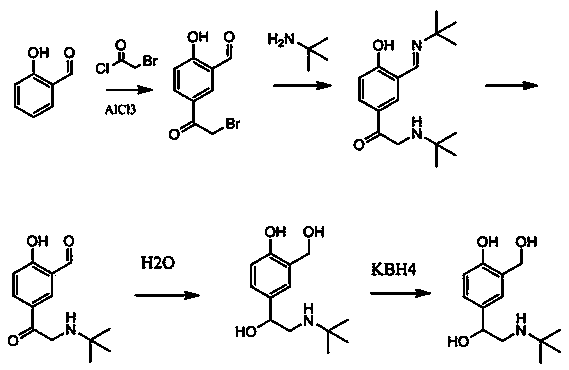
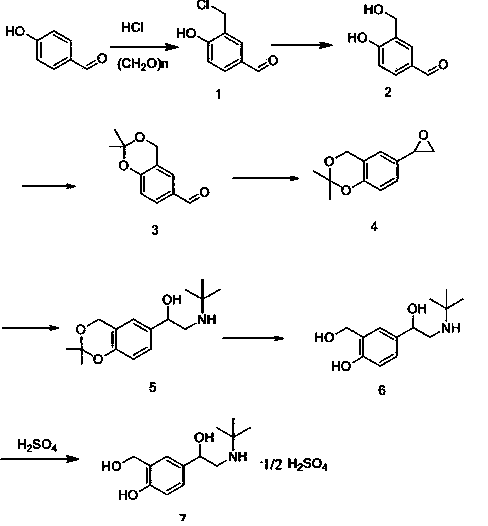
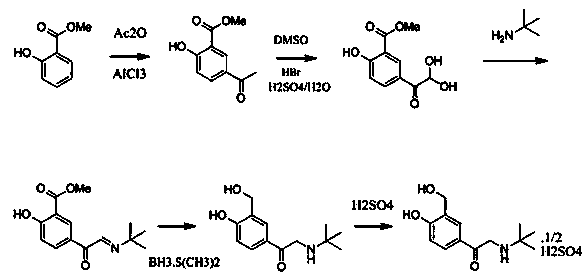
New process for synthesizing salbutamol and sulfate of salbutamol
A technology for salbutamol and a new process is applied in the field of synthesizing salbutamol and its sulfate, can solve the problems of low yield, troublesome post-processing, many side reactions, etc., and achieves the effects of easy purification, reduced product cost, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Preparation of Compound 1
[0054] Add 40ml of concentrated hydrochloric acid, 10g of p-hydroxybenzaldehyde, and 4.8g of paraformaldehyde into the reaction bottle, stir and react at 25-30°C for 8 hours, filter, and wash the obtained filter cake with 20ml of water, and 14g of the obtained red tide product is directly used for Next reaction.
[0055] (2) Preparation of Compound 2
[0056] Add 30 ml of water, 45 ml of tetrahydrofuran, and 14 g of compound (1) to the reaction bottle, add 6.4 g of sodium bicarbonate under stirring, stir and react at 25-30 ° C for 8 hours, let stand to separate layers, and continue to use the upper layer of tetrahydrofuran Wash with 20ml of water, dry and concentrate, and 10g of the obtained concentrated raffinate is directly used in the next reaction
[0057] (3) Preparation of compound 3
[0058] Add 70ml of acetone, 0.4ml of concentrated sulfuric acid, 10g of compound (2) to the reaction flask, stir and react at 25-30°C for 8 ...
Embodiment 2
[0068] (1) Preparation of Compound 1
[0069] Add 60ml of concentrated hydrochloric acid, 15g of p-hydroxybenzaldehyde, and 7.2g of paraformaldehyde into the reaction bottle, stir and react at 20-25°C for 10 hours, filter, and wash the obtained filter cake with 20ml of water, and 22g of the obtained red tide product is directly used for Next reaction.
[0070] (2) Preparation of Compound 2
[0071] Add 45 ml of water, 45 g of tetrahydrofuran, and 22 g of compound (1) in the reaction bottle, add 9.6 g of sodium bicarbonate under stirring, stir and react at 25-30 ° C for 10 hours, let stand to separate layers, and the upper layer of tetrahydrofuran layer continues Wash with 30ml of water, dry and concentrate, and 16g of the obtained concentrated residue is directly used in the next reaction
[0072] (3) Preparation of compound 3
[0073] Add 110ml of acetone, 0.7ml of concentrated sulfuric acid, and 16g of compound (2) into the reaction flask, stir and react at 25-30°C ...
Embodiment 3
[0083] (1) Preparation of Compound 1
[0084] Add 80ml of concentrated hydrochloric acid, 20g of p-hydroxybenzaldehyde, and 9.6g of paraformaldehyde into the reaction bottle, stir and react at 20-25°C for 12 hours, filter, and wash the obtained filter cake with 20ml of water, and 25g of the obtained red tide product is directly used for Next reaction.
[0085] (2) Preparation of Compound 2
[0086] Add 50 ml of water, 50 ml of tetrahydrofuran, and 25 g of compound (1) in the reaction bottle, add 6.8 g of sodium carbonate under stirring, stir and react at 25-30 ° C for 12 hours, let stand to separate layers, and continue to use 20 ml of tetrahydrofuran for the upper layer Washed with water, dried and concentrated, and 20 g of the obtained concentrated raffinate was directly used in the next step reaction
[0087] (3) Preparation of compound 3
[0088] Add 80ml of acetone, 1ml of concentrated sulfuric acid, and 20g of compound (2) into the reaction flask, stir and react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com