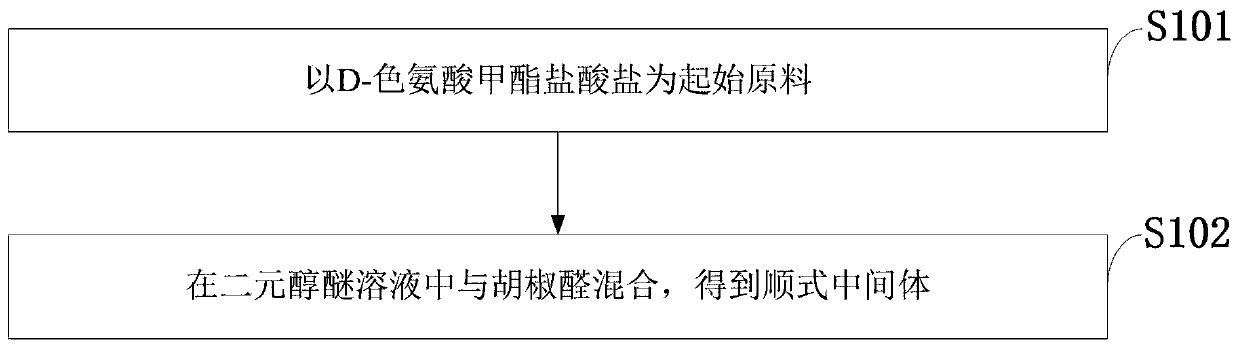
Patents
Literature
63 results about "Tryptophan methyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
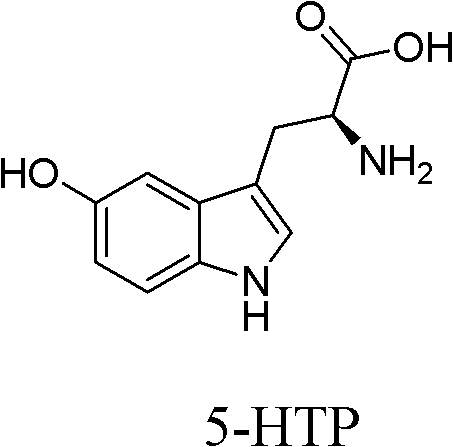
L-Tryptophan methyl ester hydrochloride 98% CAS Number 7524-52-9. Empirical Formula (Hill Notation) C 12 H 14 N 2 O 2 · HCl . Molecular Weight 254.71 . Beilstein Registry Number 4240280 . EC Number 231-385-5. MDL number MFCD00066134. eCl@ss 32160406 . PubChem Substance ID 24862436
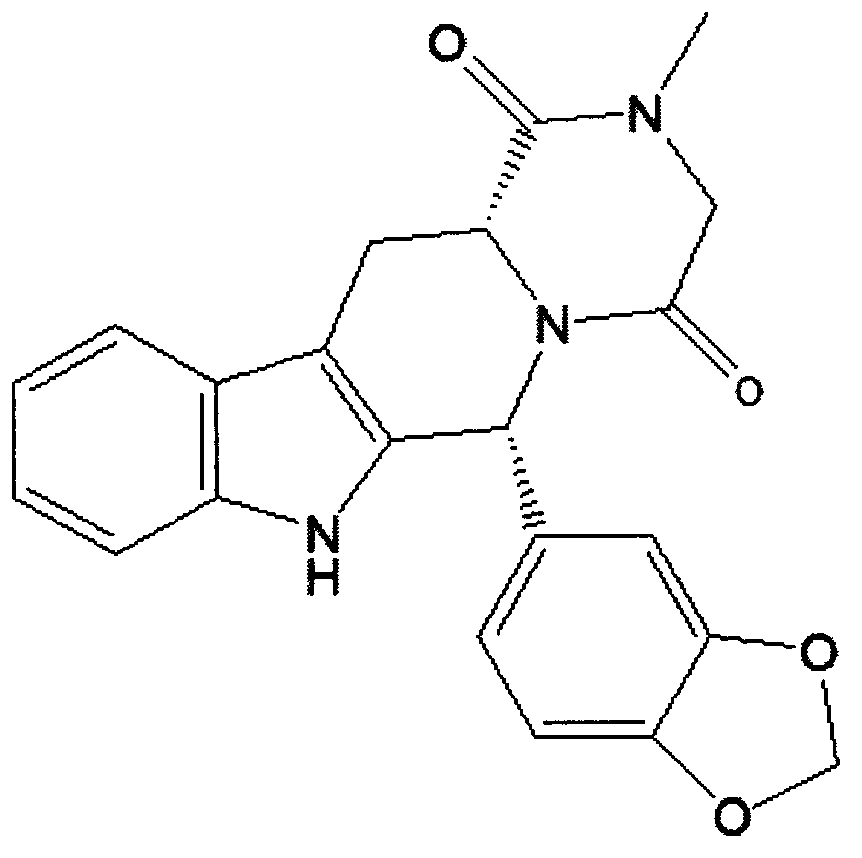
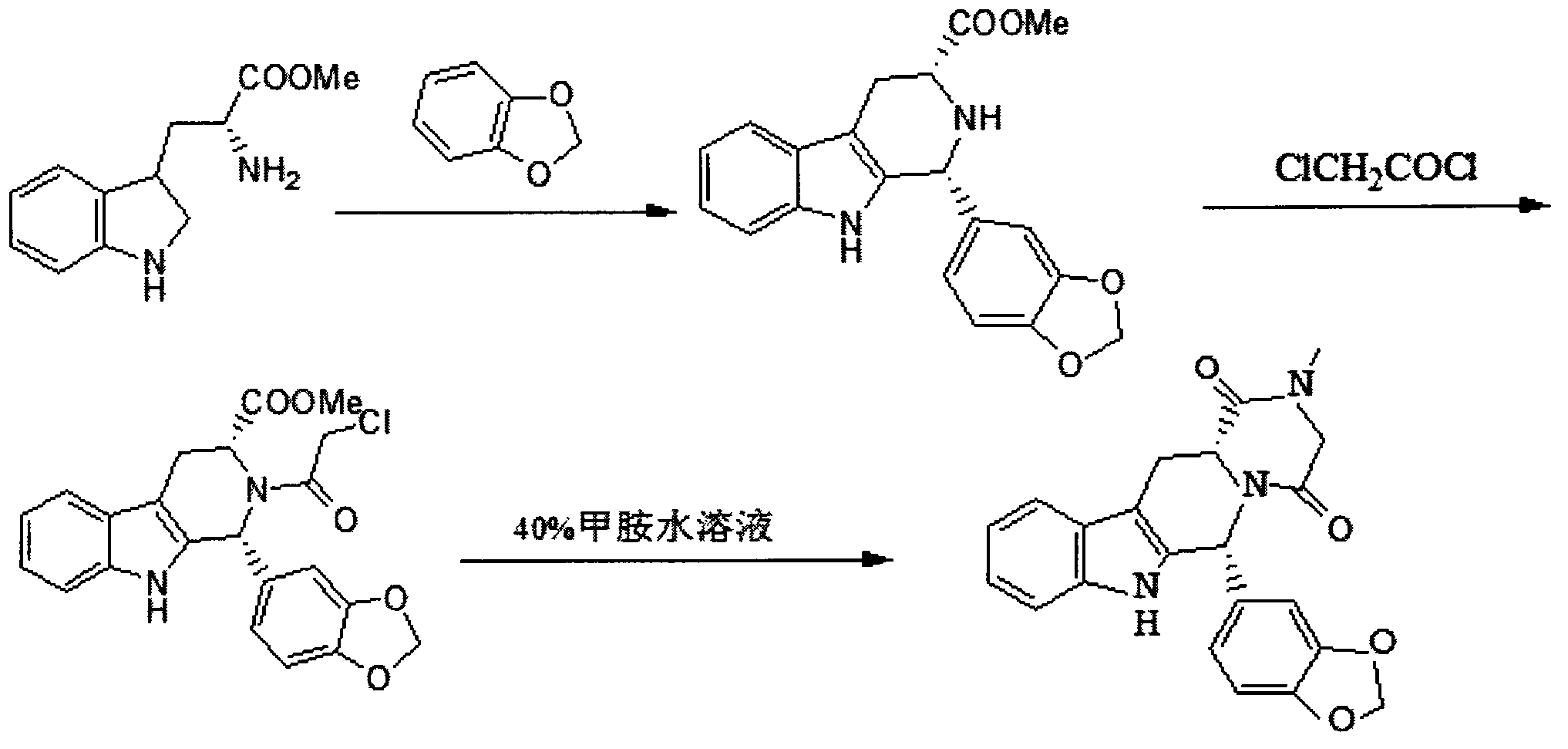
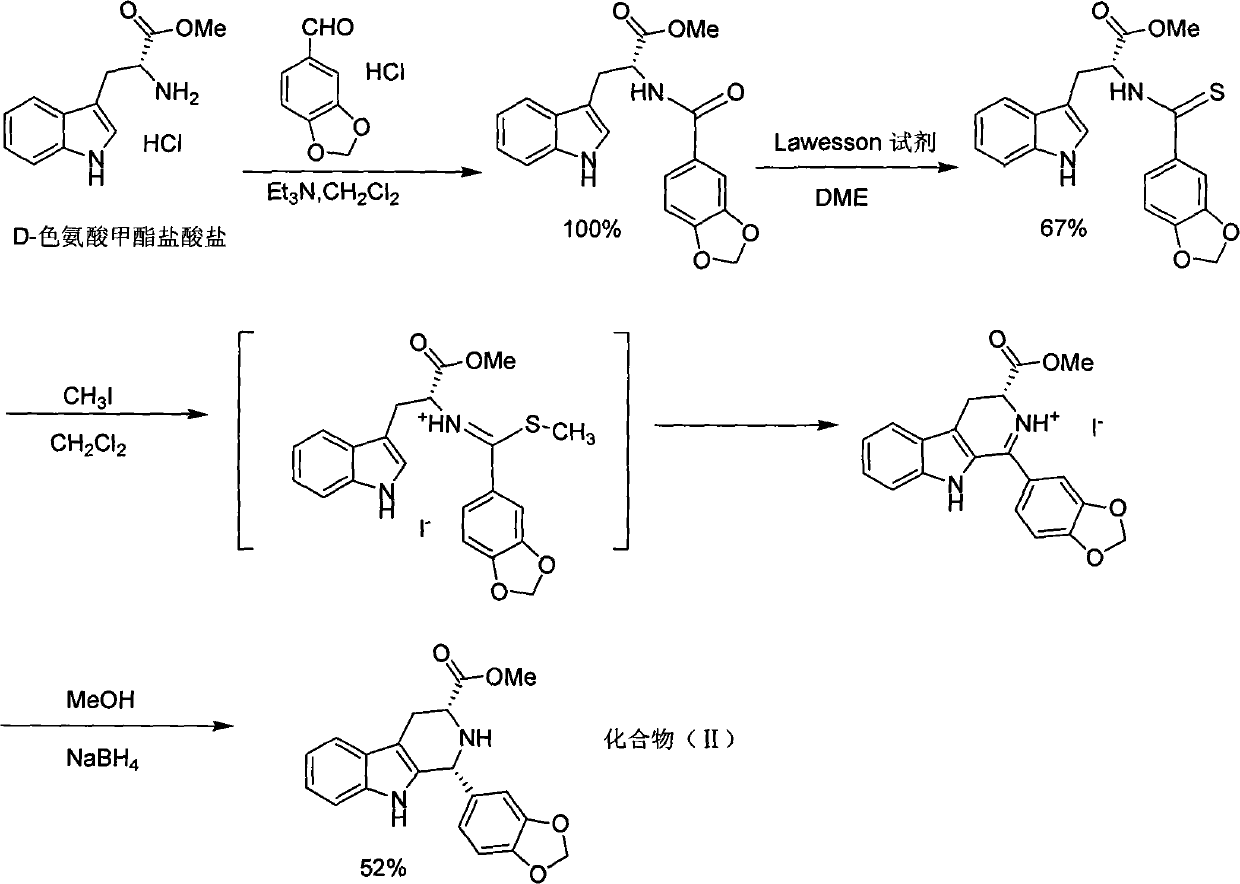
Simple preparation process of tadalafil
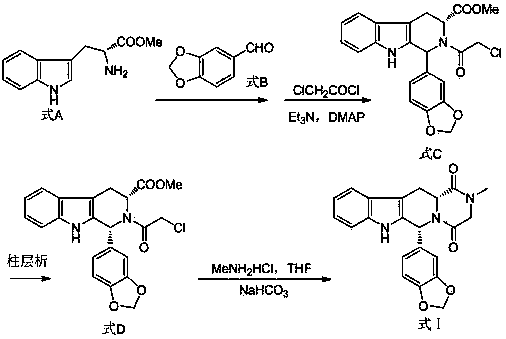
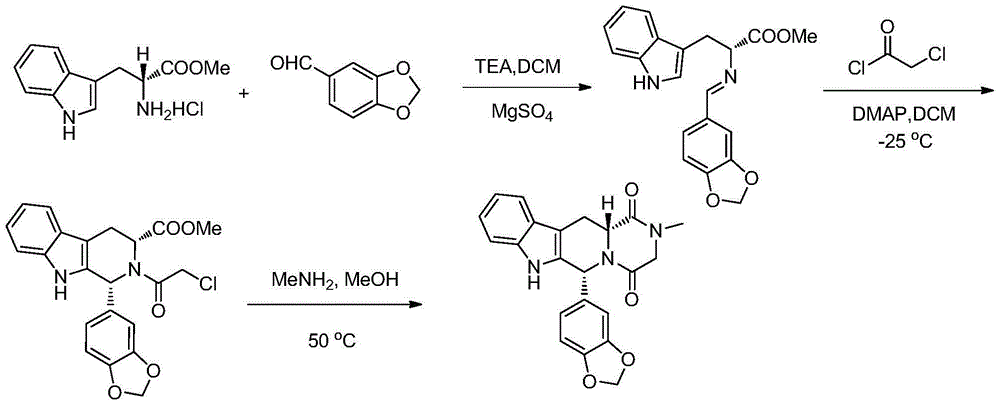
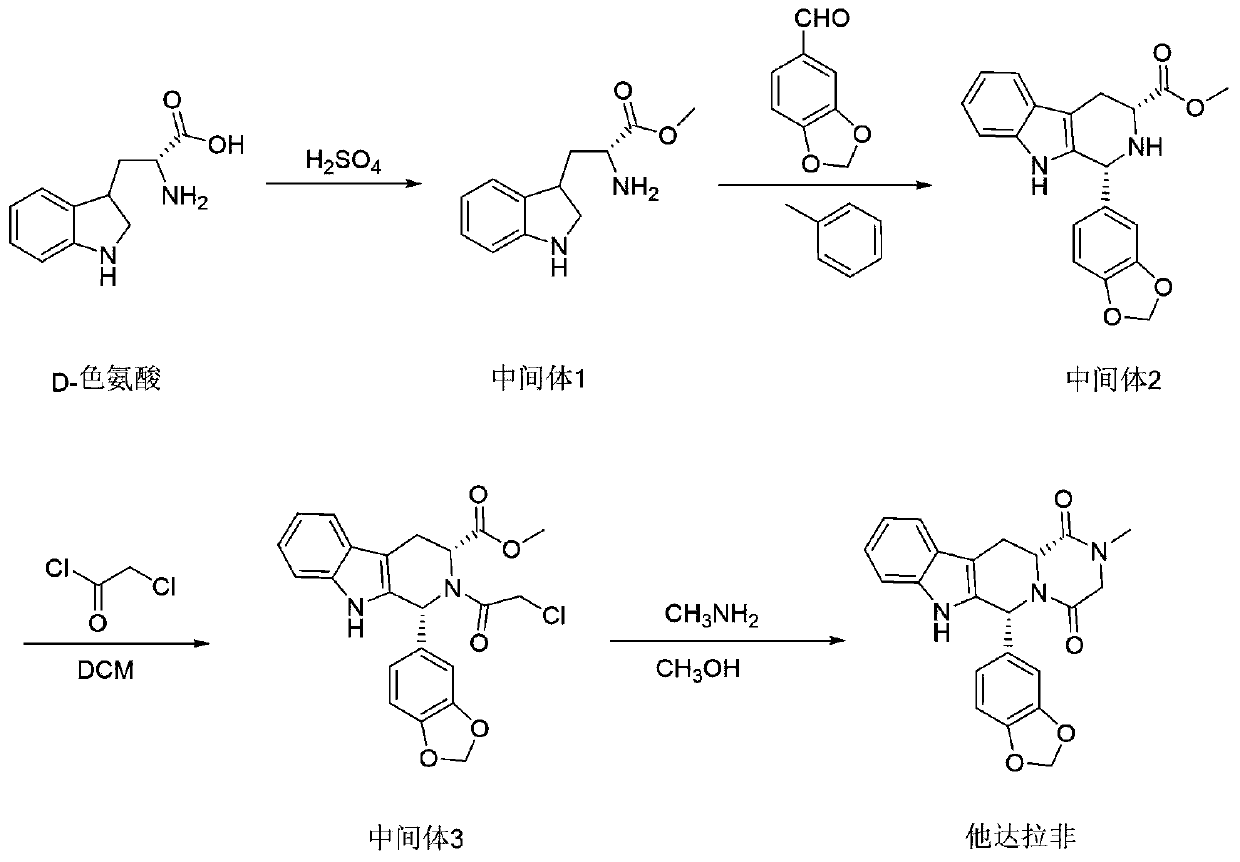
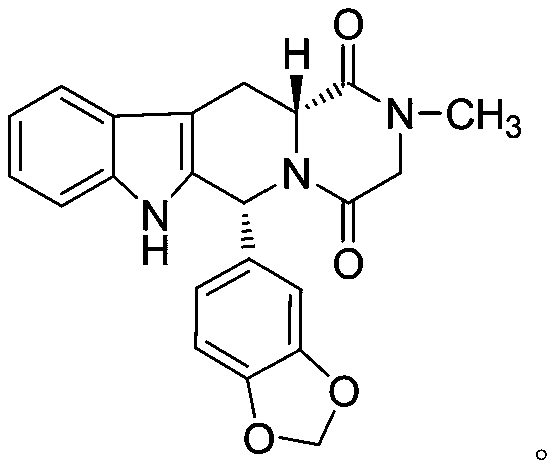
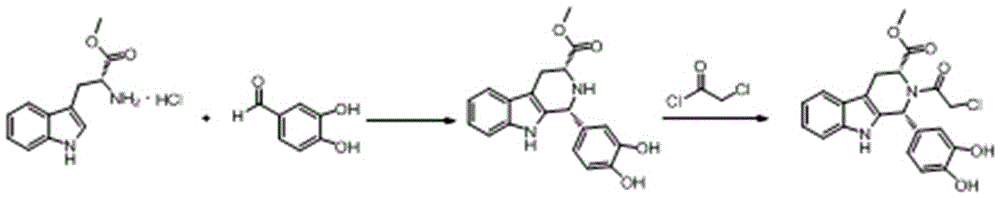
The invention relates to a preparation method of tadalafil. A product is obtained through condensation and cyclization, chloromethylation and aminolysis cyclization reaction based on D-tryptophan methyl ester hydrochloride and heliotropin as starting preparation materials. The simple preparation process is characterized in that condensation and cyclization are used for solving an isomer problem caused by Pictet-Spengler reaction by using isopropanol or nitromethane as a solvent; the yield of ethyl acetate in the aminolysis cyclization reaction is obviously improved; the aminolysis cyclization route includes three preparation steps, wherein the reaction yield of each step is high, the relevant impurities are easy to separate, the reaction conditions are simple, the production period is shorter, and toxic and highly corrosive reagents are not used, and therefore, the simple preparation process is safe and environment-friendly and easy to industrially produce, so that the high-purity qualified products are obtained.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Preparation method of tadalafil
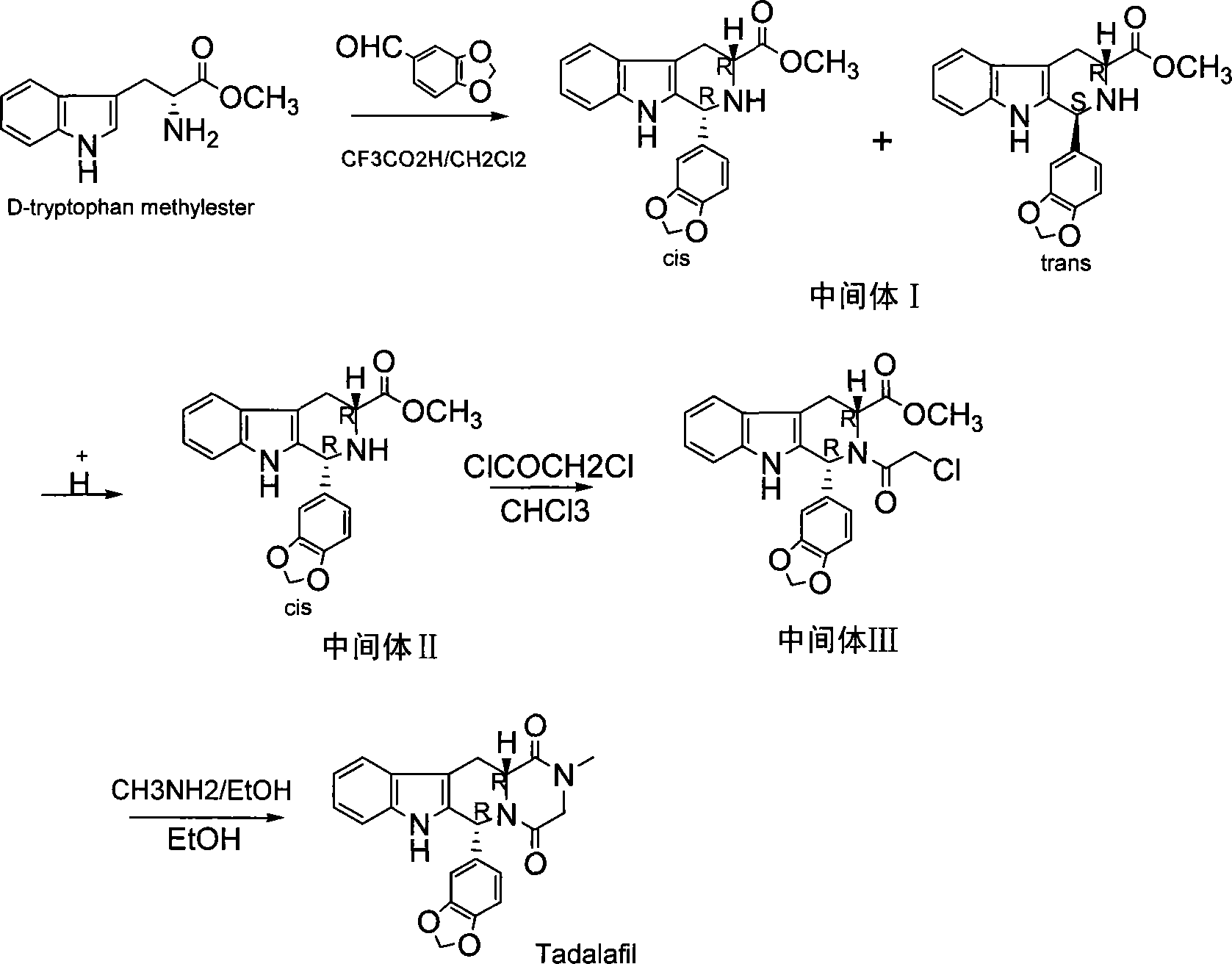
The invention discloses a preparation method of tadalafil. The concrete preparation method is used for successfully synthesizing tadalafil by taking L-tryptophan methyl ester hydrochloride as an initial raw material and utilizing the characteristics that an ortho-position of an ester group has the chiral inversion property under an alkaline condition, the reaction between ester group and Pictet-Spengler is reversible reaction, and the ester group is easily converted into a cis-form product with relatively small solubility, therefore, the preparation method is a brand new technology. The price of L-tryptophan methyl ester hydrochloride is only less than 1 / 5 of that of D-tryptophan methyl ester hydrochloride, so that the cost of the overall route is greatly lower than that of the prior art, and then the preparation method is suitable for industrial production.
Owner:优标易站(苏州)电子商务有限公司
Preparing method of phosphodiesterase 5 inhibitor tadalafil
ActiveCN103980275AGood removal effectHigh chiral purityOrganic chemistryPhosphodiesterase 5 inhibitorTadalafil
