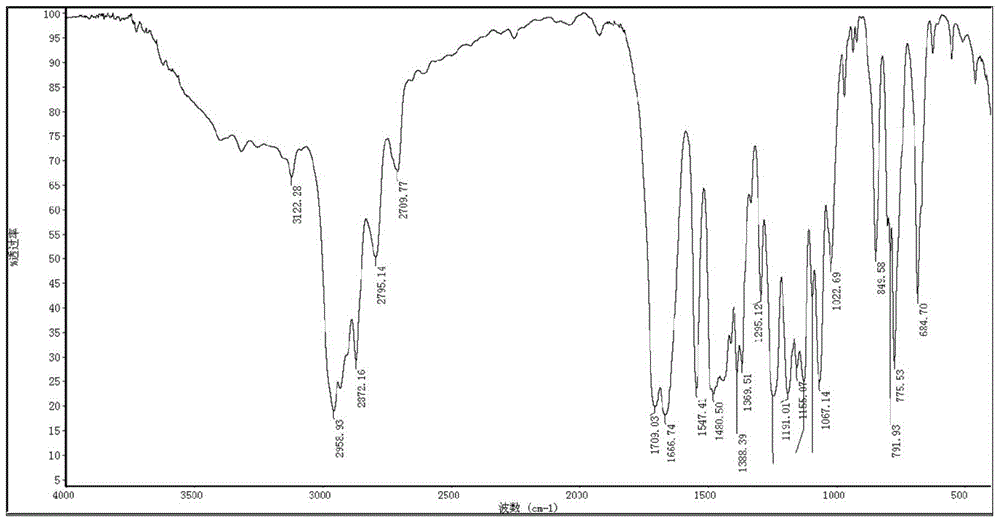
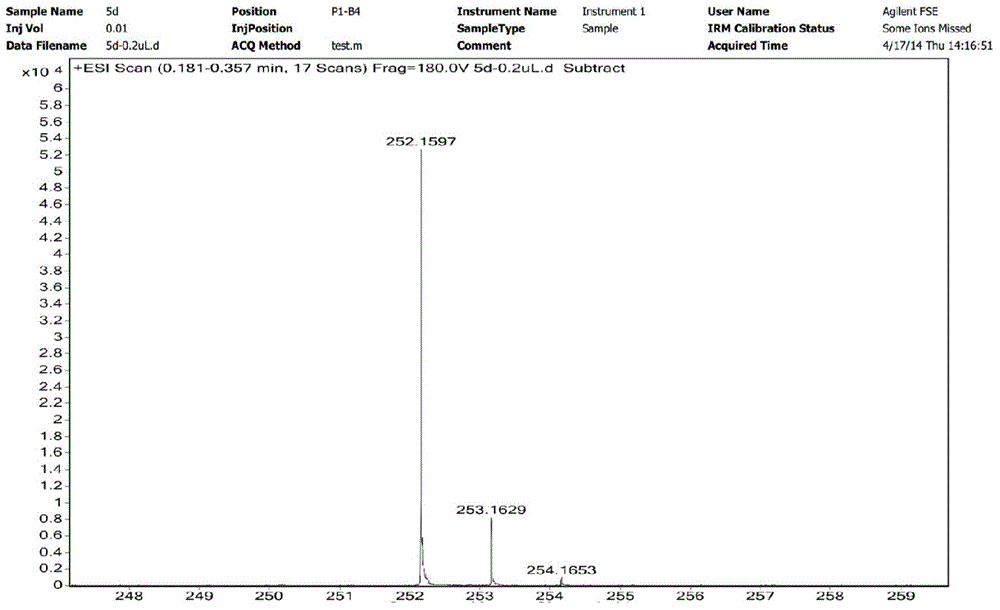
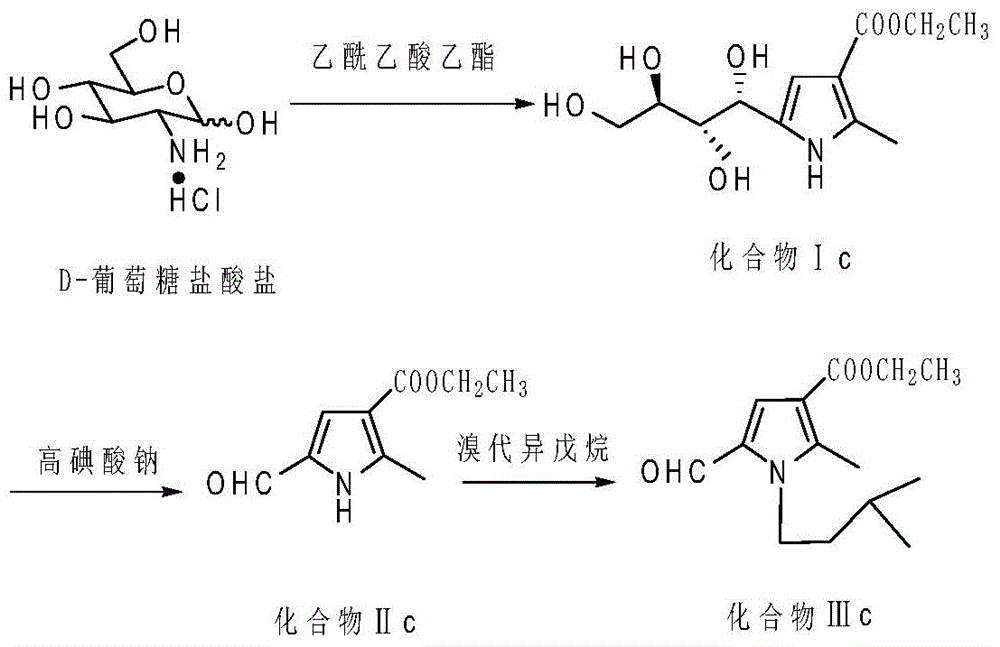
Patents
Literature
233 results about "Acetoacetic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
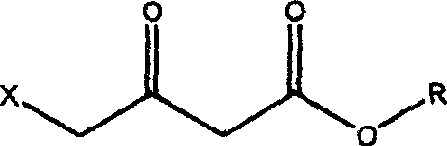
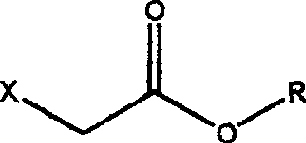
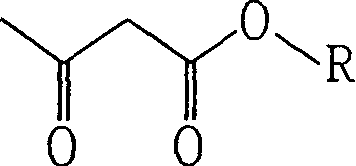
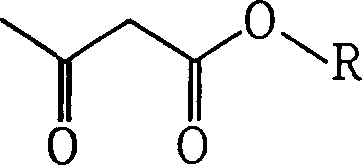
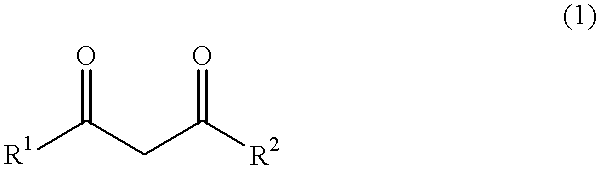
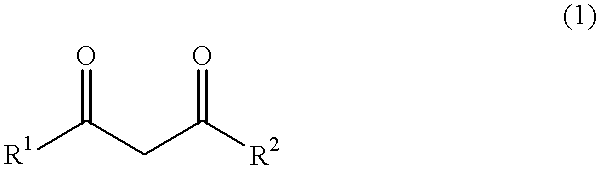
Acetoacetic acid (also diacetic acid) is the organic compound with the formula CH₃COCH₂COOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes. Acetoacetic acid is a weak acid.
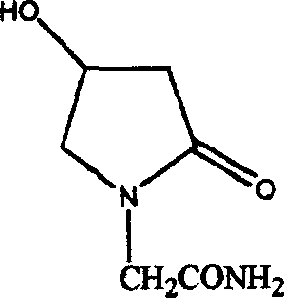
Method of preparing 4-hydroxy pyrrolidone-2-acetamine
InactiveCN1513836ARaw materials are cheap and easy to getLow costOrganic chemistryAcetic acidAlkaline earth metal
A process for preparing 4-hydroxypyrrolidone-2-acetylamine used as brain function improver includes reacting between 4-haloacetoacetic acid derivative and the azide of alkali metal or alkali-earth metal to obtain 4-azoacetoacetic acid derivative, hydrogenating, cyclizing, and ammoniating. Its advantages are high quality and output rate of product, and low cost.
Owner:SUZHOU HOPE TECH
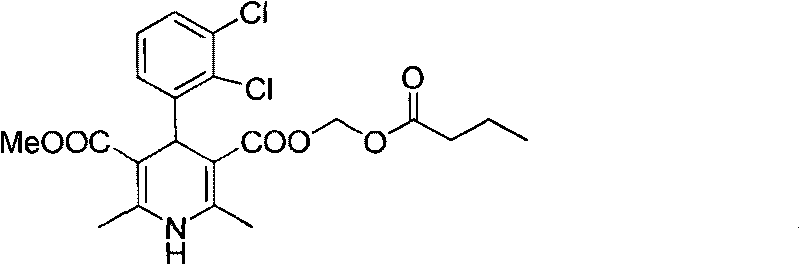
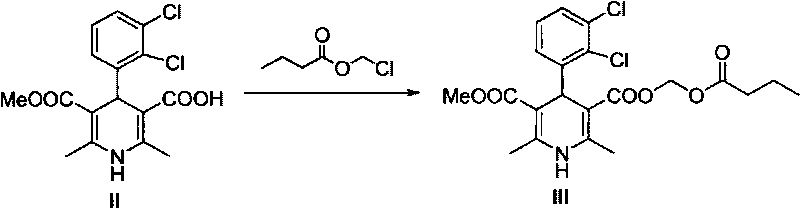
Method for preparing butyrate clevidipine
Owner:SUN YAT SEN UNIV +1
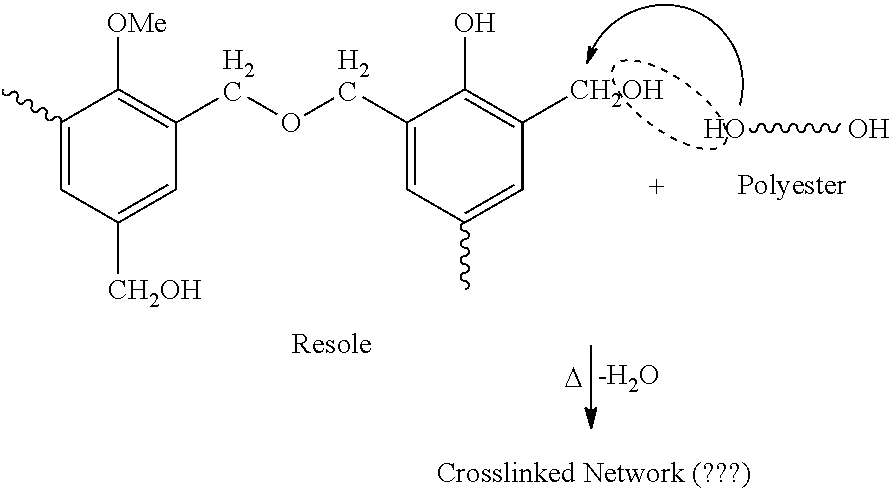
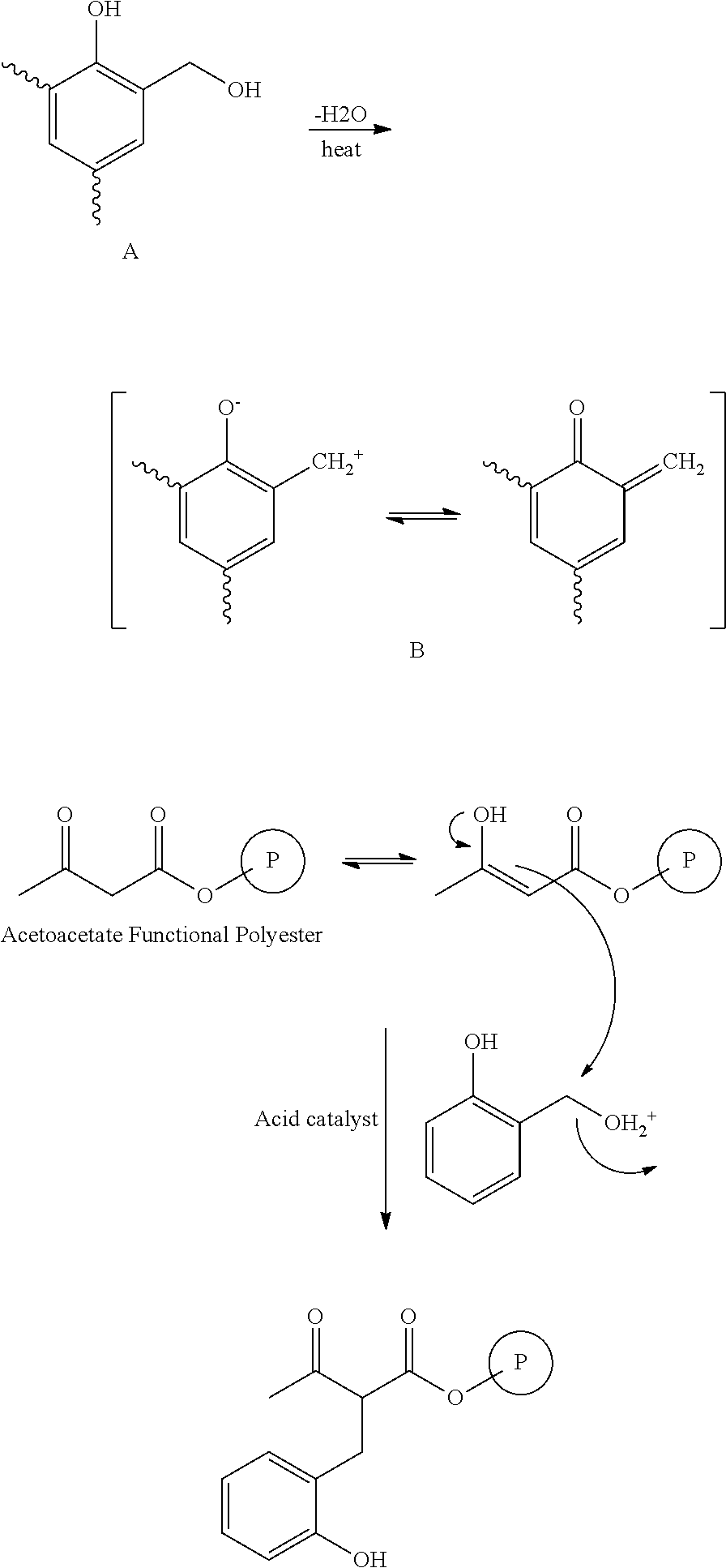
Thermosetting compositions based on phenolic resins and curable poleyester resins made with diketene or beta-ketoacetate containing compounds
ActiveUS20160137877A1Good solvent resistanceEffective cross-linkingPretreated surfacesPolyester coatingsCompound aPolymer science
A thermosetting composition having:I. a curable polyester resin containing beta-ketoacetate moieties without vinyl unsaturations; andII. a phenolic resin having at least one methylol group.The curable polyester resin can be made by reacting a polyester resin with a compound containing a beta-ketoacetate moiety that does not contain a vinyl unsaturation such as alkyl acetoacetate compounds or diketene compounds.The curable polyester resin can be dispersed in water or dissolved in a solvent and is suitable for waterborne or solventborne coating compositions. Phenolic based crosslinking coating compositions that contain these curable polyester resins cure well with phenolic resin crosslinking compounds.
