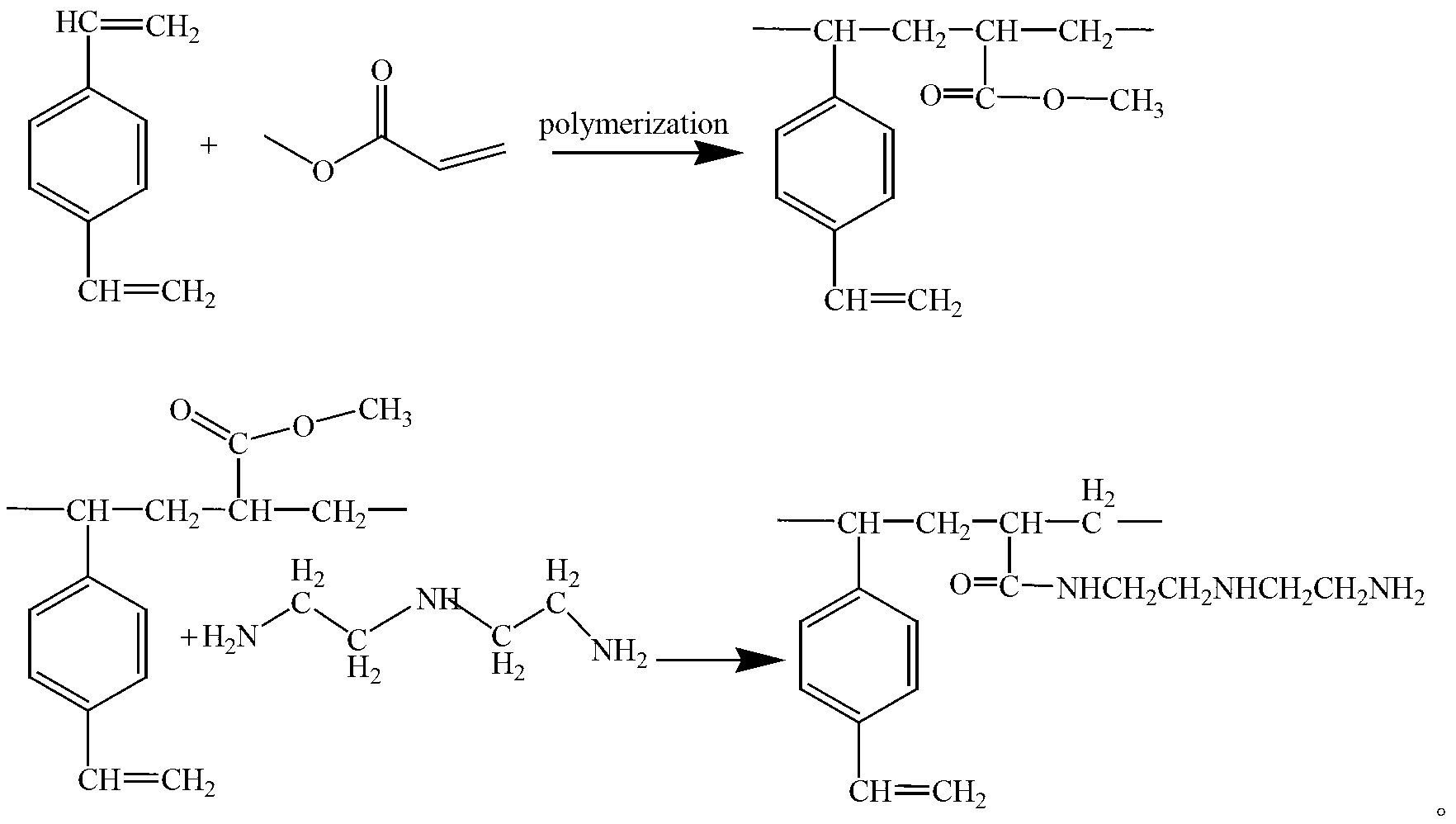
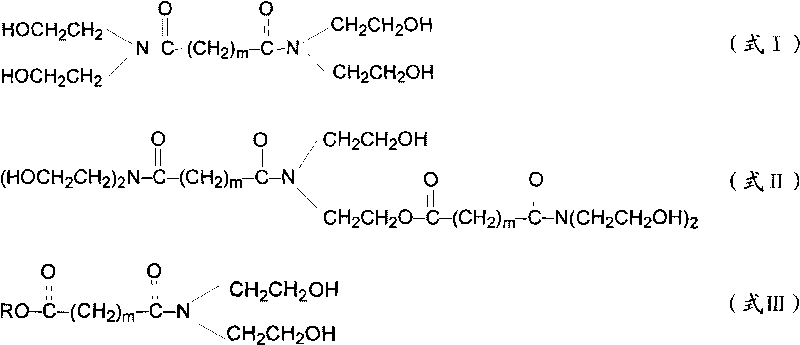
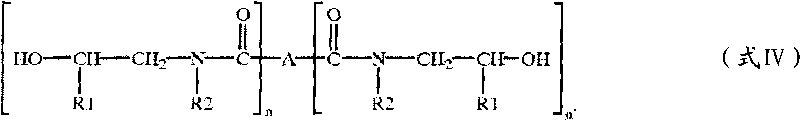
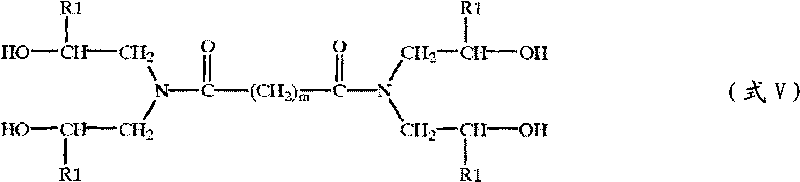
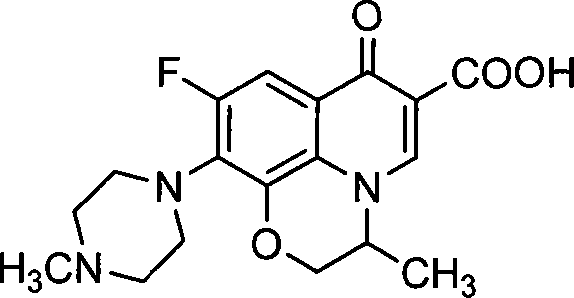
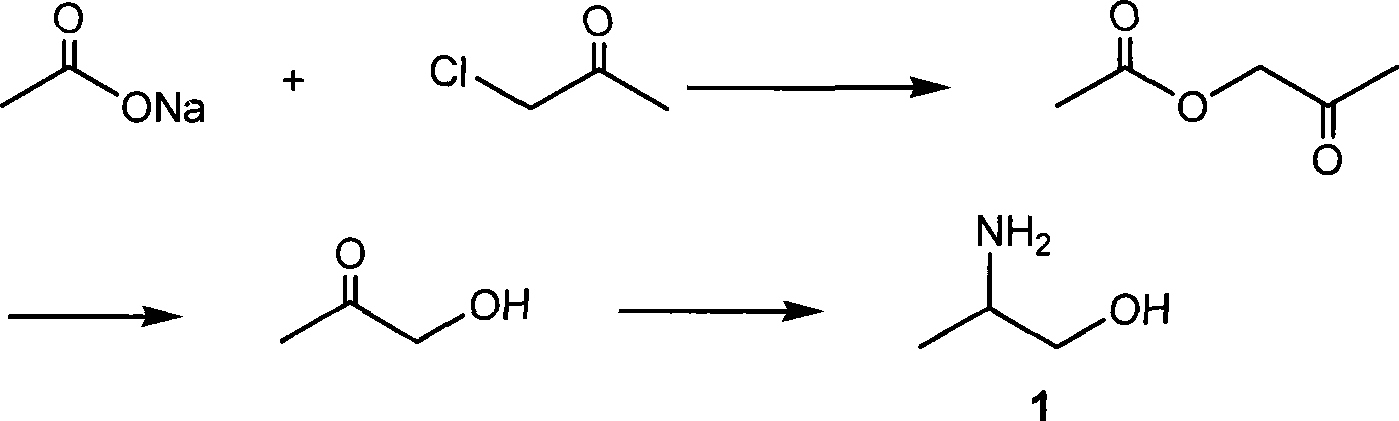
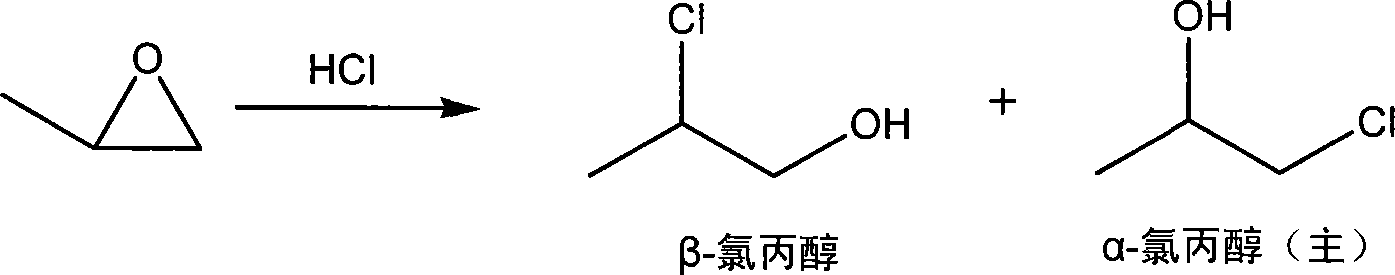
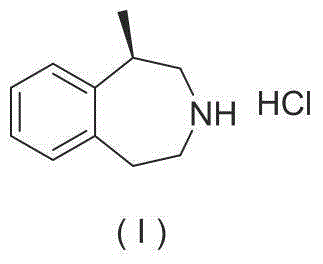
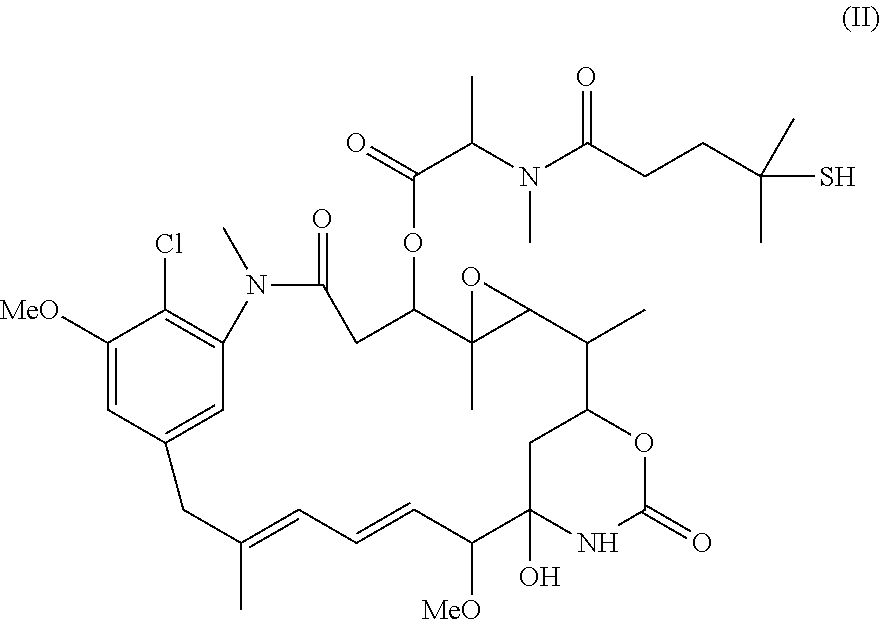
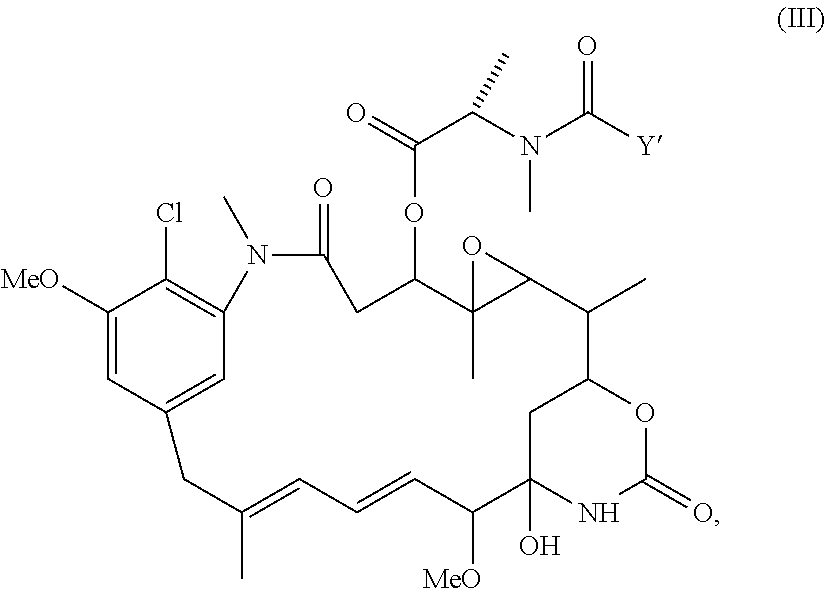
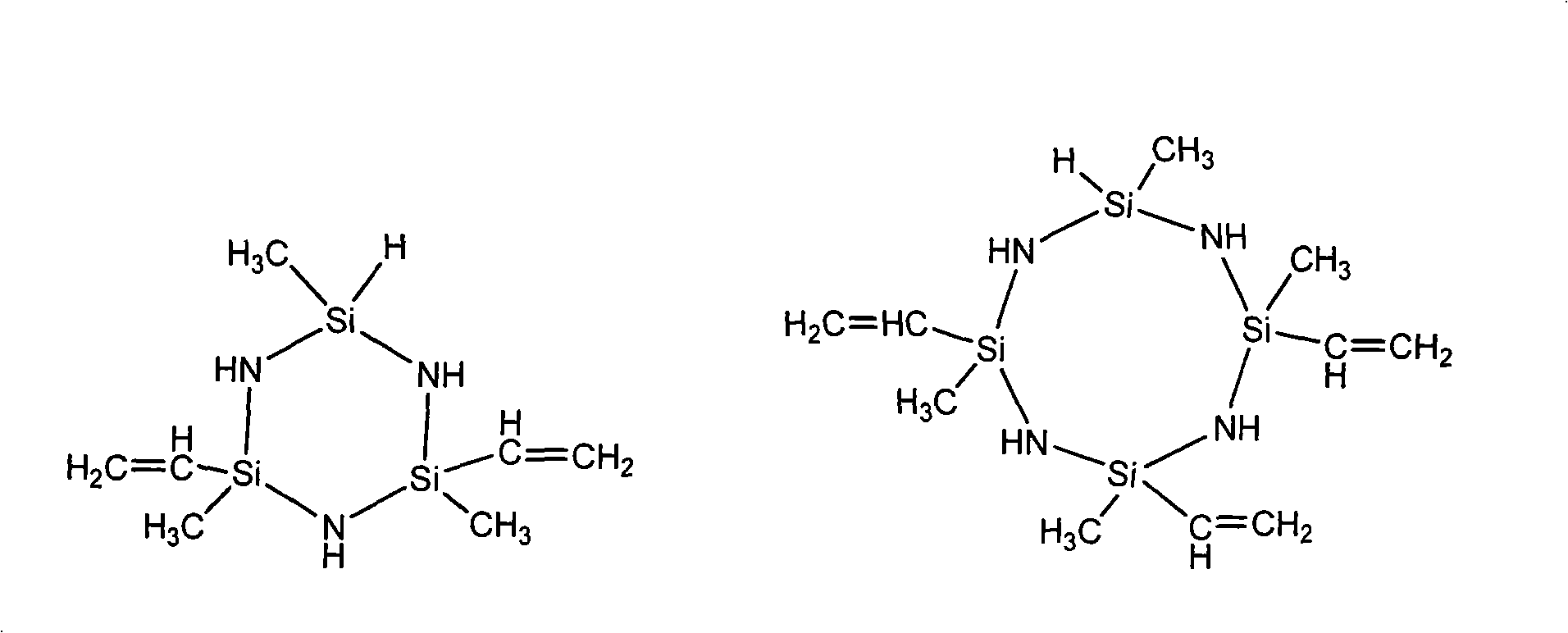
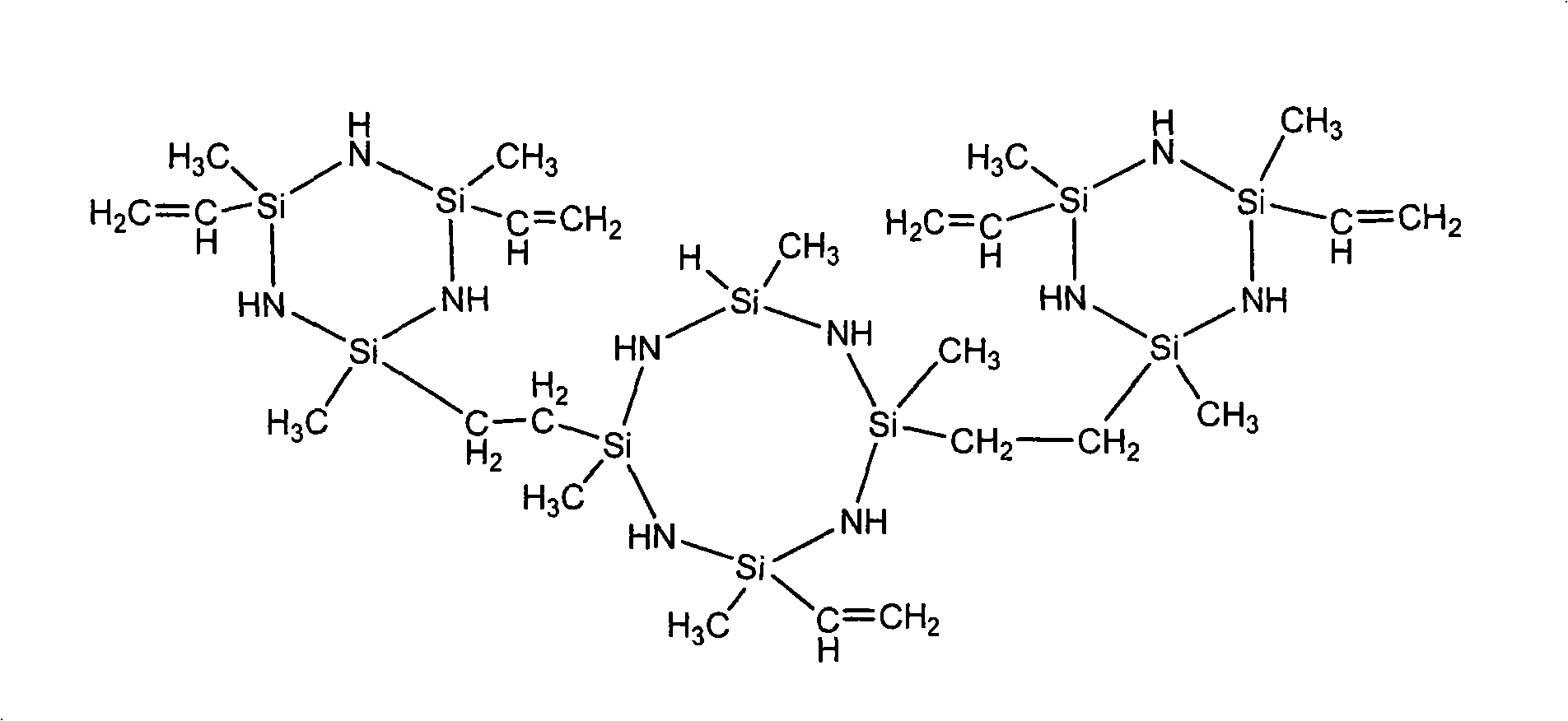
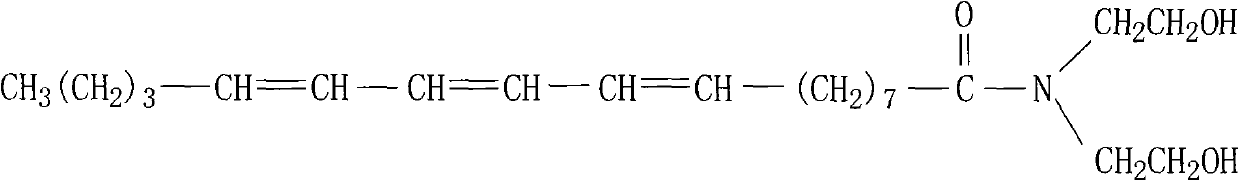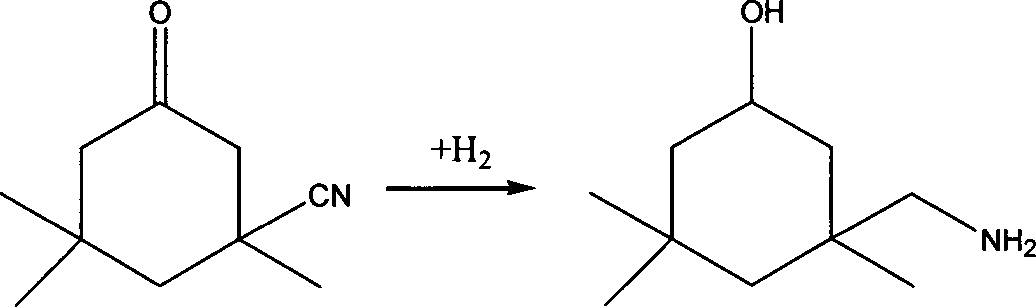Patents
Literature
618 results about "Aminolysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aminolysis (/am·i·nol·y·sis/ amino meaning "contains NH₂ group", and lysis meaning "to unbind") is any chemical compound reacts with a molecule of ammonia or an amine and causes a molecule to split into two parts, containing the addition of (or substitution by) an amino group —NH—. The subset of aminolysis reactions involving ammonia is known as ammonolysis.
Process for preparing 2-oxindoles and N-hydroxy-2-oxindoles
InactiveUS6469181B1Avoid wastingImprove economyCarboxylic acid nitrile preparationOrganic compound preparationReaction intermediateCarboxylic salt
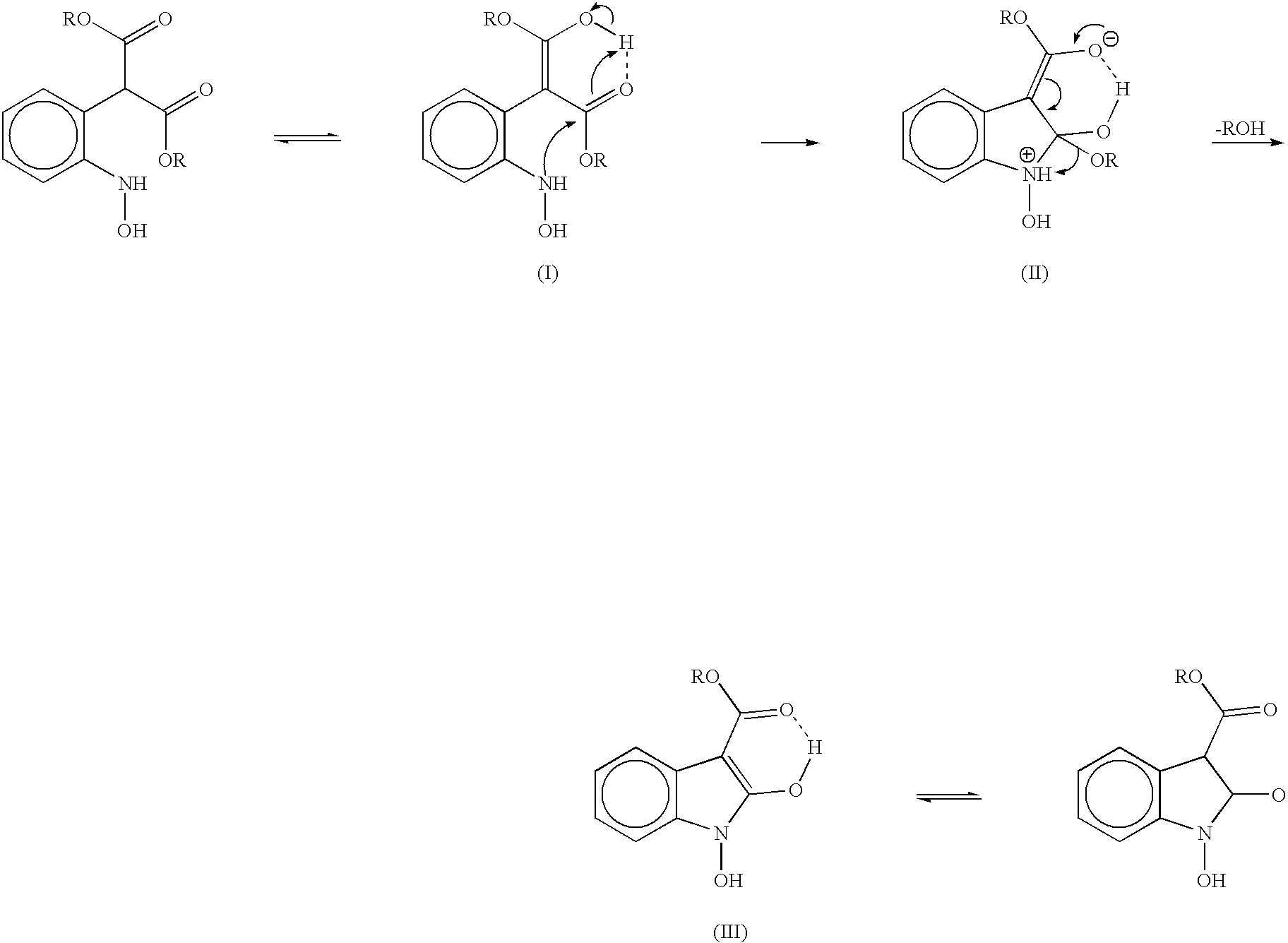
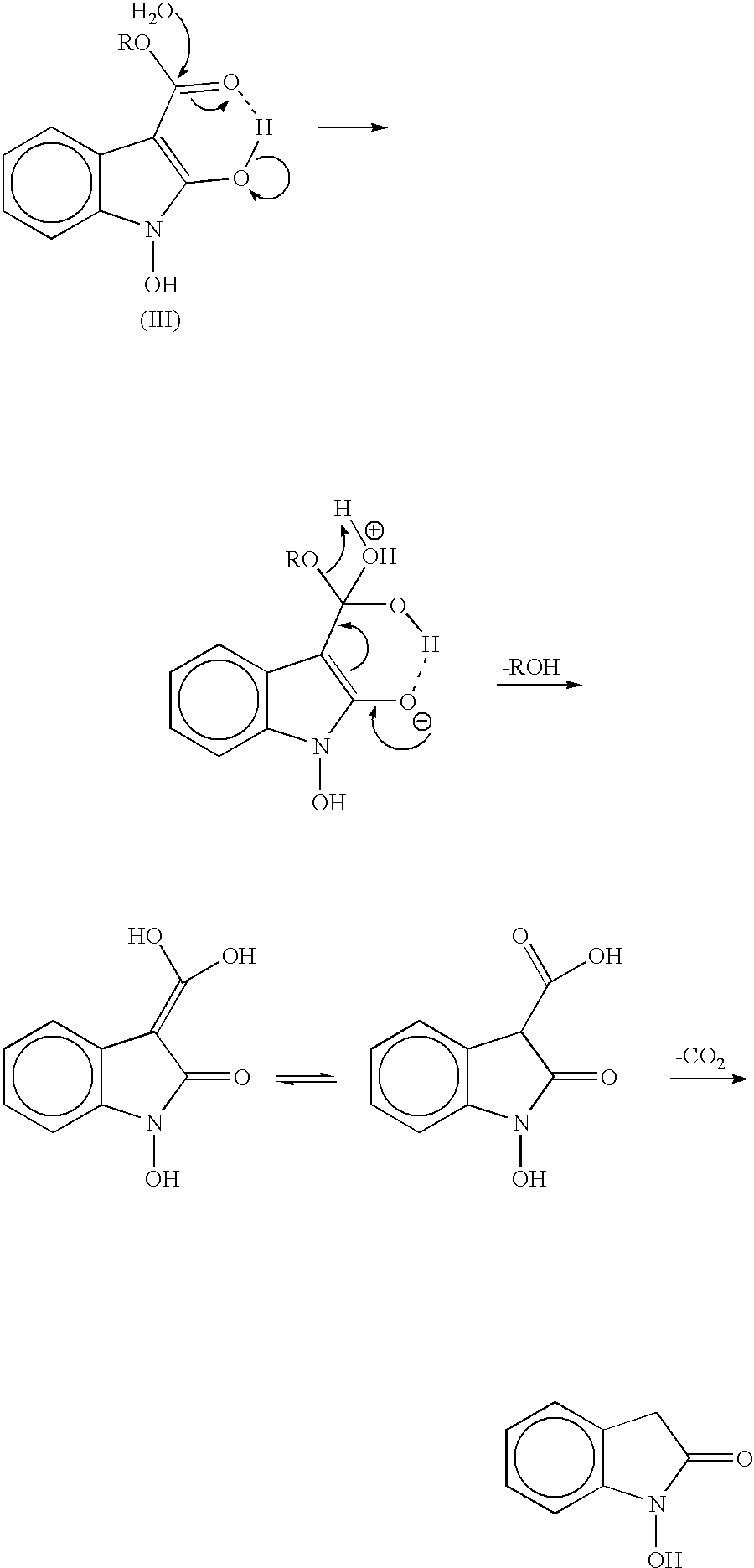
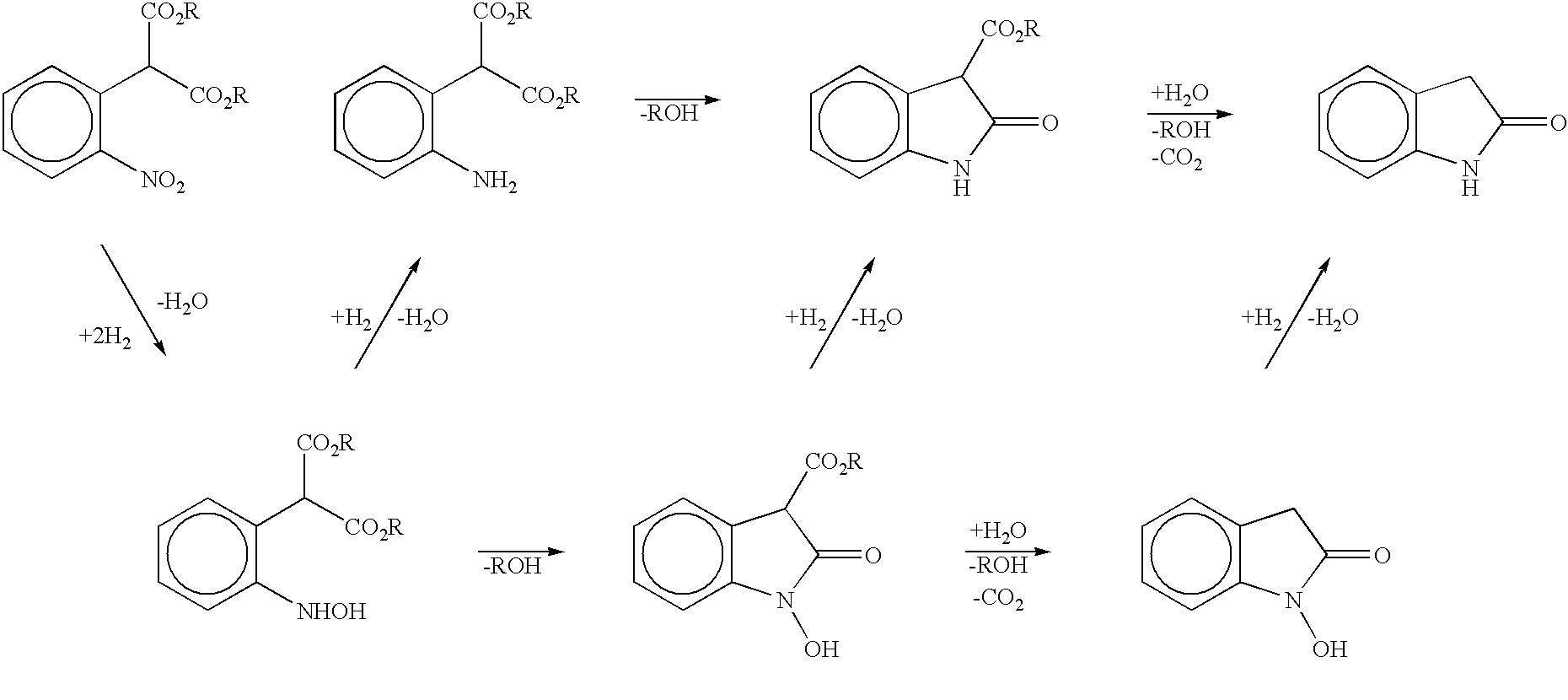
The present invention provides a processes, having practical utility, for preparing 2-oxindoles, N-hydroxy-2-oxindoles, or mixtures thereof comprising: catalytically hydrogenating a 2-nitroarylmalonate diester to produce a 2-(N-hydroxyamino)arylmalonate diester, a 2-aminoarylmalonate diester, or mixtures thereof as a first reaction intermediate; cyclizing, by intramolecular aminolysis of one ester group, the first reaction intermediate to produce a N-hydroxy-2-oxindole-3-carboxylate ester, 2-oxindole-3-carboxylate ester, or mixtures thereof as a second reaction intermediate; and hydrolyzing and decarboxylating the remaining ester group of the second reaction intermediate to produce the N-hydroxy-2-oxindole, the 2-oxindole, or mixtures thereof, wherein the cyclization reaction and the hydrolysis and decarboxylation reaction are conducted in situ with the catalytic hydrogenation reaction without isolation of said reaction intermediates.
Owner:CATALYTICA PHARMA
Hydroxylated tung oil and ester group-aminated preparation method thereof
InactiveCN101845367AIncrease profitReduce dependenceFatty acid chemical modificationChemical synthesisPetroleum product
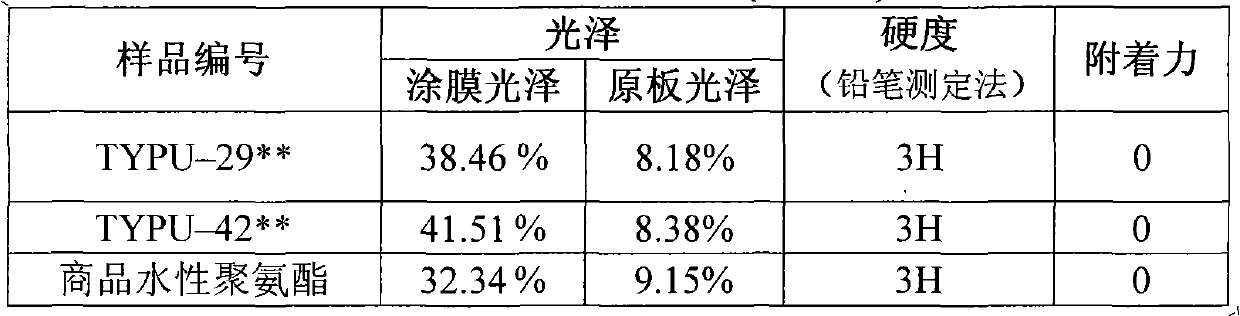
The invention discloses a hydroxylated tung oil and an ester group-aminated preparation method, belonging to the technical field of organic chemosynthesis. In the method, under a certain condition, ester groups in the tung oil and diethanol amine are subjected to aminolysis reaction on the premise of reserving conjugated double bonds, thus the tung oil is hydroxylated to mainly produce a compound containing terminated hydroxyl and three conjugated double bonds. The structural formula of the compound is shown in the specification. The aminolysis reaction product plays an important role in developing and producing processes of leading the tung oil into waterborne polyurethane, not only can combine the advantages of the tung oil and the polyurethane, but also can improve the utilization ratio of tung oil resources as special local products in China and decrease the dependence on petroleum products; and simultaneously, the method has the advantages of simplicity, practicability, high conversion rate and convenience in industrialized production.
