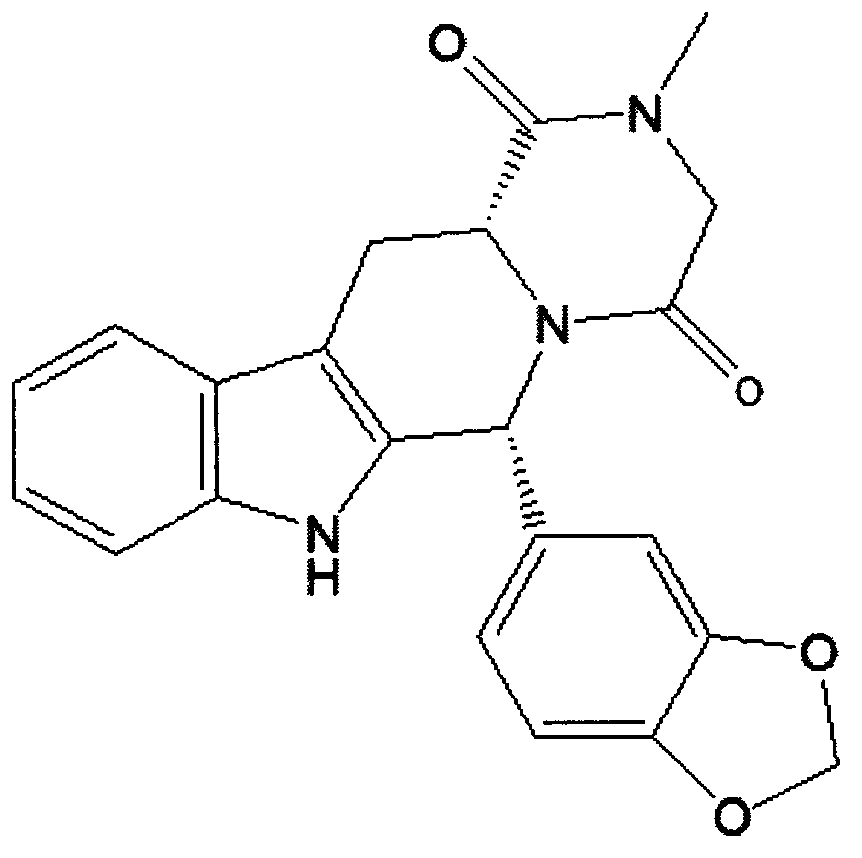
Simple preparation process of tadalafil
A tadalafil, multiple technology, applied in the field of organic synthesis, can solve the problems of low reaction yield isomers, cumbersome operation, long reaction route, etc., to achieve the effect of shortening the production cycle, improving process efficiency and shortening the route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
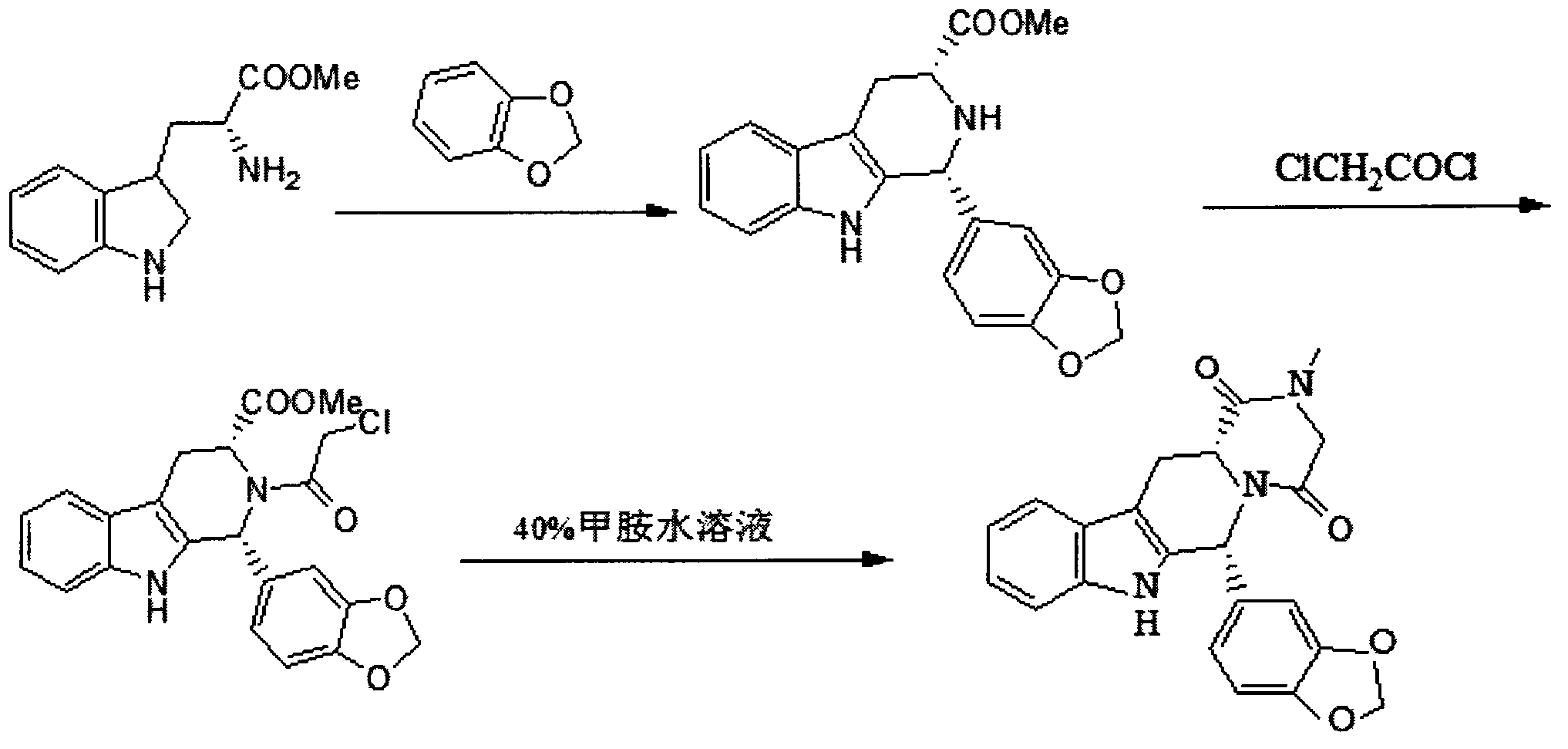
Embodiment 1
[0035] Preparation of cis-tetrahydrocarline intermediate
[0036] D-tryptophan methyl ester hydrochloride 20g, piperonal 10.2~12.2ml, isopropanol 160~200ml in a 250ml three-necked flask equipped with a reflux device and a thermometer, heat and reflux and stir for 9~10h, after the reaction is complete, stir Cool down to room temperature, filter, and dry the filter cake to obtain a pale yellow cis-tetrahydrocarline intermediate with a molar yield of 94-96%.
Embodiment 2
[0038] Preparation of cis-tetrahydrocarbazine intermediate
[0039] D-tryptophan methyl ester hydrochloride 20g, piperonal 10.2~12.2ml, nitromethane 160~200ml in a 250ml three-necked flask equipped with a reflux device and a thermometer, heat and reflux and stir for 9~10h, after the reaction is over, stir Cool to room temperature, filter, and dry the filter cake to obtain a pale yellow cis-tetrahydrocarline intermediate with a molar yield of 90-92%.
Embodiment 3
[0041] Preparation of chloroacetyl intermediate
[0042] Stir 160-200ml of ethyl acetate and 33-43g of potassium carbonate in a 250ml three-necked flask equipped with a thermometer for about 30min, then put in the cis-tetrahydrocarline intermediate prepared in the previous step, and control the temperature in an ice bath below 3-5°C Slowly add chloroacetyl chloride (8.8-10.6ml) diluted 5-6 times with ethyl acetate dropwise, continue the reaction for 1-1.5h, then rise to room temperature and react for about 1.5h, the reaction is complete, add about 400ml of water, extract, The organic phase was concentrated under reduced pressure to become a brown powder solid with a molar yield of 78-80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com