The invention relates to a preparing method of a phosphodiesterase 5 inhibitor tadalafil. D-methyl tryptophanate hydrochloride is adopted as an initial raw material, and is subjected to cyclization with heliotropin, N-acylation, aminolysis-cyclization, and other reactions to obtain a tadalafil crude product. The tadalafil crude product is recrystallized to obtain a tadalafil finished product. The method has characteristics of mild reaction conditions, short reaction time, high yield, good product stability and convenience for industrial production.
Owner:湖北省医药工业研究院有限公司
Improved tadalafil preparation method
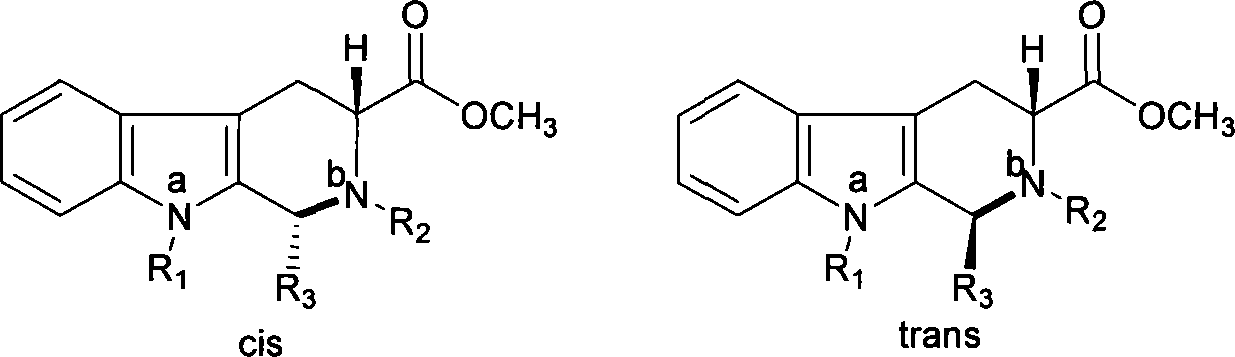
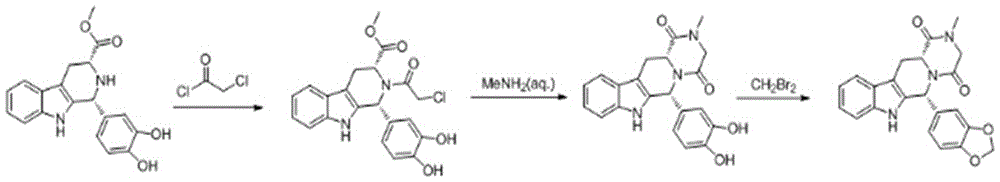
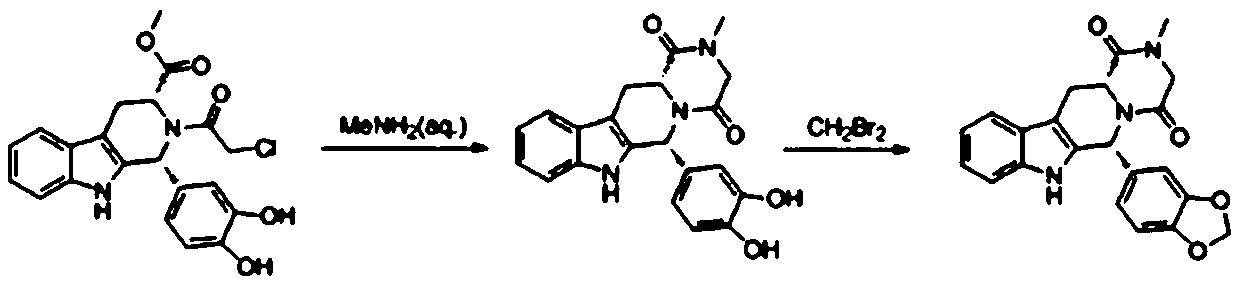
The invention belongs to the field of preparation of chemical raw medicaments, and more in particular relates to an improved preparation method for a phosphodiesterase 5 inhibitor tadalafil. A specific synthesis route is shown in the specification. The method comprises the following steps of performing Pictet-Spengler cyclization reaction and chloroacetyl chloride acylation on starting reactants (D-tryptophan methyl ester hydrochloride and piperonal) to obtain an intermediate product, directly performing subsequent reaction on the intermediate product without purification, preparing an intermediate 1-(1,3-benzodioxol-5-yl)-2-(chloracetyl)-2,3,4,9-tetrahydro-1H-pyridino-[3,4,-B]indol-3-thiophenate methyl by using a one-pot reaction method, performing column chromatography purification to obtain a single cis-isomer, and finally reacting the single cis-isomer with methylamine hydrochloride in the presence of an inorganic base to obtain the tadalafil.
Owner:ANHUI WANBANG MEDICAL TECH
Method for synthesizing phosphodiesterase 5 inhibitor tadanafil
InactiveCN101205228AIncreased Stereospecific YieldThe separation method is simpleOrganic chemistryDiketoneBenzene
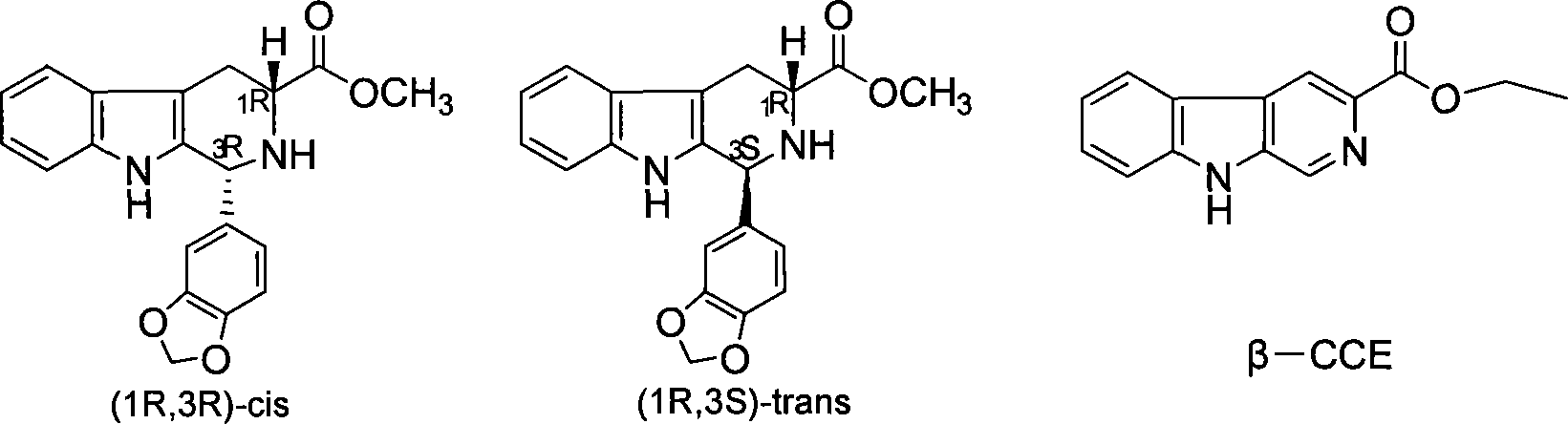
The invention relates to a synthesis method for (6R, 12aR)-6-(1, 3-benzodioxane heterocyclas-5-base)-2-methyl-2, 3, 6, 7, 12, -12a-hexahydro pyrazinyl [1', 2':1, 6] pyridyl [3, 4-b] indole-1, 4-diketone in the technical field of the pharmaceutical engineering. With the D-methyl tryptophan and piperonal as raw materials, the cis-1, 3-disubstituted carboline intermediates are prepared and generated through the Pictet-Spengler cyclization and then the products called tadalafil are obtained through the acylation ammoniation of the 3-secondary amino group and the final MTX substitution and condensation close-loop. By adopting the acidic condition protic solvents in the invention, the trans-1, 3-disubstituted carboline intermediates generated through the Pictet-Spengler cyclization, which is a key step in the existing process, are converted into those with a cis-configuration, thus greatly enhancing the stereospecific configuration yield of the carboline intermediates so as to enhace the overal yield of the tadalafil with reaching the goal of optimizing and improving the synthesis process.
