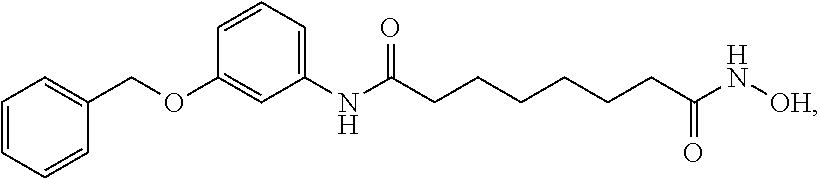
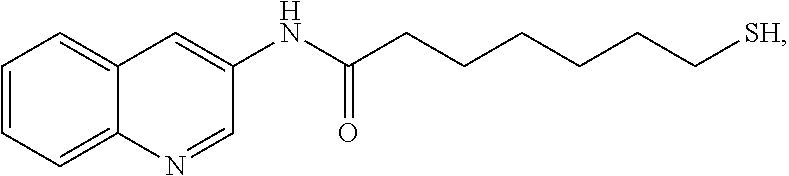
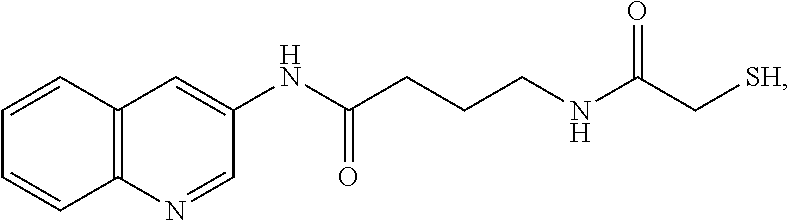
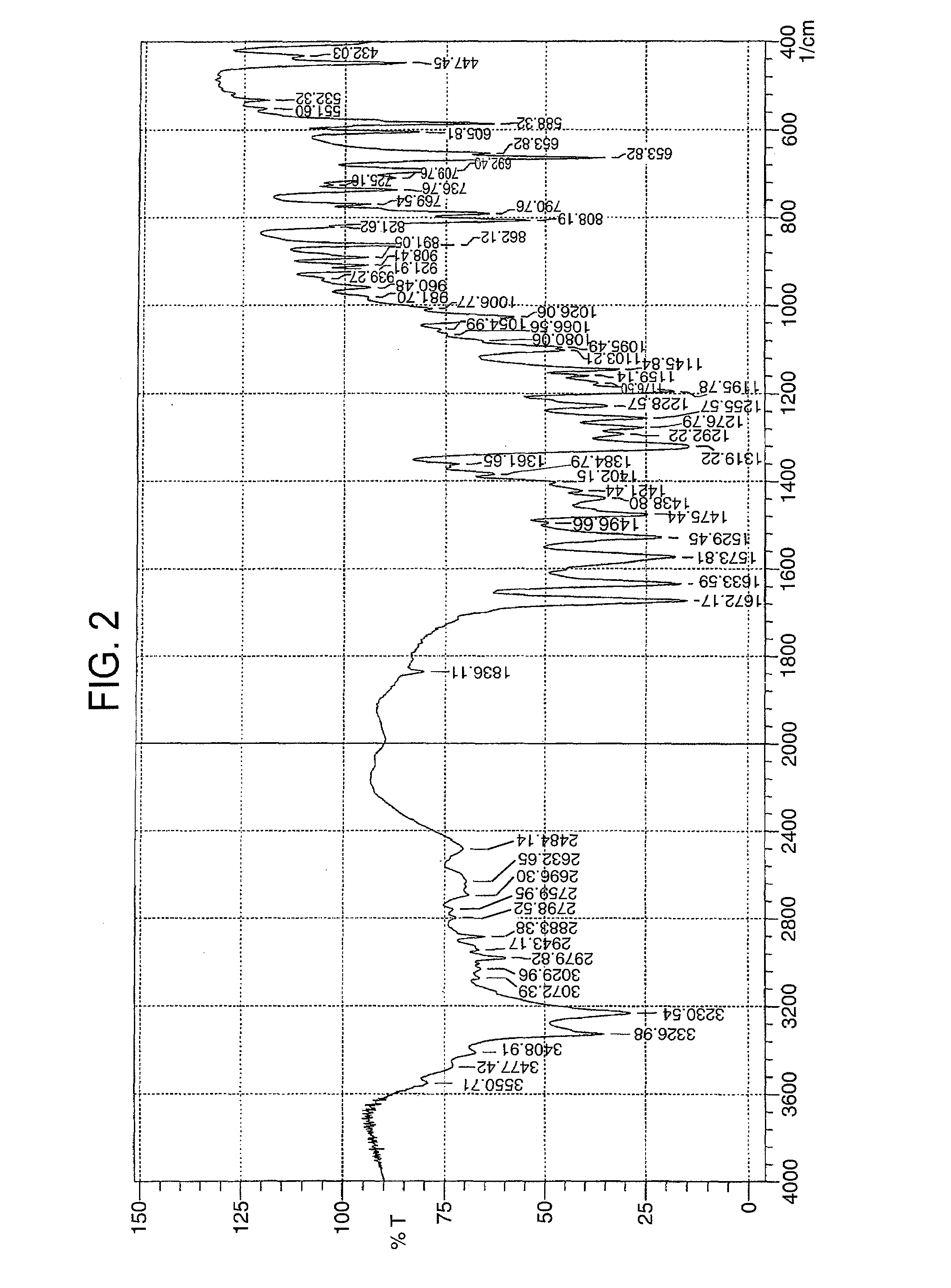
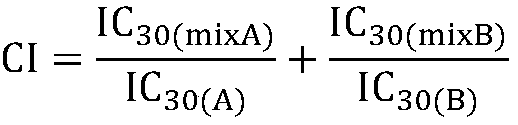Patents
Literature
108 results about "Sunitinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat certain types of cancer (kidney, pancreas, and intestinal). It is also used to treat people who are at high risk of the kidney cancer coming back again after having kidney surgery.
Methods for treating or preventing colorectal cancer
InactiveUS20110104256A1Organic active ingredientsPeptide/protein ingredientsFormyltetrahydrofolic AcidsSunitinib
The present invention provides, for example, methods for treating or preventing colorectal cancer with an anti-IGF1R antibody in association with sunitinib or a combination of leucovorin and 5-fluorouracil.
Owner:MERCK SHARP & DOHME CORP
Medicinal composition for treating non-small cell lung cancer and application thereof
InactiveCN103948689AReduce dosageLow toxicityAntineoplastic agentsHeavy metal compound active ingredientsSalvia miltiorrhizaCarboplatin
The invention discloses a medicinal composition for treating non-small cell lung cancer. The medicinal composition comprises a target medicament, a chemotherapeutic medicament and a traditional Chinese medicament, wherein the target medicament is one or more of bortezomib, imatinib, gefitinib and sunitinib; the chemotherapeutic medicament is one or more of 5-fluorouracil, carboplatin, epirubicin, adriamycin and fludarabine; and the traditional Chinese medicament is one or more of salvia miltiorrhiza, astragalus membranaceus, sappanwood, Chinese pulsatilla root and portulaca oleracea. The medicinal composition has a remarkable synergetic treatment effect when being used for treating the non-small cell lung cancer, and can be used for remarkably strengthening the cancer-inhibition effect compared with treatment of a single medicament, so that the medicament dosage can be reduced, and the toxic and side effects of chemotherapeutic medicaments can be reduced.
Owner:NORTHWEST A & F UNIV
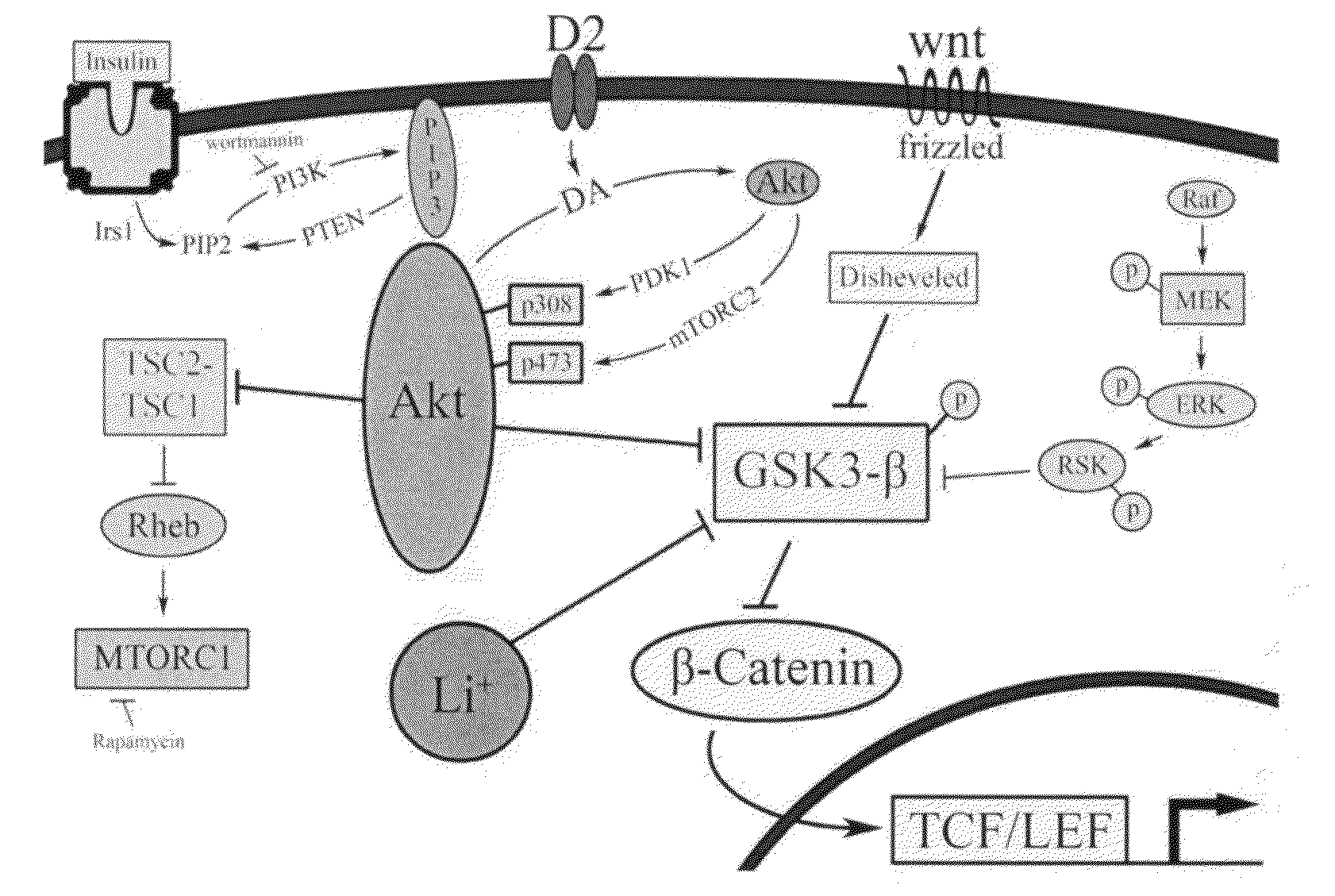
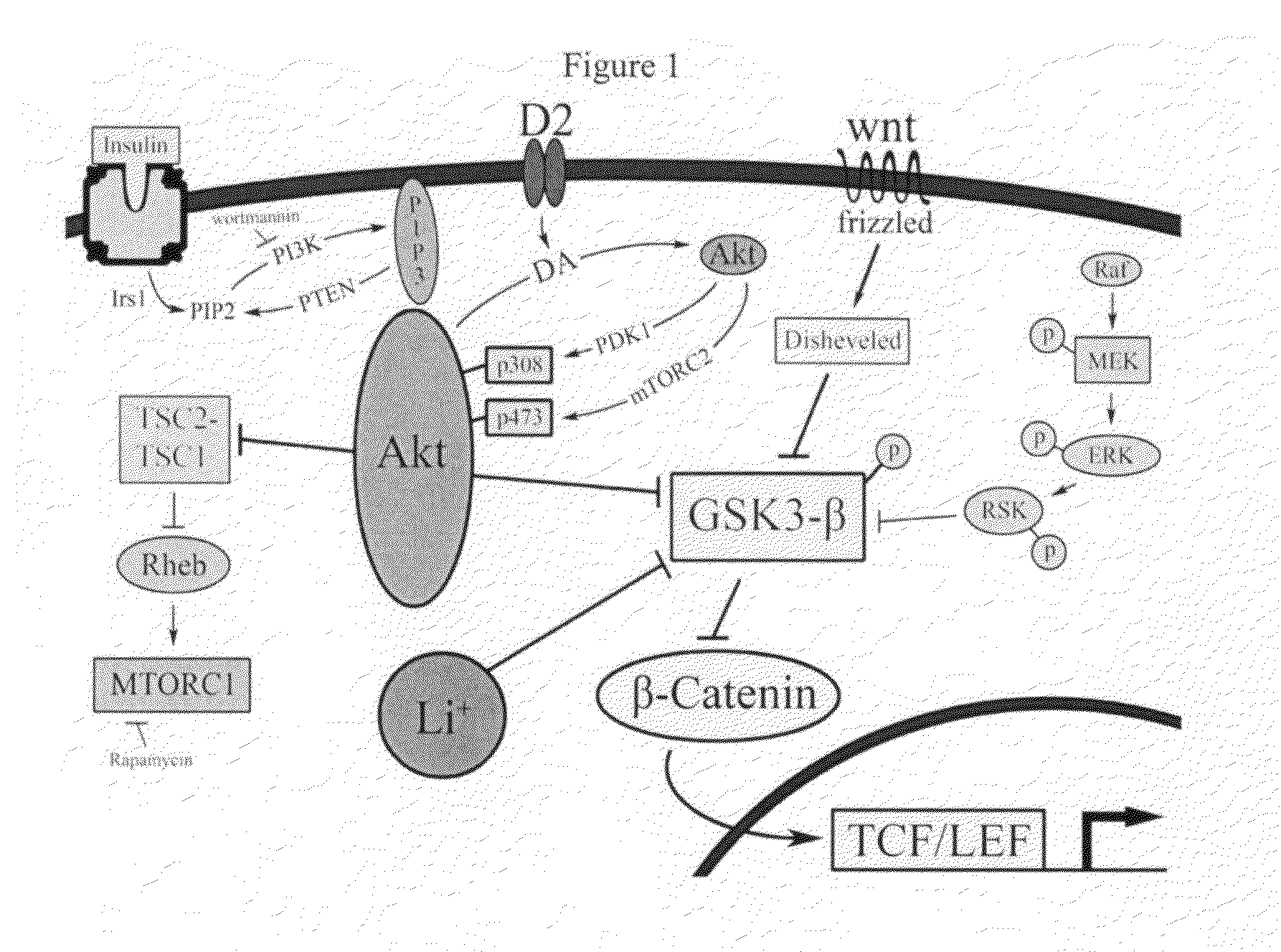
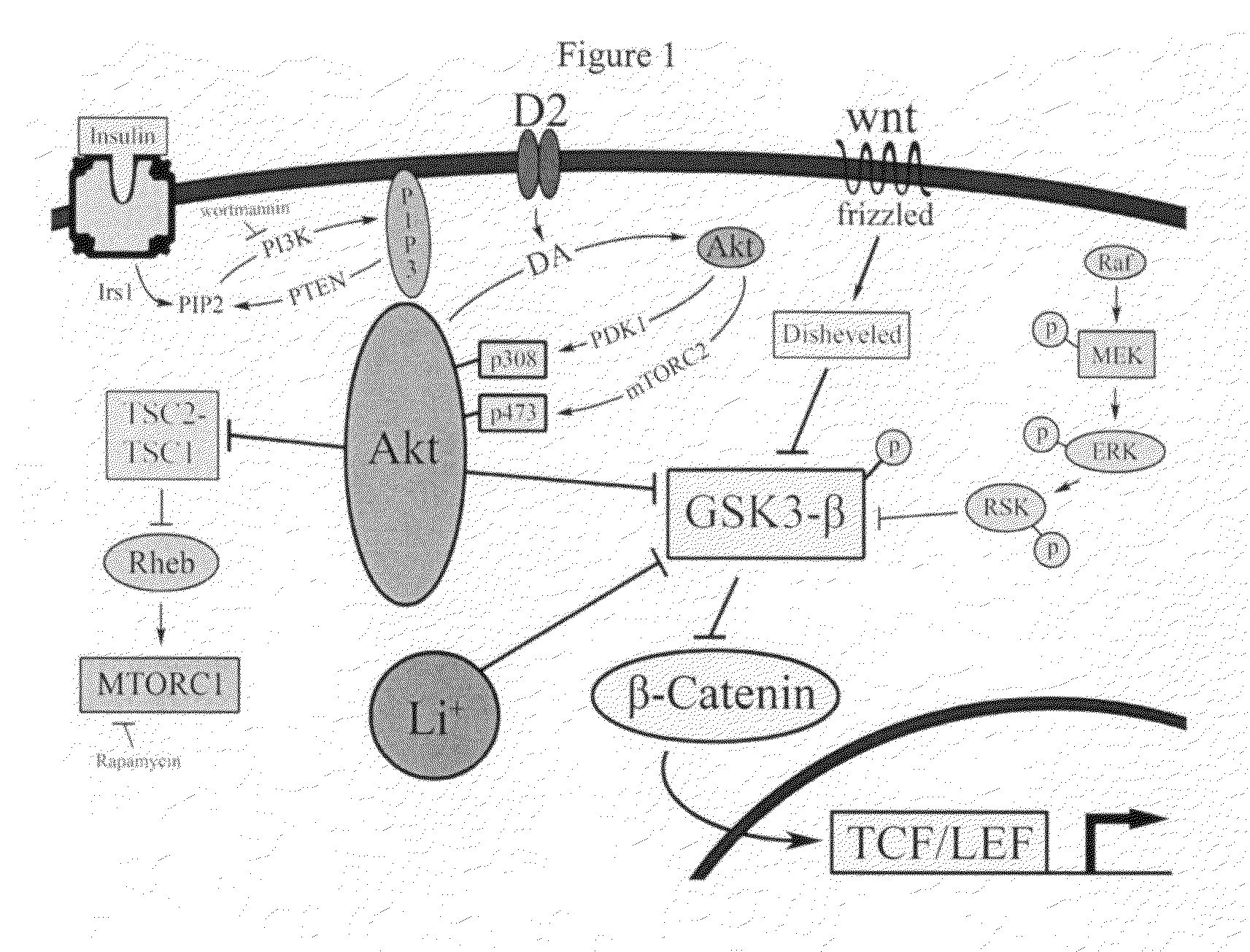
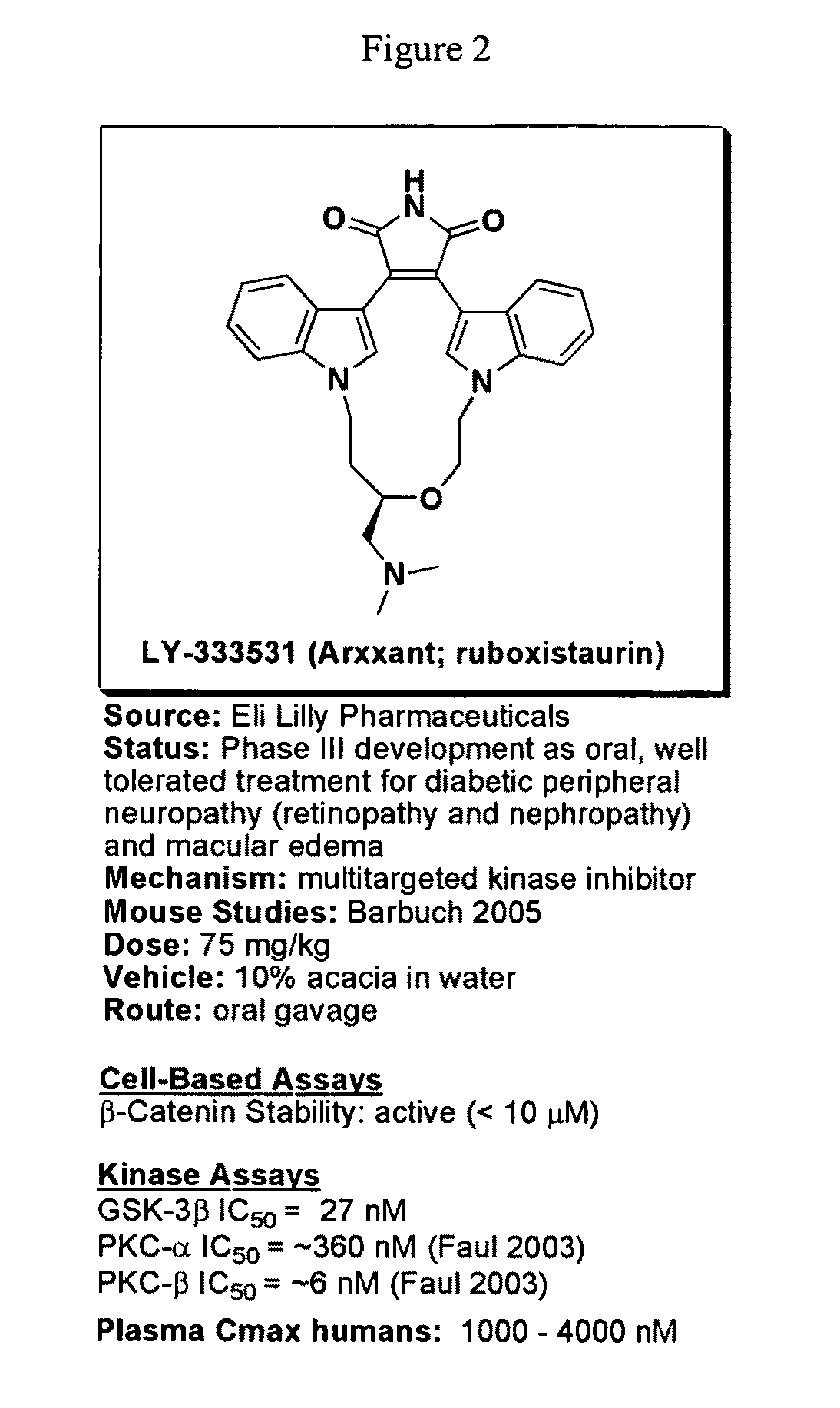
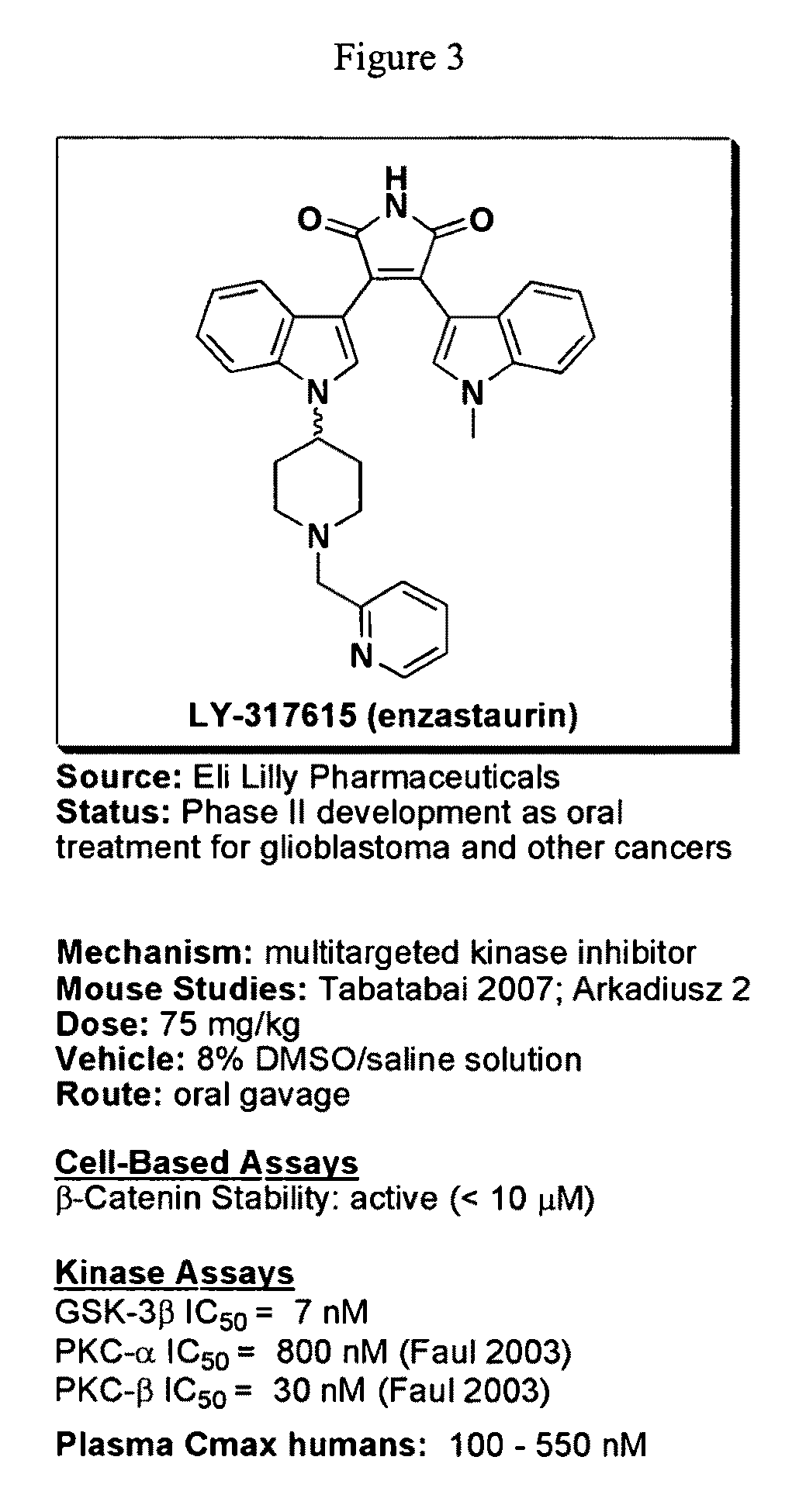
Uses of chemicals to modulate GSK-3 signaling for treatment of bipolar disorder and other brain disorders
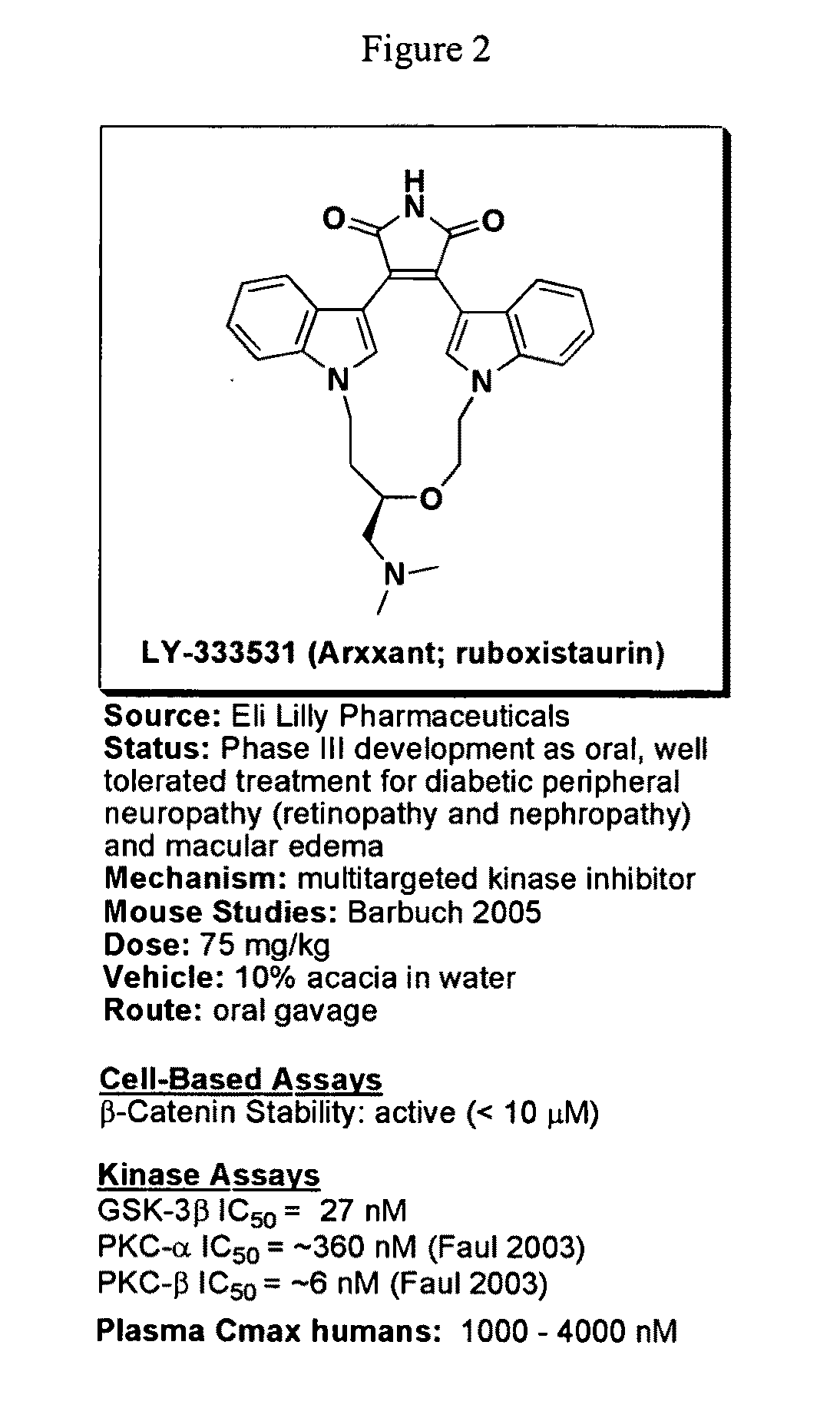
Aspects of this invention are related, at least in part, to the use of chemical compounds able to inhibit GSK-3 and / or to stabilize β-catenin and formulations thereof. Some aspects of this invention relate to compositions comprising such compounds. Some aspects of the invention provide methods of using such compounds and / or compositions in the treatment of subjects having a neurological disease and / or psychiatric disorder. Some aspects of this invention provide methods of using ruboxistaurin, enzastaurin, sunitinib, midostaurin, lestaurtinib, 7-hydroxystaurosporine, and / or Chir99021 in the treatment of subjects having a neurological disease and / or psychiatric disorder. In some embodiments, compounds are administered in combination with Lithium.
