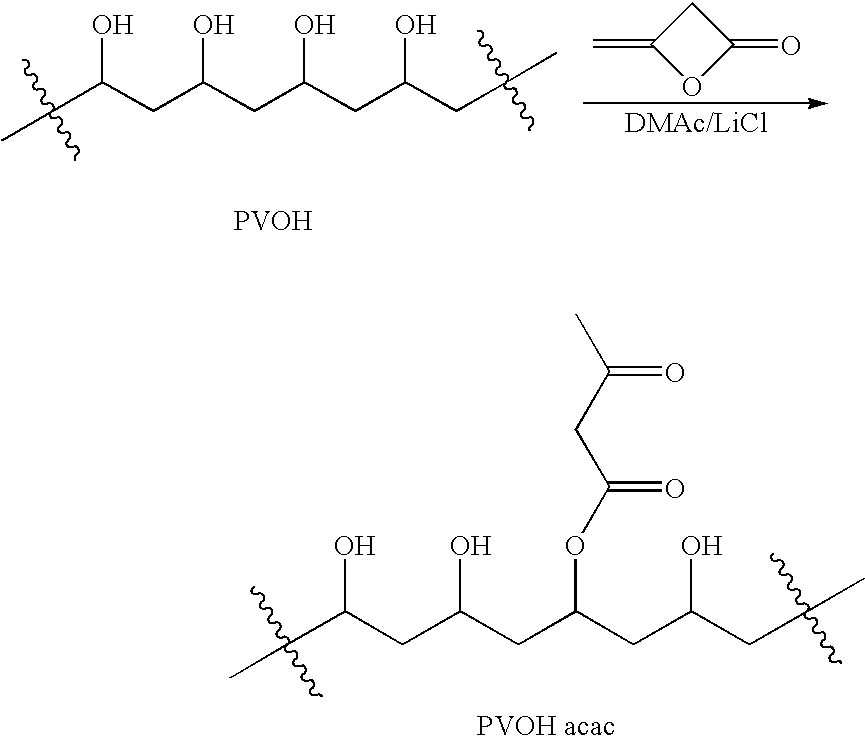
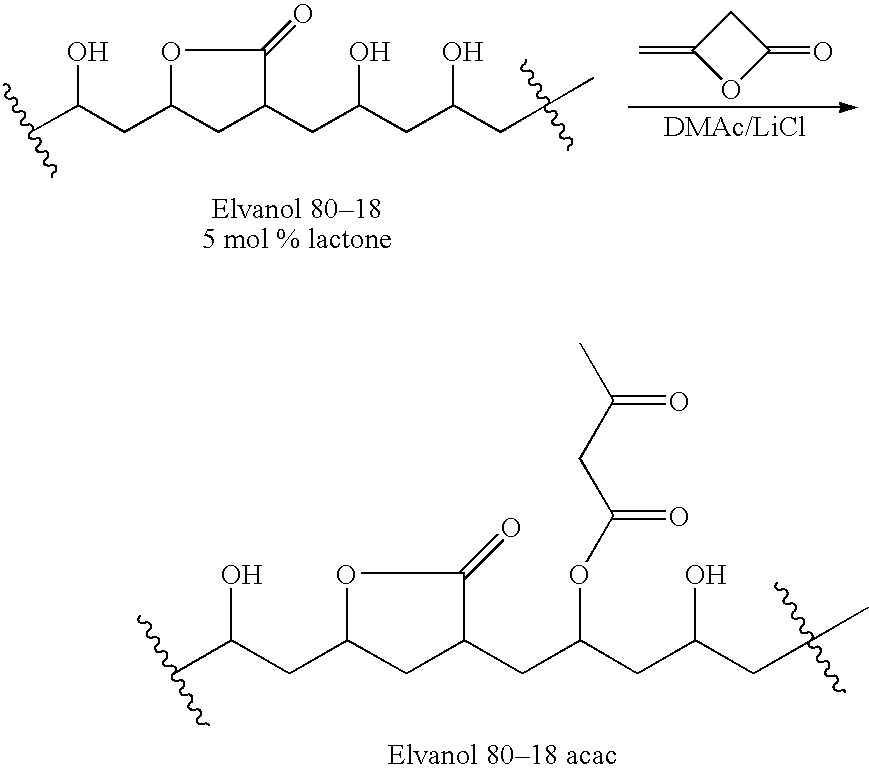
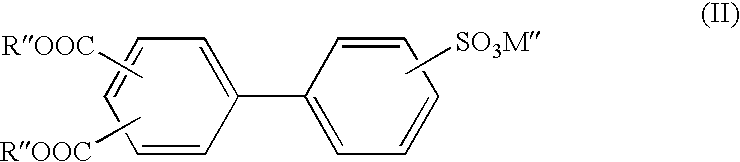
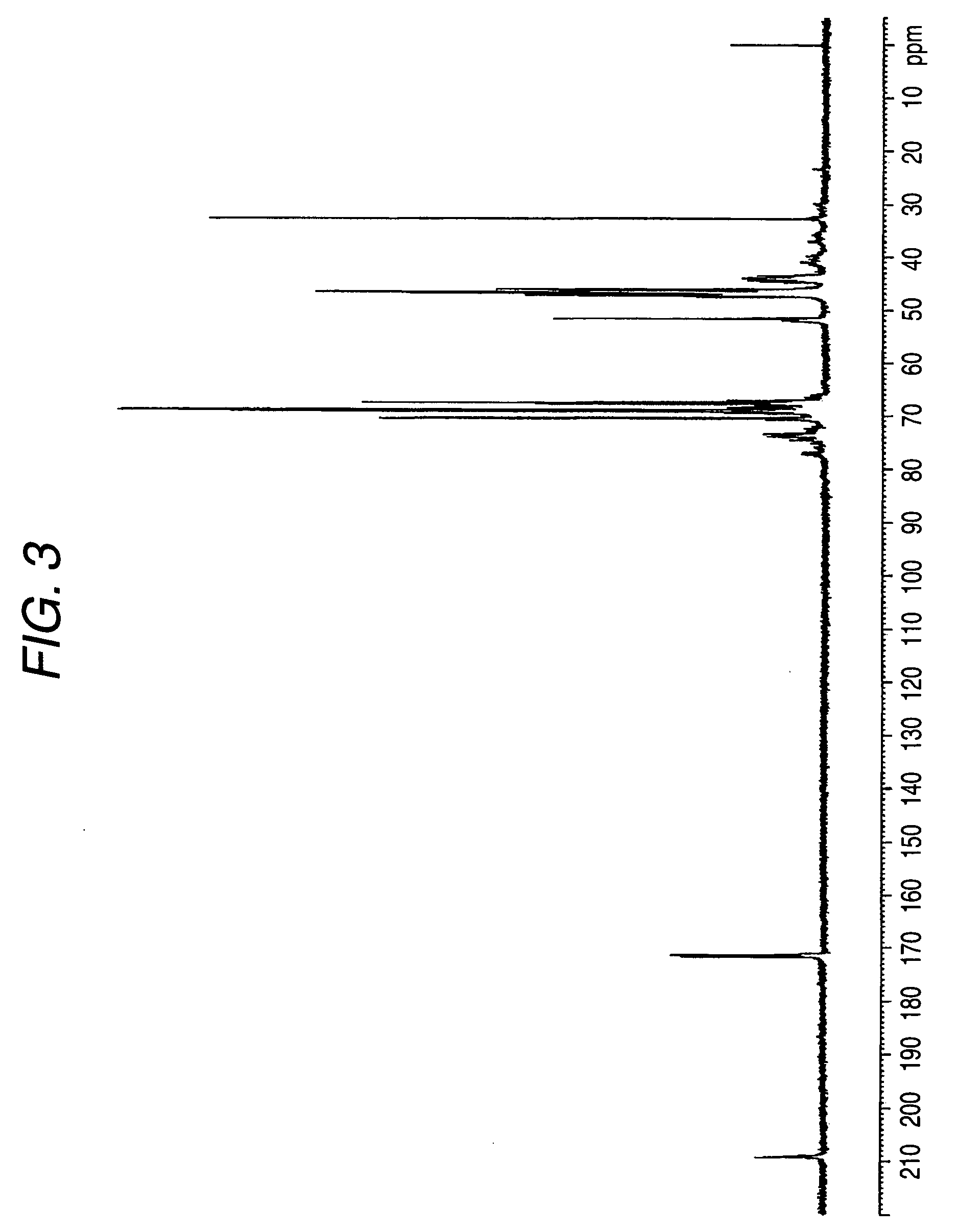
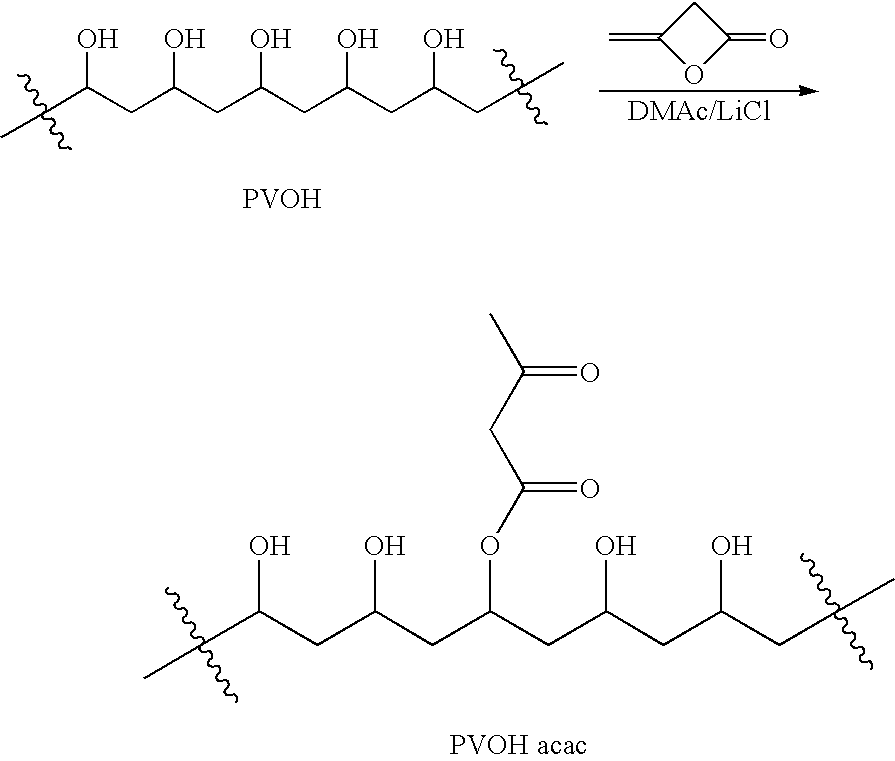
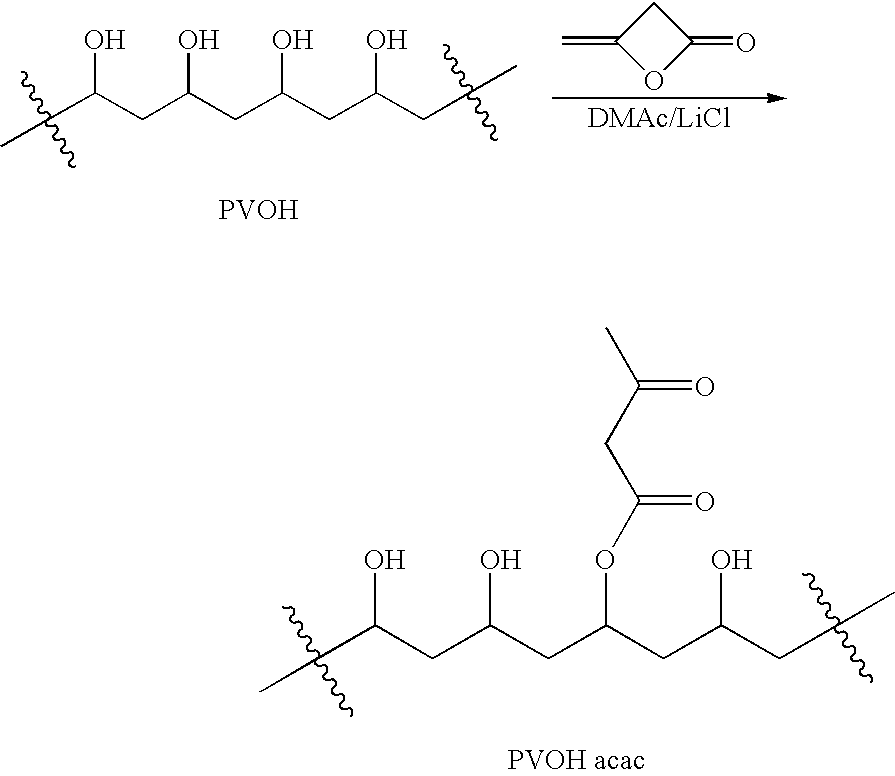
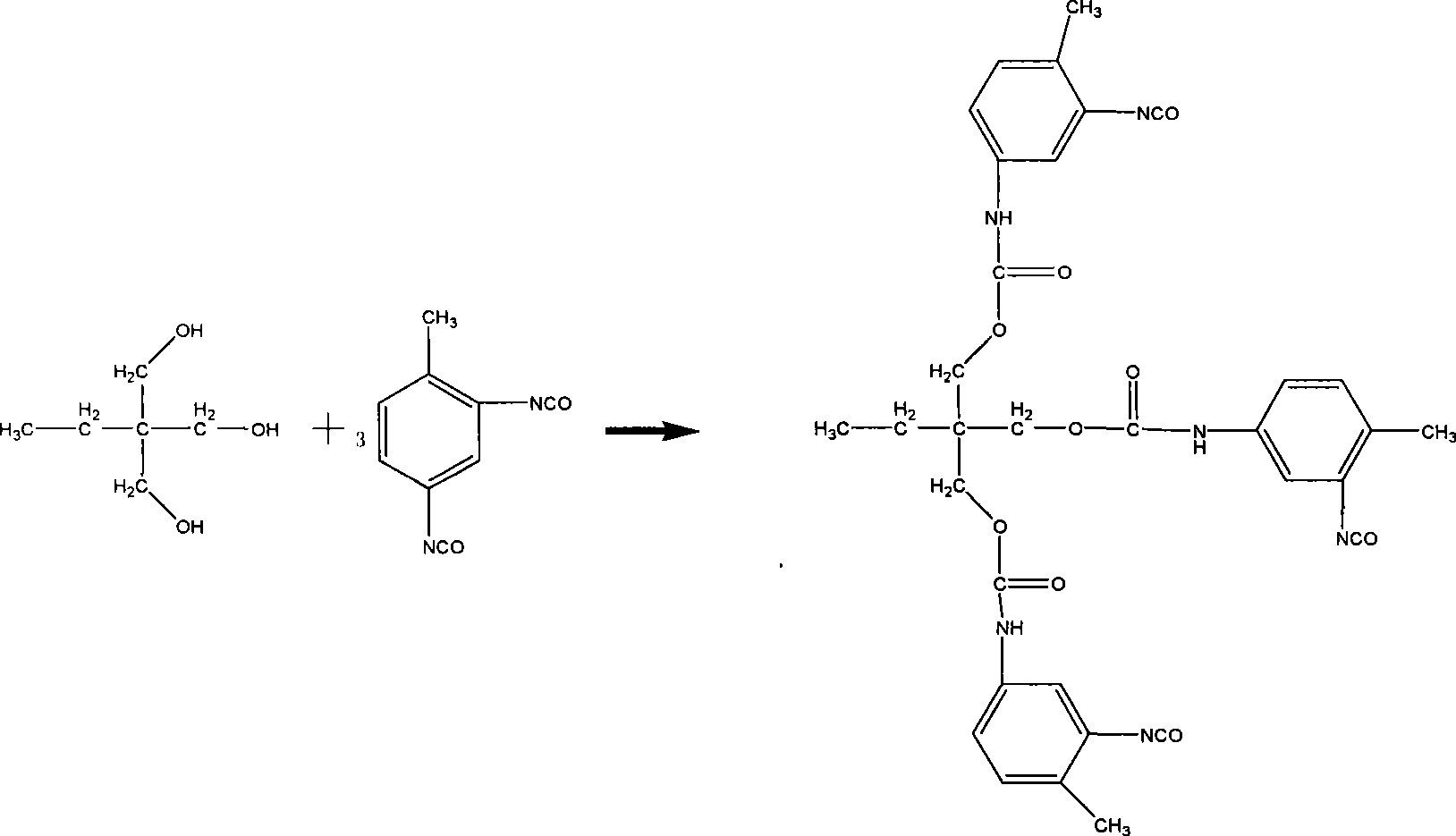
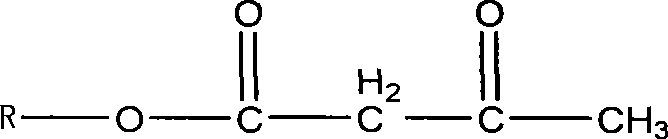
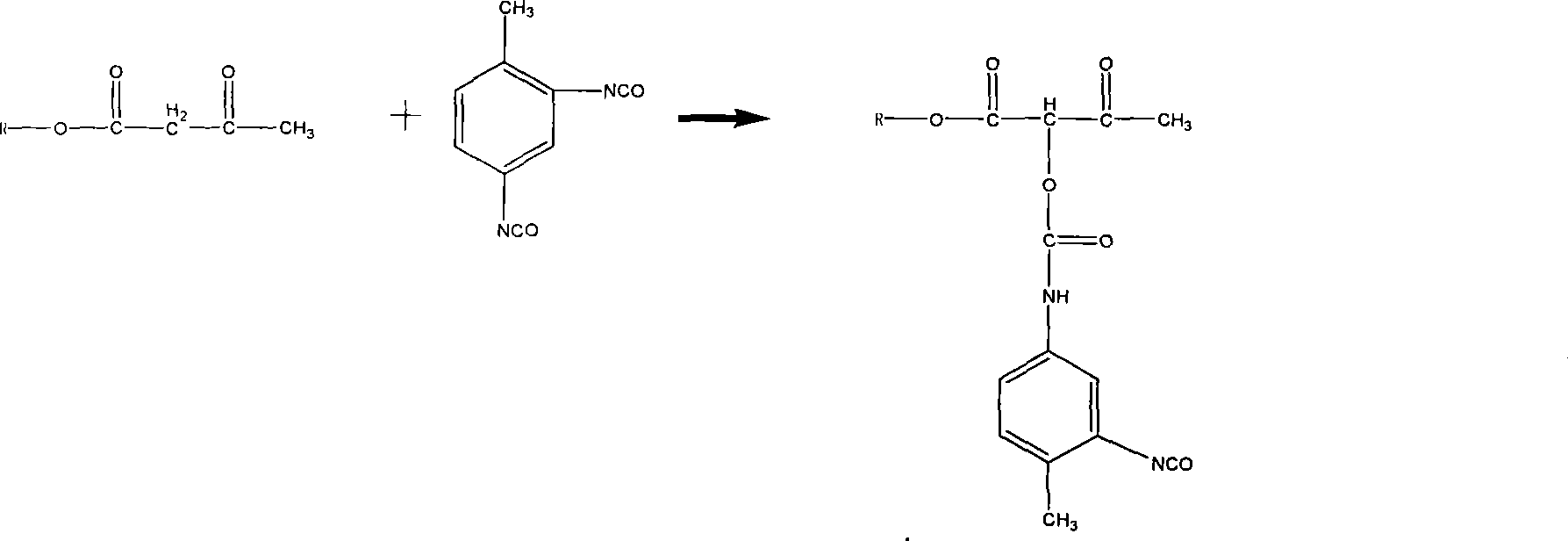
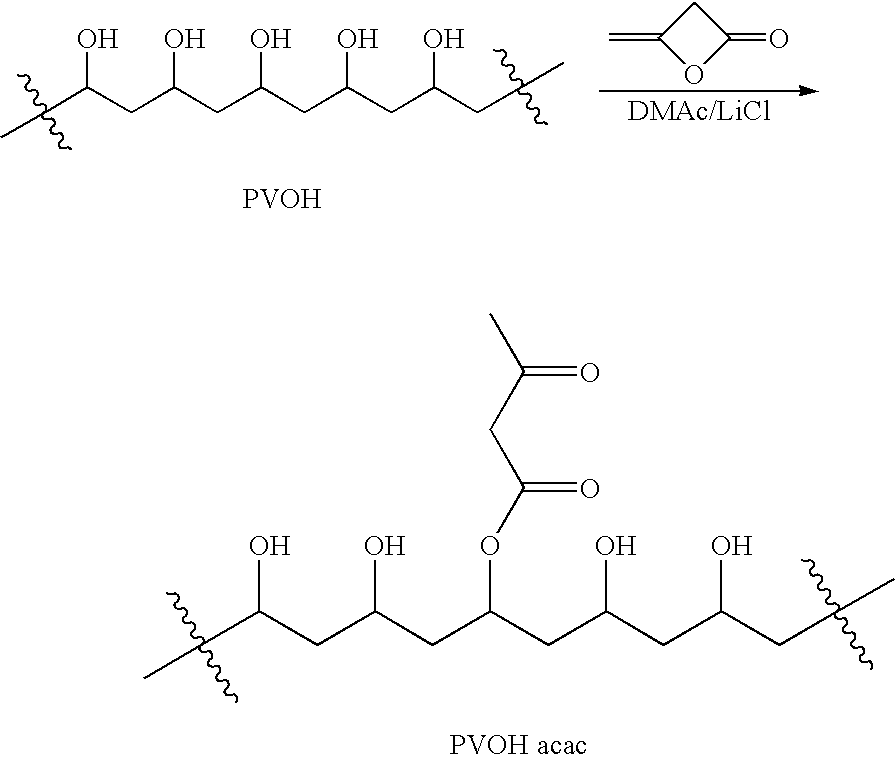
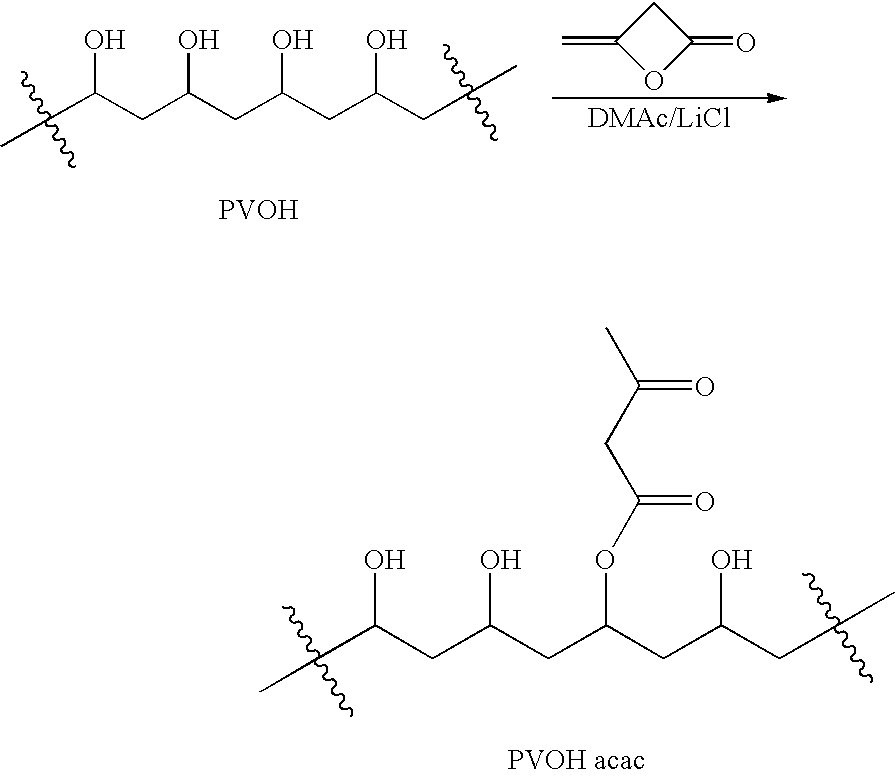
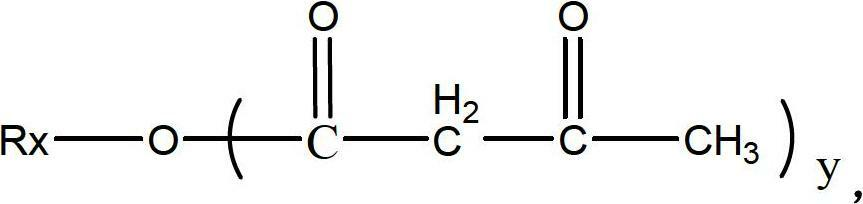
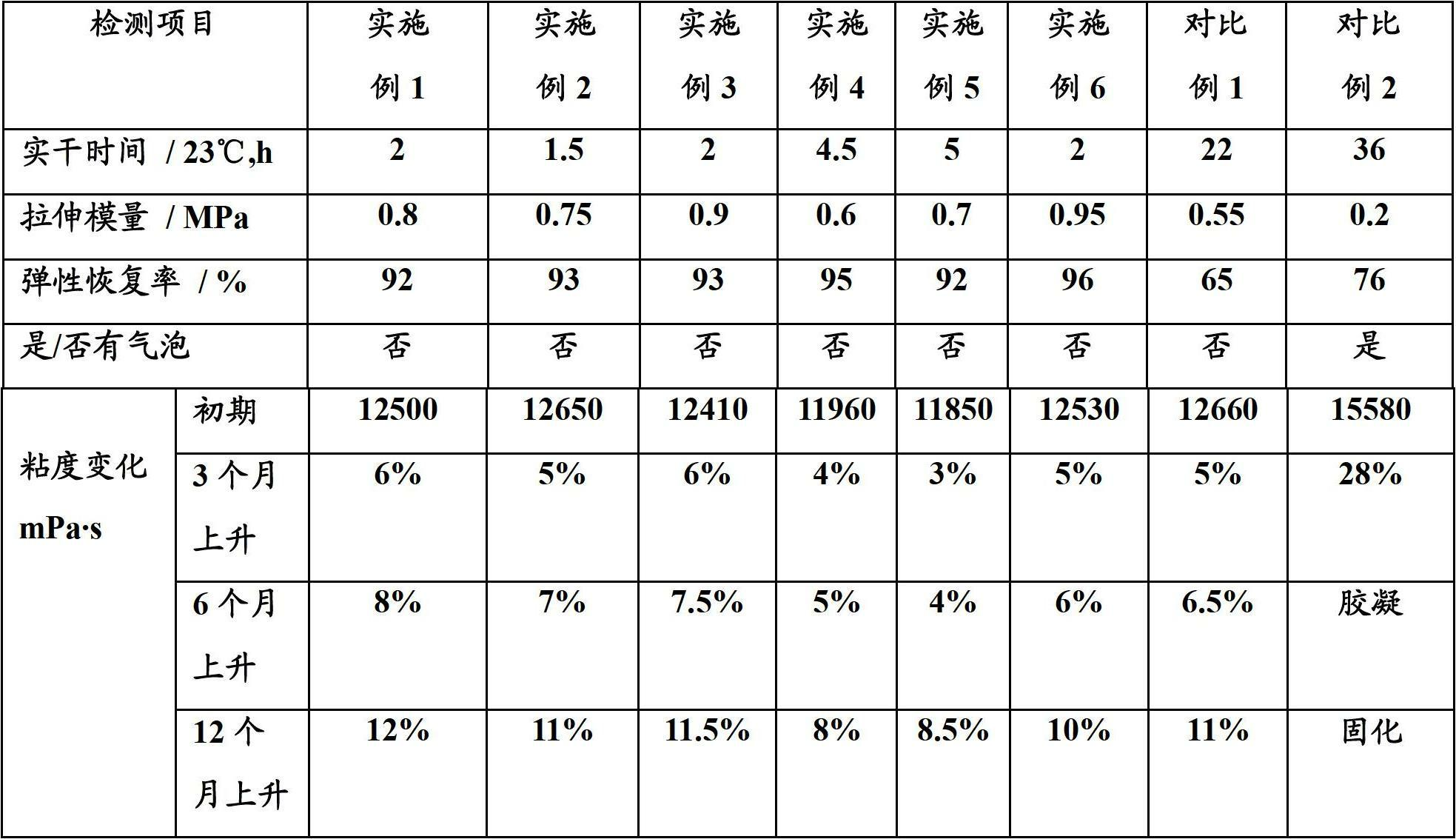