Owner:SHANGHAI JIAO TONG UNIV
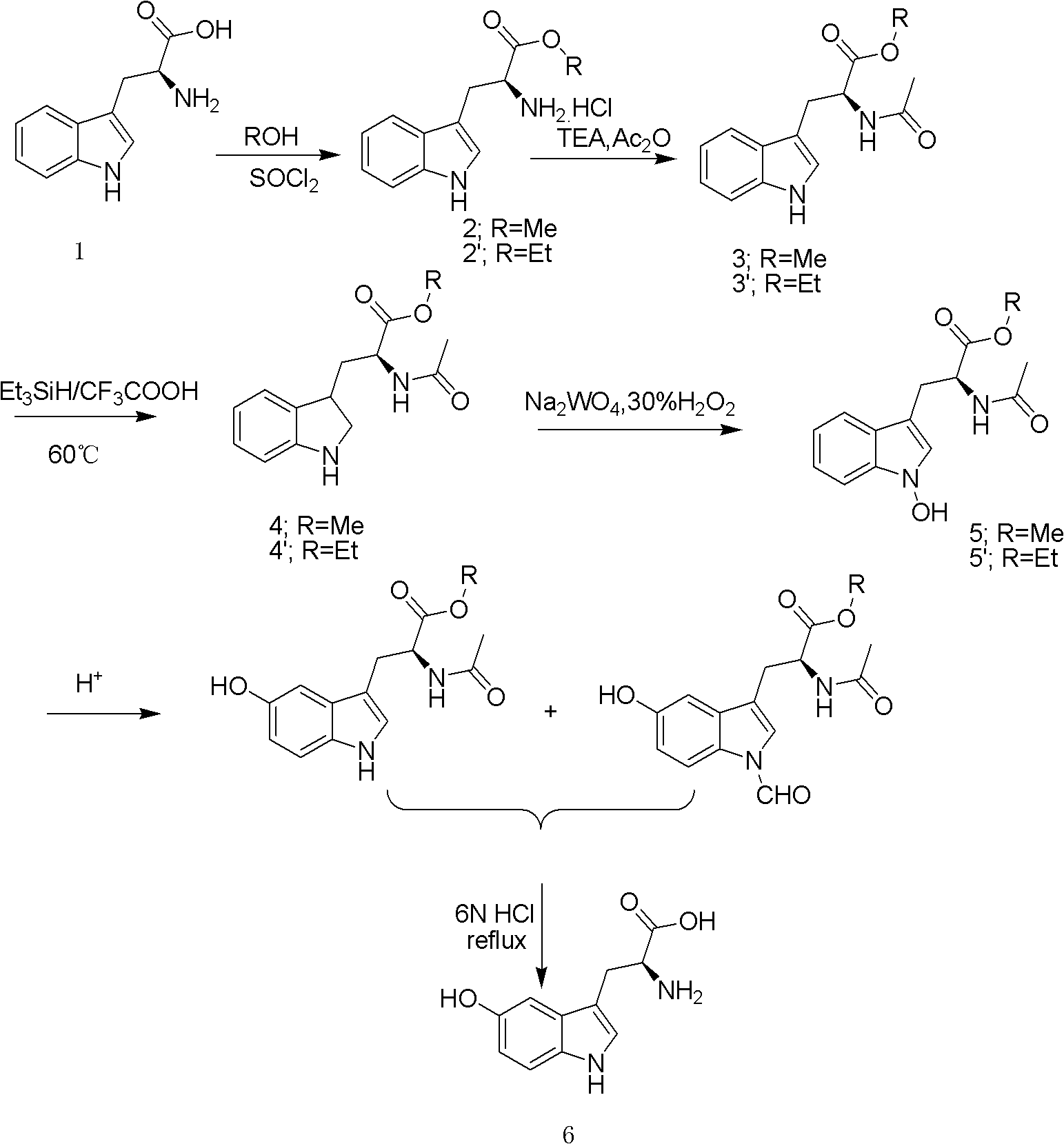
Preparation method of levo-5-hydroxytryptophan
The invention provides a preparation method of levo-5-hydroxytryptophan. The method comprises the following steps: performing methyl esterification / ethyl esterification on L-tryptophan to obtain L-tryptophan methyl / ethyl ester hydrochloride, performing dehydrochlorination under the alkaline condition to obtain L-tryptophan methyl / ethyl ester, performing acetylation to obtain N-acetyl-L-tryptophanmethyl / ethyl ester, reducing an indole ring under a triethylsilane-trifluoroacetic acid reduction system, oxidizing 1-position nitrogen of the indole ring under a sodium tungstate-30% hydrogen peroxide system, finally removing acetyl protection group to obtain levo-5-hydroxytryptophan under the acidic condition, further cooling and crystallizing, then enabling mother liquor to pass through a macroporous adsorption resin column, performing concentration, cooling and crystallization, and combining obtained levo-5-hydroxytryptophan crystals. The process has the advantages of being low in price of raw materials, being easy to obtain the raw materials, being simple in reaction operation, being high in yield, being good in product quality, being low in environmental pollution and the like, and is suitable for industrial large-scale production, the purity of the obtained product can achieve 99.2%, and the total yield is above 45%.
Owner:FOSHAN PRIZEN MEDICAL TECH
Preparation method of tadalafil
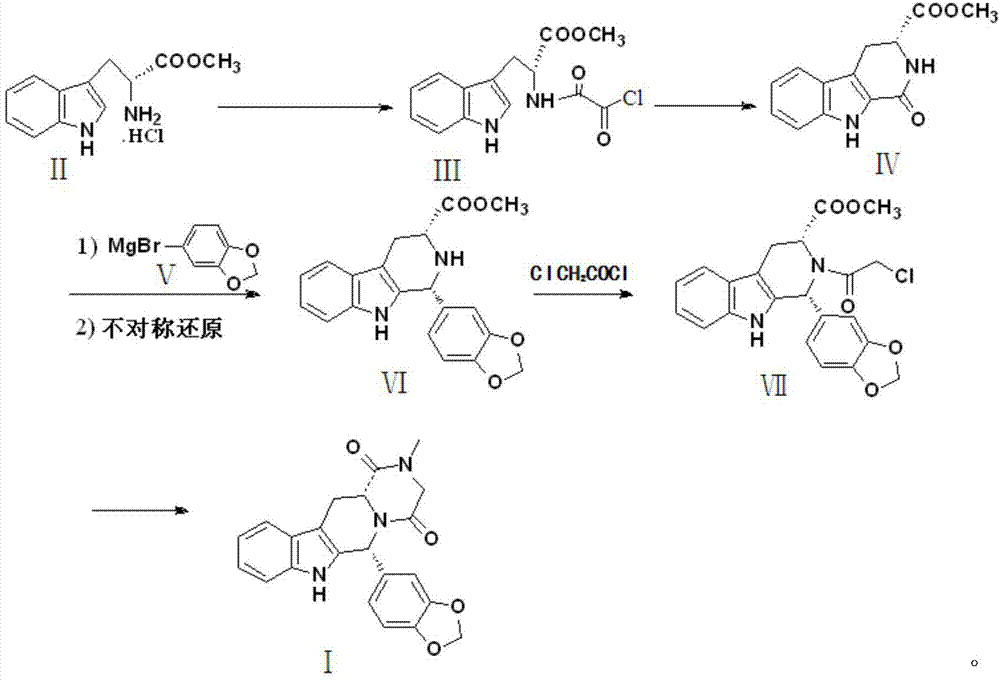
The invention discloses a preparation method of tadalafil, starting material D-Tryptophan methyl ester hydrochloride is reacted with oxalyl chloride to obtain an intermediate III, and the final product tadalafil (I) is obtained through cyclization, Grignard reaction, asymmetric reduction, substitution and condensation reaction. The use of national control chemical piperonal is avoided, an intermediate VI can be highly-selectively obtained by the asymmetric reduction, and the method has the advantages of simple postprocessing, short synthesis steps and high product total yield, and is suitable for industrialized production.
Owner:SHANDONG YUXIN PHARMA CO LTD
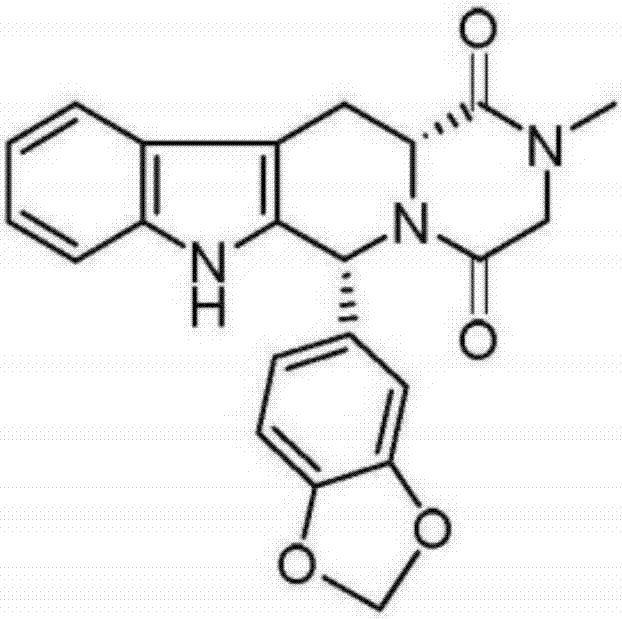
Spiro indole diketopiperazine alkaloid, and synthesis method and application of spiro indole diketopiperazine alkaloid
ActiveCN103435622AReduce dosageSave the reaction substrateAntibacterial agentsAntimycoticsSchotten–Baumann reactionSynthesis methods
The invention relates to spiro indole diketopiperazine alkaloid, and a synthesis method and an application of the spiro indole diketopiperazine alkaloid. The synthesis method comprises the steps that L-tryptophan methyl ester hydrochloride and fatty aldehyde perform Pictet-Spengler reaction to form enantiomeric hydrochloride, an inducer for inducing asymmetric conversion through crystallization induces the asymmetric conversion to form a single-configuration product, the single-configuration product performs Schotten-Baumann reaction to form an amido bond, then a spiral structure is obtained by NBS (N-bromosuccinimide) rearrangement, a protecting group is removed from the spiral structure under base catalysis, and the spiral structure is subjected to ring closing to form the spiro indole diketopiperazine alkaloid. The method has the advantages that the method is low in cost, simple and convenient in path, high in yield and easy to process, an end product and a natural product are the same in configuration, etc. The prepared spiro indole diketopiperazine alkaloid can be further applied to preparation of antibiotics.
Owner:SHAANXI UNIV OF SCI & TECH
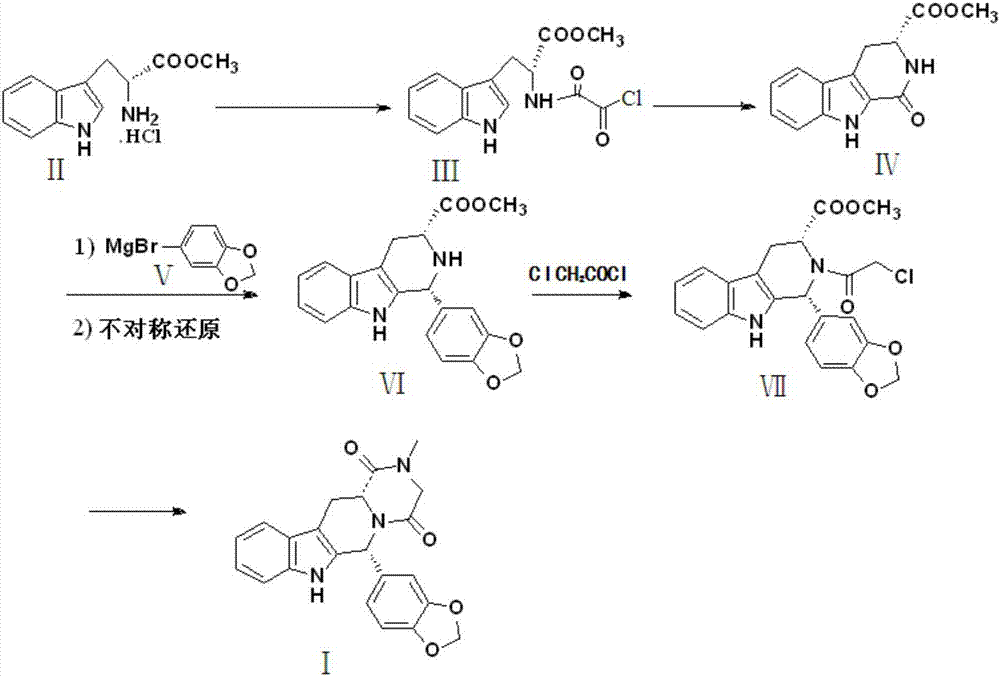
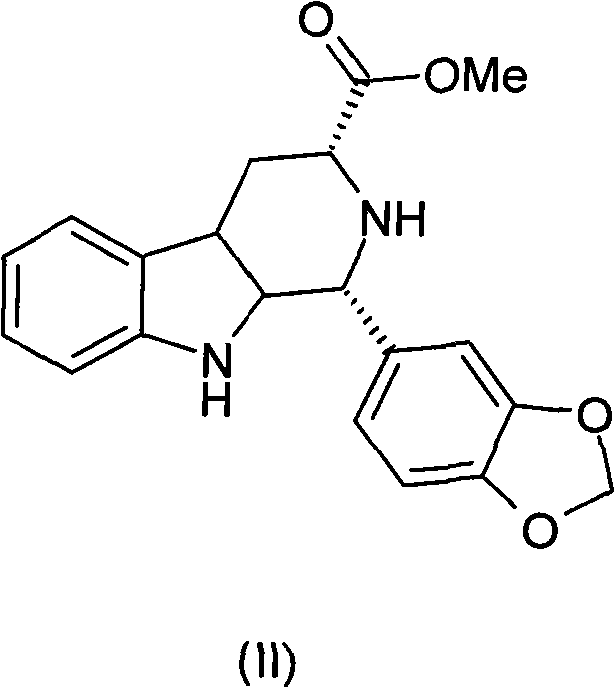
Preparation method of tadalafil intermediate
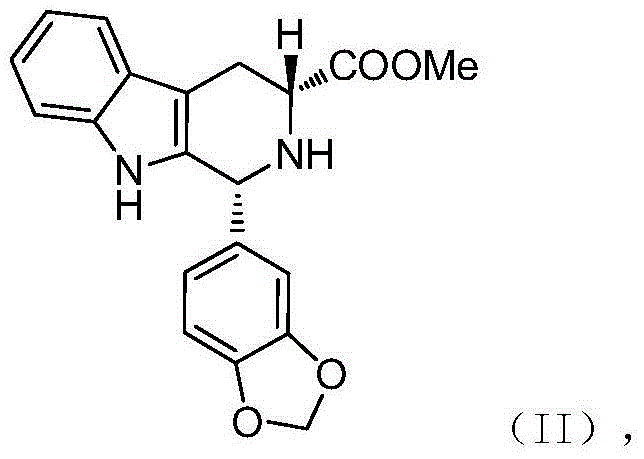
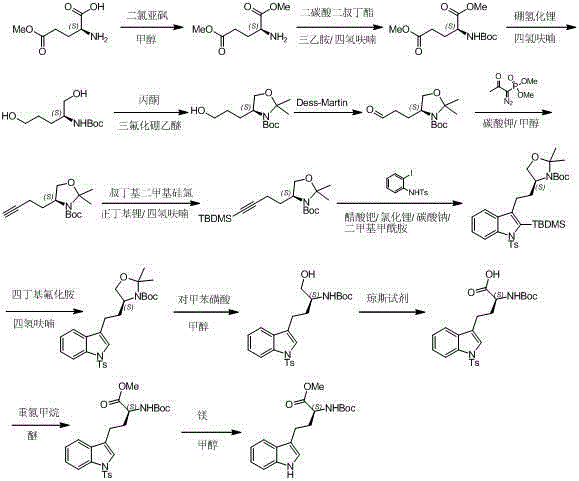
The invention relates to an improved method of preparing a compound (II). The improved method comprises the following steps: (the formula is shown in the description) 1, mixing D-tryptophan, methanol and toluene, adding thionyl chloride dropwise, and then carrying out reaction at the temperature of 70-85 DEG C to obtain D-tryptophan ester hydrochloride, wherein the structure is shown in a formula (III):(the formula is shown in the specification); and 2, mixing the D-tryptophan ester hydrochloride, a compound (IV) and a nitrile solvent, uniformly stirring, then raising the temperature to 75-85 DEG C, and carrying out reaction, (the formula is shown in the description). The improved preparation method of the compound shown by the D-tryptophan ester hydrochloride (II) has the following beneficial effects: 1, the dosage of the thionyl chloride in the first step is greatly reduced, so that the risk and the corrosion of the reaction are lowered, the post-processing is simple, and the yield is above 90 percent; 2, in the second step, the nitrile solvent is adopted to improve the stereoselectivity, and the enantiomer extra amount (ee %) is above 99 percent.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of tadalafil and intermediate of tadalafil
ActiveCN110437228AAvoid it happening againReduce lossesOrganic chemistry methodsPhosphodiesteraseTadalafil
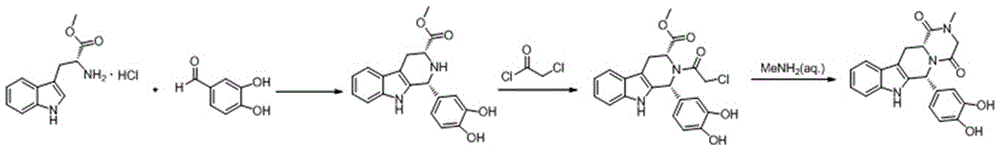
The invention relates to a preparation method of a selective and reversible inhibitor tadalafil of cyclic guanosine monophosphate (cGMP) specific phosphodiesterase 5 (PDE5). The method comprises the following steps: carrying out an esterification reaction on D-tryptophan as an initial raw material and methanol under catalysis of sulfuric acid to generate D-tryptophan methyl ester (an intermediate1); carrying out a Pictet-Spengler (P-S) reaction on the D-tryptophan methyl ester and heliotropin to prepare (1R,3R)-1-(1,3-benzodioxol-5-yl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester hydrochloride (an intermediate 2); carrying out an amidation reaction on the (1R,3R)-1-(1,3-benzodioxol-5-yl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester hydrochloride and chloroacetyl chloride to prepare (1R,3R)-1-(1,3-benzodioxol-5-yl)-2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester (an intermediate 3); andfinally carrying out a cyclization reaction on the (1R,3R)-1-(1,3-benzodioxol-5-yl)-2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester and a methylamine alcohol solution to obtain the tadalafil. The method provided by the invention has the advantages of easily available raw materials, simple operation, greenness, environmental protection and low costs, andis suitable for industrial production.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES +1
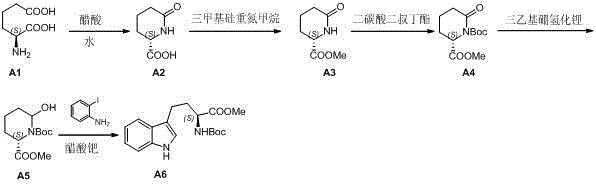
Preparation method of L-N-Boc-high tryptophan methyl ester
InactiveCN102911106AEasy to operateSimple post-processingOrganic chemistryPtru catalystPyrrolidinones
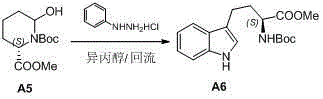
The invention discloses a preparation method of L-N-Boc-high tryptophan methyl ester, which can be mainly used for solving the problems that the reaction steps are more, the cost is low, the operation is difficult, and the single chirality of a final compound can not be ensured in an existing synthesizing method. The preparation method comprises the steps of conducting cyclization reaction to generate L-2-pyrrolidone-6-formic acid 1 by taking L-2-amino adipic acid as initial materials under the action of glacial acetic acid and water; 2. conducting an esterification reaction on the compound 1 and trimethyl silicon diazomethane to obtain L-2-pyrrolidone-6-methyl ester 2; 3. protecting N in the compound 2 by using Boc, then conducting the reduction reaction due to the action of a reducing agent, namely lithium triethylborohydride, and reducing carbonyl in the L-2-pyrrolidone-6-methyl ester protected by N-tert-butylcarbazate; and 4. finally synthesizing the high tryptophan methyl ester protected by the L-N-tert-butylcarbazate by two methods: synthesizing classical fisher benzazole in one method, and removing one molecular water by L-2-pyrrolidinol-6-methyl ester and iodoaniline, rearranging, and conducting Heck reaction under the action of a palladium catalyst to obtain the L-N-Boc-high tryptophan methyl ester in the other method.