Owner:MASSACHUSETTS INST OF TECH +1
Method for synthesizing sunitinib alkali
InactiveCN101333215AAvoid hard to getAvoid the disadvantages of using excess aminesOrganic chemistryAntineoplastic agentsEthylenediamine5-fluoroindole
Owner:NANJING UNIV OF TECH
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
Methods for treating sunitinib-resistant carcinoma and related biomarkers
InactiveUS20090285832A1Stimulate endothelial cell proliferationBiocideMicrobiological testing/measurementAbnormal tissue growthSunitinib
A method of determining whether a tumor will be non-responsive to sunitinib therapy including detecting whether IL-8 or MMP12 expression levels in the tumor are elevated as compared to a control, that is, a tumor having elevated IL-8 or MMP12 levels will be non-responsive to sunitinib. Also, a method for inhibiting the proliferation or causing the death of a sunitinib-resistant tumor cell by contacting the cell with an agent that inhibits IL-8 or MMP12 activity and with sunitinib, whereby proliferation of the tumor cell is inhibited or the tumor cell dies. Further, the invention includes compositions and kits that include an IL-8 inhibitor or an MMP12 inhibitor and sunitinib, and related methods of treatment.
Owner:VAN ANDEL RES INST
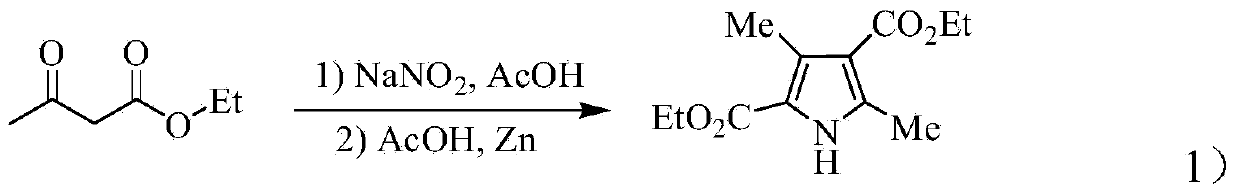
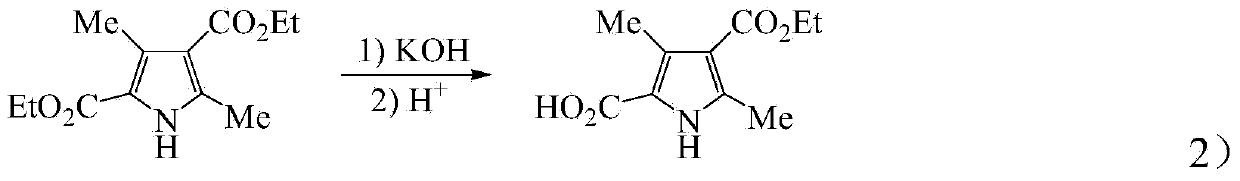
Process for synthesizing sunitinib
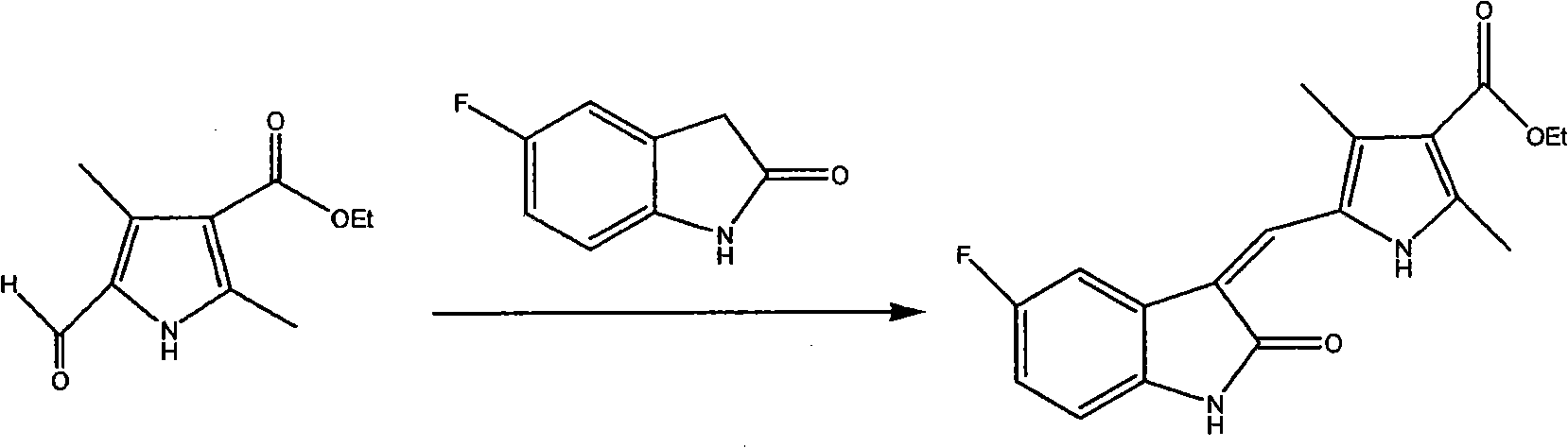
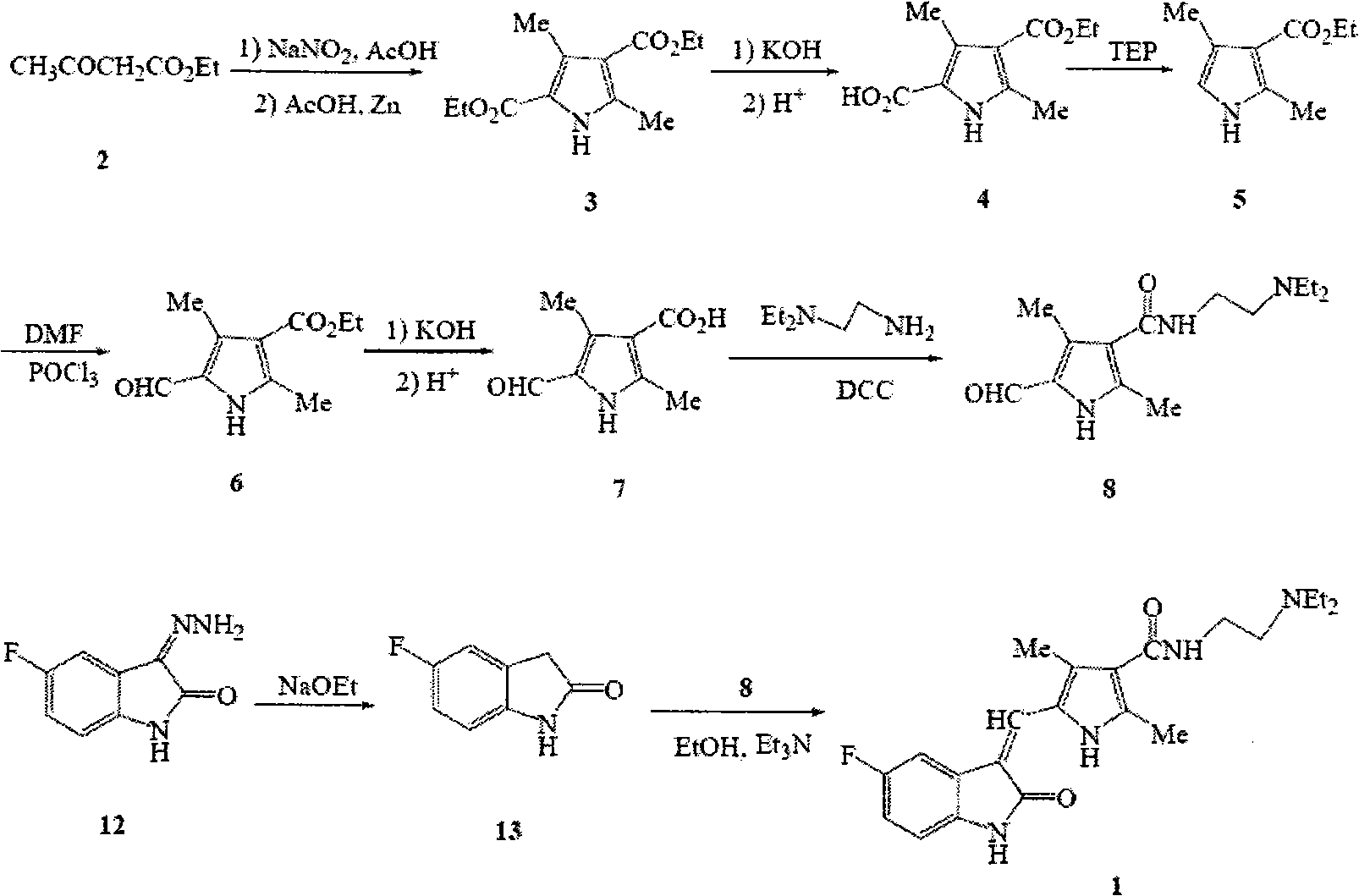
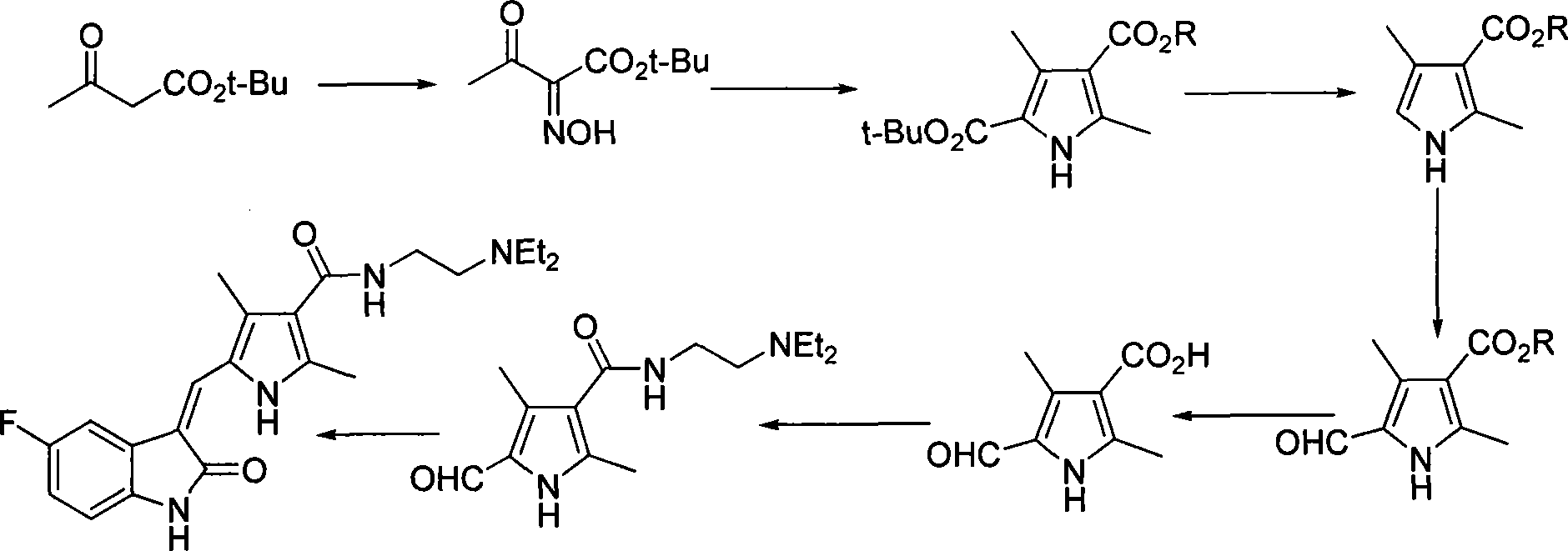
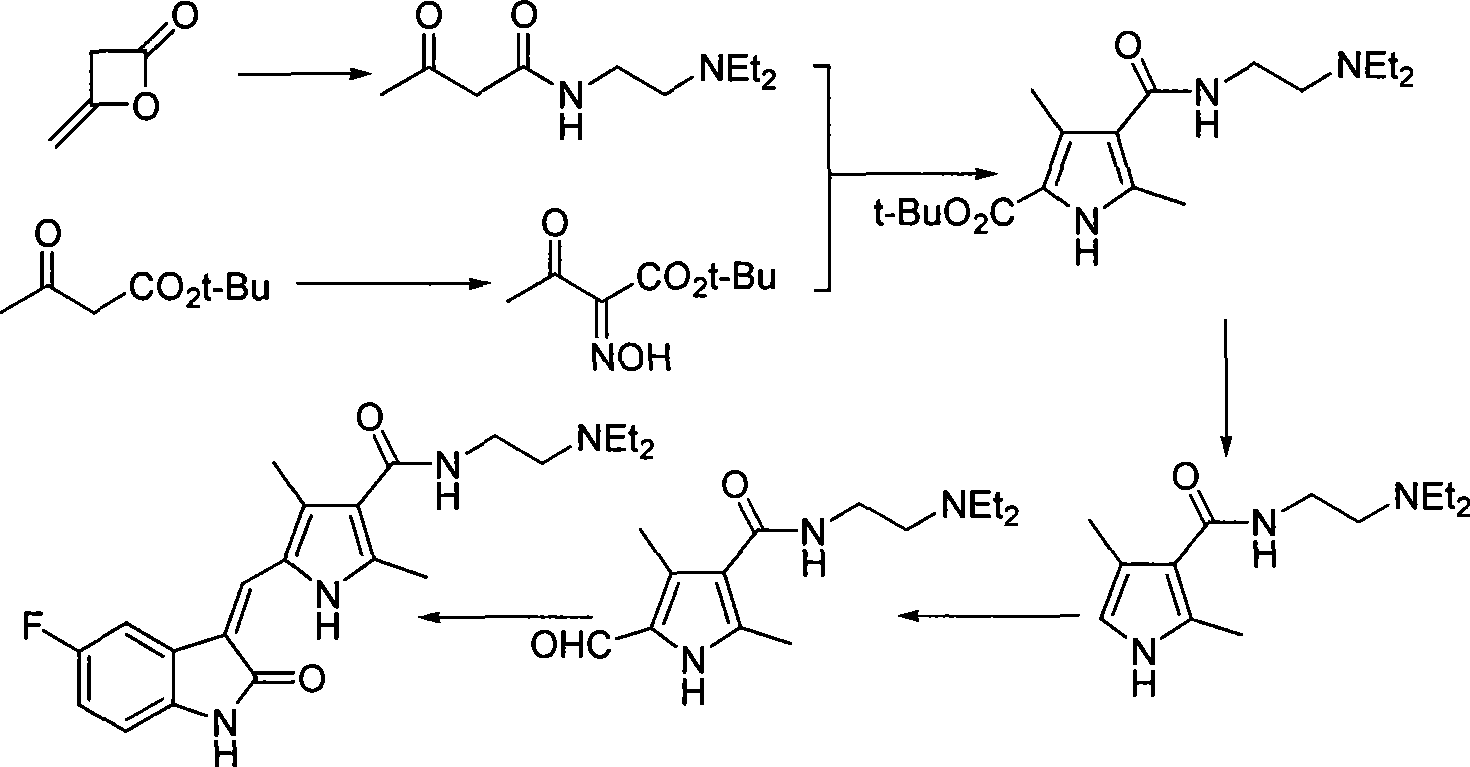
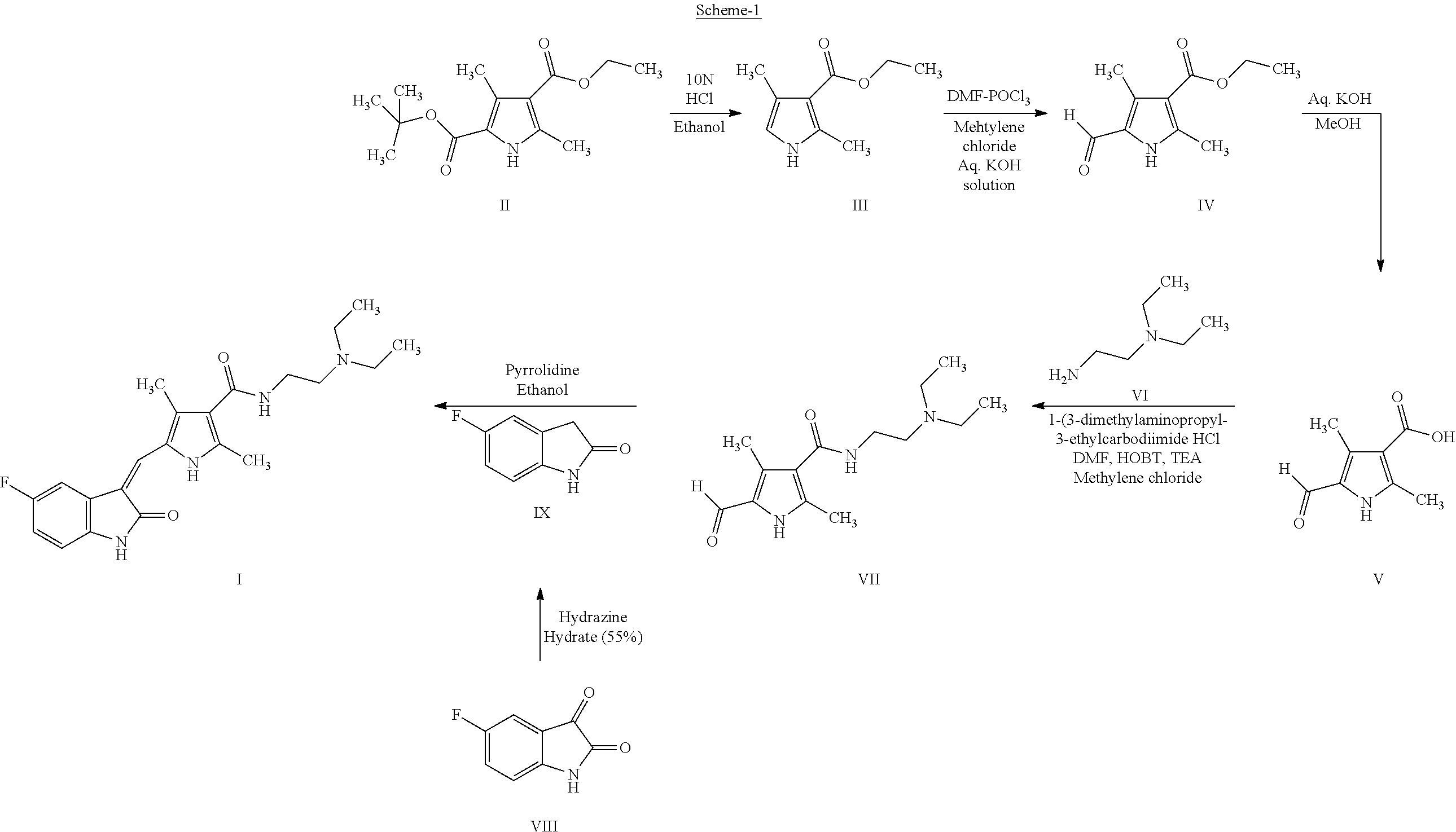
The invention discloses a method for synthesizing sunitinib, which comprises the following steps that: tert-butyl acetoacetate and acetylacetic ester are taken as initial raw materials, and tetra-substituted pyrrole is obtained through a nitrosification reduction reaction; then 2,4-dimethyl pyrrole-3-formic acid is obtained through the hydrolysis and is amidated; then an aldehyde group is utilized on position 5 of pyrrole through a Vilsmeier-Hacck reaction; and finally the obtained product and 5-fluorooxindole react to obtain the sunitinib. The method has low cost and simple operation, and is favorable for industrial production.
