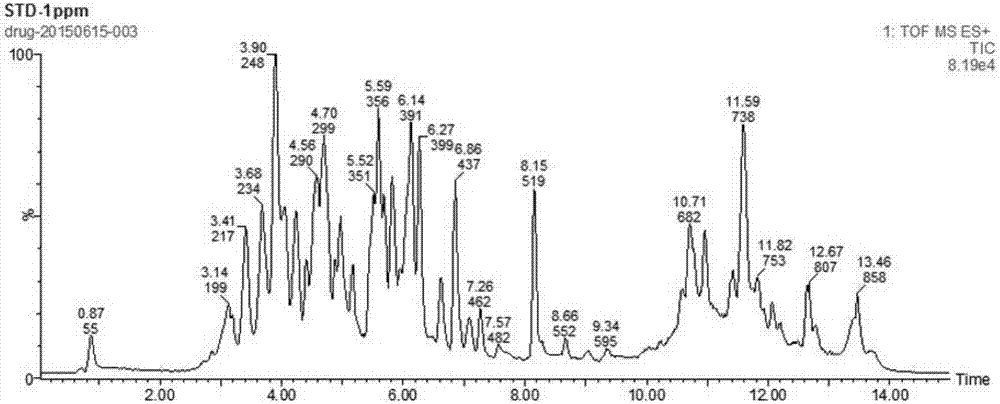
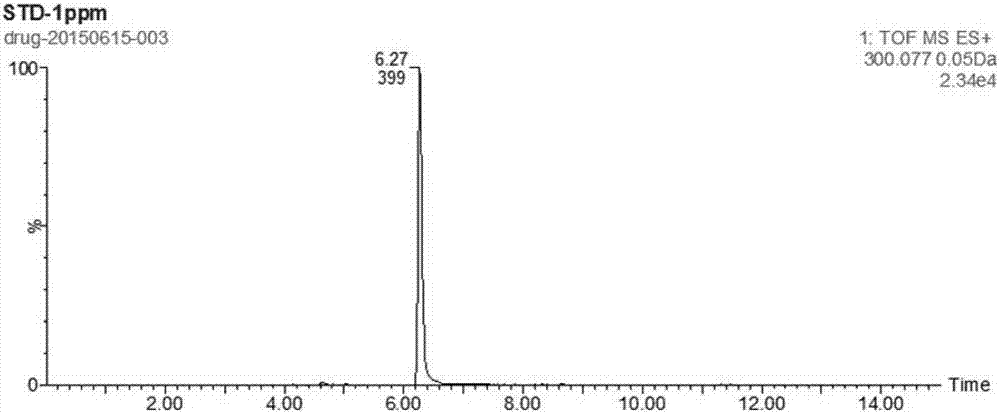
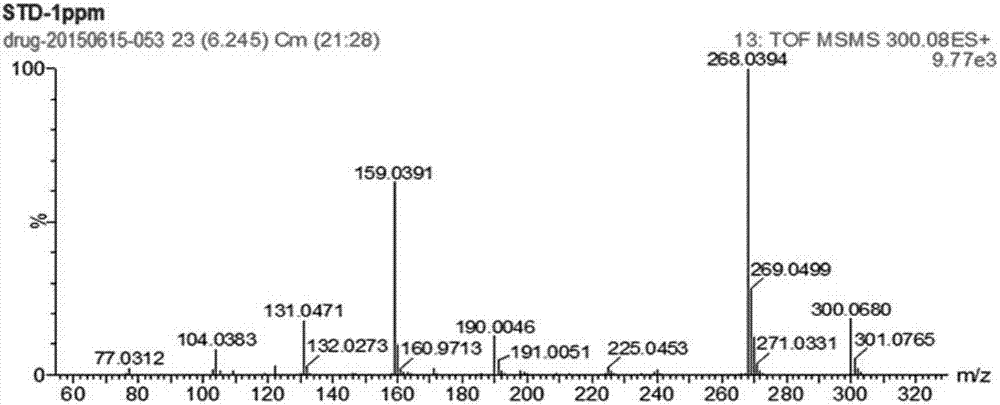
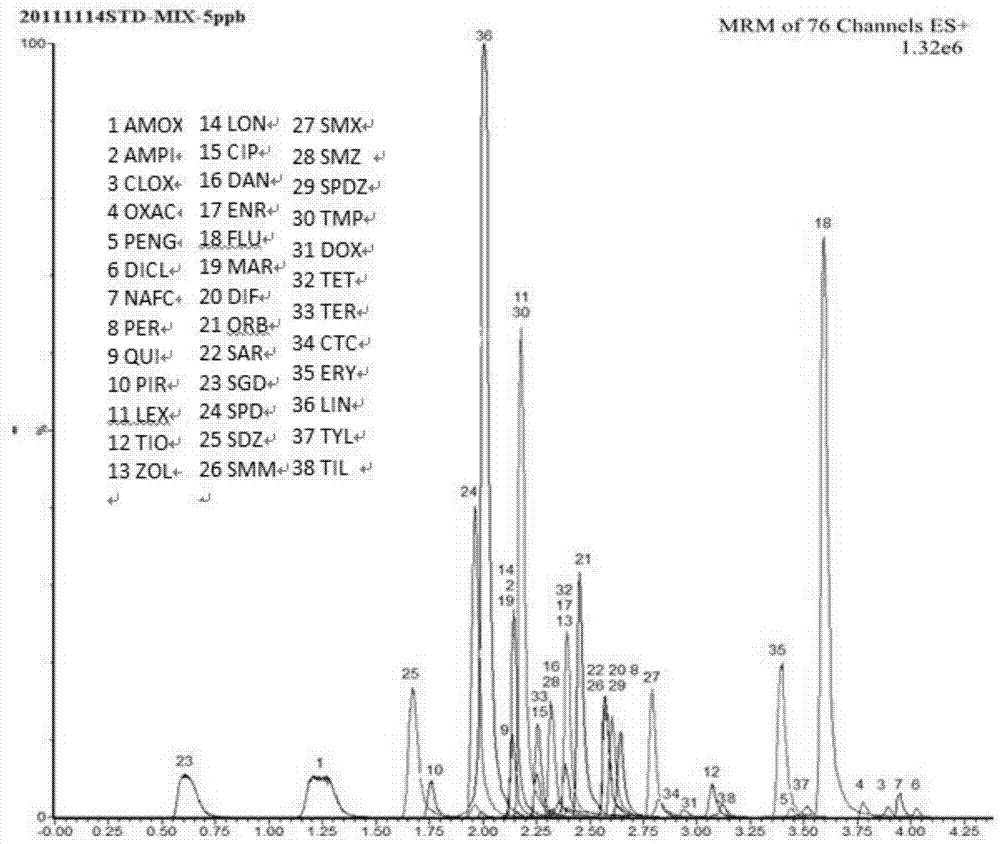
Patents
Literature
651 results about "Veterinary drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Veterinary prescription drugs are those drugs restricted by federal law to use by or on the order of a licensed veterinarian [Section 503 (f) Food, Drug, and Cosmetic Act]. The law requires that the drug sponsor label such drugs with the statement: "Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian."
Method for determining multiresidue of veterinary drugs in animal-derived foods
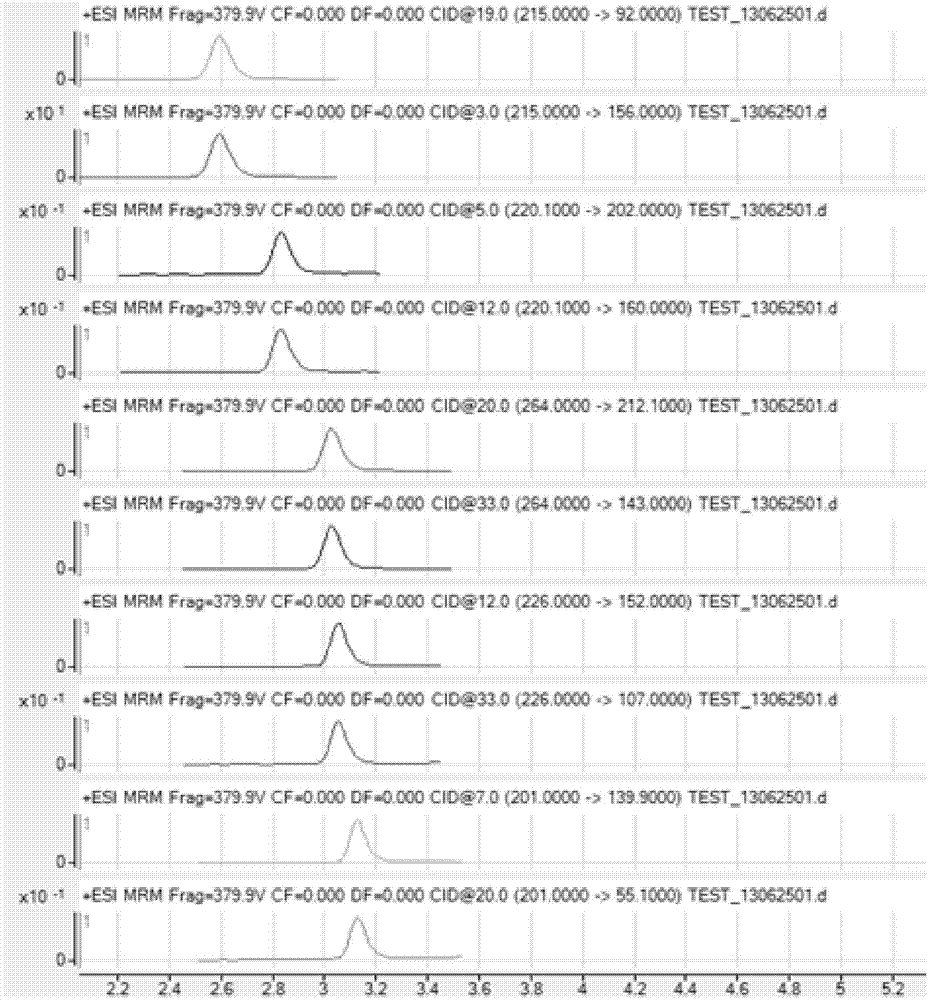
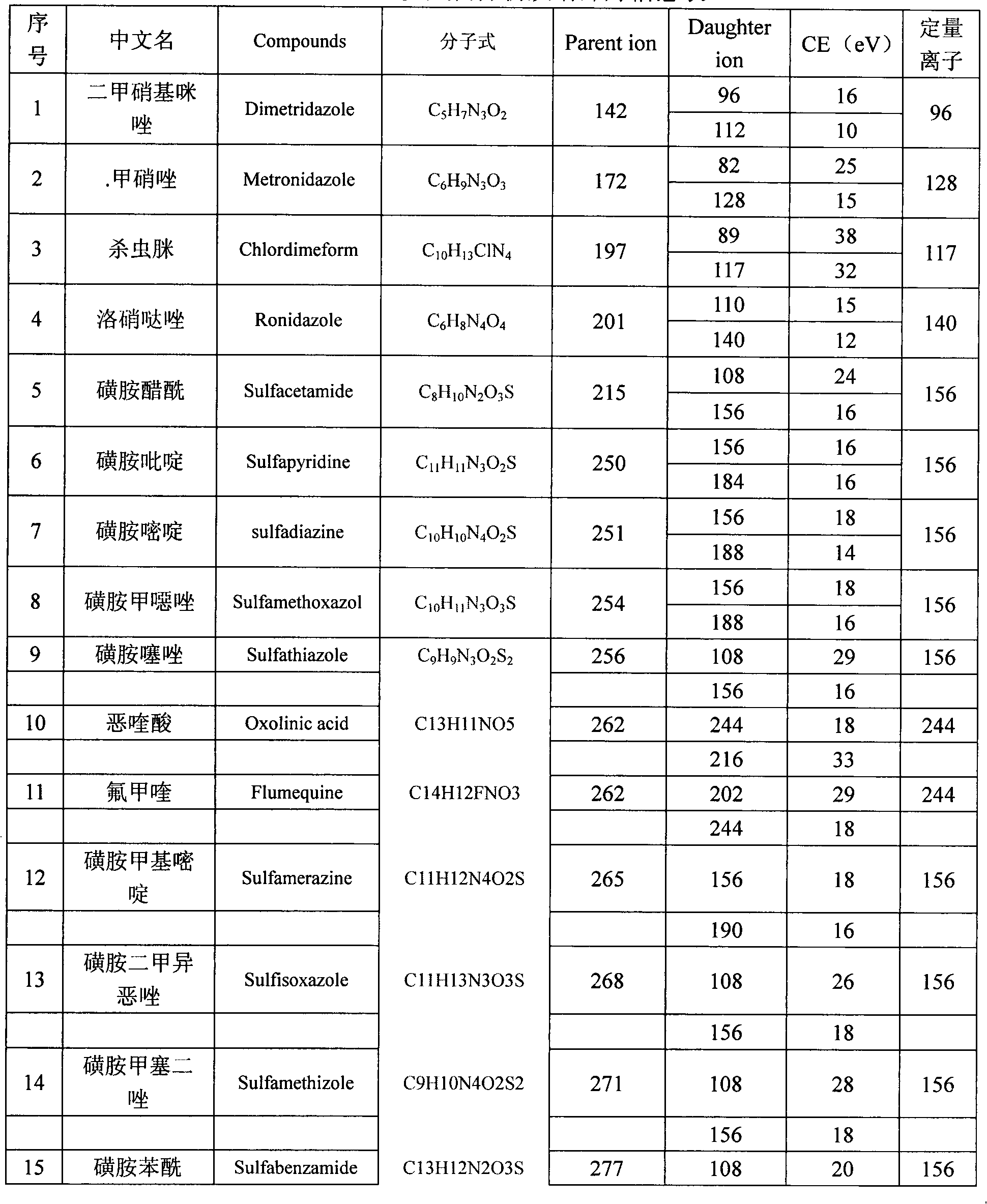
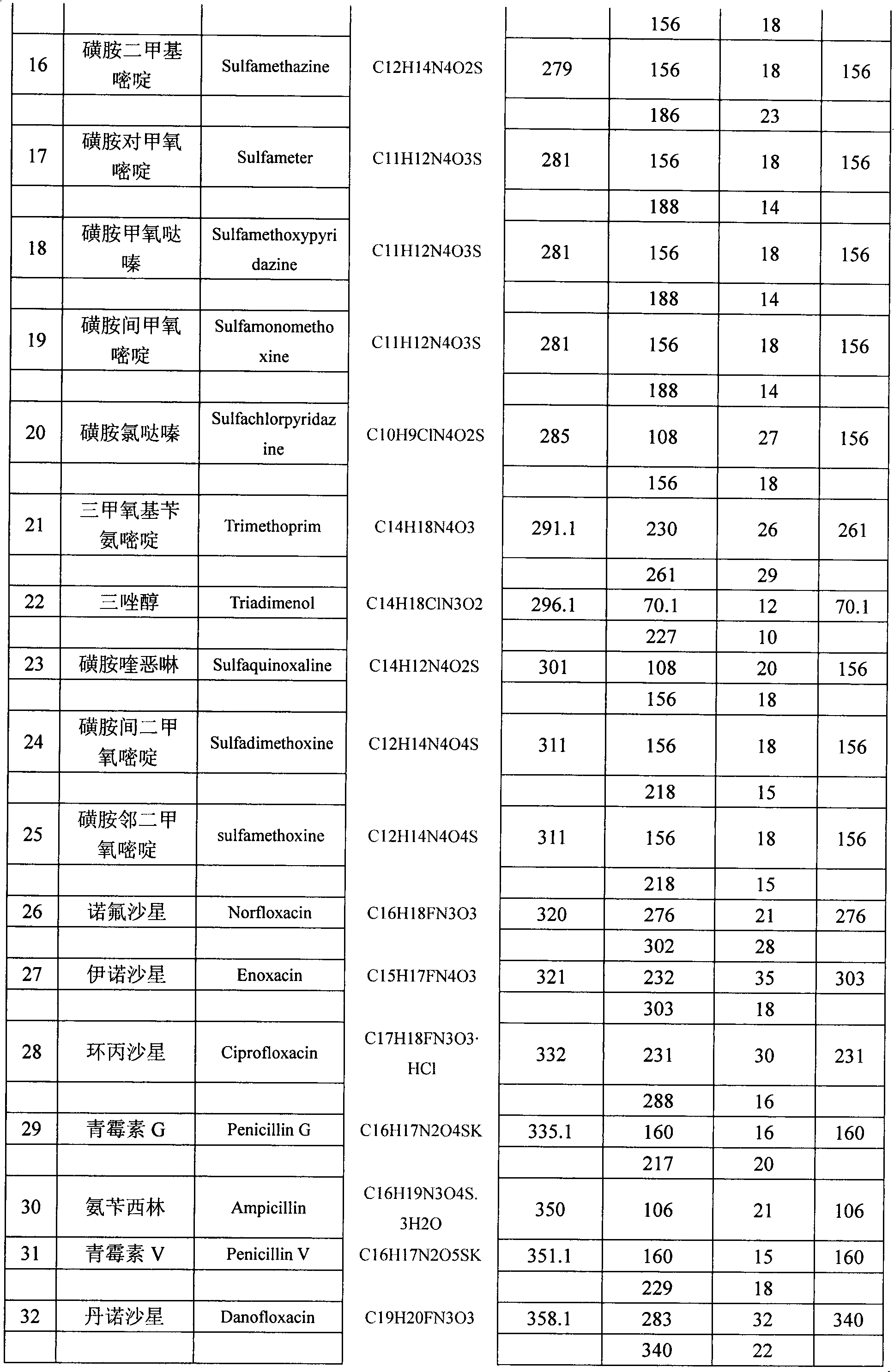
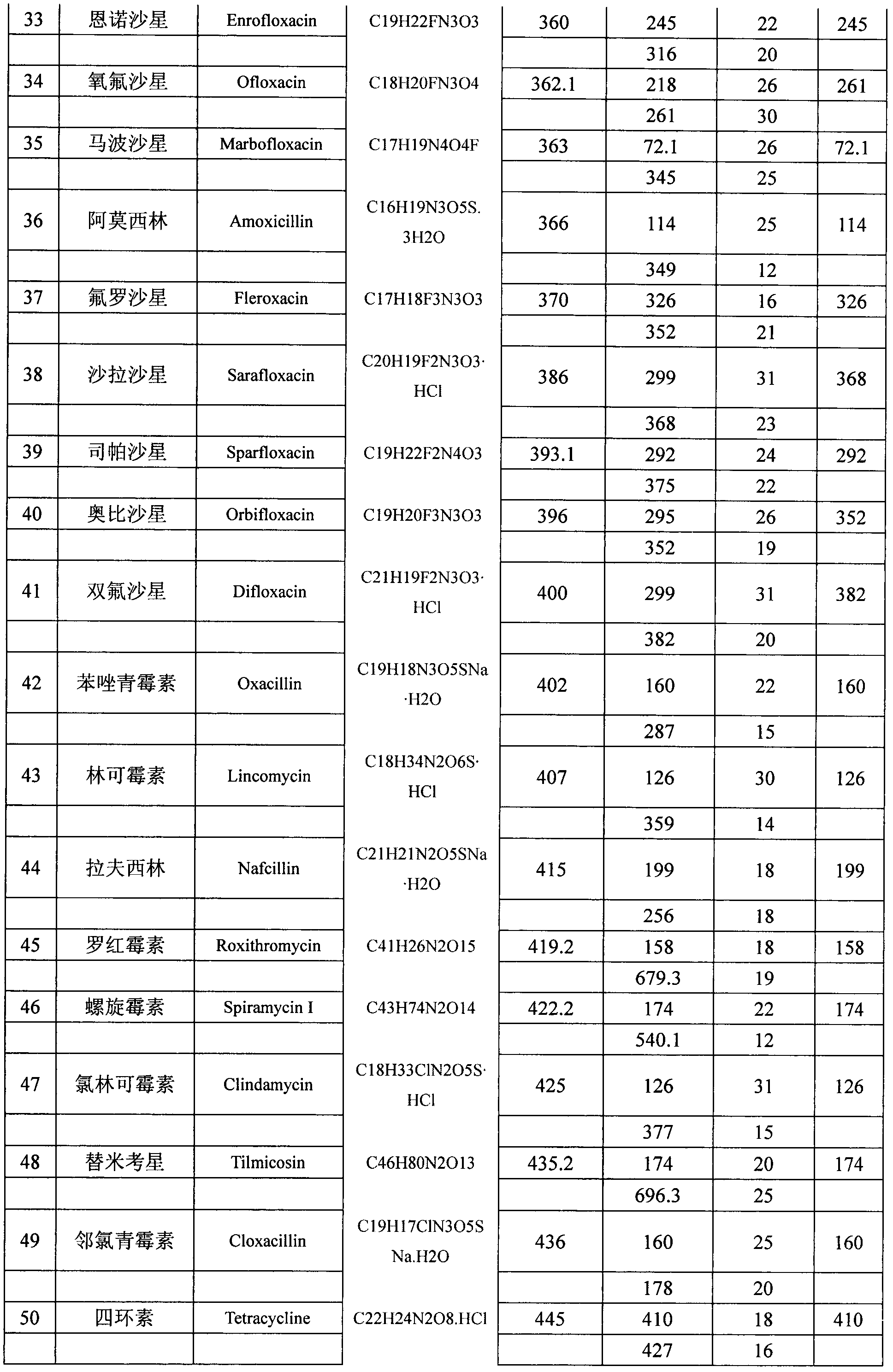
The invention belongs to the technical field of food detection methods, and particularly relates to a method for determining multiresidue of veterinary drugs in animal-derived foods. The method comprises the following steps of weighing a sample, adding an acetic acid-acetonitrile solution, carrying out vibrating extraction, centrifuging to obtain acetonitrile extracting solution, loading the extracting solution obtained by centrifuging into a centrifuge tube with a mixed filler, carrying out vibrating centrifugation, sucking purified liquid, concentrating by blowing nitrogen, diluting to constant volume and detecting by UPLC / MS / MS (ultra performance liquid chromatography-tandem mass spectrometry). The method disclosed by the invention is firstly applied in detecting animal-derived foods and 107 kinds of veterinary drugs such as sulfonamides, quinolones, beta agonists in animal products are subjected to fast qualitative and quantitative detection, the detection limit is completely capable of meeting the detection requirements and the detection efficiency is greatly promoted.