Owner:EASTMAN CHEM CO
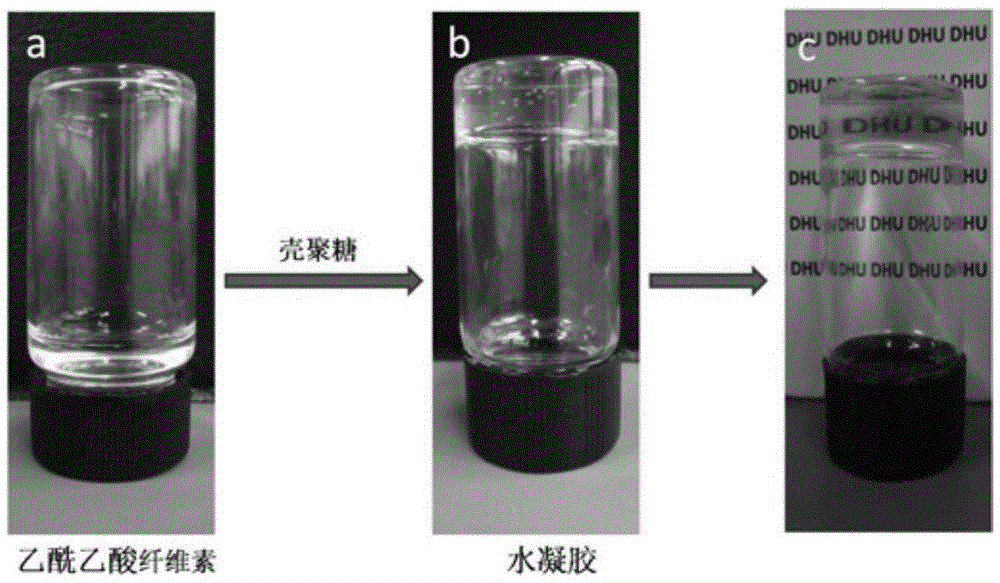
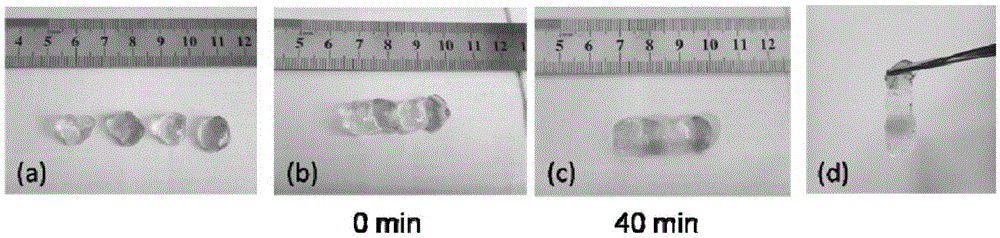
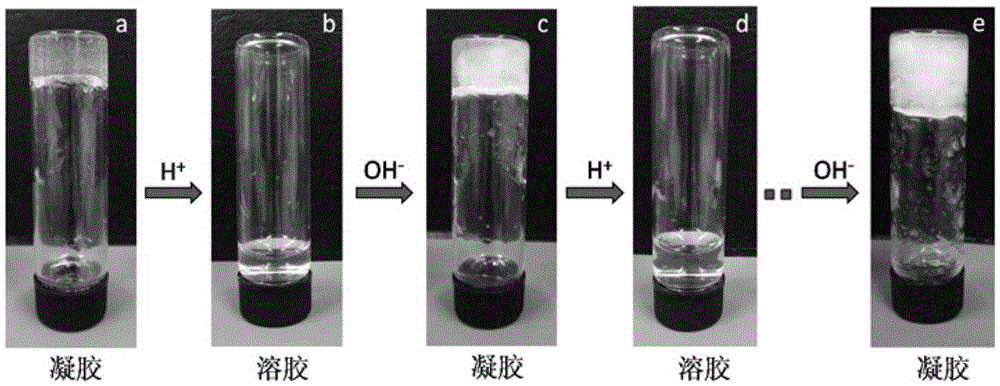
Preparing method for self-healing polysaccharide hydrogel
ActiveCN105622961AThe reaction process is simpleEasy to operatePharmaceutical non-active ingredientsProsthesisCelluloseSelf-healing
The invention relates to a preparing method for self-healing polysaccharide hydrogel. The preparing method comprises the steps that cellulose is added into ionic liquid to be dissolved, the mixture is then cooled to the room temperature, and a cellulose solution is obtained; the cellulose solution is heated and dripped with tert-butyl acetoacetate under the protection condition of introducing nitrogen, a reaction is carried out at the constant temperature, the solution is then cooled to the room temperature, purified and subjected to vacuum drying, and acetoacetic acid cellulose is obtained; under the room temperature, a chitosan solution is added into the acetoacetic acid cellulose solution, oscillated and mixed, and the self-healing polysaccharide hydrogel is obtained. The polysaccharide hydrogel prepared through the method has the self-healing performance, and has the pH responsiveness at the same time. The preparing method is simple in process, rich in raw material and suitable for modification of most polysaccharide derivatives; meanwhile, due to the good biocompatibility of cellulose and chitosan, the prepared polysaccharide hydrogel has good application prospects in the fields such as tissue engineering repair, medicine controlled release and biological bionics.
Owner:DONGHUA UNIV
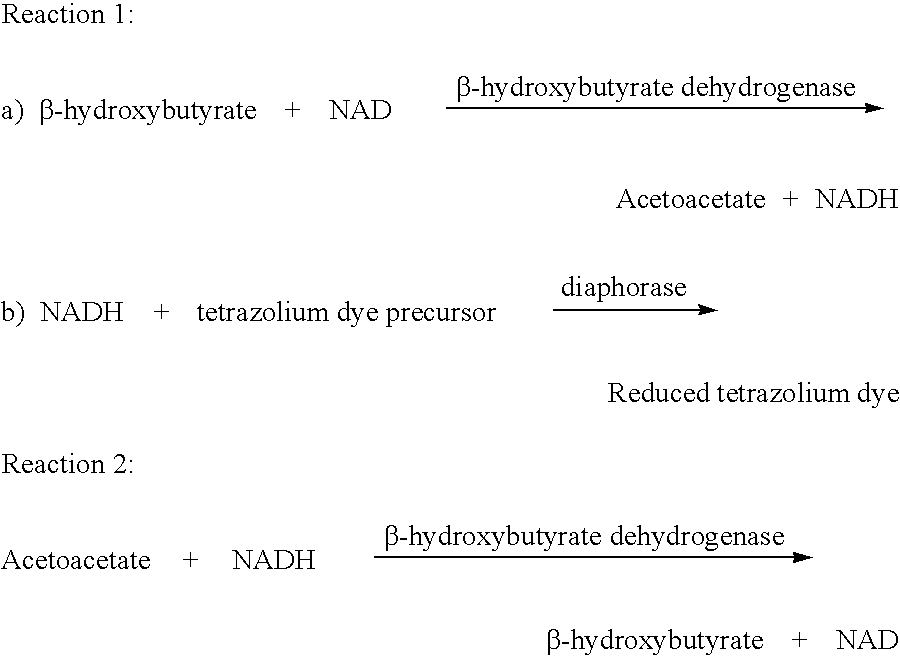
Method and test strips for the measurement of fat loss during weight loss programs
InactiveUS20040043376A1Great advantageEasy to manufactureMicroorganismsMicrobiological testing/measurementPhysiologyAcyl CoA dehydrogenase
Disposable test strips and a wet chemistry method for measuring each of beta-hydroxybutyrate alone, combined beta-hydroxybutyrate and acetoacetate or total ketone bodies (i.e., beta-hydroxybutyrate, acetoacetate and acetone) in human bodily fluid samples, including but not limited to urine, saliva or sweat are described. The test strips need only be dipped in the sample and can be used by anyone in almost any milieu. Measurement can be made electrochemically, spectrophotometrically, fluorometrically or by comparision to a color standard. Combined acetoacetate and beta-hydroxybutyrate which account for 97-98% of total ketone bodies and may be measured in a cyclic reaction that occurs at pH about 7.0 to about 8.3 with beta-hydroxybutyrate dehydrogenase, (beta-HBD), nicotinamide adenine dinucleotide, a tetrazolium dye precursor and an electron mediator. Using this reaction, false positive results obtained from urine samples taken from patients on sulfhydryl drugs are avoided. beta-HBD from some sources was found to cause false negative results in samples (e.g. urine) containing high chloride content due to chloride inhibition of beta-HBD. Using a simple test for chloride inhibition, it was found that beta-HBD from Alcaligenes is not so inhibited. Using either beta-HBD that is not inhibited by chloride or using 10-20 times the normal concentration of this enzyme eliminates false negatives in samples having substantial chloride content, such as urine, both in the reaction described above and in other reactions disclosed for measuring each of beta-hydroxybutyrate alone, combined beta-hydroxybutyrate and acetoacetate and total ketone bodies, all of which reactions occur in the pH range of about 8.6 to about 9.5.
Owner:GUPTA SURENDRA
Antiseptic solutions containing silver chelated with polypectate and edta
ActiveUS20060240122A1Improve long-term stabilityExtended antimicrobial effectiveness of solutionInorganic/elemental detergent compounding agentsBiocideBetaineAdditive ingredient
A liquid antiseptic and cleanser having improved long-term stability includes at least the following principal ingredients: deionized water; silver ion, polypectate, and ethylenediaminetetraaceticacid (EDTA). Presently preferred embodiments of the technology also include glycerine; 1,2-propanediol (a.k.a. propylene glycol); at least one surfactant from any of the families of alkylsulfates, sulfonates, alkanolamides, betaines, amine oxides, sarcosinates and sulfosuccinates; and a buffering compound sufficient to achieve a pH value within a range of 7.2 to 7.8.