Owner:FUJIAN SOUTH PHARMA CO LTD
Processes for preparing sunitinib and salts thereof
Methods for preparing sunitinib or salts thereof are described using novel intermediates and chemical pathways. One such intermediate is:
Owner:TEVA PHARM USA INC
Renal cancer sunitinib drug-resistant cell system and establishing method thereof
InactiveCN104988122AHigh scientific researchIncrease production capacityMicrobiological testing/measurementMicroorganism based processesCancer cellKidney cancer
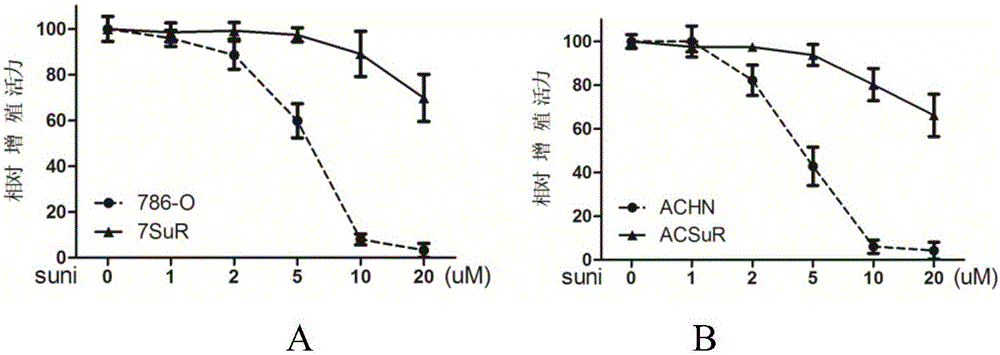
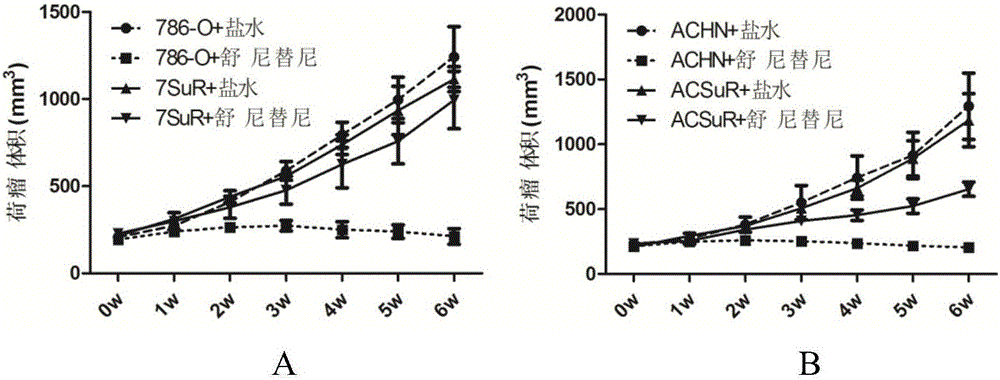
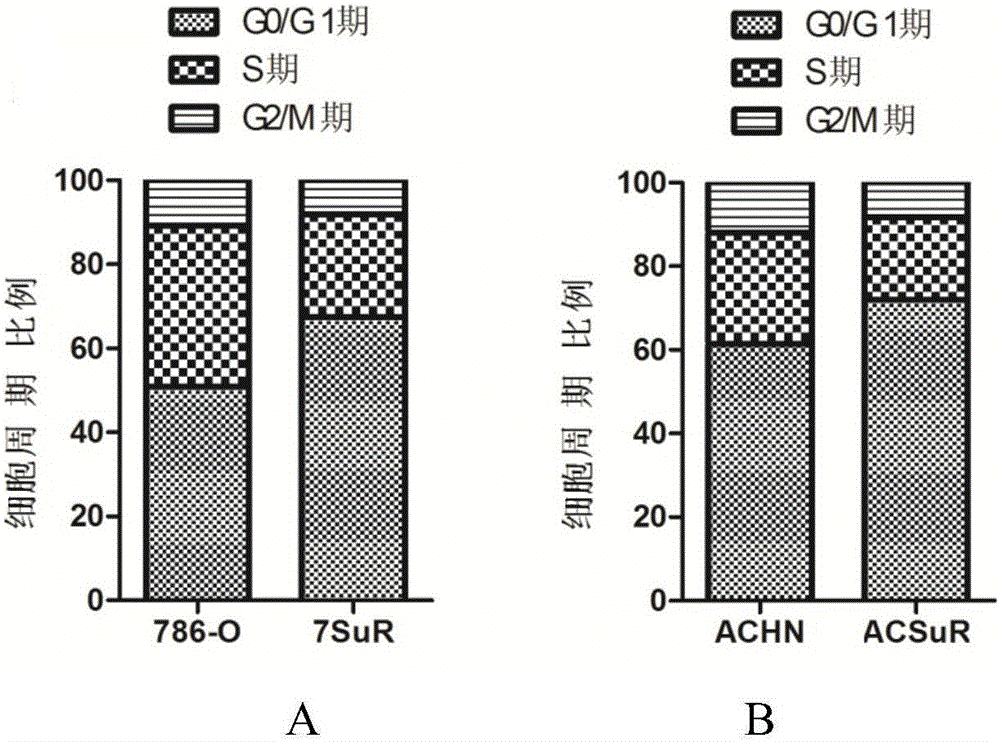
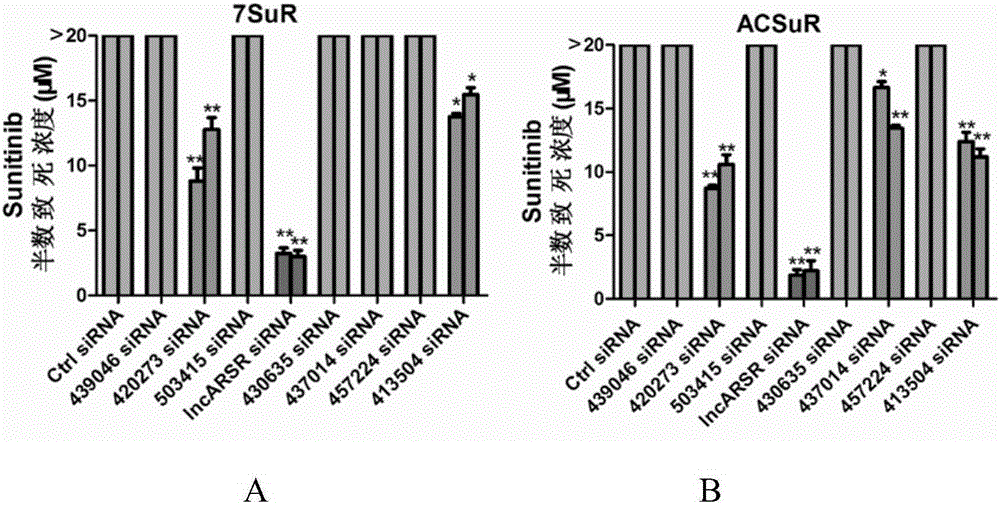
The invention relates to the technical field of biomedicine, and in particular to a renal cancer sunitinib drug-resistant cell system and an establishing method and application of the renal cancer sunitinib drug-resistant cell system. 786-O cells and ACHN cells of a human renal clear cell carcinoma system are adopted as objects for establishing a subcutaneous tumor-bearing nude mouse model; the clinical conventional dose of the sunitinib is used for treating a tumor-bearing nude mouse; and through multiple times of in-vivo continuous passage and drug pressure screening, the renal cancer drug-resistant cell system free of restraints of the sunitinib in vitro and vivo is finally obtained through separate culture and named as 7SuR and ACSuR. The established renal cancer sunitinib drug-resistant cell system can better simulate clinical drug resistance and provides an important platform for studying renal cancer targeted drug resistance mechanism, reversing renal cancer cell drug resistance and developing and evaluating new anti-cancer drugs.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
7-azaindolinyl-2-one compounds and preparation method thereof
ActiveCN104876928AImprove securitySmall toxicityOrganic active ingredientsOrganic chemistrySunitinibMedicinal chemistry
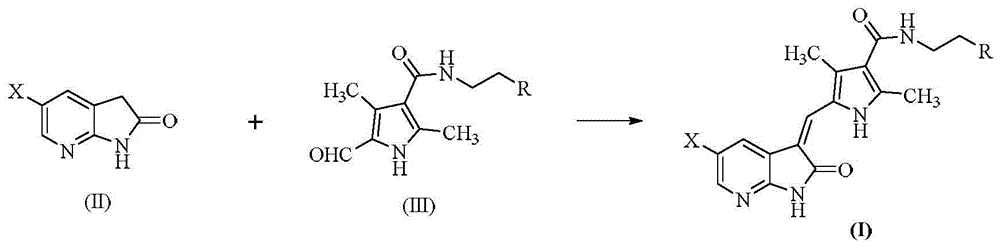
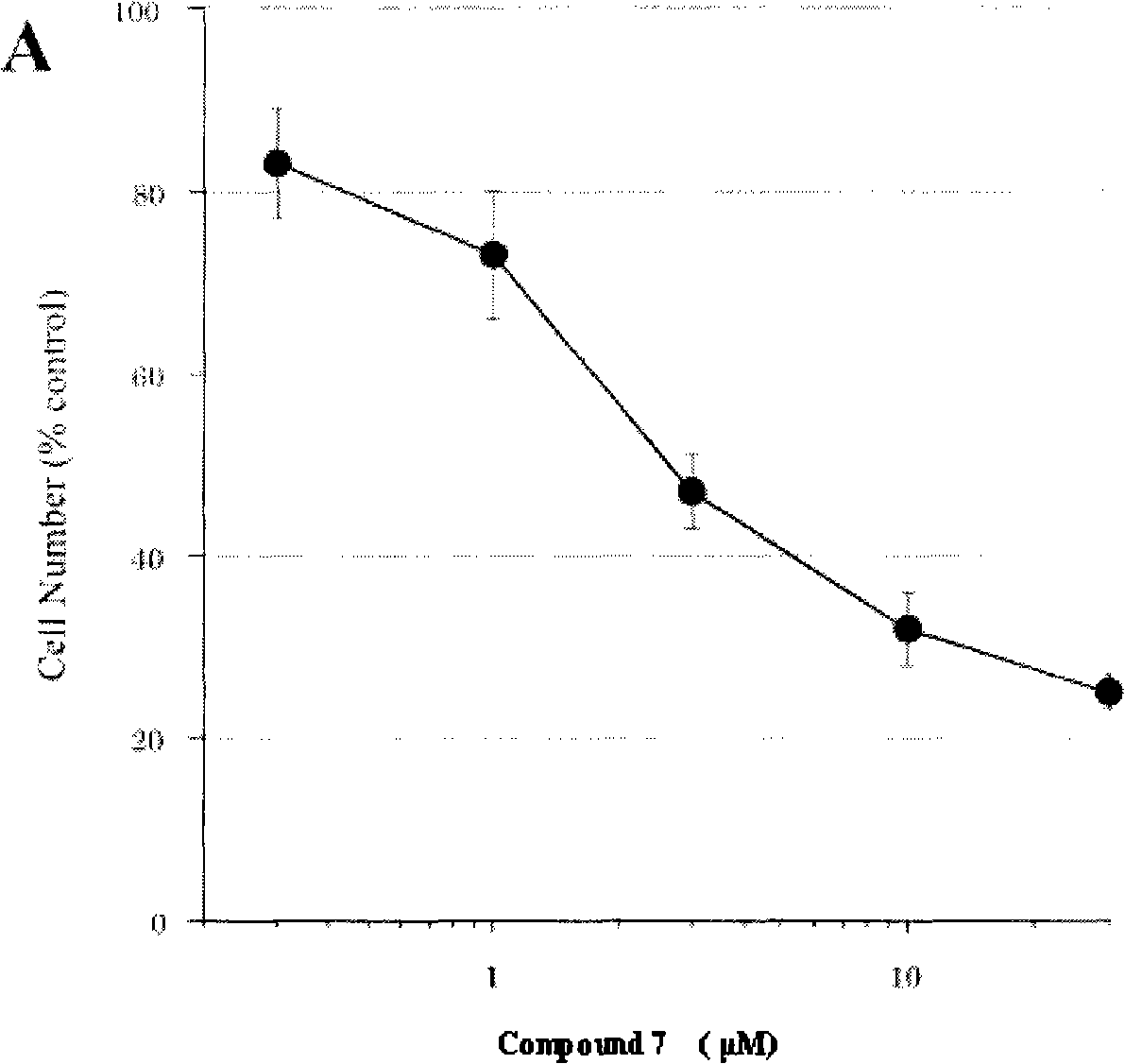
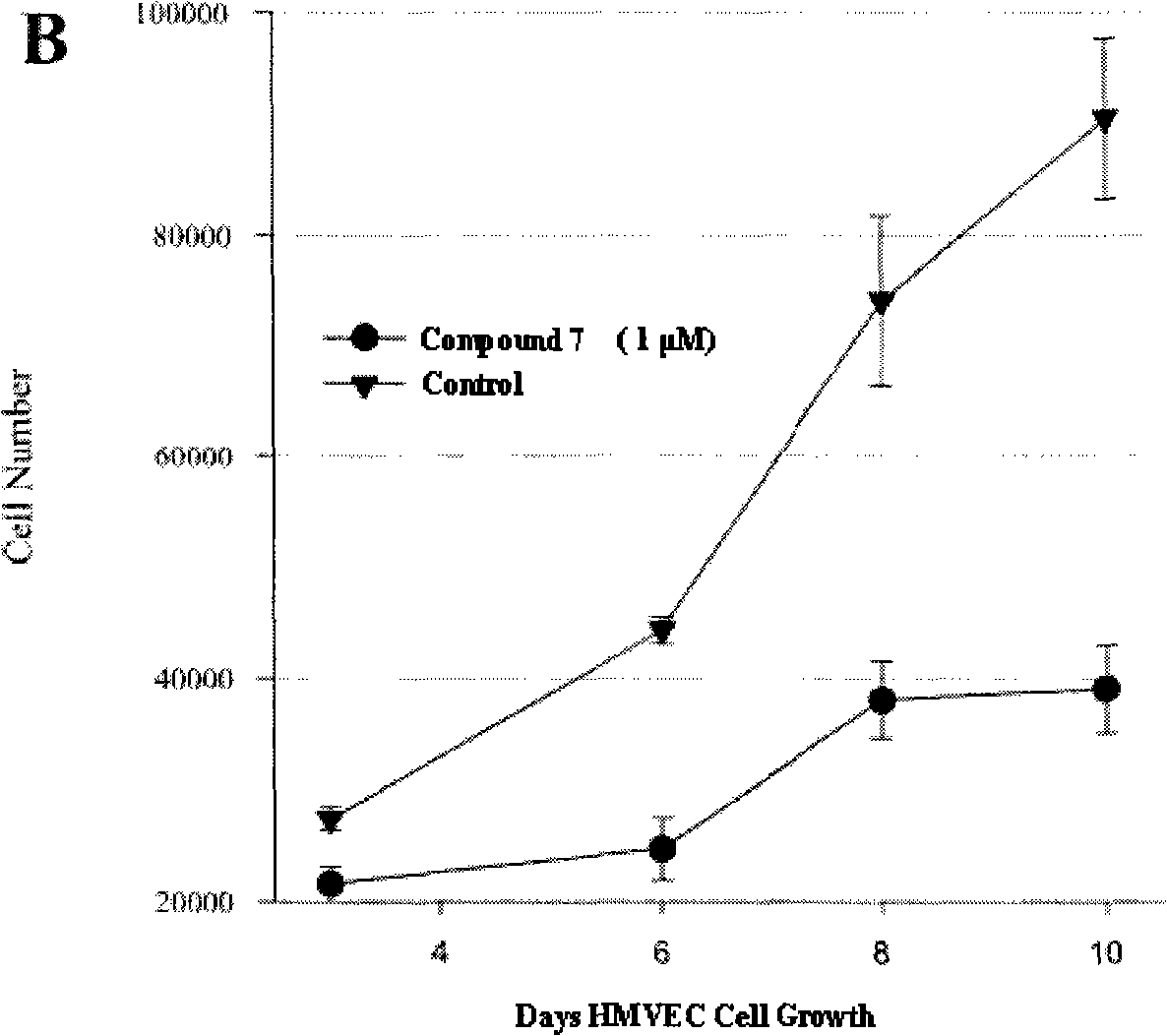
The invention relates to 7-azaindolinyl-2-one compounds disclosed as Formula, a preparation method and medical application thereof, and an antineoplastic drug composition using the compounds as effective components. In the 7-azaindolinyl-2-one compounds, the 5- substituent group at the parent nucleus is halogen, and the 3- substituent group is 2,4-dimethylpyrryl-5-ethylene containing various amino formacyl groups. Compared with the similar antineoplastic drug-sunitinib, the 7-azaindolinyl-2-one compounds have higher antineoplastic activity.
Owner:ZHEJIANG STARRY PHARMA +1
Medicine composition containing tyrosine kinase restraining agent
InactiveCN101081207APharmaceutical delivery mechanismPharmaceutical non-active ingredientsDocetaxelWhole body
The anticancer medicine composition containing tyrosine kinase inhibitor is slow released injection and slow released implant. The effective anticancer components include tyrosine kinase inhibitor selected from Erbitux, Iressa, Tarceva, Sunitinib, Trastuzumab, etc, and / or composition selected from Docetaxel, deacetyl taxol, taxol, etc. The slow releasing supplementary material is selected from p(LAEG-EOP), p(DAPG-EOP), etc. The released injection and slow released implant may be injected or set in tumor for slow releasing to maintain effective medicine concentration for over 50 days, and has obviously lowered systemic reaction on the medicine and capacity of enhancing the chemotherapeutic and radiotherapeutic effect.
Owner:JINAN KANGQUAN PHARMA TECH
Substituted 2-indoline ketone derivatives as well as preparation method and uses thereof
ActiveCN101255154AImprove solubilityHigh anticancer activityOrganic active ingredientsSugar derivativesSolubilityKetone
The invention relates to a substituted 2-indolinone derivate, a preparation method and the applications thereof in antineoplastic. The substituted 2-indolinone derivate of the invention has a chemical general structure shown in the drawing, wherein X represents one of halogen and hydrogen, or nitro and alkoxyl; R1 and R2 are any one selected from hydrogen, alkyl, cycloalkyl, aryl and heteroary respectively; A represents any one of glycoside and isomer thereof, hydroxy and alkyl, and hydroxy and alkoxyl group. The substituted substituted 2-indolinone derivate of the invention generates nucleoside analogue of sunitinib by saccharification reaction or alkylation reaction of 2-indolinone, greatly increases the solubility, the antitumor activity and the virus resistance.