Owner:烟台杰科检测服务有限公司
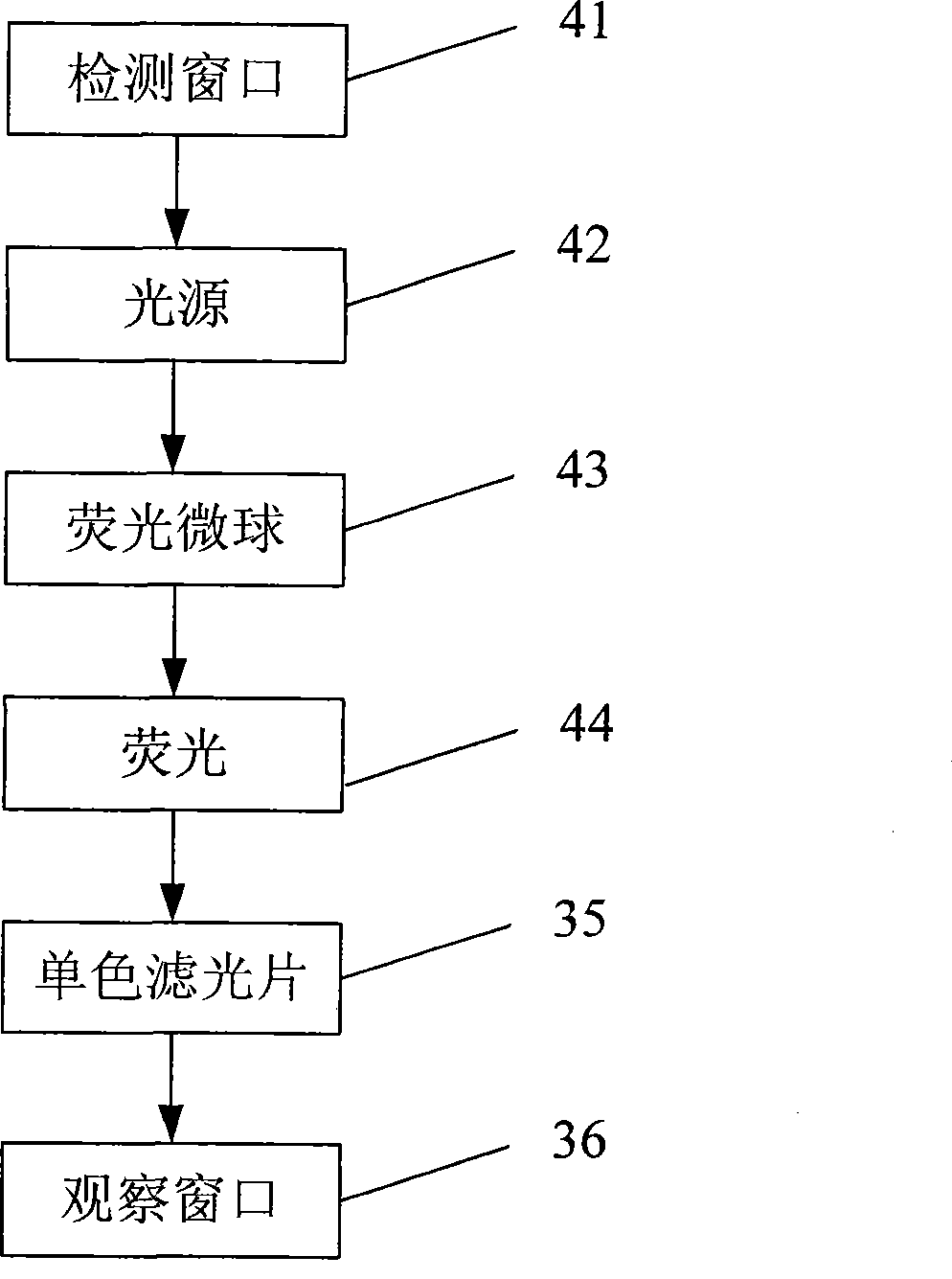
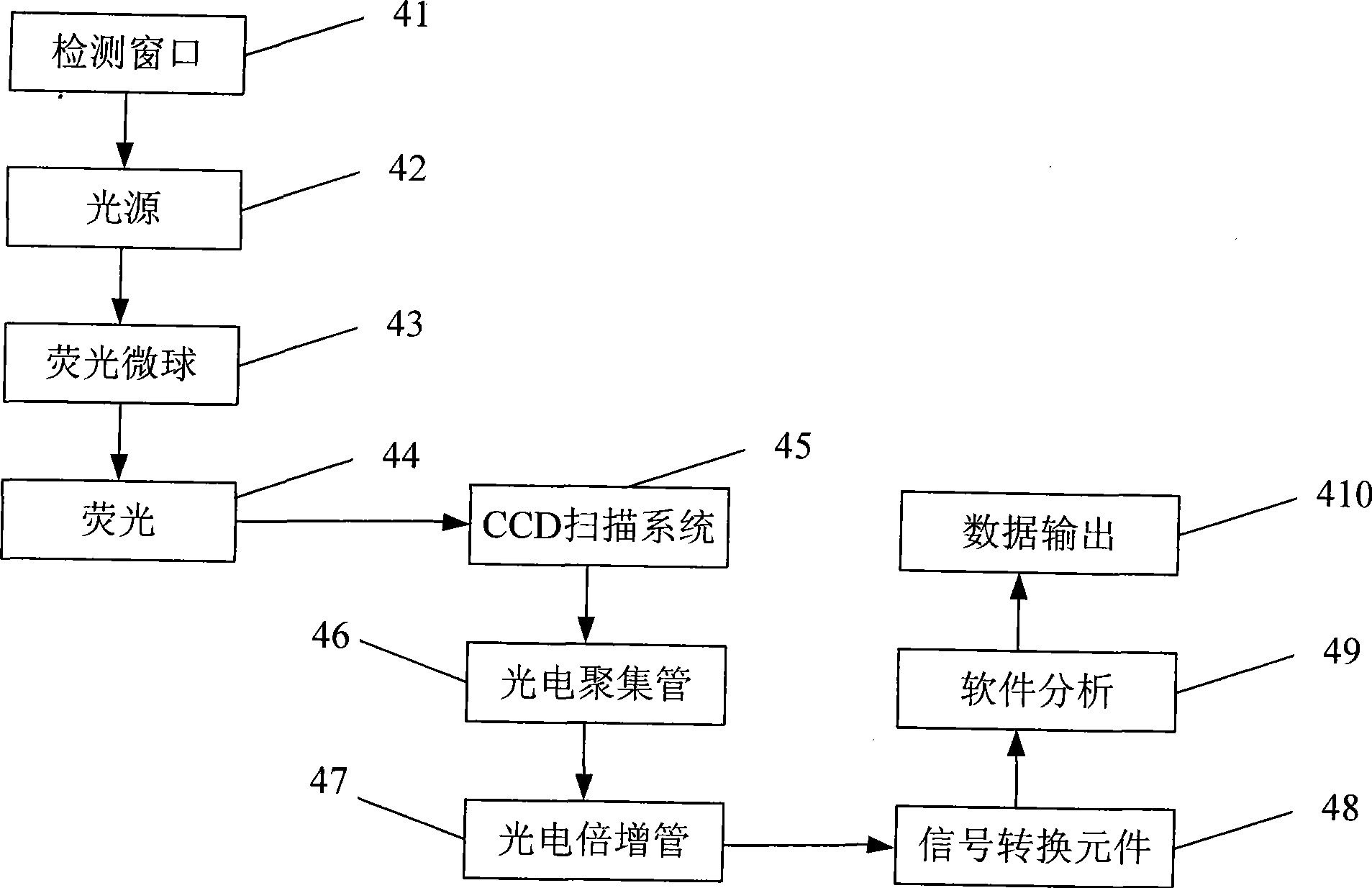
Fluorescent micro-ball immune chromatography test paper strip for detecting residual animal medicine and preparing method thereof
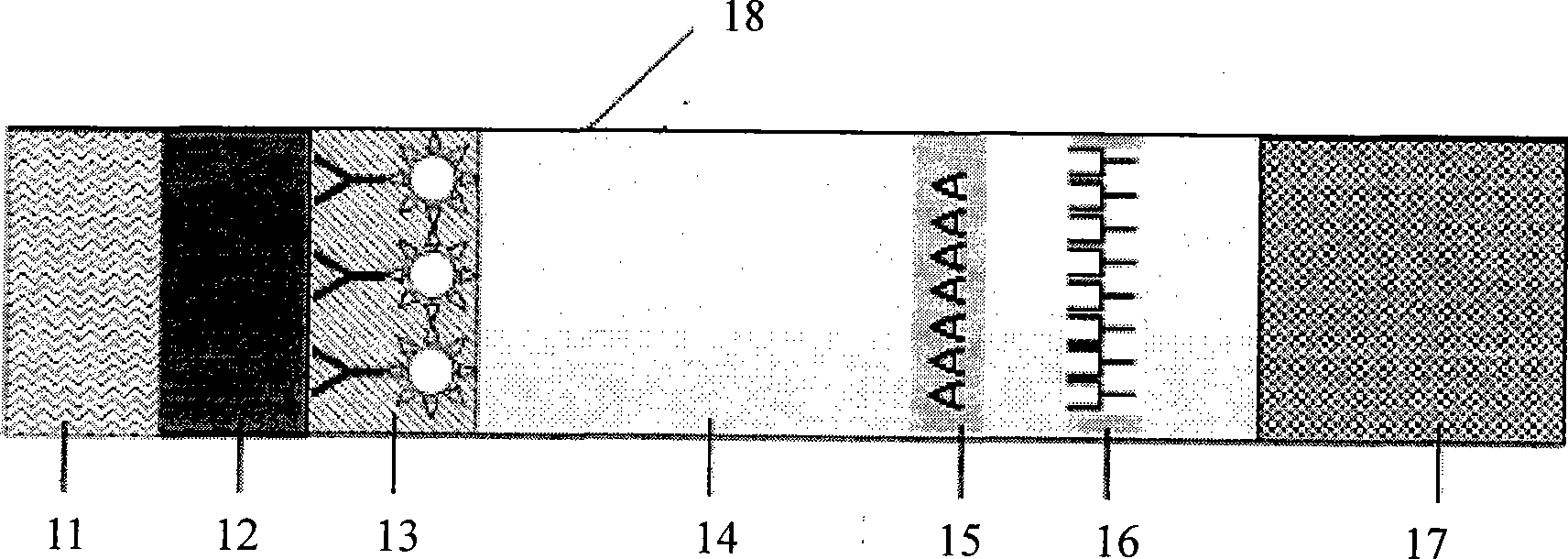
The invention discloses a test paper strip for the fluorescent bead immunochromatography of veterinary medicine remained in a detected sample and a preparation method thereof. A filter paper, a sample pad, a fiberglass membrane, a pyroxylin membrane and water sucking paper are sequentially stuck on a base plate in related joint; fluorescent beads are sprayed on the pyroxylin membrane for marking a veterinary medicine resistant molecule monoantibody; the pyroxylin membrane coated with a veterinary medicine molecule holoantigen is used as a detection region; the pyroxylin membrane coated with an anti-mouse antibody is used as a quality control region; and the test paper strip is prepared through the following steps: (1) the preparation of the pyroxylin membrane; (2) the preparation of a fluorescent bead pad; and (3) the assembling of the test paper strip. In the detecting process, emitted spectrums pass through a proper optical filter device; and all the emitted spectrums are collected through CCD scanning technology, are congregated, are multiplied and are analyzed through a fluorescence analyzer and relevant software to obtain a quantized fluorescence signal, thereby realizing quantitative detection. The test paper strip is mainly used for the qualitative detection and the quantitative detection of all the veterinary medicine classes in food safety detection.
Owner:WUXI ZODOLABS BIOTECH
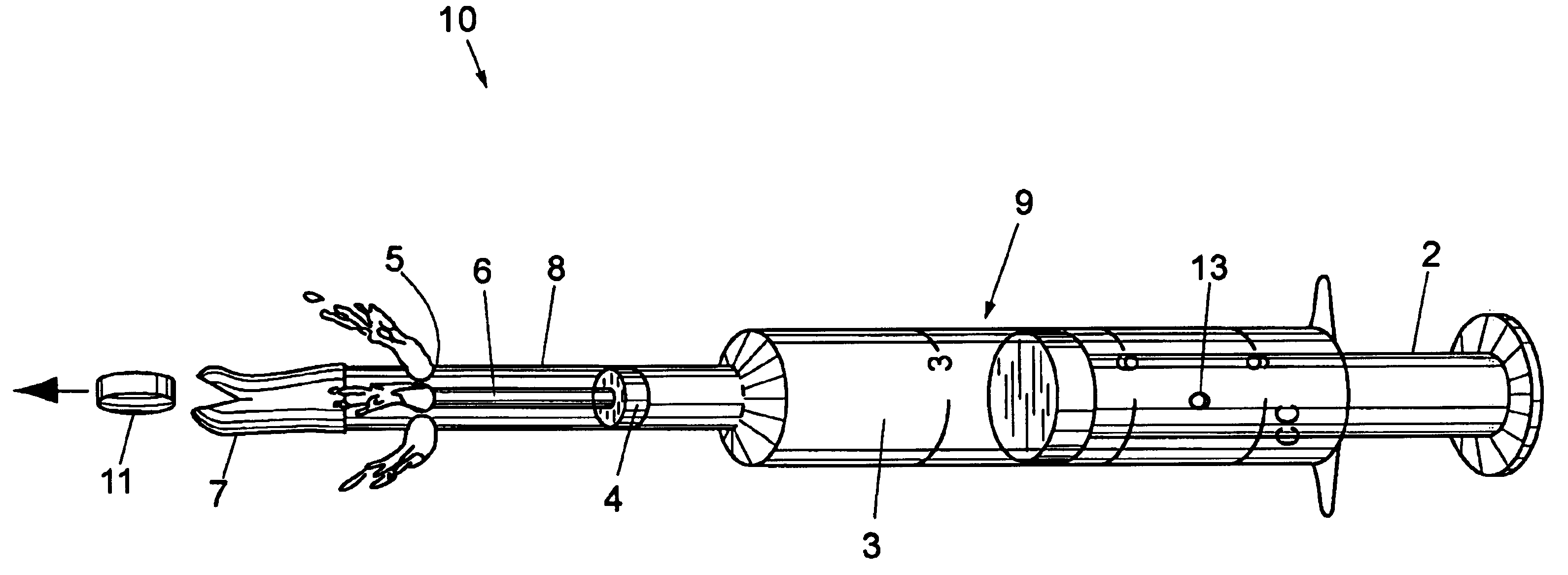
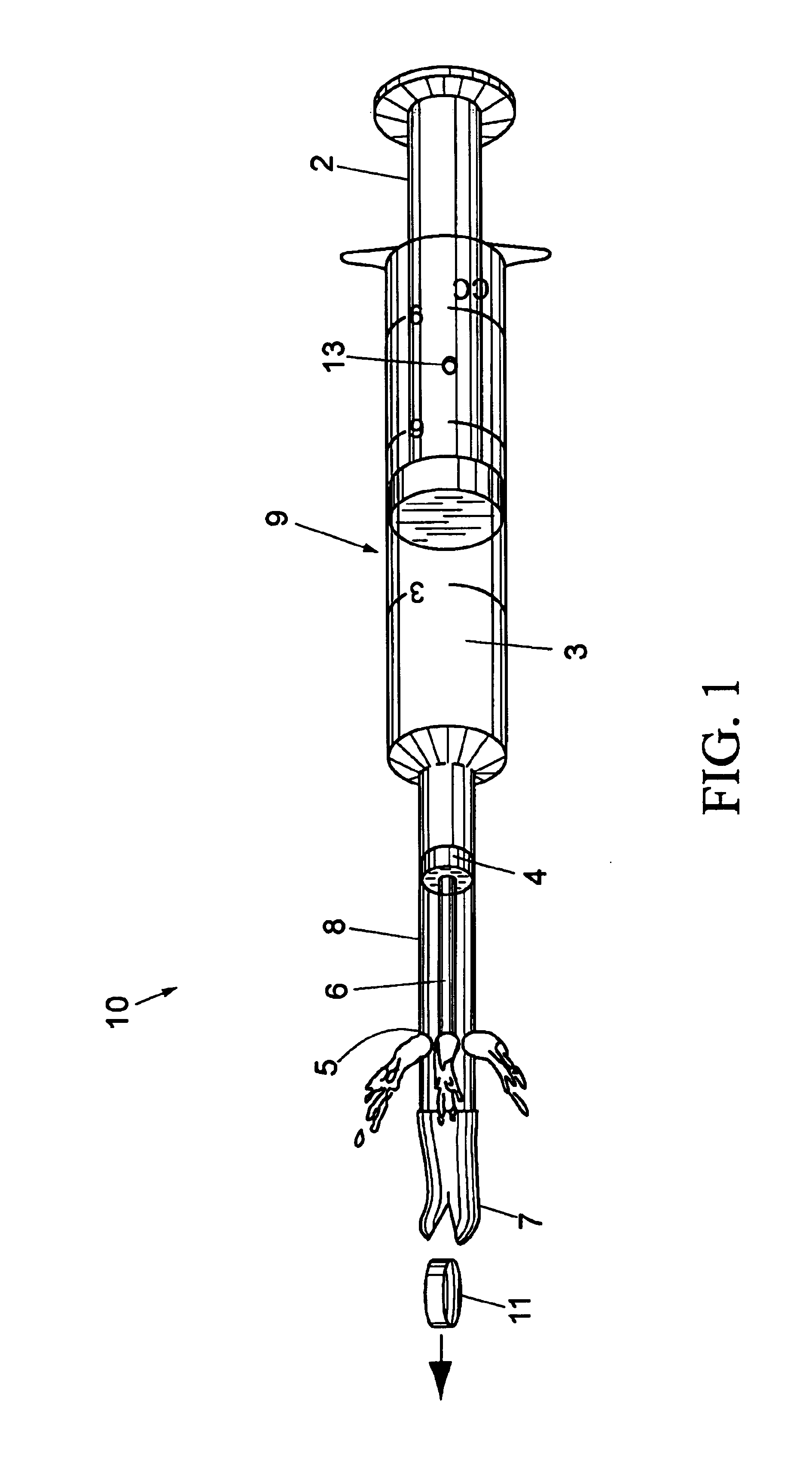
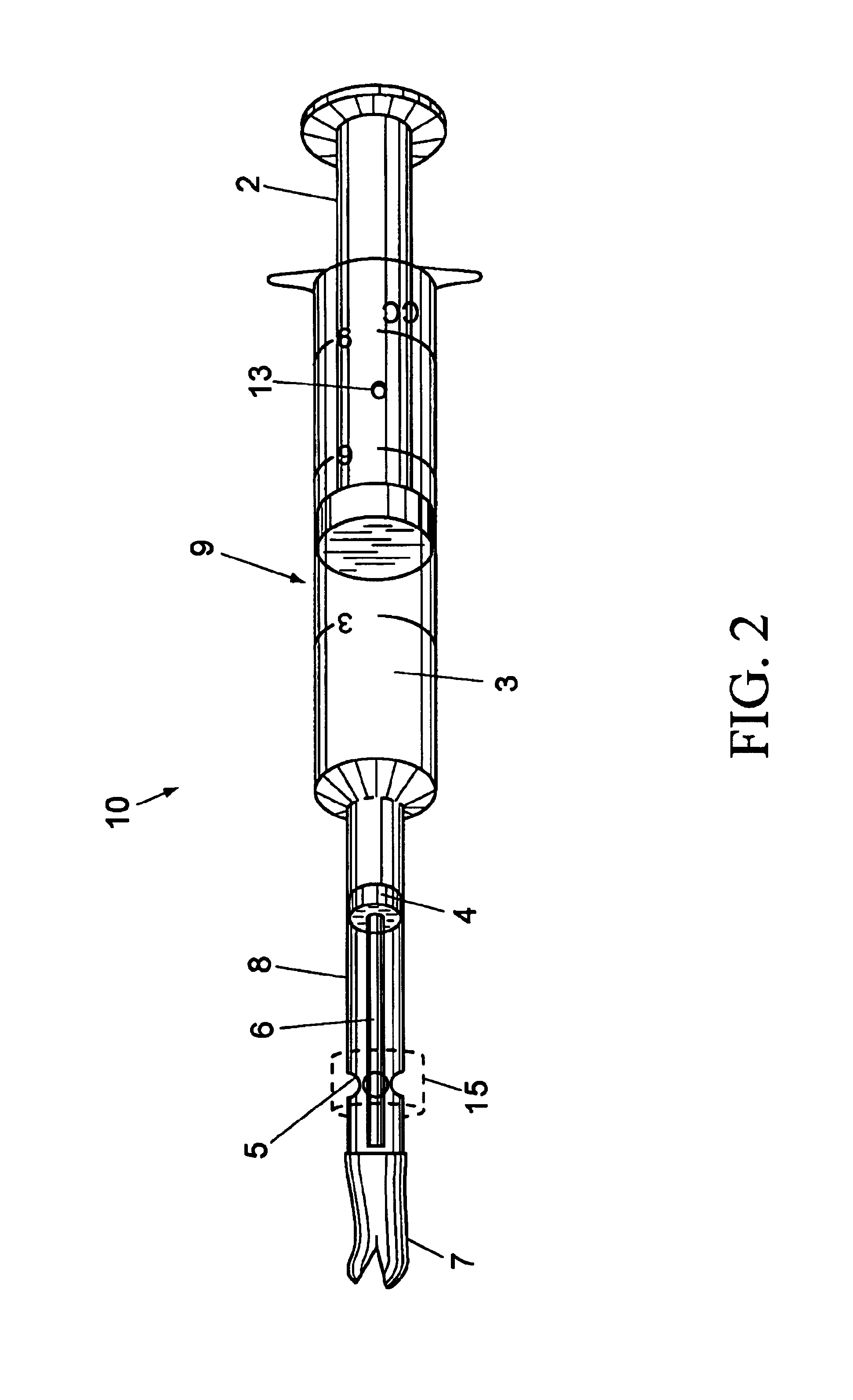
Veterinary pill and capsule delivery device
InactiveUS6960183B2Promote peristalsisCorrect swallowInfusion devicesSurgeryBiomedical engineeringWater release
A veterinary pill / capsule delivery device comprised of a dispensing head for holding a pill or capsule, the dispensing head being attached to the end of a syringe component for ejecting the pill or capsule out from the dispensing head into the animal's mouth while at the same time injecting a quantity of water into the mouth. The syringe component includes a push-rod that protrudes into dispensing head for ejecting the pill / capsule therefrom, plus water release holes near the dispensing head for simultaneously jetting water out of the syringe component into the animal's mouth, thereby compelling the animal to swallow the pill.
