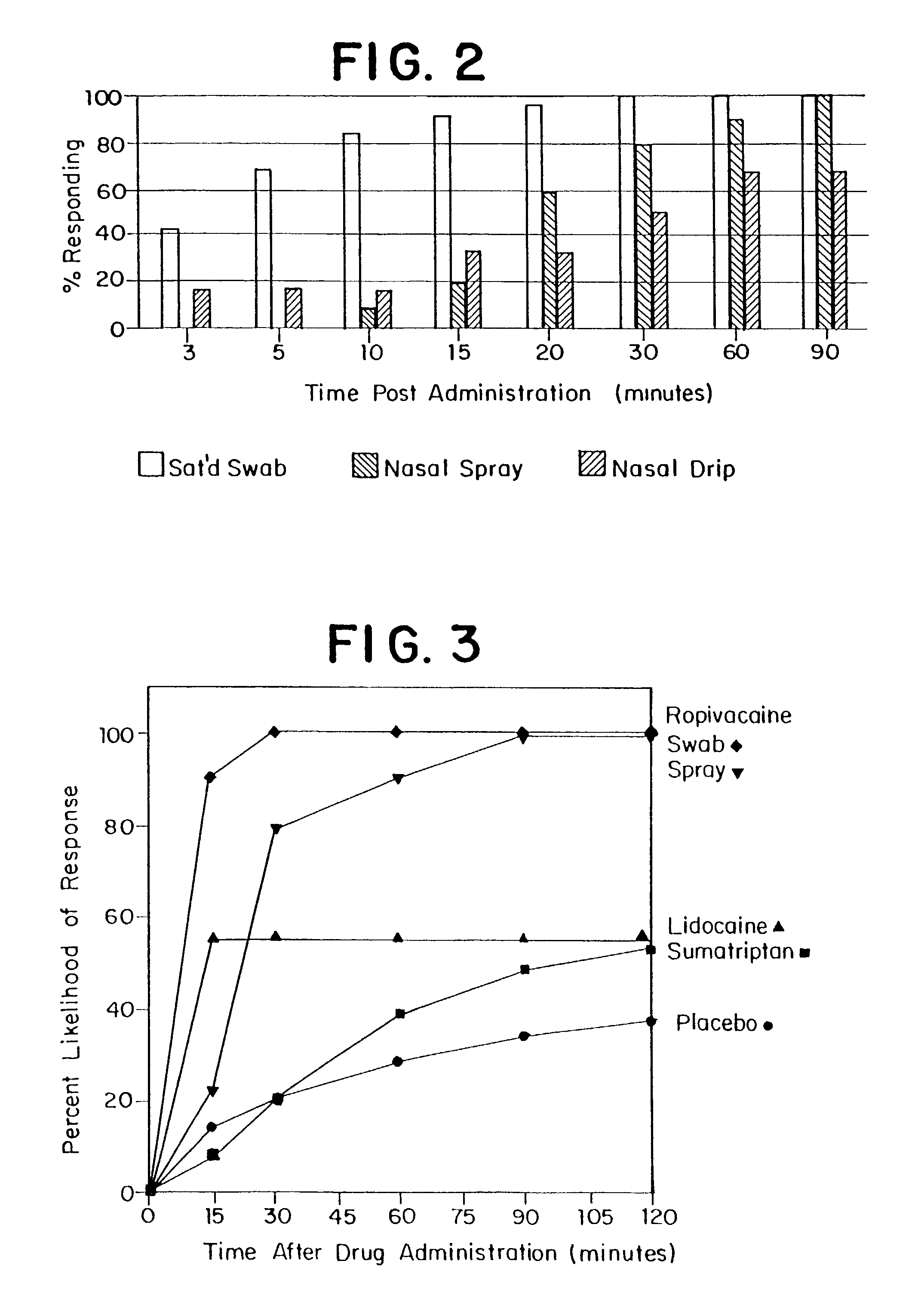
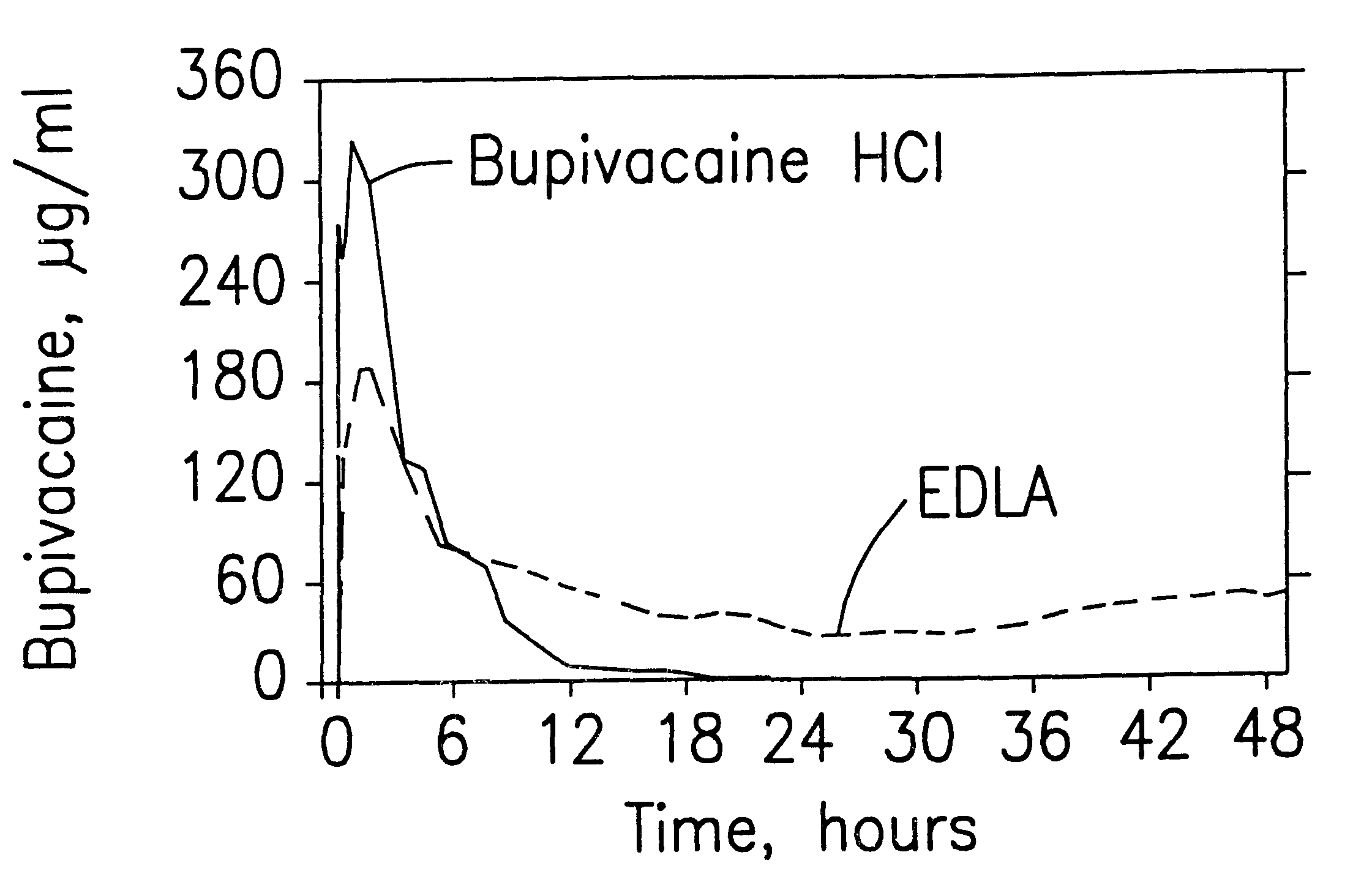
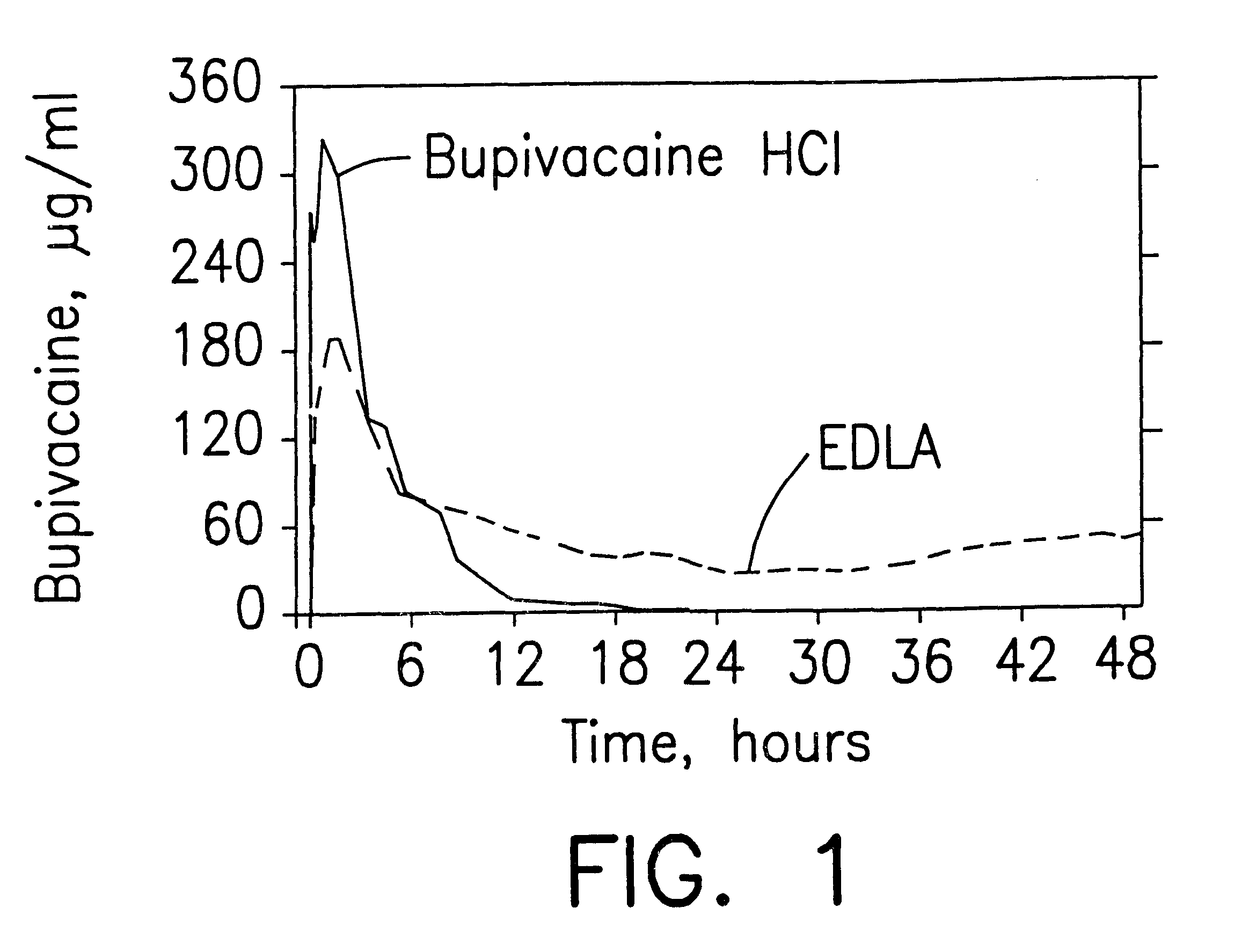
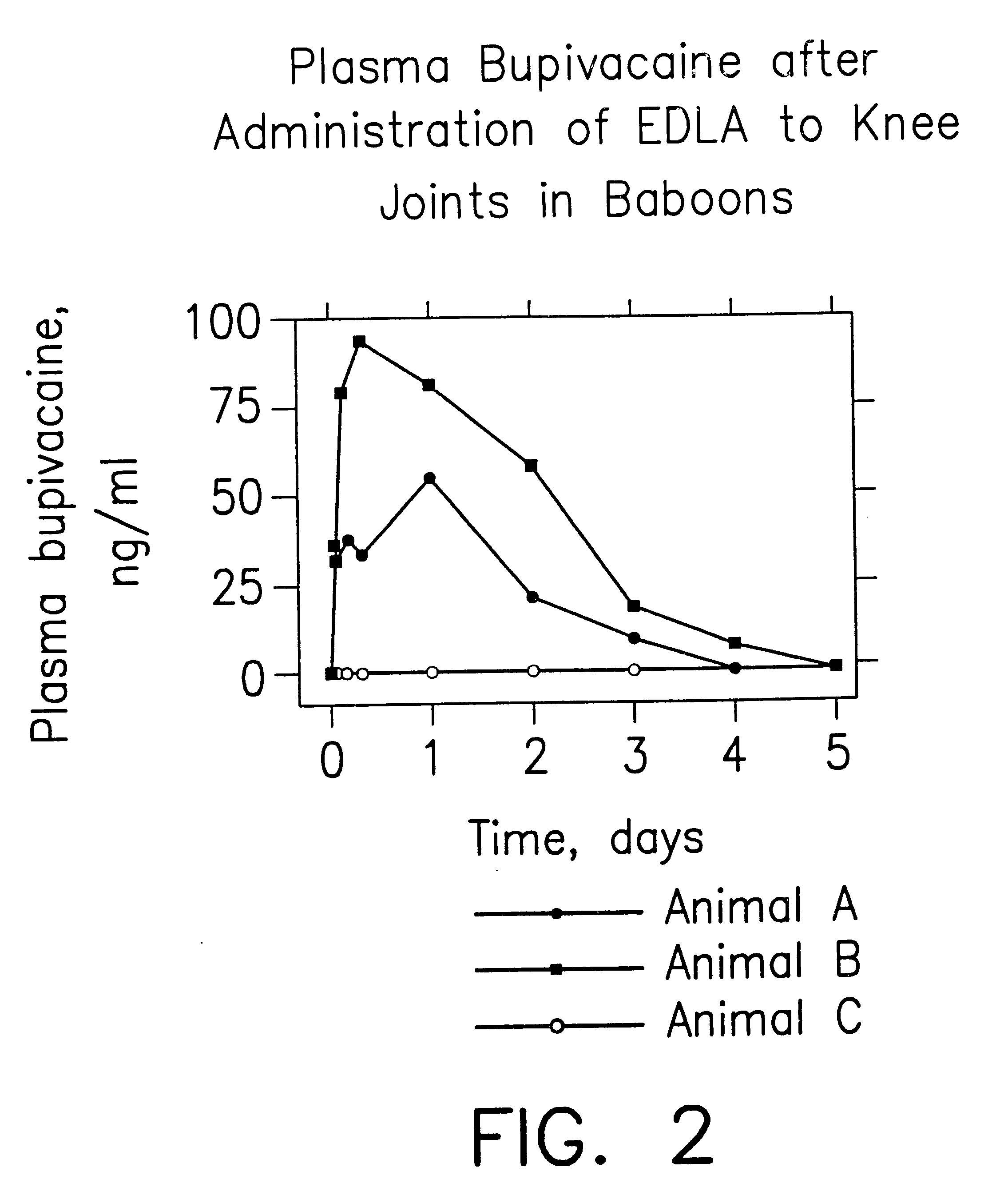
Patents
Literature
466 results about "Local anesthetic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A local anesthetic (LA) is a medication that causes absence of pain sensation. When it is used on specific nerve pathways (local anesthetic nerve block), paralysis (loss of muscle power) also can be achieved.
