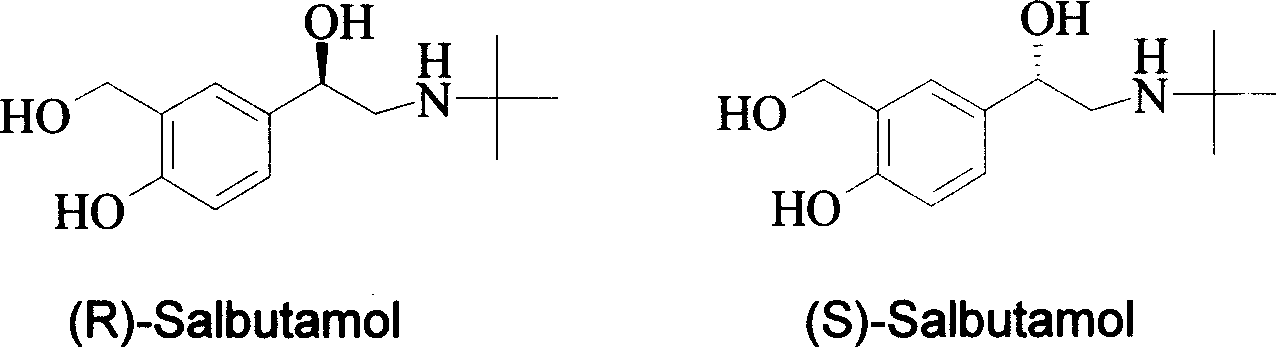Patents
Literature
227 results about "Salbutamol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
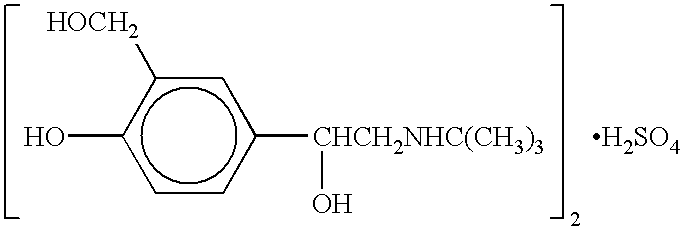
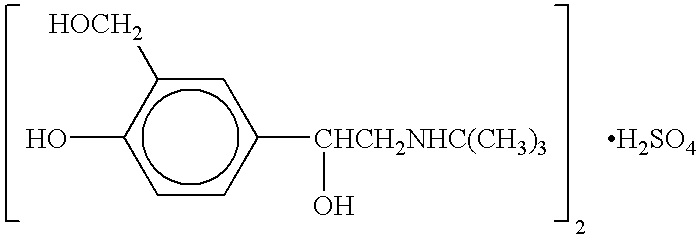
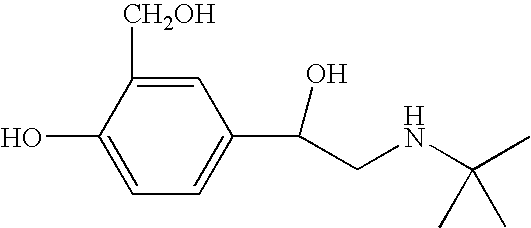
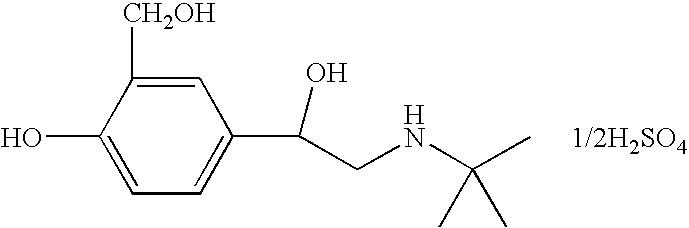
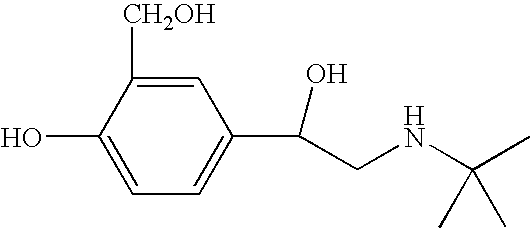
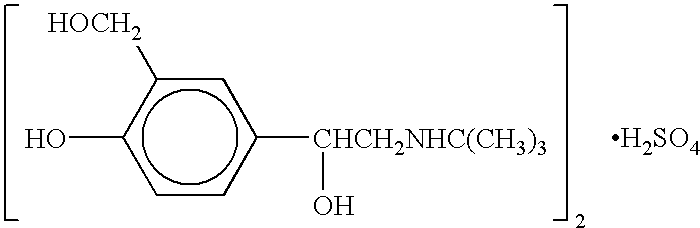
Albuterol (also known as salbutamol) is used to treat wheezing and shortness of breath caused by breathing problems (e.g., asthma, chronic obstructive pulmonary disease).
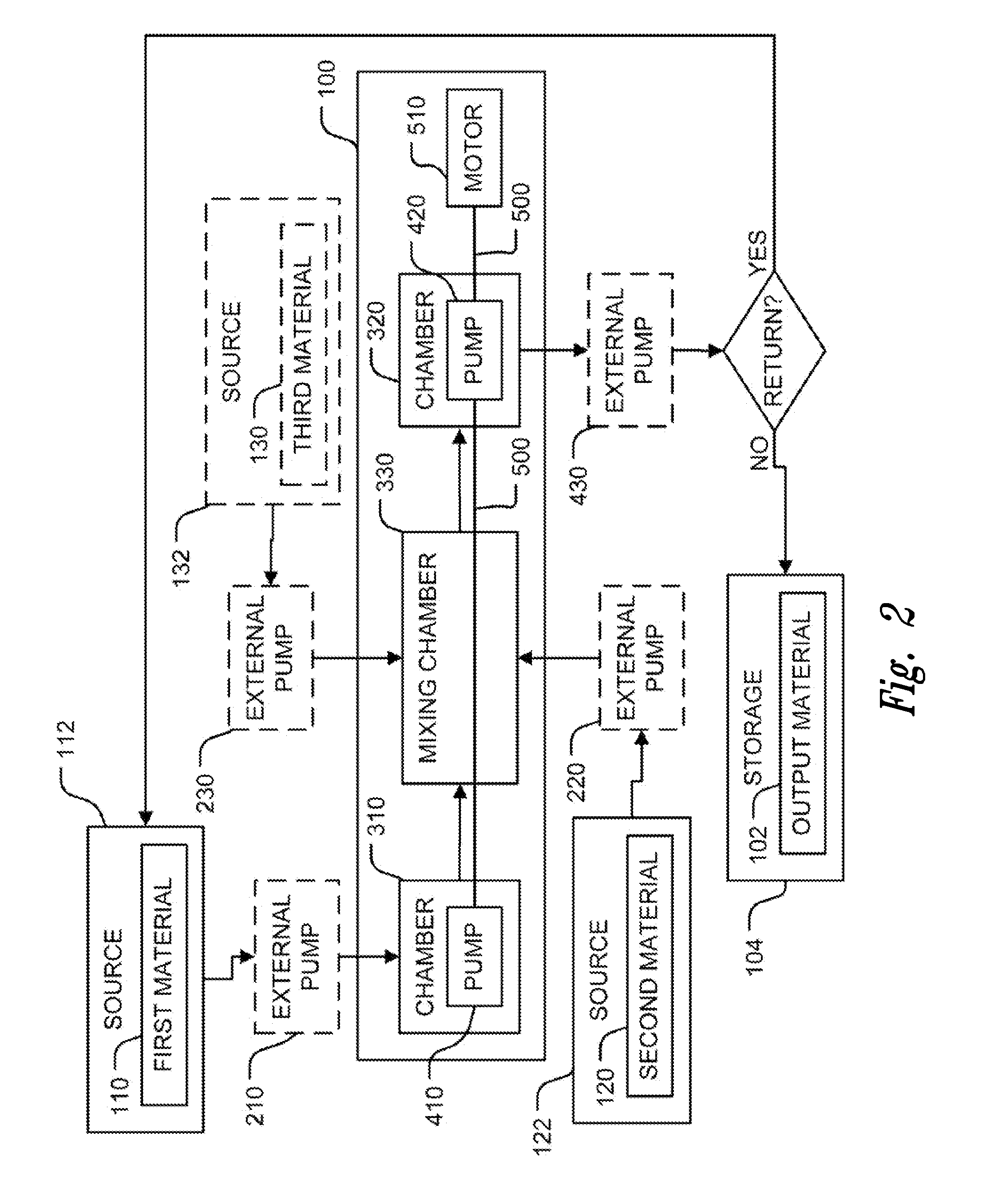
Compositions and methods for treating cystic fibrosis
InactiveUS20100303917A1Increased mucus secretionReduce airflowBiocidePowder deliveryTobramycinCell membrane
Provided are electrokinetically-altered fluids (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide, upon contact with a cell, modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for using same in treating cystic fibrosis or a symptom thereof. The electrokinetically-altered fluid compositions and methods include electrokinetically-altered fluids optionally in combination with other therapeutic agents (e.g., antibiotics, albuterol, budesonide, etc.). Particular embodiments comprise use and / or synergy with tobramycin for treating bacterial infection, and use and / or synergy with a bronchiodilator. In certain aspects, the methods comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins (like, membrane receptors, including but not limited to G protein coupled receptors, and intercellular junctions).
