Sunitinib formulations and methods for use thereof in treatment of ocular disorders
a technology of sunitinib and ocular disorders, which is applied in the direction of capsule delivery, drug composition, nervous disorder, etc., can solve the problems of tumor shrinkage, tumor vascularization and cancer cell death reduction, etc., and achieve the effect of preventing optic nerve damag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
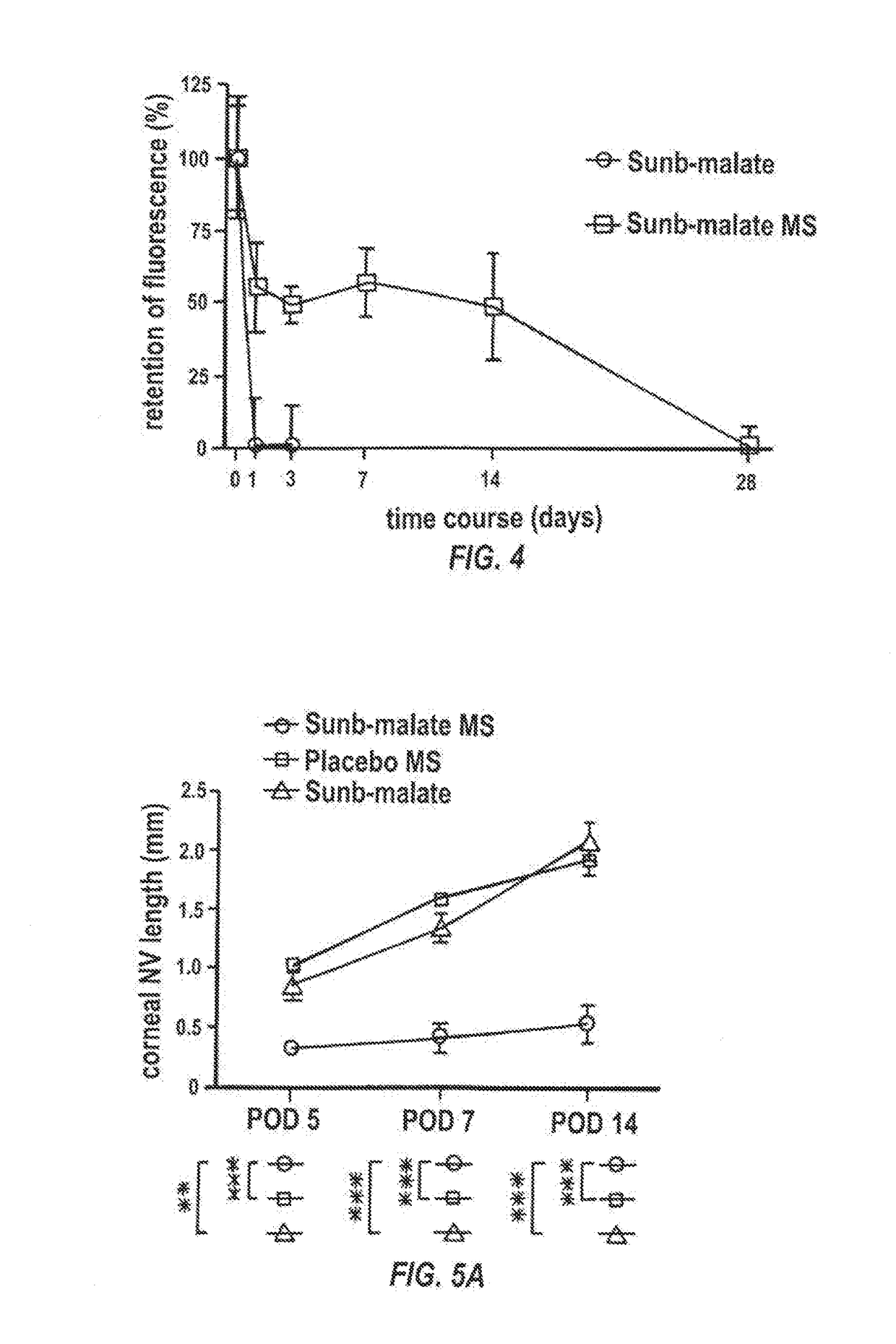
Effects of Surfactants, the Form of Sunitinib, and the Overall Alkalinity on the Drug Loading And In Vitro Release Profiles of Microparticles (Mps)
[0205]Materials and Methods
[0206]Materials—Two Forms of Sunitinib
[0207]Two forms of sunitinib were used, i.e., sunitinib malmate and sunitinib free base, both acquired from LC Lab (Woburn, Mass., USA).