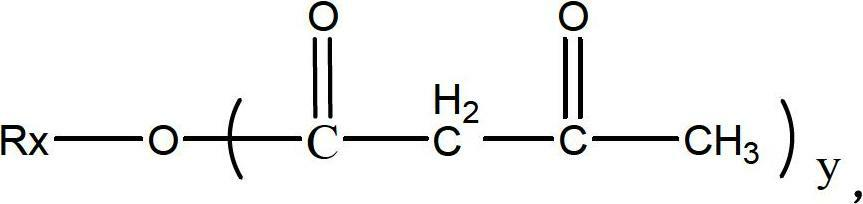
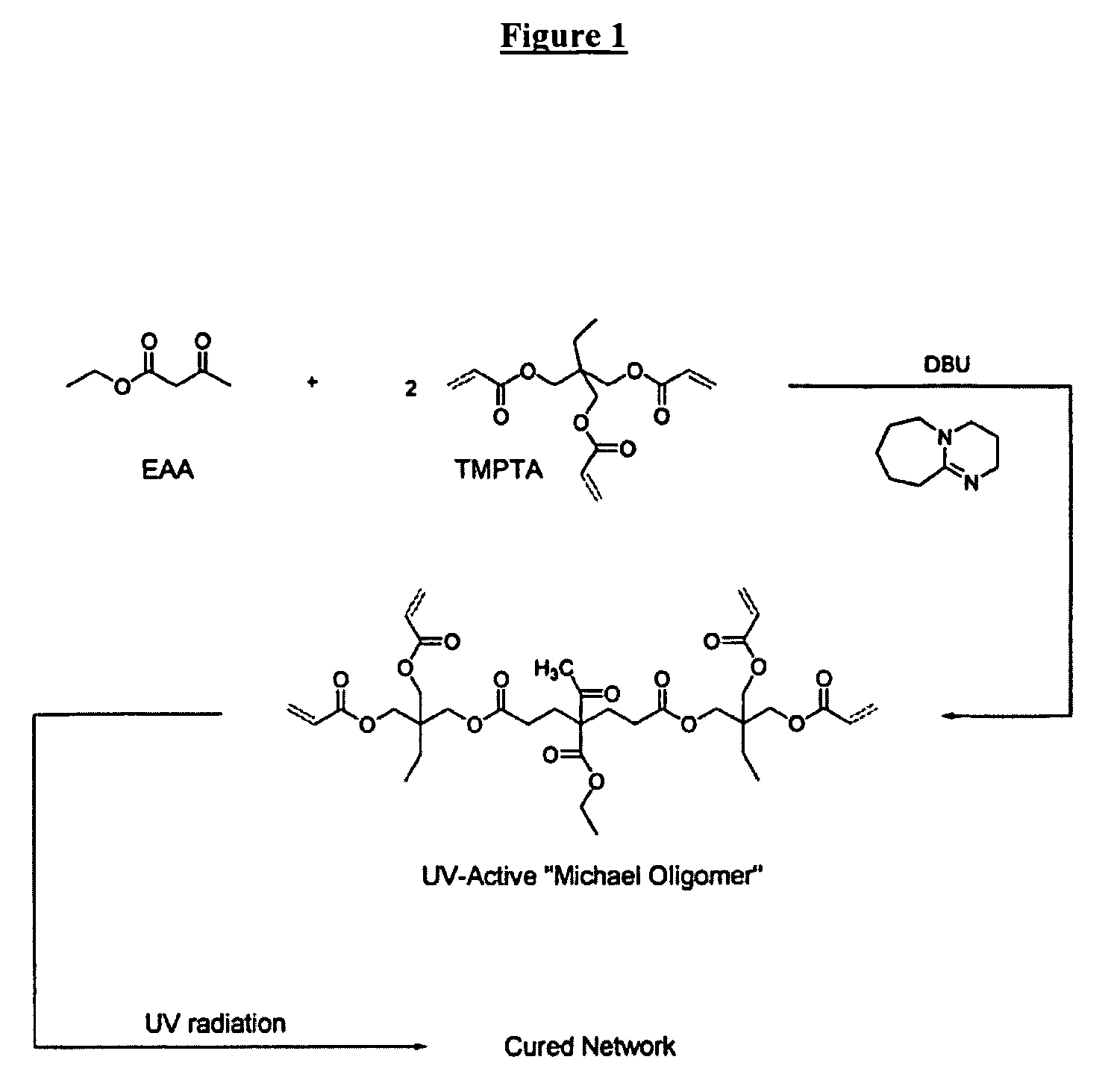
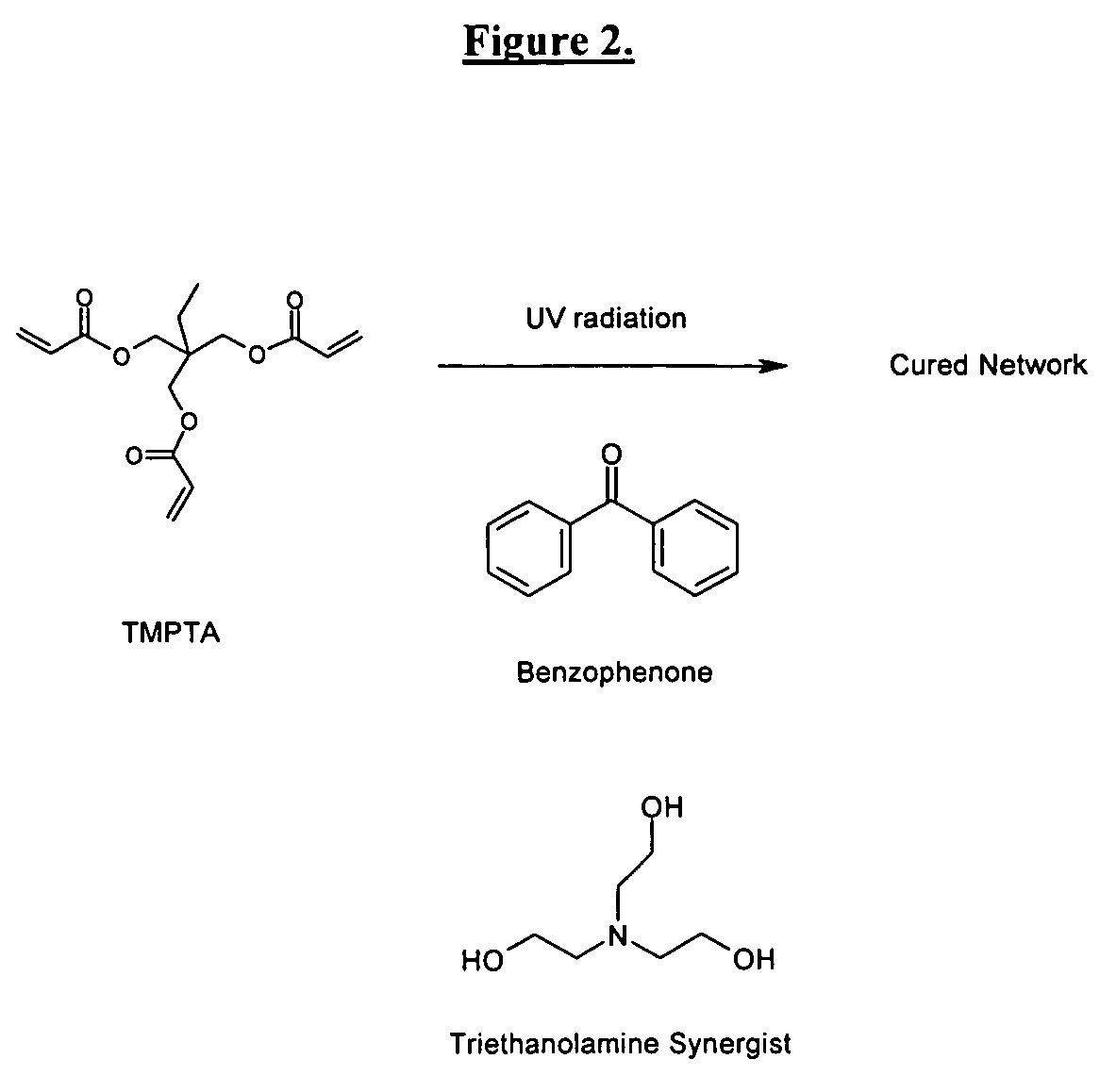
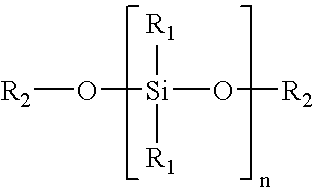
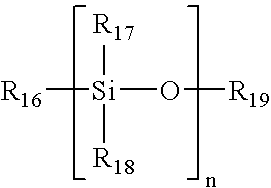
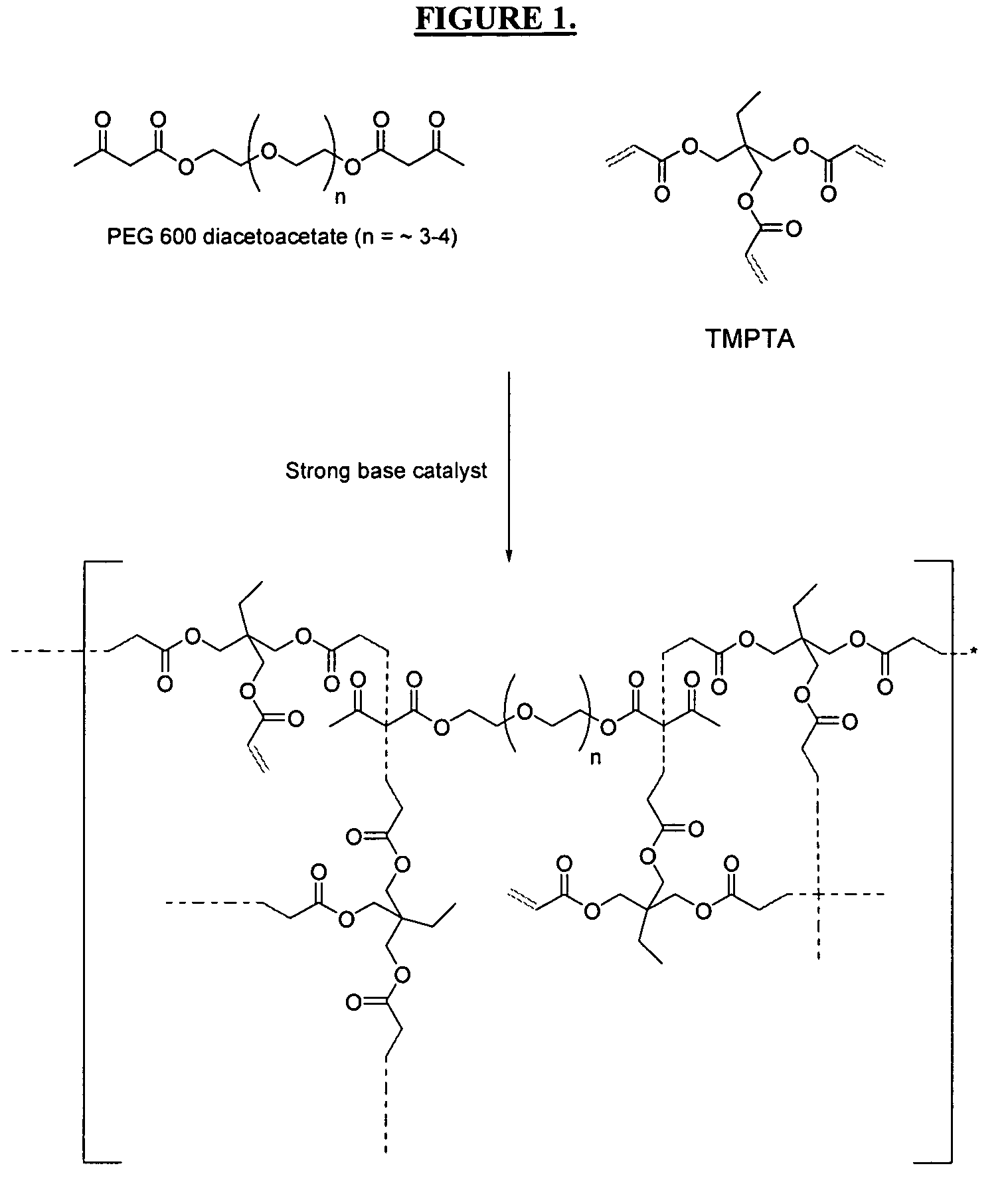
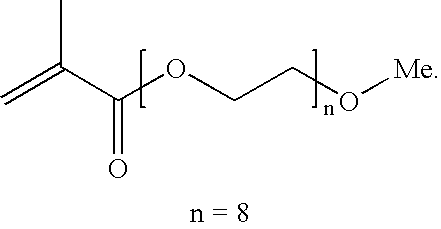
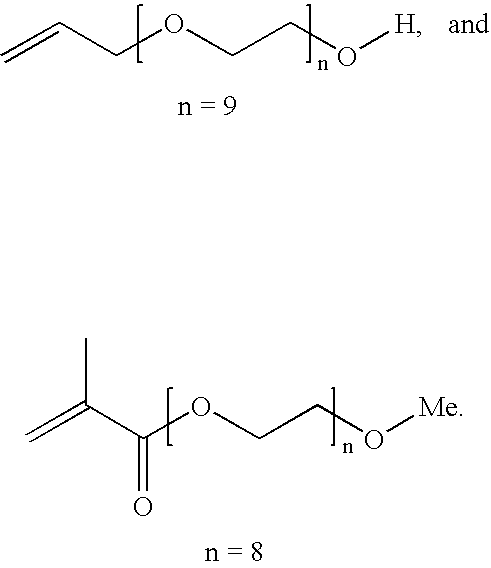
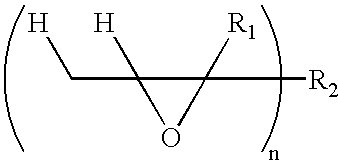
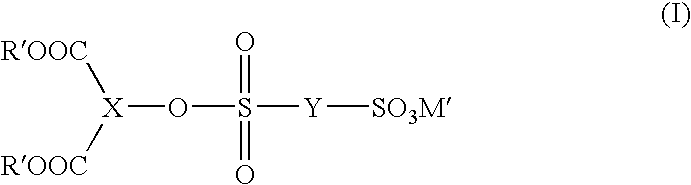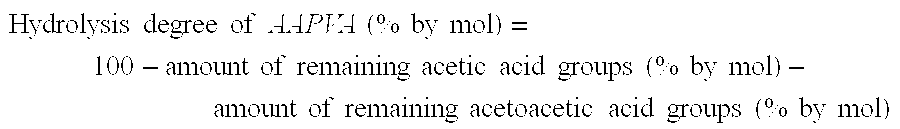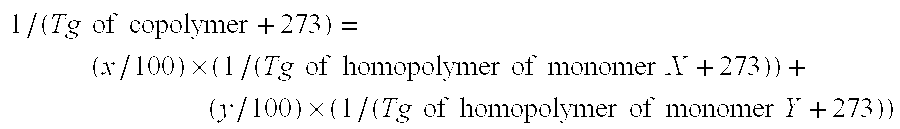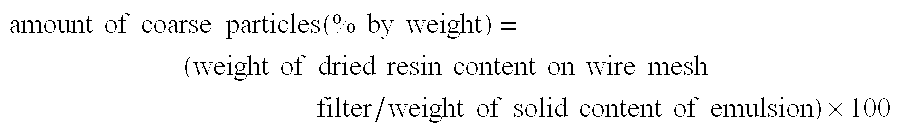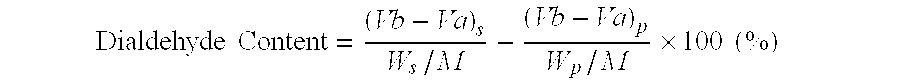