Owner:REVALESIO CORP
Compositions and methods for treating cystic fibrosis
Particular aspects provide electrokinetically-generated fluids (e.g., electrokinetically-generated gas-enriched fluids and solutions), and therapeutic compositions and methods comprising use thereof in treating at least one symptom of cystic fibrosis. In particular embodiments, at least one symptom of cystic fibrosis treated by the present invention include inhibition of Pseudomonas infection, synergy with tobramycin (including TOBI) for use against bacterial infection, and synergy with a bronchiodilator. In particular embodiments, the electrokinetically-generated fluids or therapeutic compositions and methods comprise combination with other therapeutic agents (e.g., antibiotics, albuterol, budesonide, etc.). In certain aspects, the methods comprise regulating or modulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins such as membrane receptors, including but not limited to G protein coupled receptors, and intercellular junctions (e.g., tight junctions, gap junctions, zona adherins and desmasomes).
Owner:REVALESIO CORP
Aerosol formulations of albuterol and 1,1,1,2-tetrafluoroethane
Aerosol formulations substantially free of chlorofluorocarbons, for oral and / or nasal administration are described. The formulations comprise 1,1,1,2 tetrafluoroethane, a medicament, optionally an excipient and optionally a surfactant. Methods of treatment utilizing the formulations also are described.
Owner:SCHERING CORP
Albuterol inhalation solution, system, kit and method for relieving symptoms of pediatric asthma
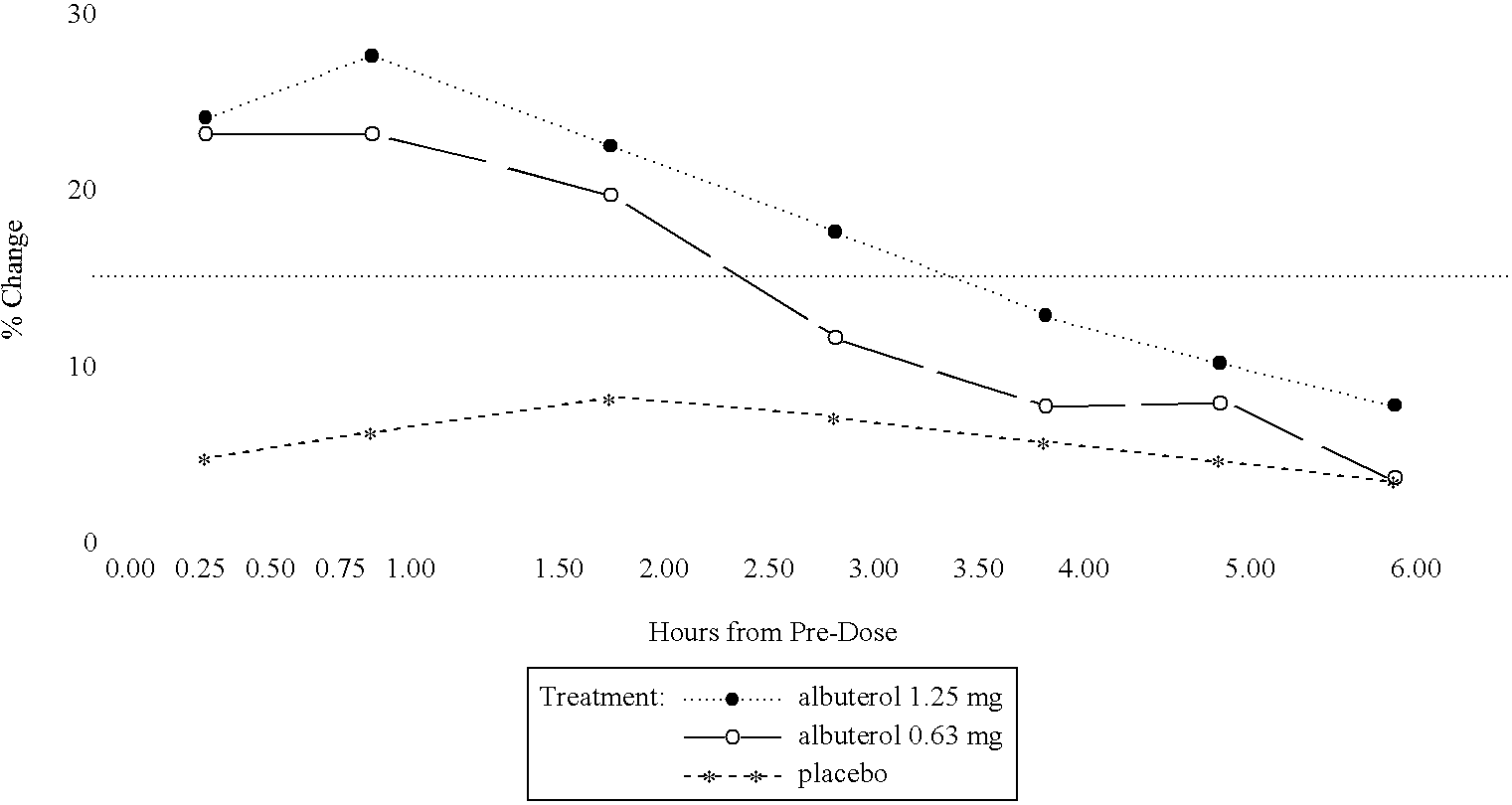
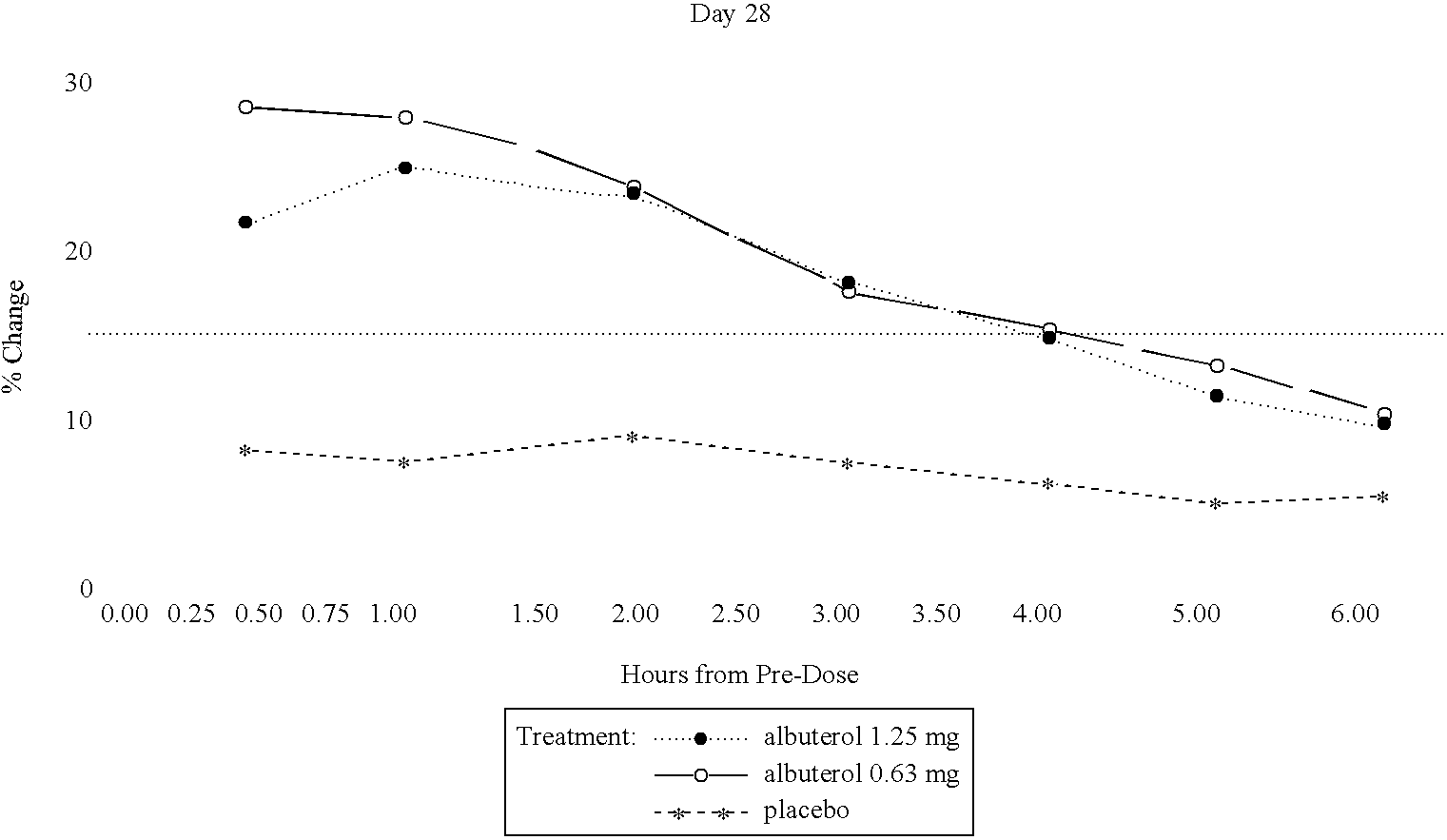
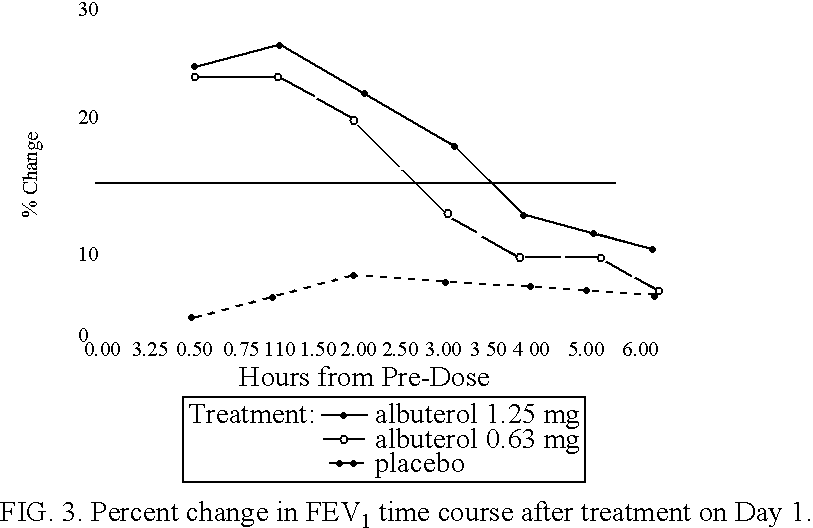
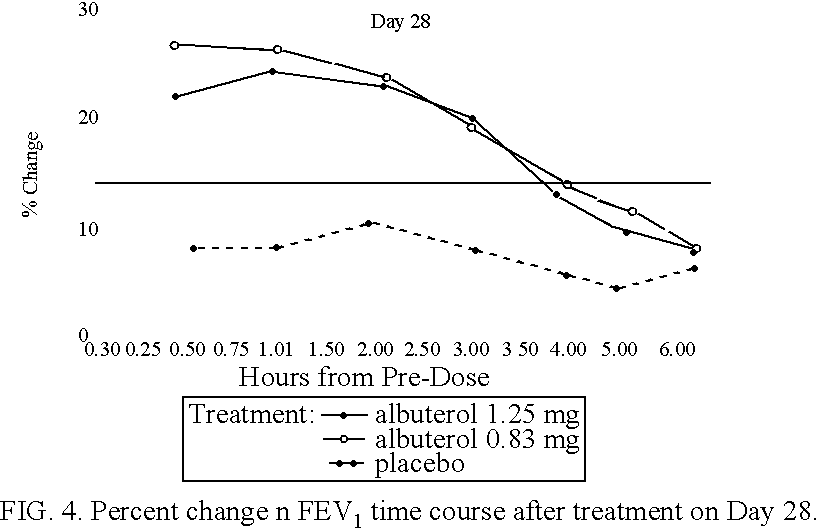
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.63 mg or about 1.25 mg albuterol.
Owner:MYLAN SPECIALTY
Method of decreasing fat deposits and body weight in mammals and birds
InactiveUS20050113456A1Reduce riskIncreased intraocular pressureBiocideOrganic active ingredientsSalbutamolPhysiology
A method is disclosed utilizing an optically pure eutomer of salbutamol for reducing body fat and / or body weight in mammals and birds. A food composition including the eutomer is also disclosed.
Owner:ALTERAGON
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20030191151A1Relieve bronchospasmBiocideDispersion deliveryBenzalkonium chlorideSalbutamol
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:CHAUDRY IMTIAZ +1
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS6632842B2Relieve bronchospasmBiocidePowder deliveryBronchospasmObstructive Pulmonary Diseases
Owner:MYLAN SPECIALTY
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20030149007A1Relieve bronchospasmBiocidePowder deliveryBronchospasmObstructive Pulmonary Diseases