Owner:长治市三宝生化药业有限公司
Kit for detecting lncARSR in serum and application of kit in detection of kidney cancer sunitinib drug resistance
InactiveCN105256036AAvoid ineffective treatmentGood prospects for translational medicineMicrobiological testing/measurementSerum igeKidney cancer
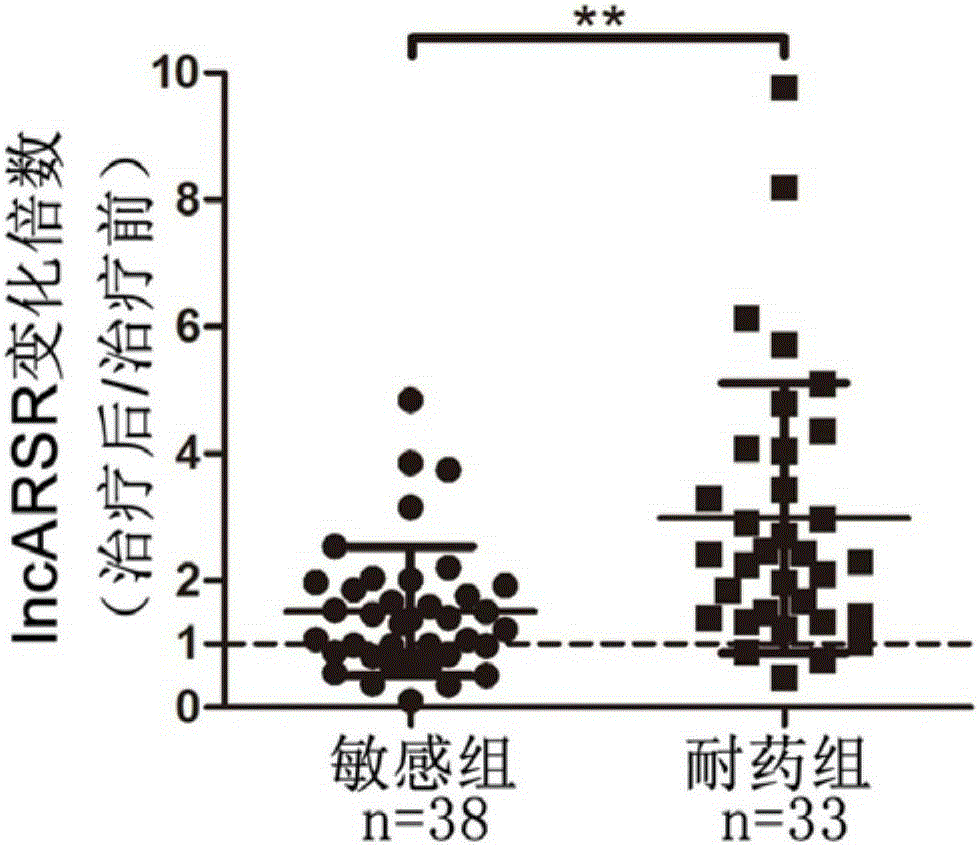
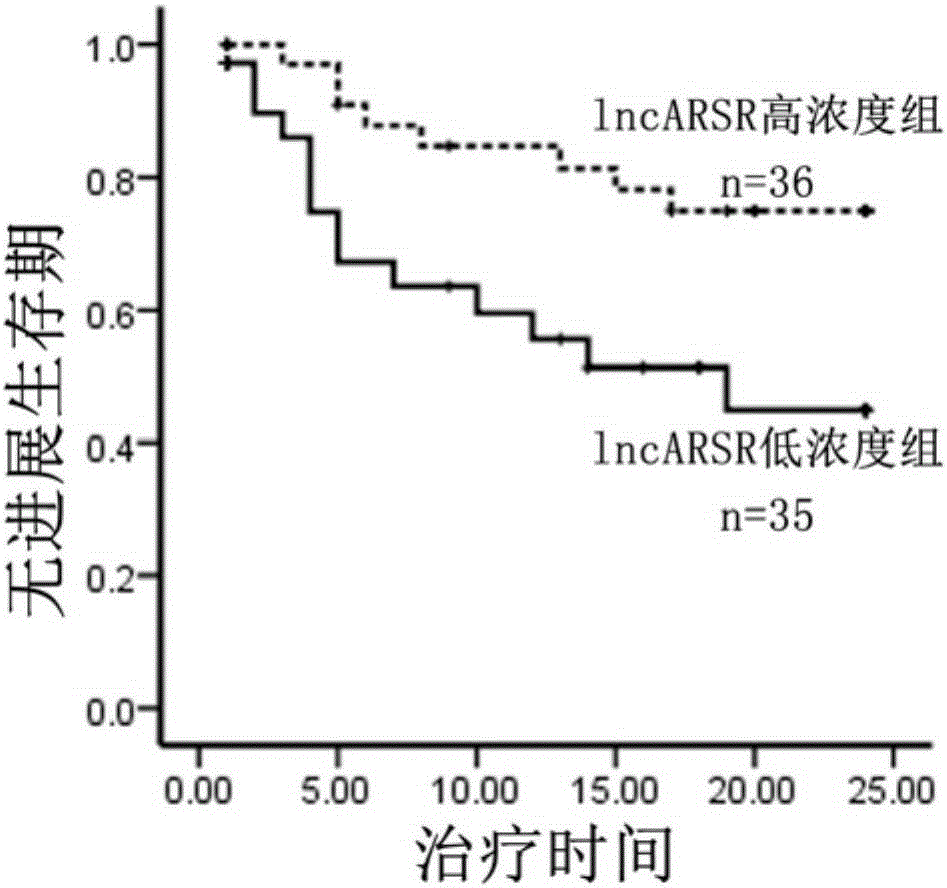
The invention relates to the technical field of medical and biological detection, and provides application of lncARSR in preparation of a kidney cancer sunitinib drug resistance detecting marker, and application of lncARSR in preparation of a kidney cancer sunitinib drug resistance detecting reagent or kit. The invention further establishes a method for detecting lncARSR in human serum, and provides the kit for detecting lncARSR in serum. By detecting serum of kidney cancer patients receiving sunitinib treatment, it is found that the content of lncARSR in serum of the drug resistance patient is obviously higher than that of lncARSR in serum of the sensitive patient. The kit is beneficial for monitoring the clinical curative effect of sunitinib medicine, thereby avoiding further invalid treatment.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Process For Preparing A 3-Pyrrole Substituted 2-Indolinone Malate Salt
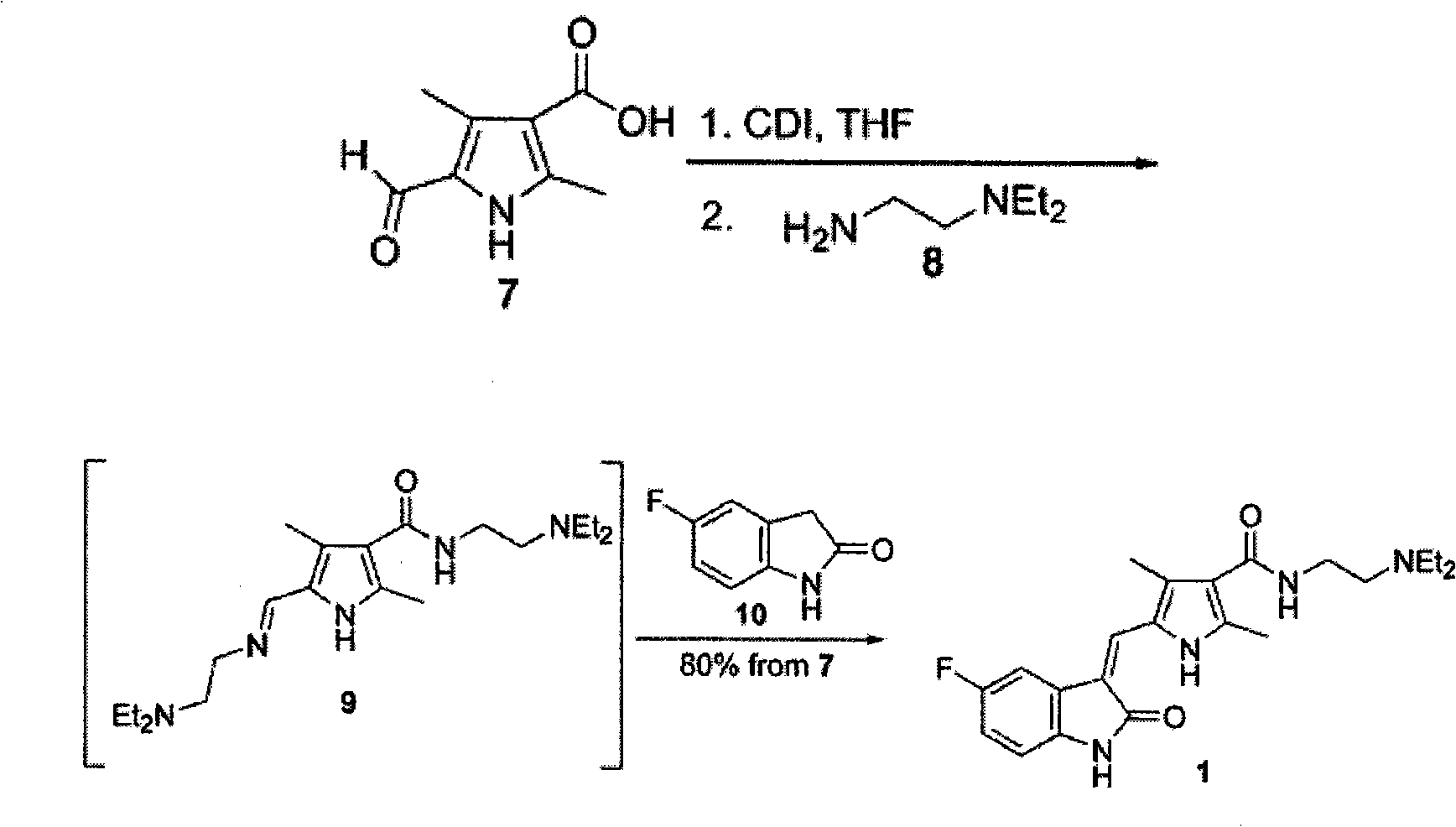
The invention relates to the malic acid salt of N-[2-(diethylamino)ethyl]-5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxamide, to the use thereof as an intermediate for preparing the malic acid salt of sunitinib, and to pharmaceutical compositions comprising said malic acid salt of sunitinib.
Owner:MEDICHEM
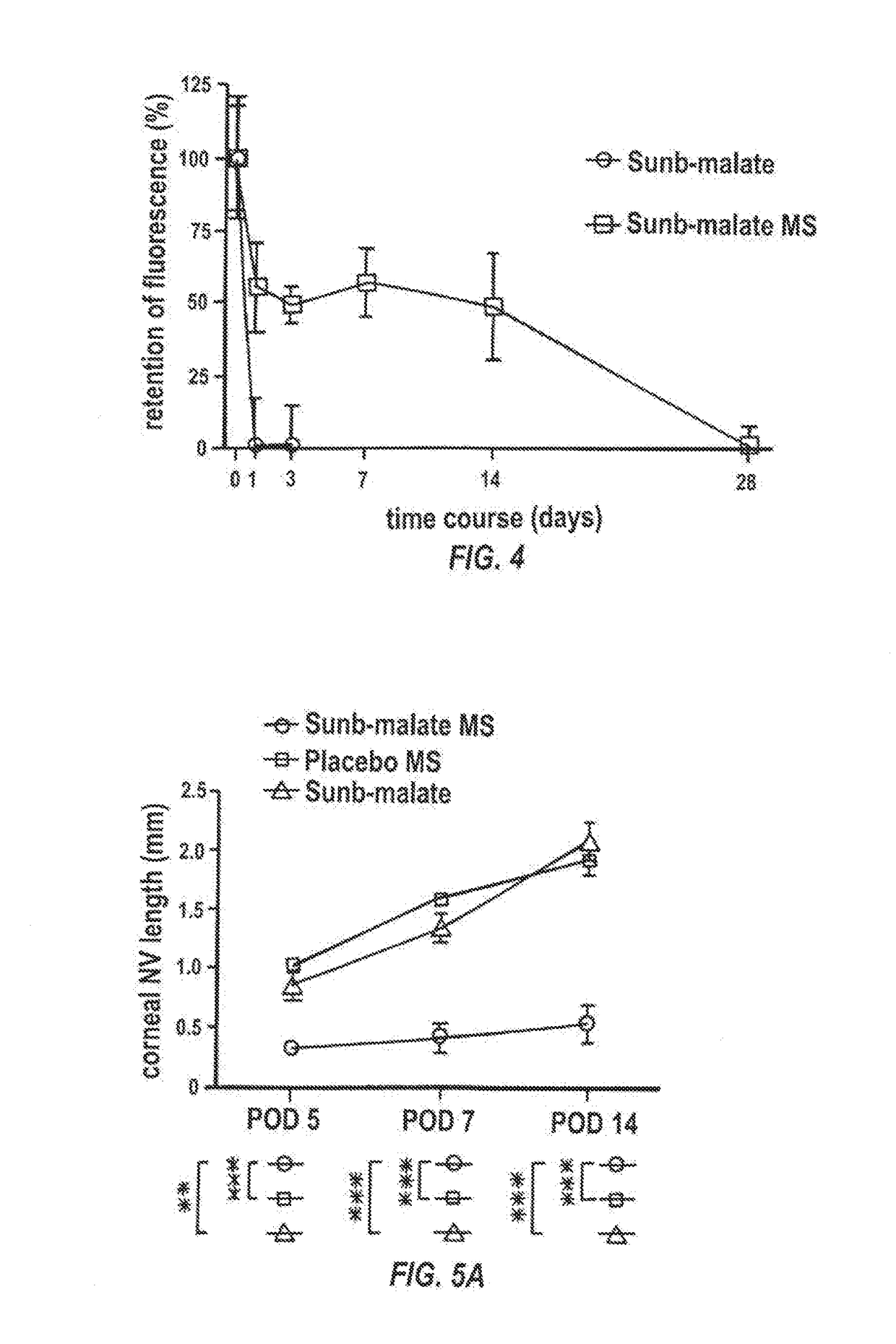
Sunitinib formulations and methods for use thereof in treatment of ocular disorders
InactiveUS20170273901A1Avoid nerve damageIncreasing encapsulation and incorporationOrganic active ingredientsSenses disorderDiseaseCytotoxicity
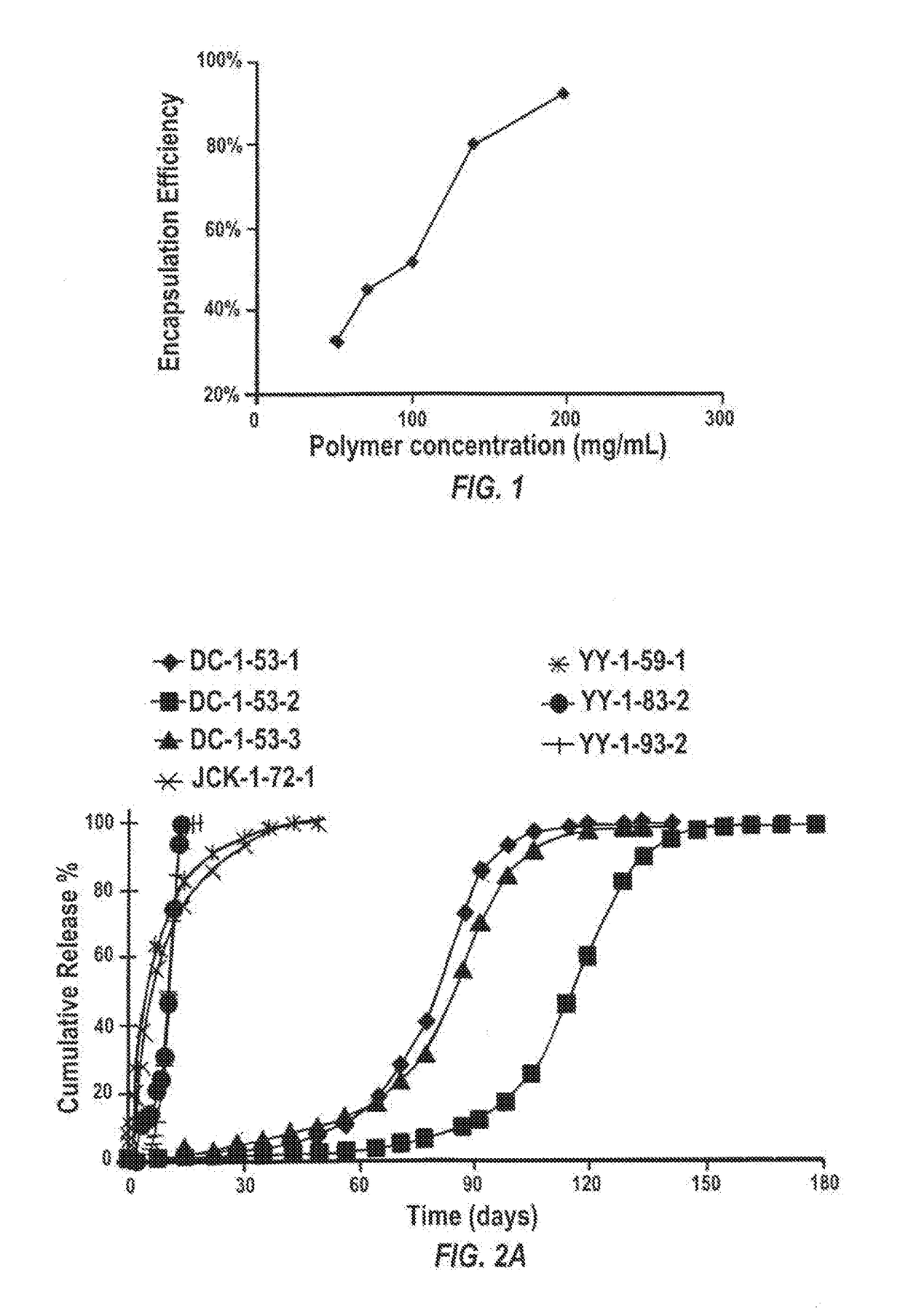
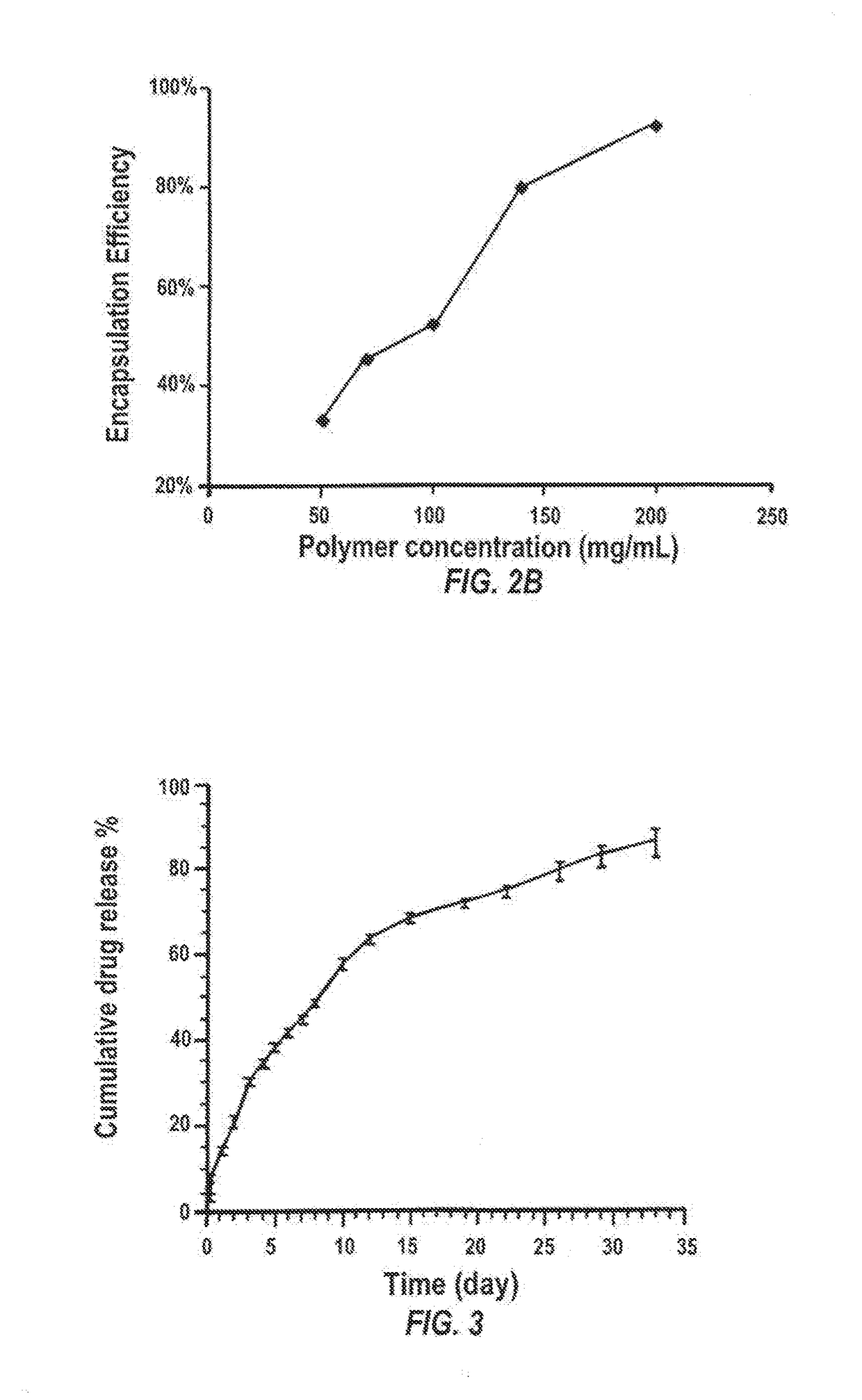
Methods for increasing the encapsulation or incorporation of Sunitinib into polymeric matrices have been developed. The resulting formulations provide for more sustained controlled release of sunitinib or its analog or a pharmaceutically acceptable salt thereof. Increased loading is achieved using an alkaline solvent system. The pharmaceutical compositions can be administered to treat or prevent a disease or disorder in or on the eye of a patient associated with vascularization, such as corneal neovascularization and acute macular degeneration. Upon administration, the sunitinib or its analog or salt is released over an extended period of time at concentrations which are high enough to produce therapeutic benefit, but low enough to avoid unacceptable levels of cytotoxicity.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Pharmaceutical combinations
Pharmaceutical combinations comprising an α5β1 antagonist in combination with a tyrosine kinase inhibitor. In some embodiments, the α5β1 antagonist is volociximab. In some embodiments, the tyrosine kinase inhibitor is sunitinib or a pharmaceutically acceptable salt thereof. The invention also relates to methods for treating cancer by administering the pharmaceutical combinations to a subject.