Owner:HIGH & NEW TECH RES CENT OF HENAN ACAD OF SCI
Magnetic microsphere resin for removing nitrate nitrogen selectively, and preparation method thereof
ActiveCN102430433AHigh base exchange capacityAccelerated settlementWater/sewage treatment by ion-exchangeAnion exchangersOil phaseStructural formula
The invention discloses magnetic microsphere resin for removing nitrate nitrogen selectively, and a preparation method thereof, and belongs to the field of ion exchange resin. The resin consists of a resin skeleton, and magnetic granules wrapped in the resin skeleton; and the resin skeleton has a basic structural formula shown in the specification, wherein B is a quaternary ammonium salt group for adsorbing the nitrate nitrogen selectively, the saturation magnetization intensity of the quaternary ammonium salt group is between 5 and 30 emu / g, the exchange capacity of strong base is between 3.0 and 4.5 mmol / g, the exchange capacity of weak base is between 0.5 and 1.5 mmol / g, and the average grain diameter of the resin is between 50 and 500 micrometers. The resin is synthesized by a suspension polymerization method; acrylate monomers are mixed with a pore-forming agent and an initiator to form an oil phase; after being mixed with the magnetic granules uniformly, the mixture is subjectedto suspension polymerization with an aqueous phase mixed with a dispersing agent to form the magnetic polymer granules, and the magnetic polymer granules are subjected to aminolysis and alkylation toform the resin serving as a finished product. The resin can adsorb negative ions such as nitrates, nitrites and the like in a water body selectively, so the magnetic microsphere resin has a bright application prospect in fields of drinking water treatment, groundwater remediation and the advanced treatment of urban domestic sewage.
Owner:NANJING UNIV +1
Thickened oil viscosity depressant as well as preparation method and application thereof
ActiveCN102719234AImprove liquidityUnique Viscosity ReductionDrilling compositionBoiling pointChain length
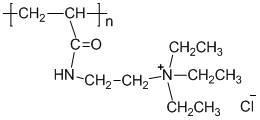
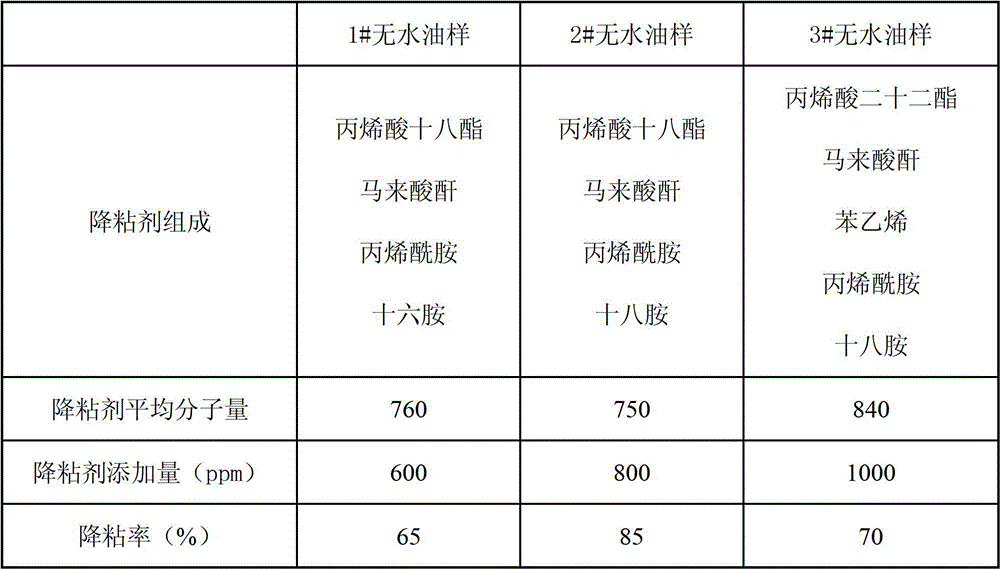
The invention discloses a thickened oil viscosity depressant as well as a preparation method and an application thereof. The viscosity depressant is obtained by carrying out aminolysis reaction on a terpolymer or a quadripolymer with an alkyl primary amine with the chain length of C12-C18, wherein the terpolymer is formed by three monomers including acrylate with the chain length of C18-C22, maleic anhydride and acrylic amide; the quadripolymer is formed by four monomers including the acrylate with the chain length of C18-C22, the maleic anhydride, styrene and the acrylic amide; and the number-average molecular weight of the viscosity depressant is 300-1000, the smelting point is 50-70 DEG C and the boiling point is 300-550 DEG C. The thickened oil viscosity depressant disclosed by the invention has unique viscosity depression and resistance reduction effects on thickened oil; by only adding 500-1000 ppm of the thickened oil viscosity depressant, the viscosity depression ratio of the thickened oil with the viscosity of 14000-24000 mPa s at 50 DEG C reaches up to 85%; and the thickened oil viscosity depressant has an obvious application effect and positive meanings.