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:MYLAN SPECIALTY
Oral solid preparation containing ambroxol hydrochloride and salbutamol active components
InactiveCN101099729APlace stableEasy to carryPowder deliveryOrganic active ingredientsDiseaseRespiratory tract disease
The present invention discloses an oral solid preparation containing ambroxol hydrochloride and salbutamol active components. It is formed from ambroxol hydrochloride, salbutamol and auxiliary material according to a certain mixing ratio. Said oral solid preparation has the obvious synergistic action for remitting the symptoms of dyspnea, etc. due to respiratory tract obstruction diseases of bronchial asthma, chronic bronchitis and pulmonary emphysema, etc.
Owner:GUANGZHOU LIXIN PHARM CO LTD
Stabilized albuterol compositions and method of preparation thereof
InactiveUS20040109826A1Extended shelf lifeBiocideOrganic active ingredientsSalbutamolBuffering agent
Stabilized albuterol compositions are provided. The compositions are aqueous inhalation compositions containing albuterol; a buffer, such as citric acid; and a metal chelator, such as EDTA.
Owner:DEY
Clenobuterol hydrochloride, salbutamol and paylean three joint inspection card and method for processing detecting sample
The invention relates to a clenobuterol hydrochloride, salbutamol and paylean three joint inspection card and a method for processing a detecting sample, and belongs to the technical field of detection of a beta-receptor stimulating agent, wherein a test strip is arranged inside a shell of the three joint inspection card, and the clenobuterol hydrochloride, salbutamol and paylean three joint inspection card is formed by the adhesion of a sample gasket, a colloidal gold membrane, a cellulose nitrate membrane and a water absorbing membrane to a bearing backboard in turn; the colloidal gold membrane is a glass fiber membrane of a colloidal gold marker containing a clenobuterol hydrochloride antibody, a salbutamol antibody and a paylean antibody; three detection strips are arranged on the cellulose nitrate membrane and contain clenobuterol hydrochloride protein conjugate, salbutamol protein conjugate and paylean protein conjugate respectively; and a quality control strip containing an anti-rabbit antibody or an anti-mouse antibody is arranged. The clenobuterol hydrochloride, salbutamol and paylean three joint inspection card has the advantages of simultaneously detecting the clenobuterol hydrochloride, the salbutamol and the paylean in urine or feed, animal tissue, meat and liver. The inspection card is easy to prepare and quick and convenient to use, saves the detection cost, and has accurate result.