Owner:FACET BIOTECH CORP
Composition containing protein kinase inhibitor and resveratrol
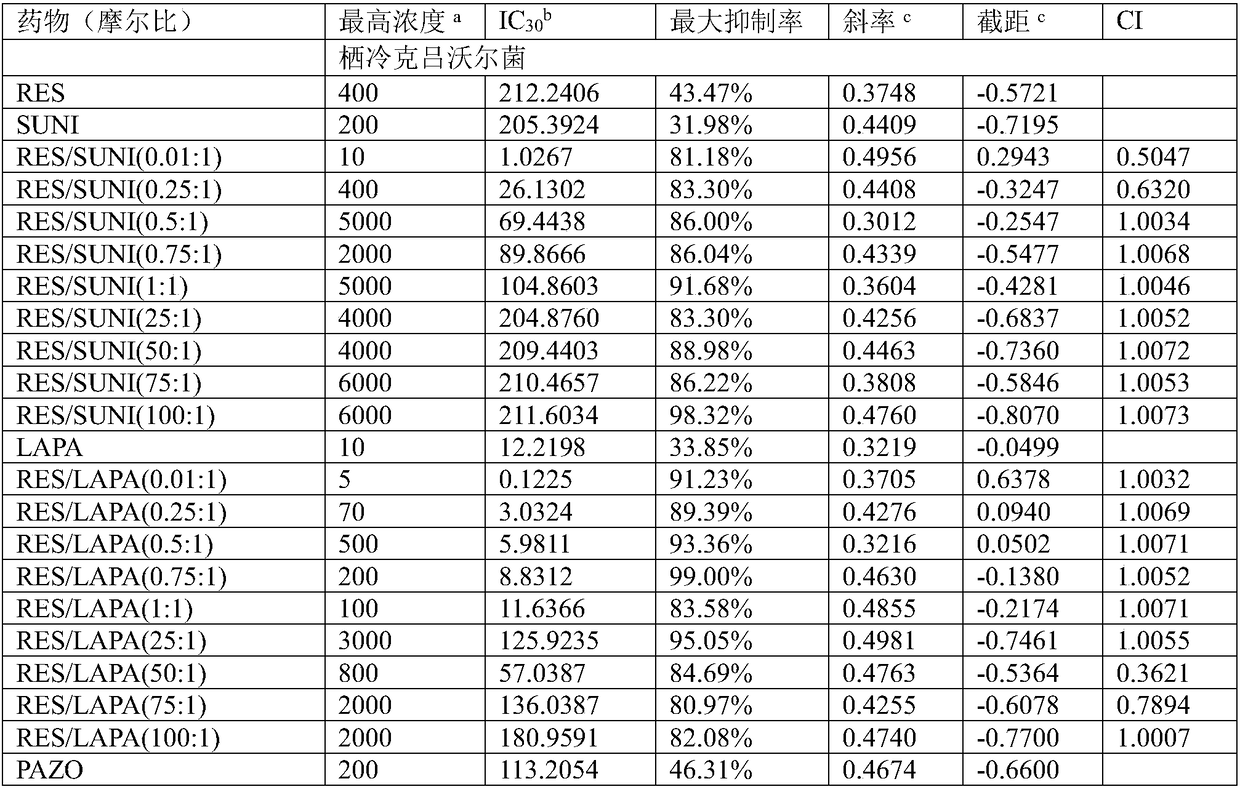
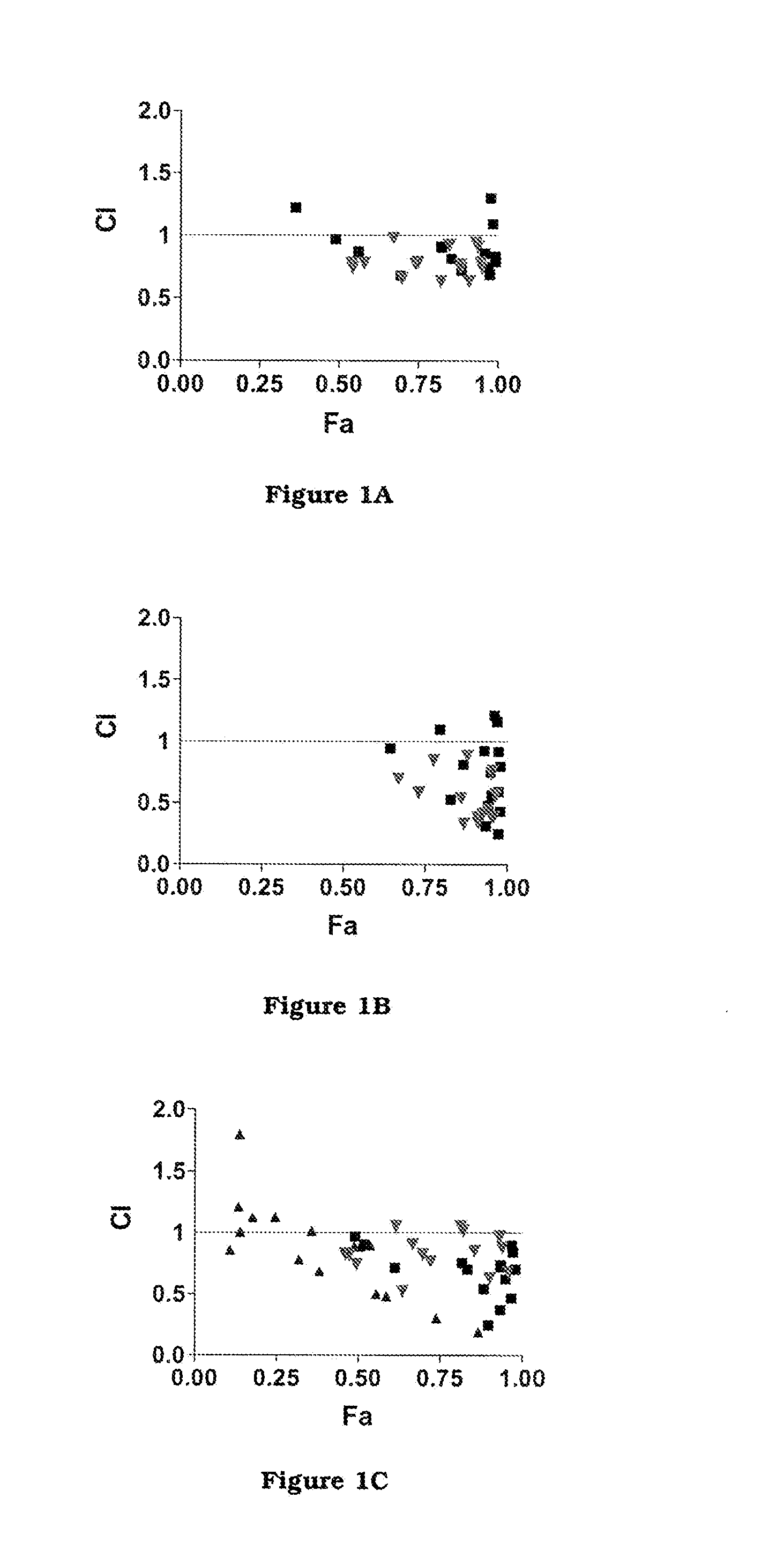
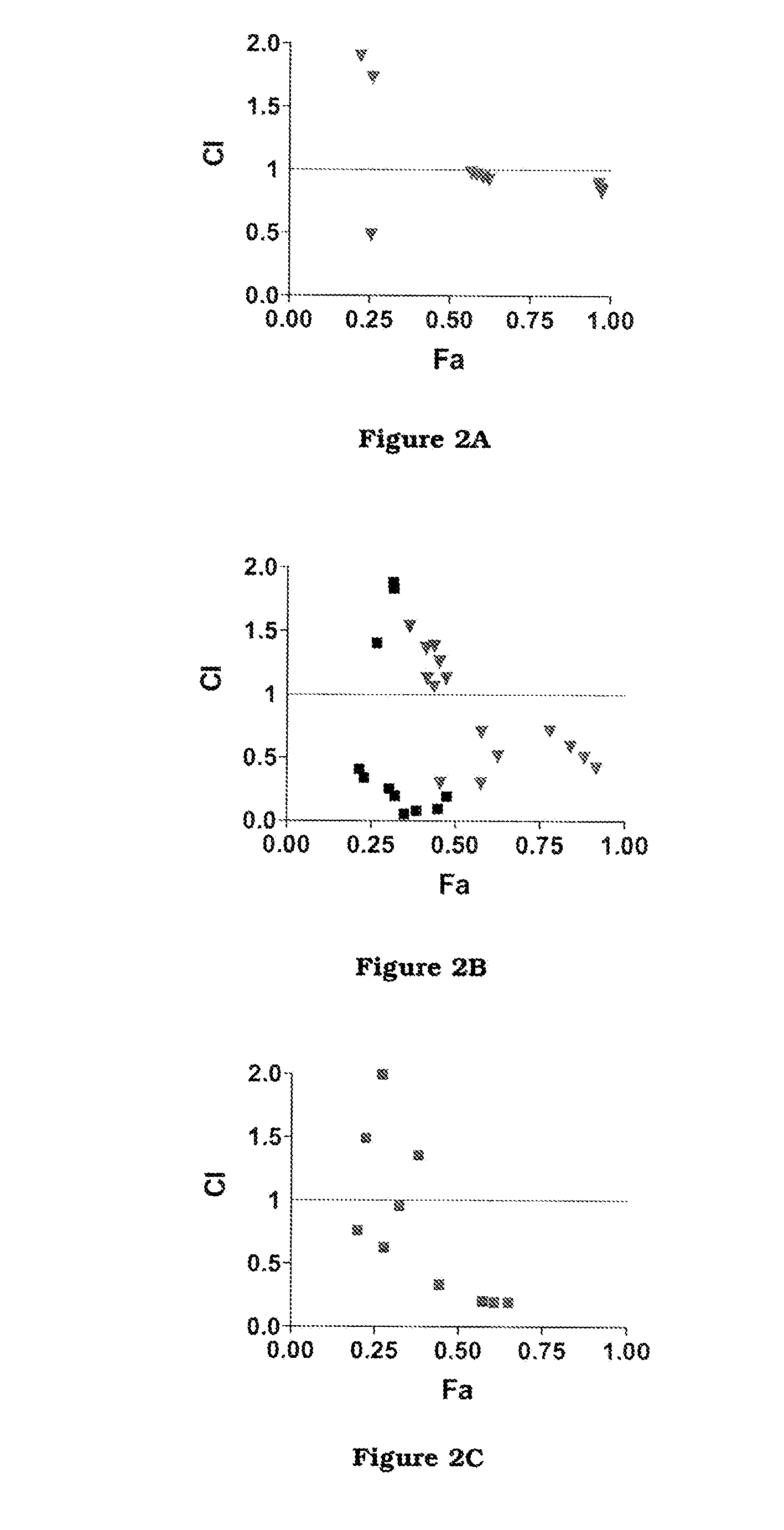
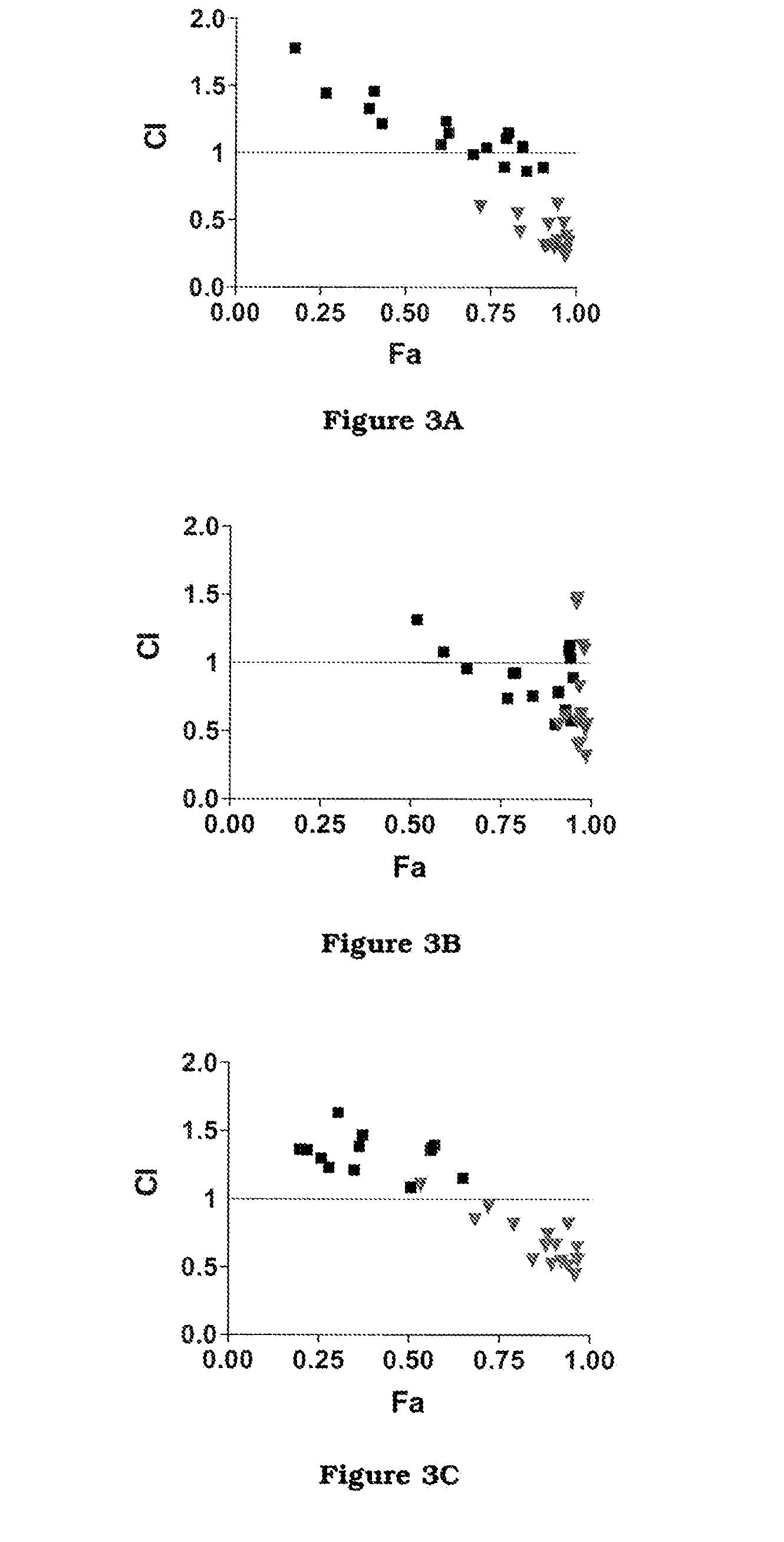
The invention firstly provides a composition containing a protein kinase inhibitor and resveratrol. The composition is characterized in that the protein kinase inhibitor is one, or its pharmaceutically-acceptable salt or solvate or pharmaceutically-acceptable salt solvate, of sunitinib, lapatinib, pazopanib, afatinib, bosutinib, crizotinib, axitinib, and regorafenib; the molar ratio of resveratrolto the protein kinase inhibitor is (0.01-100):1. It is discovered through in-vitro antibacterial tests that the composition of the protein kinase inhibitor and resveratrol, in the molar ratio of (0.01-100):1, and can provide synergic antibacterial action against Kluyvera cryocrescens and other bacteria (interaction index CI<1 upon 30% of inhibition).
Owner:黄泳华
Process for the preparation of high purity sunitinib and its pharmaceutically acceptable salt
InactiveUS20110092717A1Easily transferable technologyOrganic active ingredientsOrganic chemistrySunitinibMethyl group
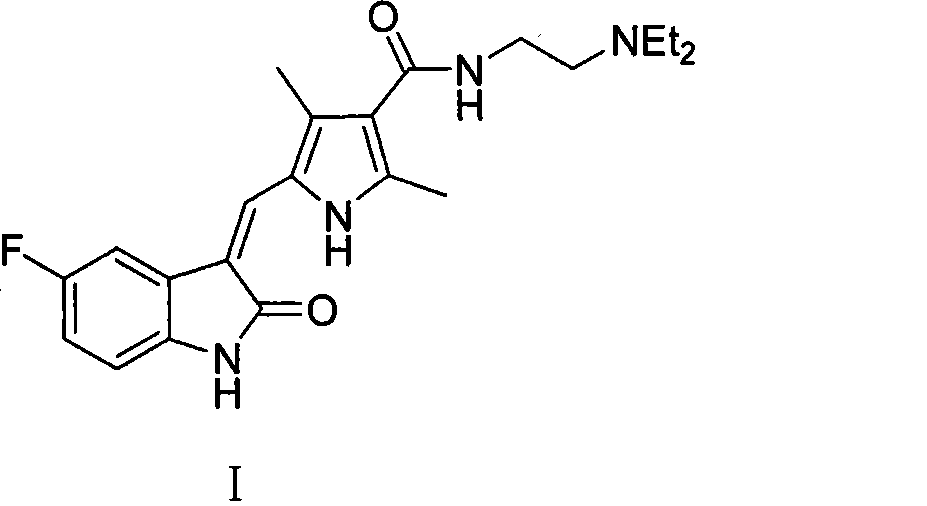
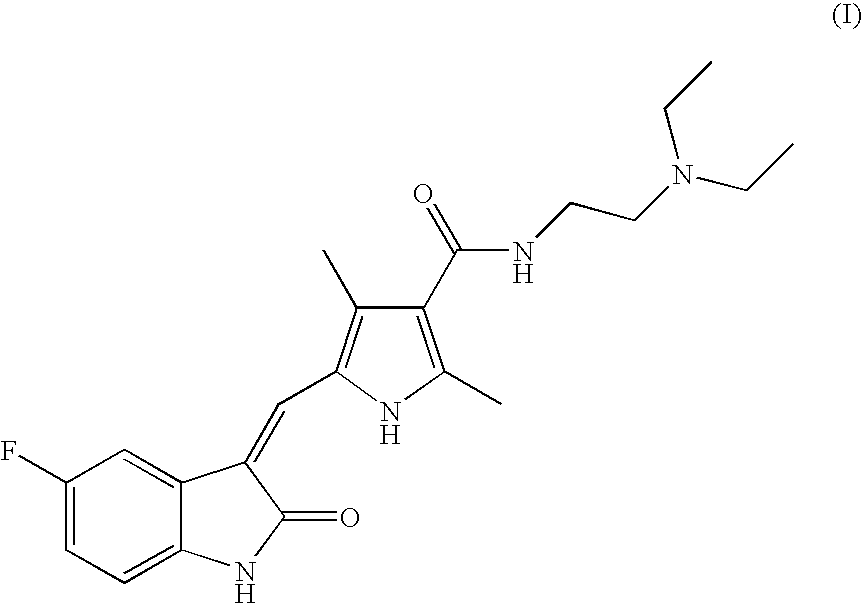
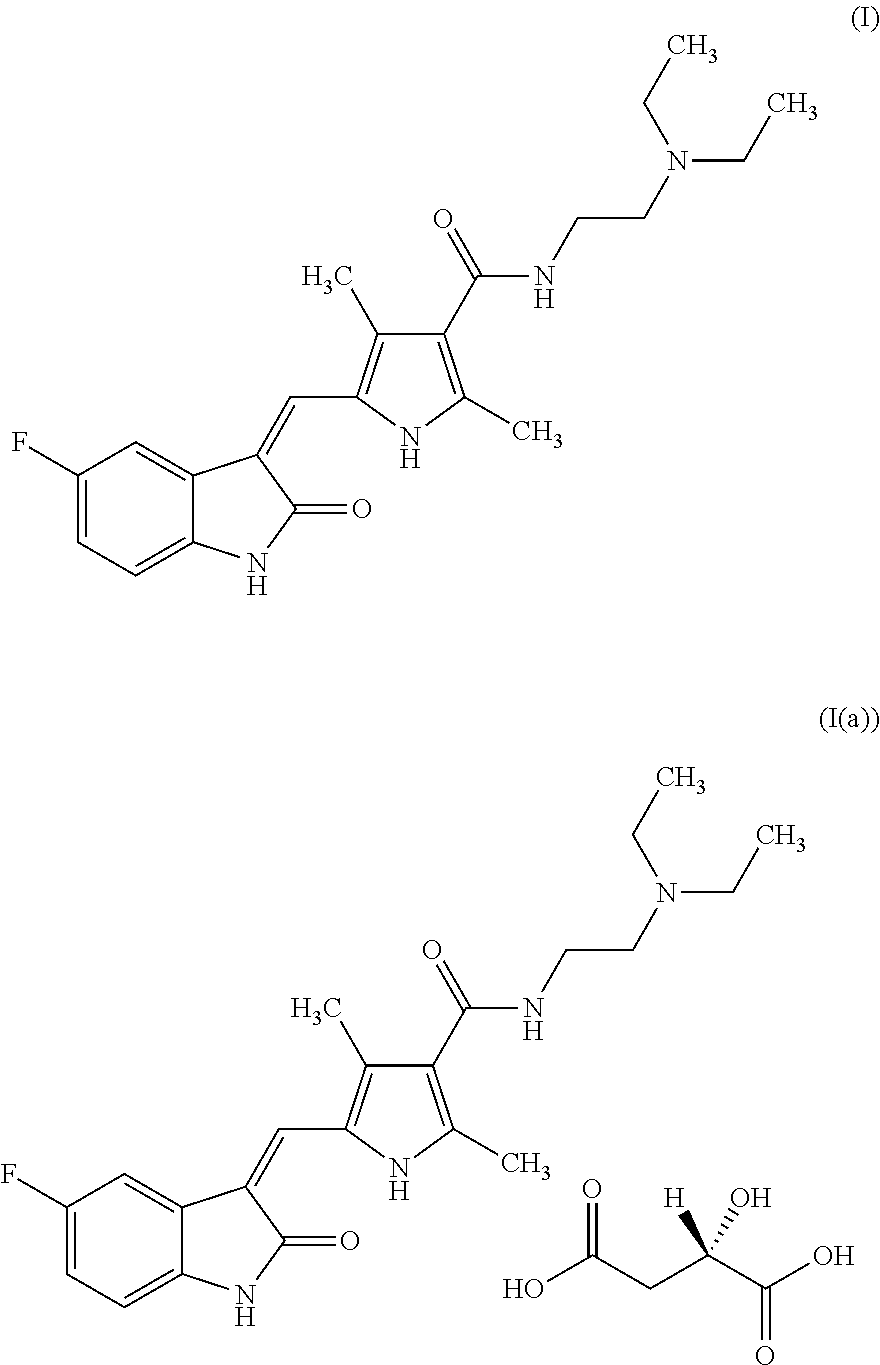
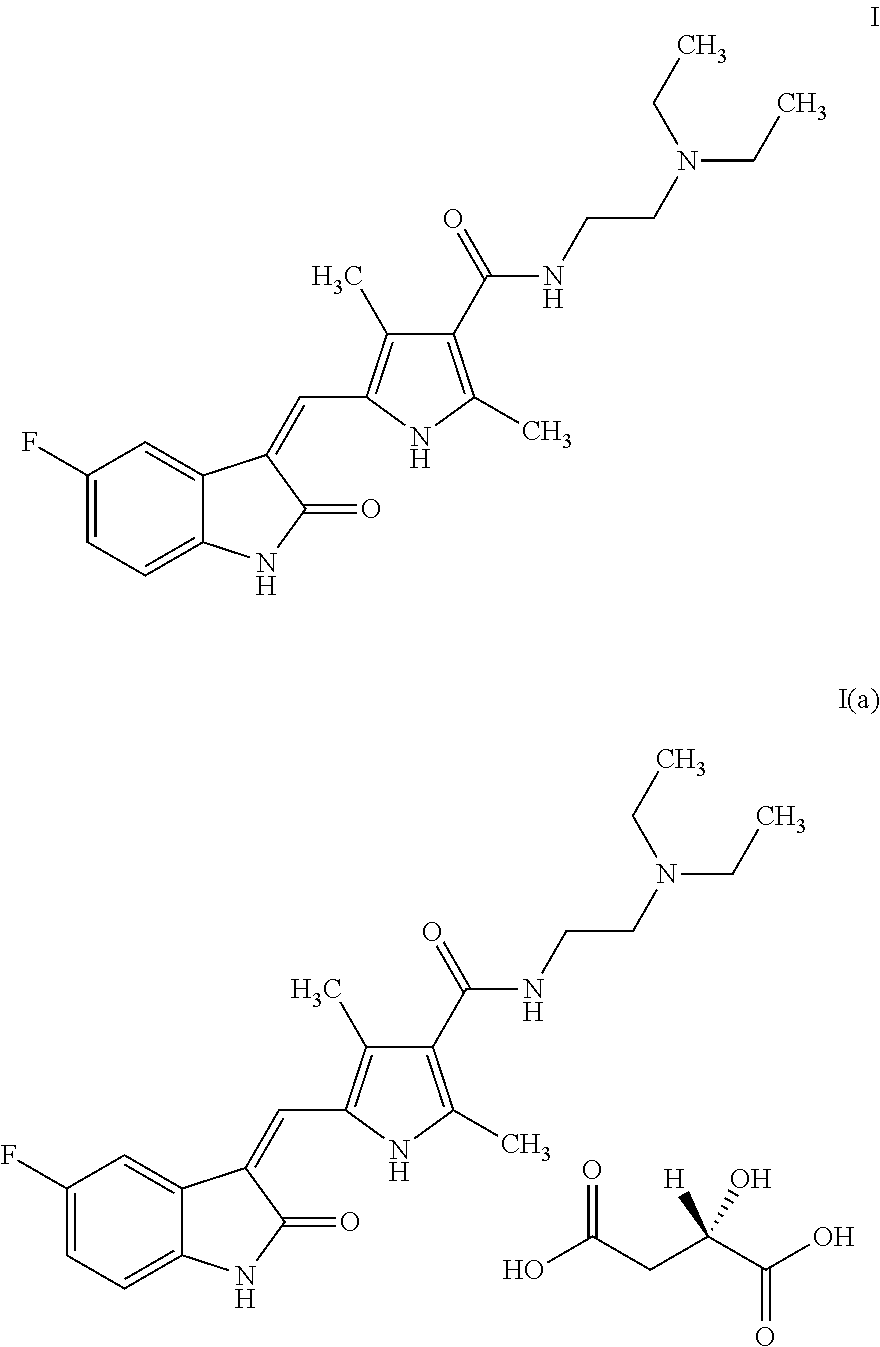
The present invention relates to an improved process for the preparation of N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidine)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide—Sunitinib base of formula (I) and its pharmaceutically acceptable malate salt of formula (I(a)).
