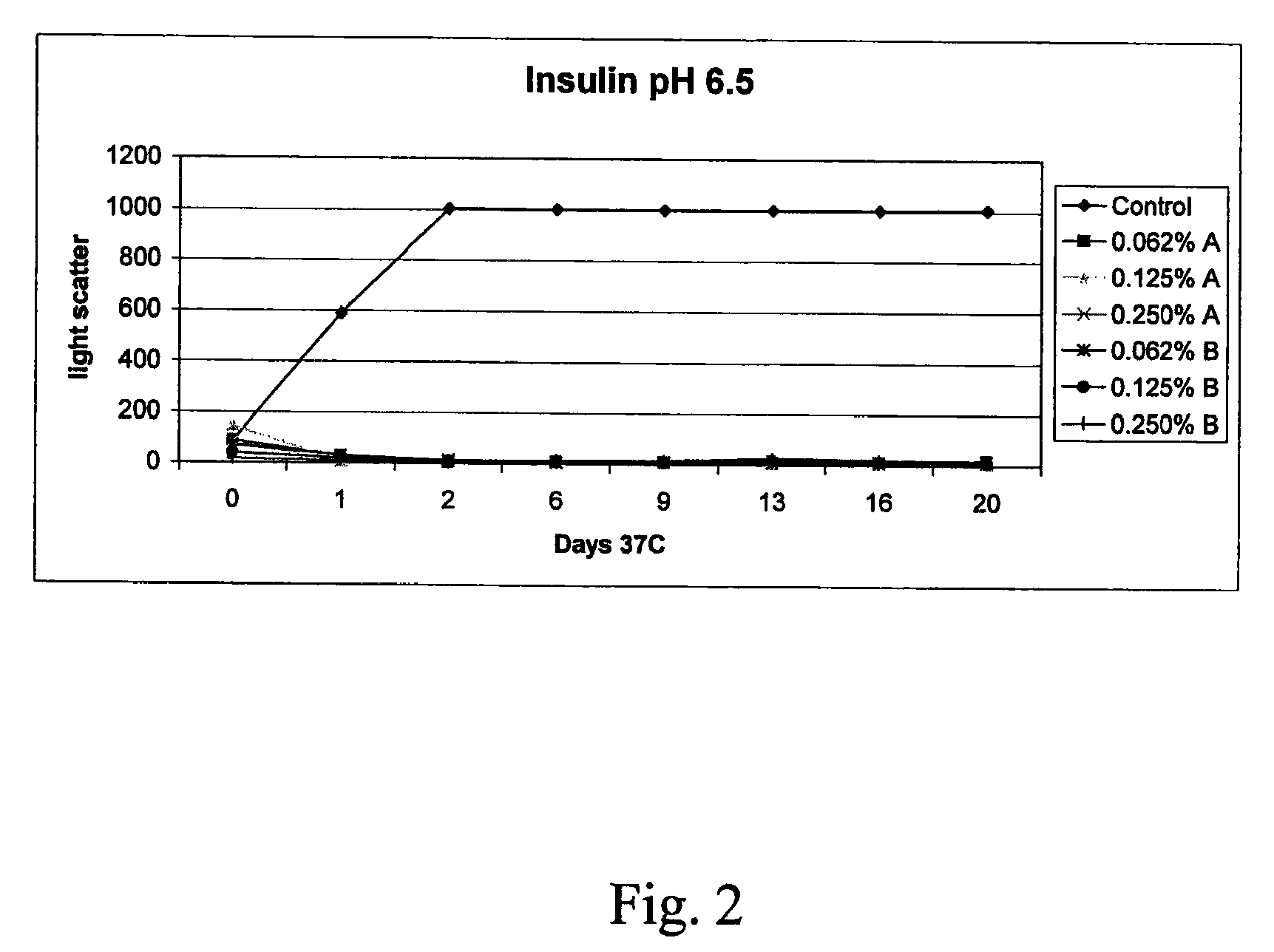
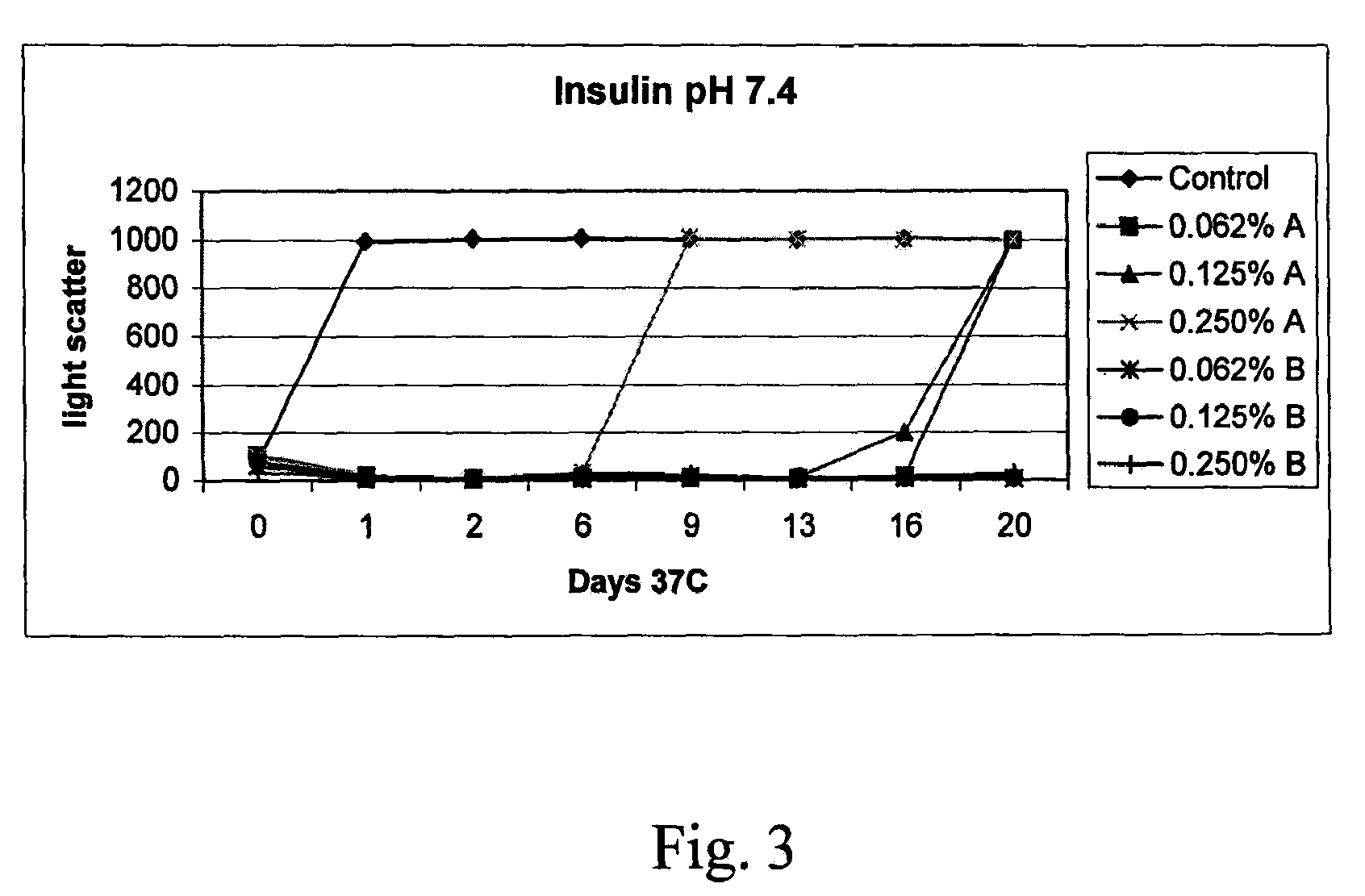
The present invention relates to alkylglycoside-containing compositions and methods for increasing the stability, reducing the aggregation and immunogenicity, increasing the biological activity, and reducing or preventing fibrillar formation of a peptide, polypeptide, or variant thereof, for example interferons, such as interferon-alpha, interferon-beta, interferon-gamma, and variants thereof.
Owner:AEGIS THERAPEUTICS LLC
Compositions and Methods for Inducing the Activation of Immature Monocytic Dendritic Cells
InactiveUS20080254537A1Antibacterial agentsTumor rejection antigen precursorsDendritic cellBiological activation
The present invention provides methods for inducing the maturation of immature dendritic cells (DC) and for activating those cells without the use of a dendritic cell maturation agent. The activated DC can be used for inducing an antigen specific T cell response. Methods of the invention can also comprise the addition of a directional maturation agent, such as interferon gamma, to induce a Th-I and / or Th-2 bias in the response obtained. The present invention also provides dendritic cell populations useful for activating and for inducing antigen specific T cells. Similarly, activated antigen specific T cell populations, and methods of making the same are provided.