Owner:无锡安迪生物工程有限公司
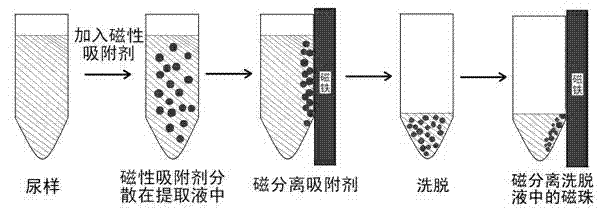
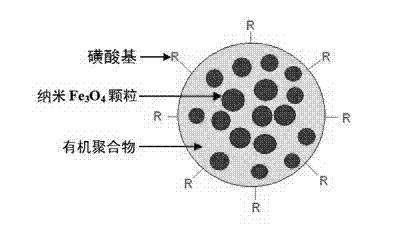
Method for enriching clenbuterol, ractopamine and salbutamol in urine sample of breeding livestock
InactiveCN103076416AEfficient enrichmentThe pre-processing process is simpleComponent separationPretreatment methodPolystyrene
The present invention discloses a method for enriching clenbuterol, ractopamine and salbutamol in a urine sample of breeding livestock, and relates to the field of analytical chemistry, particularly to a sample pretreatment method. The method comprises: carrying out a sample pretreatment, adding sulfonic group surface-modified polystyrene superparamagnetic nanometer beads or sub-micron beads to adsorb, carrying out elution, and collecting the eluent, wherein the elution solution can be directly used for liquid chromatography-mass spectrometry to quantitatively analyze contents of clenbuterol, ractopamine and salbutamol. The method has characteristics of simple operation process, high extraction recovery rate, less elution solvent use amount, and no requirement of rotary evaporation.
Owner:NANCHANG UNIV
Kit for fluorescence quantitative detection of salbutamol and preparation method of fluorescence labeling liquid
The invention discloses an immunochromatography kit for fluorescence quantitative detection of salbutamol and a preparation method of a fluorescence labeling liquid. The kit comprises a test strip and a fluorescence labeling liquid, wherein the test strip is formed by sequentially lapping and sticking a sample pad, a nitrocellulose coating film and absorbent paper on a bottom plate; the nitrocellulose coating film comprises a detection area and a quality control area; the detection area is coated with an SAL-BSA conjugate; the quality control area is coated with anti-rabbit IgG; and the fluorescence labeling liquid contains a fluorescence labeling SAL antibody and a fluorescence labeling rabbit IgG. Compared with an immune colloidal gold labeling test strip, the kit disclosed by the invention has the advantages of higher sensitivity, accurate quantification and the like; and the operation is faster and more convenient than that of an enzyme coupling method.
Owner:GUANGZHOU WONDFO BIOTECH
Compositions and methods for treating asthma and other lung disorders
InactiveUS20120114702A1Sufficient amountAntibacterial agentsPowder deliveryObstructive Pulmonary DiseasesOxygen
Provided are compositions and methods for treating lung or respiratory disorders or conditions characterized by airflow obstruction or limitation, or symptoms thereof (e g, asthma, rhinitis, allergic rhinitis, and chronic obstructive pulmonary disease (CaPO) and CaPO-associated conditions (e g, bronchitis, emphysema, asthma), emphysema, pneumonia, bronchitis, in-fluenza, SARS, tuberculosis, and whooping cough (pertussis), and the like) comprising administering a therapeutic composition comprising at least one electrokinetically altered fluid comprising an ionic aqueous solution of charge-stabilized oxygen containing nanostructures as disclosed herein, or comprising administering a nonelectrokinetic superoxygenated aqueous solution The methods preferably comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins (e g, membrane receptors, (e g, G protein-coupled receptors, and intercellular junctions)) Additional aspects include therapeutic compositions, and combination treatment methods comprising administration of electrokinetically generated fluid in combination with at least one additional therapeutic agent (e g, albuterol, etc).
Owner:REVALESIO CORP
Combination of a beta-2-adrenoceptor agonists and an aminosugars and their use for the treatment immunomodulatory disorders
InactiveUS20050130935A1Suppresses hypersensitivitySuppresses inflammatory reactionBiocideNervous disorderDiseaseAdrenergic
The invention relates to combinations of an aminosugar and a beta-2-adrenoceptor agonist, such as salbutamol, for the treatment of diseases associated with hypersensivity and inflamation, in particular hypersensivity skin diseases. The aminosugar is preferably a monosaccharide derivative.
Owner:ASTION DEV
Tiotropium aerosol inhalant and its preparation method
