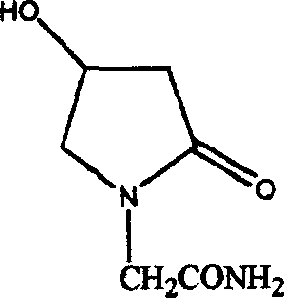
Method of preparing 4-hydroxy pyrrolidone-2-acetamine
A technology for hydroxypyrrolidone and pyrrolidone, which is applied in the field of preparing 4-hydroxypyrrolidone-2-acetamide, can solve the problems that the purity cannot be fully used for medicine, cannot meet the requirements of industrial scale production, and the product composition is complicated, and the raw materials are cheap and the cost is achieved. Low cost, easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
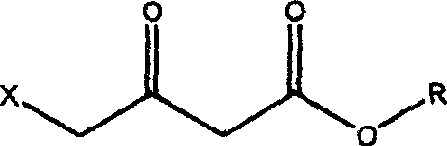
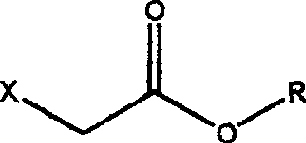
[0029] 1, Preparation of ethyl 4-azidoacetoacetate
[0030] 1000 ml of N,N-dimethylformamide and 167 g (1.0 mol, purity 98.5%) of ethyl 4-chloroacetoacetate were added to the reactor. Sodium azide 110g (2.0mol). The mixture was heated to 100-110°C while stirring, and reacted for 2 hours. After the reaction, the reaction mixture was cooled, and the solvent N,N-dimethylformamide was distilled and recovered under reduced pressure. Add 1000ml of water to the distillation residue, and extract with 1200ml of ethyl acetate (divided into three times, successively 600ml, 400ml, 200ml), combine several times of ethyl acetate, dry with anhydrous magnesium sulfate, and recover ethyl acetate by distillation under reduced pressure. The remainder is ethyl 4-azidoacetoacetate.
[0031] 2, Preparation of 4-hydroxyl-2-pyrrolidone
[0032] Dissolve the ethyl 4-azidoacetoacetate synthesized above in 1000ml of methanol, heat while stirring, control the temperature of the reaction mixture at 40...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com