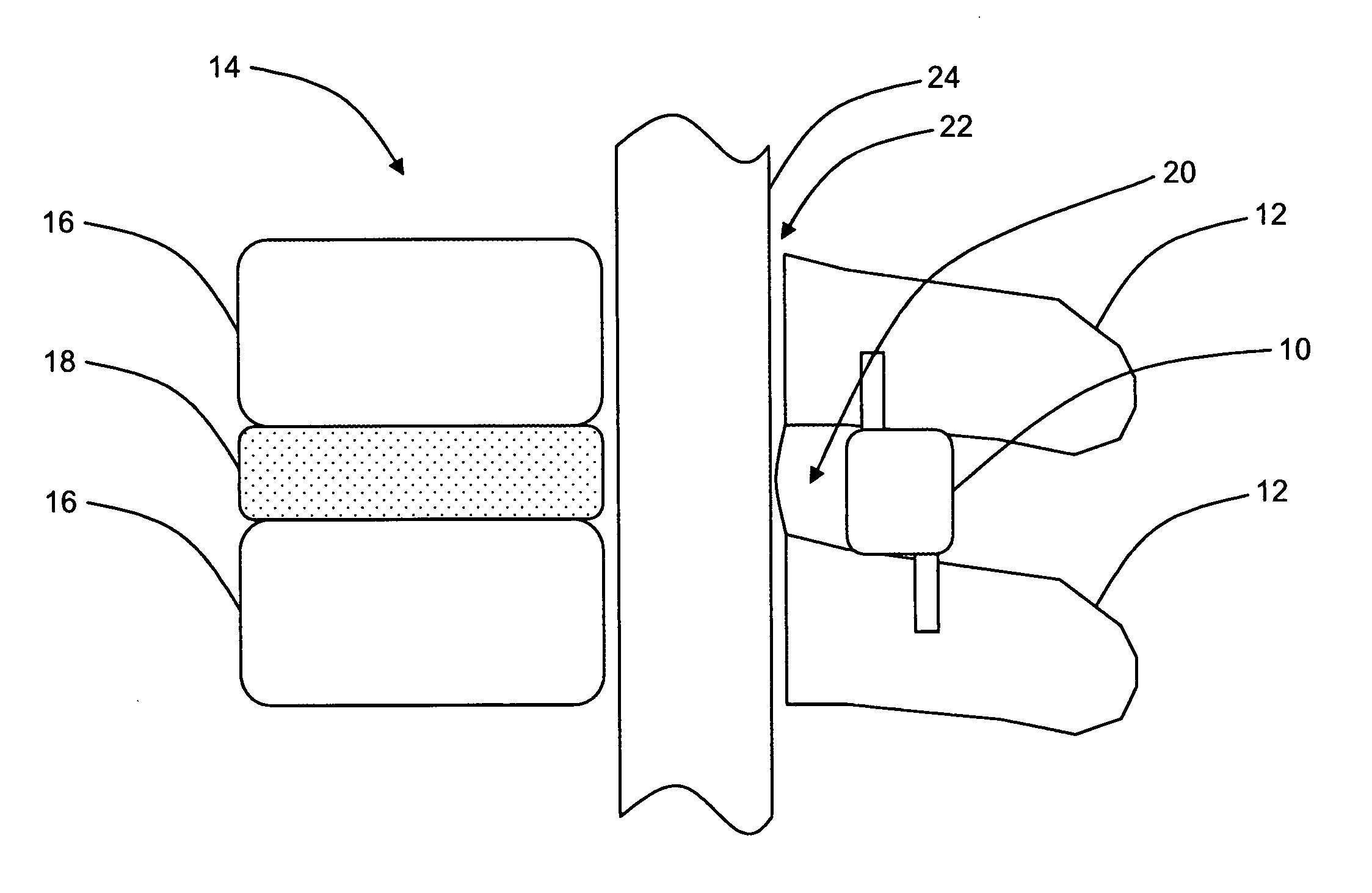
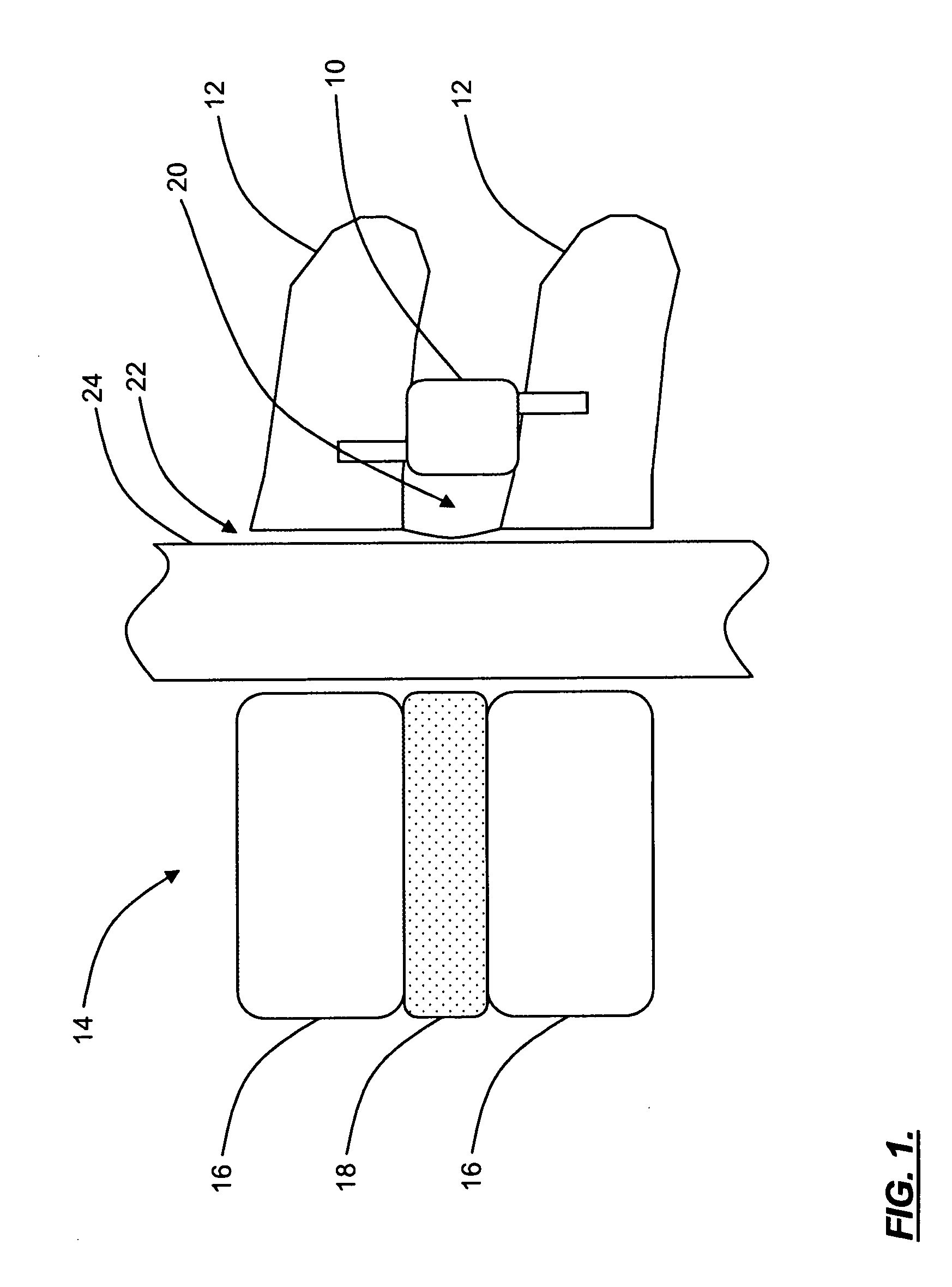
Electrosurgery with infiltration anesthesia
InactiveUS20050267455A1Reliable formationAnaesthesiaSurgical instruments for heatingElectrical resistance and conductanceElectrosurgery
Method for carrying out the recovery of an intact volume of tissue wherein a delivery cannula tip is positioned in confronting adjacency with the volume of tissue to be recovered. The electrosurgical generator employed to form an arc at a capture component extending from the tip is configured having a resistance-power profile which permits recovery of the specimen without excessive thermal artifact while providing sufficient power to sustain a cutting arc. For the recovery procedure, a local anesthetic employing a diluent which exhibits a higher resistivity is utilized and the method for deploying the capture component involves an intermittent formation of a cutting arc with capture component actuation interspersed with pauses of duration effective to evacuate any accumulation or pockets of local anesthetic solution encountered by the cutting electrodes.
Owner:INTACT MEDICAL
Devices and methods for treating pain associated with tonsillectomies
Described here are devices and methods for treating one or more conditions or symptoms associated with a tonsil procedure. In some variations, a drug-releasing device may be at least partially delivered to one or more tonsillar tissues before, during, or after a tonsil procedure. In some variations, the drug-releasing device may be configured to be biodegradable. In other variations, the drug-releasing device may comprise one or more hemostatic materials or one or more adhesives. The drug-releasing device may be configured to release one or more drugs or agents, such as, for example, one or more analgesics, local anesthetics, vasoconstrictors, antibiotics, combinations thereof and the like.
Owner:INTERSECT ENT INC
Electrosurgery with infiltration anesthesia
InactiveUS7004174B2AnaesthesiaVaccination/ovulation diagnosticsElectrical resistance and conductanceElectrosurgery
Method for carrying out the recovery of an intact volume of tissue wherein a delivery cannula tip is positioned in confronting adjacency with the volume of tissue to be recovered. The electrosurgical generator employed to form an arc at a capture component extending from the tip is configured having a resistance-power profile which permits recovery of the specimen without excessive thermal artifact while providing sufficient power to sustain a cutting arc. For the recovery procedure, a local anesthetic employing a diluent which exhibits a higher resistivity is utilized and the method for deploying the capture component involves an intermittent formation of a cutting arc with capture component actuation interspersed with pauses of duration effective to evacuate any accumulation or pockets of local anesthetic solution encountered by the cutting electrodes.
Owner:COVIDIEN AG
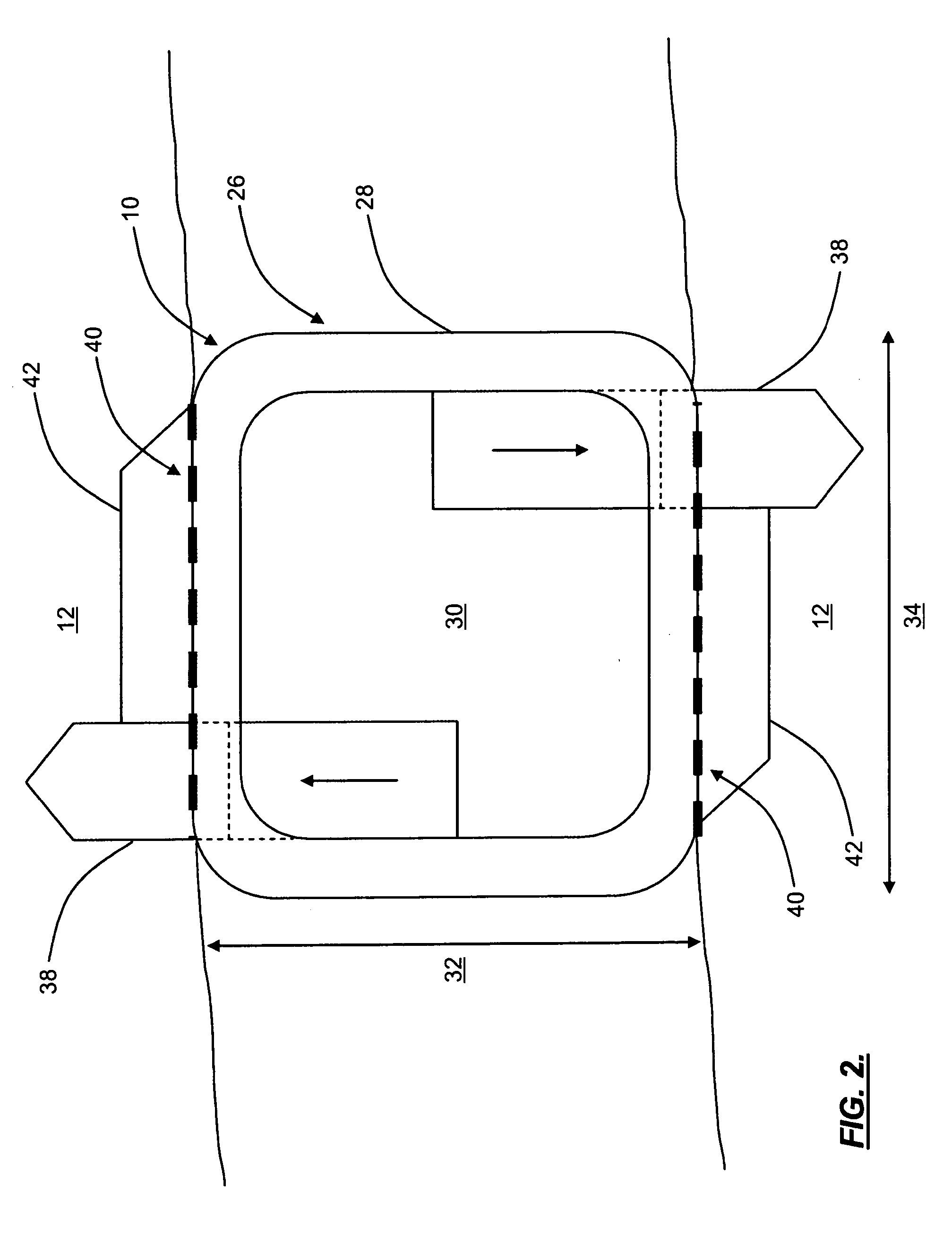
Interspinous distraction devices and associated methods of insertion
InactiveUS20060106397A1Securely holdInternal osteosythesisJoint implantsDistractionMinimally invasive procedures
In various embodiments, the present invention provides a plurality of novel interspinous distraction devices and associated methods of insertion. The interspinous distraction devices of the present invention are designed and configured to effectively treat such conditions as lumbar spinal stenosis and degenerative disc disease. Advantageously, the interspinous distraction devices of the present invention may be inserted through conventional open procedures, typically requiring a relatively large incision and a general anesthetic, or through novel minimally-invasive procedures, typically requiring only a local anesthetic. These novel minimally-invasive procedures and related enabling devices are also disclosed and described herein.
Owner:ZIMMER BIOMET SPINE INC
Interspinous distraction devices and associated methods of insertion
In various exemplary embodiments, the present invention provides a plurality of novel interspinous distraction devices and associated methods of insertion. The interspinous distraction devices of the present invention are designed and configured to effectively treat such conditions as lumbar spinal stenosis and degenerative disc disease. Advantageously, the interspinous distraction devices of the present invention may be inserted through conventional open procedures, typically requiring a relatively large incision and a general anesthetic, or through novel minimally-invasive procedures, typically requiring only a local anesthetic. These novel minimally-invasive procedures and related enabling devices are also disclosed and described herein.