InactiveCN1557308APromote absorptionImprove complianceOrganic active ingredientsAerosol deliveryIrritationSodium cromoglicate
Owner:SHANGHAI PUKANG PHARMA +2
Albuterol inhalation solution, system, kit and method for relieving symptoms of pediatric asthma
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.63 mg or about 1.25 mg albuterol.
Owner:MYLAN SPECIALTY
A group of oligonucleotide aptamers for identifying clenbuterol hydrochloride, salbutamol and ractopamine with high specificity
ActiveCN105886512AEasy accessAccurate acquisitionBiological testingDNA/RNA fragmentationAptamerFluorescence
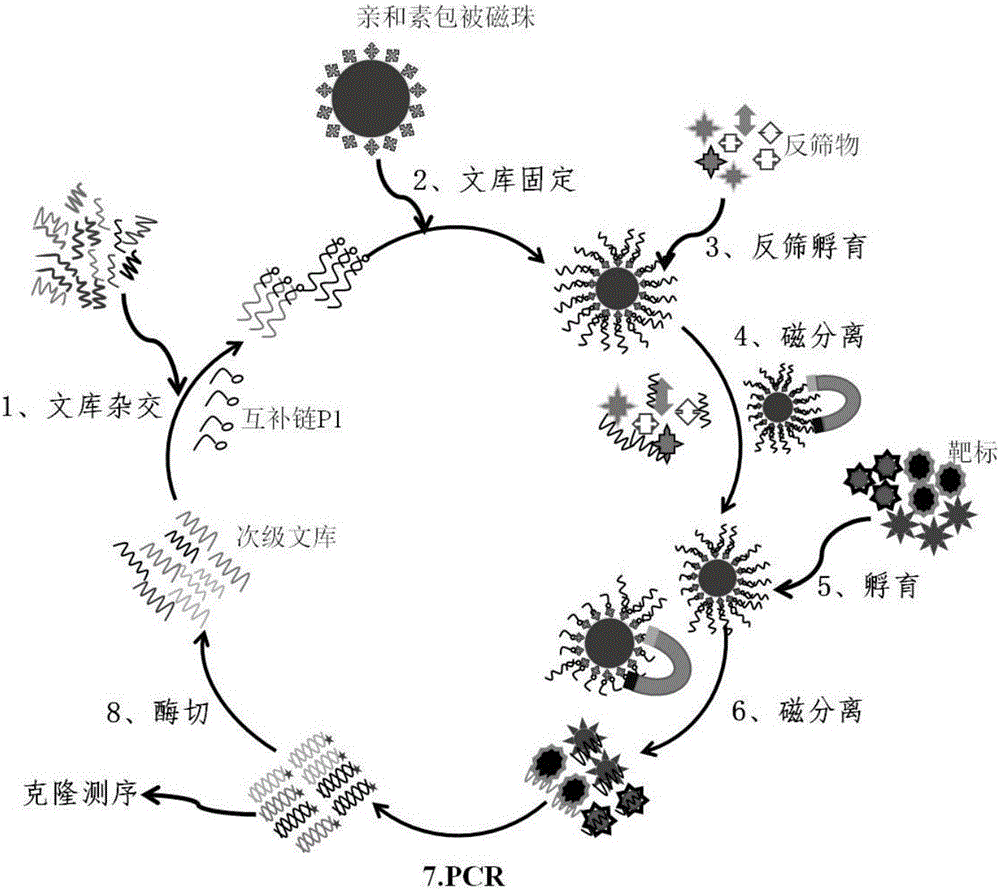
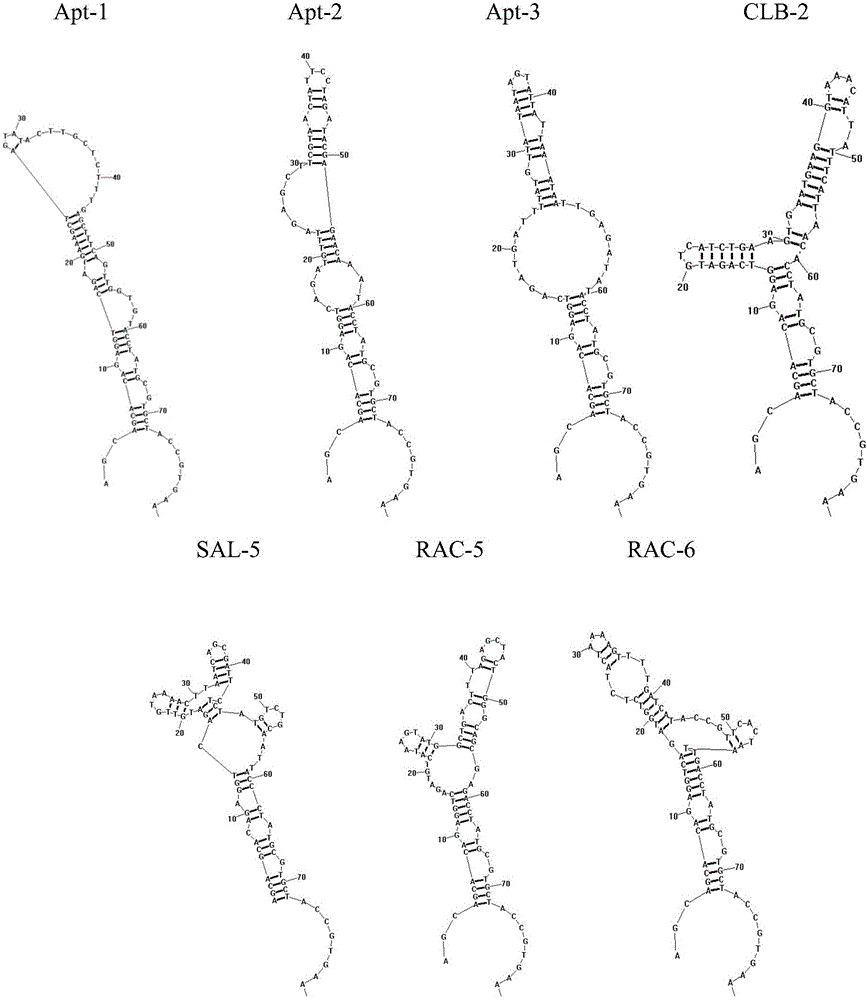
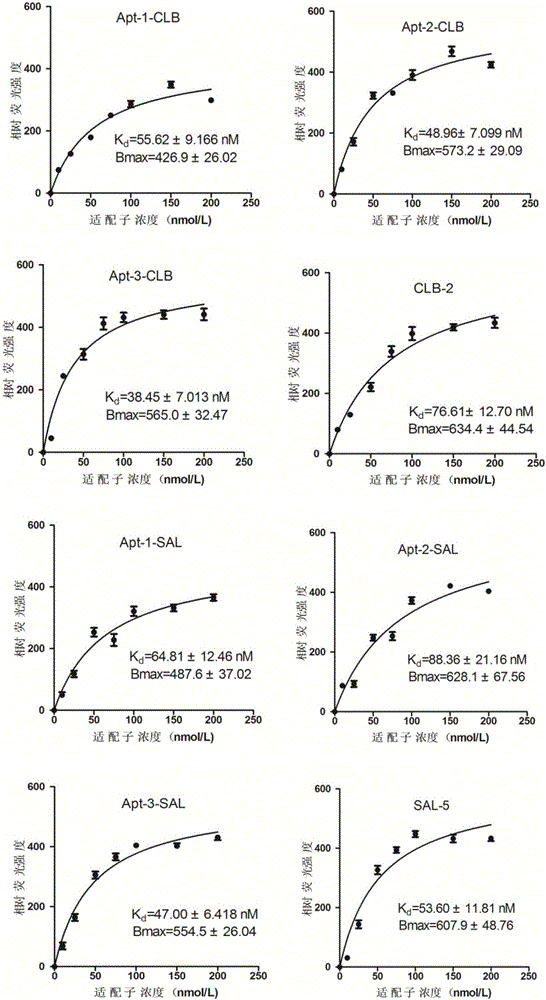
The invention provides a group of oligonucleotide aptamers Apt-1, Apt-2 and Apt-3 which are capable of simultaneously identifying clenbuterol hydrochloride and salbutamol, an oligonucleotide aptamer CLB-2 which is capable of identifying the clenbuterol hydrochloride with high specificity, an oligonucleotide aptamer SAL-5 which is capable of identifying the salbutamol with high specificity and two oligonucleotide aptamers RAC-5 and RAC-6 which are capable of identifying ractopamine with high specificity. Through an SELEX (Systematic Evolution of Ligands by Exponential Enrichment) technology based on Fe3O4 magnetic nanoparticle separation, a random oligonucleotide library is immobilized on avidin-enveloped magnetic nanoparticles by virtue of a complementary chain of a biotinylation marker, and the oligonucleotide aptamers, which are high in specific affinity, are finally obtained by conducting screening by 16 turns. The aptamers are broad in application prospect; and the aptamers, by virtue of marker function genes or fluorescent dyes, are applicable to detection of the clenbuterol hydrochloride, the salbutamol and the ractopamine in food; therefore, a new choice is provided for existing detection methods which depend on antibodies.
Owner:JIANGNAN UNIV
Preparation method of electrochemical immunosensor for detecting salbutamol quickly
InactiveCN101915792AFacilitates electron transferFixedBiological testingMaterial electrochemical variablesSalbutamolCarbon nanotube
The invention discloses a preparation method of an electrochemical immunosensor for detecting salbutamol quickly, which belongs to the technical field of chemical detection. In the preparation method, a glassy carbon electrode is modified by carbon nanotubes, polythionine and nano gold in turn, and the electrochemical immunosensor is obtained by a self-assembly process. The invention can prepare the electrochemical immunosensor which has high sensitivity and stability and can detect salbutamol on site quickly.
Owner:SHANGHAI JIAO TONG UNIV
Synthesis method of specific salbutamol artificial antigen
InactiveCN103145831AStrong specificityHigh sensitivityOvalbuminSerum albuminOrtho positionSynthesis methods
The invention discloses a synthesis method of a specific salbutamol artificial antigen, belonging to the technical field of biological chemical engineering. The synthesis method disclosed by the invention comprises the following steps of: activating salbutamol through a formaldehyde solution, and connecting 6-aminocaproic acid in the ortho-position of a phenolic hydroxyl group to obtain a salbutamol hapten; and coupling a carboxyl group on the salbutamol hapten with an amino group on a carrier protein to obtain the salbutamol artificial antigen. The synthesis method disclosed by the invention can make up for the insufficiencies and defects of the existing salbutamol antigen synthesis technologies, the salbutamol artificial antigen with high specificity is obtained, the specificity of a produced antibody is high, and the sensitivity is high; and experimental results show that the antiserum titre of an animal immunized by using the salbutamol artificial antigen disclosed by the invention can achieve 80000, the detection limit is 0.5ng / mL, and the half-inhibitory concentration IC50 is 5ng / mL. The antigen or the antibody disclosed by the invention can be used for establishing an enzyme-linked immunosorbent analytical method and a colloidal gold test strip rapid assay method so as to rapidly detect the residues of the salbutamol in a food and further realize broad application prospects.