Owner:NORTHWEST BIOTHERAPEUTICS INC
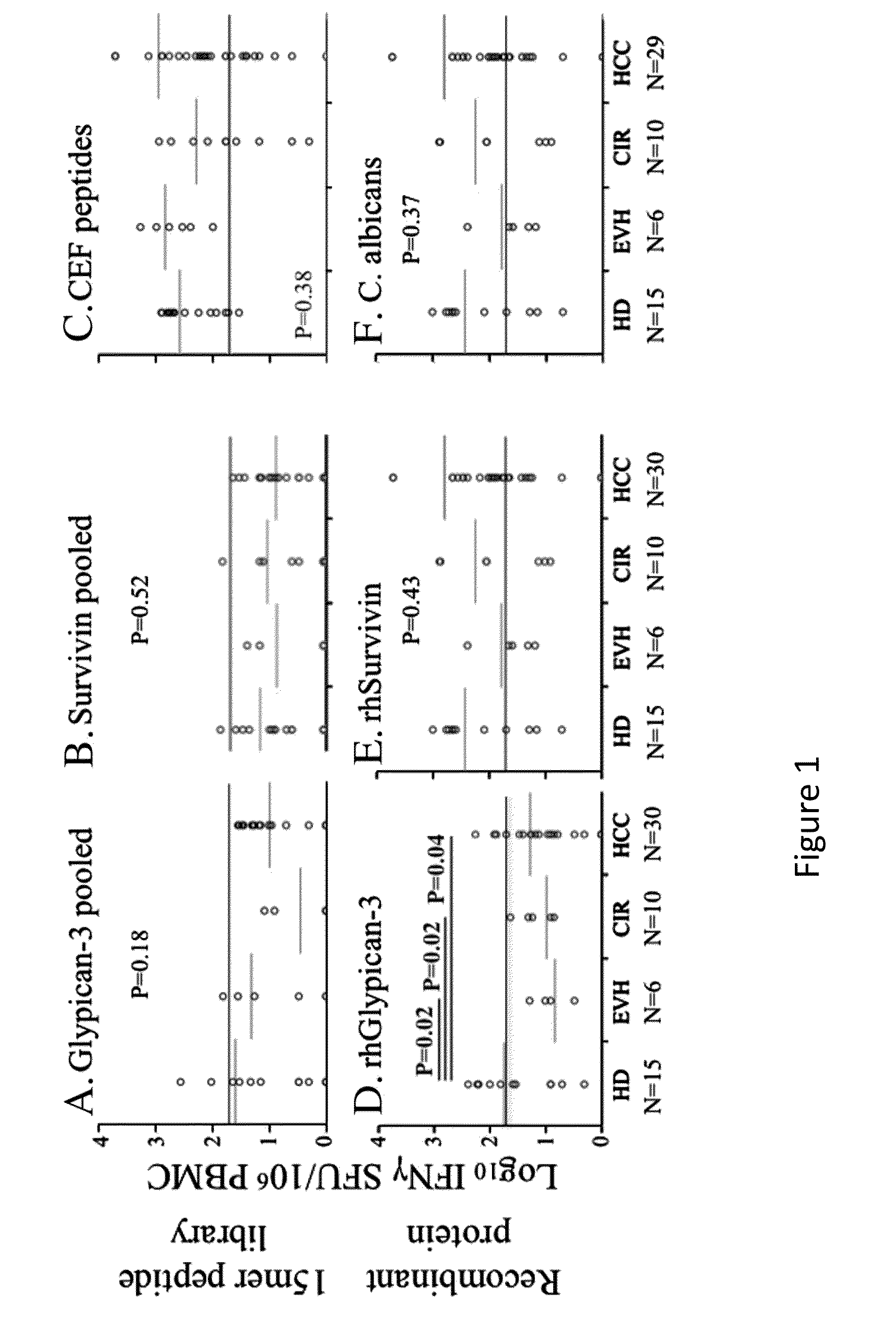
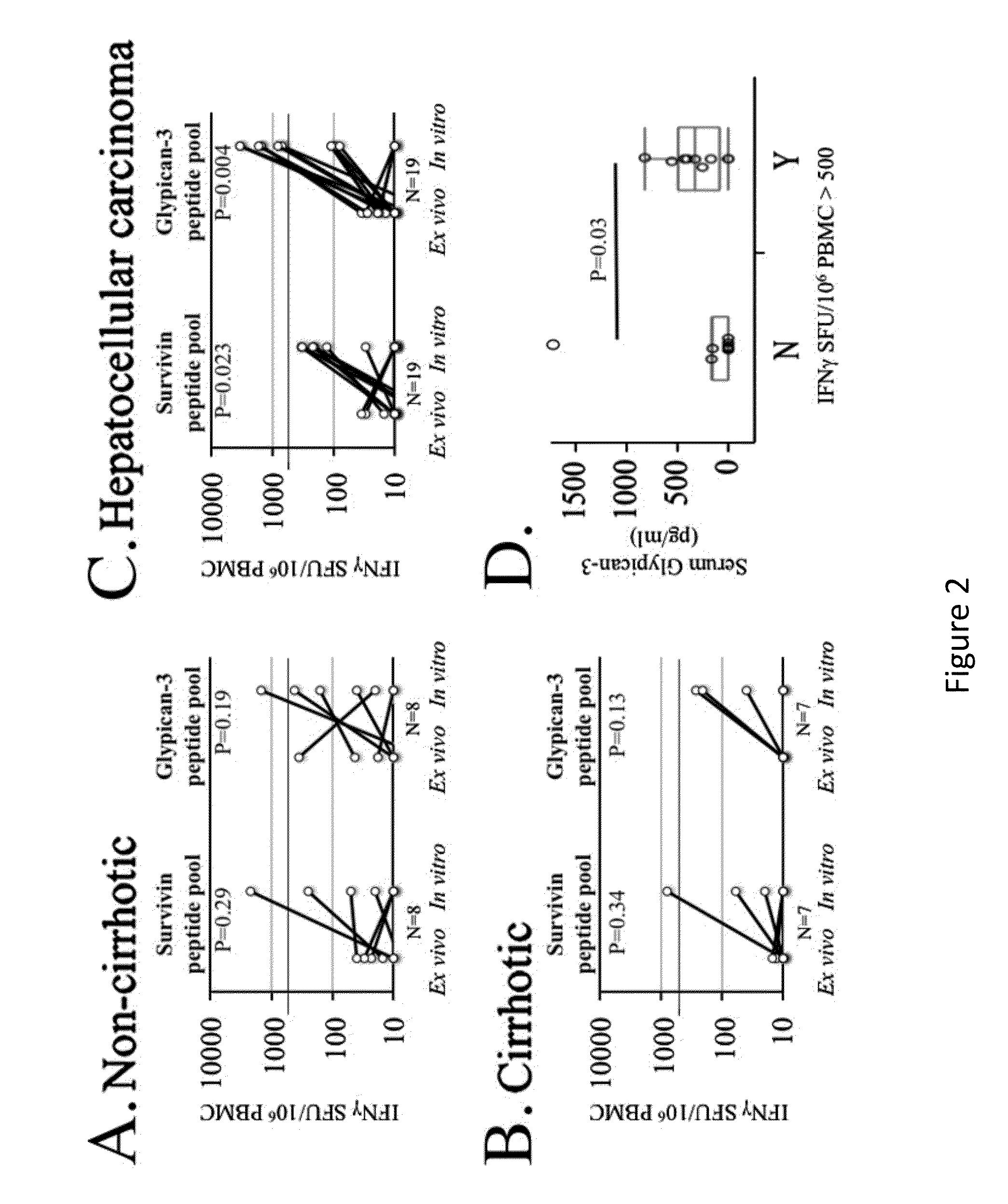
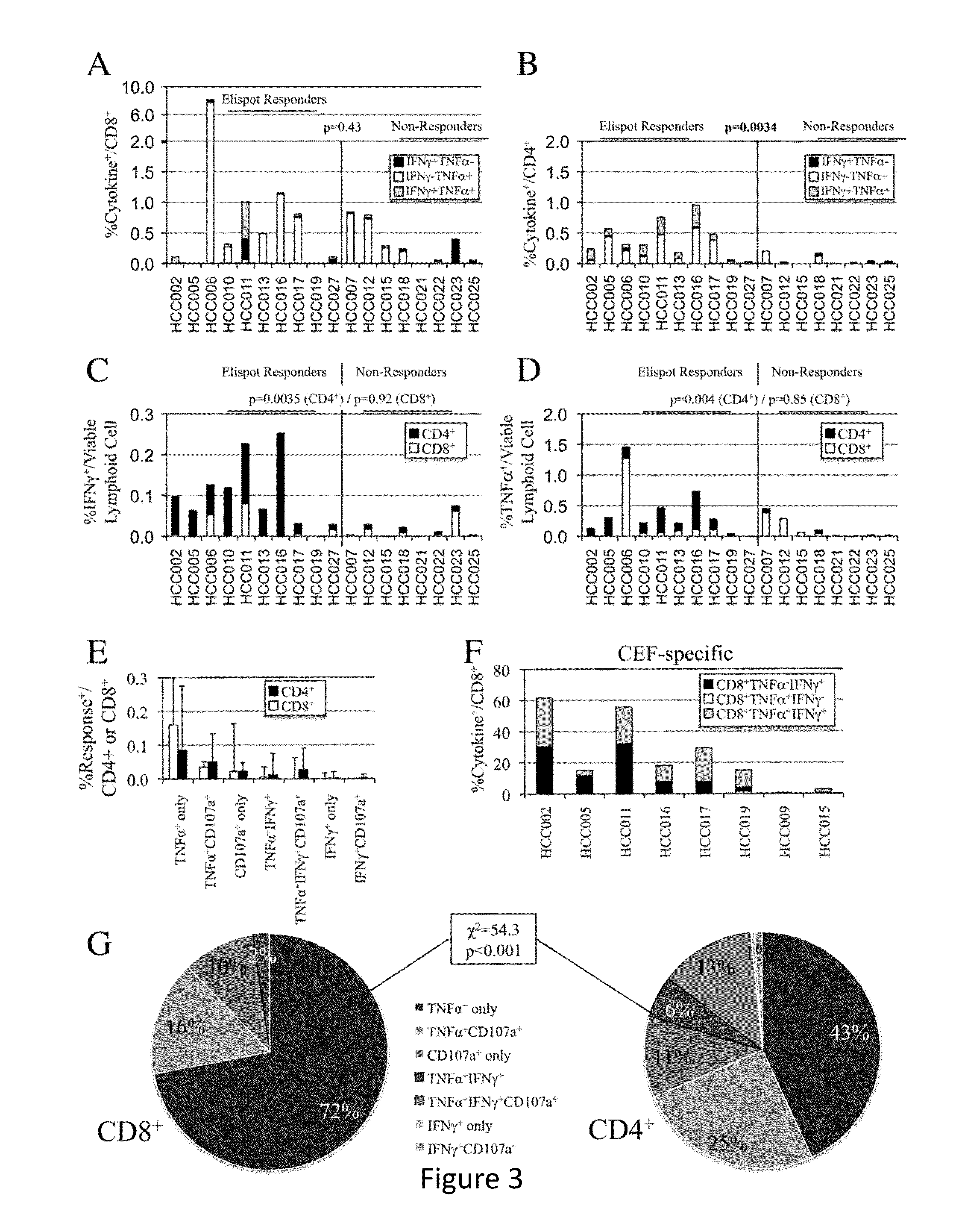
Expansion of Interferon-Gamma-Producing T-Cells Using Glypican-3 Peptide Library
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Genetic adjuvants for immunotherapy
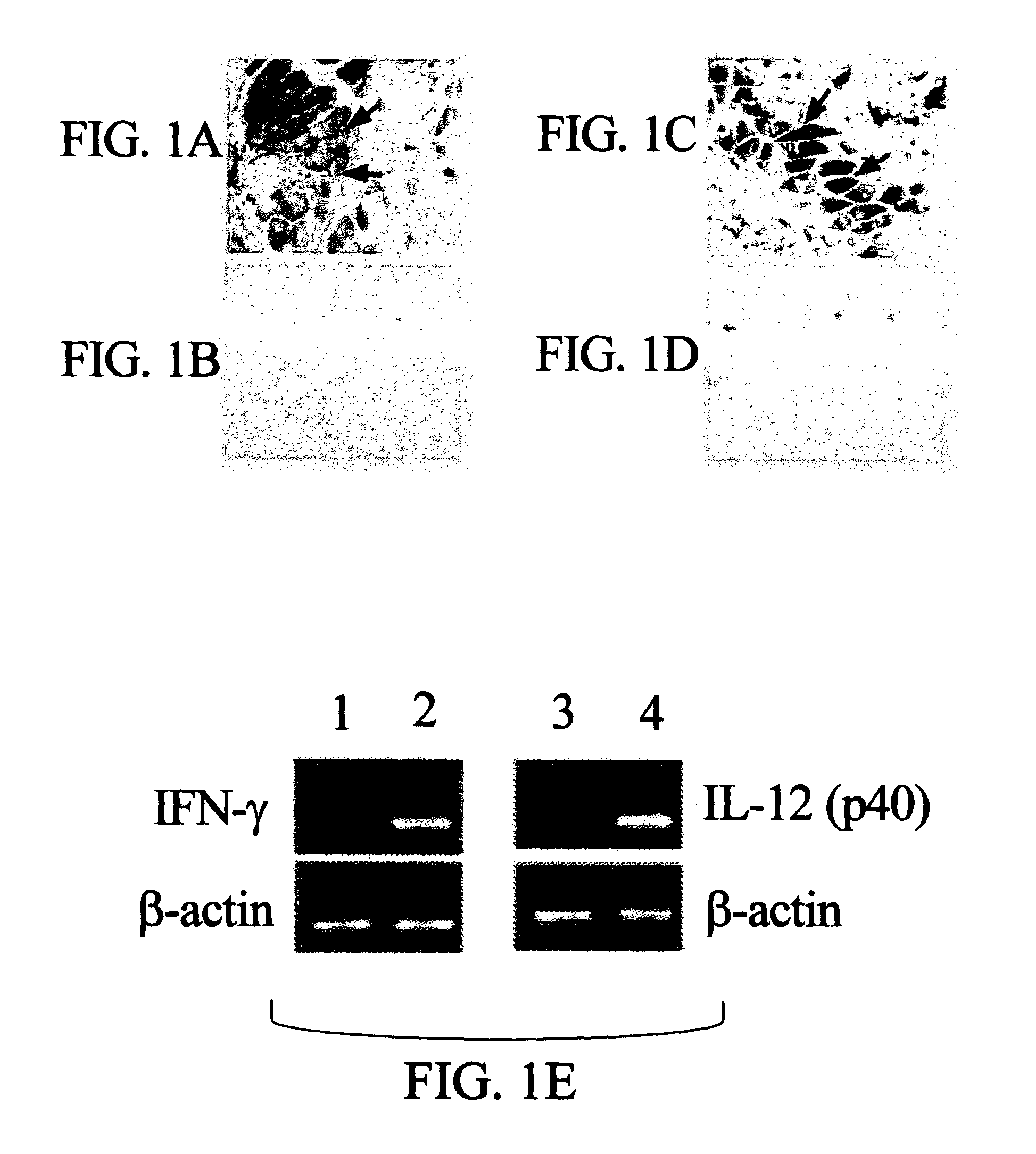
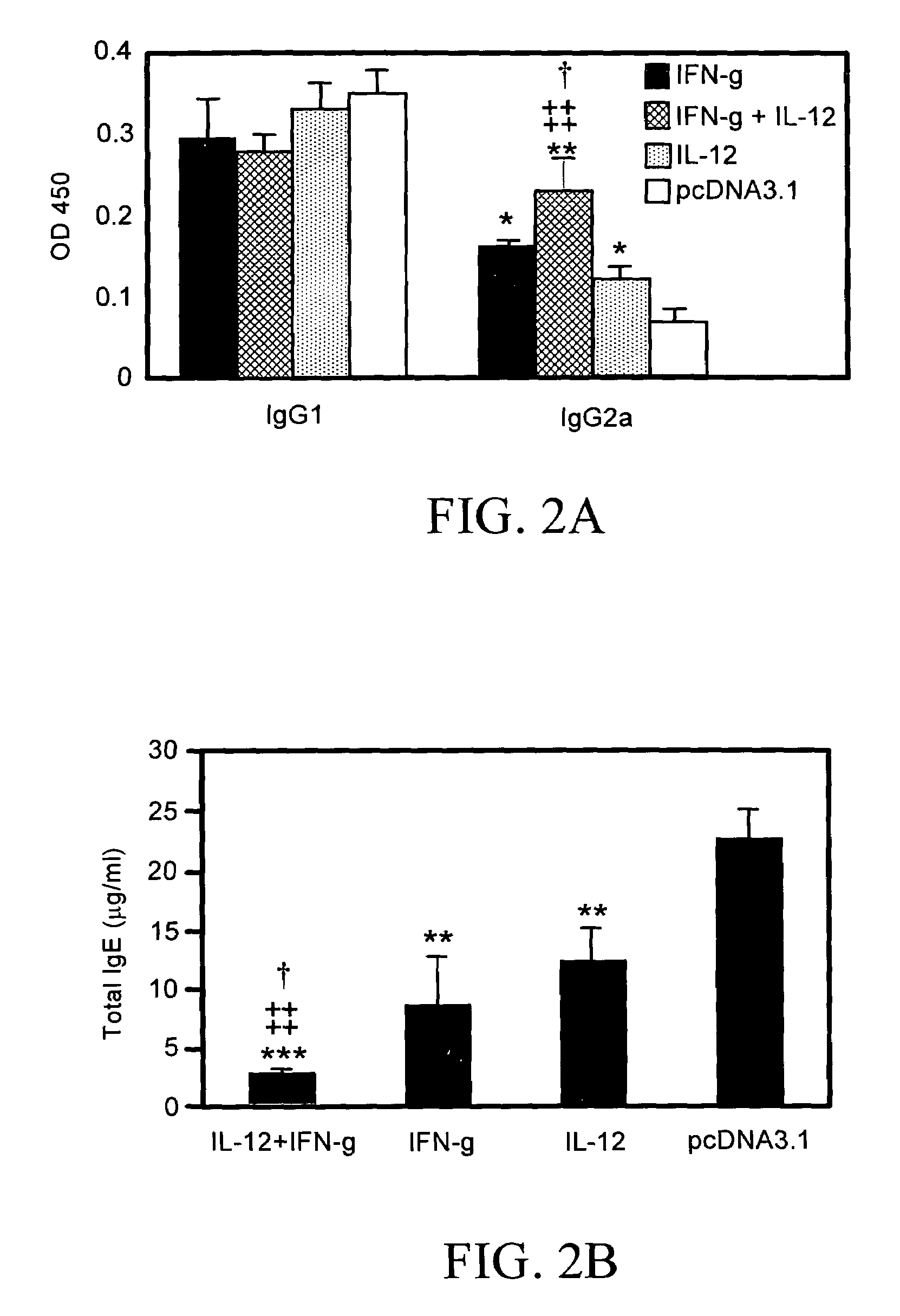
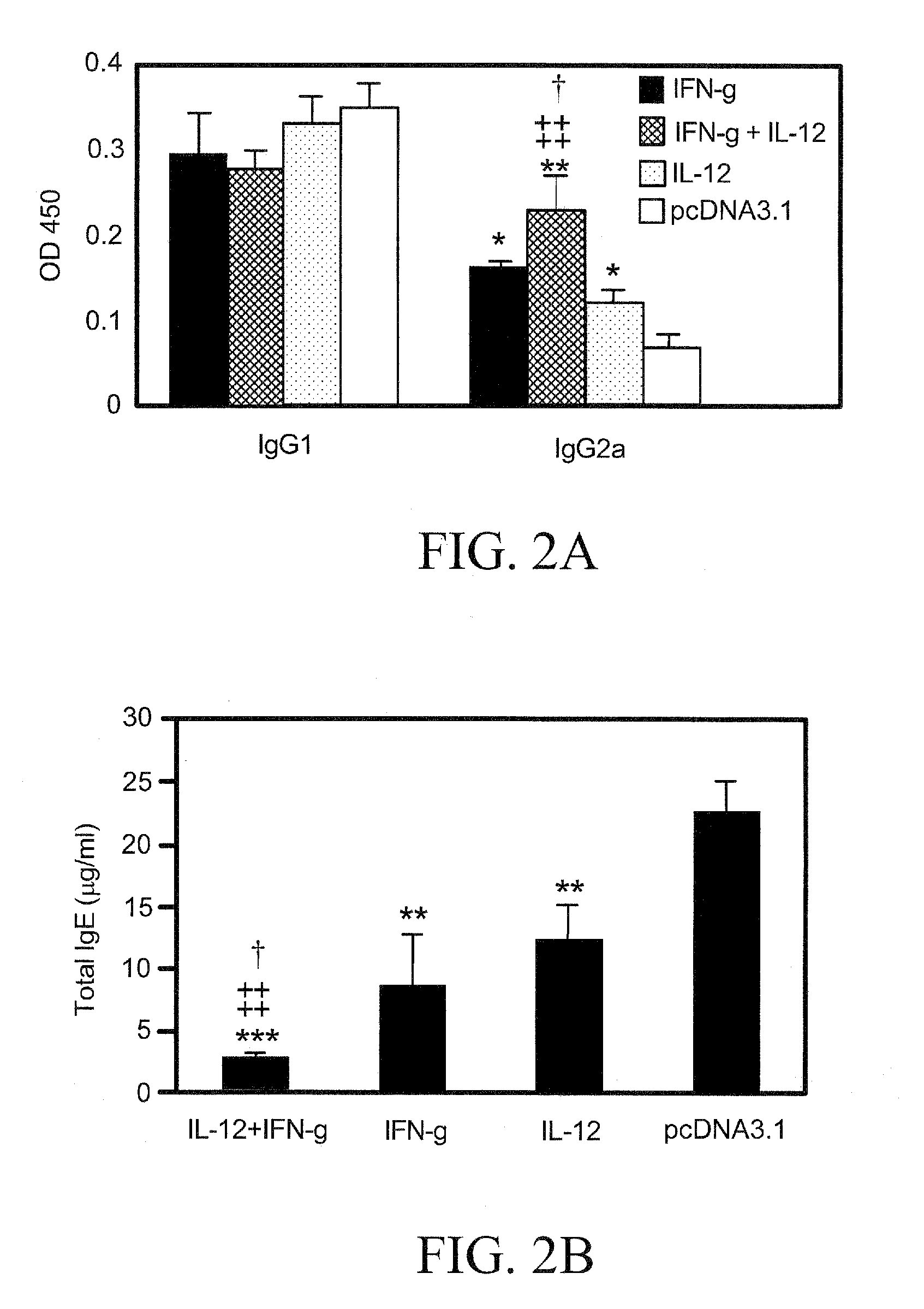
The present invention pertains to methods and pharmaceutical compositions for modulating an immune response. The method of the present invention involves administration of an effective amount of nucleic acid molecules encoding interleukin-12 (IL-12), interferon-gamma (IFN-γ), or a combination thereof, to a patient in need of such treatment. The pharmaceutical compositions of the invention contain nucleic acid molecules encoding IL-12 and / or IFN-γ and an operably-linked promoter sequence. In another aspect, the present invention concerns expression vectors containing a nucleotide sequence encoding IL-12 and IFN-γ, and an operably-linked promoter sequence. In another aspect, the present invention concerns cells generally modified with a nucleotide sequence encoding IL-12 and IFN-γ.