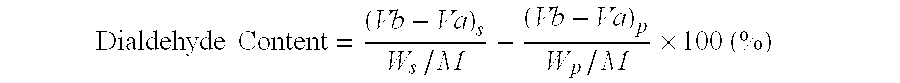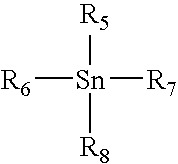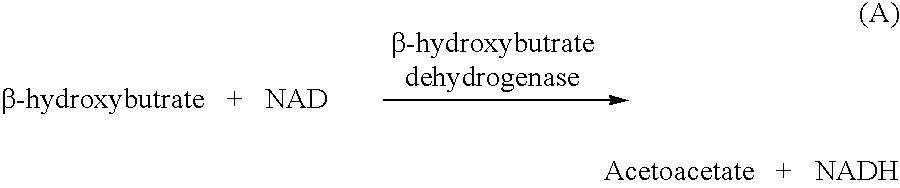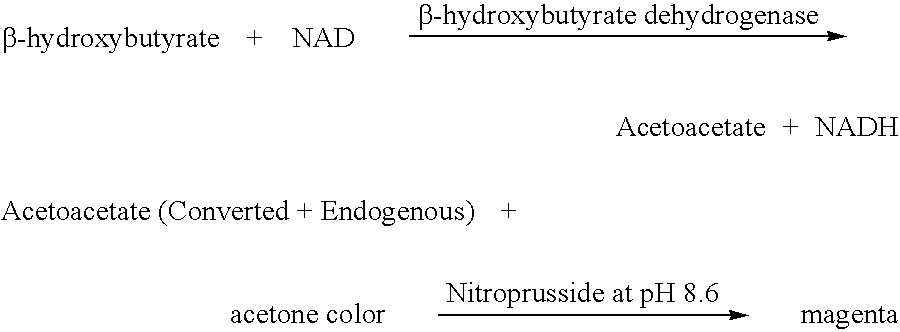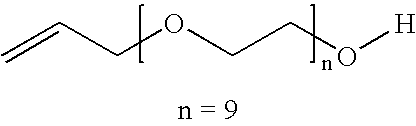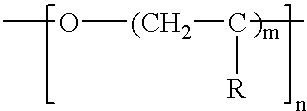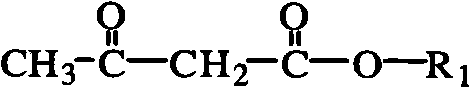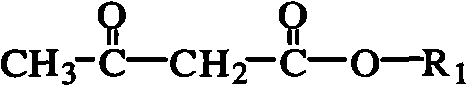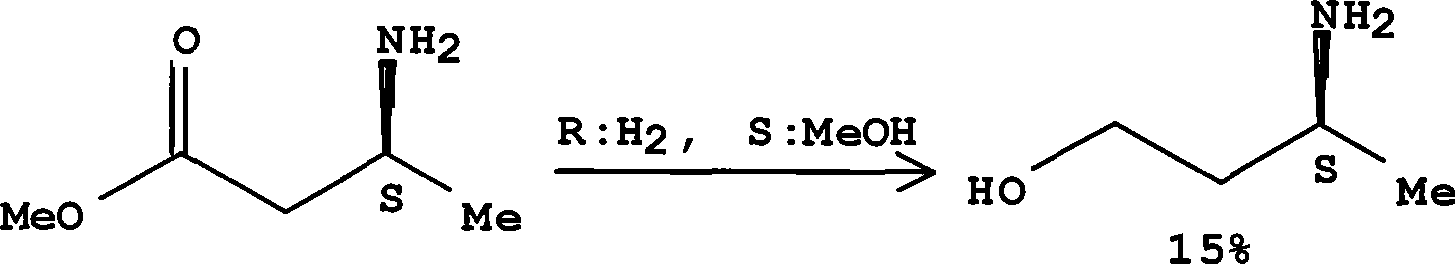Patents
Literature
251 results about "Acetoacetates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
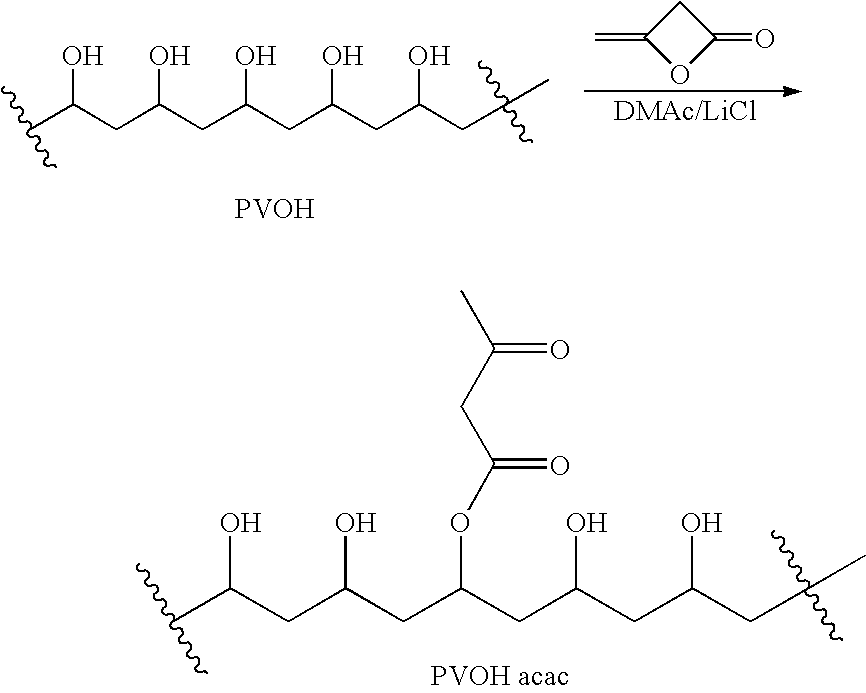
Salts and derivatives of acetoacetic acid.