Owner:SHANGHAI UNIV OF ENG SCI
Immune magnetic microsphere and preparing process and usage thereof
InactiveCN1667413ALarge specific surface areaHigh magnetic contentMaterial analysisAntigenPolyvinyl alcohol
This invention relates to an immunological magnetic microballoon, the process method of which comprises following steps: fully mixing oleic acid coating Fe3O4 magnetofluid and polymer monomer, cross linker and initiating agent to form oil phase; floating them in polyvinyl alcohol aqueous phase; polymerizing emulsion and suspension to process magnetic polymer microballoon; then introducing functional group on microballon surface through aminolysis reaction.The magnetic microballon is able to couple special immune genin on its surface, then these special immune genin further identify corresponding antibody, antigen and bioepiderm or directly use amidogen on immune magnetic microballon surface to identify antibody, antigen and bioepiderm able to react with amidogen.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Acrylic-acid high-capacity primary-amino chelate resin for trapping copper ions and preparation method thereof
ActiveCN103159888ALarge adsorption capacityImprove adsorption capacityOther chemical processesWater contaminantsWastewaterAminolysis
The invention discloses an acrylic-acid high-capacity primary-amino chelate resin for trapping copper ions and a preparation method thereof, belonging to the field of polyamine resin wastewater treatment. The structural unit of the primary-amino chelate resin disclosed by the invention is disclosed in the specification, wherein x represents a repetitive structure unit of different aminolysis reagents. The preparation method disclosed by the invention has the advantages of wide material sources, low cost, simple operation steps and controllable synthesis conditions; and the synthesized resin has superhigh adsorption capacity for copper ions.
Owner:NANJING UNIV
Production technology of beta-hydroxyalkylamide
ActiveCN101704762AAvoid hydrolysisHigh purityOrganic compound preparationCarboxylic acid amides preparationDistillationAminolysis
The invention discloses a production technology of beta-hydroxyalkylamide. The method comprises the following steps: taking carboxylic acid alkyl ester and excessive beta-alkanolamine as raw materials, conducting aminolysis reaction while heating and stirring, then adding volatile acid to break alkali catalyst, further raising temperature, constantly removing byproducts and the unreacted beta-alkanolamine by adopting the combination of bubbling and sweeping by inert gas and the reduced pressure distillation process, and then emptying, cooling and grinding to directly obtain a high-purity beta-hydroxyalkylamide powder product. The production technology purifies products by replacing the traditional organic solvent crystallization technology with the combination of bubbling and sweeping by the inert gas and the reduced pressure distillation process, needs not to add acetone, methanol and other organic solvents and can not only obtain the high-purity beta-hydroxyalkylamide product, but also avoid the consumption of crystallized solvent and the pollution thereof to the environment, thus being a novel production technology with low material consumption and low pollution.