Owner:NATCO PHARMA LTD
Anticancer composition containing tyrosine kinase inhibitor
InactiveCN101077334APharmaceutical delivery mechanismMacromolecular non-active ingredientsMiltefosineWhole body
The anticancer composition containing tyrosine kinase inhibitor is slow released injection and slow released implanted preparation. Its effective anticancer component is the composition of Erbitux, Iressa, Tarceva, Sunitinib, Trastuzumab and other tyrosine kinase inhibitor, and Edelfosine, miltefosine, perifosine, ilmofosine and other phosphoinositide-3-kinase inhibitor. Its slow releasing supplementary material is selected from p(LAEG-EOP), p(DAPG-EOP), other polyphosphate copolymer, poly(erucic acid dipolymer-sebacic acid), etc. The anticancer composition may injected or set into tumor and can maintain the effective medicine concentration for over 50 days with obviously reduced general reaction and capacity of raising the treating effect of chemotherapeutic and / or radiotherapeutic medicine.
Owner:JINAN KANGQUAN PHARMA TECH
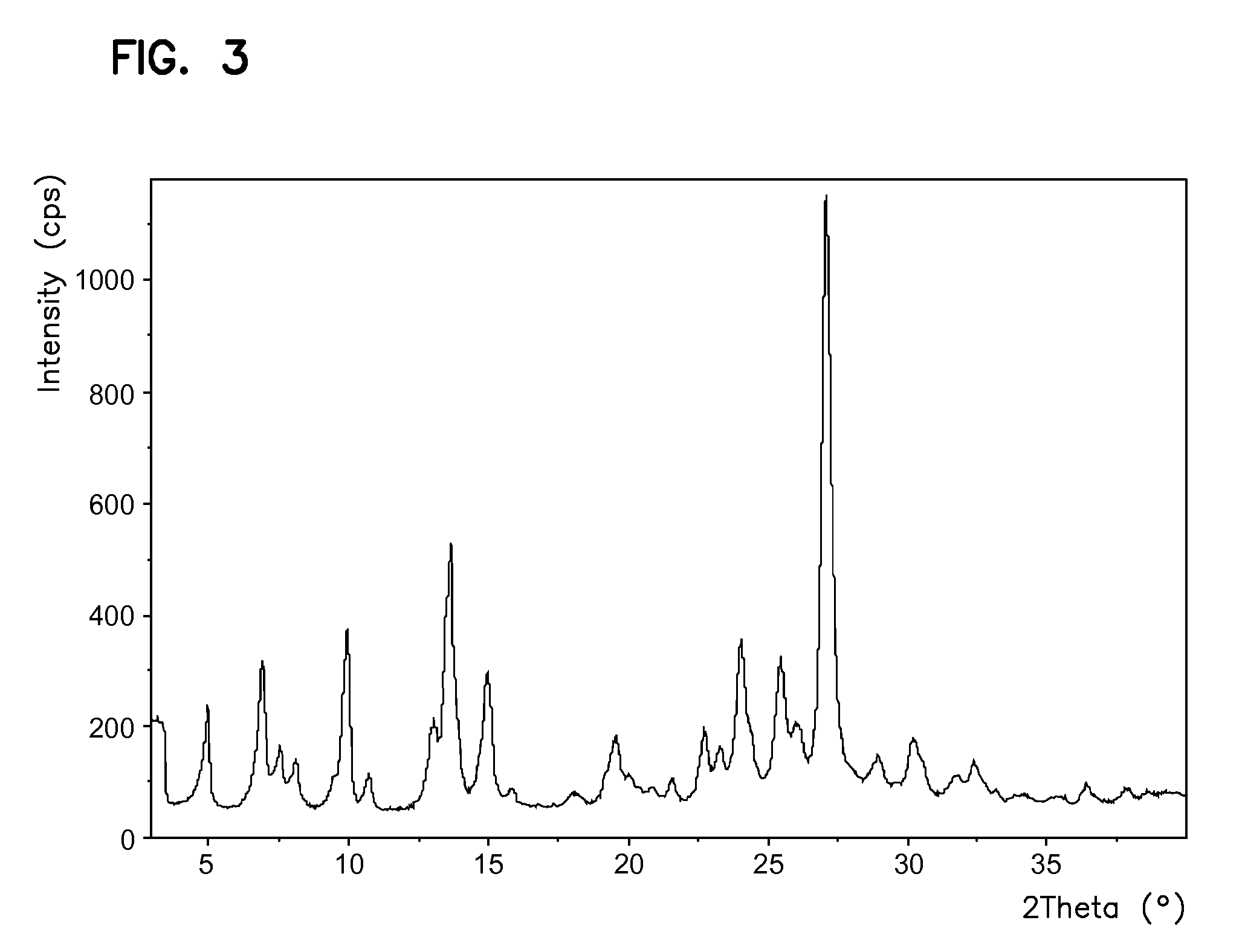
New crystal form of tini-type medicines
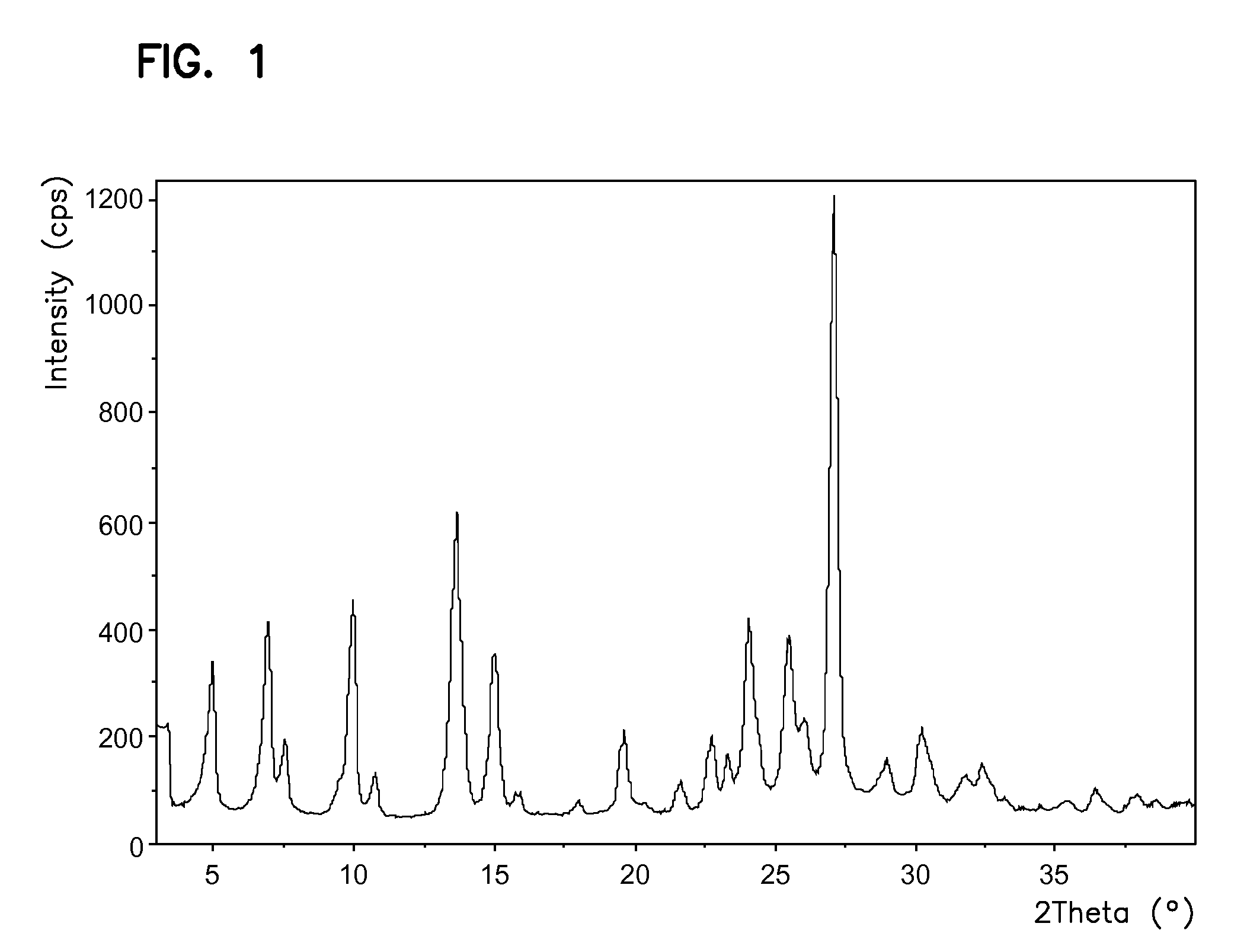
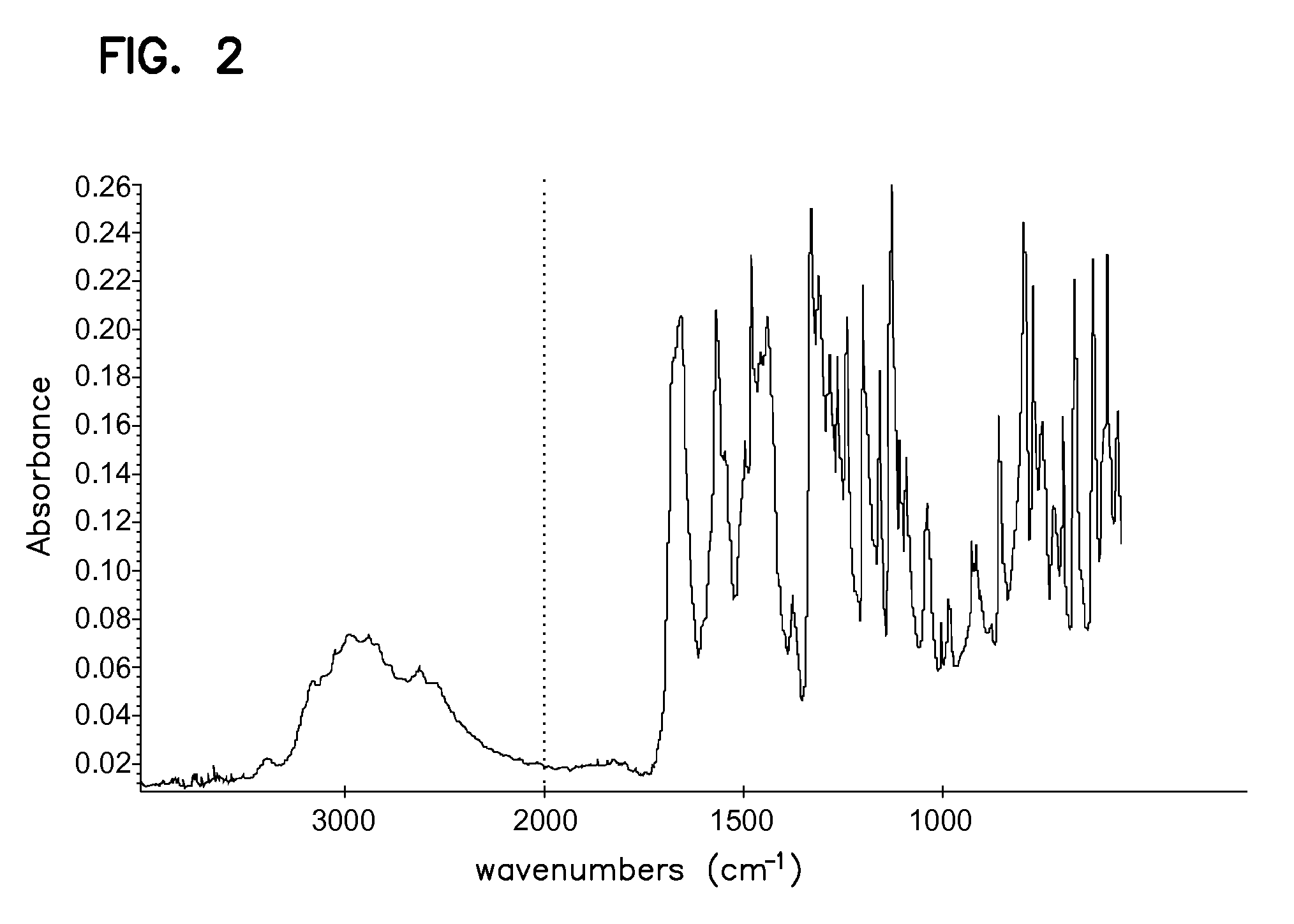
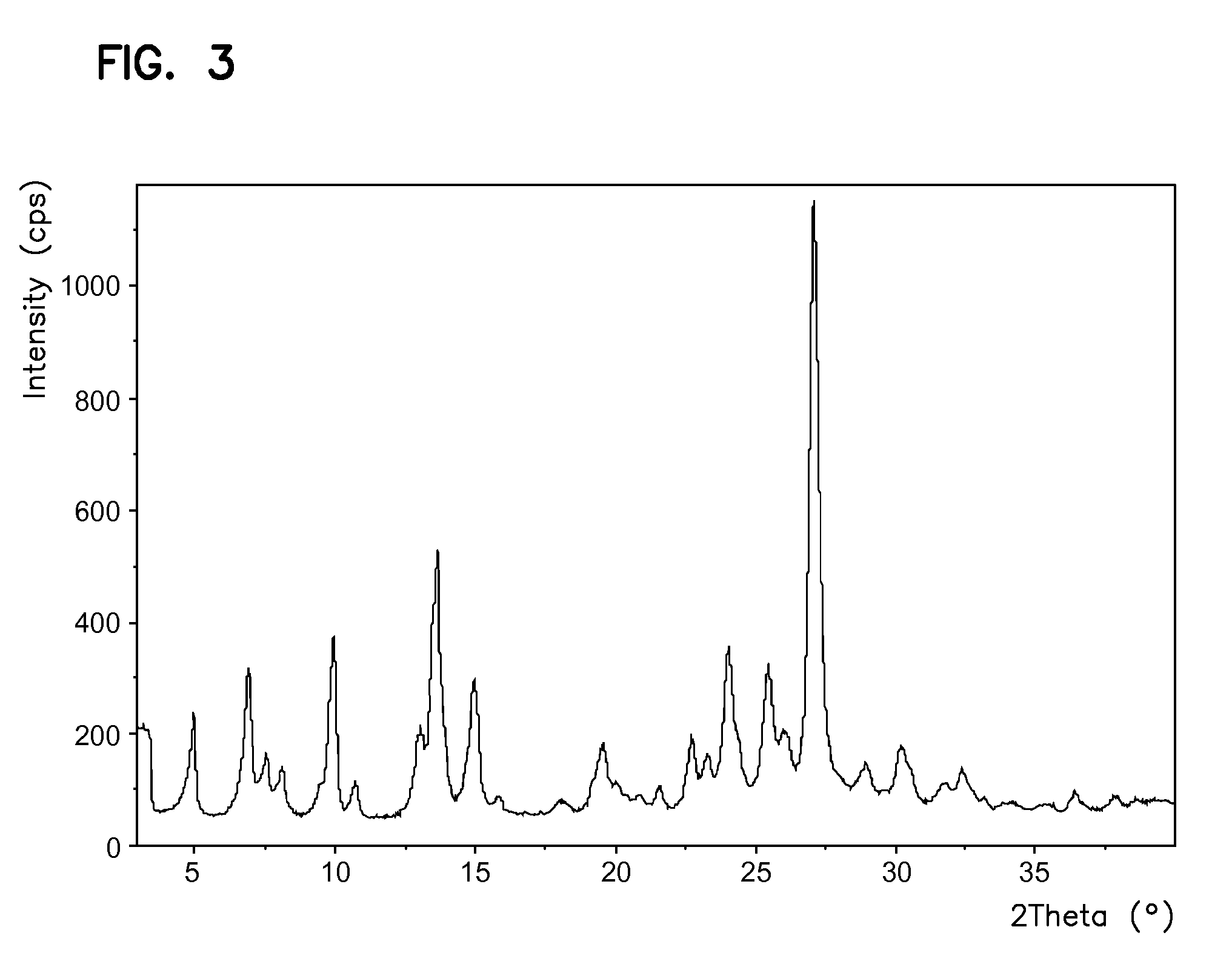
The invention relates to the field of medicine chemistry. On one hand, the invention relates to a new crystal form of sunitinib, and the crystal form of the sunitinib is a crystal form B or a crystal form C basically pure. On the other hand, the invention relates to a new crystal form of sunitinib L-malate, and the crystal form of the sunitinib L-malate is a crystal form D basically pure. The new crystal forms are non-moisture-absorbing and are beneficial to operation and / preparation of preparations in industrial production. The invention also provides a preparation method of the crystal forms.
Owner:SUNSHINE LAKE PHARM CO LTD
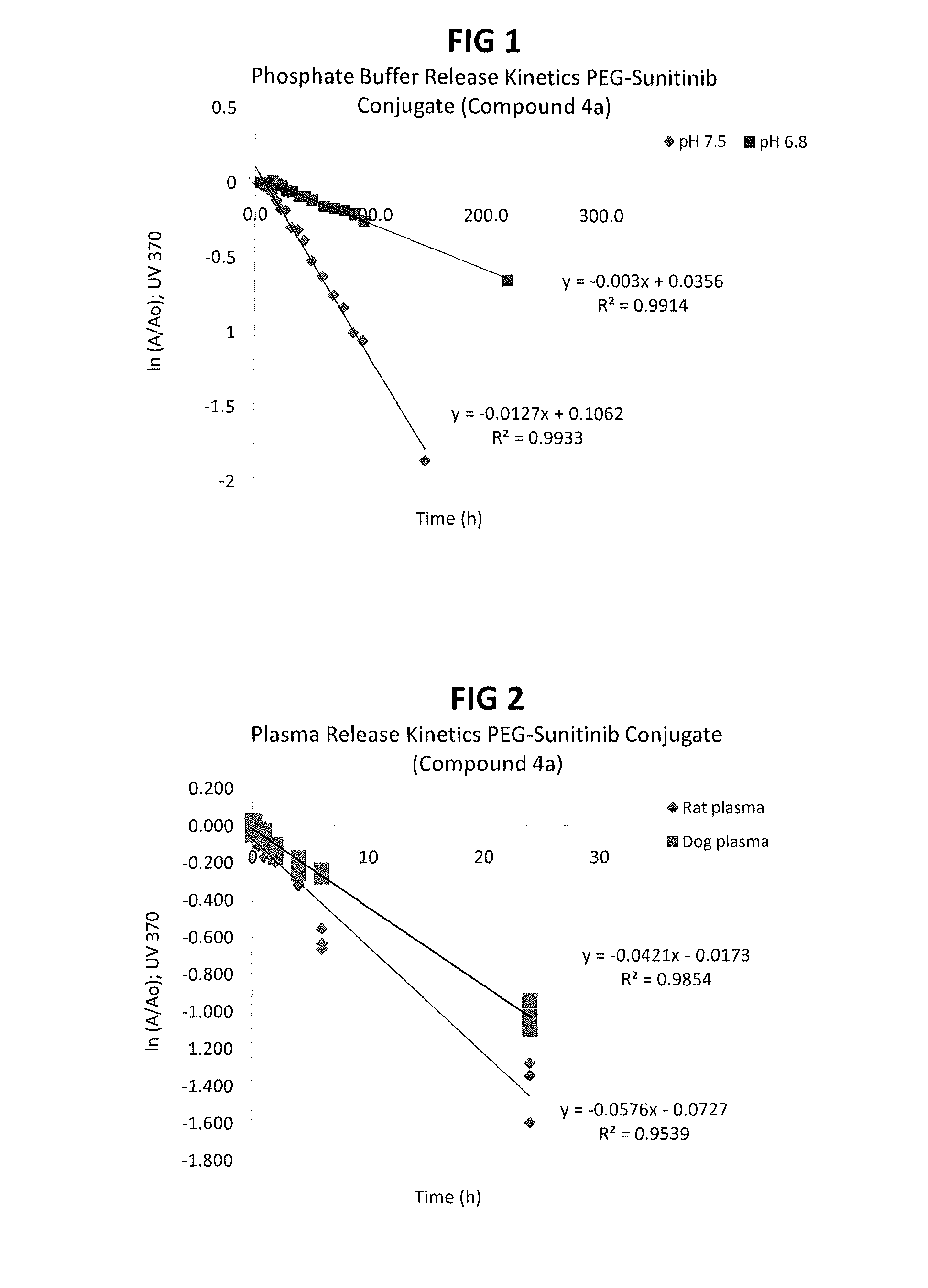
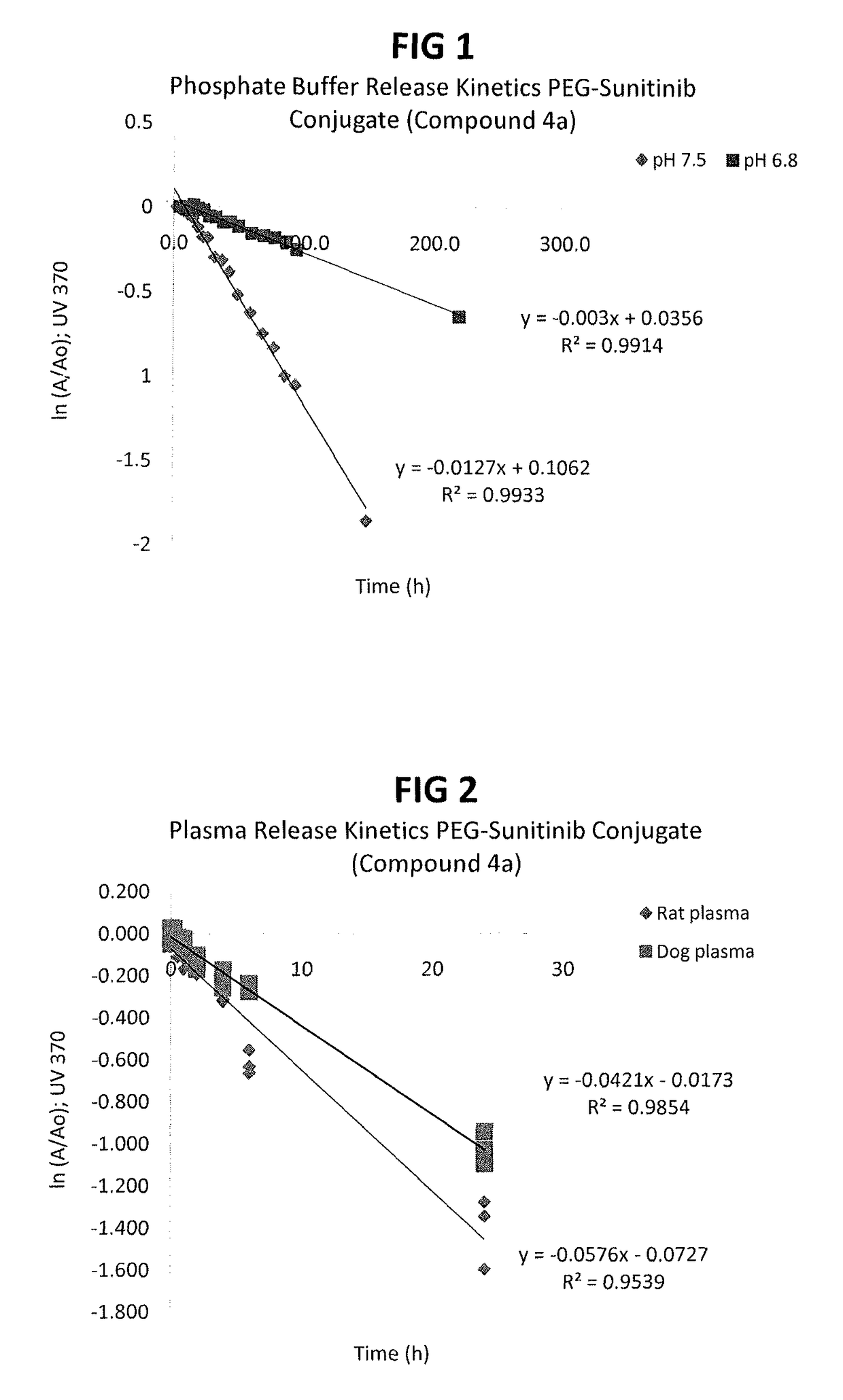
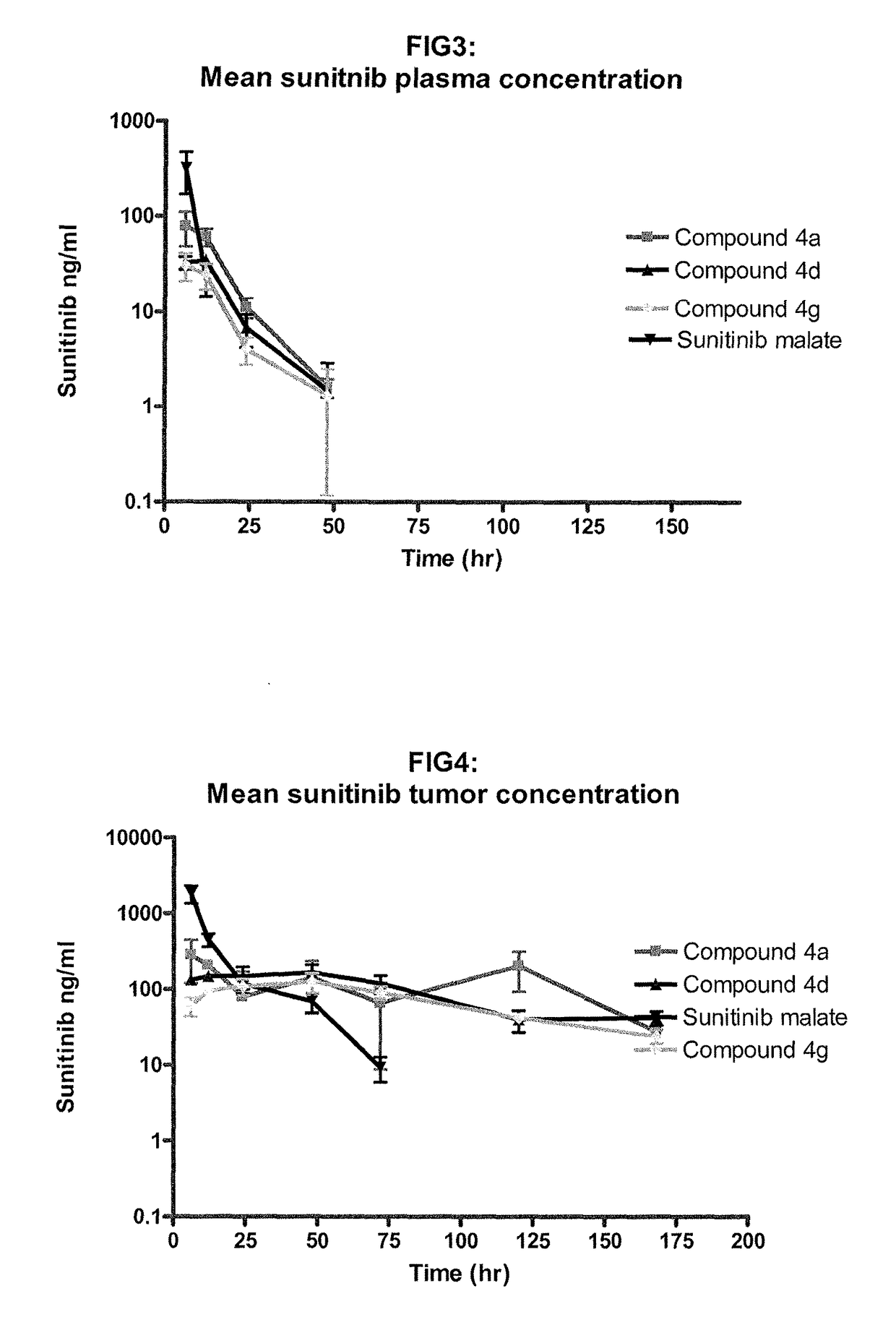
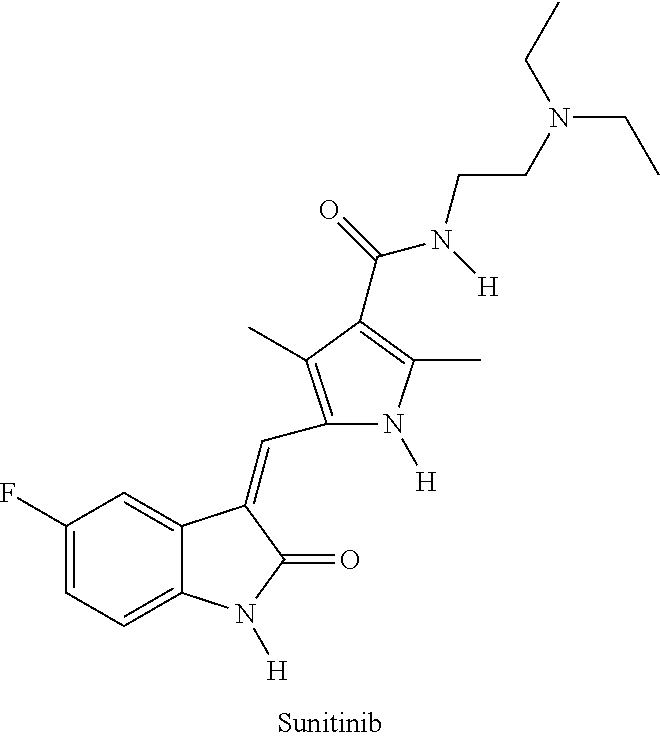
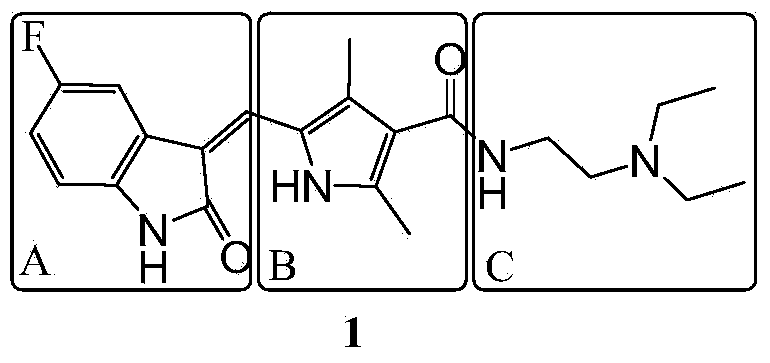
Polymer-sunitinib conjugates
Owner:NEKTAR THERAPEUTICS INC
Method for preparing sunitinib
The invention relates to a method for preparing sunitinib. The method comprises the steps of dissolving 5-fluoro-1,3-indoline-2-ketone and N-(2-diethylin ethyl)-2,4-dimethyl-5-formyl group-1H-pyrrole-3-formamide into methylbenzene, then carrying out backflow reaction for 2.5-3.5 hours with piperidine as a catalyst, cooling to room temperature, carrying out suction filtering, and washing and drying filter cakes obtained by suction filtration through petroleum ether, so as to obtain the sunitinib, wherein the N-(2-diethylin ethyl)-2,4-dimethyl-5-formyl group-1H-pyrrole-3-formamide is prepared through hot melting and decarboxylation of 3,5-dimethyl-1H-pyrrole-4-carbethoxy-2-carboxylic acid, Vilsmeier-Haack formylation, hydrolysis reaction and amidation. According to the method, an intermediate of the sunitinib is prepared and synthesized through a solvent-free method, so that the overall yield of the sunitinib is greatly increased; in addition, the technology for elementary reaction is optimized; furthermore, the raw materials are easy to obtain, and by optimizing all reaction steps in the synthetic process, the elementary reaction yield of each step is increased, the total yield of the sunitinib is increased, and thus the synthetic cost of the sunitinib is lowered.