Owner:SOUTH FLORIDA UNIVESITY OF
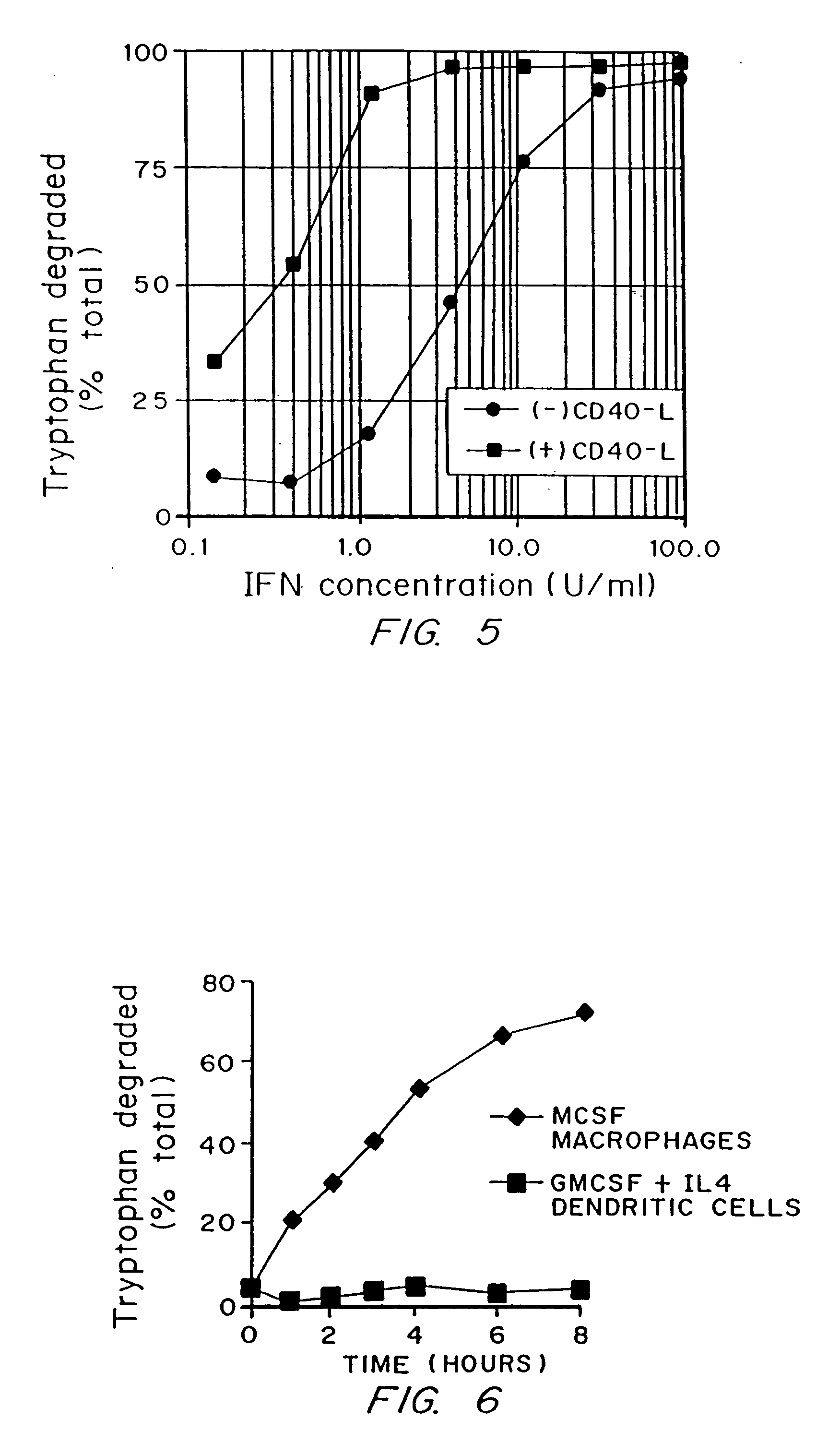
Cell Population Having Immunoregulatory Activity, Method for Isolation and Uses
InactiveUS20090130067A1BiocidePeptide/protein ingredientsAutoimmune conditionConnective tissue fiber
The present invention provides a population of connective tissue derived cells that respond to interferon-gamma (IFN-γ) by expressing indolamine-2,3-dioxygenase (IDO) for use in preventing, treating or ameliorating one or more symptoms associated with disorders in which modulation of a subject's immune system is beneficial, including, but not limited to, autoimmune diseases, inflammatory disorders, and immunologically mediated diseases including rejection of transplanted organs and tissues.
Owner:TIGENIX SAU +1
Efficient CIK amplifying method
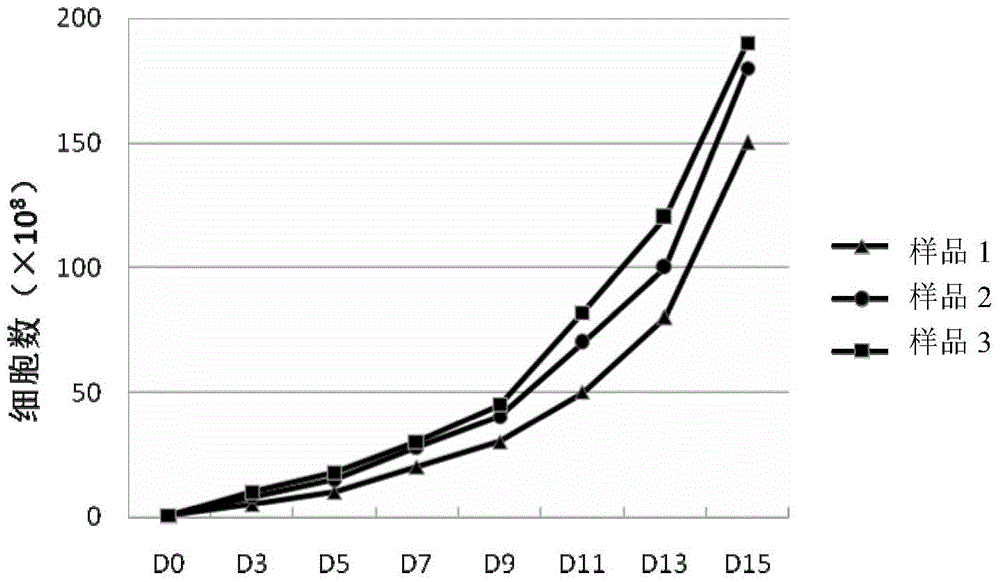
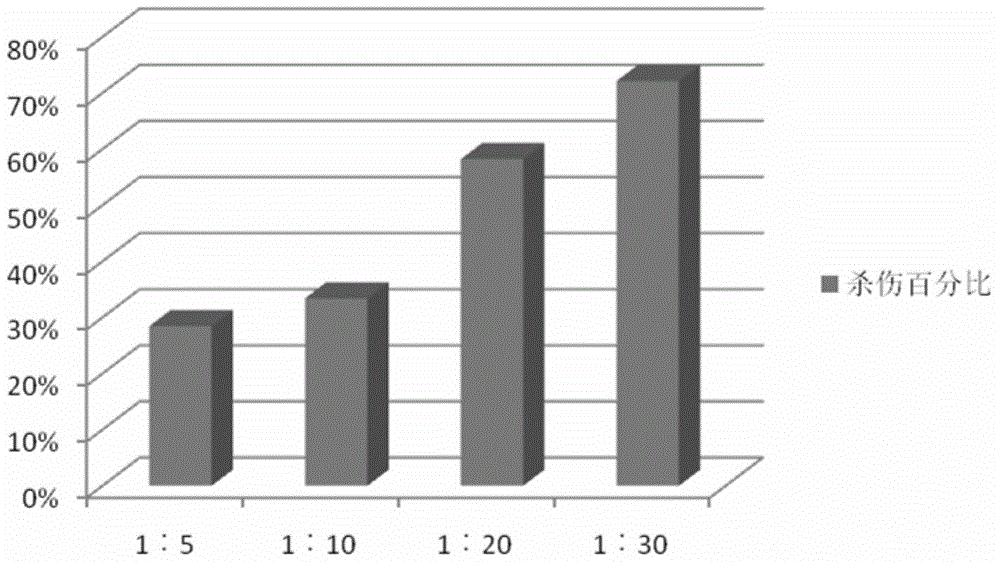
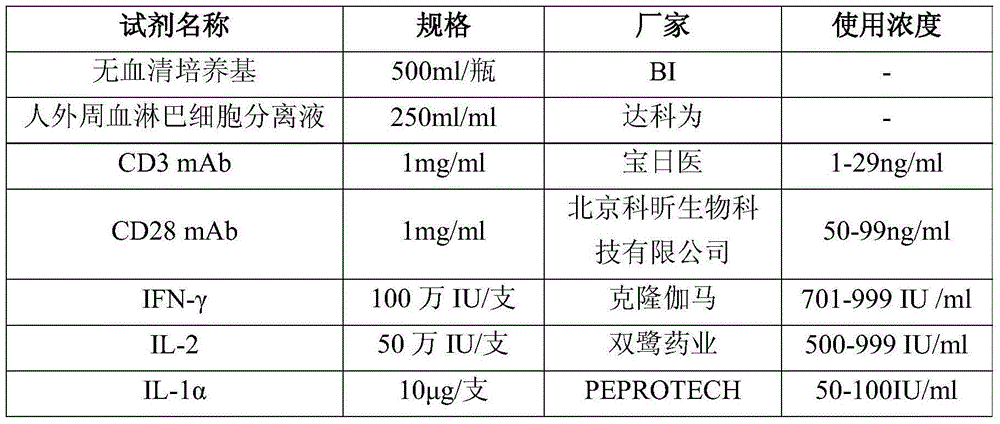
The invention discloses an efficient CIK amplifying method, in particular to a method for cell populations, namely cytokine-induced killer cells utilizing in-vitro cell factors for efficiently inducing mononuclear cell expressions CD3 and CD56 of peripheral blood, wherein the cell populations have killing functions. The cultivation method for the CIK efficiently expressing CD3+CD56 comprises the following steps that the peripheral blood of a healthy person or a patient is collected in a sterile mode, after the peripheral blood is diluted through a saline solution with the same volume, a Ficoll lymphocyte separating medium is used for separating mononuclear cells, in the CIK cell inducing process, CD3 monoclonal antibodies (CD3mAb), CD28 monoclonal antibodies (CD28mAb), interferon-gamma (IFN-gamma), interleukin-2(IL-2) and interleukin 1alpha (IL-1alpha) are added, cultivation is carried out for 13-16 days to obtain cells, a CIK cell preparation is prepared, and flow cytometry detection and microorganism detection are carried out. According to the CIK cultivation method, the CIK number can be increased to be 6*10<9> or over 6*10<9> in two weeks, the cell survival rate can be increased to be 99% or over 99%, and the double-positive proportion of the CD3+CD56+cells reaches 30% or over 30%.