Owner:六安捷通达新材料有限公司
Preparation method of Ticagrelor intermediate
ActiveCN102796007AEasy to prepareEasy to operatePreparation by rearrangement reactionsChemical synthesisTicagrelor
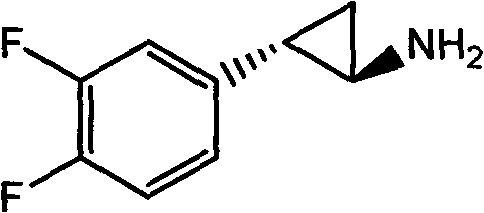
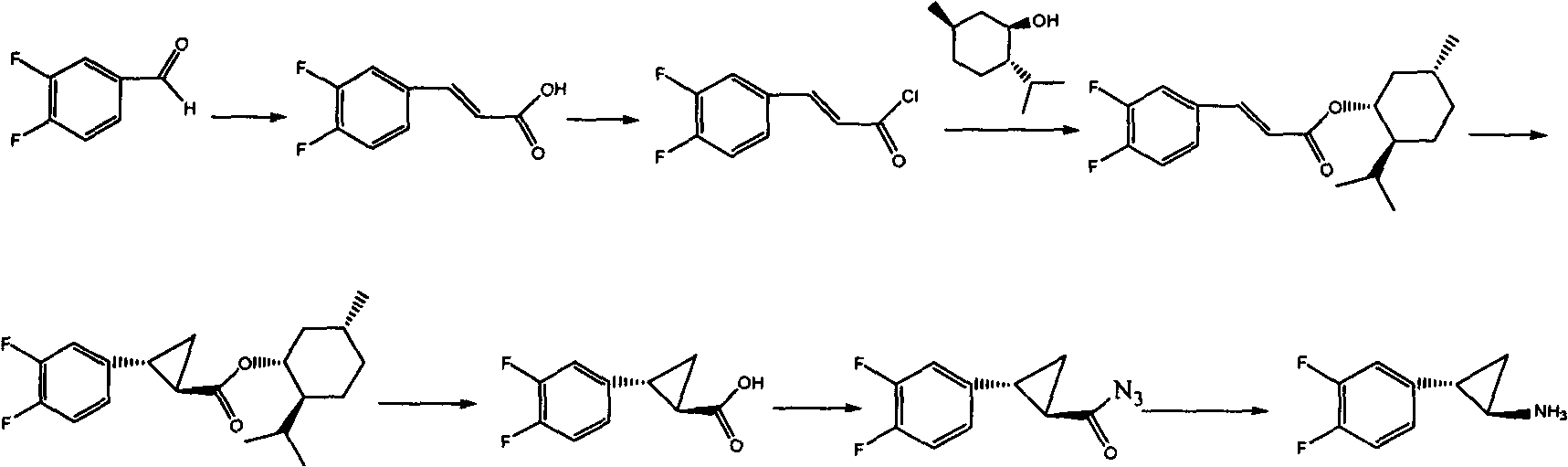
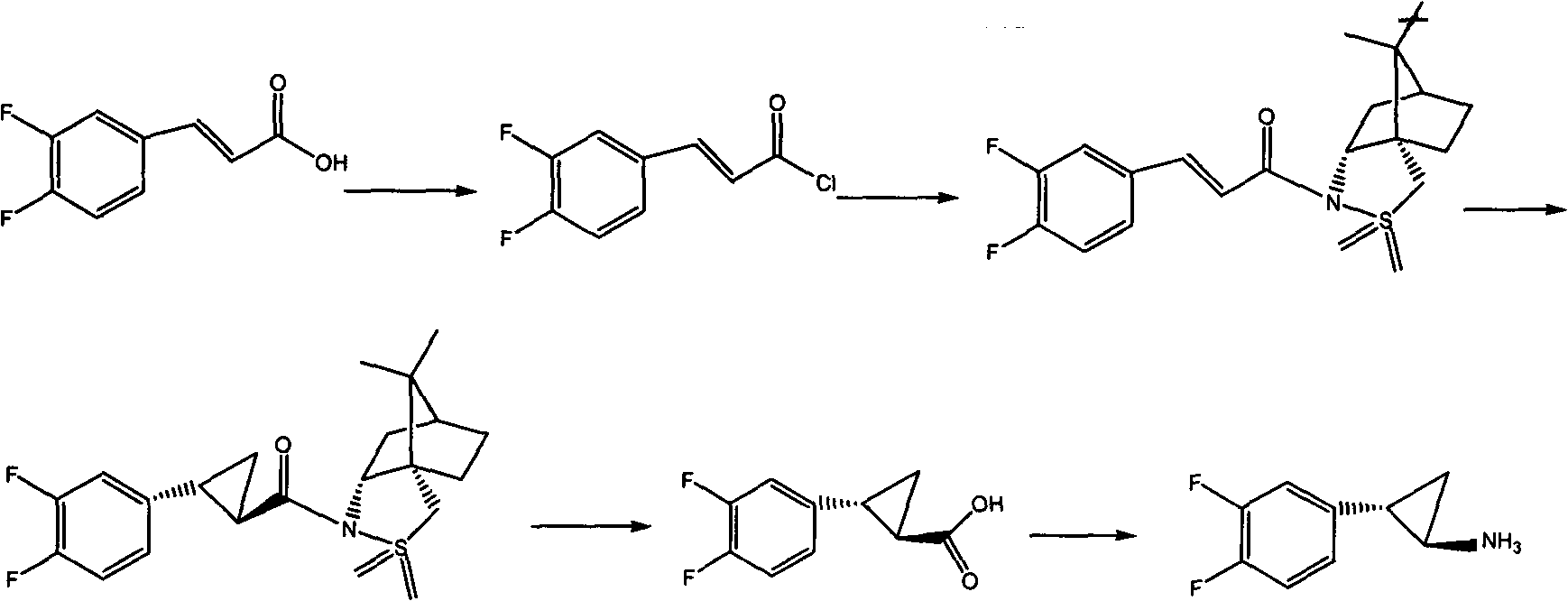
The invention relates to the medicine chemical synthesis field, and especially discloses a preparation method of a Ticagrelor intermediate. The preparation method comprises the following steps: 1) taking 3,4-difluorobenzaldehyde (I) as an initial raw material, reacting with a phosphorus ylide material liquid to obtain (E)-3-(3,4-difluorophenyl)-2-acrylic acid ester (II); 2) performing a Simons-Smith asymmetric cyproteronethe reaction on the (E)-3-(3,4-difluorophenyl)-2-acrylic acid ester (II) to obtain trans-(1R,2R)-2-(3,4-difluorophenyl) cyclopropanecarboxylic acid ester (III); 3) performing aminolysis on the trans-(1R,2R)-2-(3,4-difluorophenyl) cyclopropanecarboxylic acid ester to obtain trans-(1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxamide (IV); and 4) performing a Huffman rearrangement reaction on the trans-(1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxamide (IV) to obatain the Ticagrelor intermediate (V). The method of the invention has the advantages of simple process, convenient operation, mild reaction condition and easy control, low cost and easy acquisition of raw material, high product yield and product purity, and is adapted to large scale industrial production.
Owner:JINAN RUIFENG PHARMA +2
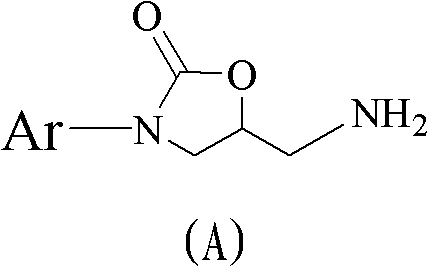
Method for preparing 5(S)-aminomethyl-3-aryl-2-oxazolidinone
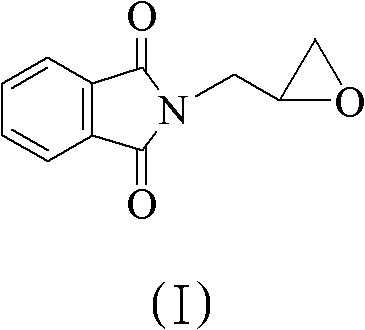
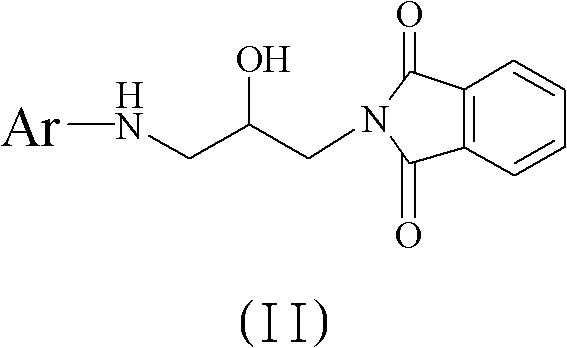
The invention discloses a method for preparing 5(S)-aminomethyl-3-aryl-2-oxazolidinone. The method comprises the following steps of: reacting (S)-glycidyl phthalimide and aryl aniline to prepare an addition compound; and finally preparing the 5(S)-aminomethyl-3-aryl-2-oxazolidinone through cyclization reaction and aminolysis reaction. The method has the advantages of high total yield and low cost; all intermediates and a product synthesis method have the characteristics of convenience in recovery and purification; and the purity of a final product of the method is at least over 99.9 percent.
Owner:OMEGA MEDICAL TAIWAN
Diesel oil pour-point depressant and preparation method and application thereof
InactiveCN104530305ASolve the problem of poor broad spectrumComposite strongLiquid carbonaceous fuelsMethacrylatePtru catalyst
The invention discloses a diesel oil pour-point depressant and a preparation method thereof, wherein the diesel oil pour-point depressant is prepared by the steps: carrying out an aminolysis reaction on a methacrylate maleic anhydride copolymer and higher fatty amine for 6 h with toluene as a solvent and p-toluenesulfonic acid as a catalyst and at a reflux temperature, adding methanol having the same volume as the reaction liquid volume into the obtained reaction liquid, producing a white precipitate, removing a supernatant, washing the obtained white precipitate with toluene, carrying out methanol precipitation to remove the catalyst p-toluenesulfonic acid, then controlling the temperature at 50 DEG C, and carrying out vacuum drying to obtain the diesel oil pour-point depressant. The diesel oil pour-point depressant solves the problems that a (methyl) acrylate pour-point depressant has poor adaptability with diesel oils with different carbon chains, at the same time, makes the reaction of maleic anhydride more diversified, provides another possibility for combination of a maleic anhydride pour-point depressant with the (methyl) acrylate pour-point depressant, effectively improves the condensation point and the cold filter plugging point of No.0 diesel oil, and enables the cold filter plugging point to be superlatively reduced by 11-12 DEG C.
Owner:SHANGHAI INST OF TECH
Apixaban preparation method
The invention discloses an Apixaban preparation method. The Apixaban preparation method comprises that 1, an intermediate I and an intermediate II undergo a [3+2] cyclization addition reaction under the alkali action to produce a compound B, and the compound B undergoes a morpholine ring removal reaction under the acid condition to produce a compound C, 2, the compound C is reduced by iron powder to form a corresponding amino compound D, 3, the amino compound D and 5-chlorovaleryl chloride undergo an amidation reaction under the triethylamine action to produce a compound E, 4, the compound E undergoes a cyclization reaction under the strong base action to produce a compound F, 5, the compound F undergoes a hydrolysis reaction under the strong base action to produce a corresponding carboxyl compound G, and 6, the carboxyl compound G and CDI undergo a reaction to produce an active intermediate H and the active intermediate H and ammonia water undergo an aminolysis reaction to produce the desired compound A. The Apixaban preparation method has simple processes, does not need strict reaction conditions, has low equipment requirements, has high reaction yield, utilizes stable intermediates thereby solving intermediate storage problems, and effectively improves product purity.
Owner:河北凯威恒诚制药有限公司
Nanometer micelle capable of intelligently releasing medicine as well as preparation method and application thereof
ActiveCN102391517ASuitable chain complianceImprove biological activityPowder deliveryPharmaceutical non-active ingredientsPolyethylene glycolAminolysis
The invention discloses a nanometer micelle capable of intelligently releasing medicine as well as a preparation method and an application thereof. The nanometer micelle provided by the invention comprises the components of polyethylene glycol-poly (aspartate-cysteine)-poly (aspartate-diisopropyl ethanediamine). The preparation method comprises the following steps of: firstly, synthesizing PEG-PBLA-N3 and PA-PAsp (DIP); then, taking cuprous bromide / pentamethyldiethylenetriamine as a catalyst system, and synthesizing PEG-PBLA-PAsp (DIP) through a click reaction of PEG-PBLA-N3 and PA-PAsp (DIP); and finally, carrying out an aminolysis reaction on 2-aminoethyl mercaptan to obtain the final polymer PEG-PAsp (MEA)-PAsp (DIP). The nanometer micelle provided by the invention can be used as a hydrophobic medicine carrier. The nanometer micelle is sensitive to pH value, the disulfide bond in the intermediate cross-linking layer is sensitive to a reducing agent, and the nanometer micelle has the characteristic of intelligently releasing medicine.
Owner:SUN YAT SEN UNIV
Preparation method of high-purity olaparib
The invention discloses a preparation method of high-purity olaparib. The preparation method comprises: subjecting 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazine-1-yl)methyl] benzoic acid as a starting material to activation and aminolysis crystallization to obtain high-purity olaparib, wherein the activation refers to adding carbonyldiimidazole activating agent into a solution containing 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazine-1-yl)methyl] benzoic acid to obtain active amide intermediate; with separation and purification, subjecting the active amide intermediate to direct aminolysis crystallization with 1-(cyclopropanecarbonyl)piperazine to obtain the olaparib. The purity of the olaparib prepared by the method is greater than 99.8 %, and the process is simple, high in yield, low in cost and more suitable for industrial production.