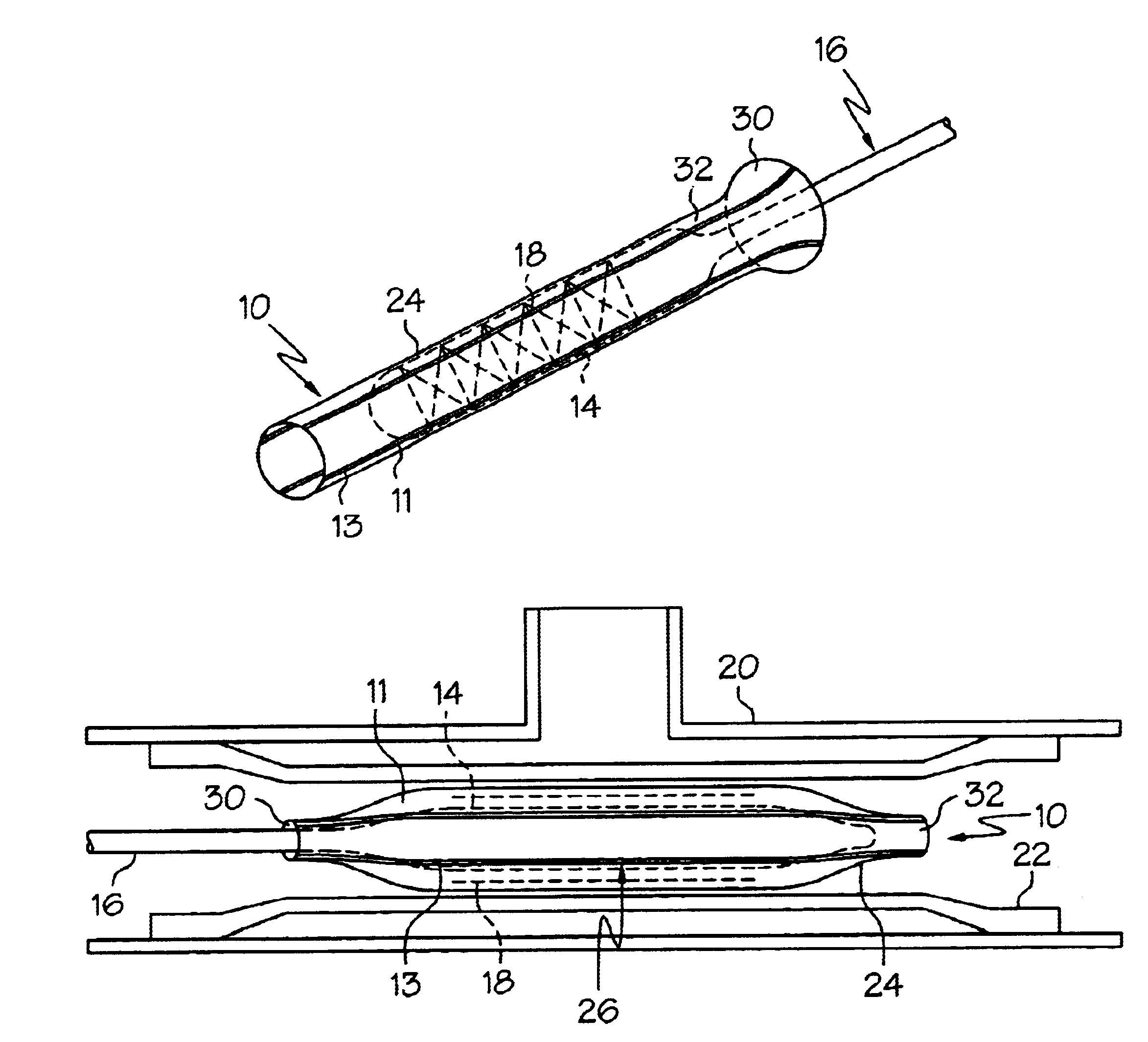
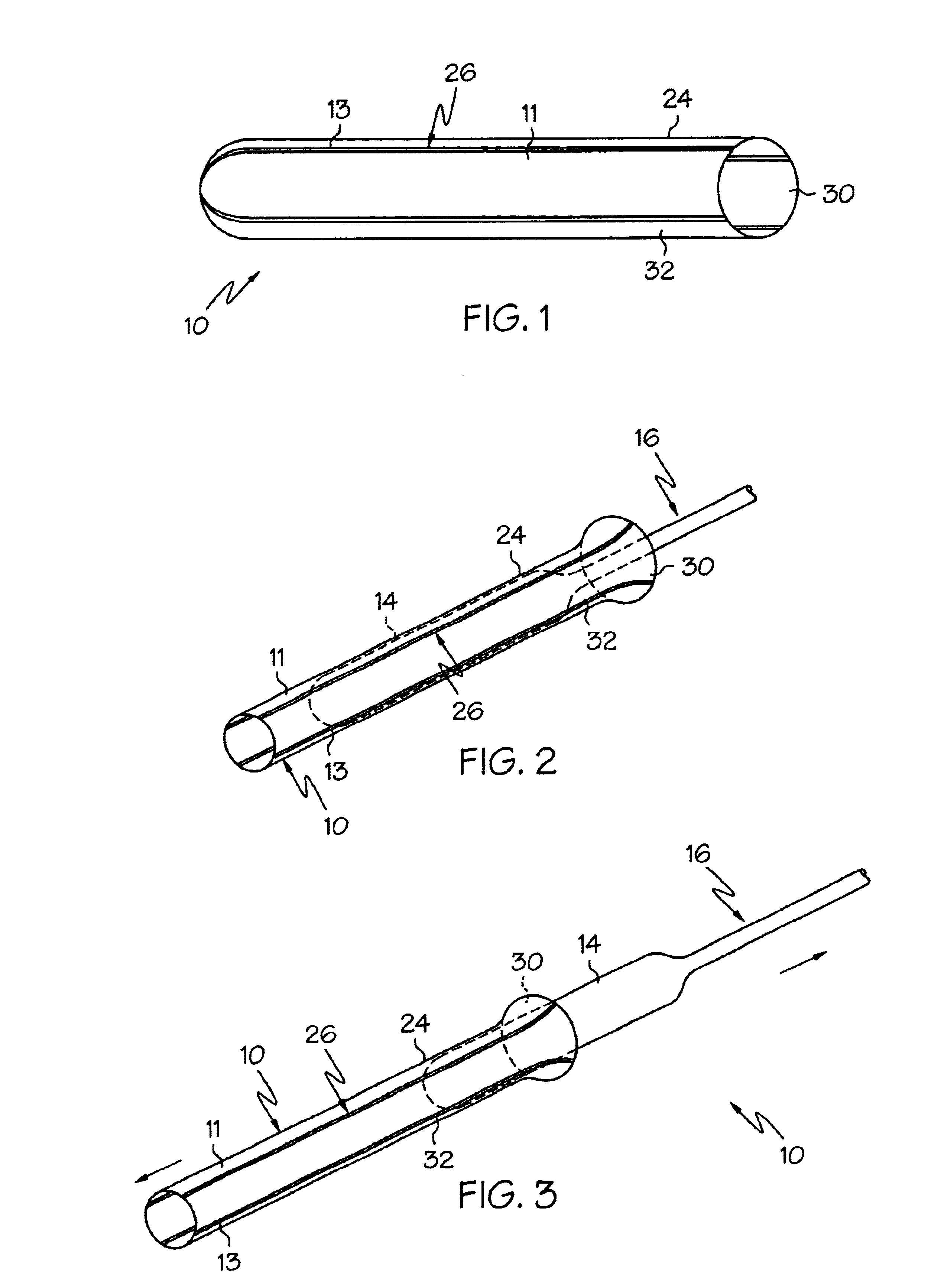
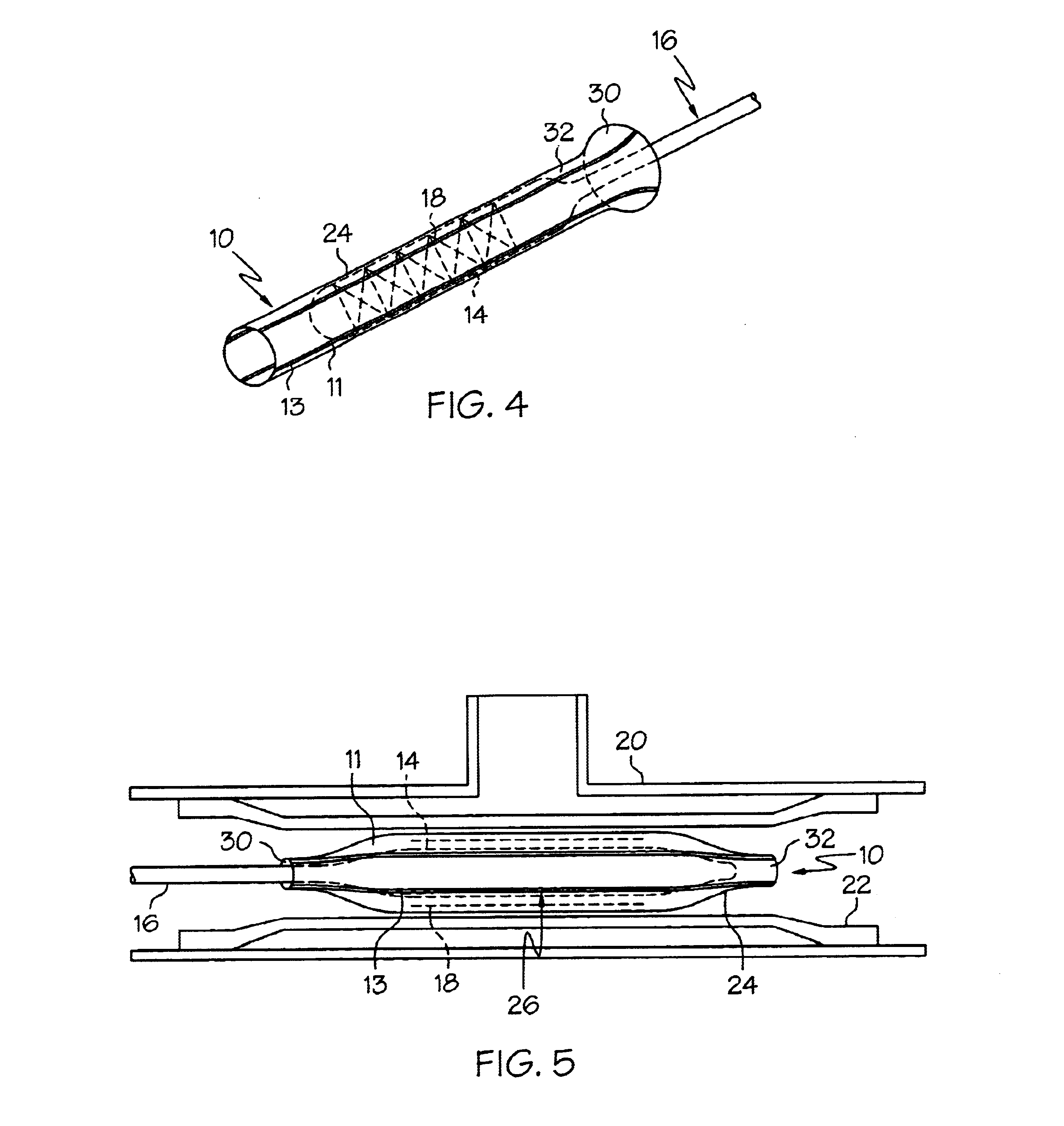
Crimpable balloon/stent protector
InactiveUS6783542B2Reduce the overall diameterIncrease flexibilityStentsBalloon catheterAcetoacetatesStructural unit
A protective sleeve for a catheter assembly comprising a tubular member composed of a first material. The first material having a first predetermined modulus of elasticity. The tubular member having at least one stripe of a second material engaged thereto. The second material having a greater modulus of elasticity than the first material. The tubular member having a loading state and being crimpable to a reduced state. In the loading state the tubular member being sized to disposingly engage the balloon of a catheter assembly wherein the balloon has a first diameter. When the tubular member is in the reduced state the tubular member is disposingly and retainingly engaged to the balloon. When the tubular member is in the reduced state the balloon has a second diameter which is less than the first diameter.
Owner:BOSTON SCI SCIMED INC
Polymer-based tissue-adhesive form medical use
Tissue adhesives formed by reacting poly(hydroxylic) compounds derivatized with acetoacetate groups and / or polyamino compounds derivatized with acetoacetamide groups with an amino-functional crosslinking compound are disclosed. The use of the tissue adhesives for medical and veterinary applications such as topical wound closure; and surgical procedures, such as intestinal anastomosis, vascular anastomosis, tissue repair, and ophthalmic procedures; drug delivery; and anti-adhesive applications are described.
Owner:ACTAMAX SURGICAL MATERIALS
Modified epoxy resin composition, production process for the same and solvent-free coating comprising the same
InactiveUS6677426B2Satisfactory curabilityImprove mechanical propertiesEpoxy resin coatingsAcetoacetatesEpoxy
There is provided a modified epoxy resin composition including a reaction product of an epoxy resin and an alkyl-substituted acetoacetate and a reaction product of an alcohol having at least one hydroxyl group in one molecule and an alkyl-substituted acetoacetate; a production process for the same; and a solvent-free coating using the same. The composition provides a solvent-free epoxy resin composition which has a low viscosity and excellent low-temperature curability.
Owner:WESTLAKE EPOXY INC
Aldehyde removal
Disclosed are filter elements constructed of a filter support material coated with an acetoacetate-functional polymeric composition that reacts with and removes aldehydes, especially formaldehyde, present in gases such as air. Also disclosed are methods for the removal of aldehydes utilizing the coated filter support materials.
Owner:EASTMAN CHEM CO
Fast-curing modified siloxane compositions
Fast-curing modified siloxane compositions comprise; (1) an alkoxy- or silanol-functional silicone intermediate, (2) at least one amine reactive ingredient selected from the group consisting of acetoacetate-functional ingredients, acrylate-functional ingredients, and mixtures thereof, (3) an epoxy-functional ingredient, (4) a curing agent selected from the group consisting of amines, aminosilanes, ketimines, aldimines and mixtures thereof, and (5) water. Other ingredients useful in forming fast-curing modified siloxane compositions of this invention include silanes, organometallic catalysts, solvents, pigments, fillers and modifying agents. The above-identified ingredients are combined and reacted to form a fully cured protective film comprising a cross-linked enamine polysiloxane and / or acrylate polysiloxane chemical structure in a reduced amount of time when compared to conventional epoxy siloxane compositions.
Owner:PPG IND OHIO INC
Aqueous emulsion and use thereof
InactiveUS20050197441A1Improve polymerization stabilityNon-fibrous pulp additionGraft polymer adhesivesAcetic acidFiber
The present invention provides an aqueous emulsion having excellent polymerization stability and standing stability and a redispersible powder. Specifically, the present invention relates to an aqueous emulsion of a polymer obtained by polymerizing an acrylic monomer in the presence of a polyvinyl alcohol containing an acetoacetic ester group; wherein the polyvinyl alcohol containing an acetoacetic ester group has block character [η] of 0.3 to 0.6, hydrolysis degree of at least 97% by mol and acetoacetic esterification degree of 0.01 to 1.5% by mol, and the value obtained by dividing the maximum value by the minimum value of the respective average acetoacetic esterification degree for each of the polyvinyl alcohol containing an acetoacetic ester group separated by particle size of 44 to 74, 74 to 105, 105 to 177, 177 to 297, 297 to 500 and 500 to 1680 μm is 1.0 to 3.0. Also, the present invention relates to a redispersible fiber obtained by drying the aqueous emulsion.
Owner:THE NIPPON SYNTHETIC CHEM IND CO LTD
Preparation method of water-based polyacrylate modified polyurethane dispersion (PUD)
The invention discloses a new method for preparing water-based polyacrylate modified polyurethane dispersion (PUD), which mainly comprises the following steps: (a) preparing an acrylate polymer or copolymer emulsion; (b) preparing a polyurethane prepolymer with carboxyl, and neutralizing the carboxyl; and (c) under the condition of high-speed stirring or other mechanical mixing, adding a polyacrylate emulsion into the polyurethane prepolymer for carrying out dispersion and chain extension. If unsaturated acetoacetate-based compounds are added when the polyacrylate emulsion is prepared, the prepared water-based polyacrylate modified PUD has self-crosslinking property.
Owner:GUANGZHOU HKUST FOK YING TUNG RES INST
Acetoacetic Ester Group-Containing Polyvinyl Alcohol-Based Resin, Resin Composition, and Uses Thereof
An object of the present invention is to provide a new acetoacetic ester group-containing polyvinyl alcohol-based resin that contains less amount of water-insoluble matter and is excellent in transparency and viscosity stability when converted into an aqueous solution, and that realizes excellent water resistance by combined use of a crosslinking agent. The invention provides an acetoacetic ester group-containing polyvinyl alcohol-based resin containing a structural unit represented by the general formula (1):wherein at least one of R1 and R2 represents an acetoacetyl group, R3, R4, R5, R6, R7 and R8 each independently represents an arbitrary substituent, and X represents a single bond or an arbitrary bonding chain.
Owner:MITSUBISHI CHEM CORP
Methods for sealing an orifice in tissue using an aldol-crosslinked polymeric hydrogel adhesive
ActiveUS20070048251A1Organic active ingredientsUnsaturated alcohol polymer adhesivesAcetoacetatesVascular anastomosis
Methods for sealing an orifice in tissue in the body of a living animal using an adhesive formed by reacting an oxidized polysaccharide with a poly(hydroxylic) compound derivatized with acetoacetate groups in the presence of a base catalyst are disclosed. Methods for using the adhesive for medical and veterinary applications such as topical wound closure; and surgical procedures, such as intestinal anastomosis, vascular anastomosis, tissue repair, and ophthalmic procedures; and drug delivery are described.
Owner:ACTAMAX SURGICAL MATERIALS
Dissolvant type polyurethane curing agent synthetic method for producing low free toluene diisocyanate content
ActiveCN101456940AReduce glossLow free toluene diisocyanate contentPolyurea/polyurethane coatingsAcetoacetatesPolyol
The invention relates to a synthesis method for preparing a solvent based polyurethane curing agent with low content of dissociated toluene di-isocyanate, which is characterized in that the reaction undergoes in two stages: stage one, the toluene di-isocyanate and polylol react in the solvent, the reaction degree is monitored by sampling and detecting the content of NCO from the reacted mixture during the reaction process; when the content of NCO is stable, the reaction reaches the endpoint of stage one; stage two, acetylacetic ester is added to the reacted mixture; after the reaction goes on, the reaction degree is monitored by sampling and detecting the content of NCO from the reacted mixture during the reaction process; when the content of NCO is stable again, the reaction reaches the endpoint of stage two, which is also the endpoint of the overall reaction; wherein, the reaction of the stage two is conducted at low temperature. The synthesis method is characterized by small investment, low content of dissociated toluene di-isocyanate monomer, low viscosity, good flexibility and stable product performance.
Owner:GUANGDONG HUARUN PAINT CO LTD
Aldol-crosslinked polymeric hydrogel adhesives
Adhesives formed by reacting an oxidized polysaccharide with a poly(hydroxylic) compound derivatized with acetoacetate groups in the presence of a base catalyst are disclosed. The use of the adhesives for medical and veterinary applications such as topical wound closure; and surgical procedures, such as intestinal anastomosis, vascular anastomosis, tissue repair, and ophthalmic procedures; and drug delivery are described. The adhesive may also be used for industrial and consumer applications.
Owner:ACTAMAX SURGICAL MATERIALS
Process for producing block copolymer pigment dispersants
The present invention is a process for producing a linear block copolymer, useful as a dispersant for pigment, wherein the block copolymer comprises acetoacetyl amine functional groups which serve as pigment anchoring groups. The acetoacetyl amine functional groups can be formed by reacting hydroxyl functional groups with an acetoacetate agent and then reacting the resulted acetoacetate functional groups with a primary amine. The linear block copolymer can be an AB, ABC, or ABA block copolymer. The linear block copolymer produced by the present invention can be useful in dispersing and stabilizing a wide range of pigments in solvent based systems, and are particularly useful in providing pigment dispersions that are used in coating compositions for automobiles and trucks, where they provide improved efficiency of pigment use, lower paint viscosity, and reduced emission of volatile organic solvent.
Owner:AXALTA COATING SYST IP CO LLC
Single-component polyurethane sealant capable of quickly curing moisture and preparation method thereof
ActiveCN102690626AFast curingWon't foamNon-macromolecular adhesive additivesOther chemical processesAcetoacetatesCyclodextrin
The invention relates to a single-component polyurethane sealant capable of quickly curing moisture and a preparation method thereof. The polyurethane sealant comprises the following raw materials in percentage by weight: 10 to 20 percent of polyisocyanate monomer, 20 to 45 percent of polyether polyol, 1 to 10 percent of acetoacetic ester sealed end polyether prepolymer, 5 to 20 percent of plasticizer, 25 to 40 percent of modified mineral powder, 1 to 5 percent of latent curing agent, 0.1 to 2 percent of cyclodextrin wrapped environment-friendly catalyst, 0.1 to 1 percent of defoaming agent and 0.1 to 1 percent of water-removing agent, wherein the water-removing agent is one or more of mono-isocyanate compound or functionalized silane compound. The single-component polyurethane sealant avoids bubble fundamentally, can be quickly cured, is stable in storage, and can be applied to construction on damp interfaces.