Owner:SHANGHAI STA PHARMA R&D CO LTD
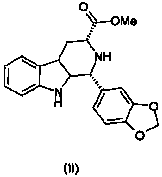
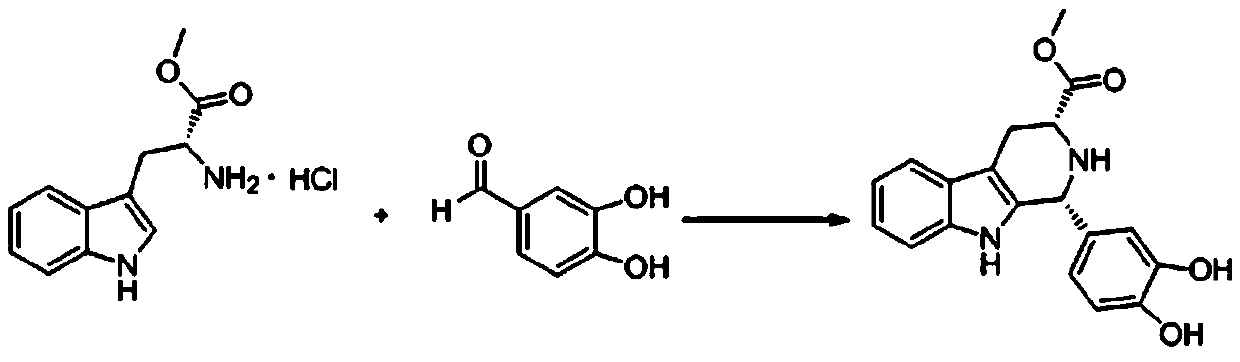
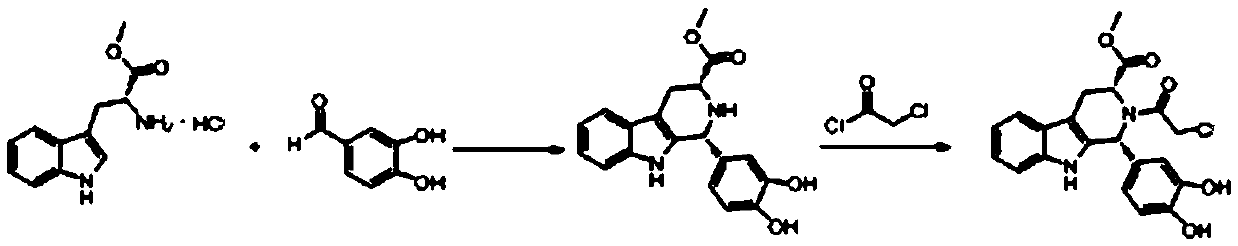
Improvement on synthesis method of tetrahydro-beta-carboline compound (II)
The invention relates to an improvement on a synthesis method of a tetrahydro-beta-carboline compound (II). The method comprises reacting D-tryptophan methyl ester hydrochloride and piperonal at 50 DEG C-150 DEG C in a certain solvent to obtain hydrochloride of a structural formula (II) compound, wherein the solvent can be nitroethane, dioxane, methylbenzene, dimethylbenzene, benzene or nitrobenzene. The yield and purity of products prepared by the method are greatly improved, and the method is especially suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
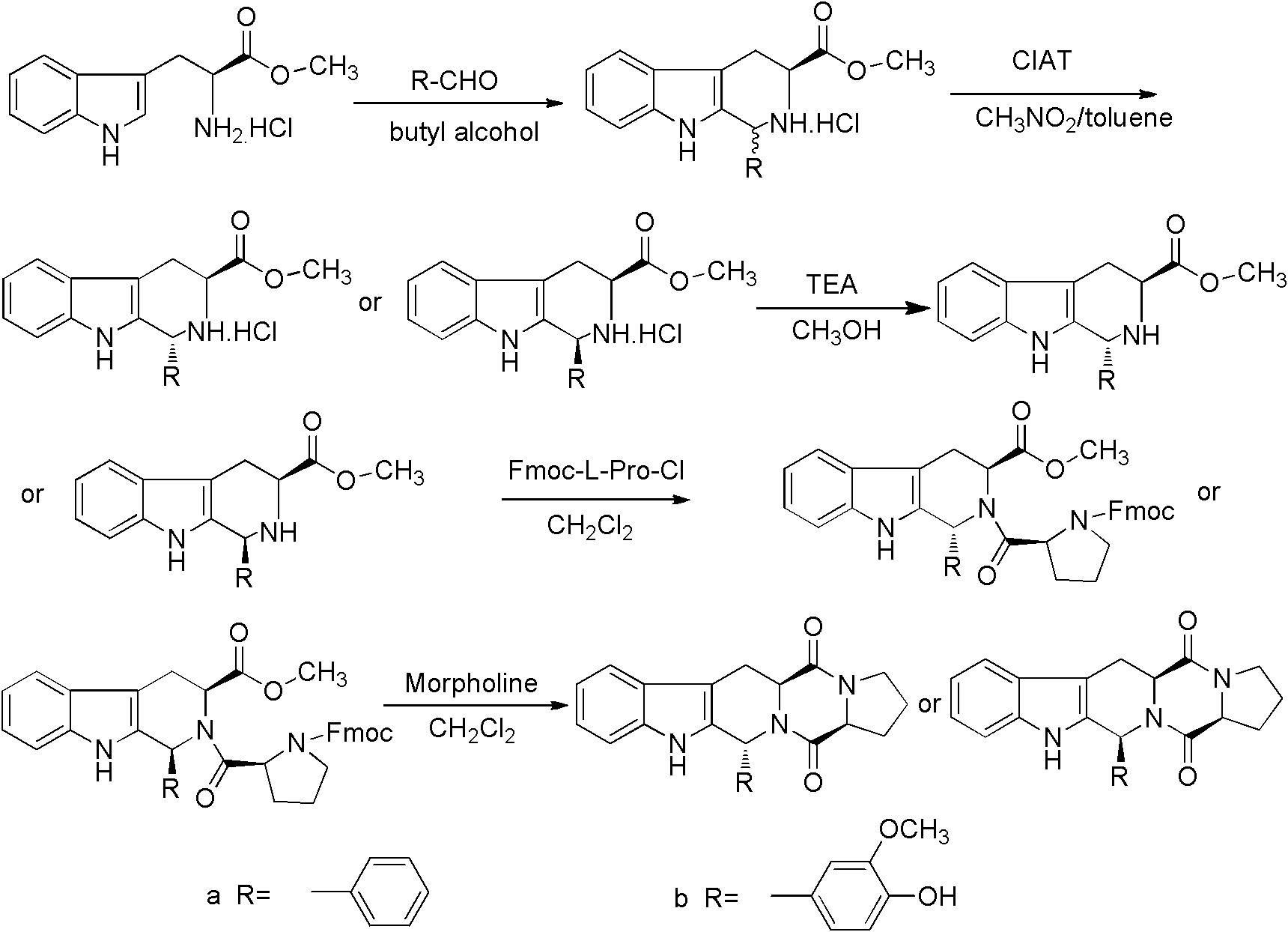
Method for synthesizing tetrahydro-beta-carboline diketopiperazine compound
InactiveCN102276608AAdvanced process routeSimple reaction conditionsOrganic chemistrySchotten–Baumann reactionDiketopiperazines
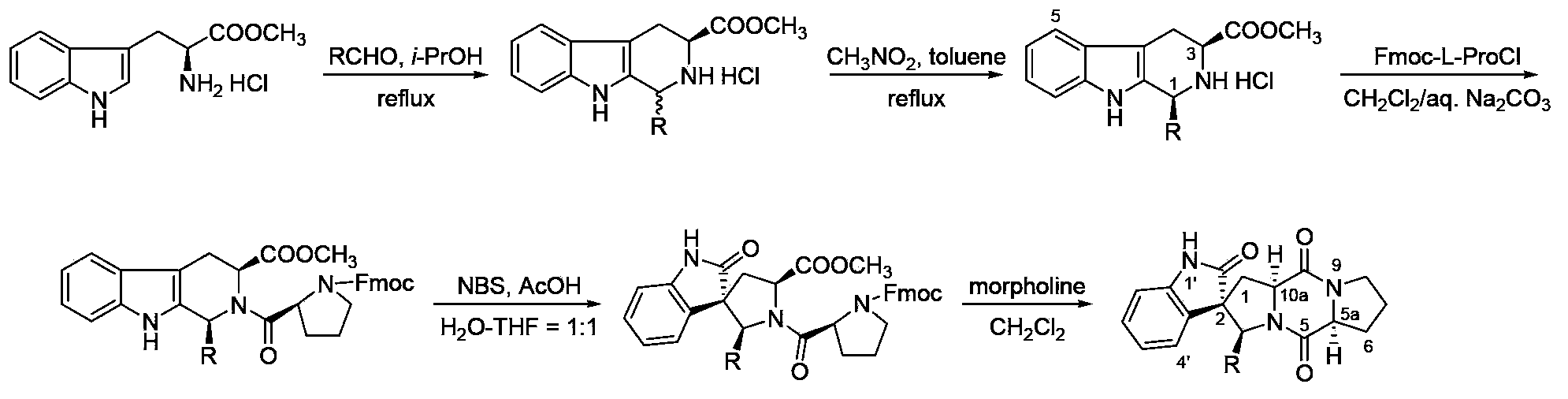
The invention provides a method for synthesizing a tetrahydro-beta-carboline diketopiperazine compound. The method comprises the following steps of: reacting Pictet-Spengler reaction on L-tryptophane methyl ester hydrochloride serving as a starting raw material with aldehyde; performing a crystallization induced asymmetric transformation (CIAT) process to obtain a tetrahydro-beta-carboline ring; performing Schotten-Baumann reaction in a two-phase solvent comprising saturated sodium carbonate and dichloromethane; and finally forming an amido bond and performing a deprotection process to obtaina target product. The method has the beneficial effects of fewer reaction steps, high transformation rate and yield, advanced technological line, simple post-treatment, easiness in purification and the like.
Owner:SHAANXI UNIV OF SCI & TECH
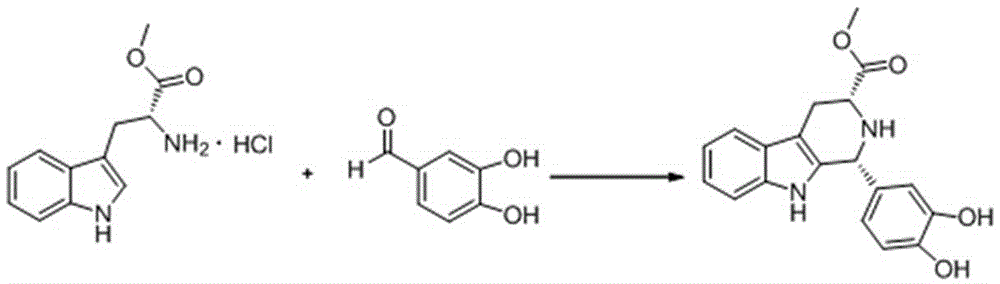
Cis-tetrahydrocarboline intermediate and synthesis method thereof, and application of cis-tetrahydrocarboline intermediate in preparing tadalafil
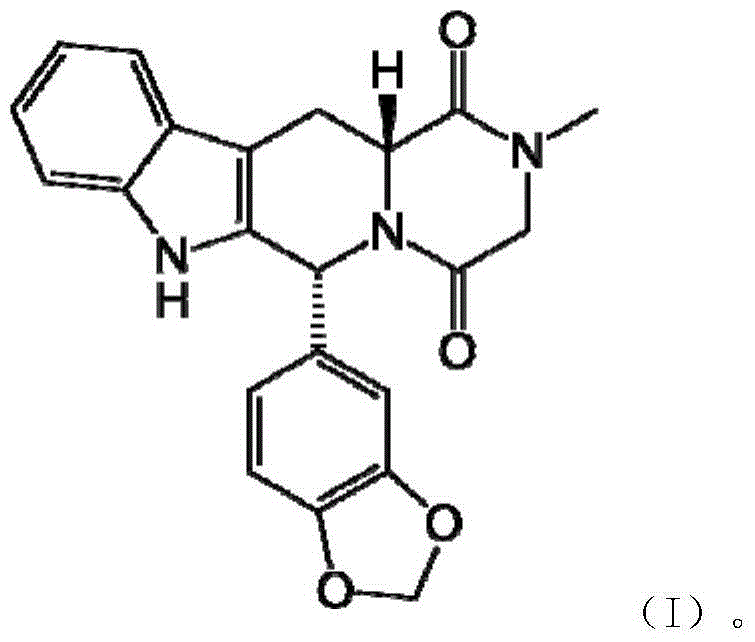
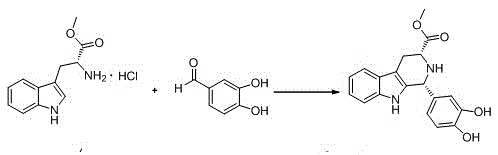
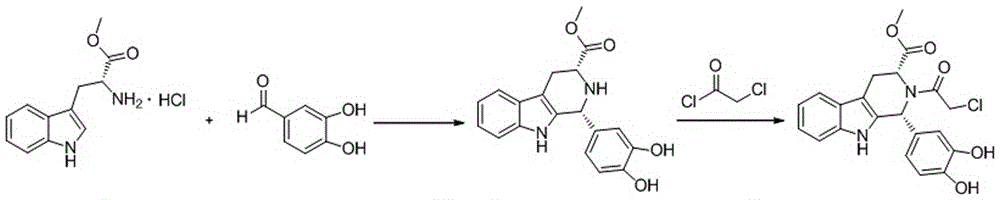
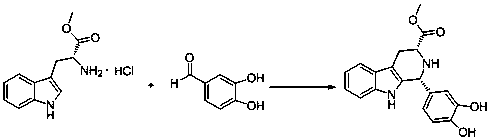
The invention discloses a cis-tetrahydrocarboline intermediate and a synthesis method thereof, and application of the cis-tetrahydrocarboline intermediate in preparing tadalafil. D-tryptophan methyl ester hydrochloride and 3,4-dihydroxybenzaldehyde are used as raw materials to prepare the cis-tetrahydrocarboline intermediate, so that the raw materials are accessible, thereby overcoming the technical defects when the police-controlled precursor chemical heliotropin is used as the raw material in the prior art. The preparation method does not need any catalyst, and can be used for directly preparing the cis-tetrahydrocarboline intermediate by using lower alcohols, nitriles or nitroparaffins as a solvent; and after the reaction finishes, simple cooling and filtration are performed to obtain the product, wherein the mole yield is 90-97%. The cis-tetrahydrocarboline intermediate can be used for preparing tadalafil; and the synthesis method is simple and easy to implement, has the advantages of stable technique and high yield, and is suitable for industrial large-scale production. The structural formula of the cis-tetrahydrocarboline intermediate is disclosed as (I).