Owner:JIANGNAN UNIV
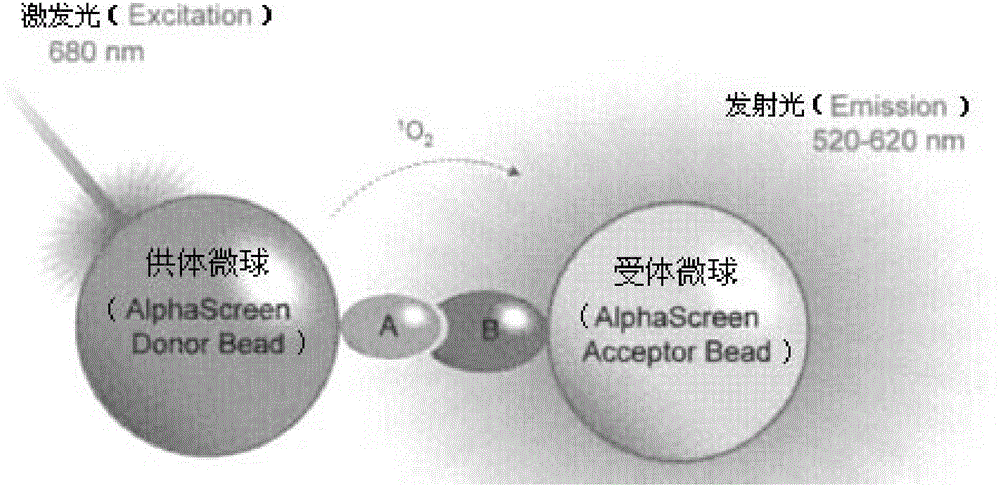
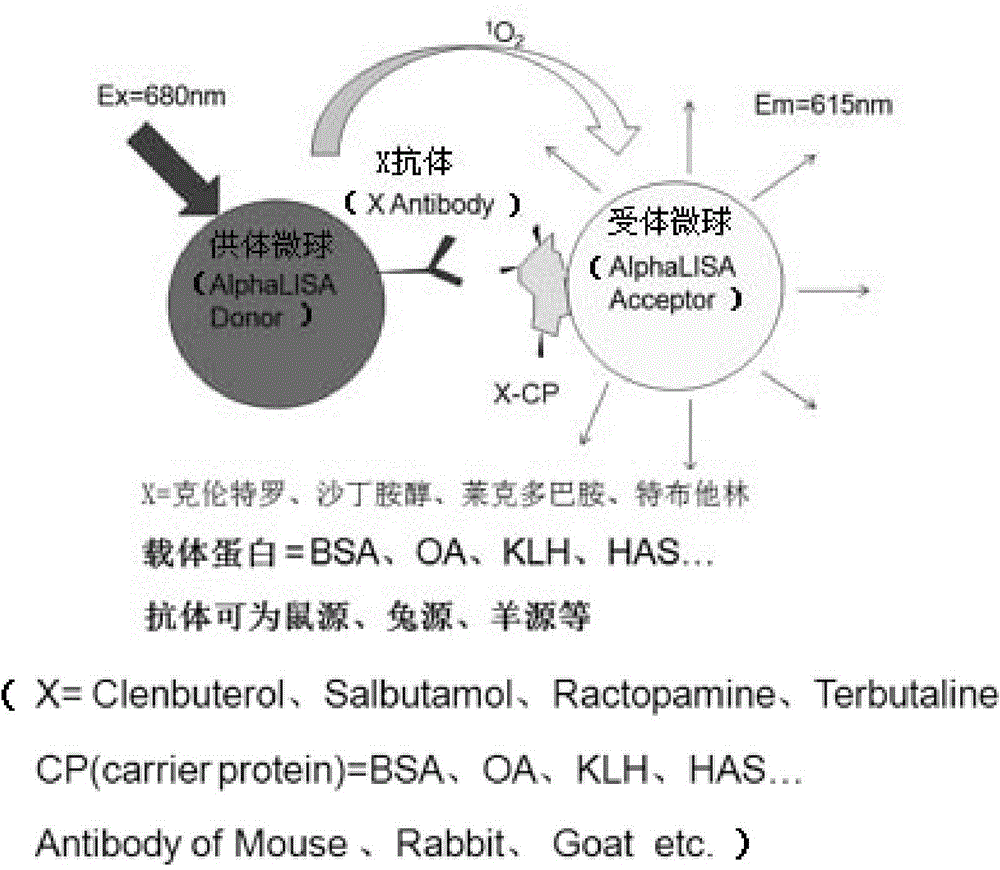
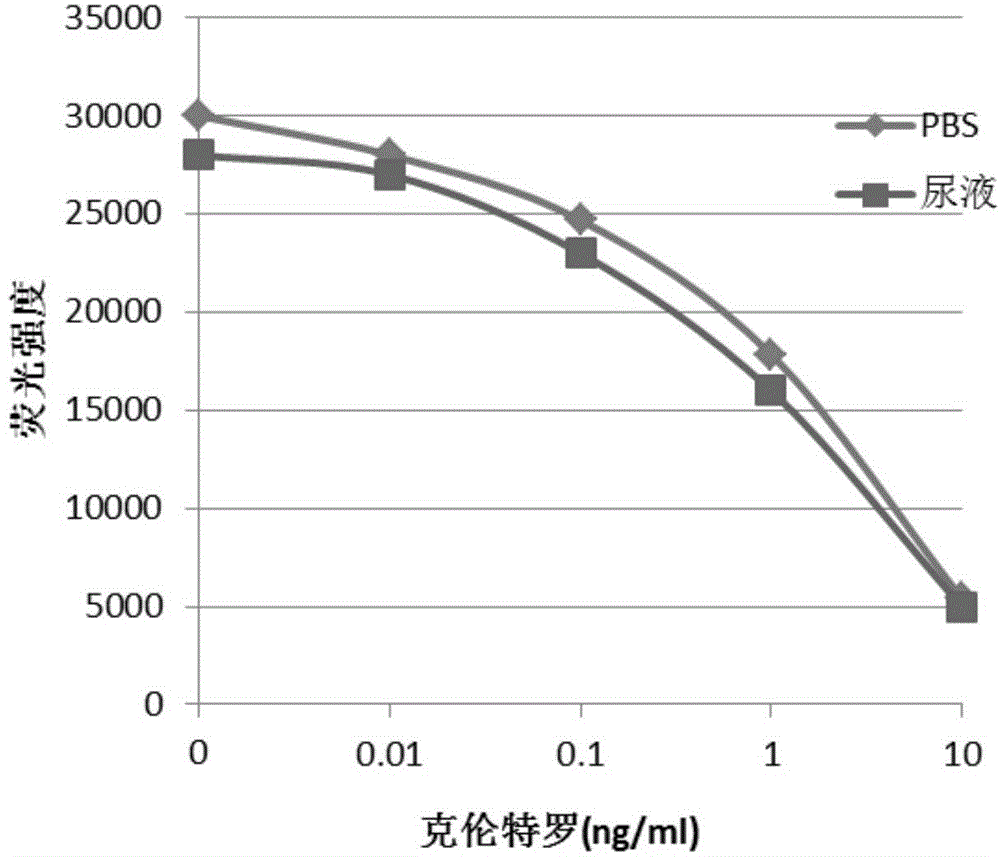
One-step homogeneous chemiluminescent detection method for micromolecule and particle used therein
InactiveCN104897652ALow costLong storage timeChemiluminescene/bioluminescenceSalbutamolCarrier protein
The invention discloses a particle used for detection of a veterinary drug micromolecule. The particle comprises a receptor particle and a donor particle and is prepared through the following steps: marking carrier protein with a veterinary drug molecule; coupling the veterinary drug molecule-marked veterinary drug with a homogeneous chemiluminescent receptor ball; and preparing a homogeneous chemiluminescent receptor ball coupled with a veterinary drug molecule antibody. The invention further discloses a one-step homogeneous chemiluminescent method for detection of the micromolecule with the particle. The one-step homogeneous chemiluminescent method is used for homogeneous, rapid and high-sensitivity detection of veterinary drug residue and for testing of the contents of frequently used veterinary drug components in feeds, e.g., clenbuterol, ractopamine, salbutamol and terbutaline.