Owner:苏州中材非金属矿工业设计研究院有限公司 +1
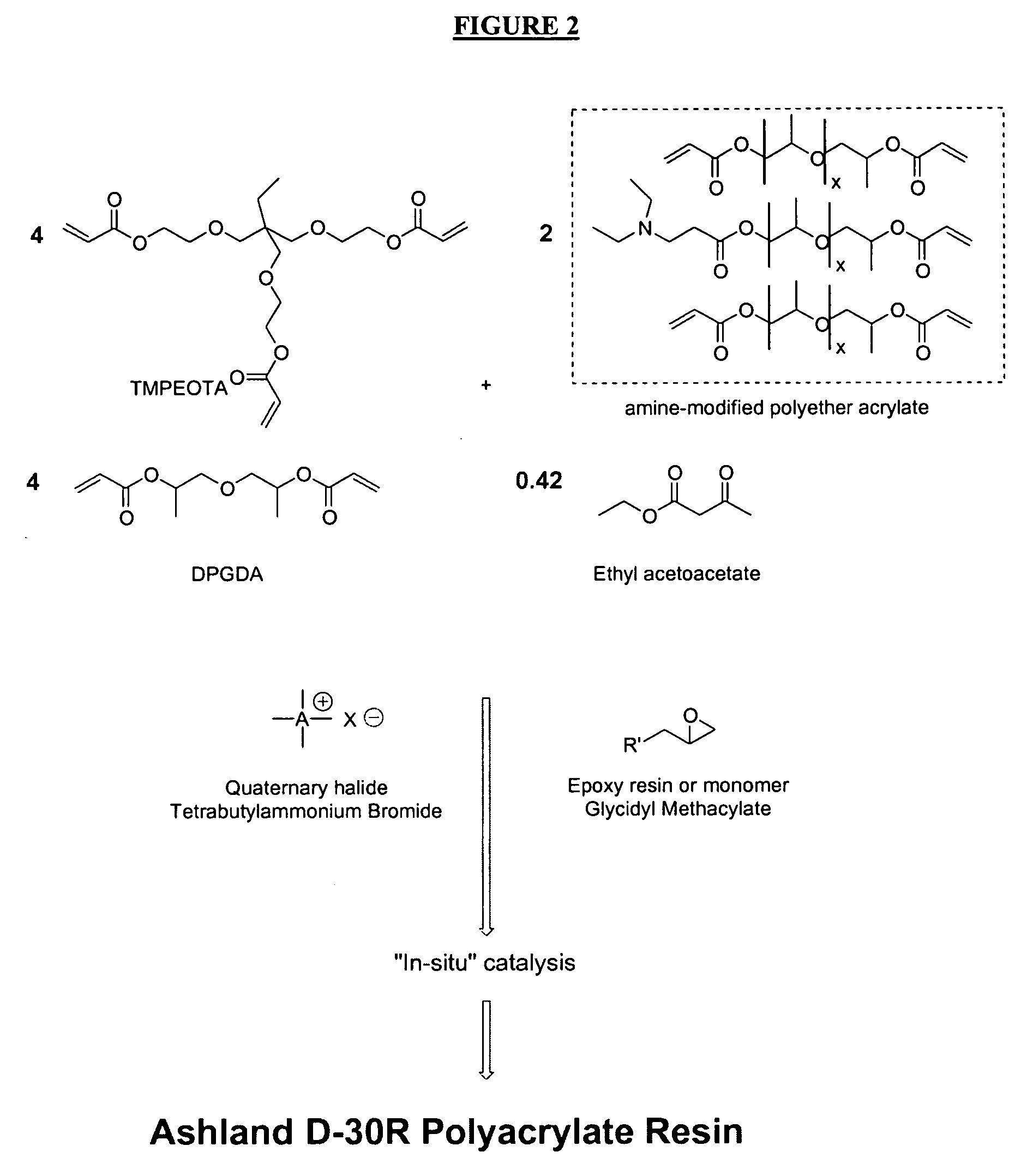
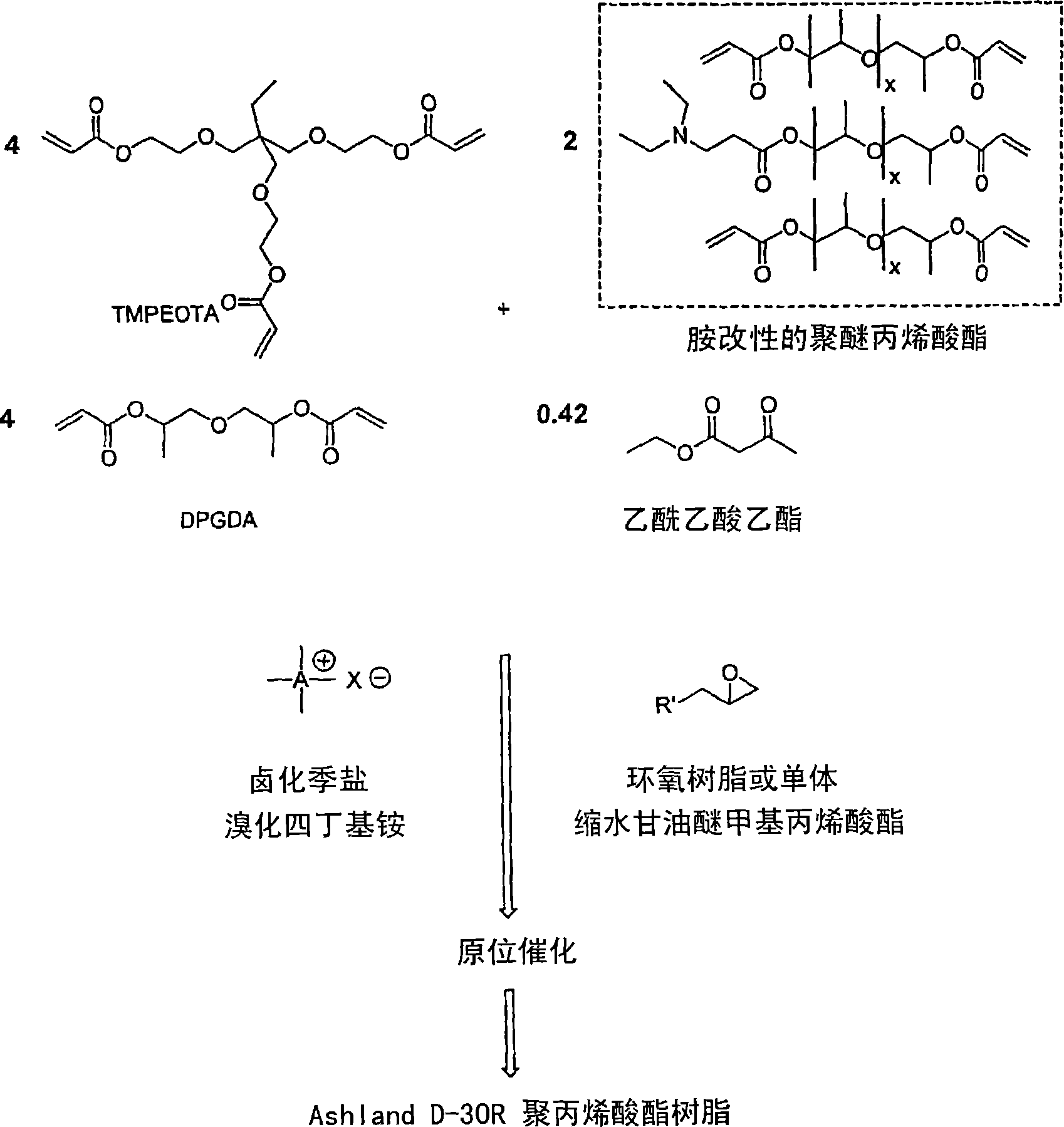
Radiation-curable coatings for metal substrates from multifunctional acrylate oligomers
The invention detailed herein comprises a family of radiation-curable coating formulations specifically for metal substrates. These coating formulations are based on multifunctional acrylate resins formed by the reaction of acrylate monomers and oligomers with ÿ-keto esters (e.g., acetoacetates), ÿ-diketones (e.g., 2, 4-pentanedione), ÿ-keto amides (e.g., acetoacetanilide, acetoacetamide), and / or other ÿ-dicarbonyl compounds that can participate in the Michael addition reaction. An essential novelty of these coating resins is that they will cure under standard UV-cure conditions without the addition of traditional photoinitiators. Other materials, both reactive (conventional acrylates) and non-reactive (e.g., solvents) may also be incorporated into the resin oligomers to enhance the coatings properties on metal substrates. These materials include a variety of acrylic monomers and oligomers, primary, secondary and tertiary amines, acid-functional monomers and oligomers, silicones, waxes and elastomers, among others. Coatings based on these novel multifunctional acrylate resins exhibit excellent adhesion and shrinkage control, flexibility, solvent resistance, scratch and mar resistance, impact resistance, color, and durability across a wide range of plastic materials. These coatings may be cured via chemical means, thermally, or by exposure to UV or electron beam radiation.
Owner:ASHLAND LICENSING & INTPROP LLC
Fast-curing modified siloxane compositions
Fast-curing modified siloxane compositions comprise; (1) an alkoxy- or silanol-functional silicone intermediate, (2) at least one amine reactive ingredient selected from the group consisting of acetoacetate-functional ingredients, acrylate-functional ingredients, and mixtures thereof, (3) an epoxy-functional ingredient, (4) a curing agent selected from the group consisting of amines, aminosilanes, ketimines, aldimines and mixtures thereof, and (5) water. Other ingredients useful in forming fast-curing modified siloxane compositions of this invention include silanes, organometallic catalysts, solvents, pigments, fillers and modifying agents. The above-identified ingredients are combined and reacted to form a fully cured protective film comprising a cross-linked enamine polysiloxane and / or acrylate polysiloxane chemical structure in a reduced amount of time when compared to conventional epoxy siloxane compositions.
Owner:PPG IND OHIO INC
Radiation-curable inks for flexographic and screen-printing applications from multifunctional acrylate oligomers
InactiveUS20050080162A1Process economyModifies its propertyLiquid surface applicatorsMixing methodsAcetoacetatesAcetic acid
The present invention relates generally to radiation-curable ink formulations, and particularly, but not by way of limitation, to a family of radiation-curable ink formulations specifically for flexographic and screen printing applications. The inventive ink formulations are based on multifunctional acrylate resins formed by the reaction of acrylate monomers and oligomers with β-keto esters (e.g., acetoacetates), β-diketones (e.g., 2,4-pentanedione), β-keto amides (e.g., acetoacetanilide, acetoacetamide), and / or other β-dicarbonyl compounds that can participate in Michael addition reactions.
Owner:ASHLAND LICENSING & INTPROP LLC
Method and test strips for the measurement of fat loss during weight loss programs
InactiveUS20040043376A1Great advantageEasy to manufactureMicroorganismsMicrobiological testing/measurementPhysiologyAcyl CoA dehydrogenase
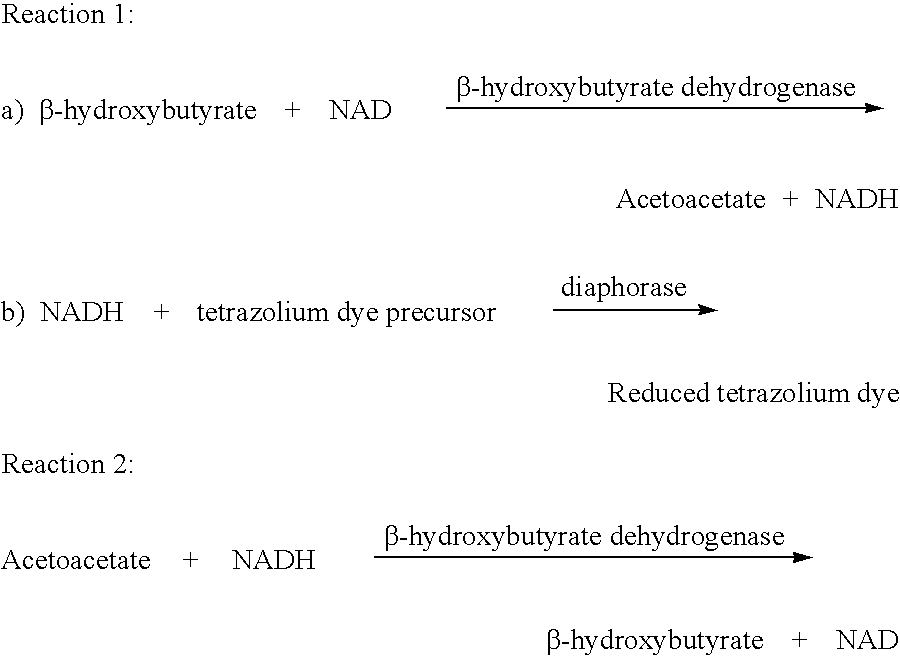
Disposable test strips and a wet chemistry method for measuring each of beta-hydroxybutyrate alone, combined beta-hydroxybutyrate and acetoacetate or total ketone bodies (i.e., beta-hydroxybutyrate, acetoacetate and acetone) in human bodily fluid samples, including but not limited to urine, saliva or sweat are described. The test strips need only be dipped in the sample and can be used by anyone in almost any milieu. Measurement can be made electrochemically, spectrophotometrically, fluorometrically or by comparision to a color standard. Combined acetoacetate and beta-hydroxybutyrate which account for 97-98% of total ketone bodies and may be measured in a cyclic reaction that occurs at pH about 7.0 to about 8.3 with beta-hydroxybutyrate dehydrogenase, (beta-HBD), nicotinamide adenine dinucleotide, a tetrazolium dye precursor and an electron mediator. Using this reaction, false positive results obtained from urine samples taken from patients on sulfhydryl drugs are avoided. beta-HBD from some sources was found to cause false negative results in samples (e.g. urine) containing high chloride content due to chloride inhibition of beta-HBD. Using a simple test for chloride inhibition, it was found that beta-HBD from Alcaligenes is not so inhibited. Using either beta-HBD that is not inhibited by chloride or using 10-20 times the normal concentration of this enzyme eliminates false negatives in samples having substantial chloride content, such as urine, both in the reaction described above and in other reactions disclosed for measuring each of beta-hydroxybutyrate alone, combined beta-hydroxybutyrate and acetoacetate and total ketone bodies, all of which reactions occur in the pH range of about 8.6 to about 9.5.
Owner:GUPTA SURENDRA
Process for production of polymer dispersions containing an acetoacetate moiety
A method of making a composition comprising reacting, in a reactor, a non-halogenated acetoacetate group containing monomer, at least one additional monomer, and a base, wherein at least a portion of the base is fed to the reactor during reaction. Also, a composition comprising an aqueous polymer dispersion that is a product of a method comprising reacting a non-halogenated acetoacetate moiety containing monomer, at least one additional monomer, and a base, wherein the base is fed to the reaction during reaction, wherein the composition has a lower viscosity than a second composition, wherein the second composition is prepared from the same materials as the composition, and the second composition is made by a method wherein the base is added to the second composition after a reaction to form the second composition.
Owner:BASF AG
Particulate filter media
InactiveUS20080134893A1Low costCombination devicesDispersed particle filtrationAcetic acidAcetoacetates
Disclosed are particulate filter media comprising a particulate or powdery acetoacetate-functional compound or composition that is effective in removing gaseous aldehydes present in gases such as air. The particulate filter media are capable of reacting with and irreversibly removing airborne aldehydes, such as formaldehyde, acetaldehyde, and acrolein. Also disclosed is a method or process for the removal of an aldehyde from a gas such as air or tobacco smoke by contacting the aldehyde-containing gas with the particulate filter media.
Owner:EASTMAN CHEM CO
Coating compositions containing highly structured macromolecules
InactiveUS6432484B1Less toxicityLow toxicityPretreated surfacesPolyester coatingsAcetoacetatesAcetic acid
The invention is directed to a two-pack high solids low VOC ambient curable coating composition, which includes cross linking and binder components. The crosslinking component includes a polyamine, a polyketimine, or a combination thereof. The polyamine is provided on an average with at least two amine functionalities per polymer chain and the polyketimine is provided on an average with at least two ketimine functionalities per polymer chain. A reactive diluent in the binder component includes a macromolecule substantially free from acrylate functionalities and having at least two acetoacetate functionalities per the macromolecule. If desired, the coating composition includes a binder polymer. The coating composition of the invention is particularly suited in automotive refinish coatings.