Owner:NICOLETTE JON R
Method for preparing and using reagent for simultaneously detecting multiple small molecular compounds
The present invention provides one kind of reagent for simultaneously detecting several kinds of small molecular compounds and its preparation process and usage. The preparation process includes the following steps: preparing coded microsphere reagent, and preparing one of the small molecular compound detecting antibody probes, including fluorescent quantum dot antibody probes, fluorescent quantum dot secondary antibody probes, fluorescent dye antibody probes and fluorescent dye secondary antibody probes. The method of detecting small molecular compounds with the antibody probe may be a direct antibody probe method or a indirect antibody probe method. The present invention may be used in detecting residual veterinary medicine, pesticide, banned drug, misused medicine and other small molecular compounds in food, agricultural product, living animal body and human body, and has the advantages of high sensitivity, high repeatability, etc.
Owner:邹明强
Method for simultaneously detecting multi-kind pesticide residues in bee products
InactiveCN101358953ASolve the problem of matrix effectFast wayComponent separationRetention timePhosphate
The present invention relates to a method of simultaneously detecting a plurality of agro-veterinary drug residues in bee products. The extracted liquid trichloroacetic acid or perchloric acid and the extracted liquid acetate, phosphate or borate solution are added into a sample; the pH value is controlled between 4.5 and 9.0; the mixed solution is centrifuged, the filtrate is added into a solid phase extraction column to be extracted, the extraction column is eluted and dried, the column is washed by oxalic acid-methanol solution, the volume of the eluent is defined by the aqueous solution of methanol, the eluent is added into liquid chromatography-tandem mass spectrometry to be analyzed and tested, the acquired chromatographic peak is contrasted with the known standard chromatographic peak of the drug, and according to the retention time and the abundance of the mass spectrum ions, the specific name of the detected drug is determined. The method only requires one pre-treatment of the sample, and thus can simultaneously extract 11 classes and more than 60 kinds of veterinary drug residues, such as sulfonamides, quinolones, macrolides, lincomycins, nitroimidazoles, beta-lactams, tetracyclines, chloromycetins, trinethoprims, chlordimeform, triadimenol and the like, the efficiency of analysis is high, and the detection cost is greatly reduced.
Owner:中华人民共和国江苏出入境检验检疫局
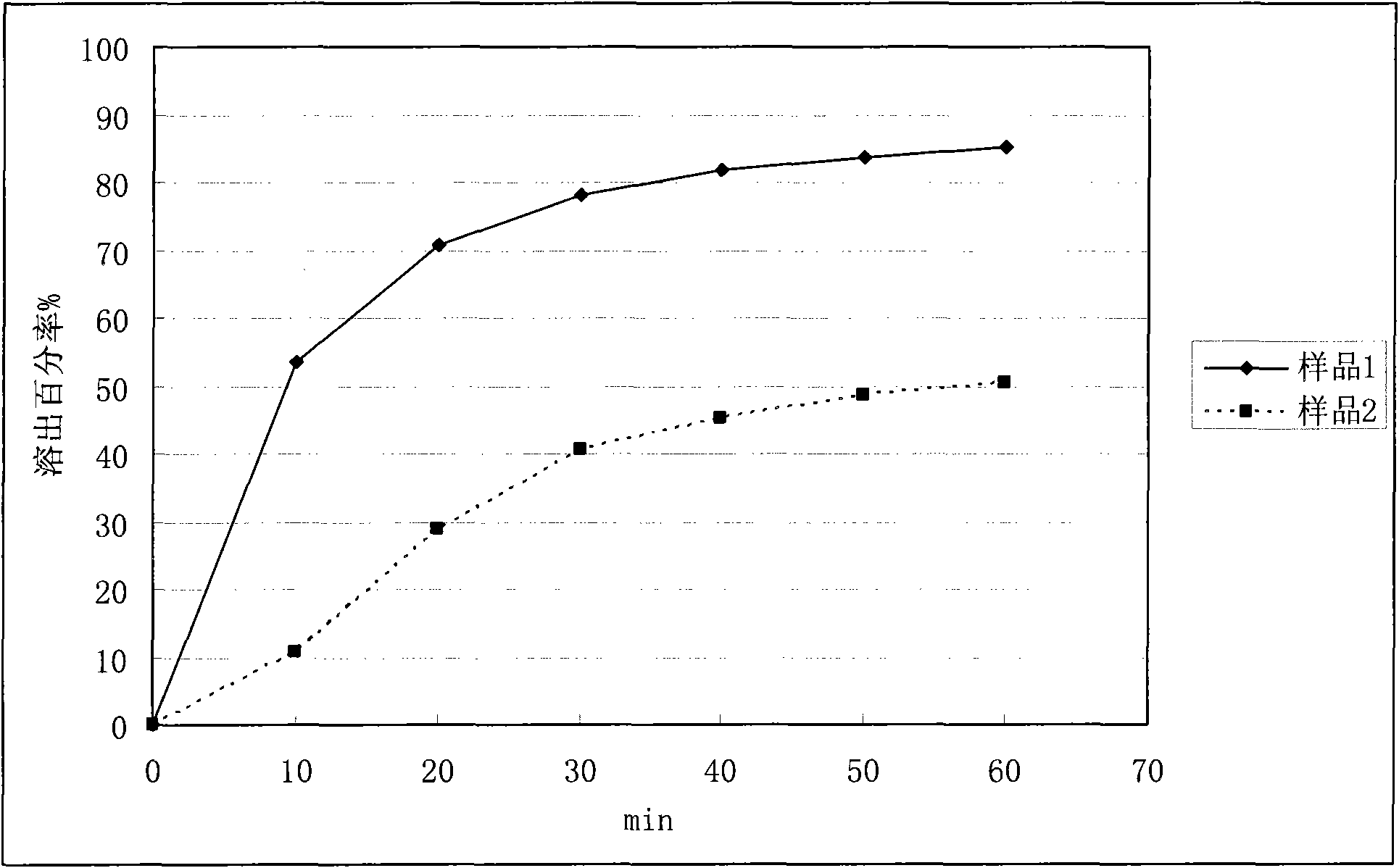
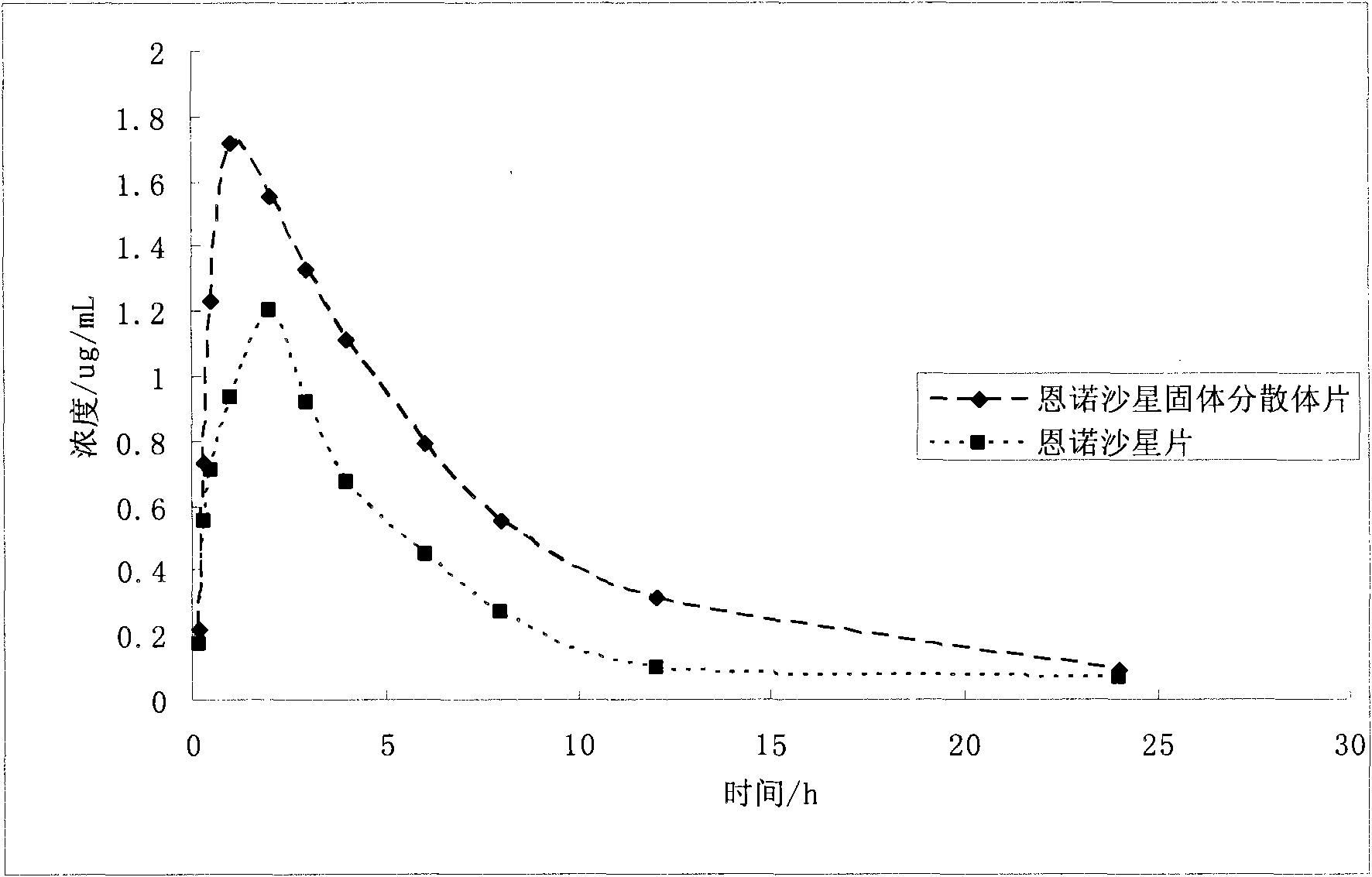
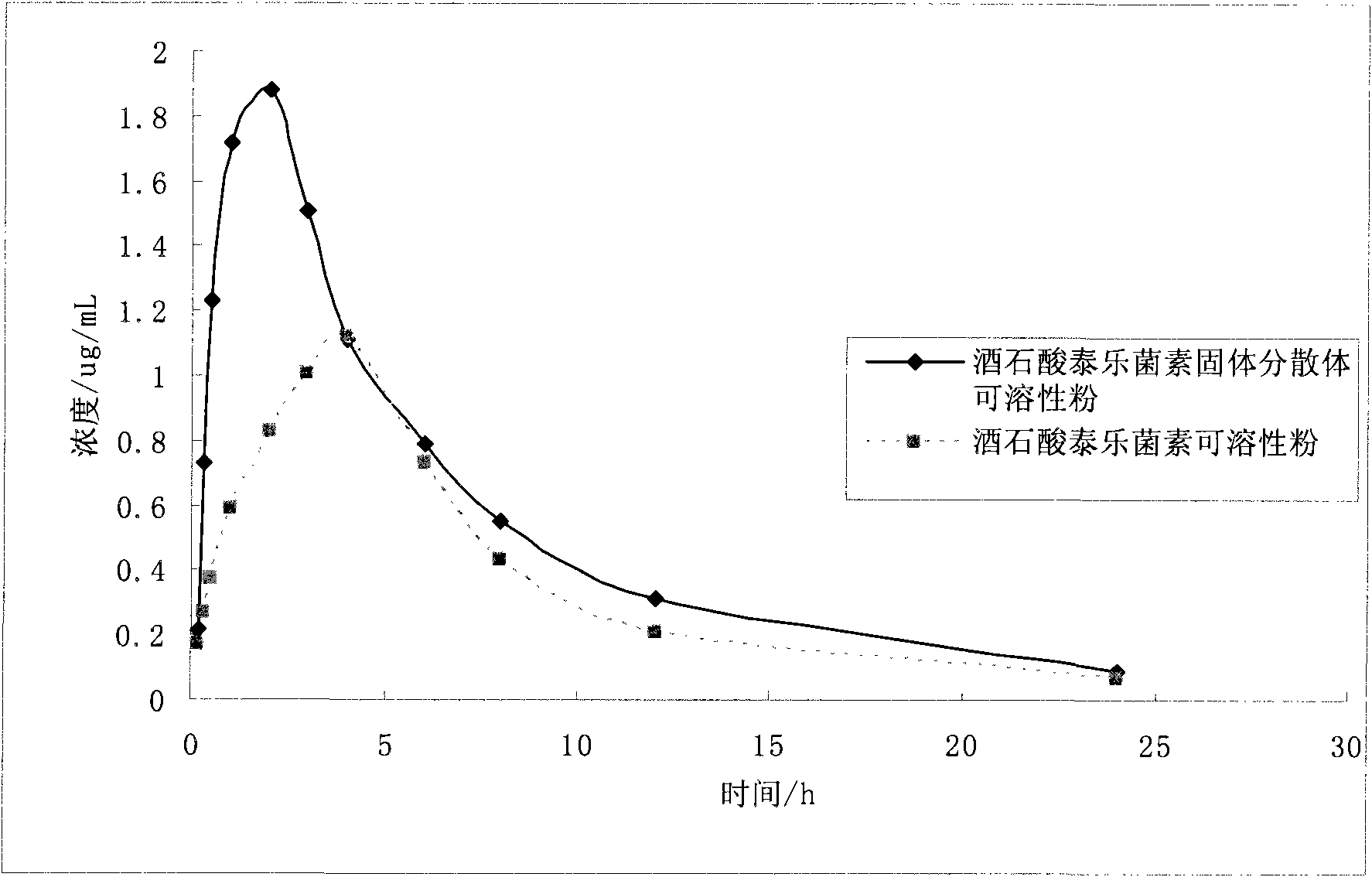
Application of solid dispersion to preparation of veterinary drugs
ActiveCN101919804AImprove palatabilityFast initial dissolutionPowder deliveryPharmaceutical non-active ingredientsInsoluble drugVeterinary medicine
The invention discloses a solid dispersion of veterinary drugs, a preparation method thereof and application of the solid dispersion to the preparation of veterinary drugs. The solid dispersion can improve the dissolving speed and the solubility of insoluble drugs, enhances the absorption and the bioavailability of drugs and covers unpleasant odor and taste of drugs. Since the effective components of the solid dispersion and the drugs prepared by the invention are covered in carriers, the stability of the drugs, including the veterinary drugs, can be improved.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE +1
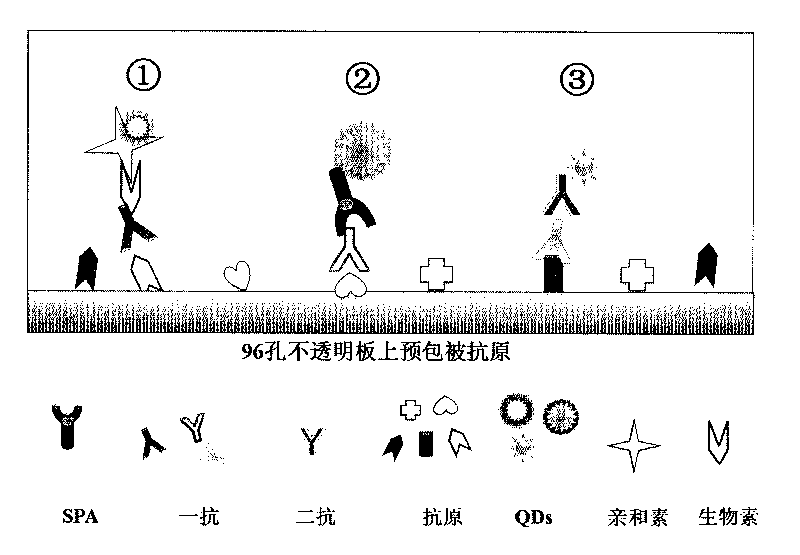
Method of detecting residue of small-molecule substance harmful to human body and a special kit
InactiveCN101762706AExcitation spectrum widthNarrow emission spectrumFluorescence/phosphorescenceHuman bodyBiology
The invention discloses a method of detecting the residue of small-molecule substances harmful to the human body and a special kit. The special kit comprises a non-transparent micro-porous plate and a light-emitting compound, wherein each hole of the non-transparent micro-porous plate is filled with a coat antigen which is simultaneously coated with three kinds of small-molecule substances. The invention makes full use of the multi-color marking function of QDs, establishes a novel kit for simple and rapid detection of the residue and a method thereof, and realizes the multi-color marking through indirect marking of polyclonal antibodies and monoclonal antibodies in the veterinary drug by coupling the QDs with different particle sizes and targets with functional groups (such as an amino) with specific surfaces. The method comprises: obtaining quantum dots with different fluorescent characteristics through separation and purification, namely, multi-color antibody markers, using the multi-color antibody markers as fluorescent probes, and establishing a reaction system for synchronous analysis of various antigen components of different kinds, thereby realizing the synchronous detection of multiple kinds of residues of the veterinary drug in animal food. Moreover, the method has the advantages of simple operation, high fluorescence intensity and long stabilization time.
Owner:CHINA AGRI UNIV
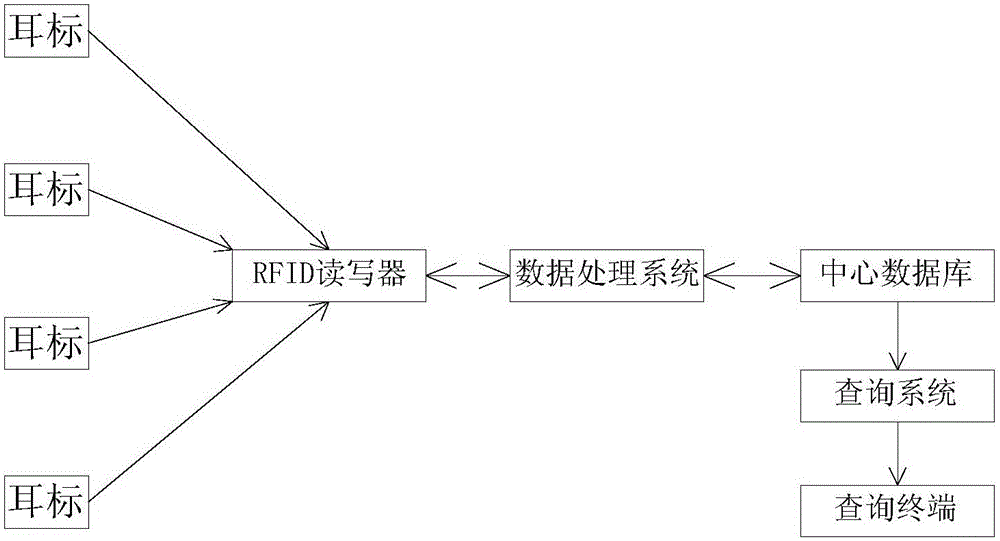
System for monitoring and traceably managing organic livestock growth, slaughter and distribution process
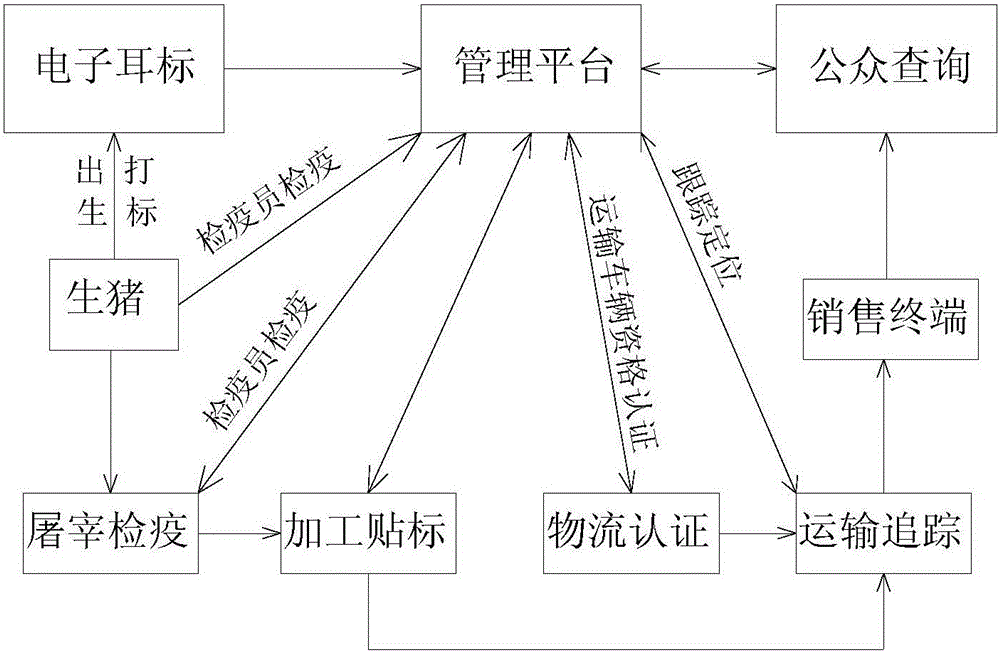
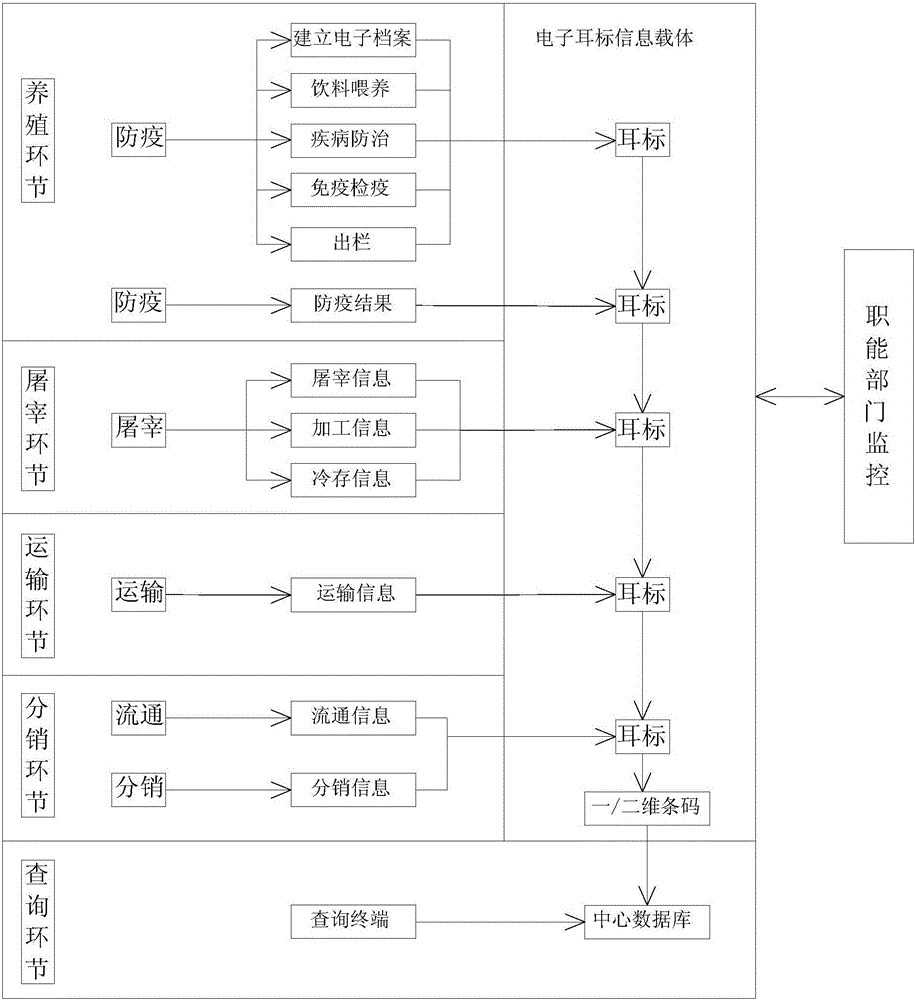
InactiveCN106204061ASlaughter preventionRealize fine feedingLogisticsCommerceDrug withdrawalDrug additive
The invention discloses a system for monitoring and traceably managing an organic livestock growth, slaughter and distribution process. The system, based on Internet of things technology, can help breeding enterprises establish perfect production files, establish data base of meat product traceability, manage safety production, and establish epidemic prevention records; helps a government supervision department achieve livestock production process supervision and control. In addition, being capable of monitoring prohibited veterinary drugs, drug additives, drug withdrawal period in real time, the system can help users avoid the use of prohibited veterinary drugs to prevent livestock slaughter in the withdrawal period and improve animal safety so as to guarantee people's health and life safety, to monitor a animal growth process and achieve fresh meat product traceability, to ensure organic and healthy ecological meat. Further, the system raises the economic benefits of farming enterprises so as to guarantee the profits of the consumers, the breeding enterprises and the government supervision department.