Owner:UNION STEMCELL & GENE ENG
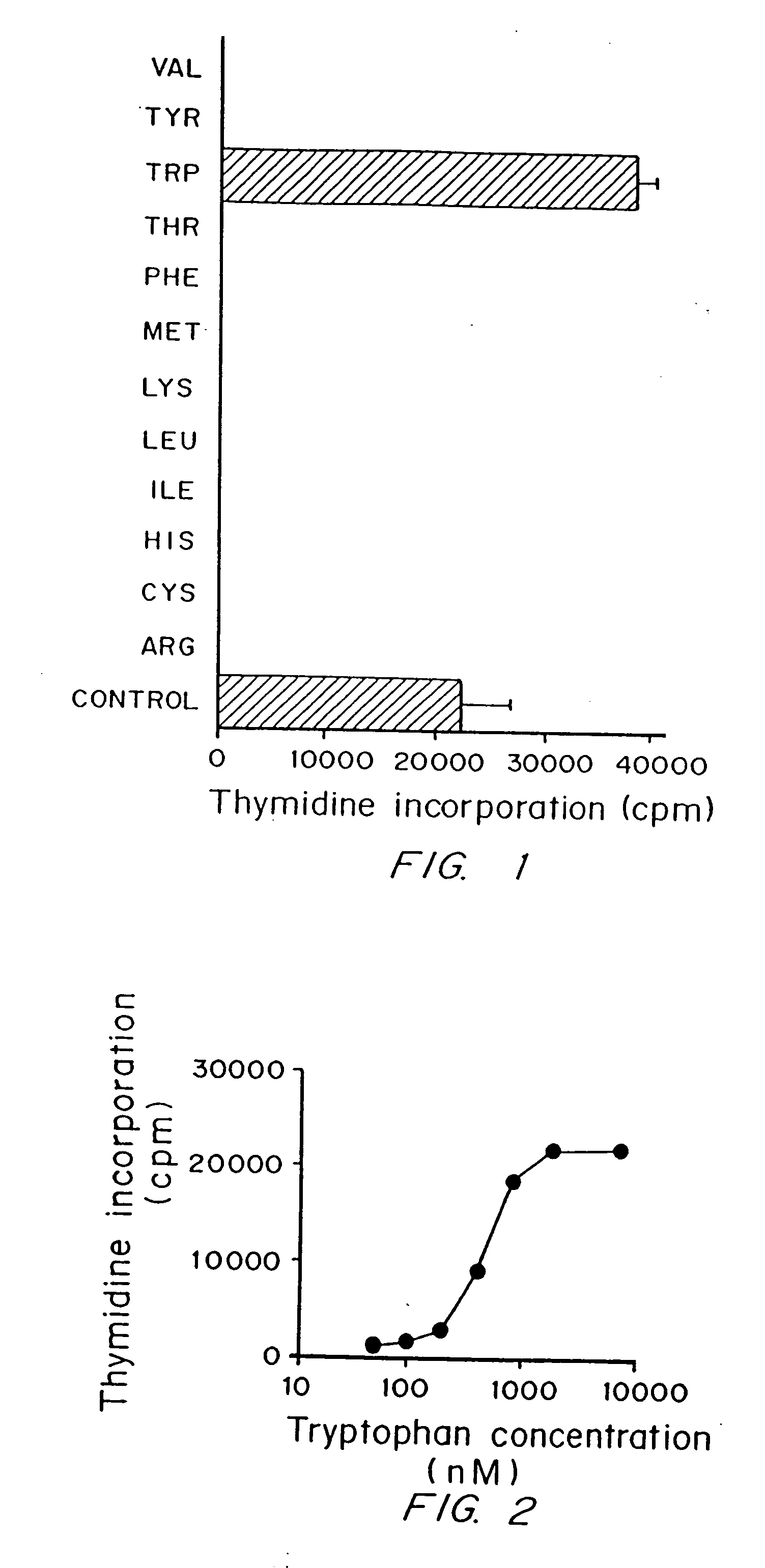
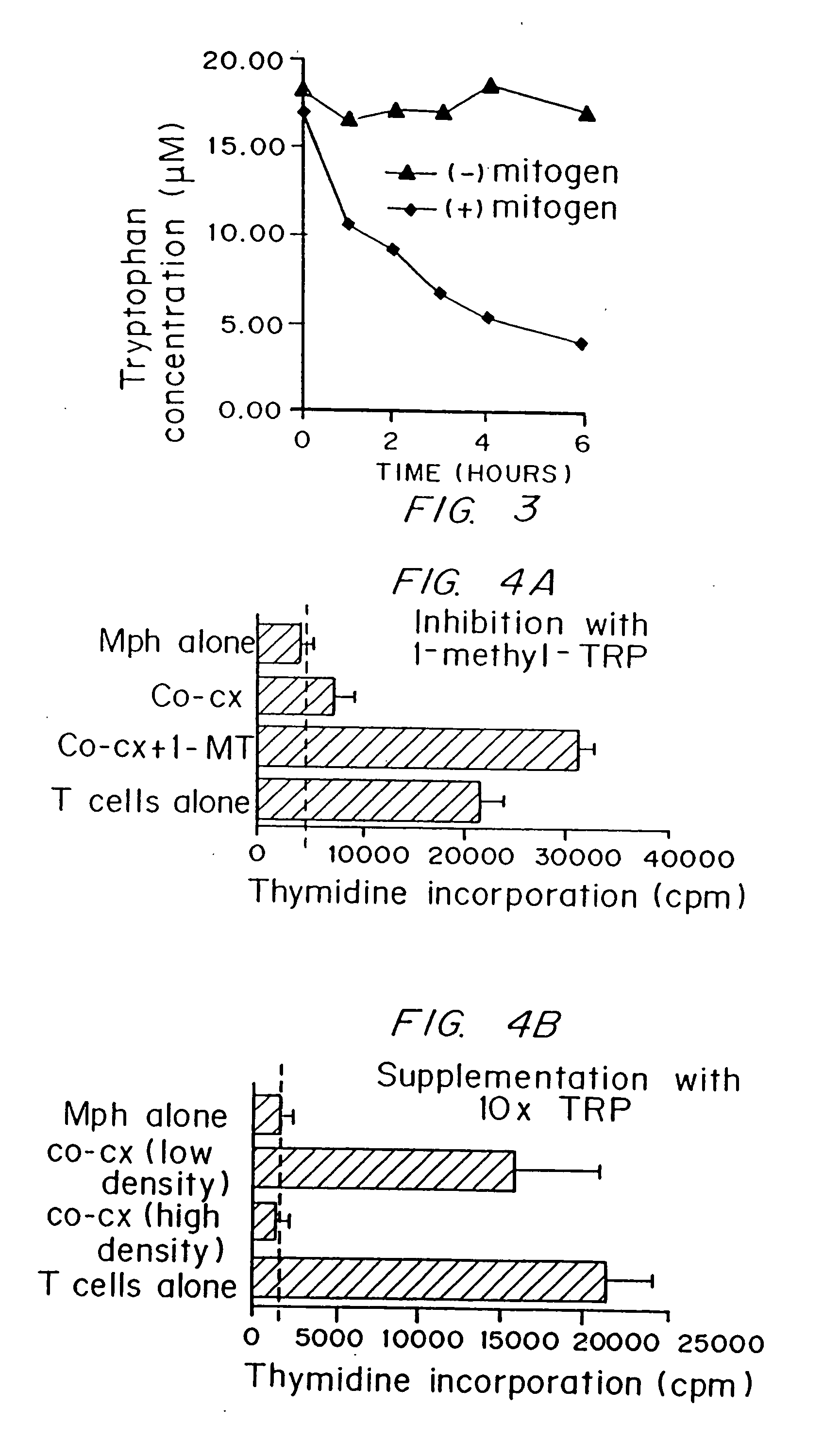
Regulation of T cell-mediated immunity by tryptophan
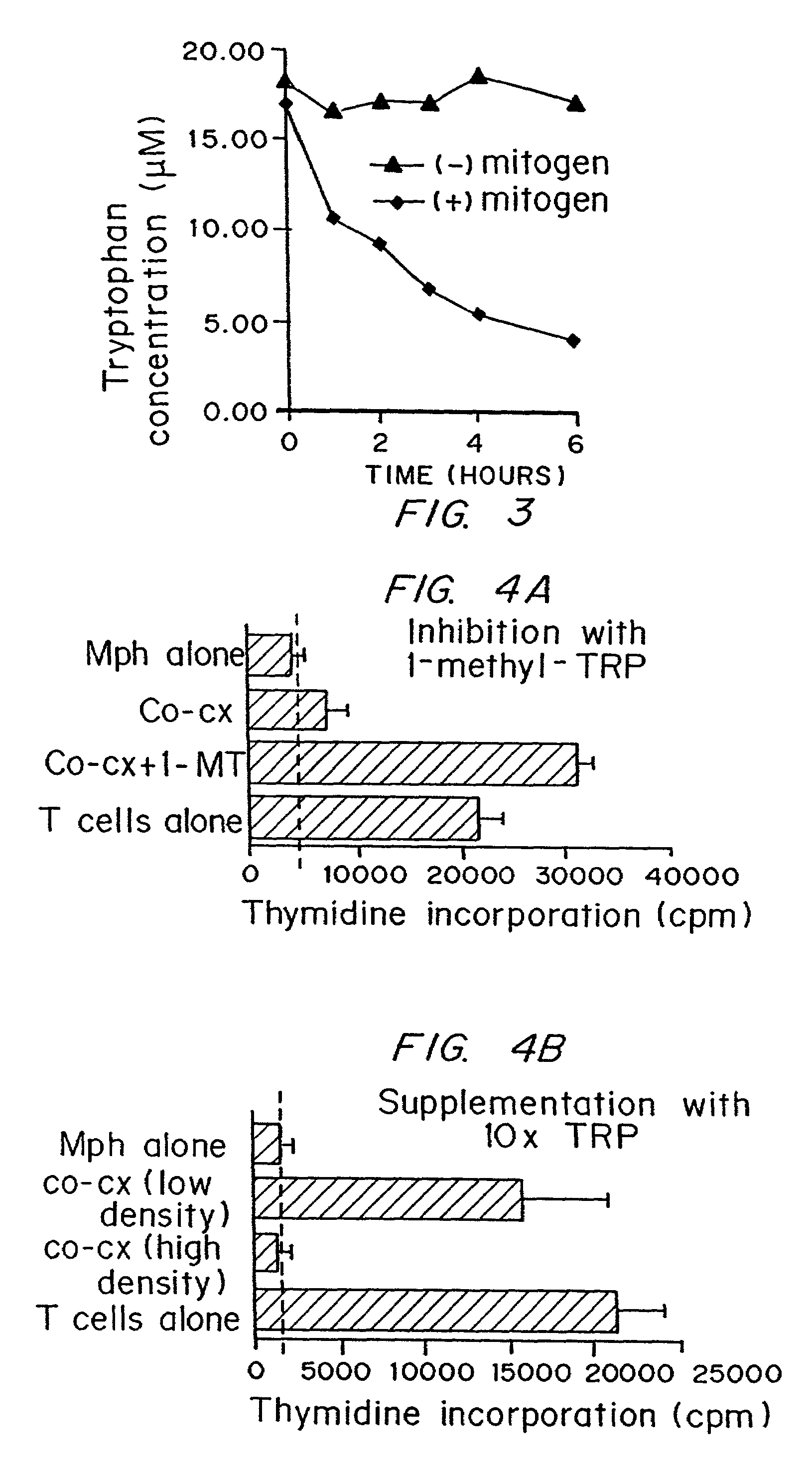
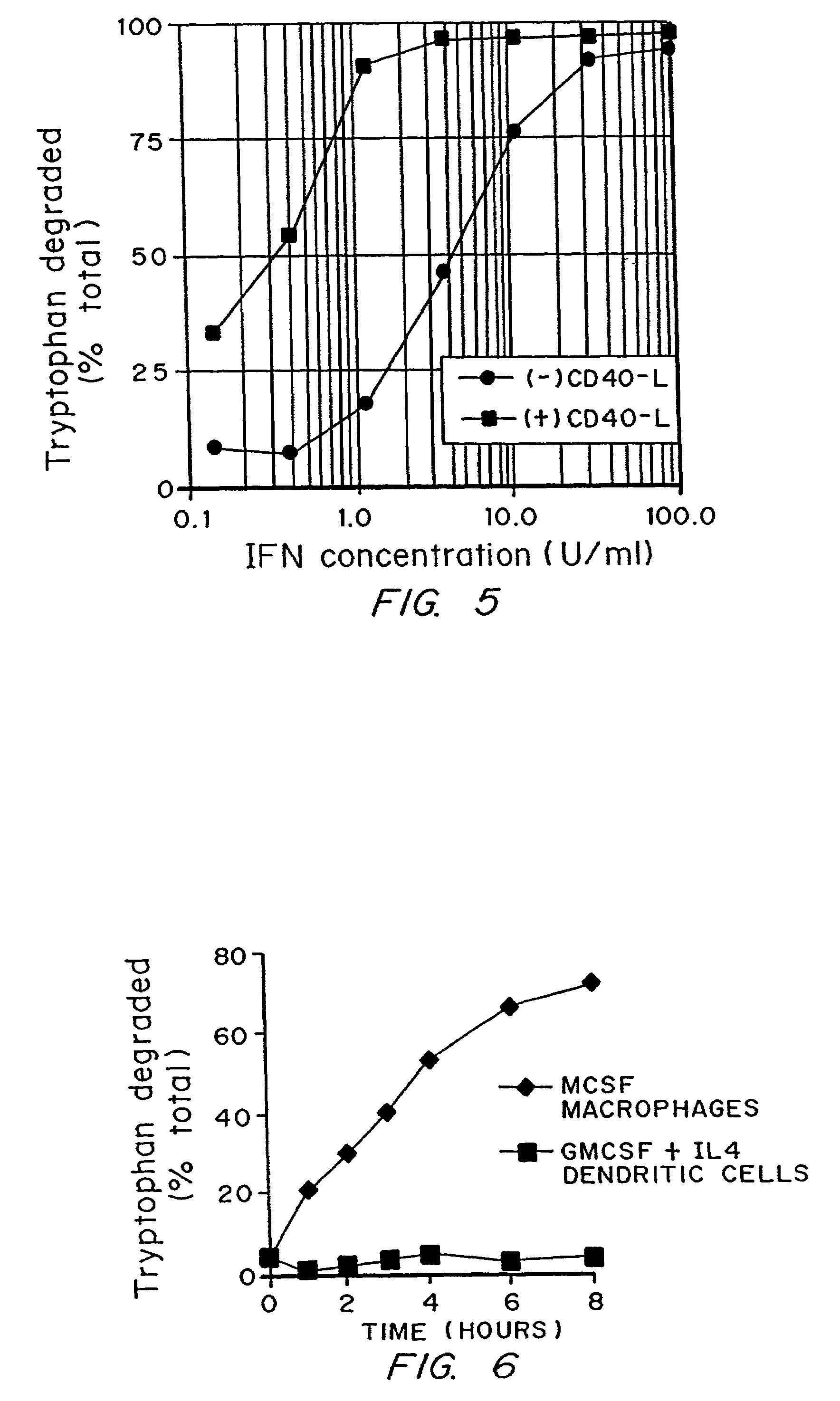
InactiveUS7160539B2Reduce viral loadAvoid destructionBiocidePeptide/protein ingredientsAntigenPregnancy
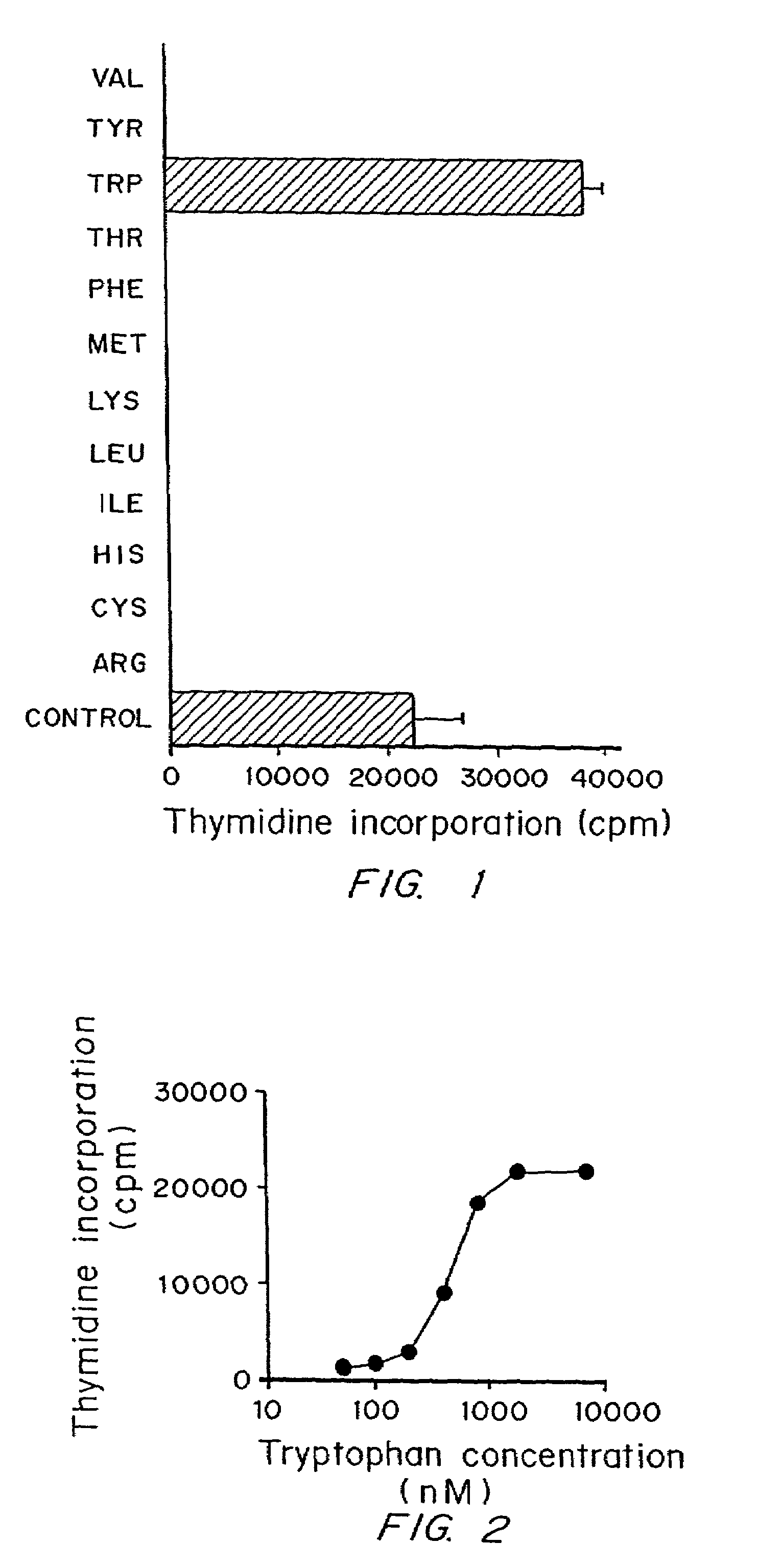
A mechanism of macrophage-induced T cell suppression is the selective elimination of tryptophan and / or increase in one or more tryptophan metabolites within the local macrophage microenvironment Studies demonstrate that expression of IDO can serve as a marker of suppression of T cell activation, and may play a significant role in allogeneic pregnancy and therefore other types of transplantation, and that inhibitors of IDO can be used to activate T cells and therefore enhance T cell activation when the T cells are suppressed by pregnancy, malignancy or a virus such as HIV. Inhibiting tryptophan degradation (and thereby increasing tryptophan concentration while decreasing tryptophan metabolite concentration), or supplementing tryptophan concentration, can therefore be used in addition to, or in place of, inhibitors of IDO. Similarly, increasing tryptophan degradation (thereby , decreasing tryptophan concentration and increasing tryptophan metabolite concentration), for example, by increasing IDO concentration or IDO activity, can suppress T cells. Although described particularly with reference to IDO regulation, one can instead manipulate local tryptophan concentrations, and / or modulate the activity of the high affinity tryptophan transporter, and / or administer other tryptophan degrading enzymes. Regulation can be further manipulated using cytokines such as macrophage colony stimulating factor, interferon gamma, alone or in combination with antigen or other cytokines.