Owner:合肥启旸生物科技有限公司
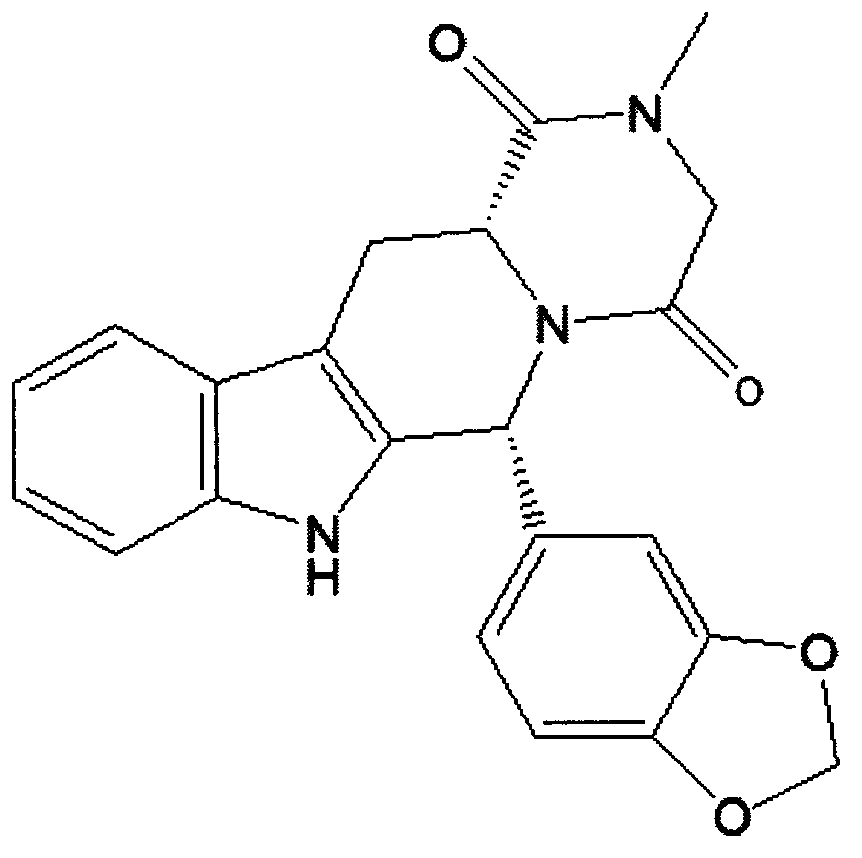
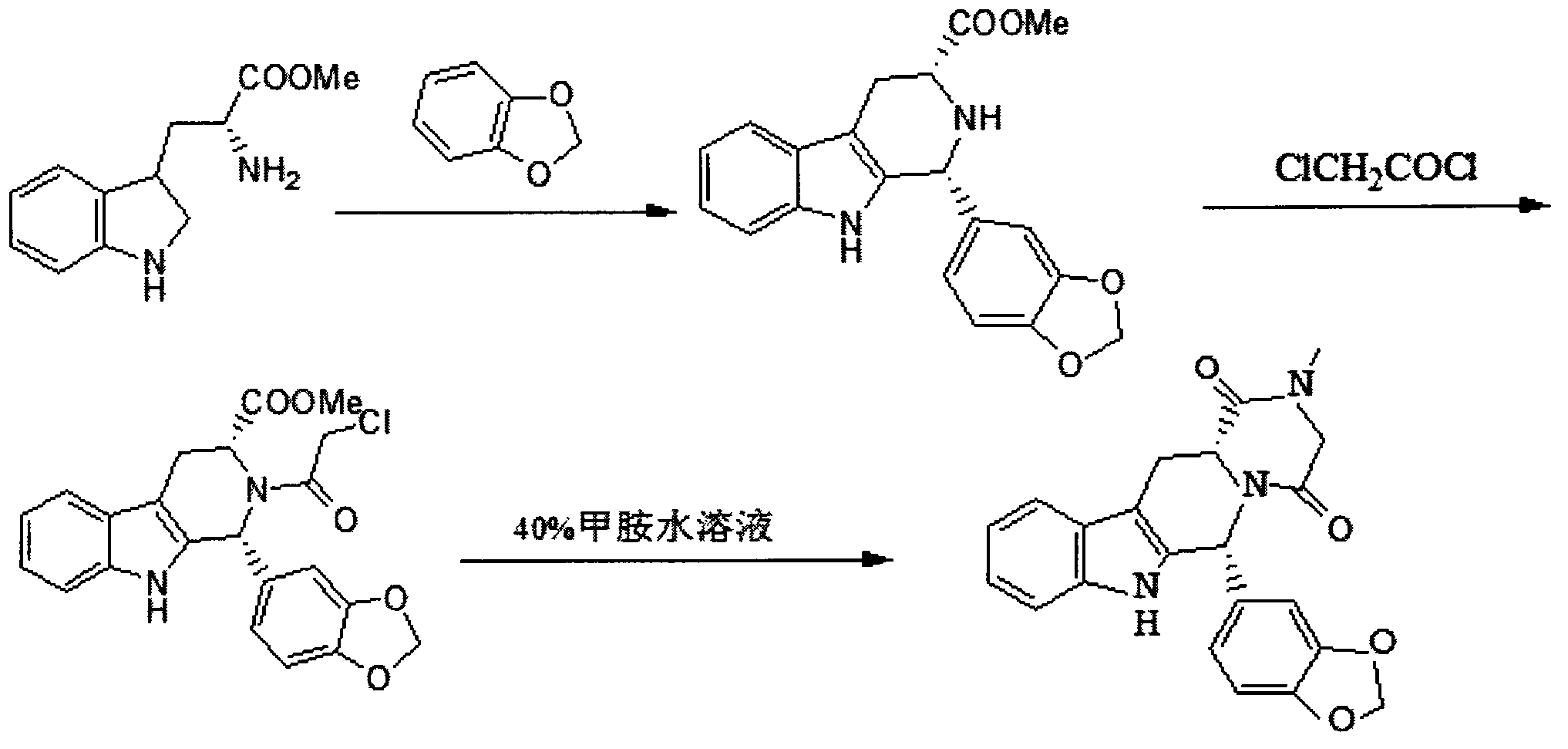
Simple preparation process of tadalafil
The invention relates to a preparation method of tadalafil. A product is obtained through condensation and cyclization, chloromethylation and aminolysis cyclization reaction based on D-tryptophan methyl ester hydrochloride and heliotropin as starting preparation materials. The simple preparation process is characterized in that condensation and cyclization are used for solving an isomer problem caused by Pictet-Spengler reaction by using isopropanol or nitromethane as a solvent; the yield of ethyl acetate in the aminolysis cyclization reaction is obviously improved; the aminolysis cyclization route includes three preparation steps, wherein the reaction yield of each step is high, the relevant impurities are easy to separate, the reaction conditions are simple, the production period is shorter, and toxic and highly corrosive reagents are not used, and therefore, the simple preparation process is safe and environment-friendly and easy to industrially produce, so that the high-purity qualified products are obtained.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
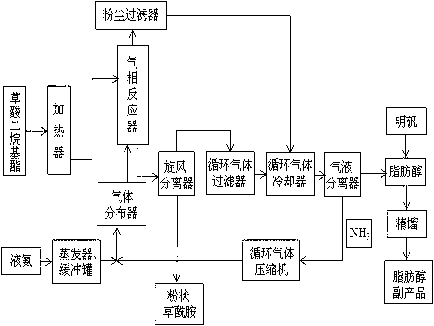
Method for continuous gas phase synthesis of oxamide
ActiveCN103288666AImprove qualityContinuous and stable industrial productionOrganic compound preparationHydroxy compound preparationGas phaseAminolysis
The invention discloses a method for continuous gas phase synthesis of oxamide. In a gas phase reactor, gas phase oxalic acid dialkyl ester and ammonia gas perform ammonolysis reaction under high temperature to generate oxamide product and corresponding fatty alcohol secondary product, and the oxamide product is collected by a cyclone separator. The method uses the gas phase continuous synthesis technology, and solves the defects of long reaction time and poor capacity of intermittent reaction in the existing oxamide preparation technology; by adopting the method, the reaction time is reduced remarkably, the reaction is continuous and steady, the oxalic ester aminolysis conversion rate is high, the quality of the obtained oxamide is high, and continuous steady industrial production of the oxamide is realized.
Owner:江苏丹化煤制化学品工程技术有限公司
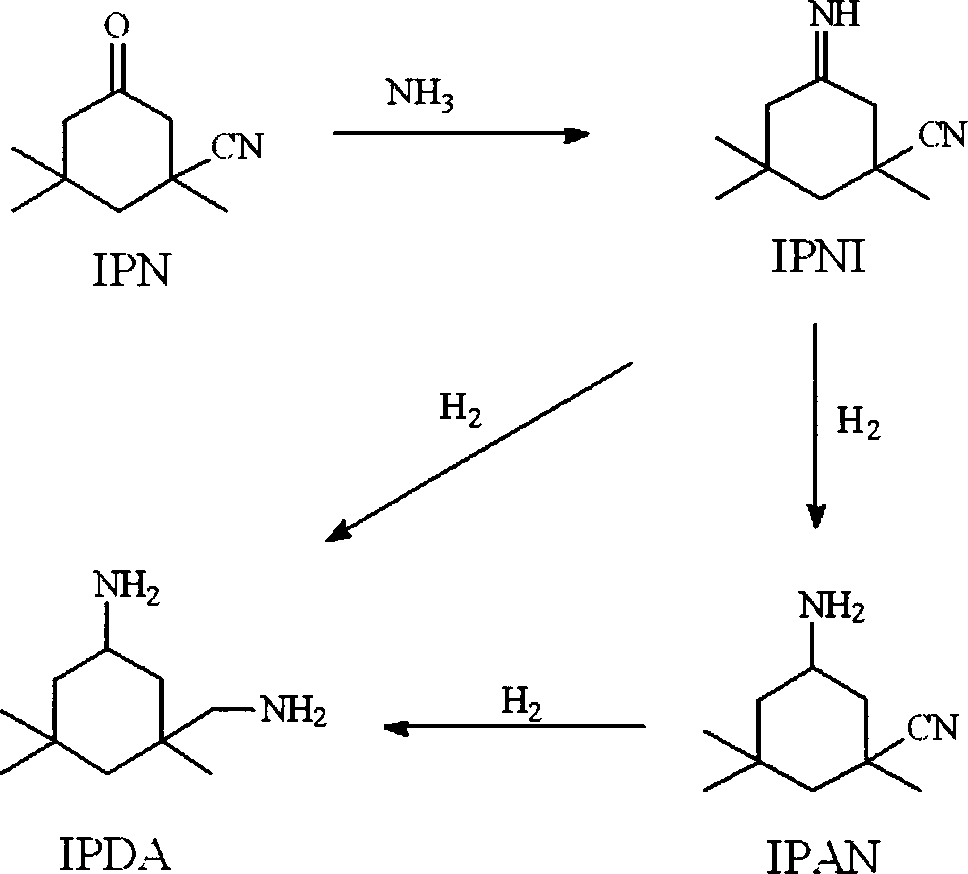
Method for preparing 3-aminomethyl-3,5,5-trimethyl cyclohexylamine
ActiveCN102976956AAvoid the hassle of separationEasy to separateOrganic compound preparationAmino compound preparationCyclohexanoneHydrogenation reaction
The invention provides a method for preparing 3-aminomethyl-3,5,5-trimethyl cyclohexylamine. The method includes: a) reacting 3-cyano-3,5,5-trimethyl cyclohexanone with excess primary amine, while removing water of the reaction, so that IPN is substantially completely converted to an imine compound; b) in the presence of an aminolysis catalyst, mixing the resulting product from step a) with liquid ammonia so as to carry out the aminolysis reaction on the imine compound to form 3-cyano-3,5,5-trimethyl cyclohexylimine and the primary amine; and c) performing the hydrogenation reaction to 3-cyano-3,5,5-trimethyl cyclohexylimine obtained in step b) to get 3-carbamoylyl-3,5,5-trimethyl cyclohexylamine in the presence of hydrogen and a hydrogenation catalyst. The method of the present invention prevents the generation of major byproducts of 3,5,5-trimethyl cyclohexanol, and 3-aminomethyl-3,5,5-trimethyl cyclohexanol in the prior art, thereby improving the yield of 3-aminomethyl-3,5,5-trimethyl cyclohexylamine.
Owner:WANHUA CHEM GRP CO LTD +1
Preparing method of phosphodiesterase 5 inhibitor tadalafil
ActiveCN103980275AGood removal effectHigh chiral purityOrganic chemistryPhosphodiesterase 5 inhibitorTadalafil
The invention relates to a preparing method of a phosphodiesterase 5 inhibitor tadalafil. D-methyl tryptophanate hydrochloride is adopted as an initial raw material, and is subjected to cyclization with heliotropin, N-acylation, aminolysis-cyclization, and other reactions to obtain a tadalafil crude product. The tadalafil crude product is recrystallized to obtain a tadalafil finished product. The method has characteristics of mild reaction conditions, short reaction time, high yield, good product stability and convenience for industrial production.
Owner:湖北省医药工业研究院有限公司
Preparation method of Niraparib
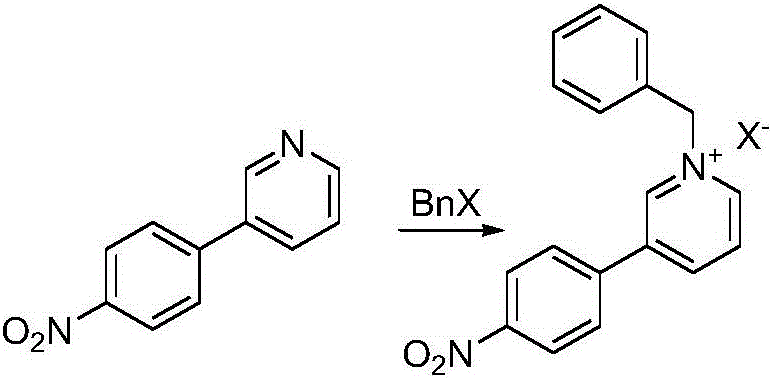
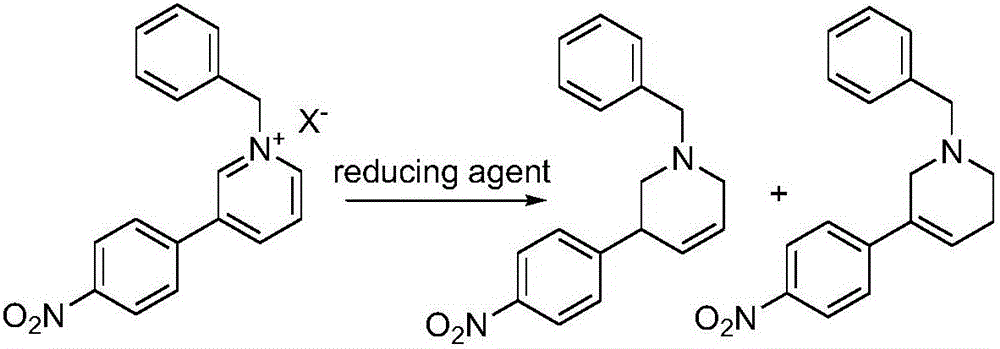
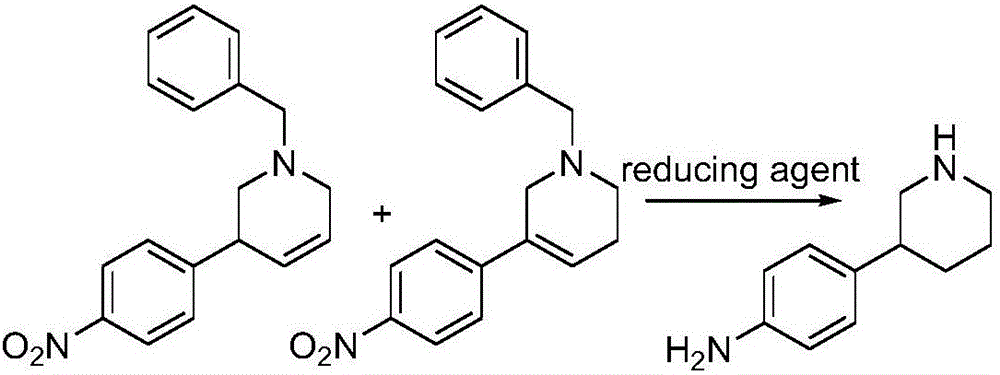
The invention discloses a preparation method of a compound 2-[4-((3S)-3-piperidin) phenyl]-2H-indazole-7-carboxamide; through the reaction of 4-nitryl phenylpyridine and benzyl halides, the benzyl quaternary ammonium salt is generated; the pyridine quaternary ammonium salt is restored selectively through sodium borohydride; under the effect of palladium reagent, the 3-(4- aminophenyl)- piperidine is obtained the (S)-3-(4- halogenated phenyl) piperidine is obtained through manually splitting the reagent, and then condensed with 3- formyl group-2- nitrobenzene methyl benzoate and forms pyrazol ring under the effect of sodium azide; through aminolysis, the Niraparib (molecule entity is: 2-[4-((3S)-3-piperidin) phenyl]-2H-indazole-7-carboxamide) is prepared.