Owner:CHUZHOU UNIV
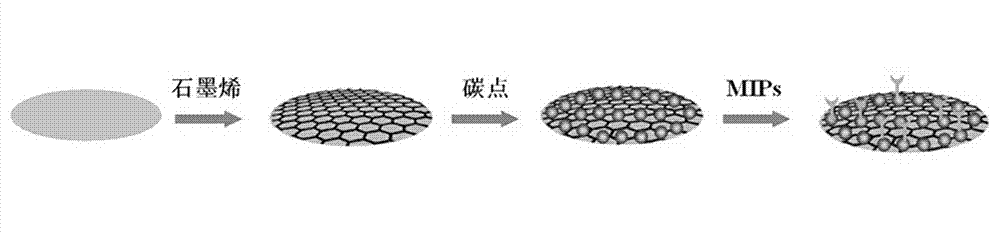
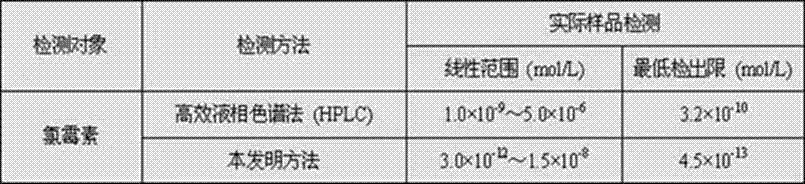
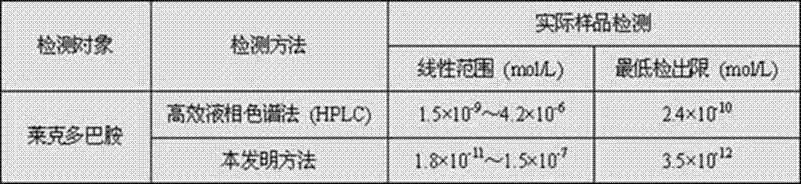
Preparation method and application of molecular imprinting electroluminescent sensor for detecting trace veterinary drug residue by taking battery as power
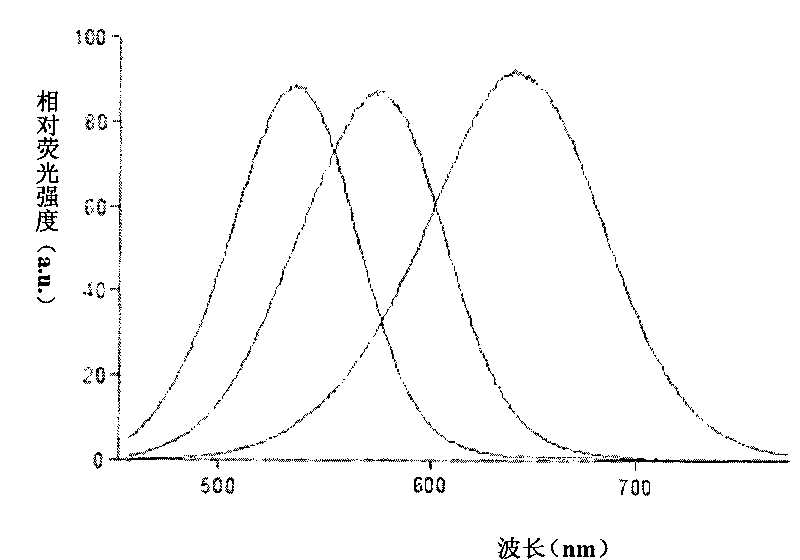
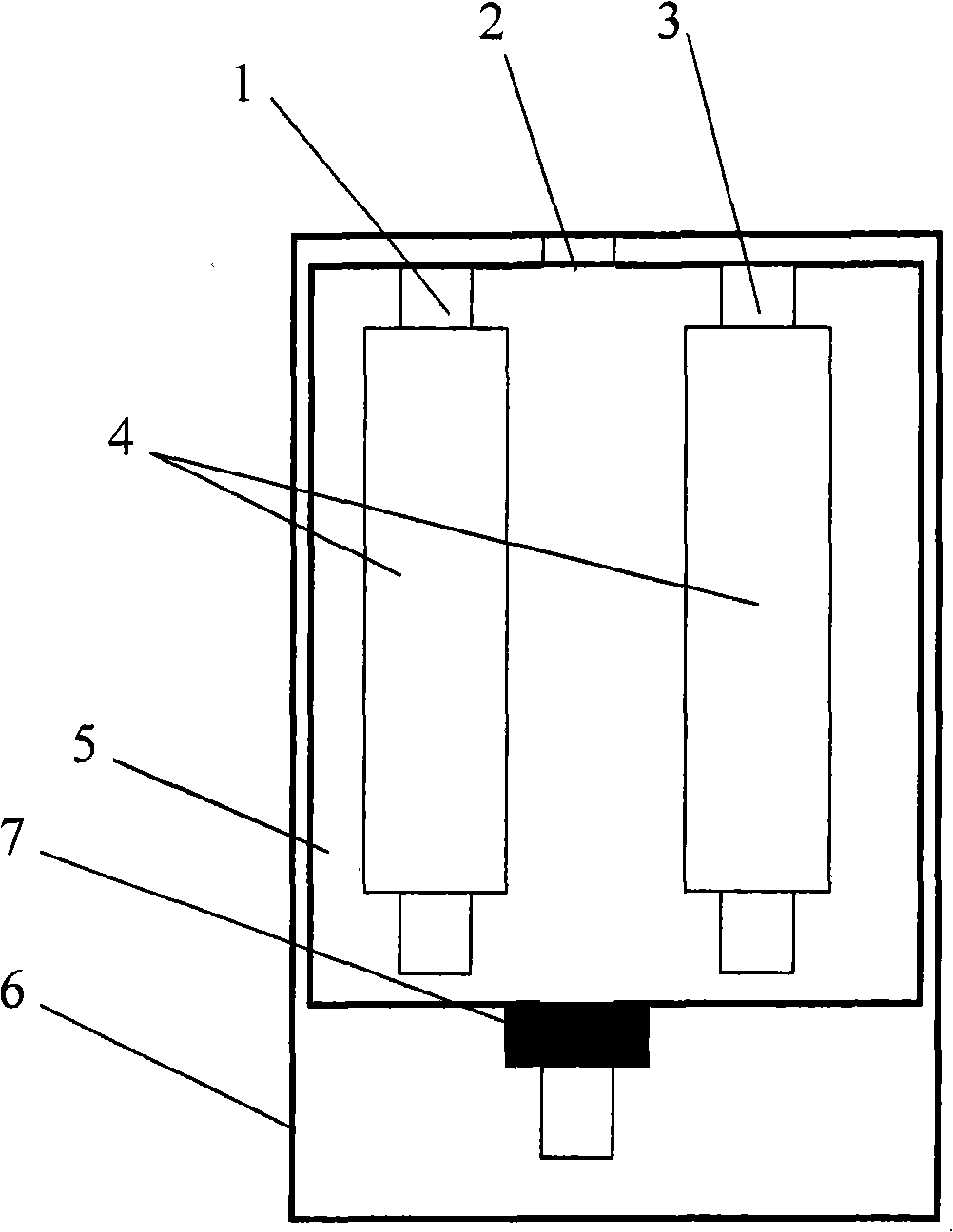
InactiveCN103115914ALow costIncreased sensitivityAnalysis by electrical excitationElectrical batterySchematic maps
The invention discloses a molecular imprinting electroluminescent sensor for detecting veterinary drug residue by taking a battery as power and a method for detecting veterinary drug residue. A preparation method of an electrode (a schematic diagram is shown in the figure) comprises the following steps of: preparing MIP (Molecularly Imprinted Polymer)s sol of the veterinary drug residue; preparing a carbon dot and preparing a graphene nano material according to documents; and modifying graphene, the carbon dot and the MIPs sol onto the surface of the electrode of the sensor. The method for detecting the trace veterinary drug residue comprises the following steps of: connecting the modified electrode to an electrogenerated chemiluminescence instrument, and detecting the veterinary drug residue in a sample extract by taking the battery as the power. The molecular imprinting electroluminescent sensor disclosed by the invention has the advantages that the specificity of the electrode is strong and the sensitivity is high and can be up to a ng grade; only 3-5 minutes are spent on a basic detection process; and the cost is low. The method for detecting the veterinary drug residue by adopting the electrode is quick and easy to operate, the reaction is automatically completed by instruments and results are automatically recorded by the instruments.
Owner:UNIV OF JINAN
Application of pink plumepoppy herb extract in veterinary drugs of economic animals
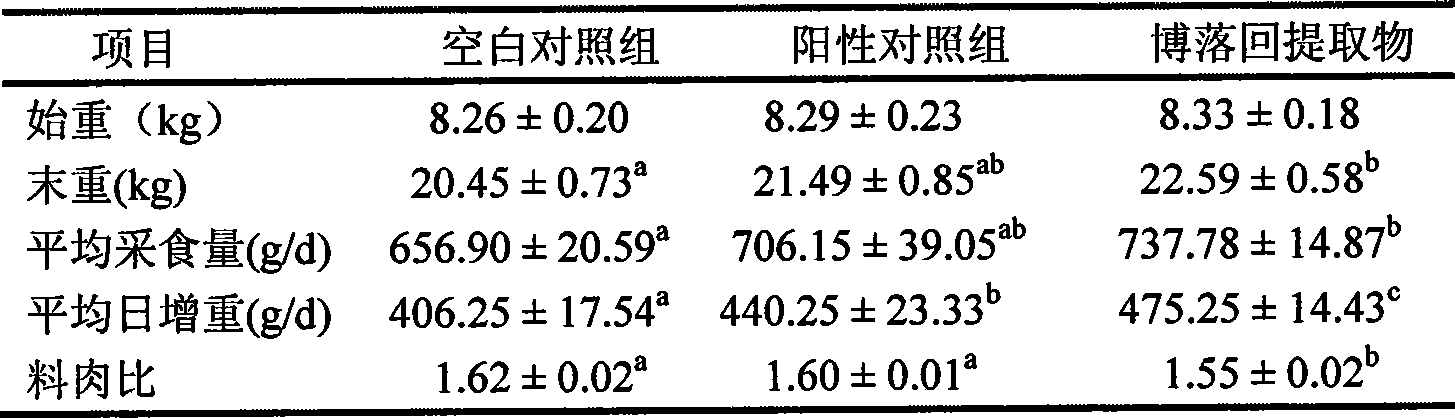
ActiveCN101530475APromote growthImprove conversion rateAntibacterial agentsAnimal feeding stuffFeed conversion ratioFeed additive
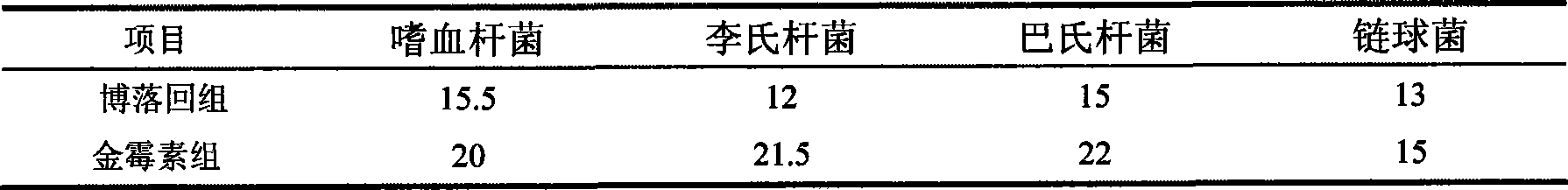
The invention relates to an application of pink plumepoppy herb extract in veterinary drugs of economic animals; the pink plumepoppy herb extract is used for preparing medical feed additives of the veterinary drugs which are used for enhancing the growth of the economic animals and increasing conversion rate of the feed or veterinary drugs which has better inhibiting action and disinfecting action to common pathogenic bacteria in livestock and poultry, such as listeria monocytogenes, haemophilus and pasteurella.
Owner:MICOLTA BIORESOURCE INC LTD
Detection method for veterinary drug residues
InactiveCN103760269AHigh sensitivityStrong specificityComponent separationFiltrationMass spectrometric
The invention provides a detection method for veterinary drug residues. The detection method comprises the following steps of (1) treating a sample: pretreating the sample to be detected, adding an extracting agent, and performing treatment by multiple steps to obtain supernate, wherein filtrate obtained by filtration on the supernate is used for UPLC-MS / MS (ultra performance liquid chromatography-mass spectrum / mass spectrum) measurement; (2) measuring the veterinary drug residues: performing UPLC-MS / MS measurement on the obtained sample under a certain measurement condition. The detection disclosed by the invention has the characteristics of high sensitivity and high specificity and can conform to the detection on the veterinary drug residues.
Owner:CHINA ANIMAL DISEASE CONTROL CENT
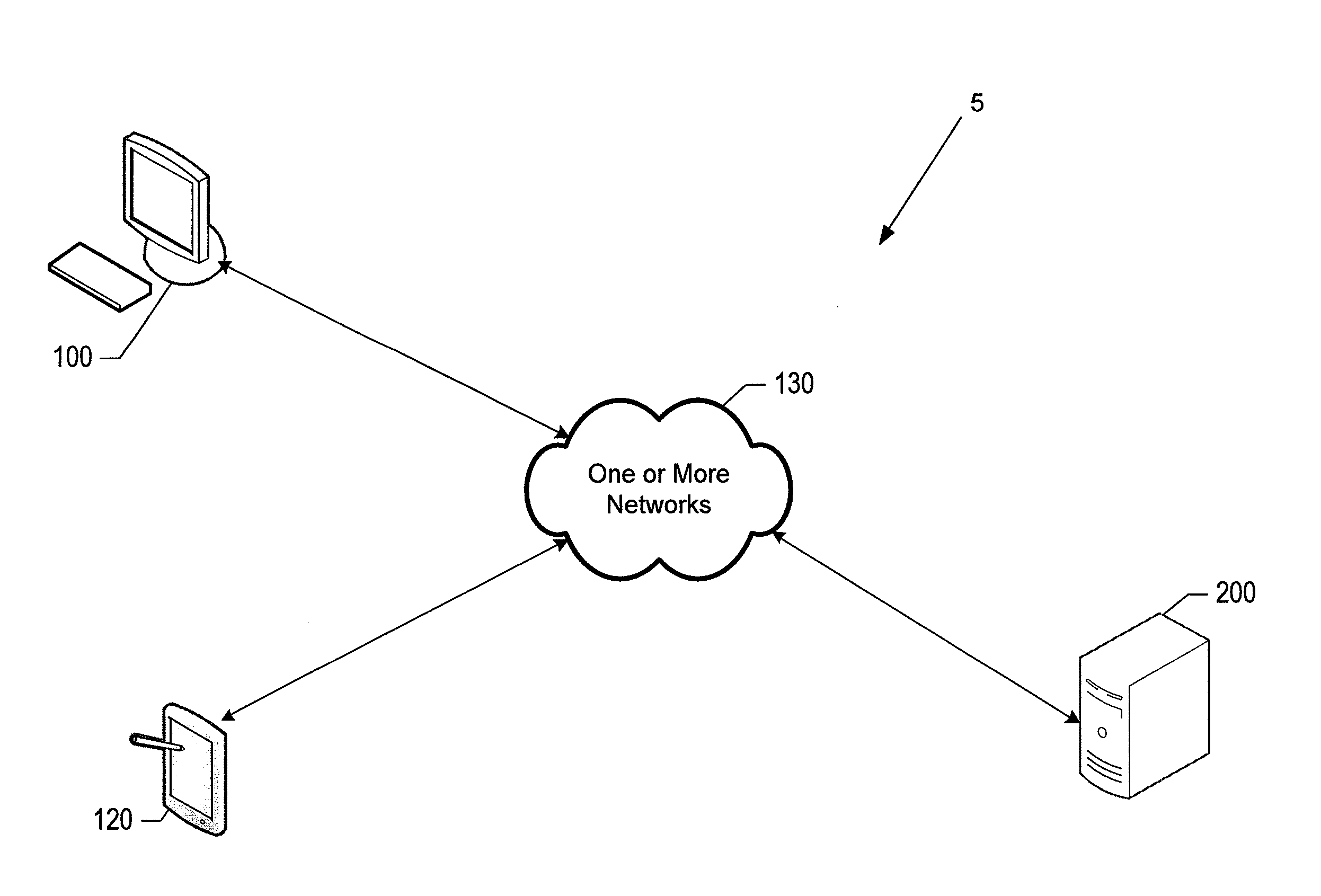
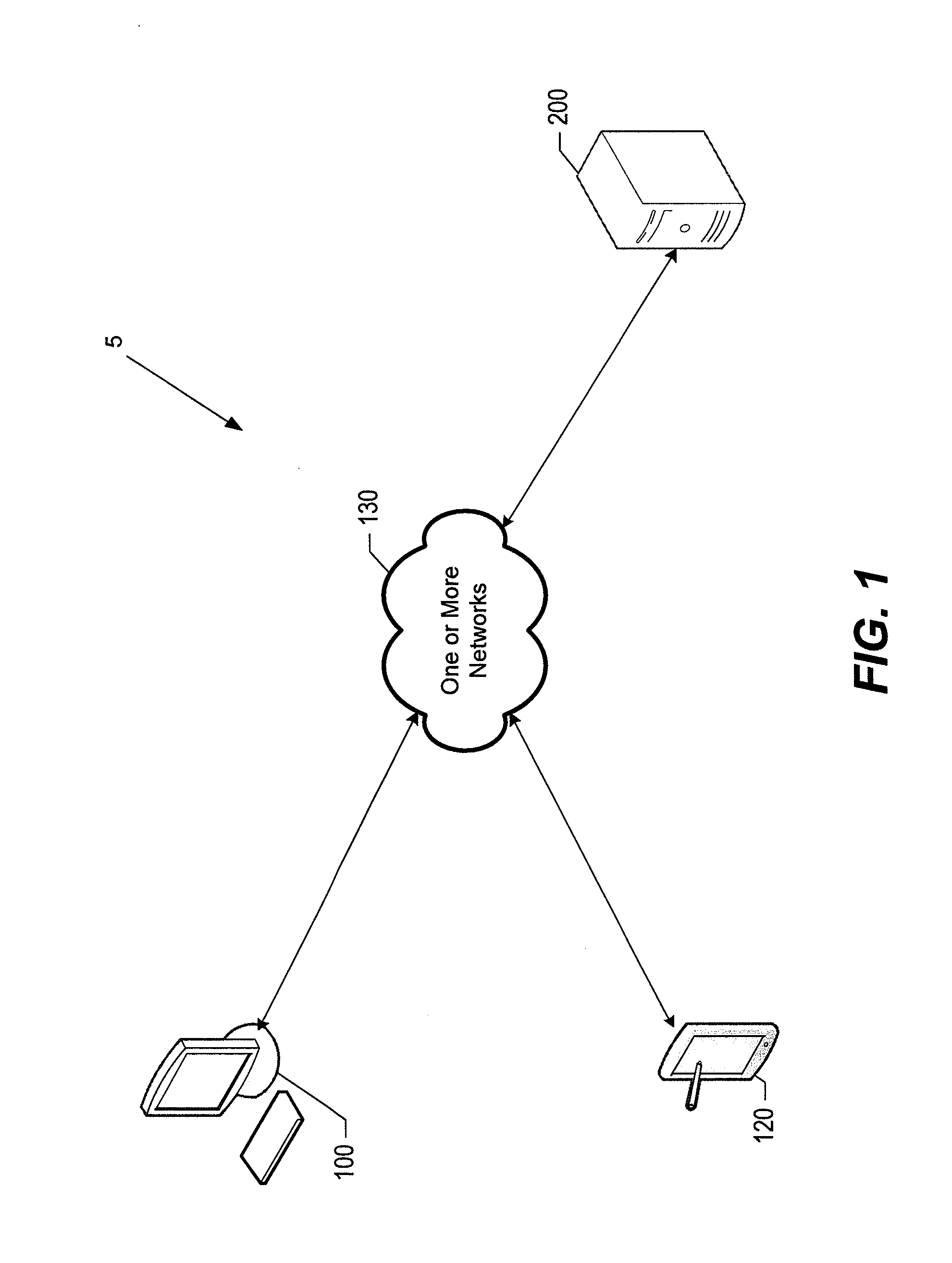
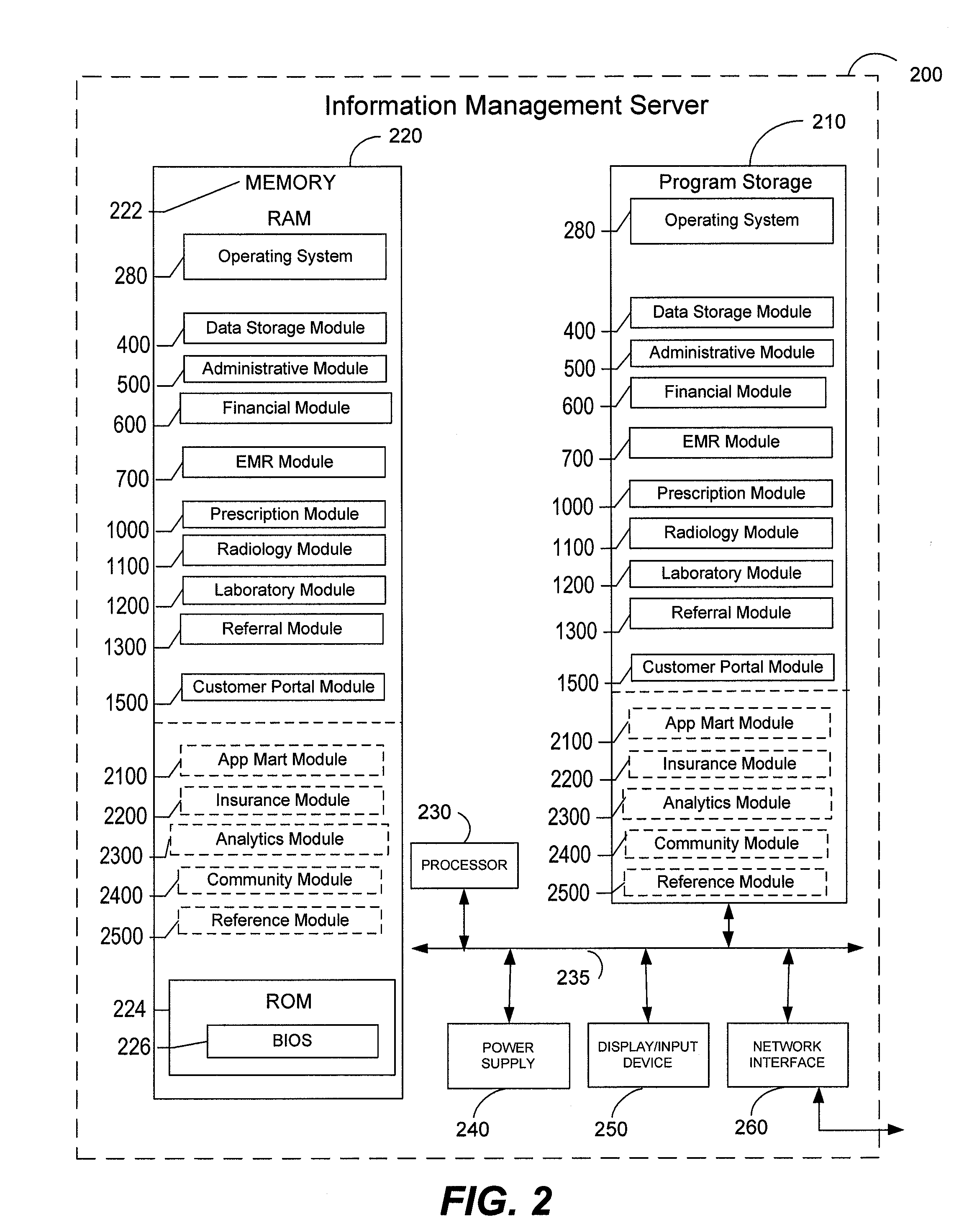
Systems and methods for facilitating consolidated management and distribution of veterinary care data
InactiveUS20130218592A1Facilitating medical treatmentFacilitating administrative activityDrug and medicationsComputer security arrangementsMedicineElectronic medical record
According to various embodiments, veterinary care management systems and associated methods are provided for managing electronic medical records and administrative data related to a veterinary care practice. The systems and methods may be used, for example, by a veterinary clinic, related specialty hospital / university facilities, and / or customers and patients thereof to seamlessly and electronically exchange any of a variety of data related to the provision of veterinary care. In certain embodiments, the systems and methods are configured for receiving data associated with at least one patient. In those and other embodiments, at least a portion of the received data is used to facilitate providing medical services for the at least one patient. Various embodiments update at least a portion of the data based upon facilitated medical services, while certain embodiments transmit the updated data, via a network, to users located at distributed sites to facilitate still further medical services or treatment.
