Method for preparing 1-substituted-beta-carboline-3-carboxylic ester
A technology of carboxylate and carboxylate, applied in the field of preparation of 1-substituted-β-carboline-3-carboxylate, which can solve the problems of high toxicity of oxidant and solvent, high oxidation reaction temperature, unsatisfactory yield, etc. , to achieve the effect of simple preparation method, convenient post-processing and easy raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
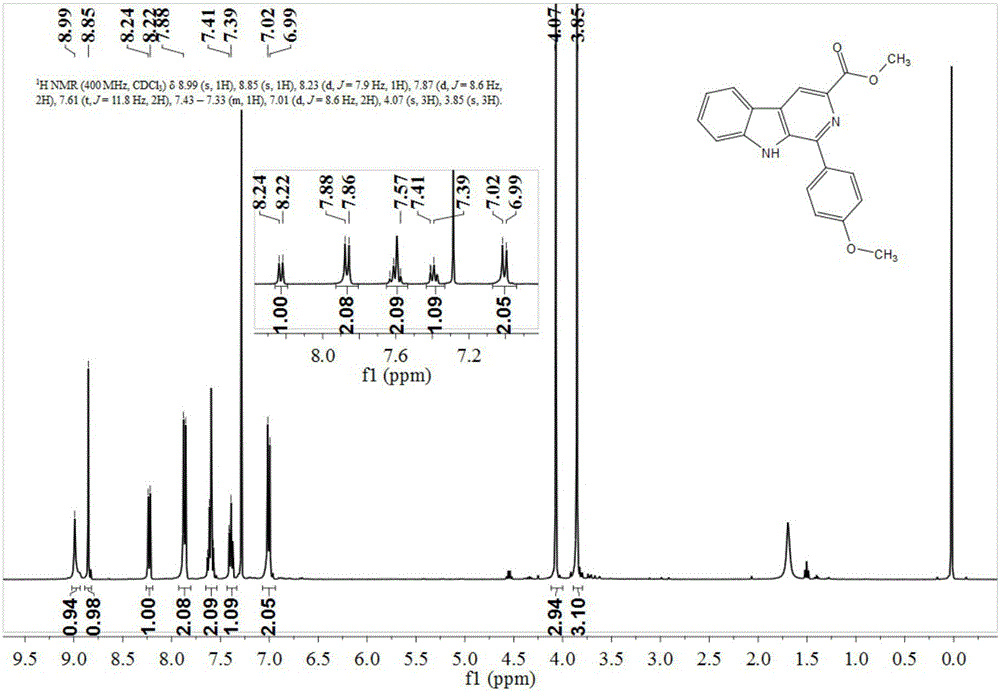
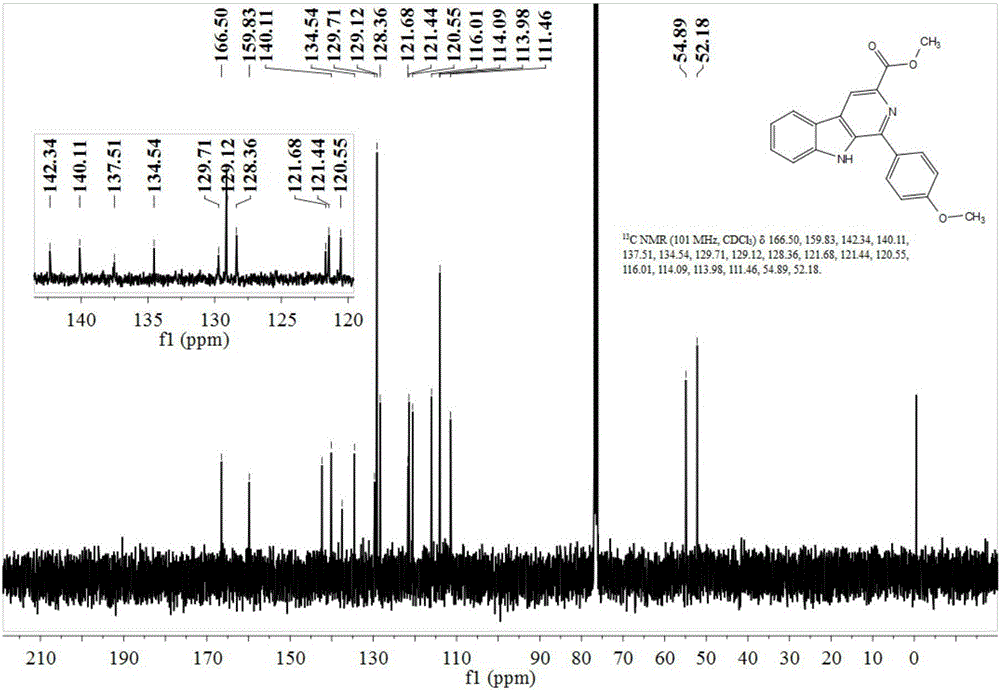
[0058] Preparation of 1-(4'-methoxy)phenyl-β-carboline-3-carboxylic acid methyl ester
[0059] 2mmol of tryptophan methyl ester was dissolved in ethanol and water mixed liquid solvent with a volume ratio of 1:2, 2.1mmol of p-methoxybenzaldehyde was added, and 0.004mmol of nano-copper oxide and 0.004mmol of nano-ferric oxide were added. As a catalyst, it was heated under reflux at 75°C for 1 hour, and the progress of the reaction was monitored by TLC. After the reaction was complete, it was concentrated and separated by column chromatography to obtain 265 mg of a light yellow solid with a yield of 89%. The structural formula of the product obtained was as follows:
[0060]
[0061] Such as figure 1 and figure 2 Shown, product NMR characterization 1 H NMR (400MHz, CDCl 3 )δ8.99(s,1H),8.85(s,1H),8.23(d,J=7.9Hz,1H),7.87(d,J=8.6Hz,2H),7.61(t,J=11.8Hz, 2H),7.43–7.33(m,1H),7.01(d,J=8.6Hz,2H),4.07(s,3H),3.85(s,3H). 13 C NMR (101MHz, CDCl 3 )δ166.50, 159.83, 142.34, 140.11, 1...