Owner:EI DU PONT DE NEMOURS & CO
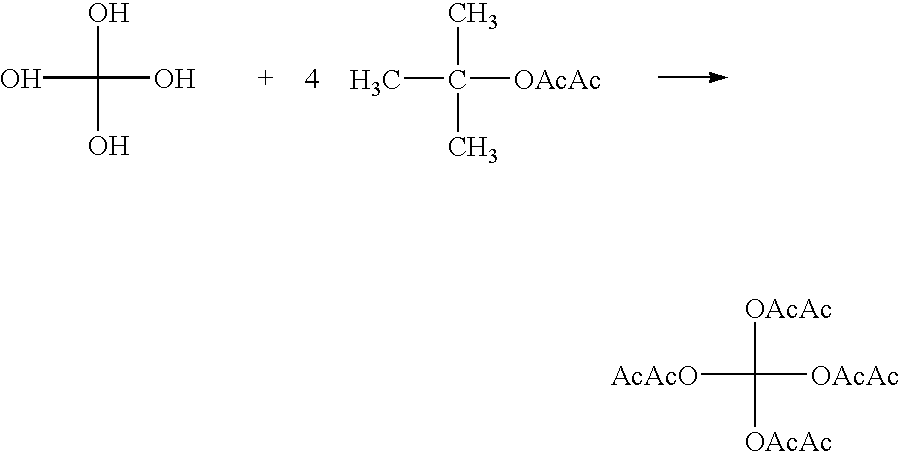
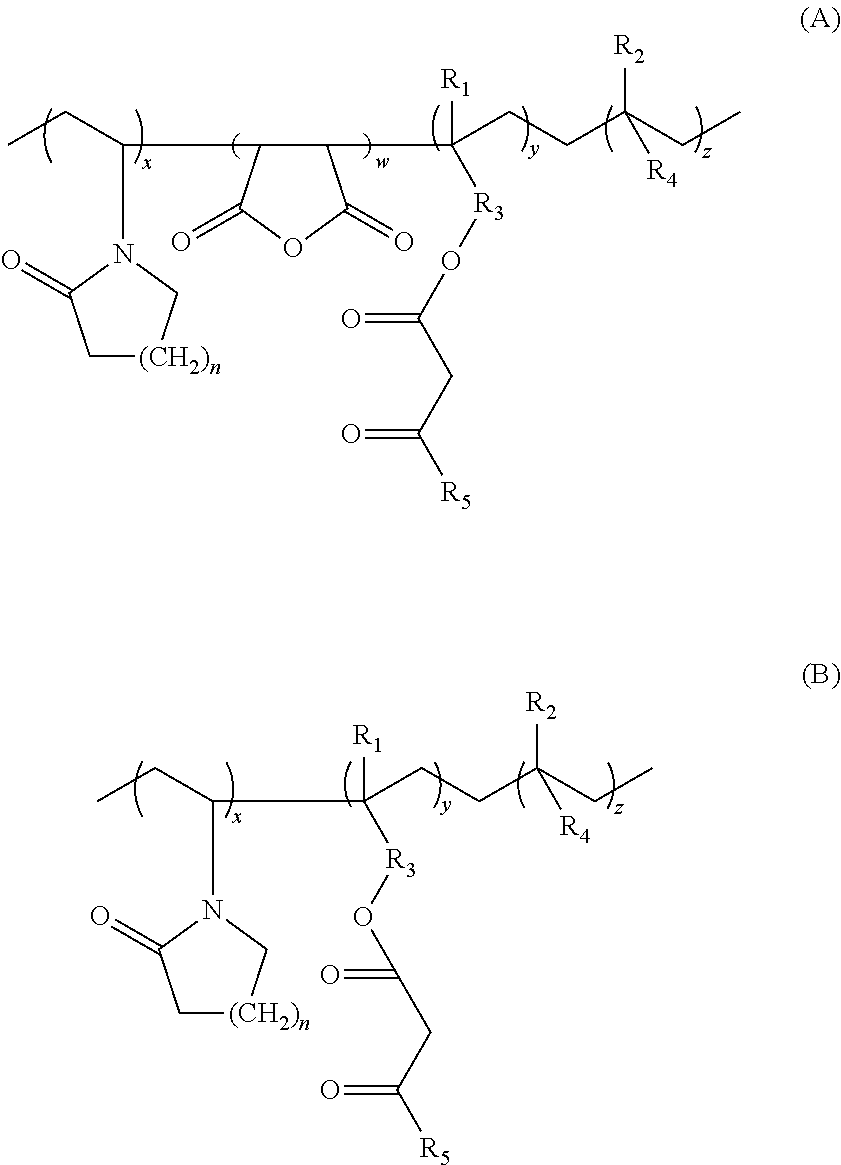
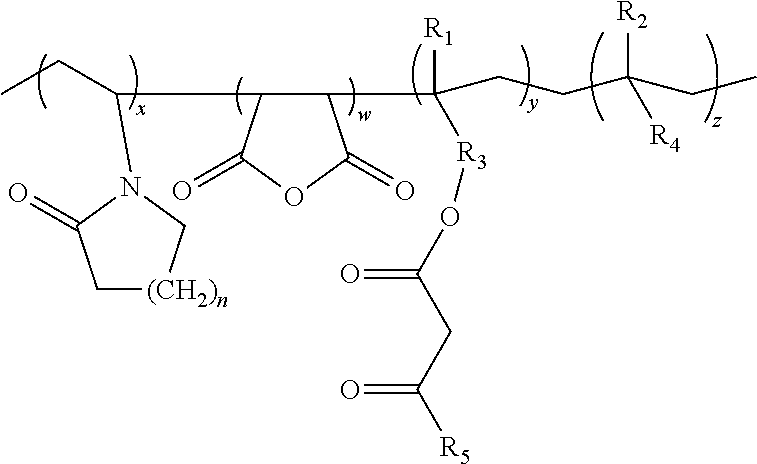
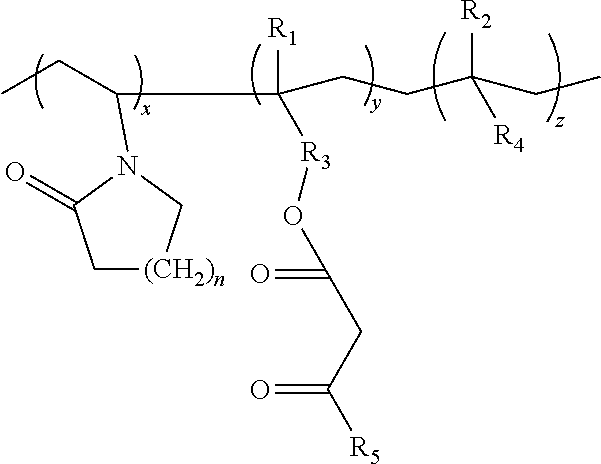
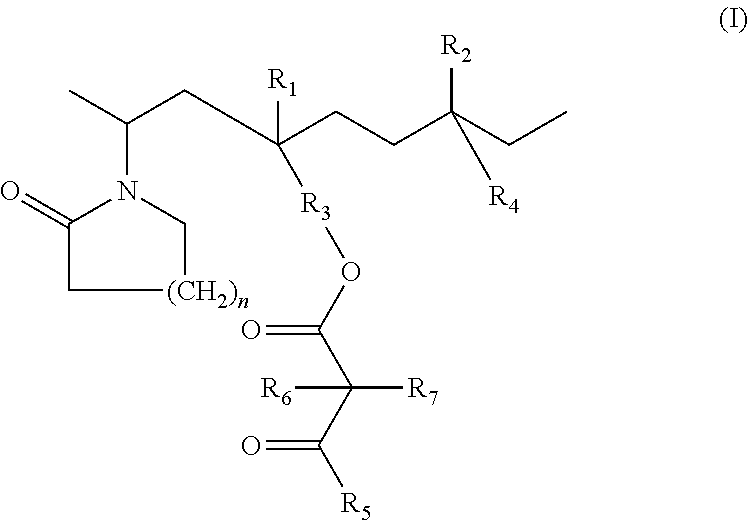
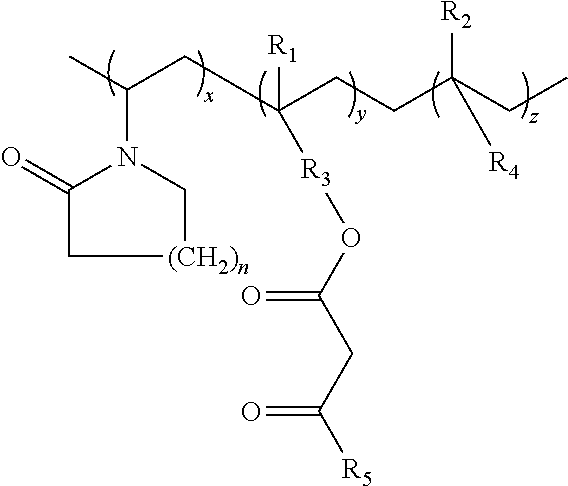
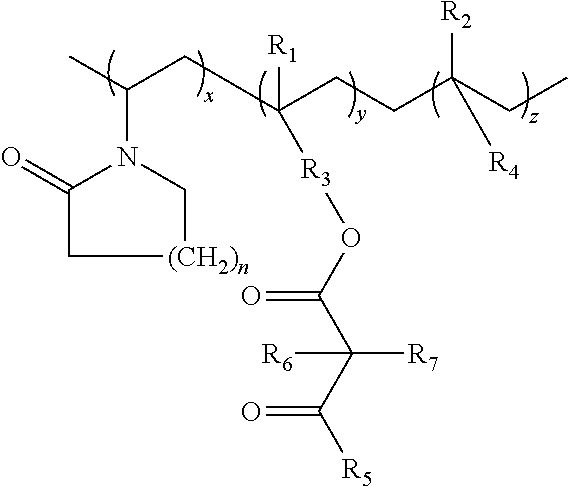
Lactamic polymer containing an acetoacetate moiety
The invention provides lactamic polymers comprising an acetoacetate moiety. The lactamic polymers may be readily functionalized and the functionalized lactamic polymers may be further derivatized to provide a wide variety of useful polymers having desirable chemical and physical properties. The lactamic polymers of the invention may be employed in a wide variety of compositions. (A); (B) wherein R1-R6, w, x, y, z, and n are described herein.
Owner:ISP INVESTMENTS LLC
Method for synthesizing acetoacetate ester compound
The invention belongs to the field of polymer materials, relating to a method for synthesizing acetoacetate ester modified polymer. The method comprises the following steps: taking polymer containing hydroxyl and micromolecule acetoacetate ester as raw materials, and performing reaction under the condition of microwave irradiation; and the equation is shown in the specification, wherein R2(OH)n is polymer containing multi-hydroxy. The invention synthesizes the polymer of which side chains or chain ends have a plurality of acetoacetate ester groups by the process of catalytic ester exchange. The synthetic method has the advantages of high efficiency, mild synthetic method and high safety. In addition, in the method, the catalyst can be added, thereby improving synthetic efficiency; the synthetic rate of the method is greatly improved compared with the prior art; and the reaction time is shortened to 2-30min.
Owner:SUN YAT SEN UNIV
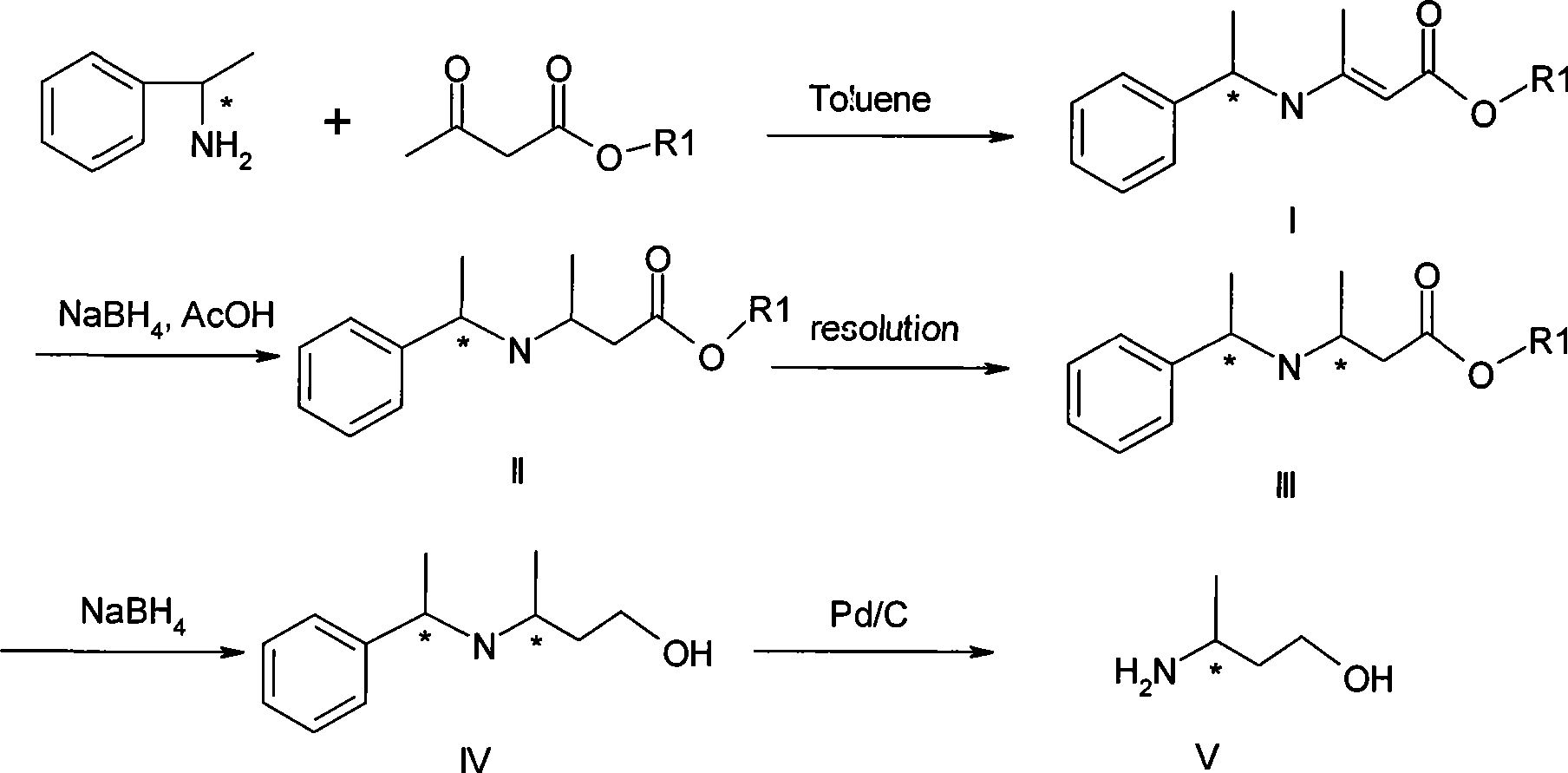
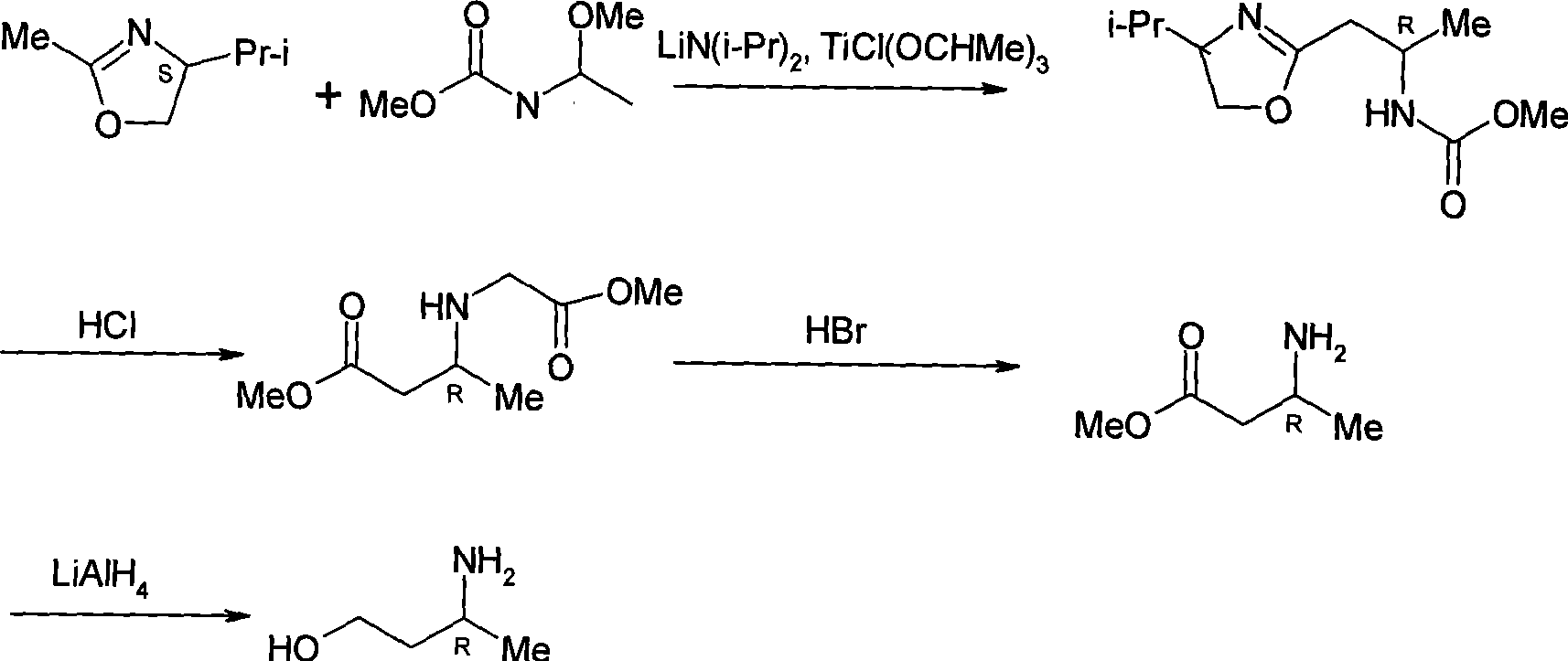
Method for preparing optically pure 3-amino butyl alcohol
ActiveCN101417954ASuitable for industrial productionStereoselectiveOrganic compound preparationAsymmetric synthesesAcetoacetatesPotassium borohydride
The invention discloses a method for preparing optical pure 3-aminobutanol, which comprises the following steps: acetylacetic ester and chiral phenethylamine react to generate 3-(1'-benzylmethylamine)-2-crotonate enantiomer, namely 3-(1'-benzylmethylamine)-2-crotonate; potassium borohydride triacetate or sodium borohydride triacetate is used to reduce the 3-(1'-benzylmethylamine)-2-crotonate enantiomer into a 3-(1'-benzylmethylamine)-2-butyric ester enantiomer; then chiral pure 3-(1'-benzylmethylamine)-2-butyric ester is obtained through salification and resolution; and the 3-(1'-benzylmethylamine)-2-butyric ester is subject to reducing debenzylation by palladium-carbon to obtain the optical pure 3-aminobutanol. The method has low cost and high product purity, and is suitable for industrialized production.
Owner:ABA CHEM SHANGHAI +1
Maleic anhydride oil modified aqueous alkyd resin, and environmentally-friendly paint prepared through using it
The invention relates to a maleic anhydride oil modified aqueous alkyd resin, and an environmentally-friendly paint prepared through using it. The maleic anhydride oil modified aqueous alkyd resin comprises 31-34mass% of soya oil acid or soybean oil or linseed oil, 25-30mass% of phthalic anhydride, 5-8mass% of benzoic acid, 12-15mass% of pentaerythritol, 12-15mass% of dimethylolpropionic acid, 8-11mass% of maleic anhydride oil, 15-25mass% of butyl ether, 5-8mass% of triethylamine, and 15-25mass% of tap water. The invention also discloses an application of an environmentally-friendly acetoacetate-cobalt drier in the aqueous alkyd resin.