Owner:CUREPET
Screening method for 122 nonprescription drugs in veterinary medicine preparations
ActiveCN107202839APromote healthy developmentRich technical meansComponent separationHigh-Throughput Screening MethodsMedicine
The invention discloses a screening method for 122 nonprescription drugs in veterinary medicine preparations. The screening method comprises the following steps specifically: preparation of a standard working solution, analysis pretreatment of a sample to be measured, establishment and qualitative analysis of a database and the like. Through a constructed high-throughput screening and qualitative detection platform, the detection of a single compound is developed into simultaneous detection of various compounds in the field, and compared with the prior art, the technical means for detecting various compounds in one substance is more perfect, so that the cost is greatly reduced, and the work efficiency is greatly improved. The method plays an important role in veterinary medicine illegal addition and bacterial drug resistance plan implemented in the department of agriculture. Sample detection is carried out in provincial animal product quality detection center of our country, and through incomplete statistics, ten thousands of samples are popularized and applied.
Owner:河南省兽药饲料监察所
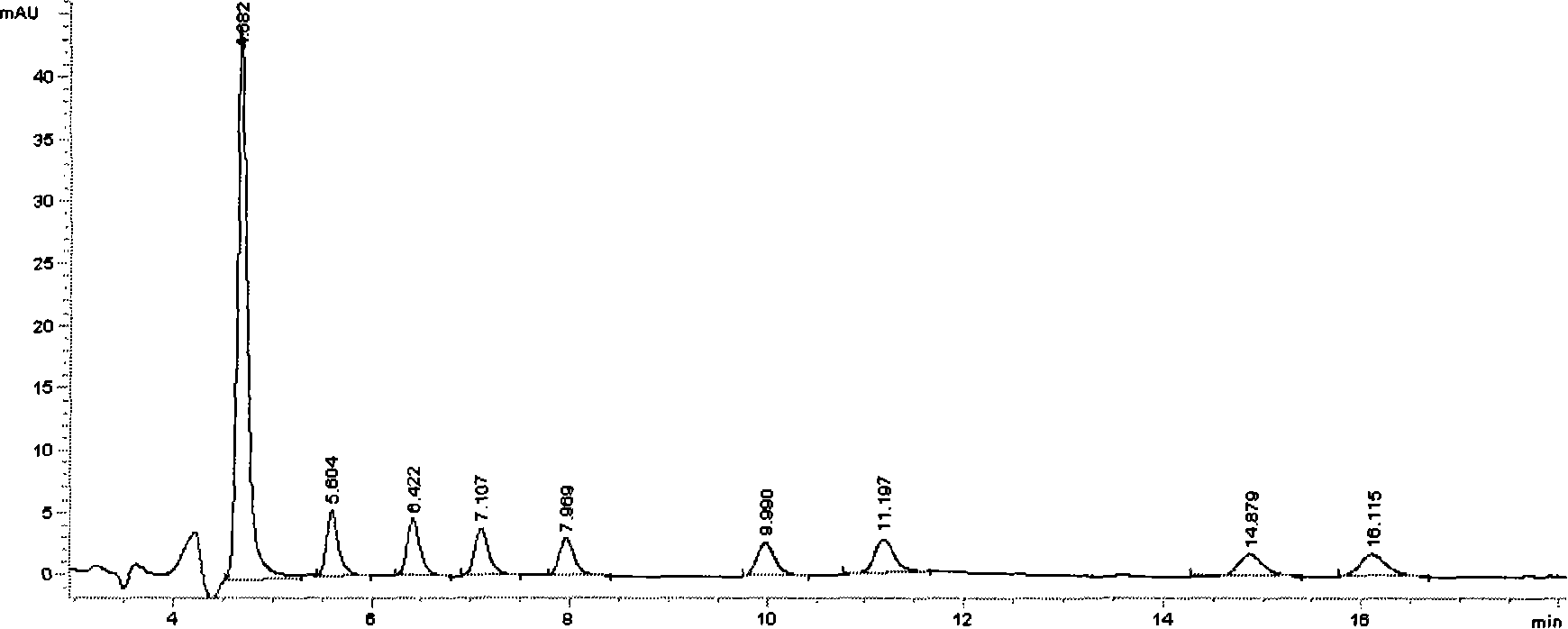
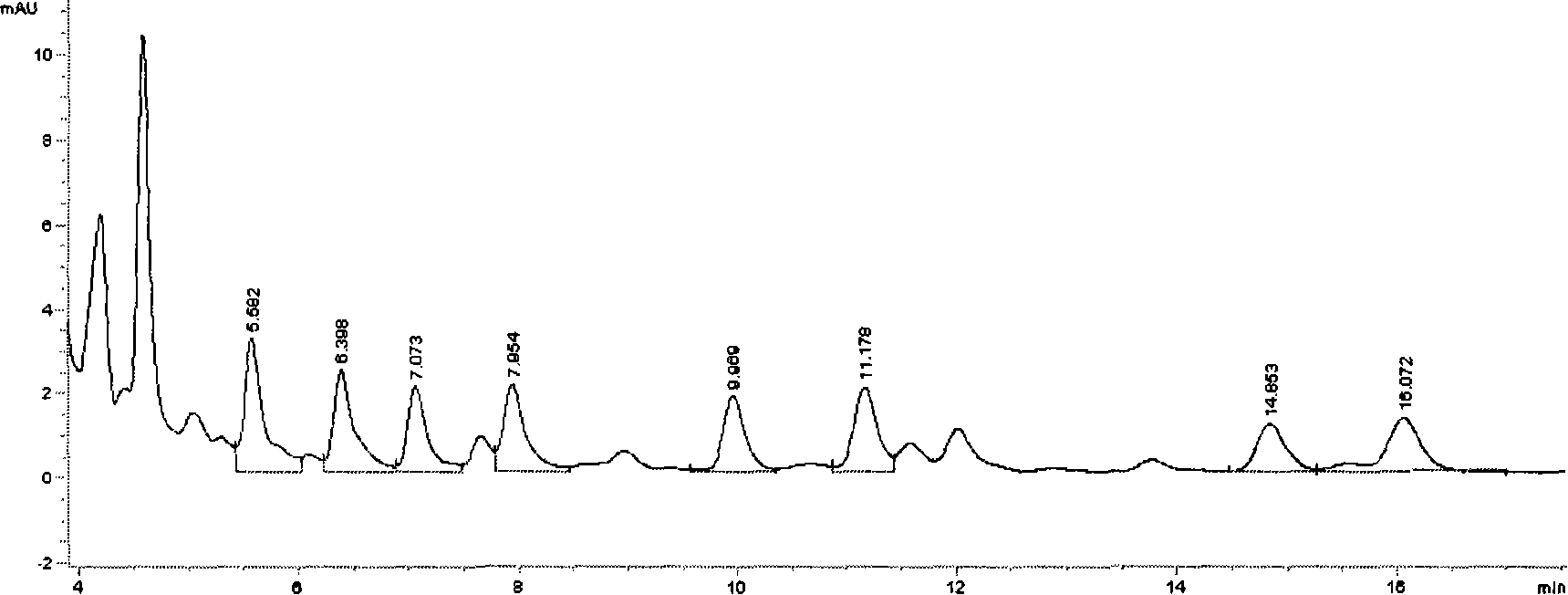
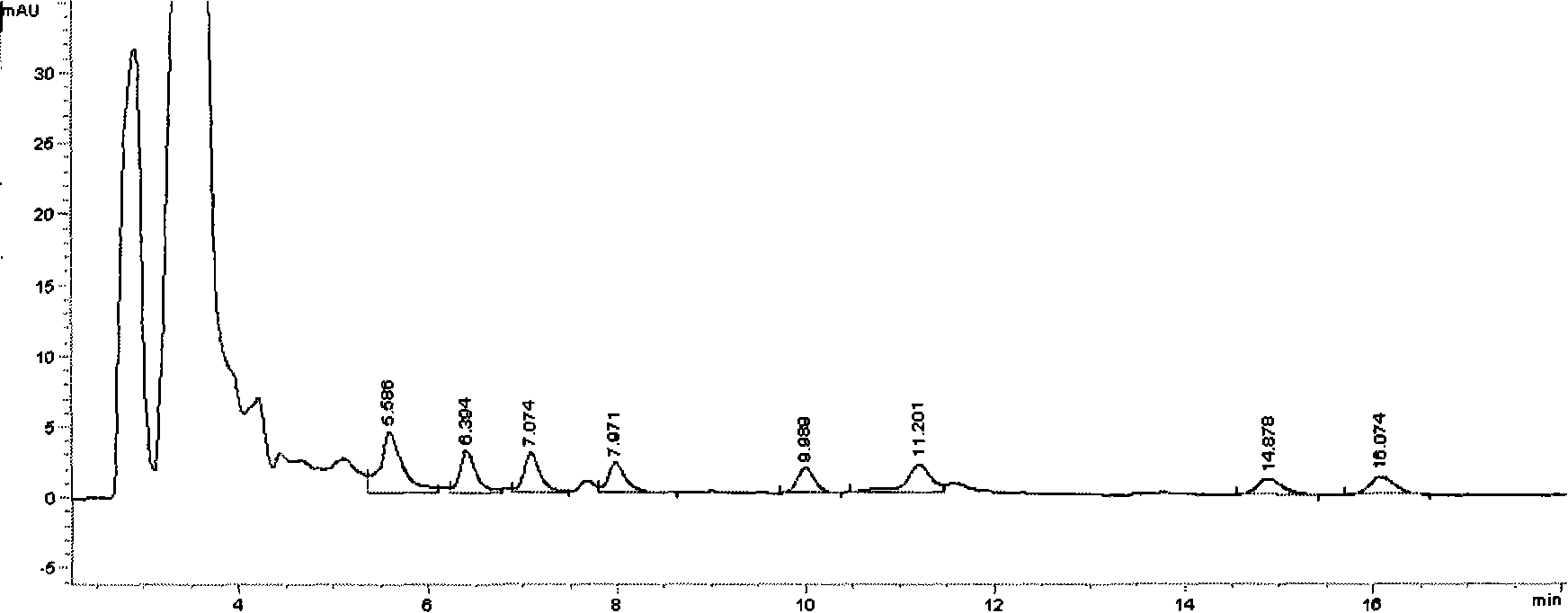
UPLC-MS/MS simultaneous flux detection method for multiclass veterinary drug residue in raw fresh milk
InactiveCN104764816ASensitive detection meansHigh sensitivityComponent separationQuantitative determinationFiltration
The invention discloses a UPLC-MS / MS simultaneous flux detection method for multiclass veterinary drug residue in raw fresh milk. The method includes the steps of: (1) extracting a veterinary drug compound in a to-be-detected sample to obtain an extracted solution; (2) concentrating the extracted solution, and then regulating the pH to 7.5-10.0 to obtain a column passing solution; (3) activating a solid phase extraction column; and (4) loading the column passing solution on the activated solid phase extraction column, leaching the solid phase extraction column with leacheate, then conducting elution with eluent, collecting the eluent, carrying out concentration, redissolving and filtration by a filter membrane, then performing qualitative and quantitative determination by UPLC-MS / MS. The method provided by the invention can simultaneously detect 38 veterinary drug residue in raw fresh milk, and has the characteristics of high sensitivity and low detection limit. The matrix standard curve correlation degree, the standard addition recovery rate of the method and the intra-day and inter-day precision are all accord with the China, European Union and international veterinary drug residue analysis method requirements. The method provided by the invention provides efficient and accurate detection means for raw fresh milk veterinary drug residue risk analysis and warning research.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI +1
Detection method for simultaneously measuring residue of tetracyclines (TCs) drugs in royal jelly
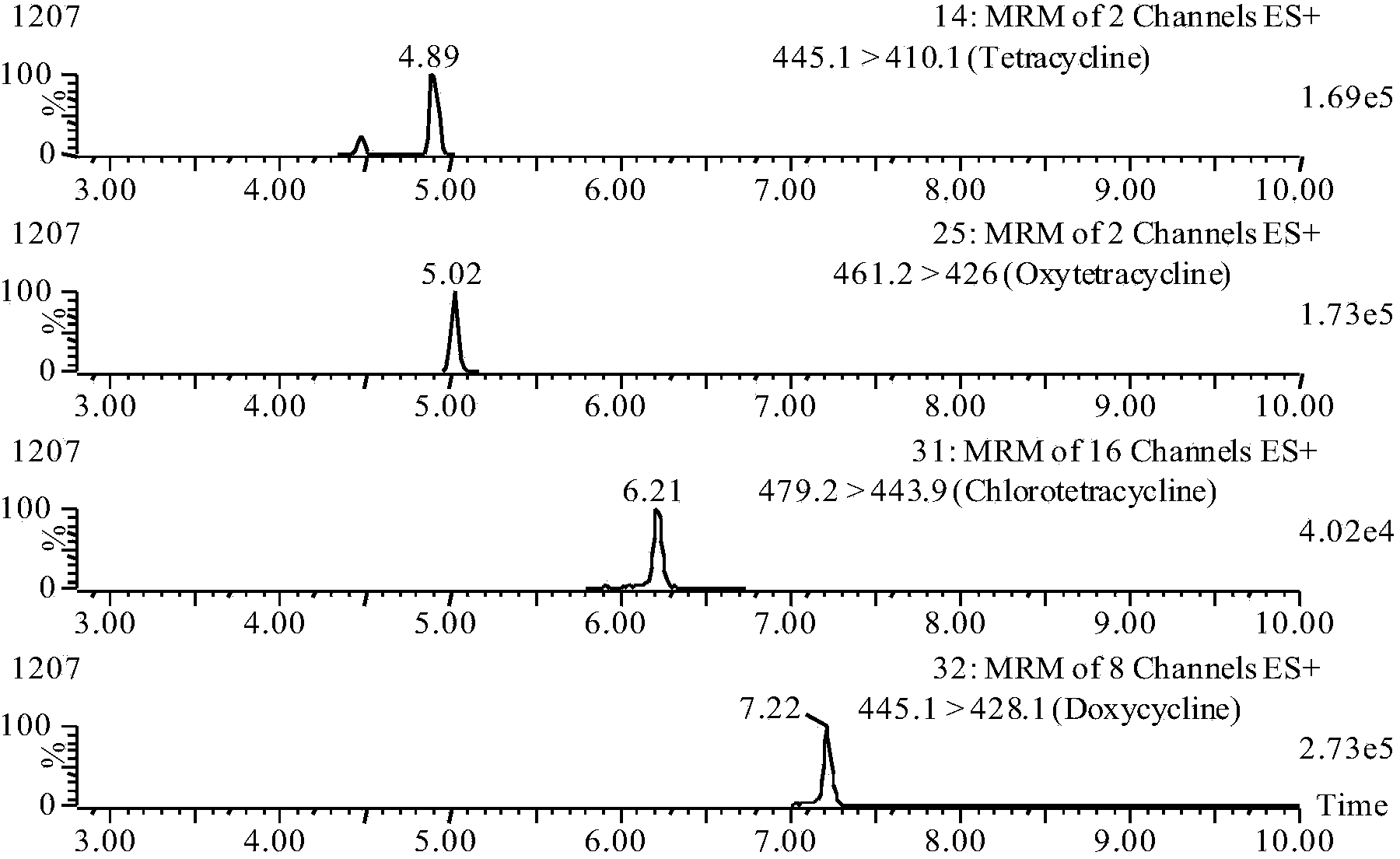
The invention relates to a detection method for simultaneously measuring the residue of tetracyclines (TCs) drugs in royal jelly, in particular to a detection method for simultaneously measuring the residue of 10 veterinary drugs including oxytetracycline, tetracycline, demethylchlortetracycline, chlorotetracycline, doxycycline, 4- epioxytetracycline, 4-epitetracycline, 4-epichlorotetracycline, minocycline and methacycline. The method comprises the following steps: precipitating protein by methanol; then, adjusting the pH value; further carrying out multi-stage purification; and simply and simultaneously measuring the residue of the TCs drugs by the liquid chromatography-mass spectrometry / mass spectrometer (LC-MS / MS). By carrying out the pre-treatment for one time, the invention can measure the residue of up to 10 TCs drugs in the royal jelly, wherein, the lower limits of detection (LLD) of the five TC antibiotics including oxytetracycline, tetracycline, demethylchlortetracycline, chlorotetracycline and doxycycline are 2.0 mug / kg; while the LLDs of the other veterinary drugs including 4- epioxytetracycline, 4-epitetracycline, 4-epichlorotetracycline, minocycline and methacycline are 10.0 mug / kg.