Owner:ZIMMER BIOMET SPINE INC
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Regional anesthetic
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
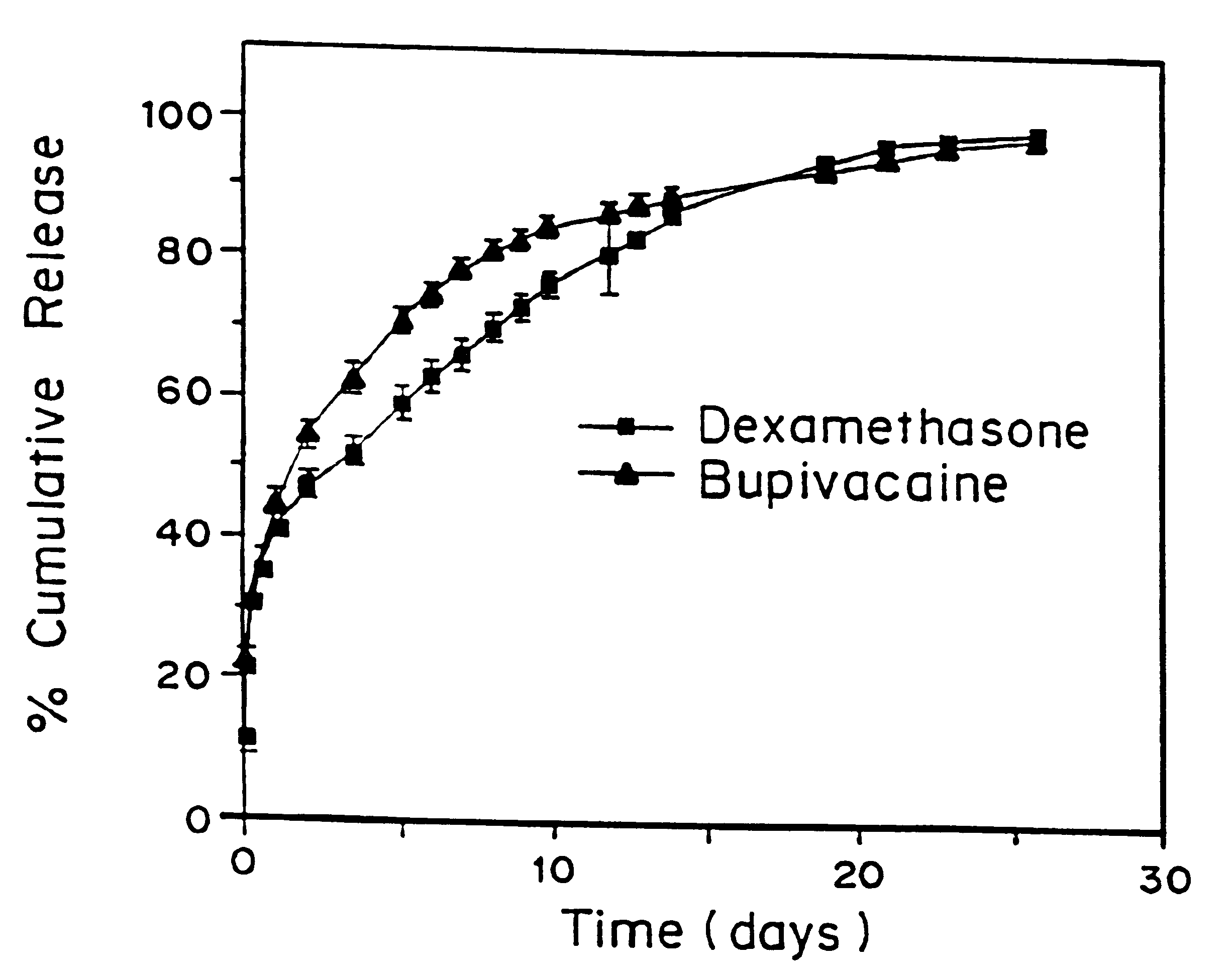
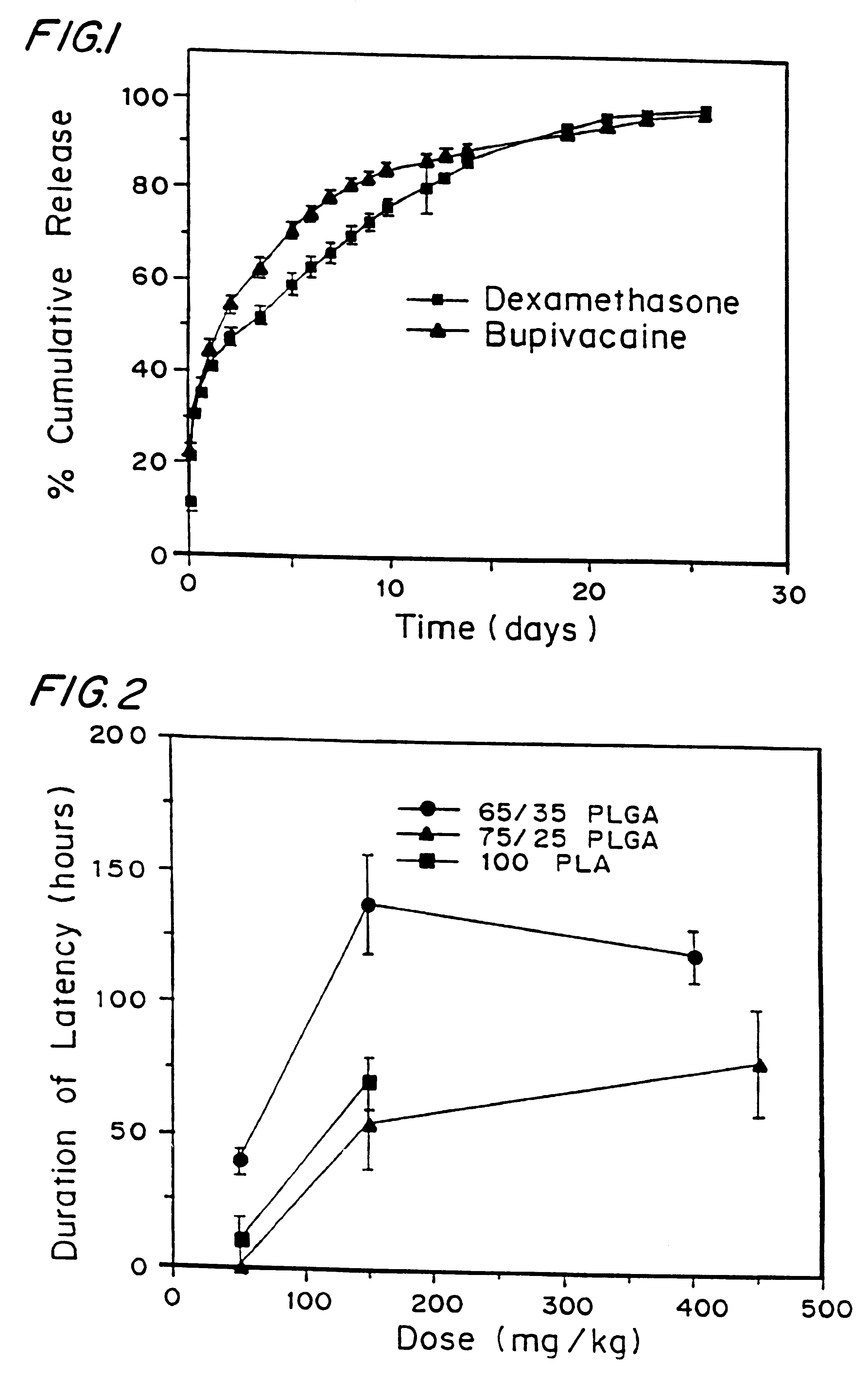
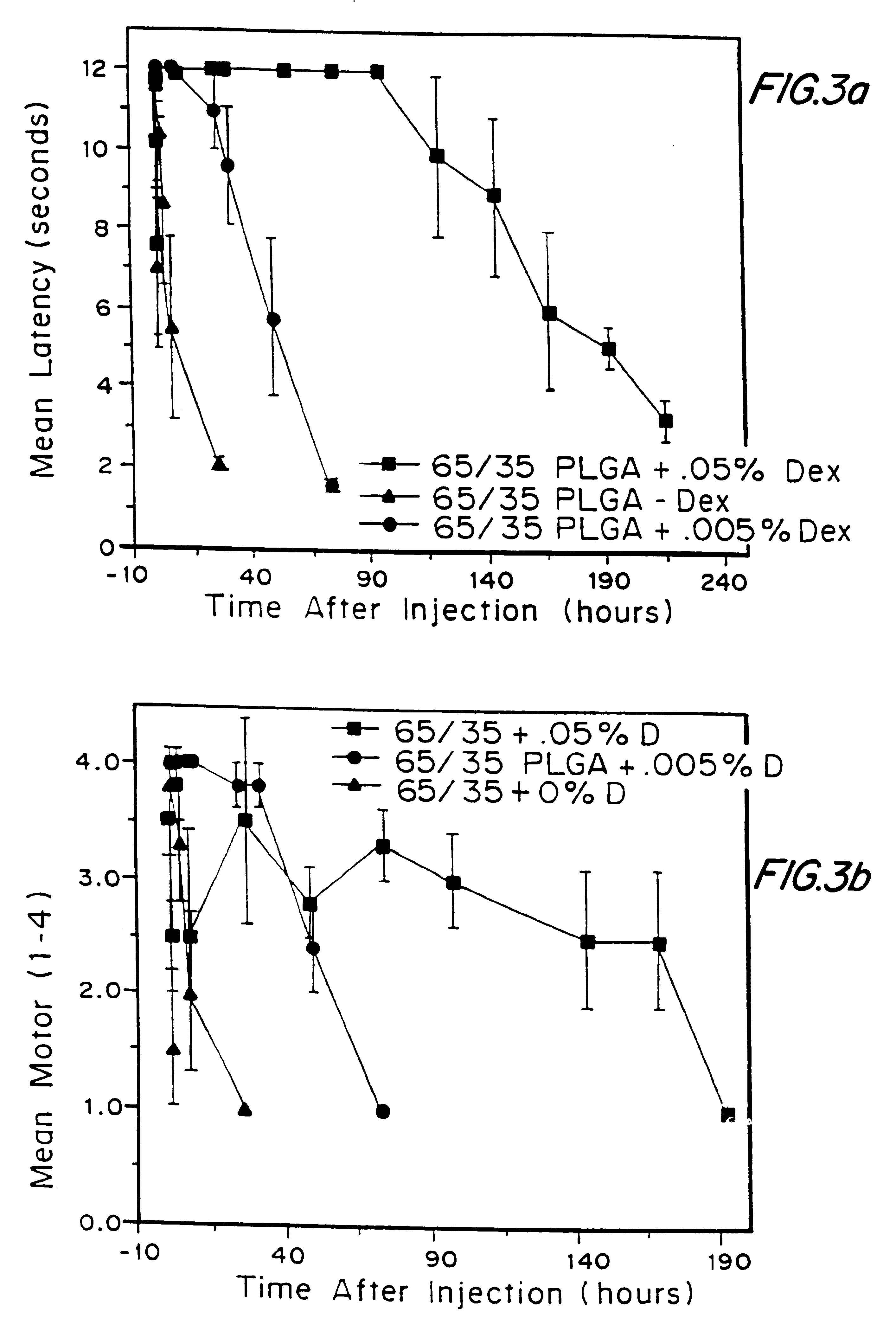
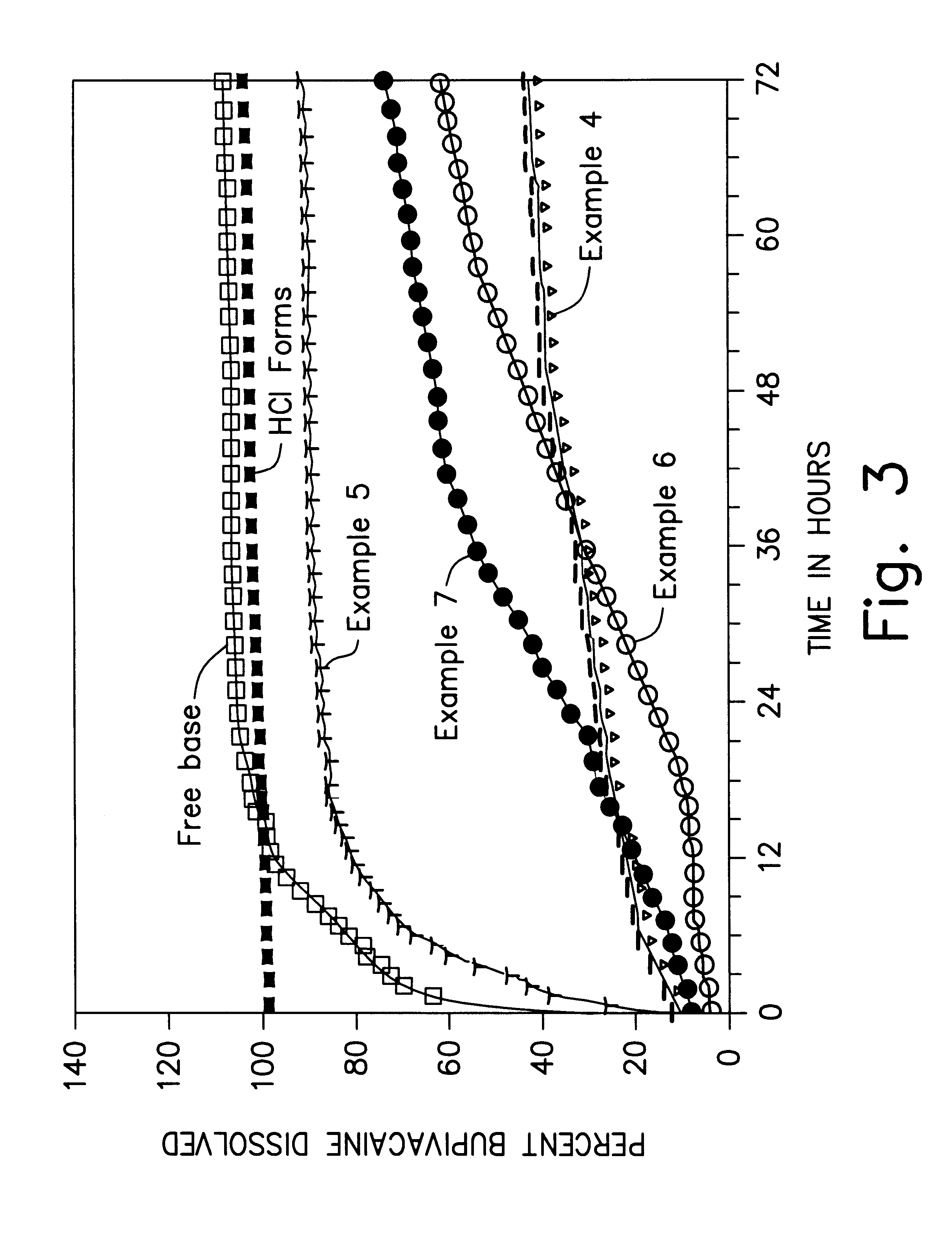
High load formulations and methods for providing prolonged local anesthesia
A formulation for inducing sustained local anesthesia in a patient comprising a substrate comprising a high load of local anesthetic by weight and an effective amount of a biocompatible, controlled release material to obtain a. reversible nerve blockade or anesthesia effect when implanted or injected in a patient, and a non-toxic glucocorticosteroid agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the glucocorticosteroid agent.
Owner:CHILDRENS MEDICAL CENT CORP
Topical compositions and methods for treating pain
InactiveUS6638981B2Avoid painComposition is stableBiocideNervous disorderNR1 NMDA receptorPreventing pain
Topical compositions and methods for treating pain. The invention provides oil-in-water emulsions comprising an antidepressant; an NMDA-receptor antagonists; a lipophilic component; water; and a surfactant. The compositions induce a local-anesthetic effect when topically administered to intact skin thereby treating or preventing pain, for example, neuropathic pain.
Owner:EPICEPT CORP
Skin resurfacing and treatment using biocompatible materials
InactiveUS20050059940A1Eliminate the problemAvoid infectionSurgeryMedical devicesHuman bodyCarrier fluid
Biocompatible materials are propelled at the skin with sufficient velocity to cause desired resurfacing of skin layers to the desired penetration depth. The materials, such as dry ice or water ice, are harmonious with the human body and thus eliminate foreign body reactions. Various materials may be used in combination, including local anesthetics and vasoconstrictors in solid or liquid form. The biocompatible solid or liquid particles are suspended in a cold carrier fluid and propelled through an insulated delivery system to the surface of the skin. The treatment of diseased skin lesions may be accomplished using the present invention as a drug delivery system.
Owner:PEARL TECHNOLOGY HOLDINGS LLC
Method for directed intranasal administration of a composition
Methods, kits, apparatus, and compositions for inhibiting a cerebral neurovascular disorder, a muscular headache, or cerebral inflammation in a human patient are provided. The methods comprise intranasally administering to the patient a pharmaceutical composition comprising a local anesthetic, and preferably a long-acting local anesthetic ingredient. A composition useful for practicing the methods of the invention is described which comprises at least one local anesthetic in a pharmaceutically acceptable carrier, wherein the composition is formulated for intranasal delivery. Cerebral neurovascular disorders include migraine and cluster headache. Muscular headaches include tension headaches and muscle contraction headaches. A kit comprising the composition and an intranasal applicator and a method of systemically delivering a pharmaceutically active agent to an animal are also included in the invention. Apparatus for directed intranasal administration of the compositions of the invention and for performing the methods of the invention are also described.
Owner:BHL PATENT HLDG
Formulations and methods for providing prolonged local anesthesia
InactiveUS6451335B1Slow in-vitro releaseRelease the local anestheticAnaesthesiaGranular deliveryControlled releaseAnesthetic Agent
A formulation for inducing sustained regional local anesthesia in a patient comprising a substrate comprising a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material prolonging the release of the local anesthetic from the substrate to obtain a reversible local anesthesia when implanted or injected in a patient, and a non-toxic augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable from the substrate without the augmenting agent. In preferred embodiments, the controlled release material is a low molecular weight, acid-terminated polymer. A further aspect of the invention is directed to such formulations which release the local anesthetic in two phases, the first a rapid "bolus" to initiate anesthesia and a second, slower release to maintain anesthesia.
Owner:EURO-CELTIQUE SA
Sterile, breathable patch for treating wound pain
InactiveUS20030082225A1Not further irritate the wound upon removalBiocideNervous disorderAnesthetic AgentIntact skin
An intradermal patch having a permeable backing coated with a polyvinylpyrrolidone-based hydrogel and containing one or more local anesthetics. The patch is breathable, non-irritating upon application and removal, soothing, and sterile. The patch is useful for treating the pain associated with non-intact skin indications.
Owner:EPICEPT CORP
Apparatus and method for the treatment of stress urinary incontinence
An apparatus and method for the treatment of stress urinary incontinence. The apparatus includes a suburethral sling having an adjustment member for adjusting the tension of the sling both during the procedure and post-procedure. The method includes using a needle to simultaneously implant the sling and to deliver a local anesthetic in the groin area while implanting the sling.