Owner:MINER E ODELL
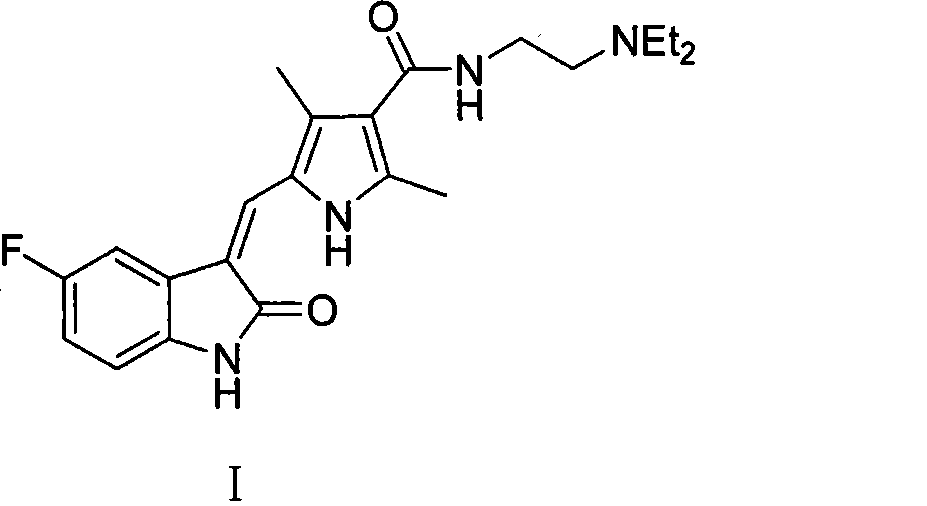
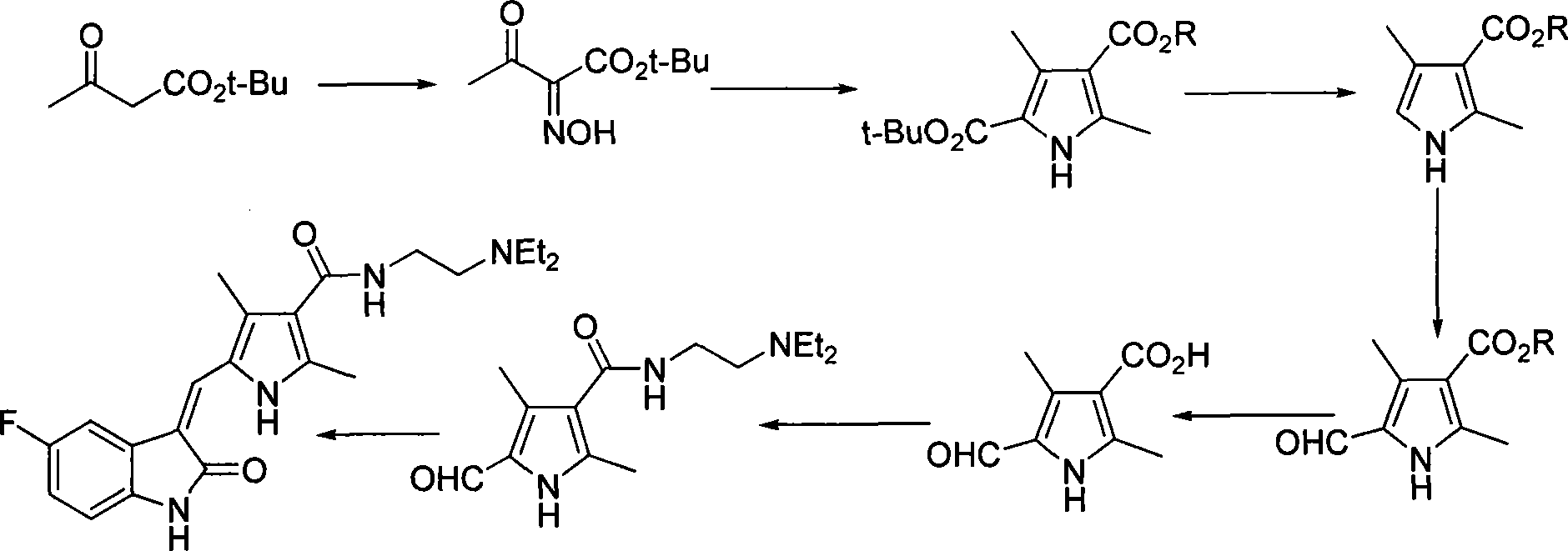
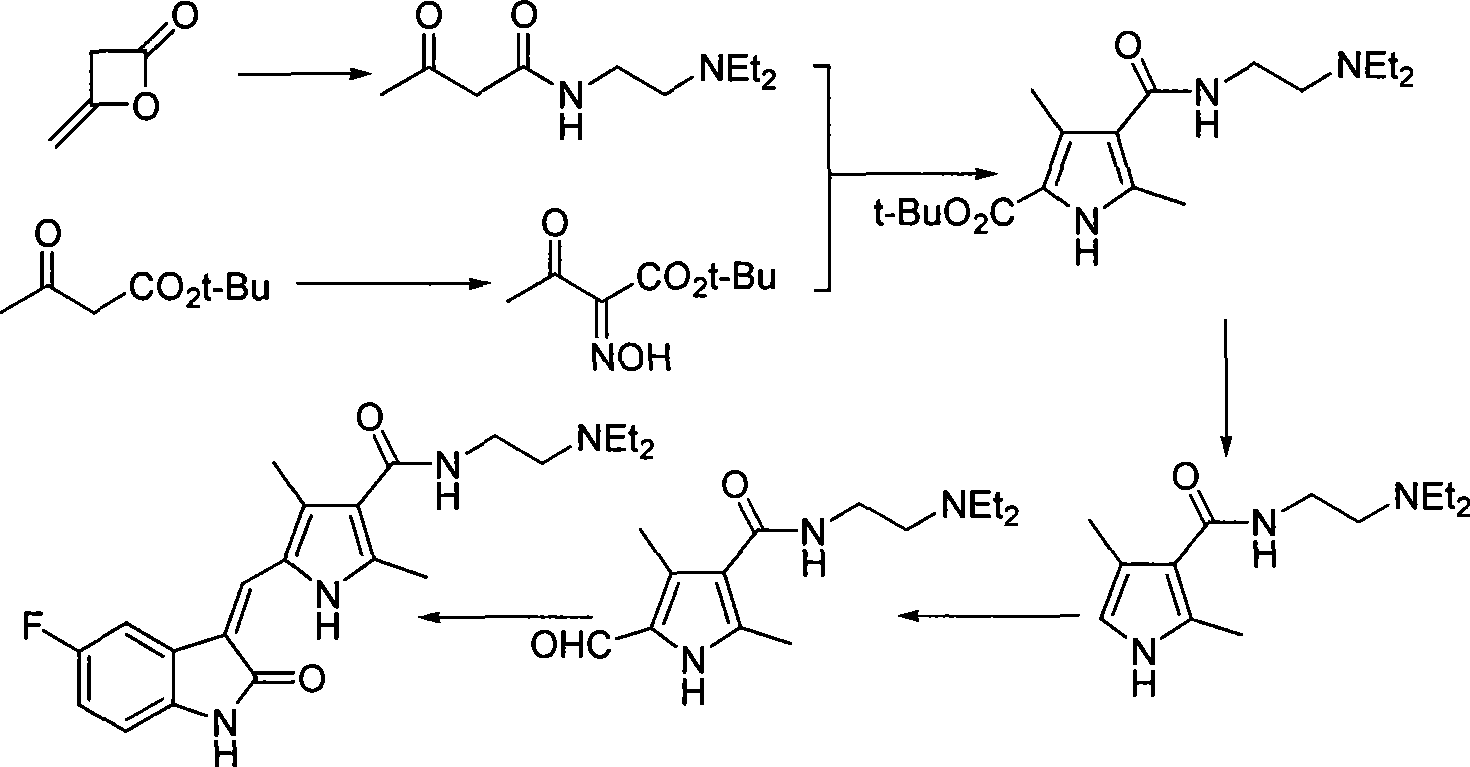
Process for synthesizing sunitinib
The invention discloses a method for synthesizing sunitinib, which comprises the following steps that: tert-butyl acetoacetate and acetylacetic ester are taken as initial raw materials, and tetra-substituted pyrrole is obtained through a nitrosification reduction reaction; then 2,4-dimethyl pyrrole-3-formic acid is obtained through the hydrolysis and is amidated; then an aldehyde group is utilized on position 5 of pyrrole through a Vilsmeier-Hacck reaction; and finally the obtained product and 5-fluorooxindole react to obtain the sunitinib. The method has low cost and simple operation, and is favorable for industrial production.
Owner:FUJIAN SOUTH PHARMA CO LTD
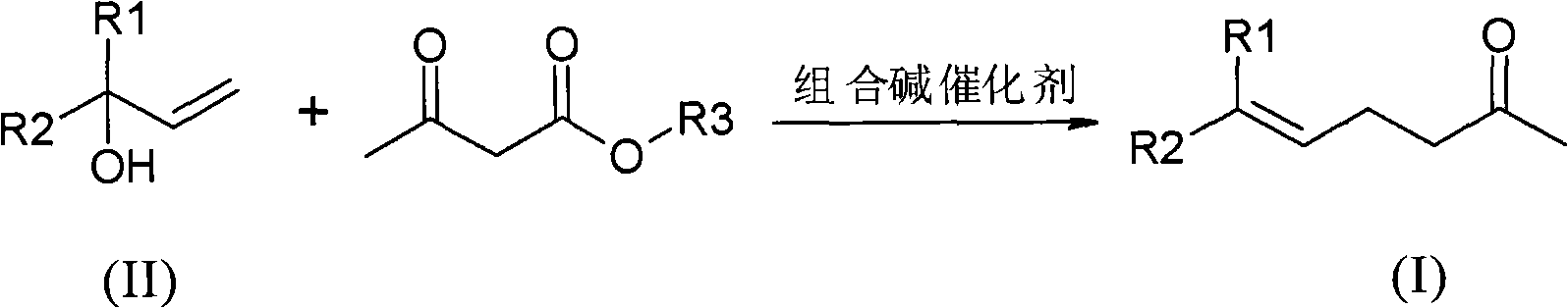
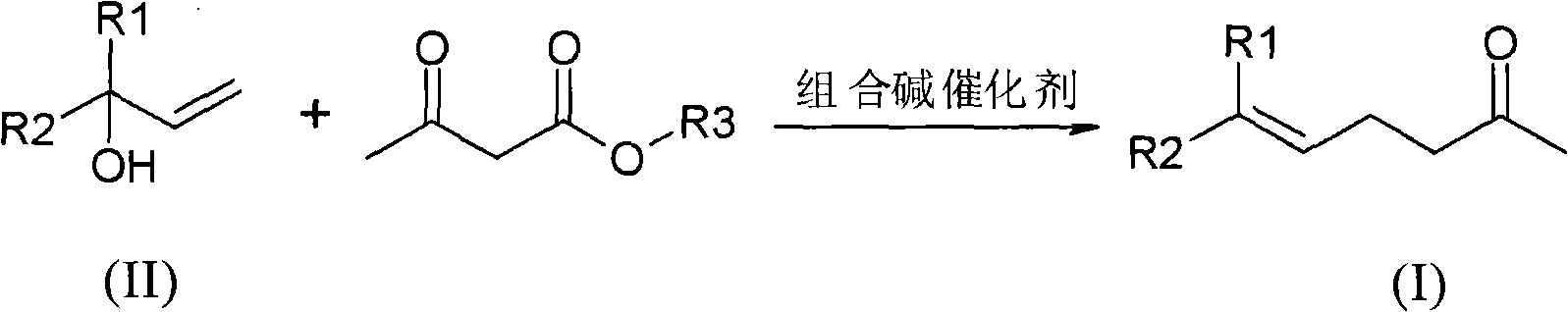
Method for preparing gamma and delta unsaturated ketone
ActiveCN102115437AEasy to recycleHigh yieldOrganic compound preparationCarbonyl compound preparationAlcoholAcetoacetic acid
The invention discloses a method for preparing gamma and delta unsaturated ketone, which comprises the steps of: leading unsaturated alcohol to have alkyl ester reaction with acetoacetic acid under the action of combined alkali catalyst, collecting products and obtaining the target product gamma and delta unsaturated ketone with high purity and high yield. The combined alkali catalyst adopted in the method is easily recovered and applied mechanically, the reaction selectivity is high, and the cost of raw materials is greatly reduced, so that the method is suitable for industrialized production.