Owner:湖南千金湘江药业股份有限公司
Method for preparing 1-substituted-beta-carboline-3-carboxylic ester
ActiveCN105884768AAvoid the defects of complex steps and unsatisfactory yieldHigh yieldOrganic chemistryReaction temperatureSolvent
The invention discloses a method for preparing 1-substituted-beta-carboline-3-carboxylic ester. Methyl tryptophan and aromatic aldehyde or a carbonyl containing cyclic compound and a catalyst are added into a solvent, separation and purification are carried out after a heating reaction, and 1-substituted-beta-carboline-3-carboxylic ester is obtained. A 1-substituted-beta-carboline-3-carboxylic ester derivative is synthesized through a one-step method with high yield. The defects that in a current synthesis reaction, a poisonous catalyst and a poisonous solvent are adopted, the reaction temperature is high, the steps are complex, and the yield is not ideal are avoided.
Owner:SHAANXI UNIV OF SCI & TECH
Tetrahydro carboline derivative modified with two amino acids and preparation method and application thereof
InactiveCN102250127ACtiveSimple methodOrganic active ingredientsDipeptide ingredientsAntithrombotic AgentHydrogen
The invention discloses a tetrahydro carboline derivative modified with two amino acids and a preparation method and an application thereof. According to the invention, L-tryptophan methyl ester is allowed to react with 1,1,3,3-tetramethoxy propane by a microwave reaction; the obtained product is modified by amino acids to obtain a series of (1R, 3S)-1-[1-(1R, 3S)-tetrahydro beta-carboline-3-formyl amino acid-1-yl-methyl]-tetrahydro beta-carboline-3-formyl amino acid derivatives and intermediates thereof; and the derivatives are evaluated for in vitro antiplatelet aggregation activity and in vitro antithrombotic activity. The method of the invention is simple; the used raw materials are easily available, safe and cheap; the obtained product has antiplatelet aggregation activity and antithrombotic activity, which indicates the clinical application prospects of (1R, 3S)-1-[1-(1R, 3S)-tetrahydro beta-carboline-3-formyl amino acid-1-yl-methyl]-tetrahydro beta-carboline-3-formyl amino acidas an antithrombotic agent.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
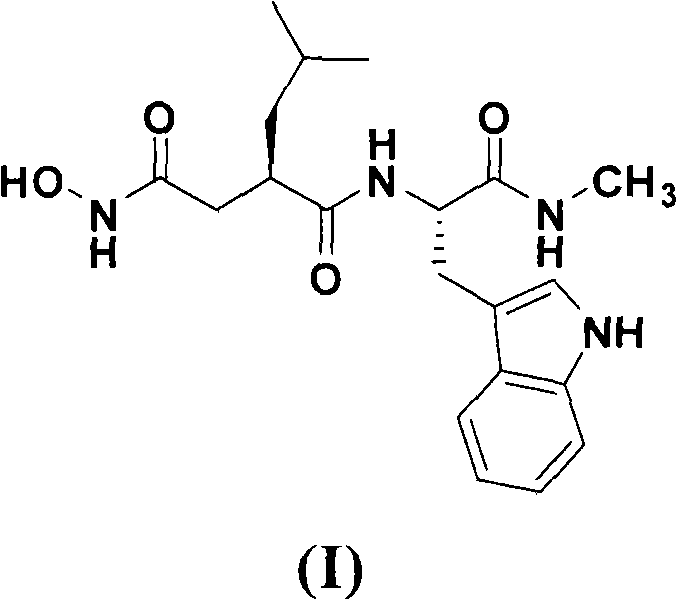
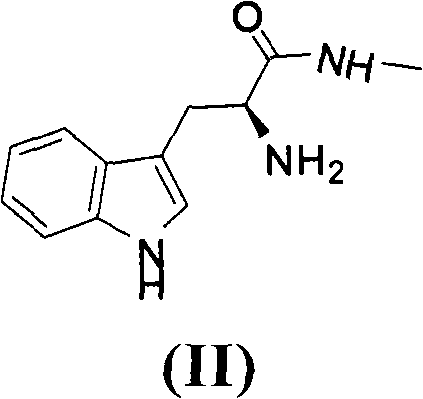
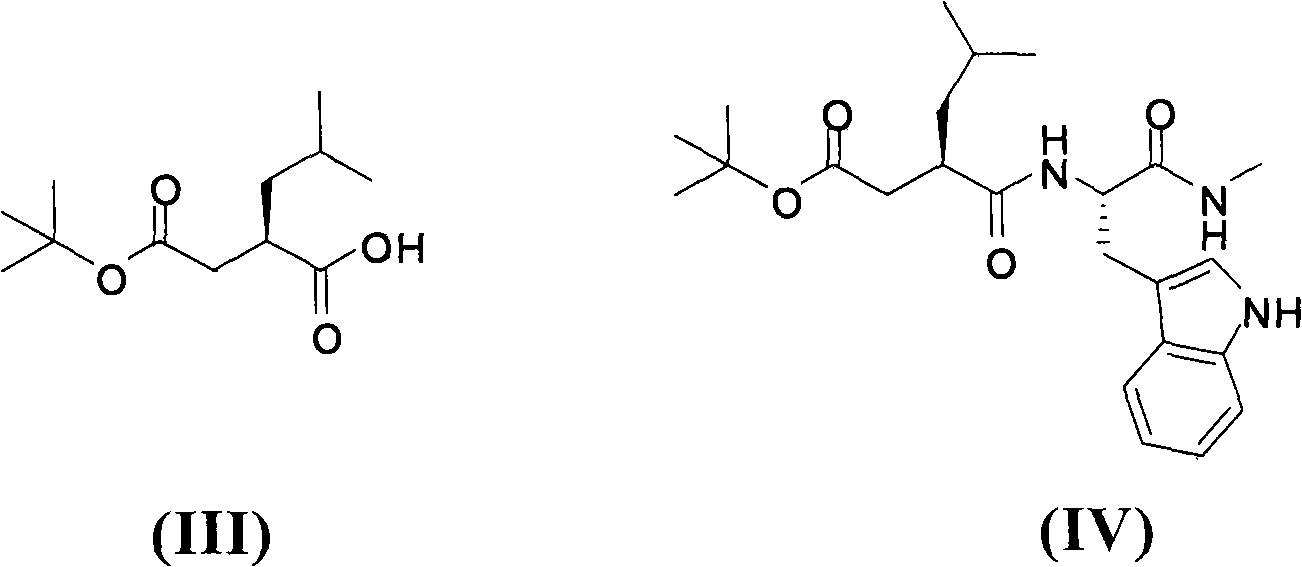
Method for preparing ilomastat
The invention relates to a new method for preparing ilomastat, which comprises the following steps of: reacting a tryptophan methyl ester hydrochloride with a methylamine to prepare tryptophanyl methylamine; performing a condensation reaction between the tryptophanyl methylamine and a tert-butyl acetate succinate derivative to prepare a midbody of a formula (IV); removing the tert-butyl from the midbody of formula (IV) to form a midbody of a formula (V); and performing a condensation reaction between the midbody of formula (V) and a free hydroxylamine to form the ilomastat. The method of the invention is simple in the process and low in the cost.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Compound acyl intermediate as well as synthetic method thereof and application thereof in preparing tadalafil
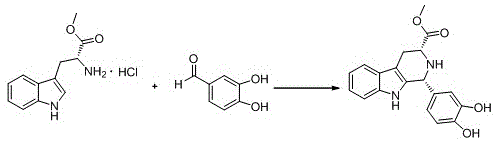
The invention discloses a compound acyl intermediate as well as a synthetic method and application thereof in preparing tadalafil. The synthetic method comprises the following steps: preparing (1R, 3R)-methyl-1-(3,4-dihydroxyphenyl)-2,3,4,9-tetrahydro-1H-pyridino[3,4-b]indol-3-carboxylic acid (compound I) by taking D-tryptophan methyl ester hydrochloride and 3,4-dihydroxy benzaldehyde as raw materials, and allowing the compound I to have chloroacetylation with chloroacetyl chloride to generate the compound acyl intermediate. By adopting the method, raw materials are easy to get, so that the technical weakness caused by using the poisonable chemical heliotropin controlled by the public security department in the prior art as a raw material is overcome. The preparation process requires no catalyst, and the yield is high. The obtained compound acyl intermediate can be used for preparing the tadalafil, and is simple and easy, stable in process, low in cost, and suitable for industrialized mass production. The structural formula of the compound acyl intermediate is shown as formula (II): (see the description) (II).
Owner:湖南千金湘江药业股份有限公司
A synthetic method of tadalafil
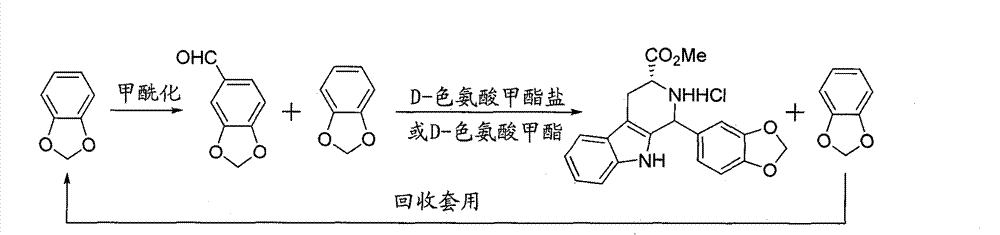
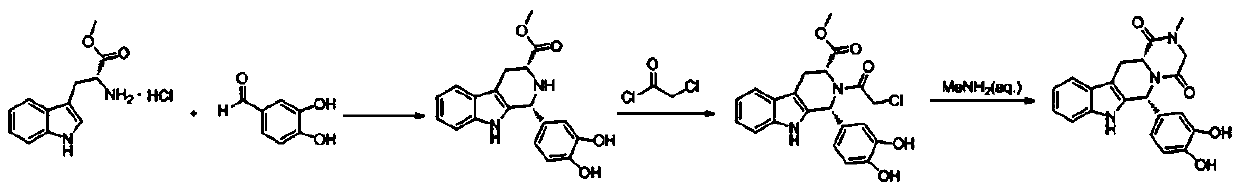
ActiveCN105524062AEasy to recycleThe three-step reaction conditions are not harshOrganic chemistryNitroalkaneAminolysis
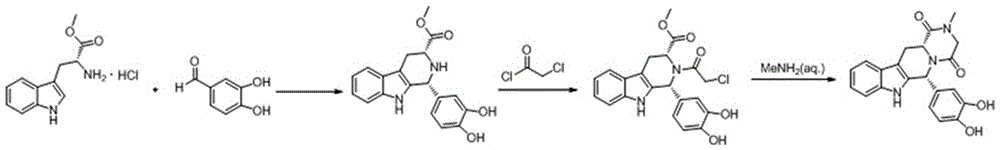
A synthetic method of tadalafil is disclosed. The method adopts 3,4-dihydroxybenzaldehyde and methyl D-tryptophanate hydrochloride as initial raw materials, and prepares a product by condensation-cyclization, chloroacetylation, aminolysis-cyclization and methyl cyclization. The 3,4-dihydroxybenzaldehyde is adopted as the raw material. Nitrile, lower alcohol and nitroalkane are adopted as solvents for the condensation-cyclization. Ethyl acetate or dichloromethane is adopted as a solvent for the chloroacetylation. Lower alcohol which is cheap is adopted as a solvent for the aminolysis-cyclization. Compared with other routes, the method is characterized by simple and convenient separation and purification, simple reaction conditions, a high yield of each step (with the yield of each step being higher than 80%), a stable process and a short production period. The method is free of heliotropin with which narcotics can be prepared easily, is free of column chromatography and other purification processes and is suitable for industrial production.
Owner:湖南千金湘江药业股份有限公司
The synthetic method of tadalafil
ActiveCN105524062BEasy to recycleThe three-step reaction conditions are not harshOrganic chemistryTadalafilNitroalkane
A synthetic method of tadalafil is disclosed. The method adopts 3,4-dihydroxybenzaldehyde and methyl D-tryptophanate hydrochloride as initial raw materials, and prepares a product by condensation-cyclization, chloroacetylation, aminolysis-cyclization and methyl cyclization. The 3,4-dihydroxybenzaldehyde is adopted as the raw material. Nitrile, lower alcohol and nitroalkane are adopted as solvents for the condensation-cyclization. Ethyl acetate or dichloromethane is adopted as a solvent for the chloroacetylation. Lower alcohol which is cheap is adopted as a solvent for the aminolysis-cyclization. Compared with other routes, the method is characterized by simple and convenient separation and purification, simple reaction conditions, a high yield of each step (with the yield of each step being higher than 80%), a stable process and a short production period. The method is free of heliotropin with which narcotics can be prepared easily, is free of column chromatography and other purification processes and is suitable for industrial production.
Owner:湖南千金湘江药业股份有限公司
Method for producing D-tryptophan
The invention discloses a method for producing D-tryptophan. The method comprises the following steps: 1) L-tryptophan is stirred in methanol and cooled to 10 DEG C, thionyl chloride is added dropwise; after the adding, the temperature rises to 20 DEG C, heat insulation is performed for 5 h, methanol is concentrated, water is added, the pH is adjusted to 7-8 with ammonia water, L-tryptophan methyl ester is crystallized and filtered and dried for standby application; 2) L-tryptophan methyl ester is dissolved in the methanol, D-tartaric acid and 3% of trimethylacetaldehyde are added at room temperature, the temperature rises to 70 DEG C, backflow is performed for 5 h, cooling is performed, and double salt is obtained; 3) the double salt is dissolved in water, the pH is adjusted to 7-8 with ammonia water, and D-tryptophan methyl ester is obtained through crystallization and filtration; 4) D-tryptophan methyl ester is dissolved in water at room temperature, the pH is adjusted to over 12 with 20% of sodium hydroxide, heat insulation is performed for 4 h, crude D-tryptophan is obtained through crystallization and filtration and stirred and washed for 1 h with water, and the product D-tryptophan is obtained through centrifugation. The method belongs to asymmetric conversion and is simple to operate, high in yield and low in cost.