Owner:BOSTON SCI SCIMED INC
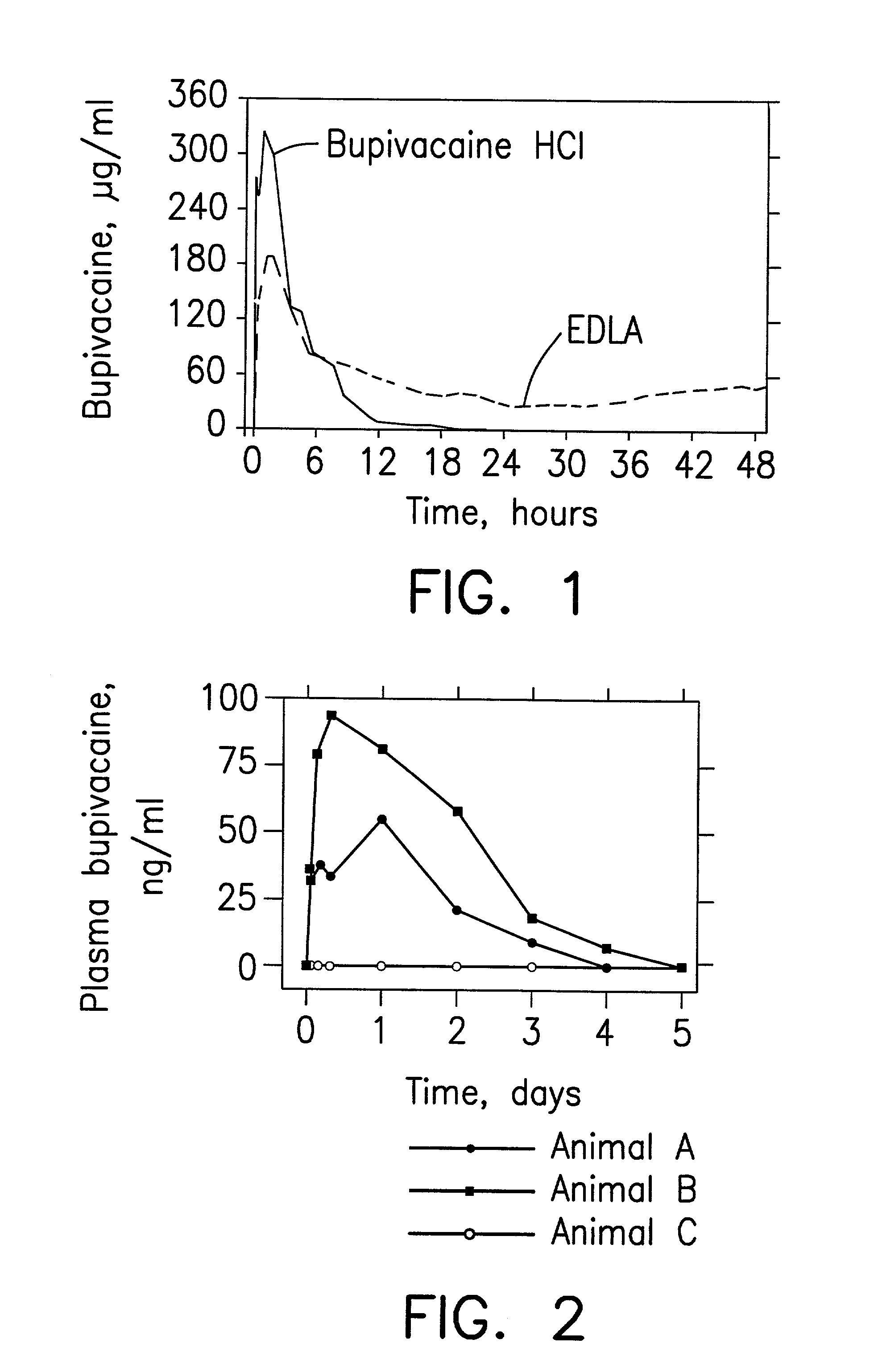
Sustained-release liposomal anesthetic compositions
InactiveUS8182835B2High acceptabilityImprove encapsulationInorganic non-active ingredientsAnaestheticsHalf-lifeMaximum tolerated dose
Owner:PACIRA PHARMA INC
Methods for providing safe local anesthesia
InactiveUS6699908B2Reduce riskImproved therapeutic indexBiocidePeptide/protein ingredientsAnesthetic AgentControlled release
Methods and formulations for inducing substantially safer local anesthesia in a patient are provided. The methods comprise administering, to a patient in need thereof, a substrate containing a local anesthetic and an effective amount of a biocompatible, biodegradable, controlled release material to safely obtain a reversible nerve blockade when implanted or injected in a patient.
Owner:PURDUE PHARMA LP
Topical compositions and methods for treating pain
InactiveUS20030082214A1Treating and preventing painAvoid painBiocideNervous disorderPreventing painNR1 NMDA receptor
Topical compositions and methods for treating pain. The invention provides oil-in-water emulsions comprising an antidepressant; an NMDA-receptor antagonists; a lipophilic component; water; and a surfactant. The compositions induce a local-anesthetic effect when topically administered to intact skin thereby treating or preventing pain, for example, neuropathic pain.
Owner:EPICEPT CORP
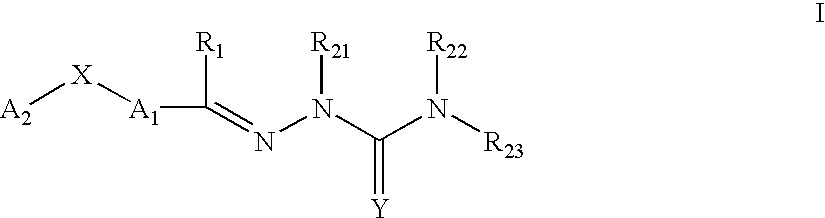
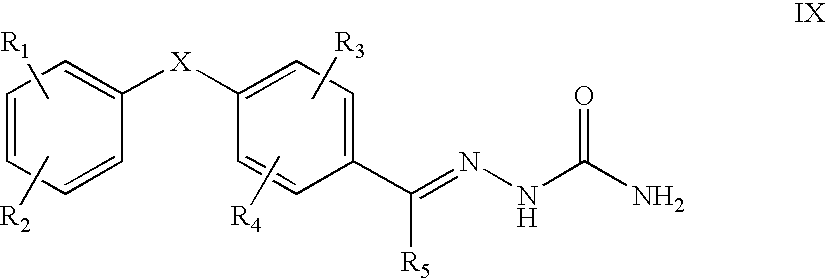
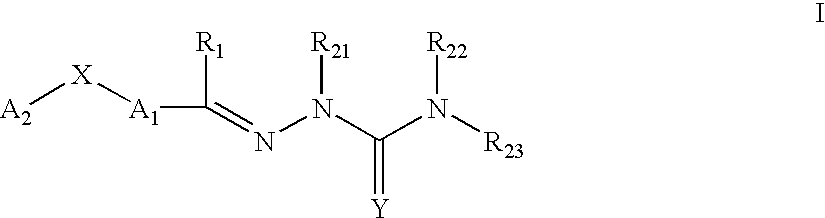
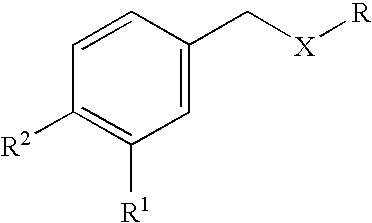
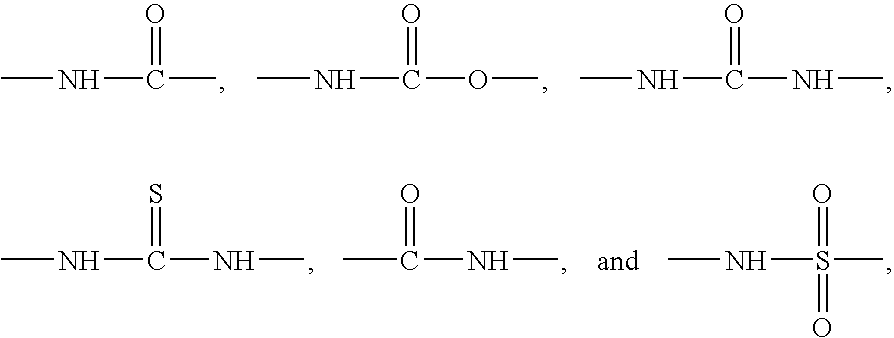
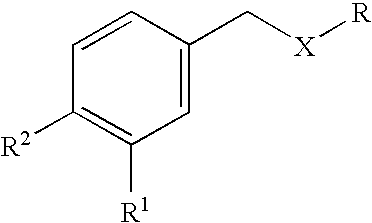
Carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones and the use thereof
This invention is related to carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones represented by Formula I: ##STR1## or a pharmaceutically acceptable salt or prodrug thereof, wherein: Y is oxygen or sulfur; R.sub.1, R.sub.21, R.sub.22 and R.sub.23 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl; or R.sub.22 and R.sub.23, together with the N, form a heterocycle; A.sub.1 and A.sub.2 are independently aryl, heteroaryl, saturated or partially unsaturated carbocycle or saturated or partially unsaturated heterocycle, any of which is optionally substituted; X is one or O, S, NR.sub.24, CR.sub.25 R.sub.26, C(O), NR.sub.24 C(O), C(O)NR.sub.24, SO, SO.sub.2 or a covalent bond; where R.sub.24, R.sub.25 and R.sub.26 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl. The invention also is directed to the use of carbocycle and heterocycle substituted semicarbazones and thiosemicarbazones for the treatment of neuronal damage following global and focal ischemia, for the treatment or prevention of neurodegenerative conditions such as amyotrophic lateral sclerosis (ALS), for the treatment and prevention of otoneurotoxicity and eye diseases involving glutamate toxicity and for the treatment, prevention or amelioration of pain, as anticonvulsants, and as antimanic depressants, as local anesthetics, as antiarrhythmics and for the treatment or prevention of diabetic neuropathy and urinary incontinence.
Owner:COCENSYS
Pharmaceutical dental formulation for topical application of metronidazole benzoate, chlorhexidine gluconate and local anesthetic
Pharmaceutical dental gel preparation comprising of metronidazole benzoate, chlorhexidine gluconate, and local anesthetic as the active ingredient; glycol as the solvent medium; a carboxyvinyl polymer, cross-linked polymer of acrylic acid copolymerized with polyalkylsucrose as a gelling agent.
Owner:J B CHEM & PHARMA
Treatment of retinal detachment
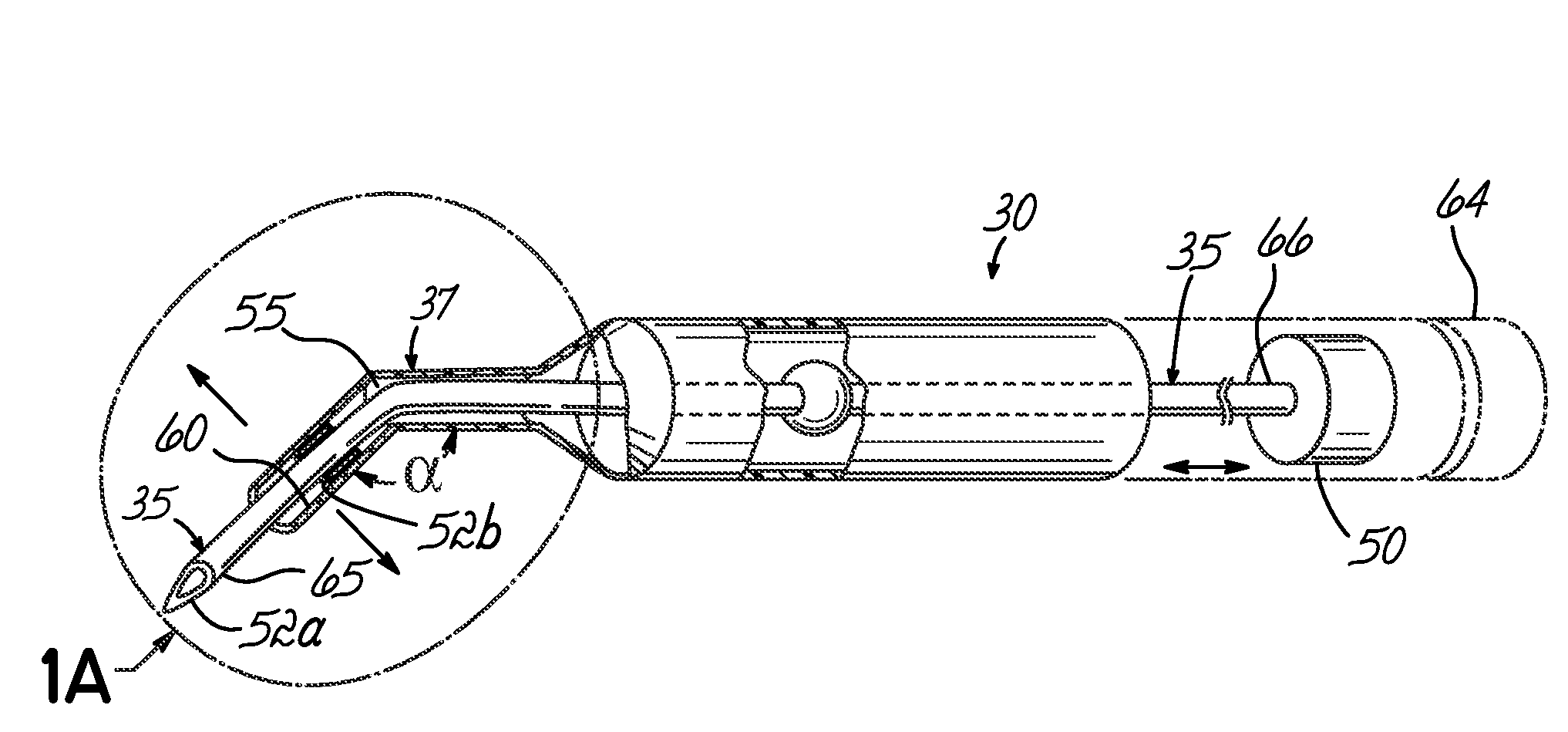
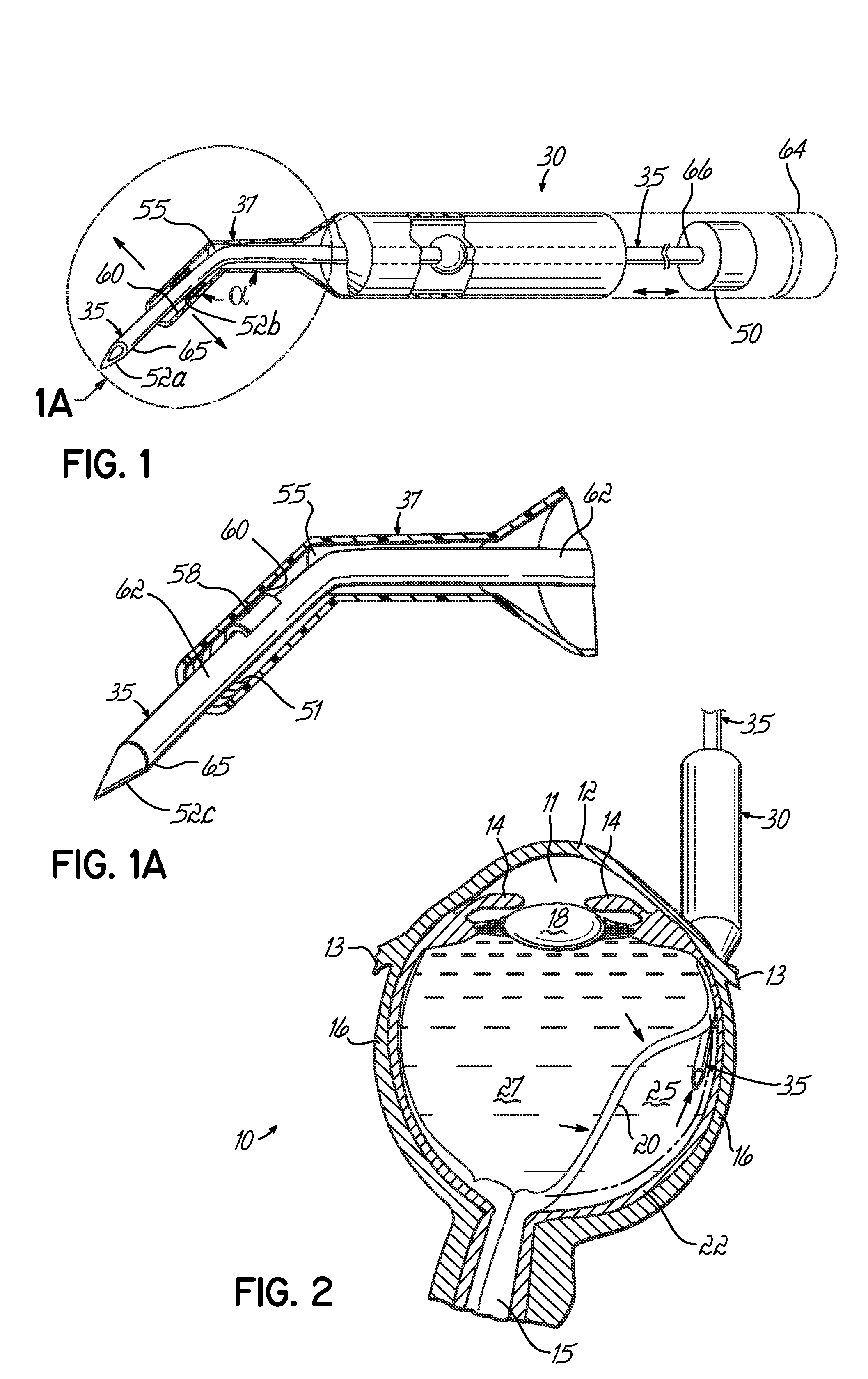
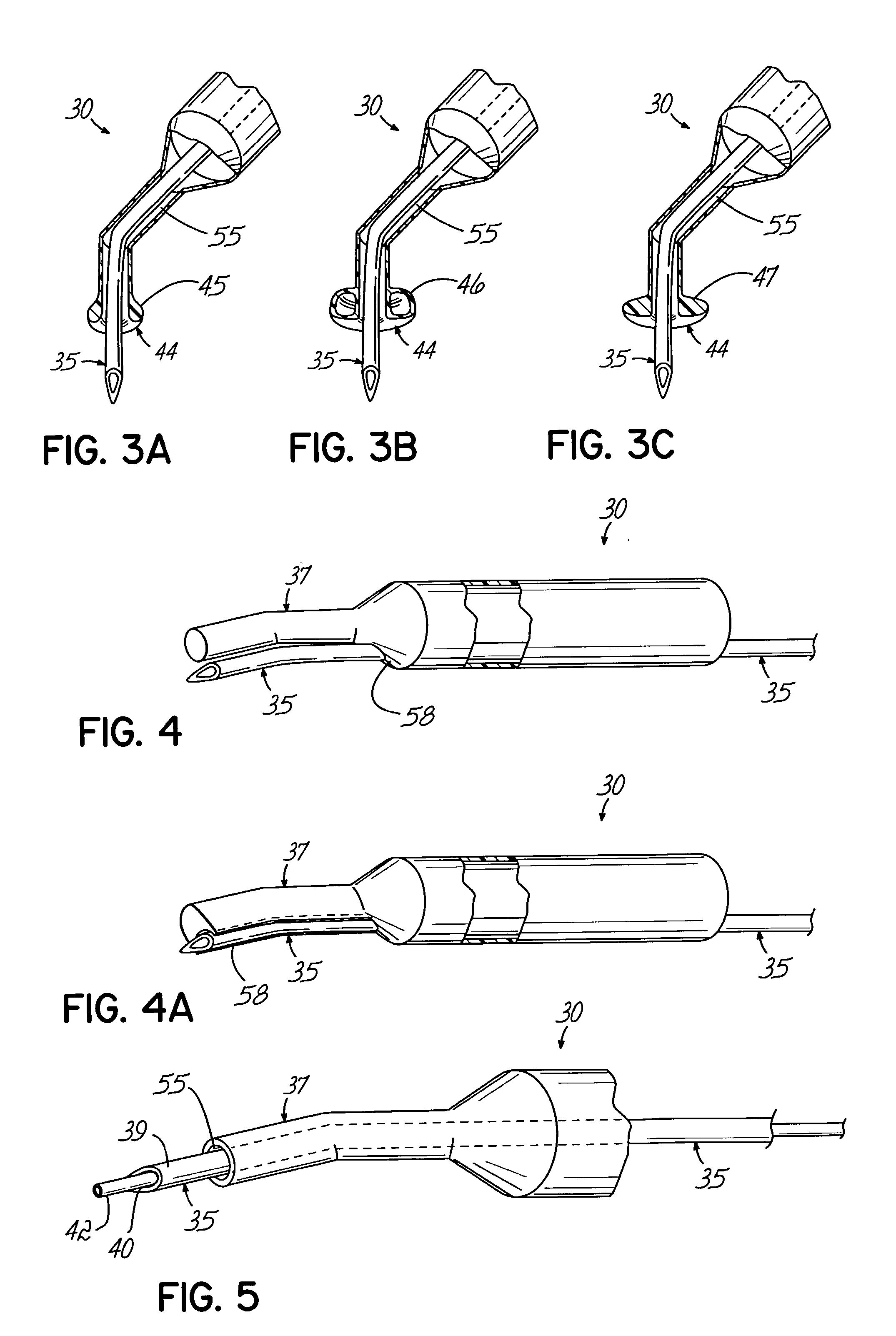
An apparatus and minimally invasive method for removing fluid from a subretinal space to allow a detached retina to flatten. An apparatus, comprising a fluid withdrawal device and a guide for advancing and placing the device, is positioned on the exterior eye surface at the detachment site. Various embodiments of the apparatus are disclosed. Using the guide, the surgeon advances the device into the fluid-filled space and drains fluid, allowing the retina to flatten. Additional injection of saline or gas into the vitreous cavity normalizes intraocular pressure, and the patient is ambulatory immediately afterward. Unlike other retinal attachment techniques, in the inventive procedure the patient receives only a local anesthetic, and has no restraint on head movement.