Owner:SHANGHAI HEGNO PHARMA HLDG +1
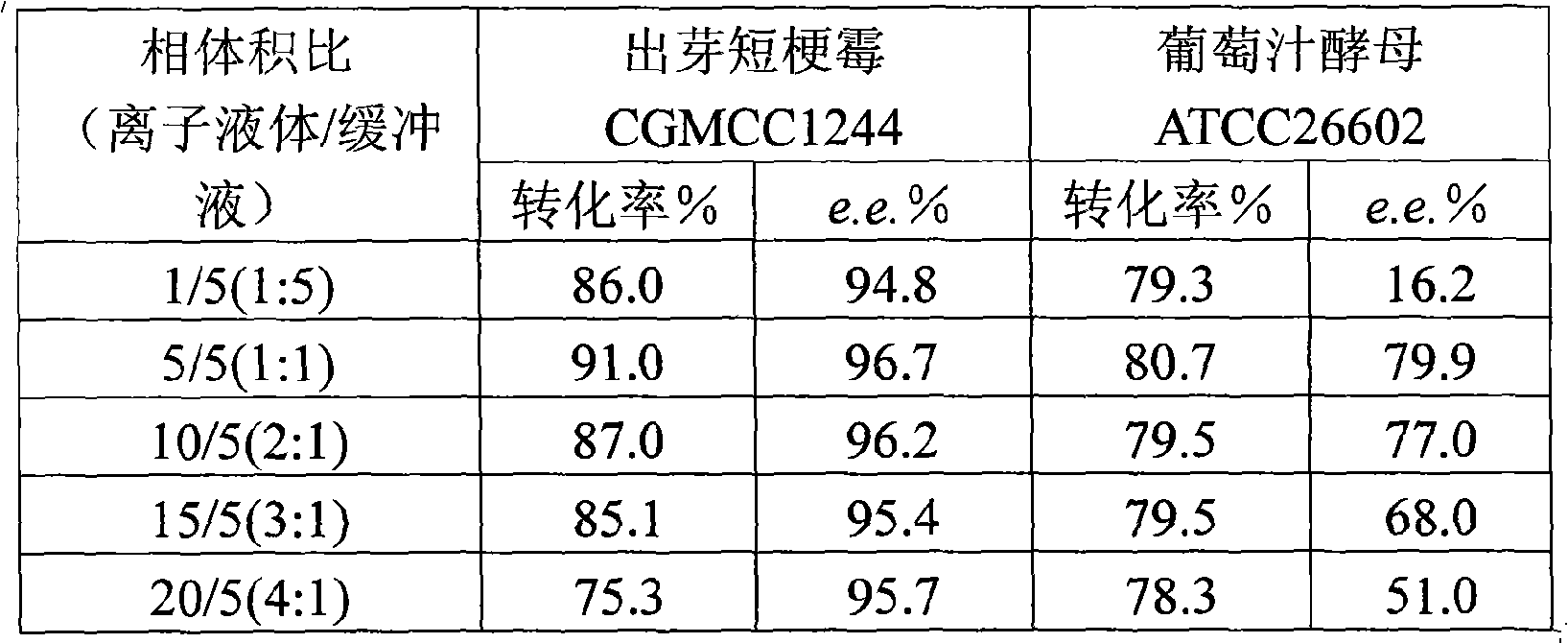
Method for biological catalysis of unsymmetrical reduction carbon based compound in water/ion liquid diphasic system
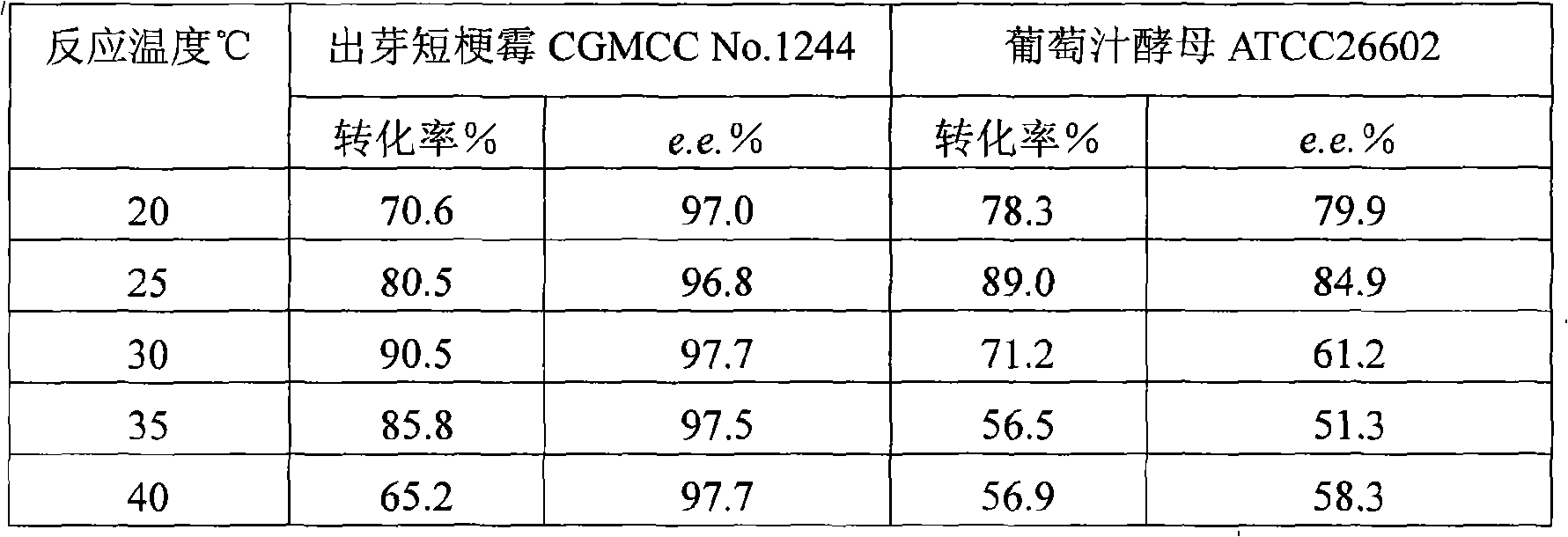
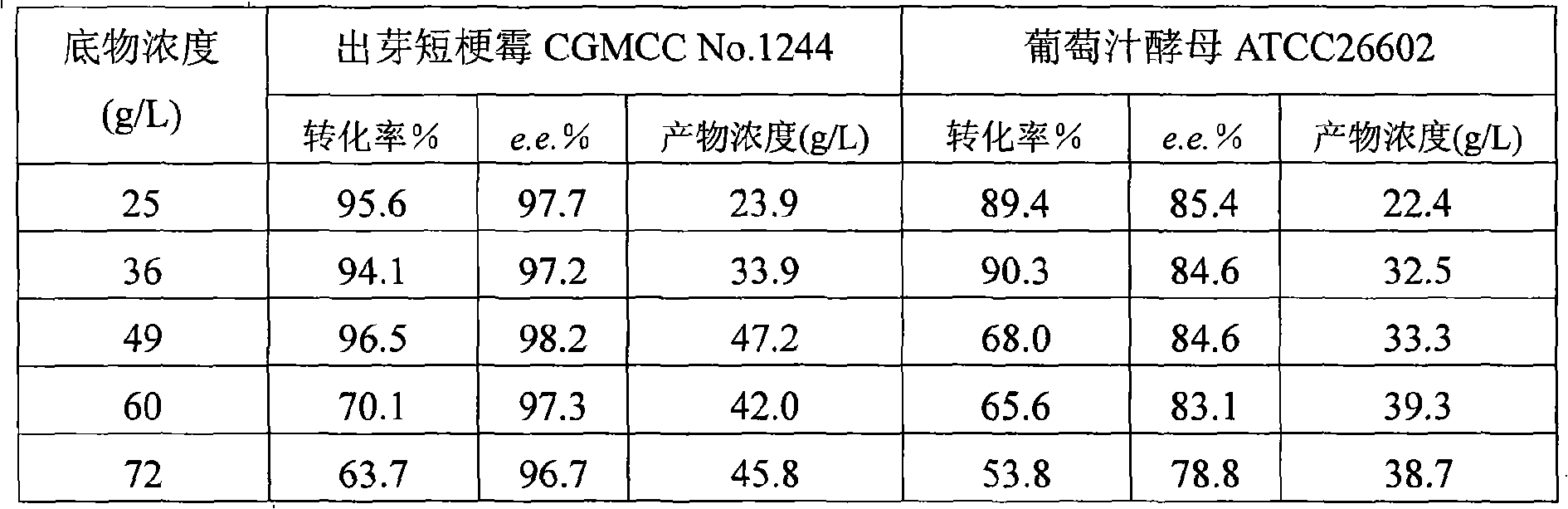
InactiveCN101319236AEasy to separateEasy to recycleMicroorganism based processesFermentationHydroxybutyric acidBiocompatibility Testing
The invention discloses a method for asymmetrically reducing a carbonyl compound by biocatalysis in a water / ion liquid biphasic system, which belongs to the biochemical engineering technical field. The method takes a selective strain producing carbonyl reductase as a starting strain, and prochiral ketone as a substrate to perform reducing preparation of corresponding chiral alcohol in the water / ion liquid biphasic system; the method comprises the following steps that: Aureobasidium pullulans CGMCC No.1244 is used to catalyze 4-chloro-acetoacetic acid ethyl ester to perform the asymmetrical reduction preparation of (S)-4-chloro-3-hydroxybutyric acid ethyl ester, and Saccharoinyces uvarum ATCC 26602 is used to catalyze 4,4,4-trifluoroacetoacetate to perform the asymmetrical reduction preparation of (R)-4, 4, 4-trilfluoro-3-hydroxybutyrate. The method shows the advantages of no toxicity, no smell, difficult volatilization, good biocompatibility, no environmental pollution, simple product separation, easy recovery, repeated use and so on, of ion liquid. At the same time, the method improves the transformation rate, the concentration and an enantiomeric excess value of a reaction product, and quickens the process of the reaction.
Owner:JIANGNAN UNIV
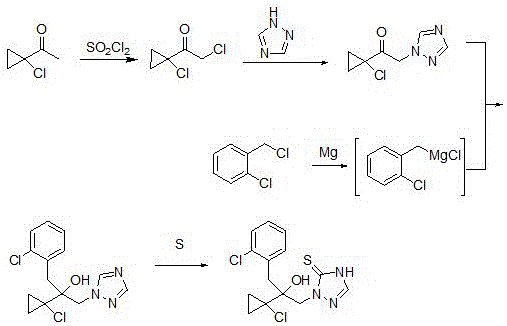
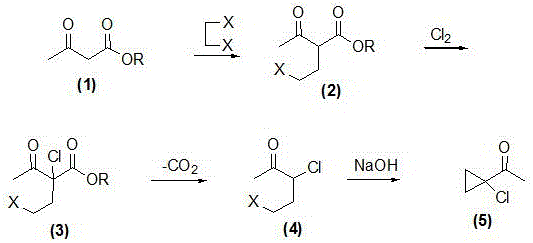
Synthetic method of prothioconazole midbody 1-chloro-1-acetyl cyclopropane
ActiveCN106278850AEasy to recycleHigh reaction yieldOrganic compound preparationCarboxylic acid esters preparationAlkyl transferQuaternary ammonium cation
The invention relates to a synthetic method of a prothioconazole midbody 1-chloro-1-acetyl cyclopropan, which relates to the technical field of chemical production. The synthetic method comprises the following steps: mixing alkyl acetoacetate and 1,2-dihaloalkane or ethylene glycol disulfonate, performing mono-alkylation reaction, after chloration reaction, mixing with a strong acid, performing hydrolysis, and obtaining 3,5-dichloropentane-2-ketone or 3-chloro-4- sulfonate pentane-2-ketone; and finally taking quaternary ammonium salt as a catalyst, mixing the 3,5-dichloropentane-2-ketone or 3-chloro-4-sulfonate pentane-2-keton and alkali liquor, performing ring closing reaction, and obtaining 1-chloro-1-acetyl cyclopropane. By adopting the synthetic method, the cost is reduced, three wastes (waste water, waste gas and waste residues) are reduced, safety and reliability are realized, and the reaction yield is high.