Owner:NANJING REDWOOD FINE CHEM CO LTD
Key intermediate and synthesis method thereof, and application of key intermediate in preparing tadalafil
ActiveCN105541840ABroaden and improve efficiencyProcess stabilityOrganic chemistryTadalafilSynthesis methods
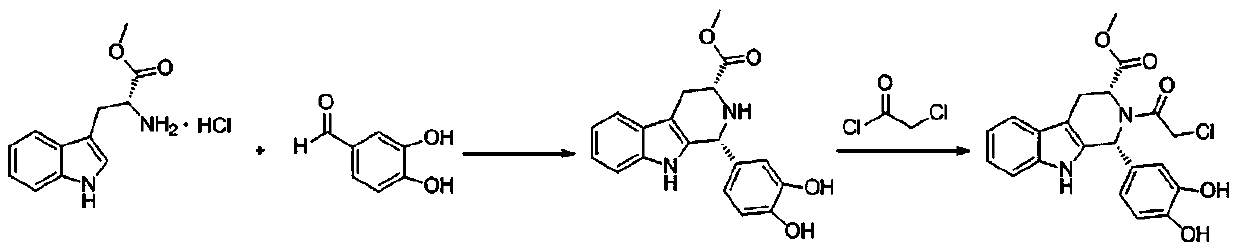
The invention discloses a key intermediate and a synthesis method thereof, and application of the key intermediate in preparing tadalafil. The synthesis method comprises the following steps: by using D-tryptophan methyl ester hydrochloride and 3,4-dihydroxybenzaldehyde as raw materials, carrying out condensation cyclization, chloracetylation and aminolysis cyclization to generate the key intermediate for preparing tadalafil. The method has the advantage of accessible raw materials, and overcomes the technical defects due to the use of the police-controlled precursor chemical heliotropin as the raw material in the prior art. The method has the advantages of no need of any catalyst and high yield in the preparation process. The obtained key intermediate can be used for preparing tadalafil. Thus, the synthesis method is simple and easy to implement, has the advantages of stable technique and low cost, and is suitable for industrial large-scale production. The structural formula of the key intermediate is disclosed as Formula (III).
Owner:湖南千金湘江药业股份有限公司
A kind of method for preparing tadalafil
The invention discloses a preparation method of tadalafil. The concrete preparation method is used for successfully synthesizing tadalafil by taking L-tryptophan methyl ester hydrochloride as an initial raw material and utilizing the characteristics that an ortho-position of an ester group has the chiral inversion property under an alkaline condition, the reaction between ester group and Pictet-Spengler is reversible reaction, and the ester group is easily converted into a cis-form product with relatively small solubility, therefore, the preparation method is a brand new technology. The price of L-tryptophan methyl ester hydrochloride is only less than 1 / 5 of that of D-tryptophan methyl ester hydrochloride, so that the cost of the overall route is greatly lower than that of the prior art, and then the preparation method is suitable for industrial production.
Owner:优标易站(苏州)电子商务有限公司
Preparation method and application of tadalafil cis-intermediate
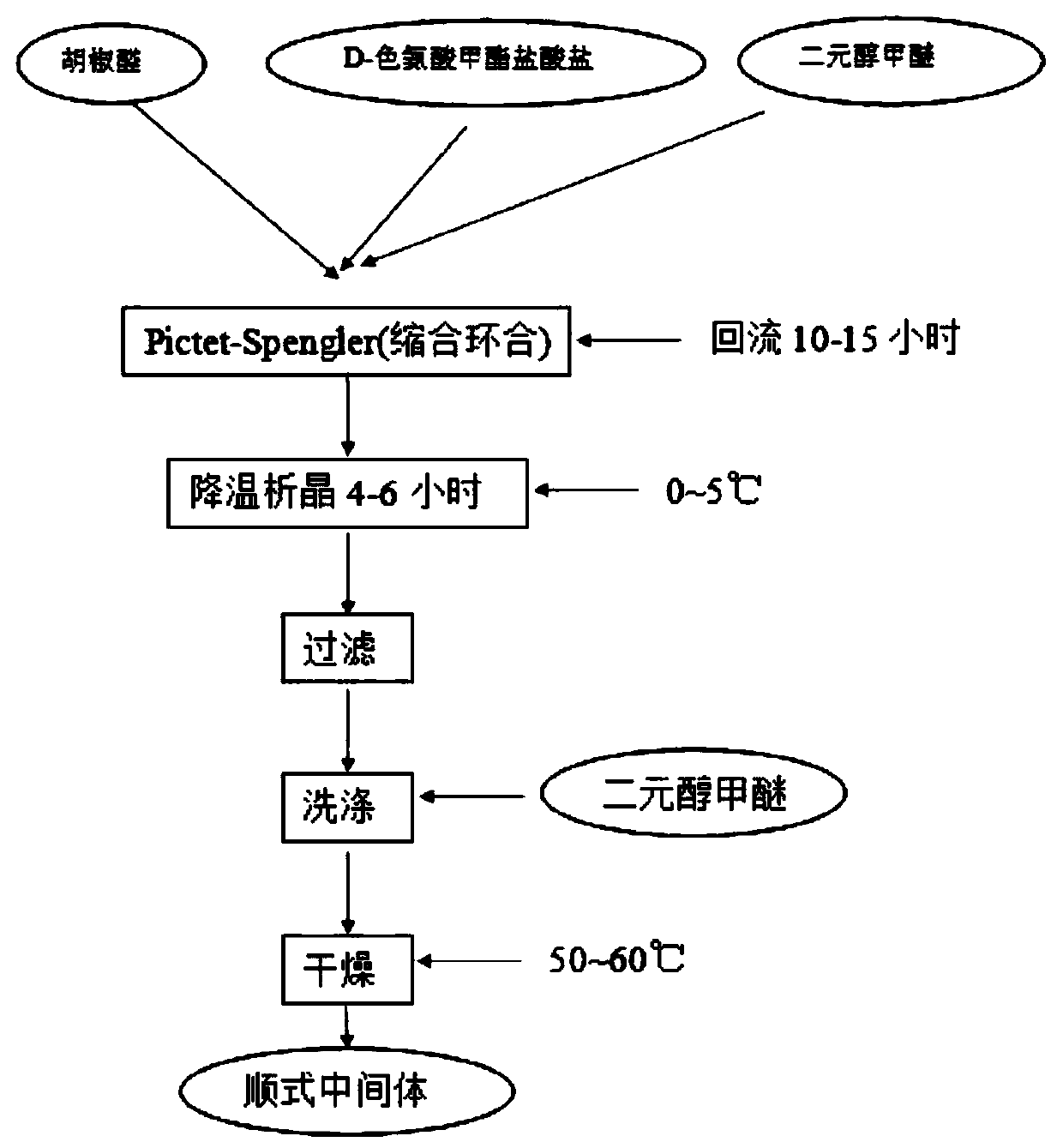
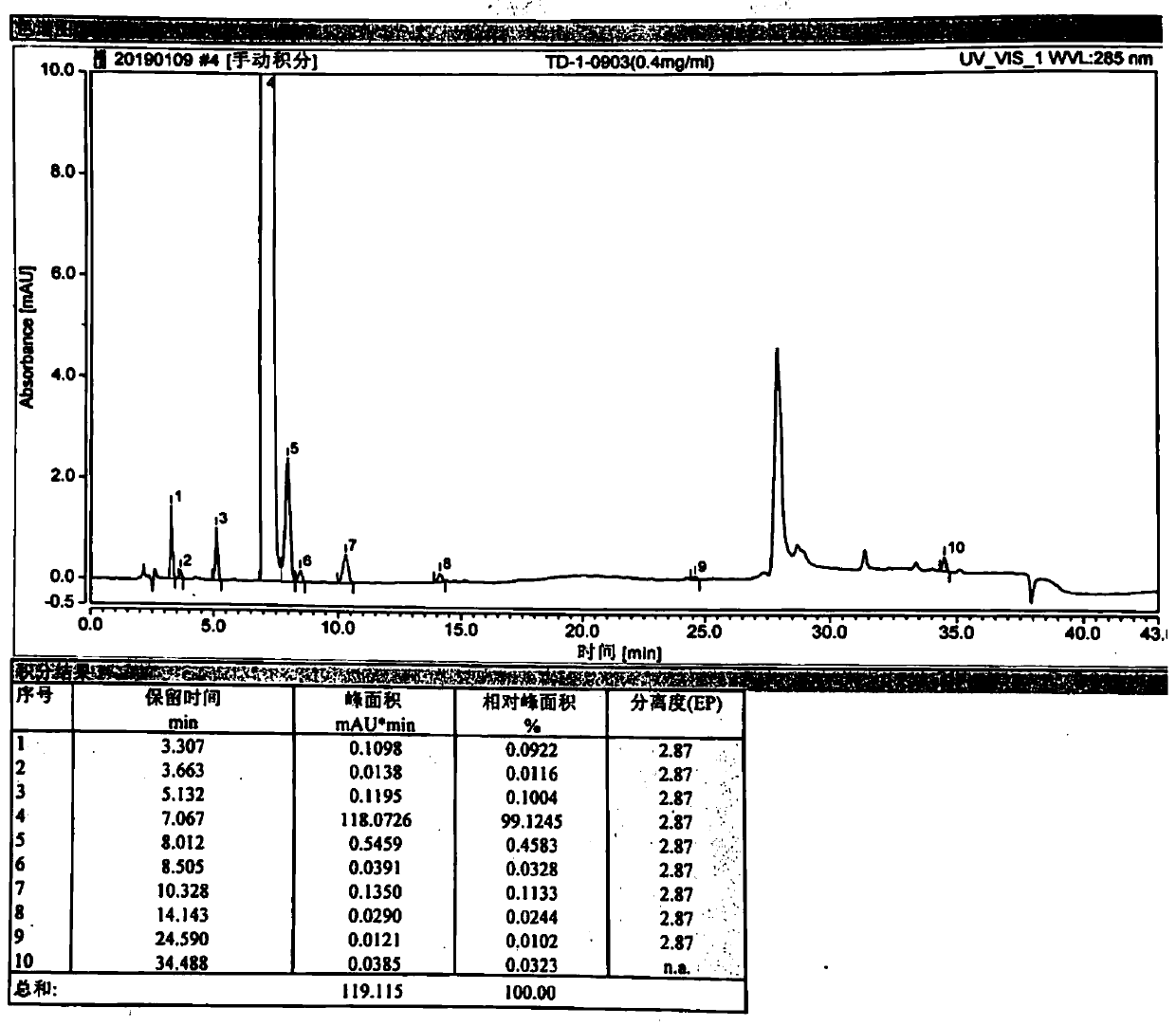
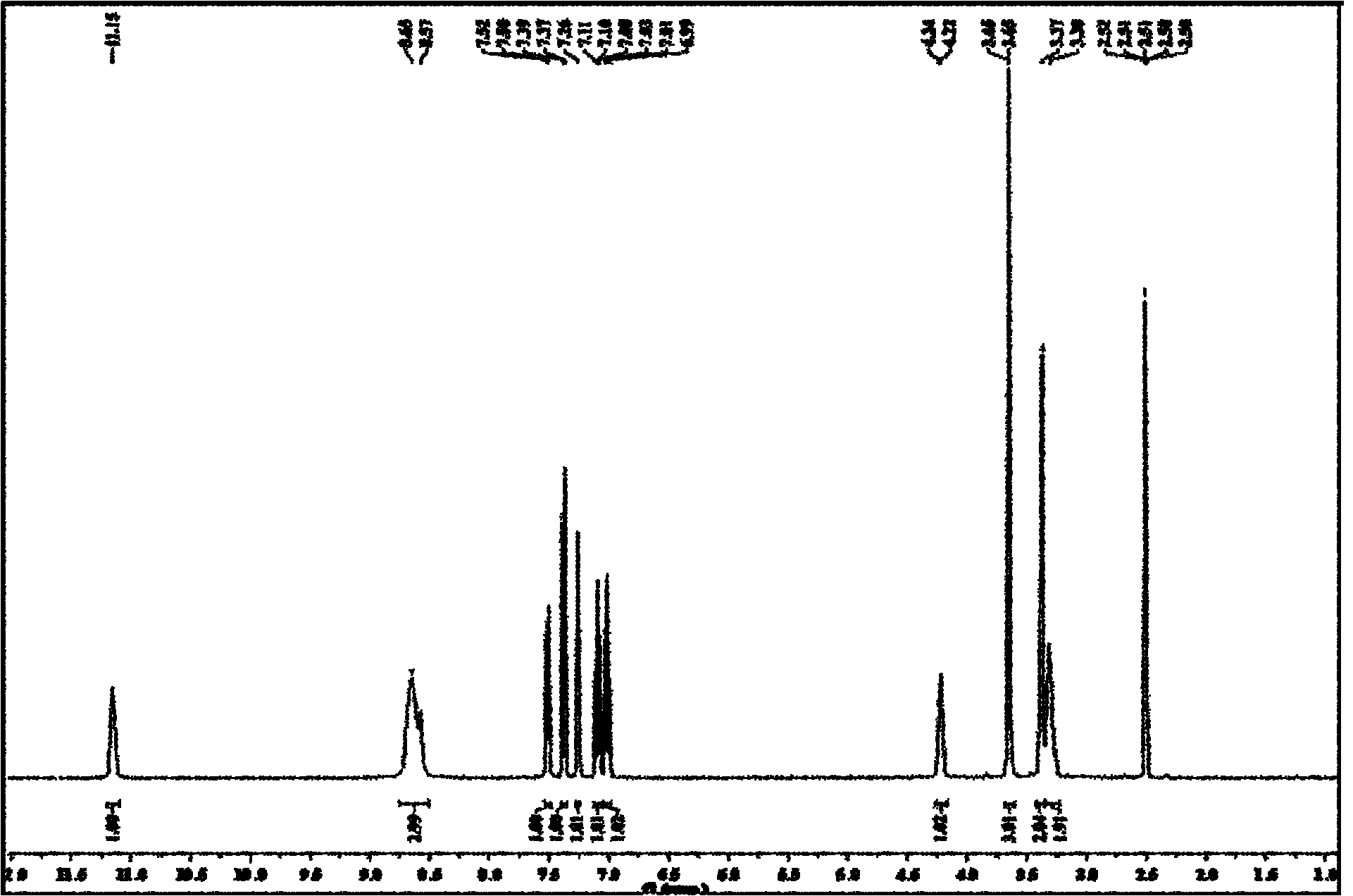
InactiveCN110606847AHigh purityShort synthesis timeOrganic chemistryRespiratory disorderTadalafilButanediol
The invention belongs to the technical field of medicinal chemistry, and discloses a preparation method and an application of a tadalafil cis-intermediate, which comprises the following steps: by using D-tryptophan methyl ester hydrochloride as an initial raw material, mixing the raw material with heliotropin in a glycol ether solution to obtain a cis-intermediate, wherein the ratio of the D-tryptophan methyl ester hydrochloride to the heliotropin is 1:(1.1-1.5), and the ratio of the D-tryptophan methyl ester hydrochloride to the glycol ether is 1g to 4ml; the glycol ether is one or a mixtureof more of ethylene glycol monomethyl ether, propylene glycol monomethyl ether, butanediol monomethyl ether, propylene glycol dimethyl ether, ethylene glycol dimethyl ether and butanediol dimethyl ether. The method disclosed by the invention is short in synthesis time, simple to operate and high in yield, and the prepared cis-intermediate is high in purity. The intermediate does not need to be refined and can be directly used for preparing tadalafil.