Owner:PEYMAN GHOLAM A
System and method for preventing wrong-site surgeries
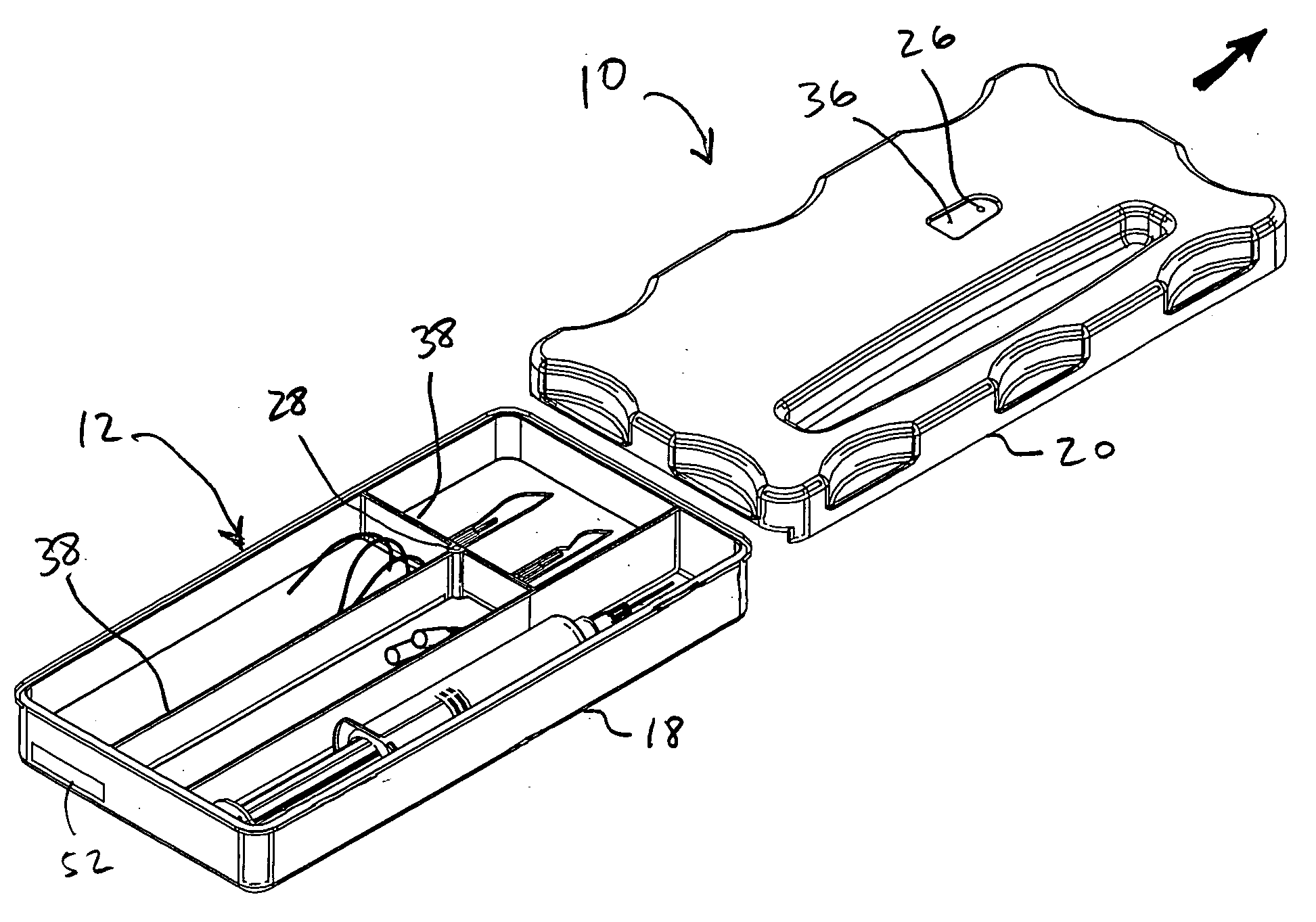
ActiveUS20060096877A1Preventing wrong-site surgeryPreventing wrong-site surgeriesSurgical furnitureDispensing apparatusMedical recordLocking mechanism
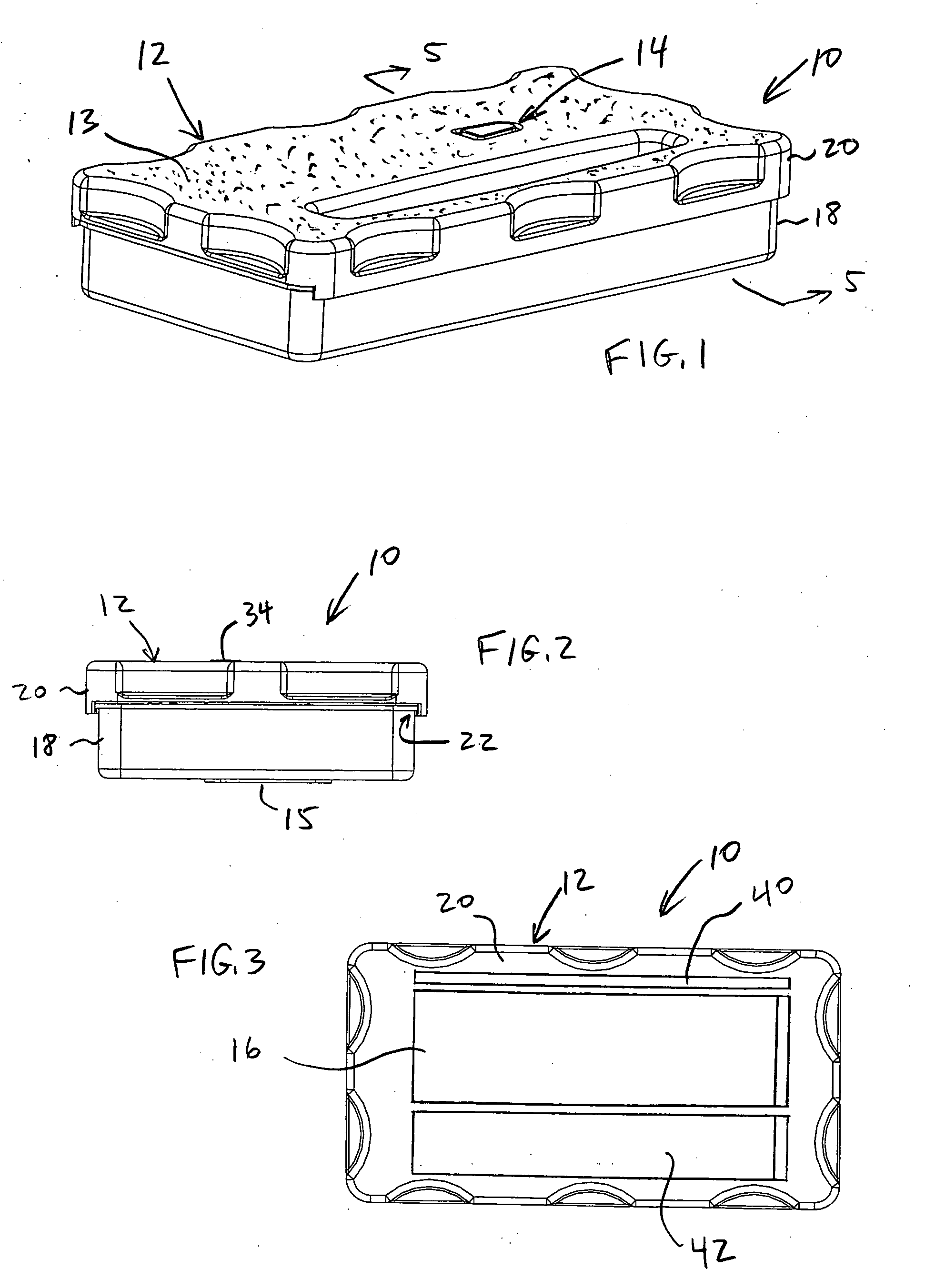
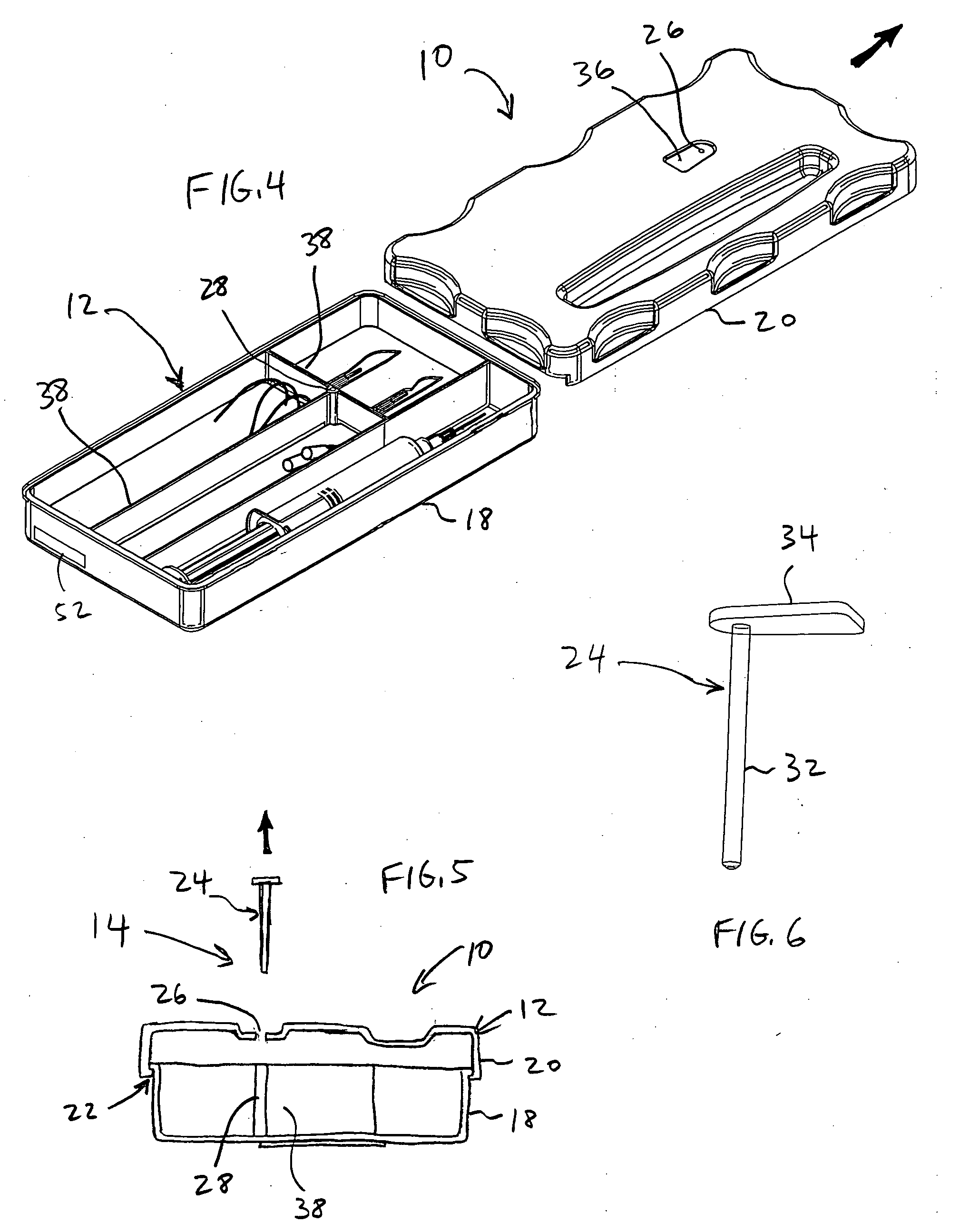
A container holds at least one surgical implement, has a lock mechanism, and has a signature label that impedes access to the surgical implement until the correct surgical site is confirmed. A method of using the container includes the steps of confirming the correct surgical site, signing the label and removing it from the container, placing the label in the medical record, unlocking the container, removing the implement, and beginning the surgery, wherein the surgical team is forced to pause to confirm the correct surgical site before starting the surgery. Preferably, the container top may be removed and placed between the surgeon and surgical technician to define a no-hands “neutral zone” to avoid being stuck by the sharps. Also, the container preferably includes compartments for storing used sharps and / or a local anesthetic-loaded syringe, and the top may be replaced and secured for safely disposing of the sharps after the surgery.