Owner:MEDICAL COLLEGE OF GEORGIA FOUND
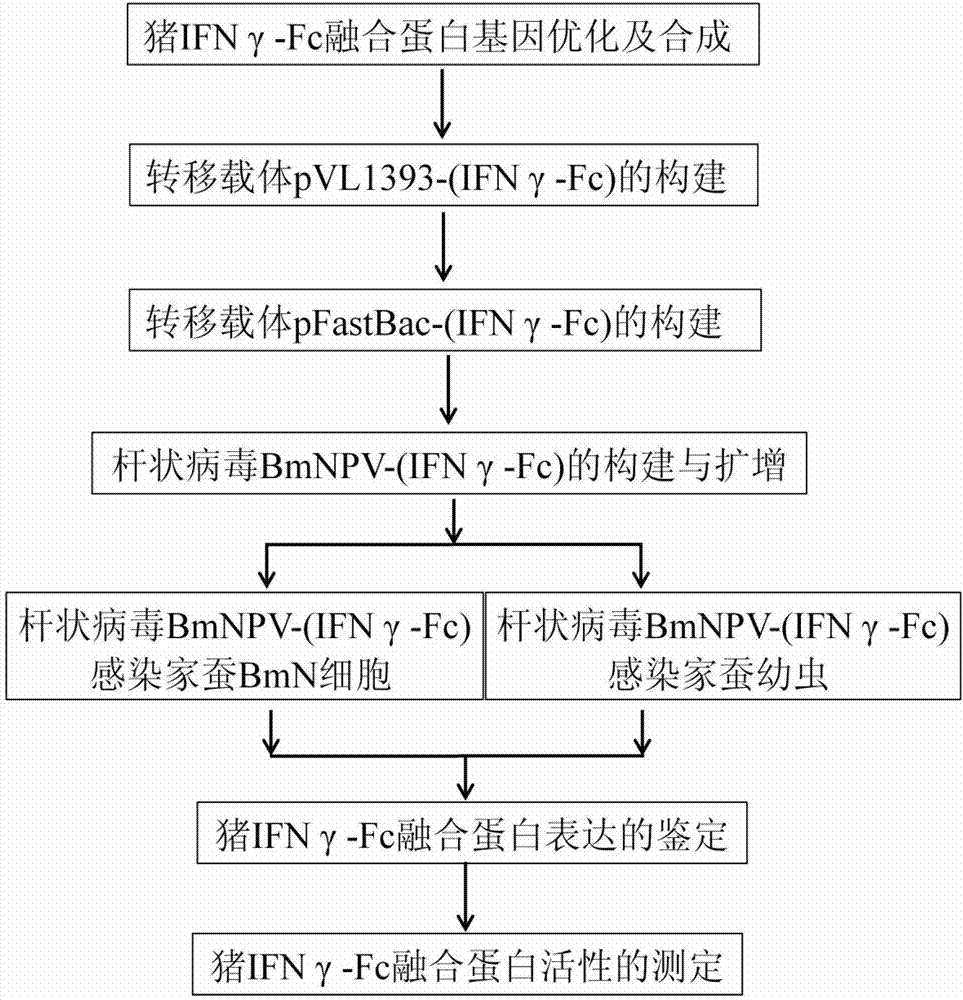
Pig IFN (interferon) gamma-Fc fusion protein as well as coding gene and expression method of pig IFN (interferon) gamma-Fc fusion protein
ActiveCN103087200AReduce manufacturing costPrecise structurePeptide/protein ingredientsAnimal feeding stuffBiotechnologyProtein target
The invention provides a pig IFN (interferon) gamma-Fc fusion protein optimized according to a silkworm reaction system and a coding gene of the pig IFNgamma-Fc fusion protein and a method of expressing pig IFNgamma-Fc fusion protein by using a silkworm bioreactor, and belongs to the field of a biological genetic engineering. The pig interferon gamma serving as a non-special broad spectrum antiviral biological agent has a wide medicinal prospect in a veterinary drug field; but like most of genetic engineering veterinary drugs, the pig interferon gamma also has the problems such as insufficient output, expensive price, irregular quality, short acting time and the like. A target protein with a high expression efficiency and strong activity is obtained by expressing the pig IFNgamma-Fc fusion protein optimized by using a silkworm expression system. In addition, the acting time of the pig interferon gamma is prolonged by addition of an Fc segment, so that the overall immune regulation effect is enhanced, and purification at a later stage is facilitated, thus a pig IFNgamma-Fc fusion protein preparation produced by using a genetic engineering method is possible, and a condition is also created for developing a novel feed containing the fusion protein.
Owner:江苏晶红生物医药科技股份有限公司
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
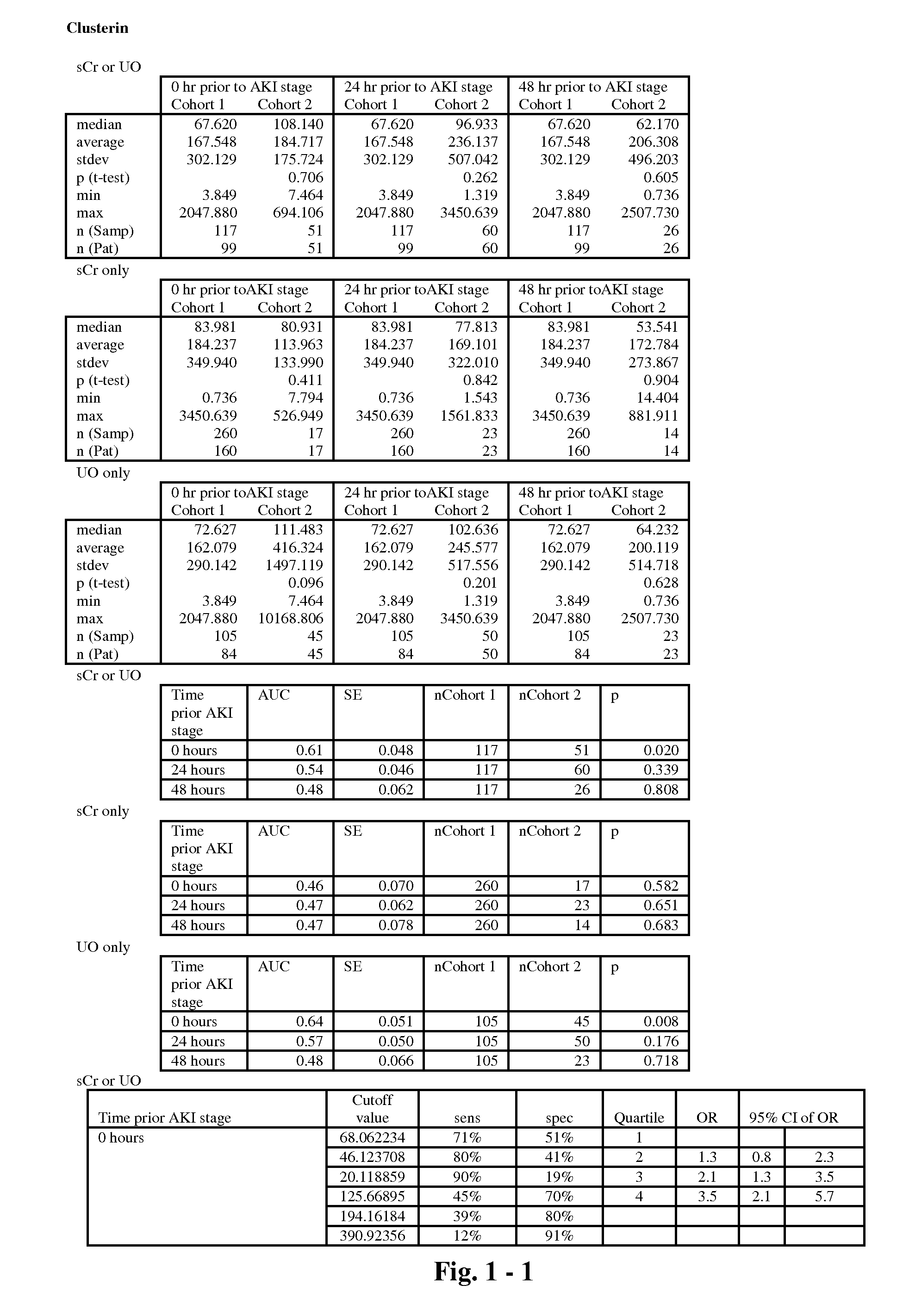
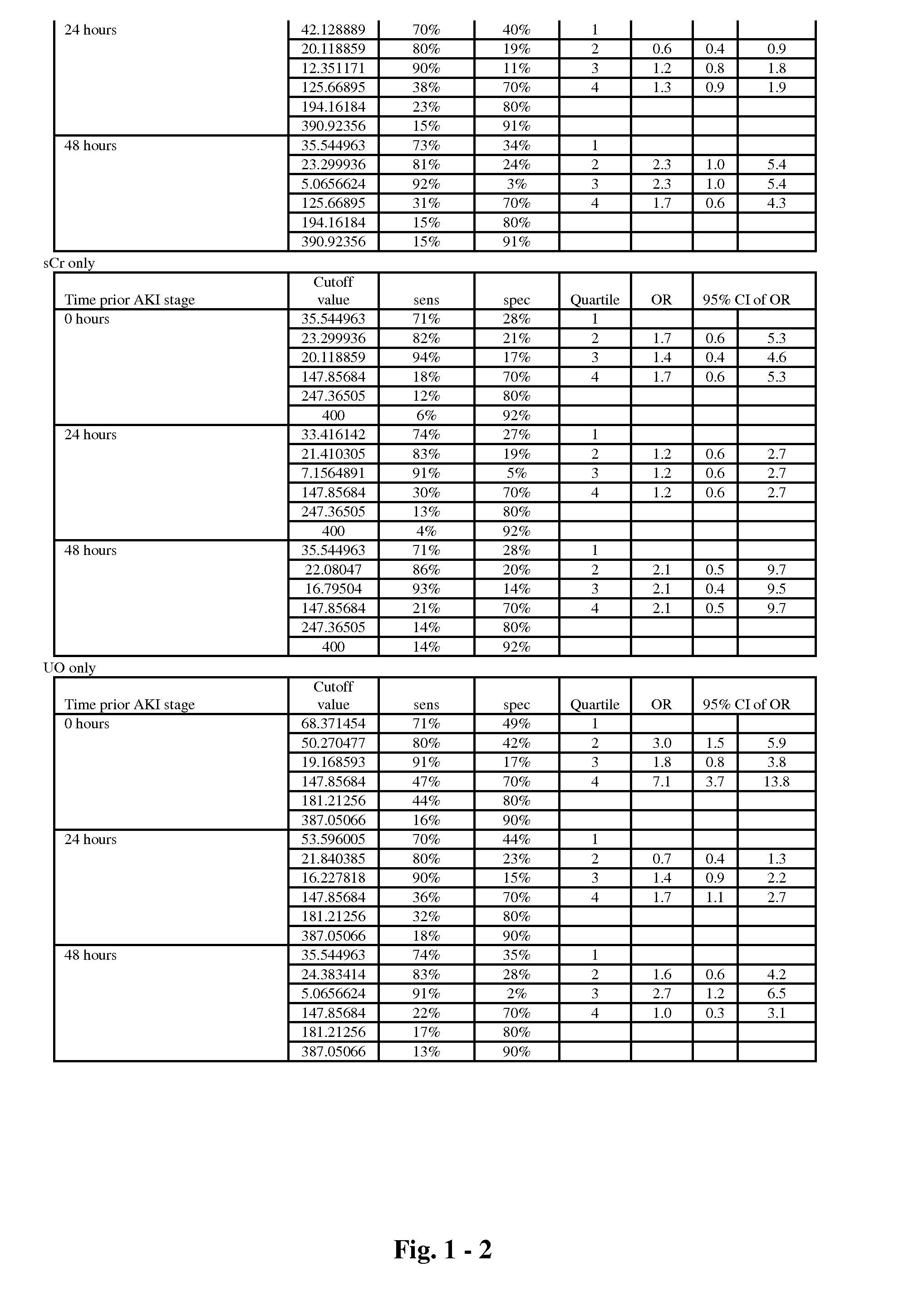
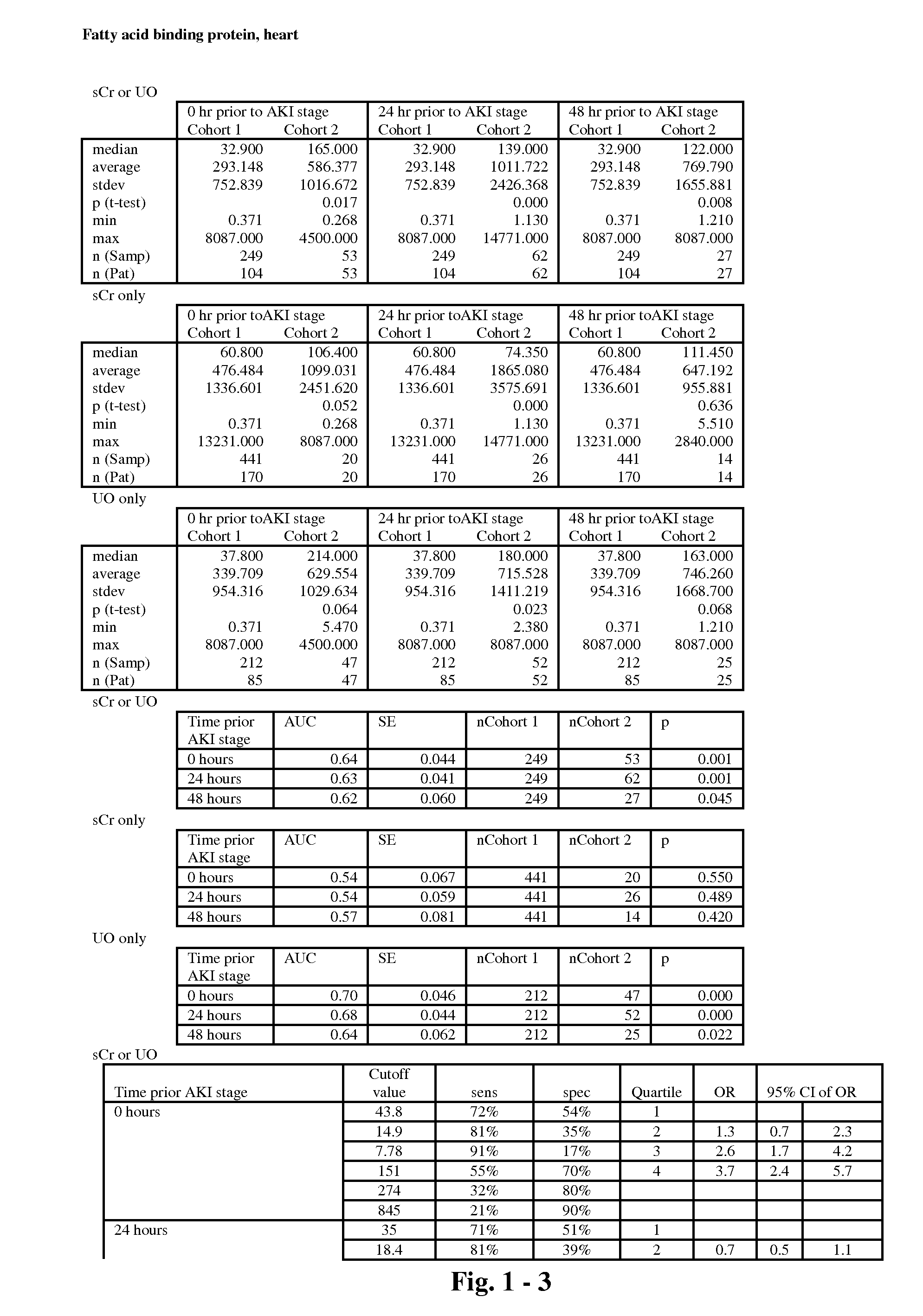
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Clusterin, Heart-type fatty acid binding protein, Hepatocyte growth factor, Interferon gamma, Interleukin-12 subunit beta, Interleukin-16, Interleukin-2, 72 kDa type IV collagenase, Matrix metalloproteinase-9, Midkine, and Serum amyloid P-component as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Anti-interferon gamma antibodies and methods of use thereof
The invention relates to fully human antibodies, and fragments thereof, that bind to human interferon gamma (hIFNγ), thereby modulating the interaction between IFNγ and its receptor, IFNγ-R, and / or modulating the biological activities of IFNγ. The invention also relates to the use of such anti-IFNγ antibodies in the prevention or treatment of immune-related disorders and in the amelioration of a symptom associated with an immune-related disorder.
Owner:SWEDISH ORPHAN BIOVITRUM AG
Novel pharmaceutical composition of interferon gamma or pirfenidone with molecular diagnostics for the improved treatment of interstitial lung diseases
InactiveUS20060270618A1Faster and successful treatmentPromote circulationOrganic active ingredientsBioreactor/fermenter combinationsInterstitial lung diseaseMortality rate
The present invention relates to a novel pharmaceutical composition comprising interferon-γ or pirfenidone and a diagnostic array of candidate polynucleotides for the improved treatment of lung diseases, especially for all forms of interstitial lung diseases. This invention describes the combination of molecular diagnosis and clinical therapy as a novel medication principle for reduction of mortality and improvement of disease management in interstitial lung diseases.