[0208]Poly (D, L-lactic-co-glycolic acid (PLGA, 50:50), 2A was acquired from Alkermes, Waltham, Mass., US; poly (D, L-lactic-co-glycolic acid (PLGA, 50:50), 2A from Lakeshore Biomaterials, Birmingham, Ala., US; poly (D, L-lactic-co-glycolic acid (PLGA, 50:50), 4A from Lakeshore, Biomaterials, Birmingham, Ala., US; poly (D, L-lactic-co-glycolic acid (PLGA, 75:25), PURASORB PDLG 7502A from PURAC, Netherlands; polyethylene glycol-Poly (D, L-lactic-co-glycolic acid), PEG-PLGA (5K, 45K), PEG 10%, PLGA 50:50, from Jinan Daigang Biomaterials Co, Ltd., Jinan, Shandong, China and purified by dissolved in chloroform and precipitated in ether. PLA, pol...
example 2
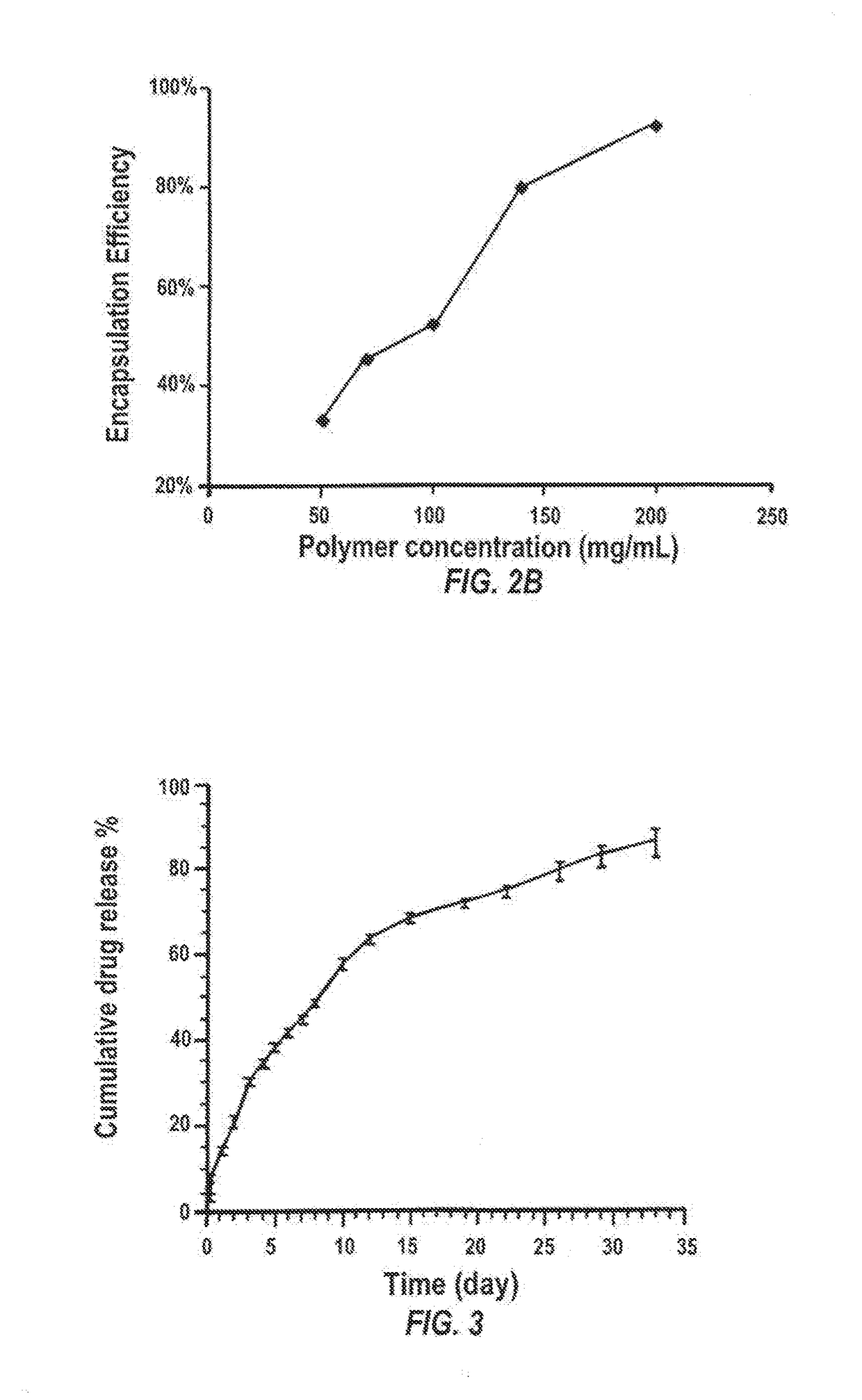
Preparation and in Vitro Release Profiles of Nanoparticles (NPs) Encapsulating Sunitinib
[0250]Materials and Methods
[0251]Formulation IDs
[0252]For ease of identification, nanoparticles prepared in an aqueous phase containing 1% of PVA in PBS (pH 7.4) according to different formulations were each given an ID, e.g. NP-n (n is a number from 21 to 27).[0253]NP-21: 100 mg PLGA (2A, Lakeshore biomaterials) was dissolved in 1 mL methylene chloride. 20mg sunitinib malmate was added in 250 μl DMSO and then poured into 1% PVA. 5 mL was sonicated 3 mins and poured into 80 mL 0.1% PVA, stirred for 2 hours, particles collected, washed with double distilled water, and freeze dried.[0254]NP-22: 100 mg PLGA (2A, Lakeshore biomaterials) was dissolved in 1 mL methylene chloride. 20 mg sunitinib malmate was added in 250 μl DMSO and then poured into 1% PVA in PBS(7.4). 5 mL was sonicated for 3 mins and poured into 80 mL 0.1% PVA, stirred for 2 hours, particles collected, washed with double distilled wat...