Owner:STARTBOX
Topical anesthetic for rapid local anesthesia and method of applying a topical anesthetic
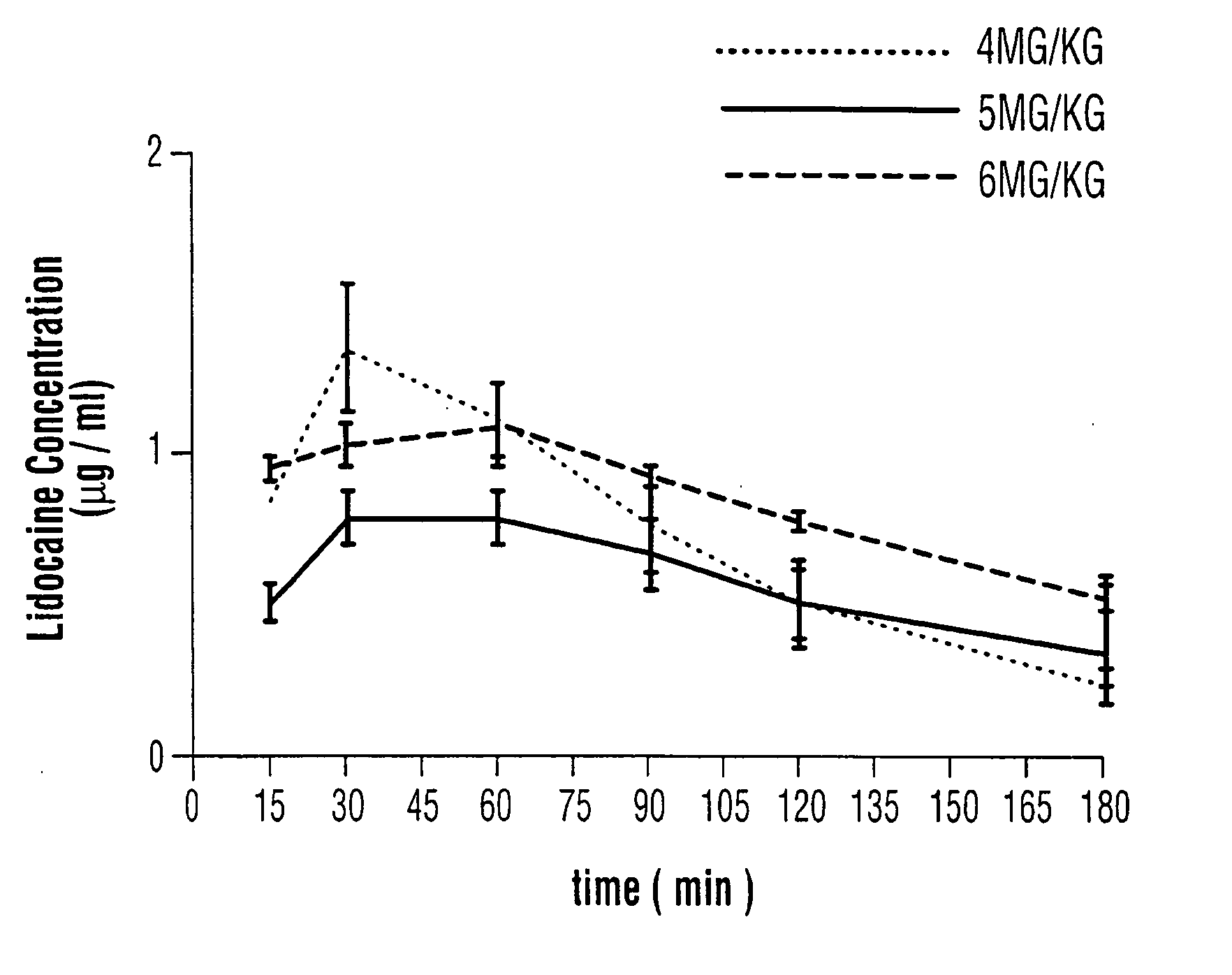
The invention relates to a drug delivery system for the topical administration of anesthetic agents. For example, a topical anesthetic for rapid local anesthesia is provided. The topical anesthetic includes an anesthetic, volatile and non-volatile solvents, and an optional thickener. In addition, a method is taught for applying the topical anesthetic to the face of a patient without occlusion. The anesthetic is applied topically to an area for injection such that the dermatological procedure (cosmetic injections) can be performed in fifteen minutes.
Owner:JUVENTIO
Topical anesthesia of the urinary bladder
InactiveUS20050238733A1Reduce concentrationReduce absorptionBiocideInorganic active ingredientsSodium bicarbonateCystoscopy
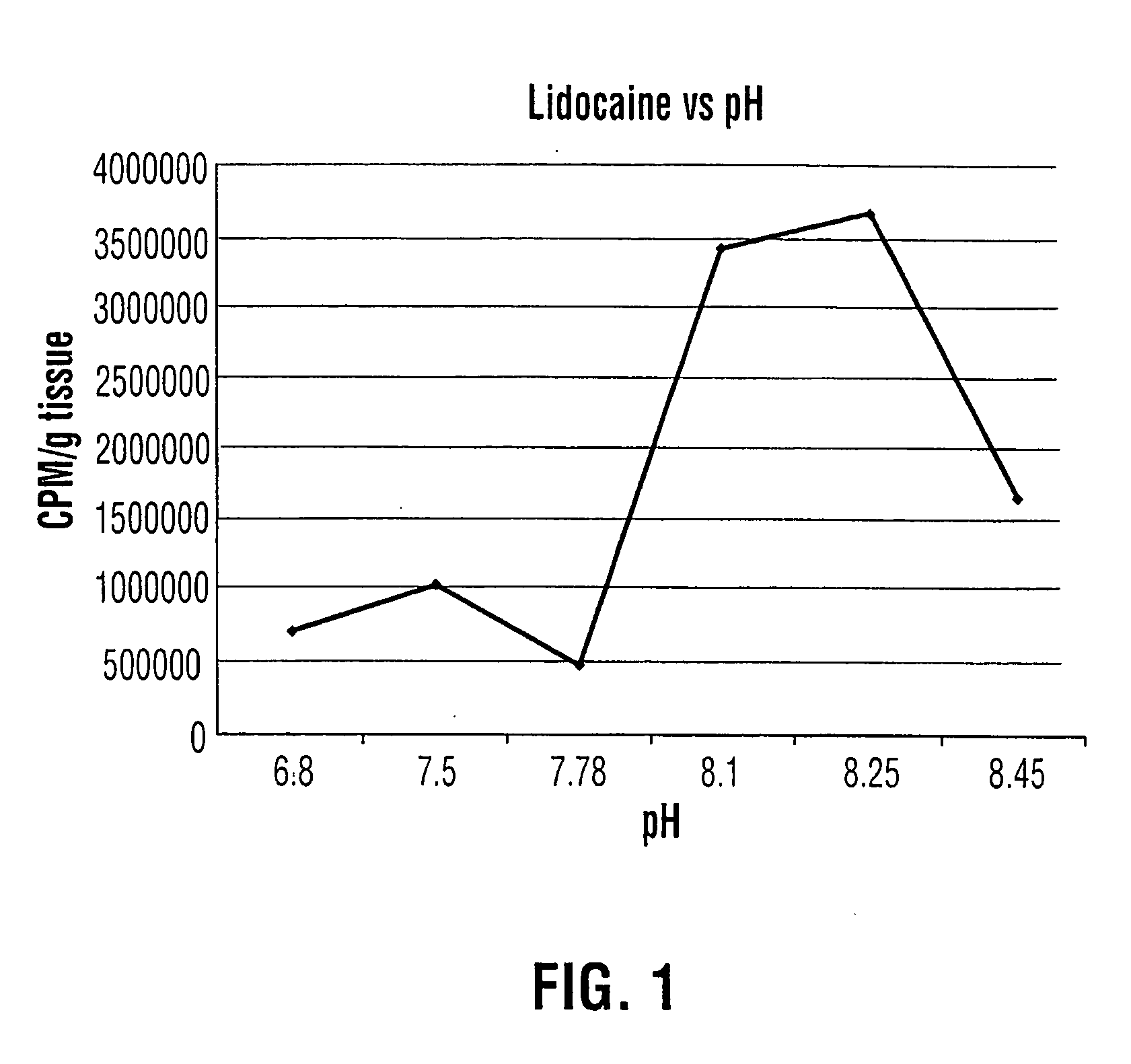
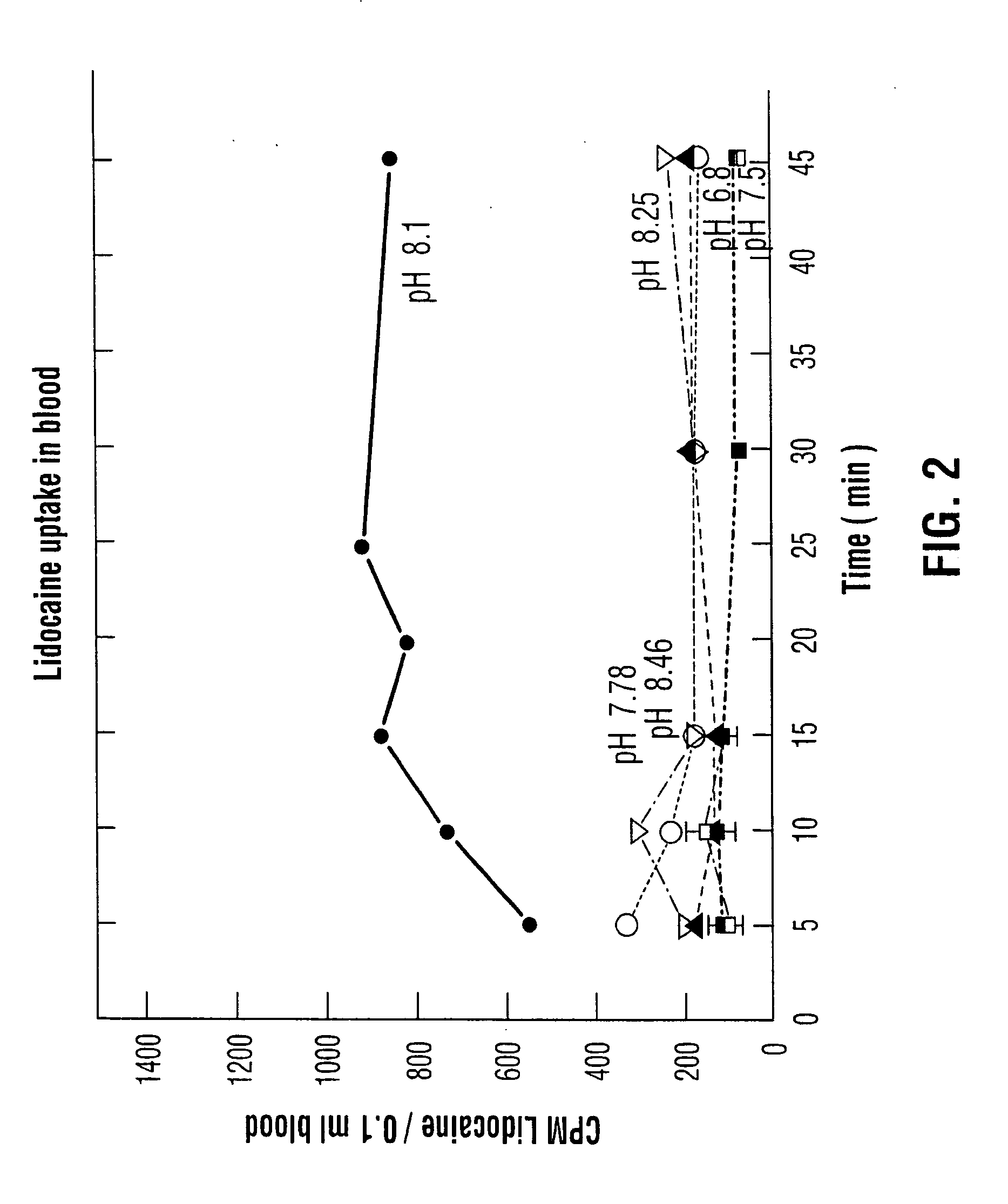
An aqueous solution of local anesthetic is instilled into the urinary bladder in sufficient concentration with the addition of an alkalinizing agent such as sodium bicarbonate to elevate the intra-vesical pH to approximately 8.0. The combination is left in situ in the bladder for at least fifteen minutes to allow time for absorption of the base form of the local anesthetic. This method provides safe and effective topical anesthesia to allow pain-free cystoscopic biopsy and cautery of bladder lesions such as bladder cancer, and provides a means to treat inflammatory conditions of the bladder such as chronic interstitial cystitis and acute bacterial cystitis.