Owner:王芳贵
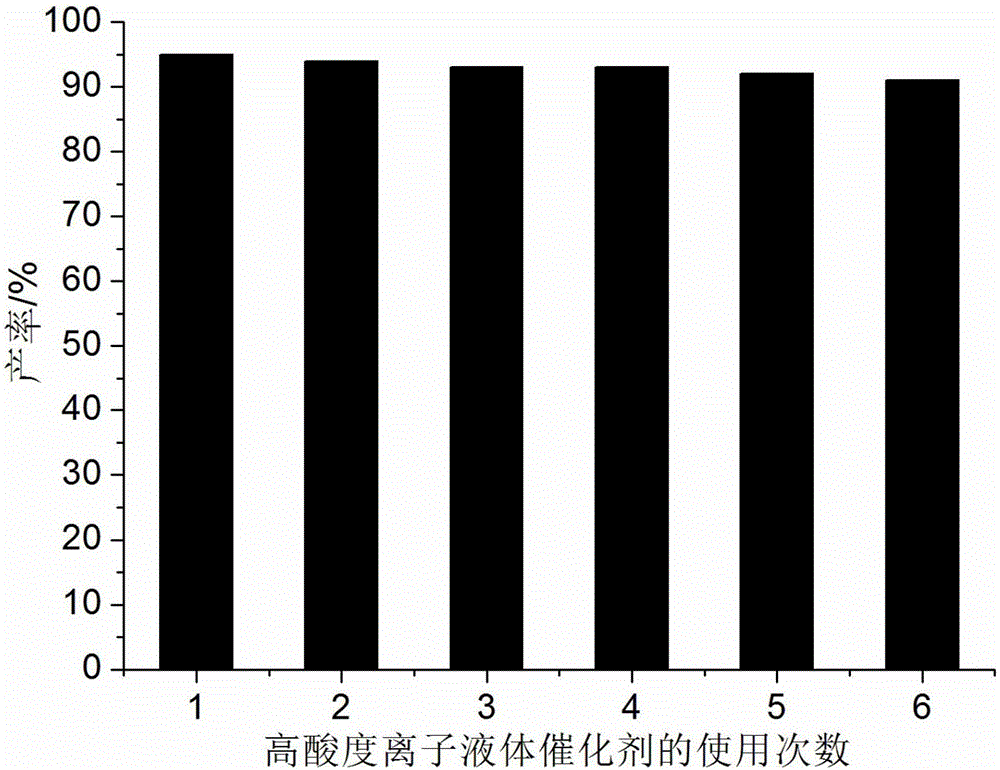
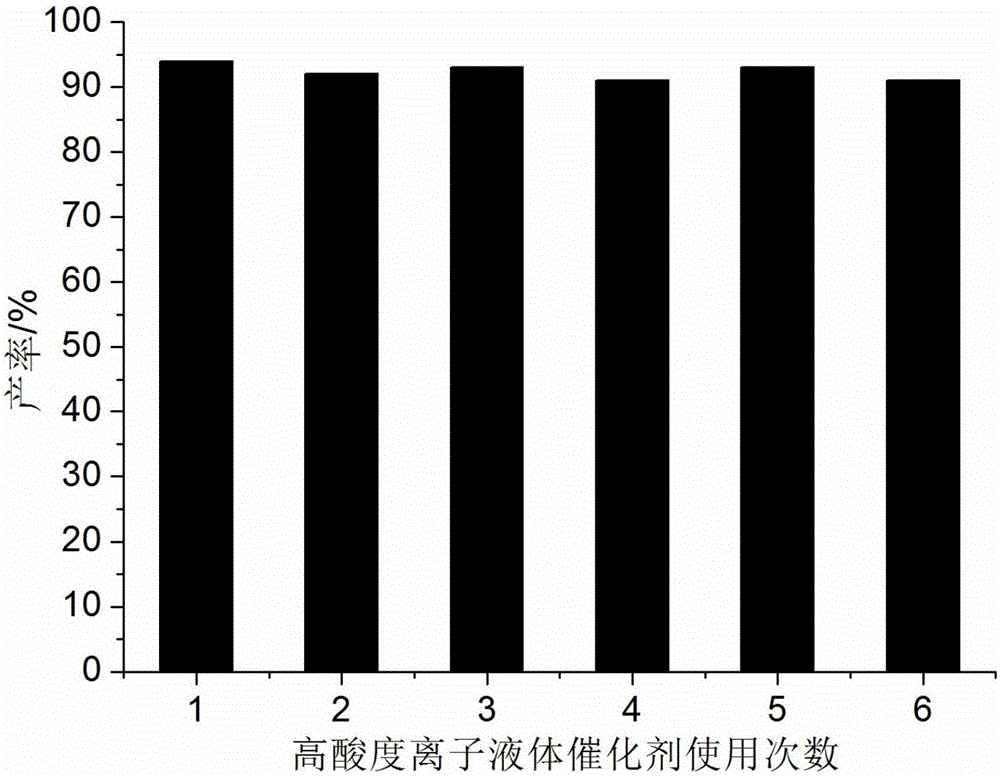
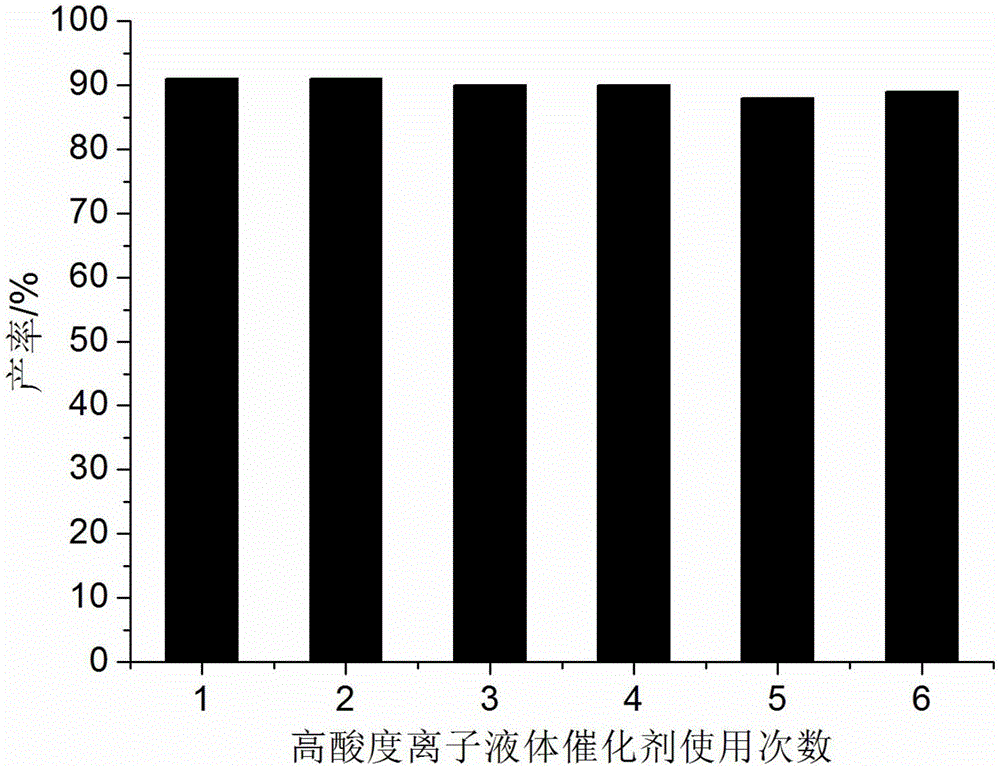
Method for preparing hexahydroquinoline derivatives through high-acidity ionic liquid catalysis one-pot method
ActiveCN105130890AIncrease acidityHigh catalytic activityOrganic chemistryAcetoacetatesOrganic synthesis
The invention discloses a method for preparing hexahydroquinoline derivatives through a high-acidity ionic liquid catalysis one-pot method and belongs to the technical field of organic synthesis. In a preparation reaction, the molar ratio of aromatic aldehyde, 5, 5- dimethyl-1, 3-cyclohexanedione, acetoacetic ester and ammonium acetate is 1 to 1 to 1 to 1-1.5, the molar weight of a high-acidity ionic liquid catalyst is 2%-3% that of the aromatic aldehyde, the volume dose of the reaction solvent ethyl alcohol metered in milliliter is 5-7 times the molar weight of the aromatic aldehyde metered in millimole, the reaction pressure is one atmosphere pressure value, a backflow reaction is conducted for 5-20 minutes, the mixture is cooled to room temperature after the reaction is finished, a great amount of solid is separated out, suction filtration is conducted, and the hexahydroquinoline derivatives are obtained after the obtained filter residues are dried in a vacuum mode. Compared with a preparing method adopting other acidic ionic liquid as the catalyst, the method has the advantages that the catalyst is biodegraded easily, little in consumption, low in price and easy to obtain, the whole preparing process is high in raw material utilization rate and easy and convenient to operate, and industrial large-scale production is facilitated.
Owner:兰陵财金产业发展有限公司
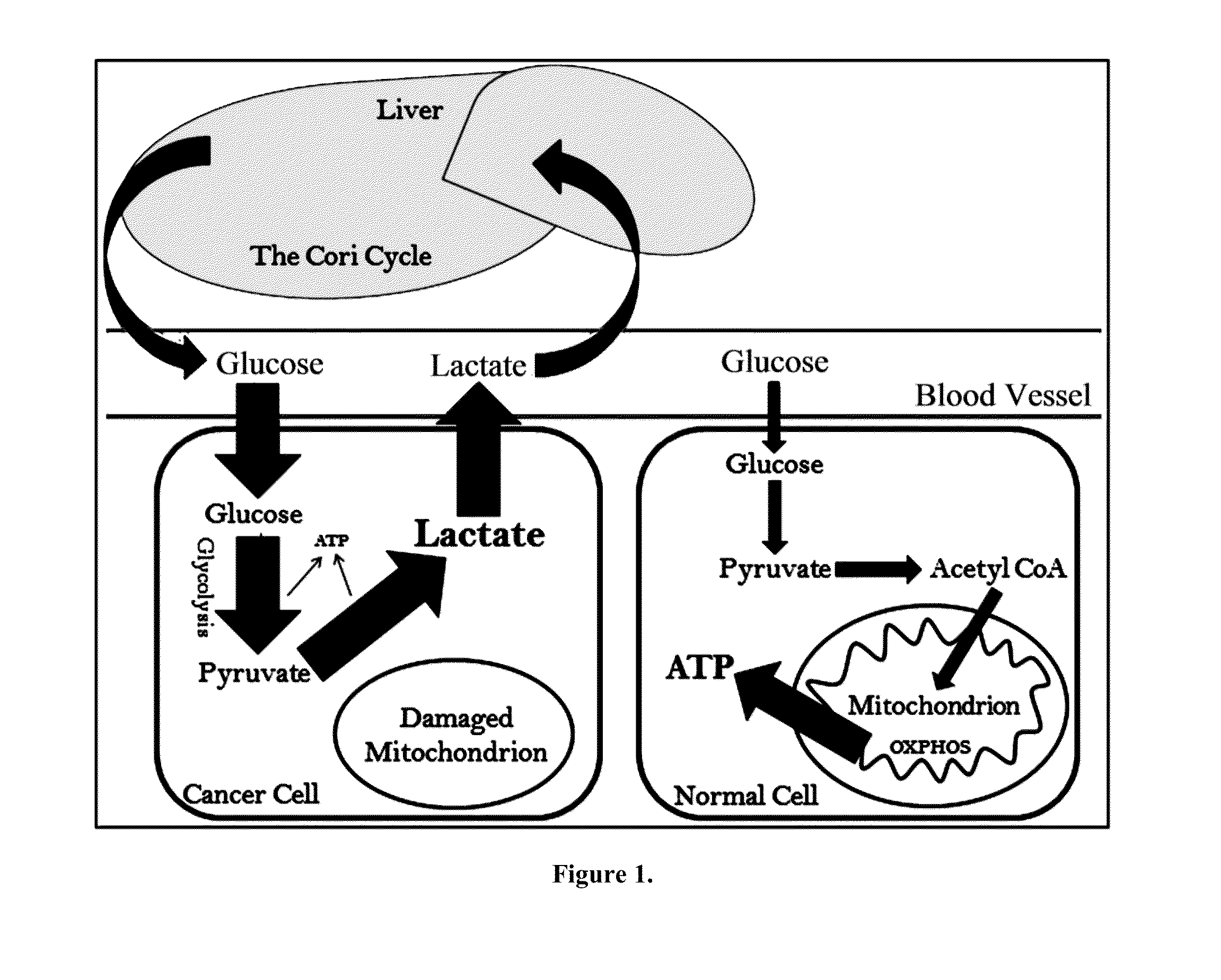
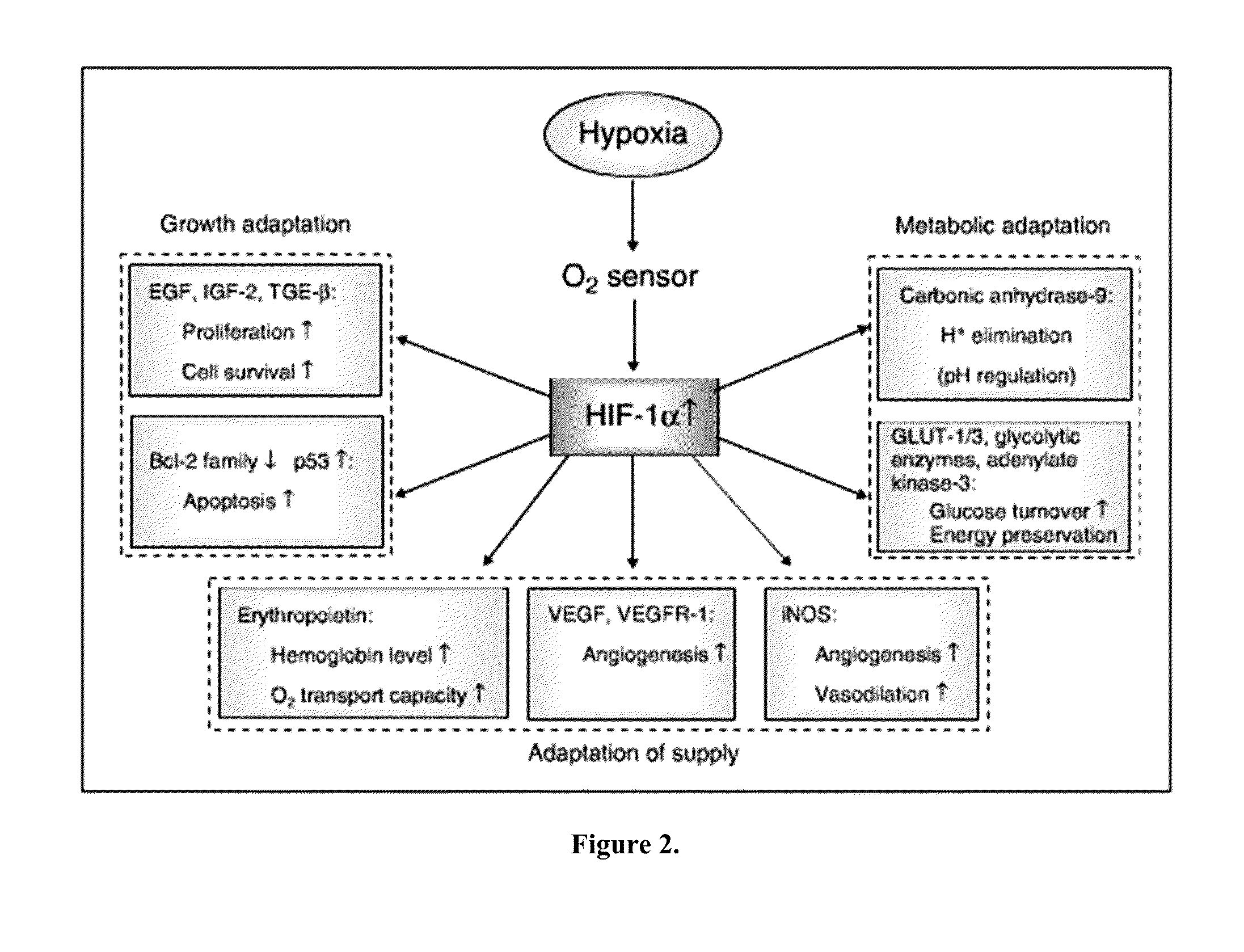
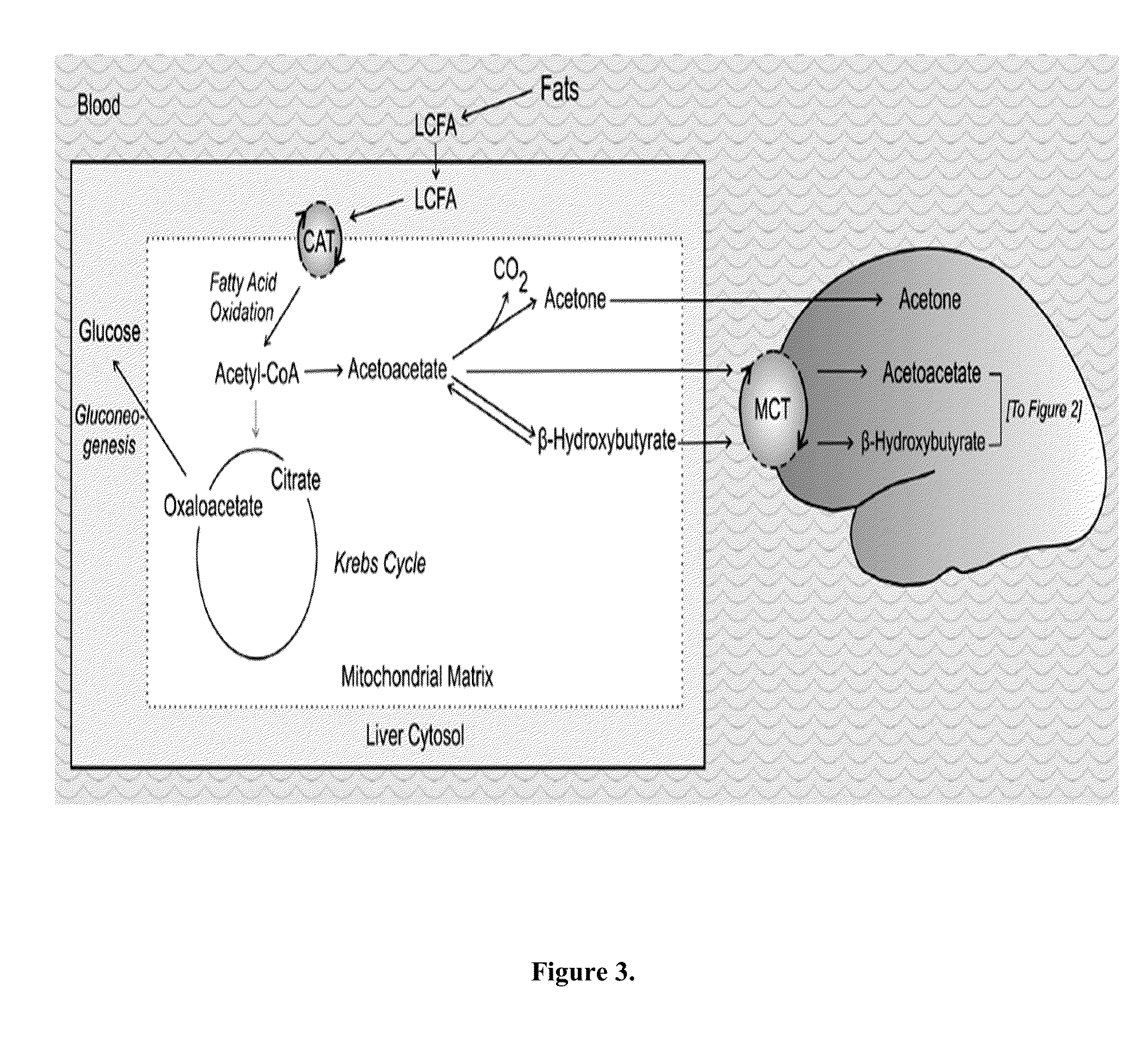
Cancer with metabolic therapy and hyperbaric oxygen
InactiveUS20140072654A1Raise the possibilityIncrease productionBiocidePeroxide active ingredientsDiseaseAcetoacetates
The present invention demonstrates the therapeutic use of ketone esters for seizure disorders, Alzheimer's disease malignant brain cancer, and other cancers, which are associated with metabolic dysregulation. The administration of a ketogenic diet, such as ketone esters, while concurrently subjecting the patient to a hyperbaric, oxygen-enriched environment resulted in therapeutic ketosis. Optionally, the hyperbaric, oxygen-enriched environment is 100% oxygen at 2.5 ATA absolute. The ketone esters may be derived from acetoacetate and can include R,S-1,3-butanediol acetoacetate monoester, R,S-1,3-butanediol acetoacetate diester, or a combination of the two. The treatment may further include administering at least 10% ketone supplementation, such as acetoacetate, adenosine monophosphate kinase, 1,3-butanediol, or ketone ester, to the patient.
