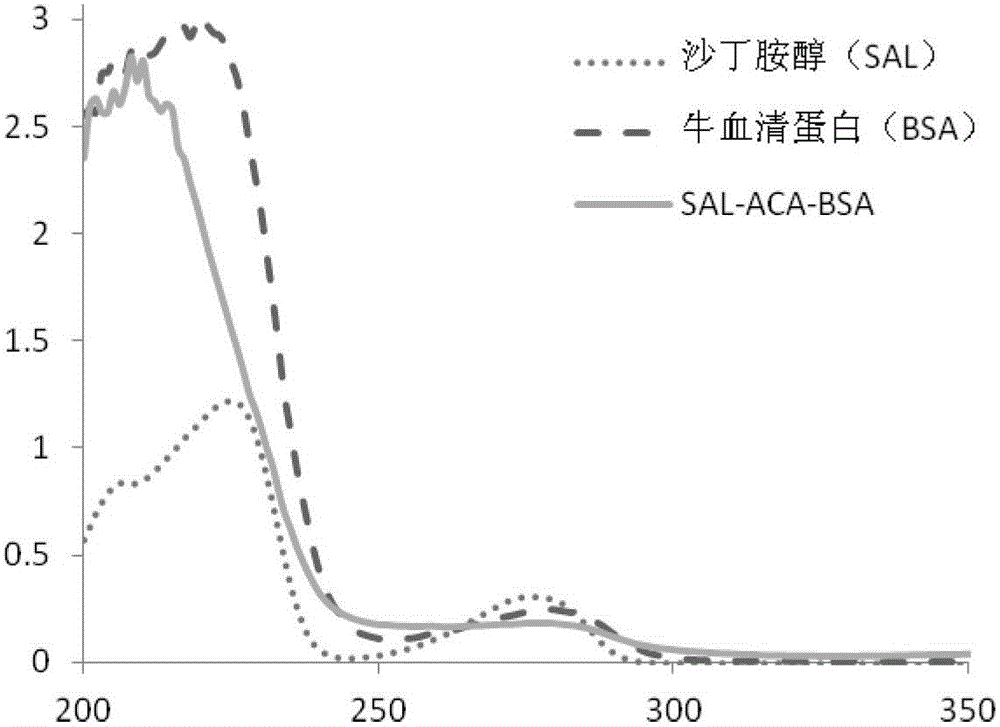
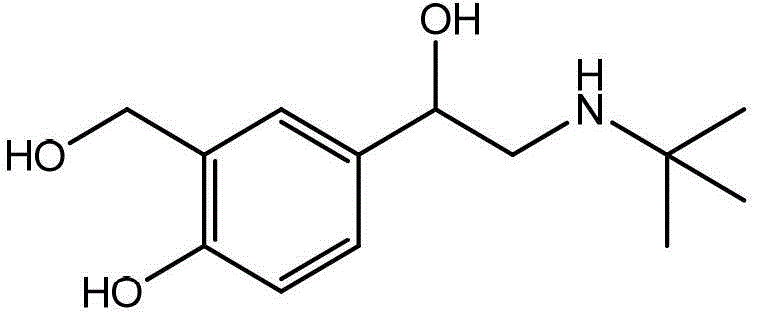
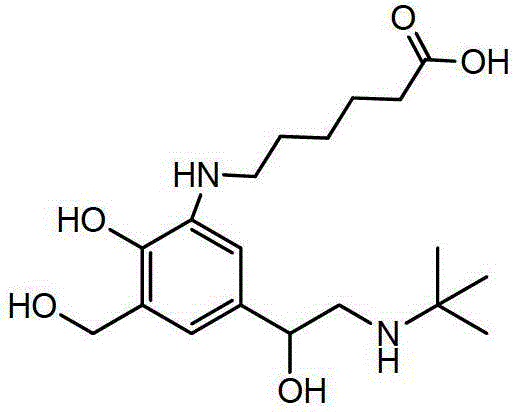
Synthesis method of specific salbutamol artificial antigen
A technology of albuterol and artificial antigen, which is applied in chemical instruments and methods, specific peptides, animal/human proteins, etc., to achieve high specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of albuterol hapten
[0040] 200mg (0.836mmol) of albuterol was dissolved in 15mL of ethanol. At room temperature, 99μL of 37% formaldehyde solution was added dropwise, and the reaction was stirred for 30min. Then, 110mg (0.836mmol) of 6-aminocaproic acid was added, and the reaction was continued for 6h. 6M HCl was slowly added dropwise to the reaction solution to adjust the pH to 2-3, 10 mL of ethyl acetate was added to extract three times, the organic phase was collected, and dried by rotary evaporation at 37° C. to obtain the albuterol hapten.
Embodiment 2
[0041] Embodiment 2, the preparation of albuterol artificial antigen
[0042] Take 25mg (0.0678mmol) albuterol hapten, add 2mL N,N-dimethylformamide (DMF) to dissolve, then add N-hydroxysuccinimide (NHS), 1-ethyl-3-(3- Dimethylaminopropyl) carbodiimide (EDC) (the molar ratio of hapten, NHS, EDC) is 1:1.5:2), at 4°C, mix well in the dark, stir for 60min, and then at room temperature Reaction 12h. Take 56mg (0.00084mmol) of bovine serum albumin (the molar ratio of hapten to bovine serum albumin is 80:1), add 10mL of 0.1M pH9.6 carbonate buffer. The activated hapten solution was slowly added dropwise to the bovine serum albumin solution, and reacted for 24 hours at room temperature. Dialyze with PBS buffer solution for 2 days, during which the PBS buffer solution was changed 4 times to obtain salbutamol artificial antigen.
Embodiment 3
[0043] Embodiment 3, the preparation of albuterol artificial antigen
[0044]Take 25 mg (0.0678 mmol) of albuterol hapten, add 2 mL of N,N-dimethylformamide (DMF) to dissolve, and pre-cool at 0°C for 30 minutes. At 0°C, add tri-n-butylamine and isobutyl chloroformate (the molar ratio of hapten, tri-n-butylamine, and isobutyl chloroformate is 1:1.2:1.2), and react at 0°C for 1 hour. Take 56mg (0.00084mmol) of bovine serum albumin (the molar ratio of hapten to bovine serum albumin is 80:1), add 10mL of 0.1M pH9.6 carbonate buffer, and pre-cool at 0°C for 30min. At 0°C, the activated hapten solution was slowly added dropwise to the bovine serum albumin solution, reacted at 0°C for 1 hour, and then reacted at room temperature for 24 hours. Dialyze with PBS buffer solution for 2 days, during which the PBS buffer solution was changed 4 times to obtain salbutamol artificial antigen.
PUM
| Property | Measurement | Unit |
|---|---|---|
| reaction rate constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com