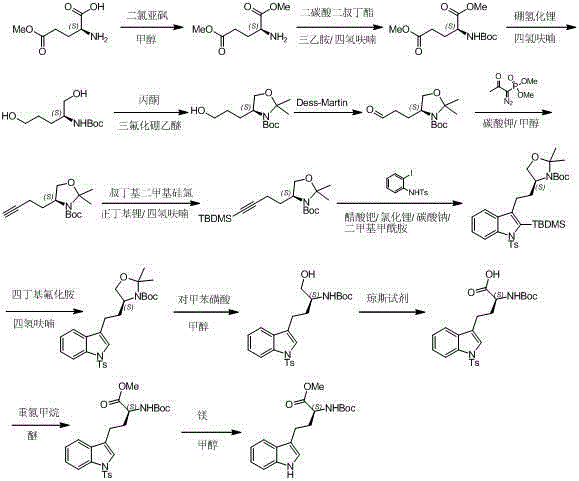
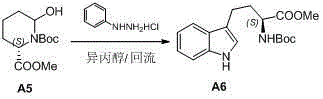
Preparation method of L-N-Boc-high tryptophan methyl ester
A high-tryptophan methyl ester and methyl ester technology, applied in organic chemistry and other fields, can solve the problems of unguaranteed single chirality of the final compound, high cost of raw materials and labor, long reaction steps, etc., to achieve cheap and easy-to-obtain raw materials, Low cost and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
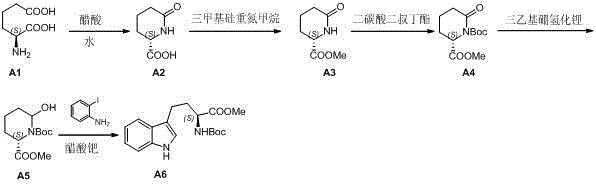
[0020] Preparation of L-2-pyrrolidone-6-carboxylic acid
[0021]
[0022] Steps:
Embodiment 1
[0023] Example 1 : Add L-2-aminoadipic acid (1.61 g, 10 mmol) to acetic acid (30 mL) and water (5 mL). The resulting mixture was reacted at 80°C for about 12 hours. After the reaction of raw materials was monitored by LC-MS, the reactants were concentrated to obtain L-2-pyrrolidone-6-carboxylic acid (1.21 g, yield: 84.6%), which could be used in the next reaction without purification.
Embodiment 2
[0024] Example 2 : Add L-2-aminoadipic acid (1.61 g, 10 mmol) to acetic acid (30 mL) and water (5 mL). The resulting mixture was reacted at 50°C for about 12 hours. After the reaction of the raw materials was monitored by LC-MS, the reactant was concentrated to obtain L-2-pyrrolidone-6-carboxylic acid (0.20 g, yield: 14.0%), which was used in the next reaction without purification.
[0025] 1 HNMR (DMSO, Bruker Avance 400 MHz):δ 1.623 (m, 2H), 1.749 (t, J=7.2Hz, 1H), 1.902 (dd, J =8.8Hz, J =4.4 Hz, 1H), 2.110 (m, 2H), 3.924 (m, 2H), 7.4(s, 1H).
[0026] Preparation of L-2-pyrrolidone-6-methyl ester
[0027]
[0028] Steps:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com