Owner:XI AN JIAOTONG UNIV
Processes for preparing sunitinib and salts thereof
Methods for preparing sunitinib or salts thereof are described using novel intermediates and chemical pathways. One such intermediate is:
Owner:TEVA PHARMA IND LTD
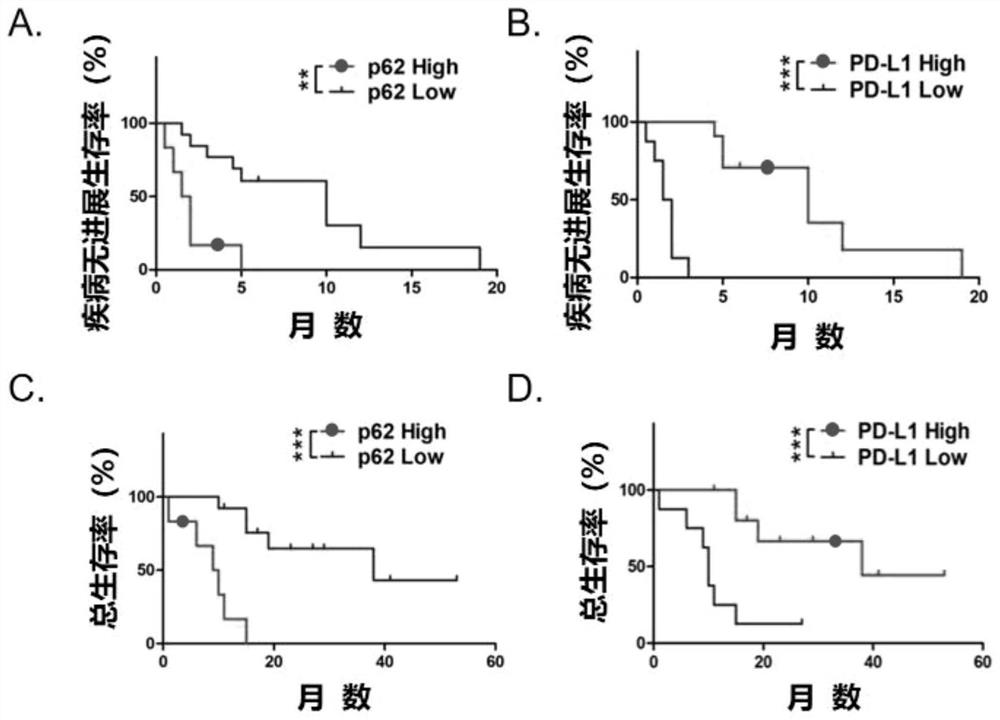
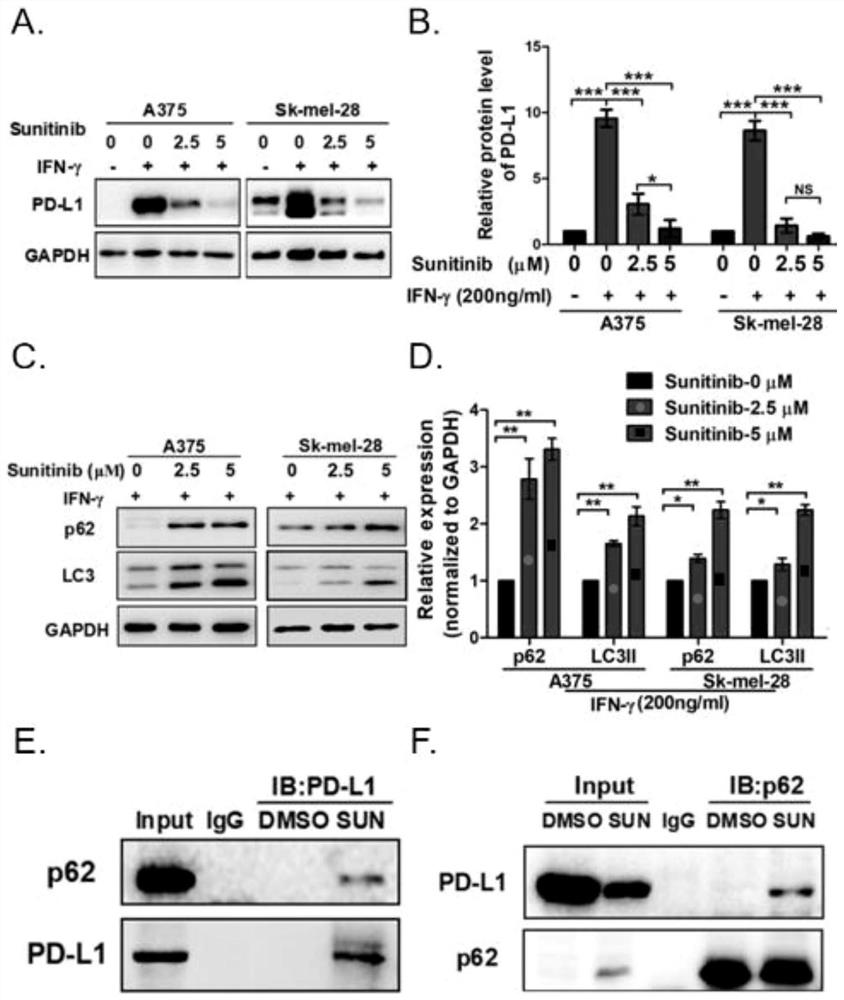
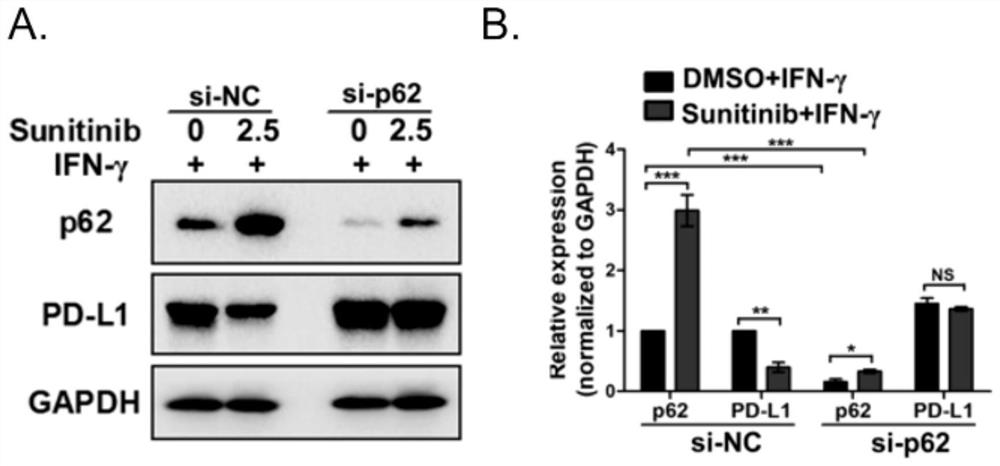
Application of p62/SQSTM1 in preparation of PD-L1/PD-1 monoclonal antibody tumor immunotherapy drug
The invention provides application of p62 / SQSTM1 as a biomarker and a target site detection reagent as well as application of an inhibitor of p62 / SQSTM1 in PD-L1 / PD-1 monoclonal antibody tumor immunotherapy Drug, and also discloses application of combination of sunitinib and CTLA-4 monoclonal antibody drugs in preparation of tumor immunotherapy adjuvant drugs, wherein the tumor is preferably melanoma or lung cancer. The invention provides a new research and development direction of PD-L1 / PD-1 monoclonal antibody tumor immunodetection or immunotherapy adjuvant drugs, and develops a new drug combination mode.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Antitumoral Treatments
InactiveUS20110015135A1Successful useOrganic active ingredientsHeavy metal active ingredientsCisplatinSunitinib
The present invention relates to combinations of PM02734 with another anticancer drug selected from Cisplatin, Gemcitabine, Paclitaxel, Oxaliplatin, 5-Fluorouracil, Trabectedin, Rapamycin, and Sunitinib, and the use of these combinations in the treatment of cancer.
Owner:PHARMA MAR U
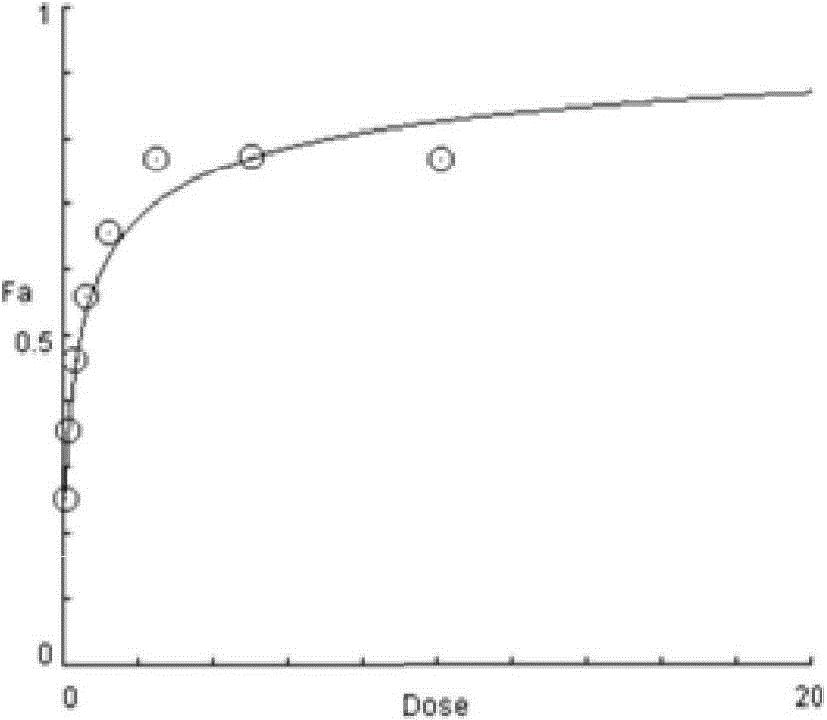
Methods for predicting a cancer patient's response to sunitinib
InactiveUS20110207119A1Avoiding unnecessary treatmentAvoid treatmentMicrobiological testing/measurementMaterial analysisRegimenActive agent
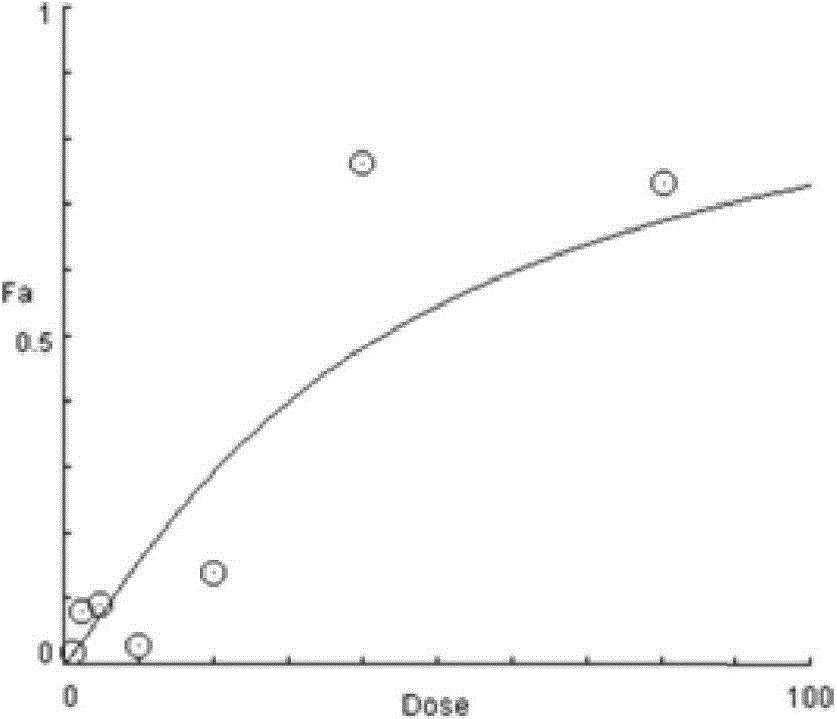
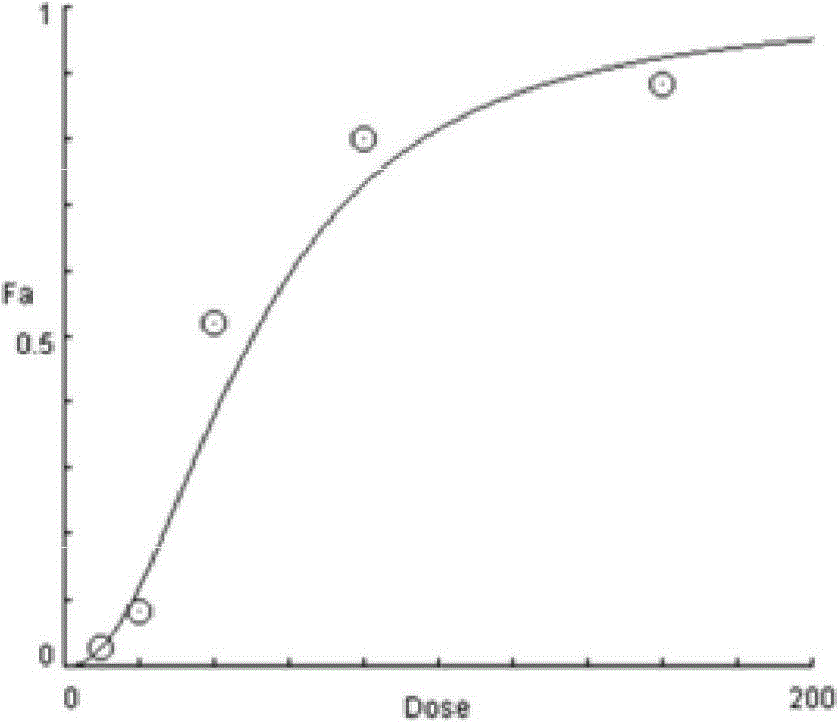
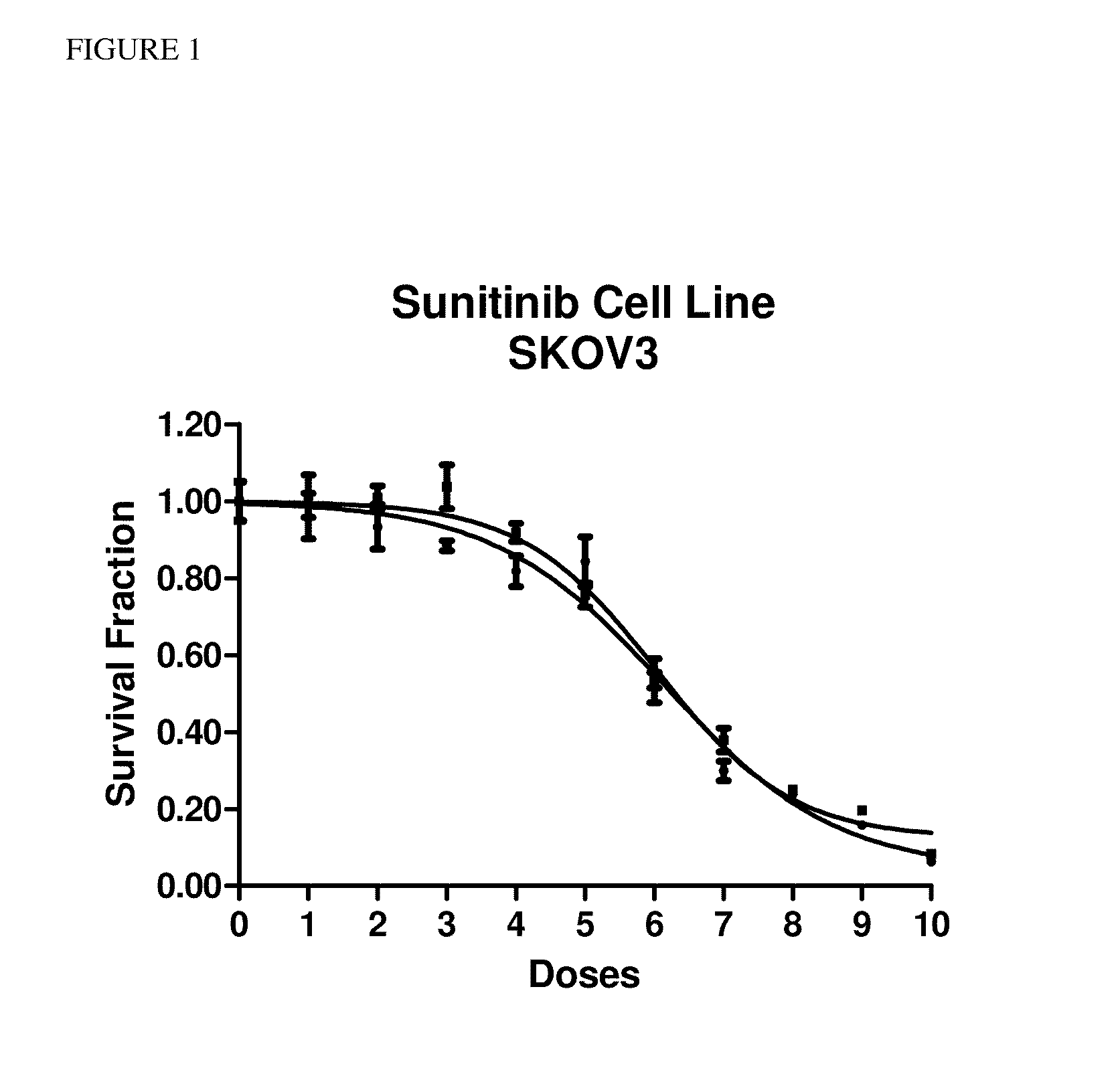
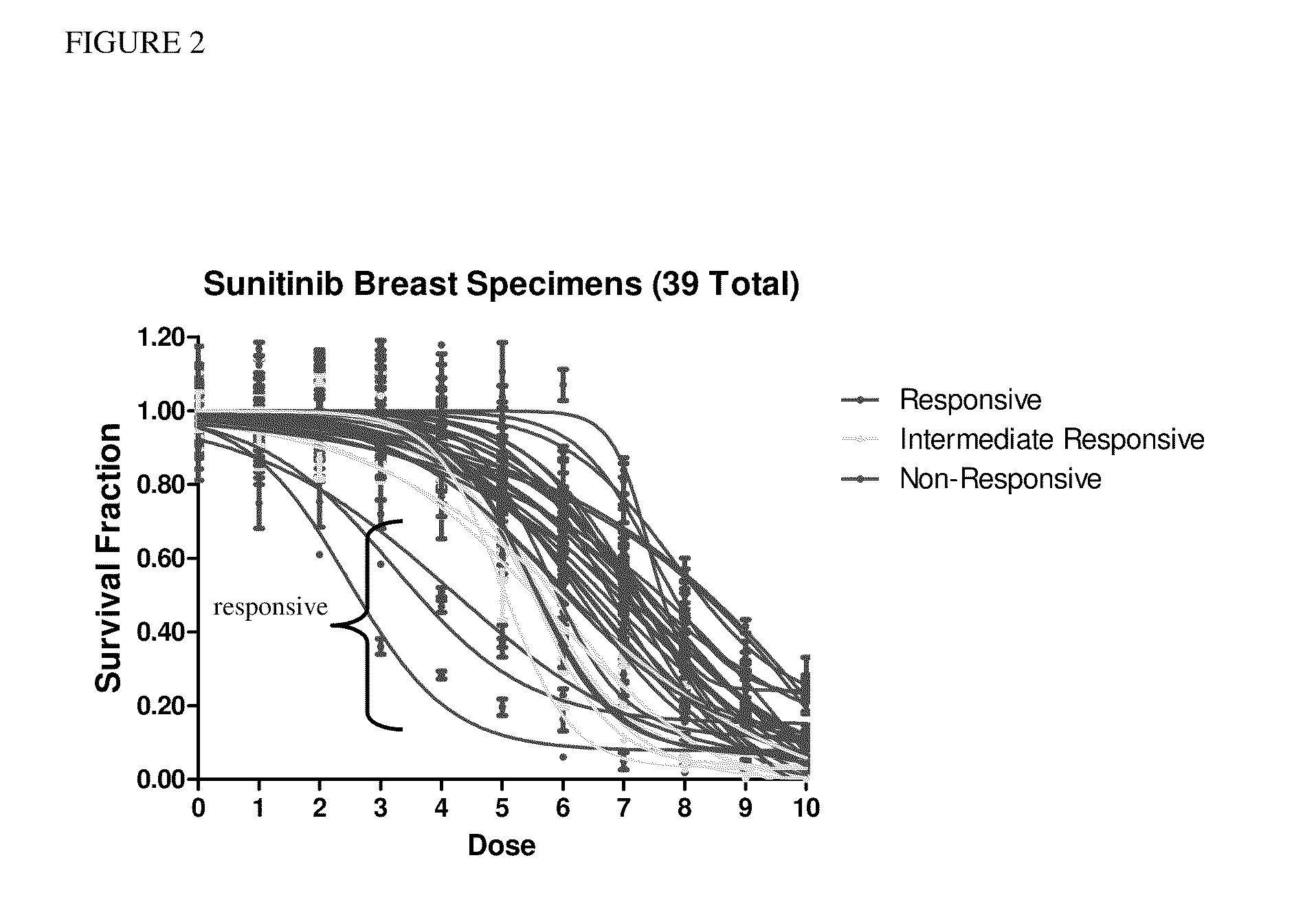
The present invention provides methods for individualizing chemotherapy for cancer treatment, and particularly for evaluating a patient's responsiveness to sunitinib prior to treatment. The method comprises expanding malignant cells in culture from a patient's tumor specimen, contacting the cultured cells with one or more active agents including sunitinib, and evaluating or quantifying the response to the active agent(s). The result of the assay is a dose response curve, which may be evaluated using algorithms described herein, so as to quantitatively assess drug sensitivity. The in vitro response to the drug as determined by the method of the invention is correlative with the patient's in vivo response upon receiving sunitinib during chemotherapeutic treatment, in the course of standardized or individualized chemotherapeutic regimen.