Owner:HANGZHOU JINXI BIOLOGICAL TECH
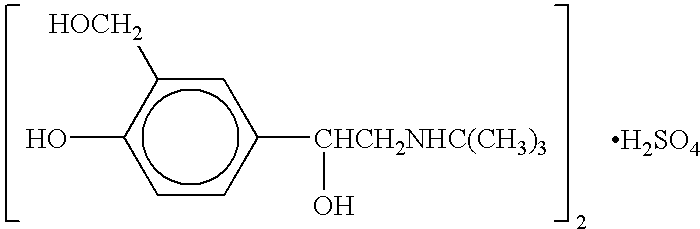
New process for synthesizing salbutamol and sulfate of salbutamol
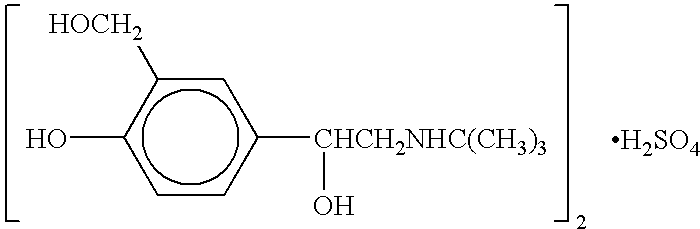
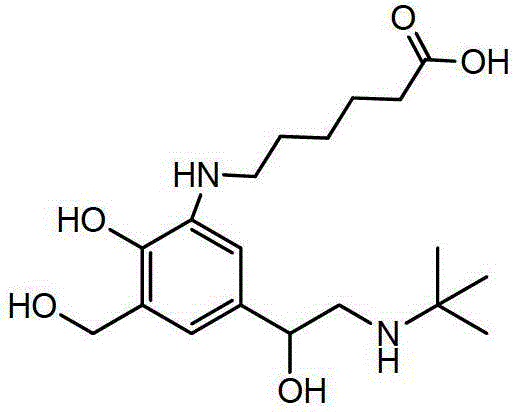
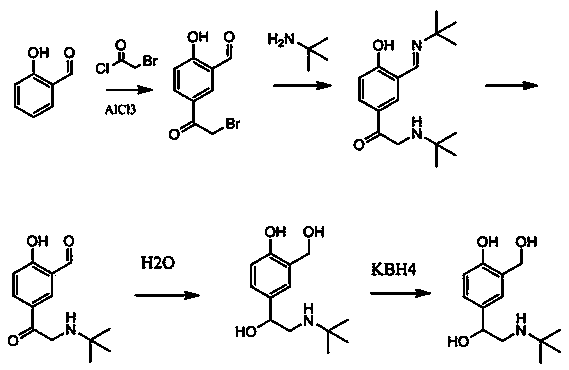
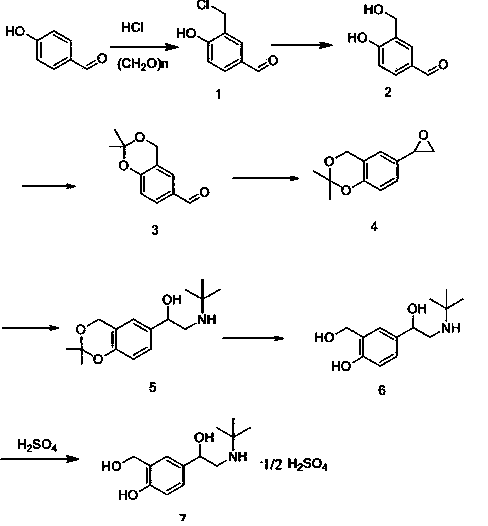
ActiveCN103951568AMild reaction conditionsEasy to purifyOrganic compound preparationAmino-hyroxy compound preparationBenzaldehydeAminolysis
The invention discloses a new process for synthesizing salbutamol. The process comprises the steps that (1) chloromethylation reaction: reactants namely p-hydroxy benzaldehyde and paraformaldehyde react under an acidic condition to generate a compound 1; (2) hydrolysis reaction: the compound 1 is subjected to hydrolysis reaction under a weakly alkaline condition to generate a compound 2; (3) propylidene protective reaction: dihydroxy of a reactant 2 is subjected to propylidene protection under catalysis of concentrated sulfuric acid; (4) epoxidation reaction: a reactant 3 reacts to obtain a compound 4 by using the effect of strong base under the effects of a ylide reagent and a phase transfer catalyst; (5) aminolysis ring-opening reaction: the compound 4 is heated and refluxed in tert-butylamine, and is subjected to aminolysis ring-opening reaction to obtain a compound 5; (6) hydrolysis de-protective reaction: the compound 5 is subjected to hydrolysis reaction under the acidic condition to obtain salbutamol. The new process disclosed by the invention is mild in reaction condition, easy in purification, and simple and easily-available in raw materials; the total molar yield of the process reaches up to 45%, and the product quality reaches up to more than 99.5%.
Owner:SUZHOU HOMESUN PHARMA
Method for quantitatively detecting salbutamol by using CdTe quantum dots
ActiveCN104792762AEnables trace detectionHigh sensitivityFluorescence/phosphorescenceSolution systemQuantum dot
The invention provides a method for quantitatively detecting salbutamol by using CdTe quantum dots. The method comprises the following steps: uniformly mixing the CdTe quantum dots with surfaces modified by mercaptoacetic acid with a substance to be detected, namely salbutamol, in a phosphate buffer solution, and then detecting fluorescent strength of a system by adopting a molecular fluorometer to realize trace detection of salbutamol because that in a weakly alkaline solution system, the CdTe quantum dots with the surfaces modified with carboxyl groups having negative charges and target molecules having positive charges, namely salbutamol, form a new compound system through electrostatic interaction so as to cause fluorescence quenching. Detection results show that salbutamol has relatively high sensitivity and a relatively wide linear range against the fluorescence quenching of the CeTe quantum dots, so that the changes in the fluorescent strength of the CdTe quantum dots are used for realizing the quantitative detection of salbutamol; and furthermore, the method for quantitatively detecting salbutamol by using the CdTe quantum dots, provided by the invention, is very simple in experimental operation, the detection time is short and the analysis cost is low.
Owner:INST OF AGRI PROD QUALITY SAFETY & STANDARD JIANGXI ACAD OF AGRI SCI
Albuterol and ipratropium inhalation solution, system, kit and method for relieving symptoms of chronic obstructive pulmonary disease
InactiveUS20030203930A1Relieve bronchospasmBiocideDispersion deliveryBronchospasmObstructive Pulmonary Diseases
The present invention relates to a dual bronchodilator inhalation solution, system, kit and method for relieving bronchospasm in patients suffering from chronic obstructive pulmonary disease (COPD). In one alternative embodiment, the solution of the present invention is a prepackaged, sterile, premixed, premeasured single unit dose of albuterol and ipratropium bromide for patients suffering from COPD. The present solution may be free of antimicrobial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 2.50 mg albuterol and about 0.50 mg ipratropium bromide.
Owner:CHAUDRY IMTIAZ +1
Ternary detection test bar of beta-stimulants albuterol, clenobuterol hydrochloride and ractopamine and preparation method thereof
InactiveCN101915841ASimple methodThe result is accurateColor/spectral properties measurementsBiological testingGlass fiberSalbutamol
The invention relates to a ternary detection test bar of beta-stimulants albuterol, clenobuterol hydrochloride and ractopamine and a preparation method thereof. The problem of detecting the beta-stimulants the albuterol, the clenobuterol hydrochloride and the ractopamine simultaneously is solved. A parent material PVC plate is sequentially provided with a diversion glass fiber, a carrier glass fiber, a nitrocellulose membrane and a suction cotton pulp plate from front to back; the diversion glass fiber and the suction cotton pulp plate are covered with membranes; the carrier glass fiber absorbs a monoclonal antibody colloidal gold marker resistant to albuterol protein conjugates, clenobuterol hydrochloride protein conjugates and ractopamine protein conjugates; and the intermediate nitrocellulose membrane is provided with a rabbit anti-mouse polyclonal antibody quality control line and three detection lines which are respectively coated with the albuterol protein conjugates, the clenobuterol hydrochloride protein conjugates and the ractopamine protein conjugates. By the invention, the beta-stimulants the albuterol, the clenobuterol hydrochloride and the ractopamine can be effectively detected simultaneously with convenience, quickness and accurate result.
Owner:HENAN ACAD OF SCI INST OF BIOLOGY LIABILITY
Albuterol time controlling pulse slow release oral preparation and its preparation method
An orally taken salbutamol able to release active components in the sequence: predefined time lag, fast pulse release, and slow release is composed of a slow-release microsphere containing salbutamol, a coated quick release layer, and a coated time-lagging layer.
Owner:SHANGHAI INST OF PHARMA IND
Albuterol inhalation solution, system, kit and method for relieving symptoms of pediatric asthma
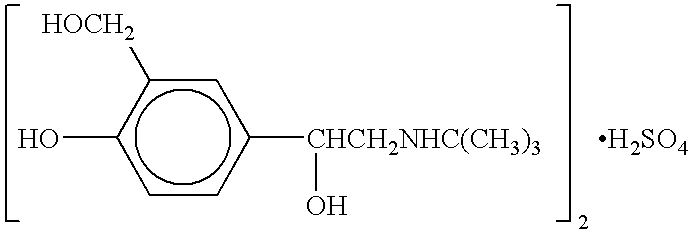
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.75 mg or about 1.5 mg albuterol sulfate.
