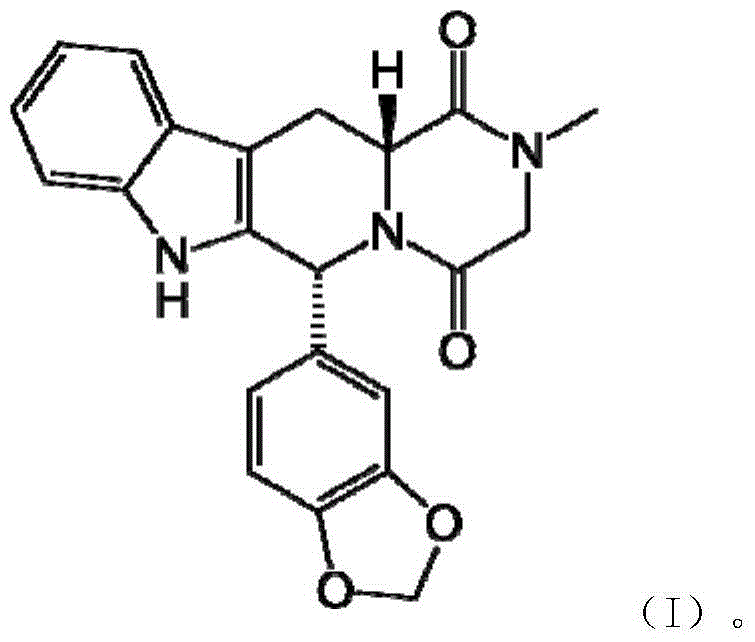
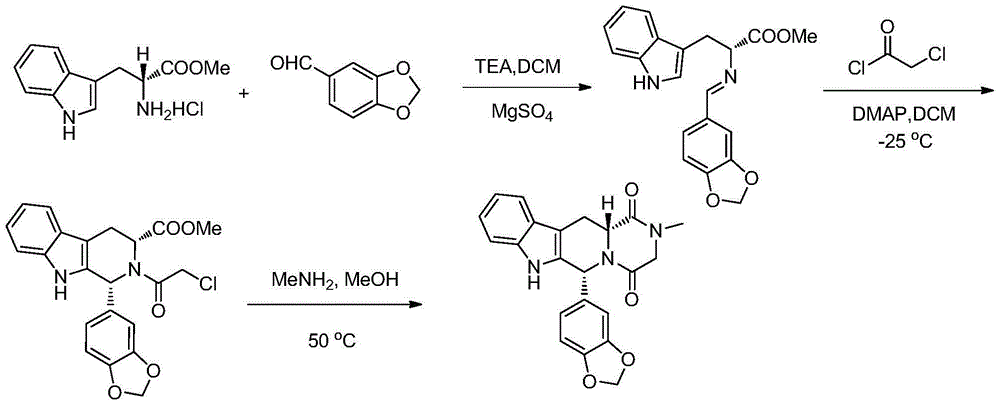
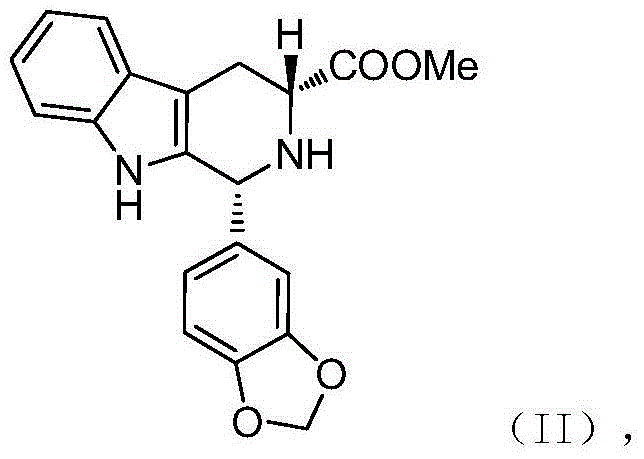
Preparation method of tadalafil intermediate
A technology of tryptophan and hydrochloride, which is applied in the direction of organic chemistry, can solve the problems of cumbersome post-processing, violent reaction, and high cost, and achieve the effects of improving stereoselectivity, simple post-processing, and reducing risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1D
[0026] The preparation of embodiment 1D-tryptophan methyl ester hydrochloride
[0027] Add D-tryptophan (50g) to a mixed solution of toluene (650ml) and methanol (105ml) and stir evenly, slowly add thionyl chloride (38.6g) dropwise at 0°C, and heat up to 75 After reflux reaction at ~80°C for 4 hours, the reaction is over, the reaction solution is cooled to 0°C and stirred for 2 hours, the solid precipitates, filtered, the filter cake is washed with toluene, and vacuum-dried at 50°C for 6h to obtain D-tryptophan methyl Ester hydrochloride, white powder solid. Yield 95%, HPLC purity 99%.
Embodiment 2
[0028] The preparation method of embodiment 2 compound (II)
[0029]
[0030] Into the reaction bottle, add quantitative D-tryptophan methyl ester hydrochloride (50g), compound (IV) (32.5g), acetonitrile (500ml), stir evenly, heat up to 75-85°C, reflux for 16h, HPLC detects that the reaction is progressing; after the reaction, cool down to 25±5°C, stir and crystallize for 4h; filter with suction, wash the filter cake with a small amount of acetonitrile, and dry it in vacuum at 50°C for 6-8h to obtain a white solid with a yield of 94.2%, the compound The HPLC purity of (II) was 98.175%, and the enantiomeric excess (ee%) was 99.12%.
Embodiment 3
[0031] Embodiment 3HPLC detection condition
[0032] 1. The analysis methods of central control and intermediates are as follows:
[0033]
[0034]
[0035] 2. Compound (II) chiral isomer analysis method:
[0036] Chromatographic column: CHIRAL PAK AS-H (4.6*250mm, 5μm);
[0037] Mobile phase: n-hexane-ethanol (containing 0.1% DEA) = 40:60 (on-line mixing);
[0038] Flow rate: 0.5ml / min; Column temperature: 30°C; Wavelength: 225nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com