Owner:UNIV OF SOUTH FLORIDA
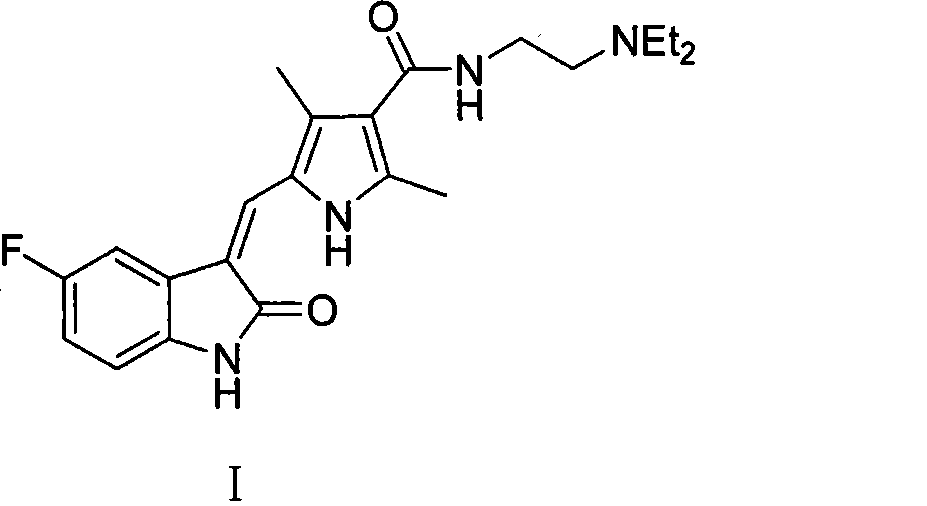
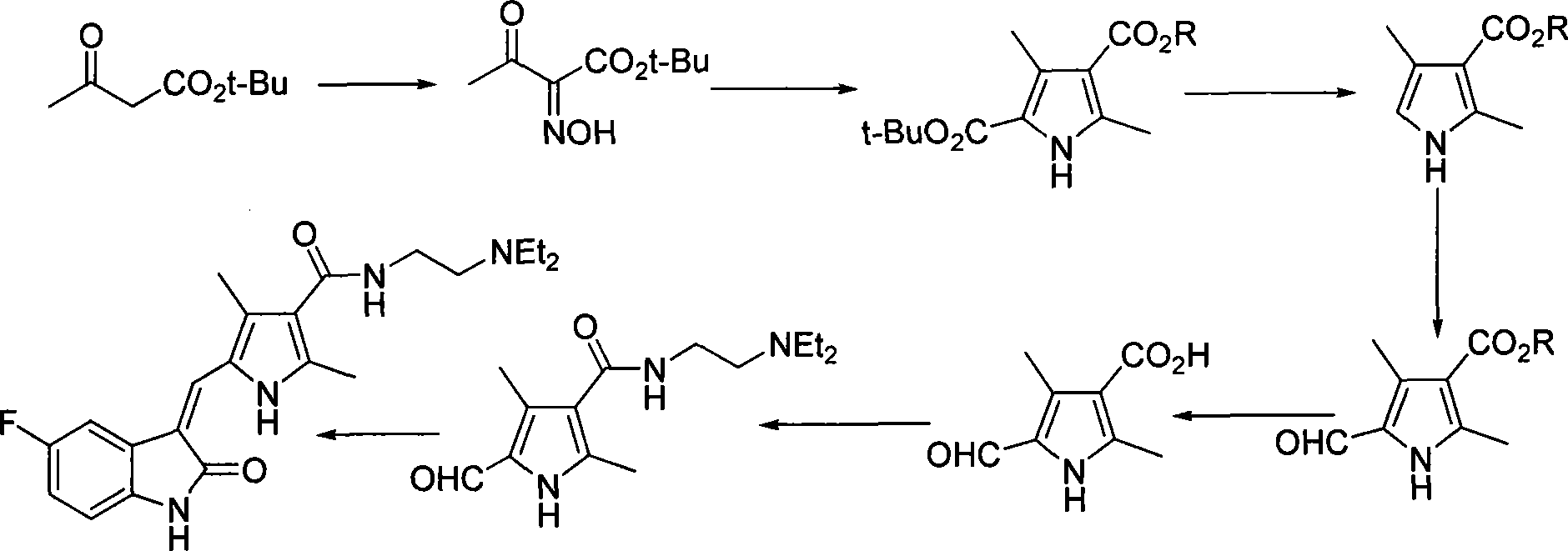
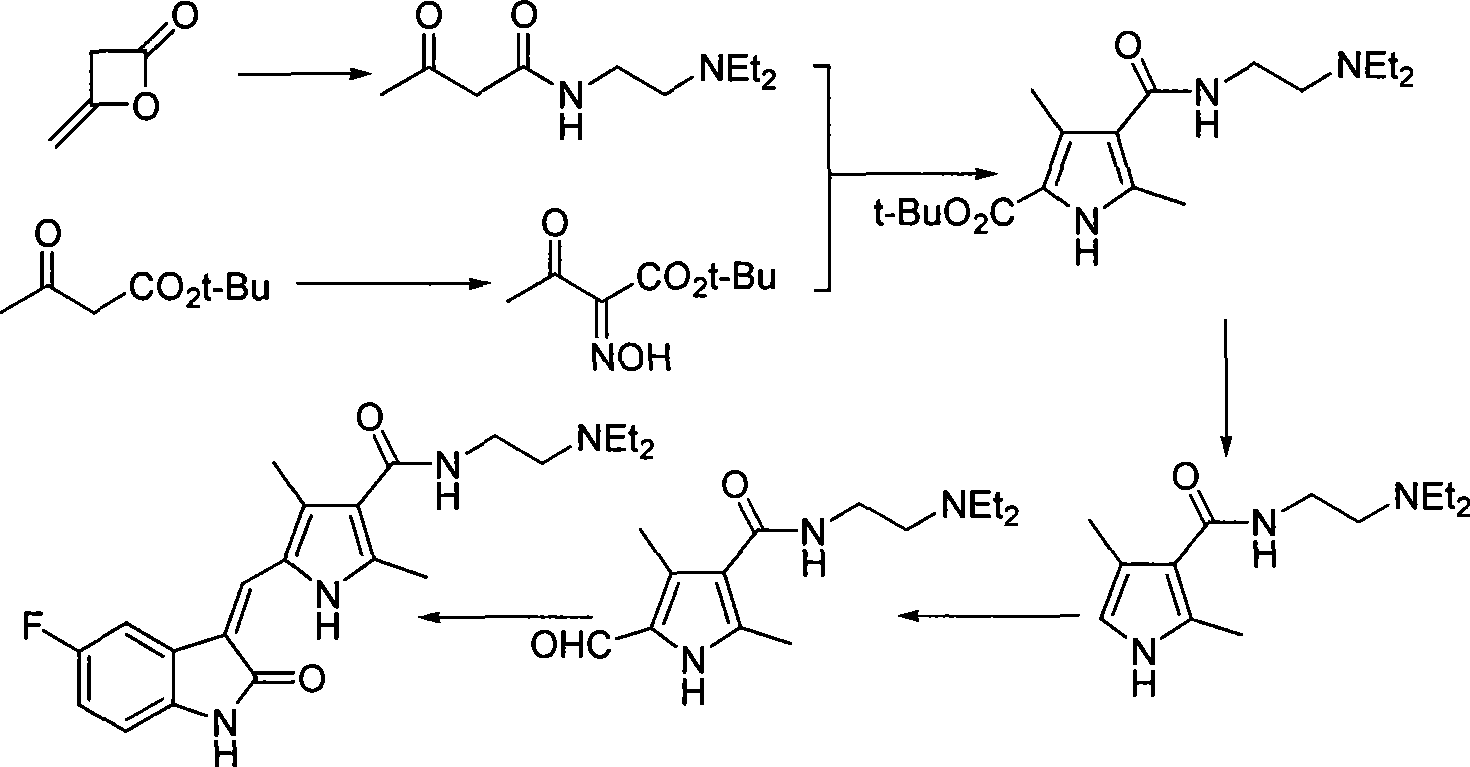
Process for synthesizing sunitinib
The invention discloses a method for synthesizing sunitinib, which comprises the following steps that: tert-butyl acetoacetate and acetylacetic ester are taken as initial raw materials, and tetra-substituted pyrrole is obtained through a nitrosification reduction reaction; then 2,4-dimethyl pyrrole-3-formic acid is obtained through the hydrolysis and is amidated; then an aldehyde group is utilized on position 5 of pyrrole through a Vilsmeier-Hacck reaction; and finally the obtained product and 5-fluorooxindole react to obtain the sunitinib. The method has low cost and simple operation, and is favorable for industrial production.
Owner:FUJIAN SOUTH PHARMA CO LTD
Lactamic polymer containing an acetoacetate moiety
The present invention provides lactamic polymers containing an acetoacetate moiety. The lactamic polymers may be readily functionalized and the functionalized lactamic polymers may be further derivatized to provide a wide variety of useful polymers having desirable chemical and physical properties. The lactamic polymers of the present invention may be employed in a wide variety of compositions. Formula (I) wherein R1-R7, x, y, z, and n are described herein.
Owner:ISP INVESTMENTS INC
Radiation-curable inks for flexographic and screen-printing applications from multifunctional acrylate oligomers
InactiveCN1894291AChange rheologyChange adhesive propertiesInksThin material handlingAcetic acidAcetoacetates
The present invention relates generally to radiation-curable ink formulations, and particularly, but not by way of limitation, to a family of radiation-curable ink formulations specifically for flexographic and screen printing applications. The inventive ink formulations are based on multifunctional acrylate resins formed by the reaction of acrylate monomers and oligomers with ss-keto esters (e.g., acetoacetates), ss-diketones (e.g., 2, 4-pentanedione), ss-keto amides (e.g., acetoacetanilide, acetoacetamide), and / or other ss-dicarbonyl compounds that can participate in Michael addition reactions.
Owner:ASHLAND LICENSING & INTPROP LLC
Preparation method of high-purity palbociclib
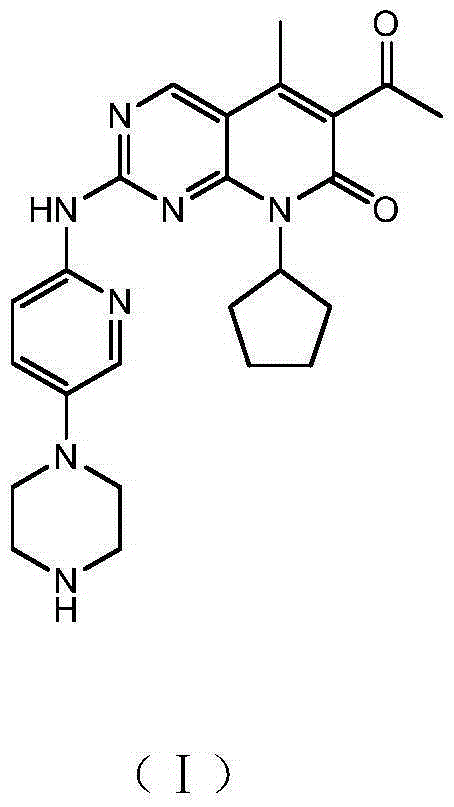
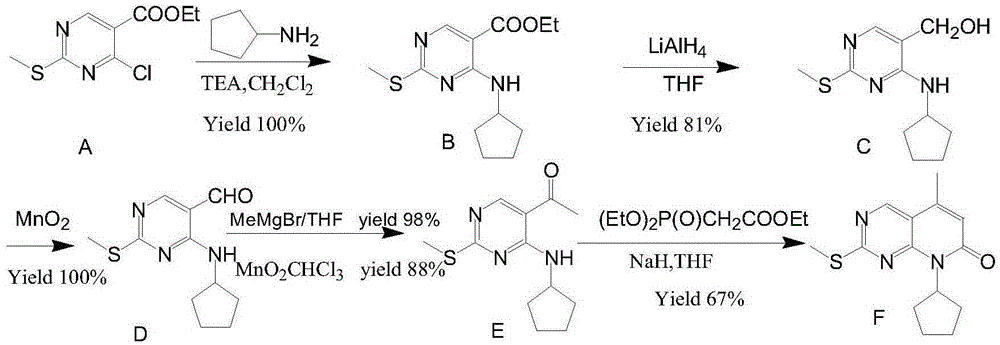
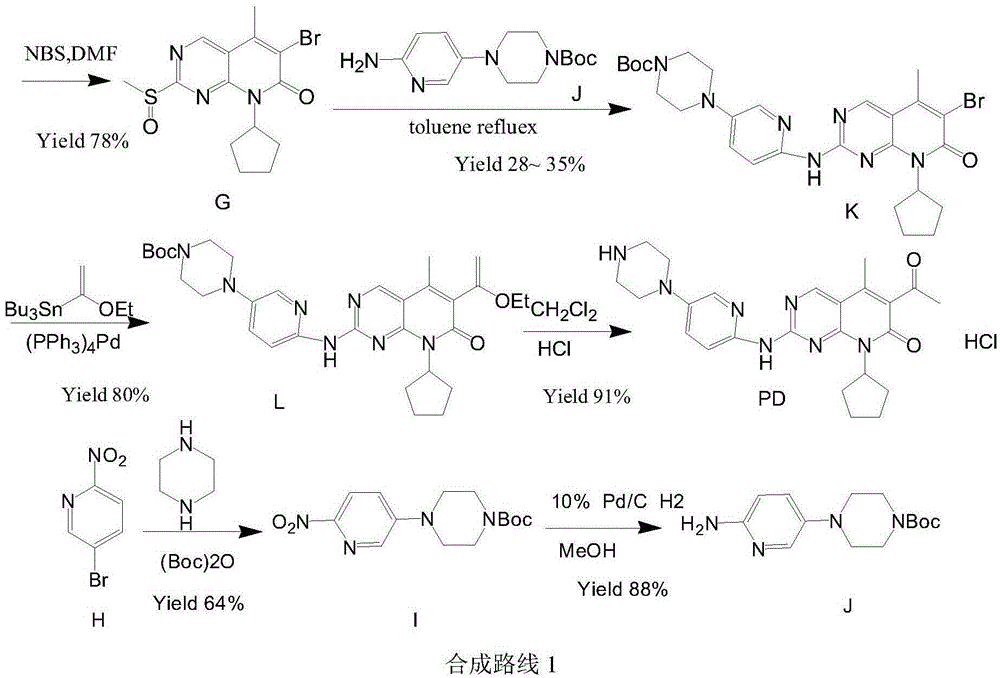
ActiveCN106608876ARaw materials are cheap and easy to getShort processOrganic chemistryBulk chemical productionAcetoacetatesKetone
The invention relates to a preparation method of high-purity palbociclib. The method comprises using acetoacetate for reflux dehydration in the function of an acid catalyst to prepare 2-acetyl-3-methyl-2-diethyl (methyl) glutaconate (III), condensing the compound (III) and trimethyl orthoformate for methanol removal to obtain 2-acetyl-3-methyl-4-methoxymethylene-2-diethyl (methyl) glutaconate (IV), cyclizing the compound (IV) and N-(5-(4-tertbutoxycarbonyl-1-hexahydropiperazinyl)-2-pyridyl) guanidine (V) with pyrimidine to obtain 2-acetyl-3-[[5-(4-tertbutoxycarbonylpiperazinyl-1-yl) pyridine-2-yl] amino]-3H-4-one-dihydropiperazinyl-2-ethyl (methyl) butenyl ester (VI), and finally cyclizing the compound (VI) and cyclopentylamine to remove Boc protecting groups, thereby obtaining palbociclib. The preparation method is cheap and available in raw materials, short in technical process, simple and convenient to operate, good in reaction selectivity, and high in product yield and purity and is green and eco-friendly.
Owner:XINFA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com