Owner:NANJING CORE TECH CO LTD
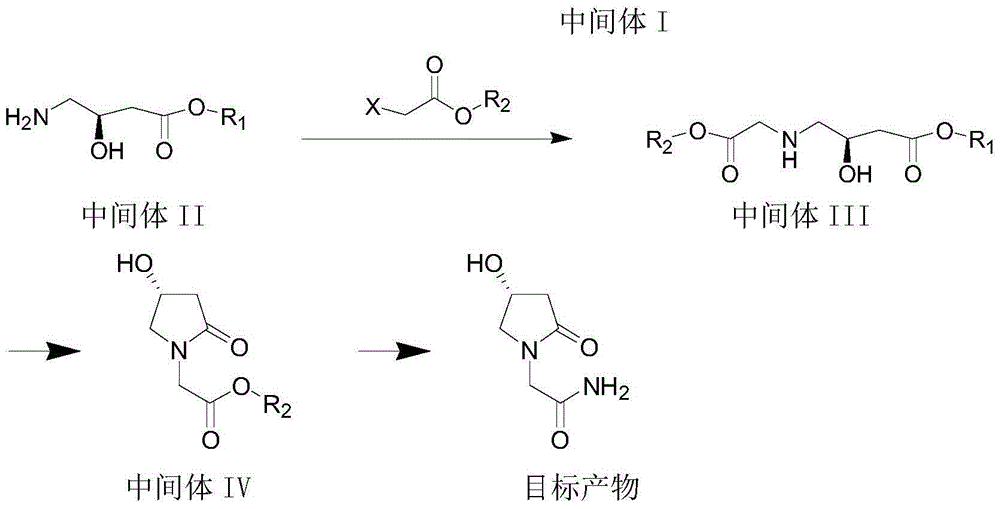
Preparation method for (R)-4-hydroxy-2-oxo-1-pyrrolidine acetamide
The invention provides a preparation method for (R)-4-hydroxy-2-oxo-1-pyrrolidine acetamide. The preparation method comprises the following steps: (1) with R-4-chloro-3-hydroxy butyrate as an initial raw material, subjecting the initial raw material and an azido reaction agent to an azido reaction so as to obtain an intermediate I; (2) subjecting the intermediate I to a reduction reaction so as to obtain an intermediate II; (3) subjecting the intermediate II and halogenated acetate to a condensation reaction so as to obtain an intermediate III; (4) subjecting the intermediate III to a ring closure reaction so as to obtain an intermediate IV; and (5) subjecting the intermediate IV to an aminolysis reaction so as to obtain (R)-4-hydroxy-2-oxo-1-pyrrolidine acetamide. The preparation method can prepare a (R)-4-hydroxy-2-oxo-1-pyrrolidine acetamide product with ideal yield of at least more than 38%, and a novel synthetic route is opened up for (R)-4-hydroxy-2-oxo-1-pyrrolidine acetamide.
Owner:CHONGQING RUNZE PHARM CO LTD
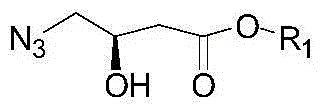
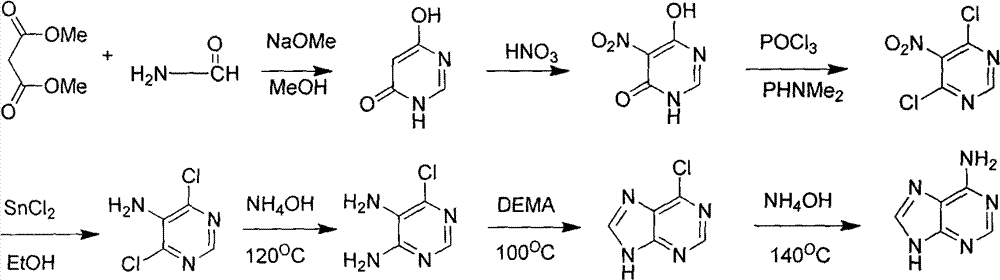
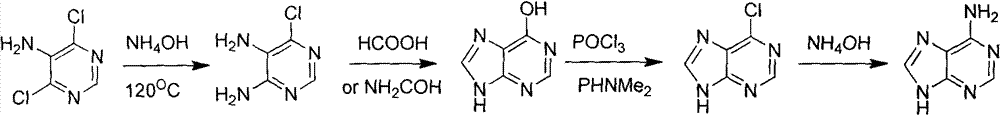
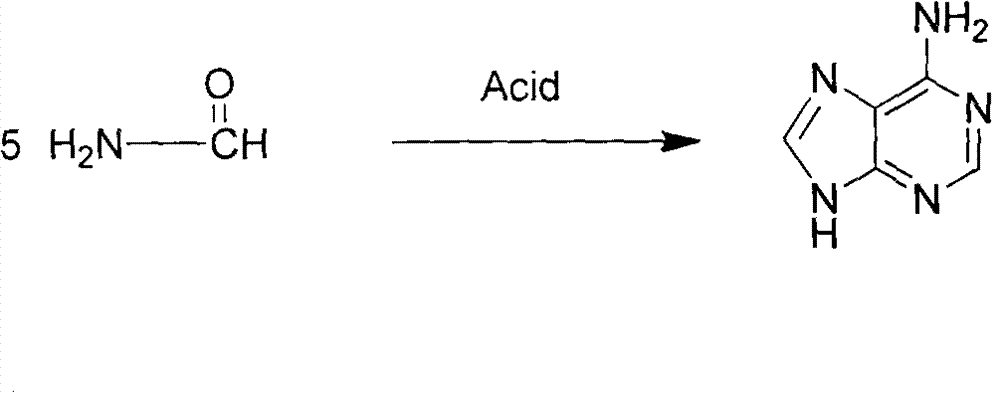
Novel chemical synthesis method for adenine
InactiveCN102887899AShort route stepsIncrease productivityOrganic chemistryChemical synthesisAcetic anhydride
The invention discloses a method for synthesizing adenine represented by a formula (I), wherein the method comprises the following steps of making formamide react with diethyl malonate in an ethanol solution of sodium ethoxide to obtain a raw material 4,6-dihydroxypyrimidine represented by a formula (VI); nitrifying the 4,6-dihydroxypyrimidine to obtain 4,6-dihydroxy-5-nitropyrimidine represented by a formula (V); carrying out chlorination reaction on the 4,6-dihydroxy-5-nitropyrimidine (V) to obtain 4,6-dichloro-5-nitropyrimidine represented by a formula (IV), carrying out aminolysis reaction on the 4,6-dichloro-5-nitropyrimidine (IV) and an saturated aminoethanol solution to obtain 4,6-diamino-5-nitropyrimidine represented by a formula (III); carrying out catalytic hydrogenation on the 4,6-diamino-5-nitropyrimidine (III), and reducing a nitro group to obtain 4,5,6-triaminopyrimidine represented by a formula (II); making the 4,5,6-triaminopyrimidine (II) react with ethyl orthoformate in acetic anhydride to obtain the adenine represented by the formula (I). The method provided by the invention has the advantages of cheap and easily-obtained raw materials, mild reaction conditions, single product, high total yield, low production cost and easiness in industrial production.
Owner:YANGZHOU UNIV
Method for aminolysis of fluorosilicone compounds and separation of fluorine and silicon
InactiveCN1884077AOvercoming the disadvantages of separation methodsImprove conversion rateSilicon halogen compoundsAmmonium halidesPhosphoric acidSilicon tetrafluoride
The invention discloses a separating method of aminolysis and hydrofluosilicic element of silicofluoride, which comprises the following steps: 1. adding solid ammonium fluosilicate or silicon tetrafluoride gas in the saturated ammonium fluoride circulating reacting liquid; putting raw material amino to proceed aminolysis reaction; 2. reducing slurry temperature; screening the reacted slurry; 3. filtering the screened silica suspension and ammonium fluoride crystal slurry to obtain saturated ammonium fluoride solution, silica solid and ammonium fluoride crystal; returning to former procedures as the circulating reacting liquid; washing the ammonium fluoride crystal; drying through normal drying method to obtain high-purity product. The invention can improves technological and economical index, which is fit for wet-producing phosphoric acid, phosphate fertilizer and other phosphor businesses.
Owner:GUIYANG KAILIN FERTILIZER CO LTD
Method of synthesizing 2-aminopropanol
InactiveCN101033193AReduce usageEmission reductionOrganic compound preparationAmino-hyroxy compound preparationCycloadditionAminolysis
This invention provides a synthesizing method for aminopropanediol including the following steps: 1, taking propylene epoxide and 15-38% HCl solution as the raw materials to carry out ring opening addition reaction without solvent or in an organic solvent1 under -5-85deg.C to refine and collect beta-propyl alcohol chloride, 2, beta-propyl alcohol chloride and excessive liquid ammonia carry out aminolysis without solvent or in organic solvent2 catalyzed by hydrohalogenic salt under 50-200deg.C to get said aminopropanediol.
Owner:ZHEJIANG UNIV
Preparation method of lorcaserin hydrochloride
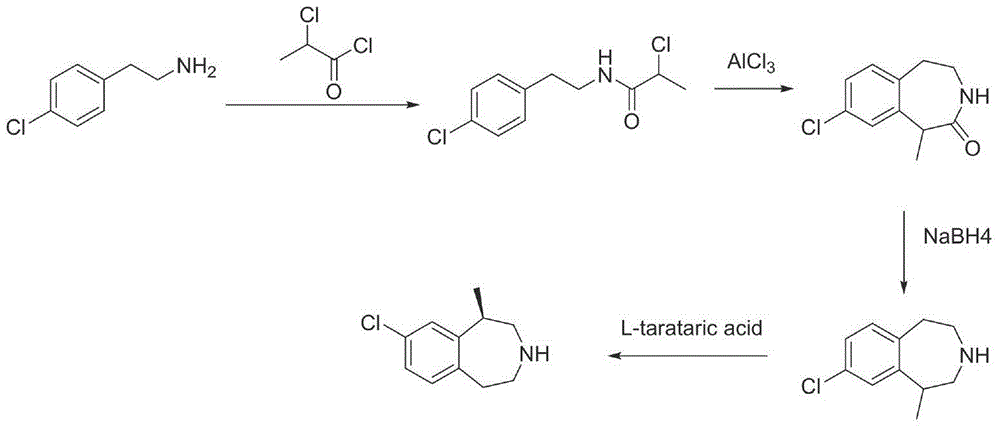
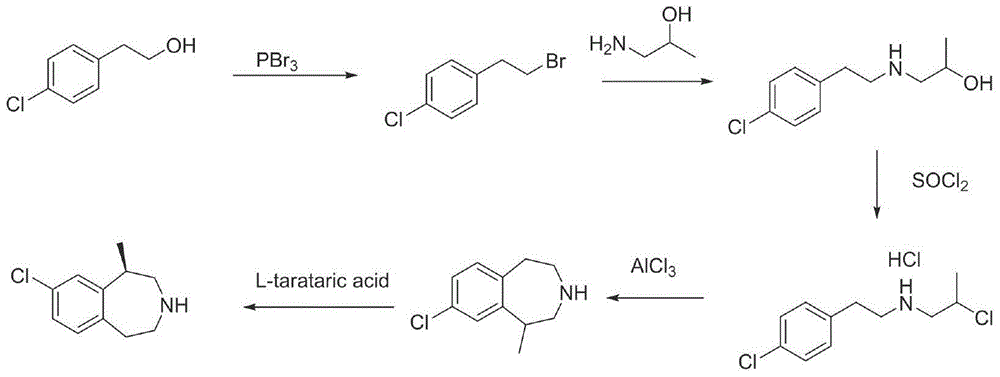
The invention provides a preparation method of lorcaserin hydrochloride, namely (R)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride. The method comprises the following steps: taking p-chlorophenyl acetate as raw material, performing aminolysis with isopropanol amine, then performing chlorination, reduction and Friedel-Crafts alkylation, further splitting by L-tartaric acid, and performing salt formation to get the lorcaserin hydrochloride. The invention provides the preparation method of the lorcaserin hydrochloride. The method has the advantages of low cost, simplicity in operation and higher product purity; according to the process disclosed by the invention, the continuous operation can be performed, and the production cycle is greatly reduced; meanwhile a reagent is low in price and easy to obtain, the operation is simple, and the post-treatment is simple and convenient, so that the preparation method is a brand new and economic synthesis method which can realize industrial production.