Owner:YANGZHOU TIANCHEN FINE CHEM
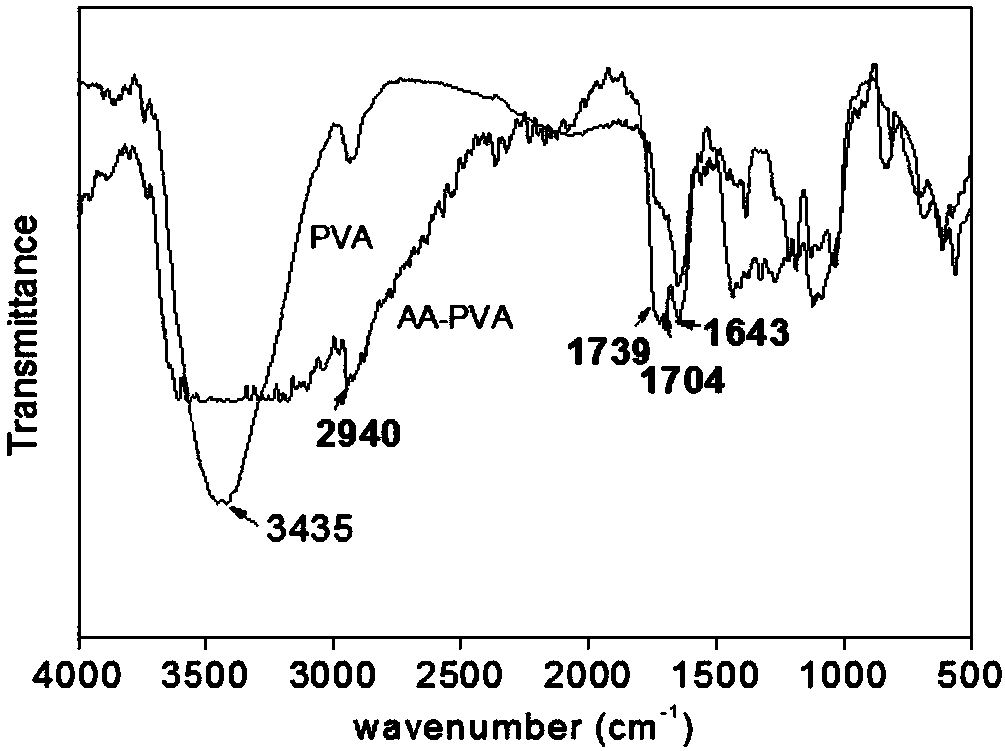
Method for preparing alumina aerogel
InactiveCN108328635AHigh densityHigh strengthAluminium oxide/hydroxide preparationSupercritical dryingAluminum Ion
The invention relates to a method for preparing alumina aerogel. Firstly, methyl acetoacetate and polyvinyl alcohol are used as raw materials; concentrated sulfuric acid is used as a catalyst; throughester exchange reaction, acetoacetic acid groups are grafted on polyvinyl alcohol to prepare a macromolecule complexing agent; secondly, massive alumina aerogel with high density, high intensity, lowshrinkage rate, high porosity, high specific surface area and concentrated hole diameter distribution is obtained through preparation by using aluminium chloride hexahydrate as an inorganic phase precursor, using the macromolecule complexing agent as an additive, using deionized water as a solvent and using propylene oxide as a network gel inducting agent through the sol-gel process and the supercritical drying and roasting processes. The macromolecule complexing agent is added into the sol-gel process as a gel guiding agent and a dispersant, so that metal aluminum ion sol is uniformly dispersed in the solvent and is restrained by a macromolecule chain segment into a core; the primary particle aggregation mode is changed, so that the microstructure of the aerogel is changed. Meanwhile, the density and the intensity of the gel are enhanced.
Owner:SHANGHAI INST OF TECH
Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid
The invention provides a method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid, which comprises the following steps of: reacting 4-chloro-acetoacetic acid ester and 2-aminopyridine in an organic solvent in the presence of an acid binding agent to obtain 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid ester; and hydrolyzing the 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid under the acid or alkaline condition to obtain the 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid. In the method, raw materials have high stability and low price and are readily available; deadly toxic cyanides or high corrosion and unstable bromides are not used; the reaction step is short; the operation is simple; the method is safe and environment-friendly; special equipment is not needed; and the method is suitable for industrial production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Flexible self-healing conductive hydrogel sensor and preparation method thereof
InactiveCN110698693AGood tensile mechanical propertiesImprove fatigue resistanceFluid pressure measurement using ohmic-resistance variationForce measurement using piezo-resistive materialsPolyvinyl alcoholMaterials science
The invention relates to a flexible self-healing conductive hydrogel sensor and a preparation method thereof. According to the preparation method, the flexible self-healing conductive hydrogel sensoris prepared through following steps: blending an acrylamide solution and an acetoacetated polyvinyl alcohol solution, initiating polymerization to obtain a flexible precursor hydrogel, and then soaking the flexible precursor hydrogel in a ferric trichloride solution to obtain a finished product. The prepared hydrogel has excellent sensing performance and can be successfully used for monitoring human motion or physiological signals, meanwhile, the hydrogel device has good fatigue durability and self-healing performance, and therefore the requirements of sensing devices on the service life and signal stability can be met.
Owner:DONGHUA UNIV
Impact-resisting toughening PP/PBT plastic alloy material and preparation method thereof
InactiveCN105111586AStrong impact resistanceGood toughening effectPolytetramethylene terephthalateElastomer
The invention discloses an impact-resisting toughening PP / PBT plastic alloy material. The material is prepared from, by weight, 55-80 parts of polypropylene, 20-40 parts of polybutylene terephthalate, 8-23 parts of polytetrahydrofuran, 15-28 parts of acetoacetic acid allyl ester, 10-30 parts of acrylic elastomer, 5-15 parts of antioxidants, 5-18 parts of toughening agents and 10-35 parts of talcum powder. The invention further discloses a preparation method of the impact-resisting toughened PP / PBT plastic alloy material. The prepared impact-resisting toughened PP / PBT plastic alloy material has the good impact resistance and toughening performance, and the application range of the PP / PBT plastic alloy material can be widened.
Owner:SUZHOU FAST INFORMATION TECH CO LTD
Acetacetic acid alkyl ester metal chelate coating drier and production thereof
A drier of acetacetic acid alkyl ester metal chelate paint and its production are disclosed. The procedure is carried out by reacting alkyl alcohol with diketene to obtain acetacetic acid alkyl ester chelant, and synthesizing the chelant with inorganic metal salt to obtain drier of acetacetic acid alkyl ester metal chelate. Its advantages include solid storage, light color, non-toxic, various raw material resources, simple process and more output.
Owner:上海市涂料研究所有限公司
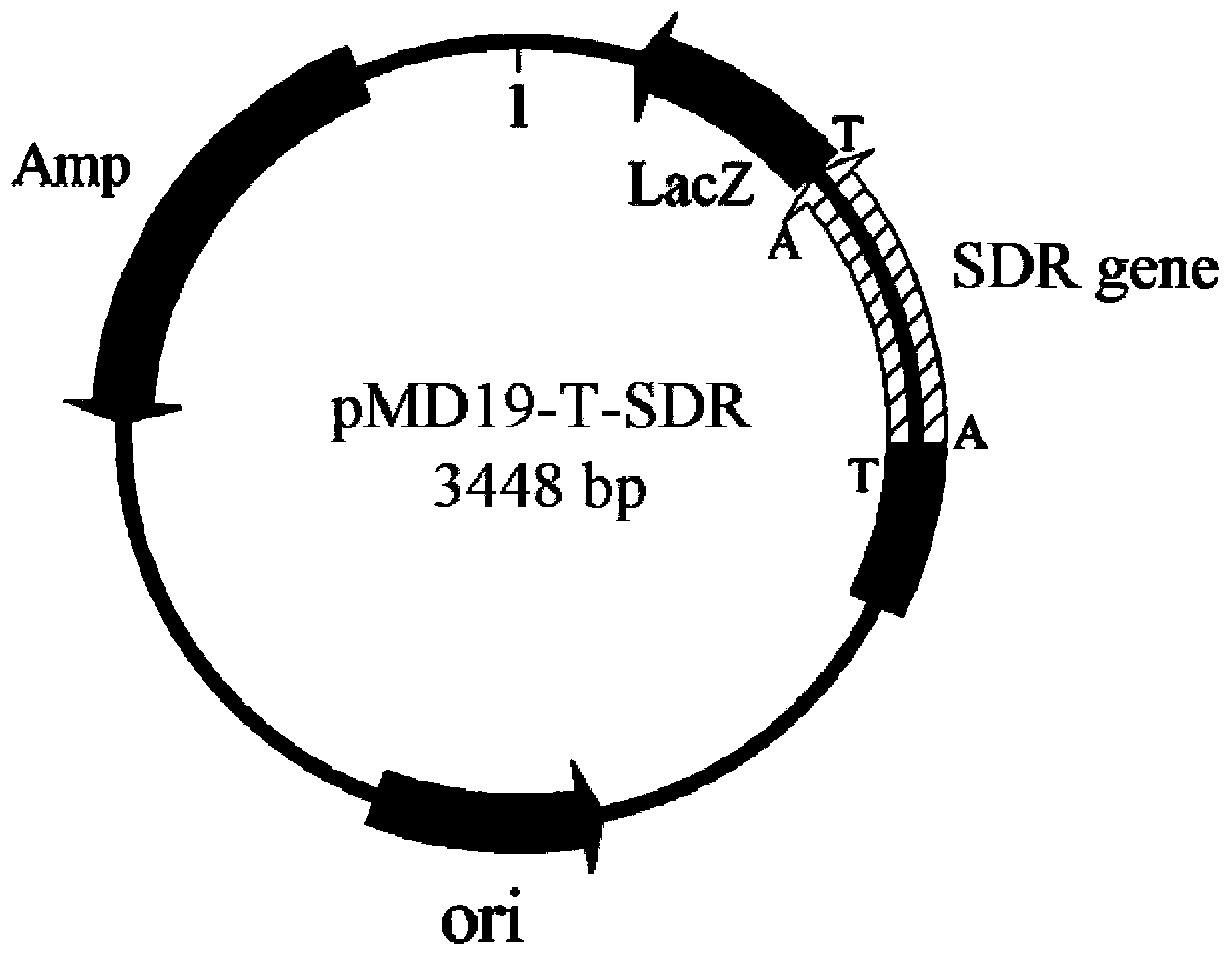
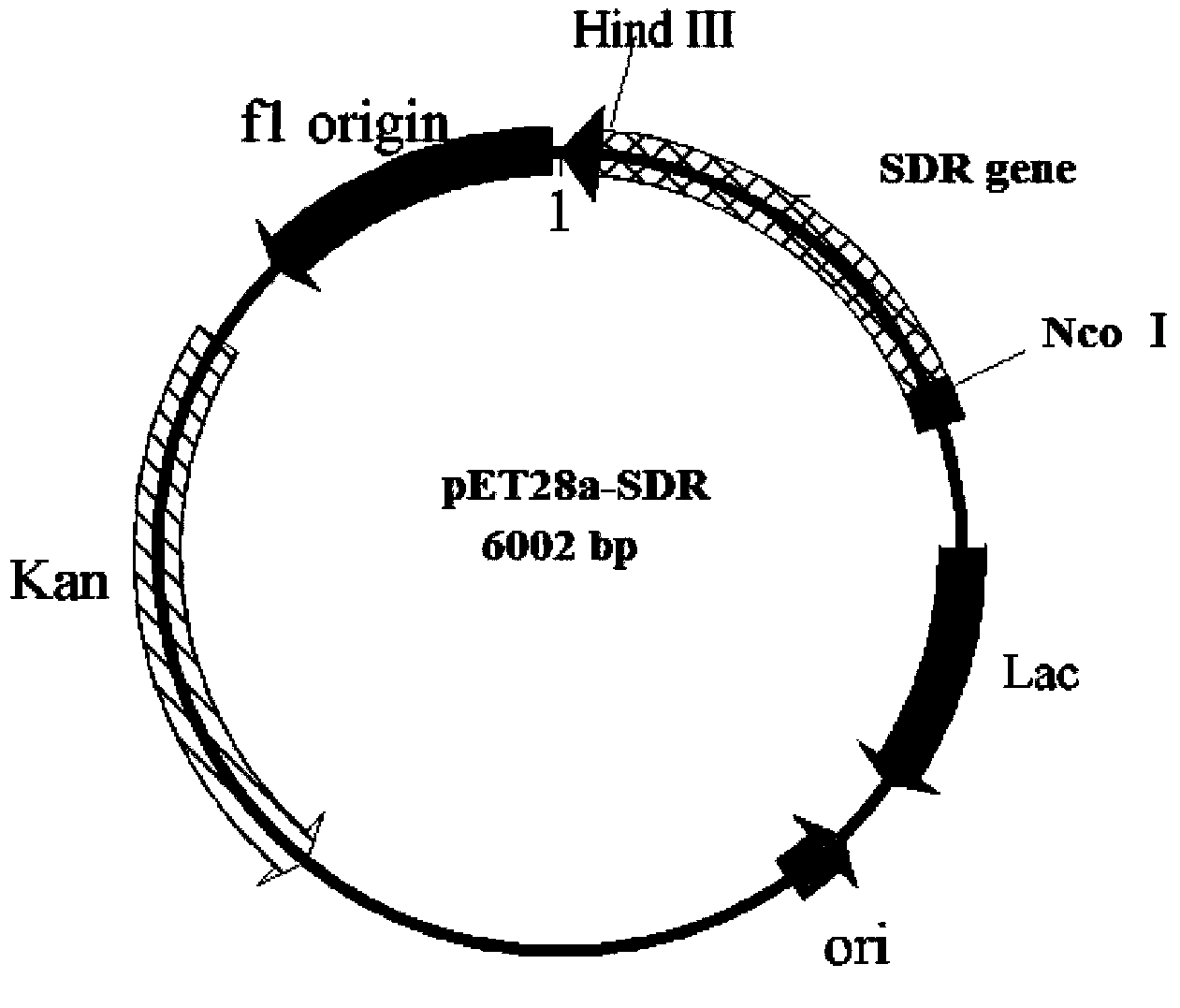
Leifsonia xyli HSO904-based short chain dehydrogenase, and encoding gene, carrier, engineering bacteria and application thereof
The invention discloses a leifsonia xyli HSO904-based short chain dehydrogenase, and an encoding gene, a carrier, engineering bacteria and application thereof. A gene of the leifsonia xyli HSO904-based short chain dehydrogenase has more than 90% of homology of a nucleotide sequence shown in SEQ ID NO. 1. A colon bacillus BL21 / pET28a (+)-SDR prepared by the recombination of the short chain dehydrogenase is used as an enzyme source, 3, 5-bis-trifluoro methyl acetophenone, trifluoromethyl acetophenone, 4-hydroxyl-2-butanone, acetoacetic ester, 4-chloro ethyl acetoacetate, acetoacetic acid tert-butyl acetate and the like are used as substrates to prepare corresponding chiral compounds such as (R)-3, 5-bis-trifluoromethyl phenethyl alcohol, trifluoromethyl benzaldehyde ethanol, 2-hydroxyl-butyl alcohol, 3-hydroxy ethyl butyrate, 4-chloro-3-hydroxy ethyl butyrate and 3-hydroxy butyric acid tert-butyl acetate through a catalytic asymmetric reduction reaction.