Owner:DEY
Method for asymmetrical hydrogen transfer of alpha-imino keton for synthesizing chirality salbutamol
InactiveCN1733701AEfficient Synthesis of Chiral SalbutamolGood choiceOrganic compound preparationAmino-hyroxy compound preparationMethyl salicylateKetone
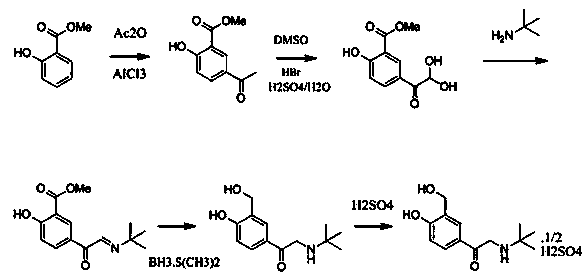
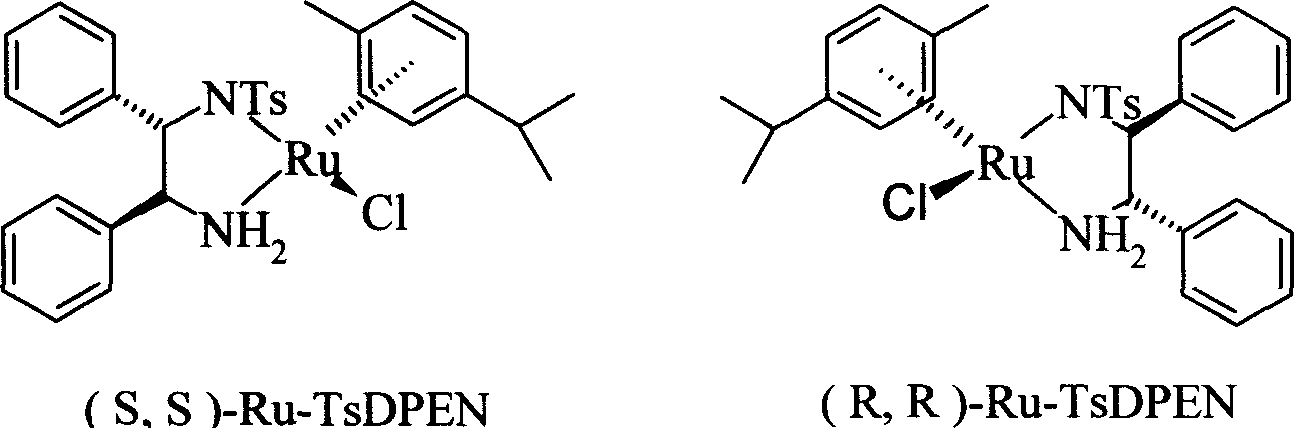
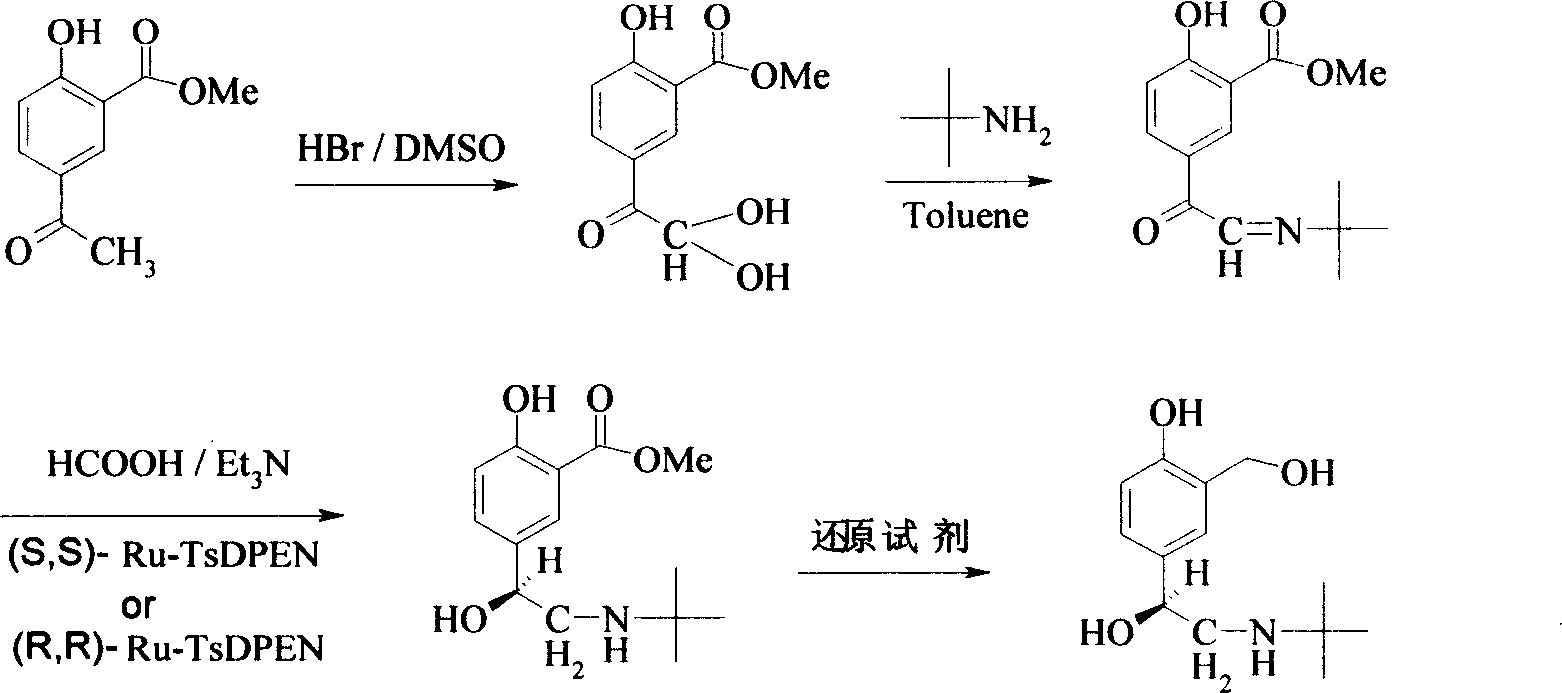
Disclosed is a method for asymmetrical hydrogen transfer of alpha-imino keton for synthesizing chirality salbutamol which comprises, using 5-acetyl methyl salicylate as raw material, oxidizing with oxidation agent into 5-(1,1-)dihydroxy)-acetyl-2-hydroxybenzoate, then reacting with tert-butylamine, obtaining alpha-imine ketone compound. Then using (S,S)-Ru-TsDPEN or (R,R)-Ru-TsDPEN as catalyst, subjecting the alpha-imine ketone compound to asymmetrical hydrogen migration in formylic acid, triethylamine and inert organic solvent system, thus obtaining the optically pure product of (R) or (S)-5-[2-[(1,1-dimethylethyl)amino]-1-ethoxyl]-2-hydroxybenzoate, finally deoxidizing the (R) or (S)-5-[2[(1,1-dimethylethyl)amino]-1-ethoxyl]-2-hydroxybenzoate to obtain the optically pure (R) or (S) chiral salbutamol.
Owner:EAST CHINA NORMAL UNIV
Levalbuterol hydrochloride orally disintegrating tablet and preparation method thereof
InactiveCN101103963ALittle side effectsGood curative effectOrganic active ingredientsPill deliveryBronchospasmAsthmatic bronchitis
The invention relates to a levalbuterol hydrochloride orally disintegrating tablet and the preparation method; wherein the levalbuterol hydrochloride orally disintegrating tablet comprises an active pharmaceutical ingredient, the levalbuterol hydrochloride, and pharmaceutical necessities. The pharmaceutical necessities include a disintegrant, a filling agent, a masking agent and a corrective. The raw material ingredients calculated by weight ratio are 0.79-2.5 per cent levalbuterol hydrochloride, 50-60 percent disintegrant, 20-40 per cent filling agent, 1.58-10 per cent masking agent and 10-12 per cent corrective, which are made into the orally disintegrating tablets by direct powder compression process. The tablets of the invention is taken easily without water, disintegrated quickly and takes effect quickly, high in bioavailability, less stimulatory function to gastrointestinal tract, and applicable to prevention and cure of bronchial asthma, asthmatic bronchitis, and bronchospasm of an emphysema patient.
Owner:HARBIN UNIV OF COMMERCE
Albuterol inhalation soultion, system, kit and method for relieving symptoms of pediatric asthma
InactiveUS20030140920A1Relieve bronchospasmRespiratorsOrganic active ingredientsBronchospasmSalbutamol
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.63 mg or about 1.25 mg albuterol.
Owner:DEY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com