Embodiment 2
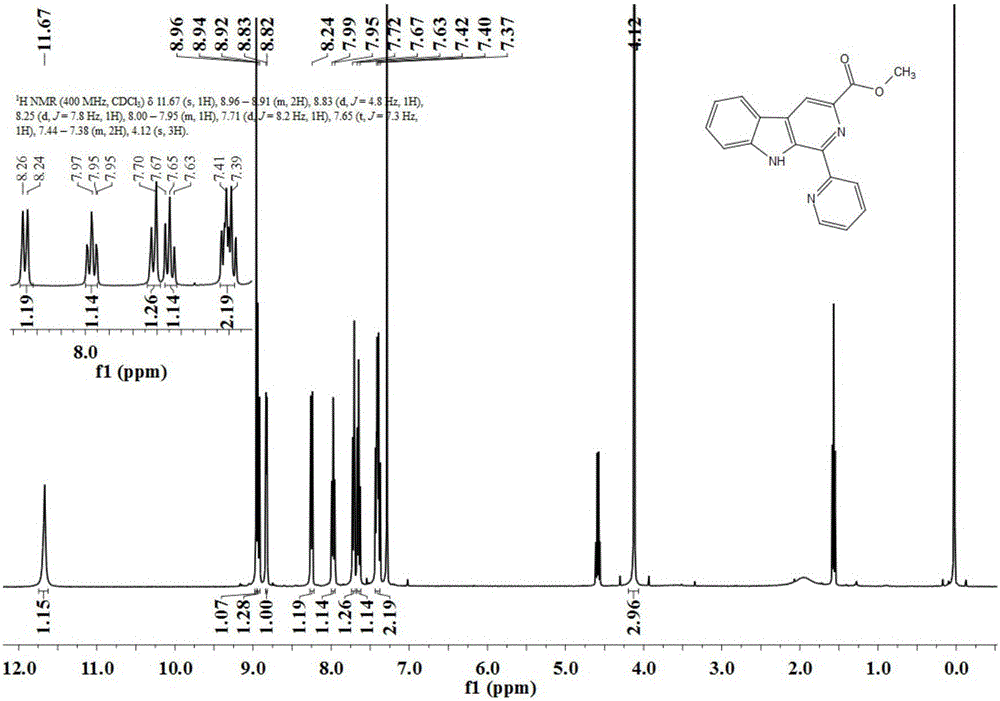
[0063] Preparation of 1-(2'-pyridine)-β-carboline-3-carboxylic acid methyl ester
[0064] Dissolve 2mmol of tryptophan methyl ester in a methanol-water mixture solvent with a volume ratio of 5:1, add 2.4mmol of dipyridylbenzaldehyde, use 0.006mmol of nanometer ferric oxide as a catalyst, and heat at 40°C Reflux for 30 hours, monitor the progress of the reaction with TLC, concentrate after the reaction is complete, and separate through column chromatography to obtain 515 mg of a light yellow solid with a yield of 86%. The structural formula of the resulting product is as follows:
[0065]
[0066] Such as image 3 and Figure 4 Shown, product NMR characterization: 1 H NMR (400MHz, CDCl 3 )δ11.67(s,1H),8.96–8.91(m,2H),8.83(d,J=4.8Hz,1H),8.25(d,J=7.8Hz,1H),8.00–7.95(m,1H ), 7.71(d, J=8.2Hz, 1H), 7.65(t, J=7.3Hz, 1H), 7.44–7.38(m, 2H), 4.12(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ166.24, 156.53, 147.72, 140.50, 137.07, 136.69, 136.15, 135.66, 130.18, 128.51, 123.10, 121.62, 12...
Embodiment 3
[0068] Preparation of 1-(4'-pyridine)-β-carboline-3-carboxylic acid methyl ester
[0069] Dissolve 2mmol of tryptophan methyl ester in ethanol and water mixture solvent with a volume ratio of 1:5, add 2.2mmol of dipyridylbenzaldehyde, use 0.008mmol of nano-cerium oxide as a catalyst, and heat to reflux at 50°C for 20 Hour, monitor the progress of reaction with TLC, concentrate after the reaction is complete, separate through column chromatography, obtain 500mg light yellow solid, productive rate is 84%, and gained product structural formula is as follows:
[0070]
[0071] Such as Figure 5 and Figure 6 As shown, the NMR characterization of the product: 1 H NMR (400MHz, CDCl 3 )δ9.74(s,1H),8.95(d,J=10.5Hz,1H),8.72(d,J=5.5Hz,2H),8.26(d,J=7.9Hz,1H),7.87(d, J=5.8Hz, 2H), 7.74–7.58(m, 2H), 7.47–7.39(m, 1H), 4.08(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ166.04, 149.85, 145.12, 140.54, 139.19, 137.86, 134.81, 130.21, 129.02, 122.65, 121.60, 121.36, 121.01, 117.55, 111.68, 52.37....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com