Owner:艾吉泰康(嘉兴)生物科技有限公司
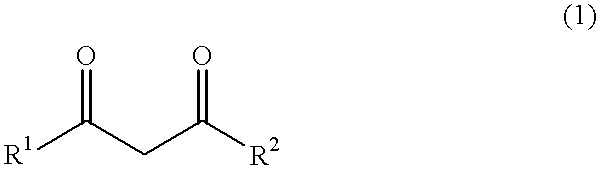
Process for the preparation of alkaline earth metal salts of beta-diketo compounds
InactiveUS6376719B1High purityLow purityOrganic compound preparationCarboxylic acid esters preparationAcetoacetatesAlkaline earth metal
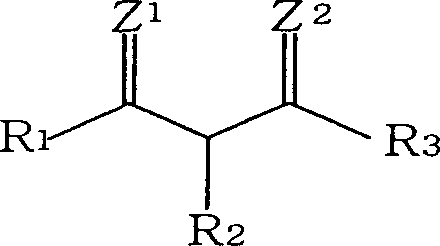
An alkaline earth metal salt of beta-diketo compound is produced using 1 mol of a powdery alkaline earth metal compound and 2.04 mol or more of an aliphatic beta-diketo compound. The reaction may be carried out while supplying both components to a reactor continuously or intermittently, or may be carried out by adding one component to the other continuously or intermittently. The highest temperature during the reaction may be at 50° C. or higher. The reaction mixture may be aged, and then dried at a temperature of at 100 to 180° C. in an atmosphere of an inert gas. The alkaline earth metal compound may be calcium hydroxide, magnesium hydroxide, or barium hydroxide. The aliphatic beta-diketo compound may be represented by the following formula, particularly an acetoacetic acid ester or acetylacetone.According to the production method as mentioned above, highly stable alkaline earth metal salts of beta-diketo compounds of high quality can be produced.
Owner:DAICEL CHEM IND LTD
Oligomer diacetyl acetate alkylene diester metal chelate coating drier and preparation and application
InactiveCN1955238ASimilar drying performanceWide variety of sourcesSiccativesVegetable oilMetal chelate
This invention is an oligomerization diacetic acid alkylene diester metal chelate compound, and it is a neotype oxidoreduction polymerizing siccative which can be used in coating industry. It not only can be used in solvent coating containing dry vegetable oil, but also can be used in waterborne coating after making into aqueous dispersions. The diacetic acid alkylene diester metal chelate agent is made by the reaction of dihydric alcohol and diethenone under accelerant, and then this metal chelate agent reacts with inorganic metallic salt to obtain oligomerization diacetic acid alkylene diester metal chelate compound siccative. The drying properties of this siccative are similar to traditional metallic soap siccative in solvent coating and waterborne coating. But it is different from traditional metallic soap siccative, beacuase this invention is oligomerization metal chelate polymer, which can be preserved in solid. It is of light color and luster, low toxicity, extensive synthesis material, simple preparation and higher yield, and so it is suitable for industrial production.
Owner:上海市涂料研究所有限公司
Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes
ActiveCN103420896APromote safe productionEasy to operateOrganic chemistryTert-Butyloxycarbonyl protecting groupKetone
The invention discloses a preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptane. The method comprises the following steps: reacting benzoylamide acetoacetate as a raw material with 1,2-dichloroethane to obtain 3-cyclopropyl benzoylamide acetoacetate, brominating 3-cyclopropyl benzoylamide acetoacetate by NBS (n-bromosuccinimide) to obtain 1-bromo-3-cyclopropyl benzoylamide acetoacetate, cyclizing under alkaline conditions to obtain 5-benzyl-5-aza-spiro[2,4]heptane-4,7-diketone, further reacting with hydroxylamine hydrochloride to form an oxime compound-4-oxo-5-benzyl-7-oximido-5-aza-spiro[2,4]heptane, reducing by NaBH4 and boron trifluoride diethyl etherate to obtain 5-benzyl-7-amino-5-aza-spiro[2,4]heptane, performing chiral resolution by a resolving agent-L-camphorsulfonic acid to obtain 5-benzyl-7(S)-amino-5-aza-spiro[2,4]heptane, and reacting with di-tert-butyl dicarbonate ester to obtain 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptane. According to the method, an intermediate body-carbonyl does not need protection, raw materials are easy to get, a process route is simple, and the method is suitable for industrial production.
Owner:苏州楚凯药业有限公司
Method for measuring beta-hydroxybutyric acid by using enzyme colorimetric reaction, matched kit and application thereof
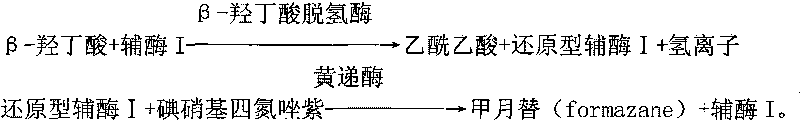
ActiveCN101726463ASmall sample sizeShort reaction timeMicrobiological testing/measurementColor/spectral properties measurementsBeta-Hydroxybutyric acidAbsorbance
The invention provides a method for measuring beta-hydroxybutyric acid by using enzyme colorimetric reaction, a matched kit and application thereof. Under the system of Tris-HCL buffer solution, serum beta-hydroxybutyric acid and coenzyme I are dehydrogenized under the catalysis of beta-hydroxybutyric acid dehydrogenase to generate acetoacetic acid and reduced coenzyme I, the generated reduced coenzyme I and iodonitrotetrazole chloride are reacted under the catalysis of diaphorase to generate a red substance formazane of which the highest absorbance is 505 nanometers, and then the content of the beta-hydroxybutyric acid in a biological sample is quantified by measuring the change of the absorbance of the red product at the wavelength of 505 nanometers. The method can linearly measure the concentration range (0 to 4.5 mmol / L) of the beta-hydroxybutyric acid in the biological sample, the measurement is used for judging the human ketosis for acid poisoning diagnosis, the reagent used by the method is liquid dual-reagent, and the quantity of the sample required for measuring the beta-hydroxybutyric acid is little; moreover, the reaction time during measuring is short, the operation is simple, and the method is suitable for mass detection.
Owner:NINGBO RUI BIO TECH
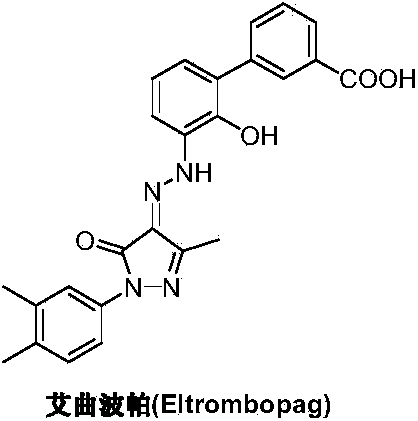
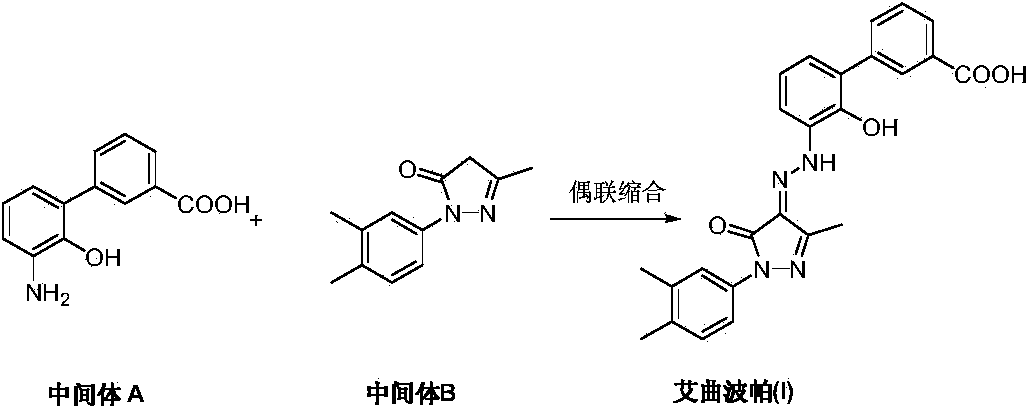
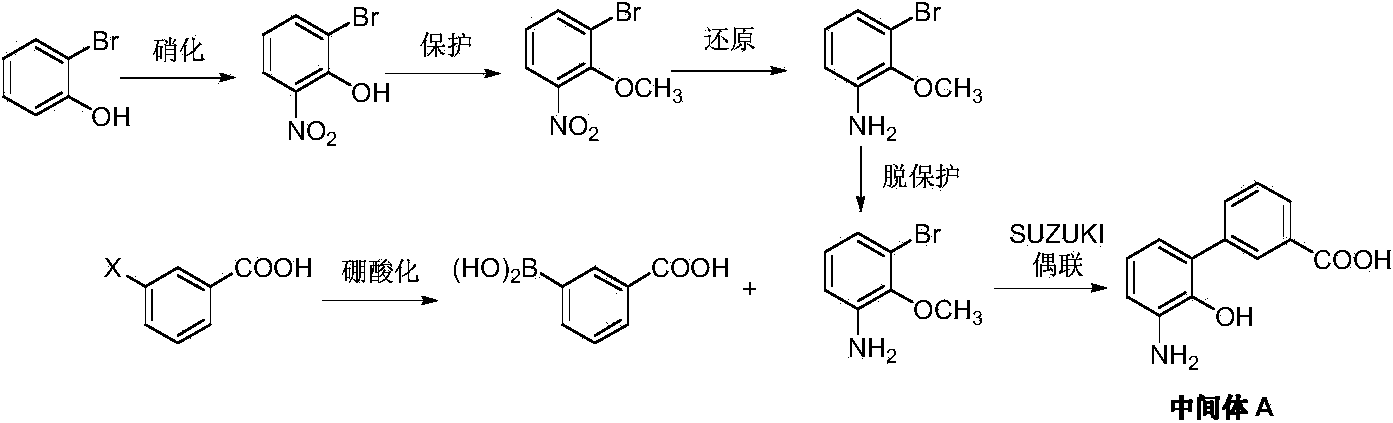
Preparation method of eltrombopag olamine
ActiveCN103819406ARaw materials are easy to getHigh purityOrganic active ingredientsOrganic chemistryHydrazine compoundEltrombopag Olamine
The invention discloses a preparation method of eltrombopag olamine (Eltrombopag). The preparation method comprises the following steps: carrying out azo coupling reaction by using 3'-amino-2'-hydroxy-biphenyl-3-carboxylic acid (II) and acetoacetic acid alkyl ester to obtain (Z)-2-[3'-(2'-hydroxyl-3-hydroxy-biphenyl) hydrazono]-3-oxo acid alkyl ester (III); carrying out condensation cyclization reaction on an intermediate (III) and 3,4-dimethyl benzene hydrazine to prepare the eltrombopag olamine (I). The method is concise in process, available in raw material, economical and environmental friendly, and beneficial to achievement of industrialization, and economic and technological development of the eltrombopag olamine crude drug can be facilitated.