Owner:PRECISION THERAPEUTICS
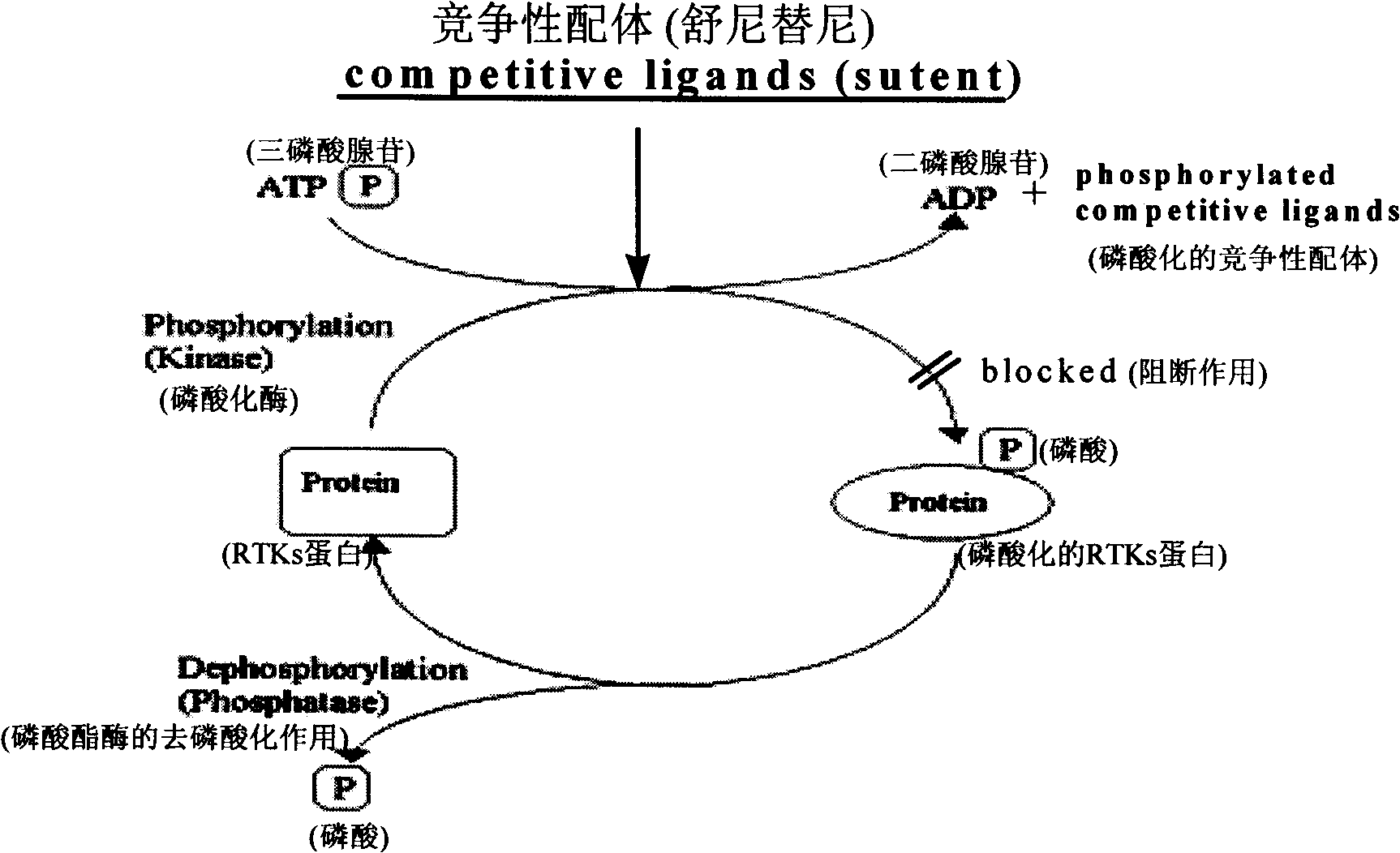
Novel methods for predicting the responsiveness of a patient affected with a tumor to a treatment with a tyrosine kinase inhibitor
InactiveUS20130131136A1Prediction of responsivenessSure easyOrganic active ingredientsElectrolysis componentsPatient affectedSunitinib
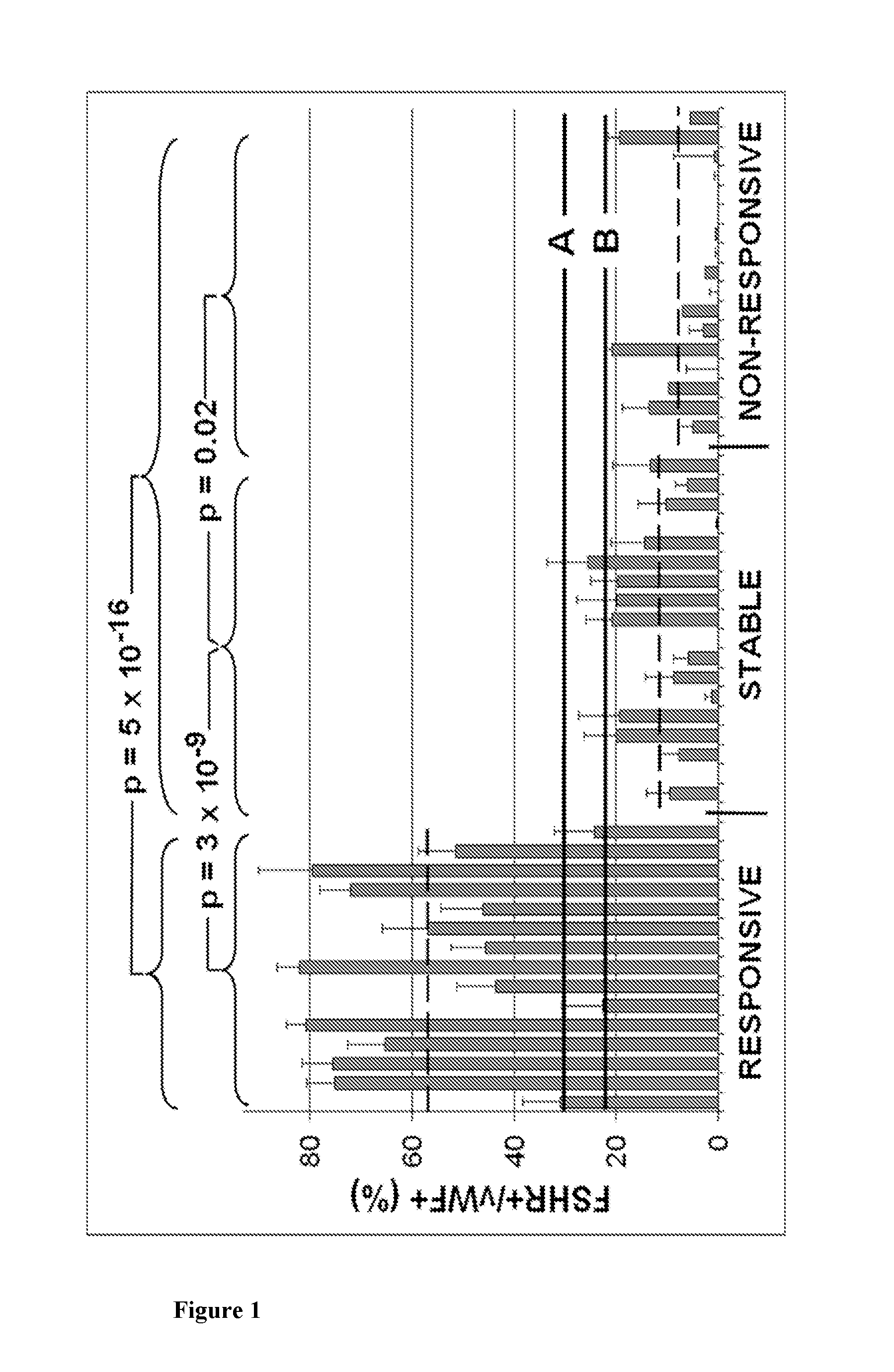
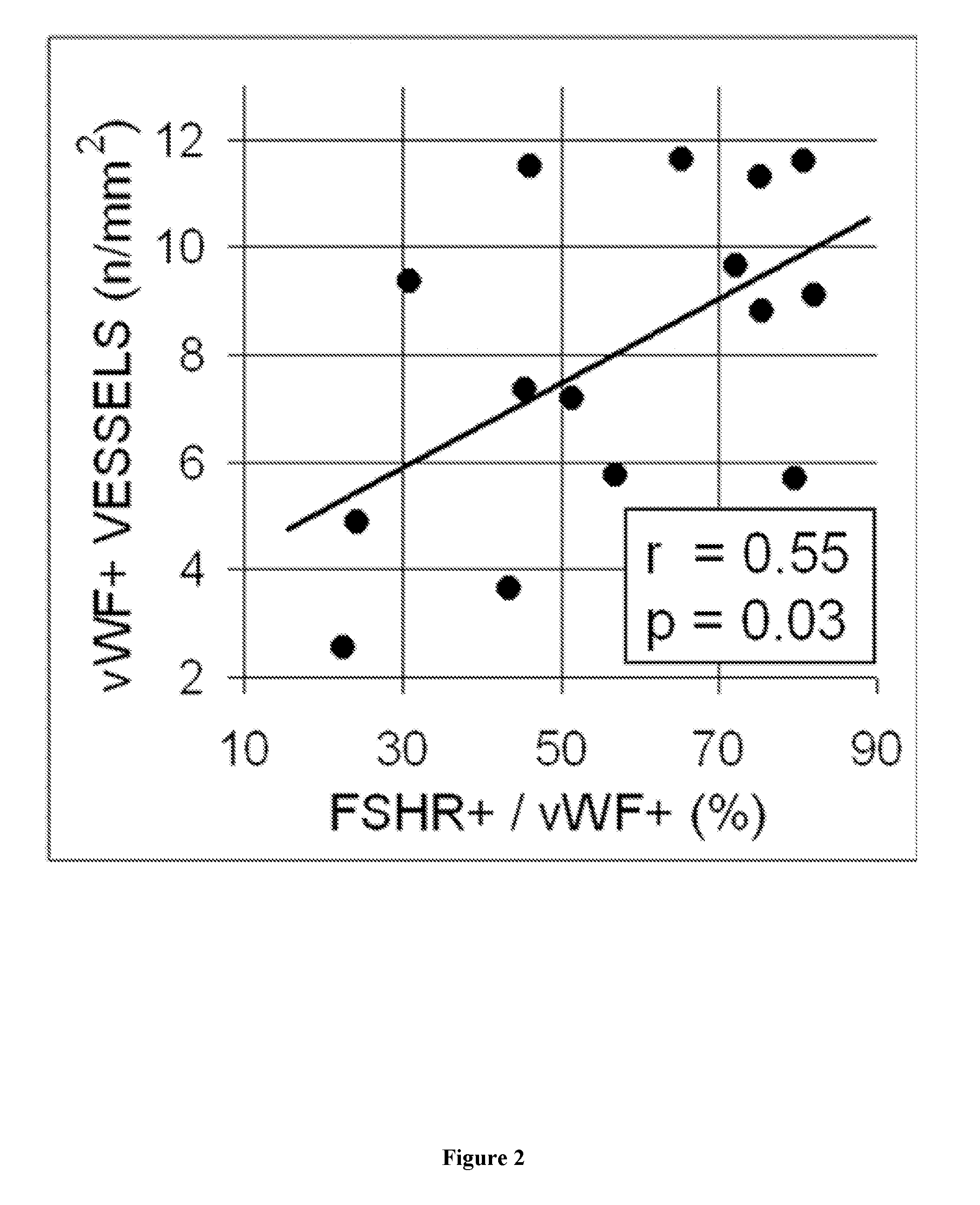
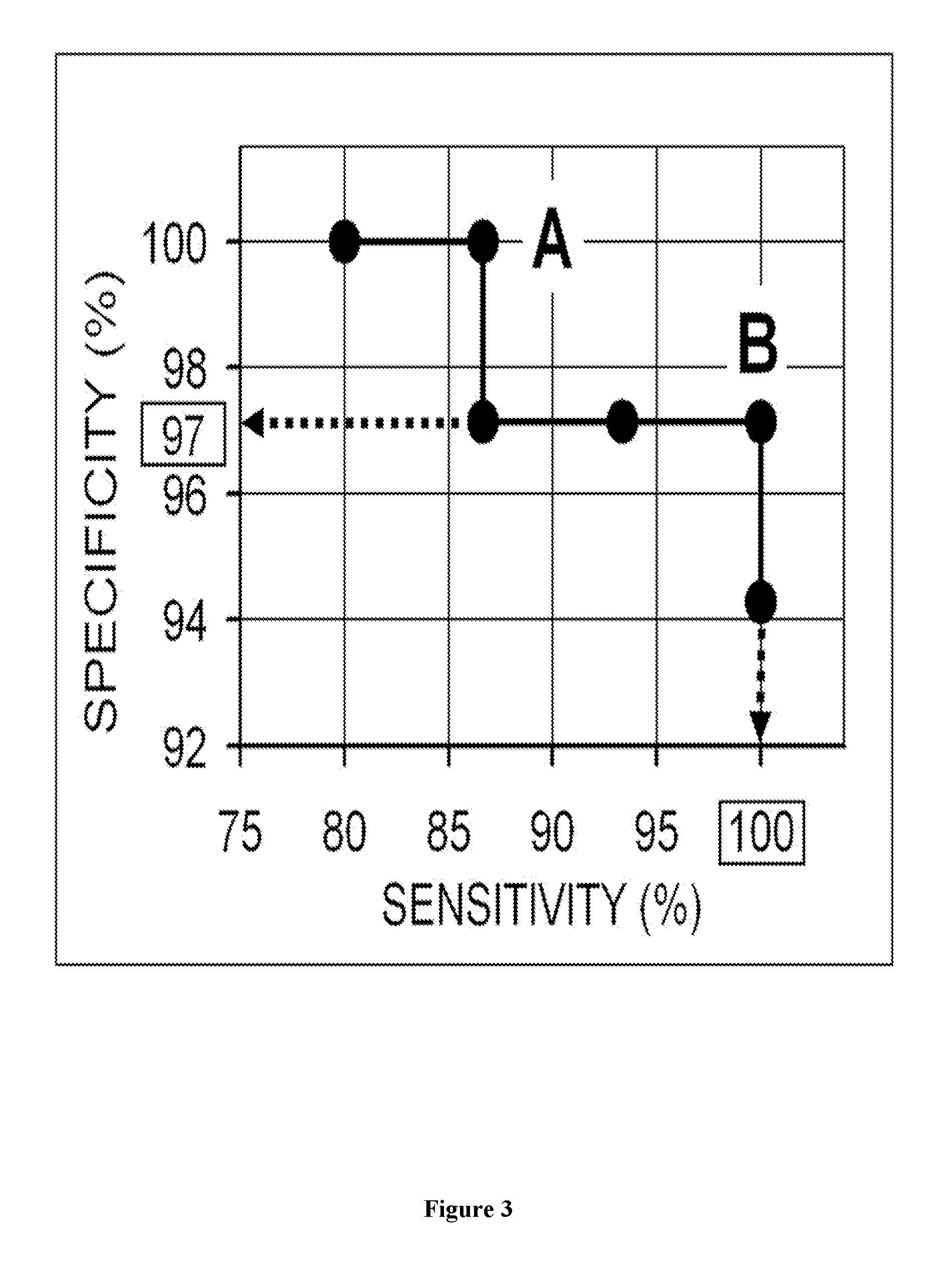
The present invention relates to a method for predicting the responsiveness of a patient affected with a tumor to a treatment with a tyrosine kinase inhibitor such as sunitinib. More specifically, the method of the invention comprises a step of determining the expression level of one marker consisting of the FSHR in a biological sample obtained from said patient, and more specifically in the blood endothelial cells from the tumors.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Uses of chemicals to modulate GSK-3 signaling for treatment of bipolar disorder and other brain disorders
Aspects of this invention are related, at least in part, to the use of chemical compounds able to inhibit GSK-3 and / or to stabilize β-catenin and formulations thereof. Some aspects of this invention relate to compositions comprising such compounds. Some aspects of the invention provide methods of using such compounds and / or compositions in the treatment of subjects having a neurological disease and / or psychiatric disorder. Some aspects of this invention provide methods of using ruboxistaurin, enzastaurin, sunitinib, midostaurin, lestaurtinib, 7-hydroxystaurosporine, and / or Chir99021 in the treatment of subjects having a neurological disease and / or psychiatric disorder. In some embodiments, compounds are administered in combination with Lithium.
Owner:MASSACHUSETTS INST OF TECH +1
Renal cell carcinoma treatment medicine sunitinib-PLGA/Fe3O4 composite microsphere and preparation method thereof
ActiveCN105434399AIncrease concentrationProlong the action timeOrganic active ingredientsInorganic non-active ingredientsCancer cellActive agent
The invention discloses a renal cell carcinoma treatment medicine sunitinib-PLGA / Fe3O4 composite microsphere and a preparation method thereof. The method comprises the following steps: dissolving PLGA in an organic solvent, adding a surfactant and a pore forming agent, uniformly stirring and mixing, and adding magnetic nano-Fe3O4 particles to prepare a compound solution; slowly adding sunitinib into 120-200ml of a sodium carboxymethyl cellulose aqueous solution having a concentration of 1.2 percent by mass, and uniformly stirring and mixing to prepare a solution 1; and slowly dispersing the compound solution into the solution 1, stirring to sufficiently volatize the organic solvent, and drying the solid at 25-30 DEG C in vacuum for 24-32 hours to prepare the sunitinib-PLGA / Fe3O4 composite microsphere. The sunitinib-PLGA / Fe3O4 composite microsphere has a regular pore diameter and relatively high entrapment efficiency, and has a clinical application prospect in the target cancer cell killing and cancer treatment aspects.
Owner:HUNAN ER KANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com