Owner:HENRY RICHARD
Injectable biodegradable polymer compositions for soft tissue repair and augmentation
InactiveUS20100260703A1Pharmaceutical delivery mechanismUrinary disorderInjectable polymersActive agent
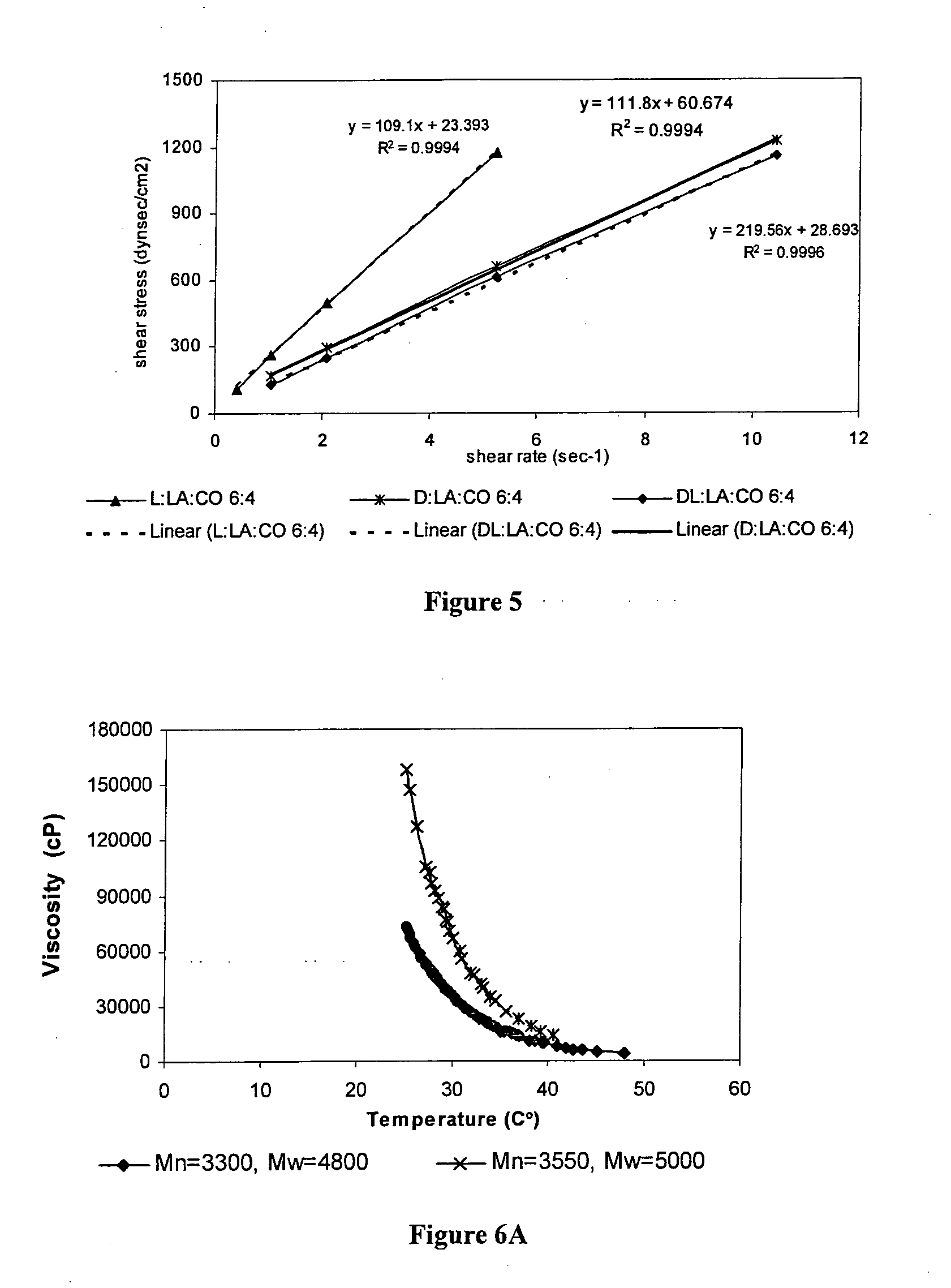
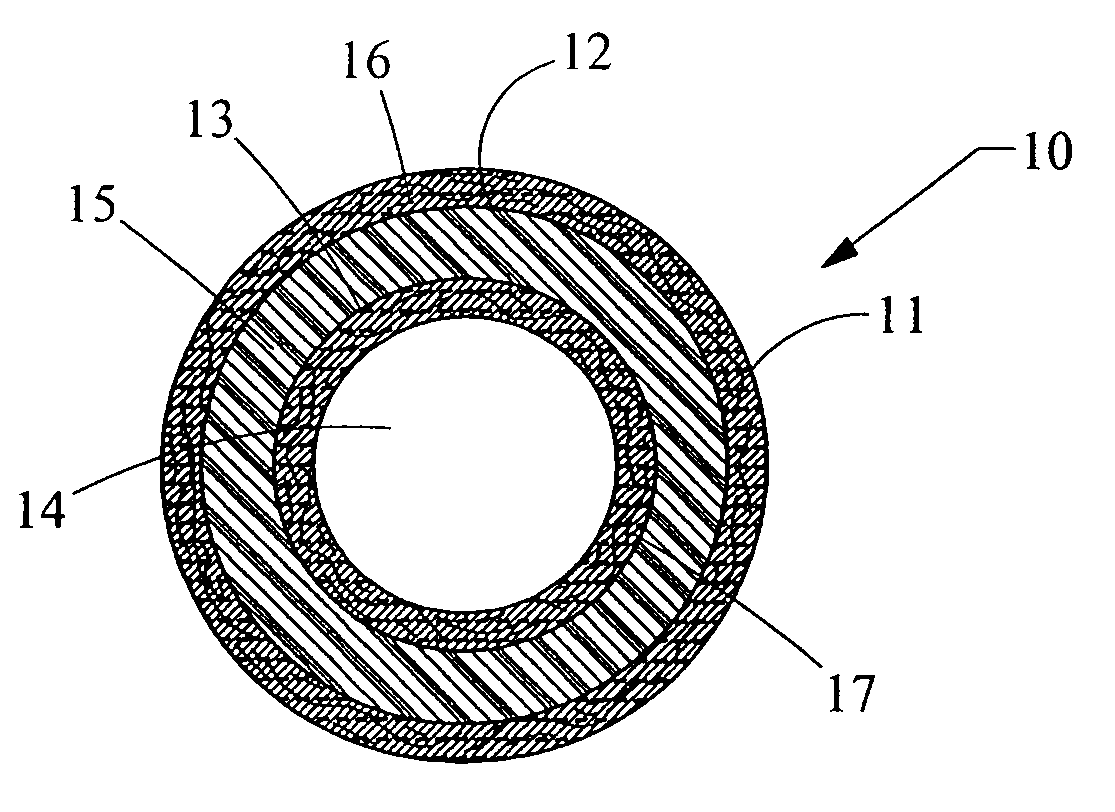
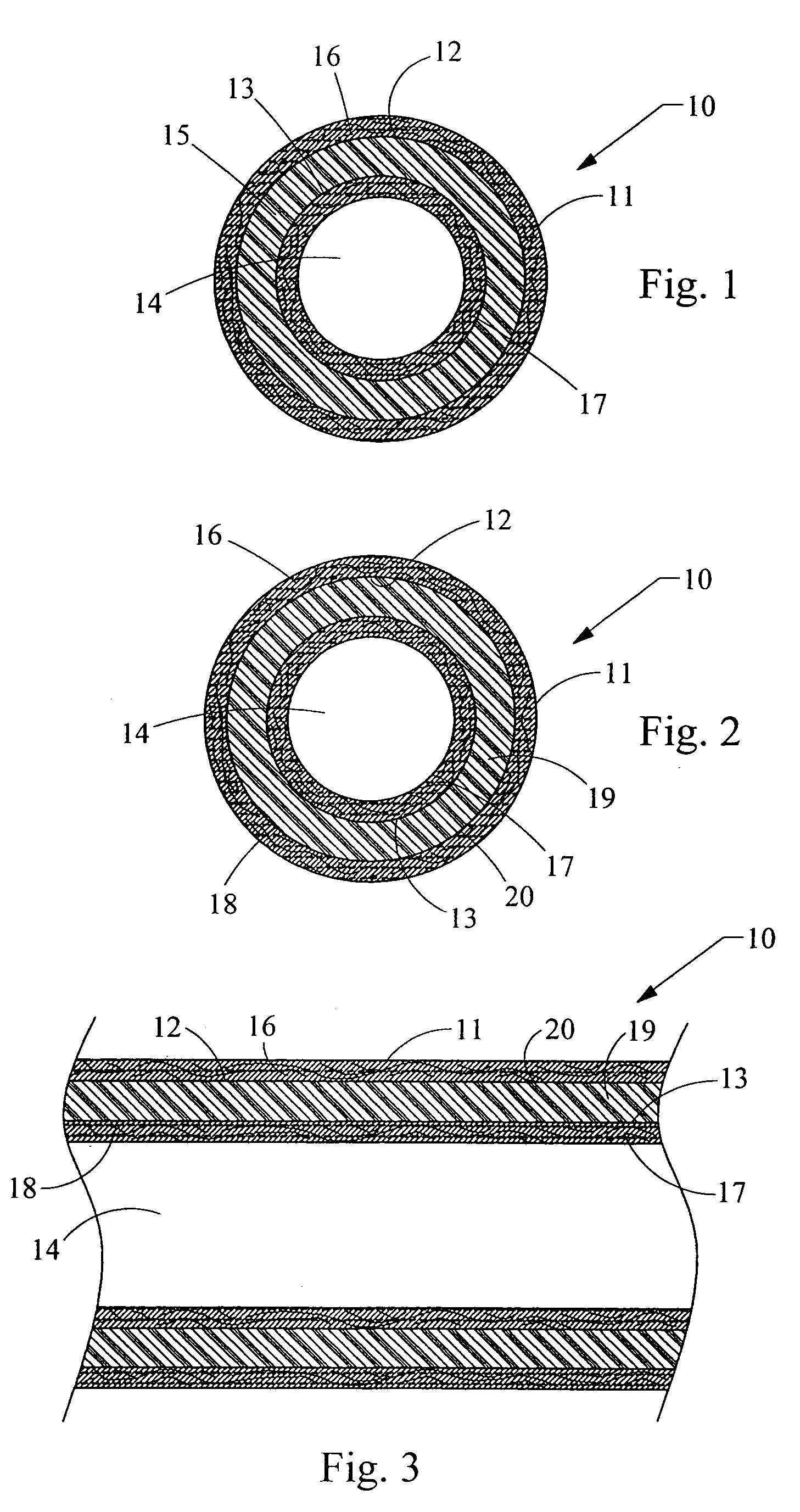
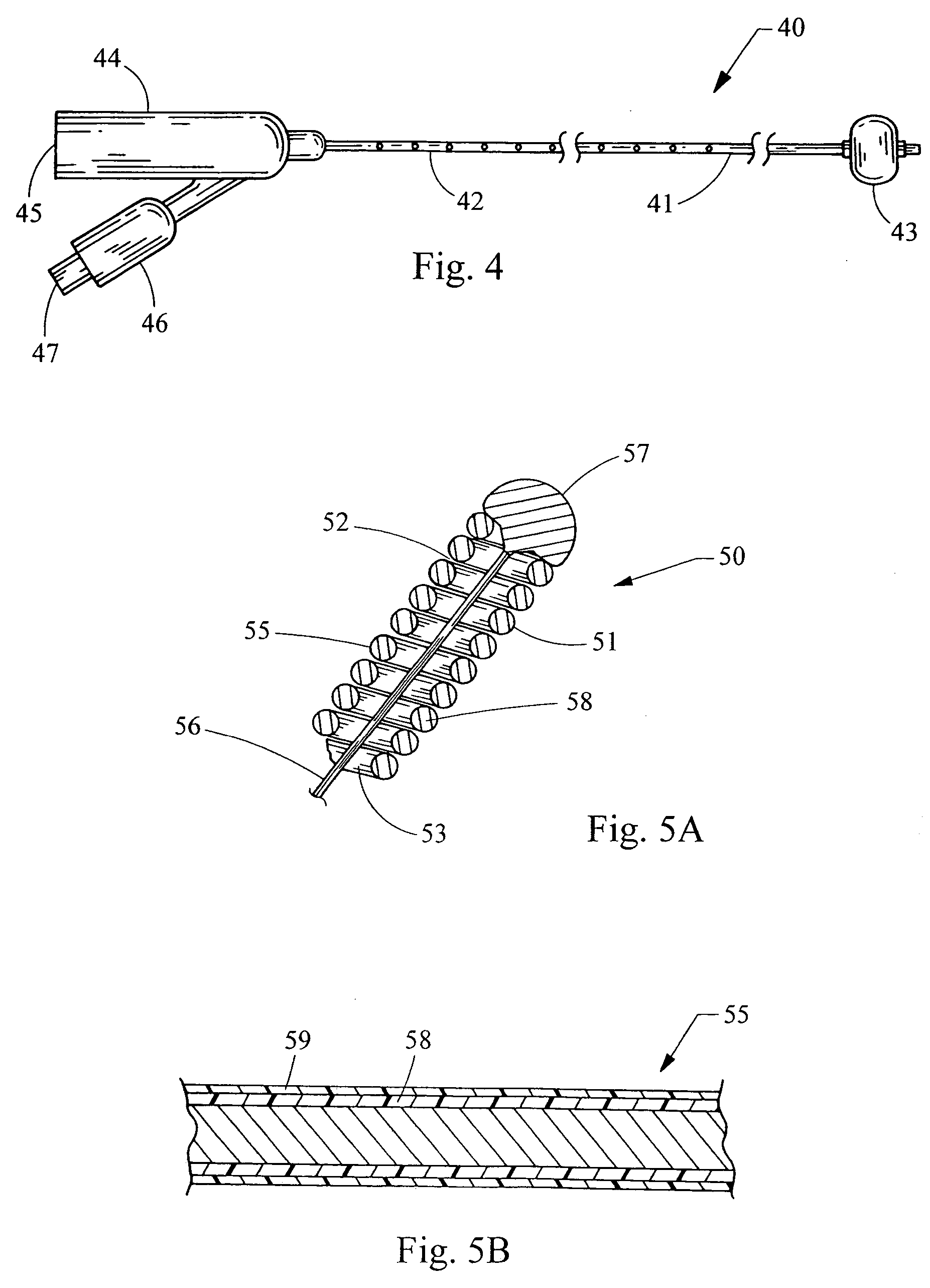
Methods for soft tissue repair and / or augmentation using injectable, biodegradable polymers are described herein. In one embodiment, the polymer compositions are liquid or pastes at room temperature. In a preferred embodiment, the polymer composition contains liquid or pasty hydroxy fatty acid-based copolyesters, polyester-anhydrides, or combinations thereof. The viscosity of the polymers increases upon contact with bodily fluid to form a solid or semisolid implant suitable for soft tissue repair and / or augmentation. In another embodiment, the polymer composition contains particles of a polymer stereocomplex. One or more active agents may be incorporated into the polymer compositions. Suitable classes of active agents include local anesthetics, anti-inflammatory agents, antibiotics, analgesics, growth factors and agents that induce and / or enhance growth of tissue within the filled cavity or control the growth of a certain type of tissue, and combinations thereof. The polymer compositions may also contain one or more additives or excipients that modify the physical and / or mechanical properties of the polymer. The polymer compositions are typically administered by injection. The injectable polymers can be used for a variety of soft tissue repair and augmentation procedures.
Owner:POLYGENE LTD
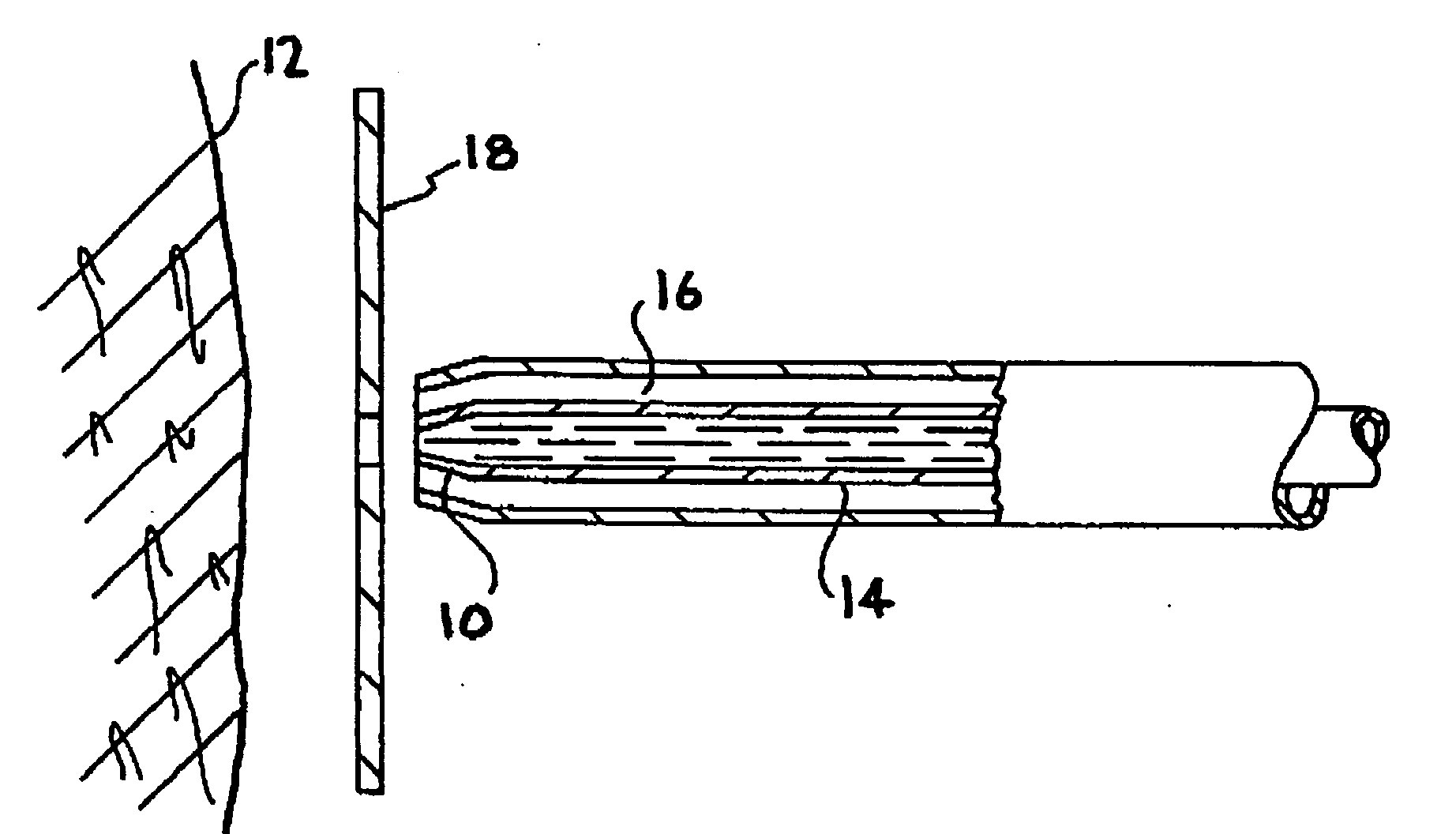
Implantable medical device with analgesic or anesthetic
An implantable medical device such as a catheter with a controlled-release outer layer including a pharmacologically active ingredient for helping to relieve pain associated with the implantation of the device. The device includes a base material with an outer bioactive material layer including, for example, an analgesic or a local anesthetic.
Owner:VANCE PROD INC D B A COOK UROLOGICAL INC
Formulations for the treatment of pain
Formulations and methods are provided for the treatment of pain, and neuropathic pain in particular. The formulations are eutectic mixtures of a capsaicinoid and a local anesthetic agent and / or an anti-pruritic agent.