Owner:山东佰盛能源科技有限公司
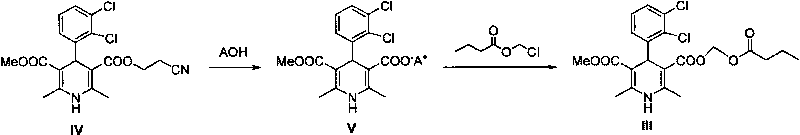
Method for preparing cilnidipine used as dihydropyridine calcium antagonist
InactiveCN101602709AShort reaction cycleEasy post-processingOrganic chemistryCardiovascular disorderSolventMethoxyethyl acetate
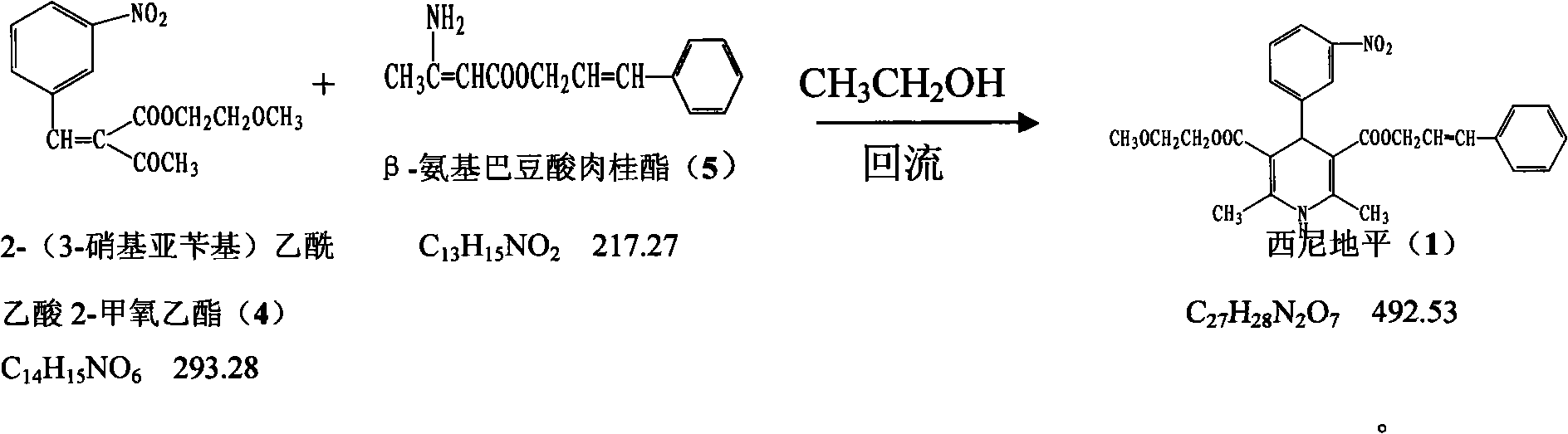
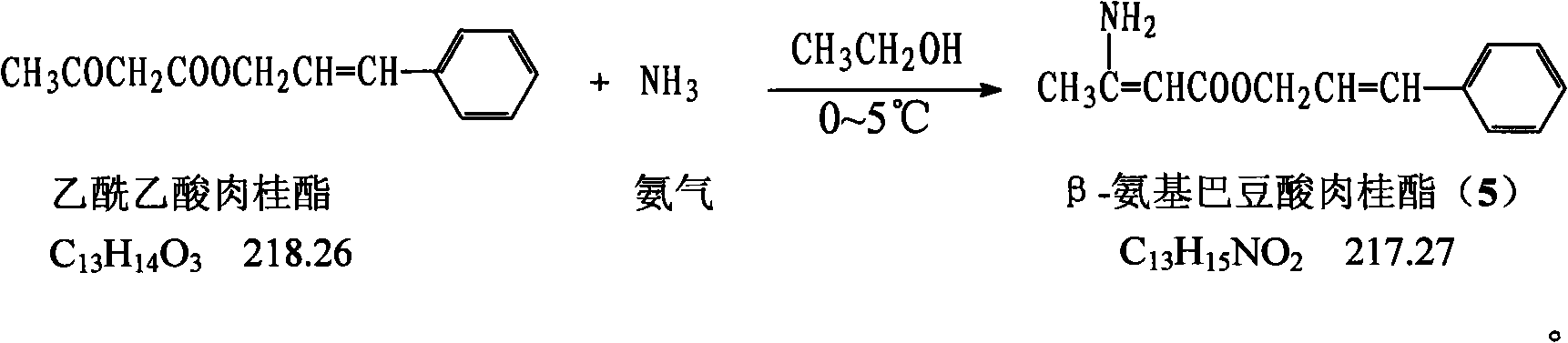
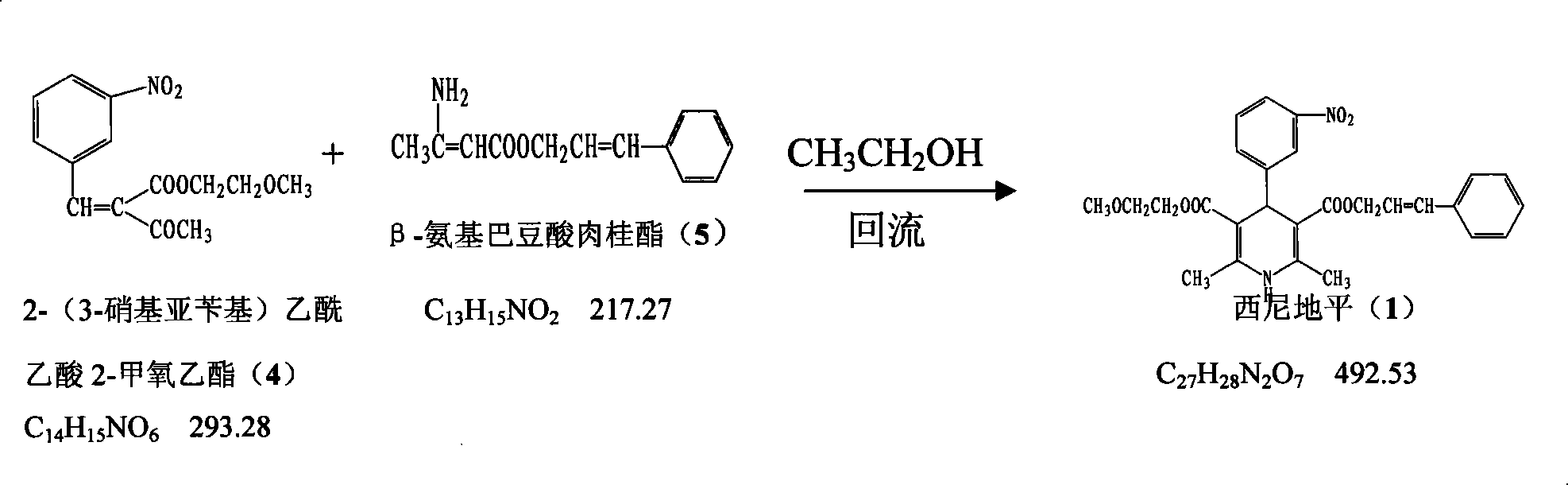
The invention provides a method for preparing cilnidipine used as a dihydropyridine calcium antagonist, which comprises the following steps: 2-(3-nitrobenzyl) acetoacetic acid 2-methoxyethyl acetate 4 and beta-amino crotonic acid cinnamic ester 5 react in an alcohol solvent to obtain a coarse product of cilnidipine 1, and the coarse product of the cilnidipine 1 is recrystallized to obtain the cilnidipine 1. The method has the advantages of short reaction period, simple and convenient after-treatment, simple synthetic route, moderate process condition, and the like and is suitable for industrial production.
Owner:上海医药科技发展有限公司
Acrylate emulsion and application thereof
The invention relates to an acrylate emulsion. Acetoacetoxy ethyl methacrylate is taken as a functional monomer, an acetoacetic acid group reacts with amine to generate enamine in the neutralizing stage of synthesis of the emulsion, enamine participates in later curing crosslinking, the shrinking percentage of the functional monomer is low, and internal stress during curing shrinking of a film is relieved. The surface of a polyester film is coated with the acrylate emulsion, permeation and spreading of a coating solution on a function coating are facilitated after high-temperature horizontal stretching and curing, the adhesion force between the function coating and the polyester film is high, and shedding is prevented. The optical polyester film with the surface coating can be used for the fields of a reflecting film, a hardening film, a window film and the like, and the film reprocessing demands are met.
Owner:HEFEI LUCKY SCI & TECH IND
Water-based acrylate pressure-sensitive adhesive and preparation method thereof
InactiveCN113999632AHigh solid contentNo pollution in the processEster polymer adhesivesMethacrylateDiacetonamine
The invention discloses a water-based acrylate pressure-sensitive adhesive and a preparation method thereof. The pressure-sensitive adhesive is prepared from the following raw materials in parts by weight: 0.5 to 5 parts of acrylic acid or methacrylic acid, 30 to 50 parts of acrylate monomer, 1 to 10 parts of crosslinking functional monomer, 0.1 to 1 part of initiator, 0.5 to 5 parts of emulsifier, 0.01 to 0.5 part of buffering agent, 0.05 to 3 parts of molecular weight regulator, 0.5 to 10 parts of tackifying resin, 0.5 to 5 parts of pH regulator, 45 to 60 parts of deionized water and 0.5 to 5 parts of water-based additive, wherein the cross-linking functional monomer is at least one of beta-carboxyethyl acrylate, diacetone acrylamide, glycidyl methacrylate, acetoacetic acid ethyl methacrylate, methacrylamide ethyl ethylene urea, ethylidene urea ethyoxyl methacrylate and trimethylolpropane trimethacrylate; and the emulsifier comprises an anionic emulsifier and a nonionic emulsifier, and the weight percentage of the anionic emulsifier in the emulsifier is at least 10%. The water-based acrylate pressure-sensitive adhesive has the advantages of high peel strength, high retention, high adhesion and wide use temperature range.
Owner:ETERNAL CHEM (CHINA) CO LTD
Weather-resistant polyvinyl chloride color master batch
The invention discloses a weather-resistant polyvinyl chloride color master batch. The weather-resistant polyvinyl chloride color master batch is prepared from the following raw materials in parts by weight: 150 to 165 parts of polyvinyl chloride, 8 to 14 parts of an ethylene-octene block copolymer, 3 to 6 parts of alkyl dimethyl betaine, 15 to 28 parts of acetoacetic acid allyl ester, 3 to 7 parts of barium titanate, 4 to 7 parts of nanometer magnesia, 4 to 8 parts of nanosilicon dioxide, 5 to 8 parts of tri(2,4-ditert-butylphenyl) phosphite ester, 4 to 5 parts of triethanolamine and 2 to 6 parts of potassium peroxodisulfate. The weather-resistant polyvinyl chloride color master batch provided by the invention has good weather resistance, and is low in processing cost and simple in preparation technology; and when the color master batch is applied to coloring of PVC products, the color master batch has the advantages that the color stability is improved and the color difference is little, and the problems that an existing PVC color master batch is unstable in color and relatively large in color difference during coloring are overcome.