Owner:SICHUAN INDAL INST OF ANTIBIOTICS CHINA NAT PHARMA GROUP CORP
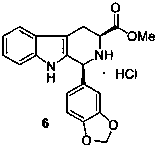
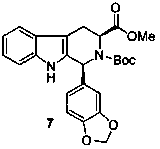
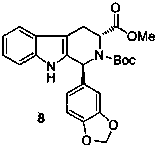
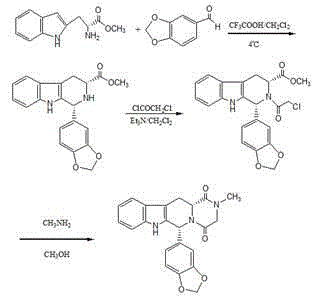
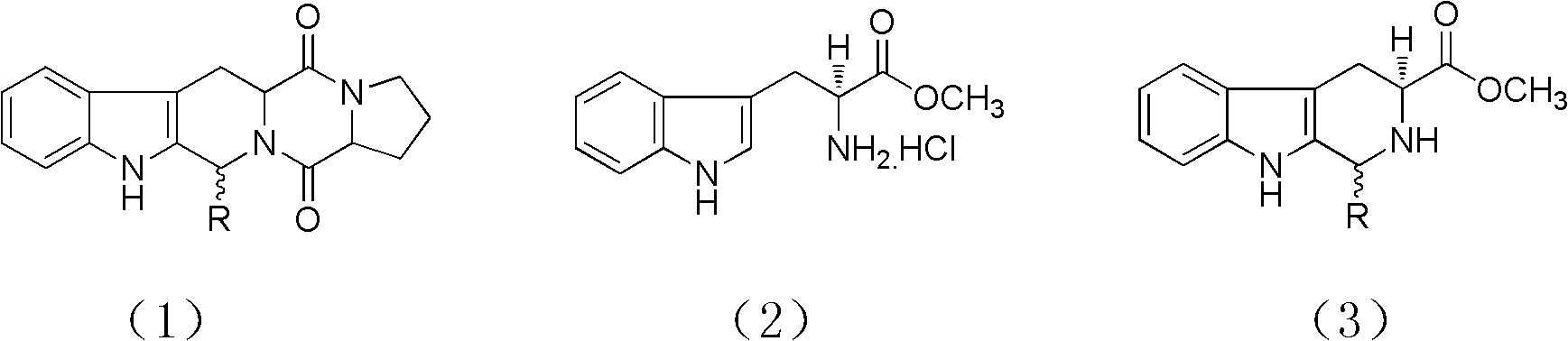
Asymmetric synthesis of 1-aryl-1H-pyridine[3,4-b]indole-3-carboxylic acid methyl ester derivatives
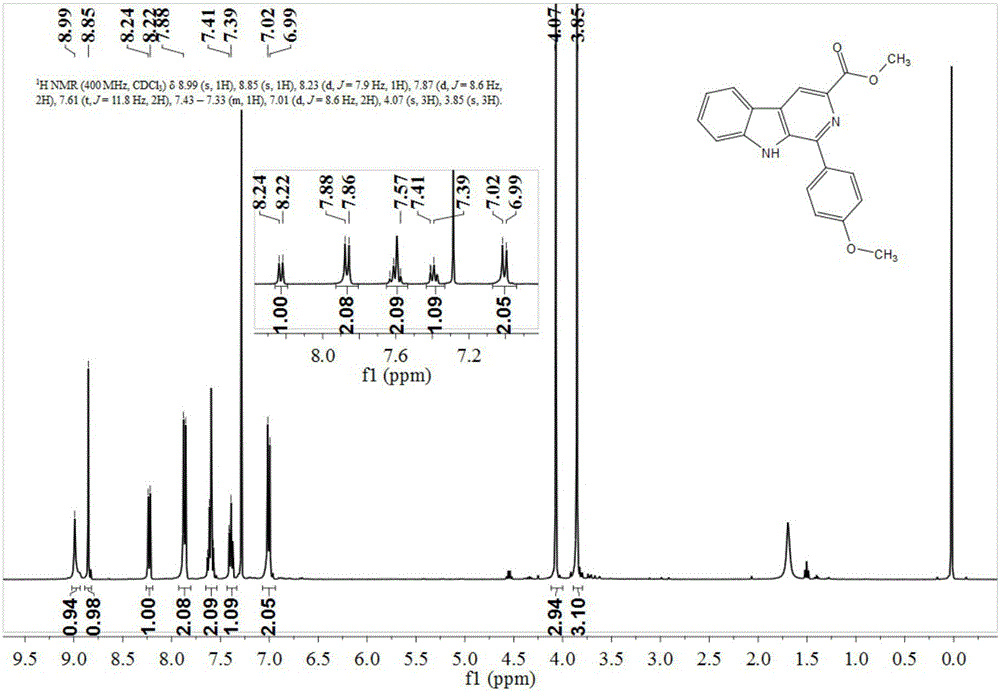
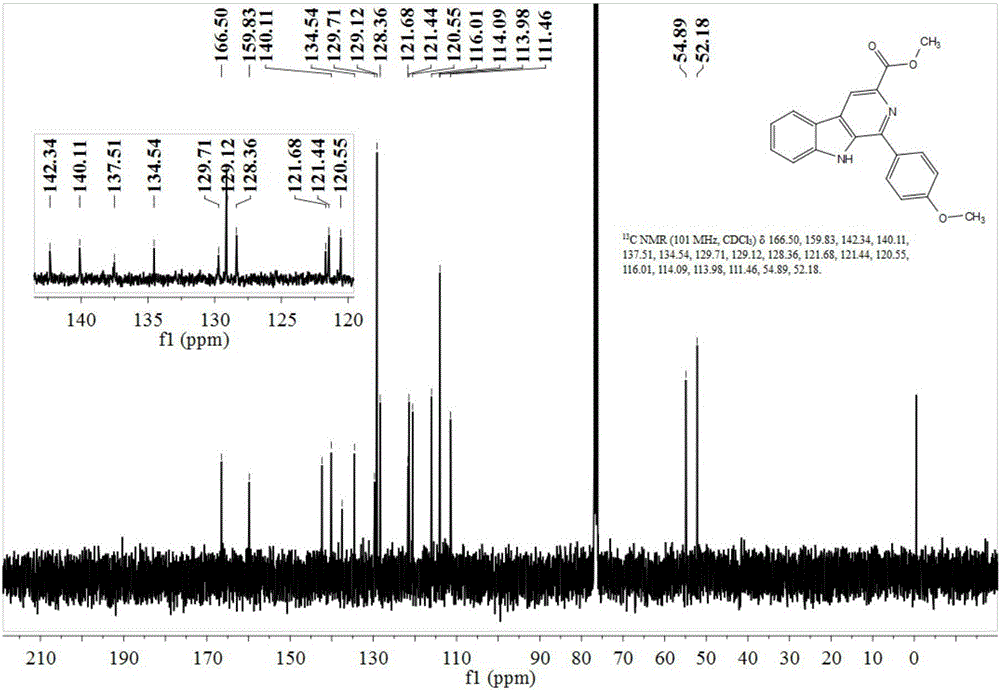
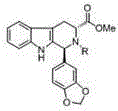
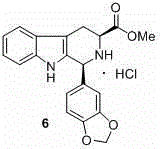
The invention discloses an asymmetric synthesis method for synthesizing 1-aryl-1H-pyridine[3,4-4]indole-3-carboxylic acid methyl ester derivatives containing two chiral centers from (R)methyl-2-amino-3-(1H-indol-3-yl)propanoate and aldehyde as main starting materials under catalysis of a catalyst, namely, chiral Lewis acid formed by a chiral tridentate ligand compound L and Ti(O-iPr)4. Compared with the prior art, the method has the advantages that the reaction yield is increased and the reaction non-correspondence selectivity is remarkably improved.
Owner:北京华素制药股份有限公司
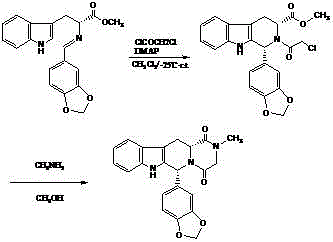
Non-enantioselective Synthesis of 1-aryl-1h-pyridino[3,4-b]indole derivatives
ActiveCN107417685BOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsArylSynthesis methods
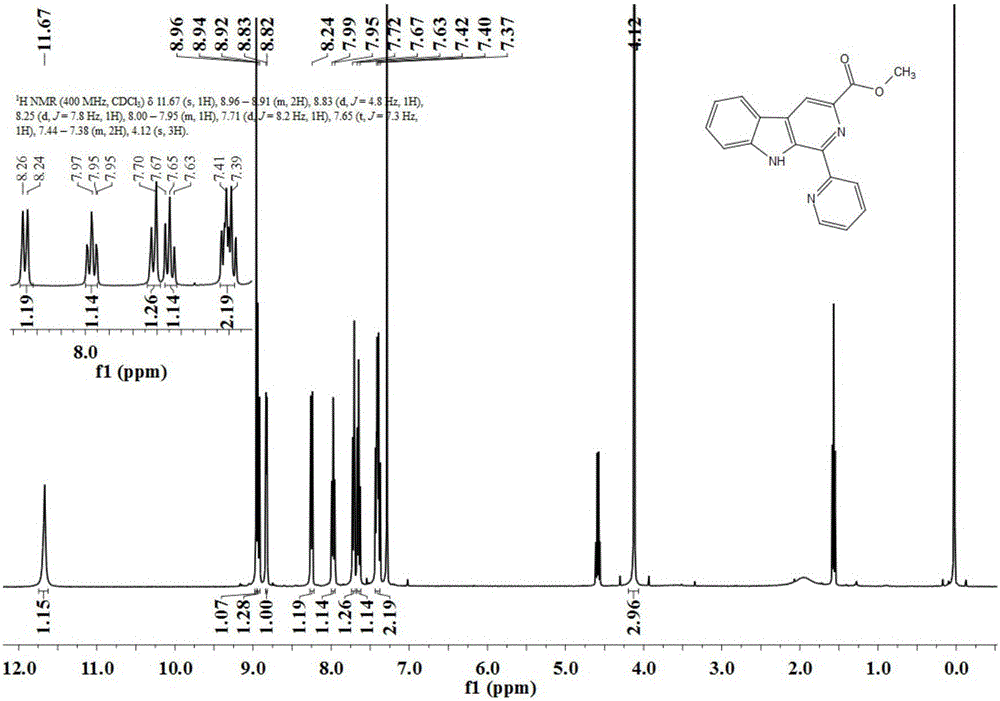
The invention discloses an asymmetric synthesis method for synthesizing 1-aryl-1H-pyridine[3,4-b]indole-3-carboxylic acid methyl ester derivatives containing two chiral centers by taking (R)-tryptophan methyl ester and aldehyde as main starting raw materials, taking chiral Lewis acid Salen-Mn as a catalyst and taking (R)-alpha-methyl-4-nitrophenylacetic acid as a chiral additive. Compared with the prior art, the reaction yield is improved and the non-correspondence selectivity of the reaction is remarkably improved.
Owner:嘉兴慧泉生物科技有限公司
Method for preparing N-substituted-L-methyl tryptophan ester
InactiveCN102050777AImprove utilization efficiencyComplete restorationOrganic chemistrySodium borohydrideReaction speed
The invention provides a method for preparing N-substituted-L-methyl tryptophan ester, comprising the following steps: 1) carrying out methyl-esterification on L-tryptophan to obtain L-methyl tryptophan ester hydrochloride; 2) removing hydrochloric acid from the L-methyl tryptophan ester hydrochloride under the alkaline condition to obtain L-methyl tryptophan ester; 3) carrying out a reaction on the L-methyl tryptophan ester and aromatic aldehyde to obtain indole aromatic imine; and 4) reducing the indole aromatic imine into secondary amine by taking sodium borohydride as a reducing agent. The process has the characteristics of cheap and easily obtained raw materials, no need of purification on intermediate products, fast reaction speed, high yield, less environmental pollution, objective economic and practical values and wide application prospect in the pharmaceutical synthesis field, and is simple in reaction operation and easy in final treatment.
Owner:SHAANXI UNIV OF SCI & TECH
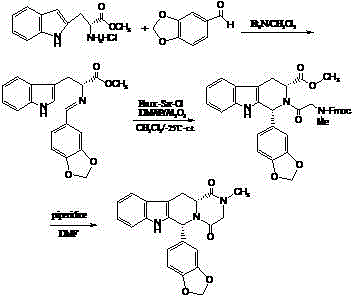
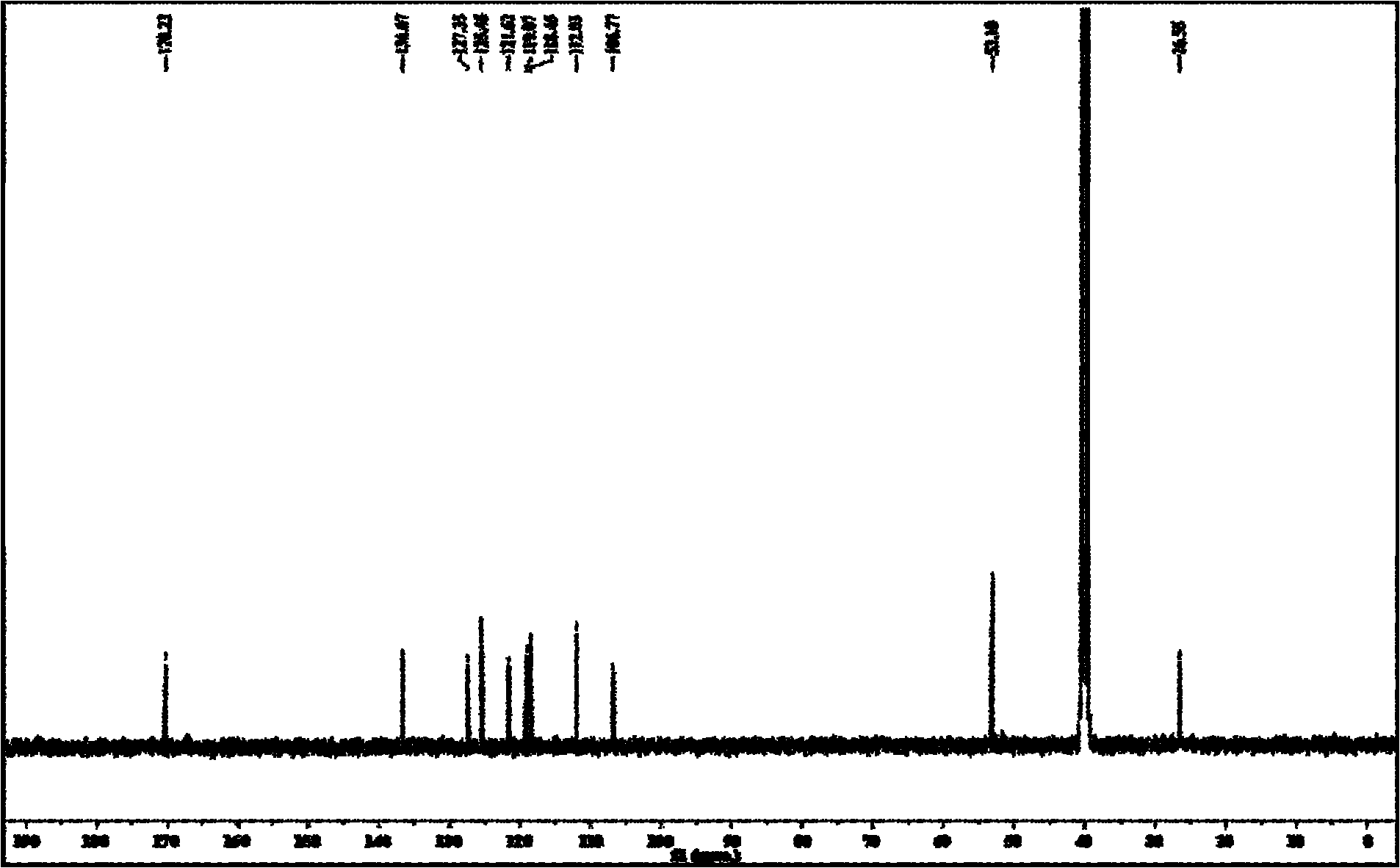
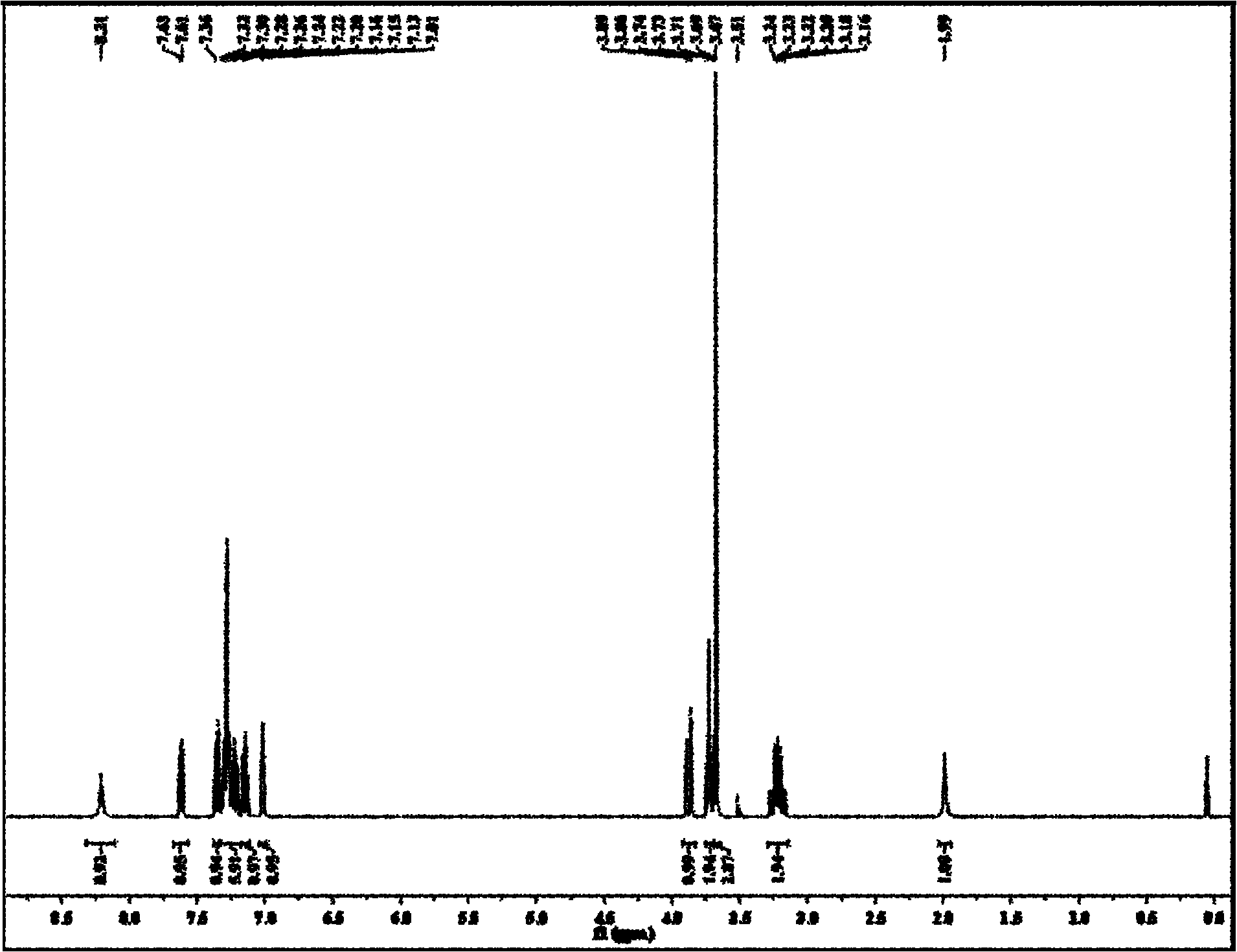
Non-correspondence selective synthesis of 1-aryl-1H-pyridine[3,4-b]indole derivative
ActiveCN107417685AOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsArylSynthesis methods
The invention discloses an asymmetric synthesis method for synthesizing 1-aryl-1H-pyridine[3,4-b]indole-3-carboxylic acid methyl ester derivatives containing two chiral centers by taking (R)-tryptophan methyl ester and aldehyde as main starting raw materials, taking chiral Lewis acid Salen-Mn as a catalyst and taking (R)-alpha-methyl-4-nitrophenylacetic acid as a chiral additive. Compared with the prior art, the reaction yield is improved and the non-correspondence selectivity of the reaction is remarkably improved.