Owner:ACORDA THERAPEUTICS INC
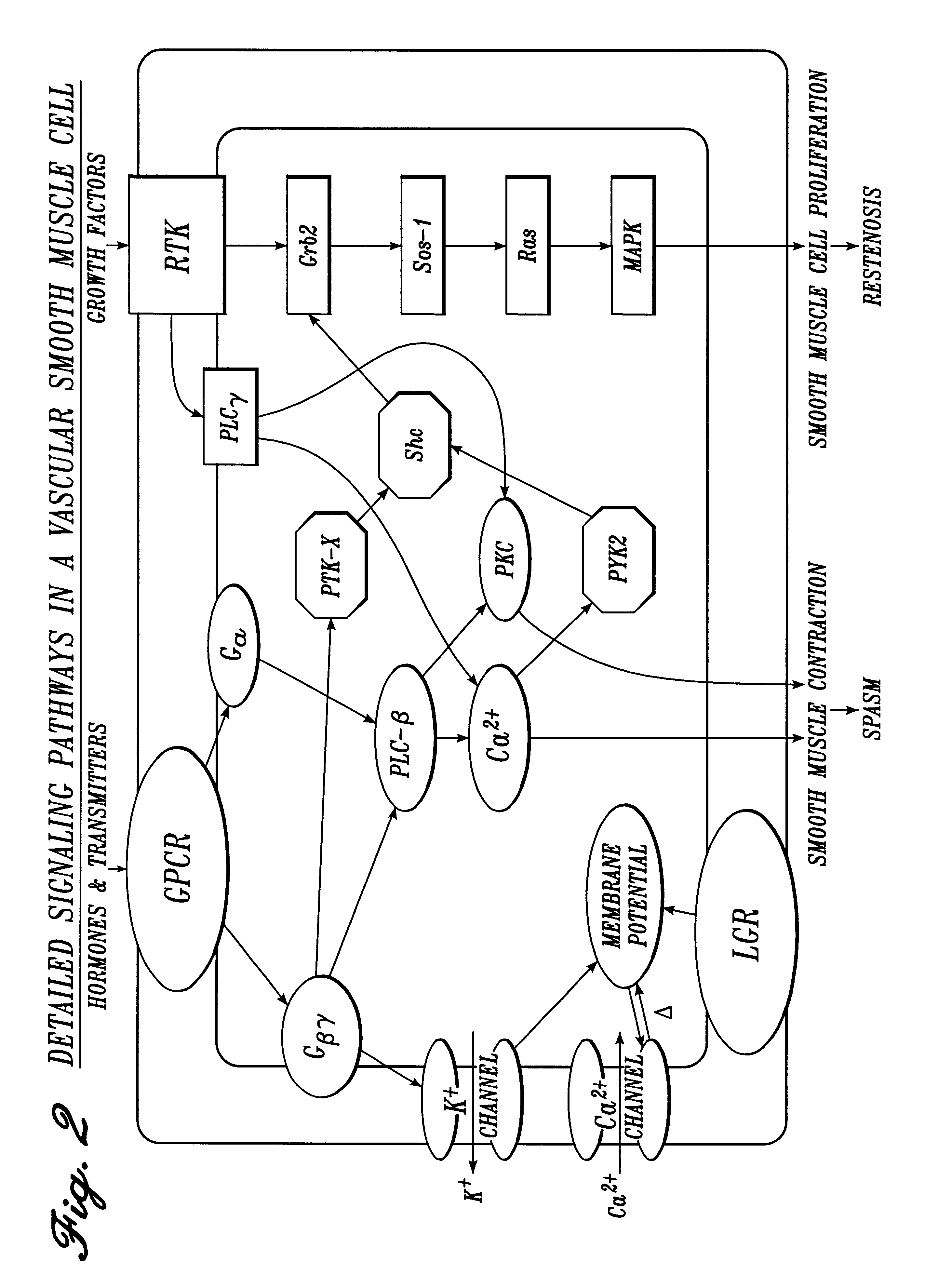
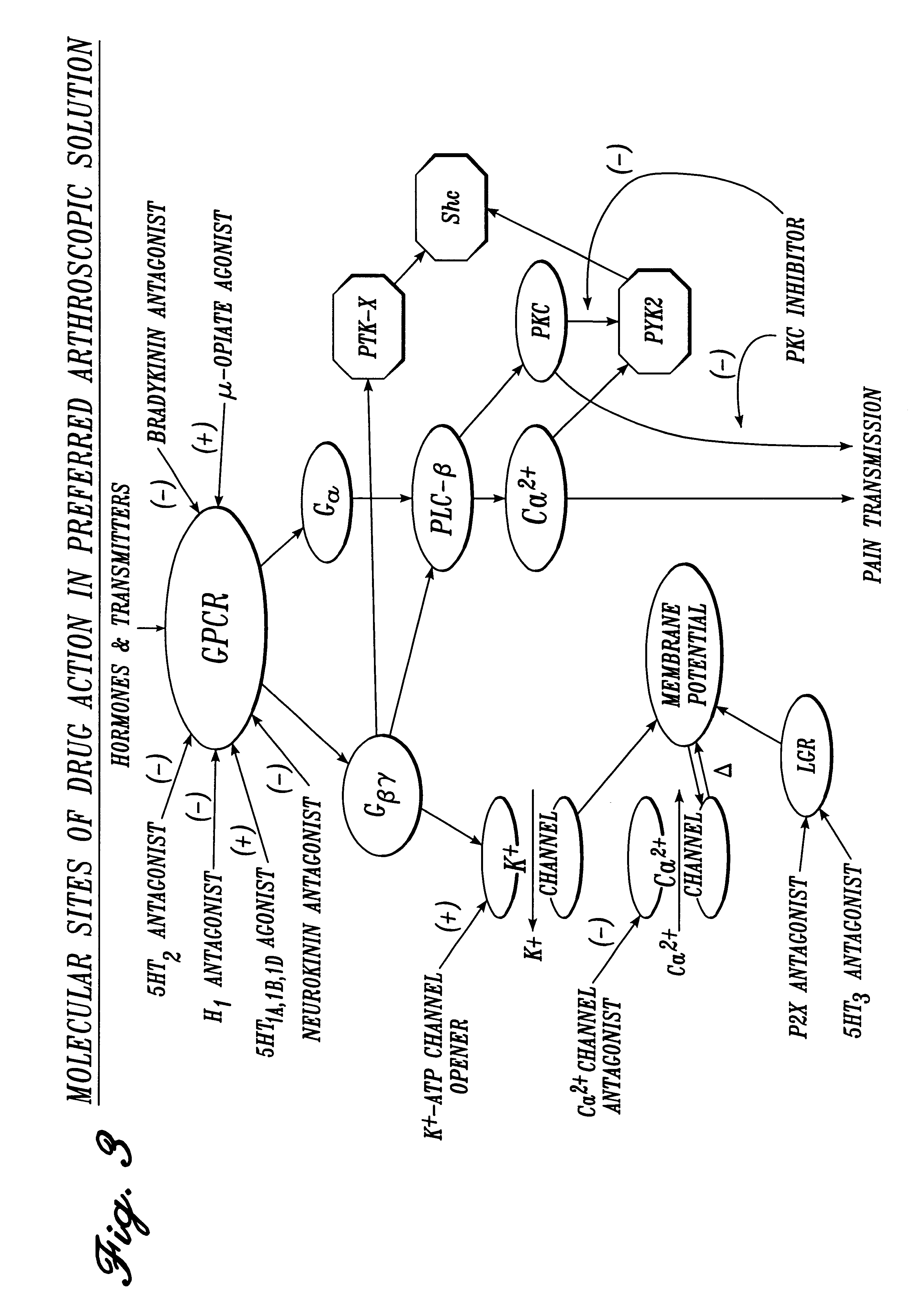
Irrigation solution and method for inhibition of pain, inflammation, spasm and restenosis
InactiveUS6413961B1Limit their usefulnessLow costBiocideNervous disorderPercent Diameter StenosisMuscle spasm
A method and solution for perioperatively inhibiting a variety of pain, inflammation, spasm and restenosis processes resulting from cardiovascular or general surgical, therapeutic and diagnostic procedures. The solution preferably includes multiple pain and inflammation inhibitory agents, including at least one local anesthetic agent, and spasm inhibitory agents at dilute concentration in a physiologic carrier, such as saline or lactated Ringer's solution. Specific preferred embodiments of the solution of the present invention for use in cardiovascular and general vascular procedures also include anti-restenosis agents.
Owner:OMEROS CORP
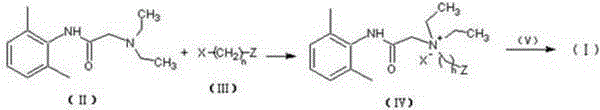
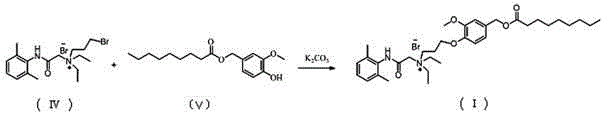
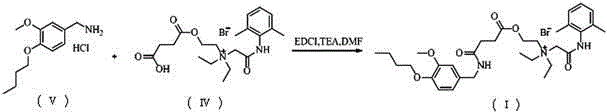
N-diethylaminoacetyl-2,6-dimethylaniline derivatives, preparation method and applications thereof
The invention provides N-diethylaminoacetyl-2,6-dimethylaniline derivatives, a preparation method and applications thereof. The structure of the derivatives is represented by the formula (I). The preparation method comprises the following steps: carrying out reactions between N-diethylaminoacetyl-2,6-dimethylaniline and halogenated compounds to obtain a corresponding quaternary ammonium salt intermediate, and then subjecting the quaternary ammonium salt intermediate to react with corresponding 2-methoxyl-4-subsituted phenol derivative raw material so as to obtain the N-diethylaminoacetyl-2,6-dimethylaniline derivatives. The derivatives can be used as a long-acting local anesthetic drug or a pain relieving drug capable of separating the motion and the feeling, can exert a reversible and lasting local anesthetic effect in organism bodies, do not affect motor function in a certain dosage range, and have an ideal retardant effect on separation of motion and feeling.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Prolonged anesthesia in joints and body spaces
InactiveUS20020054915A1Enhance and prolong local anesthesiaGood effectPeptide/protein ingredientsAnaestheticsAnesthetic AgentMicroparticle
Sustained release local anesthetic formulations are administered intra articularly and / or into body spaces / cavities. The formulation is preferably a plurality of injectable microparticles including a local anesthetic and an effective amount of a biocompatible, biodegradable, sustained release material prolonging the release of the local anesthetic and optionally and a pharmaceutically acceptable, i.e., non-toxic, augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable without the augmenting agent.
Owner:PURDUE PHARMA LP
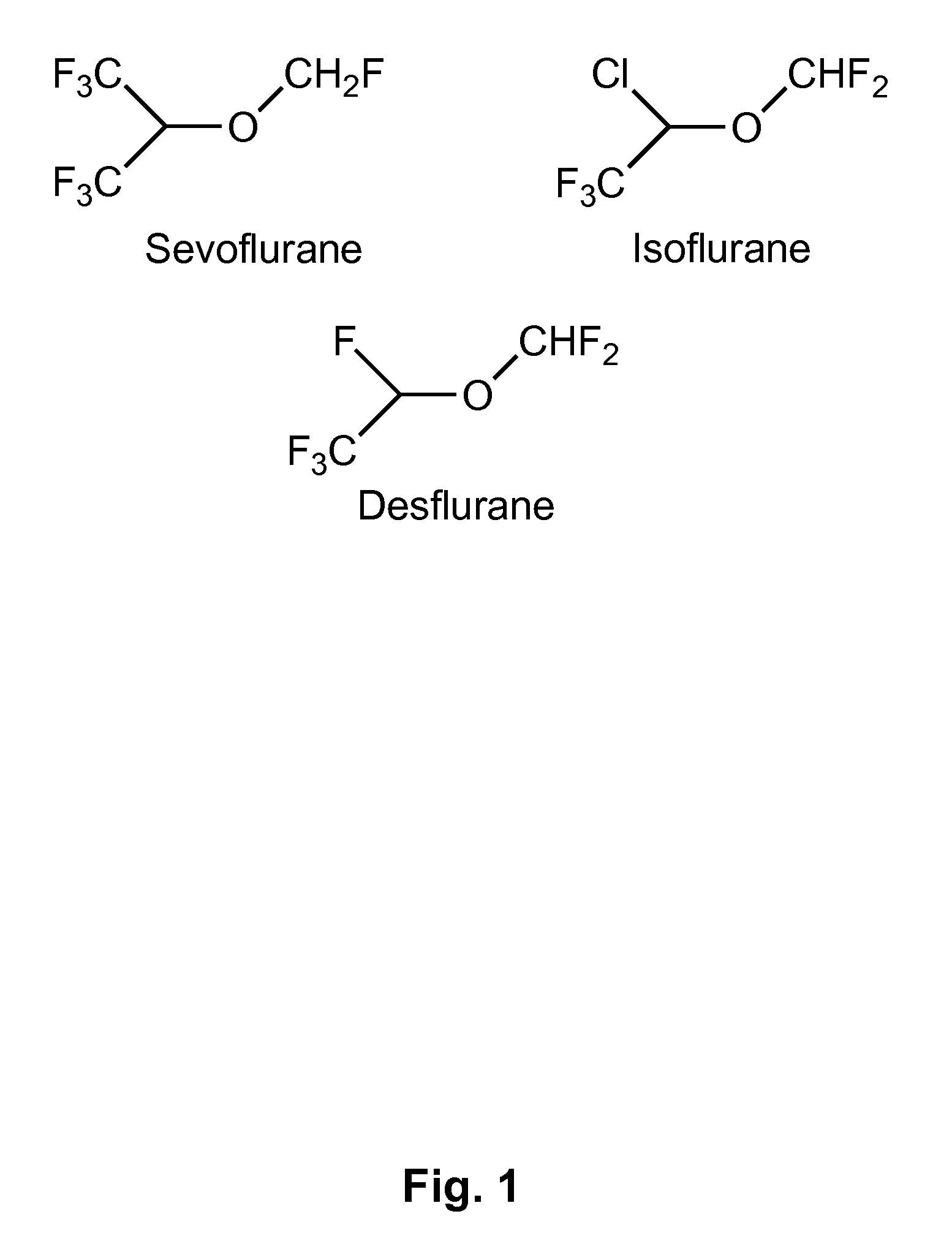
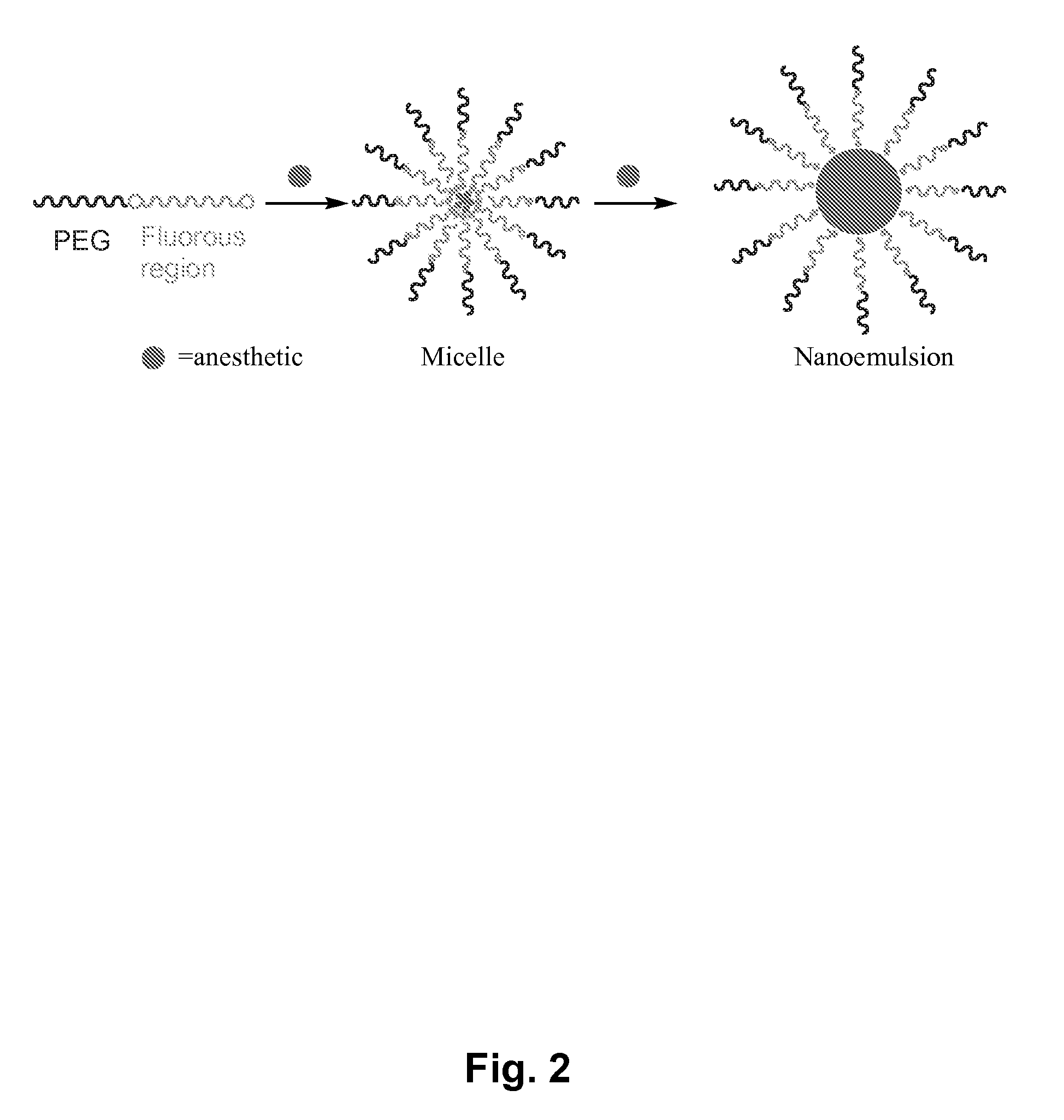
Fluoropolymer-based emulsions for the intravenous delivery of fluorinated volatile anesthetics
InactiveUS20080234389A1Maintenance can be inducedReduce interfacial tensionBiocideNervous disorderAnesthetic AgentEmulsion
The present invention provides therapeutic formulations, including therapeutic emulsions and nanoemulsions, and related methods for the delivery of fluorinated therapeutic compounds, including an important class of low boiling point perfluorinated and / or perhalogenated volatile anesthetics. Emulsion-based fluorinated volatile anesthetic formulations compatible with intravenous administration are provided that are capable of delivering and releasing amounts of fluorinated volatile anesthetic compounds effective for inducing and maintaining anesthesia in patients. Intravenous delivery of the present emulsion-based fluorinated volatile anesthetic formulations permits anesthetic levels in a patient to be selectively adjusted very rapidly and accurately without the need to hyperventilate patients and without the use of irritating agents.
Owner:WISCONSIN ALUMNI RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com