Owner:THE INSPECTION & QUARANTINE TECH CENT ZHEJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
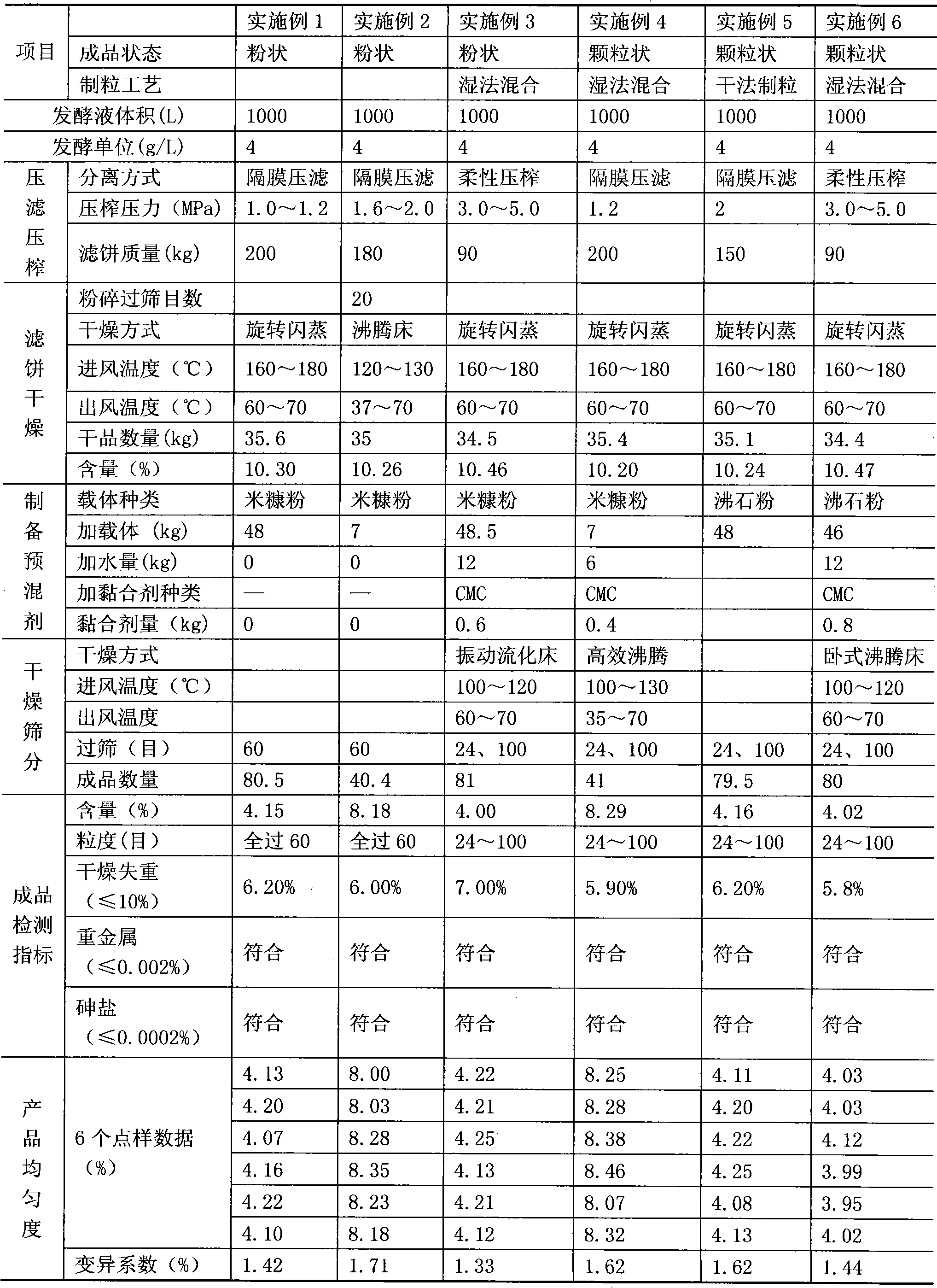
Preparation method of enramycin premix
ActiveCN101862443AReduce pollutionReduce manufacturing costAntibacterial agentsPowder deliveryOrganic solventEnramycin
The invention relates to a preparation method of an enramycin premix, comprising the following steps of: carrying out high-pressure filtration / squeeze on enramycin fermentation liquor prepared by using a conventional method; separating the fermentation liquor; then carrying flash drying on an obtained enramycin filter cake and then crushing; adding a powdery carrier to fine mycelium powder according to the purity and the end-product requirement content of a dried product so as to regulate the concentration of the fine mycelium powder; evenly mixing to obtain a powdery enramycin premix; and pelleting the powdery enramycin premix into a granulated enramycin premix according to demands. The preparation process of the preparation method does not need to refine and extract enramycin, does not consume organic solvents and acid and base, and has less pollution on the environment, and the production cost is largely reduced; the content and the uniformity of the obtained product meet the quality standard requirements of imported veterinary drugs, and the stability of the product meets the specified requirements; moreover, dangerous solid wastes, such as antibiotic bacterium residues and the like, are not generated in the production and preparation process, thereby greatly reducing the pollution to the environment and the later-period management cost.
Owner:濮阳泓天威药业有限公司
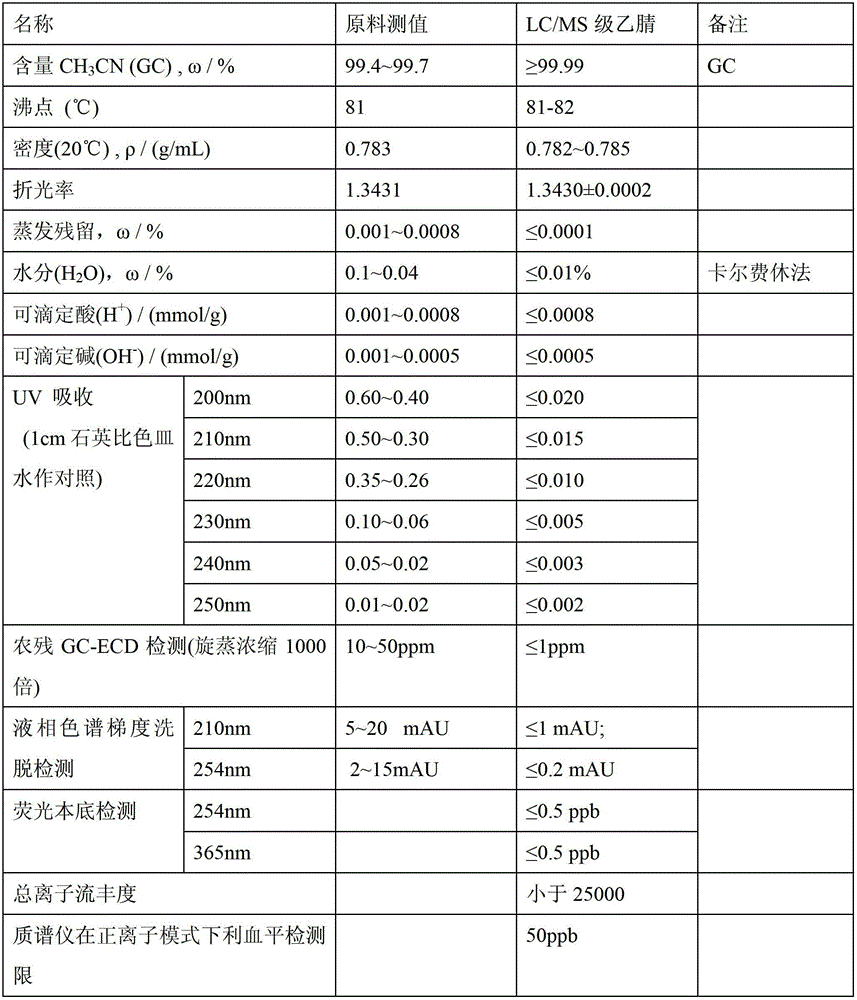
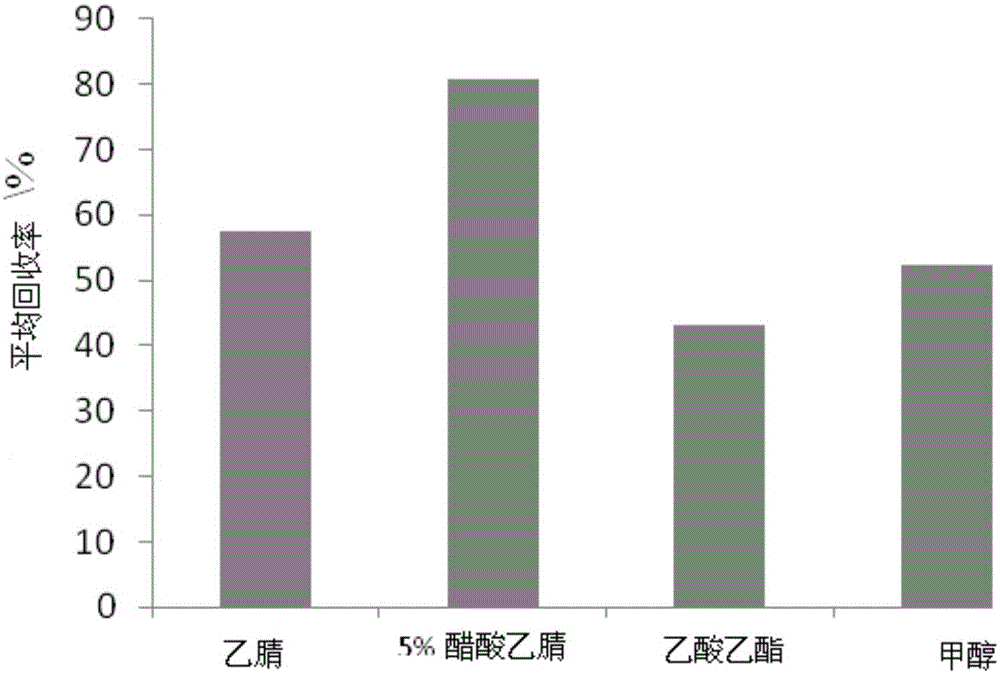
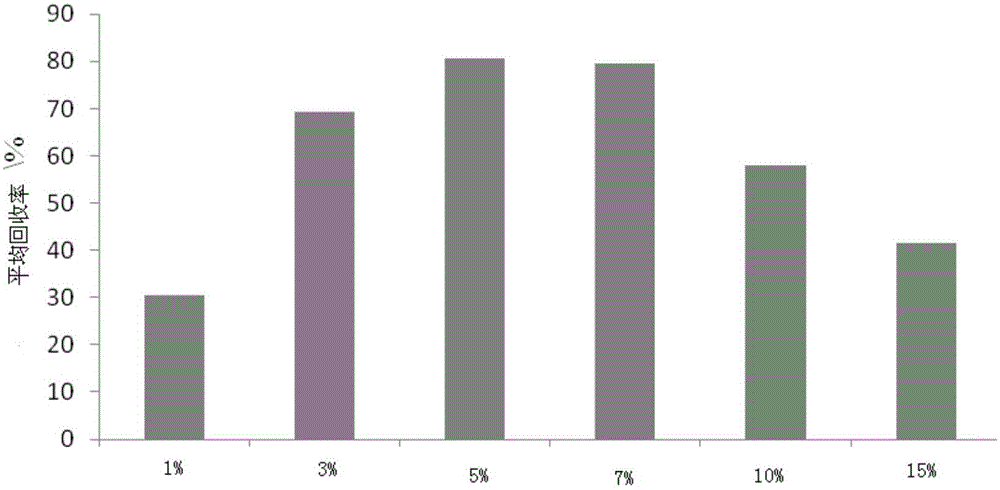
Preparation method for liquid chromatography mass spectrometry (LC-MS)-grade high-purity acetonitrile reagents
InactiveCN102746187AHigh recovery rateSimple and fast operationCarboxylic acid nitrile purification/separationGas chromatography–mass spectrometrySorbent
The invention relates to a preparation method for liquid chromatography mass spectrometry (LC-MS)-grade high-purity acetonitrile reagents. The method includes steps: subjecting raw acetonitrile to adsorption by the aid of adsorbents such as activated carbon and activated alumina, oxidizing for purification, rectifying, dehydrating and drying by the aid of a molecular sieve, filtering by the aid of a microfiltration membrane, filling nitrogen for packaging, and obtaining the LC-MS-grade high-purity acetonitrile reagents. By the method, the recovery rate is more than 98%, the yield is more than 95%, the purity reaches to more than 99.99%, application requirements of clients on scientific researches and subject studies of the LC-MS-grade high-purity acetonitrile reagents can be met, domestic vacancies of production of the LC-MS-grade high-purity acetonitrile reagents can be filled, dependency on foreign reagents is lowered, high-quality reagents are provided for growing wide application of LC-MS quantitative analysis in high-tech fields such as biomedical research and development, clinic, food safety detection, pesticide residue analysis, veterinary drug residues, legal examiners, criminal investigations, doping detection, drug testing, agriculture and environment protection.
Owner:天津康科德医药化工有限公司
Simultaneous screening and detection method of plurality of types of veterinary drug residues in solid animal-derived foods
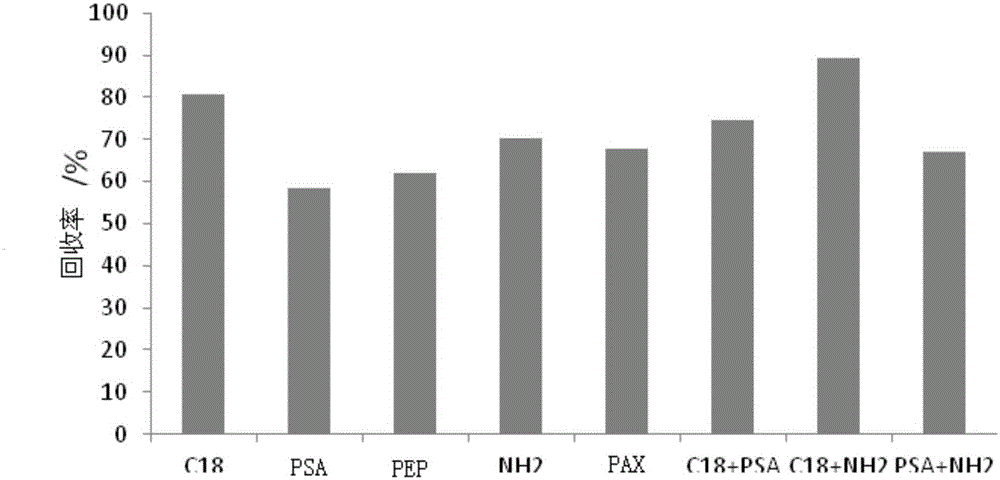
The invention relates to a simultaneous screening and detection method of a plurality of types of veterinary drug residues in solid animal-derived foods. The simultaneous screening and detection method comprises the following steps: after taking and crushing a sample to be detected, adding an acetonitrile acetate solution and a Na2EDTA buffering solution into the sample to be detected; meanwhile, adding anhydrous sodium sulfate and sodium chloride, and homogenizing and extracting; adding an adsorbent containing C18 and NH2 into extracting-obtained liquid supernatant to carry out purification; then determining by using high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. The screening method provided by the invention can be used for simultaneously screening and detecting the plurality of types of veterinary drug residues in the animal-derived foods, has the characteristics of rapidness, simplicity and convenience, flexibility, accuracy and the like, can be used for carrying out an accurate qualitative analysis on target veterinary drugs in the sample, and also can be used for carrying out quantitative detection. The method is applicable to rapid detection of the plurality of types of veterinary drug residues in batch samples. Aiming at the solid animal-derived foods, the impact on the target veterinary drugs by sample substrates is reduced, and the extraction effect is good and the recycling rate is high.
Owner:中华人民共和国临沂出入境检验检疫局
QuEchERS method package for efficiently and homogeneously extracting residues of pesticide and veterinary drug
ActiveCN103698195AEasy to operateEasy to joinPreparing sample for investigationSilica gelAmmonium sulfate
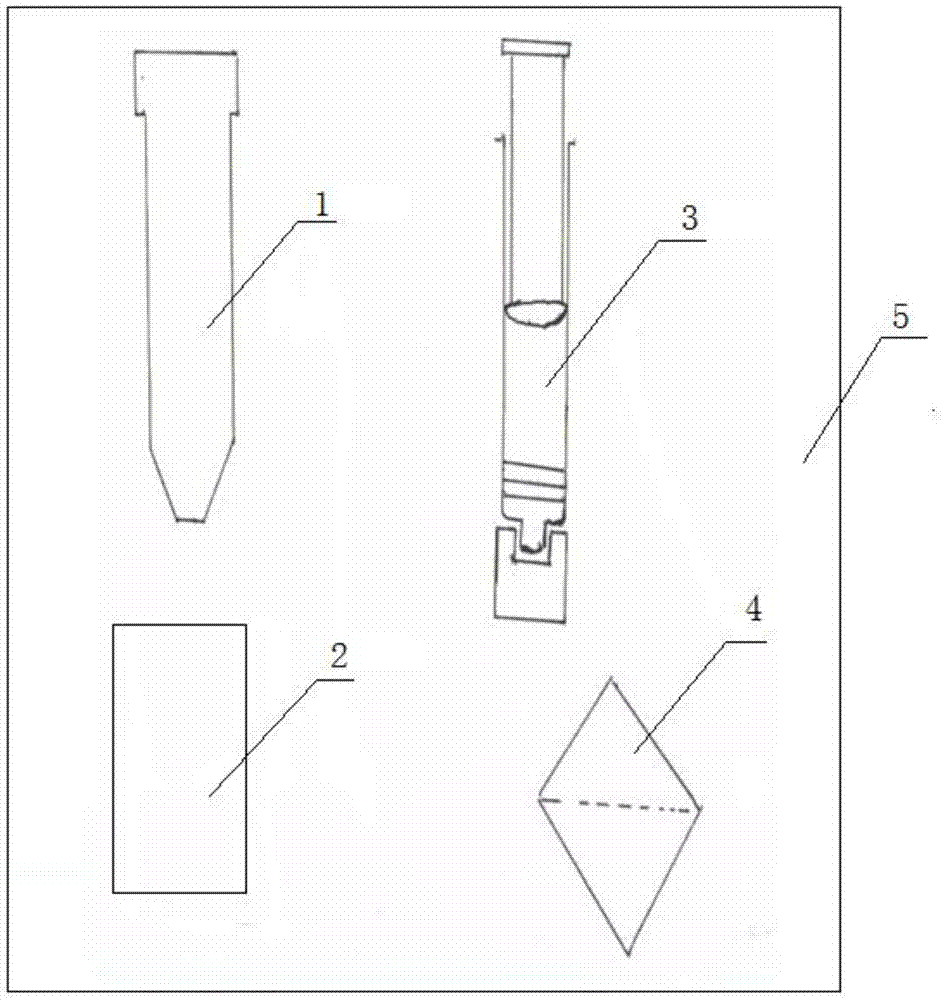
The invention discloses a QuEchERS method package for efficiently and homogeneously extracting residues of a pesticide and a veterinary drug. The QuEchERS method package comprises an extraction pipe containing ammonium sulfate, a buffer salting-out phase-splitting salt package containing a mixture of anhydrous magnesium sulfate and sodium chloride, a needle cylinder type membrane filter containing a mixture of PSA, graphitized carbon black and a C18 reverse phase silica gel filler, and a salting package tool, wherein the PSA is an N-propylethylendiamine-modified silica gel filler and the C18 reverse phase silica gel filler is an octadecyl-modified reverse phase silica gel filler. According to the method, the technical feature of the combination of extraction, salting-out phase-splitting and dehydration purification is fully utilized, devices, materials and operating steps are reasonable to form, the structure is simple, and the operation is easy and convenient.
Owner:山东青云实验耗材有限公司
Method for preparing traditional Chinese herb feed additive and its glagctogogue powder preparation
InactiveCN101164429ASuitable for mass productionEasy to useAnimal feeding stuffSexual disorderMedical prescriptionTraditional medicine
The present invention belongs to the field of Chinese medicine feed additive technology, and relates to a preparation method of Chinese medicine feed additive. Said preparation method includes the following steps: according to its prescription using partial Chinese medicinal materials, adding water and decocting them to obtain liquid medicine, filtering and concentrating to obtain extract, spray-drying said extract, pulverizing and sieving to obtain the extract powder for stand-by; drying the residue remained after the above-mentioned partial Chinese medicinal materials are decocted, pulverizing and sieving to obtain second extract powder for stand-by; pulverizing residual Chinese medicinal materials and sieving to obtain third extract powder; mixing all the extract powders so as to obtain the invented Chinese medicine feed additive-lactogenesis power preparation capable of obtaining good effect for stimulating milk secretion.