Owner:SUZHOU HUIHE PHARMA
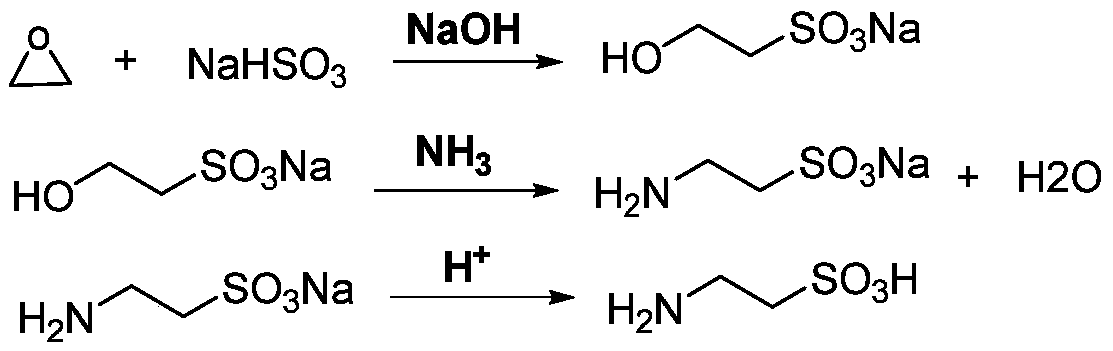
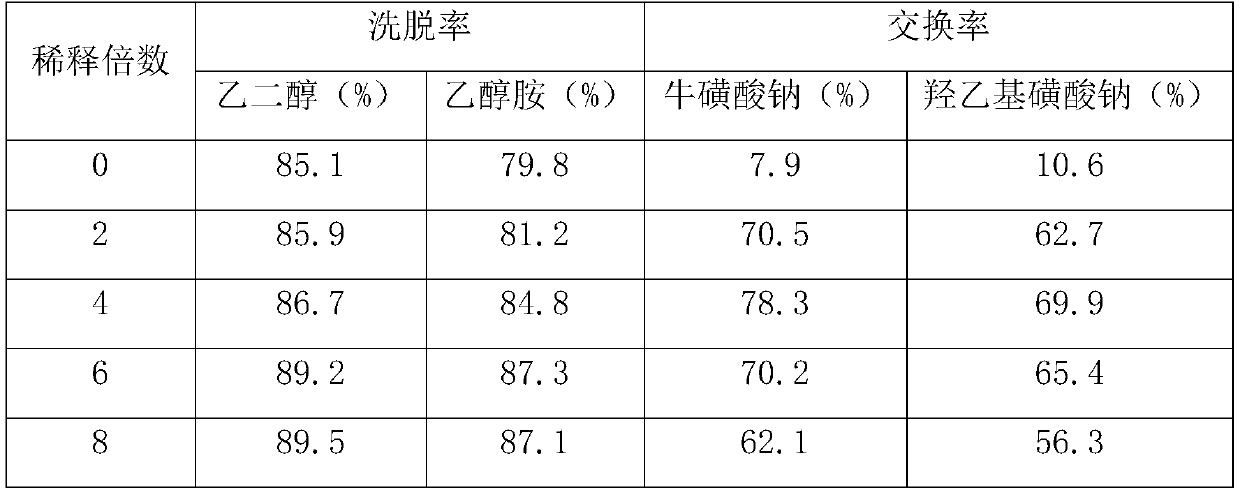
Method for preparing taurine
InactiveCN110452136AHigh yieldSimple production processOrganic compound preparationSulfonic acid preparationSolubilityOrganic solvent
The invention relates to a method for preparing taurine. The method comprises the following steps: performing treatment on a solution containing an alkali metal salt of 2-hydroxyethanesulphonic acid through an acidic cation exchange resin column to acidify the alkali metal salt of the 2-hydroxyethanesulphonic acid to form 2-hydroxyethanesulphonic acid, and performing collection to obtain an eluate; and performing recrystallization on the eluate to obtain the 2-hydroxyethanesulphonic acid, performing aminolysis on the 2-hydroxyethanesulphonic acid, and performing acidification to obtain the taurine. In the process of removing impurities in the taurine reaction system provided by the invention, no other organic solvent is introduced to remove the impurities, the acidic cation exchange resincolumn is adopted to acidify the alkali metal salt of the 2-hydroxyethanesulphonic acid and an alkali metal salt of the taurine to form an acid with lower solubility, and organic impurities and / or electrically neutral impurities are easy to separate by recrystallization; and the method provided by the invention can improve a conversion rate of the alkali metal salt of the 2-hydroxyethanesulphonicacid, has a simple production process, can reduce production energy consumption, and facilitates environmental protection.
Owner:HUBEI GRAND LIFE SCI & TECH CO LTD
Synthesizing process of fatty diglycollic amide
InactiveCN100999482ASimple processLess side effectsOrganic compound preparationCarboxylic acid amides preparationPotassium fluorideFiltration
This invention relates to a synthesis method of fatty acids diethanolamine. It takes fatty acid and diethanolamine as raw materials. The method includes follow steps: first fatty acids and a certain amount of diethanolamine for dehydration and condensation reaction, then adding the remaining diethanolamine and alkaline catalyst for aminolysis reaction, the final using activated clay for adsorption treatment, and through filtration to obtain fatty acid diethanolamine. The weight proportion between fatty acids and diethanolamine is 1:0.5 ~ 0.6; account for weight, 30 ~ 70% diethanolamine add in dehydration and condensation reaction, and the remaining add in aminolysis reaction; its alkaline catalyst is one of the alkali metal hydroxide, potassium fluoride and sodium borohydride, account for 0.3 to 2.0% of total weight of fatty and diethanolamine.
Owner:王伟松
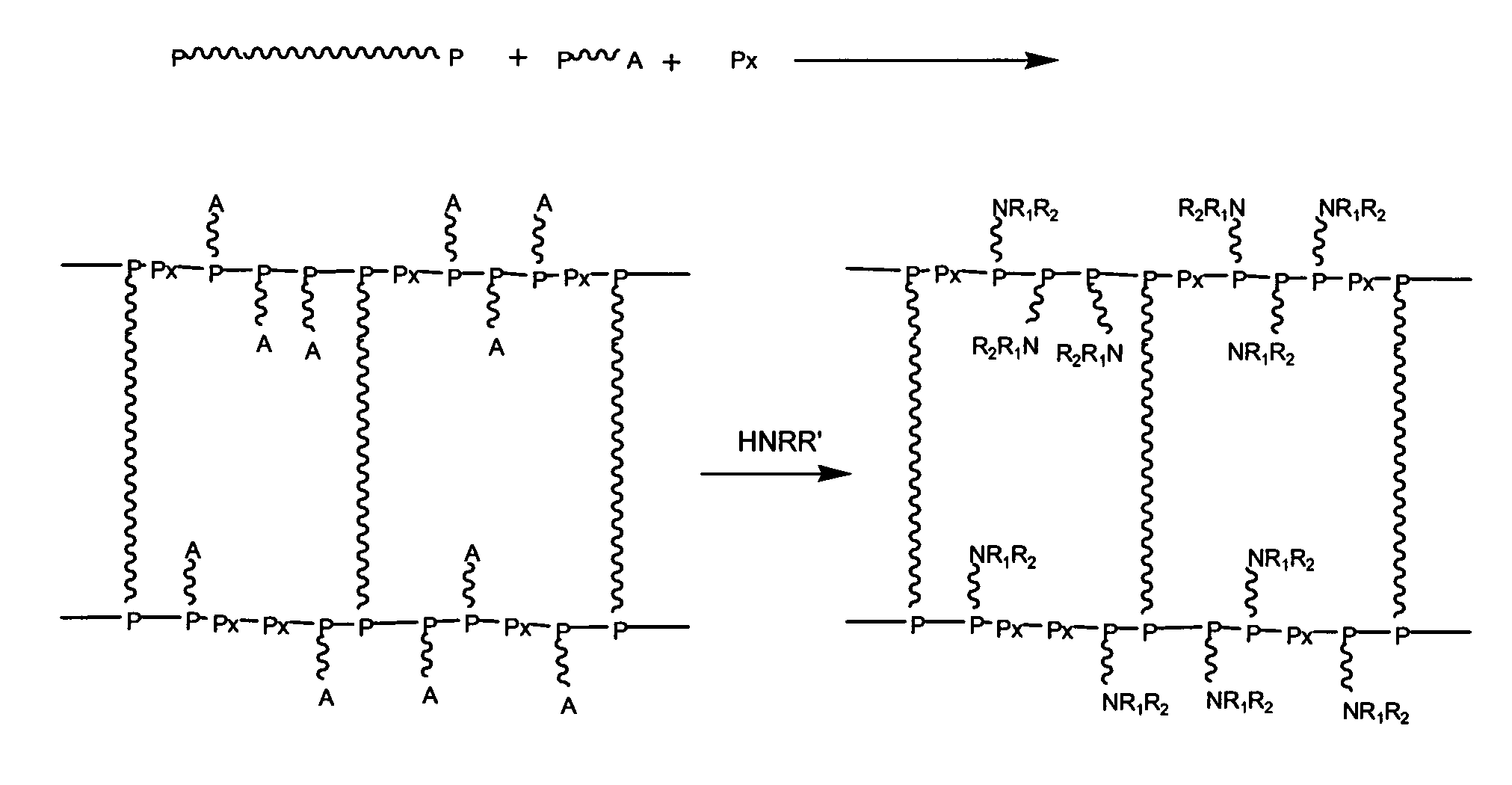
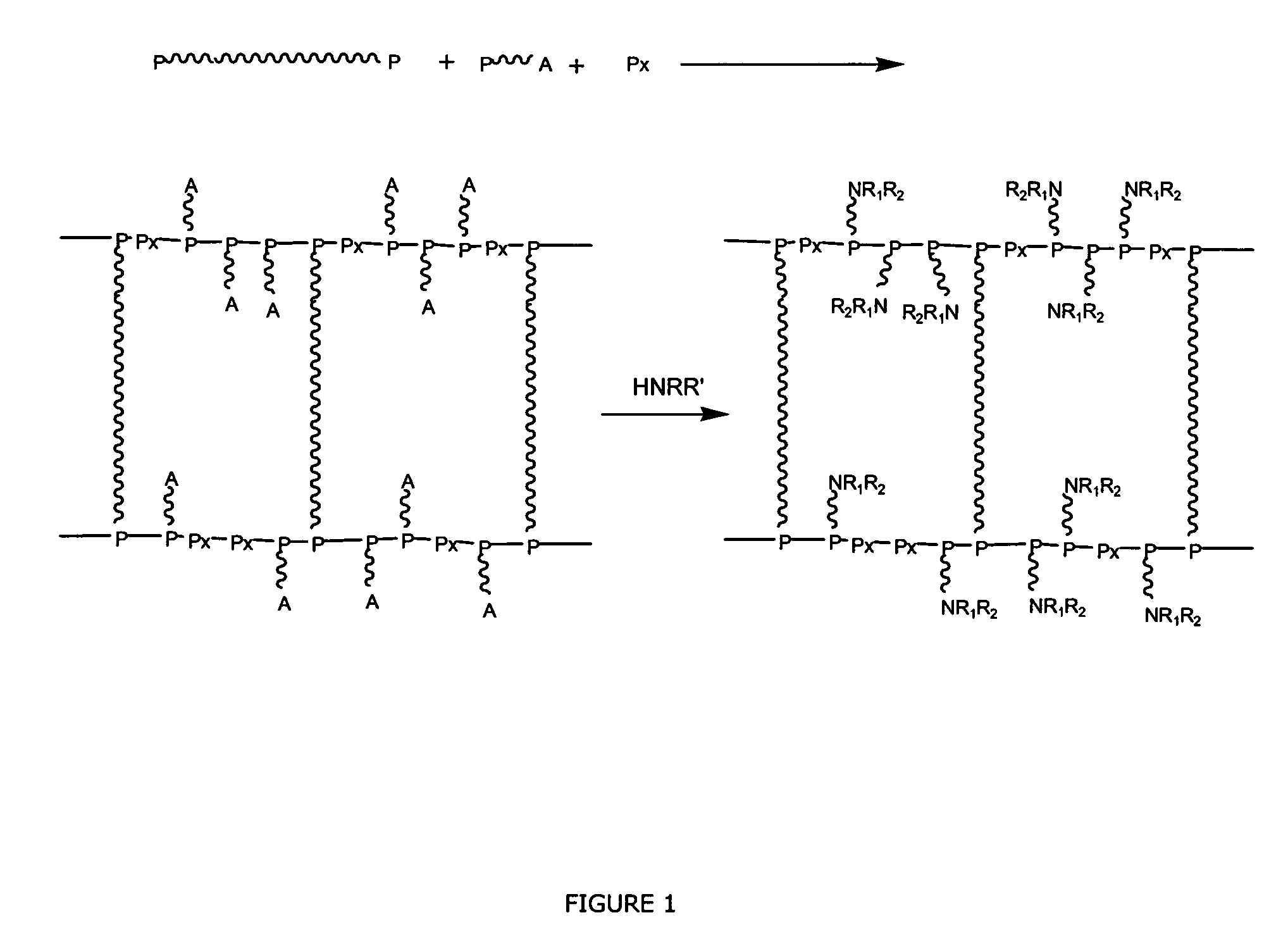
Polyether Polymer Matrix
The present invention relates to polymer resins, methods for their generation and uses thereof. In one aspect the present invention is directed to a resin obtainable by aminolysis of a precursor resin, wherein the precursor resin is obtainable by polymerisation of i) polydisperse di- or oligofunctional vinyl or cyclic ether compounds and ii) aminolytically sensitive, mono-functional vinyl or cyclic ether compounds.