Owner:MONDOBIOTECH LICENSING OUT AG +1
Method and composition for treatment of renal disease with antibodies and their equivalents
InactiveUS20070218063A1Improve the quality of lifeMore pathogenetic treatmentPeptide/protein ingredientsImmunoglobulins against animals/humansInterleukin 6Interleukin 10
A method for treating renal disease includes administering to a patient suffering from renal disease an effective amount of antibody or of a functional equivalent thereof to at least two of urea, creatinine, tumor necrosis factor alpha, interferon gamma, interleukin 6 and interleukin 1 beta. Soluble cytokine receptors also can be employed. Antibody, functional equivalent thereof or soluble cytokine receptor to interleukin 10 or interleukin 13 also can be administered. The method can be used as a supplement to or as partial or complete replacement for dialysis. A pharmaceutical composition includes antibody or functional equivalent thereof to urea, creatinine, or both; antibody, functional equivalent or soluble cytokine receptor to tumor necrosis factor alpha, interferon gamma, interleukin 6, interleukin 1 beta or any combination thereof; and, optionally, antibody, functional equivalent or soluble cytokine receptor to interleukin 10, interleukin 13 or both. The composition can be included in a kit.
Owner:SKURKOVICH BORIS +2
Genetic adjuvants for immunotherapy
Owner:UNIV OF SOUTH FLORIDA
Regulation of T cell-mediated immunity by tryptophan
InactiveUS20070077234A1Reduce loadAvoid destructionBiocidePeptide/protein ingredientsAntigenMetabolite
Owner:GEORGIA HEALTH SCI UNIV RES INST
CD106-positive cells, and identification and preparation method and application thereof
ActiveCN102539736APrevent rejectionSuppress immune responseSkeletal/connective tissue cellsEmbryonic cellsAdipogenesisAutoimmune disease
The invention discloses an identification and preparation method and application CD106-positive mesenchymal stem cells. The method comprises the following steps: identifying and isolating CD106-positive cells from mononuclear cells isolated from bone marrow, placenta or other tissues through an immunological method, carrying out adherent culture, and collecting adherent CD106-positive cells; or carrying out adherent culture of mononuclear cells isolated from bone marrow, placenta or other tissues, collecting adherent mesenchymal cells, and isolating the CD106-positive mesenchymal stem cells when the count of the obtained cells is large enough. The CD106-positive cells are mesenchymal stem cells with adherent growth, express mesenchymal stem cell-related immunological phenotype, and have osteogenesis, adipogenesis and other differentiation potencies. Compared with CD106-negative mesenchymal stem cells, the CD106-positive cells have higher immunosuppression capacity, can better inhibit proliferation of IFN-gamma (interferon-gamma) secretion of lymphocytes, and can be used for treating graft versus host disease (GVHD) and autoimmune diseases.
Owner:北京汉氏干细胞科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com