Novel synthetic method of beta-tetrahydrocarboline compound by using pentamethyleneamine as raw material
A technology of tetrahydrocarboline and piperonyl ring, applied in the new synthesis field of beta-tetrahydrocarboline compounds, can solve the problems of high cost of derivatives, low product content, low yield, etc., avoiding social problems and cheap raw materials The effect of easy availability and lower production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
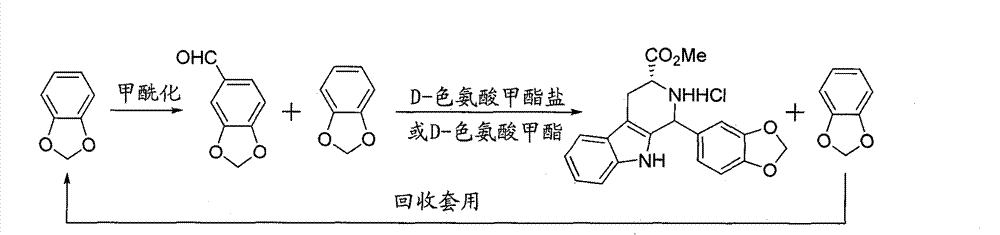
[0017] 1) Add N,N-dimethylformamide (7.5g, 0.1025mol) into a 250mL three-necked flask, and add phosphorus oxychloride (16.3g, 0.1063mol) dropwise at no more than 10°C. , add piperonylcycline (6.1g, 0.049mol), slowly heat up under stirring, after heating for 8 hours, wash and separate the mixed solution of piperonylcycline and piperonal;
[0018] 2) In a 500mL three-necked flask, add D-tryptophan methyl ester hydrochloride (12.5g, 0.049mol) into the mixture in the previous step, then add 150mL of nitroethane, reflux for 4 hours, cool, pump 15.6g of yellow-white solid was obtained by filtration, which was β-tetrahydrocarboline compound, and the yield was 82.5%;
[0019] 3) The filtrate is distilled under reduced pressure, and the obtained pepper ring is applied mechanically, and added into the first step.
Embodiment approach 2
[0021] 1) Add N-methylaniline (10.8g, 0.1025mol) into a 250mL three-necked flask, add phosphorus oxychloride (16.3g, 0.1063mol) dropwise at room temperature, after the addition is complete, add piperonylcycline (6.1g, 0.049mol), heating after stirring, separates the mixed solution of piperonylcycline and piperonal;
[0022] 2) In a 500mL three-neck flask, add D-tryptophan methyl ester hydrochloride (12.5g, 0.049mol) to the mixture in the previous step, then add 100mL of nitroethane, reflux for 8 hours, cool, and filter with suction Obtained 13.6g yellow-white solid, yield rate is 78.5%;
[0023] 3) The mother liquor is recovered by distillation to recover the pepper ring, which is applied mechanically and added to the first step.
Embodiment approach 3
[0025] 1) Add N-methylaniline (15g, 0.205mol) into a 500mL three-necked flask, add phosphorus oxychloride (32.6g, 0.213mol) dropwise at room temperature, after the addition is complete, add piperonylcycline (6.1g, 0.049 mol), heated after stirring, separated to obtain the mixed solution of piperonylcycline and piperonal;
[0026] 2) In a 500mL three-necked flask, add D-tryptophan methyl ester hydrochloride (25g, 0.1mol) to the mixed solution in the previous step, then add 200mL of nitroethane, reflux for 10 hours, cool, and filter with suction to obtain 28g yellow-white solid, the yield is 79.5%;
[0027] 3) The mother liquor is recovered by distillation to recover the pepper ring, which is applied mechanically and added to the first step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com