Owner:TIANJIN RINGPU BIO TECH
Fast high-precision detecting method for animal medicine residue in food
The invention provides a fast high-accuracy method for detecting agricultural and veterinary products residues in food, including following four steps: (1) matrix pre-treatment; (2) adding analysis protecting agent; (3) introducing sample and detecting on machine; (4) data processing. The detecting method, under the condition that GC / MS instrument detecting sensitivity is no changed, and with large volume injection, could reduce pre-treatment sample amount sharply. At the same time the invention has the advantages of low analysis cost, short analysis time, and uses the standard solution containing analysis protecting agent as standard working solution to draw standard curve for quantifying agricultural and veterinary products residues in various kinds of vegetable, fruit and corn substrates, and quantifies agricultural and veterinary products residues in various kinds of substrates with one standard curve, and has good applicability, the sensibility, accuracy and precision are all in accordance with command of agricultural and veterinary products multi-residues technology, and is suitable for detecting various kinds of agricultural and veterinary products' residues in food quickly and sensitively.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
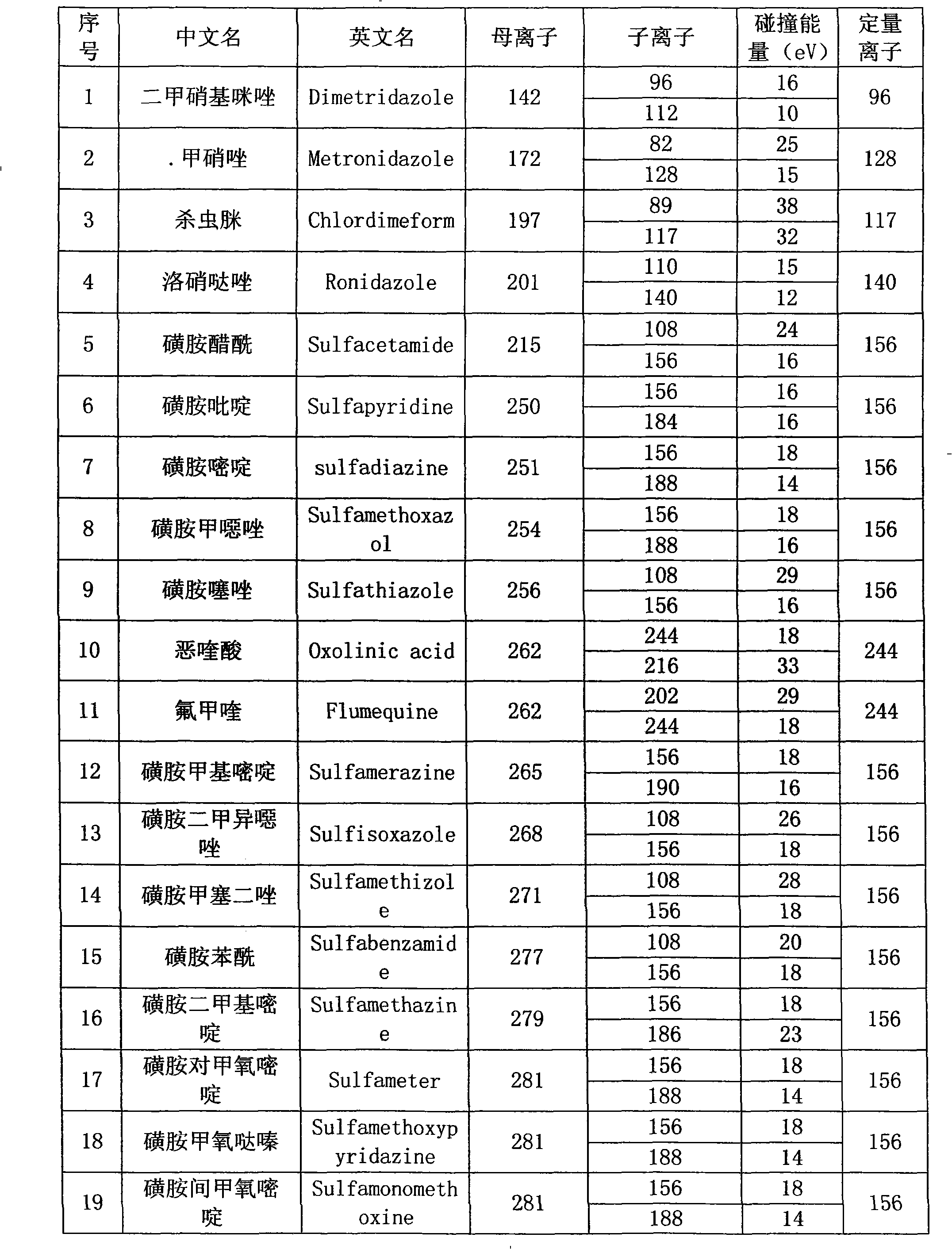
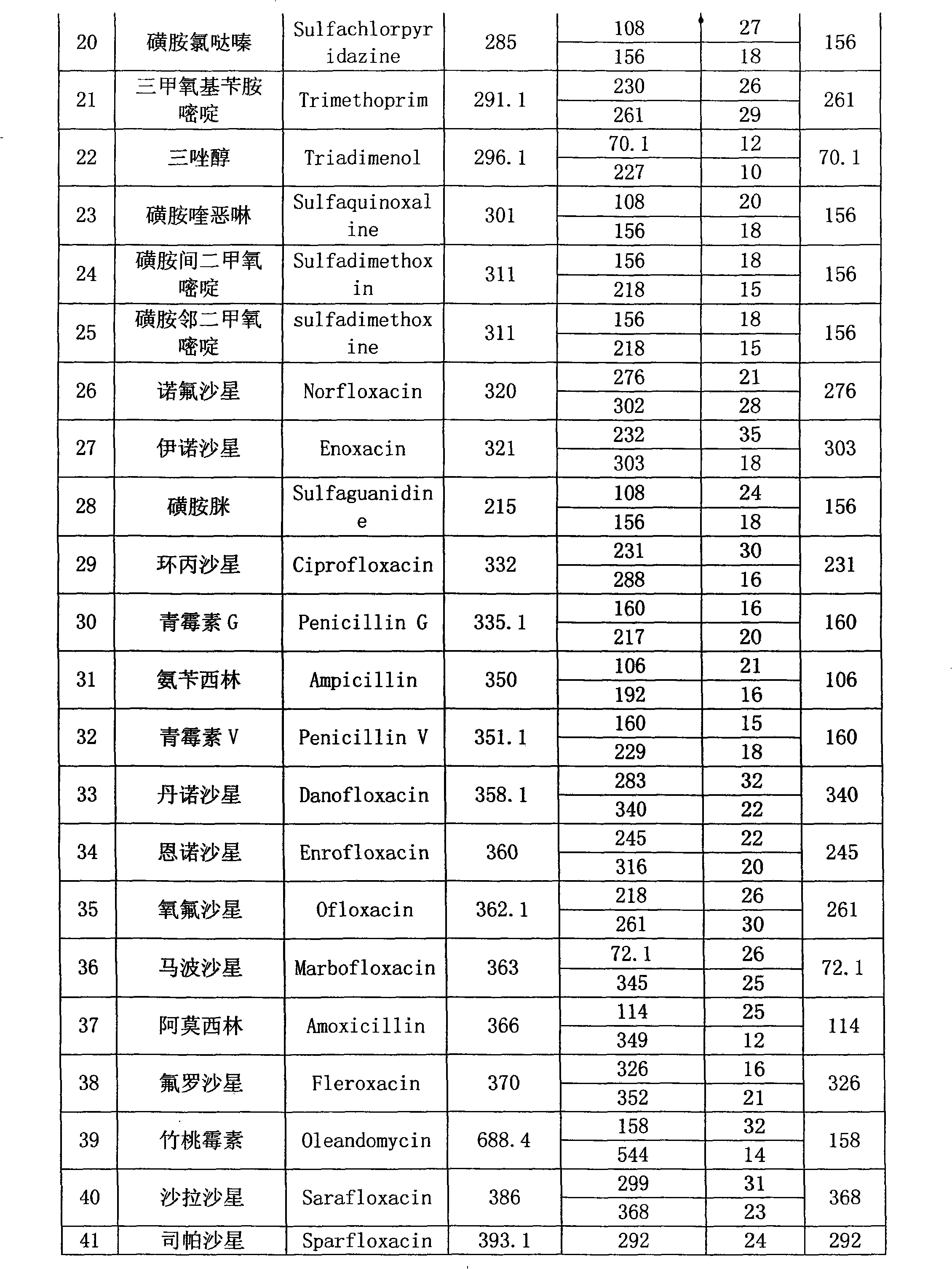
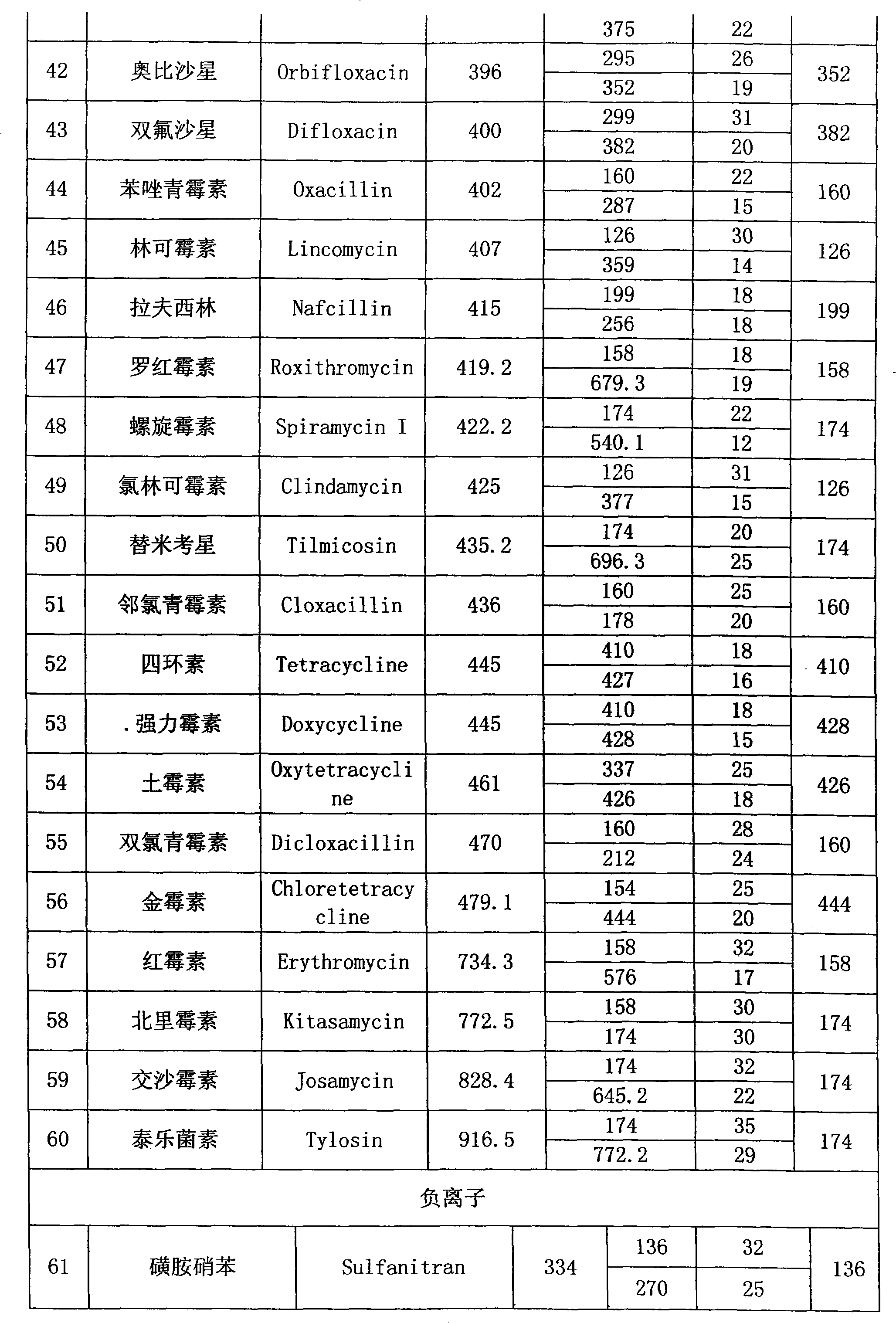
Detection method of residual quantities of various veterinary drugs in culturing or slaughtering environment
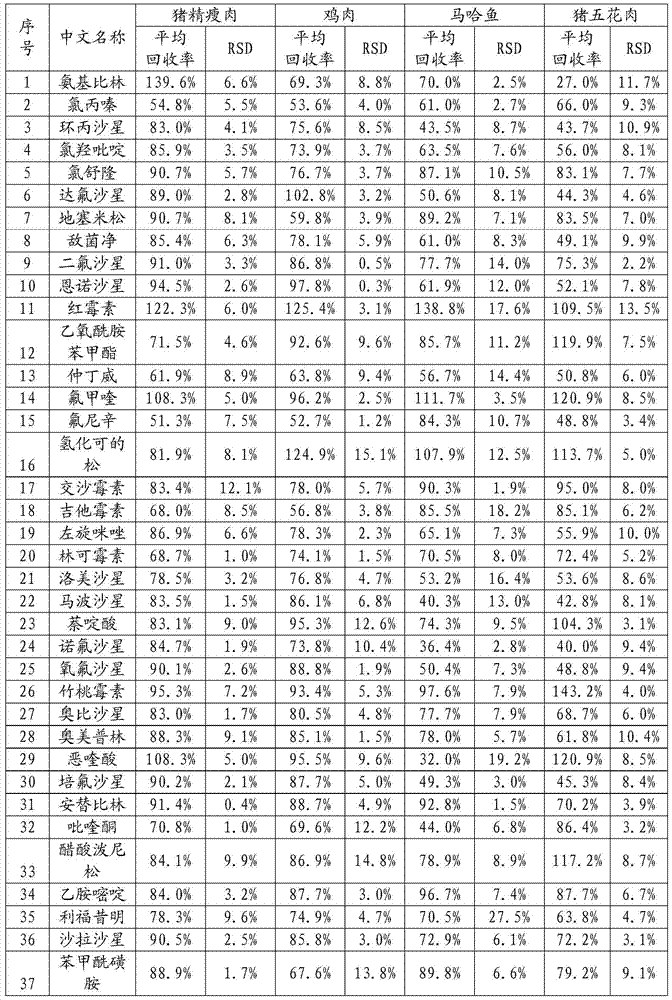
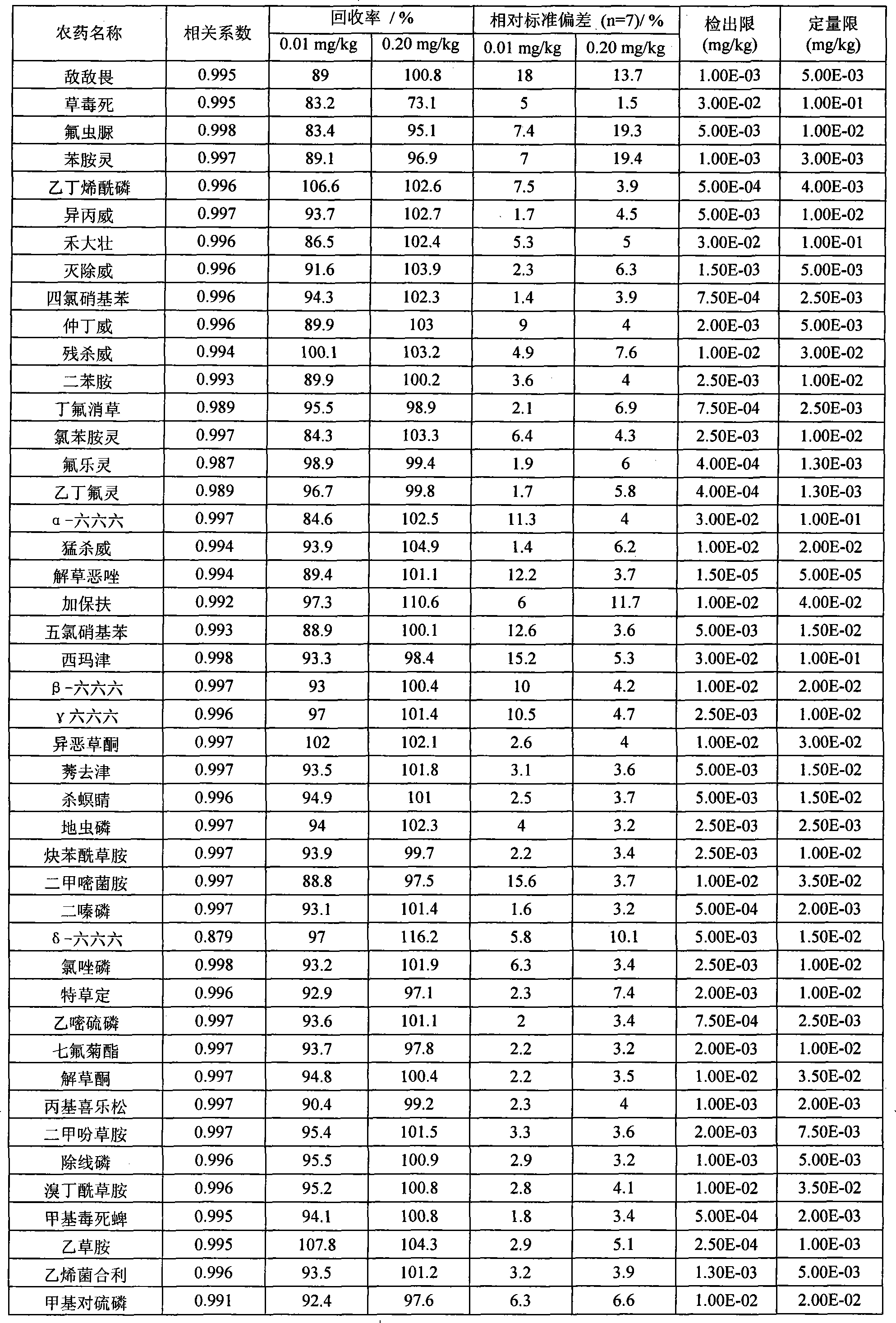
The invention provides a detection method of residual quantities of various veterinary drugs in a culturing or a slaughtering environment. With the method, rapid screening and quantitative detection can be carried out upon 61 drugs of 9 categories. The drugs include chlormycetin, beta lactams (penicillins), quinolones, sulfonamides, trimethoprims, macrolides, tetracyclines, and nitroimidazoles. According to the invention, a soil sample or an environmental water sample is added into a phosphate buffering solution; a filtrate obtained through centrifugation is extracted in a solid phase extraction column, and is eluted; the extraction column is dried by blowing, and is washed by using a methanol solution; an obtained eluent is titrated by using a methanol solution; a chromatogram peak of the sample is detected by using liquid chromatography-tandem mass spectrometry; the chromatogram peak is compared with a standard chromatogram peak, such that a specific variety of the detected drug is accurately determined. According to the invention, the sample solution is subject to liquid chromatography-tandem mass spectrometry multi-reaction monitoring selected ion analysis. Through internal standard correction, the recovering rate is 70-120%, and a relative standard deviation RSD is no larger than 18%. Compared with existing technologies, the analysis efficiency is improved by at least 5 times, and the detection cost is 30% of that of existing technologies.
Owner:ANIMAL AND PLANT & FOOD DETECTION CENTER JIANGSU ENTRY EXIT INSPECTION AND QUARANTINE BUREAU
Immune colloidal gold detecting card of carbendazin and preparation method of immune colloidal gold detecting card
The invention discloses an immune colloidal gold detecting card of carbendazin and a preparation method of the immune colloidal gold detecting card, and relates to the technical field of animal source food veterinary drug residue detection. A test strip in a shell of the detecting card is composed of a PVC glue plate, a sample cushion, a colloidal gold conjugate pad, a coating film and a water absorbing cushion, wherein a colloidal gold film is a glass cellulose membrane containing carbendazin monoclonal antibodies, the coating film is a nitrocellulose film and is provided with a line T and a line C, the line T is wrapped by carbendazin protein conjugates, and the line C is wrapped by goat-anti-mouse IgG antibodies. The detecting card can be effectively used for rapidly detecting carbendazin and is convenient to use, rapid and accurate in result.
Owner:NANJING YITE BIOTECH
Animal source food sulfonamide residual medium solid phase dispersion-highly effective liquid phase chromatography determination method
InactiveCN101241114ANot easy to changeGuaranteed repeatabilityComponent separationSulfur drugHplc mass spectrometry
The present invention relates to 'method for examining solid-phase dispersion -high performance liquid chromatogram of residues substrate of sulfonamides in animal derived food' which belongs to the field of food safety-veterinary drug residues. The method comprises of chromatogram examining steps of substrate solid-phase dispersion and high performance liquid, wherein, the mobile phase of sodium dihydrogen phosphate-acetonitrile is 50mmol / L in high performance liquid chromatogram examining method, the volume ratio of sodium dihydrogen phosphate and acetonitrile is 70:30, because the sodium dihydrogen phosphate solution is buffer solution which has a certain PH buffer capability and makes the PH value stable, the stability of pH value of HPLC mobile phase is ensured, and repeatability and reproducibility of analysis result are ensured also, at the same time, the classical process of extraction, separation, concentration and purification are simplified, the operation is simple, the milk combination phenomenon is eliminated. The method of present invention has the merits of ideal precision and sensitivity, and environment protecting by using the less toxicity organic solvent eluting analyte employed.
Owner:BEIJING JINXIU DADI AGRI CO LTD
Silk screen printing electrode, manufacturing technique and use thereof
InactiveCN101344501AEasy to makeGood chemical stabilityMaterial electrochemical variablesWire gauzePesticide residue
The invention discloses a screen painting electrode, which comprises a printing sizing agent that is used on a substrate and adopts a mixture of titanium powder and nano titanium oxide, a working electrode that is prepared through a screen painting technique, separately processed by chemical modification and provided with an electrode specification layer that is solidified by printing ultraviolet lights of photocureable insulating slurry, an auxiliary electrode that realizes the printing by adopting a carbon powder printing ink and a silver and silver chloride sizing agent on another substrate, and a silver chloride reference electrode; all electrode plates are assembled together by a silicon rubber. The invention further discloses a preparation method of the screen painting electrode and a plurality of application methods of the screen painting electrode. The invention can be widely applied in various electrochemical analysis fields, and is especially applicable to the heavy metal ion measurement with stripping voltammetry as well as the pesticide residue and veterinary drug residue measurement with adsorption wave voltammetry in food analysis and environment monitoring.
Owner:CENT SOUTH UNIV
Method for simultaneously detecting residues of 64 veterinary drugs in aquatic products
The invention discloses a method for simultaneously detecting residues of 64 veterinary drugs in aquatic products. The method comprises the following steps: performing pretreatment on samples so as towell reduce the interference effect of a substrate on a target compound; and detecting the treated samples by adopting ultra-high performance liquid chromatography-quadrupole rod / electrostatic fieldorbitrap high resolution mass spectrum. The method disclosed by the invention is capable of simultaneously detecting residue of 14 categories of 64 veterinary drugs in aquatic products. The method islow in detection cost, short in cycle, excellent in stability and high in accuracy and is applicable to daily rapid screening of the residues of the veterinary drugs in the aquatic products on a largescale.