Owner:嘉兴慧泉生物科技有限公司
Novel synthetic method of beta-tetrahydrocarboline compound by using pentamethyleneamine as raw material
InactiveCN102952132ARaw materials are cheap and easy to getReduce manufacturing costOrganic chemistryFormylation reactionChemical products
The invention discloses a novel synthetic method of a beta-tetrahydrocarboline compound by using pentamethyleneamine as a raw material, belonging to the synthesis field of medical intermediates. The invention aims to provide a novel method for preparing the beta-tetrahydrocarboline compound by using pentamethyleneamine as the raw material through formylation reaction in a two-steps cascade reaction manner, wherein the first step of reaction is used for preparing a mixture containing heliotropin and pentamethyleneamine, and the mixture is simply treated but not separated and then directly carries out second step of P-S reaction with D-tryptophan methyl ester or a salt of D-tryptophan methyl ester for cascade-preparing the beta-tetrahydrocarboline compound. Compared with the traditional synthetic method, the synthetic method is a cascade reaction, thereby avoiding purchasing and storing heliotropin which is a precursor chemical product and omitting a complex purification step. The novel synthetic method has the advantages of reducing the cost, saving expenses, having low requirements on production equipment and environment conditions, and achieving high yield and is suitable for industrial production.
Owner:SHANDONG UNIV OF SCI & TECH
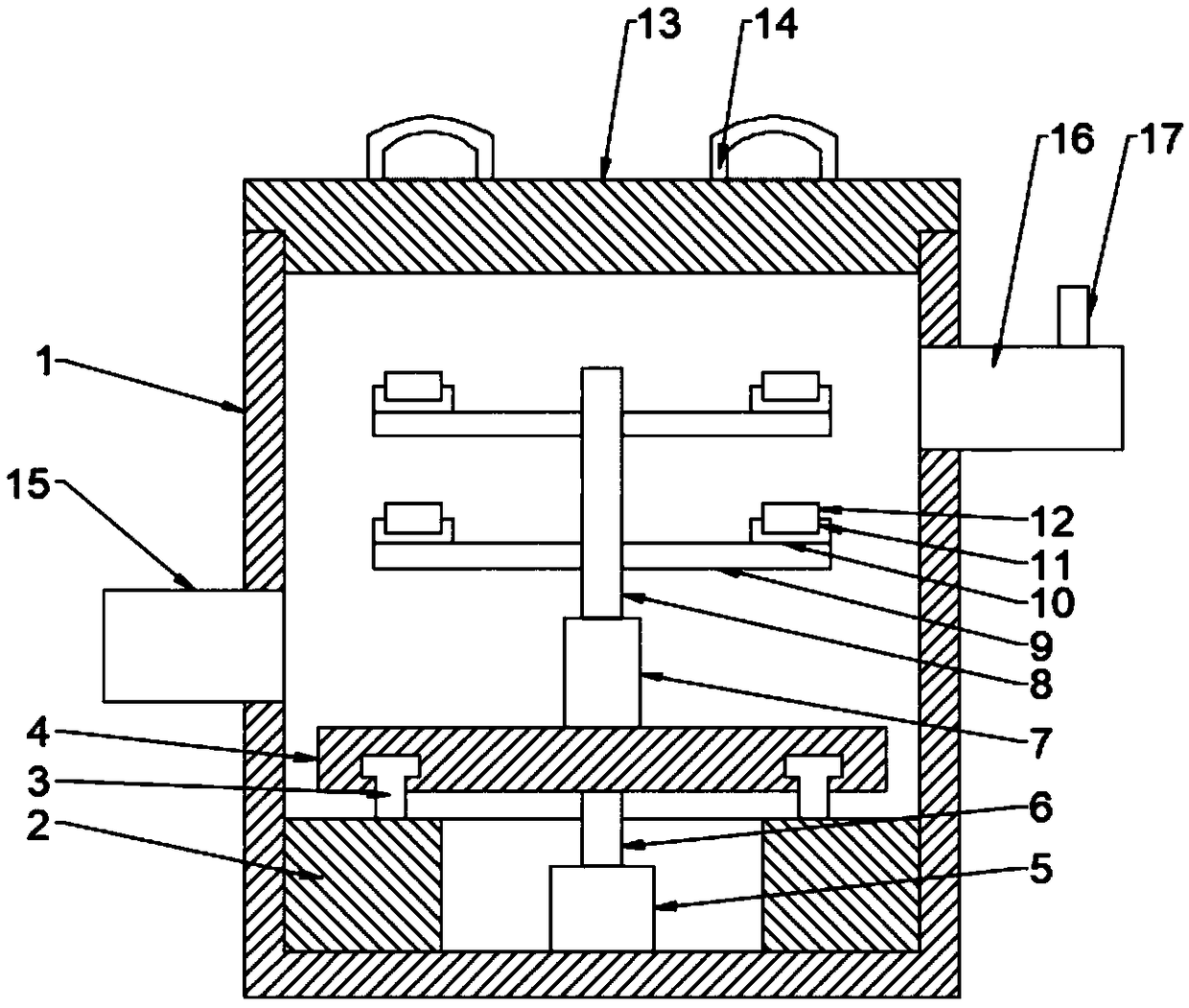
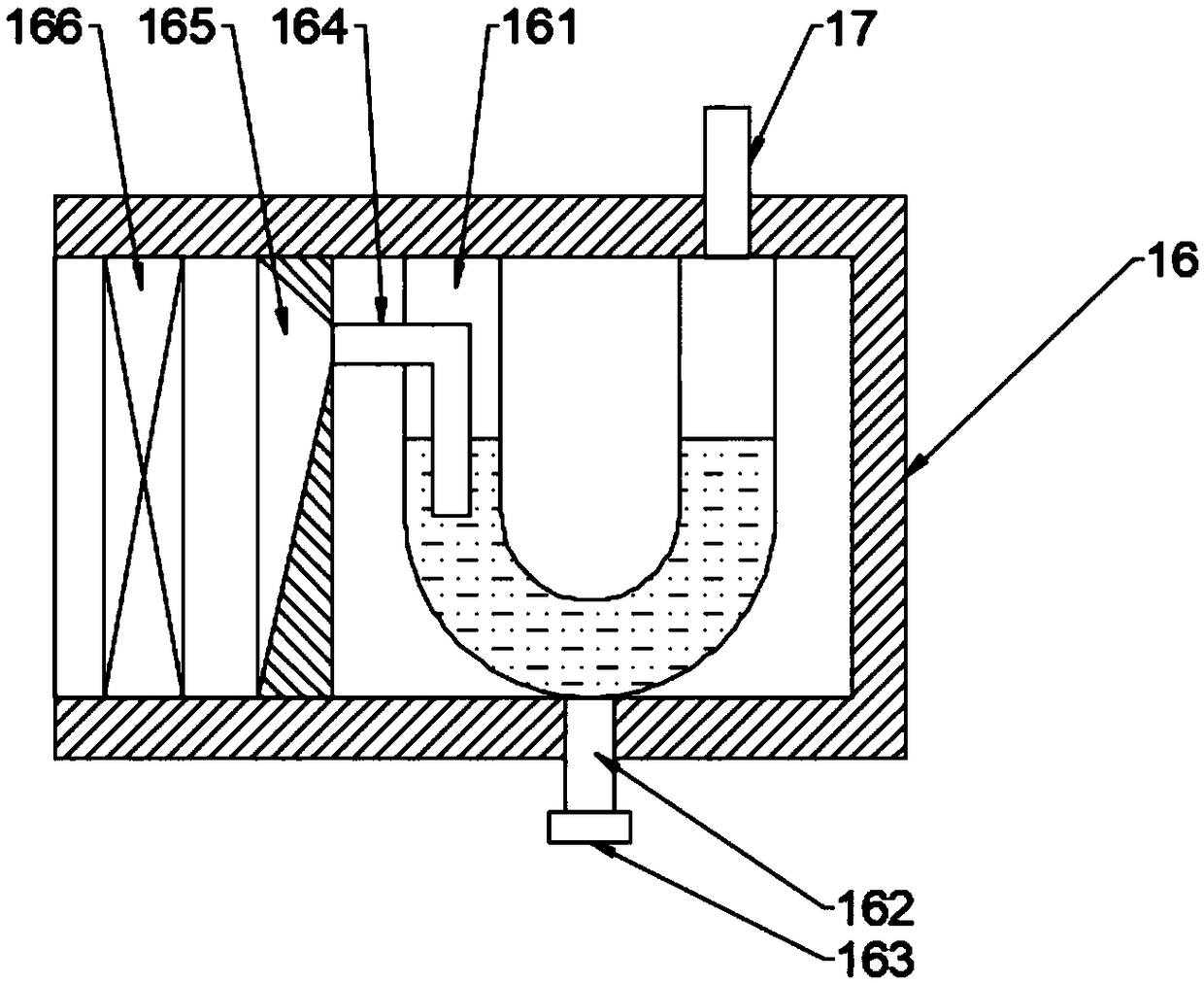
D-tryptophan methyl ester hydrochloride drying device based on lifting and discharging technology
InactiveCN109405489AProtection securityEnsure safetyDrying gas arrangementsDrying chambers/containersEngineeringEster hydrochloride
The invention discloses a D-tryptophan methyl ester hydrochloride drying device based on a lifting and discharging technology. A box body is of a cylinder structure with an opening in the top end, a supporting table is arranged on the edge of the bottom wall of the inner cavity of the box body, and a guide rail is arranged at the top end of the supporting table; a base is arranged at the top end of the guide rail, the guide rail is rotationally connected to the base, an electric telescopic device is arranged in the center of the top end surface of the base, a plurality of supporting rods are uniformly arranged on the outer circle surface of a telescopic rod on the electric telescopic device, and base plates are arranged on one sides, away from the telescopic rod, of the top end surfaces ofthe supporting rods; and a clamping cavity is formed in the top end of each base plate, and a placing plate is arranged in each clamping cavity. According to the device, the telescopic rod is jackedup through the electric telescopic device, so that all the base plates are supported to be separated from the box body, and loading and unloading of the placing plates are completed; a worker finishesloading and unloading above the box body, the position is close to the box body, and the safety of the worker can be protected to the largest extent; and the acid gas in the box body can be timely discharged through an exhaust bucket, the residual of the polluted gas in the box body can be reduced, and the safety of the worker can be guaranteed.
Owner:ZHENGZHOU YUANRAN BIOLOGY TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Asymmetric synthesis of 1-aryl-1H-pyridine[3,4-b]indole-3-carboxylic acid methyl ester derivatives Asymmetric synthesis of 1-aryl-1H-pyridine[3,4-b]indole-3-carboxylic acid methyl ester derivatives](https://images-eureka.patsnap.com/patent_img/5d456563-b1e7-4eed-ad4f-4340bdc8d21b/102362DEST_PATH_IMAGE007.png)
![Asymmetric synthesis of 1-aryl-1H-pyridine[3,4-b]indole-3-carboxylic acid methyl ester derivatives Asymmetric synthesis of 1-aryl-1H-pyridine[3,4-b]indole-3-carboxylic acid methyl ester derivatives](https://images-eureka.patsnap.com/patent_img/5d456563-b1e7-4eed-ad4f-4340bdc8d21b/132634DEST_PATH_IMAGE004.png)
![Asymmetric synthesis of 1-aryl-1H-pyridine[3,4-b]indole-3-carboxylic acid methyl ester derivatives Asymmetric synthesis of 1-aryl-1H-pyridine[3,4-b]indole-3-carboxylic acid methyl ester derivatives](https://images-eureka.patsnap.com/patent_img/5d456563-b1e7-4eed-ad4f-4340bdc8d21b/137413DEST_PATH_IMAGE001.png)
![Non-enantioselective Synthesis of 1-aryl-1h-pyridino[3,4-b]indole derivatives Non-enantioselective Synthesis of 1-aryl-1h-pyridino[3,4-b]indole derivatives](https://images-eureka.patsnap.com/patent_img/6b71df30-a603-4a82-9a59-0f425c599efa/RE-DEST_PATH_IMAGE001.png)
![Non-enantioselective Synthesis of 1-aryl-1h-pyridino[3,4-b]indole derivatives Non-enantioselective Synthesis of 1-aryl-1h-pyridino[3,4-b]indole derivatives](https://images-eureka.patsnap.com/patent_img/6b71df30-a603-4a82-9a59-0f425c599efa/110640DEST_PATH_IMAGE003.png)
![Non-enantioselective Synthesis of 1-aryl-1h-pyridino[3,4-b]indole derivatives Non-enantioselective Synthesis of 1-aryl-1h-pyridino[3,4-b]indole derivatives](https://images-eureka.patsnap.com/patent_img/6b71df30-a603-4a82-9a59-0f425c599efa/117014DEST_PATH_IMAGE001.png)
![Non-correspondence selective synthesis of 1-aryl-1H-pyridine[3,4-b]indole derivative Non-correspondence selective synthesis of 1-aryl-1H-pyridine[3,4-b]indole derivative](https://images-eureka.patsnap.com/patent_img/b5bebc46-becb-47e2-ab61-4e064e45e49c/110640DEST_PATH_IMAGE003.png)
![Non-correspondence selective synthesis of 1-aryl-1H-pyridine[3,4-b]indole derivative Non-correspondence selective synthesis of 1-aryl-1H-pyridine[3,4-b]indole derivative](https://images-eureka.patsnap.com/patent_img/b5bebc46-becb-47e2-ab61-4e064e45e49c/117014DEST_PATH_IMAGE001.png)
![Non-correspondence selective synthesis of 1-aryl-1H-pyridine[3,4-b]indole derivative Non-correspondence selective synthesis of 1-aryl-1H-pyridine[3,4-b]indole derivative](https://images-eureka.patsnap.com/patent_img/b5bebc46-becb-47e2-ab61-4e064e45e49c/24075DEST_PATH_IMAGE001.png)