Owner:NOVO NORDISK AS
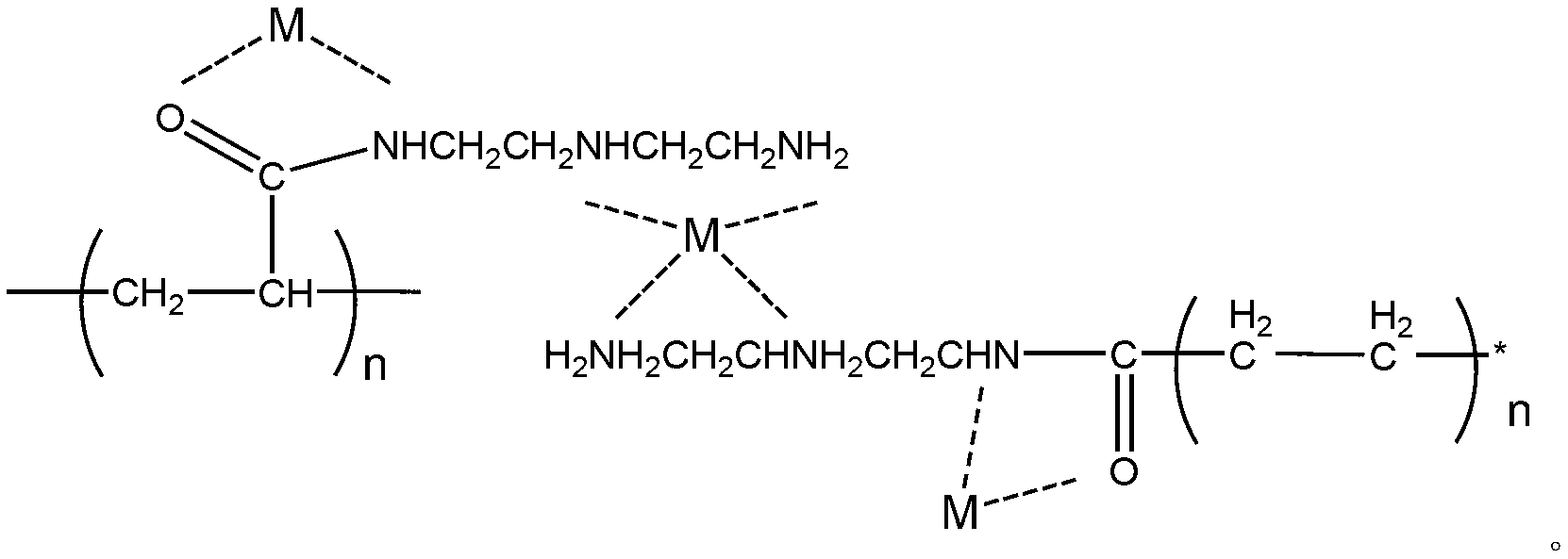
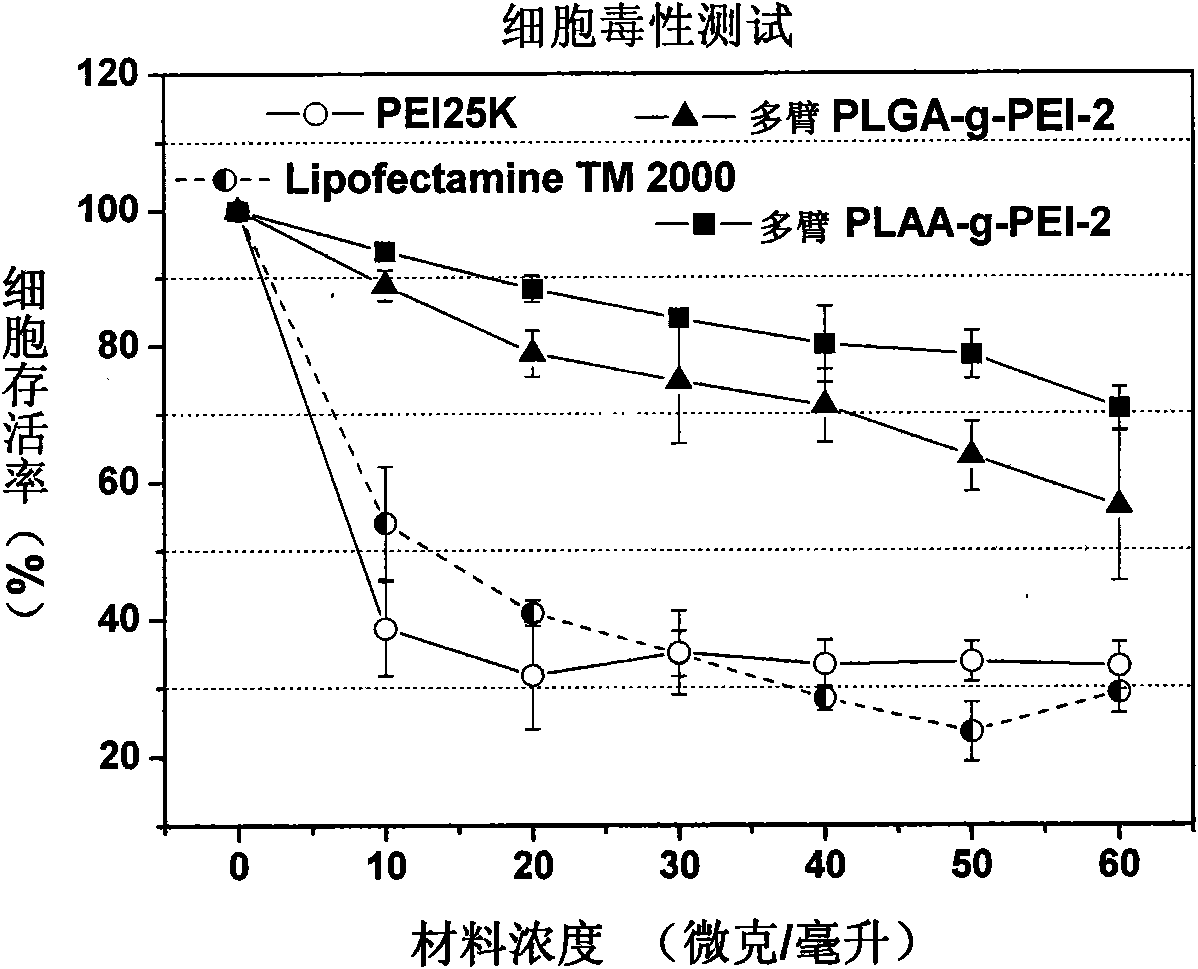
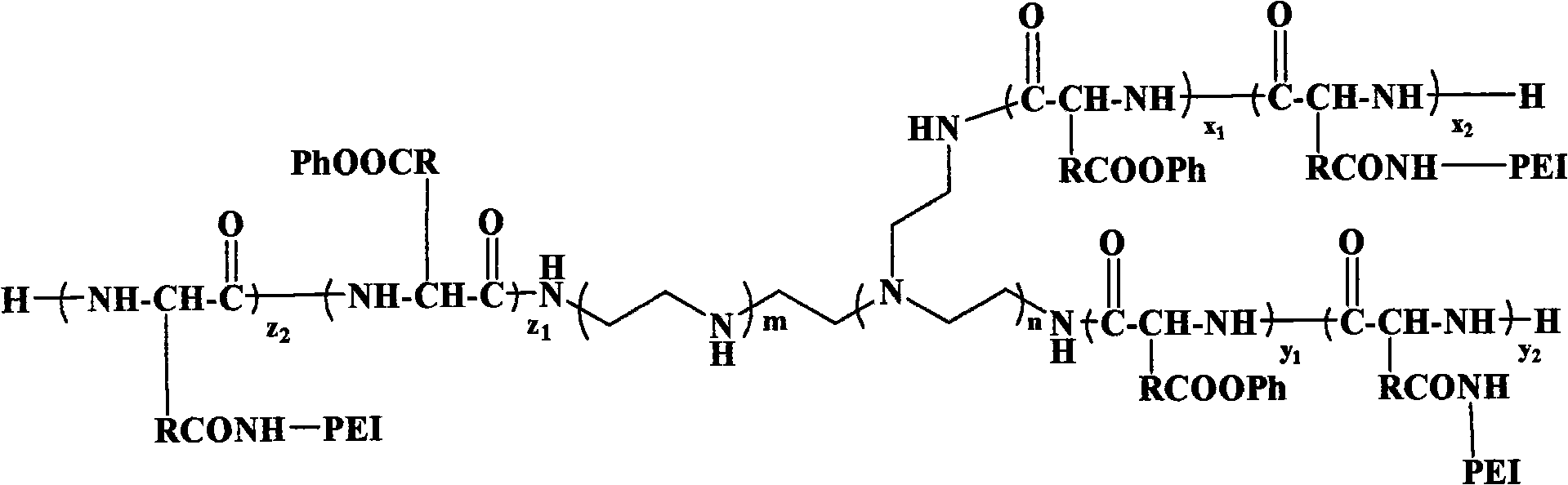
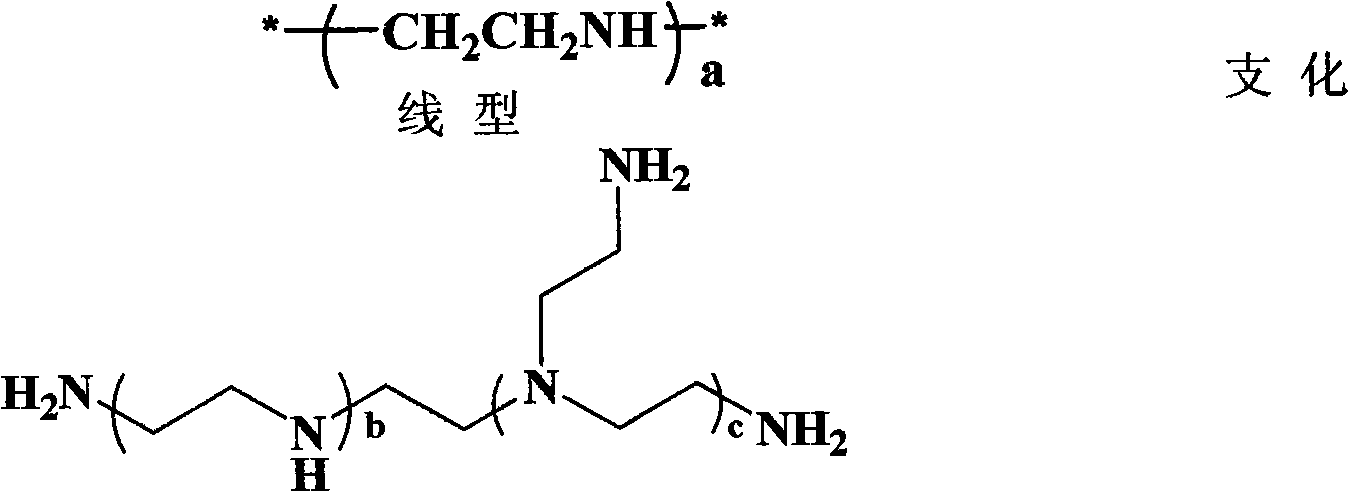
Multi-arm polyamino acid (ester) grafted polyethyleneimine copolymer, preparation method and application in gene delivery
InactiveCN101575416ALow toxicityHigh transfection efficiencyOther foreign material introduction processesVector-based foreign material introductionGene deliveryCytotoxicity
The invention relates to a multi-arm polyamino acid (ester) grafted polyethyleneimine copolymer, a preparation method and application in gene delivery. The method includes the steps of: dissolving polyamine initiator in organic solvent and initiating the ring opening polymerization of alpha-amino acid-N-carboxylic acid anhydride protected by phenmethyl under anhydrous and oxygen free conditions to obtain the multi-arm polyamino acid (ester); and then causing the full or partial aminolysis reaction to occur between benzyl ester at the lateral chain of the multi-arm polyamino acid (ester) and amino group of polyethyleneimine to form the grafted copolymer. The polymer is a novel high-efficiency polycation gene vector, integrates the properties of polyethyleneimine and polyamino acid and is high in transfection efficiency, and the highest transfection efficiency to the mediate luciferase plasmid of Chinese Hamster Ovary epithelial cell is ten times than that of the Lipofectamine2000 of the commercial transfection reagent in US Invitrogen biomax under the same conditions; cytotoxicity is small; and cell survival rate is over 80% within the best transfection rate range, thus having broad application prospect.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
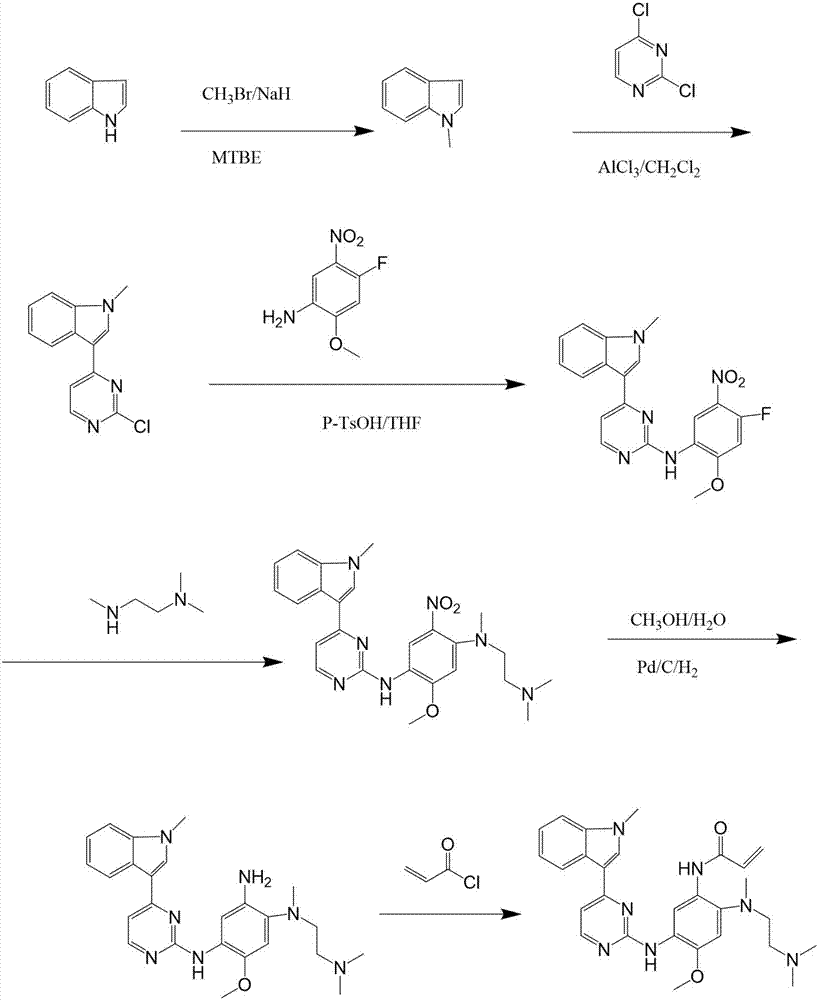
Preparation method of osimertinib
The invention relates to a preparation method of osimertinib. The method comprises the steps: taking 5-fluorine-2-nitroanisole as a starting material, performing aminolysis, reduction, nitration and Lewis acid reaction, carrying out chlorination with N-methyl indol, performing nitryl reduction and amidation to form a product, and finally performing salification. The yield of each step of the technology is higher and reaches above 80%; the method is simple to operate and suitable for amplified production; and the purity is high.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Use of n-hydroxysuccinimide to improve conjugate stability
InactiveUS20140350228A1Improve stabilityAntibody ingredientsImmunoglobulinsBiochemical engineeringCombinatorial chemistry
The invention provides processes for manufacturing cell-binding agent-cytotoxic agent conjugates of improved stability in the presence of exogenous NHS. In some embodiments, the inventive process comprises the addition of a molar ratio of exogenous NHS with respect to the amount of NHS generated during the modification reaction as a result of hydrolysis / aminolysis of the bifunctional linker.
Owner:IMMUNOGEN INC
Liquid ethylene based polysilazane resin and preparation thereof
The invention relates to a preparation method of liquid vinyl poly-silazane resin. The technique is characterized in that: by controlling the proportion of aminolysis-raw material methyl vinyl dichlorsilane and methyl hydrogen dichlorsilane in the preparation method, a hexahydric or octatomic ring-shaped vinyl silazane mixture which is provided with a Si-H bond and 2 to 3 vinyl double bonds on a ring body is synthesized; and by the additive reaction of platina-series catalyst catalyzed silicon hydride and the vinyl double bonds, the vinyl poly-silazane resin which has a branched structure and proper viscosity is polymerized by the micro-molecule silazane mixture. The vinyl poly-silazane resin prepared by the method of the invention has the advantages that: the yield thereof can achieve 44-50 percent of the usage of chlorosilane, the M thereof can be up to 4500 shown by the analysis of gel permeation chromatography (GPC) and the molecular weight dispersion coefficient thereof is within 1.5-3.4. In addition, the viscosity is within 300-2000mPa question mark s( 25 DEG C, tested by a rotation viscometer).
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com