Owner:INSPECTION AND QUARANTINE TECHNOLOGY CENTER ZHONGSHAN ENTRY EXIT INSPECTION AND QUARANTINE
Digital rapid food safety detection system
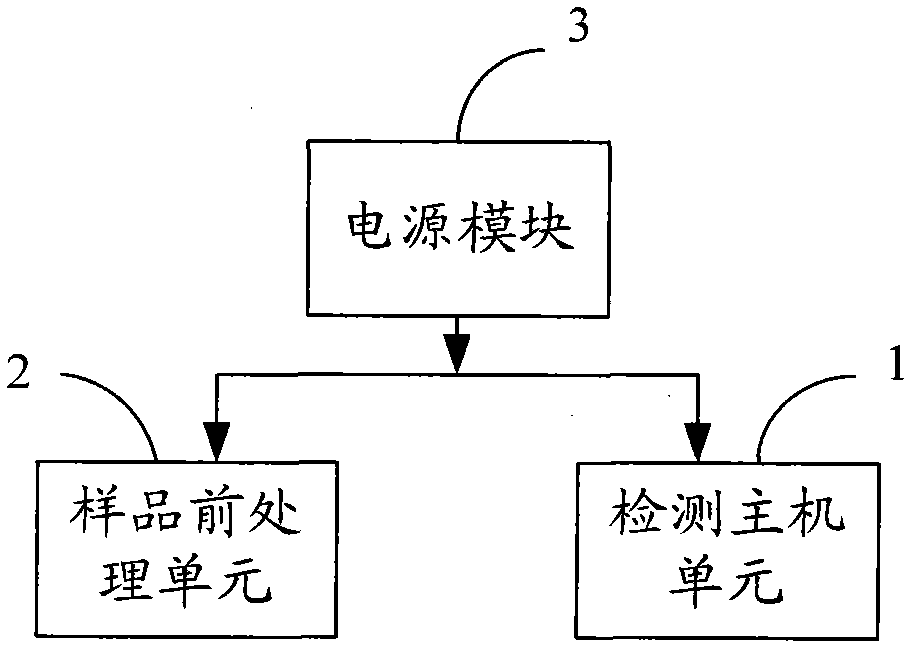
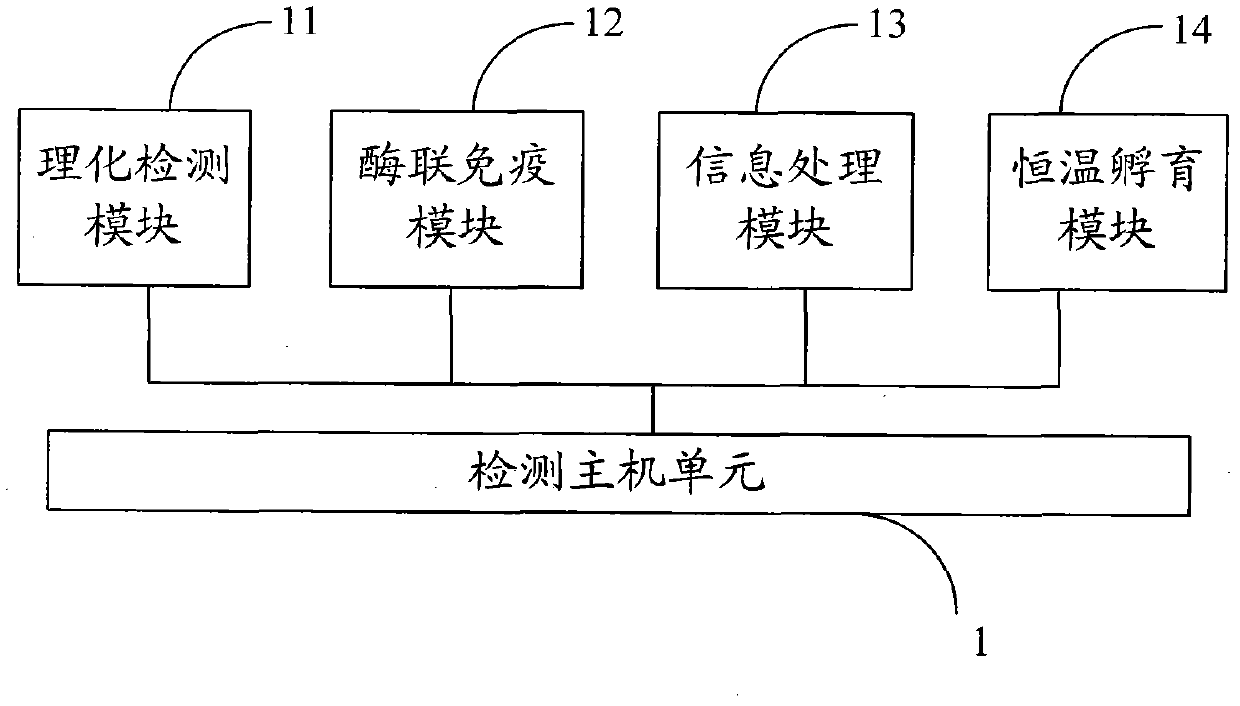
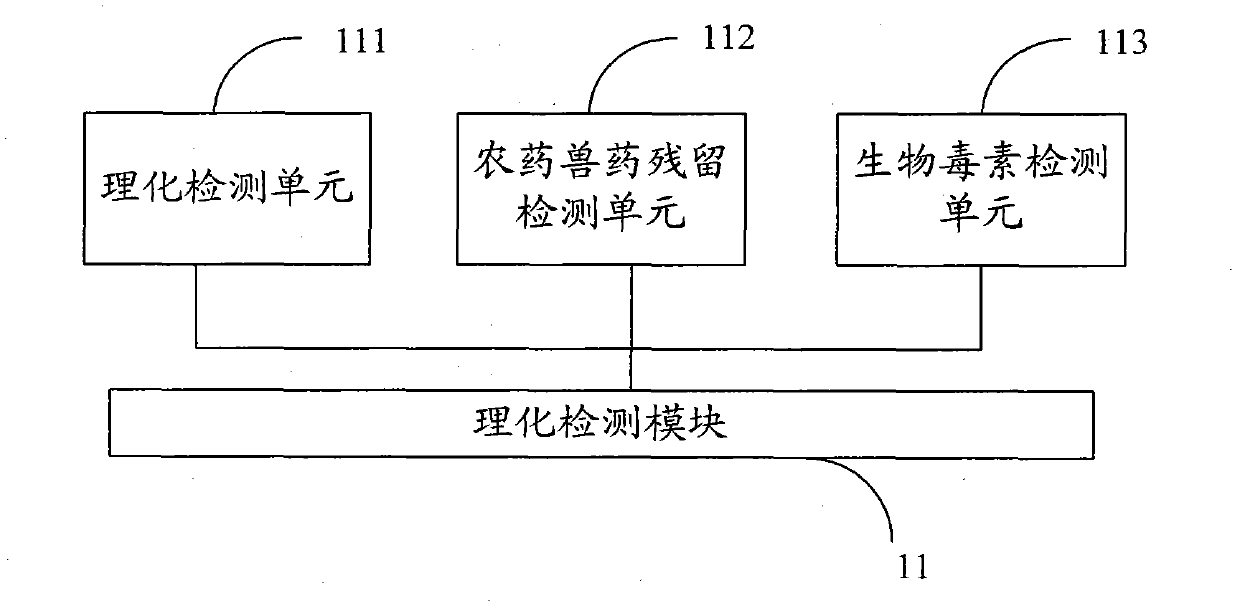
ActiveCN103323584AMeet the needs of security routine testingThe detection process is fastColor/spectral properties measurementsBiological testingInformatizationTemperature control
The invention belongs to the field of rapid food safety detection, and provides a digital rapid food safety detection system, which mainly comprises a detection host machine unit, a sample pretreatment unit and a power supply module, can be provided for detecting a plurality of physical and chemical items, pesticide and veterinary drug residues, and biotoxins, has characteristics of rapid detection, high sensitivity, high informatization degree, and sample treatment-sample detection-informatization management integration, is an optimal selection for safe and rapid on-site detection, meets requirements of enterprise laboratories on food safety routine detection, and has strong promotion and application values. In addition, the detection host machine unit has the following characteristics that: temperature control precision and rotation speed control precision are high, functions of precise timing, short shock inching, temperature calibration and power-down recovery are provided, three functions such as constant temperature maintaining, oscillation and timing can be manually switched, the machine with a plurality of uses can be achieved, and equipment utilization can be increased.
Owner:北京倍肯恒业科技发展股份有限公司
Animal feed mold removal agent and preparation method thereof
InactiveCN106689982AImprove adsorption capacityStable formAnimal feeding stuffAccessory food factorsMycotoxinEnzyme
The invention discloses an animal feed mold removal agent and a preparation method thereof and belongs to the technical field of husbandry veterinary drugs. According to a key point of the technical scheme, the animal feed mold removal agent is prepared from, by weight parts, 50-100 parts of live protection and kidney protection ingredient, 150-200 parts of mycotoxin adsorbent, 150-300 parts of probiotics, 100 parts of biological enzyme and 350-500 parts of nutritive additive. The invention further discloses the method for preparing the animal feed mold removal agent. The animal feed mold removal agent obtained according to the method can adsorb various mycotoxins in the intestinal tract, is high in adsorption capacity, wide in applied range and stable in form and can be completely and quickly decomposed and excreted by animal organisms.
Owner:XINXIANG TIANXIANG PHARMA CO LTD
Consumable veterinary medicine delivery device
InactiveUS20070298077A1High viscoelasticityLow viscosityAnimal feeding stuffAccessory food factorsOral medicationMedicine
A medicine delivery device for oral administration of a medication, the device comprising an edible outer shell including a container piece comprised of a wall bounded by an outer surface, an inner surface, and a mating surface, and a recessed cavity formed within the inner surface; and a cap piece comprised of a cap wall partially bounded by a mating surface; and an edible paste comprising a first portion disposable on at least one of the mating surfaces of the container piece and the cap piece, and a second portion disposable within the recessed cavity of the container piece. A medicament is embedded in the second portion of edible paste, and the mating surfaces of the cap piece and the container piece are joined by the first portion of the edible paste, thereby forming a medicament cavity within the edible outer shell, within which the medicament is contained.
Owner:JONES BRADLEY E
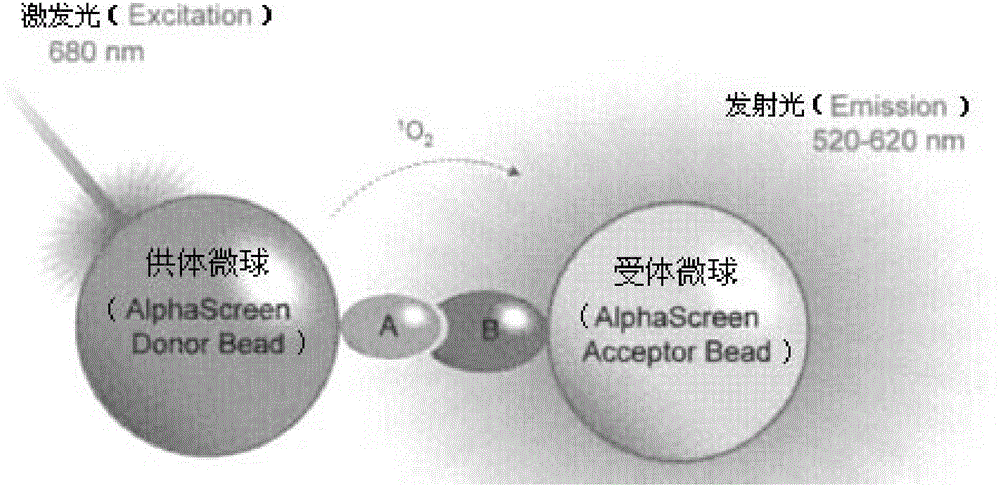
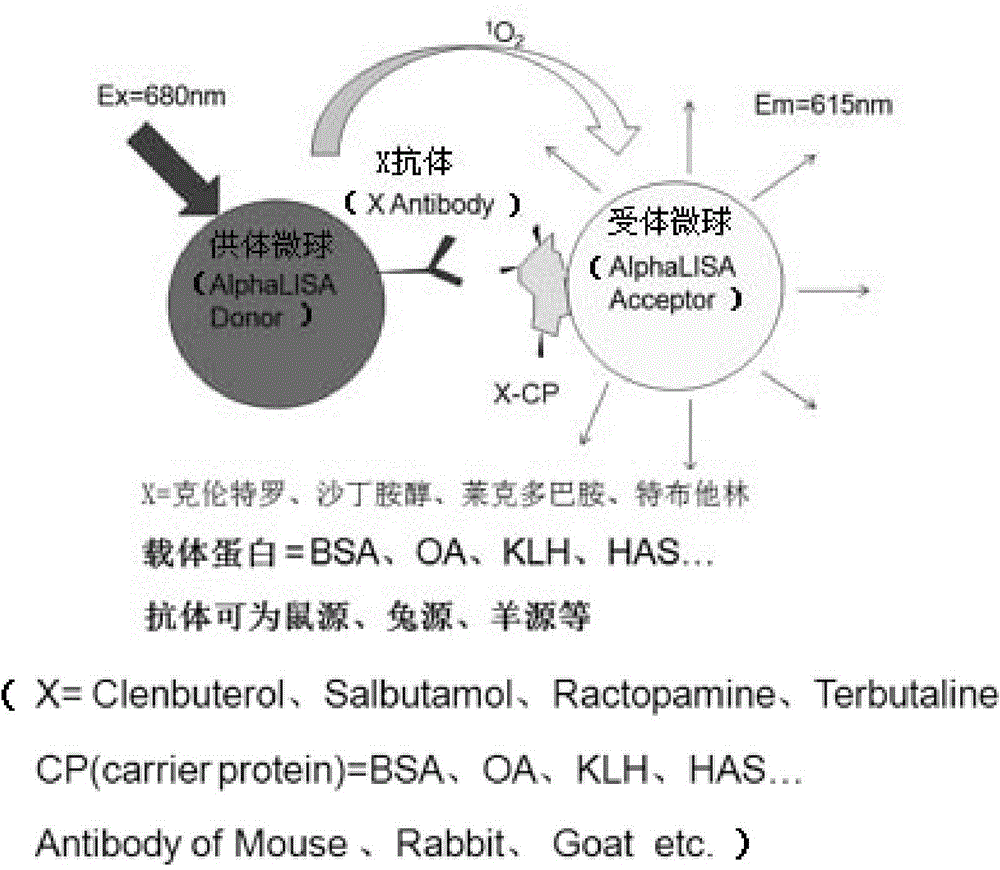
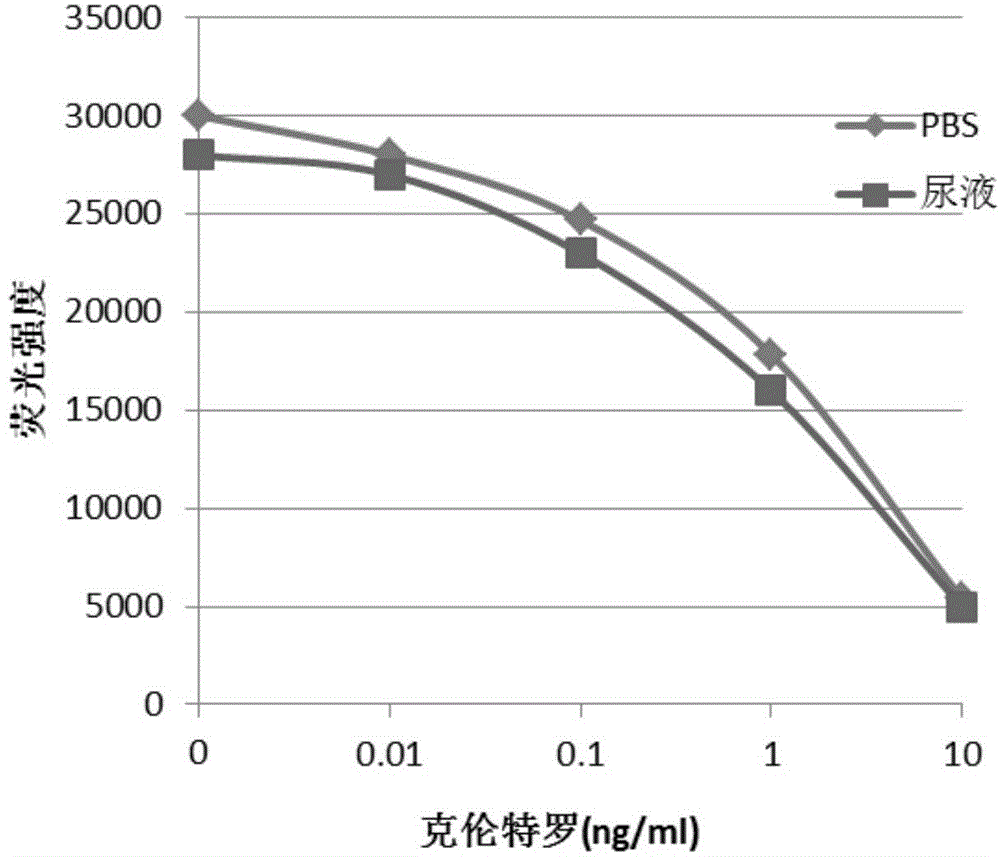
One-step homogeneous chemiluminescent detection method for micromolecule and particle used therein
InactiveCN104897652ALow costLong storage timeChemiluminescene/bioluminescenceSalbutamolCarrier protein
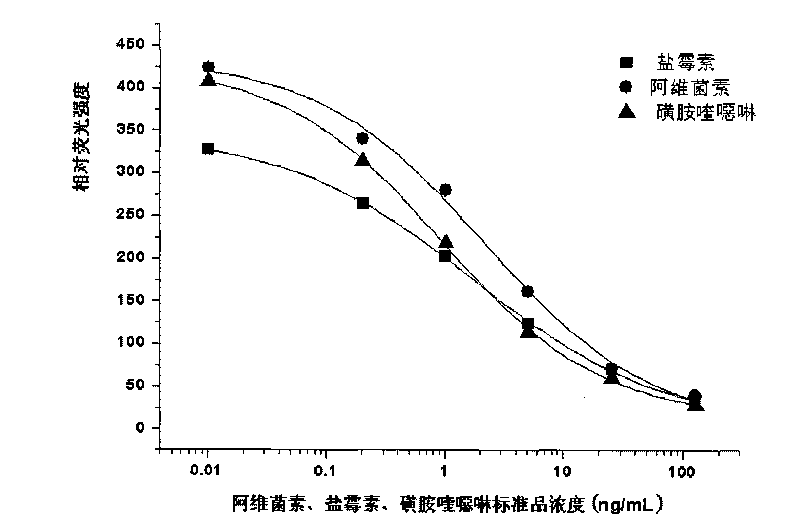
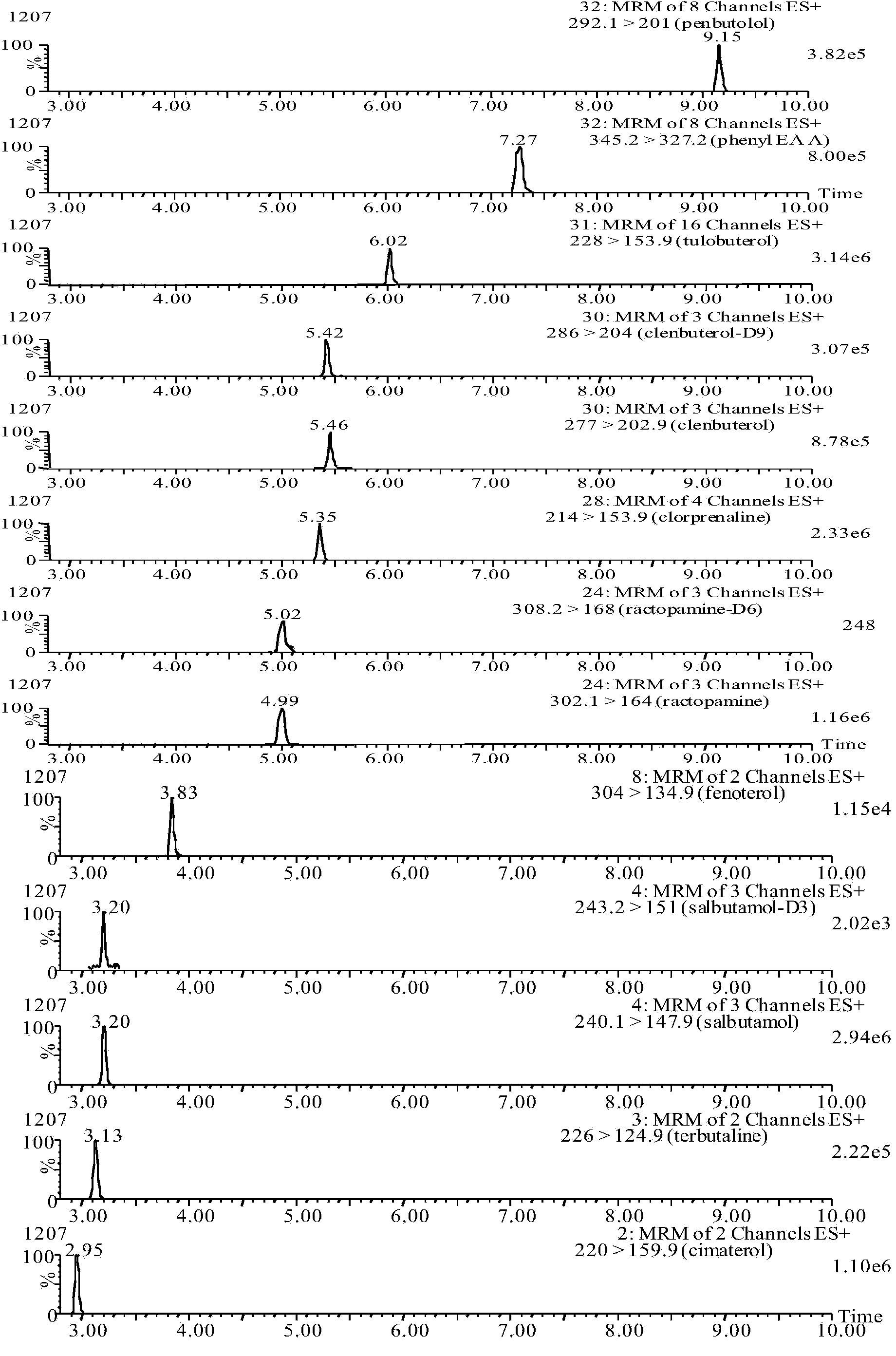
The invention discloses a particle used for detection of a veterinary drug micromolecule. The particle comprises a receptor particle and a donor particle and is prepared through the following steps: marking carrier protein with a veterinary drug molecule; coupling the veterinary drug molecule-marked veterinary drug with a homogeneous chemiluminescent receptor ball; and preparing a homogeneous chemiluminescent receptor ball coupled with a veterinary drug molecule antibody. The invention further discloses a one-step homogeneous chemiluminescent method for detection of the micromolecule with the particle. The one-step homogeneous chemiluminescent method is used for homogeneous, rapid and high-sensitivity detection of veterinary drug residue and for testing of the contents of frequently used veterinary drug components in feeds, e.g., clenbuterol, ractopamine, salbutamol and terbutaline.
Owner:HANGZHOU JINXI BIOLOGICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com