example 3
Effect of Aqueous Ph on Encapsulation Efficiency of Sunitinib
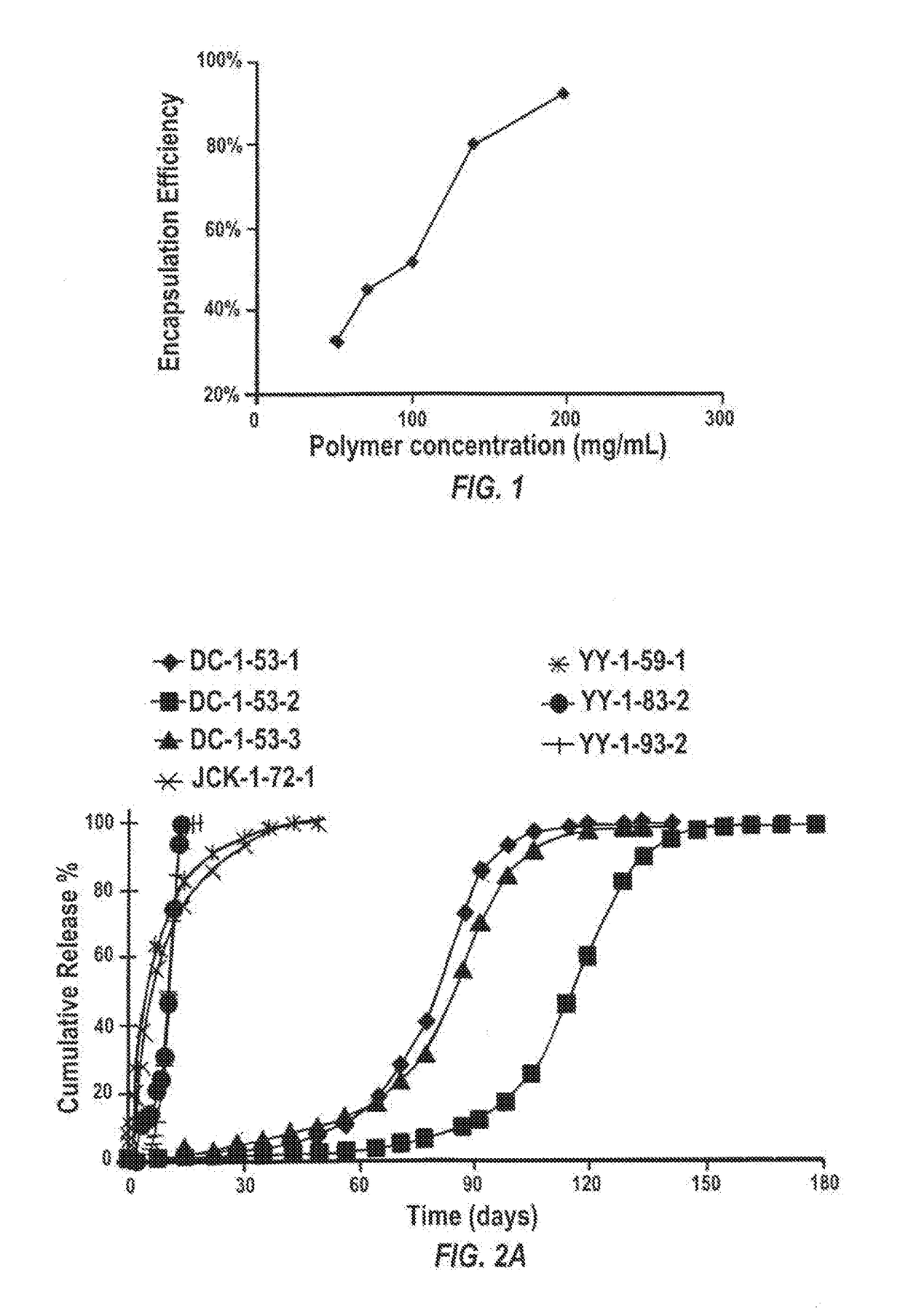
[0261]Materials and Methods
[0262]Polymer microparticles of PLGA and / or a diblock copolymer of PLGA and PEG covalently conjugated to PLGA (Mw 45 kDa) (PLGA45k-PEG5k) with or without sunitinib malate were prepared using a single emulsion solvent evaporation method. Briefly, PLGA and / or PLGA-PEG were first dissolved in dichloromethane (DCM) and sunitinib malate was dissolved in dimethyl sulfoxide (DMSO) at predetermined concentrations. The polymer solution and the drug solution were mixed to form a homogeneous solution (organic phase). The organic phase was added to an aqueous solution of 1% polyvinyl alcohol (PVA) (Polysciences, Mw 25 kDa, 88% hydroplyzed) and homogenized at 5,000 rpm for 1 min using an L5M-A laboratory mixer (Silverson Machines Inc., East Longmeadow, MA) to obtain an emulsion.
[0263]The solvent-laden microparticles in the emulsion were then hardened by stirring at room temperature for >2 hr to allow the DCM ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com