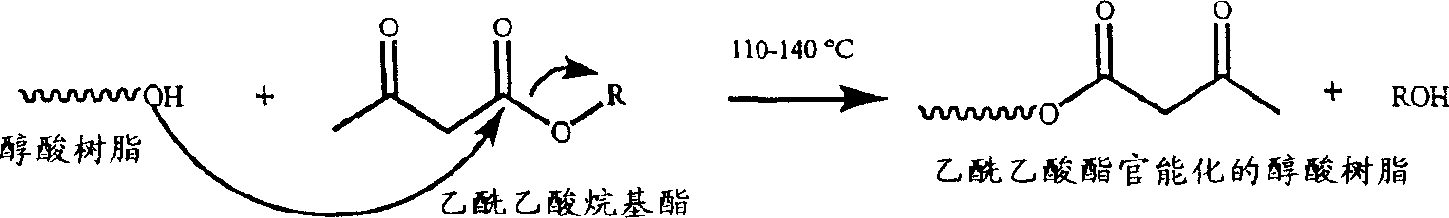
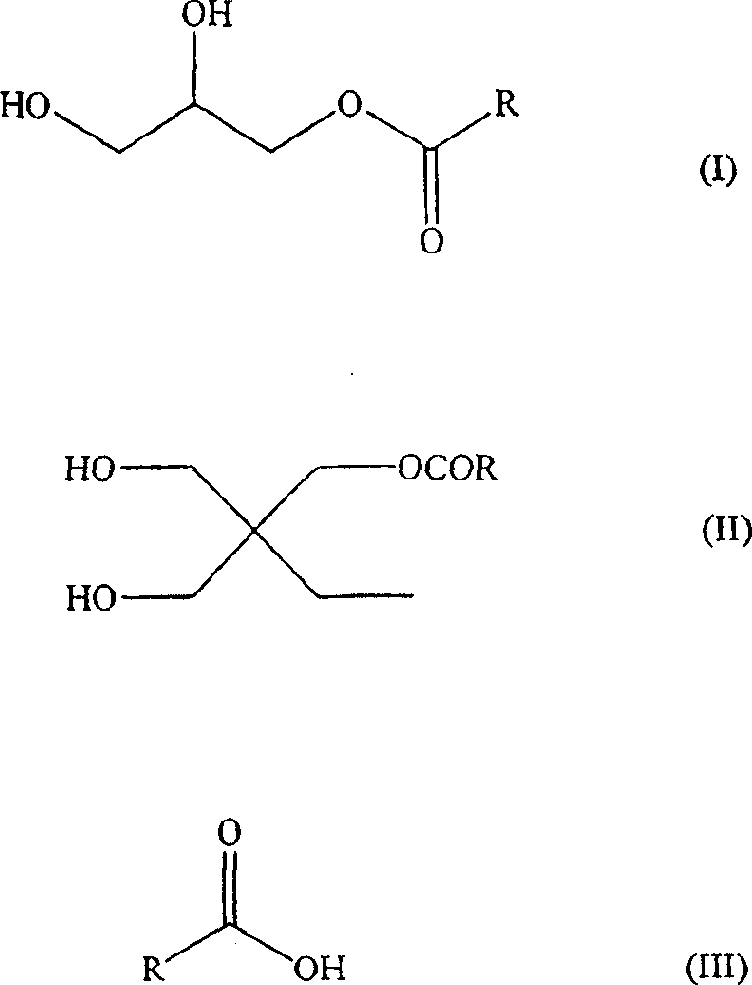
Waterborne acetoacetate-functionalized alkyd coating
A technology of acetoacetate and acetoacetate, applied in the field of water-based coating compositions, can solve problems such as insufficient curing and consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the preparation of water-based alkyd resin 1
[0063] The NPG / SIP addition was first prepared by reacting the following components in a three-necked round bottom flask equipped with a mechanical stirrer, water vapor-jacketed partial condenser, Dean-Stark trap, nitrogen inlet, and water condenser Compounds: neopentyl glycol (NPG) (827 grams, 7.95 moles), 5-sodiumsulfoisophthalic acid (SIP) (536 grams, 2.00 moles), water (91.9 g) and acid catalyst FASCAT4100 (1.10 g) . The reaction temperature was gradually increased from 110 to 150°C over a period of 45 minutes and the distillate was collected in a Dean-Stark trap. The reaction was allowed to continue for 3 hours at 150-180°C and 4.5 hours at 190°C until an acid number of 3.0 mgKOH / g was reached. A part of the obtained product was used in the following steps.
[0064] Into a separate reactor equipped with the same configuration as above were charged neopentyl glycol (NPG) (48.4 g, 0.47 mol), the above NP...
Embodiment 2
[0066] Preparation of acetoacetate-functionalized alkyd resin 1
[0067] Into a three necked round bottom flask equipped with a mechanical stirrer, steam-jacketed partial condenser, Dean-Stark trap, water condenser and nitrogen inlet was charged the alkyd resin of Example 1 (150 g) and acetyl tert-Butyl acetate (t-BAA) (16.7 g, 0.105 mol). The reaction mixture was stirred at 100°C for 30 minutes, then at 110°C for 1.5 hours while collecting distillate in a Dean-Stark trap. The reaction was continued at 120°C for 1 hr, at 130°C for 30 min, and at 140°C for 30 min, during which time a total of 7 ml of distillate was collected. The mixture was then cooled and the resulting resin collected.
Embodiment 3
[0069] paint formulation
[0070] Acetoacetate-modified alkyd resin 1 (10.0 g) prepared in Example 2 was mixed with water (14.6 g), desiccant blend (0.34 g) and Silwet L-77 (OSI Specialties) (0.06 g) to prepare a coating formulation. A control formulation was also prepared using the unmodified resin of Example 1. A desiccant blend was prepared by mixing Zirconium HYDRO-CEM (12%, OMG Americas) (26.9 g), Cobalt HYDRO-CURE II (OMG Americas) (13.1 g) and Ethylene Glycol Monobutyl Ether (EB) (10.0 g) thing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com