Owner:苏州曙帆信电子科技有限公司
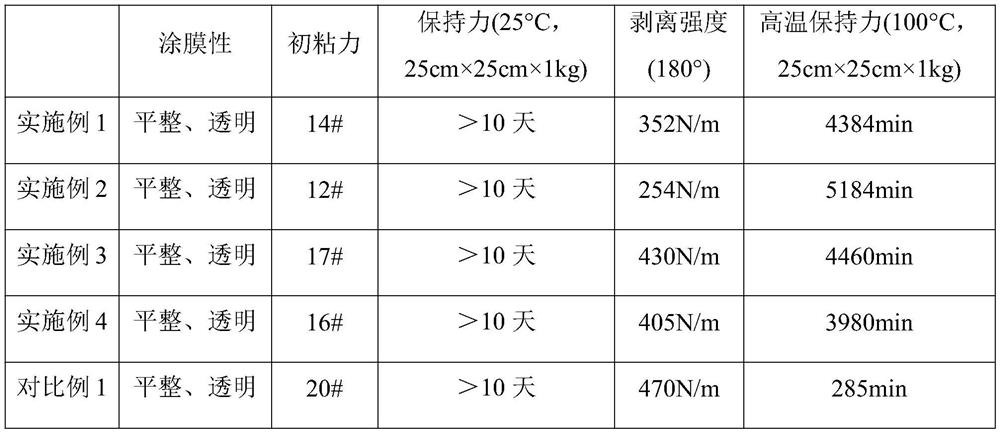
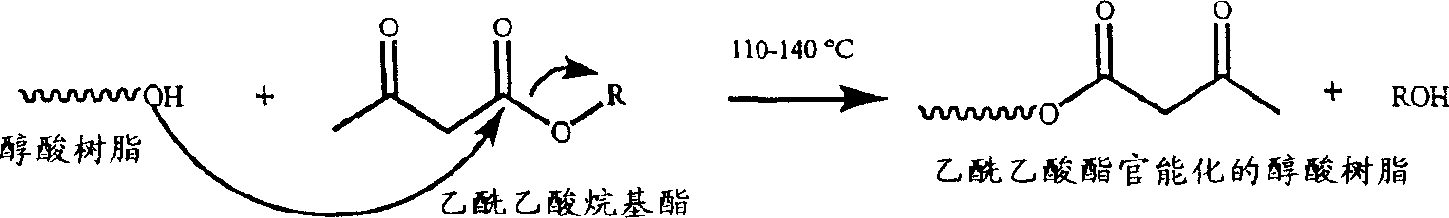
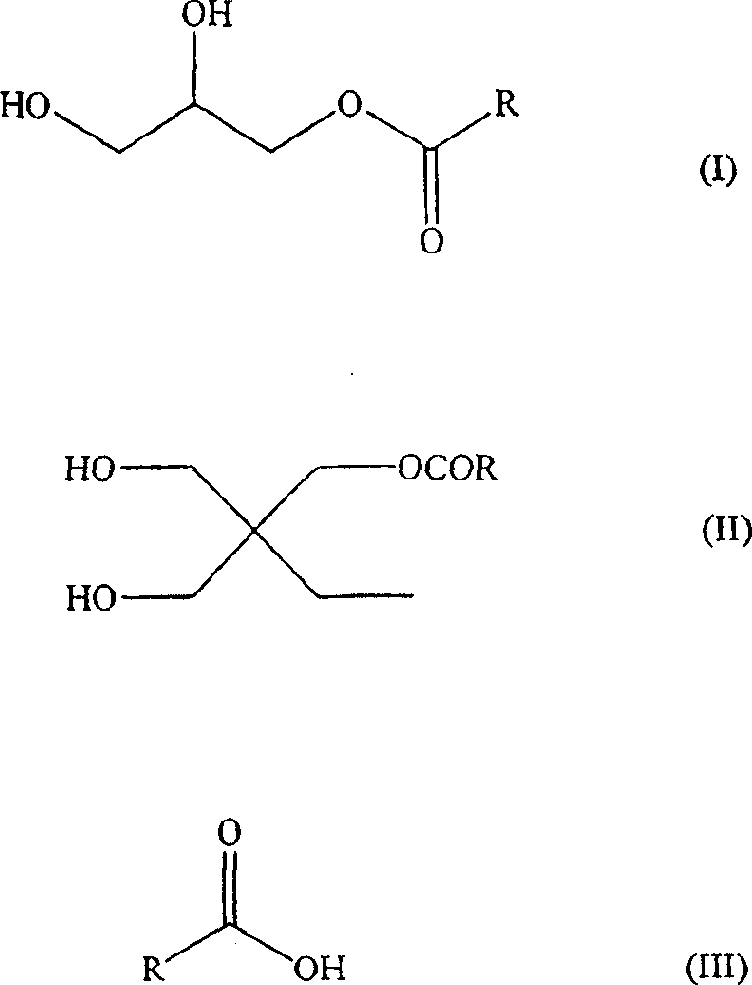
Waterborne acetoacetate-functionalized alkyd coating
A waterborne acetoacetate-functionalized alkyd coating composition is disclosed which includes an acetoacetate-functionalized alkyd resin, at least one drier, and water. The acetoacetate-functionalized alkyd resin is the reaction product of (i) an alkyd resin, and (ii) an alkyl acetoacetate. Also disclosed is a method of preparing a waterborne acetoacetate-functionalized alkyd coating composition which includes the step of contacting an acetoacetate-functionalized alkyd resin with at least one drier in the presence of water.
Owner:EASTMAN CHEM CO
Hydrocarbon oil demetalization agent and method for hydrocarbon oil demetalization
ActiveCN105733657AHigh removal rateWeak corrosiveRefining with non-metalsDewatering/demulsification with chemical means2-ketobutyric acidKeto acid
The invention relates to a hydrocarbon oil demetalization agent and a method for hydrocarbon oil demetalization. The hydrocarbon oil demetalization agent is a keto acid, wherein the keto acid is any of alpha-keto acid, beta-keto acid and gamma-keto acid, and the alpha-keto acid is one of pyruvic acid, 2-ketobutyric acid, phenylpyruvic acid and benzoyl formic acid; the beta-keto acid is acetoacetic acid; and the gamma-keto acid is 4-ketovaleric acid. The method comprises the steps of carrying out mixed contact on hydrocarbon oil, water, the keto acid, which serves as the demetalization agent, and a demulsifier, and then, carrying out electric desalting, so as to achieve oil-water separation and remove metals from the hydrocarbon oil. A molecule of the demetalization agent disclosed by the invention contains a difunctional group, can be in chelation with the metals and can also be in complexation with the metals, so that the demetalization agent has a high removal ratio to the metals in the hydrocarbon oil; according to the demetalization agent, the acidity is between the acidity of organic acids and the acidity of inorganic acids, the corrosiveness is lower than that of the inorganic acids, and the demetalization rate is higher than that of the organic acids; and meanwhile, the demetalization agent is free of elements such as phosphorus, sulfur and nitrogen and thus cannot cause adverse effects on subsequent processing.
Owner:PETROCHINA CO LTD +1
Dustproof and bactericidal type aqueous coating material for glass doors and preparation method thereof
InactiveCN104342003AImprove the bactericidal effectImprove dustproofPaints with biocidesPolyurea/polyurethane coatingsPolymer sciencePolyvinyl alcohol
The invention discloses a dustproof and bactericidal type aqueous coating material for glass doors. The dustproof and bactericidal type aqueous coating material is characterized by being prepared from the following raw materials in parts by weight: 34-38 parts of aqueous polyurethane resin, 28-34 parts of acrylic epoxy resin, 8-12 parts of modified styrene-acrylic emulsion, 0.4-0.8 part of polyvinyl alcohol, 1-3 parts of diethylene glycol monoethyl ether, 0.3-0.6 part of alcohol-amine dipyrophosphate acyloxy glycolate titanate, 0.2-0.5 part of polyether modified polyorganosiloxane, 0.2-0.4 part of cellaburate, 0.3-0.6 part of dioctyl sodium sulfosuccinate, 0.4-0.7 part of acetoacetoxy ethyl methacrylate, 0.6-1.2 parts of climbazole, 3-5 parts of ethylene glycol, 0.5-1.0 part of graphene, 1-2 parts of pigment and 10-15 parts of deionized water. According to the aqueous coating material disclosed by the invention, on the basis of high adhesion, water resistance and alcohol acid resistance, the modified styrene-acrylic emulsion is added, so that the bactericidal, dustproof and anti-electrostatic effects of the coating material are improved; added climbazole has spectrum sterilization property, so that the quality of the coating material is further improved; and the coating material disclosed by the invention is simple in operation and low in production cost, does not contain benzene solvents and heavy metals, is harmless to human bodies and environment, is environment-friendly and safe and is suitable for being popularized.
Owner:凤阳徽亨商贸有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
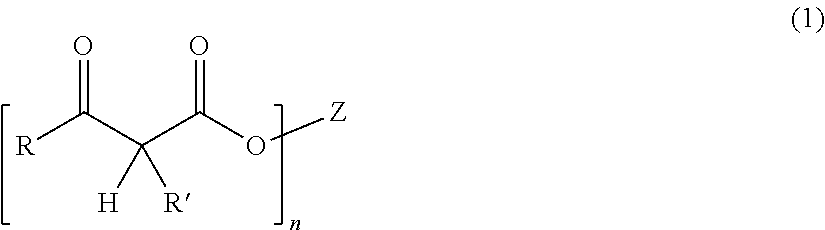
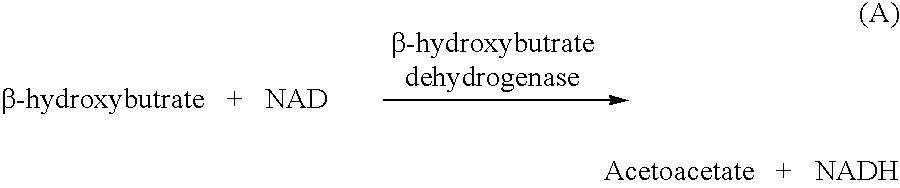
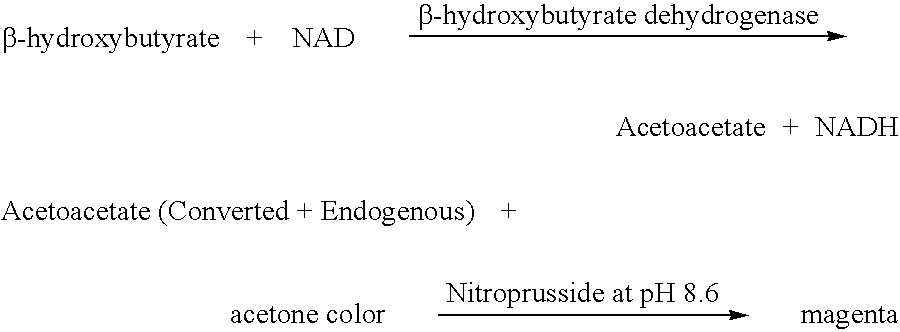

![Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid](https://images-eureka.patsnap.com/patent_img/d95228d2-e0a0-422d-ad7c-d5c58d8b3ed6/BSA00000334200700013.PNG)
![Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid](https://images-eureka.patsnap.com/patent_img/d95228d2-e0a0-422d-ad7c-d5c58d8b3ed6/BSA00000334200700021.PNG)
![Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid Method for preparing 2-[imidazo[1,2-a]pyridine-3-yl]acetic acid](https://images-eureka.patsnap.com/patent_img/d95228d2-e0a0-422d-ad7c-d5c58d8b3ed6/BSA00000334200700022.PNG)


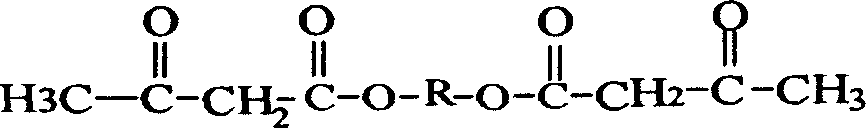
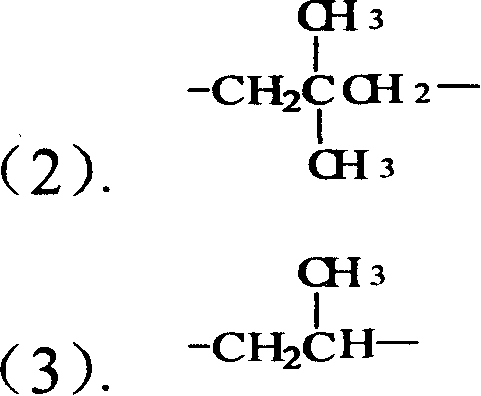
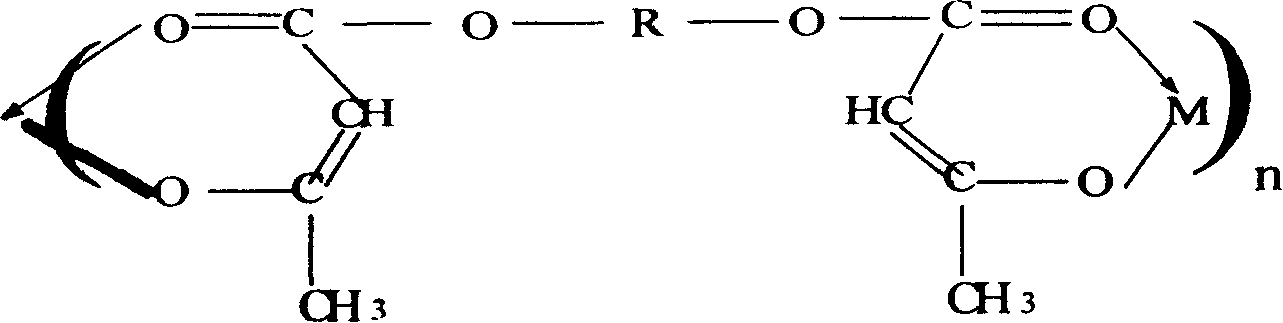
![Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes](https://images-eureka.patsnap.com/patent_img/c4e5bc10-490b-4118-8859-0b9646c67500/BSA0000093282790000011.png)
![Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes](https://images-eureka.patsnap.com/patent_img/c4e5bc10-490b-4118-8859-0b9646c67500/BSA0000093282790000021.png)
![Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes Preparation method for 5-benzyl-7(S)-t-butyloxycarborylamino-5-aza-spiro[2,4]heptanes](https://images-eureka.patsnap.com/patent_img/c4e5bc10-490b-4118-8859-0b9646c67500/BSA0000093282790000031.png)